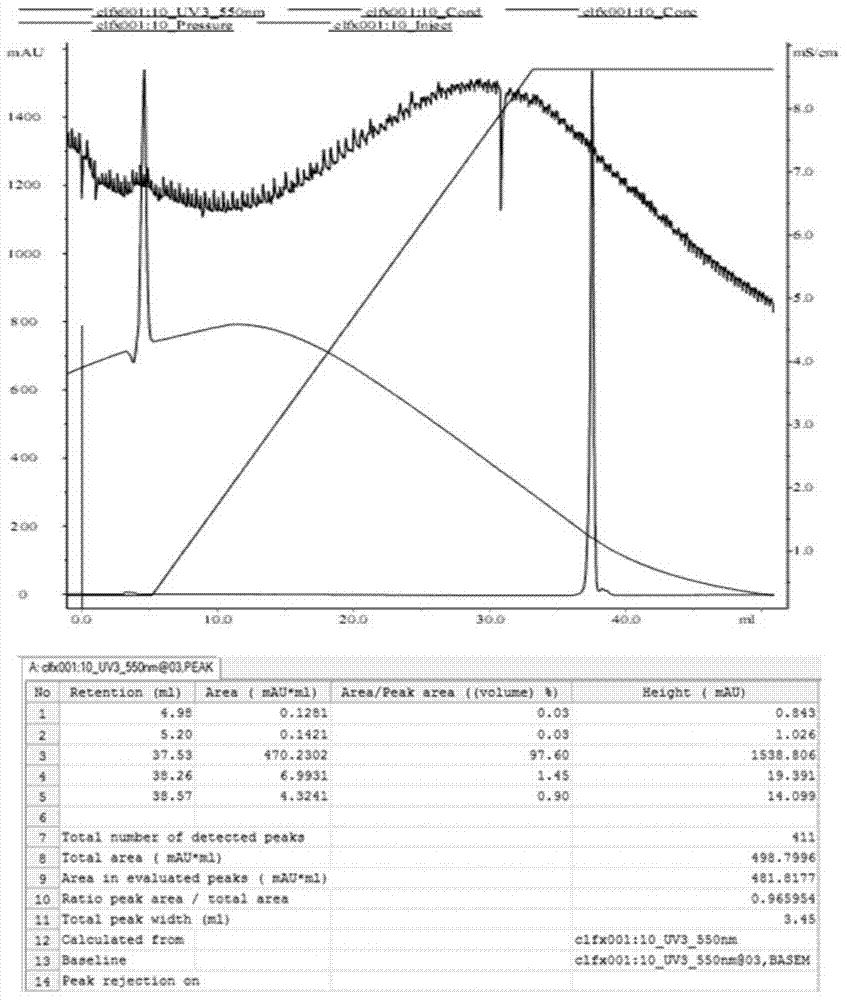
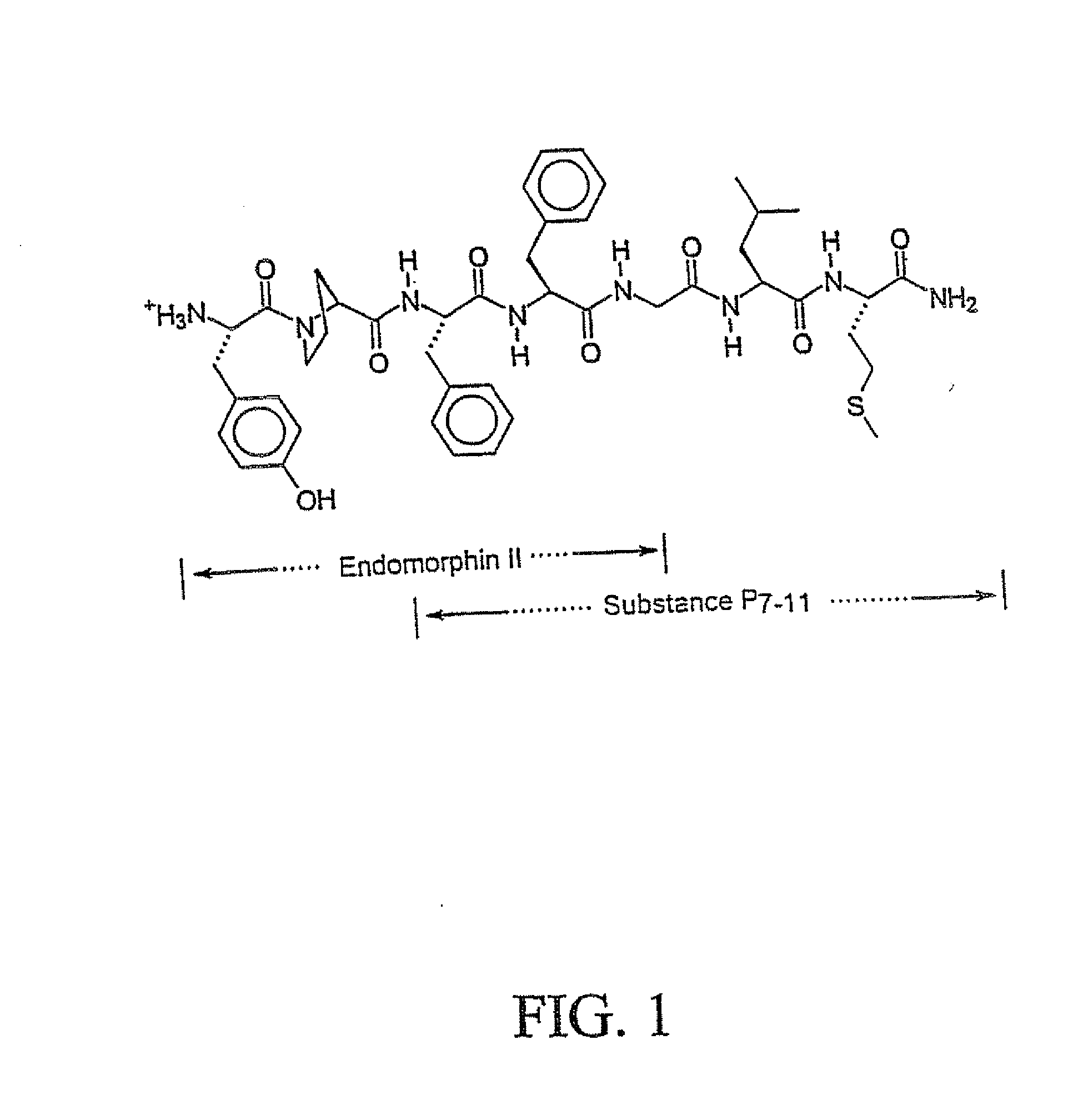
Patents
Literature
30results about "Tachykinins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
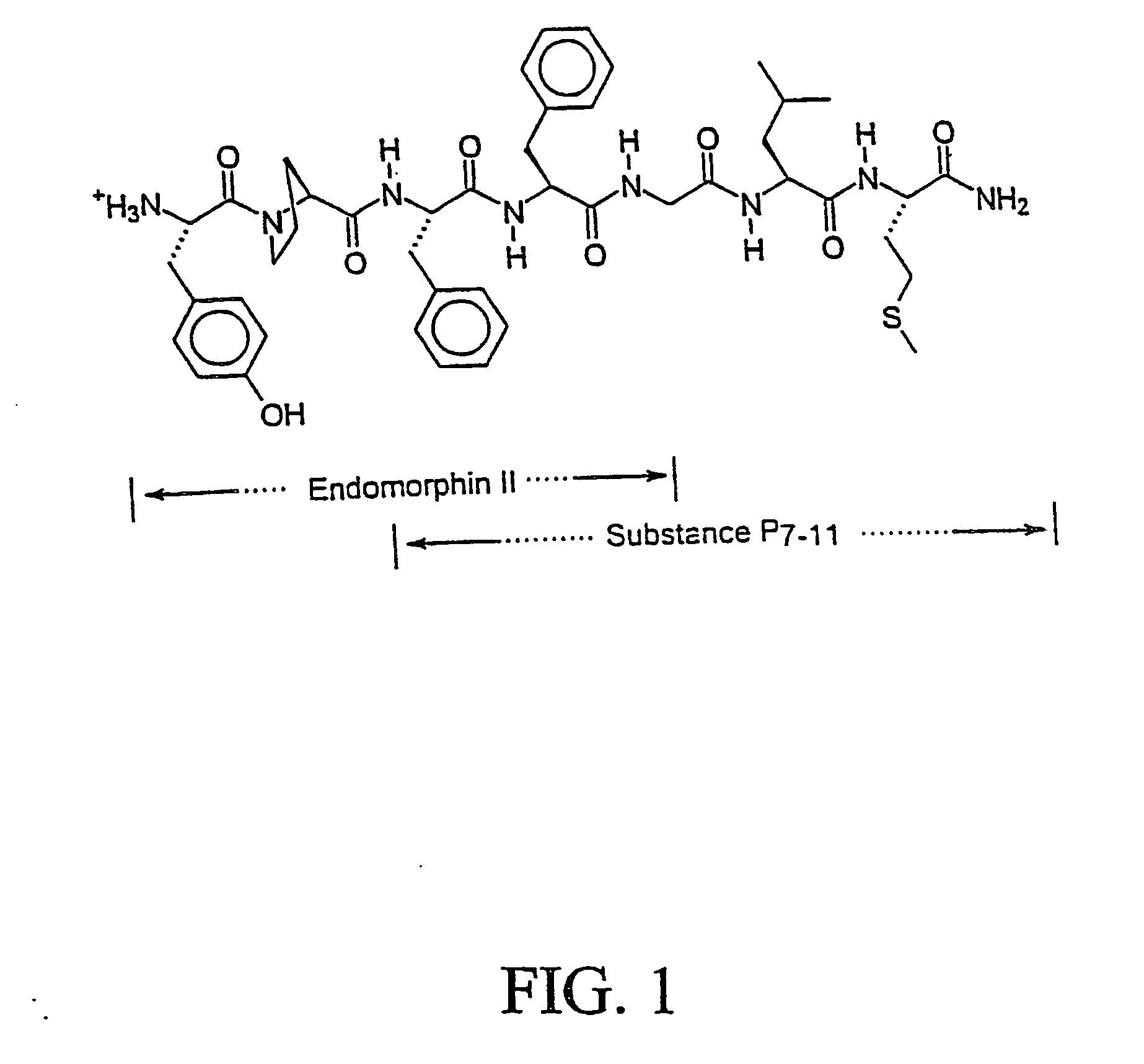
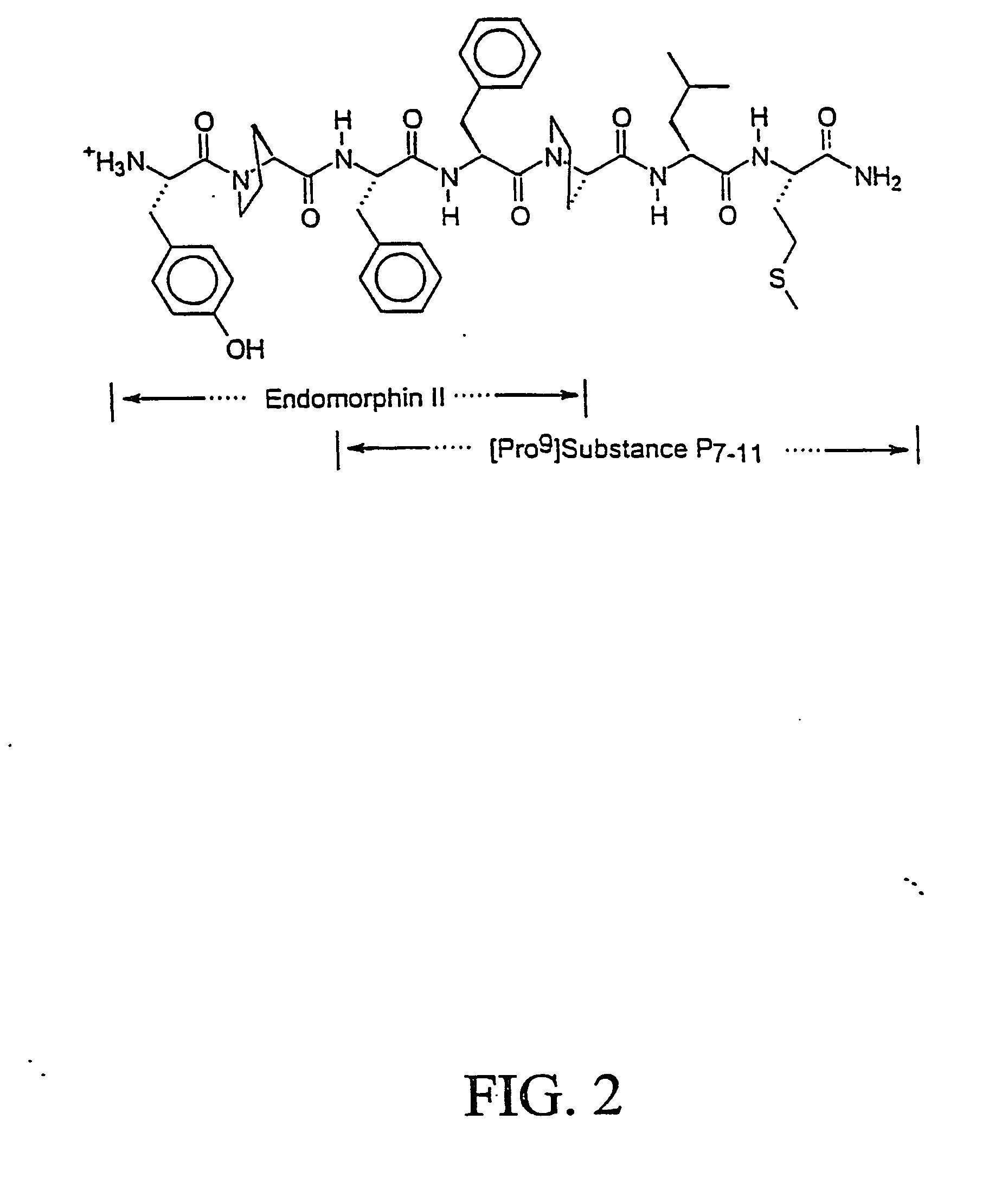
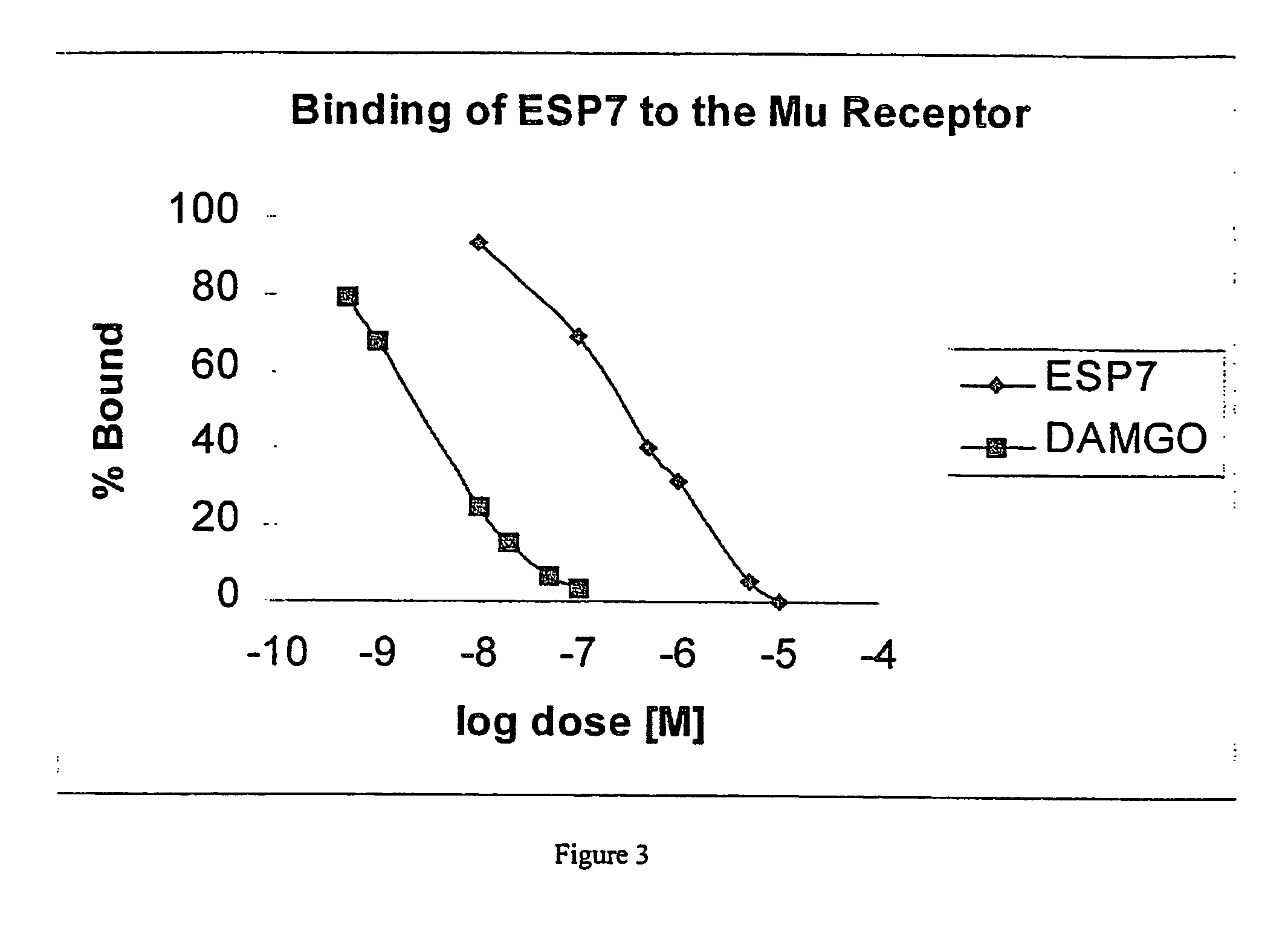
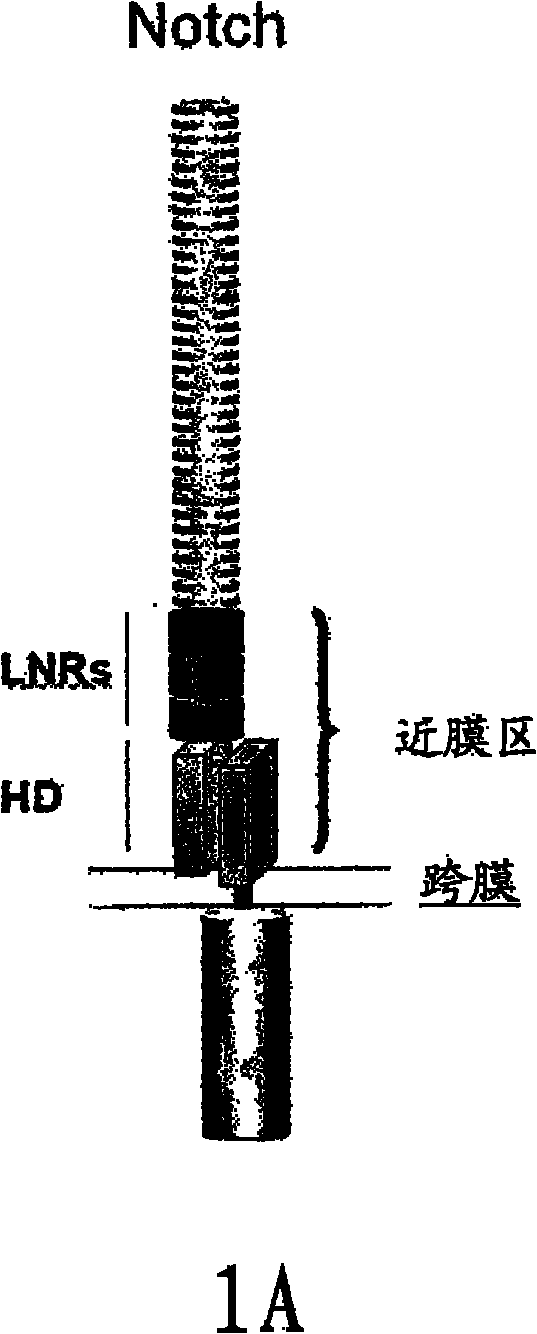
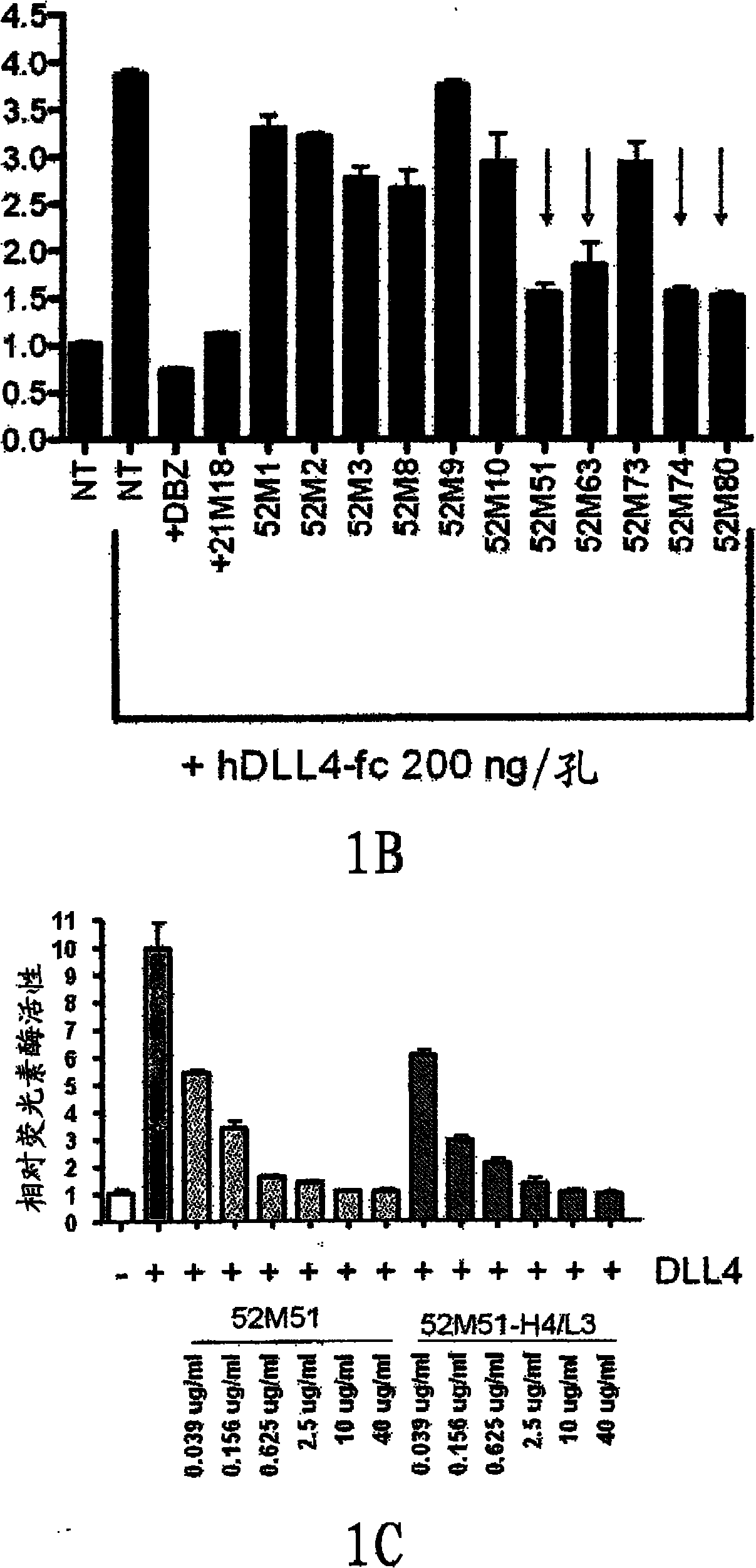
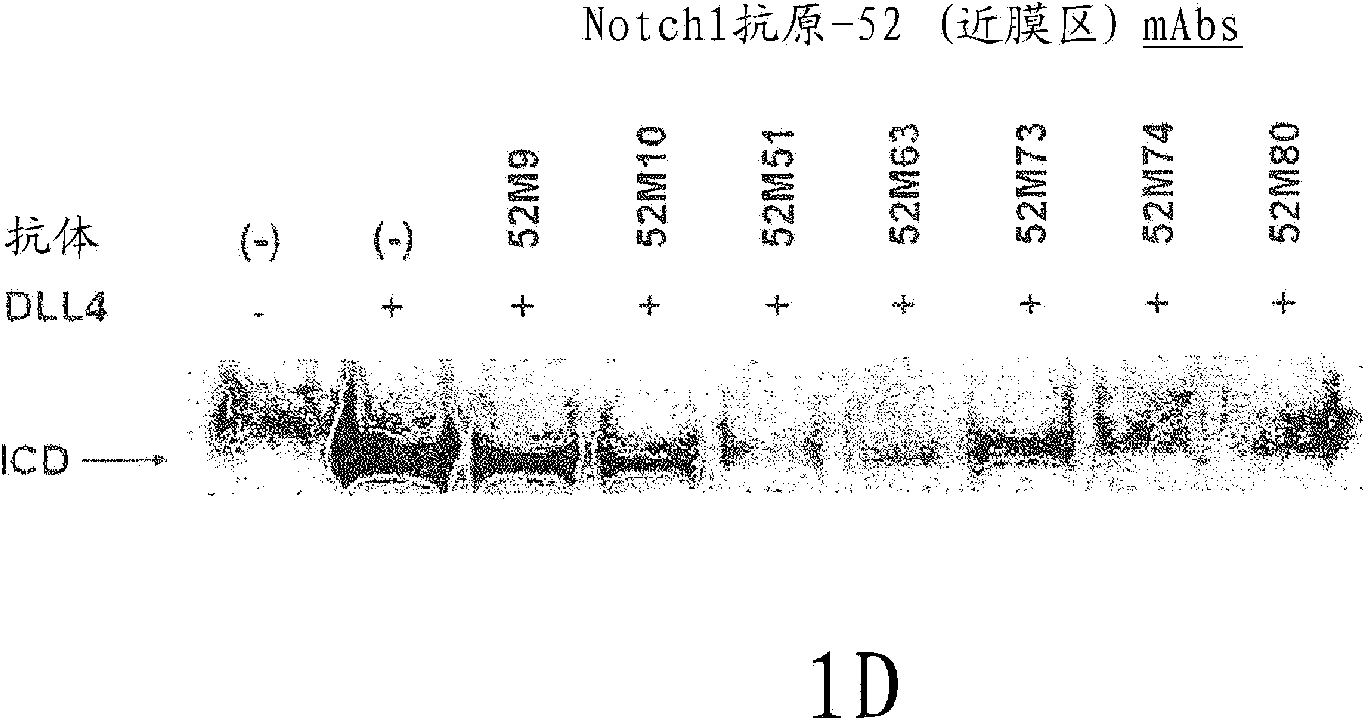
NOTCH1 receptor binding agents and methods of use thereof
InactiveCN102112490AOrganic active ingredientsImmunoglobulins against growth factorsWilms' tumorCancer research
The present invention discloses means and methods for the diagnosis, characterization, prognosis and treatment of cancer and specifically targeting cancer stem cells. The present invention provides an antibody that specifically binds to a non-hgand binding membrane proximal region of the extracellular domain of a human Notch receptor and inhibits tumor growth, and a method of treating cancer, wherein the method comprises administering the antibody for study subjects.
