PHARMACEUTICAL COMPOSITION FOR TREATING BONE DISEASES WHICH COMPRISES PROTEIN COMPRISING Frizzled1, Frizzled2 OR Frizzled7 EXTRACELLULAR CYSTEINE-RICH DOMAIN
a technology of protein and therapeutic agent, applied in the field of bone disease therapy, can solve the problems of increasing increasing the burden on society, medical, economic, etc., and reducing the number of people with osteoporosis, so as to improve the quality of life, increase the number of bone mass and reduce the effect of bone mass, bone density, and/or bone strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of USmFZD7crd-hFcm KI Chimeric Mouse
[0196]In accordance with the method described in the examples of WO 2006 / 78072, a pUSmFZD7crd-hFcm KI vector was prepared from mouse FZD7-cDNA (a 1,719-bp sequence comprising a region from an initiation codon to a termination codon, SEQ ID NO: 1) and human IgG1 Fc mutant-cDNA (a 702-bp sequence comprising a region from a linker sequence to a termination codon inserted to bind to the FZD7 extracellular cysteine-rich domain, SEQ ID NO: 3).
[0197]The mouse FZD7 signal sequence, a CRD (the cystein-rich-domain), and a region located downstream of a CRD comprising the 7-transmembrane domain in SEQ ID NO: 1 are marked by a single underline, a solid box, and a double underline, respectively, based on the information regarding the GenBank Accession Numbers: NM—008057.2 and NP—032083.2.
