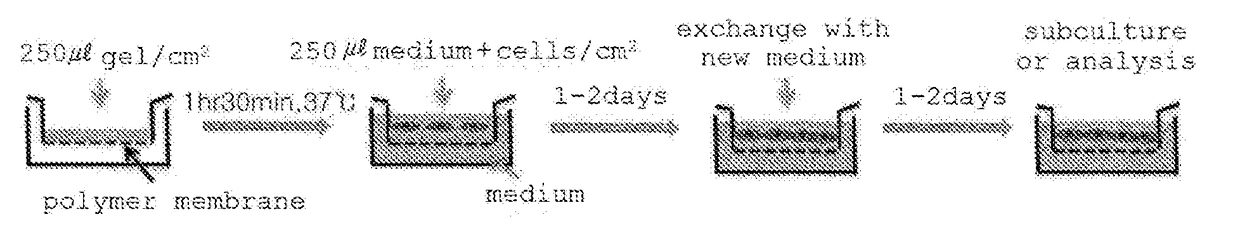
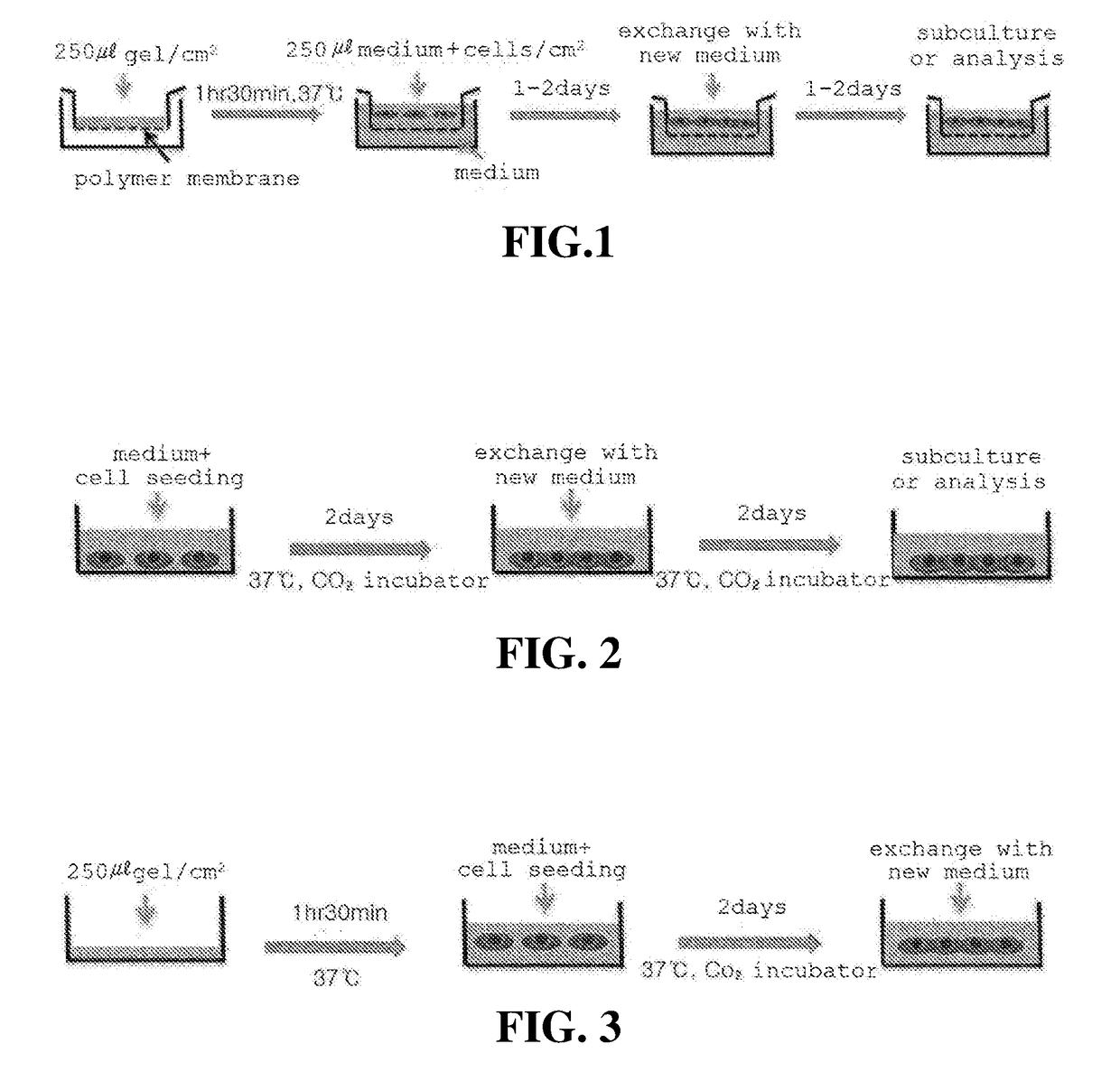
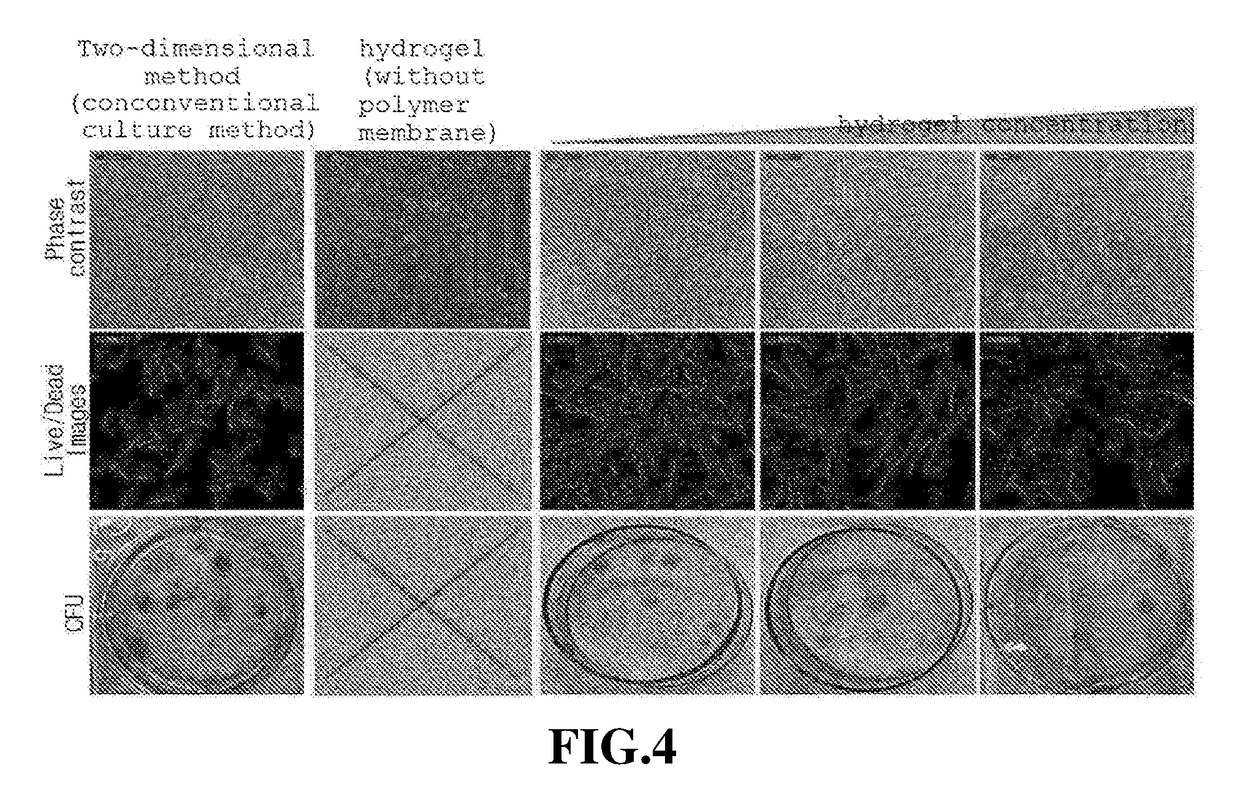
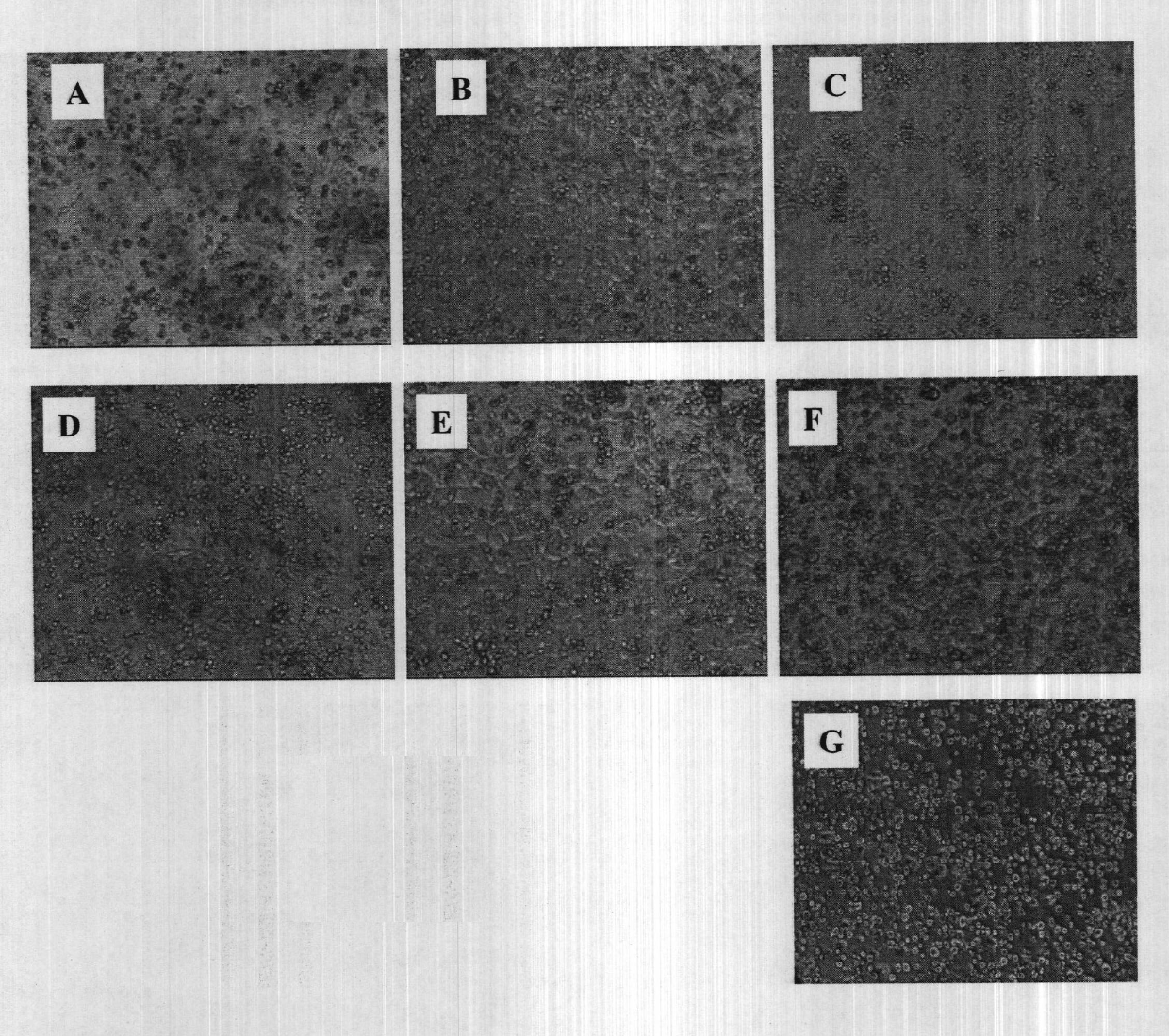
Patents
Literature
55 results about "Xeno free" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of Culturing Retinal Pigmented Epithelium Cells, Including Xeno-Free Production, RPE Enrichment, and Cryopreservation
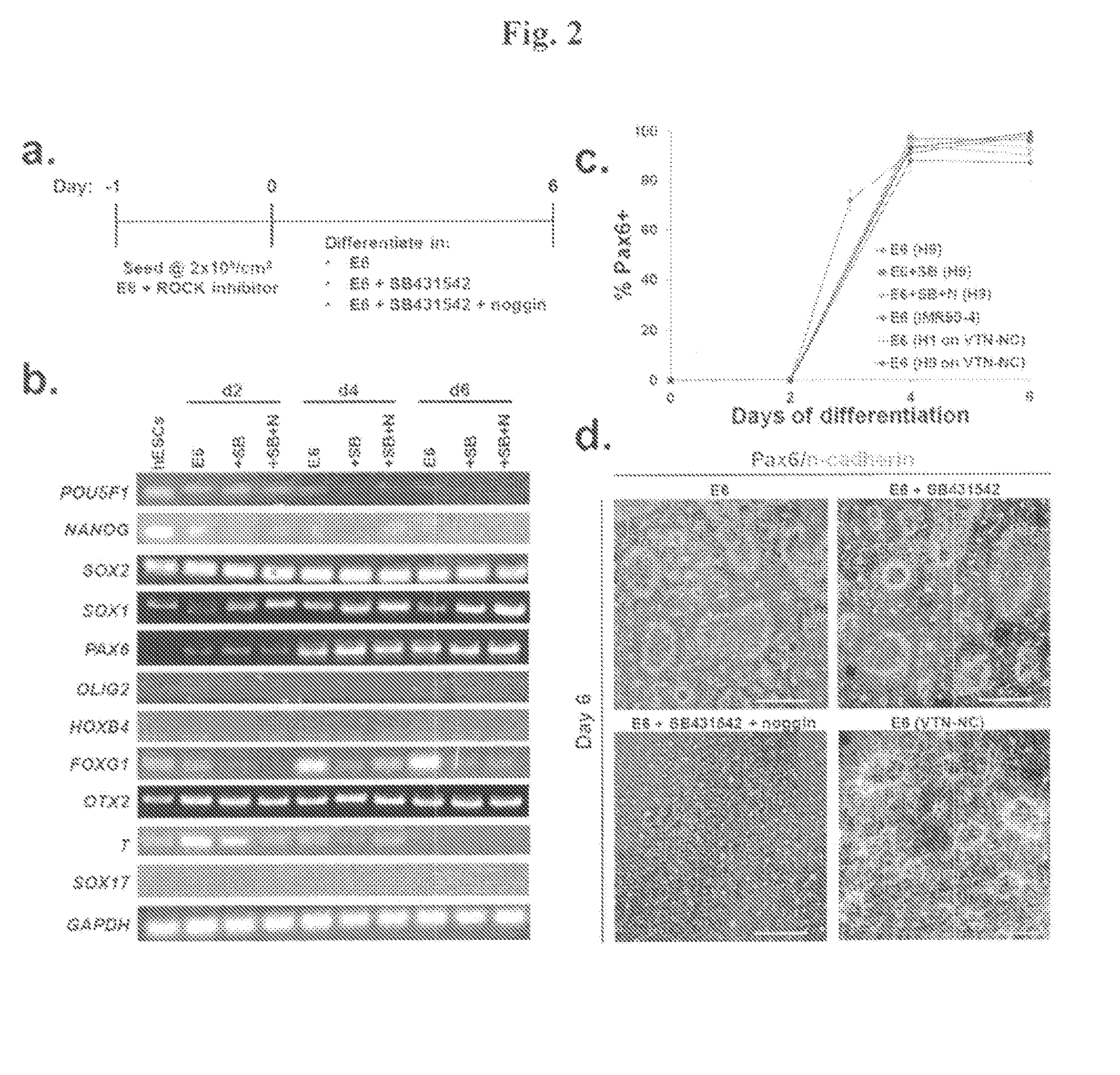
The production of high quality retinal pigmented epithelium (RPE) cells is necessary for research and potential therapeutic uses. Especially desirable are methods for the production of RPE cells using xeno-free culture conditions. Disclosed herein are novel methods for the production of RPE cells from pluripotent cells with high yields, including xeno-free production methods. Also provided are methods of efficiently isolating RPE cells from cultures containing heterogeneous cell types, allowing for substantially pure RPE cell cultures to be established. Additionally, novel methods for the cryopreservation of RPE cells are provided.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Media for culturing stem cells
Well-defined, xeno-free culture media which comprise a TGF-beta isoform or the chimera formed between IL6 and the soluble IL6 receptor (IL6RIL6), which are capable of maintaining stem cells, and particularly, human embryonic stem cells, in an undifferentiated state are provided. Also provided are cell cultures comprising the culture media and the stem cells and methods of expanding and deriving embryonic stem cells in such well-defined, xeno-free culture media. In addition, the present invention provides methods of differentiating ESCs or EBs formed therefrom for the generation of lineage specific cells.
