Patents
Literature
55 results about "Plasma glucose level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
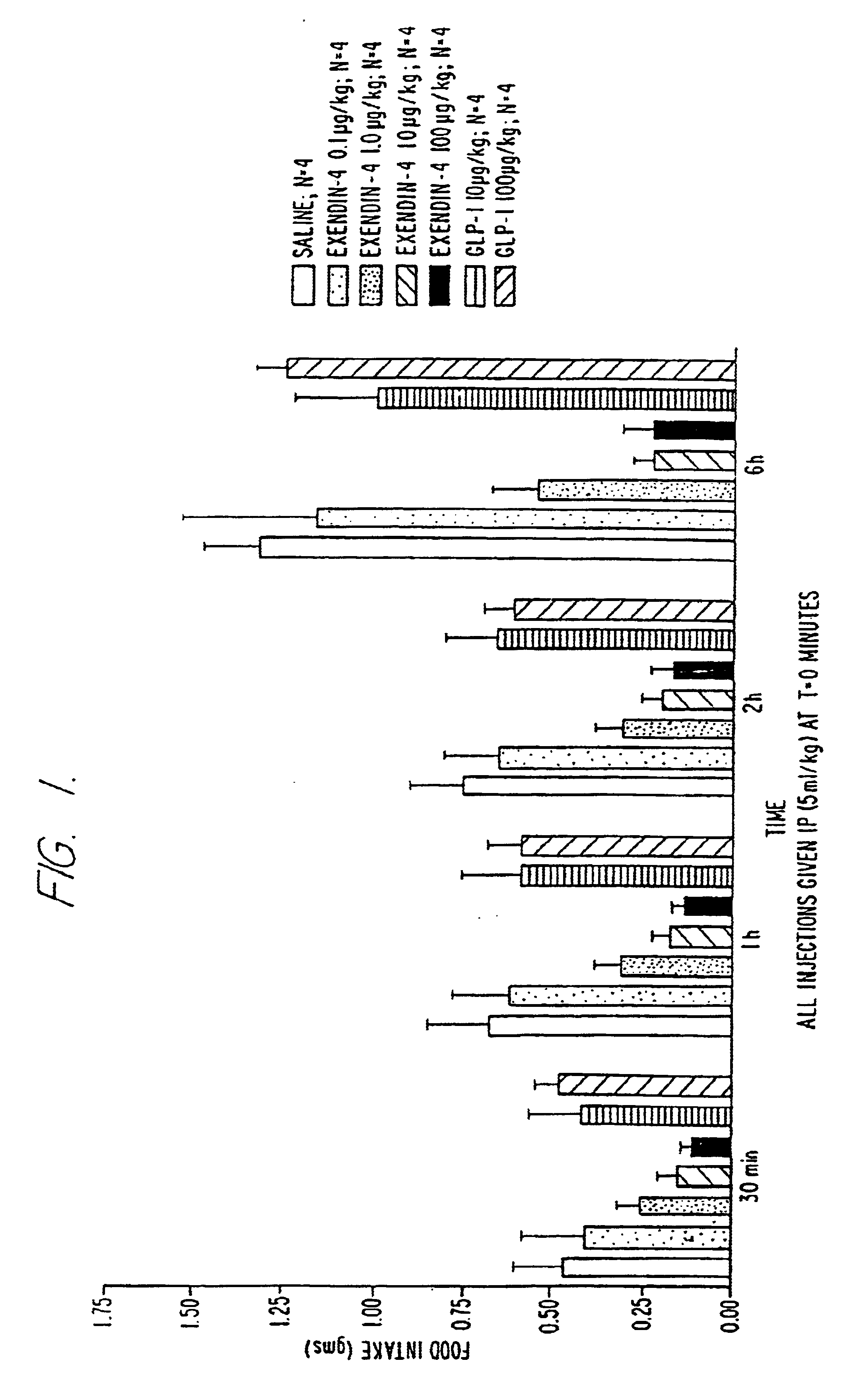
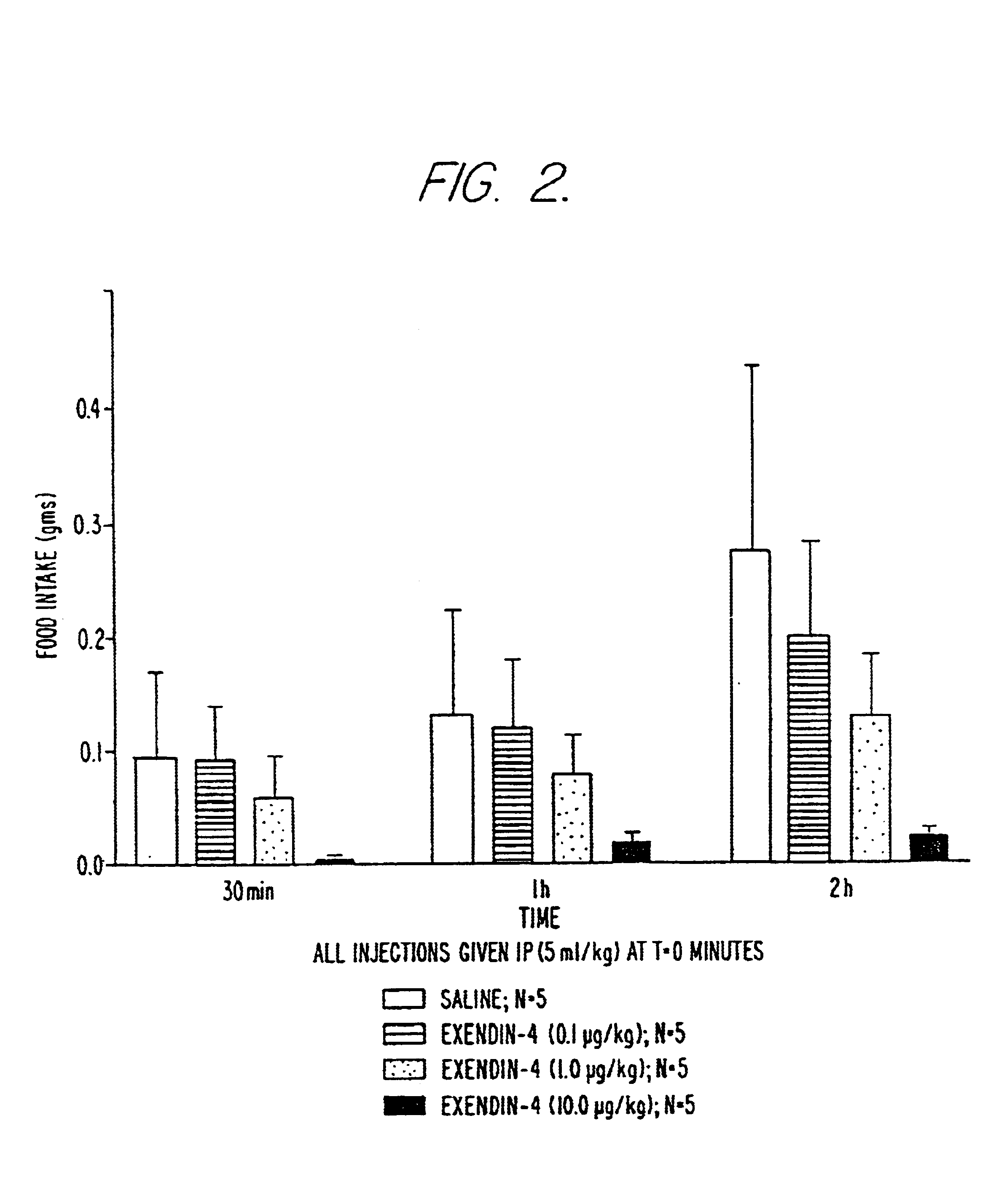
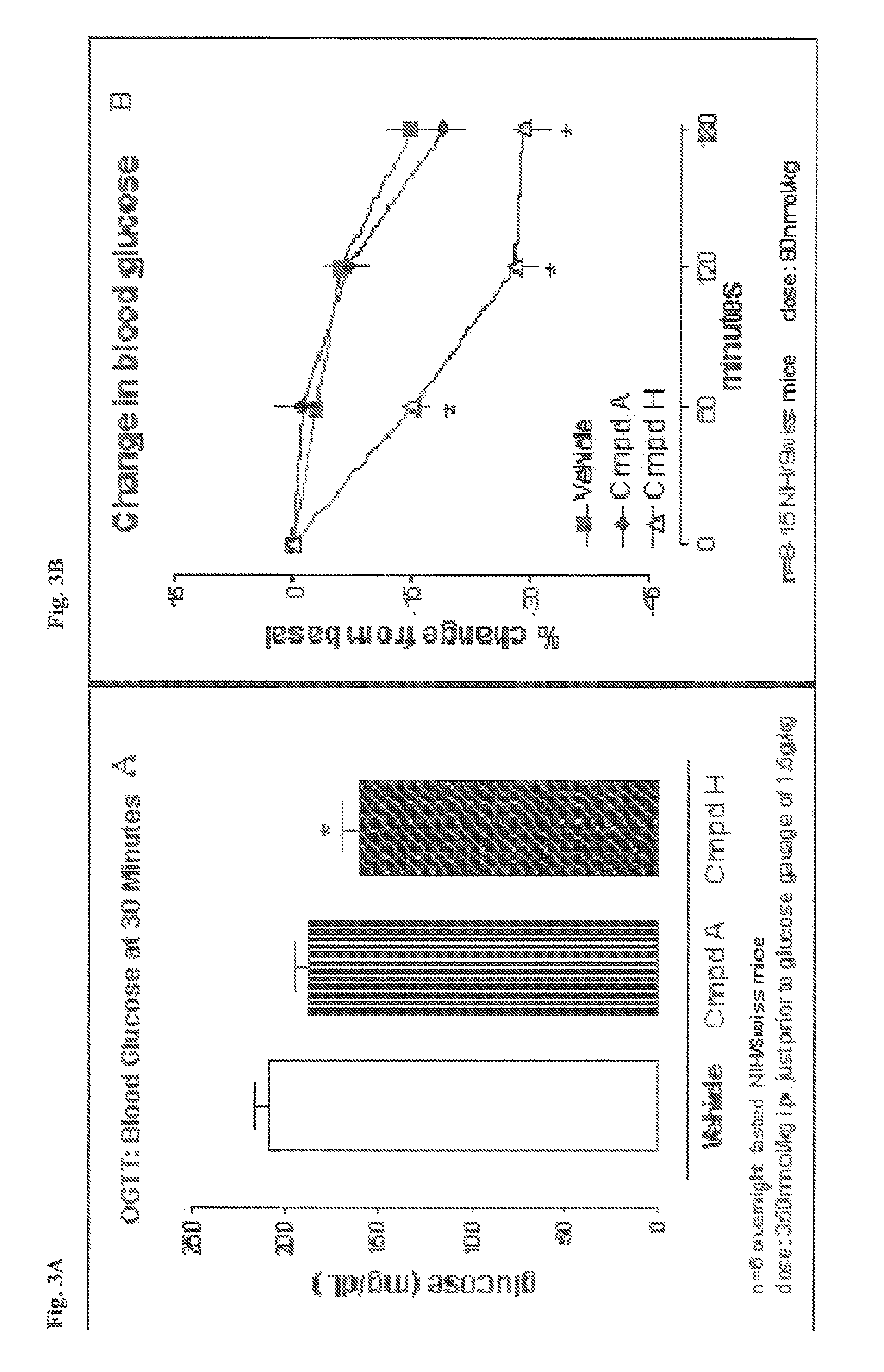
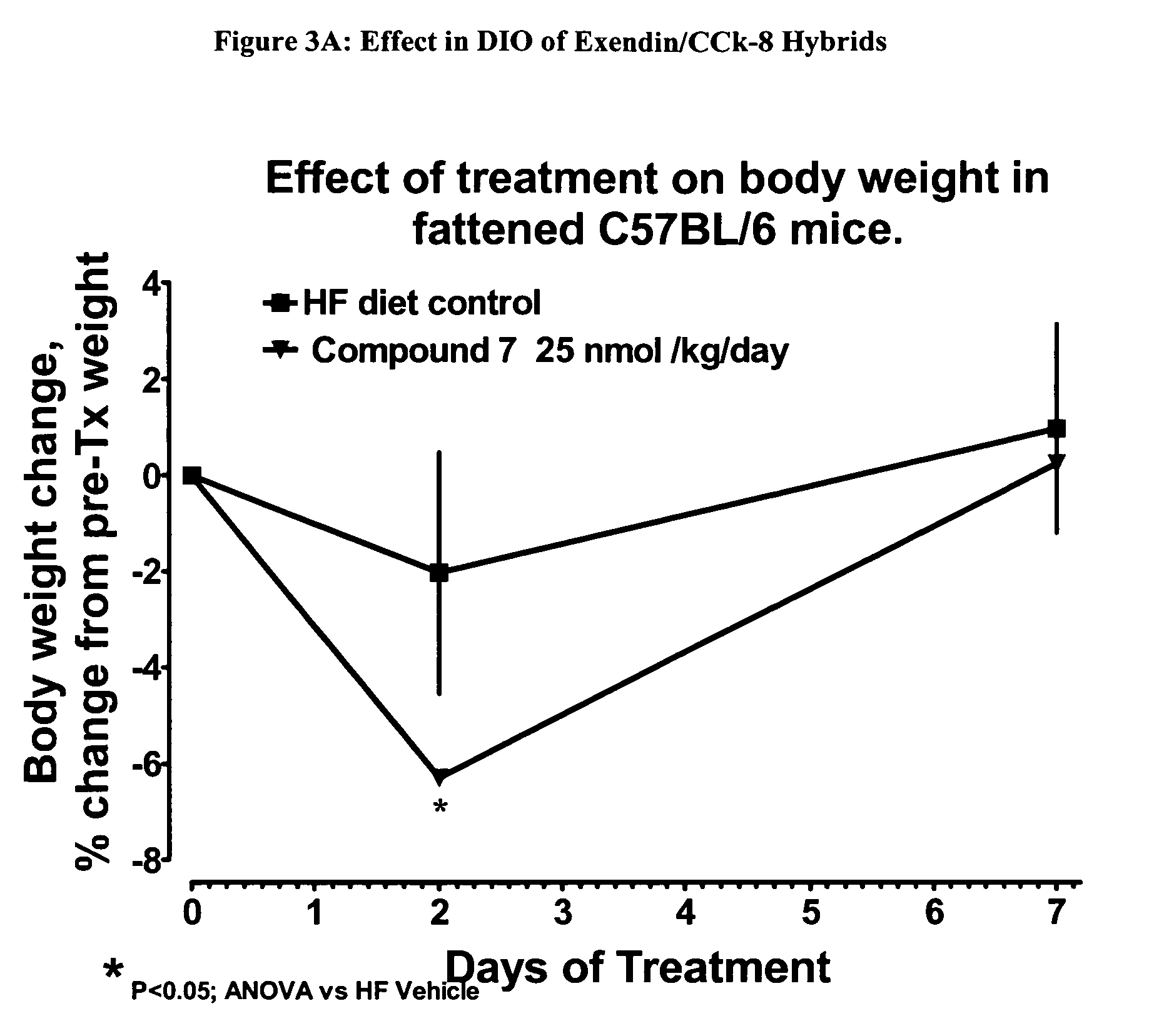
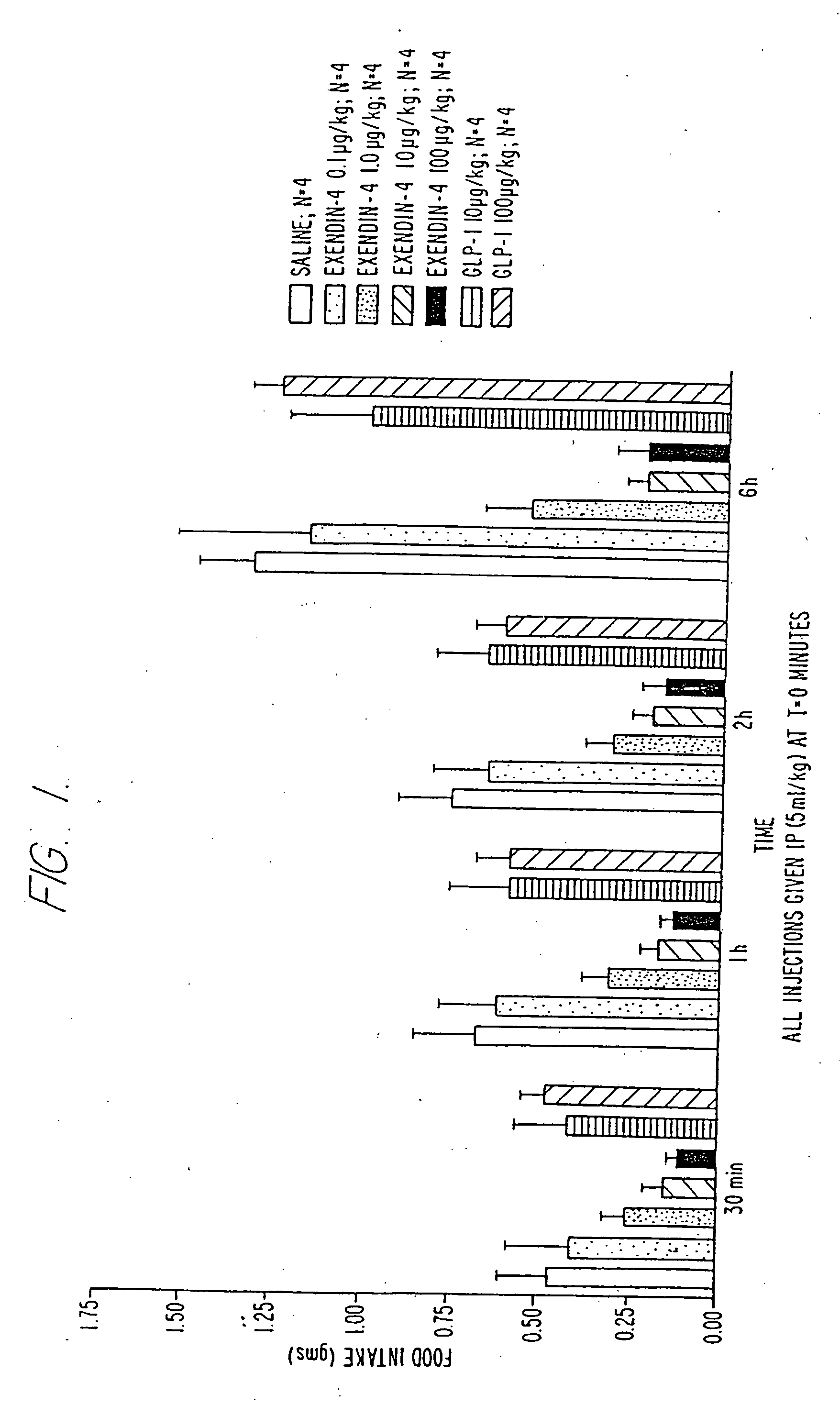
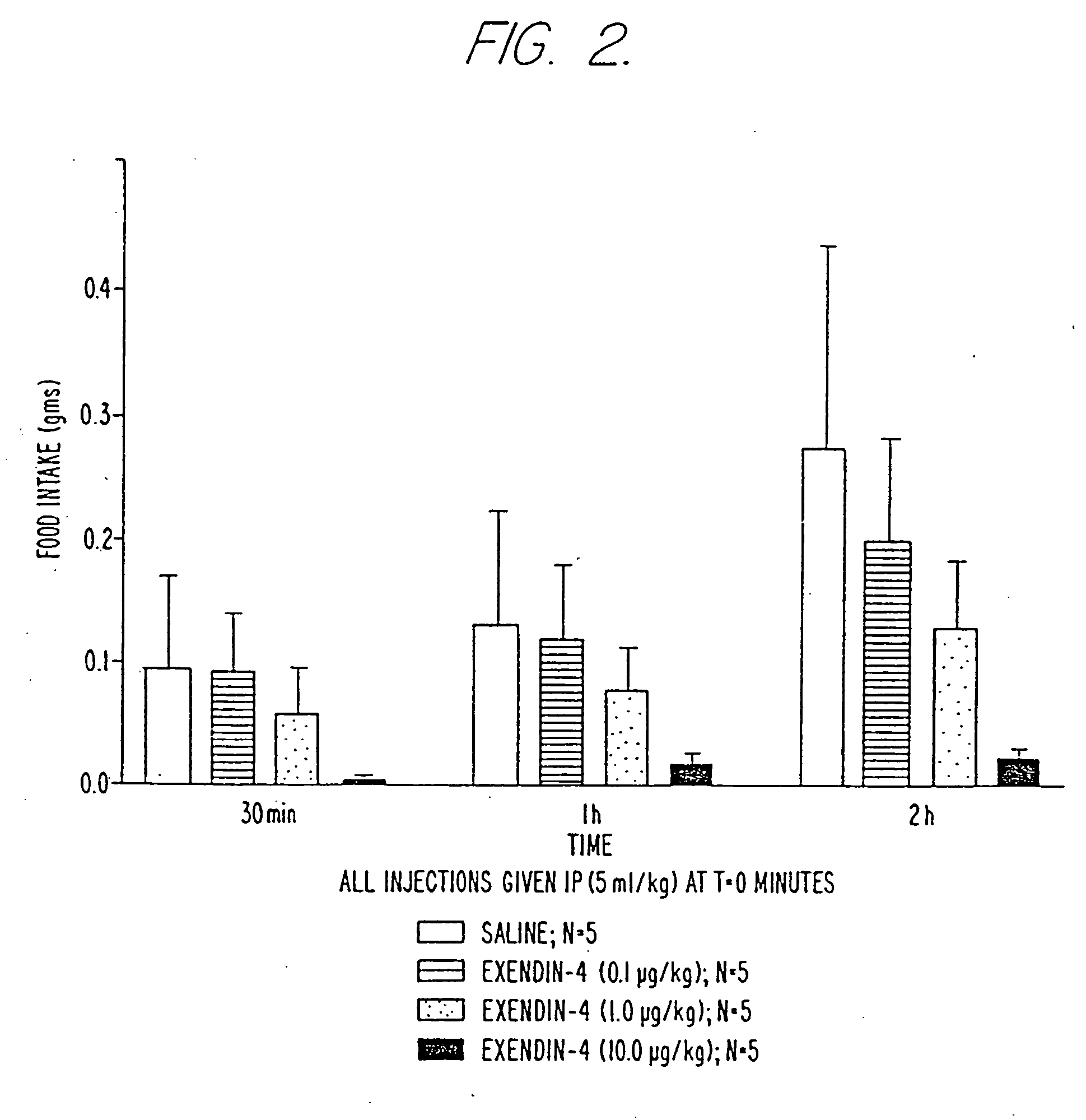
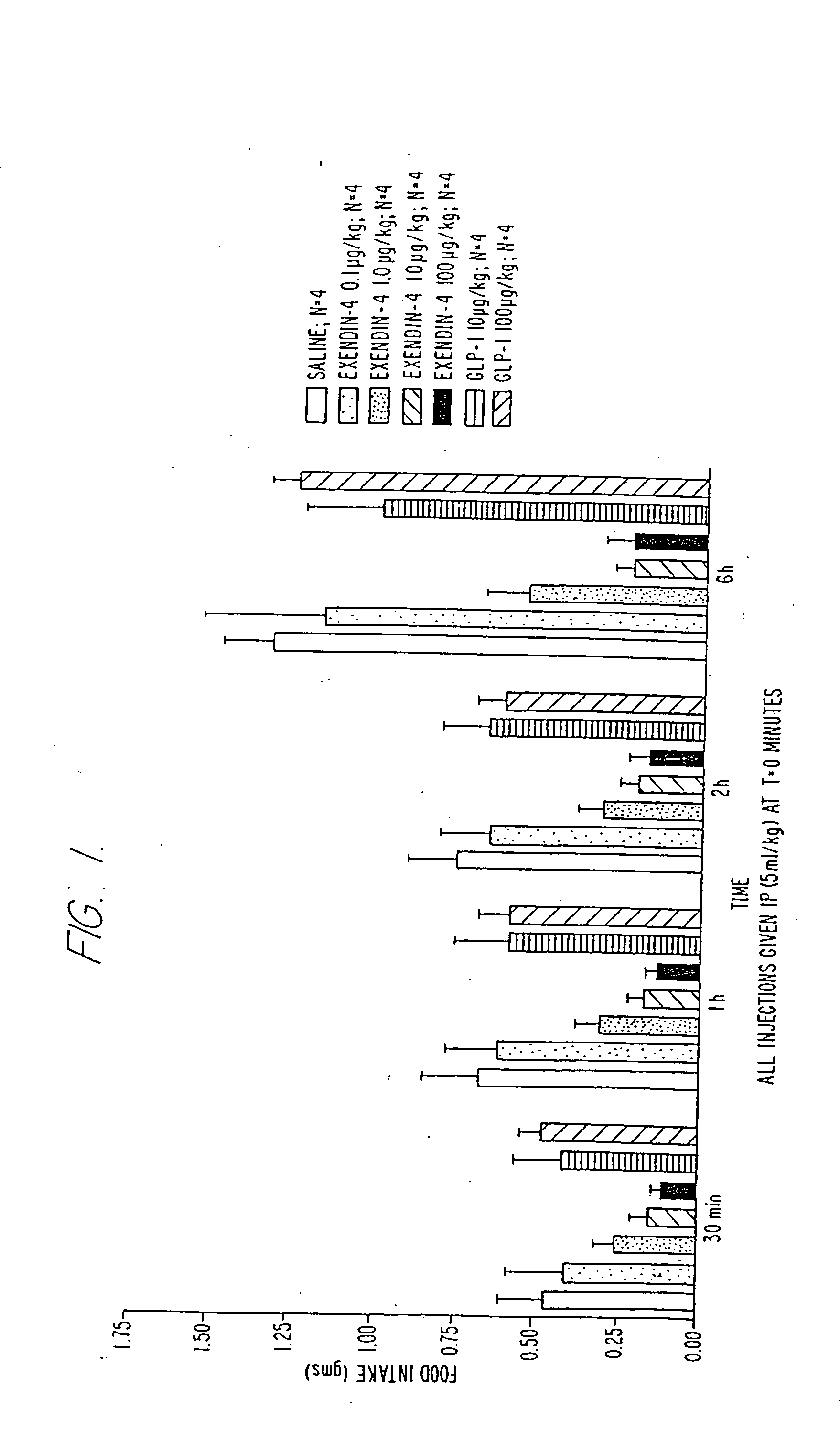
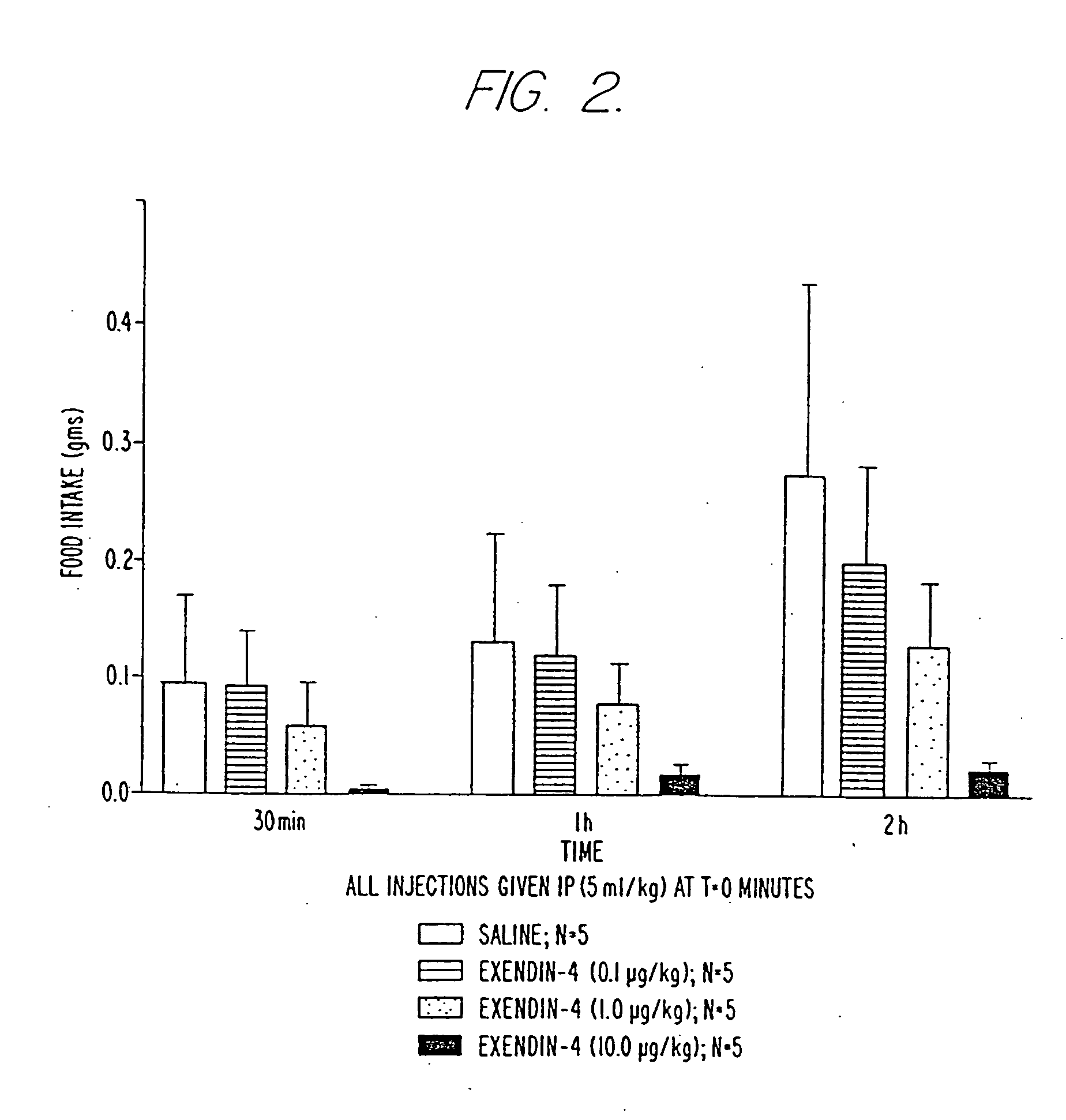
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
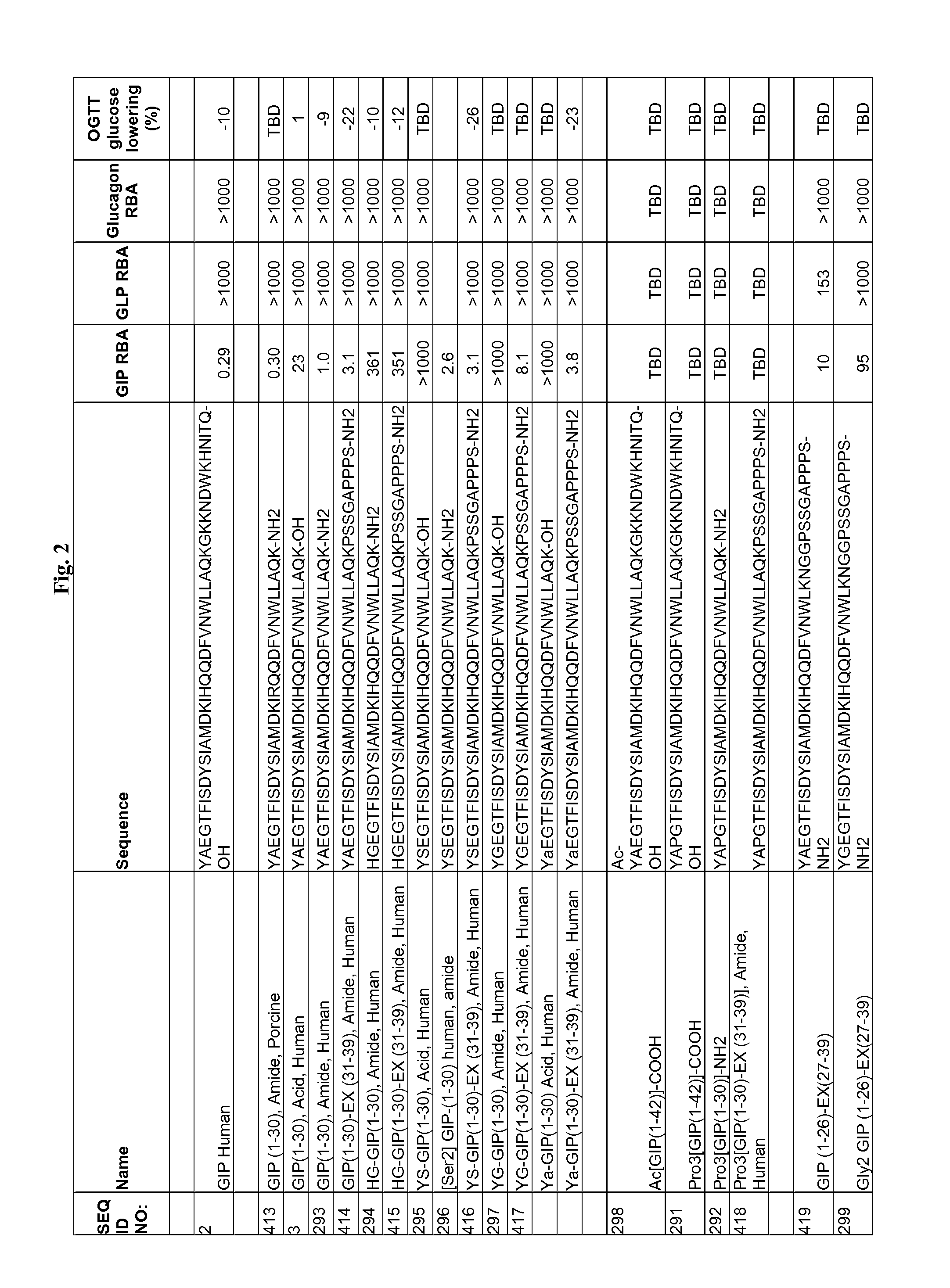
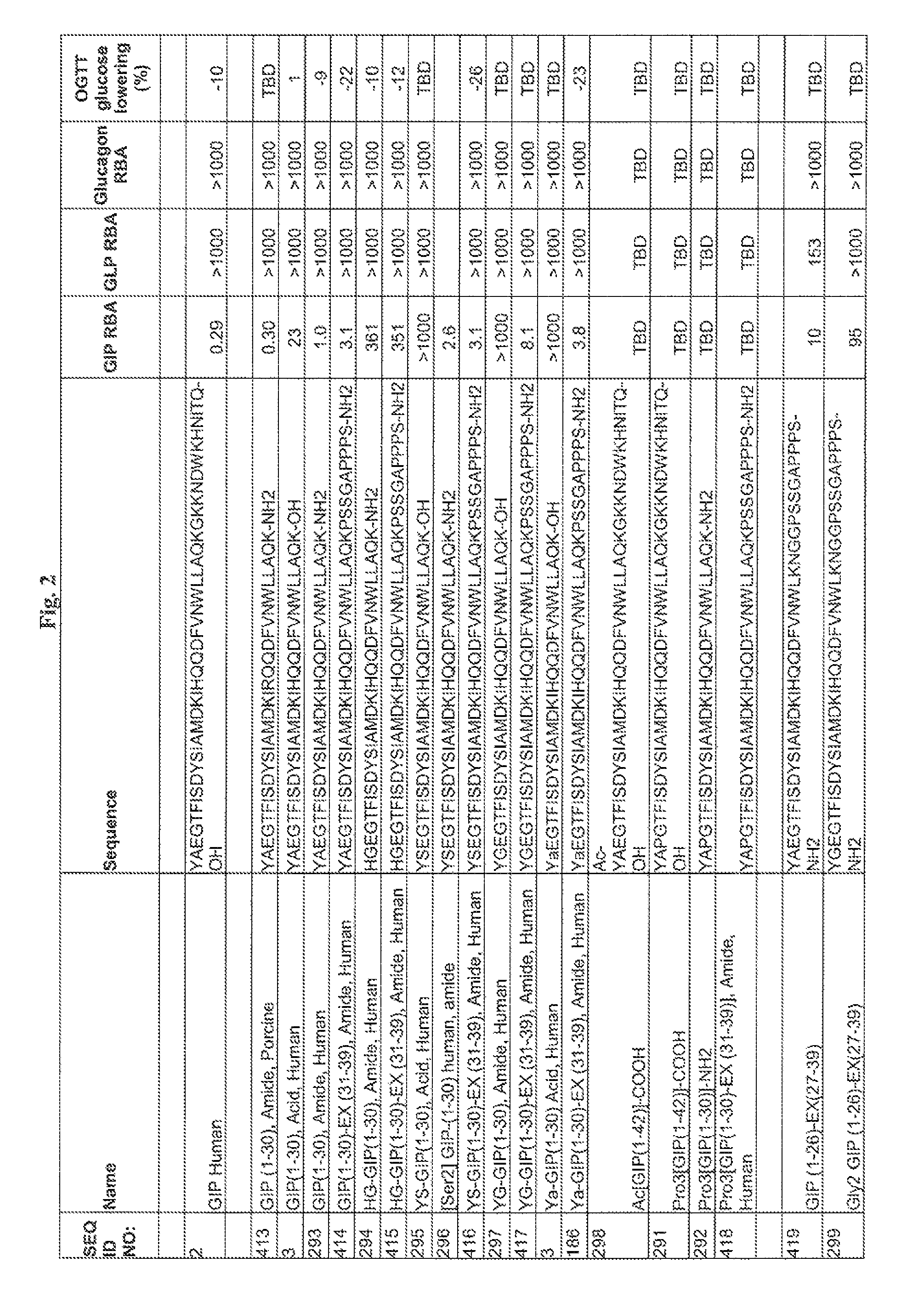
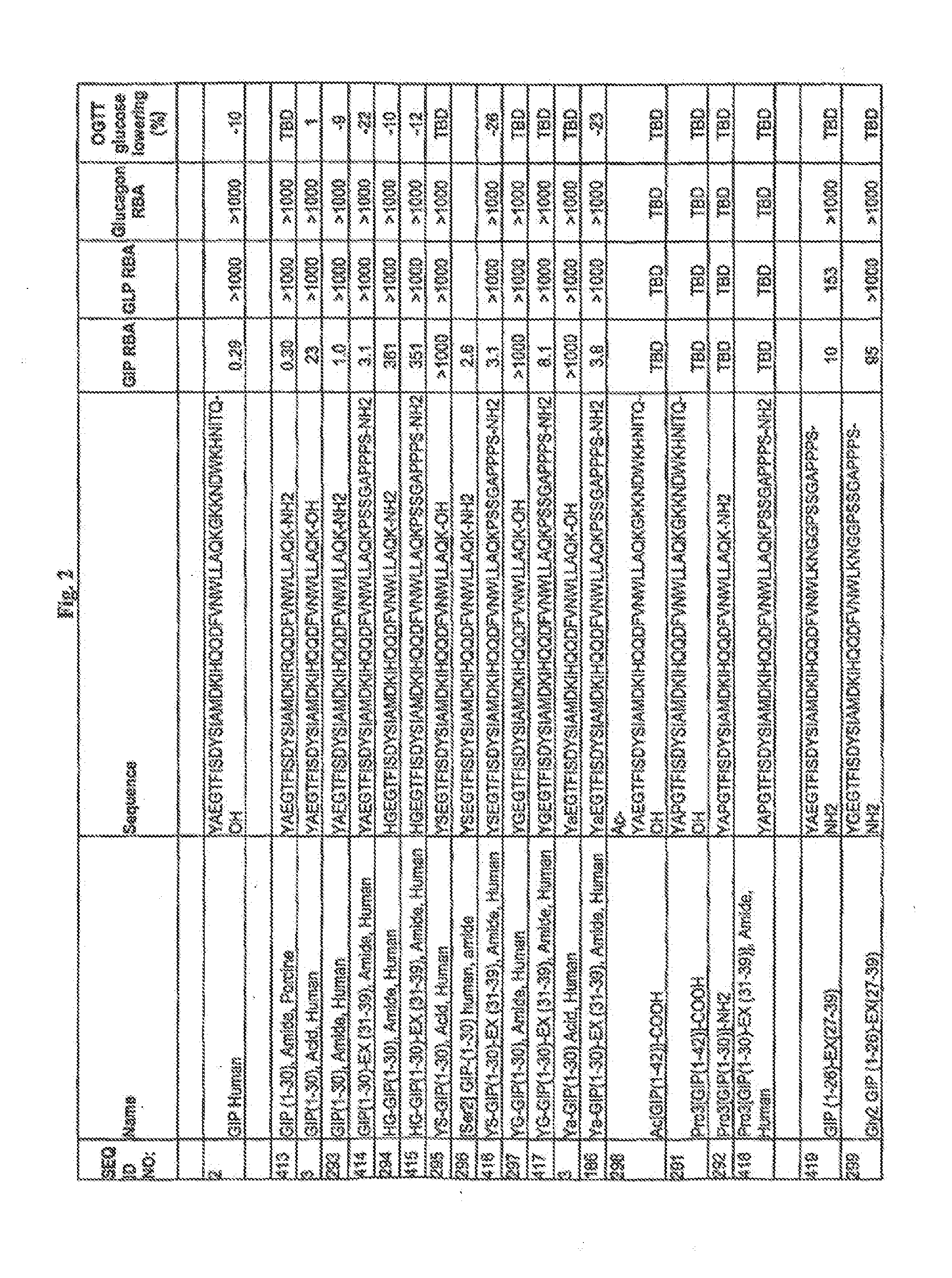
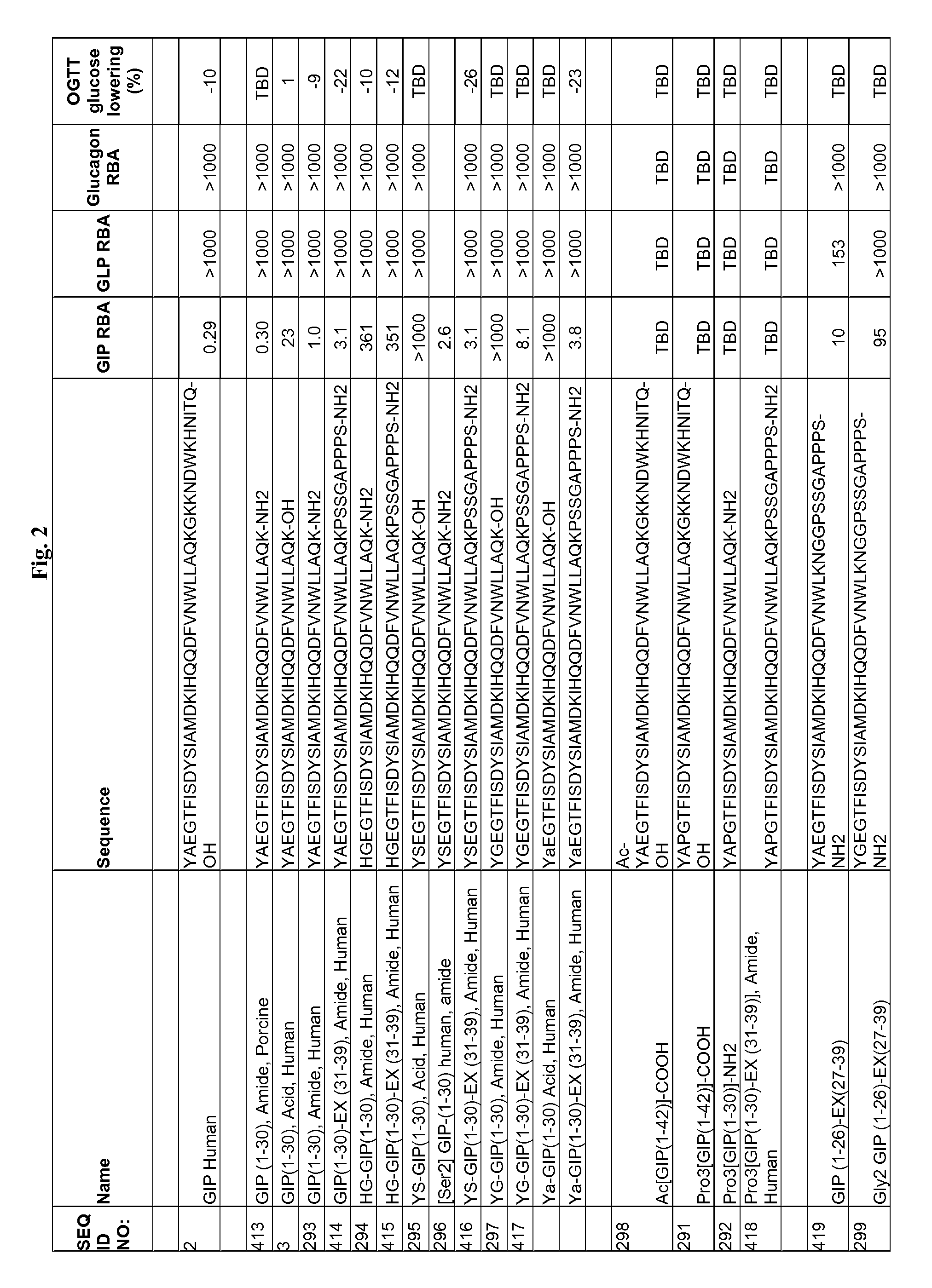
Gip analog and hybrid polypeptides with selectable properties
ActiveUS20080312157A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsMetabolism disorderDyslipidemiaFeeding disability
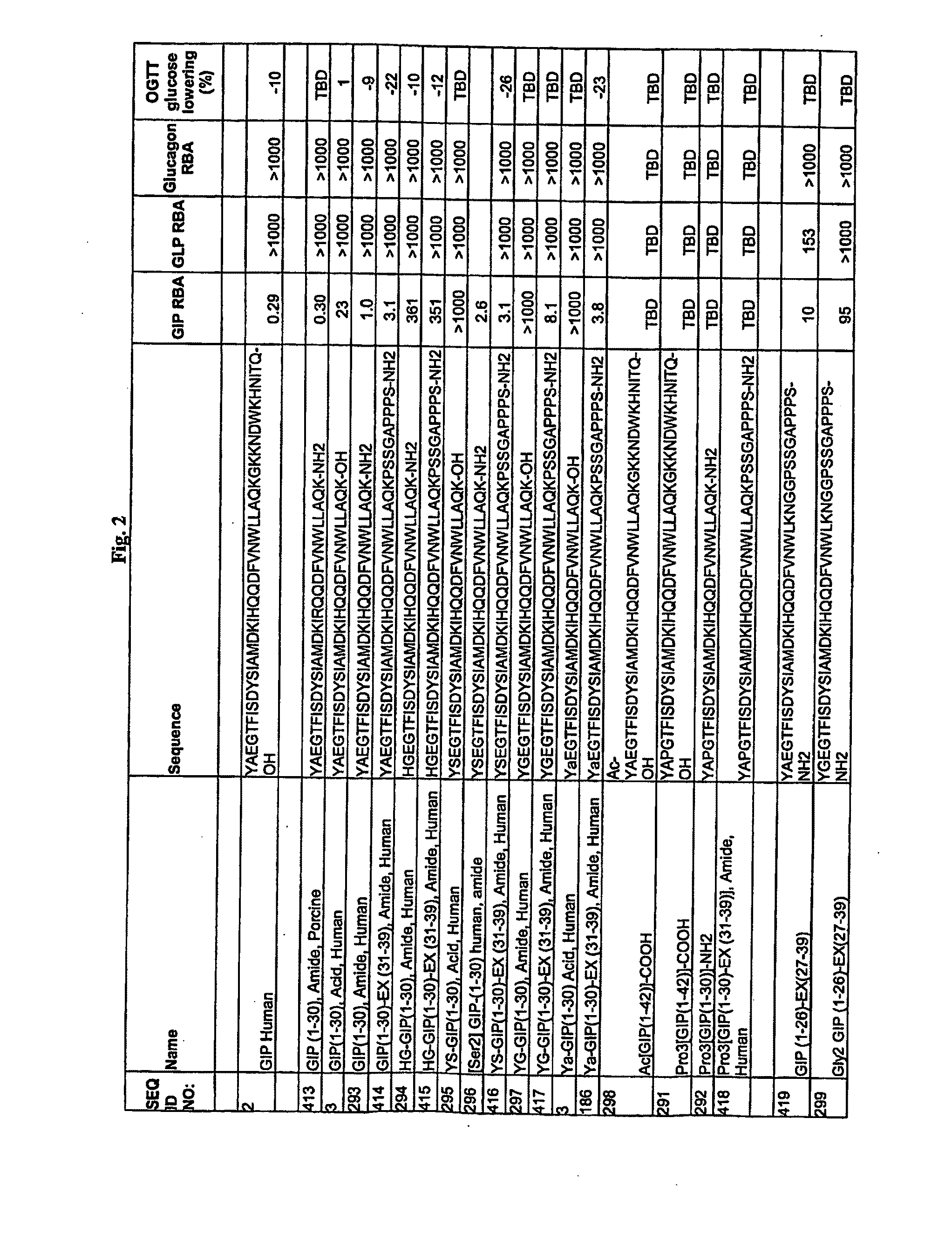
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Gip analog and hybrid polypeptides with selectable properties
