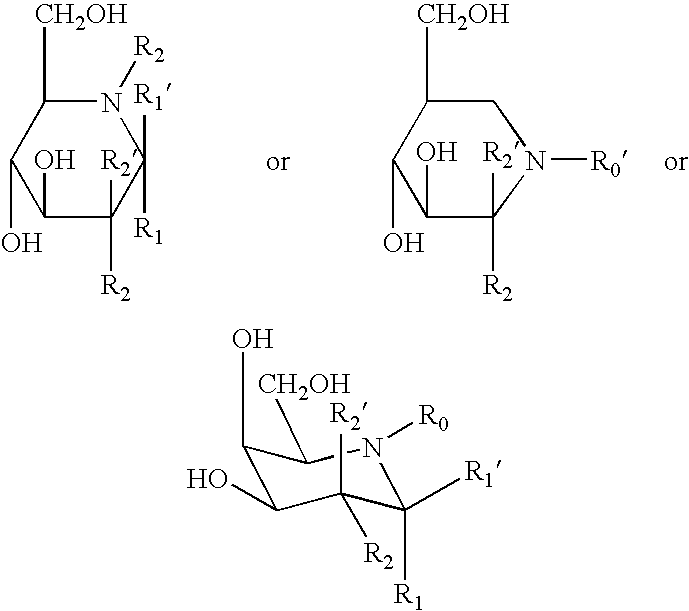
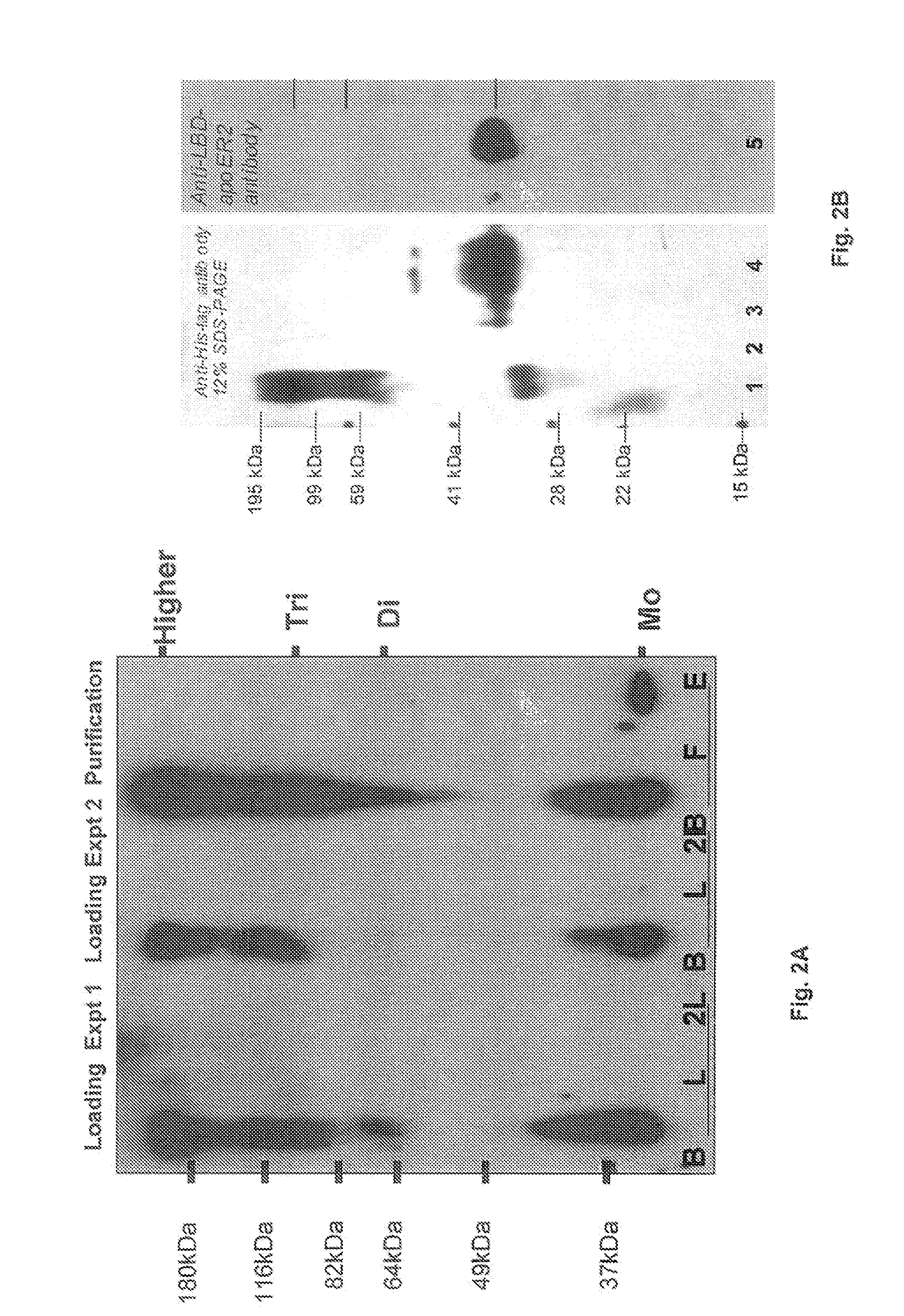
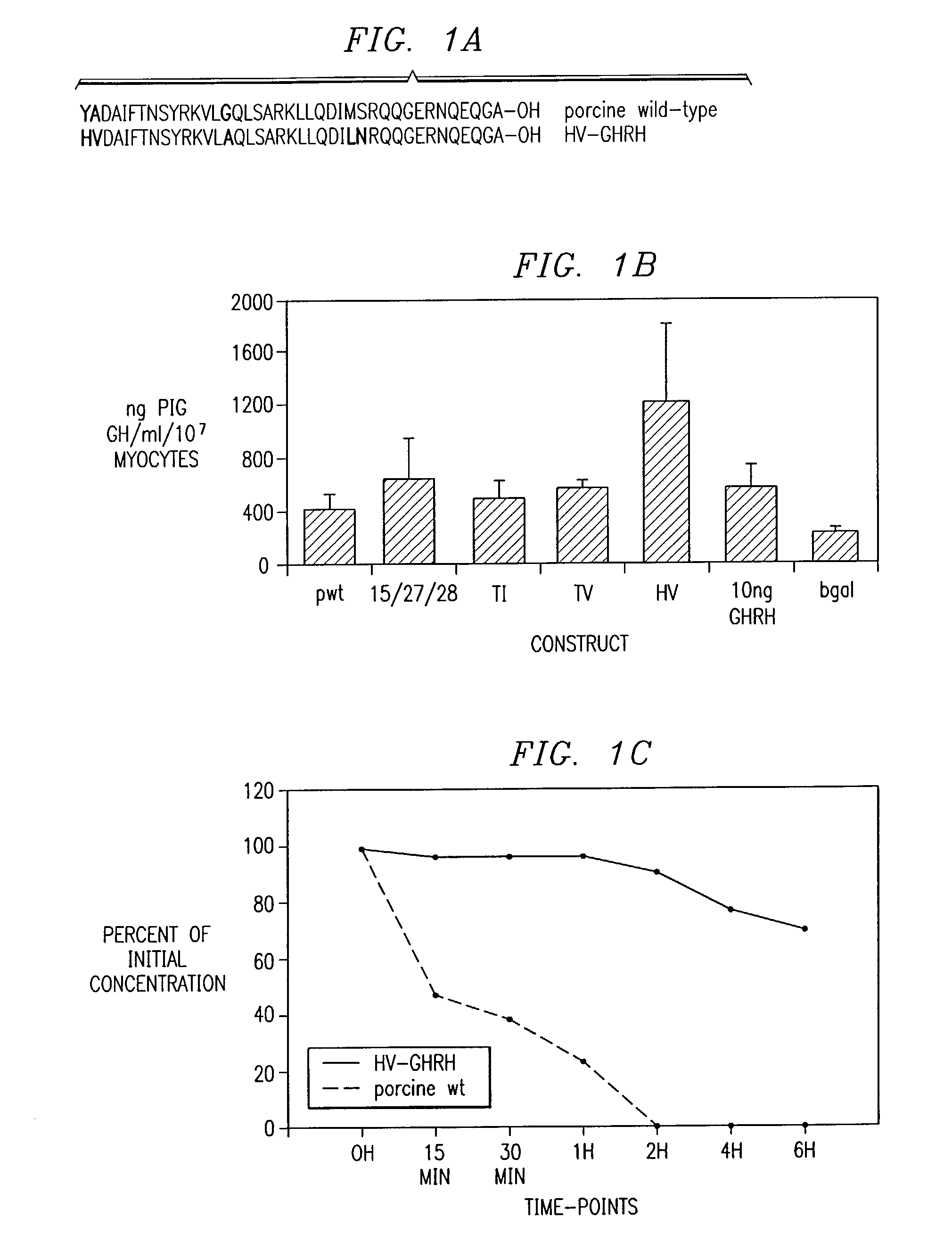
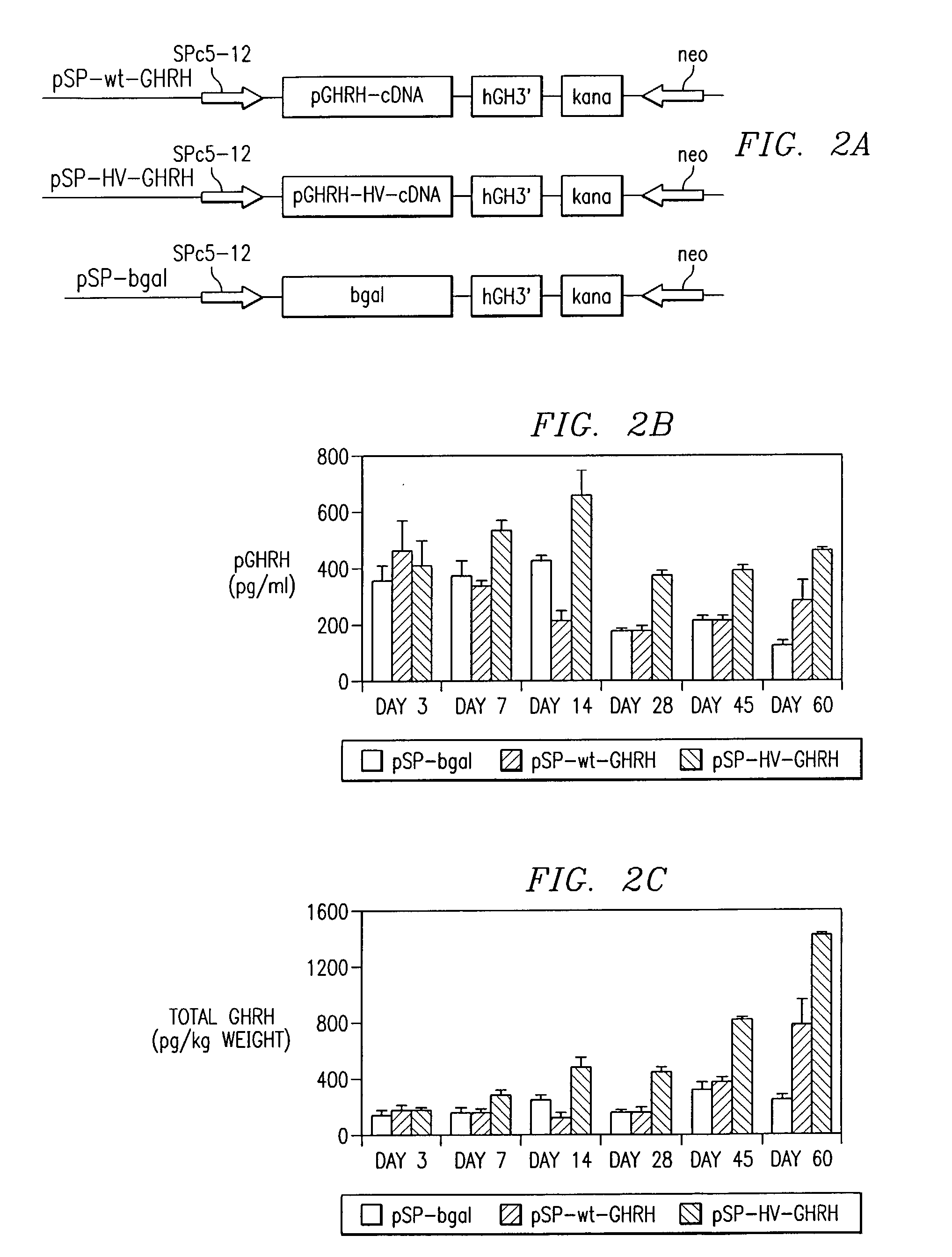
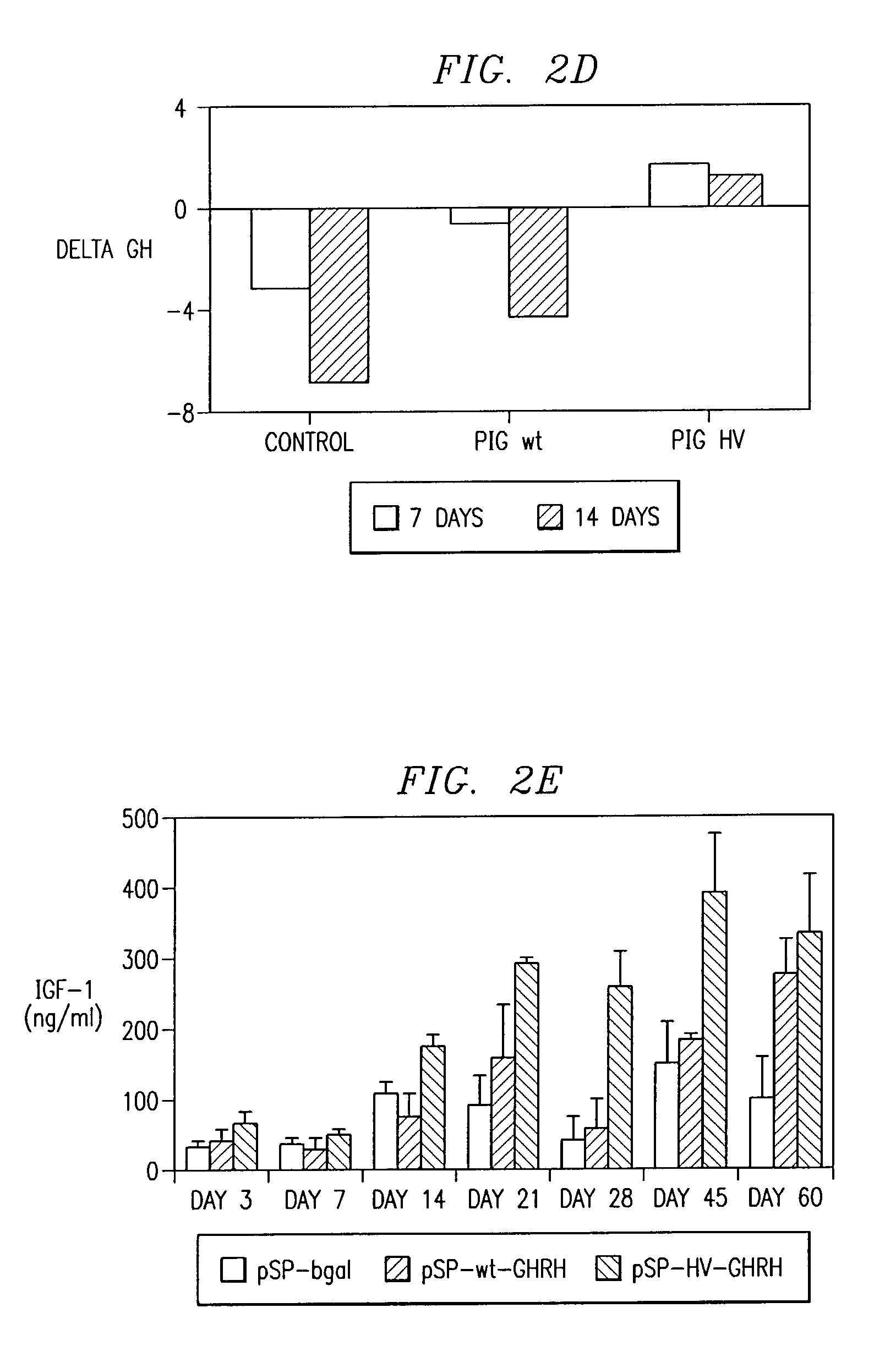
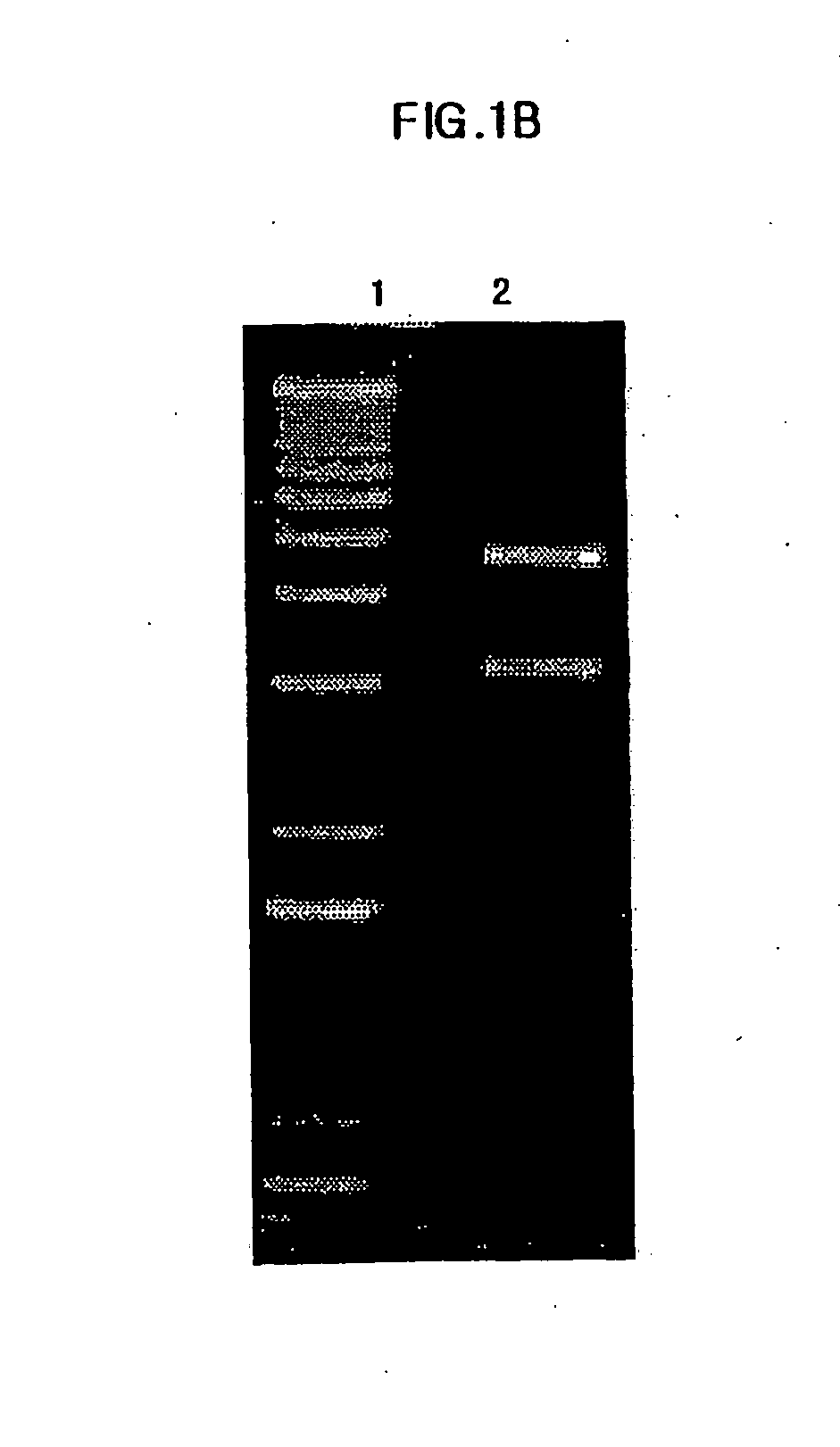
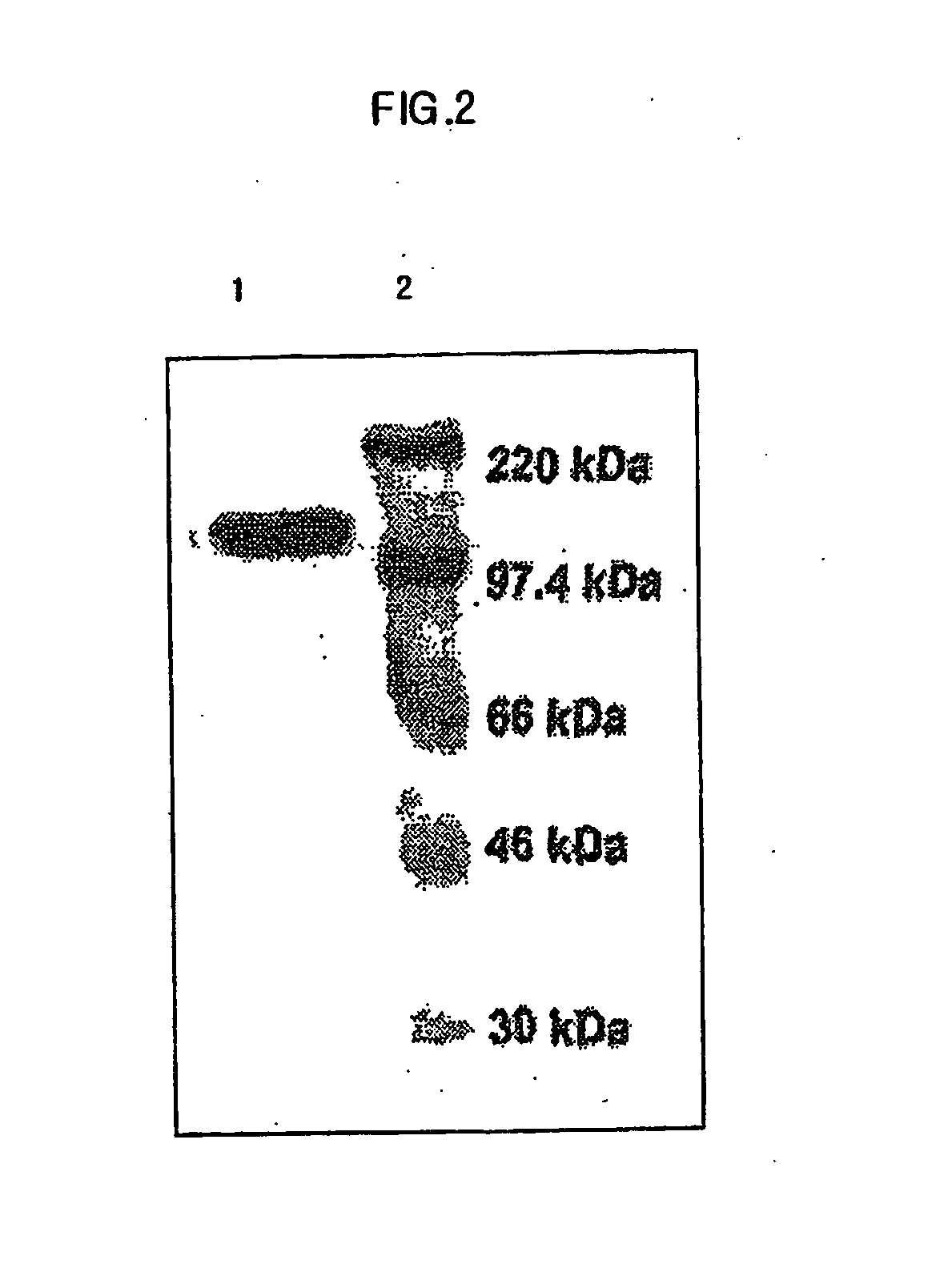
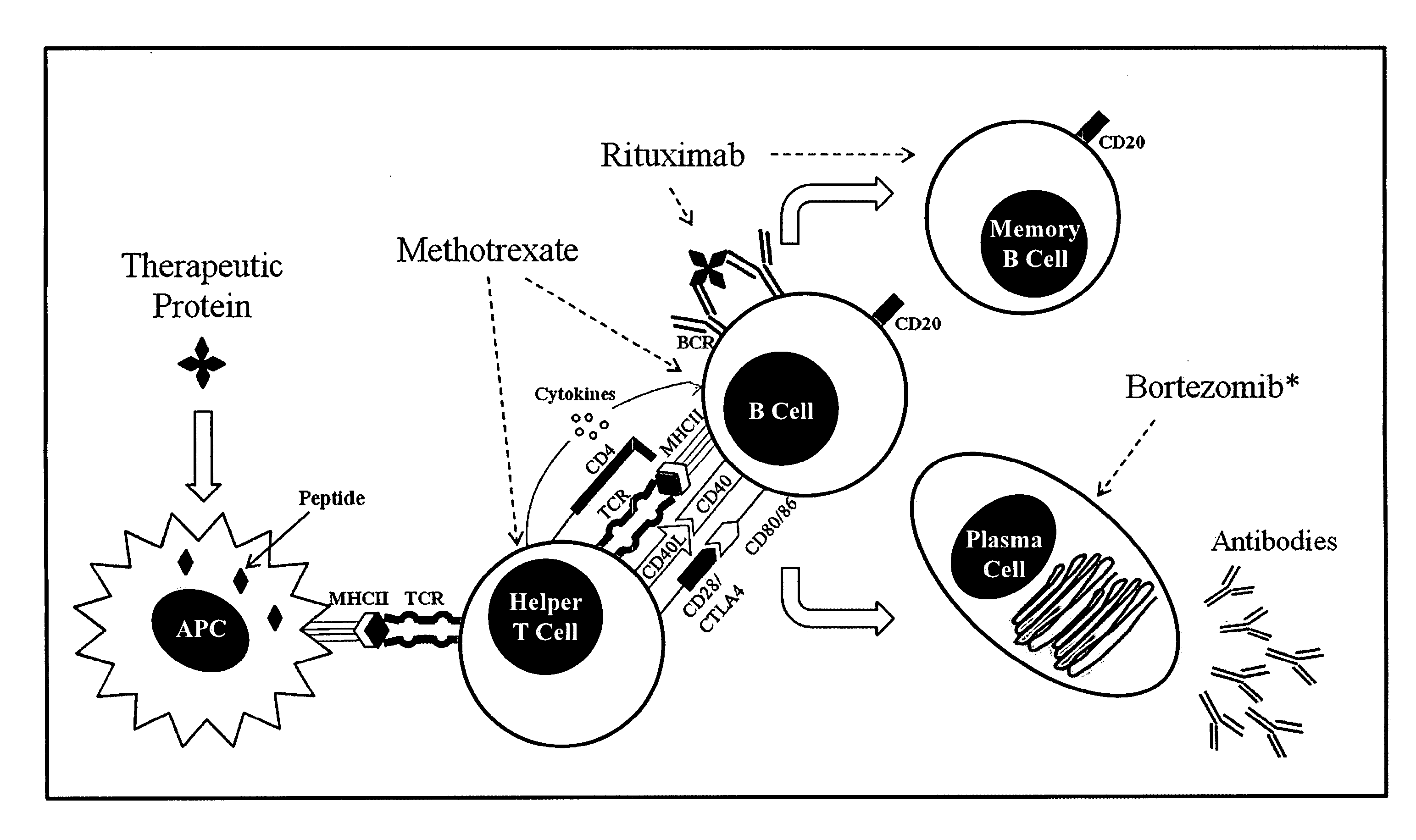
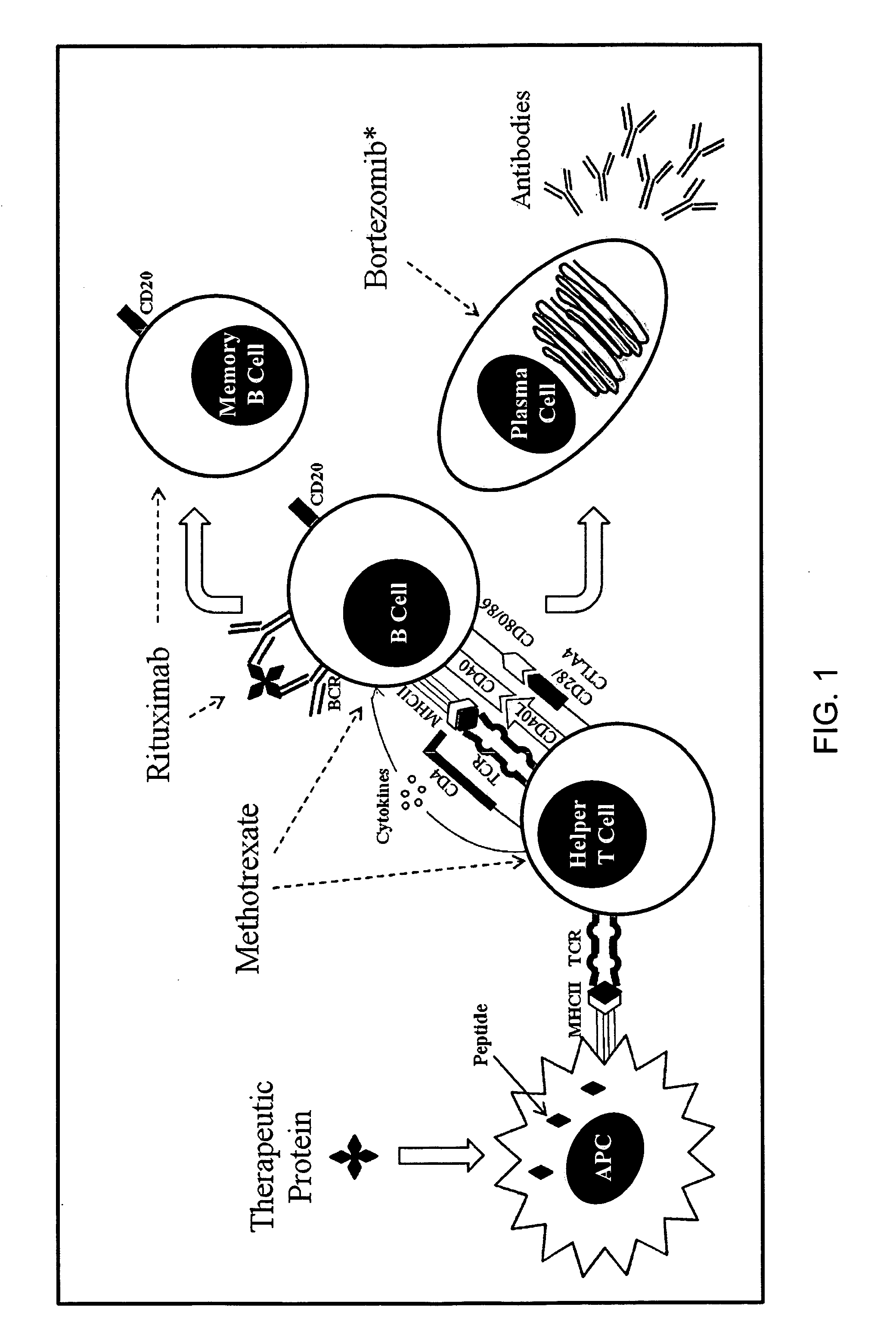
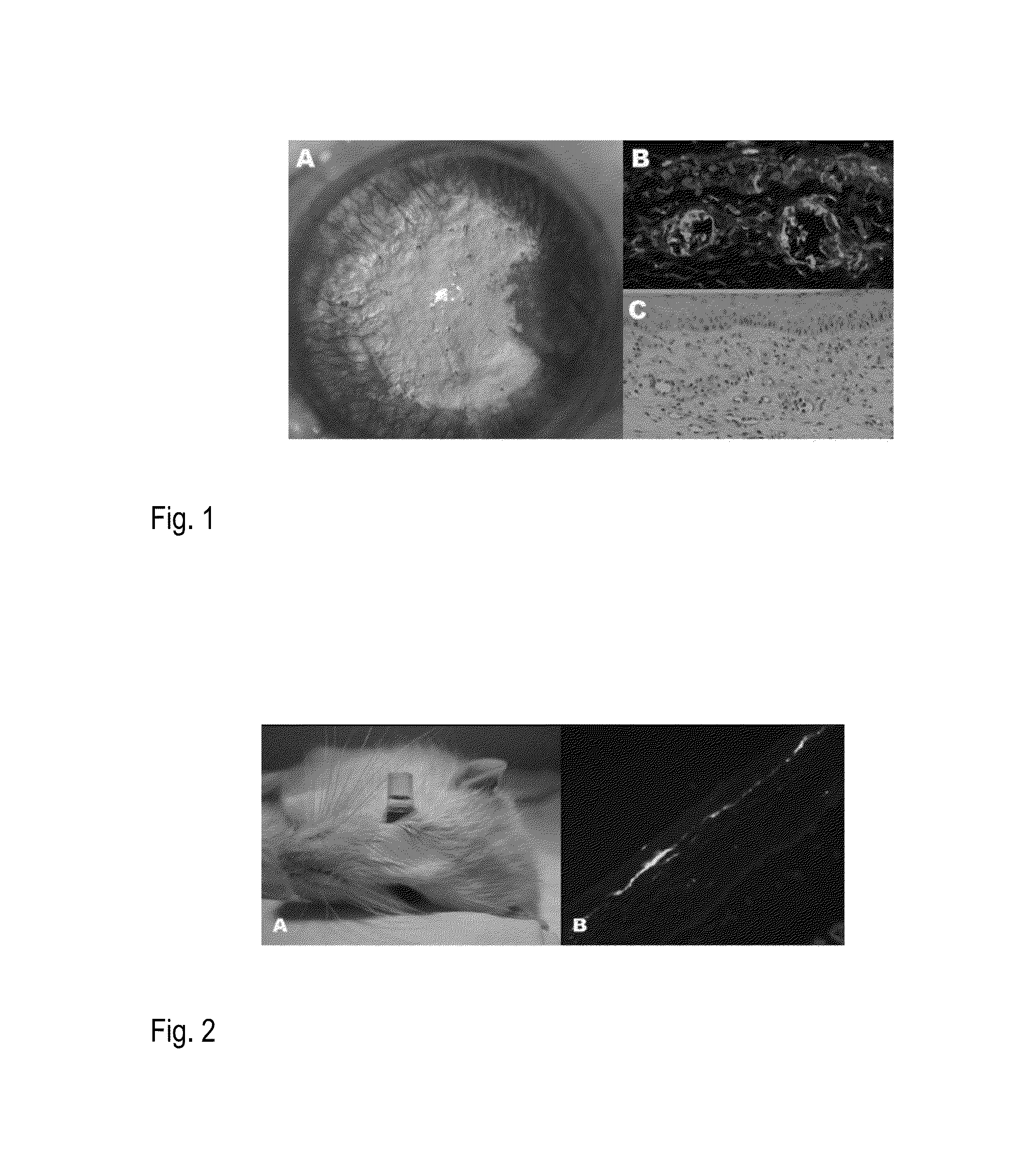
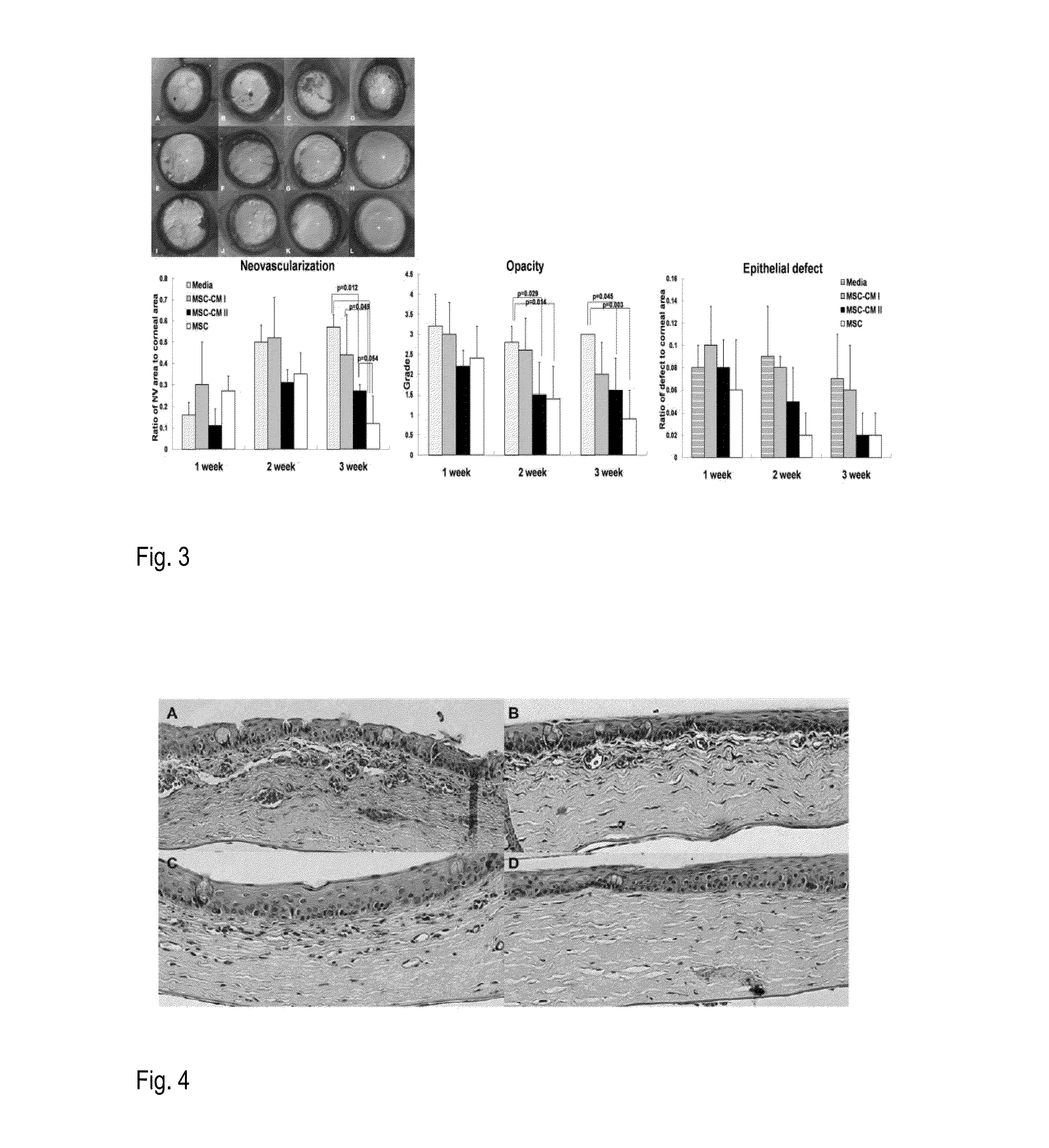
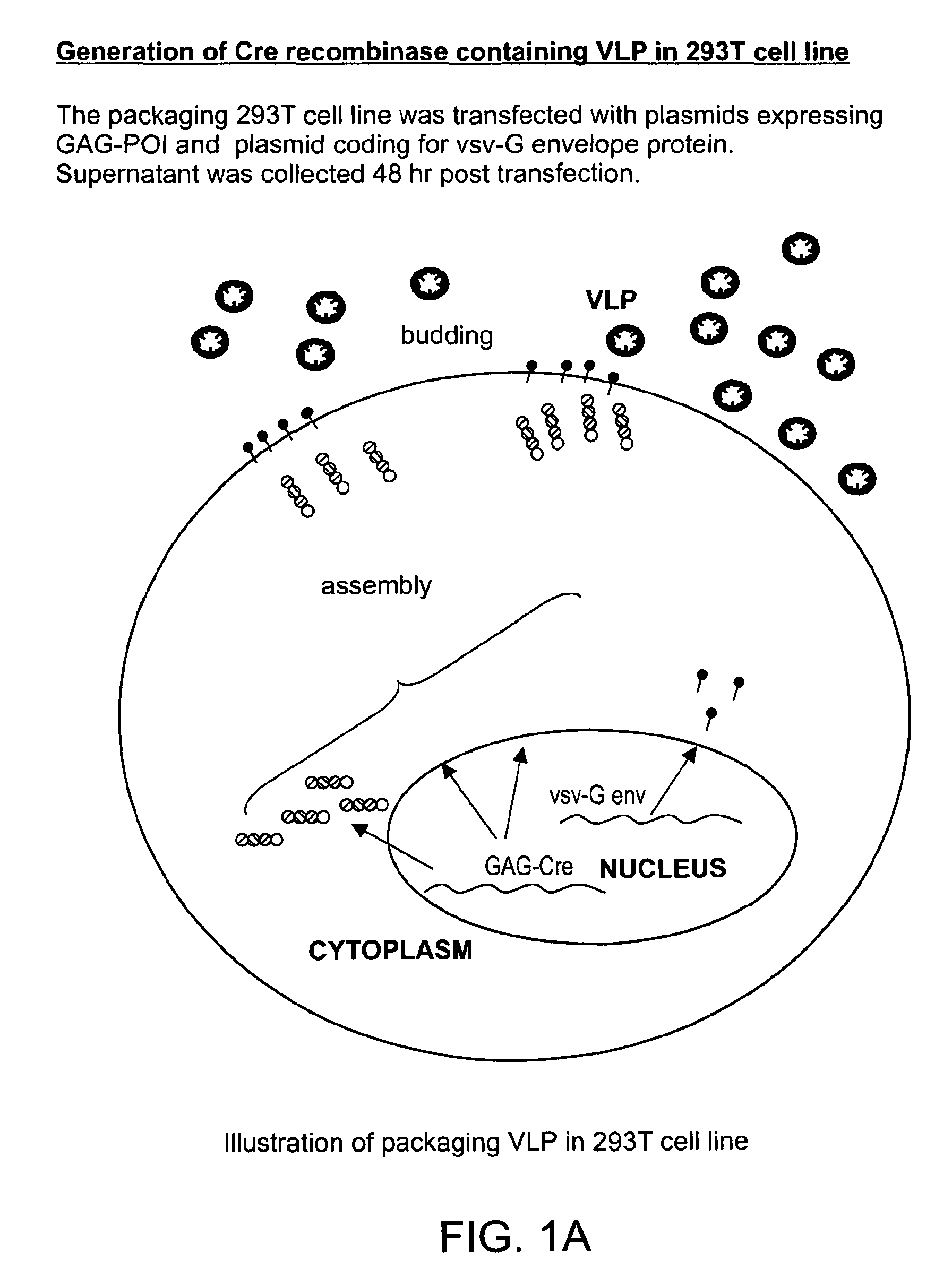
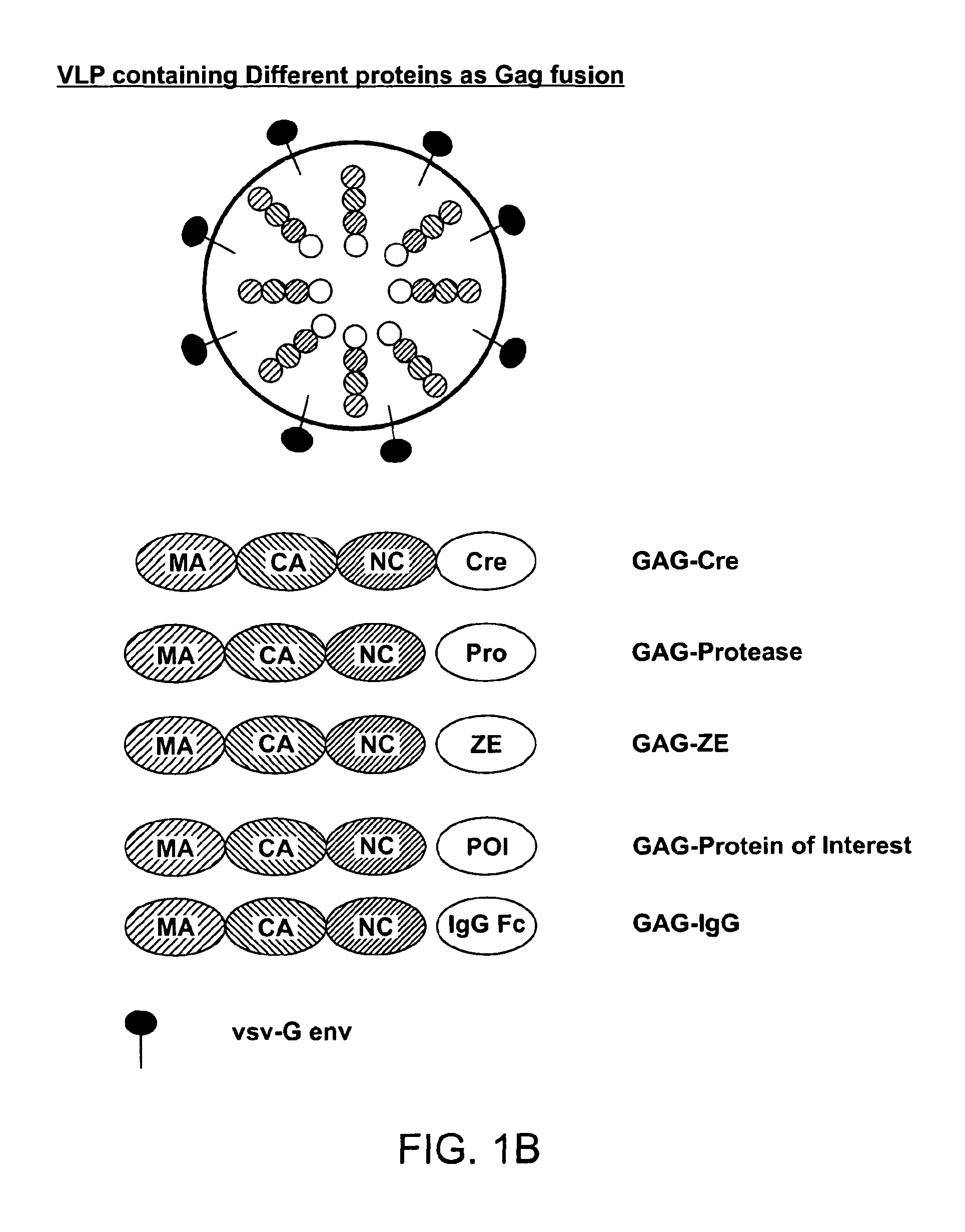
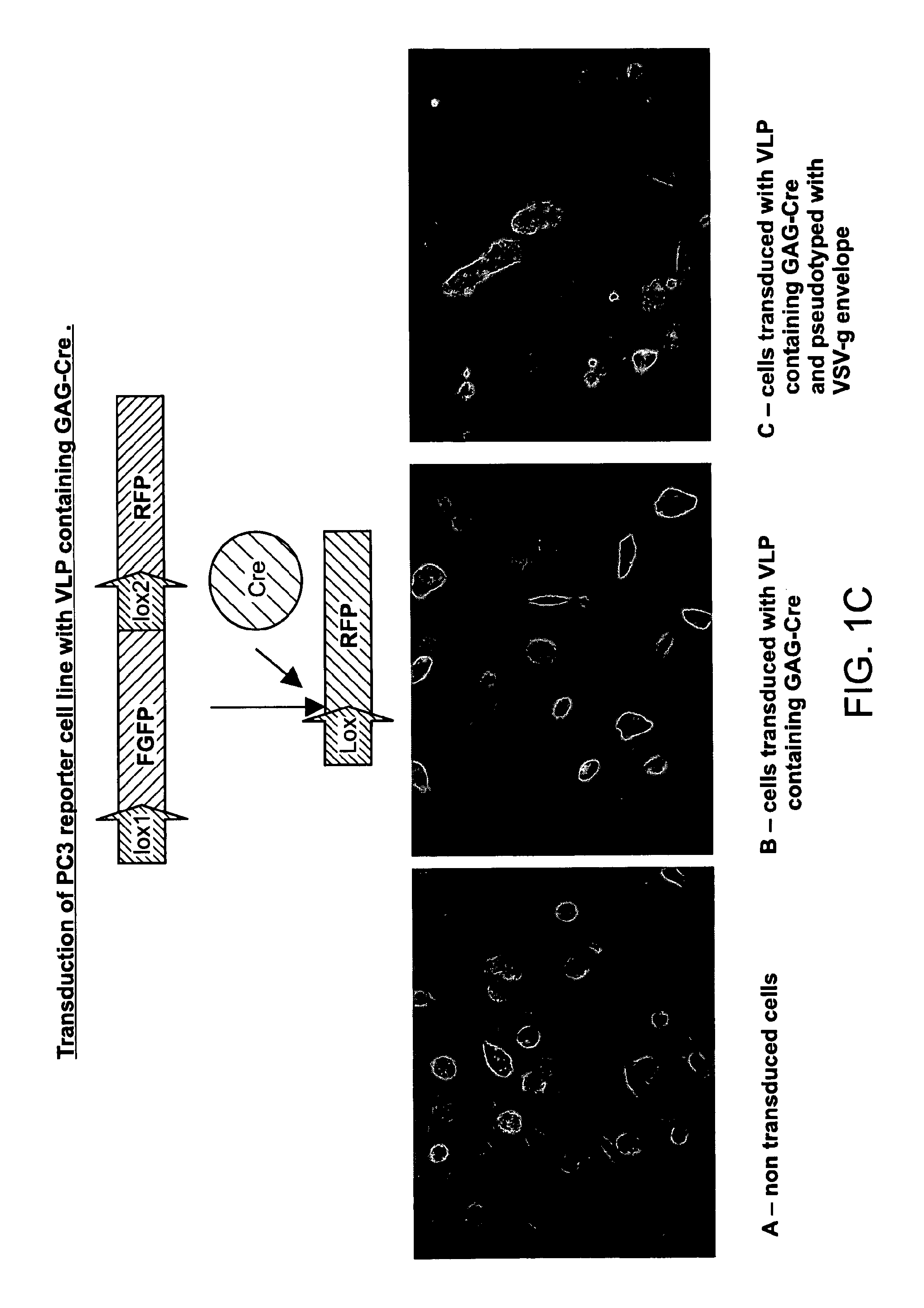
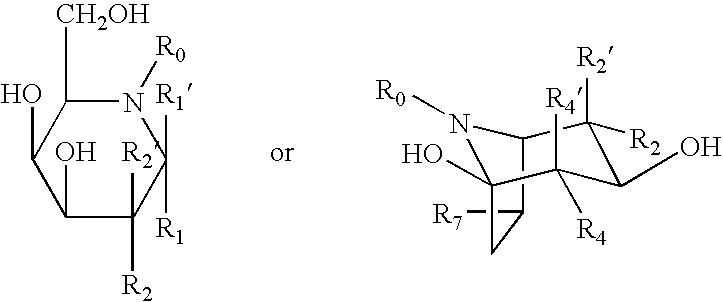Patents
Literature
52 results about "Protein replacement therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein replacement therapy is a medical treatment that supplements or replaces a protein in patients in whom that particular protein is deficient or absent. There have been significant advances in this treatment. PRT is being tested in clinical trials with the diseases Progeria and Epidermolysis bullosa dystrophica as a potential treatment. For patients with Epidermolysis bullosa dystrophica there has been promising results.
Modified RNA with decreased immunostimulatory properties
ActiveUS20160235864A1Reduce innate immune responseIncrease mRNA levelsOrganic active ingredientsPeptide/protein ingredientsVaccine ImmunogenicityRNA
The present invention provides a method for providing modified mRNAs of reduced immunogenicity and / or immunostimulatory capacity for use in protein replacement therapy. The invention further provides modified mRNAs and pharmaceutical compositions comprising the modified mRNAs according to the invention for use in protein replacement therapy.
Owner:CUREVAC SE
Combination therapy for treating protein deficiency disorders
InactiveUS20070178081A1Improve stabilityExtended shelf lifeBiocidePeptide/protein ingredientsCombined Modality TherapyActive site
This application provides methods of improving protein replacement therapy by combining protein replacement therapy with active site-specific chaperones (ASSC) to increase the stability and efficiency of the protein being administered. The application further provides stable compositions comprising the purified protein and an ASSC, and methods of treatment by administering the compositions.
