Patents
Literature
44 results about "MMP1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Matrix metalloproteinase-1 (MMP-1) also known as interstitial collagenase and fibroblast collagenase is an enzyme that in humans is encoded by the MMP1 gene. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-1 was the first vertebrate collagenase both purified to homogeneity as a protein, and cloned as a cDNA.
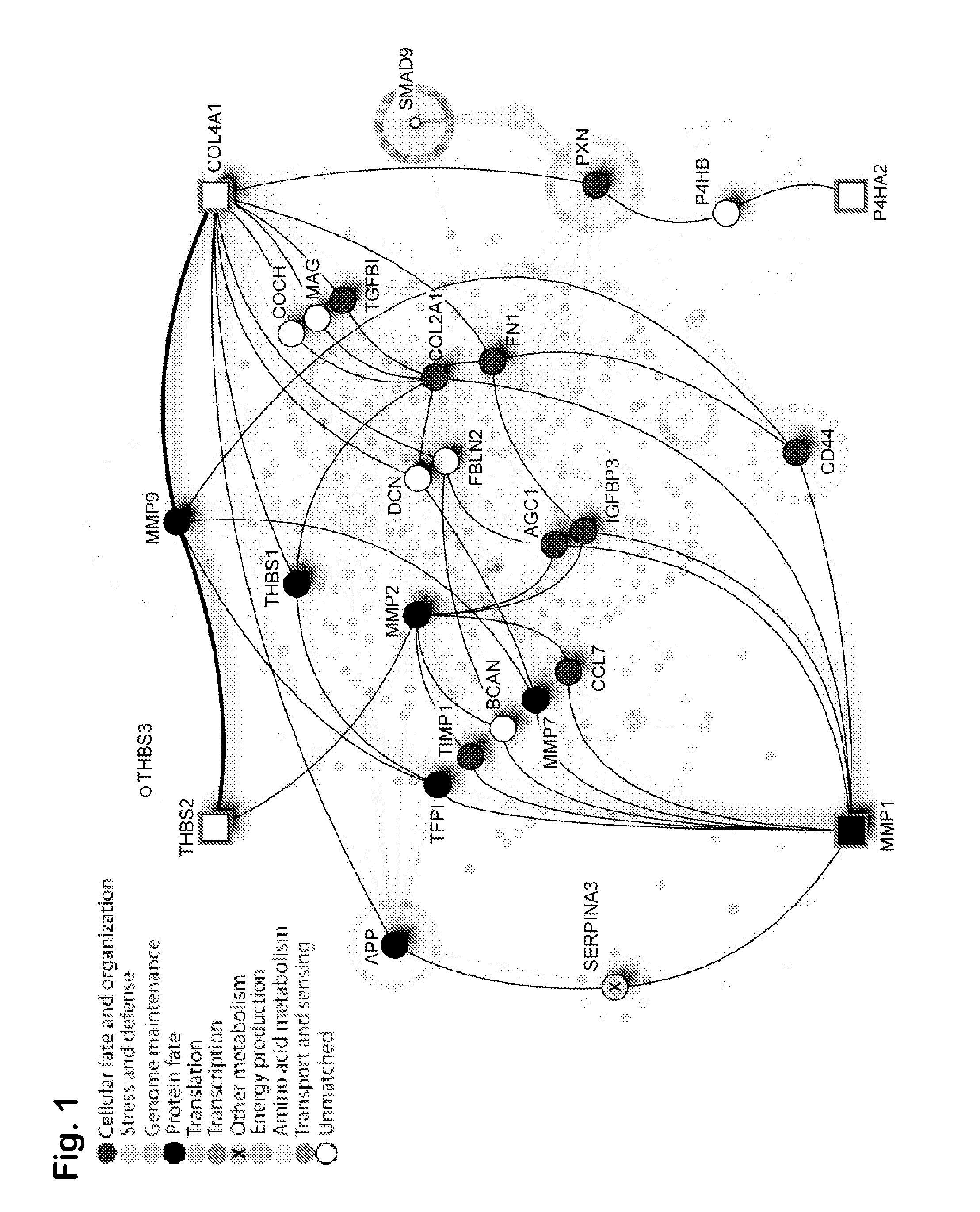
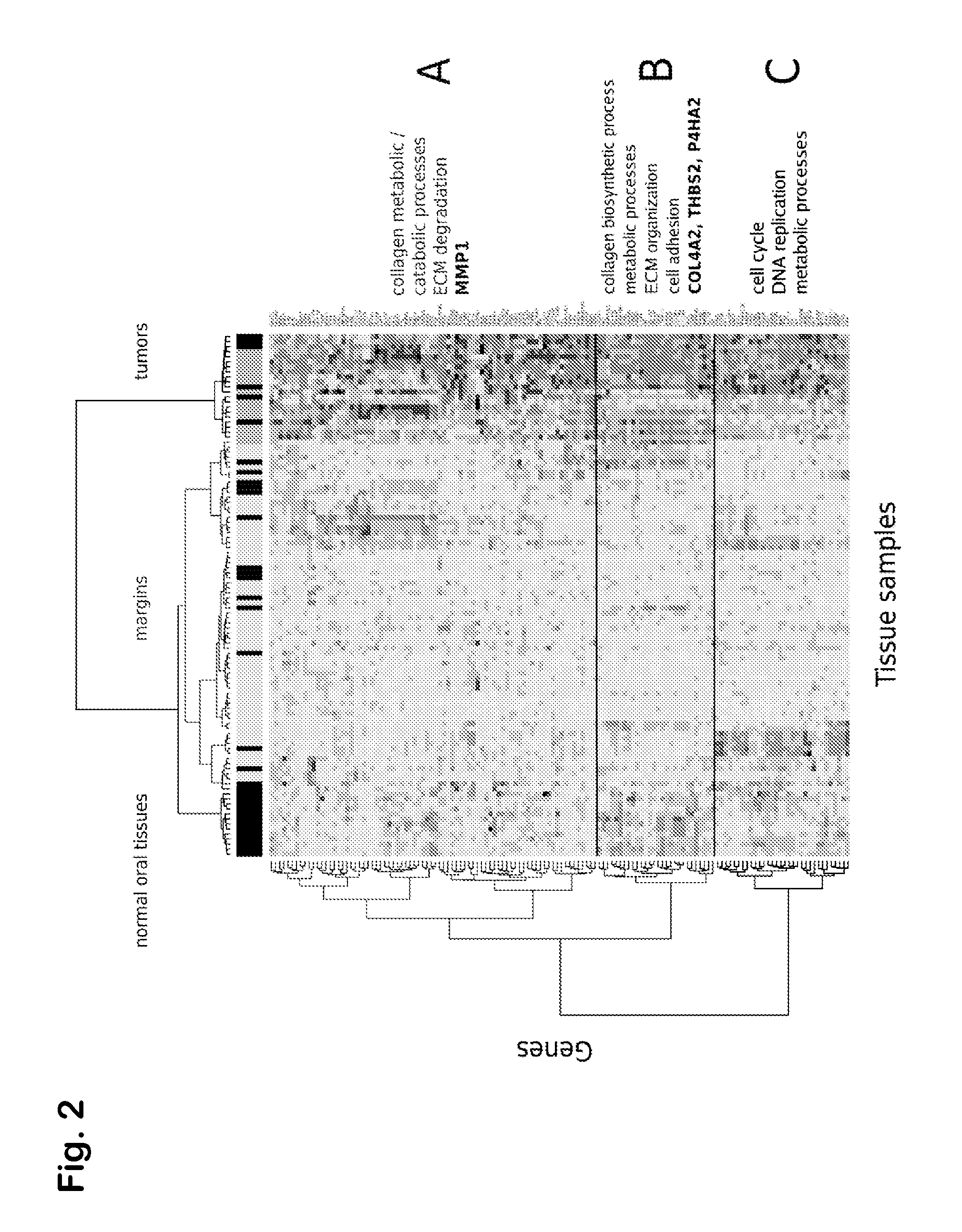
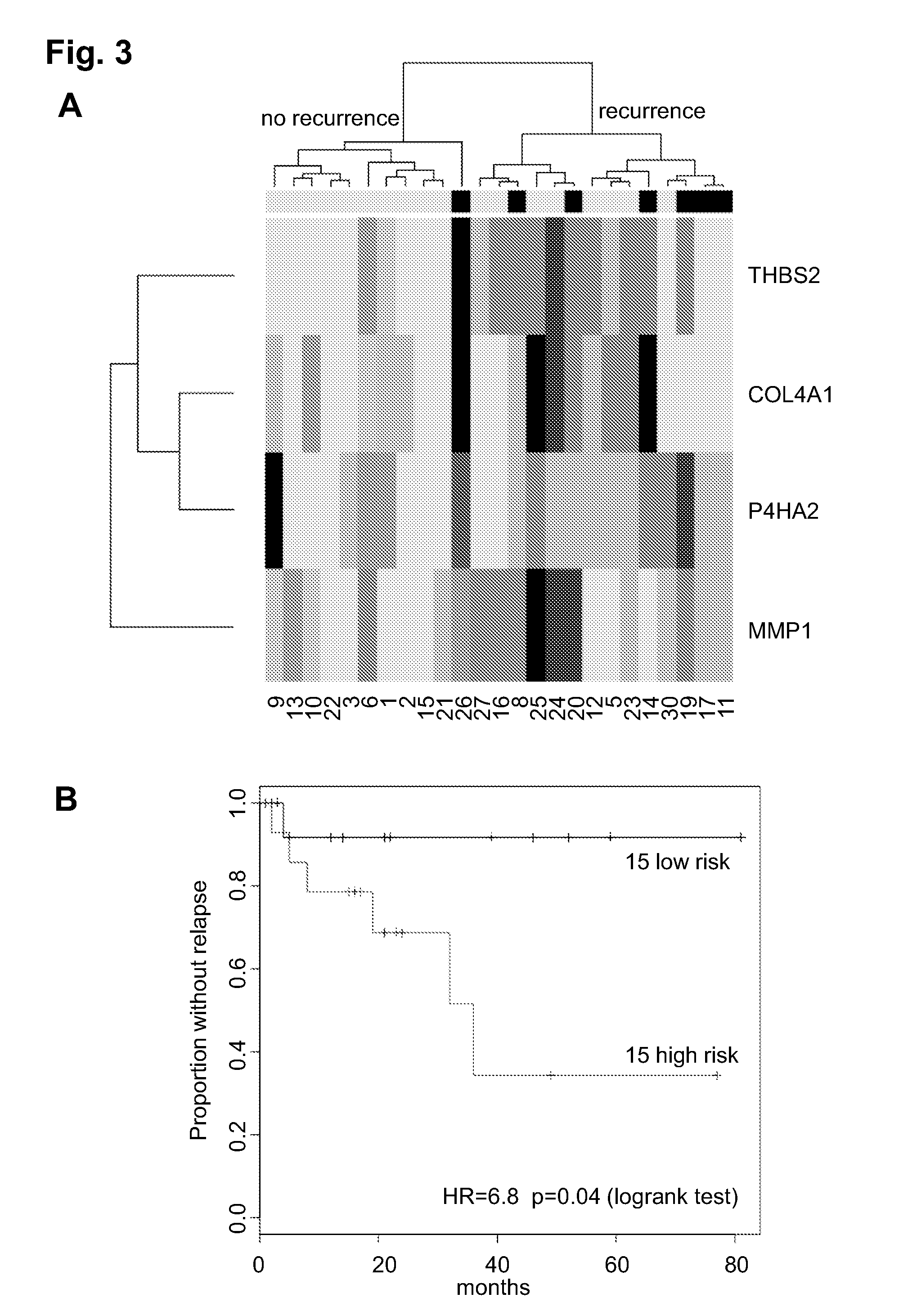
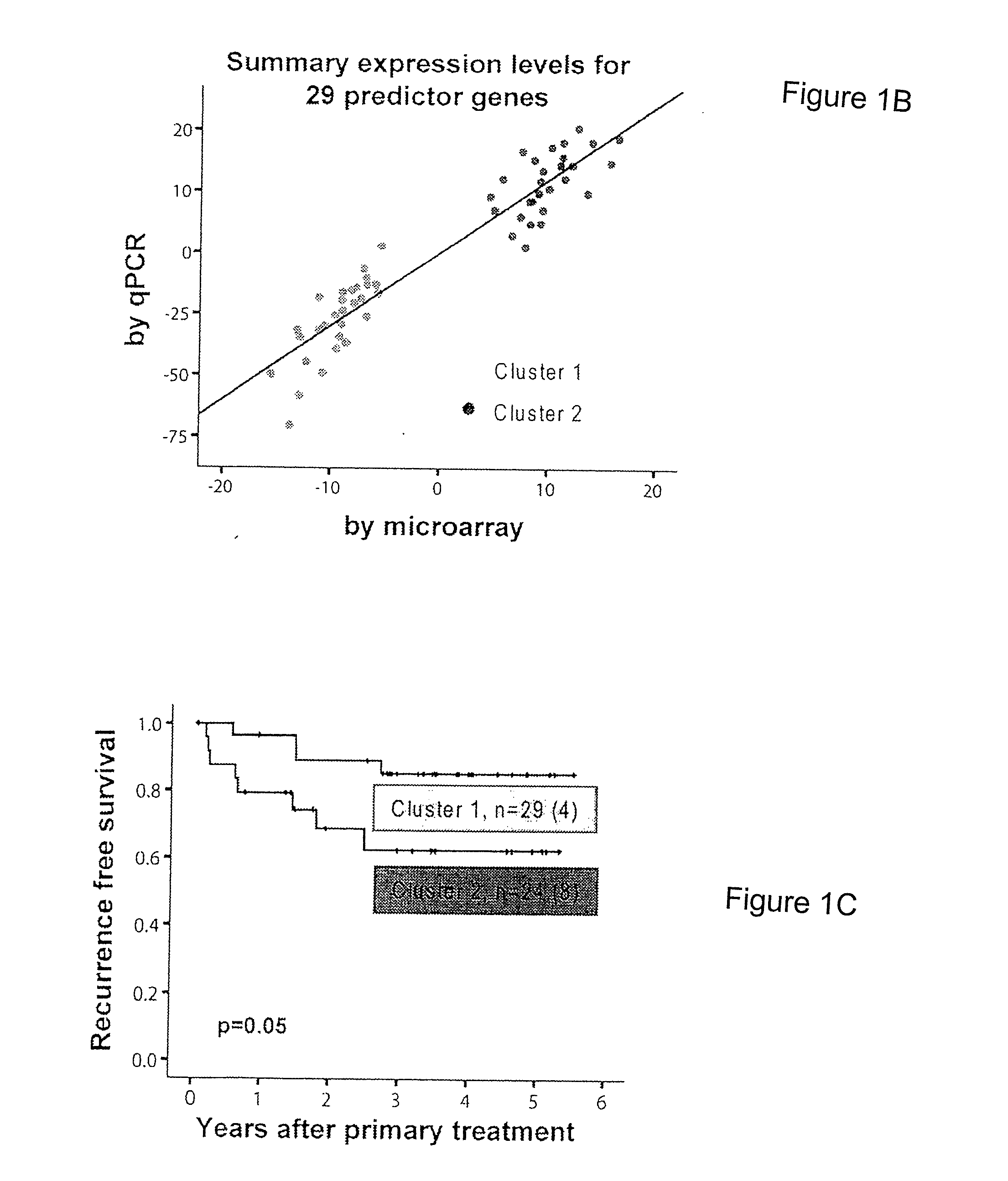
Prognostic signature for oral squamous cell carcinoma
InactiveUS20130303826A1Raise the possibilityImprove expression levelMicrobiological testing/measurementLibrary screeningPrognostic signatureSquamous Carcinomas
The present disclosure describes methods and compositions for diagnosing or predicting likelihood of a OSCC recurrence in a subject having undergone OSCC resection comprising: a) determining an expression level of one or more biomarkers selected from Table 4, 5 and / or 7, optionally MMP1, COL4A1, THBS2 and / or P4HA2 in a test sample from the subject, the one or more biomarkers comprising at least one of THBS2 and P4HA2, and b) comparing the expression level of the one or more biomarkers with a control, wherein a difference or a similarity in the expression level of the one or more biomarkers between the test sample and the control is used to diagnose or predict the likelihood of OSCC recurrence in the subject In particular, the present disclosure describes methods and compositions using a four-gene biomarker signature that can predict recurrence of oral squamous cell carcinoma in subjects that have histologically normal surgical resection margins.
