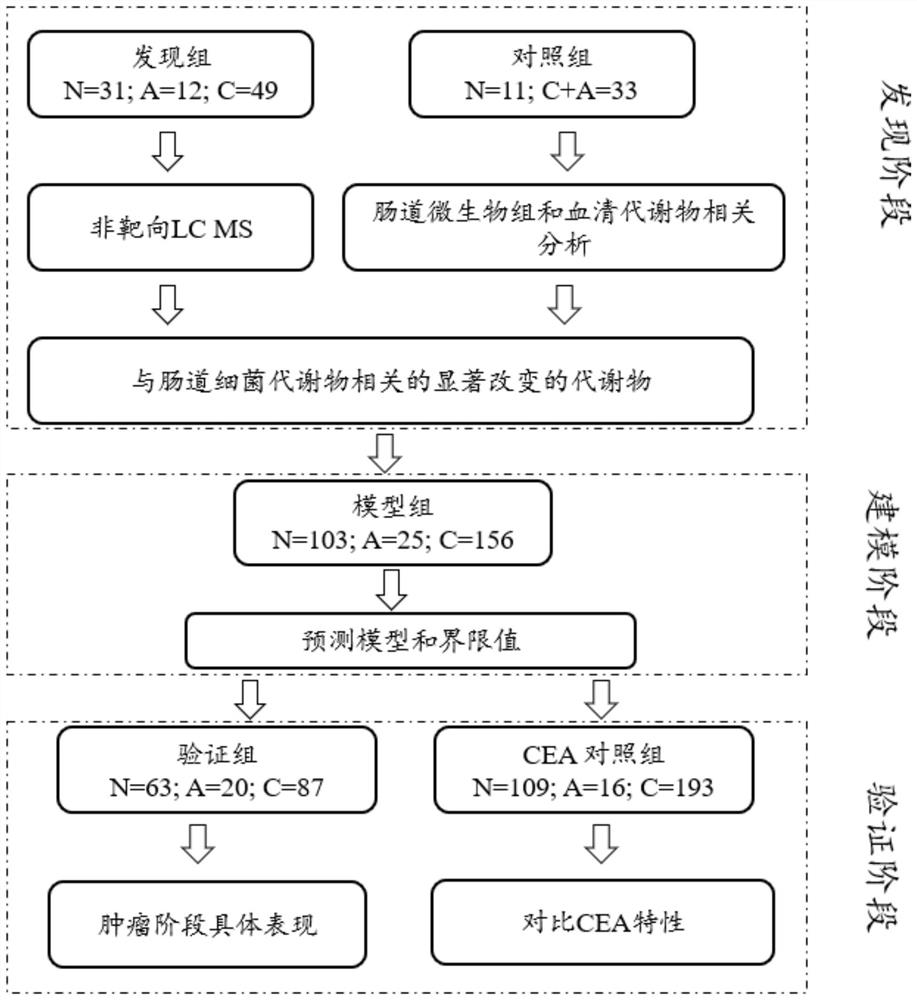
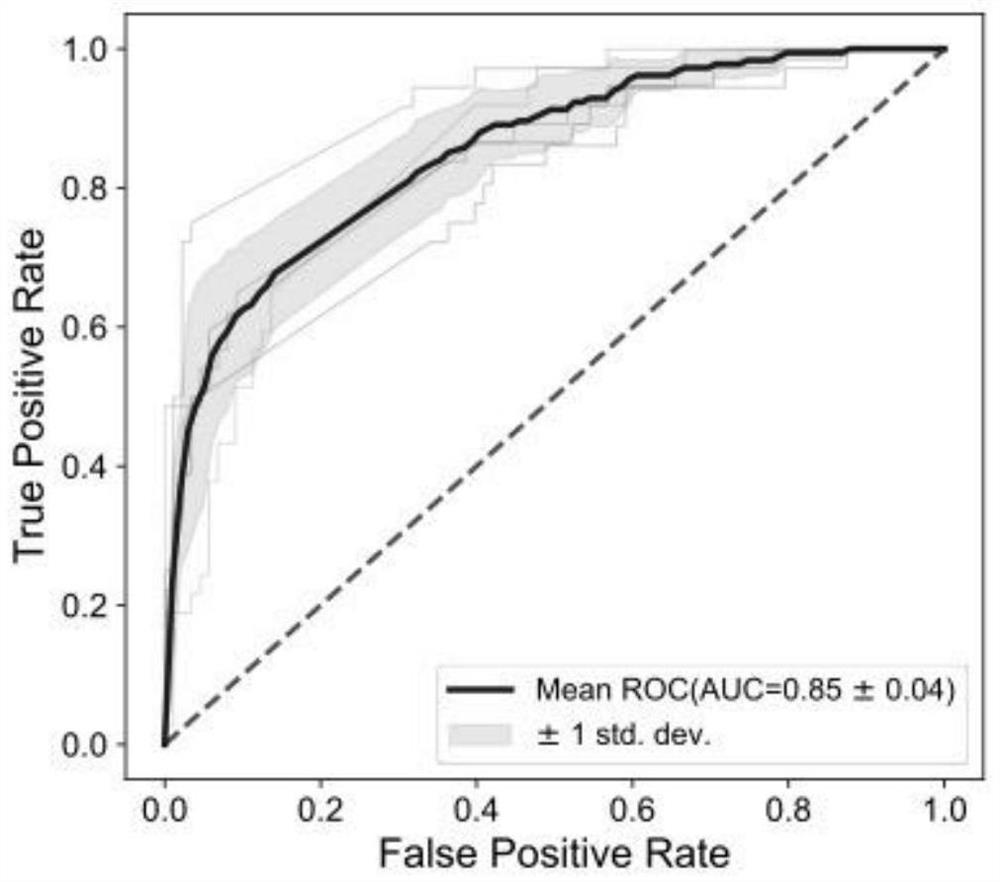
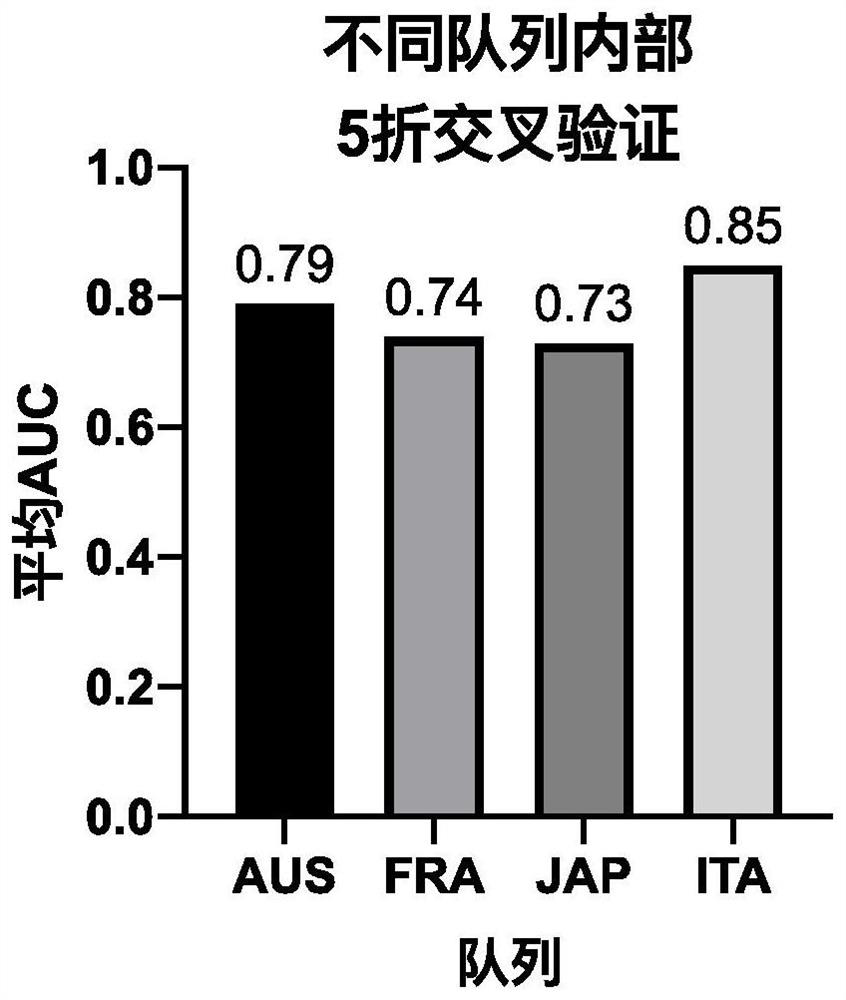
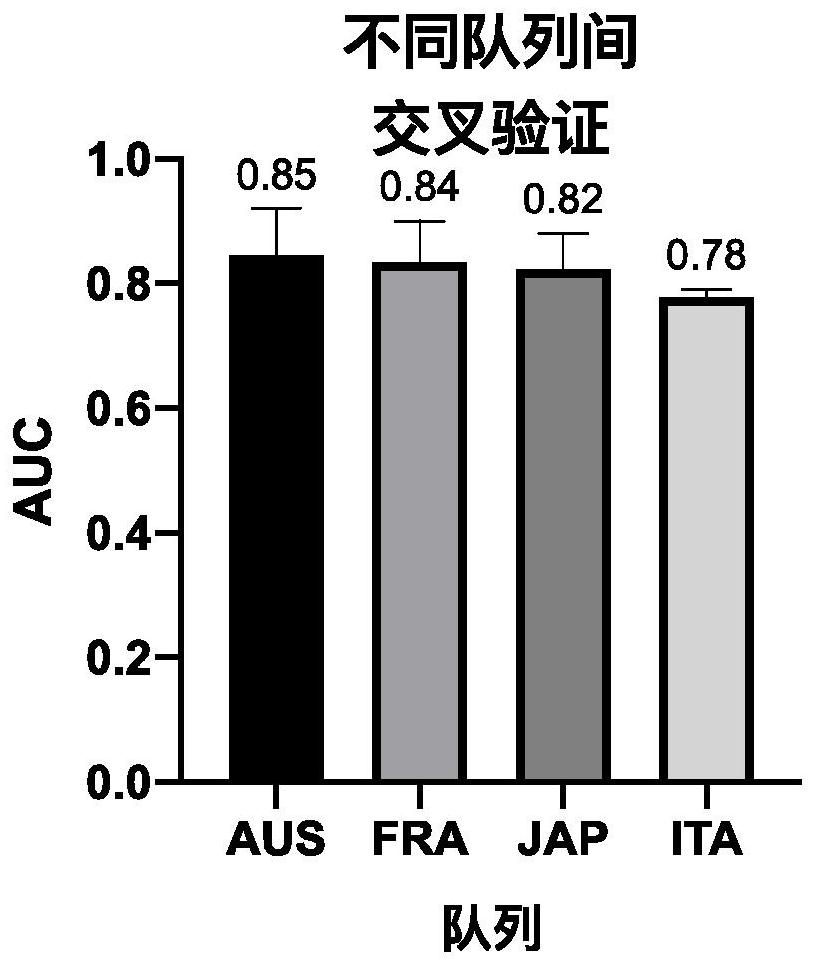
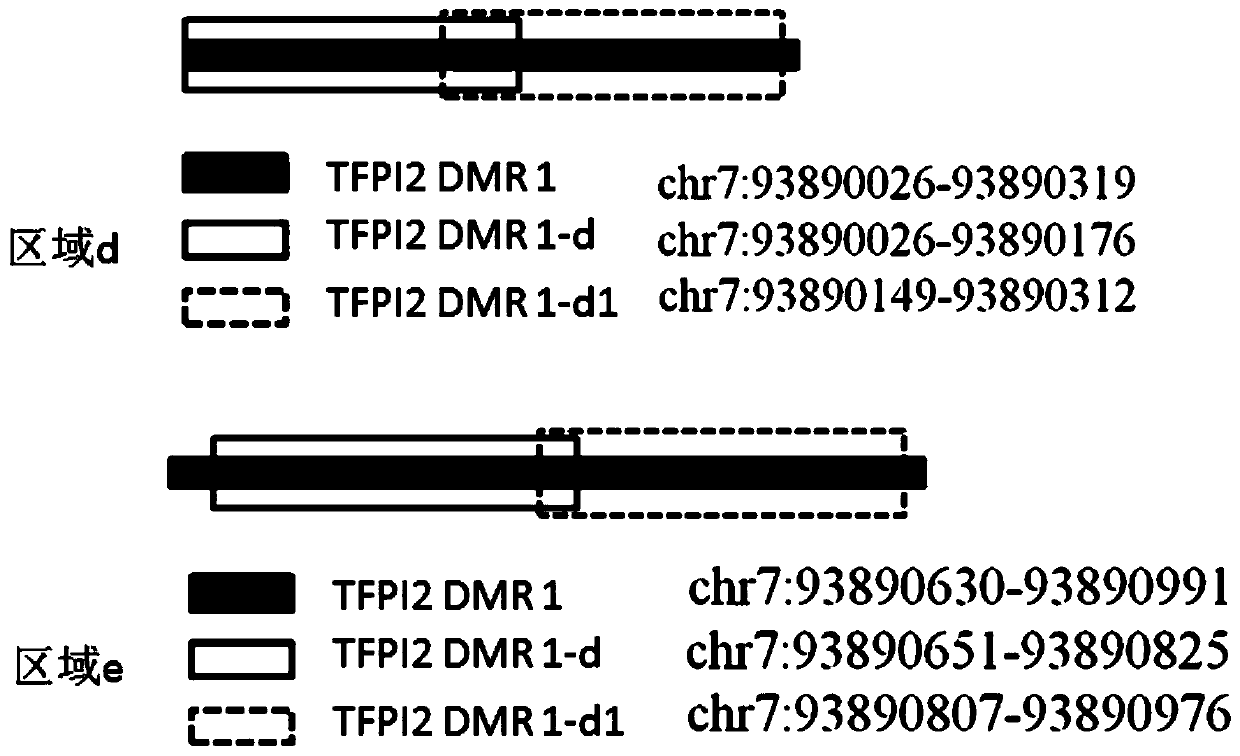
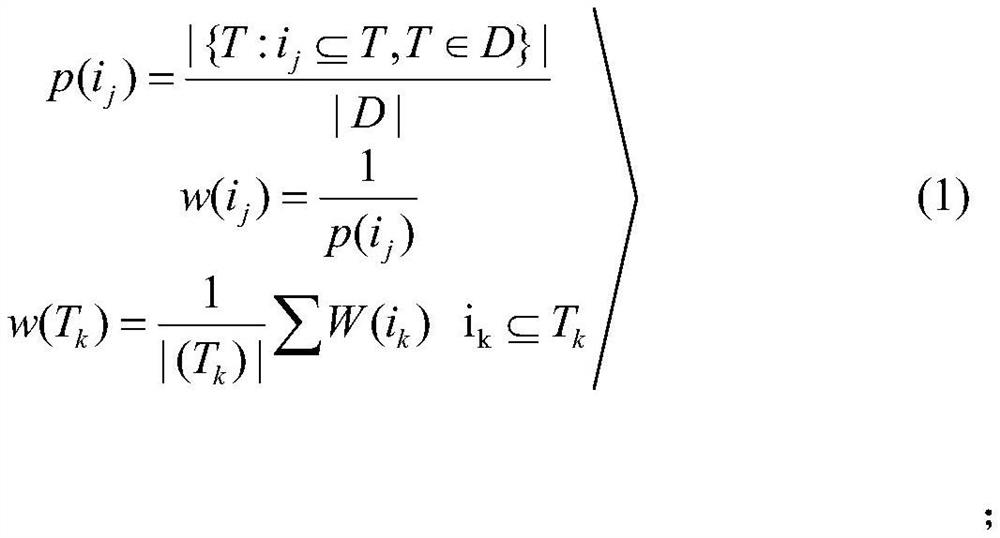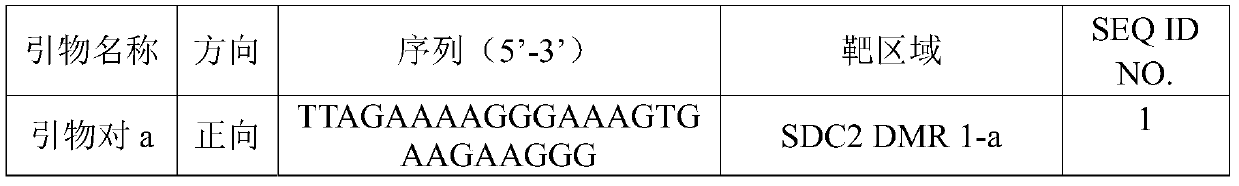Patents
Literature
30 results about "Rectal adenoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The colorectal adenoma is a benign glandular tumor of the colon and the rectum. It is a precursor lesion of the colorectal adenocarcinoma ( colon cancer ). [1] [2] [3]
Bile preparations for colorectal disorders
The present disclosure relates to methods and compositions to ameliorate or treat at least one symptom of colorectal cancer and / or adenomatous polyposis coli (APC). For example, some embodiments of the methods and compositions may reduce recurrence of colorectal adenomas and / or extend the life of a subject having colorectal cancer and / or APC. Some embodiments of the disclosure include maintaining a the total body weight in a subject having colorectal cancer and / or APC. According to some embodiments, a method of the disclosure may include administering a bile acid composition to a subject. A bile acid composition may include, in some embodiments, an aqueous solution that is free or substantially free of precipitates or particles. A aqueous solution may include (1) a bile acid, an aqueous soluble derivative of a bile acid, a bile acid salt, and / or 7-ketolithocholic acid, (2) a carbohydrate, and (3) water. An aqueous composition may further include an alkali.
