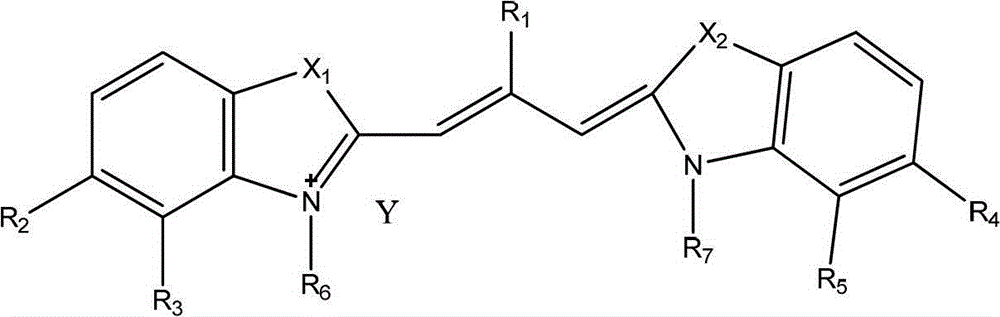
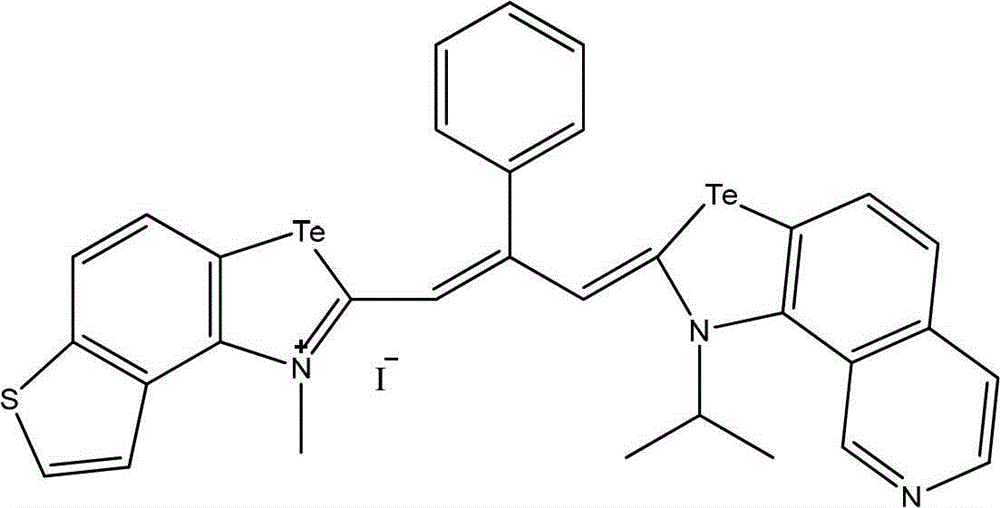
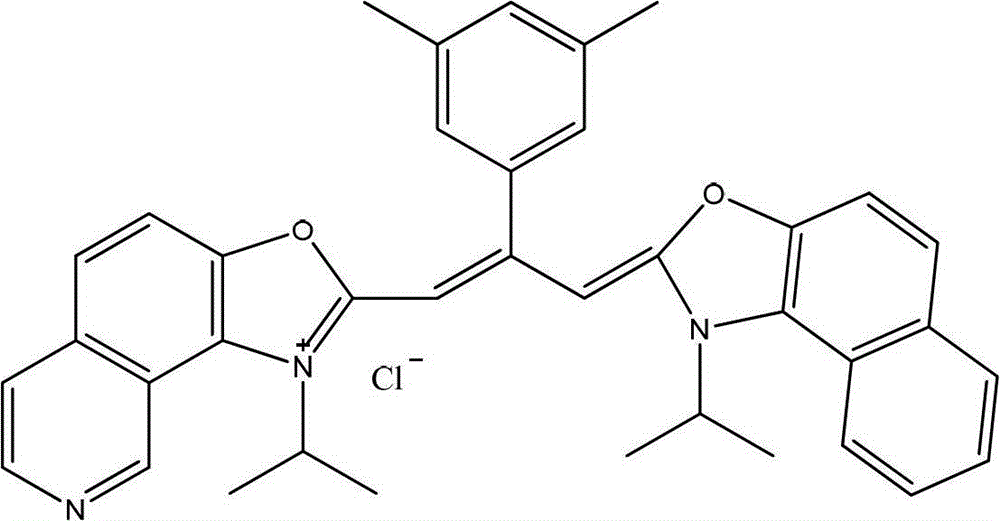
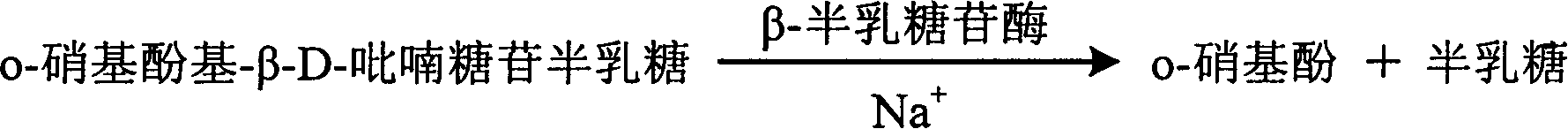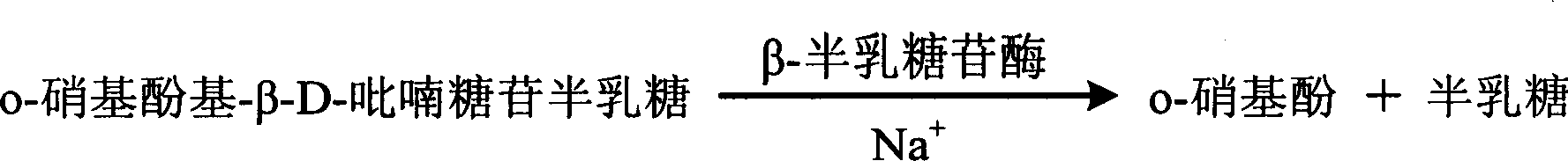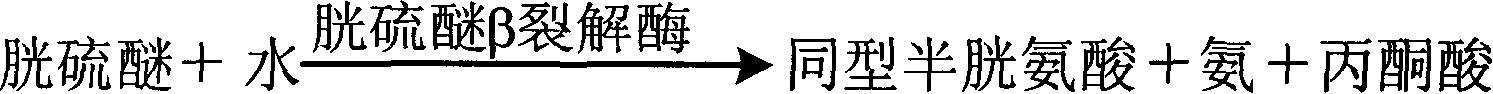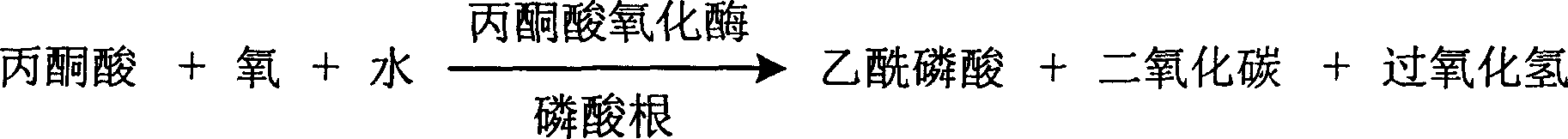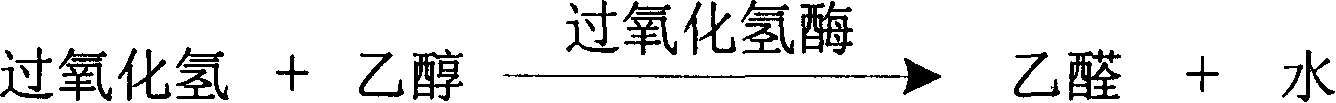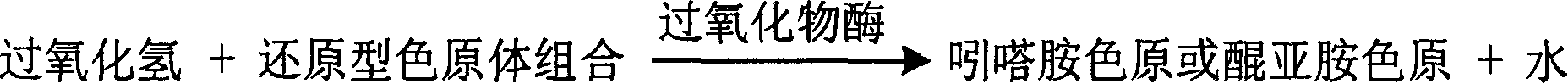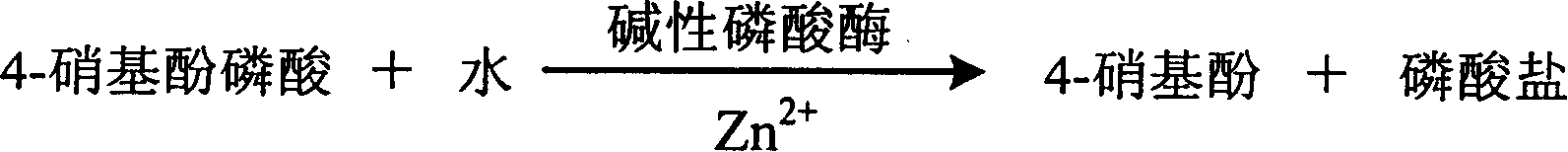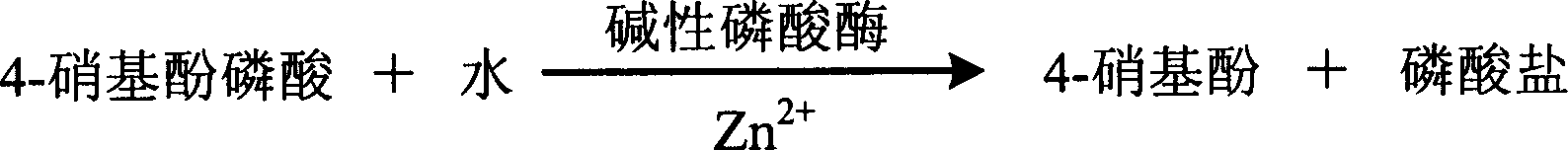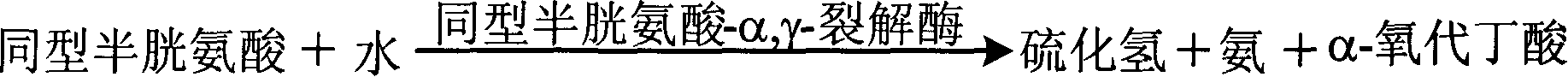Patents
Literature
45results about How to "Guaranteed application test results" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
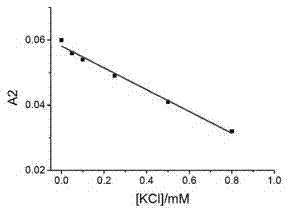
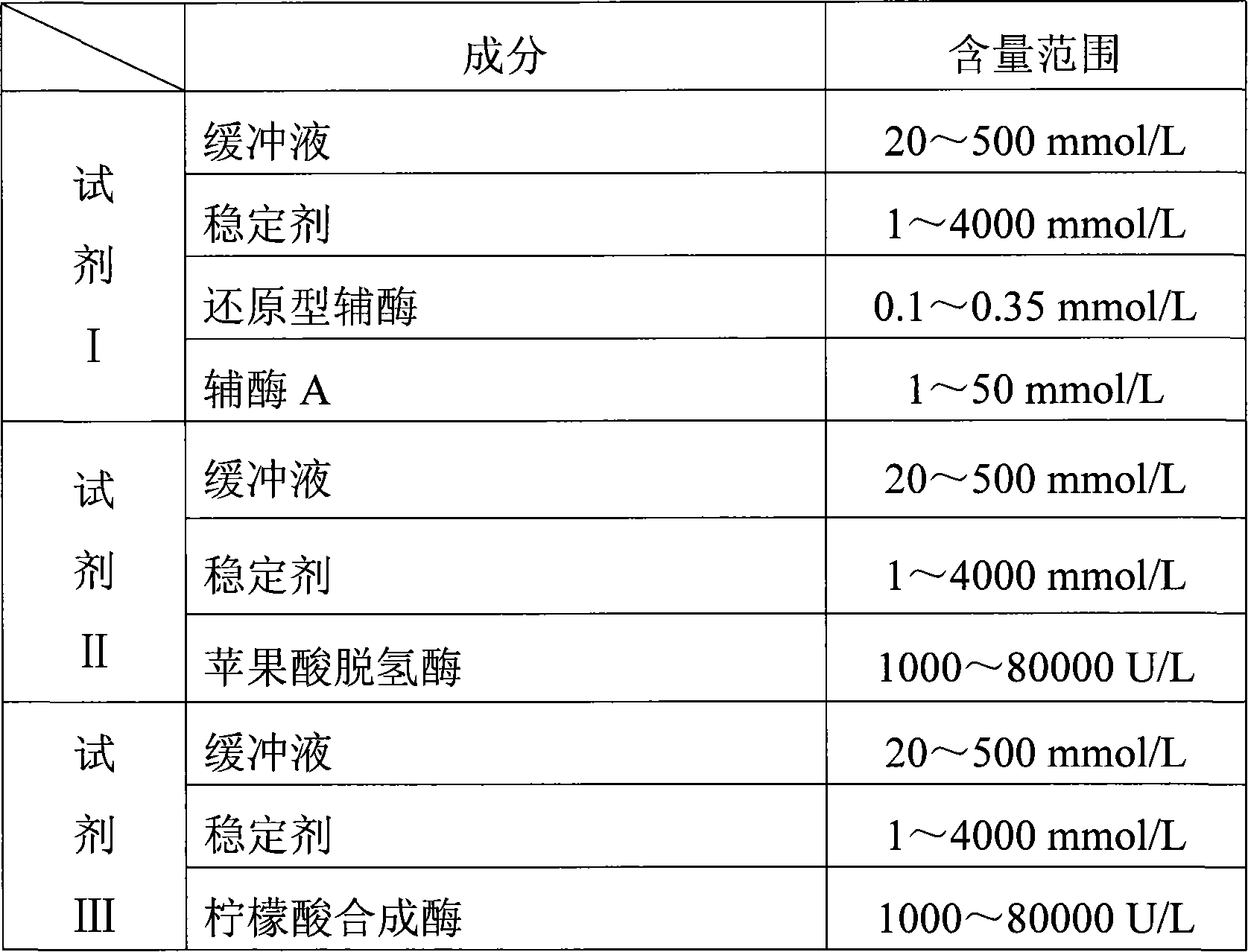
Potassium ion concentration detection method
ActiveCN102866148AStrong specificityRealize visual detectionMethine/polymethine dyesMaterial analysis by observing effect on chemical indicatorCyaninePotassium ions
The invention relates to a potassium ion concentration detection method. The characteristic for forming the conversion of a G-four chain deoxyribonucleic acid (DNA) structure by controlling potassium ions and the characteristic for recognizing the conversion of the G-four structure through cyanine dye supramolecular aggregate are utilized, a sample to be tested is placed into DNA and cyanine dye mixed solution capable of forming the G-four chain, and the concentration range of the potassium ions in the sample can be semiquantitatively judged according to the color of the solution. Due to adopting the method, the visible detection of the potassium ion concentration can be realized, and detection test paper can be developed. A reagent is simple in ingredients, the reaction process is simple, no special or additional instrument is needed, the detection cost is low, and the method is convenient for being popularized to use in the industry.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Potassium ion concentration detection method
ActiveCN102735664AStrong specificityRealize visual detectionMethine/polymethine dyesMaterial analysis by observing effect on chemical indicatorCyaninePotassium ions
