Implantation of encapsulated biological materials for treating diseases
a technology of encapsulated biological materials and diseases, applied in the field of diseases and their composition, can solve the problems of not being able to be transported into the cells where it is needed and utilized, wasting the appearance of many patients with poorly controlled insulin-dependent diabetes, and unable to meet the needs of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolating Islet Cells in Mice
[0241]Donor mice [C57BL / 6] with an age range of 18 weeks old and average size of 33 grams were obtained from supplier. Pancreas was exposed with euthanasia laparotomy. The pancreata were distended with Sigma collagenase, Type V. The pancreata were removed and kept in cold collagenase during transport to the isolation laboratory. The isolation process combined 30 pancreata for the digestion process. The digestate was washed with 10% fetal bovine serum in RPMI and centrifuged. The COBE was prepared for purification and a continuous gradient marker was used to make the gradient densities. The gradient was loaded into the COBE and the pancreata digestate was loaded on top to perform the purification process. The purified islets were collected and washed in RPMI media. The islets were cultured in T75 flasks in modified ICM media supplemented with 10% fetal bovine serum until ready for encapsulation.
Isolating Islet Cells in Primates
[0242]Juvenile Cynomolgus pr...
example 2
Preparation of Conformal Coating Materials
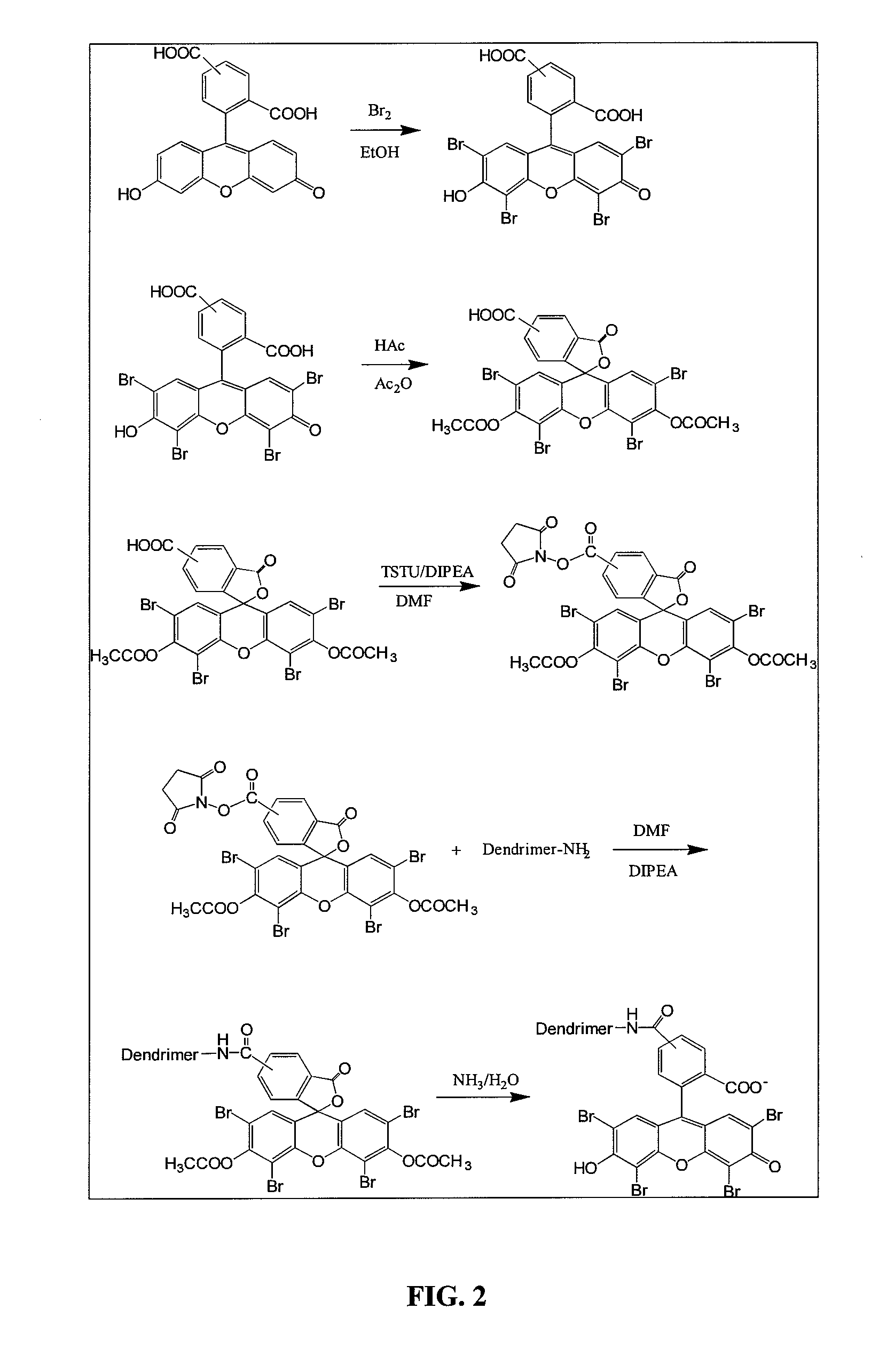
[0243]Depending on the type of cell being encapsulated, the cells were coated directly by a conformal coating or enclosed in a matrix, such as alginate, and then coated with a permselective PEG capsule. FIG. 2 illustrates the synthesis of dendrimer eosin Y conjugate, Dendrimer-EY, a preferred embodiment of this coating, and described as follows.
