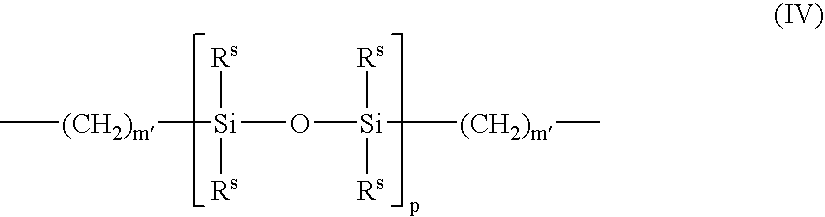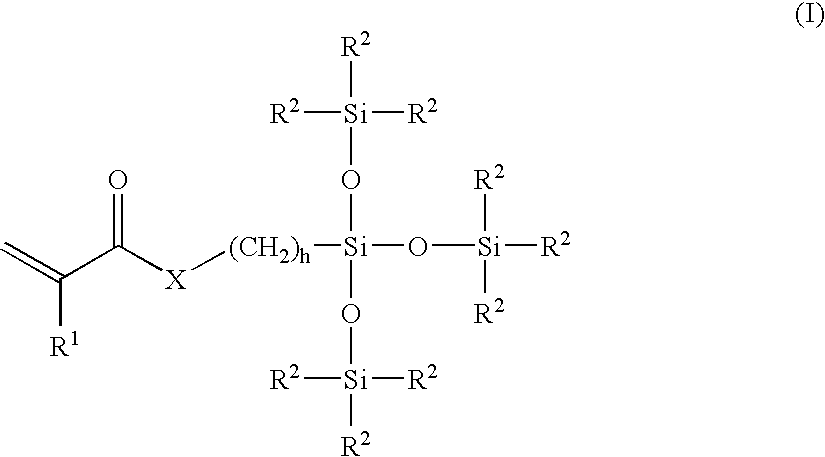Patents
Literature
148results about "Using protective liquids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
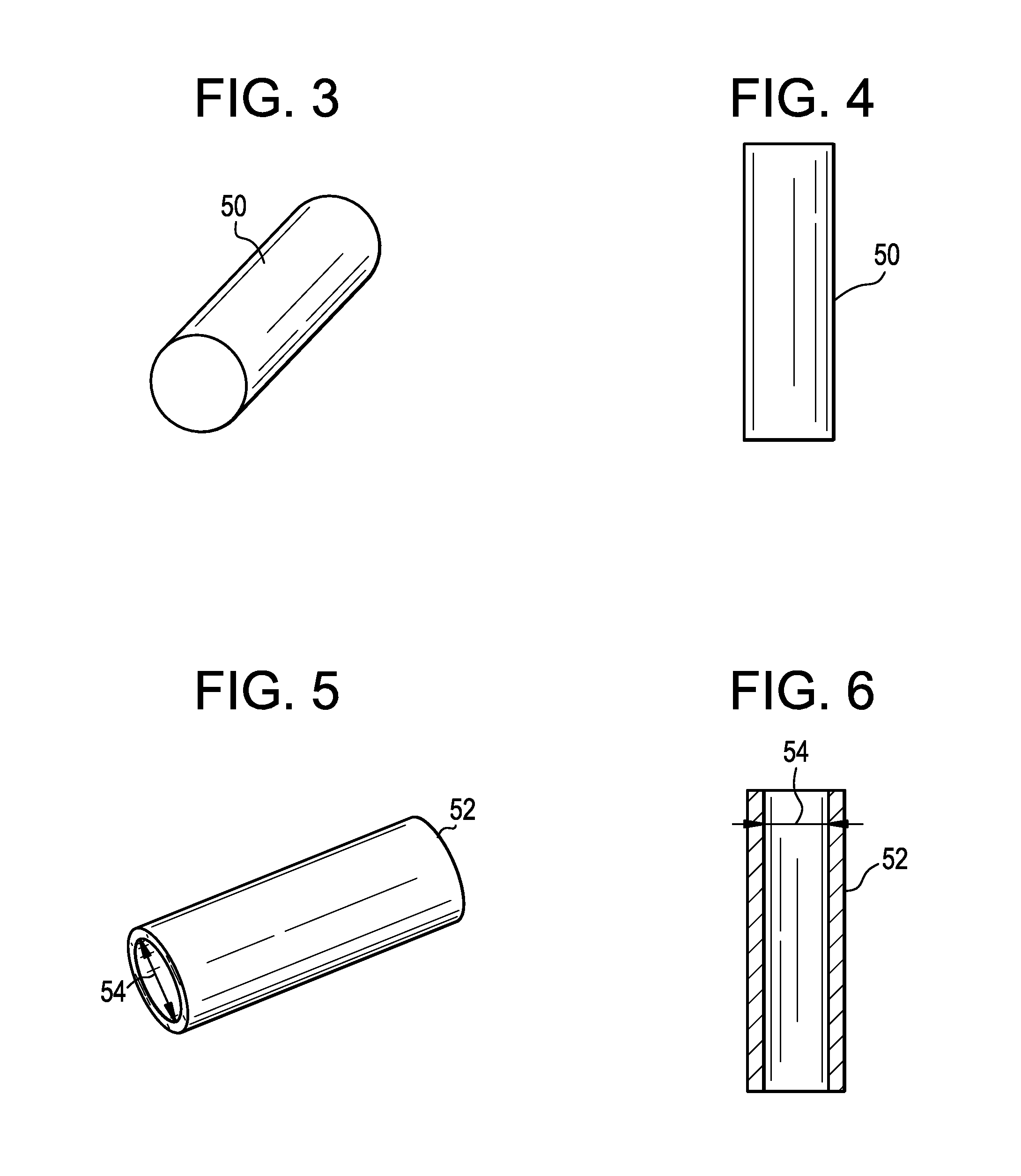
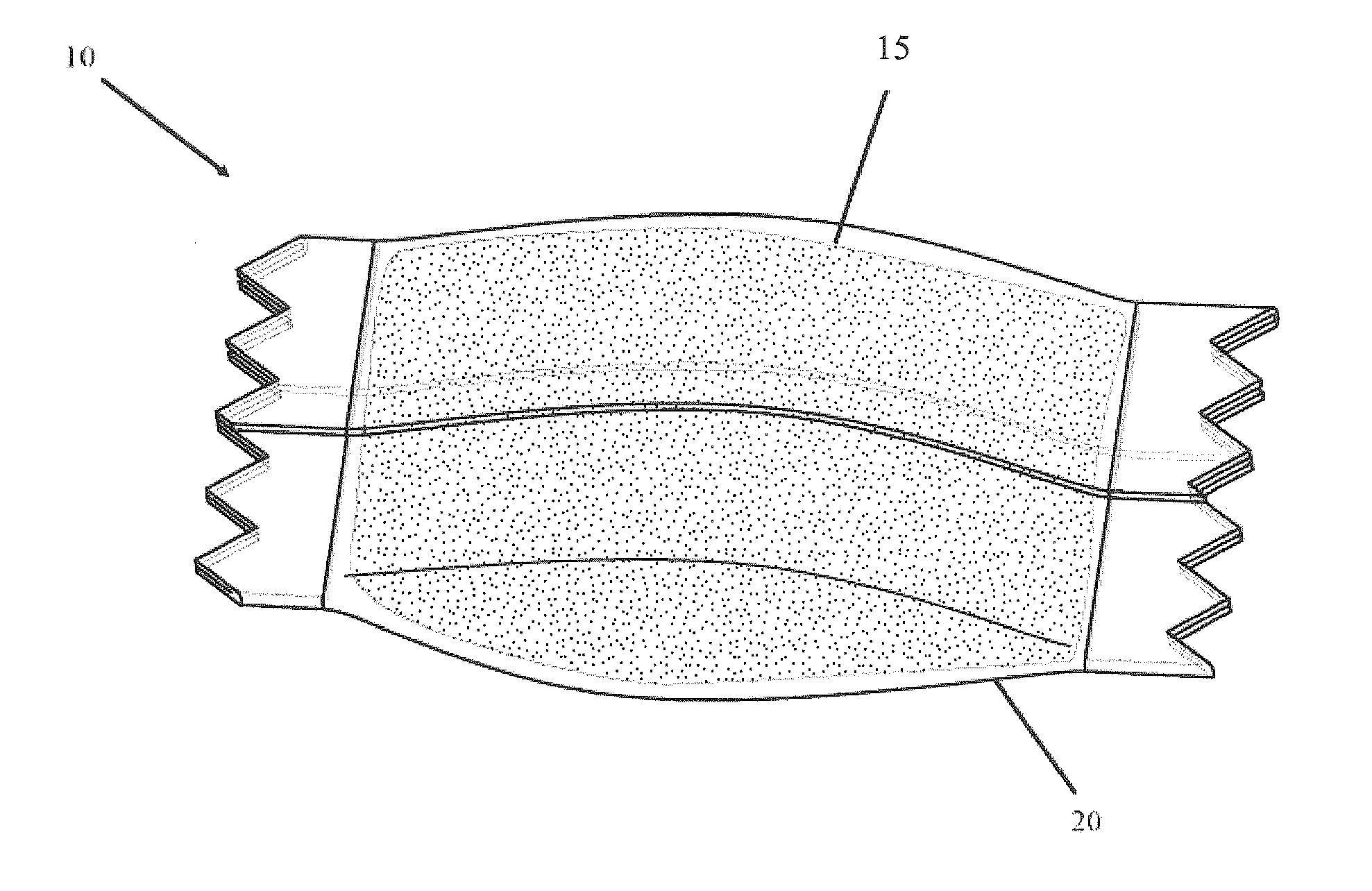
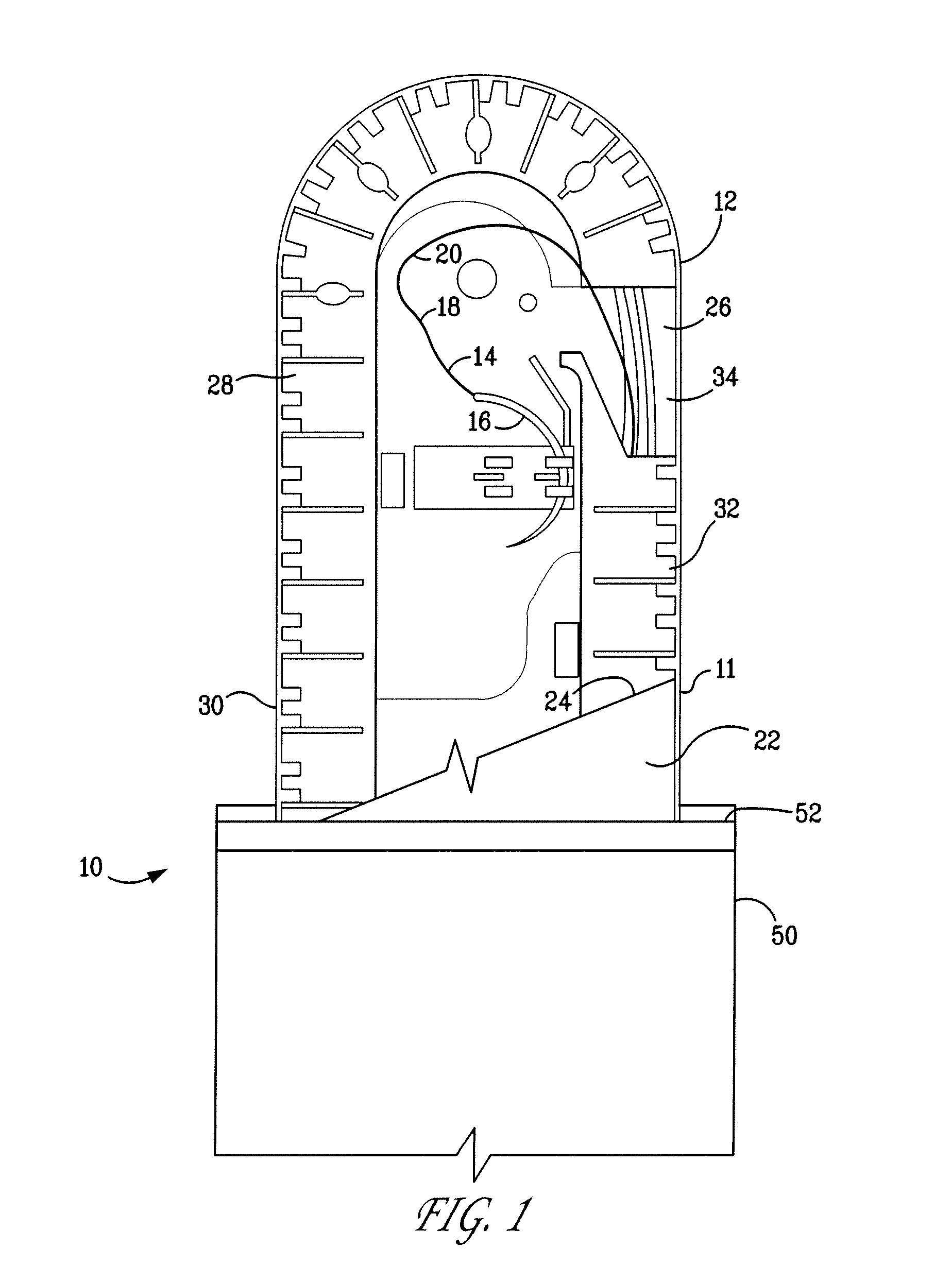
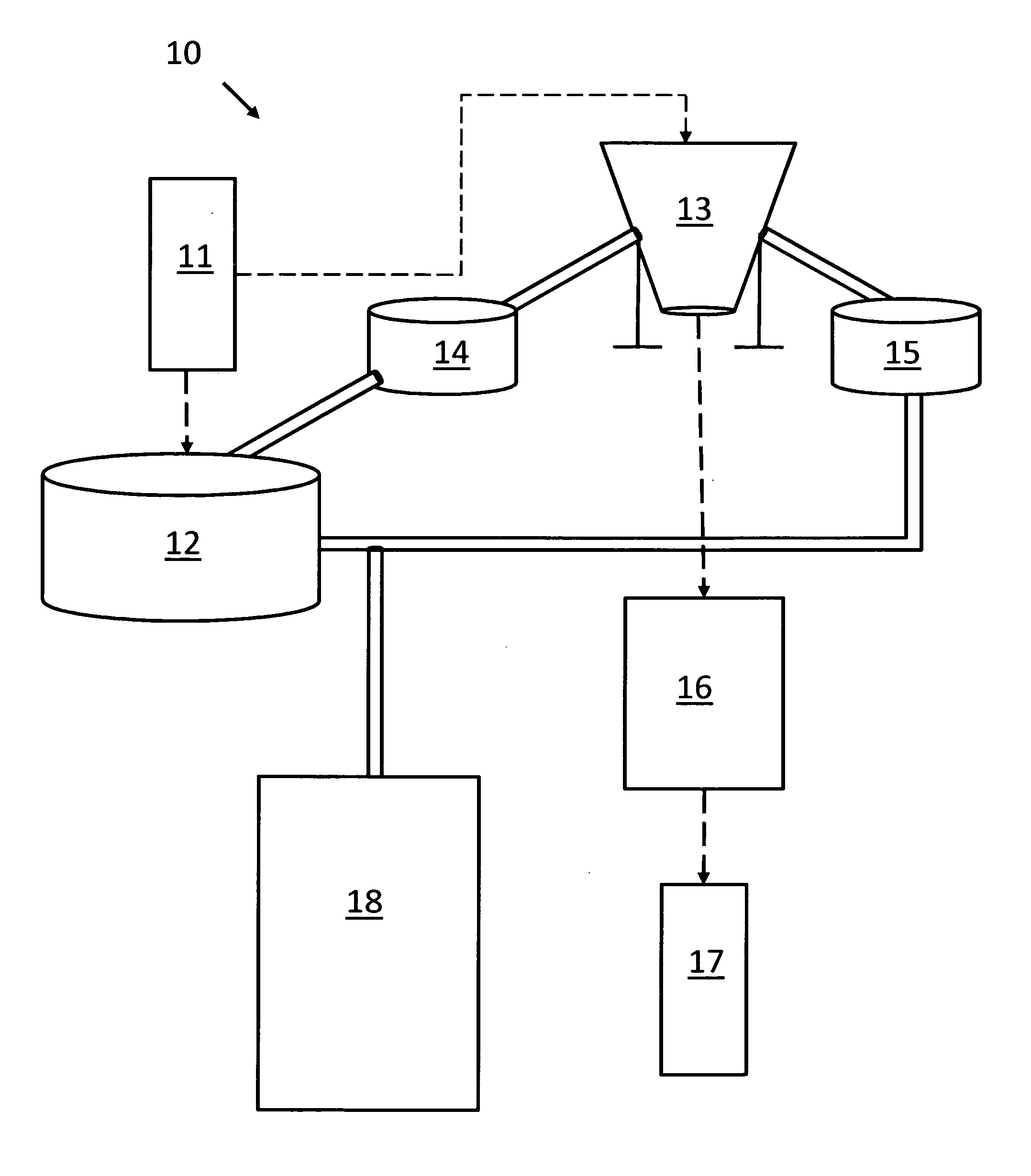
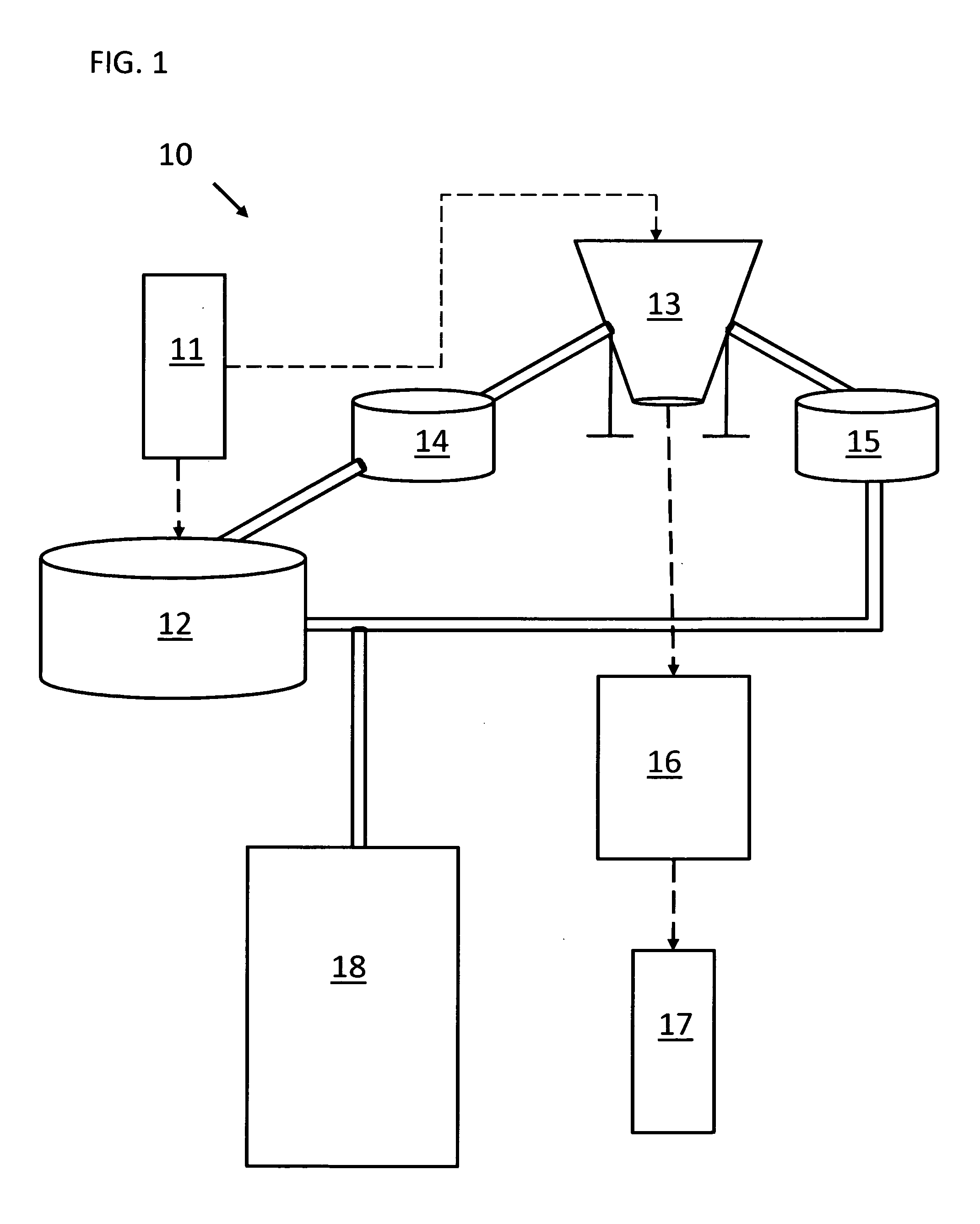
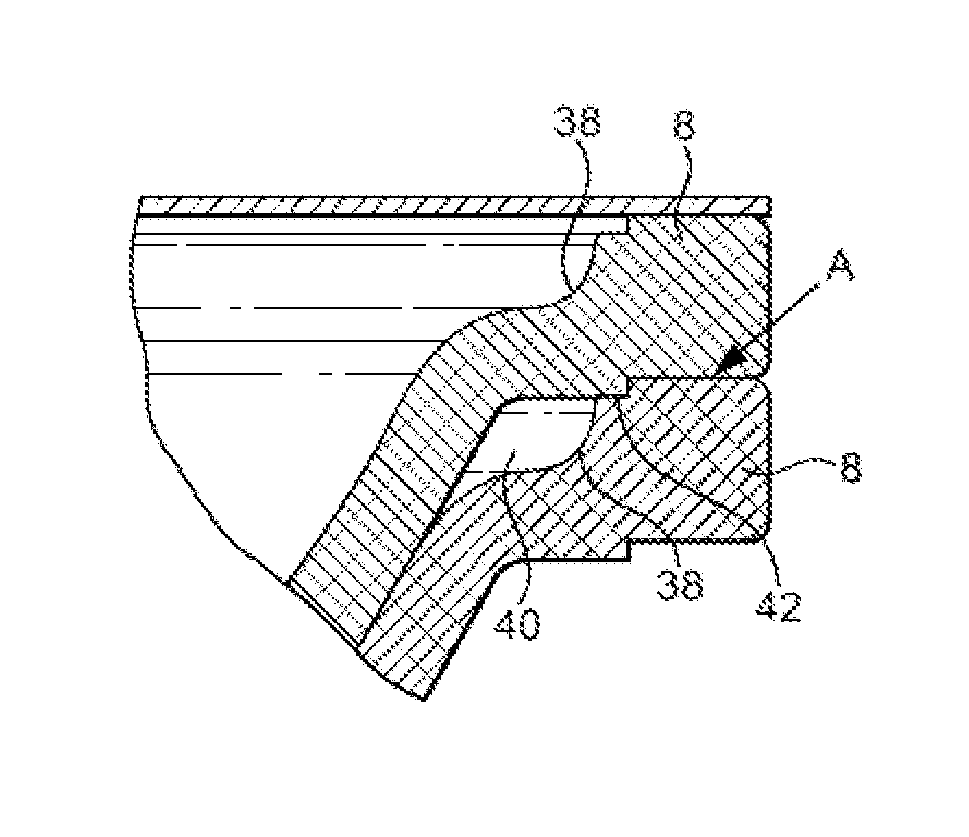
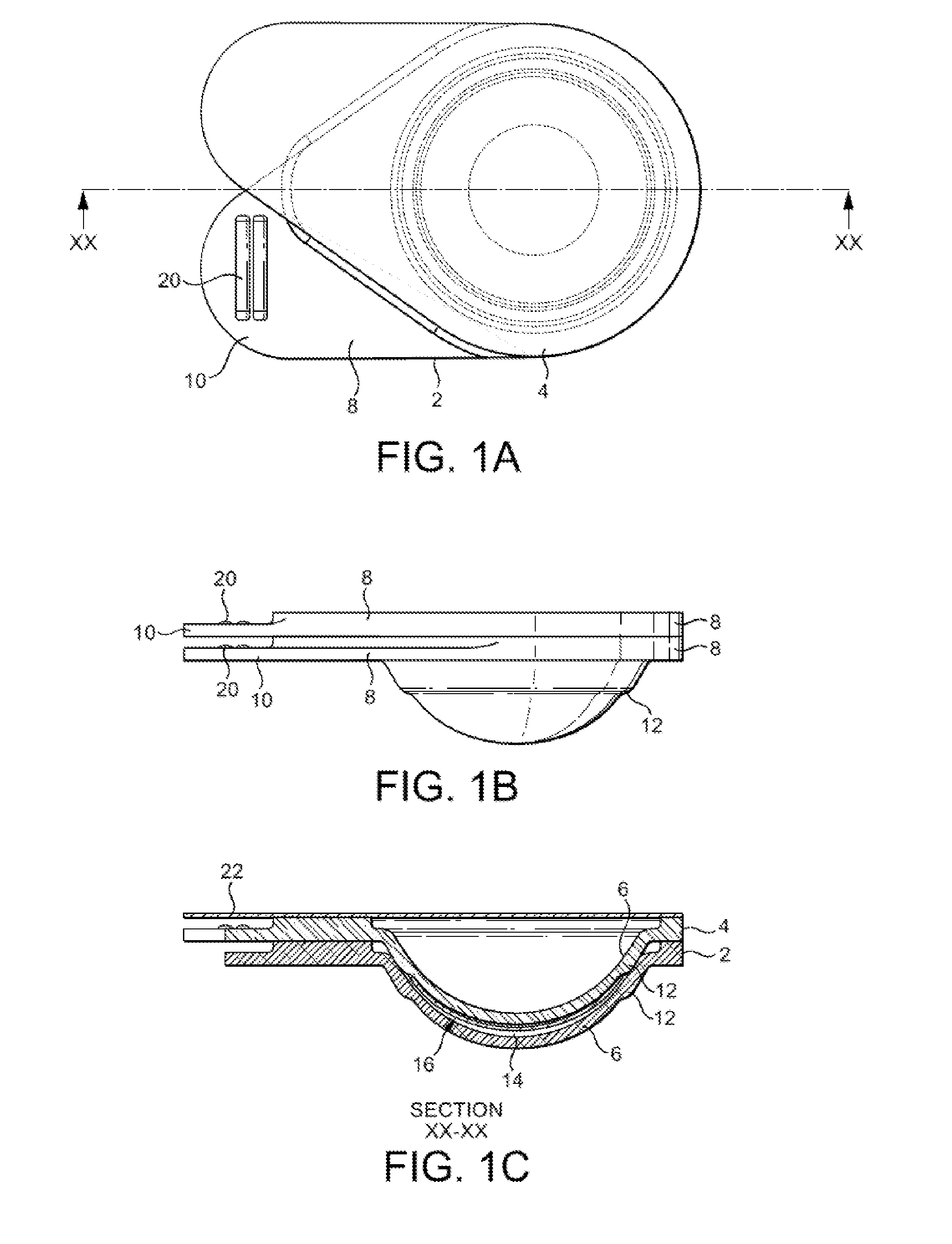
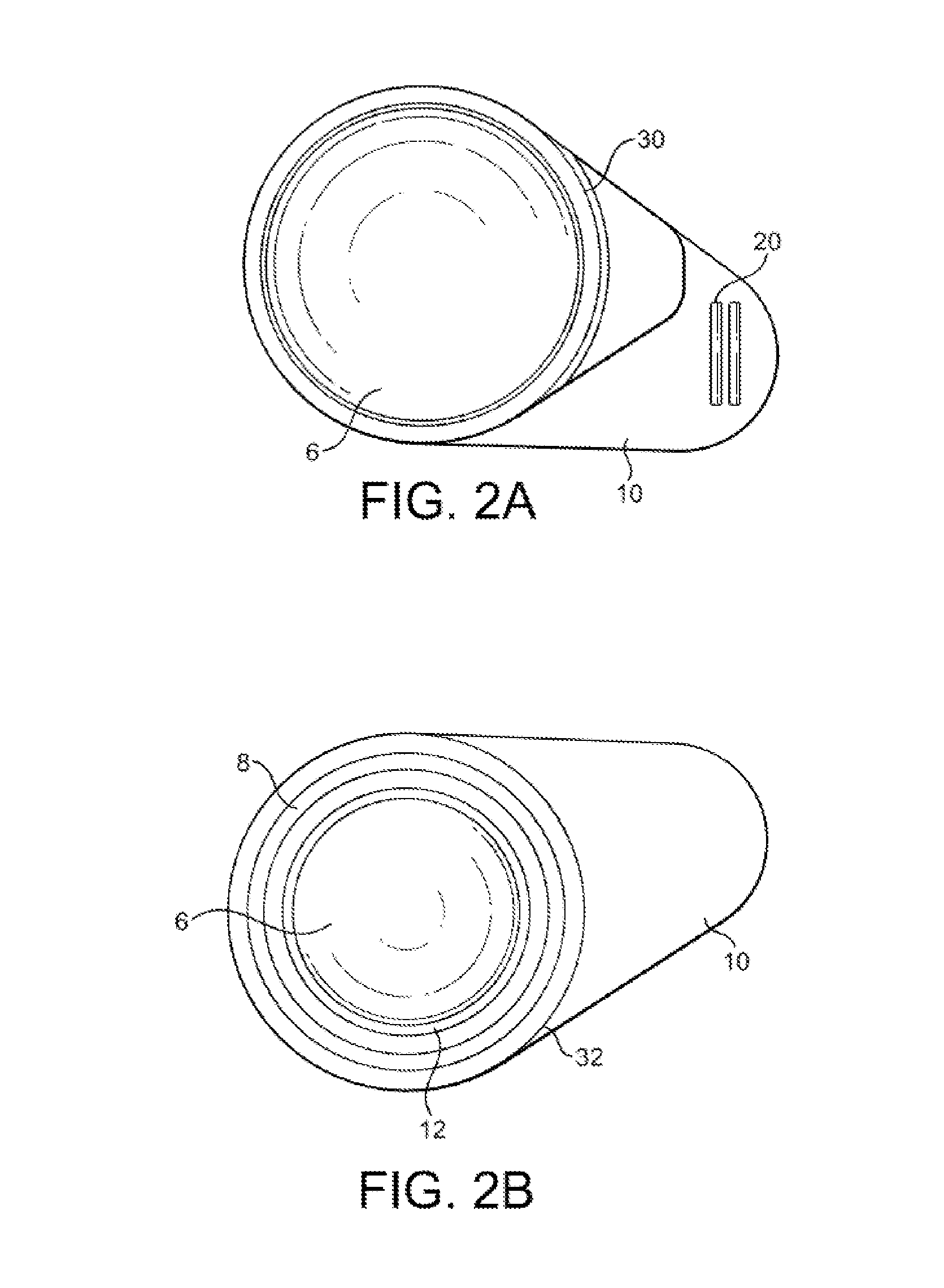
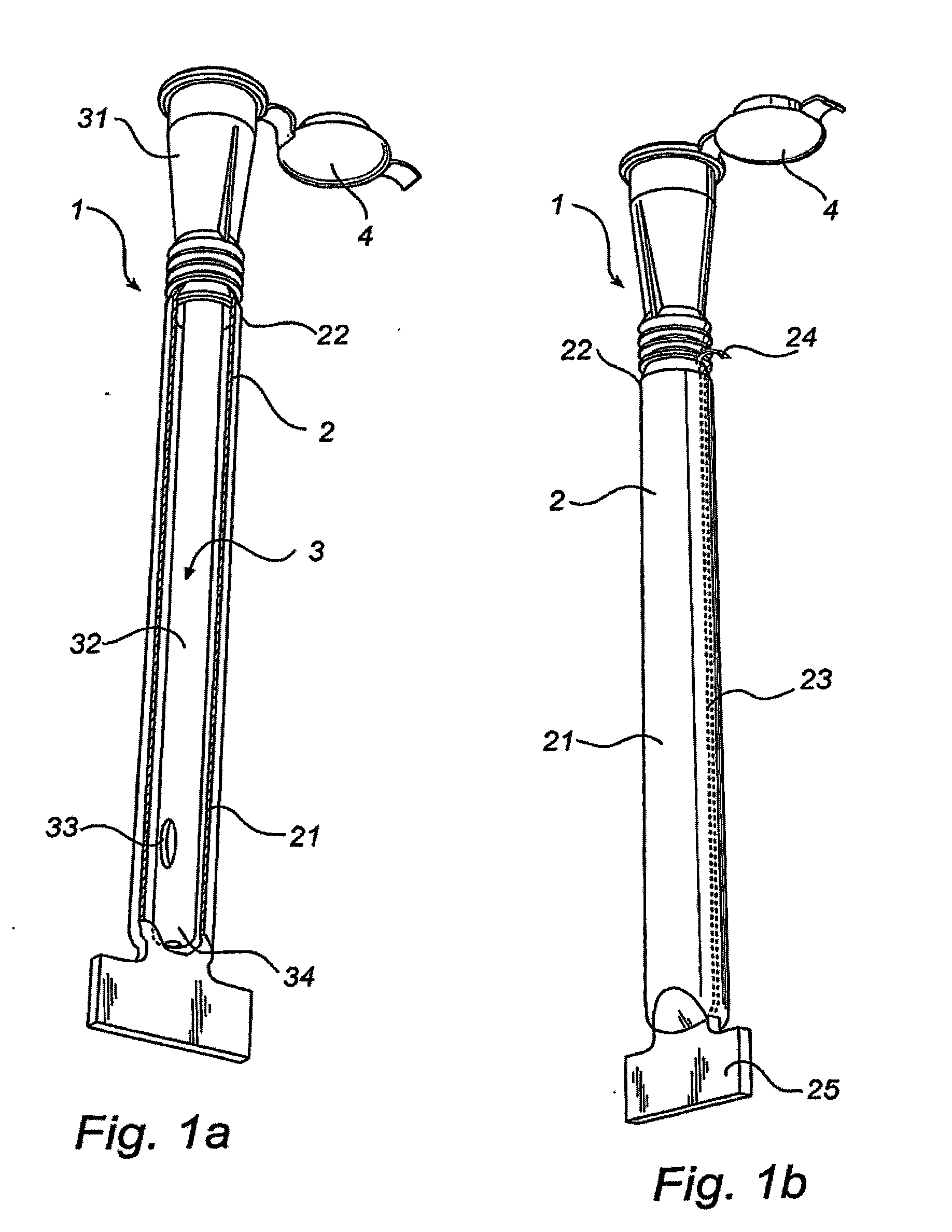