The invention relates to a potassium ion concentration detection method. According to the method, characteristics comprising that potassium ion regulation allows G-quadruplex DNA structure transformation to be formed and cyanine dye supermolecular aggregates identify the G-quadruplex DNA structure transformation are utilized, a sample to be detected is added to a mixed solution of G-quadruplex DNA and the cyanine dye, the absorbance value at 560-590nm, 500-540nm or 610-670nm or the fluorescence intensity value at 580-640nm is determined, and the corresponding potassium ion concentration value can be obtained through finding the value corresponding to the absorbance value or the fluorescence intensity value on a standard curve. The method has a high specificity, so the method is not affected by sodium ions in the sample; and reagent components is simple, and the reaction process is simple, so errors generated by operations can be effectively reduced, and the test accuracy is high. The method can be rapidly realized through a common ultraviolet-visible absorption detector, a spectrophotometer or a fluorescent spectrometer without special or extra instruments, so the detection cost is low, thereby the popularization and the application of the method in industries are convenient.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Potassium ion concentration detection kit and system thereof
ActiveCN102735623AStrong specificityRealize visual detectionMethine/polymethine dyesMaterial analysis by observing effect on chemical indicatorPotassium ionsReagent
The invention relates to a potassium ion concentration detection kit and a system thereof. The kit and the system, which utilize characteristics comprising that potassium ion regulation allows G-quadruplex DNA structure transformation to be formed and cyanine dye supermolecular aggregates identify the G-quadruplex DNA structure transformation, have high specificities, and are not affected by sodium ions in a sample. Reagent components are simple and the reaction process is simple, so errors generated by operations can be effectively reduced, and the test accuracy is high. The kit and the system of the invention allow rapid detection to be realized without special or extra apparatuses, and the detection cost is low, so the popularization and the application of the kit and the system are convenient.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Determination method of proangiotension transferase activity and proangiotension transferase diagnosis kit
InactiveCN1786185AActive responseReflect activityMicrobiological testing/measurementAngiotensin-converting enzymeBiochemistry