Owner:FAN JIAN QIANG
Stable formulations of purified proteins
InactiveUS20060153829A1Improve stabilityExtended shelf lifeBiocidePeptide/protein ingredientsProtein replacement therapyMedicine
This application provides methods of improving protein replacement therapy by combining protein replacement therapy with active site-specific chaperones (ASSC) to increase the stability and efficiency of the protein being administered. The application further provides stable compositions comprising the purified protein and an ASSC, and methods of treatment by administering the compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
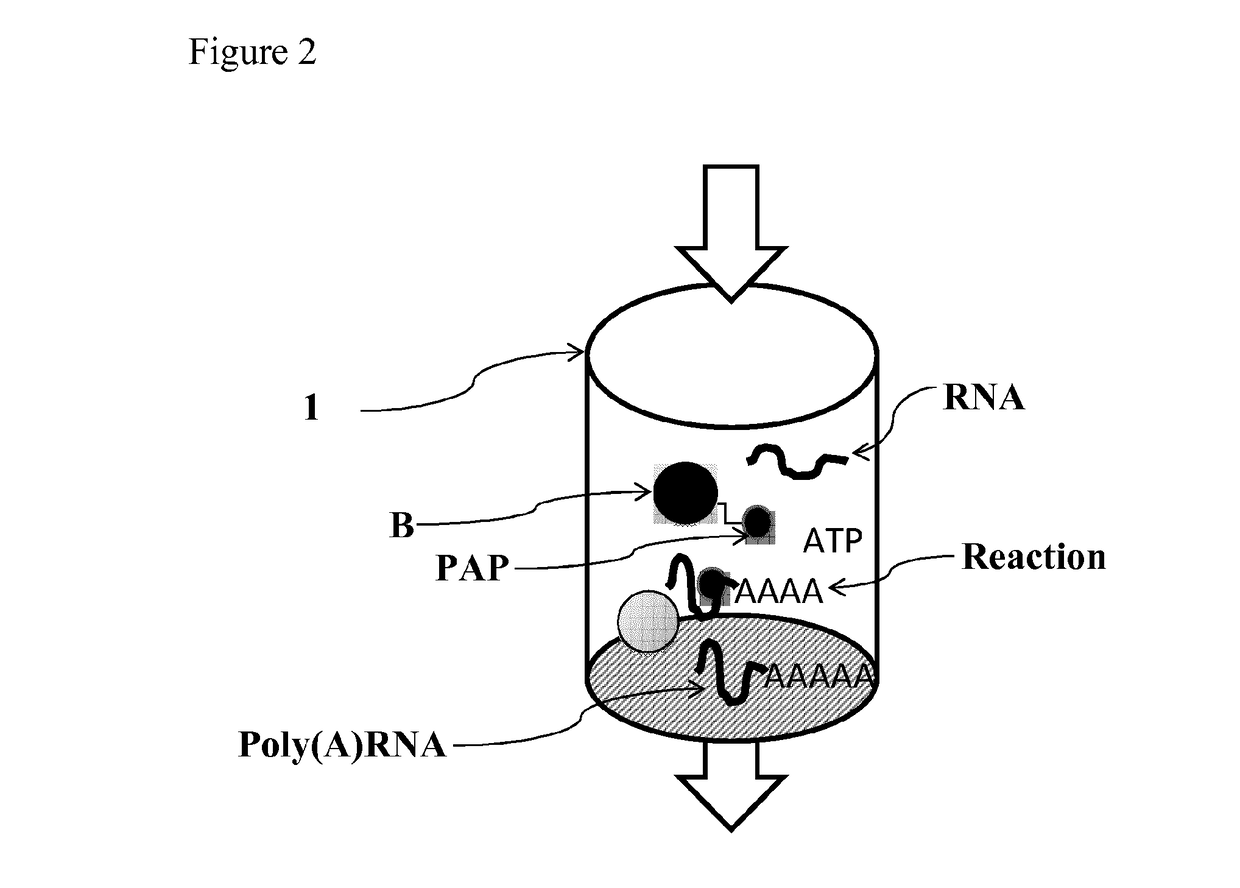
Immobilized poly(n)polymerase
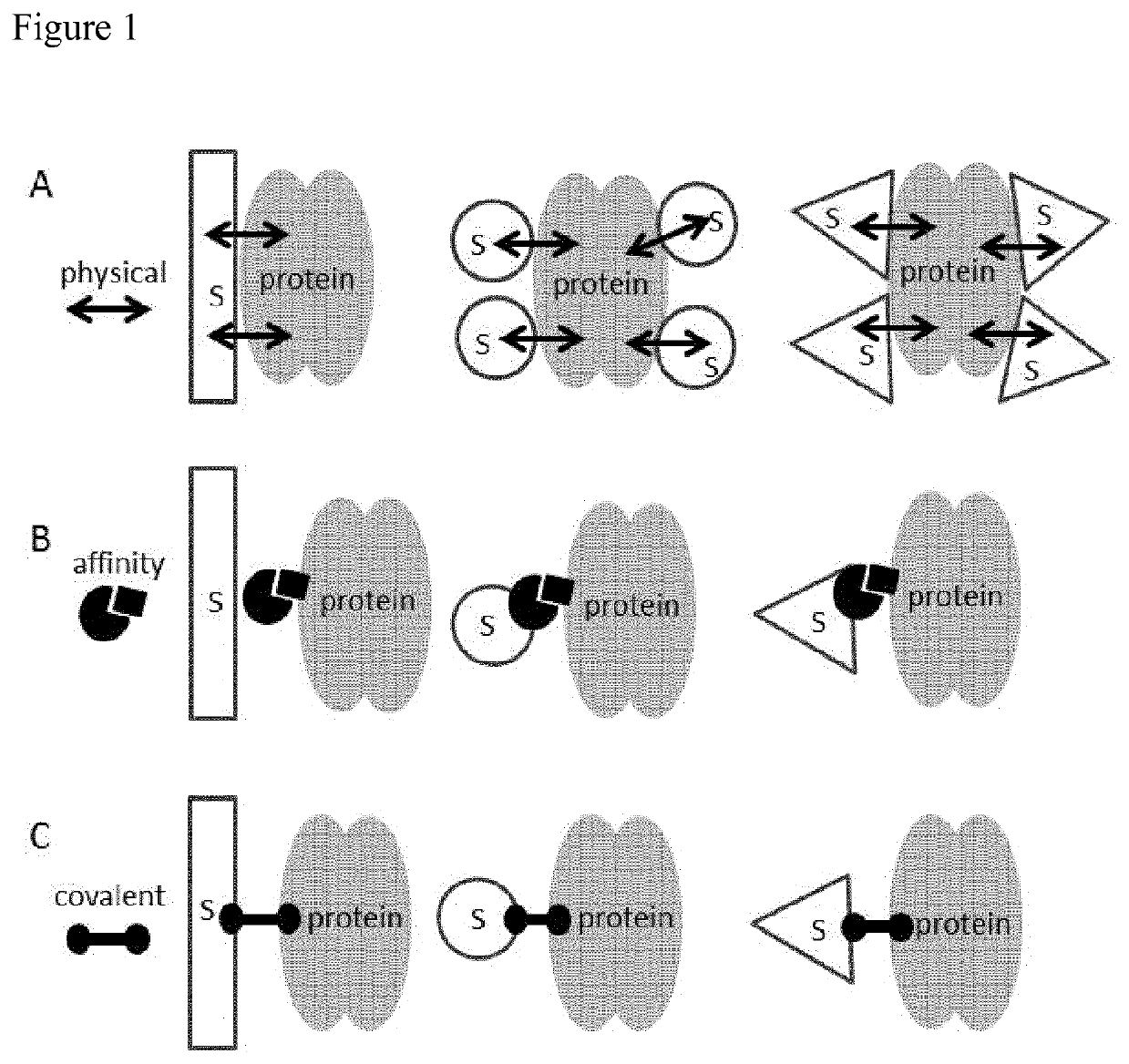
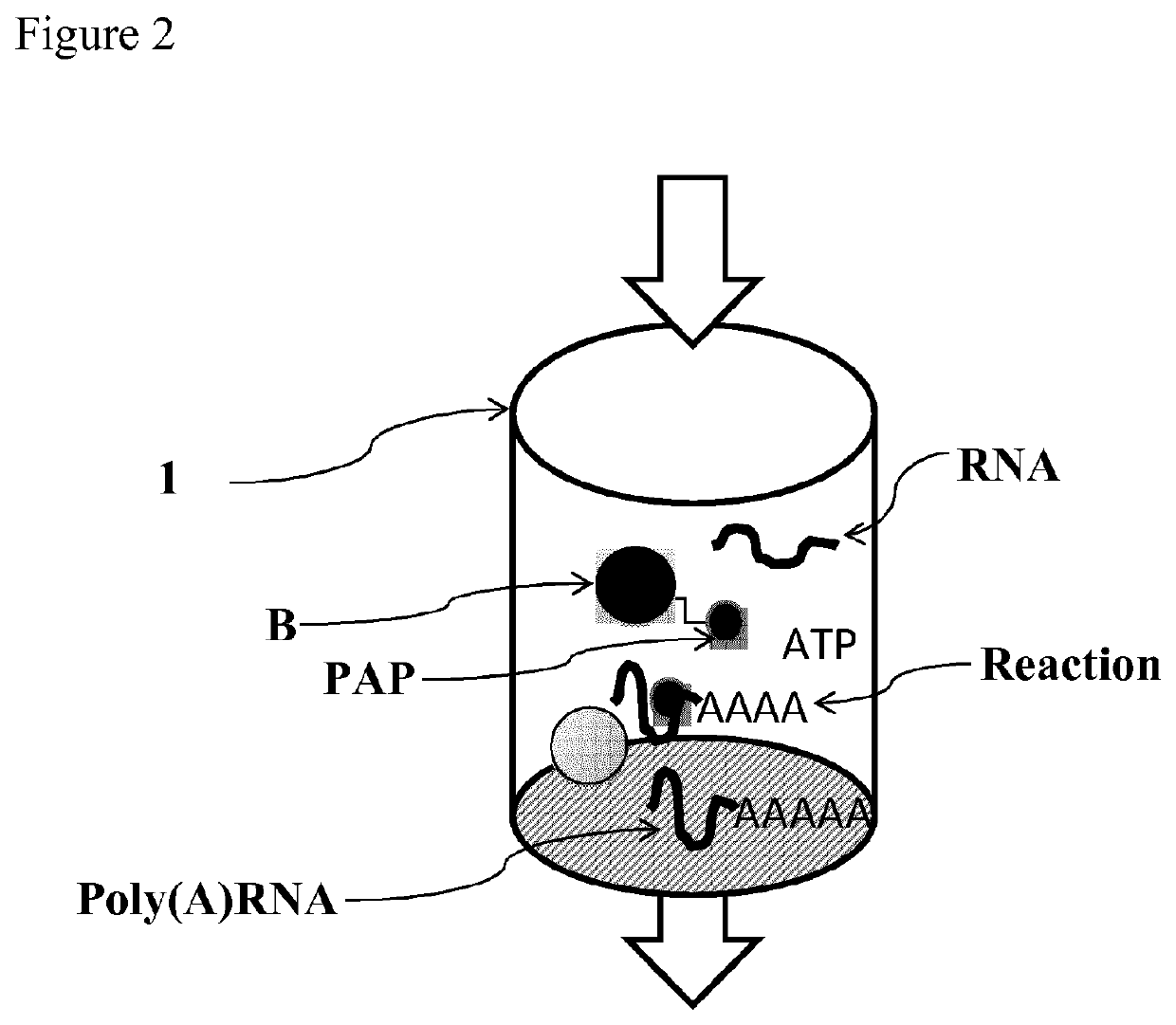
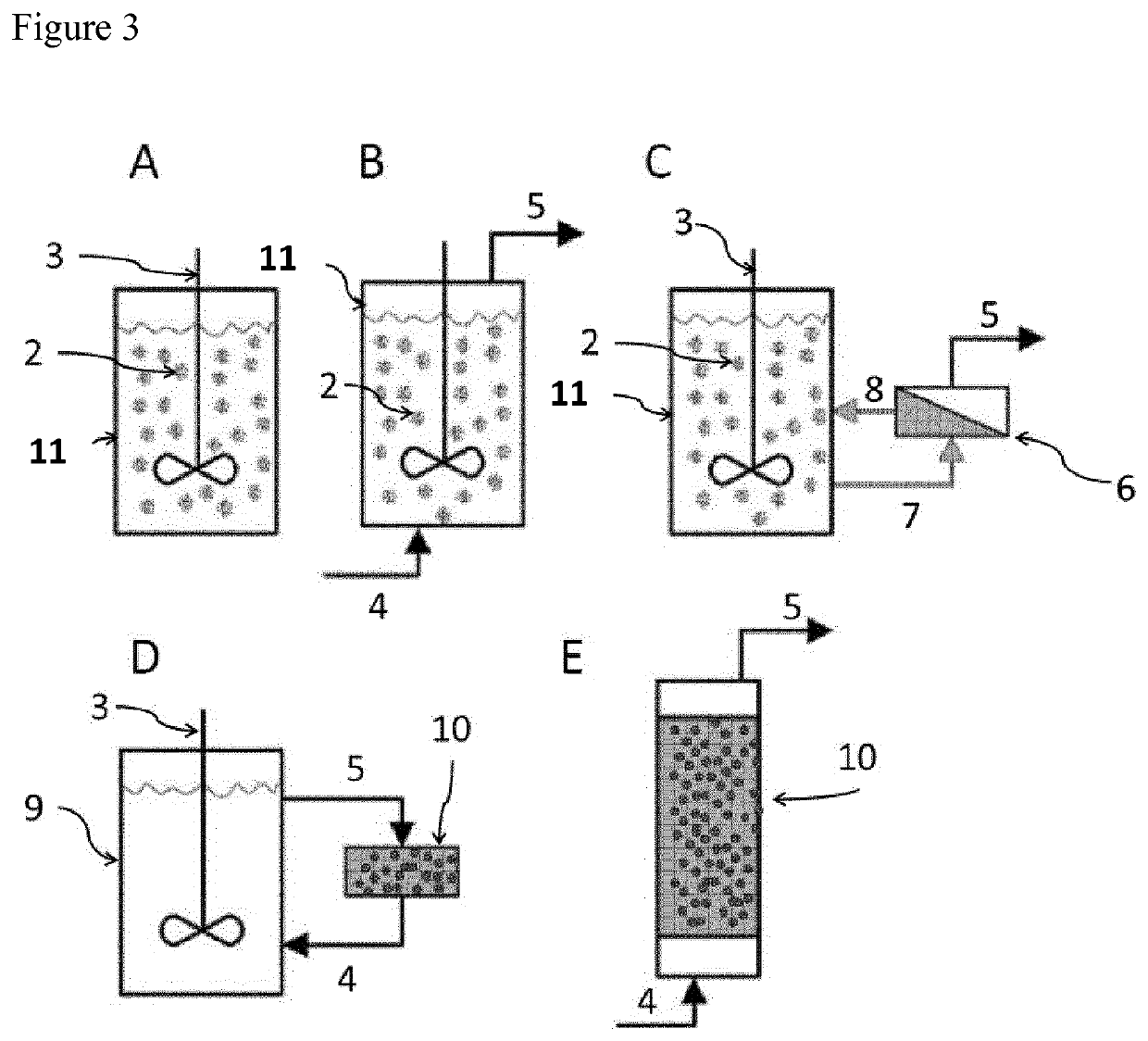
ActiveUS20180142275A1Improve stabilityMore time-effectiveMicrobiological testing/measurementChemical industryVaccinationRNA - Ribonucleic acid
The present invention relates to an immobilized poly(N)polymerase (PNP), methods of producing said PNP and uses thereof. Further disclosed is an enzyme reactor and kit comprising the PNP for producing polynucleotidylated ribonucleic acid poly(N)RNA)molecules which are useful in gene therapy, immunotherapy, protein replacement therapy and / or vaccination.
Owner:CUREVAC SE
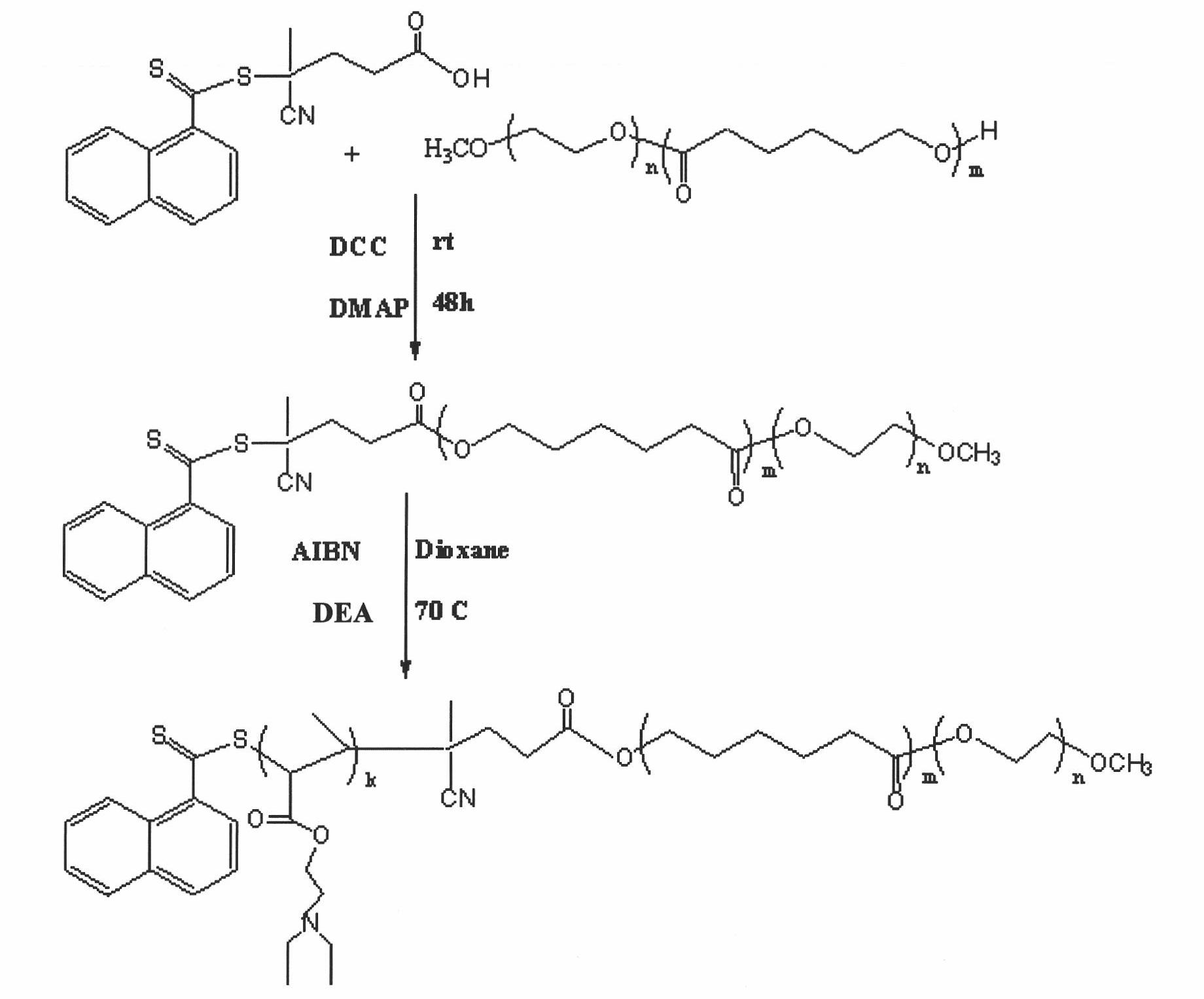
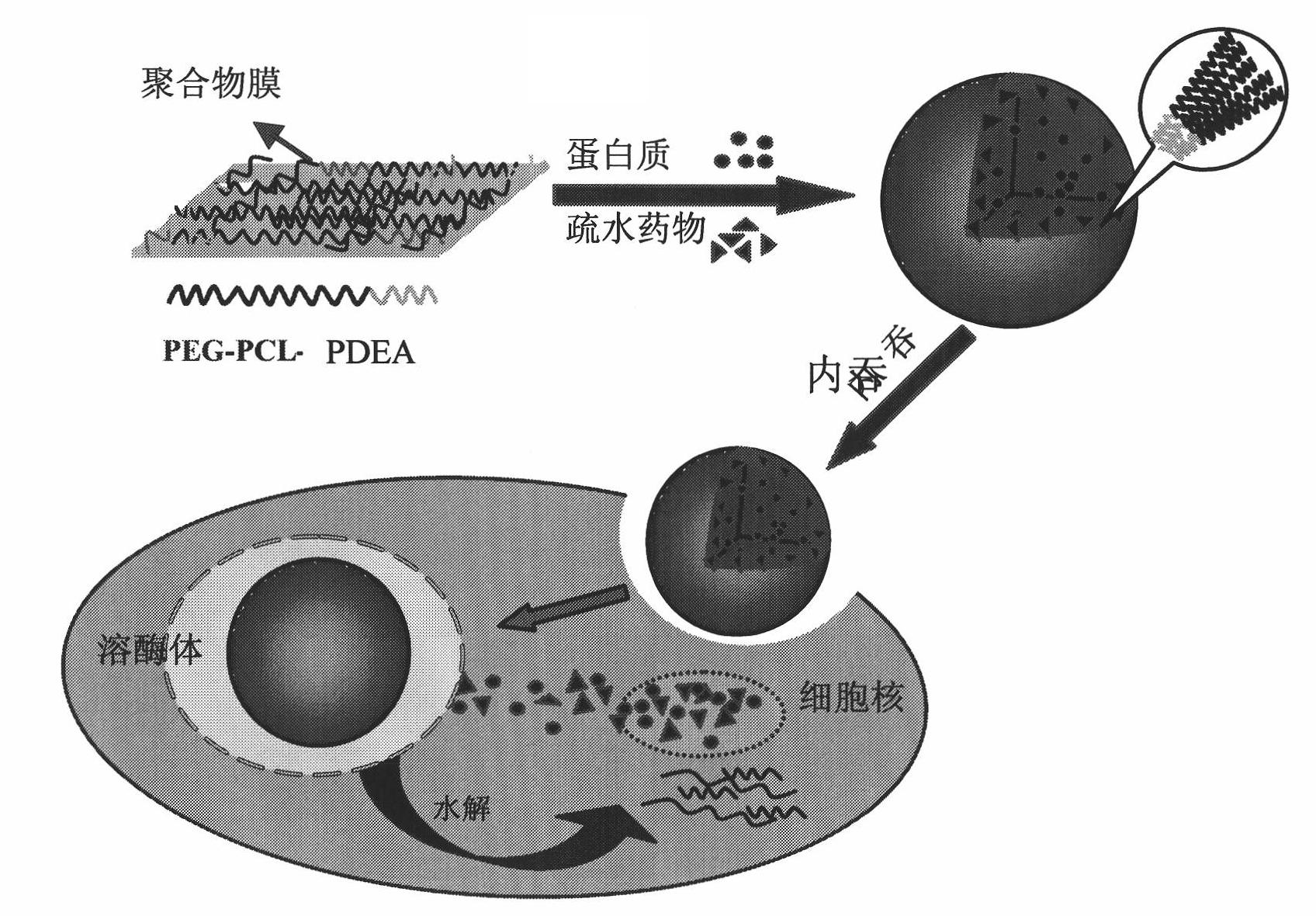
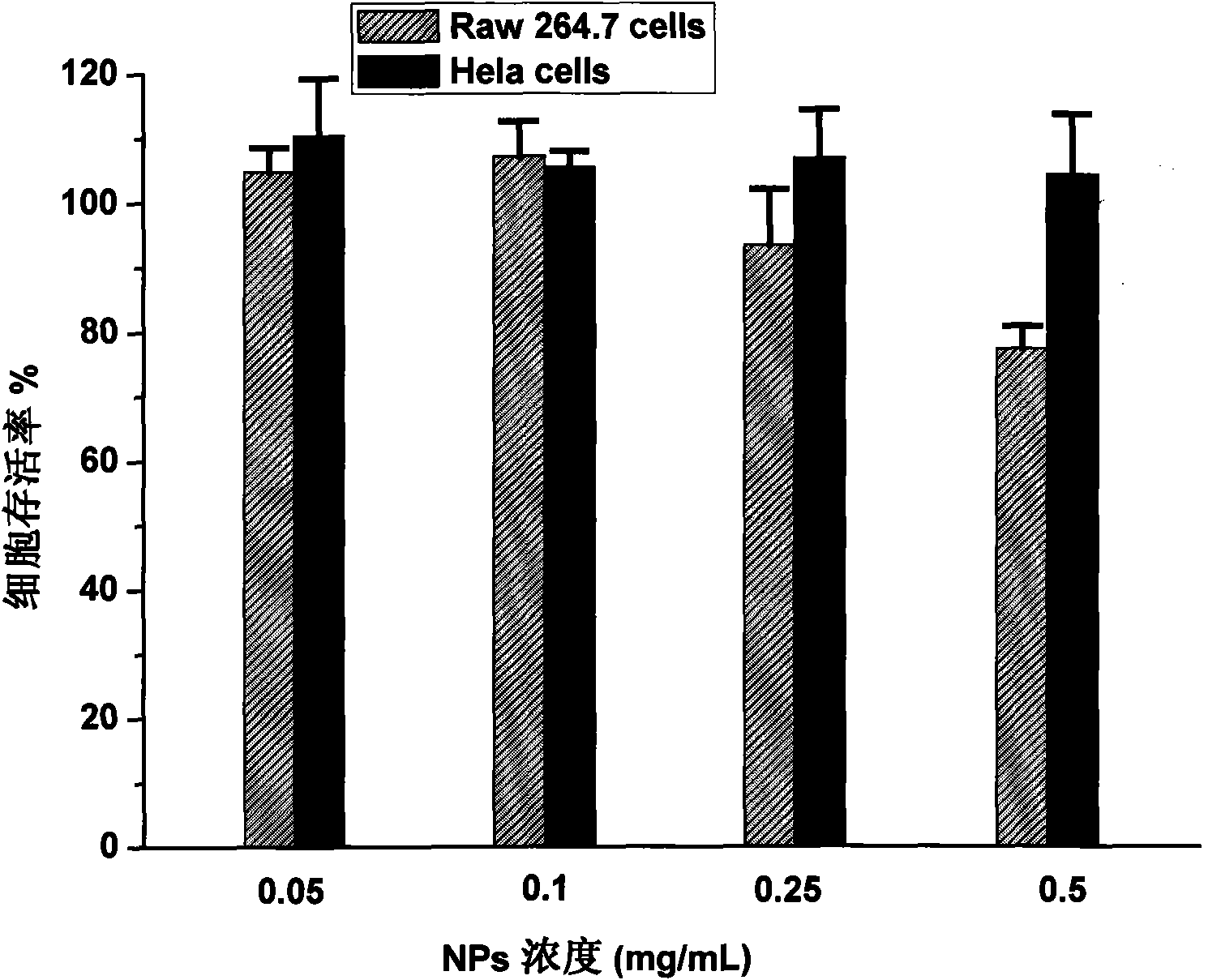
Biodegradable polymer vesicles and preparation and application thereof
InactiveCN101792516AEfficient packagingEliminate drug resistancePharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
The invention relates to a medicament carrier and a preparation method thereof, in particular to a medicament delivery system comprising biodegradable polymer vesicles with asymmetric membranes. The invention discloses the biodegradable polymer vesicles and preparation and application thereof. The biodegradable polymer vesicles are prepared from A-B-C type block polymer, wherein a block A is polyethylene glycol (PEG) distributed on outer surfaces of the vesicles; a block B is hydrophobic biodegradable polymer to form the nucleuses of the vesicles; and a block C is polyelectrolyte distributed on inner walls of the vesicular membranes and used for efficiently loading medicaments with opposite electric charge. The biodegradable polymer vesicles are formed in aqueous solution directly, can efficiently load protein and polypeptide medicaments, nucleic acid medicaments and micro-molecular medicaments, and are expected to be applied to the protein therapy and the combination therapy of cancers.