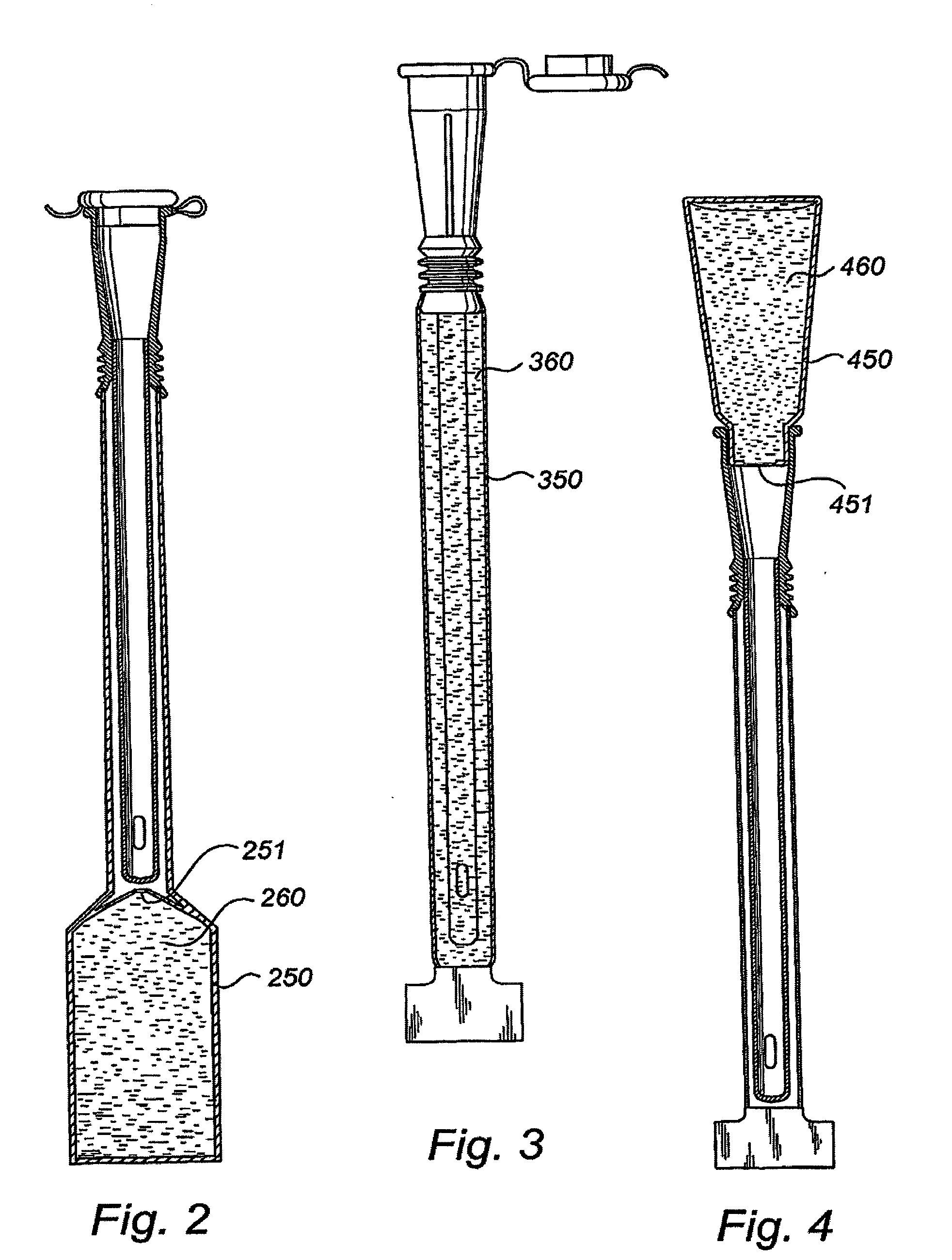
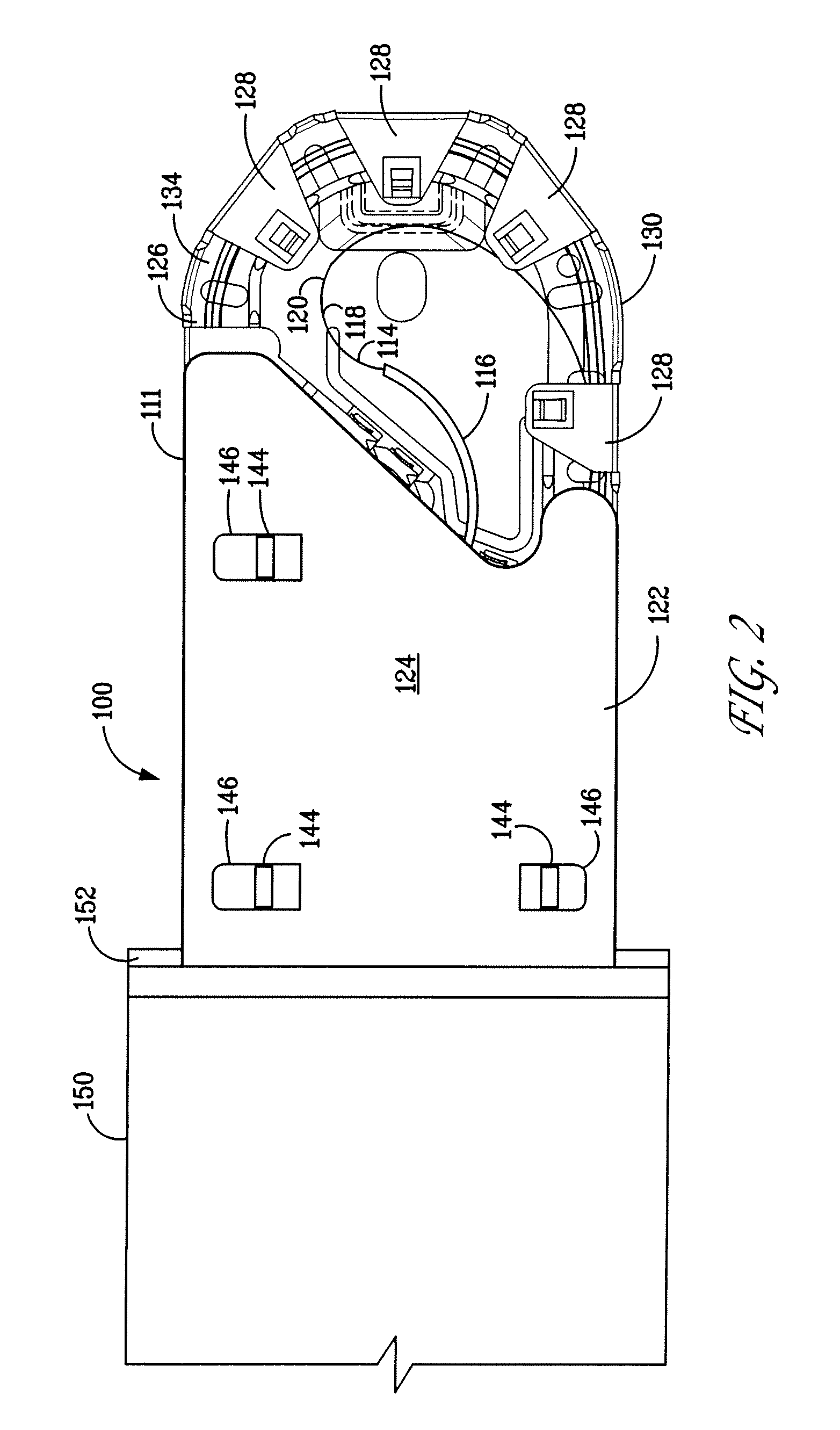
Packaging system
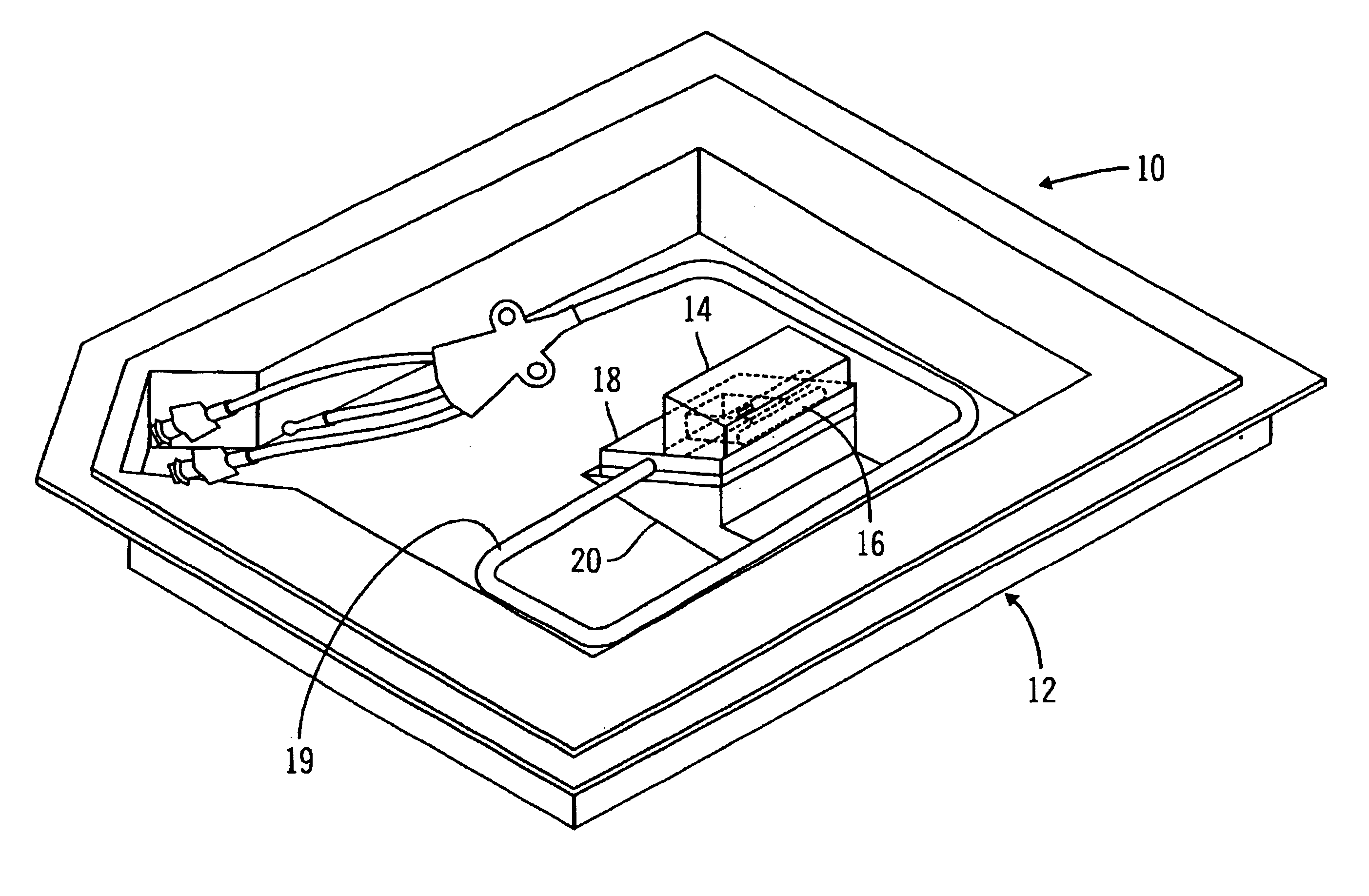
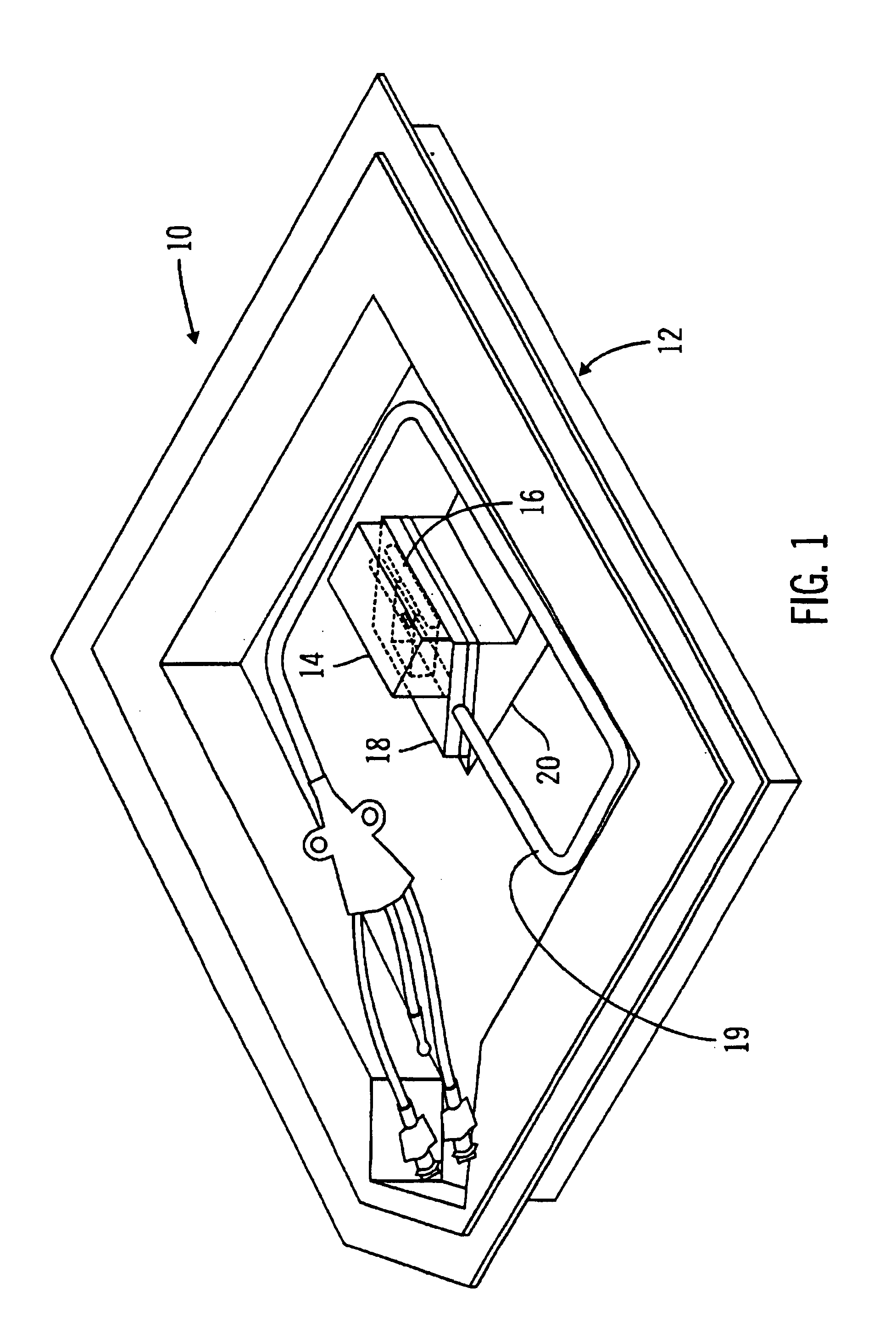
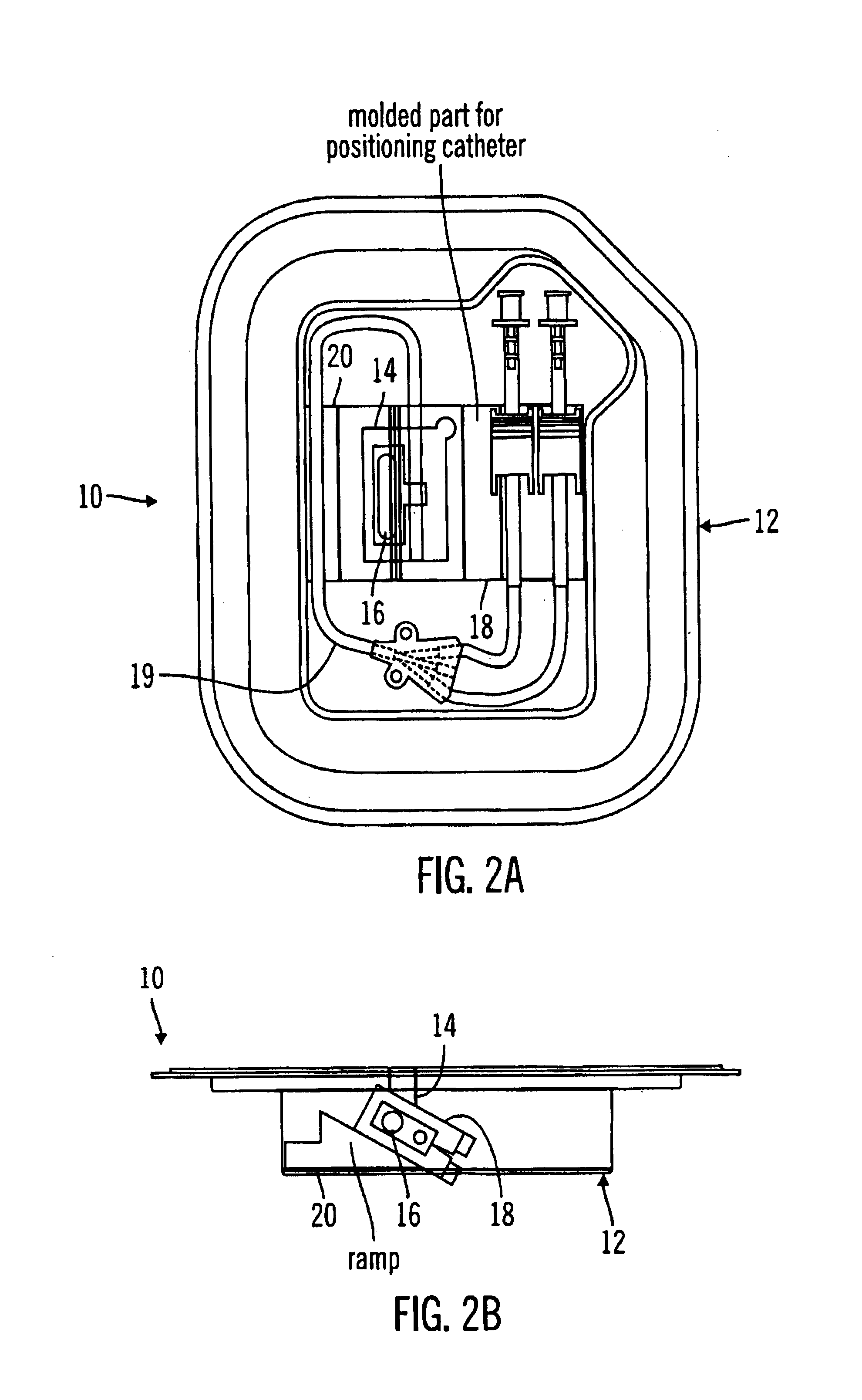
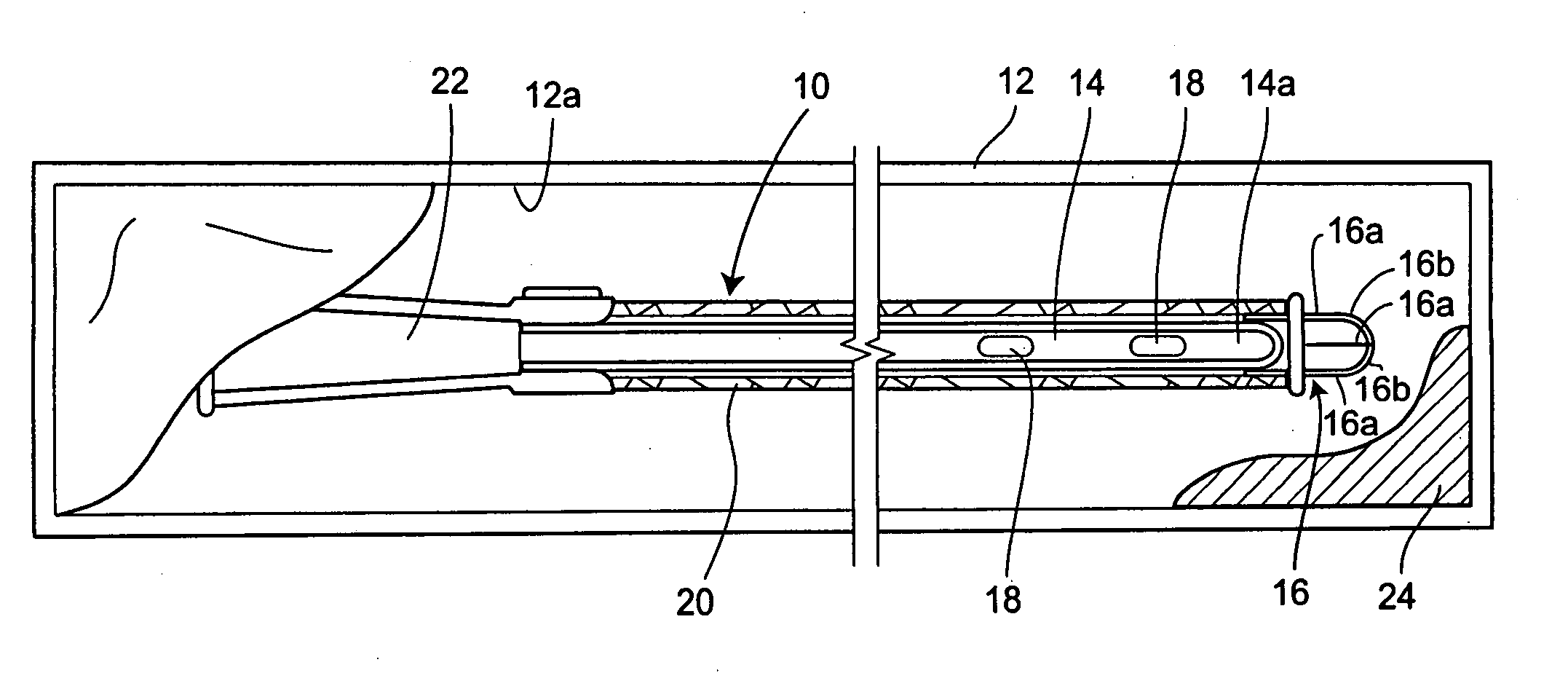
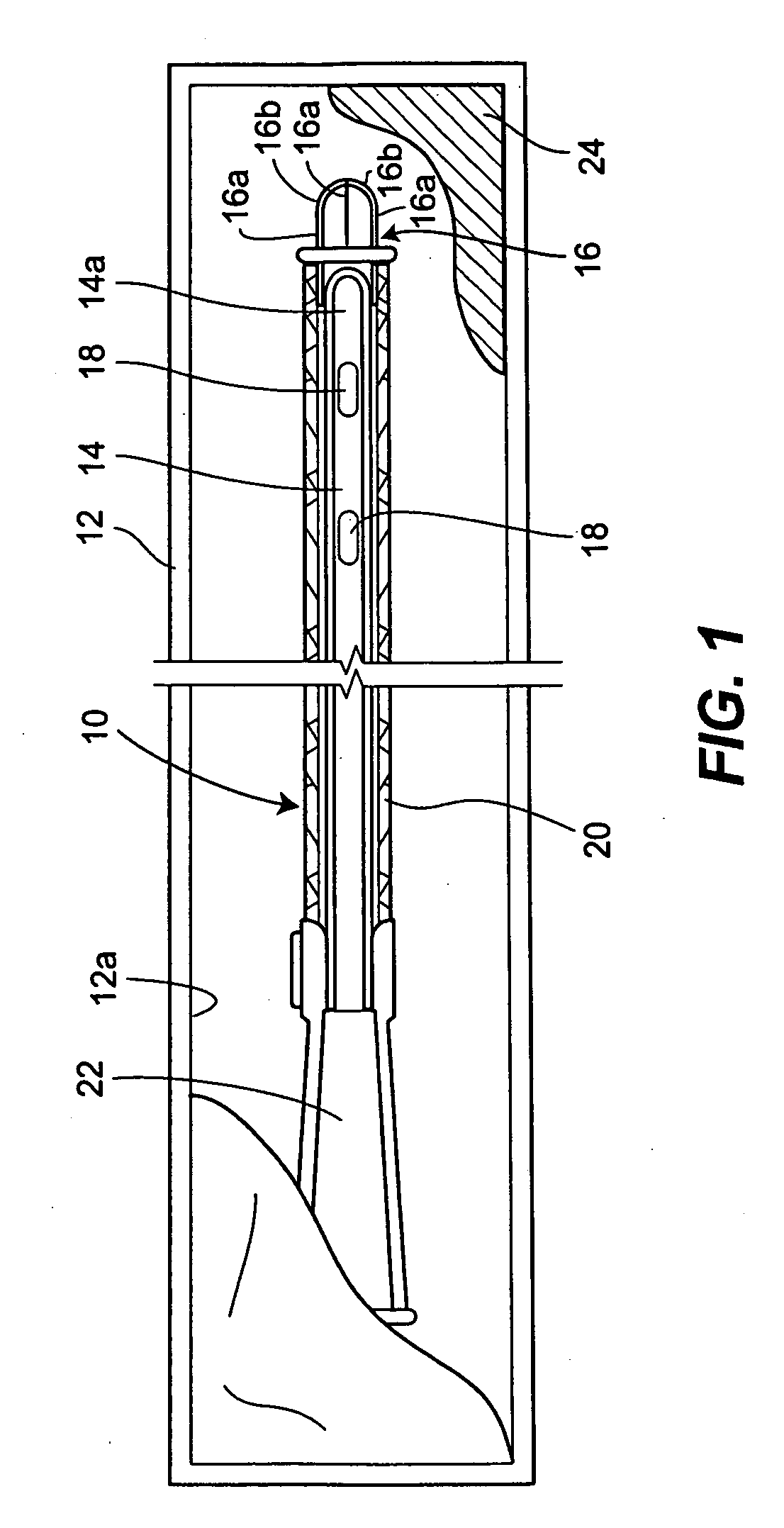
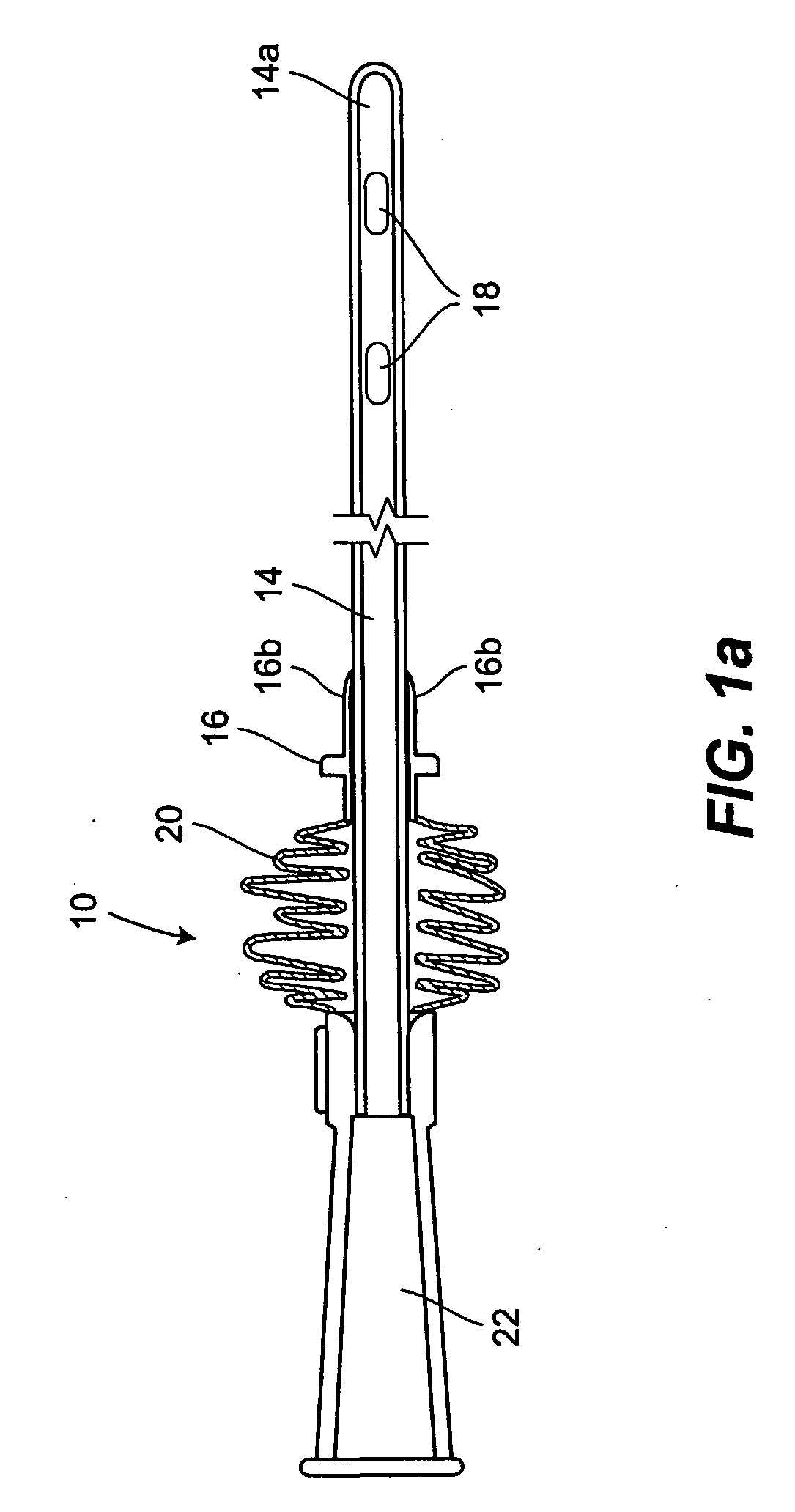
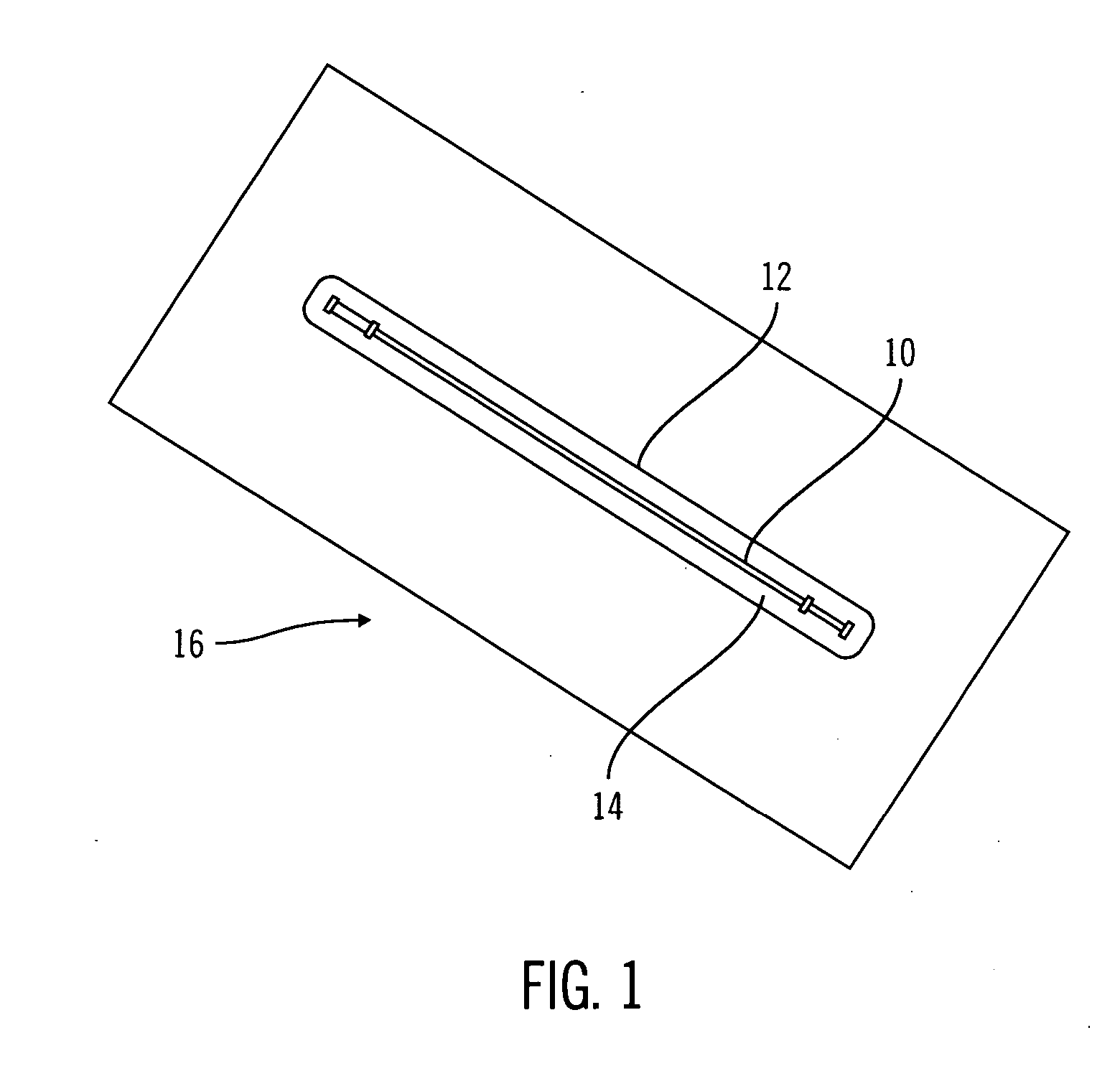
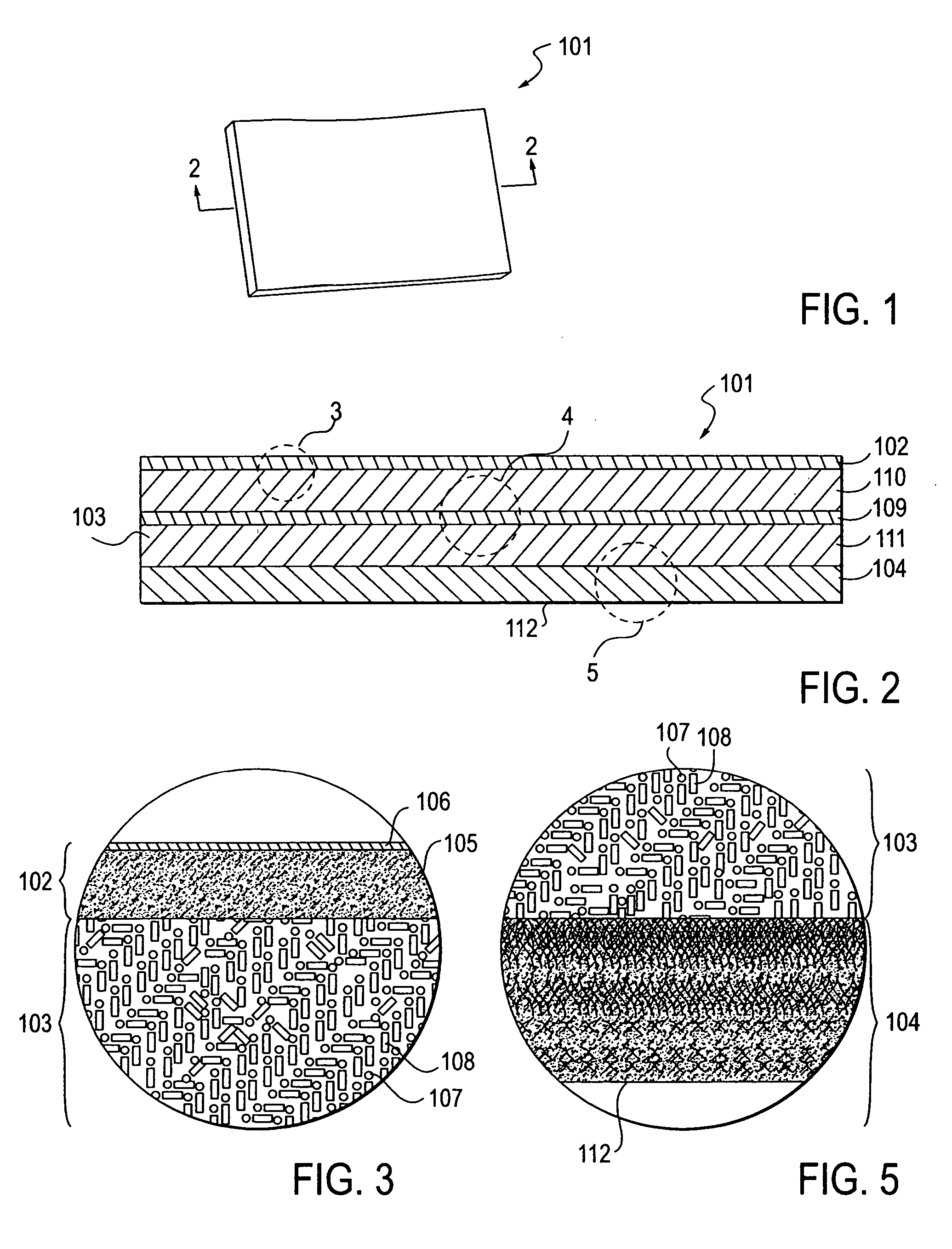
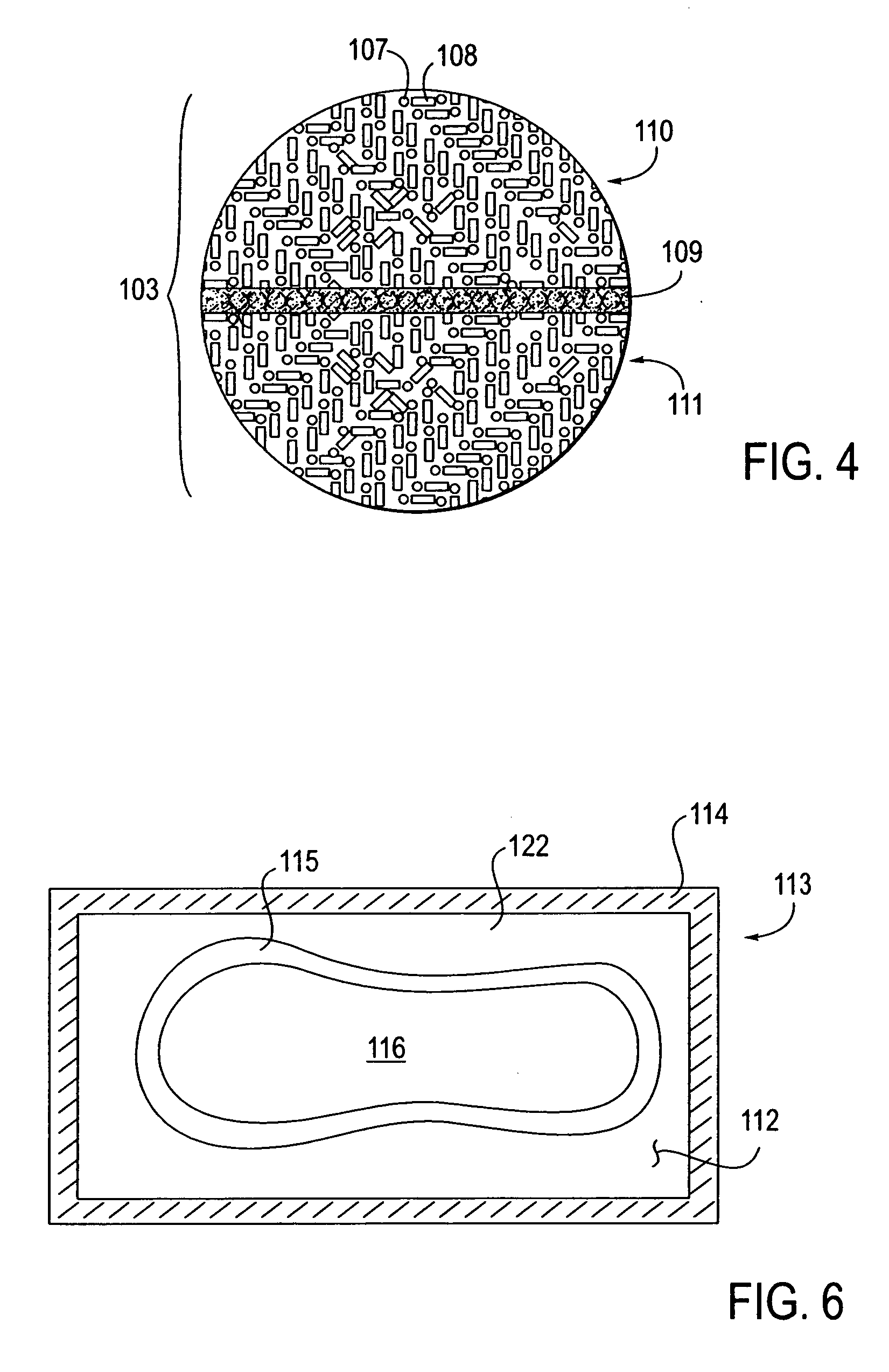
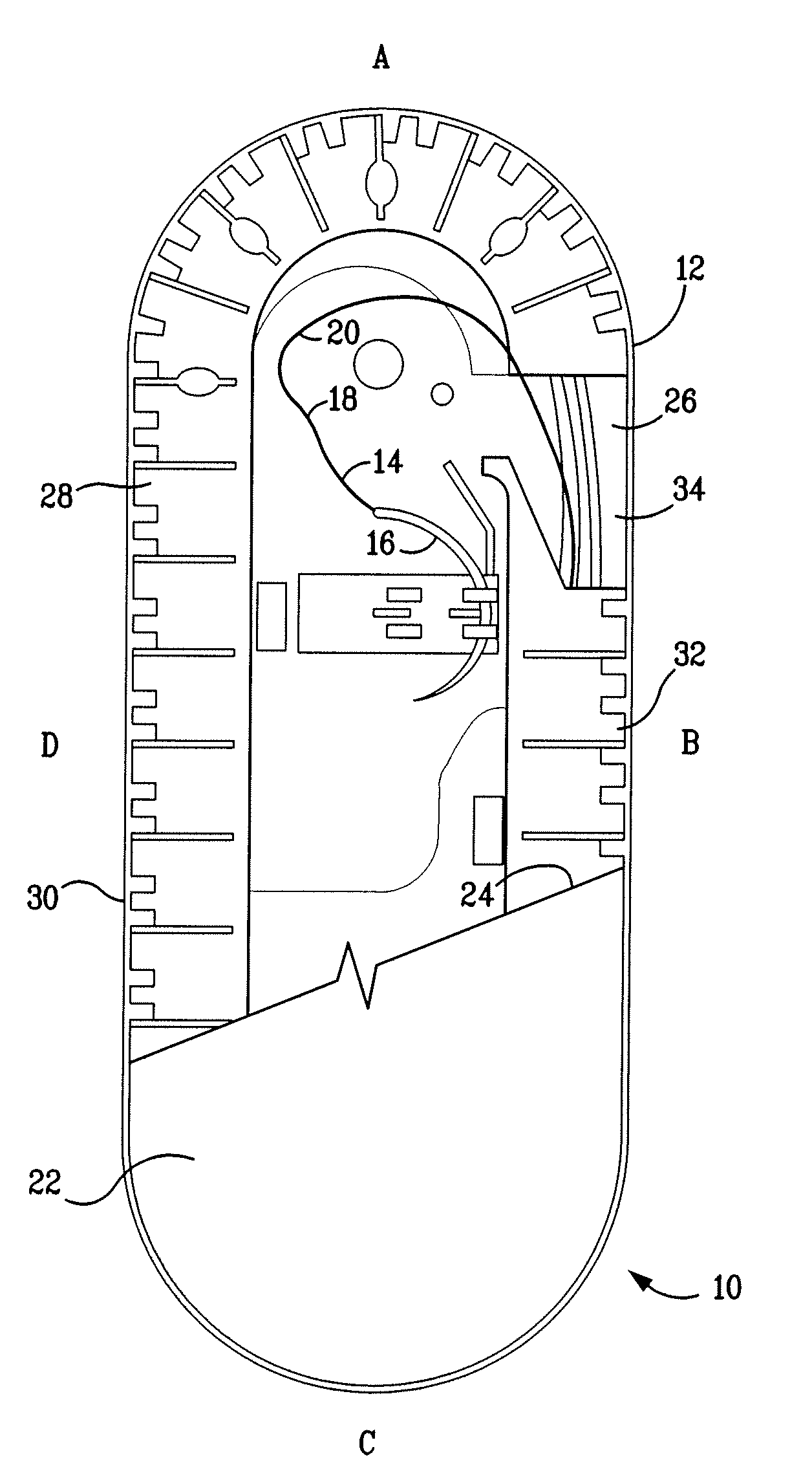
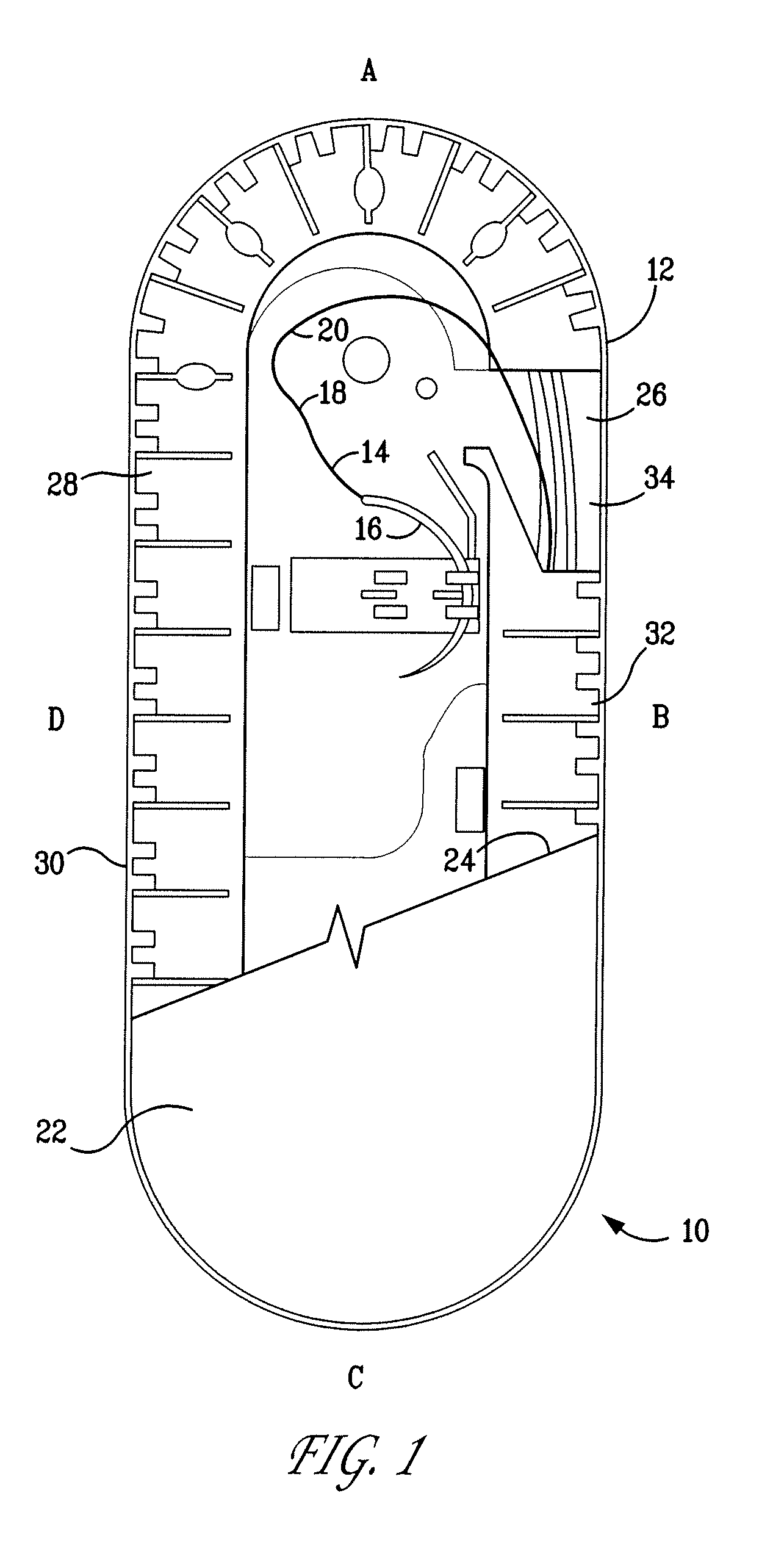
