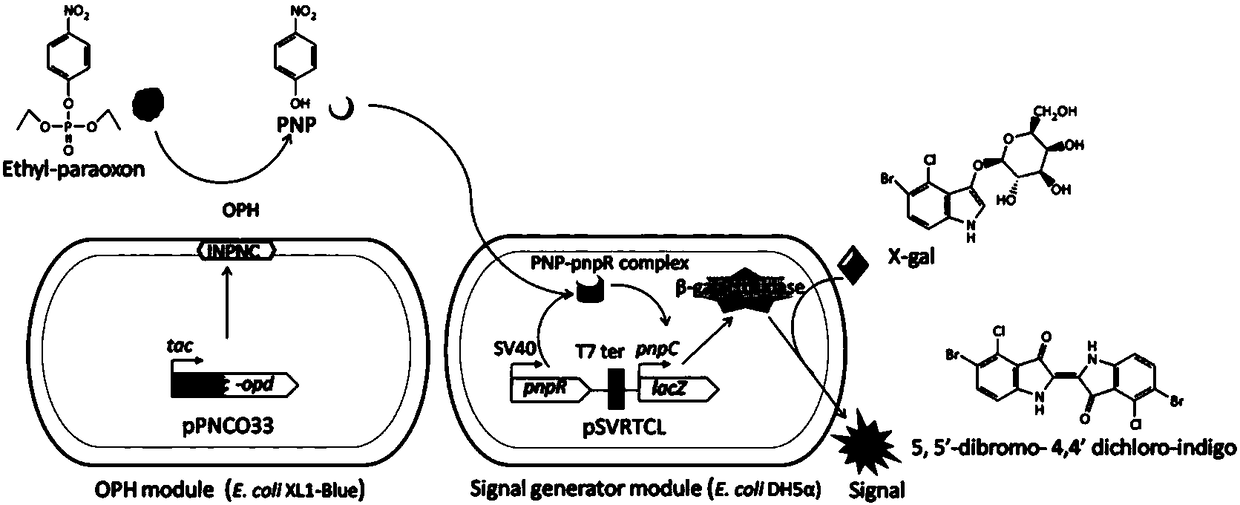
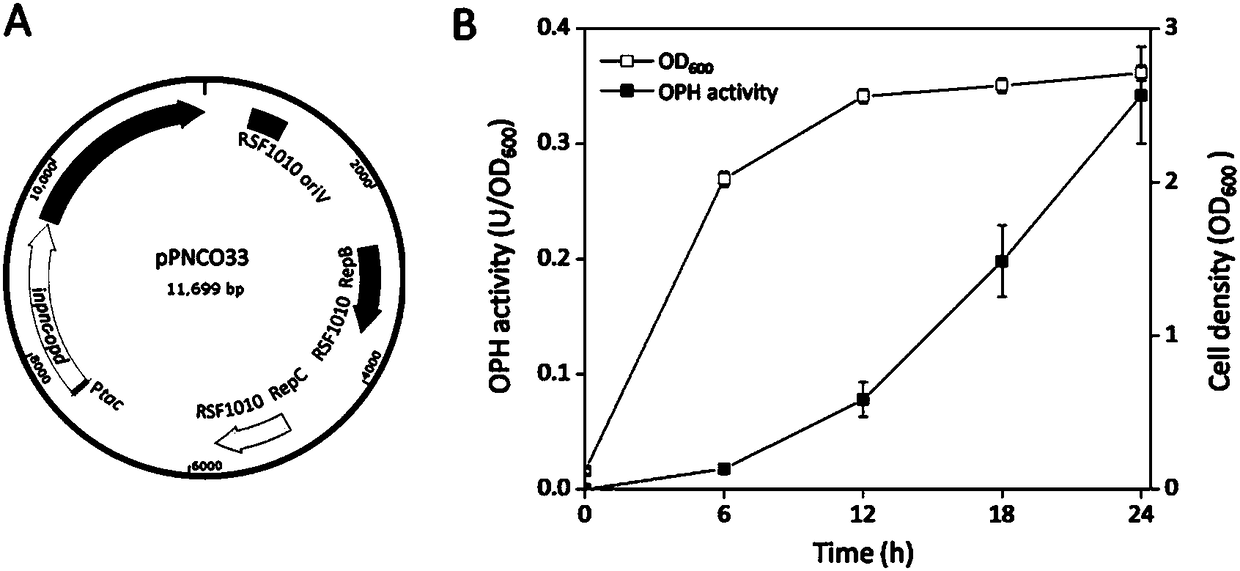
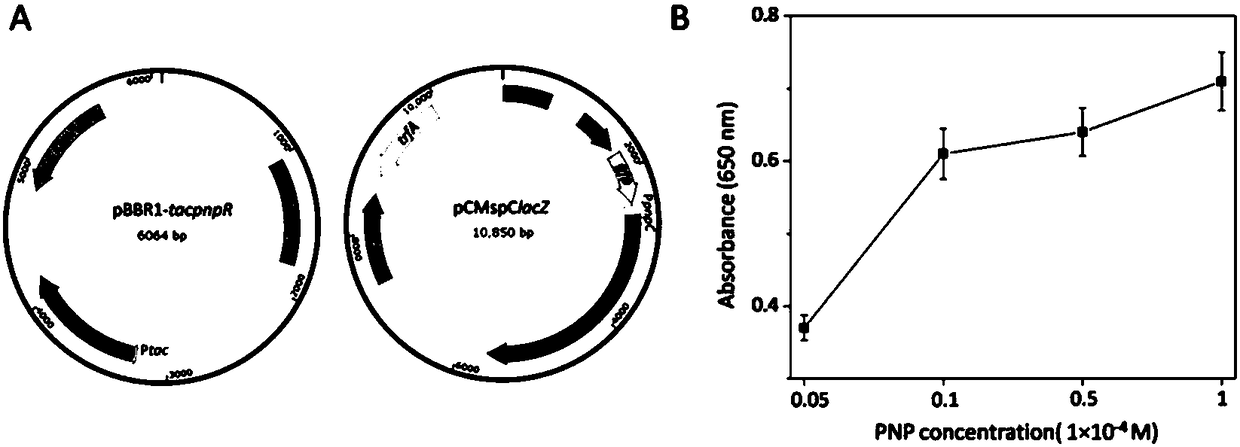
Method for detecting organophosphorus pesticide by flora-based sensing system
A technology for organophosphorus pesticides and sensing systems, which is applied in the field of detection of organophosphorus pesticides based on bacterial flora-based sensing systems. The requirements of the instrument, the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. The bacterial strains, medium and reagents used in this embodiment
[0044] Escherichia coli XL1-Blue (Clontech, USA) and Escherichia coli DH5α (Invitrogen, USA) were used for all cloning and protein expression steps. Bacteria were cultured in 2YT medium (16 g / L tryptone, 10 g / L yeast extract and 5 g / L sodium chloride).
[0045] Dissolve p-nitrophenol (PNP) and Ops (such as ethyl paraoxon, methyl paraoxon, ethyl parathion, methyl parathion, fenitrothion and EPN) in acetonitrile to prepare concentrations 1×10 -1 M stock solution.
[0046] Test chemicals were purchased from Sigma-Aldrich Company. Whatman Universal filter paper (medium speed, creped, diameter = 9.0 cm), animal tissue peptone, beef extract, gelatin, L-sodium ascorbate, D-(+)-sodium pentafluoride, L-glutamic acid monosodium salt Hydrate, ampicillin, kanamycin, tetracycline and anhydrous N,N-dimethylformamide (DMF) were purchased from Promega (Madison, WI, USA). HPLC grade acetonitrile, sodium chlorid...
Embodiment 2
[0074] Embodiment 2, the preparation of filter paper sensing strip