Owner:UNIV HEALTH NETWORK
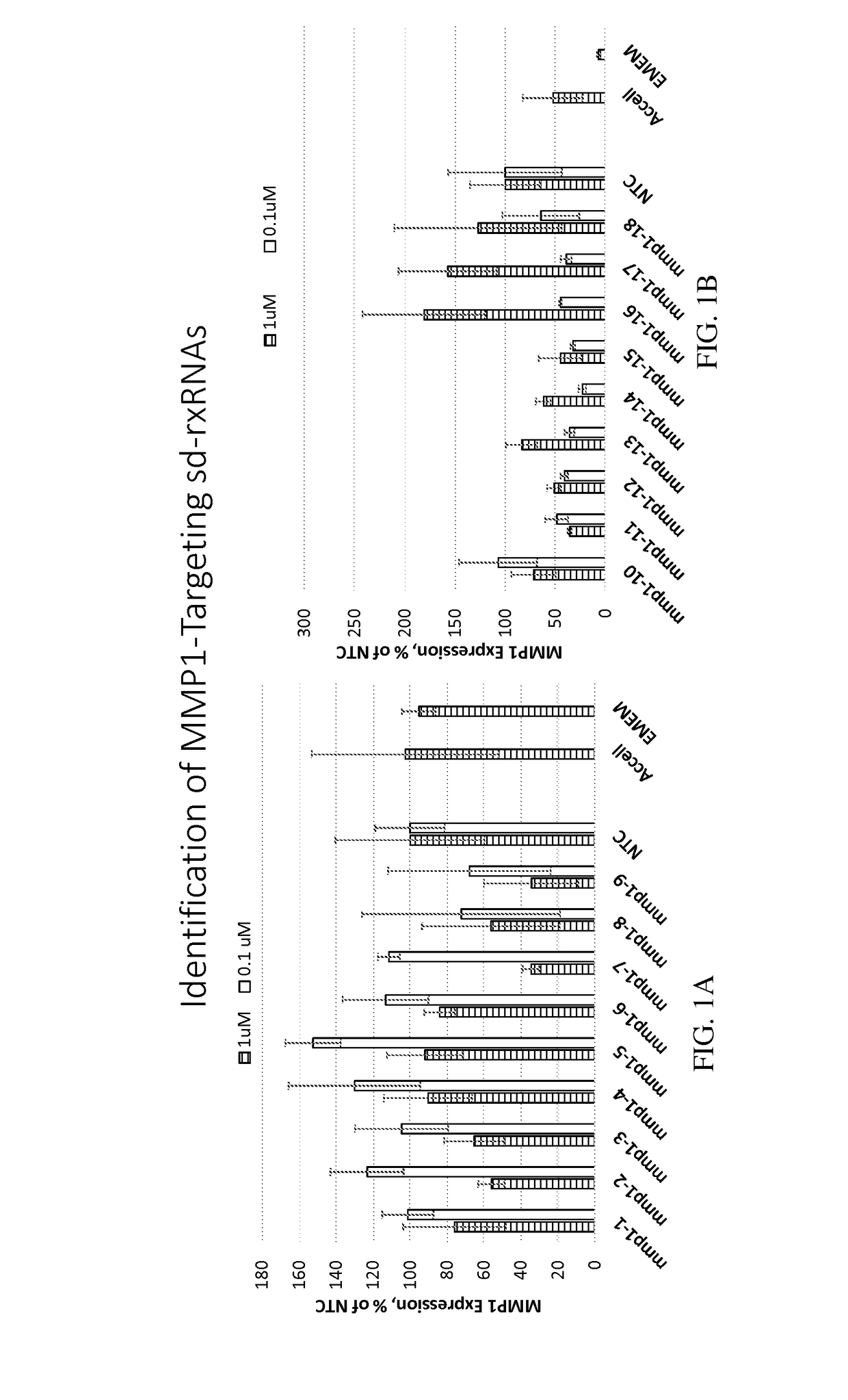
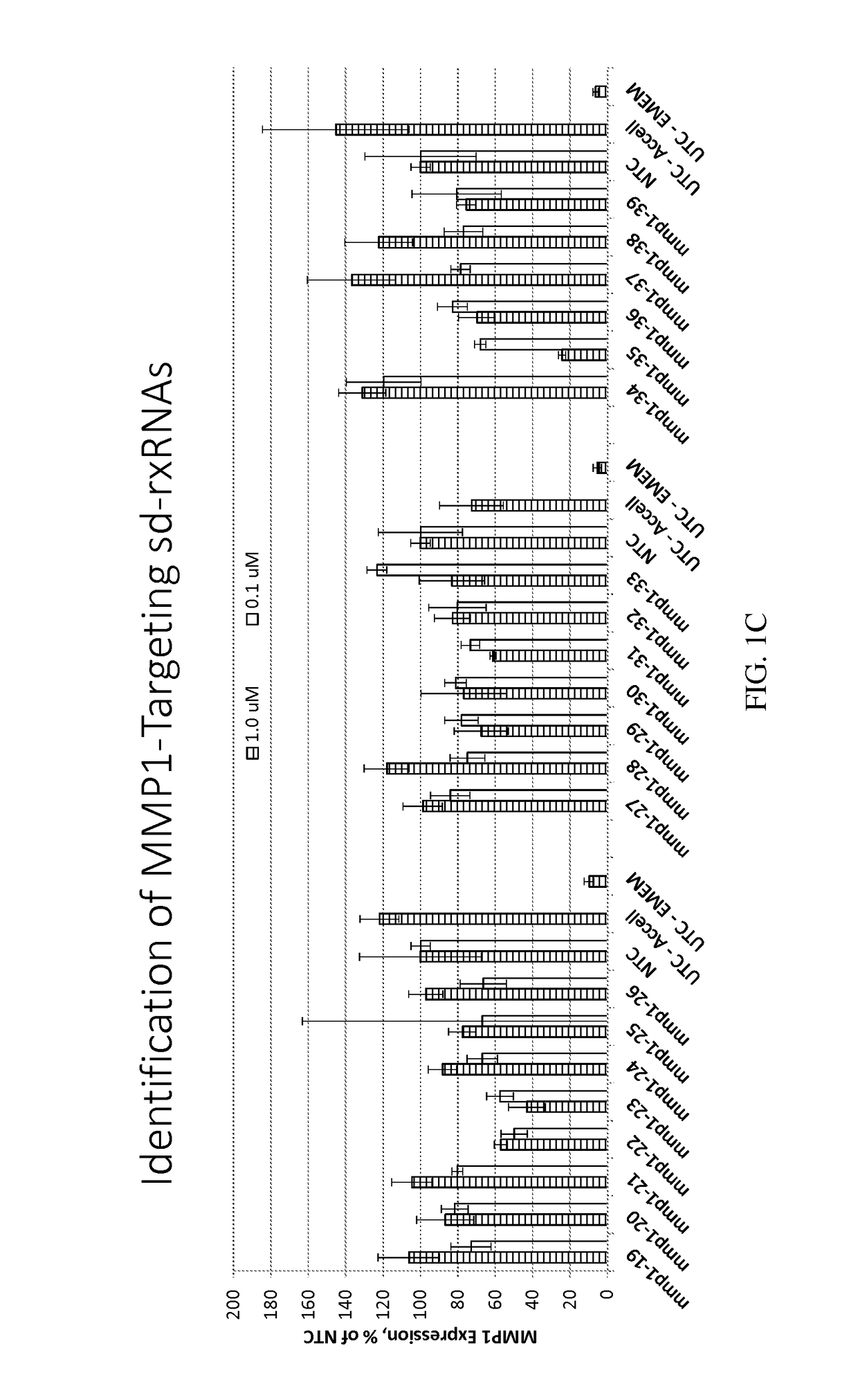
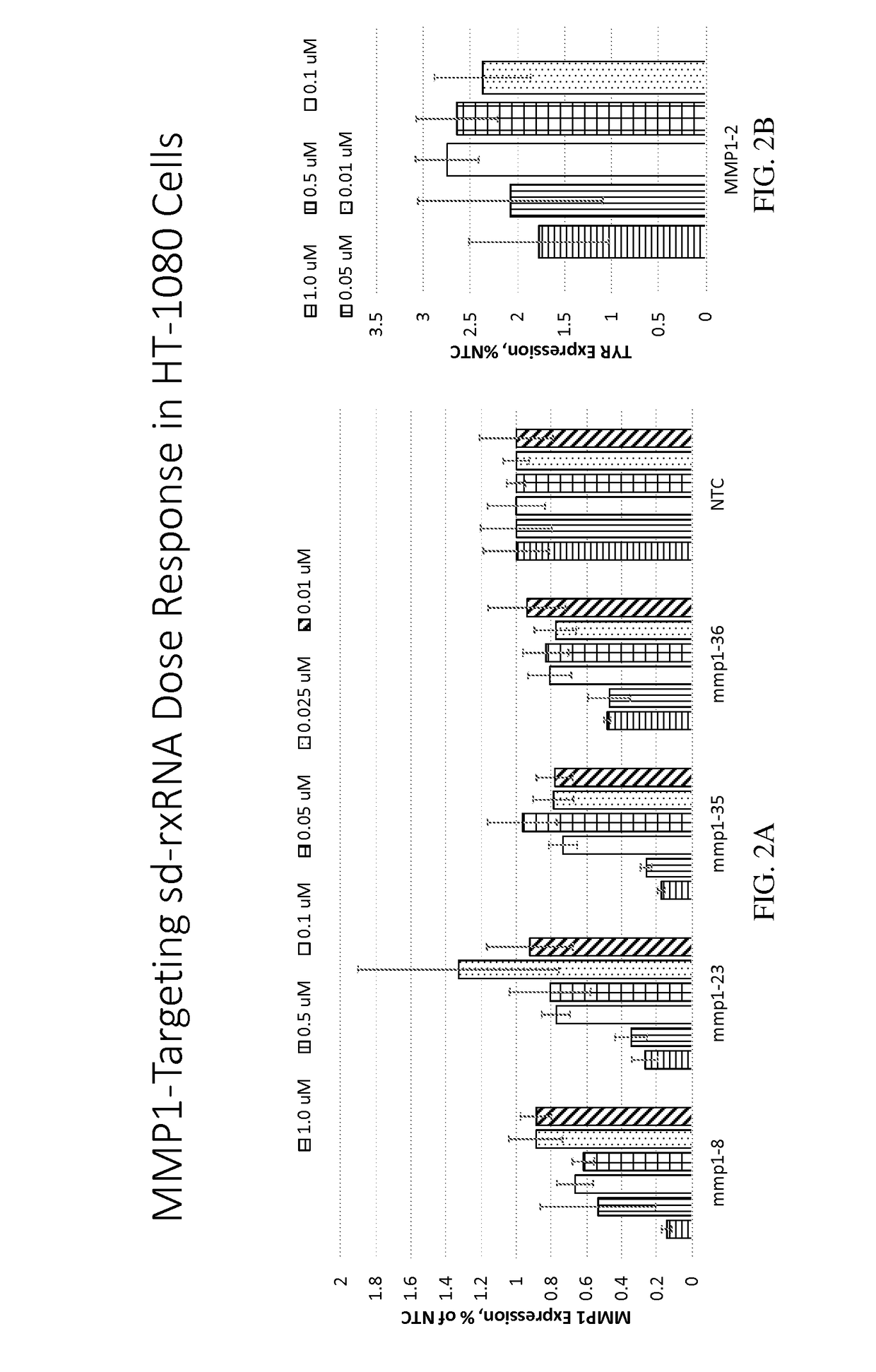
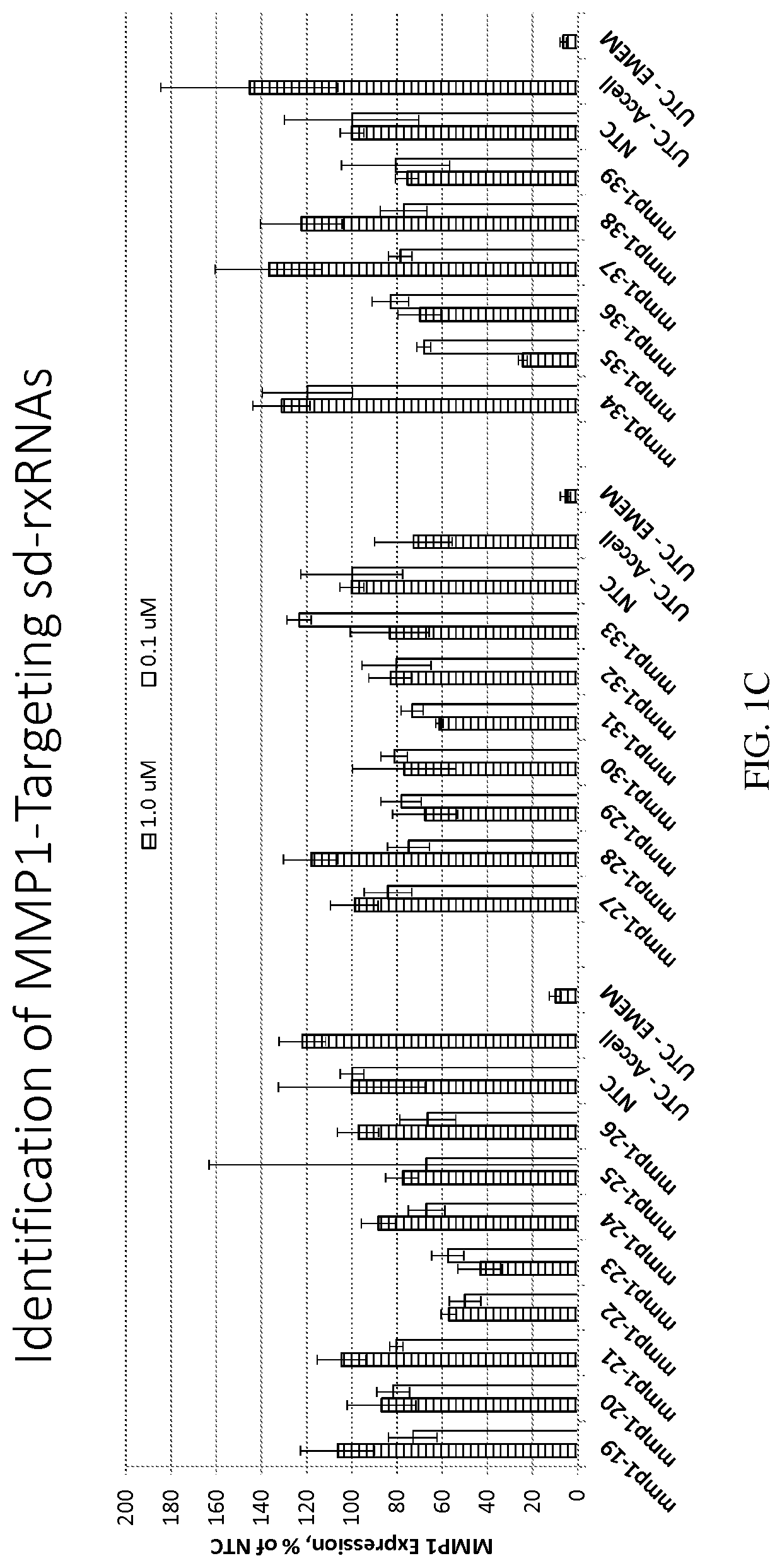
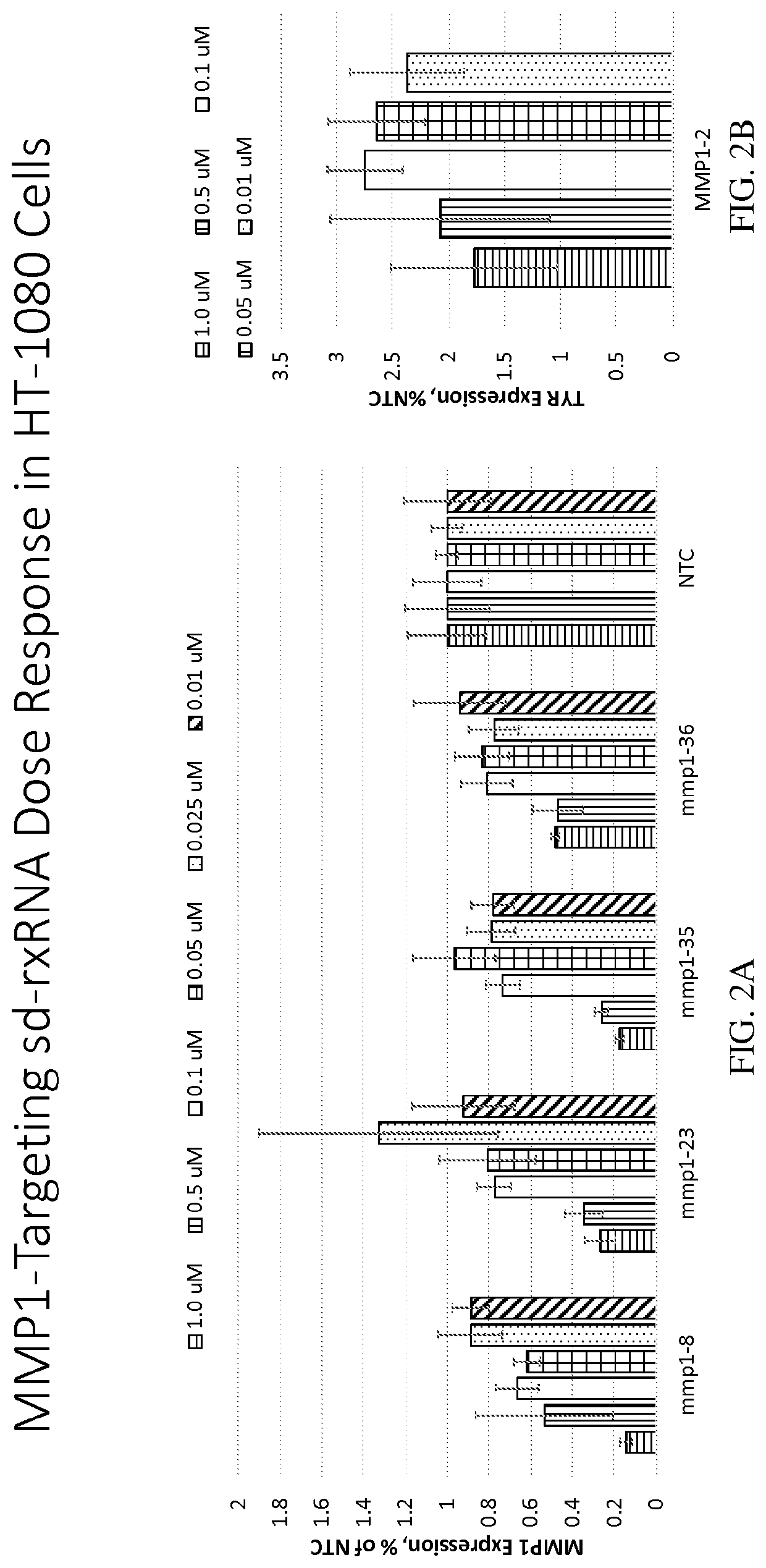
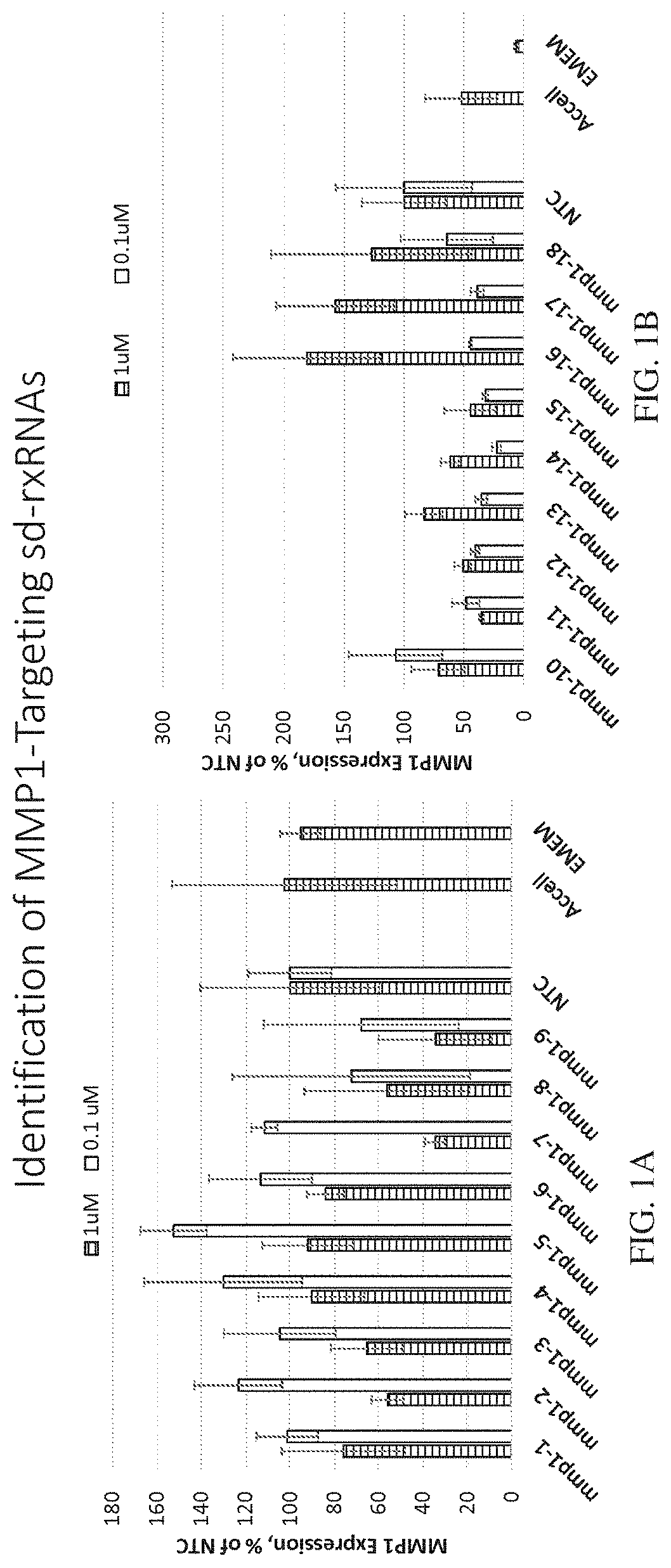
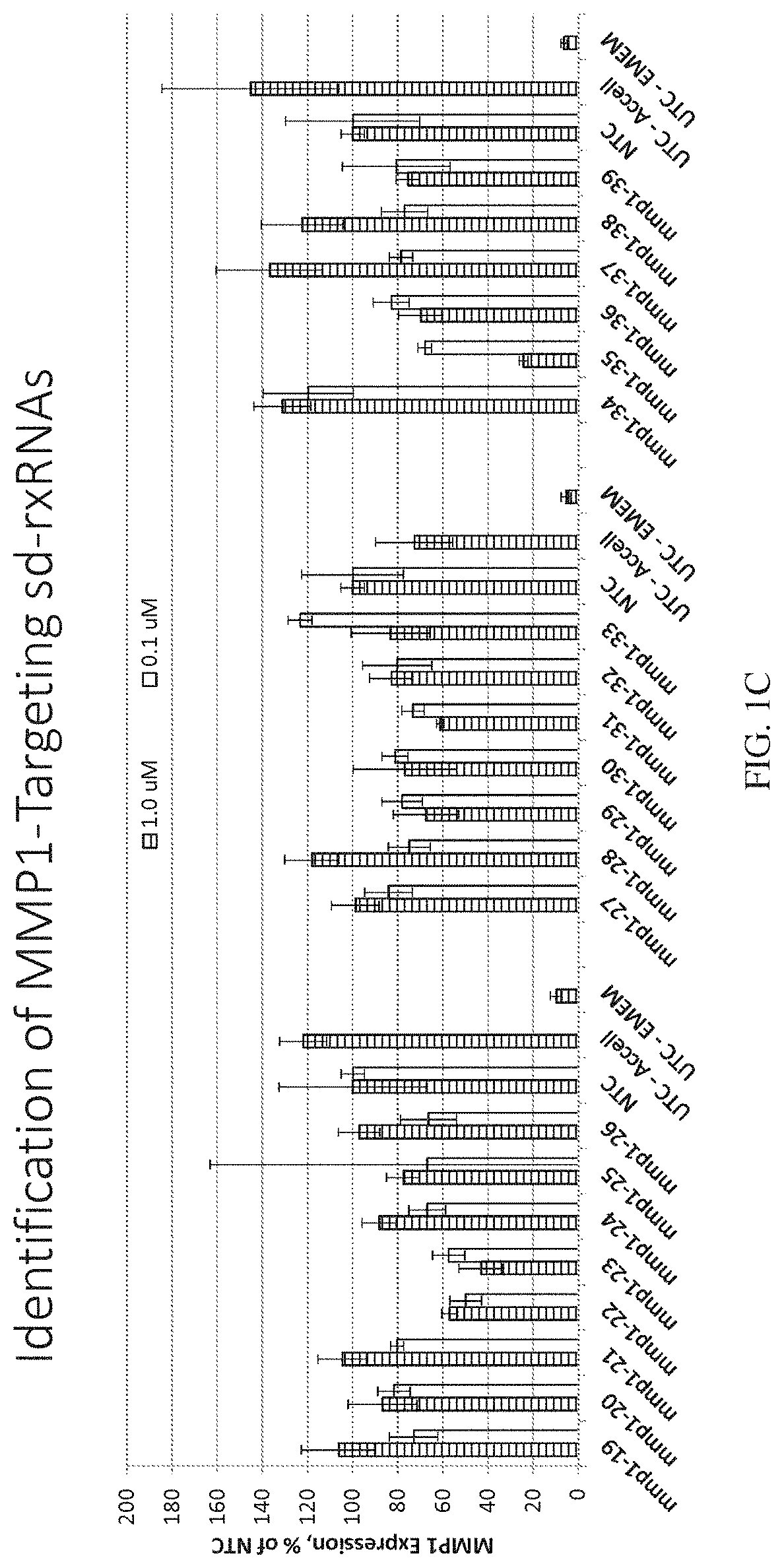
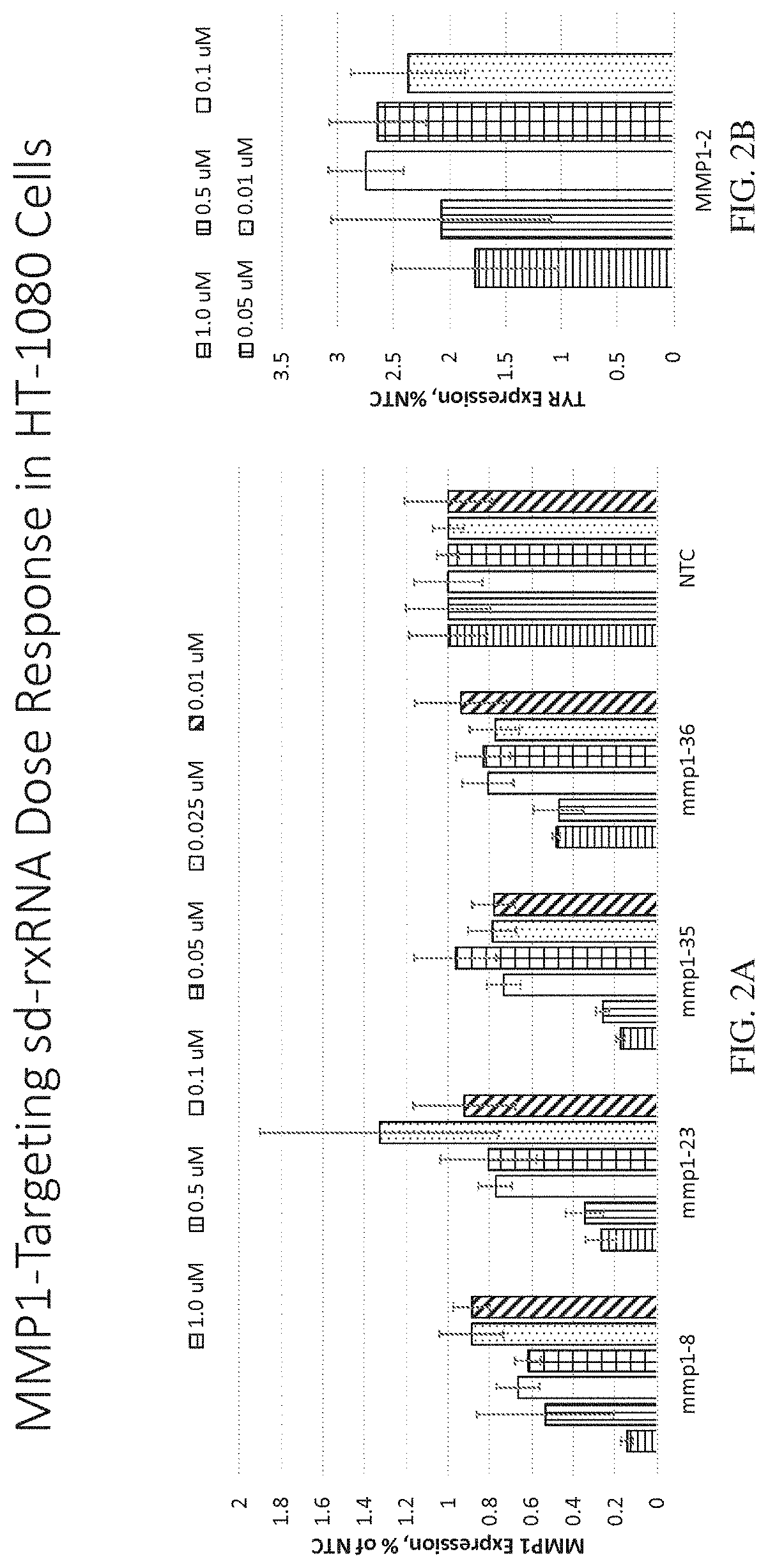
Methods for treating aging and skin disorders using nucleic acids targeting tyr or mmp1
Owner:PHIO PHARMA CORP
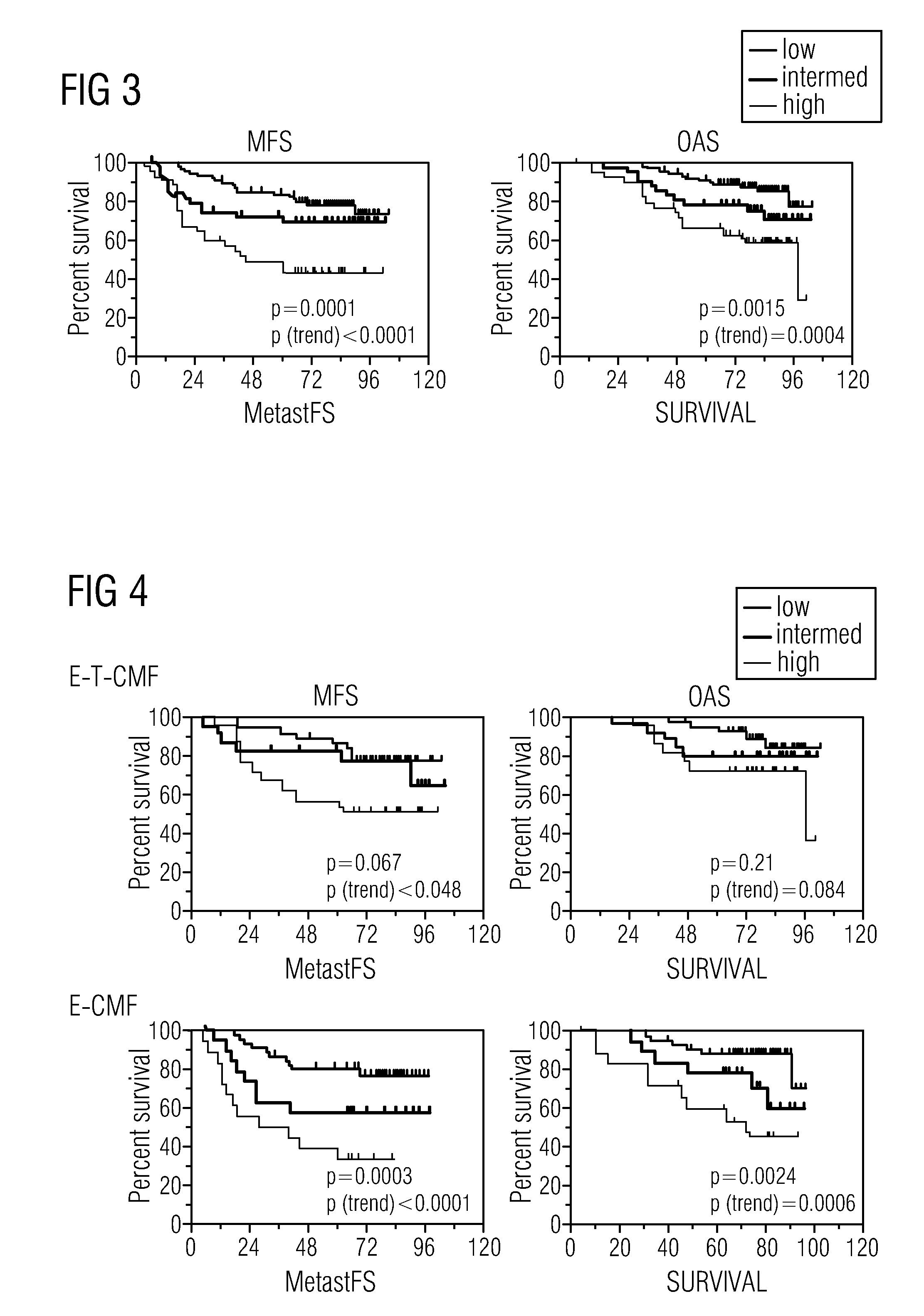
Algorithms for outcome prediction in patients with node-positive chemotherapy-treated breast cancer
InactiveUS20110166838A1Reduce chanceReduce deathMicrobiological testing/measurementBiostatisticsCentromere protein JSOX4
The invention relates to methods for predicting an outcome of cancer in a patient suffering from cancer, said patient having been previously diagnosed as node positive and treated with cytotoxic chemotherapy, said method comprising determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CAl2, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF; ABCB1, ABCG2, ADAM15, AKR1C1, AKR1C3, AKT1, BANF1, BCL2, BIRC5, BRMS1, CASP10, CCNE2, CENPJ, CHPT1, EGFR, CTTN, ERBB3, ERBB4, FBLN1, FIP1L1, FLT1, FLT4, FNTA, GATA3, GSTP1, Herstatin, IGF1R, IGHM, KDR, KIT, CKRT5, SLC39A6, MAPK3, MAPT, MKI67, MMP7, MTA1, FRAP1, MUC1, MYC, NCOA3, NFIB, OLFM1, TP53, PCNA, PI3K, PPERLD1, RAB31, RAD54B, RAF1, SCUBE2, STAU, TINF2, TMSL8, VGLL1, TRA@, TUBA1, TUBB, TUBB2A.
