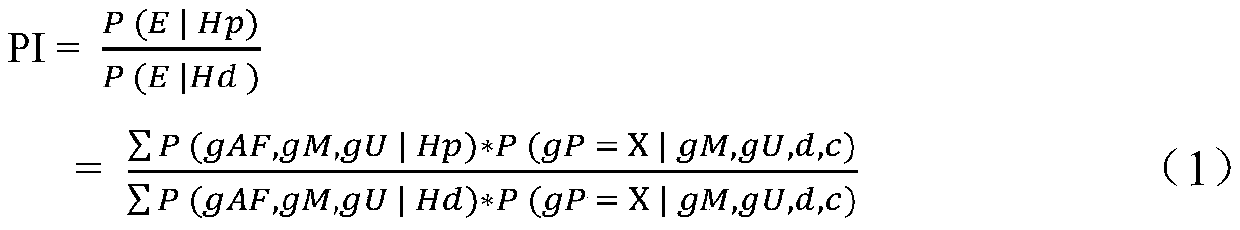Patents
Literature
42 results about "Peripheral plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peripheral Blood Plasma. Human peripheral blood plasma contains a variety of cytokines, chemokines, and growth factors that affect the proliferation and function of immune cells, which allows peripheral blood plasma to be applicable in a wide variety of therapeutic uses.
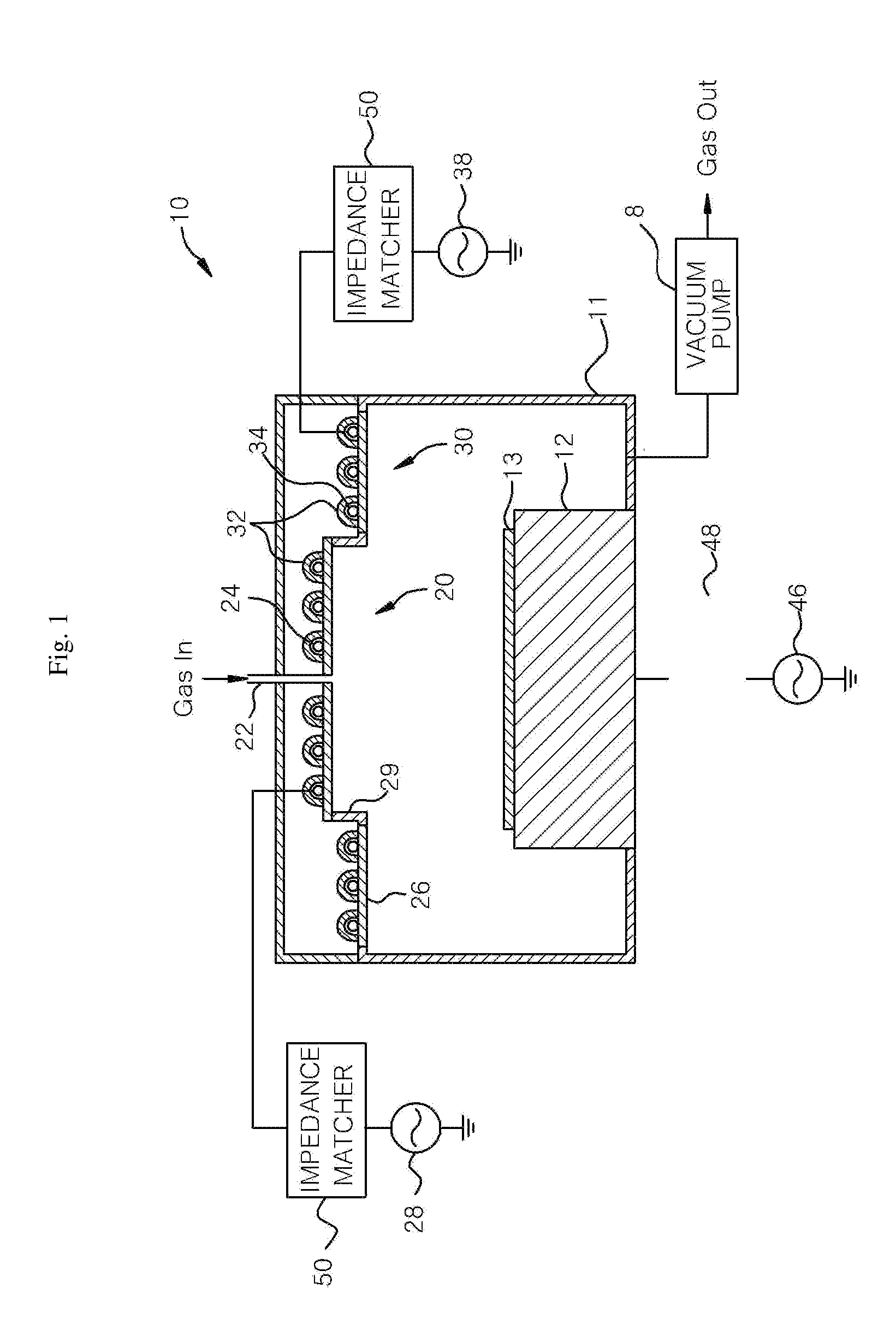
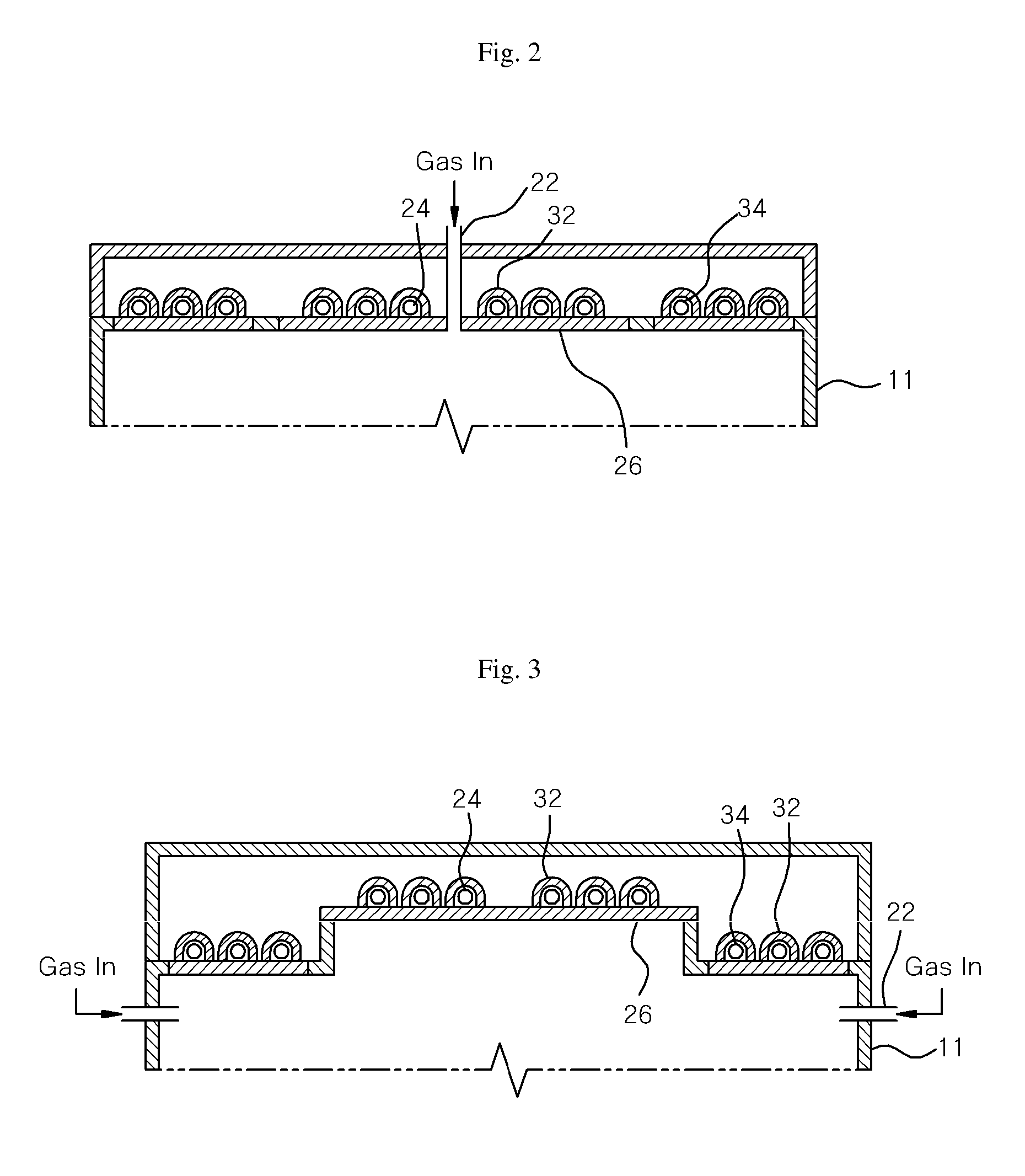
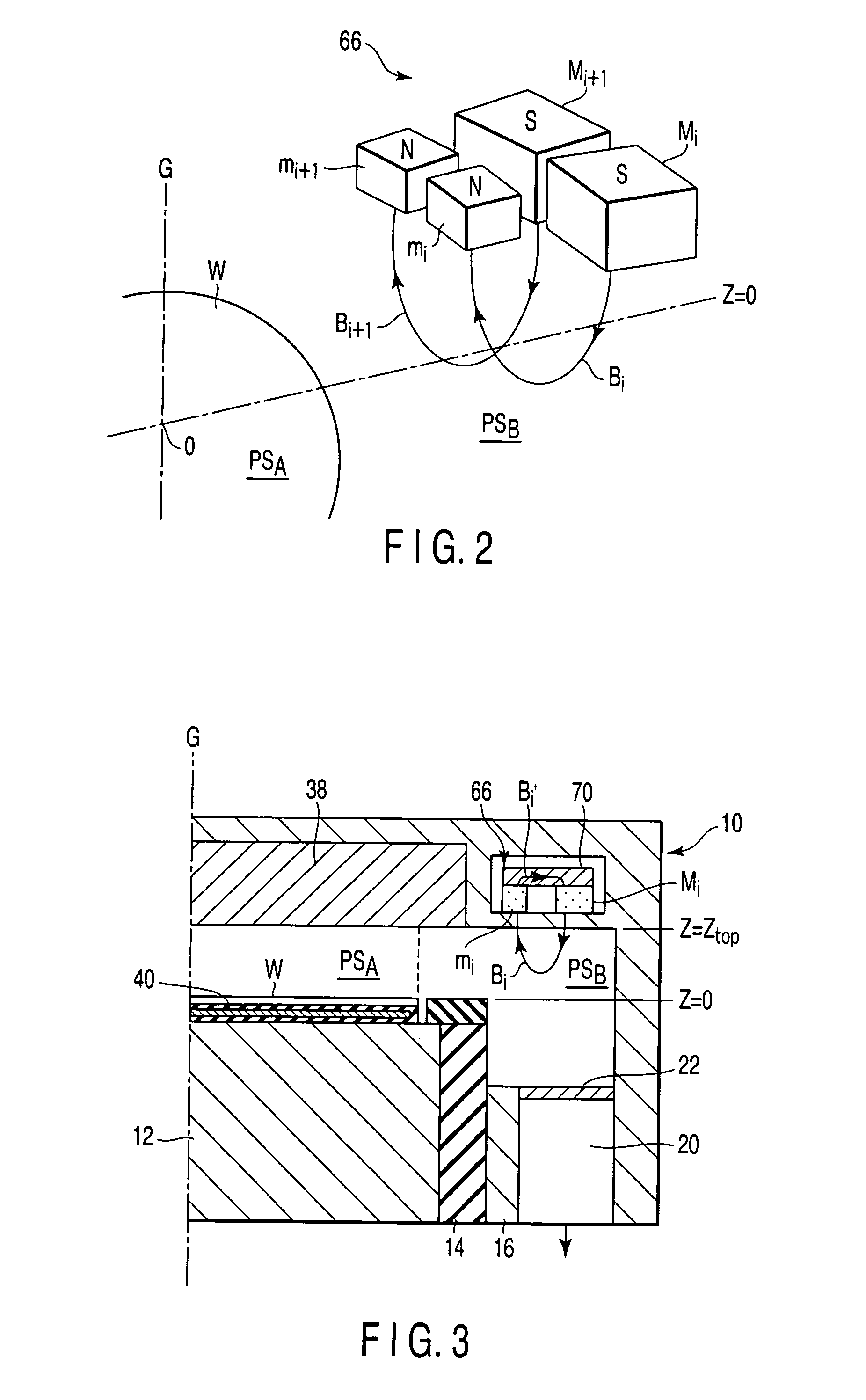
Plasma processing apparatus and method
ActiveUS7846293B2Easy to startStably sustain electric dischargeCellsElectric discharge tubesForce linesPeripheral plasma
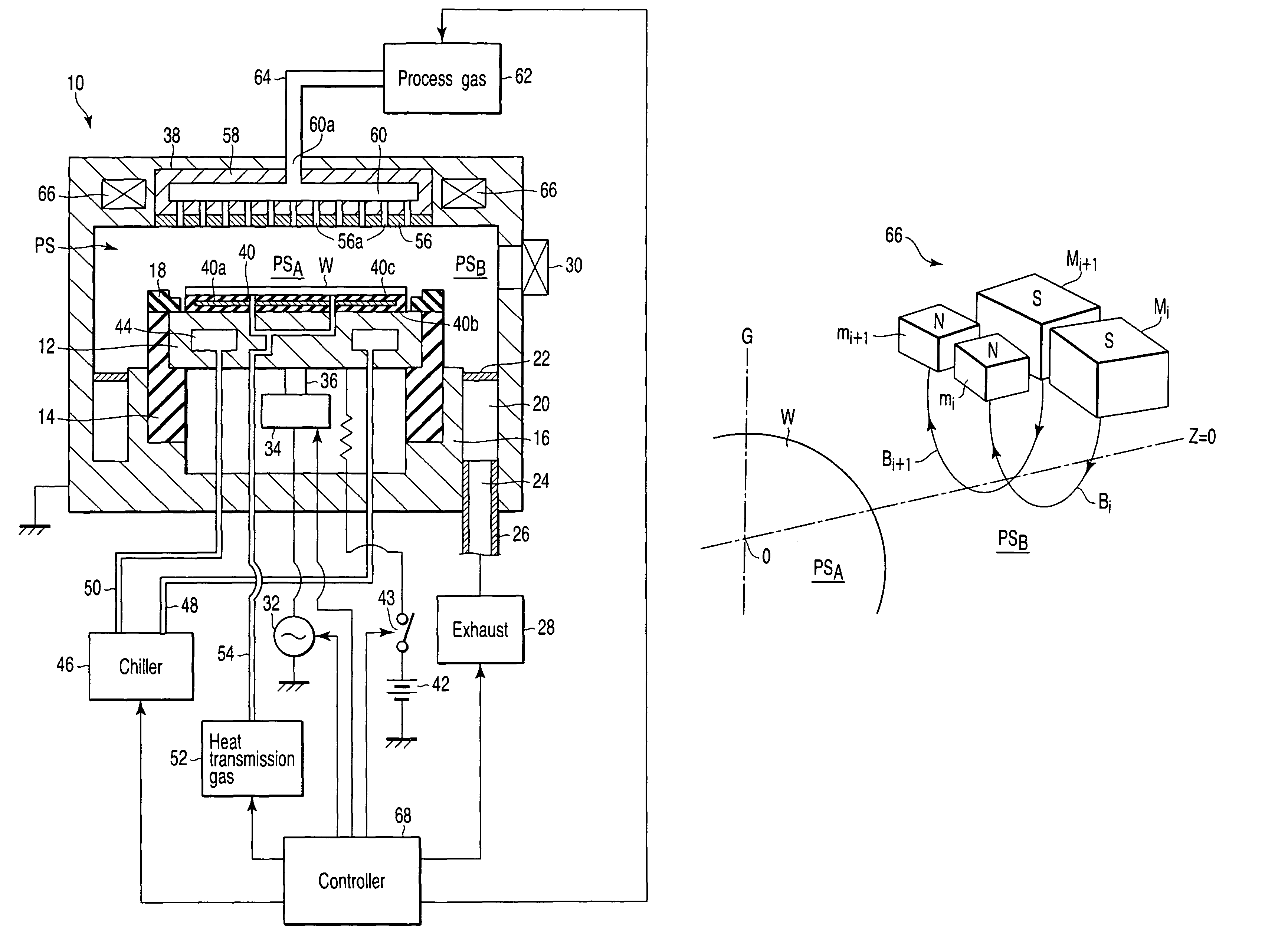
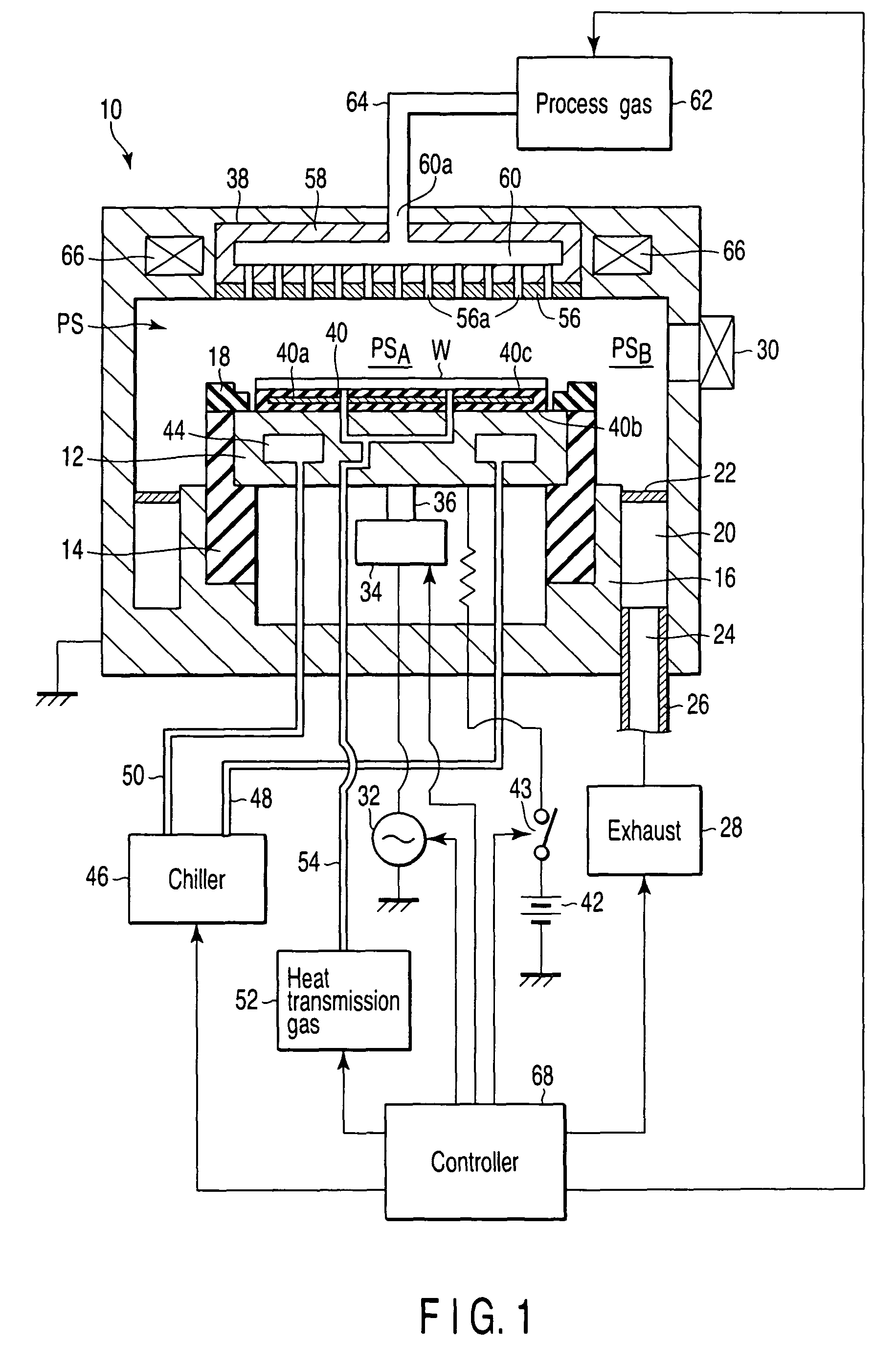
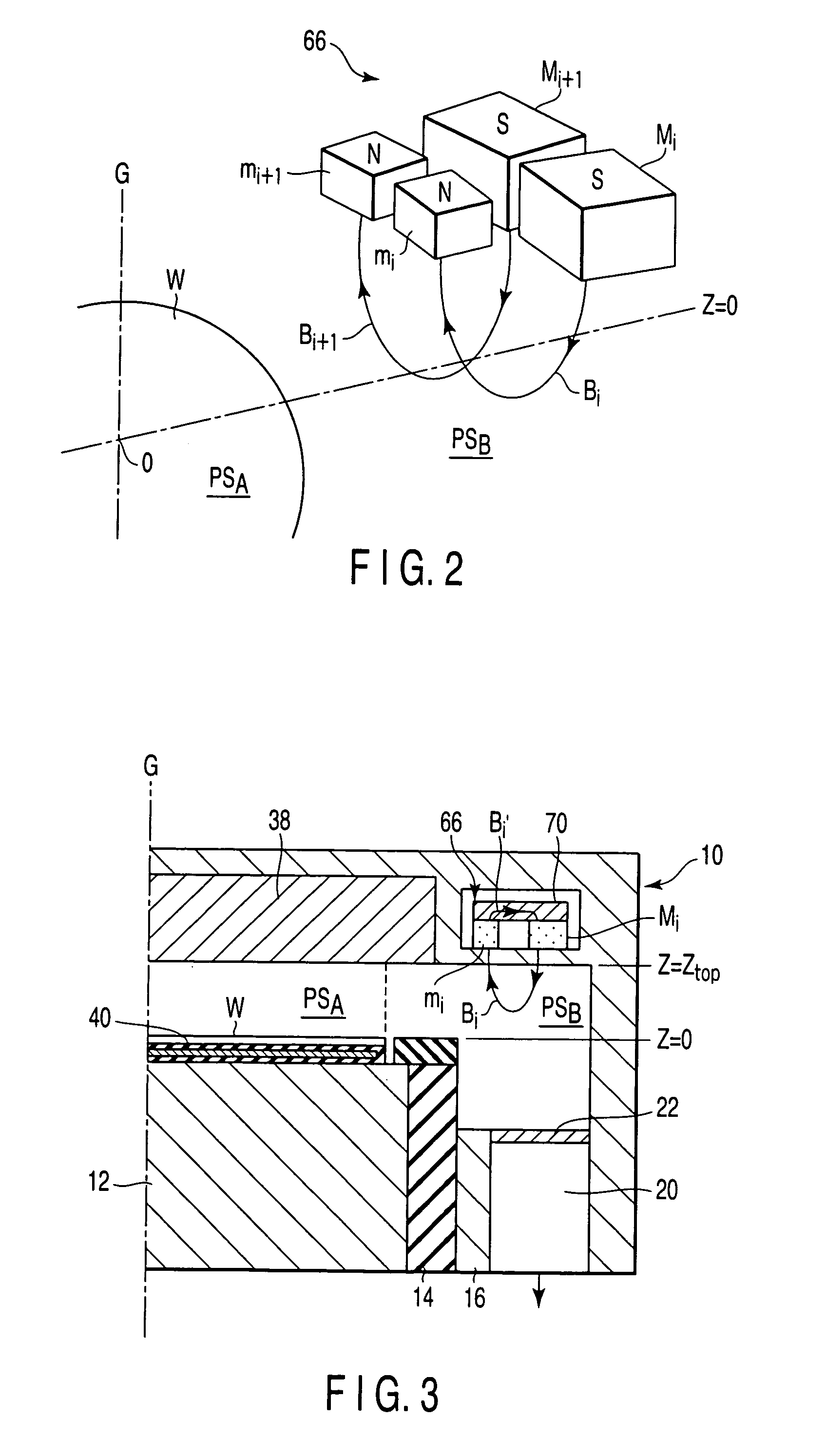
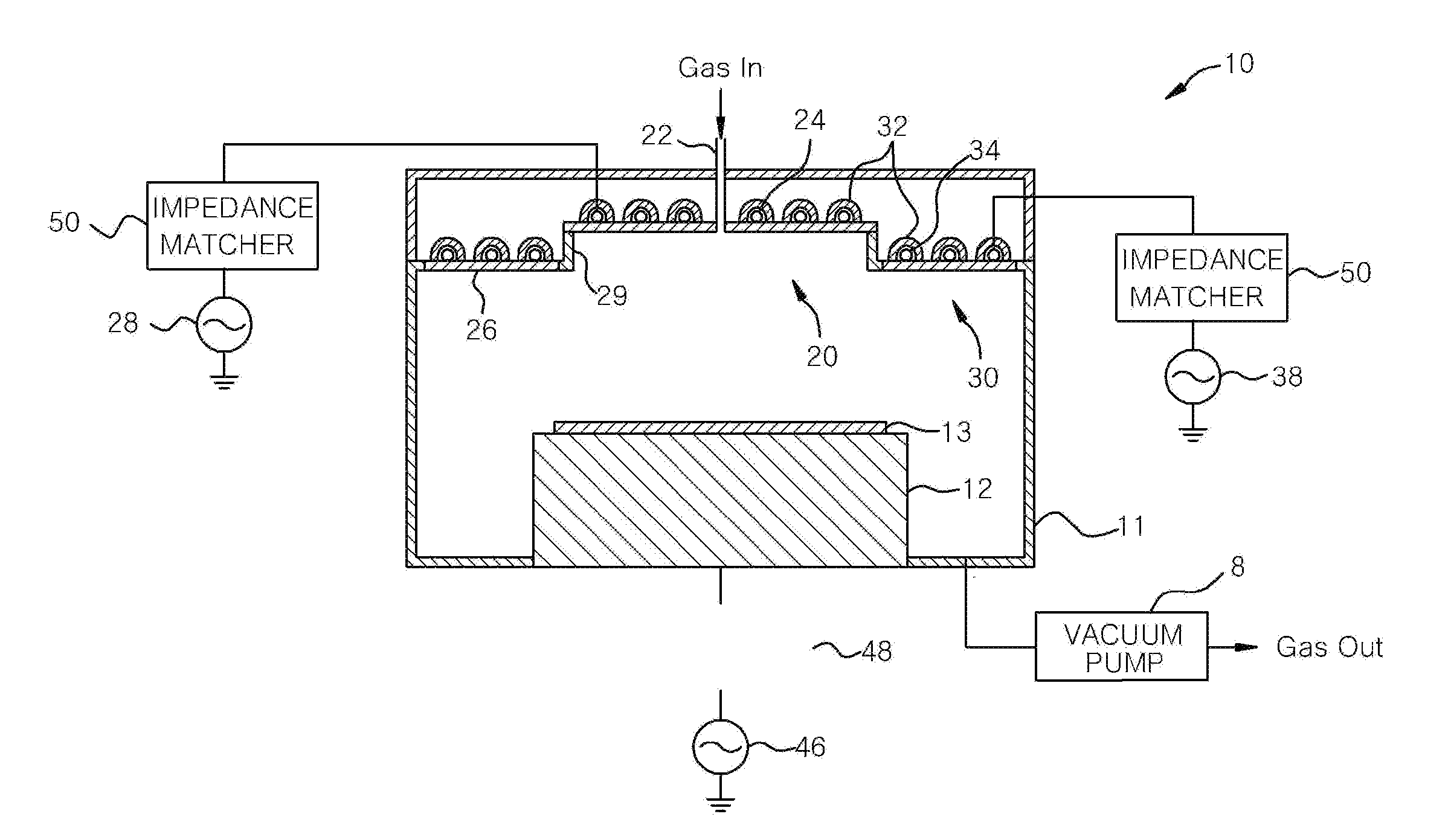
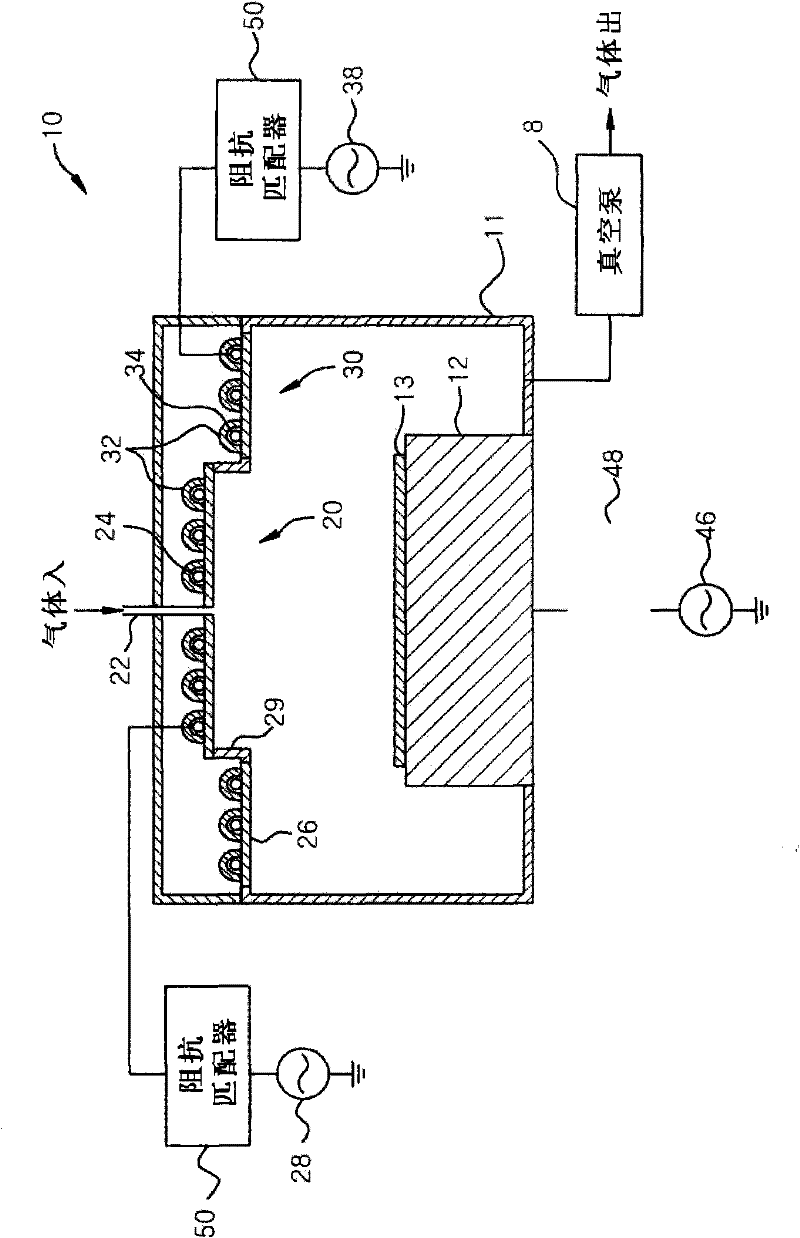
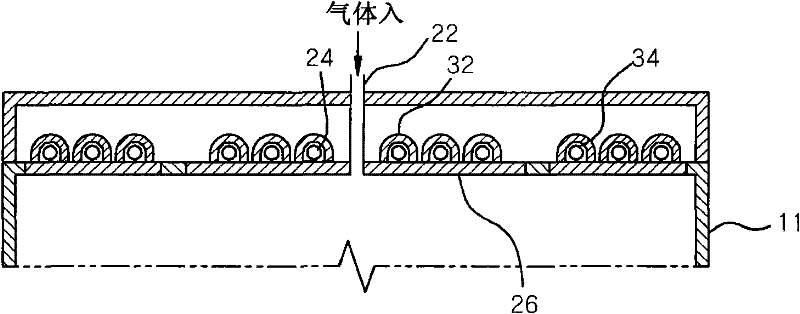
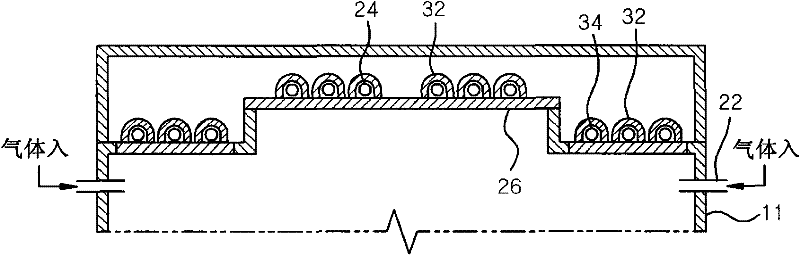
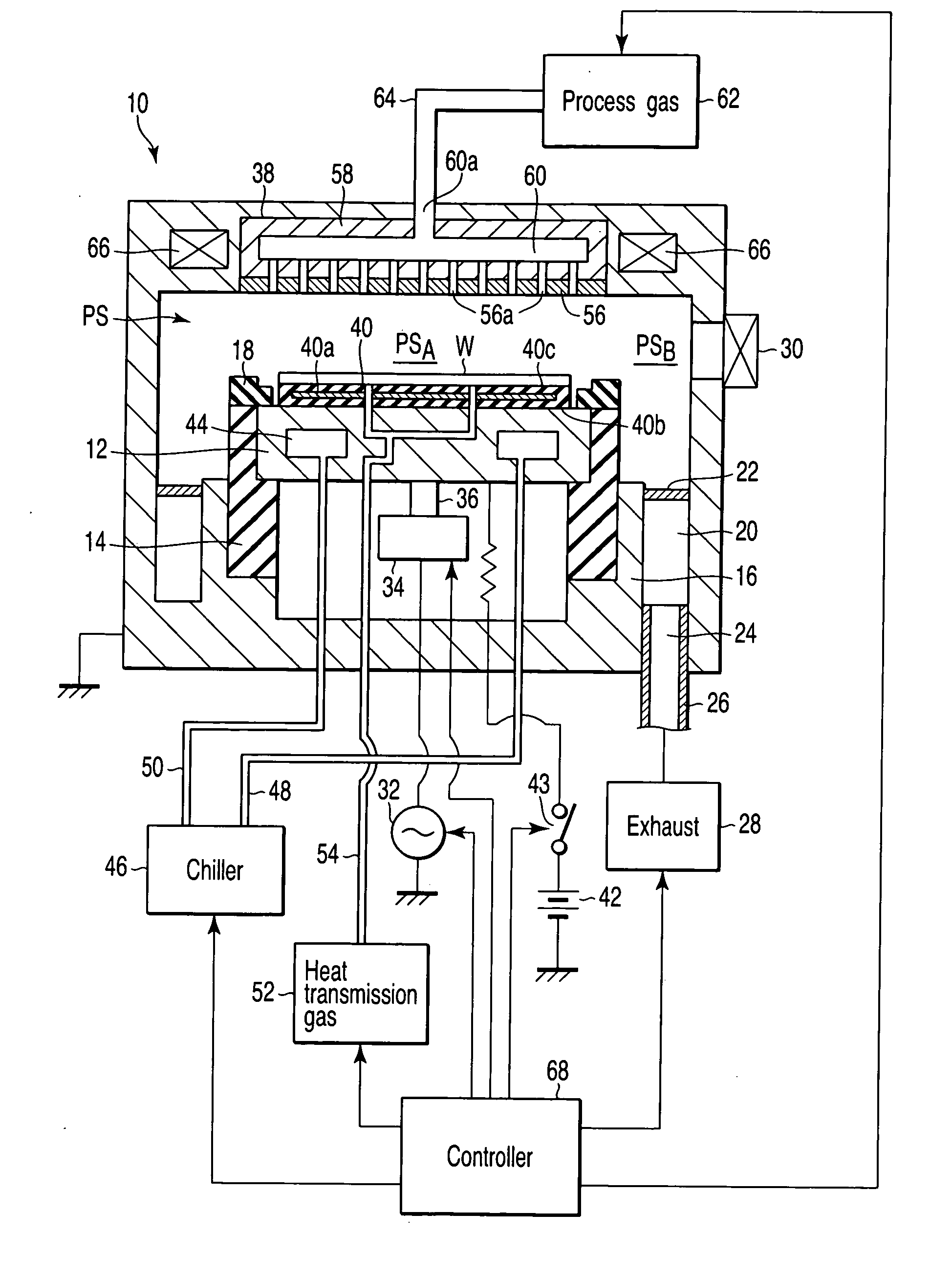
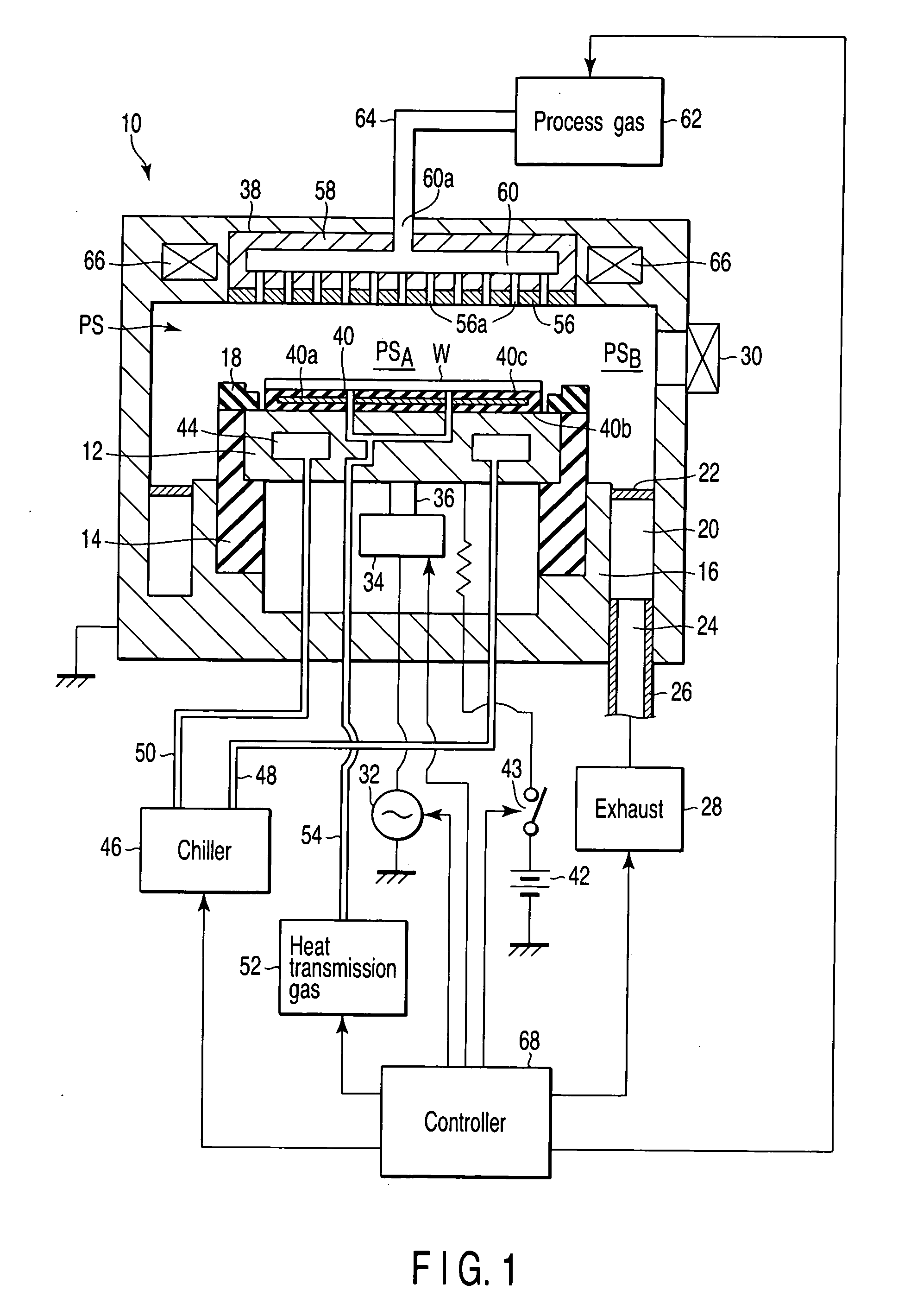
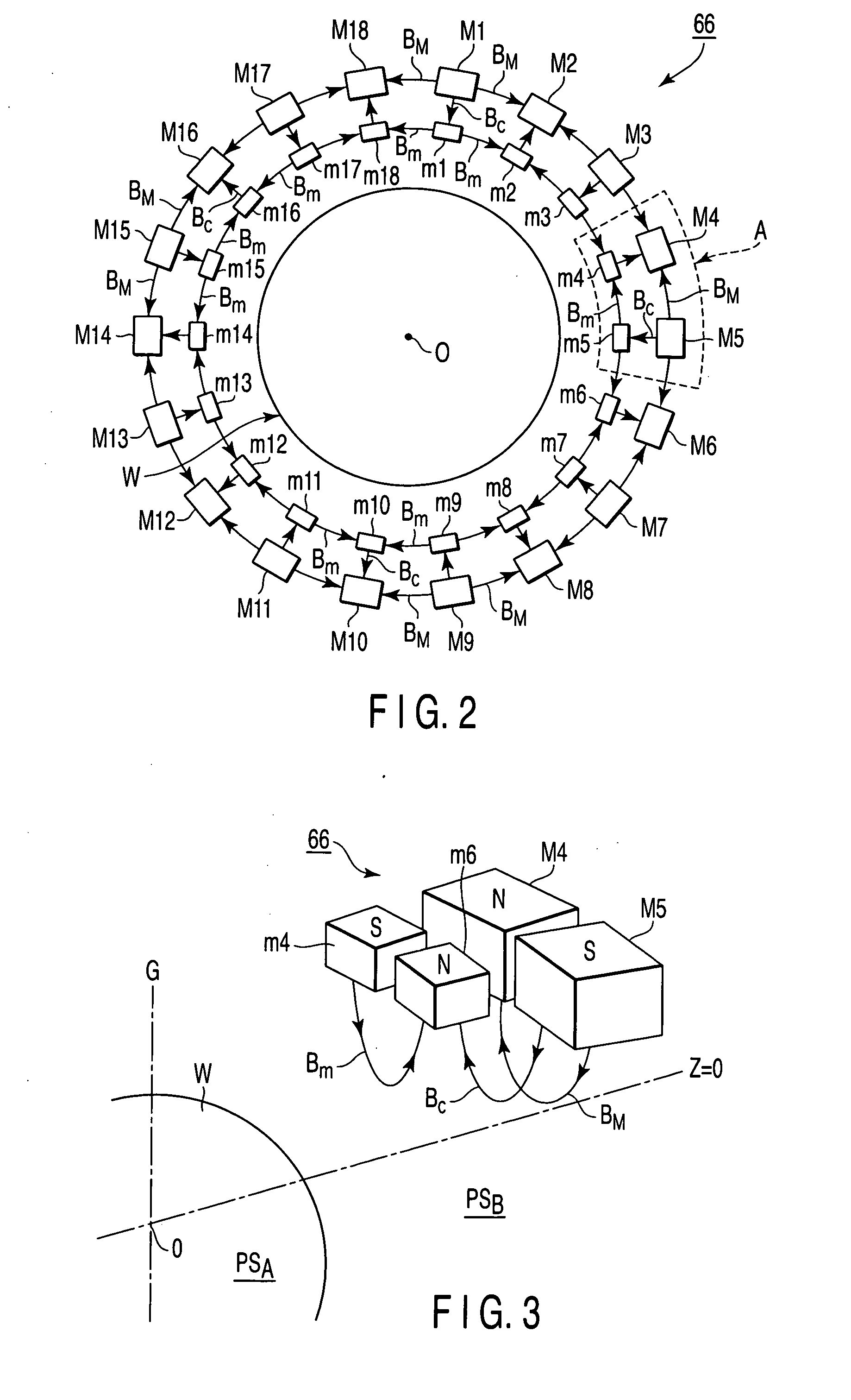
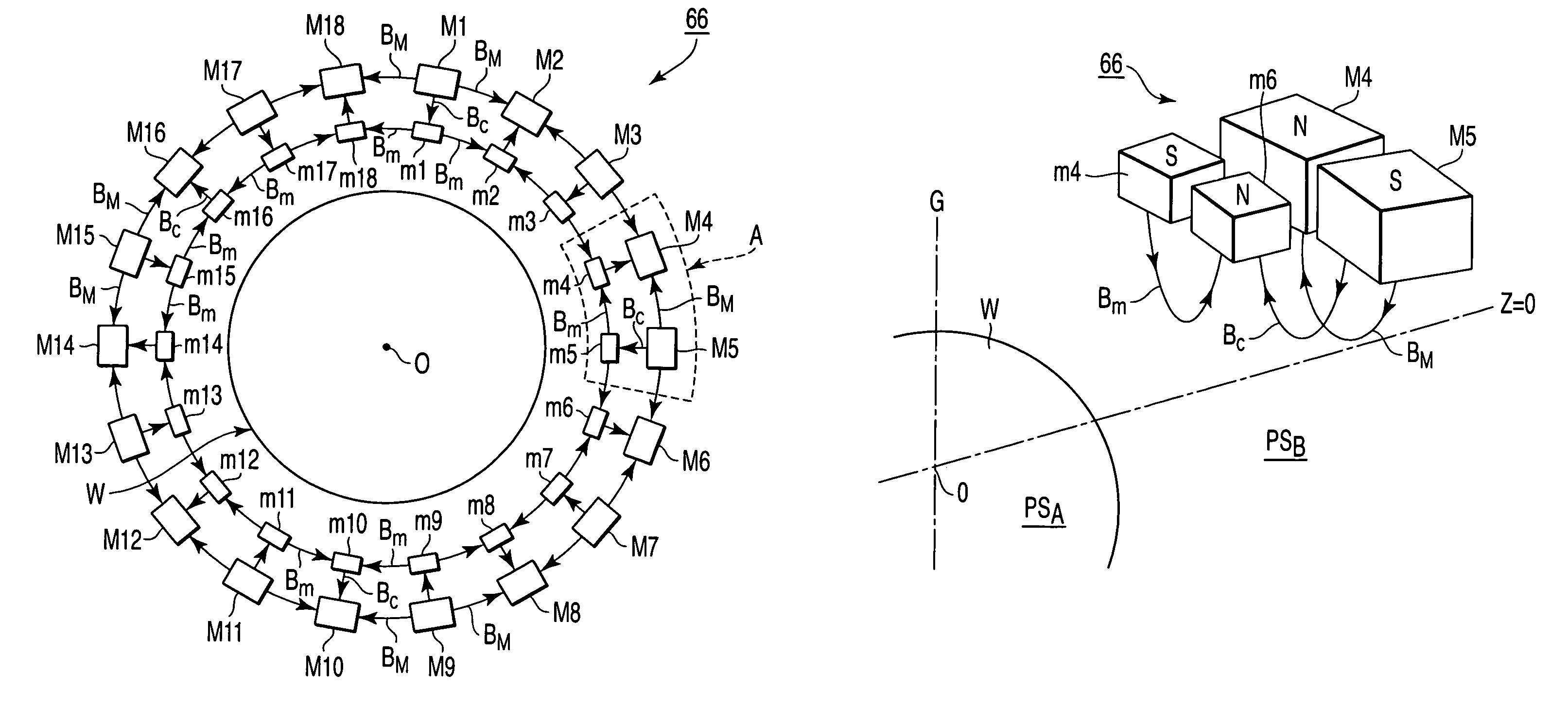
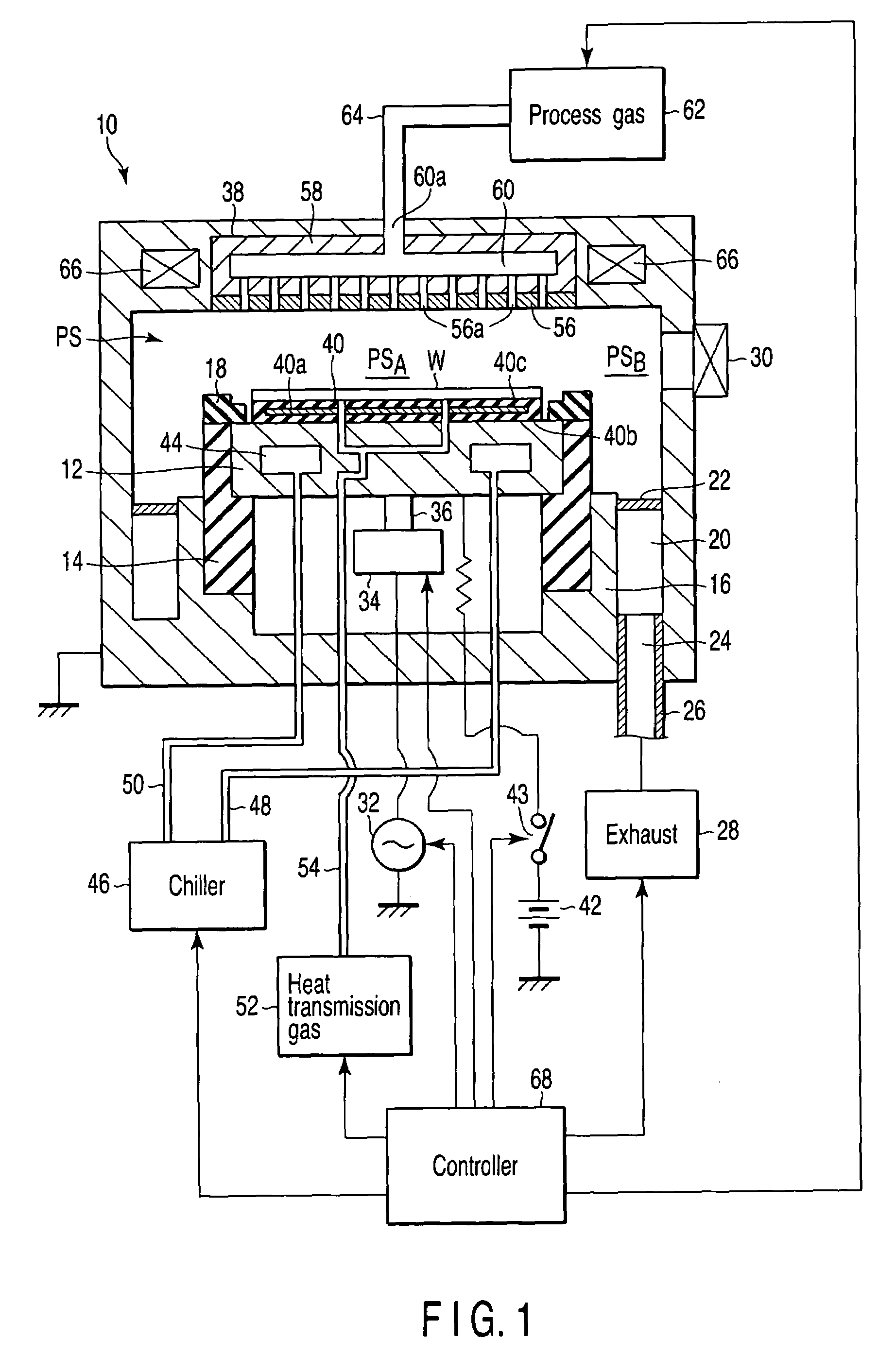
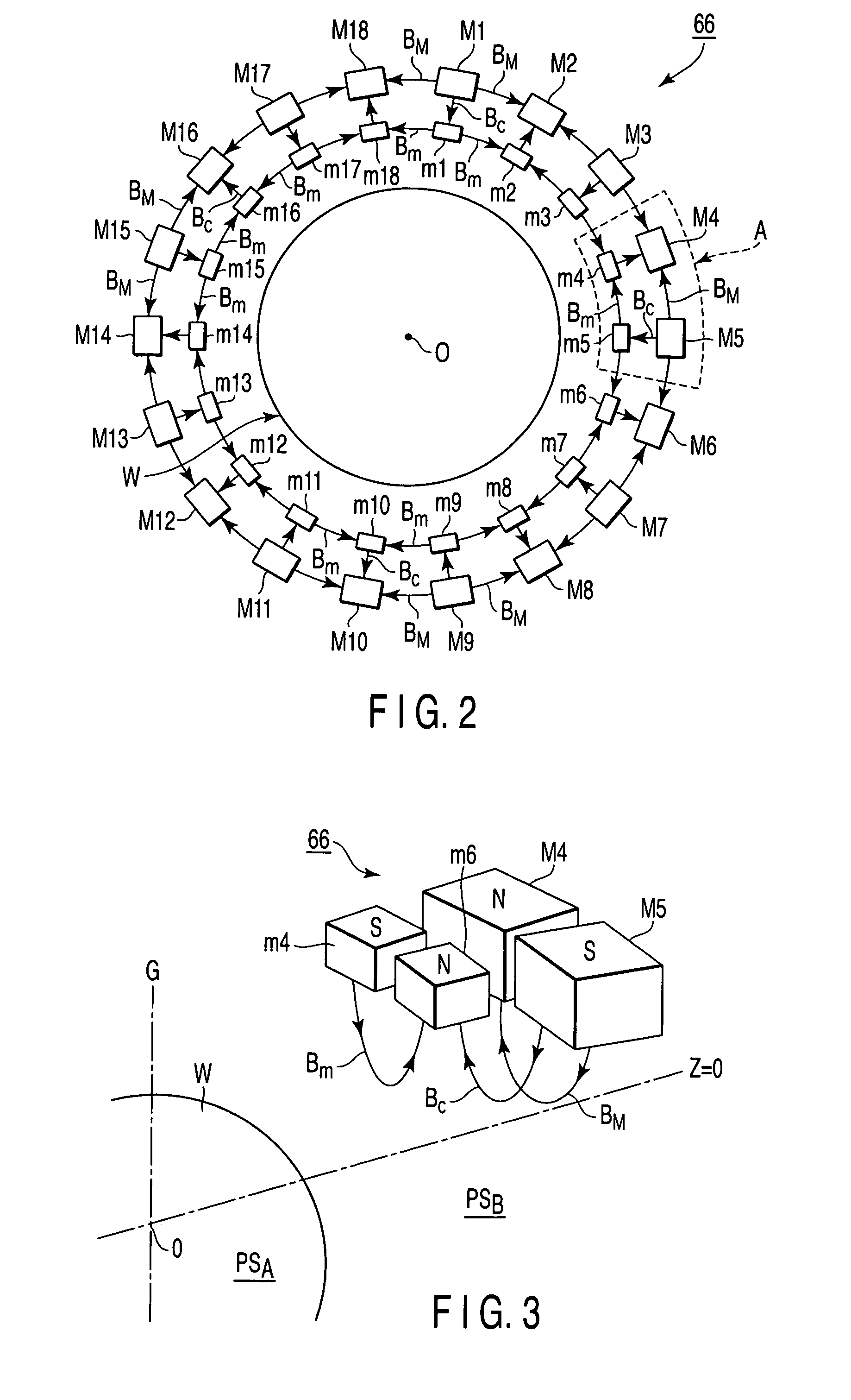
A plasma processing apparatus includes a worktable in a process chamber to horizontally place a target substrate thereon. A plasma generation space is defined above and around the worktable within the process chamber. The plasma generation space includes a peripheral plasma region and a main plasma region respectively located outside and inside an outer edge of the target substrate placed on the worktable. The apparatus further includes a magnetic field forming mechanism configured to form a magnetic field within the peripheral plasma region. The magnetic field includes magnetic force lines extending through the peripheral plasma region between a start position and an end position, at least one of which is located radially inside a sidewall of the process chamber.
Owner:TOKYO ELECTRON LTD
Multi inductively coupled plasma reactor and method thereof
InactiveUS20110204023A1Improve processing efficiencyElectric discharge tubesDecorative surface effectsInductively coupled plasmaPlasma reactor
Disclosed is a multi-inductively coupled plasma reactor and method thereof. In a multi-inductively coupled plasma reacting method, an etching method to increase a specific portion of a substrate to be processed includes etching a specific portion of a substrate to be processed; and depositing a passivation layer on a surface of the specific portion etched, wherein the etching and depositing steps are repeatedly proceeded, and one of both steps is executed when there is plasma