Patents
Literature
41 results about "HRT - Hormone replacement therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
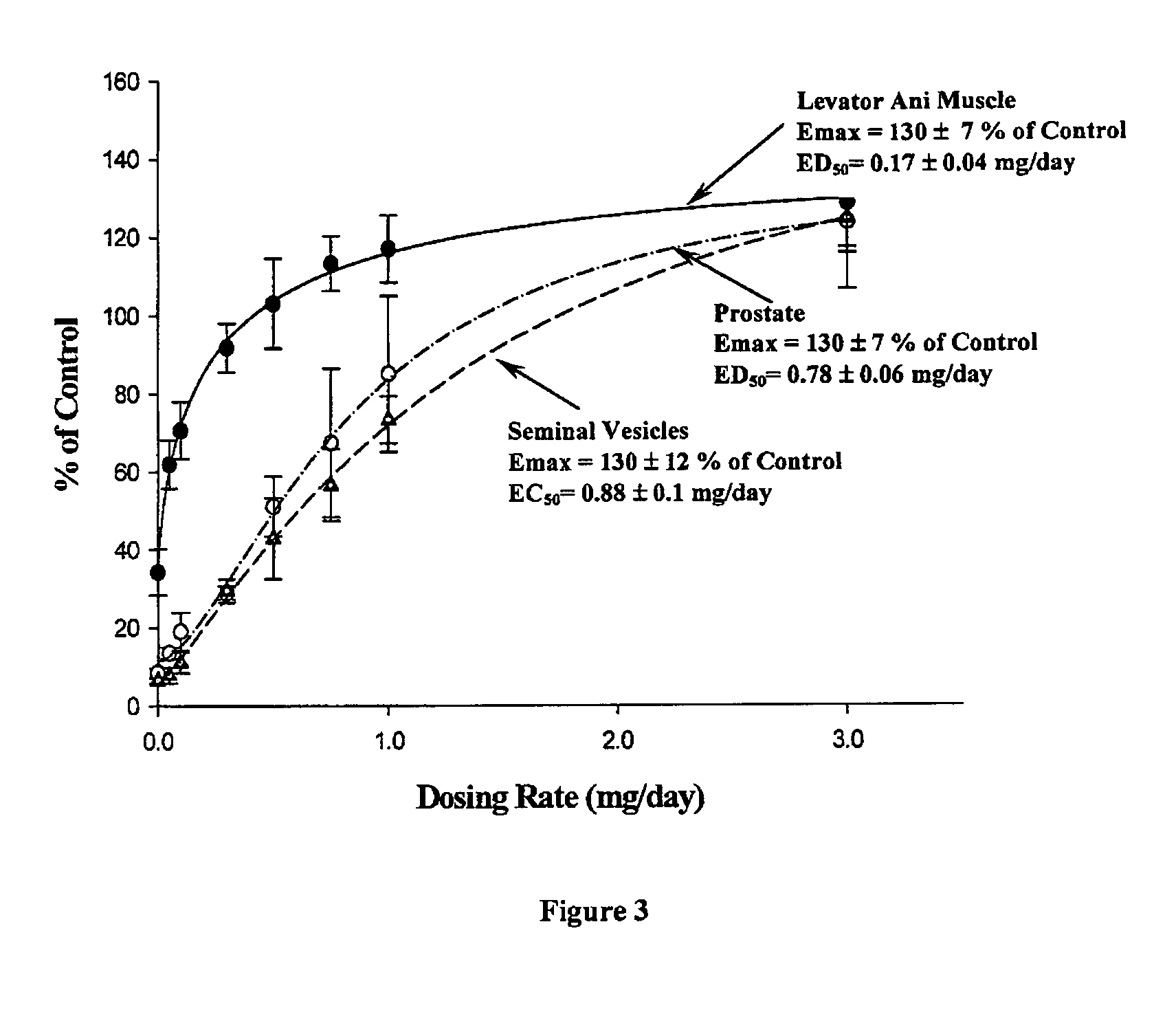
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
Pharmaceutical composition for use in hormone replacement therapy
ActiveUS8048869B2Interaction can be difficultReliable efficacyBiocideOrganic active ingredientsAnti-ProgestinPresent method
One aspect of the invention is concerned with a method of hormone replacement therapy, which method comprises administering to a person in need of such a therapy an effective amount of an estrogenic component selected from the group consisting of: substances represented by the formulain which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alokxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method, and mixtures thereof; said composition containing virtually no progestogen or anti-progestin.Another aspect of the invention relates to a drug delivery system for enteral or parenteral administration that contains at least 1 μg of the aforementioned estrogenic component and virtually no progestogen or anti-progestin.
