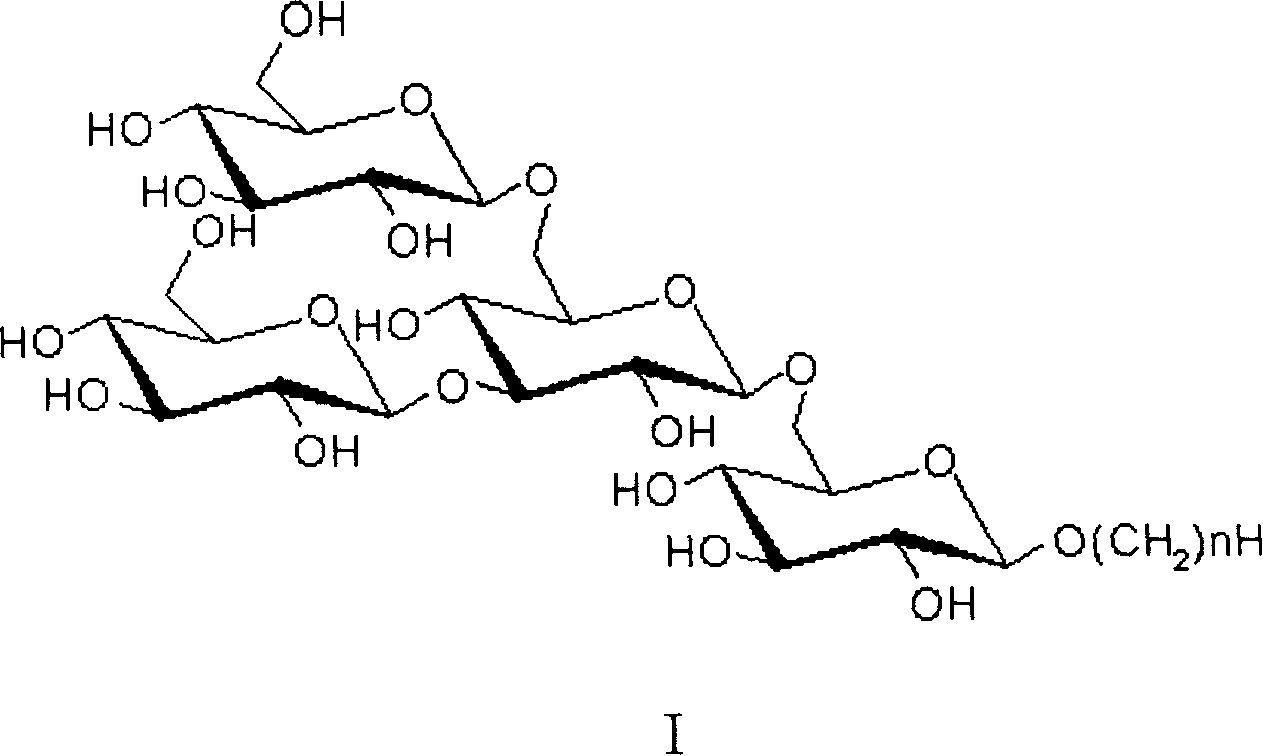
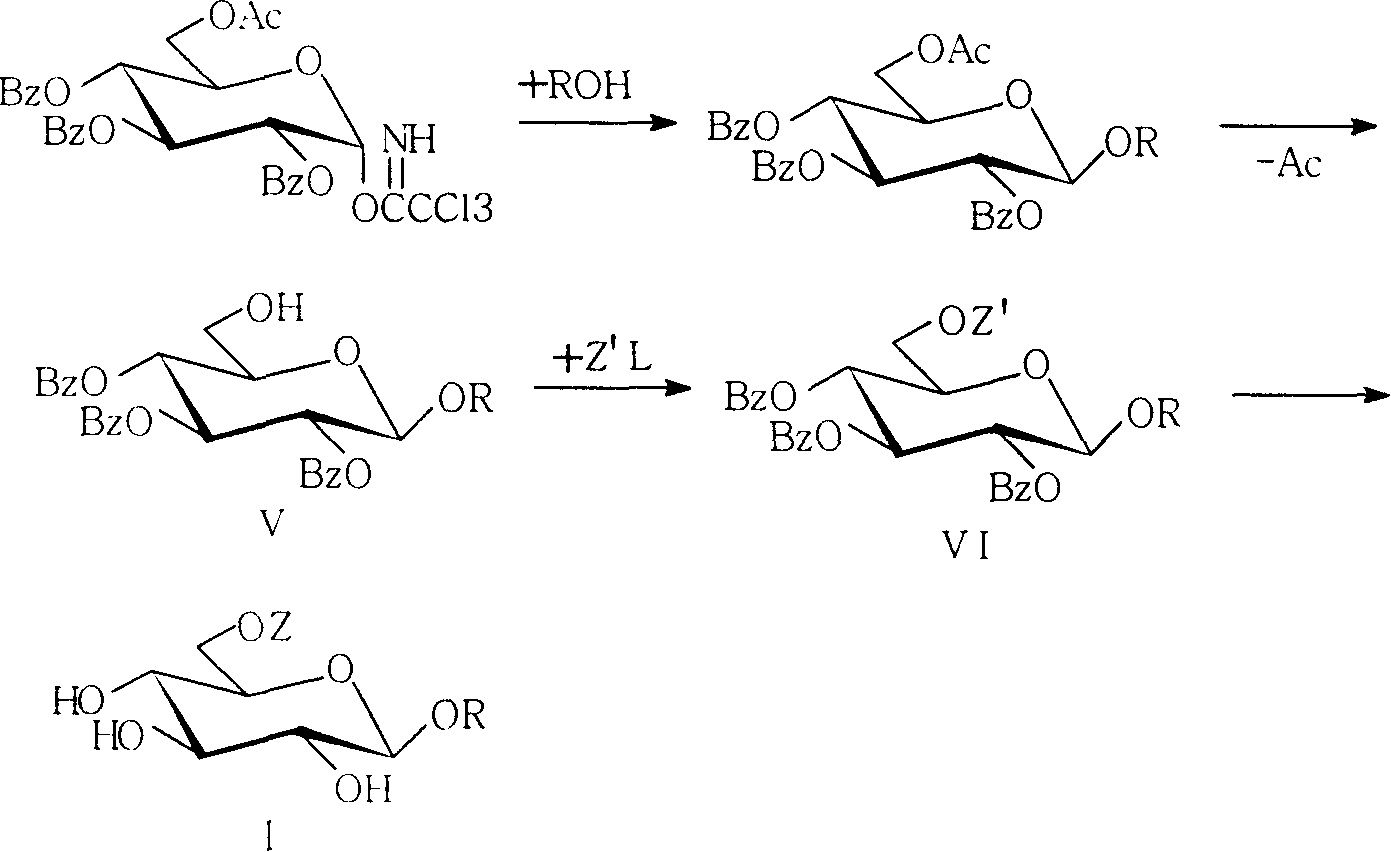
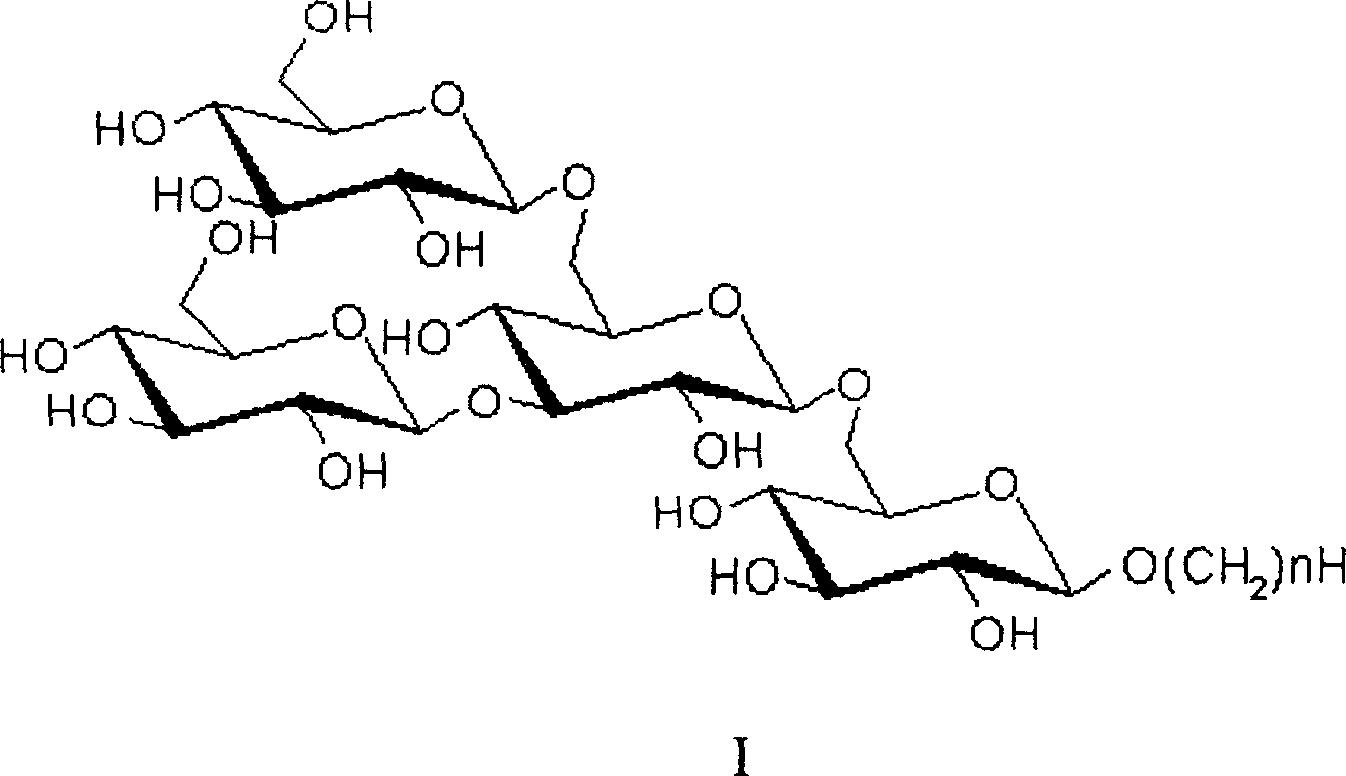
Active dextran tetra saccharide alkyl glycoside and its prepn process and application
A technology of glutrans alkyl glycosides and compounds, which is applied in the field of glucan tetrasaccharide alkyl glycoside compounds, can solve the problems of increasing the difficulty of patient recovery, damaging the vitality of patients, killing tumor cells, etc., and achieving simple structure and high quality. The effect of controllability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of tetrasaccharide II
[0023] 1. Preparation of monosaccharide receptor 3
[0024]
[0025] (1), preparation of monosaccharide donor 1
[0026] In the reactor, add D-glucose (3.60g, 20.0mmol), trityl chloride (6.69g, 24.0mmol) and 40ml of pyridine, heat to 70°C, stir for 8 hours, cool to 0°C, add benzene Formyl chloride (10.3mL, 88mmol), stirred at room temperature for 24 hours, added 100ml of dichloromethane, followed by water, 1N HCl, saturated NaHCO 3 / H 2 O wash, anhydrous Na 2 SO 4 Dry, spin dry, add 100ml of dichloromethane, 100ml of methanol, 0.2ml of acetyl chloride, stir at room