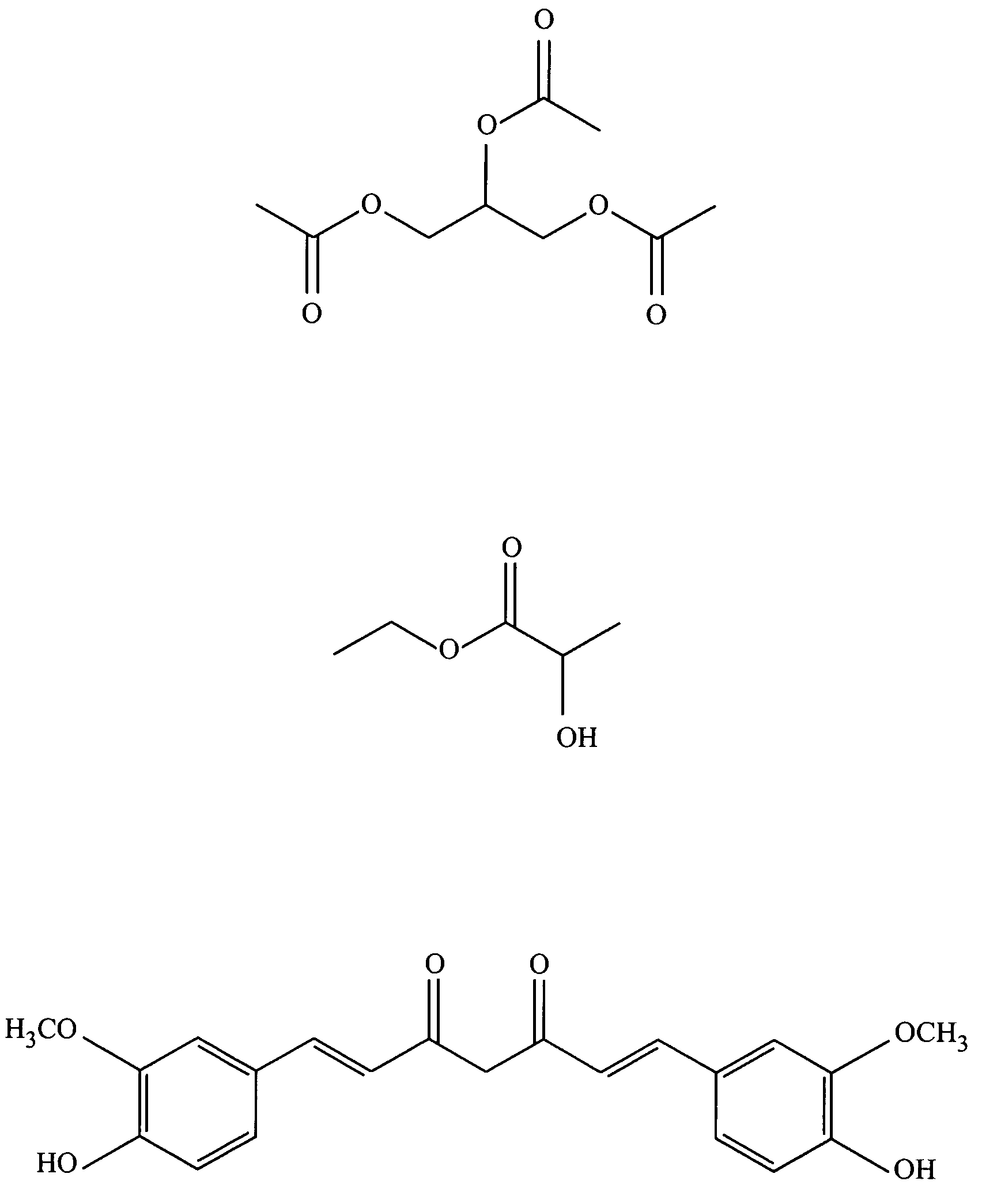
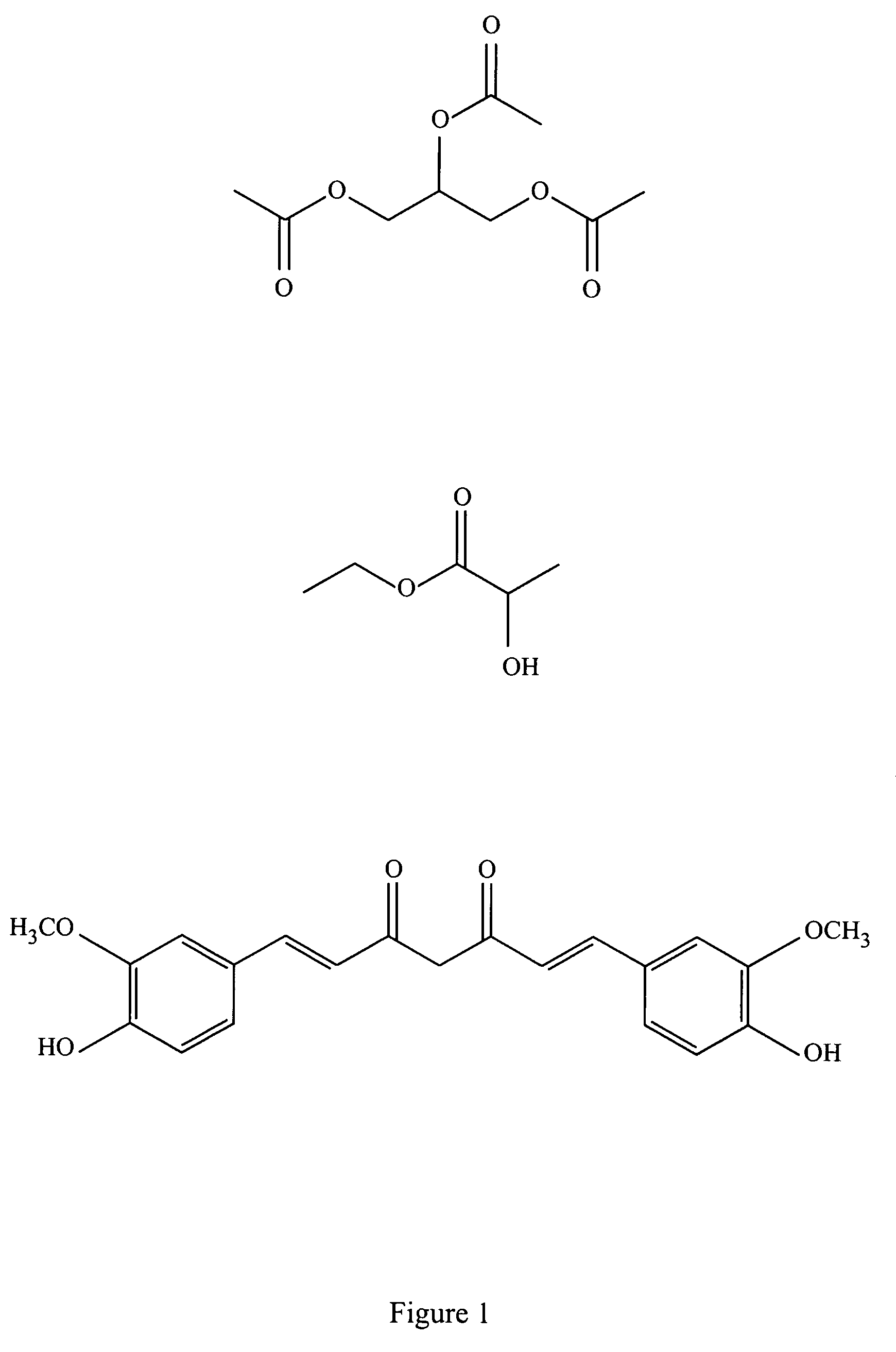
Patents
Literature
30 results about "Research studies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A scientific study of nature that sometimes includes processes involved in health and disease. For example, clinical trials are research studies that involve people. These studies may be related to new ways to screen, prevent, diagnose, and treat disease.
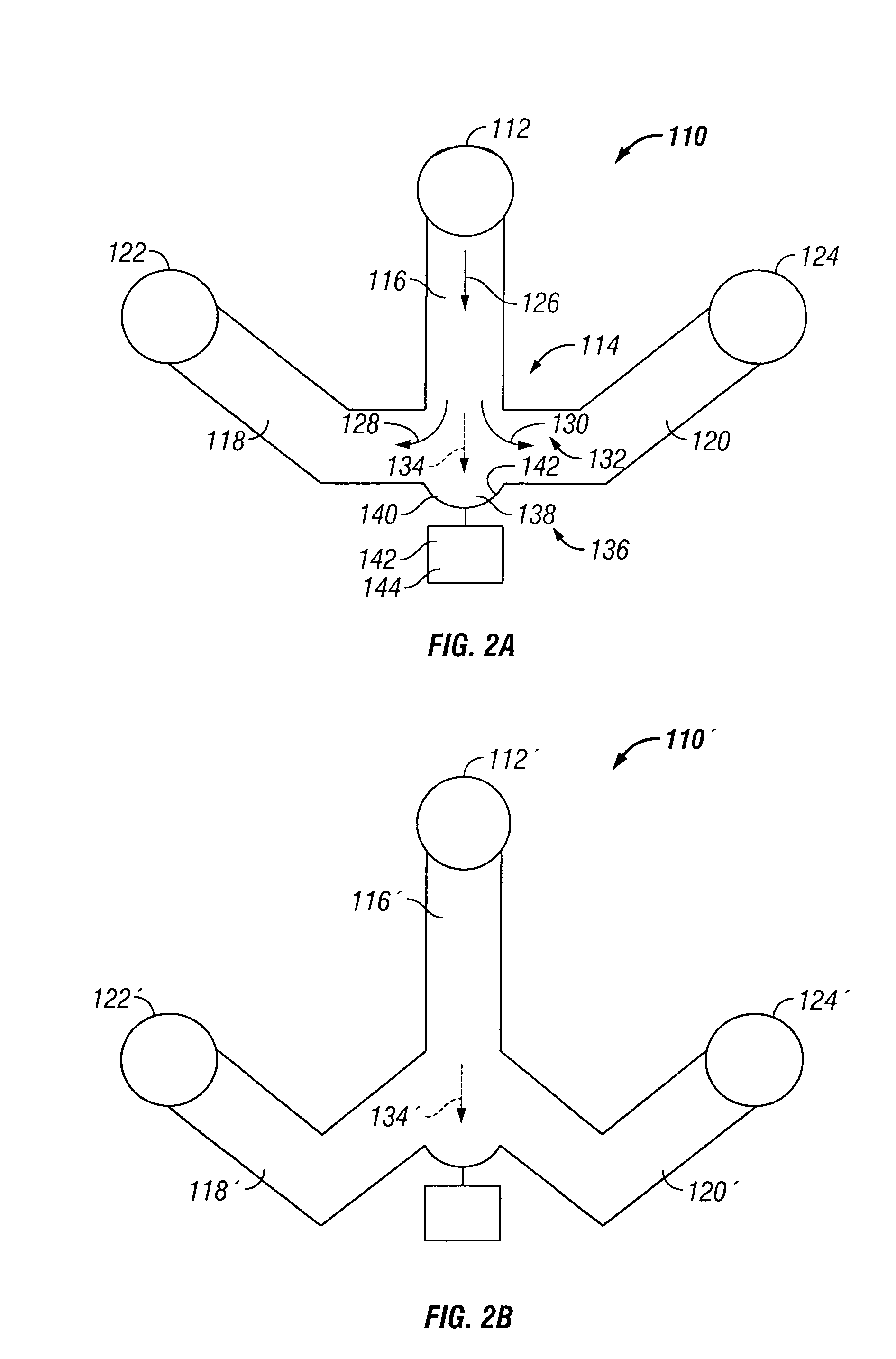
Microfluidic particle-analysis systems
ActiveUS7312085B2Bioreactor/fermenter combinationsBiological substance pretreatmentsReady to useMixed group
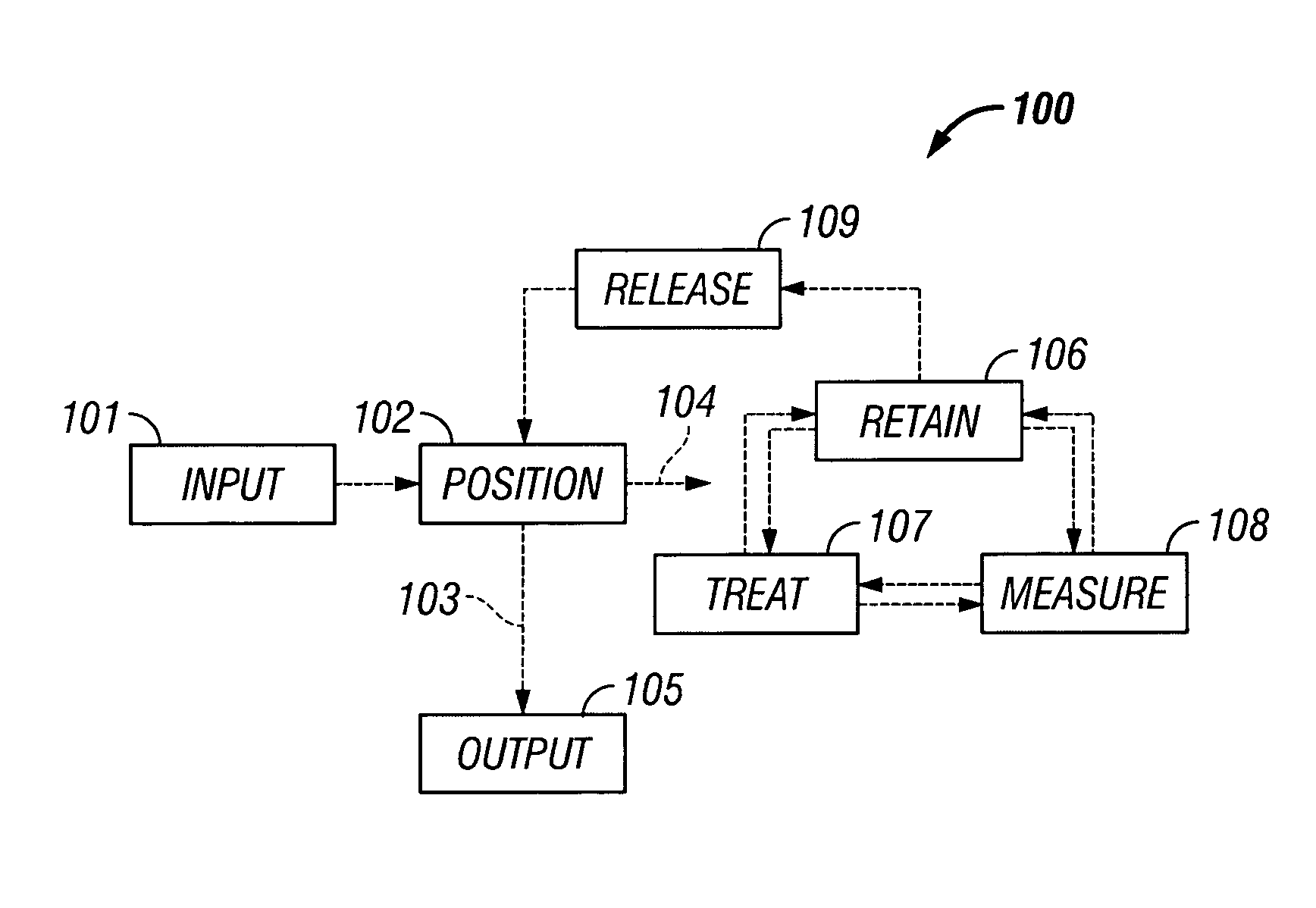
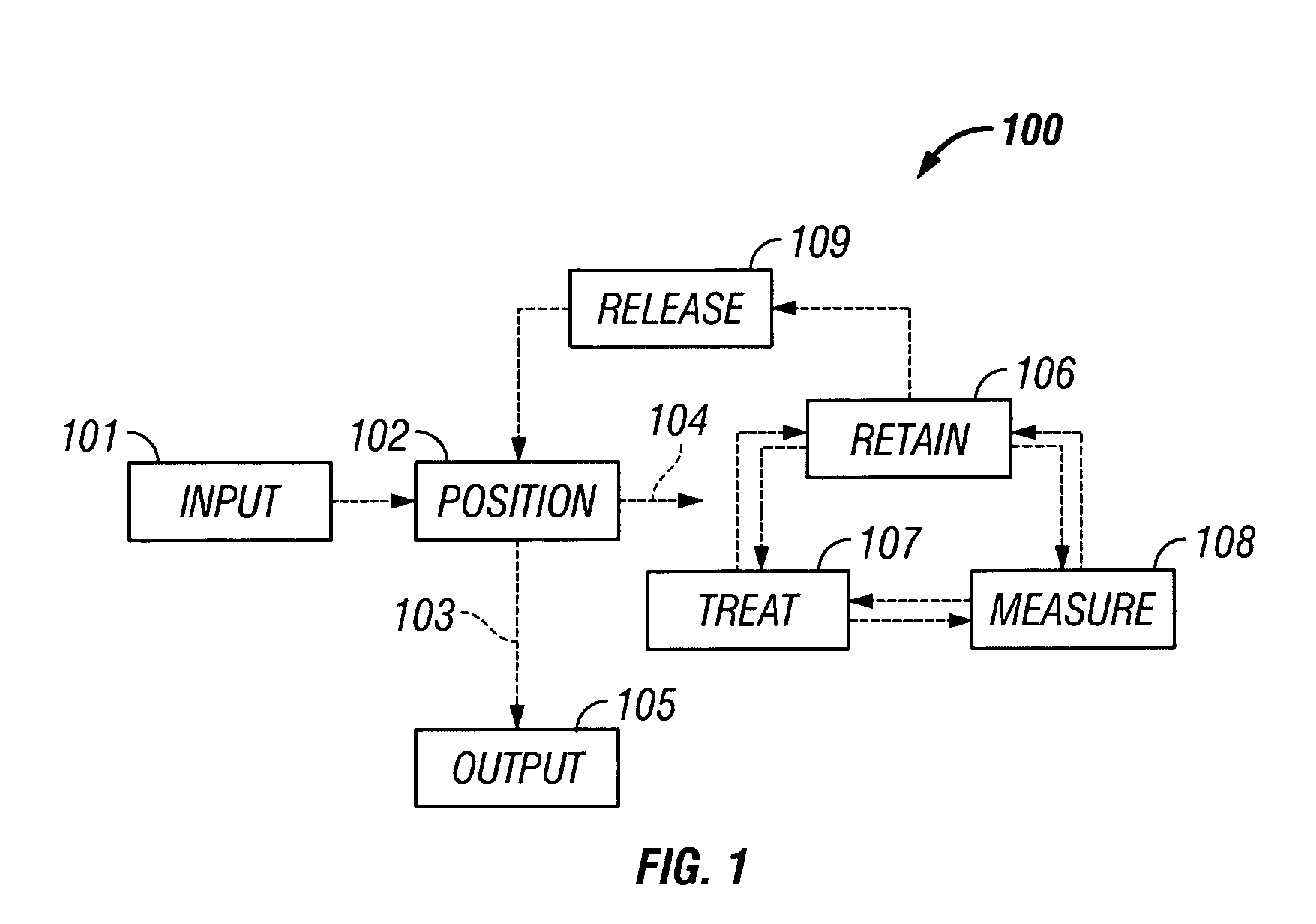
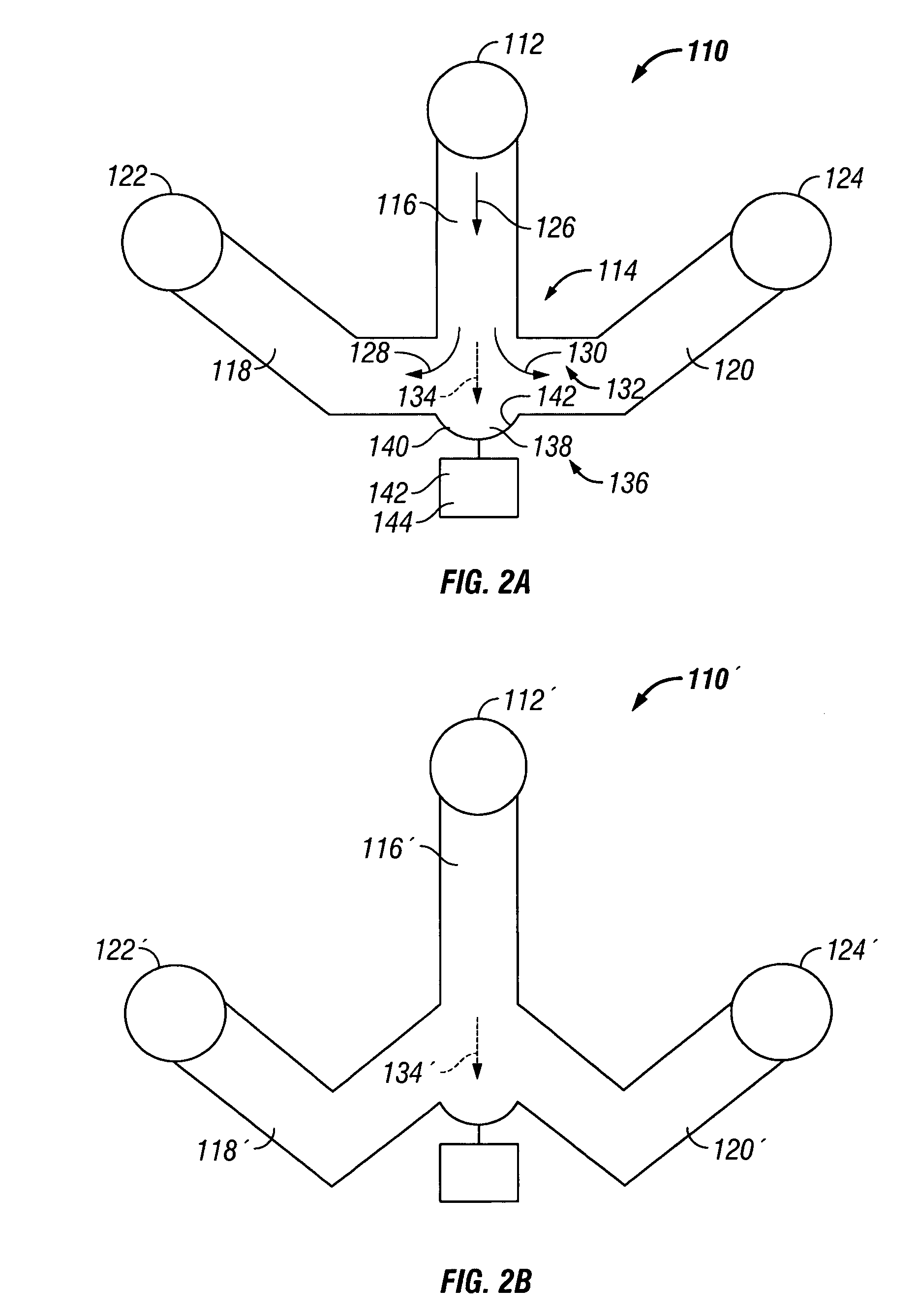
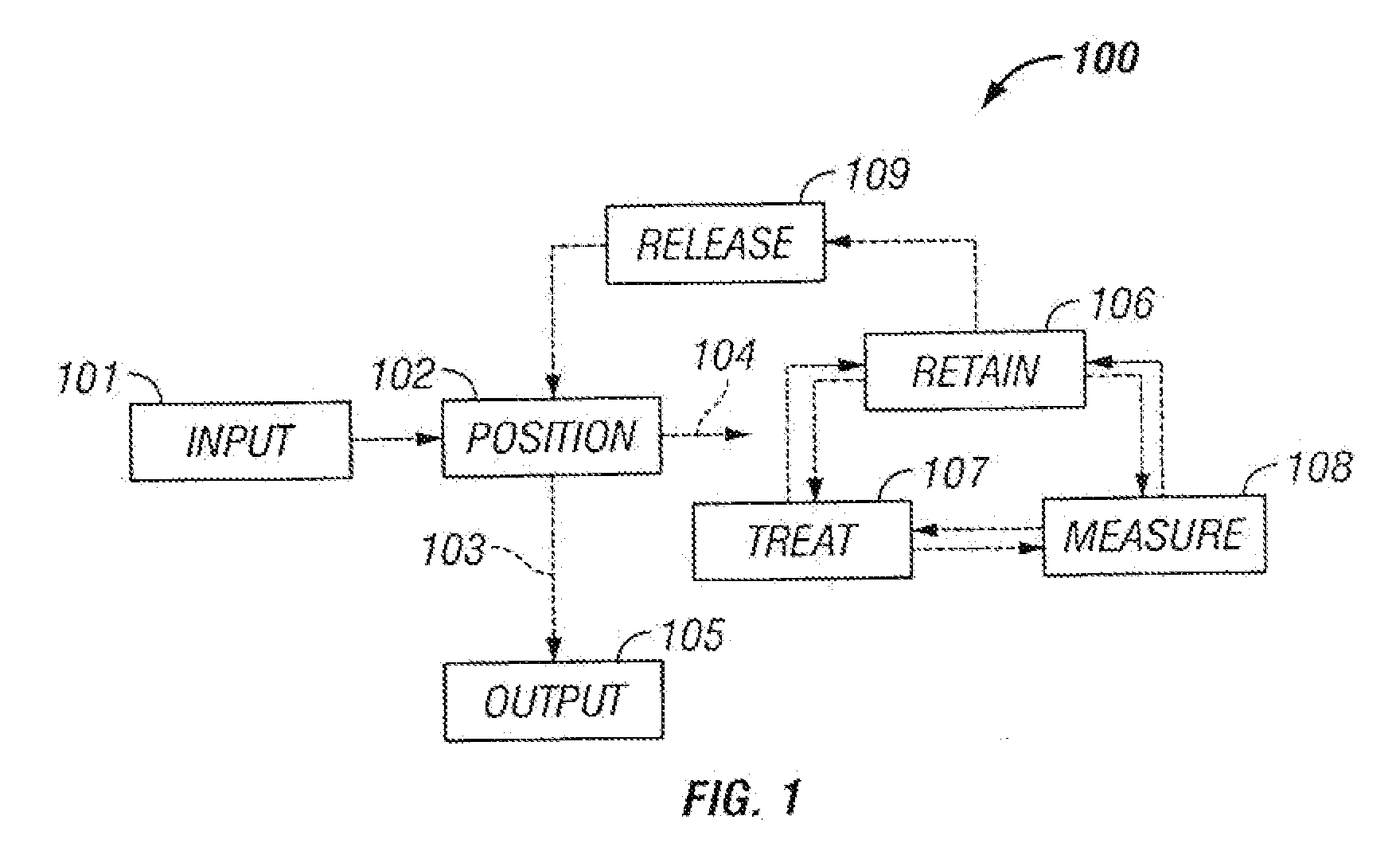
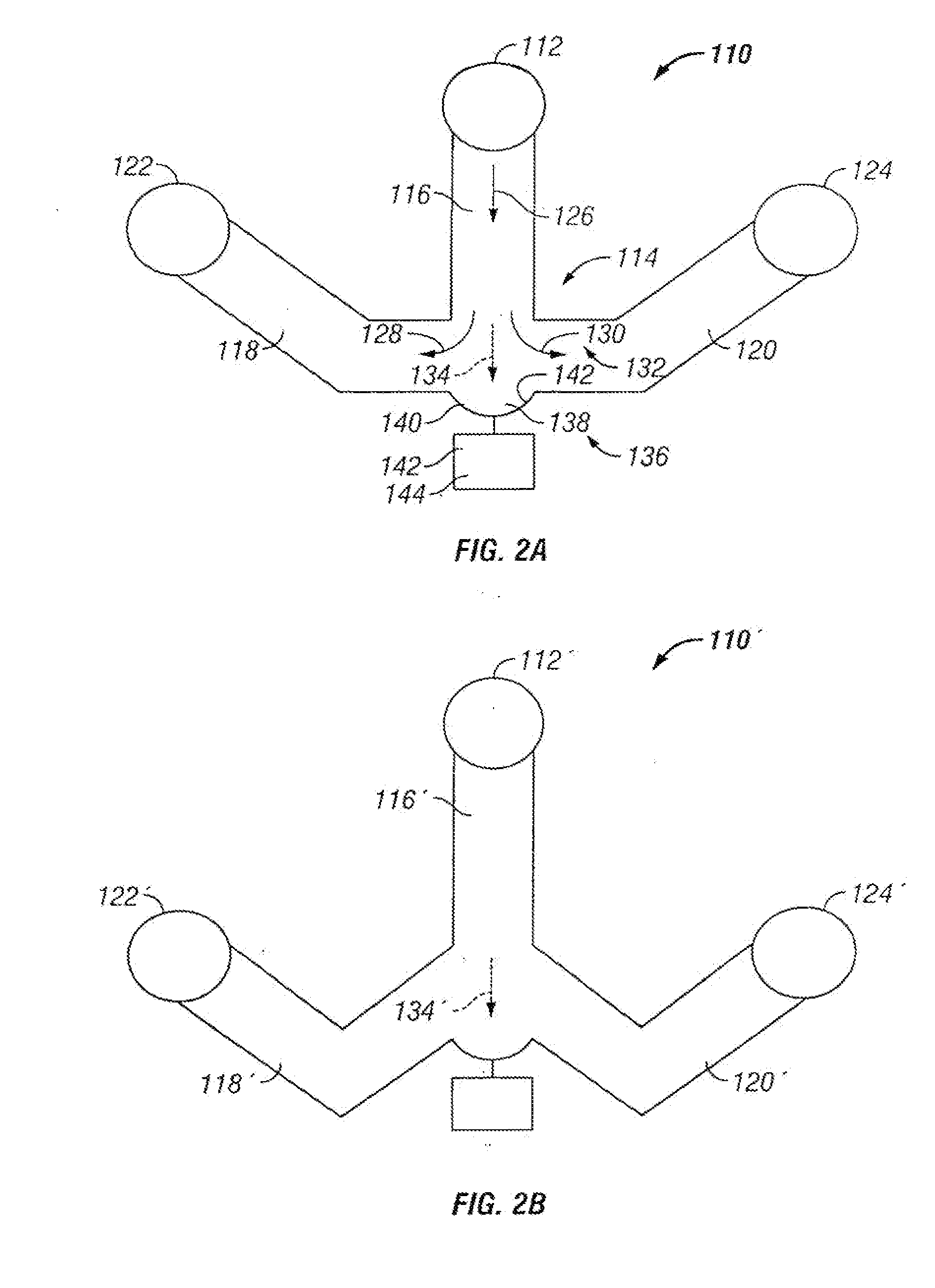
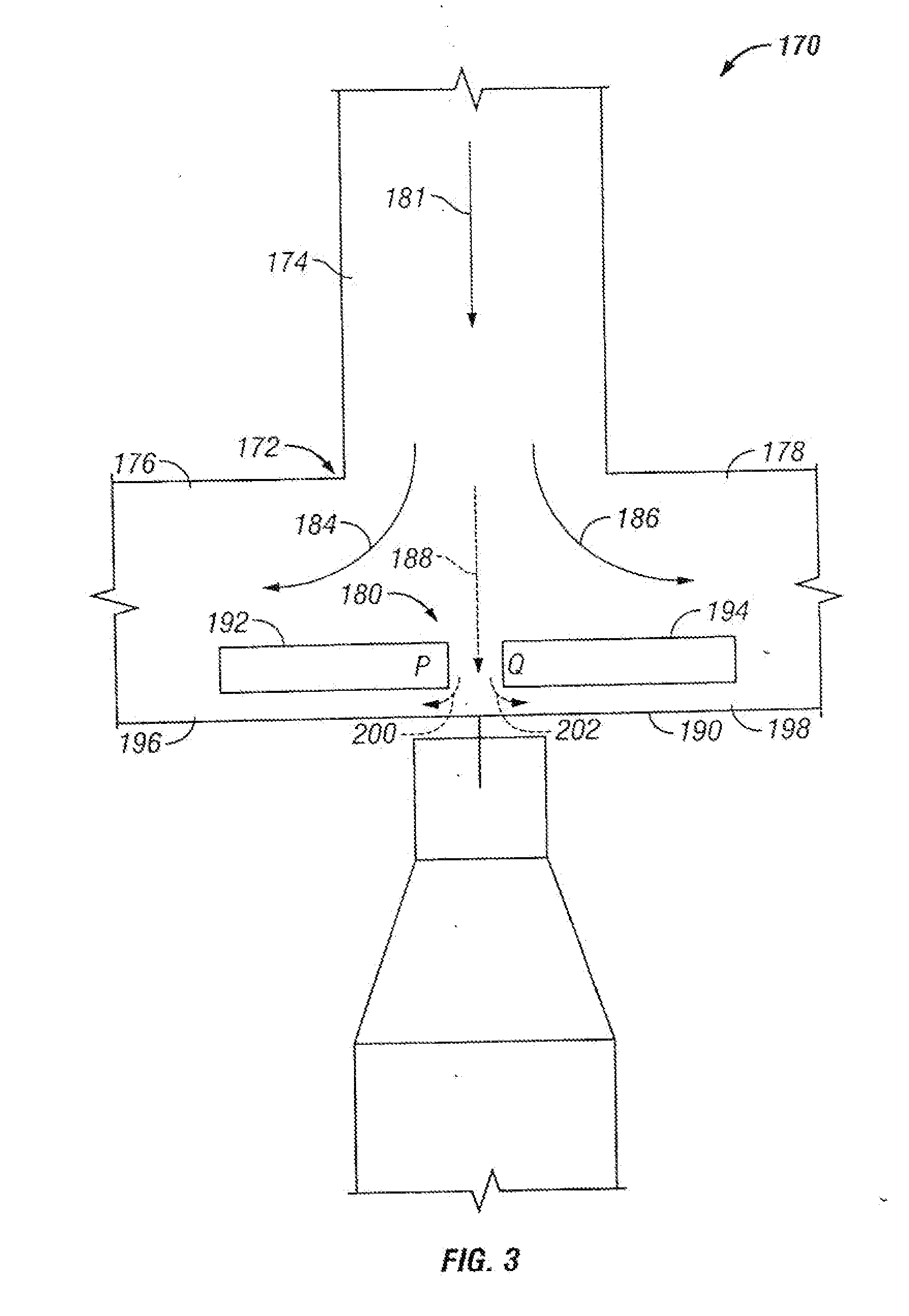
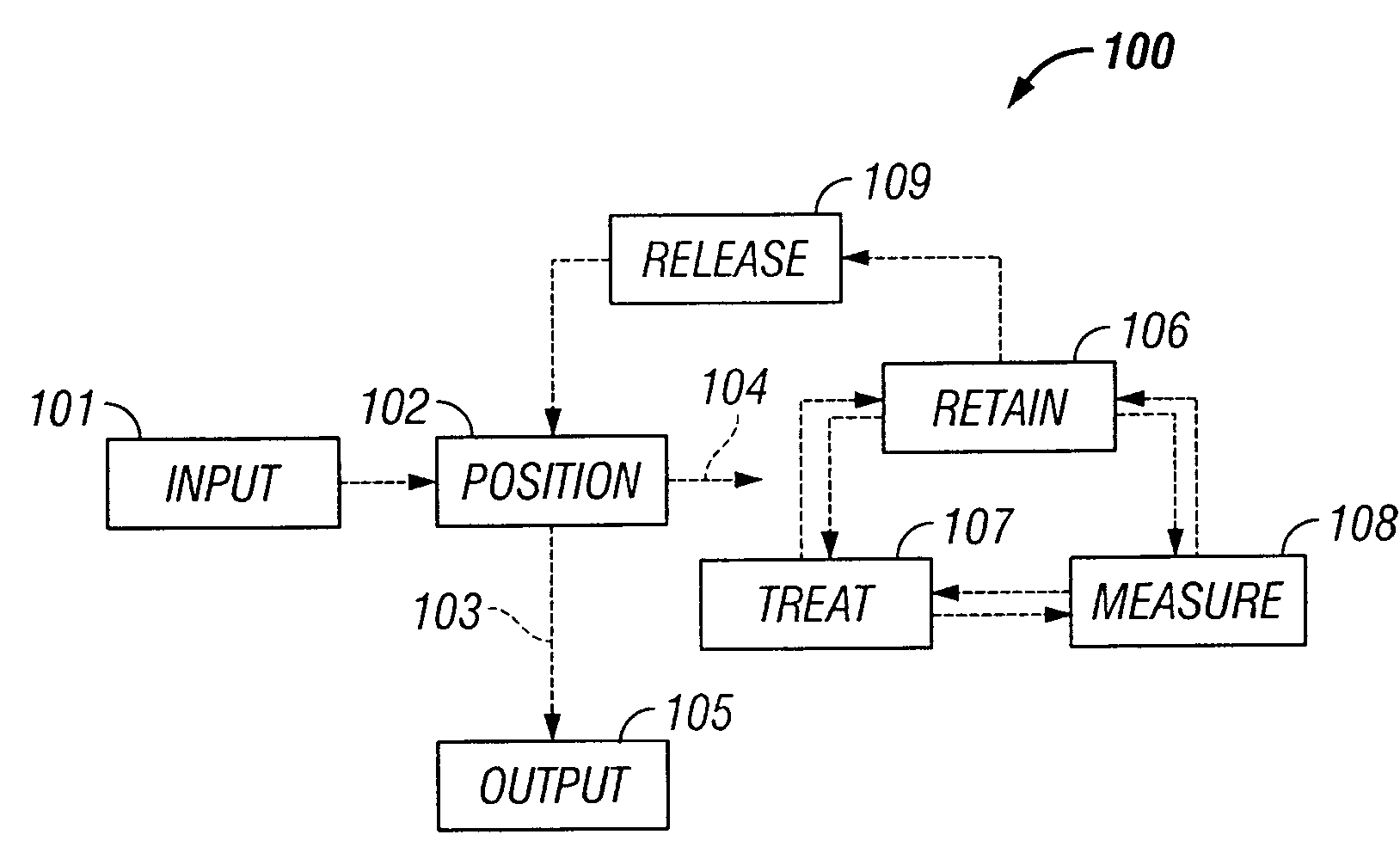
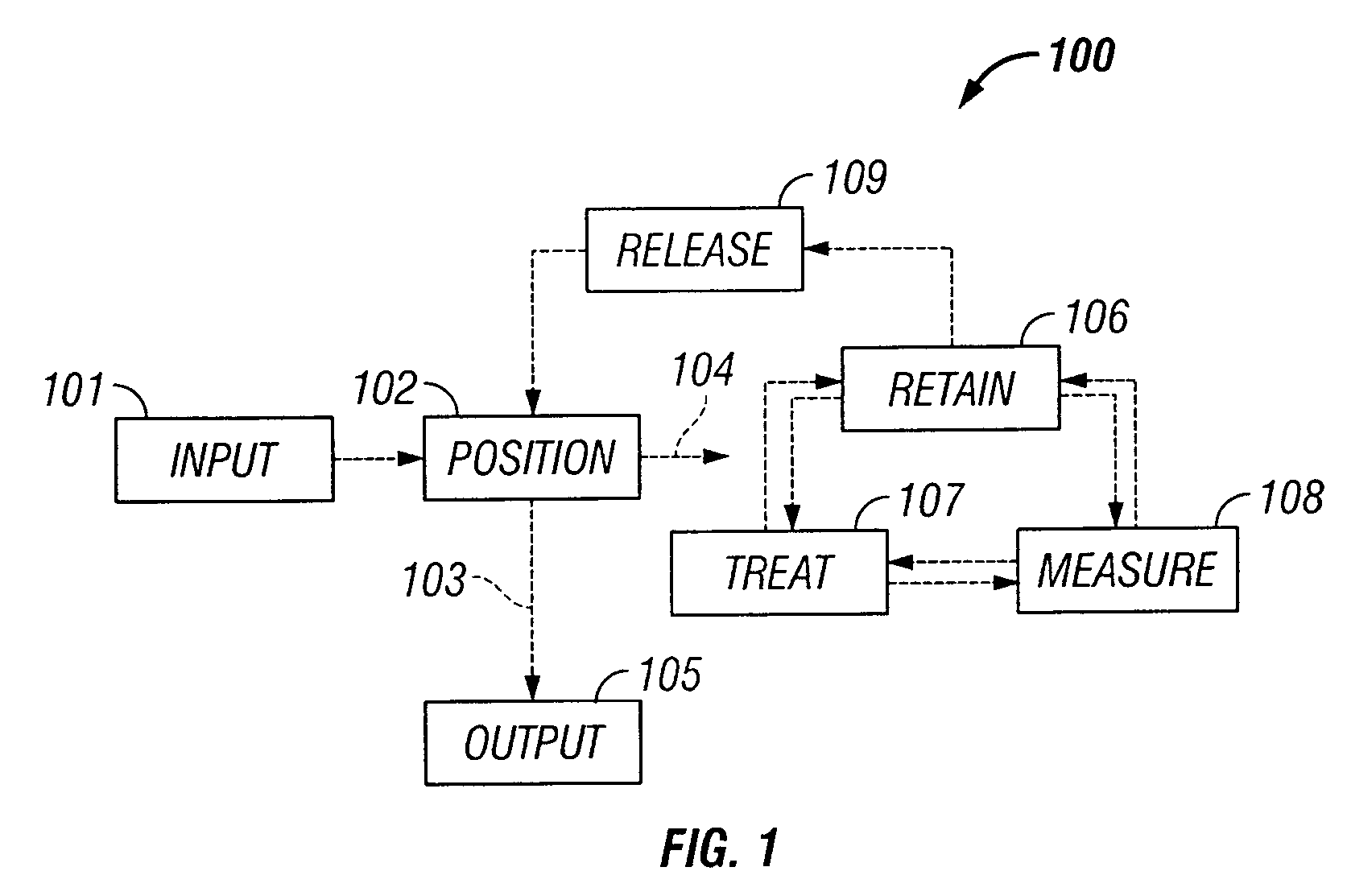
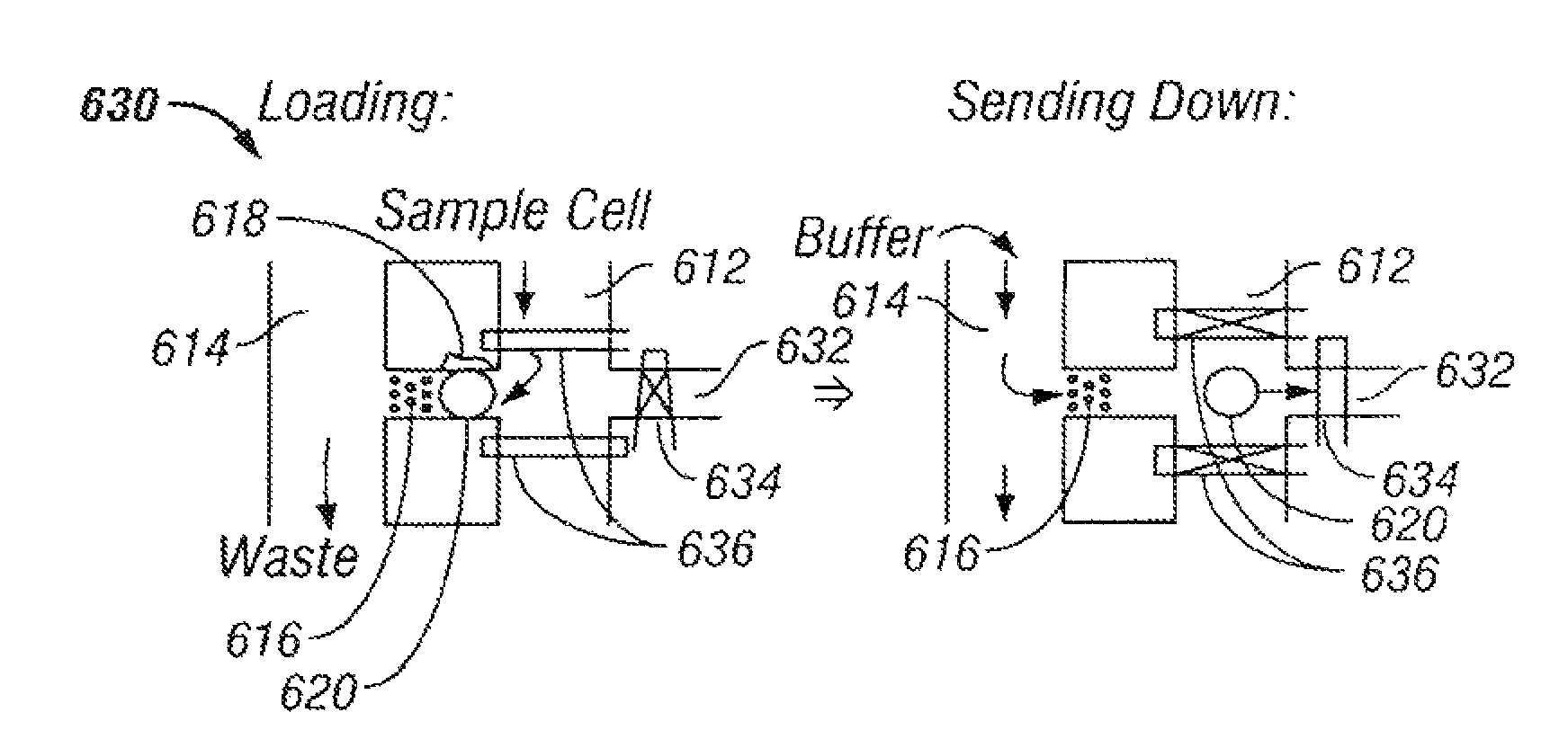
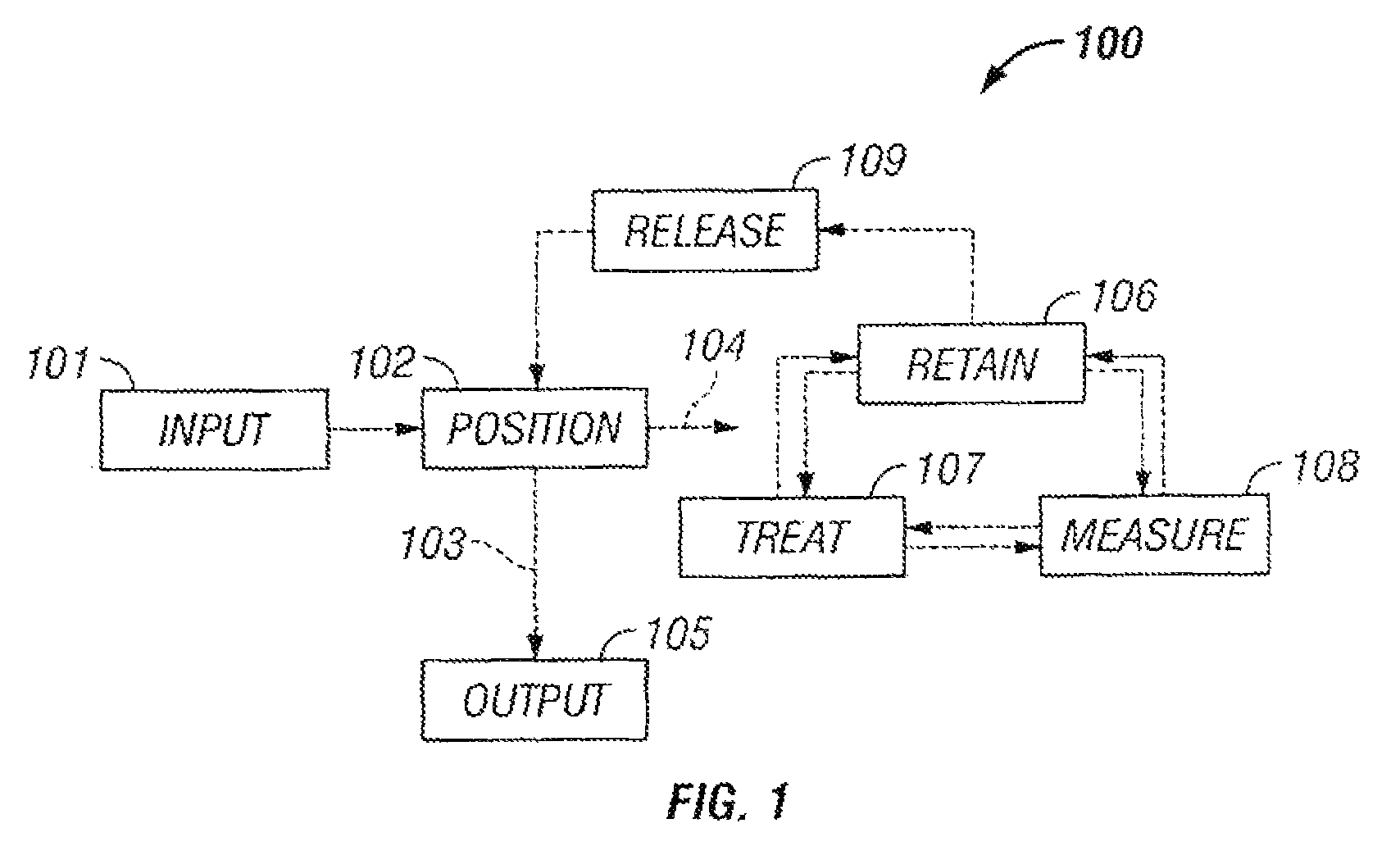
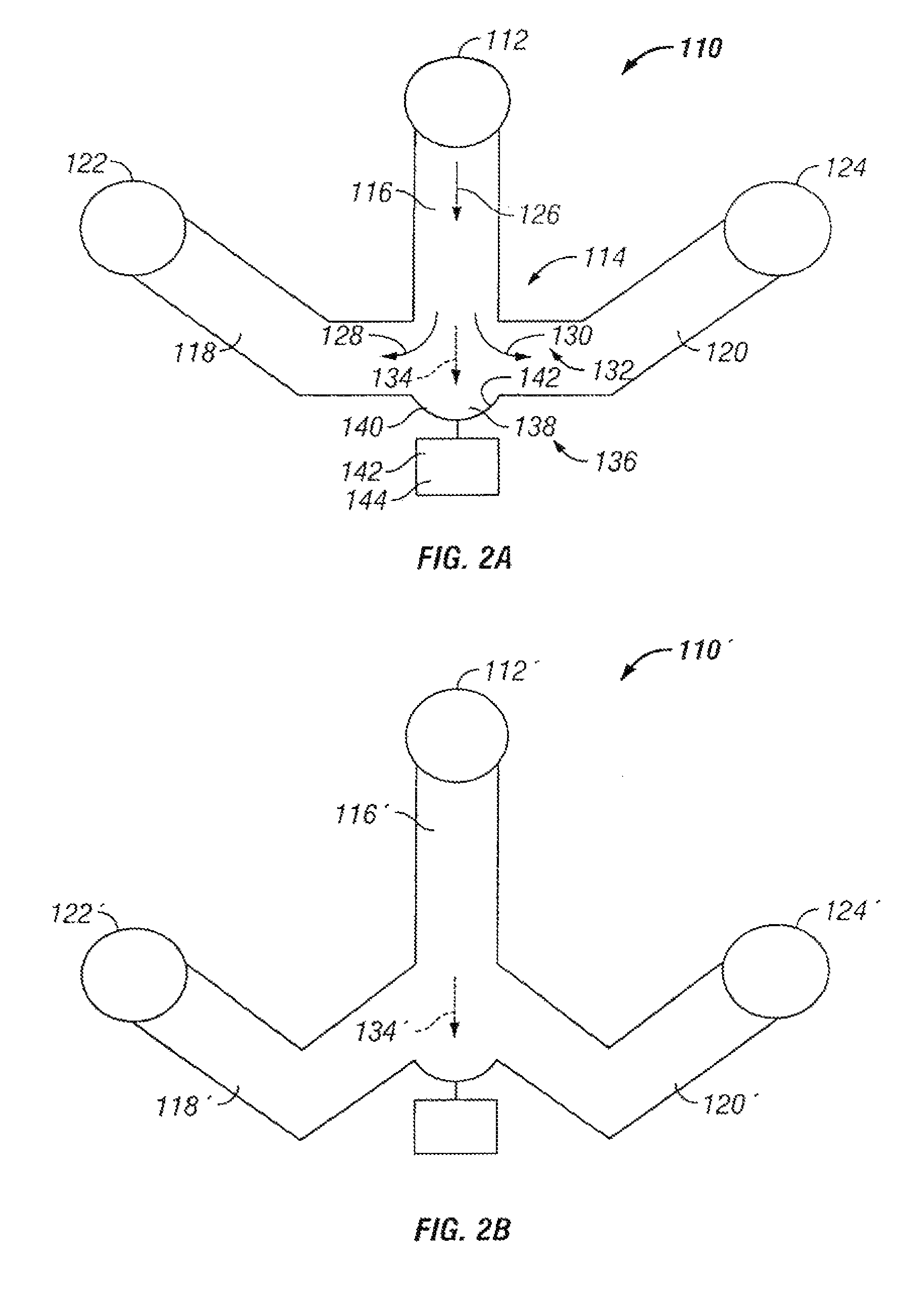
The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or detection of particles, such as cells and / or beads. The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or analysis of particles, such as cells, viruses, organelles, beads, and / or vesicles. The invention also provides microfluidic mechanisms for carrying out these manipulations and analyses. These mechanisms may enable controlled input, movement / positioning, retention / localization, treatment, measurement, release, and / or output of particles. Furthermore, these mechanisms may be combined in any suitable order and / or employed for any suitable number of times within a system. Accordingly, these combinations may allow particles to be sorted, cultured, mixed, treated, and / or assayed, among others, as single particles, mixed groups of particles, arrays of particles, heterogeneous particle sets, and / or homogeneous particle sets, among others, in series and / or in parallel. In addition, these combinations may enable microfluidic systems to be reused. Furthermore, these combinations may allow the response of particles to treatment to be measured on a shorter time scale than was previously possible. Therefore, systems of the invention may allow a broad range of cell and particle assays, such as drug screens, cell characterizations, research studies, and / or clinical analyses, among others, to be scaled down to microfluidic size. Such scaled-down assays may use less sample and reagent, may be less labor intensive, and / or may be more informative than comparable macrofluidic assays.
Owner:STANDARD BIOTOOLS INC
Microfluidic particle-analysis systems
InactiveUS20100120077A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAssayMixed group
The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or detection of particles, such as cells and / or beads. The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or analysis of particles, such as cells, viruses, organelles, beads, and / or vesicles. The invention also provides microfluidic mechanisms for carrying out these manipulations and analyses. These mechanisms may enable controlled input, movement / positioning, retention / localization, treatment, measurement, release, and / or output of particles. Furthermore, these mechanisms may be combined in any suitable order and / or employed for any suitable number of times within a system. Accordingly, these combinations may allow particles to be sorted, cultured, mixed, treated, and / or assayed, among others, as single particles, mixed groups of particles, arrays of particles, heterogeneous particle sets, and / or homogeneous particle sets, among others, in series and / or in parallel. In addition, these combinations may enable microfluidic systems to be reused. Furthermore, these combinations may allow the response of particles to treatment to be measured on a shorter time scale than was previously possible. Therefore, systems of the invention may allow a broad range of cell and particle assays, such as drug screens, cell characterizations, research studies, and / or clinical analyses, among others, to be scaled down to microfluidic size. Such scaled-down assays may use less sample and reagent, may be less labor intensive, and / or may be more informative than comparable macrofluidic assays.