formed by a central plasma source and a peripheral plasma source. According to the multi-inductively coupled plasma reactor and method thereof of the invention, it is possible that plasma is uniformly processed on the entire area of the substrate since the central plasma source and the peripheral source are provided separately. Further, it is possible to form an independent multiple plasma area without electrical interference in the plasma reactor using the interference prevention electrode grounded between the central plasma source and the peripheral plasma source. Further, the plasma formed by the central plasma source and the peripheral plasma source is used to deeply etch a specific portion of the substrate to be processed.
Owner:GENERAL CO LTD
Plasma processing apparatus and method
ActiveUS20050224337A1Easy to startStably sustain electric dischargeCellsElectric discharge tubesForce linesPeripheral plasma
A plasma processing apparatus includes a worktable in a process chamber to horizontally place a target substrate thereon. A plasma generation space is defined above and around the worktable within the process chamber. The plasma generation space includes a peripheral plasma region and a main plasma region respectively located outside and inside an outer edge of the target substrate placed on the worktable. The apparatus further includes a magnetic field forming mechanism configured to form a magnetic field within the peripheral plasma region. The magnetic field includes magnetic force lines extending through the peripheral plasma region between a start position and an end position, at least one of which is located radially inside a sidewall of the process chamber.
Owner:TOKYO ELECTRON LTD
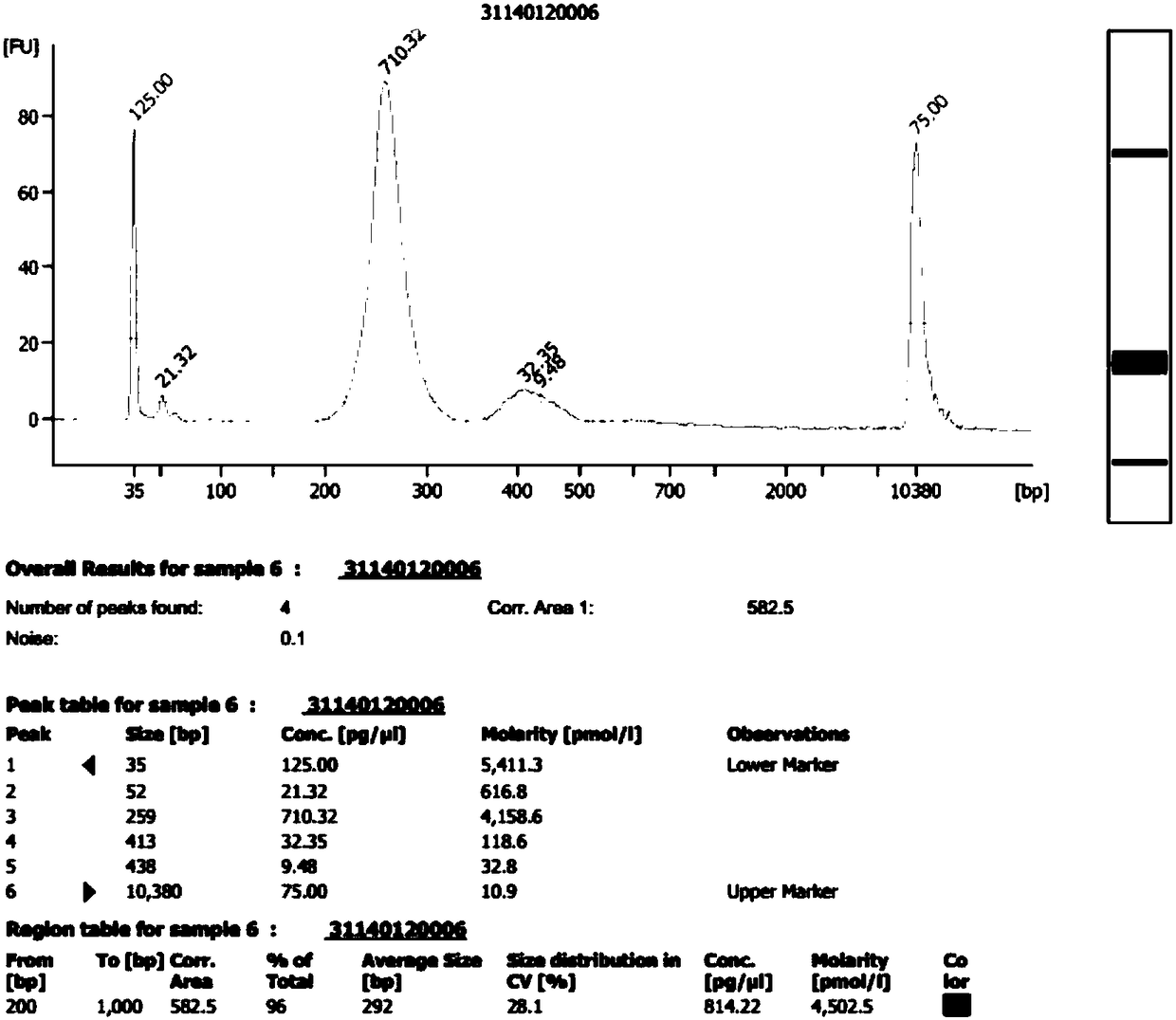
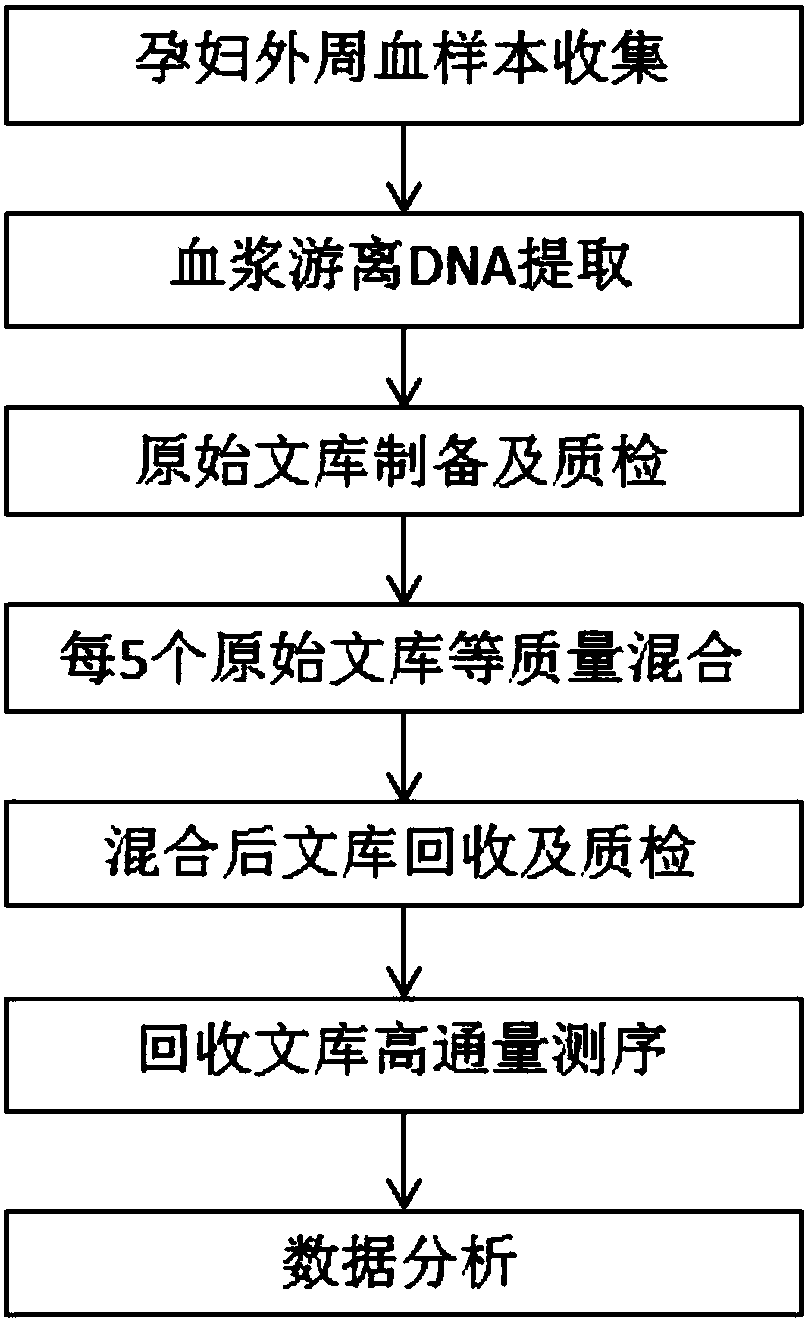
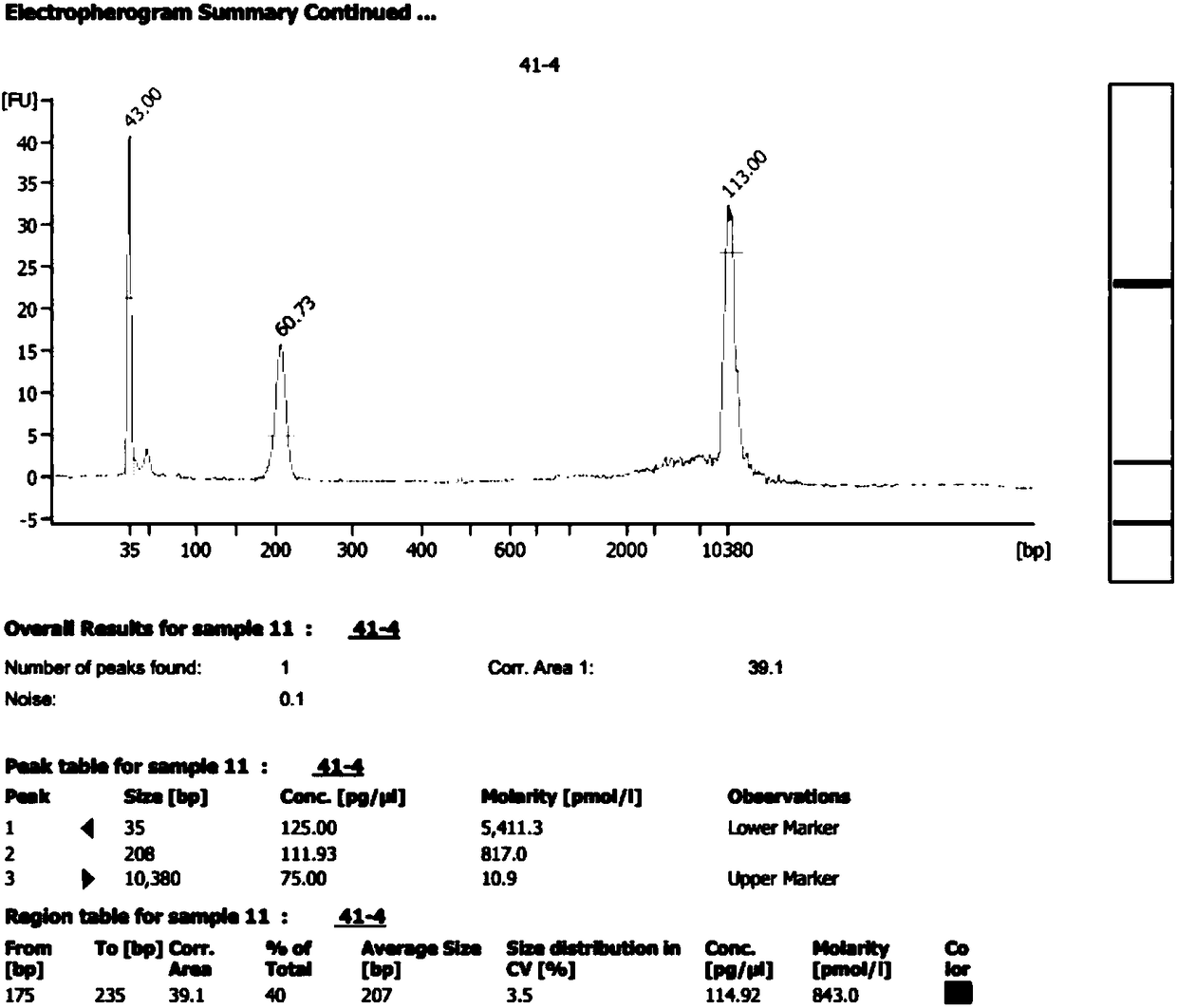
Method for improving proportion of fetal free DNA in maternal plasma free DNA sequencing library
ActiveCN105926043AIncrease the proportion of free DNA contentImprove throughputMicrobiological testing/measurementLibrary creationBlood plasmaBiology
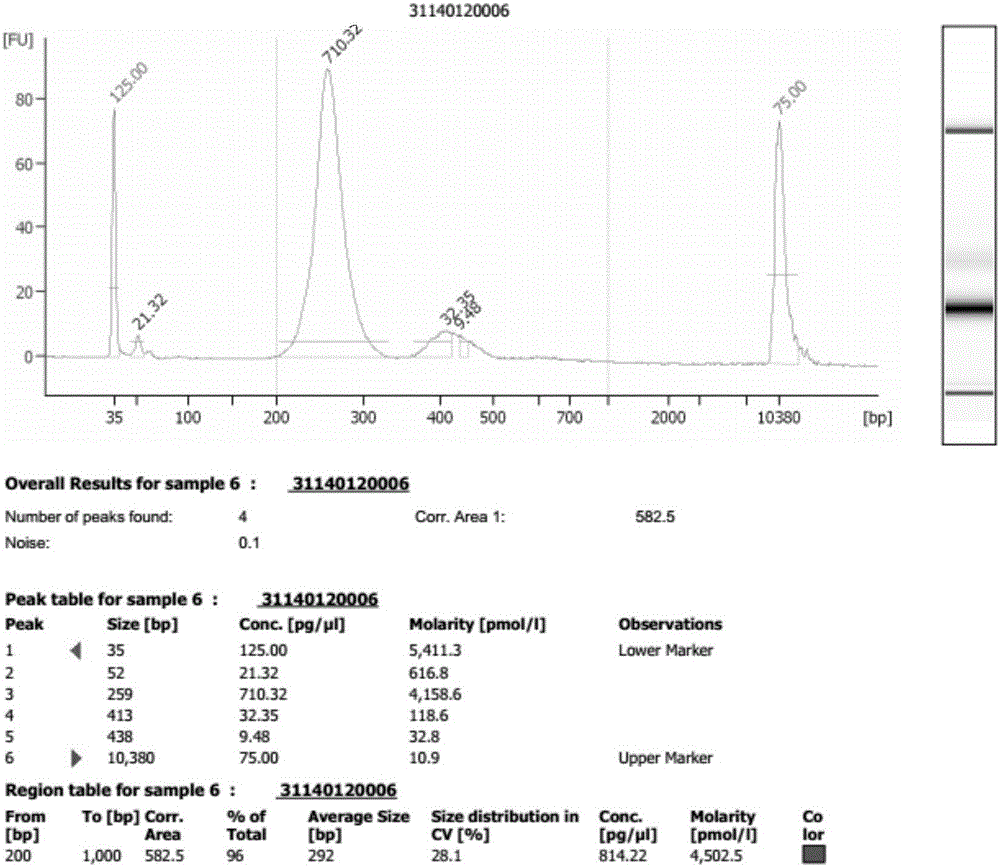
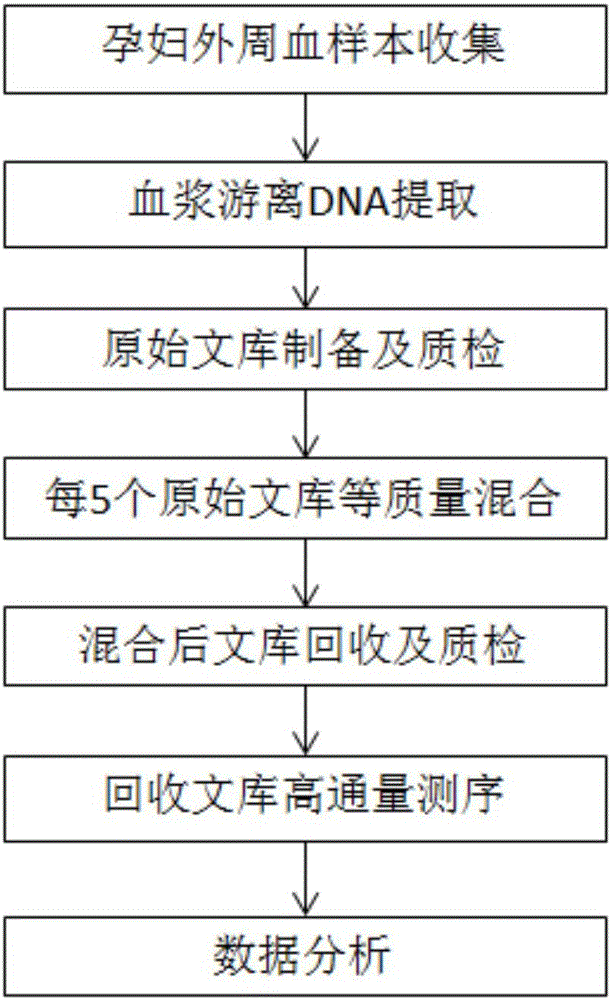
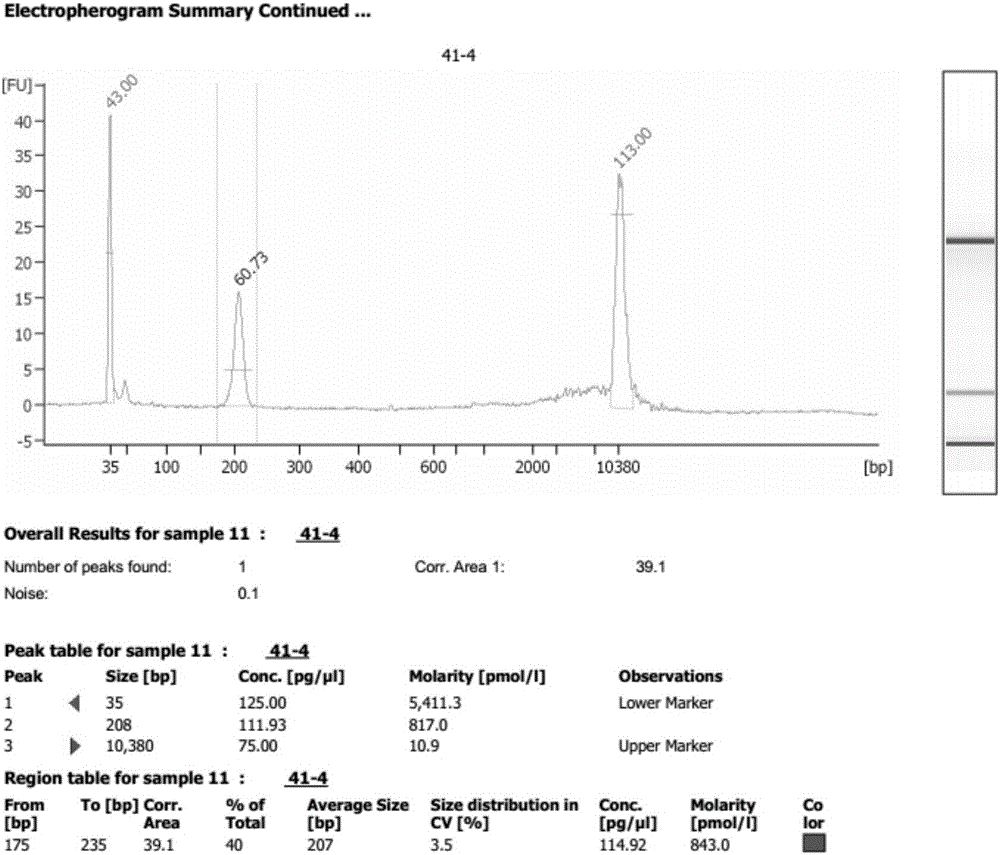
The invention discloses a method for improving a proportion of fetal free DNA in a maternal plasma free DNA sequencing library. The method comprises the following steps: extracting maternal plasma free DNA; adding specific sequence tags at two ends of the maternal plasma free DNA so as to obtain plasma free DNA with maternal individual identification markings; subjecting the plasma free DNA with the maternal individual identification markings to PCR amplification so as to obtain an original