InactiveUS20090036364A1Increased insulin secretionDecreasing bone loss boneSenses disorderNervous disorderDyslipidemiaPhysiology
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
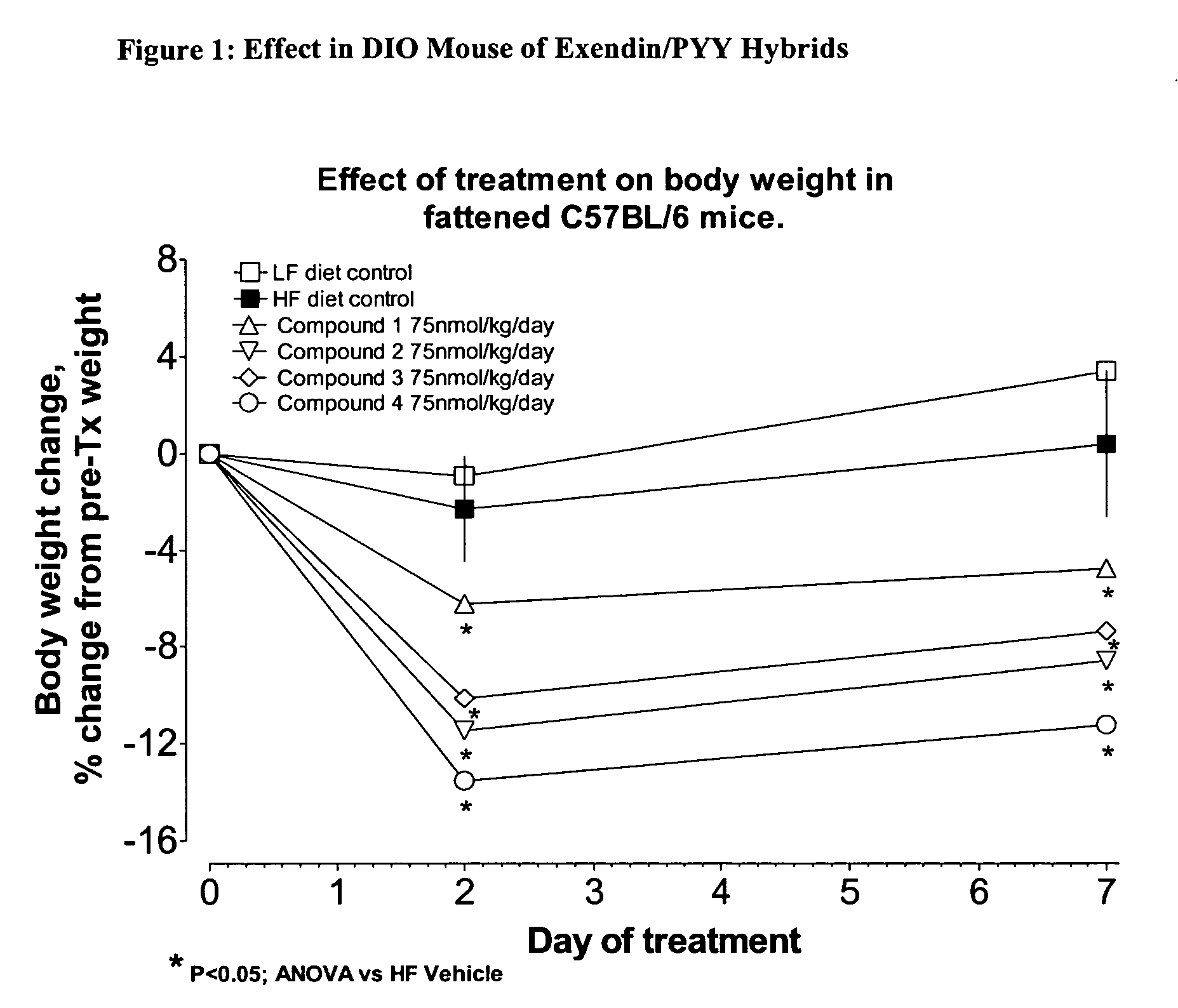
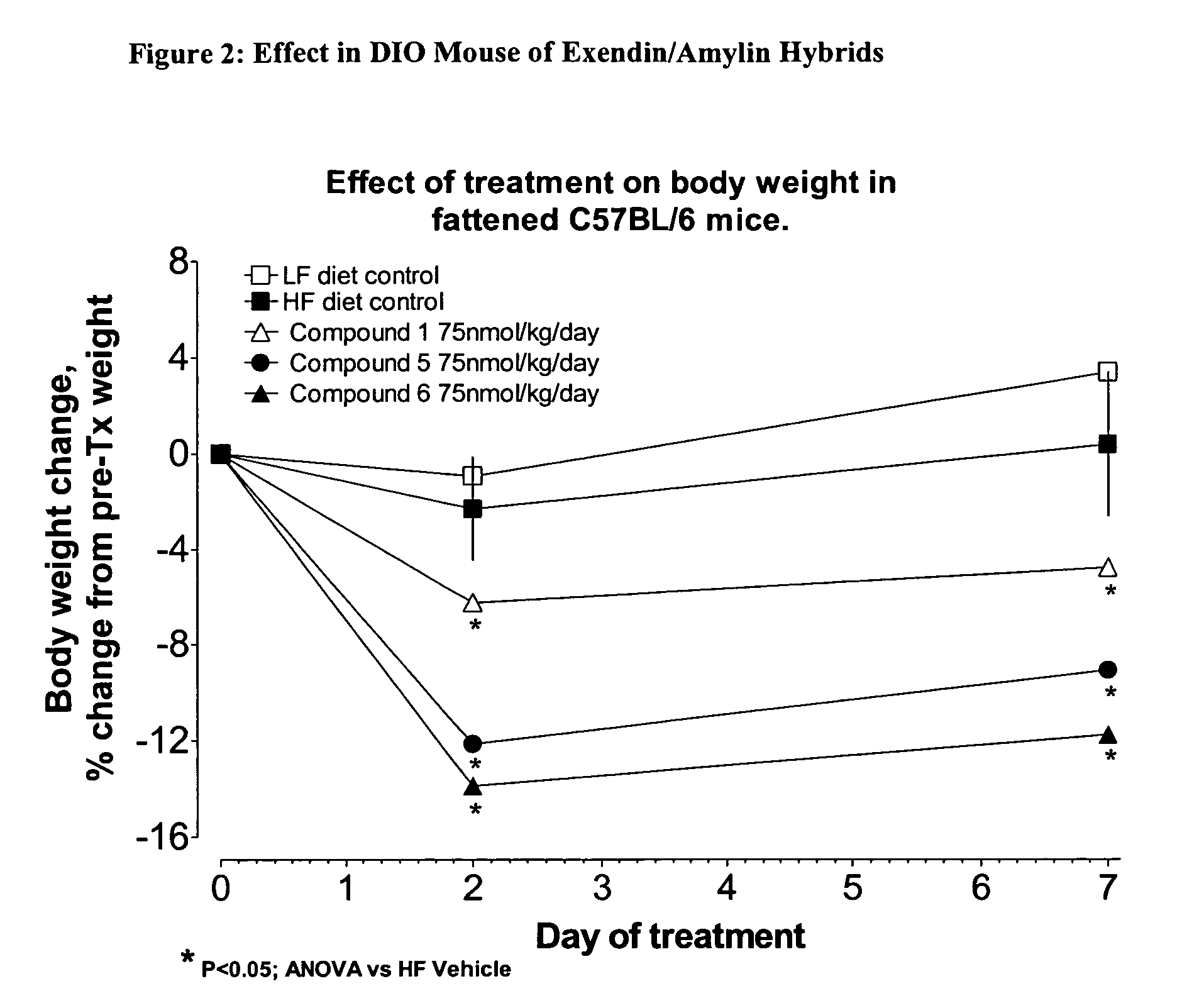
Hybrid polypeptides with selectable properties
InactiveUS20060094652A1Reverse glucose intoleranceIncrease beta cell massPeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaBlood plasma
The present invention relates generally to novel, selectable hybrid polypeptides useful as agents for the treatment and prevention of metabolic diseases and disorders which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, such as diabetes and diabetes-related conditions. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:AMYLIN PHARMA INC
GIP analog and hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Hybrid polypeptides having glucose lowering activity