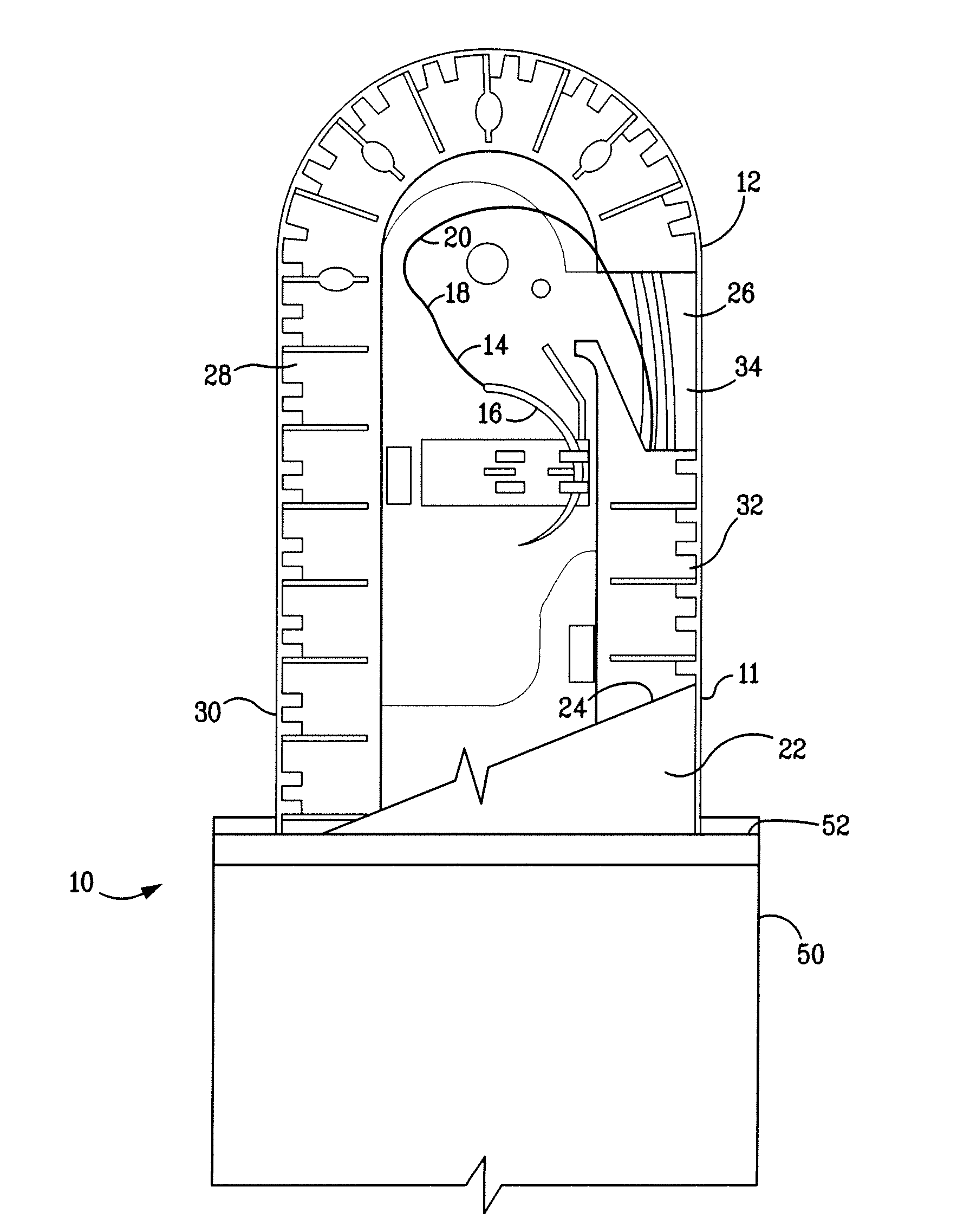
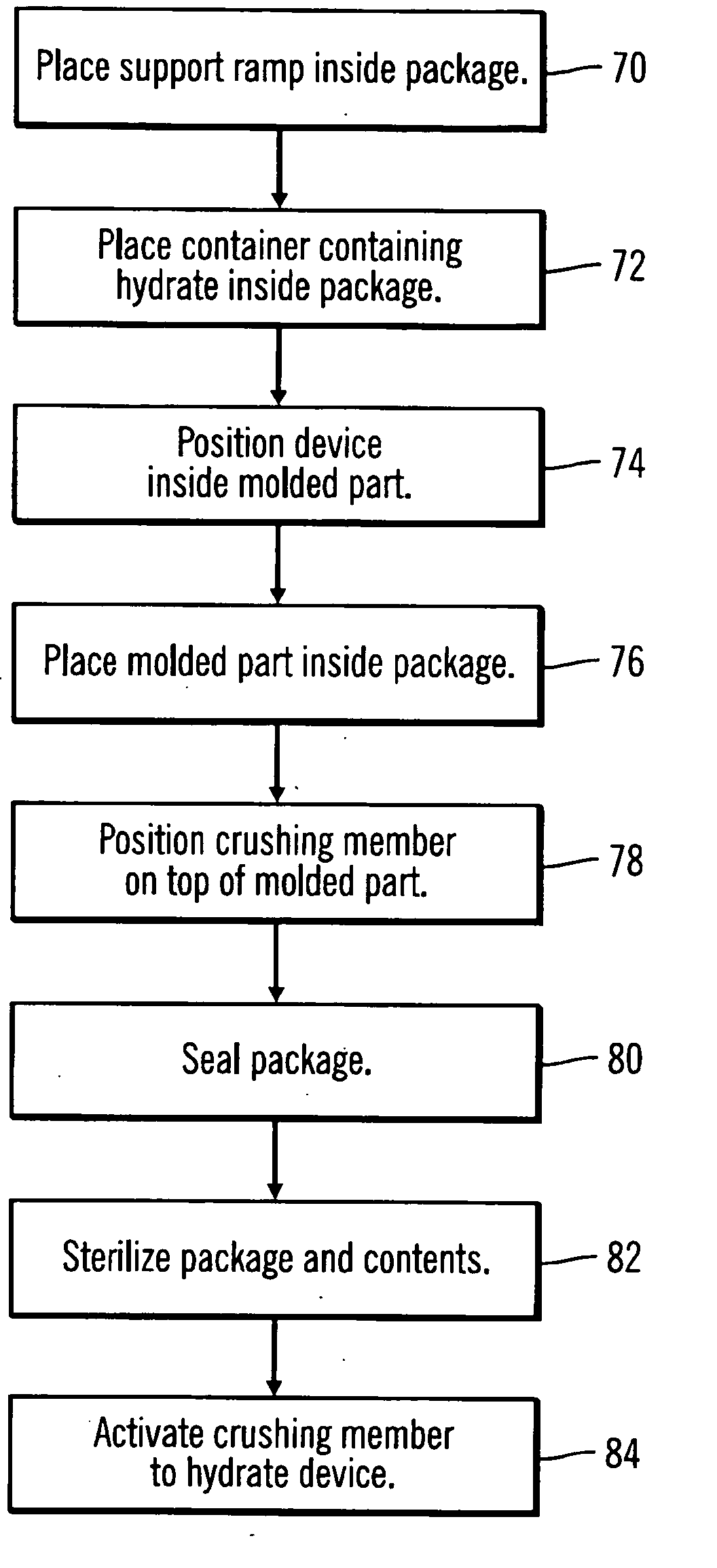
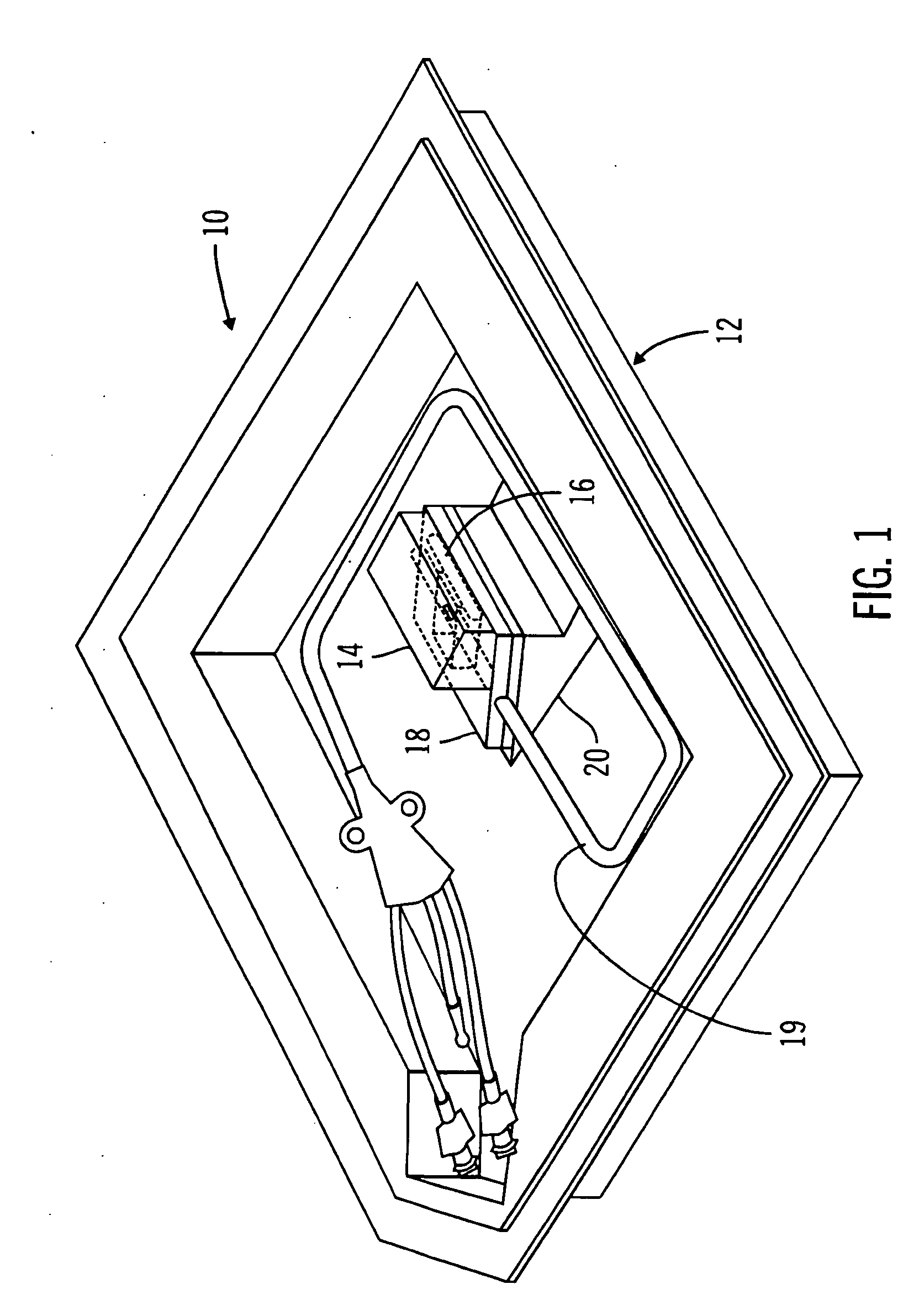
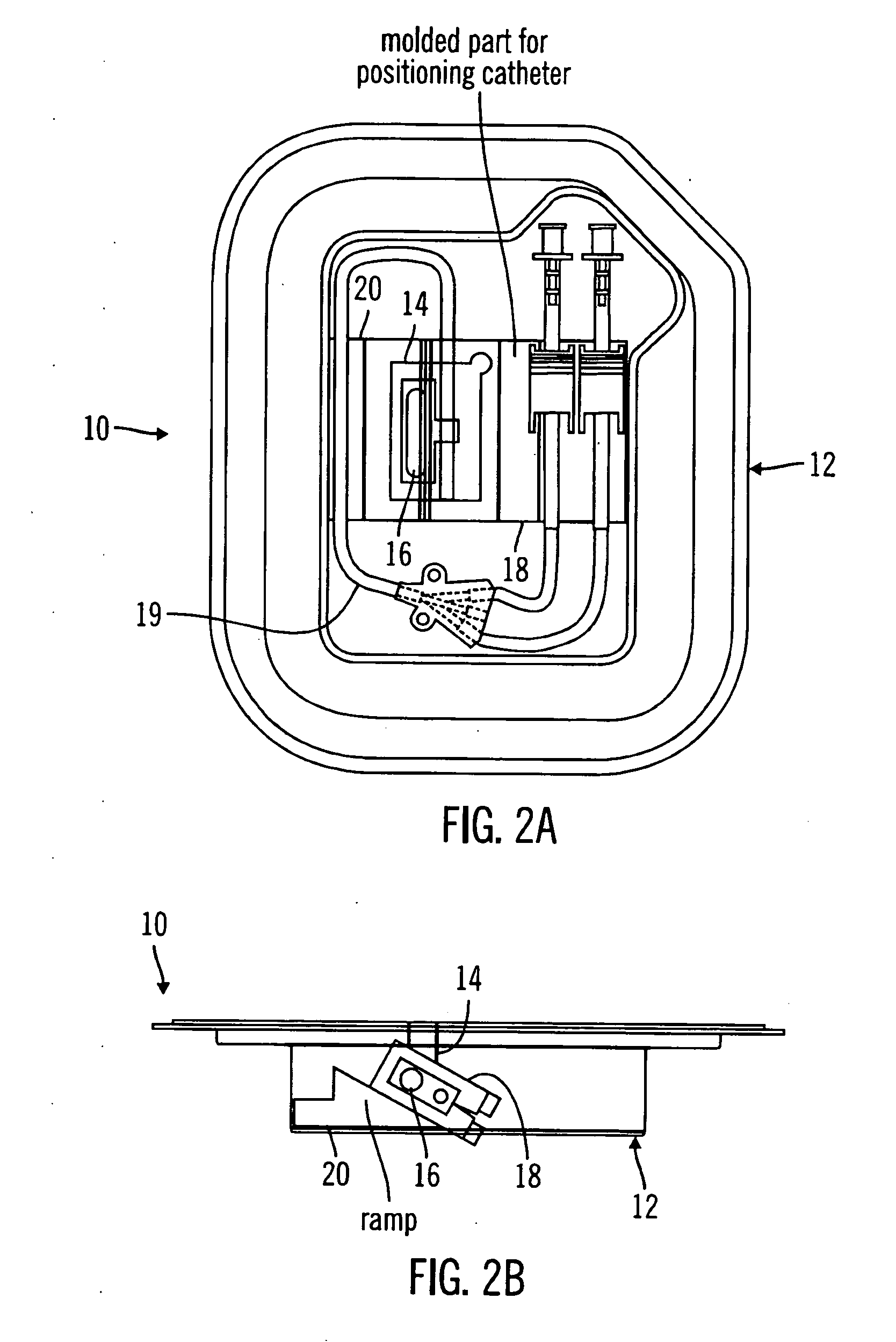
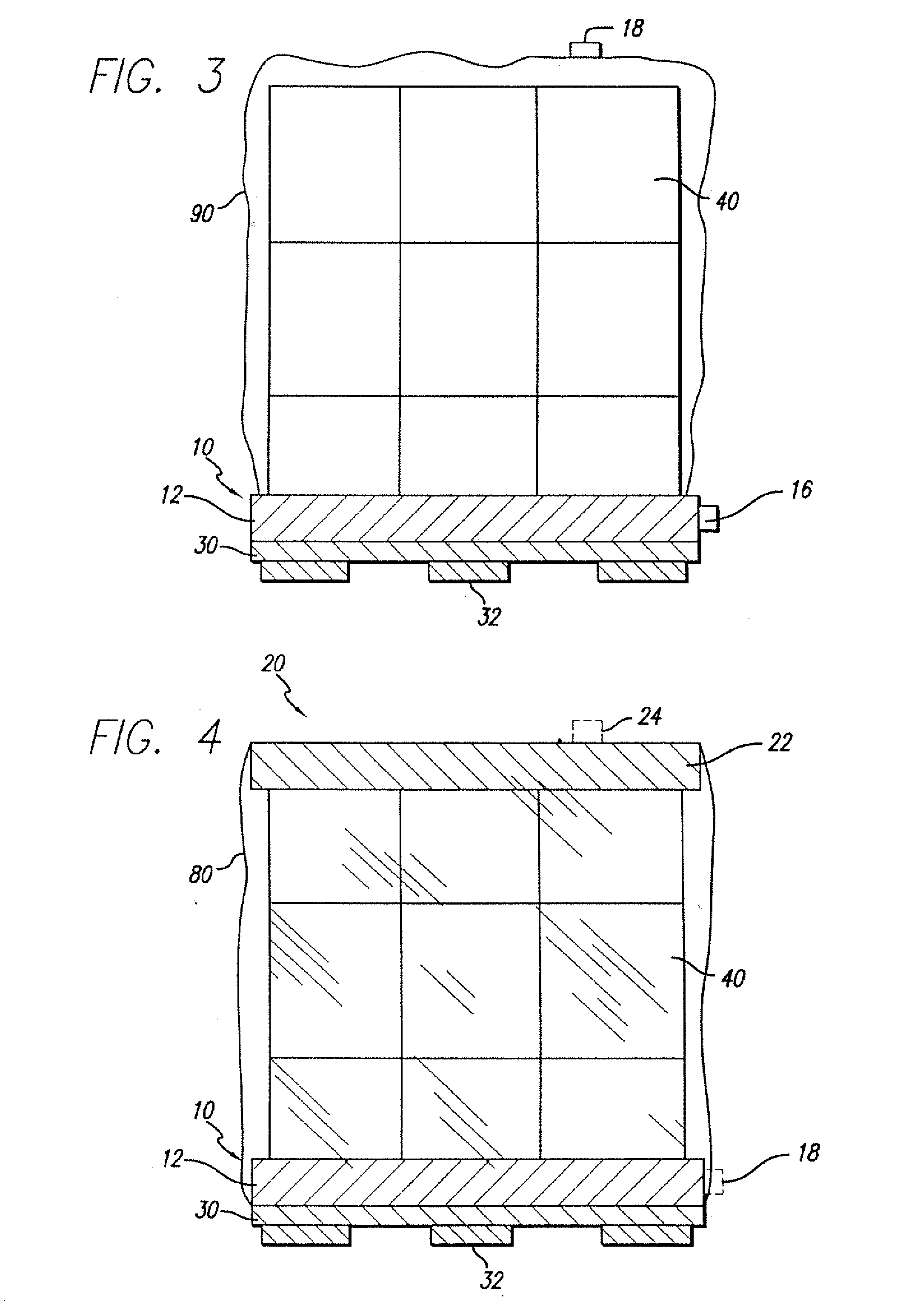
A packaging system for hydrating sterile devices without comprising the integrity of the sterilization. The packaging system may include an enclosure for enclosing a device requiring hydration, a container containing a hydrate, a base located within the interior of the enclosure and an activating member located within the interior of the enclosure. The container and the device may be located within a receptacle. The receptacle may rest on the base and the activating member may be affixed on top of the receptacle. A force may be exerted on an exterior portion of the enclosure such that the activating member pushes on the receptacle and crushes or ruptures the container. The hydrate located within the container is then released to the device, thereby hydrating the device without breaking the seal of the enclosure. The sterilized environment is therefore maintained and the device is hydrated.
