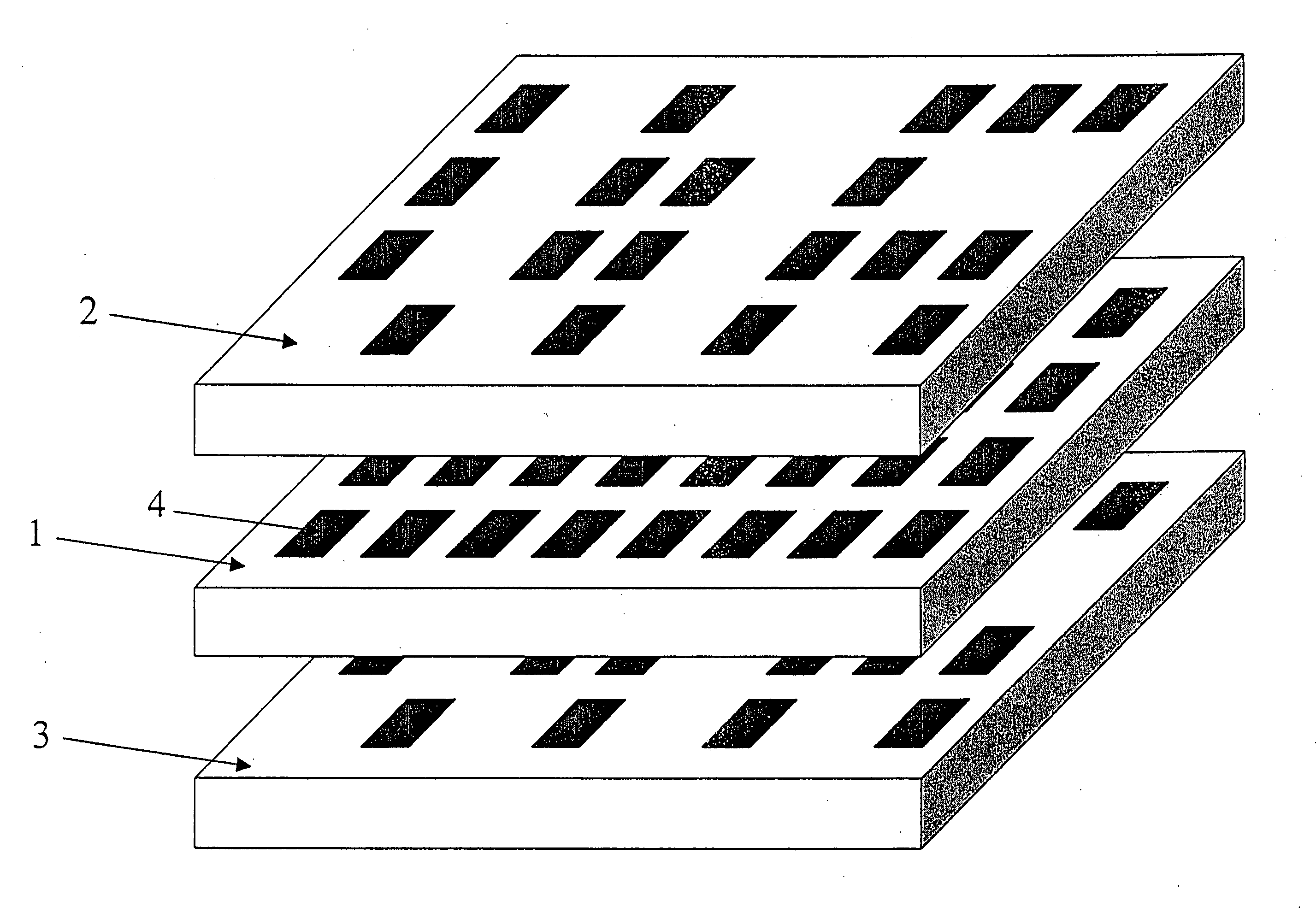
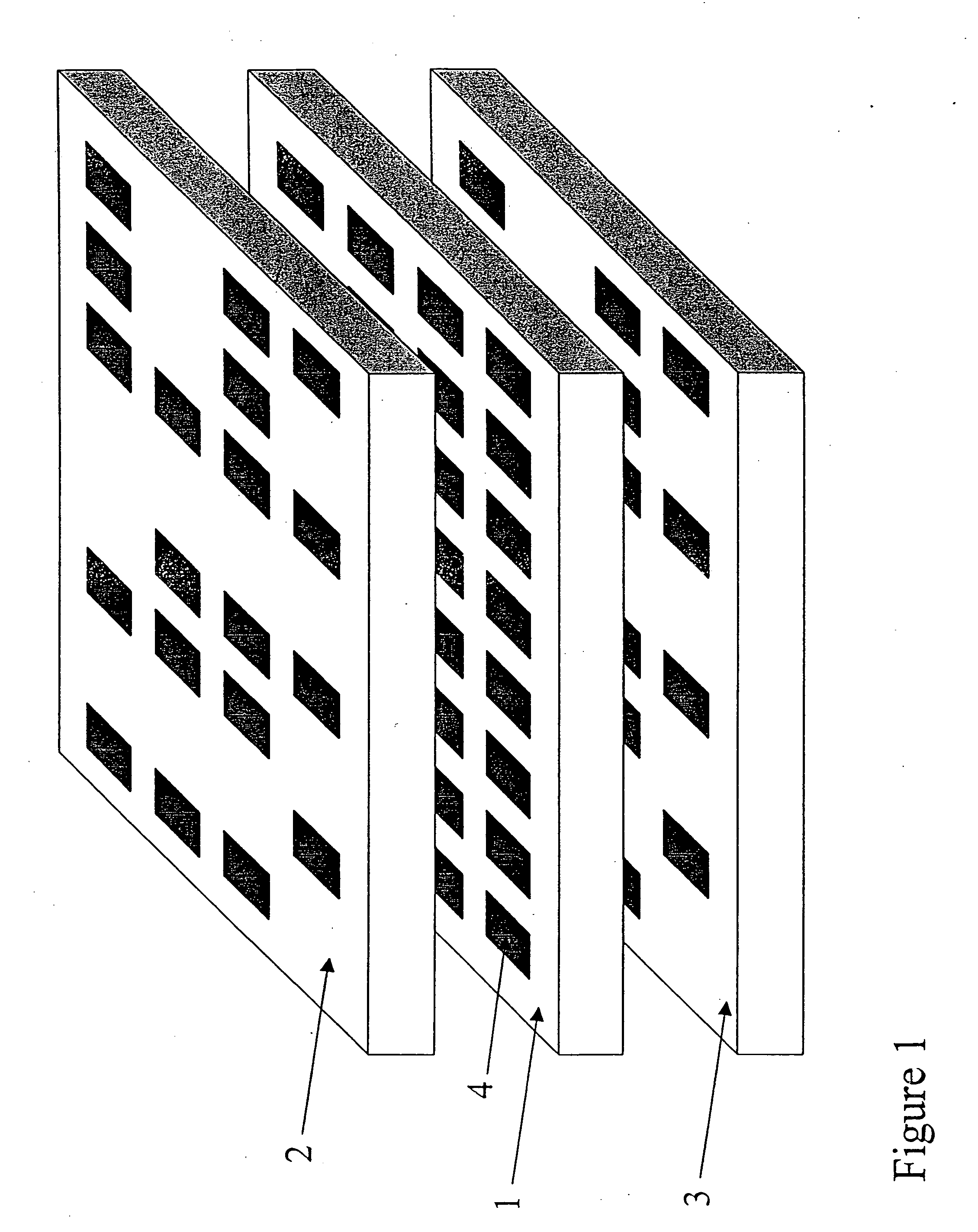
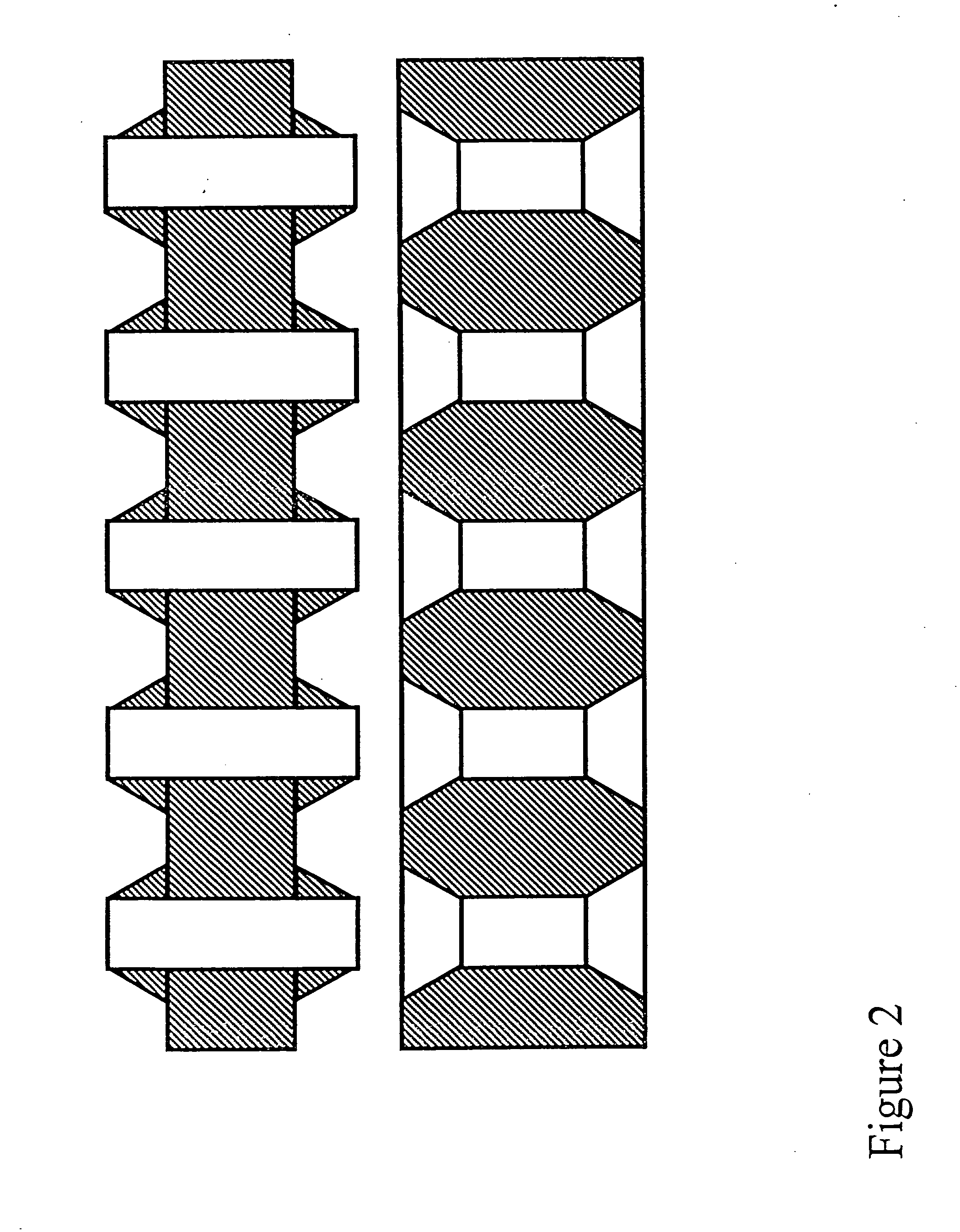
Apparatus for assay, synthesis and storage, and methods of manufacture, use, and manipulation thereof
a technology of apparatus and liquid, applied in the field of apparatus for assay, synthesis and storage, and methods of manufacture, use, and manipulation thereof, can solve the problems of limiting the utility of higher density plates, sample volume loss, and conventional liquid handling techniques such as pipetting, piezoelectric droplet dispensing, etc., to achieve precise and known spatial location, amplification of signal, and preventing cross-contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0327]A 50,000-channel through-hole array is fabricated from silicon. Using the gene database, series of 80 spatial filter top masks with another 80 identical bottom masks are fabricated. Arrays are aligned and derivatized with 3-glycidoxypropyltrimethoxysilane in order to provide a free functional group coating on the interior surfaces of the through-holes.