Owner:柳署弘
Leveraging sequence-based fecal microbial community survey data to identify composite biomarker for colorectal cancer
PendingCN110637097AMicrobiological testing/measurementSequence analysisFecesIntestinal microorganisms
The present disclosure provides fecal microbial markers for diagnosing colorectal cancer and colorectal adenoma. The present disclosure also provides methods for diagnosing colorectal cancer and colorectal adenoma using these intestinal microbial markers.
Owner:SECOND GENOME +1
Kit for screening colorectal adenomas (CRA)
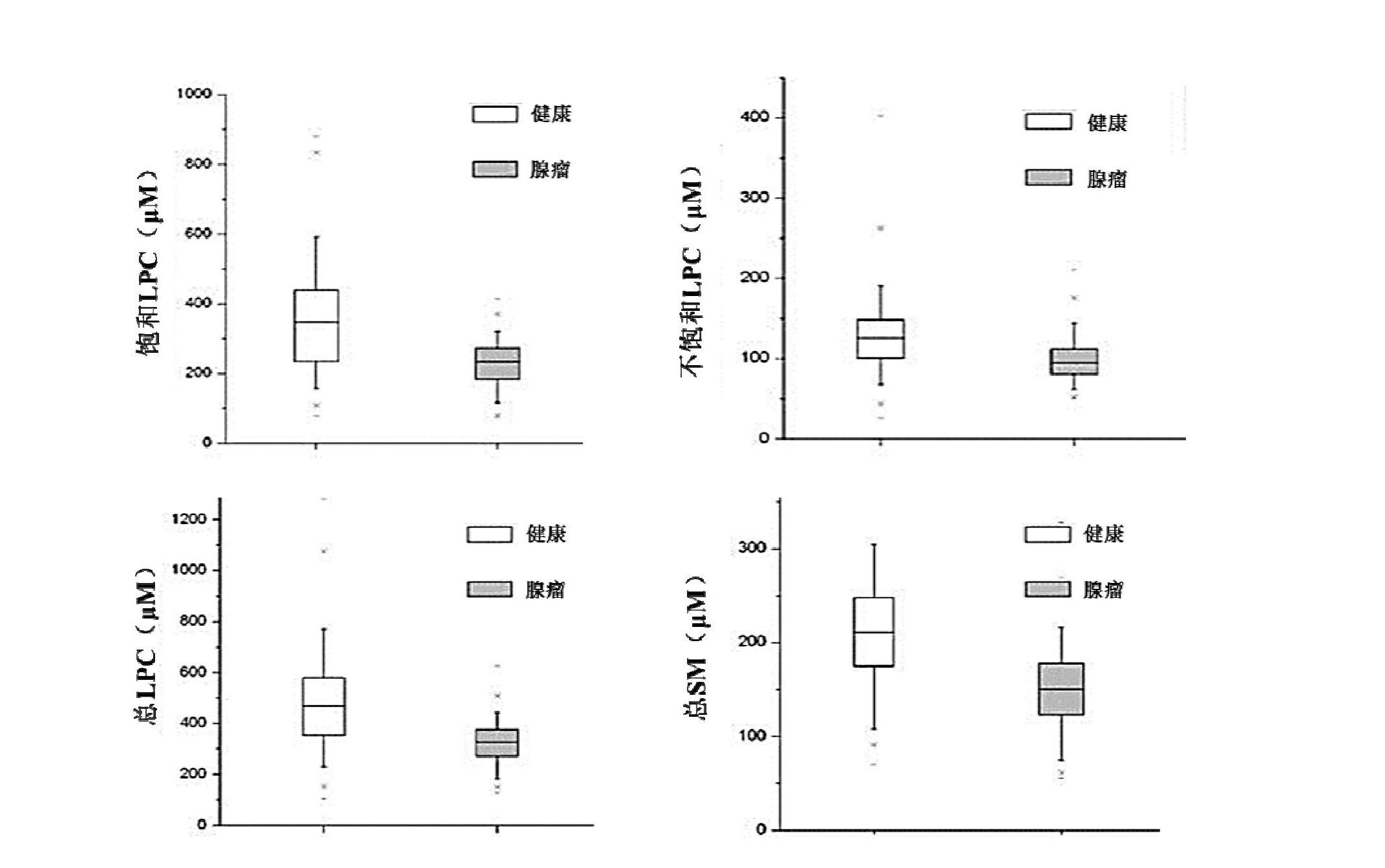
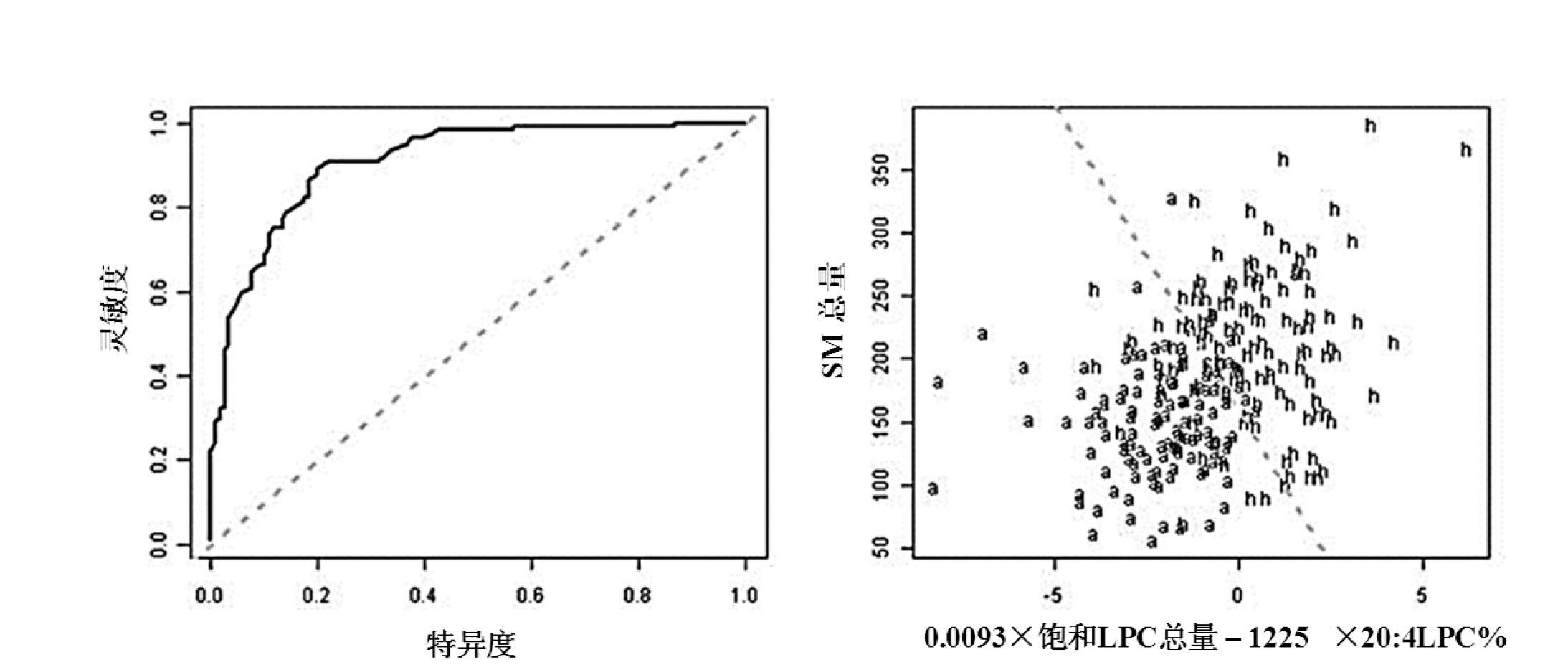
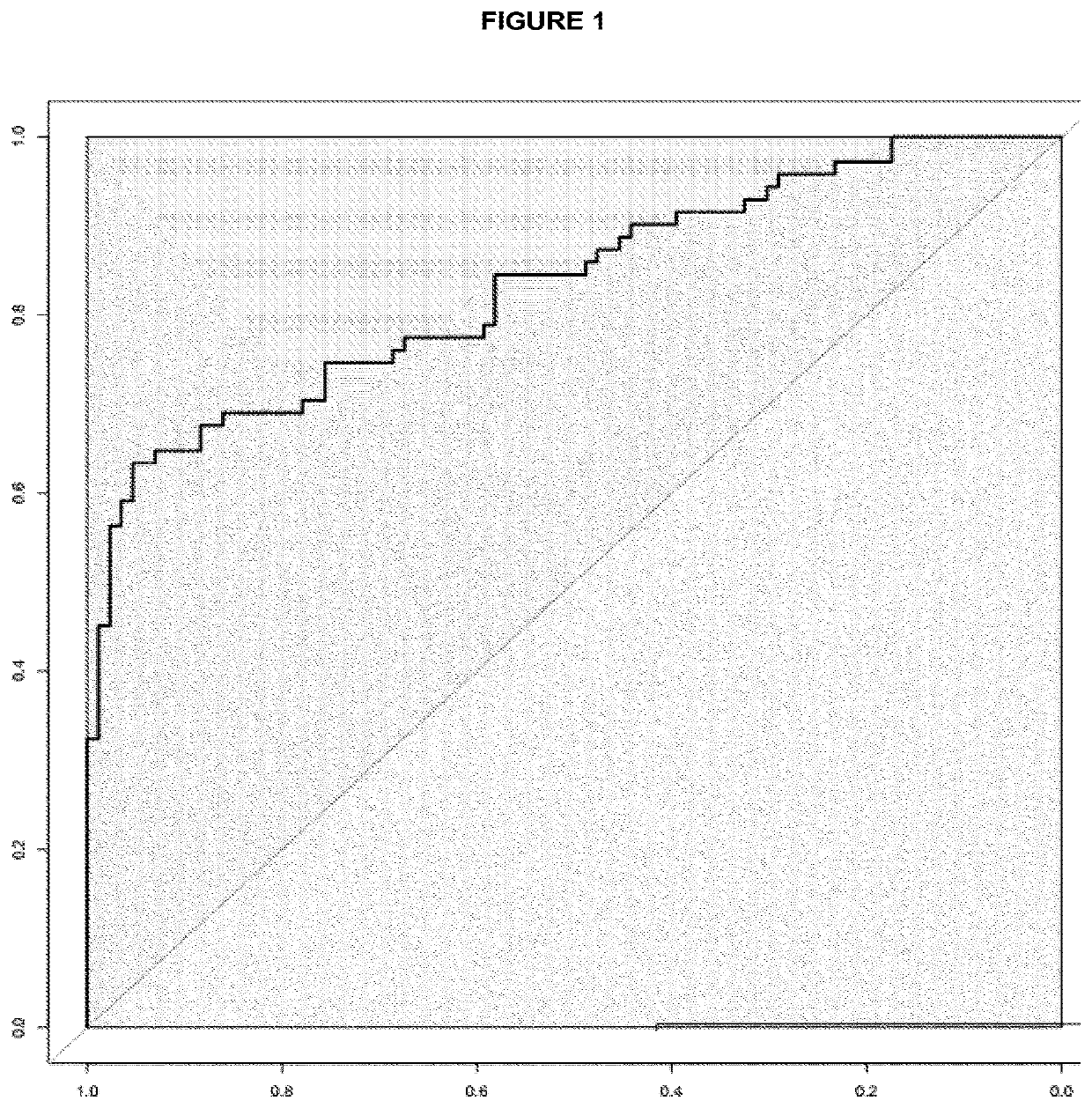
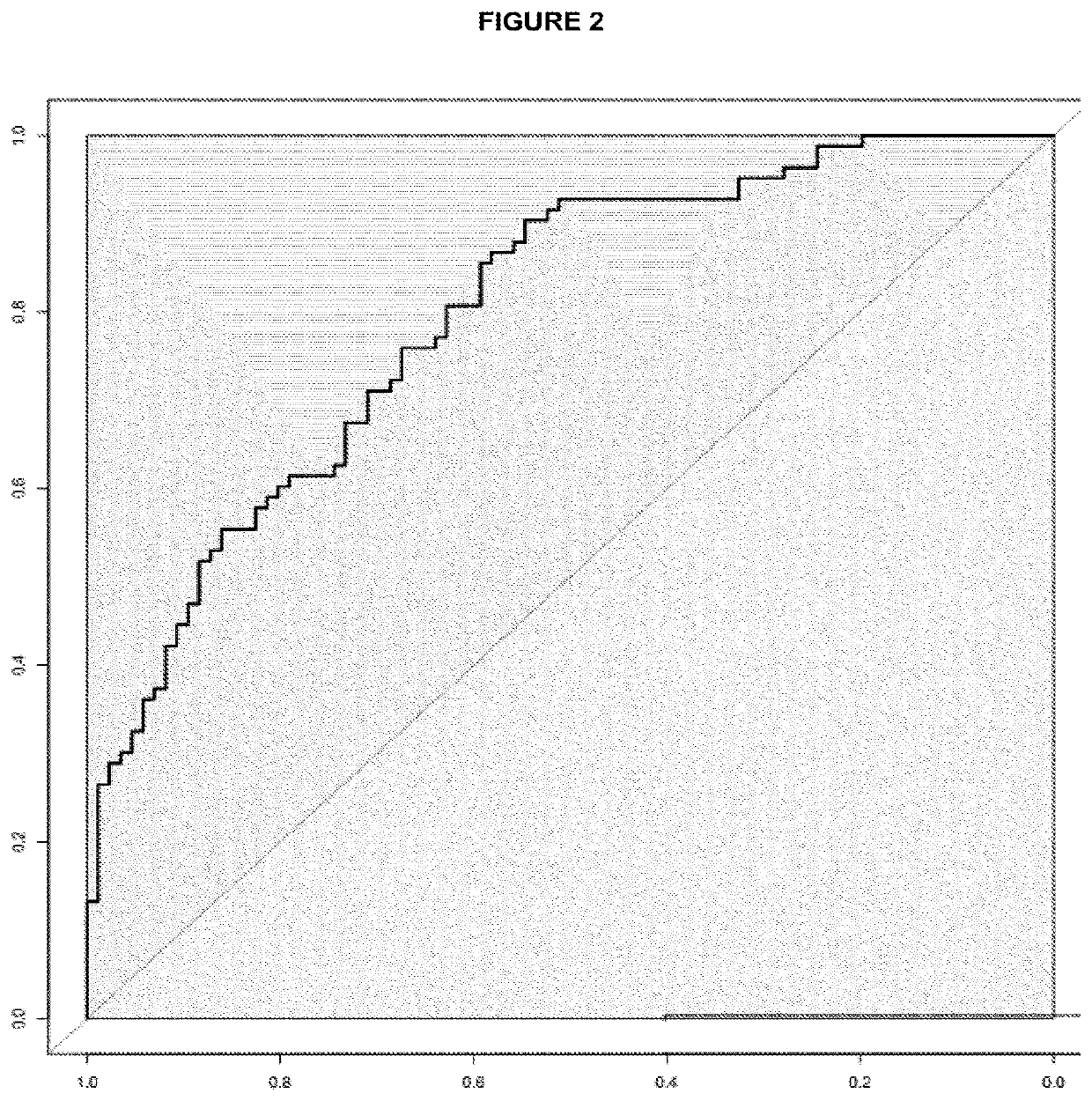
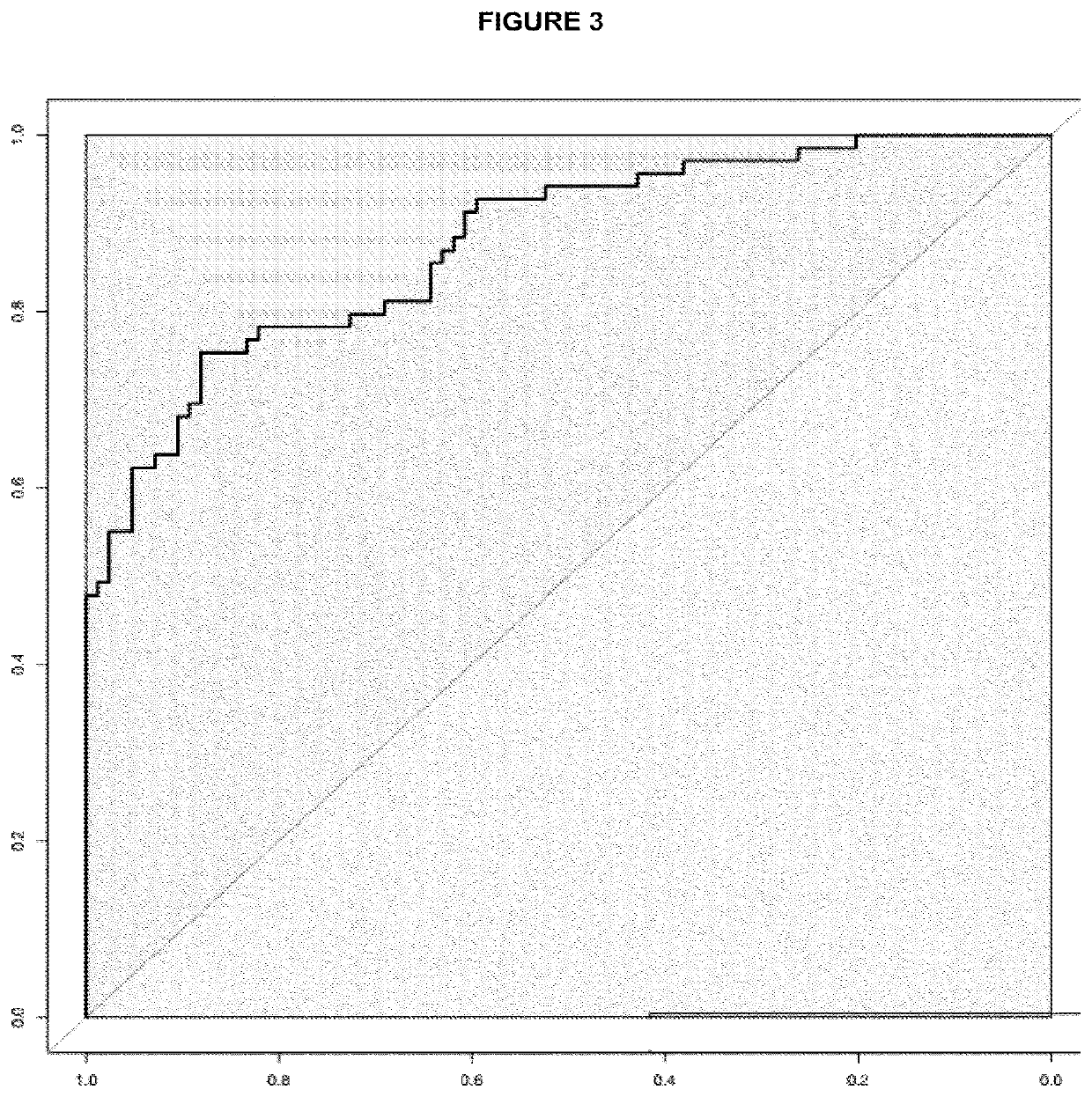
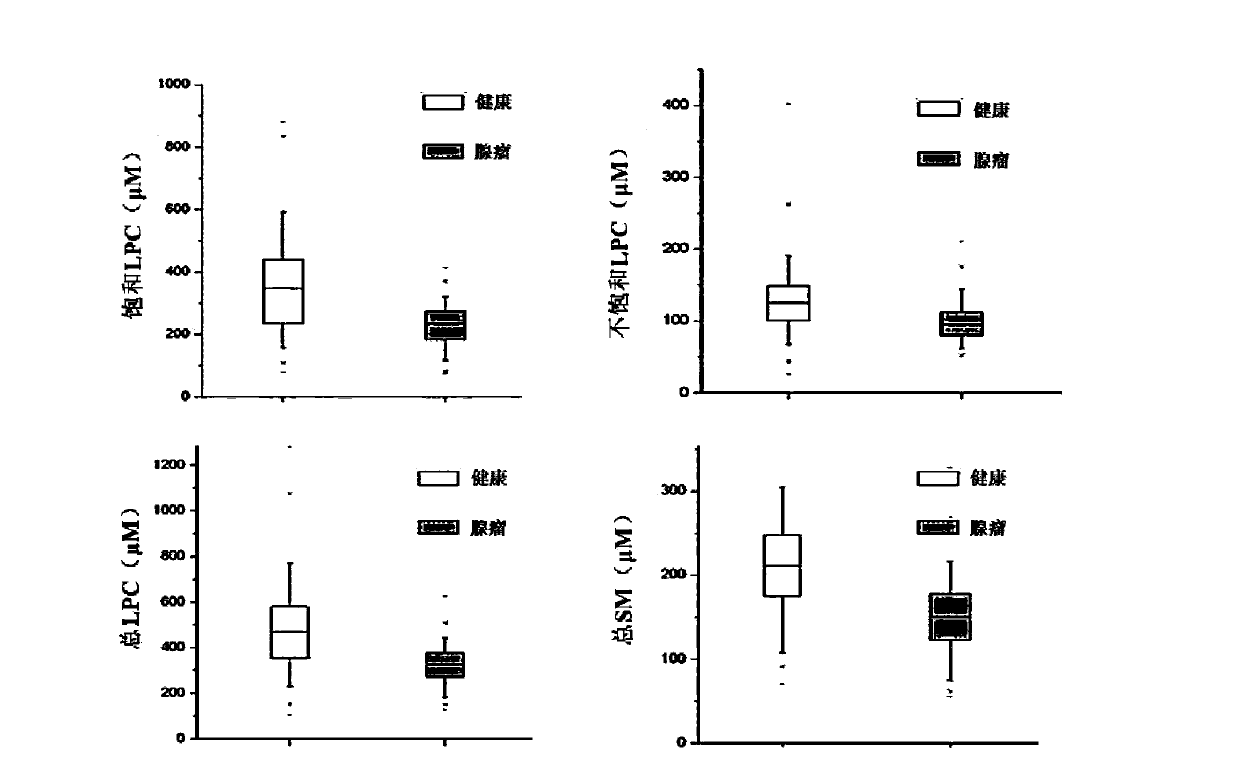
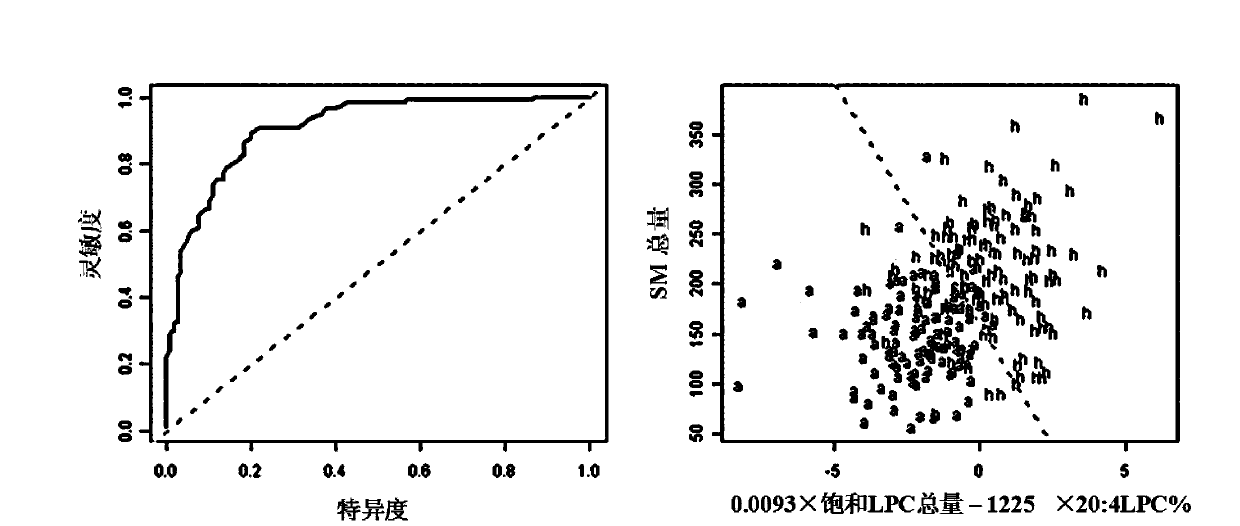
The invention discloses a kit for screening colorectal adenomas (CRA). The kit for screening or screening CRA in an aided manner comprises a device for detecting the contents of phospholipids and a comparison card established by the contents of phospholipids, wherein the phospholipids include 16:0SM, 18:0SM, 14:0LPC, 16:0LPC, 18:2LPC, 18:1LPC, 18:0LPC, 20:4LPC, 20:0LPC, 22:6LPC and 22:0LPC. A molecular model for distinguishing healthy controls from CRA patients is established by taking saturated LPC, 20:4LPC and SM as markers, and the sensitivity and specificity of the model in distinguishing the CRA are respectively 89% and 80%.
Owner:BEIJING NORMAL UNIVERSITY
Biomarkers for detecting colorectal cancer or adenoma and methods thereof
ActiveCN113711044AMechanical/radiation/invasive therapiesComponent separationSerum samplesDiagnostic biomarker
A group of diagnostic biomarkers usable for diagnosis of colorectal cancer or colorectal adenoma. A method for detecting colorectal cancer or colorectal adenoma using the group of diagnostic biomarkers is also provided. For example, the method is a non-invasive approach that may utilize serum samples for detecting colorectal cancer. Moreover, the method for detecting colorectal cancer may detect colorectal cancer of different stages (e.g., pre-cancer stage, early stage, middle stage, late stage).
