Patents
Literature
50results about How to "Unit cell volume reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium iron phosphate anode material with activating aluminum and barium
InactiveCN102376954AShape is easy to controlIncrease the diffusion coefficientCell electrodesLithium iron phosphateChemical compound
The invention provides a lithium iron phosphate anode material with activating aluminum and barium, wherein the chemical formula can be expressed as follows: LiAlxBayFePO4, wherein X=0.002-0.005, and y=0.0003-0.003; the mol ratio of Li, Al, Ba, Fe and P is as follows: 1 mol of Li: 0.002-0.005 mol of Al: 0.0003-0.003 mol of Ba: 1 mol of Fe: 1 mol of P; after a small quantity of substituted aluminum and barium are doped, the shape and the grain diameter of a product can be preferably controlled, a stable lithium iron phosphate chemical compound can be obtained, the crystal lattice can be activated, the diffusion coefficient of lithium ions can be improved, and the first discharge capacity can reach 155.52mAh / g; the electric potential of a charge / discharge platform is about 3.5V relative to a lithium electrode, the initial discharge capacity exceeds 164mAh / g, and the capacity is attenuated by about 3.0% after 100 times of charge / discharge circulation; compared with the embodiment which is not doped with LiFePO4, the specific capacity and the cyclical stability can be greatly improved; and as the price of Ba is lower than the price of Li by more than 100 times, the production cost can be reduced by more than 10 times.
Owner:张雅静
Preparing method for aluminium and barium activated lithium iron phosphate positive electrode material
ActiveCN102376952AShape is easy to controlGood for particle size controlCell electrodesCharge dischargeLithium electrode
The invention relates to a preparing method for an aluminium and barium activated lithium iron phosphate positive electrode material, which comprises the following steps of: mixing raw materials of a lithium source, an iron source, a phosphate radical source, an aluminium source and a barium source of the positive electrode material according to proportions of 1 mol of Li to 0.002-0.005 mol of Alto 0.0003 mol of Ba to 1 mol of Fe to 1 mol of P, carrying out high-speed ball milling for 20h at a revolving speed of 200 r / min in an absolute ethyl alcohol medium, drying in the temperature of 105 DEG C to 120 DEG C to obtain a precursor, placing the precursor obtained by drying in a high temperature furnace, and calcining for 24h in the high temperature of 500 DEG C to 750 DEG C at a common pure nitrogen atmosphere, thereby obtaining the aluminium and barium activated lithium iron phosphate positive electrode material of the invention. A small quantity of substituted calcium and barium doped in the obtained material are beneficial to the control of the appearance and the grain size of a product and the obtaining of the stable lithium iron phosphate compound; the crystal lattice of the positive electrode material is activated, thereby increasing diffusion coefficients of lithium ions; the initial discharge capacity of the obtained positive electrode material reaches 155.52mAh / g; a relative lithium electrode potential of a charge-discharge platform of the positive electrode material is about 3.5V; after the initial discharge capacity of the positive electrode material exceeds 164mAh / g and the positive electrode material undergoes 100 times of charge-discharge circulations, the capacity is attenuated by about 3.0%; and compared with the reference embodiment of the undoped LiFePO4, the positive electrode material has the advantages that the specific capacity and the circulation stability are increased greatly; and as the price of the barium is higher than that of lithium by more than a hundred times, the production cost can be reduced by more than ten times.
Owner:桐乡乐维新材料有限公司
Method for preparing antimony and barium activated lithium iron phosphate cathode material
ActiveCN102364734AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphatePhosphate
The invention discloses a method for preparing an antimony and barium activated lithium iron phosphate cathode material. The method comprises the following steps of: mixing a lithium source, an iron source, a phosphate group source, an antimony source and a barium source which serve as raw materials according to the ratio of Li to Sn to Ba to Fe to P of 1:(0.00002-0.00005):(0.0003-0.003):1:1, performing high-speed ball milling in an absolute ethanol medium at the rotating speed of 200r / min for 20 hours, drying at the temperature of between 105 and 120 DEG C, and thus obtaining a precursor; and putting the dried precursor into a high temperature furnace, calcining in a common pure nitrogen atmosphere at the temperature of between 500 and 750 DEG C for 24 hours, and thus obtaining the antimony and barium activated lithium iron phosphate cathode material. A small amount of antimony and barium is doped, so that control over the appearance and the particle size of a product is facilitated, a stable lithium iron phosphate compound is obtained, the crystal lattice of the material is activated, a lithium ion diffusion coefficient is improved, and the specific capacity and the circulating stability of the material are greatly improved.
Owner:桐乡乐维新材料有限公司
Preparation method of magnesium-aluminum-vanadium co-doped lithium cobalt oxide positive electrode material
PendingCN114368790AWon't breakHigh voltageSecondary cellsPositive electrodesLithium carbonateVanadate
The invention discloses a preparation method of a magnesium-aluminum-vanadium co-doped lithium cobalt oxide positive electrode material, which comprises the following steps: a, cobalt oxide, lithium carbonate, magnesium oxide, aluminum oxide and ammonium vanadate raw materials are uniformly mixed, the molar ratio of the lithium element to the cobalt element is 1.04-1.06, the mass fraction of the magnesium oxide is 0.05-0.2%, the mass fraction of the aluminum oxide is 0.05-0.5%, the mass fraction of the ammonium vanadate is 0.05-0.5%, and the molar ratio of the lithium element to the cobalt element is 1.04-1.06; the mass fraction of the ammonium vanadate is 0.05%-0.5%; b, putting the raw materials mixed in the step a into calcining equipment at 500-1080 DEG C, sintering for 2-20 hours at a constant temperature, cooling to room temperature after sintering, and taking out; and c, crushing and sieving the product obtained in the step b to obtain the magnesium-aluminum-vanadium co-doped lithium cobalt oxide positive electrode material. By doping magnesium, aluminum and vanadium elements, the stable cycle performance of lithium cobalt oxide under 4.5 V voltage is improved, and the capacity is better under high-voltage charging and discharging conditions.
Owner:GEM JIANGSU COBALT IND CO LTD
Preparation method of selenium and barium activated lithium iron phosphate anode materials
ActiveCN102386406AImprove conductivityShape is easy to controlCell electrodesElectrode potentialNiobium
The invention relates to a preparation method of selenium and barium activated lithium iron phosphate anode materials, which includes mixing raw materials of a lithium source, an iron source, a phosphoric acid root source, a selenium source and a barium source according to the following ratio by mole: 1 part of lithium, 0.00002 to 0.00005 part of selenium, 0.0003 to 0.003 part of barium, 1 part of iron and 1 part of phosphoric acid root, milling the raw materials in an absolute ethyl alcohol medium for 20 hours through a high-speed ball with 200r / mimn rotating speed, drying the raw materials under the temperature between 105 DEG C and 120 DEG C to obtain a precursor, placing the precursor obtained by drying in a high-temperature furnace and conducting high-temperature calcination on the precursor under the temperature between 500 DEG C and 750 DEG C for 24 hours in an atmosphere of common pure nitrogen to obtain the niobium and barium activated lithium iron phosphate anode materials. Due to the fact that a small quantity of selenium and barium is mingled for replacement, appearance and particle diameter of products can be favorably controlled to obtain stable lithium iron phosphate compound. Lattice of the products is activated, diffusion coefficient of lithium ions is improved, and first discharging capacity of obtained materials reaches 160.52mAh / g. Electrode potential of a charging-discharging platform is about 3.5V relative to lithium, initial discharging capacity surpasses 168mAh / g, and the capacity attenuates by about 1.2% after 100 times of charging-discharging circulation. Compared with unmingled LiFePO4 contrast embodiment, specific capacity and circulation stability are greatly improved.
