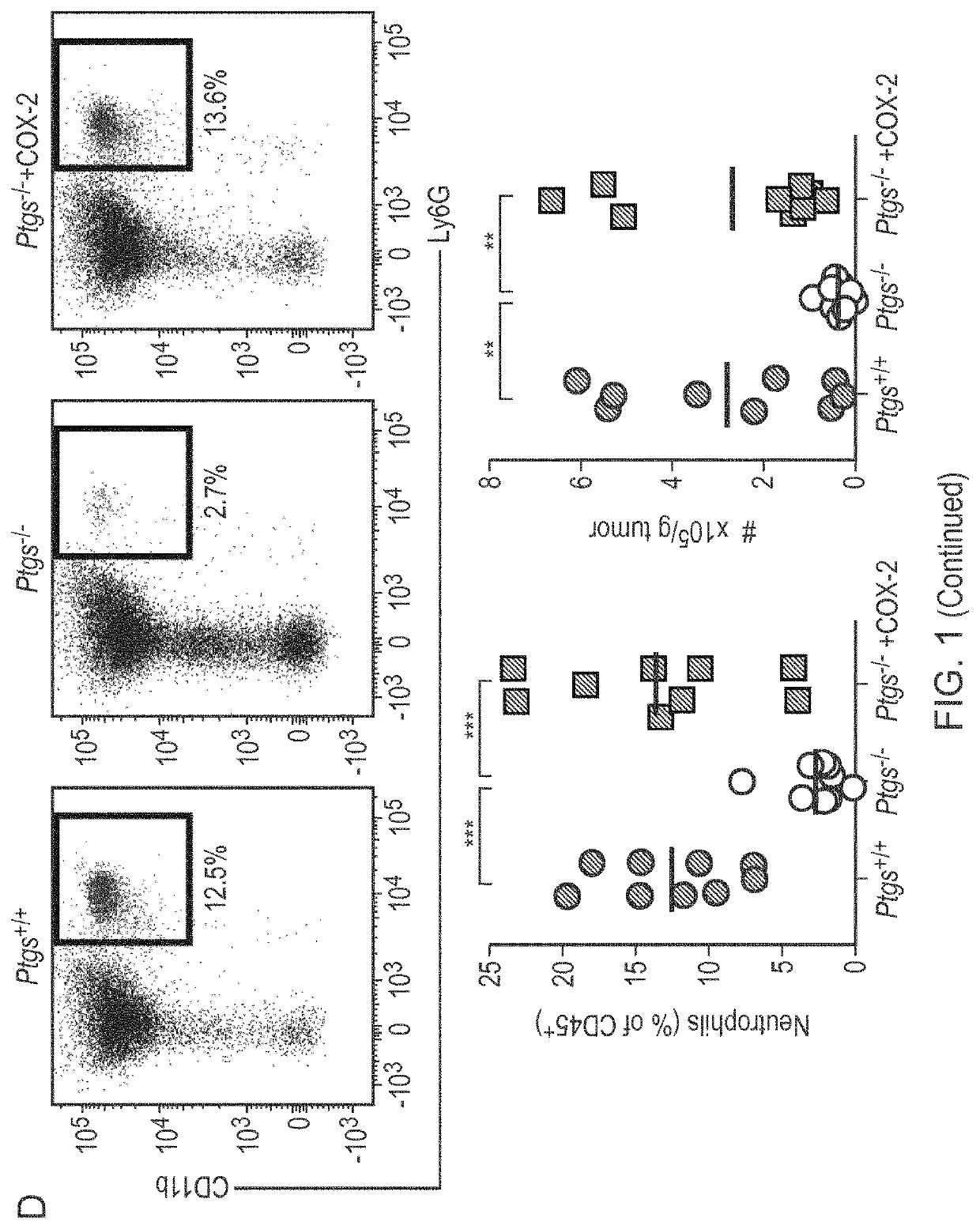
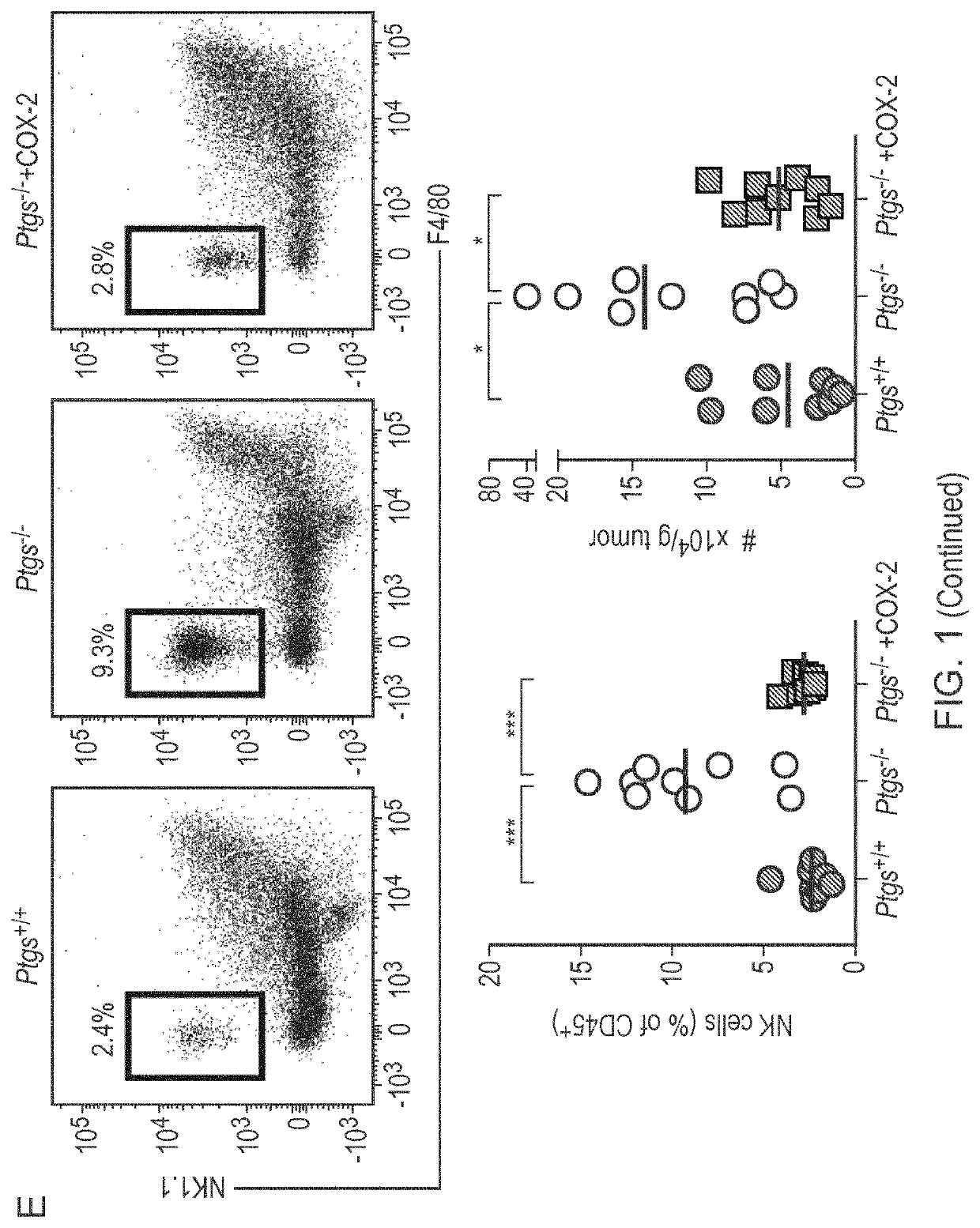
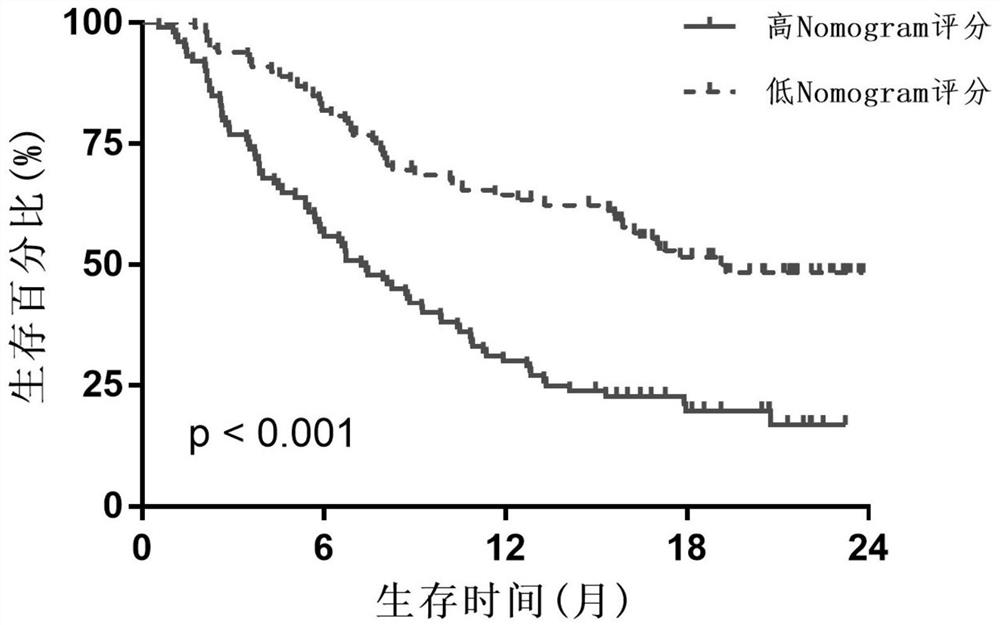
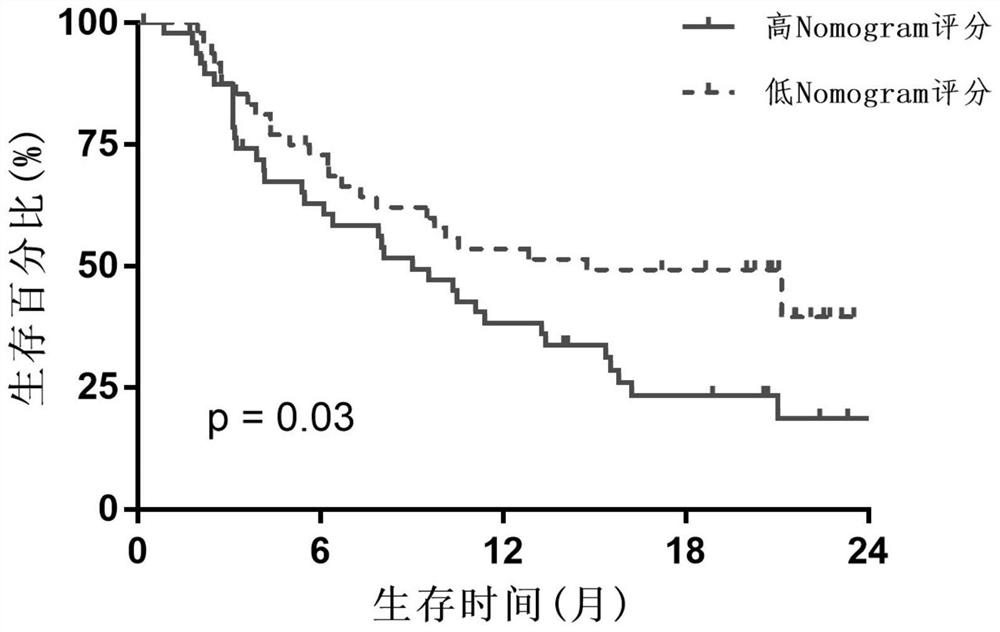
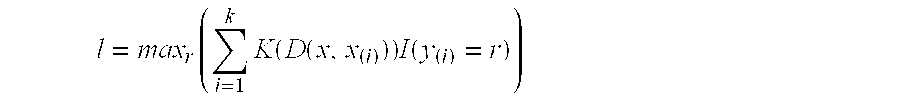Patents
Literature
41 results about "CXCL9" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemokine (C-X-C motif) ligand 9 (CXCL9) is a small cytokine belonging to the CXC chemokine family that is also known as Monokine induced by gamma interferon (MIG). The CXCL9 is one of the chemokine which plays role to induce chemotaxis, promote differentiation and multiplication of leukocytes, and cause tissue extravasation.
Methods for Detecting and Treating Rhinovirus Infection
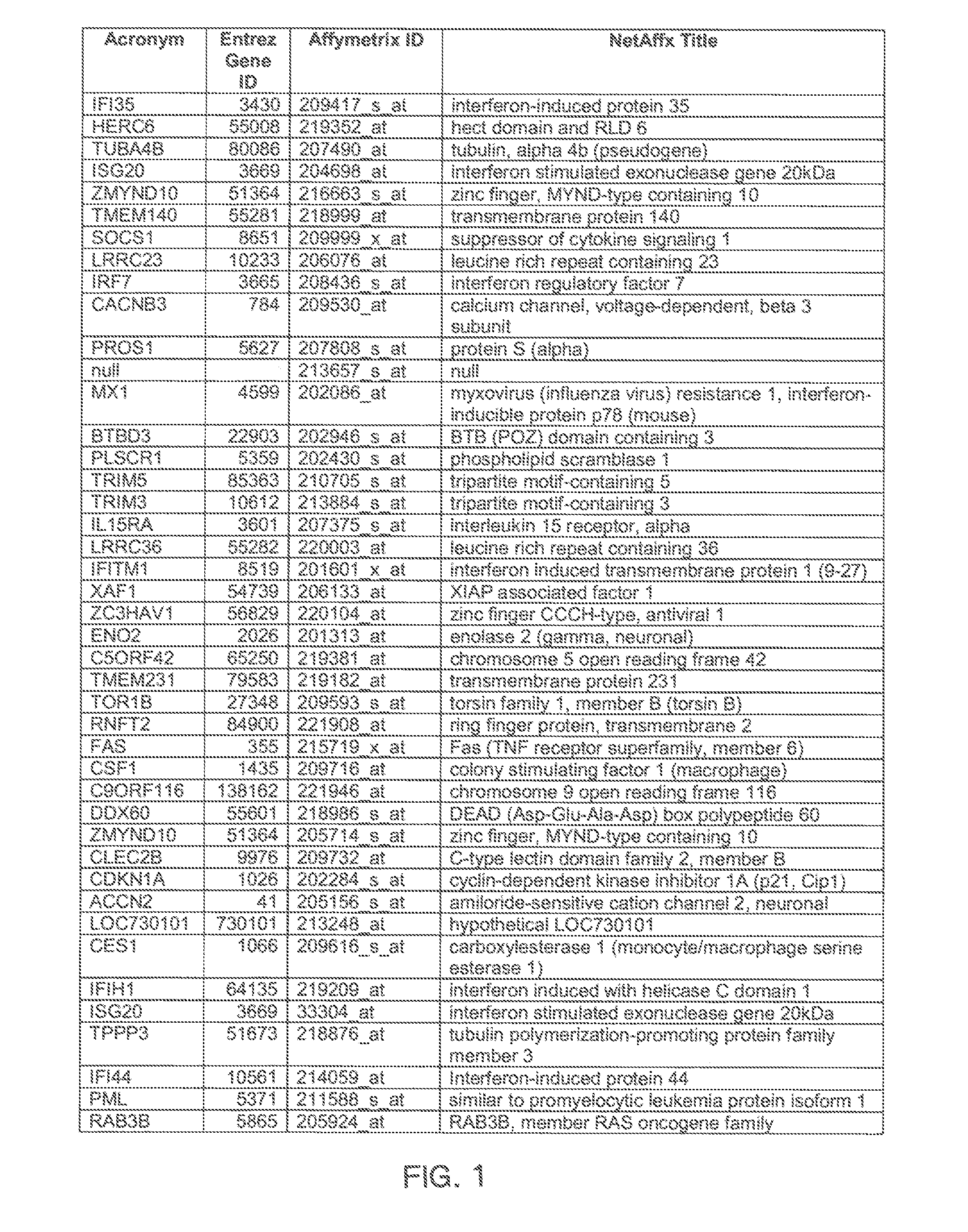
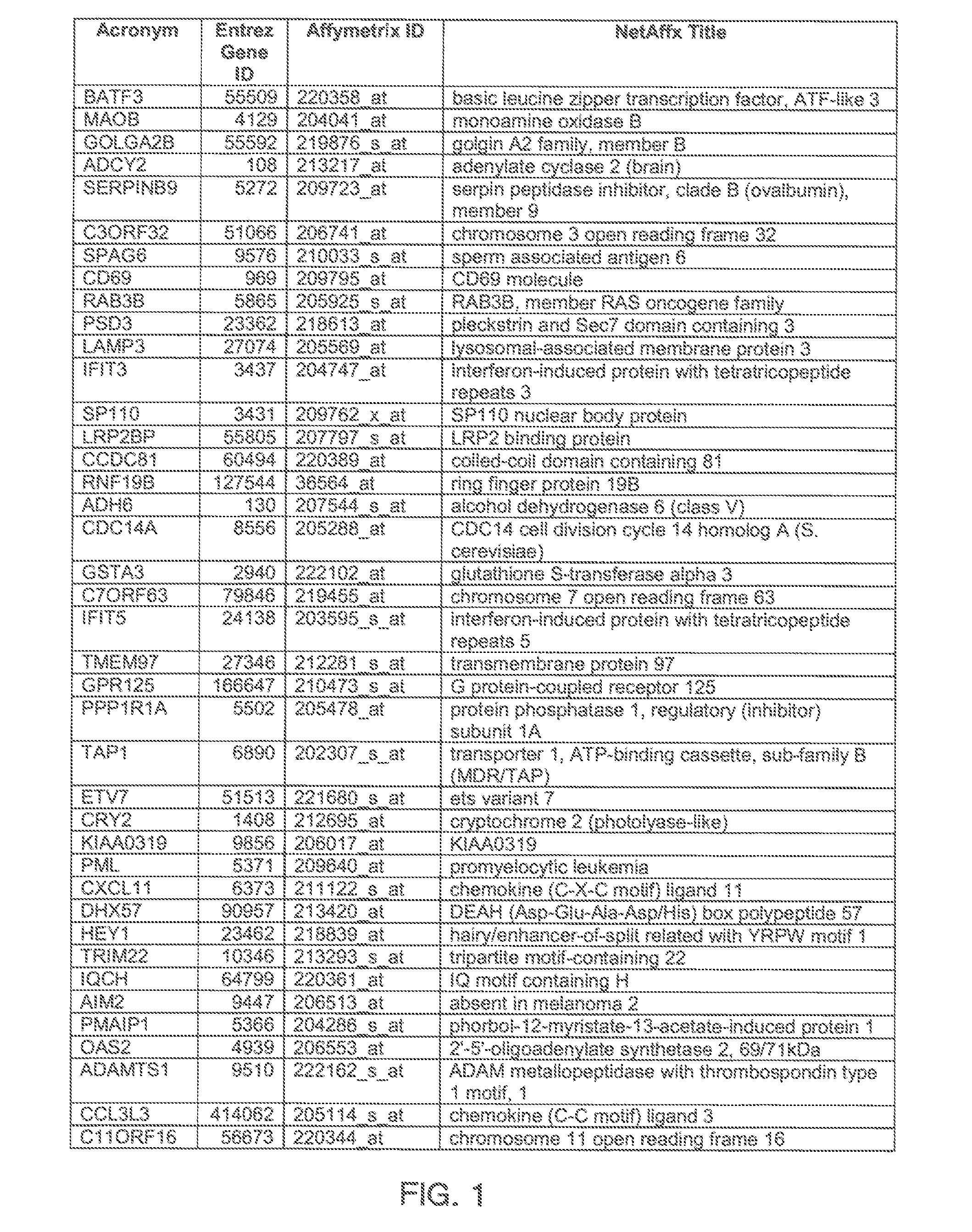
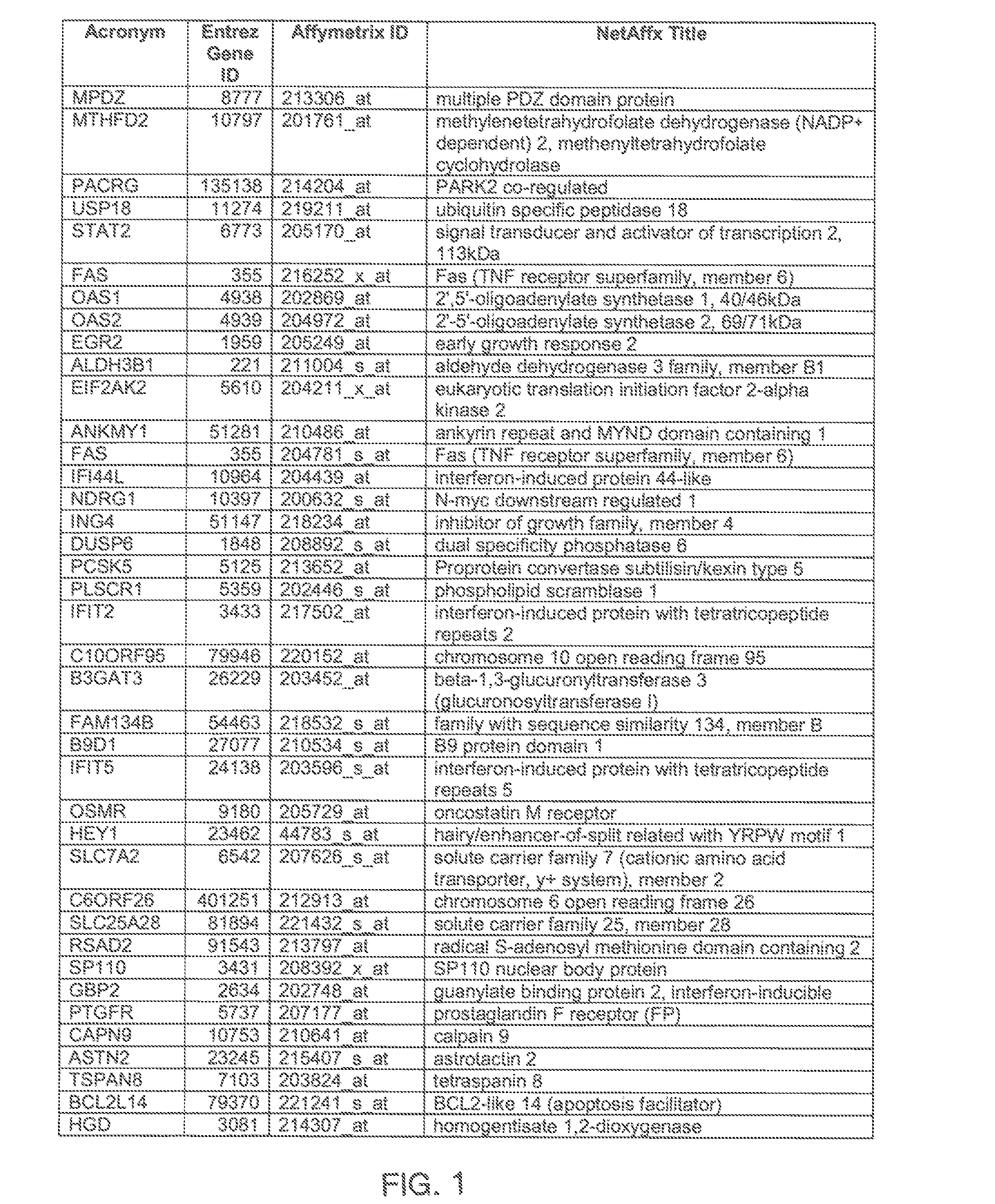
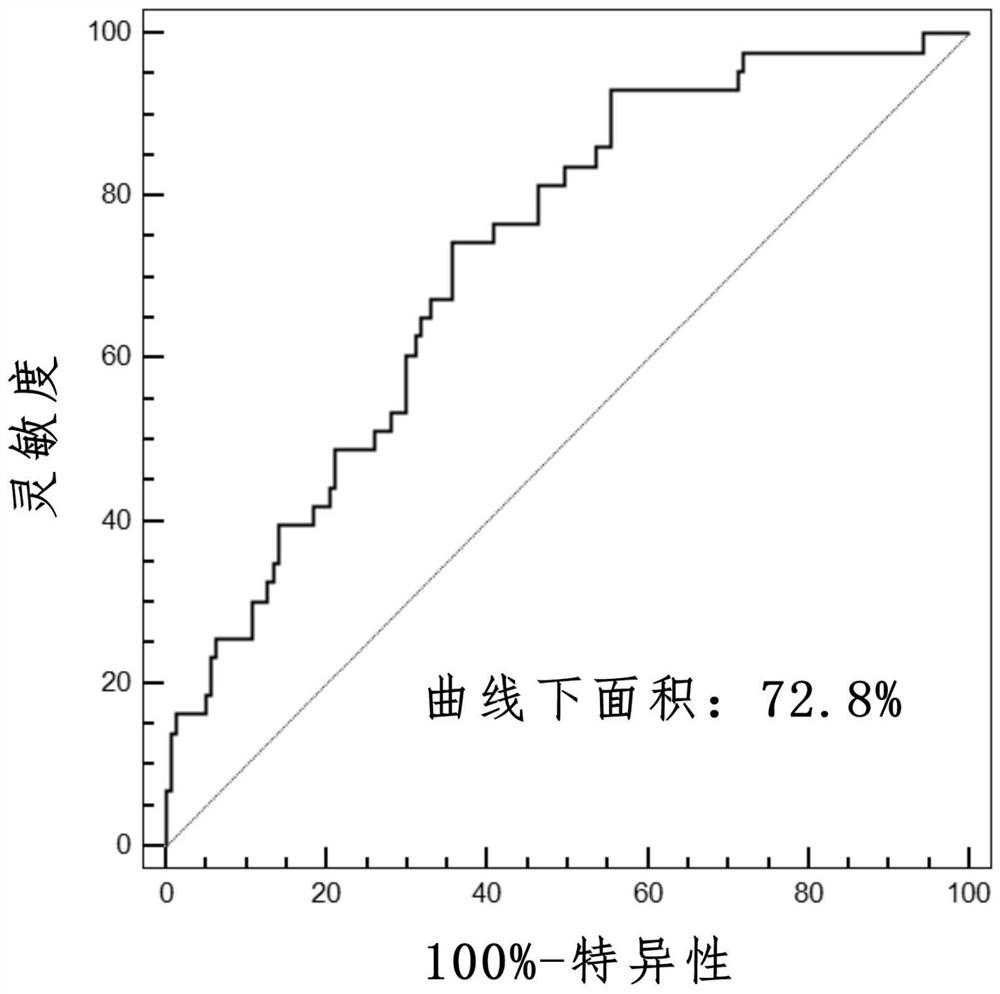
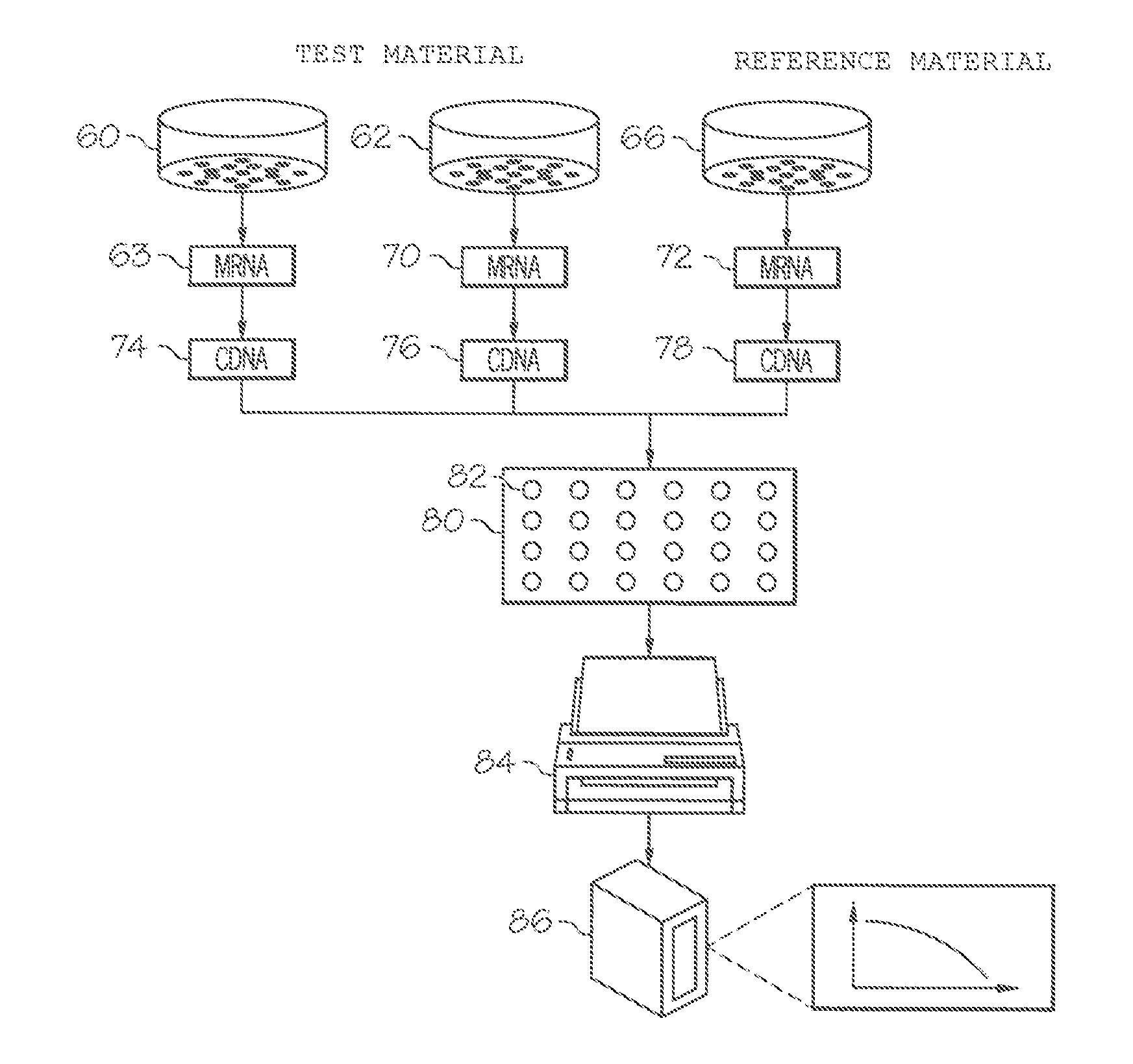
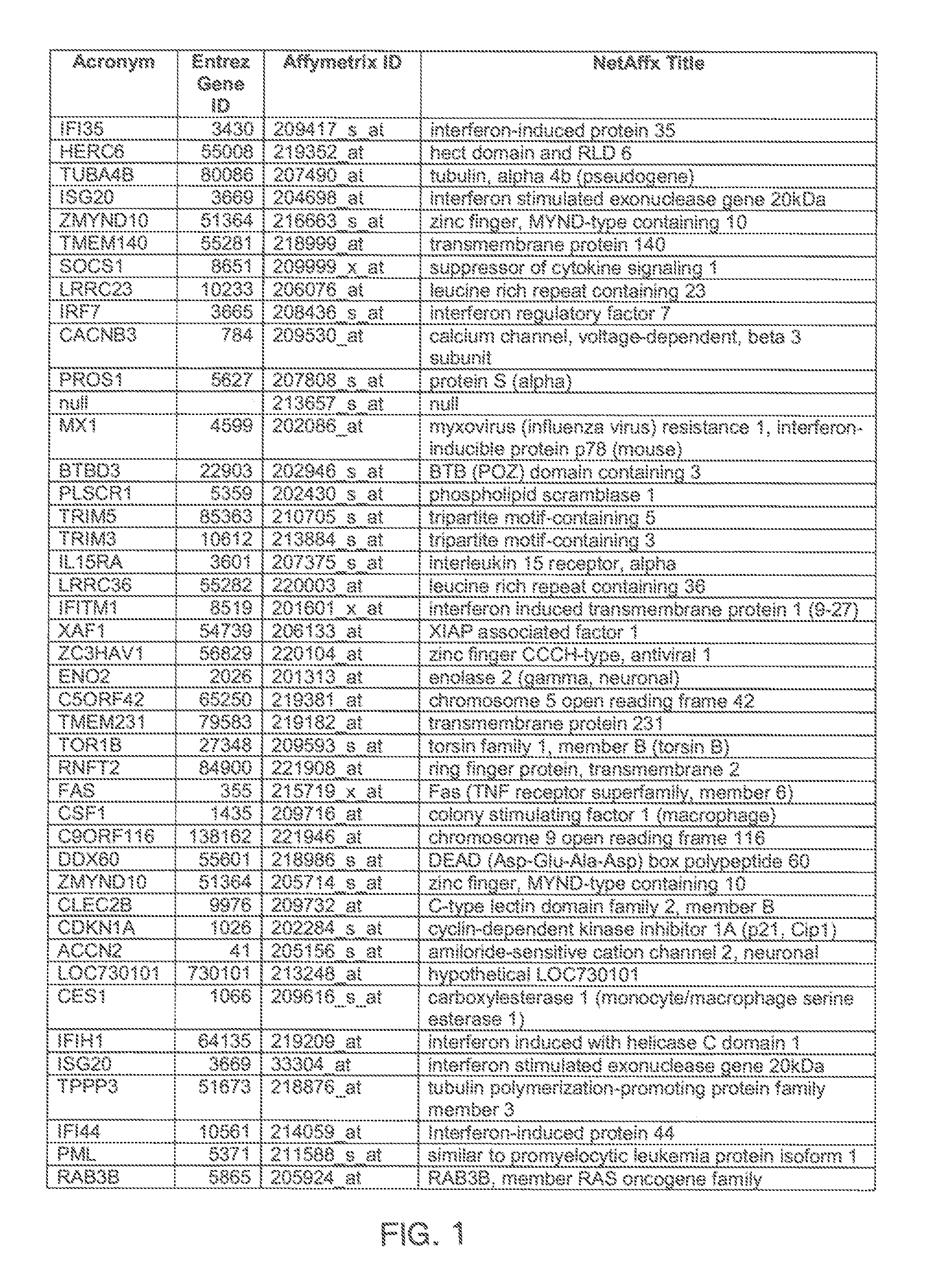
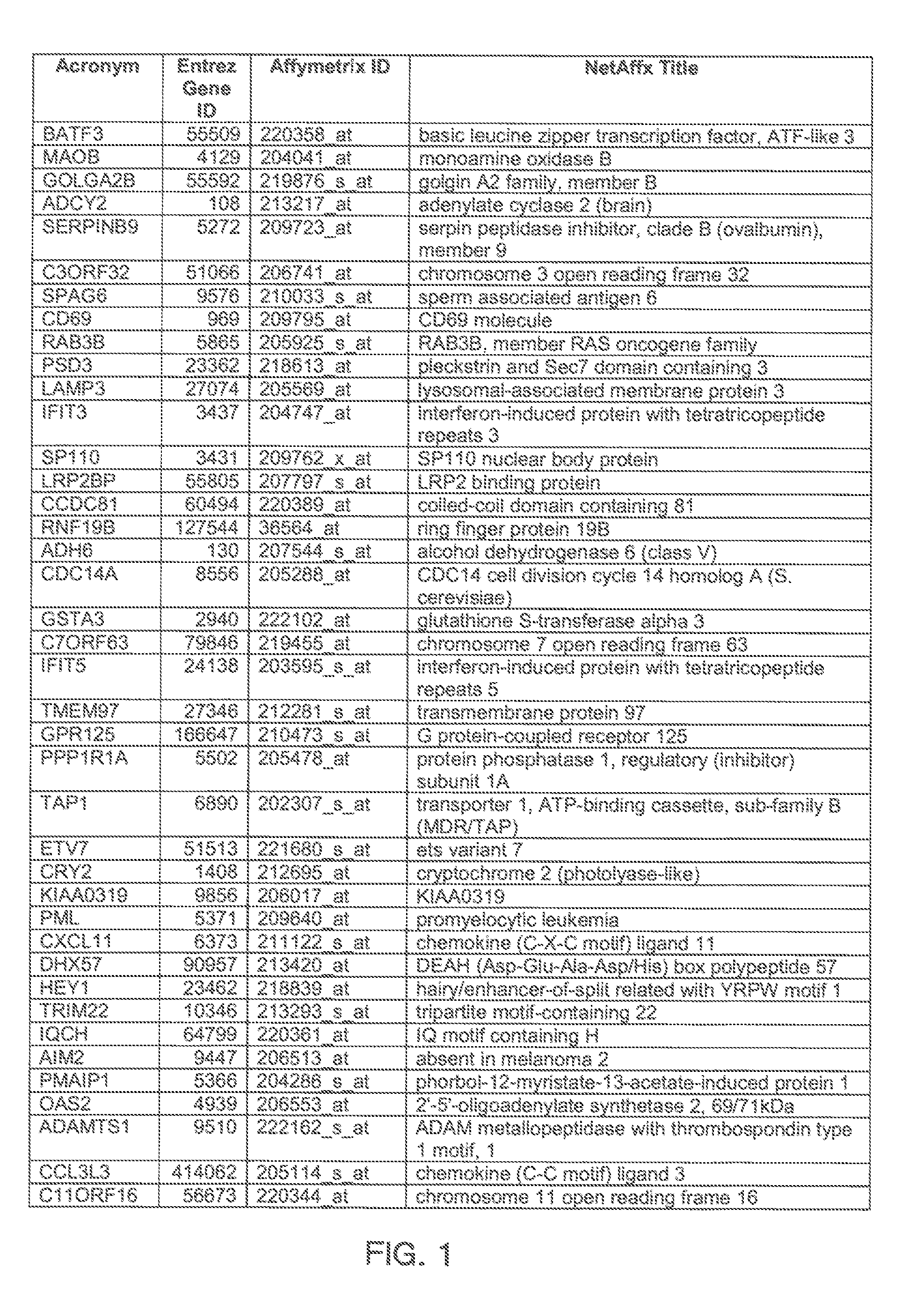
The invention provides a method for evaluating the activity of an agent for treating rhinovirus infection or a symptom thereof, a method of detecting or monitoring rhinovirus infection, and a method of treating rhinovirus infection or a symptom thereof. Various embodiments comprise measuring expression of (i) one or more genes selected from the group consisting of CRY2, B3GAT3, C10ORF95, and BATF3, and (ii) one