The present invention relates generally to novel, selectable hybrid polypeptides useful as agents for the treatment and prevention of metabolic diseases and disorders which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, such as diabetes and diabetes-related conditions. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
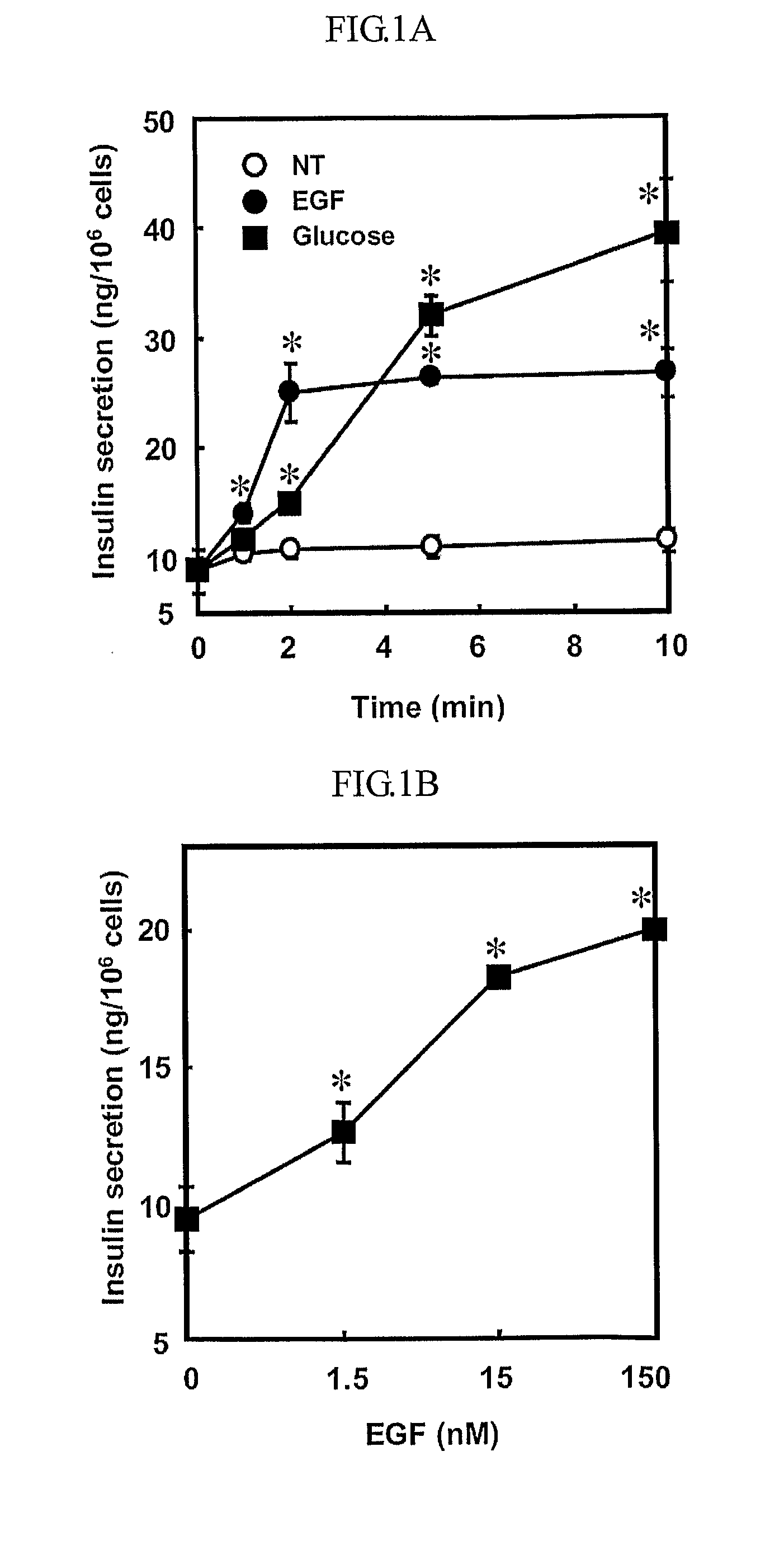
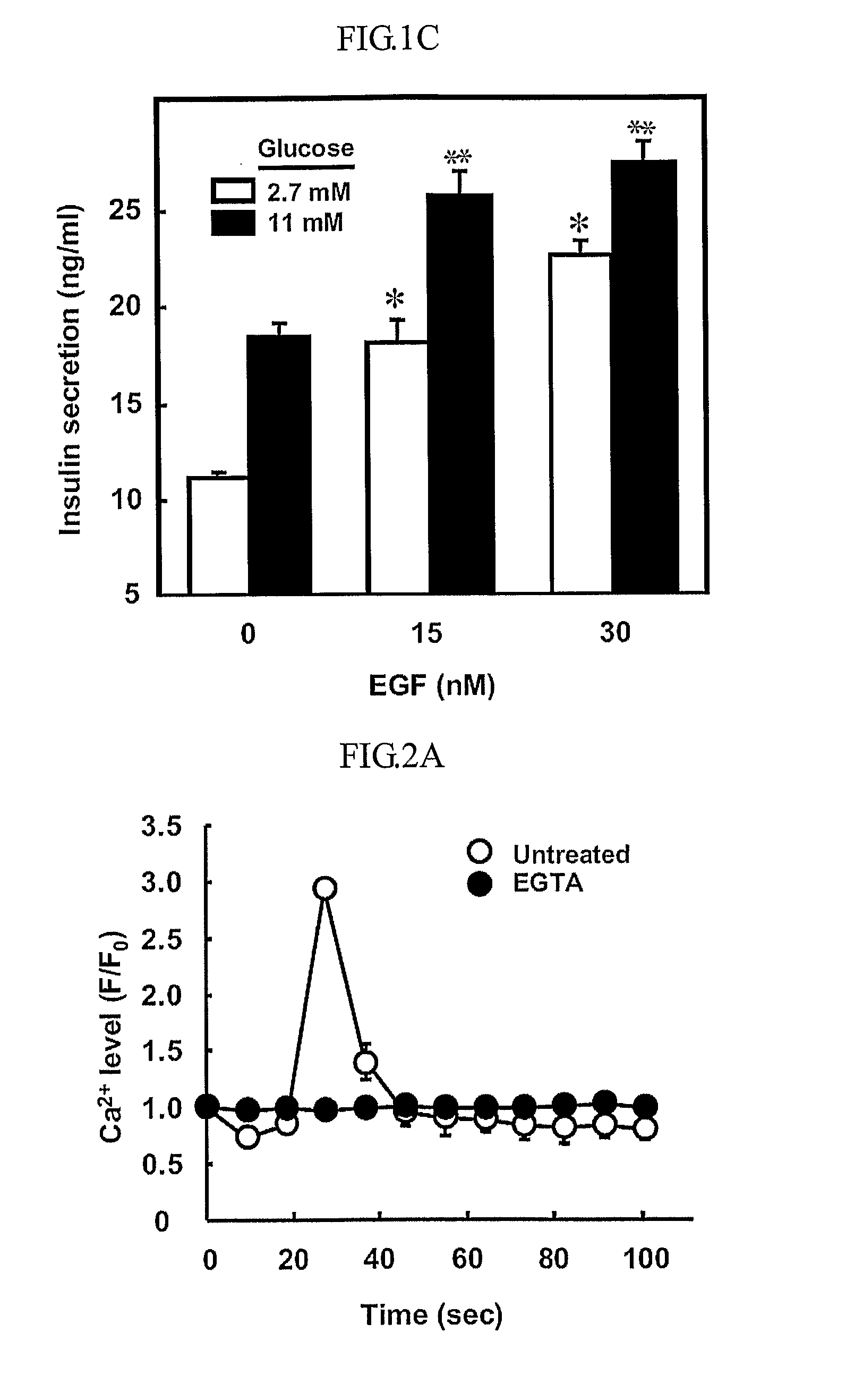
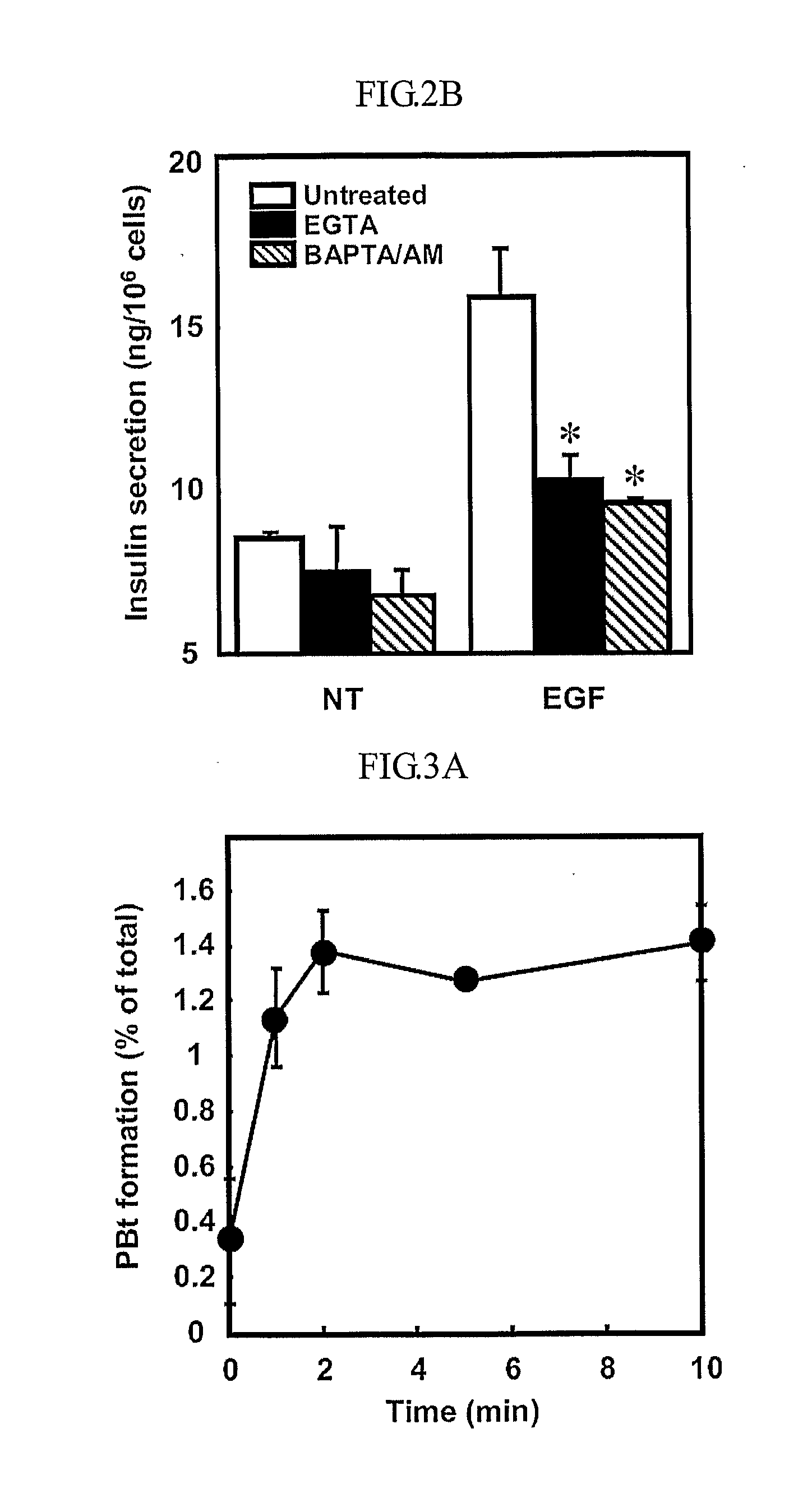
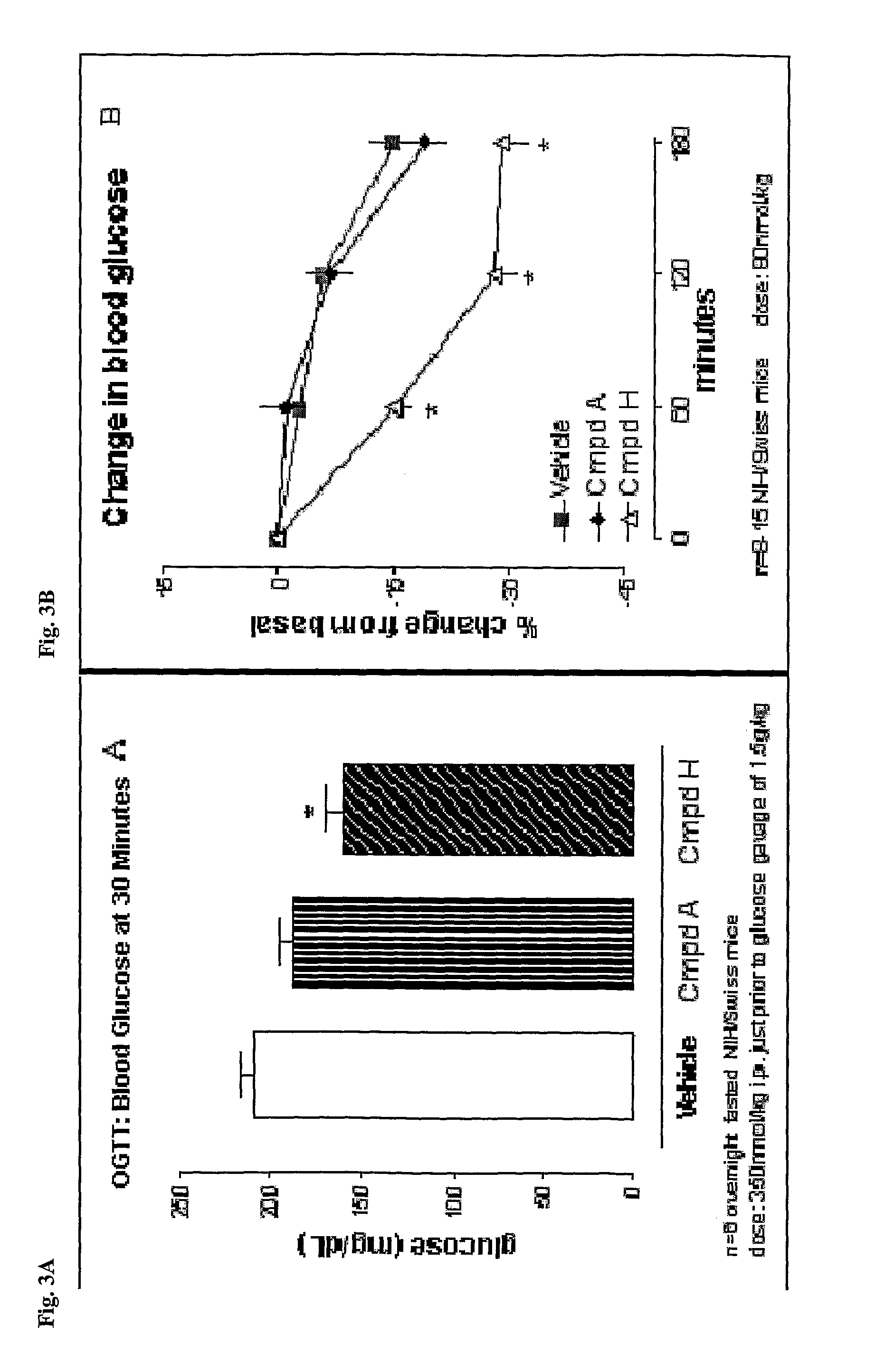
Epidermal growth factor increasing insulin secretion and lowering blood glucose
InactiveUS20090312250A1Lower Level RequirementsEGF treatmentPeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
The present inventors show that a brief exposure to EGF stimulates insulin secretion glucose-independently via a Ca2+ influx- and PLD2-dependent mechanism. Furthermore, the present invention shows that EGF is a novel secretagogue that lowers plasma glucose levels in normal and diabetic mice, suggesting the potential for EGF treatment in diabetes.