Owner:桐乡乐维新材料有限公司
Lithium manganese silicate/carbon composite material doped with alkaline-earth metal ions and preparing method thereof
ActiveCN107546386AImproved magnification performanceHigh activityMaterial nanotechnologyCell electrodesCarbon compositesAlkaline earth metal
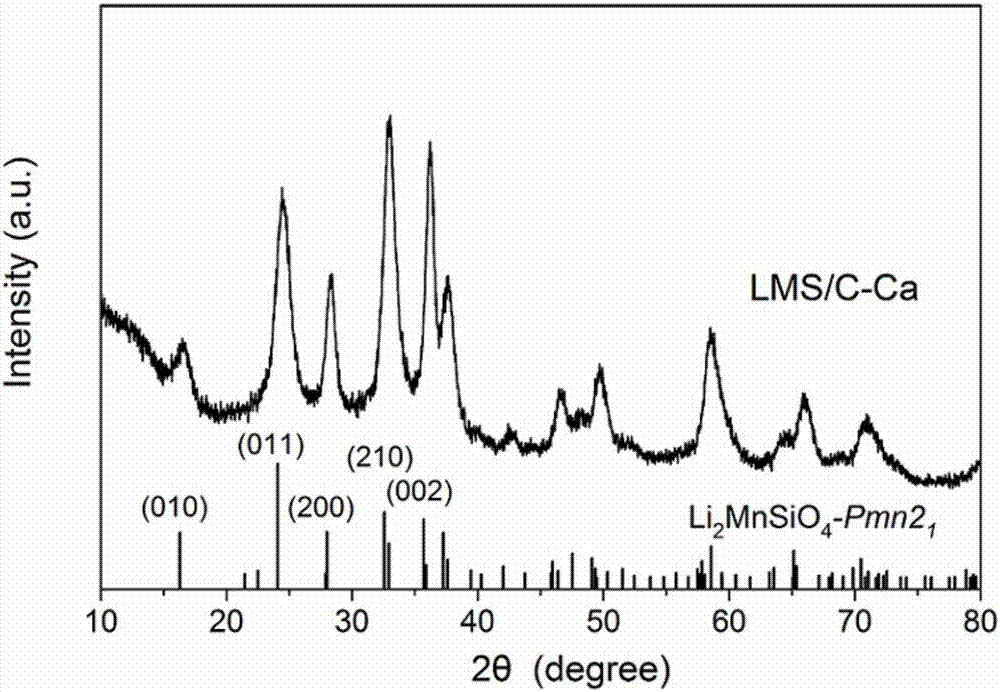
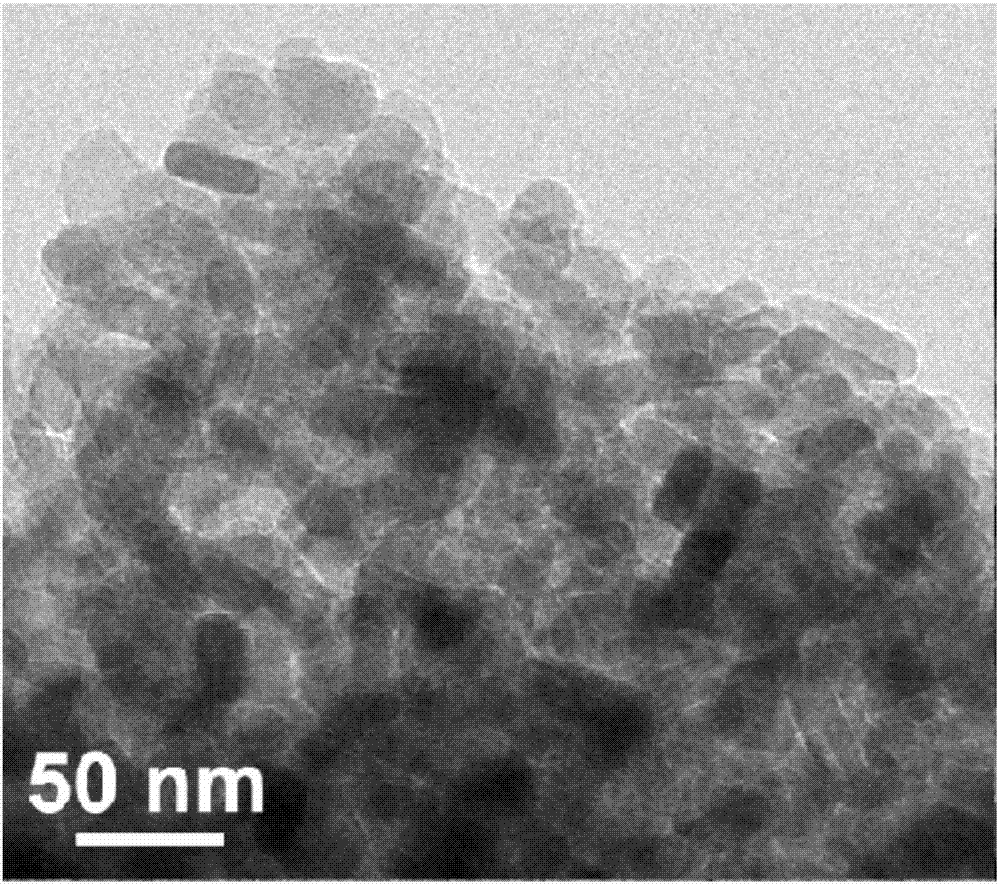
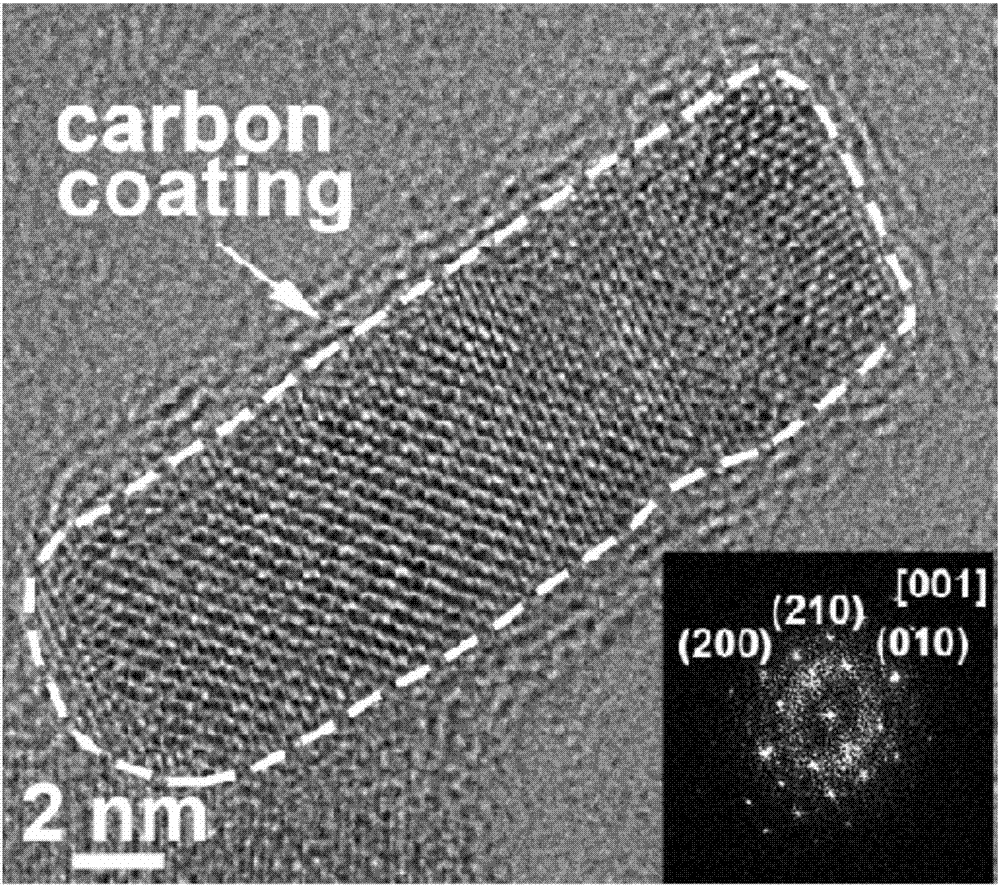
The invention provides a lithium manganese silicate / carbon composite material doped with alkaline-earth metal ions and a preparing method thereof. The chemical formula of the material is Li2Mn(1-x)MxSiO4 / C, wherein 0<x<=0.10, and M is selected from one of Mg, Ca, Sr or Ba. The material displays [001] spots with axes under a high-resolution transmission electron microscopy, and is prepared based on the principle of a hydrothermal method. The lithium manganese silicate / carbon composite material is uniform in shape and high in degree of crystallinity, [001] crystal faces are exposed, and due todoping of alkaline-earth metal elements, the lattice constants of the material are changed; meanwhile, all the elements show the features that concentrations are distributed in a gradient mode, thus promoting of electrical conductivity is facilitated, and the material has excellent electrochemical performance under high magnification.