Owner:SIVIDON DIAGNOSTICS
Method for distinguishing between head and neck squamous cell carcinoma and lung squamous cell carcinoma
ActiveUS20070264644A1Sugar derivativesMicrobiological testing/measurementLung squamous cell carcinomaCXCL13
The present invention is a method distinguishing between head and neck squamous cell carcinoma and lung squamous cell carcinoma. In particular, a 10-gene classifier has been identified which can be used to distinguish between primary squamous cell carcinoma of the lung and metastatic head and neck squamous cell carcinoma. These genes include CXCL13, COL6A2, SFTPB, KRT14, TSPYL5, TMP3, KLK10, MMP1, GAS1, and MYH2. A panel of one or more of these genes, or proteins encoded thereby, can be used for early diagnosis and selection of an appropriate therapeutic treatment.
Owner:WISTAR INST THE A CORP OF PA +1
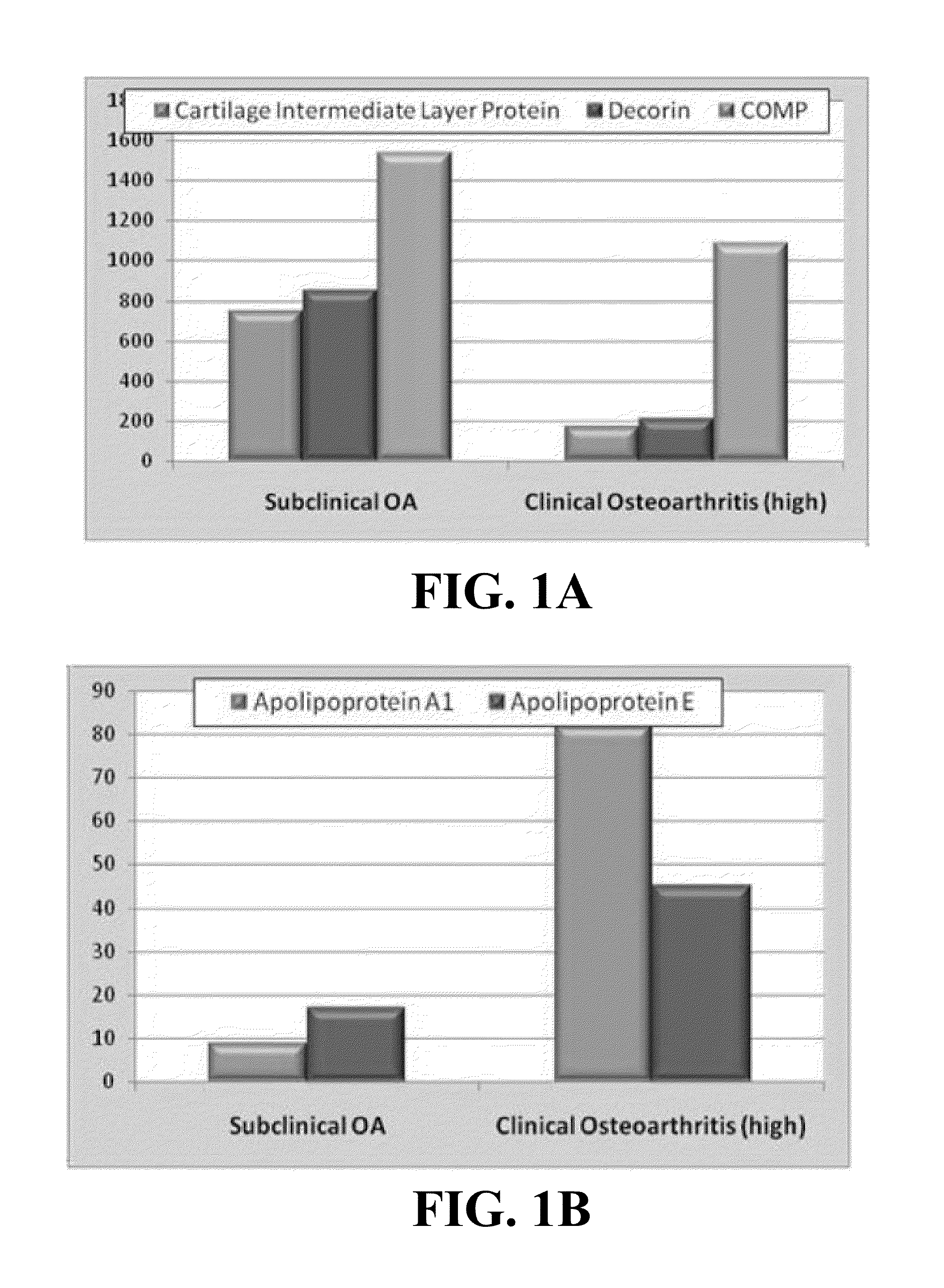
Biomarkers of osteoarthritis
Owner:UNIVERSITY OF MISSOURI
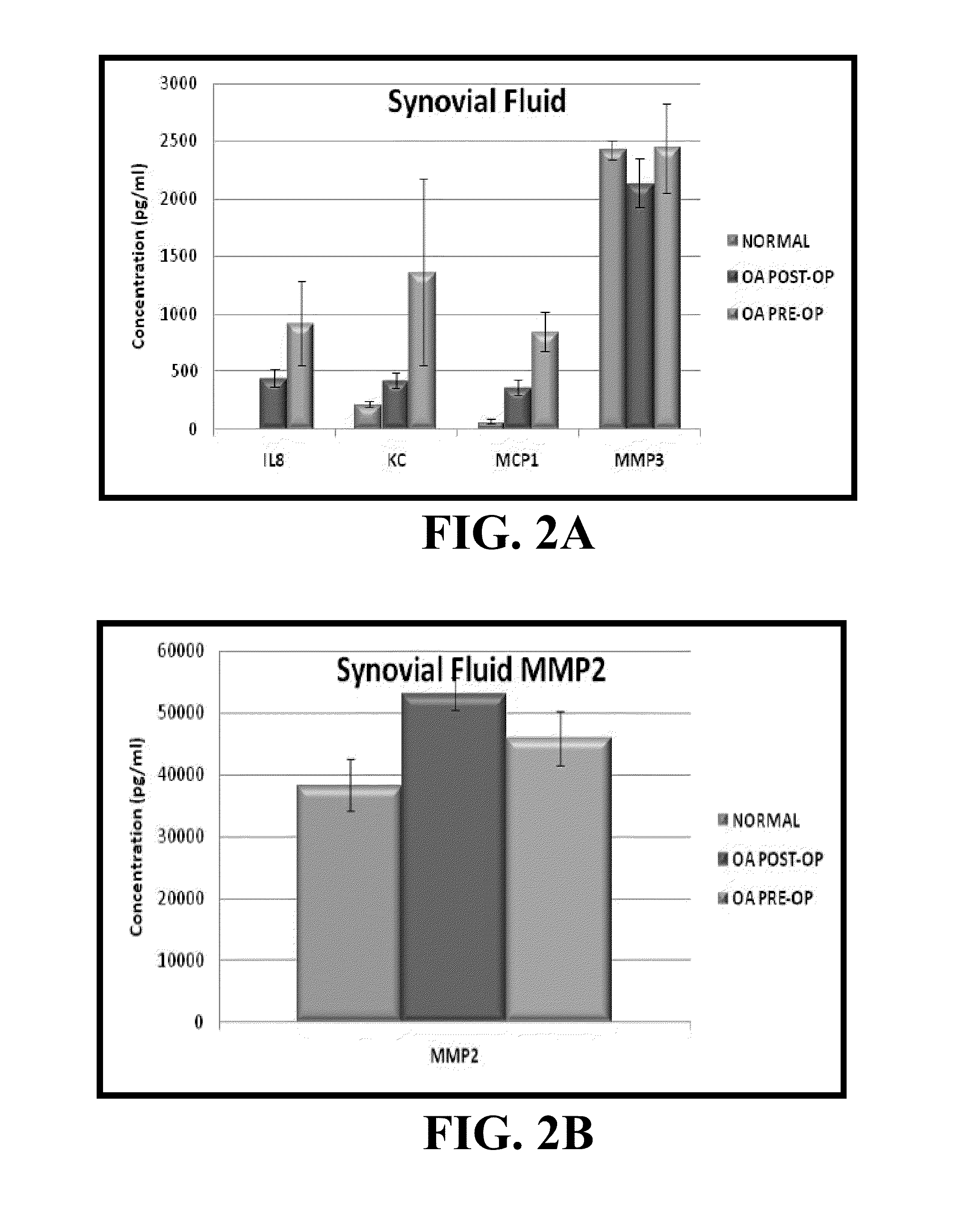
Biomarkers For Predicting Progressive Joint Damage
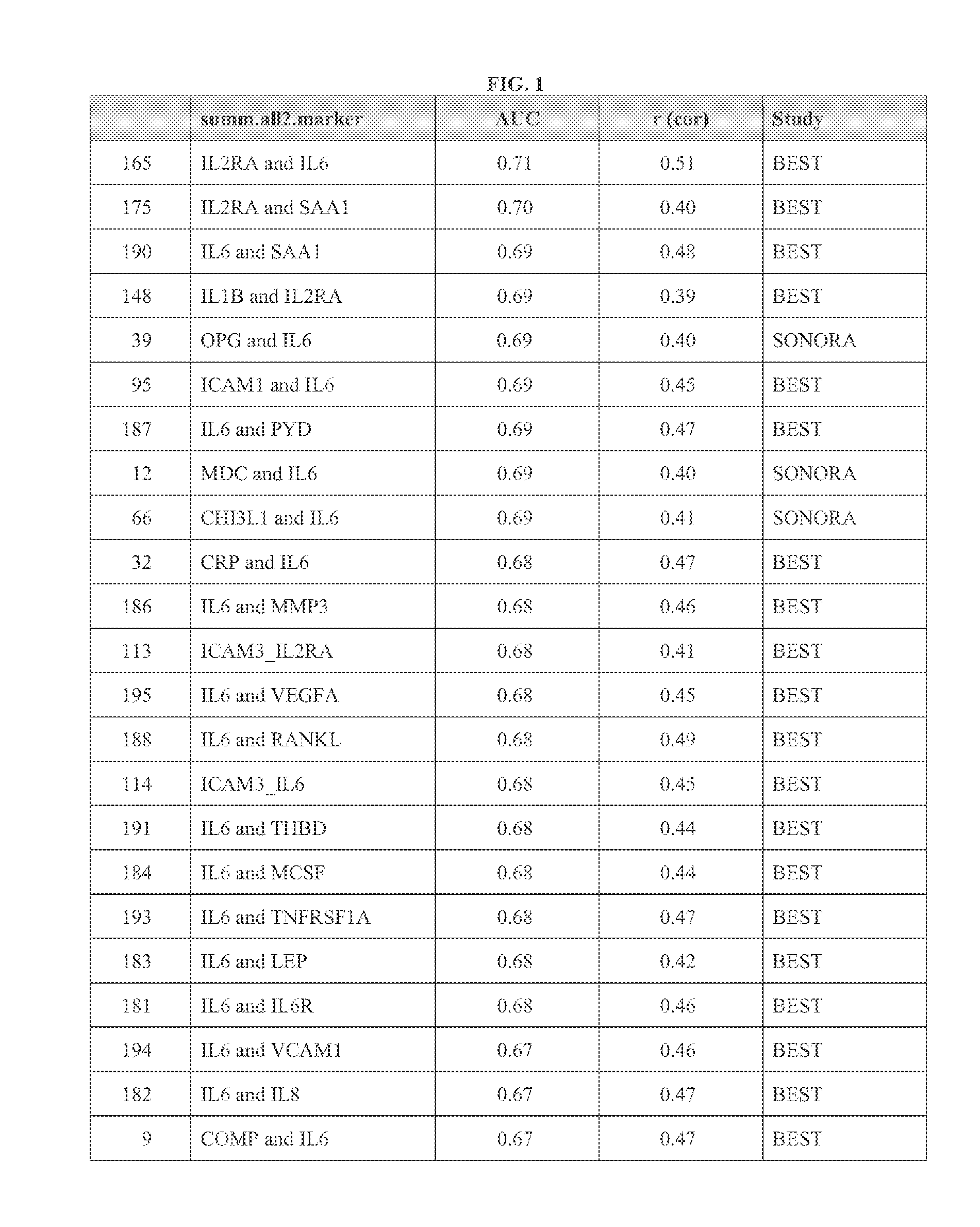
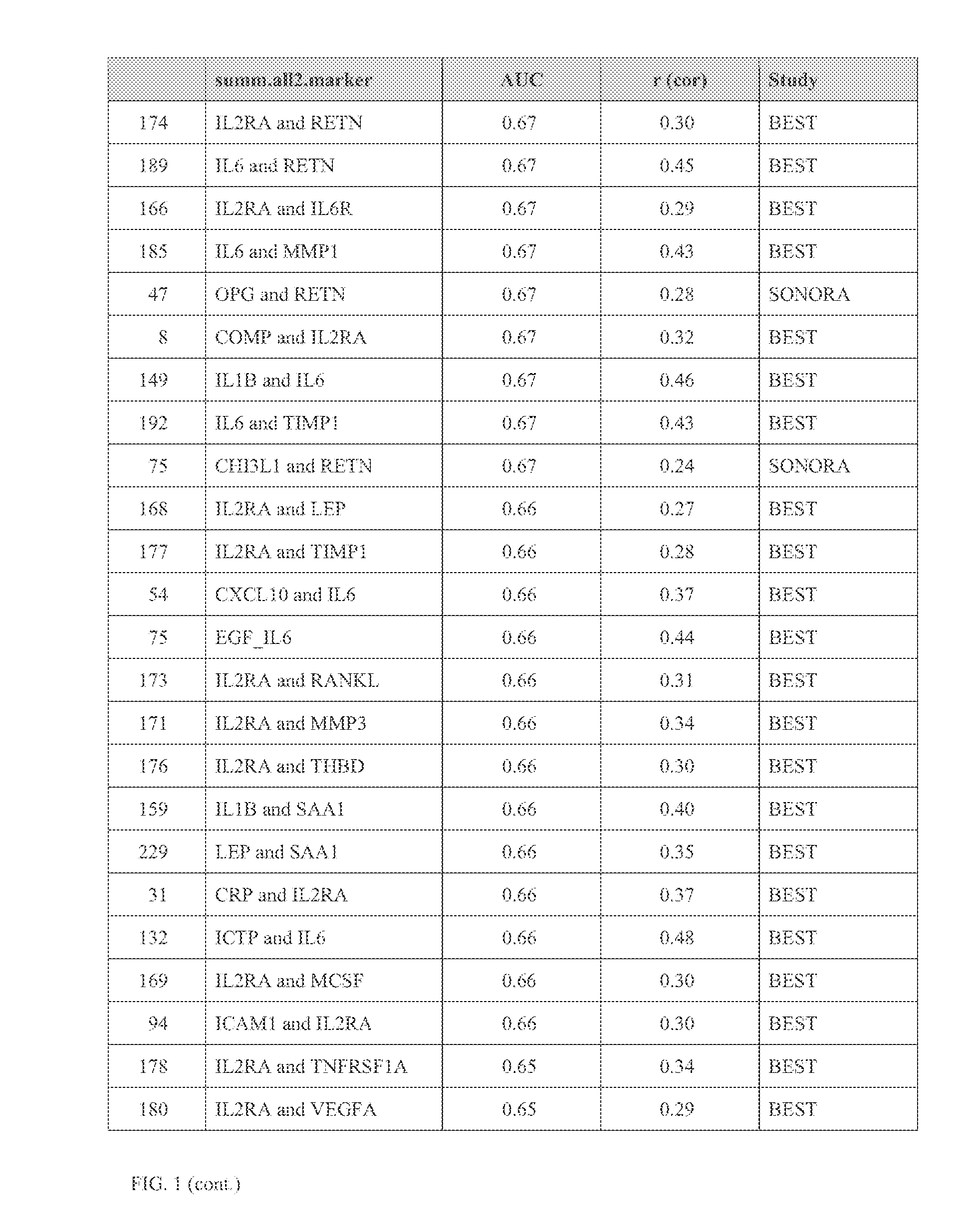
A method scores a sample, by receiving a first dataset associated with a first sample obtained from a first subject, wherein said first dataset comprises quantitative data for at least two markers selected from the group consisting of: CCL22; CHI3L1; COMP; CRP; CSF1; CXCL10; EGF; ICAM1; ICAM3; ICTP; IL1B; IL2RA; IL6; IL6R; IL8; LEP; MMP1; MMP3; PYD; RETN; SAA1; THBD; TIMP1; TNFRSF11B; TNFRSF1A; TNFSF11; VCAM1; and VEGFA; and determining a first SDI score from said first dataset using an interpretation function, wherein the first SDI score provides a quantitative measure of the rate of change in joint structural damage in said first subject.