library of maternal individual plasma free DNA; and recovering the maternal original library in a selected length range so as to obtain a recovered library used for high-throughput sequencing, or mixing the maternal original libraries of a plurality of different individual pregnant women so as to build the recovered library. The method provided by the invention can accurately detect a sample with excessively low content of maternal peripheral plasma fetal free DNA, reduces the sequencing data volume of every sample, improves the sample throughput of a sequencing reaction, reduces detection cost, and facilitates large-scale popularization.
Owner:SUZHOU BASECARE MEDICAL DEVICE CO LTD
Multi inductively coupled plasma reactor and method thereof
InactiveCN102163538AUniform treatmentElectric discharge tubesSemiconductor/solid-state device manufacturingInductively coupled plasmaPlasma reactor
Disclosed is a multi-inductively coupled plasma reactor and method thereof. In a multi-inductively coupled plasma reacting method, an etching method to increase a specific portion of a substrate to be processed includes etching a specific portion of a substrate to be processed; and depositing a passivation layer on a surface of the specific portion etched, wherein the etching and depositing steps are repeatedly proceeded, and one of both steps is executed when there is plasma formed by a central plasma source and a peripheral plasma source. According to the multi-inductively coupled plasma reactor and method thereof of the invention, it is possible that plasma is uniformly processed on the entire area of the substrate since the central plasma source and the peripheral source are provided separately. Further, it is possible to form an independent multiple plasma area without electrical interference in the plasma reactor using the interference prevention electrode grounded between the central plasma source and the peripheral plasma source. Further, the plasma formed by the central plasma source and the peripheral plasma source is used to deeply etch a specific portion of the substrate to be processed.
Owner:ACN CO LTD
Plasma processing apparatus and method
ActiveUS20050224344A1Easy to startStably sustain electric dischargeCellsElectric discharge tubesForce linesRadial plane
A plasma processing apparatus includes a worktable in a process chamber to horizontally place a target substrate thereon. A plasma generation space is defined above and around the worktable within the process chamber. The plasma generation space includes a peripheral plasma region and a main plasma region respectively located outside and inside an outer edge of the target substrate placed on the worktable. The