Preparation method of silibinin nano-cage type nanocrystalline injection
A technology of silibinin and nanocrystals, which is applied in the field of medicine, can solve the problems of difficulty in exerting drug effects, increasing the risk of liver and spleen damage, and weakening the treatment effect, so as to improve relative bioavailability, increase average residence time, prolong The effect of blood circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Formula and preparation method of silibinin nanocage nanocrystal injection of the present invention
[0032] Formula and preparation of silibinin nanocage nanocrystal injection: Weigh 40mg of silibinin and dissolve it in 10ml of methanol, stir magnetically at room temperature until completely dissolved to obtain 4mg ml -1 The methanol solution of silibinin was used as the solvent phase. Weigh 40mg PEG-CS dissolved in 50ml ultrapure water as the anti-solvent phase. Use a syringe with a microporous filter (0.45 μm) to draw 4 ml of the anti-solvent phase solution and inject it into the vial, and in addition take a syringe with a microporous filter (0.45 μm) to draw 1 ml of silibinin methanol solution and quickly inject it into the bottle containing In the vial of the anti-solvent phase, after magnetic stirring for 1 min, add 0.8 mg PEG-PCL carrier into the solution, then add copper sulfate and sodium ascorbate in sequence, after standing for 20 min, ultraso...
Embodiment 2
[0034] Example 2 The process and preparation performance detection test of silibinin nanocage nanocrystal injection
[0035] (1) Repeated inspection
[0036] Three batches of silibinin nanocage nanocrystal injection were prepared under the prescription process conditions of Example 1, and the average particle size, particle size distribution (PDI) and Zeta potential were measured respectively, and the results are shown in Table 1. The optimized nanocrystal formulation process conditions have good repeatability.
[0037] Table 1 Repeatability investigation of silibinin nanocage nanocrystal injection process
[0038] batch
Average particle size (nm)
PDI
Zeta potential (mV)
1
126.34
0.114
-26.81
2
132.51
0.125
-27.54
3
121.64
0.119
-29.96
RSD(%)
4.30
4.62
5.87
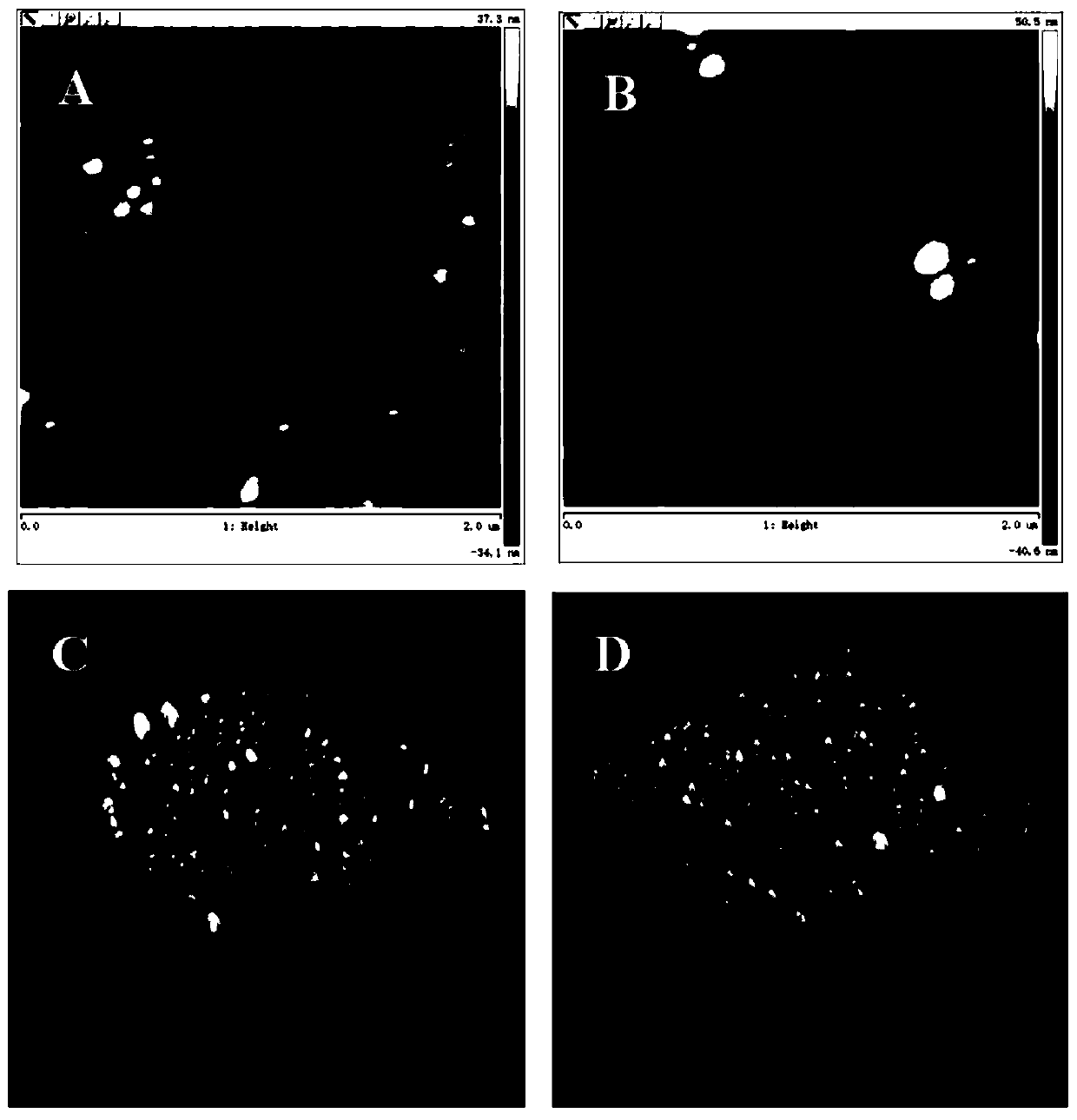
[0039] (2) Morphological analysis
[0040] Select two mica sheets, stick the surface layer off with adhesive tape, then drop the ...
