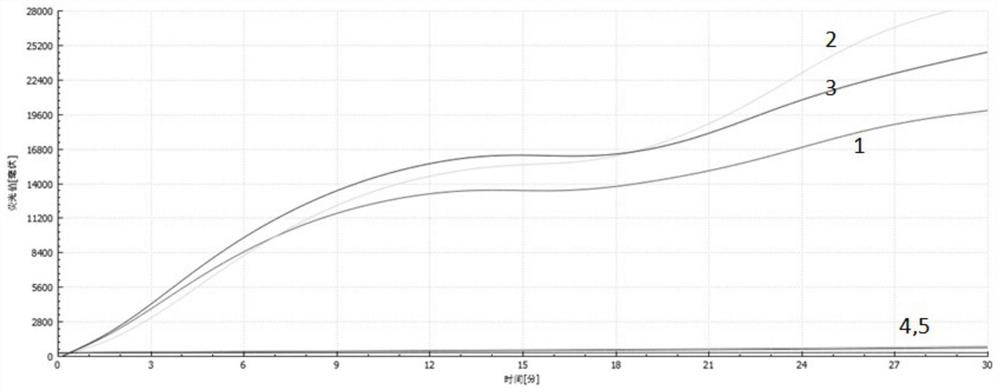
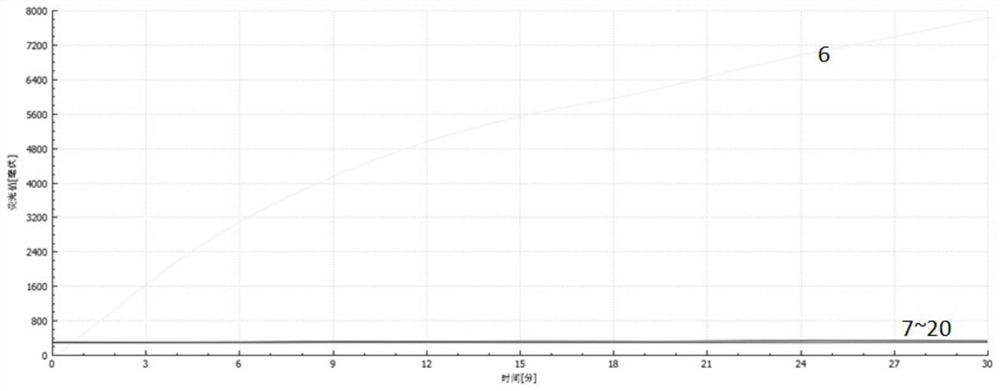
Patents
Literature
32 results about "STX2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Syntaxin-2, also known as epimorphin, is a protein that in humans is encoded by the STX2 gene. The product of this gene belongs to the syntaxin/epimorphin family of proteins. The syntaxins are a large protein family implicated in the targeting and fusion of intracellular transport vesicles. The product of this gene regulates epithelial-mesenchymal interactions and epithelial cell morphogenesis and activation. Alternatively spliced transcript variants encoding different isoforms have been identified. When the N terminus is on the cytosolic face it acts as a t-SNARE involved in intracellular vesicle docking and is called Syntaxin-2. When flipped inside out, i.e. N terminus hangs out on the extracellular surface (by some nonclassical secretion pathway) it acts as a versatile morphogen and is called epimorphin. This membrane protein enjoys the double choice of another form of topological alternatives of being targeted to either apical or basolateral surface of an epithelial cell in a regulated way depending on various contexts. When expressed by mesenchymal cells it can instruct epithelial morphogenesis at epithelial mesenchymal interfaces.
Methods and compositions based on shiga toxin type 2 protein
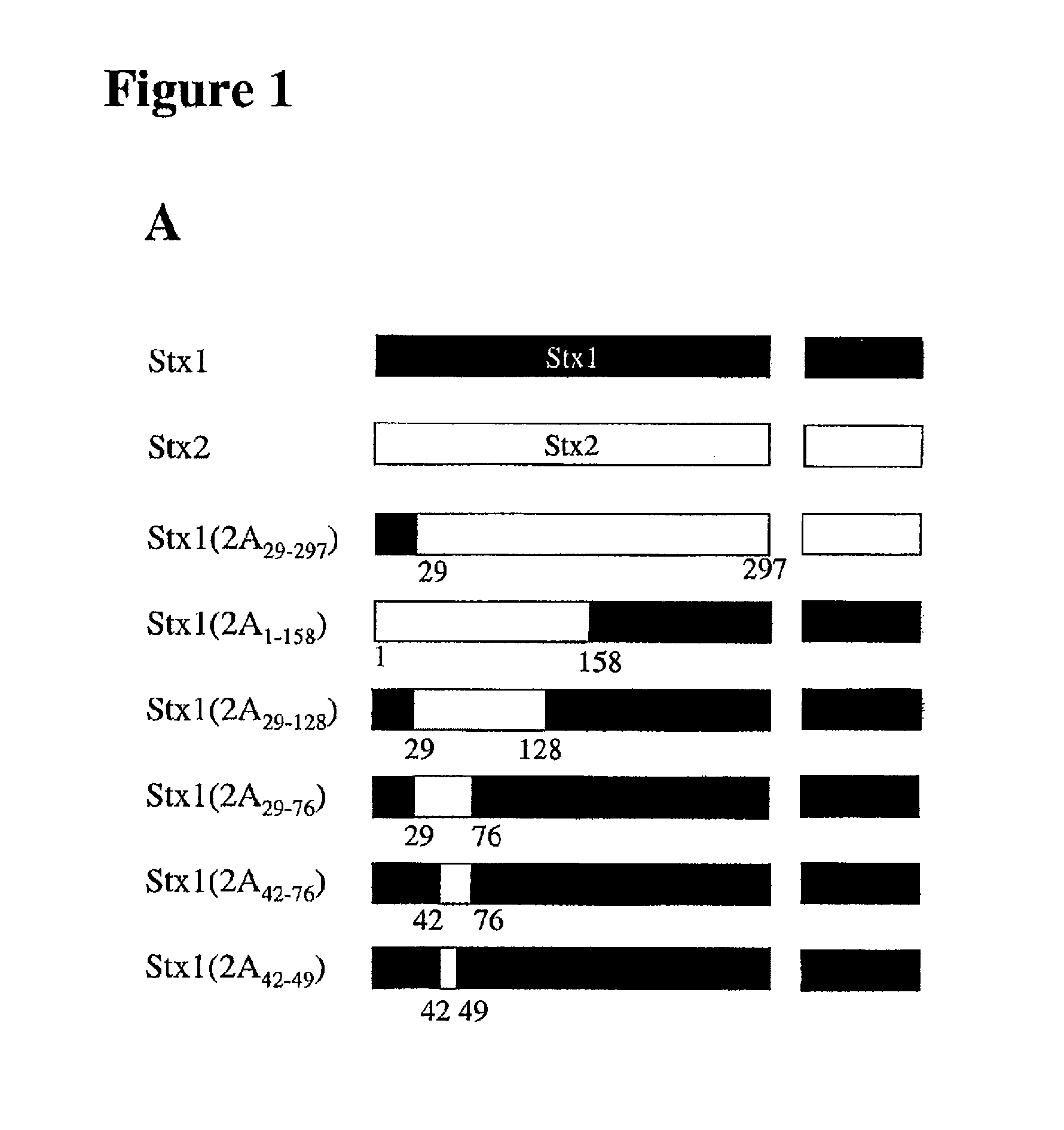
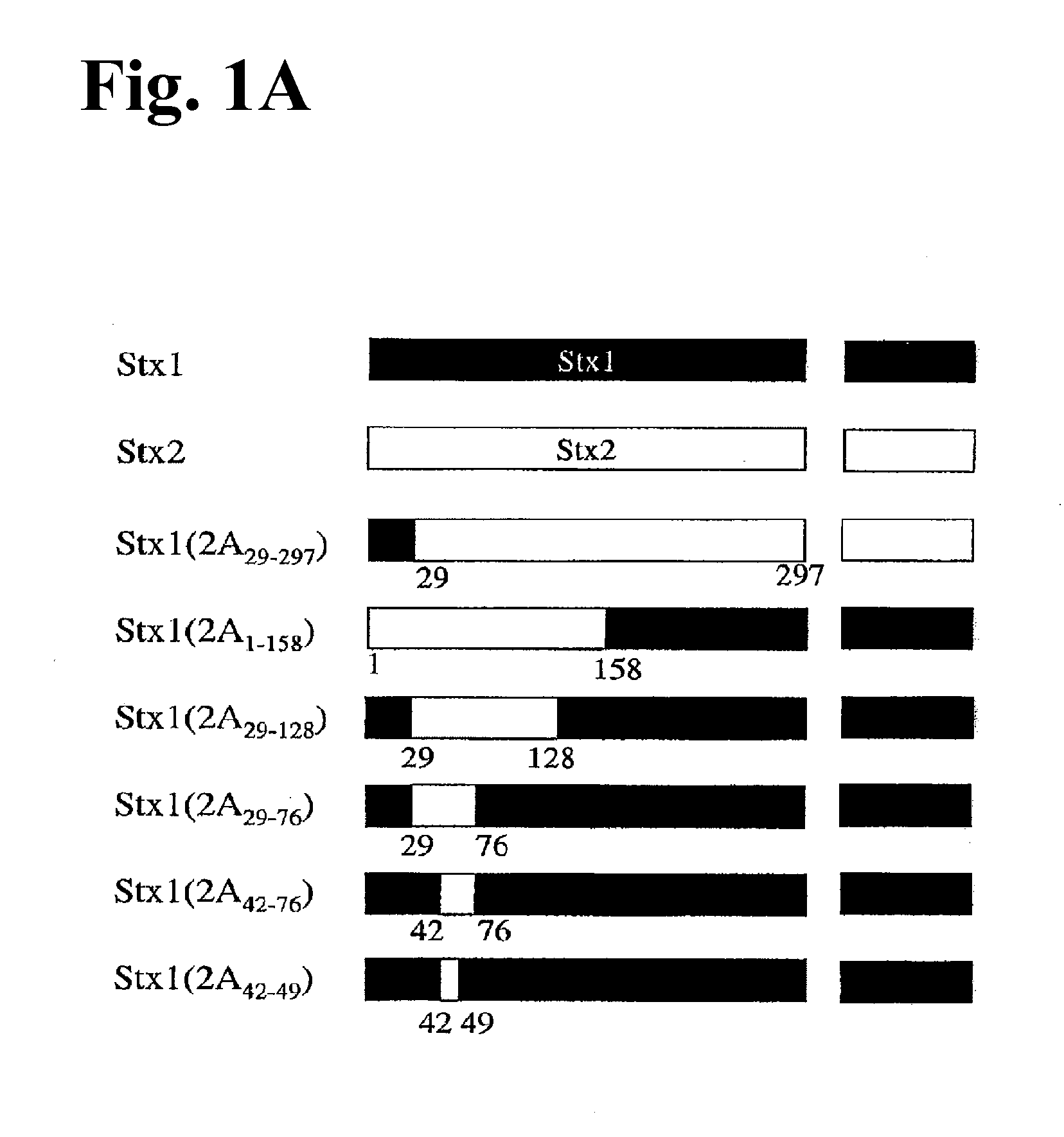
The invention is based on the discovery of the epitope in the Stx2 protein for the 11 E1O antibody. The invention features compositions containing non-full length Stx2 polypeptides that include the 11 E1O monoclonal antibody epitope. The invention also features methods of producing anti-Stx2 antibodies specific for the 11 E1O epitope of the Stx2 protein. Additionally, the invention features methods for treating a subject having, or at risk of developing, a Shiga toxin associated disease (e.g., hemolytic uremia syndrome and diseases associated with E. coli and S. dysenteriae infection) with a polypeptide that includes the 11 E1O epitope or with an anti-Stx2 antibody developed using the methods of the invention. Furthermore, the invention features the detection of Stx2 in a sample using the antibodies developed using the methods of the invention.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Loop-mediated isothermal amplification fast detecting reagent kit and method for O157:H7 coliform
InactiveCN101487057AShort detection cycleShort timeMicrobiological testing/measurementMicroorganism based processesAntigenLoop-mediated isothermal amplification
The invention provides a quick detecting kit that is used for loop-mediated isothermal augmentation of O157:H7 coliform, designs LAMP primers respectively for O157:H7 O-antigen encoding rfbE, Shiga-like toxin stx2 and H flagellar antigen encoding flic gene, provides a quick detecting kit and a detecting method with strong specificity and high sensitivity that are used for loop-mediated isothermal augmentation of O157:H7 coliform, and has following essential advantages: (1) expensive and precise instruments and equipments are unnecessary, and only a metal pyrolyzer / temperature-constant water bathing device is required; (2) the detecting kit and the detecting method consume less time, and can finish reaction in about 1 hour at constant temperature; (3) a result can be determined by observing color changes of a reaction system with naked eyes under white light, thus having simple and convenient operation; and (4) the method is simple, easy, quick, specific and sensitive, and has wider application prospect in quick detection aspects.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Gene chip and kit for detecting diarrheagenic escherichia coli in food and clinical samples
InactiveCN101760514AImprove accuracyGood repeatabilityMicrobiological testing/measurementEnteroinvasive Escherichia coliSolid phases
The invention provides a gene chip and a kit for detecting diarrheagenic escherichia coli in food and clinical samples. The gene chip comprises a solid-phase carrier and an oligonucleotide probe fixed on the solid-phase carrier, wherein the oligonucleotide probe contains DNA fragments and the complementary DNA or RNA sequence selected from eae genes of enteropathogenic escherichia coli (EPEC), lt, STap and STah genes of enterotoxigenic escherichia coli (ETEC), stx1 and stx2 of enterohaemorrhagic escherichia coli (EHEC), ipaH genes of enteroinvasive escherichia coli (EIEC), escherichia coli O157wzy genes and escherichia coli 16s genes. The invention further provides the kit containing the gene chip. The utilization of the gene chip and the kit for detecting the diarrheagenic escherichia coli has the advantages of simple operation, high accuracy and strong repeatability.