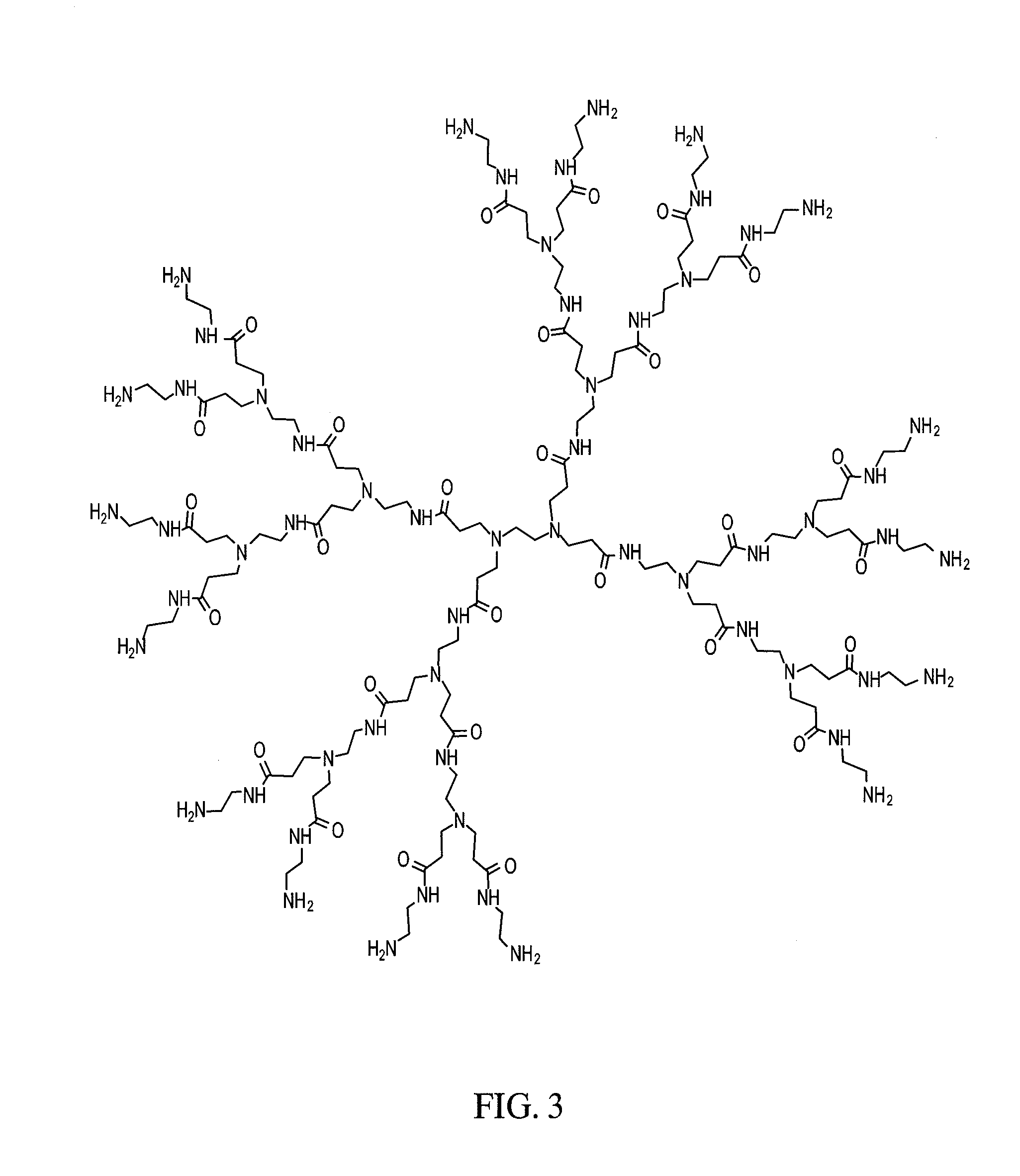
[0244]The dendrimer used for encapsulation was PAMAM Dendrimer generation 4, which was purchased from Dendritech (FIG. 3). 5(6)-Carboxyeosin was made by bromination of 5(6)-Carboxyfluorescein. The hydroxyl group and 1-carboxyl group were then protected by forming an acetate. The protected 5(6)-Carboxyeosin was activated by N,N,N′N′-Tetramethyl-O—(N-Succinnimidyl)uranium tetrafluoroborate (TSTU). Without further purification, the activated 5(6)-Carboxyeosin diacetate was mixed with PAMAM Dendrimer to form Dendrimer-EY conjugate. The protection group was then removed by reacting with aqueous ammonia. Th...