Owner:TECHNION RES & DEV FOUND LTD
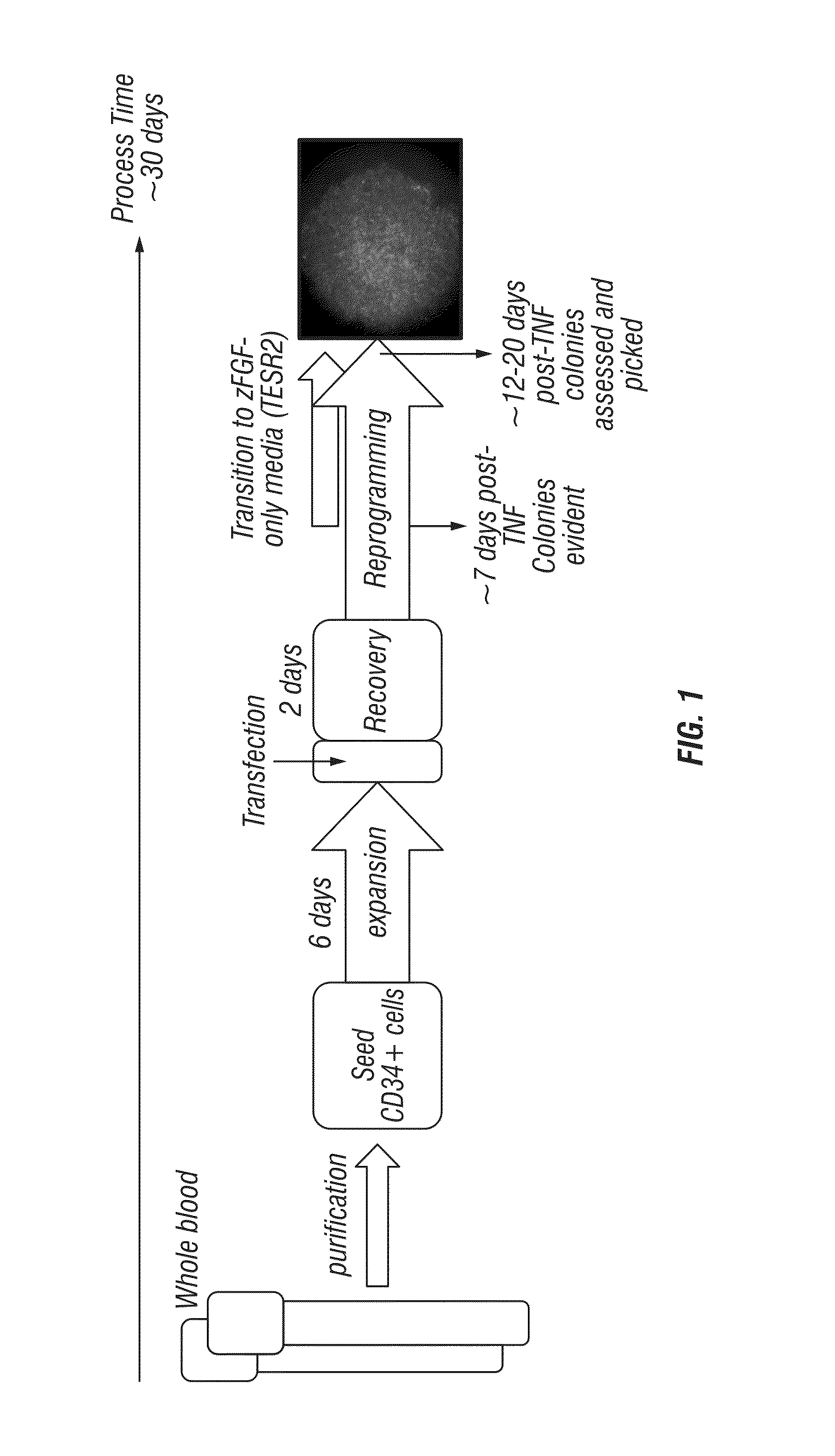
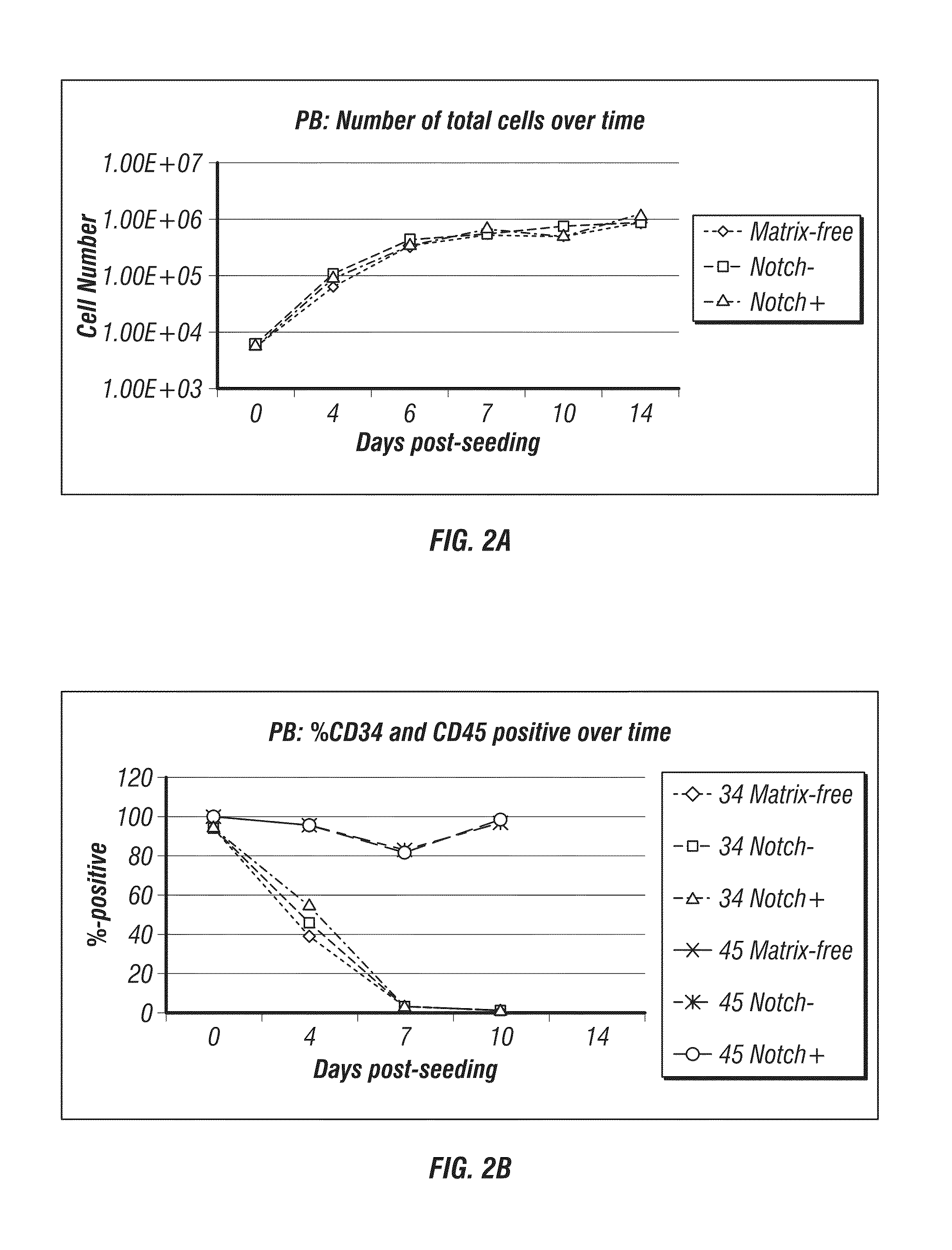
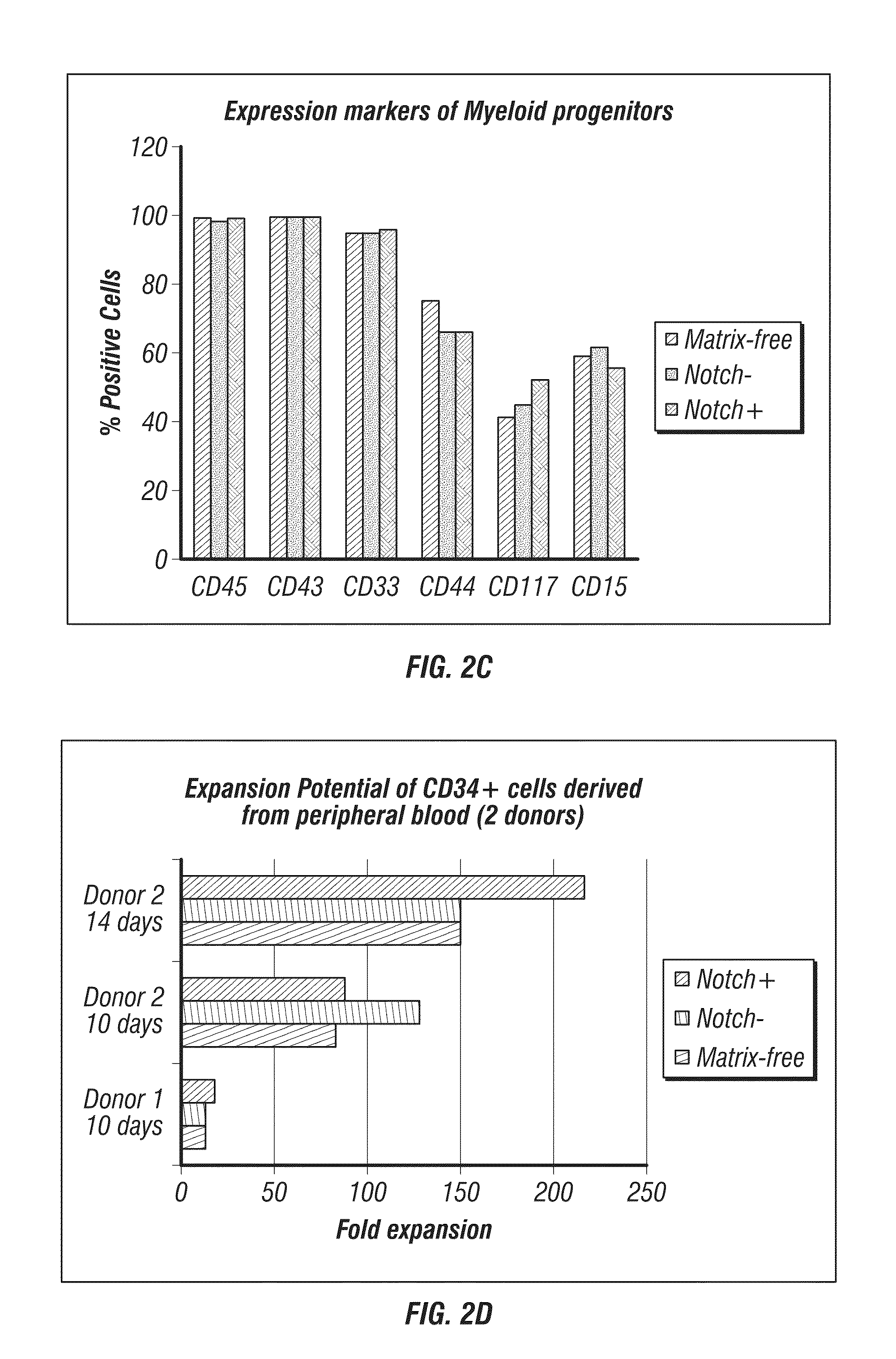
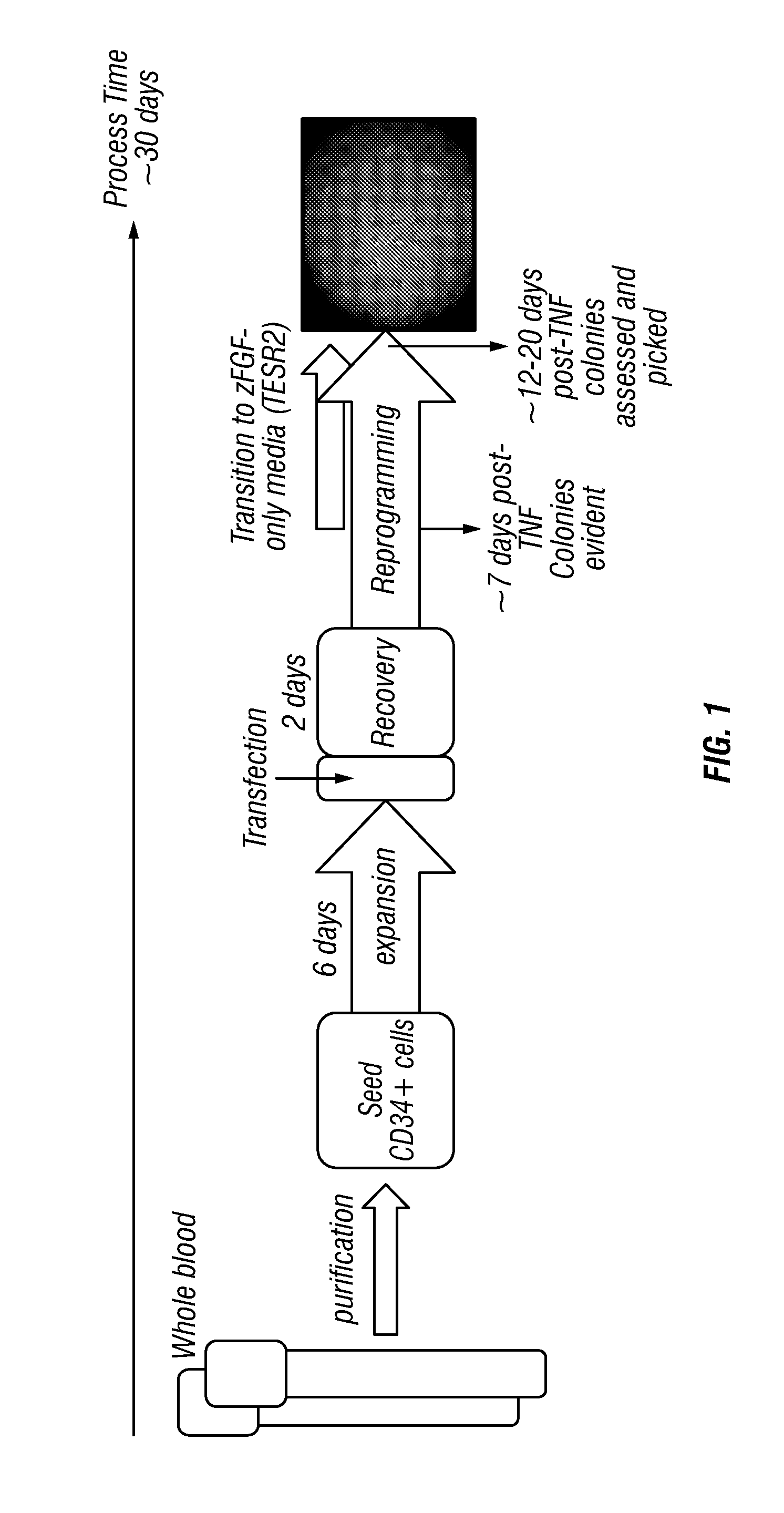
Generation of induced pluripotent stem cells from small volumes of peripheral blood
ActiveUS8691574B2Improve efficiencyLower the volumeGenetically modified cellsCulture processProgenitorReprogramming
Owner:FUJIFILM CELLULAR DYNAMICS INC
Generation of induced pluripotent stem cells from small volumes of peripheral blood
ActiveUS20120009676A1Improve processing efficiencyLower the volumeGenetically modified cellsCulture processProgenitorReprogramming
Methods and compositions relating to the production of induced pluripotent stem cells (iPS cells) are disclosed. For example, induced pluripotent stem cells may be generated from peripheral blood cells, such as human blood progenitor cells, using episomal reprogramming and feeder-free or xeno-free conditions. In certain embodiments, the invention provides novel methods for improving overall reprogramming efficiency with low number of blood progenitor cells.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Simplified basic media for human pluripotent cell culture
ActiveUS20120202291A1Maximize percentageCulture processArtificial cell constructsCellular viabilityBiology
Owner:WISCONSIN ALUMNI RES FOUND
Human Mesenchymal stem cells and preparation thereof
InactiveUS20100105132A1Artificial cell constructsSkeletal/connective tissue cellsMesenchymal stem cellXeno free
The present invention provides a process of isolation, proliferation and / or maintenance of mesenchymal stem cells (MSCs). The invention further provides a culture medium for proliferation and / or maintenance of human mesenchymal stem cells in xeno-free conditions. The culture medium provided in the present invention proliferates and / or maintains mesenchymal stem cell expansion while maintaining a multipotent phenotype.