Owner:ESTETRA SRL
Skin permeation enhancement composition for transdermal hormone delivery system
Owner:AGILE THERAPEUTICS
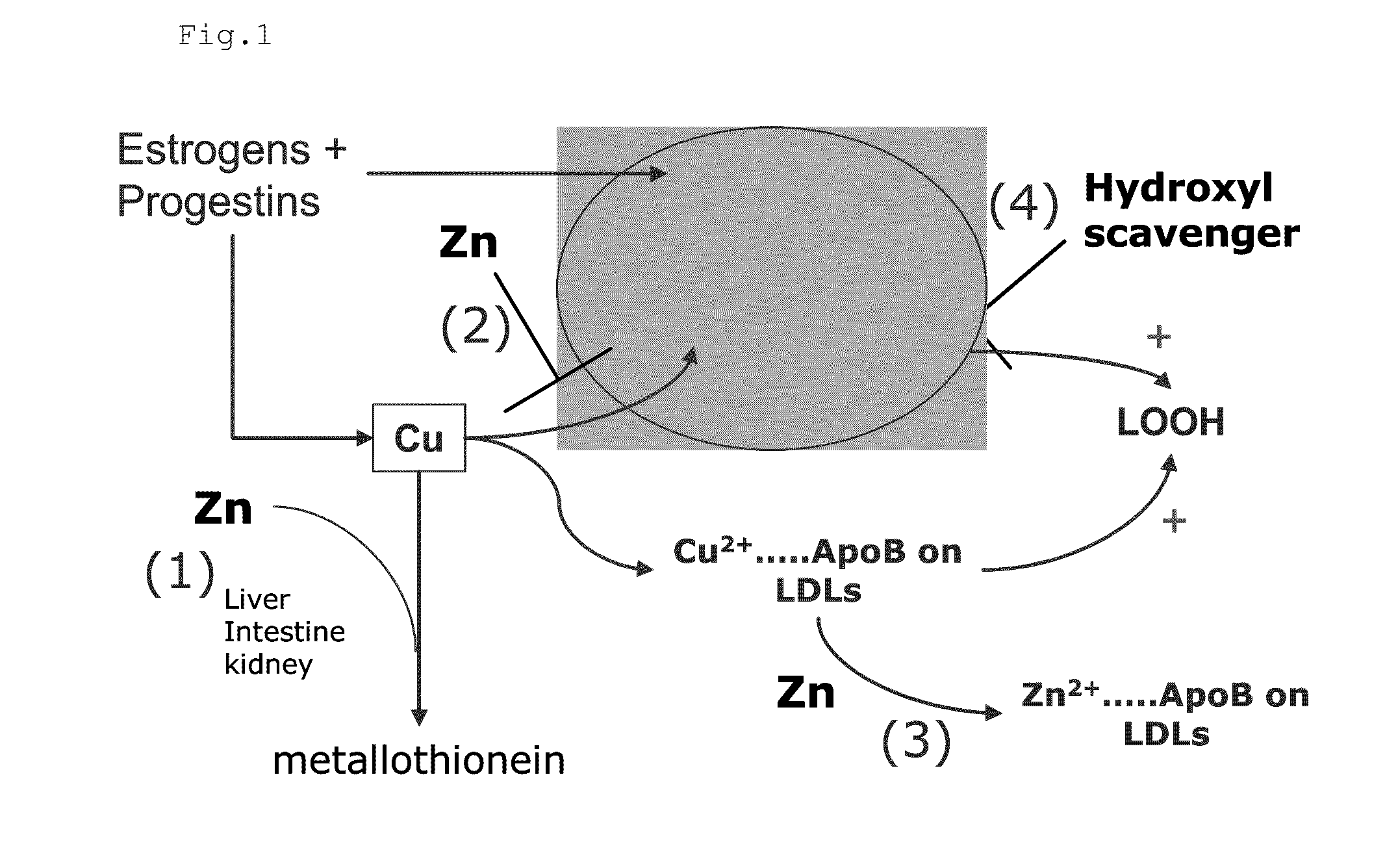
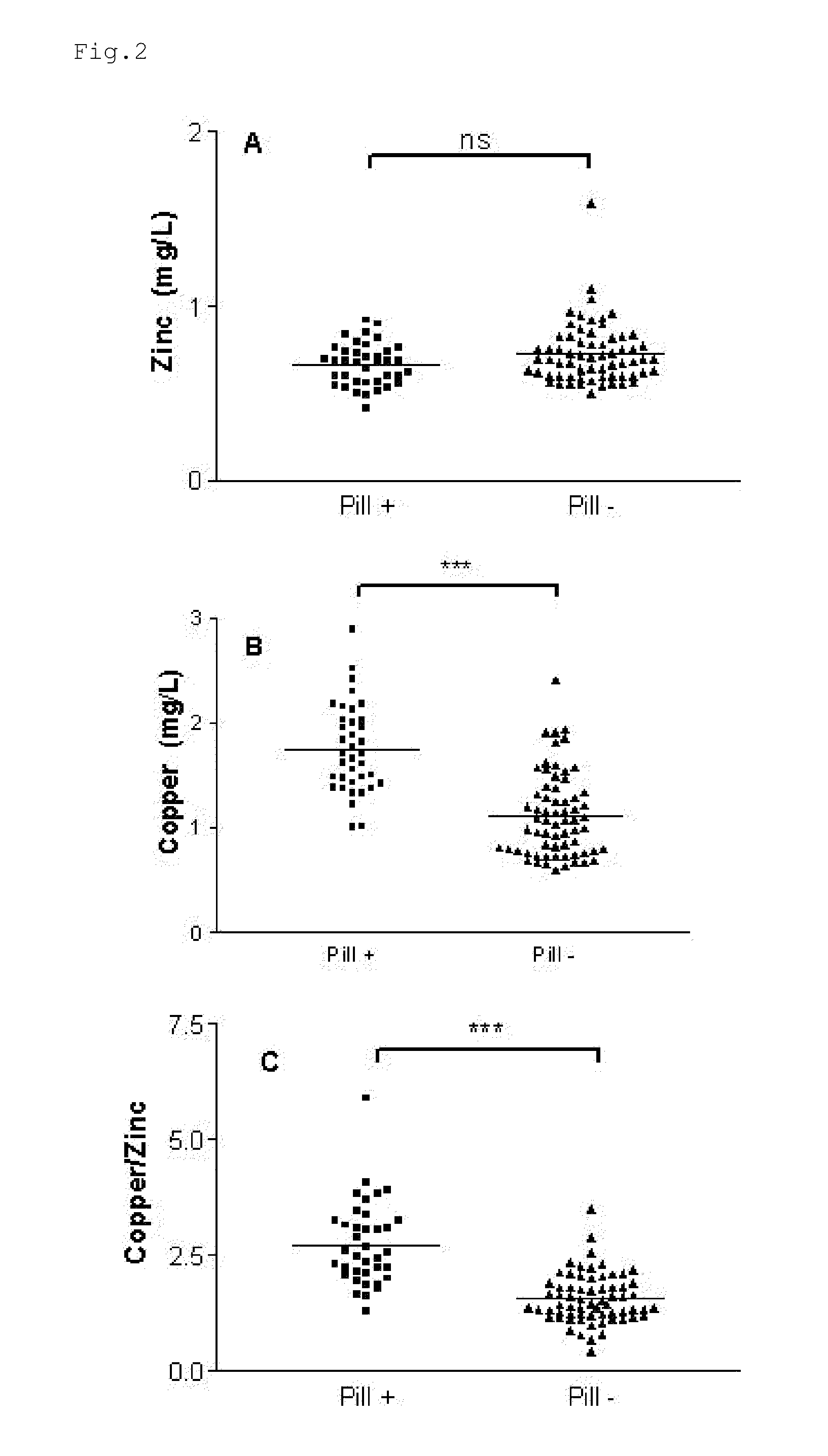
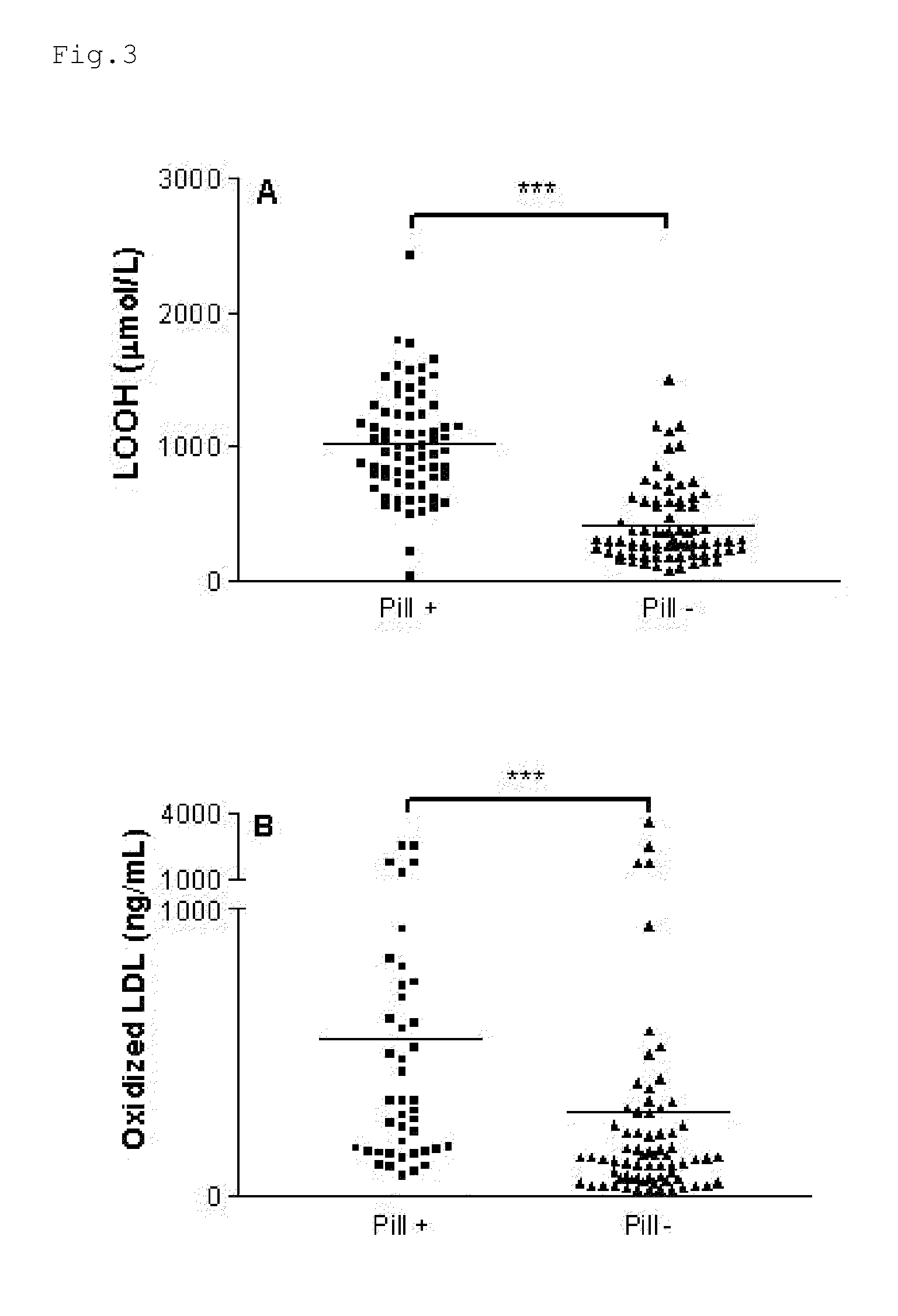
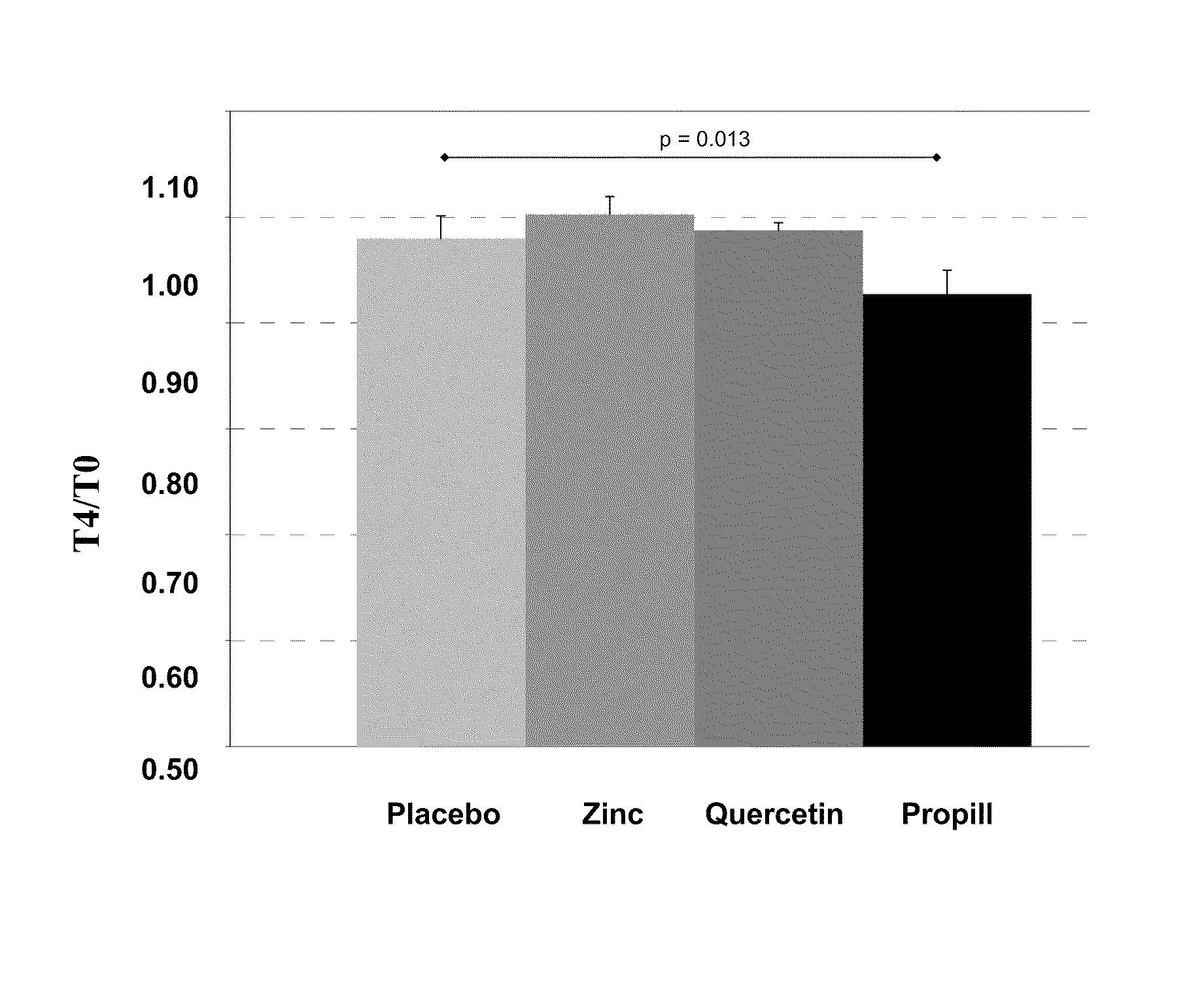
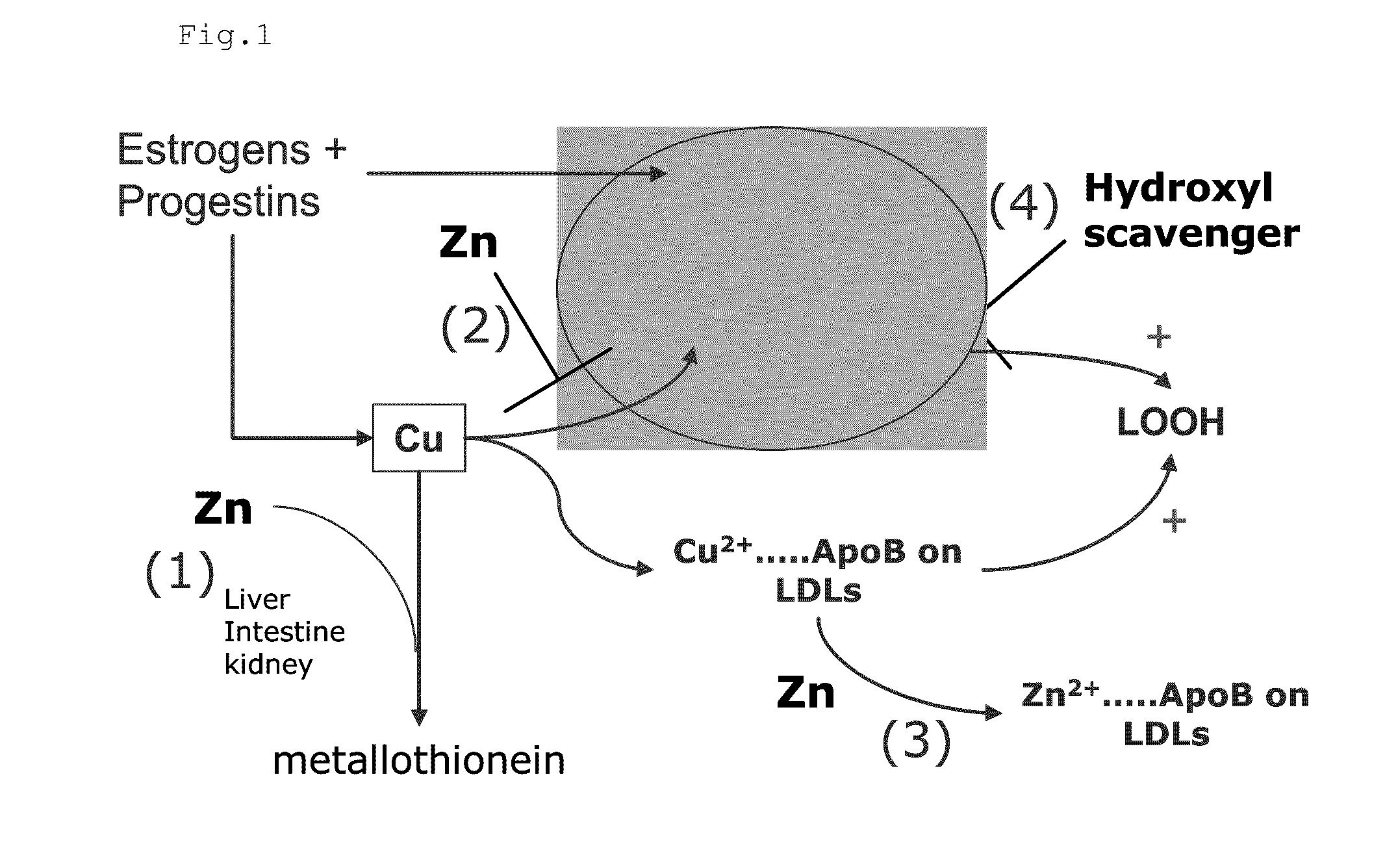
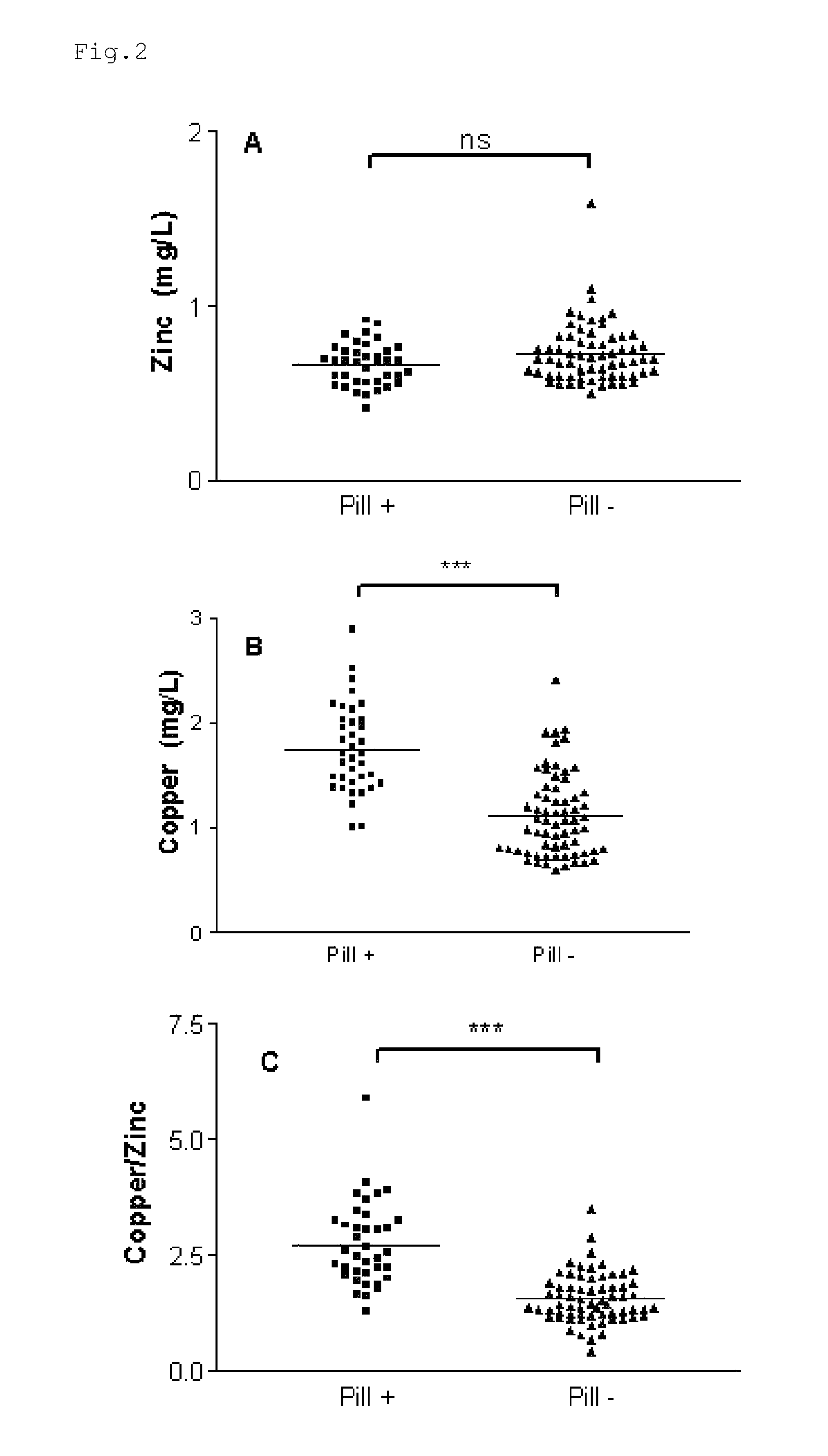
Composition for the treatment of oxidative stress
InactiveUS20120009276A1Preventing and or reducing increased lipid peroxidationEnhanced lipid peroxidationBiocideHeavy metal active ingredientsScavengerHormone replacement
This invention is based on the observed oxidative stress and increased risk on cardiovascular diseases in subjects with increased lipid peroxidation, in particular with women using oral contraceptives and in hormone replacement therapies. The invention provides compositions and combinations, particularly useful in preventing and or reducing the increased lipid peroxidation in subjects in need thereof. These compositions are based on the synergistic combination of zinc and / or a hydroxyl radical scavenger in reducing lipid peroxidation.