Owner:CENT SOUTH UNIV
Preparation method of copper-barium-activated lithium iron phosphate anode material
ActiveCN102509798AImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium iron phosphateAlcohol
The invention relates to a preparation method of a copper-barium-activated lithium iron phosphate anode material. The preparation method comprises the following steps of: mixing raw materials, such as a lithium source, an iron source, a phosphate radical source, a copper source and a barium source according to a mol ratio of 1 of Li to Cu to Ba to Fe to P of 1: (0.00002-0.00005):0.0003:1:1; performing high-speed ball milling at a rotating speed of 200r / min for 20 hours in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; and placing the precursor obtained by drying into a high-temperature furnace, and calcining the precursor at 500-750 DEG C for 24 hours in an ordinary pure nitrogen atmosphere to obtain the copper-barium-activated lithium iron phosphate anode material. Because a small amount of substituting copper and barium are doped, the shape and the particle size of the copper-barium-activated lithium iron phosphate anode material can be conveniently controlled to obtain a stable lithium iron phosphate compound, the crystal lattice of the lithium iron phosphate compound is activated, the diffusion coefficient of lithium ions is increased, and the first-time discharge capacity of the obtained material reaches 160.52mAh / g; the electric potential of a charging and discharging platform of the copper-barium-activated lithium iron phosphate anode material relative to a lithium electrode is about 3.5V, the initial discharge capacity is more than 168mAh / g, and after 100 times of charging and discharging circulation, the capacity is approximately attenuated by about 1.2 percent; and compared with a contrast embodiment without doping of LiFePO4, the copper-barium-activated lithium iron phosphate anode material has the advantage that: the specific capacity and the cycle stability are greatly improved.
Owner:桐乡乐维新材料有限公司
Ultralow-demagnetization-rate high-temperature samarium-cobalt permanent magnet material and preparation method thereof
PendingCN111863368APrevent oxidationReduce volatilityMagnetic materialsPermanent magnet manufactureRare-earth elementSamarium
The invention discloses an ultralow-demagnetization-rate high-temperature samarium-cobalt permanent magnet material and a preparation method thereof. The stoichiometric formula of the samarium-cobaltpermanent magnet material is Sm(Co1-x-y-vFexCuyZrv)z, wherein x is equal to 0.09-0.13, y is equal to 0.12-0.18, v is equal to 0.033-0.04, z is equal to 7.69-8.3, and z is the ratio of the total atomicweight of the transition group elements Co, Fe, Cu and Zr to the atomic weight of the rare earth element Sm. The samarium-cobalt permanent magnet material prepared by the method is good in high-temperature resistance, and has extremely low demagnetization rate at the use temperature of 500 DEG C.
Owner:HANGZHOU PERMANENT MAGNET GRP
Multi-metal composite oxide coated modified lithium manganate positive electrode material and preparation method thereof
InactiveCN113594445ASufficient lithiumUnit cell volume reductionCell electrodesSecondary cellsPhysical chemistryManganate
A multi-metal composite oxide coated modified lithium manganate positive electrode material takes lithium manganate as a matrix, wherein the surface of the matrix is coated with a Li(M1)beta(M2)gammaO2 coating layer, beta is larger than 0 and smaller than or equal to 1, gamma is larger than 0 and smaller than or equal to 0.5, and M1 is at least one of Mn, Co and Ni; and M2 is at least one of Al, Mg, Zr, Ti, Sr, Y, W, Bi, La, Sd, Ba, Ce, V, Se, Mo, Nb and B. The preparation method comprises the following steps of: mixing the raw materials according to a stoichiometric ratio for preparing a lithium manganate matrix, and calcining the mixture at the temperature not lower than 900 DEG C to obtain a first calcined product; and mixing the calcined product with a M1-containing compound and a M2-containing compound, and calcining to obtain the multi-metal composite oxide coated modified lithium manganate positive electrode material. The modified lithium manganate positive electrode material disclosed by the invention not only has long cycle life, especially has relatively outstanding cycle performance under a high-temperature condition, but also has a high capacity level.
Owner:HUNAN SHANSHAN ENERGY TECH CO LTD
Germanium and barium activated lithium iron phosphate anode material
InactiveCN102347485AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphateCharge discharge
The invention provides a germanium and barium activated lithium iron phosphate anode material. The chemical formula of the material can be represented as LiGexBay FePO4, wherein x is equal to 0.0000-0.00005 and y is equal to 0.0003-0.003; the mol ratio of Li, Ge, Ba, Fe and P is as follows: Li to Ge to Ba to Fe to P is equal to 1 mol to 0.00002-0.00005 mol to 0.0003-0.003 mol to 1 mol to 1 mol; as less substituted germanium and barium is doped, the appearance and the grain diameter of a product are easy to control and a stable lithium iron phosphate compound is obtained; the crystal lattice of the lithium iron phosphate compound is activated, the lithium ion diffusion coefficient is improved and the initial discharge capacity reaches 160.52 mAh / g; the potential of a charge-discharge platform corresponding to a lithium electrode is about 3.5 V, the initial discharge capacity exceeds 168 mAh / g and the capacity is attenuated by about 1.2% after 100 times of charge-discharge cycles; and compared with the undoped LiFePO4 comparing embodiment, the specific capacity and the cycling stability of the anode material are greatly improved.