The invention relates to angiotensin converting enzyme activity measuring method and its diagnosis kit. It is applied artificial synthesis substrate FAPGG to directly react with angiotensin converting enzyme to form furyl-acryloyl-phenylalanine, and to measure its activity by 340nm absorbance lowering speed. The method has high specificity, good accuracy. And it can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
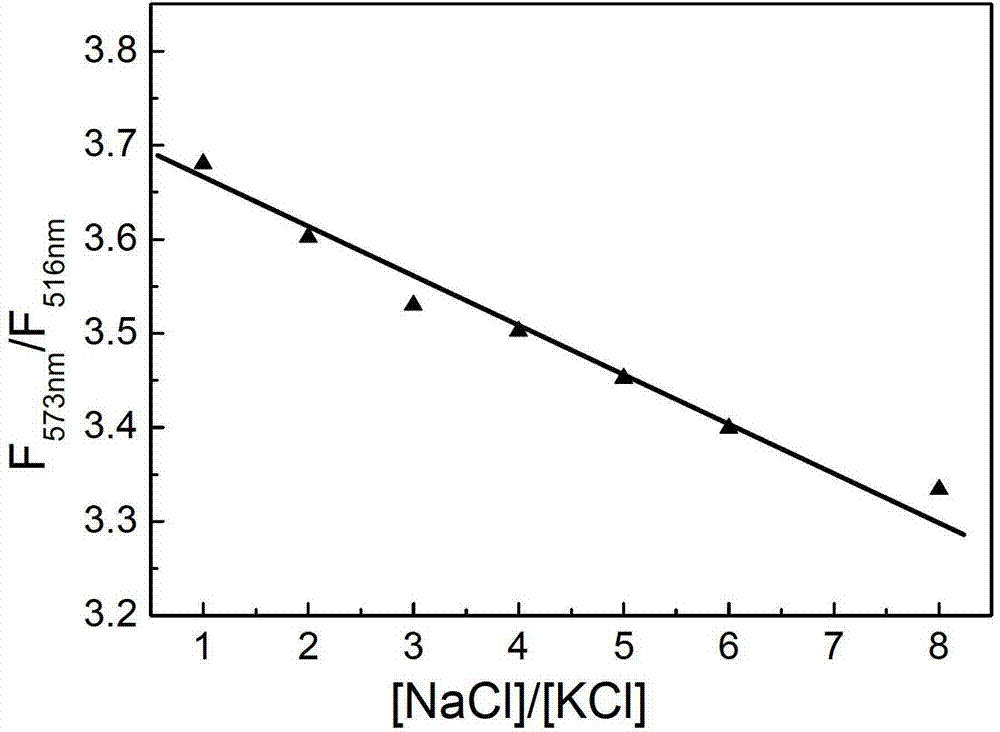
Sodium/ potassium ion ratio detecting method, system and kit
ActiveCN103048301AStrong specificityReduce testing costsFluorescence/phosphorescenceConcentration ratioChain structure
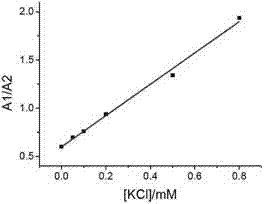
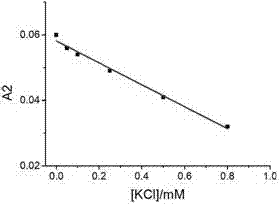
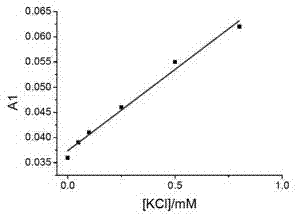
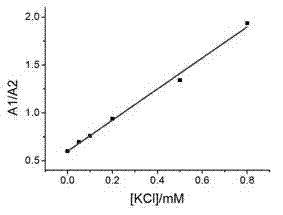
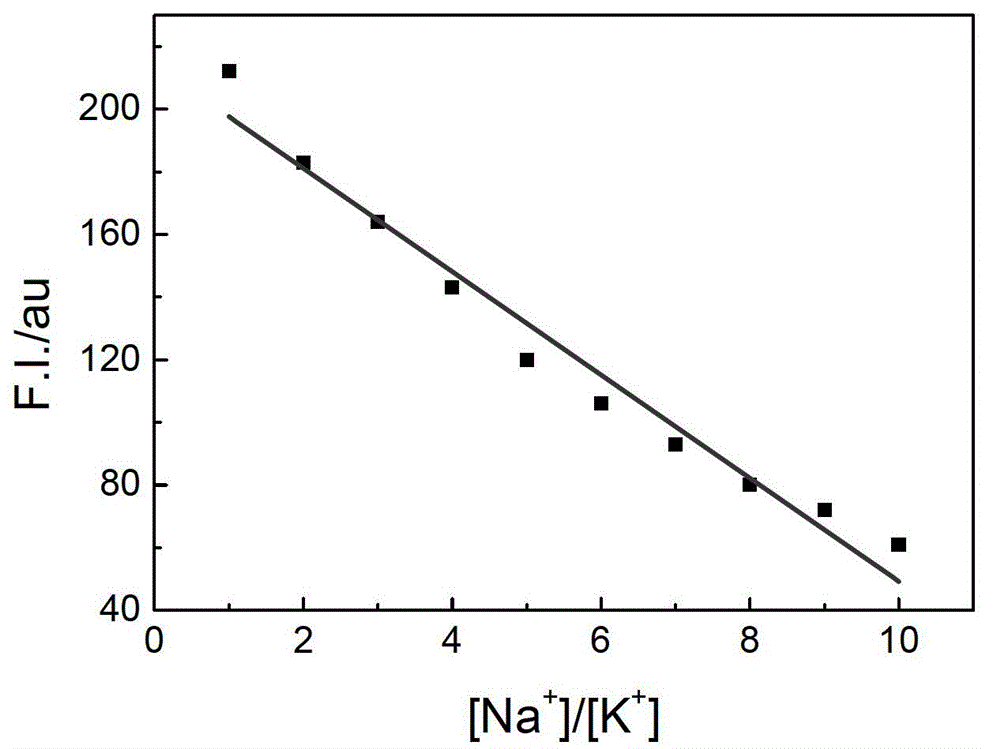
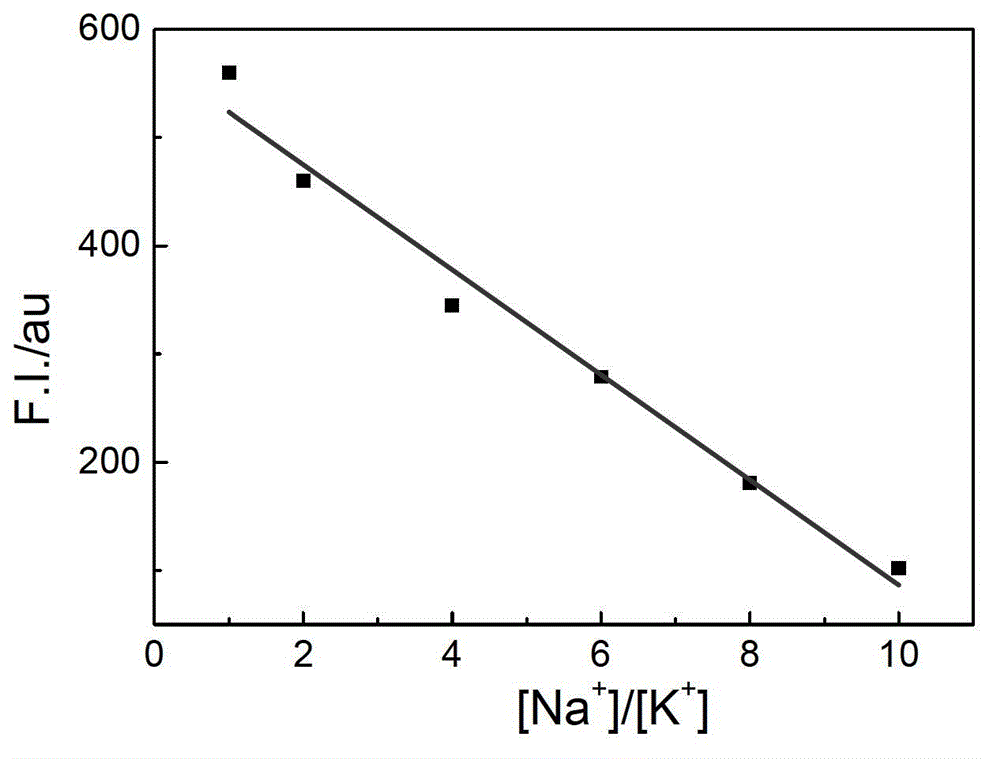
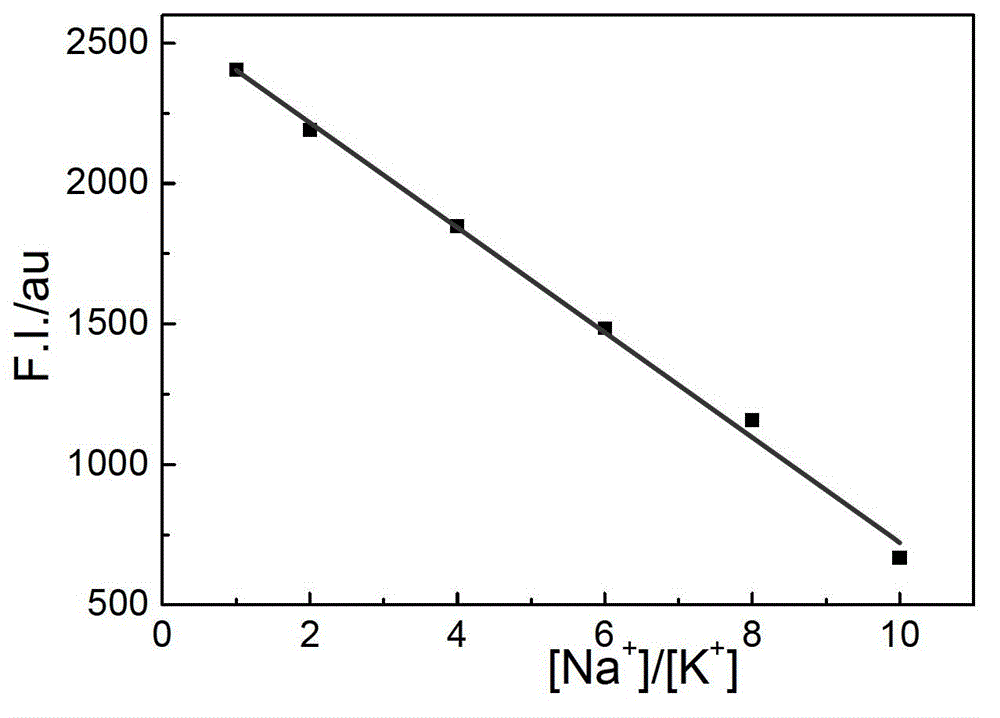
The invention relates to a sodium / potassium ion ratio detecting method, a sodium / potassium ion ratio detecting system and a sodium / potassium ion ratio detecting kit. The invention relates to a method for detecting a sodium / potassium ion ratio concentration. The method comprises the following steps: (1) preparing multiple solution samples with different sodium / potassium ion ratios; (2) putting the multiple solution samples under a fluorescence spectrophotometer, and testing fluorescence intensity values at a first wavelength and a second wavelength; (3) acquiring a standard curve of the sodium / potassium ion ratios; (4) adding DNA molecules with fluorescently-labeled groups and can form different G-four chain structures in the presence of sodium and potassium ions respectively into a liquid sample to be tested, and regulating pH value of the sample so as to obtain a test solution; (5) putting the test solution under the fluorescence spectrophotometer, and testing fluorescence intensity values at the first wavelength and the second wavelength; and (6) finding a corresponding sodium / potassium ion concentration ratio of the test solution in the standard curve of the sodium / potassium ion ratios according to a ratio of the fluorescence intensity value tested at the first wavelength to the fluorescence intensity value tested at the second wavelength in the step (5).