Owner:PROBIOX
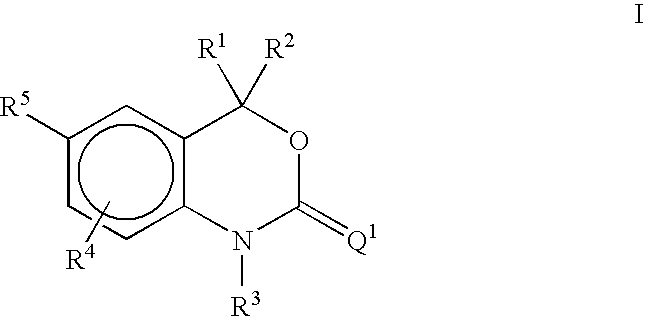
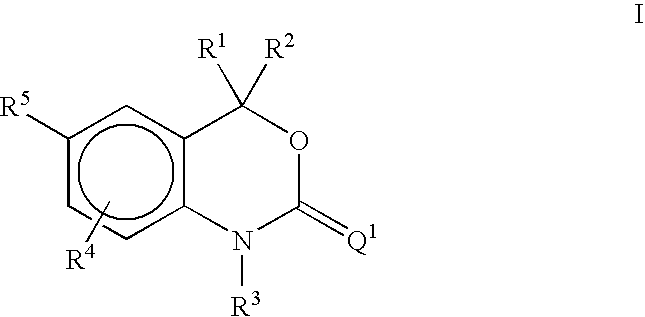
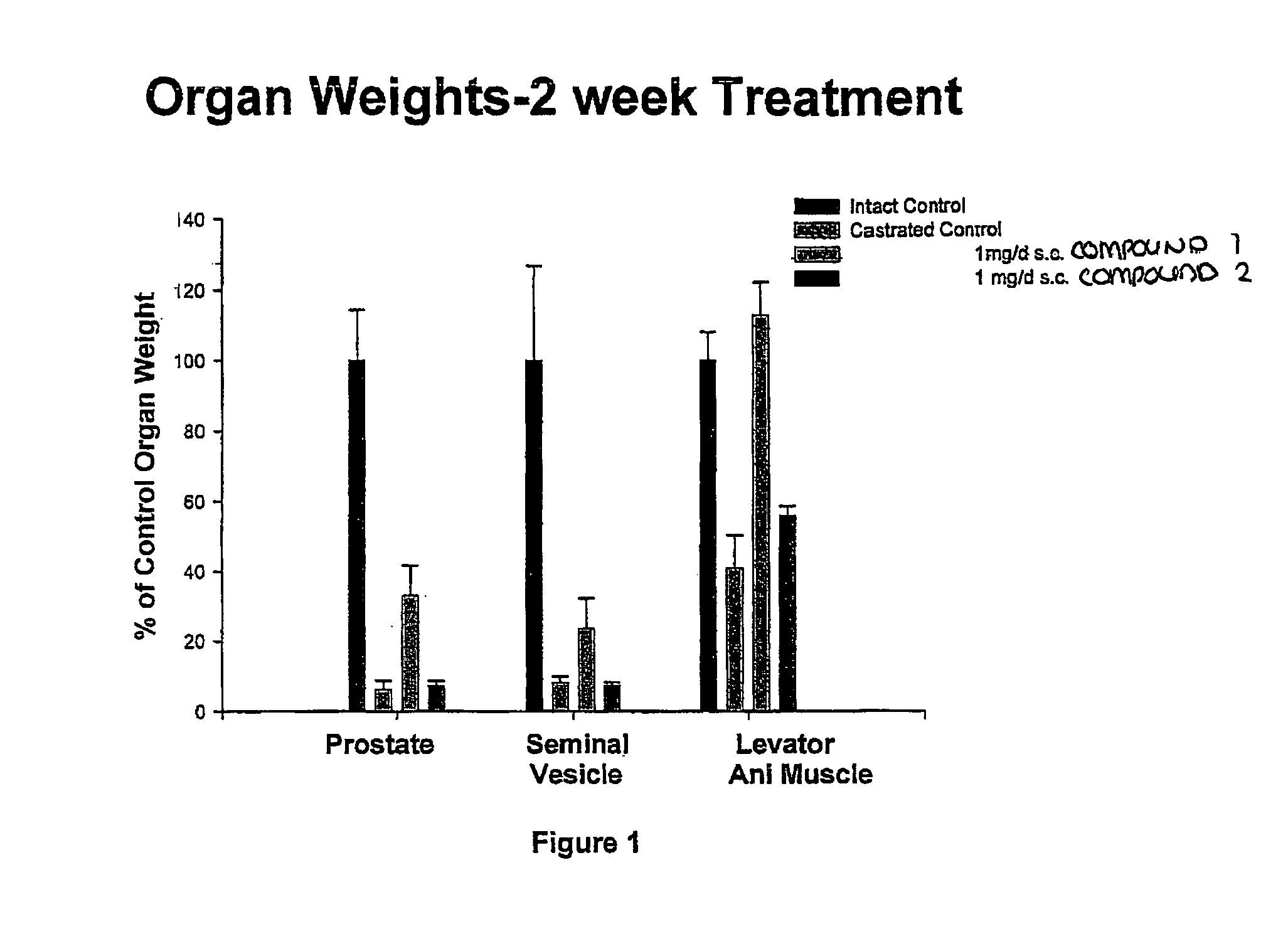
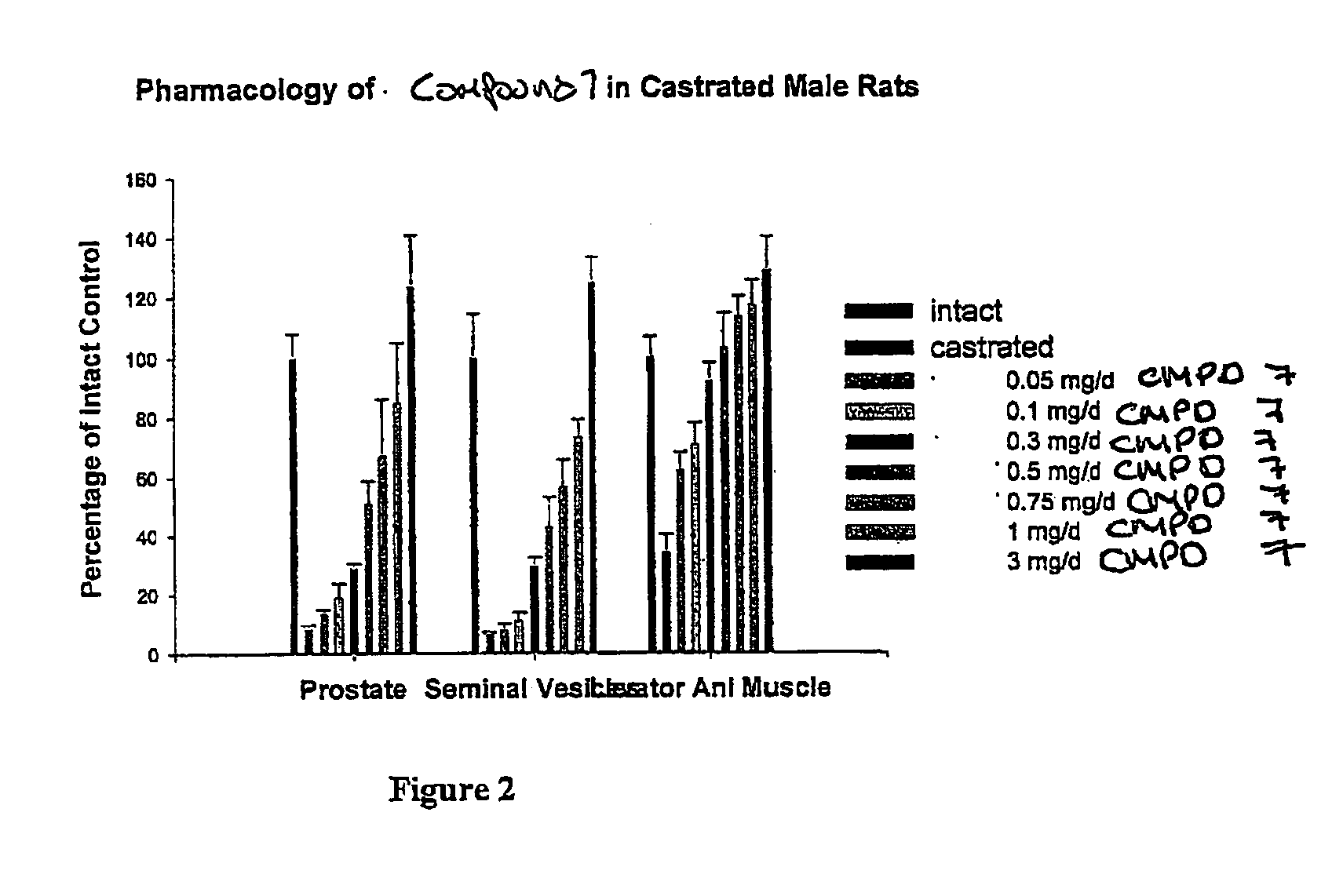
Methods of treating hormone-related conditions using cyclothiocarbamate derivatives
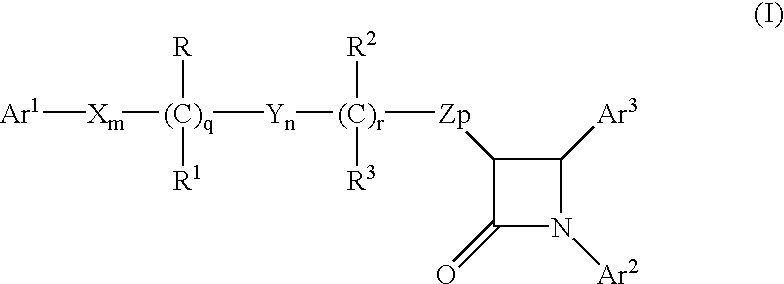
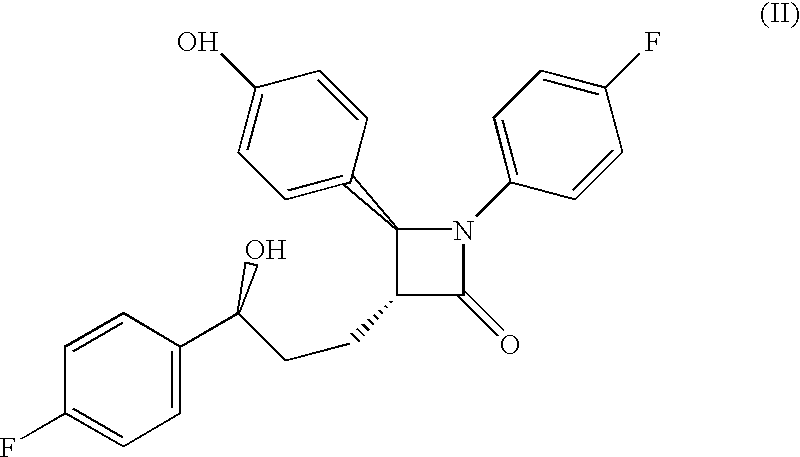
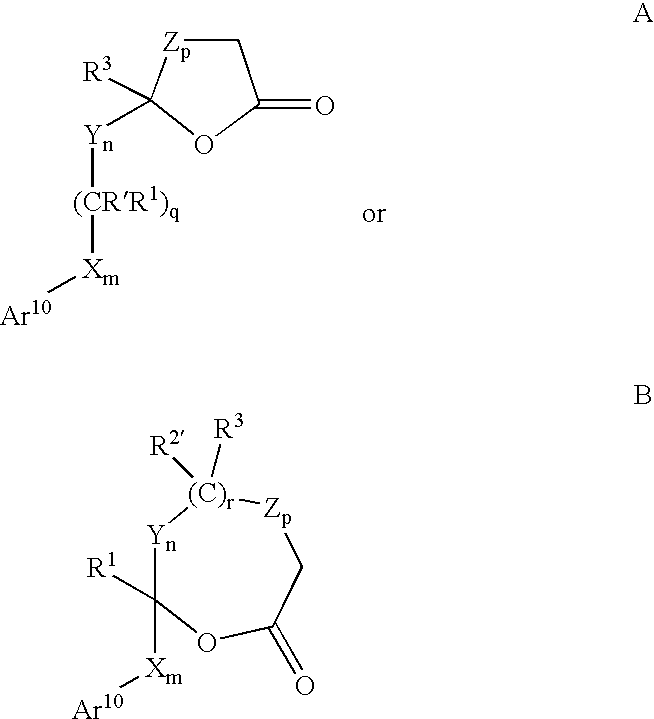
The present invention provides methods of inducing contraception which includes delivering to a female a composition containing a compound of formula I or formula II, or tautomers thereof, in a regimen which involves delivering one or more of a selective estrogen receptor modulator, wherein formula I is:and wherein R1–R5 and Q1 are defined as described herein. Methods of providing hormone replacement therapy and for treating carcinomas, dysfunctional bleeding, uterine leiomyomata, endometriosis, and polycystic ovary syndrome is provided which includes delivering a compound of formula I and a selective estrogen receptor modulator are also described.
Owner:WYETH
Multi-substitued selective androgen receptor modulators and methods of use thereof
InactiveUS7803970B2Reduce incidenceDecreasing regressionBiocideUrea derivatives preparationAging maleHyperplasia
Owner:UNIV OF TENNESSEE RES FOUND
Hormone replacement therapy method
InactiveUS20020142028A1Increase and decrease doseWeaken energyOrganic active ingredientsAerosol deliveryDrugPhysician roles
Varying the daily dose of either or both of the estrogen and the progestogen administered for hormone replacement therapy (HRT) is readily and inexpensively accomplished, without the necessity of the physician prescribing a new product each time the daily dose of the estrogen or progestogen is changed, by administering preferably transdermally the estrogen and the progestogen contained in separate extrudable pharmaceutical compositions from a dispenser which contains means, preferably adjustable only by the attending physician or dispensing pharmacist, for varying the volume of either or both of the respective compositions which is dispensed as a single dose from the dispenser in response to a defined digital dispensing manipulation of the dispenser thereby facilitating optimal compliance to a combination of HRT with individually adjusted dosages of the estrogen and progestogen.
Owner:BAYER SCHERING PHARMA AG
Combinations of hormone replacement therapy composition(s) and sterol absorption inhibitor(s) and treatments for vascular conditions in post-menopausal women
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one hormone replacement therapy composition; and (b) at least one sterol absorption inhibitor which can be useful for treating vascular conditions in post-menopausal women and lowering plasma levels of sterols or 5α-stanols.
