Preparation method of photo-crosslinked chitosan-methacrylic acid nanoparticles
A technology of methacrylic acid and methacrylic anhydride, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problem of unsuitable encapsulation of bioactive macromolecules and chemical crosslinking. To solve the problems of drug cytotoxicity, enhance mucous membrane adhesion, etc., achieve good biocompatibility and degradability, protect protein, and promote absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis and characterization of chitosan-methacrylic acid
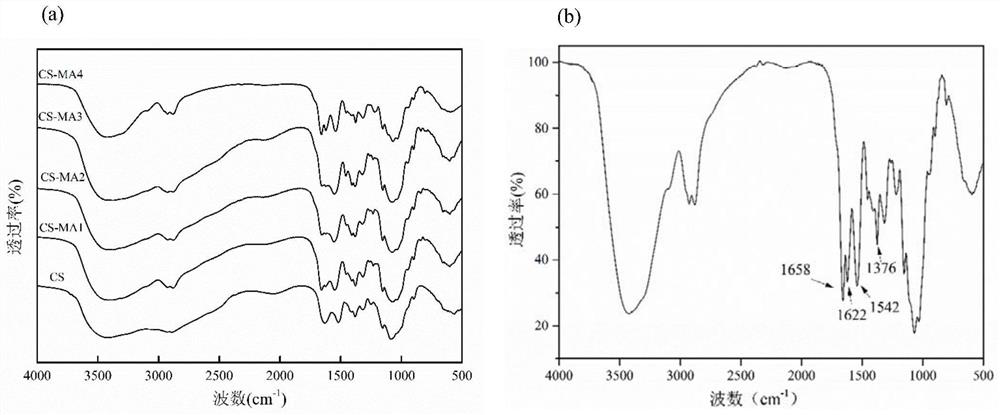
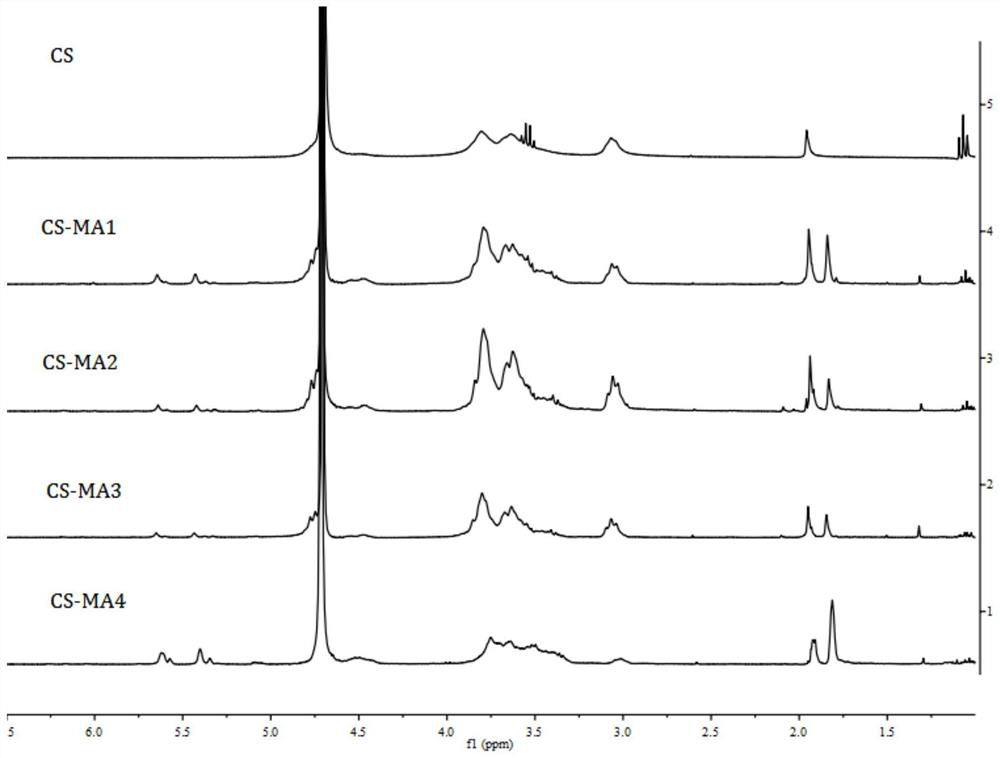
[0044] Synthesis steps: Weigh 0.5g of chitosan hydrochloride and dissolve them in 25mL of deionized water, stir evenly to obtain a clear chitosan solution, add 2-methacrylic acid drop by drop to the chitosan solution under strong stirring Anhydride, dark stirring reaction 24h under different conditions, as shown in table 1, 2-methacrylic anhydride in reaction conditions 1,2,3 and 4 and chitosan sugar unit mol ratio and temperature of reaction are respectively: 0.4: 1 and 25°C, 1:1 and 25°C, 5:1 and 25°C, 5:1 and 60°C, the resulting reaction products are sequentially recorded as CS-MA1, CS-MA2, CS-MA3, CS-MA4, and the reaction is over Finally, the reaction solution was dialyzed in ultrapure water for 3-4 days until excess 2-methacrylic anhydride in the reaction solution was removed, and the dialysate was freeze-dried to obtain a white flocculent solid.