Owner:CRESCENDO BIOSCI +1
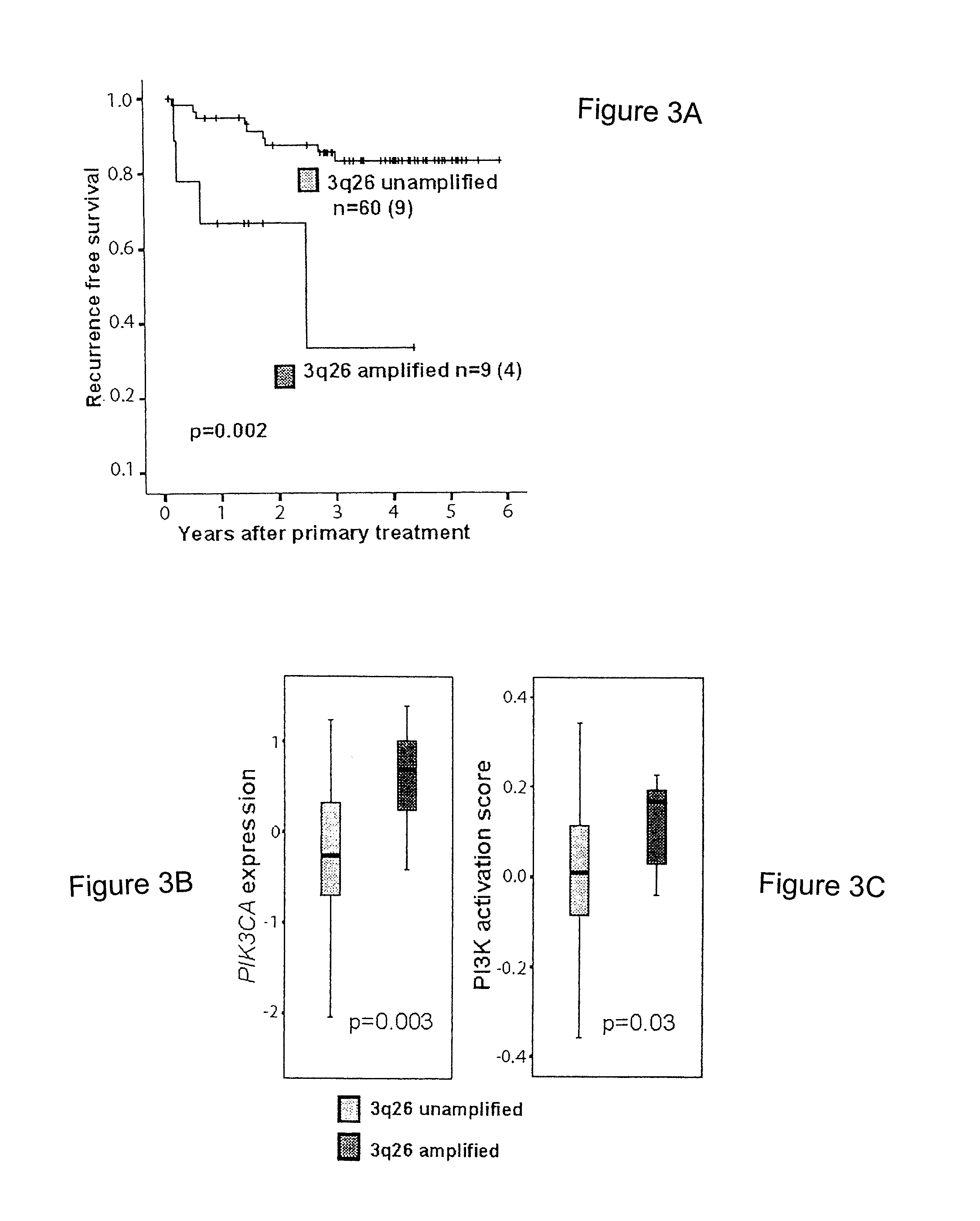
Prognostic Marker for Endometrial Carcinoma
InactiveUS20110217701A1Microbiological testing/measurementDisease diagnosisNon small lung cancerRegimen
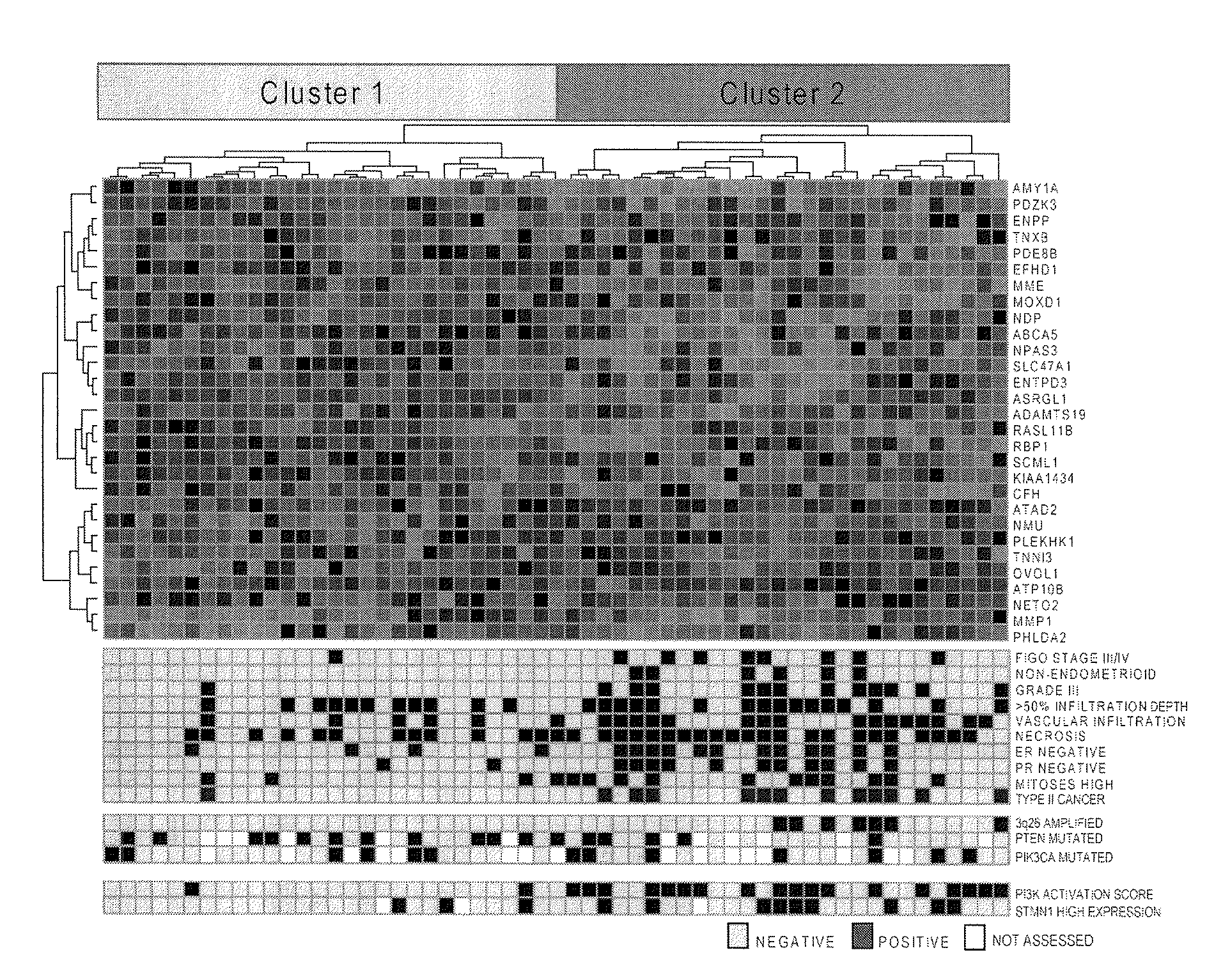
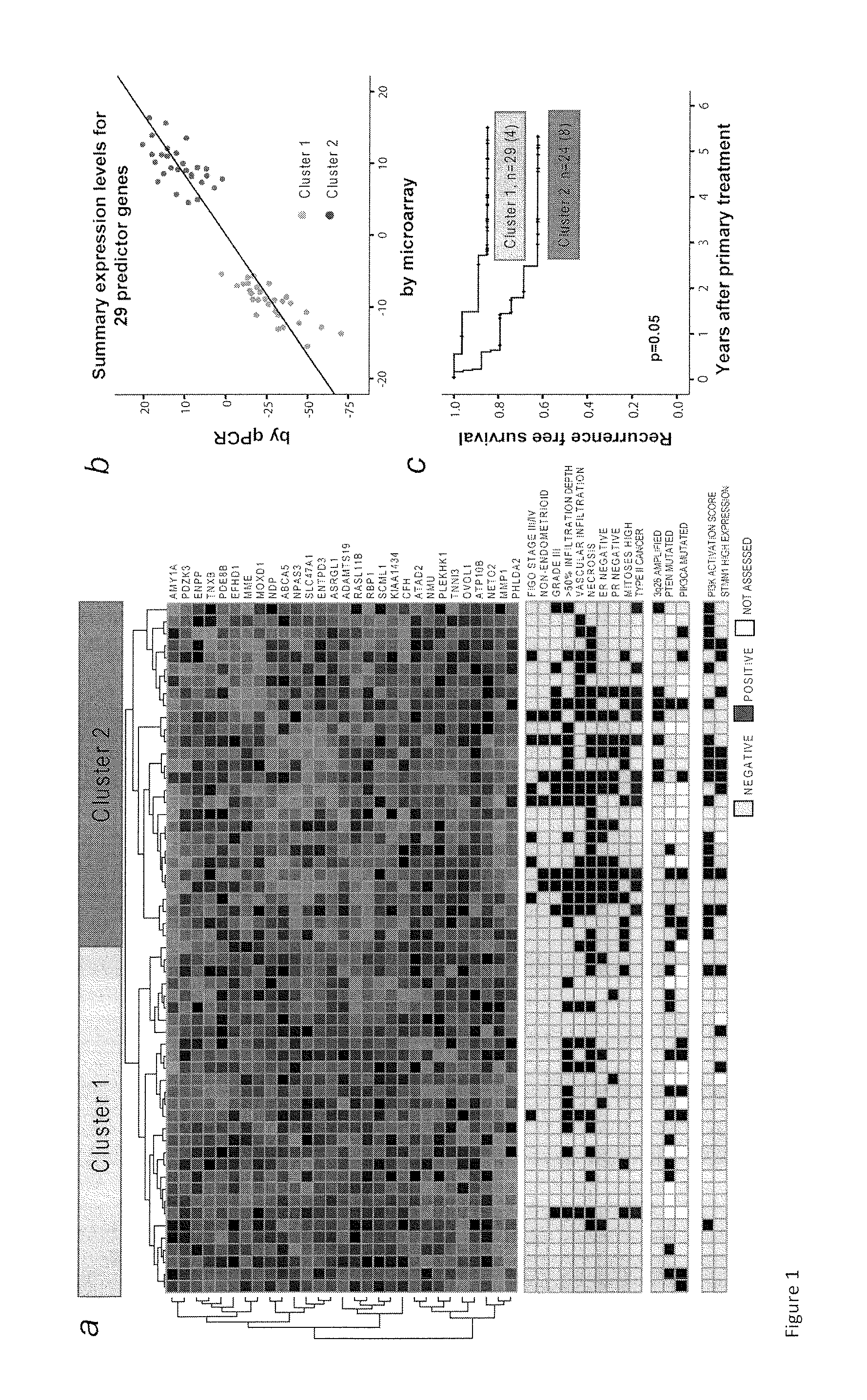
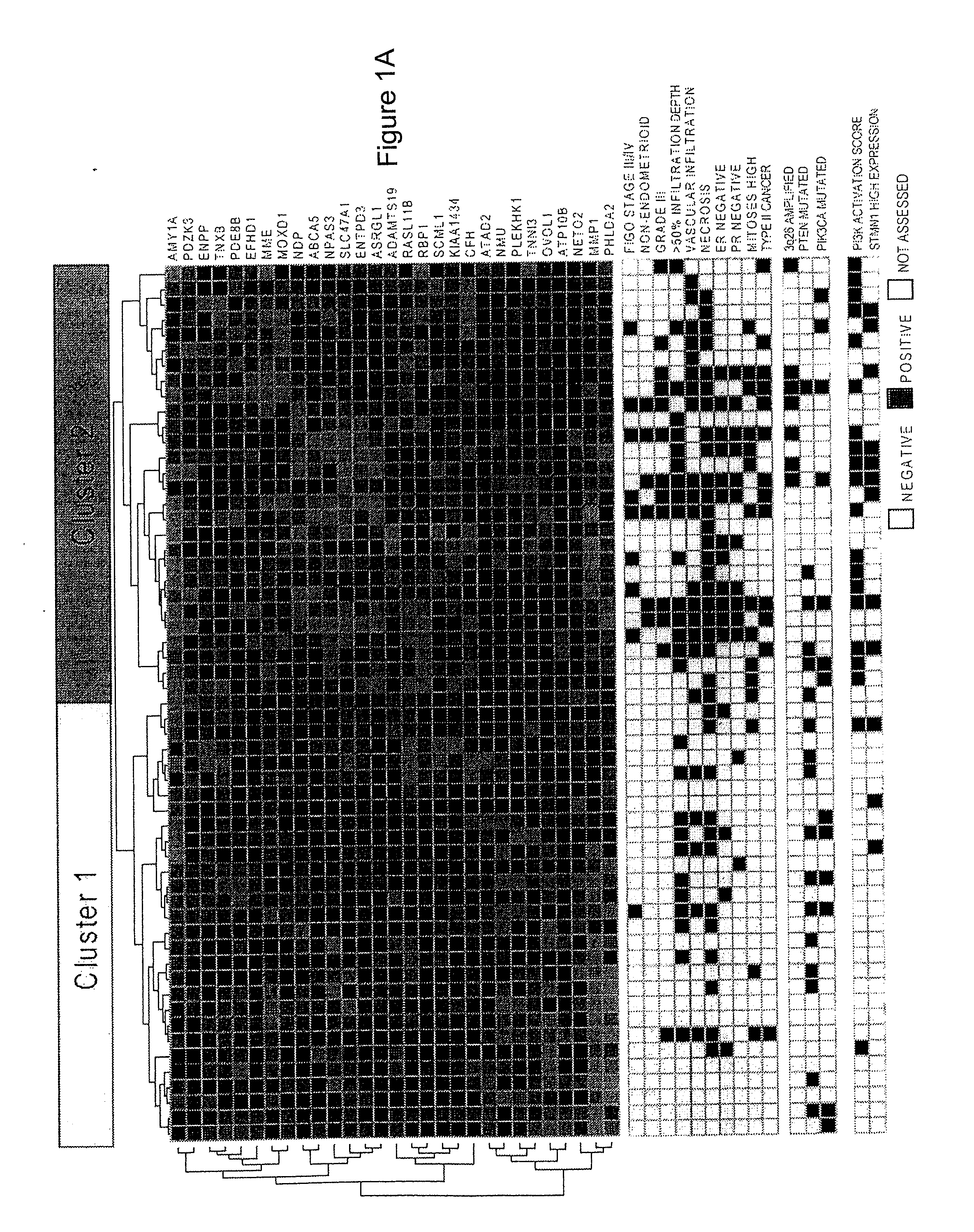
The present invention relates to a method for diagnosis of different stages of endometrial cancer in an individual. Further, the present invention relates to a method for evaluating the probability of survival for an individual suffering from endometrial carcinoma. In another aspect, the present invention relates to the stratification of therapy regimen of endometrial tumor, ovarian cancer, breast cancer, non-small lung cancer or hormone refractory prostate cancer therapy in an individual or monitoring therapeutic efficacy in an individual suffering from the same based on the expression status of STMN1 gene or protein. Moreover, the present invention relates to a kit for use in any of the above referenced methods comprising a means for determining amplifications and deletions of chromosomal regions 3q26.32 and 12p12.1, determining alterations of the gene expression profile of the genes (gene signature): upregulation of the genes PLEKHK1, ATP10B, NMU, MMP1, ATAD2, NETO2, TNNI3, PHLDA2, OVOL1 and down-regulation of the genes: NDP, KIAA1434, MME, CFH, MOXD1, SLC47A1, RBP1, PDE8B, ASRGL1, ADAMTS19, EFHD1, ABCA5, NPAS3, SCML1, TNXB, ENTPD3, AMY1A, ENPP, RASL11B, PDZK3, or the expression status of the STMN1 gene or protein, respectively. Finally, the present invention provides a method for predicting the response to taxanes in an individual suffering from a disease treated with the taxanes based on the expression status of the STMN1 gene or protein.
Owner:BERGEN TEKNOLOGIOVERFORING
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS20130323720A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisOrganism
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Molecular markers for cancer prognosis
InactiveUS20120053842A9Reduce overtreatmentMicrobiological testing/measurementBiostatisticsDiseaseSOX4
The present invention relates to methods for prediction of an outcome of neoplastic disease or cancer. More specifically, the present invention relates to a method for the prediction of breast cancer by determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CA12, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF.