Owner:SCHERING CORP
Micronized tanaproget and compositions containing same
The present invention provides compositions, desirably pharmaceutical compositions, containing micronized tanaproget. The compositions can also contain microcrystalline cellulose, croscarmellose sodium, anhydrous lactose, and magnesium stearate; or can contain microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, povidone, and magnesium stearate. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefore Additional uses include stimulation of food intake.
Owner:WYETH LLC
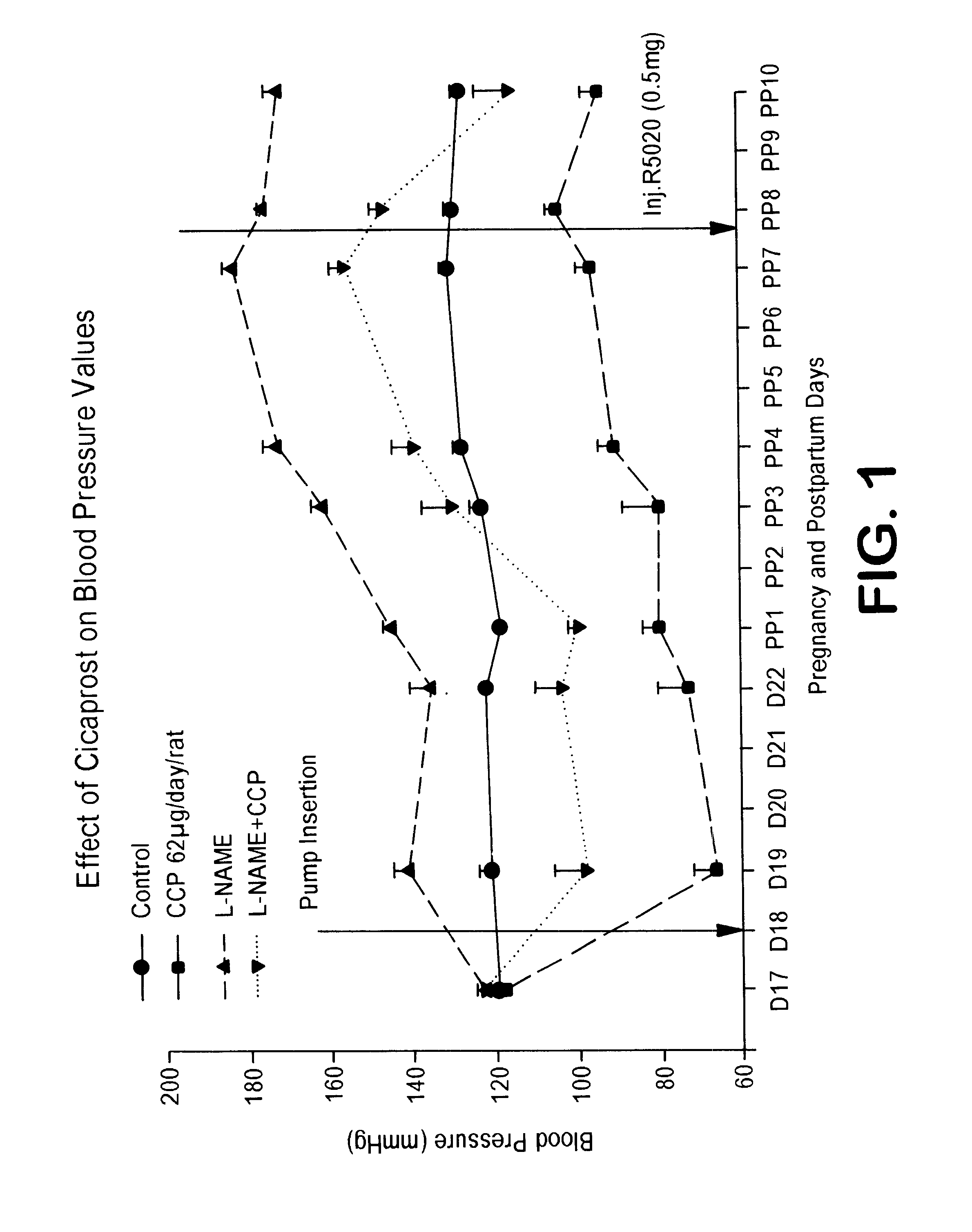
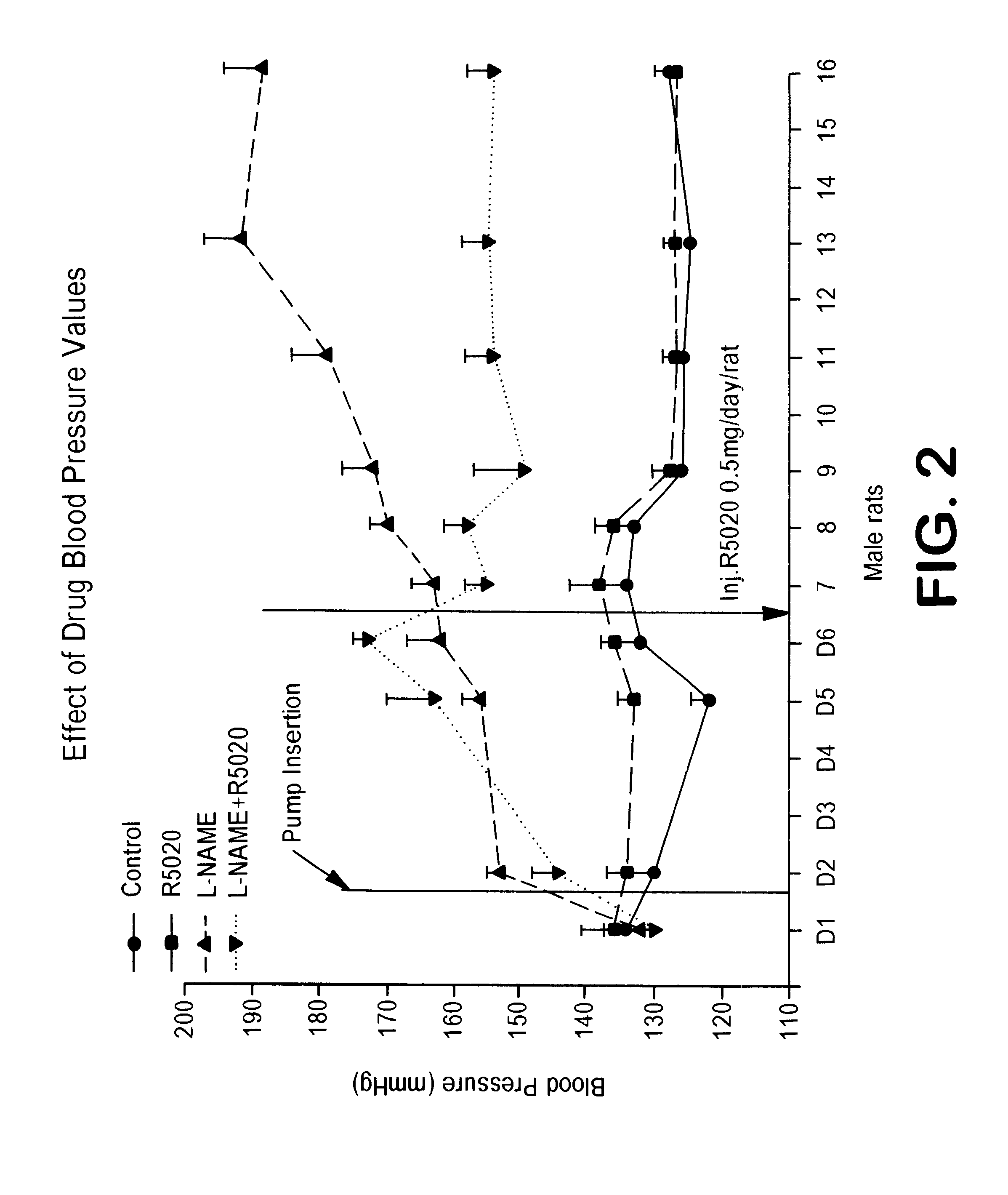
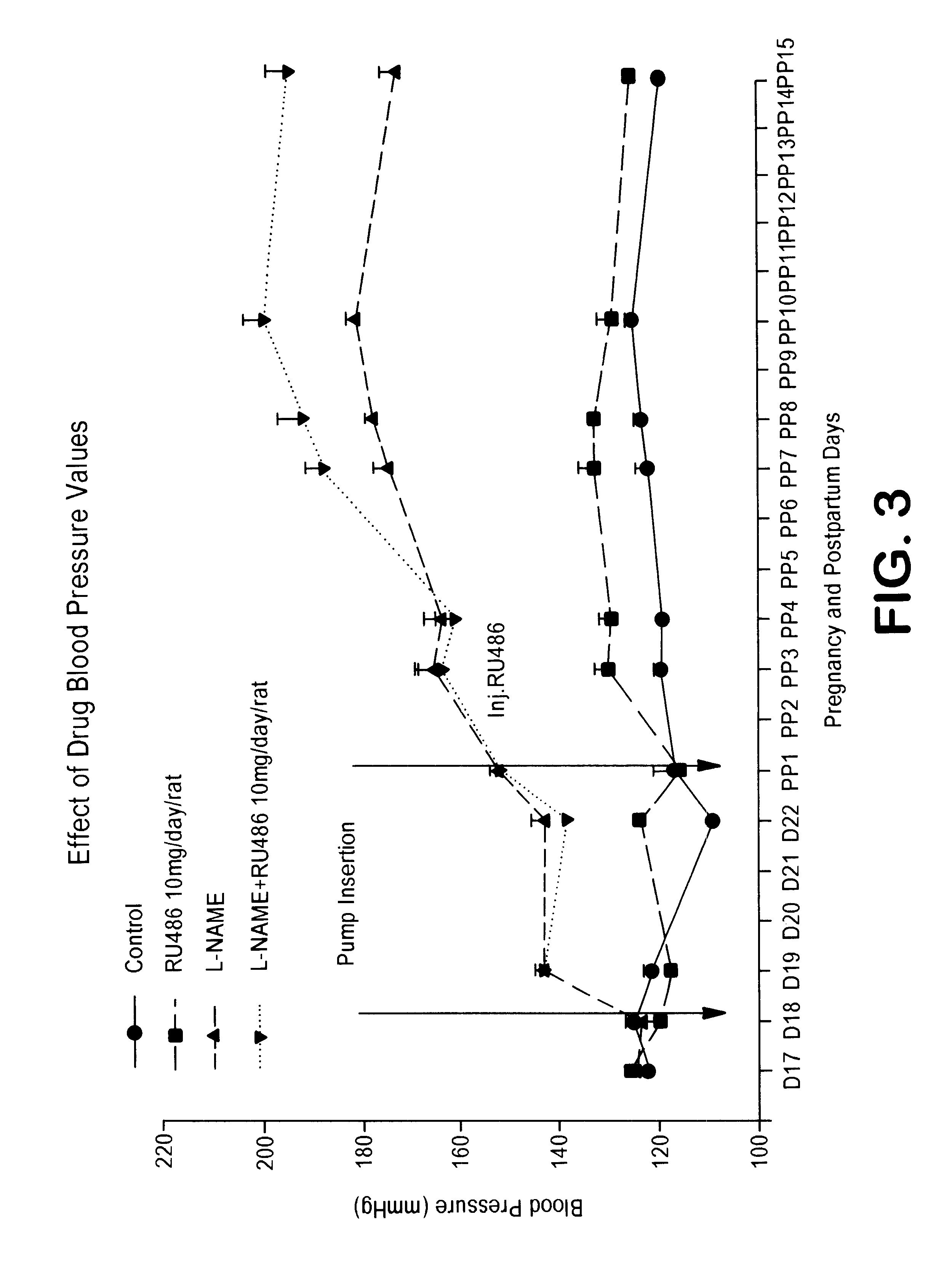
Combination of prostacyclin with an estrogen or progestin for the prevention and treatment of atherosclerotic vascular disease including preeclampsia and for the treatment of hypertension, and for hormone replacement therapy
Cardiovascular disease, including preeclampsia in pregnant women and hypertension in both women and men, are prevented or treated by administering thereto prostacyclin or a prostacyclin analog in combination with one or both of an estrogen and a progestin, which combination is also useful for HRT in peri- and post-menopausal women.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
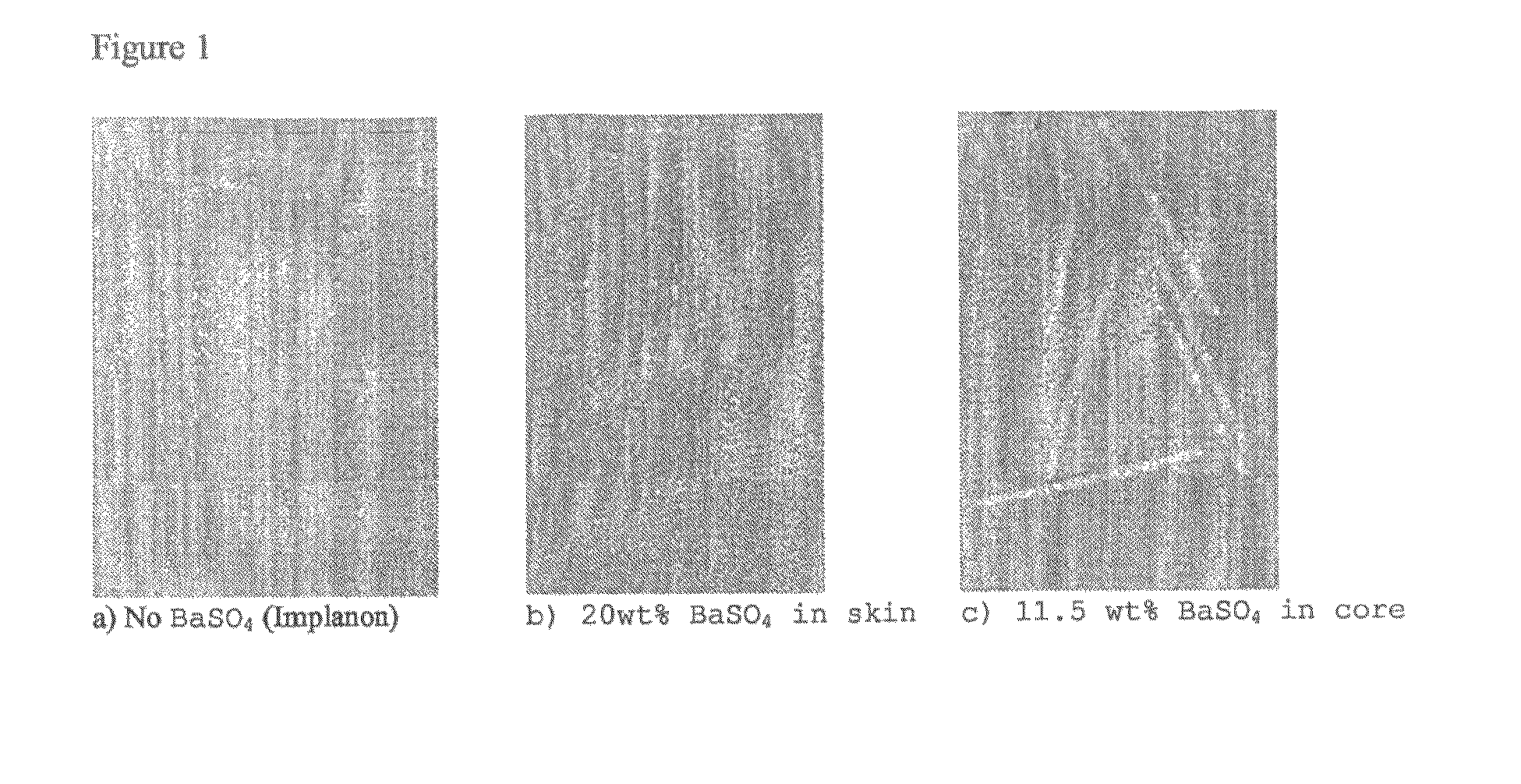
X-ray visible drug delivery device
ActiveUS8722037B2Organic active ingredientsPeptide/protein ingredientsHormone replacementPharmaceutical drug
Owner:MERCK SHARP & DOHME BV
X-Ray Visible Drug Delivery Device
ActiveUS20080112892A1Organic active ingredientsPharmaceutical delivery mechanismHormone replacementPharmaceutical drug
Owner:MERCK SHARP & DOHME BV
Composition for the treatment of oxidative stress
InactiveUS8586629B2Enhanced lipid peroxidationReduce lipid peroxidationBiocideHeavy metal active ingredientsScavengerHormone replacement
Owner:PROBIOX
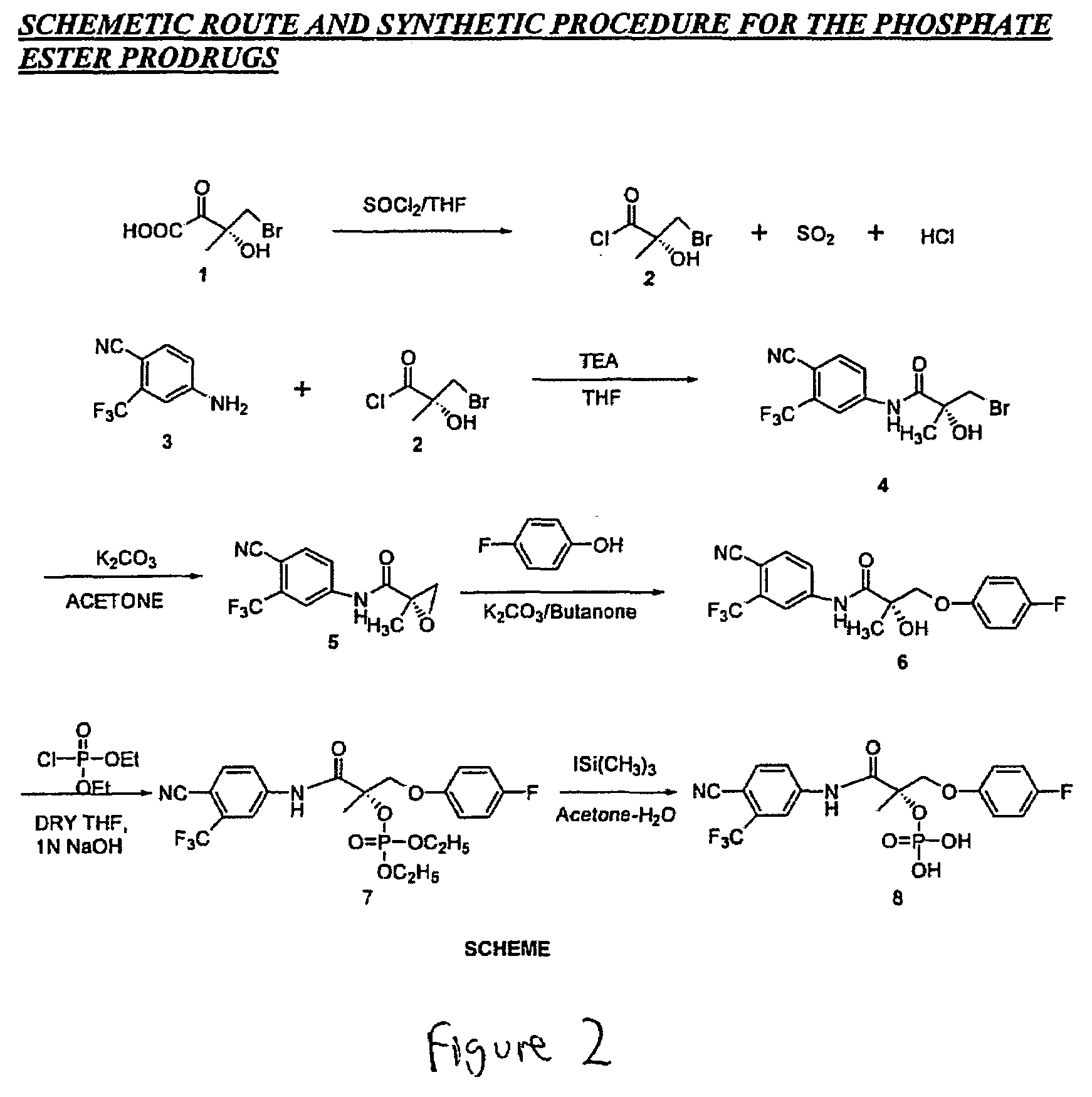
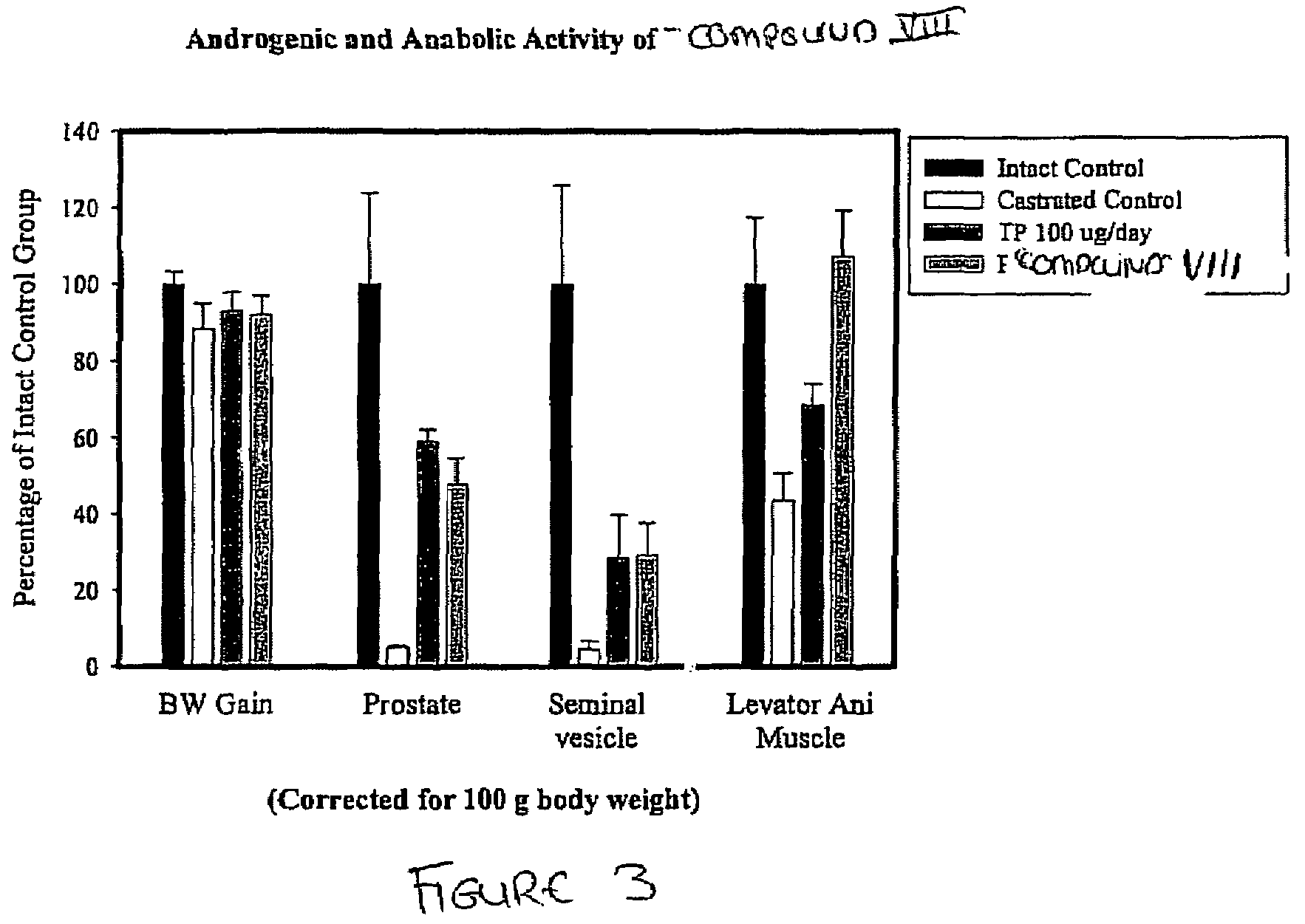
Prodrugs of selective androgen receptor modulators and methods of use thereof
InactiveUS7595402B2Suppressing spermatogenesisInhibit productionBiocideSenses disorderDysostosisSelective androgen receptor modulator
The present invention provides prodrugs of selective androgen receptor modulators (SARMs), and their use in treating or reducing the incidence of osteoporosis, a variety of hormone-related conditions, conditions associated with Androgen Decline in Aging Male (ADAM); conditions associated with Androgen Decline in Female (ADIF), and muscular wasting conditions, obesity, dry eye conditions, and prostate cancer. The prodrugs are also useful in oral androgen replacement therapy and male contraception.