Owner:STEMPEUTICS RES PRIVATE
Methods of preparing feeder cells-free, xeno-free human embryonic stem cells and stem cell cultures prepared using same
ActiveUS20060051862A1Culture processCell culture mediaStem cell cultureHuman embryonic stem cell line
The present invention is of methods of establishing and propagating human embryonic stem cell lines using feeder cells-free, xeno-free culture systems and stem cells which are capable of being maintained in an undifferentiated, pluripotent and proliferative state in culture which is free of xeno contaminants and feeder cells.
Owner:TECHNION RES & DEV FOUND LTD
Methods of preparing feeder cells-free, xeno-free human embryonic stem cells and cell cultures prepared using same
ActiveUS7592175B2Nervous system cellsBlood/immune system cells3D cell cultureHuman embryonic stem cell line
The present invention is of methods of establishing and propagating human embryonic stem cell lines using feeder cells-free, xeno-free culture systems and stem cells which are capable of being maintained in an undifferentiated, pluripotent and proliferative state in culture which is free of xeno contaminants and feeder cells.
Owner:TECHNION RES & DEV FOUND LTD
Methods of culturing retinal pigmented epithelium cells, including xeno-free production, RPE enrichment, and cryopreservation
The production of high quality retinal pigmented epithelium (RPE) cells is necessary for research and potential therapeutic uses. Especially desirable are methods for the production of RPE cells using xeno-free culture conditions. Disclosed herein are novel methods for the production of RPE cells from pluripotent cells with high yields, including xeno-free production methods. Also provided are methods of efficiently isolating RPE cells from cultures containing heterogeneous cell types, allowing for substantially pure RPE cell cultures to be established. Additionally, novel methods for the cryopreservation of RPE cells are provided.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Simplified Compositions and Methods for Generating Neural Stem Cells From Human Pluripotent Stem Cells
Simplified methods and compositions for directed differentiation of human pluripotent stem cells into neural stem cells are described. Methods and compositions for deriving neural stem cells from human pluripotent stem cells under defined, xeno-free conditions are also described.
Owner:WISCONSIN ALUMNI RES FOUND
Methods of producing rpe cells
ActiveUS20150299653A1Fast and economically efficient scale-upFacilitate scientificBiocideCulture processClinical gradeLaminin
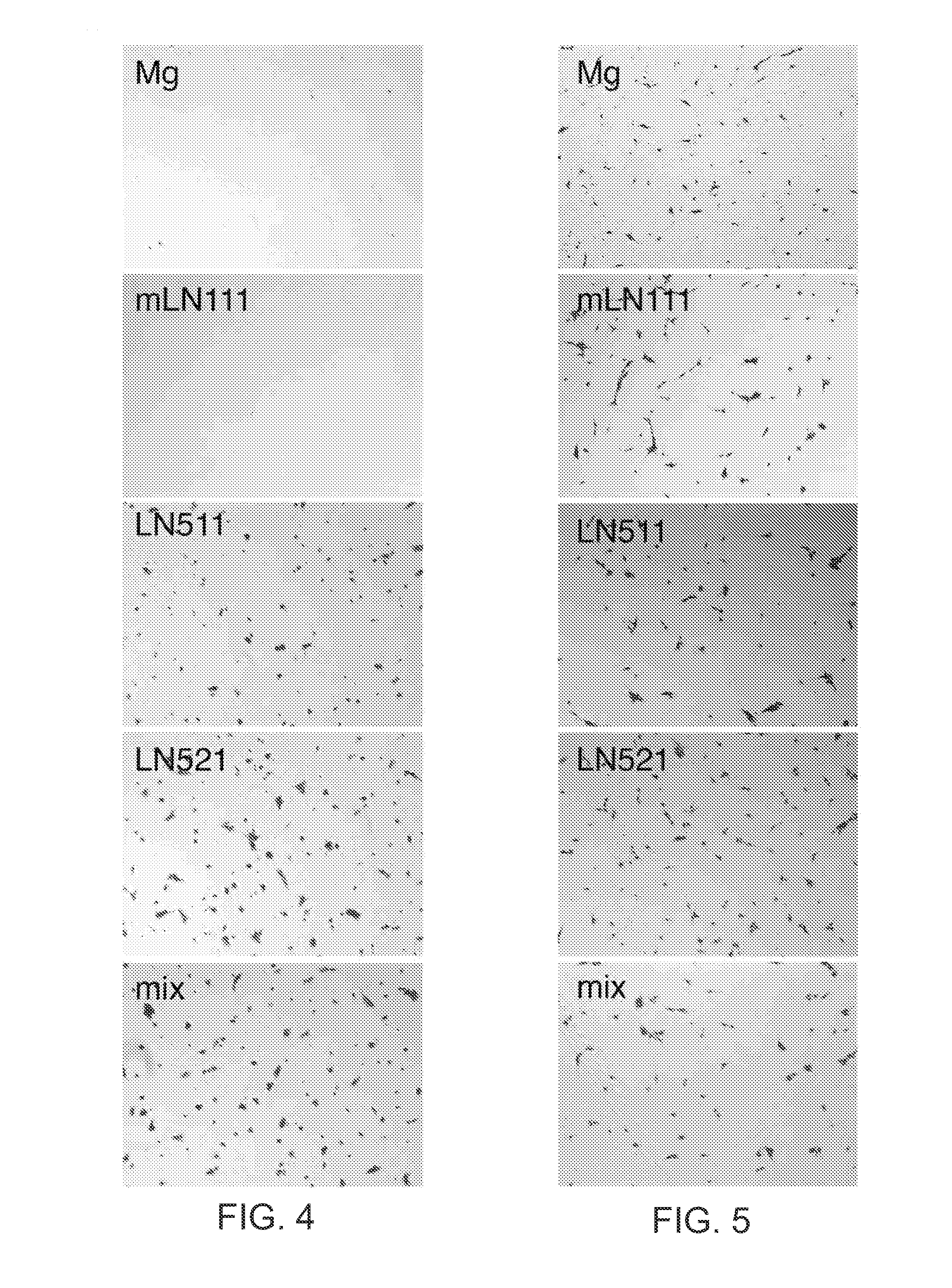
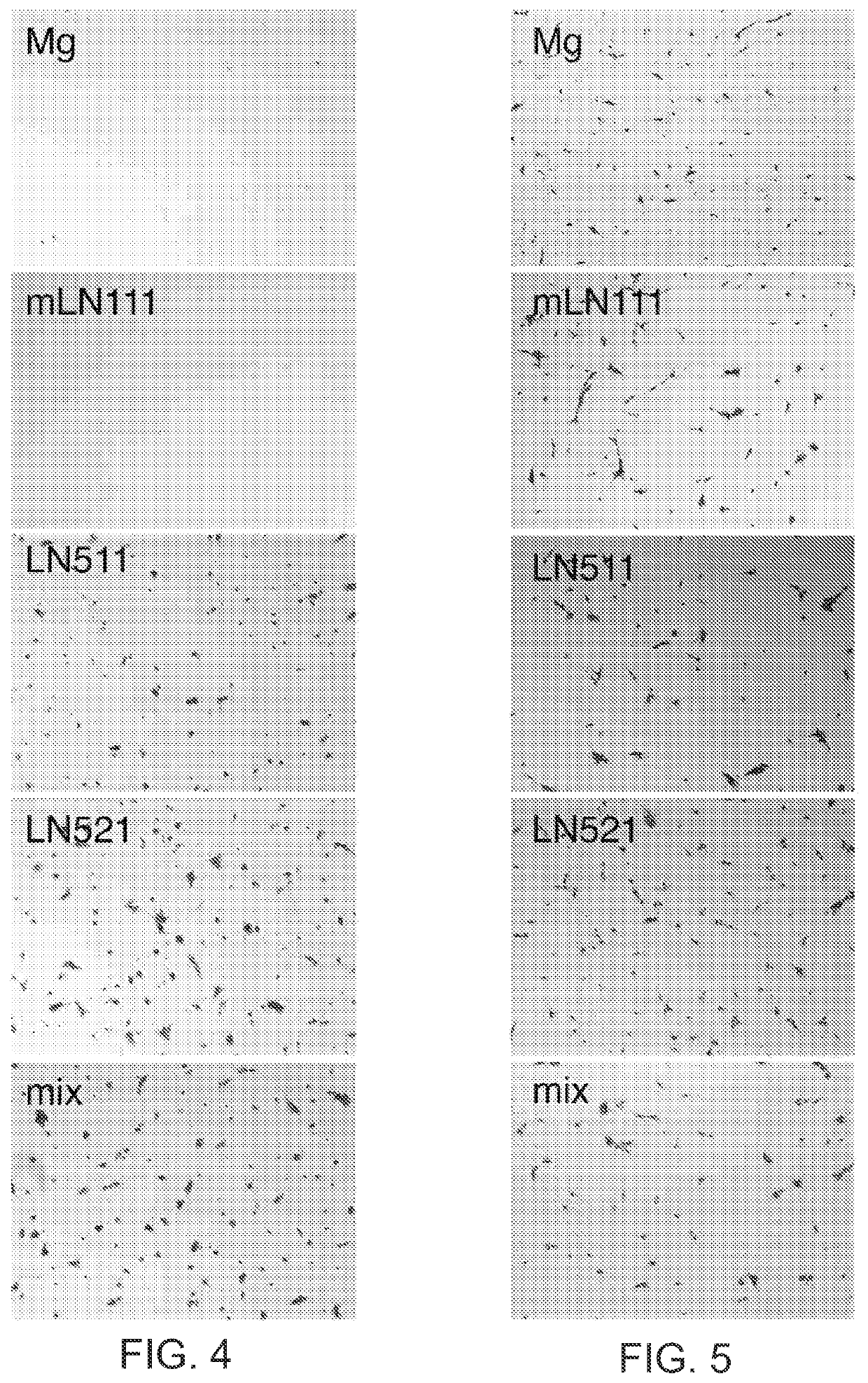
The present disclosure relates to the use of laminin-521 in obtaining retinal pigment epithelium (RPE) cells. Pluripotent human embryonic stem cells are cultured on plates coated with recombinant laminin-521 (laminin-11), in totally defined and xeno-free conditions. A first cell culture medium contains a growth factor, and a second cell culture medium does not contain growth factor. The stem cells are first exposed to the first cell culture medium, then exposed to the second cell culture medium for a longer time period. After a number of weeks, clinical grade RPE cells are obtained from the stem cells.