Owner:SIVIDON DIAGNOSTICS
Reagent for auxiliarily diagnosing lung cancer lymph node metastasis
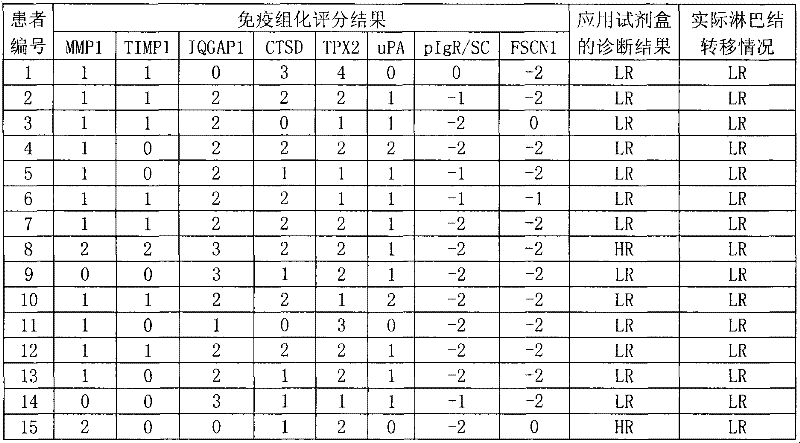
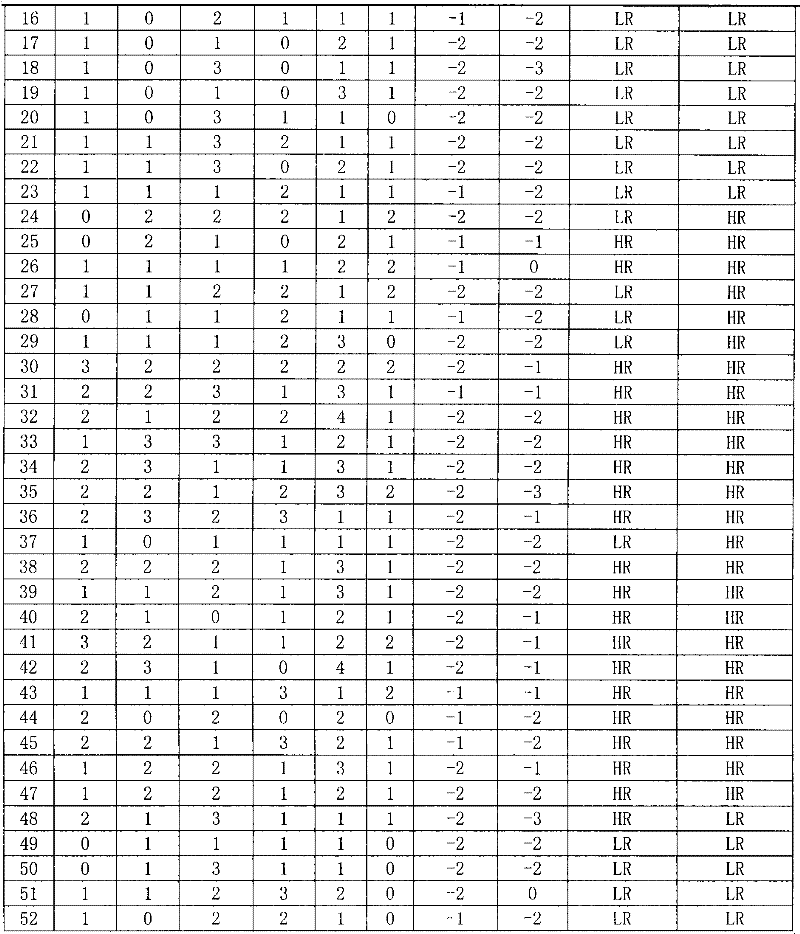
InactiveCN102445543AIncrease credibilityPracticalBiological testingStainingPolymeric immunoglobulin receptor
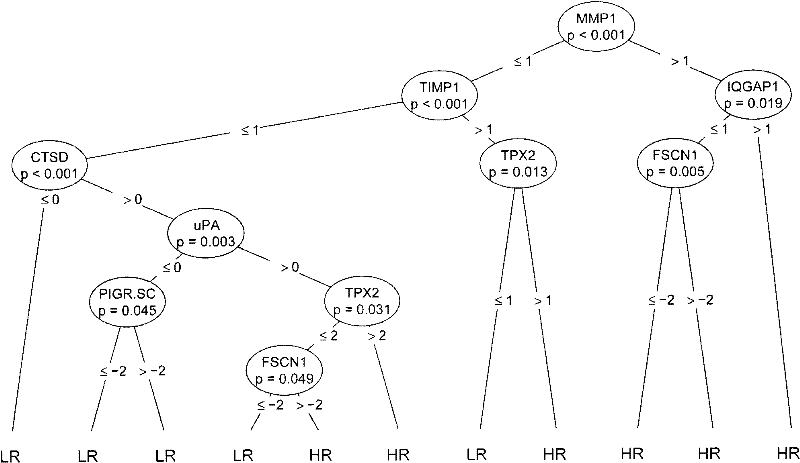
The invention discloses a reagent for auxiliarily diagnosing lung cancer lymph node metastasis, comprising 8 antibodies which are used for detecting 8 protein markers which are MMP1 (matrix metalloproteinase-1), TIMP1 (tissue inhibitor of metalloproteinases metallopeptidase inhibitor 1), IQGAP1 (IQ motif containing GTPase activating protein 1), TPX2 (targeting protein for Xklp2), uPA (Urokinase-type plasminogen activator), Cathepsin-D, Fascin and pIgR / SC (polymeric immunoglobulin receptor / secretory component). By adopting the 8 antibodies and an immunohistochemical staining result, lung cancer lymph node metastasis can be auxiliarily diagnosed, and the reagent is expected to be used for the risk estimation of lung squamous cell cancer lymph node metastasis and the prognostic prediction. The reagent has high creditability, strong practicability and clinical use value based on the clinical routine immunohistochemical staining technology when the reagent is used for auxiliary diagnosis.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Anti-aging composition and preparation and application thereof
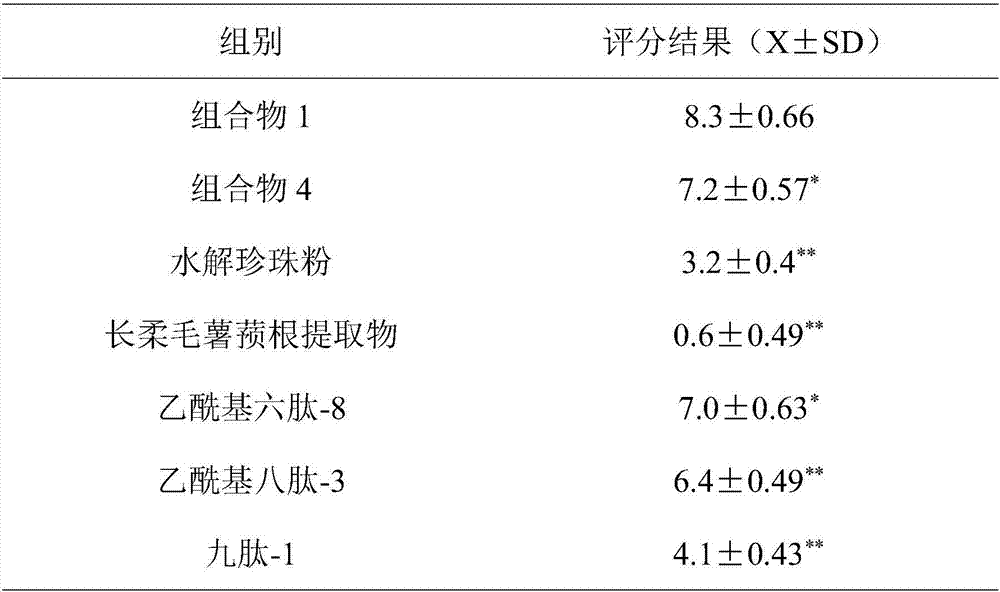
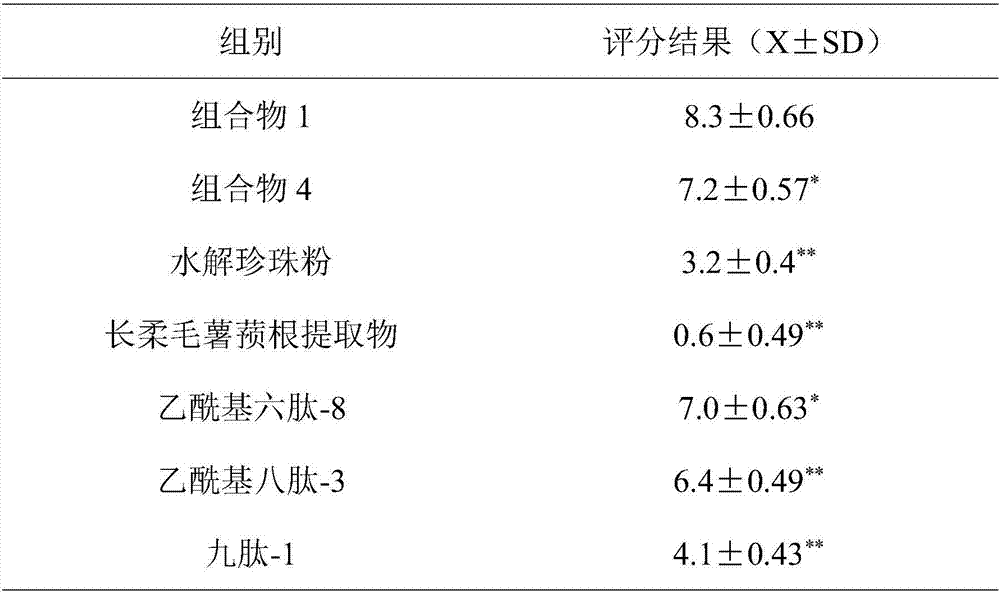
ActiveCN107441017ASignificant anti-aging effectEasy to removeCosmetic preparationsToilet preparationsCollagen Type IIIHuman skin fibroblast
The invention belongs to the field of cosmetics and particularly relates to an anti-aging composition and preparation and application thereof. The anti-aging composition is prepared from the following ingredients in parts by weight: 1 to 3 parts of hydrolyzed pearl powder, 1 to 3 parts of dioscorea villosa root extract, 0.5 to 2 parts of acetyl hexapeptide-8, 0.5 to 2 parts of acetyl octapeptide-3 and 0.5 to 2 parts of nonapeptide-1. Experiments prove that the anti-aging composition disclosed by the invention has an extremely obvious removing effect on quiescent wrinkles, dynamic wrinkles and posture extrusion wrinkles, meanwhile can obviously promote multiplication of human skin fibroblast, extremely obviously promotes synthesis of I and III type collagen and extremely obviously inhibits expression of MMP1 and MMP3.
Owner:佐登妮丝(广州)美容化妆品有限公司
Methods for treating aging and skin disorders using nucleic acids targeting Tyr or MMP1
Owner:PHIO PHARMA CORP
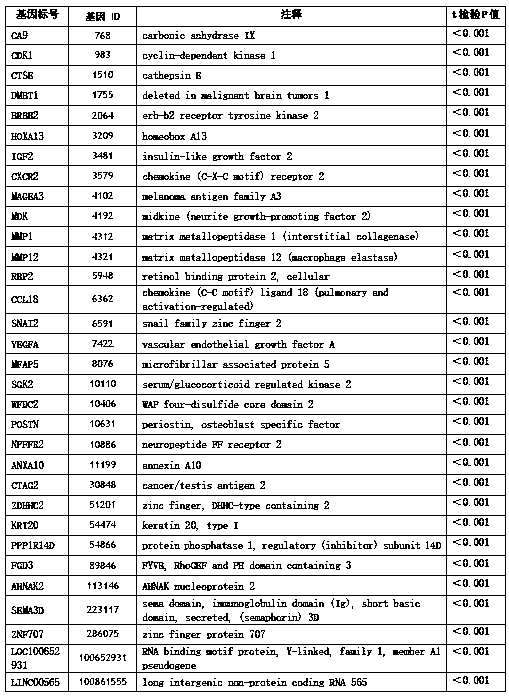
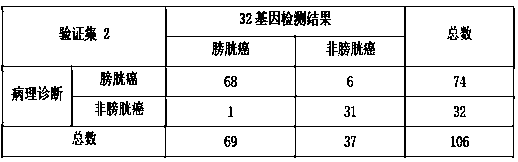
Set of genes used for bladder cancer detection and application thereof
ActiveCN108866194AHigh yieldHigh purityMicrobiological testing/measurementBiostatisticsBladder cancerCCL18
The invention discloses a set of genes used for bladder cancer detection and application. The set of genes used for bladder cancer detection comprise the following 32 genes: a CA9 gene, a CDK1 gene, aCTSE gene, a DMBT1 gene, an ERBB2 gene, an HOXA13 gene, an IGF2 gene, a CXCR2 gene, an MAGEA3 gene, an MDK gene, an MMP1 gene, an MMP12 gene, a RBP2 gene, a CCL18 gene, an SNAI2 gene, a VEGFA gene, an MFAP5 gene, an SGK2 gene, a WFDC2 gene, a POSTN gene, an NPFFR2 gene, an ANXA10 gene, a CTAG2 gene, a ZDHHC2 gene, a KRT20 gene, a PPP1R14D gene, an FGD3 gene, an AHNAK2 gene, an SEMA3D gene, a ZNF707 gene, a LOC100652931 gene and an LINC00565 gene. High accuracy and objective result interpretation by adopting a detection kit of the set of genes for bladder cancer detection are proven clinically; meanwhile, as non-invasive detection, compared with existing cystoscopy, the detection kit has the advantage that the compliance of a patient is greatly improved, and the detection kit has importantclinical significance to early detection and postoperation monitoring of bladder cancer.
Owner:HANGZHOU CANHELP GENOMICS TECH CO LTD
Molecular markers for cancer prognosis
InactiveUS20110172928A1Reduce the impactStrong specificityMicrobiological testing/measurementBiostatisticsDiseaseSOX4
The present invention relates to methods for prediction of an outcome of neoplastic disease or cancer. More specifically, the present invention relates to a method for the prediction of breast cancer by determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CA12, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF.
Owner:SIVIDON DIAGNOSTICS
Method for screening anti-breast cancer metastasis compounds, and applications of related compounds
ActiveCN107841532AMicrobiological testing/measurementKetone active ingredientsLymphatic SpreadAnti breast cancer
The present invention provides a method for screening anti-breast cancer metastasis compounds by using a tumor metastasis gene map. The method comprises: randomly selecting at least one gene from 18 genes such as MMP1, FSCN1, PTGS2, EREG, ANGPTL4, VCAM1, CXCR4, CXCL1, RARRES3, ID1, TNC, LY6E, LTBP1, C10orf116, KRTHB1, KYNU, MAN1A1 and NEDD9; and designing probes, and selecting compounds for converting the expression levels of the genes in breast cancer cells after metastasis to the expression levels of the genes in in-situ carcinoma cells or normal cells. According to the present invention, the 20 compounds are selected through the method, and the cytological level and animal level experiment results demonstrate that the compounds or the combinations thereof exhibit the anti-breast cancermetastasis activity to different degrees.