Owner:ONCTERNAL THERAPEUTICS INC
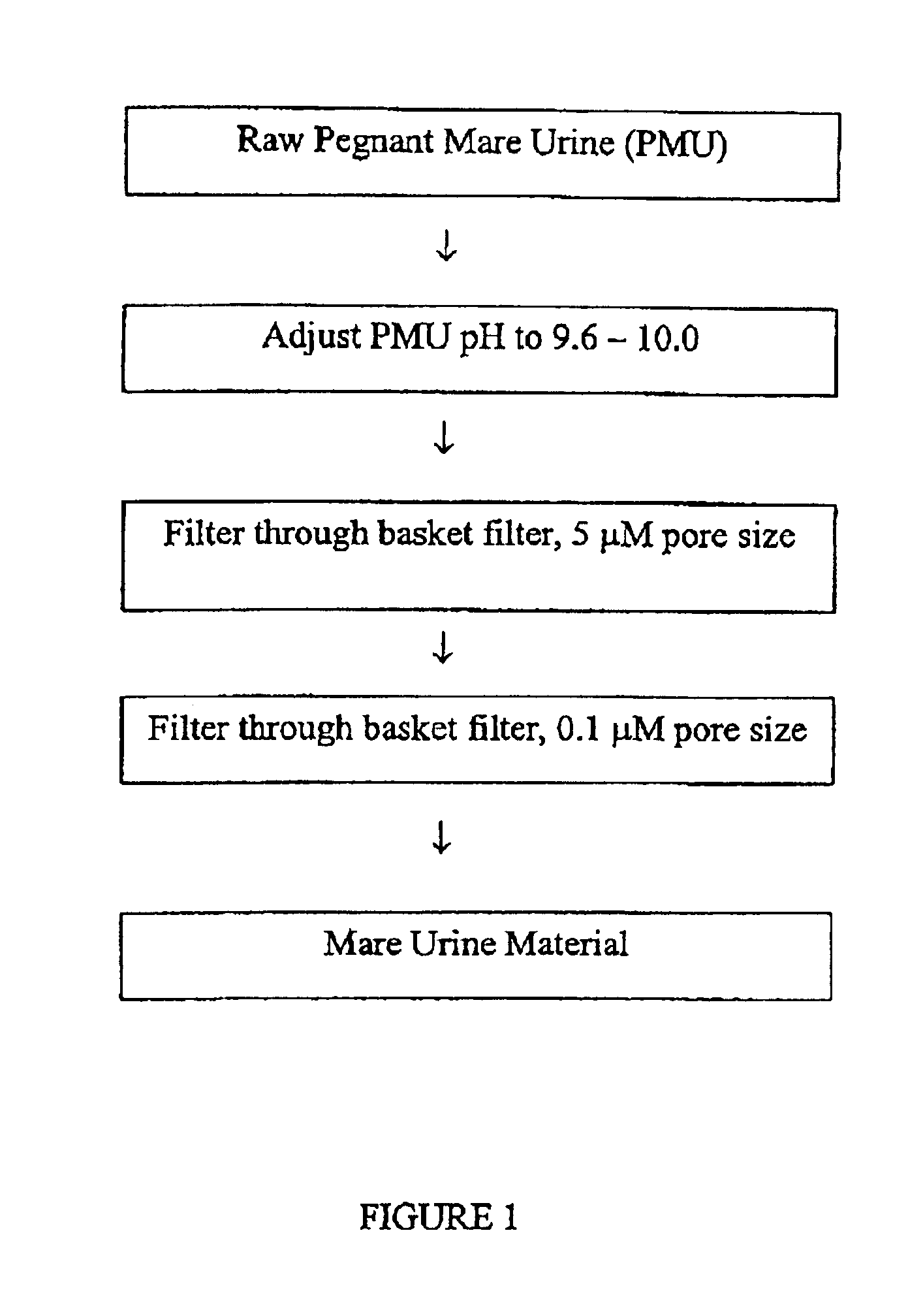
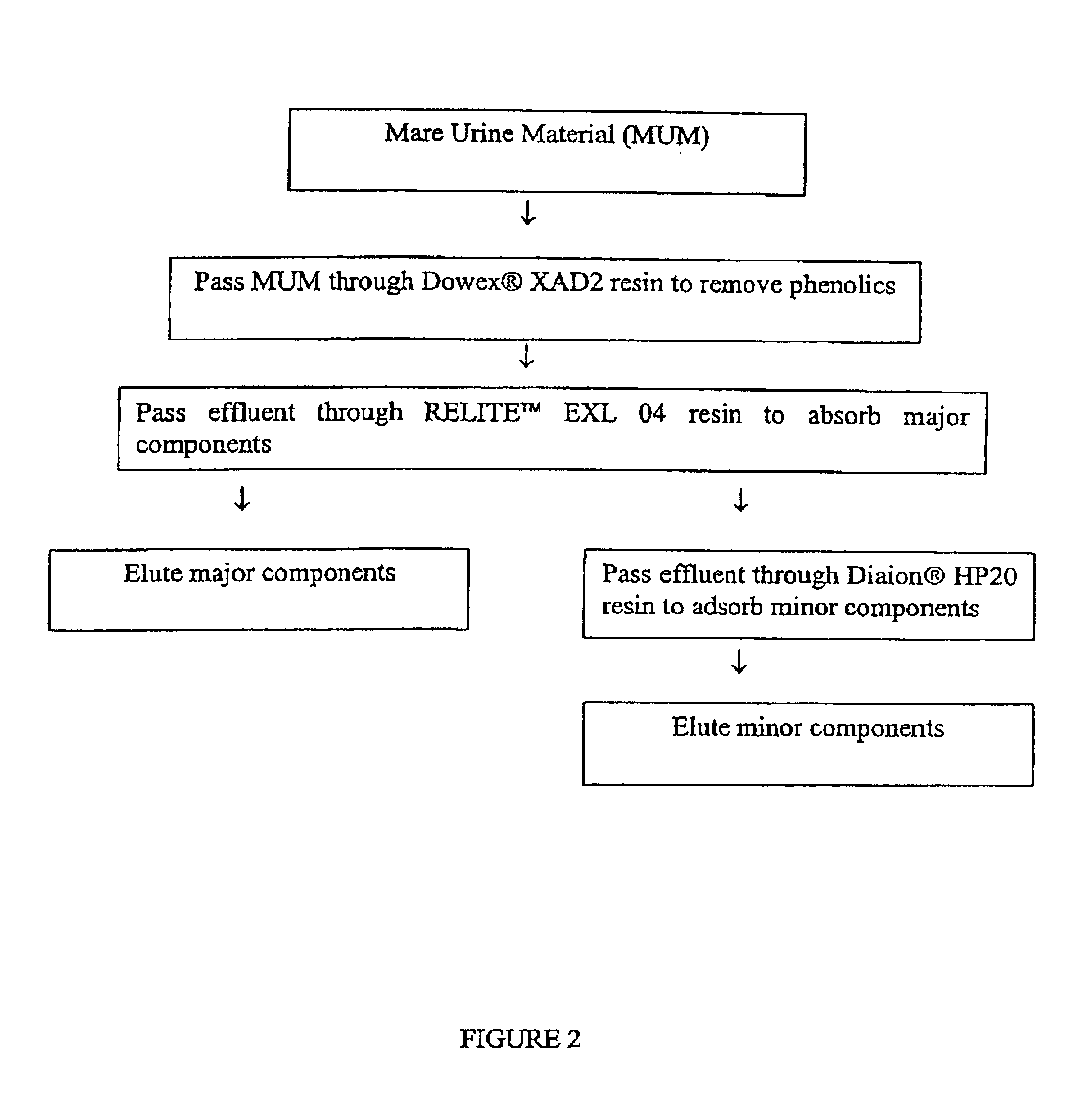
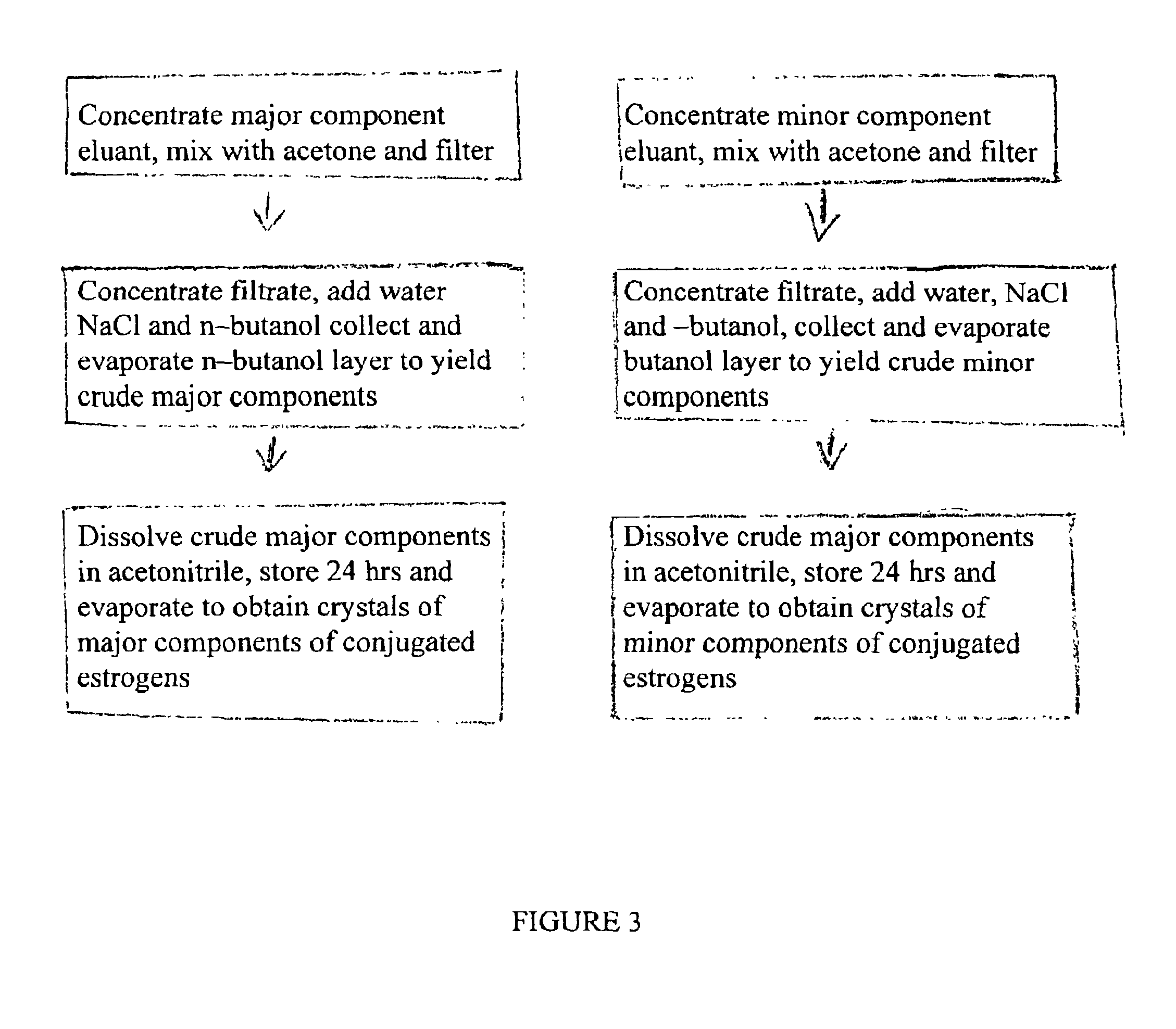
Process for isolating conjugated estrogens
The present invention relates to a process for extracting conjugated estrogens from pregnant mare urine. The present invention further relates to a process for obtaining a natural mixture of conjugated estrogens. The mixture of conjugated estrogens can be used to prepare products for estrogen replacement therapy or hormone replacement therapy. More specifically, the process for extracting conjugated estrogens from PMU comprises the steps of (a) contacting a mare urine material (MUM) with a resin to adsorb phenolic components, (b) contacting the phenolics-depleted MUM of step (a) with a resin to adsorb the stone and equilin, the major estrogen components, (c) containing the major estrogen-depleted MUM of step (b) with a resin to adsorb the minor estrogen components, (d) separately desorbing the major estrogen components and the minor estrogen components from the resins used in steps (b) and (c), and (e) separately treating the desorbed material form step (d) to obtain crystals of the major estrogen components and the minor estrogen components.