[0075] Sensing paper strips were prepared according to the aforementioned flora-based biosensing system. Briefly, the two cell types were suspended in a 1:1 ratio in sterile dry protection solution pre-warmed at 37°C. 5 μL of the cell suspension was spotted on Whatman filter paper strips (0.6×4 cm), and the strips were dried in a laminar air oven for 10 minutes, followed by vacuum drying at 20° C. in a lyophilizer. The filter paper sensor strips were then stored at 4°C until use.
Embodiment 3
[0076] Embodiment 3, the mensuration of the dose-response OPs curve on the filter paper sensing strip
[0077] Dilute ethyl paraoxon (concentration of 1×10- 3 -1×10 -9 M). Take 100 μL of each standard solution and add to the culture tube containing 900 μL 2YT medium and prepared filter paper strips. The culture tubes were then incubated statically at 28°C for 2 hours. The paper strips were then removed from the culture tubes and kept between layers of plastic wrap to prevent drying. Subsequently, 10 μL of X-gal substrate solution (50 mg / mL) dissolved in DMF was carefully added to the site containing the sensor cells, and color development was allowed to proceed for 90 minutes. Such as Figure 7 As shown in A, the cell-retained area of the paper strip changes from colorless to blue as the concentration of ethyl paraoxon increases.
[0078] In addition to visual observation of the developed blue color, color intensity was also measured using ImageJ software (NIH, Bethesd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com