System and method for preparing high-purity vanadium electrolytic solution through efficient clean chlorination method
A chlorination method and high-purity technology, which is applied in the field of high-purity vanadium electrolyte preparation by an efficient and clean chlorination method, can solve the problems of environmental pollution, unfavorable production costs, and inability to use economically and efficiently, so as to reduce pollution and save production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
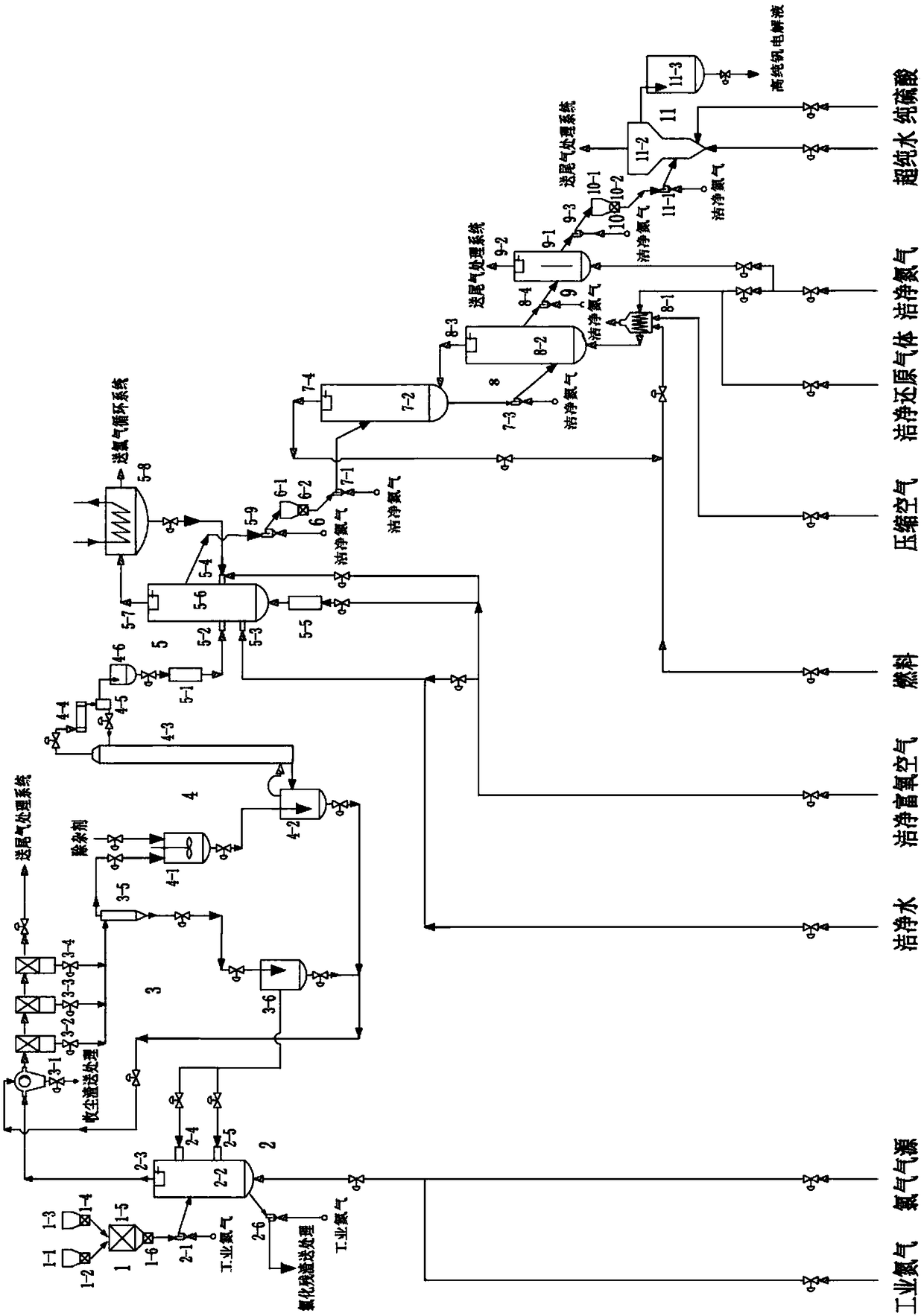
[0112] A system for preparing high-purity vanadium electrolyte by an efficient and clean chlorination method, such as figure 1 As shown, it includes feeding section 1, chlorination section 2, dedusting and rinsing section 3, purification section 4, catalytic oxidation section 5, catalytic oxidation product feeding section 6, fluidized bed preheating section 7, reduction roasting section 8, cooling flow Chemical bed section 9, high-purity low-valent vanadium oxide feeding section 10 and liquid-solid fluidized bed dissolving section 11;
[0113] Feeding section 1 includes industrial-grade vanadium oxide silo 1-1, industrial-grade vanadium oxide star feeder 1-2, carbon source silo 1-3, carbon source star feeder 1-4, and mixer 1-5 and mixer star feeder 1-6;
[0114] Chlorination section 2 includes fluidized chlorination furnace feeder 2-1, fluidized chlorination furnace 2-2, chlorination furnace cyclone separator 2-3, first slurry nozzle 2-4, second slurry nozzle 2- 5 and chlori...
Embodiment 2
[0136] A kind of method based on the high-efficiency clean chlorination method of system described in embodiment 1 prepares high-purity vanadium electrolyte, comprises the following steps:
[0137] The industrial-grade vanadium oxide in the industrial-grade vanadium oxide silo 1-1 and the carbon source in the carbon source silo 1-3 are respectively fed by the industrial-grade vanadium oxide star feeder 1-2 and the carbon source star The machine 1-4 enters the mixer 1-5 for mixing at the same time; the mixed material enters the boiling chlorination furnace 2-2 through the star feeder 1-6 of the mixer and the feeder 2-1 of the boiling chlorination furnace in turn. The chlorine from the chlorine gas source main pipe and the nitrogen of the industrial nitrogen main pipe enter in the described ebullient chlorination furnace 2-2 through the air inlet of the boiling chlorination furnace 2-2 bottom, so that the industrial-grade vanadium oxide and carbon source maintain flow Chlorine g...
Embodiment 3
[0146] In this example, powdered industrial-grade vanadium pentoxide (purity: 98.50%) is used as raw material, and the method described in Example 2 is used to prepare a high-purity vanadium electrolyte. The processing capacity of industrial-grade vanadium pentoxide is 80kg / h. After chlorination, dedusting and rinsing, vanadium oxychloride purification, catalytic oxidation, fluidized reduction, and fluidized bed dissolution, the high-purity vanadium pentoxide with an average valence of 3.0 is prepared. Vanadium electrolyte.
[0147] In the boiling chlorination furnace 2-2, the amount of petroleum coke added in the chlorination process is 30% of the quality of the industrial-grade vanadium pentoxide powder, and the chlorination operating temperature is 600 ° C. The gas velocity of the mixed gas of chlorine and nitrogen is 3.0m / s, and the mole fraction of chlorine in the mixed gas of chlorine and nitrogen entering the boiling chlorination furnace 2-2 is 20%; The impurity remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com