Owner:ONCOMED PHARMA INC
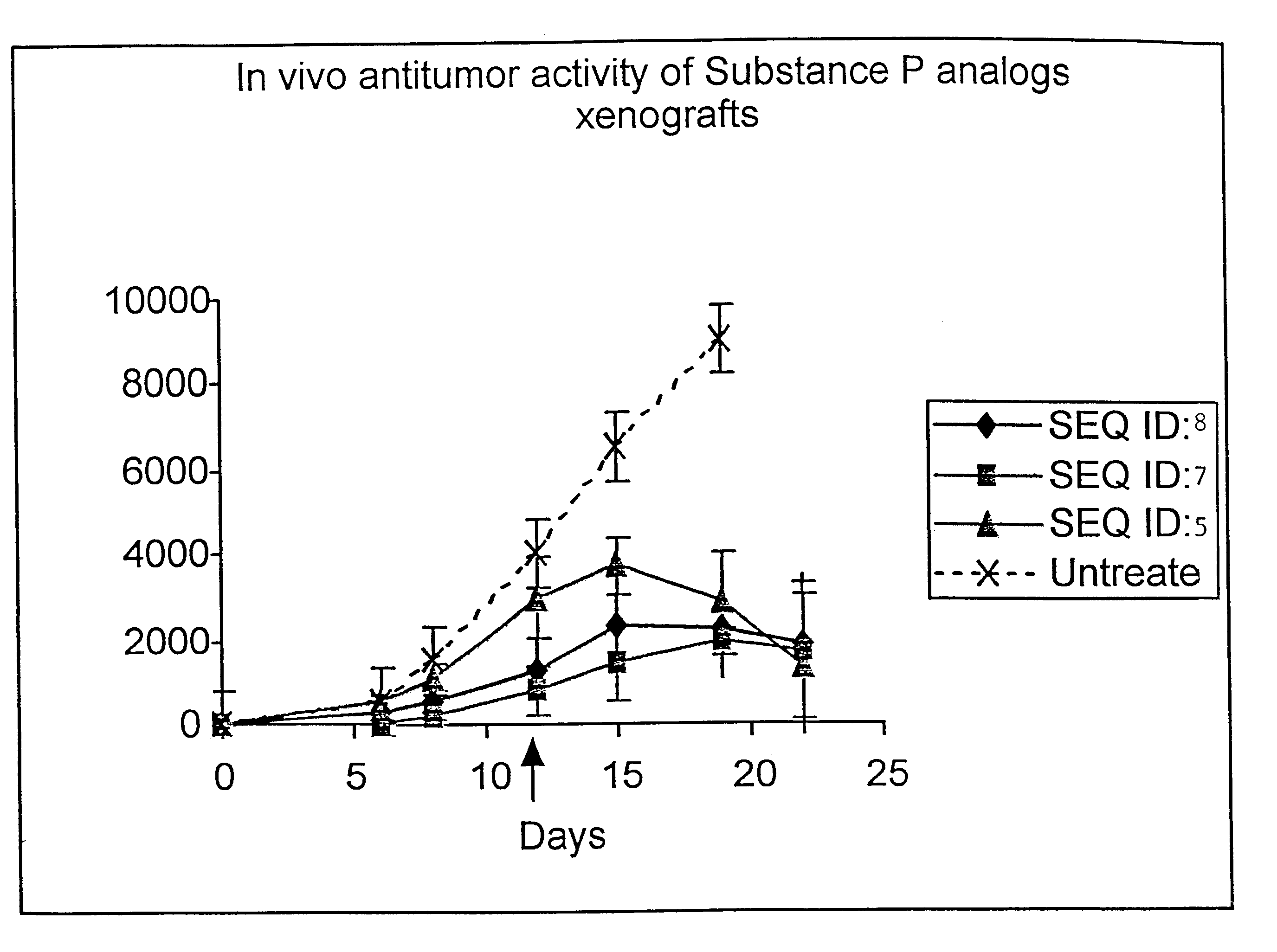
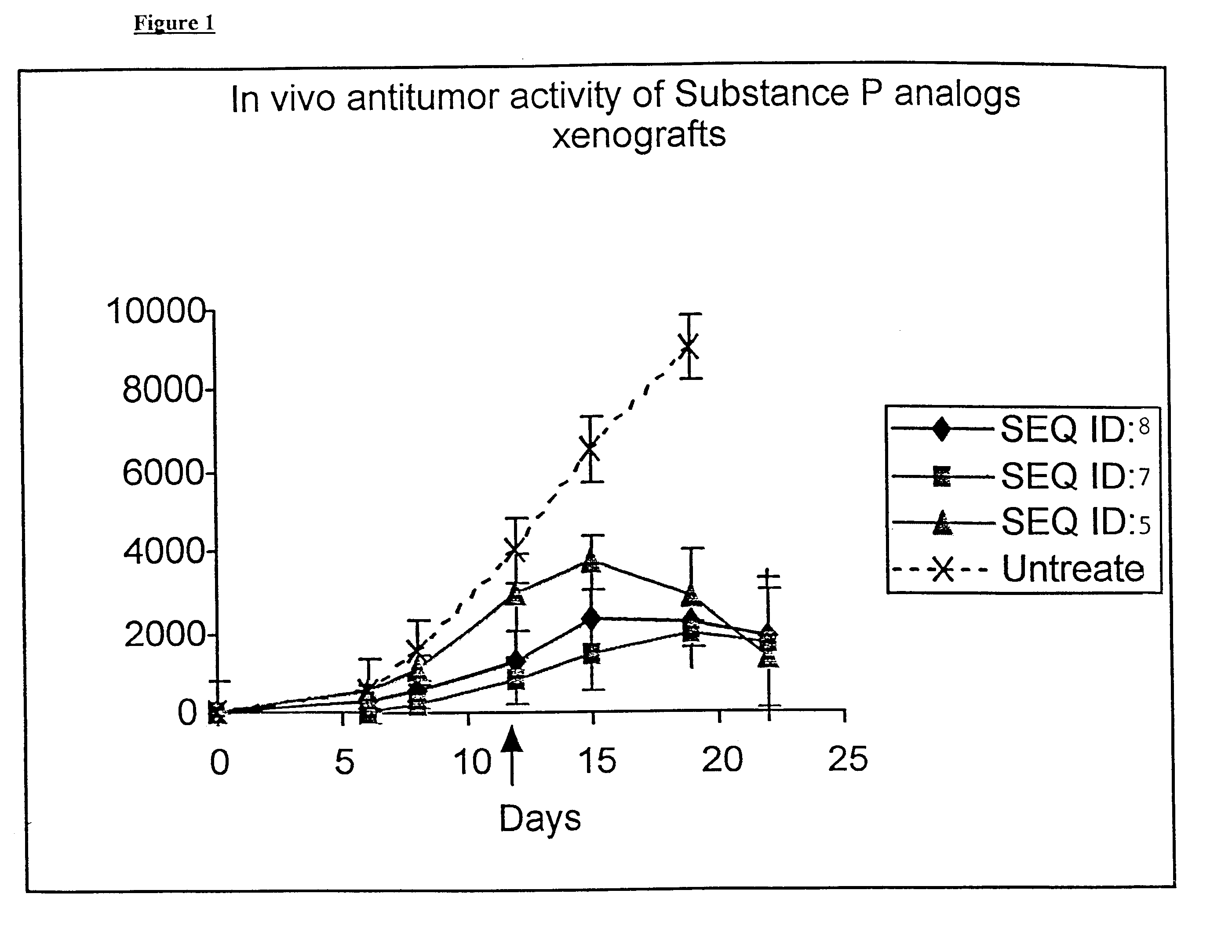
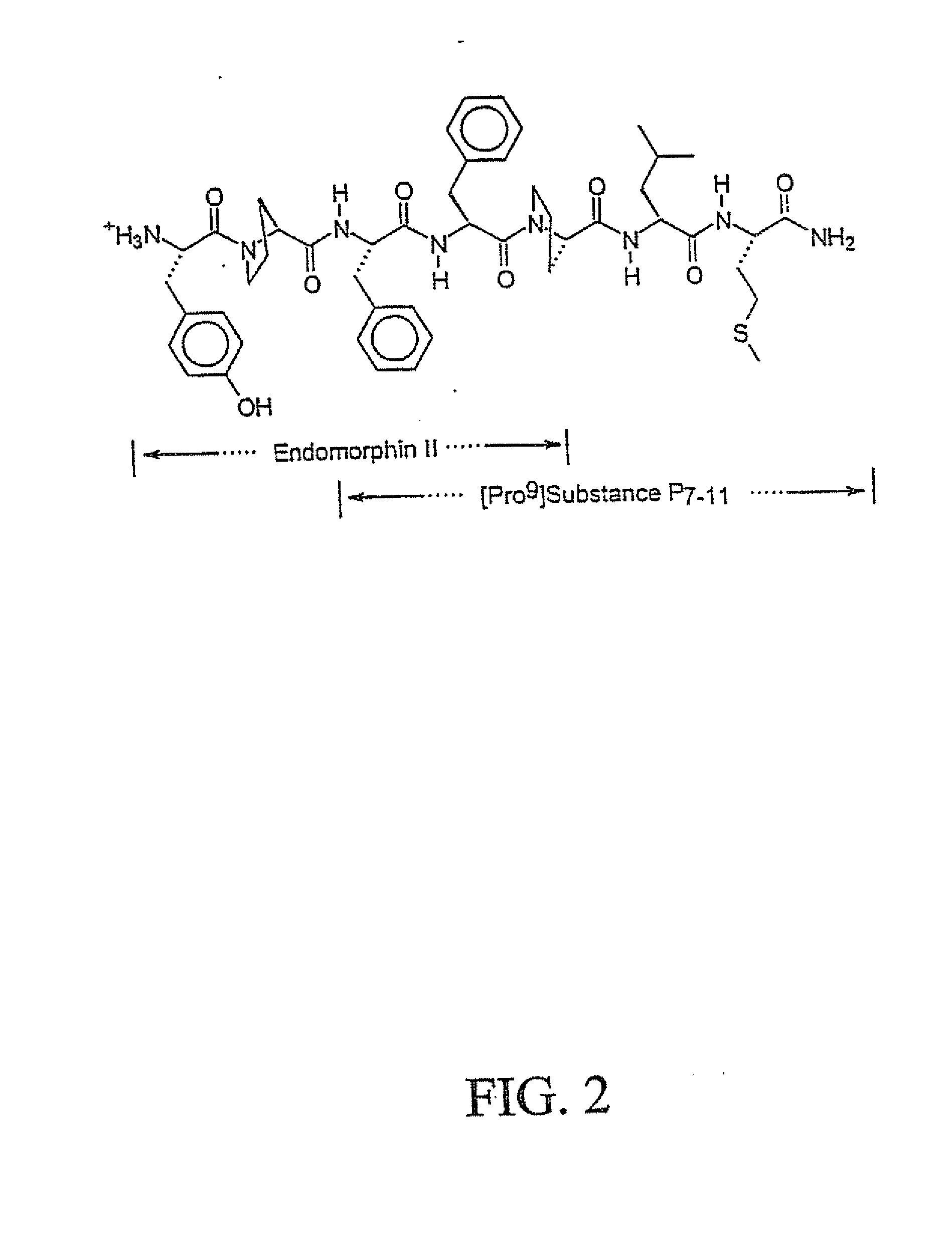
Substance P analogs for the treatment of cancer
The present invention encompasses novel synthetic peptide analogs that are antagonists to Substance P, substance P like peptides and related peptides and are useful for the treatment of cancer. The invention particularly relates to the design and synthesis of the novel substance P antagonist analogs incorporating alpha,alpha-dialkylated amino acids in a site specific manner. The invention encompasses methods for the generation of these peptides, compositions containing these peptides and pharmacological applications of these peptides specifically in the treatment and prevention of cancer.
