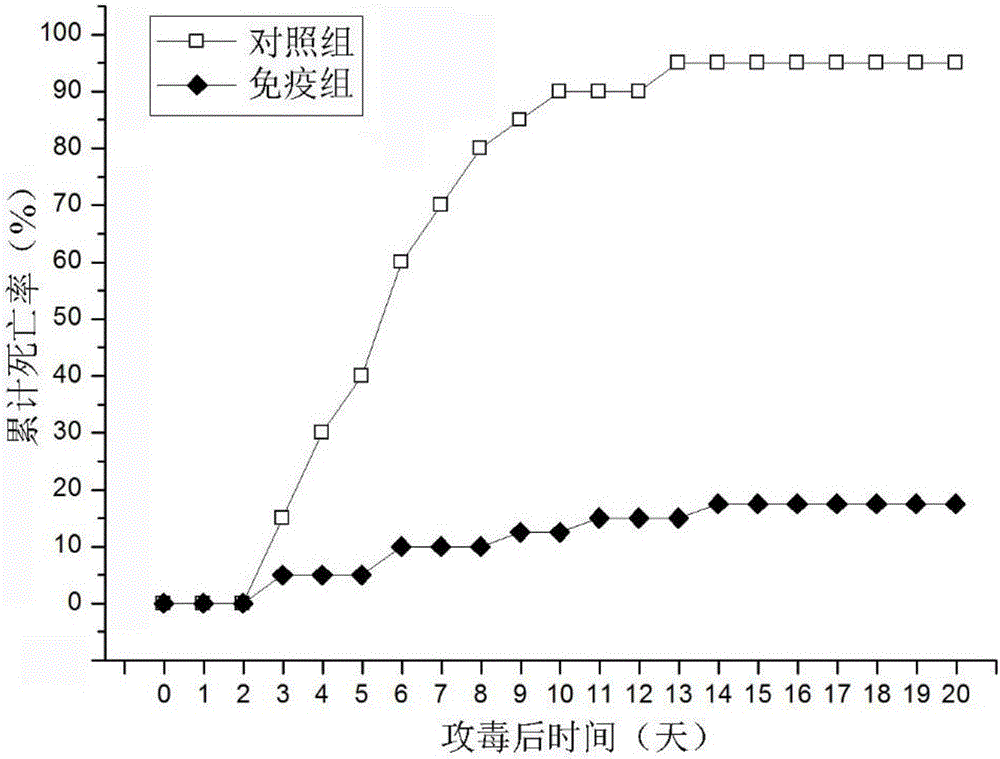
Edwardsiella tarda subunit oral microencapsule vaccine for aquatic product
A subunit vaccine and microcapsule technology, which is applied in the direction of microcapsules, vaccines, and capsule delivery, can solve the problems of physiological damage to fish bodies and difficulty in implementing small-scale fish bodies, and achieve good immune effects, good immune enhancement effects, and convenient delivery. The effect of large-scale promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Isolation of Edwardsiella tarda strains and analysis of their virulence characteristics
[0021] (1) The diseased flounder was taken from Shandong Huangdao Fish Farm. The average weight of the diseased fish was (200±5) g, and the average body length was (20±5) cm. The main symptoms were ascites, protruding anus, liver and kidney swelling.
[0022] (2) Take dying diseased fish with typical ascites symptoms, pick some tissues from liver, kidney, ascites and other lesions under aseptic conditions, streak them on a common nutrient agar plate, culture them at 28°C for 24 hours, and pick out those with the same shape. The dominant colonies were purified and cultured until pure cultures were obtained. The formula of ordinary nutrient agar medium is: yeast extract 3g, peptone 10g, sodium chloride 15g, distilled water 1L, pH 7.6-7.8, agar 20g.
[0023] (3) The isolated pure cultures of each bacterial strain were expanded on ordinary nutrient agar plates. After cultu...
Embodiment 2
[0028] Embodiment 2: the prokaryotic recombinant expression of GAPDH, outer membrane protein ompW and flagellin fliC of Edwardsiella tarda bacterial strain ET-1008
[0029] (1) Sequence amplification of the target expressed protein: according to the gene sequences of Genbank blunt Edwardian GAPDH, ompW and fliC, respectively design their specific primers (see Table 1). Using the bacterial genome extraction kit, the extracted bacterial genomic DNA was used as a template, and the forward and reverse primers of each gene were used for PCR amplification. The PCR reaction system and reaction conditions were: 50 μl reaction system, containing 37 μl H 2 O, 5 μl 10×Taq buffer, 4 μl dNTP (2.5 mM), 1 μl each of forward and reverse primers, 1 μl TaqDNA Polymerase and 1 μl DNA template; Extend for 1 min at °C for 30 cycles, and finally extend for 8 min at 72 °C. The PCR product was detected by 1% agarose gel electrophoresis, and the gel imaging system was photographed and observed. The r...
Embodiment 3
[0036] Embodiment 3: the extraction of Edwardsiella tarda lipopolysaccharide (LPS)
[0037] (1) Bacterial culture: use a sterile inoculation loop to pick a small amount of preserved ET-1008 colonies and streak them on a common nutrient agar plate, wash the well-grown bacterial lawn with a liquid medium, and transfer it to a medium containing 40ml In a 100ml Erlenmeyer flask, culture overnight at 28°C with shaking (160rpm). Add 7ml of bacterial solution into a 10ml centrifuge tube, centrifuge at 5000r / min for 10min at 4°C to collect the bacteria, wash with normal saline three times, wash with 7ml of 50mmol / L phosphate buffer (pH 7.0, containing 5mmol / L EDTA, 20mmol / L MgCl 2 ) to suspend bacteria at a concentration of 1×10 8 CFU / ml.
[0038] (2) Preparation of LPS by hot phenol water method: Ultrasonic crushing was carried out under ice bath conditions, and the set time was 5 minutes, of which 4s were on and 4s were off; the bacterial liquid was transferred to a 250ml beaker,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com