or more genes selected from the group consisting of RNFT2, BTG4, PSD3, CAPN9, SULT1E1, HEY1, LRRC36, RAB3B, ALDH3B1, FAM134B, FAS, PLSCR1, CLEC2B, HAS2, MX1, SP110, GBP1, IFIT3, IFIT1, CXCL9, CXCL10, and CXCL11, from at least one biological sample to produce a gene expression profile, and comparing the gene expression profile to a reference gene expression profile. Systems, computer readable media, compositions, and methods for maintaining or improving respiratory health also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
Biomarkers for inflammatory disease and methods of using same
InactiveUS20160000936A1Low abundanceIncrease doseCompounds screening/testingMicrobiological testing/measurementBiomarker (petroleum)Combination therapy
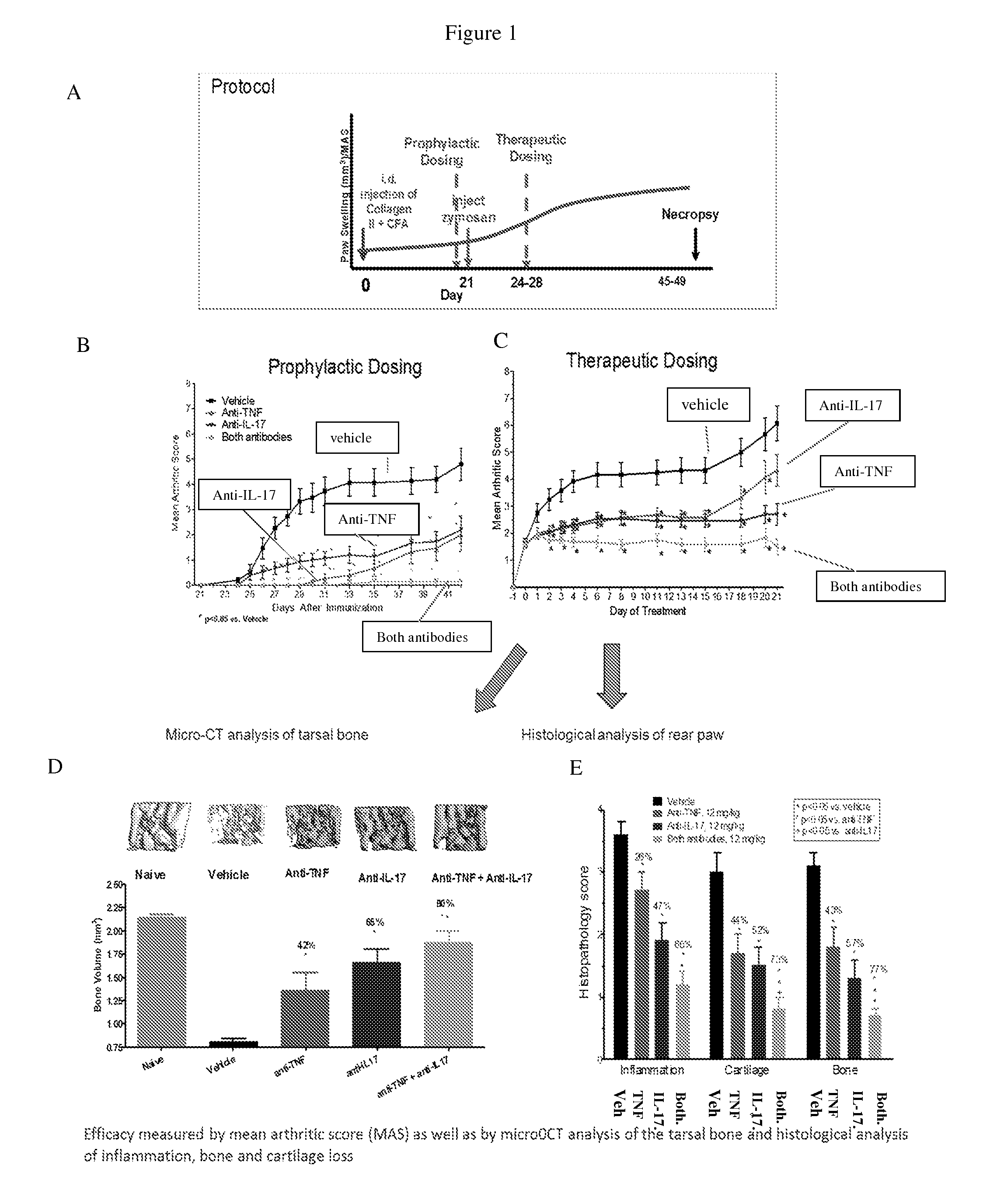
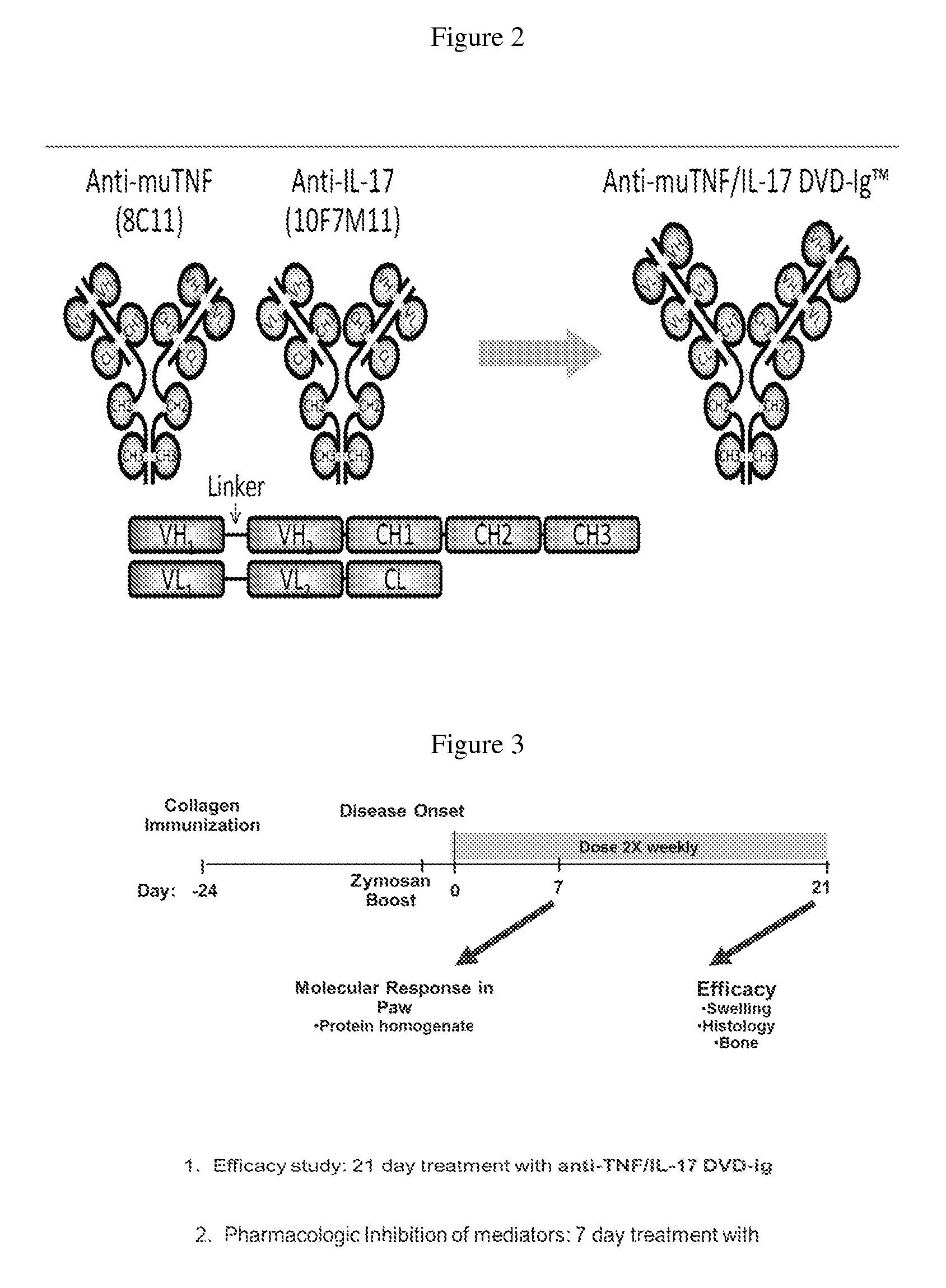
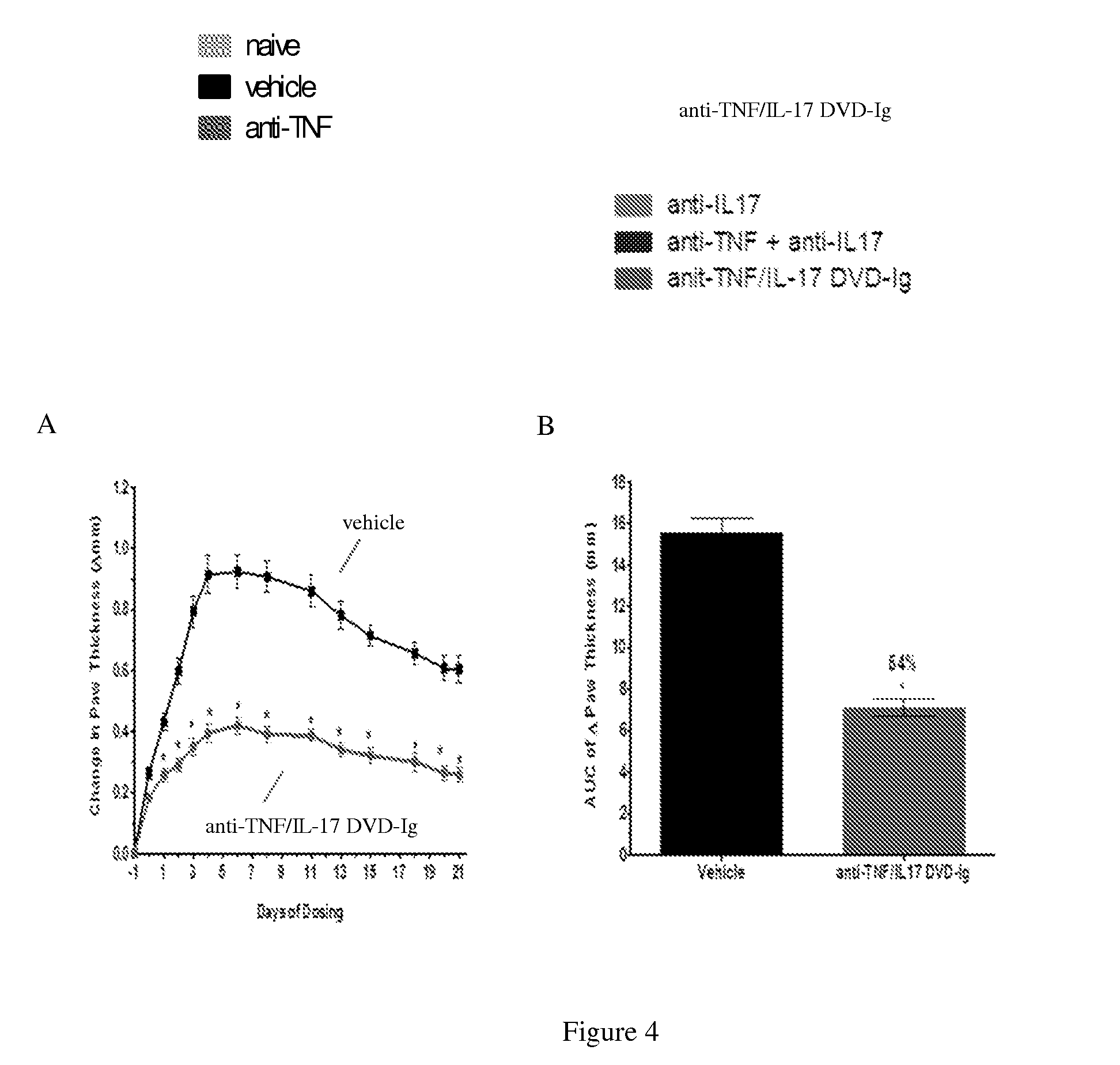
The invention provides methods for predicting the efficacy of anti-TNF and anti-IL-17 combination therapies in the treatment of a subject suffering from inflammatory disease by determining the level LIF, CXCL1, CXCL2, CXCL4, CXCL5, CXCL8, CXCL9, CXCL10, CCL2, CCL23, IL-1β, IL-1Ra, TNF, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IFNγ, CXCR1, CXCR4, CXCR5, GM-CSF, GM-CSFR, G-CSF, G-CSFR protein or nucleic acid, or a homolog, portion or derivative thereof markers in a sample derived from the subject.