Owner:STANDARD BIOTOOLS INC
Microfluidic particle-analysis systems
InactiveUS7452726B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMixed groupScale down
The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or detection of particles, such as cells and / or beads. The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or analysis of particles, such as cells, viruses, organelles, beads, and / or vesicles. The invention also provides microfluidic mechanisms for carrying out these manipulations and analyses. These mechanisms may enable controlled input, movement / positioning, retention / localization, treatment, measurement, release, and / or output of particles. Furthermore, these mechanisms may be combined in any suitable order and / or employed for any suitable number of times within a system. Accordingly, these combinations may allow particles to be sorted, cultured, mixed, treated, and / or assayed, among others, as single particles, mixed groups of particles, arrays of particles, heterogeneous particle sets, and / or homogeneous particle sets, among others, in series and / or in parallel. In addition, these combinations may enable microfluidic systems to be reused. Furthermore, these combinations may allow the response of particles to treatment to be measured on a shorter time scale than was previously possible. Therefore, systems of the invention may allow a broad range of cell and particle assays, such as drug screens, cell characterizations, research studies, and / or clinical analyses, among others, to be scaled down to microfluidic size. Such scaled-down assays may use less sample and reagent, may be less labor intensive, and / or may be more informative than comparable macrofluidic assays.
Owner:FLUIDIGM CORP
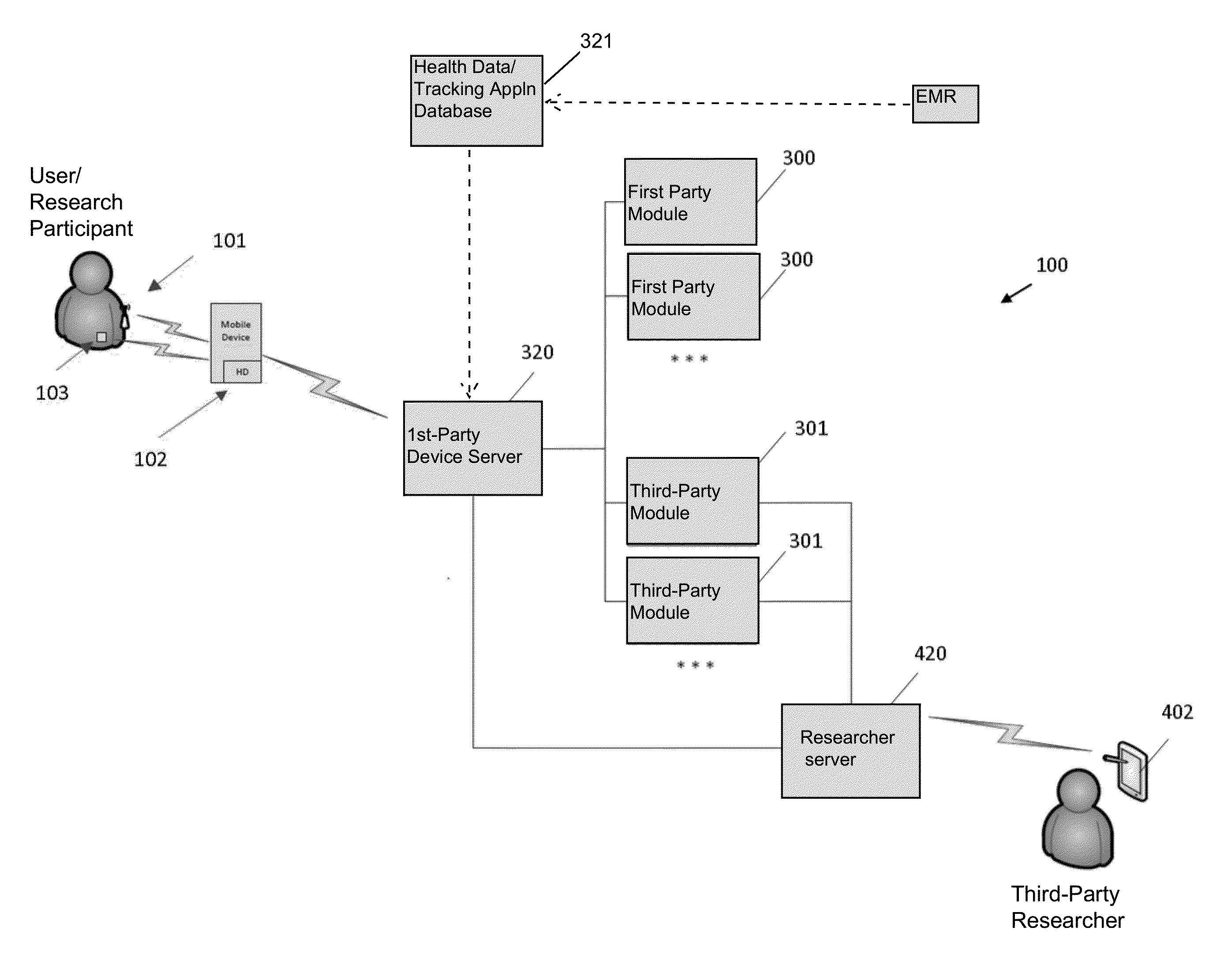
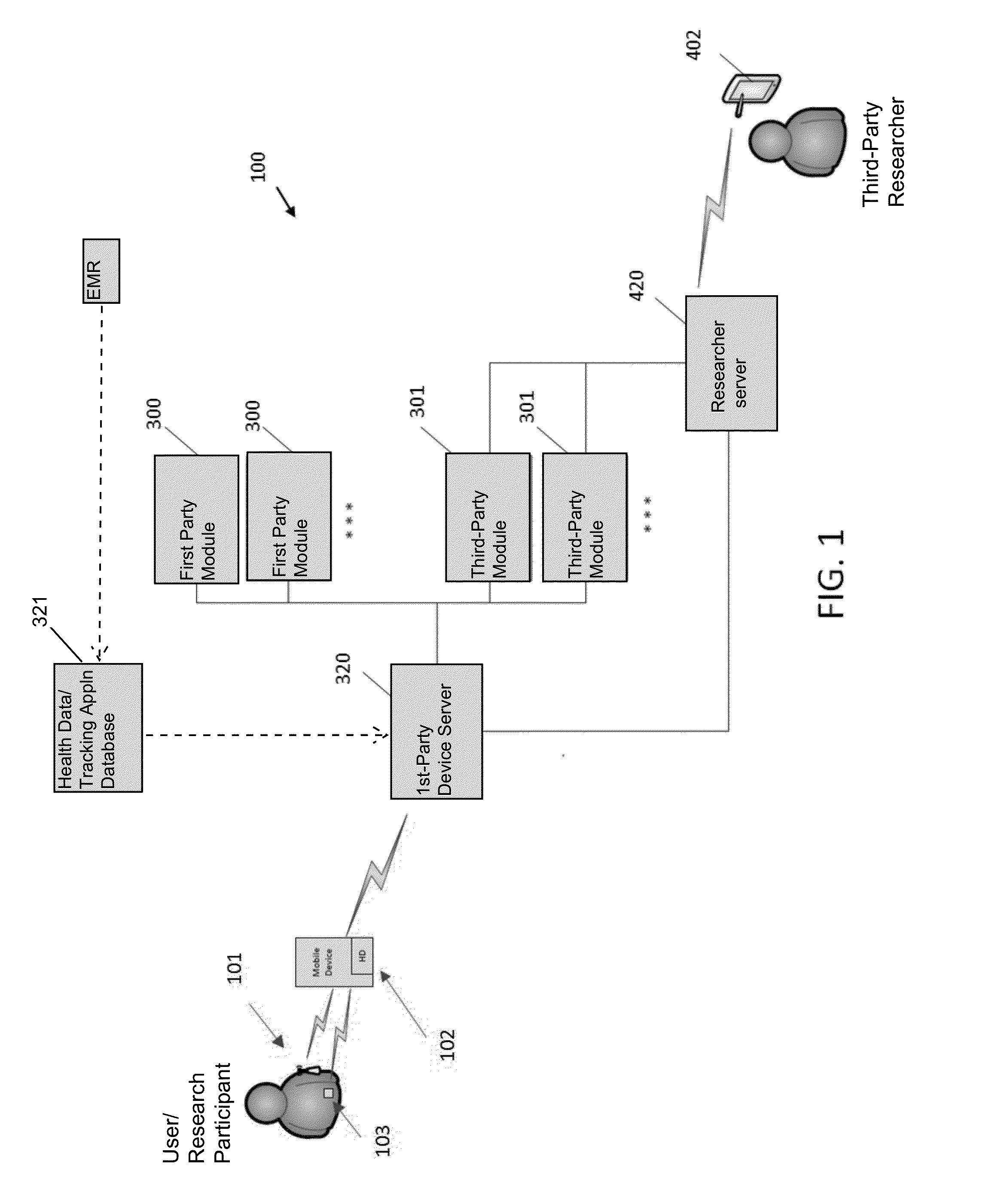
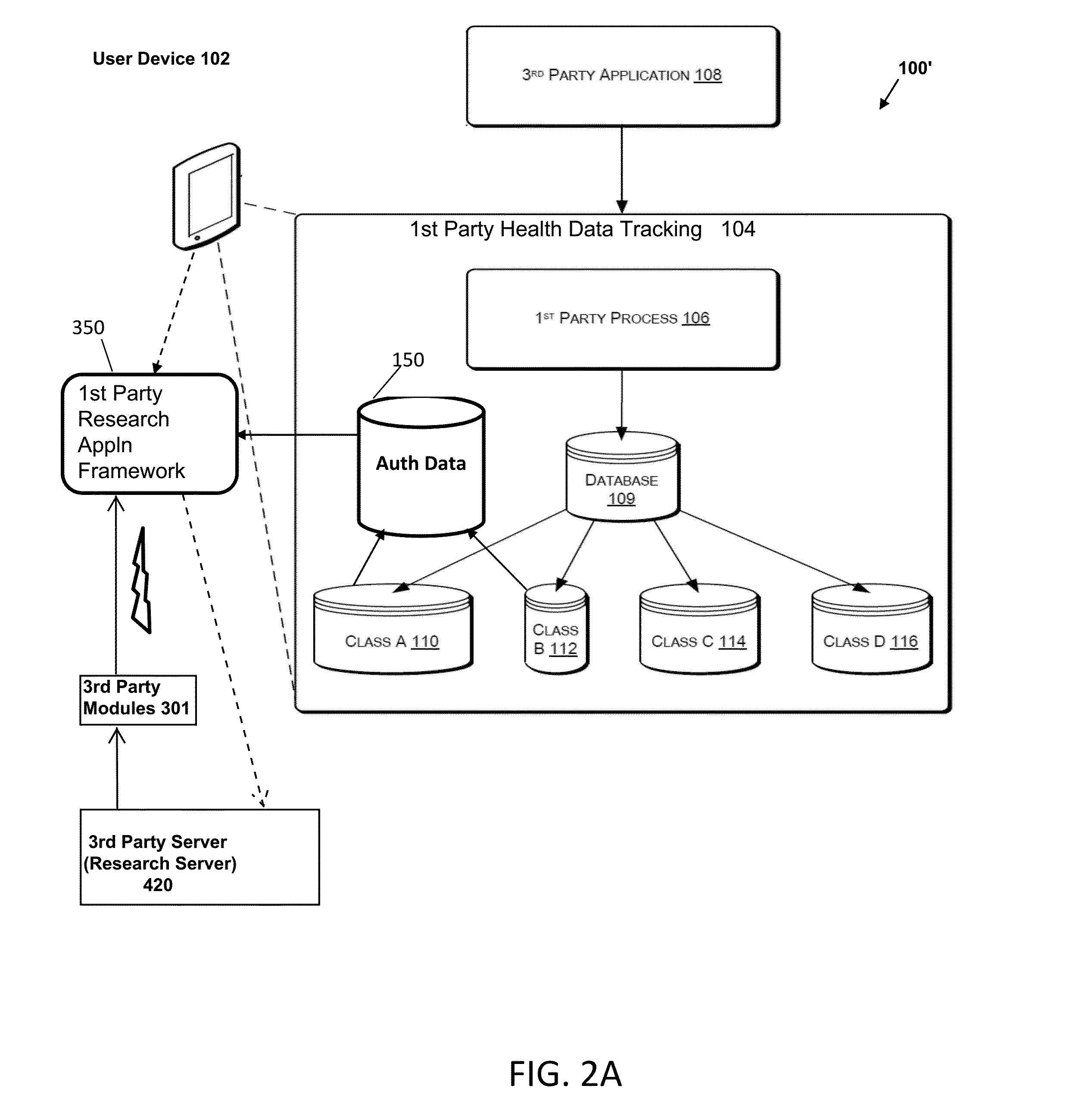
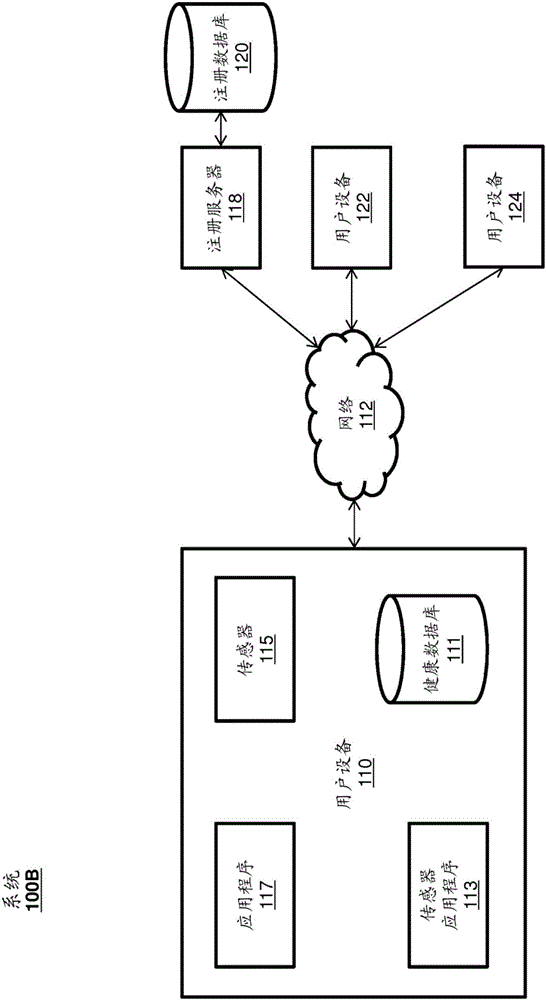
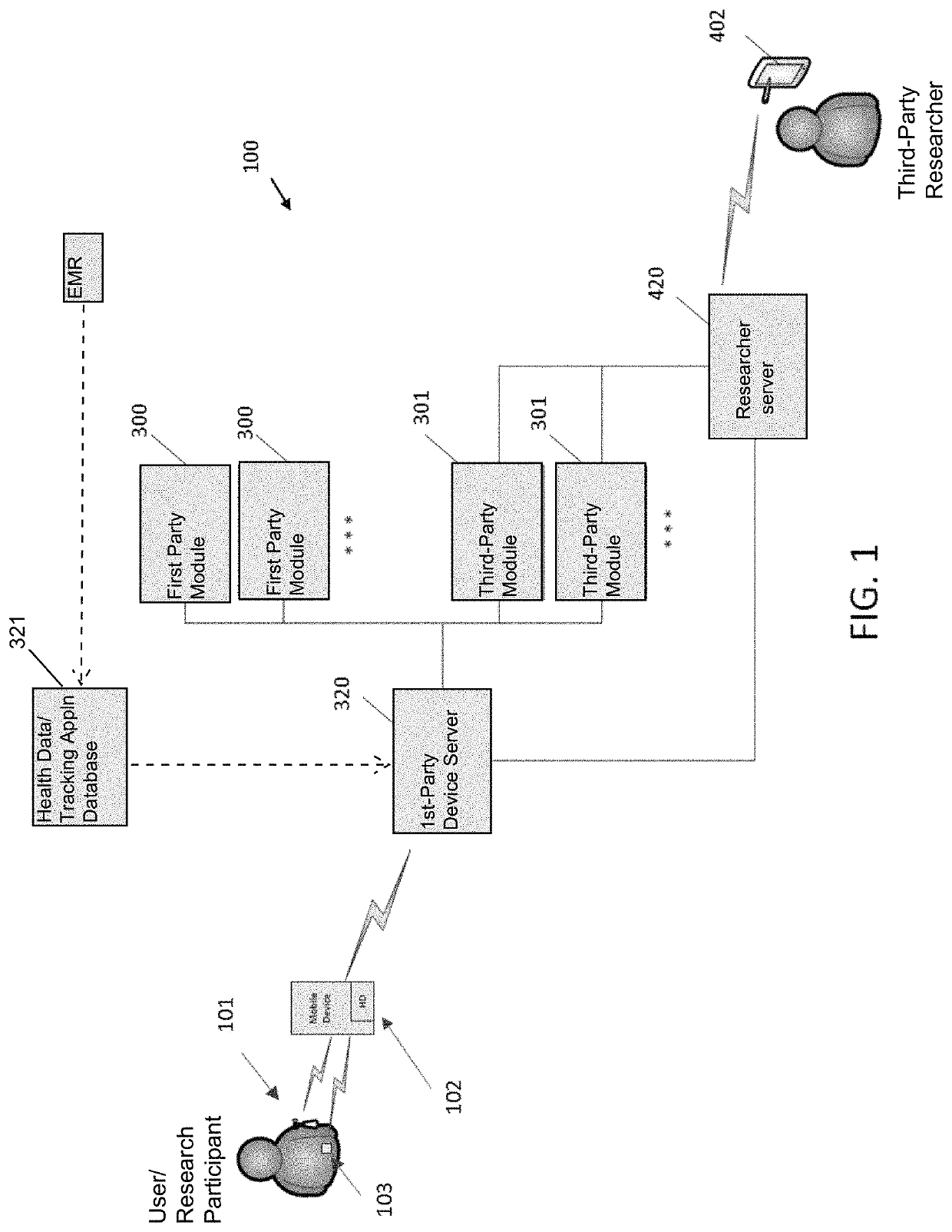
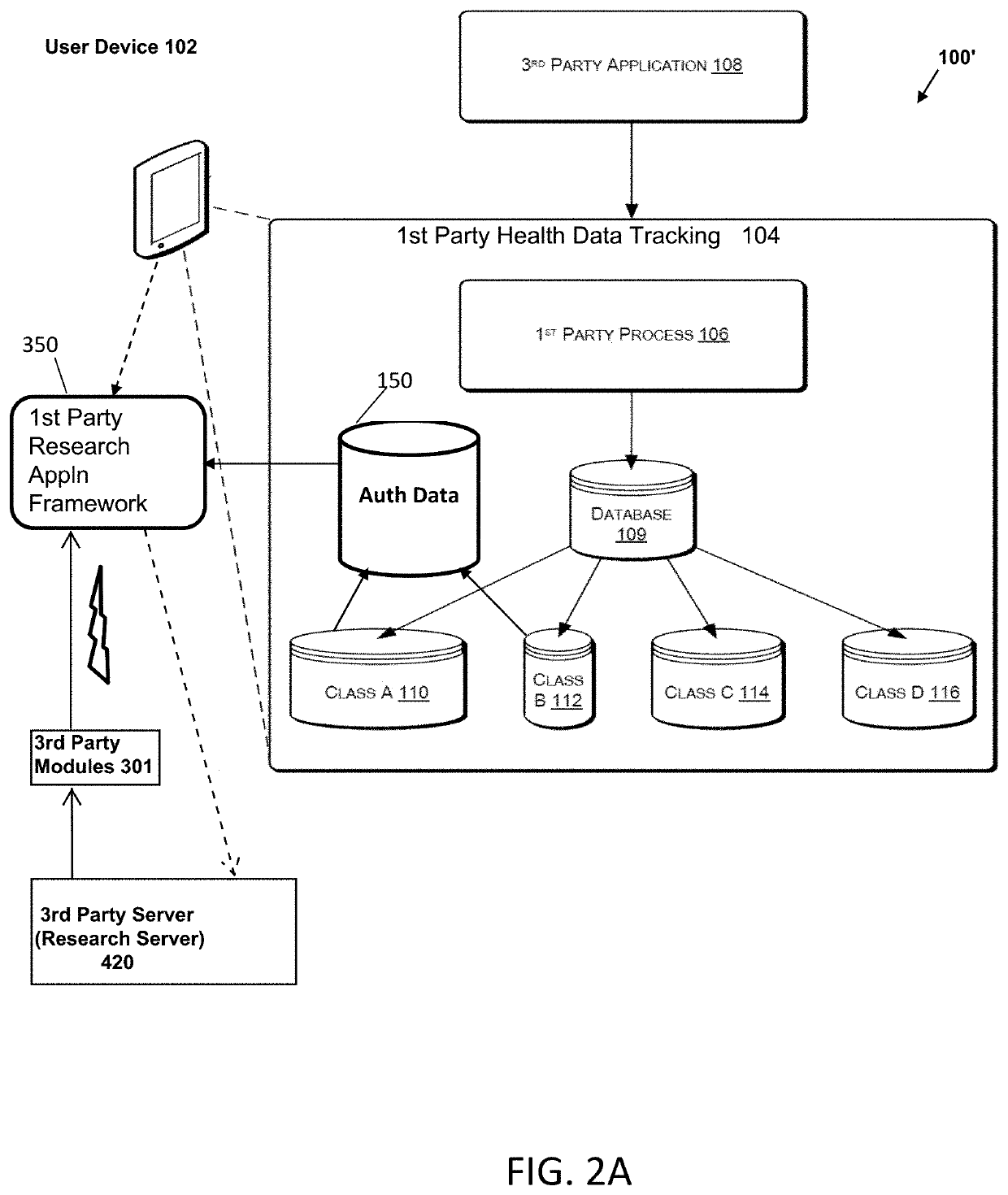
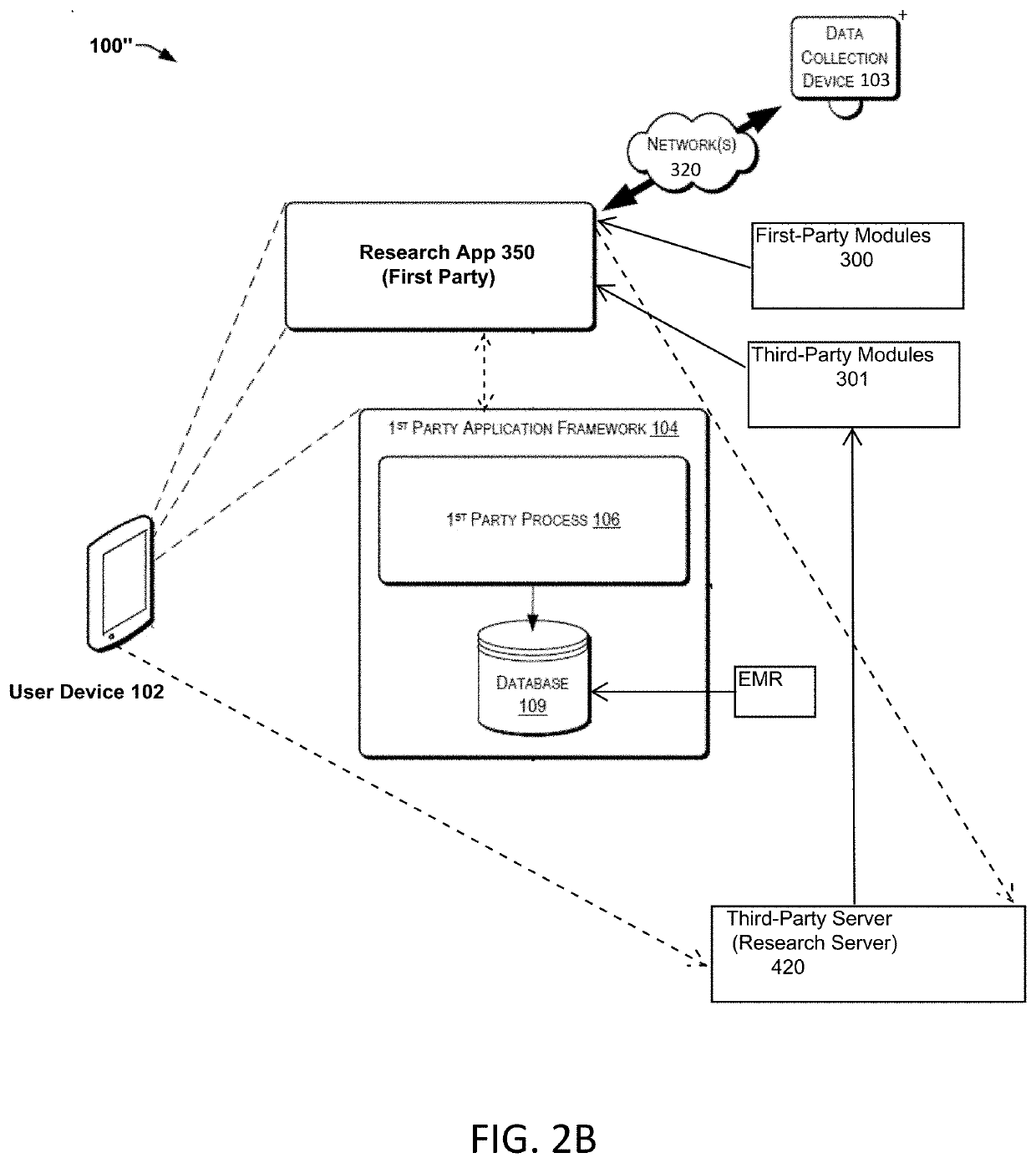
Systems and Methods for Facilitating Health Research
Methods and systems for facilitating health research through enhanced communication between research participants and researchers. Methods include communication of information related to a research study to a portable computing device of a research participant by use of an application framework and one or more modules. The one or more modules may be provided with the application framework or may include one or more modules from third-party researchers so as to allow standardization of communication from multiple research studies and differing research facilities.
Owner:APPLE INC
Microfluidic particle-analysis systems
InactiveUS8658418B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAssayMixed group