Owner:DABUR PHARM LTD
Antiangiogenic drugs
InactiveUS6492330B1Avoid multiplicationPeptide/protein ingredientsVasoactive intestinal peptideTumor angiogenesisPharmaceutical drug
The invention relates to the use of peptides individually or in combination, for treating and / or preventing angiogenesis. It also relates to the use of peptide analogs or a combination of peptides referred to as MuJ-7 as anticancer drugs in restricting the tumor growth and spread by inhibiting tumor angiogenesis. MuJ-7, in addition inhibits metastasis through its antiangiogenic activity in all cancers. The invention also relates to a pharmaceutical composition containing either individual peptides or in combination, and methods of treatment of human beings and animals for curing and / or preventing angiogenesis.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY +1
Disulfide conjugated cell toxins and methods of making and using them
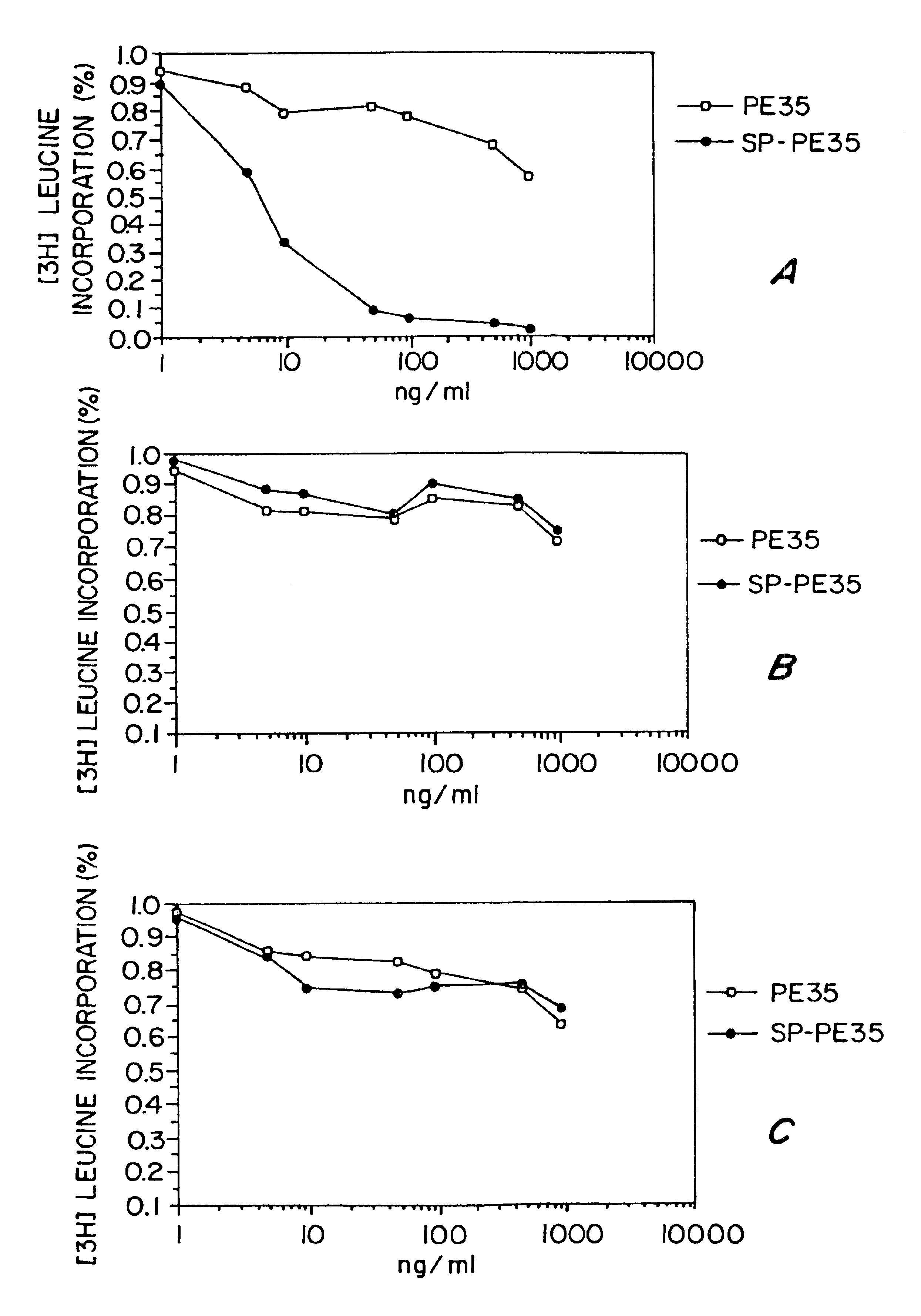
The invention pertains to the discovery of novel disulfide linked cell toxins which can ablate NK-1 receptor expressing cells. These toxins are used as pharmaceutical compositions for the ablation of NK1 receptor expressing cells and comprise a substance P (SP)-Pseudomonas exotoxin disulfide linked conjugate. The invention also includes methods of making and using these toxins and pharmaceutical compositions.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SE
Genetic polymorphisms in the preprotachykinin gene
The present invention relates to a method of correlating single nucleotide polymorphisms in the pre-tachykininogen (NKNA) gene with the efficacy and compatibility of pharmaceutically active compounds administered to human subjects. The present invention also relates to a method of determining the efficacy and compatibility of a pharmaceutically active compound administered to a human individual, the method comprising detecting at least one single nucleotide polymorphism in the NKNA gene. The method is based on the detection of specific single nucleotide polymorphisms in the NKNA gene and the determination of the efficacy and compatibility of pharmaceutically active compounds in human subjects with reference to the NKNA gene polymorphisms. The present invention also relates to isolated nucleic acids comprising polymorphisms as defined herein in their sequence, to nucleic acid primers and oligonucleotide probes capable of hybridizing to such nucleic acids, to nucleic acids comprising one or more of such primers and probes A diagnostic kit for detecting NKNA gene polymorphisms, relating to a pharmaceutical pack containing an NK-1 receptor antagonist and instructions for administering the drug to a human individual subject to polymorphism detection, and also to a stored NKNA gene polymorphism A computer readable medium of state sequence information.
Owner:F HOFFMANN LA ROCHE & CO AG
Regulation of Specific Spinal Neurons Regulating Pain Transmission
ActiveUS20140255376A1Long-lasting and/or stable and/or reversiblePolypeptide with localisation/targeting motifTachykininsPeptide ligandNeuron
A chimeric toxin is disclosed comprising a peptide ligand specifically targeting neurons involved in pain processing; and a clostridial neurotoxin light chain, wherein the ligand is linked to the light chain. The methods of preparing such chimeric toxin and the method of using the chimeric toxin to regulate pain transmission are also disclosed.
Owner:RGT UNIV OF CALIFORNIA +1
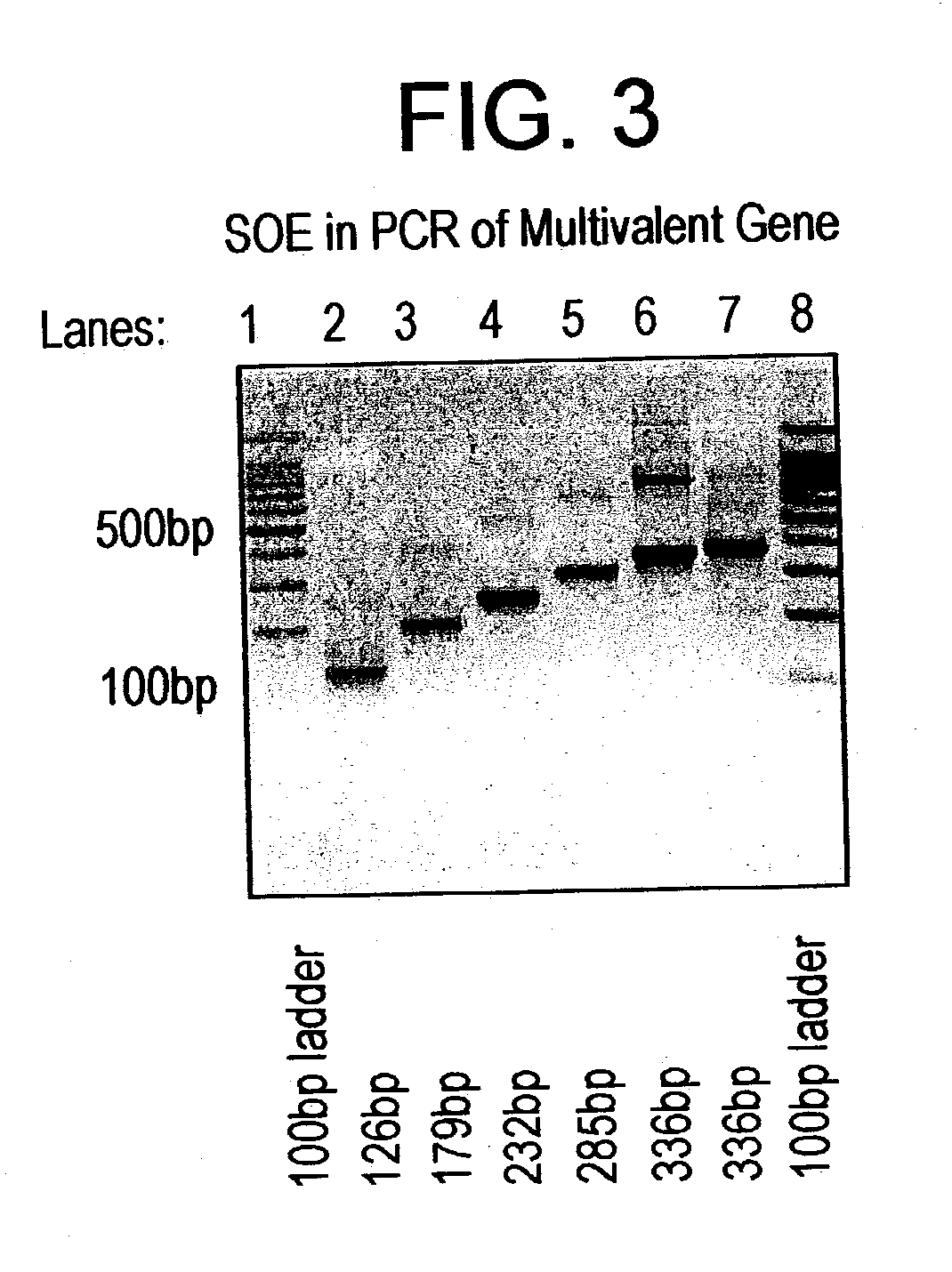
Multivalent synthetic vaccine for cancer
InactiveUS20030082201A1Preventing, inhibiting, or modulating the hypersecretion of VIPBacteriaAntibody mimetics/scaffoldsBombesinVasoactive intestinal peptide
Multivalent vaccine comprising peptides from vasoactive intestinal peptide, bombesin, Substance P and epidermal growth factor are described. A method of constructing a multivalent gene for use in various expressions vectors and the protein recombinantly expressed in the prokaryotic expression systems are also described.