Owner:INST OF CHEM CHINESE ACAD OF SCI
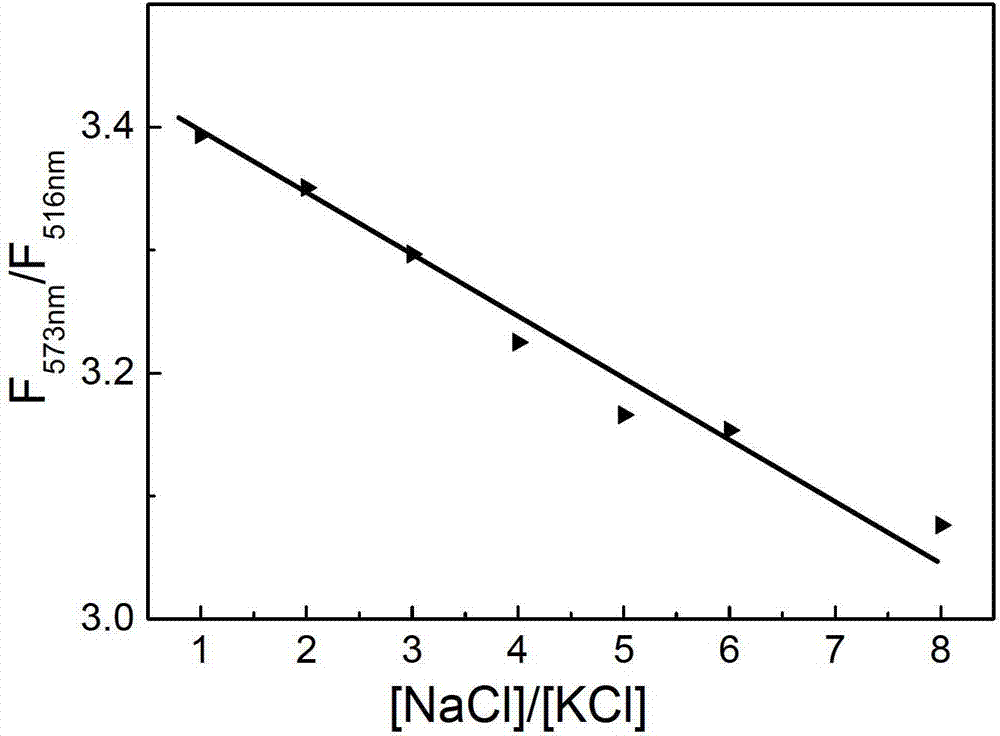
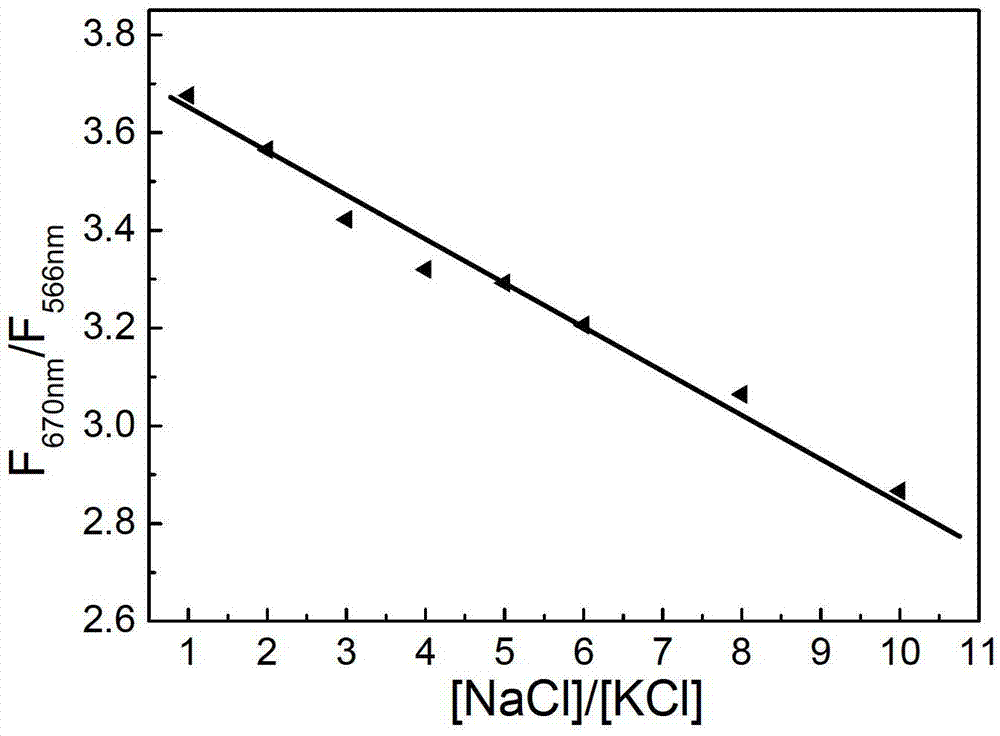
Method for detecting ratio of sodium ions to potassium ions, kit and system
ActiveCN103063629AStrong specificityRealize visual detectionMethine/polymethine dyesFluorescence/phosphorescencePotassium ionsLength wave
The invention relates to a method for detecting a ratio of sodium ions to potassium ions, a kit and a system. The invention relates to a method for detecting mol ratio of sodium ions to potassium ions in solution, comprising the following steps: (1) preparing a plurality of solution samples according to difference of ratios of sodium ions to potassium ions, wherein each solution sample contains cyanine dye with the same concentration and DNA molecules capable of respectively forming different G-quadruplex structures with the existence of sodium ions and potassium ions; (2) detecting fluorescence intensity level where wavelength is collected; (3) plotting by using the ratio of sodium ions to potassium ions in each solution sample as a horizontal ordinate or a vertical ordinate and using the fluorescence intensity level where wavelength is collected detected in the step (2) as a horizontal ordinate or a vertical ordinate so as to obtain a specification curve of ratios of sodium ions to potassium ions; (4) adding the DNA molecules in a liquid sample to be detected, and adjusting the pH value so as to obtain a test solution; (5) detecting the fluorescence intensity level of the wavelength where the wavelength is collected; and (6) finding out corresponding ratio of sodium ions to potassium ions in the test solution in the specification curve of ratios of sodium ions to potassium ions.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Enzymatic method for determining sodium ion content and sodium ion diagnosis kit
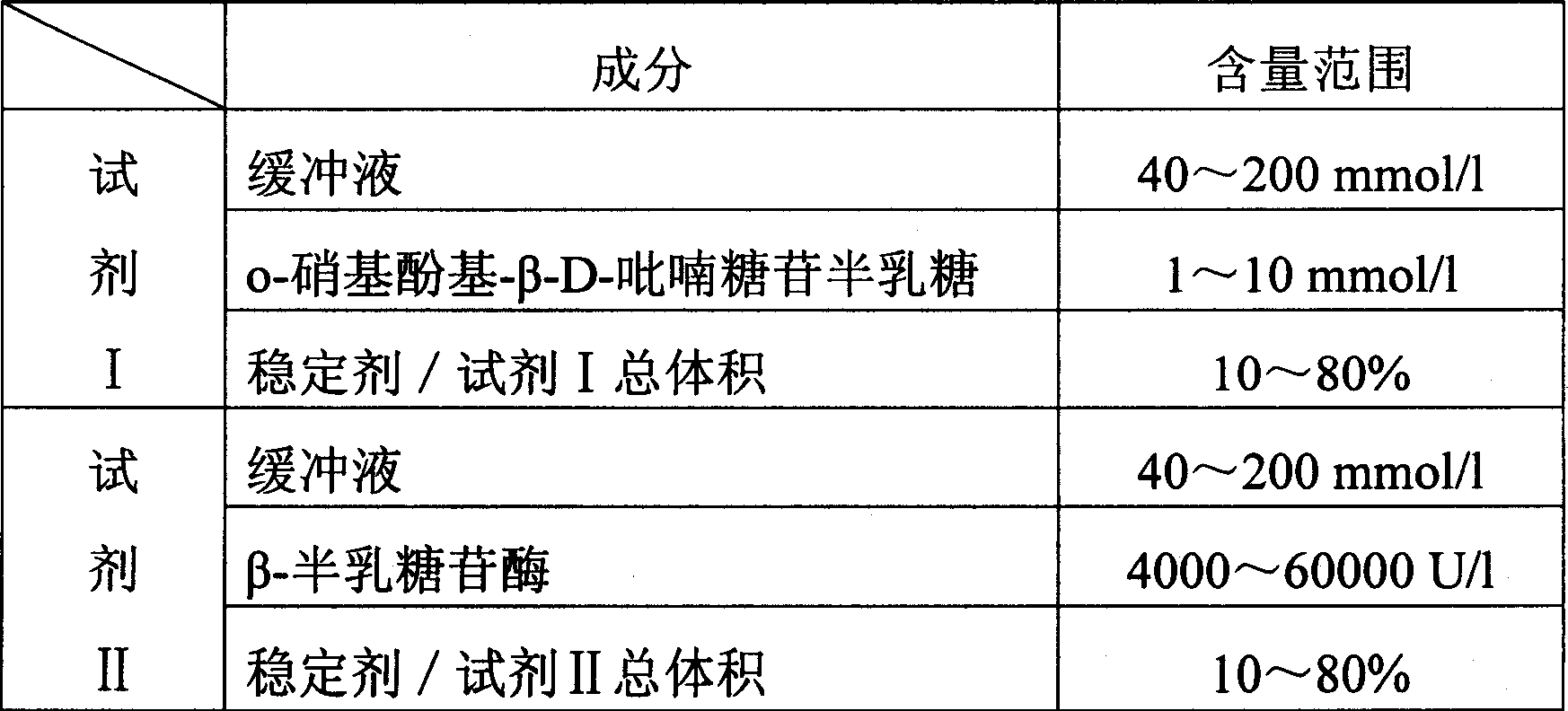
InactiveCN1786186AQuantitative reflection of contentHighlight substantive featuresMicrobiological testing/measurementIon contentPotassium
The invention relates to enzyme method measuring sodium ion content method and its diagnosis kit. It is adopted galactosidase reaction colorimetry to use sodium ion to activate beta-galactosidase enzymolysis o-nitrophenol group-beta-D- pyranoside galactose to make 405nm dominant wavelength absorbance rise. Its absorbance amplitude of fluctuation is proportional to sample sodium ion. The method has high specificity, good accuracy. The diagnosis kit can be made into bi-agent to reduce cross influence. The method can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Measuring method of homotype cysteine concentration and homotype cysteine diagnostic reagent kit
InactiveCN1912583AThe test result is accurateFree from pollutionMicrobiological testing/measurementColor/spectral properties measurementsLactate dehydrogenaseCysteine thiolate
A method for determining concentration of homotype cysteine includes synthesizing cystathionine B synthase, homotype cysteine and serine to be cystathionine; cracking cystathionine by cystathionine B lyase to change it back to be homotype cysteine and to generate ammonia and pyruvic acid; using some chemicals to act on ammonia to let reduction coenzyme be oxidized to be oxidation coenzyme for determining out absorbance dropping speed of reduction coenzyme at 340nm then estimating out concentration size of homotyp cysteine. Its diagnosis kit can be prepared in double dosages for decreasing cross influence of various compositions.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
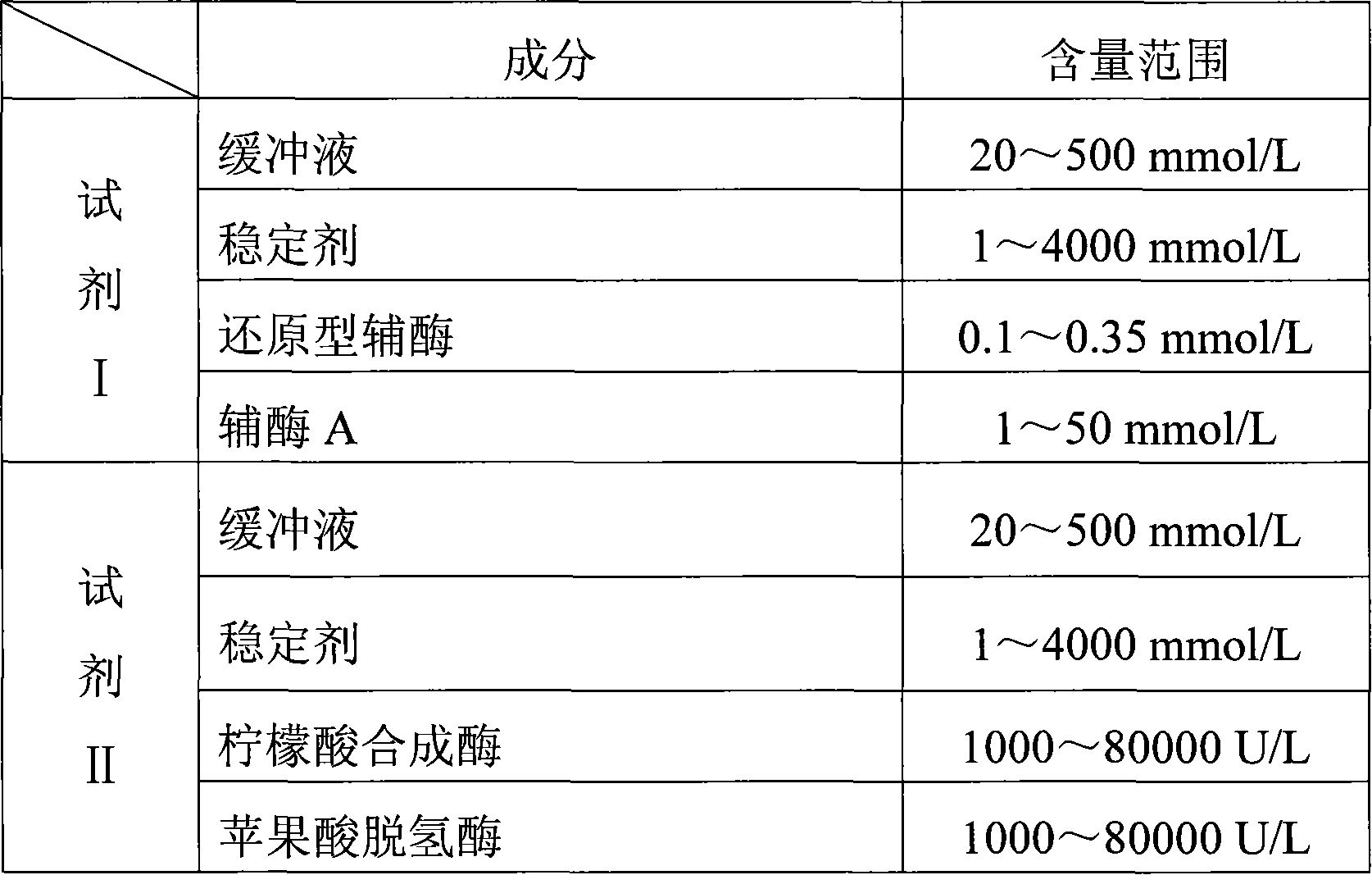
Method for measuring citric acid concentration and citric acid diagnose reagent kit
InactiveCN101082575AFree from pollutionHigh precisionMicrobiological testing/measurementColor/spectral properties measurementsCITRATE ESTERCoupling