Owner:TIANJIN BIOCHIP TECH CO LTD +1
A set of oligonucleotide probe for detecting intestinal hemorrhage type colibacillus and vibrio cholerae
InactiveCN1661113AAccurate detectionEfficient detectionMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseFood poisoning
A set of oligonucleotide probe for detecting the enterohemorrhagic E. coli O157:H7 and cholera vibrio O139 is designed on the Shiga-like toxin generating gene stx1 or stx2 and the beta-glucuronidase gene uidA of said enterohemorrhagic E coli O157:H7 and the enterotoxin A subset (ctxA), toxicity coordinate pilus A subset (tcpA) and glucosyltransferase (LPS gt) genes of cholera vibrio O139, and has high sensibility and specificity. It can be used to detect the enterohemorrhagic E. coli O157:H7, cholera vibrio O139 and other pathogenic genes from the specimen.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Multiple qPCR (quantitative Polymerase Chain Reaction) method for rapidly detecting five kinds of diarrheogenic Escherichia coli, kit and application thereof
InactiveCN107760766AComprehensive detection coverageAvoid missing detectionMicrobiological testing/measurementDNA/RNA fragmentationEscherichia coliFluorescence
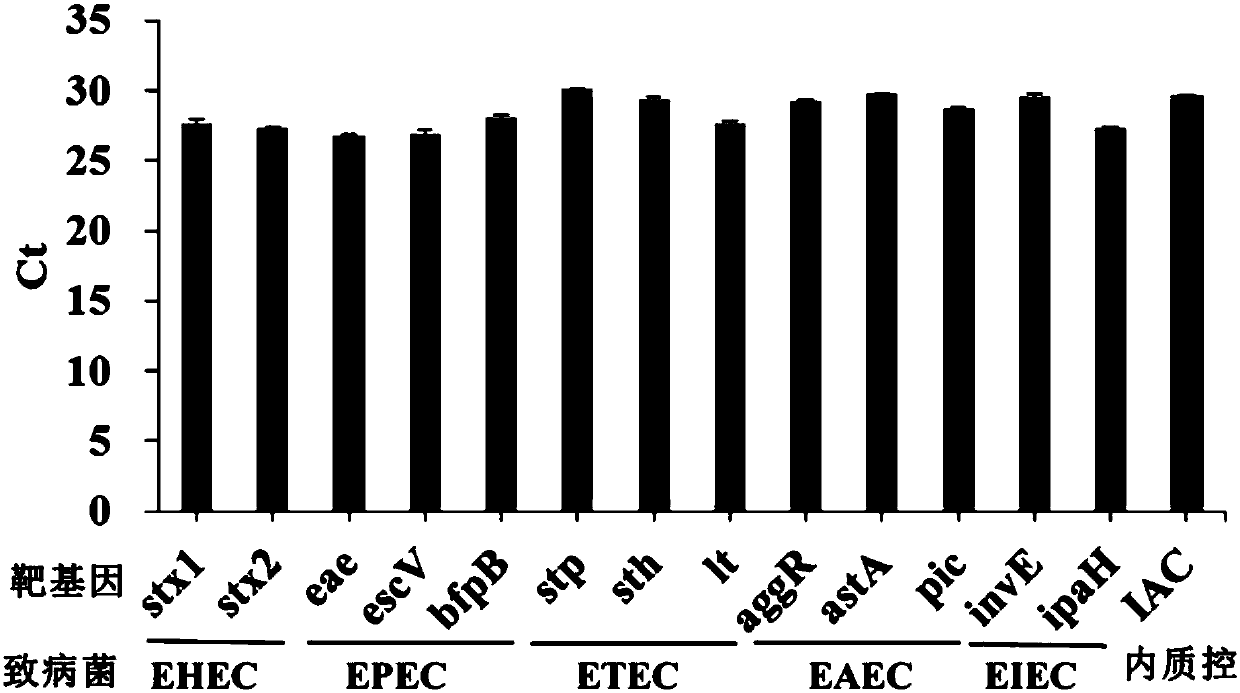
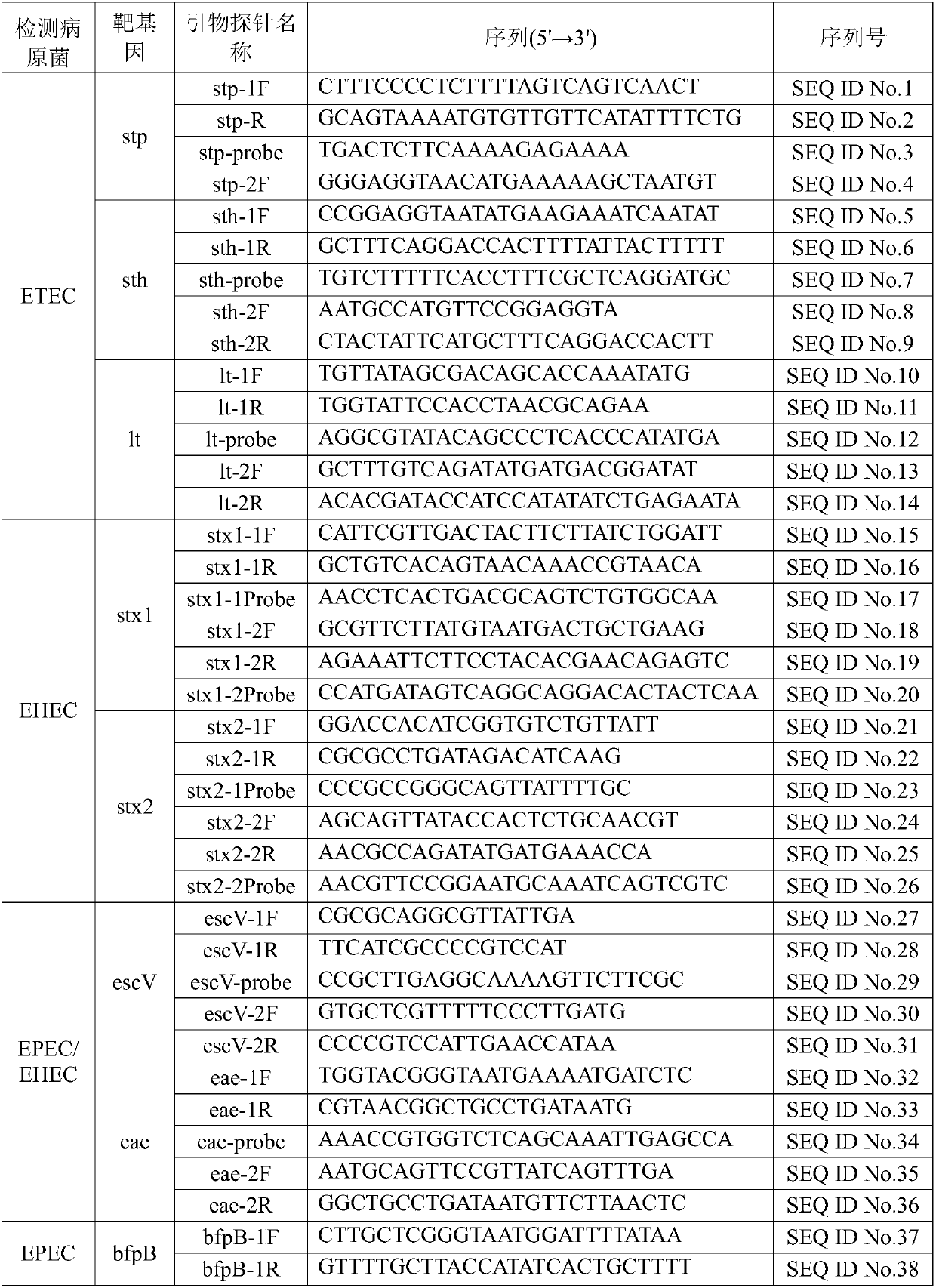
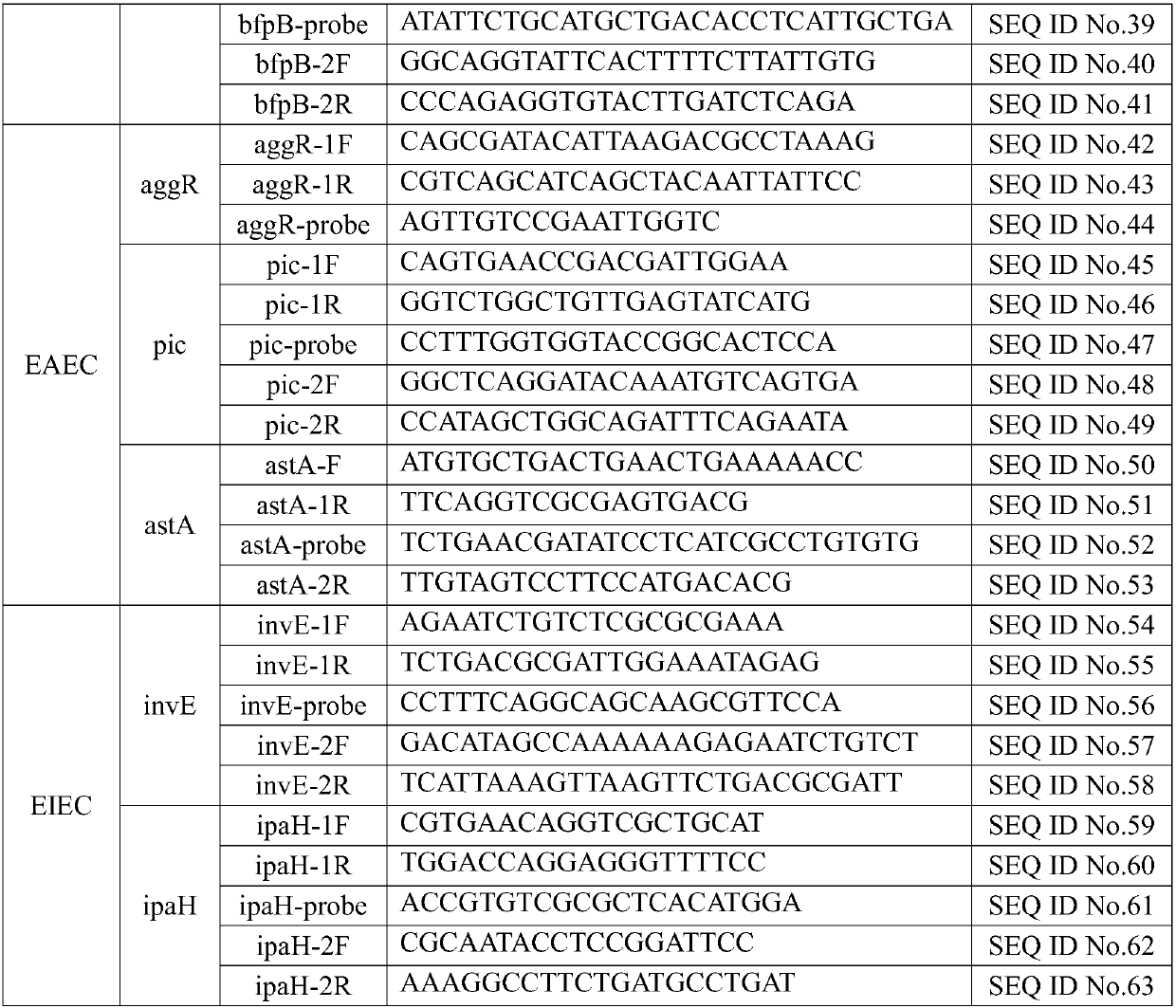
The invention discloses a kit for rapidly detecting five kinds of diarrheogenic Escherichia coli through multiple qPCR (quantitative Polymerase Chain Reaction). The kit comprises primer and probe groups capable of respectively performing specific amplification on eae genes of EPEC or EHEC, escV genes of EPEC or EHEC, bfpB genes of EPEC, stx1 or stx2 genes of EHEC lt, sth or stp genes of ETEC, aggR, astA or pic genes of EAEC and internal quality control IAC genes. Moreover, the invention further discloses a method and application for rapidly detecting the five kinds of diarrheogenic Escherichiacoli by using the kit. A method for simultaneously detecting the five kinds of diarrheogenic Escherichia coli in foods in the same reaction tube by virtue of fourteen real-time fluorescence quantitative PCR is realized, all characteristic genes in the national standard of the five DEC (diarrheogenic Escherichia coli) can be totally detected, and phenomena such as missing detection and false negative are avoided. The kit has the advantages of being short in detection time, simple and convenient to operate, easily decipherable in results, high in sensitivity, excellent in specificity and the like.
