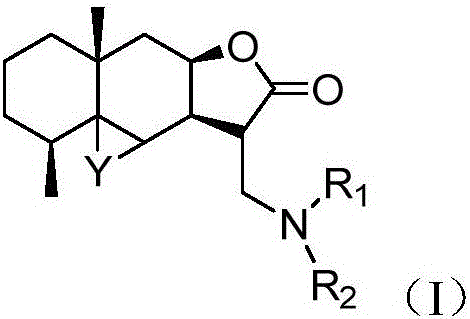
Alantolactone derivative and salts thereof
A technology of inulin and derivatives, which can be applied in drug combinations, endocrine system diseases, digestive system, etc., can solve the problems of poor water solubility, low bioavailability, short half-life, etc., and achieve improved survival and high tumor inhibition rate , the effect of excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
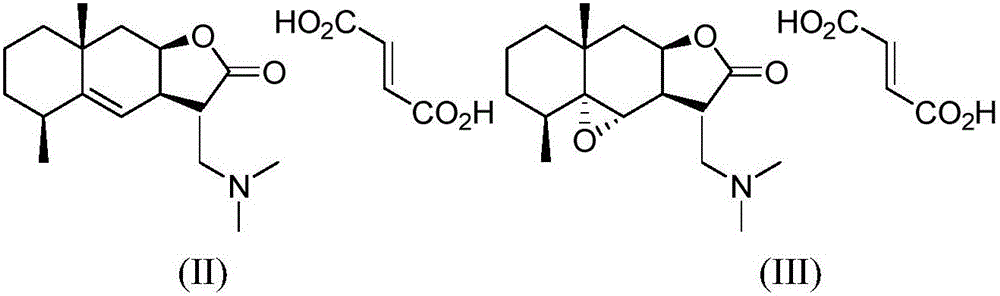
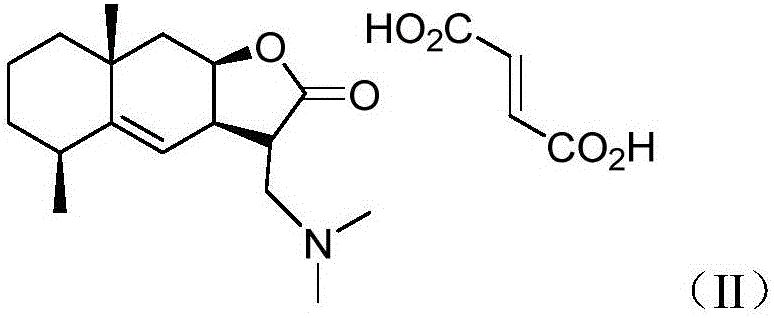
[0024] Preparation of compound (II)
[0025]
[0026] 1) Preparation of compound (I-II)
[0027]
[0028] Inulin (300.0mg, 1.29mmol) was dissolved in dichloromethane (50mL), and dimethylamine hydrochloride (1.6g, 19.4mmol) and potassium carbonate (5.2g, 37.4mmol) were successively added to the above mixed system ), the system was heated to reflux for 3 hours, the solid was removed by filtration, the filtrate was washed with water, dried and concentrated, and purified by column chromatography (petroleum ether: ethyl acetate=1:1) to obtain compound (I-II) (white solid, 236.0g, Yield: 50%).
[0029] 2) Preparation of compound (II), dissolving compound (I-II) (200.0mg, 0.72mmol) obtained in step 1) in methanol (7mL), adding fumaric acid (86.7mg, 0.74mmol ), stirring and reacting for 0.5 hour, spin off the solvent, add ethyl acetate to dissolve, and filter to obtain compound (II) (white solid, 246.4mg, yield: 87.3%)
[0030] Elemental analysis determines the molecular form...
Embodiment 2
[0033] Preparation of compound (III)
[0034]
[0035] 1) Preparation of compound (I-III)
[0036]
[0037]Inulin (2.0g, 8.6mmol) was dissolved in dichloromethane (10mL), and a solution of peroxybenzoic acid (2.1g, 10.3mmol) in dichloromethane (20mL) was slowly added dropwise to the above system. React for 3 hours, quench the reaction with saturated aqueous sodium thiosulfate solution, wash the organic layer with saturated aqueous sodium bicarbonate (20mL×3), dry and concentrate, and purify by column chromatography (petroleum ether: ethyl acetate = 5:2) to obtain the compound (I-III) (white solid, 2.0g, yield 94%)
[0038] 2) Preparation of compound (III)
[0039] The compound (I-III) (300.0mg, 1.29mmol) obtained in the previous step was dissolved in dichloromethane (50mL), and dimethylamine hydrochloride (1.6g, 19.4mmol) and carbonic acid were added to the above mixed system in turn. Potassium (5.2g, 37.4mmol), the system was heated to reflux for 3 hours, the solid w...
Embodiment 3
[0043] In vitro cancer cell inhibitory effect of inulin derivative salts
[0044] The inhibitory effect of inulin derivative salts on cancer cells was detected by tetramethylazolidine blue (MTT) colorimetric method.
[0045] MTT colorimetric method experimental steps: Inoculate cancer cells in the logarithmic growth phase into 96-well cell culture plates at a density of 5×104 cells per milliliter, and adjust the zero wells to normal culture medium without cells. After 12 hours, the medium containing inulin derivatives with different concentration gradients was replaced, and the normal medium was replaced in the zero-adjusted wells. Five replicate wells were set for each concentration gradient, and cultured in a 37°C, 5% CO2 incubator. After 24 hours, the cell state and growth changes were observed under a microscope. After 48 hours, add tetramethylazolazolium blue (KG Bio, 5 mg / mL) solution to each well, continue to cultivate at 37°C, 5% CO2 incubator, suck out the medium aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com