EGCG and gamma-aminobutyric acid composition, and preparation method and applications thereof
A technology of aminobutyric acid and composition, which is applied in the directions of drug combination, pill delivery, cardiovascular system diseases, etc., can solve the problems of poor stability of EGCG, adverse effects on product preparation, storage, circulation, and restricted compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
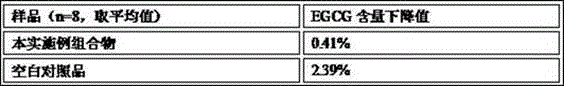
Embodiment 1
[0142] The compound tablet of embodiment 1, EGCG and gamma-aminobutyric acid and preparation thereof
[0143] The prescription quantity is calculated by weight percentage and contains:
[0144] EGCG 5%~25%, preferably 15%,
[0145] γ-aminobutyric acid 7%~35%, preferably 25%,
[0146] Mannitol 20%~45%, preferably 37%,
[0147] Calcium hydrogen phosphate 8% to 25%, preferably 15%,
[0148] Sodium carboxymethyl starch 1%~10%, preferably 5%,
[0149] 2.5% hydroxypropyl cellulose aqueous solution Appropriate amount, preferably 2% based on hydroxypropyl cellulose,
[0150] Magnesium stearate 0.5% to 2%, preferably 1%;
[0151] Its preparation method includes: passing EGCG, γ-aminobutyric acid, mannitol, calcium hydrogen phosphate, and sodium carboxymethyl starch respectively through 80 mesh sieves, preferably 100 mesh, and weighing EGCG and mannitol Mix evenly to get A mixed powder, and mix γ-aminobutyric acid and calcium hydrogen phos...
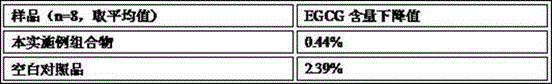
Embodiment 2
[0156] Compound granule of embodiment 2, EGCG and gamma-aminobutyric acid and its preparation
[0157] The prescription quantity is calculated by weight percentage and contains:
[0158] EGCG 1%~6%, preferably 3%,
[0159] γ-aminobutyric acid 1%~10%, preferably 5%,
[0160] Granule auxiliary material 85%~98%, preferably fructose 92%;
[0161] Wherein the granule excipients include one or more of the following ingredients: fructose, xylitol, fructooligosaccharides, xylooligosaccharides, polydextrose, mannitol, sucrose, invert sugar (i.e. an equal mixture of glucose and fructose) ), glucose, resistant dextrin, sorbitol, maltose, isomalt, mannose, solid polyethylene glycols (such as polyethylene glycol-4000, polyethylene glycol-12000, polyethylene glycol Alcohol-6000, polyethylene glycol-2000, etc.), dextrin, cyclodextrin, maltodextrin, hydroxypropyl-β-cyclodextrin, microcrystalline cellulose, carboxymethyl cellulose, hydroxypropyl methylcellulose Vegetarian...
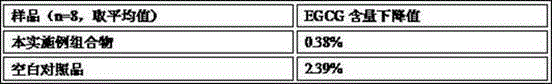
Embodiment 3
[0167] The compound capsule of embodiment 3, EGCG and gamma-aminobutyric acid and preparation thereof
[0168] The prescription quantity is calculated by weight percentage and contains:
[0169] EGCG 10%~30%, preferably 15%,
[0170] γ-aminobutyric acid 15%~35%, preferably 30%,
[0171] Mannitol 15%~40%, preferably 36%,
[0172] Pregelatinized starch 8%~25%, preferably 12%,
[0173] Sodium carboxymethyl starch 1%~10%, preferably 5%,
[0174] The ethanol solution of polyvinylpyrrolidone is an appropriate amount, preferably 1% with polyvinylpyrrolidone,
[0175] Magnesium stearate 0.5% to 2%, preferably 1%;
[0176] Its preparation method comprises: passing EGCG, γ-aminobutyric acid, mannitol, pregelatinized starch and sodium carboxymethyl starch through 80 mesh sieve respectively, preferably passing through 100 mesh sieve, after weighing according to the prescription amount, firstly mix EGCG and Mix γ-aminobutyric acid evenly to obtain mixed powder A, which is ready for u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com