Method for preparing glycolate from carbohydrates
A technology for carbohydrates and glycolic acid esters, which is applied in the field of one-step catalytic production of glycolic acid esters from carbohydrates in an alcohol solution through a vanadium-containing or molybdenum-containing catalyst, and can solve problems such as unfavorable large-scale industrial production, unfavorable industrialized production, and inconvenient operation. , to achieve good catalytic cycle performance, abundant and renewable resources, and good industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
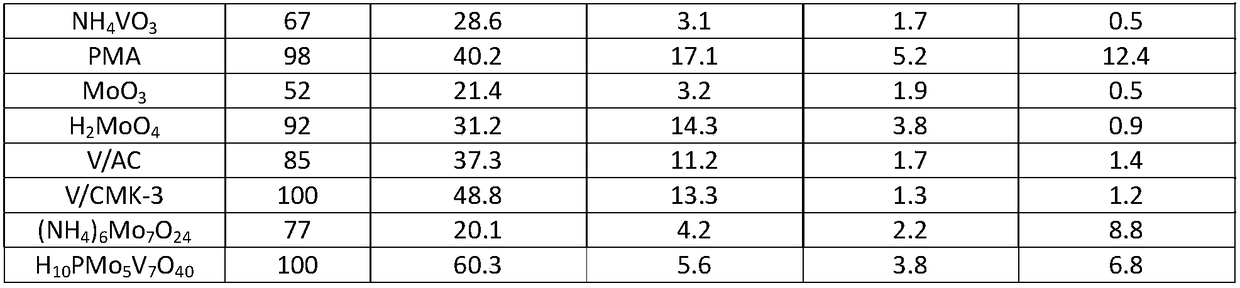
[0030] h 10 PMo 5 V 7 o 40 Catalyst preparation
[0031] First put the 3.18g V 2 o 5 Add dropwise 30 mL of cold 10% H 2 o 2 solution to form a deep red vanadium peroxide (V) compound; gradually heated to room temperature, the solution decomposes and releases oxygen to form 0.0125mol / L orange H 6 V 10 o 28 Solution, then add excess concentrated phosphoric acid to solidify, forming dark brown H9 PV 14 o 42 solution;
[0032] The freshly prepared H 9 PV 14 o 42 The solution was gradually added dropwise to the boiling water containing 3.60g MoO 3 in the phosphoric acid suspension, stirred for 30min, MoO 3 Slowly dissolve into the mixed solution to form a solution, and obtain the heteropolyacid H 10 PMo 5 V 7 o 40 .
[0033] The obtained solid was filtered, washed, dried at about 110°C, and roasted at 300°C for 2 hours before use.
Embodiment 2
[0035] Preparation of V / AC: Weigh 50g of activated carbon (AC), 250mL of 33wt% nitric acid, place in a 500mL three-neck flask, treat in a water bath at 80°C for 24h, wash until neutral, and dry at 120°C for 24h. Pour 1g of pretreated AC into 0.23g of ammonium metavanadate (NH 4 VO 3 ) in an aqueous solution of 120°C after being dried in an oven, the catalyst precursor was subjected to temperature-programmed reduction in hydrogen. The heating rate of min was raised to 900 ° C and maintained for 1 h, and the hydrogen flow rate was 120 mL / min. The theoretical loading of V in the prepared catalyst was 10wt%.
Embodiment 3
[0037] Preparation of V / CMK-3: The preparation process was similar to Example 2, except that the AC carrier was replaced by CMK-3. The preparation process of CMK-3 is as follows: dissolve 1.25g sucrose in 5g deionized water, add 0.14g concentrated sulfuric acid, add 1g SBA-15 to the obtained mixed solution, soak at room temperature for 6h, and then dry at 100°C and 160°C for 6h respectively . The obtained beige powder was dipped again in a solution consisting of 0.8 g of sucrose, 0.09 g of concentrated sulfuric acid and 5 g of deionized water, and the drying step was repeated. Transfer to a tube furnace at a constant temperature of 900°C for 6 hours under a nitrogen atmosphere to completely carbonize the sucrose. Add 50ml of 4wt% hydrofluoric acid to the obtained black powder, stir at room temperature for 2h, filter / wash, repeat this process three times to completely remove silica, and finally dry overnight in an oven at 120°C to obtain ordered mesoporous carbon CMK -3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com