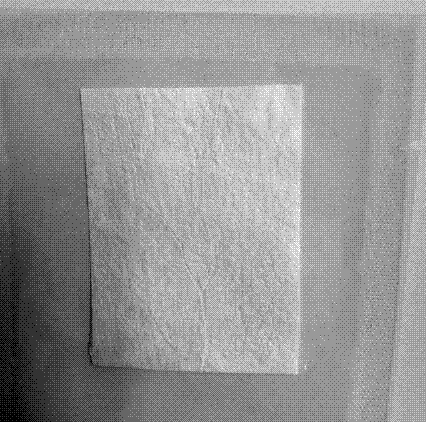
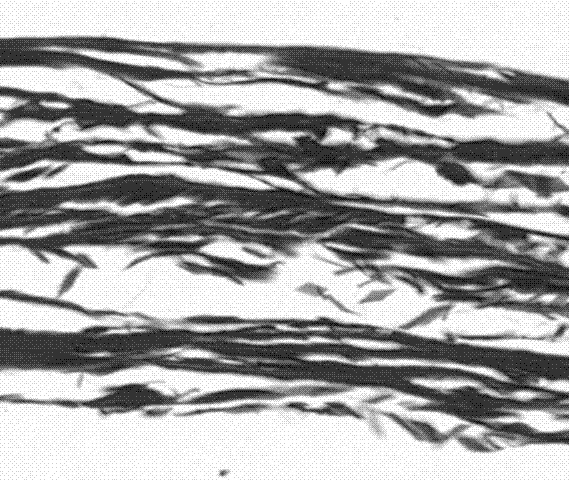
Dura-mater biological patch and preparation method thereof
A biological patch, dura mater technology, applied in tissue regeneration, medical science, prosthesis, etc., can solve the problems of poor decellularization and degreasing, high trypsin concentration, and insufficient adhesion, etc., to prevent cerebrospinal fluid. Effects of leakage, low immune rejection, and synchronized degradation rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Pretreatment
[0041] Freshly slaughtered porcine small intestine tissue was cleaned and soaked in 0.5% acetic acid solution for 30 minutes. The ratio of porcine small intestine to acetic acid solution was 1:5, and the mucosal layer, muscular layer and serosa layer of porcine small intestine and jejunum were removed by physical scraping , Lymph nodes, the submucosa was separated, cut into pieces, and washed 3 times with purified water.
[0042] (2) Disinfection
[0043] Use a mixed aqueous solution containing 1.0% peracetic acid and 15% ethanol, the ratio of the SIS material to the mixed aqueous solution is 1:10, and immerse at room temperature for 100 minutes under ultrasonic conditions for disinfection. Afterwards, it was ultrasonically washed 3 times with purified water.
[0044] (3) Degreasing
[0045] Use ethanol with a concentration of 95%, the ratio of SIS material to ethanol is 1:10, and soak at room temperature for 2 hours under ultrasonic conditions. A...
Embodiment 2
[0053] Embodiment 2: The sample prepared in embodiment 1 is subjected to physical performance detection, chemical performance detection, histological detection, growth factor detection, biological performance detection and animal test.
[0054] 1. Physical performance testing
[0055] 1) Water resistance
[0056] Method: Test according to the method of "YY / T 0471.3-2004 Test Method for Contact Wound Dressing Part 3: Water Resistance".
[0057] The result: water-repelling properties.
[0058] 2) Suture strength
[0059] Method: Use 3-0 non-absorbable suture to suture at the center of both sides of the patch at a distance of 2mm from the edge, fix the other end of the suture and the other end of the sample on the two ends of the tension meter respectively, and stretch it at a speed of 20mm / min. Record the maximum force until the suture is torn. Three batches of samples were tested according to the above method.
[0060] Results: The suture strength was in the range of 3N~5N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| suture strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com