Owner:李杰
Preparation method of copper-barium-activated lithium iron phosphate anode material
ActiveCN102509798BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium electrodeHigh heat
The invention relates to a preparation method of a copper-barium-activated lithium iron phosphate anode material. The preparation method comprises the following steps of: mixing raw materials, such as a lithium source, an iron source, a phosphate radical source, a copper source and a barium source according to a mol ratio of 1 of Li to Cu to Ba to Fe to P of 1: (0.00002-0.00005):0.0003:1:1; performing high-speed ball milling at a rotating speed of 200r / min for 20 hours in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; and placing the precursor obtained by drying into a high-temperature furnace, and calcining the precursor at 500-750 DEG C for 24 hours in an ordinary pure nitrogen atmosphere to obtain the copper-barium-activated lithium iron phosphate anode material. Because a small amount of substituting copper and barium are doped, the shape and the particle size of the copper-barium-activated lithium iron phosphate anode material can be conveniently controlled to obtain a stable lithium iron phosphate compound, the crystal lattice of the lithium iron phosphate compound is activated, the diffusion coefficient of lithium ions is increased, and the first-time discharge capacity of the obtained material reaches 160.52mAh / g; the electric potential of a charging and discharging platform of the copper-barium-activated lithium iron phosphate anode material relative to a lithium electrode is about 3.5V, the initial discharge capacity is more than 168mAh / g, and after 100 times of charging and discharging circulation, the capacity is approximately attenuated by about 1.2 percent; and compared with a contrast embodiment without doping of LiFePO4, the copper-barium-activated lithium iron phosphate anode material has the advantage that: the specific capacity and the cycle stability are greatly improved.
Owner:桐乡乐维新材料有限公司
Preparation method of manganese and barium activated lithium iron phosphate as cathode material
ActiveCN102437332BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium iron phosphatePhysical chemistry
The invention discloses a preparation method of manganese and barium activated lithium iron phosphate as a cathode material. The preparation method comprises the steps of: mixing raw materials including a lithium source, a manganese source, a barium source, an iron source and a phosphate source according to the proportion of 1mol:(0.00002-0.00005)mol:(0.0003)mol:1mol:1mol; then, performing high-speed ball milling at the rotation speed of 200r / min for 20h in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; placing the precursor obtained through drying in a high-temperature furnace; and calcinating for 24h at the high temperature of 500-750 DEG C in a common pure nitrogen atmosphere to obtain the manganese and barium activated lithium iron phosphate as the cathode material. Because a small amount of substitutive manganese and barium are doped, the shape and the particle size of a product are favorably controlled to obtain a stable lithium iron phosphate compound, of which the crystal lattice is activated, the lithium ion diffusion coefficient is increased, and the initial discharge capacity of the obtained cathode material reaches 160.52mAh / g; a charge-discharge platform of the cathode material is about 3.5V relative to a lithium electrode potential, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2% after 100 times of charge-discharge cycles; and compared with a control embodiment, namely undoped LiFePO4, the cathode material is greatly improved in specific capacity and cyclic stability.
Owner:桐乡乐维新材料有限公司
Preparation method of zirconium, barium activated lithium iron phosphate anode material
ActiveCN102509794BShape is easy to controlIncrease the diffusion coefficientCell electrodesPhosphorus compoundsLithium iron phosphatePhysical chemistry
The invention provides a preparation method of a zirconium, barium activated lithium iron phosphate anode material, characterized in that: raw materials of lithium source, iron source, phosphate radial source, zirconium source and barium source are mixed according to the proportion of 1mol Li:0.00002-00005mol Zr:0.0003-0.003mol Ba:1mol Fe:1mol P, ball-milled for 20h in absolute ethyl alcohol medium at high rotation speed of 200r / minn and then dried at 105-120 DEG C to obtain a precursor, the dried precursor is placed into a high-temperature furnace and calcined for 24h at high temperature of 500-750 DEG C in a common pure nitrogen atmosphere to obtain the zirconium, barium activated lithium iron phosphate anode material. Because the zirconium, barium activated lithium iron phosphate anode material is doped with a small amount of zirconium, barium, the shape and particle diameter of the products are controlled, the stable lithium iron phosphate compound is obtained, the crystal lattice is activated, the diffusion coefficient of the lithium ion is increased and the first discharge capacity of the material reaches 160.52mAh / g; the electric potential of a discharge and charge platform is about 3.5V relative to the lithium electrode, the first discharge capacity exceeds 168mAh / g, the capacity attenuates by about 1.2% after 100 times of charge and discharge cycle. Compared with the non-doped LiFePO4 contrast embodiment, the capacity and the cycle stability of the doped LiFePO4 are relatively greatly improved.
Owner:桐乡乐维新材料有限公司
Preparation method of cobalt/barium activated lithium iron phosphate anode material
ActiveCN102386402BImprove conductivityShape is easy to controlCell electrodesLithium iron phosphatePhosphoric acid
The invention relates to a preparation method of a cobalt / barium activated lithium iron phosphate anode material, which comprises the following steps: mixing a lithium source, an iron source, a phosphate radical source, a cobalt source and a barium source at a ratio of 1mol Li : (0.00002-0.00005) mol Co : 0.0003 mol Ba : 1mol Fe : 1 mol P, performing high-speed ball milling in an anhydrous alcohol medium at a rotation speed of 200 r / min for 20 h, drying at 105-120 DEG C to obtain a precursor, placing the precursor obtained via the drying into a high-temperature furnace, and performing high-temperature calcination in a nitrogen atmosphere at 500-750 DEG C for 24 h to obtain the cobalt / barium activated lithium iron phosphate anode material. The doping of a small amount of cobalt / barium is helpful for controlling the morphological form and particle size of product and obtaining stable lithium iron phosphate compound, the crystal lattice of the cobalt / barium activated lithium ironphosphate anode material is activated so as to improve the lithium ion diffusion coefficient, and the initial discharge capacity of the obtained material is up to 160.52 mAh / g; the lithium electrode potential of the charging / discharging platform for the method is about 3.5 V, the initial discharging capacity is over 168 mAh / g, and the capacity after 100 cycles of charging / discharging is attenuated by about 1.2%; and comparing with the non-doped LiFePO4 comparison example, the specific capacity and cycle stability of the obtained material are greatly improved.
Owner:桐乡乐维新材料有限公司
Preparation method of germanium and barium activated lithium iron phosphate as cathode material
InactiveCN102437328BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsPhosphoric acidNitrogen gas
Owner:浙江远志新材料有限公司
Preparation method of titanium and barium activated lithium iron phosphate as cathode material
ActiveCN102437329BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium iron phosphatePhysical chemistry
The invention discloses a preparation method of titanium and barium activated lithium iron phosphate as a cathode material. The preparation method comprises the steps of: mixing raw materials including a lithium source, a titanium source, a barium source, an iron source and a phosphate source according to the proportion of 1mol:(0.00002-0.00005)mol:(0.0003-0.003)mol:1mol:1mol; then, performing high-speed ball milling at the rotation speed of 200r / minm for 20h in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; placing the precursor obtained through drying in a high-temperature furnace; and calcinating for 24h at the high temperature of 500-750 DEG C in a pure nitrogen atmosphere to obtain the titanium and barium activated lithium iron phosphate as the cathode material. Because a small amount of substitutive titanium and barium are doped, the shape and the particle size of a product are favorably controlled to obtain a stable lithium iron phosphate compound, of which the crystal lattice is activated, the lithium ion diffusion coefficient is increased, and the initial discharge capacity of the obtained cathode material reaches 160.52mAh / g; a charge-discharge platform of the cathode material is about 3.5V relative to a lithium electrode potential, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2% after 100 times of charge-discharge cycles; and compared with a control embodiment, namely undoped LiFePO4, the cathode material is greatly improved in specific capacity and cyclic stability.