Owner:SUZHOU UNIV
Use of rapamycin to inhibit immune response and induce tolerance to gene therapy vector and encoded transgene products
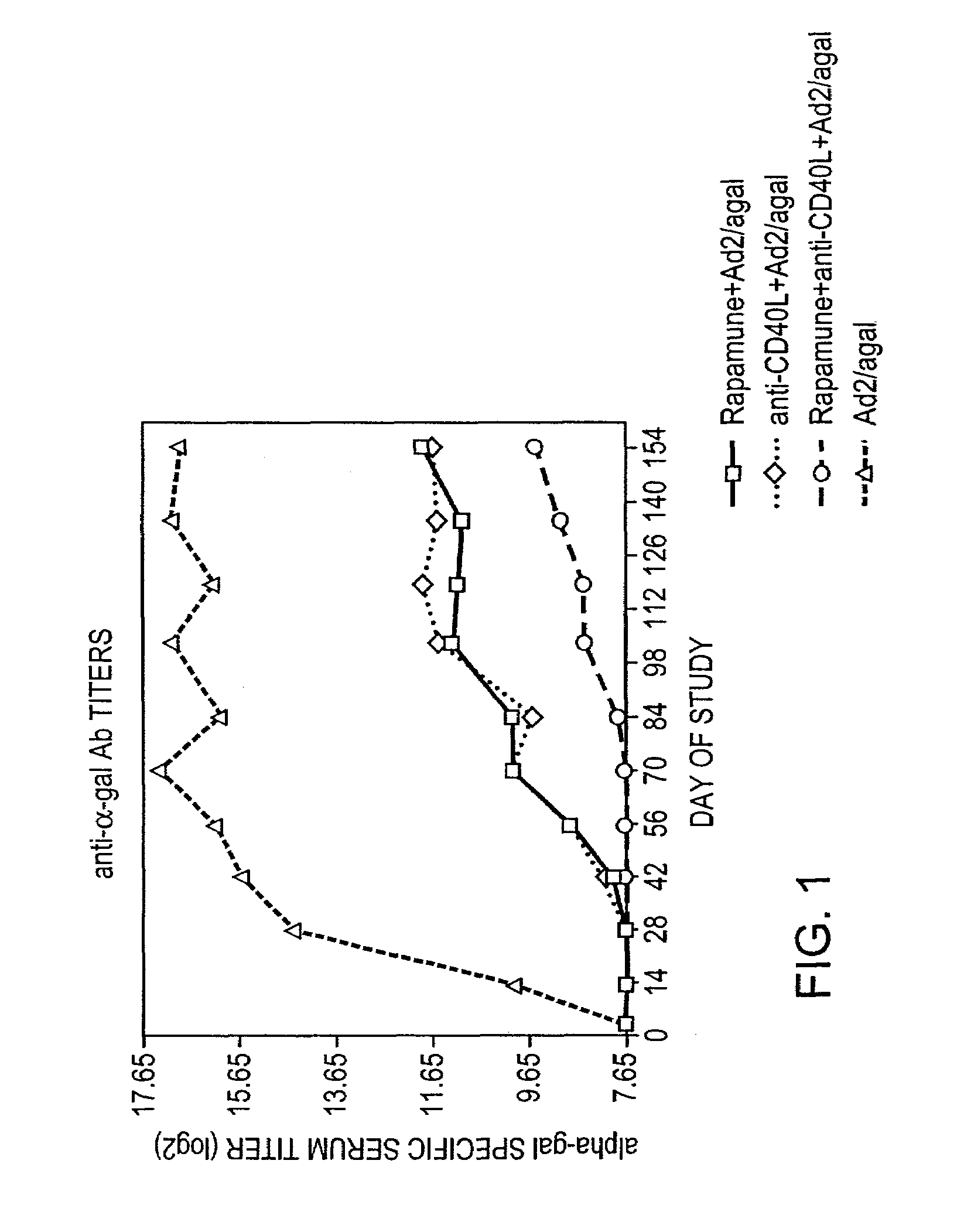
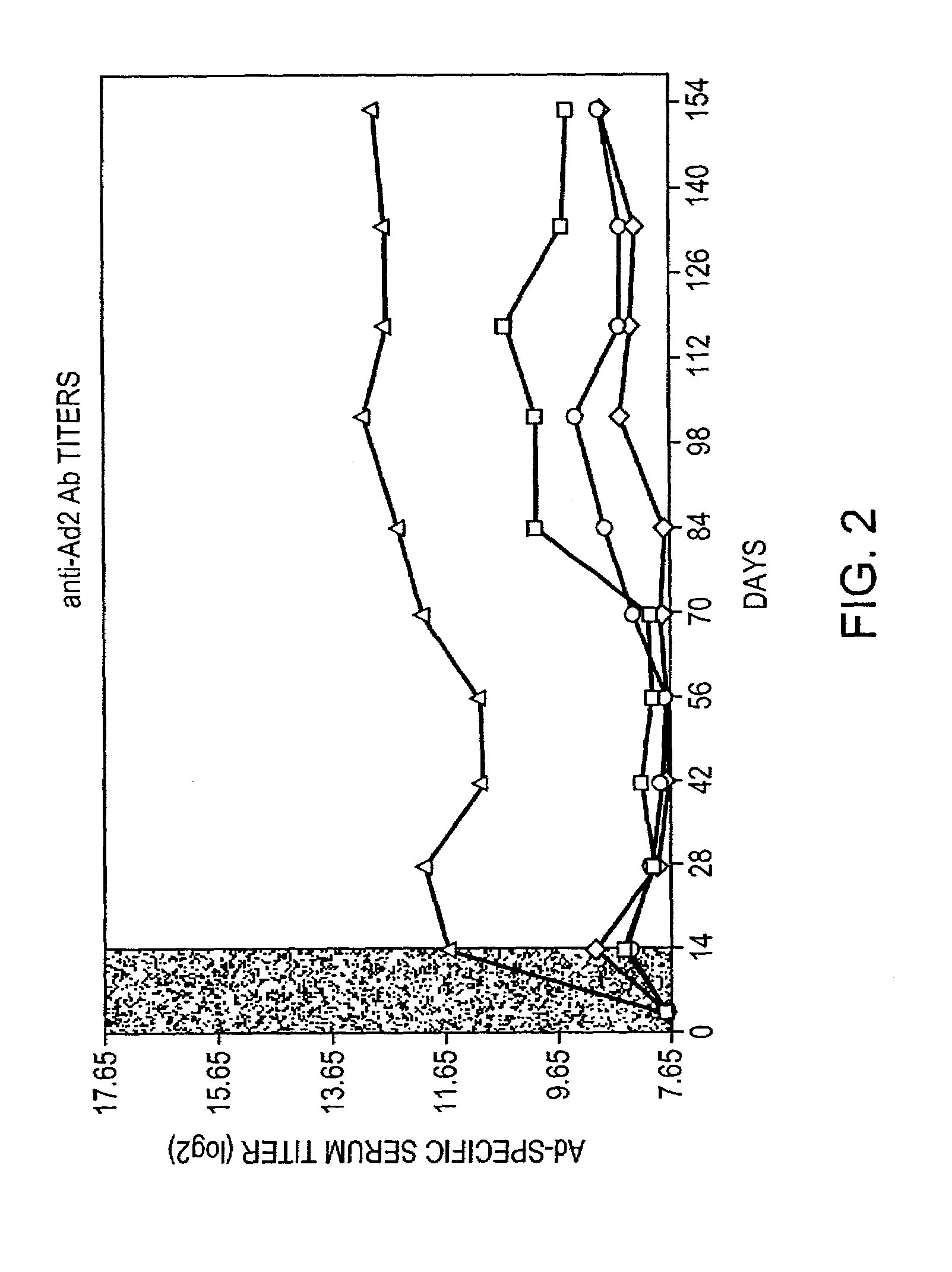
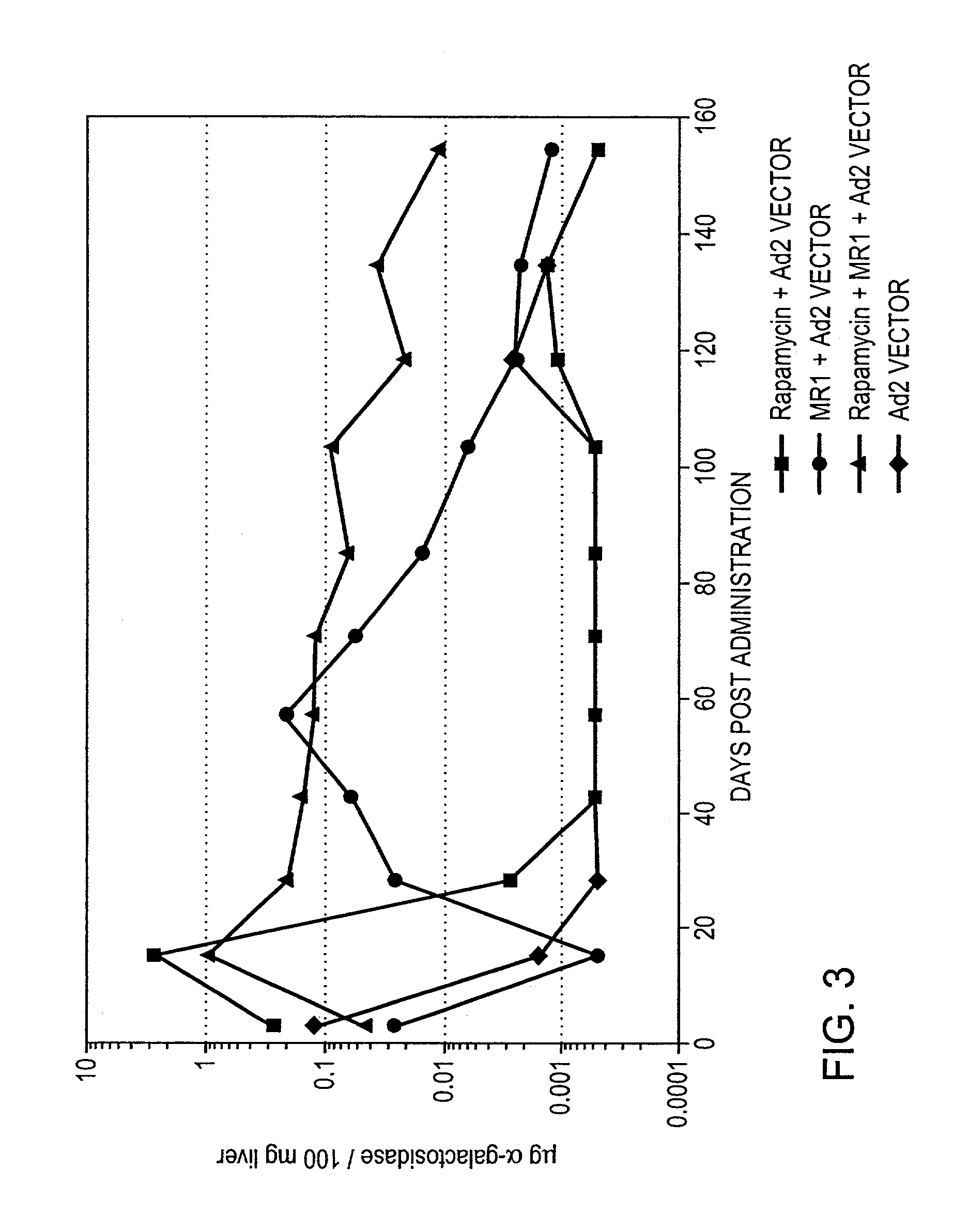
Disclosed are methods for transient co-administration of rapamycin together with a gene therapy vector encoding a transgene. The present invention is directed to inhibiting the immune response of a host to the administered gene therapy vector and encoded transgene product, thus allowing persistent transgene expression and repeated administration of the gene therapy product to the host. The present invention is also of relevance in genetic disease patients that mount immune responses to protein replacement therapies in which case the present invention provides for transient co-administration of rapamycin together with protein replacement therapy. In a further aspect of the invention, co-administration of rapamycin could inhibit a secondary immune response in a host that has been pre-immunized with the gene therapy vector or pre-immunized with the protein product encoded by the transgene.
Owner:VASCO DATA SECURITY INTERNATIONAL
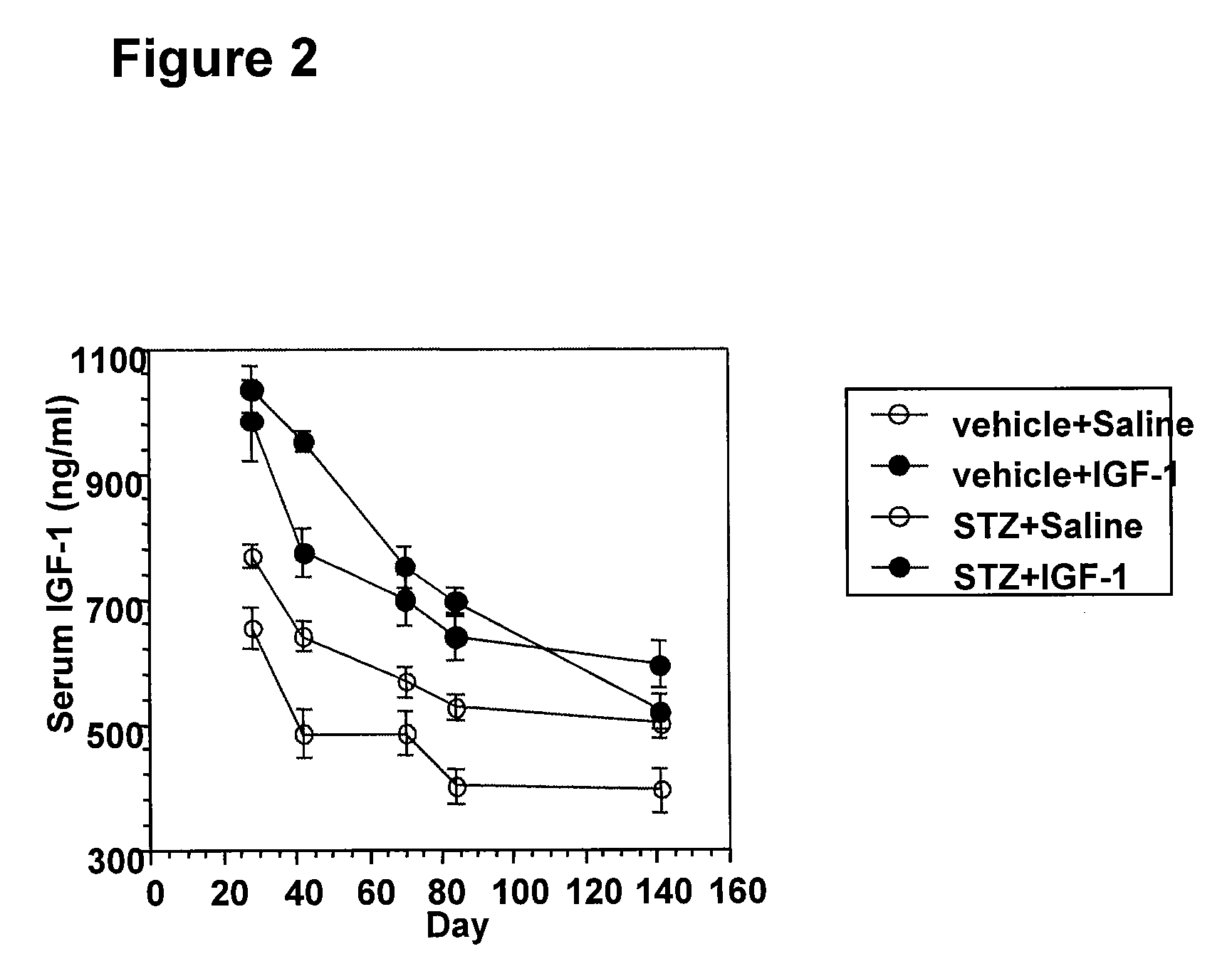
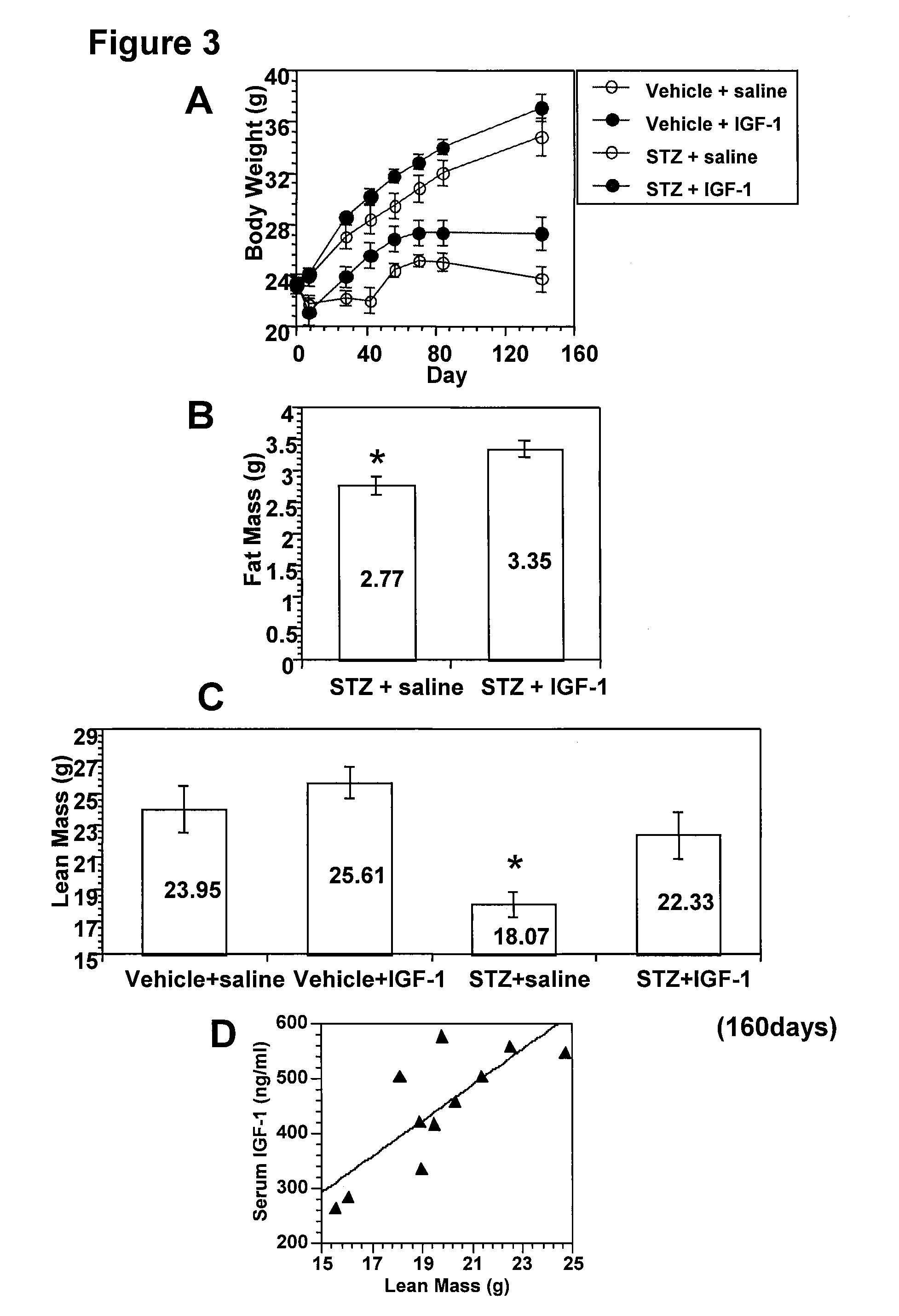
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
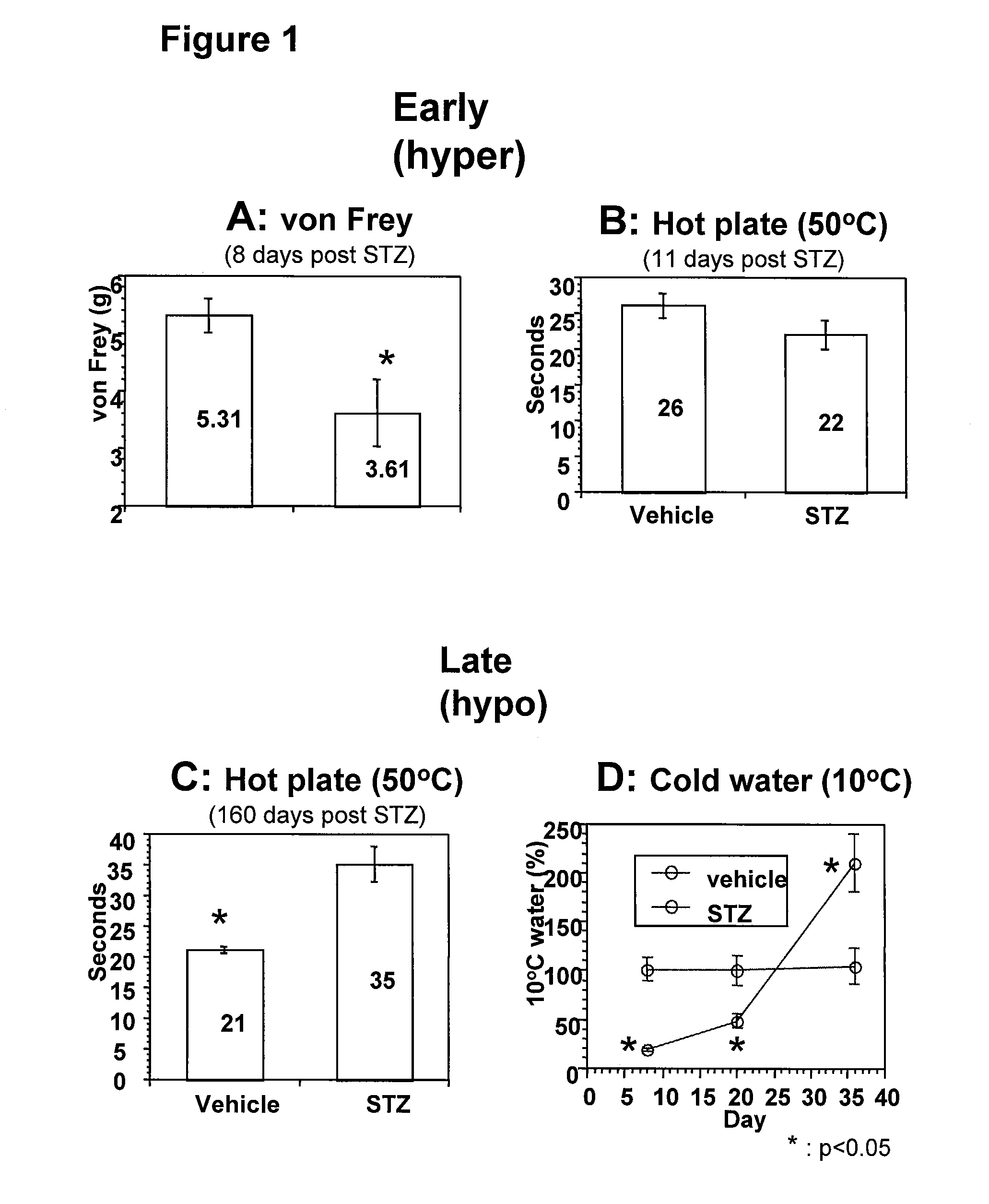
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
Method and composition for a protein transduction technology and its applications
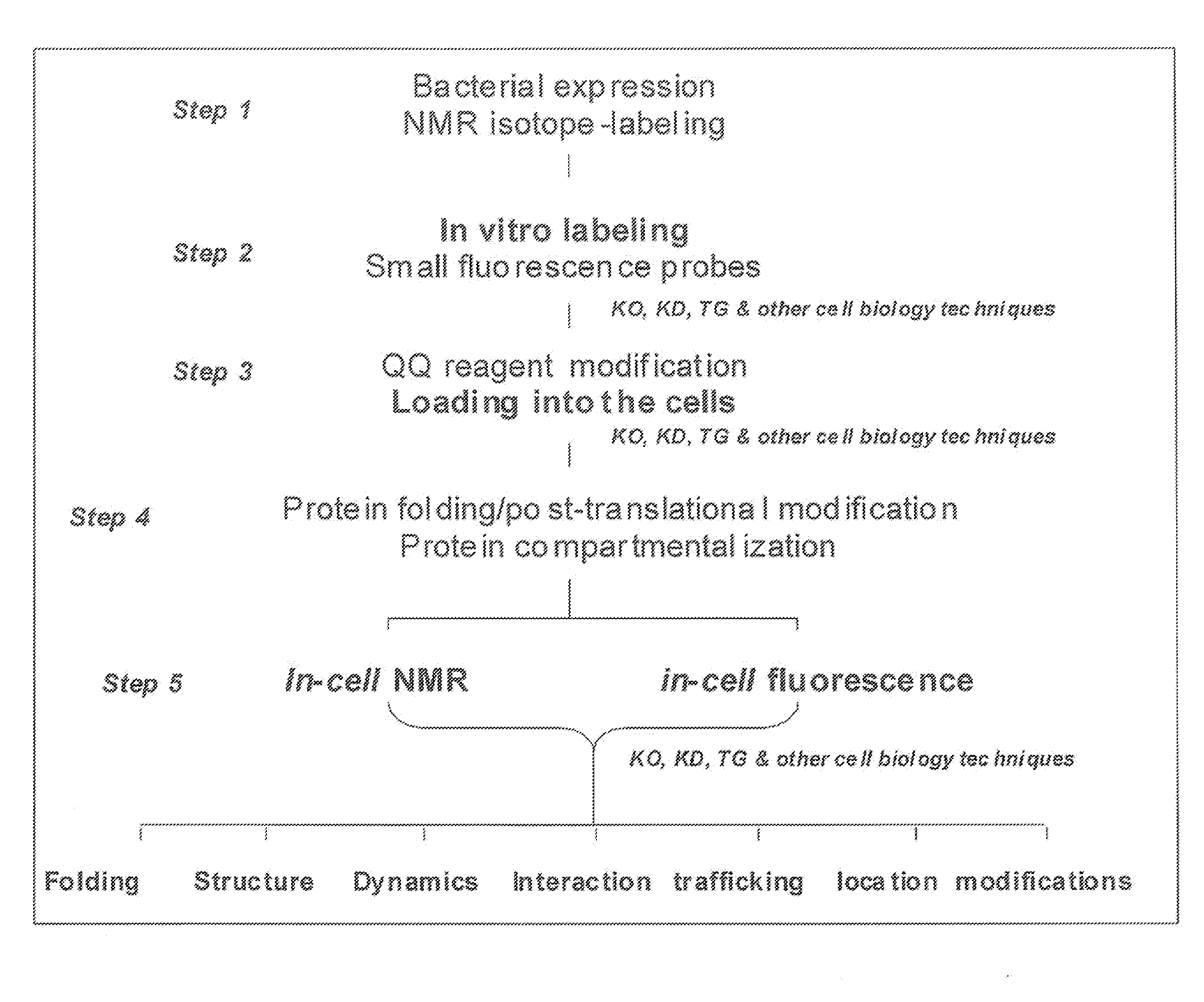
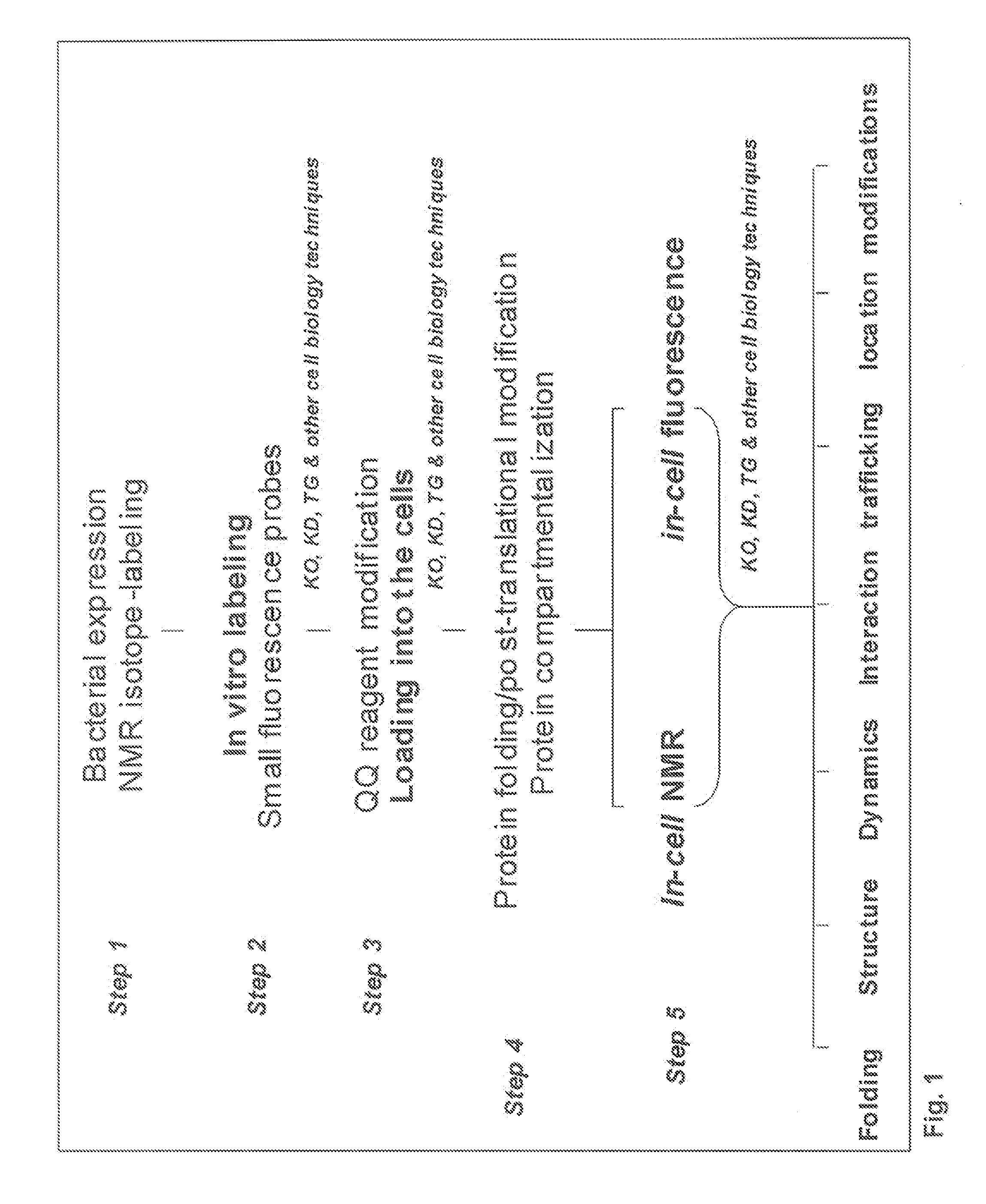
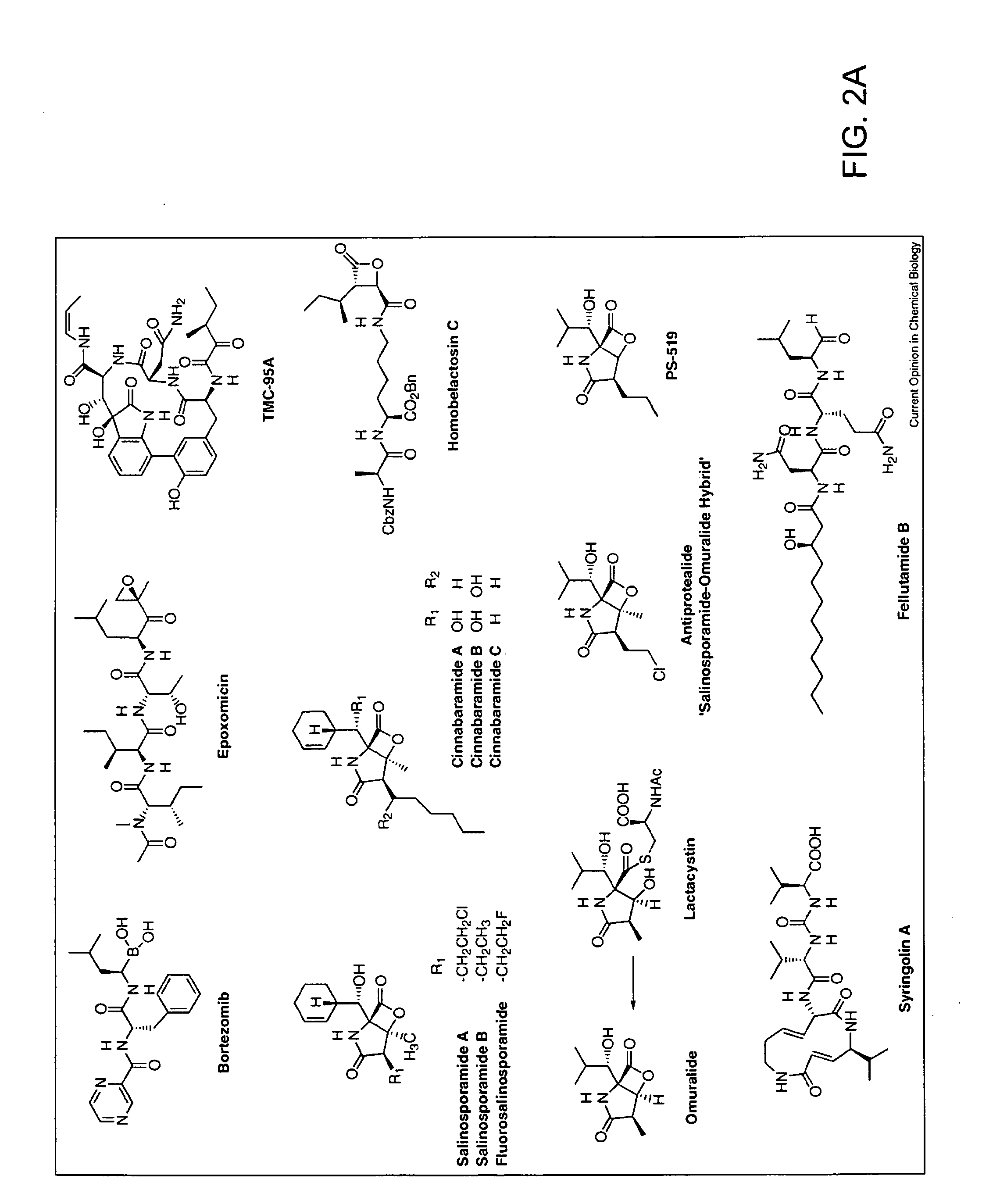
ActiveUS20090298111A1Efficient deliveryBest protein transduction efficiencyPeptide/protein ingredientsMicroencapsulation basedSpectroscopyFluorophore
A protein transduction method for efficiently delivery of exogenous proteins into mammalian cells is invented, which has the capability of targeting different cellular compartments and protection from degradation of the delivered proteins from cellular proteases. A composition for treat proteins has cation reagents, lipids