Owner:DABUR RESEARCH FOUNDATION
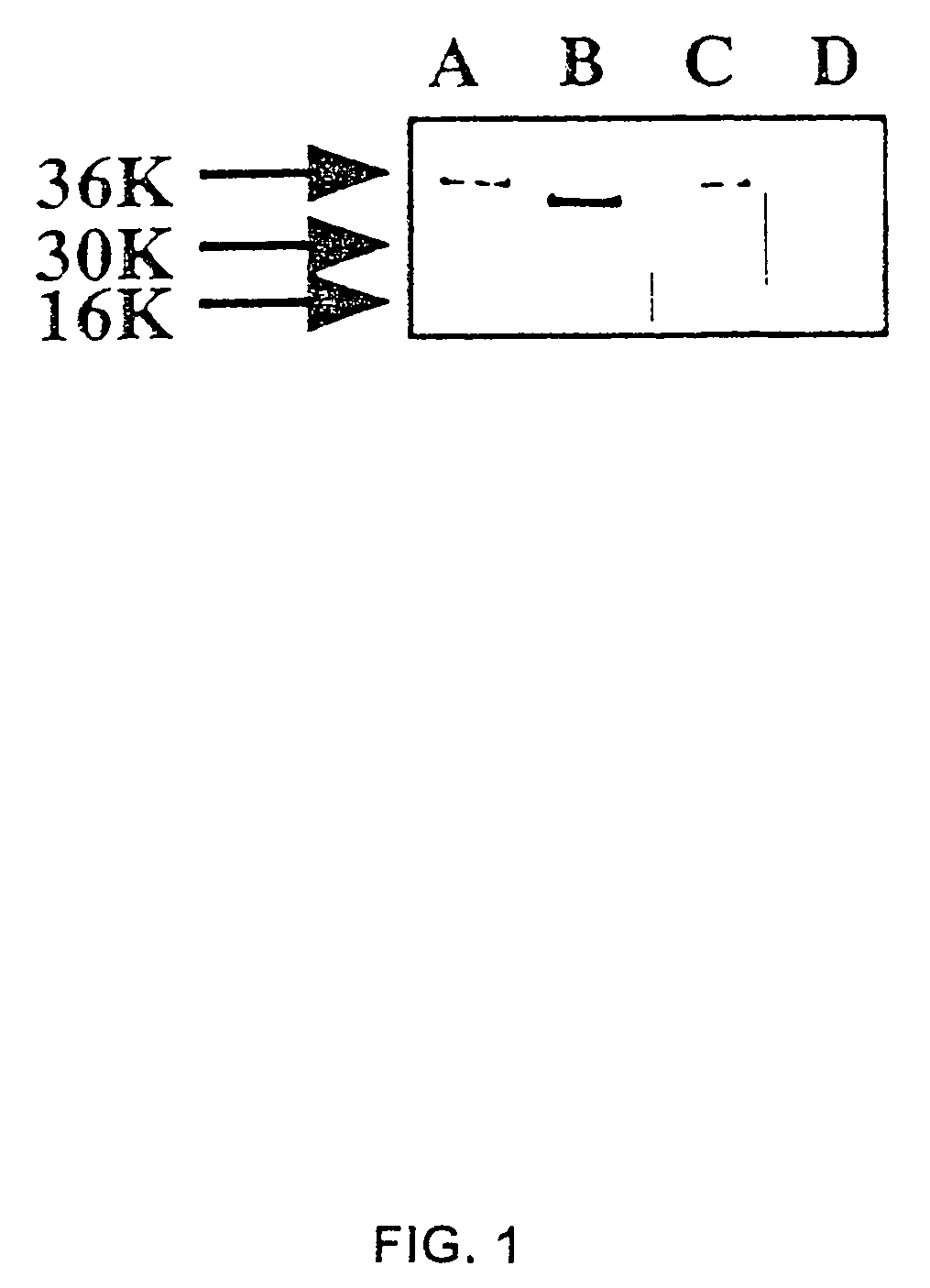
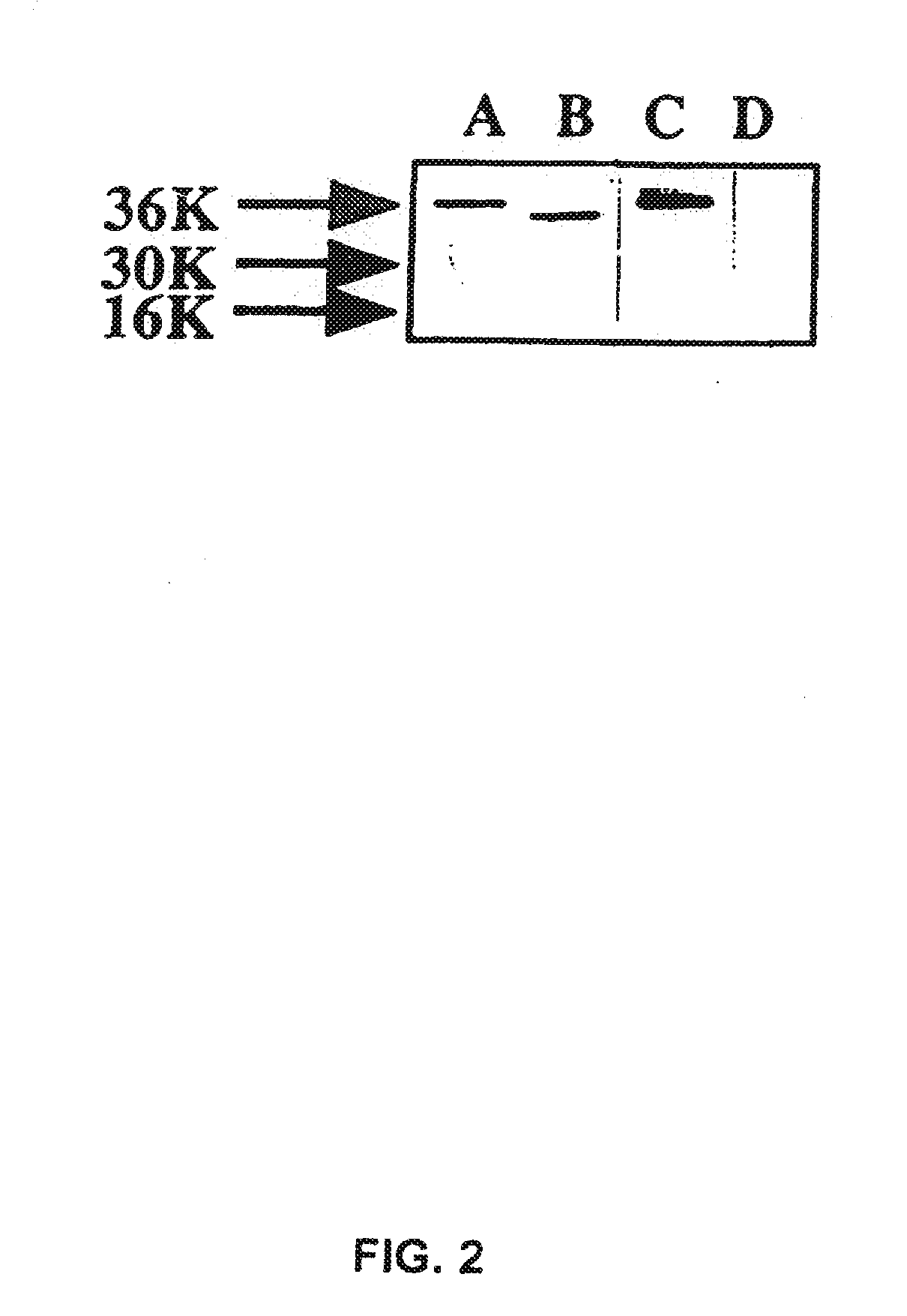
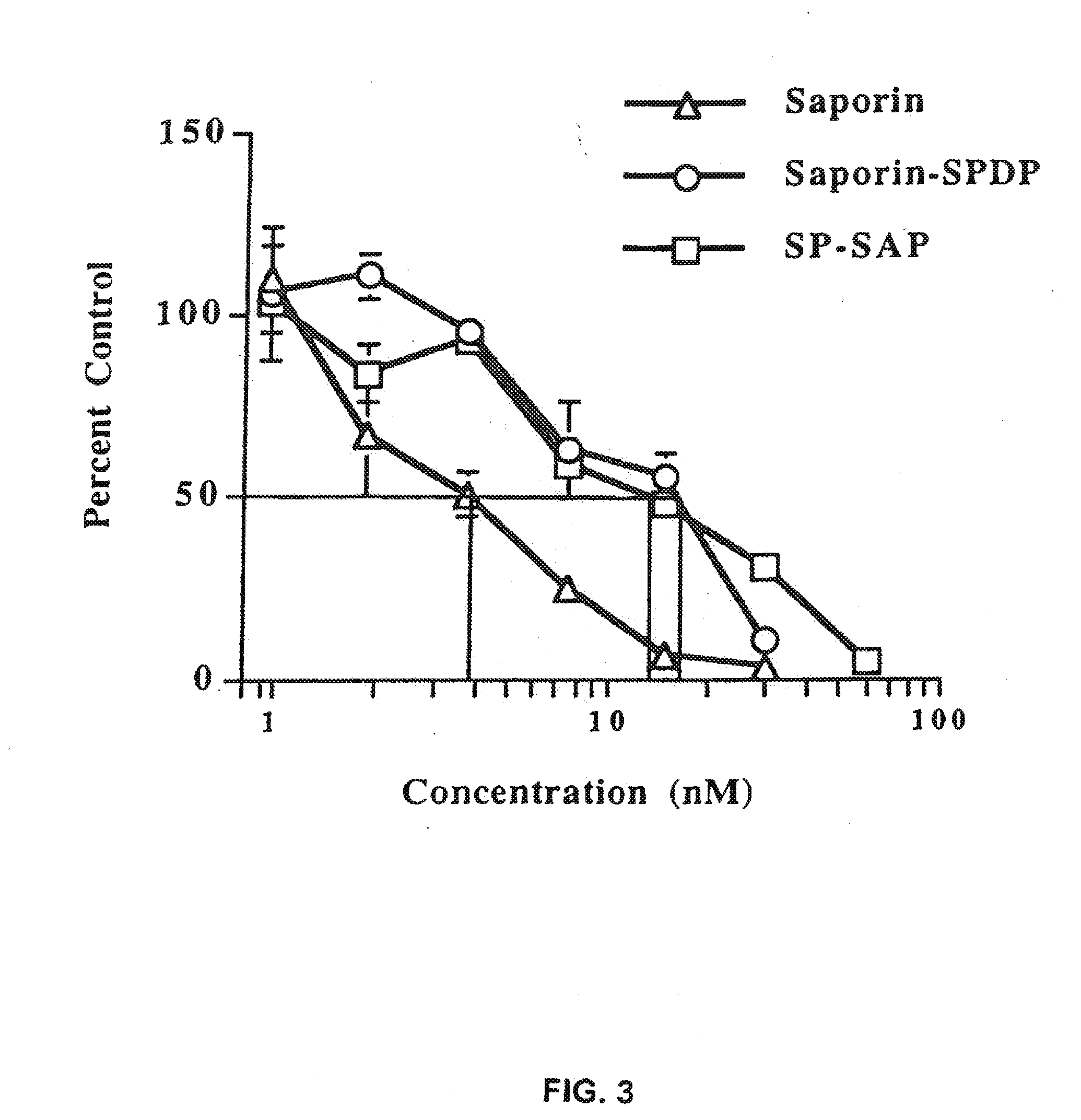
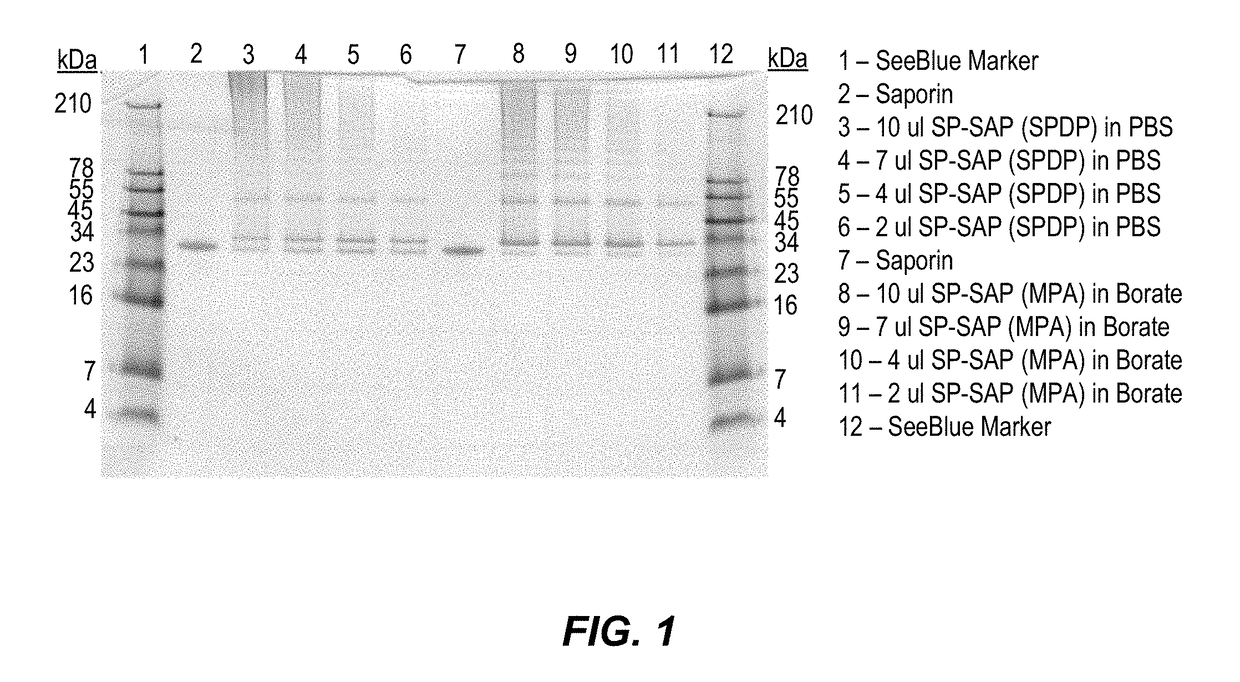
Substance P-saporin (SP-SAP) conjugates and methods of use thereof
InactiveUS7741435B2Reduce perceptionReduce painNervous disorderPeptide/protein ingredientsSaporinDisease
This invention provides a conjugate comprising Substance P, or an analog thereof, and a protein, such as Saporin, that inhibits protein synthesis.This invention provides a method of reducing the perception of pain by a subject comprising administering to the subject an effective amount of the pharmaceutical composition of the conjugate comprising Substance P, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, so as to reduce the perception of pain by the subject.This invention provides a method of selectively destroying NK-1R-expressing neuronal cells in a subject comprising administering to the subject an effective amount of the conjugate comprising Substance P, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, so as to selectively destroy NK-1R-expressing neuronal cells.Lastly, this invention provides a method for treating a NK-1R-associated disorder in a subject, which comprises administering to the subject an amount of the pharmaceutical composition comprising substance P, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, in an effective amount to treat the disorder associated with the NK-1R.
Owner:ADVANCED MARKETING SYST
Regulation of specific spinal neurons regulating pain transmission via chimeric toxins
ActiveUS9447405B2Long-lasting and/or stable and/or reversiblePeptide/protein ingredientsPharmaceutical delivery mechanismPeptide ligandToxin
A chimeric toxin is disclosed comprising a peptide ligand specifically targeting neurons involved in pain processing; and a clostridial neurotoxin light chain, wherein the ligand is linked to the light chain. The methods of preparing such chimeric toxin and the method of using the chimeric toxin to regulate pain transmission are also disclosed.
Owner:RGT UNIV OF CALIFORNIA +1
Monoclonal antibody that binds human notch2 and notch3
ActiveUS8980260B2Block ligand activationReducing tumorigenicityOrganic active ingredientsImmunoglobulins against growth factorsMonoclonal antibodyExtracellular Structure
The present invention relates to Notch-binding agents and Notch antagonists and methods of using the agents and / or antagonists for treating diseases such as cancer. The present invention provides antibodies that specifically bind to a non-ligand binding region of the extracellular domain of one or more human Notch receptor, such as Notch2 and / or Notch3, and inhibit tumor growth. The present invention further provides methods of treating cancer, the methods comprising administering a therapeutically effective amount of an antibody that specifically binds to a non-ligand binding region of the extracellular domain of a human Notch receptor protein and inhibits tumor growth.