Owner:桐乡乐维新材料有限公司
Preparation method of strontium and barium activated lithium iron phosphate positive pole material
ActiveCN102354755AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphateAlcohol
The invention discloses a preparation method of a strontium and barium activated lithium iron phosphate positive pole material. The preparation method is characterized by comprising the following steps: mixing a lithium source, an iron source, a phosphate radical source, and a strontium source utilized as a raw material according to the ratio of 1:(0.00002-0.00005):(0.0003-0.003):1:1; in an absolute ethyl alcohol medium, carrying out high-speed ball-milling for 20 hours at the rotation rate of 200r / mimn and drying at the temperature of 105-120 DEG C, thereby obtaining a front body, and placing the drying obtained front body in a high temperature furnace; and carrying out high-temperature calcination for 24 hours at 500-750 DEG C in a common pure nitrogen atmosphere on to obtain the strontium, barium activating lithium iron phosphate positive pole material. According to the invention, strontium and the barium are replaced because of the doping, the feature and the particle diameter of a product are effectively controlled, thereby obtaining a stable lithium iron phosphate compound, the crystal lattice of the lithium iron phosphate compound is activated, the lithium ion diffusion coefficient is improved, and the loading capacity achieves 160.52 m A h / g in the first time; a charge-discharge platform relative to lithium electrode potential is around 3.5V, the initial discharge capacity exceeds 168 m A h / g, and the capacity decays around 1.2% after the charging and discharging are circled for100 times; compared with the unmixed LiFePO4 contrast embodiment, the specific capacity and the cyclical stability are greatly improved.
Owner:桐乡乐维新材料有限公司
Preparation method of selenium and barium activated lithium iron phosphate anode materials
ActiveCN102386406BImprove conductivityShape is easy to controlCell electrodesElectrode potentialO-Phosphoric Acid
The invention relates to a preparation method of selenium and barium activated lithium iron phosphate anode materials, which includes mixing raw materials of a lithium source, an iron source, a phosphoric acid root source, a selenium source and a barium source according to the following ratio by mole: 1 part of lithium, 0.00002 to 0.00005 part of selenium, 0.0003 to 0.003 part of barium, 1 part of iron and 1 part of phosphoric acid root, milling the raw materials in an absolute ethyl alcohol medium for 20 hours through a high-speed ball with 200r / mimn rotating speed, drying the raw materials under the temperature between 105 DEG C and 120 DEG C to obtain a precursor, placing the precursor obtained by drying in a high-temperature furnace and conducting high-temperature calcination on the precursor under the temperature between 500 DEG C and 750 DEG C for 24 hours in an atmosphere of common pure nitrogen to obtain the niobium and barium activated lithium iron phosphate anode materials. Due to the fact that a small quantity of selenium and barium is mingled for replacement, appearance and particle diameter of products can be favorably controlled to obtain stable lithium iron phosphate compound. Lattice of the products is activated, diffusion coefficient of lithium ions is improved, and first discharging capacity of obtained materials reaches 160.52mAh / g. Electrode potential of a charging-discharging platform is about 3.5V relative to lithium, initial discharging capacity surpasses 168mAh / g, and the capacity attenuates by about 1.2% after 100 times of charging-discharging circulation. Compared with unmingled LiFePO4 contrast embodiment, specific capacity and circulation stability are greatly improved.
Owner:桐乡乐维新材料有限公司
Beryllium-barium activated lithium iron phosphate cathode material
InactiveCN102386398AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphatePhysical chemistry
The invention provides a beryllium-barium activated lithium iron phosphate cathode material which has a general chemical formula of LiBexBay FePO4, wherein, x is in a range of 0.00002 to 0.00005, y is in a range of 0.0003 to 0.003, and the mol ratio of Li, Be, Ba, Fe and P satisfies the following equation: 1 mol Li: 0.000020-0.00005 mol Be: 0.0003-0.003 mol Ba: 1 mol Fe: 1 mol P. Doping of a small amount of substituting beryllium and barium is favorable for controlling the morphology and particle size of a product and for obtaining a stable lithium iron phosphate compound, crystal lattice of the cathode material is activated, the diffusion coefficient of lithium ions is improved, and the initial discharge capacity of the cathode material is up to 160.52 mAh; the charge and discharge platform of the cathode material has a potential of about 3.5 V relative to a lithium electrode and has an initial discharge capacity of more than 168 mAh / g, and the capacity of the charge and discharge platform attenuates about 1.2% after 100 cycles of charge and discharge; compared to undoped LiFePO4 in contrast embodiments, the doped lithium iron phosphate cathode material in the invention has greatly improved specific capacity and cycling stability.
Owner:李安平
Method for preparing vanadium and barium activated lithium iron phosphate anode material
ActiveCN102361083AImprove conductivityShape is easy to controlCell electrodesAlcoholLithium iron phosphate
The invention relates to a method for preparing a vanadium and barium activated lithium iron phosphate anode material, which comprises the steps of mixing lithium source raw materials, iron source raw materials, phosphate radical source raw materials, vanadium source raw materials and barium source raw materials in the proportion of 1mol Li: 0.00002-0.00005mol V: 0.0003-0.003mol Ba: 1mol Fe: 1molP prior to ball milling in an absolute ethyl alcohol medium at a high revolution speed of 200r / min for 20h, obtaining a precursor after drying at the temperature ranging from 105 DEG C to 120 DEG C, and placing the obtained precursor by means of drying into a high-temperature furnace prior to being calcinated in an ordinary pure nitrogen atmosphere at the high temperature ranging from 500 DEG C to 750 DEG C for 24h, so that the vanadium and barium activated lithium iron phosphate anode material is obtained. By the aid of a small amount of substituted vanadium and barium which are doped, the shape and the grain size of a product can be controlled beneficially, and a stable lithium iron phosphate compound is obtained, so that crystal lattices of the stable lithium iron phosphate compound are activated, lithium-ion diffusion coefficient is increased, the first discharge capacity is as high as 160.52mAh / g, a charge-discharge platform is about 3.5V relative to a lithium electrode potential, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2% after one hundred times of cycle. Compared with an embodiment of undoped LiFePO4, the vanadium and barium activated lithium iron phosphate anode material is higher in specific capacity and cyclical stability.
Owner:桐乡乐维新材料有限公司
Method for preparing zinc and barium activated lithium iron phosphate cathode material
ActiveCN102509793BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium iron phosphatePhysical chemistry
The invention relates to a method for preparing a zinc and barium activated lithium iron phosphate cathode material. Raw materials for preparing the zinc and barium activated lithium iron phosphate cathode material include lithium sources, ferrum sources, phosphate radical sources, zinc sources and barium sources, all the raw materials are mixed according to a proportion of 1 mol Li : 0.00002-0.00005 mol Zn : 0.0003-0.003 mol Ba : 1 mol Fe : 1 mol P, performed with ball milling at high speed with rotation speed at 200 r / min in absolute ethyl alcohol media, dried at 105-120 DEG C to obtain a precursor, and the precursor obtained through drying is placed in a high temperature furnace to be roasted for 24 hours at 500-750 DEG C high temperature in nitrogen atmosphere so as to obtain the zinc and barium activated lithium iron phosphate cathode material. Due to the fact that a small amount of doping replaces zinc and barium, control on shape and particle size of products is facilitated, stable lithium iron phosphate compound can be obtained, crystal lattice of the lithium iron phosphate compound is activated, lithium-ion diffusion coefficient is enlarged, and first discharge capacity of obtained materials reaches 160.52 mAh / g. A charge-discharge platform of the zinc and barium activated lithium iron phosphate cathode material is about 3.5 V relative to electric potential of lithium electrodes, first discharge capacity of the charge-discharge platform surpasses 168 mAh / g, and capacity of the charge-discharge platform is reduced to about 1.2% after 100 times of charge-discharge circulation. Compared with a lithium iron phosphate (LiFePO4) comparison embodiment without doping, the zinc and barium activated lithium iron phosphate cathode material is greatly improved in both specific capacity and cycle stability.