Owner:POSTECH ACAD IND FOUND
DPP-IV Resistant GIP Hybrid Polypeptides with Selectable Properties
ActiveUS20110136737A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaFeeding disability
The Present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Dpp-iv resistant gip hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:LEVY ODILE ESTHER +15
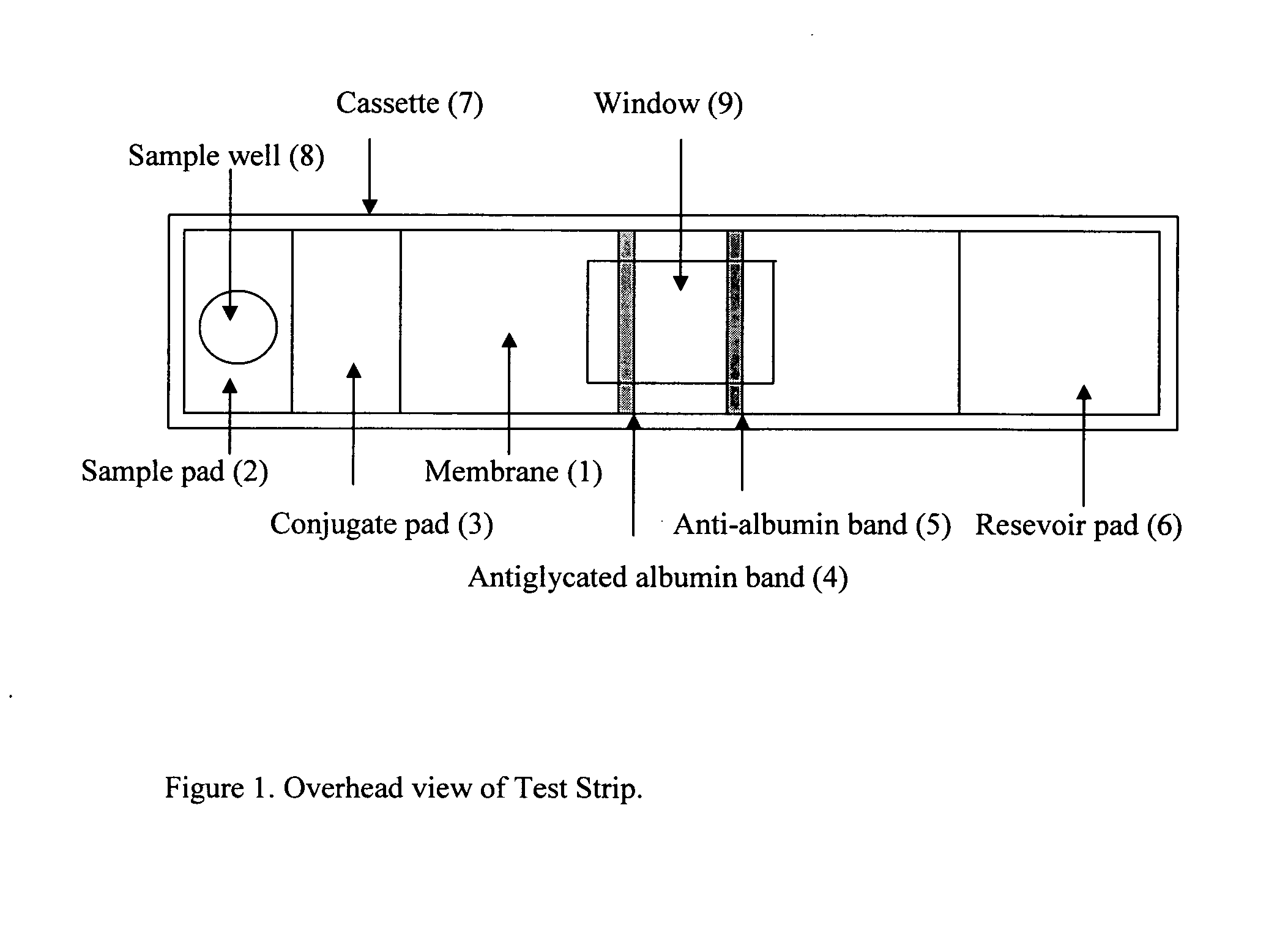
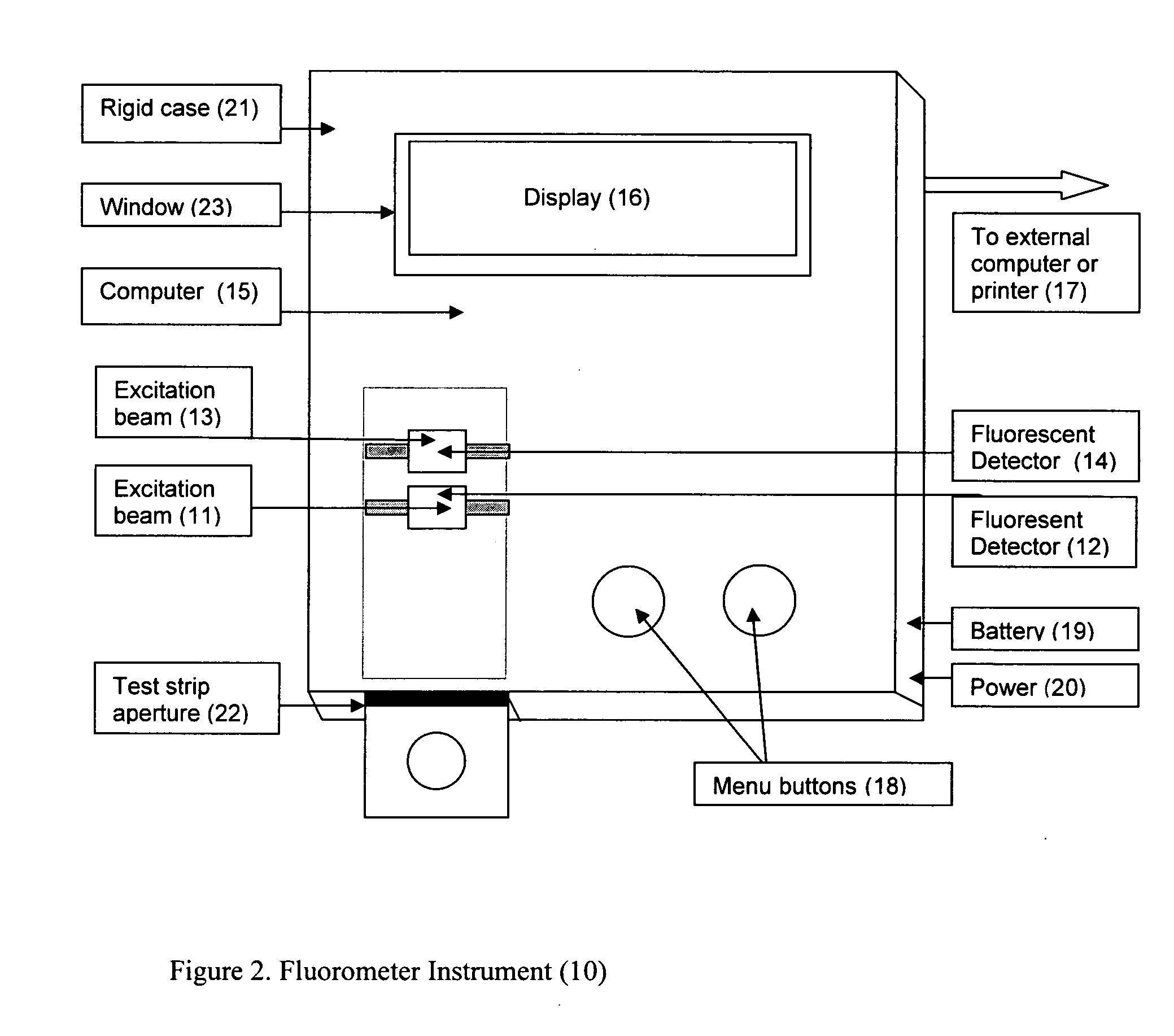
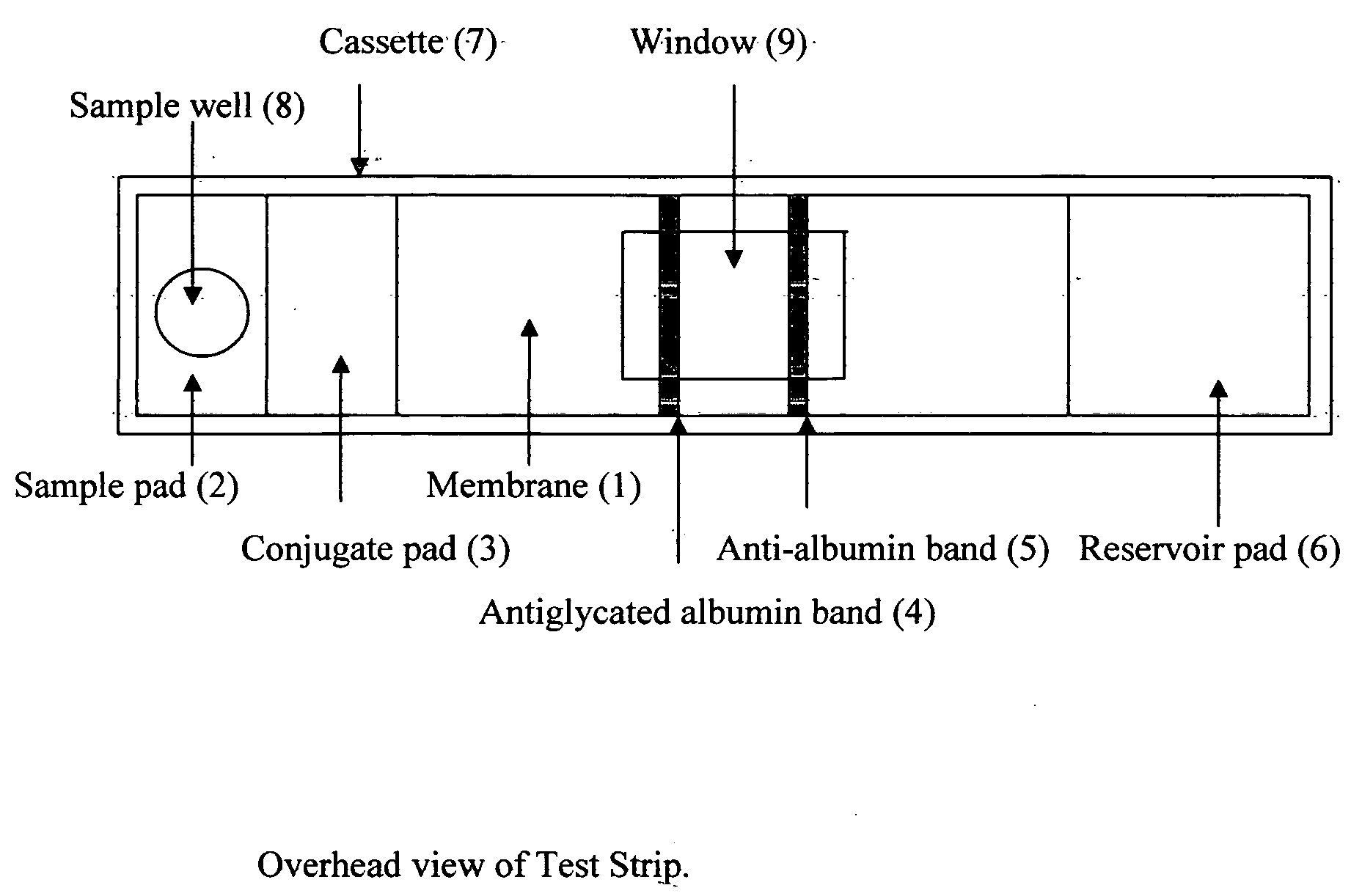
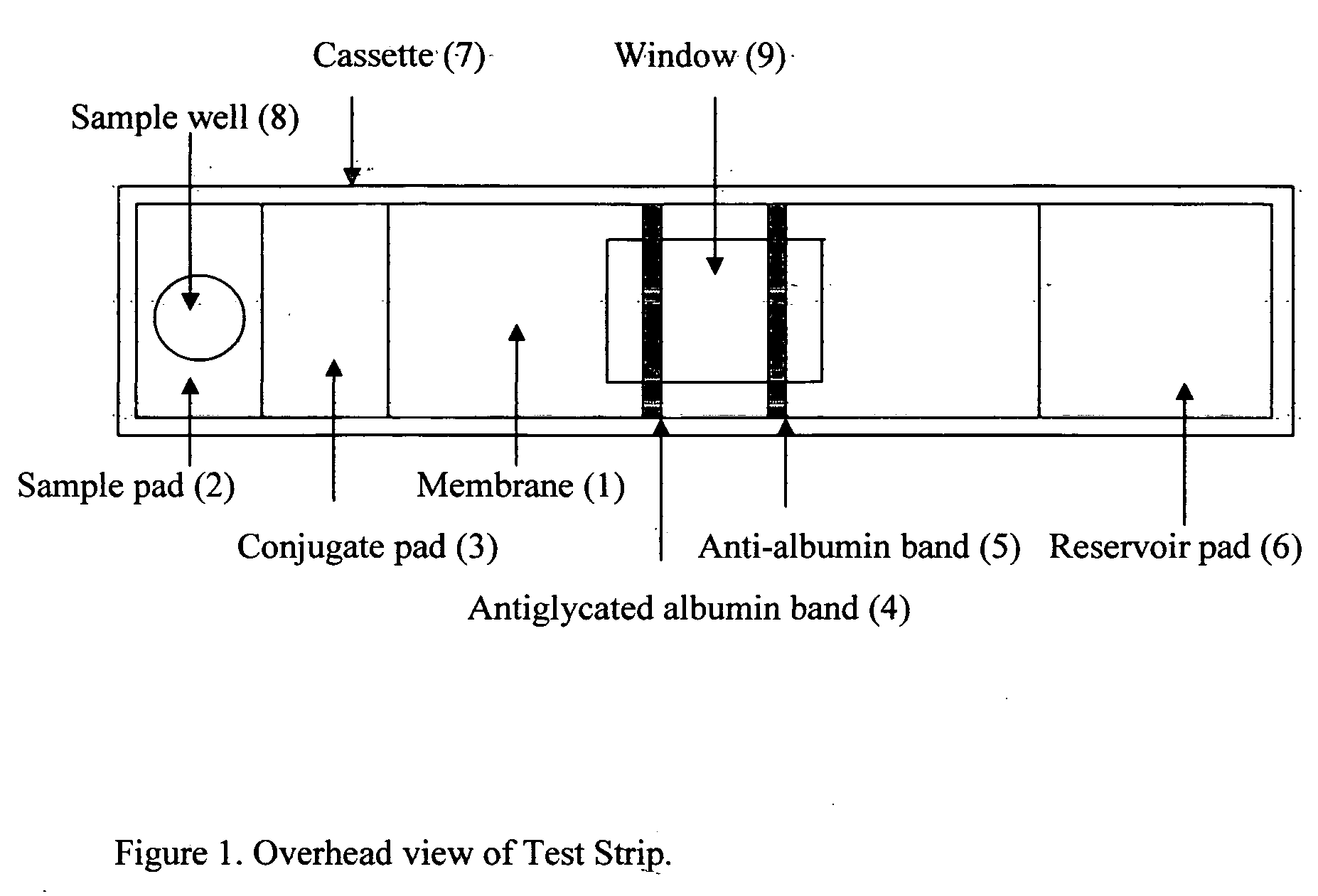
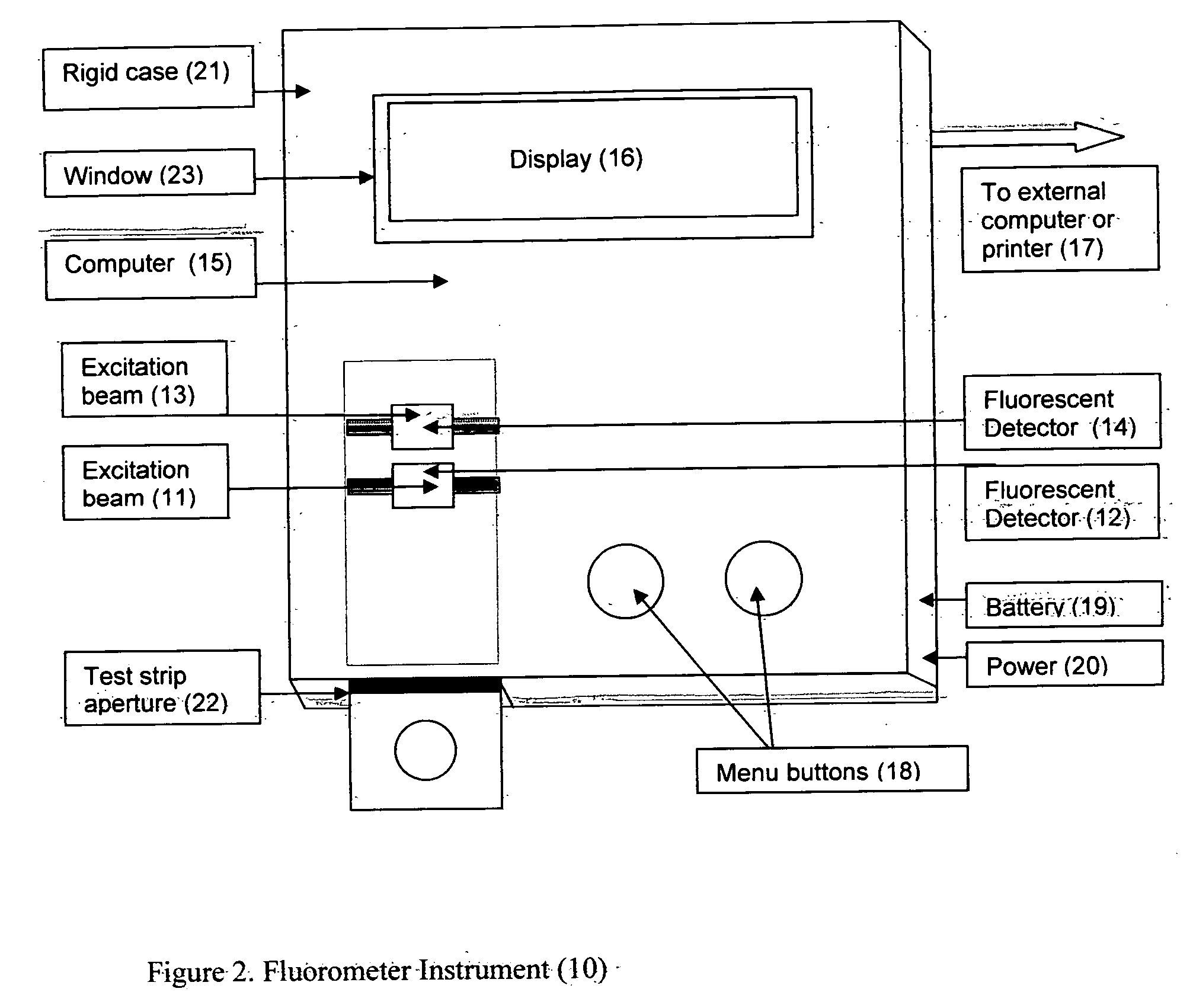
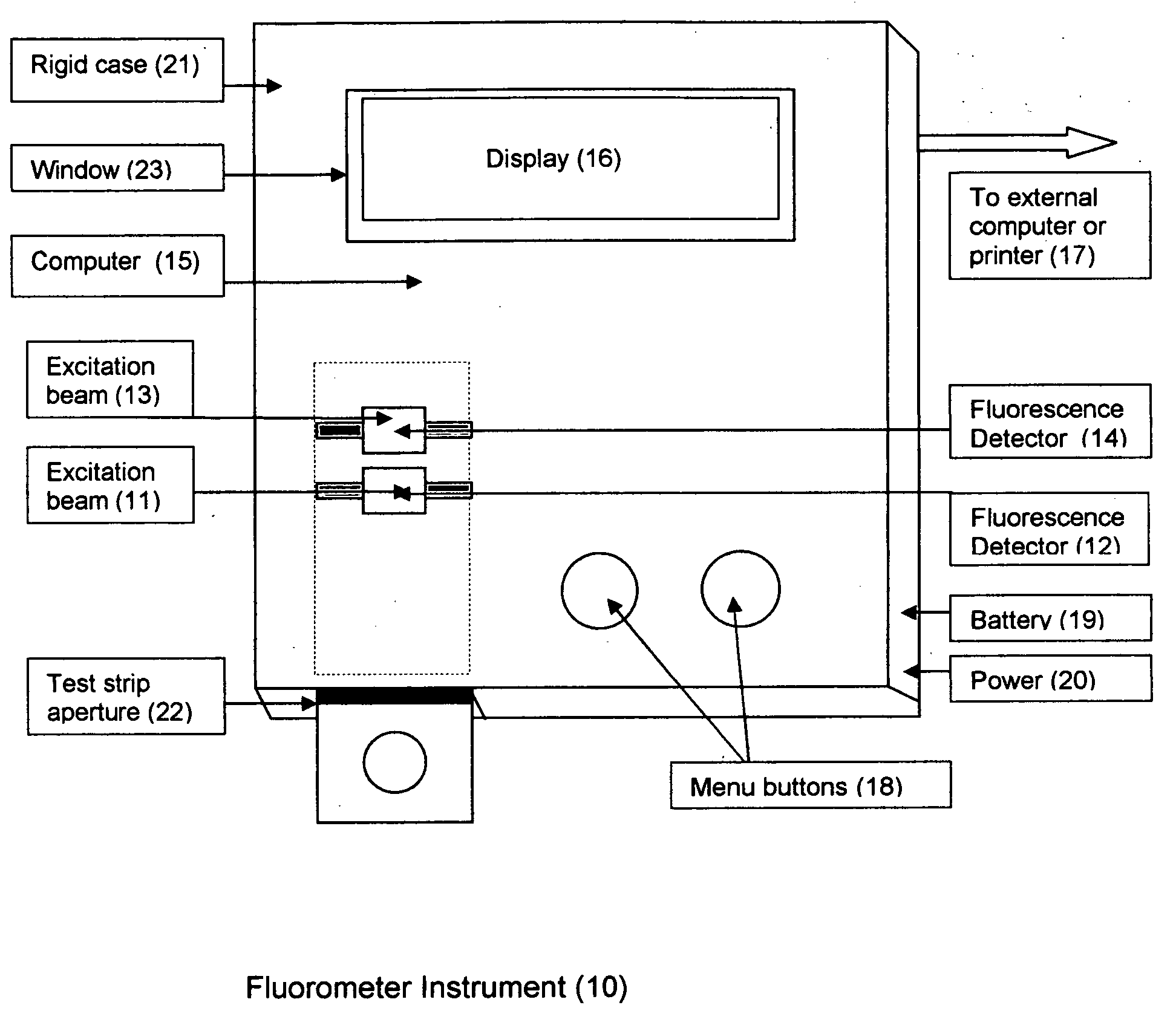
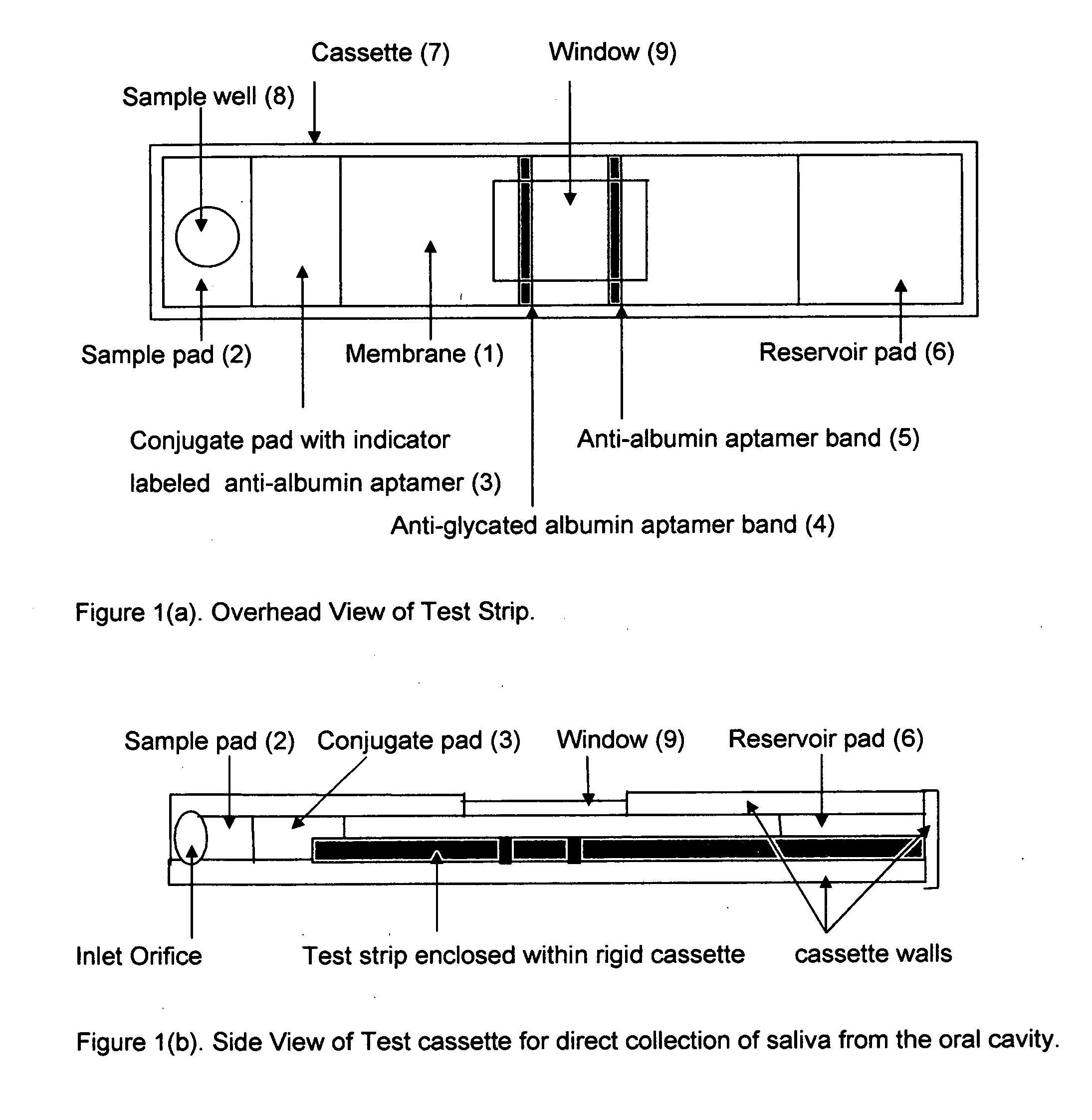
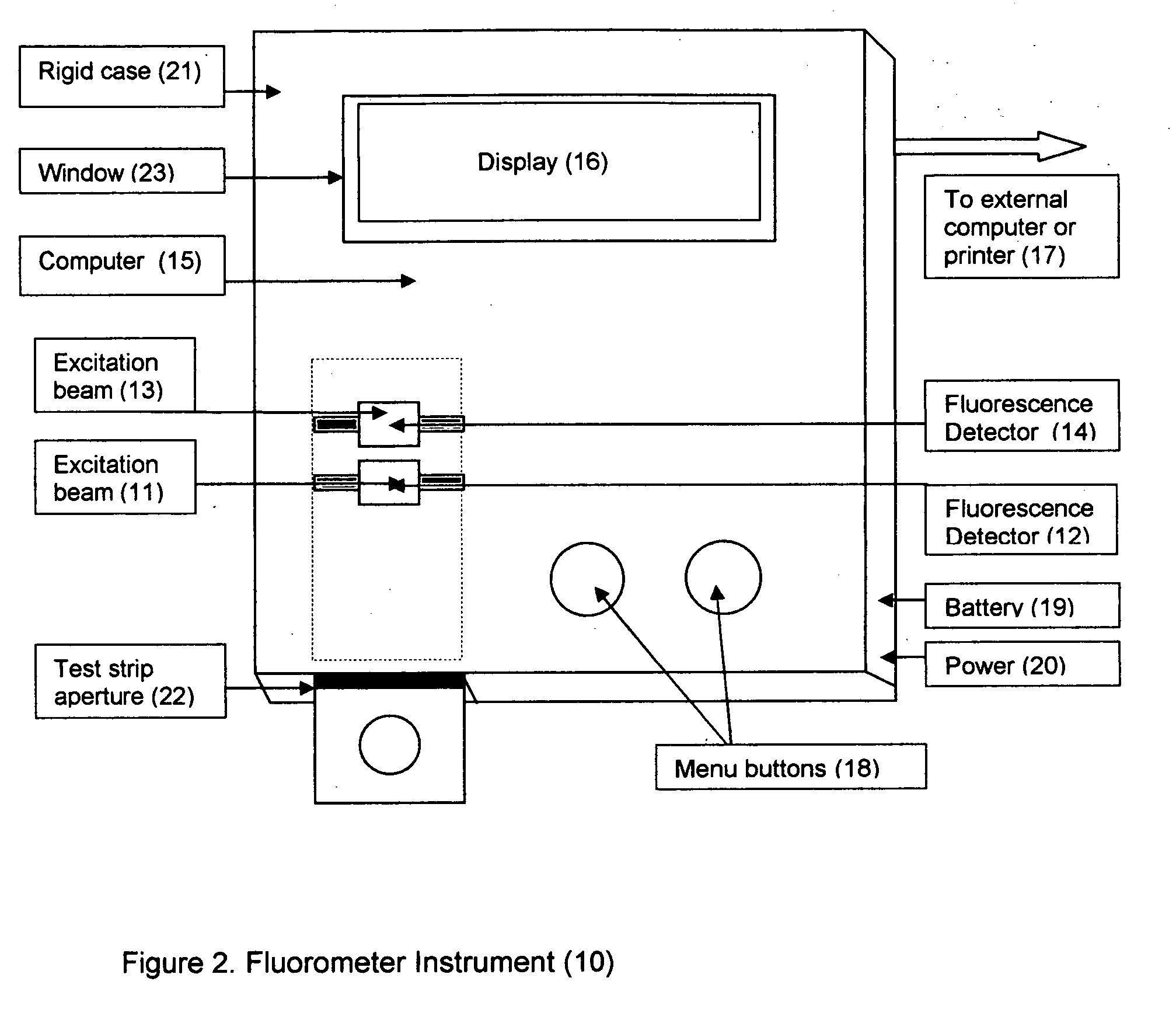
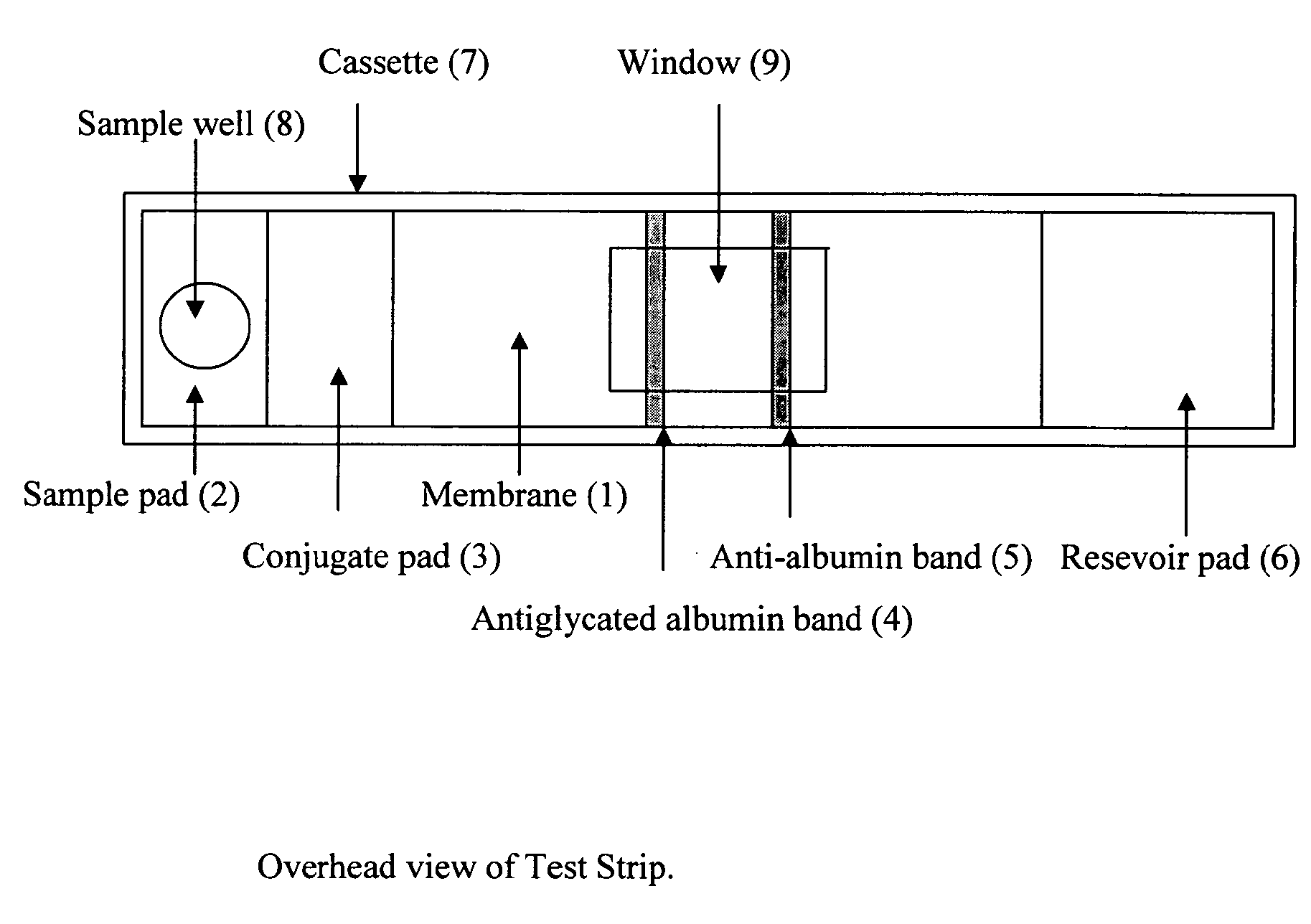
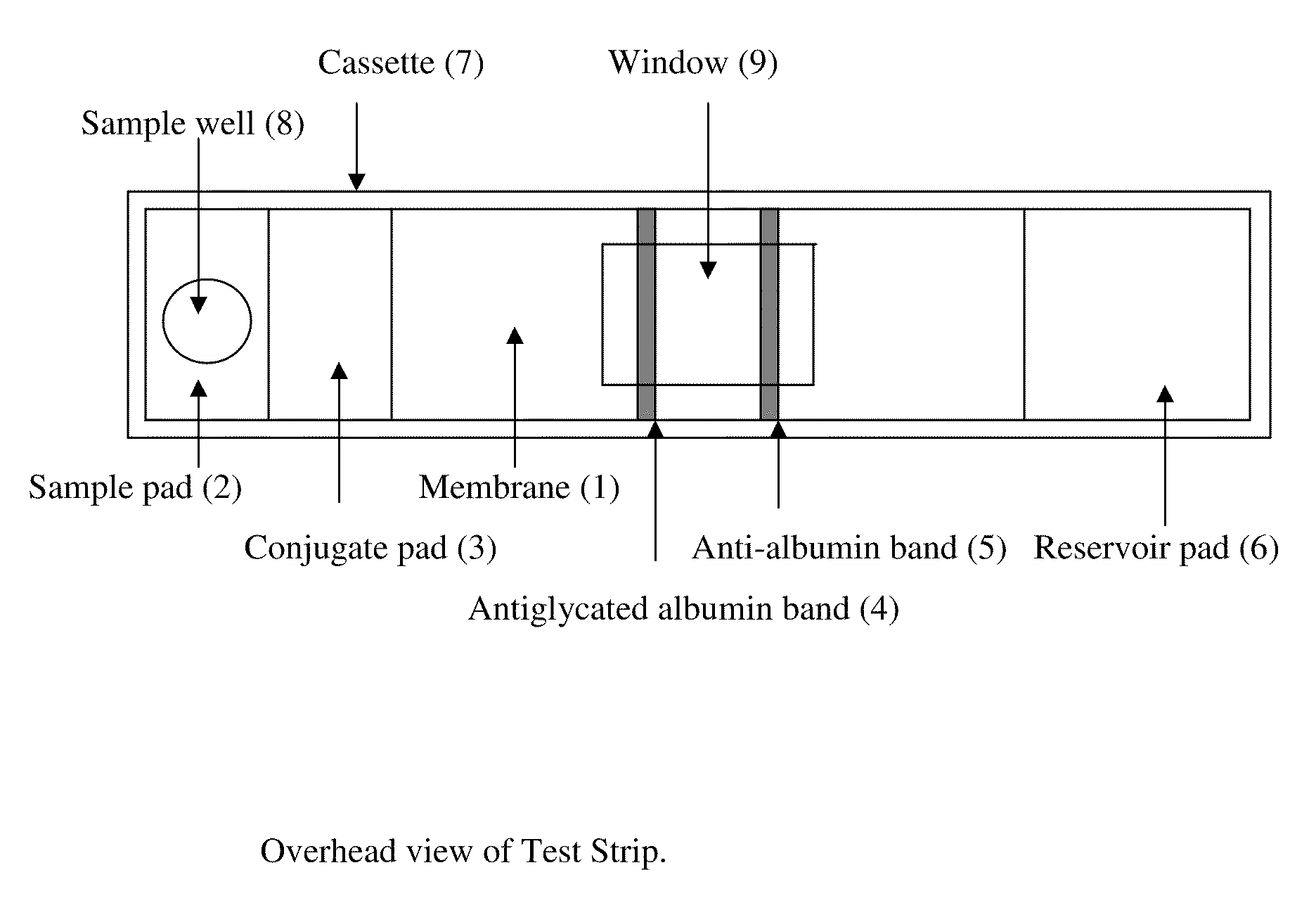
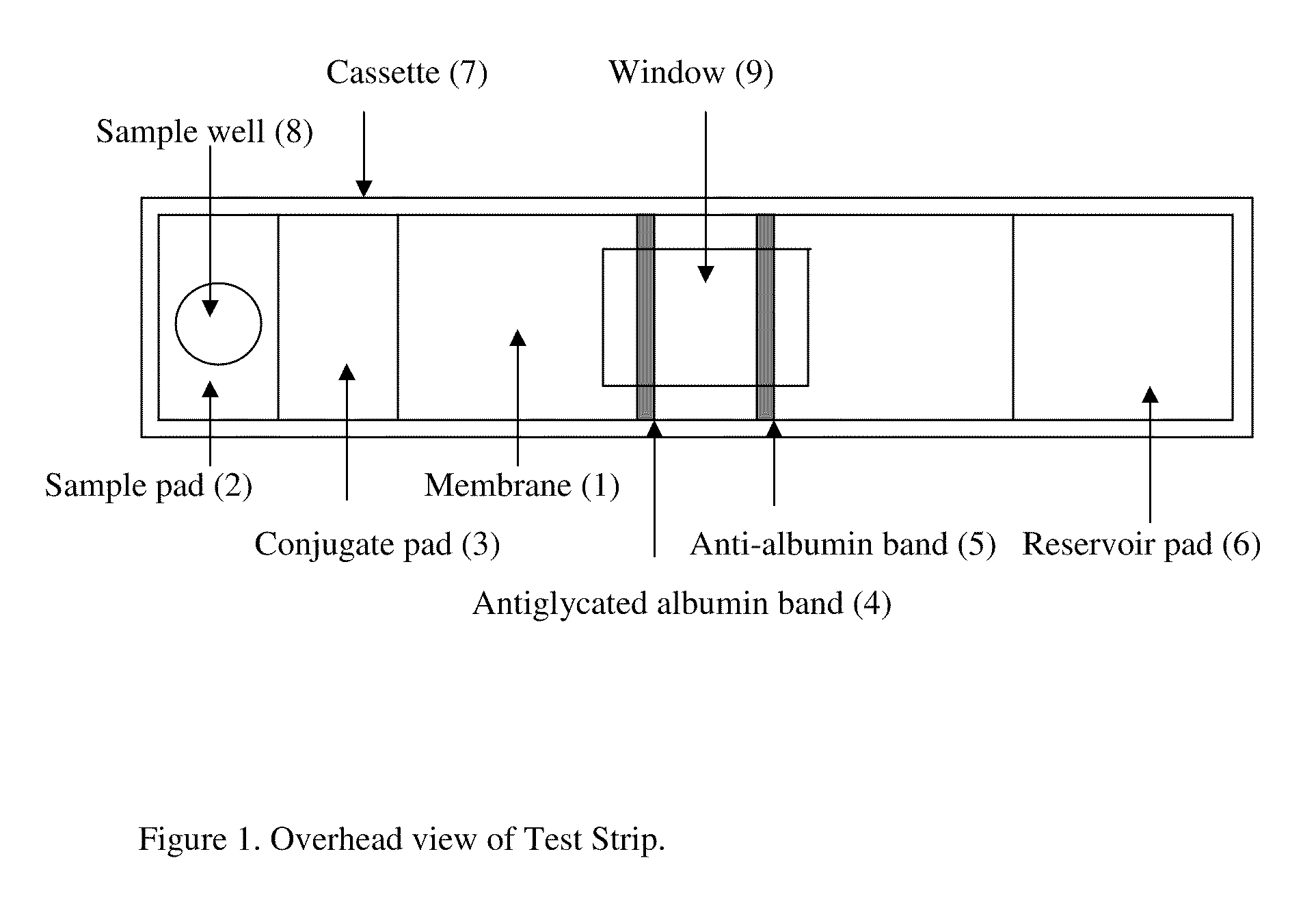
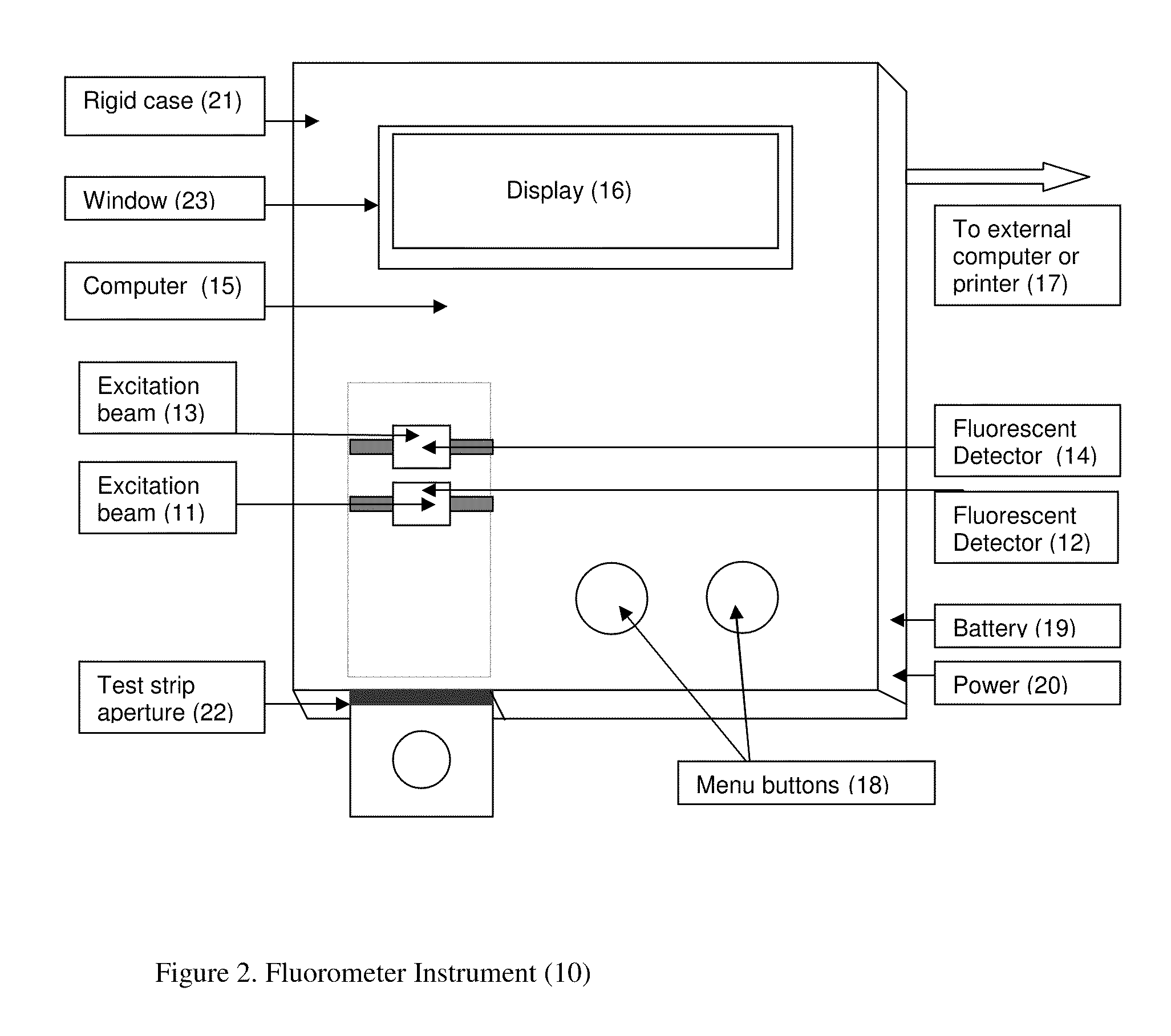
Rapid test for glycated albumin in saliva
InactiveUS20060270060A1Accurate assessmentBioreactor/fermenter combinationsBiological substance pretreatmentsMeasuring instrumentPlasma Albumin
This invention describes a rapid immunochromatographic assay for measuring the ratio of glycated albumin to total albumin in saliva. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:SMITH HENRY JOHN
Gip analog and hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP +1
Pharmaceutical compositions containing exendins
InactiveUS20050215469A1Reduce appetiteReduce cardiac riskPeptide/protein ingredientsMetabolism disorderBlood plasmaInsulin resistance