Owner:BIOLAMINA
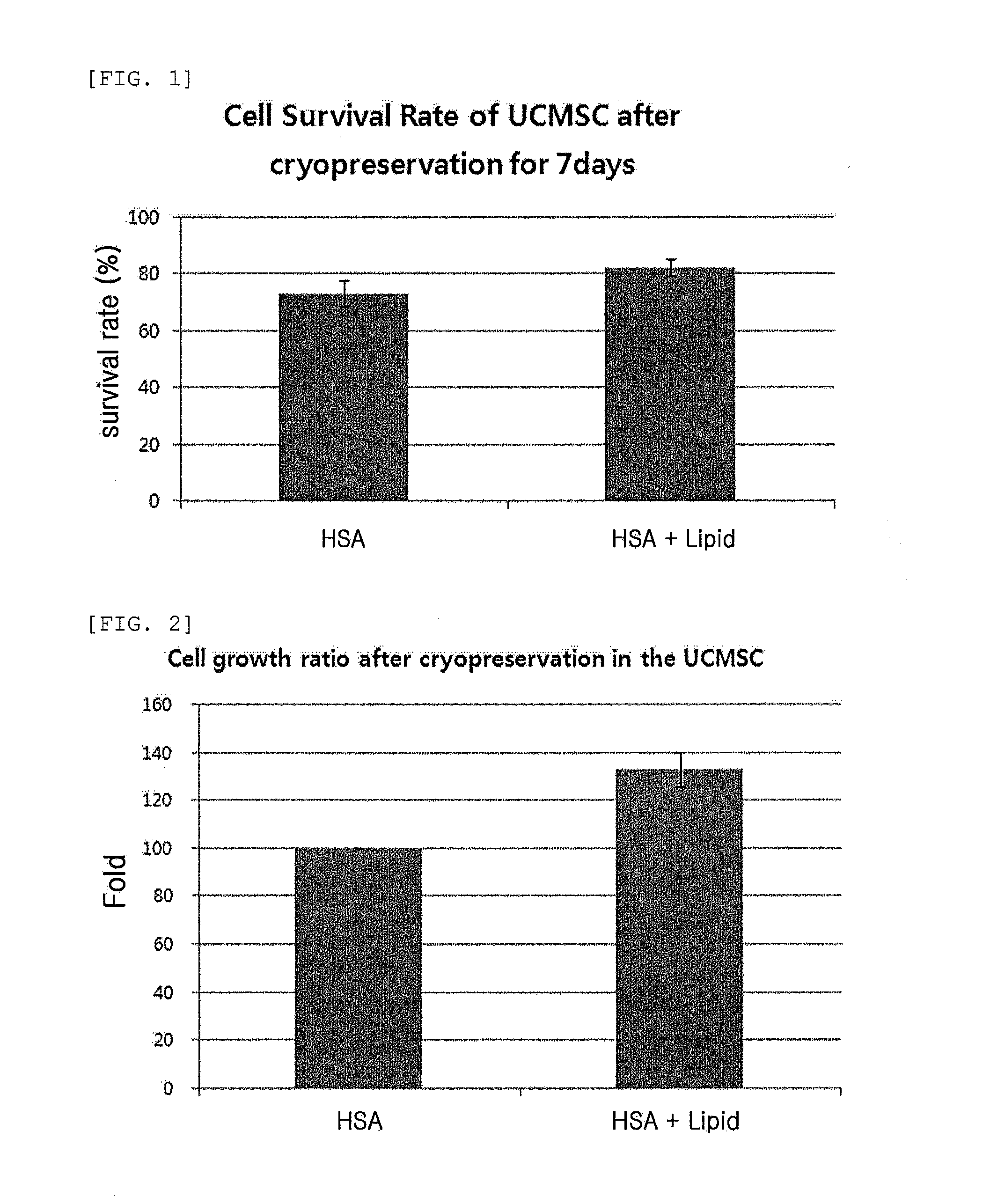
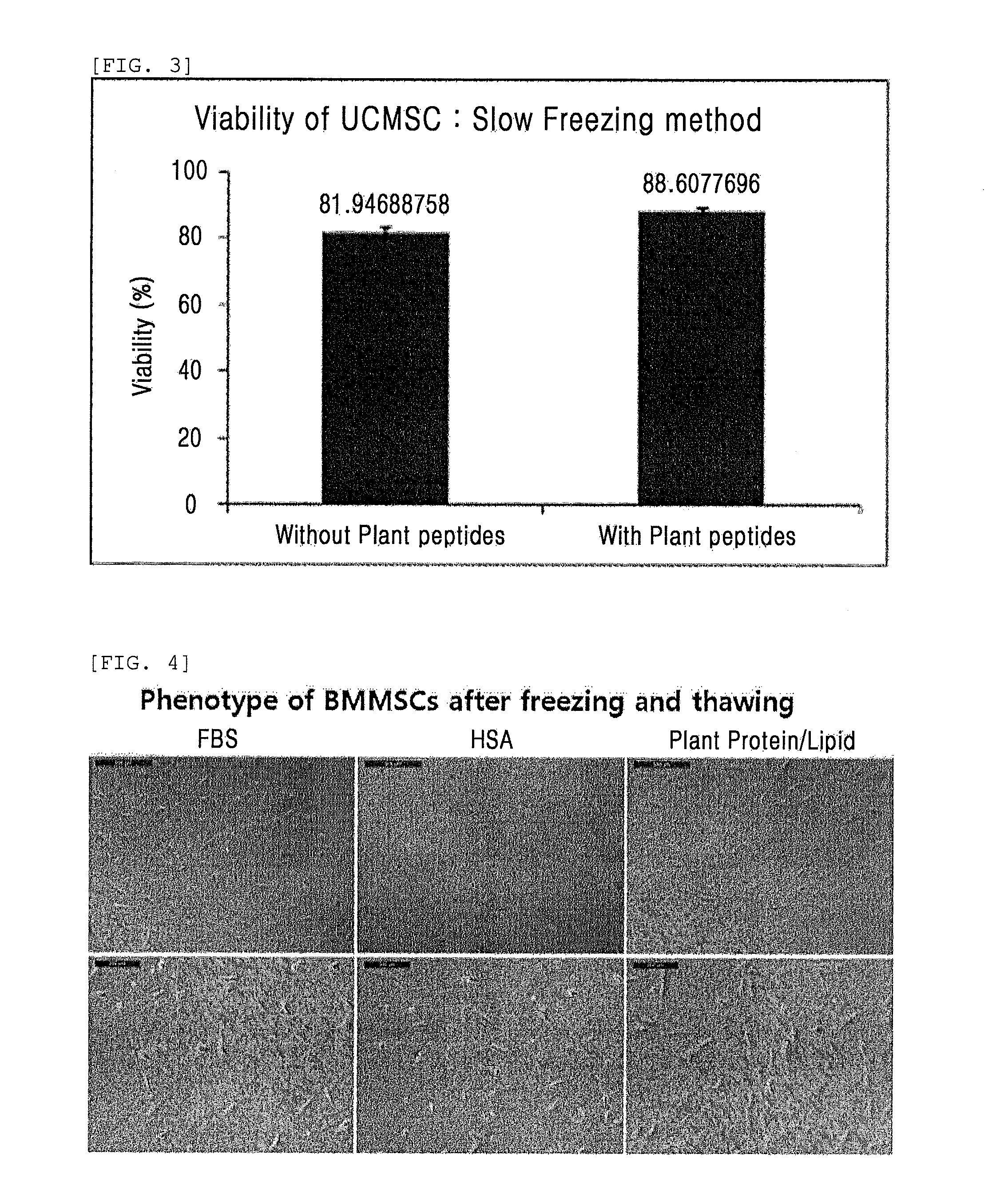
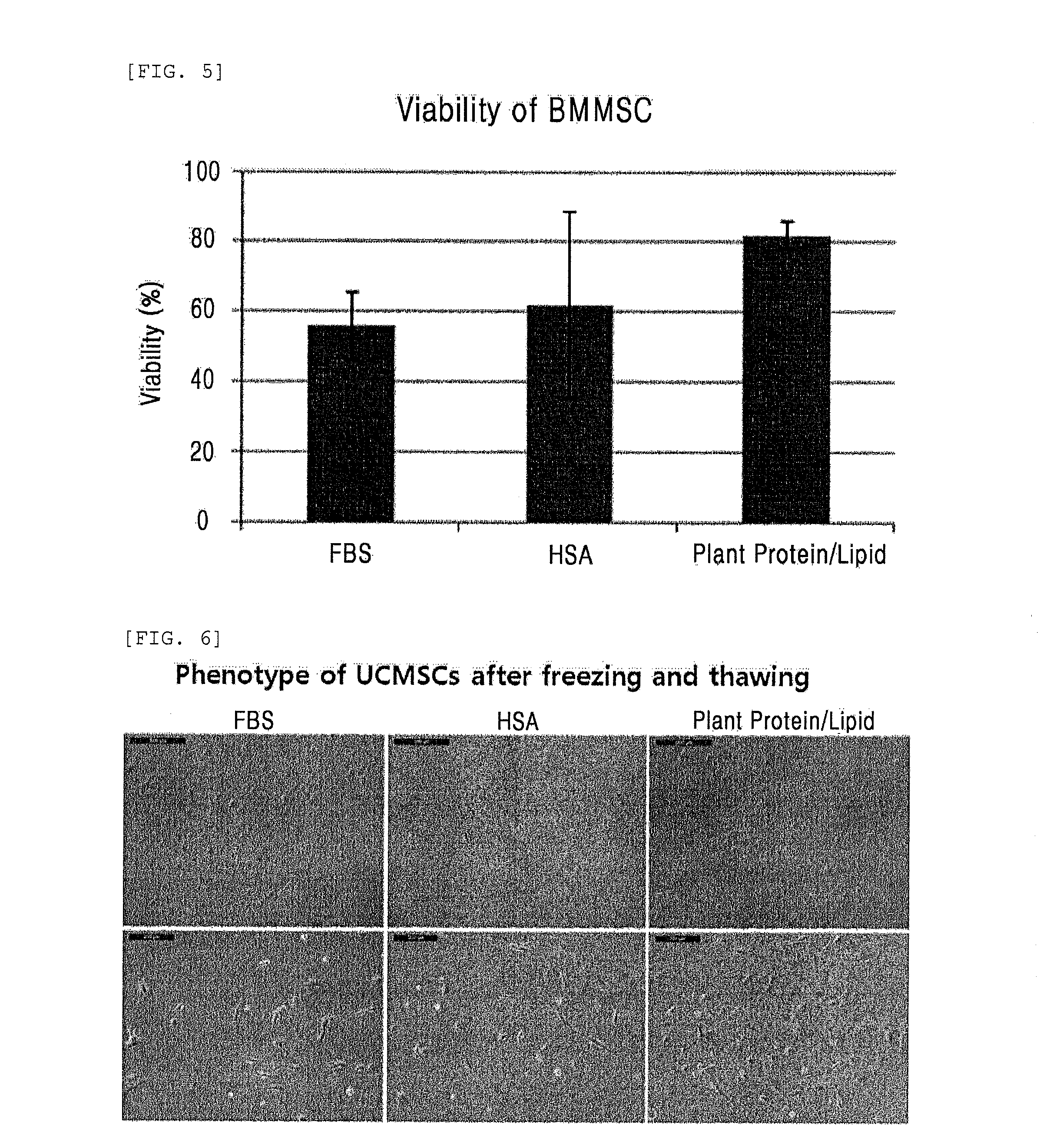
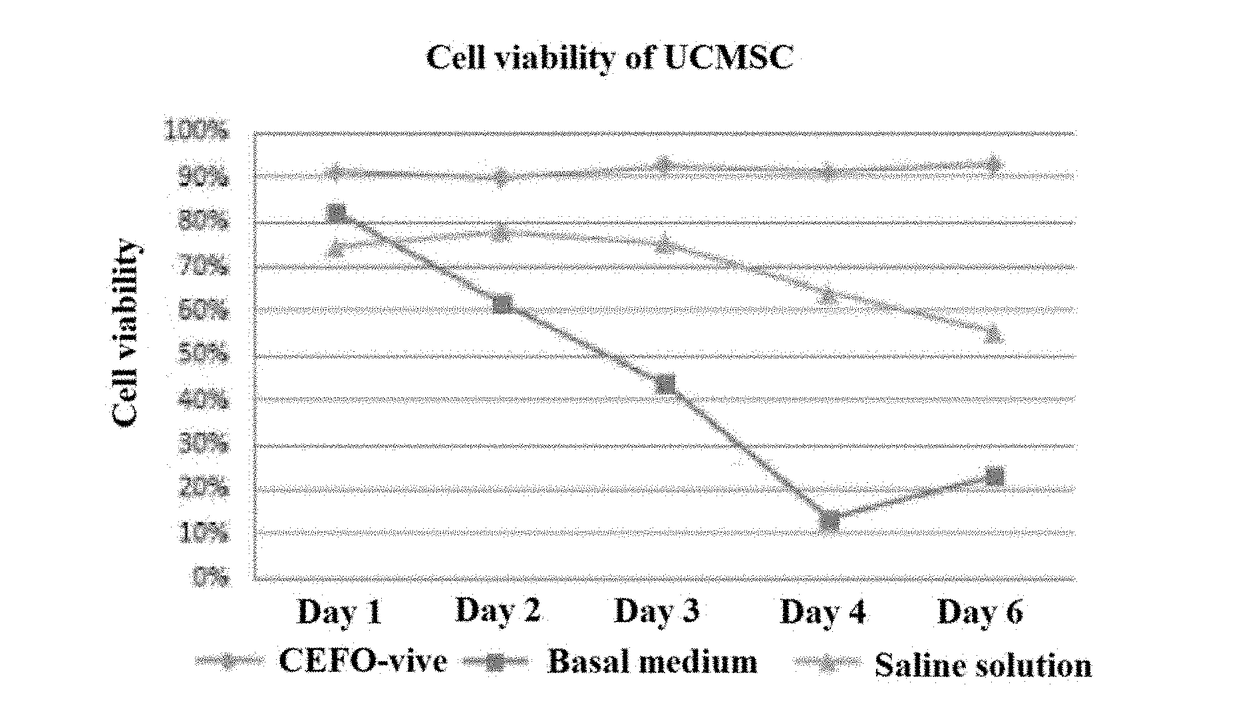
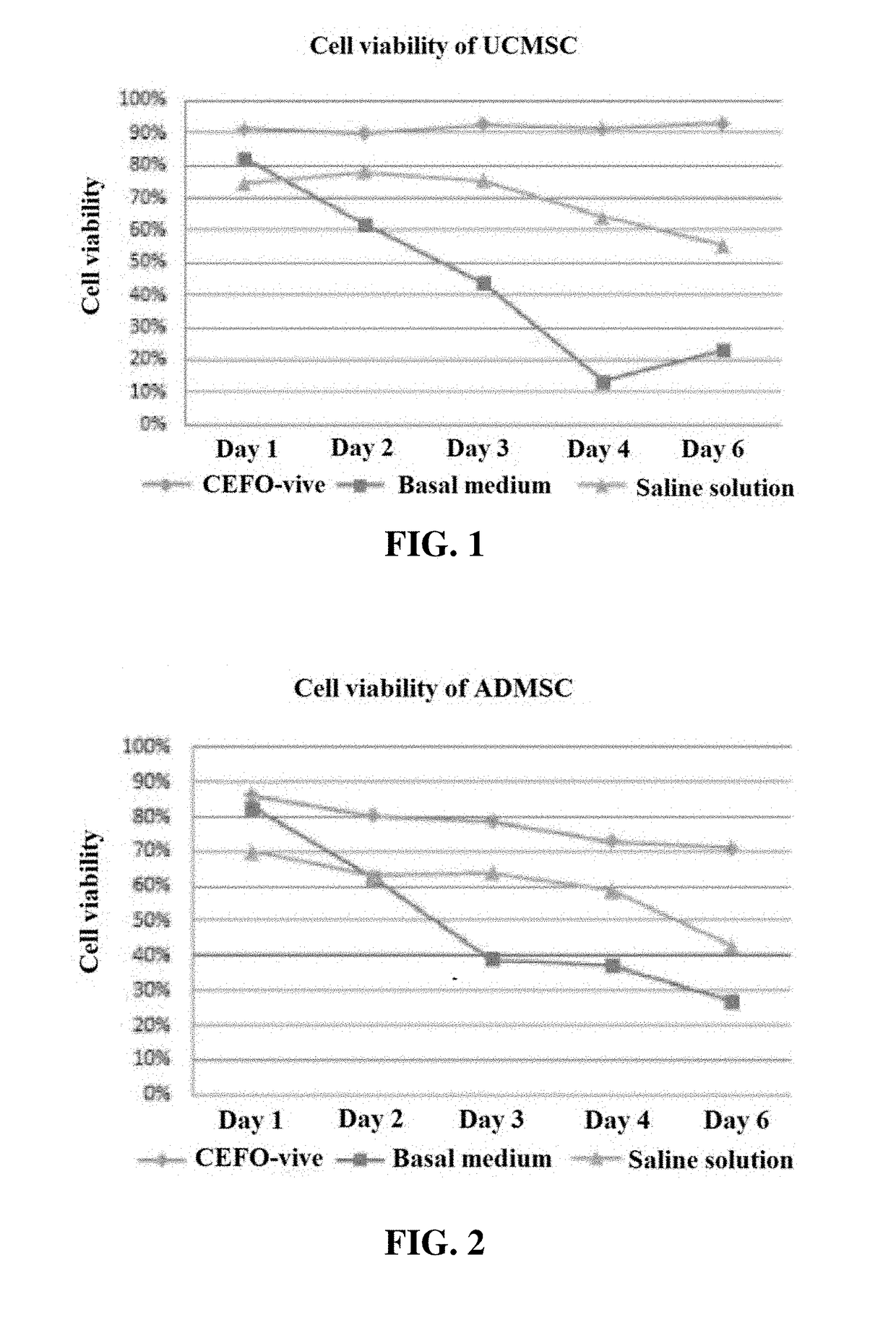
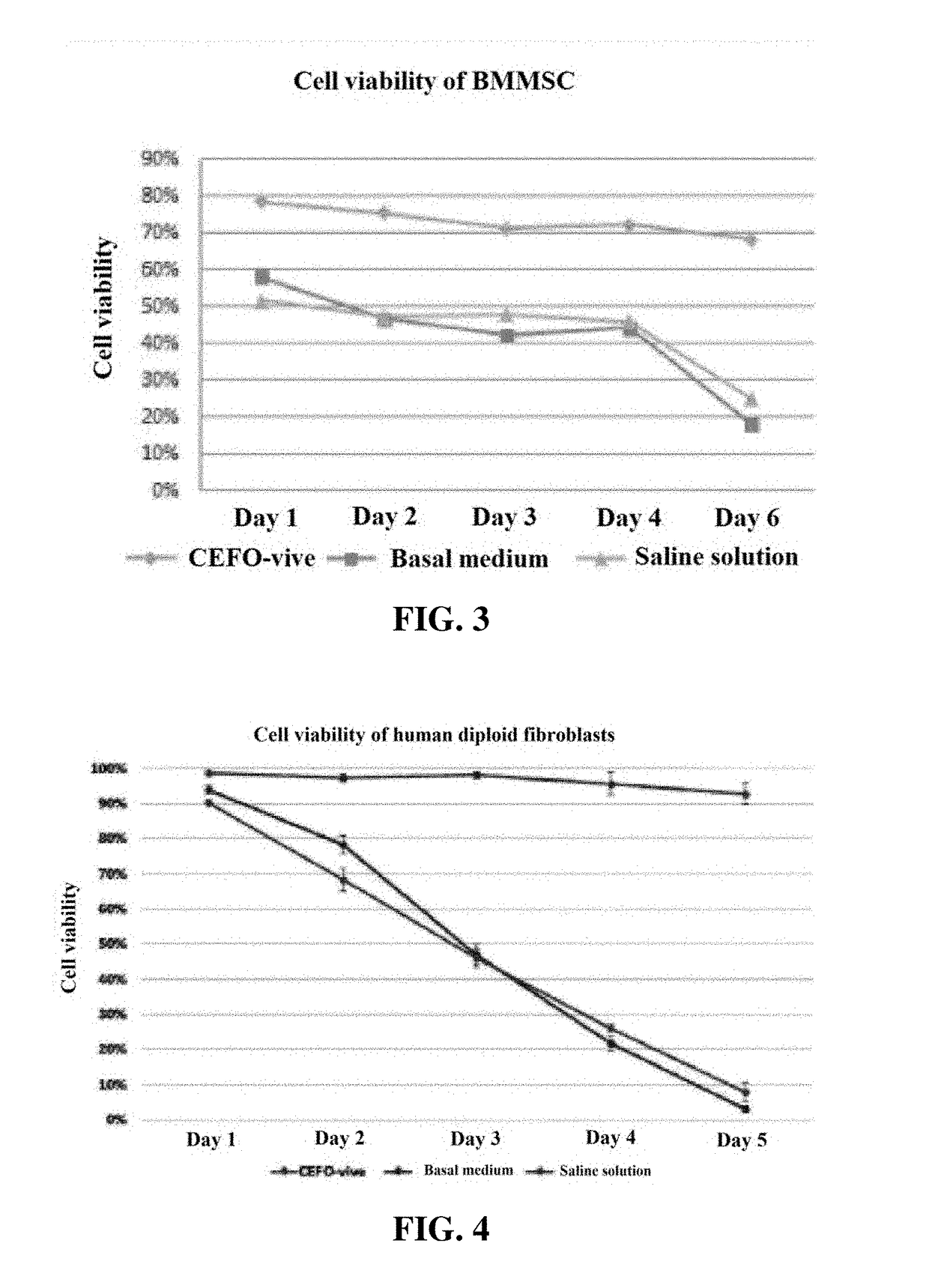
Composition comprising plant-derived recombinant human serum albumin, lipids, and plant protein hydrolysates as active ingredients for cryopreservation of stem cells or primary cells
ActiveUS20150327537A1Minimize damageImprove survival rateDead animal preservationEmbryonic cellsLipid formationHydrolysate
The present invention relates to a composition including plant-derived recombinant human serum albumin, lipids, and plant protein hydrolysates as active ingredients for cryopreservation of stem cells or primary cells, and use thereof, more particularly, to a composition including plant-derived recombinant human serum albumin, lipids, and plant protein hydrolysates as active ingredients for cryopreservation of stem cells or primary cells, in which the albumin, lipids, and plant protein hydrolysates are present in sufficient quantities to reduce cell