Owner:ABBVIE INC
Anti-CXCL9, anti-CXCL 10, anti-CXCL 11, anti-CXCL 13, anti-CXCR3 and anti-CXCR5 agents for inflammatory disorder
A method for detecting an inflammatory disease in a subject is disclosed. The method comprises the steps of (a) detecting a level of expression of one or more inflammatory disease markers in a biological sample obtained from the subject; and (b) comparing the level of expression of said one or more inflammatory disease markers in the biological sample to a normal level of expression of the one or more inflammatory disease markers, wherein the one or more inflammatory disease markers comprise one or more markers selected from the group consisting of CXCL9, CXCLIO, CXCLl 1, CXCLl 3, CXCR3 and CXCR5. Also disclosed are a method for monitoring the course of treatment for an inflammatory disease in a subject and a kit for detecting an inflammatory disease in a subject.
Owner:JYANT TECH
Methods and kits for determining drug sensitivity in patients infected with hcv
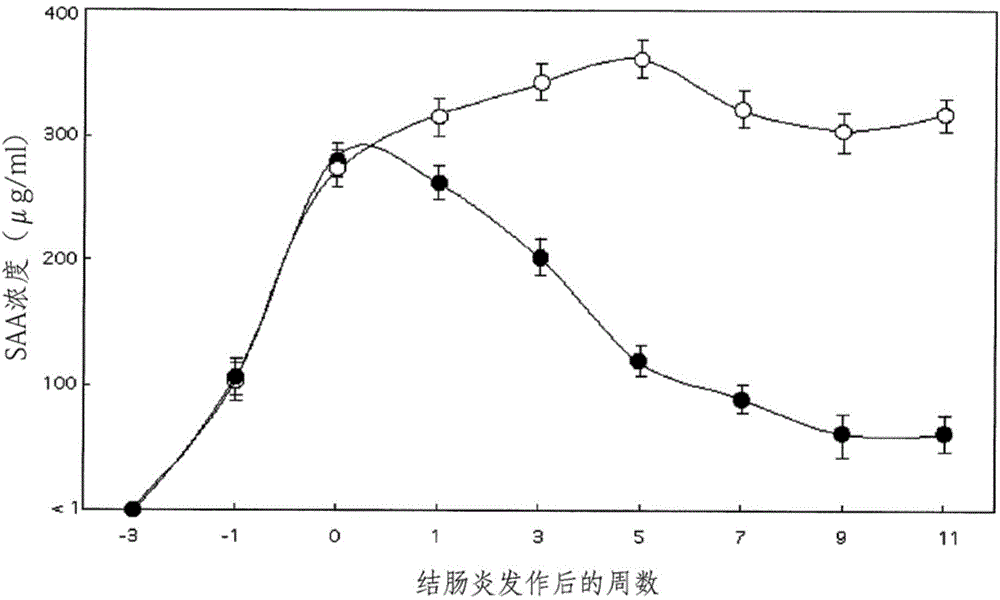
An in vitro method for determining whether a patient infected with HCV is a responder or non-responder to the treatment with interferon-alpha and ribavirin. More specifically, the method includes a step of determining the expression level of the IFI27, CXCL9 and G1P2 genes in a biological sample.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Anti-cxcl9, anti-cxcl10, anti-cxcl11, anti-cxcl13, anti-cxcr3 and anti-cxcr5 agents for inhibition of inflammation
InactiveCN104870013AAvoid interactionInhibit biological activityAntibacterial agentsNervous disorderDiseaseAntibody fragments
Methods for preventing or inhibiting inflammation in a subject are disclosed. In one aspect, the method comprises administering to a subject diagnosed with an inflammatory disease an effective amount of an anti-inflammatory agent that (1) inhibits the expression of CXCL9, CXCL10, CXCL11, CXCL13, CXCR3 and / or CXCR5, or (2) inhibits the interaction between CXCR3 and CXCL9, CXCL10 or CXCL11, or between CXCR5 and CXCLl 3, or (3) inhibits a biological activity of CXCL9, CXCL10, CXCL11, CXCL13, CXCR3 and / or CXCR5, wherein the agent comprises an antibody, antibody fragment, short interfering RNA (siRNA), aptamer, synbody, binding agent, peptide, aptamer-siRNA chimera, single stranded antisense oligonucleotide, triplex forming oligonucleotide, ribozyme, external guide sequence, or an agent-encoding expression vector.
Owner:JYANT TECH
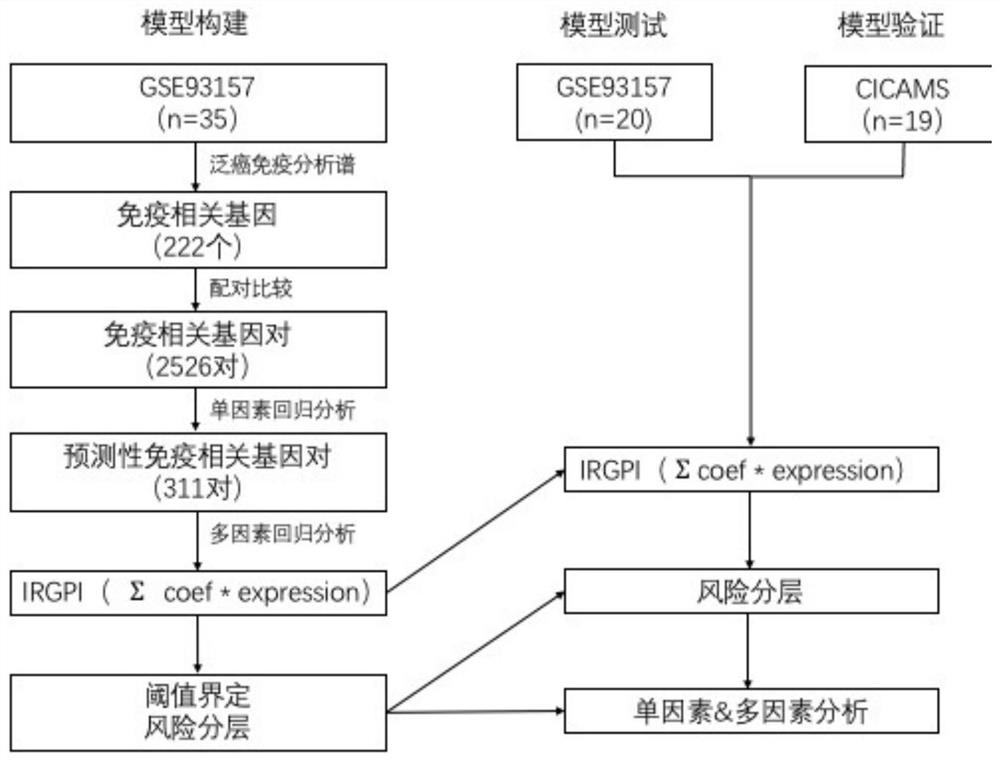
Application of scoring system based on immune gene pair in prediction of immunotherapy effect of non-small cell lung cancer patient
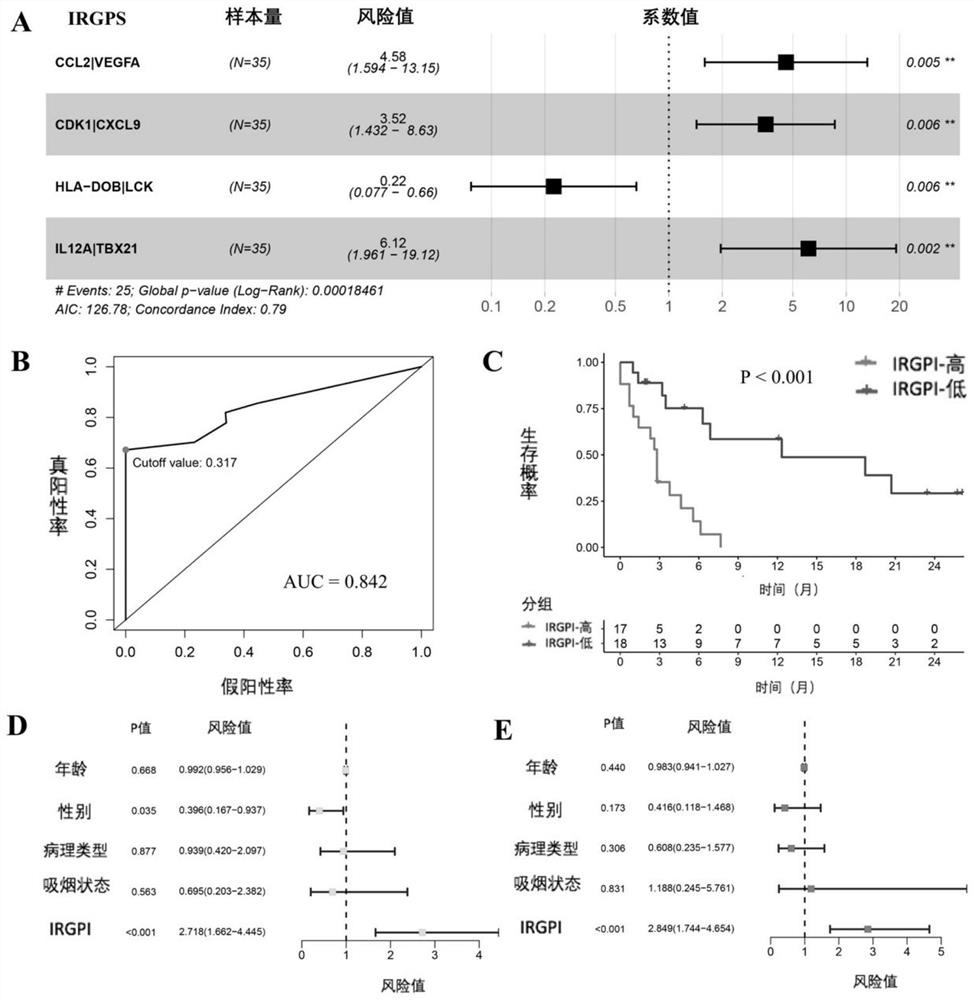
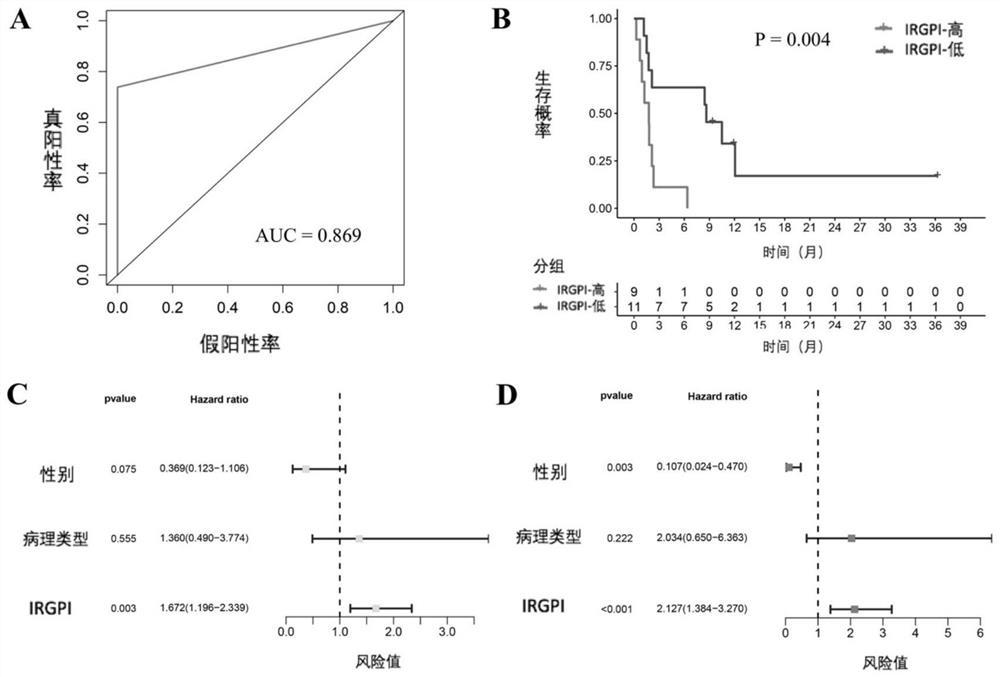
The invention discloses an application of a scoring system based on an immunogene pair in prediction of immunotherapy curative effect and prognosis of a non-small cell lung cancer patient. The scoring system disclosed by the invention is carried out based on the following four immune related gene pairs: CCL2 and VEGFA genes, CDK1 and CXCL9 genes, HLA-DOB and LCK genes, and IL-12A and TBX21 genes. The IRGP index (IRGPI) calculated based on an immune gene pair scoring system constructed by the invention is significantly related to the progression-free lifetime of a non-small cell lung cancer patient receiving anti-PD-1 immunotherapy. Therefore, the four immune-related gene pairs and the scoring system constructed according to the four immune-related gene pairs can be used for performing curative effect and prognosis prediction on the non-small cell lung cancer patient receiving anti-PD-1 immunotherapy.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Combination of biomarkers for the prognosis of response or non-response to an Anti-hcv treatment
ActiveUS20130316332A1Improve forecastMicrobiological testing/measurementDisease diagnosisHcv treatmentHigh probability
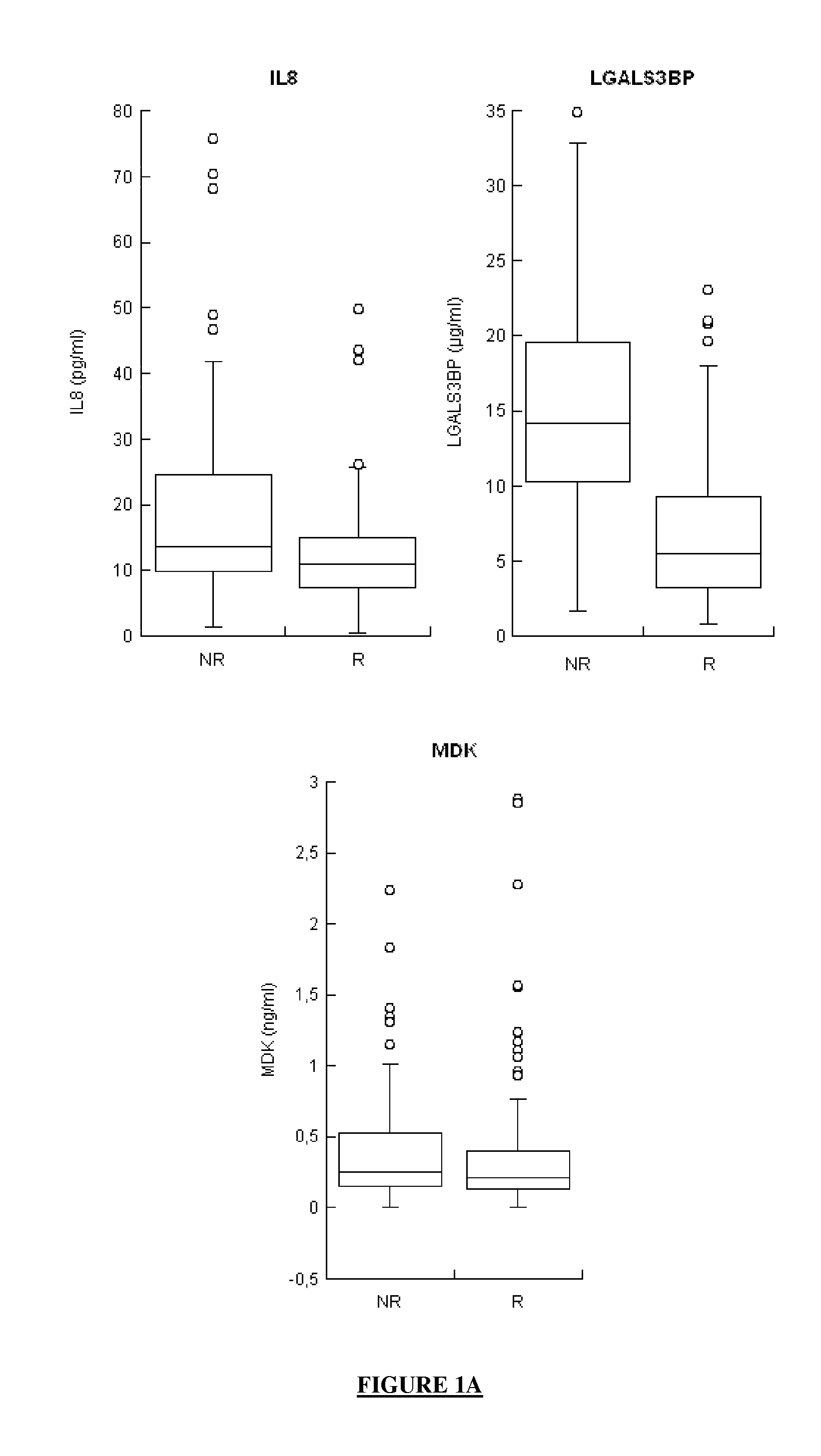
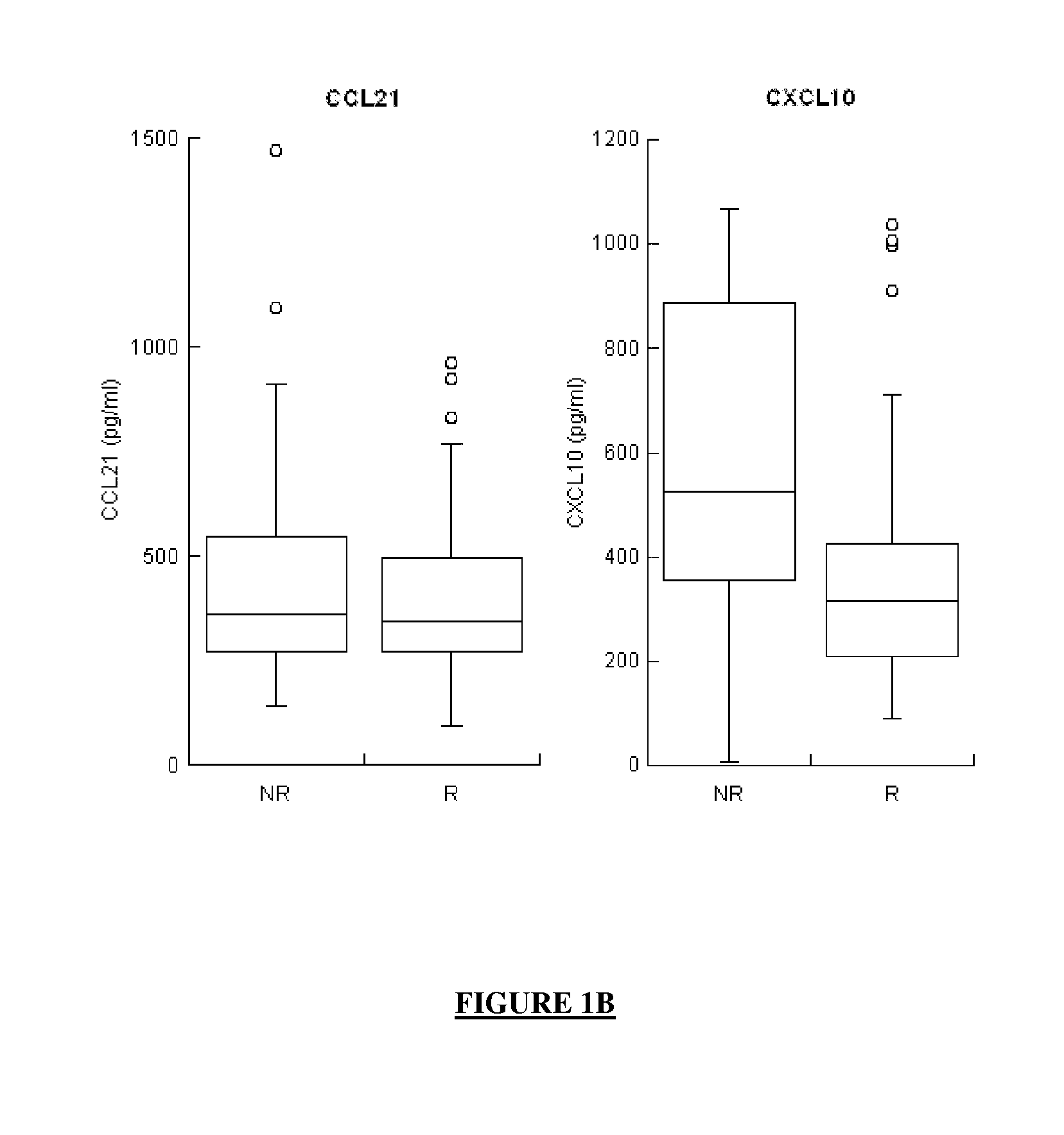
The application concerns means for predicting whether a subject infected with one or more HCVs has a high probability of responding to an anti-HCV treatment which will comprise the administration of interferon and of ribavirin or whether, in contrast, that subject has a high probability of not responding to that anti-HCV treatment. The means of the invention in particular involve assaying the levels of expression of selected genes, said selected genes being:at least one gene from among MBL2, LGALS3BP and IL8, andat least one gene from among G1P2, CCL21 and CXCL10, andoptionally, at least one gene from among AFP, CRP, CXCL11, CXCL6, CXCL9, FGF7, MDK, MMP2, SFN, TGFB2 and VEGFD.