The invention relates to a kind of enzyme colorimetric method and a method of the coupling method measuring the density of citric acid and a diagnosis reagent box applying the citrate synthetase and coupling malic dehydrogenase enzymatic reaction continues monitoring method / ratio colorimetric method. The reaction of citrate synthetase enzymolysis citric acid generates oxaloacetic acid and then through the effect of coupling malic dehydrogenase at last oxidizes the reduced coenzyme (there is a absorption peak at the site of 340nm) becoming the coenzyme (there is a absorption peak at the site of 340nm) so we can assay the degree / speed of the fall-way absorbance at the place of 340nm. It can measure and calculate the density of citric acid by measuring the degree / speed of the fall-way absorbance at the place of 340nm. The method has high specificity and it is not polluted by the endogenous and exogenous object and the test result is precise and accurate. The invention can get the array result by the ultraviolet / visible light analytic instrument so it is convenience to extend and apply.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Detection kit for potassium ion concentration
ActiveCN102866149AStrong specificityRealize visual detectionAnalysis using chemical indicatorsMethine/polymethine dyesCyaninePhysical chemistry
The invention relates to a detection kit for potassium ion concentration. The method comprises the steps of: adding a sample to be detected into a mixed solution of DNA with a G-four chain body and cyanine dye by means of the characteristic that the G-four chain body DNA structural transformation can be formed by regulating potassium ion and the characteristic that cyanine dye supermolecular aggregate identifies the G-four chain body structural transformation; and semi-quantitatively judging the potassium ion concentration range in the sample according to color of the solution. Visual detection of potassium ion concentration can be realized by using the kit which can be realized, and the detection kit can be developed as a detection strip. The kit is simple in components, simple in reaction process, low in detection cost and convenient for being popularized and applied in the industry, and special instruments or extra instruments are not needed.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Detection method, system and kit of microalbuminuria
ActiveCN103115904AStrong specificityOperation is not affectedMethine/polymethine dyesFluorescence/phosphorescencePotassium ionsLength wave
The invention relates to a detection method, a system and a kit of microalbuminuria. The method for detecting the concentration of microalbuminuria in a solution comprises the following steps of: (1) preparing a plurality of solution samples with different concentrations of microalbuminuria, wherein each solution sample contains cyanine dyes with same concentration; (2) detecting fluorescence intensity values of an acquisition wavelength part; (3) obtaining a standard curve of the microalbuminuria concentration; (4) adding the cyanine dyes and a buffer solution containing potassium ions or sodium ions in a liquid sample to be detected, so that the concentration and the pH value of the cyanine dyes in the liquid solution to be detected is consistent with those of the solution sample in the step (1), so as to obtain a test solution and record a dilution ratio of the liquid sample to be detected; (5) detecting the fluorescence intensity values of the acquisition wavelength part; and (6) finding the microalbuminuria concentration value of the corresponding test solution in the standard curve, and calculating the microalbuminuria concentration of the sample to be detected, wherein the acquisition wavelength is in the range of 550 to 650 nanometers.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Process for determining content of carbon dioxide and kit for diagnosing carbon dioxide therefor
InactiveCN1786694AHighlight substantive featuresSignificant progressAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsUltravioletAbsorbance
The invention relates to measuring method for carbon dioxide content and diagnosing kit. The invention directly uses carbon dioxide( bicarbonate radical) and pyruvic acid to produce malic acid by decarboxylation of malic dehydrogenase and oxidizes reduced coenzyme into oxidized type coenzyme, measuring the decent rate of dominant wavelength 340nm absorbance could quantitatively response the carbon dioxide content in sample, thus, the strength of bicarbonate radical could be extrapolated. The method has high specificity, has no pollution from inside and outside material, and accurate test result. The diagnose kit is made up into bi-agent or tri-agent that could reduce cross influence from each component and keeps stability of the agent that is convenient for long-term storage. The method could quickly test on commonness ultraviolet / visible light, analysis or semi-transfer / full automatically biochemical study instrument, and doesn't need particularity or extra instrument. The testing cost is cheap, and is convenient for popularization.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method for detecting N-acetyl-beta-amino glucosaccharase activity and diagnosis kit therefor