death caused by freezing while maintaining animal-free and xeno-free conditions during long-term preservation of stem cells or primary cells; and to a method for cryopreservation of stem cells or primary cells using the composition.
Owner:CEFO
Stem cell defined media for xeno-free and feeder free conditions and uses thereof
ActiveUS20130059377A1More cost attractiveEvoke undesirable immune responseCulture processArtificial cell constructsTricarboxylic acidXeno free
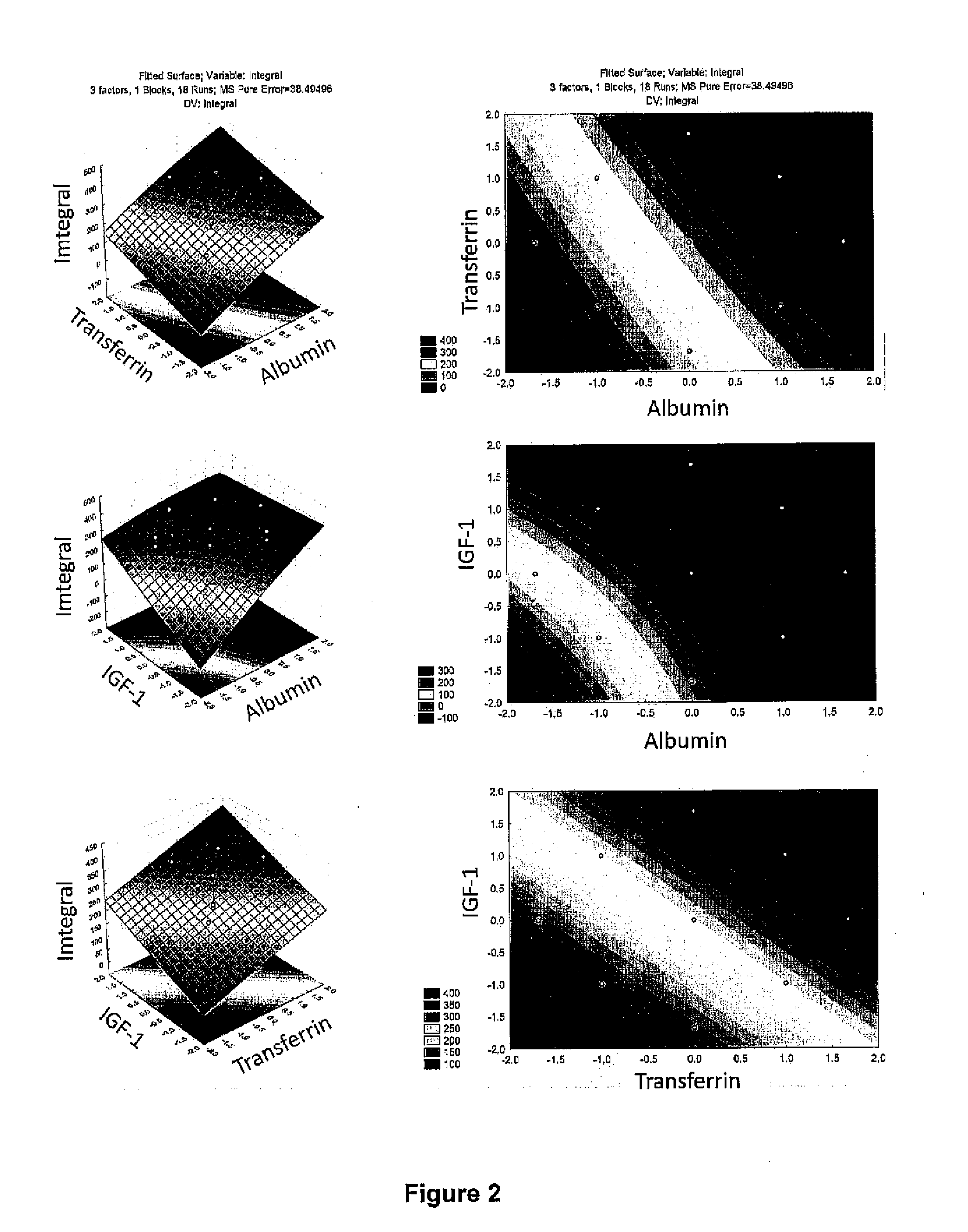
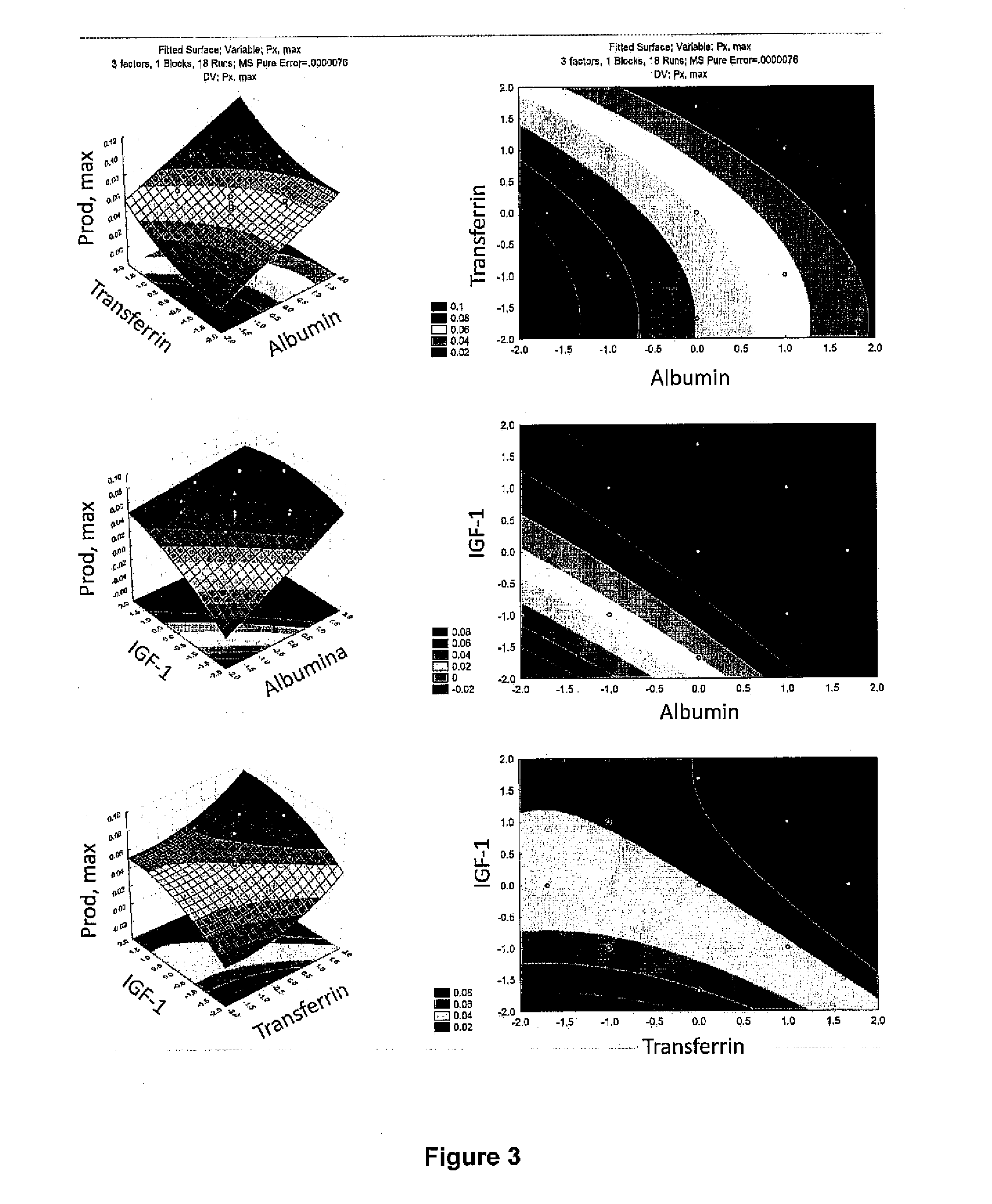
The invention provides a defined low protein culture medium for maintaining cells in an undifferentiated state, the medium comprising: a basal medium, an organic acid from the tricarboxylic acid cycle, nonessential amino acids, a combination of growth factors selected from the group consisting of FGF-2 protein, an IGF-1 protein or insulin, a Transferrin protein, and a TGF beta 1 protein, wherein the medium is essentially feeder-free, essentially xeno-free, essentially free of beta-mercaptoethanol, and essentially free of animal-derived or human-derived proteins.