Owner:MEREO BIOPHARMA 5 INC
Previns as specific inhibitors and therapeutic agents for Botulinum toxin B and Tetanus neurotoxins
The compounds of the invention are generally described by the formula: B1Z*2B3Z*4X*5Q6F7X8X9X10X11 (1), B1X2X3X4X5Q6F7X8X9X10X11 (2), or B1Z2B3X4Z5Q6F7Z8X9X10X11 (3) and the salts, esters, amides, and acyl forms thereof. Each position represented by a letter indicates a single amino acid residue: B is a basic of polar / large amino acid or a modified form thereof; X is a small or hydrophobic amino acid or a modified form thereof; X* is a small or polar / large amino acid or a modified form thereof; Z is a polar / large or hydrophobic amino acid or a modified form thereof; Z* is Proline or a polar / large of hydrophobic amino acid or a modified form thereof. As described below, one or more of the peptide linkages between the amino acid residues may be replaced by a peptide linkage mimic. These compounds may be used as molecular building blocks to create compounds that are optimized for inhibiting the protease activity of Botulinum b and tetanus toxins.
Owner:ARMY MEDICAL RES & MATERIEL COMMAND UNITED STATES AS REPRESENTED BY THE DEPT OF THE
Antagonists of neurokinin B in fish reproduction
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
P substance peptide probe capable of specific recognition of neurokinin-1 receptor protein, and preparation and application thereof
InactiveCN104744568AImprove bindingHigh sensitivityTachykininsPeptide preparation methodsFluorescenceKinin
The invention provides a P substance peptide probe capable of specific recognition of a neurokinin-1 receptor protein. the P substance peptide probe is a polypeptide dimer or a polypeptide polymer, the monomer is formed by a P substance amino acid sequence MLGFFQQPKPR, a bifunctional chelating group, a fluorescence group and a terpyridyl group connected through lysine. The novel peptide probe is used for the preparation of a polypeptide dimer and polypeptide polymer with function of diagnosing multi tumors. The polypeptide dimer and polypeptide polymer marks metal Gd<3+> to a polypeptide molecule by the bifunctional chelating group; the marked P substance peptide probe and the NK-1 receptor protein specifically bind and concentrate to the tumor site in vivo; and magnetic imaging and optical imaging technology are utilized for detection of the neurokinin-1 receptor protein to improve the sensitivity of the detection.
Owner:EAST CHINA UNIV OF SCI & TECH
Genetic polymorphisms in the preprotachykinin gene
InactiveUS20060228752A1Useful predictionOrganic active ingredientsSenses disorderPreprotachykininNK 1 Receptor Antagonists
The present invention relates to a method for correlating single nucleotide polymorphisms in the preprotachykinin (NKNA) gene with the efficacy and compatibility of a pharmaceutically active compound administered to a human being. The invention further relates to a method for determining the efficacy and compatibility of a pharmaceutically active compound administered to a human being which method comprises determining at least one single nucleotide polymorphism in the NKNA gene. Said methods are based on determining specific single nucleotide polymorphisms in the NKNA gene and determining the efficacy and compatibility of a pharmaceutically active compound in the human by reference to polymorphism in NKNA. The invention further relates to isolated nucleic acids comprising within their sequence the polymorphisms as defined herein, to nucleic acid primers and oligonucleotide probes capable of hybridizing to such nucleic acids and to a diagnostic kit comprising one or more of such primers and probes for detecting a polymorphism in the NKNA gene, to a pharmaceutical pack comprising NK-1 receptor antagonists and instructions for administration of the drug to human beings tested for the polymorphisms as well as to a computer readable medium with the stored sequence information for the polymorphisms in the NKNA gene.
Owner:FOERNZLER DOROTHEE +5
Method for stabilization of peptides in a biological sample
InactiveUS8173385B2Accurate measurementSimple methodTachykininsPeptide/protein ingredientsStable stateReagent
Owner:KYOWA MEDEX CO LTD
Method for stabilization of peptides in a biological sample
InactiveUS20090035803A1Accurate measurementSimple methodPeptide/protein ingredientsTachykininsClinical testsReagent
Owner:KYOWA MEDEX CO LTD
Substance P analog having progenitor cell or stem cell recruiting activity and method for progenitor cell or stem cell recruiting using the same
A substance P analog having a progenitor cell or stem cell recruiting activity and a method of recruiting progenitor cells or stem cells using the substance P analog are disclosed. The substance P analog has an effect of recruiting endogenous progenitor cells or stem cells to a wound or disease-occurring site. Thus, the disclosure also describes its use in recruiting progenitor cells or stem cells and a method of regenerating or treating a damaged organ or tissue, or a method of healing a wound.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Substance p-saporin (sp-sap) conjugates and methods of use
This invention provides a conjugate comprising Substance P, or an analog thereof, and a protein, such as Saporin, that inhibits protein synthesis.This invention provides a method of reducing the perception of pain by a subject comprising administering to the subject an effective amount of the pharmaceutical composition of the conjugate comprising Substance 9, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, so as to reduce the perception of pain by the subject.This invention provides a method of selectively destroying NK-1R-expressing neuronal cells in a subject comprising administering to the subject an effective amount of the conjugate comprising Substance P, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, so as to selectively destroy NK-1R-expresssing neuronal cells.Lastly, this invention provides a method for treating a NK-1R-associated disorder in a subject, which comprises administering to the subject an amount of the pharmaceutical composition comprising substance P, or an analog thereof, and a protein such as Saporin that inhibits protein synthesis, in an effective amount to treat the disorder associated with the NK-1R.
Owner:ADVANCED MARKETING SYST
Antagonists of fish reproduction
Peptide-based neurokinin antagonists of fish reproduction are disclosed. Compositions comprising antagonists of fish neurokinin and methods of inhibiting or delaying puberty, fish maturation or reproduction processes using these compounds are also provided.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Substance p polypeptide probe specifically recognizing neurokinin-1 receptor protein and its preparation and application
InactiveCN104744568BImprove bindingHigh sensitivityTachykininsPeptide preparation methodsFluorescenceMetal chelate
The present invention provides a substance P polypeptide probe that specifically recognizes neurokinin-1 receptor protein. The substance P polypeptide probe is a polypeptide dimer or a polypeptide polymer, and its monomer consists of a substance P amino acid sequence MLGFFQQPKPR, A bifunctional chelating group, a fluorescent group and a terpyridine group are connected through lysine. The polypeptide probe is a new type of polypeptide dimer and polypeptide polymer used to prepare various tumor diagnostic functions. The polypeptide dimer and polypeptide polymer label metal Gd3+ to the polypeptide through a bifunctional chelating group Molecularly, the labeled Substance P polypeptide probe in vivo specifically binds to the NK-1 receptor protein and concentrates on the tumor site, and uses magnetic imaging and optical imaging techniques to detect the neurokinin-1 receptor protein to improve detection sensitivity sex.
Owner:EAST CHINA UNIV OF SCI & TECH
NK-1 Receptor Mediated Delivery of Agents to Cells
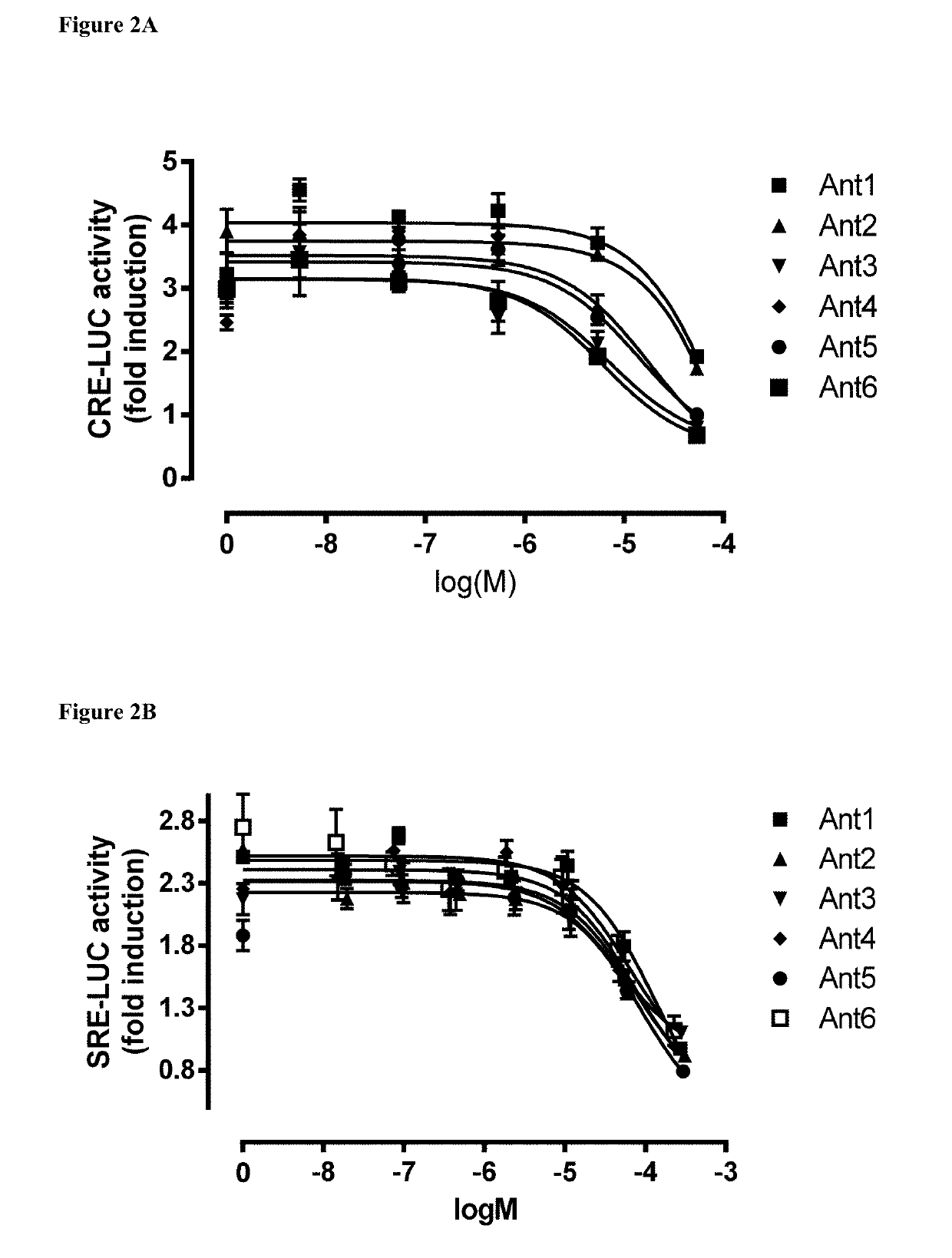
Provided herein are conjugates including a targeting vehicle coupled to an agent. The targeting vehicle includes a tachykinin receptor ligand and a reactive moiety. Conjugates including a tachykinin receptor ligand attached to an antibody or fragment thereof that is specific for an intracellular target are also provided. Also provided are methods of delivering agents to cells expressing tachykinin receptors, methods of delivering antibodies or fragments thereof to an intracellular extra-endosomal target, and methods of arresting cell growth or introducing cell death of a cancer cell.
Owner:UNIVERSITY OF CHICAGO
Antimicrobial compounds
The invention features an antimicrobial composition comprising a substance P peptide and methods of inhibiting growth of a microorganism by contacting the microorganism with a substance P peptide. Bacterial and fungal pathogens are inhibited by the substance P compositions.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS
Agonists of Tacr2
The present disclosure relates to agonists of Tacr2, e.g., peptide agonists of Tacr2, and methods of using the same for the treatment of insulin resistance, obesity, and / or diabetes. The present disclosure also relates to the use of said Tacr2 agonists for increasing the energy consumption in an individual.
Owner:UNIVERSITY OF COPENHAGEN
Antagonists of fish reproduction
Peptide-based neurokinin antagonists of fish reproduction are disclosed. Compositions comprising antagonists of fish neurokinin and methods of inhibiting or delaying puberty, fish maturation or reproduction processes using these compounds are also provided.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
NK-1 Receptor Mediated Delivery of Agents to Cells
Provided herein are conjugates including a targeting vehicle coupled to an agent. The targeting vehicle includes a tachykinin receptor ligand and a reactive moiety. Conjugates including a tachykinin receptor ligand attached to an antibody or fragment thereof that is specific for an intracellular target are also provided. Also provided are methods of delivering agents to cells expressing tachykinin receptors, methods of delivering antibodies or fragments thereof to an intracellular extra-endosomal target, and methods of arresting cell growth or introducing cell death of a cancer cell.