Owner:GWP BIOTECHNOLOGIES INC
Detection reagent for shiga toxin family gene of entero-hemorrhagic Escherichia coli
InactiveUS7345161B2Superior speed and specificitySugar derivativesMicrobiological testing/measurementEscherichia coliBase J
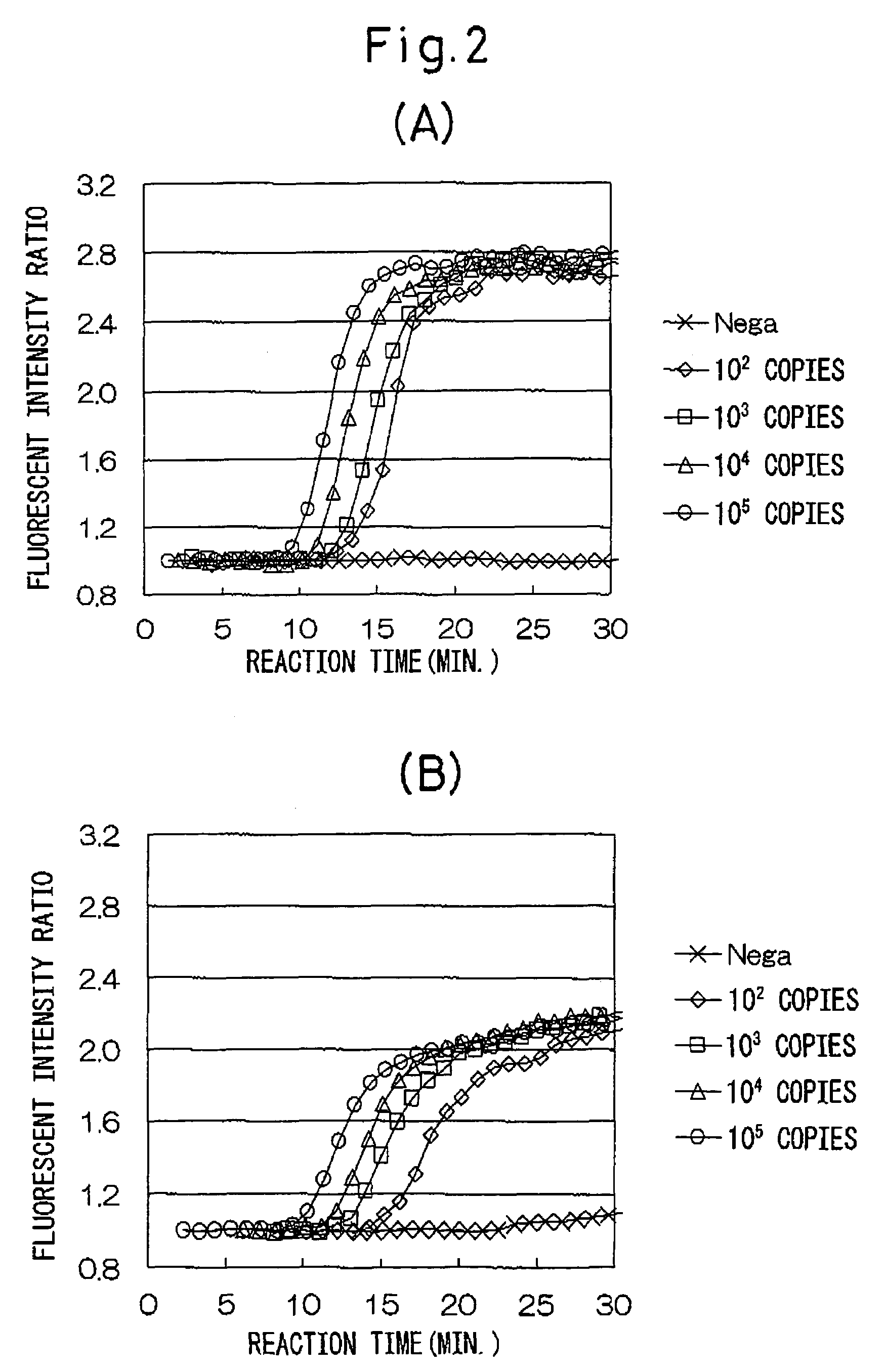
The present invention provides a combination of oligonucleotides preferable for composing a rapid and specific gene testing reagent for Shiga toxin family gene type 2 (stx) of entero-hemorrhagic Escherichia coli (EHEC). More specifically, the present invention provides a method for detecting stx2 RNA of EHEC by specifically amplifying only stx2 RNA using a primer having a sequence homologous or complementary to a base sequence specific for stx2 gene of EHEC and located at sites free of alterations between genotypes, and an oligonucleotide that binds to a specific site of stx2 RNA.
Owner:TOSOH CORP
Polypeptide TF1 for inhibiting activity of shiga toxin (stx) 2 and coding gene and application of stx2
The invention discloses a polypeptide TF1 for inhibiting activity of shiga toxin (stx) 2 and a coding gene and application of stx2. The polypeptide provided by the invention is named as TF1 (also P1), and the amino acid sequence of the polypeptide is shown in the sequence 1 in a sequence table. The polypeptide P1 can be prepared into medicines to prevent and / or treat diseases caused by the stx2 or pathogenic bacteria producing the stx2.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Methods and compositions based on shiga toxin type 2 protein
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Multiplex PCR detection method and kit for Shiga toxin-producing Escherichia coli and application thereof