Owner:TSINGHUA UNIV
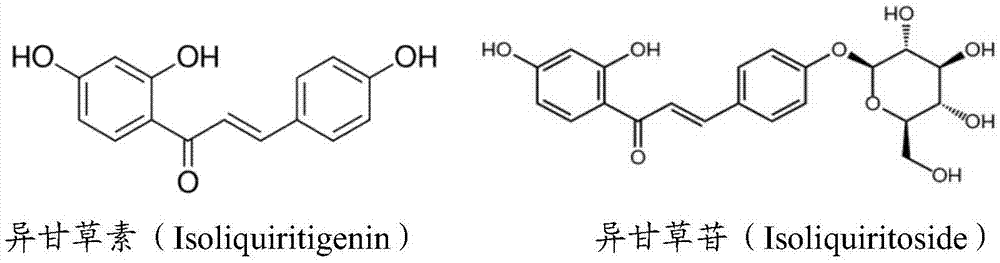
Application of isoliquiritigenin or isoliquiritoside to preparation of anti-aging cosmetic products with high safety performance
ActiveCN107028782AInhibit apoptosisReduced expression levelCosmetic preparationsToilet preparationsProcollagen iApoptosis
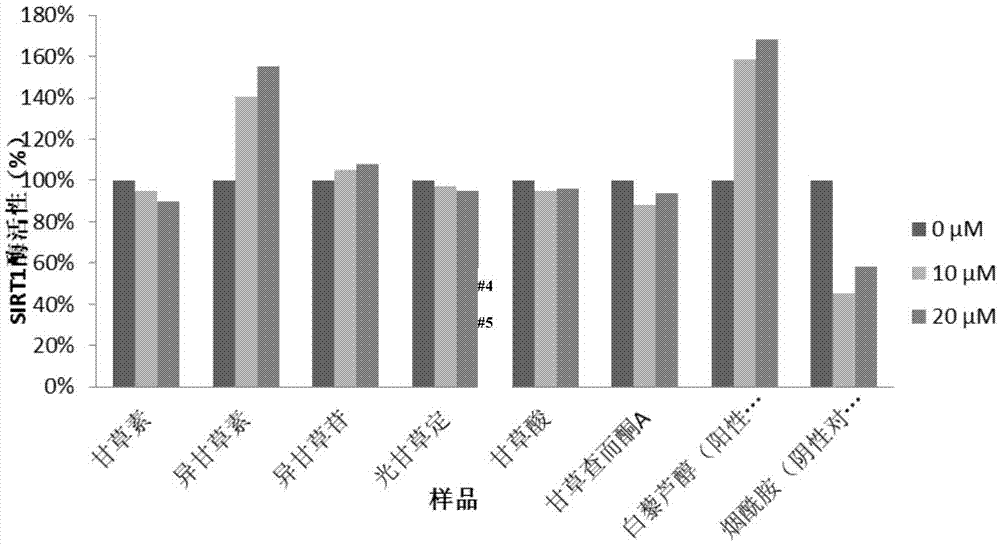
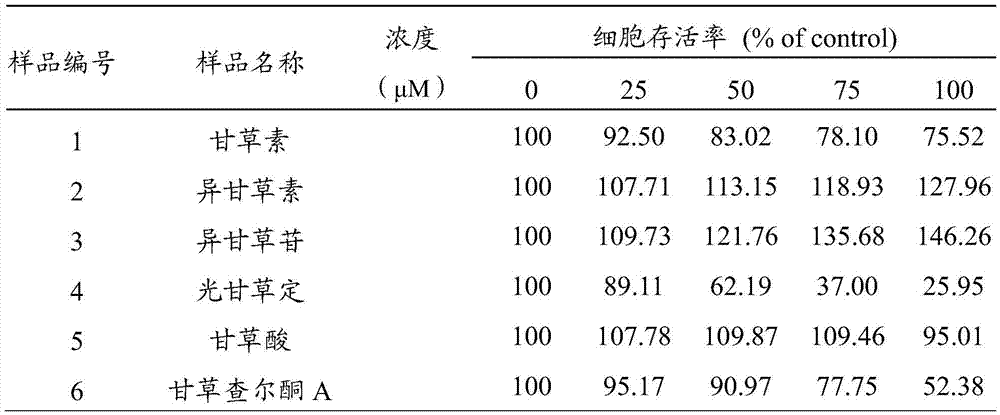
The invention discloses application of isoliquiritigenin or isoliquiritoside to preparation of anti-aging cosmetic products with high safety performance. Researches prove that isoliquiritigenin or isoliquiritoside can improve the expression level of the procollagen I alpha 1 chain (Col1A1) in human skin fibroblasts, reduce the expression level of matrix metalloproteinase 1 (MMP1), and promote the expression level of the silent information regulator 1 (Sirt1); therefore, isoliquiritigenin or isoliquiritoside can inhibit human skin fibroblast apoptosis and promote synthesis of collagen in human skin fibroblasts, so as to resist aging. Furthermore, isoliquiritigenin or isoliquiritoside has low toxicity to human skin fibroblasts. Researches show that isoliquiritigenin or isoliquiritoside is of great application value in preparation of anti-aging cosmetic products with high safety performance.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Recombinant halomonas, construction method of recombinant halomonas and application of recombinant halomonas in preparation of itaconic acid by catalyzing citric acid
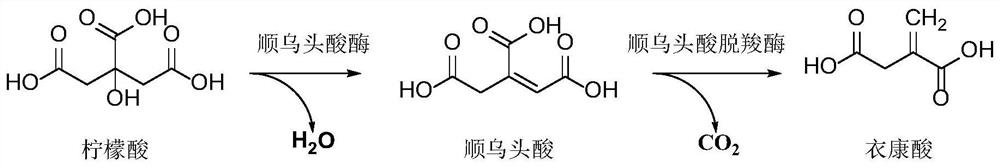
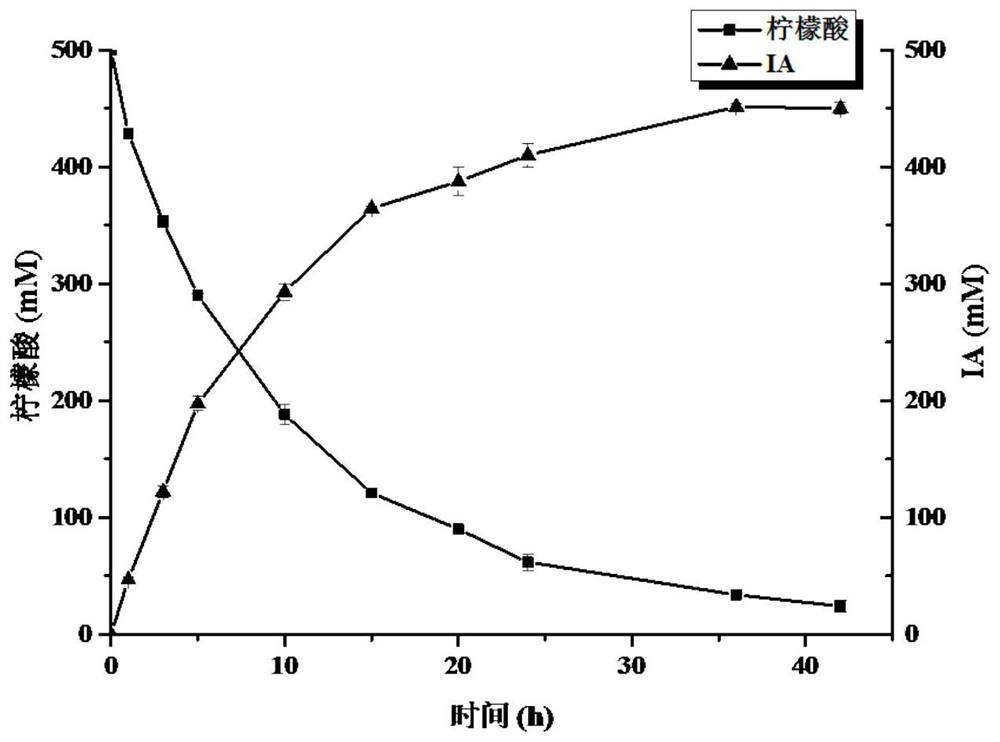
ActiveCN113481136AEasy to purifyShort reaction cycleBacteriaTransferasesHalomonas salinaItaconic acid
The invention discloses recombinant halomonas, a construction method of the recombinant halomonas and application of the recombinant halomonas to preparation of itaconic acid by catalyzing citric acid. The construction method of the recombinant halomonas is characterized by comprising the following steps of: integrating an Mmp1 RNA polymerase expression unit on a wild type halomonas sp. TD01 genome, and replacing an initiation codon ATG of an icd gene of the genome with TTG to obtain Halomonas sp. TD2.0; sequentially introducing a high-copy expression vector pN59-PMmp1-RBS-cadA-acn and a low-copy expression vector pN85-PMmp1-RBS-GroESL-PMmp1-RBS-acn into the Halomonas sp. TD2.0; and obtaining the recombinant halomonas sp. IA02. The itaconic acid can be efficiently prepared through cell catalysis of the bacterium. The recombinant halomonas is short in period, simple, convenient and high in yield.
Owner:TIANJIN UNIV +1
Combination of treating and/or preventing hemorrhoid
InactiveCN1660433APharmaceutical active ingredientsCardiovascular disorderInos inhibitorTreatment effect
A composition for preventing and / or treating piles contains at least one NO synthetase (NOS) inhibitor, especially iNOS inhibitor, at least one vascular angiogenesis inhibitor, especially VEGF inhibitor, and at least one matrix metal proteinase (MMP) inhibitor, especially MMP1, MMP3, MMP9 and / or MMP12 inhibitor.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Methods for treating aging and skin disorders using nucleic acids targeting tyr or mmp1
ActiveUS20210348169A1Antibacterial agentsOrganic active ingredientsDermatologic disordersCell biology
Owner:PHIO PHARMA CORP
Marker for carcinoma
InactiveUS20130267440A1Microbiological testing/measurementLibrary screeningNon small lung cancerRegimen
The present invention relates to a method for diagnosis of different stages of endometrial cancer in an individual. Further, the present invention relates to a method for evaluating the probability of survival for an individual suffering from endometrial carcinoma. In another aspect, the present invention relates to the stratification of therapy regimen of endometrial tumor, ovarian cancer, breast cancer, non-small lung cancer or hormone refractory prostate cancer therapy in an individual or monitoring therapeutic efficacy in an individual suffering from the same based on the expression status of STMN1 gene or protein. Moreover, the present invention relates to a kit for use in any of the above referenced methods comprising a means for determining amplifications and deletions of chromosomal regions 3q26.32 and 12p12.1, determining alterations of the gene expression profile of the genes (gene signature): upregulation of the genes PLEKHK1, ATP10B, NMU, MMP1, ATAD2, NETO2, TNNI3, PHLDA2, OVOL1 and down-regulation of the genes: NDP, KIAA1434, MME, CFH, MOXD1, SLC47A1, RBP1, PDE8B, ASRGL1, ADAMTS19, EFHD1, ABCA5, NPAS3, SCML1, TNXB, ENTPD3, AMY1A, ENPP, RASL11B, PDZK3, or the expression status of the STMN1 gene or protein, respectively. Finally, the present invention provides a method for predicting the response to taxanes in an individual suffering from a disease treated with the taxanes based on the expression status of the STMN1 gene or protein.
Owner:BERGEN TEKNOLOGIOVERFORING AS
New application of inhibitor of matrix metal metal protease (MMP) in use for treating hemorrhoid
InactiveCN1660429APharmaceutical delivery mechanismPharmaceutical active ingredientsProteinase activityMedicine
An application of the matrix metal proteinase (MMP) inhibitor, especially the inhibitor of 1(MMP1), 9 (MMP9) or 12 (MMP12), in preparing the medicines for preventing and / or treating piles is disclosed.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
A kind of anti-aging composition and its preparation and application
ActiveCN107441017BSignificant anti-aging effectEasy to removeCosmetic preparationsToilet preparationsWrinkle skinAdditive ingredient
The invention belongs to the field of cosmetics and particularly relates to an anti-aging composition and preparation and application thereof. The anti-aging composition is prepared from the following ingredients in parts by weight: 1 to 3 parts of hydrolyzed pearl powder, 1 to 3 parts of dioscorea villosa root extract, 0.5 to 2 parts of acetyl hexapeptide-8, 0.5 to 2 parts of acetyl octapeptide-3 and 0.5 to 2 parts of nonapeptide-1. Experiments prove that the anti-aging composition disclosed by the invention has an extremely obvious removing effect on quiescent wrinkles, dynamic wrinkles and posture extrusion wrinkles, meanwhile can obviously promote multiplication of human skin fibroblast, extremely obviously promotes synthesis of I and III type collagen and extremely obviously inhibits expression of MMP1 and MMP3.
Owner:佐登妮丝(广州)美容化妆品有限公司
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS9624541B2Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisBiomarker (petroleum)
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Skin gene detection method
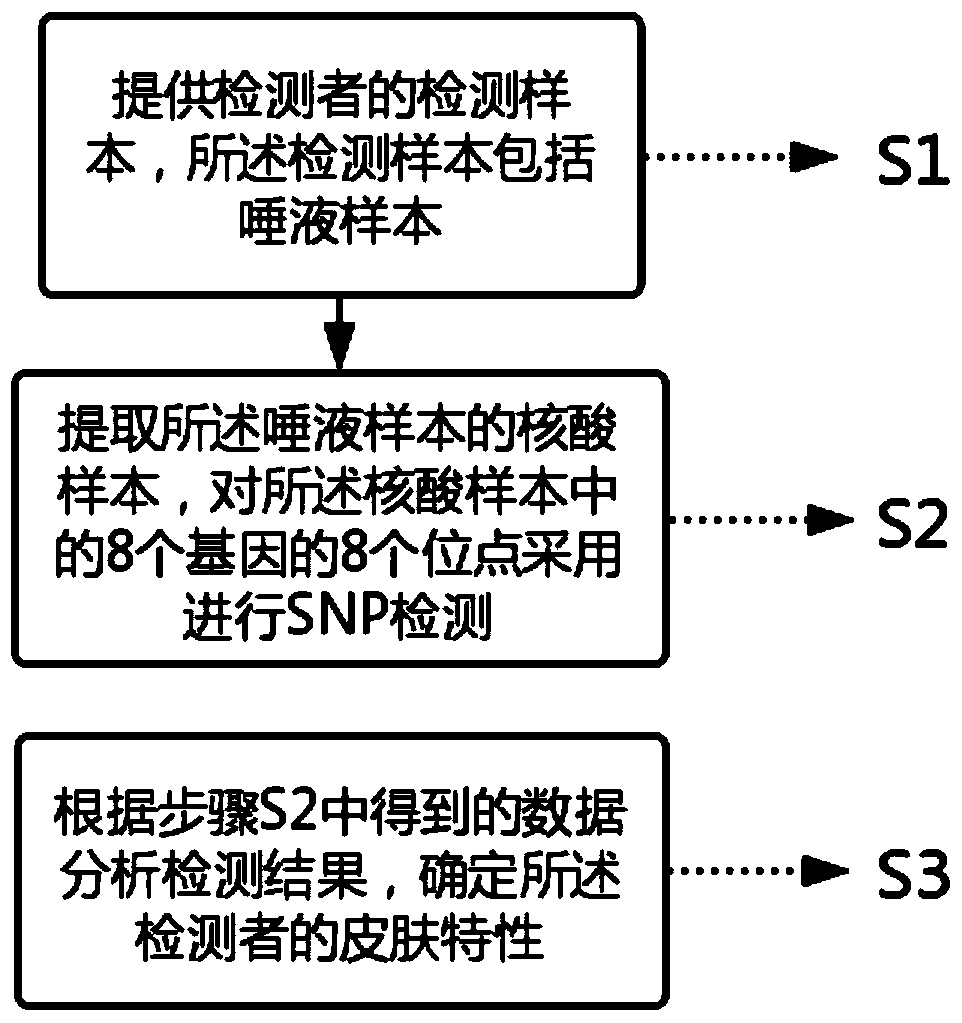
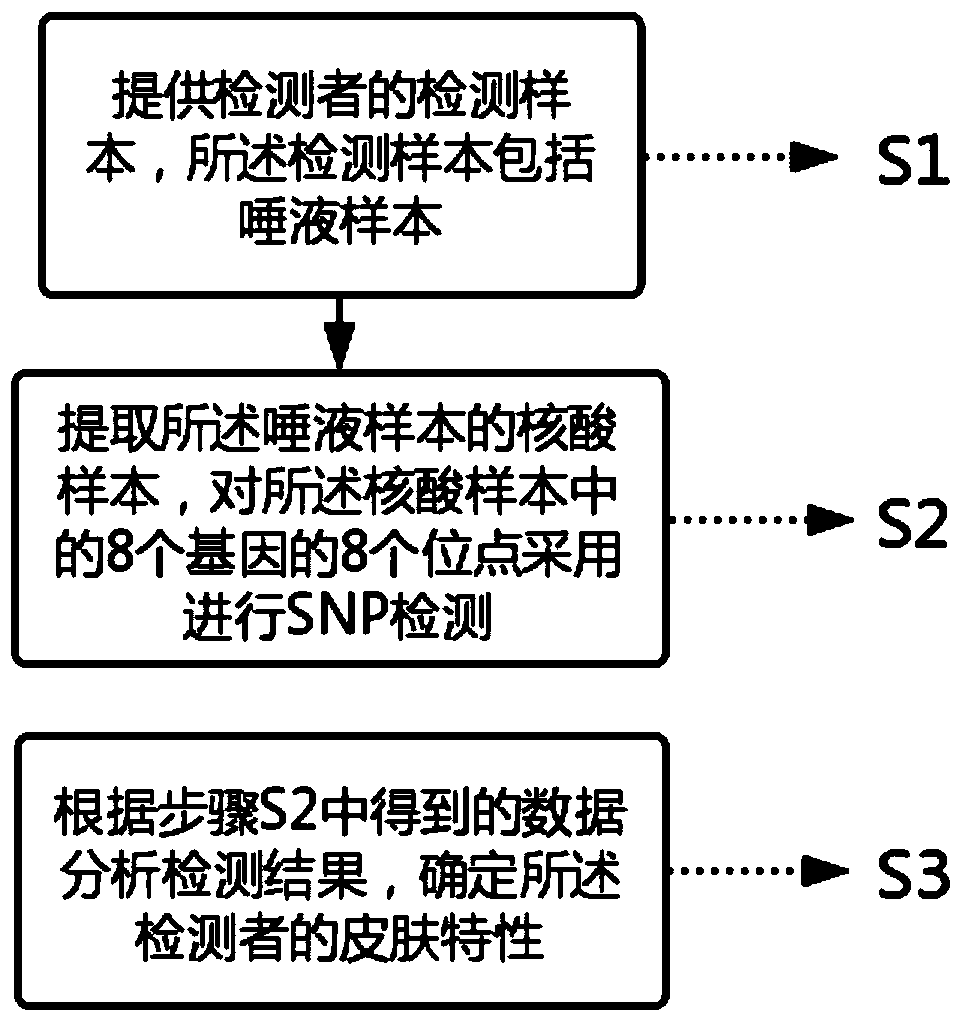
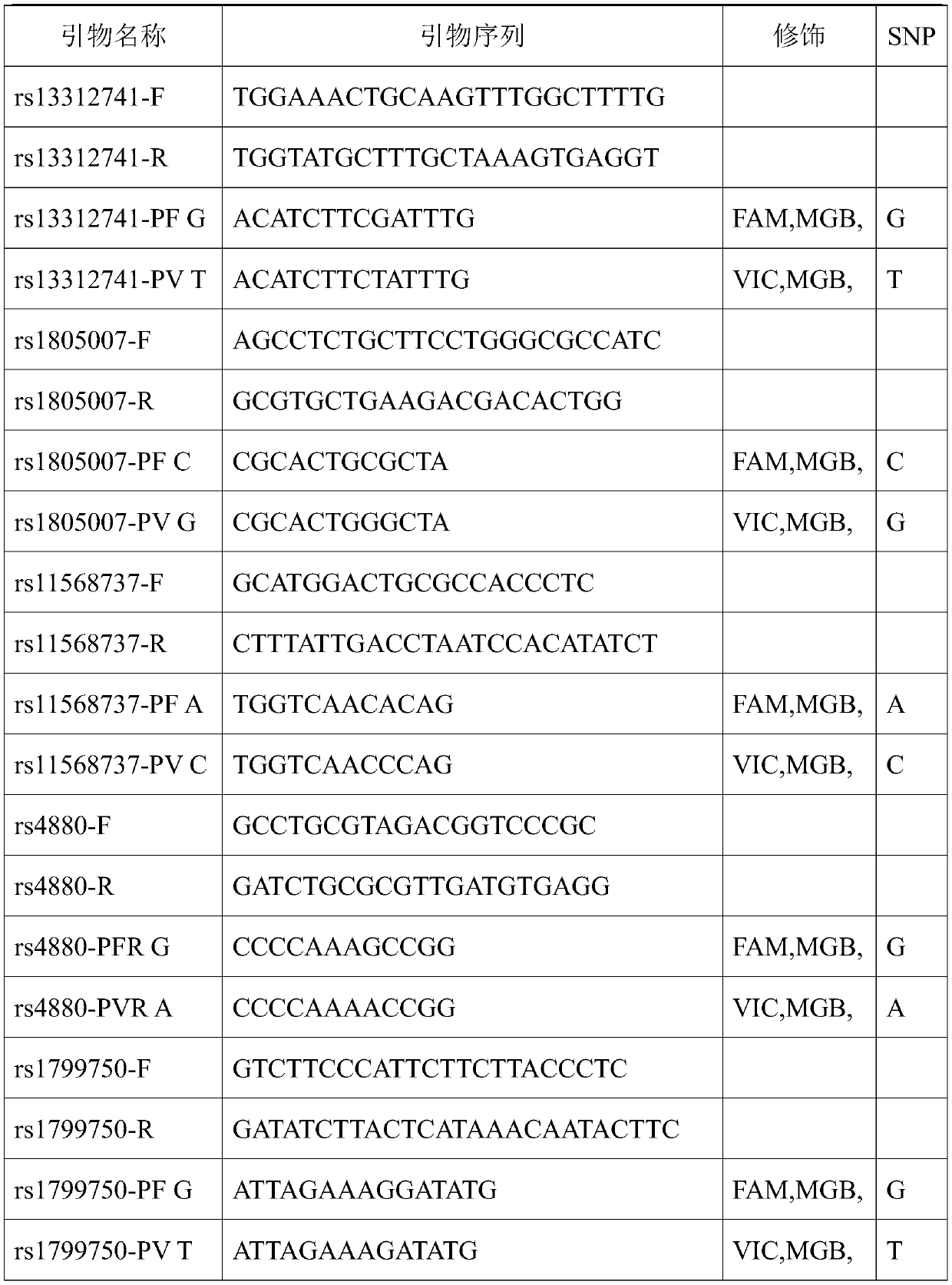
The invention relates to a skin gene detection method. The method includes the following steps: S1, providing a detection sample of a detector, wherein the detection sample comprises a saliva sample;S2, extracting a nucleic acid sample of the saliva sample, performing SNP detection on 8 sites of 8 genes in the nucleic acid sample, wherein the 8 genes and sites respectively are rs13312741 site ofa TYR gene, rs1805007 site of an MC1R gene, rs11568737 site of an SLC45A2 gene, rs4880 site of an SOD2 gene, rs1799750 site of an MMP1 gene, rs222747 site of a TRPV1 gene, rs61816761 site of an FLG gene and rs11103631 site of an AQP3 gene; and S3, analyzing the detection results according to the data obtained in the S2, and determining the skin characteristic of the detector. The gene detection method can carry out characteristic detection on the skin at the gene level, can reflect the real condition of the skin of a detector, and can carry out skin care in a targeted way for a long time.