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
Use of exendins and agonists thereof for lowering plasma lipid
InactiveUS20050101537A1Reduce appetiteReduce cardiac riskPeptide/protein ingredientsMetabolism disorderBlood plasmaInsulin resistance
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:AMYLIN PHARMA INC
Rapid test for glycated albumin in blood
InactiveUS20070015291A1Accurate assessmentBiological material analysisMeasuring instrumentBlood plasma
This invention describes a rapid assay for measuring the ratio of glycated albumin to total albumin in blood. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. The ratio of glycated albumin to total albumin in blood will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads; calculates and displays the final result: The results of tests performed over a period of time are stored in the instrument's memory presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:EPINEX DIAGNOSTICS
Aptamer based point-of-care test for glycated albumin
InactiveUS20090042237A1Accurate assessmentBioreactor/fermenter combinationsRadiation pyrometryPoint of careMeasuring instrument
This invention describes a point-of-care or home use device for measuring the ratio of glycated albumin to total albumin in saliva and other body fluids. Diabetics and prediabetics may have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:EPINEX DIAGNOSTICS
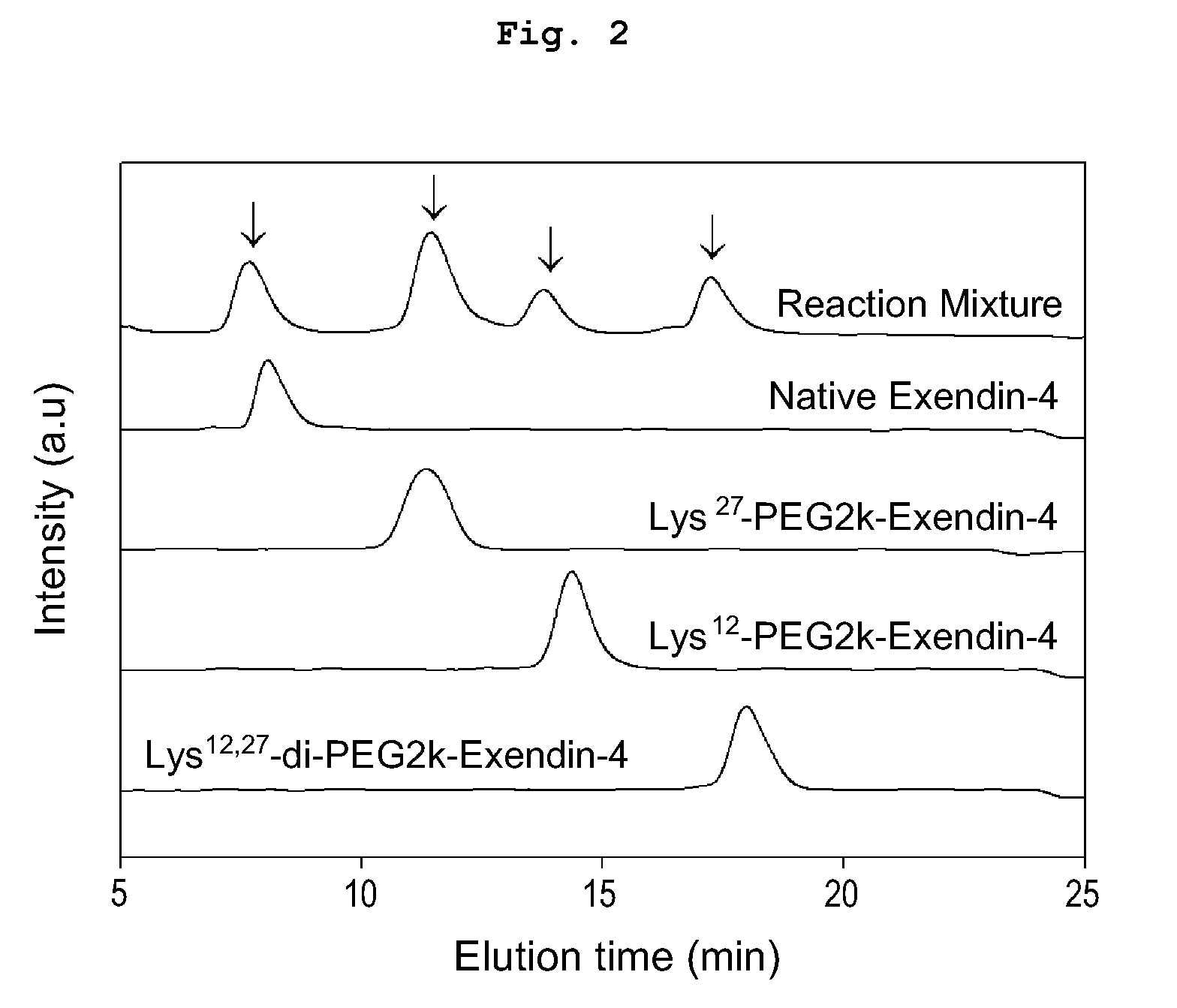