apparatus further includes a magnetic field forming mechanism configured to form first, second, and third magnetic fields within the peripheral plasma region. The first magnetic field includes magnetic force lines extending along a vertical first cylindrical plane. The second magnetic field includes magnetic force lines extending along a vertical second cylindrical plane located inside the first cylindrical plane. The third magnetic field includes magnetic force lines extending along vertical radial planes located between the first and second cylindrical planes.
Owner:TOKYO ELECTRON LTD +1
Method for monitoring secondary drug resistance of lung cancer patient to tyrosine kinase inhibitor through ddPCR technology
PendingCN105838777AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTyrosine-kinase inhibitorMonitoring and control
The invention provides a method for monitoring secondary drug resistance of a lung cancer patient to a tyrosine kinase inhibitor through ddPCR technology, and an application of the method to monitoring and control of the tyrosine kinase inhibitor in a process of treating a lung cancer patient. The method comprises: firstly, extracting DNA from peripheral plasma of a lung cancer patient, then detecting a mutation condition of a drug-resistant mutation site T790M of EGFR in the DNA through adoption of ddPCR technology, and finally judging whether the patient produces drug resistance according to the mutation situation.
Owner:上海张江转化医学研发中心有限公司 +2
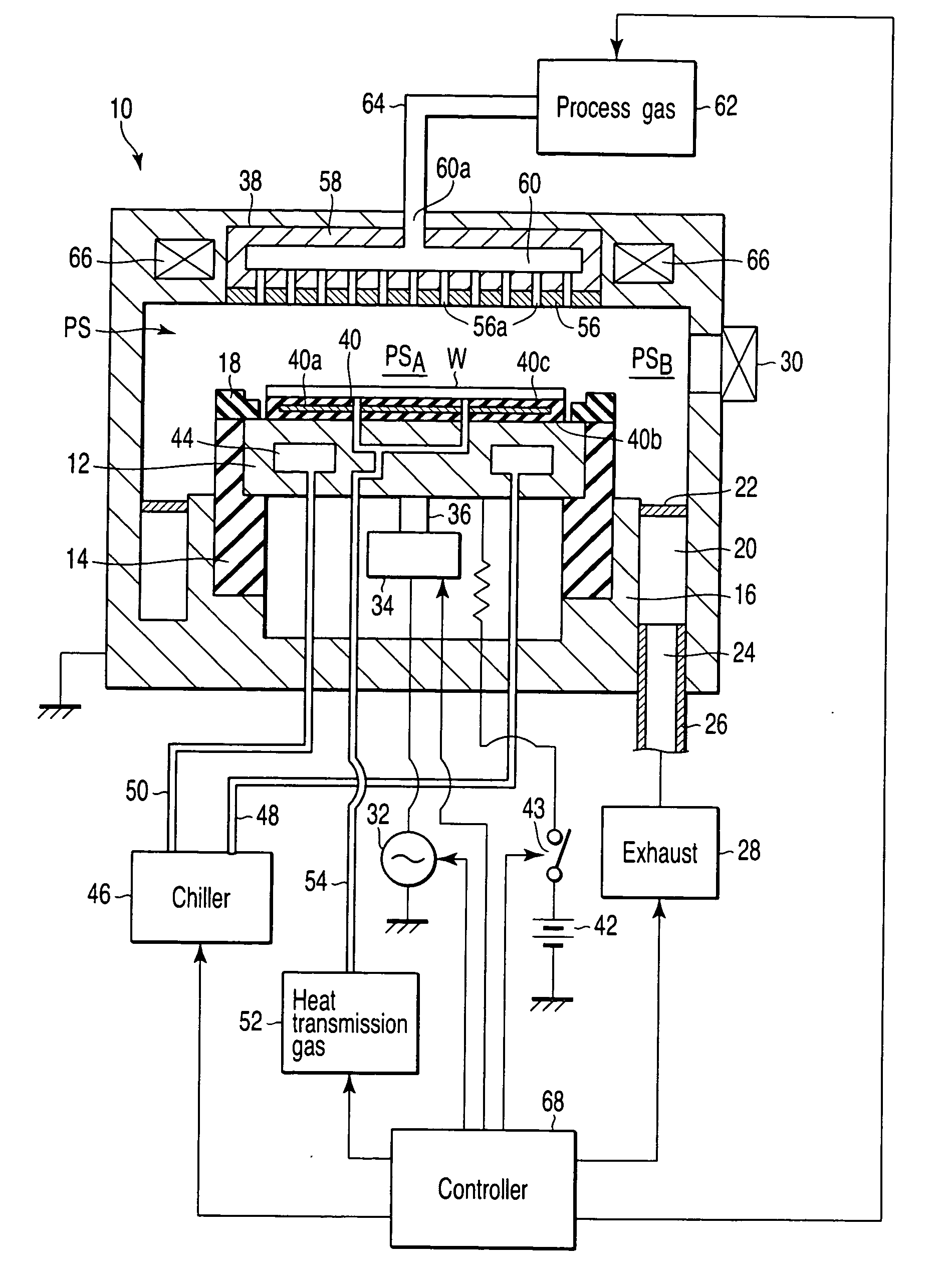
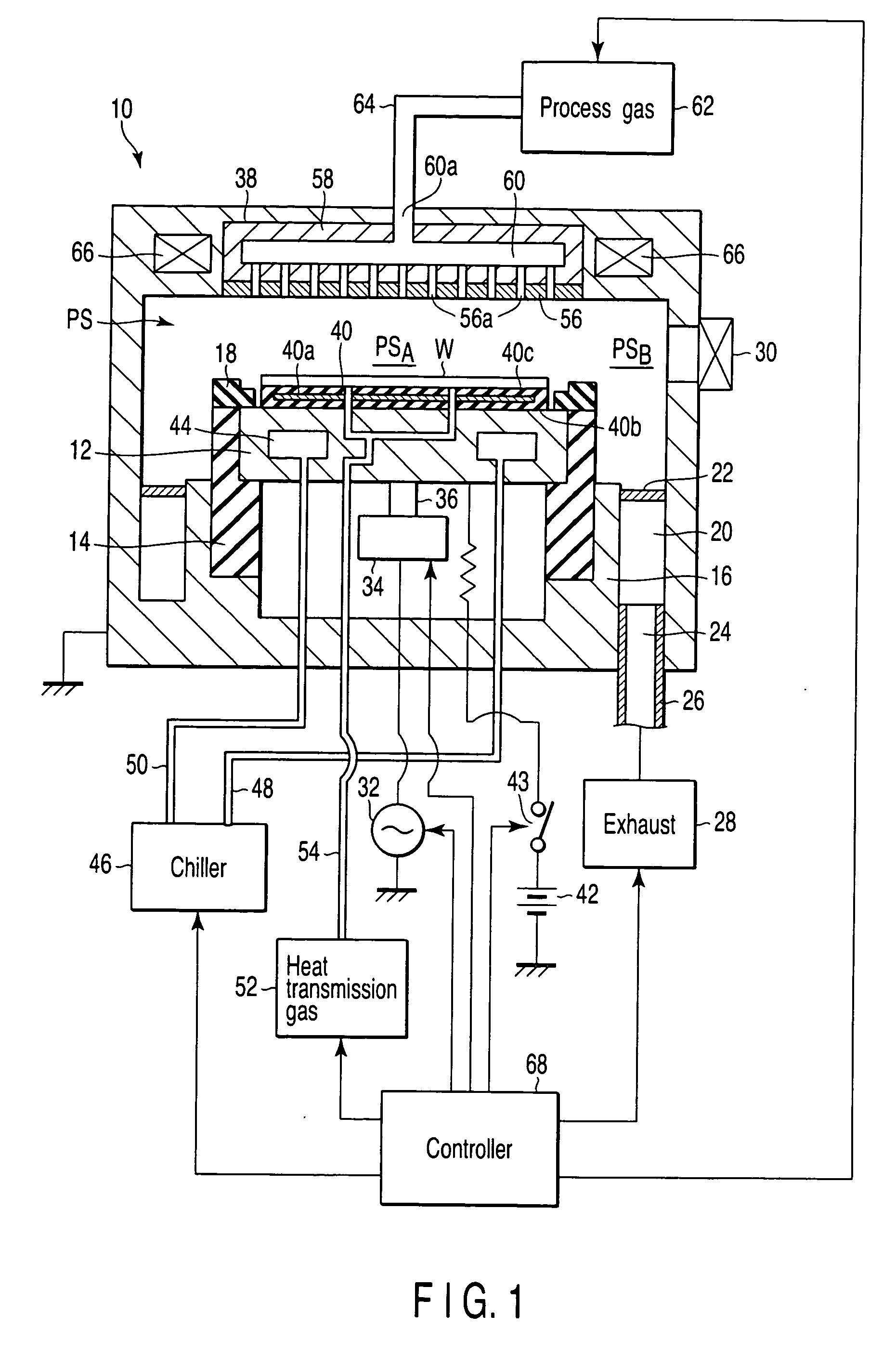
Plasma processing apparatus and method
ActiveUS7419567B2Easy to startStably sustain electric dischargeCellsElectric discharge tubesForce linesRadial plane
A plasma processing apparatus includes a worktable in a process chamber to horizontally place a target substrate thereon. A plasma generation space is defined above and around the worktable within the process chamber. The plasma generation space includes a peripheral plasma region and a main plasma region respectively located outside and inside an outer edge of the target substrate placed on the worktable. The apparatus further includes a magnetic field forming mechanism configured to form first, second, and third magnetic fields within the peripheral plasma region. The first magnetic field includes magnetic force lines extending along a vertical first cylindrical plane. The second magnetic field includes magnetic force lines extending along a vertical second cylindrical plane located inside the first cylindrical plane. The third magnetic field includes magnetic force lines extending along vertical radial planes located between the first and second cylindrical planes.