Embodiment 3
[0043] Example 3 Preliminary stability study of silibinin nanocage nanocrystal injection
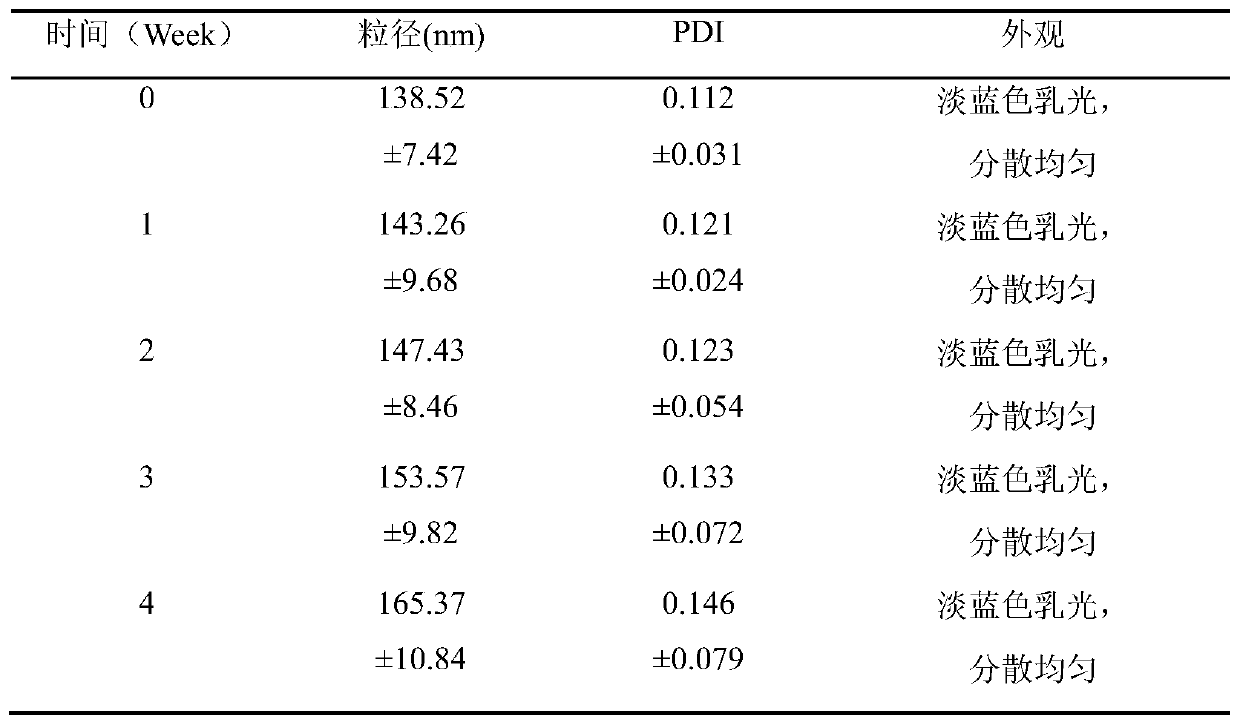
[0044] In order to investigate the stability of the liquid preparation - silibinin nanocrystals, the silibinin nanocage nanocrystal injection prepared in Example 1 was stored at different temperatures (4°C and 25°C) for one month, and laser The nano particle size analyzer takes samples at 1, 2, 3, and 4 weeks respectively, observes the appearance change and measures the particle size and PDI. Each sample is measured 3 times, and the average value is calculated and the data is recorded.
[0045] Table 2 Stability test of silibinin nanocage nanocrystal injection at 4°C
[0046]
[0047] Table 3 Stability test of silibinin nanocage nanocrystal injection at 25°C
[0048]
[0049]
[0050] According to the test results, the silibinin nanocage nanocrystal injection maintained good stability under the storage condition of 4°C; precipitation. It can be concluded that the silibinin ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com