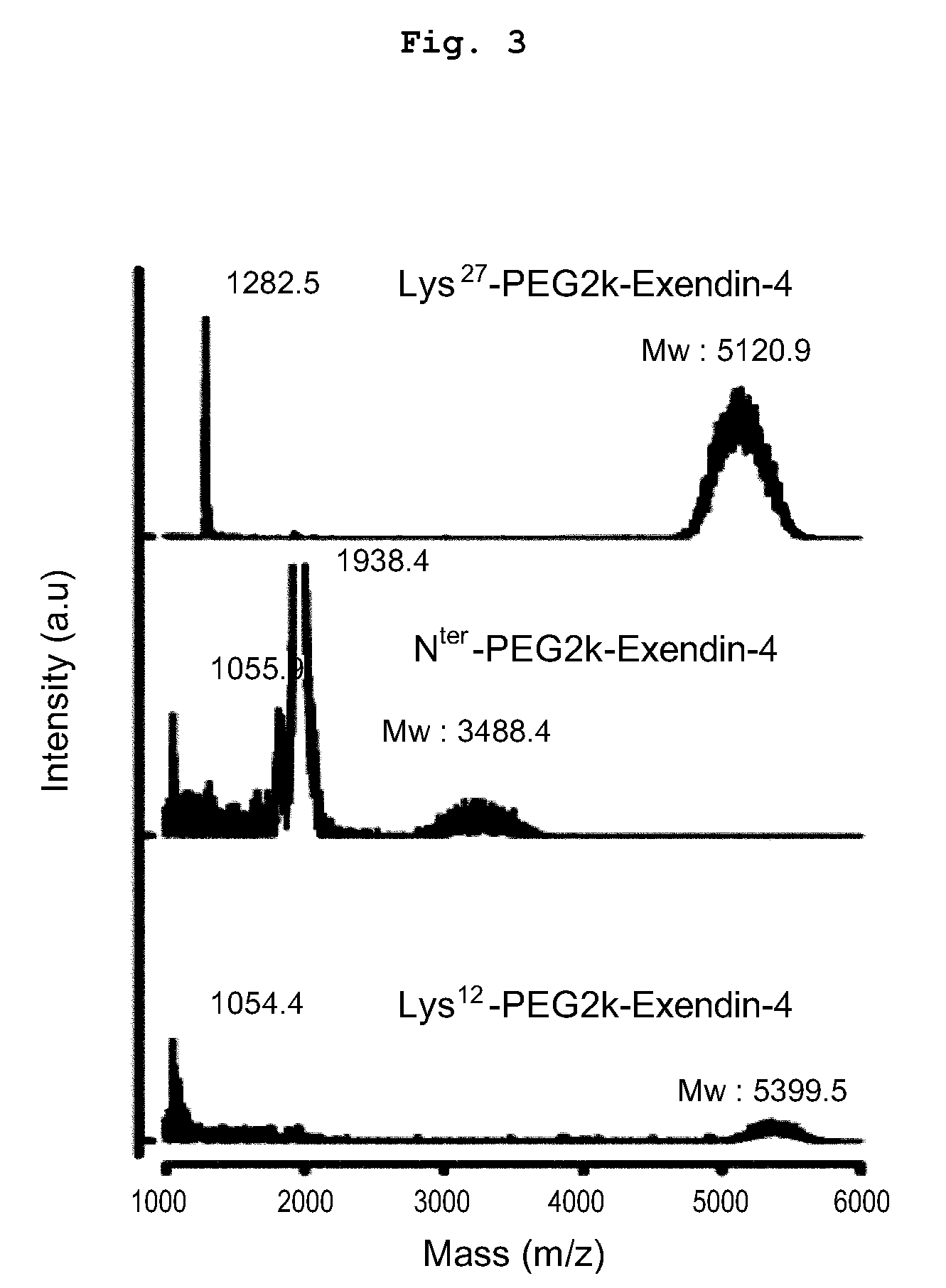
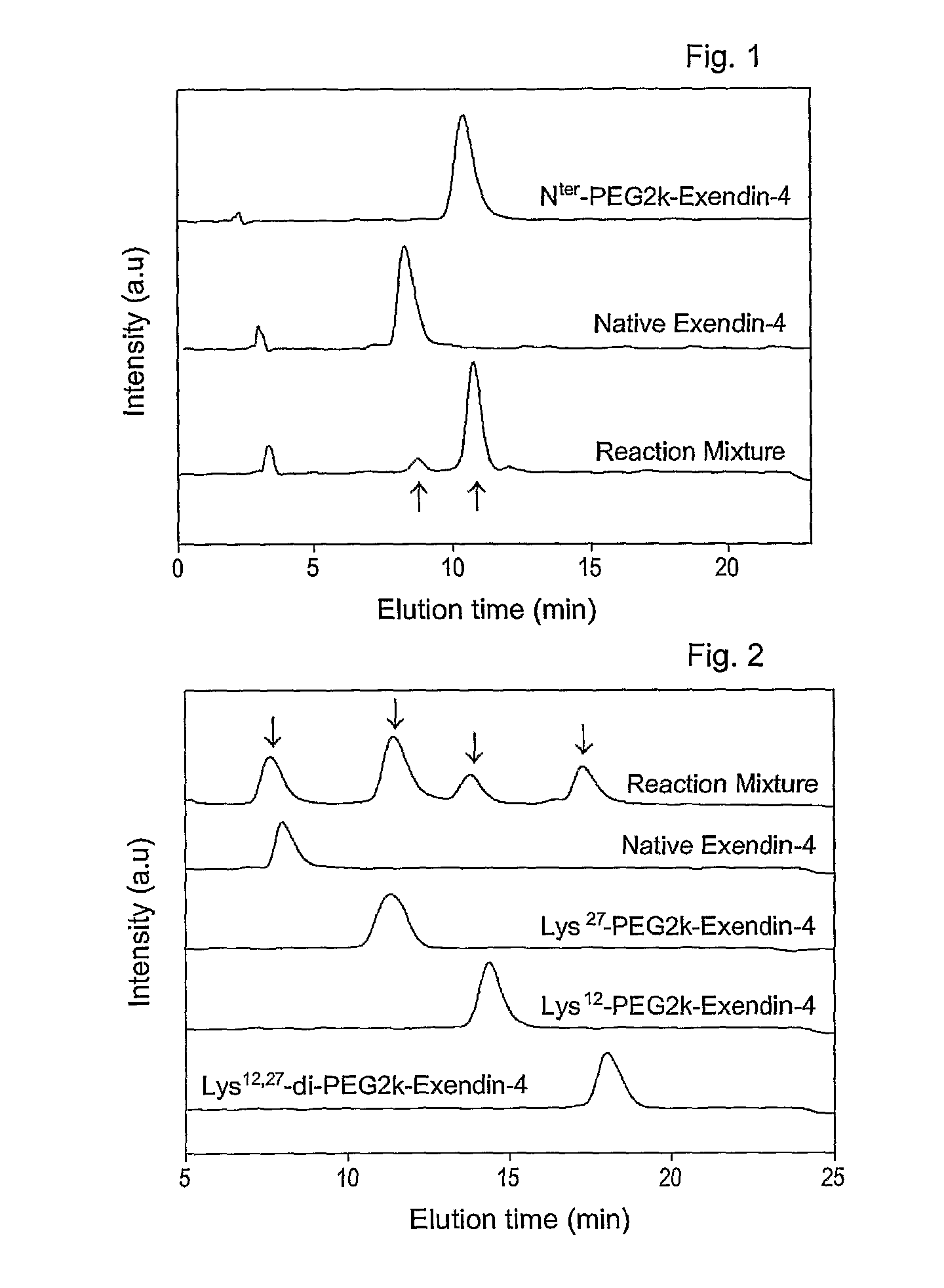
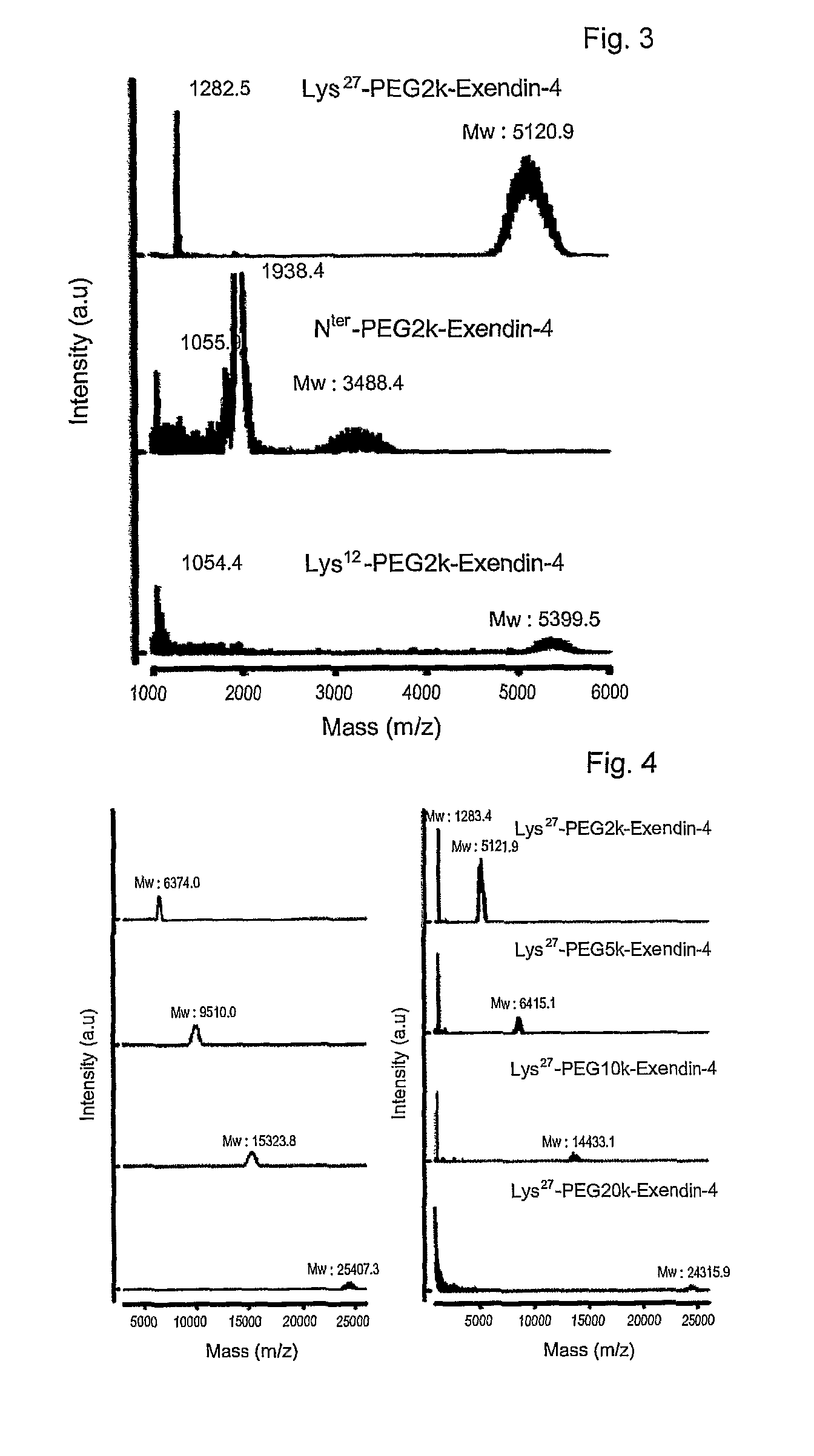
Mono modified exendin with polyethylene glycol or its derivatives and uses thereof
ActiveUS20100137558A1Extended half-lifeMinimize side effectsPeptide/protein ingredientsMetabolism disorderSide effectHalf-life
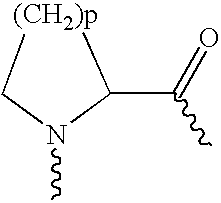
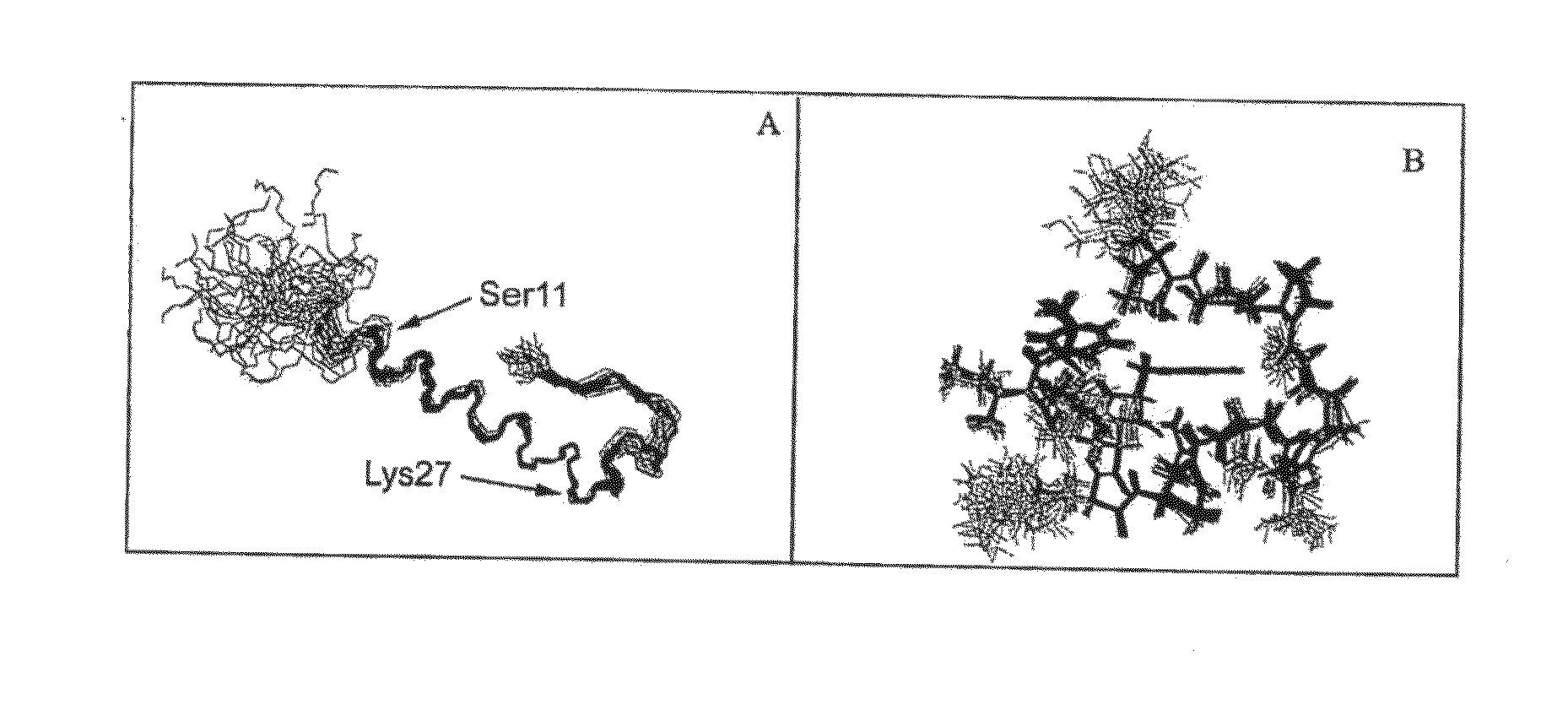
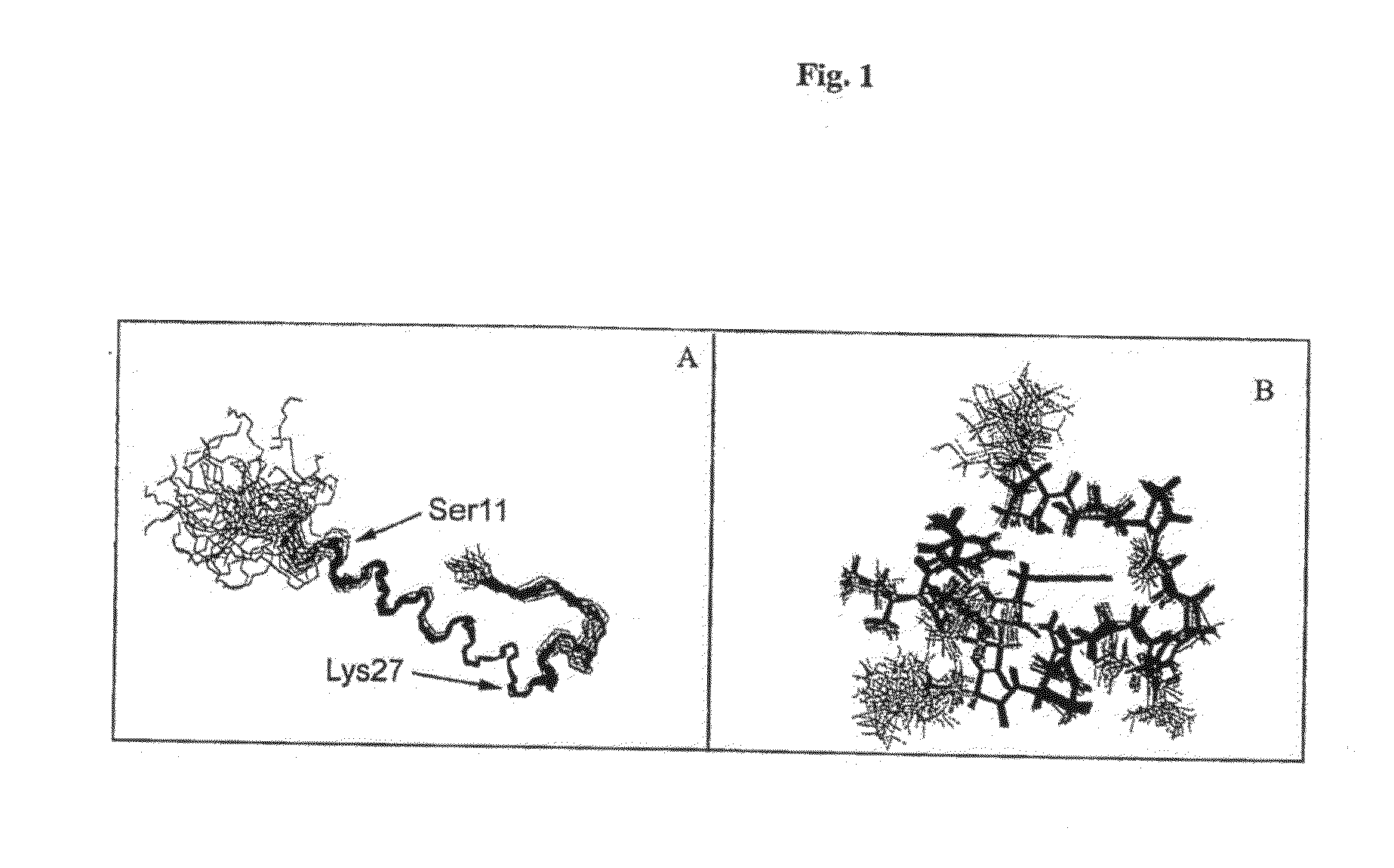
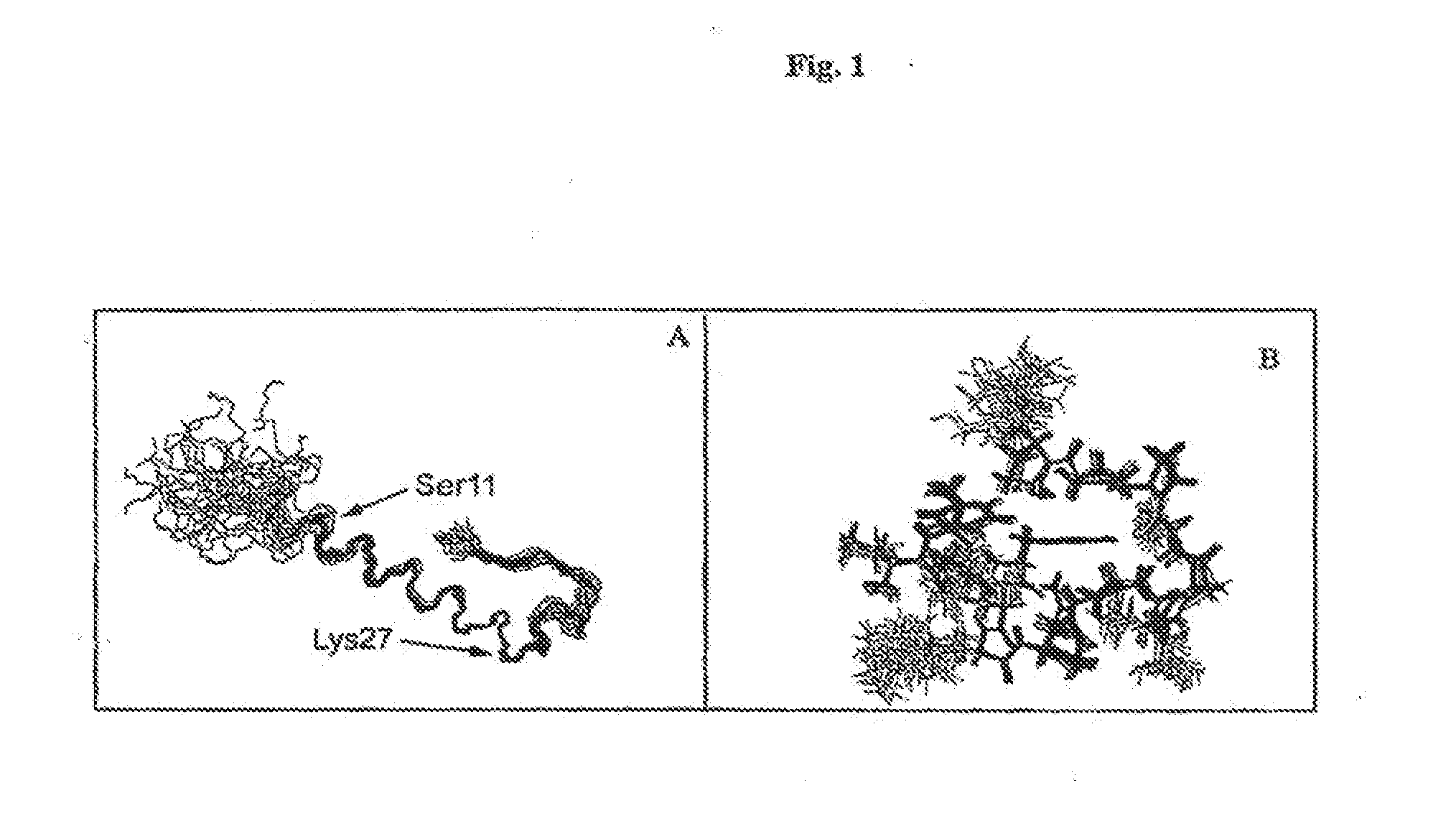
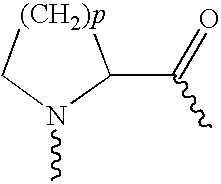
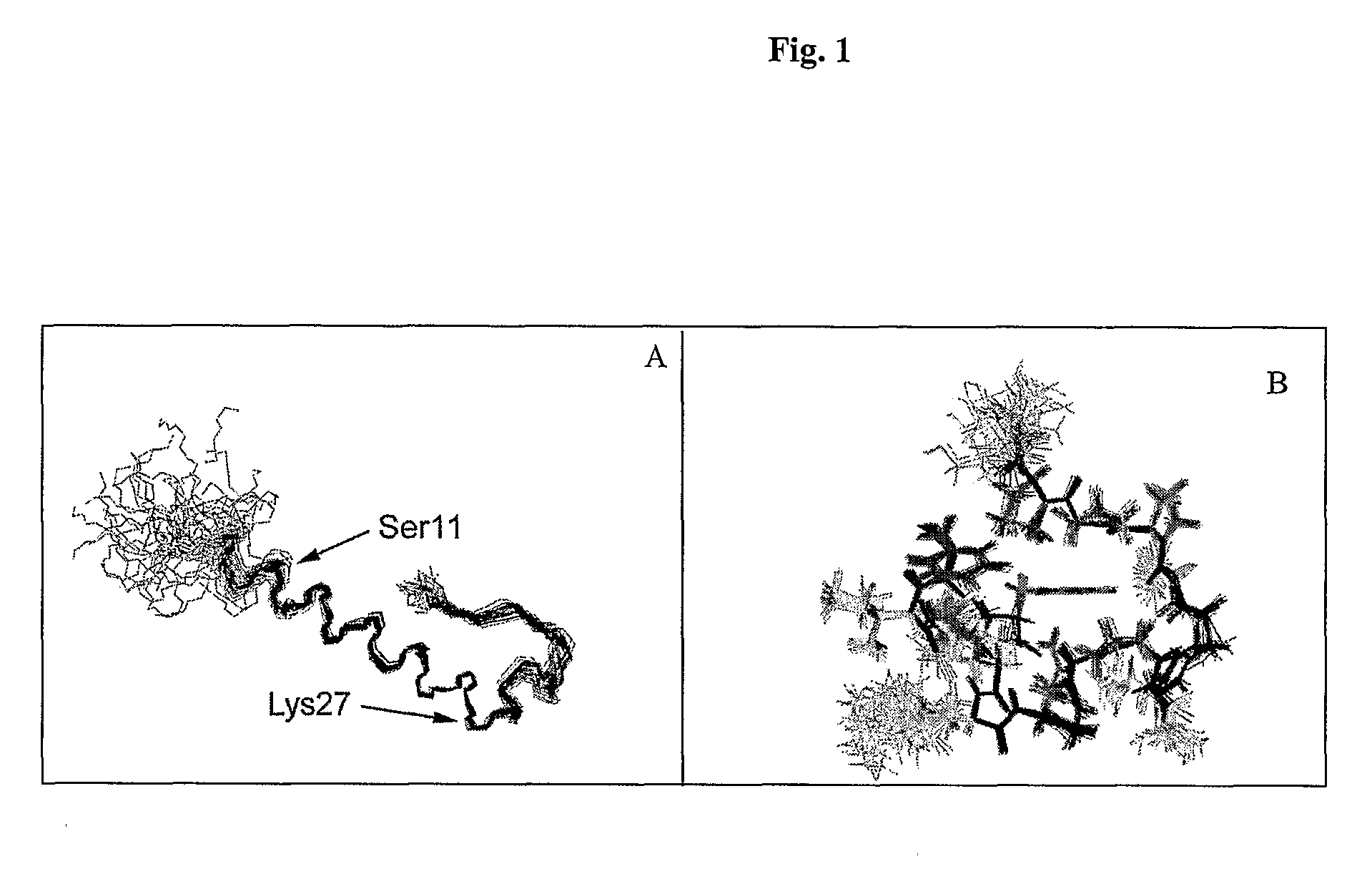
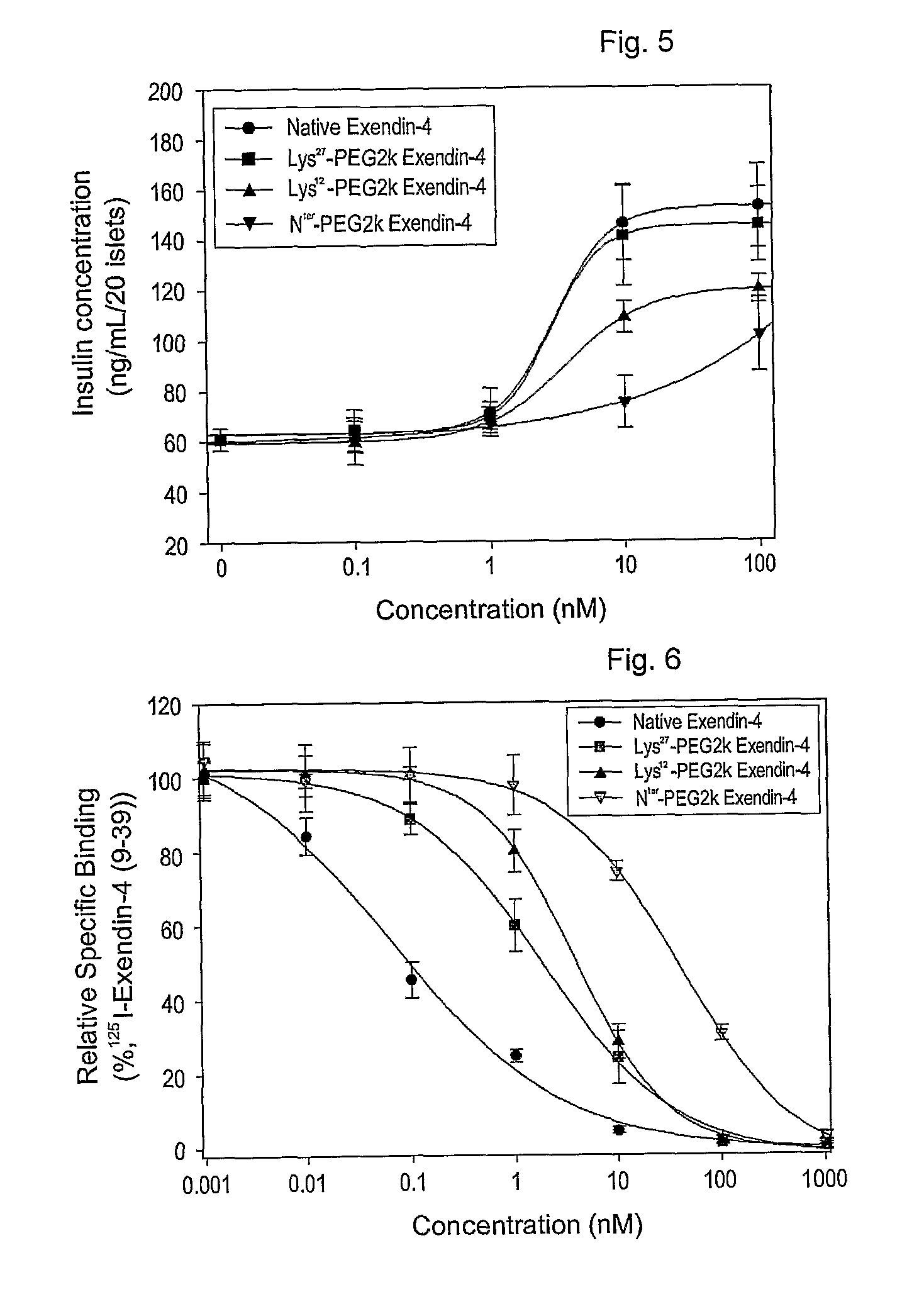
Disclosed herein are exendin singly modified with polyethylene glycole or a derivative thereof, a method for the preparation of the same, and uses thereof. Exendin modified at lysine (27) with polyethylene glycol shows biological activity similar to that of natural exendin, but is improved in half life. In addition, the modification position and the number of PEG or its derivative are restricted so as to minimize the side effects caused by a variety of combinations of such factors. The exendin is useful in the prevention and treatment of diseases caused by the over-secretion of insulin, or diseases caused due to a decrease in plasma glucose level, the inhibition of gastric or intestinal motility, the promotion of satiety, or the inhibition of food intake, especially diabetes, obesity and irritable colon syndrome.