InactiveCN103343164AReduce infectionStrong specificityMicrobiological testing/measurementMicroorganism based processesEscherichia coliStaining
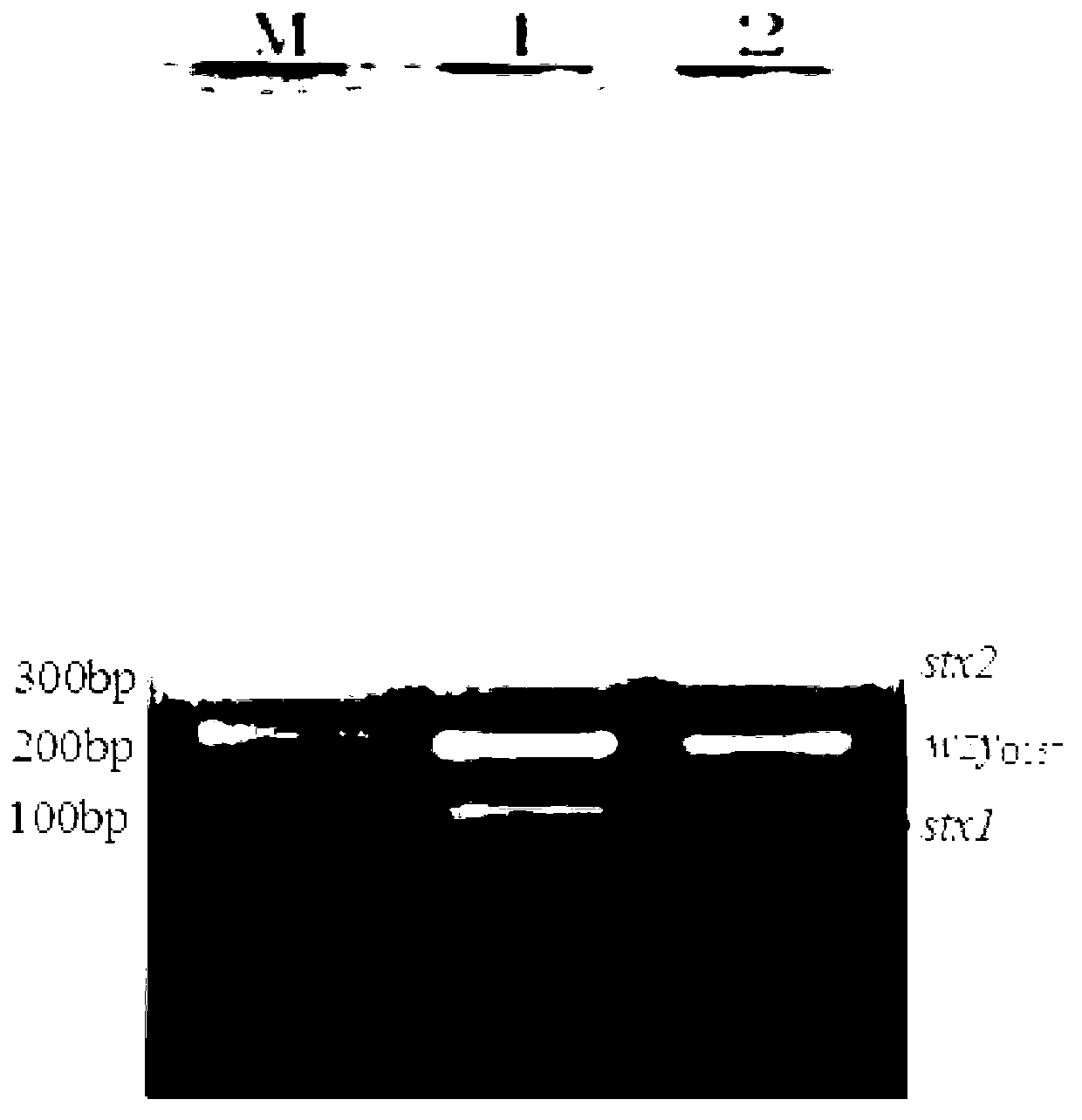
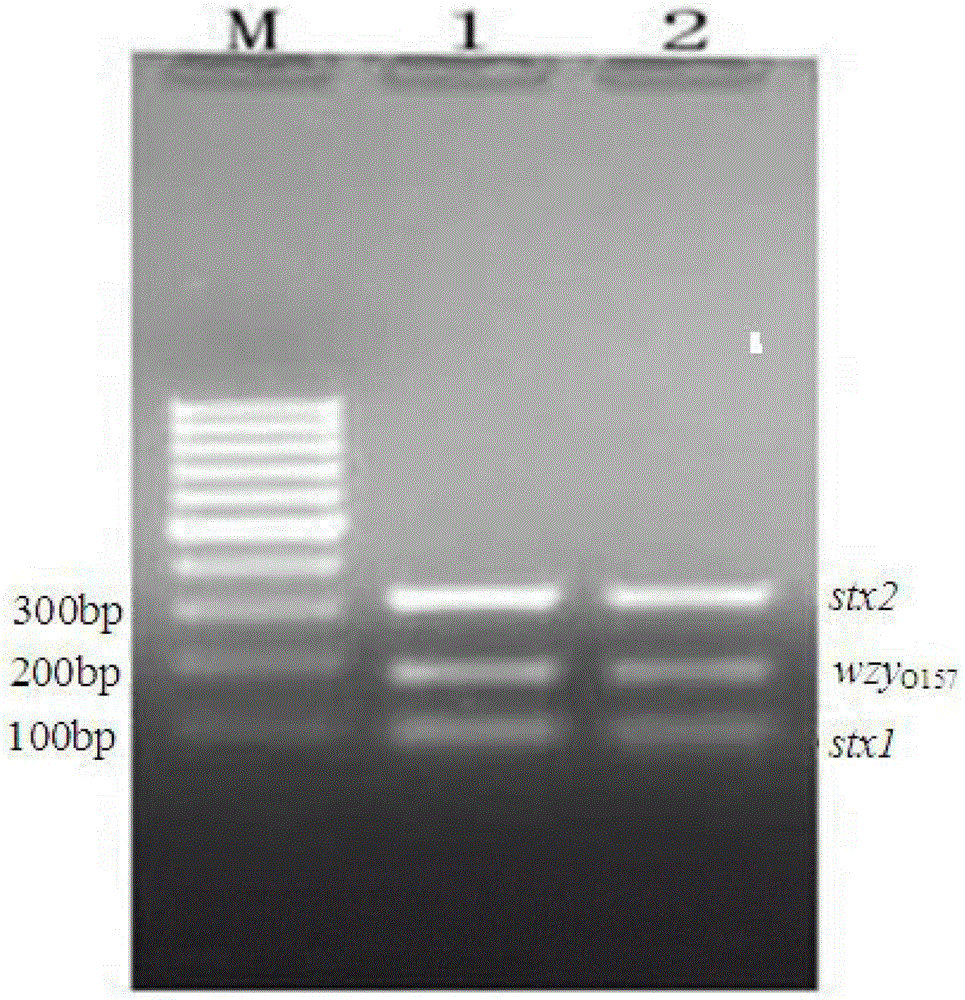
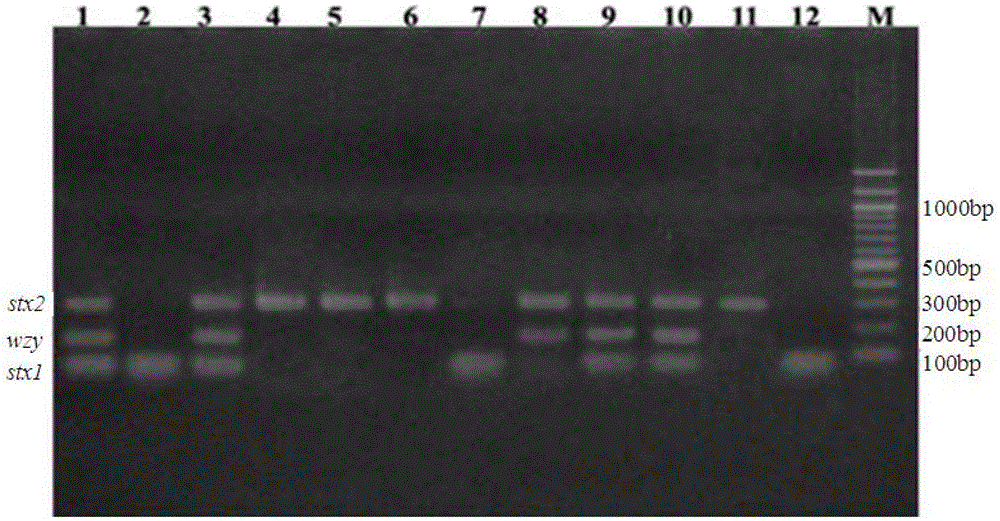
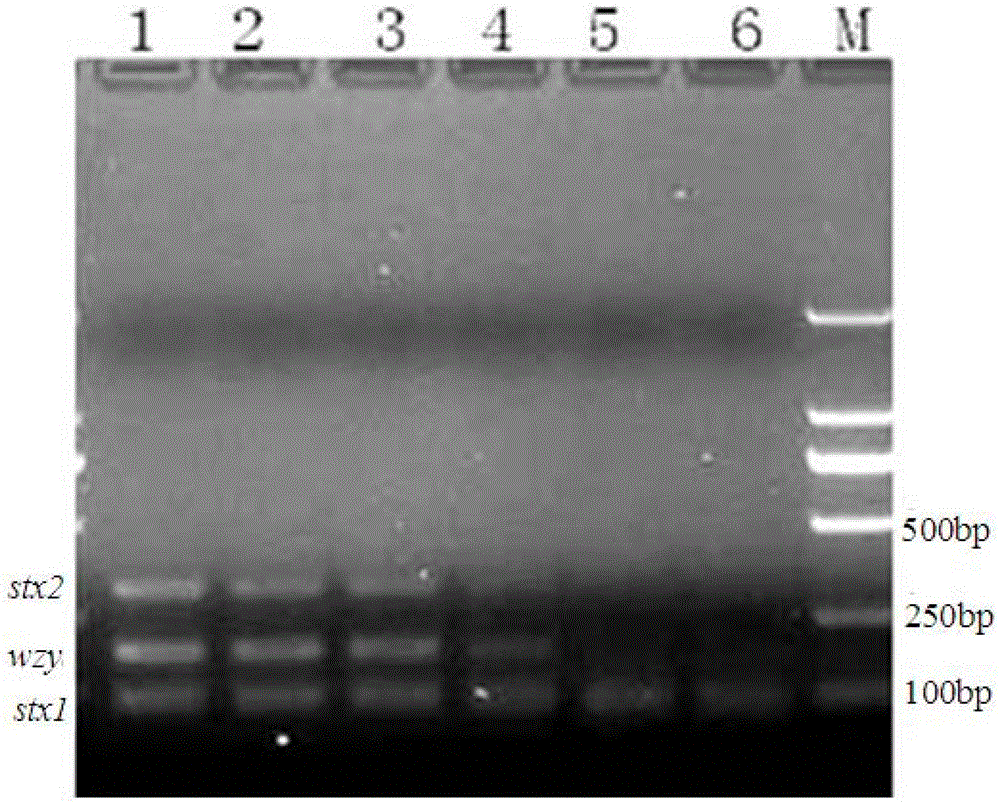
The invention discloses a multiplex PCR detection method and kit for Shiga toxin-producing Escherichia coli and application thereof. The method and the kit thereof can simultaneously detect the genes consisting of stx1, stx2 and wzyO157 through a triplex PCR reaction, electrophoresis staining and ultraviolet visualization, subtypes consisting of stx1 and stx1c can be amplified by using a detection primer for the stx1 gene, subtypes consisting of stx2, stx2c and stx2d can be amplified by using a detection primer for the stx2 gene, and an O157 serotype strain can be identified by using a detection primer for the wzyO157 gene. According to the invention, the method and the kit can effectively detect stx and main variants thereof and can be used for distinguishing O157 from other non-O157 Escherichia coli, for directly detecting Shiga toxin-producing Escherichia coli in a food sample and for identifying a clinical strain and a food isolate.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Methods, compositions, and kits for treating shiga toxin associated conditions
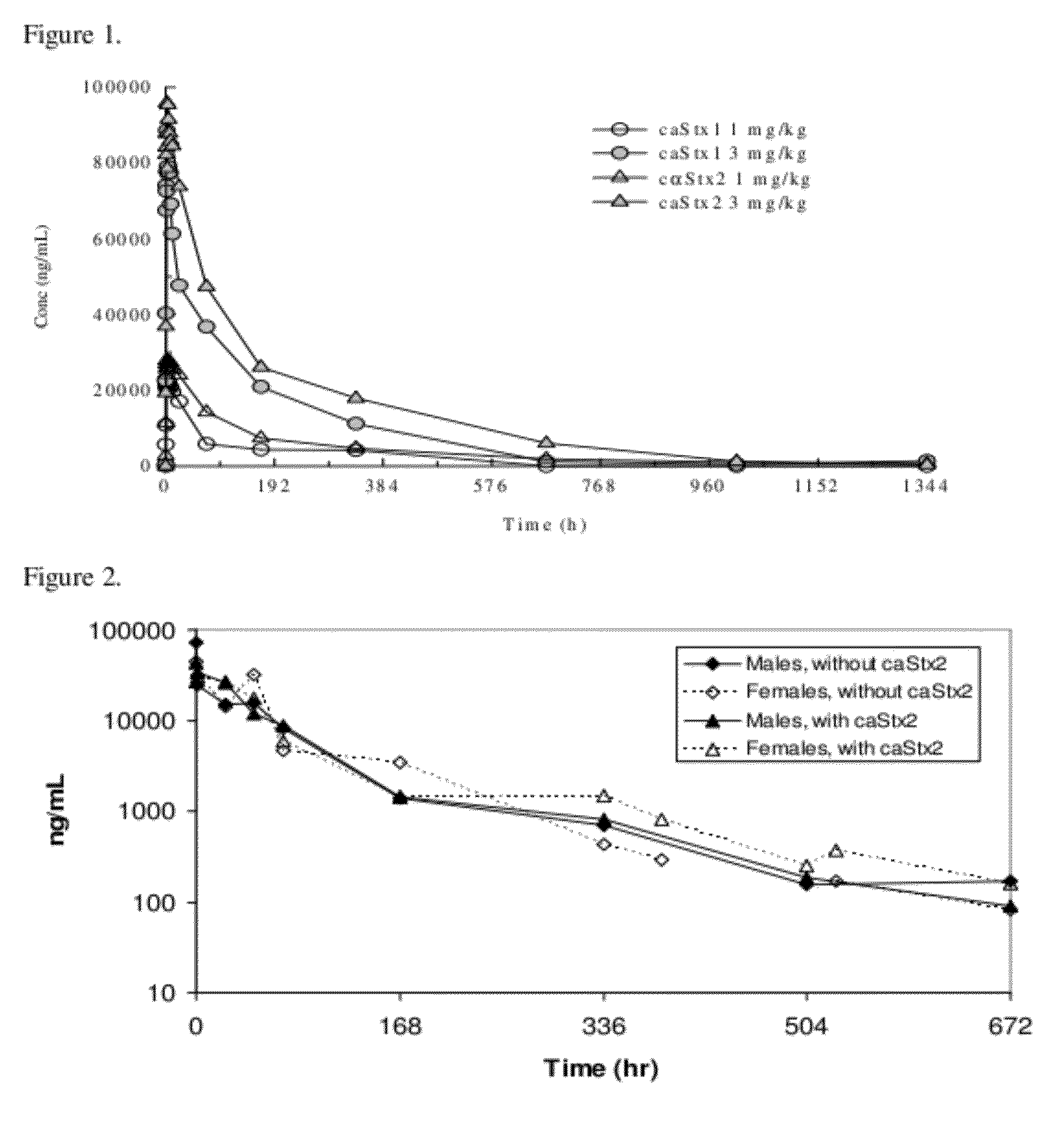
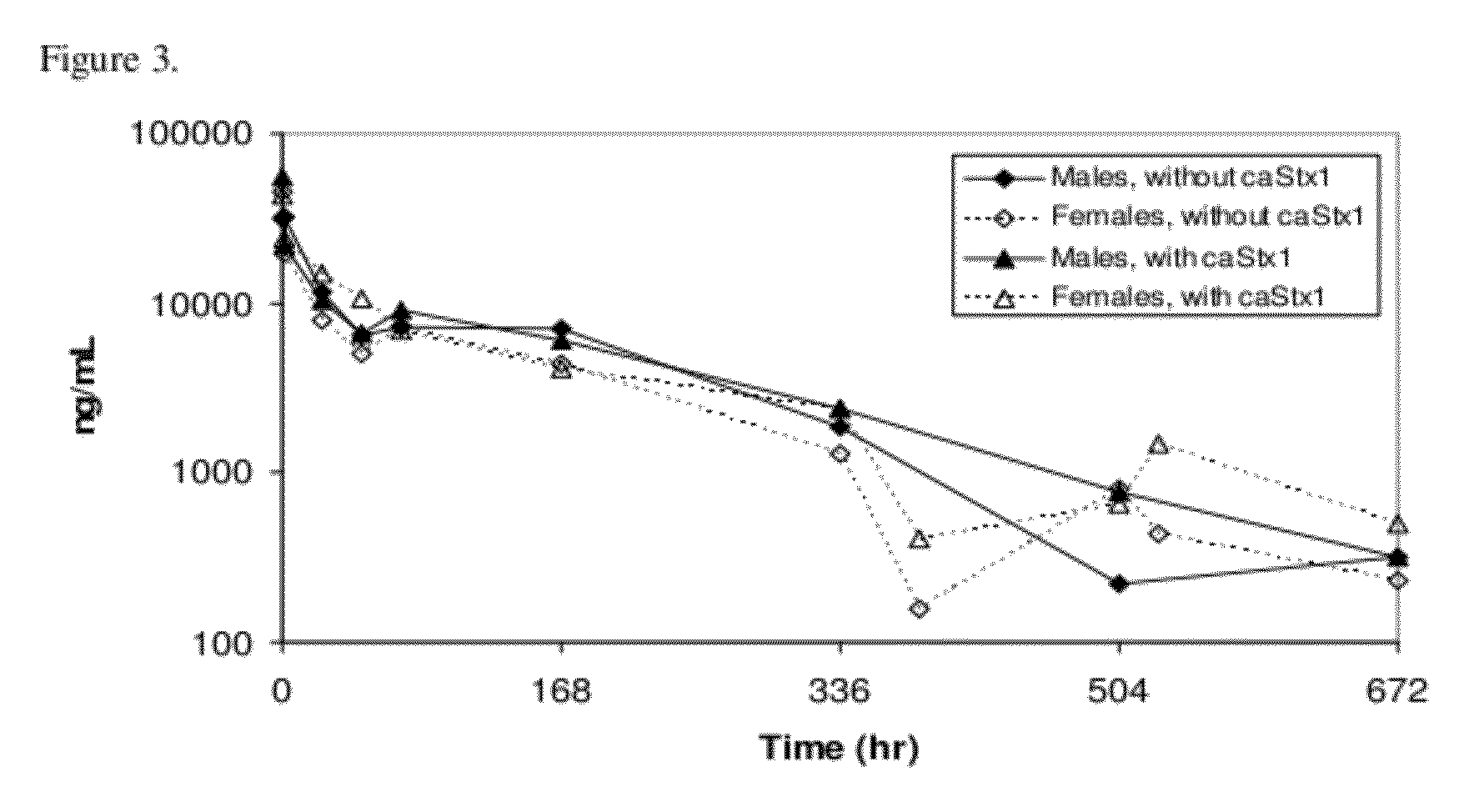
The invention features methods, compositions, and kits for treating a subject having a Shiga toxin associated disease with chimeric anti-Shiga Toxin 1 (cαStx1) and anti-Shiga Toxin 2 (cαStx2) antibodies.