Owner:TOKYO ELECTRON LTD +1
Method for detecting EGFR L858R locus in lung cancer by ddPCR and application thereof
InactiveCN109762878ALow nucleic acid contentLow Mutation Sequence ContentMicrobiological testing/measurementWild typeBlood plasma
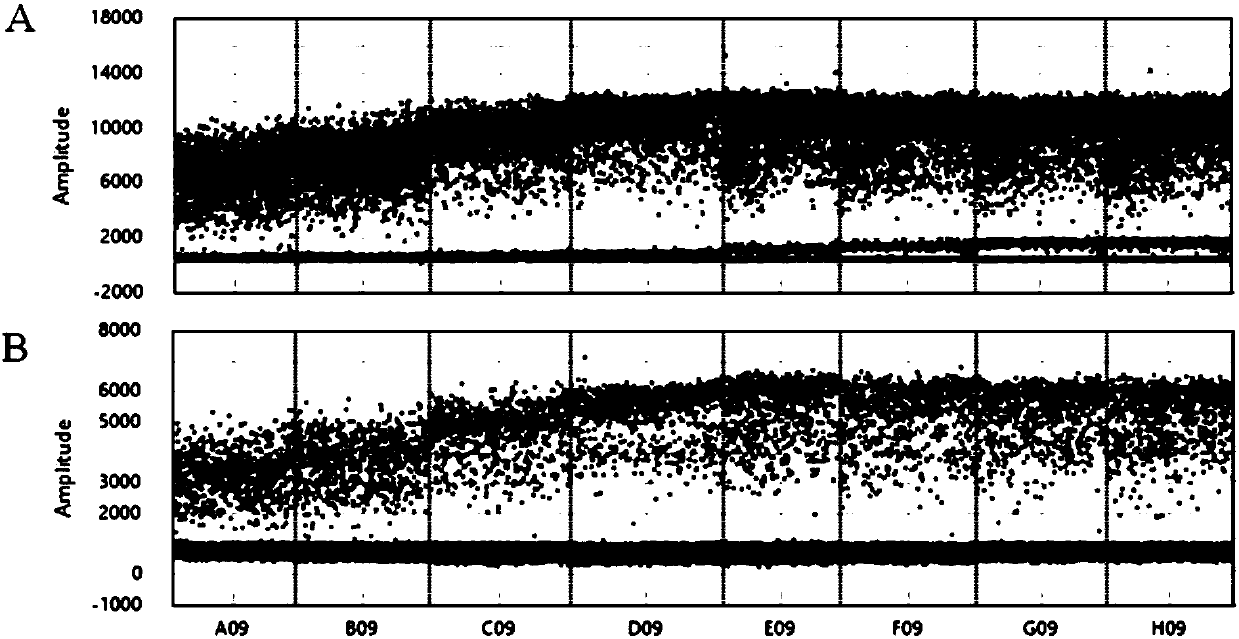
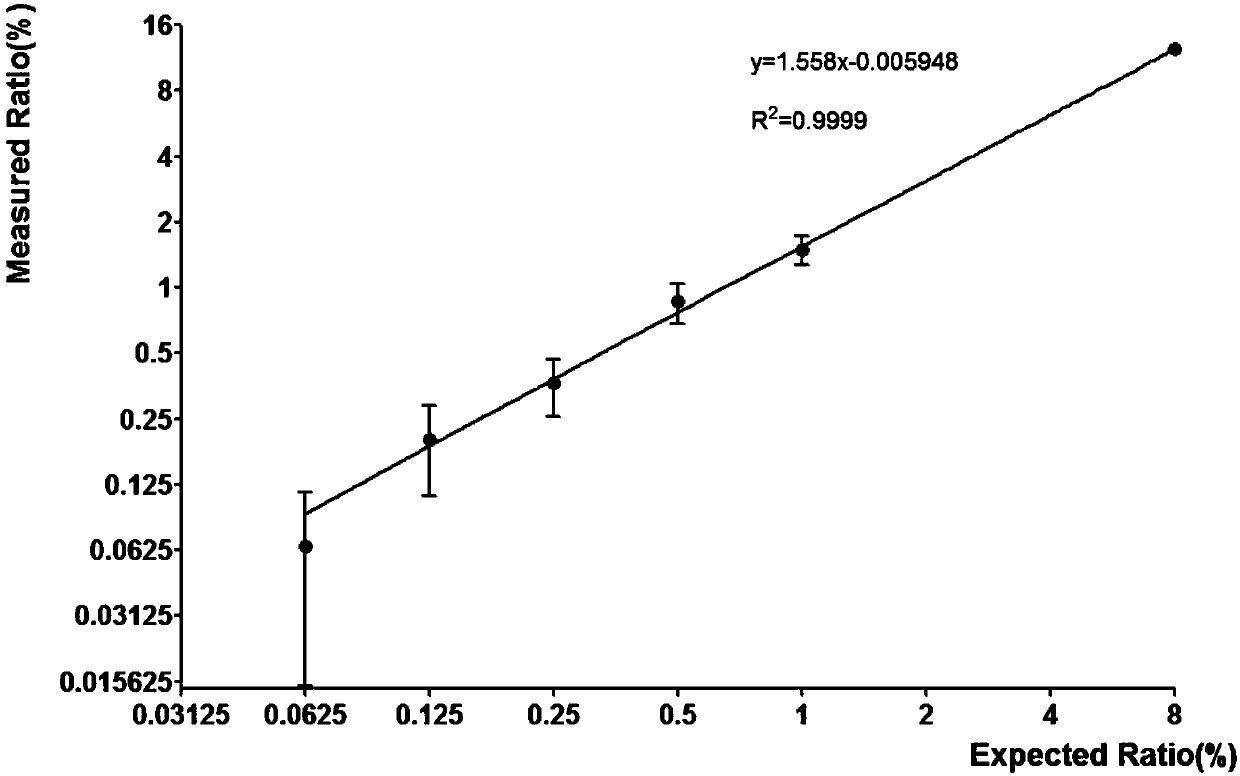
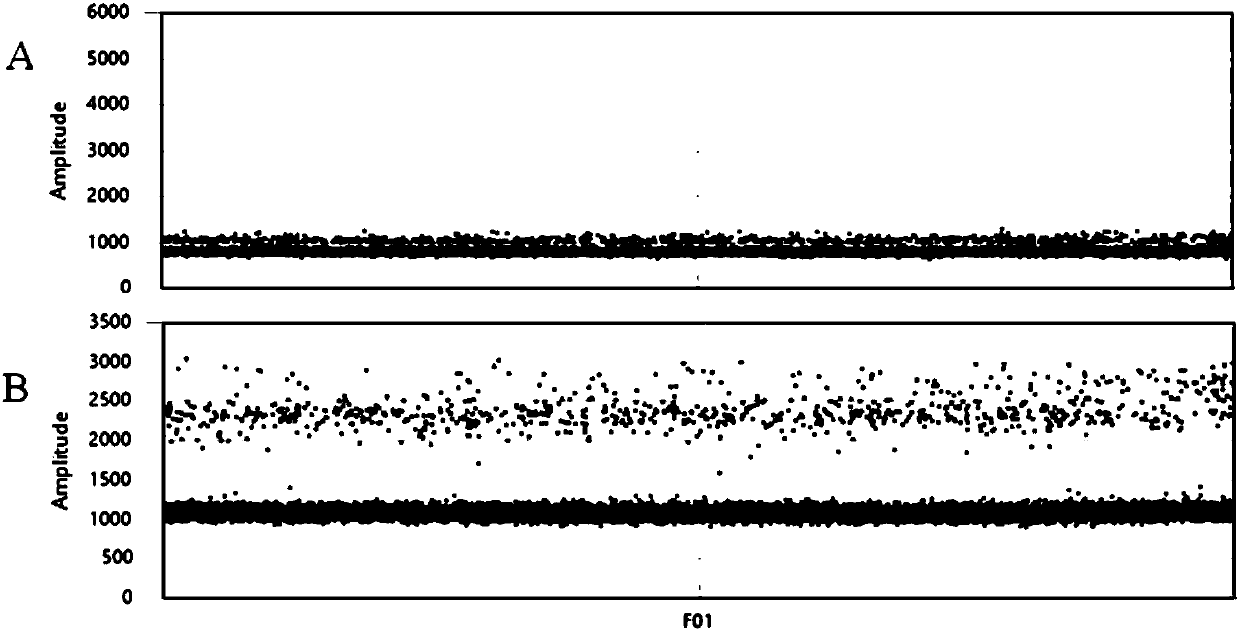
The invention discloses a method for detecting an EGFR L858R locus in lung cancer by ddPCR and an application thereof. According to the sequence of an EGFR gene, a pair of amplified primers includingan upstream primer and a downstream primer are designed; according to the mutation sequence of L858R (2573T>G, COSM6224), a probe for detecting a wild type sequence and a probe for detecting a mutation sequence are designed. The primers and the probes are used for ddPCR and used for detecting and analyzing important loci related to targeted medication for lung cancer in ctDNA. Firstly, DNA is extracted from peripheral plasma of lung cancer patients, and the mutation condition of the mutation locus L858R of EGFR is detected by the ddPCR technology, so as to guide the patients to use drugs. Themethod can be used for diagnosis of early-stage lung cancer such as stage I, II and III A of tumor, can detect 0.0625% EGFR gene L858R mutation, and has the sensitivity reaching 10 times or more thatof conventional methods.
Owner:铭时医疗科技(宁波)有限公司
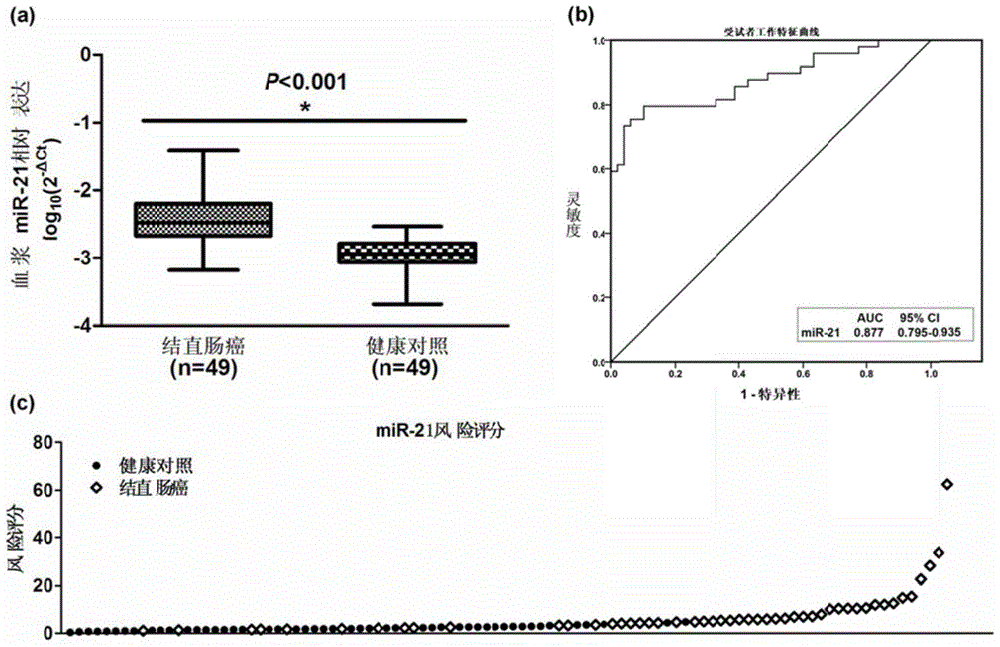
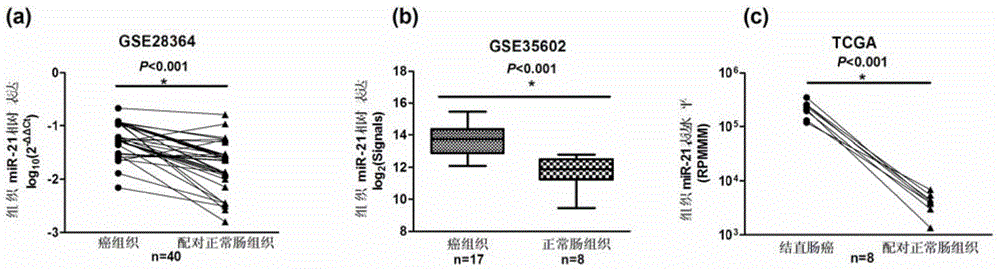
Plasma miRNA biomarker related to colorectal cancer and application thereof
InactiveCN104630357AClinically instructiveSignificant clinical practical valueMicrobiological testing/measurementDifferentially expressed mirnasBlood plasma
The invention provides a plasma miRNA biomarker related to colorectal cancer diagnosis and an application thereof, and belongs to the field of biotechnology and oncology. The plasma miRNA biomarker related to colorectal cancer diagnosis is plasma miR-21. The present study suggests that plasma miRNAs are mainly from secretion of tumor tissues or are induced by death and dissociation of tumor cells, but the miRNAs with differential expression in the tissues do not absolutely experience differential expression in the plasma, and some miRNAs are even reverse. Although the miR-21 has been determined to be capable of being used as the marker in colorectal cancer tissues, results in the reports of peripheral plasma samples are not uniform, the innovation of the invention lies in that the expression trend uniformity of the plasma miR-21 and the tissue miR-21 is determined, the plasma miR-21 can be used as a diagnostic marker and is non-invasive, so that the plasma miR-21 can be applied to future clinic.
Owner:NANJING MEDICAL UNIV
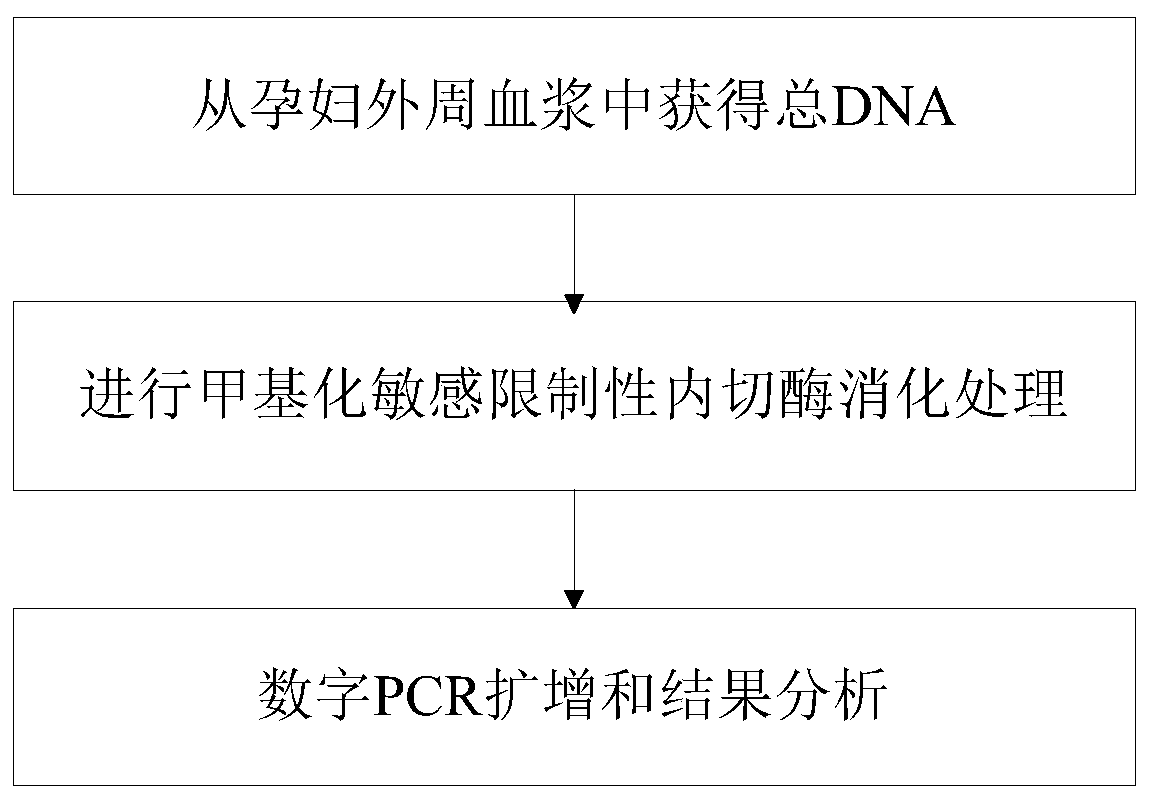
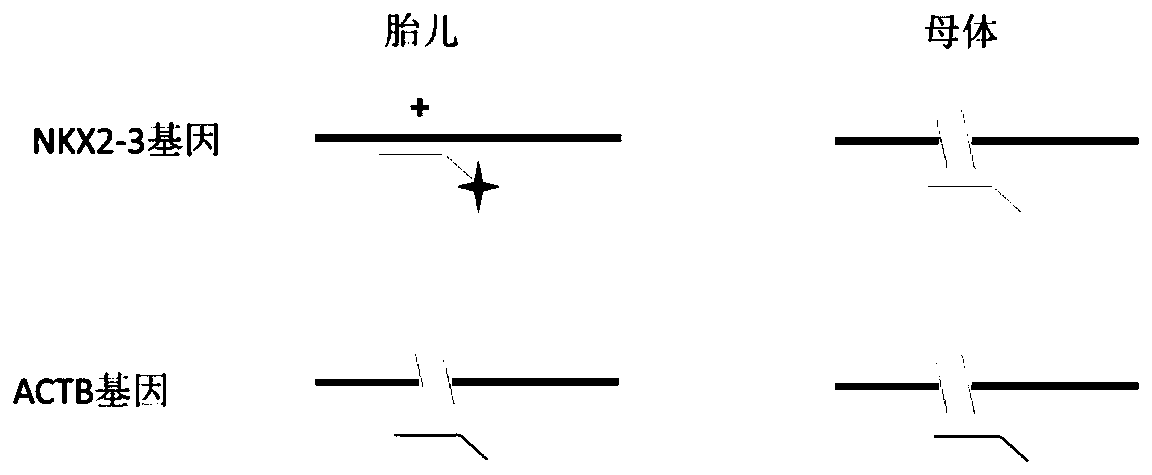
Method for detecting absolute copy number of fetal free DNA in maternal plasma on basis of digital PCR and kit for detecting absolute copy number of fetal free DNA in maternal plasma on basis of digital PCR
PendingCN111154841AThe experimental system is stableThe experimental method is simple and reliableMicrobiological testing/measurementReference genesAntepartum diagnosis
Owner:江苏圣极基因科技有限公司
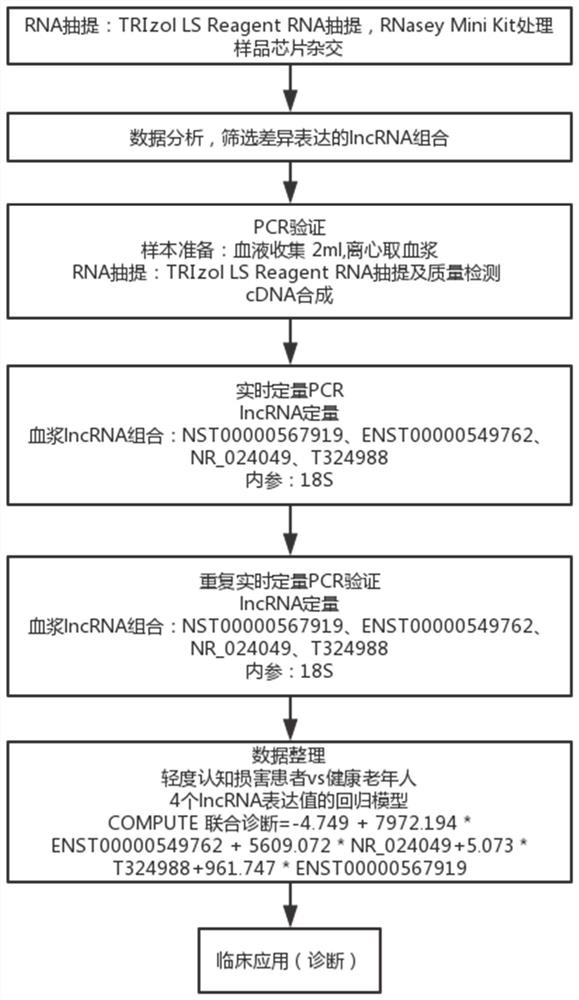
MCI diagnostic marker, MCI diagnostic kit and corresponding detection method
ActiveCN111690739AHigh diagnostic valueBreakthroughMicrobiological testing/measurementDiseaseRNA - Ribonucleic acid
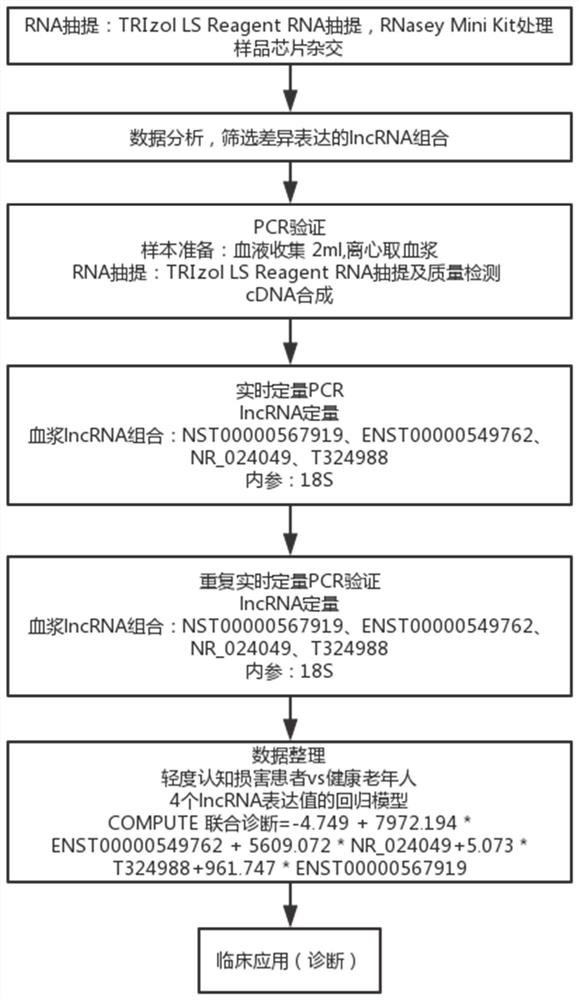
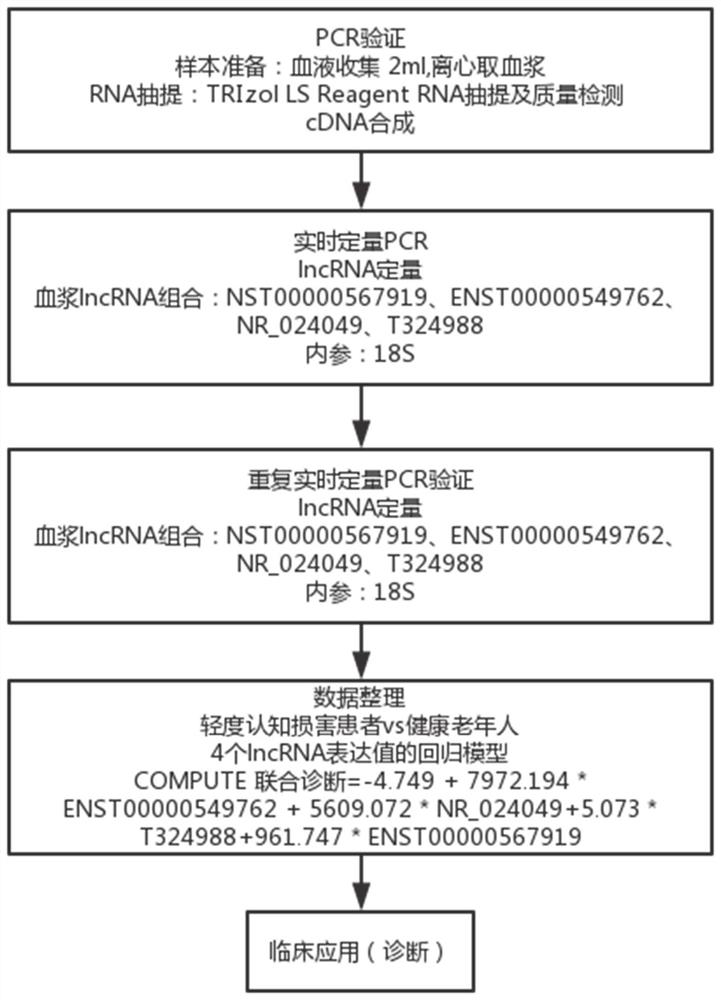
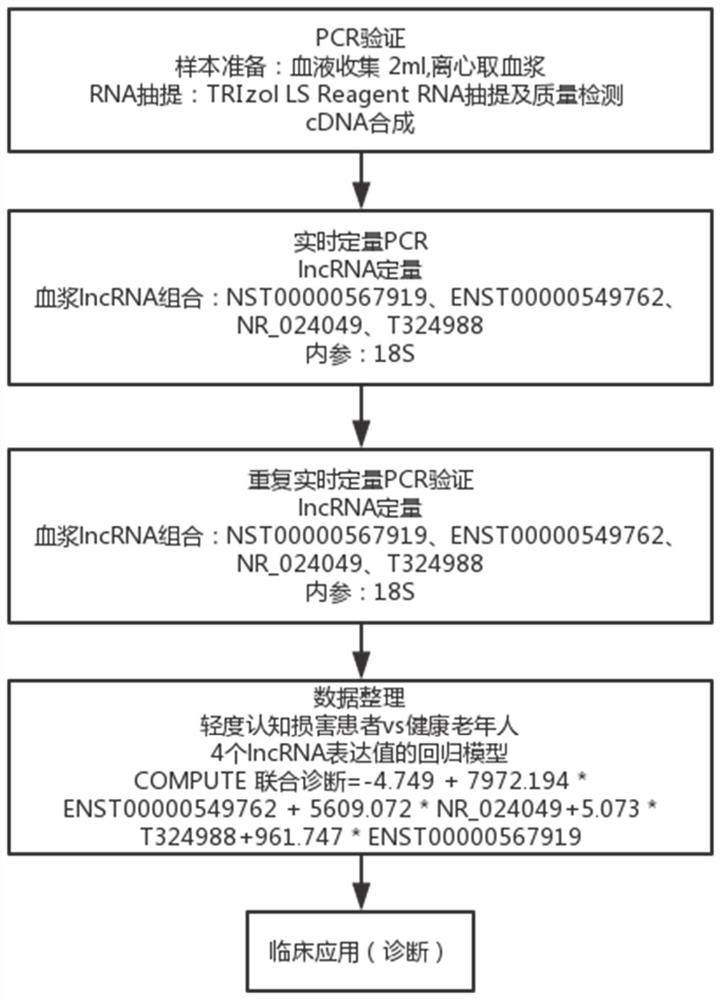
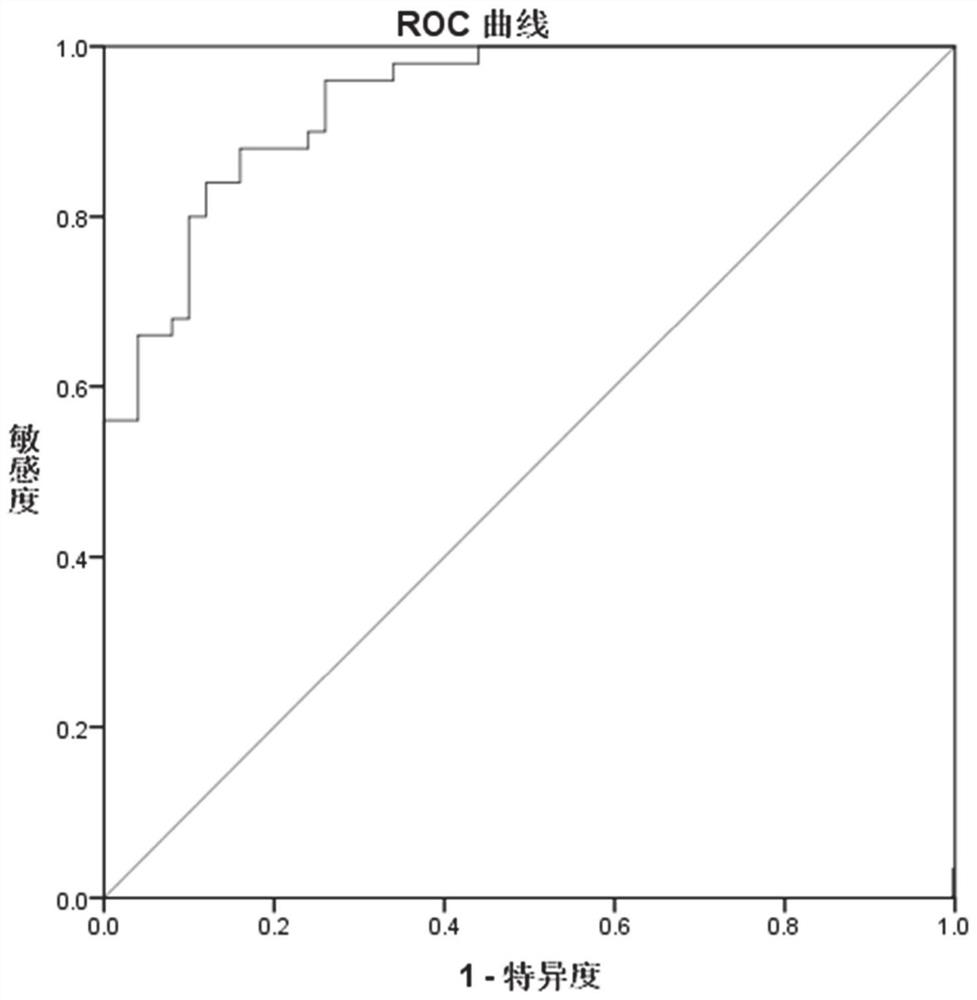
The invention relates to a diagnostic marker for precursor Mild Cognitive Impairment (MCI) of Alzheimer's disease. The marker is plasma long-chain non-coding ribonucleic acid (lncRNA), and the plasmalncRNA comprises one or more of ENST00000549762, NR_024049, T32498 or ENST00000567919. The invention further relates to application of the marker and a corresponding kit. The MCI diagnostic marker, the MCI diagnostic kit and the corresponding detection method have the advantages that the peripheral blood lncRNA biomarker with high diagnostic value for MCI is found for the first time, the effectivemarker is provided for low-damage MCI diagnosis through peripheral plasma, and early diagnosis and early intervention of Alzheimer's disease are facilitated.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT)