example 3
Encapsulation of Islets
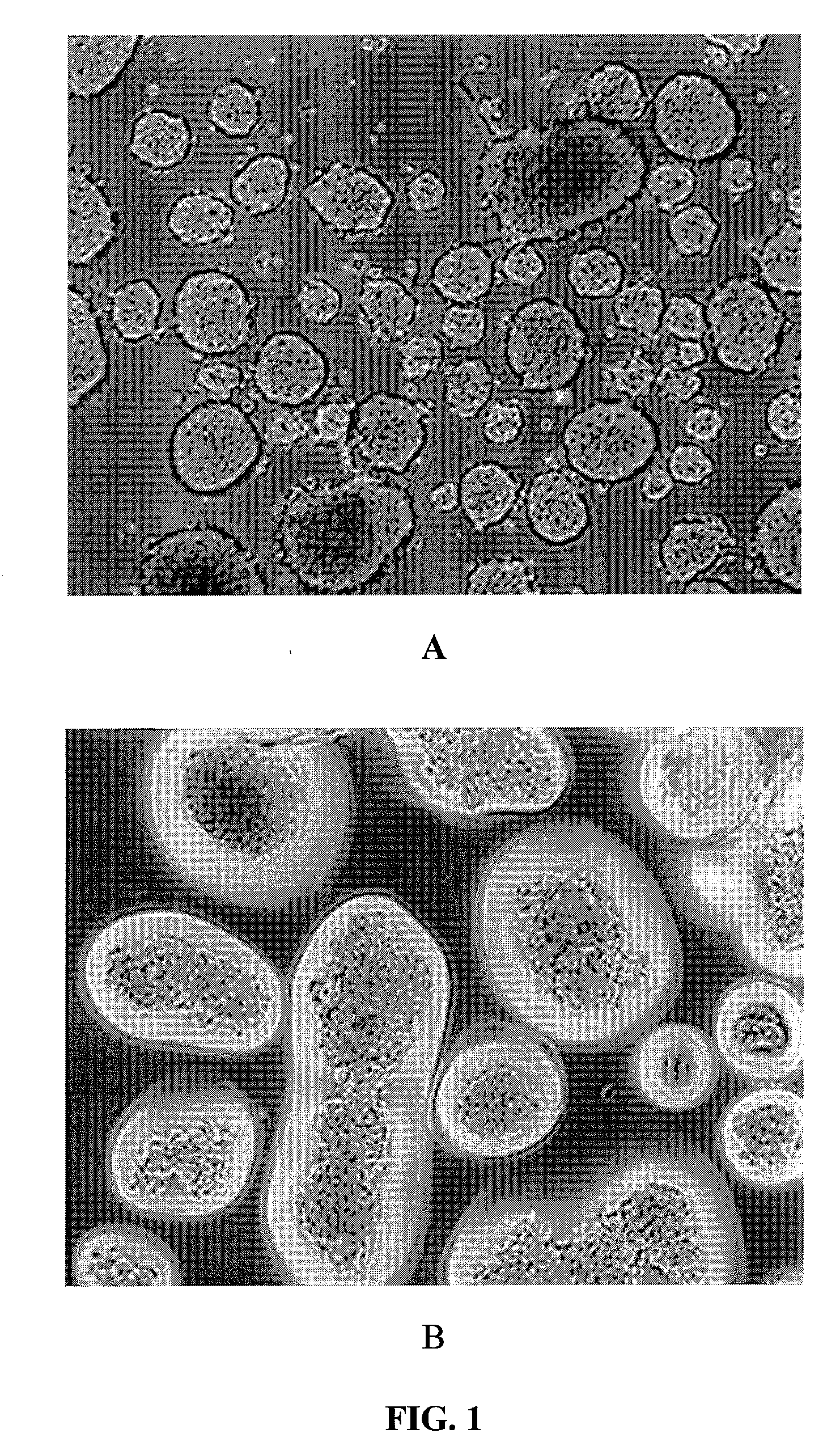
Encapsulating Mouse Islets
[0249]A preferred method of coating mouse islets is described as follows. Fifteen milliliters of 20 mM low ionic HEPES buffer (containing 1.8 mM CaCl2 and 260 mM Manitol, pH=7.0) was added to a 15 ml conical tube, containing 10 μl of islets. The supernatant was removed after centrifugation. 15 ml of Den-EY solution (0.1 mg / ml to 0.4 mg / ml in low ionic HEPES buffer) was added into the pellet and the tube was kept horizontal for 10-30 minutes at room temperature. The stained islets were washed twice with low ionic 20 mM HEPES buffer, which was sparged with Argon for at least 30 minutes. The stained islet pellet was mixed with 10 ml of photoactive polymer solution, which was also sparged with Argon and pre-equilibrated in a 8° C. waterbath for at least 30 minutes. The photoactive polymer solution was made in 20 mM HEPES buffer, pH=8.0, which contained up to 20% PEG, 100 mM TEoA, 32 mg / ml AMPS and 2 μl / ml NVP, and 13% Nycodenz. The suspen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com