and enhancers in a carrier. The method can be used in a number of ways including: production of large quantities of properly folded, post-translationally modified proteins using mammalian cell machinery, a in-cell fluorescence spectroscopy and imaging using small molecule fluorophores and a in-cell NMR spectroscopy using living mammalian cells. The method permits cell biology at atomic resolution that is physiologically and pathological relevant and permits protein therapy to treat human diseases. The method can also be used to deliver exogenous protein inside mammalian cells, wherein the exogenous proteins follow a similar secretion pathway as that of the endogenous protein.
Owner:WAYNE STATE UNIV
Polynucleotides encoding a super-active porcine growth hormone releasing hormone analog
InactiveUS7166461B2Improve growth performanceEasy to useBiocidePeptide/protein ingredientsDiseaseGrowth deficiency
Inadequate growth due to deficiencies in growth hormone (GR), growth hormone releasing hormone (GHRH), or genetic diseases can be ameliorated utilizing recombinant protein therapy with a novel GHRH analog having a sequence (SEQ ID NO:1). Also included is (1) a method of treating growth hormone-related deficiencies associated with the growth hormone pathway; (2) a method for treating growth hormone-related deficiencies associated with genetic disease; (3) a method to improve growth performance in an animal; (4) a method of treating an animal having a growth deficiency disease; (5) a method of increasing the efficiency of an animal used for food; and (6) a method to enhance growth in an animal.
Owner:BERGAN RONALD
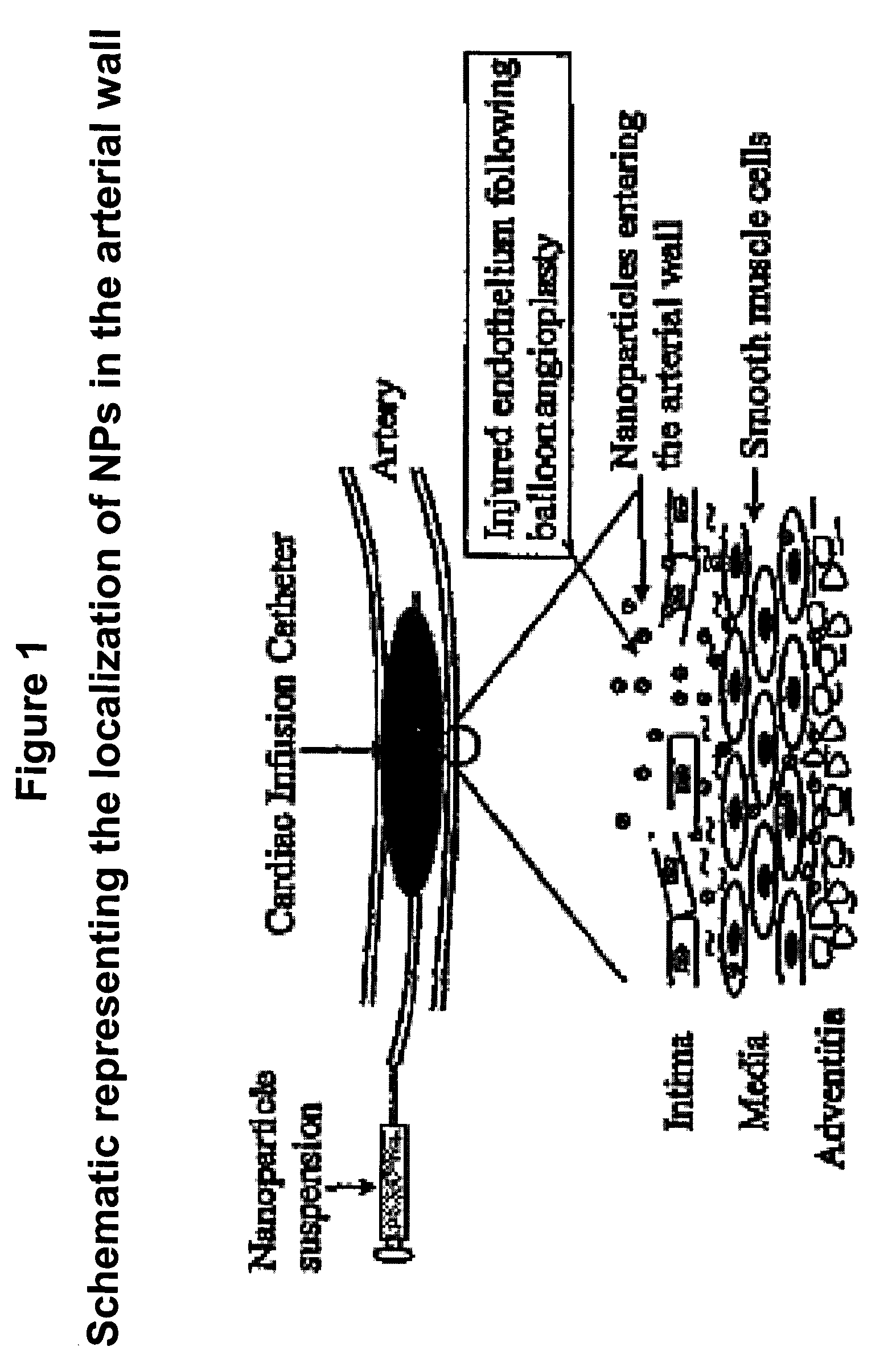
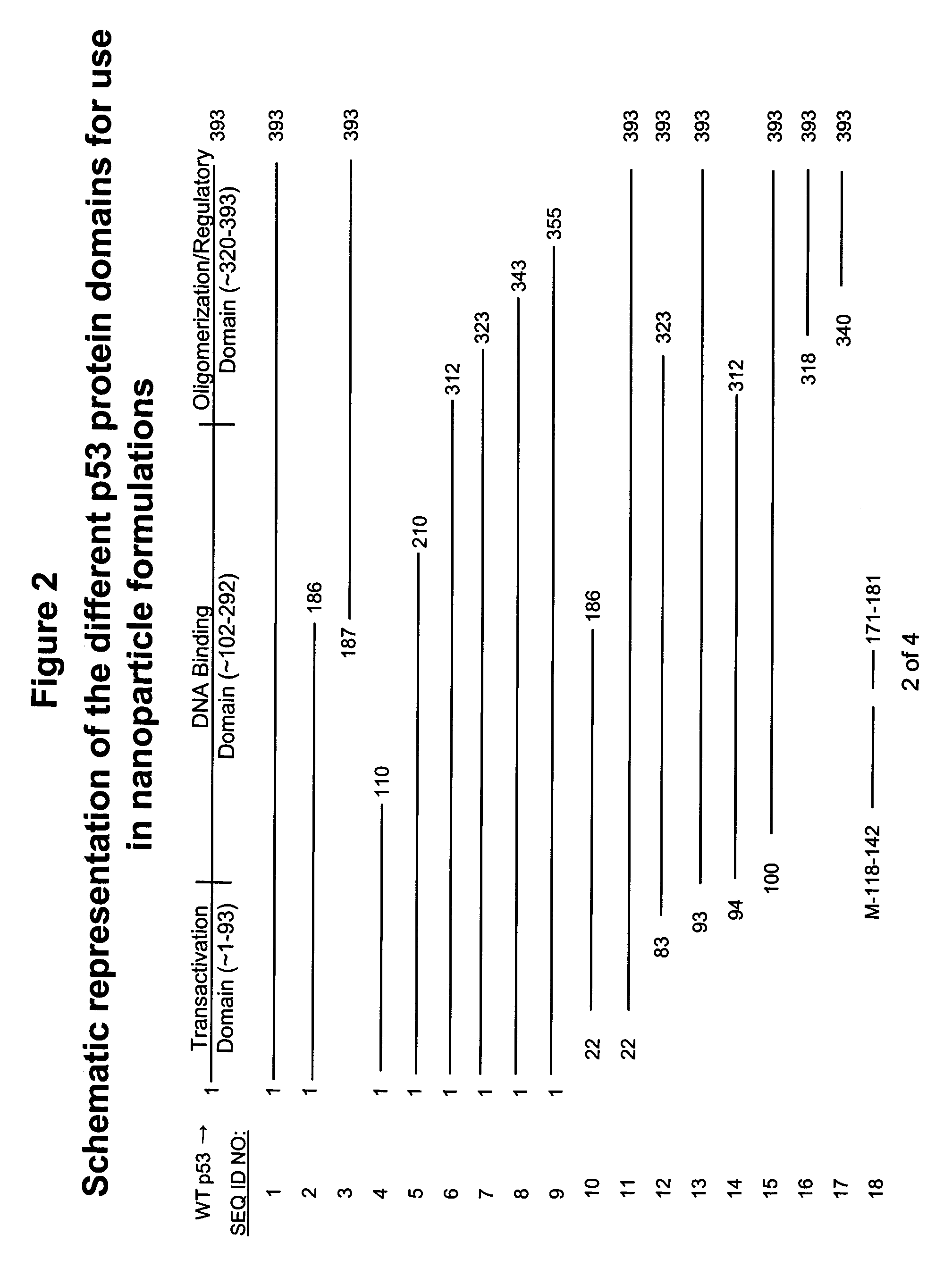
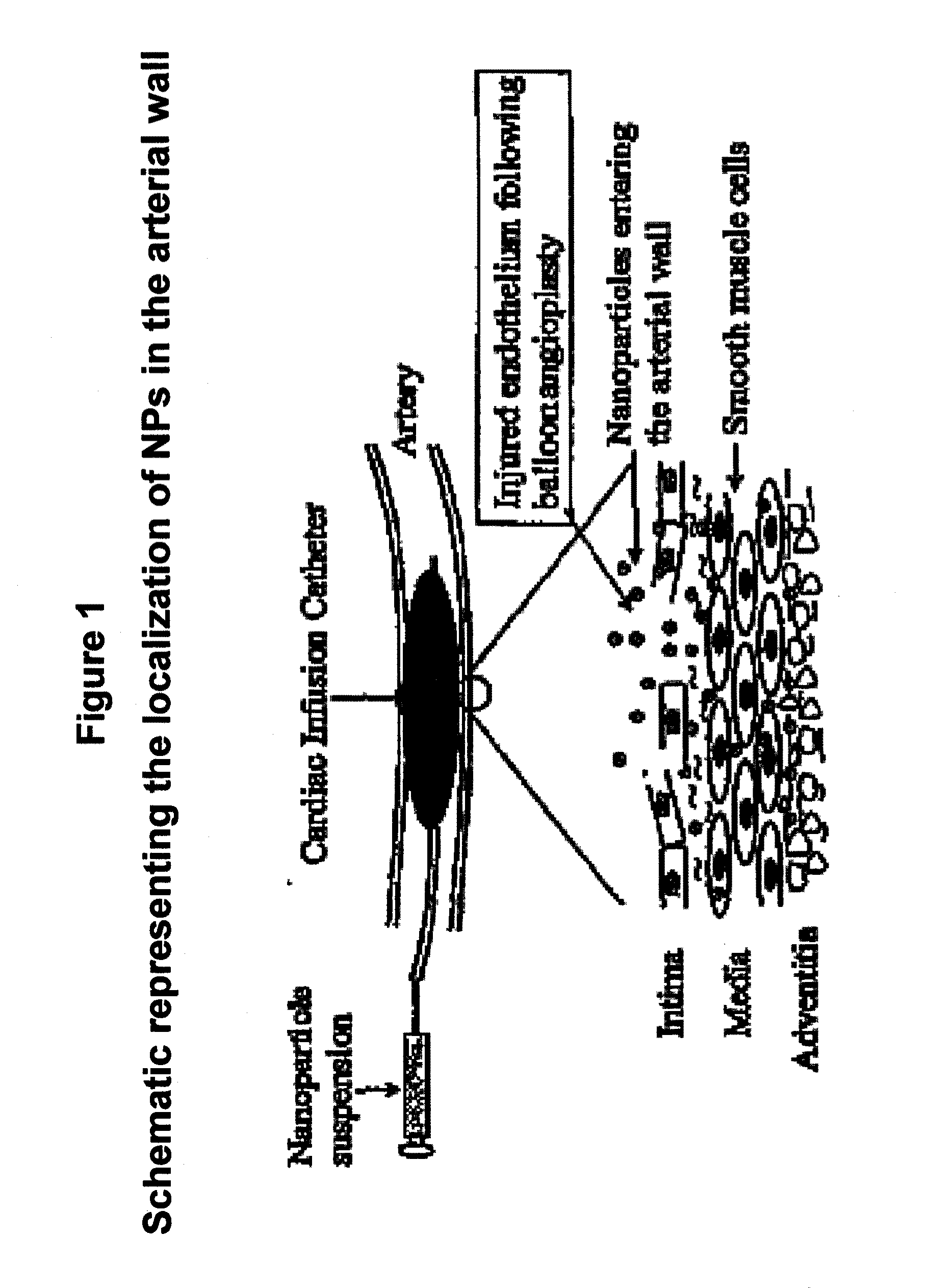
Apoptosis-Modulating Protein Therapy for Proliferative Disorders and Nanoparticles Containing the Same
InactiveUS20090018078A1Inhibit inflammationPeptide/protein ingredientsAntipyreticProtein replacement therapyNanoparticle
Protein containing nanoparticles and methods of use thereof for the treatment of proliferative disorders are disclosed.