Owner:MEDTRONIC MIMIMED INC
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20130118127A1Increased durabilitySpectales/gogglesPackage sterilisationHydrophilic coatingPolymer science
The invention is related to a cost-effective method for making a silicone hydrogel contact lens having a crosslinked hydrophilic coating thereon. A method of the invention involves autoclaving, in a sealed lens package, a silicone hydrogel contact lens having a base coating of polyacrylic acid thereon in an aqueous solution in the presence of a water-soluble, crosslinkable hydrophilic polymeric material having epoxide groups, for a period of time sufficient to covalently attach the crosslinkable hydrophilic polymeric material onto the surface of the silicone hydrogel contact lens through covalent linkages each formed between one epoxide group and one of the carboxyl groups on and / or near the surface of the silicone hydrogel contact lens.
Owner:ALCON INC
Vapor hydration of a hydrophilic catheter in a package
ActiveUS20060163097A1Little and no possibility of liquid spillageEasy to handleDispensing apparatusDiagnosticsHydrophilic coatingBiological activation
Owner:HOLLISTER INCORPORAED
Silicone hydrogel lens with a grafted hydrophilic coating
ActiveUS8409599B2Easy to implementMinimal orDental implantsPackage sterilisationHydrophilic coatingRoom temperature
The invention provides a cost-effective method for applying a hydrophilic coating onto a silicone hydrogel contact lens based on Fenton chemistry. The hydrophilic coating is covalently attached onto the contact lens at room temperature without UV irradiation. The invention also provides silicone hydrogel contact lenses having a hydrophilic coating obtained according to the method of the invention.
Owner:ALCON INC
Sterile device and method for producing same
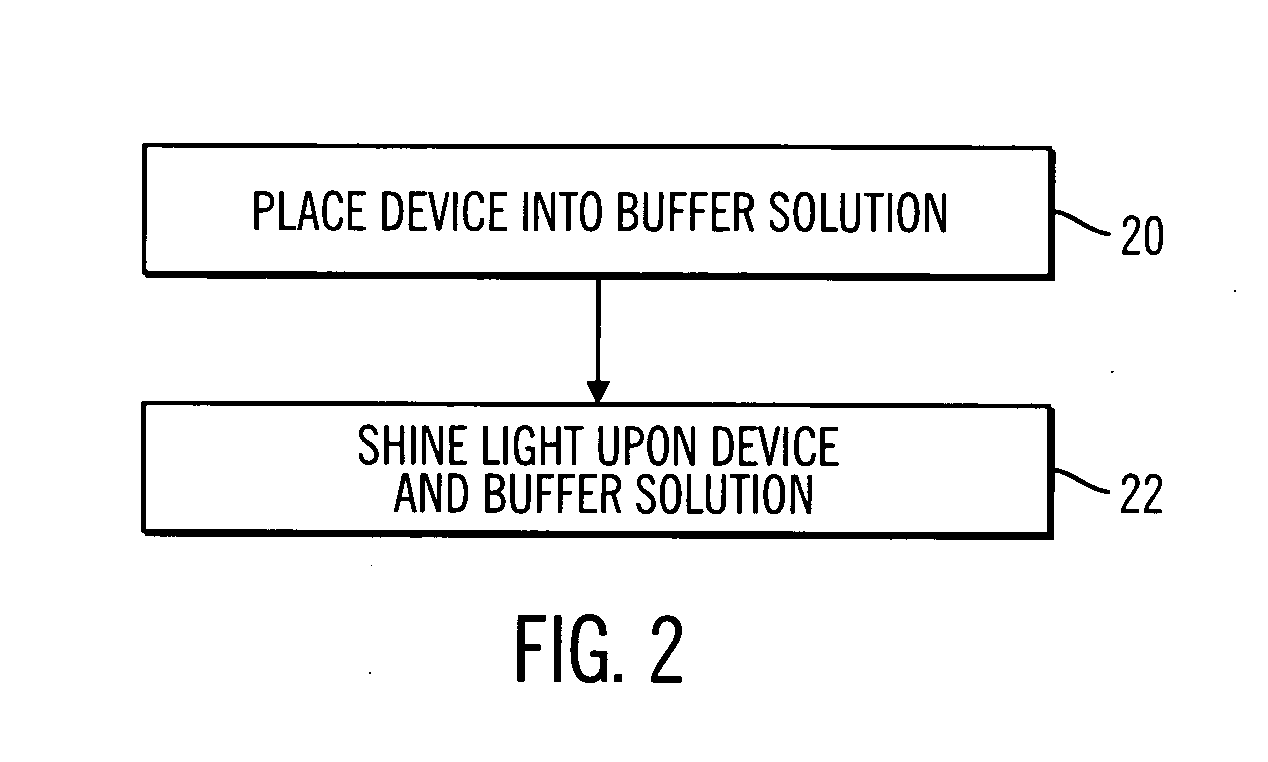
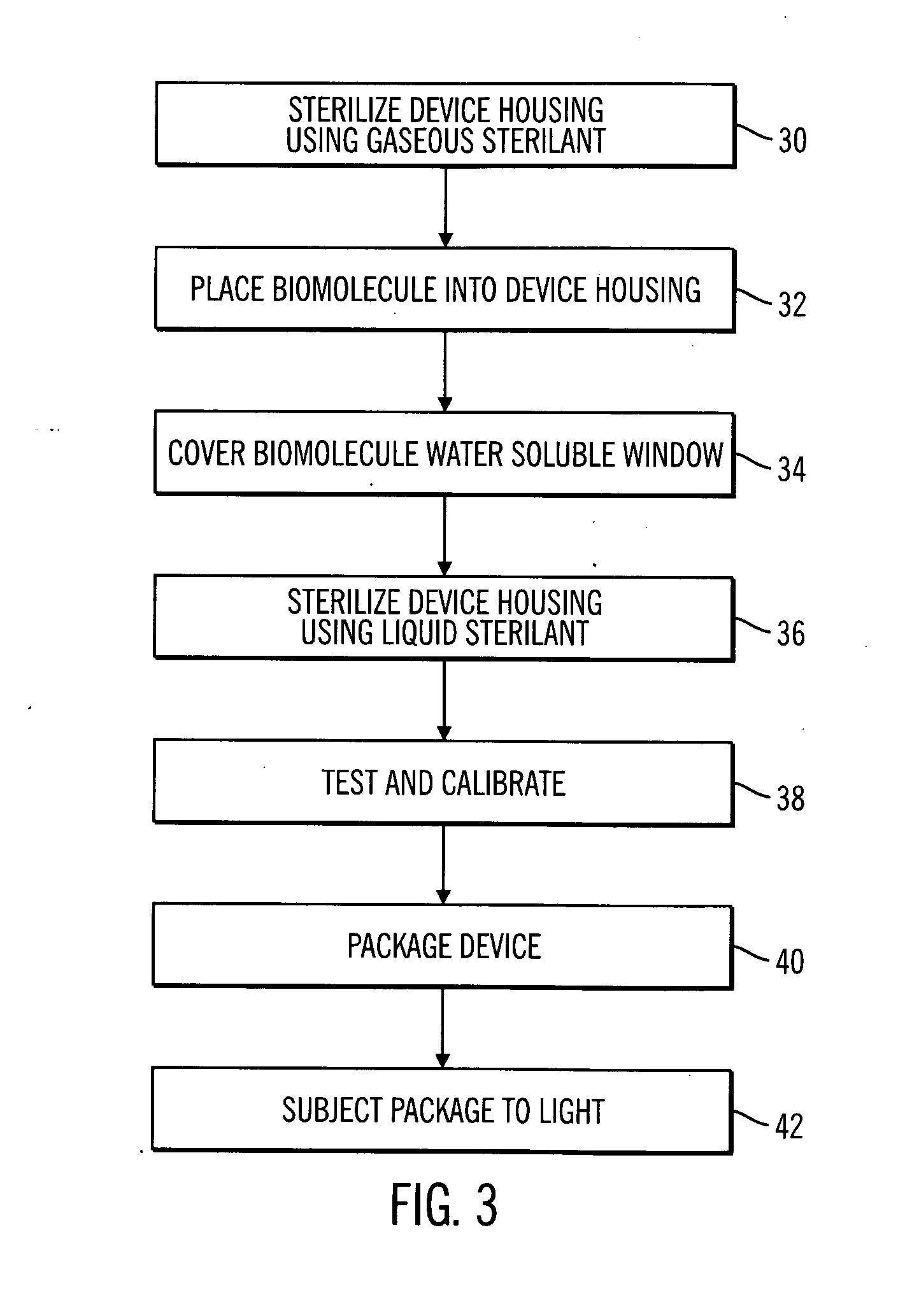
A sterile device immersed in a sterile buffer and a method for providing same. The sterile device may be a medical device such as a biosensor having a biomolecule as a sensing element such as, for example, a glucose oxidase enzyme. The buffer may be a bicarbonate solution. Both the device and the buffer may be packaged and stored over long term while maintaining sterilization. The sterilization method may comprise a combination of gaseous, liquid and light sterilization.
Owner:MEDTRONIC MIMIMED INC
Wound dressing and apparatus for forming same
A multi-layered wound dressing includes a moisture-retaining portion for enhancing the healing of a wound. The wound dressing includes an intermediate layer that has both water soluble and water insoluble fibers. An apparatus that includes a cutting tool and a reservoir of liquid to pre-moisten a portion of the dressing may be used to manufacture the dressings.
Owner:POLYREMEDY
Ethanol-free gel formulation cartridge for e-vaping device
A cartridge for an e-vaping device includes an ethanol-free gel formulation. The ethanol-free gel formulation includes a vapor former, water, and a biopolymer. The biopolymer may be included in an amount ranging from about 0.01% by weight based on the weight of the ethanol-free gel formulation to about 2.0% by weight based on the weight of the ethanol-free gel formulation. The biopolymer may be one or more of agar, kappa carrageenan, gelatin, sodium alginate, gellan gum, pectin, and combinations thereof. The cartridge also includes a heater configured to heat liquid from the gel formulation to a temperature sufficient to release a liquid / semi-liquid component from the gel, which component thereupon forms a vapor.
Owner:AKRIA CLIENT SERVICES LLC
Oral pouch products
A pouched product configured for insertion into the mouth of a user of that product is provided herein. The pouched product can include an outer water-permeable pouch defining a cavity containing a composition adapted for oral use and having a surface area, wherein the outer water-permeable pouch can include a nonwoven web including a first plurality of continuous filament heat sealable binder fibers oriented substantially parallel to each other in a first direction, a second plurality of continuous filament heat sealable binder fibers oriented substantially parallel to each other in a second direction, wherein the first direction and the second direction are substantially perpendicular to each other, and wherein the first plurality of continuous filament heat sealable binder fibers and the second plurality if continuous filament heat sealable binder fibers are bonded with a heated lamination process.
Owner:R J REYNOLDS TOBACCO COMPANY
Silicone hydrogel lens with a grafted hydrophilic coating
ActiveUS20110102736A1Easy to implementMinimal orPackage sterilisationSpecial surfacesHydrophilic coatingRoom temperature
The invention provides a cost-effective method for applying a hydrophilic coating onto a silicone hydrogel contact lens based on Fenton chemistry. The hydrophilic coating is covalently attached onto the contact lens at room temperature without UV irradiation. The invention also provides silicone hydrogel contact lenses having a hydrophilic coating obtained according to the method of the invention.
Owner:ALCON INC
Packaged antimicrobial medical device and method of preparing same
ActiveUS20100078336A1Prevent bacterial growthSuture equipmentsSurgical furniturePolymer resinEngineering