Owner:中精普康(北京)医药科技有限公司
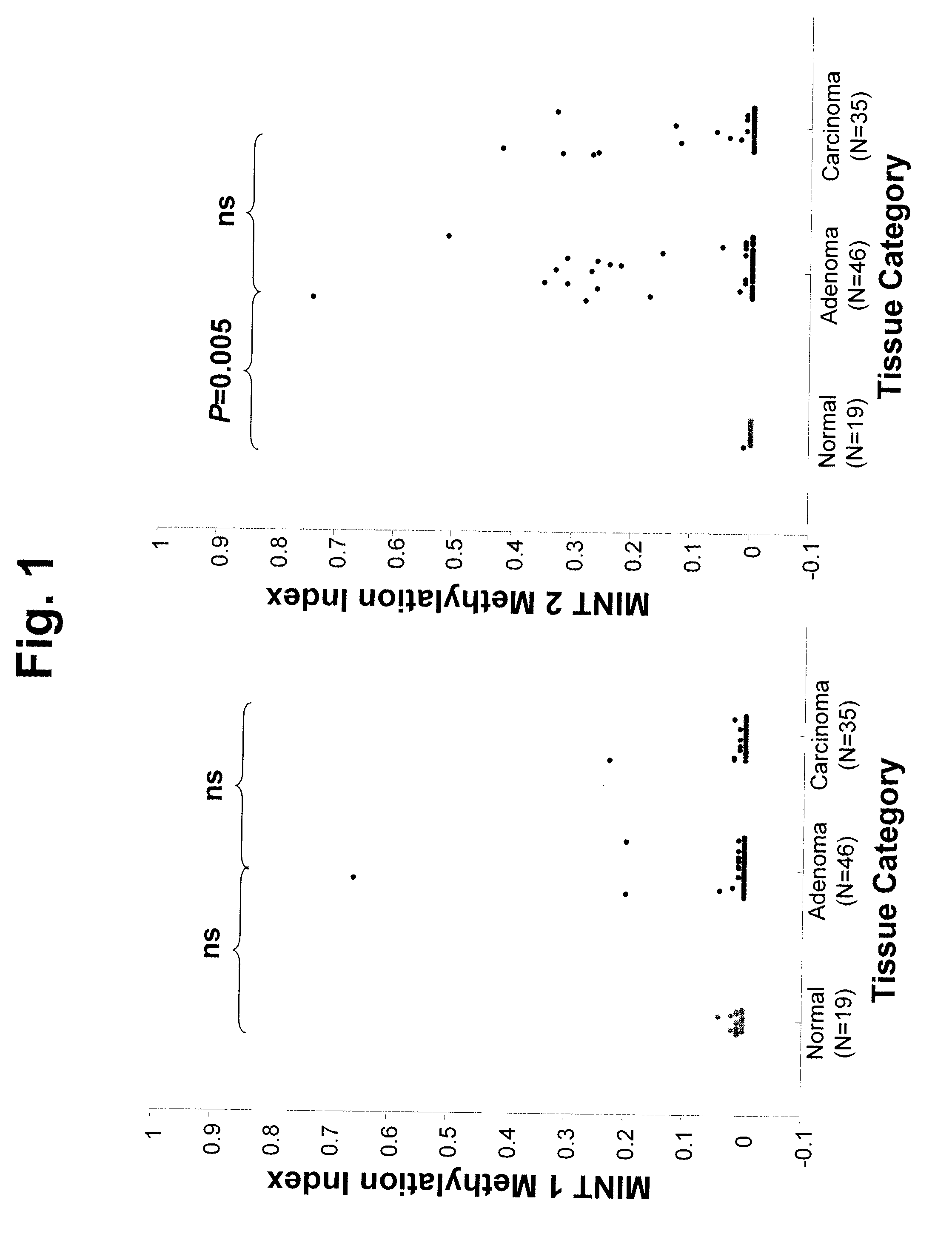
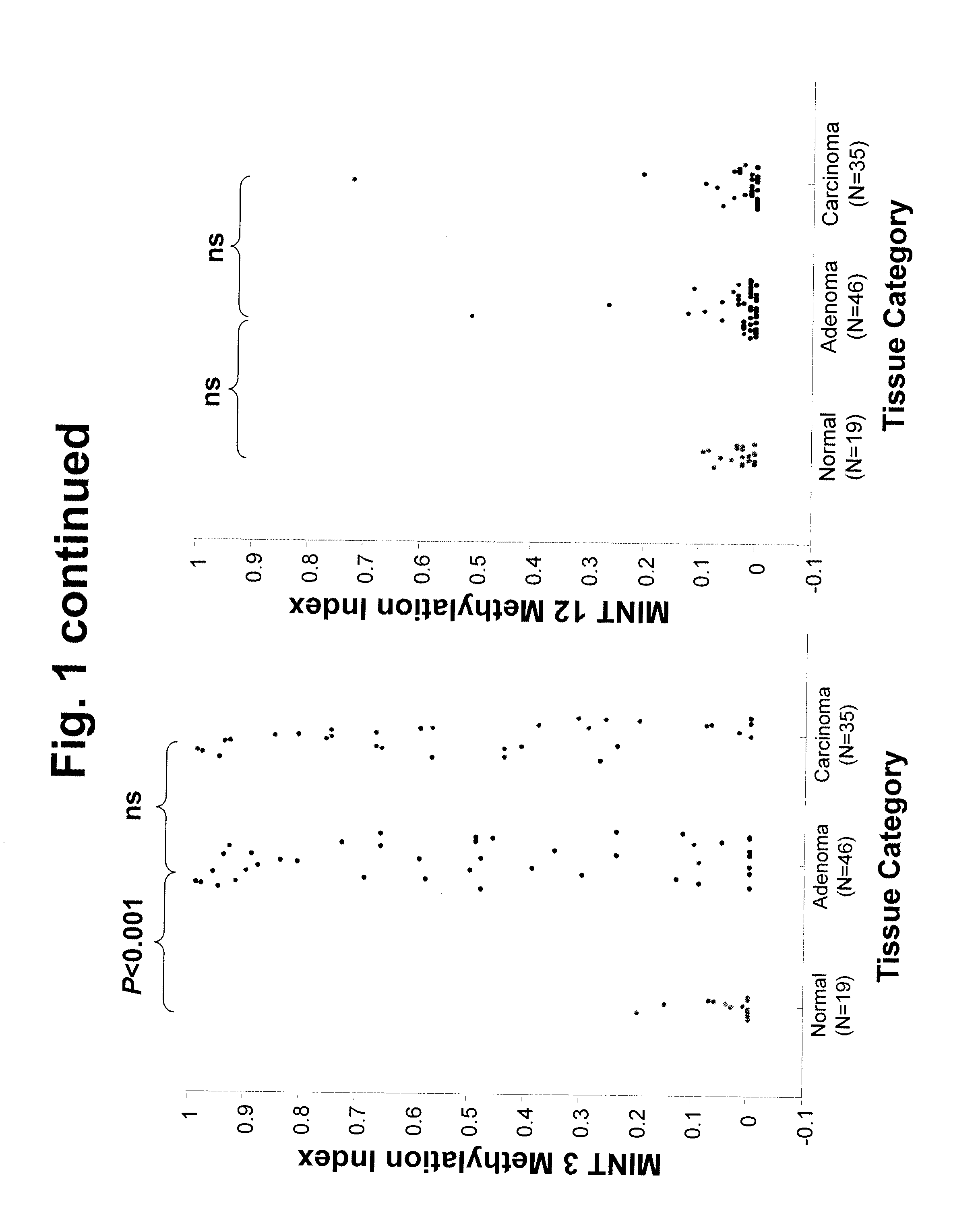
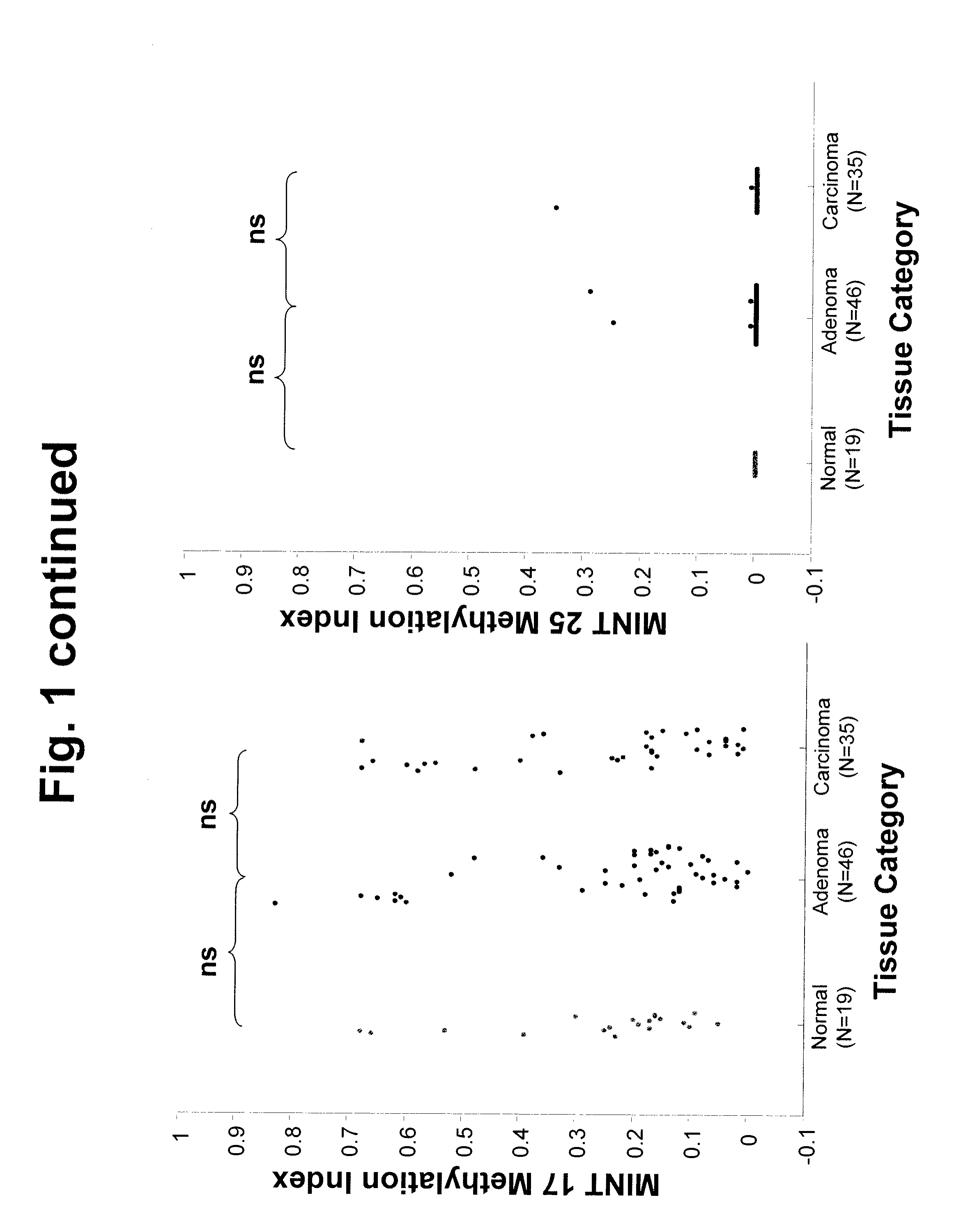
Use of Methylation Status of MINT Loci as a Marker for Rectal Cancer
InactiveUS20100003689A1Increased riskShort overall survivalMicrobiological testing/measurementDNA methylationMalignancy
The invention relates to methods for predicting the outcome of rectal cancer and stratifying a rectal cancer treatment according to the level of DNA methylation at MINT 1, 3, 12, or 17. Also disclosed is a method of detecting rectal adenoma or malignancy based on the level of DNA methylation at MINT 2, 3, or 31.
Owner:JOHN WAYNE CANCER INST
Traditional Chinese medicine composition and application thereof
ActiveCN113713057AImprove immunityEnhance anti-inflammatory and anti-oxidantDigestive systemImmunological disordersLicorice rootsBULK ACTIVE INGREDIENT
The invention relates to a traditional Chinese medicine composition for preventing and treating colorectal adenoma, colorectal adenocarcinoma and relapse after endoscopic resection of colorectal adenoma. The traditional Chinese medicine composition is characterized in that the traditional Chinese medicine composition is prepared from the following main active ingredients in parts by weight: 1-300 parts of poria cocos, 1-300 parts of Chinese yam, 1-300 parts of coix seed, 1-300 parts of Chinese actinidia root, 1-250 parts of duchesnea indica, 1-250 parts of sargentgloryvine stem, 1-250 parts of elecampane and 1-120 parts of honey-fried licorice root. The invention further provides application of the traditional Chinese medicine composition in preparation of medicines for improving immunity of organisms and application of the traditional Chinese medicine composition in preparation of medicines for preventing and treating colorectal adenoma, colorectal adenocarcinoma and relapse after endoscopic resection of the colorectal adenoma.
Owner:段鲜红
Exosome RNA molecular marker combination for diagnosis of colorectal adenoma and application thereof
ActiveCN111455053AImprove complianceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationExosomeRNA marker
The invention relates to the field of biological detection, in particular to molecular detection of organisms, more particularly to a group of exosome RNA markers associated with colorectal adenoma and an application thereof.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
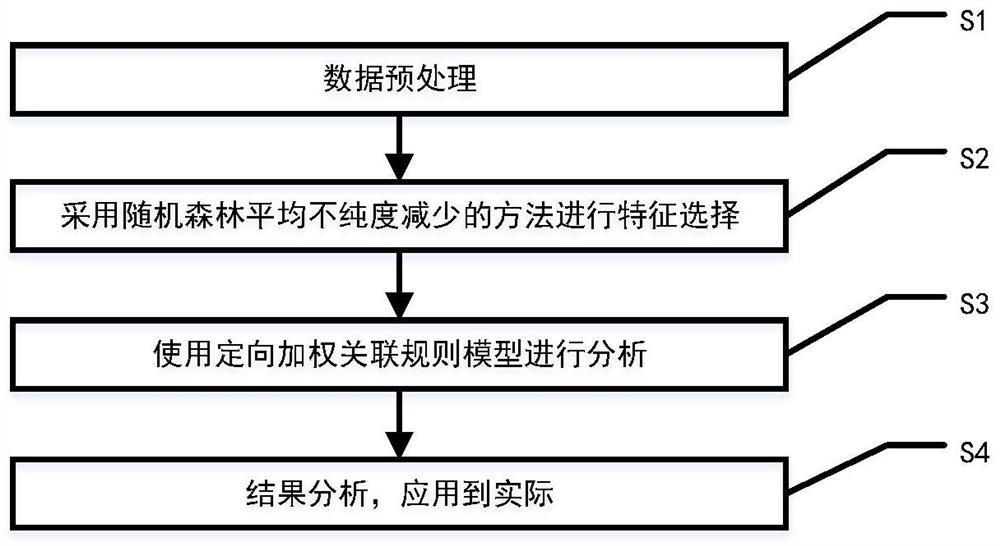
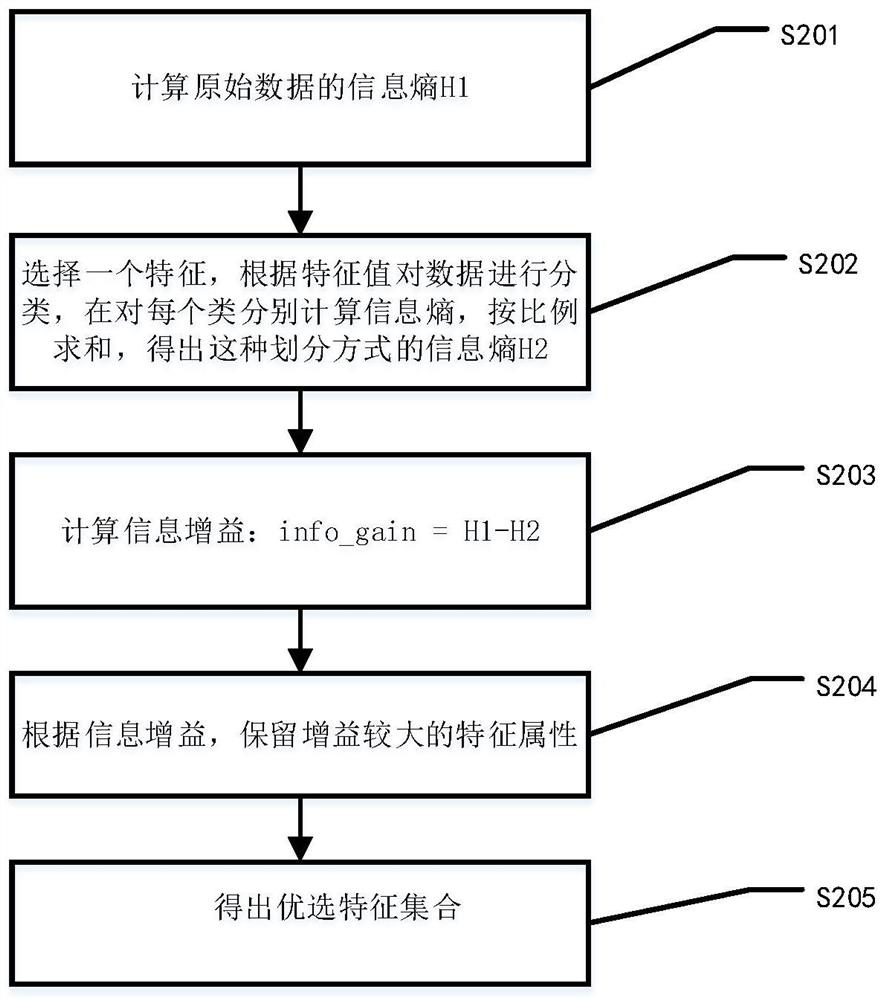
Method for screening risk factors of diffuse colorectal adenoma based on directional weighted association rule model
The invention discloses a method for screening risk factors of diffuse colorectal adenoma based on a directional weighted association rule model, and belongs to the field of data mining. The method comprises the following steps: firstly, preprocessing data; secondly, performing feature extraction by adopting a feature selection method for reducing the average impure degree of the random forest, and determining an optimal division node by utilizing information gain to obtain an optimal feature set; then, inputting the optimal feature set into a directional weighted association rule model to generate a strong association rule; and finally, bringing the risk factors contained in the strong association rule into a risk factor set, and communicating with experts. Compared with the prior art, the method mainly provides a directional weighted association rule model to screen the risk factors of colorectal adenoma, affirms the significance of living dietary habit factors in the etiology of colorectal adenoma, finds out unfound high-risk factors in the previous research, and provides a set of method worthy of reference for finding out the risk factors of colorectal adenoma.