[0328]A stack of ten coated platens is aligned. Alignment is verified by observing the optical transmission of the various channels. Mask #1 is aligned with the top of the stack and an identical mask aligned in the same orientation and the bottom of the stack. Mask #1 is constructed such that the positions in the mask that corresponded to a gene in the database with an adenosine in the first position are open and allow flow through the through-holes in those addressable positions. The stack is rinsed with dry acetonitrile. A phosphoramidite monomer at a concentration of 0.1 M acetonitrile and tetrazole is added by ...
example 2
Catalyst Screening
[0332]A recombinant enzyme library is screened in a dense array of through-holes against a fluorogenic substrate. Genetically diverse E. coli containing the gene for the protease subtilisin with a poly histidine tag is created by mutagenesis. A dilute solution of the bacteria is added to a nickel-coated array such that there is an average of 1 to 2 bacteria per through-hole. The bacteria are allowed to grow to the log phase and replicate plated (as described above). The bacteria in the array are lysed by heating, allowing the tagged subtilisin to attach to the nickel coated walls of the array. Another array containing the fluorogenic substrate and reaction buffer (Boehringer) in each well is stacked with the first array. The stack is immediately placed in a CCD camera-based fluorescent imaging system, and the rate of increase in fluorescence intensity is measured for each through-hole. The enzyme with the fastest rate is selected and the corresponding bacteria in t...
example 3
High Throughput Screening with Beads
[0333]A 100,000 member combinatorial library immobilized via a photocleavable linker on 10 micron diameter beads is purchased from Affymax. A platen is prepared with 100,000 through-holes of a diameter such that only one bead fits in each hole. The beads are washed in PBS, suspended in a solution containing a fluorogenic substrate for the enzyme to be screened, and spread over the platen with a rubber spatula. An ultraviolet lamp is used to decouple the members of the library from the beads. A second platen filled with a solution of the enzyme in a reaction buffer is aligned and stacked on top of the first chip. The stack is immediately imaged by epi-fluorescence to determine the rate of increase in fluorescence intensity as a function of channel position. Those holes that exhibit decreased enzyme rates are selected for further analysis as drug leads.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com