The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or detection of particles, such as cells and / or beads. The invention provides systems, including apparatus, methods, and kits, for the microfluidic manipulation and / or analysis of particles, such as cells, viruses, organelles, beads, and / or vesicles. The invention also provides microfluidic mechanisms for carrying out these manipulations and analysis. These mechanisms may enable controlled input, movement / positioning, retention / localization, treatment, measurement, release, and / or output of particles. Furthermore, these mechanisms may be combined in any suitable order and / or employed for any suitable number of times within a system. Accordingly, these combinations may allow particles to be sorted, cultured, mixed, treated, and / or assayed, among others, as single particles, mixed groups of particles, arrays of particles, heterogeneous particle sets, and / or homogeneous particle sets, among others, in series and / or in parallel. In addition, these combinations may enable microfluidic systems to be reused. Furthermore, these combinations may allow the response of particles to treatment to be measured on a shorter time scale than was previously possible. Therefore, systems of the invention may allow a broad range of cell and particle assays, such as drug screens, cell characterizations, research studies, and / or clinical analysis, among others, to be scaled down to microfluidic size. Such scaled-down assays may use less sample and reagent, may be less labor intensive, and / or may be more informative than comparable macrofluidic assays.
Owner:STANDARD BIOTOOLS INC
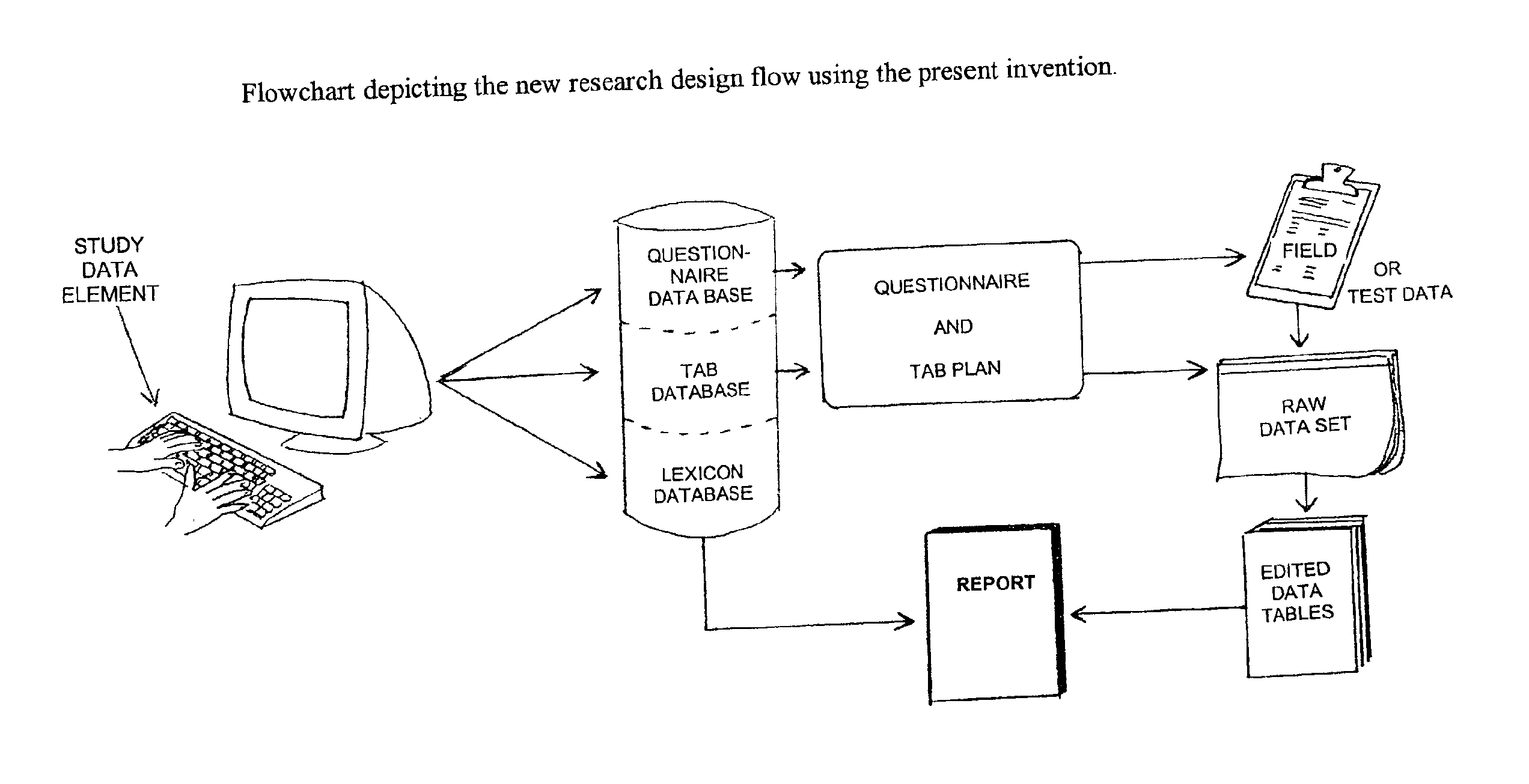
Method and apparatus for the design and analysis of market research studies
InactiveUS6865578B2Less timeEnsure qualityMarket predictionsOffice automationProgram planningHeuristic
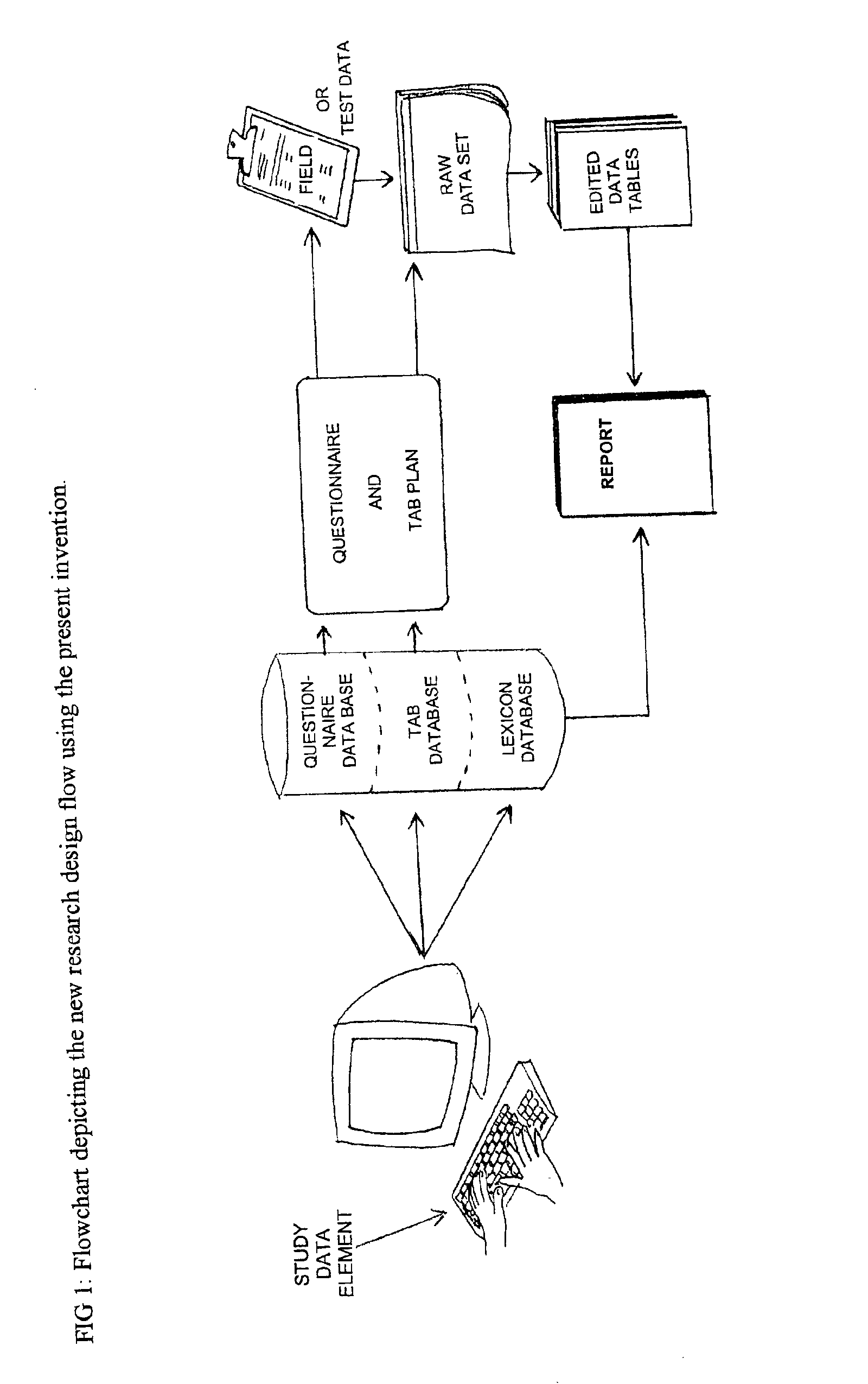
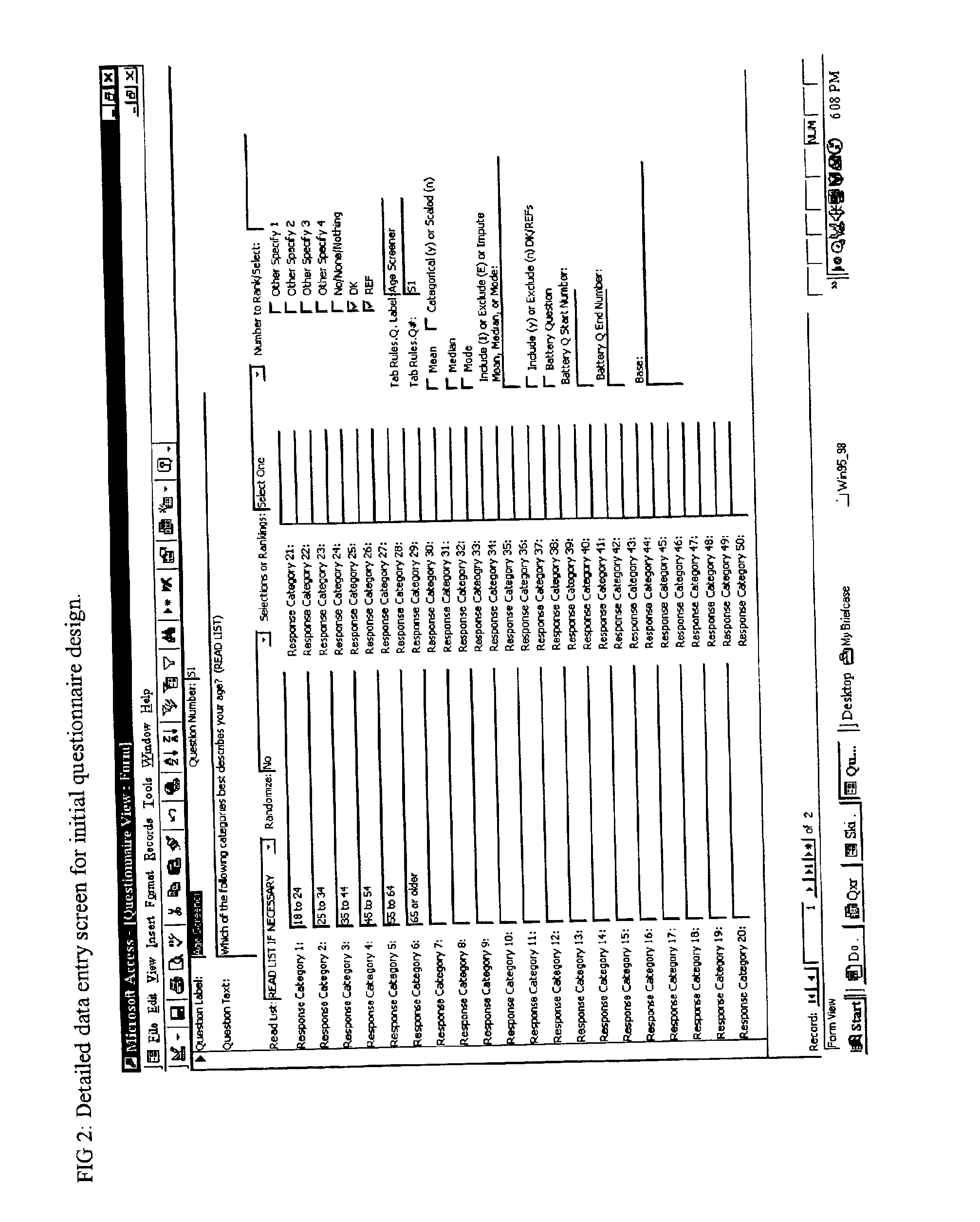
This invention automates the most common processes in market research study design and analysis. By combining a database framework designed to hold all the data elements used in a market research survey, codifying heuristics actually used by analysts to make decisions about survey analysis, and building a lexicon to communicate summary results to lay readers. First, this provides a stringent framework for designing a questionnaire and study plan which will lead to clear tab plans and analysis plans. Second, by automating the research design and analysis process, it eliminates the human error associated with the intricacies of questionnaire design, tab plan design, and research report writing. Thirdly, it also greatly reduces the time necessary to rerun an entire report based on simple changes to any study element definitions to a few hours, a process formerly taking days or weeks. Lastly, it reduces the need for experienced researchers in the research design and analysis process, freeing analysts to spend a greater proportion of their time working on the more mentally challenging and demanding work of synthesizing the market research survey to produce credible findings, rather than the redundant work of data tabulation and summary reporting.
Owner:HAYS WESLEY JOSEPH
System and method for recruiting subjects for research studies and clinical trials over the internet
InactiveUS20140289001A1Computer-assisted medical data acquisitionComputer security arrangementsLimited accessResearch Object
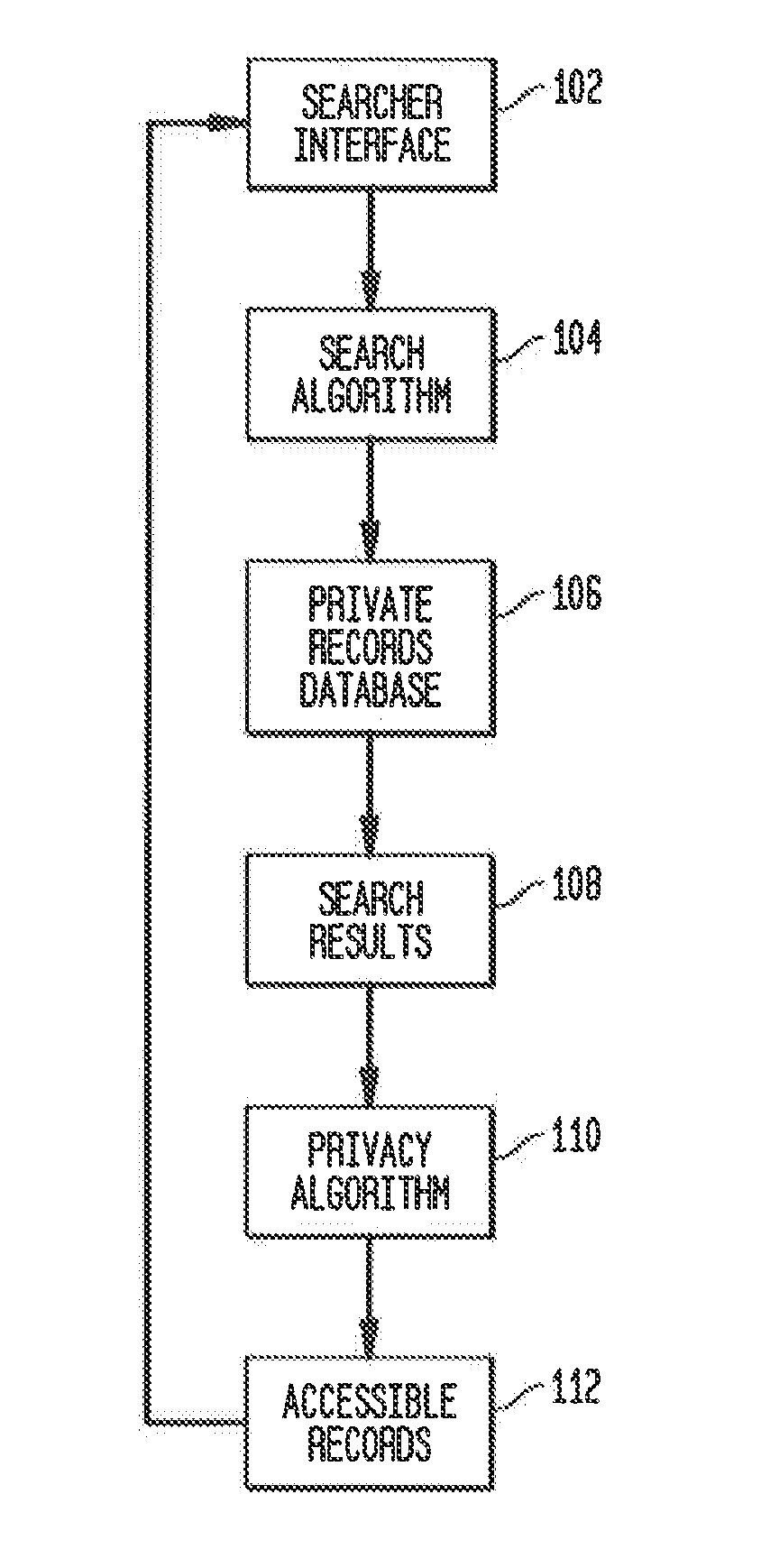
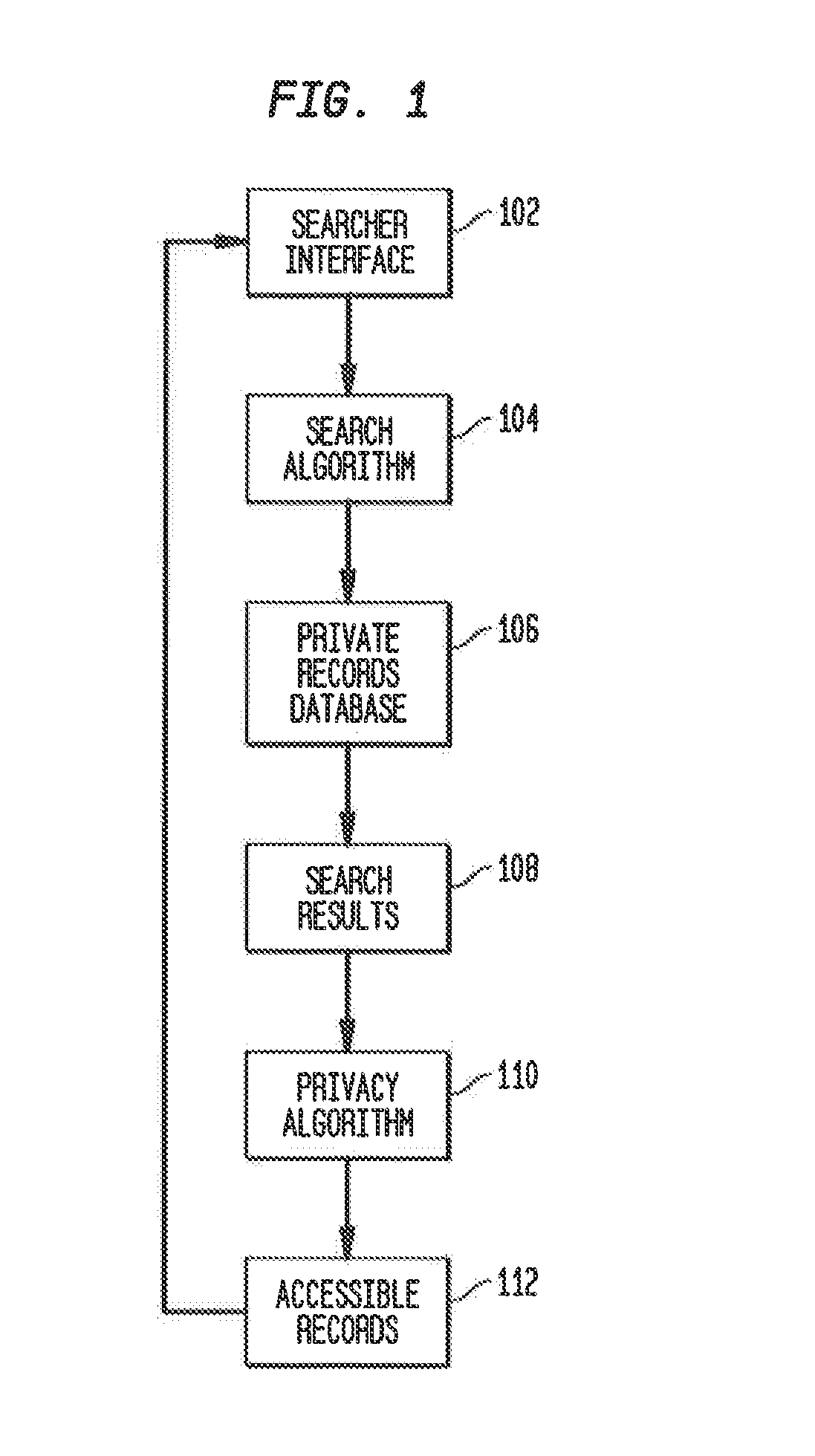
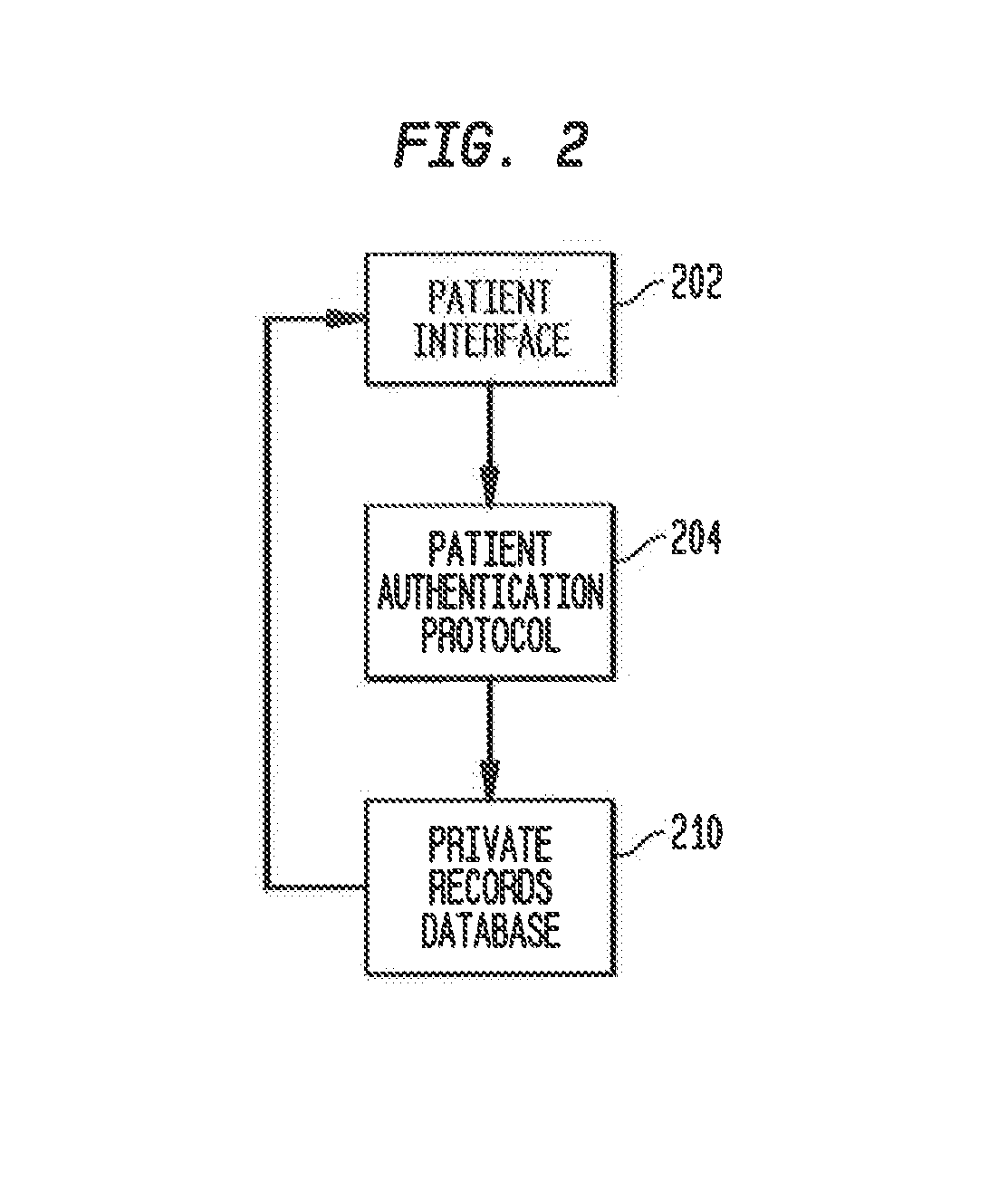
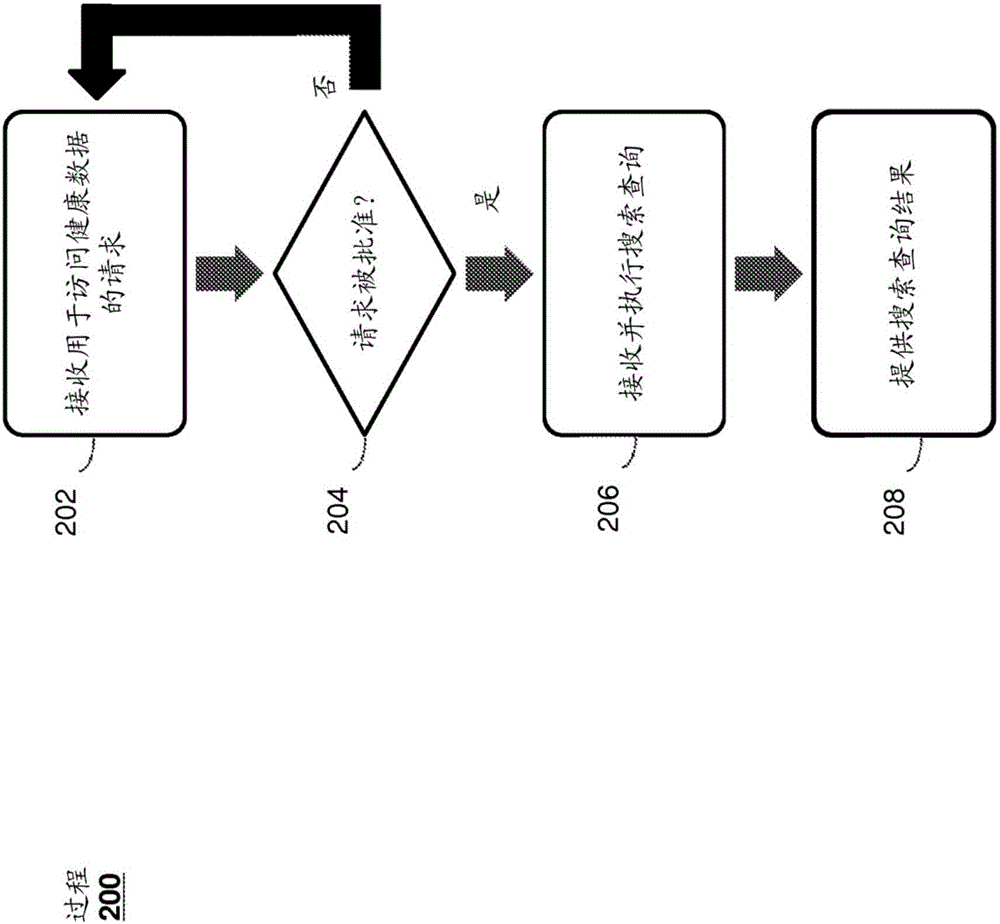
A system and method for managing private records and other confidential information is provided, including a patient interface that allows patients to configure privileges for various parties to access various private records or portions thereof. A database can be accessed via a privacy engine, which respects the configured privileges and allows searching for private records. A plurality of user interfaces is provided to allow records managers, patients, and interested parties to access selected private information. A clinical trials interface allows limited access to a private records database for interested parties to identify germane clinical trials and research studies. A recruitment interface allows conductors of research studies to access private records to locate appropriate research subjects.