Owner:SHANGHAI MARITIME UNIVERSITY
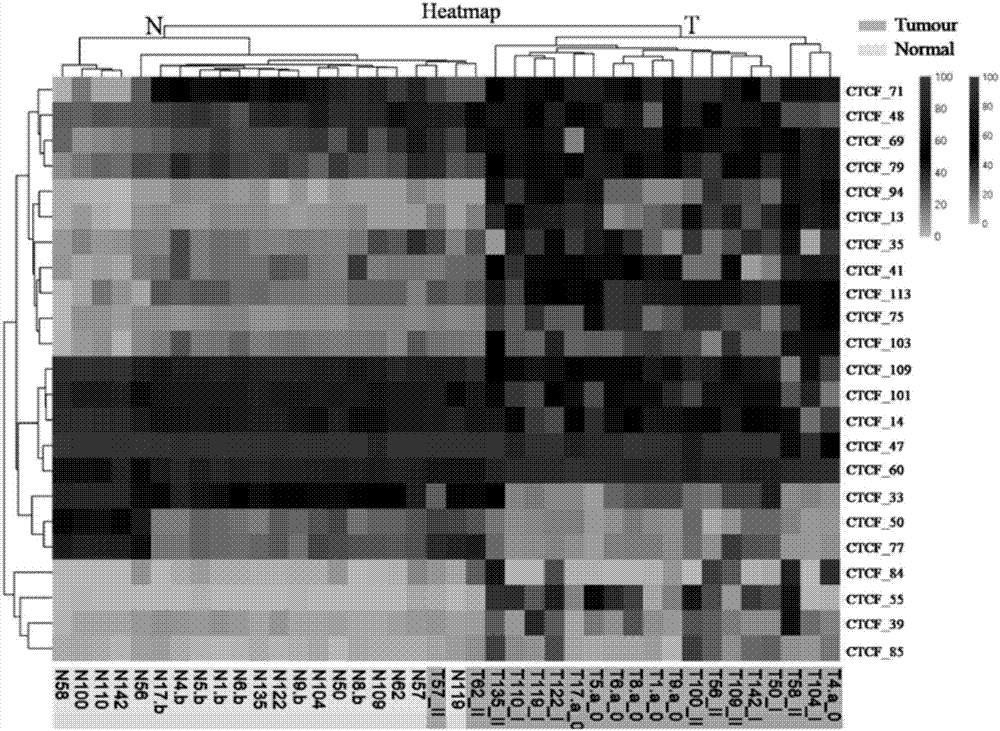
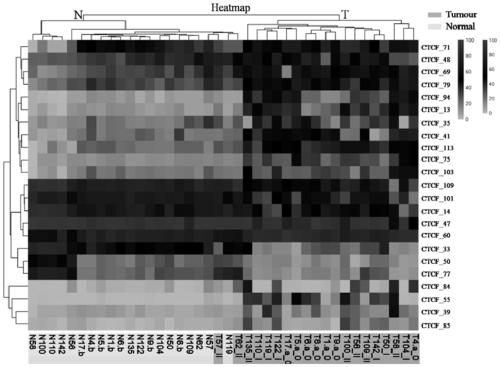
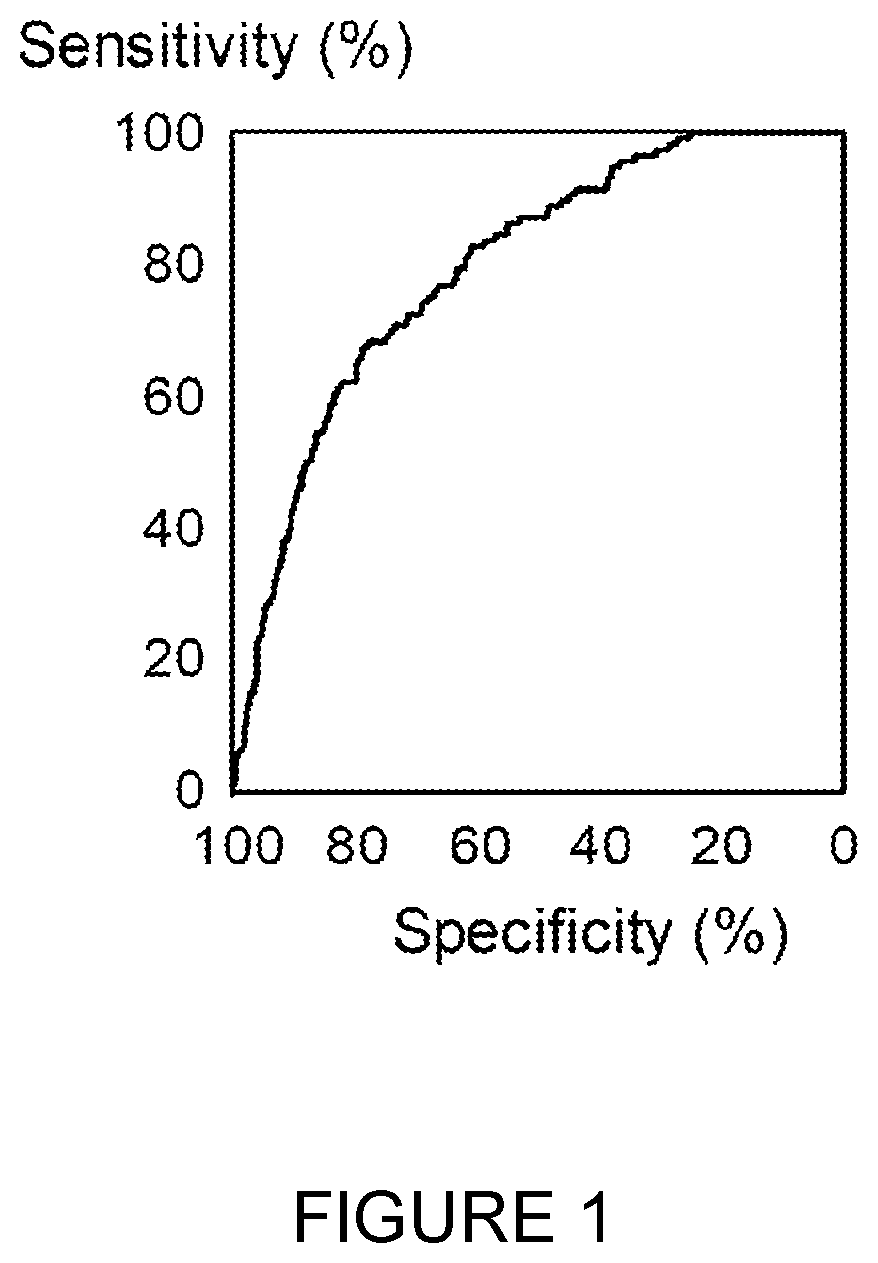
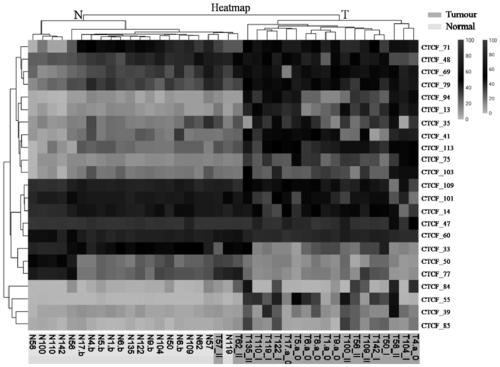
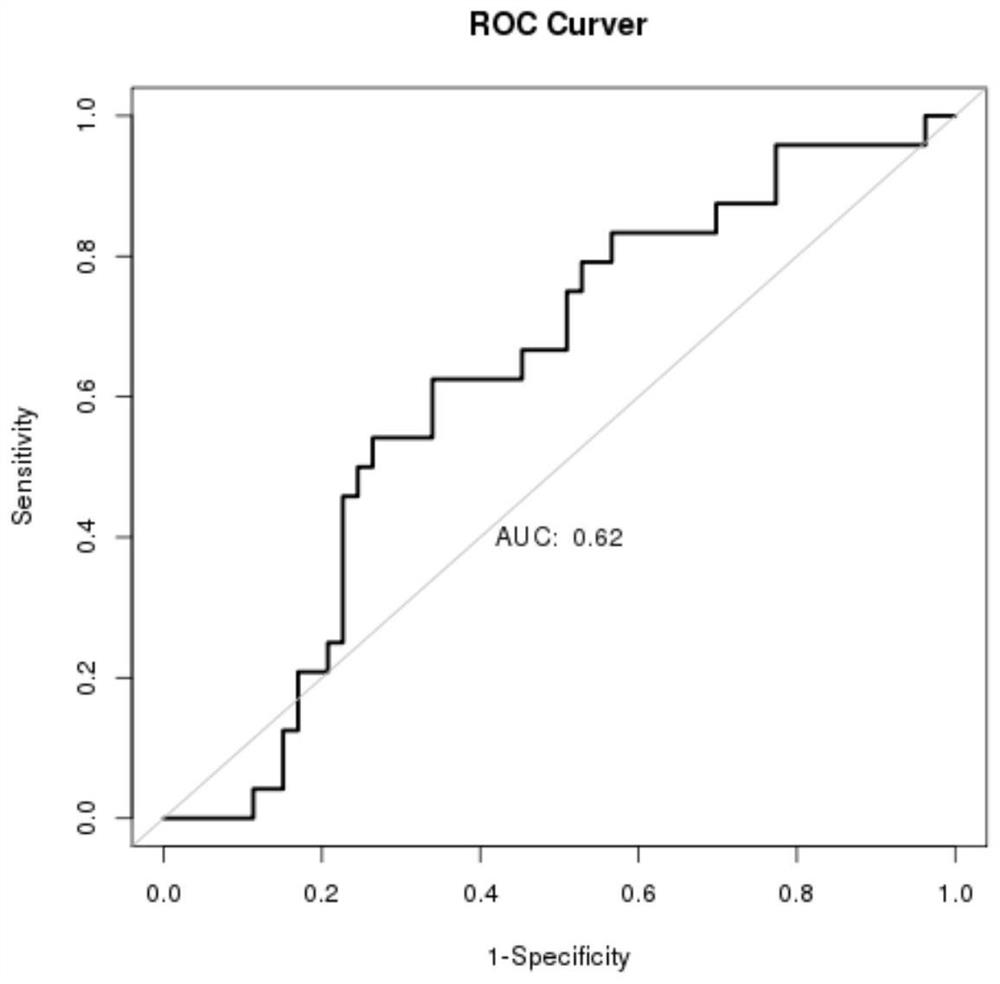
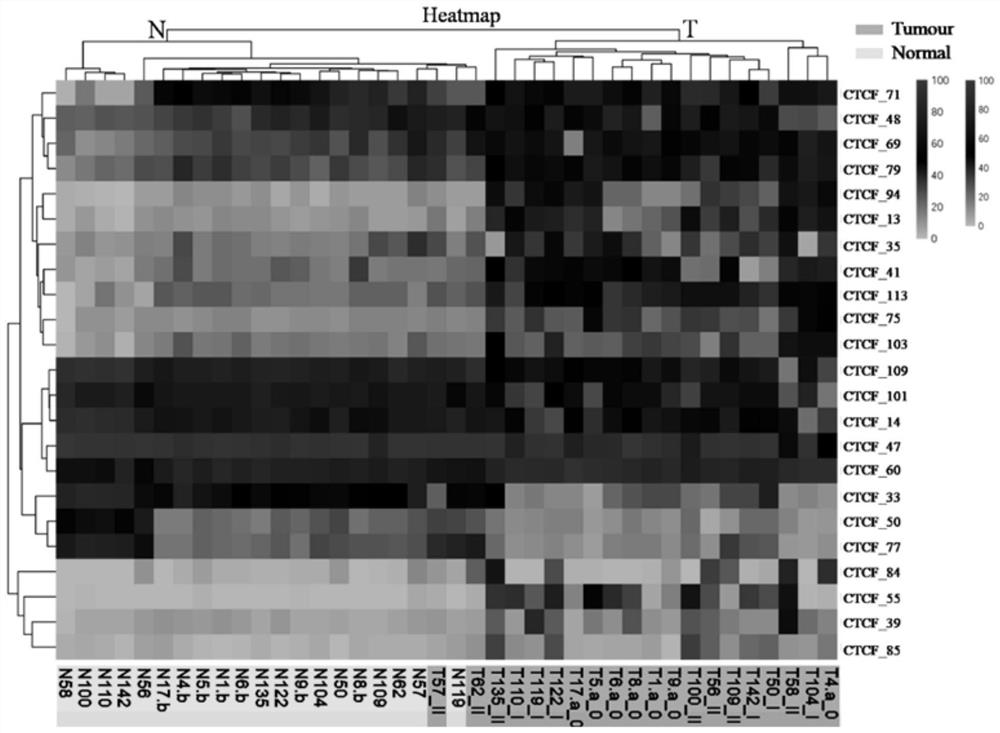
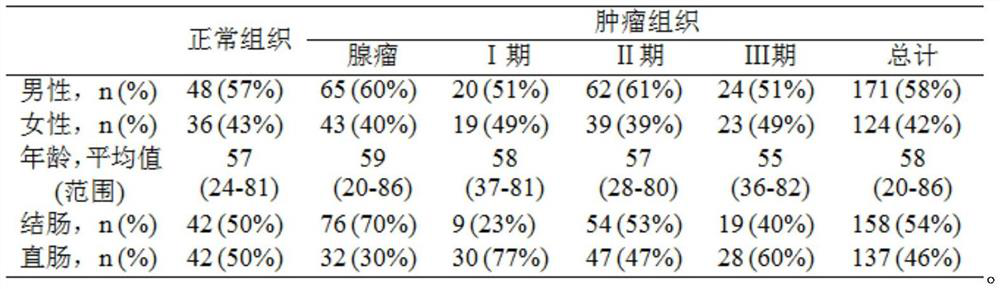
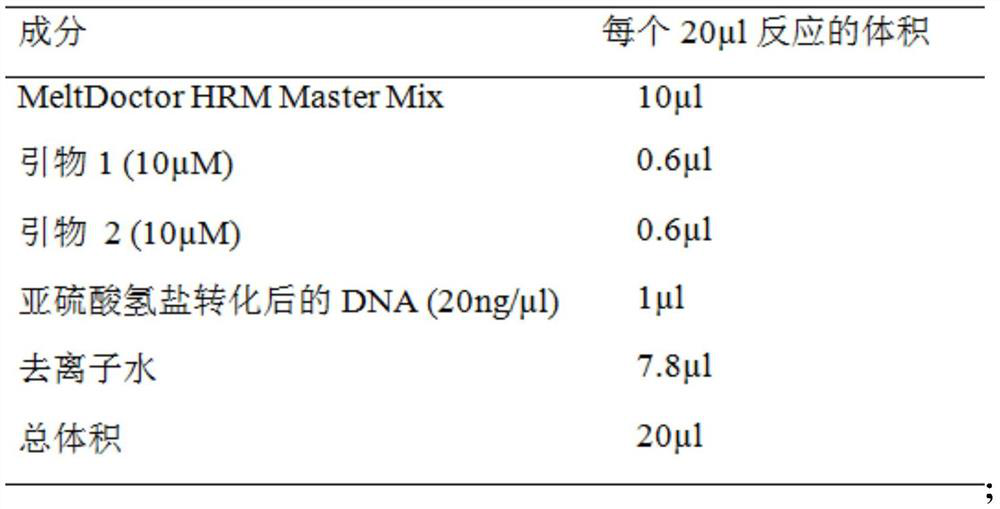
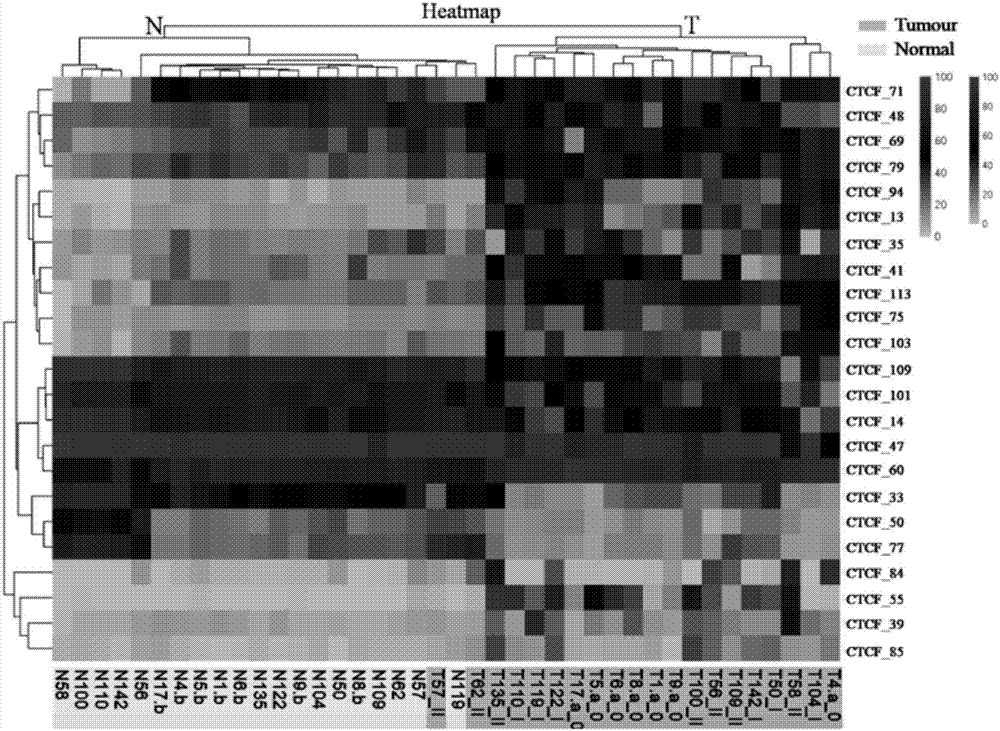
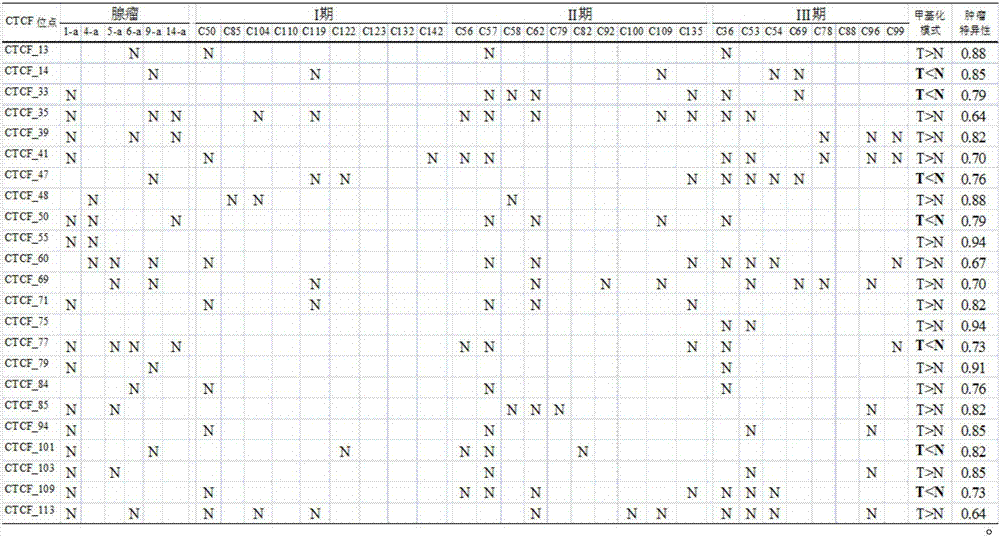
Application of DNA binding site CTCF_113 of multifunctional transcriptional regulatory factor CTCF
ActiveCN107227366AImprove accuracyMicrobiological testing/measurementDNA methylationParanasal Sinus Carcinoma
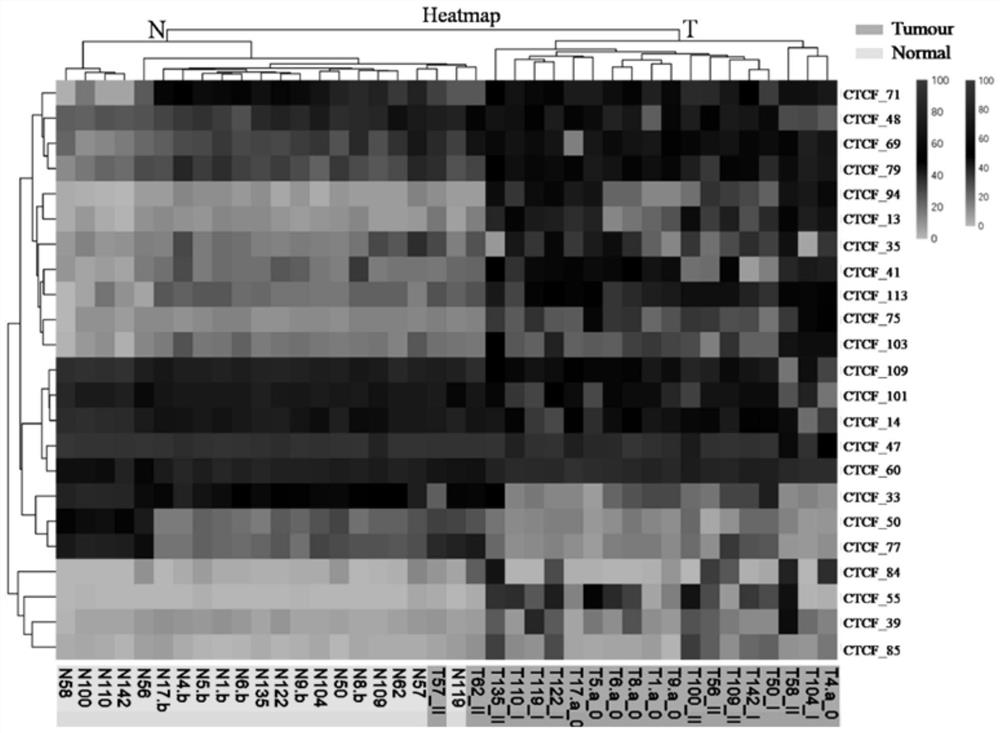
The invention discloses a novel purpose of a DNA binding site CTCF_113 of a multifunctional transcriptional regulatory factor CTCF, i.e., the application thereof to prevention of a kit for early diagnosis of colorectal carcinoma. The binding site has the nucleotide sequence shown as SEQ ID NO:1. The accuracy of the CTCT binding site provided by the invention for detecting rectal adenoma and colorectal cancer is obviously higher than that of the reported DNA methylation molecular markers; the DNA binding site of the CTCF can be likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular subtyping (such as CIMP subtyping). Generally, the invention provides a novel idea for finding the more effective tumor early stage diagnosis, treatment effect monitoring, prognosis effect evaluation and molecular subtyping molecular markers, and has the important significance on tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
In vitro method for identifying colorectal adenomas or colorectal cancer
InactiveUS20190316212A1Accurately screening and diagnosing subjectsMicrobiological testing/measurementDiseaseColo-rectal cancer
The present invention refers to an in vitro method for identifying patients at risk of suffering from colorectal cancer and / or colorectal adenomas, preferably advanced colorectal adenomas, based on measuring the expression profile or level of some miRNAs, e.g. miR-15b, which are up-regulated or over-expressed in patients suffering from said diseases.
Owner:ADVANCED MARKER DISCOVERY SL
Prescription for clearing and spleen tonifying and application thereof in preparation of medicine for preventing postoperative recurrence of colorectal adenoma
InactiveCN112773871ALow recurrence rateRelief of clinical symptomsAntineoplastic agentsPlant ingredientsSmoked PlumRadix Astragali seu Hedysari
The invention discloses a prescription for clearing and spleen tonifying and application thereof in preparation of a medicine for preventing postoperative recurrence of colorectal adenoma. The prescription comprises the following traditional Chinese medicines in parts by weight: 13-17 parts of fried codonopsis pilosula; 8-12 parts of fried radix scutellariae; 13- 17 parts of roasted rhizoma atractylodis macrocephalae; 8-10 parts of poria cocos; 13-17 parts of raw radix astragali seu hedysari; 13-17 parts of radix paeoniae rubra; 13-17 parts of raw coix seeds; 28-32 parts of smoked plum; 8-12 parts of divaricate saposhnikovia root; 4-8 parts of pericarpium citri reticulatae; 8-12 parts of radix achyranthis bidentatae; and 4-8 parts of raw licorice. The prescription for clearing and spleen tonifying provided by the invention can effectively reduce the postoperative recurrence rate of colorectal adenoma and effectively relieve the postoperative clinical symptoms of colorectal adenoma, so that the prescription has the prospect of being developed into the medicine for preventing postoperative recurrence of colorectal adenoma.
Owner:江阴市中医院
Exosome RNA for colorectal adenoma diagnosis and application thereof
ActiveCN111485021AImprove complianceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationExosomeAdenoma
The invention relates to the field of biological detection, in particular to biological molecular detection, and more particularly relates to a group of exosome RNA molecular markers related to colorectal adenoma and application thereof.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Application of the DNA-binding site CTCF_33 of the multifunctional transcriptional regulator CTCF
ActiveCN107227365BImprove accuracyMicrobiological testing/measurementDNA methylationParanasal Sinus Carcinoma
The invention discloses a new purpose of a DNA binding site CTCF_33 for a multifunctional transcriptional regulatory factor CTCF, i.e., the application thereof to prevention of a kit for early diagnosis of colorectal carcinoma. The binding site has the nucleotide sequence shown as SEQ ID NO:1. The accuracy of the CTCT binding site provided by the invention for detecting rectal adenoma and colorectal cancer is obviously higher than that of the reported DNA methylation molecular markers; the DNA binding site of the CTCF can be likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular subtyping (such as CIMP subtyping). Generally, the invention provides a novel idea for finding the more effective tumor early stage diagnosis, treatment effect monitoring, prognosis effect evaluation and molecular subtyping molecular markers, and has the important significance on tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
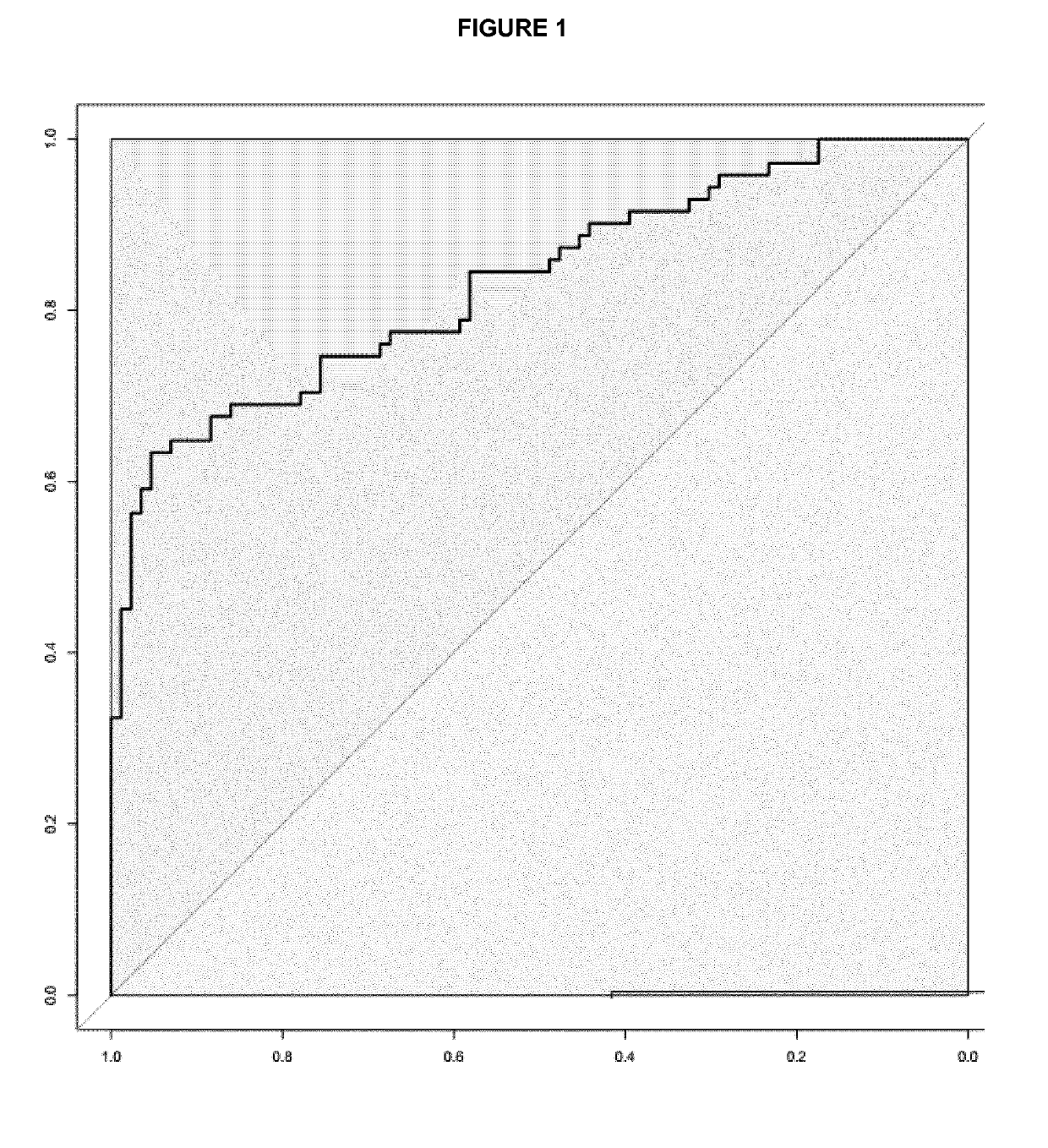
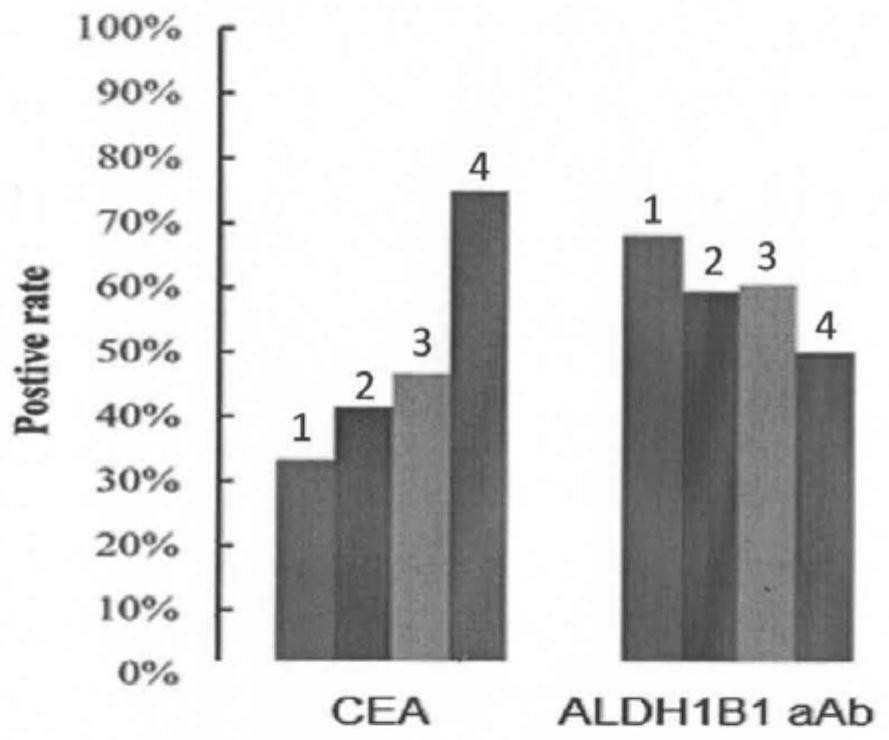
Serum ALDH1B1 autoantibody quantitative detection kit and application thereof
ActiveCN111735946AImprove detection valueIncreased sensitivityBiological material analysisAgainst vector-borne diseasesAntigenSerum autoantibodies
The invention discloses a serum ALDH1B1 autoantibody quantitative detection kit and an application thereof. The kit comprises a solid-phase carrier, a recombinant ALDH1B1 antigen protein coated on thesolid-phase carrier, an enzyme-labeled secondary antibody, a standard substance, a chromogenic substrate, a washing solution and a reaction stop solution. The kit can be used for specifically and quantitatively detecting the level of the ALDH1B1 antibody in a serum sample, and is obviously superior to a serum marker CEA which is clinically used at present in the aspects of early screening and diagnosis of colorectal cancer and high-grade colorectal adenoma sensitivity.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Colorectal adenoma biomarker, kit and screening method of biomarker
PendingCN114369673AStrong specificityGood screening efficiencyMicrobiological testing/measurementInstrumentsCelluloseMutation frequency
The invention discloses a colorectal adenoma biomarker, a kit and a screening method of the biomarker. The colorectal adenoma biomarker comprises the following components: an internal organ Odorbacter splanchnicus62174, Bacteroides cellulolyticus 58046, Alistipes shahii62199 and the like, and is characterized in that the internal organ Odorbacter splanchnicus62174, the internal organ Bacteroides cellulolyticus 58046, the internal organ Alistipes shahii62199, the internal organ Alistipes shahii62199, the internal organ Alistipes shahii62199 and the internal organ Alistipes shahii62199 and the internal organ Alistipes shahii62199 and the internal organ Alistipes The tumor biomarker screening method comprises the following steps: S1, acquiring microorganism sequencing data and clinical information data of a disease control group and a normal control group, and preprocessing; s2, screening the preprocessed microbial sequencing data, and annotating SNV levels of strains with enough sequencing depth and coverage to obtain information such as SNV mutation frequency of each strain; s3, performing difference analysis on the microbial SNV data of the disease patient and the healthy control group to obtain SNV sites with significant difference in different strains; s4, screening the differential SNV loci, and determining an optimal SNV biomarker; in conclusion, the biomarker and the screening method provided by the invention can be used for better early diagnosis and treatment of cancers, and have important significance and clinical application value.