Owner:DR REDDYS LAB NEW YORK
Pharmaceutical compositions of testosterone
ActiveUS20190307772A1Crystal formation is minimized and preventedOrganic active ingredientsPharmaceutical delivery mechanismMedicineHormone replacement
The present invention provides stable pharmaceutical compositions, comprising a pharmaceutically effective amount of testosterone or a pharmaceutically acceptable ester thereof, a pharmaceutically acceptable oil vehicle, and a stabilizing amount of benzyl alcohol, for example, about 1% to 3% weight / volume of benzyl alcohol. The present invention also provides a process for stabilizing testosterone-containing pharmaceutical compositions by ageing them at a temperature of about 20° C. to about 60° C. for at least 48 hours, e.g., prior to secondary packing and labeling. These compositions were stable over the shelf life of the product, without exhibiting crystal formation, even upon storing at temperatures of about 2° C. to about 8° C. Other aspects of the invention relate to methods for making such pharmaceutical compositions, and methods of using such pharmaceutical compositions for hormone replacement therapy, e.g., in a male patient having a condition associated with symptoms of deficiency or absence of endogenous testosterone.
Owner:SLAYBACK PHARMA LLC
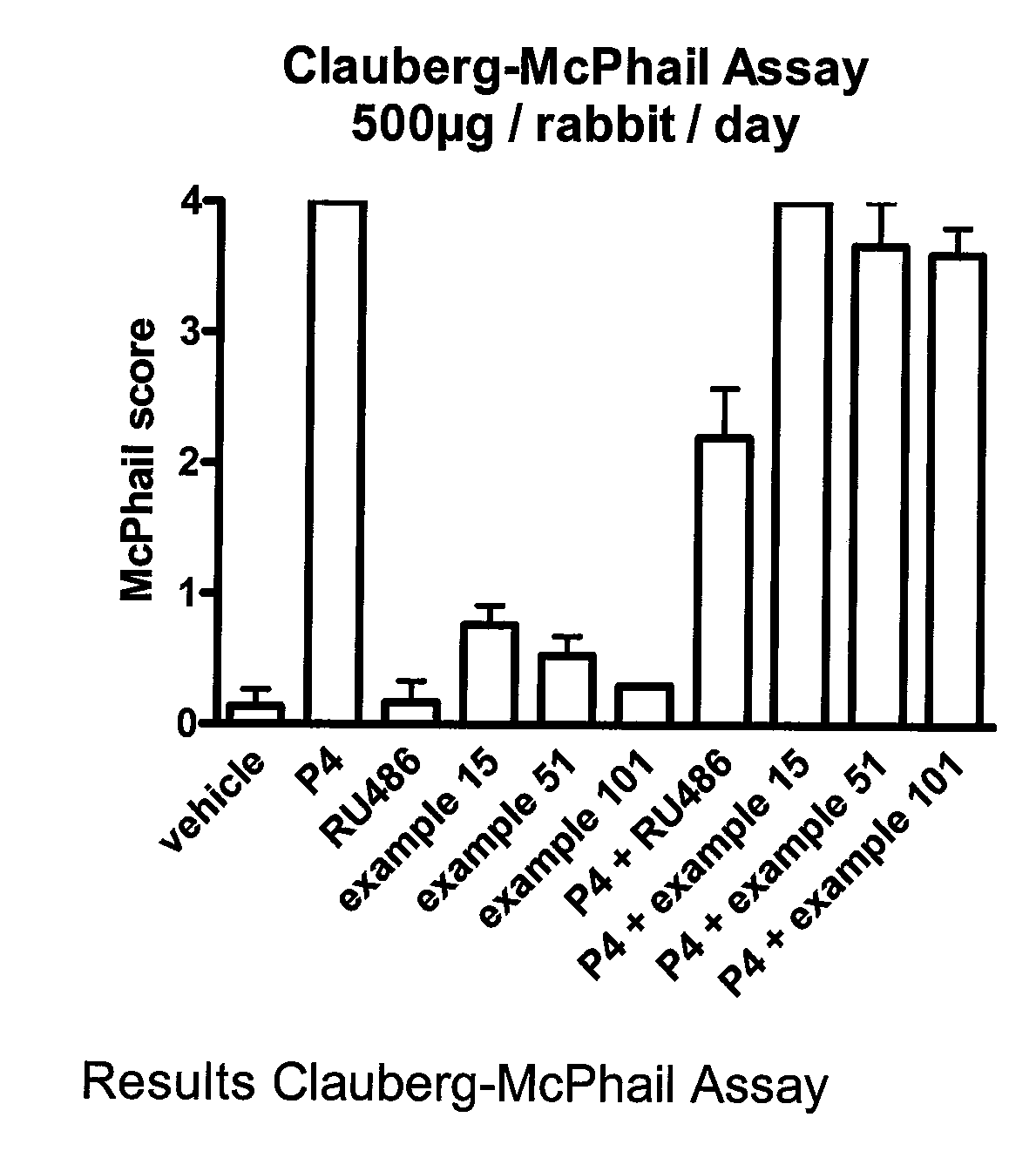
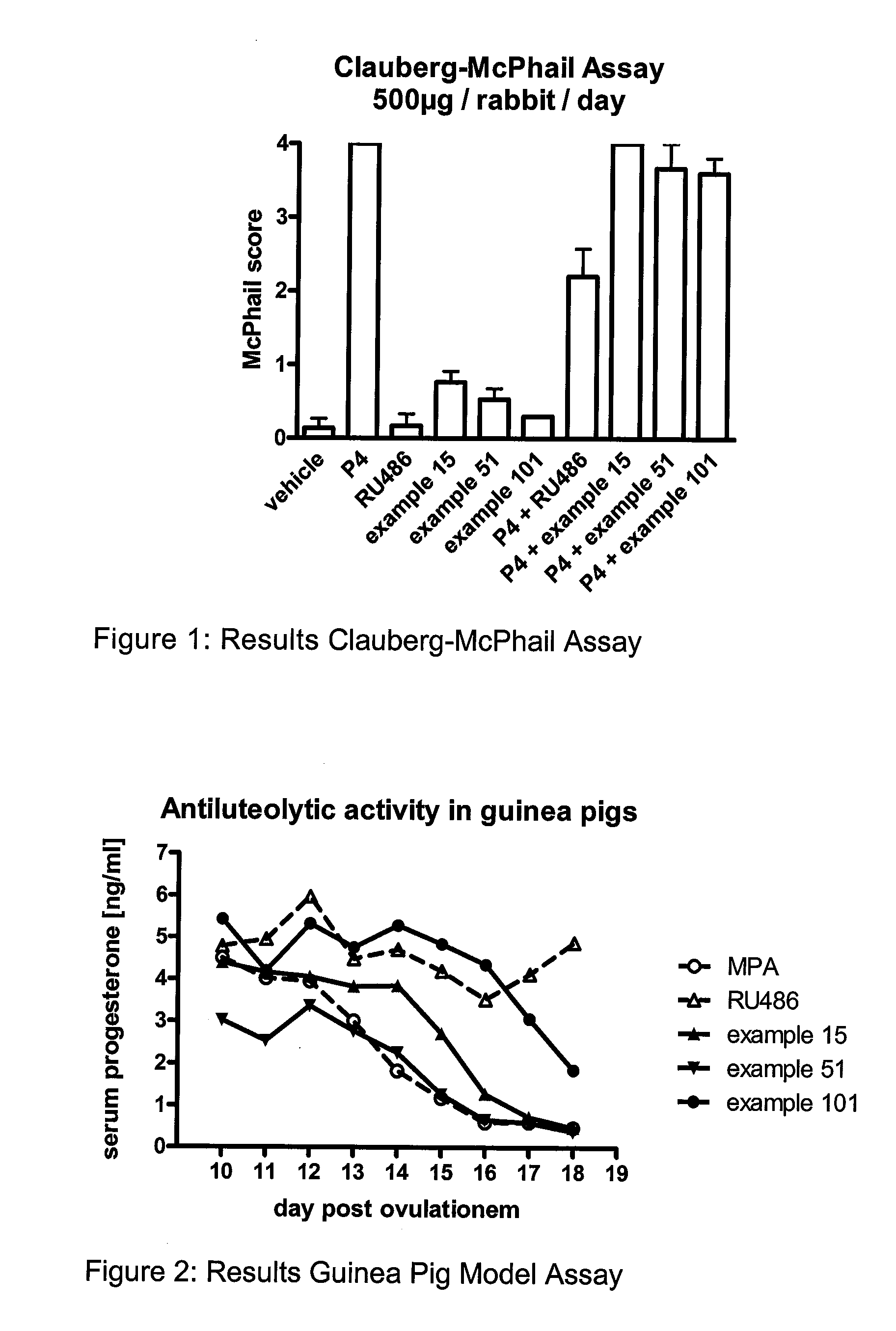
C11 Modified Retrosteroids as Progesterone Receptor Modulator Compounds
InactiveUS20080249075A1Modulate PREfficient modulationOrganic active ingredientsMicrobiological testing/measurementGynecological disordersProgesterone receptor modulators
Retrosteroidal compounds corresponding to formula I, representing progesterone receptor modulators, and their production, and pharmaceutical preparations containing these compounds. These compounds are useful in the treatment of benign gynecological disorders such as endometriosis and uterine fibroids, as well as for female birth control and for hormone replacement therapy.
Owner:SOLVAY PHARMA GMBH
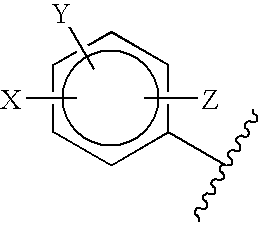
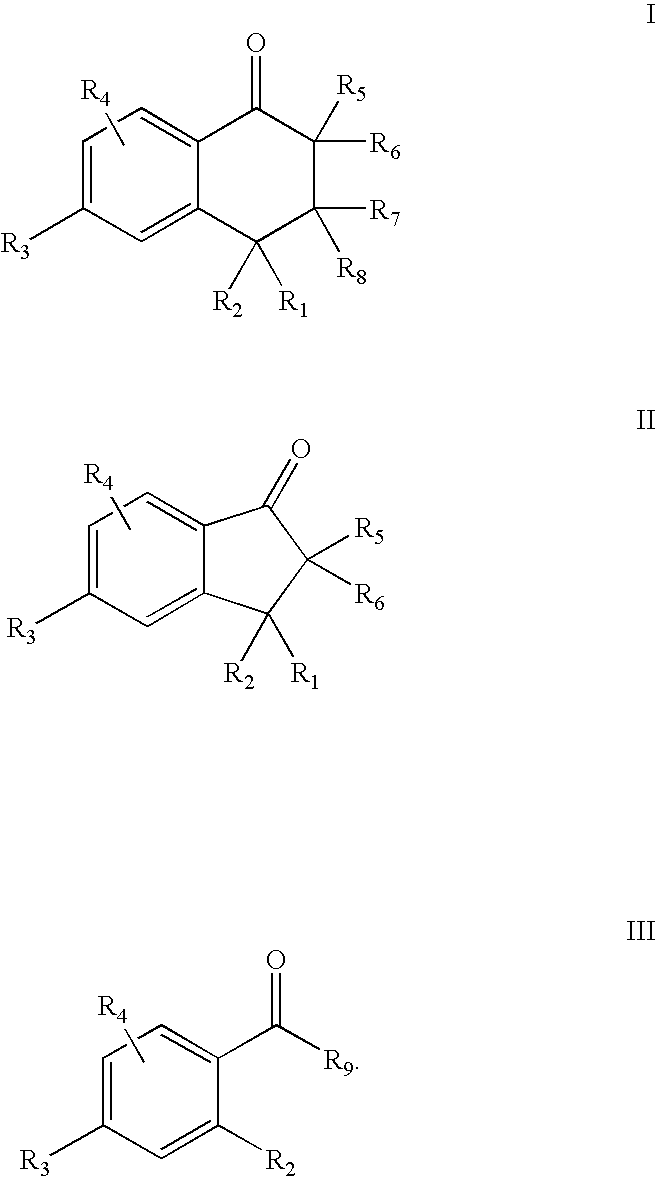
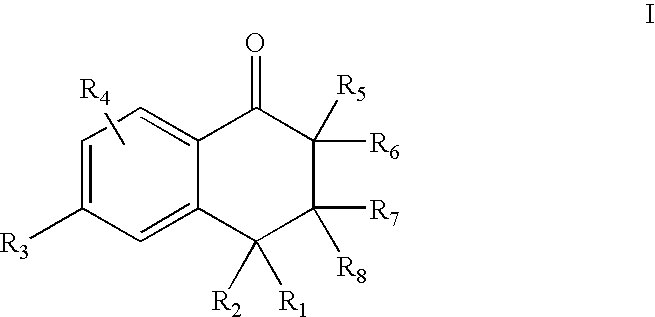
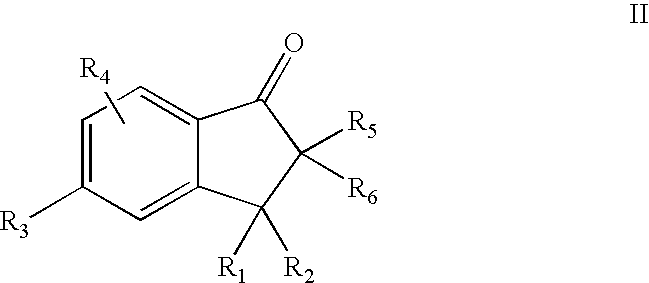
5-Aryl-indan-1-one and analogs useful as progesterone receptor modulators
Compounds of formula I or II are provided, wherein R1-R8 are defined herein, and pharmaceutical compositions and kits containing these compounds. Also provided are methods of inducing contraception, providing hormone replacement therapy, treating cycle-related symptoms, or treating or preventing benign or malignant neoplastic disease using the compounds of formula I, formula II, or formula III, wherein R1-R9 are defined herein:
Owner:WYETH LLC
Transdermal Hormone Delivery System: Compositions And Methods
A transdermal hormone delivery system (THDS) is disclosed. The THDS is useful for control of fertility and as therapy for a variety of diseases and conditions treatable by robust delivery of progestin and estrogen hormones, particularly the progestin, levonorgestrel. The THDS comprises a backing layer, an adjoining adhesive polymer matrix comprising an effective amount of at least a progestin hormone, delivery of which is enhanced by one or more skin permeation enhancing agents present in pre-determined amounts. The THDS is capable of providing effective daily doses of progestin and estrogen hormones from a small surface area in contact with the skin, e.g., less than 20 square centimeters. Methods of fertility control and various types of hormone replacement therapy utilizing the THDS are also disclosed.