A method of making a packaged antimicrobial suture. The method includes the steps of providing a containment compartment molded from a polymeric resin comprising a polymeric material and an antimicrobial agent, positioning a suture within the containment compartment, the suture comprising one or more surfaces; covering the containment compartment having the suture in an outer package cover having an inner surface, and subjecting the outer package, the containment compartment and the suture to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the containment compartment to the suture, while retaining an effective amount of the antimicrobial agent on the containment compartment, thereby substantially inhibiting bacterial colonization on the suture and the containment compartment. A packaged antimicrobial suture is also provided.
Owner:ETHICON INC
Use of moisture impervious packaging units and package for absorbent articles comprising moisture-sensitive additives
InactiveUS6854600B1Good effectImprove impermeabilityContainers for flexible articlesFlexible coversEngineeringTampon
Active additives in absorbent articles, such as sanitary napkins, panty liners, tampons, incontinence protectors and diapers have been found to lose their properties due to taking-up moisture during storage and transportation for instance, when conventional packaging materials are used. The invention relates to the use of a moisture impervious film material for packaging an absorbent article comprising one or more active moisture-sensitive additives. Packaging is effected in film material that has low vapor and gas permeability and in packaging unites that have tight joins or seams.
Owner:ESSITY HYGIENE & HEALTH AB
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20150166205A1Reduce concentrationLow costPackage sterilisationOptical articlesHydrophilic coatingTime efficient
The present invention generally relates to a cost-effective and time-efficient method for applying a crosslinked hydrophilic coating onto a silicone hydrogel contact lens to reduce its positively charged preservatives such as PHMB uptake and to improve its hydrophilicity and lubricity.
Owner:ALCON INC
Packaged antimicrobial medical device having improved shelf life and method of preparing same
ActiveUS20100163435A1Extended shelf lifeInhibition of colonizationSuture equipmentsSurgical furnitureMedicineBacterial colonization
A method of making a packaged antimicrobial suture having improved shelf life. The method comprising the steps of providing an inner package having a source of antimicrobial agent, providing an adsorbent material effective to adsorb a portion of the antimicrobial agent over time, positioning a suture within the inner package, the suture comprising one or more surfaces, covering the inner package with an outer package having an inner surface and subjecting the suture, the inner package and the inner surface of the outer package to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the suture and the inner package, thereby substantially inhibiting bacterial colonization on the suture and the inner package, wherein the packaged antimicrobial suture exhibits improved shelf life. A packaged antimicrobial suture and a method of increasing the shelf life of a packaged antimicrobial medical device are also provided.
Owner:ETHICON INC
Packaging solutions
InactiveUS20080148689A1Avoid pollutionUniform coatingPackage sterilisationOther accessoriesPolyolAnionic polymers
The present invention is directed to new and improved packaging systems for storing ophthalmic devices such as contact lenses and to methods for packaging such ophthalmic devices with aqueous packaging solutions to improve the comfort of the lens during wear. In particular, the present invention is directed to a packaging system for storing an ophthalmic device in an aqueous packaging solution comprising an anionic polymer and a non-ionic polyol. Such solutions can be retained on the surface of an unused lens for extended periods of time, resulting in surface modification that persists in the eye, which may provide significant improvement in the wetting properties of fresh contact lenses used for the first time and, moreover, even several hours after lens insertion, preventing dryness and improving lubricity.
Owner:BAUSCH & LOMB INC
Contact Lens Packaging
ActiveUS20150129437A1Reduce the possibilityDead volumeDispensing apparatusPackage sterilisationEngineeringContact lens
Owner:CONTACT LENS PRECISION LAB
Packaging Solutions
InactiveUS20100162663A1Preserve sterilityImprove the lubrication effectPackage sterilisationLavatory sanitoryEngineeringContact lens
Owner:BARCLAYS BANK PLC AS SUCCESSOR AGENT
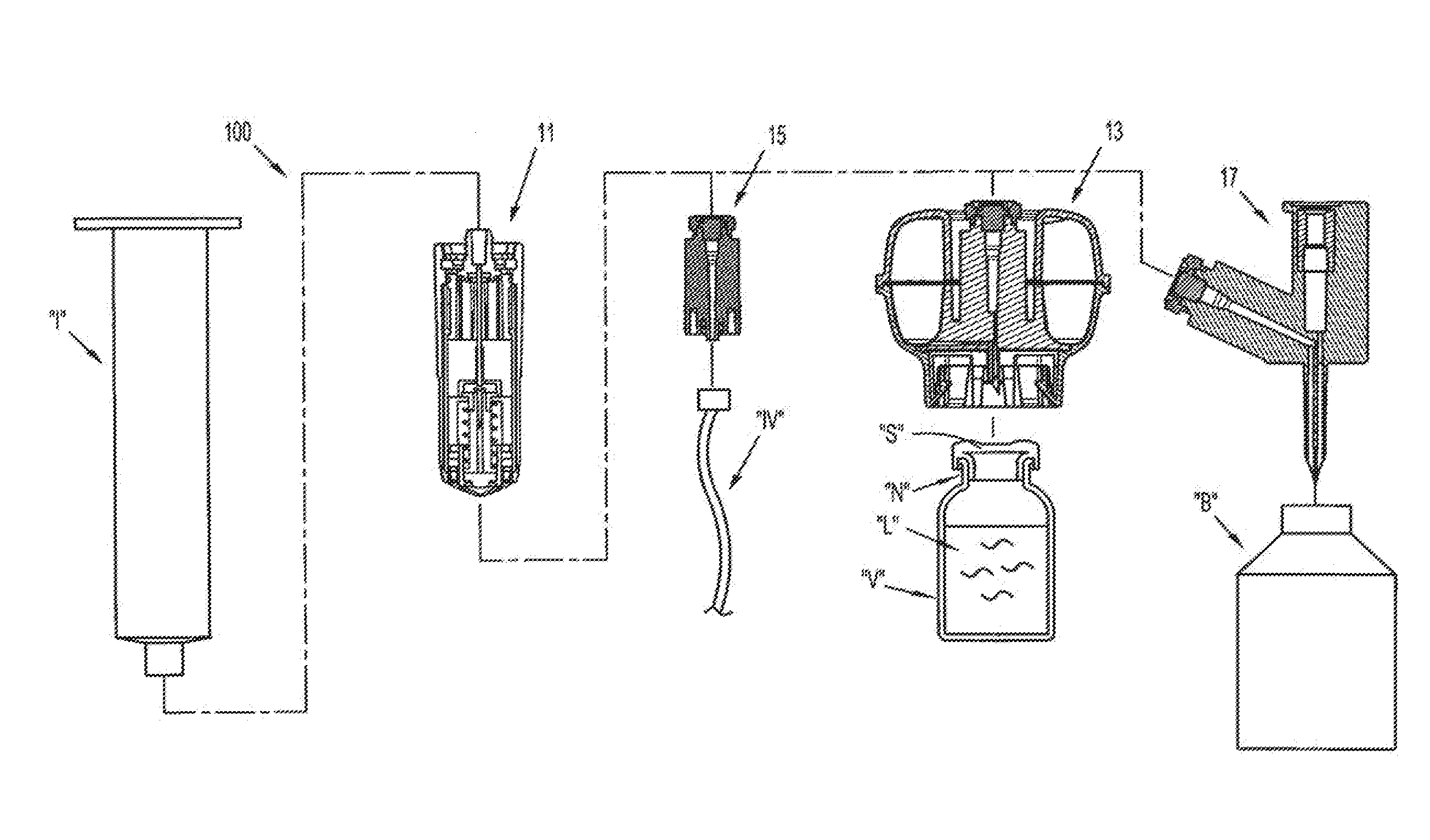
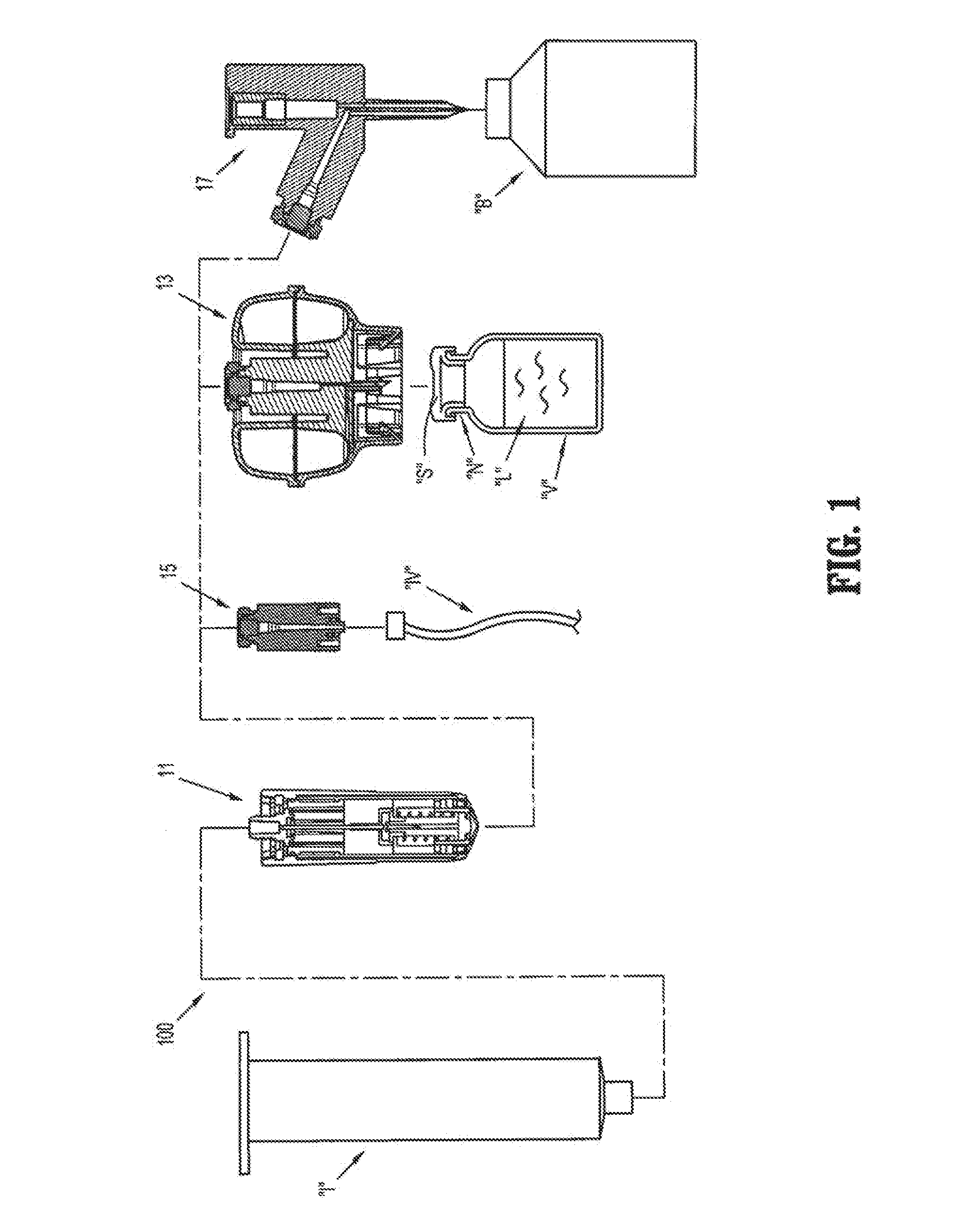
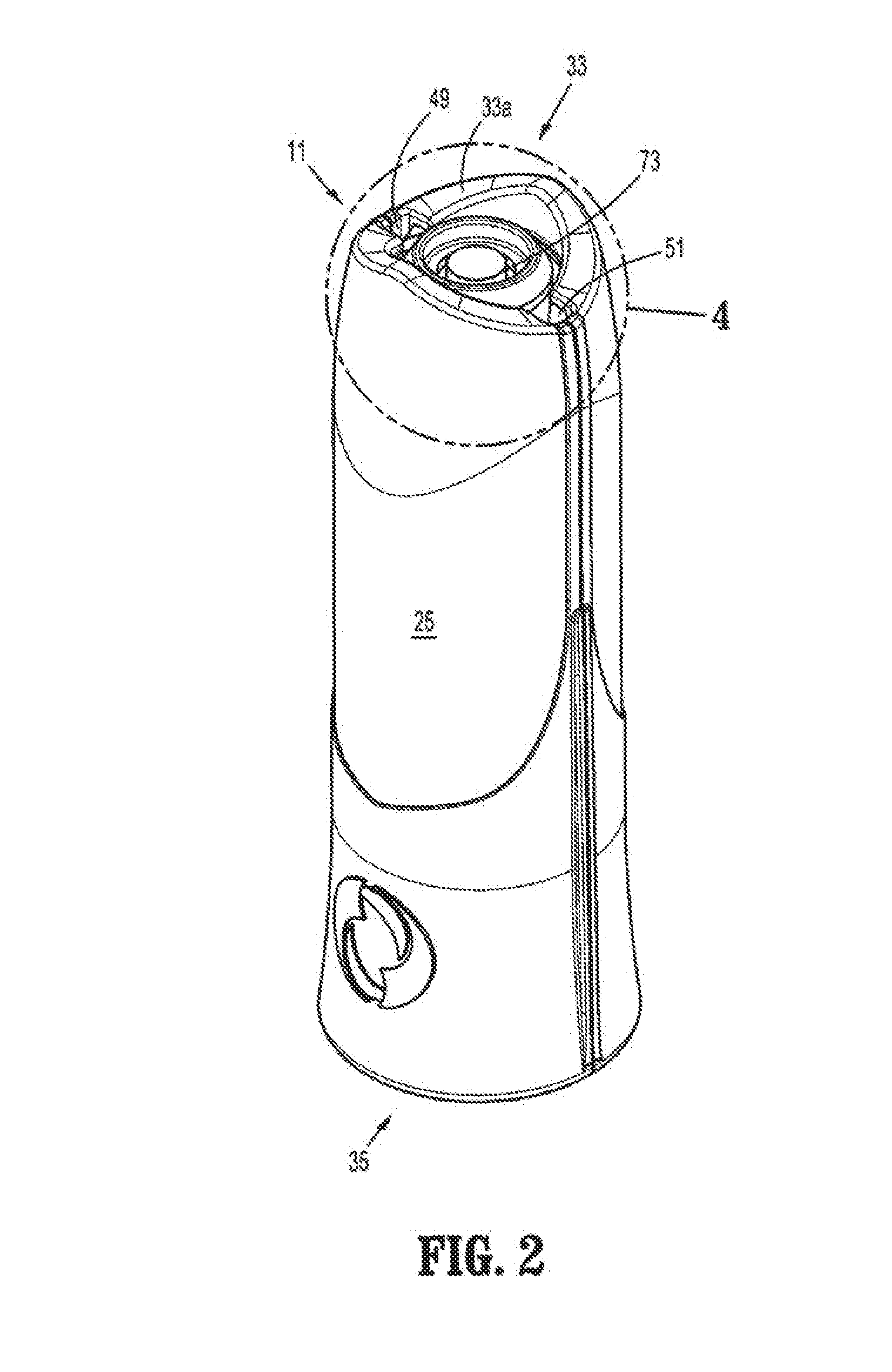
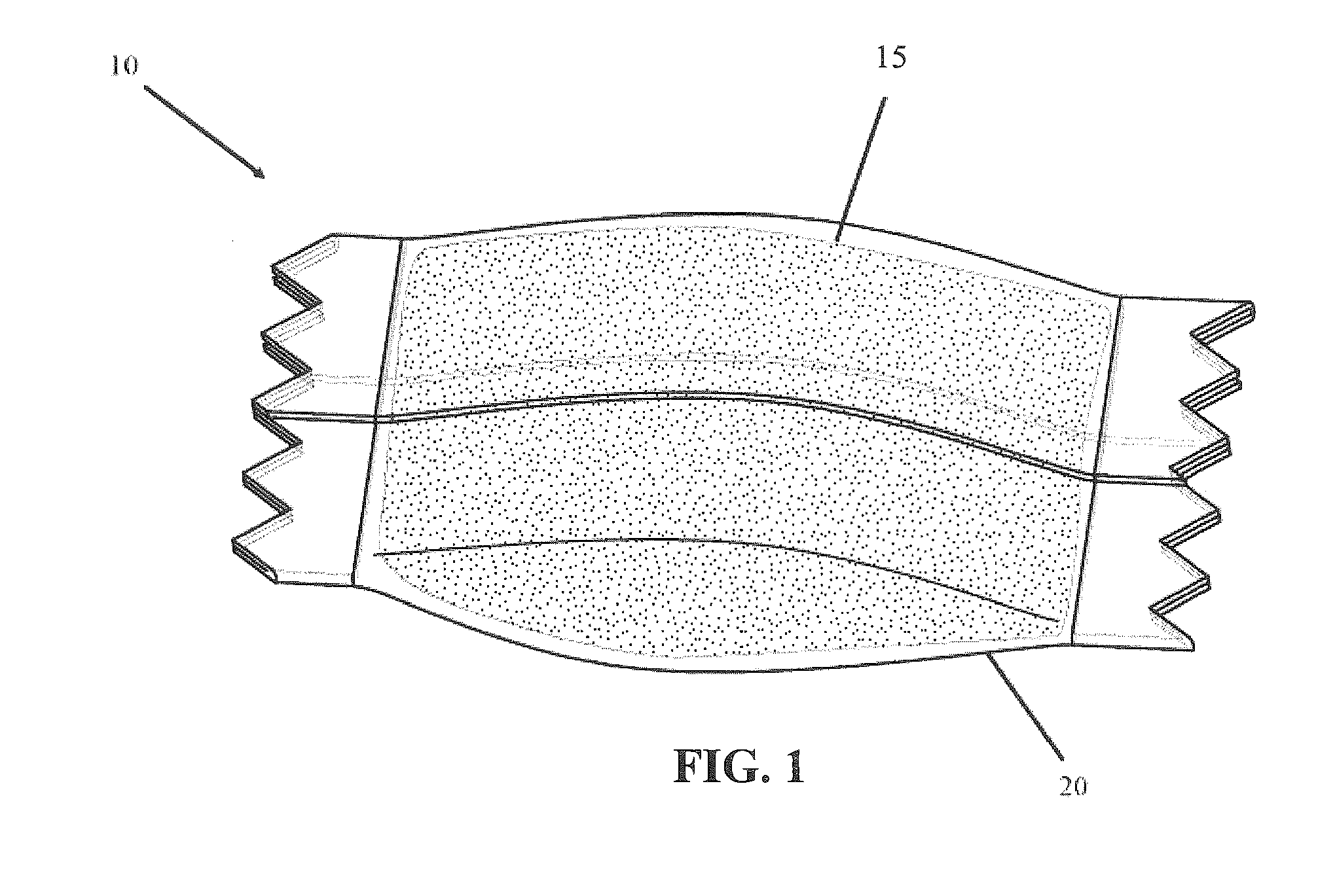
Packaging system
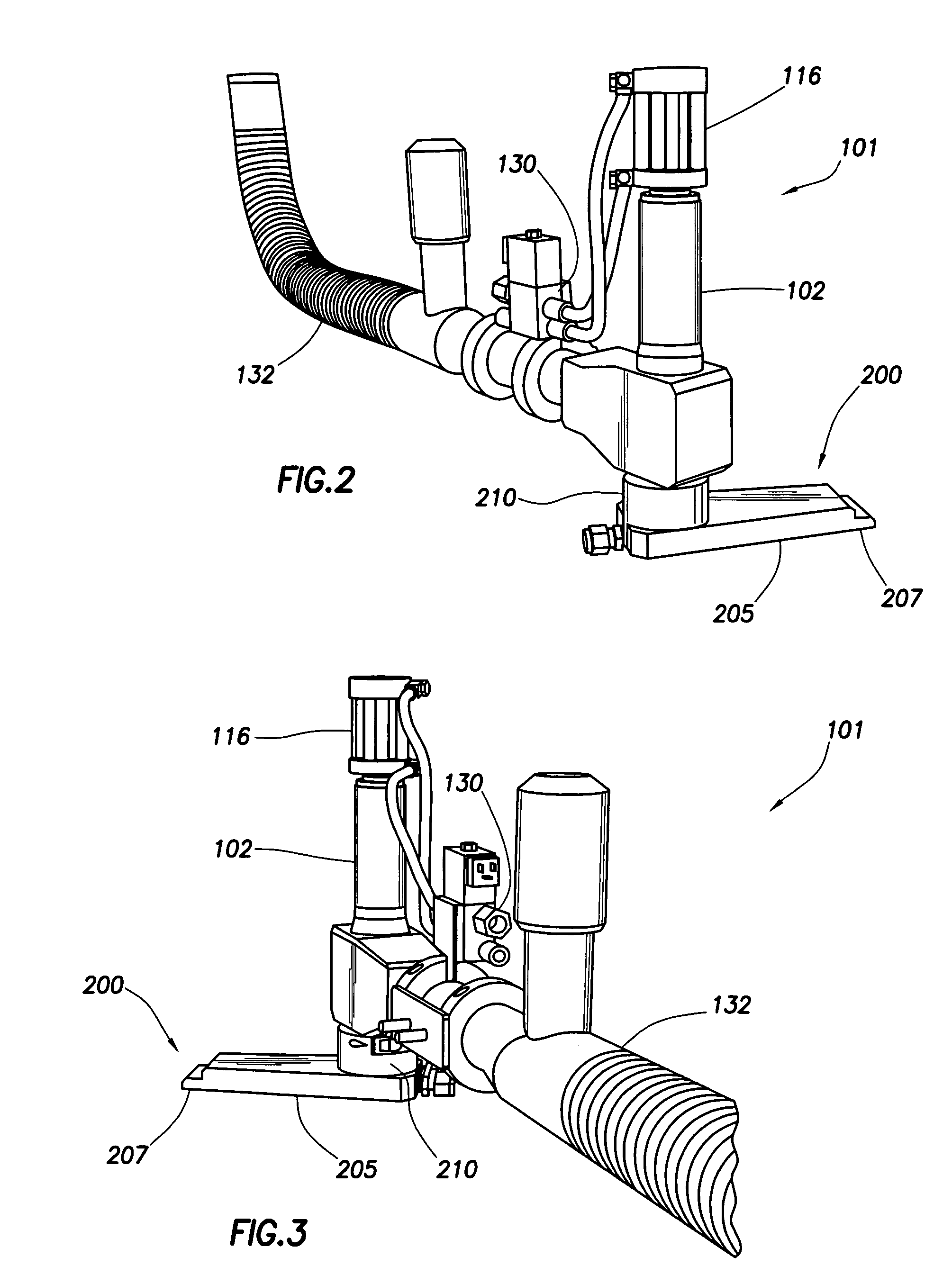
InactiveUS20050223679A1Little or no shelf lifeSurgical furnitureDiagnosticsEngineeringMechanical engineering
A packaging system for hydrating sterile devices without comprising the integrity of the sterilization. The packaging system may include an enclosure for enclosing a device requiring hydration, a container containing a hydrate, a base located within the interior of the enclosure and an activating member located within the interior of the enclosure. The container and the device may be located within a receptacle. The receptacle may rest on the base and the activating member may be affixed on top of the receptacle. A force may be exerted on an exterior portion of the enclosure such that the activating member pushes on the receptacle and crushes or ruptures the container. The hydrate located within the container is then released to the device, thereby hydrating the device without breaking the seal of the enclosure. The sterilized environment is therefore maintained and the device is hydrated.