Owner:BIO RAD EURO GMBH +4
Plasmid constructs for treating cancer and methods of use
PendingCN113412334APolypeptide with localisation/targeting motifElectrotherapyOncologyElectroporation
Described are expression cassettes encoding CD3-half-BiTE, CXCL9 and CTLA-4 scFv. The described expression cassettes can be used to treat cancer in a subject. Methods of delivering the expression cassettes to a tumor by direct intratumoral injection and electroporation are also described.
Owner:安克塞克医疗公司
Method for acquiring information on condition of patient with interstitial pneumonia and use thereof
PendingCN111863122AMedical automated diagnosisDisease diagnosisInterstitial lung diseaseInterstitial pneumonias
The invention relates to a method for acquiring information on a condition of a patient with interstitial pneumonia and application thereof. The present invention addresses the problem of providing ameans capable of identifying the condition of a patient suffering from interstitial pneumonia. The method comprises steps of measuring at least one biomarker in a biological sample of an interstitialpneumonia patient, the biomarker including at least one selected from CXCL9 and CXCL10, a measurement result of the biomarker serving as an index for discrimination between IPAF and CTD-ILD.
Owner:SAPPORO MEDICAL UNIVERSITY +1
Immunotherapy of targeted transportation of chemotactic factor and cytokine via mesenchymal stem cell
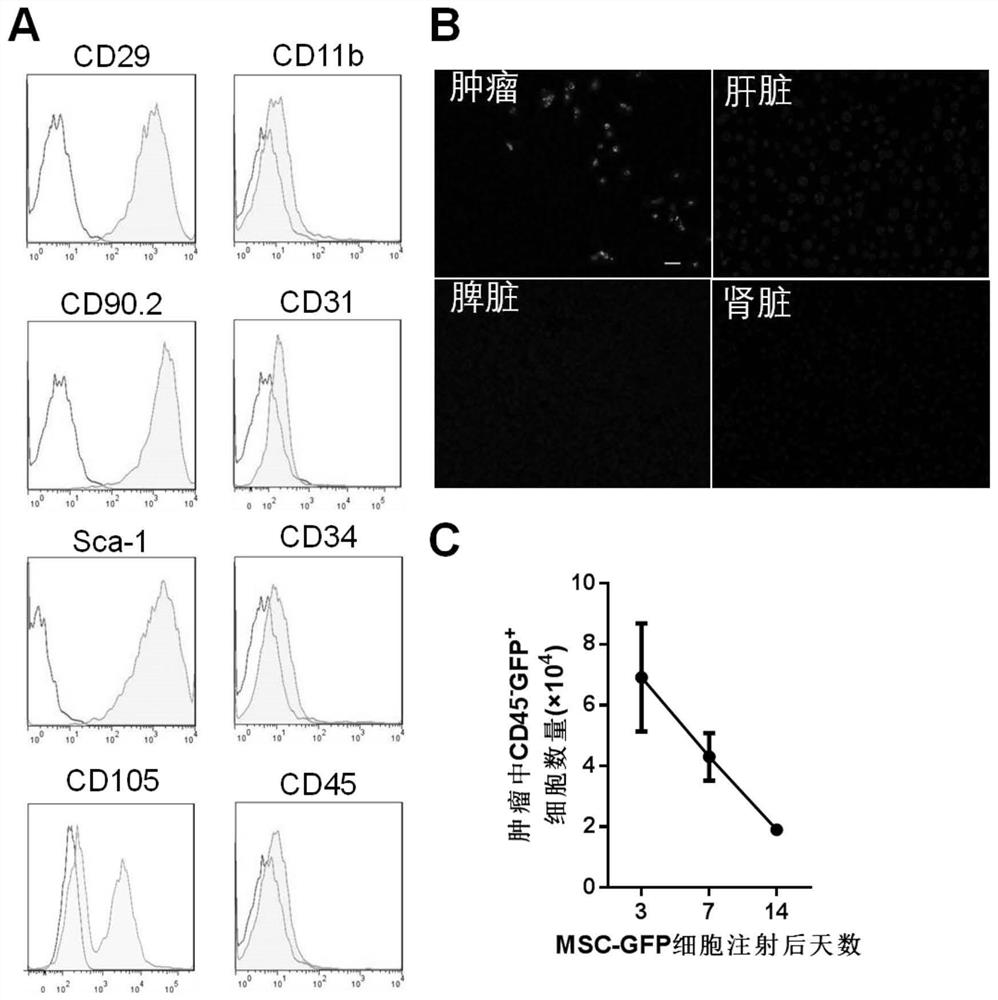
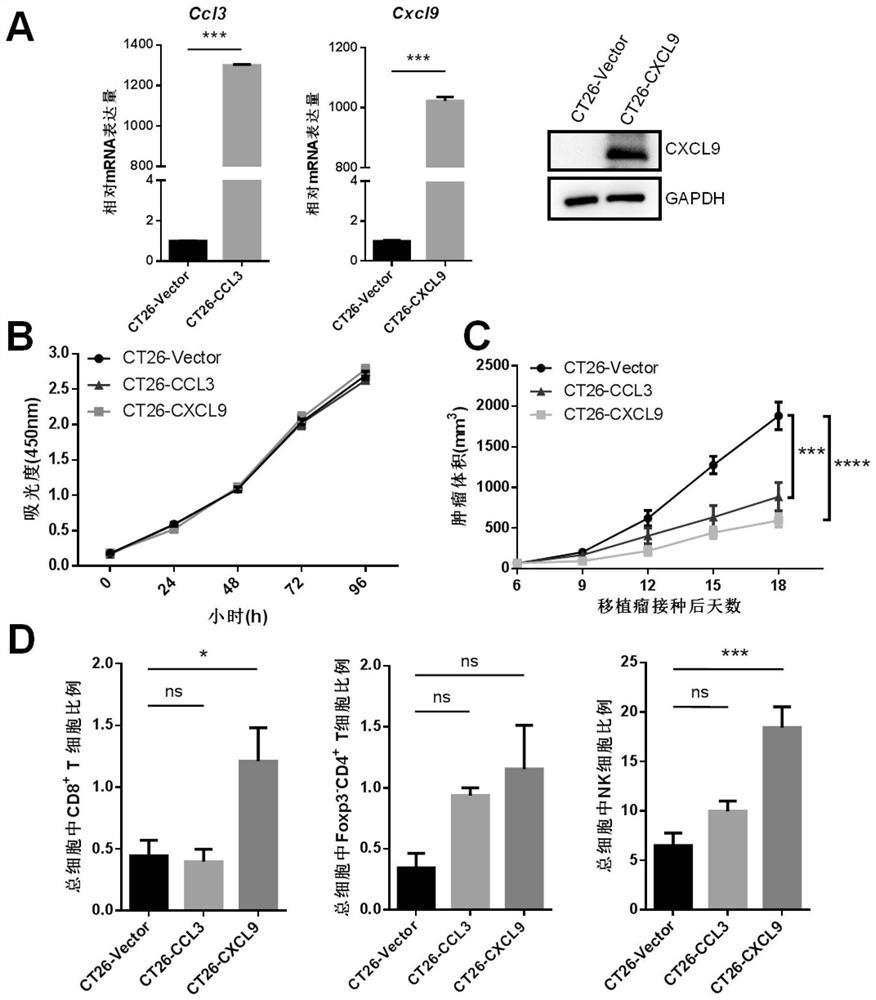
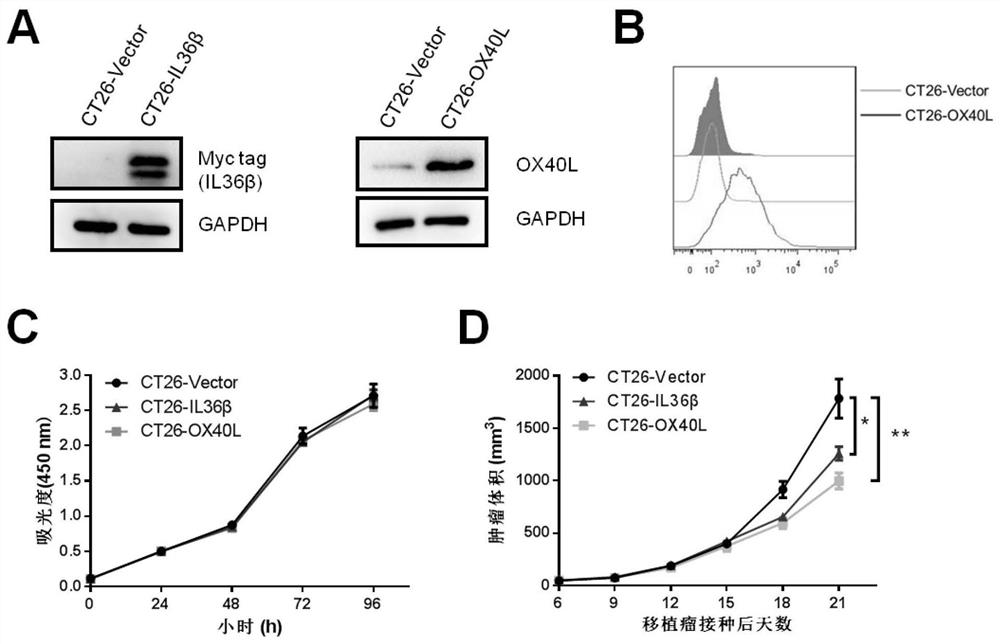
The invention provides immunotherapy for targeted transportation of a chemotactic factor and a cytokine via a mesenchymal stem cell. Specifically, the invention provides the mesenchymal stem cell. Themesenchymal stem cell expresses an immunostimulatory factor, wherein the immunostimulatory factor is selected from the group consisting of CCL3, CCL19, CCL21, XCL1, CXCL9, OX40L, 4-1BBL, GITRL, CD40L, and combinations of the above immunostimulatory factors. The mesenchymal stem cell disclosed by the invention can specifically attract and activate immune cells for killing tumor tissues at a tumorpart, and the mesenchymal stem cell and chemotactic factor and / or cytokine have a synergistic effect, so the mesenchymal stem cell has an immune curative effect which is more efficient and low in sideeffect and is remarkably enhanced in tumor tissue killing capability, especially colorectal cancer cell killing capability.