Owner:SHELTON ROBERT
System and method for recruitment of candidates for clinical trials while maintaining security
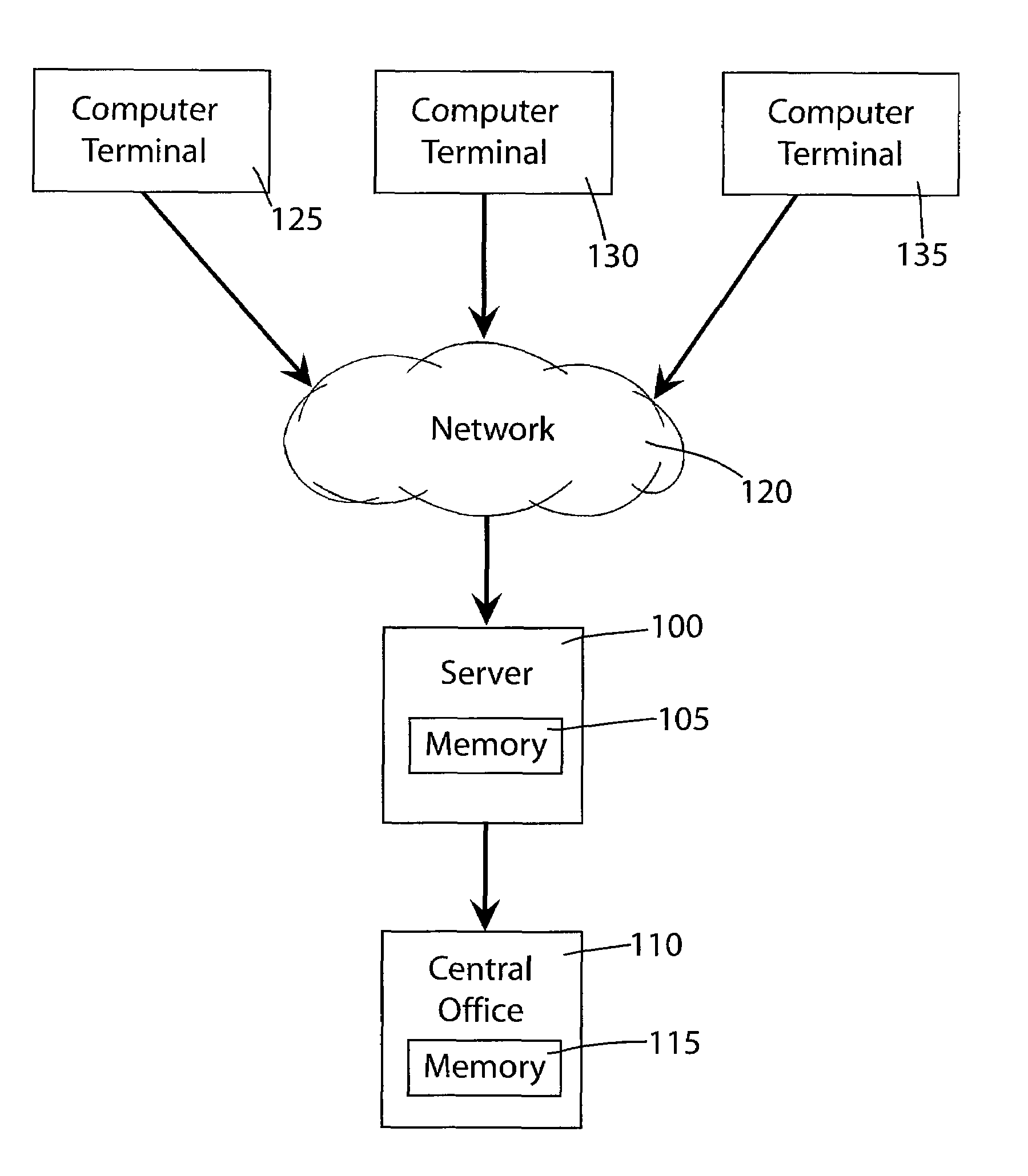
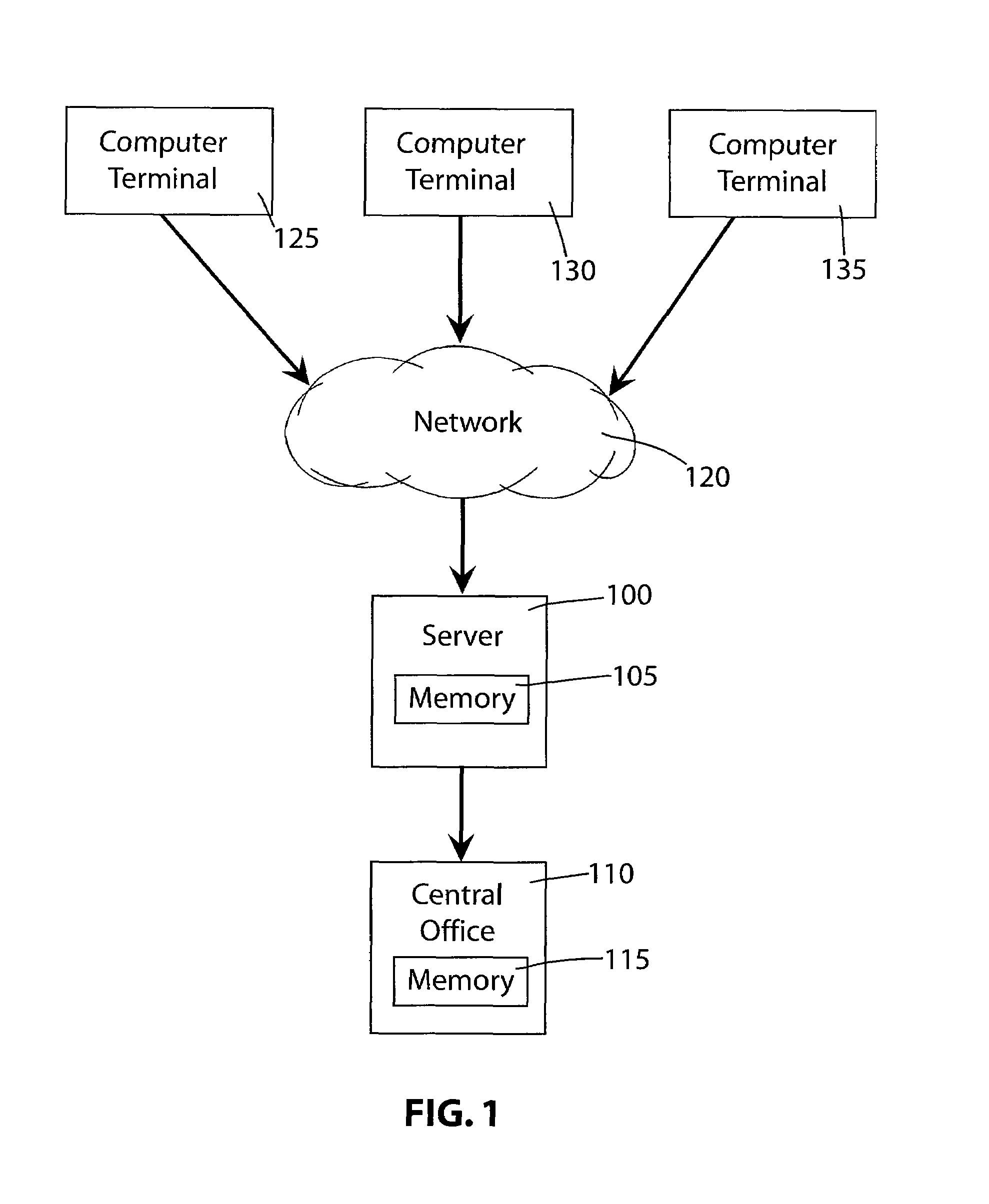
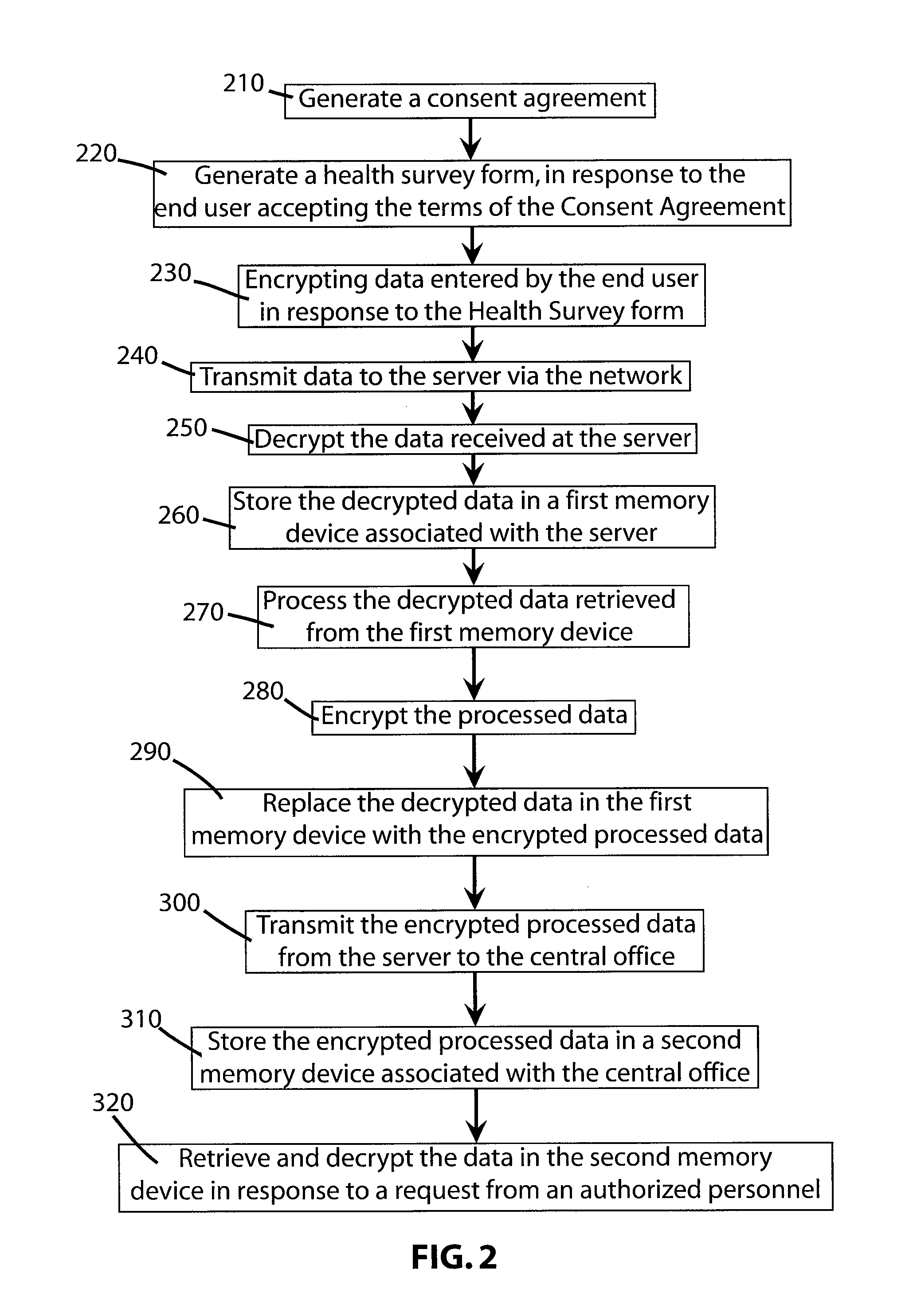
An on-line click wrap electronic agreement that a user must agree to prior to being registered as a volunteer and considered as a potential candidate in a clinical trial or research study. The agreement authorizes the release of the end user's medical and / or personal information to representatives of the clinical trials and research studies for which the volunteer may be considered as a potential candidate. After receiving the end user's consent to the click wrap agreement an electronic survey form is generated by a secure server and displayed at the end user's computer terminal. Responses by the end user to the survey form are kept secure as much as possible while being transmitted from the computer terminal across the network to the server and while stored and accessed only by authorized personnel at the central office.
Owner:BODY HEALTH RESOURCES CORP
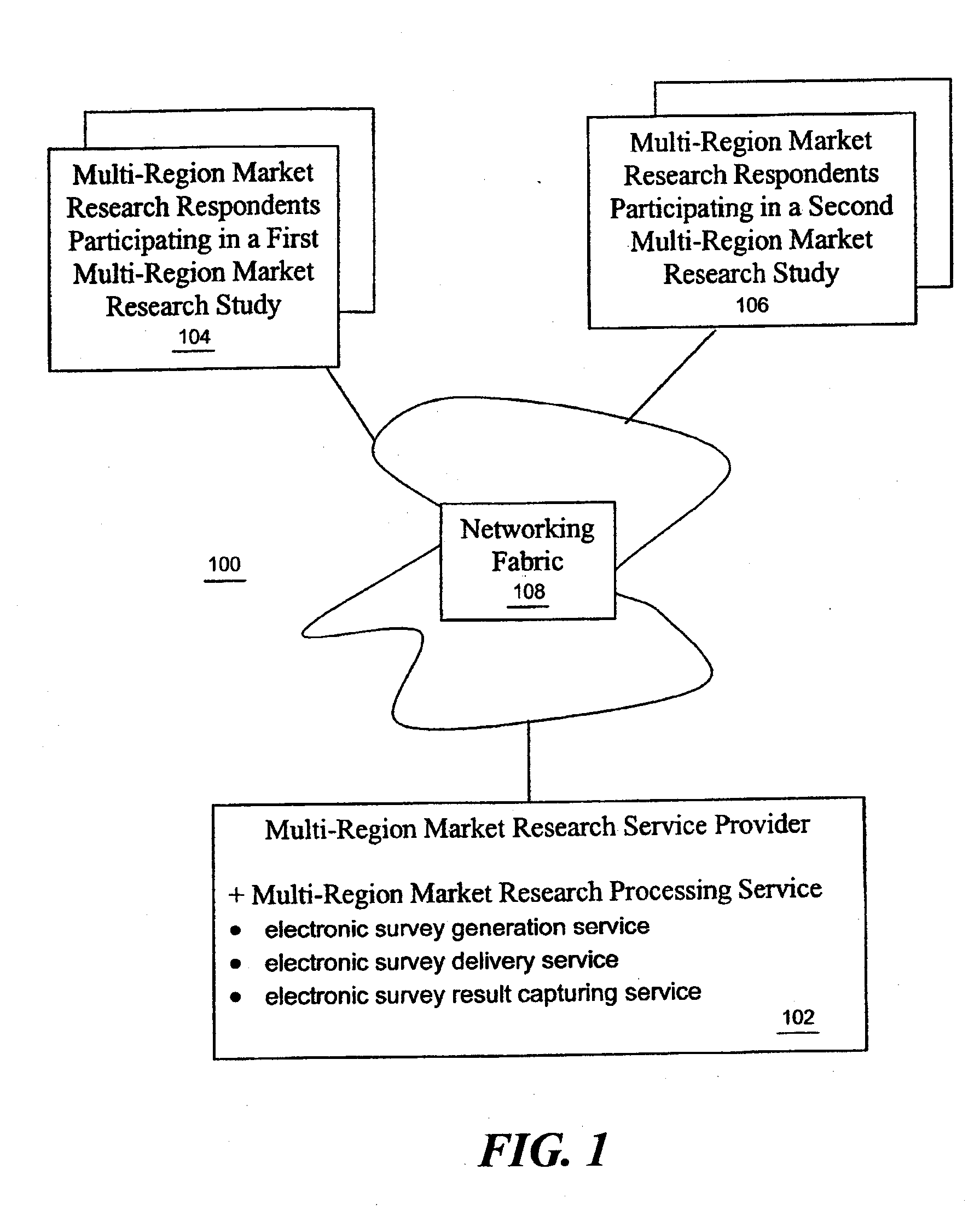
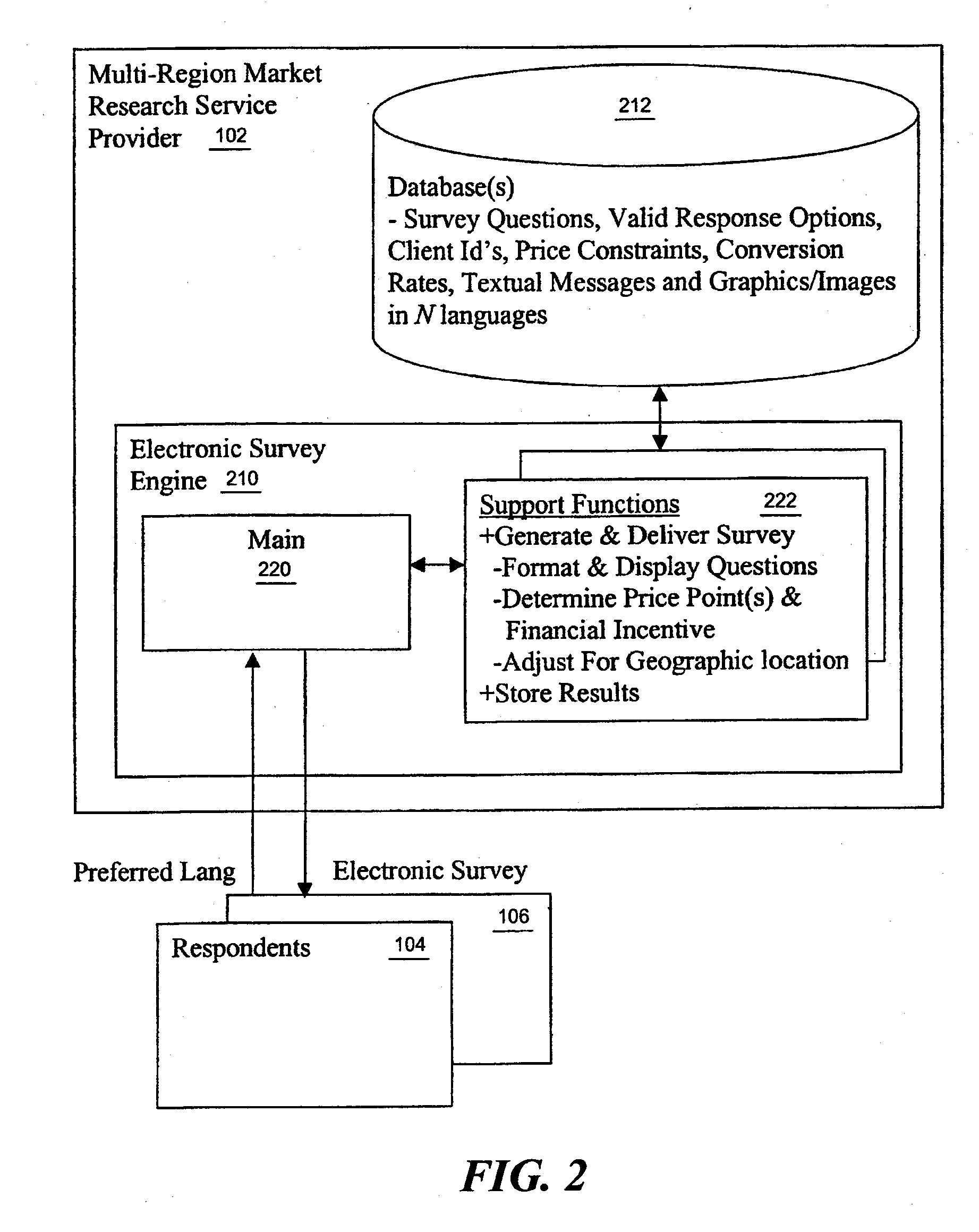
Multi-region market research study processing
A market research service is provided with a study creation service that includes a number of functions to support the creation of multi-region market research studies. In one embodiment, the functions include a function in support of implicit country and / or city selection within a region. In another embodiment, the functions include a function in support of providing a real time cost estimate for the study being created. In yet another embodiment, the functions include a function in support of auto notification to translators providing translation services for translating study elements (such as questions, messages, pick lists and concepts) into supported target languages. In yet another embodiment, the functions include a function in support of on-line check in of the translated study elements. In yet another embodiment, the functions include a function in support of on-line monitoring of translation status.