InactiveCN1995975AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsDiagnostic agentMedicine
The active feature inspection for acetylglucosamidase and its diagnostic agent box uses its fostering of continuous monitoring method, enzymolysising dual chlorineor chlorine acetylglucosamidase reaction, through the absorbance elevating speed of the 405nm, inspecting its active feature. The agent box comprises the bumper solution, synthesized nitrobenzene betaacetylglucosamidase and stabilizer to make dual agent by the diagnostic agent box to reduce cross impact of all kinds of components. Through visible light analyzer, the result is precise, free from outside contamination, easy for promotion.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778955AReflect contentHighlight substantive featuresMicrobiological testing/measurementUltravioletWavelength
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Monoamine oxidase activity determination method and monoamine oxidase diagnostic kit
InactiveCN1789427AStrong specificityQuantitatively reflect the activityMicrobiological testing/measurementMonoamine oxidase APeroxidase
The invention relates measuring method of monoamine oxidase activity and its diagnostic kit, comprising the following steps: using the benzylamine, p-toluidine red-ª‰-azo naphthol, butyl amine, amyl amine, ª‰-phenylethylamine, tyramine and other aminated compounds as substrate, it reacted with monoamine oxidase to produce auricome, then auricome carried out coupling reaction with hydrogen peroxidase and aldehyde dehydrogenase, carrying out the reaction to turn the oxidation type coenzyme to reduction type coenzyme, detecting the ascending velocity of absorbance of main wavelength 340nm to reflect the activity of monoamine oxidase in sample by definite quantity. The method has high specificity, good accuracy. The diagnostic kit is made to double agents or tri-agent to reduce the across impact and keep the agent stability and be good for long term storage. The method can be rapidly detected by the ultra-violet / visible light analysis meter or semi-automatic / full-automatic biochemical analysis meter, so the method is easy to spread and use.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Monoamine oxidase activity determination method and monoamine oxidase diagnostic kit
InactiveCN1789426AStrong specificityThe test result is accurateMicrobiological testing/measurementQuinoneHydrogen peroxide
The invention relates measuring method of monoamine oxidase activity and its diagnostic kit. The method comprises the following steps: carrying out reaction with benzylamine, tyramine and monoamine oxidase to produce the hydrogen dioxide, then taking it with peroxidase to carry out enzyme coupling reaction, oxygenizing the achromatic color reduction type chromogen to become the quinone imines chromogen or indamine chromogen dye, detecting the change of absorbance of main wavelength 400-600nm to reflect the activity of monoamine oxidase in sample by definite quantity. The method has high specificity and good accuracy. The diagnostic kit is made to double agents or tri-agent to reduce the across impact and keep the agent stability and be good for long term storage. The method can be rapidly detected by the ultra-violet / visible light analysis meter or semi-automatic / full-automatic biochemical analysis meter, so the method is easy to spread and use.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778941AHighlight substantive featuresSignificant progressMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of inorganic Phosphates and its diagnosis reagent box. Producing hydroperoxide by reacting pyruvate oxidase with pyruvate under the existence of Inorganic Phosphates , then causing enzyme-coupled reaction with peroxidase and oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length 400ú¡600nm absorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778956AStrong specificityThe test result is accurateMicrobiological testing/measurementUltravioletWavelength