Owner:THALLION PHARMA
Enterohemorrhagic Escherichia coli stx2 gene detection kit and its application method
InactiveCN102286620AHigh yieldHigh yield is high; 4. detection kit sensitivity of the present inventionMicrobiological testing/measurementSTX2Enterobacteriales
The present invention provides a detection kit for E.coli stx2 gene. The E.coli stx2 gene detection kit includes two pairs of primers designed based on the loop-mediated constant temperature amplification technology with the stx2 gene as the target gene. : inner primer FIP / BIP and outer primer F3 / B3. The enterohaemorrhagic Escherichia coli stx2 gene detection kit of the present invention has more comprehensive detection effect and low missed detection rate.
Owner:GUANGZHOU HUAFENG BIOTECH +1
Nontoxic shiga-like toxin mutant compositions and methods
Disclosed are nontoxic mutants of Shiga-like toxin (Stx1 or Stx2), nucleic acids encoding them, compositions containing the mutants and methods of using the mutants in connection with hemolytic euremic syndrome (HUS). Also disclosed are methods of treating HUS using L3 protein fragments, the nontoxic Stx1 or Stx2 mutants, or combinations thereof.
Owner:RUTGERS THE STATE UNIV
Major virulence factor detection and verocytontoxin type 2 subtype from clinical e. coli isolates using a one-step multiplex pcr
InactiveUS20060051751A1Sugar derivativesMicrobiological testing/measurementEscherichia coliVirulent characteristics
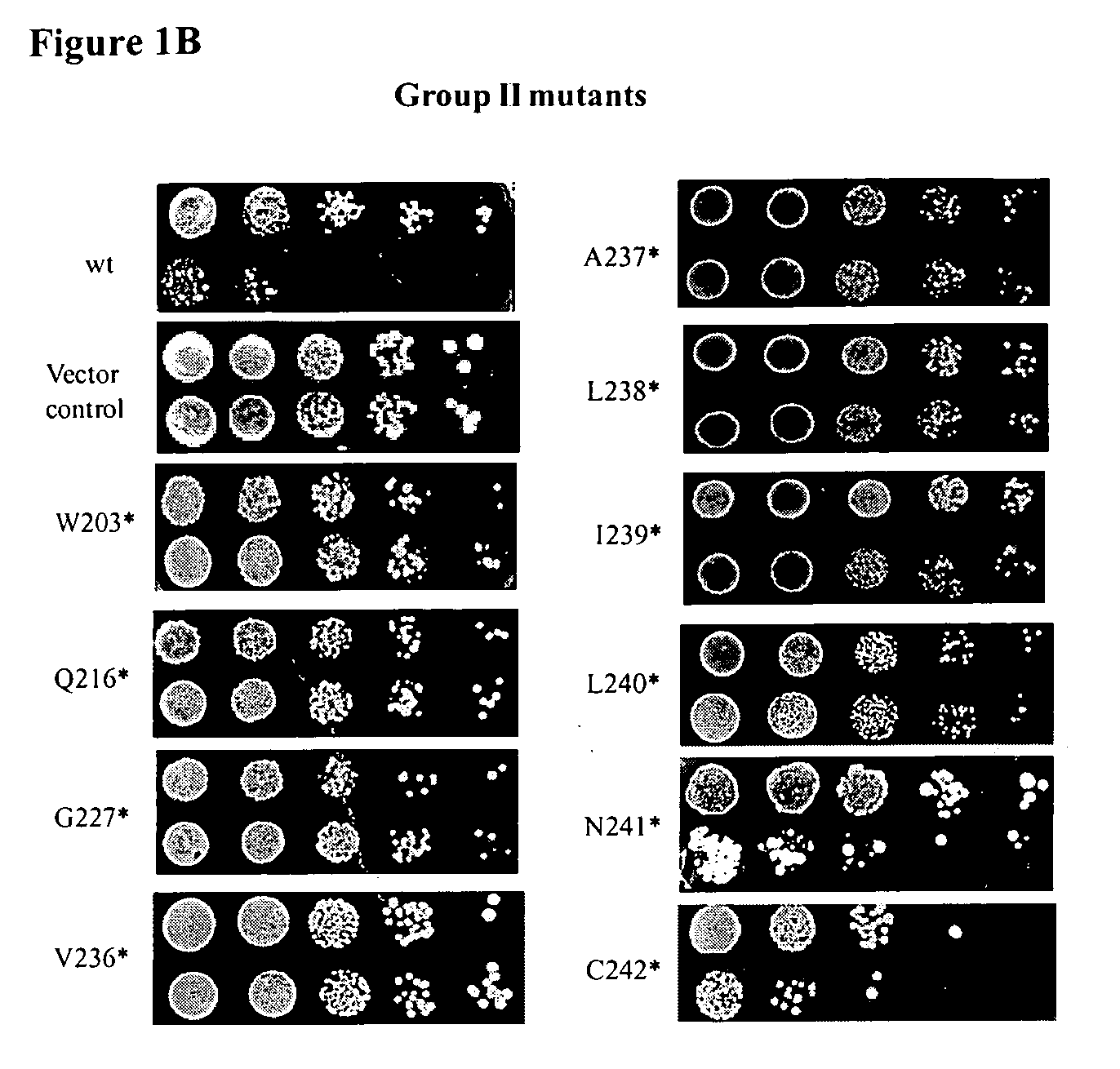
A single kit comprising 3 multiplex PCR assays that can detect in E. coli the presence of the 8 virulence genes: eaeA, EHEC-HlyA, Stx1 (VT1), Stx2 (VT2), Stx2c (VT2c), Stx2d (VT2d), Stx2e (VT2e) and Stx2f (VT2f) is described. In addition, the kit can detect the two critical serotypes (O157 and H7) and identify the species (Escherichia coli) simultaneously using a one step reaction. Following evaluation in our hands, this PCR kit has been used to detect the above 11 components of disease-causing E. coli in a fast, accurate, reliable and specific fashion. These kits can be used on bacterial isolates and has the potential for use directly on foods and environmental samples.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
RPA primer composition and method for detecting shiga toxin-producing Escherichia coli
InactiveCN111500753AHigh-throughput and convenient test resultsQuick checkMicrobiological testing/measurementMicroorganism based processesBiotechnologyEscherichia coli
The invention discloses an RPA (recombinase polymerase amplification) primer composition and method for detecting shigella toxin-producing Escherichia coli. The primer composition is used for detecting nine gene subtypes, namely, stx1a, stx1c, stx1d, stx2a, stx2b, stx2c, stx2d, stx2e and stx2g and comprises a primer pair with sequences shown as SEQ ID NO: 1 and SEQ ID NO: 4 and a primer pair withsequences shown as SEQ ID NO: 9 and SEQ ID NO: 12. The method provided by the invention has the advantages of simple operation, high specificity and high sensitivity, is suitable for on-site rapid detection, can complete nucleic acid detection within 20 min, and can provide a rapid screening result for supervision and inspection of listed products and emergency treatment of supervision departments. Meanwhile, the primer and probe combination designed and screened for the first time can effectively detect nine stx gene subtypes, namely stx1a, stx1c, stx1d, stx2a, stx2b, stx2c, stx2d, stx2e andstx2g genes. And in combination with a centrifugal disc type micro-fluidic chip technology, rapid and high-throughput detection on STEC in foods, medicines and livestock breeding products can be realized within an extremely small volume (5 microliters ).
Owner:SHANGHAI INST FOR FOOD & DRUG CONTROL
Primer and probe group for detecting enterohemorrhagic escherichia coli, kit and PCR (polymerase chain reaction) detection method
InactiveCN105779585AReliable detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEscherichia coliFluorescence
The invention relates to a primer and probe group for detecting enterohemorrhagic escherichia coli, a kit and a PCR (polymerase chain reaction) detection method. Based upon PCR detection on stx1 gene, stx2 gene and eae gene in the enterohemorrhagic escherichia coli, the primer and probe group, which adopts real-time fluorescence PCR detection, is completely consistent with the genotype of an escherichia coli strain in amplification of the strain and is free from specific amplification; therefore, the primer and probe group, which adopts real-time fluorescence PCR detection, has the advantages of being excellent in specificity and real-time fluorescence PCR on on three genes in the enterohemorrhagic escherichia coli, namely the stx1 gene, the stx2 gene and the eae gene; and meanwhile, the corresponding PCR detection method has the characteristics of being strong in specificity, high in sensitivity, rapid and reliable in detection and simple and convenient to operate.