Owner:桐乡乐维新材料有限公司
Copper and barium activated lithium iron phosphate cathode material
InactiveCN102361082AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphatePhysical chemistry
The invention discloses a copper and barium activated lithium iron phosphate cathode material. The chemical general formula of the material is LiCuxBayFePO4, wherein x is between 0.00002 and 0.00005; Y is between 0.0003 and 0.003; and the molar ratio of Li to Cu to Ba to Fe to P is 1:0.00002-0.00005:0.0003-0.003:1:1. A small amount of substitutional copper and a small amount of barium are doped, so the control of the morphology and particle size of a product is facilitated to obtain a stable lithium iron phosphate compound; crystal lattices of the material are activated, the lithium ion diffusion coefficient is improved, and the first discharge capacity of the material is 160.52mAh / g; the relative lithium electrode potential of a charge and discharge plateau of the material is about 3.5V, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2 percent after charge and discharge cycle for 100 times; and compared with an undoped LiFePO4 contrast embodiment, the invention has the advantages that: the specific capacity and cycle stability are greatly improved.
Owner:张爱萍
Method for enhancing compactness of BaMnO4 material
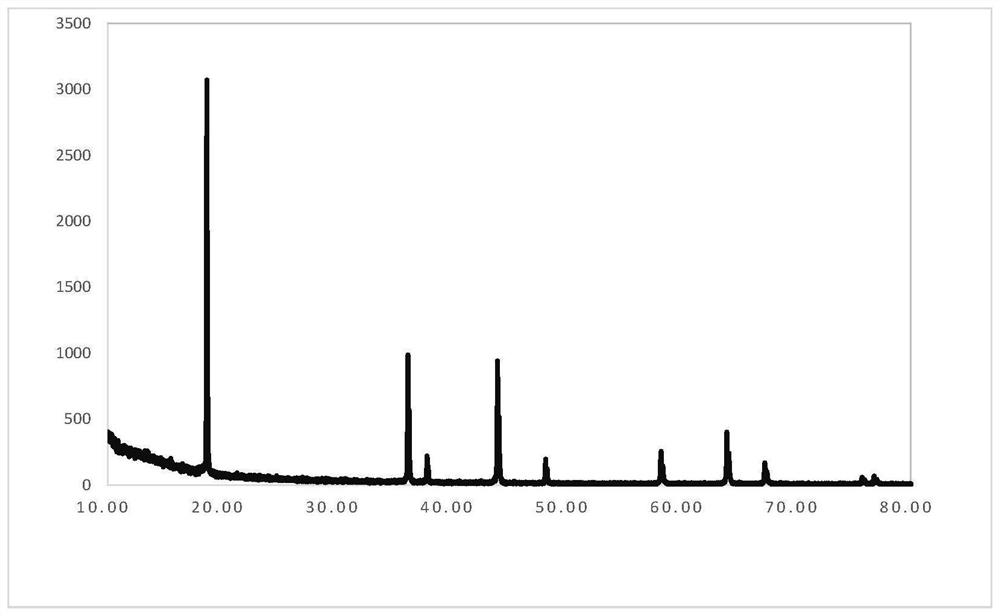
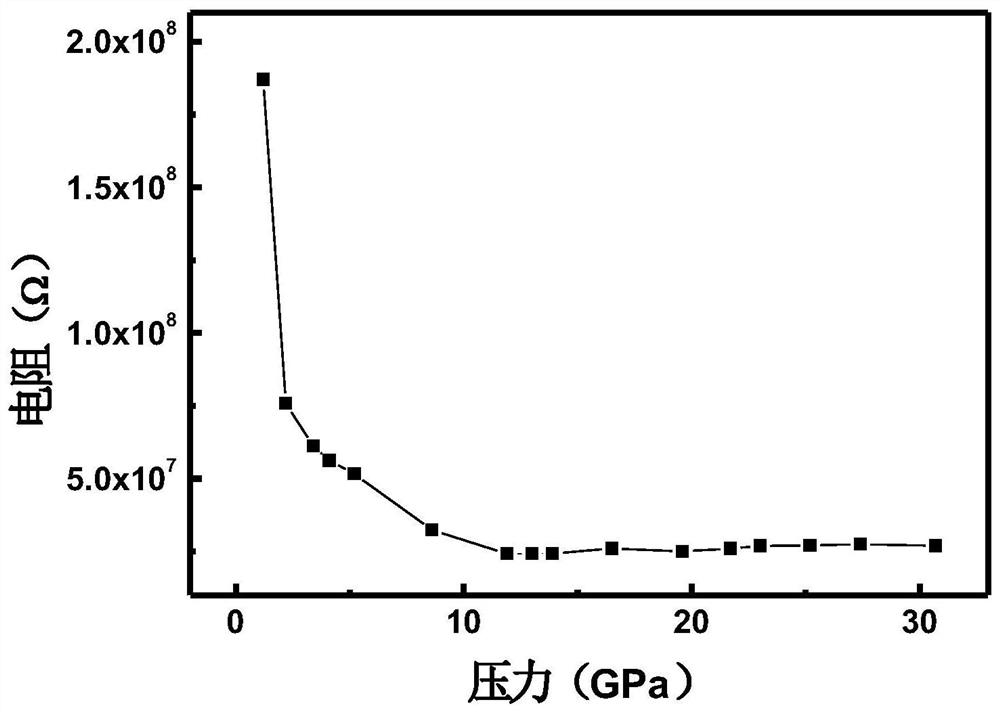
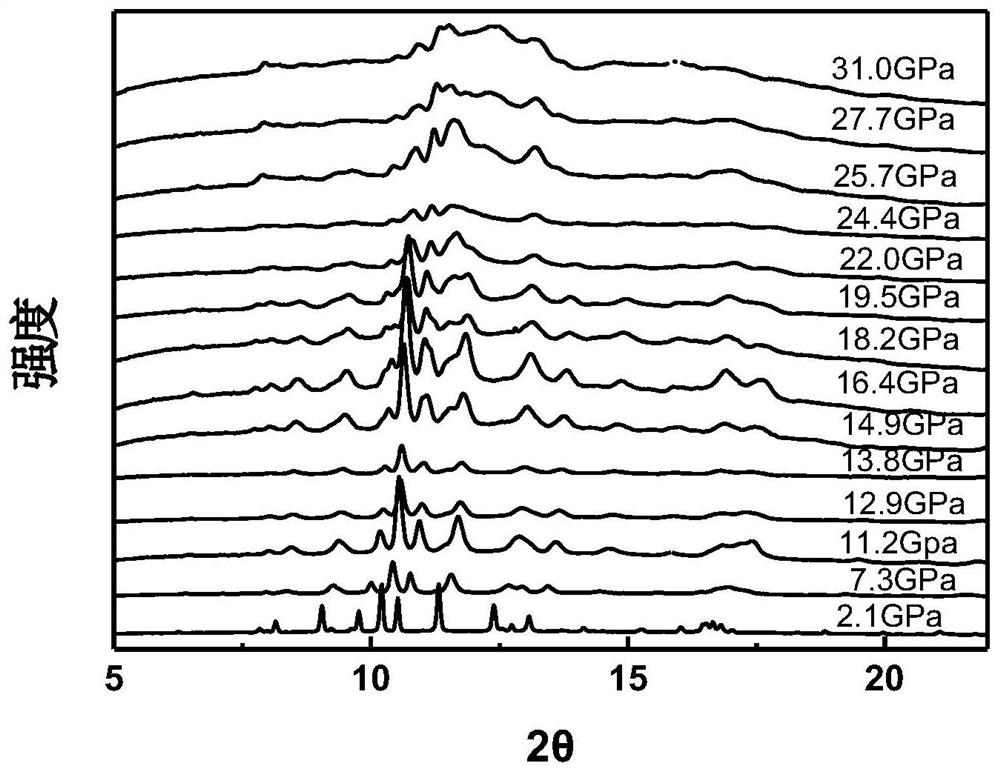
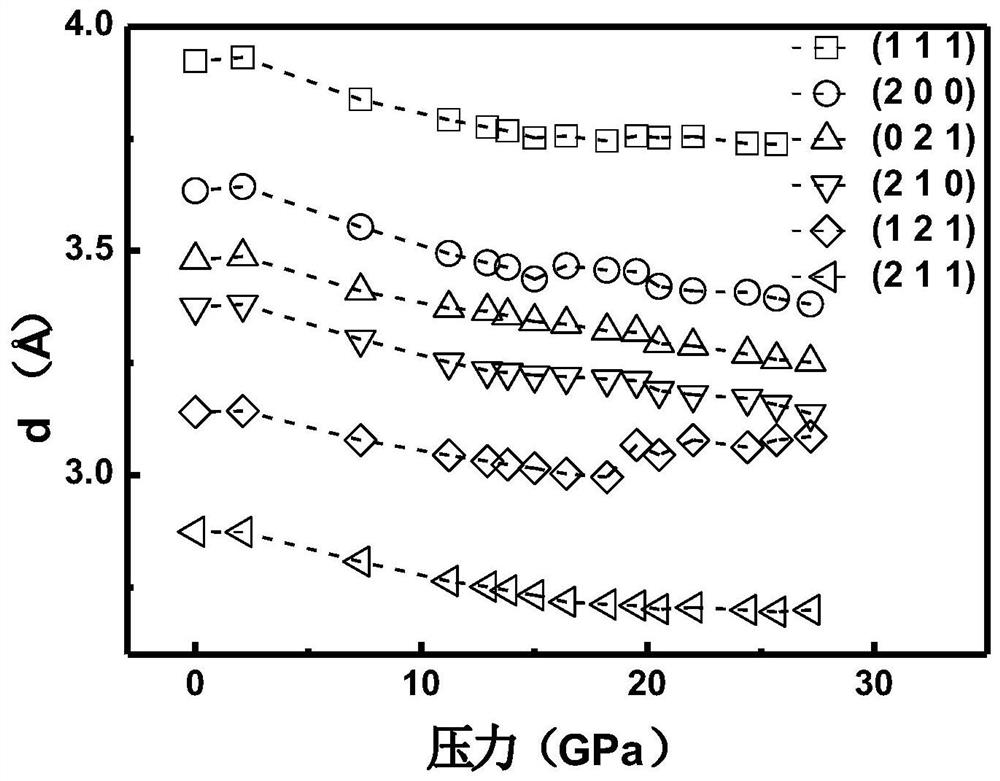
InactiveCN111994961AUnit cell volume reductionImprove densification performanceManganates/permanganatesFluid pressure measurement by optical meansEngineeringHigh pressure
The invention discloses a method for enhancing the structural compactness of a BaMnO4 material, and belongs to the technical field of high-pressure regulation and control of material compactness. 1.2-31 GPa of pressure is applied to a BaMnO4 sample in a diamond anvil cell under the condition of room temperature to obtain the BaMnO4 material with enhanced compactness. According to the preparation method disclosed by the invention, the cell volume of BaMnO4 can be reduced to 304.85 angstroms to the maximum extent, so that the material compactness of the BaMnO4 is effectively improved. The methodprovided by the invention is simple to operate and good in repeatability, and provides a new direction for application of BaMnO4 in the technical fields of sewage treatment and novel positive electrode active materials.
Owner:JILIN UNIV
Calcium and barium activated lithium iron phosphate positive pole material
InactiveCN102354753AShape is easy to controlGood for particle size controlCell electrodesLithium iron phosphateCharge discharge