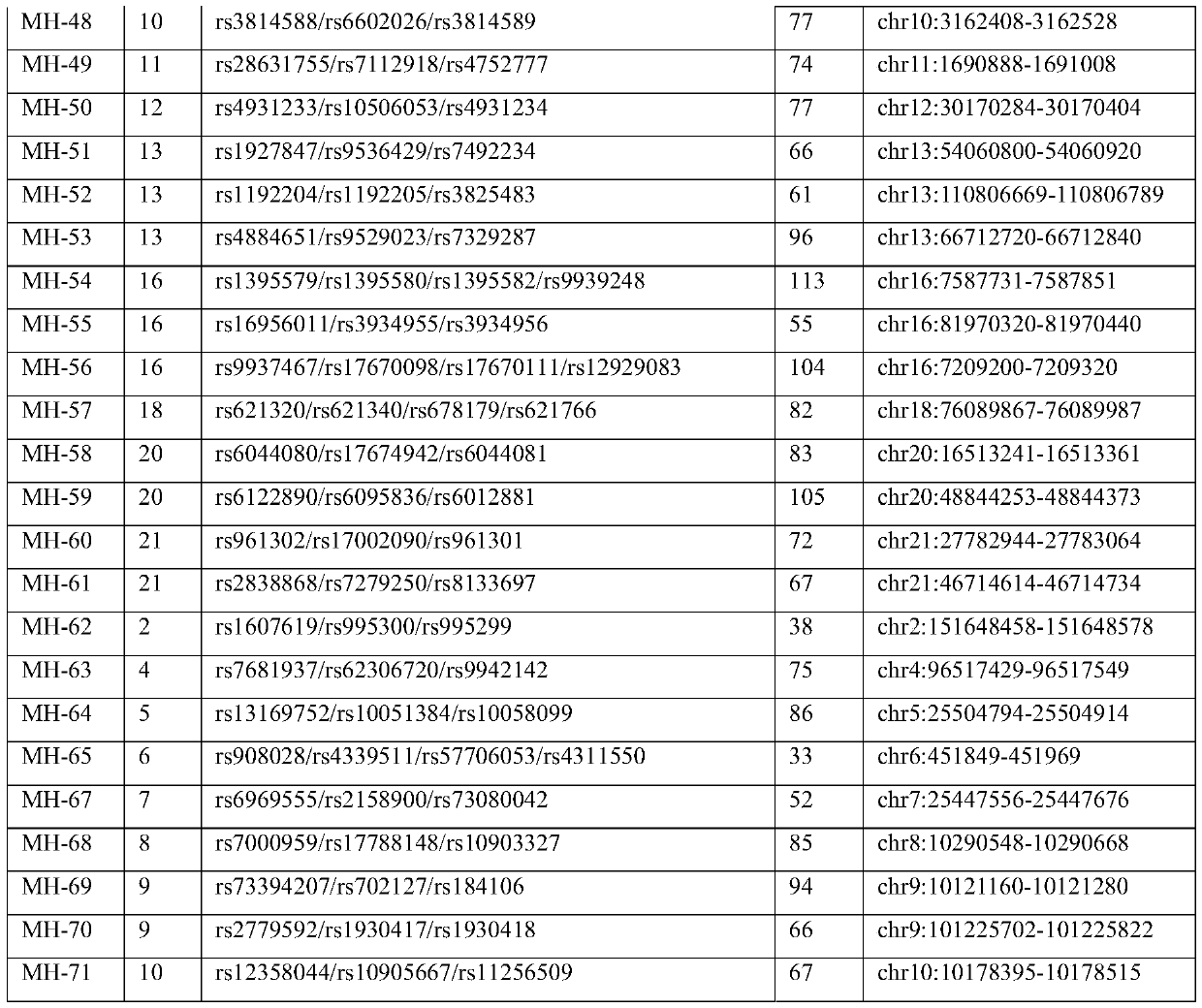
Microhaplotype genetic marker combination and method for noninvasive prenatal paternity relationship judgment
ActiveCN111518917ACause traumaSimple and fast operationMicrobiological testing/measurementProteomicsObstetricsPhysiology
The invention discloses a microhaplotype genetic marker combination and method for noninvasive prenatal paternity relationship judgment. The microhaplotype genetic marker combination for noninvasive prenatal paternity relationship judgment is provided firstly; and a calculation model of a fetal paternity index (PI) is built based on a Bayesian principle according to a sequencing result in combination with a high-throughput sequencing technology by taking microhaplotypes uniformly distributed on autosomes as genetic markers so as to judge a prenatal paternity relationship of gemellary pregnancy. According to the method, only 10ml of peripheral blood of a mother needs to be provided, and free DNA extracted from peripheral plasma of the mother already contains free DNA of a fetus, so that themother and the fetus only need one sample. Because only the venous blood of the pregnant woman needs to be extracted, the operation is simple and convenient, and the pregnant woman and the fetus cannot be wounded; and identification can be carried out after 7 weeks of pregnancy, and a detection result is consistent with that of a conventional STR typing method, so that the application prospect isrelatively great.
Owner:SUN YAT SEN UNIV
Method for monitoring and controlling drug resistance of colorectal cancer patient to panitumumab/cetuximab through ddPCR technology
PendingCN105838778AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMedicinePanitumumab
The invention provides a method for monitoring and controlling drug resistance of a colorectal cancer patient to panitumumab / cetuximab through ddPCR technology, and an application of the method to monitoring and controlling of panitumumab / cetuximab in a process of treating colorectal cancer. The method comprises: firstly extracting DNA from peripheral plasma of a colorectal cancer patient, then detecting a mutation situation of a drug-resistant mutation site G13D of KRAS in the DNA through ddPCR technology, and finally judging if the patient produces drug resistance according to the mutation situation.
Owner:SHANGHAI BIOTECAN PHARMA +2
Method for monitoring and controlling drug resistance of gastrointestinal stromal tumor patient to imatinib/sunitinib through ddPCR technology
PendingCN105838779AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum igeStromal tumor
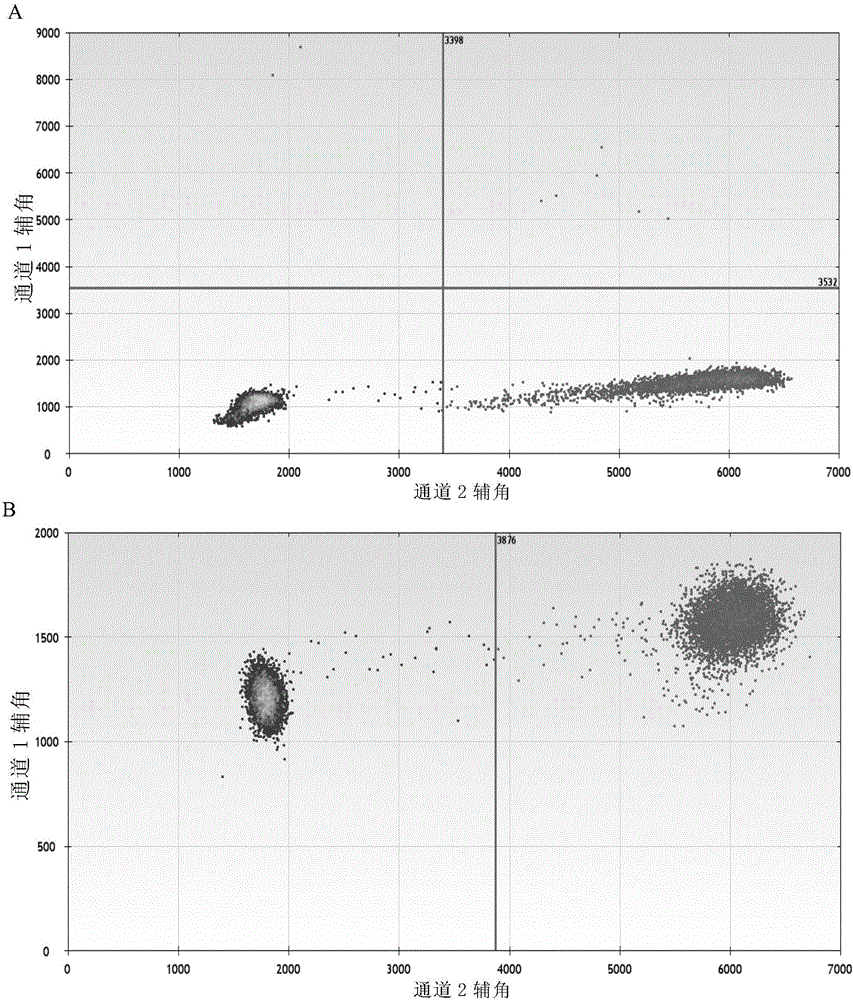
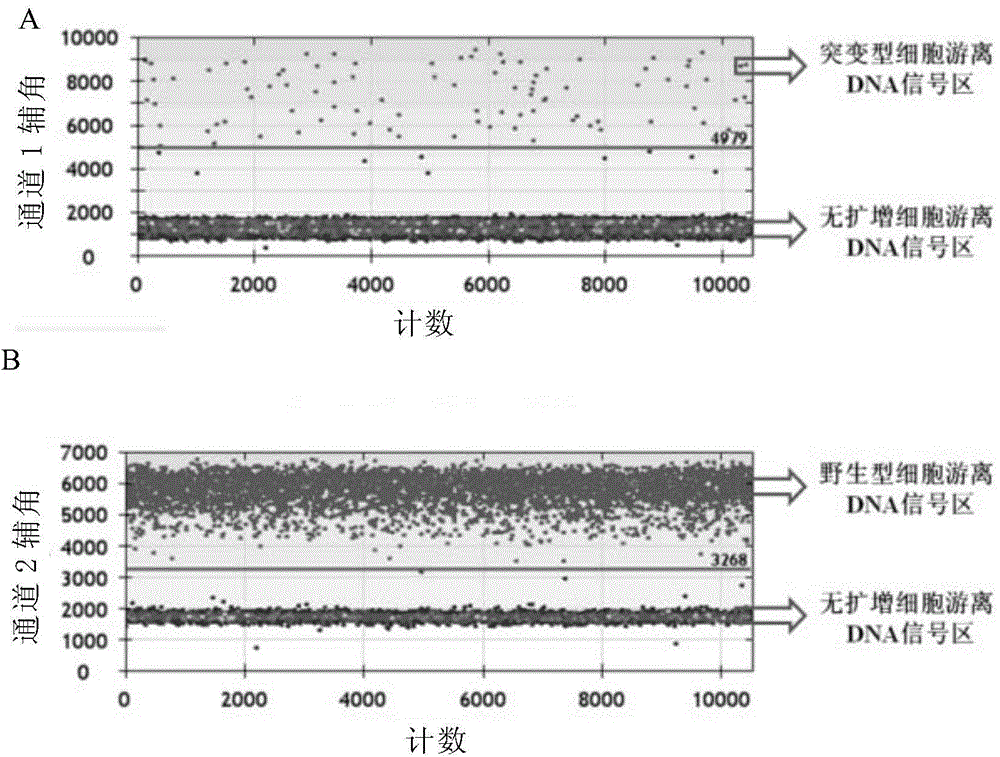
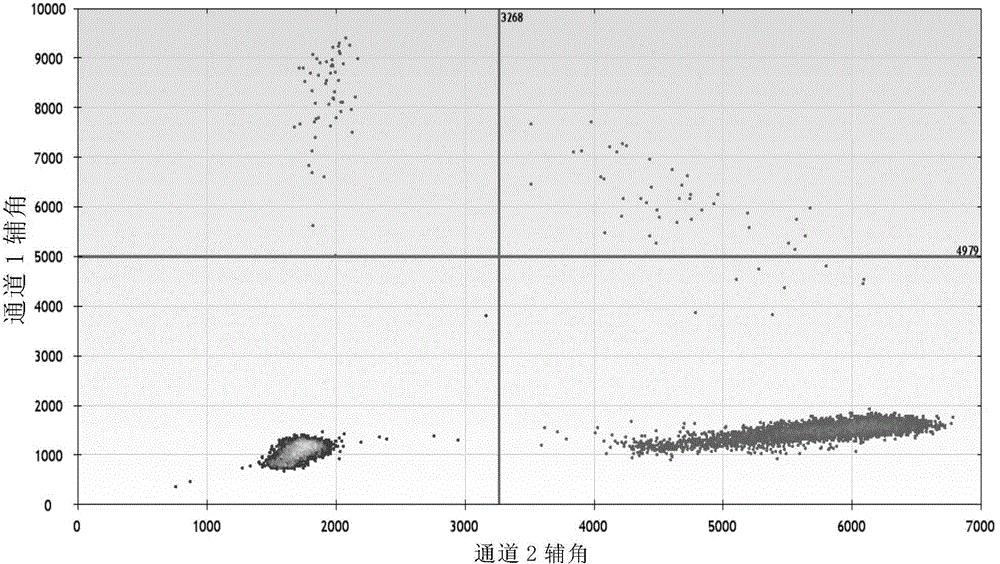
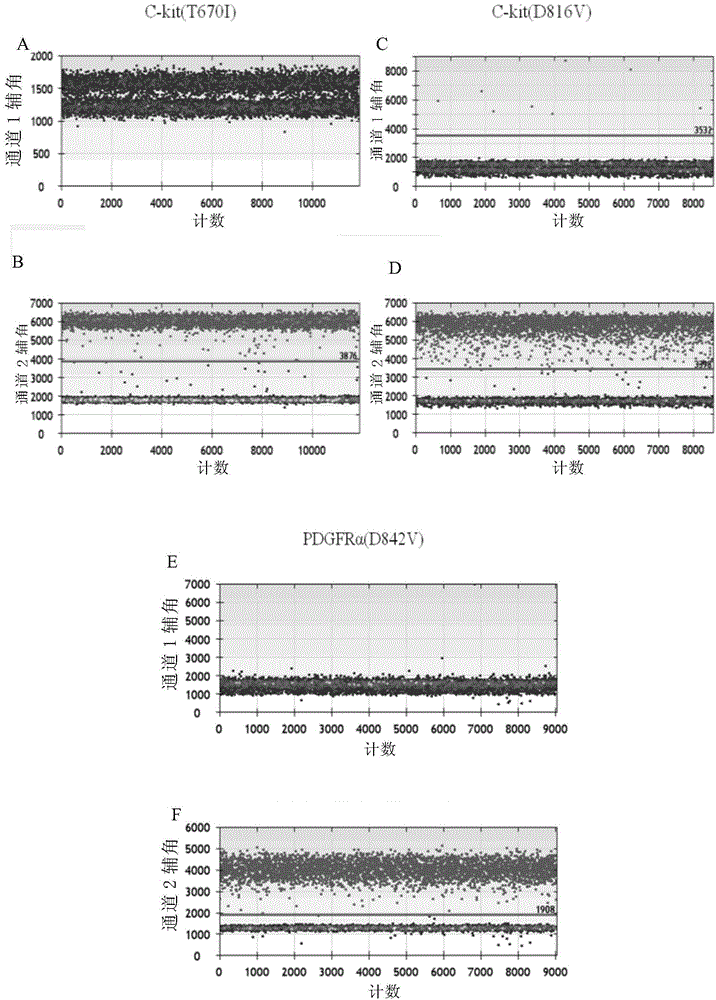
The invention discloses a method for monitoring and controlling drug resistance of a gastrointestinal stromal tumor patient to imatinib / sunitinib through ddPCR technology. The method comprises: extracting DNA from peripheral plasma or serum of a gastrointestinal stromal tumor patient, detecting a mutation situation of a drug-resistant mutation site T670I, D816V, or D842V of C-kit or PDGFR[alpha] in the DNA through ddPCR technology, and judging if the patient produces drug resistance according to the mutation situation.
Owner:SHANGHAI BIOTECAN PHARMA +2
Method for identifying prenatal parental power relationship
PendingCN110724732AReduced sample size requirementsRich choiceMicrobiological testing/measurementBiostatisticsGynecologyObstetrics
The invention relates to a method for identifying prenatal parental power relationship. Through cyclization amplification enlargement, only peripheral 10ul-1.2ml of plasma of a mother needs to be provided, and free DNA extracted from the peripheral plasma of the mother always contains free DNA of a foetus, so that only one part of samples of the mother and the foetus can be needed. Only the veinalblood of a pregnant woman needs to be extracted, the operation is simple and convenient, and the blood volume is small, so that the pregnant woman and the foetus cannot be damaged, and identificationcan be performed after pregnancy for 10 weeks.