Owner:珠海出入境检验检疫局检验检疫技术中心
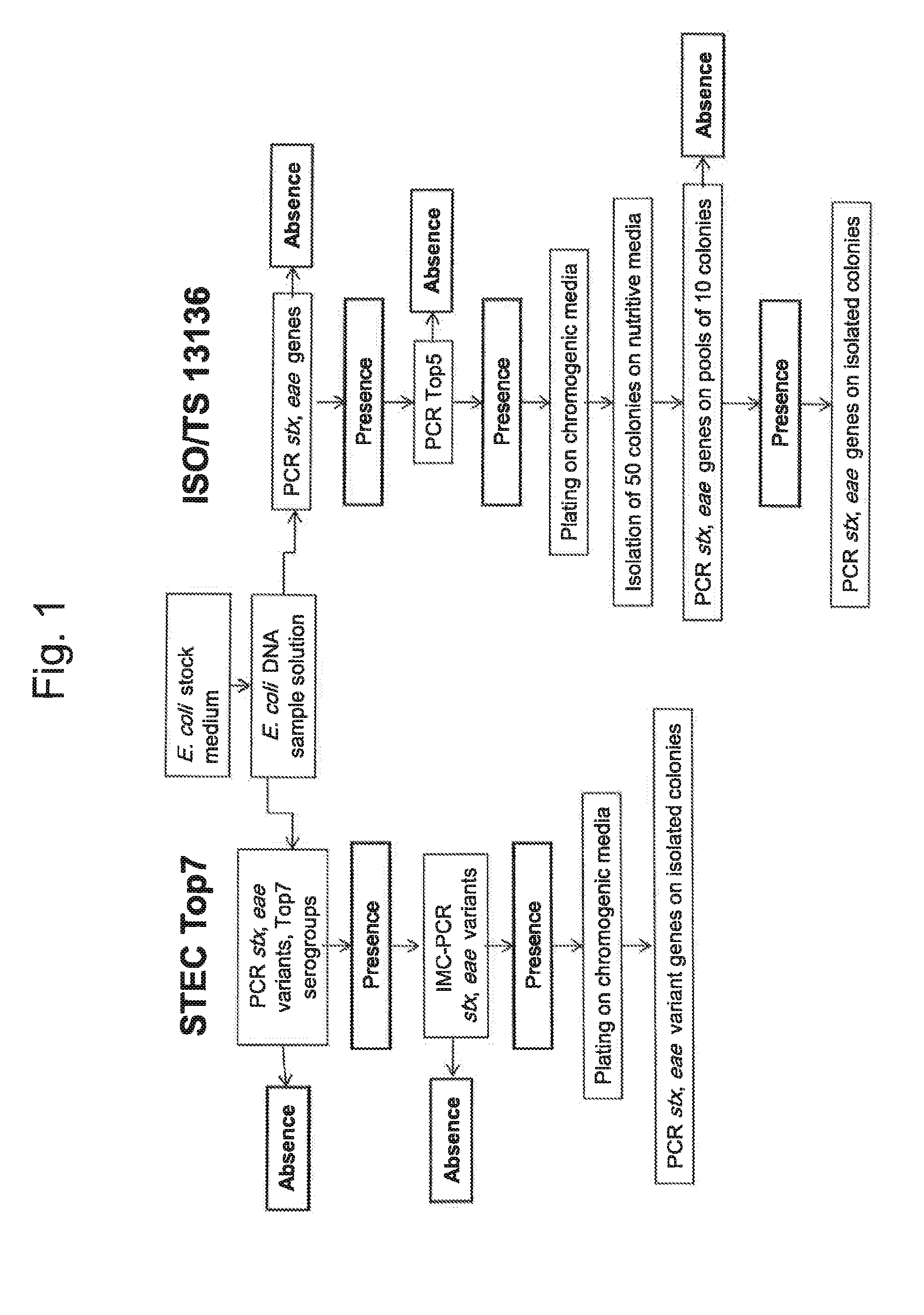
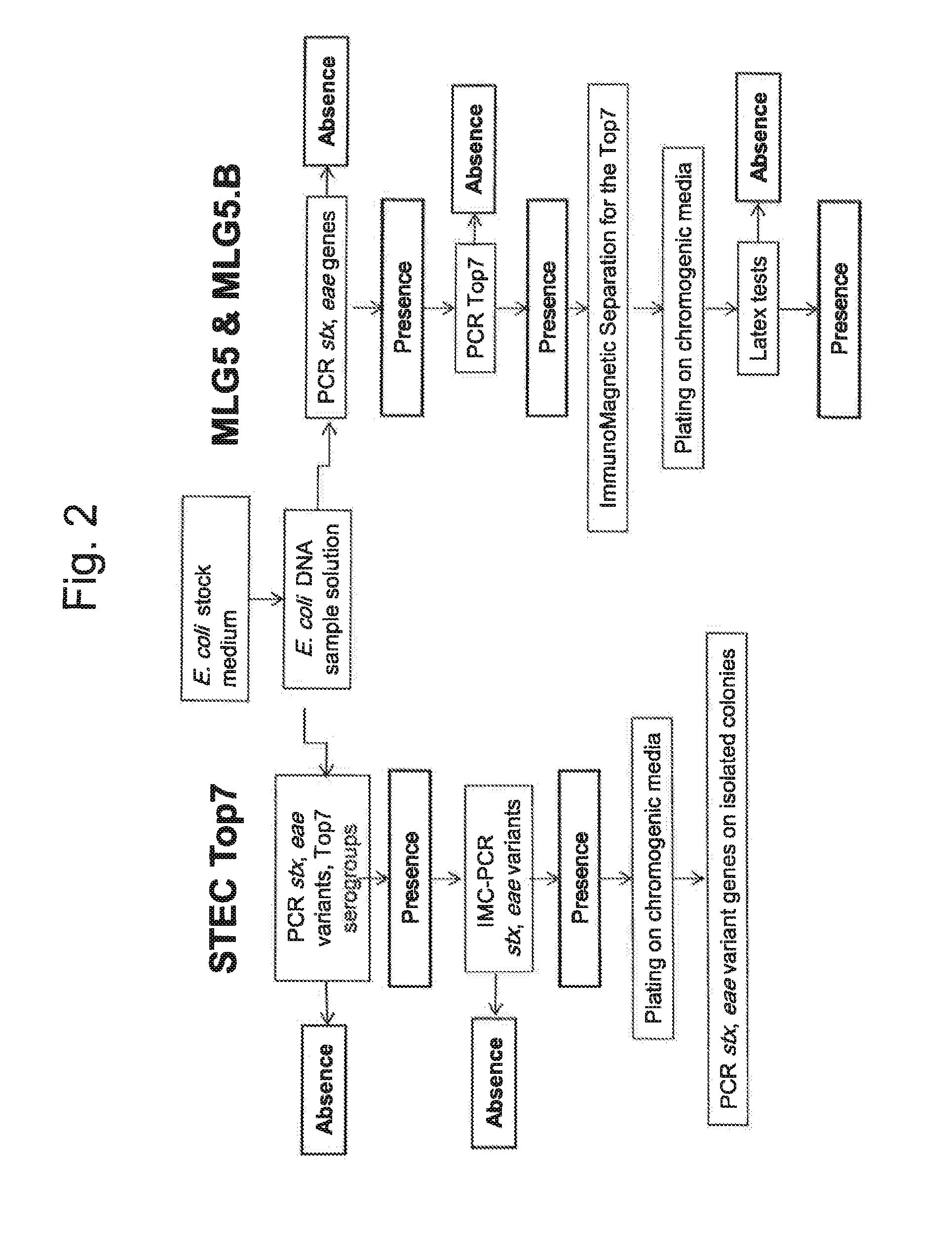
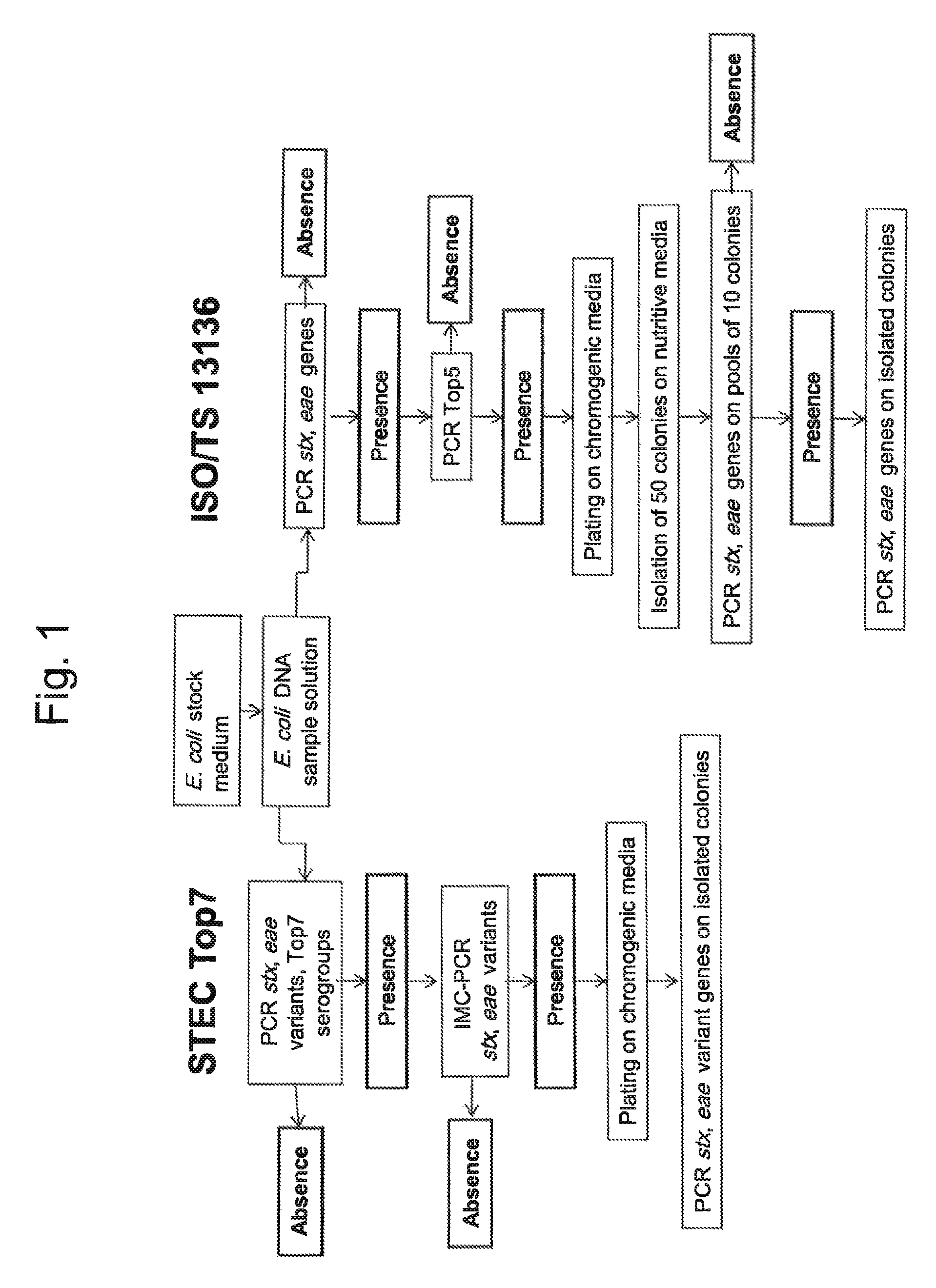
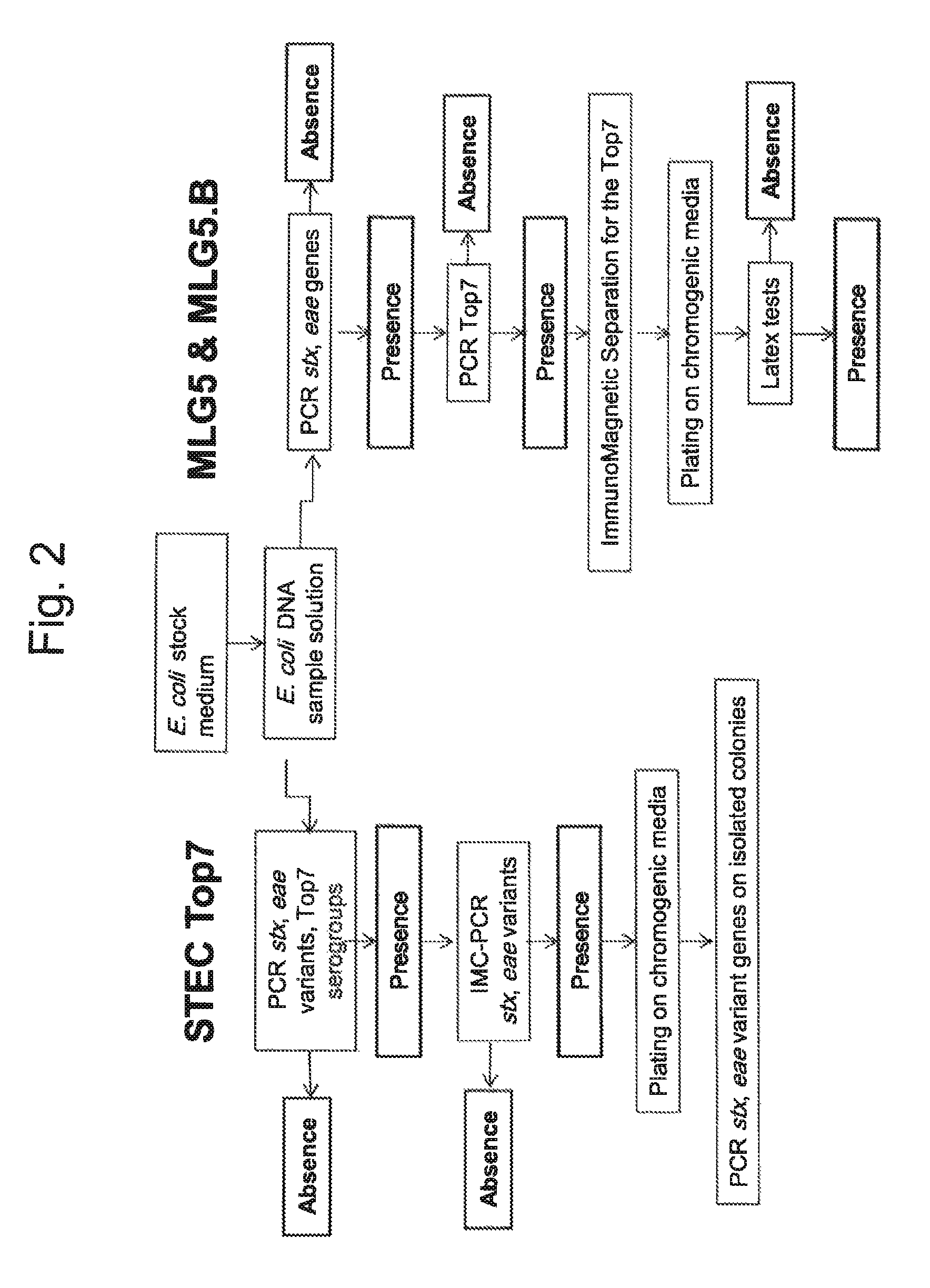
Method for determining the presence or absence of shiga toxin-producing escherichia coli (STEC) in a food sample
ActiveUS20150072343A1Reliable resultsShort timeBioreactor/fermenter combinationsBiological substance pretreatmentsEscherichia coliSerum ige
A method for detecting the presence or absence of pathogenic STEC in food samples includesincubating the food sample in a culture medium to obtain an E. coli stock medium;lysing the E. coli to obtain a E. coli DNA; andsubjecting the DNA to PCR, and amplifying the following genes or fragments thereof:stx1 and / or stx2 encoding Shiga toxin 1 and Shiga toxin 2,for each STEC serogroup to be determined, the subtype of the eae gene of that serogroup encoding an intimin, andfor each STEC serogroup to be determined, a biomarker gene specific for that serogroup;and, if stx1 and / or stx2 and the eae gene and the specific biomarker gene for at least one serogroup are amplified in the PCRs:verifying the presence of the one or more specific serogroups of STEC by PCRs on concentrates of the E. coli stock medium.
Owner:PALL GENEDISC TECH
Method for determining the presence or absence of shiga toxin-producing Escherichia coli (STEC) in a food sample
ActiveUS9580757B2Short timeReliable resultsMicrobiological testing/measurementEscherichia coliSalmonella serotype typhi
A method for detecting the presence or absence of pathogenic. STEC in food samples includesincubating the food sample in a culture medium to obtain an E. coli stock medium;lysing the E. coli to obtain a E. coli DNA; andsubjecting the DNA to PCR, and amplifying the following genes or fragments thereof:stx1 and / or stx2 encoding Shiga toxin 1 and Shiga toxin 2,for each STEC