Owner:MEDTRONIC MIMIMED INC
Liquid delivery system with horizontally displaced dispensing point
A method and apparatus is provided for the efficient and controllable delivery of cryogen liquid droplets into thin walled containers before they are sealed, the pressurization of the sealed container caused by the evaporation of the liquid cryogen causing the walls of the container to stiffen. Discharge of the droplets immediately upstream of the container sealing station is facilitated using a horizontal displacement assembly to transport metered droplets from a liquid dosing unit to the point of injection above the container. The horizontal displacement assembly may be provided with internal heaters to prevent freeze up, and a sensor to confirm droplet discharge. It may also be provided with a separate source of heated nitrogen gas, which can be used to back purge the dispensing unit should it become clogged, to melt any frozen liquid occlusions which may have formed in the cryogen supply line. In one embodiment, the solenoid used to actuate the piston regulating the opening and closing of the needle valve, which meters the dispensing of droplets, is mounted in thermal contact with said piston, this placement of the solenoid serving to cool the piston and thus prevent overheating in the case of rapid cycling.
Owner:CHART INC
System and method for reprocessing animal bedding
ActiveUS20100212262A1Eliminate needBeneficially usedPackage sterilisationDough shapingFecesEngineering
A process and system reprocesses soiled animal bedding material commingled with animal manure. The material is to remove a preponderance of the manure. The remaining soiled bedding is washed in water and a cleaning agent, rinsed and, optionally, bleached to restore color and appearance. The resulting material is dewatered and dried to reduce its moisture content and kill any remaining pathogens. After being cleaned and sanitized, the material may be reused as bedding or further processed into pellets or other products, such as manufactured fire logs.
Owner:EQUINE ECO GREEN LLC
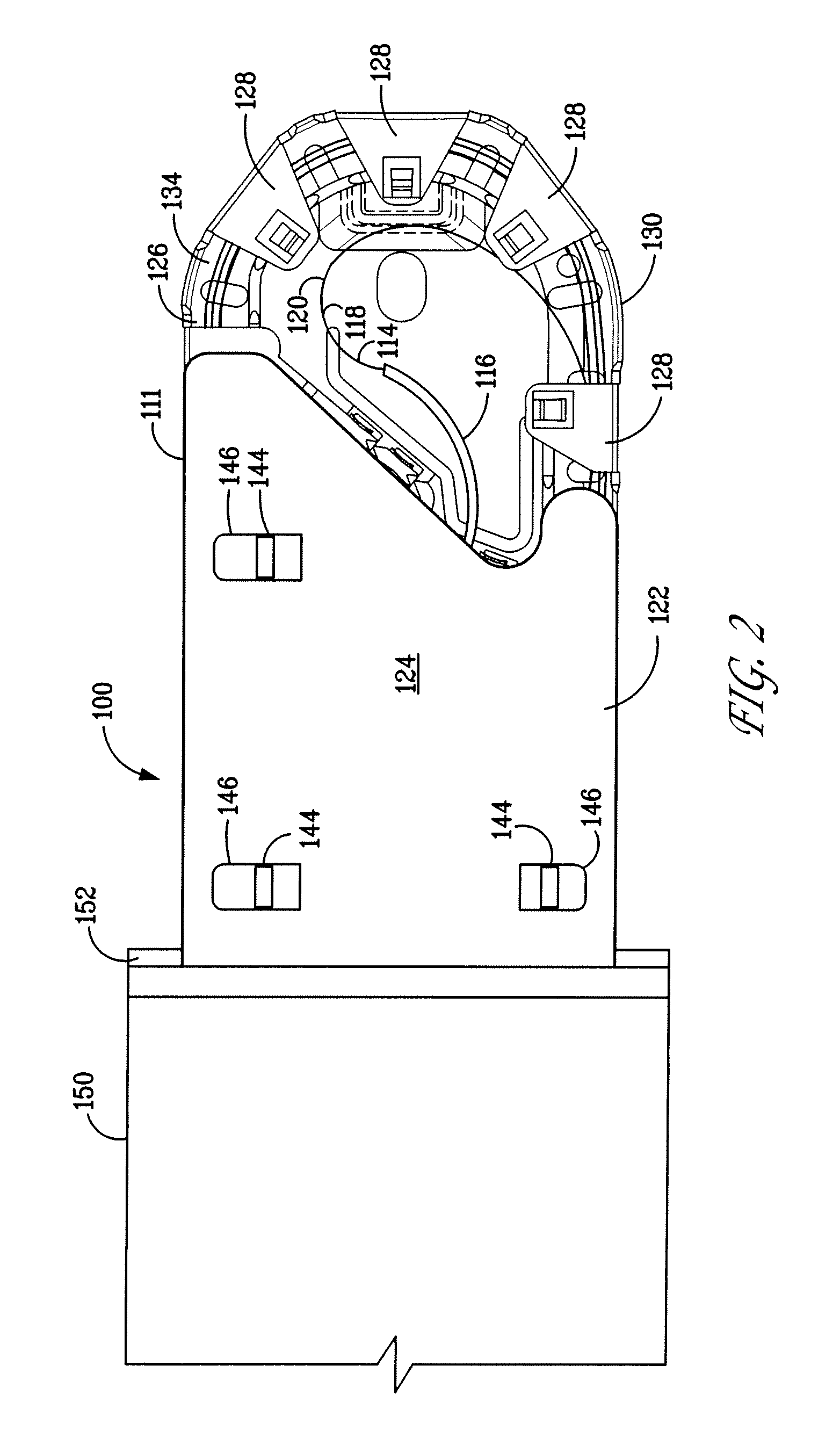
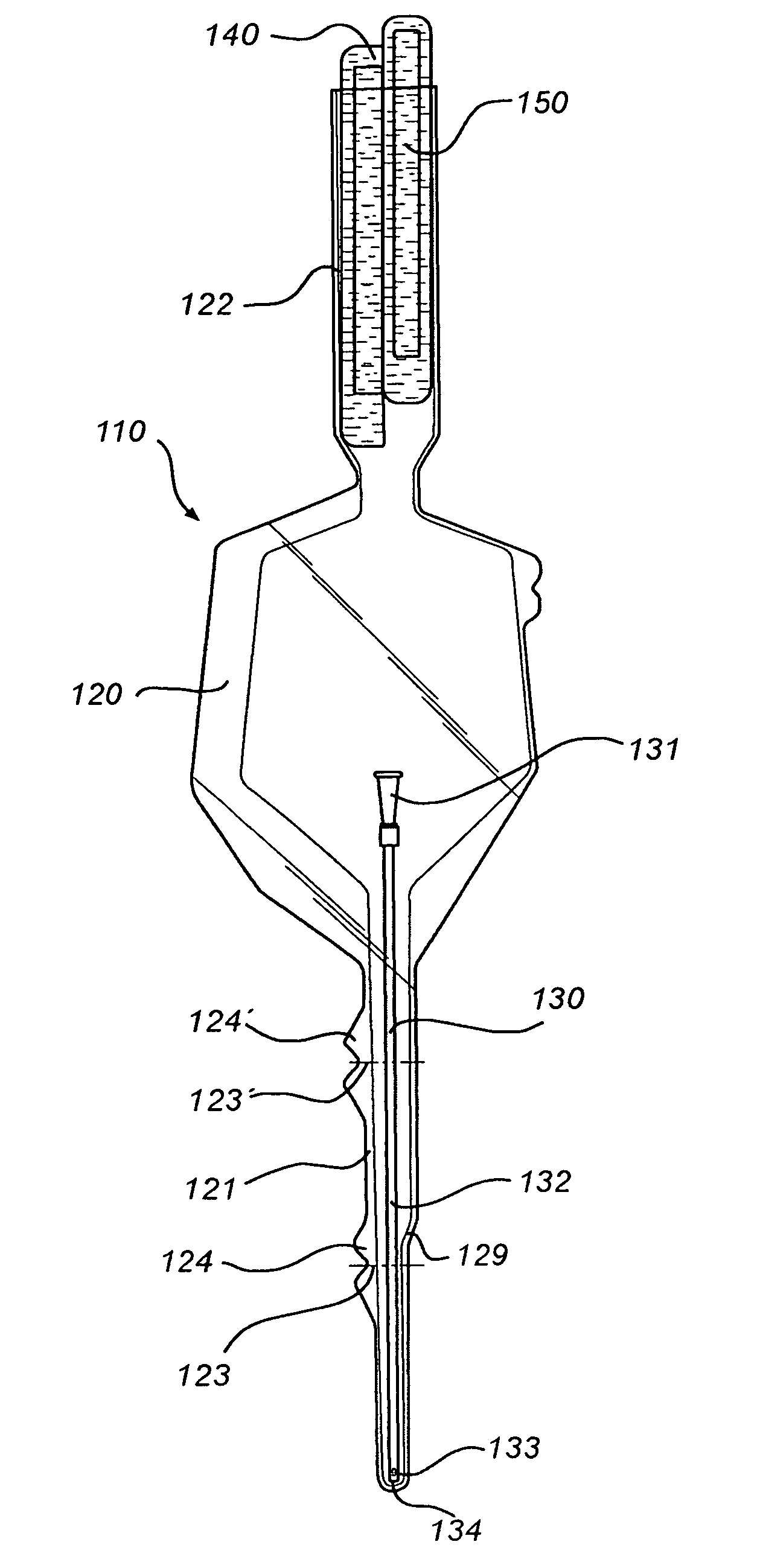
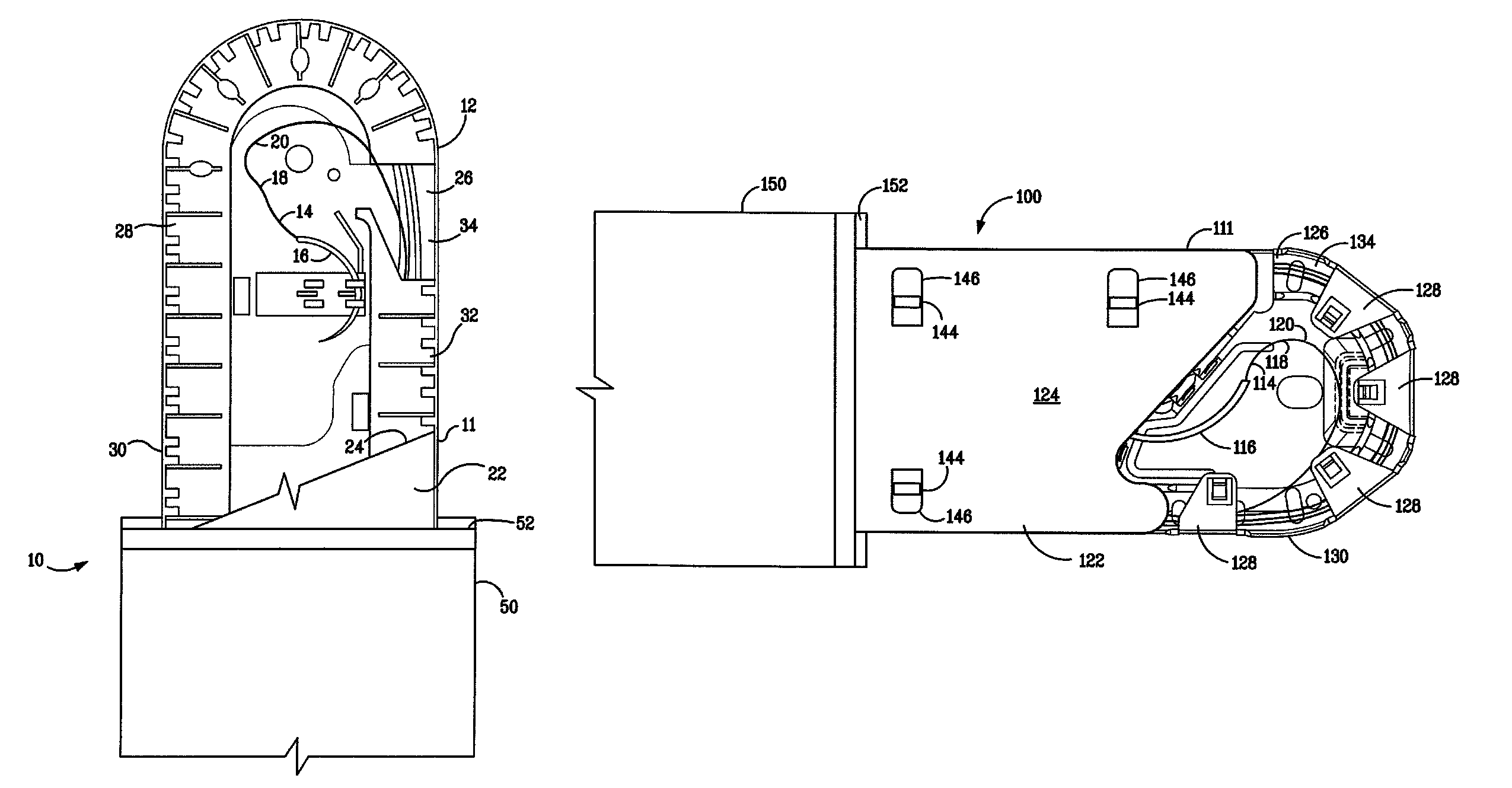
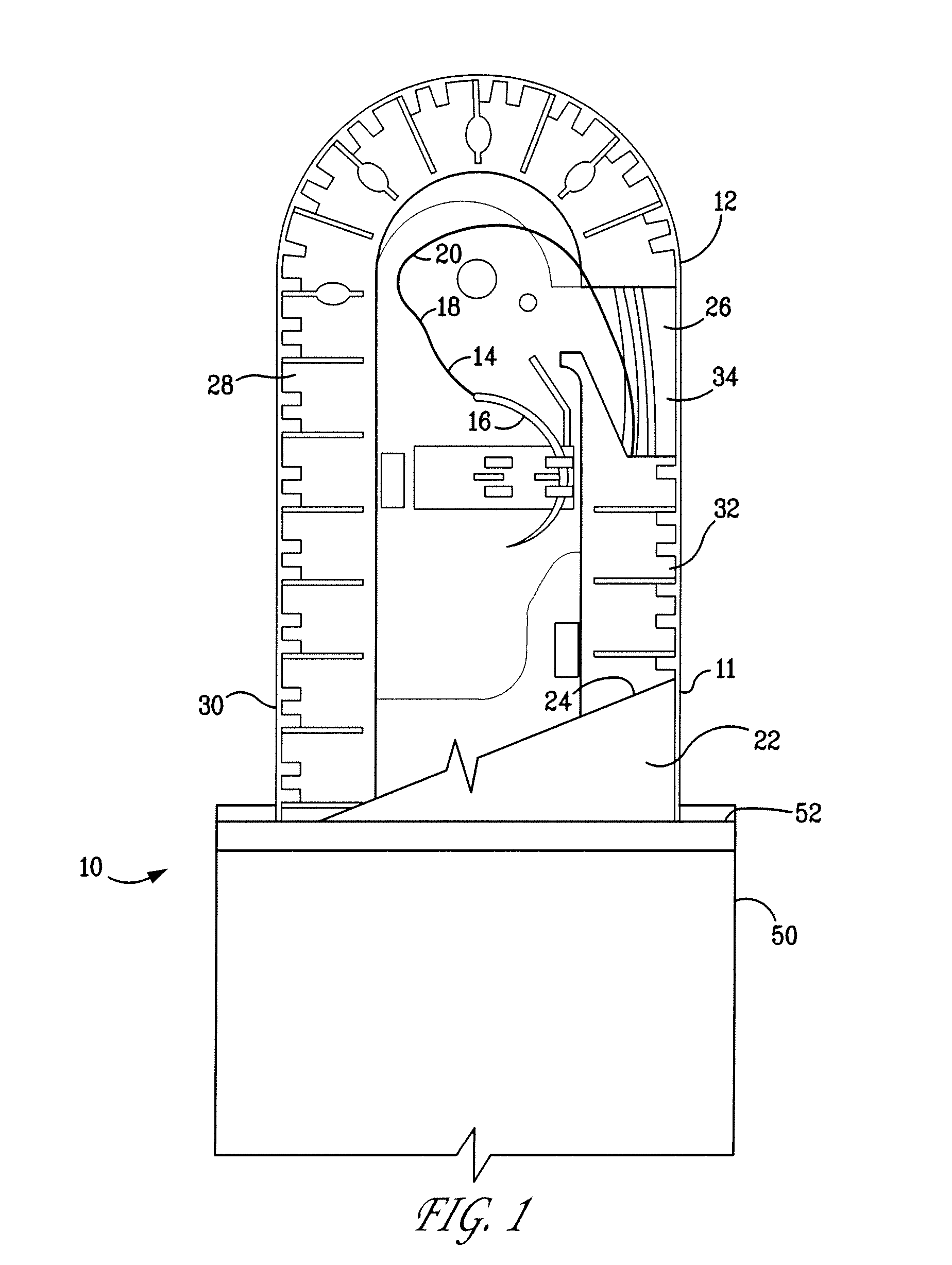
Catheter receptacle provided with an antimicrobial compound
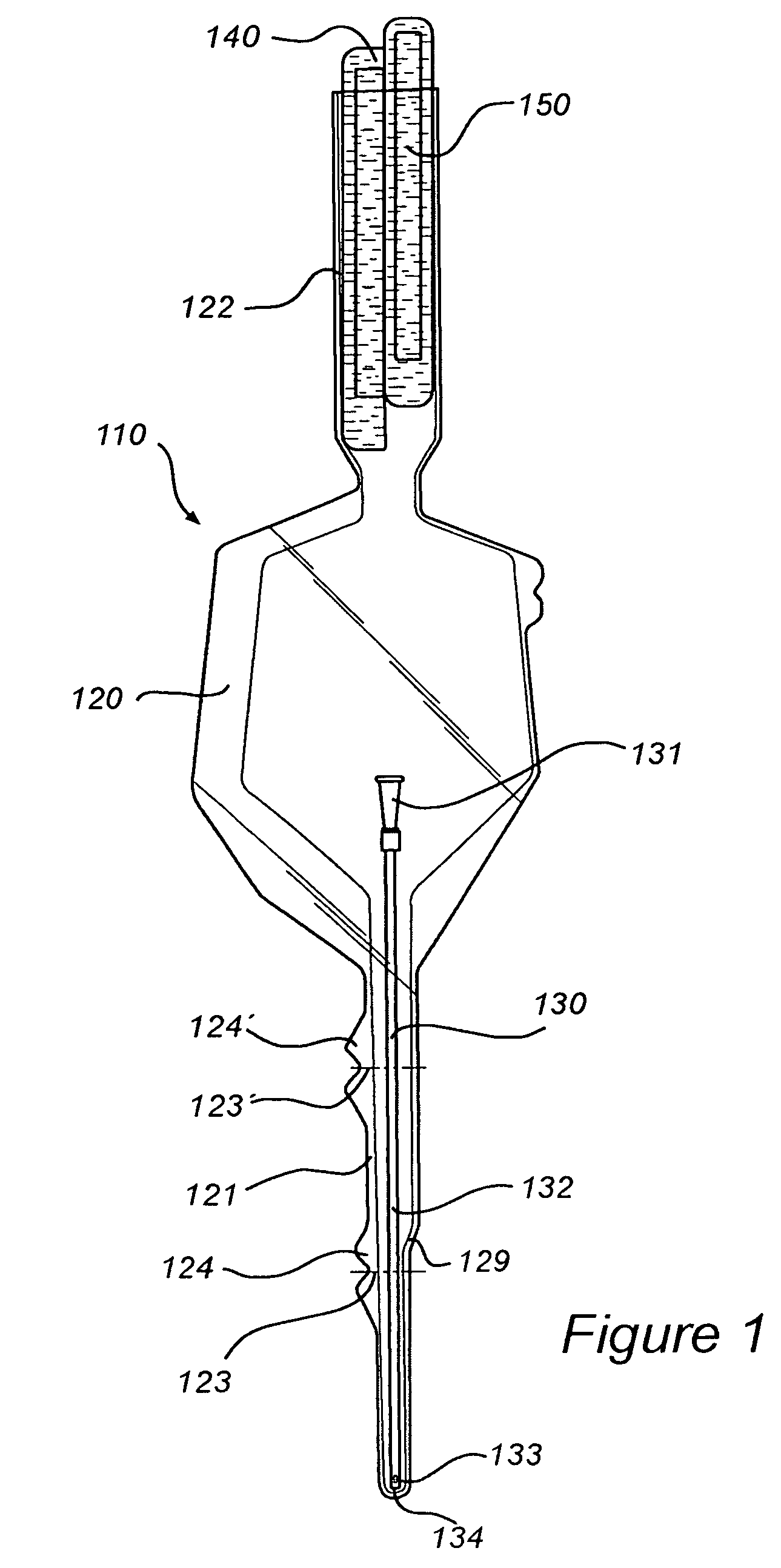
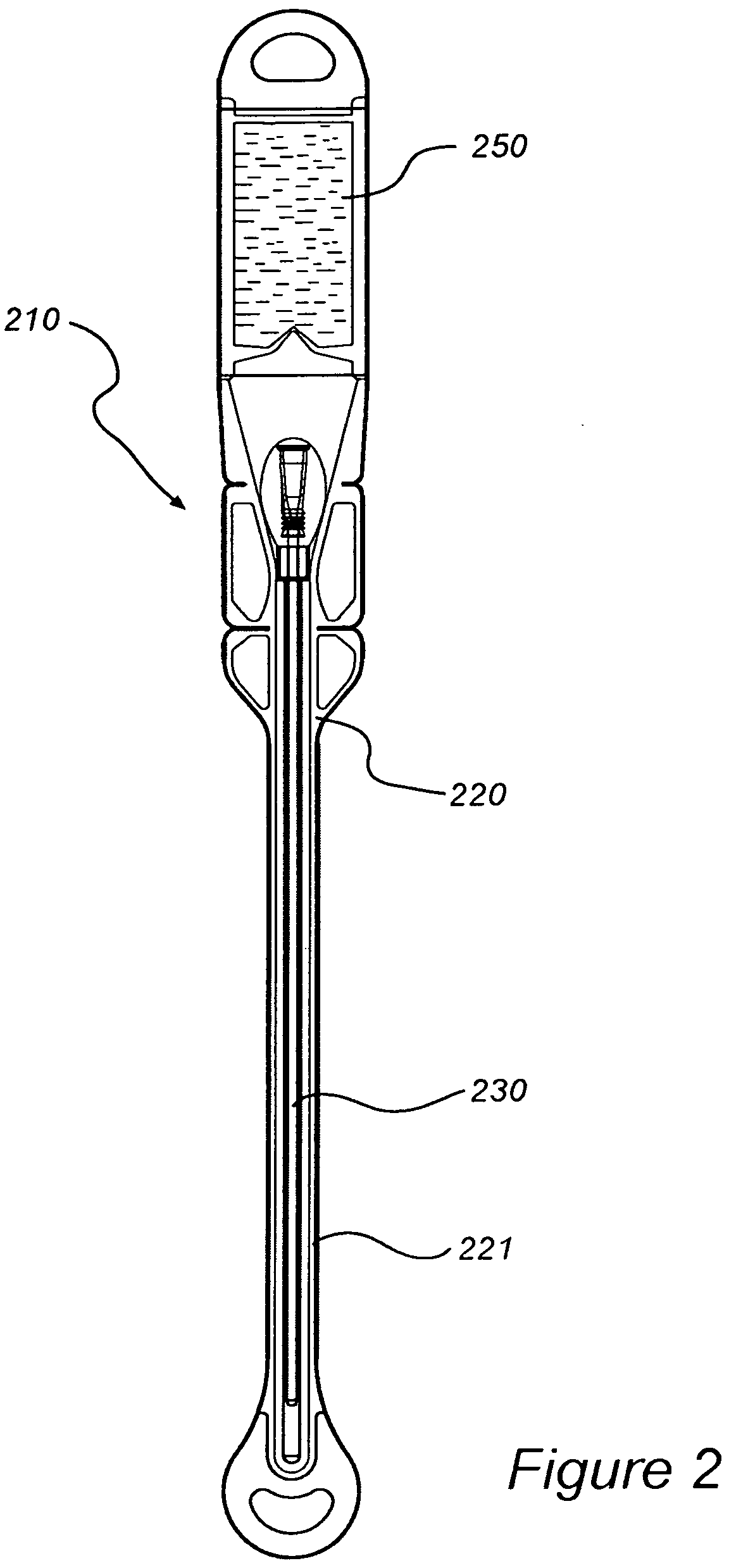
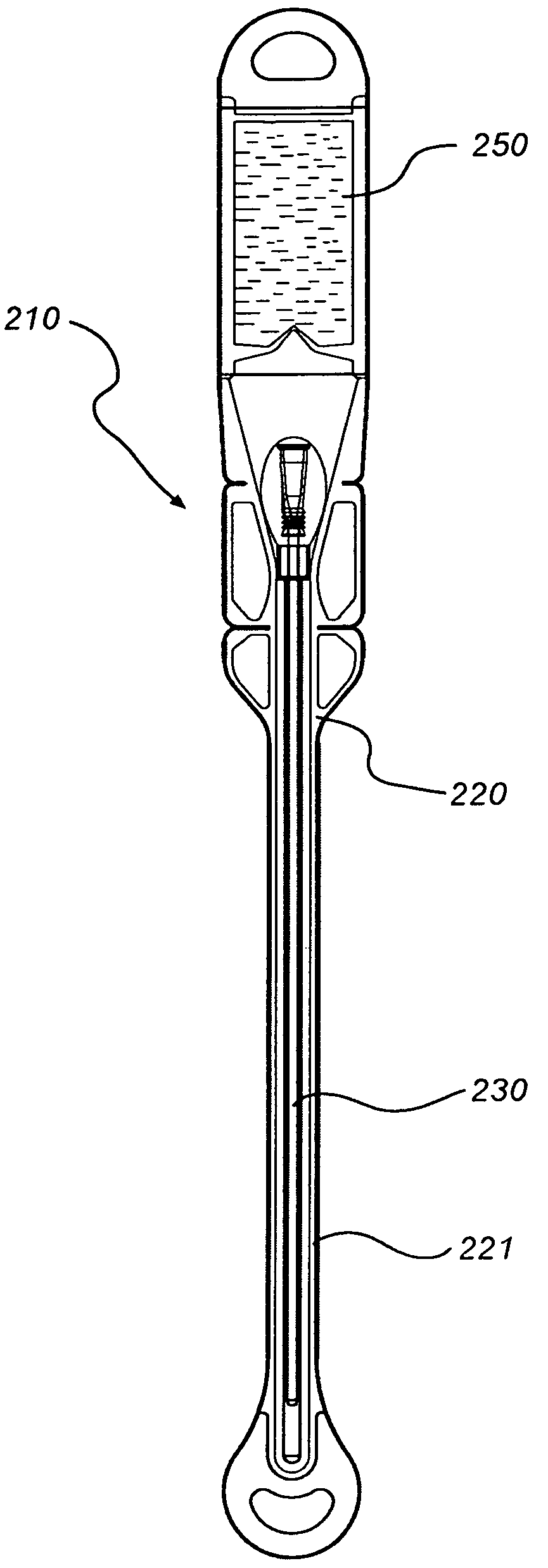
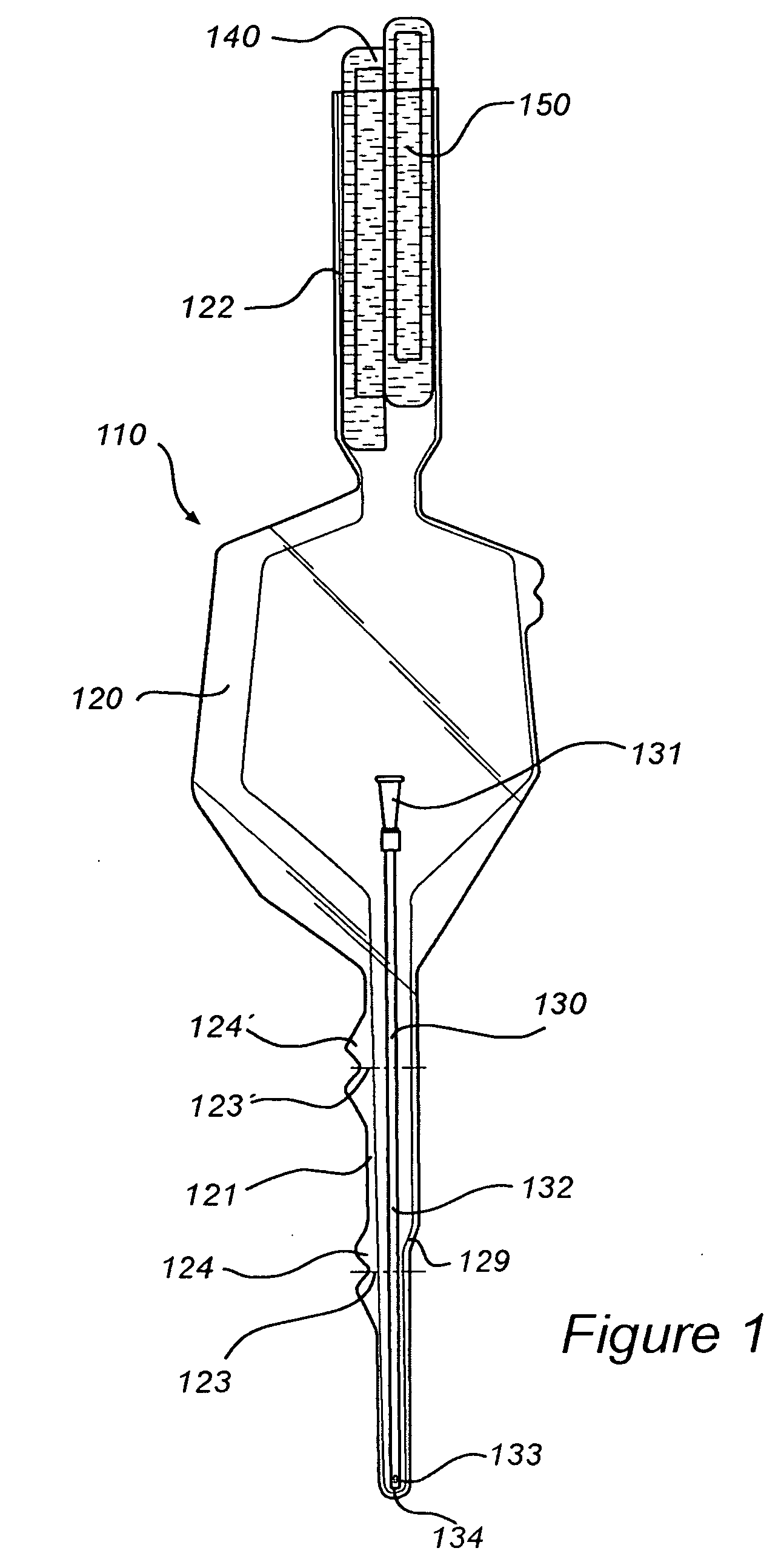
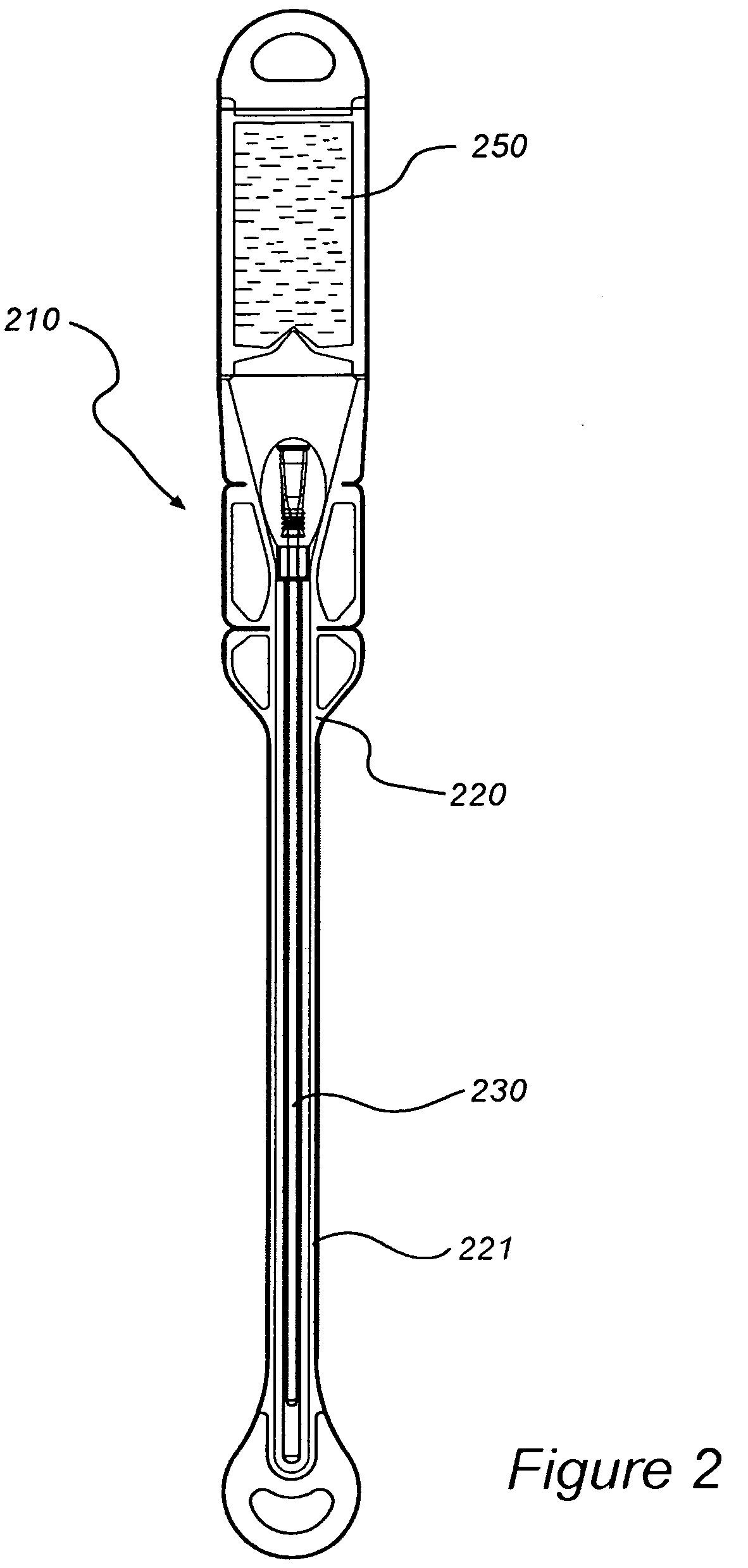
InactiveUS8127922B2Inhibit transferSuitable antimicrobial effectivenessDispensing apparatusDiagnosticsAntimicrobial compoundCatheter device
A catheter assembly (110, 210) comprising a catheter (130, 230) and a receptacle (120, 220) is disclosed. The receptacle is arranged to accommodate at least part of said catheter and having at least one opening (123) for withdrawal of said catheter. Further, the receptacle is provided with an antimicrobial compound (301) at least in the vicinity of the opening and at least on the outer surface of the receptacle, said antimicrobial compound inhibiting microbes from being transferred to said catheter while being withdrawn through said at least one opening.
Owner:ASTRA TECH SE
Packaging Solutions
InactiveUS20090173045A1Preserve sterilityImprove the lubrication effectPackage sterilisationOther accessoriesCarboxylic acidPolymer
Packaging systems for storing ophthalmic devices such as contact lenses and to methods for packaging such ophthalmic devices with solutions to improve the comfort of the lenses during wear are disclosed. A packaging system includes an ophthalmic device stored in an aqueous packaging solution comprising a hydrophilic polymer having one or more non-ethylenically-unsaturated carboxylic acid terminal groups.
Owner:LAI YU CHIN +1
Catheter receptacle provided with an antimicrobial compound
InactiveUS20090101531A1Inhibit transferSuitable antimicrobial effectivenessDispensing apparatusDiagnosticsAntimicrobial compoundCatheter device
A catheter assembly (110, 210) comprising a catheter (130, 230) and a receptacle (120, 220) is disclosed. The receptacle is arranged to accommodate at least part of said catheter and having at least one opening (123) for withdrawal of said catheter. Further, the receptacle is provided with an antimicrobial compound (301) at least in the vicinity of the opening and at least on the outer surface of the receptacle, said antimicrobial compound inhibiting microbes from being transferred to said catheter while being withdrawn through said at least one opening.
Owner:ASTRA TECH SE
Contact lens packaging
ActiveUS9173463B2Reduce the possibilityDead volumePackage sterilisationFlat article dispensingEngineeringContact lens
Owner:CONTACT LENS PRECISION LAB
Catheter assembly
InactiveUS20090200186A1Less bulkyLess materialDispensing apparatusDiagnosticsFluid compartmentsWeakness
A catheter assembly is provided comprising a catheter including a catheter tube and a connector arranged in one end of the catheter tube. The catheter is preferably a urinary, hydrophilic catheter. Further, the assembly comprises a catheter receptacle, a compartment accommodating the wetting fluid, the compartment being an integrated part of the receptacle but is separated from the cavity, the separation between the wetting fluid compartment and the cavity accommodating the catheter providing a rupturable sealed enclosure with at least one point of weakness.