Owner:BOARD OF RGT UNIV OF NEBRASKA
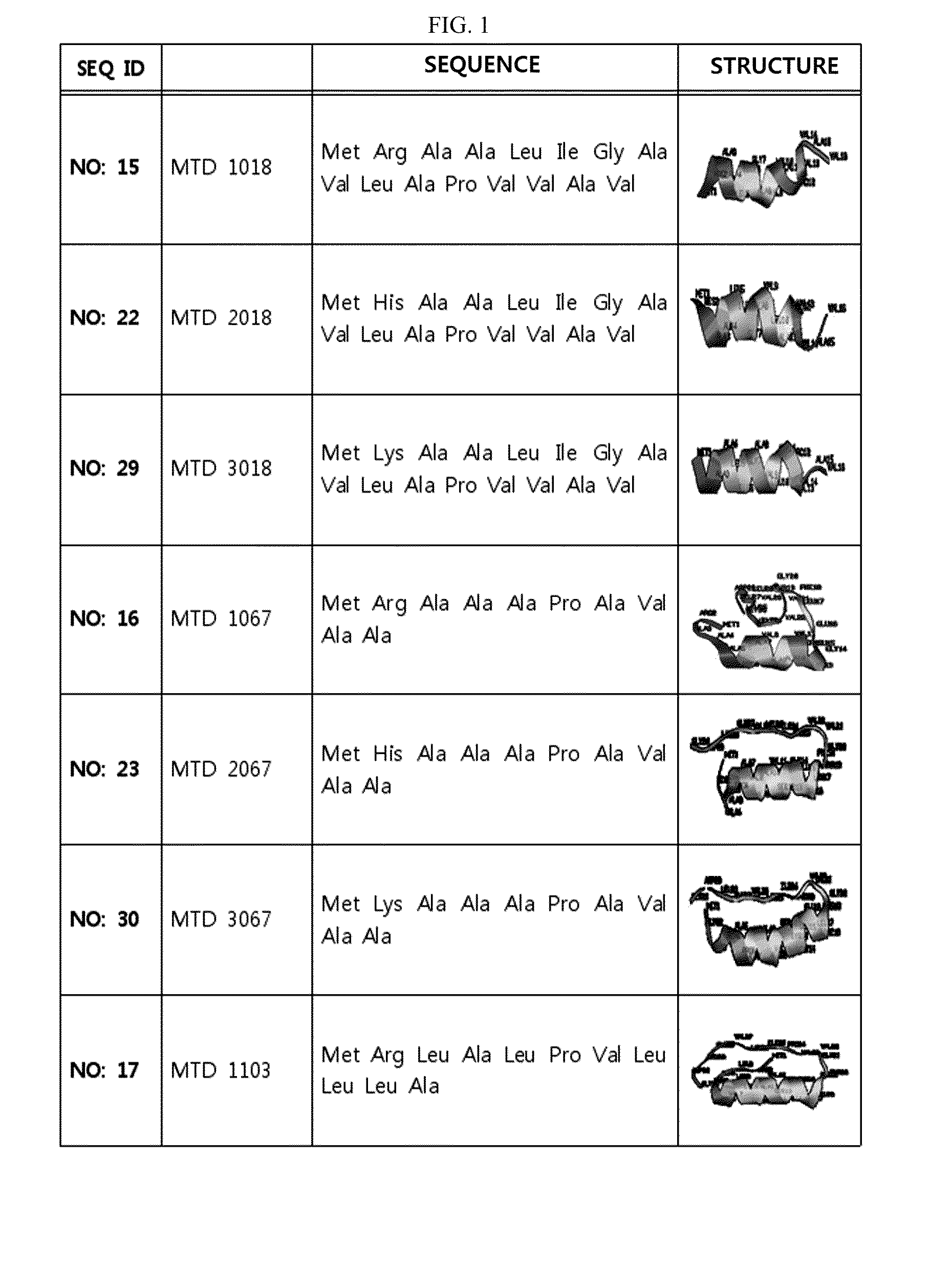
Development of Novel Macromolecule Transduction Domain with Improved Cell Permeability and Method for Using Same
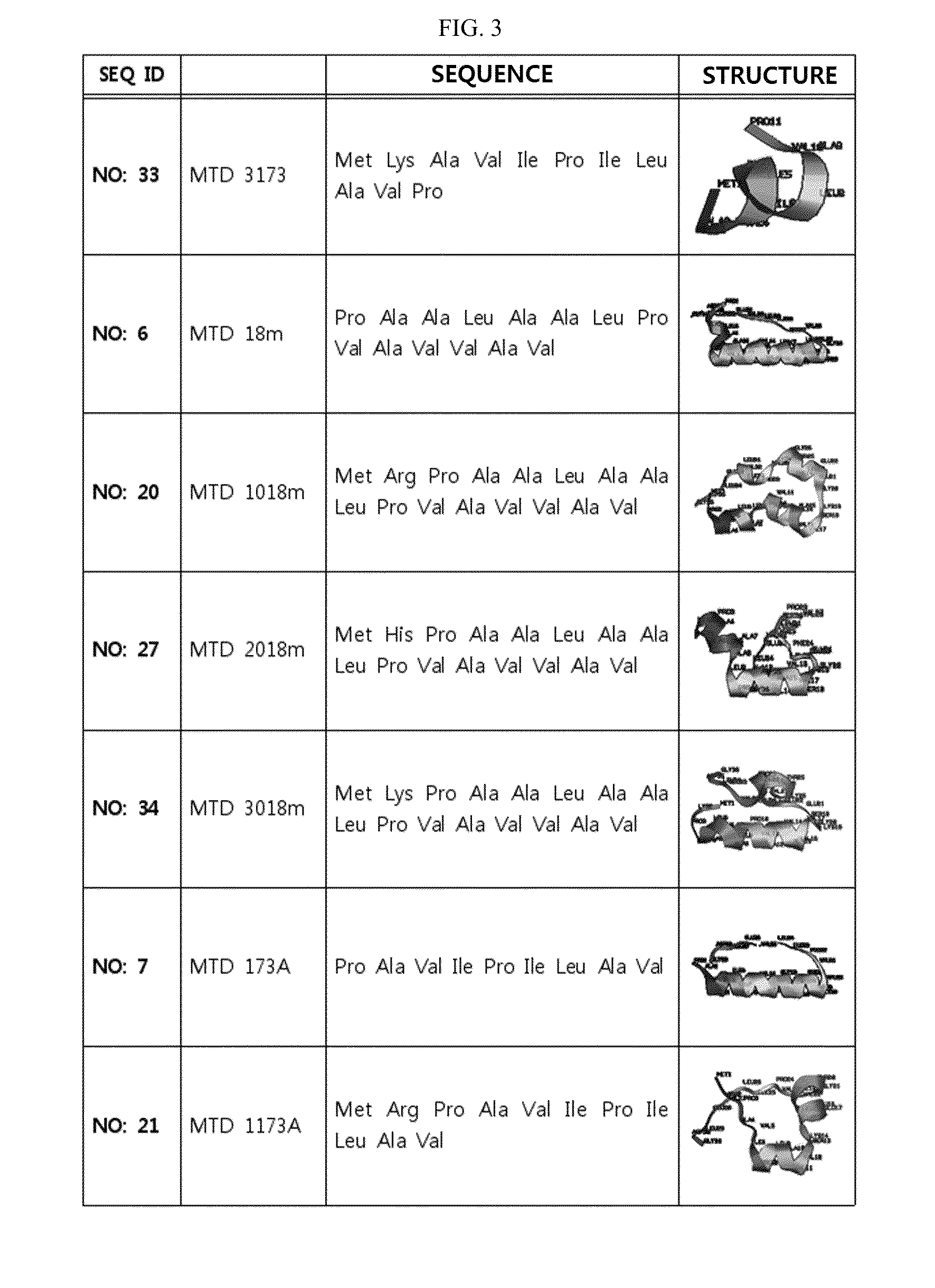
ActiveUS20140329737A1Good cell permeabilityMaintain activityCosmetic preparationsOrganic active ingredientsCell membraneOrganism
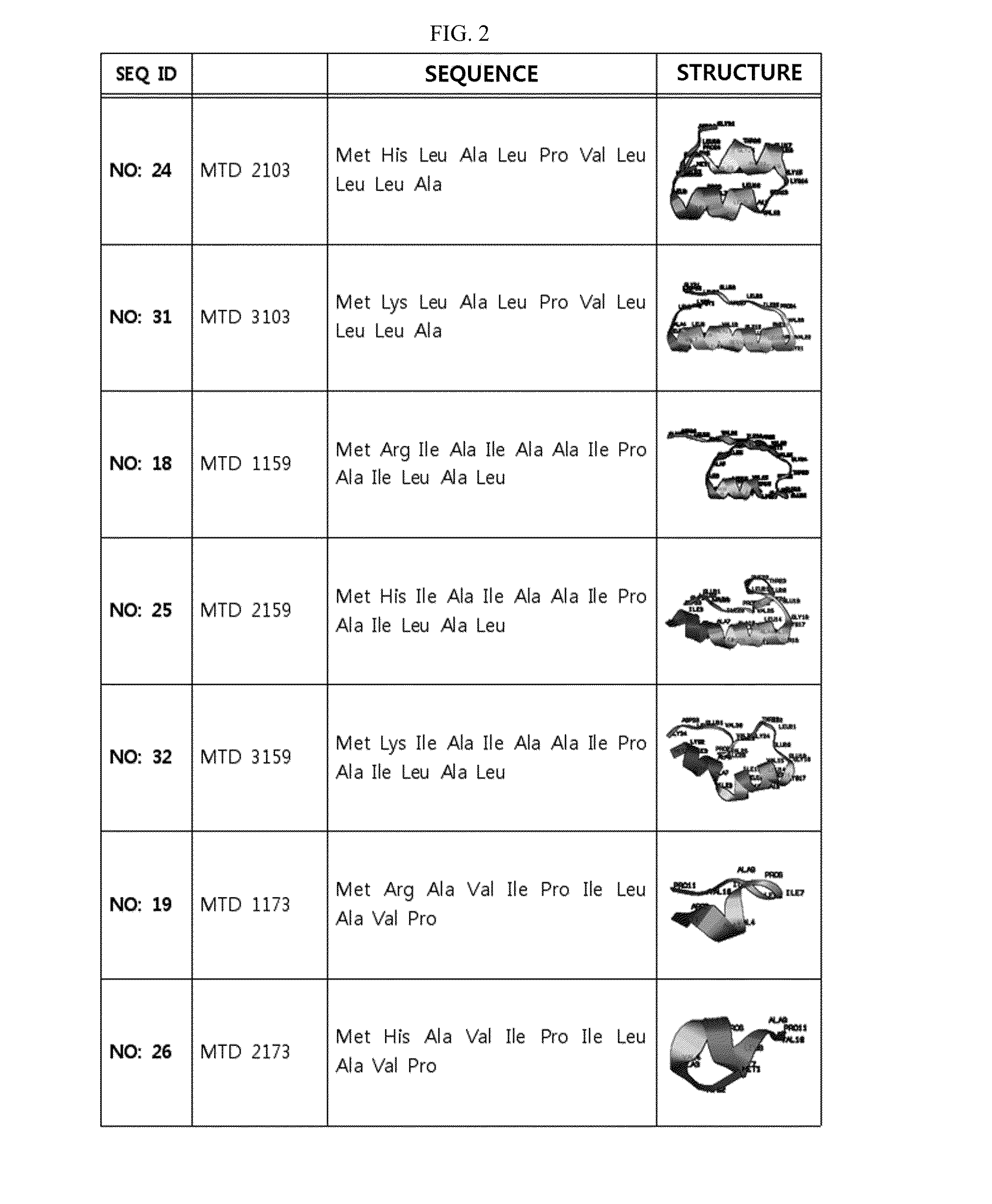
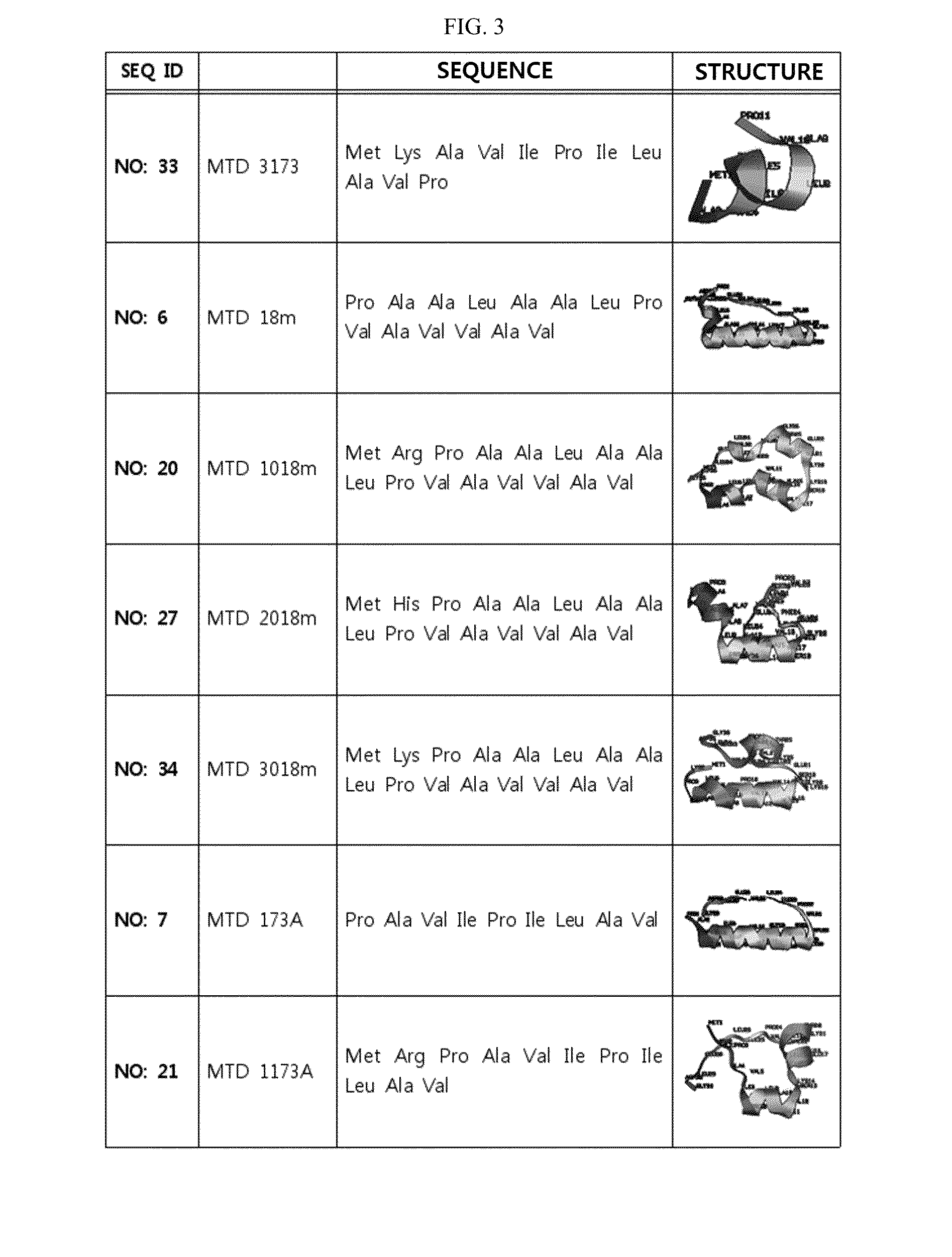
The present invention relates to an improved macromolecule transduction domain (MTD), which facilitates permeating the cell membrane of a biologically active molecule, having enhanced cell permeability. Specifically, an improved MTD according to the present invention, compared to an existing MTD, can transmit various types of biologically active molecule from inside the body and inside a test tube more effectively, and thus can be effectively used in a method to genetically alter a biologically active molecule so as to have cell permeability or in a method to transport a biologically active molecule into a cell, or the like. Additionally, the improved MTD can be very useful in development of new drugs and incrementally modified drugs as uses of the improved MTD are possible in drug delivery systems, recombinant protein vaccines or DNA / RNA therapeutic agents, gene or protein therapies, and pharmacologically or medically useful protein production or medical, pharmacological and pharmaceutical compositions.
Owner:PROCELL THERAPEUTICS
Methods and compositions for protein delivery
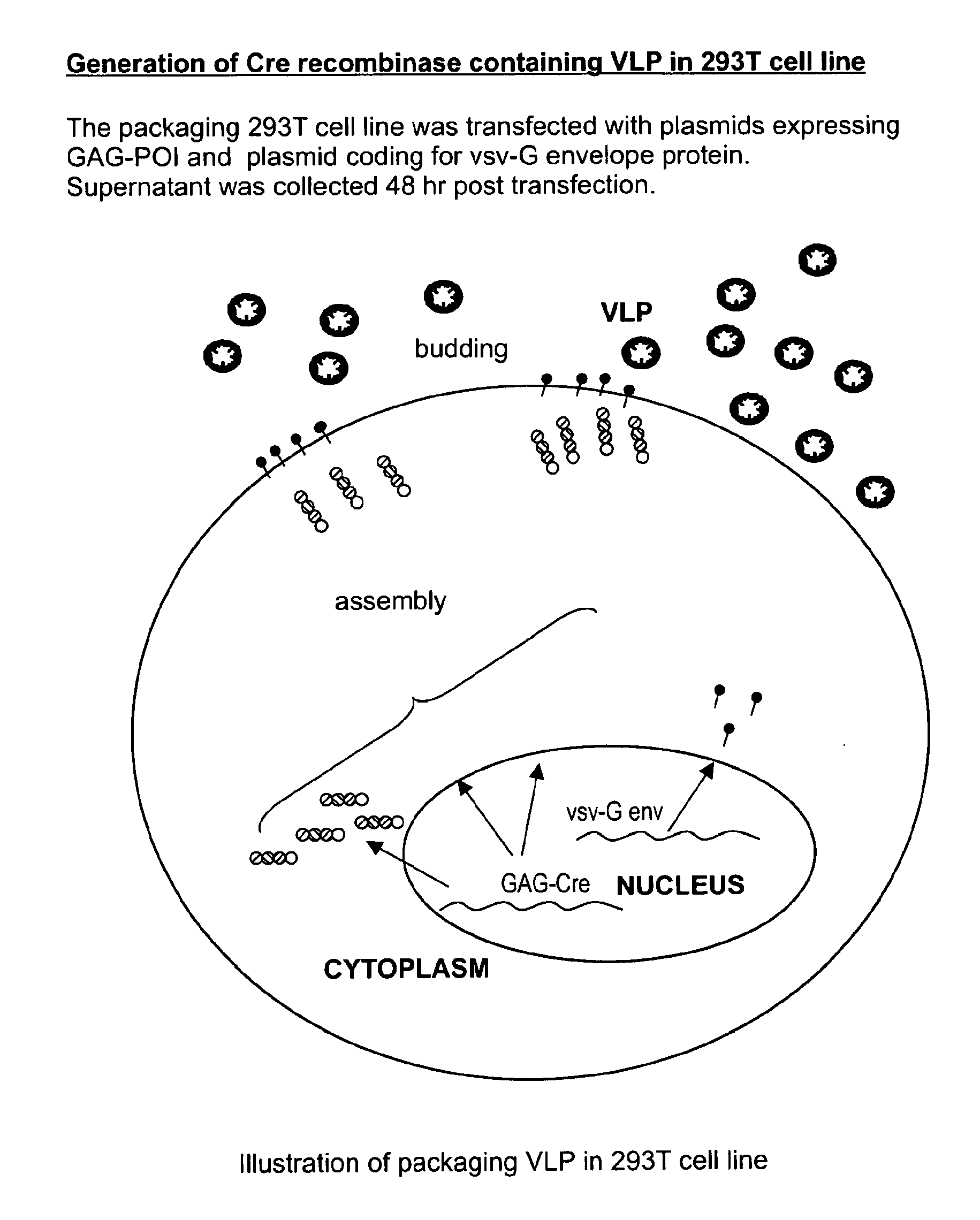
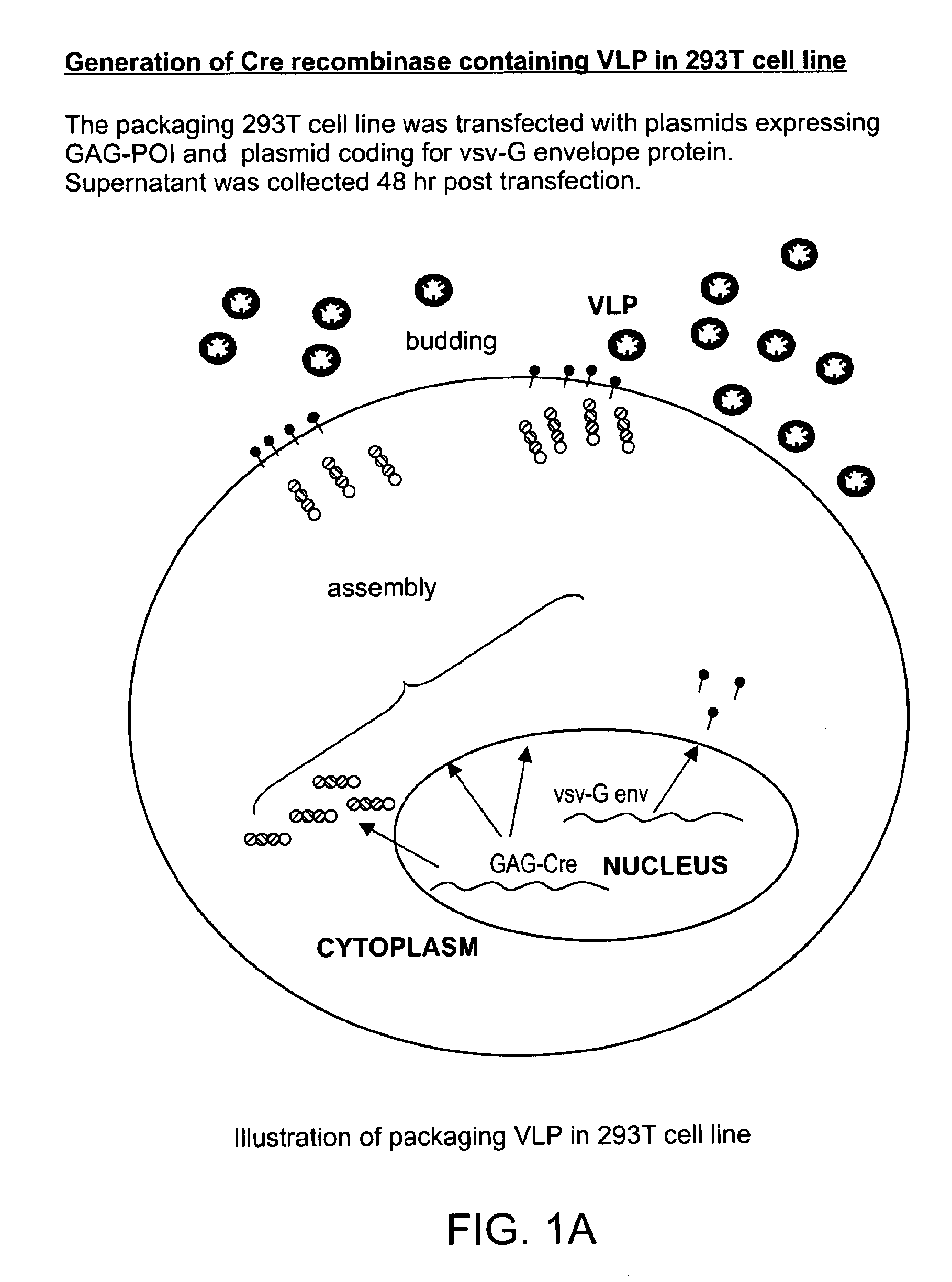
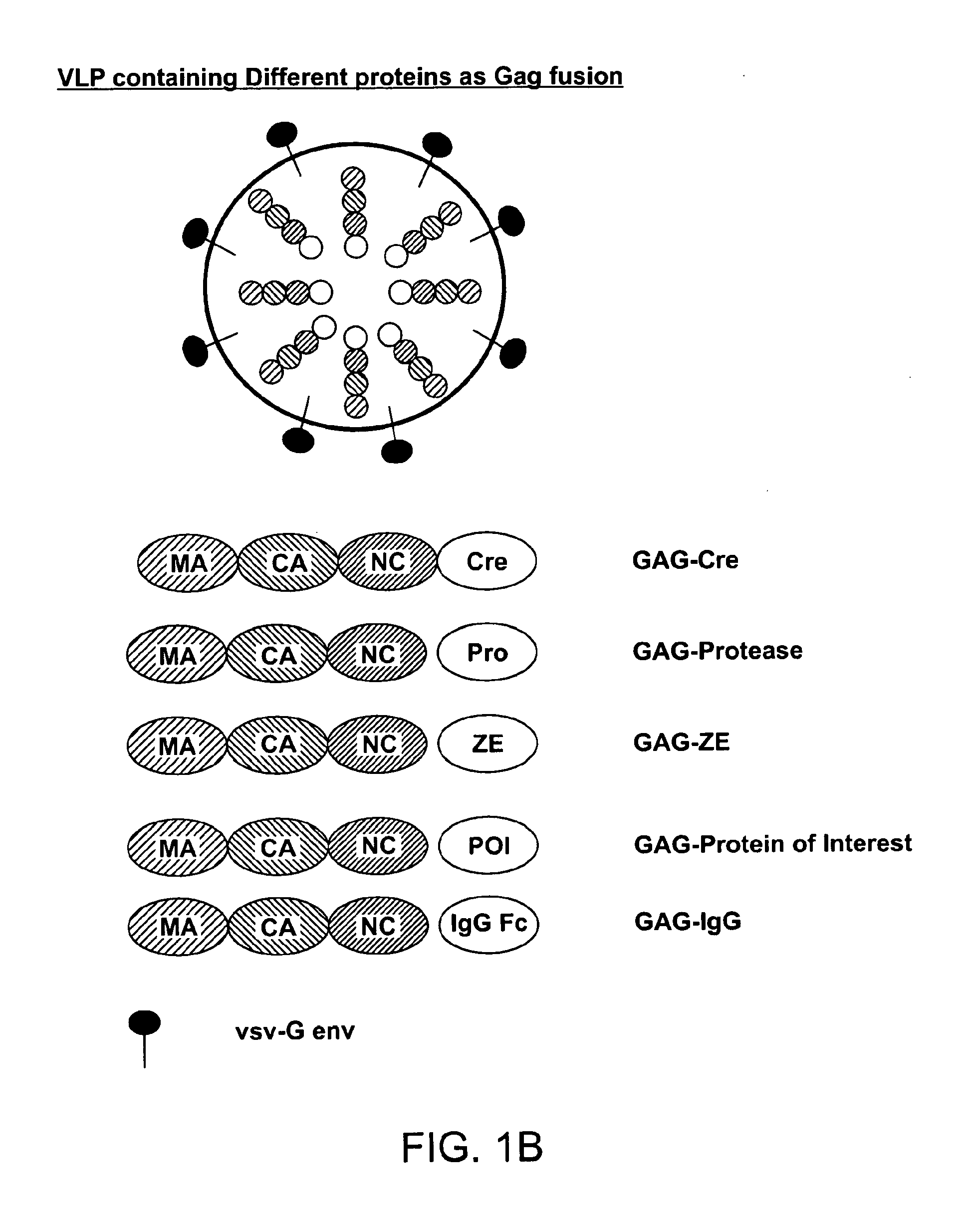
The present invention provides methods and compositions for protein delivery. The invention features virus like particles, methods of making virus like particles and methods of using virus like particles to deliver proteins to a cell, to provide protein therapy and to treat diseases or disorders. The invention also features methods of targeting a protein to a cell, methods of protein therapy and methods of treating diseases or disorders using a TUS protein, a NLS or NES identified from full length TUS.
Owner:UNITED STATES OF AMERICA
Protein therapy for treatment of retinal diseases
InactiveUS20180236035A1High expressionEasy transferHormone peptidesPeptide/protein ingredientsMammalApoptosis
The present invention encompasses methods, compositions, and devices for treating an ocular disease, disorder or condition in a mammal. The invention includes polypeptides that possess anti-inflammatory, anti-apoptotic, immune modulatory and anti-tumorigenic properties, and their application in the treatment of eye disease, particularly diseases of the retina. In particular aspects, the invention includes administration of a therapeutic polypeptide such as a stanniocalcin family member protein for the treatment of an eye disease. Also included are fusion proteins and cells stimulated or modified to express the therapeutic polypeptides as set forth herein.