Owner:RGT UNIV OF CALIFORNIA +1
Method for preparing induced pluripotent stem cells using synthetic peptide
ActiveUS20170275594A1Peptide-nucleic acidsGenetically modified cellsHuman Induced Pluripotent Stem CellsPeptide
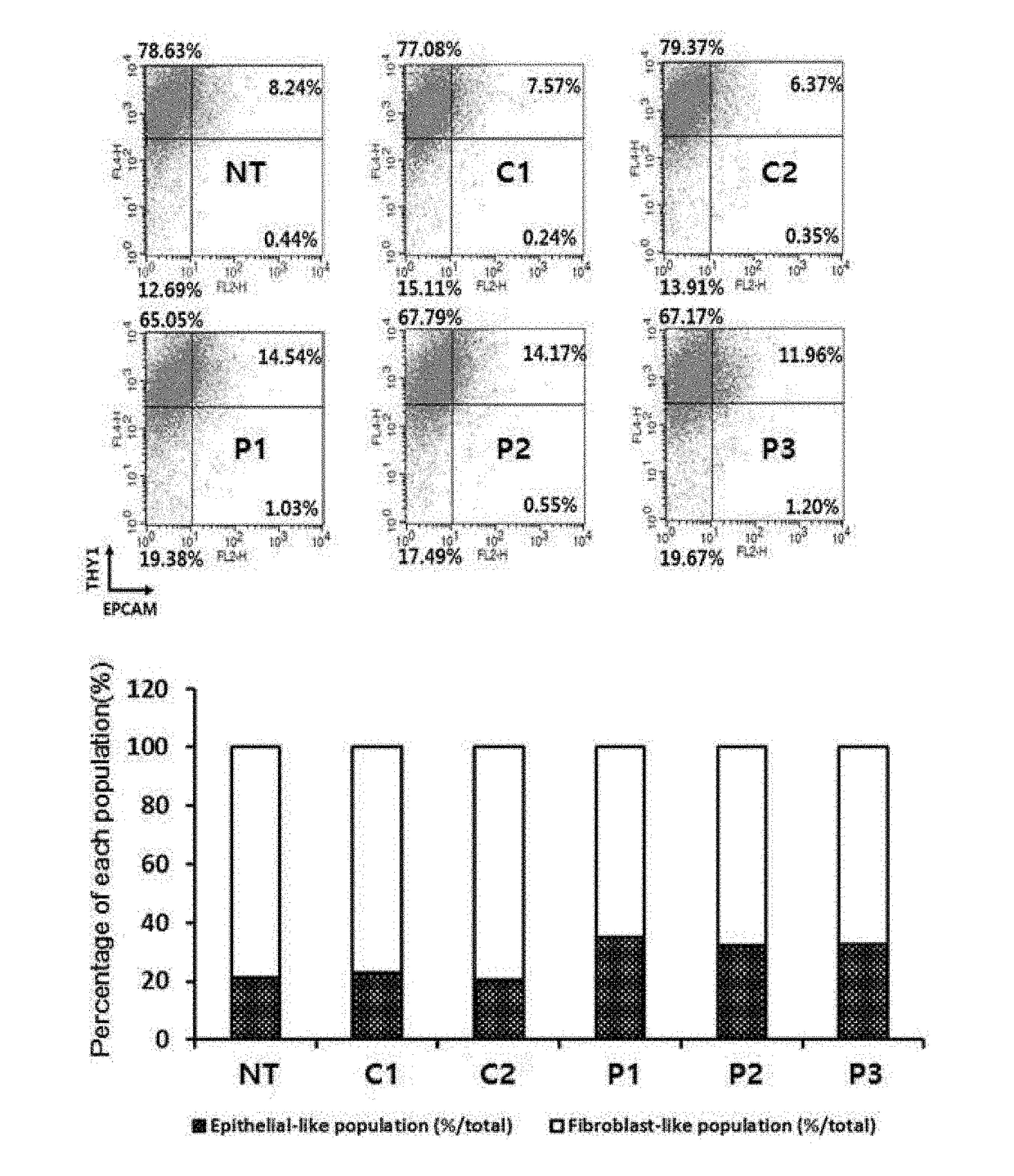
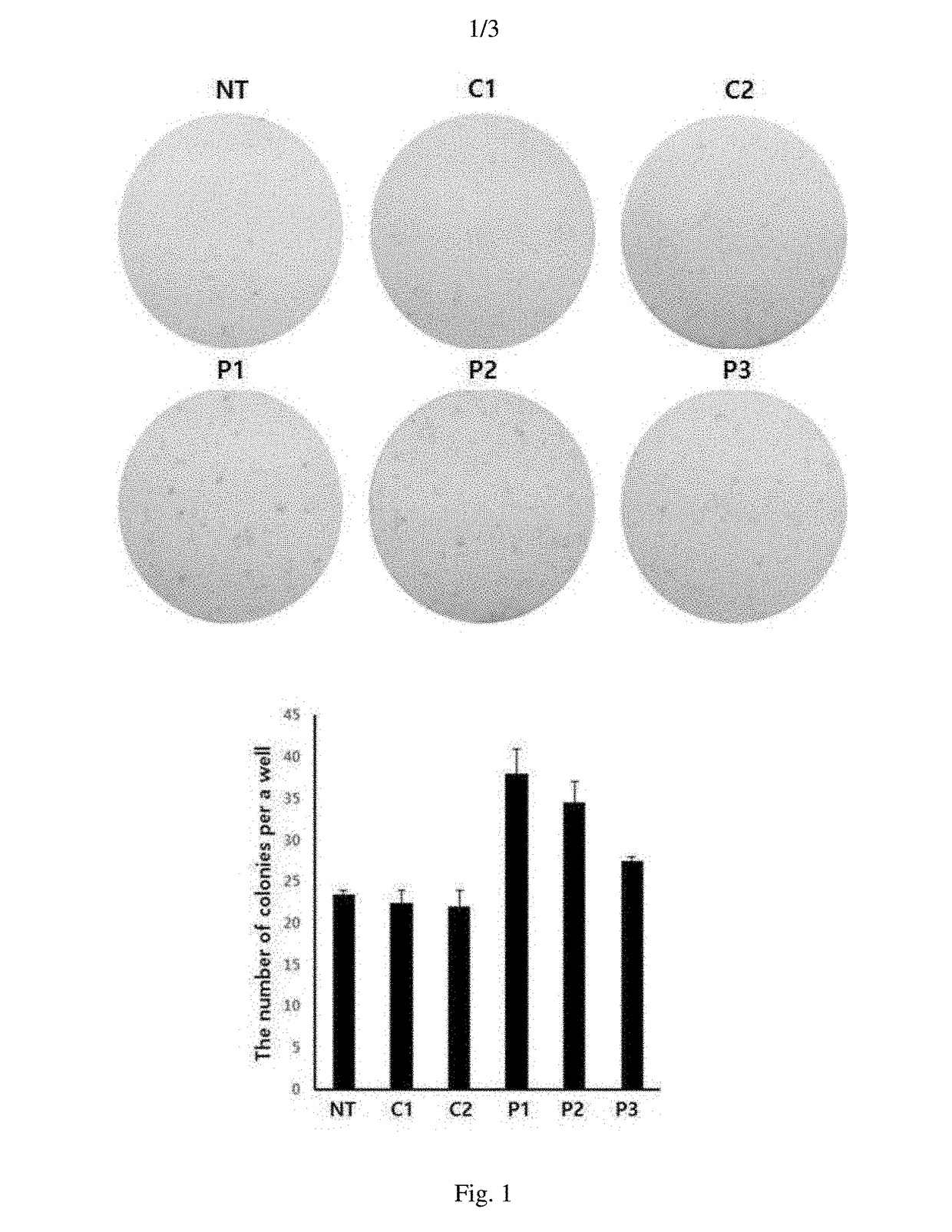
Provided is a method of preparing induced pluripotent stem cells using a synthetic peptide, and more particularly, to a method of preparing induced pluripotent stem cells using a peptide capable of inhibiting the activity of NF-κB and promoting mesenchymal-epithelial transition (MET). Since undifferentiated multipotent stem cells may be efficiently prepared under xenopathogen-free or feeder cell-free conditions without requiring co-culture with animal serum or xenogeneic cells, the method for preparing the induced pluripotent stem cells using the synthetic peptide according to the present disclosure is very useful for developing stem cell therapeutic agents that are clinically applicable.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Cell culture media, kits and methods of use
ActiveUS20130029414A1Efficient cultivationCulture processCell culture mediaHematopoietic cellCell culture media
Albumin-supplemented and xenogeneic product-free cell culture media, cell culture media supplements, and cell culture media kits for the support of primary culture of normal non-hematopoietic cells of mesodermal origin suitable for both research and clinical applications.
Owner:MOSCATELLO DAVID
Xeno-free generation of tissue-specific progenitor cells
Owner:WISCONSIN ALUMNI RES FOUND
Defined conditions for human embryonic stem cell culture and passage
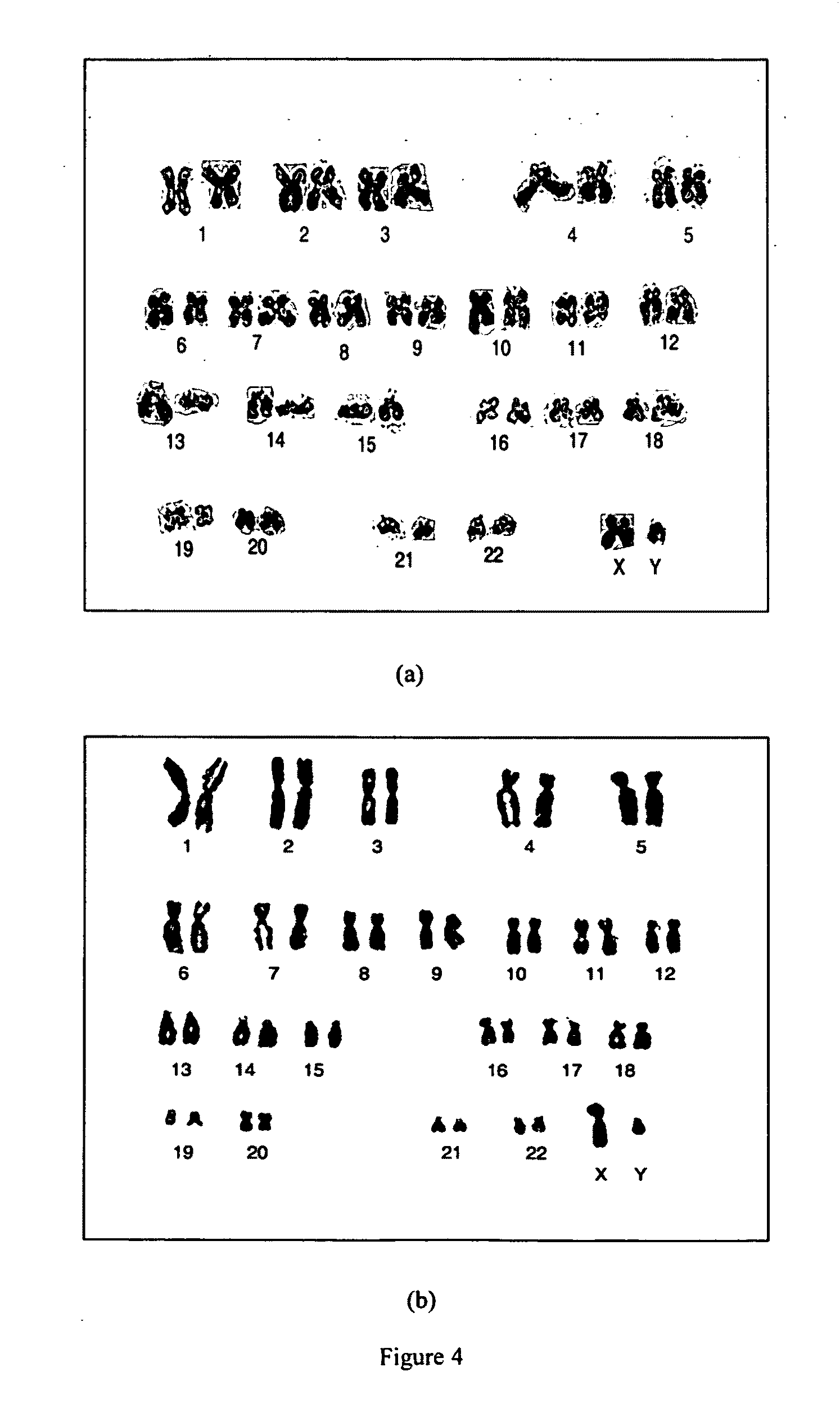
The invention relates to human pluripotent cells. More specifically, the invention provides a chemically defined xeno-free culture system that allows for long term expansion of human pluripotent cells. This culture system allows for human pluripotent cell lines to be maintained in the pluripotent state for an extended time while maintaining a normal karyotype and the ability to differentiate into all three germ layers.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for Obtaining Xeno-Free Hbs Cell line
InactiveUS20100129906A1Easy to detectAvoid damageCell dissociation methodsNervous disorderGerm layerXeno free
A method for obtaining a stable xeno-free hBS cell line, xeno-free hBS cell lines obtained according to said method and use thereof. The method comprises the steps of:i) removing the zona pellucida from a blastocyst to obtain trophectoderm-enclosed inner cell mass by a xeno-free procedure,ii) at least partly removing the trophectoderm to obtain isolated inner cell mass cells by a xeno-free procedure,iii) placing the inner cell mass cells on a layer of human feeder cells in a xeno-free medium,iv) co-culturing of the inner cell mass cells with human feeder cells for a time period of from about 5 days to about 50 days in a xeno-free medium,v) releasing the inner cell mass cells or cells derived thereof from trophectoderm overgrowth, if any, by a xeno-free procedure,vi) selectively, transferring the inner cell mass cells or cells derived thereof to a fresh layer of human feeder cells in a xeno-free medium to obtain xeno-free hBS cells,vii) propagating the xeno-free hBS cells by co-culturing with human feeder cells in a xeno-free medium to obtain a xeno-free hBS cell line.The xeno-free hBS cell line is suitable for use in medicine and in in vitro testing.
Owner:CELLARTIS AB (SE)
Retinal ganglion cells and progenitors thereof
PendingUS20170274019A1Improve homogeneityIncrease differentiationSenses disorderCell culture mediaProgenitorXeno free
Methods are provided for the production of retinal ganglion (RG) progenitor cells and mature RG cells from pluripotent stem cells optionally under feeder-free conditions, and further optionally under xeno-free conditions. Additionally provided are compositions of RG progenitor cells and mature RG cells, as well as methods of use thereof including therapeutic use thereof. Exemplary methods may produce substantially pure populations and cultures of RG progenitor cells and mature RG cells.