Owner:TONGJI UNIV
Application and kit of detection reagents for detecting colorectal cancer-related gene methylation
ActiveCN110343764BHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationOncologyAdenoma
The invention discloses an application and a kit of detection reagents for detecting colorectal cancer-related gene methylation, and relates to the technical field of gene detection. The kit disclosed in the present invention includes detection reagents for detecting the methylation of colorectal cancer-related genes, by performing methylation detection on specific regions in the SDC2 gene and TFPI2 gene, and comparing the methylation detection results of these regions As the basis for auxiliary diagnosis of colorectal cancer, it has higher sensitivity and specificity, and provides a more accurate and reliable reference for early screening and diagnosis of colorectal cancer or colorectal adenoma.
Owner:WUHAN AIMISEN LIFE TECH CO LTD
Biomarker for detecting colorectal cancer or adenoma and method thereof
PendingCN114807332AMedical simulationMechanical/radiation/invasive therapiesSerum samplesDiagnostic biomarker
The present disclosure provides a set of diagnostic biomarkers useful for diagnosing colorectal cancer or colorectal adenoma, and also provides a method for detecting colorectal cancer or colorectal adenoma using the diagnostic biomarker set. For example, the method provided by the present disclosure is a non-invasive method that can detect colorectal cancer using a serum sample. In addition, the method for detecting colorectal cancer can detect colorectal cancer in different stages (e.g., pre-cancerous, early, mid-term, and advanced stages).
Owner:IBRAINBABY
Combination test for colorectal cancer
InactiveUS20200182875A1Microbiological testing/measurementDisease diagnosisOncologyFecal occult blood
The present invention relates to methods of detecting and / or screening for colorectal cancer (CRC), a colorectal adenoma or a polyp in a patient comprising identifying patients found to be positive for fecal occult blood and further testing for one or more additional factors. Said methods can be used to assess the suitability of a patient for colonoscopy.
Owner:BELGIAN VOLITION SPRL
Application of the DNA-binding site CTCF_94 of the multifunctional transcriptional regulator CTCF
The invention discloses a new use of a DNA binding site CTCF_94 of a multifunctional transcriptional regulator CTCF. The use is an application of the DNA binding site CTCF_94 in the preparation of a kit for early diagnosis of colorectal cancer. The nucleotide sequence of the binding site is represented by SEQ ID NO:1. The colorectal adenoma detection accuracy and the colorectal cancer detection accuracy of the CTCF binding site are obviously higher than those of reported DNA methylation molecule markers; and the DNA binding site of the CTCF is also possible to become a biomarker for tumor therapy efficacy monitoring, tumor prognosis effect evaluation and tumor molecule typing (such as CIMP typing). The use provides a new idea for finding effective molecular markers for early diagnosis of tumors, therapeutic effect monitoring, prognostic effect evaluation and molecular typing, and is of great significance to prevent and treat tumors.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
In vitro method for identifying colorectal adenomas or colorectal cancer
PendingUS20220220562A1Accurately screening and diagnosing subjectsMicrobiological testing/measurementDiseaseOncology
Owner:ADVANCED MARKER DISCOVERY SL
Kit for screening colorectal adenomas (CRA)
The invention discloses a kit for screening colorectal adenomas (CRA). The kit for screening or screening CRA in an aided manner comprises a device for detecting the contents of phospholipids and a comparison card established by the contents of phospholipids, wherein the phospholipids include 16:0SM, 18:0SM, 14:0LPC, 16:0LPC, 18:2LPC, 18:1LPC, 18:0LPC, 20:4LPC, 20:0LPC, 22:6LPC and 22:0LPC. A molecular model for distinguishing healthy controls from CRA patients is established by taking saturated LPC, 20:4LPC and SM as markers, and the sensitivity and specificity of the model in distinguishing the CRA are respectively 89% and 80%.
Owner:BEIJING NORMAL UNIVERSITY
A kind of biomarker and method thereof for detecting colorectal cancer or adenoma
ActiveCN113711044BMechanical/radiation/invasive therapiesComponent separationSerum samplesDiagnostic biomarker
The present disclosure provides a panel of diagnostic biomarkers useful for diagnosing colorectal cancer or colorectal adenoma, and also provides methods of detecting colorectal cancer or colorectal adenoma using the panel of diagnostic biomarkers. For example, the method provided by the present disclosure is a non-invasive method that can detect colorectal cancer using a serum sample. In addition, the method for detecting colorectal cancer can detect colorectal cancer at different stages (eg, precancerous, early, intermediate, advanced).
Owner:中精普康(北京)医药科技有限公司
Application of the DNA-binding site CTCF_113 of the multifunctional transcriptional regulator CTCF
ActiveCN107227366BImprove accuracyMicrobiological testing/measurementDNA methylationParanasal Sinus Carcinoma
The invention discloses a novel purpose of a DNA binding site CTCF_113 of a multifunctional transcriptional regulatory factor CTCF, i.e., the application thereof to prevention of a kit for early diagnosis of colorectal carcinoma. The binding site has the nucleotide sequence shown as SEQ ID NO:1. The accuracy of the CTCT binding site provided by the invention for detecting rectal adenoma and colorectal cancer is obviously higher than that of the reported DNA methylation molecular markers; the DNA binding site of the CTCF can be likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular subtyping (such as CIMP subtyping). Generally, the invention provides a novel idea for finding the more effective tumor early stage diagnosis, treatment effect monitoring, prognosis effect evaluation and molecular subtyping molecular markers, and has the important significance on tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Exosomal RNA for the diagnosis of colorectal adenoma and its application
ActiveCN111485021BImprove complianceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationExosomeAdenoma
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Application of the DNA-binding site CTCF_13 of the multifunctional transcriptional regulator CTCF
The invention discloses a novel use of a DNA (deoxyribonucleic acid) binding site CTCF_13 of a multifunctional transcription regulation factor CTCF, i.e. application thereof to preparation of a kit for early diagnosis of colorectal neoplasms. The nucleotide sequence of the binding site is shown as SEQ ID NO:1. The accuracy of the binding site CTCF, provided by the invention, in detection of either colorectal adenomas or the colorectal neoplasms is remarkably higher than that of a reported DNA methylated molecular marker; moreover, the DNA binding site of the CTCF is also likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular typing (such as CIMP typing). In brief, a novel concept is provided for seeking for a more effective molecular marker for early diagnosis of tumors, treatment effect monitoring, prognosis effect evaluation and molecular typing, and is significant for tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Combination of exosomal RNA molecular markers for the diagnosis of colorectal adenoma and its application
ActiveCN111455053BImprove complianceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationRNA markerAdenoma
The present invention relates to the field of biological detection, more specifically to biological molecular detection, and more specifically to a group of exosome RNA markers related to colorectal adenoma and applications thereof.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
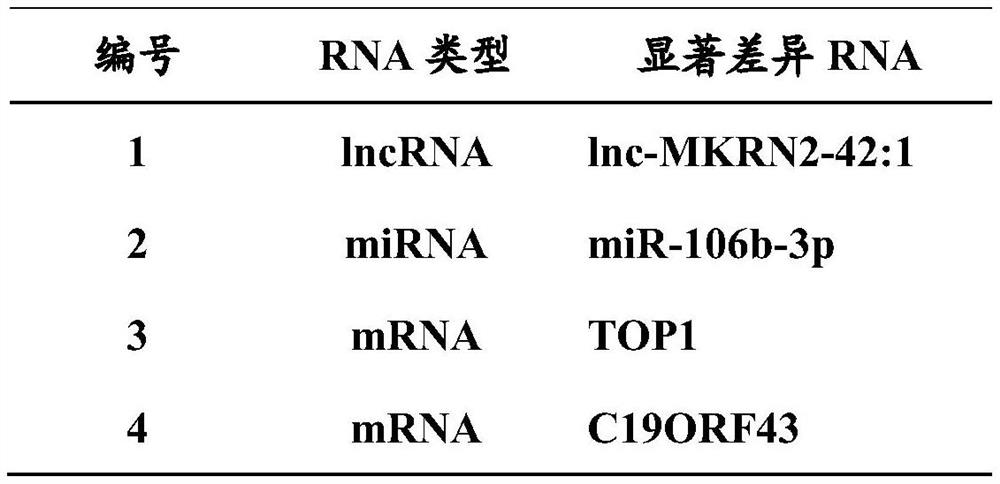
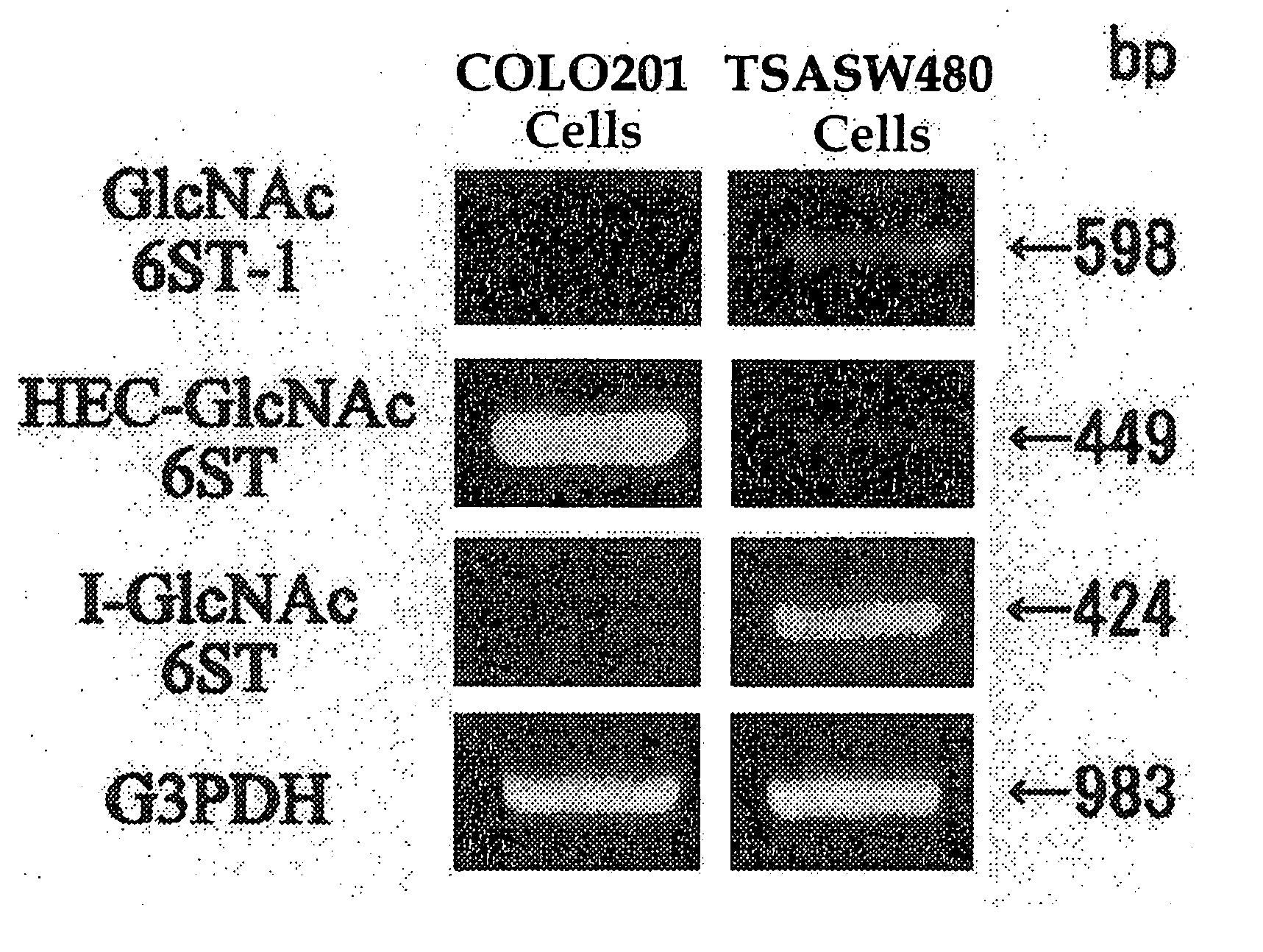
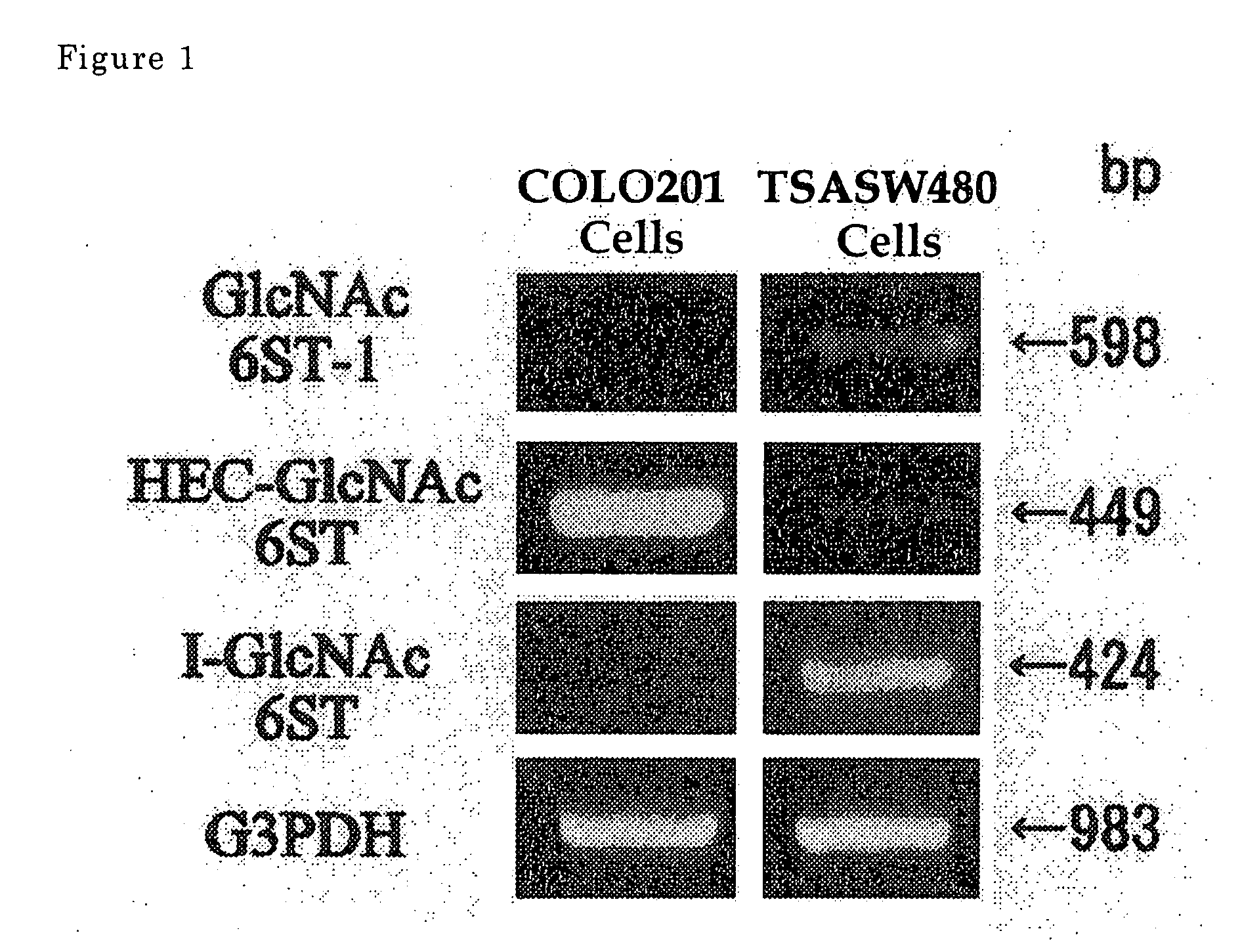
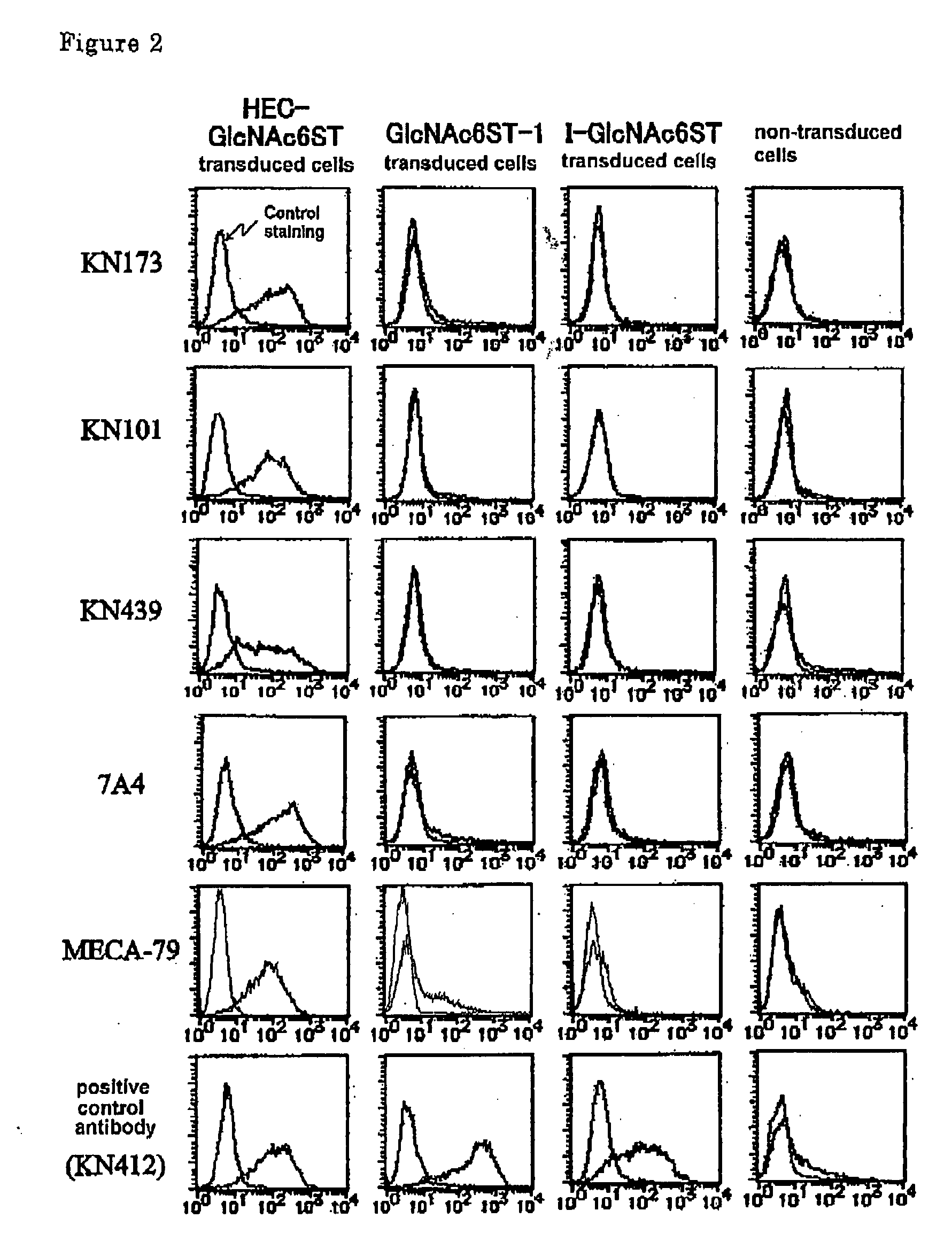
Method For Examining Carcinoma And Adenoma
[PROBLEMS] To provide examination methods and reagents able to detect efficiently cancer patients and patients at high risk of cancer. [MEANS FOR SOLVING PROBLEMS] Significant differences in the distribution of GlcNAc-6-sulfotransferase isozymes, sulfation enzymes of sugar residues, between non-carcinoma tissues and carcinoma tissues or adenoma tissues were discovered. The discovery is evidently applicable to detect carcinomas and adenomas (except colorectal carcinomas and colorectal adenomas) specifically by assaying a certain range of GlcNAc-6-sulfated sugar residue groups in tissues of patients and in fecal samples. Examination of carcinomas and adenomas is possible by the use of antibodies reacting specifically with GlcNAc-6-sulfated sugar residues specifically synthesized by enzymes present in carcinoma and adenoma tissues.
Owner:LOCAL GOVERNMENT OF AICHI PREFECTURE +2
Application of DNA binding site CTCF_33 for multifunctional transcriptional regulatory factor CTCF
ActiveCN107227365AImprove accuracyMicrobiological testing/measurementDNA methylationParanasal Sinus Carcinoma
The invention discloses a new purpose of a DNA binding site CTCF_33 for a multifunctional transcriptional regulatory factor CTCF, i.e., the application thereof to prevention of a kit for early diagnosis of colorectal carcinoma. The binding site has the nucleotide sequence shown as SEQ ID NO:1. The accuracy of the CTCT binding site provided by the invention for detecting rectal adenoma and colorectal cancer is obviously higher than that of the reported DNA methylation molecular markers; the DNA binding site of the CTCF can be likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular subtyping (such as CIMP subtyping). Generally, the invention provides a novel idea for finding the more effective tumor early stage diagnosis, treatment effect monitoring, prognosis effect evaluation and molecular subtyping molecular markers, and has the important significance on tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Marking composition for detecting colorectal adenoma and early diagnosis reagent thereof
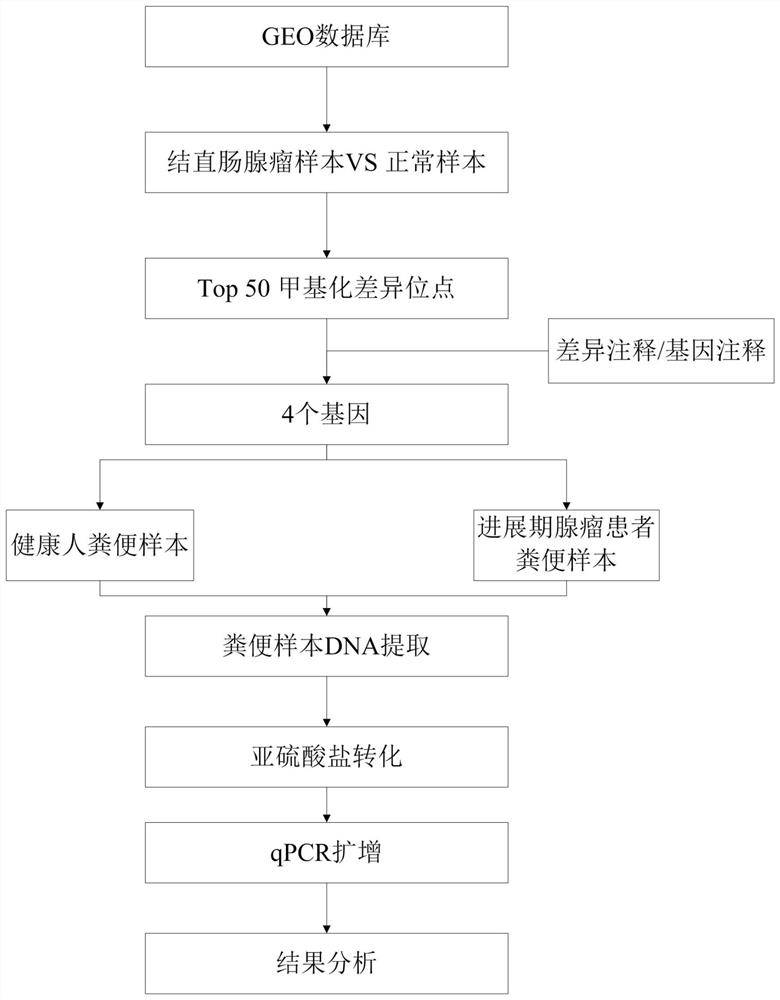
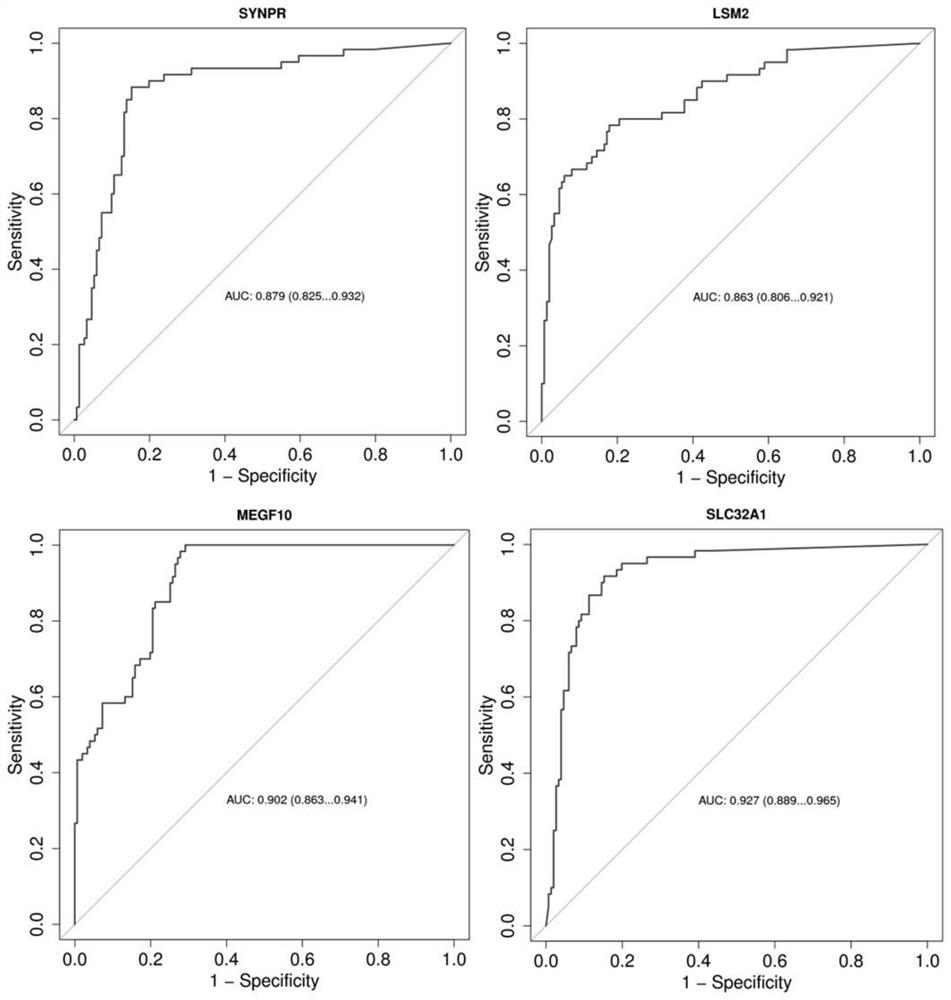
PendingCN114561465ACause painMake an impactMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationAdenoma
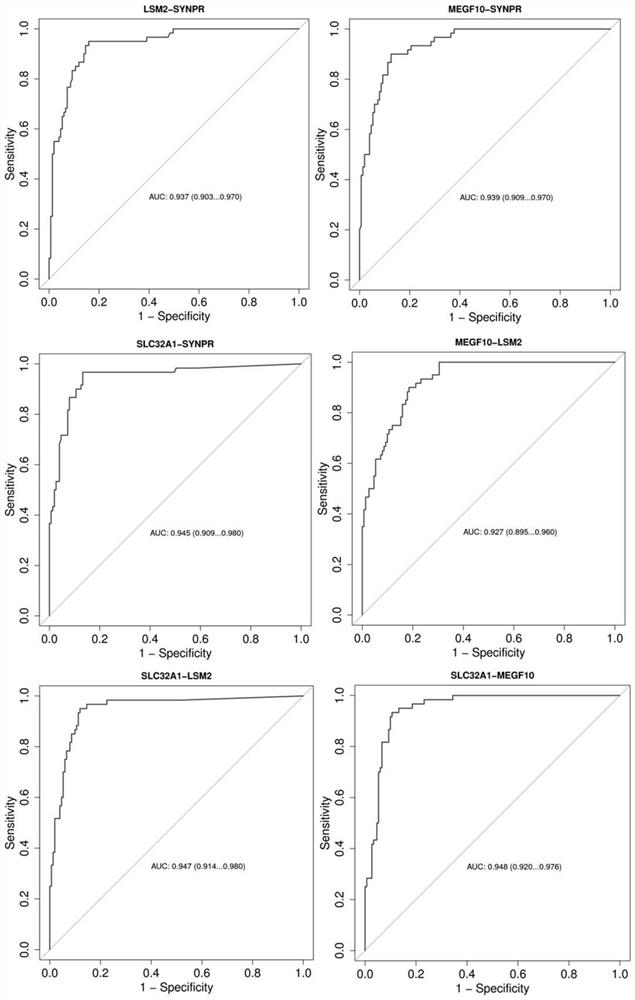
The invention belongs to the field of molecular biology, and particularly relates to a tumor marker combination for detecting colorectal progression stage adenoma and a reagent for early diagnosis of colorectal progression stage adenoma. According to the method, excrement is taken as a detection sample, target gene DNA methylation is taken as a molecular marker, the target gene methylation is one or more of SYNPR, MEGF10, LSM2 and SLC32A1 genes, and the methylation level of the target gene methylation is detected. Results show that the provided reagent for detecting single or combined SYNPR, MEGF10, LSM2 and SLC32A1 genes can well distinguish colorectal progression stage adenoma from normal human samples, and can be used for screening and early diagnosis of colorectal progression stage adenoma. The invention also relates to a specific primer and a specific probe for detecting methylation of the SYNPR gene, the MEGF10 gene, the LSM2 gene and the SLC32A1 gene.
Owner:SHANGHAI REALBIO TECH CO LTD +1
Application of the DNA-binding site CTCF_55 of the multifunctional transcriptional regulator CTCF
The invention discloses novel application of a DNA binding site CTCF_55 of a multifunctional transcriptional regulation factor CTCF, and particularly relates to application of the DNA binding site CTCF_55 in preparation of a kit for early diagnosis of a colorectal tumor. The nucleotide of the binding site is shown as SEQ ID NO: 1. The accuracy of the CTCF binding site provided by the invention for detecting colorectal adenomas or a colorectal cancer is obviously higher than that of a reported DNA methylated molecular marker; furthermore, the DNA binding site of the CTCF may be a biological marker for monitoring the tumor treatment effect, estimating the prognosis effect and tumor molecular parting (such as CIMP parting). In conclusion, the invention provides a new idea for searching a more effective molecular marker for early diagnosis of the tumor, monitoring of the treatment effect, estimation of the prognosis effect and the molecular parting, and the application has important significance for prevention and treatment of the tumor.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com