Owner:SCOTT & WHITE HEALTHCARE
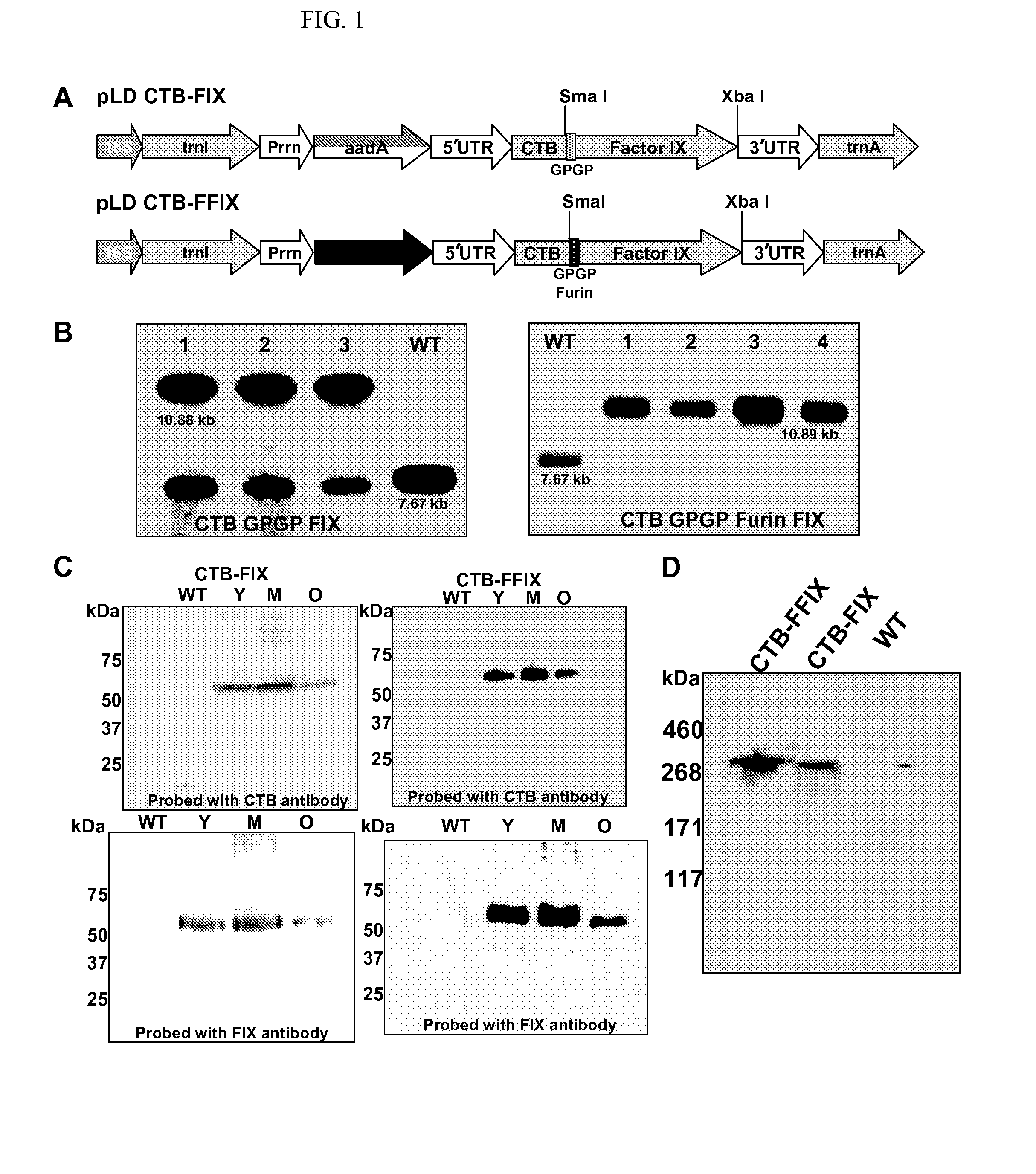
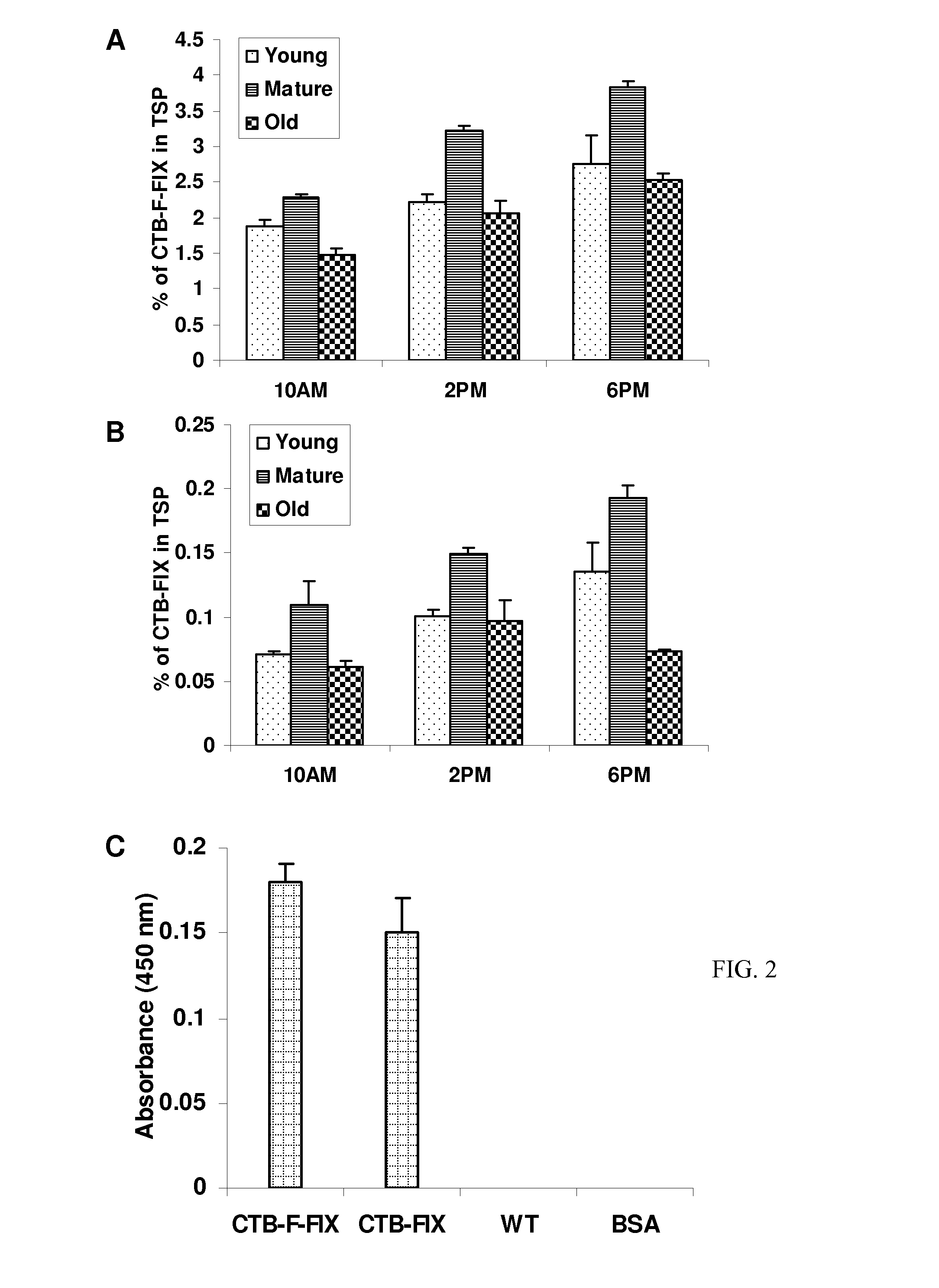
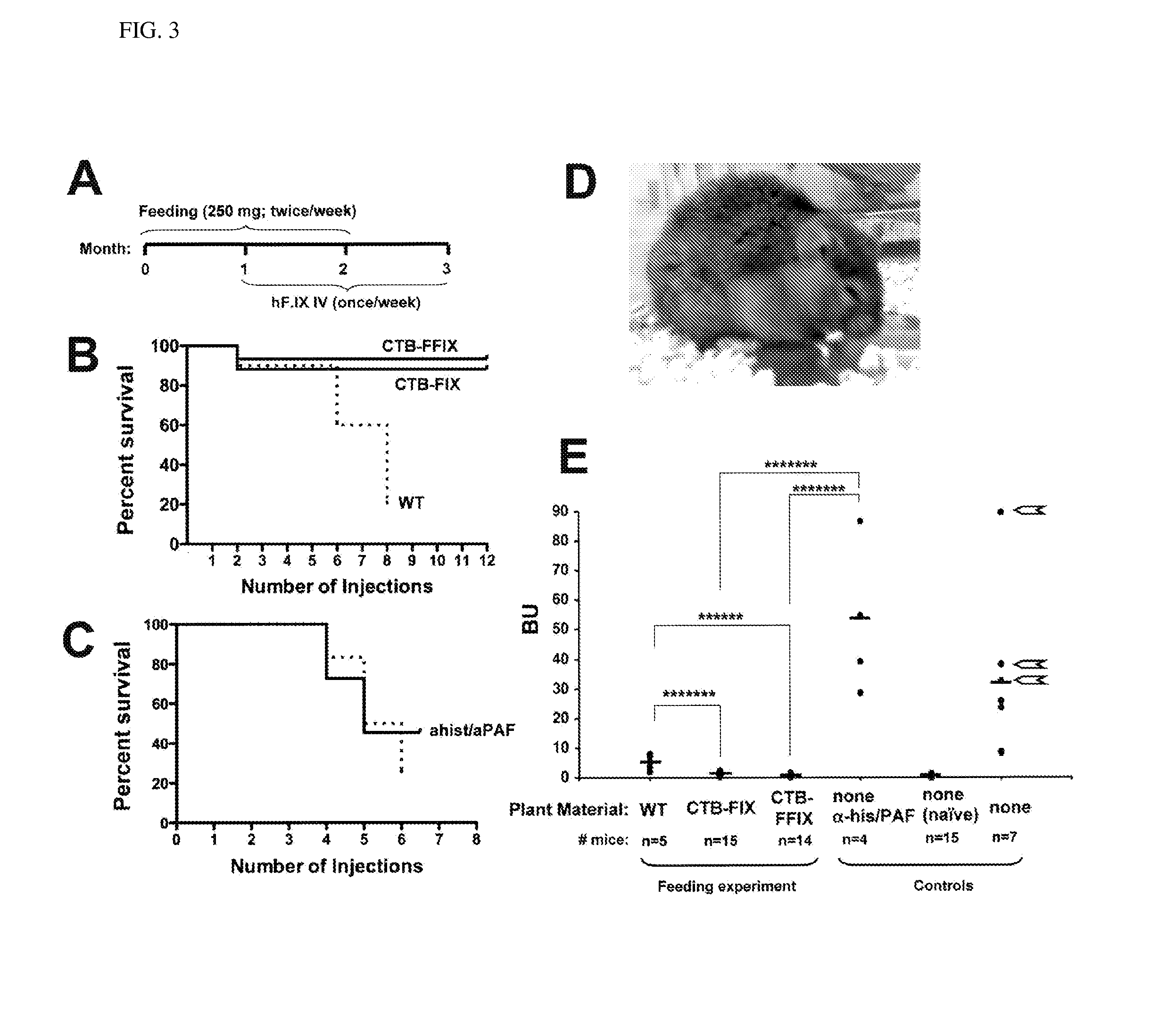
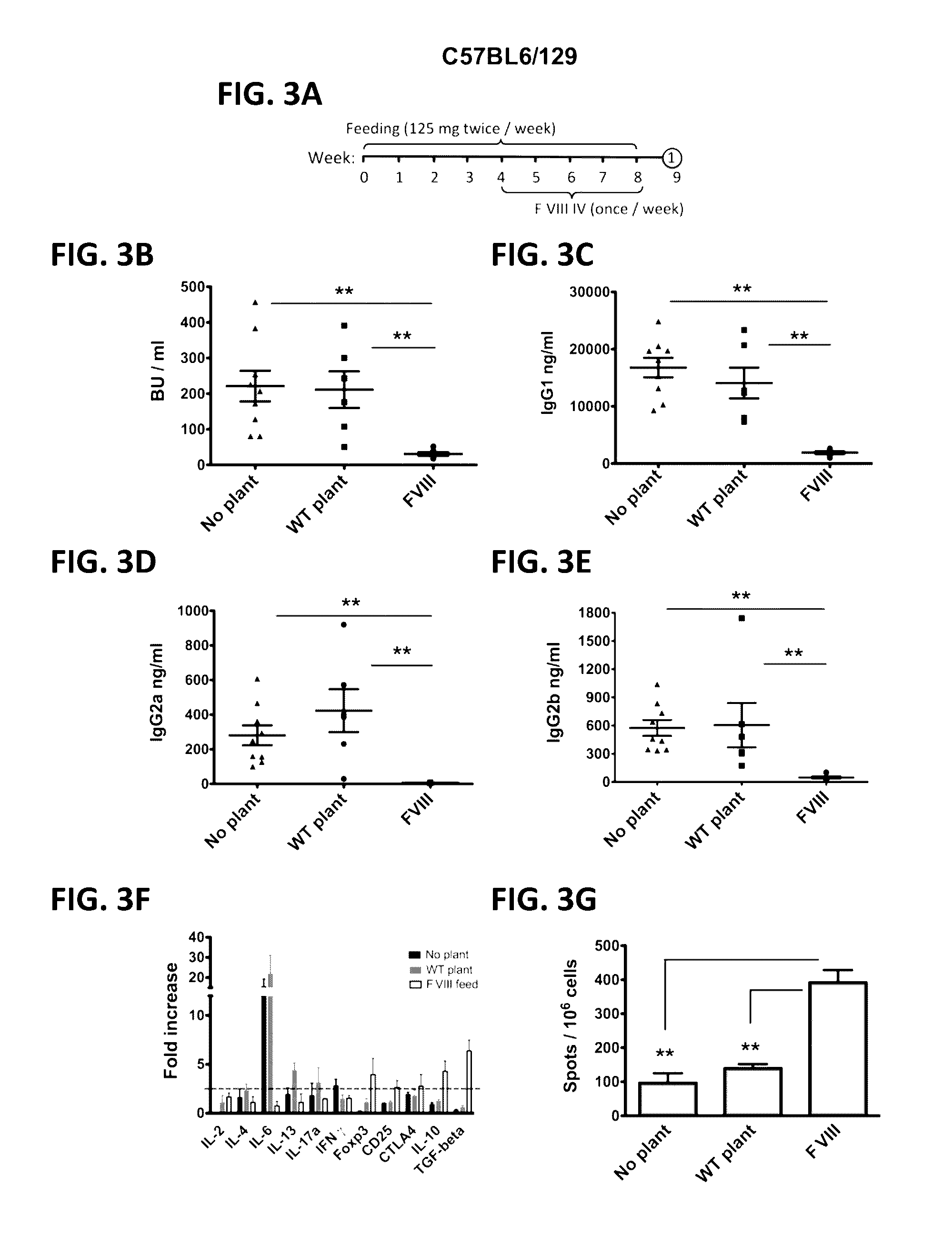
Administration of plant expressed oral tolerance agents
Protein replacement therapy for patients with hemophilia or other inherited protein deficiencies is often complicated by pathogenic antibody responses, including antibodies that neutralize the therapeutic protein or that predispose to potentially life-threatening anaphylactic reactions by formation of IgE. Using murine hemophilia B as a model, we have developed a prophylactic protocol against such responses that is non-invasive and does not include immune suppression or genetic manipulation of the patient's cells. Oral delivery of coagulation factor IX (F. IX) expressed in chloroplasts, bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies in protein replacement therapy. Inhibitor titers were mostly undetectable and up to 100-fold lower in treated mice when compared to controls. Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after 4 to 6 exposures to intravenous F. IX protein. While only 20-25% of control animals survived after 6-8 F. IX doses, 90-95% of tolerized mice survived 12 injections without signs of allergy or anaphylaxis. This high-responder strain of hemophilia B mice represents the first hemophilic animal model to study anaphylactic reactions. The plant material was effective over a range of oral antigen doses (equivalent to 5-80 μg recombinant F.IX / kg), and controlled inhibitor formation and anaphylaxis long-term, up to 7 months. Oral antigen administration caused a deviant immune response that suppressed formation of IgE and inhibitory antibodies. This cost-effective and efficient approach to oral delivery of protein antigens to the gut should be applicable to several genetic diseases that are prone to pathogenic antibody responses during treatment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
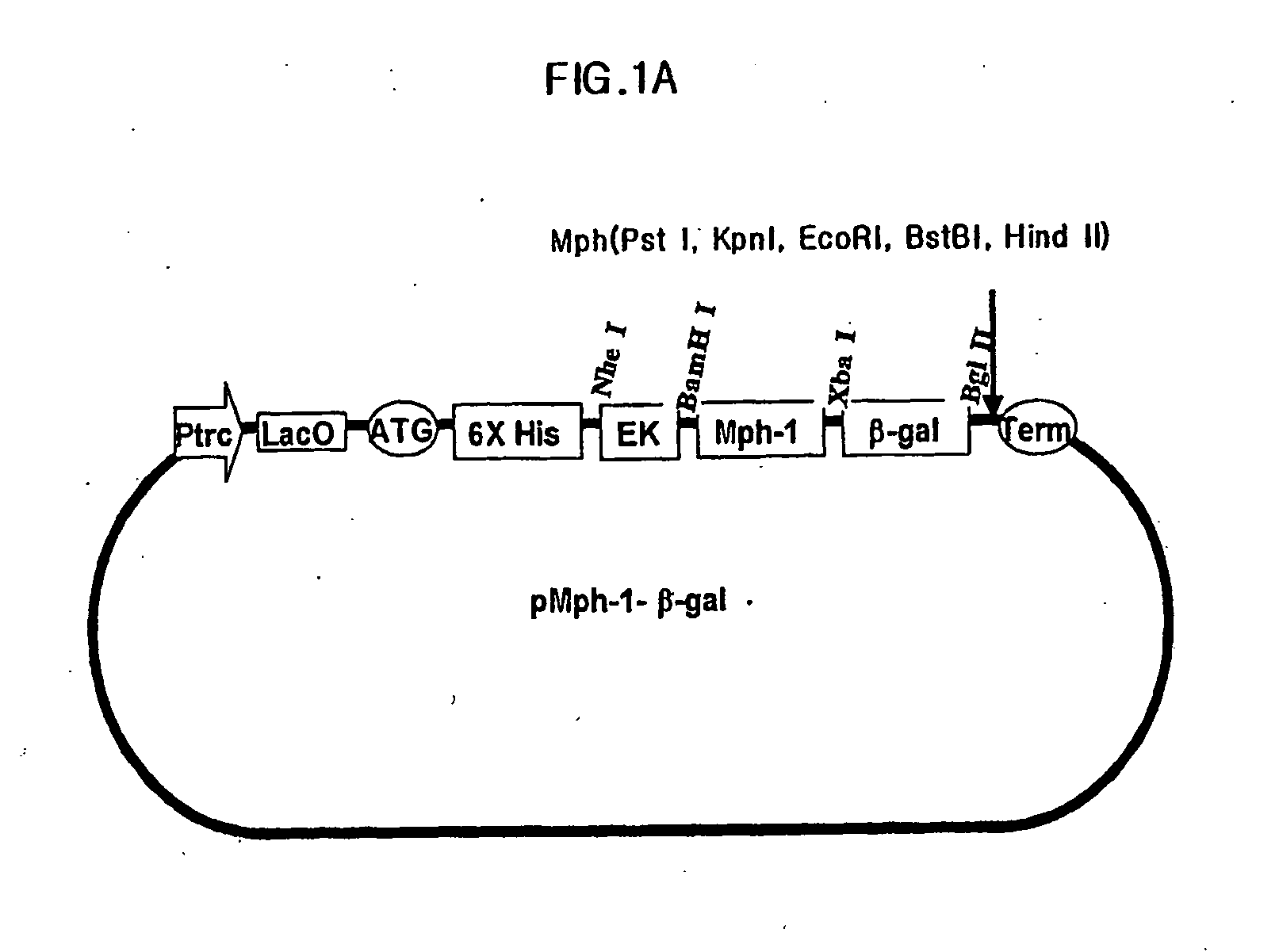
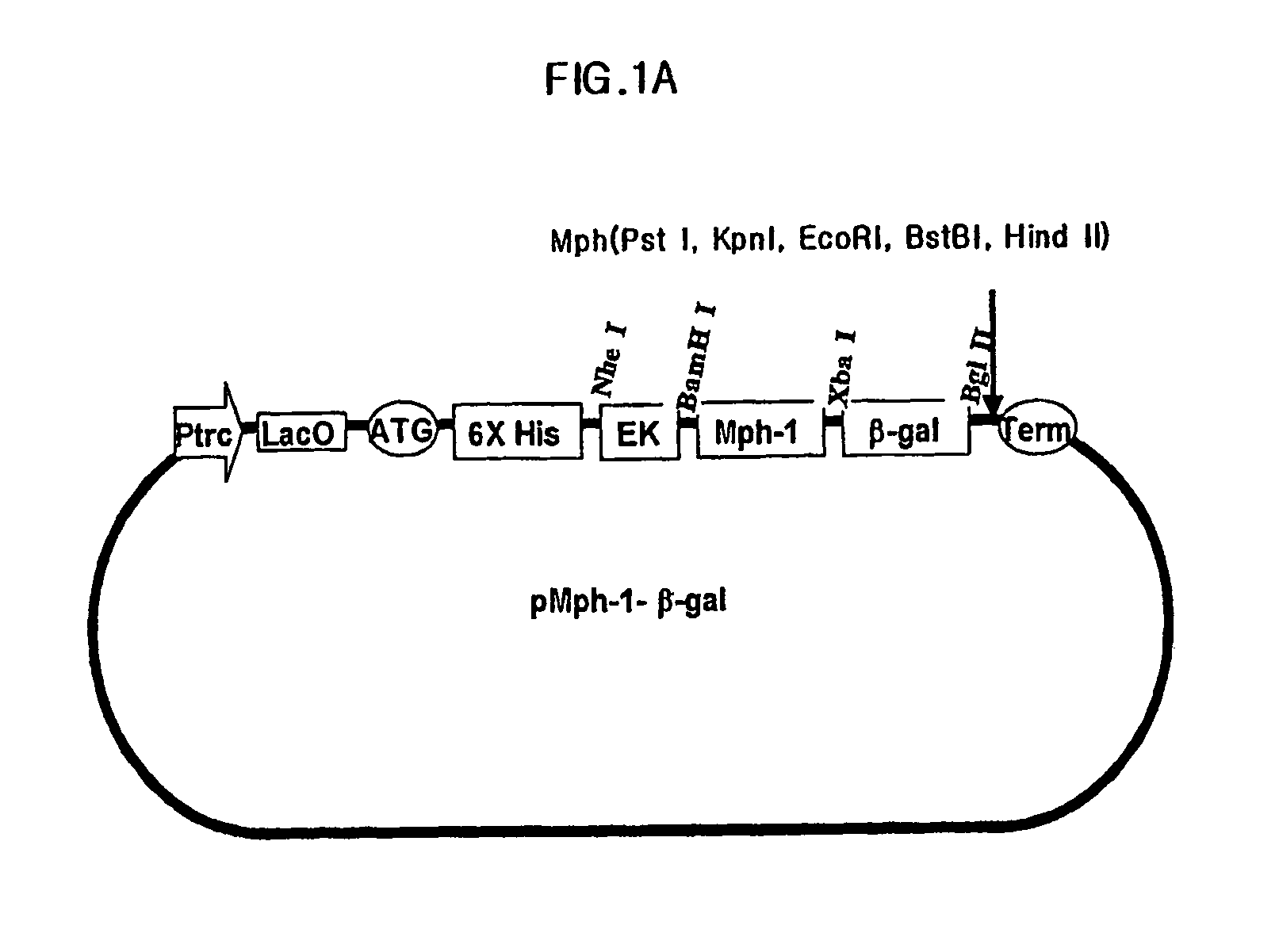
Biomolecule transduction motif Mph-1-BTM and the use thereof
This invention relates to a novel Biomolecule Transduction Motif (BTM), Mph-1 peptide, which has the potential to transduce many biological response modifiers effectively into the cytoplasm, intracellular organelles or nucleus of prokaryotic or eukaryotic cells in vivo and in vitro, and the related technological methods using Mph-1 BTM. This Mph-1 BTM can be used in the development of new recombinant protein vaccines or DNA / RNA vaccines, gene and protein therapy, production of pharmacologically or medicinally useful proteins, or pharmaco-medicinal drug therapy.
Owner:FORHUMANTECH CO LTD
Method of treating patients undergoing protein replacement therapy, gene replacement therapy, or other therapeutic modalities
ActiveUS20130052189A1Preventing and reducing antibody titerInhibition formationBiocideDipeptide ingredientsDiagnostic Radiology ModalityOligomer
The present invention relates, in general, to a method of treating patients undergoing enzyme replacement therapy (ERT) or other therapy involving the administration of a proteinaceous therapeutic agent as well gene replacement therapy with non-viral or viral vectors, or other therapeutic modality or modalities, used alone or in combination, which involve the administration of exogenous substances for potential therapeutic benefit, including, but not limited to DNA vaccines, siRNA, splice-site switching oligomers (SSOs) as well as RNA-based nanoparticles (RNPs) and nanovaccines. The invention further relates to compounds and compositions suitable for use in such methods.
Owner:SYNPAC NORTH CAROLINA INC
Method and composition for a protein transduction technology and its applications
ActiveUS8722348B2Efficient deliveryImprove efficiencyPeptide/protein ingredientsMicroencapsulation basedSpectroscopyFluorophore
A protein transduction method for efficiently delivery of exogenous proteins into mammalian cells is invented, which has the capability of targeting different cellular compartments and protection from degradation of the delivered proteins from cellular proteases. A composition for treat proteins has cation reagents, lipids and enhancers in a carrier. The method can be used in a number of ways including: production of large quantities of properly folded, post-translationally modified proteins using mammalian cell machinery, a in-cell fluorescence spectroscopy and imaging using small molecule fluorophores and a in-cell NMR spectroscopy using living mammalian cells. The method permits cell biology at atomic resolution that is physiologically and pathological relevant and permits protein therapy to treat human diseases. The method can also be used to deliver exogenous protein inside mammalian cells, wherein the exogenous proteins follow a similar secretion pathway as that of the endogenous protein.
Owner:WAYNE STATE UNIV
Protein Therapy for Treatment of Eye Diseases
The present invention encompasses methods, compositions, and devices for treating an ocular disease, disorder or condition in a mammal. The invention includes polypeptides that possess anti-inflammatory, anti-apoptotic, immune modulatory and anti-tumorigenic properties, and their application in the treatment of eye disease, particularly diseases of the retina. In particular aspects, the invention includes administration of a therapeutic polypeptide such as a stanniocalcin family member protein for the treatment of an eye disease. Also included are fusion proteins and cells stimulated or modified to express the therapeutic polypeptides as set forth herein.
Owner:SCOTT & WHITE HEALTHCARE +1
Modified RNA with decreased immunostimulatory properties
ActiveUS10898584B2Lower immune responseOrganic active ingredientsPeptide/protein ingredientsMedicinePharmaceutical drug
Owner:CUREVAC SE
Development of novel macromolecule transduction domain with improved cell permeability and method for using same
ActiveUS9259481B2Good cell permeabilityMaintain activityOrganic active ingredientsCosmetic preparationsCell membraneDNA
The present invention relates to an improved macromolecule transduction domain (MTD), which facilitates permeating the cell membrane of a biologically active molecule, having enhanced cell permeability. Specifically, an improved MTD according to the present invention, compared to an existing MTD, can transmit various types of biologically active molecule from inside the body and inside a test tube more effectively, and thus can be effectively used in a method to genetically alter a biologically active molecule so as to have cell permeability or in a method to transport a biologically active molecule into a cell, or the like. Additionally, the improved MTD can be very useful in development of new drugs and incrementally modified drugs as uses of the improved MTD are possible in drug delivery systems, recombinant protein vaccines or DNA / RNA therapeutic agents, gene or protein therapies, and pharmacologically or medically useful protein production or medical, pharmacological and pharmaceutical compositions.