Owner:D&D PHARMATECH INC
Rapid test for glycated albumin in saliva
InactiveUS20100167306A1Accurate assessmentMicrobiological testing/measurementBiological material analysisMeasuring instrumentPlasma Albumin
This invention describes a rapid immunochromatographic assay for measuring the ratio of glycated albumin to total albumin in saliva. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:SMITH HENRY JOHN
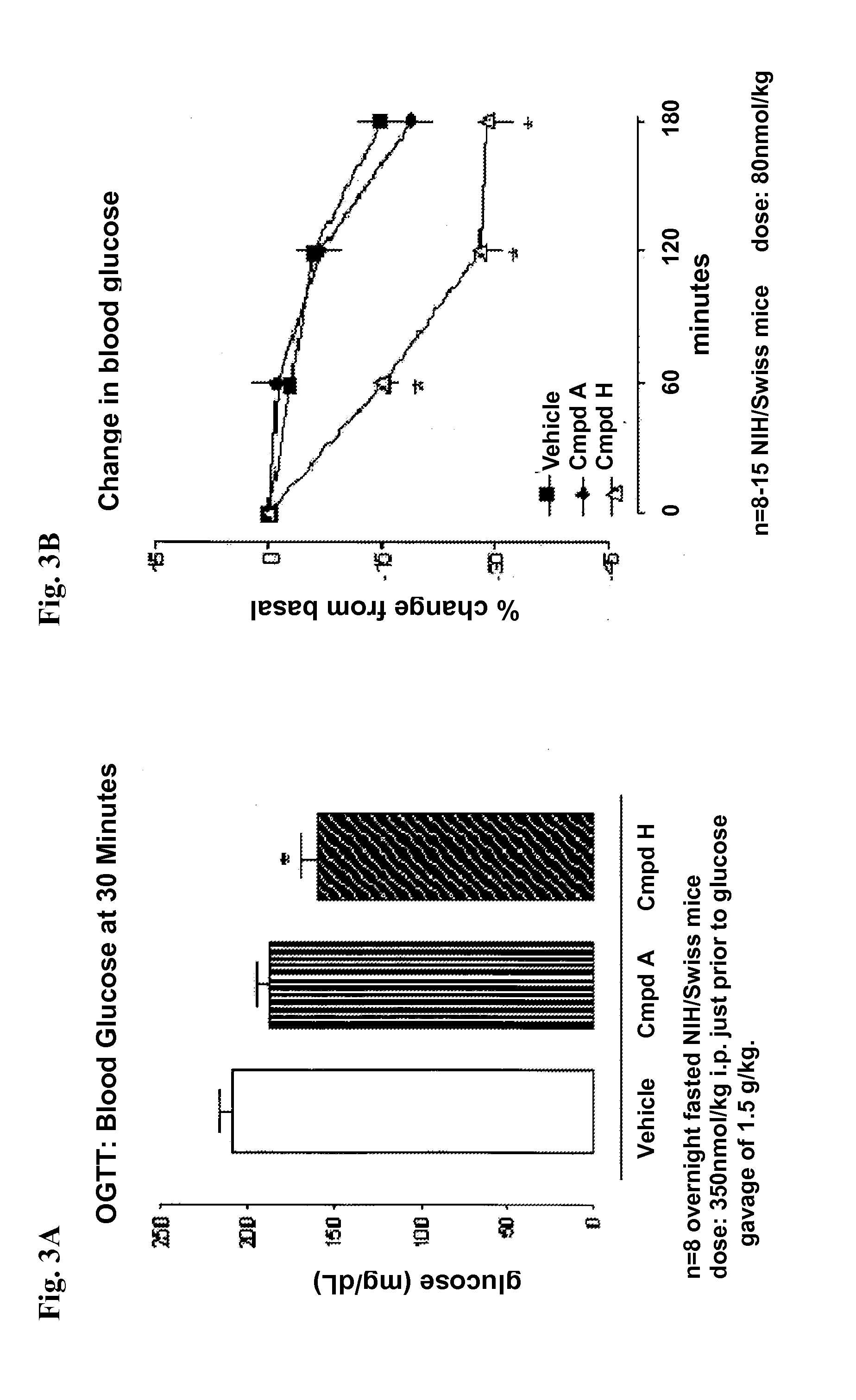
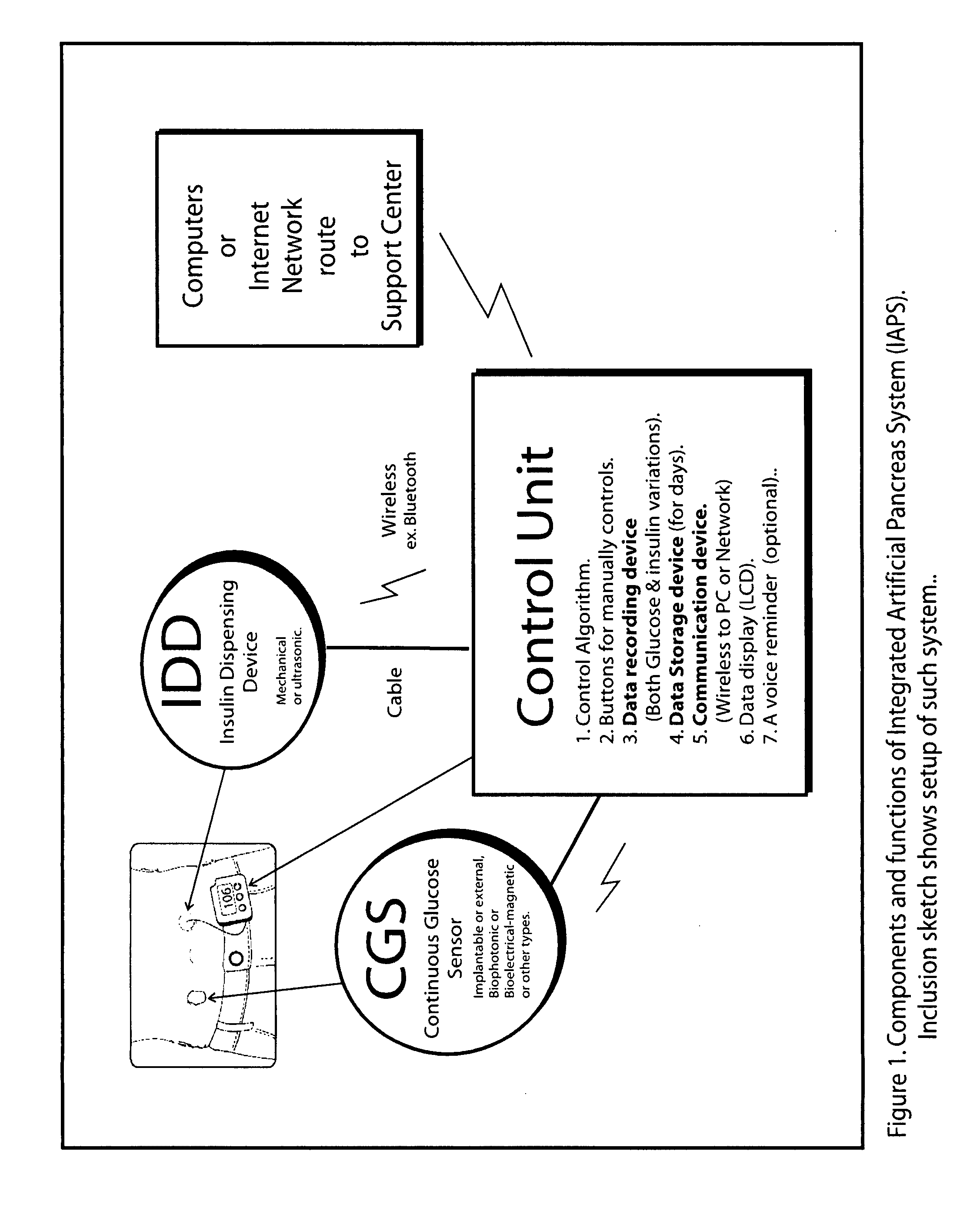
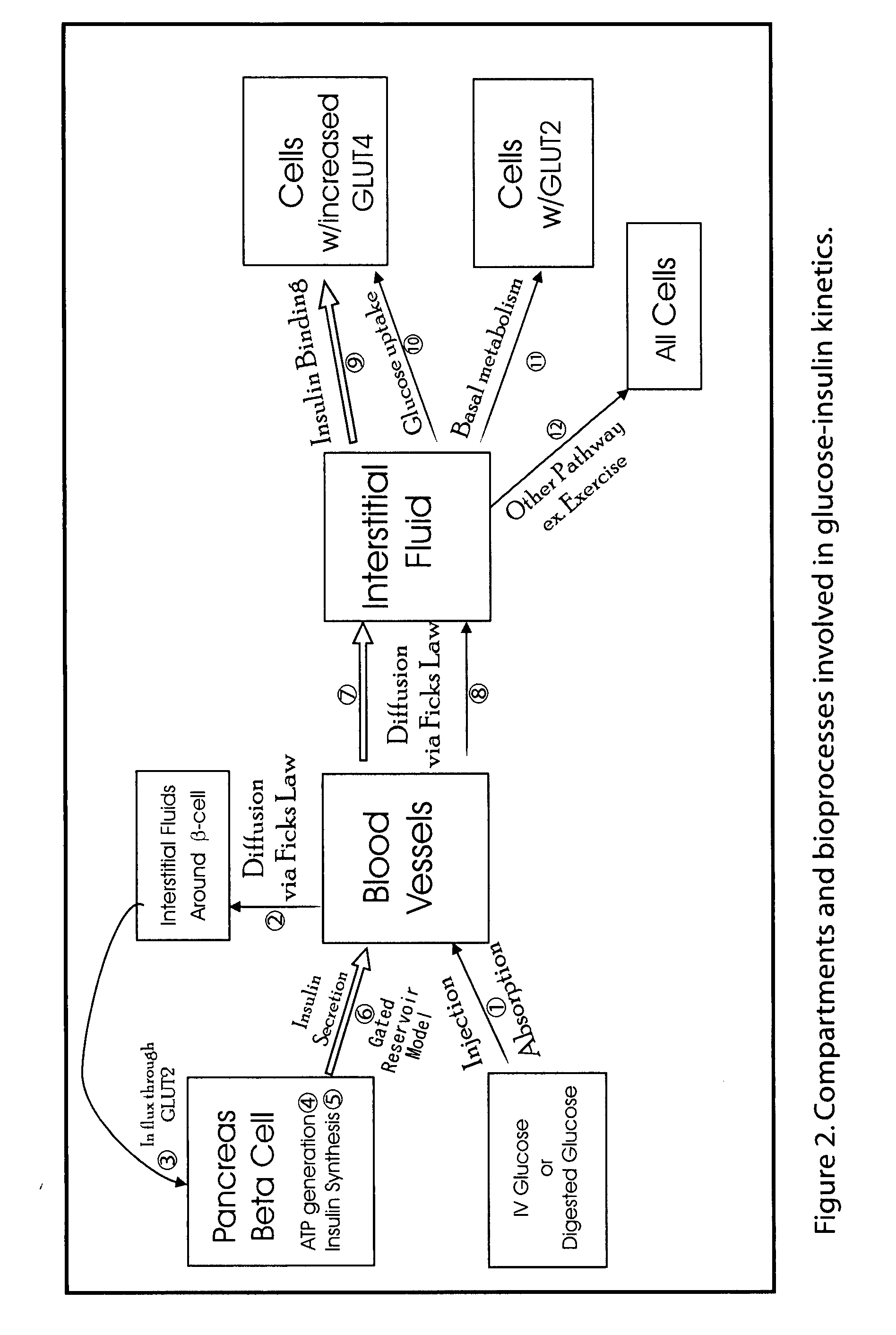
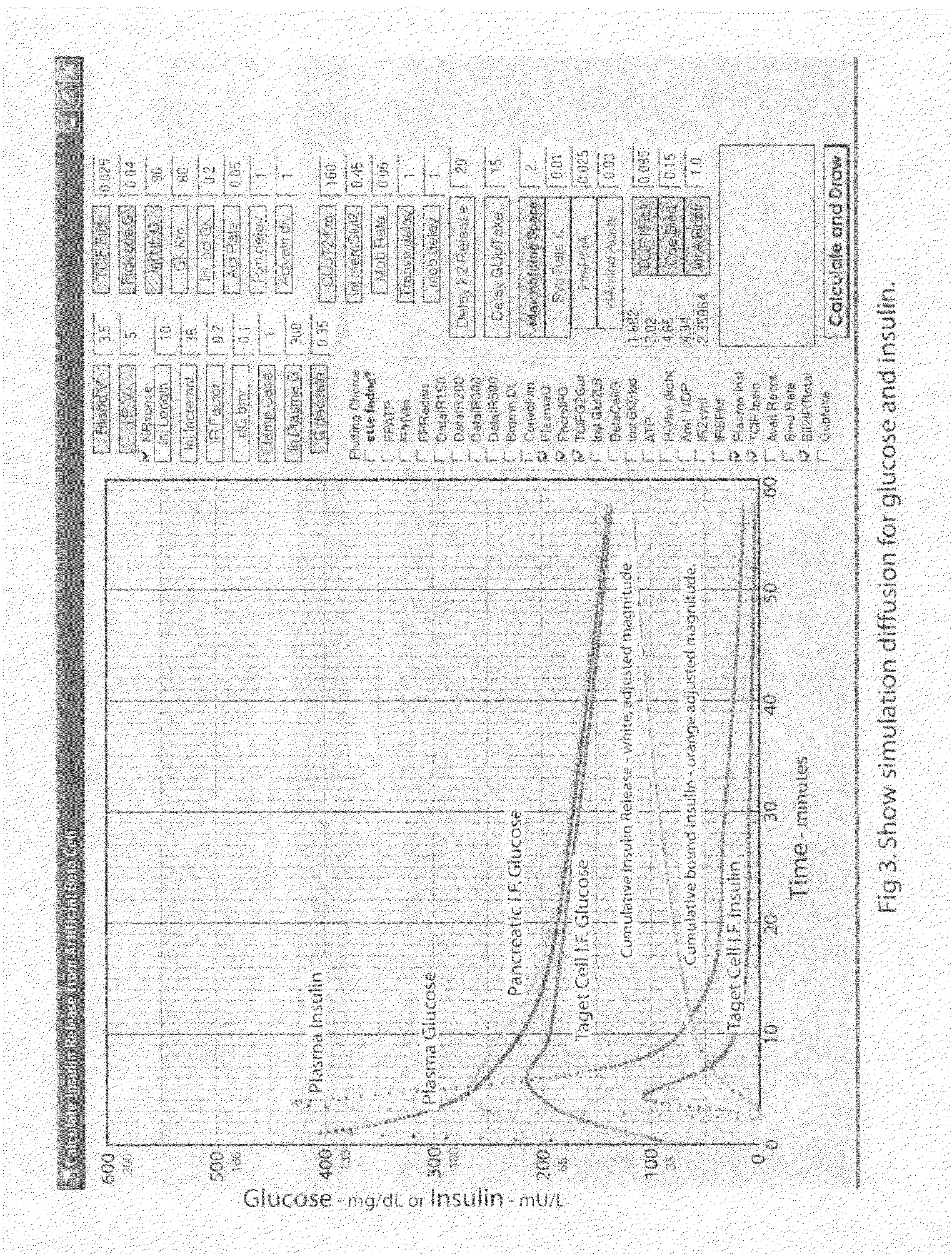
Beta cell mimicking control algorithm for artificial pancreas
The invention is a method of controlling an artificial pancreas. The method includes reading and recording a current plasma glucose level. The method includes computing glucose in beta cell if a current plasma glucose level is greater than a threshold value, and continuing the step of reading and recording a current plasma glucose level if a current plasma glucose level is less than a threshold value. The method includes calling ATP functions for interpolation. The method includes calculating a holding capacity. The method includes calculating a synthesized insulin amount. The method includes comparing amount of insulin with the holding capacity. The method includes releasing insulin if the amount of insulin is greater than the holding capacity, and not releasing insulin if the amount of insulin is less than the holding capacity.
Owner:CHANG SYHHONG
GIP analog and hybrid polypeptides with selectable properties
InactiveUS8404637B2Convenient treatmentReduce gastric emptyingSenses disorderNervous disorderDyslipidemiaFeeding disorder
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Rapid test for glycated albumin in blood
InactiveUS20090246801A1Accurate assessmentBiological material analysisBiological testingMeasuring instrumentBlood plasma
This invention describes a rapid assay for measuring the ratio of glycated albumin to total albumin in blood. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. The ratio of glycated albumin to total albumin in blood will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:MEDYTOX SOLUTIONS
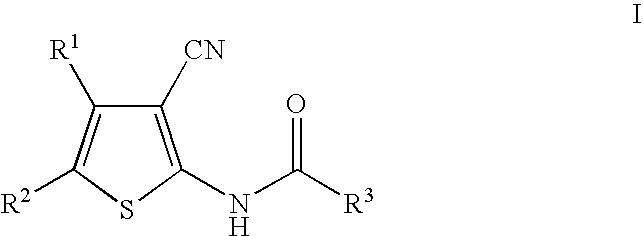
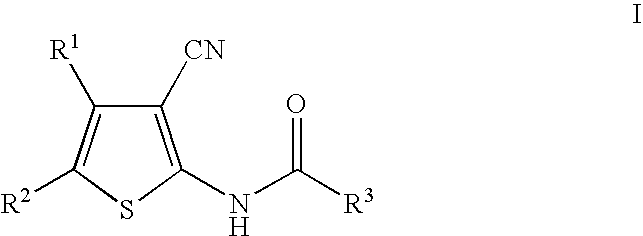
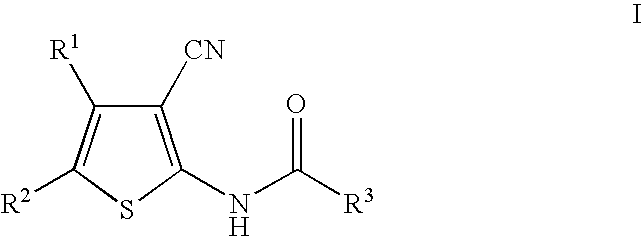
Cyanothiophene derivatives, compositions containing such compounds and methods of use
The present invention addresses substituted cyanothiophene derivatives of the formula I:as well as compositions containing such compounds and methods of treatment. The compounds in the present invention are glucagon antagonists. The compounds block the action of glucagon at its receptor and thereby decrease the levels of plasma glucose providing a treatment of diabetes.
Owner:MERCK SHARP & DOHME CORP
Animal model for type II diabetes mellitus and Syndrome X and methods and uses thereof
InactiveUS20070271620A1Relieve stressNo pressureMetabolism disorderMedical devicesPhysiologyPancreatic A Cells
The invention provides a method for generating a type II diabetes mellitus and / or Syndrome X model in pigs. A method of the invention comprises partially destructing pancreatic beta-cells in pigs. From these pigs, a pig is preferably selected that comprises a fasting plasma glucose level higher than 6 mmol / L. The invention further provides a pig according to the invention and uses thereof.