Owner:UNIVERSITY OF CHICAGO
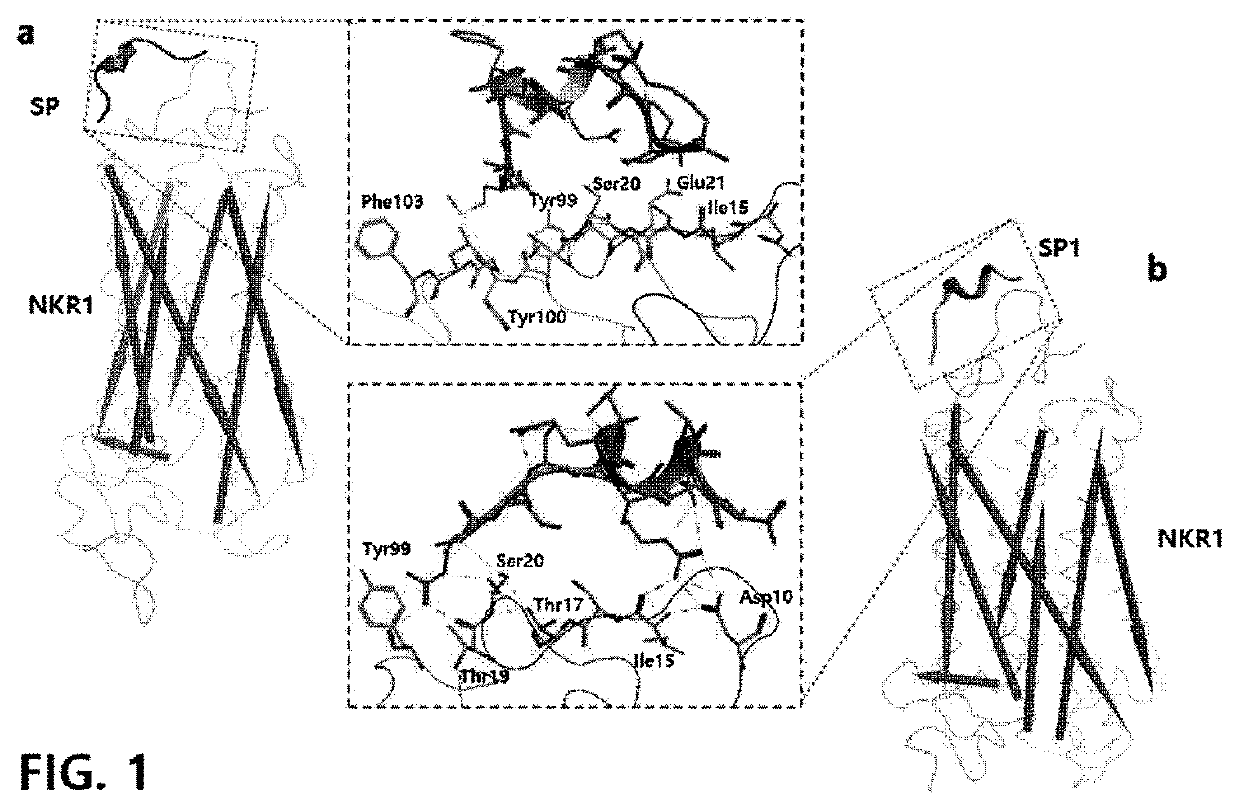
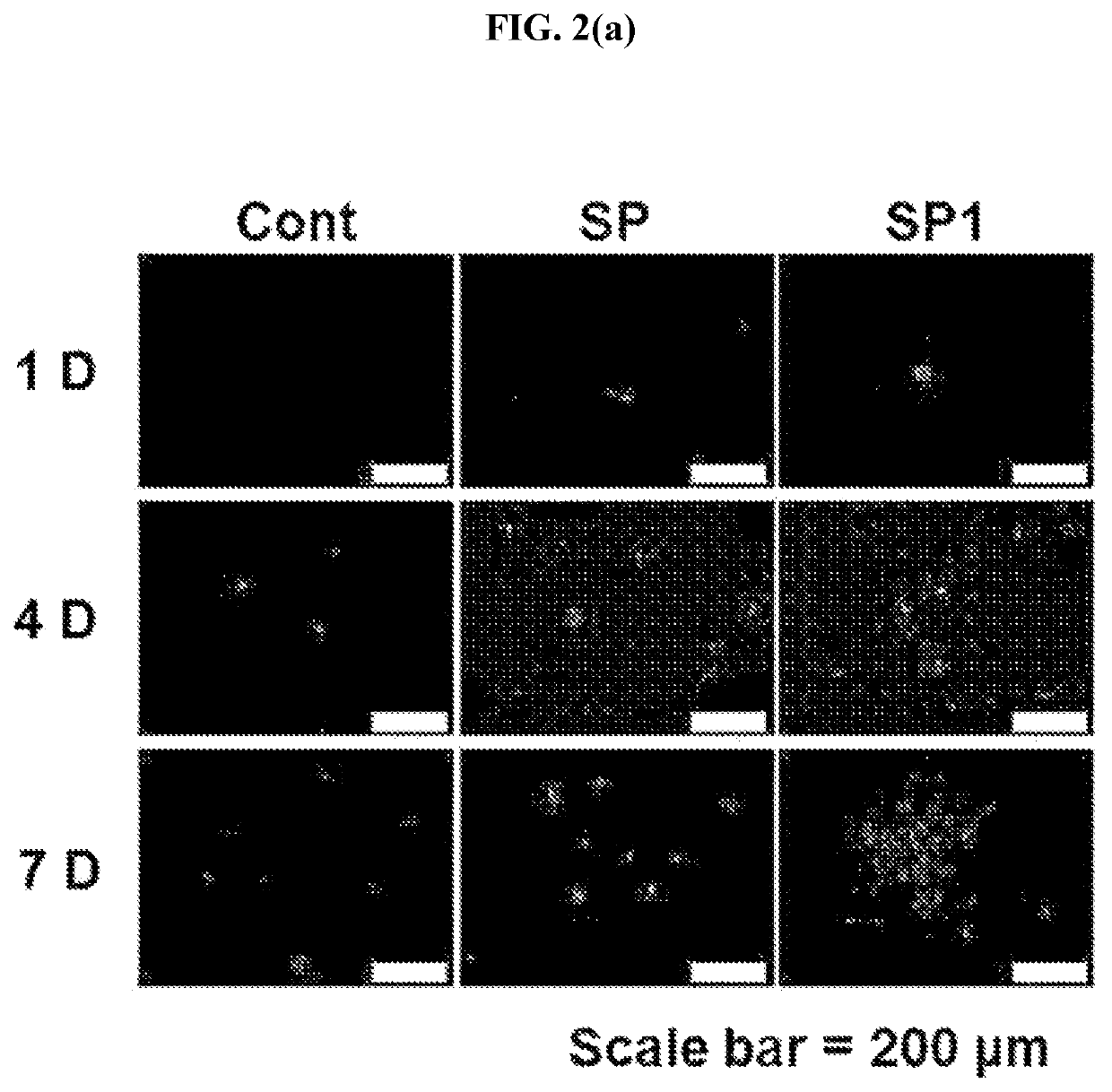
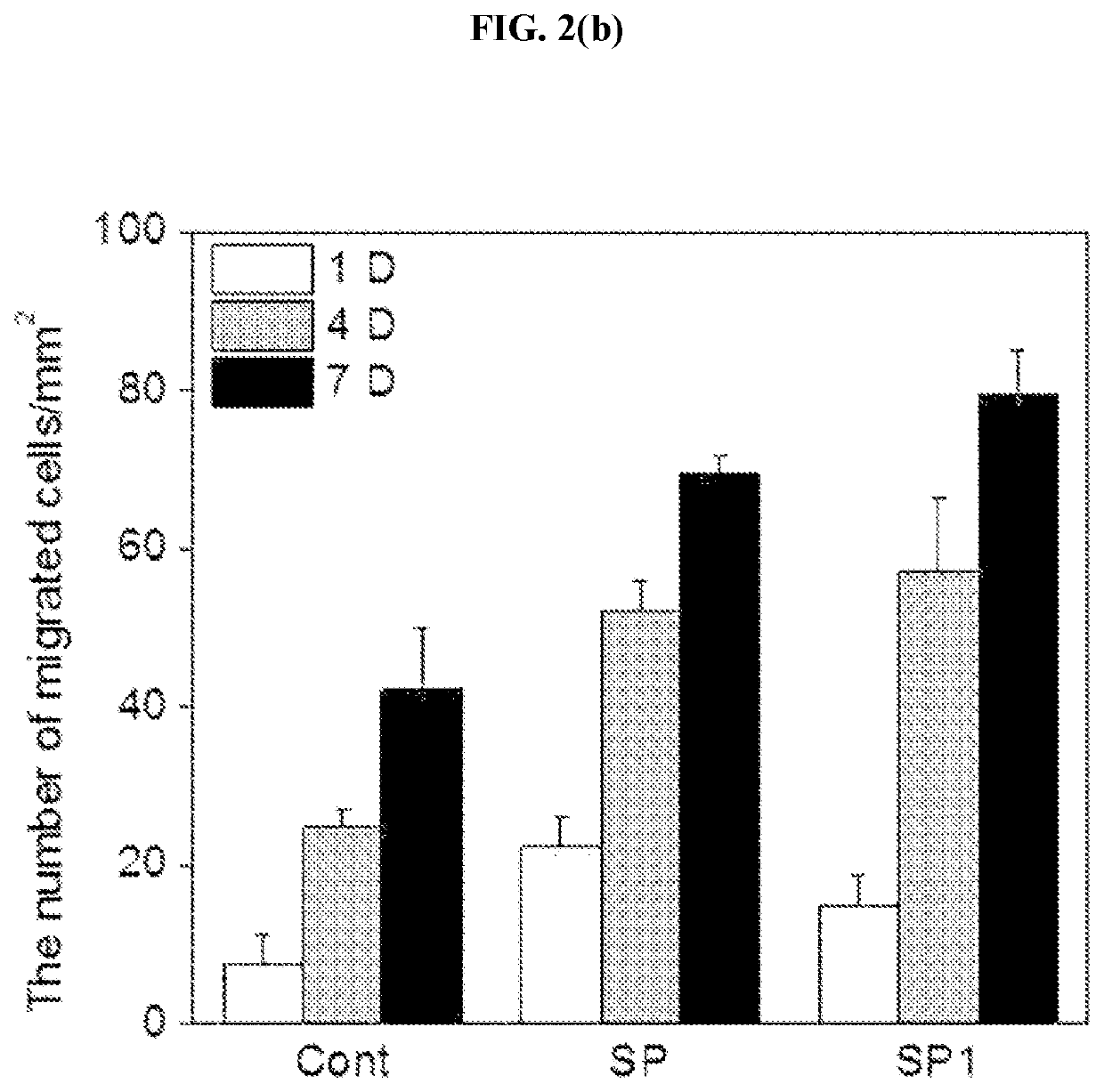
Method of treating a wound, comprising the step of administering a composition including a substance P
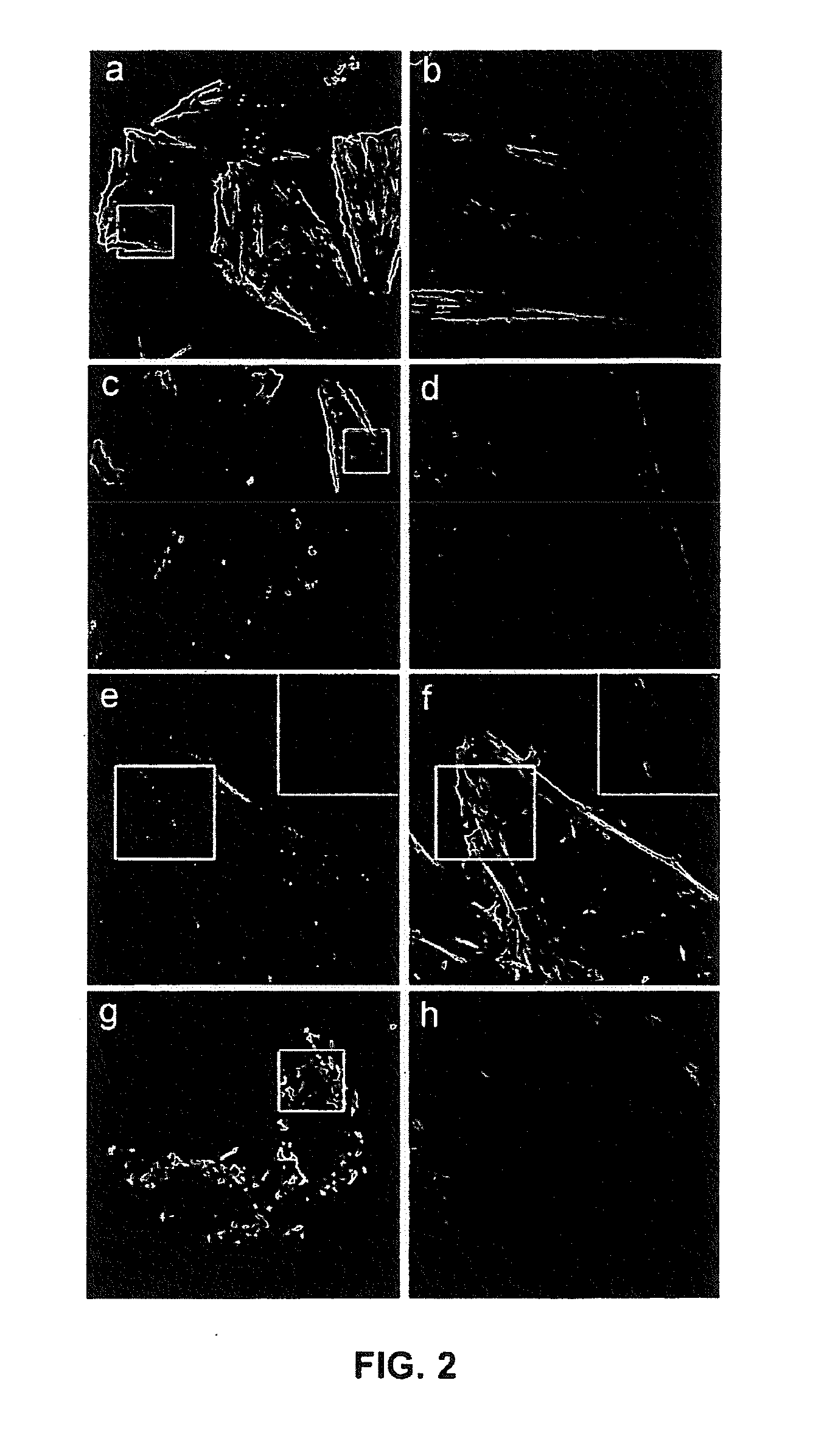
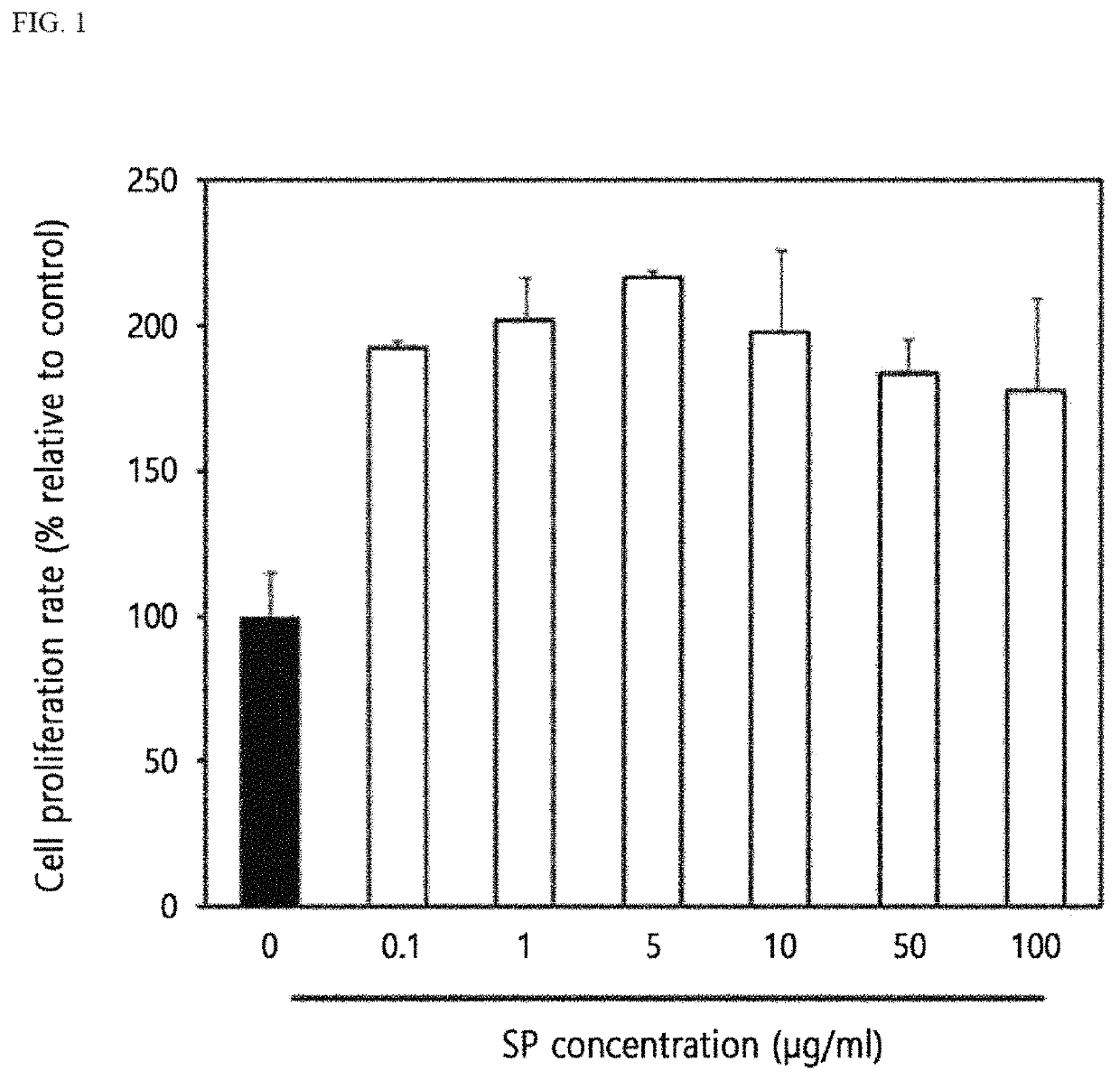
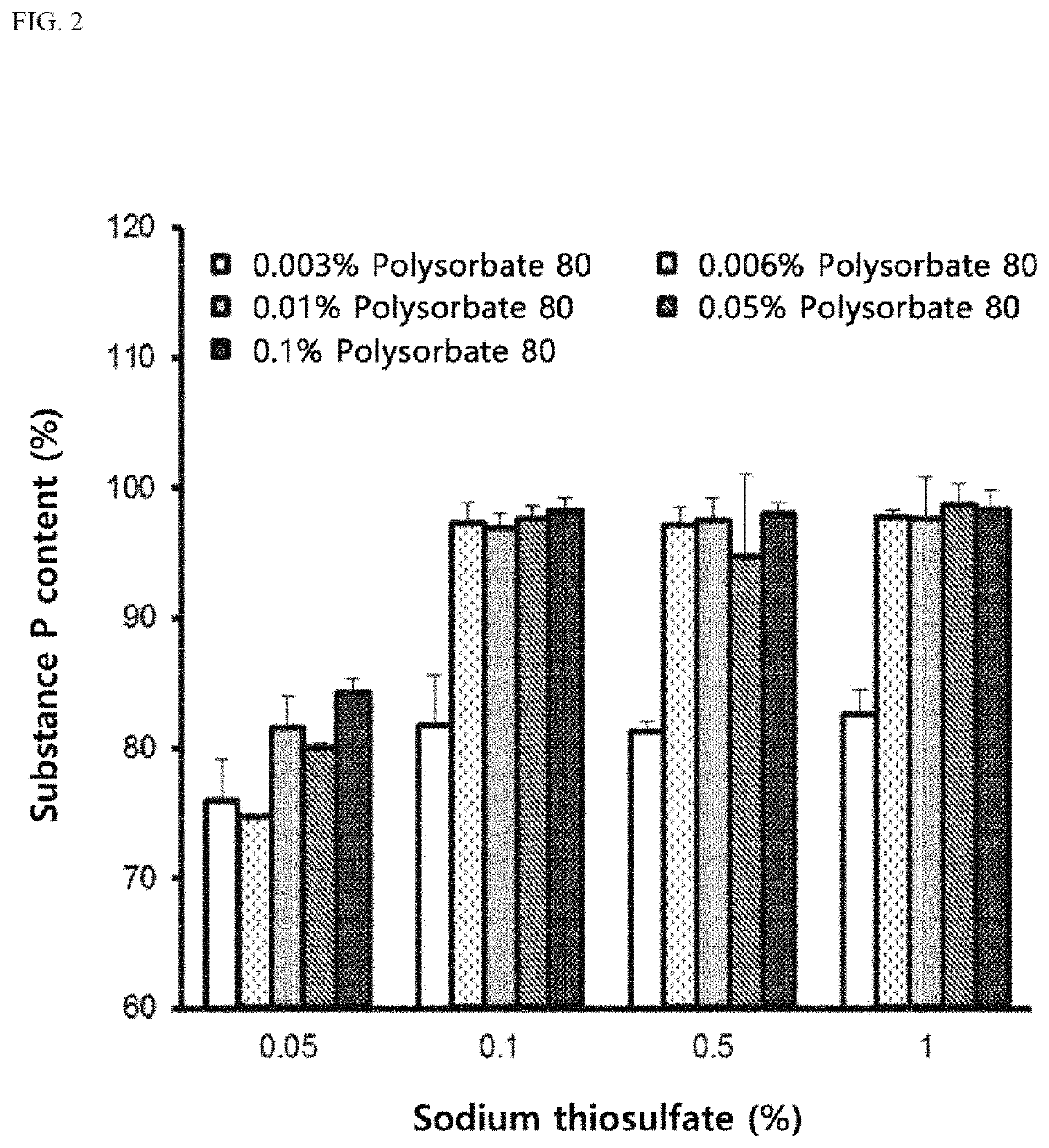
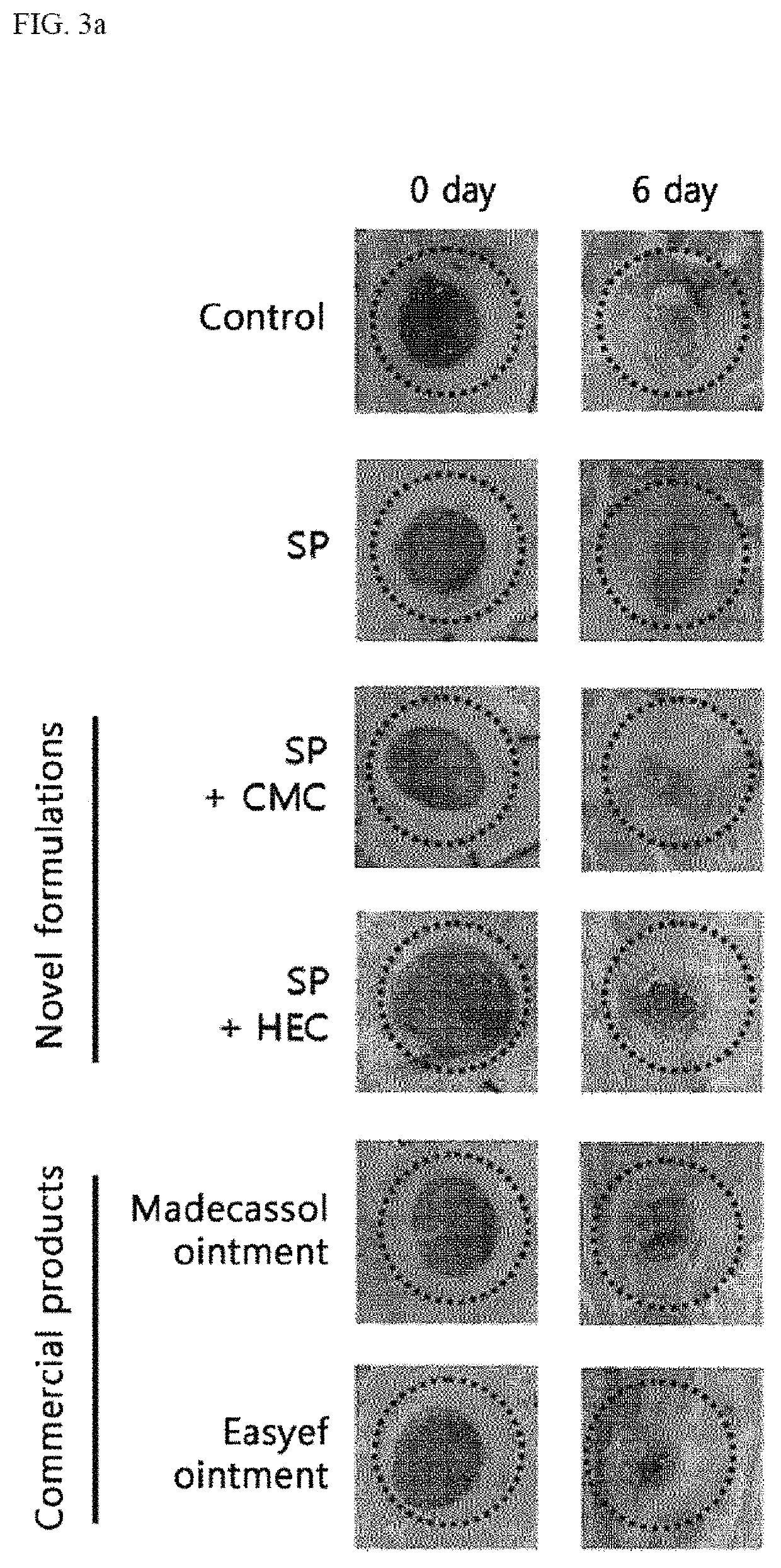
ActiveUS10596224B2Good effectPromote wound healingTachykininsTachykinin ingredientsActive agentSurface-active agents
Provided are a pharmaceutical composition for wound healing including a surfactant, an antioxidant, a thickener, and SP, a method of treating a wound including administering the pharmaceutical composition to a subject, and a quasi-drug composition for wound healing including a surfactant, an antioxidant, a thickener, and substance.The pharmaceutical composition of the present invention reduces a wound size, generates new blood vessels, shows dermal and epidermal regeneration effects, matures granulation tissues, and synthesizes collagen, and thus may be used for wound healing.
Owner:BIO SOLUTION CO LTD
Preprotachykinin Enhancer Elements
InactiveUS20080250511A1Reduce painful inflammatory symptomIncrease transcriptional activityVectorsSugar derivativesPreprotachykinin aPreprotachykinin
Provided are previously uncharacterised enhancer elements from the preprotachykinin-A (PPTA) which shows transcriptional enhancement activity in Substance P (SP) expressing cells. These are termed ECR1 and ECR2. The invention provides nucleic acids comprising these sequences and variants or fragments thereof, plus methods and materials based thereon, such as transformed host cells, transgenic animals, and screening and expression systems.
Owner:THE UNIV COURT OF THE UNIV OF ABERDEEN REGENT WALK
Non-Cleavable Substance P Conjugates and Methods of Use Thereof
Described herein are methods for treating disorders that relate to neurons that express the neurokinin-1 receptor (NK-1R) in a subject which comprises administering to the subject an effective amount of the pharmaceutical composition of the non-cleavable conjugate comprising a molecule that is recognized and internalized by the NK-1R, and a molecule that is taken inside the cell to kill or temporarily alter the cell.
Owner:VEIOVE ANIMAL HEALTH INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com