Owner:ADVANCED CELL TECH INC
Xeno-free culture conditions for human embryonic stem cells and methods thereof
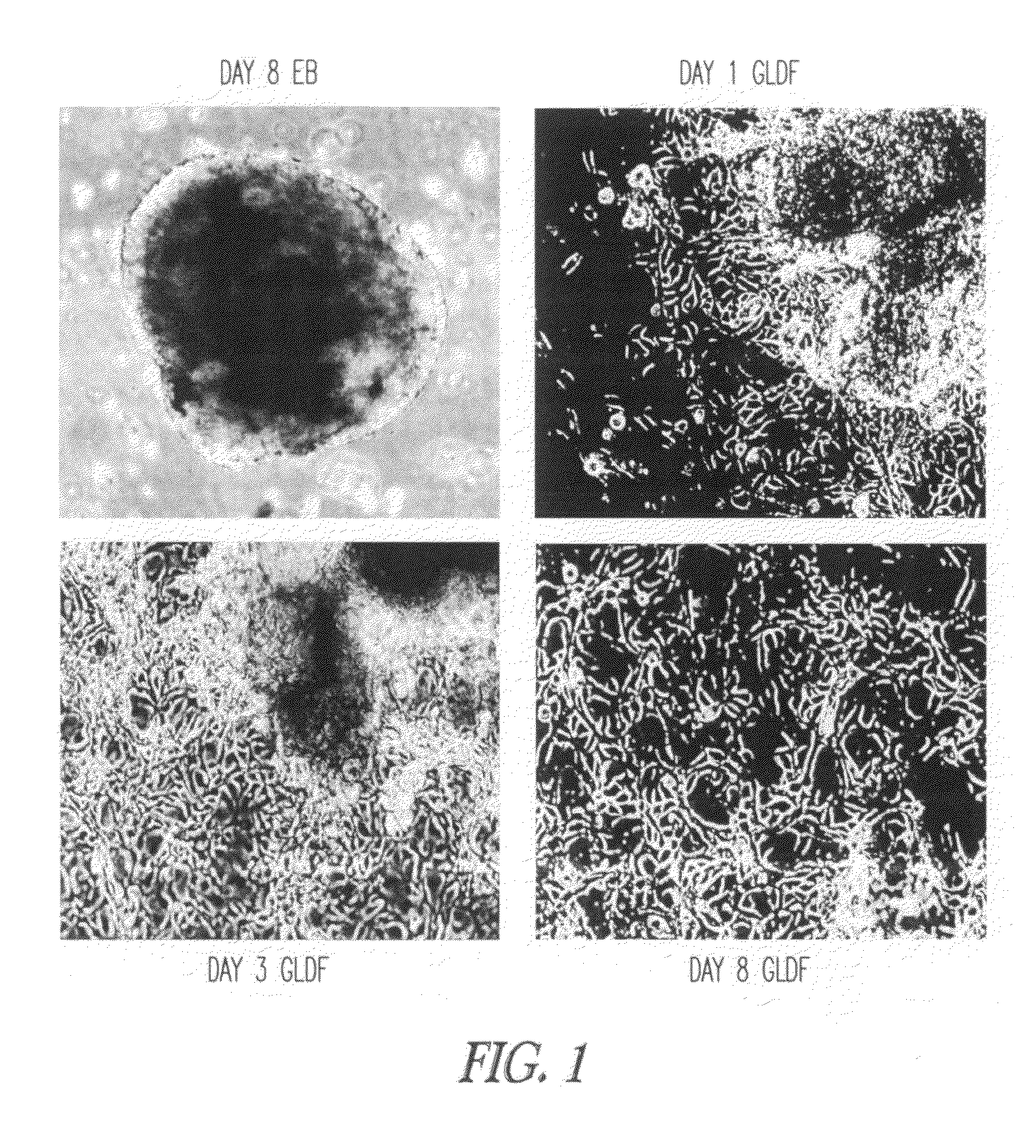
The present disclosure provides novel culture system and methods for culturing and propagating hESCs in a substantially undifferentiated state for several passages. The ability to grow such cells without differentiation has important applications for therapeutic uses of ES cells for treating human disorders using tissue transplantation and / or gene therapy techniques. In particular, the disclosure further relates to conditioned medium obtained from human germ lineage derived feeder cells (GLDF). The hESC lines are derived, cultured and propagated in substantially undifferentiated state using the conditioned media from GLDF cells of the disclosure. In particular, the disclosure relates to the xeno-free derivation, culture and propagation of hESCs using conditioned medium of GLDF cells obtained thereof.
Owner:STEMPEUTICS RES PRIVATE
Stem cell cryopreservation protective agent, preparation method and application
PendingCN111587877ALow biosecurityPossibility of clinical application is lowDead animal preservationWhole blood productCell free
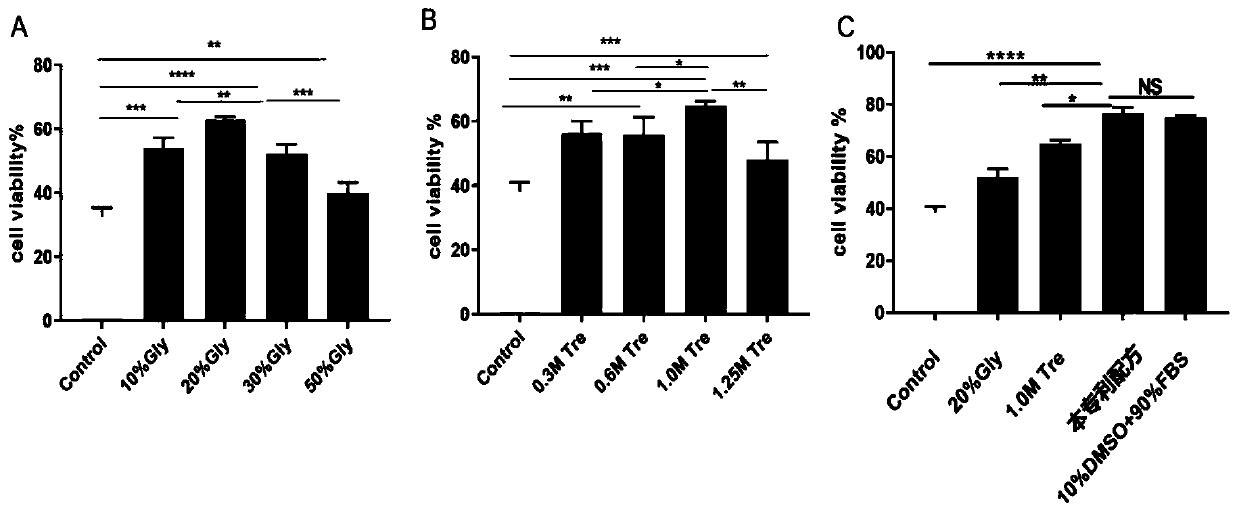
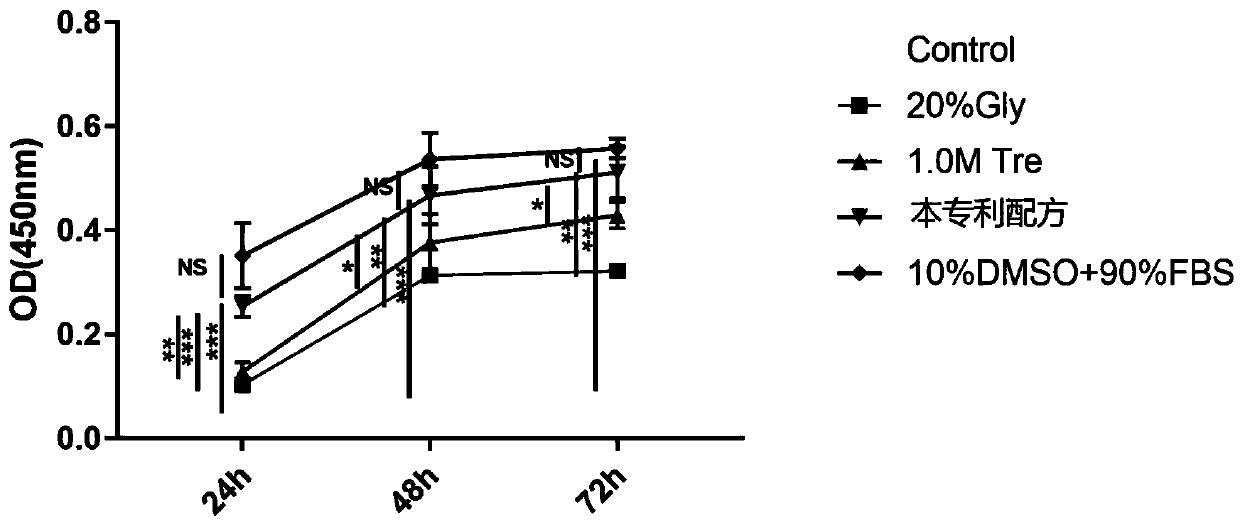
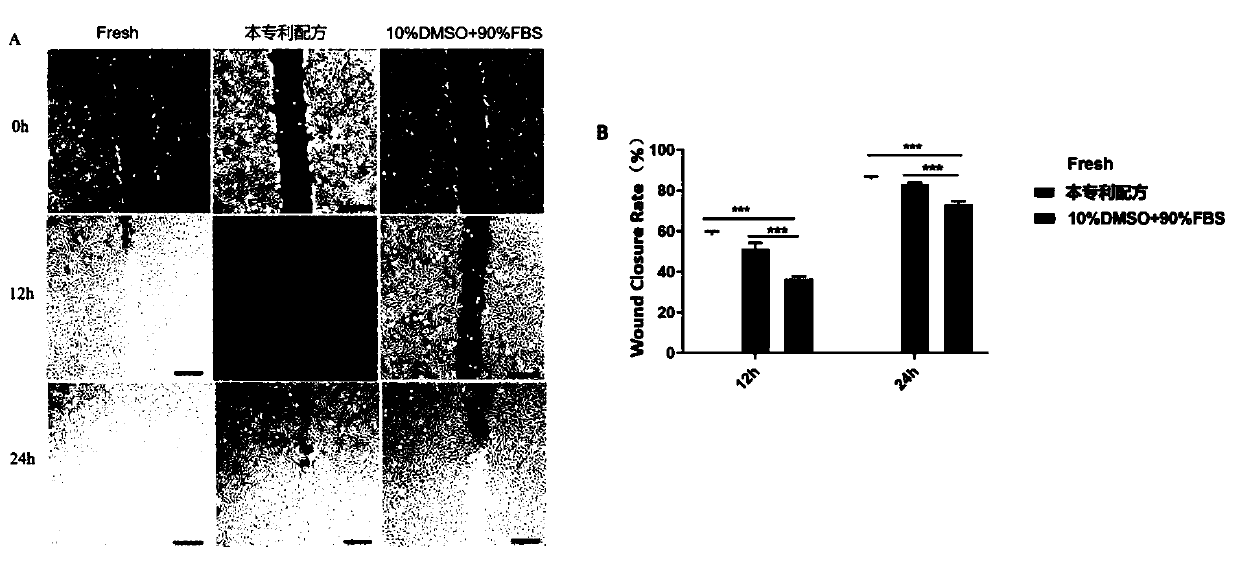
The invention provides a stem cell cryopreservation protective agent which comprises the following components 10-30 vol% of glycerinum, 0.25-1.5 mol% of trehalose and a solvent being normal saline ora phosphate buffer solution. The stem cell cryopreservation protective agent provided by the invention is a cryopreservation protective agent with effective cryopreservation activity and function of stem cells, is efficient, non-toxic and free of xenogenic antigen, and has higher biological safety and clinical application possibility; no high-molecular polymer or biotoxic substance is used, and reagents used in the method have no cytotoxicity and are easy to elute; according to the invention, no blood product from any heterologous source exists, no heterologous antigen exists, and the possibility of hypersensitive reaction is low; compared with an existing cell cryopreservation protective agent, the cell cryopreservation protective agent is low in cost and large in large-dose application possibility; operation is convenient, and popularization is easy.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Methods of producing RPE cells
The present disclosure relates to the use of laminin-521 in obtaining retinal pigment epithelium (RPE) cells. Pluripotent human embryonic stem cells are cultured on plates coated with recombinant laminin-521 (laminin-11), in totally defined and xeno-free conditions. A first cell culture medium contains a growth factor, and a second cell culture medium does not contain growth factor. The stem cells are first exposed to the first cell culture medium, then exposed to the second cell culture medium for a longer time period. After a number of weeks, clinical grade RPE cells are obtained from the stem cells.