Owner:STICHTING DLO
Antioxidant-Enriched Fruit Extracts and Uses Thereof in the Treatment and Prevention of Diabetes and Obesity
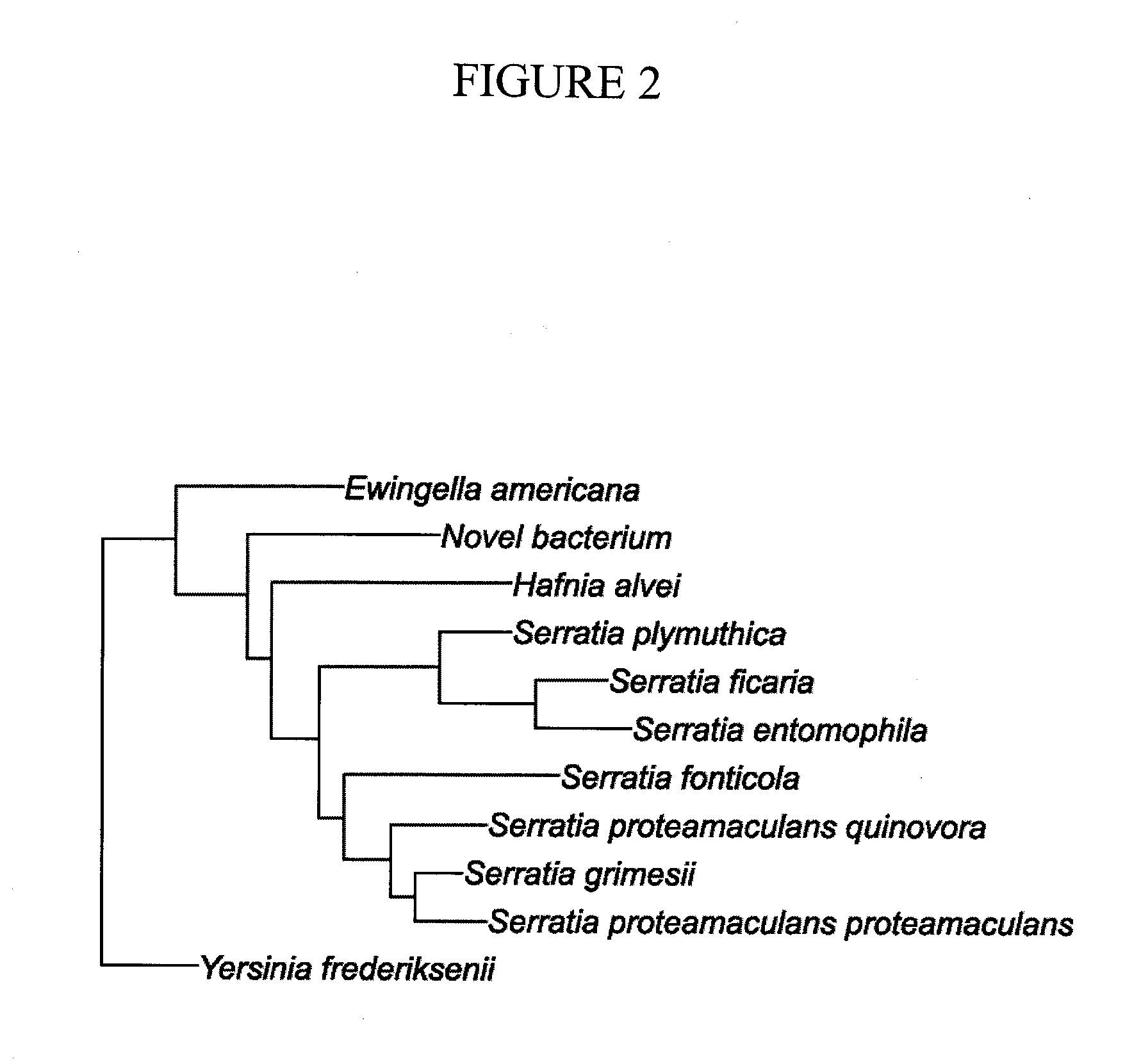
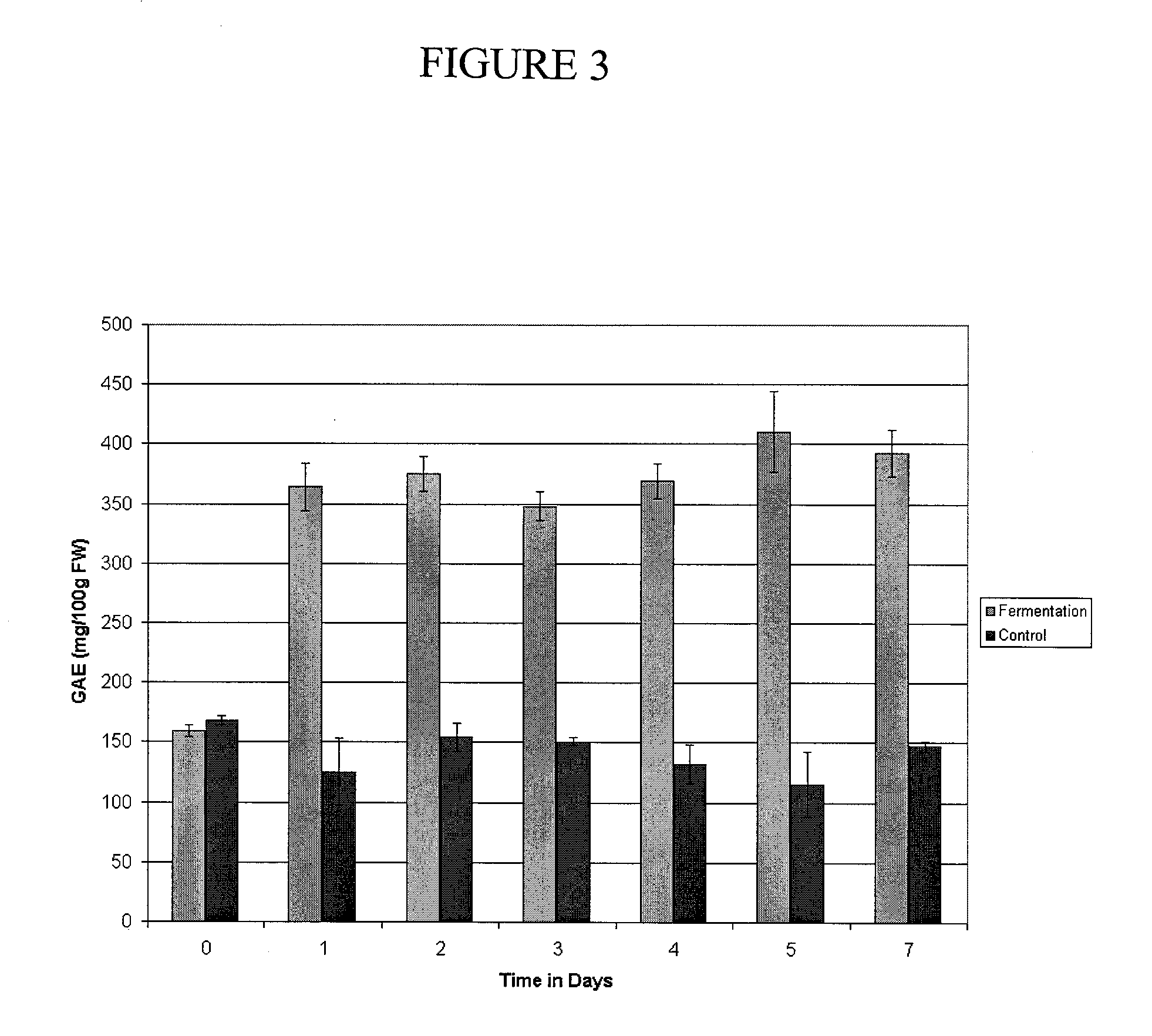
A method of treating or preventing diabetes or obesity that comprises administering an antioxidant-enriched composition produced by fermenting fruit extracts with the bacteria Serratia vaccinii. The antioxidant-enriched composition is capable of decreasing plasma glucose levels, increasing circulating adiponectin levels and decreasing hyperphagia in a subject.
Owner:UNIVERSITY OF OTTAWA
Mono modified exendin with polyethylene glycol or its derivatives and uses thereof
ActiveUS8420598B2Extended half-lifeMinimize side effectsPeptide/protein ingredientsMetabolism disorderDiseaseSide effect
Disclosed herein are exendin singly modified with polyethylene glycole or a derivative thereof, a method for the preparation of the same, and uses thereof. Exendin modified at lysine (27) with polyethylene glycol shows biological activity similar to that of natural exendin, but is improved in half life. In addition, the modification position and the number of PEG or its derivative are restricted so as to minimize the side effects caused by a variety of combinations of such factors. The exendin is useful in the prevention and treatment of diseases caused by the over-secretion of insulin, or diseases caused due to a decrease in plasma glucose level, the inhibition of gastric or intestinal motility, the promotion of satiety, or the inhibition of food intake, especially diabetes, obesity and irritable colon syndrome.
Owner:D&D PHARMATECH INC
Synthetic apolipoprotein e mimicking polypeptides and methods of use
The present invention provides methods for using synthetic apolipoprotein E (ApoE)-mimicking peptides. Also disclosed are provides methods for using synthetic apolipoprotein E (ApoE)-mimicking peptides to reduce plasma glucose levels. Methods of using the disclosed apolipoprotein E (ApoE)-mimicking peptides to treat diabetes and diabetic complications are also disclosed.
Owner:UAB RES FOUND
Application of salvia miltiorrhiza extracts in preparing protein tyrosine phosphatase 1B inhibitor and drug for preventing and/or treating type 2 diabetes
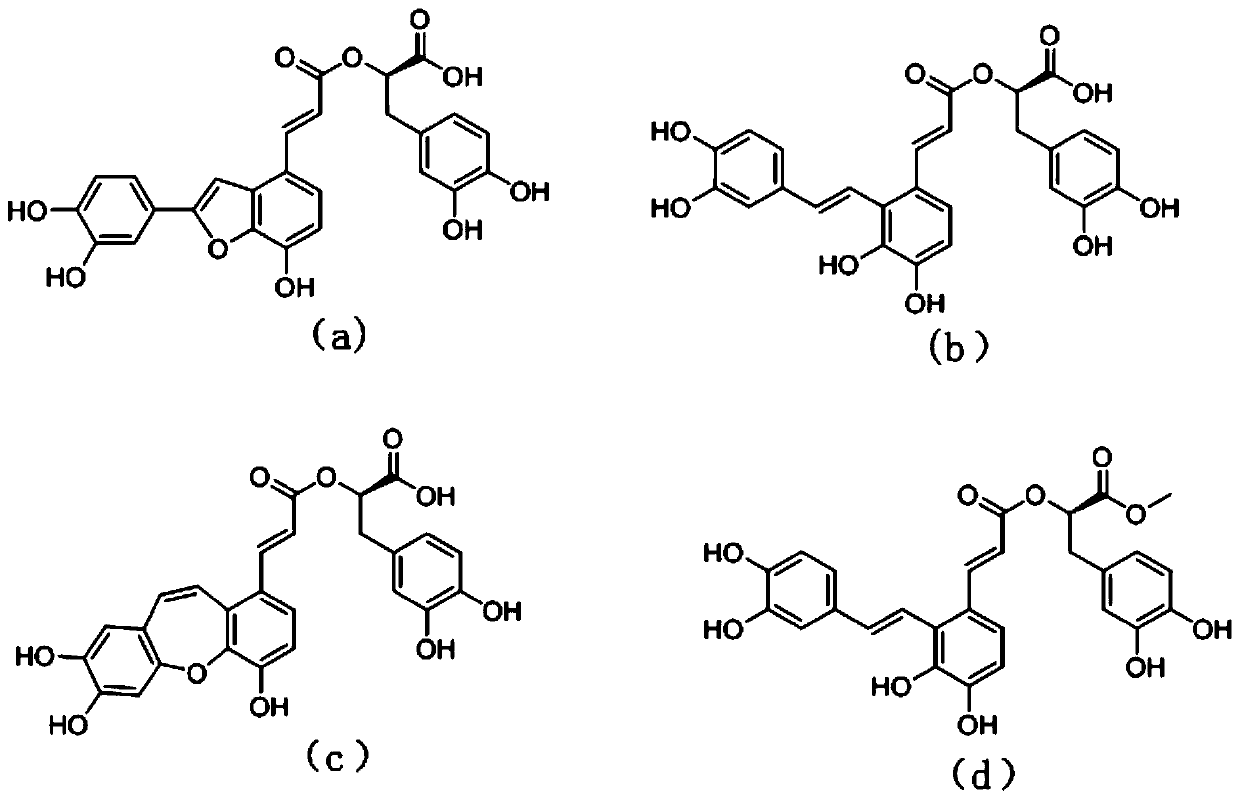
The invention discloses application of salvia miltiorrhiza extracts in preparing a protein tyrosine phosphatase 1B inhibitor and a drug for preventing and / or treating type 2 diabetes. The salvia miltiorrhiza extracts salvianolic acid C, salvianolic acid A, danshinolic acid C or salvianolic acid A methyl ester can significantly inhibit the activity of protein tyrosine phosphatase 1B, further increase the glucose uptake rate, reduce the plasma glucose levels and prevent and / or treat the type 2 diabetes. The salvianolic acid C, the salvianolic acid A, the danshinolic acid C or the salvianolic acid A methyl ester are used as active ingredients to make a pharmaceutical preparation which can be used as the protein tyrosine phosphatase 1B inhibitor to prevent and / or treat the type 2 diabetes.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
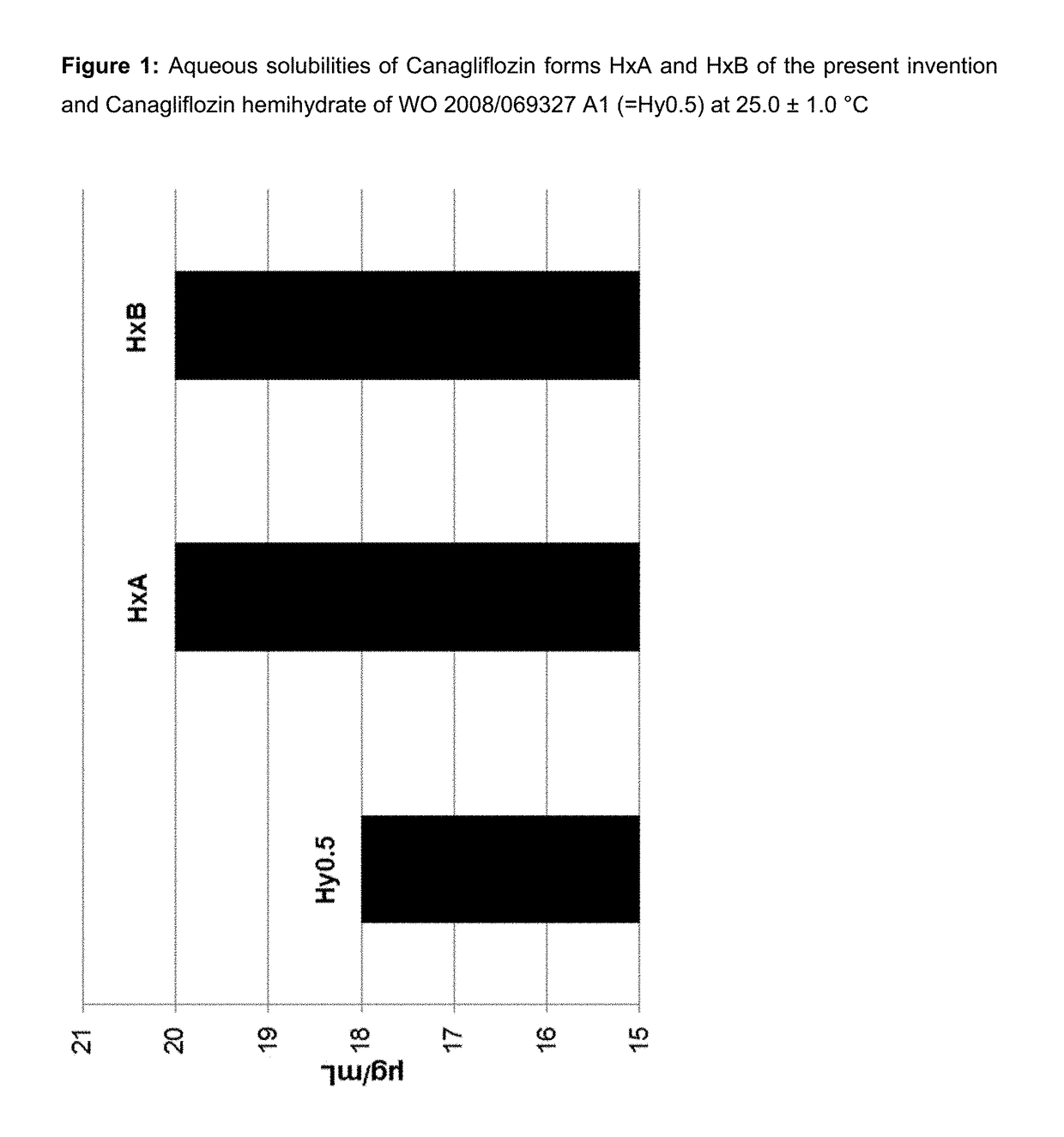
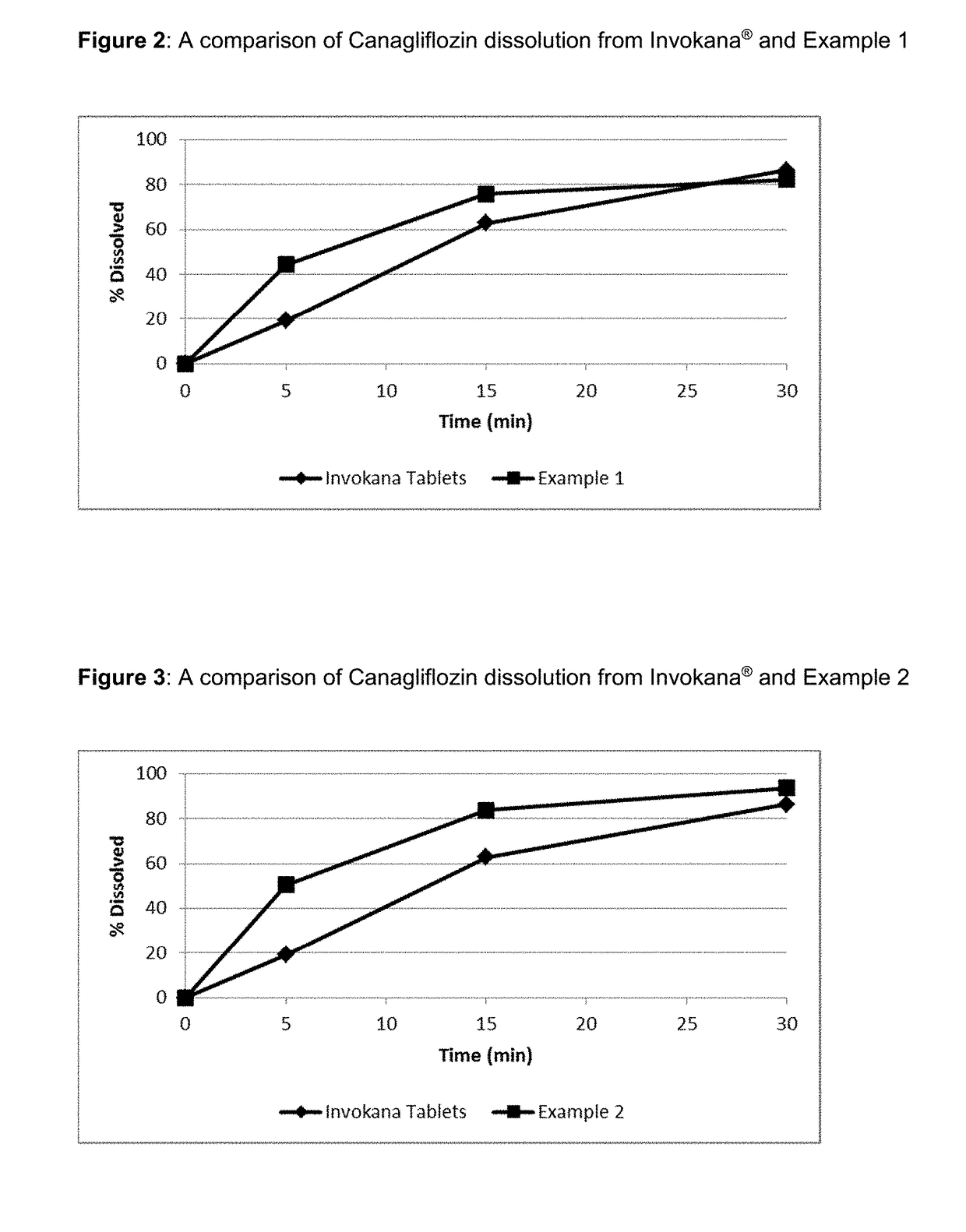
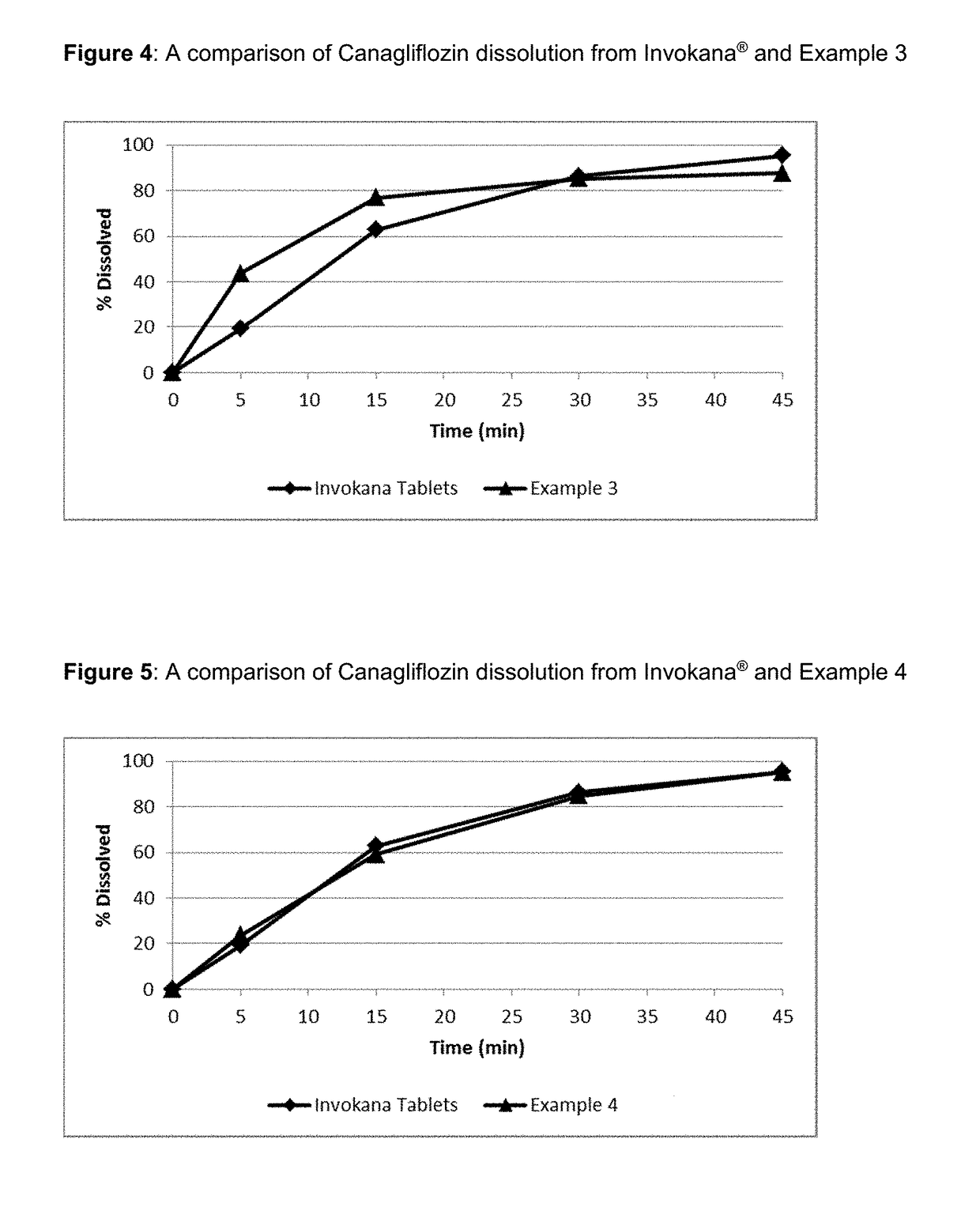
Pharmaceutical Compositions Comprising Canagliflozin
InactiveUS20170258761A1Improve solubilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderMedicinePharmaceutical industry
The present invention belongs to the field of pharmaceutical industry and relates to a dry pharmaceutical composition comprising Canagliflozin, as well as to a process for preparing the same. Such dry pharmaceutical composition is useful as a medicament, especially for the normalization of plasma glucose levels.
Owner:SANDOZ AG
Novel antidiabetic furostanolic saponin rich (FSR) fraction from fenugreek seeds
InactiveUS20120071427A1Effective treatmentEasily and conveniently formulatedBiocideOrganic active ingredientsOral treatmentAdditive ingredient
The present invention discloses a novel anti-diabetic composition extracted from fenugreek seeds. The same comprises a furostanolic-saponin-rich fraction (>70%) with approximately 30% protodioscin as one of the bioactive components. Pre-clinical studies in rats indicated significant glucose lowering effect of the fraction (31.5%) as compared to control after two weeks of oral treatment. Clinical studies in human volunteers indicated suitability of a dosage form of 500 mg given once or twice daily as anti-diabetic agent either alone or in combination with standard, synthetic anti-diabetic drugs such as metformin and glipizide in controlling plasma glucose levels.
Owner:GOEL PAWAN KUMAR
Functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus
InactiveCN104056271AWorsening fatty liverThe role of exacerbating type 2 diabetes diseaseMetabolism disorderGenetic material ingredientsIntraperitoneal routeStaining
The invention discloses functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus. Studies on a TRAF 5 gene by a high-fat diet (HFD) induced model discover that both the body weight and fasting plasma glucose level of an HFD bred TRAF gene knock-out mouse are lower than those of a WT mouse; glucose tolerance tests by intraperitoneal injection discover that the glucose tolerance of the TRAF5 gene knock-out mouse is remarkably reinforced; results of on liver gross appearance, liver weight, liver / body weight ratio and lipid component pathological staining indicate that the TRAF5-KO mouse fatty liver lesion in the HFD group is remarkably improved, the lipid accumulation is remarkably reduced, and the TRAF5 gene knock-out has the effects of remarkably improving fatty acid and type-II diabetes mellitus. Against the effects, the TRAF5 gene knock-out can be used as a medicinal target for screening and treating fatty liver and / or type-II diabetes mellitus, and the inhibitor of the TRAF5 gene knock-out can be used for preparing medicaments for treating fatty liver and / or type-II diabetes mellitus.
Owner:WUHAN UNIV
Preparation method of oral liquid for treating diabetes
InactiveCN107929341AMaintain ultrastructureAvoid thickeningMetabolism disorderPharmaceutical delivery mechanismAdditive ingredientFiltration
The invention relates to the technical field of health caring, in particular to a preparation method of oral liquid for treating diabetes. Firstly, ligularia intermedia and Chinese aralis are cleanlycleaned; polysaccharide substances and total saponin substances in the ligularia intermedia and the Chinese aralis can effectively prevent the continuous rise of the plasma glucose level; the ultra microstructure of the kidney is maintained; the substrate membrane thickening is avoided, so that the filtration function of the glomerulus is normal; through the joint fermentation of lactobacilli andsaccharomycetes, ingredients for maintaining the blood glucose are converted into beneficial substances capable of being easily digested and absorbed by the human body; the molecular weight of the effective medicine ingredients is reduced; new microbial metabolism secondary products and other probiotics are generated; under the condition of co-existence of the saccharomycetes and the lactobacilli,lactic acid is converted into lactose; the lactose is decomposed to be converted into galactose; the synergistic effect is achieved; the content of ingredients, with the blood glucose reduction effect, in the fermented ligularia intermedia and Chinese aralis is improved; the diabetes is better treated in an auxiliary way.
Owner:蒋文明
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com