Owner:MONSTER ROBERT W
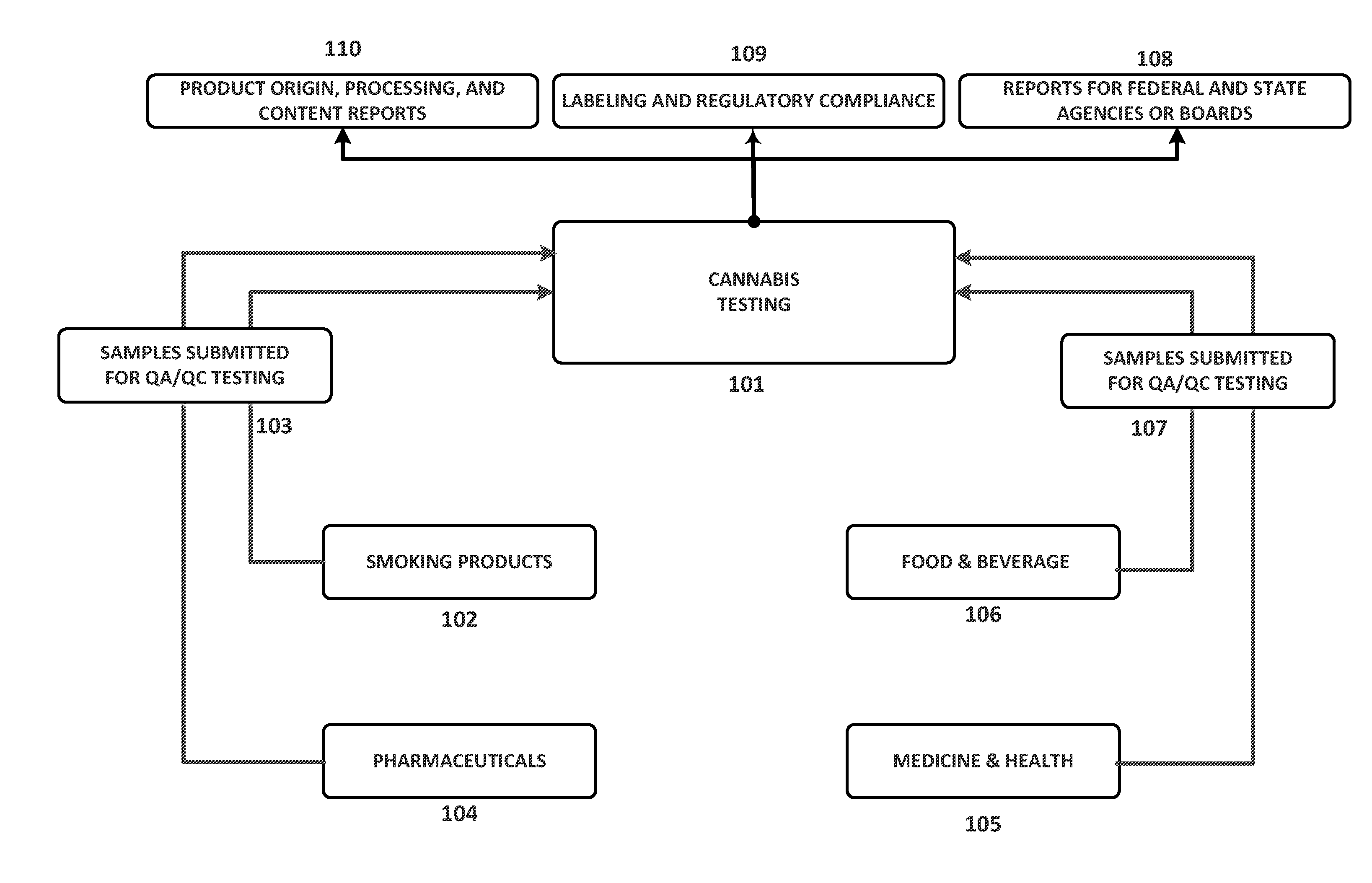
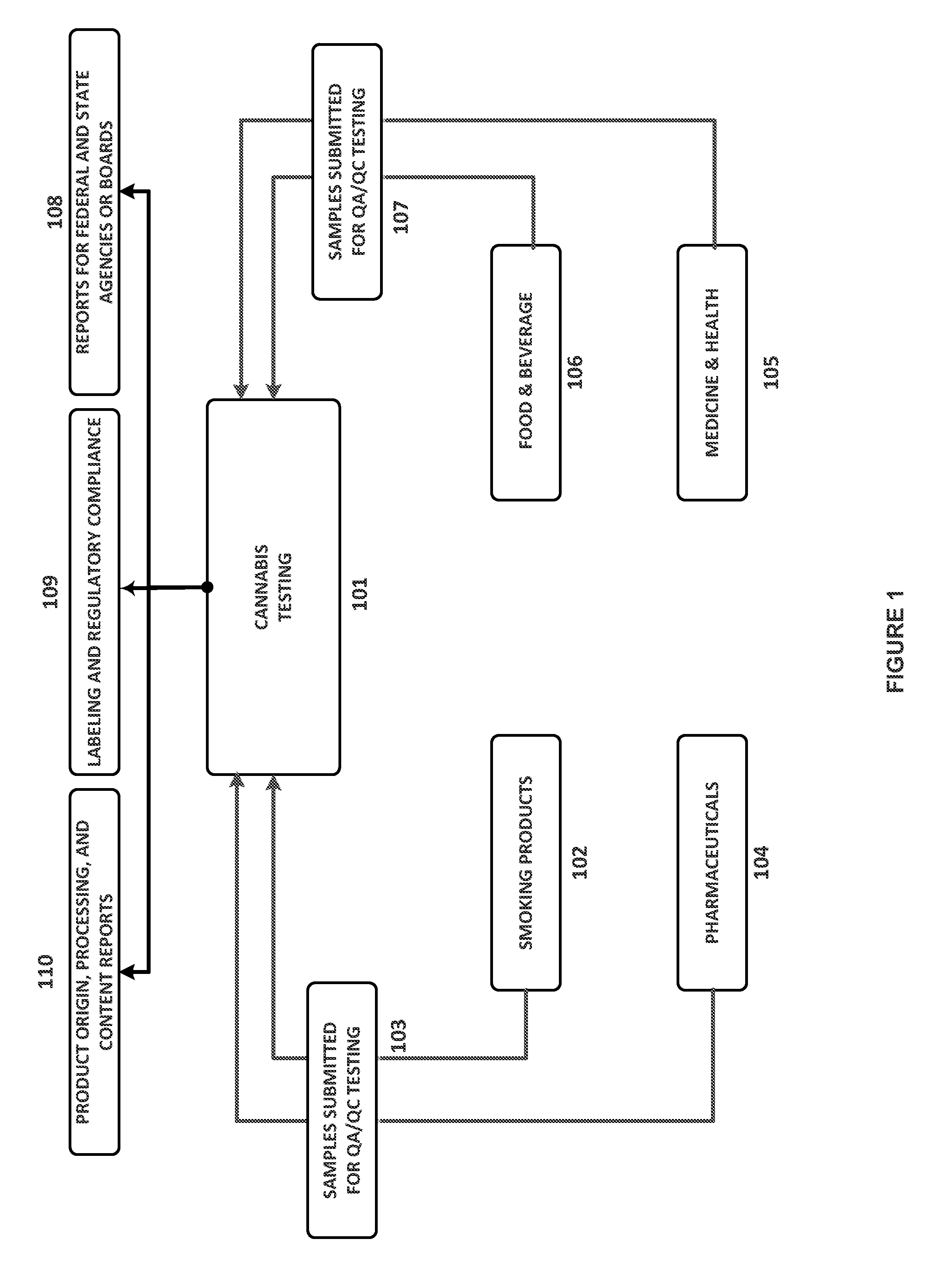
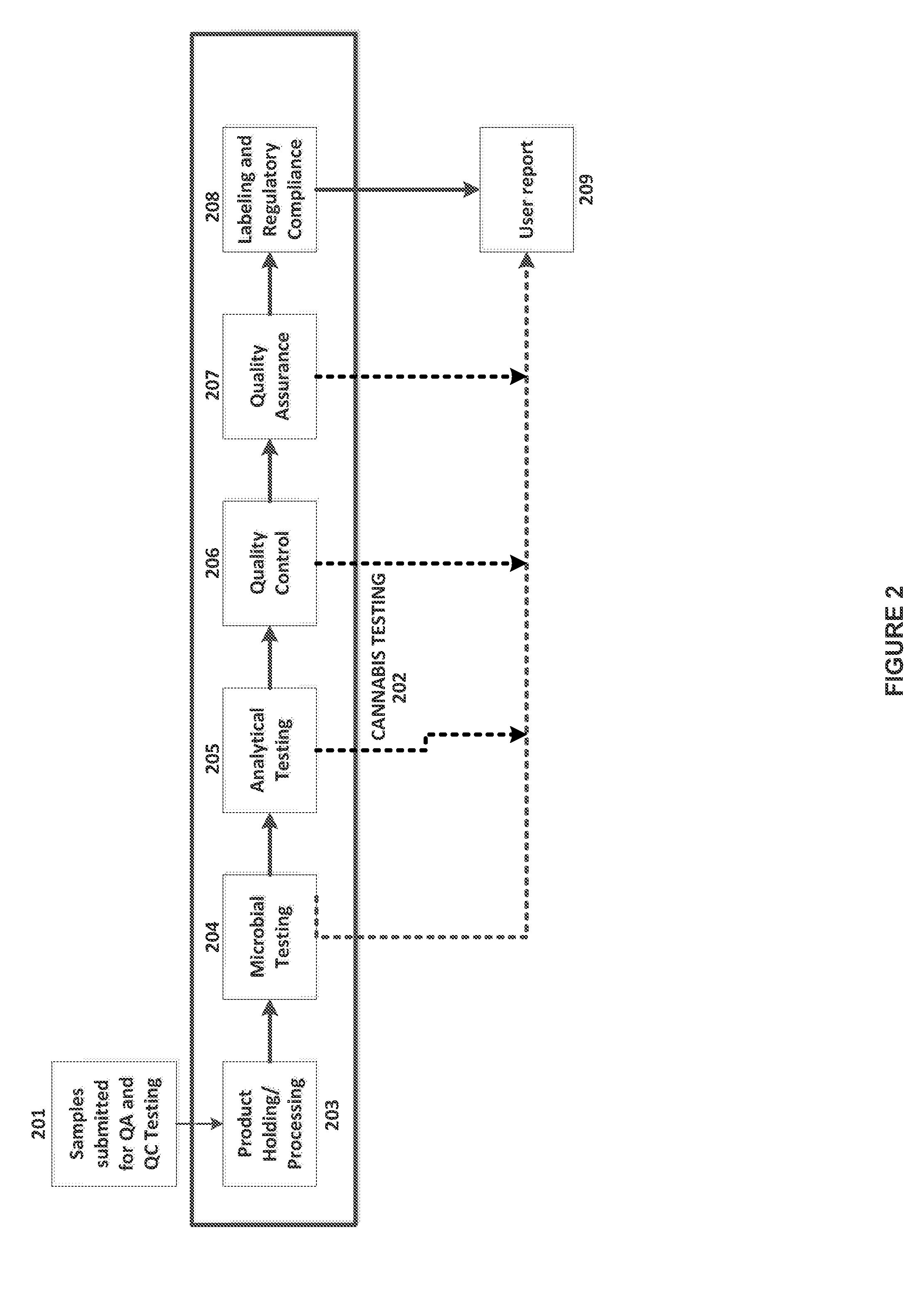
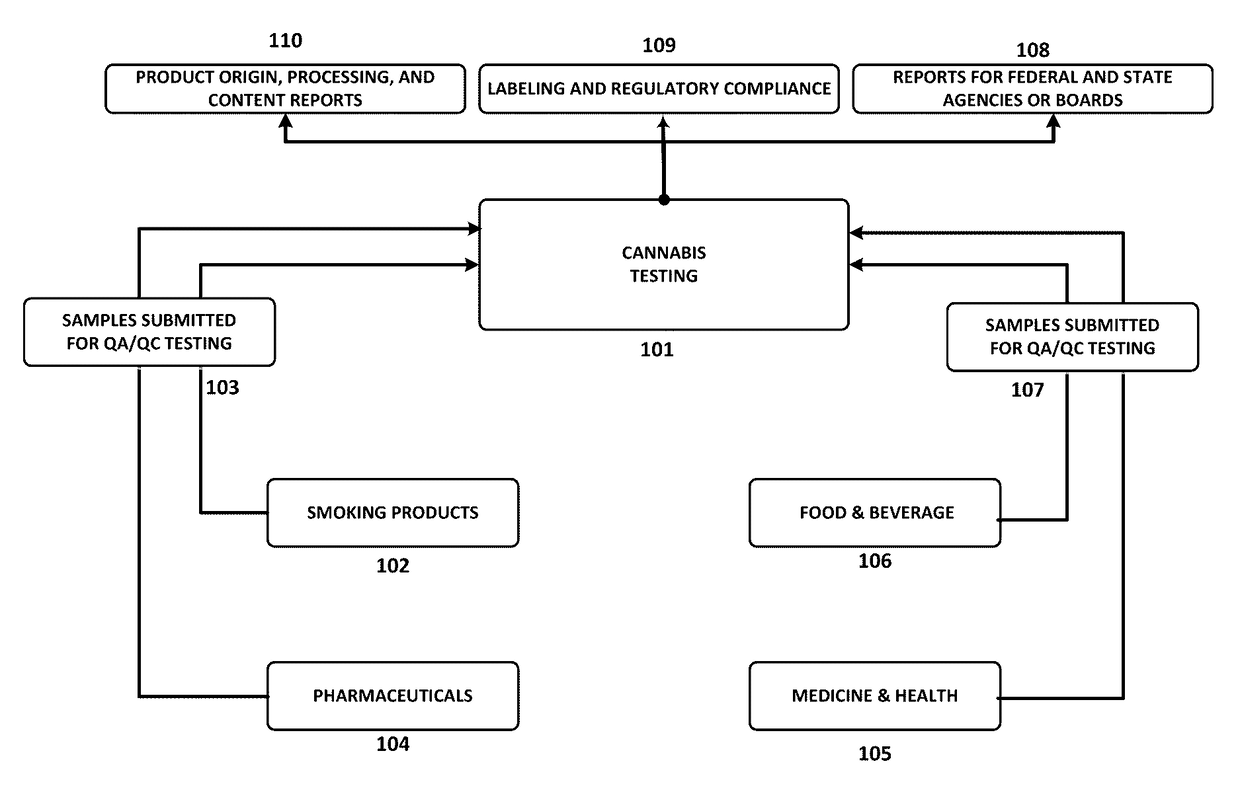
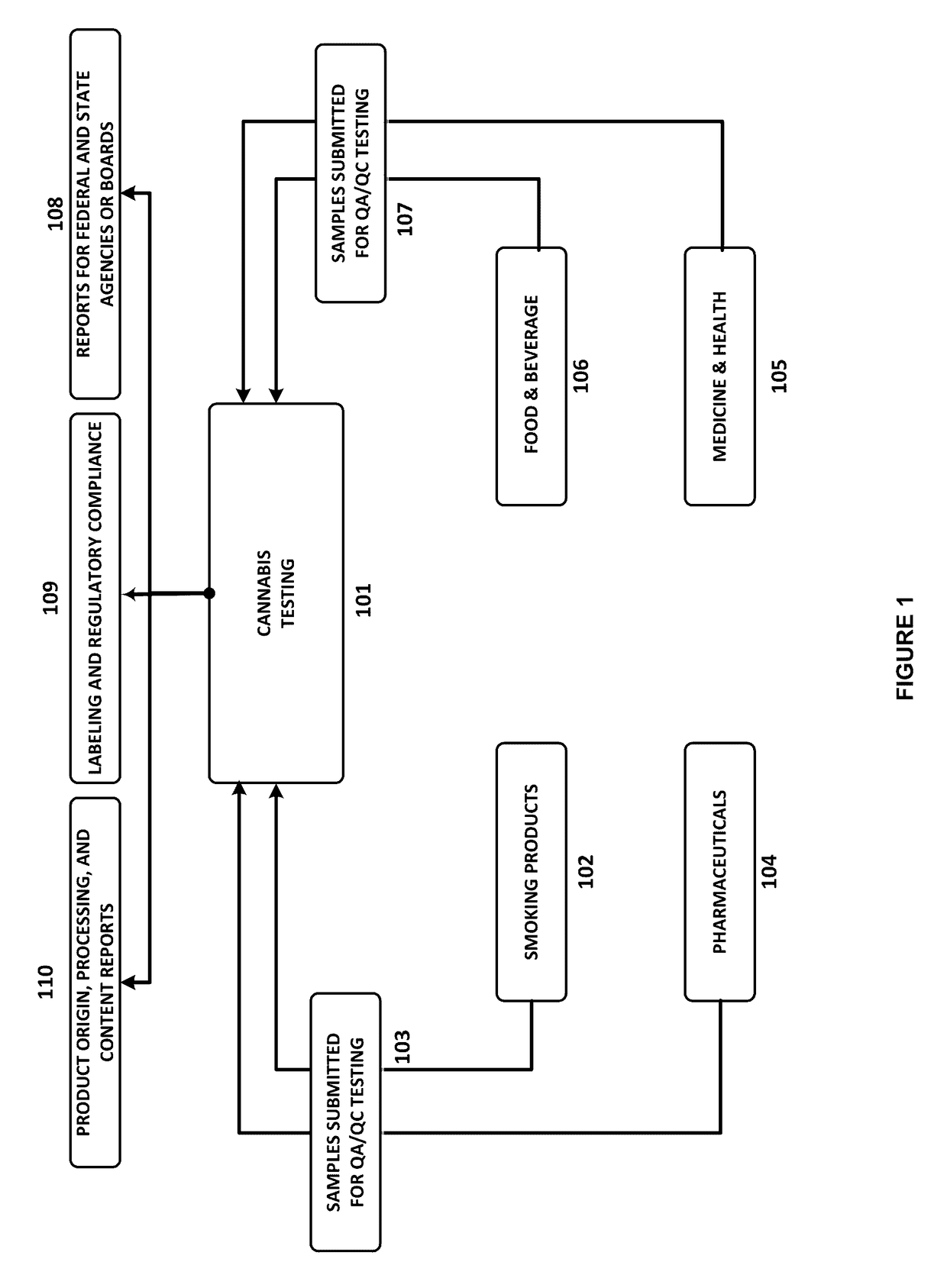
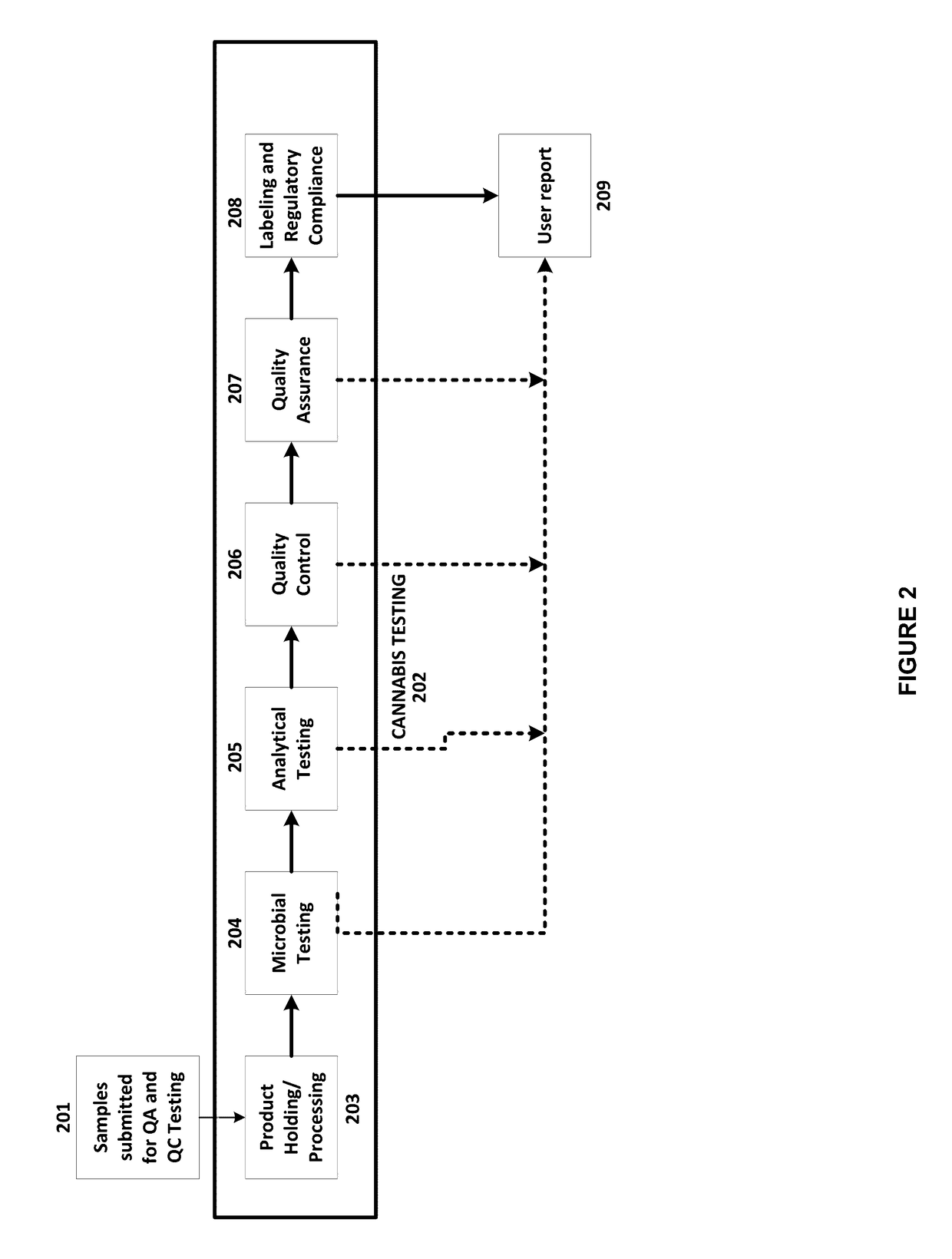
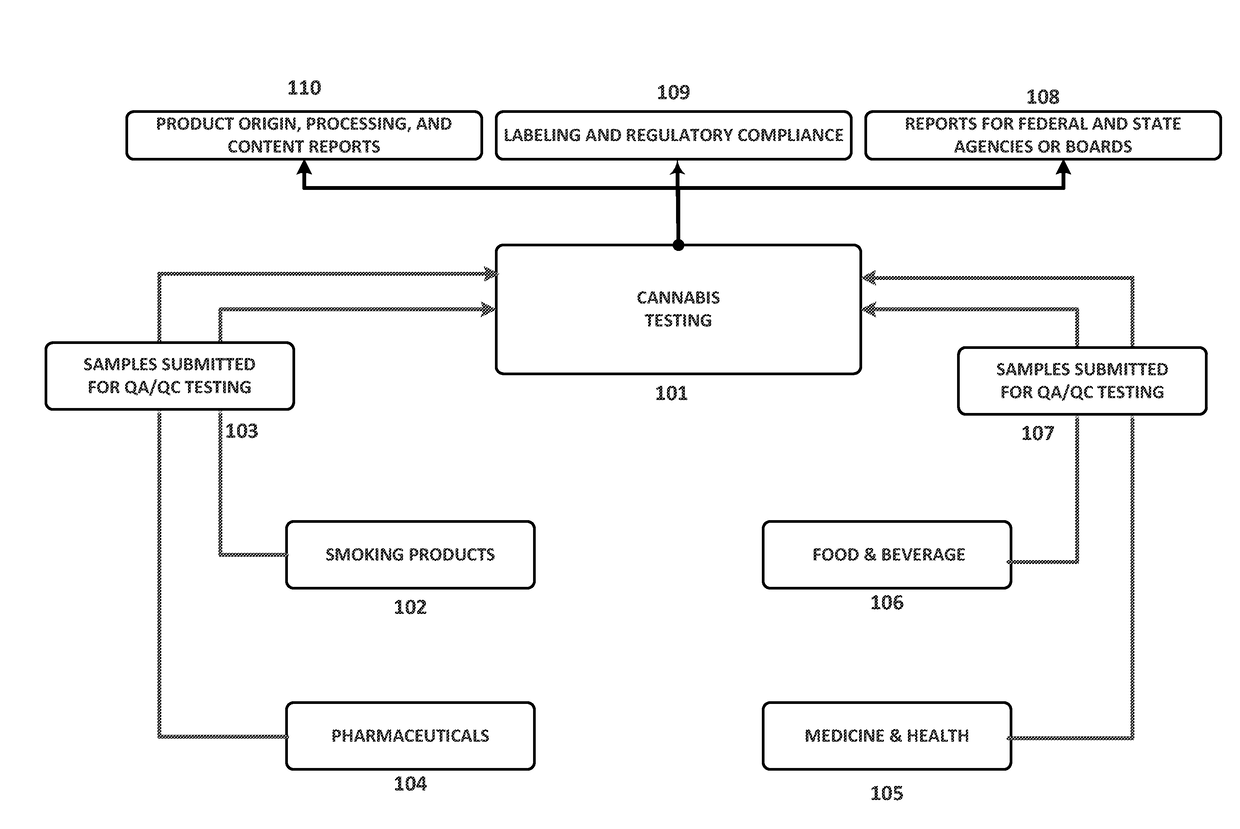
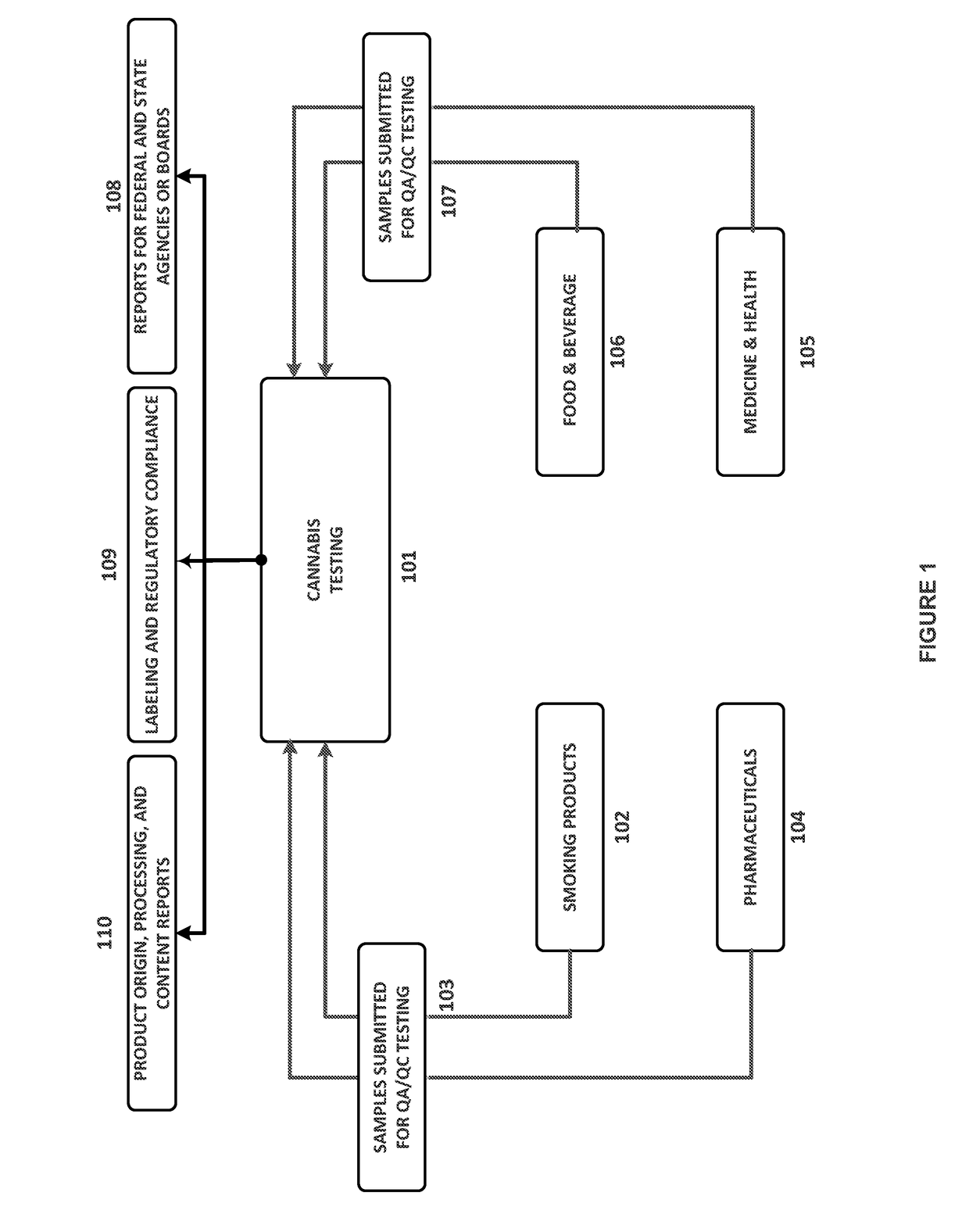
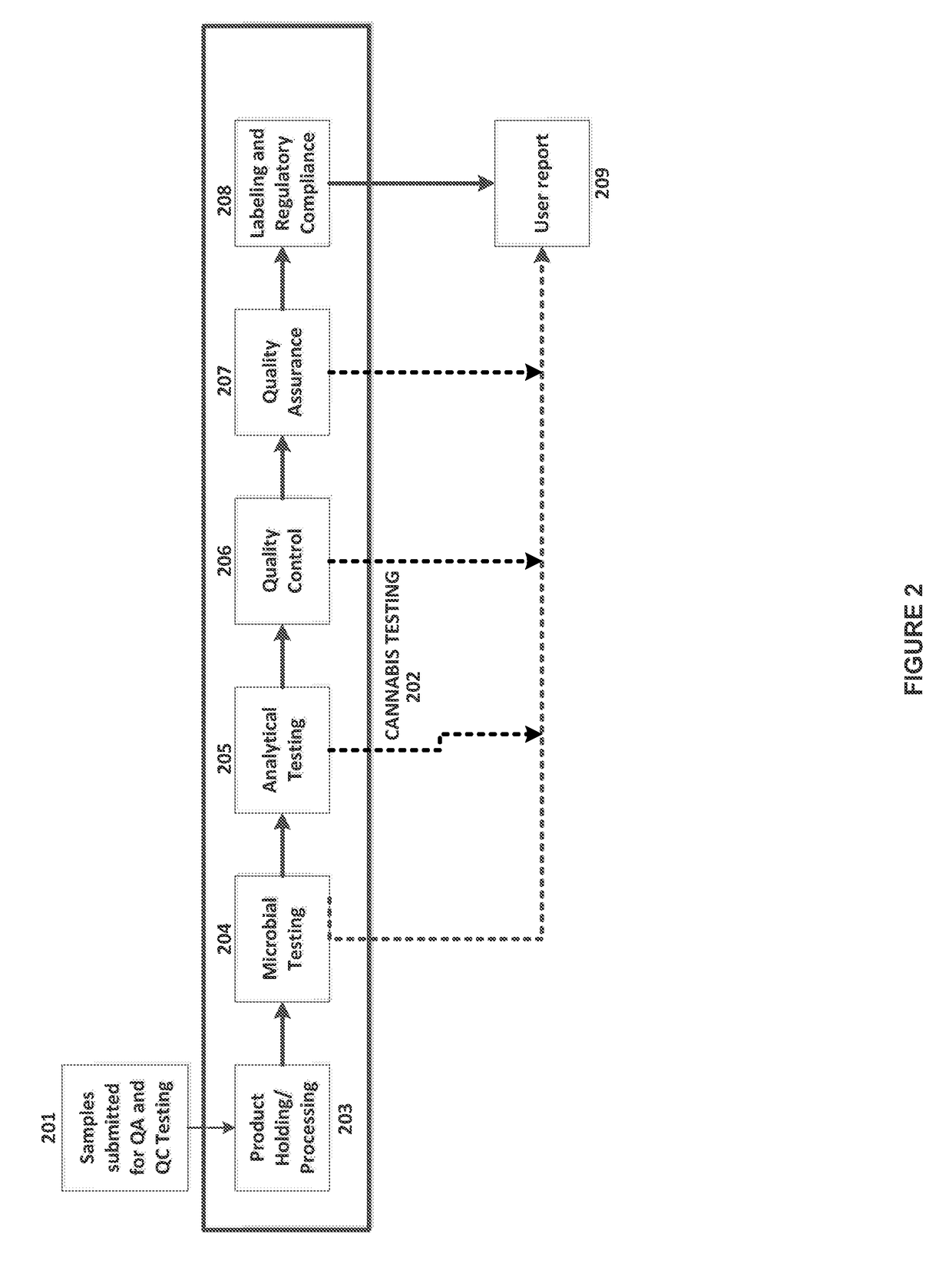
Integrated Systems and Methods of Evaluating Cannabis and Cannabinoid Products for Public Safety, Quality Control and Quality Assurance Purposes
ActiveUS20150219610A1Threaten healthThreaten safetyWeb data indexingDigital data processing detailsCannabisThird party
Embodiments of the invention include integrated systems and methods of evaluating cannabis and cannabinoid products for public safety, quality control and quality assurance purposes for research and public use purposes. Embodiments of the invention facilitate suppliers and consumers of a cannabis and / or cannabinoid product to evaluate the origin, efficacy, potency, and quality of the product. Embodiments of the invention also include cannabis processing center where samples of the products are analyzed for research studies to test and set parameters for third parties. Embodiments of the invention include, for example, analyzing cannabis products to determine quality and quantity of desired components and undesired components, determining concentrations of cannabinoids in the product, and comparing measures of components in the product against regulations. Embodiments of the invention further include, for example, an online testing facility for determine whether cannabis products meet a plurality of regulations or guidelines.
Owner:VYRIPHARM ENTERPRISES LLC +1
Integrated systems and methods of evaluating cannabis and cannabinoid products for public safety, quality control and quality assurance purposes
Embodiments of the invention include integrated systems and methods of evaluating cannabis and cannabinoid products for public safety, quality control and quality assurance purposes for research and public use purposes. Embodiments of the invention facilitate suppliers and consumers of a cannabis and / or cannabinoid product to evaluate the origin, efficacy, potency, and quality of the product. Embodiments of the invention also include cannabis processing center where samples of the products are analyzed for research studies to test and set parameters for third parties. Embodiments of the invention include, for example, analyzing cannabis products to determine quality and quantity of desired components and undesired components, determining concentrations of cannabinoids in the product, and comparing measures of components in the product against regulations. Embodiments of the invention further include, for example, an online testing facility for determine whether cannabis products meet a plurality of regulations or guidelines.
