Preparation of a core-shell UV fluorescent molecularly imprinted material and its application in the detection of sulfonamide
A fluorescent molecular imprinting and ultraviolet technology, which is applied in the direction of fluorescence/phosphorescence, material excitation analysis, and other chemical processes, can solve the problems affecting the dispersion and optical stability of imprinted materials, the reproducibility of detection results, and the particle size of the quantum dot core. Small and other problems, to achieve the effect of uniform shape, stable optical properties, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
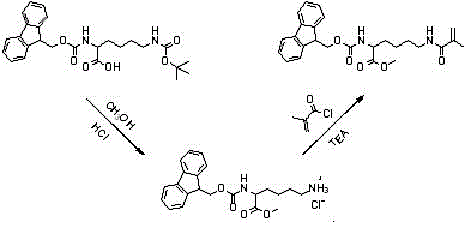
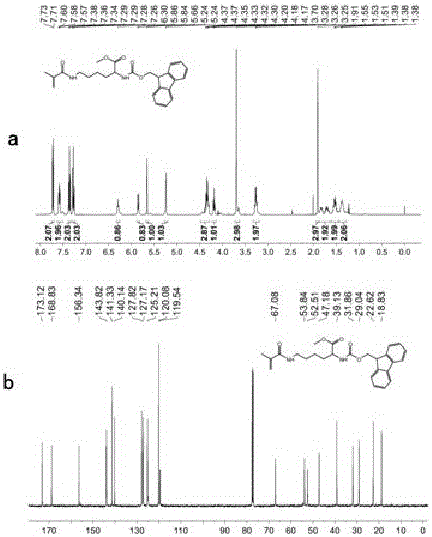
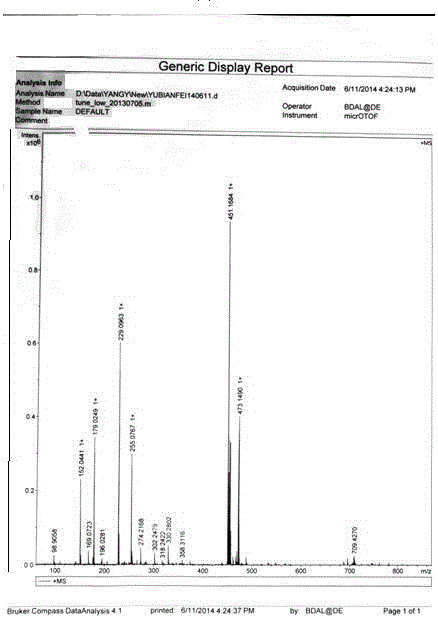
[0034] Preparation of fluorescent functional monomer: Add 2.5 g of N-fluorenylmethylformyl-N-tert-butylformyl-lysine (FMOC-Lys-BOC) into 40 ml of methanol saturated with hydrogen chloride gas, and stir at room temperature for 2 After 1 hour, it was suction filtered, washed 3 times with 25 ml of methanol, and dried to obtain FMOC-protected lysine methyl ester hydrochloride. Take 3 mmol of the raw material that has completely removed BOC and add it to 25 ml of anhydrous dichloromethane (DCM). After adding 3.3 mmol of methacryloyl chloride, add 6 mmol of anhydrous triethylamine (TEA) dropwise. After 1 hour of reaction, add 25 ml of Diluted in dichloromethane (DCM), then washed successively with hydrochloric acid with pH = 1 and saturated brine, the organic phase was dried over anhydrous sodium sulfate, concentrated and purified by column (ethyl acetate: petroleum ether = 1:2), The product was spin-dried to obtain white crystals of N-fluorenylmethylformyl-N-methacryloyl-lysine met...
Embodiment 2
[0040] Five batches of fluorescent imprinted materials doped with different functional monomers were prepared by bulk polymerization (from which the optimal raw material ratio was screened out): Taking the fluorescent imprinted materials doped with methacrylic acid (MAA) functional monomer as an example, 0.15 Add 1 mmol template molecule SMZ and 0.45 mmol MAA to a 3 ml vial with a cap, then add 1.5 ml N,N-dimethylformamide (DMF) to dissolve, stir magnetically for 1 h, then add fluorescent monomer FMOC-Lys-MC , stirred at room temperature for 1 hour after bubbling nitrogen gas, and left overnight. Then add 1.5 mmol of cross-linking agent ethylene glycol dimethacrylate (EGDMA) and 6 mg of initiator azobisisobutyronitrile (AIBN) in sequence, stir at 60°C for 20 hours after nitrogen blowing, grind and sieve, then Soxhlet wash (methanol: acetic acid = 8:1) until the supernatant has no UV absorption of template molecules, dry it under vacuum at 60°C for use. This material number is...
Embodiment 3
[0042] Preparation of methacrylic silicon sphere carrier: mix 80 ml of ethanol, 6 ml of water, and 3 ml of ammonia water, add 8 ml of tetraethyl orthosilicate (TEOS) dropwise under stirring, react at room temperature for 8 hours, and centrifuge with ethanol Wash twice, then disperse the resulting silicon spheres into 60 ml of ethanol, add 0.5 ml of water and 0.1 ml of ammonia water, add 1 ml of methacrylpropyltrimethoxysilane (MPS) dropwise under stirring, and react for 12 hours Centrifuge and wash with ethanol for 3 times, and vacuum-dry at 50°C to obtain 2.28 g of methacrylylated silicon spheres, which are refrigerated for use.
[0043] Add 0.15 mmol of the template molecule SMZ and 0.15 mmol of the fluorescent monomer FMOC-Lys-MC into 25 ml of acetonitrile for ultrasonic dissolution, and stir at room temperature for 5 hours after bubbling with nitrogen, then add 1.5 mmol of cross-linking agent EGDMA and 300 mg of methacryl 5 ml of acetonitrile solution of silicon spheres, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com