serogroup to be determined, the subtype of the eae gene of that serogroup encoding an intimin, andfor each STEC serogroup to be determined, a biomarker gene specific for that serogroup;and, if stx1 and / or stx2 and the eae gene and the specific biomarker gene for at least one serogroup are amplified in the PCRs:verifying the presence of the one or more specific serogroups of STEC by PCRs on concentrates of the E. coil stock medium.
Owner:PALL GENEDISC TECH
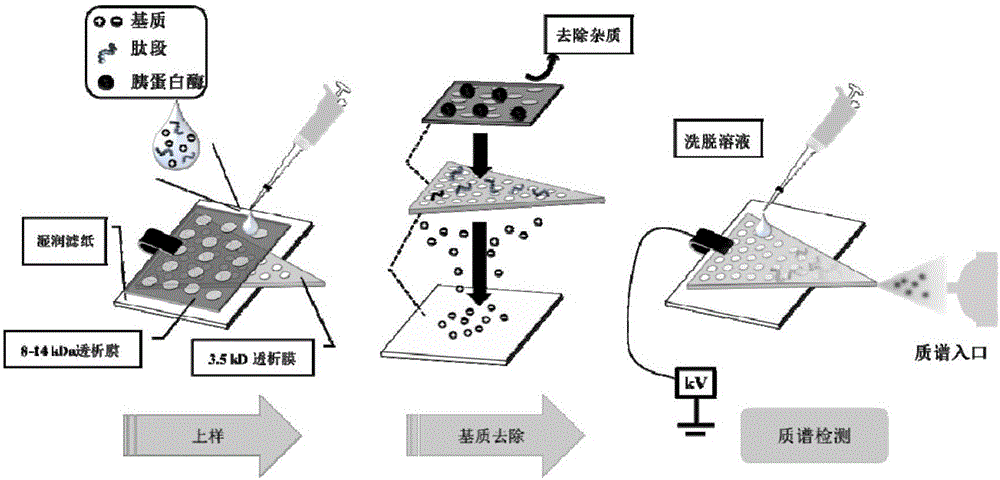
Qualitative and quantitative detection method for bacterium AB5 enterotoxin proteins
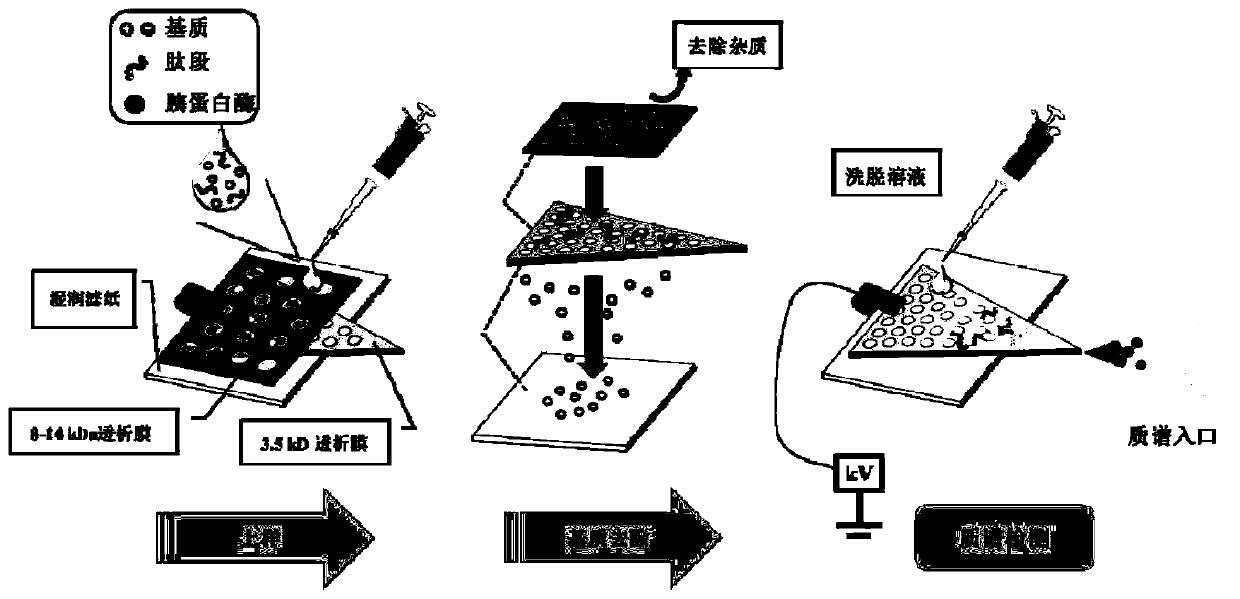
InactiveCN106841638ASave on desalination stepsHigh detection sensitivityMaterial analysis by electric/magnetic meansBiological testingUltrafiltrationEnterotoxin
The invention relates to a qualitative and quantitative detection method for bacterium AB5 enterotoxin proteins. The method comprises the following steps: extracting a protein in a to-be-detected sample by adopting ultrafiltration so as to obtain a sample protein; performing enzyme digestion on the sample protein; detecting AB5 enterotoxin specific peptide fragments by adopting membrane electrospray ionization mass spectrometry, and qualitatively and quantitatively determining existence of AB5 enterotoxin coding genes on the peptide fragment level, wherein the AB5 enterotoxins respectively refer to Ctx, LT, Stx1 and Stx2 of which sequences are respectively shown as SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.5 and SEQ ID NO.7. With the adoption of the membrane electrospray ionization mass spectrometry, matrix effects caused by small molecules introduced by enzyme digestion are effectively removed, and a desalting step in the conventional mass spectrometry process is saved, so that the method disclosed by the invention can realize rapid detection at extremely high sensitivity.