Owner:VYRIPHARM ENTERPRISES LLC +1
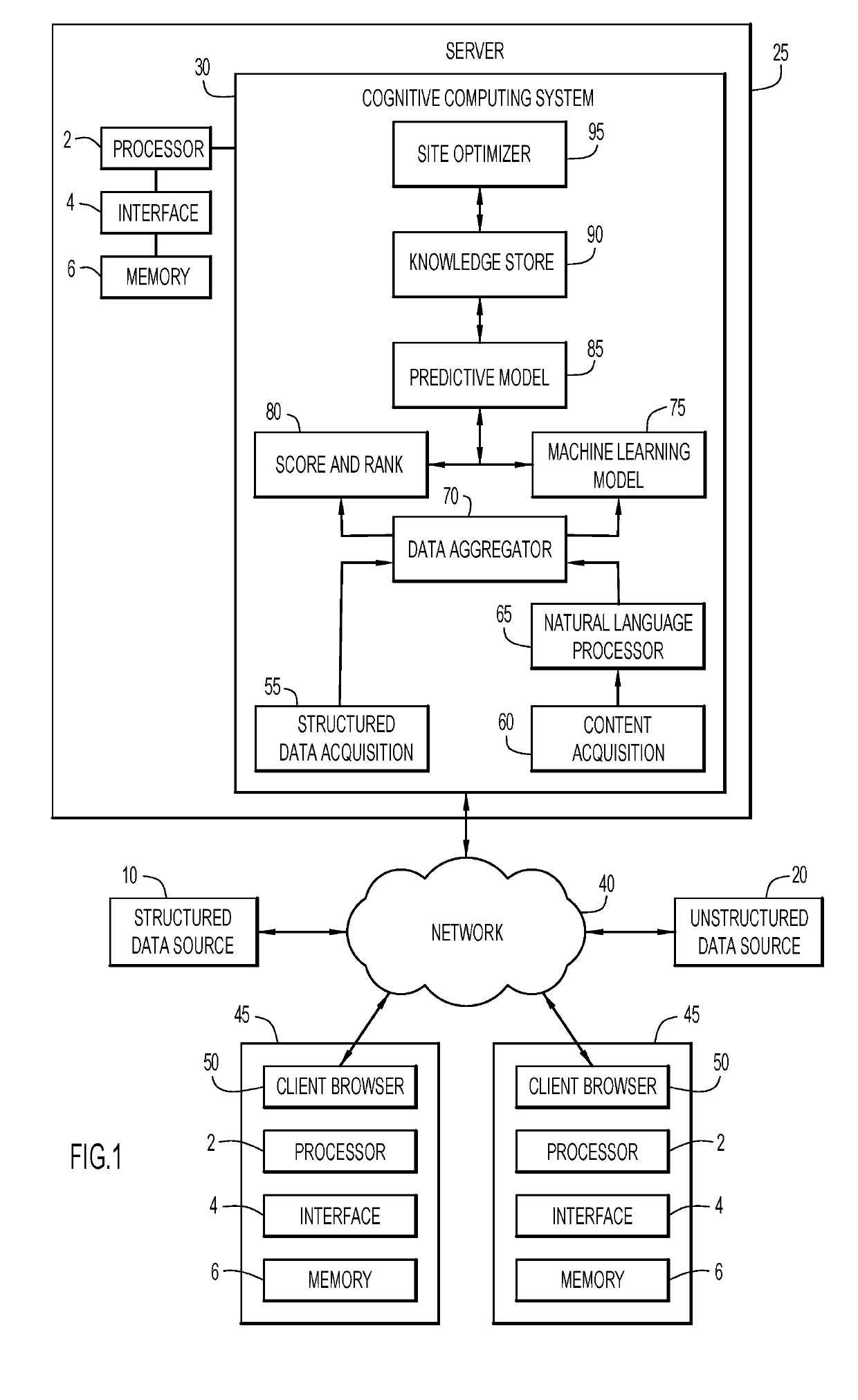
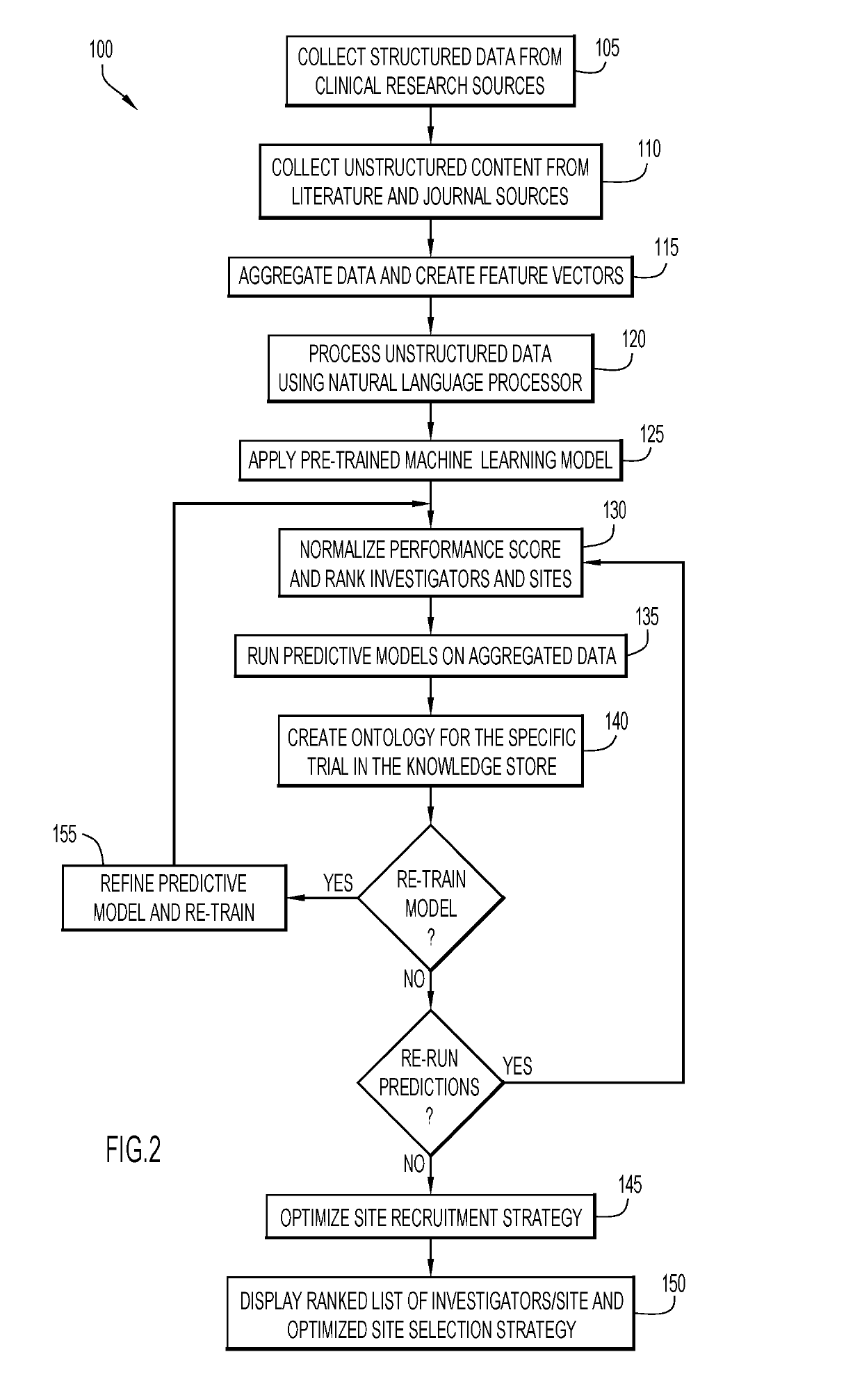
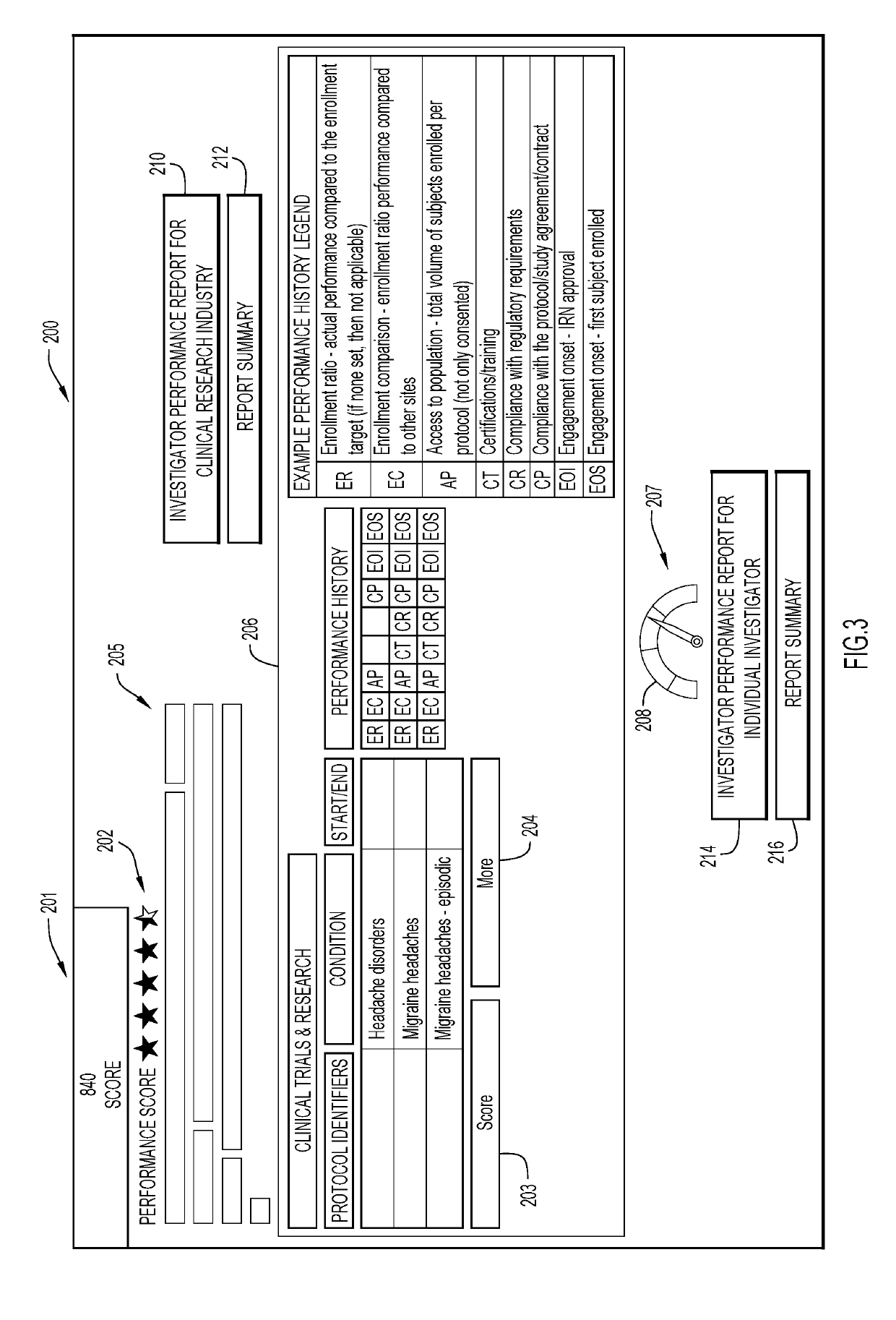
Monitoring clinical research performance
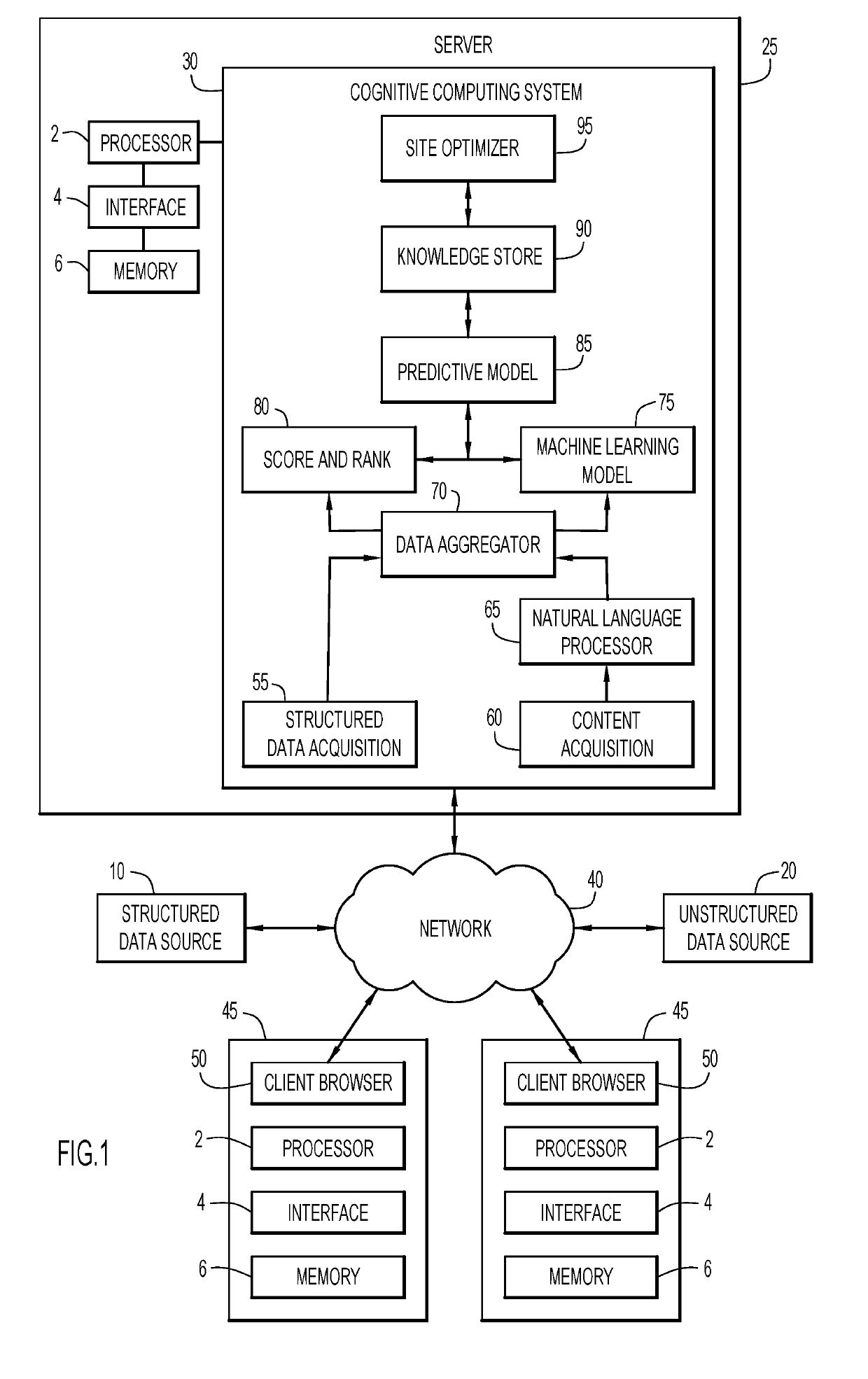
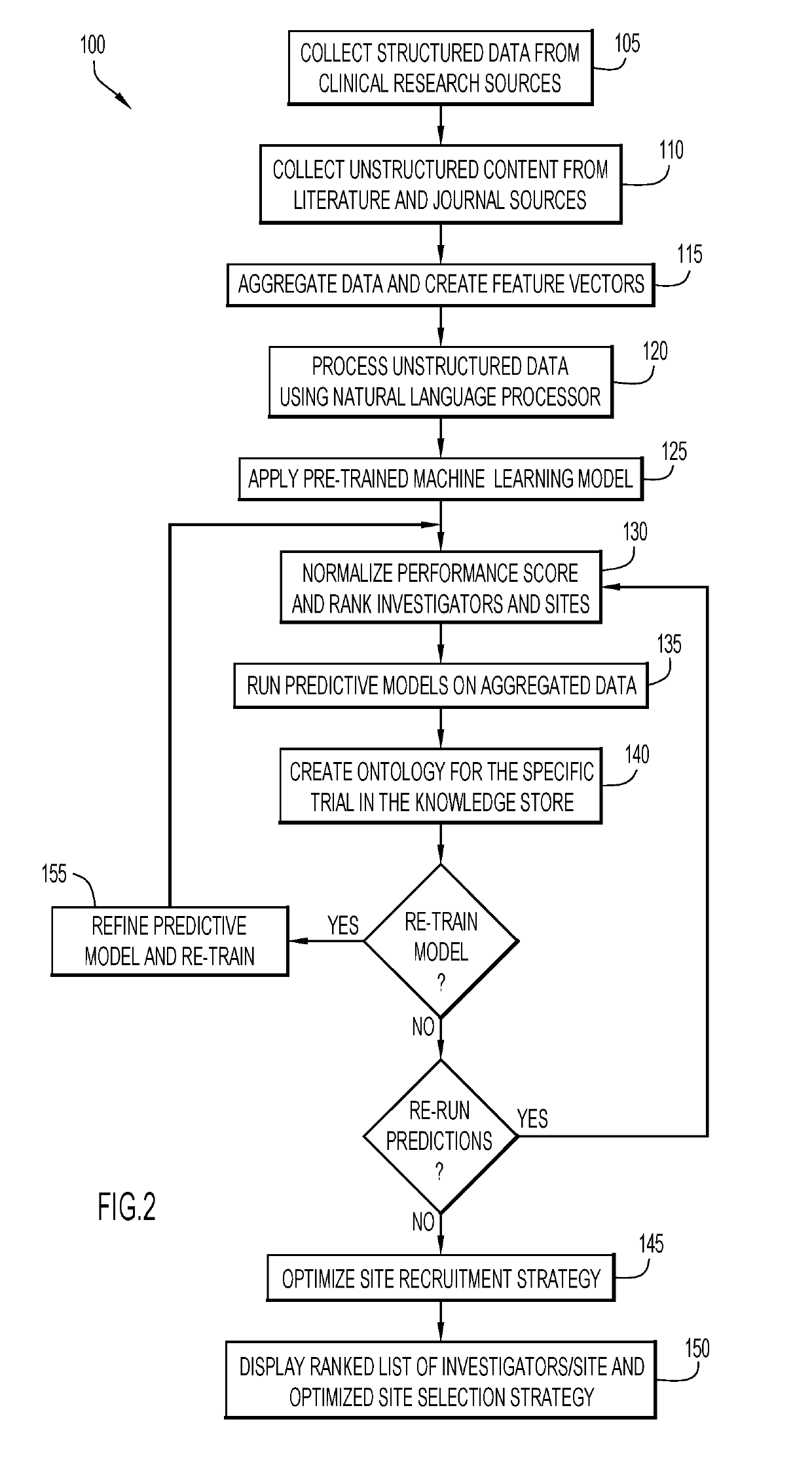
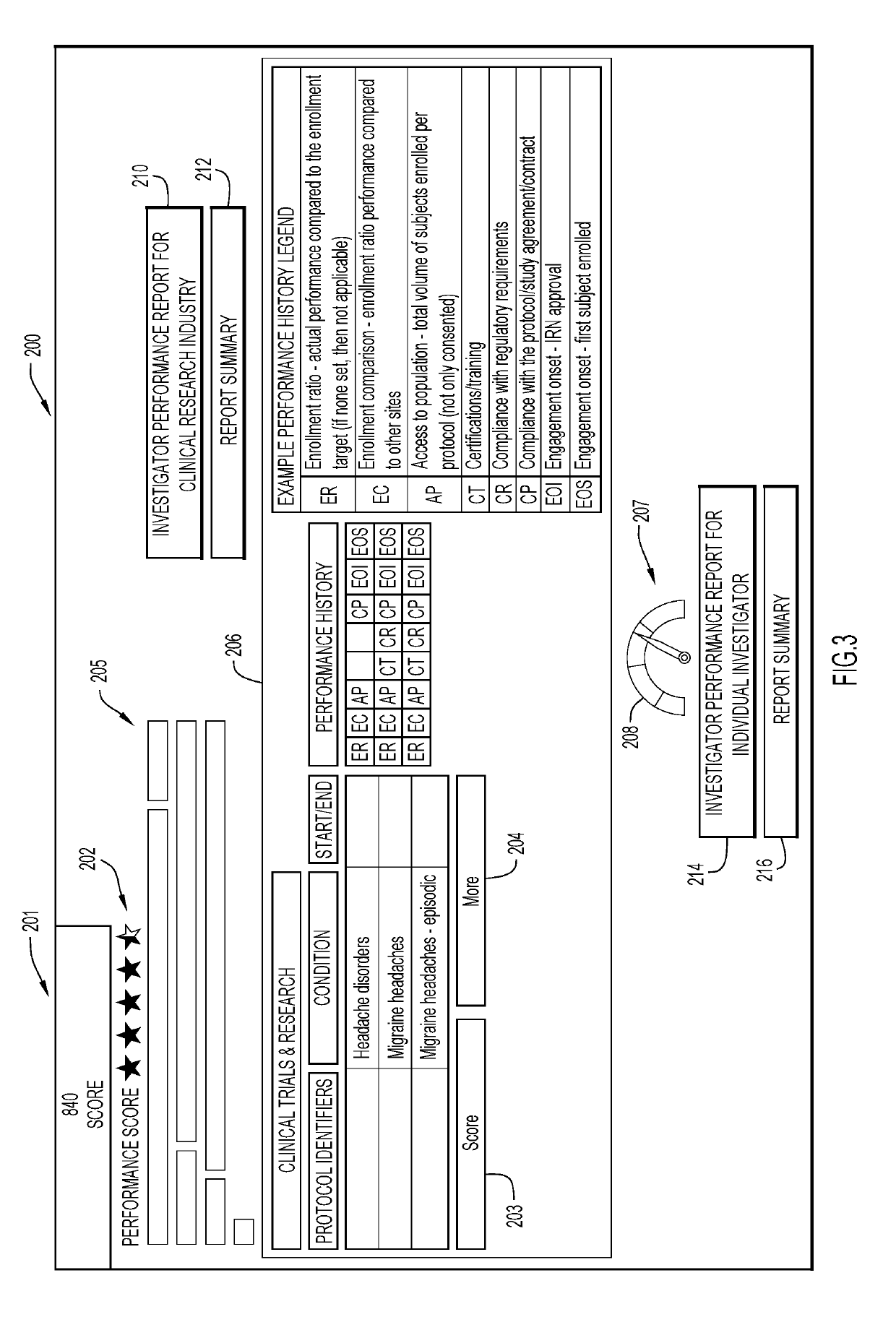
ActiveUS20190304575A1Health-index calculationHealthcare resources and facilitiesClinical researchData mining
A computer-implemented method, system, and computer program product monitors clinical research performance. One or more metrics of clinical research performance for investigator / provider / research sites across research studies are collected. The metrics include performance area, characteristic of the performance area with one or more attributes, point values for each attribute, and weight value for the characteristic. A performance score is produced for each of the entities based on the one or more metrics. A machine learning model is trained to determine performance scores based on the produced performance score for each of the entities. A request for entities is processed by applying performance scores from the machine learning model and appropriate corresponding data to a predictive model to determine resulting performance scores, rank and / or match for each of the one or more entities for a given protocol and / or assessment trigger. Actions are performed based on the resulting performance scores, rank and / or match.
Owner:MERATIVE US LP
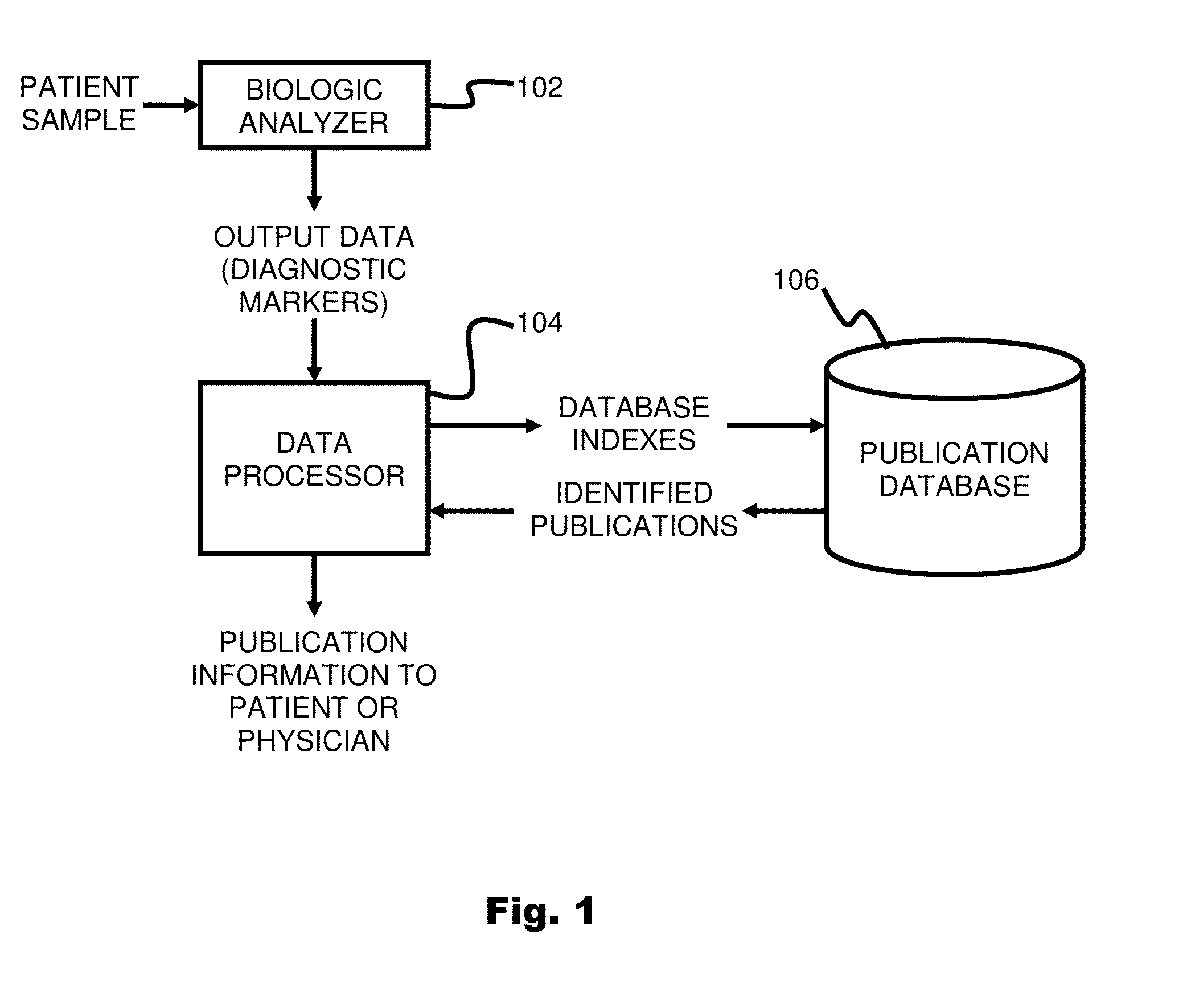
System and method for targeting relevant research activity in response to angiogenic regulator analyses
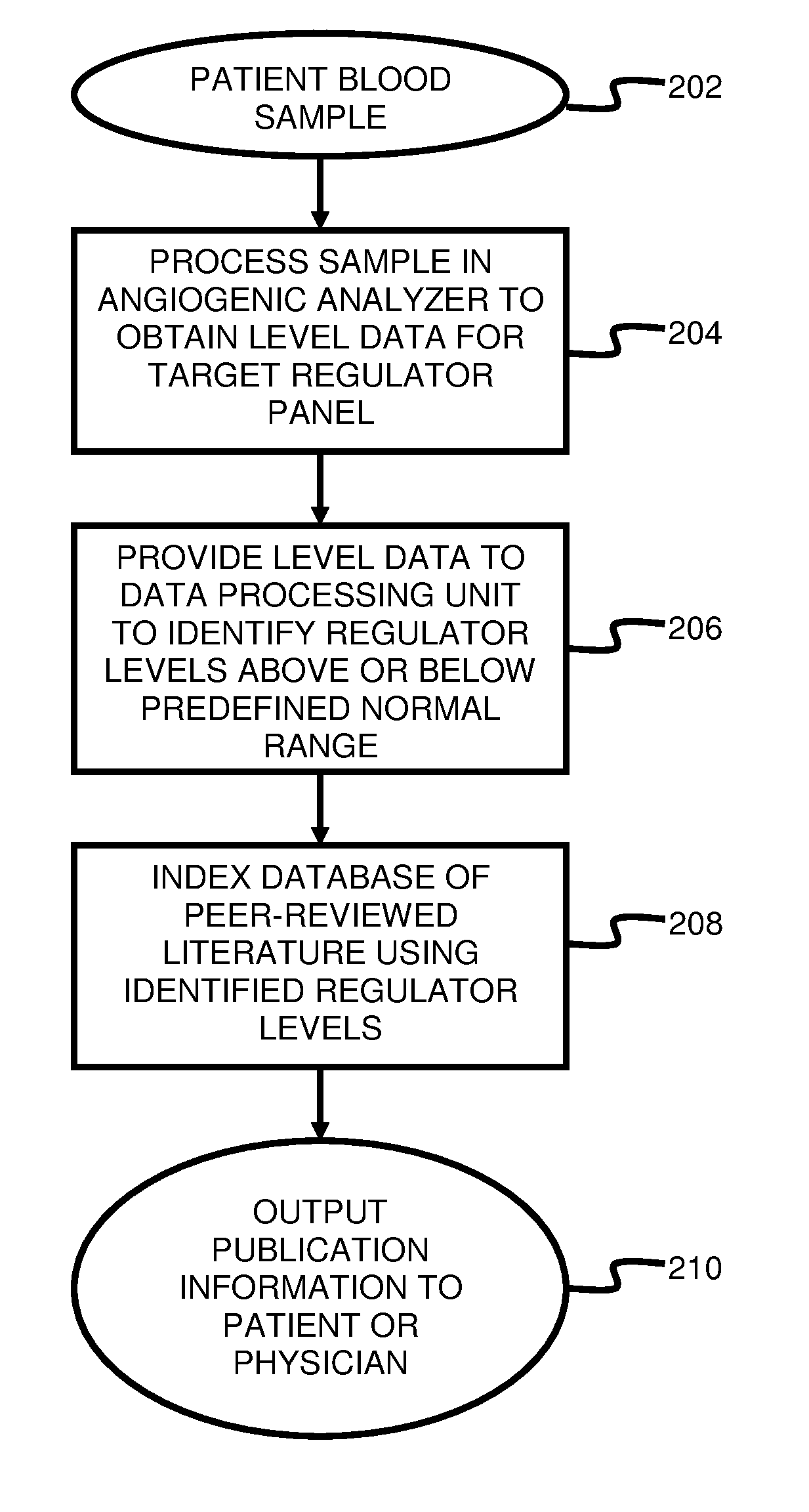
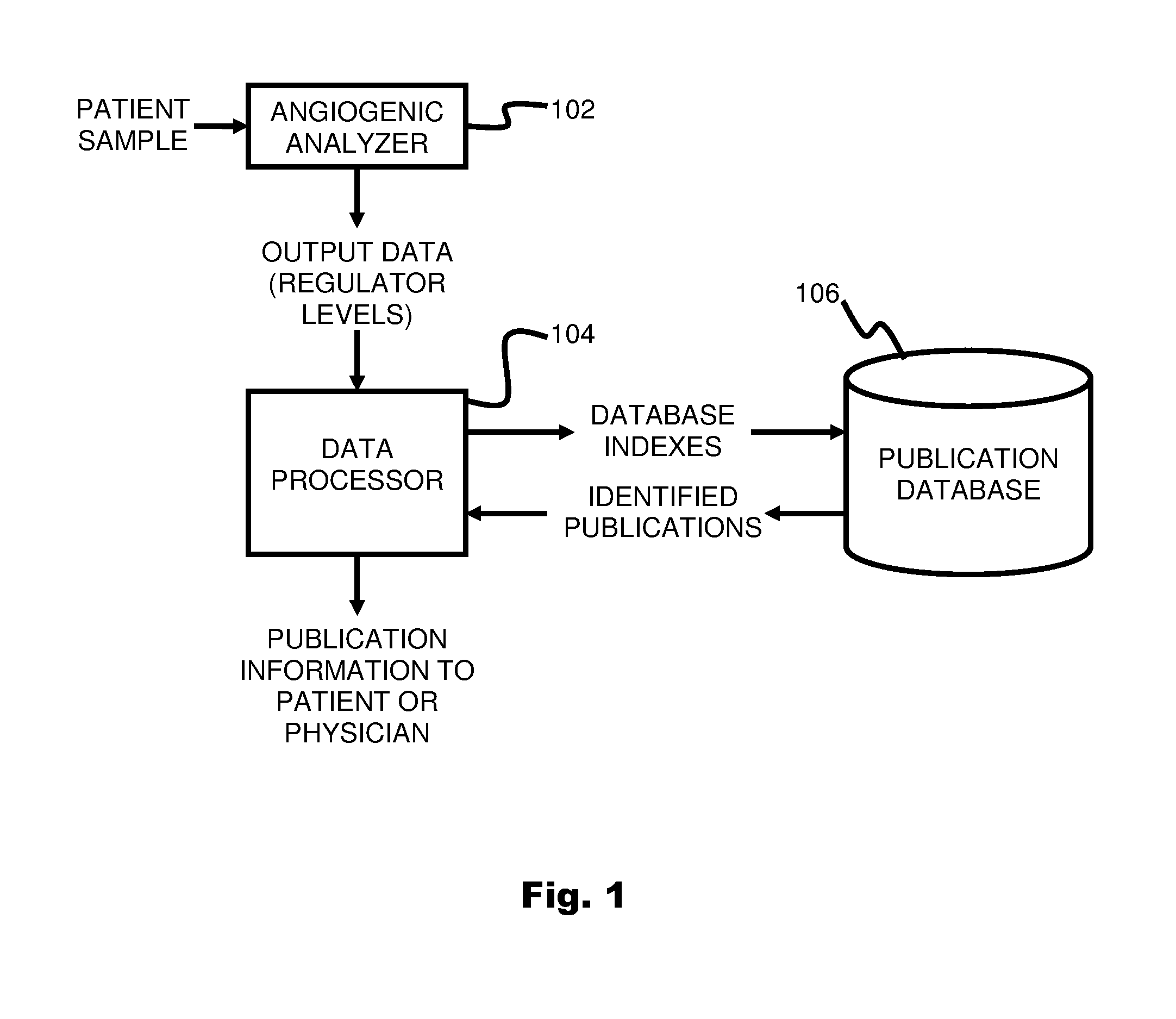
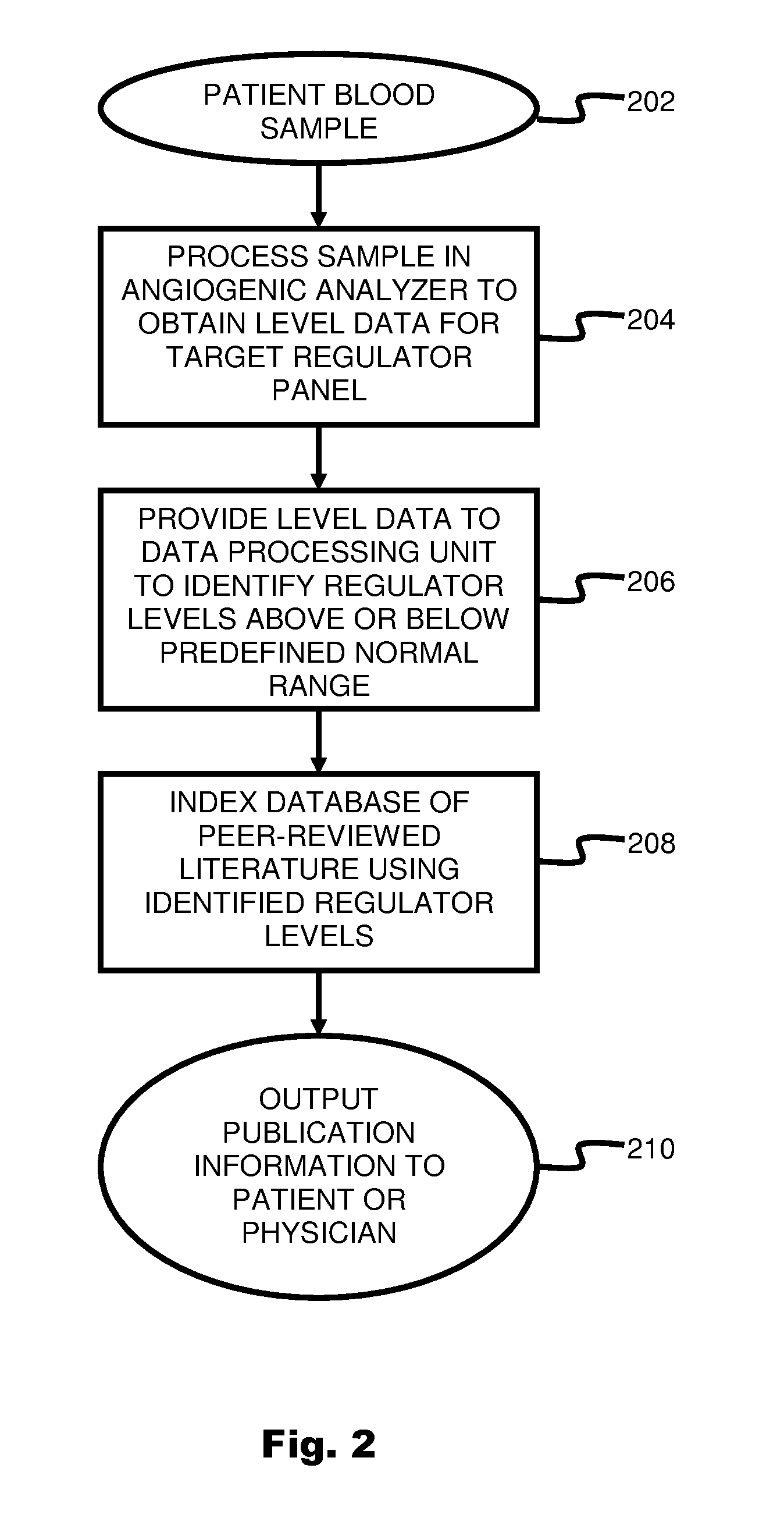
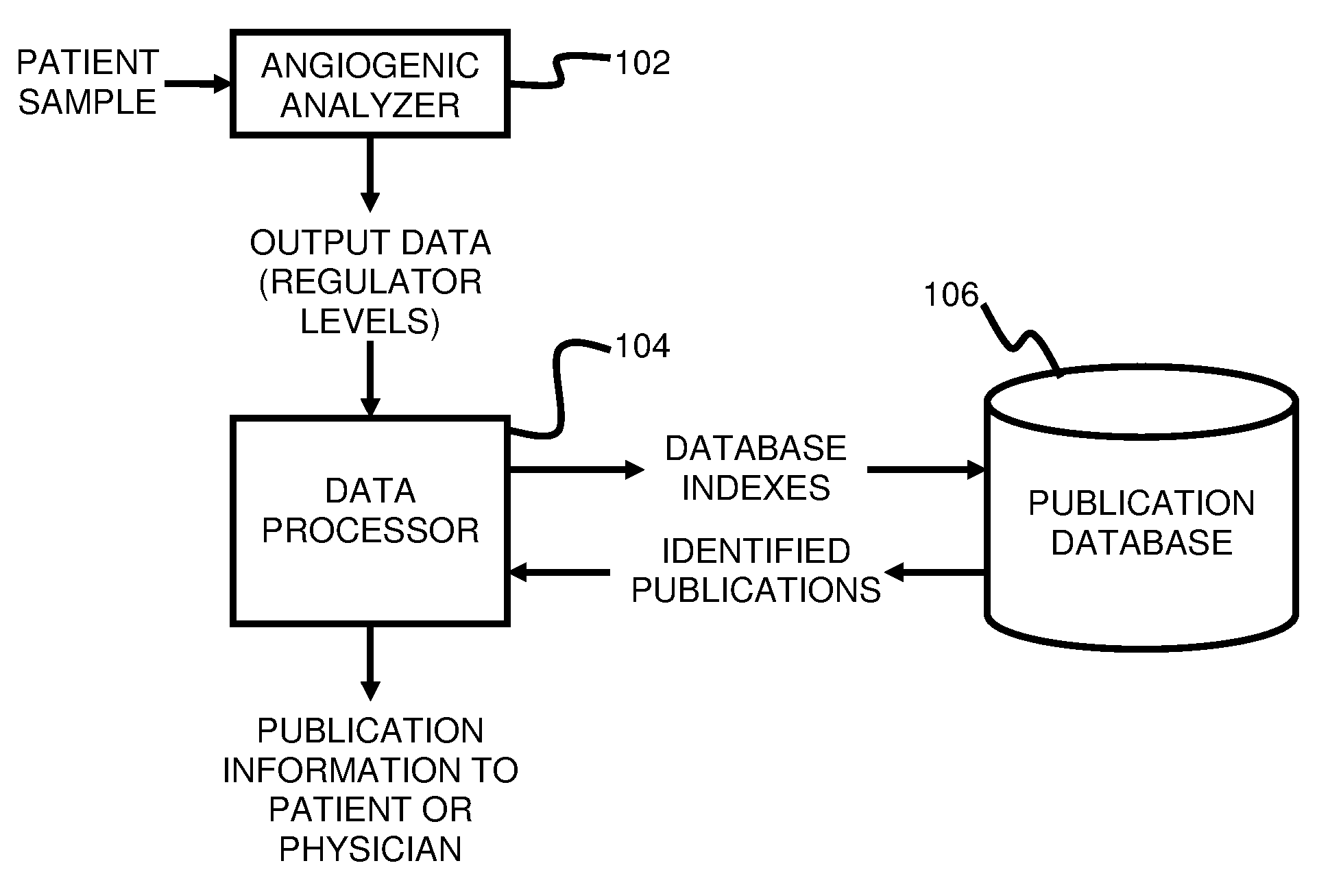
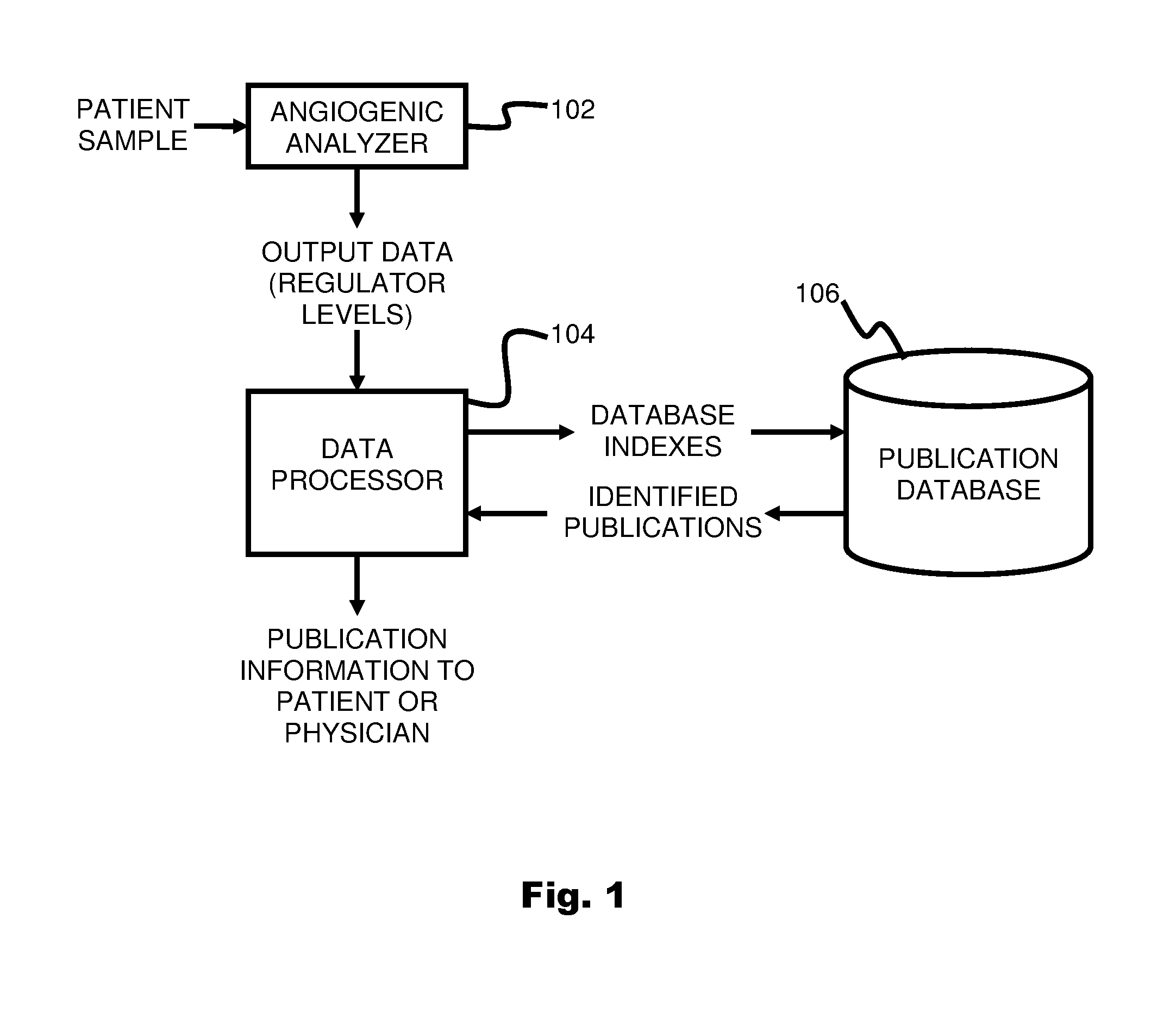
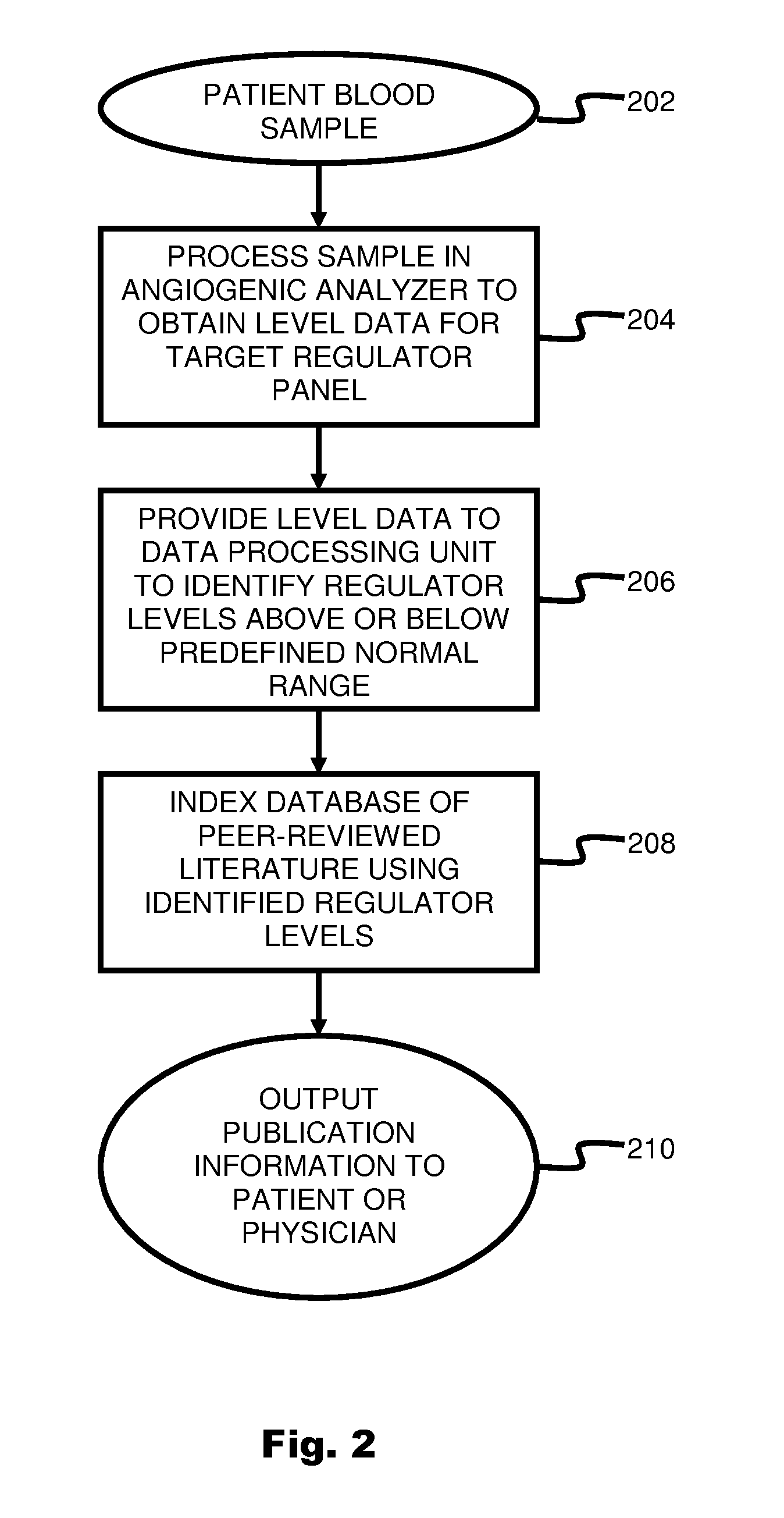
ActiveUS20110231104A1Highly targeted and unique mannerEfficacy of treatmentDisease diagnosisProteomicsAdditional diagnosesCorrelational study
A system and method for targeting relevant research activity for clinical application in response to angiogenic regulator analyses. An angiogenic analysis is performed on a patient blood sample in order to detect the level of each of at least ten angiogenic regulators. The levels of the tested regulators are used as indexes to identify relevant peer-reviewed research publications from among a large database of articles. The most relevant peer-reviewed literature reporting research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of angiogenic regulators for various disease states are then provided to the patient and / or to the patient's physician, optionally in conjunction with a summarization of the treatment recommendations gleaned from the provided literature. The customized information delivery provides the patient and physician a range of published peer-reviewed therapeutic options and published research studies for moderating the out of range regulators to within normal range or other diagnostic significant range.