Owner:SHANGHAI JIAO TONG UNIV
Diagnostic and therapeutic methods for cancer
PendingCN110621787AOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesEfficacy
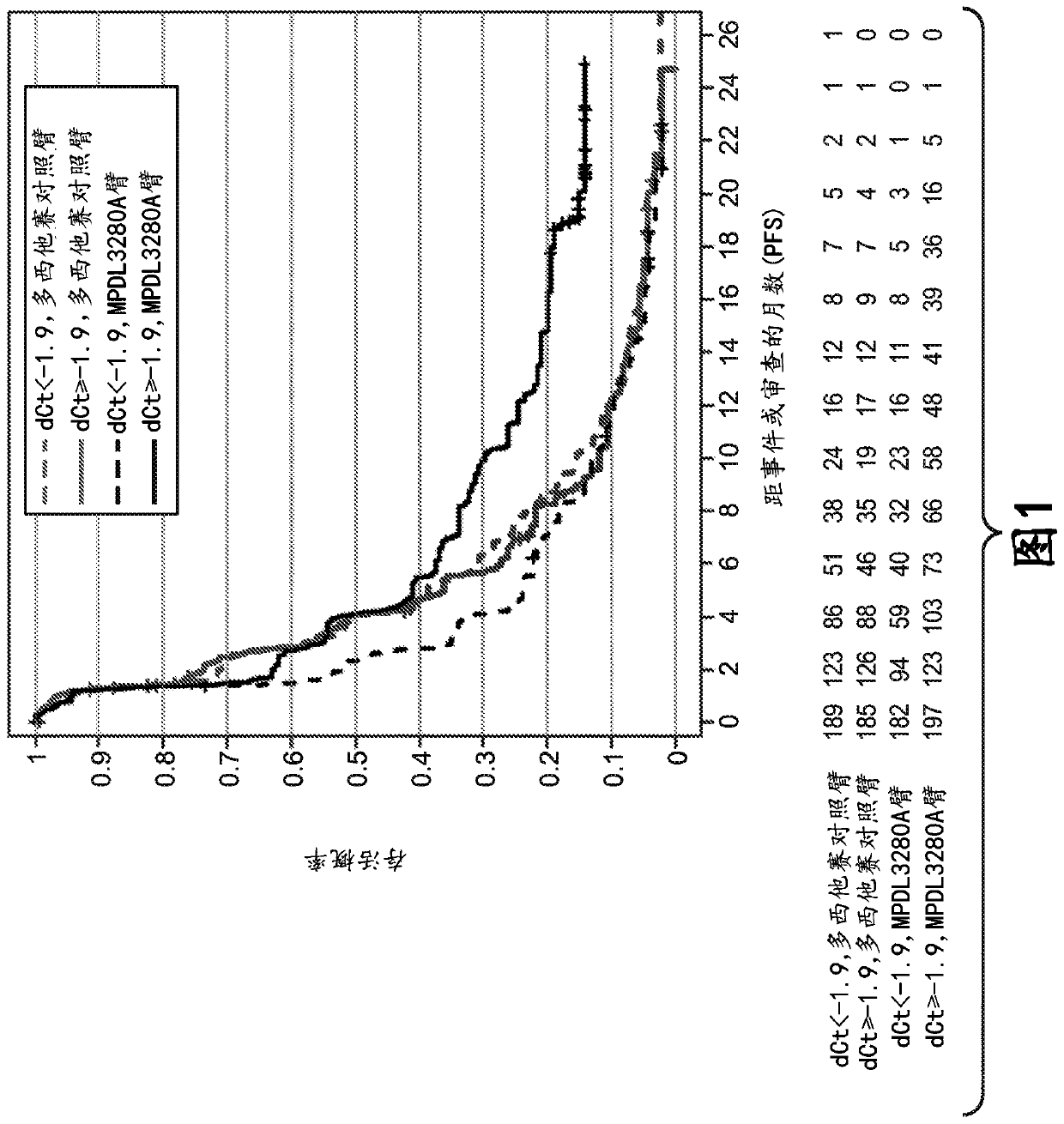
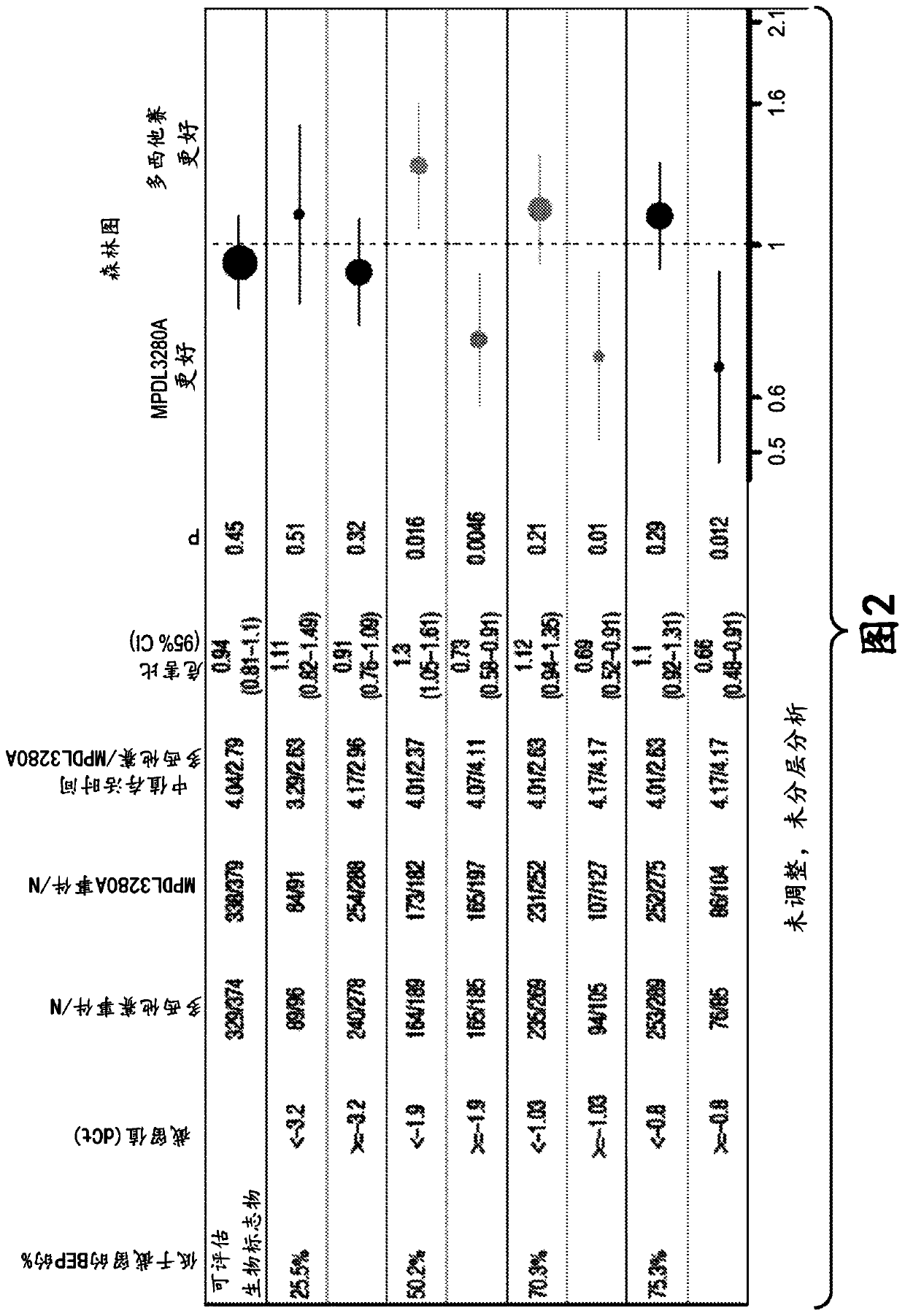
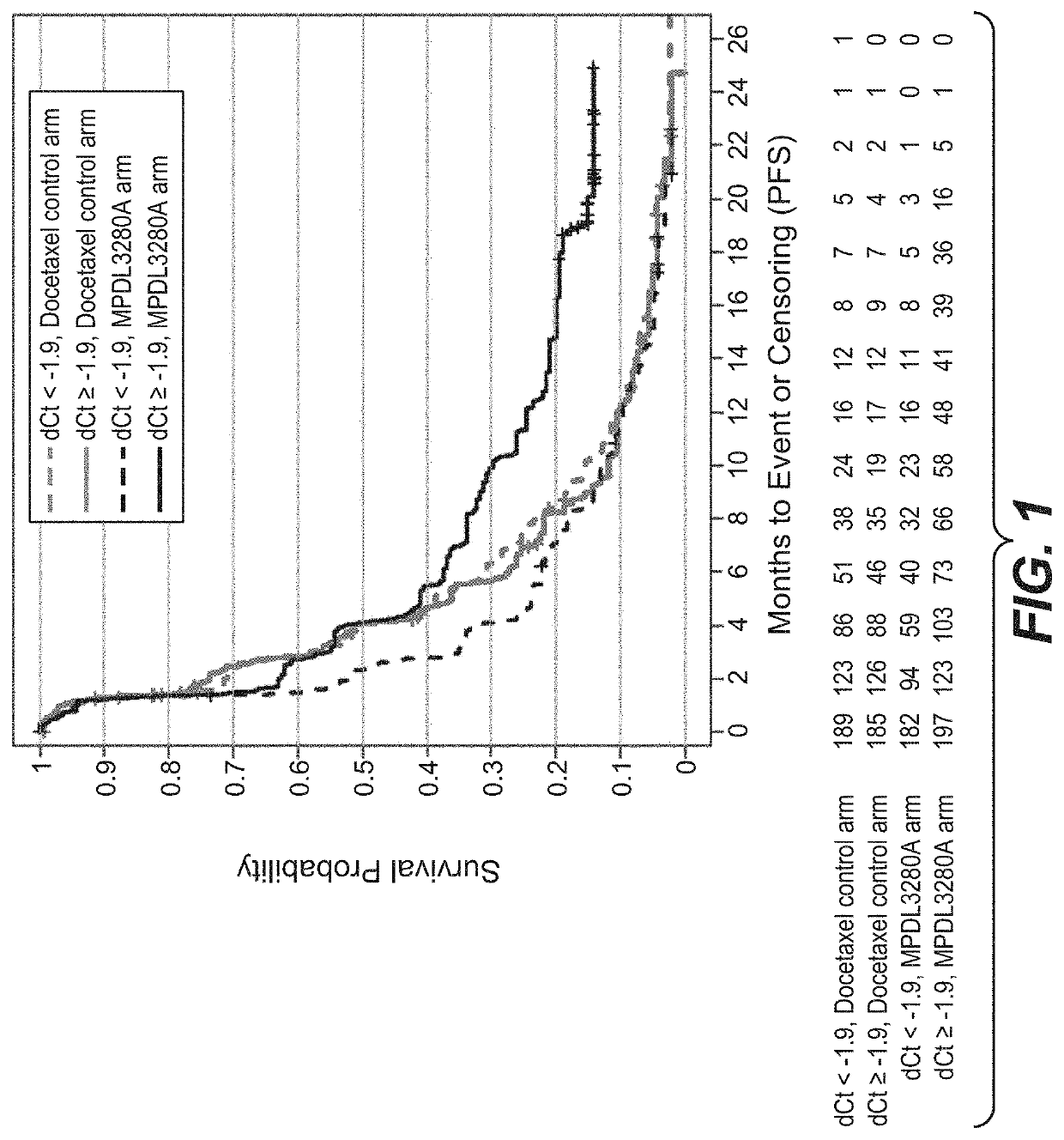
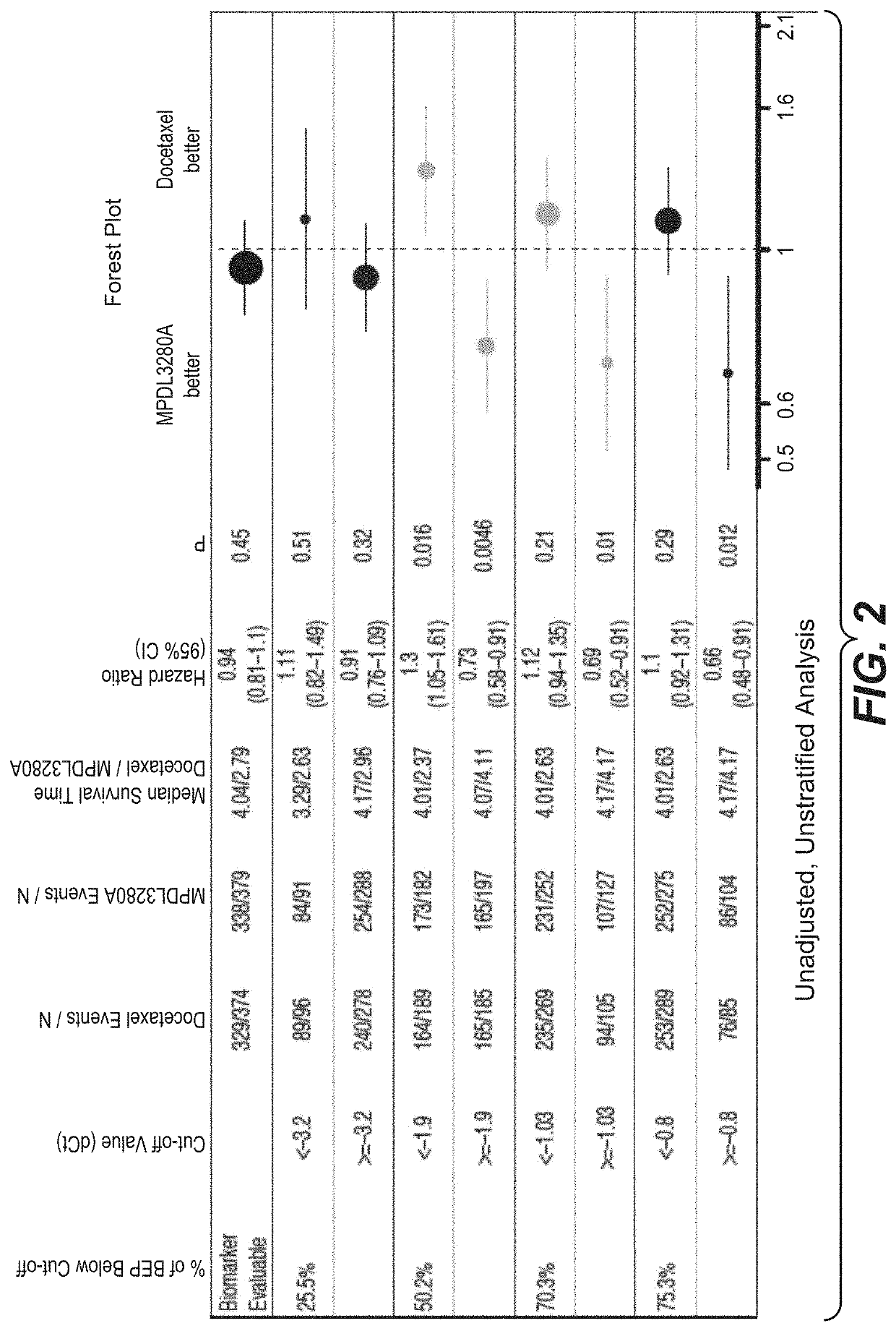
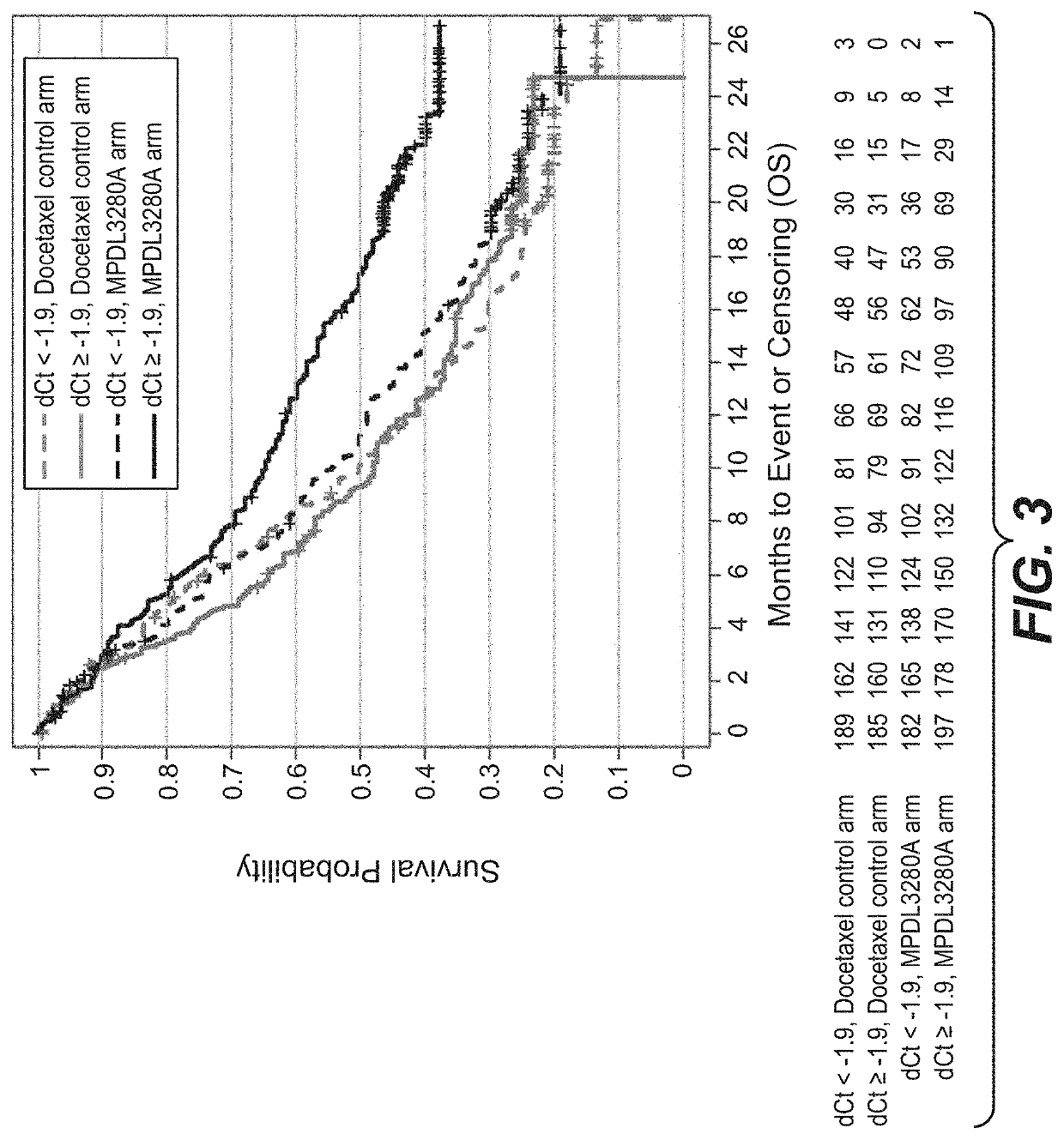
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:F HOFFMANN LA ROCHE & CO AG
Application of IRF1 (Interferon Regulating Factor 1) in regulating and controlling immune regulation effect of mesenchymal stem cells and product
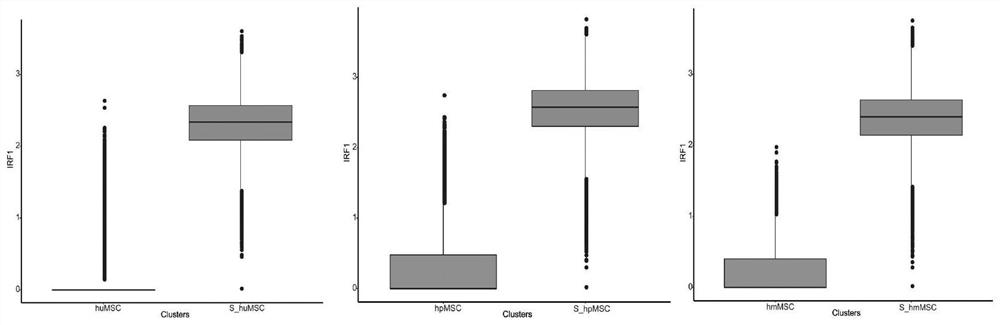
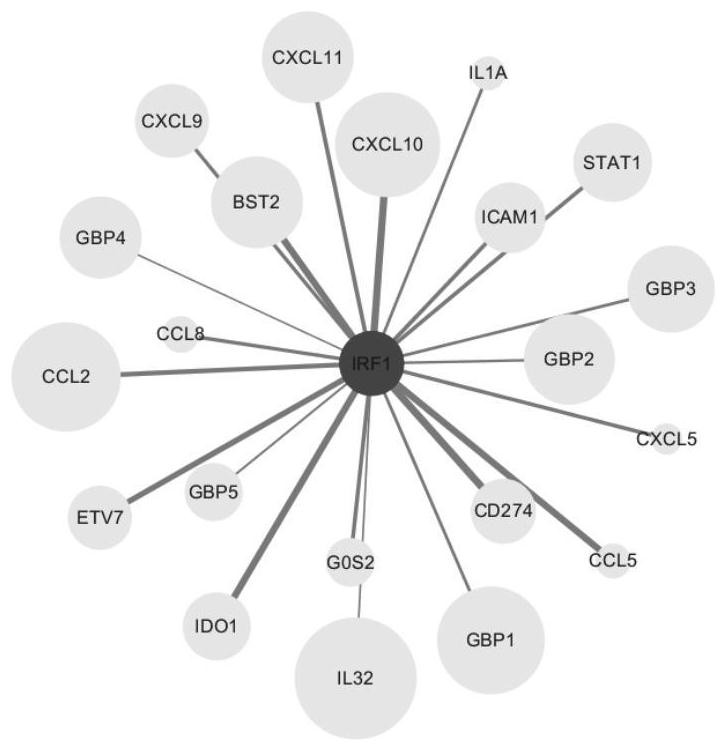
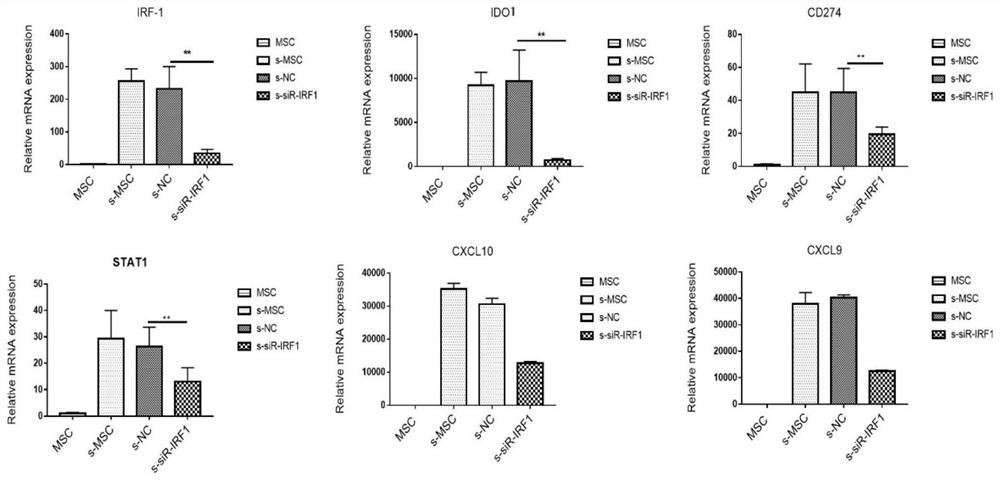
ActiveCN113980896ARegulation of proliferationComplements immune plasticityPeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The invention discloses an application of an Interferon Regulating Factor 1 (IRF1) in regulating and controlling the immunomodulatory effect of human placenta, amnion and umbilical cord derived mesenchymal stem cells. The immunomodulatory effect is the effect of inhibiting T cell proliferation. The biological characteristics of human amniotic membrane, placenta and umbilical cord derived mesenchymal stem cells are researched by utilizing a single cell sequencing technology, and the IRF1 is found to be highly expressed in the placenta, amniotic membrane and umbilical cord derived mesenchymal stem cells in an inflammatory microenvironment. Transcription factor analysis and in-vitro experimental research further prove that the IRF1 can regulate and control the expression of MSC immunosuppressive molecules IDO1, CD274 and STAT1 and chemotactic factors CXCL9 and CXCL10, and in-vitro T cell proliferation experiments find that the IRF1 can regulate and control the T cell proliferation inhibition effect of the umbilical cord MSC, so that an important theoretical basis and a therapeutic target are provided for treatment of autoimmune diseases.