Owner:ICDC CHINA CDC
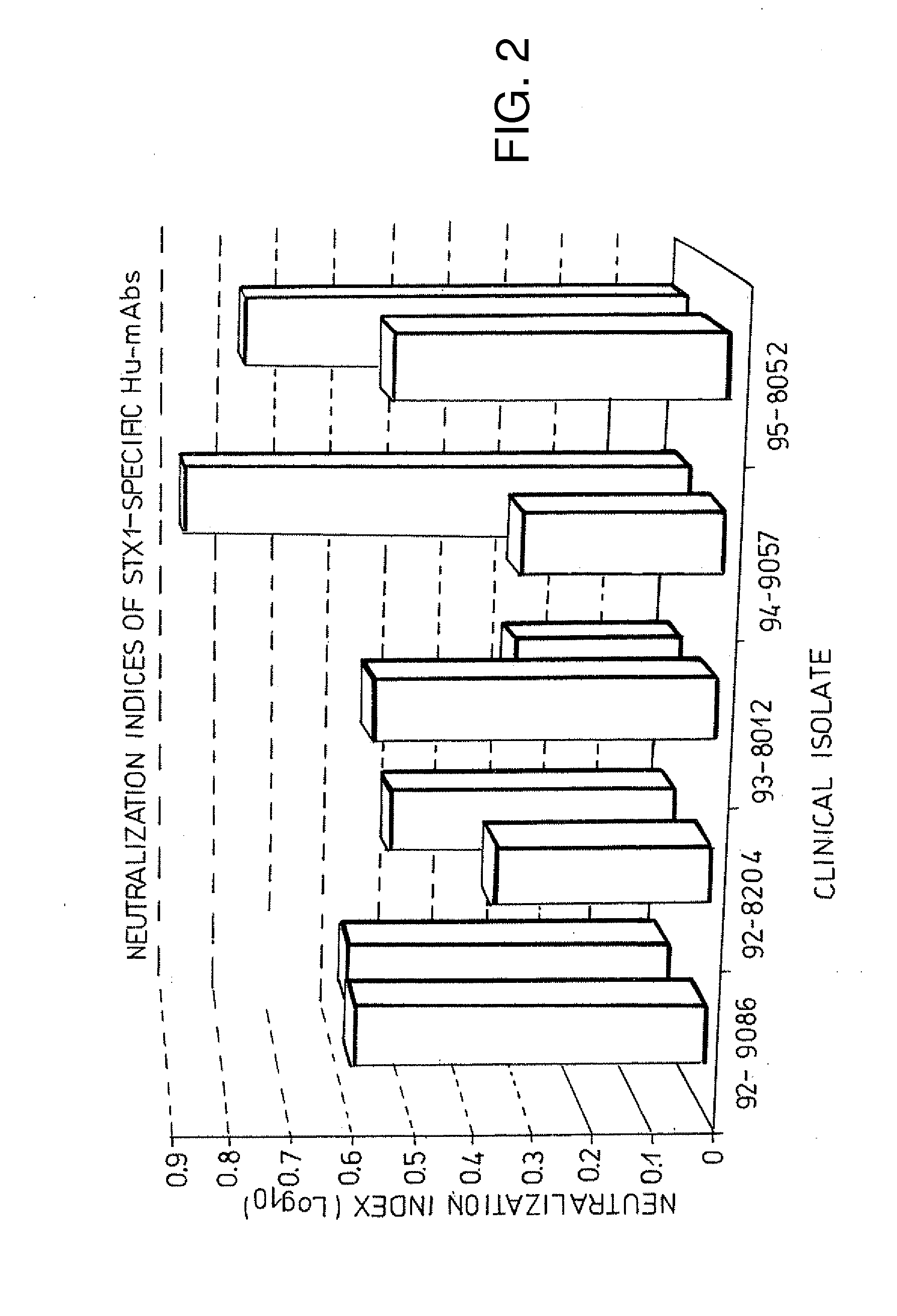
High affinity monoclonal antibodies for detection of Shiga toxin 2 (STX2)
High affinity monoclonal antibodies against Stx2 and hybridomas that produce such antibodies are described. The antibodies may be used in a kit for detecting Stx2 and variants thereof in a sample as well as neutralization of Shiga toxin in vivo.
Owner:US SEC AGRI
Methods and compositions based on shiga toxin type 2 protein
The invention is based on the discovery of the epitope in the Stx2 protein for the 11 E1O antibody. The invention features compositions containing non-full length Stx2 polypeptides that include the 11 E1O monoclonal antibody epitope. The invention also features methods of producing anti-Stx2 antibodies specific for the 11 E1O epitope of the Stx2 protein. Additionally, the invention features methods for treating a subject having, or at risk of developing, a Shiga toxin associated disease (e.g., hemolytic uremia syndrome and diseases associated with E. coli and S. dysenteriae infection) with a polypeptide that includes the 11 E1O epitope or with an anti-Stx2 antibody developed using the methods of the invention. Furthermore, the invention features the detection of Stx2 in a sample using the antibodies developed using the methods of the invention.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
CPA detection primer for escherichia coli shiga toxin type II, kit and method thereof
PendingCN110951896AQuick checkoutAccurate detectionMicrobiological testing/measurementMicroorganism based processesBiotechnologyEscherichia coli
The invention discloses a CPA detection primer for escherichia coli shiga toxin type II, a kit and a method thereof. The CPA detection primer is designed for the specific target sequence stx2 of the escherichia coli shiga toxin type II, a primer sequence is shown as SEQ ID NO.1-5, and the primer ensures the reliability of the detection result. The invention further provides a CPA detection kit forthe escherichia coli shiga toxin II type. The CPA detection kit has the advantages of high sensitivity, good applicability, good specificity, simplicity, convenience and rapidness in operation, accurate and reliable result, low detection cost and the like. The CPA detection primer or the CPA detection kit is used for carrying out a cross primer isothermal amplification reaction on a to-be-detected sample, a result can be directly judged and read by naked eyes through fluorescent dye, whether the to-be-detected sample contains the escherichia coli shiga toxin type II or not can be rapidly andaccurately detected, and the kit is particularly suitable for small and medium-sized units and field detection.
Owner:SOUTH CHINA UNIV OF TECH +1
Methods, compositions, and kits for treating shiga toxin associated conditions
The invention features methods, compositions, and kits for treating a subject having a Shiga toxin associated disease with chimeric anti-Shiga Toxin 1 (cαStx1) and anti-Shiga Toxin 2 (cαStx2) antibodies.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Shiga toxin-producing Escherichia coli multiplex PCR detection method, kit and application
InactiveCN103343164BEfficient detectionStrong specificityMicrobiological testing/measurementMicroorganism based processesEscherichia coliStaining
The invention discloses a multiplex PCR detection method and kit for Shiga toxin-producing Escherichia coli and application thereof. The method and the kit thereof can simultaneously detect the genes consisting of stx1, stx2 and wzyO157 through a triplex PCR reaction, electrophoresis staining and ultraviolet visualization, subtypes consisting of stx1 and stx1c can be amplified by using a detection primer for the stx1 gene, subtypes consisting of stx2, stx2c and stx2d can be amplified by using a detection primer for the stx2 gene, and an O157 serotype strain can be identified by using a detection primer for the wzyO157 gene. According to the invention, the method and the kit can effectively detect stx and main variants thereof and can be used for distinguishing O157 from other non-O157 Escherichia coli, for directly detecting Shiga toxin-producing Escherichia coli in a food sample and for identifying a clinical strain and a food isolate.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Qualitative and quantitative detection method of bacterial ab5 enterotoxin protein
InactiveCN106841638BQuick checkSave on desalination stepsMaterial analysis by electric/magnetic meansBiological testingEnzyme digestionUltrafiltration
The invention relates to a qualitative and quantitative detection method for bacterium AB5 enterotoxin proteins. The method comprises the following steps: extracting a protein in a to-be-detected sample by adopting ultrafiltration so as to obtain a sample protein; performing enzyme digestion on the sample protein; detecting AB5 enterotoxin specific peptide fragments by adopting membrane electrospray ionization mass spectrometry, and qualitatively and quantitatively determining existence of AB5 enterotoxin coding genes on the peptide fragment level, wherein the AB5 enterotoxins respectively refer to Ctx, LT, Stx1 and Stx2 of which sequences are respectively shown as SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.5 and SEQ ID NO.7. With the adoption of the membrane electrospray ionization mass spectrometry, matrix effects caused by small molecules introduced by enzyme digestion are effectively removed, and a desalting step in the conventional mass spectrometry process is saved, so that the method disclosed by the invention can realize rapid detection at extremely high sensitivity.
Owner:ICDC CHINA CDC
Primer, probe, kit and detection method for detecting real-time fluorescence RAA of shiga toxin-producing escherichia coli
PendingCN112159855ASensitive methodSimple methodMicrobiological testing/measurementMicroorganism based processesShiga bacillus DysenterySTX2
The invention provides a primer, a probe, a kit and a detection method for detecting real-time fluorescence RAA of shiga toxin-producing escherichia coli. The primer sequences of an stx1 gene are respectively shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of an stx1 gene probe is shown as SEQ ID NO.3; the primer sequences of an stx2 gene are respectively shown as SEQ ID NO.4 and SEQ ID NO.5, and the sequence of the stx2 gene probe is shown as SEQ ID NO.6; and the kit comprises the primer, the probe, a buffer solution and purified water. The specificity of the real-time fluorescence RAAdetection method reaches 100%, the sensitivity reaches 1.6*10<3> cfu / mL, a result can be obtained within 15 min, and the real-time fluorescence RAA detection method is faster, more sensitive, simplerand more convenient than a real-time fluorescence PCR method, can be used for disease monitoring and food safety detection, and is suitable for popularization and application in primary laboratories.
Owner:PLANTS & ANIMALS & FOOD TESTING QUARANTINE TECH CENT SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Human neutralizing antibodies aganst hemolytic uremic syndrome
InactiveUS20090068176A1Less side effectsPeptide/protein ingredientsAntibody ingredientsDiseaseHigh doses
Human and humanized monoclonal antibodies which binds specifically to subunit A of Shiga like toxin II have been developed which are effective to prevent or ameliorate one or more symptoms of HUS in a human. Effective dosages for treatment or prevention range from approximately 0.1 to 5.0 mg of antibody / kg of patient weight. The examples demonstrate the preferred dosage ranges based on the pig model, and what is being tested in phase I clinical trials. Antibodies are preferably transfused over a period of two hours, although this will depend on the patient and the disease state at the time of treatment. Preferred dosages for treatment of humans are between 0.1 mg / kg-5.0 mg / kg of 5C120, or an equivalent dosage of another antibody to subunit A of STX2. In the most preferred embodiments, dosages of 0.1 mg / kg, 0.5 mg / kg, or 5.0 mg / kg of 5C12 (low dose, anticipated therapeutic dose based on animal data and high dose) are administered.
Owner:TRUSTEES OF TUFTS COLLEGE
Method For Screening Toxin Neutralizing Peptide, STX2 Inhibiting Peptide And Verotoxin Neutralizing Agent
InactiveUS20090029864A1Easy to synthesizeInhibition is effectiveAntibacterial agentsPeptide/protein ingredientsSTX2Peptide library
A screening method comprises the following steps;(1) specifying a receptor binding site by introduction of mutation, and(2) specifying a binding site-specific peptide motif on the basis of an amino acid selection ratio by contrast between a peptide motif bound to a wild-type subunit and a peptide motif bound to a mutant functionally deficient in the binding site according to a peptide library method. A peptide which inhibits a toxin whose receptor binding portion has a subunit structure is screened.Accordingly, an STX2 inhibitor in which an STX2 inhibiting peptide is incorporated in a molecular nuclear structure portion having three molecules of lysine (Lys) peptide-linked thereto and which is easy to synthesize and can inhibit verotoxin is provided.
Owner:JAPAN SCI & TECH CORP
Gene chip and kit for detecting diarrheagenic escherichia coli in food and clinical samples
InactiveCN101760514BImprove accuracyGood repeatabilityMicrobiological testing/measurementNucleotideEnteroinvasive Escherichia coli
The invention provides a gene chip and a kit for detecting diarrheagenic escherichia coli in food and clinical samples. The gene chip comprises a solid-phase carrier and an oligonucleotide probe fixed on the solid-phase carrier, wherein the oligonucleotide probe contains DNA fragments and the complementary DNA or RNA sequence selected from eae genes of enteropathogenic escherichia coli (EPEC), lt, STap and STah genes of enterotoxigenic escherichia coli (ETEC), stx1 and stx2 of enterohaemorrhagic escherichia coli (EHEC), ipaH genes of enteroinvasive escherichia coli (EIEC), escherichia coli O157wzy genes and escherichia coli 16s genes. The invention further provides the kit containing the gene chip. The utilization of the gene chip and the kit for detecting the diarrheagenic escherichia coli has the advantages of simple operation, high accuracy and strong repeatability.
Owner:TIANJIN BIOCHIP TECH CO LTD +1
Method for screening toxin neutralizing peptide, STX2 inhibiting peptide and verotoxin neutralizing agent
InactiveUS9103820B2Easy to synthesizeInhibition is effectiveAntibacterial agentsPeptide/protein ingredientsSTX2Peptide library
Owner:JAPAN SCI & TECH CORP
Detection reagent for shiga toxin family gene of entero-hemorrhagic escherichia coli
InactiveUS20060115823A1Superior speed and specificitySugar derivativesMicrobiological testing/measurementBase JEscherichia coli
The present invention provides a combination of oligonucleotides preferable for composing a rapid and specific gene testing reagent for Shiga toxin family gene type 2 (stx) of entero-hemorrhagic Escherichia coli (EHEC). More specifically, the present invention provides a method for detecting stx2 RNA of EHEC by specifically amplifying only stx2 RNA using a primer having a sequence homologous or complementary to a base sequence specific for stx2 gene of EHEC and located at sites free of alterations between genotypes, and an oligonucleotide that binds to a specific site of stx2 RNA.
Owner:TOSOH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com