The invention discloses a calcium and barium activated lithium iron phosphate positive pole material. The general formula of the positive pole material is LiCaxFeyPO4, wherein x=0.002-0.005, y=0.0003-0.003, the mol ratio of Li, Ca, Ba, Fe and P is 1.0:(0.002-0.005):(0.0003-0.003):1:1; as the doping of a small amount of Ca and Ba for substitution, the feature and the particle diameter of a product are effectively controlled, thereby obtaining a stable lithium iron phosphate compound, the crystal lattice of the lithium iron phosphate compound is activated, the lithium ion diffusion coefficient is improved, and the loading capacity achieves 155.52 m A h / g in the first time; a charge-discharge platform relative to lithium electrode potential is around 3.5V, the initial discharge capacity exceeds 164 m A h / g, and the capacity decays around 3.0% after charging and discharging are circled for 100 times; compared with the unmixed LiFePO4 comparing embodiment, the specific capacity and the cyclical stability are greatly improved; and as the price of Ba is over hundreds of times lower than that of Li, the manufacturing cost can be reduced by more than 10 times.
Owner:张雅静
Preparation method of fluorine and yttrium-doped lithium ferrous silicate composite material for lithium-ion battery
InactiveCN108063227AIncreased diffusion rateExtended service lifeCell electrodesSecondary cellsPorous grapheneTube furnace
The invention discloses a preparation method of a fluorine and yttrium-doped lithium ferrous silicate composite material for a lithium-ion battery. The method comprises the following steps of (1) preparing a fluorine and yttrium-doped lithium iron silicate precursor; (2) preparing porous graphene; and (3) mechanically mixing the porous graphene with the precursor, mixing evenly in a ball milling manner and then burning in a tube furnace in helium atmosphere to obtain porous graphene-coated fluorine-doped lithium ferrous silicate. According to the prepared lithium ferrous silicate composite material for the lithium-ion battery, lithium ferrous silicate is modified by adopting fluorine and a rare-earth material yttrium, so that the cycling stability of the material is improved; and sinteringcoating is also carried out on the fluorine and yttrium-doped lithium ferrous silicate by adopting the porous graphene, so that the conductivity of the material is further improved. Therefore, when the composite material is applied to the lithium-ion battery, the lithium-ion battery has high specific capacity and relatively long service life.