Owner:BIOLAMINA
Three-dimensional cell culture system and cell culture method using same
ActiveUS20170283767A1Improve adhesionPromote growthDead animal preservationMammal material medical ingredientsHigh cellPlant Sources
Owner:CEFO
Methods for cell culture using a synthetic defined collagen mimetic surface
InactiveCN102586172AMicrobiological testing/measurementArtificial cell constructsCell adhesionXeno free
Owner:CORNING INC
Xeno-free and a feeder free self-renewal extracellular matrix for long-term maintenance of undifferentiated human pluripotent stem cells and method of synthesizing the same
InactiveUS20140220680A1Culture processArtificial cell constructsCell-Extracellular MatrixECM Protein
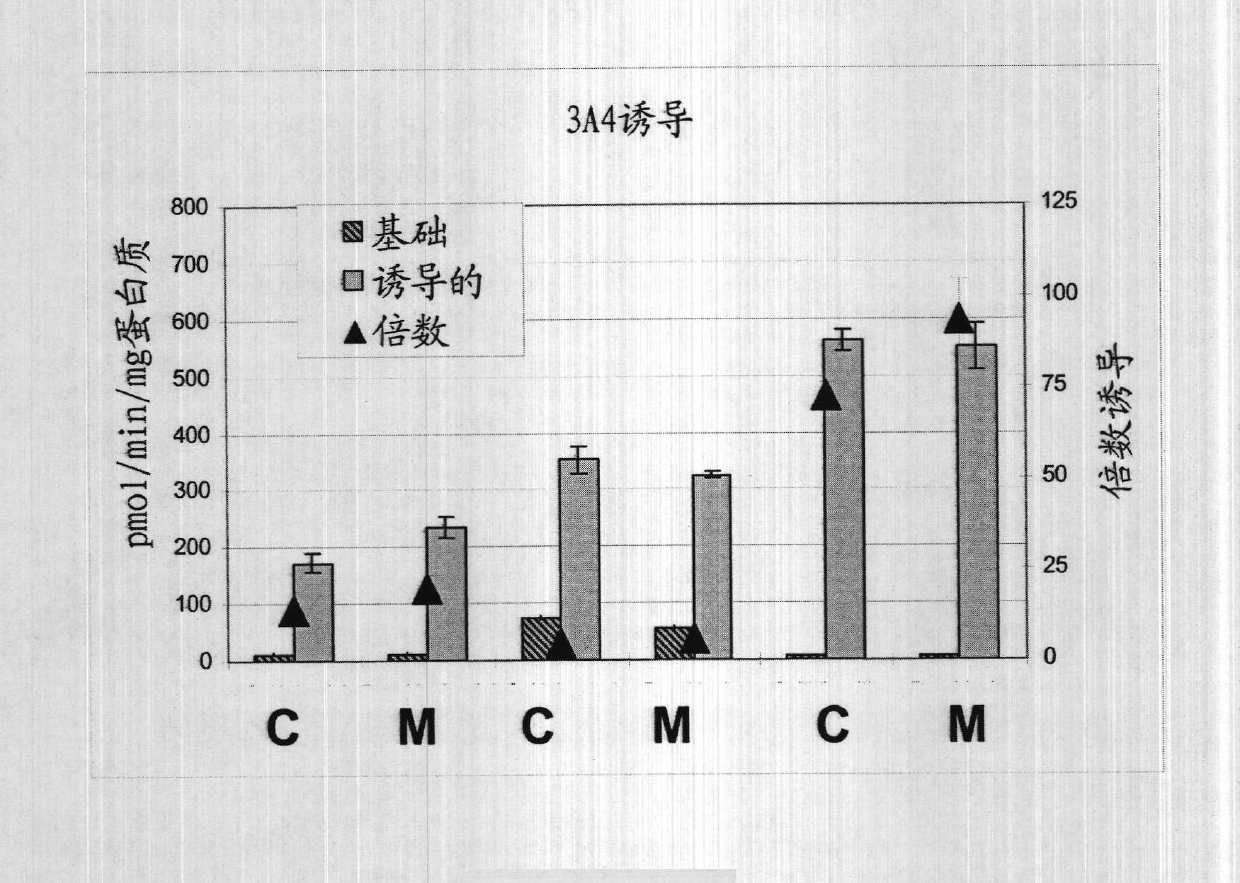
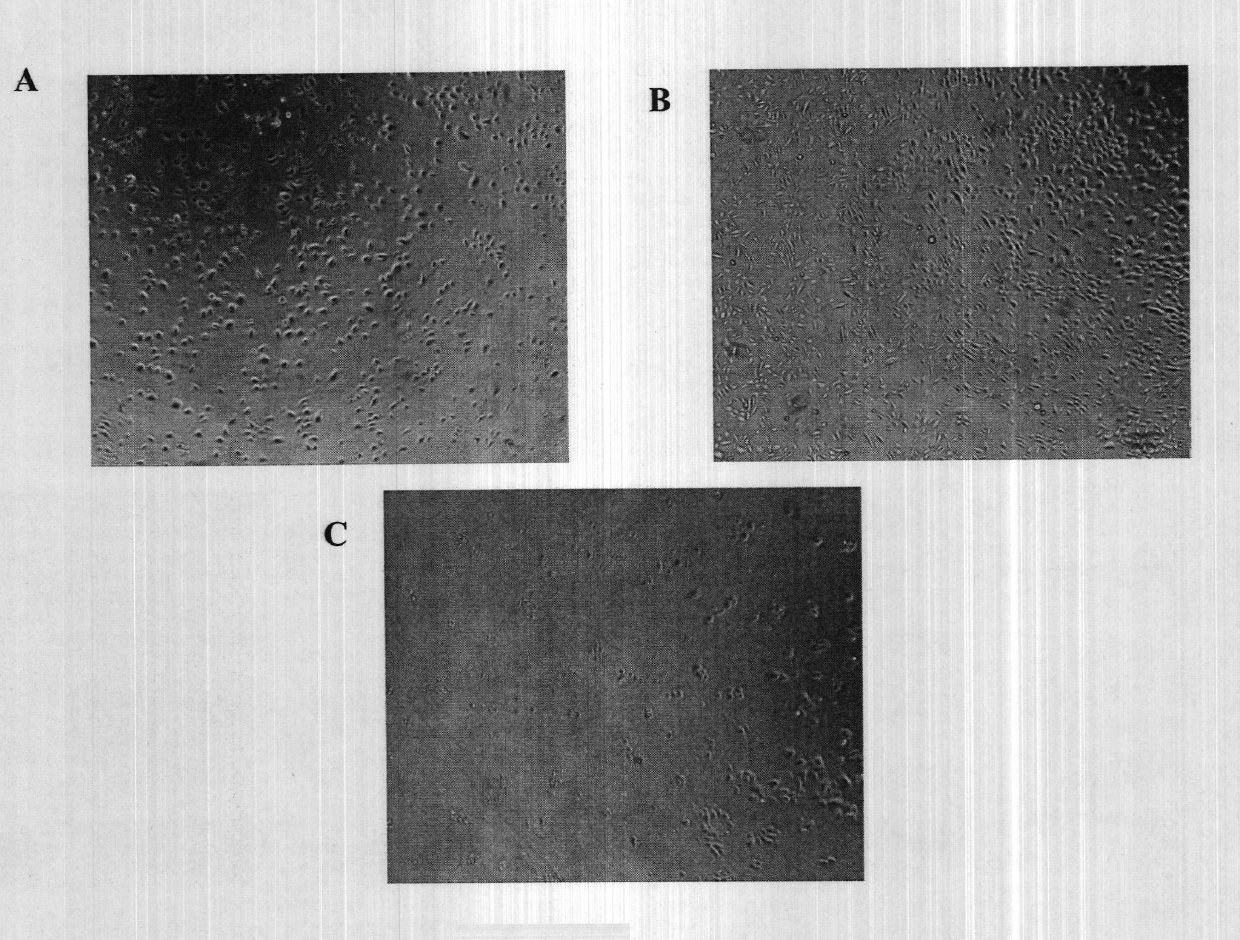
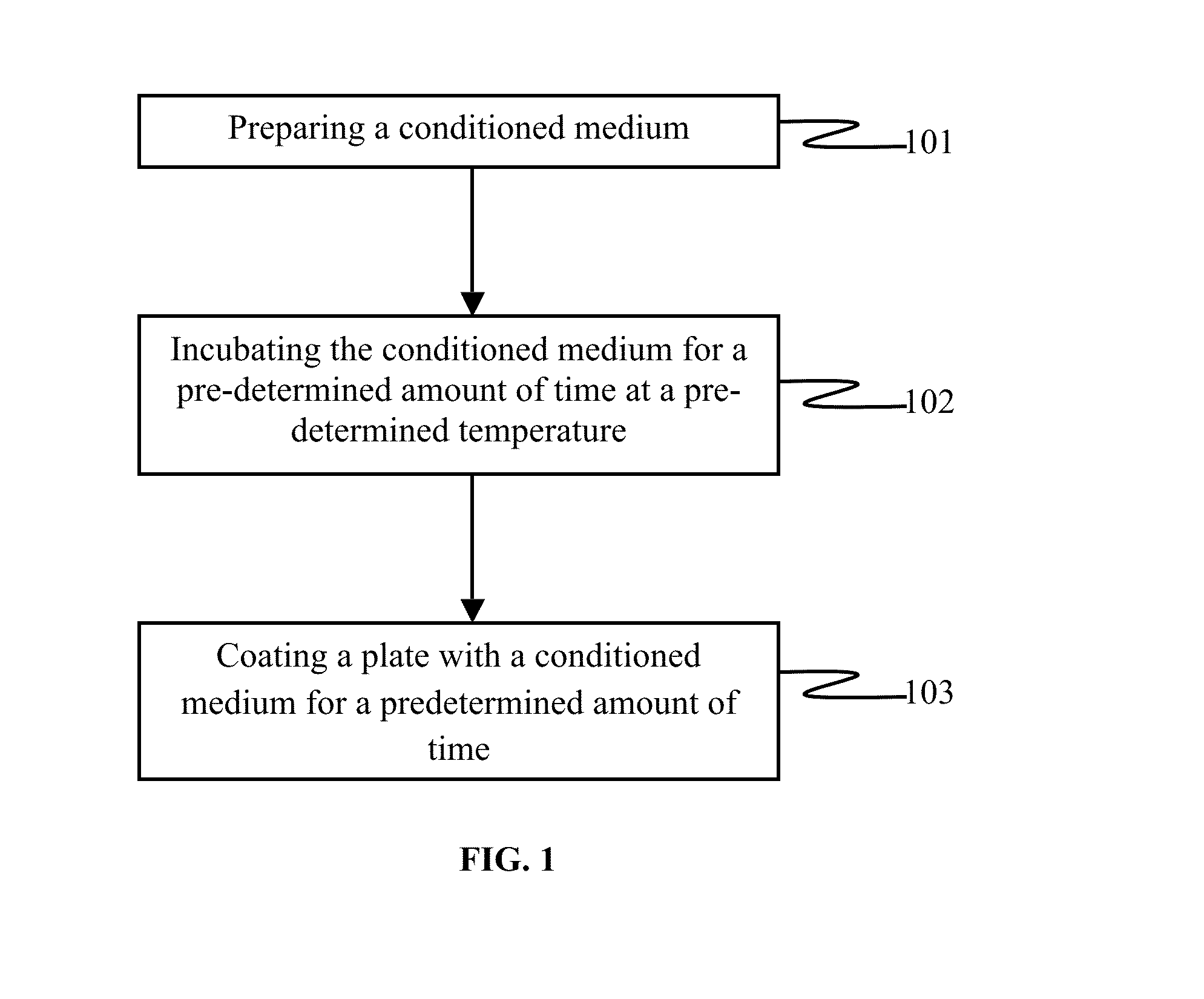
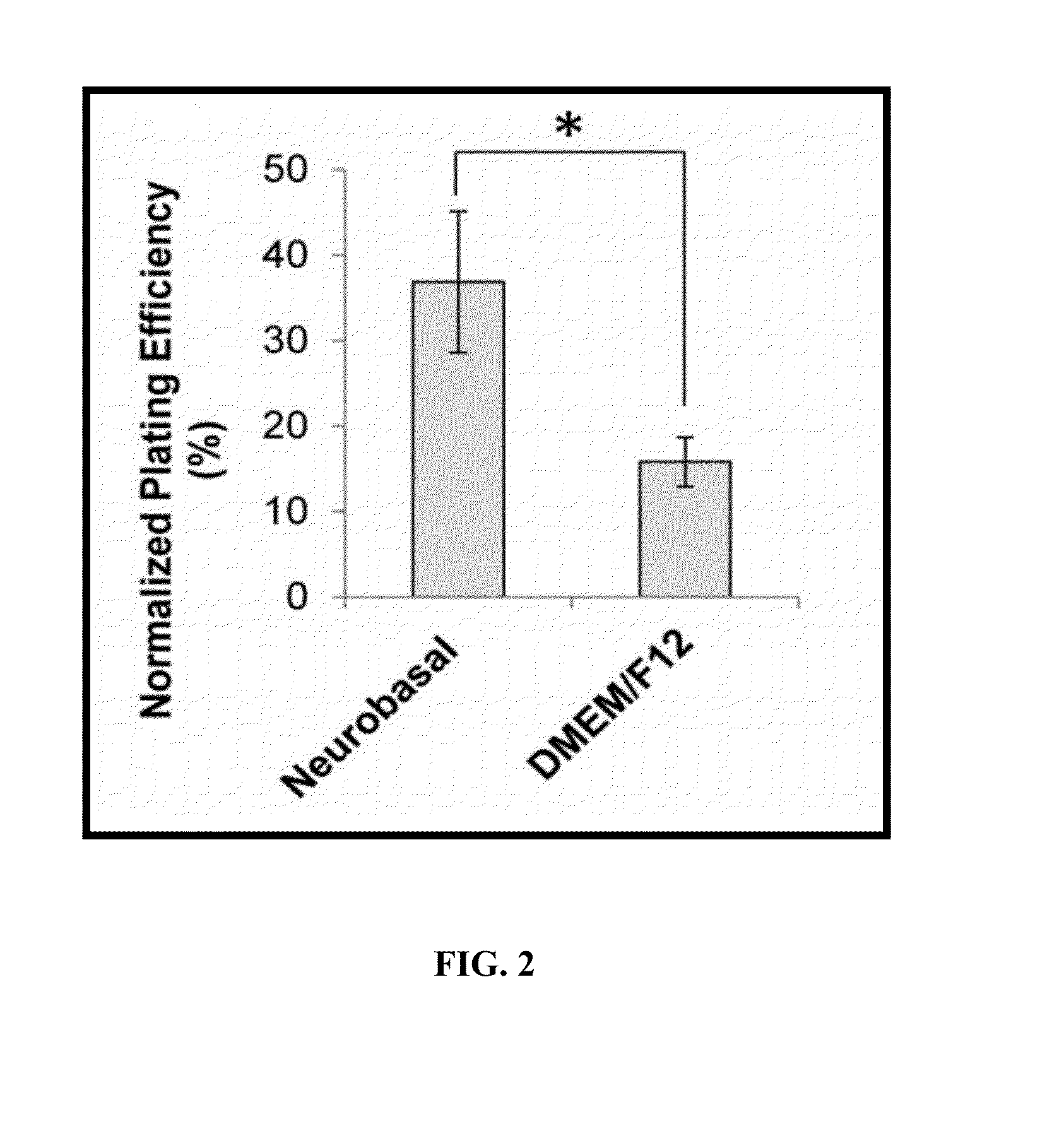
The embodiments herein provide a xeno-free and a feeder free self-renewal extracellular matrix for long-term maintenance of undifferentiated Human Embryonic Stem Cells (hESCs) and undifferentiated Human Pluripotent Stem Cells (hPSCs) and a method of synthesizing the same. The extracellular matrix includes a conditioned medium comprising a neurobasal medium, a DMEM / F12 medium, a plurality of additives and a plurality of feeder cells. The plurality of additives are L-glutamine, β-mercaptoethanol, nonessential amino acids and insulin-transferrin-selenite. The feeder cells are Human Dermal Fibroblasts (HDFs). The conditioned medium may be added with a Rho-associated Coiled Kinase (ROCK) inhibitor Y-27632. The extracellular matrix is in the form of a gel. The method comprises preparing a conditioned medium and incubating the conditioned medium for 24 hours or 72 hours at 37° C. The method comprises coating a plate with the prepared conditioned medium for 5 min or 15 min.
Owner:ROYAN RES
Preparation method and application of treatment-grade mesenchymal stem cells based on induced pluripotent stem cells
PendingCN110628712ASuitable for mass productionShort training periodCulture processAntinoxious agentsDiseaseTissue repair
The invention discloses a preparation method of iPSCs-derived MSCs. The preparation method comprises the following steps of culturing iPSCs; digesting the iPSCs, and performing suspension culture on digested iPSCs cells so as to form an embryoid body; adding an induced culture medium combination A in the suspension culture process of the embryoid body for induction and disintegration; and after the embryoid body is disintegrated, performing direct adherence culture, or after the embryoid body is digested, performing adherence culture, or after the embryoid body is digested, performing sortingculture to obtain the MSCs. The invention also discloses an application of the iPSCs-derived MSCs. The novel method for quickly preparing the iPSCs-derived MSCs disclosed by the invention has the characteristics that xenogeneic exogenous substances do not exist, the suspension culture is performed, the culture period is shorter, the operation is simple, infinite amplification is achieved, the efficiency is high, and the preparation method is more suitable for large-scale production. The obtained iPSCs-derived MSCs conforms to essential features of the MSCs through identification, besides, hasfunctions being similar to those of bone marrow derived MSCs, and can be used for tissue repair and treatment of immunization relevant diseases.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
Method for inducing iPSCs or ESCs to be differentiated into brown fat cells
PendingCN110592004AShort training periodEasy to operateMetabolism disorderCulture processSuspension cultureAdherent Culture
The invention relates to a method for inducing iPSCs or ESCs to be differentiated into brown fat cells. The invention discloses a preparation method of iPSCs-derived MSCs. The method comprises the following steps: cultivating the iPSCs; digesting the iPSCs and performing suspension culture on the digested iPSCs cells to form an embryoid body; adding inducing medium combination A in the suspensionculture process of the embryoid body to induce differentiation; and directly performing adherent culture after the embryoid body is differentiated, or performing adherent culture after digestion or performing sorting culture after digestion to obtain the MSCs. The invention also discloses application of the iPSCs-derived MSCs. The novel method for rapidly preparing the iPSCs-derived MSCs has the characteristics of no foreign matters, suspension culture, shorter culture period, simplicity in operation, infinite amplification and the like, has high efficiency and is more suitable for large-scaleproduction. The iPSCs-derived MSCs accord with the basic characteristic of the MSCs through identification, have a function similar to the marrow-derived MSCs, and can be applied to tissue repair andtreatment on immune-related diseases.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
Composition for preserving cells, containing, as active ingredients, plantderived recombinant human serum albumin and plant peptides
ActiveUS20170226476A1Improve the level ofStable storageNervous system cellsDead animal preservationPlant SourcesBULK ACTIVE INGREDIENT
Owner:CEFO
Media for culturing stem cells
Owner:TECHNION RES & DEV FOUND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com