Owner:陈洪亮
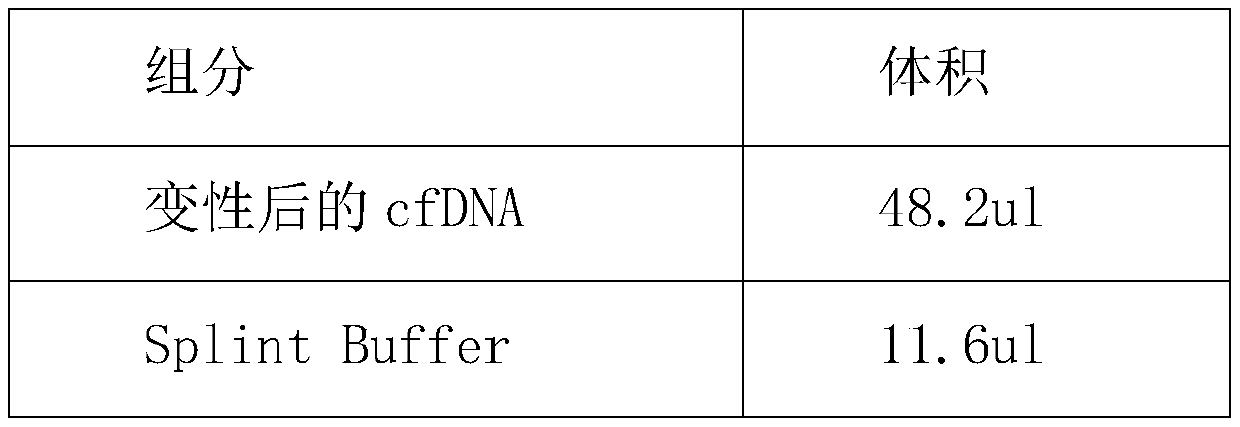
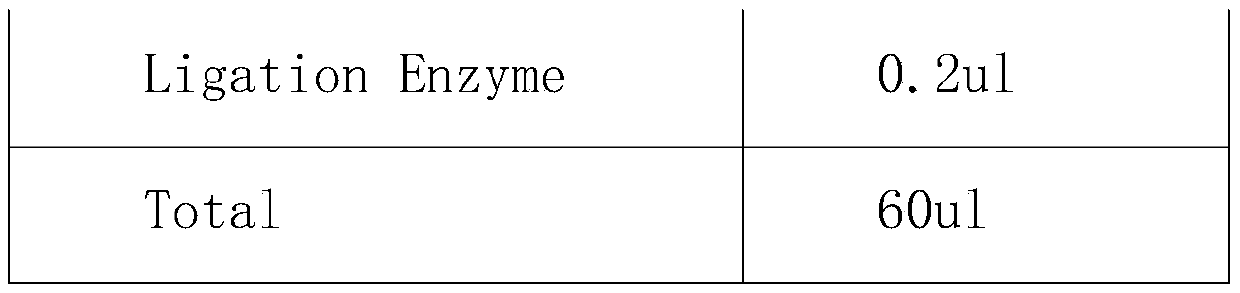
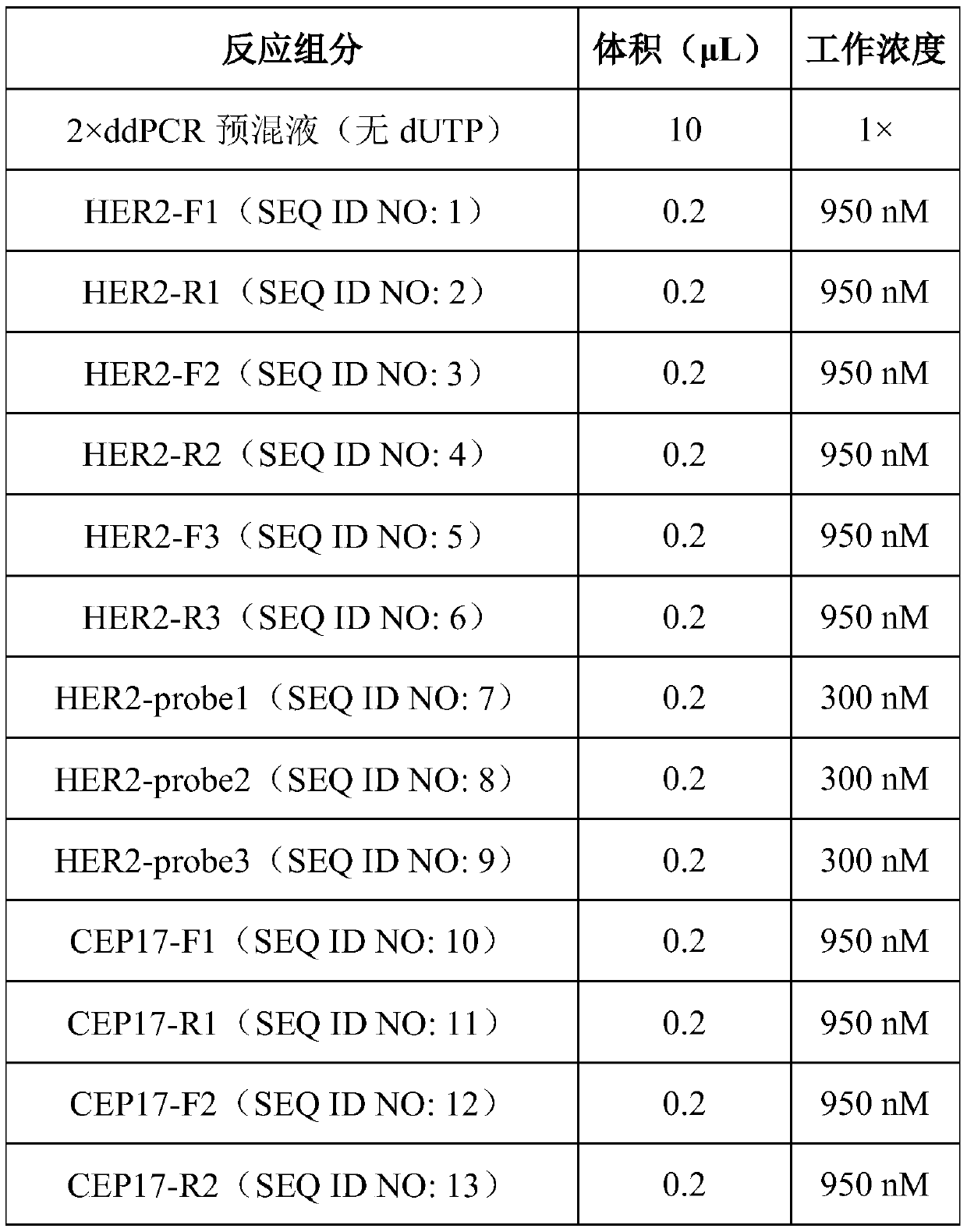
HER2 (human epidermal growth factor receptor-2) detection kit and detection method and application thereof
PendingCN110129448AHigh sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationReference genesBlood plasma
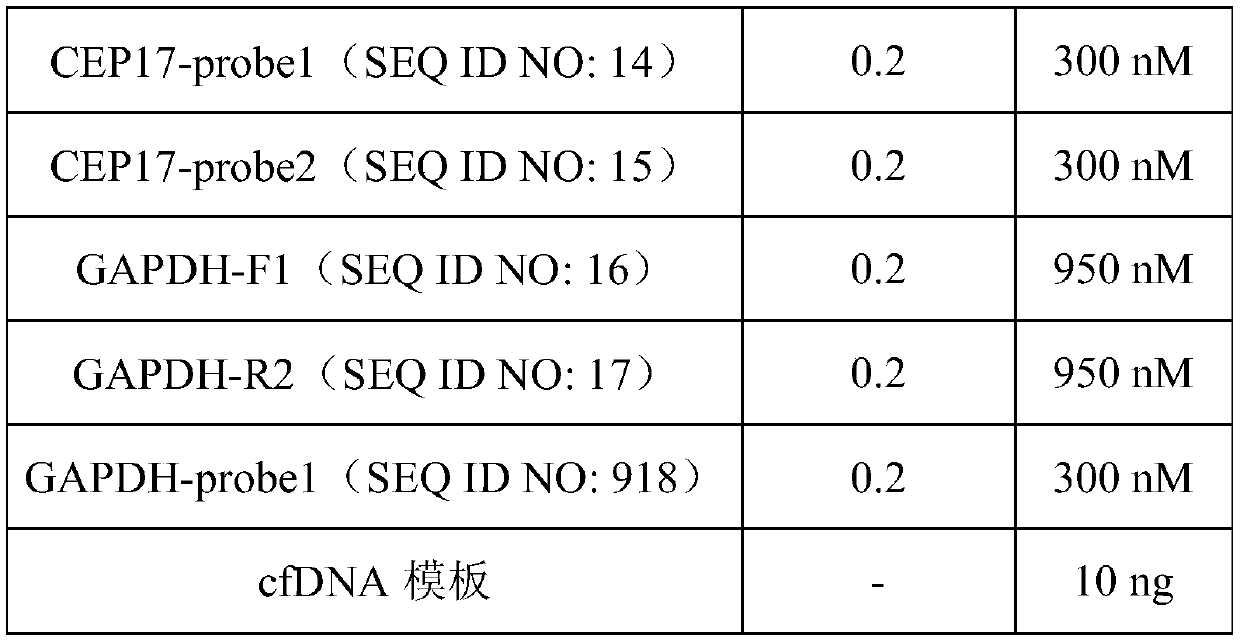
The invention provides an HER2 (human epidermal growth factor receptor-2) detection kit and a detection method and application thereof. Primers of HER2 are shown in SEQ ID NO.:1-6; probes of the HER2are shown in SEQ ID NO.:7-9. The primers and the probes are designed for HER2 genes, which cooperates with primers and probes of internal reference genes CEP17 and GAPDH, the amplification mutation ofDNA genes in paraffin embedded pathological section or cfDNA genes in peripheral plasma of patients with breast cancer is detected quantitatively according to the ratio of HER2 genes to the internalreference genes, and key information is provided for targeted drug use; the HER2 detection kit and the detection method and application thereof have the advantages of absolute quantification, short detection cycle, high specificity, high accuracy, high sensitivity and the like.
Owner:深圳海普洛斯医学检验实验室
Application of miR-145-3p in preparing medicines for preventing or treating multiple myeloma disease
ActiveCN106420791APromote apoptosisPromote autophagyOrganic active ingredientsMicrobiological testing/measurementDiseaseAutophagic death
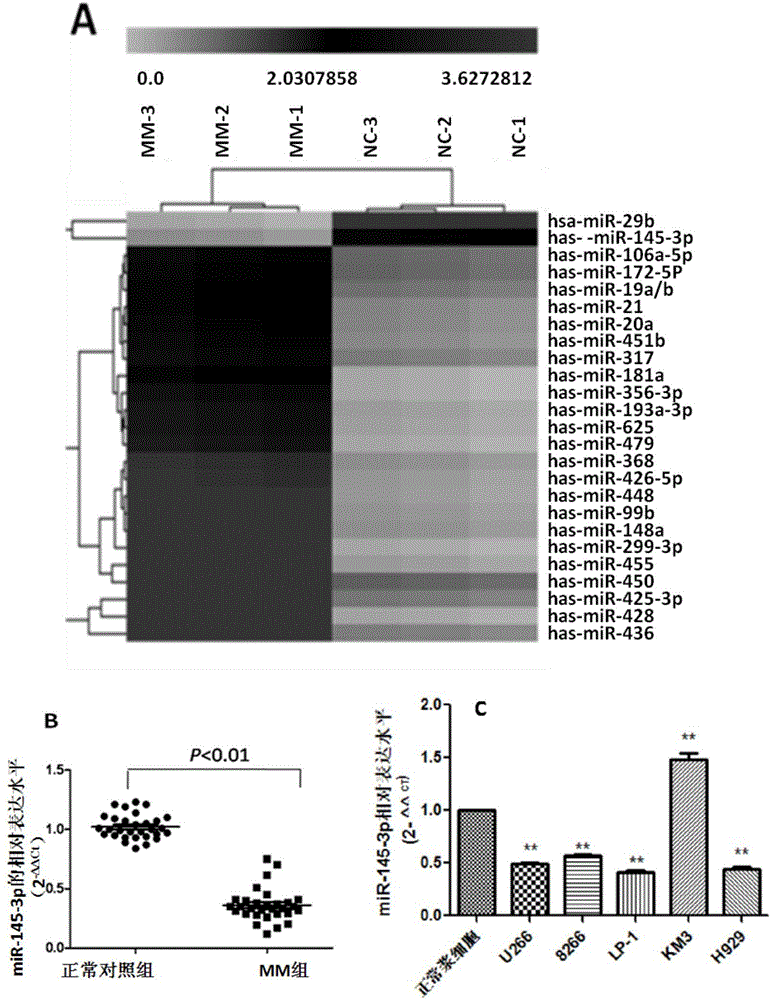
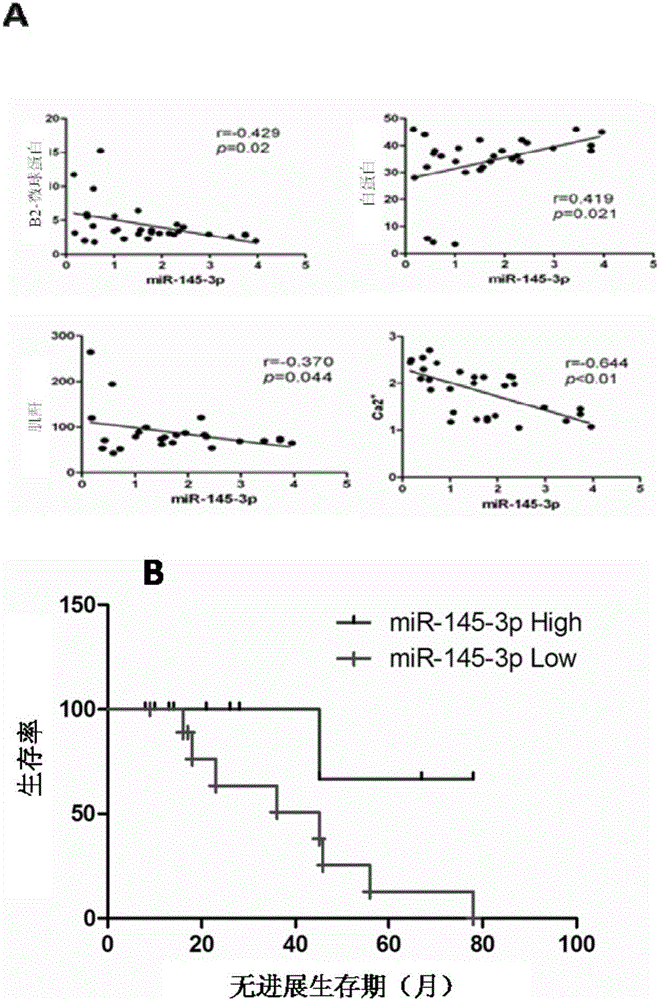
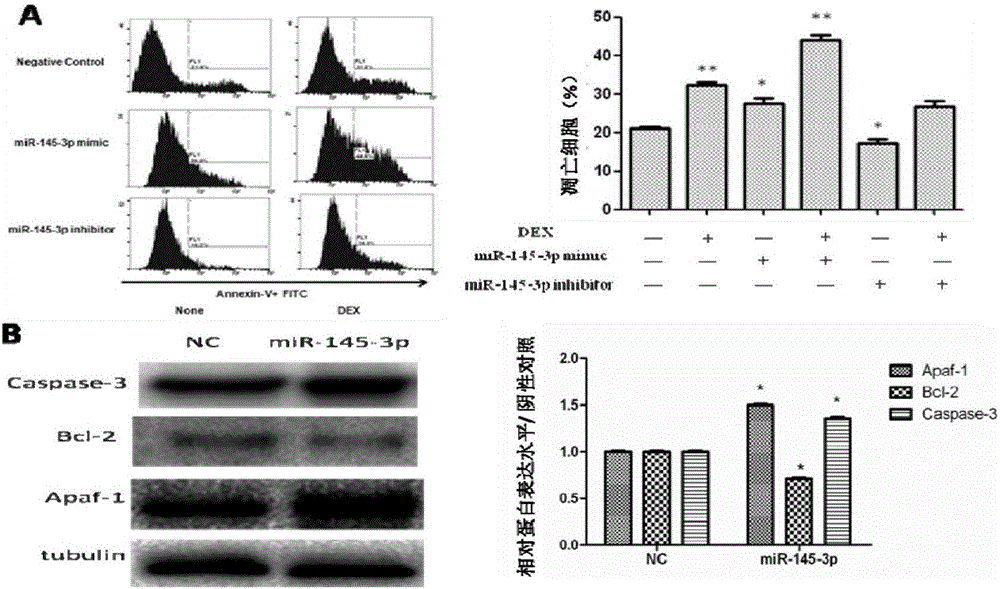
The invention relates to the technical field of biological medicines. According to the invention, peripheral plasma of a patient with multiple myeloma is subjected to microRNAs expression profile analysis, and based upon qPCR technical verification, it discovers that the expression level of miR-145-3p in the peripheral plasma of the patient with the multiple myeloma is significantly reduced in comparison that in normal and healthy people. In addition, by conducting miR-145-3p over-expression and miR-145-3p silencing and by observing cell apoptosis and autophagy levels, results show that the miR-145-3p is capable of promoting cell apoptosis and autophagy. The invention provides a novel serum marker for the diagnosis of the multiple myeloma disease and also provides a novel target for the prevention and treatment of the multiple myeloma disease.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
Antibody composition for detecting treatment effect of multiple myeloma as well as kit and application thereof
ActiveCN112698037ARelieve painImprove work efficiencyDisease diagnosisIndividual particle analysisTreatment effectAntiendomysial antibodies
The invention discloses an antibody composition for detecting the treatment effect of multiple myeloma as well as a kit and application thereof, and the antibody composition scheme provided by the invention comprises a flow antibody combination which is never clinically used for detecting peripheral blood tumor plasma cells in the past, stipulates the number of detected cells and standard operation steps, and defines the degree to which the sensitivity needs to be reached. Peripheral blood is adopted to replace a bone marrow specimen, the pain of a patient receiving bone marrow puncture can be greatly relieved, peripheral plasma cell detection can be repeatedly operated, flexibility and convenience are achieved, the workload of clinicians is greatly relieved, and efficiency is improved.
Owner:BEIJING JISHUITAN HOSPITAL
Method for monitoring secondary drug resistance to imatinib (Glivec)/nilotinib through ddPCR technology
PendingCN105838781AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationChronic granulocytic leukemiaMonitoring and control
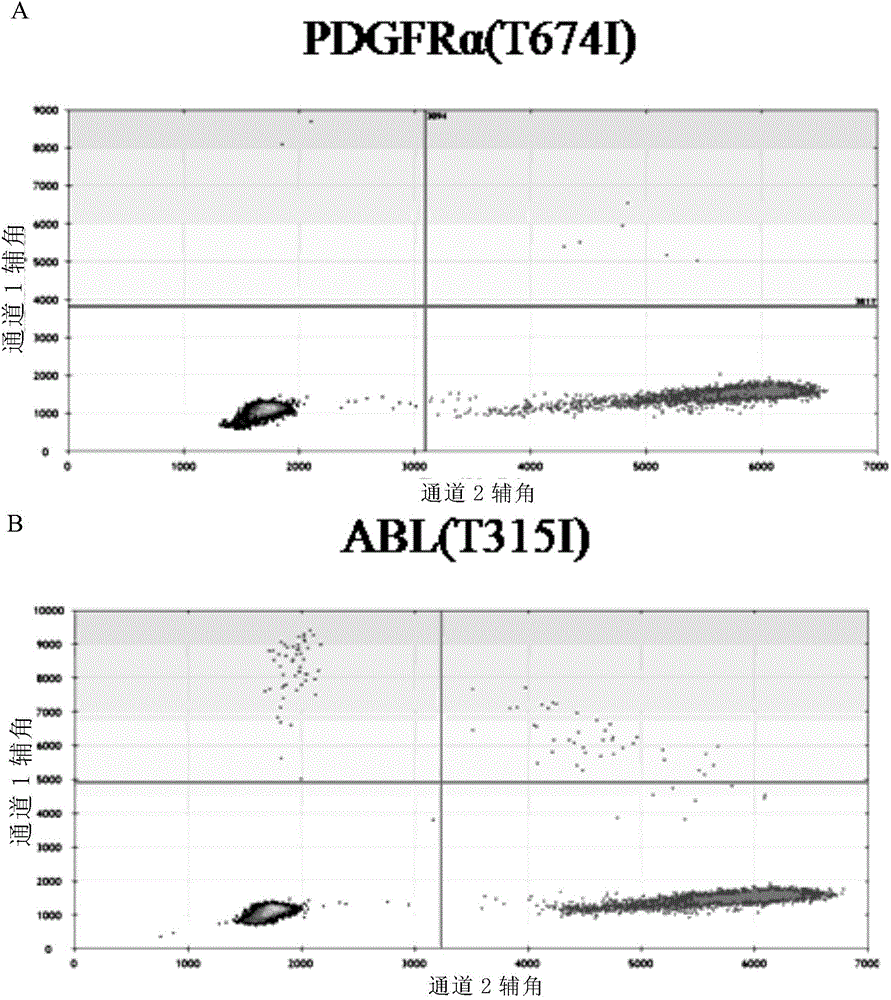
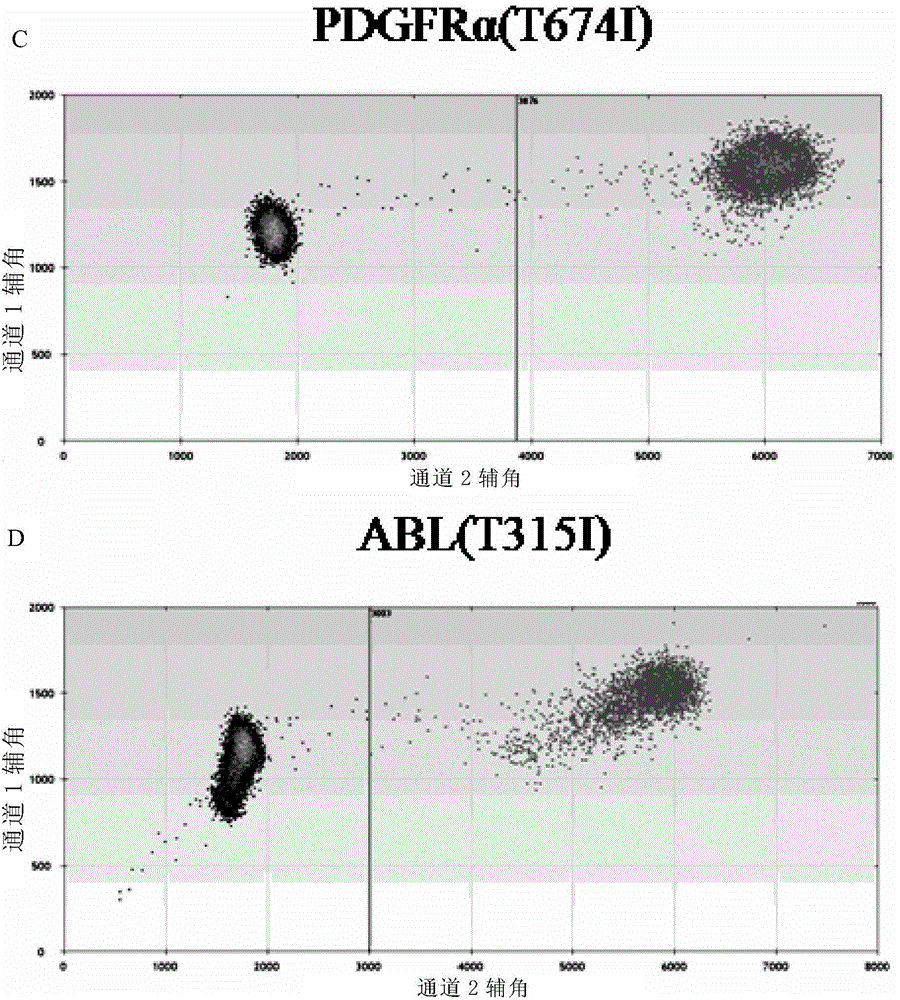
The invention provides a method for monitoring secondary drug resistance to imatinib (Glivec) / nilotinib through ddPCR technology, and an application of the method to monitoring and control of imatinib (Glivec) / nilotinib in a process of treating chronic granulocytic leukemia. The method comprises: firstly, extracting DNA from peripheral plasma of a patient suffering from chronic granulocytic leukemia, then detecting a mutation situation of a drug-resistant mutation site T315I of an ABL gene or a drug-resistant mutation site T674I of a PDGFR[alpha] gene in the DNA through ddPCR technology, and finally judging if the patient produces drug resistance according to the mutation situation.