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing glucose-6-phosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with phosphoglucose dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778943AContent reflectionHighlight substantive featuresMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing hypoxanthine by reacting nucleoside phosphorylase with carnine under the existence of Inorganic Phosphates in the sample of plasma, serum and so on, then producing urate and hydroperoxide by reactinghypoxanthine withxanthine oxidase, and then reacting bimolecular hydroperoxide with peroxidaseand oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length400ú¡600nmabsorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
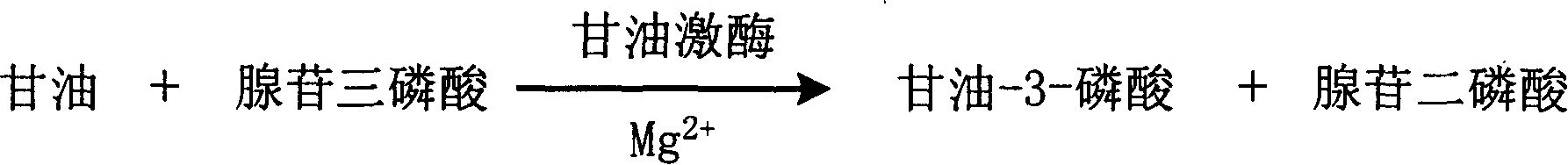
InactiveCN1778962AStrong specificityThe test result is accurateMicrobiological testing/measurementGlycerolUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase, hydroperoxide enzyme and aldehyde dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the ascending range of dominant wave-length 340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778961AStrong specificityThe test result is accurateMicrobiological testing/measurementUltravioletGlycerol
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase, peroxidase and oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the ascending range of 400ú¡600nmabsorbance and finally measuring the content of Inorganic Phosphates This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method for measuring diethyl p-nitrophenyl phosphate esterase active concentration and diagnostic kit
InactiveCN1975383AHighlight substantive featuresSignificant progressMicrobiological testing/measurementColor/spectral properties measurementsAcetic acidDiethyl p-nitrophenyl phosphate
This invention disclosed a determination method for paraoxonase activity and the reagent box. The procedures are as follows: react with phenylacetate to produce acetic acid, oxidate the deoxidize style coenzyme to coenzyme, measure the coenzyme at 340nm absorbency, calculate the activity and concentration. The reagent box contains buffer, deoxidize style coenzyme, phenylacetate and stabilizer. The result obtained by this method was precise and accurate.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
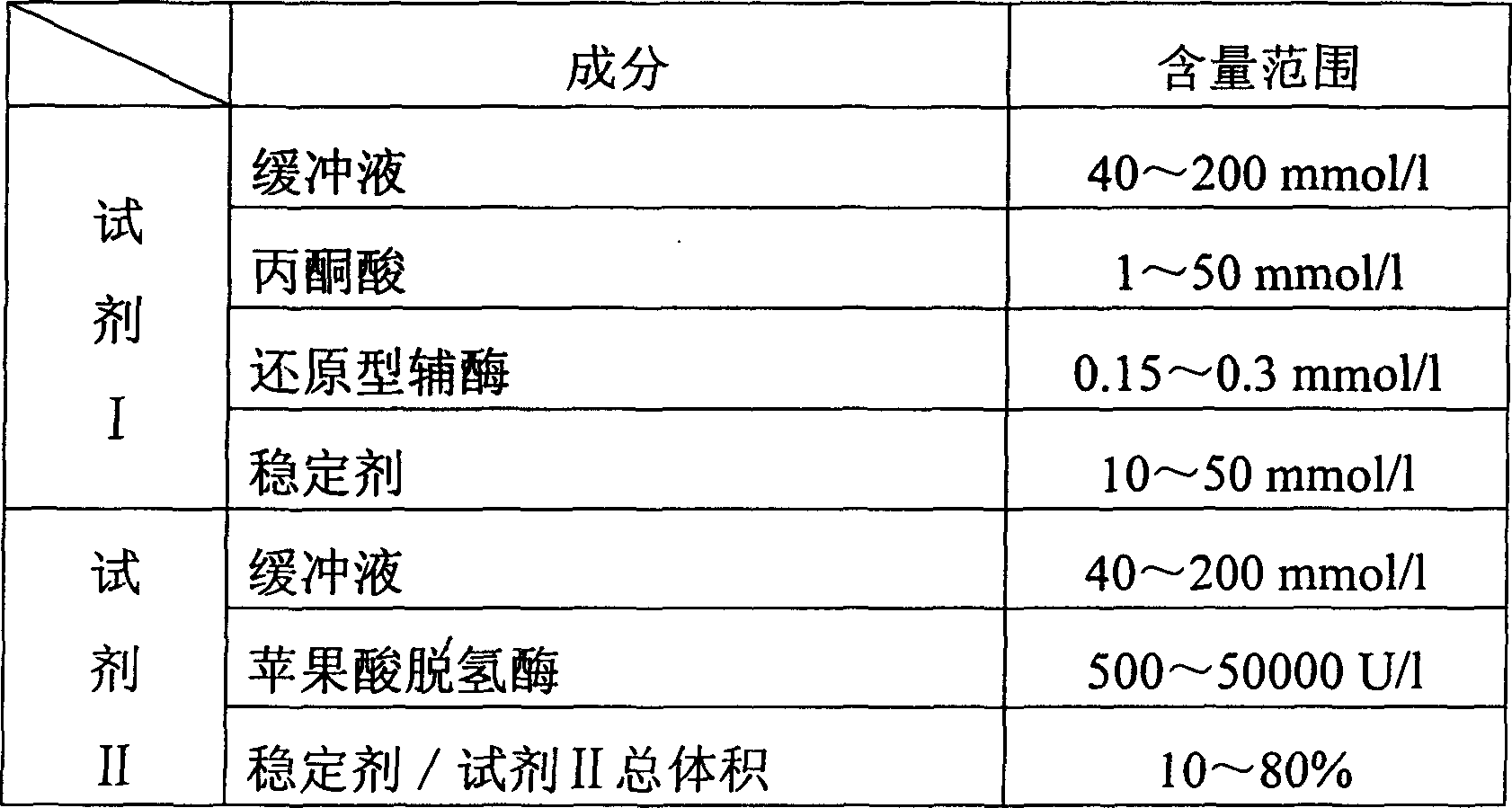
Enzymatic method for determining potassium ion content and potassium ion diagnosis kit
InactiveCN1786187AStrong specificityThe test result is accurateMicrobiological testing/measurementIon content6-Phosphogluconolactone
The invention relates to enzyme method measuring kalium ion content method and its diagnosis kit. It is utilized kalium ion activation pyruvate kinase specificity to couple hexokinase, glucose phosphate dehydrogenase, 6-phosphogluconolactone and phosphogluconate dehydrogenase to react, reduce dichotomy type oxidizing coenzyme to dichotomy reducing type. To measure dominant wavelength 340nm absorbance rising speed can quantificationally reflect sample kalium ion content. The method has high specificity, good accuracy. The diagnosis kit can be made into bi-agent or tri-agent to reduce cross influence. The method can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778957AHigh activityStrong specificityMicrobiological testing/measurementLactate dehydrogenaseUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing adenosine diphosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with pyruvate kinase and lactate dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778954AReflect contentHighlight substantive featuresMicrobiological testing/measurementPhosphoenolpyruvate carboxylaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing carbon dioxide by reacting pyruvate oxidase with pyruvate under the activation ofInorganic Phosphates in the sample of plasma or serum and so on, and producing oxaloacetic acid by reacting carbon dioxide and phosphoenolpyruvate under the existence of phosphoenolpyruvate carboxylase, and then transferring oxidized coenzyme to reduced coenzyme by reacting oxaloacetic acid and malic acid dehydrogenase. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of Inorganic Phosphates. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Process for determining content of zinc ion by enzyme method and kit for diagnosing zine ion thereof
