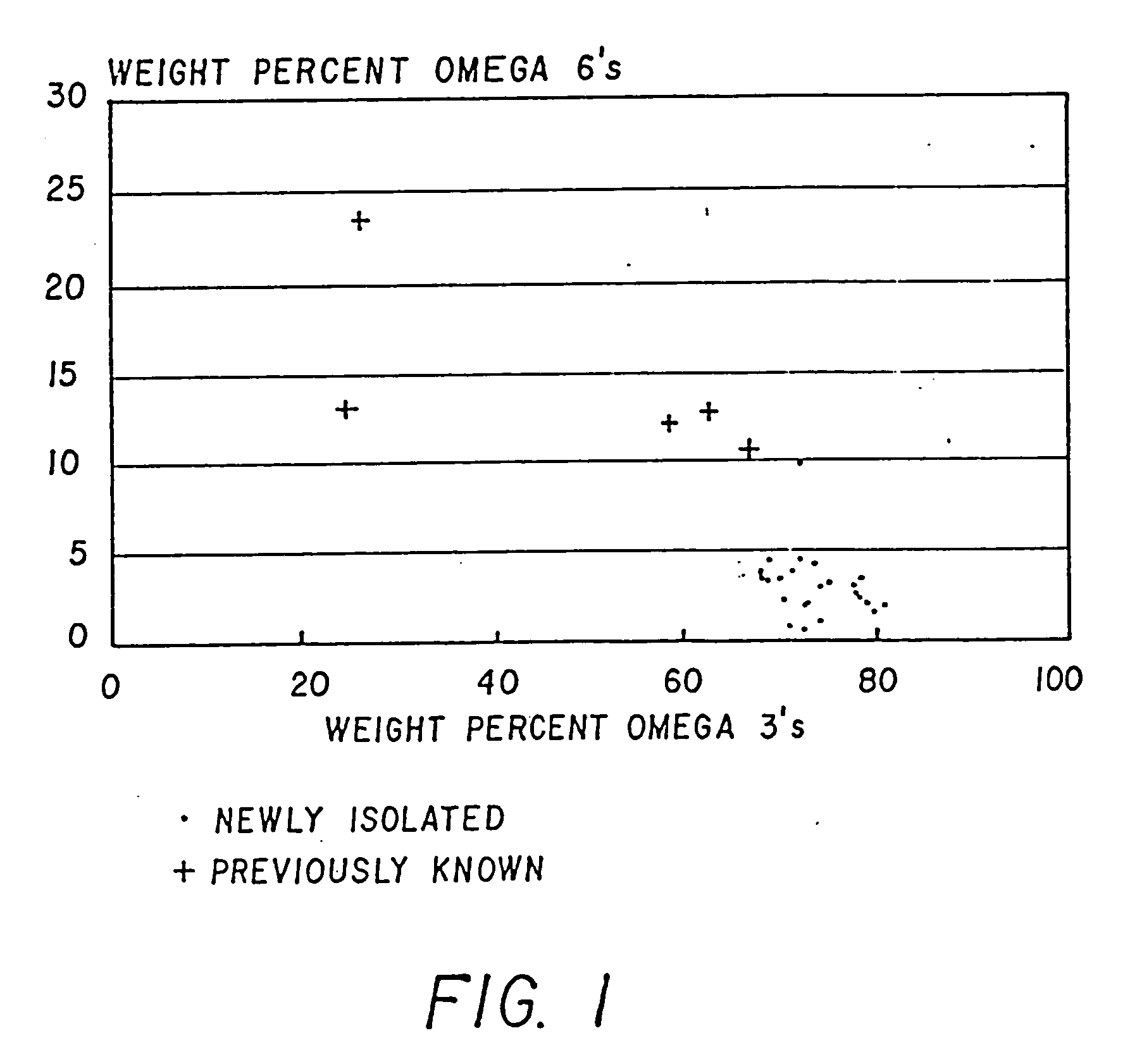
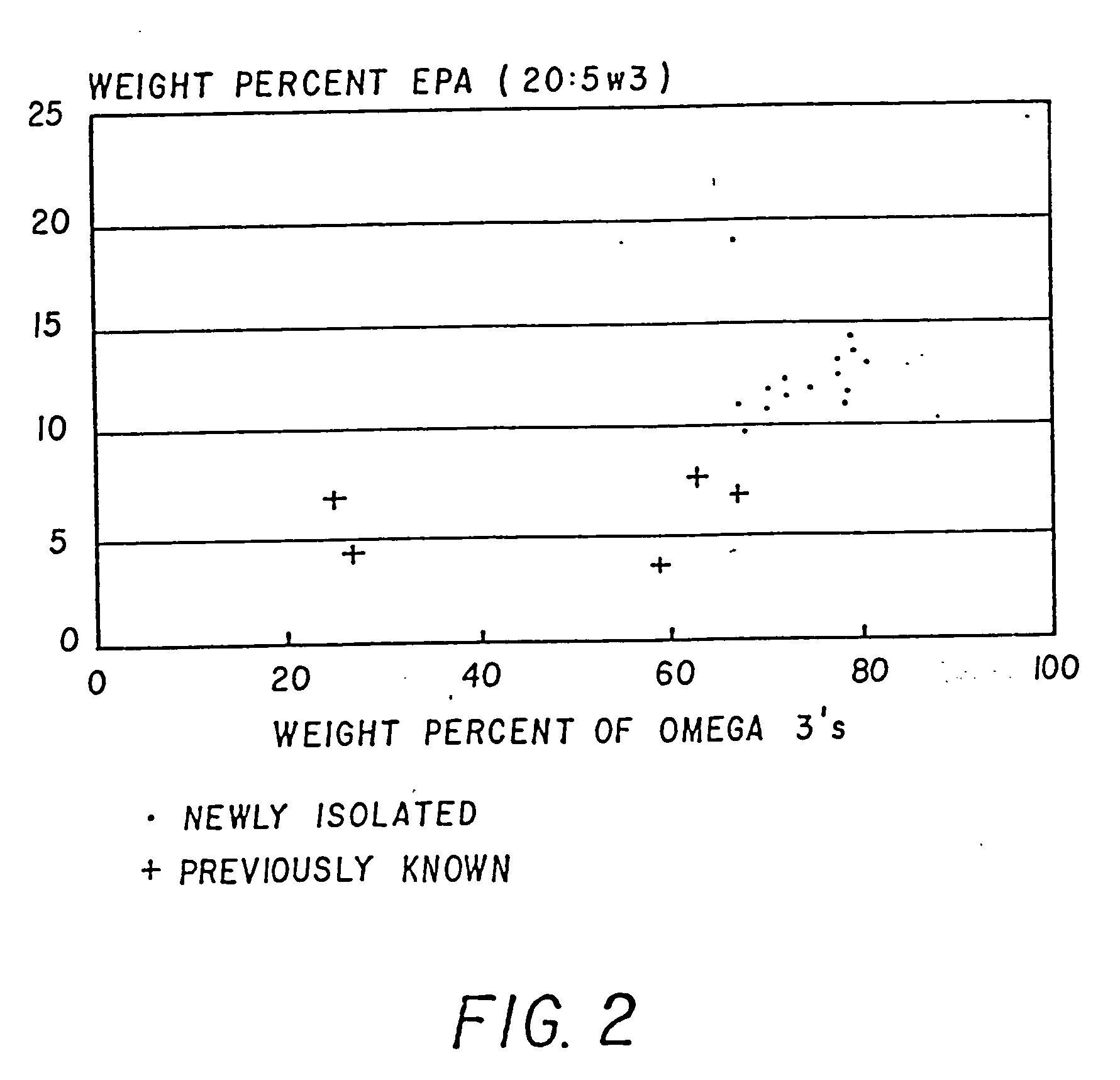
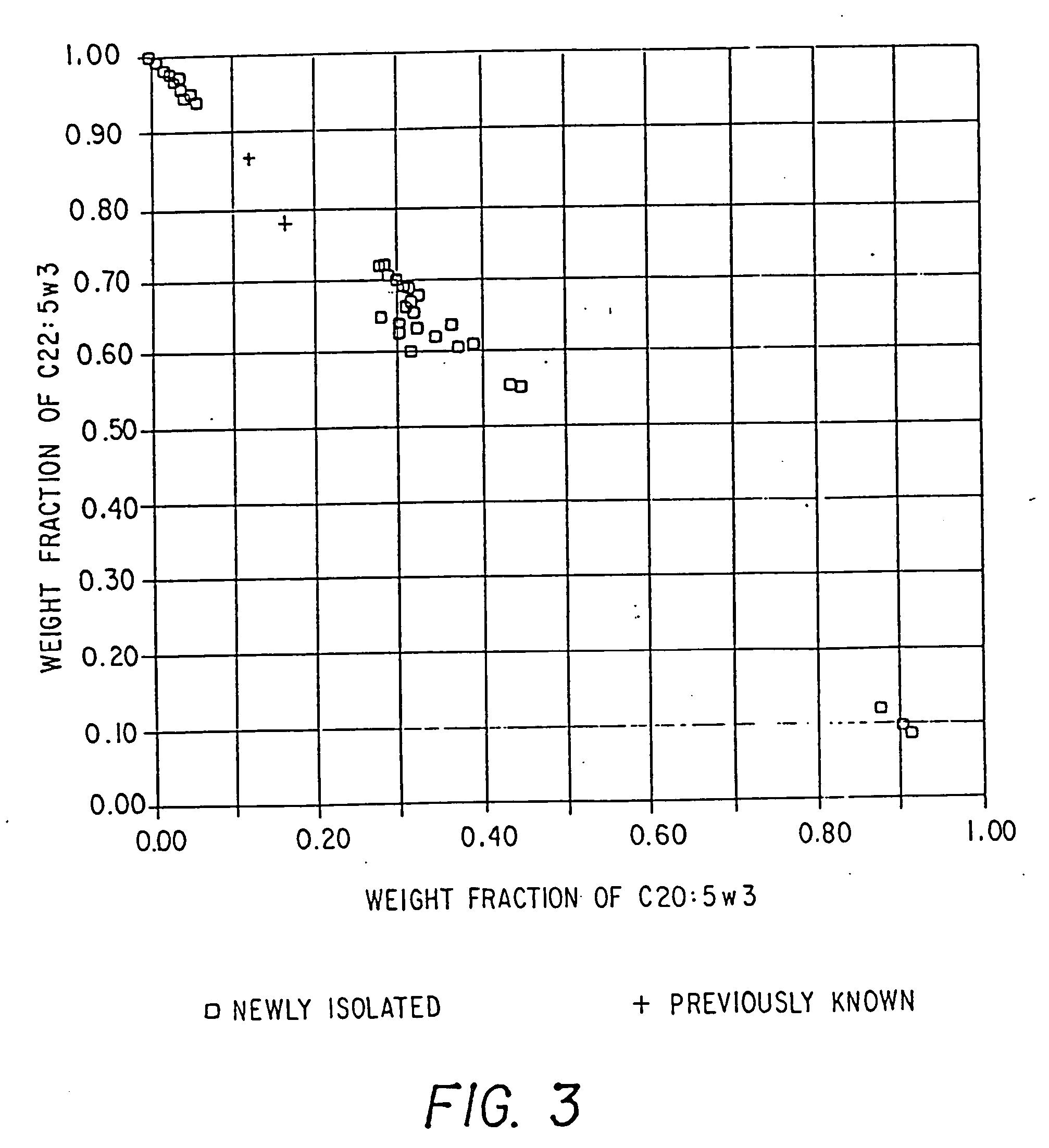
Process for the heterotrophic production of microbial products with high concentrations of omega-3 highly unsaturated fatty acids
a technology of highly unsaturated fatty acids and heterotrophic organisms, which is applied in the field of heterotrophic organisms, can solve problems such as health effects that cannot be eliminated, and achieve the effects of extending shelf life, increasing the ph of fermentation medium, and increasing the lipid content of strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Collection and Screening
[0038] A 150 ml water sample was collected from a shallow, inland saline pond and stored in a sterile polyethylene bottle. Special effort was made to include-some of the living plant material and naturally occurring detritus (decaying plant and animal matter) along with the water sample. The sample was placed on ice until return to the laboratory. In the lab, the water sample was shaken for 15-30 seconds, and 1-10 ml of the sample was pipetted or poured into a filter unit containing 2 types of filters: 1) on top, a sterile 47 mm diameter Whatman #4 filter having a pore size about 25 μm; and 2) underneath the Whatman filter, a 47 mm diameter polycarbonate filter with about 1.0 μm pore size. Given slight variations of nominal pore sizes for the filters, the cells collected on the polycarbonate filter range in size from about 1.0 μm to about 25 μm.
