Preparation method and use of catalyst for glucose hydrogenolysis preparation of low carbon dihydric alcohol
A technology of glucose and catalyst, applied in the field of catalyst preparation, can solve the problems of high pressure, water pollution and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
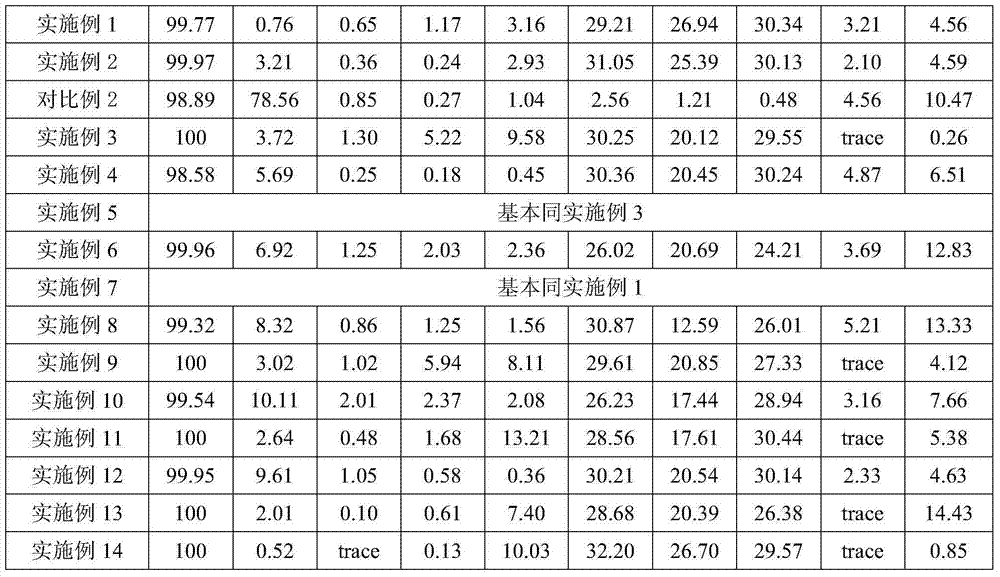
Examples
Embodiment 1
[0090] A method for preparing a catalyst that can be used for hydrogenolysis of glucose to prepare low-carbon dihydric alcohols, comprising the following steps:
[0091]1. Pretreatment of silica carrier
[0092] 1 takes by weighing 5g of silicon dioxide, and at 200°C, it is placed in an air atmosphere and roasted for 2h, taken out after cooling to room temperature, and transferred to a three-necked flask;
[0093] II Add 80mL of PEG-200 pure solution to the flask, use an electric heating mantle to raise the temperature to 120°C, heat for 2h and then cool to room temperature;
[0094] III Use a Buchner funnel to filter at room temperature for 1 hour, take out the filter residue, vacuumize it for 1 hour, then place it in a vacuum oven, dry it under vacuum at 120°C for 10 hours, and finally bake it in an air atmosphere at 500°C for 2 hours, and cool it to Packaged after room temperature for use.
[0095] 2. Loading auxiliary metals on silica
[0096] ① Take a certain amount of...
Embodiment 2
[0110] A method for preparing a catalyst that can be used for hydrogenolysis of glucose to prepare low-carbon dihydric alcohols, comprising the following steps:
[0111] 1. Pretreatment of activated carbon AC
[0112] 1 takes by weighing 5g gac, at 200 ℃, it is placed in the roasting 2h of nitrogen atmosphere, takes out after being cooled to room temperature, transfers in the three-necked flask;
[0113] II Add 80mL of pure PEG-400 solution to the flask, use an electric heating mantle to raise the temperature to 120°C, heat for 2 hours and then cool to room temperature;
[0114] III Use a Buchner funnel to filter at room temperature for 1h, take out the filter residue, vacuumize for 1h, then place it in a vacuum oven, dry it in vacuum at 120°C for 10h, and finally roast it in a nitrogen atmosphere at 500°C for 2h, and cool to Packaged after room temperature for use.
[0115] 2. Loading auxiliary metals on activated carbon
[0116] ① Take a certain amount of ammonium molybda...
Embodiment 3
[0128] A method for preparing a catalyst that can be used for hydrogenolysis of glucose to prepare low-carbon dihydric alcohols, comprising the following steps:
[0129] 1. Pretreatment of carbon fiber CNFs
[0130] I Weigh 5g of carbon fiber carrier and place it in a three-necked flask, add 80mL of nitric acid solution into the flask, use an electric heating mantle to raise the temperature to 80°C, heat for 2h and condense and reflux, then cool to room temperature; then, at 120°C in an air atmosphere Dry for 10 hours;
[0131] II Add 80mL of PEG-600 pure solution to the flask, use an electric heating mantle to raise the temperature to 120°C, heat for 2 hours and then cool to room temperature;
[0132] III is the same as embodiment 1.
[0133] 2. Support additive metal on carbon fiber
[0134] ① Dissolve a certain amount of zirconium oxynitrate precursor in deionized water to prepare a zirconium nitrate aqueous solution, and impregnate the treated CNFs carrier with an equal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com