Owner:AGILE THERAPEUTICS
Compounds and their uses for alleviating menopause-associated symptoms
ActiveUS11484539B2Quick effectAvoid exposurePharmaceutical delivery mechanismSexual disorderSide effectHormone replacement
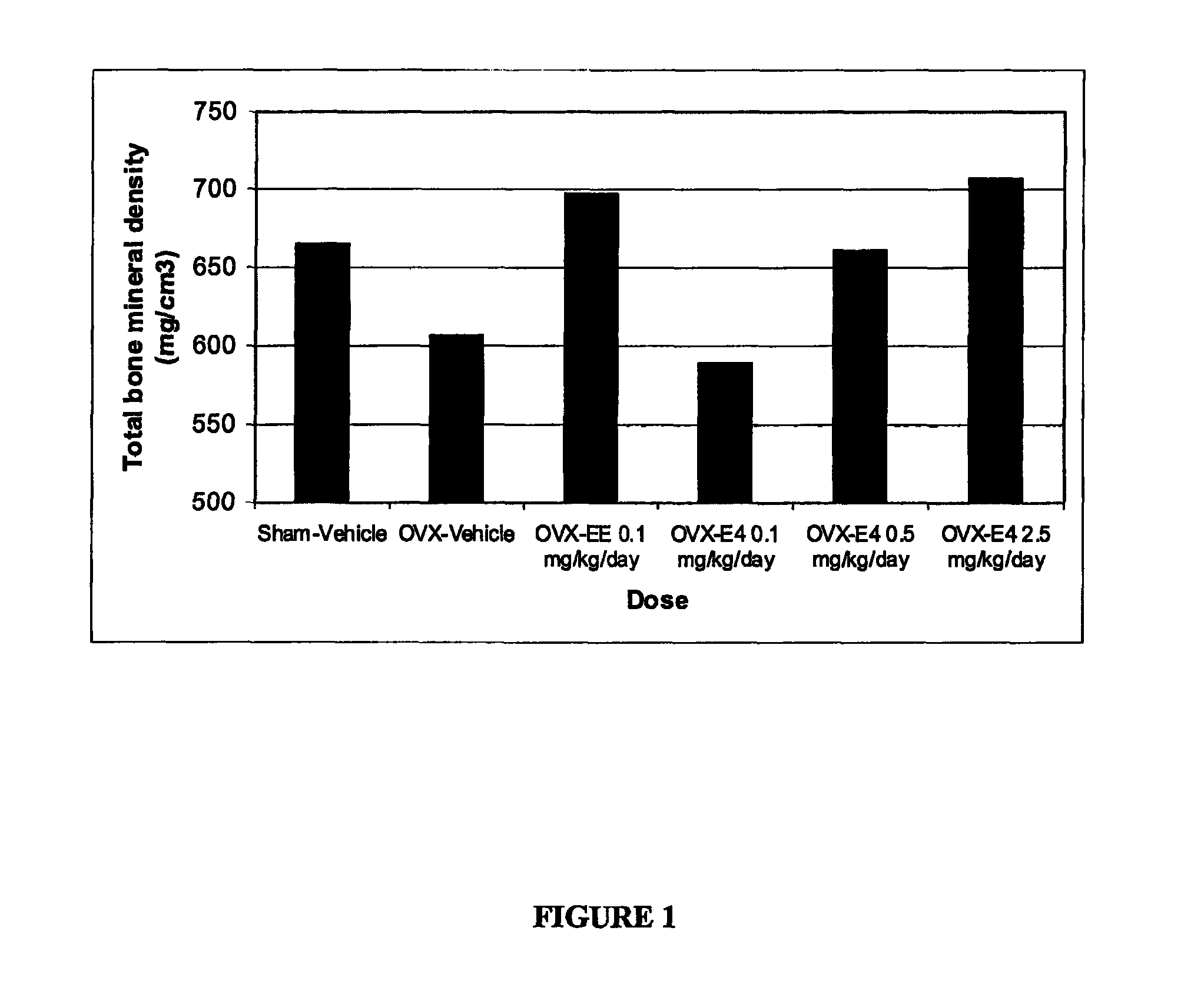
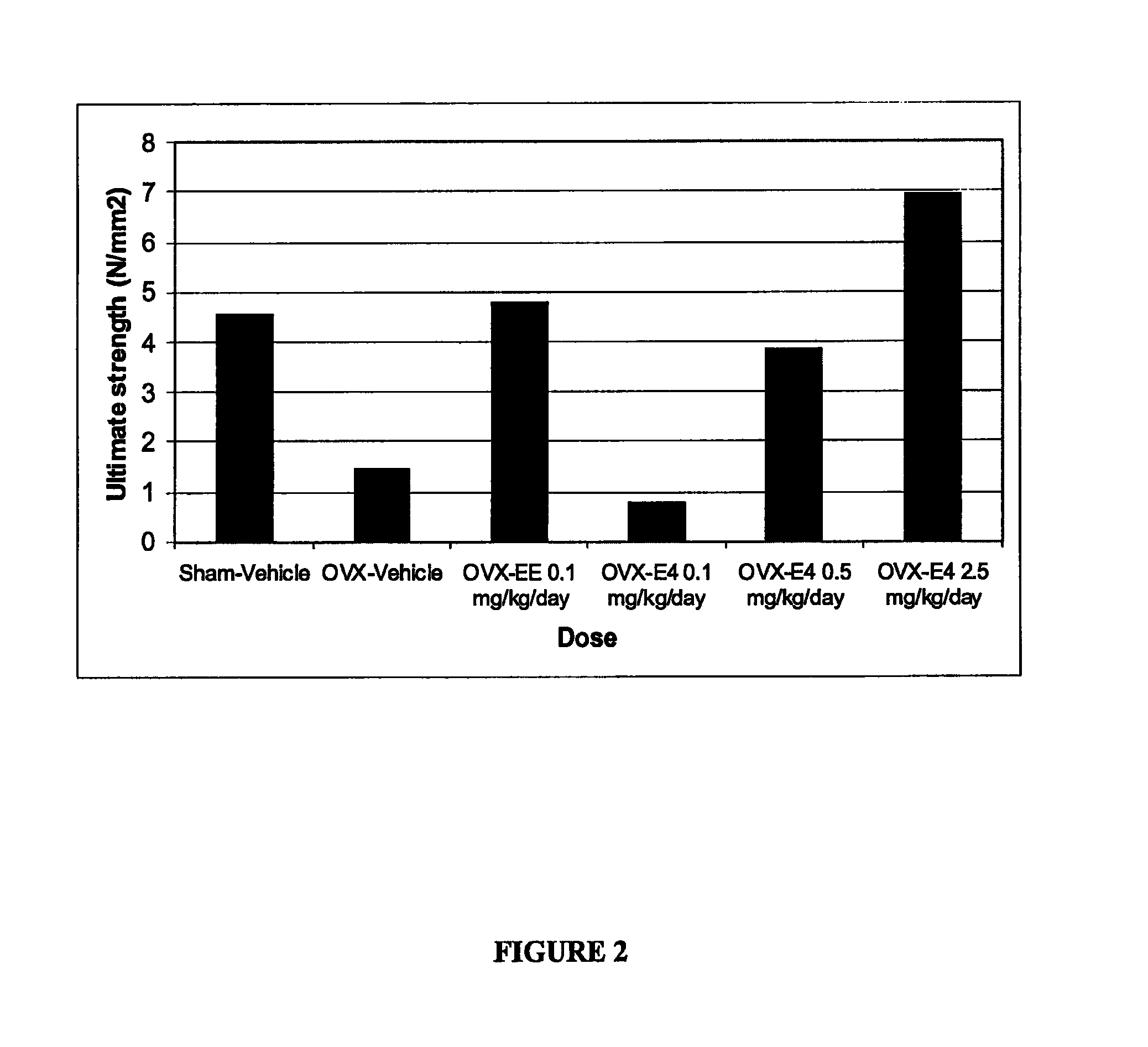
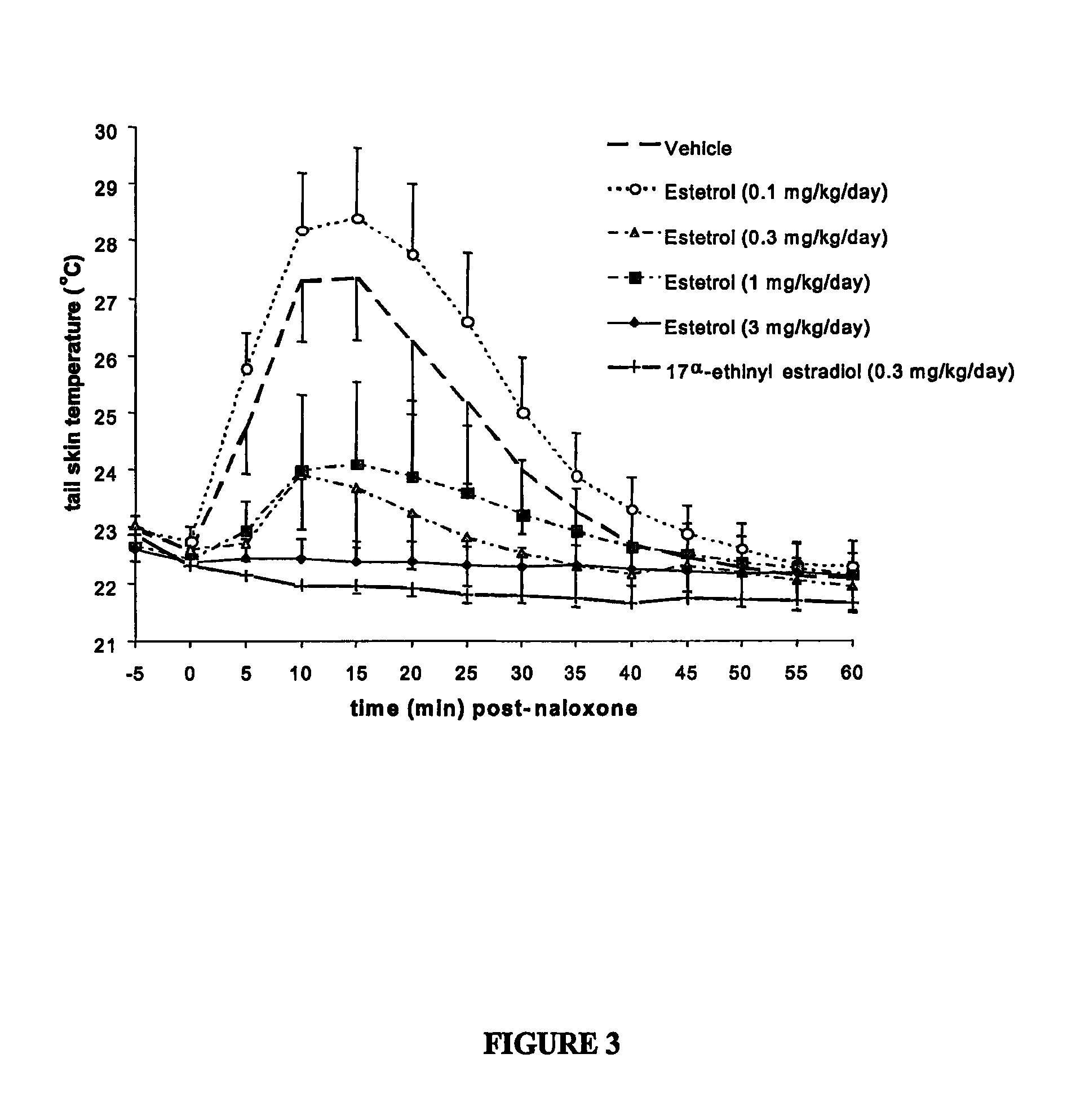
The present invention relates to a hormone replacement therapy, to the associated compounds and to the associated packaging units, for alleviating menopause-associated symptoms which is based on the administration to a female mammal of an estetrol component at a specific daily dose, optionally in combination with a progestogenic component. The therapy enjoys a statistically significant efficacy combined with a favourable profile for side effects compared to currently available methods for alleviating menopause-associated symptoms.
Owner:ESTETRA SRL
Use of conjugated estrogens in combination with trimegestone in hormone replacement therapy
This invention relates to methods and pharmaceutical compositions for providing hormone replacement therapy in perimenopausal, menopausal, and postmenopausal women through the continuous administration of combinations of conjugated estrogens and trimegestone.
Owner:WYETH LLC
Method for predicting the risk of deep vein thrombosis and pulmonary embolism associated with hormonal preparations and hormone levels
ActiveUS20200071762A1Microbiological testing/measurementDisease diagnosisHuman DNA sequencingNucleotide
Specific single nucleotide polymorphisms (SNPs) in the human genome, and their association with deep vein thrombosis (DVT) and related pathologies, such as pulmonary embolism (PE), in relation with hormonal preparations (i.e. combined contraceptives, hormone replacement therapeutics) and hormone levels (i.e. during pregnancy and post-partum).
Owner:GENE GENDER SRL
Low-dosed solid oral dosage forms for hrt
InactiveUS20130137664A1Rapid and adequate relief of moderate to severe vasomotor symptomsReduce frequencyOrganic active ingredientsBiocideDrospirenoneHormone replacement
The present invention relates to a very low-dosed dosage form for hormone replacement therapy (HRT). More particularly, the present invention concerns a solid oral dosage form comprising about 0.5 mg estradiol and about 0.25 mg drospirenone, and at least one pharmaceutically acceptable excipient. Despite the very low E2 and DRSP doses it has surprisingly been found that a high proportion of the women suffering from moderate to severe hot flushes actually respond to this treatment. Accordingly, the dosage form of the invention may be used as maintenance HRT or may be used already when HRT is initiated.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Pharmaceutical compositions of testosterone
ActiveUS11311554B2Organic active ingredientsOrganic non-active ingredientsHormone replacementPharmaceutical drug
The present invention provides stable pharmaceutical compositions, comprising a pharmaceutically effective amount of testosterone or a pharmaceutically acceptable ester thereof, a pharmaceutically acceptable oil vehicle, and a stabilizing amount of benzyl alcohol, for example, about 1% to 3% weight / volume of benzyl alcohol. The present invention also provides a process for stabilizing testosterone-containing pharmaceutical compositions by ageing them at a temperature of about 20° C. to about 60° C. for at least 48 hours, e.g., prior to secondary packing and labeling. These compositions were stable over the shelf life of the product, without exhibiting crystal formation, even upon storing at temperatures of about 2° C. to about 8° C. Other aspects of the invention relate to methods for making such pharmaceutical compositions, and methods of using such pharmaceutical compositions for hormone replacement therapy, e.g., in a male patient having a condition associated with symptoms of deficiency or absence of endogenous testosterone.
Owner:SLAYBACK PHARMA LLC
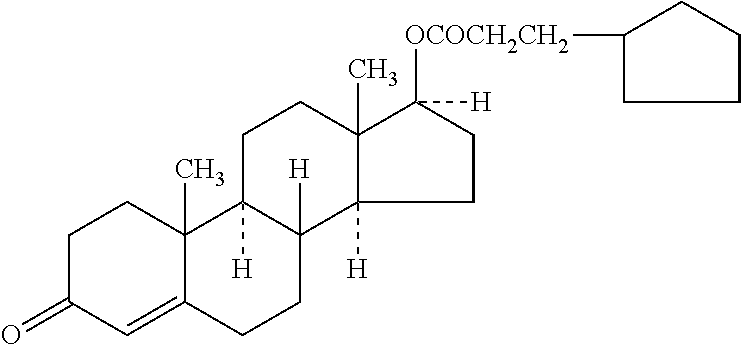
Composition and method to aid in hormone replacement therapy
ActiveUS20200297738A1Less agitationOrganic active ingredientsEmulsion deliveryJojoba oilEpiandrosterone
A pharmaceutical two-phase admixture for topical application, transdermal or transmucosal, characterized by components in two phases, a liquid and a solid, adapted for topical application, transdermal or transmucosal, to various skin and / or mucosal surface areas of the body is disclosed. The solid phase is comprised of one or more bio-identical hormones and the liquid phase is comprised of one or more excipient carrier oils. The bio-identical hormone component is comprised of one or more of Bi-Est, testosterone, progesterone, and dehydroepiandrosterone. The excipient carrier oil component is comprised of one or more of a wide range of common and rare pharmacological oils including specific formulations of jojoba oil, evening primrose oil, and borage seed oil. The solid phase bio-identical hormone component is comprised of either a standard coarse formulation or a formulation comprised of nanoparticles. The pharmaceutical admixture is especially useful in a regime of hormone replacement therapy.