[0039] The Whatman filter was removed and discarded. The polycarbonate filter was placed on solid F-1 media in a p...
example 2
Maintaining Unrestricted Growth: PO4 and Yeast Extract
[0041] Cells of Schizochytrium aggregatum (ATCC 28209) were picked from solid F-1 medium and inoculated into 50 ml of FFM medium. (Fuller et al., 1964). This medium contains: seawater, 1000 ml; glucose, 1.0 g; gelatin hydrolysate, 1.0 g; liver extract, 0.01 g; yeast extract, 0.1 g; PII metals, 5 ml; 1 ml B-vitamins solution (Goldstein et al., 1969); and 1 ml of an antibiotic solution (25 g / l streptomycin sulfate and penicillin-G). 11.0 ml of the vitamin mix (pH 7.2) contains: thiamine HCl, 200 μg; biotin, 0.5 μg; cyanocobalamin, 0.05 μg; nicotinic acid, 100 μg; calcium pantothenate, 100 μg; riboflavin, 5.0 μg; pyridoxine HCl, 40.0 μg; pyridoxamine 2HCl, 20.0 μg; p-aminobenzoic acid, 10 μg; chlorine HCl, 500 kg; inositol, 1.0 mg; thymine, 0.8 mg; orotic acid, 0.26 mg; folinic acid, 0.2 μg; and folic acid, 2.5 μg. The culture was placed on a rotary shaker (200 rpm) at 27° C. After 3-4 days, 1 ml of this culture was transferred to ...
example 3
Maintaining Unrestricted Growth: Substitution of Corn Steep Liquor for Yeast Extract
[0042] Cells of Schizochytrium sp. S31 (ATCC No. 20888) were picked from solid F-1 medium and placed into 50 ml of M-5 medium. This medium consists of (on a per liter basis): yeast extract, 1 g; NaCl, 25 g; MgSO40.7H2O, 5 g; KCl, 1 g; CaCl., 200 mg; glucose, 5 g; glutamate, 5 g; KHZPO4, 1 g; PII metals, 5 ml; A-vitamins solution, 1 ml; and antibiotic solution, 1 ml. The pH of the solution was adjusted to 7.0 and the solution was filter sterilized. Sterile solutions of corn steep liquor (4 g / 40 ml; pH 7.0) and yeast extract (1 g / 40 ml; pH 7.0) were prepared. To one set of M-5 medium flasks, the following amount of yeast extract solution was added: 1) 2 ml; 2) 1.5 ml; 3) 1 ml; 4) 0.5 ml; and 5) 0.25 ml. To another set of M-5 medium flasks the yeast extract and corn steep liquor solutions were added at the following levels: 1) 2 ml yeast extract; 2) 1.5 ml yeast extract and 0.5 ml corn steep liquor; 3)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com