Owner:PROCELL THERAPEUTICS
Apoptosis-Modulating Protein Therapy for Proliferative Disorders and Nanoparticles Containing the Same
Protein containing nanoparticles and methods of use thereof for the treatment of proliferative disorders are disclosed.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Methods and compositions for protein delivery
Owner:UNITED STATES OF AMERICA
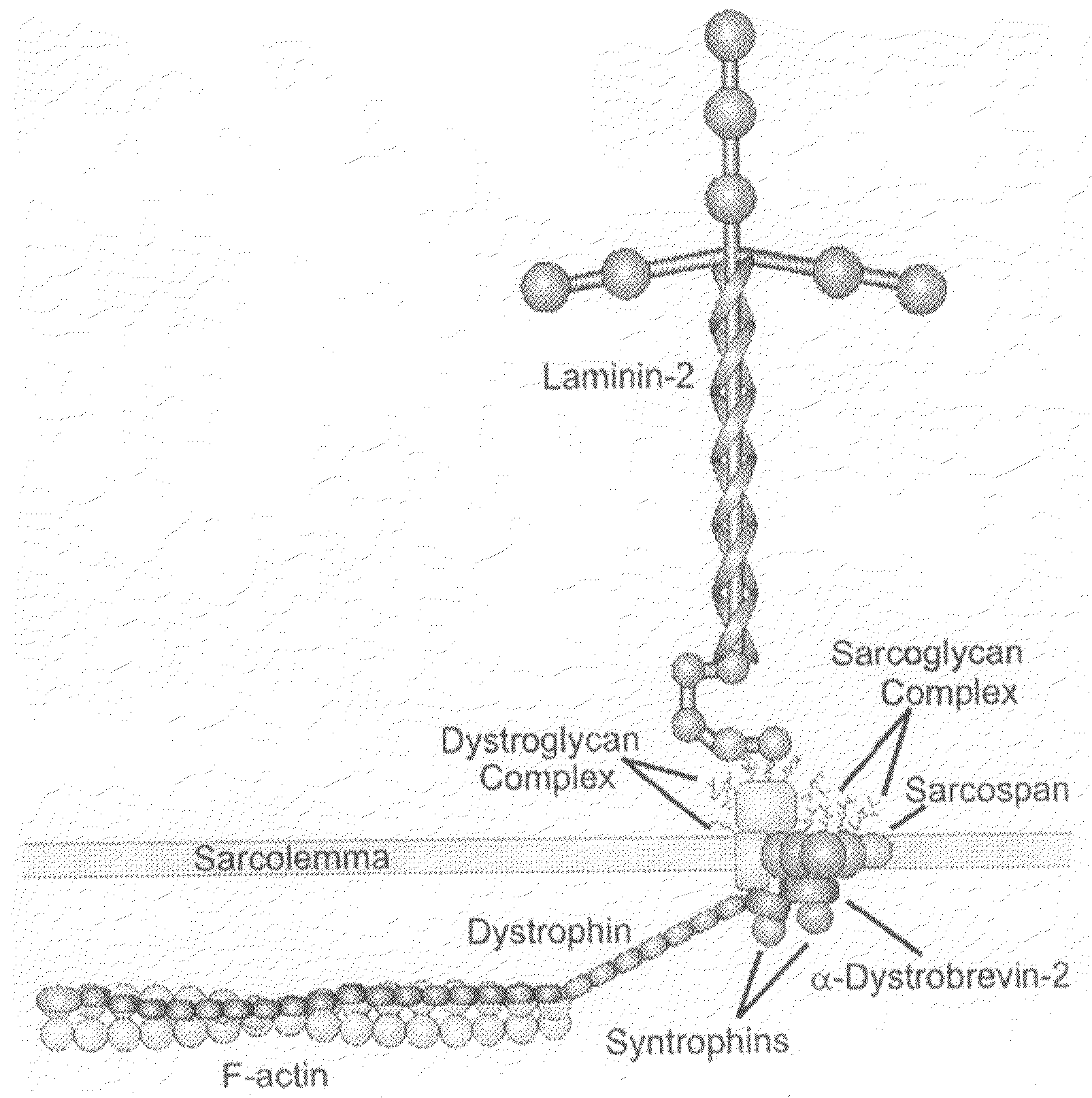
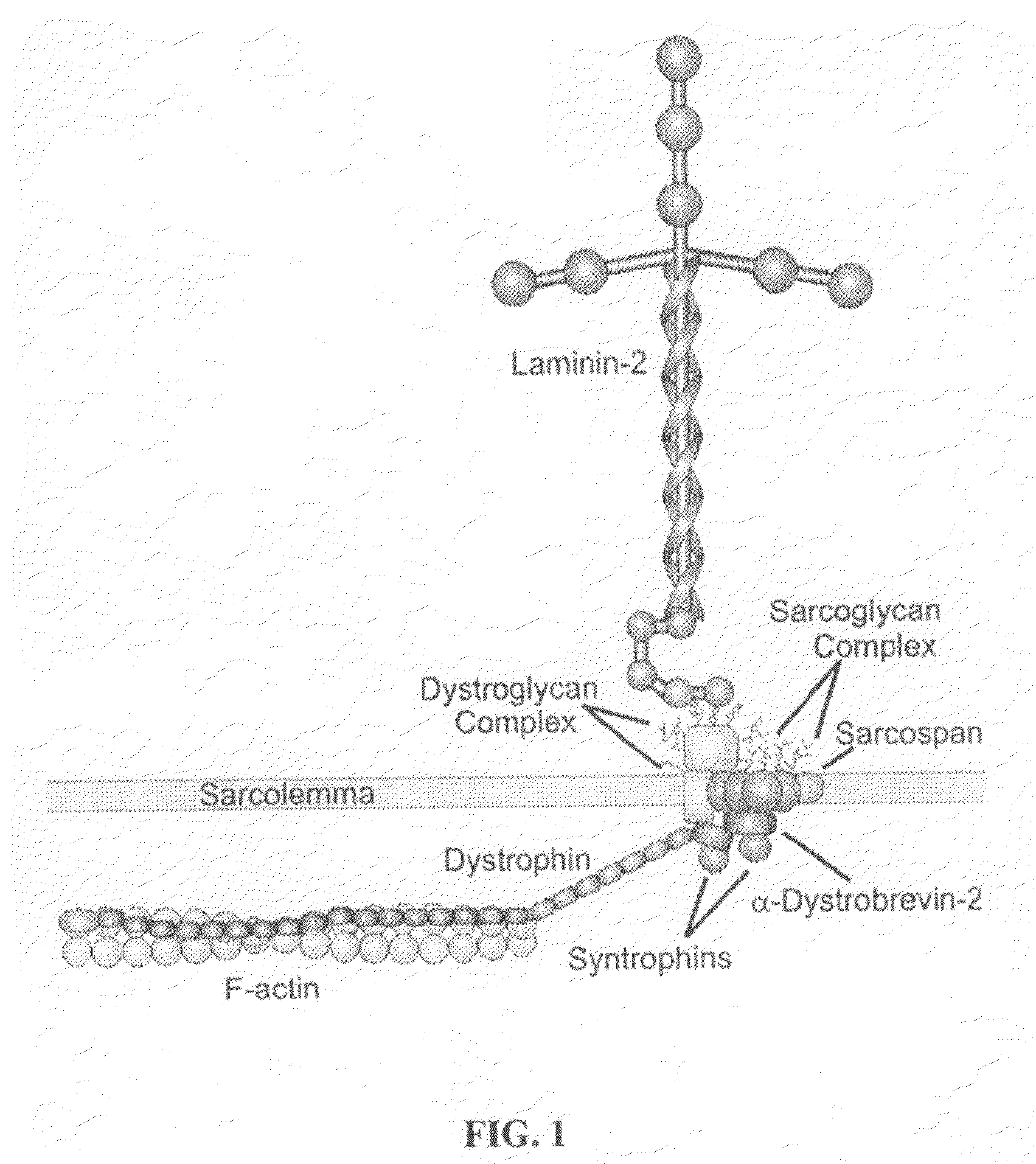
TAT-utrophin as a protein therapy for dystrophinopathies
ActiveUS20090054327A1Improve the level ofQuick purificationNervous disorderMuscular disorderMammalDuchenne's Muscular Dystrophy
Disclosed is a fusion protein including a full-length TAT-utrophin or an anti-dystrophinopathic fragment thereof, a method of treating dystrophinopathies (including Duchenne muscular dystrophy) using the fusion protein, a pharmaceutical composition for treating treating dystrophinopathies in mammals comprising the fusion protein, and nucleic acid constructs for expressing the fusion protein.
Owner:WISCONSIN ALUMNI RES FOUND
Method of Analyzing a BRCA2 Gene in a Human Subject
InactiveUS20090269814A1Increased riskIncreased susceptibilitySugar derivativesFermentationMedicineProtein therapeutics
Five novel DNA and protein sequences have been determined for the BRCA2 gene, as have been ten polymorphic sites and their rates of occurrence in the normal alleles of BRCA2. The sequences BRCA2(omi 1-5) and the ten polymorphic sites will provide greater accuracy and reliability for genetic testing. One skilled in the art will be better able to avoid misinterpretations of changes in the gene and / or protein sequence, determine the presence of a normal sequence, and of mutations of BRCA2. This invention is also related to a method of performing gene therapy with BRCA2(omi 1-5) coding sequences or fragments thereof. This invention is further related to protein therapy with BRCA2(omi 1-5) proteins or their functional equivalents.
Owner:MYRIAD GENETICS
Biomolecule transduction motif Mph-1-BTM and the use thereof
InactiveUS7700109B2Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsIn vivoOrganism
Owner:FORHUMANTECH CO LTD
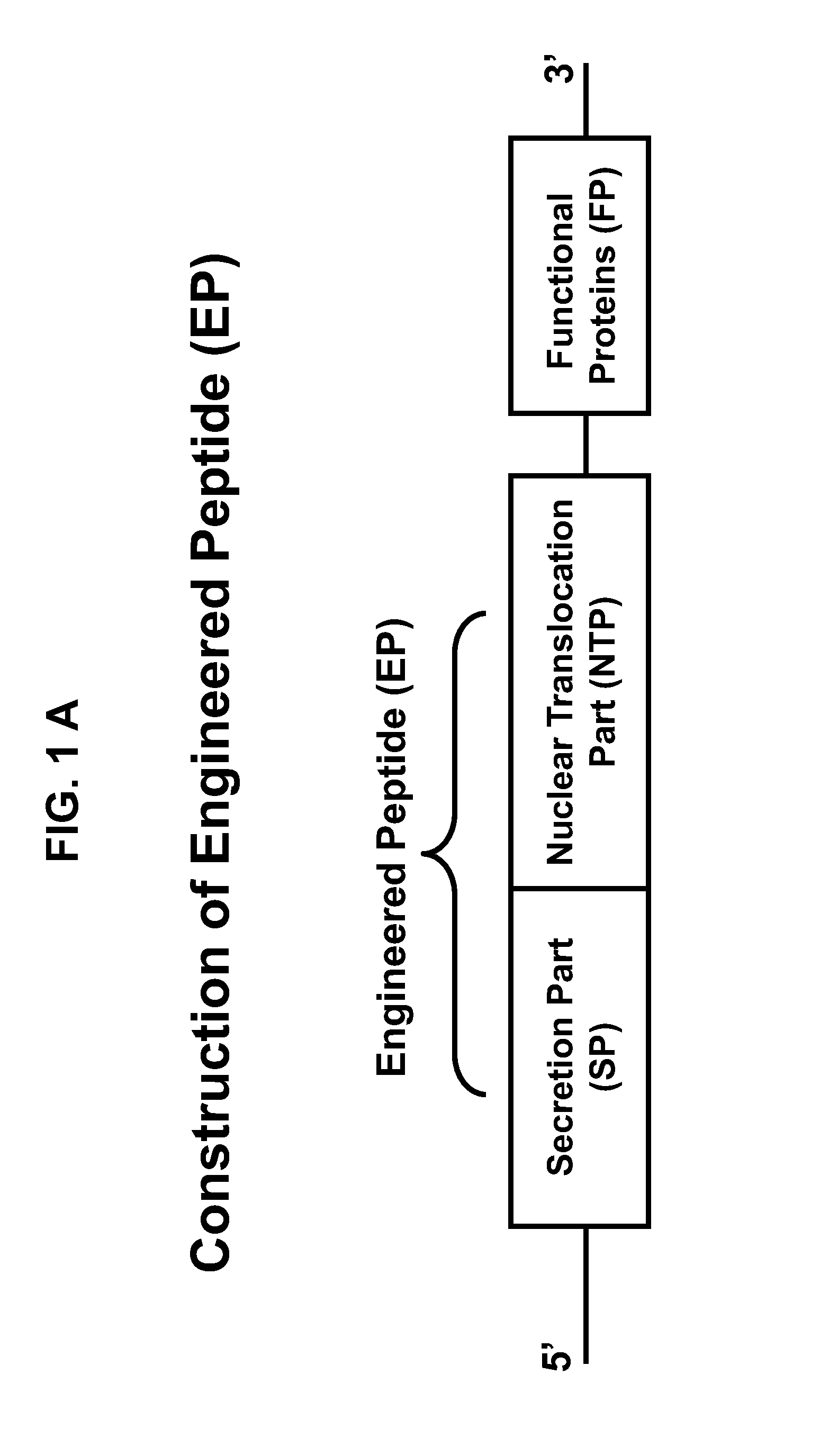
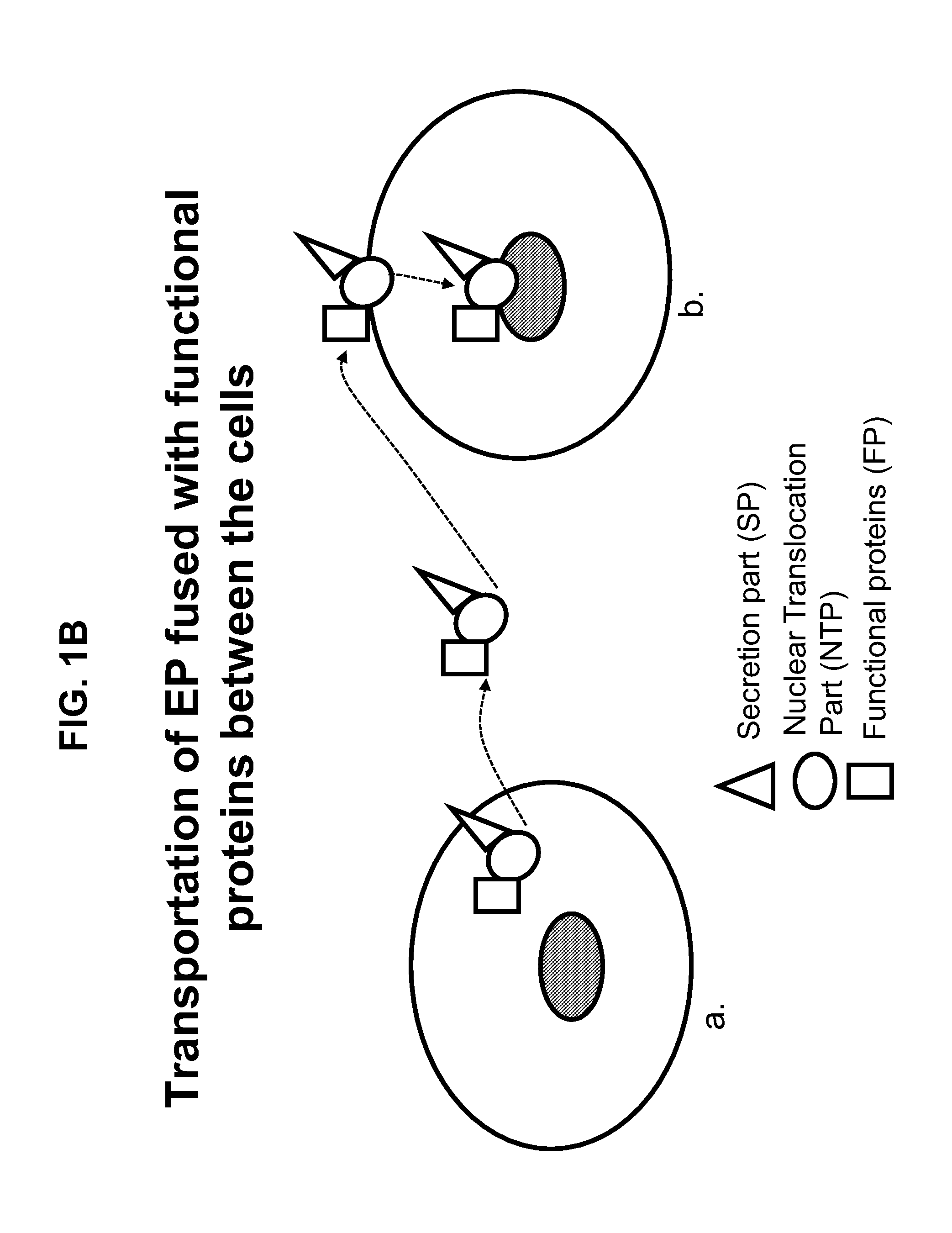
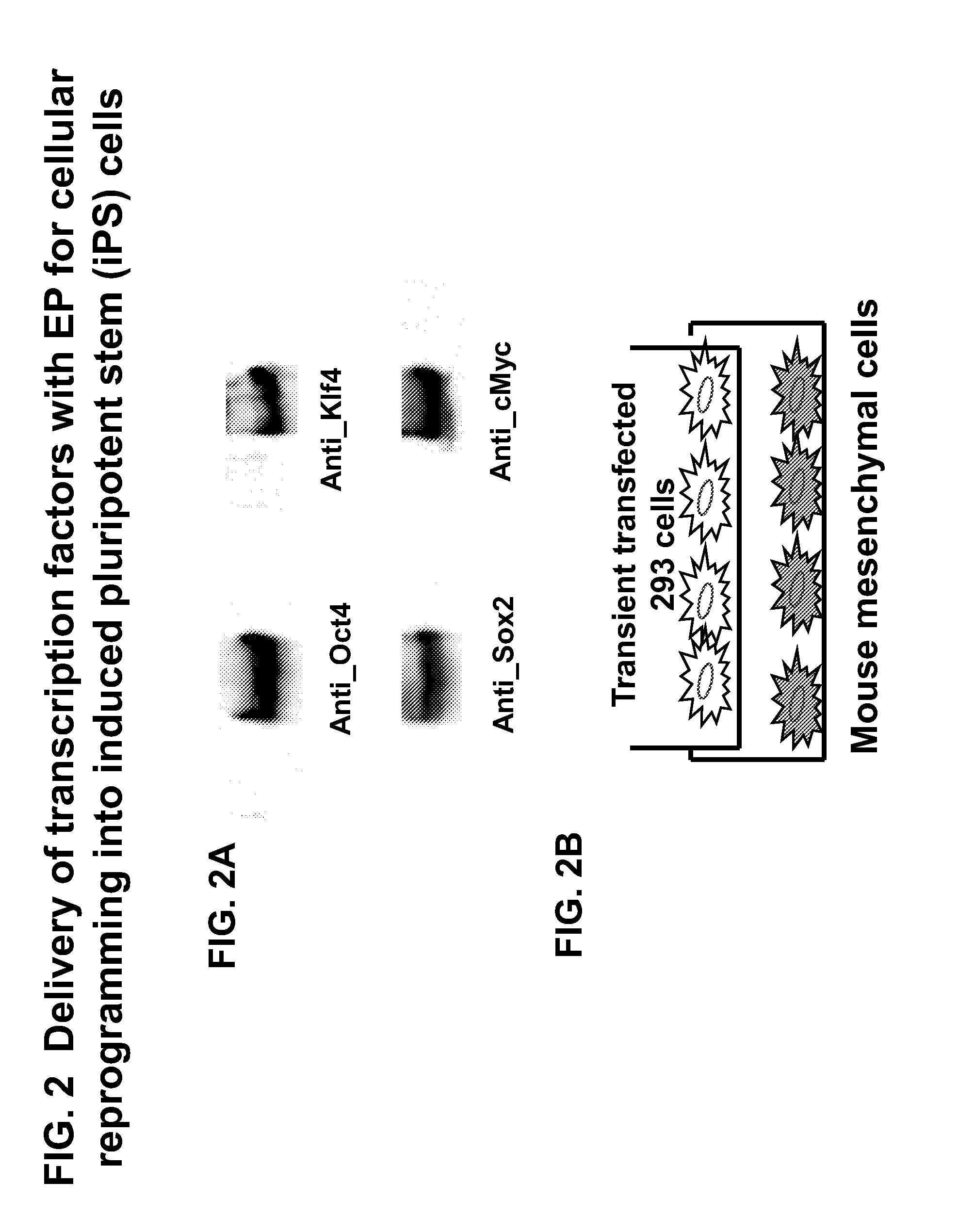
Engineered peptide (EP)-directed protein intercellular delivery system and uses thereof
InactiveUS20150023932A1Avoids cellular toxicity arouseSimple processPeptide/protein ingredientsAntibody mimetics/scaffoldsTherapeutic proteinIn vivo
The present invention provides an intercellular protein delivery system comprising an engineered peptide (EP), composed of secretion part (SP) and nuclear translocation part (NTP), a functional or therapeutic protein (FP), cells that express the fusion proteins and cells that accept the fusion proteins. The system can be used in vivo or in vitro to sustainably supply proteins of interest for cellular reprogramming, cellular differentiation and cell-based protein therapies.
Owner:FAN KE KE +1
Compositions and methods for suppression of inhibitor formation against coagulation factors in hemophilia patients
ActiveUS20160289277A1Reduce formationSuppress formationFactor VIIPeptide/protein ingredientsTherapeutic proteinPlant cell
Protein replacement therapy for patients with hemophilia or other inherited protein deficiencies is often complicated by pathogenic antibody responses, including antibodies that neutralize the therapeutic protein or that predispose to potentially life-threatening anaphylactic reactions by formation of IgE. Using murine and canine hemophilia as a model, we have developed a prophylactic protocol against such responses that is non-invasive and does not include immune suppression or genetic manipulation of the patient's cells. Oral delivery of a coagulation factor expressed in chloroplasts, bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies in protein replacement therapy. Inhibitor titers were mostly undetectable and up to 100-fold lower in treated subjects when compared to controls. Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after four to six exposures to intravenous coagulation factor protein. Finally, the method can effectively be used to reverse or reduce undesirable pre-existing inhibitor titers.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Method of Analyzing a BRCA2 Gene in a Human Subject
InactiveUS20090325237A1Increased riskIncreased susceptibilitySugar derivativesFermentationMedicineProtein therapeutics
Five novel DNA and protein sequences have been determined for the BRCA2 gene, as have been ten polymorphic sites and their rates of occurrence in the normal alleles of BRCA2. The sequences BRCA2(omi 1-5) and the ten polymorphic sites will provide greater accuracy and reliability for genetic testing. One skilled in the art will be better able to avoid misinterpretations of changes in the gene and / or protein sequence, determine the presence of a normal sequence, and of mutations of BRCA2. This invention is also related to a method of performing gene therapy with BRCA2(omi 1-5) coding sequences or fragments thereof. This invention is further related to protein therapy with BRCA2(omi 1-5) proteins or their functional equivalents.
Owner:ORE PHARMA
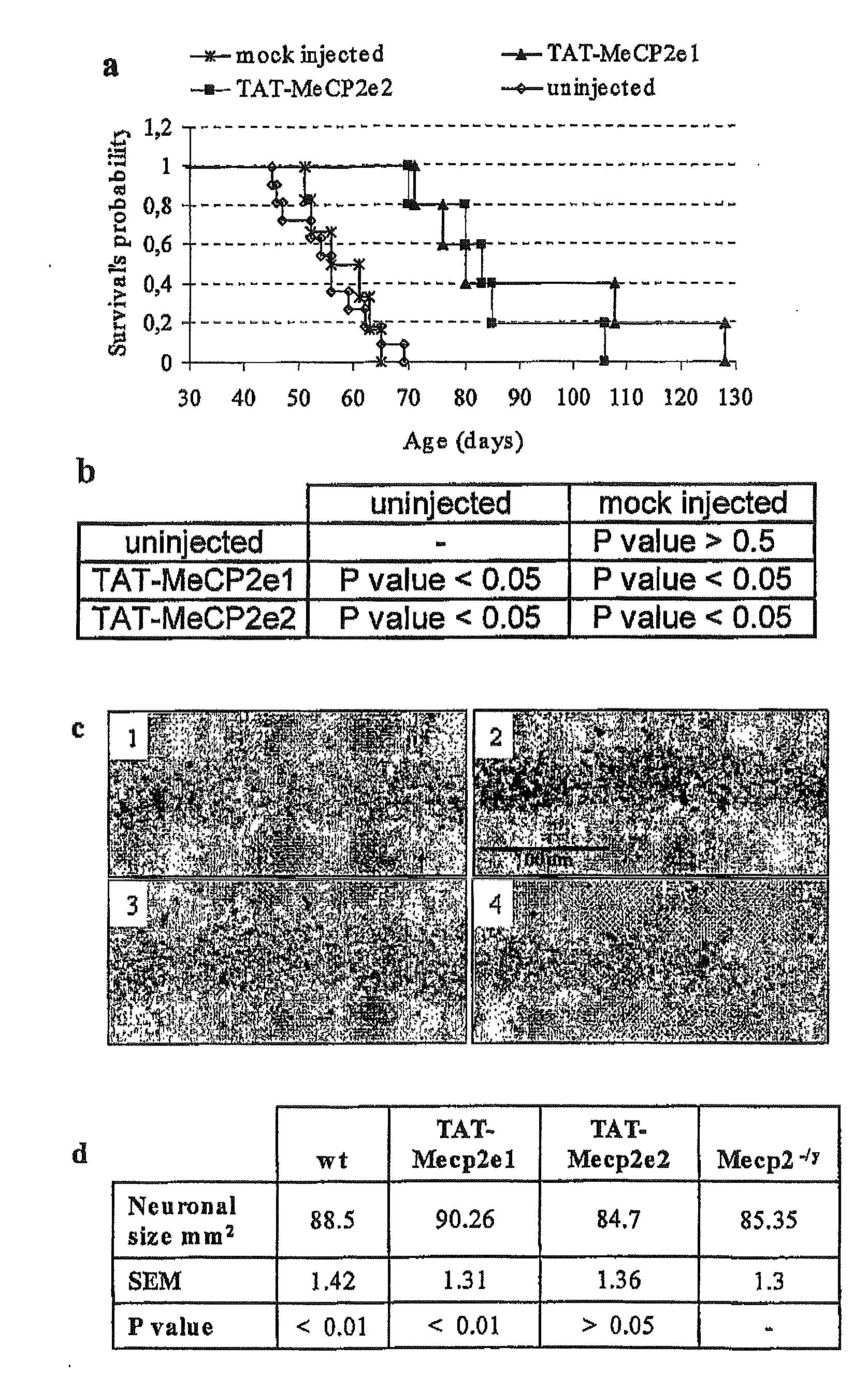
Synthetic mecp2 sequence for protein substitution therapy
ActiveUS20090233856A1Improve purification effectHigh transduction efficiencyNervous disorderPeptide/protein ingredientsDiseaseNucleic acid sequencing
The invention relates to the MeCP2 protein and its use in protein substitution therapy. More specifically, the invention relates to condon-optimized nucleic acid sequences for the expression of MeCP2 proteins, methods for creating such a nucleic acid sequence and expressing such a protein, fusions of a protein of the invention to a transduction domain, and vectors and host cells comprising a protein of the invention. Further, the invention relates to uses of nucleic acids or proteins of the invention in medicine, pharmaceutical compositions comprising nucleic acid sequences and proteins of the invention, as well as methods for the treatment, prevention, and / or therapy of neurodegenerative or neurodevelopmental diseases including Rett syndrome.
Owner:GEORG AUGUST UNIVERSITAT GOTTINGEN STIFTUNG OFFENLICHEN RECHTS +1
Immobilized poly(n)polymerase
ActiveUS11384375B2Improve stabilityMore time-effectiveMicrobiological testing/measurementChemical industryVaccinationRNA - Ribonucleic acid
The present invention relates to an immobilized poly(N)polymerase (PNP), methods of producing said PNP and uses thereof. Further disclosed is an enzyme reactor and kit comprising the PNP for producing polynucleotidylated ribonucleic acid poly(N)RNA)molecules which are useful in gene therapy, immunotherapy, protein replacement therapy and / or vaccination.
Owner:CUREVAC SE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com