Composite membrane for guided bone regeneration and preparation method of composite membrane
A technology for guiding bone regeneration and composite membranes. It is used in tissue regeneration, medical science, non-woven fabrics, etc. It can solve the problems of uneven mechanical strength, low tension resistance of collagen membranes, and collapse, and reduce the incidence of inflammation and infection. , good biocompatibility and mechanical strength, maintaining the effect of maintaining ability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
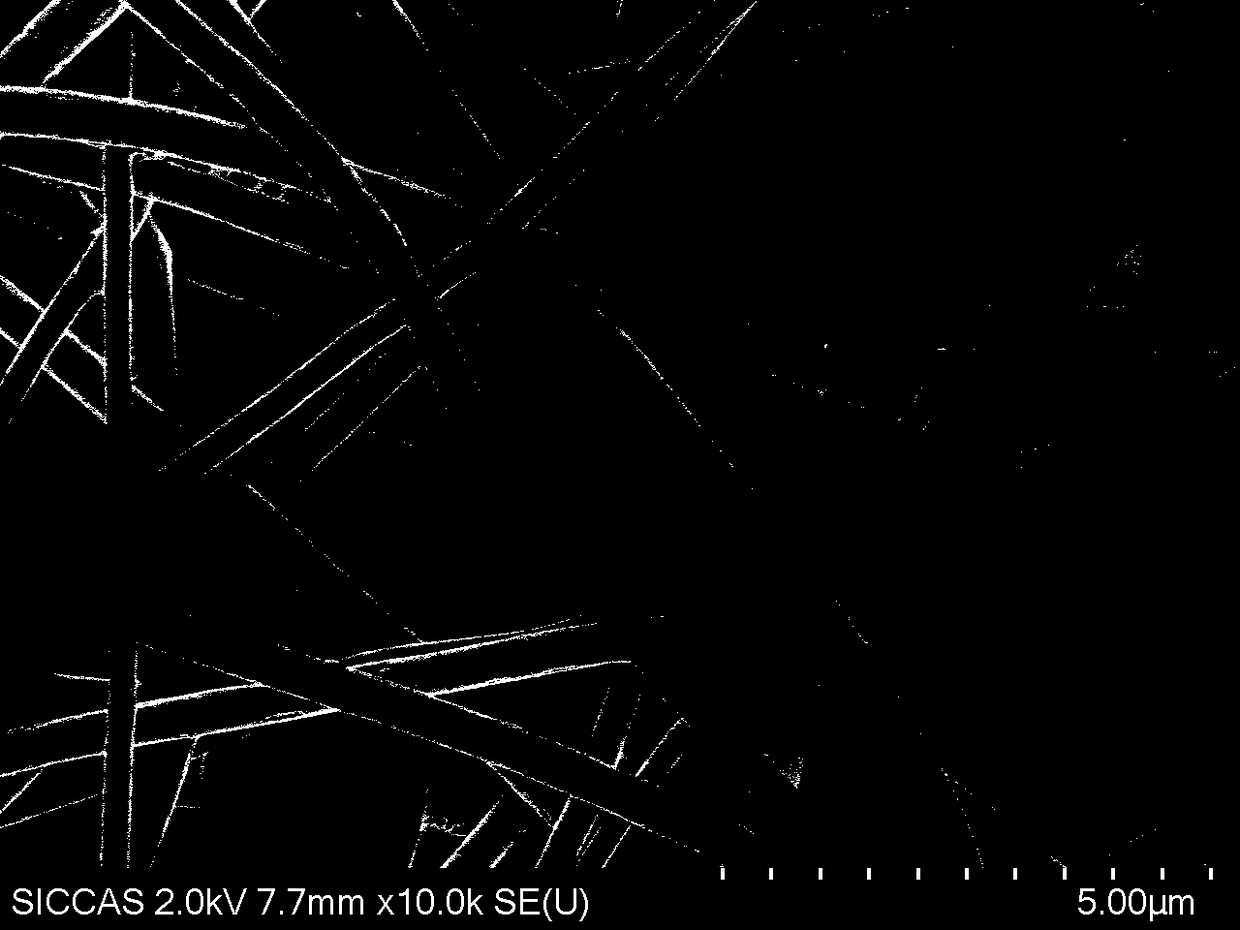
Image
Examples
Embodiment 1
[0040] Preparation of Dense Layer of Composite Regenerated Membrane
[0041] (1) Pretreatment
[0042]Freshly slaughtered porcine small intestine tissue was cleaned and soaked in 0.5% acetic acid solution for 30 minutes. The ratio of porcine small intestine to acetic acid solution was 1:5, and the mucosal layer, muscular layer and serosa layer of porcine small intestine and jejunum were removed by physical scraping , Lymph nodes, the submucosa was separated, cut into pieces, and washed 3 times with purified water;
[0043] (2) Virus inactivation
[0044] Use a mixed aqueous solution containing 1.0% peracetic acid and 15% ethanol, the ratio of the SIS material to the mixed aqueous solution is 1:10, and soak at room temperature for 100 minutes under ultrasonic conditions to inactivate the virus. Then use purified water to ultrasonically clean 3 times;
[0045] (3) Remove immunogenic substances
[0046] Use ethanol with a concentration of 95%, the ratio of SIS material to eth...
Embodiment 2
[0054] Immunogenic substance detection is carried out to the sample prepared in embodiment 1
[0055] (1) Detection method of residual cells: fixed with 10% neutral formalin, embedded in paraffin, cut into thin slices of 0.4 micron, dewaxed with xylene, dehydrated with serial alcohol, stained with hematoxylin-eosin, and microscope Observe the cell residue and matrix fiber structure;
[0056] (2) DNA content detection method: according to YY / T 0606.25-2014 "Determination of DNA residues in biological materials of animal origin: fluorescent staining method";
[0057] (3) α-Gal antigen content detection method: After the sample was fixed with paraformaldehyde, it was routinely embedded in paraffin and sectioned, with a thickness of 3 microns. Immunohistochemical reaction was carried out by using the specific affinity between biotin-labeled BSI-B4 and α-Gal antigen. Judgment of staining results: dark brown-yellow particles are strongly positive (+++), brown-yellow particles are ...
Embodiment 3
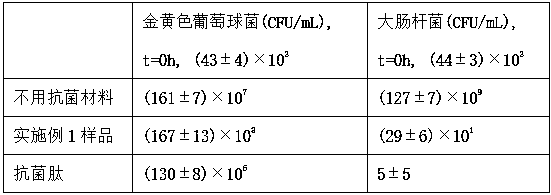
[0061] Antibacterial property detection is carried out to the sample prepared by embodiment 1
[0062] Grind the sample prepared in Example 1 in 0.01 M hydrochloric acid with a grinding rod until no particles are visible to the naked eye, and adjust its concentration to 100 mg / 10 mL. Add pepsin for digestion, pepsin:sample ratio is 1:10. Stir continuously at 25°C for 48h, then cool down to 4°C, add 1 / 10 volume of 0.1 M sodium hydroxide to adjust the pH to 7.2-7.4;
[0063] Prepare a mixed culture medium plate, and use an inoculation loop to pick a small amount of cultured Staphylococcus aureus and Escherichia coli slant medium into 5ml sterile normal saline to make a bacterial suspension. Take 1.0ml of the bacterial suspension and 1ml of the above-mentioned degraded sample and add it to a sterilized and dried petri dish, add the ordinary nutrient broth agar medium cooled to about 50°C, shake well, and wait until it is fully condensed for later use. Incubate upside down at 37...
PUM
| Property | Measurement | Unit |
|---|---|---|
| suture strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com