Owner:宁波高新区锦众信息科技有限公司
Preparation method of germanium and barium activated lithium iron phosphate as cathode material
InactiveCN102437328AImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsCharge dischargeLithium electrode
The invention discloses a preparation method of germanium and barium activated lithium iron phosphate as a cathode material. The preparation method comprises the steps of: mixing raw materials including a lithium source, a germanium source, a barium source, an iron source and a phosphate source according to the proportion of 1mol:(0.00002-0.00005)mol:(0.0003-0.003)mol:1mol:1mol; then, performing high-speed ball milling at the rotation speed of 200r / minm for 20h in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; placing the precursor obtained through drying in a high-temperature furnace; and calcinating for 24h at the high temperature of 500-750 DEG C in a nitrogen atmosphere to obtain the germanium and barium activated lithium iron phosphate as the cathode material. Because a small amount of substitutive germanium and barium are doped, the shape and the particle size of a product are favorably controlled to obtain a stable lithium iron phosphate compound, of which the crystal lattice is activated, the lithium ion diffusion coefficient is increased, and the initial discharge capacity of the obtained cathode material reaches 160.52mAh / g; a charge-discharge platform of the cathode material is about 3.5V relative to a lithium electrode potential, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2% after 100times of charge-discharge cycles; and compared with a control embodiment, namely undoped LiFePO4, the cathode material is greatly improved in specific capacity and cyclic stability.
Owner:浙江远志新材料有限公司
Boron and barium activated lithium iron phosphate anode material
InactiveCN102354754AImprove conductivityShape is easy to controlCell electrodesLithium iron phosphateNon doped
The invention discloses a boron and barium activated lithium iron phosphate anode material. The chemical general formula of the boron and barium activated lithium iron phosphate anode material can be expressed as LiBxBayFePO4, wherein x is equal to 0.00002-0.00005, y is equal to 0.0003-0.003, and the mol ratio of Li, B, Ba, Fe and P is 1:0.00002-0.00005:0.0003-0.003:1:1. Because a small quantity of replaced boron and barium is doped, the feature and the diameter diameter of a product are contributed to controlling to obtain stable lithium iron phosphate compound. The crystal lattice of the boron and barium activated lithium iron phosphate anode material is activated, a lithium ion diffusion coefficient is improved, and the first discharging volume of the anode materials reaches 160.52mAh / g; the voltage of the charging and discharging platform of the anode material is 3.5V relative to the potential of the lithium electrode, an initial discharging volume exceeds 168mAh / g, and volume is attenuated by about 1.2% after charging and discharging circulation for 100 times; and compared with an non-doped LiFePO4 contrast embodiment, the specific capacity and the cyclical stability are greatly improved.
Owner:张爱萍
Method for preparing antimony and barium activated lithium iron phosphate cathode material
InactiveCN102364733BImprove conductivityShape is easy to controlCell electrodesPhosphatePhosphoric acid
The invention discloses a method for preparing an antimony and barium activated lithium iron phosphate cathode material. The method comprises the following steps of: mixing a lithium source, an iron source, a phosphate group source, an antimony source and a barium source which serve as raw materials according to the ratio of Li to Sb to Ba to Fe to P of 1:(0.00002-0.00005):(0.0003-0.003):1:1, performing high-speed ball milling in an absolute ethanol medium at the rotating speed of 200r / min for 20 hours, drying at the temperature of between 105 and 120 DEG C, and thus obtaining a precursor; and putting the dried precursor into a high temperature furnace, calcining in a nitrogen atmosphere at the temperature of between 500 and 750 DEG C for 24 hours, and thus obtaining the antimony and barium activated lithium iron phosphate cathode material. A small amount of antimony and barium is doped, so that control over the appearance and the particle size of a product is facilitated, a stable lithium iron phosphate compound is obtained, the crystal lattice of the material is activated, a lithium ion diffusion coefficient is improved, and the material has the initial discharging capacity of 160.52mAh / g; a charging / discharging platform of the material has a relative lithium electrode potential of about 3.5V, and material has the initial discharging capacity of more than 168mAh / g and has the capacity which is reduced by about 1.2 percent after 100-time charging / discharging operation; and compared with an undoped LiFePO4 comparison embodiment, the method has the advantage that the specific capacity and the circulating stability of the material are greatly improved.
Owner:浙江远志新材料有限公司
Preparation method for magnesium/barium-activated lithium iron phosphate cathode material
ActiveCN102386403BShape is easy to controlIncrease the diffusion coefficientCell electrodesLithium iron phosphatePhosphate
The invention discloses a preparation method for a magnesium / barium-activated lithium iron phosphate cathode material. The preparation method is characterized by comprising the following steps of: mixing raw materials which are a lithium source, an iron source, a phosphate group source, a magnesium source and a barium source in the molar ratio of 1:(0.002-0.005):(0.0003-0.003):1:1, ball-milling the mixture for 20h at the high rotating speed of 200r / mimn in an absolute ethanol medium, drying the mixture at 105 to 120 DEG C to obtain a precursor, arranging the dried precursor in a high-temperature furnace, and sintering the precursor for 24h at the high temperature of 500 to 750 DEG C to obtain the magnesium / barium-activated lithium iron phosphate cathode material. Due to the doping of a small amount of substitutional magnesium / barium, so control over the appearance and grain size of the product is facilitated, a stable lithium iron phosphate compound is obtained, the crystal lattice of the compound is activated, a lithium ion diffusion coefficient is increased and the first discharging capacity of a battery adopting the obtained material reaches 155.52mAh / g. The potential of a charging and discharging platform relative to a lithium electrode is about 3.5V, the initial discharging capacity of the battery exceeds 164mAh / g, and the capacity of the battery is attenuated by about 3.0 percent after 100 charging and discharging cycles. Compared with those of the control embodiment of undoped LiFePO4, the material prepared by the method has the advantages that: specific capacity and cyclical stability are relatively more improved. The cost of barium is a hundred times lower than that of lithium, so production cost can be decreased by over ten times.
Owner:桐乡乐维新材料有限公司
Preparation method of bismuth and barium activated lithium iron phosphate as cathode material
ActiveCN102437333BImprove conductivityShape is easy to controlCell electrodesPhosphorus compoundsLithium iron phosphateAlcohol
The invention discloses a preparation method of bismuth and barium activated lithium iron phosphate as a cathode material. The preparation method comprises the steps of: mixing raw materials including a lithium source, a bismuth source, a barium source, an iron source and a phosphate source according to the proportion of 1mol:(0.00002-0.00005)mol:(0.0003-0.003)mol:1mol:1mol; then, performing high-speed ball milling at the rotation speed of 200r / minm for 20h in an absolute ethyl alcohol medium; drying at 105-120 DEG C to obtain a precursor; placing the precursor obtained through drying in a high-temperature furnace; and calcinating for 24h at the high temperature of 500-750 DEG C in a common pure nitrogen atmosphere to obtain the bismuth and barium activated lithium iron phosphate as the cathode material. Because a small amount of substitutive bismuth and barium are doped, the shape and the particle size of a product are favorably controlled to obtain a stable lithium iron phosphate compound, of which the crystal lattice is activated, the lithium ion diffusion coefficient is increased, and the initial discharge capacity of the obtained cathode material reaches 160.52mAh / g; a charge-discharge platform of the cathode material is about 3.5V relative to a lithium electrode potential, the initial discharge capacity exceeds 168mAh / g, and the capacity is attenuated by about 1.2% after 100 times of charge-discharge cycles; and compared with a control embodiment, namely undoped LiFePO4, the cathode material is greatly improved in specific capacity and cyclic stability.
Owner:桐乡乐维新材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com