Owner:ACADEMY OF MILITARY MEDICAL SCI
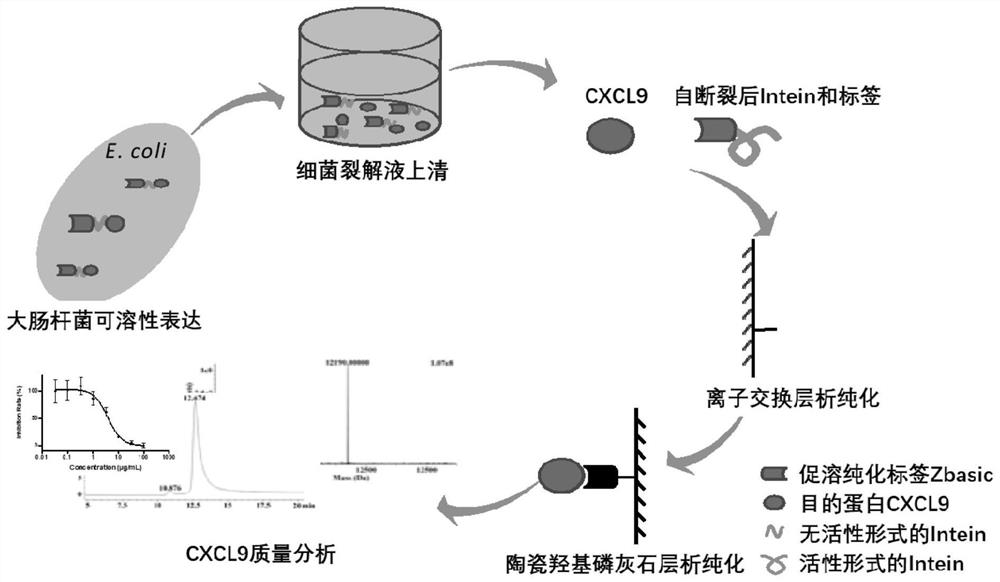
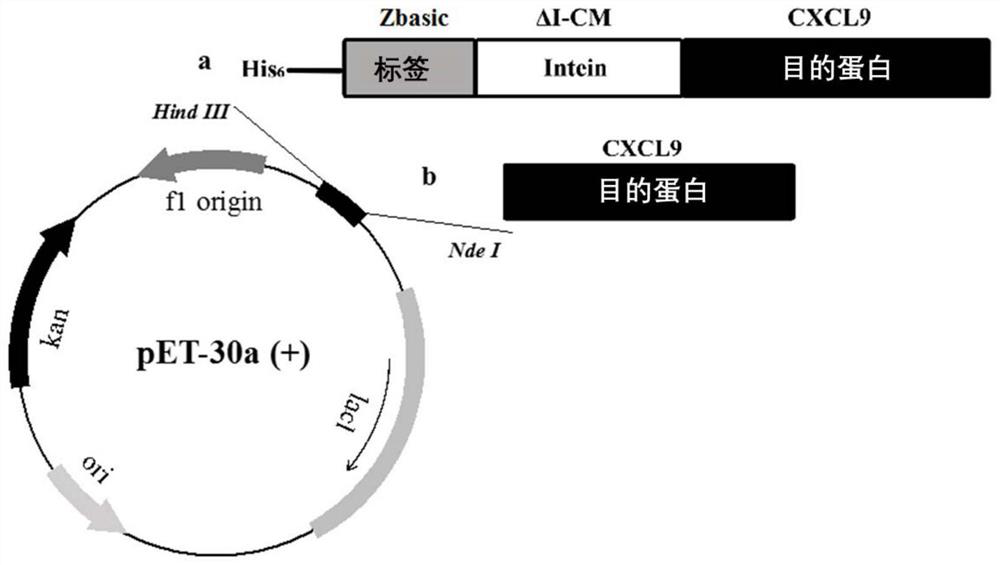
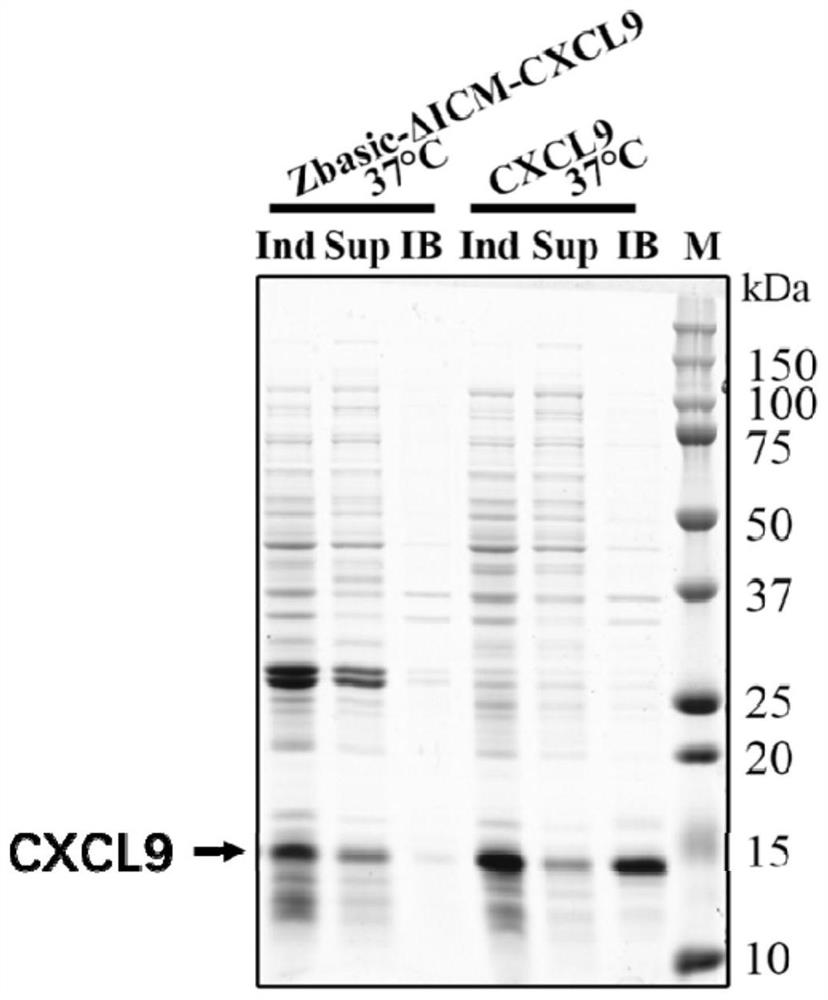
Method for expression and purification of recombinant CXCL9 (C-X-C motif chemokine 9) protein and application of method
ActiveCN108707193AQuickly achieve purificationAchieving soluble expressionChemokinesPeptide preparation methodsEscherichia coliProteinase activity
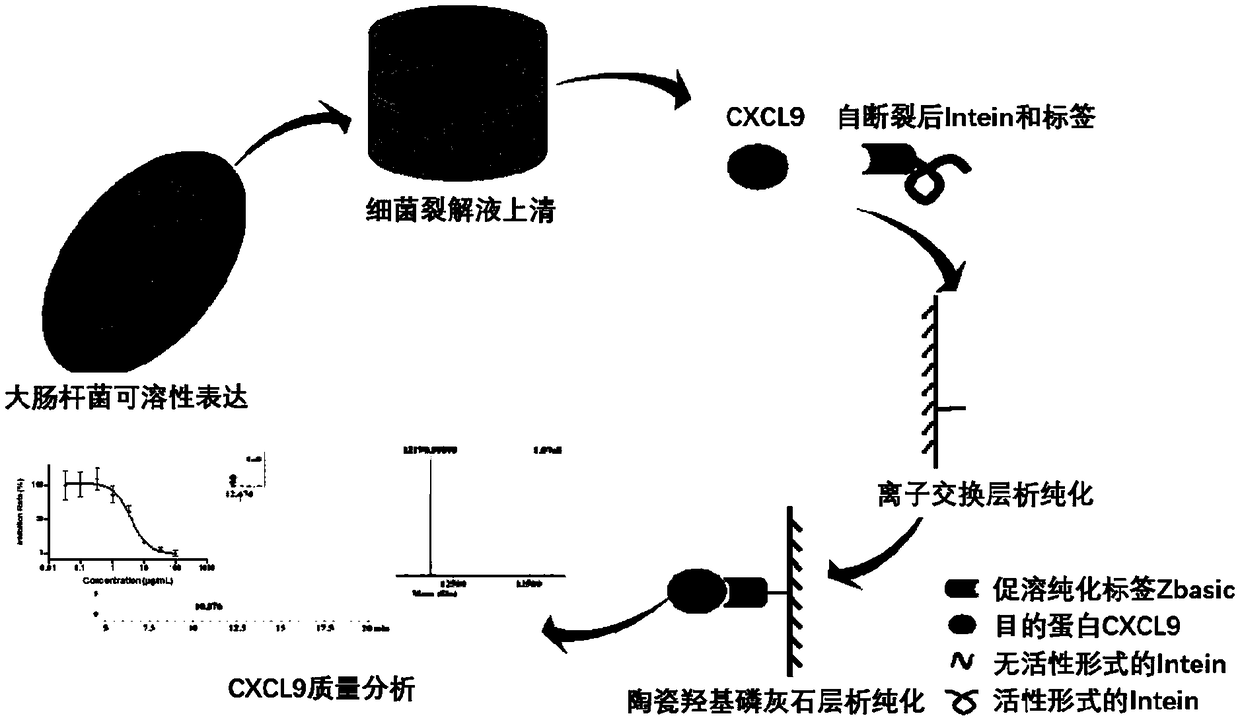
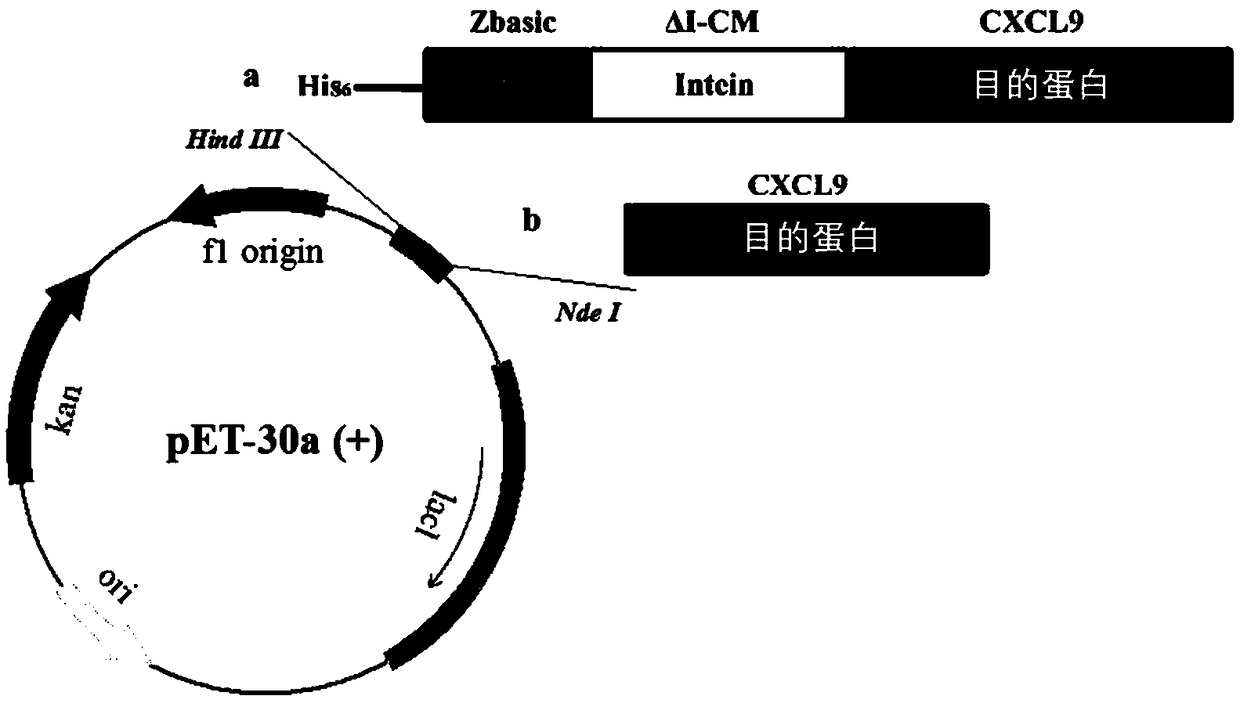
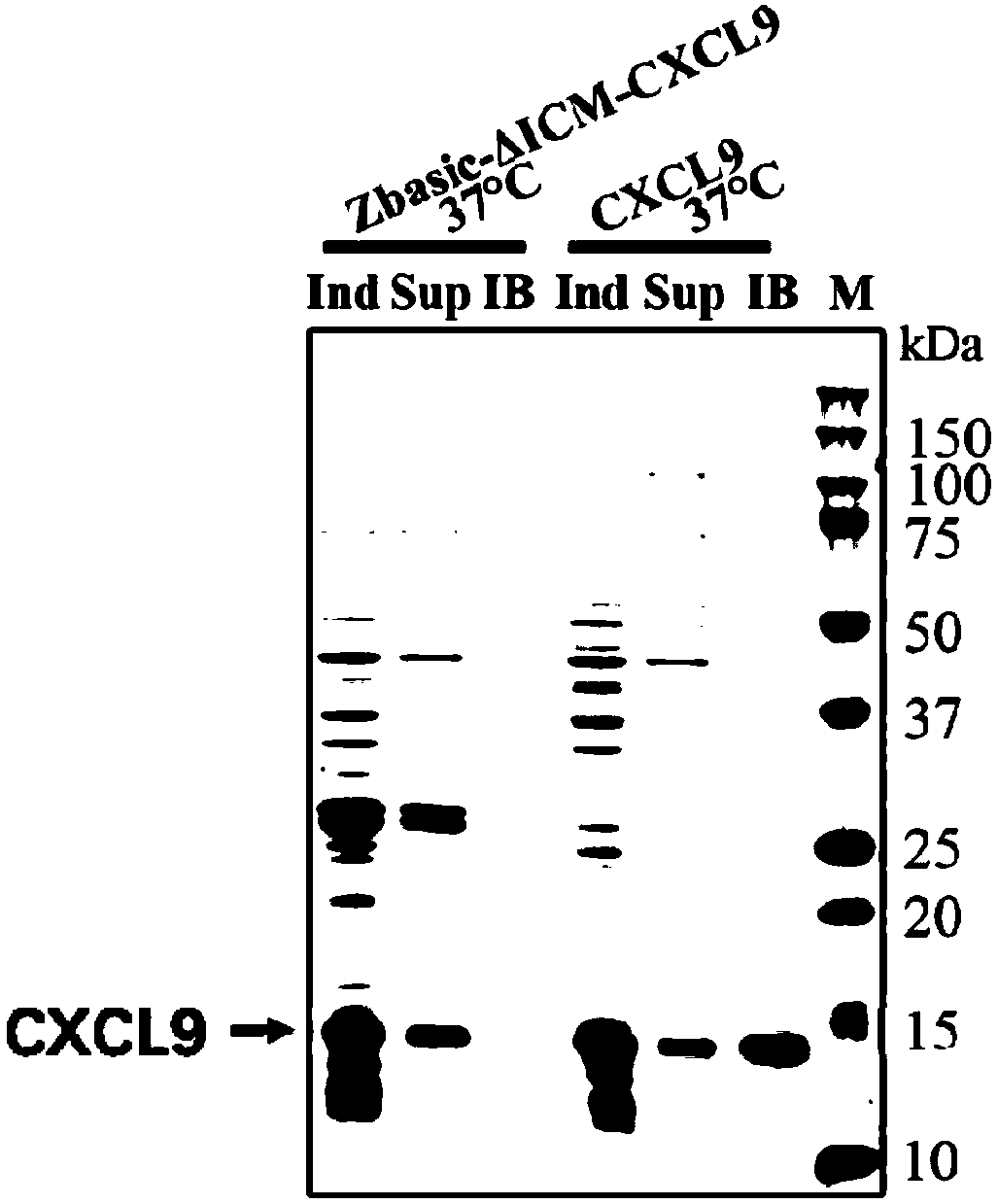
The invention relates to the technical field of biology, in particular to a method for achieving soluble expression of recombinant CXCL9 (C-X-C motif chemokine 9) protein in Escherichia coli by fusionof a solubilizing label and Intern. The target protein is expressed by fusion with the solubilizing label and the Intein with the self-shear function, and soluble protein and shear release of the target protein are realized. Compared with a known method of expression of recombinant CXCL9 protein in Escherichia coli, the method has the advantages that the process of inclusion volume degeneration renaturation can be avoided, introduction of protease is not needed, a target product with the completely naturally sequence can be obtained, and good application prospect for the enlarged-scale industrial production is achieved.
Owner:SHANGHAI JIAO TONG UNIV +1
Prognostic and treatment response predictive method
PendingUS20210139999A1Easy to sign forImprove predictive performanceMicrobiological testing/measurementBiostatisticsCCL2IL12A
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of at least 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL A in a sample obtained from the tumour of the patient; b) measuring the gene expression of at least 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained from the tumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including with immune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Marker for evaluating responsiveness and prognosis survival of advanced bladder cancer anti-tumor immunotherapy and application of marker
ActiveCN112481380AIncreased sensitivityStrong specificityMicrobiological testing/measurementAntineoplastic ImmunotherapeuticOncology
The invention relates to a marker for evaluating anti-tumor immunotherapy responsiveness and prognosis survival of advanced bladder cancer and application of the marker. The marker consists of the following six genes: CDH18, CXCL10, FOXN4, SLC6A4, CXCL9 and PCDH11X. The invention further comprises application of a reagent for detecting the expression quantity of the marker in preparation of a kitfor evaluating responsiveness and prognosis survival of advanced bladder cancer anti-tumor immunotherapy. According to the invention, full transcriptome sequencing is carried out on advanced bladder cancer specimens based on large-sample anti-tumor immunotherapy, and screening and construction are carried out after machine learning, so that the reactivity of the advanced bladder cancer patients receiving the anti-tumor immunotherapy can be efficiently and accurately predicted; effective guidance opinions can be provided for clinicians to make treatment decisions on patients with advanced bladder cancer, invalid treatment is reduced, and therefore the treatment cost and discomfort experience of the patients are reduced.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Methods for Detecting and Treating Rhinovirus Infection
The invention provides a method for evaluating the activity of an agent for treating rhinovirus infection or a symptom thereof, a method of detecting or monitoring rhinovirus infection, and a method of treating rhinovirus infection or a symptom thereof. Various embodiments comprise measuring expression of (i) one or more genes selected from the group consisting of CRY2, B3GAT3, C10ORF95, and BATF3, and (ii) one or more genes selected from the group consisting of RNFT2, BTG4, PSD3, CAPN9, SULT1E1, HEY1, LRRC36, RAB3B, ALDH3B1, FAM134B, FAS, PLSCR1, CLEC2B, HAS2, MX1, SP110, GBP1, IFIT3, IFIT1, CXCL9, CXCL10, and CXCL11, from at least one biological sample to produce a gene expression profile, and comparing the gene expression profile to a reference gene expression profile. Systems, computer readable media, compositions, and methods for maintaining or improving respiratory health also are provided.
Owner:PROCTER & GAMBLE CO
CXCL9 modified chimeric antigen receptor T (CAR-T) structure and application thereof
ActiveCN112625142APromote infiltrationPromote growthPolypeptide with localisation/targeting motifImmunoglobulin superfamilyOncologyCell therapy
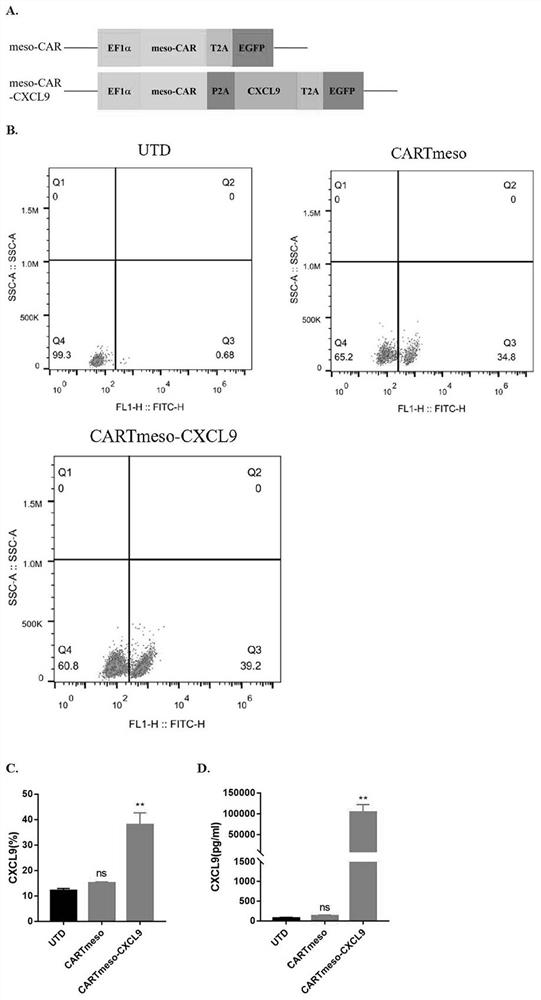
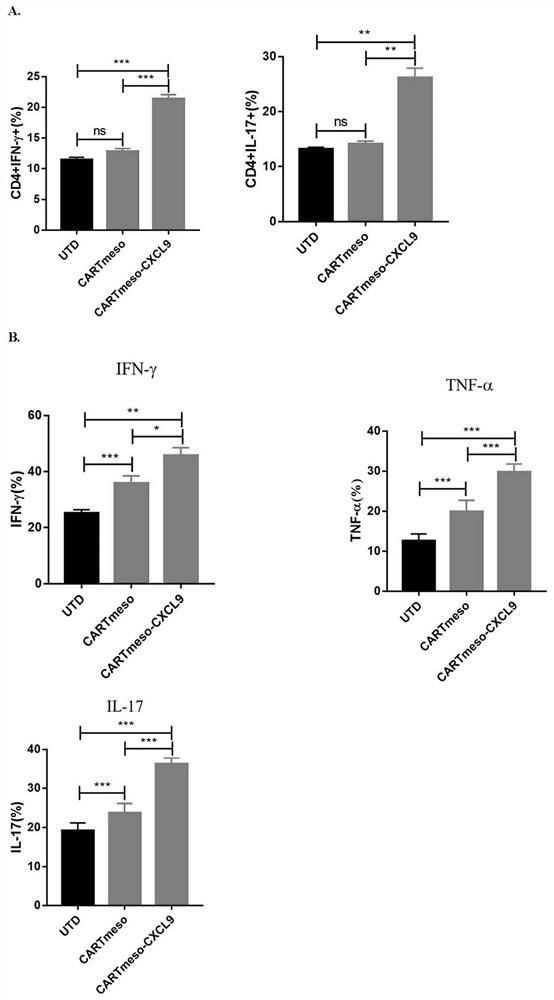
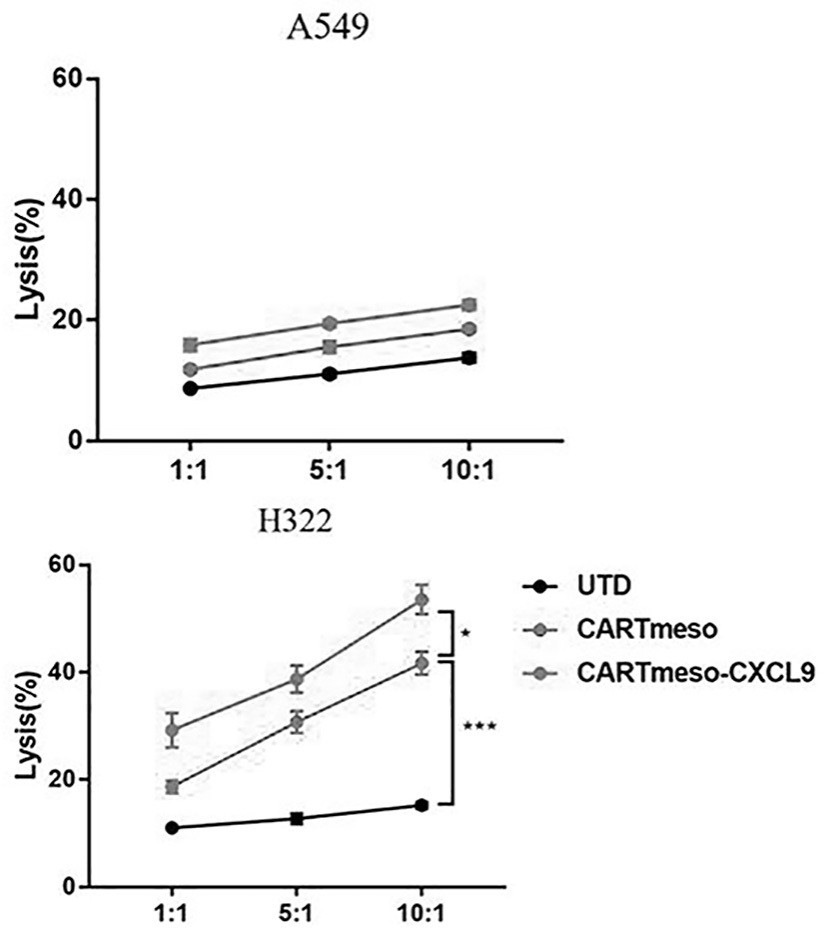
The invention belongs to the technical field of tumor immunotherapy, and particularly relates to a CXCL9 modified chimeric antigen receptor T (CAR-T) structure and application patent application matter thereof. The CXCL9 modified CAR-T structure meso-CAR-CXCL9 has a specific structure: CD8a-meso-CD28-CD3Zeta-P2A-CXCL9. In the application, inventors obtain CAR-T-CXCL9 cells based on mesothelin design by taking a lung cancer model as an example. Preliminary experiment results show that, compared with conventional CAR-T cells, the modified cells have higher cytokine secretion and cytotoxicity capacities, can obviously increase immune cell infiltration of a tumor site, and inhibit angiogenesis, and further, the modified CAR-T-CXCL9 cell therapy slows down tumor growth, increases the survival rate of mice, and shows excellent anti-tumor activity.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Screening method of tumor immunotherapy prognosis marker and application
PendingCN111863130AAssessing immunogenicityGood treatment effectProteomicsGenomicsCandidate Gene Association StudyGZMB
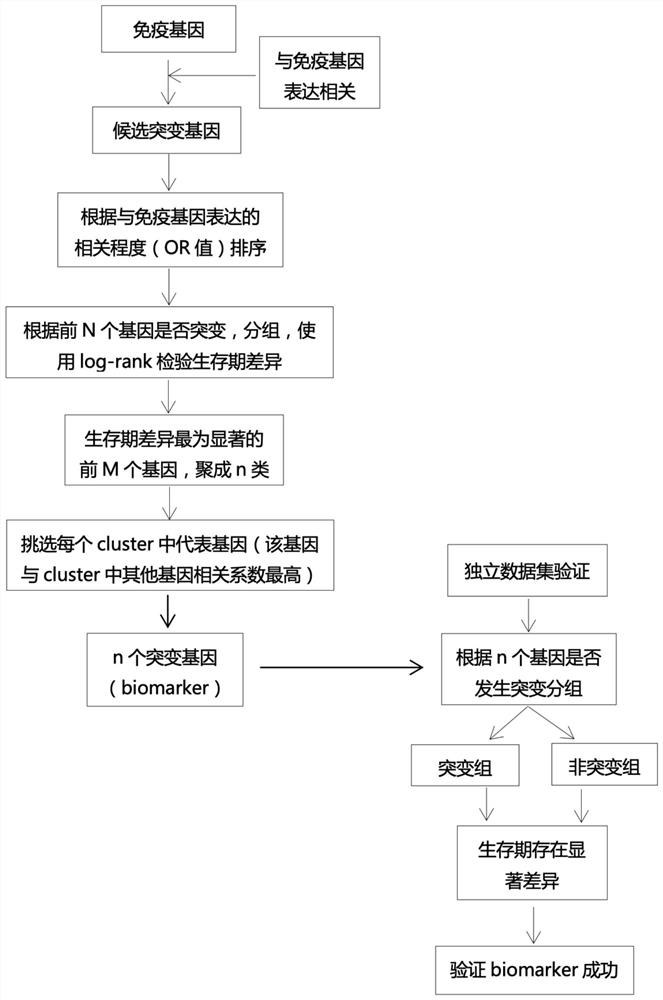
The invention relates to a screening method of a tumor immunotherapy prognosis marker. The screening method comprises the following steps: acquiring expression data, mutation data and clinical curative effect data of a candidate gene group of cancer species to be analyzed; performing statistical independence test on the mutation condition of the candidate gene group and the expression condition ofan immune genome, finding out gene mutation related to the immune genome, and sorting genes in the candidate gene group according to association degrees from high to low, wherein the immune genome comprises CD8A, GZMA, GZMB, IFN [gamma], EOMES, CXCL9, CXCL10, and TBX21; dividing the first N genes into a mutation group and a non-mutation group according to whether the first N genes are mutated ornot, performing survival analysis, and selecting M genes with most significant differences for reservation according to log-rank changes; validating the resulting genes in independent datasets to determine mutation and prognostic correlation thereof.