[0045] Infrared spectrum: Take 2 mg o...
Embodiment 2
[0053] Embodiment 2: the ultraviolet light cross-linking process of chitosan-methacrylic acid
[0054] Photocrosslinking process: Dissolve 10 mg of chitosan-methacrylic acid CS-MA1, 2, 3, 4 in 1 mL of 1% acetic acid (v / v), add 0.05% (w / v) photoinitiator Irgacure1173 ( 2-hydroxyl-2-methylpropiophenone), stir evenly, add 0.1M sodium hydroxide solution to adjust the pH of the solution=7, configure the chitosan-methacrylic acid solution that the concentration is 1% (w / v), UV light irradiation for 1min, 5min, 10min, 20min, 30min, 60min.
[0055] Discussion of the results: CS-MA1, CS-MA2, and CS-MA3 showed a partial gel state after 60 minutes of irradiation, but they were still able to flow and did not form a complete gel, while CS-MA4 completely gelled after 1 minute of irradiation, forming Gel, not flowable. The reason is that the number of double bonds in 1% CS-MA1, CS-MA2, and CS-MA3 solutions is too low to form a dense cross-linked network, so CS-MA4 with a high degree of sub...
Embodiment 3
[0056] Embodiment 3: the preparation method of nanoparticle and particle size distribution, microscopic morphology
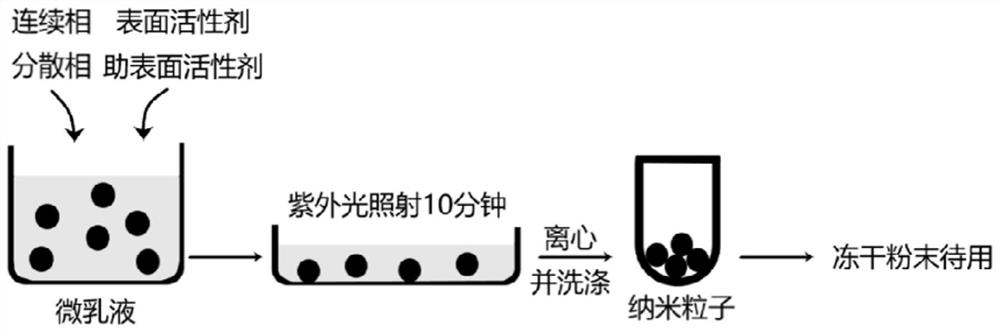
[0057] (1) Preparation method: Dissolve 10 mg chitosan-methacrylic acid CS-MA4 in 1 mL of 1% acetic acid (v / v), add 0.05% (w / v) photoinitiator Irgacure1173 (2-hydroxy-2 -methyl propiophenone), stir evenly, add 0.1M sodium hydroxide solution to adjust pH=7 of CS-MA4 solution, and prepare chitosan-methacrylic acid solution whose concentration is 1% (w / v). The chitosan-methacrylic acid solution of 1% (w / v) of 1mL is dripped in the mixed solvent of 2.75mL cyclohexane and 1mL n-hexanol, then adds surfactant Triton X-100 dropwise, until The solution becomes clear and transparent, that is, stop adding the surfactant to form a W / O microemulsion, irradiate the microemulsion under a UV lamp for 10 minutes, add ethanol to destroy the emulsion until the emulsion becomes turbid, and centrifuge at 6000 rpm for 10 minutes to obtain white nanoparticles. The nanoparticles were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com