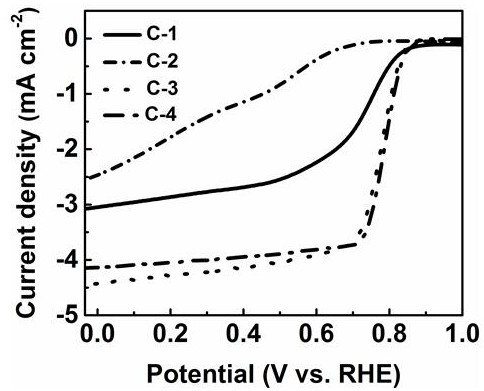
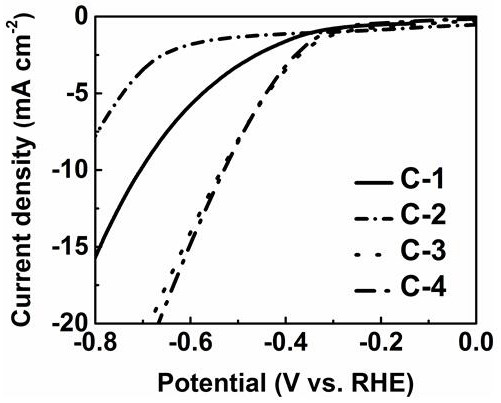
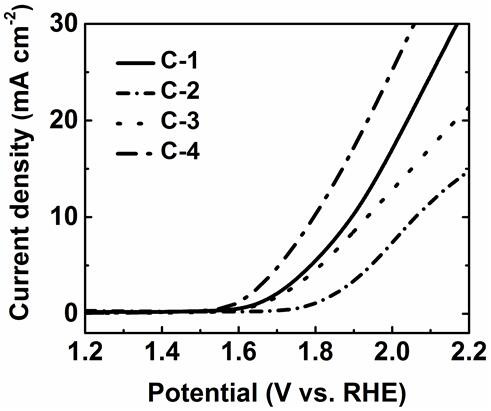
Electronegative heteroatom-transition metal co-doped carbon-based non-noble metal electrocatalyst and preparation method thereof
A transition metal and non-noble metal technology, which is applied in the field of electronegative heteroatom-transition metal co-doped carbon-based non-noble metal electrocatalyst and its preparation, can solve the gap between catalytic activity and stability, and the inability to effectively separate the catalyst from the substrate material , poor conductivity of metal phosphides, etc., to achieve the effect of improving catalytic performance, improving catalyst performance, and improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 10 mmol cyclobutanecarboxylic acid, 0.01 mmol sodium hypophosphite, 10 mmol ethylenediamine, 15 mmol thiopropionamide and 23 mmol cobalt chloride were fully stirred in 30 mL deionized water for 6 h; the resulting mixed solution was placed in The solvothermal reaction was carried out in the reaction kettle, the reaction temperature was 120 °C, and the reaction time was 10 h; then the obtained reactant was dried, placed in a tube furnace, and the temperature was raised to 600 °C at a rate of 1 °C / min in a nitrogen atmosphere. and keep the temperature down to room temperature after 2 h; the product was taken out from the tube furnace, washed with 1M dilute hydrochloric acid, then washed with deionized water until neutral, and dried to obtain the electronegative heteroatom-transition metal co-doped carbon-based non- Noble metal electrocatalysts. The obtained product is marked as C-1, and the appearance of the catalyst is black powder, which is tested by ICP-AES (inductively...
Embodiment 2
[0032] Stir 10 mmol 5-methyl-2-pyrazinecarboxylic acid, 0.8 mmol potassium dihydrogen phosphate, 20 mmol sodium dicyanamide, 5 mmol methyl propyl disulfide and 10 mmol cobalt chloride in 30 mL ethanol 14 h; the resulting mixed solution was placed in a reactor for solvothermal reaction at a reaction temperature of 125 °C and a reaction time of 15 h; then the obtained reactant was dried and placed in a tube furnace at 2 °C in a nitrogen atmosphere The heating rate was increased to 540 °C / min, and the temperature was kept constant for 3 h and then lowered to room temperature; the product was taken out from the tube furnace, washed with 1M dilute hydrochloric acid, then washed with deionized water until neutral, and dried to obtain the electronegative impurity Atom-transition metal co-doped carbon-based non-noble metal electrocatalysts. The obtained product is marked as C-2, and the appearance of the catalyst is black powder, which is tested by ICP-AES (inductively coupled atomic ...
Embodiment 3
[0035] Stir 10 mmol 4-hydroxycyclohexanecarboxylic acid, 0.1 mmol melamine phosphate, 25 mmol methylamine, 15 mmol methyl thiocyanate and 20 mmol ferric acetate in 30 mL methanol for 3 h; place the resulting mixed solution in the reaction The solvothermal reaction was carried out in a kettle, the reaction temperature was 100 °C, and the reaction time was 12 h; then the obtained reactant was dried, placed in a tube furnace, and the temperature was raised to 800 °C at a rate of 0.5 °C / min in a nitrogen atmosphere, and After constant temperature for 0.5 h, it was lowered to room temperature; the product was taken out from the tube furnace, washed with 1M dilute hydrochloric acid, then washed with deionized water until neutral, and dried to obtain the electronegative heteroatom-transition metal co-doped carbon-based non-noble metal electrocatalyst. The obtained product is marked as C-3, and the appearance of the catalyst is black powder, which is tested by ICP-AES (inductively cou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com