Owner:SHANGHAI ORIGIMED CO LTD
Methods and compositions for diagnosis and treatment of disorders in patients with elevated levels of cxcl9 and other biomarkers
Owner:SWEDISH ORPHAN BIOVITRUM AG
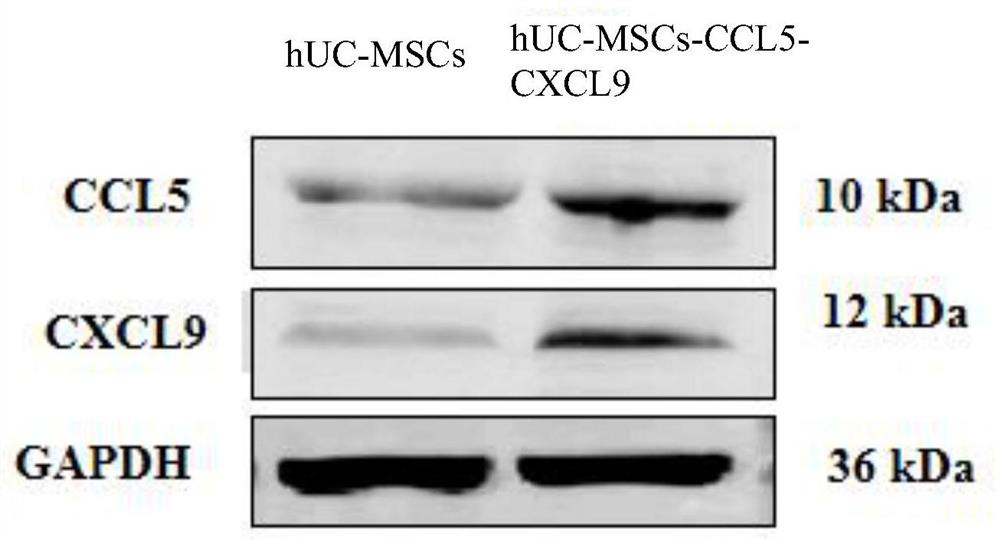
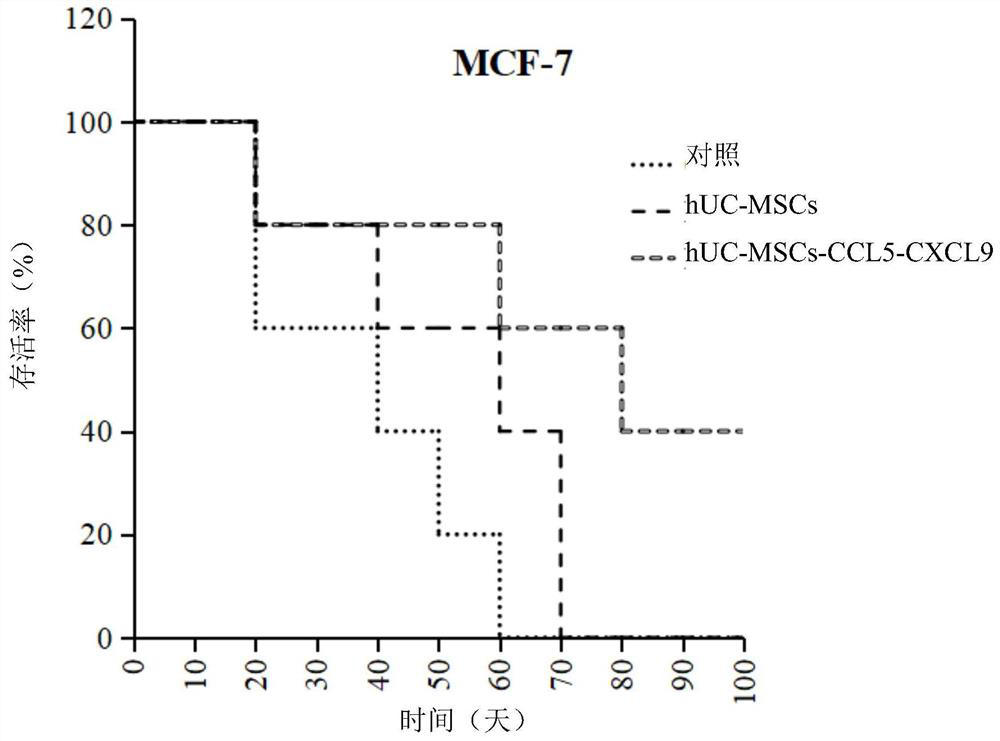
Gene modified mesenchymal stem cell and application thereof
PendingCN112029730AEffective therapeuticEffective preventionChemokinesUnknown materialsMesenchymal stem cellPharmaceutical drug
The invention provides a gene modified mesenchymal stem cell and application thereof. The gene modified mesenchymal stem cell expresses CCL5 and CXCL9 proteins. The invention also provides applicationof the gene modified mesenchymal stem cell in preparation of drugs for treating or preventing tumors, and application of the gene modified mesenchymal stem cell in preparation of vaccines for treating or preventing tumors. The scheme for treating or preventing tumors by using the gene modified mesenchymal stem cell provided by the invention can significantly prolong the lifetime of tumor-bearingmice.
Owner:深圳市芥至和生物科技有限公司
Prognostic and treatment response predictive method
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of atleast 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL1A in a sample obtained from the tumour of the patient; b)measuring the gene expression of atleast 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained fromthetumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including withimmune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
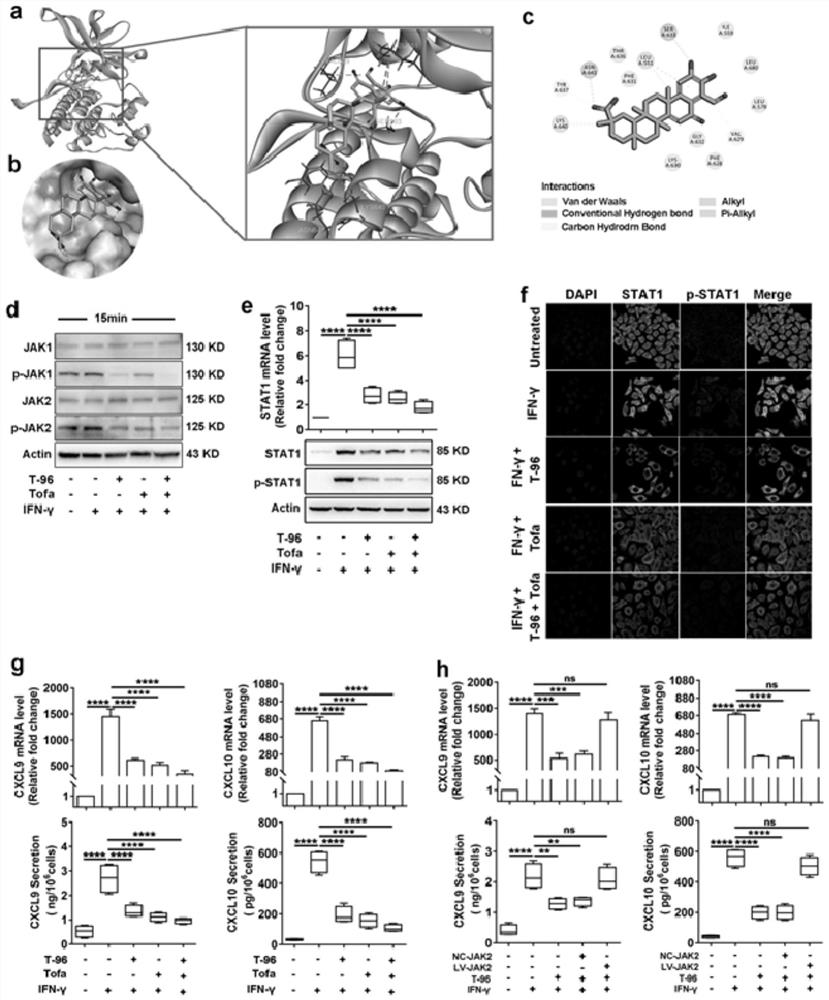
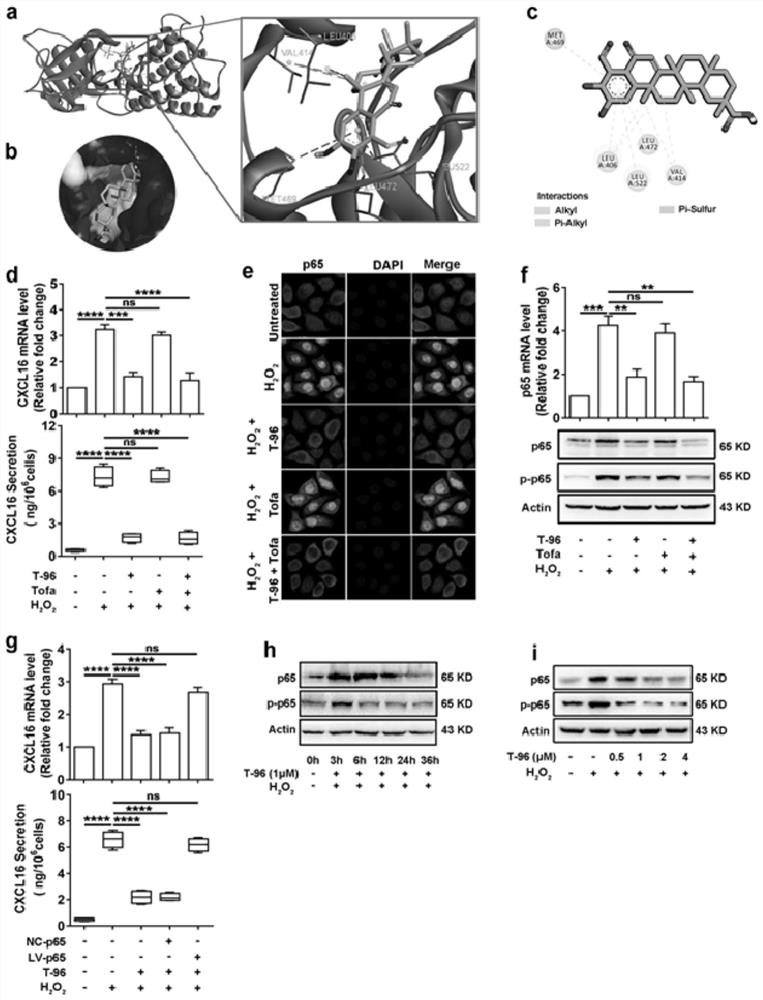
Application of demethylzeylasteral in medicine for treating leucoderma and ointment prepared from demethylzeylasteral
ActiveCN112933100AEffective therapeutic effectHigh purityOrganic active ingredientsAerosol deliveryCD8T cell
The invention belongs to the technical field of biomedicine, relates to an application of demethylzeylasteral in a medicine for treating leucoderma and an ointment prepared from demethylzeylasteral, and determines an effective treatment effect of a tripterygium wilfordii monomer demethylzeylasteral in the aspect of treating leucoderma. The active component demethylzeylasteral in the medicine is used for treating leucoderma by reducing generation and secretion of keratinocyte chemotactic factors CXCL9 / 10 / 16 under inflammatory stimulation and oxidative stress and inhibiting skin migration, activation, proliferation and differentiation of CD8 + T cells. The medicine can be prepared into an oral administration dosage form, a non-oral administration injection or an external dosage form, the prepared medicine is used for treating leucoderma, the ointment is low in toxicity and good in effect, and a foundation is laid for research and development of leucoderma targeted therapy medicines in the future.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Diagnostic and therapeutic methods for cancer
PendingUS20200157635A1Improve responsivenessResponsiveness to treatmentOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:GENENTECH INC
Kit for detection or assisted detection of tuberculous pleurisy
ActiveCN102621331BFew test samplesStrong specificityBiological testingDiseaseSecondary Lymphoid-Tissue Chemokine
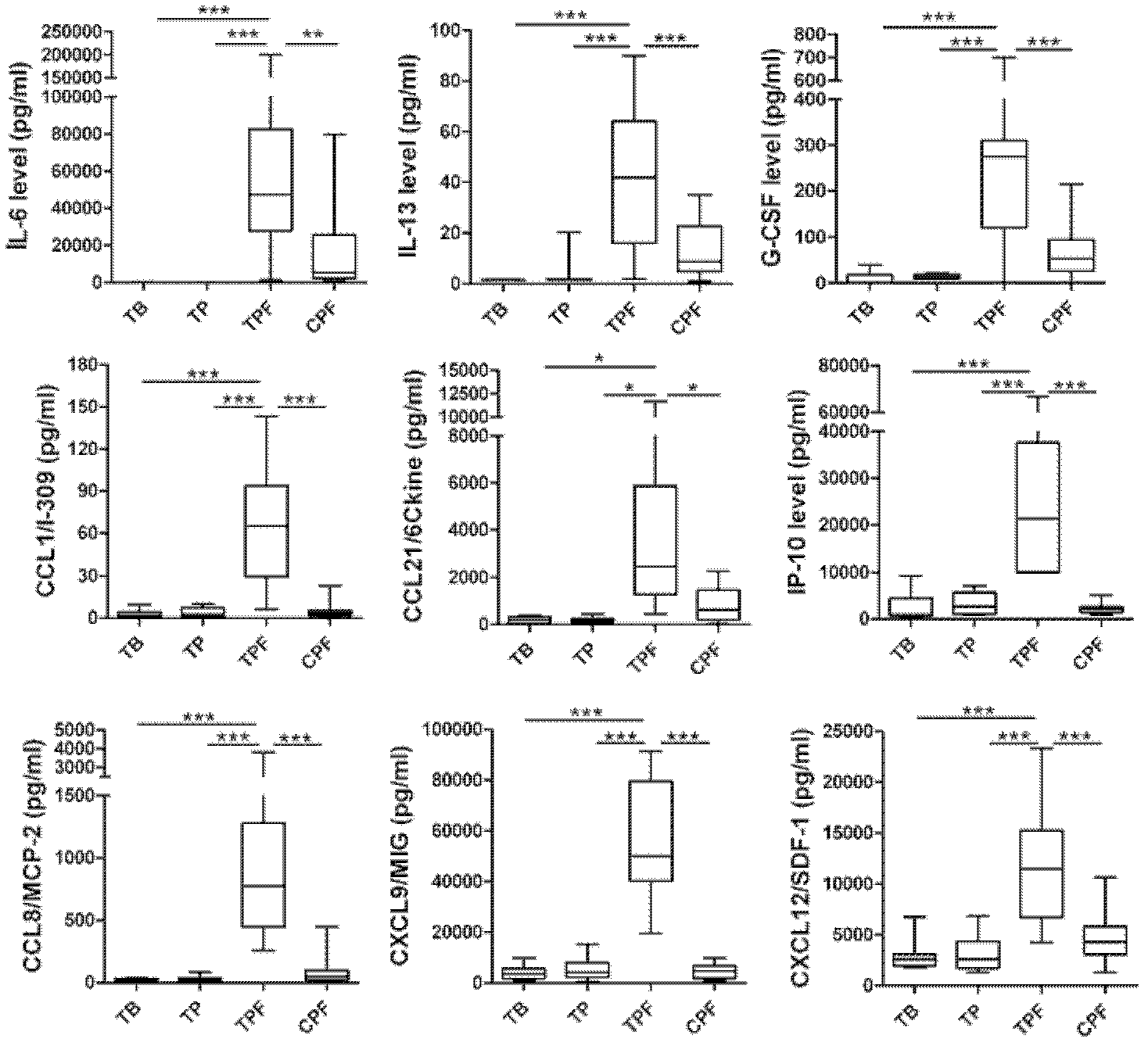
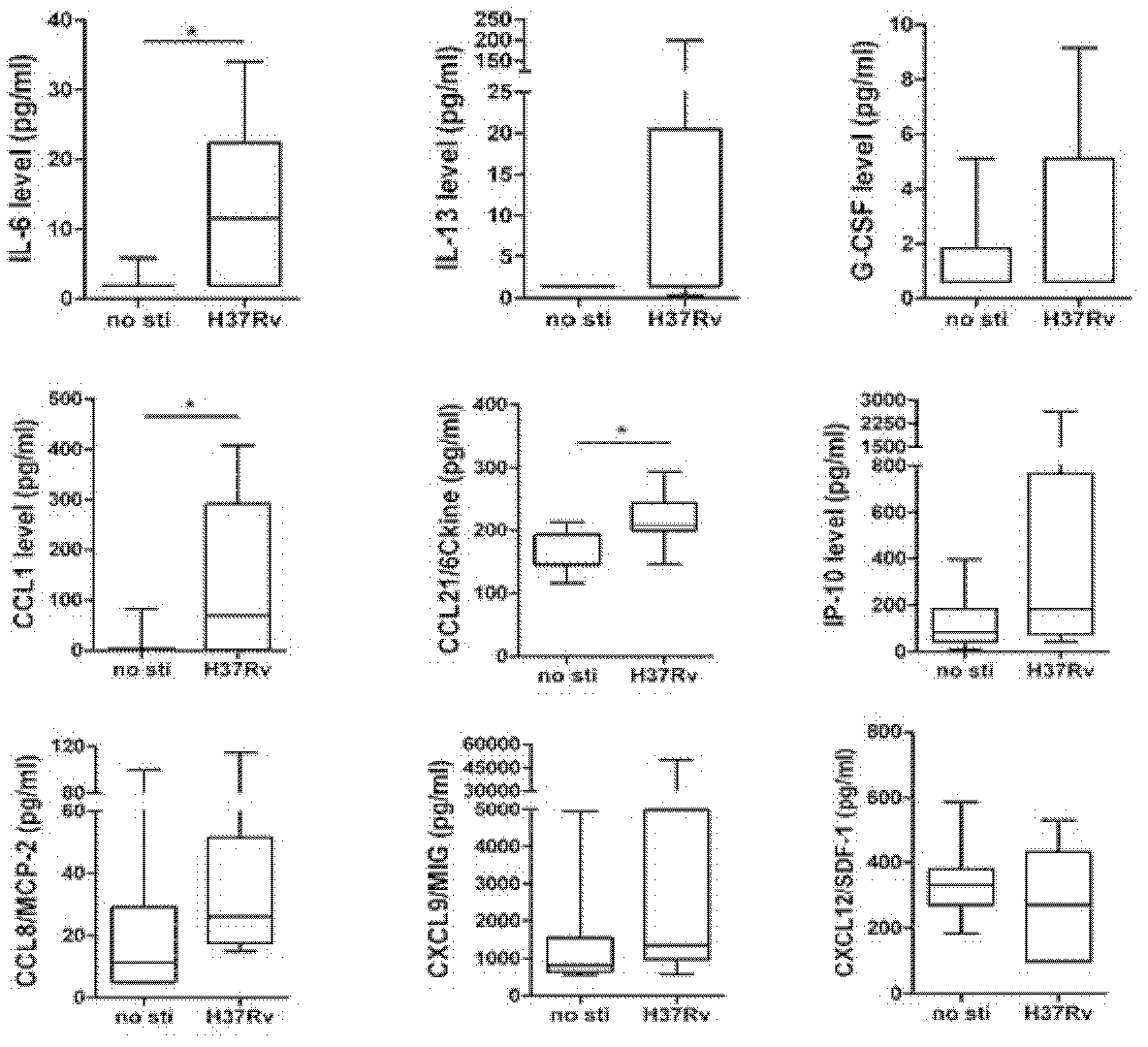
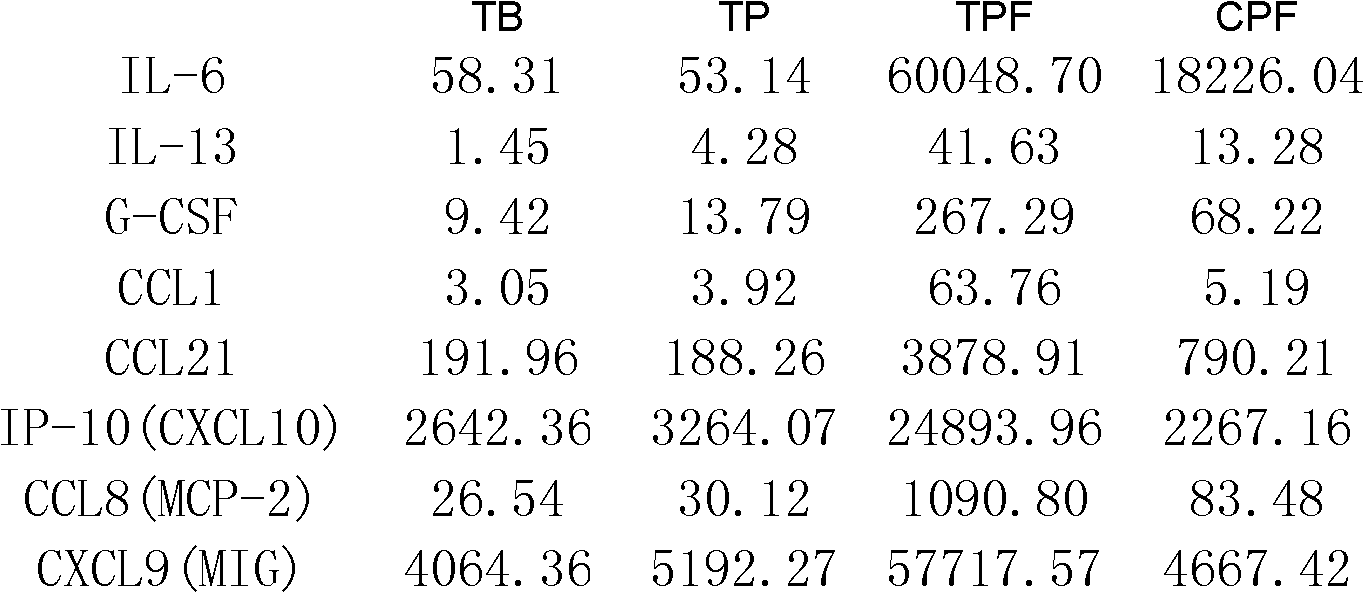
The invention discloses a kit for detection or assisted detection of tuberculous pleurisy. The kit comprises a device for detecting the expression amount of an immune factor, wherein the immune factor comprises (1) IL-6, IL-13, G-CSF, CCL1, CCL21, IP-10 (CXCL10), CCL8 (MCP-2), CXCL9 (MIG) and CXCL12 (SDF-1); or (2) at least one of IL-6, IL-13, G-CSF, CCL1, CCL21, IP-10 (CXCL10), CCL8 (MCP-2), CXCL9 (MIG) and CXCL12 (SDF-1). Experiments prove that a method for specifically identifying tuberculous pleurisy by taking hydrothorax as a detection object, utilizing a group of immunologic molecular marker, and adopting a multiple-factor microsphere detection technique is developed, and the purposes of improving the disease diagnosis accuracy rate, scientifically judging the state of illness and the like are realized by a multiple-factor combination manner.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Cytokine inhibition of eosinophils
InactiveUS20050191273A1Reduced responseReduce assemblyPeptide/protein ingredientsCytokine SuppressionEosinophilic Granulocyte
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Method for acquiring information on respiratory infection
Disclosed is a method for acquiring information on respiratory infection, the method including measuring at least one biomarker in a specimen collected from a subject suffering from respiratory infection, or from a subject suspected of having the respiratory infection, in which the biomarker includes at least one selected from the group consisting of CXCL9, CCL3, and IL-18, and a measured value of the biomarker can serve as an index of a risk of causing acute kidney injury or pulmonary fibrosis following respiratory infection.
