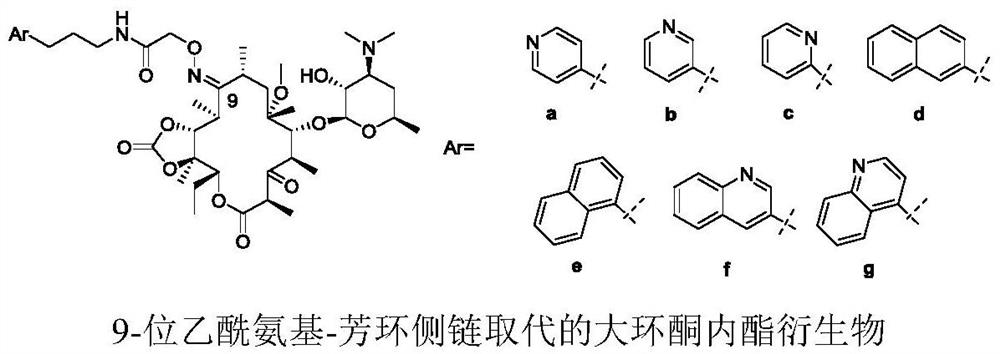
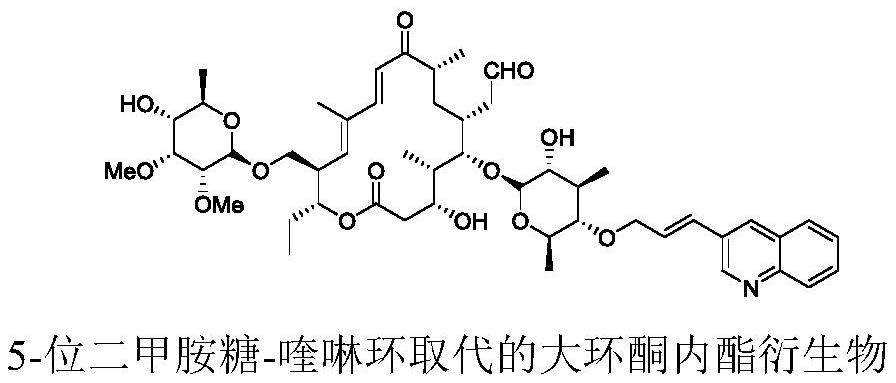
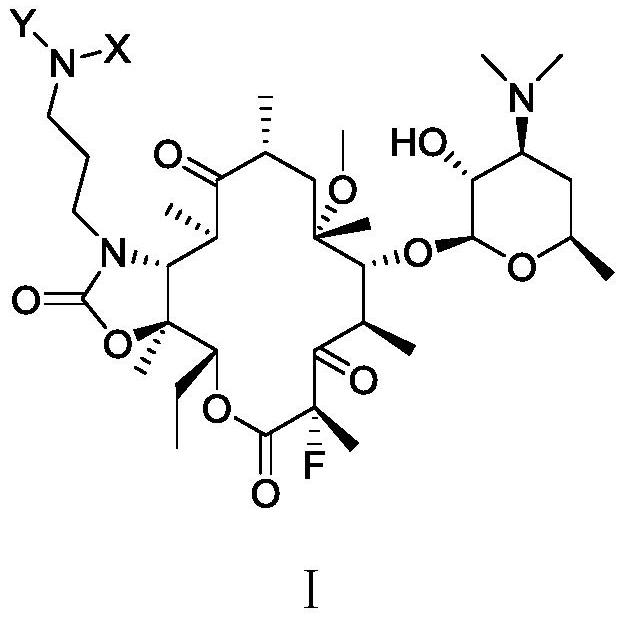
Erythromycin A ketolide antibiotic derivative, its preparation method and application
A technology of antibiotics and derivatives, applied in the field of medicine, can solve the problems of increasing and affecting the clinical application of telithromycin, and achieve the effects of high total yield, novel structure and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0074] Preparation part
[0075] The structure of the compound was obtained by H NMR spectroscopy ( 1 H NMR), carbon nuclear magnetic resonance ( 13 C NMR) and mass spectrometry (MS). Proton and Carbon NMR shifts (δ) are given in parts per million (ppm). Proton NMR spectrum is measured with Mercury-300, Mercury-400, Bruke-400 or Mercury-600 type nuclear magnetic resonance instrument, deuterated chloroform (CDCl 3 ) or heavy water (D 2 O) or deuterated dimethylsulfoxide (DMSO-d 6 ) as solvent and tetramethylsilane (TMS) as internal standard.
[0076] High-resolution mass spectrometry was determined by Agilent 1100series LC / MSD trap mass spectrometer or Theromo Exactive orbitrap plus LC / MSD mass spectrometer.
[0077] Column chromatography generally uses 160-200 mesh silica gel as the carrier.
[0078] Anhydrous solvents were all worked up by standard methods. Other reagents were commercially available analytically pure.
[0079] in,
[0080] Acetyl
[0081] Bn benzyl...
preparation example 1
[0113] The preparation of preparation example 1 raw material compound 8
[0114] The macrocyclic ketolide compound of the present invention starts from clarithromycin, adopts known literature reports or a feasible reaction method known to the public in synthetic chemistry, and obtains appropriate derivatives as raw materials of the present invention.
[0115]
[0116]Add 2.0 g, 2.675 mmol of clarithromycin to 20 ml of 1N hydrochloric acid under cooling in an ice-water bath and stir. When the reaction starts, the reaction solution becomes viscous. Continue stirring for 4 hours, and the reaction solution gradually becomes clear. TLC (CH 2 Cl 2 :CH 3 OH=10:1) to monitor the completion of the reaction, that is, to stop the reaction. Cool the reaction system in an ice-water bath, add 20% sodium hydroxide solution dropwise to the reaction solution while stirring, adjust the pH to alkaline, a large amount of white solid precipitates, filter, and the filter cake is washed with w...
preparation example 2
[0153] The synthetic route of preparation example 2 intermediate side chain a4
[0154]
[0155] Scheme 1 reagents and reaction conditions: a. Di-tert-butyl dicarbonate, sodium bis(trimethylsilyl)amide, tetrahydrofuran, room temperature, 2 hours, 92.8%; b. Nitrogen-3-bromopropyl-phthalamide Formimide, potassium carbonate, N,N-dimethylformamide, 90°C, 8 hours, 85.2%; c. Trifluoroacetic acid, dichloromethane, 0°C, 8 hours, 97.3%; d. Hydrazine hydrate, Ethanol, 4 hours, 80°C, 82.7%;
[0156] Dissolve 10.02g, 69.50mmol of 3-aminoquinoline in 150ml of dry tetrahydrofuran, place the reaction system in an ice-water bath for cooling, and slowly add 69ml of bis(trimethylsilyl) to the reaction system under the protection of argon. Base) sodium amide (THF solution of 2mol / L), then add 16.68g, 76.45mmol of di-tert-butyl dicarbonate, the reaction solution is gradually returned to room temperature, TLC (petroleum ether: ethyl acetate=1:1) monitoring When the reaction is complete, stop ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com