Owner:ASTRA TECH SE
Method of making a packaged antimicrobial suture
InactiveUS8112973B2Extended shelf lifeInhibition of colonizationSuture equipmentsSurgical furnitureMedicineBacterial colonization
Owner:ETHICON INC
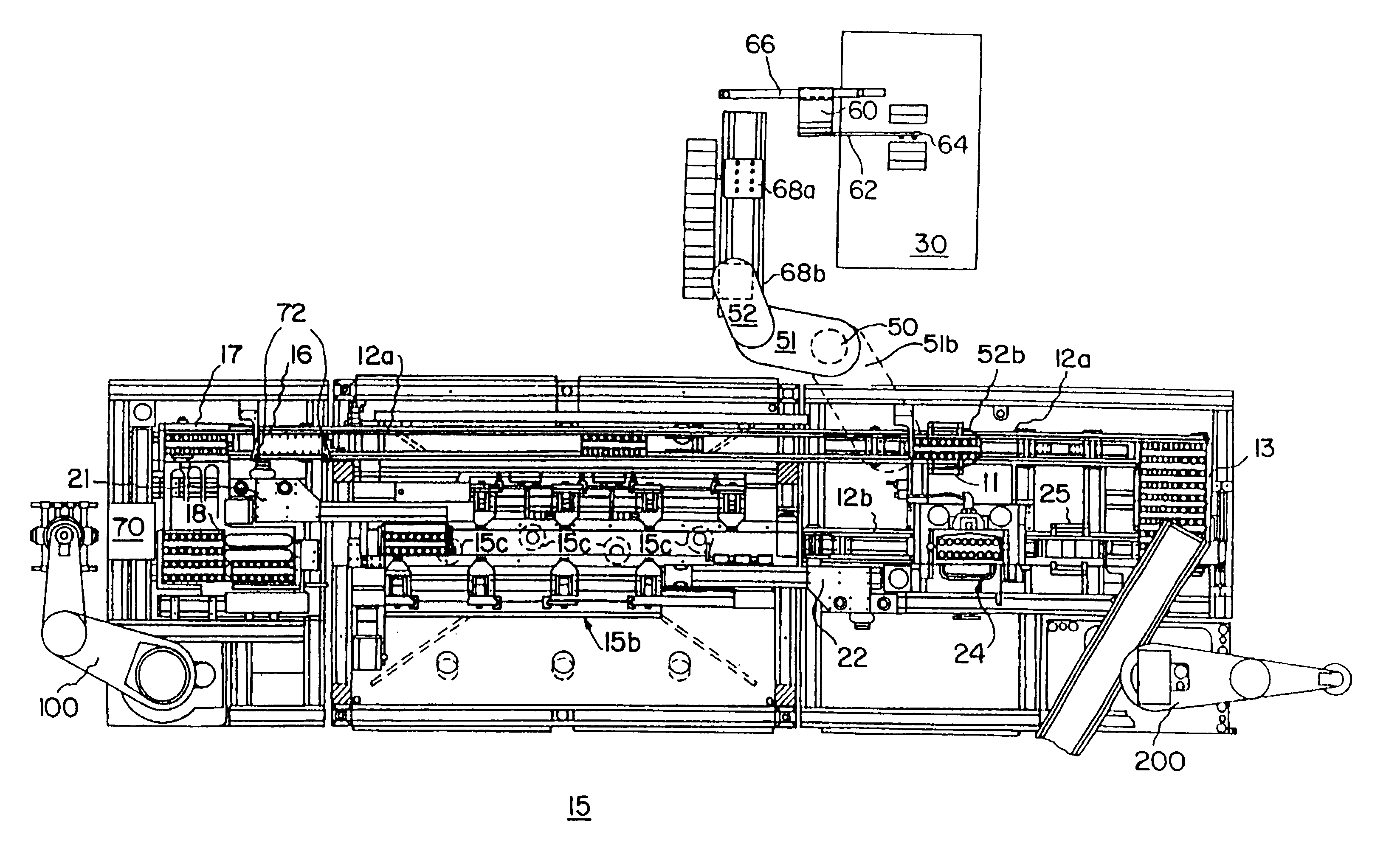
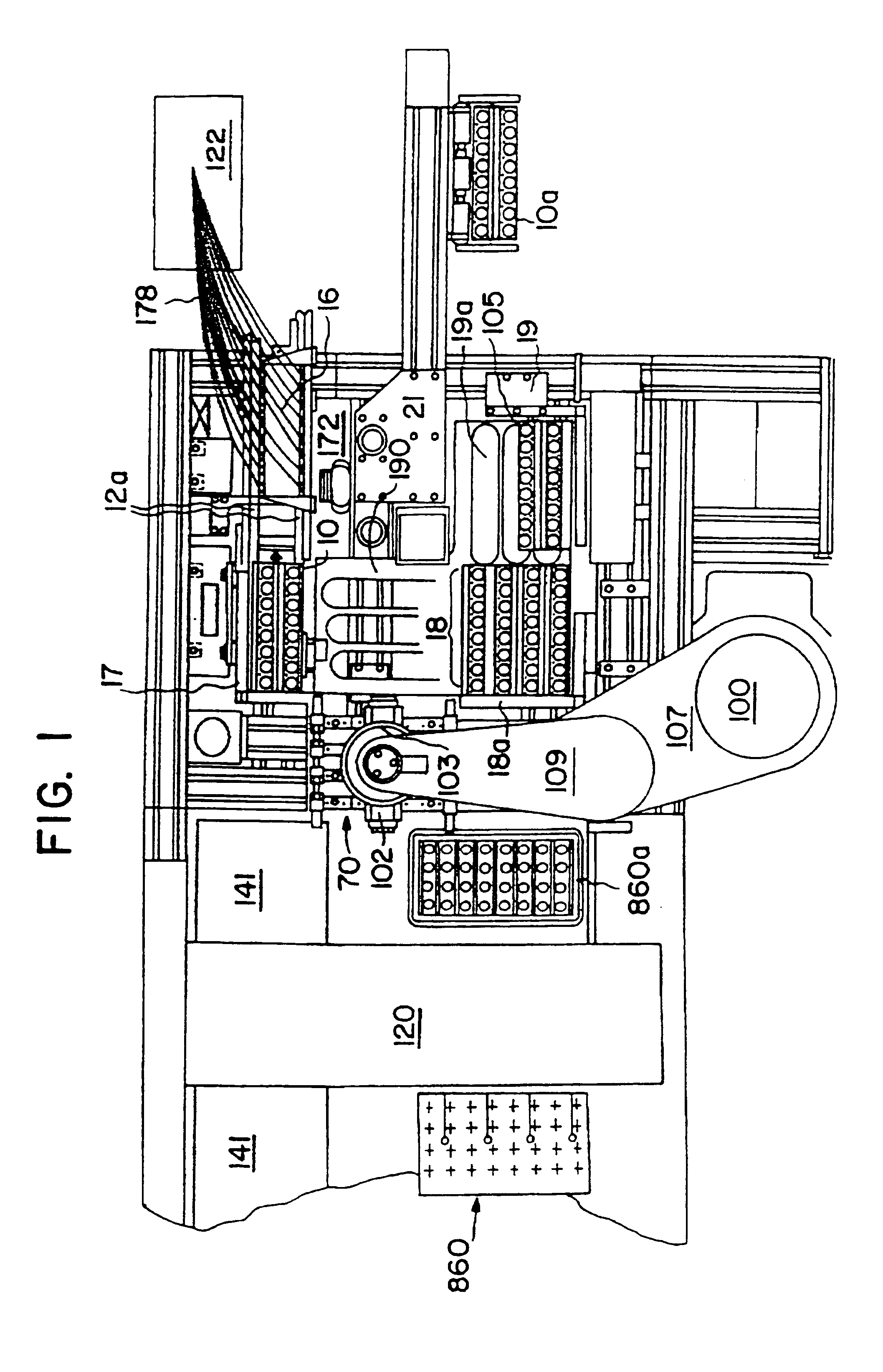
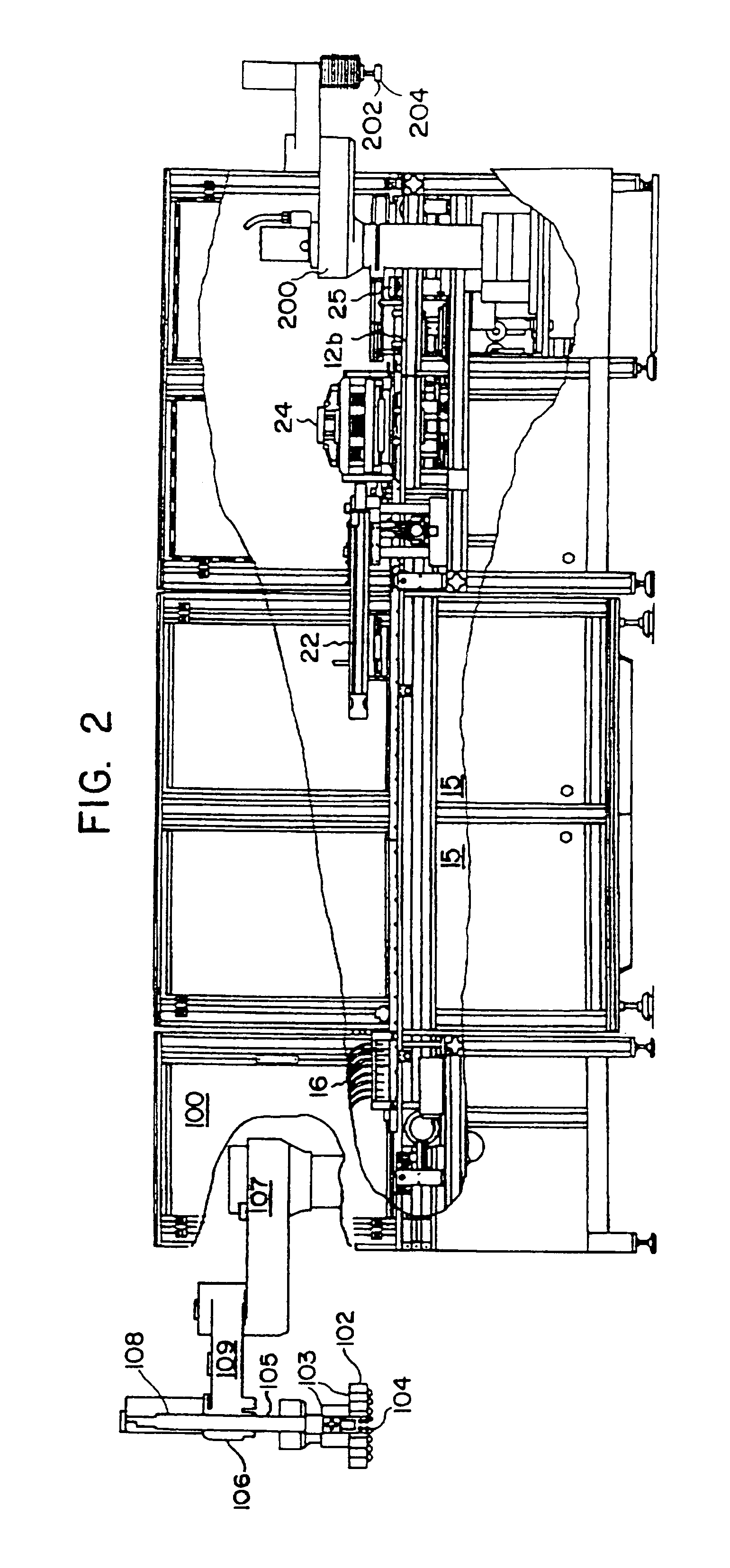
Automated apparatus and method for preparing contact lenses for inspection and packaging
InactiveUSRE37432E1Minimizes formation of air bubbleGripping headsOptical articlesSaline solutionsManufacturing line
An apparatus is provided for removing and transporting articles, such as contact lens sections from a manufacturing line to inspection and packaging stations. The lenses are deposited in a transparent plastic primary package which carries the lenses through the inspection station and becomes part of the primary package when a cover is sealed thereto. The invention includes various assemblies, including lens transfer assemblies, deionized water filling and removal assemblies, a water degassing assembly, a lens inspection assembly, and a lens package sealing assembly. The lenses are removed from pallets at a post hydration station, transported and spatially redistributed, and deposited in the primary packages disposed on a second set of pallets. The packages on the second set of pallets are filled with degassed deionized water. The contact lenses and packages are then transported to an inspection station. After inspection, the lenses and packages are transported to a water removal assembly, and then to another transfer assembly. This transfer assembly separates those lenses which passed inspection from those which did not, and places those that did in a consolidating assembly. The lenses and packages are then filled with saline solution and a foil label is then sealed thereto to form the primary package.
Owner:JOHNSON & JOHNSON VISION CARE INC
Packaging Solutions
InactiveUS20080141628A1Avoid pollutionLens cleaning compositionsPackage sterilisationEngineeringHyaluronic acid
The present invention is directed to new and improved packaging systems for storing ophthalmic devices such as contact lenses and to methods for packaging such ophthalmic devices with solutions to improve the comfort of the lenses during wear. In particular, the present invention is directed to a packaging system for storing an ophthalmic device in an aqueous packaging solution comprising hyaluronic acid or a salt thereof. Such solutions are retained on the surface of an unused lens for extended periods of time, resulting in surface modification that persists in the eye, which may provide significant improvement in the wetting properties of fresh contact lenses used for the first time and, moreover, even several hours after lens insertion, thereby preventing dryness and improving lubricity.
Owner:BAUSCH & LOMB INC
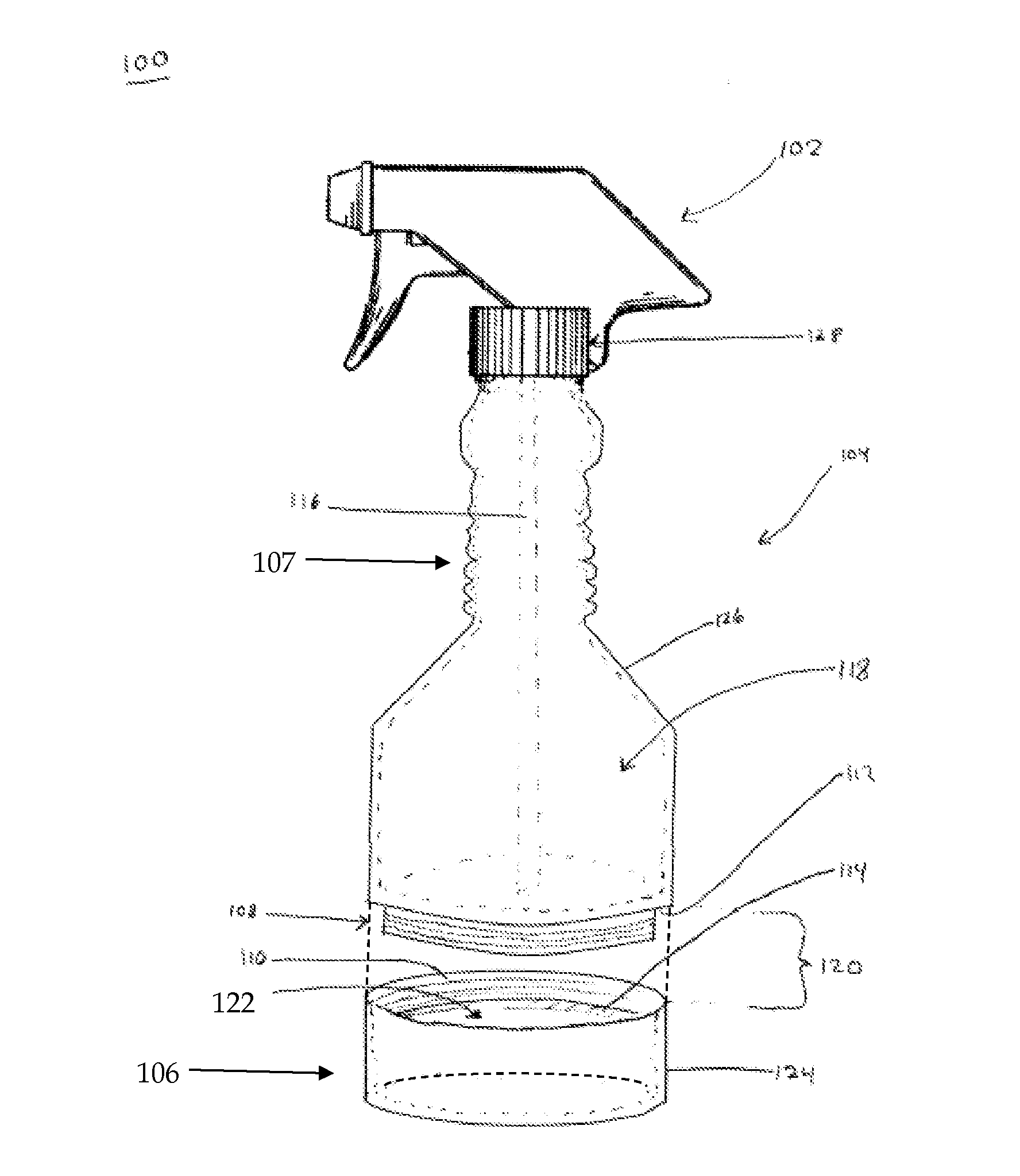
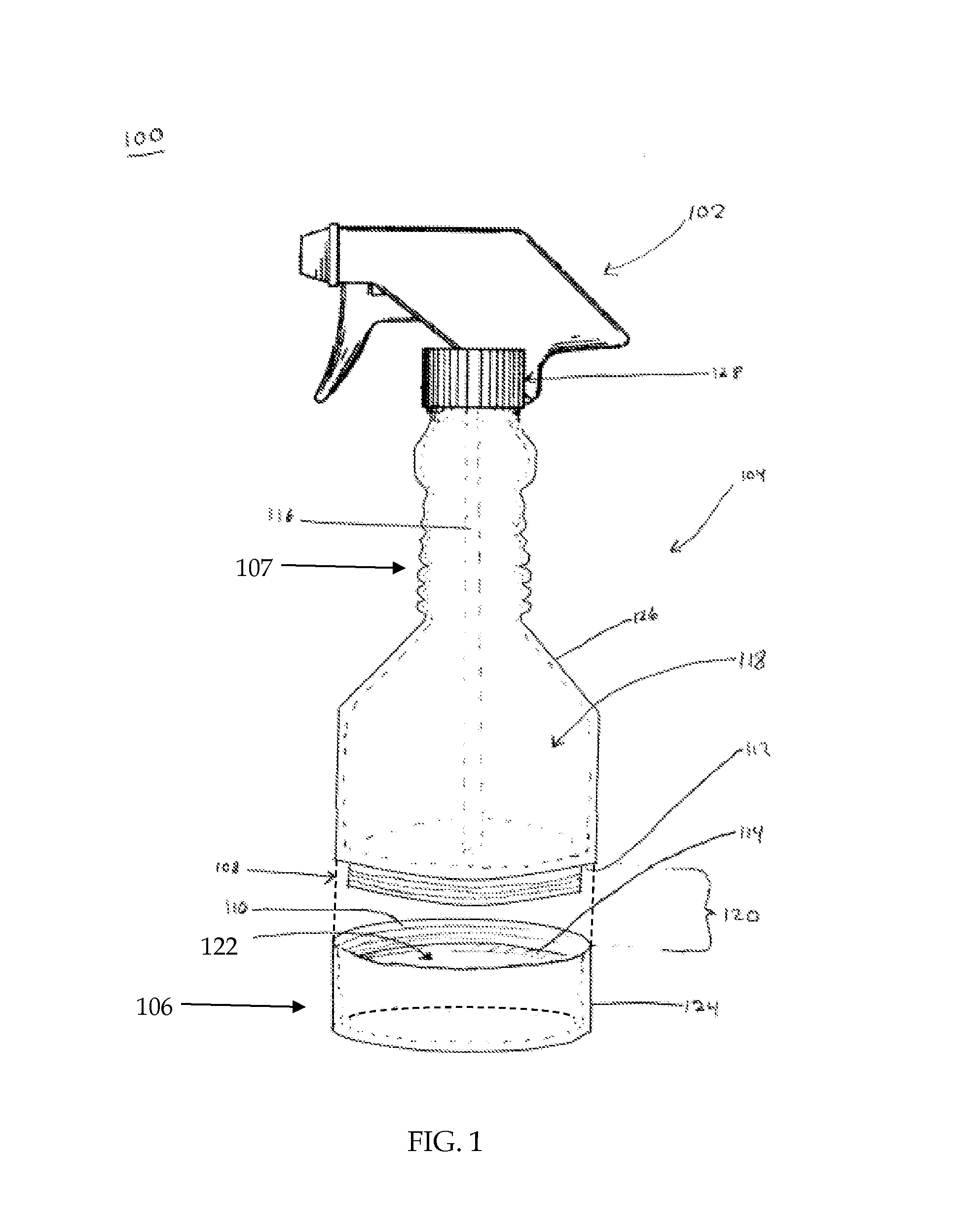
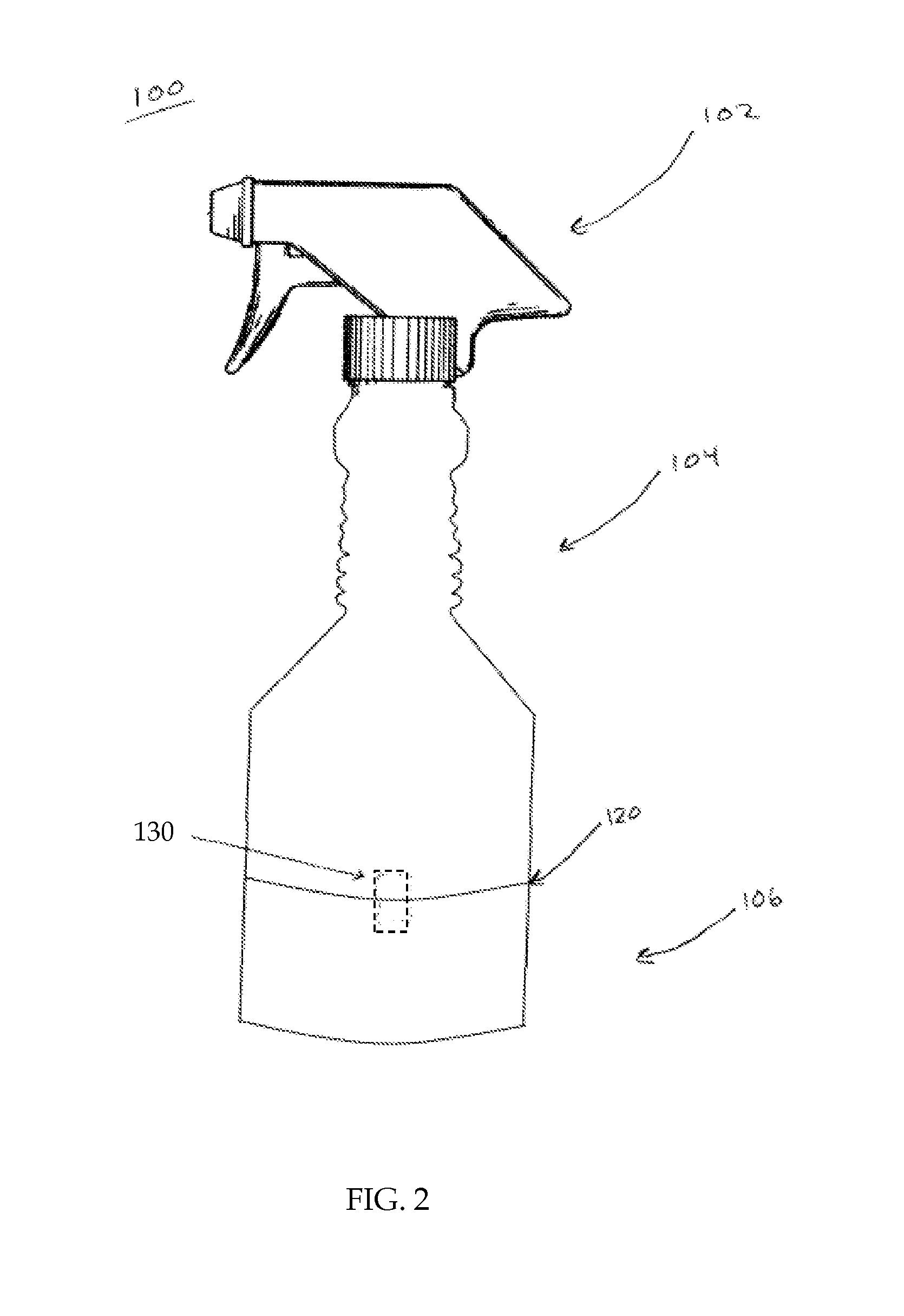
Spray bottle with storage area and methods thereof
A spray bottle is provided that may comprise a spray head for spraying liquid contained within the spray bottle; a bottle for storing the liquid, the bottle comprising a bottle attachment; a compartment for storing items, the compartment comprising a storage attachment adapted to detachably attach to the bottle attachment. In some embodiments the spray bottle apparatus may comprise a spray head for spraying liquid contained within the spray bottle; a bottle for storing the liquid, the bottle comprising a bottle attachment; a compartment for storing items, the compartment comprising a storage attachment adapted to detachably attach to the bottle attachment; and wherein the bottle attachment and the storage attachment comprise inversely threaded portions adapted to couple with each other.
Owner:STERNBERG ERIC +1
System and method for providing a regulated atmosphere for packaging perishable goods
InactiveUS20080134640A1Reduce disadvantagesPrevent and minimize gas leakageWeb rotation wrappingPackage sterilisationBiomedical engineeringAtmosphere
A method for introducing at least one substance into a sealed enclosure holding at least one product. The sealed enclosure having at least one conduit through which one of gas or fluid may flow into or out of the sealed enclosure. Air is evacuated from the sealed enclosure through the at least one conduit to create a predetermined pressure within the sealed enclosure and a predetermined quantity of the at least one substance is injected into the sealed enclosure through the at least one conduit.
Owner:THE BOWDEN GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com