Owner:SYSMEX CORP
Method for measuring a biomarker in a biological sample of an ipaf patient
ActiveUS20200341008A1Medical automated diagnosisDisease diagnosisAnti inflammatory treatmentBiologic marker
Owner:SAPPORO MEDICAL UNIVERSITY +1
Compositions and methods for characterizing solid tumors responsiveness to Anti-pd-l1 antibody monotherapy
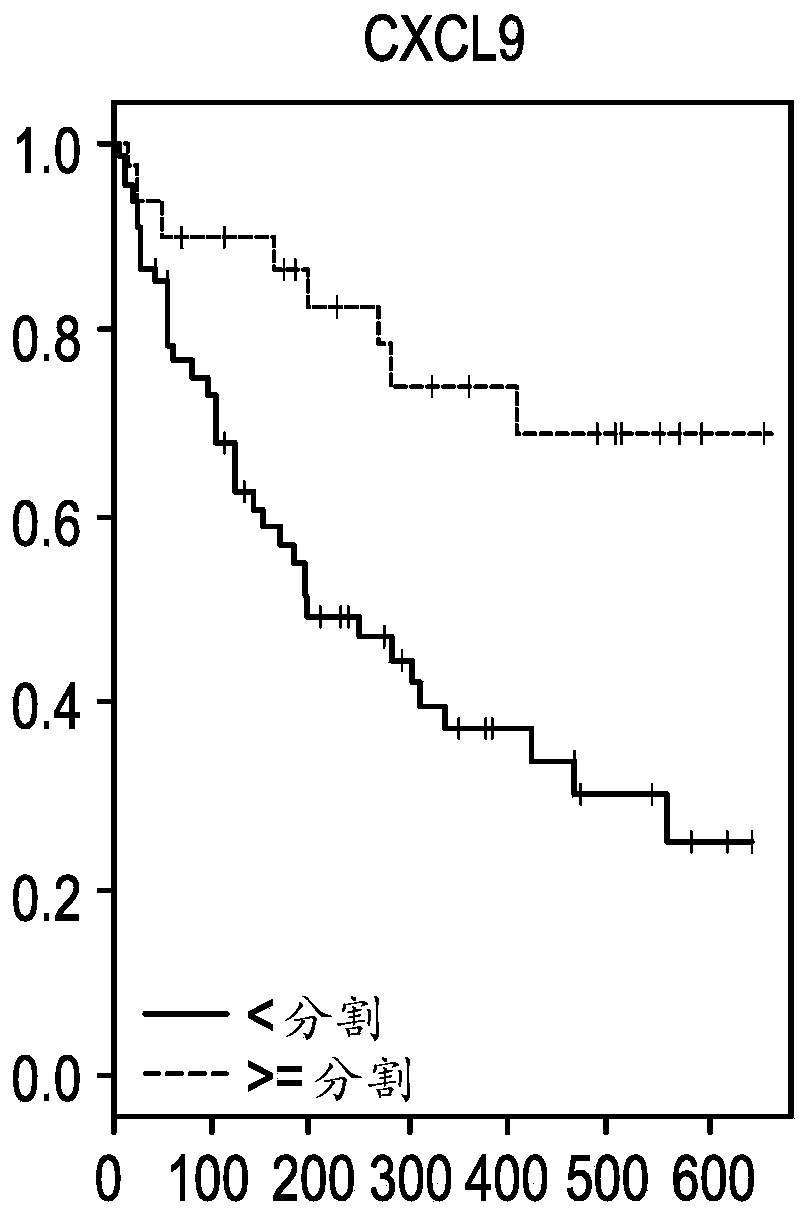
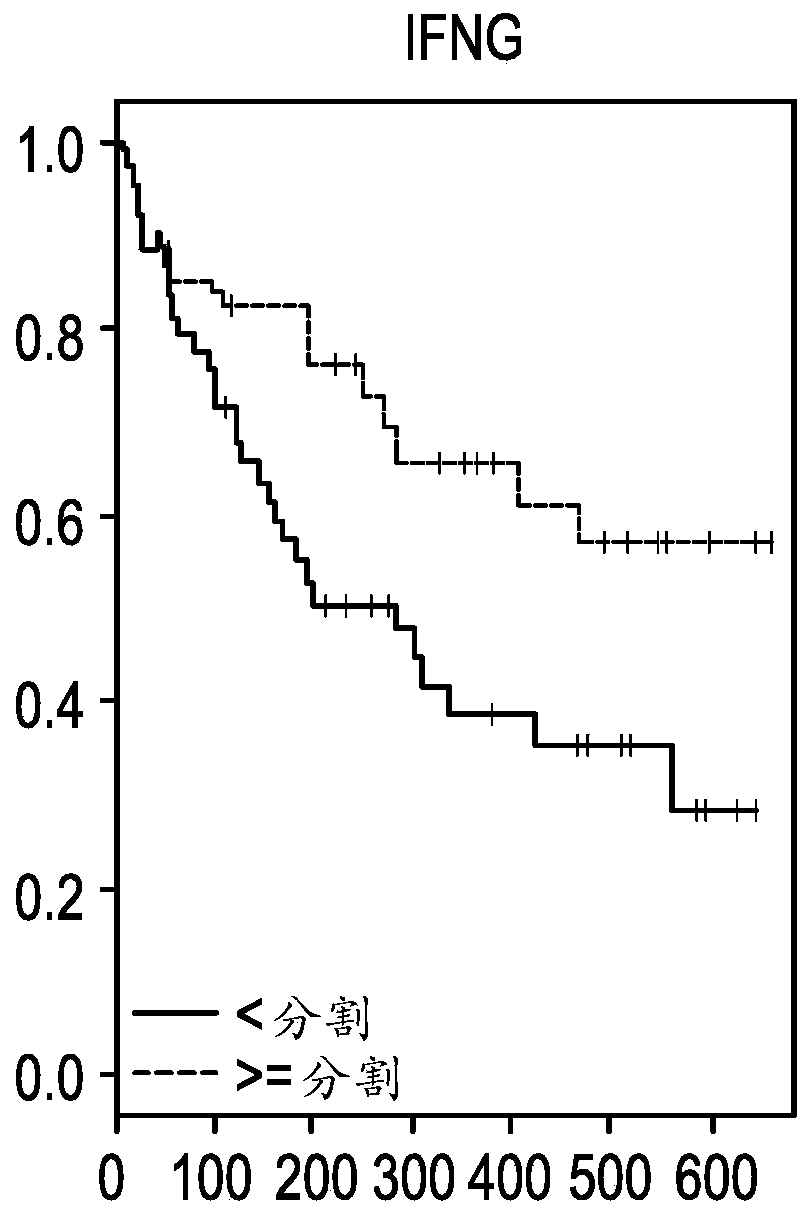
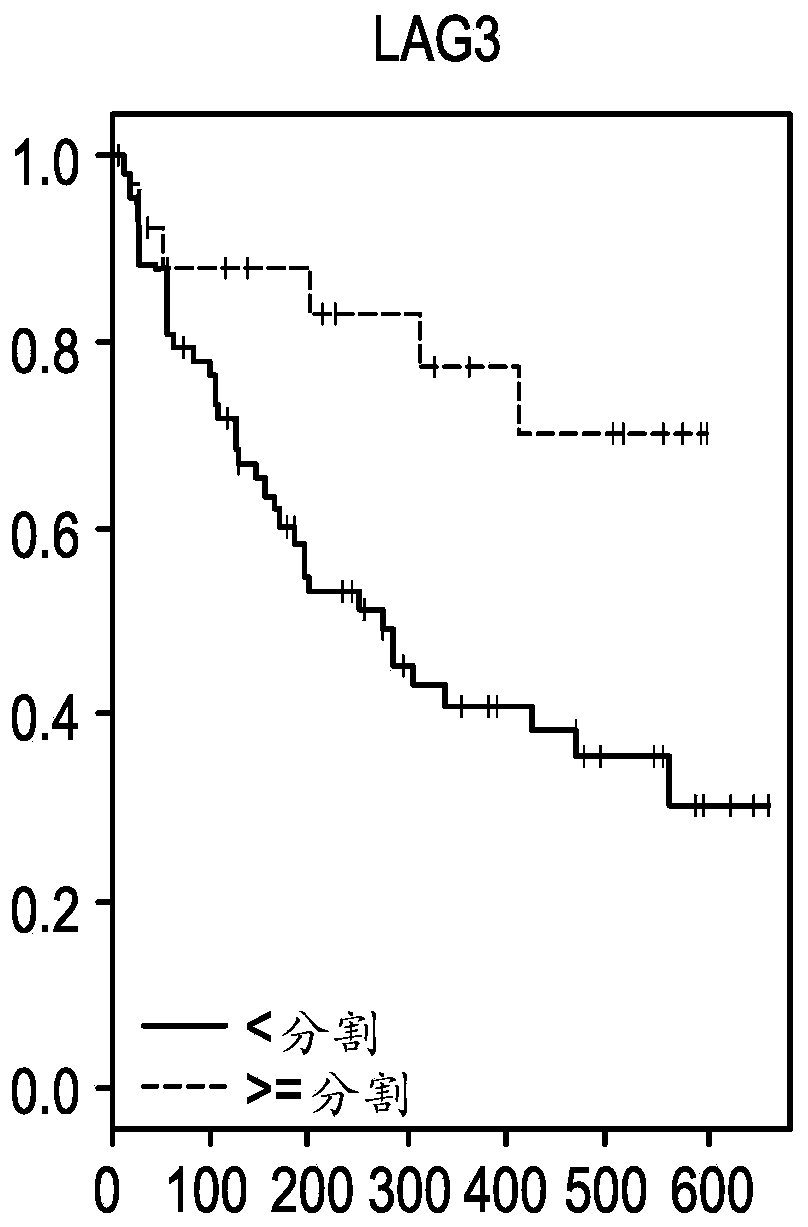
The present invention provides methods for selecting patients as having a solid tumor (e.g., bladder cancer, ovarian cancer, colorectal cancer, head and neck cancer, cervical cancer, renal cell carcinoma, and non-small cell lung cancer (NSCLC)) that is responsive to treatment with an anti-PD-L1 antibody, and methods of treating such patients. The method involves detecting expression of an IFNG polynucleotide and / or one or more of polynucleotide markers: CXCL9, CD274, and LAG3, in a biological sample of the patient.
Owner:免疫医疗有限责任公司
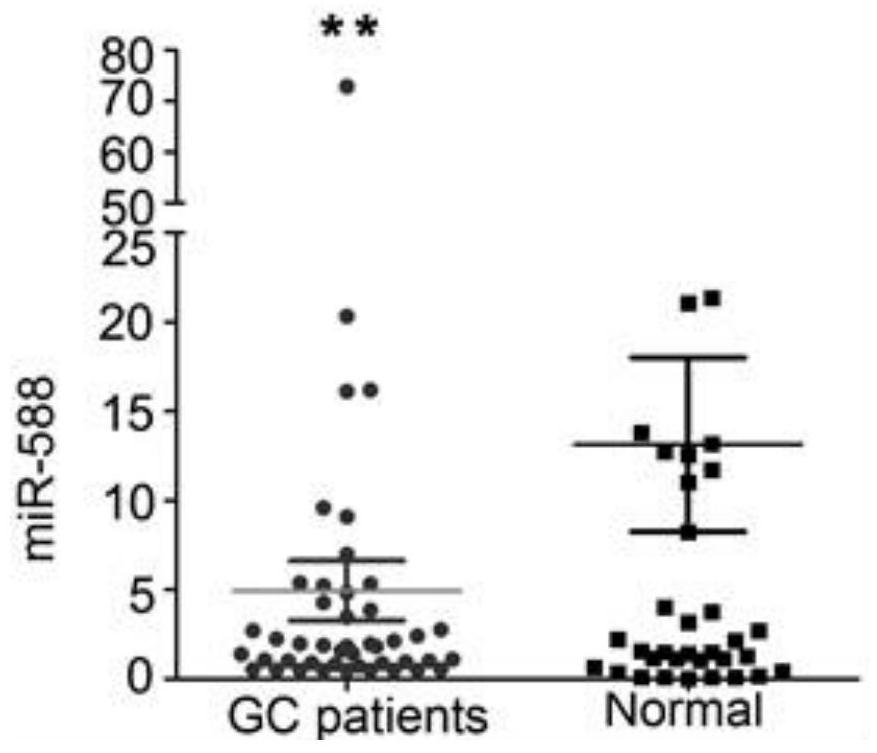
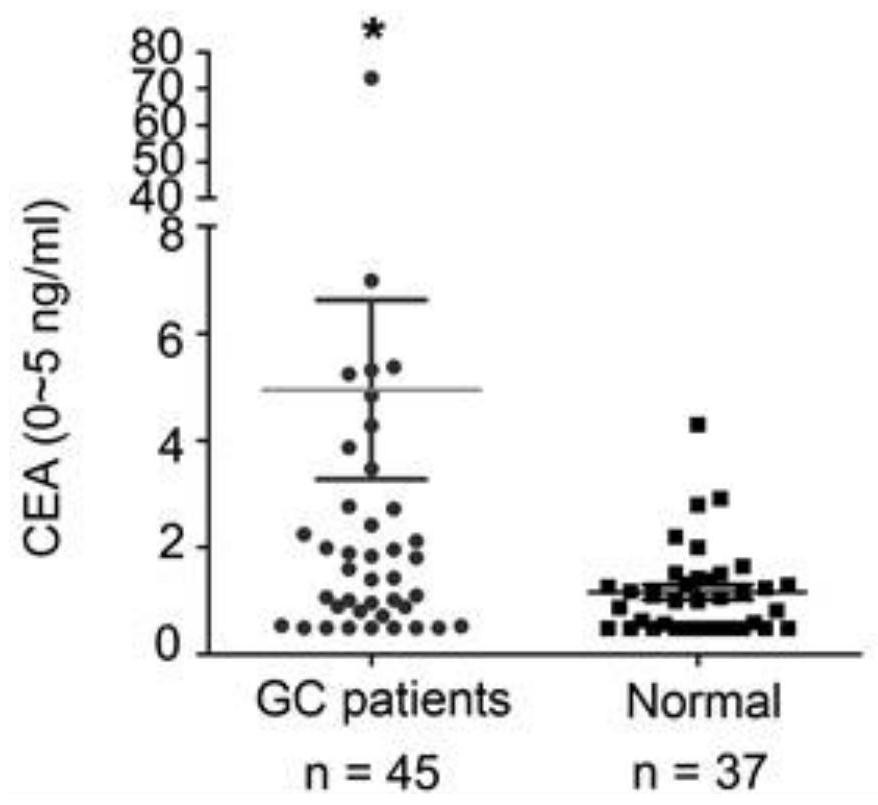
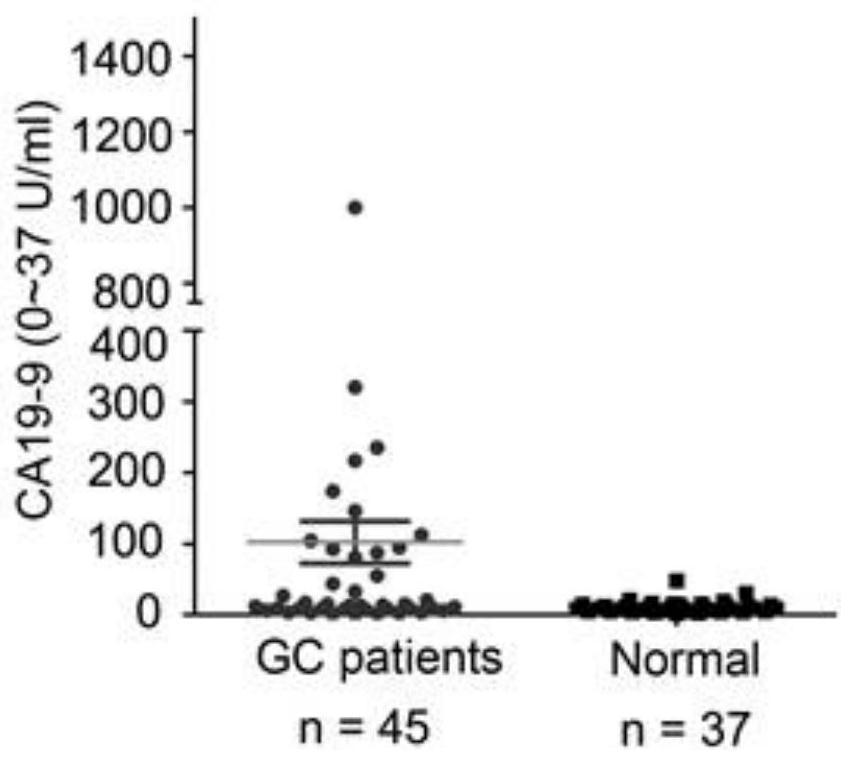
Application of miR-588 and target gene thereof in gastric cancer
PendingCN111961727AIncreased sensitivityImprove featuresOrganic active ingredientsPeptide/protein ingredientsHigh level expressionOncology
The invention discloses application of miR-588 and a target gene thereof in gastric cancer. It is found that the expression of miR-588 in serum of gastric cancer patients is remarkably lower than thatof miR-588 in serum of healthy people, the expression of miR-588 target genes CXCL5, CXCL9 or CXCL10 in gastric cancer tissues is remarkably lower than that of the healthy people. In the aspect of distinguishing the gastric cancer patients from the healthy people, MiR-588 has better sensitivity and specificity than traditional gastric cancer tumor markers CEA and CA19-9, and miR-588 can be used as a gastric cancer molecular marker; the overexpression miR-588 obviously inhibits the proliferation of gastric cancer cells in vitro and vivo and the vitro invasion by up-regulating the expression oftarget genes CXCL5, CXCL9 or CXCL10; finally, the high-level expression of the miR-588, the CXCL5, the CXCL9 or the CXCL10 is remarkably related to the longer total survival time of a patient suffering from the gastric cancer.
Owner:ZHEJIANG CANCER HOSPITAL
A method for expressing and purifying recombinant cxcl9 protein and its application
ActiveCN108707193BOvercome the disadvantage of easy formation of inclusion bodiesAchieving soluble expressionChemokinesPeptide preparation methodsEscherichia coliInclusion bodies
The invention relates to the field of biotechnology, and specifically discloses a method for realizing soluble expression of recombinant CXCL9 protein in Escherichia coli by using fusion expression of a solubilization tag and an intein (Intein) and an application thereof. In the method, the target protein is fused and expressed with a solubilizing tag and an Intein with self-cleavage function, so as to realize the soluble expression and shear release of the target protein. Compared with the known method for expressing recombinant CXCL9 protein in Escherichia coli, the method of the present invention can avoid the denaturation and renaturation process of inclusion bodies, does not need to introduce protease, and can obtain a target product with a completely natural sequence, which is useful for large-scale industrial production Very good application prospects.
Owner:SHANGHAI JIAO TONG UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com