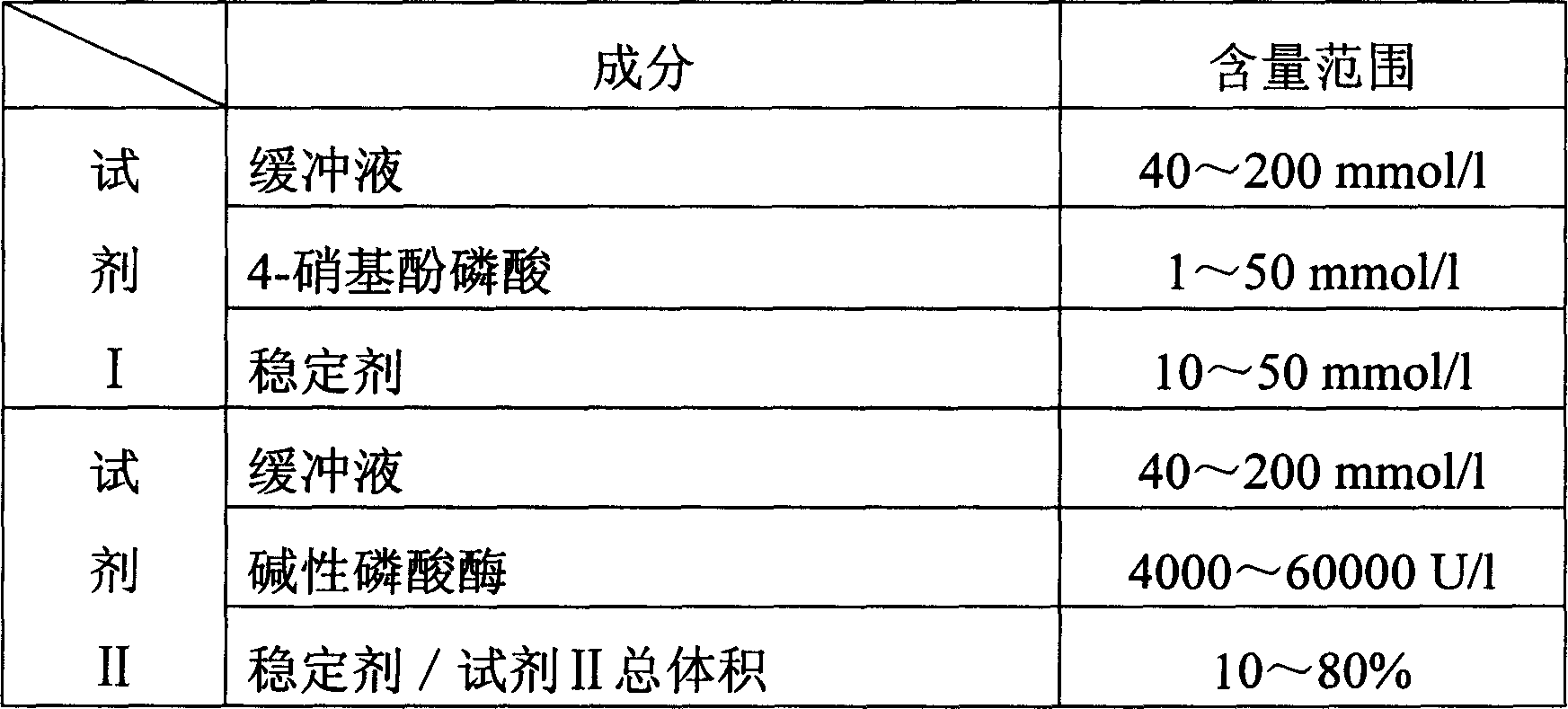
InactiveCN1786695AStrong specificityThe test result is accurateAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsUltravioletPhosphoric acid
The invention relates to enzyme method measuring zinc ion concentration and zinc ion diagnose kit. The invention uses alkaline phosphatase reaction colorimetry method that uses alkaline phosphatase to enzymolysis four-nitrophenol phosphoric acid to form into four-nitrophenol under the activation of zinc ion, by detecting the ascending speed of 405nm dominant wavelength absorbance the zinc ionic content in sample would be quantitatively responsed. The method has high specificity, has no influence from inside and outside metal material, accurate test result. The diagnose kit is made up into bi-agent or tri-agent that could reduce cross influence from each component and keeps stability of the agent that is convenient for long-term storage. The method could quickly test on commonness ultraviolet / visible light, analyzer or semi-transfer / full automatic biochemical study instrument, and doesn't need particularity or extra instrument. The testing cost is cheap, and is convenient for popularization.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778960AStrong specificityThe test result is accurateMicrobiological testing/measurementGlycerolUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778958AStrong specificityThe test result is accurateMicrobiological testing/measurementChemistryEnzyme method
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing glucose-6-phosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with phosphoglucose dehydrogenase, 6-phosphogluconic acid lactone enzyme and phosphogluconate dehydrogenase, and transferring dimolecule oxidized coenzyme to dimolecule reduced coenzyme. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Measuring method of homotype cysteine concentration and homotype cysteine diagnostic reagent kit
InactiveCN1912582AHighlight substantive featuresSignificant progressMicrobiological testing/measurementColor/spectral properties measurementsCysteine thiolateLyase
A method for determining concentration of homotype cysteine includes cracking cysteine-a and Y-lyase with cysteine to produce hydrogen sulfide, using hydrogen sulfide and some other material to resynthesize homotype cysteine being used to generate ammonia and succinate, using some chemical to act on them to let reduction coenzyme be oxidized to be oxidation coenzyme for determining out absorbance dropping speed of reduction coenzyme at 340nm then estimating out concentration size of homotype cysteine. Its diagnosis kit can be prepared in double dosage for decreasing cross influence of various compositions.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
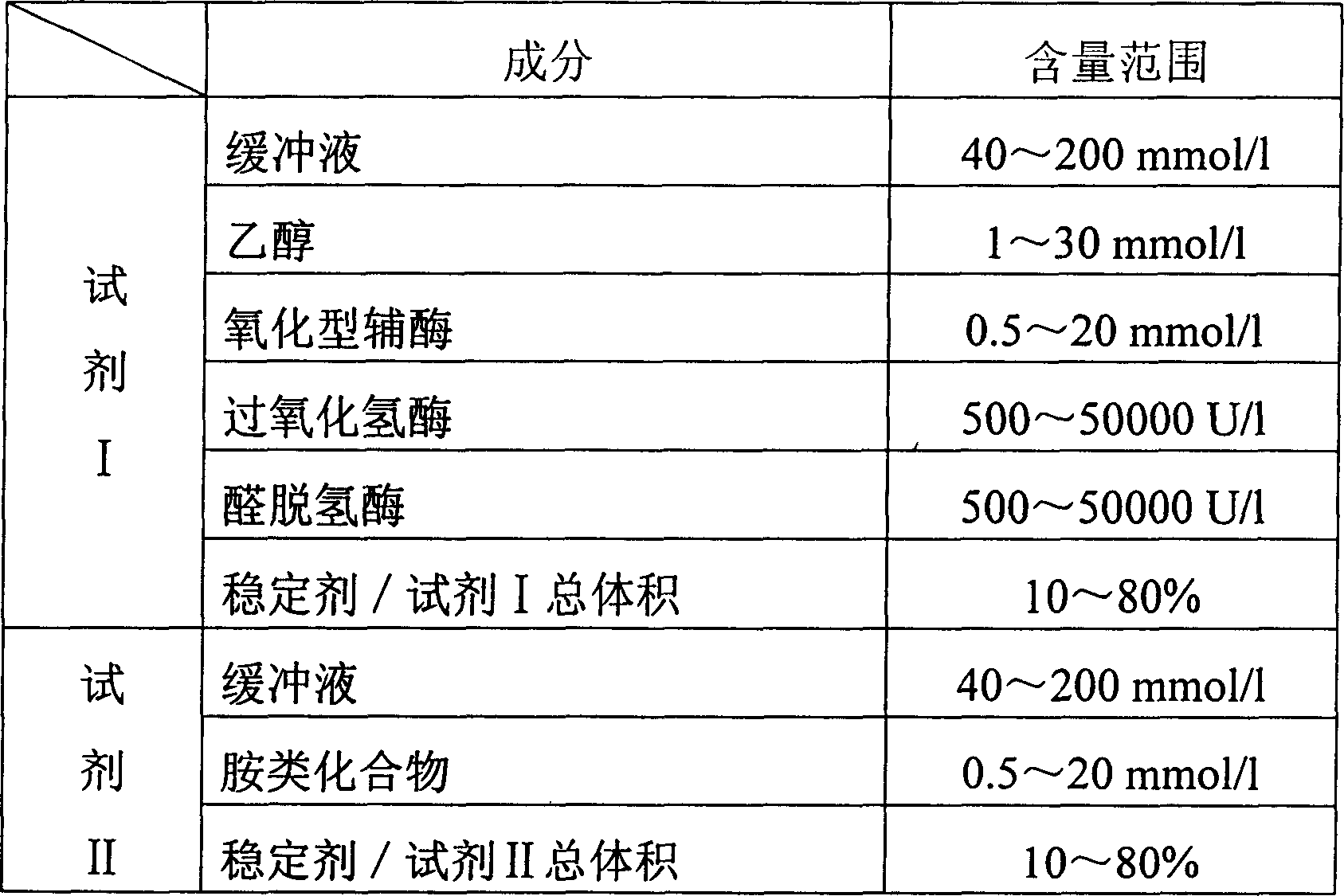
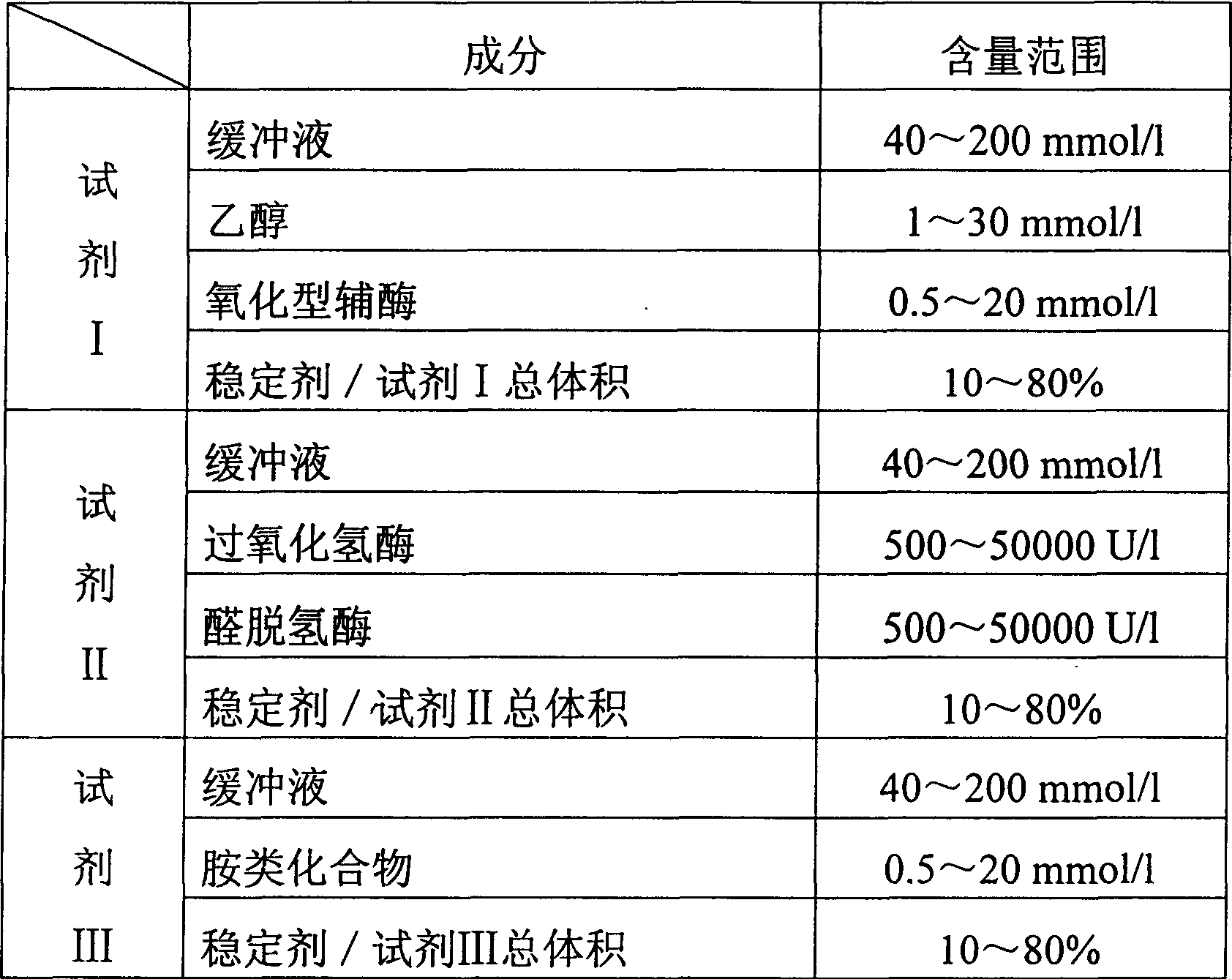
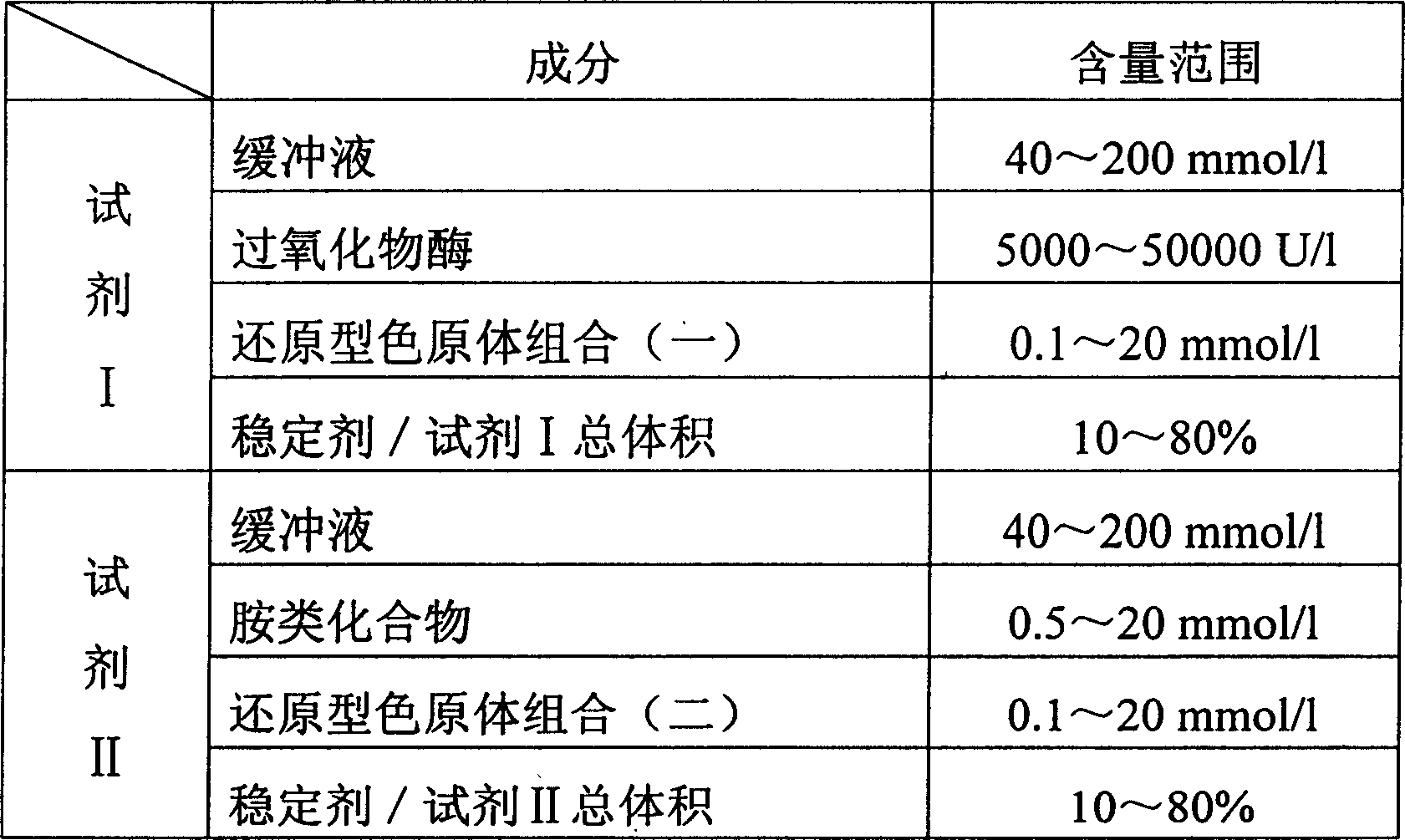
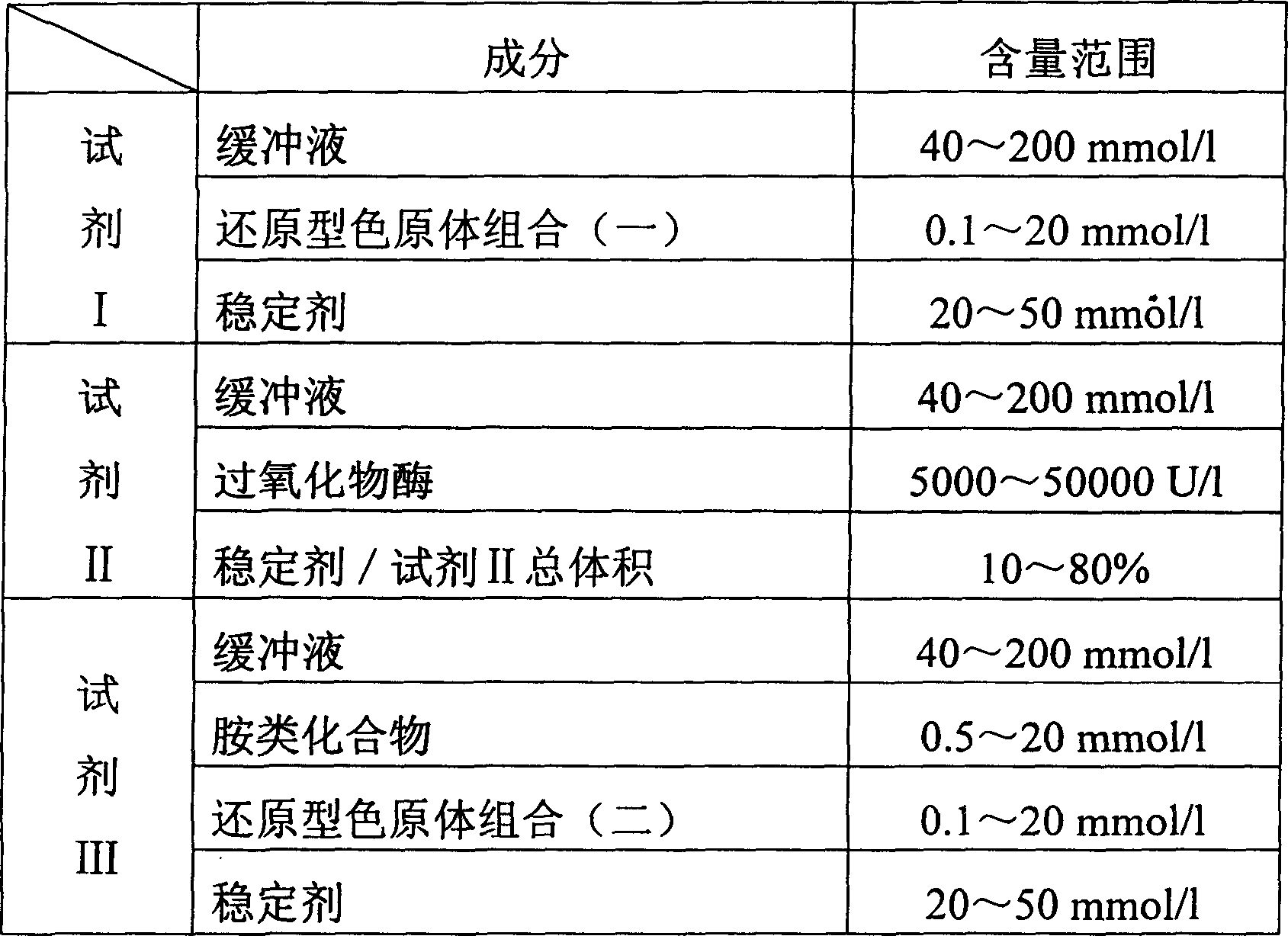
Process for determining contect of potassium ion by enzyme method and kit for diagnosing potassium ion thereof
InactiveCN1786691AStrong specificityThe test result is accurateAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsForeign matterPhosphoric acid
The invention relates to potassium ion content measuring method by enzyme method and potassium ion diagnosis kit. It is utilized potassium ion to activate pyruvate kinase to make adenosine diphosphate react with phosphoric acid enol pyruvic acid to generate adenosine triphosphate, couple hexokinase glucose phosphate dehydrogenase to react, and reduce oxidized coenzyme to reduced coenzyme, and quantitatively response potassium ion content by measuring main wavelength 340nm absorbance vertical speed. The method is not influence for sodium ion in sample, and not polluted by internal and foreign matter. And it has high specificity, precise testing result, and good accuracy. The diagnosis kit is formed to double or three reagents to reduce cross influence, keep reagent stability to be convenient for storing for long time. The method can realize fast testing by common ultraviolet / visible light analyzer or semi / full automatic biochemical analyzer. Thus its testing cost is low; and it is convenient for popularizing and applying in industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com