Owner:LAMBERT REBECCA
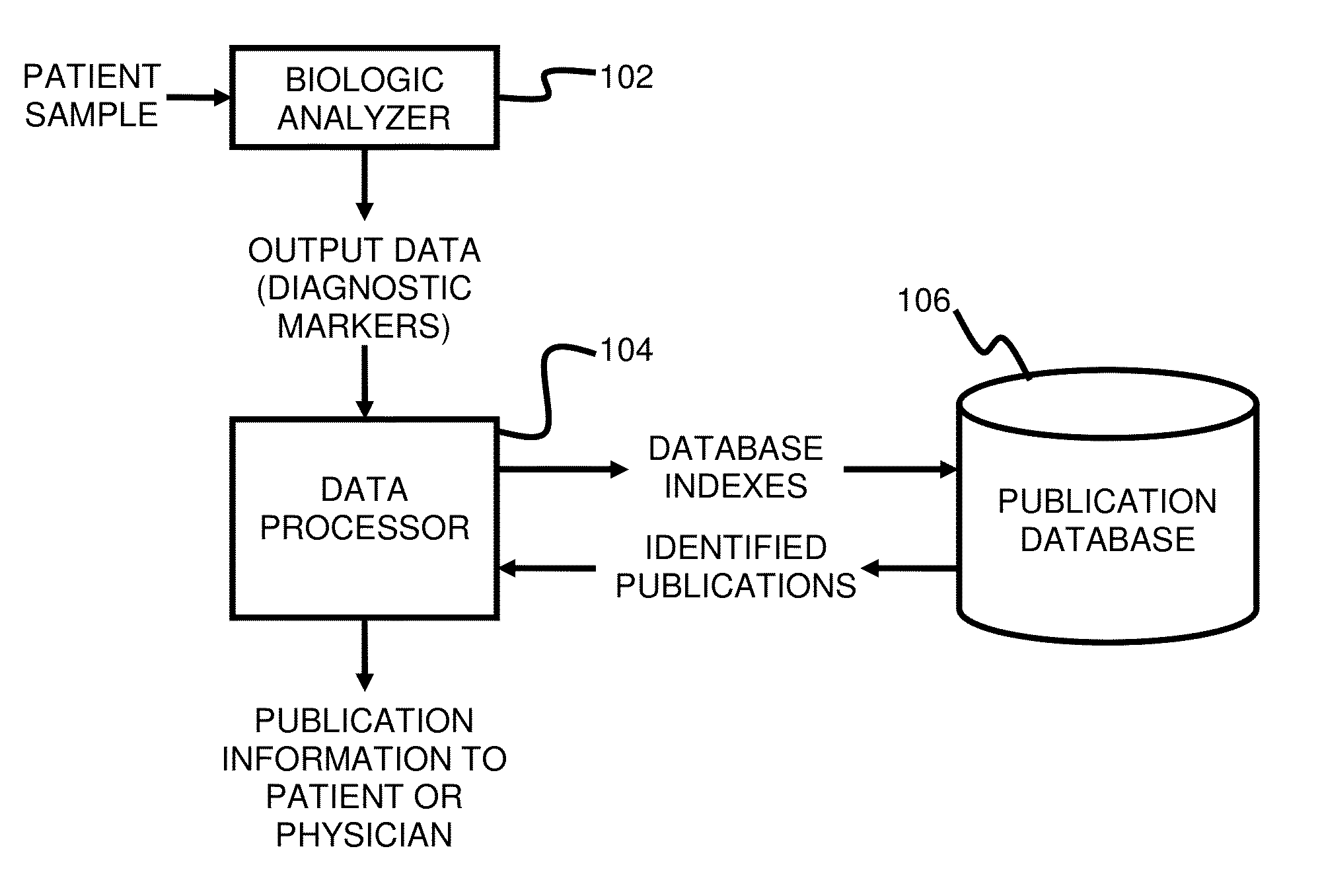
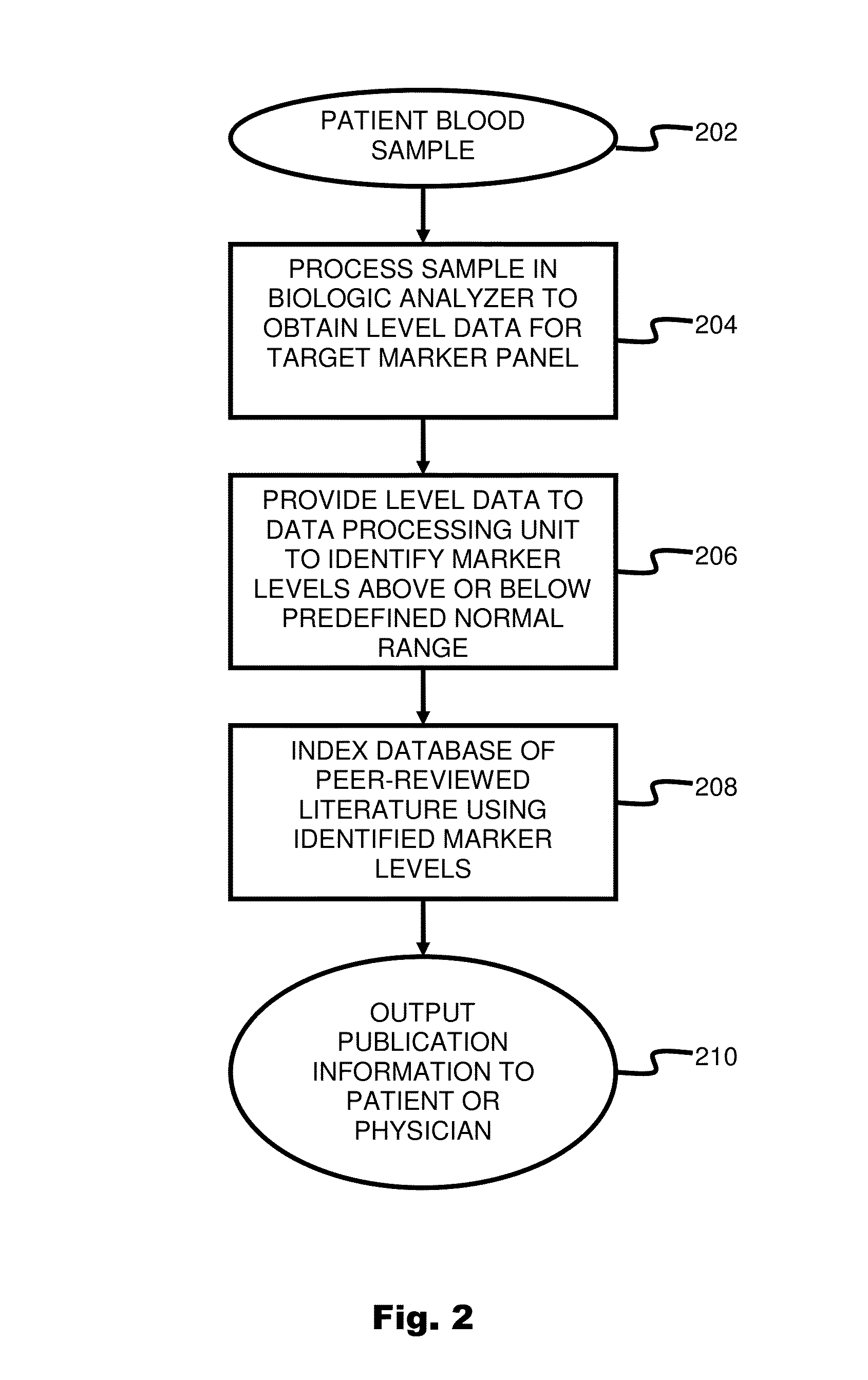
System and method for targeting relevant research activity in response to diagnostic marker analyses
InactiveUS20150046465A1Improve impactLow densityDigital data processing detailsLibrary screeningDiseaseCorrelational study
A system and method for targeting relevant research activity for clinical application in response to diagnostic markers analyses is described. Diagnostic analysis is performed to detect the level of each of at least three diagnostic markers. The levels of the tested markers are used to identify relevant publications from among a large database of articles. The most relevant literature, such as, one which reports research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of diagnostic markers for various disease states, is then provided to the patient and / or the patient's physician, optionally with a summarization of the treatment recommendations from the provided literature. The customized information delivery provides a range of published peer-reviewed therapeutic options and / or published research studies.
Owner:LAMBERT REBECCA
Integrated Systems and Methods of Evaluating Cannabis and Cannabinoid Products for Public Safety, Quality Control and Quality Assurance Purposes
Embodiments of the invention include integrated systems and methods of evaluating cannabis and cannabinoid products for public safety, quality control and quality assurance purposes for research and public use purposes. Embodiments of the invention facilitate suppliers and consumers of a cannabis and / or cannabinoid product to evaluate the origin, efficacy, potency, and quality of the product. Embodiments of the invention also include cannabis processing center where samples of the products are analyzed for research studies to test and set parameters for third parties. Embodiments of the invention include, for example, analyzing cannabis products to determine quality and quantity of desired components and undesired components, determining concentrations of cannabinoids in the product, and comparing measures of components in the product against regulations. Embodiments of the invention further include, for example, an online testing facility for determine whether cannabis products meet a plurality of regulations or guidelines.
Owner:VYRIPHARM ENTERPRISES LLC +1
Prepreg viscosity quantitative measuring method
ActiveCN108254307ASticky controlImprove the use of technologyUsing mechanical meansMaterial strength using steady shearing forcesPhysical chemistryTensile testing
The invention belongs to the field of detection technology, and relates to a quantitative measuring method of the physical property viscosity of an intermediate product prepreg of a moulded compositematerial, and especially relates to a prepreg viscosity quantitative measuring method. According to the prepreg viscosity quantitative measuring method, at least five samples with the sample materialof a product to be tested are prepared, test temperature is controlled to be 23+-2 DEG C, the relative humidity is controlled to be 65% or lower, a tensile testing machine is adopted for tension-sheartest. The quantitative measuring method is capable of controlling the viscosity of prepreg preferably, and improving pregreg using technology. Prepreg viscosity quantification test, test parameter selection and optimization, and forming of a prepreg viscosity quantitative measuring method are realized through a large amount of research studies and argumentation, prepreg viscosity fixed value control is realized through prepreg paving technology adaptability verification, and prepreg technology stability is increased.
Owner:AVIC COMPOSITES
Simulants of Toxants for Training and Testing
InactiveUS20090062386A1Realistic trainingHigh fidelity characteristicBiocideOrganic active ingredientsMedicineFluorescence
Compositions are formulated with generally regarded as safe (GRAS) ingredients for use as chemical simulants of toxants such as chemical warfare agents. The compositions can be used for training exercises, testing, and research studies and they can be applied safely to human skin. They include ultraviolet-excited fluorescent ingredients that make possible visible viewing of the simulants. The chemical simulants have good fidelity with the physical properties of toxants, for example, vapor pressure, volatility, persistence, viscosity, response to oxidative, hydrolysis, and perhydrolysis decontaminants, and they can be detected by commonly used portable instruments used to detect toxants.
Owner:CLEAN EARTH TECH
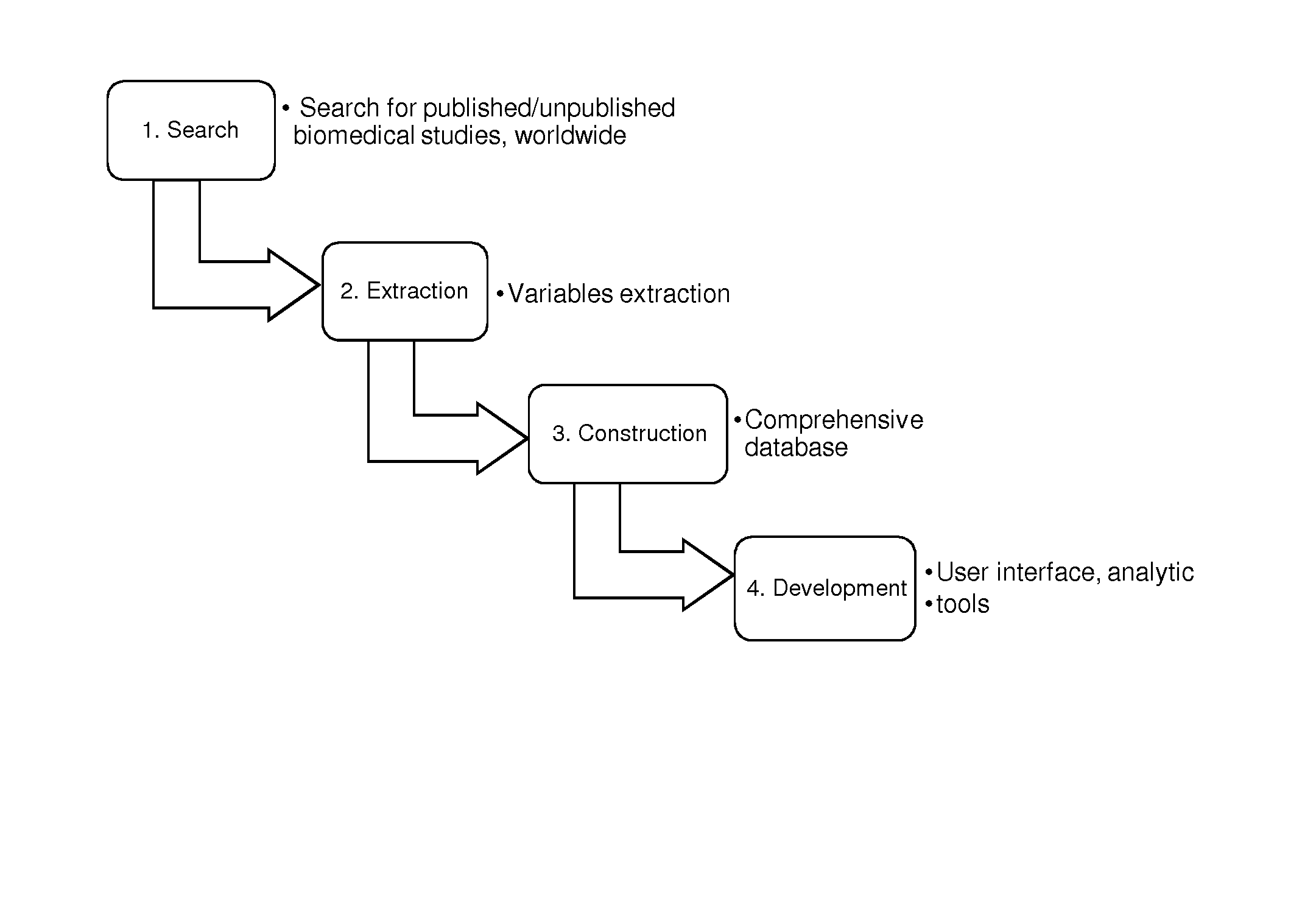
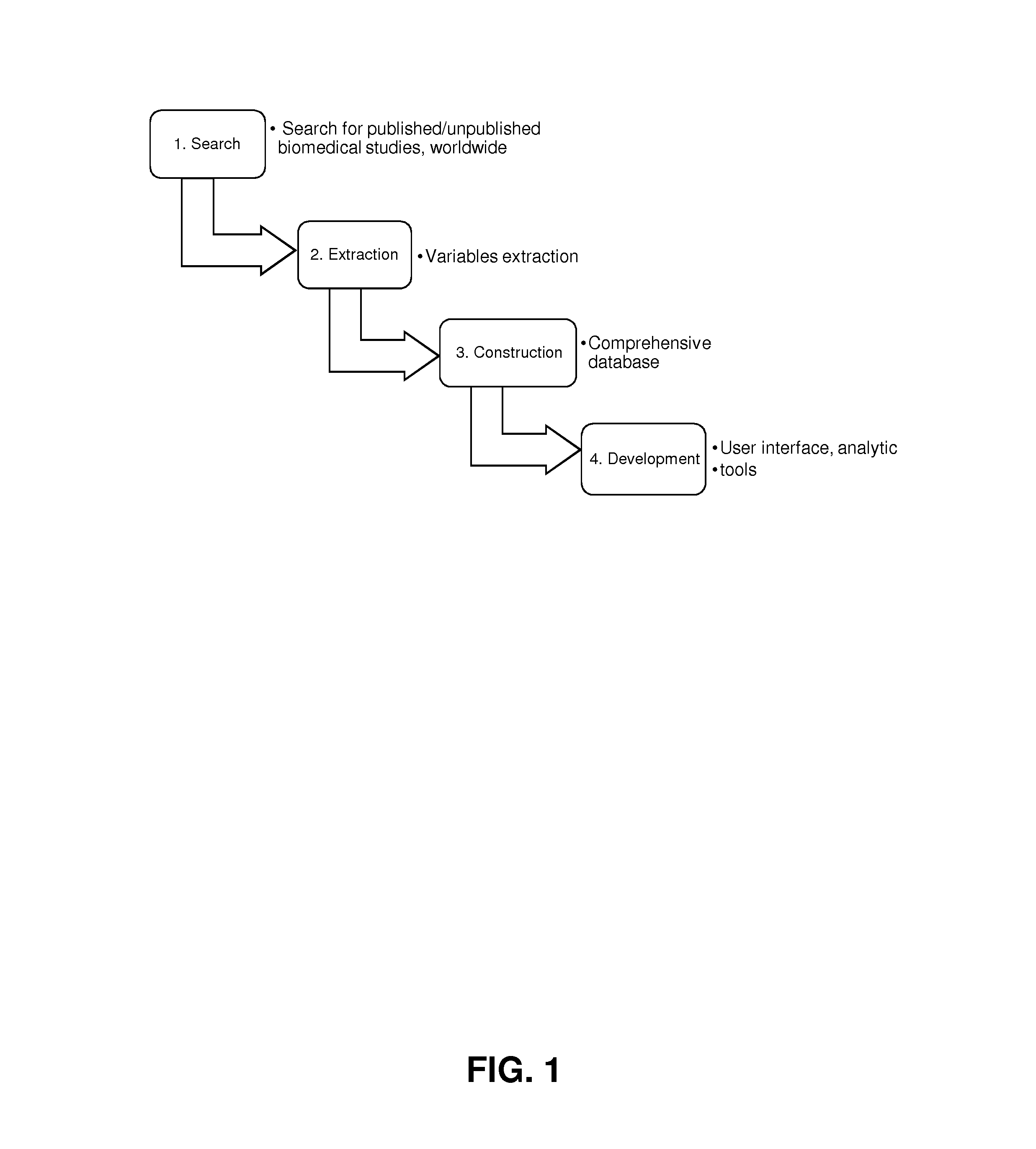
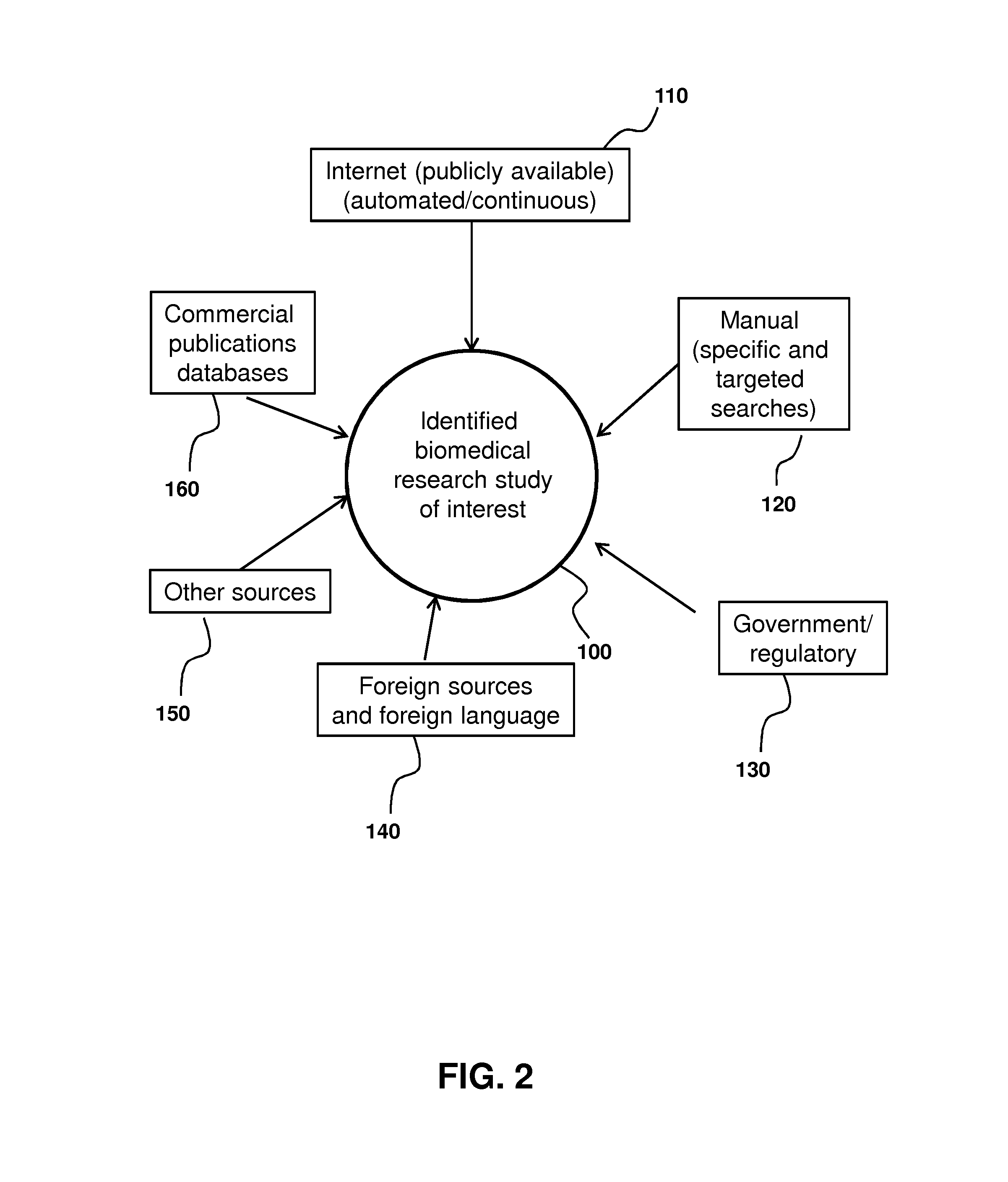
Biomedical research database development and uses
InactiveUS20170046487A1Easy to analyze and useEfficiently populateMedical data miningComputer-assisted medical data acquisitionQuality controlClinical trial
Provided are methods and systems for extracting, integrating, organizing, navigating and querying a large-scale database constructed from biomedical research studies. The database provides a highly efficient and comprehensive infrastructure for performing systematic and meta-analytic queries across a large number of studies and clinical trials from different areas of biomedical research, as well as systems and methods to build and add to such a database. Active quality control steps ensure fidelity and accuracy of standardized values obtained from a range of biomedical research studies that populate the databased described herein.
Owner:MEDAWARE SYST
Monitoring clinical research performance
A computer-implemented method, system, and computer program product monitors clinical research performance. One or more metrics of clinical research performance for investigator / provider / research sites across research studies are collected. The metrics include performance area, characteristic of the performance area with one or more attributes, point values for each attribute, and weight value for the characteristic. A performance score is produced for each of the entities based on the one or more metrics. A machine learning model is trained to determine performance scores based on the produced performance score for each of the entities. A request for entities is processed by applying performance scores from the machine learning model and appropriate corresponding data to a predictive model to determine resulting performance scores, rank and / or match for each of the one or more entities for a given protocol and / or assessment trigger. Actions are performed based on the resulting performance scores, rank and / or match.
Owner:MERATIVE US LP
Methods to predict risk for celiac disease by detecting anti-flagellin antibody levels
InactiveUS8409819B1Effective predictionEffectively stratifyDisease diagnosisBiological testingGenotypeFlagellin
The present invention provides methods, assays, and kits for predicting or stratifying the risk of celiac disease (CD) based upon HLA-DQ genotype and / or anti-flagellin antibody levels. Such risk prediction or stratification can provide benefits to family members of CD patients, to a subset of patients who are being evaluated clinically for CD, and to researchers, who can utilize this strategy to establish inclusion criteria for participation in research studies investigating potential preventive interventions.