Owner:THE MENOPAUSE METHOD INC
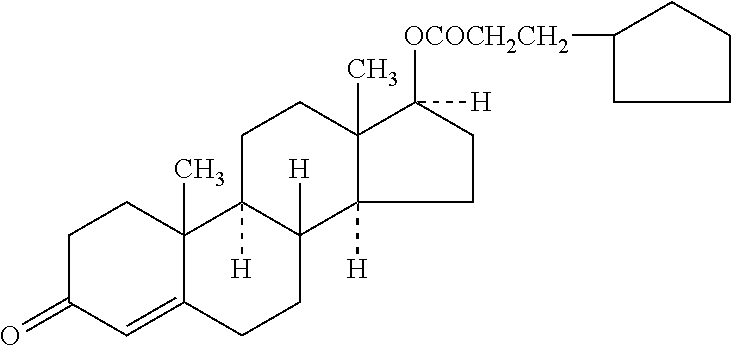
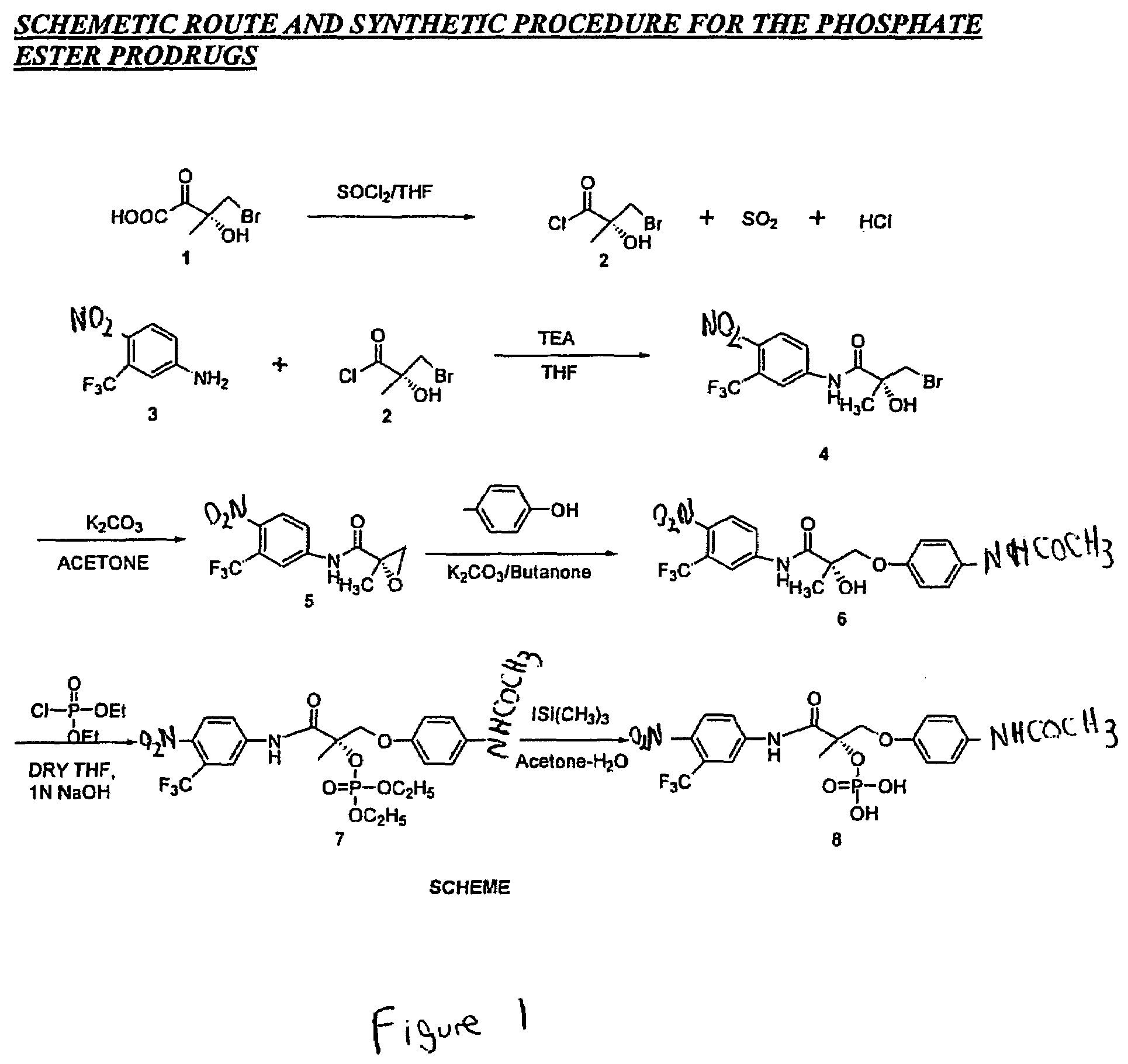
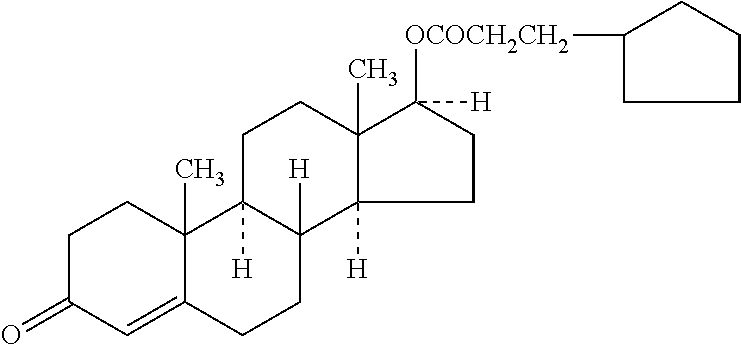
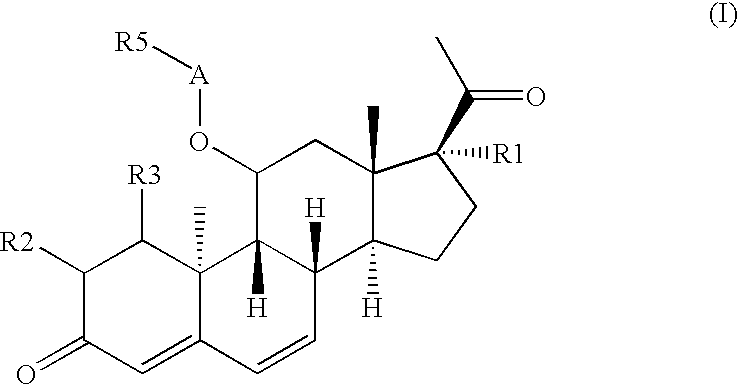
Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one
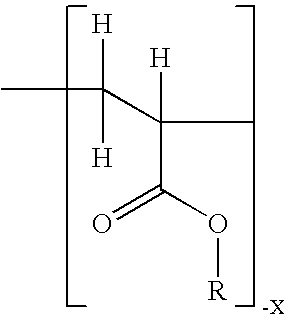
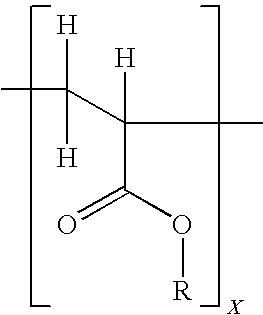
InactiveCN100384867CNegative effects of bioavailability to avoidExcellent solubility propertiesOrganic active ingredientsSteroidsPhenyl groupBiology
The invention relates to novel solid body forms of mesoprogestin 11 beta -[4E-(hydroxyiminomethyl)-phenyl]-17 alpha-methoxymethyl-17 beta-methoxy-estra-4,9-dien-3-one (oxime J867), particularly a highly pure and stable amorphous or highly crystalline form (ansolvate / anhydrate) of compound J867. The invention also relates to methods for producing said novel solid body forms and to the use thereof in pharmaceutical compositions. The novel solid body forms are characterized by exhibiting a high degree of stability. The solid body forms of oxime J 867 can, in particular, be used in the area of fertility control and in hormone replacement therapy.
Owner:合林股份公司
System and Method for Automated Dosage Calculation and Patient Treatment Life Cycle
ActiveUS20220270732A1Minimize incorrect dosage determinationGenerate revenueMedical data miningDrug and medicationsHormone replacementIntensive care medicine
A system and method for automatically calculating an accurate recommended dosage for hormone replacement therapy and automating the life cycle of a patient's treatment over time. The system and method can automatically acquire relevant patient parameters and apply a consistent formulaic approach to help reduce incorrect dosage determinations. A pellet insertion size may be determined and documented based on a calculated dosage, and an insertion side and lot numbers may be tracked and managed. In addition, corresponding revenues may be tracked and profitability may be reported for hormone replacement therapy practices.
Owner:THE SOTTOPELLE GRP
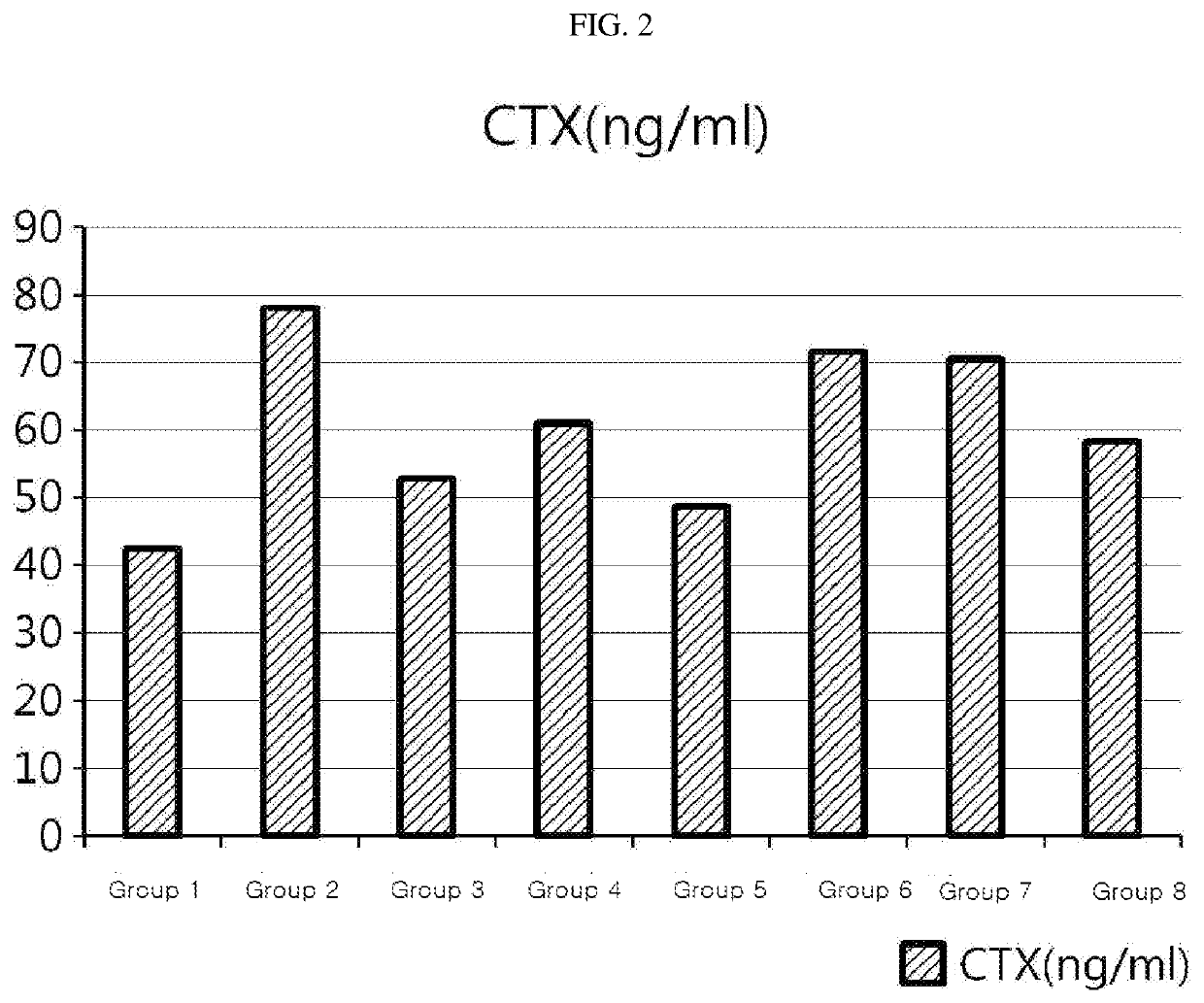
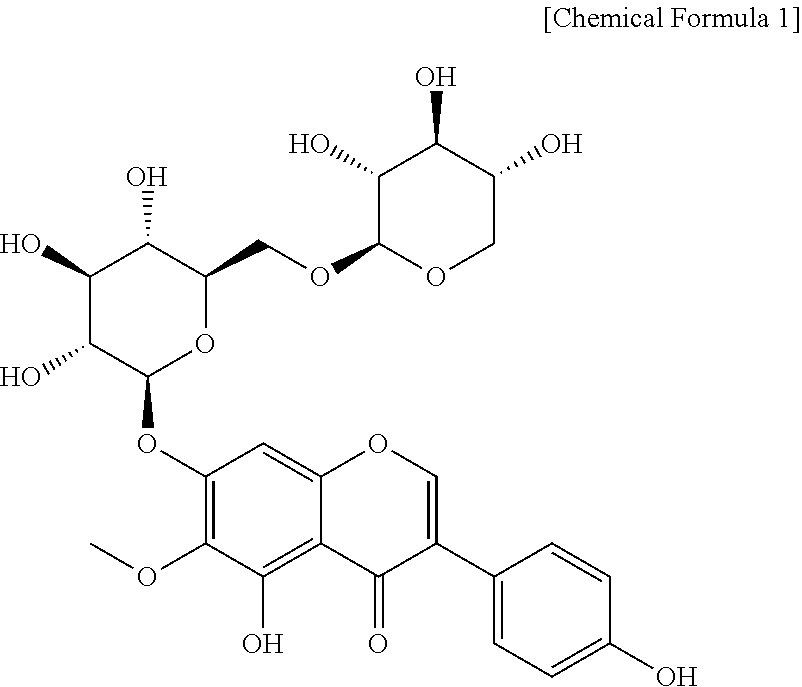
Composition, For Remedying Female Climacteric Syndrome Symptoms, Comprising Tectorigenin 7-O-Xylosylglucoside
ActiveUS20200030350A1Quick effectPreventing, remedying and/or treating sweating, facial flushingCosmetic preparationsOrganic active ingredientsPhysiologyFacial flushing
The present disclosure relates to a composition for preventing, treating or remedying female climacteric syndrome symptoms, which contains tectorigenin 7-O-xylosylglucoside. The composition according to the present disclosure shows quick effects for preventing, remedying and / or treating female climacteric syndrome symptoms, particularly facial flushing and / or osteoporosis, and thus can be utilized for the hormone replacement therapy (HRT) used for preventing or remedying climacteric syndrome symptoms.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
Compounds and their uses for alleviating menopause-associated symptoms
PendingCN112020360AAvoid exposureQuick effectPharmaceutical delivery mechanismSexual disorderSide effectHormone replacement
The present invention relates to a hormone replacement therapy, to the associated compounds and to the associated packaging units,for alleviating menopause-associated symptoms which is based on the administration to a female mammal of an estetrol component at specified daily doses, optionally in combination with a progestogenic component. The therapy enjoys a statistically significant efficacy combined with a favourable profile for side effects compared to currently available methods for alleviating menopause-associated symptoms.
Owner:ESTETRA S P R L
Pharmaceutical compositions of testosterone
ActiveUS20220202830A1Organic active ingredientsPharmaceutical delivery mechanismHormone replacementPharmaceutical drug
The present invention provides stable pharmaceutical compositions, comprising a pharmaceutically effective amount of testosterone or a pharmaceutically acceptable ester thereof, a pharmaceutically acceptable oil vehicle, and a stabilizing amount of benzyl alcohol, for example, about 1% to 3% weight / volume of benzyl alcohol. The present invention also provides a process for stabilizing testosterone-containing pharmaceutical compositions by ageing them at a temperature of about 20° C. to about 60° C. for at least 48 hours, e.g., prior to secondary packing and labeling. These compositions were stable over the shelf life of the product, without exhibiting crystal formation, even upon storing at temperatures of about 2° C. to about 8° C. Other aspects of the invention relate to methods for making such pharmaceutical compositions, as well as methods of using such pharmaceutical compositions for hormone replacement therapy, e.g., in a male patient having a condition associated with symptoms of deficiency or absence of endogenous testosterone.
Owner:SLAYBACK PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one](https://images-eureka.patsnap.com/patent_img/60215b62-278c-4c49-9b3d-2f43485807bd/C0180993900191.PNG)
![Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one](https://images-eureka.patsnap.com/patent_img/60215b62-278c-4c49-9b3d-2f43485807bd/C0180993900201.PNG)
![Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one Novel solid body forms of mesoprogestin 11-beta-[4E-(hydroxyimino methyl)-phenyl]-17-alpha-methoxy methyl-17 beta-methoxy-estra-4,9-dien-3-one](https://images-eureka.patsnap.com/patent_img/60215b62-278c-4c49-9b3d-2f43485807bd/C0180993900211.PNG)