SEQ ID NO: 1:ATGCGGGGCCCCGGCACGGCGGCGTCGCACTCGCCCCTGGGCCTCTGCGCCCTGGTGCTTGCTCTTCTGTGCGCGCTGCCCACGGACACCCGGGCTCCAGACCCACCTTTCACTGCGATGTCCCCCTCAGATGGCAGAGGCCGCTTGTCT...
example 2
Analysis of USmFZD7crd-hFcm KI Chimeric Mouse
2-1. Necropsy Finding
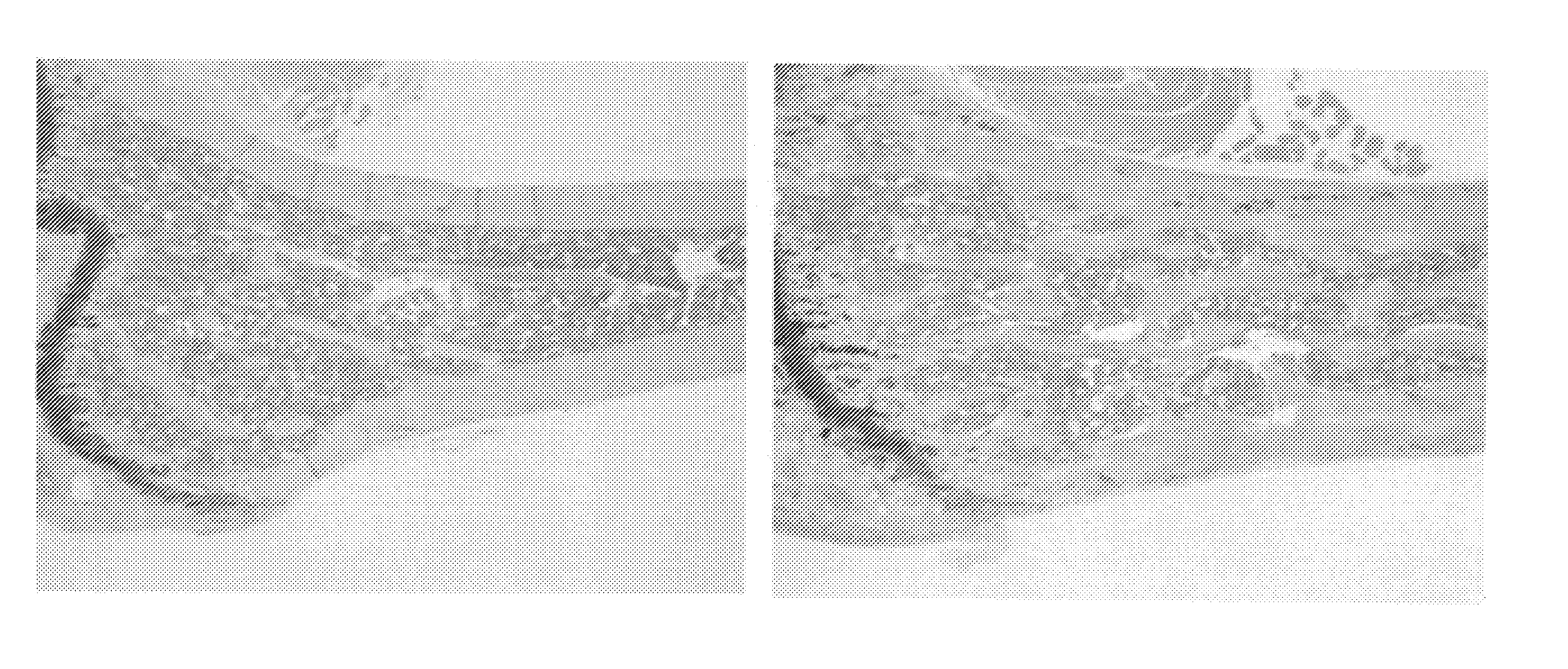
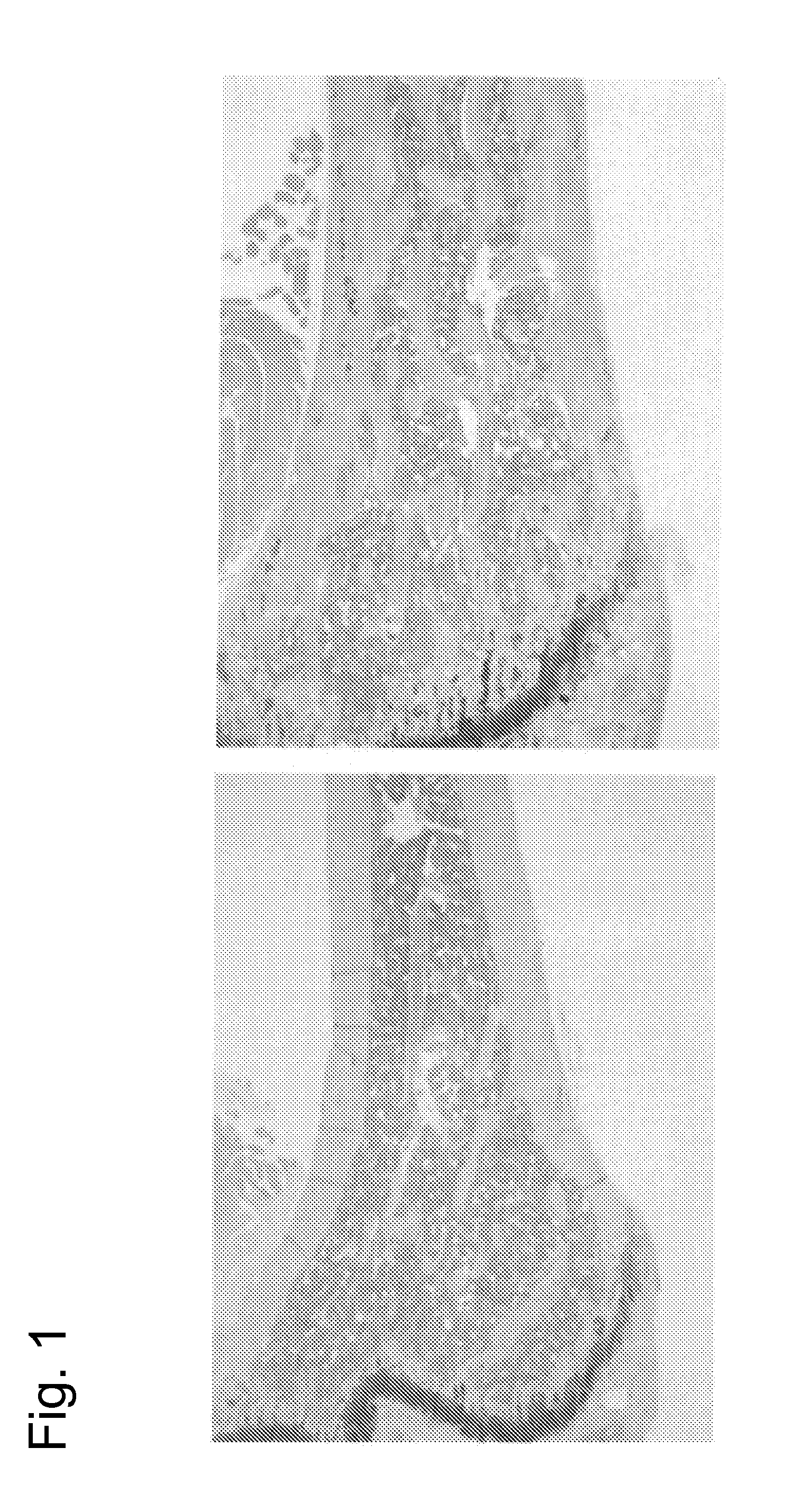
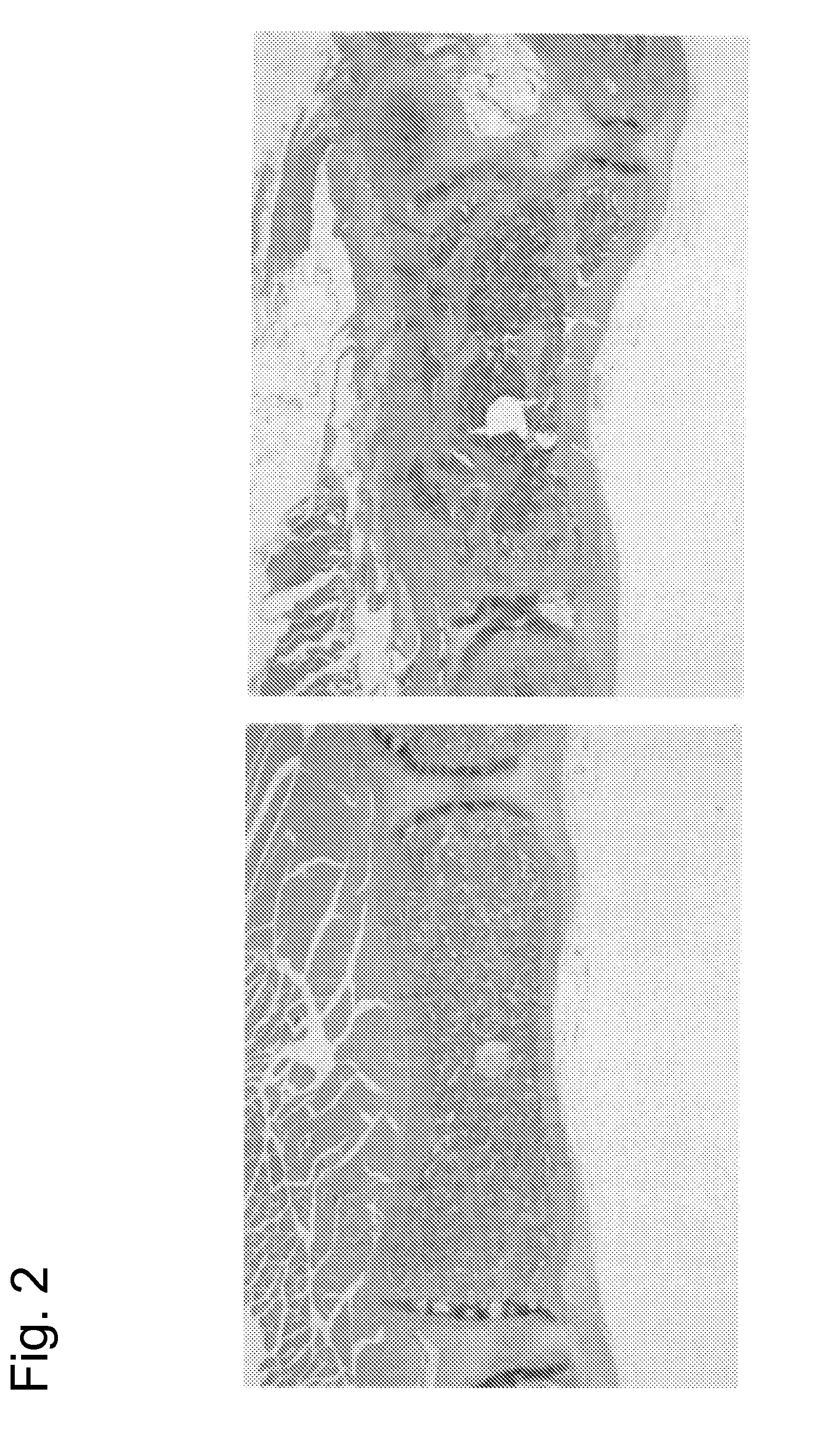
[0204]The chimeric mice prepared in Example 1 were subjected to necropsy at age of 16 weeks, and the spleen, the liver, the kidney, the adrenal gland, the stomach, the small intestine, the appendix, the large intestine, the pancreas, the mesenteric lymph node, the female / male reproductive organ, the thymic gland, the lung, the heart, the brain, the muscle, the skin, the femur, the sternum, the cranium, the spondylus, and the costa were observed. As a result, whitening of the femur, whitening of the sternum, whitening and hardening of the cranium, whitening and hardening of the spondylus, and hardening of the costa were more significantly observed as characteristic changes in the USmFZD7crd-hFcm KI chimeric mice compared with the control mice. In addition, spleen enlargement was observed in approximately a half of the USmFZD7crd-hFcm KI chimeric mice. The number of mice exhibiting changes is described below.
2-1-1. Femu...
example 3
Preparation of UShFZD7crd-hFcm KI Chimeric Mouse
[0225]A pUShFZD7crd-hFcm KI vector was prepared from human FZD7-cDNA (SEQ ID NO: 7) and human IgG1 Fc mutant-cDNA (SEQ ID NO: 3) in accordance with the method described in Example 1.
[0226]The human FZD7 signal sequence, CRD, and a region located downstream of a CRD comprising the 7-transmembrane domain in SEQ ID NO: 7 are marked by a single underline, a solid box, and a double underline, respectively, based on the information regarding the GenBank Accession Numbers: NM—003507.1 and NP—003498.1.
SEQ ID NO: 7:
[0227]The amino acid sequence encoded by SEQ ID NO: 7 (574 amino acids, SEQ ID NO: 8) is shown below.
SEQ ID NO: 8:
[0228]A polynucleotide sequence comprising a region from the initiation codon to the termination codon of the pUShFZD7crd-hFcm KI vector expression unit (SEQ ID NO: 9; a 1,462-bp sequence comprising a mouse Igκ signal sequence containing an intron region (a region marked by a single underline) substituted with the human F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com