Owner:苏州罗塞塔生物科技有限公司
Combination of biomarkers for detecting and evaluating a hepatic fibrosis
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH
Prognostic Marker for Endometrial Carcinoma
InactiveUS20130338026A1Microbiological testing/measurementLibrary screeningParanasal Sinus CarcinomaRegimen
A method for diagnosis of different stages of endometrial cancer and for evaluating the probability of survival for an individual suffering from endometrial carcinoma, and for stratification of a therapy regimen for an endometrial tumor or monitoring therapeutic efficacy in an individual suffering considers the expression status of STMN1 gene or protein. A kit for use in the methods allows for determining amplifications and deletions of chromosomal regions 3q26.32 and 12p12.1, determining alterations of the gene expression profile of the genes (gene signature): upregulation of the genes PLEKHK1, ATP10B, NMU, MMP1, ATAD2, NETO2, TNNI3, PHLDA2, OVOL1 and down-regulation of the genes: NDP, KIAA1434, MME, CFH, MOXD1, SLC47A1, RBP1, PDE8B, ASRGL1, ADAMTS19, EFHD1, ABCA5, NPAS3, SCML1, TNXB, ENTPD3, AMY1A, ENPP, RASL11B, PDZK3, or the expression status of the STMN1 gene or protein.
Owner:CARTER SCOTT L +5
Topical skin compositions for treating rosacea and skin redness
ActiveUS20200261531A1Reduce skin inflammationIncreased agonist activityCosmetic preparationsBacteria material medical ingredientsPimpleVascular dilatation
The present invention relates generally to methods of use and compositions useful for treating skin. The composition includes a combination of Phoenix dactylifera extract, tea tree oil, Myrothamnus flabellifolia extract, and saccharide isomerate. This combination can be used to create topical skin compositions that reduce rosacea, erythema, and / or inflammation, and inhibit nitric oxide synthase, increase α2A adrenergic receptor agonist activity, reduce oxidation, increase anti-oxidant capacity of a composition or of skin, inhibit cyclooxygenase-2 (COX-2) production, inhibit vascular endothelial growth factor (VEGF) production, inhibit interleukin-6 (IL-6) and interleukin-8 (IL-8) production, reduce tumor necrosis factor alpha (TNF-α) production, increase collagen stimulation, increase lysyl oxidase expression, inhibit matrix metalloproteinase 1 (MMP1) activity, increase occludin production, increase filaggrin production, and increase skin moisturization. This combination can also be used to reduce transient or persistent erythema, telangiectasia, inflammatory papules and / or pustules, transient or persistent flushing of the skin, and / or hyperplasia of a connective tissue.
Owner:MARY KAY INC
Combination of treating and/or preventing hemorrhoid
InactiveCN1660433BPharmaceutical active ingredientsCardiovascular disorderInos inhibitorTherapeutic effect
A composition for preventing and / or treating piles contains at least one NO synthetase (NOS) inhibitor, especially iNOS inhibitor, at least one vascular angiogenesis inhibitor, especially VEGF inhibitor, and at least one matrix metal proteinase (MMP) inhibitor, especially MMP1, MMP3, MMP9 and / or MMP12 inhibitor.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Topical skin compositions for treating rosacea and skin redness
ActiveUS11090352B2Reduce skin inflammationReduced activityCosmetic preparationsBacteria material medical ingredientsPimpleVascular dilatation
The present invention relates generally to methods of use and compositions useful for treating skin. The composition includes a combination of Phoenix dactylifera extract, tea tree oil, Myrothamnus flabellifolia extract, and saccharide isomerate. This combination can be used to create topical skin compositions that reduce rosacea, erythema, and / or inflammation, and inhibit nitric oxide synthase, increase α2A adrenergic receptor agonist activity, reduce oxidation, increase anti-oxidant capacity of a composition or of skin, inhibit cyclooxygenase-2 (COX-2) production, inhibit vascular endothelial growth factor (VEGF) production, inhibit interleukin-6 (IL-6) and interleukin-8 (IL-8) production, reduce tumor necrosis factor alpha (TNF-α) production, increase collagen stimulation, increase lysyl oxidase expression, inhibit matrix metalloproteinase 1 (MMP1) activity, increase occludin production, increase filaggrin production, and increase skin moisturization. This combination can also be used to reduce transient or persistent erythema, telangiectasia, inflammatory papules and / or pustules, transient or persistent flushing of the skin, and / or hyperplasia of a connective tissue.
Owner:MARY KAY INC
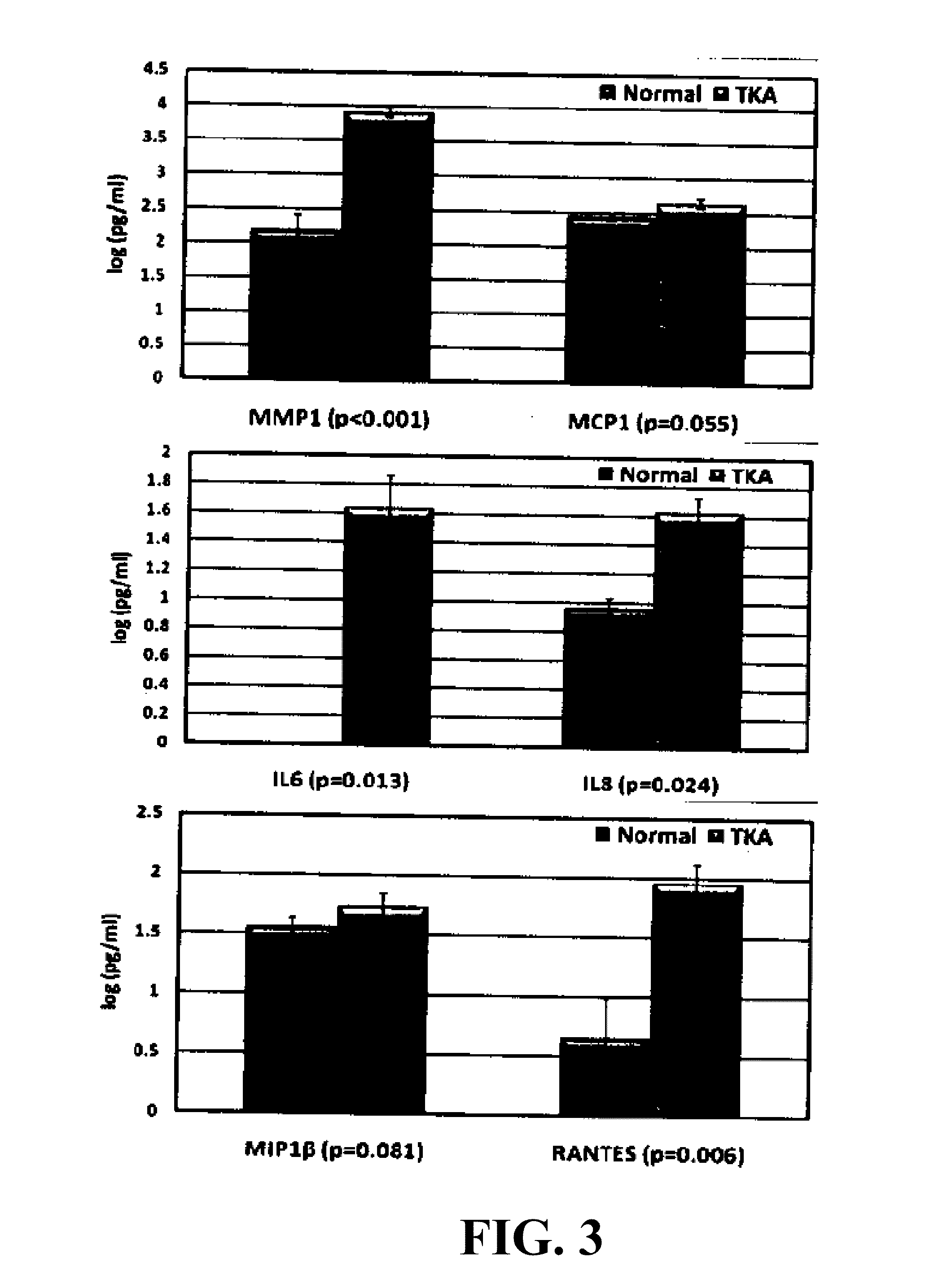
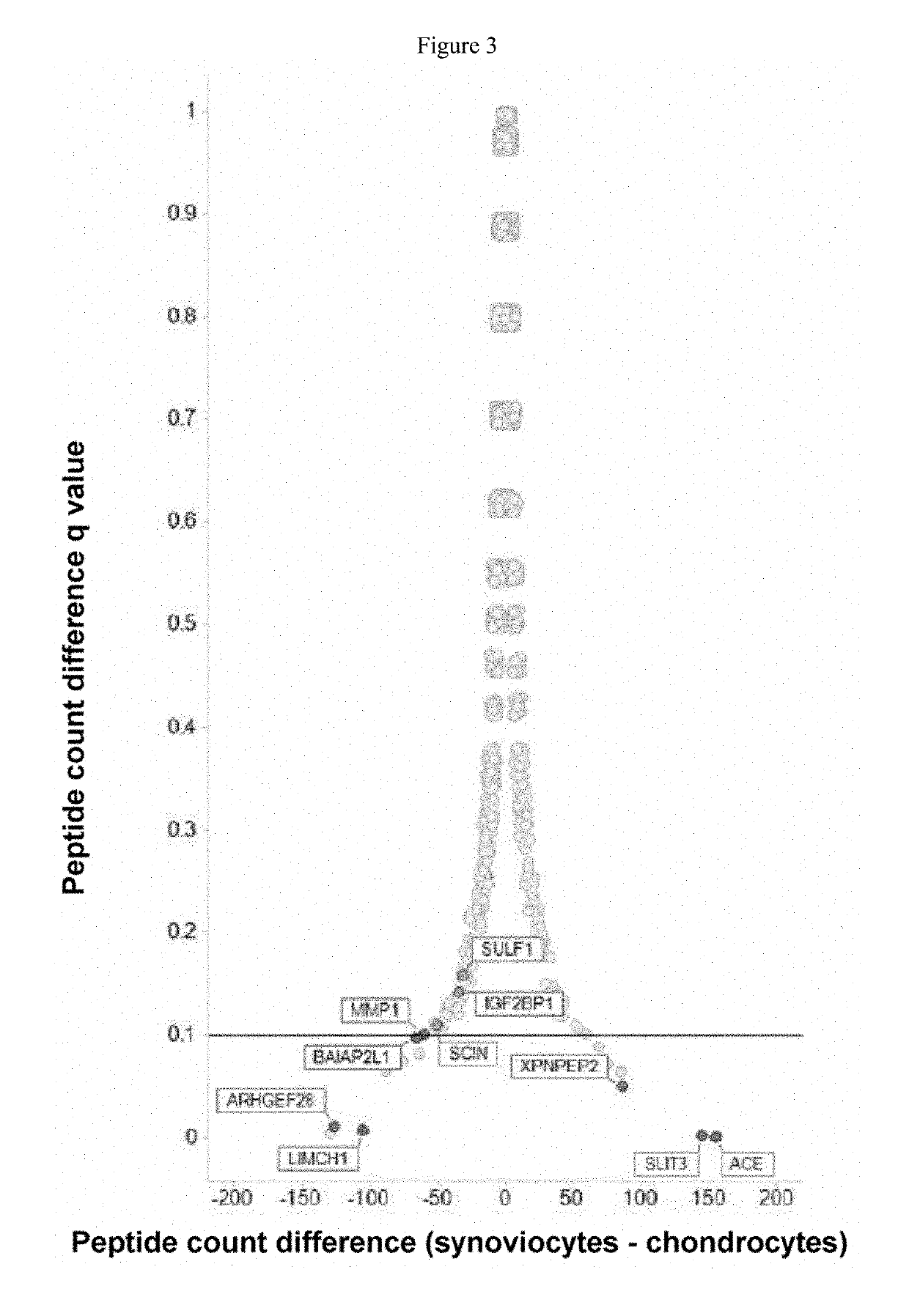
Marker and Method for Determining the Composition or Purity of a Cell Culture and for Determining In Vitro the Identity of a Cartilage Cell or Synovial Cell
InactiveUS20190161799A1Microbiological testing/measurementDisease diagnosisCartilage cellsSynovial Cell
The use of a marker for determining the composition or purity of a cell culture, wherein the marker is at least one marker selected from the group consisting of MMP1, ACE, POSTN, SYNPO, SULF1, ARH-GEF28, IGF2BP1, BAIAP2L1, LIMCH1, SCIN, DCLK1, PPL, CRABP2, NES, XPNPEP2 and SLIT3, and the expression level of the at least one marker is determined.
Owner:TETEC TISSUE ENG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com