temperature for 1 hour, neutralize with triethylamine, evaporate the solvent, add 40ml of pyridine, 30ml of acetic anhydride, and stir for 4 hours at room temperature. Spin to dry, add 90ml of tetrahydrofuran, 60ml of methanol, saturate with ammonia, and stir for 4 hours. Spin to dry, add 50ml of dichloromethane, K 2 CO 3 3g, 3ml of trichloroacetonitrile, reacted...
Embodiment 2
[0037] Preparation of tetrasaccharide III
[0038] 1. Preparation of monosaccharide receptor 7
[0039]
[0040] In the reactor, add 1 (1.29g, 1.9mmol), dodecanol (0.50g, 2.7mmol), dichloromethane 20ml, trimethylsilyl trifluoromethanesulfonate (TMSOTF) 30μl, and stir at room temperature to react 2 After hours, it was neutralized with triethylamine, concentrated and purified on a silica gel column, using petroleum ether / ethyl acetate (5 / 1) as eluent, and the corresponding components were collected to obtain 6 (1.11 g), with a yield of 83%.
[0041] In the reactor, add 6 (0.98g, 1.4mmol), 2ml of dichloromethane, 40ml of anhydrous methanol, and 0.2ml of acetyl chloride, leave it at room temperature for 5 hours, neutralize with triethylamine, distill off the solvent, and dissolve the sample in dichloromethane Purify on a silica gel column, use petroleum ether / ethyl acetate (3 / 1) as eluent, collect the corresponding components to obtain 7 (0.80g), yield 86%, [α] D -9.8° (c1.0,...
Embodiment 3
[0047] Preparation of tetrasaccharide IV
[0048] 1. Preparation of monosaccharide receptor 10
[0049]
[0050] In the reactor, add 1 (1.29g, 1.9mmol), cetyl alcohol (0.61g, 2.5mmol), dichloromethane 20ml, boron trifluoride-diethyl ether 0.5ml, stir at room temperature for 2 hours, and use triethylamine After neutralization and concentration, it was refined on a silica gel column, using petroleum ether / ethyl acetate (6 / 1) as eluent, and the corresponding components were collected to obtain 9 (1.20 g), with a yield of 83%.
[0051] In the reactor, add 9 (1.06g, 1.4mmol), 2ml of dichloromethane, 40ml of anhydrous methanol, and 0.2ml of acetyl chloride, leave it at room temperature for 5 hours, neutralize with triethylamine, distill off the solvent, and dissolve the sample in dichloromethane Purify on a silica gel column, use petroleum ether / ethyl acetate (4 / 1) as eluent, collect the corresponding components to obtain 10 (0.90g), yield 90%, [α] D .-2.0° (c1.0, CHCl 3 ); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com