Owner:PROMETHEUS BIOSCI INC
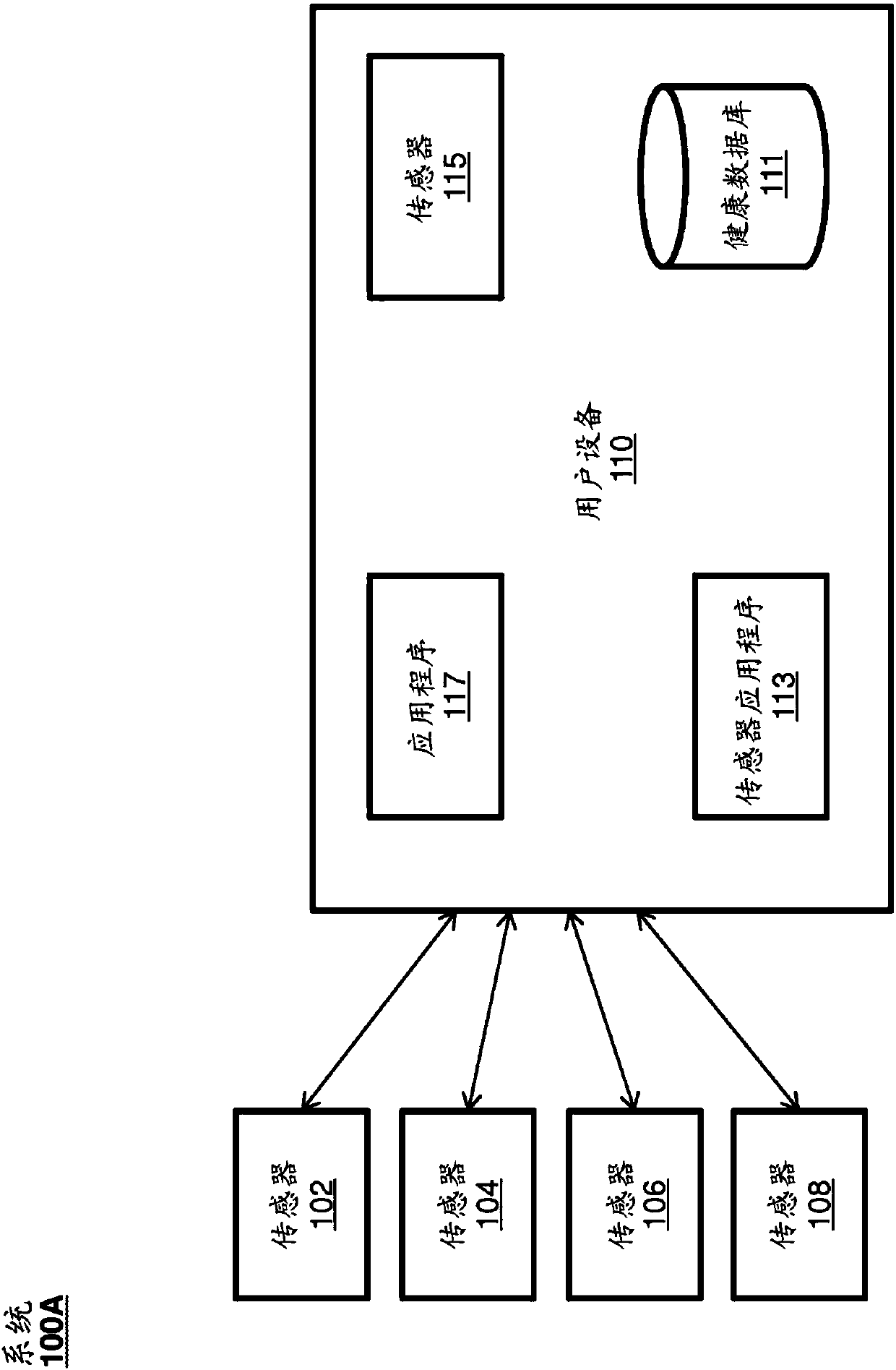
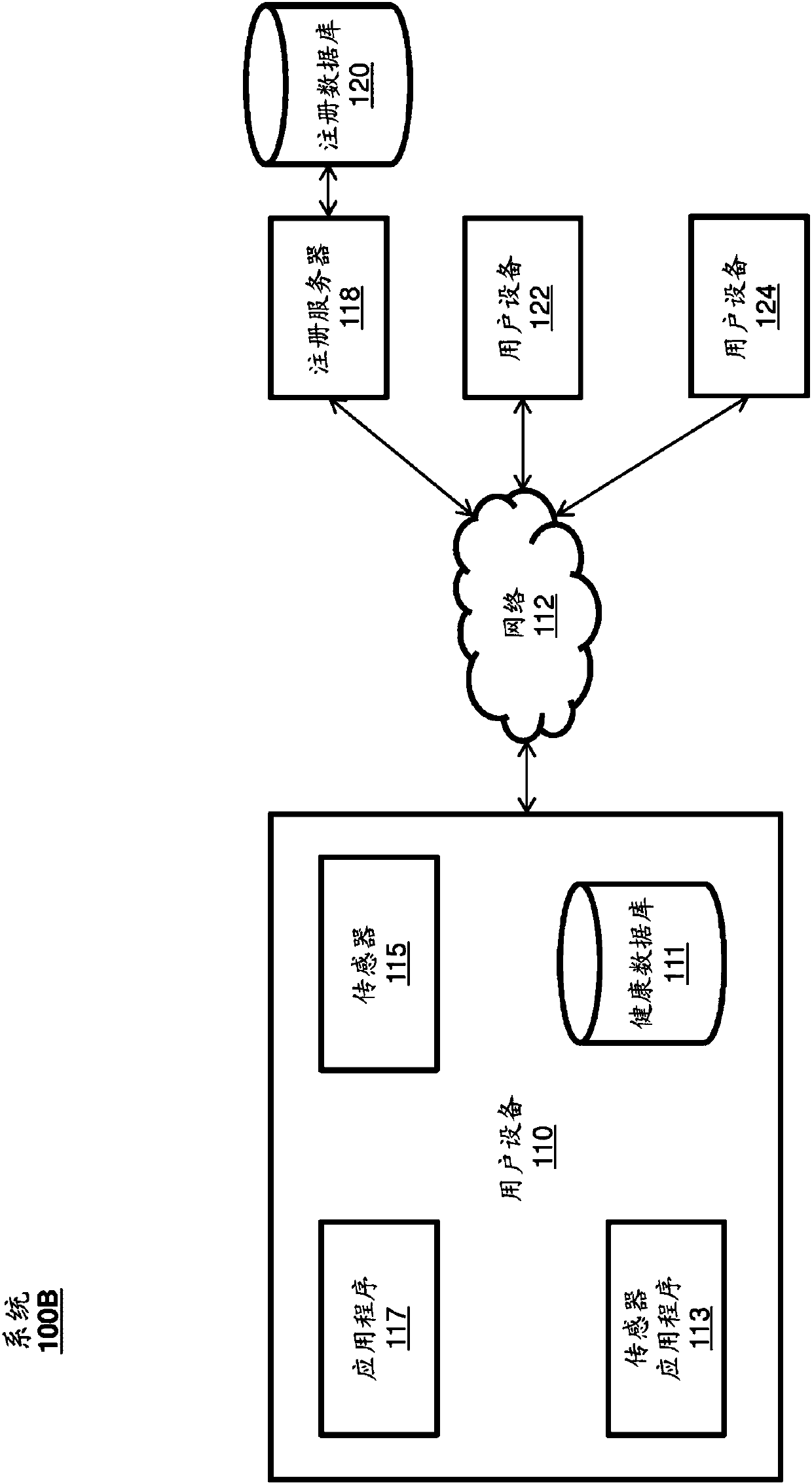
Wellness registry
The present disclosure relates to receiving and sharing wellness data. The wellness data can be received by a user device from any number of sensors external or internal to the user device, from a user manually entering the wellness data, or from other users or entities. The user device can securely store the wellness data on the user device and transmit the wellness data to be stored on a remote database. A user of the device can share some or all of the wellness data with research entities conducting research studies, friends, relatives, caregivers, healthcare providers, or the like.
Owner:APPLE INC
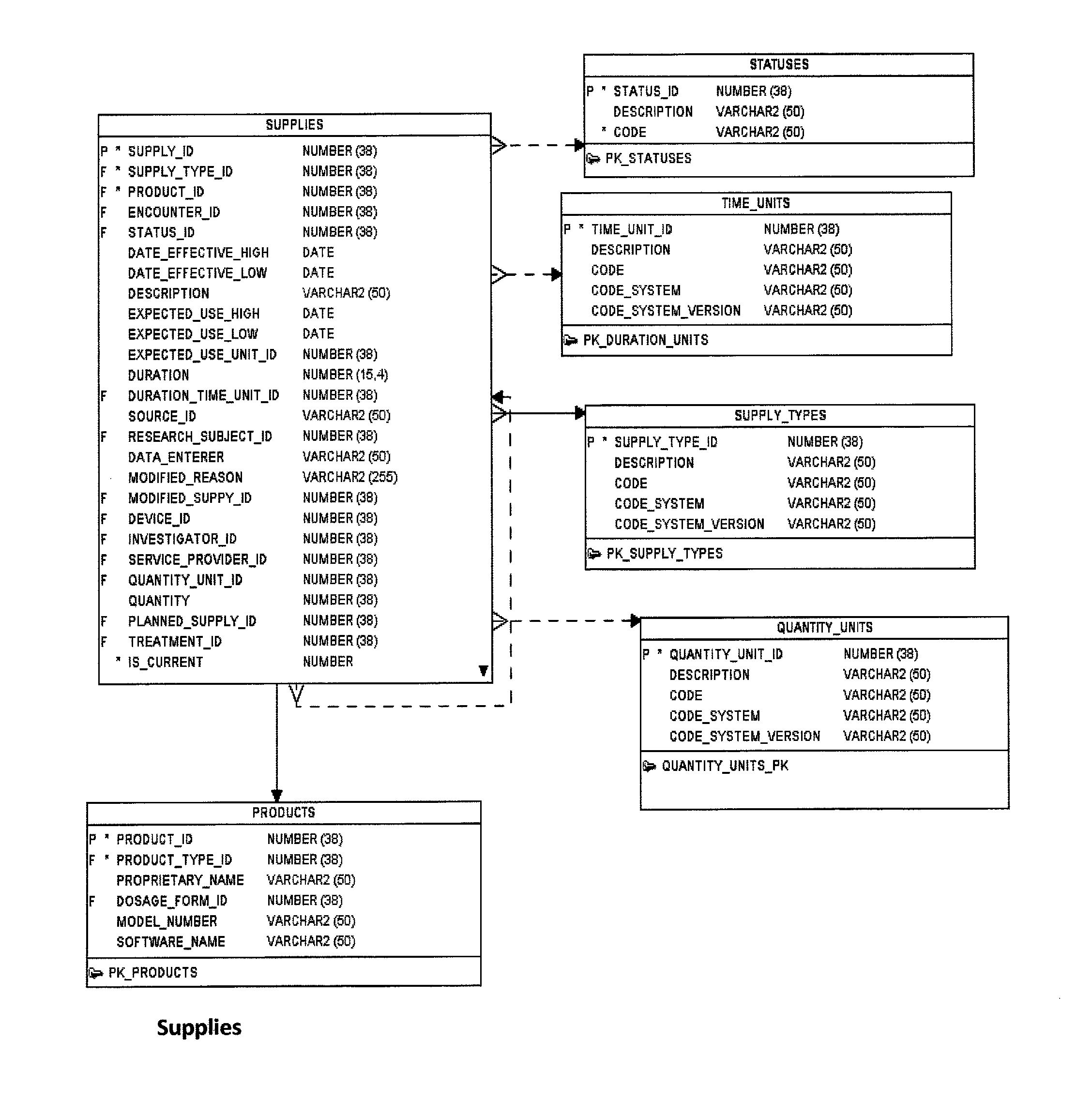
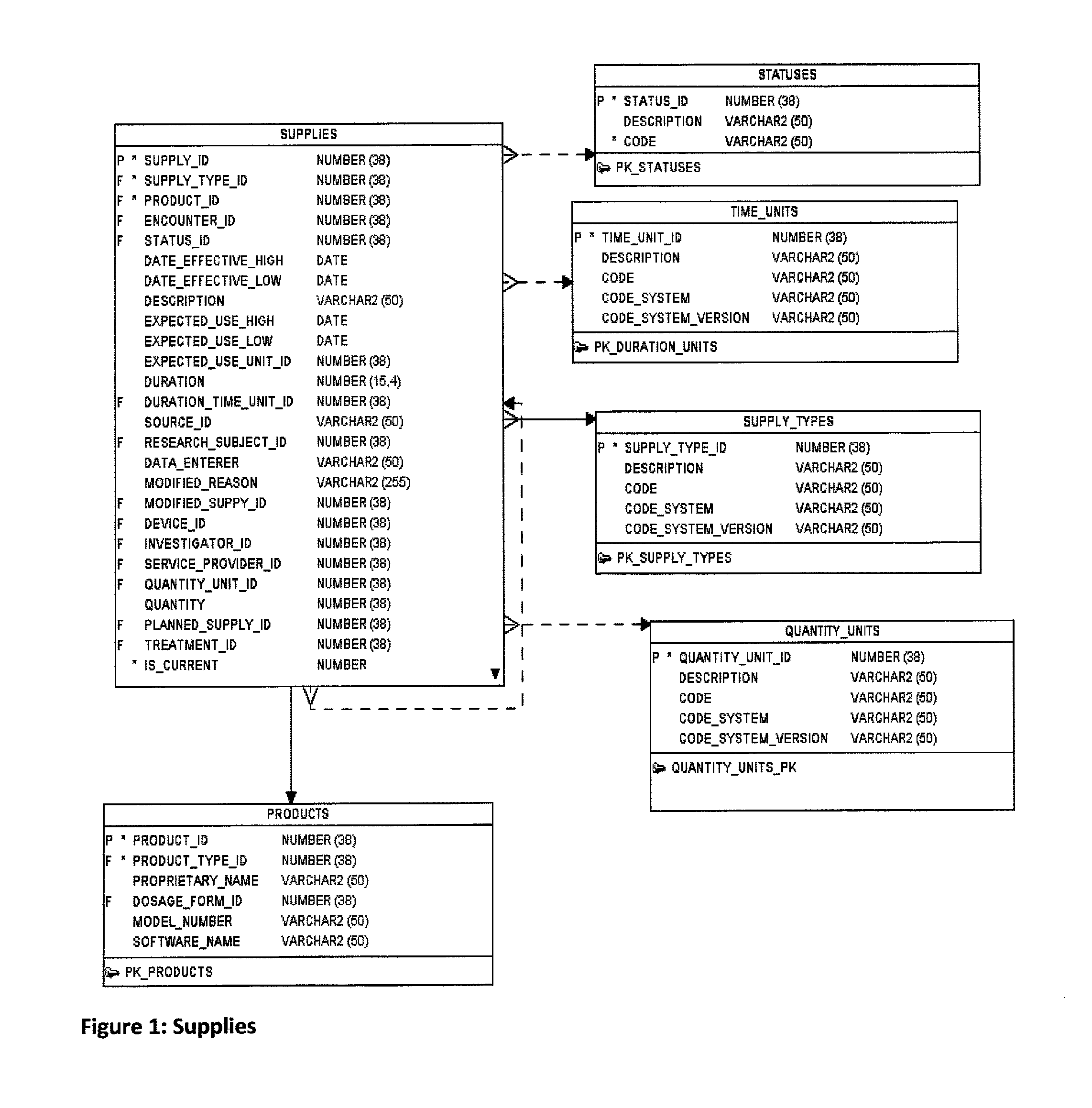
Research study database to compare different research studies and to compare actual activities compared to the protocol
ActiveUS8484254B1Quick and efficient analysisReduce data volumeDigital data processing detailsSpecial data processing applicationsDatabase schemaObject oriented databases
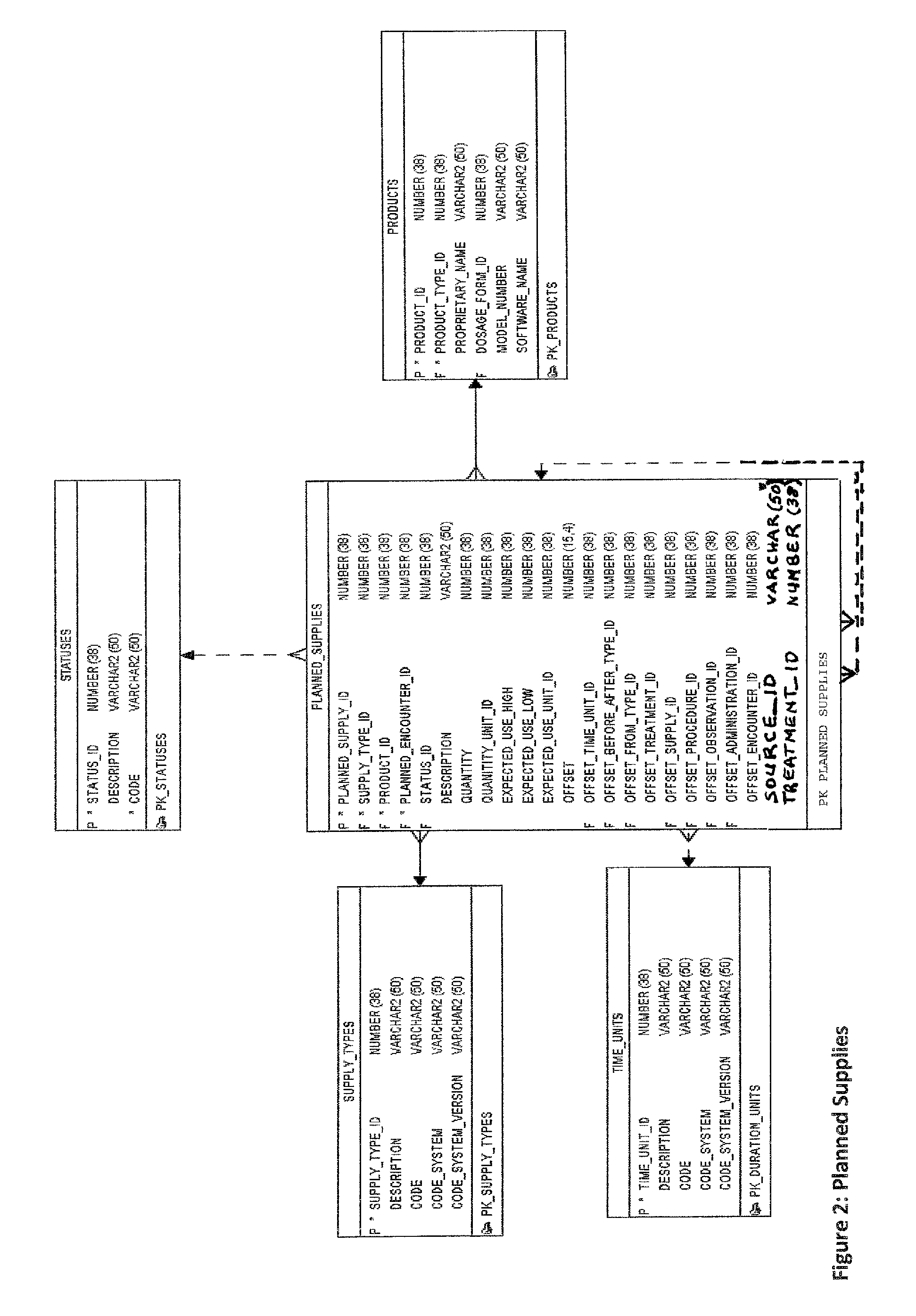
A computer is provided for processing data from a plurality of studies of investigational products in a manner that allows the data from one study to be compared to one or more other studies. Each study includes a plurality of planned activities, a plurality of actual activities, and a plurality of assessments. The computer includes a memory, a database schema and a database. The memory is configured to store an operating system which includes an object-oriented database engine. The database schema is maintained by the object-oriented database engine of the operating system. The database schema has a plurality of uniquely defined database objects. For each study, the uniquely defined database objects include respective sets of objects that store the plurality of planned activities, actual activities, and assessments. The database is populated with data associated with the plurality of planned activities, actual activities, and assessments. The respective sets of objects that store the plurality of planned activities, actual activities, and assessments for each study share common attributes and relationships. Each planned activity, actual activity, and assessment has an associated data type that is the same for different studies.
Owner:SYNCHROGENIX INFORMATION STRATEGIES LLC
System and method for targeting relevant research activity in response to angiogenic regulator analyses
ActiveUS8874378B2Highly targeted and unique mannerEfficacy of treatmentDisease diagnosisProteomicsDiseaseCorrelational study
A system and method for targeting relevant research activity for clinical application in response to angiogenic regulator analyses. An angiogenic analysis is performed on a patient blood sample in order to detect the level of each of at least ten angiogenic regulators. The levels of the tested regulators are used as indexes to identify relevant peer-reviewed research publications from among a large database of articles. The most relevant peer-reviewed literature reporting research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of angiogenic regulators for various disease states are then provided to the patient and / or to the patient's physician, optionally in conjunction with a summarization of the treatment recommendations gleaned from the provided literature. The customized information delivery provides the patient and physician a range of published peer-reviewed therapeutic options and published research studies for moderating the out of range regulators to within normal range or other diagnostic significant range.
Owner:LAMBERT REBECCA
Research study user interfaces
ActiveUS11266330B2Faster and efficient method and interfaceReduce cognitive loadAudiometeringSensorsHearing testClassical mechanics
The present disclosure generally relates to techniques and user interfaces for interacting with research studies. In some embodiments, an electronic device displays a user interface that includes a task view with active tasks from multiple research studies. In some embodiments, an electronic device, while displaying a research study user interface, displays an indication of a problem that prevents enrollment in the research study when enrollment problem criteria are met. In some embodiments, an electronic device, while performing a hearing test, suspends the test and displays a restart affordance when the ambient noise level exceeds a threshold.
Owner:APPLE INC
Dye-based handwriting protectant for written archives
The invention discloses a dye-based handwriting protectant for written archives, and the protectant provided by the invention is prepared from the following raw materials in percentage by weight: 0.8-1.6% of chitosan, 0.3-0.6% of glacial acetic acids, and 97.8-98.9% of distilled water. Lab research studies show that the dye-based handwriting protectant for written archives prepared according to the mass ratio disclosed by the invention can be used for effectively preventing dye-based handwritings from diffusion when the dye-based handwritings are contacted with water, so that the mechanical strength of the whole paper is significantly improved, and the mount and repair of archival paper subjected to protection are not affected. The dye-based handwriting protectant for written archives disclosed by the invention can be applied to the protection of easily-diffused dye-based archival handwritings.
Owner:SHAANXI NORMAL UNIV
health registration
This disclosure relates to receiving and sharing health data. The wellness data may be received by the user device from any number of sensors external or internal to the user device, from the user manually entering the wellness data, or from other users or entities. The user device may securely store the health data on the user device and transmit the health data stored on the remote database. A user of the device may share some or all of the health data with friends, relatives, caregivers, healthcare providers, research entities conducting research studies, and the like.
Owner:APPLE INC
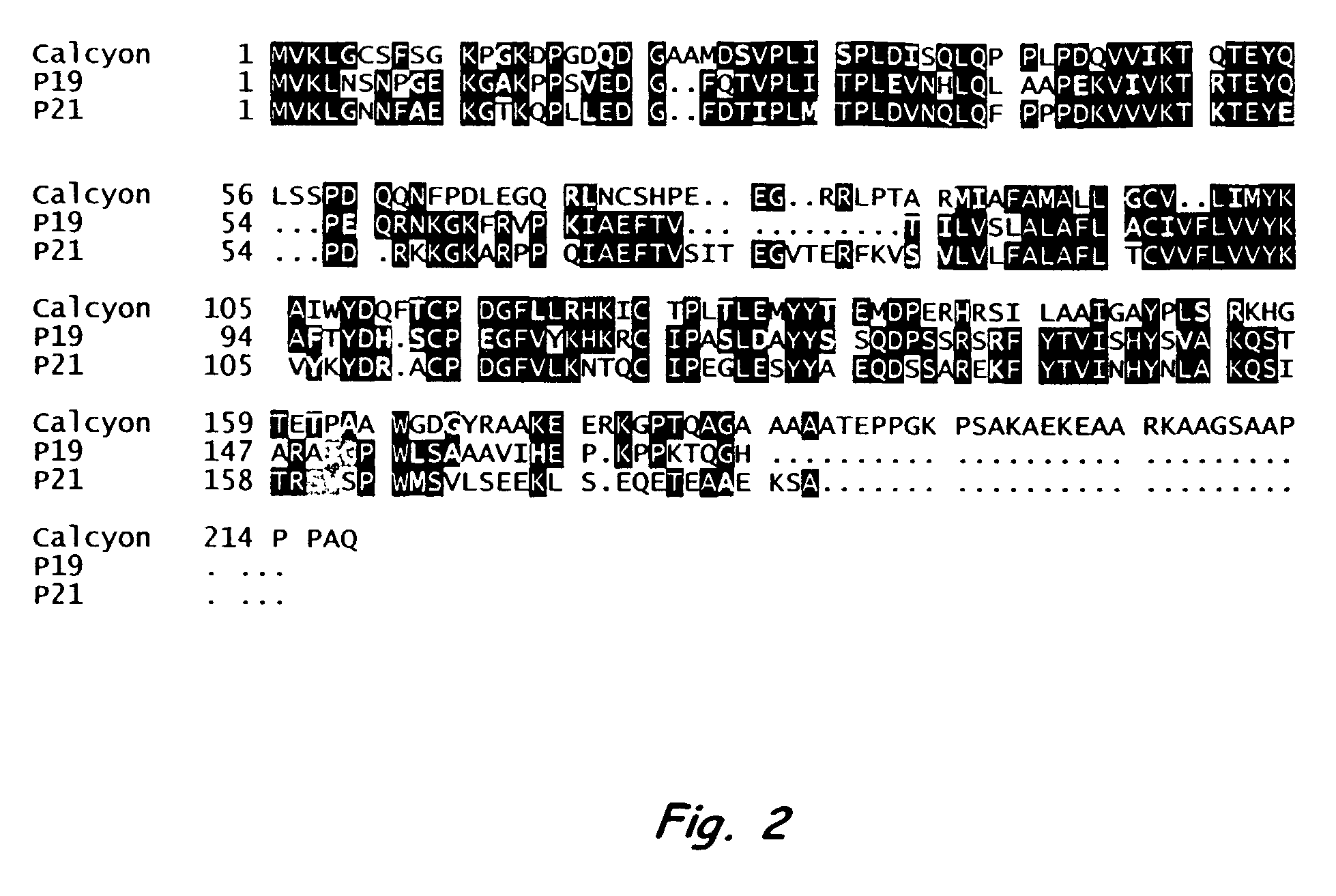
Nucleic acid encoding calcyon, a D-1 like dopamine receptor activity modifying protein
A number of cDNA clones whose products may interact with D1 receptors in vivo were identified. One of the clones, P24, was characterized further. P24 is localized in dendrites and spines of pyramidal cells in PFC. The extent of overlap between P24 expressing and D1 receptor expressing pyramidal cells appeared to be 100%. In contrast, only a limited number D1 receptor antibody labeled neurons in caudate expressed P24. P24 lowers the threshold of D1 receptor response to dopamine (DA) by an order of magnitude. Sequence similarity suggests P24 is a diverged member of the RAMP family. The P24 protein is therefore referred to as a D1 DA RAMP, calcyon. The isolated protein and nucleotide molecule encoding the protein, as well as primers for the nucleotide, are described. The protein and compounds modifying DA binding to the receptor or calcium release which is mediated by the Calcyon, are useful in research studies, drug screening, and therapeutically.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Gene markers, primers, probes and kits for detecting lung cancer
ActiveCN105671181BEasy to get materialsTraumaMicrobiological testing/measurementDNA/RNA fragmentationOncologyMonocyte
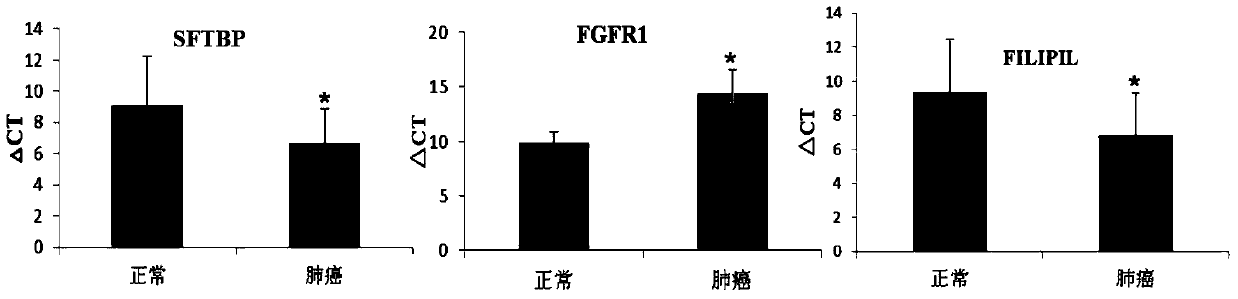
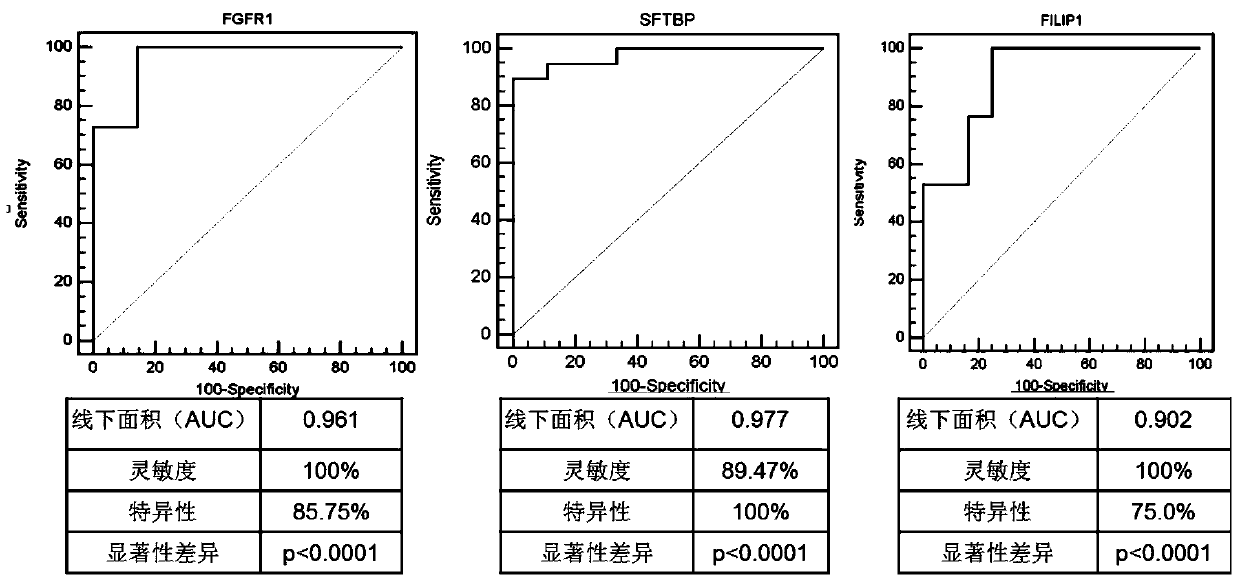
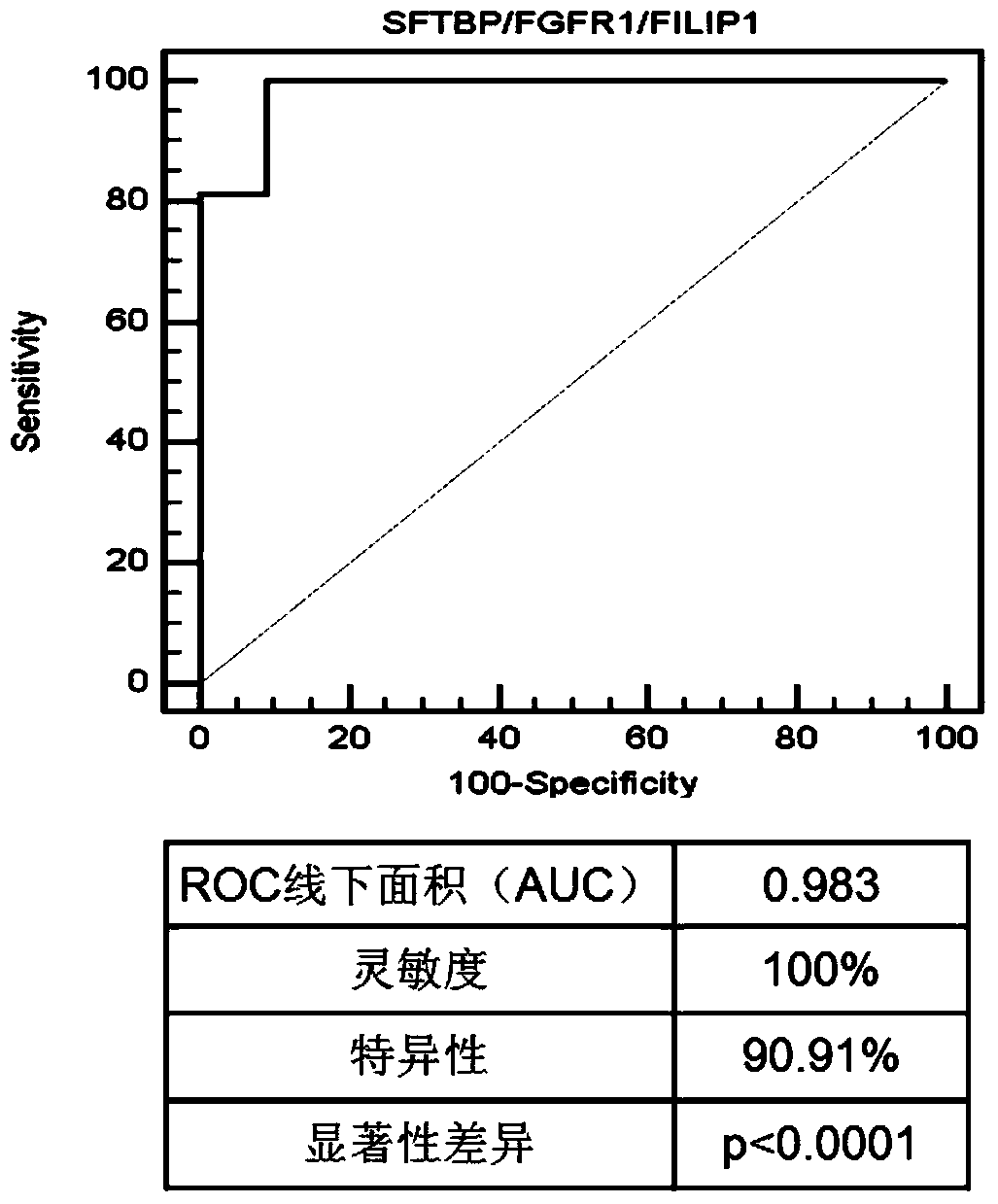
The invention discloses a genetic mark for detecting lung cancer. The genetic marker comprises an SFTPB gene, an FGFR1 gene and an FILIP1L gene. Research studies show that mRNAs (messenger ribonucleic acids) of SFTPB, FGFR1 and FILIP1L in blood mononuclear cells of patients with the lung cancer are not expressed normally, accuracy rate of diagnosis on the lung cancer reaches 98.3% after integration, and the genetic mark is much more superior to a single gene in terms of diagnosis specificity and sensitivity. Through measurement of expression quantities of the SFTPB gene, the FGFR1 gene and the FILIP1L gene in blood, diagnosis on the lung cancer (especially early lung cancer from which the patients suffer without symptoms) is achieved, so that clinical lung cancer detection rate is increased, and death rate of the patients with the lung cancer is reduced.
Owner:王义明
Systems and methods for facilitating health research
Owner:APPLE INC
Simulant of Radiological Contamination
InactiveUS20090057622A1High fidelity characteristicSatisfy safety performance requirementsConductive materialOrganic dyesParticulatesAdditive ingredient
A composition is formulated with generally regarded as safe (GRAS) ingredients for use as a non-radio-active simulant of radiological contamination such as fallout from a nuclear explosion, particulate from a radiological dispersal device, or contamination from operation of nuclear facilities. The compositions can be used for training exercises, testing, and research studies, and they can be applied safely to human skin. They include an ultraviolet (UV)-excited fluorescent ingredient that makes possible visible viewing of the simulants when illuminated by UV light. The chemical simulants have good fidelity with the physical properties of contamination, for example, adhesion, particle size, electrostatic charging, and response to decontamination technologies such as washing and vacuuming.
Owner:CLEAN EARTH TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com