Polyurethane elastomer with main chain containing ferrocene, and preparation method thereof
A polyurethane elastomer containing ferrocene technology, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems that thermal stability has not been significantly improved, and achieve outstanding mechanical properties, excellent processing performance, and excellent Effects of Thermal Stability and Heat Resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
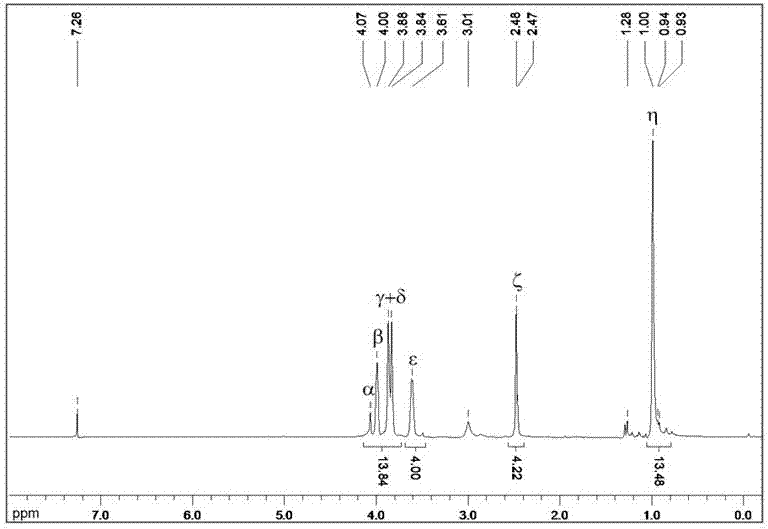
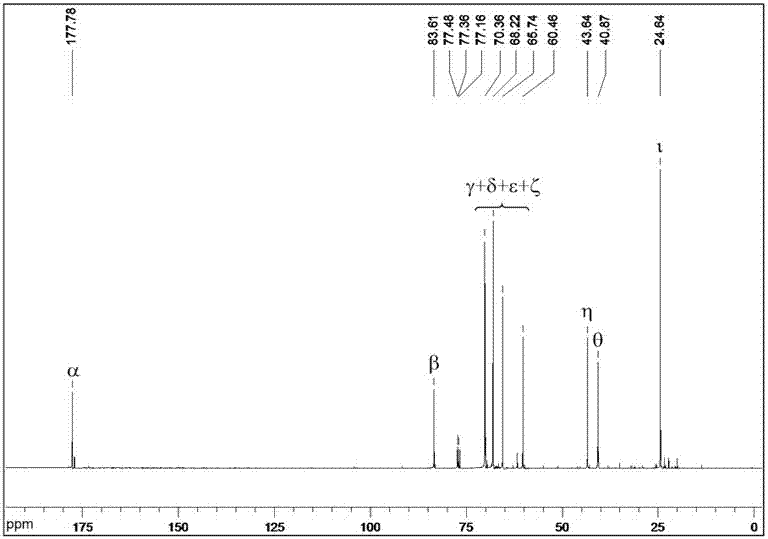
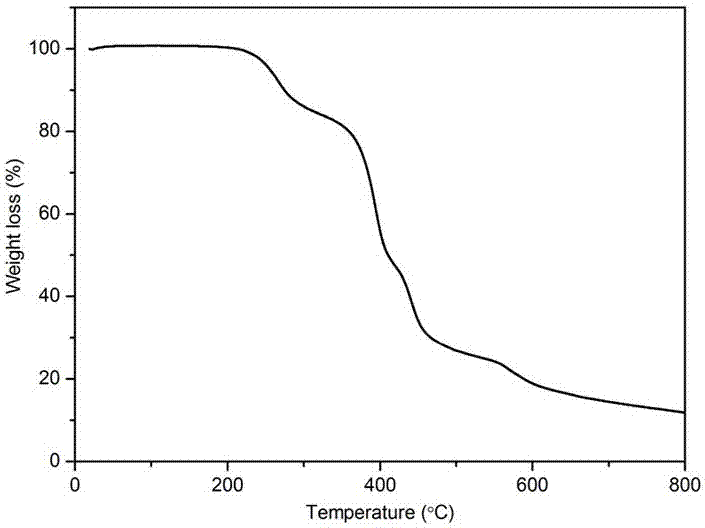
Image
Examples
Embodiment 1
[0048] 1. Chain extender A: Preparation of 1,1'-bis[2-(β-hydroxyethyl) formate-2-methyl-propyl)]ferrocene
[0049] Etherification reaction: In a 100mL dry single-necked round bottom flask, add 2.145g 1,1'-bis(hydroxymethyl)ferrocene, and then add 60.0mL mixed solvent (methanol and glacial acetic acid volume ratio 4:1) , stir to dissolve, heat up to 75°C and stir and reflux for 12h, remove the volatile solvent under reduced pressure, re-dissolve with dichloromethane, wash with saturated NaHCO 3 Wash with deionized water and dry in vacuo to obtain 2.355g of 1,1'-bis(1-methoxy-methyl)ferrocene, whose chemical structural formula is:
[0050] ;
[0051] Catalytic addition reaction: In a 250 mL dry Schlenk bottle, add 2.355 g 1,1'-bis(1-methoxy-methyl)ferrocene and 10.6 mL 1-methoxy-1-(trimethyl Siloxane)-2-methyl-1-propene, then add 120.0mL anhydrous dichloromethane and stir to dissolve; under the protection of dry nitrogen, cool the reaction system to -72 ~ -78°C, add dropwise...
Embodiment 2
[0061] 1. Chain extender B: Preparation of 1,1'-bis[2-(β-hydroxyethyl)formamide-2-methyl-propyl)]ferrocene
[0062] The specific preparation method refers to Example 1, and the raw material "ethylene glycol" in Example 1 is replaced with "ethanolamine", and the chemical structure of the chain extender B obtained is:
[0063] .
[0064] 2. Preparation of polyurethane elastomers containing ferrocene in the main chain
[0065] In a 250 mL three-necked round bottom flask, add 10.042g polytetrahydrofuran ether glycol (molecular weight: 2000), heat to 120°C, remove water under vacuum for 4 to 5 hours, then cool to room temperature, blow nitrogen, add 2.970g 4, For 4'-diphenylmethane diisocyanate, slowly heat up to 50°C and stir until uniform; then continue to heat up to 60°C, stir and react for 3 hours; cool to room temperature, add 2.418g of chain extender B, stir until dissolved, Slowly raise the temperature to 90°C and react for 2 hours. After the viscosity no longer increase...
Embodiment 3
[0068] 1. Chain extender C: Preparation of 6,6'-bis[1-methyl-2-(β-hydroxyethyl) formate-2-methyl-propyl)] dinuclear ferrocene propane
[0069] Acetylation reaction: Under the protection of an inert gas, dissolve 6.594g of dinuclear ferrocenepropane and 40mL of anhydrous dichloromethane in a 250mL two-necked round-bottomed flask, heat to 38-40°C, and reflux slightly; Add dropwise the mixed solution of 4.5mL acetic anhydride, 6.7mL boron trifluoride ethyl ether and 20mL anhydrous dichloromethane, and dropwise add in 5-6h; continue to heat and reflux for 30min, cool naturally to room temperature, and directly use 15% potassium acetate aqueous solution Perform hydrolysis, extract the organic phase, and wash with saturated NaHCO 3 Repeated washing with deionized water, vacuum drying to obtain 7.495g of dark red viscous liquid 2,2-(6,6'-diacetyl)binuclear ferrocenepropane, its structural formula is:
[0070] .
[0071] Reduction reaction: Under the protection of inert gas, mix 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com