Owner:SHANGHAI BIOTECAN PHARMA +2
Preparation of Human-Human Cell Fusion Maternal-Fetal Blood Group Incompatibility Therapy Hybrid Strain
InactiveCN109157692AIncrease the cultivation stepPromote directed differentiationOther blood circulation devicesMedical devicesDiseasePlasma Exchanges
A person who is used in the field of medicine- Preparation of human cell fusion maternal-fetal blood group incompatibility therapy hybrid strain, characterized by, Using erythropoietin as inducer, ''O'' type human bone marrow cell positive for Rh were cultured, to increase the number of directionally developing erythroid cells, human bone marrow cells and human myeloma cells were fused into hybridized cells, Erythropoietin was use to induce that directional differentiation of the hybridized cell, Hybrid cell clones were screened by HAT, The hybridization strains of Rh positive cells which canbe expanded indefinitely in vitro were screened by Rh antibody, As that adsorbent aft industrialized amplification, A low-cost and safe method for that treatment of maternal-fetal blood group incompatibility hemolytic disease without plasma exchange solution without remove plasma and its beneficial ingredients is create by absorbing pathogenic Rh antibody in peripheral plasma of pregnant women with maternal-fetal blood group incompatibility through an in vitro adsorption device which is formed by an adsorber and a blood separator.
Owner:翁炳焕
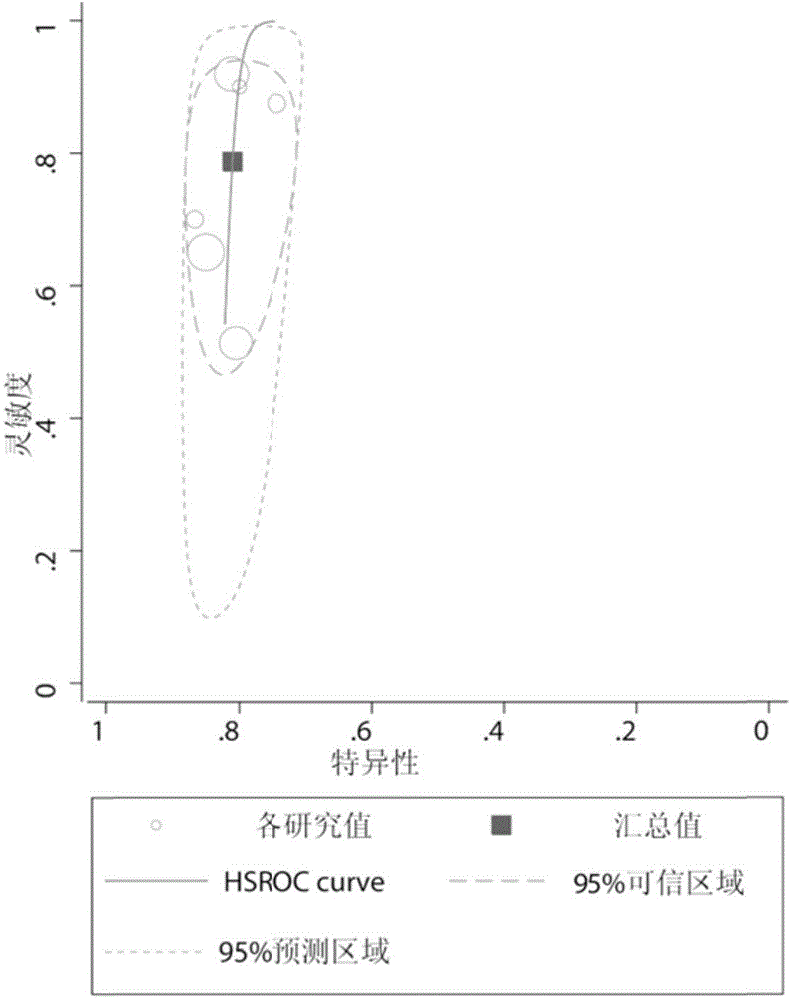
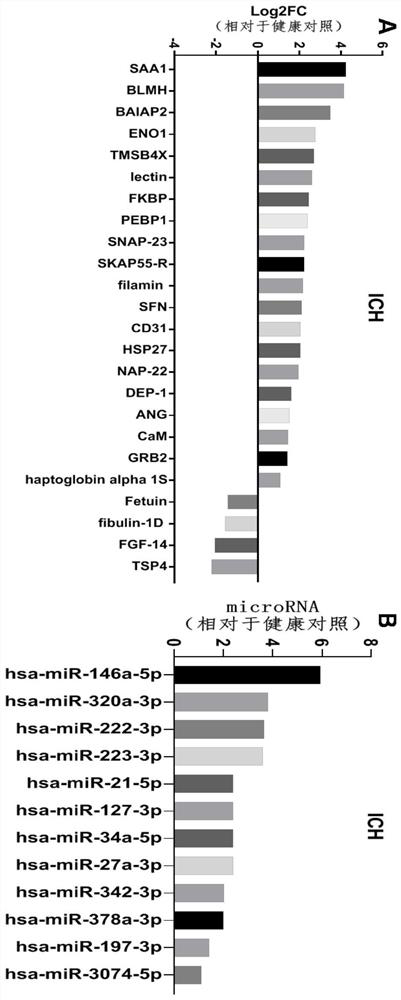
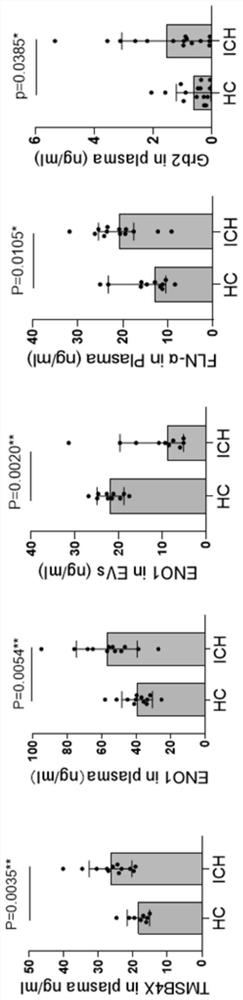
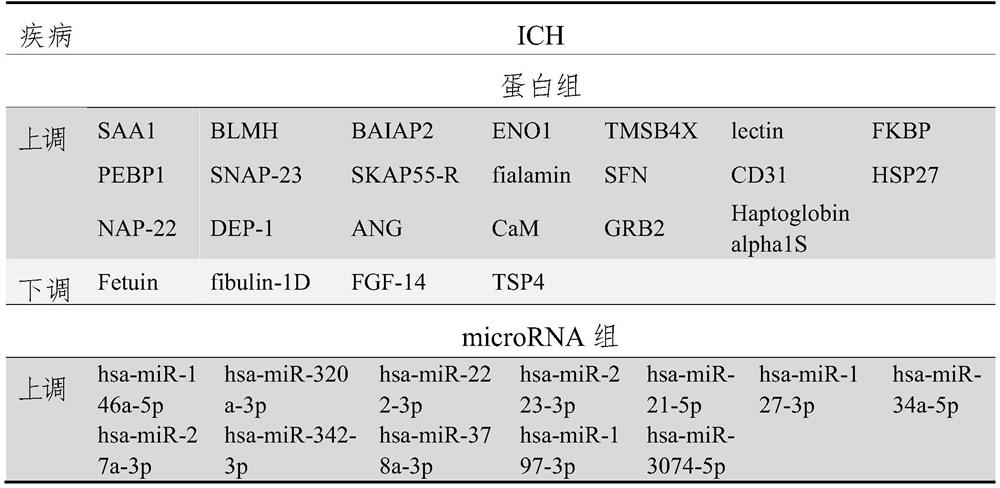
Biomarkers for ich prognostic assessment and their applications
ActiveCN113970640BMicrobiological testing/measurementDisease diagnosisBiomarker panelTransient ischemic attack (TIA)
The present disclosure provides a biomarker for ICH prognosis assessment and application thereof. Specifically, the present disclosure provides a reagent for detecting at least one peripheral plasma or exosomal protein, or at least one exosomal microRNA, in the preparation of a reagent for predicting, diagnosing or monitoring transient ischemic attack or application in the kit. The technical solutions provided by the present disclosure form a new biomarker panel that helps to better understand the pathophysiology of ICH, and will provide new opportunities for diagnosis and prognosis, thereby improving clinical services for ICH patients.
Owner:BEIJING TIANTAN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
An antibody composition for detecting the therapeutic effect of multiple myeloma, its kit and application
ActiveCN112698037BRelieve painImprove work efficiencyDisease diagnosisIndividual particle analysisPlasma cellTherapeutic effect
Owner:BEIJING JISHUITAN HOSPITAL
MCI diagnostic marker, MCI diagnostic kit and corresponding detection method
PendingCN113667737AHigh diagnostic valueBreakthroughMicrobiological testing/measurementDiseaseRadiology
The invention relates to a diagnostic marker for prophase mild cognitive function impairment (MCI) of Alzheimer's disease. The marker is a plasma long-chain non-coding ribonucleic acid (lncRNA), and the plasma lncRNA comprises one or more of ENST00000549762, NR_024049, T324988 or ENST00000567919. The invention further relates to application of the marker and a corresponding kit. The MCI diagnostic marker has the beneficial effects that the peripheral blood lncRNA biomarker with higher diagnostic value on MCI is found for the first time, an effective marker is provided for low-damage diagnosis of MCI by adopting peripheral plasma, and early diagnosis and early intervention of Alzheimer's disease are facilitated.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT)
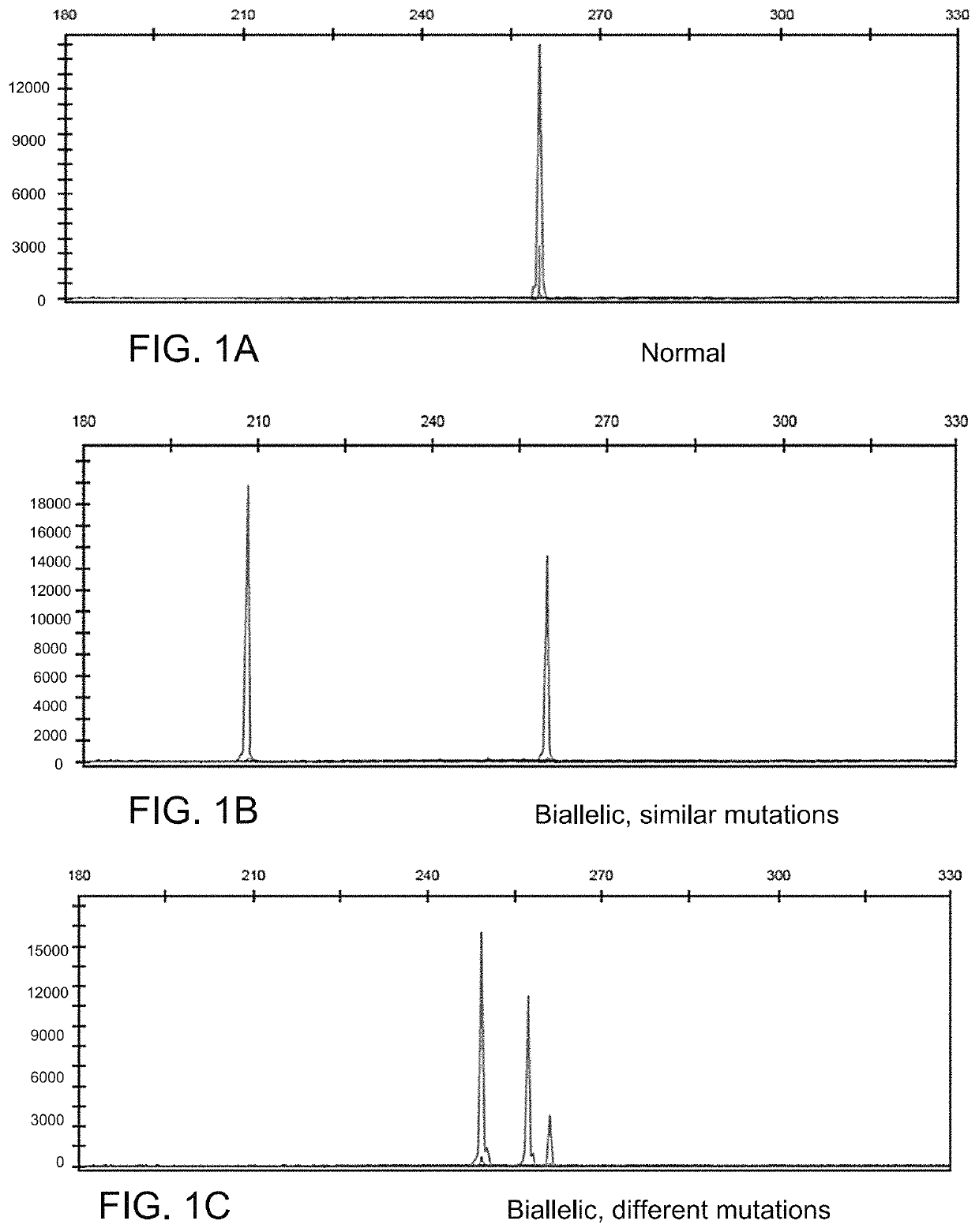
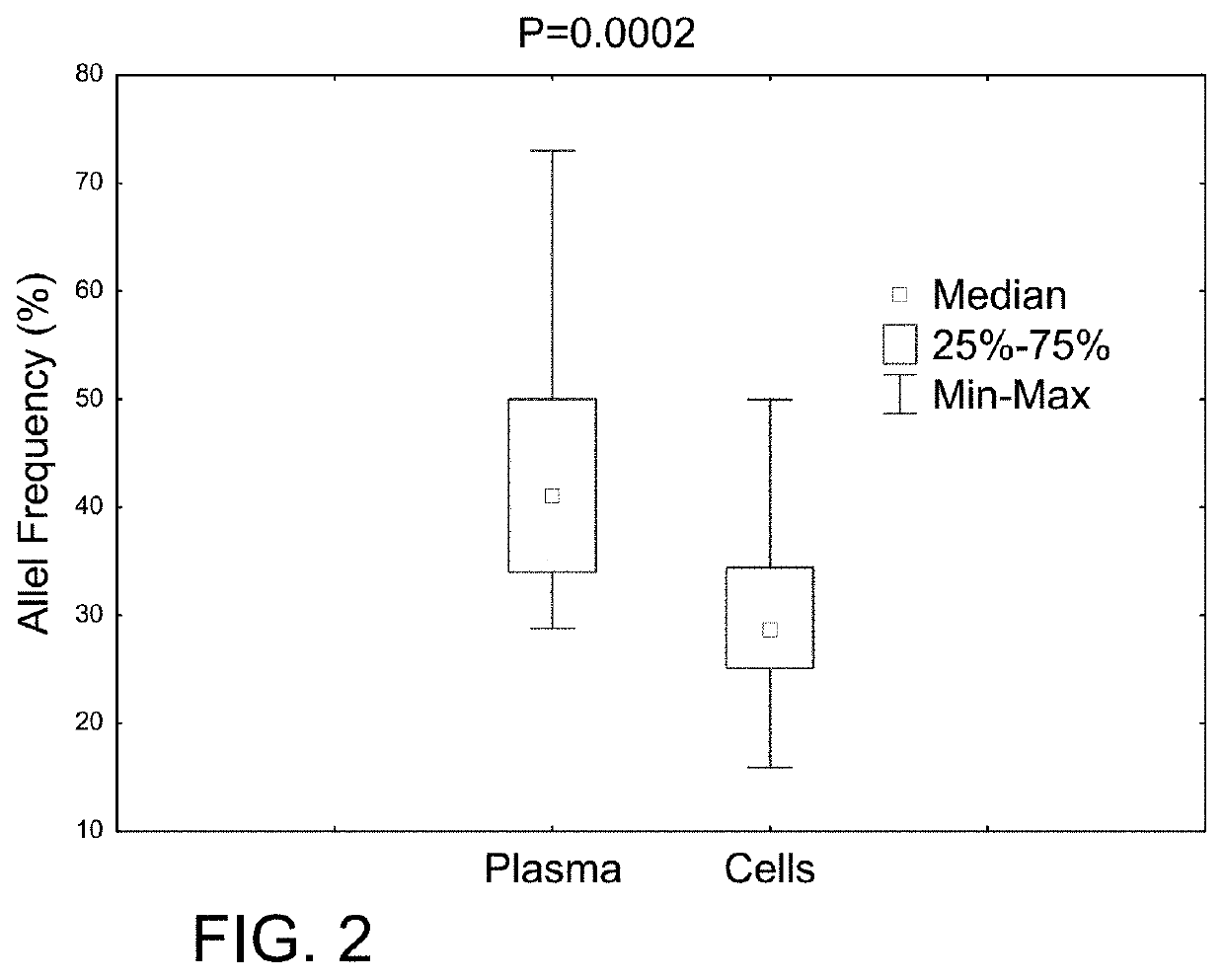
Determining tumor load and biallelic mutation in patients with CALR mutation using peripheral blood plasma
ActiveUS10550435B2Microbiological testing/measurementX-ray/gamma-ray/particle-irradiation therapyTumor LoadMyeloid hyperplasia
Compositions and fragment length analysis methods are provided for detecting CALR mutations and determining tumor load in patients with myeloproliferative neoplasms.
Owner:NEOGENOMICS LAB
Three-Dimensional Cross-Linked Scaffolds Of Peripheral Blood Plasma And Their Use
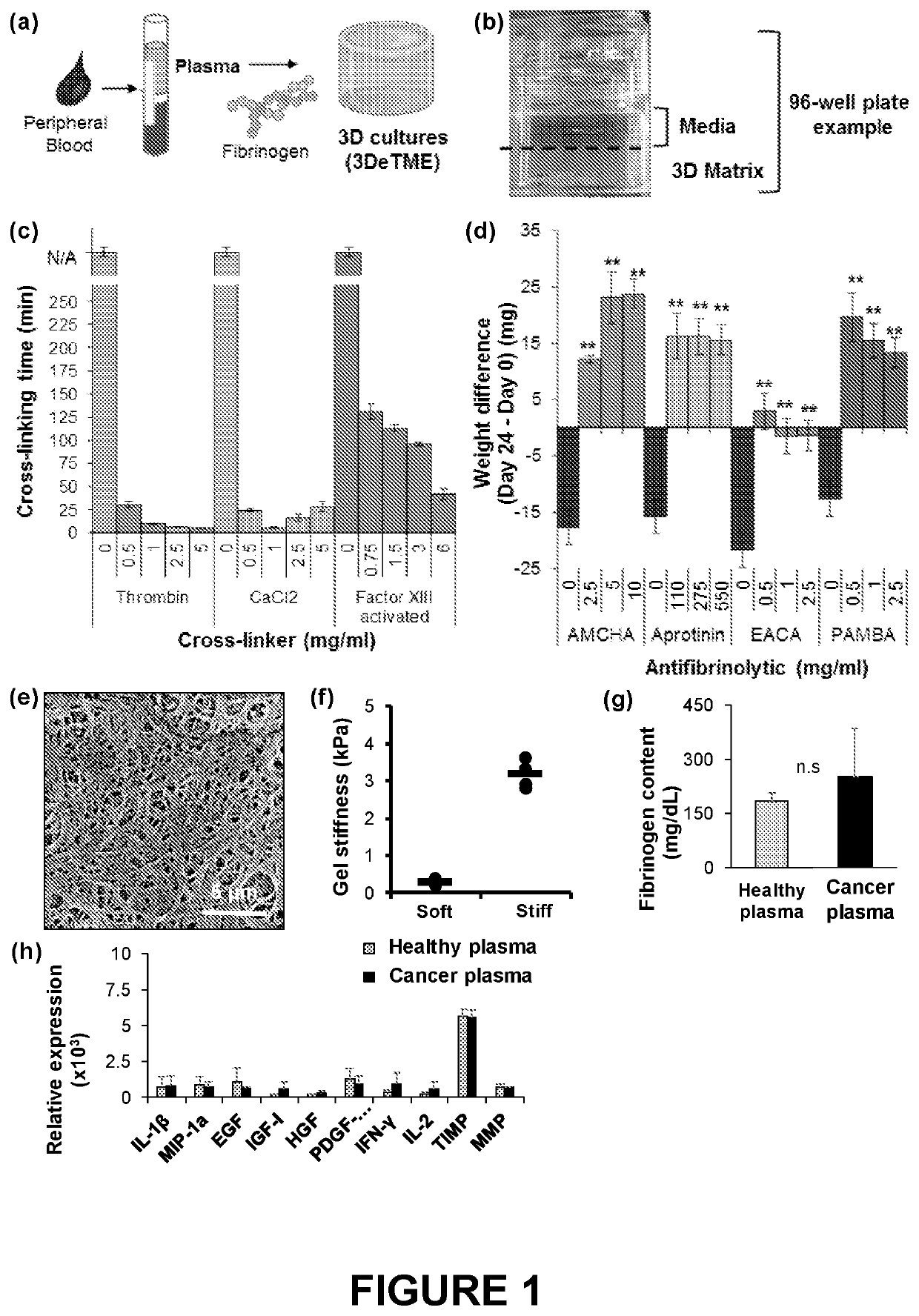
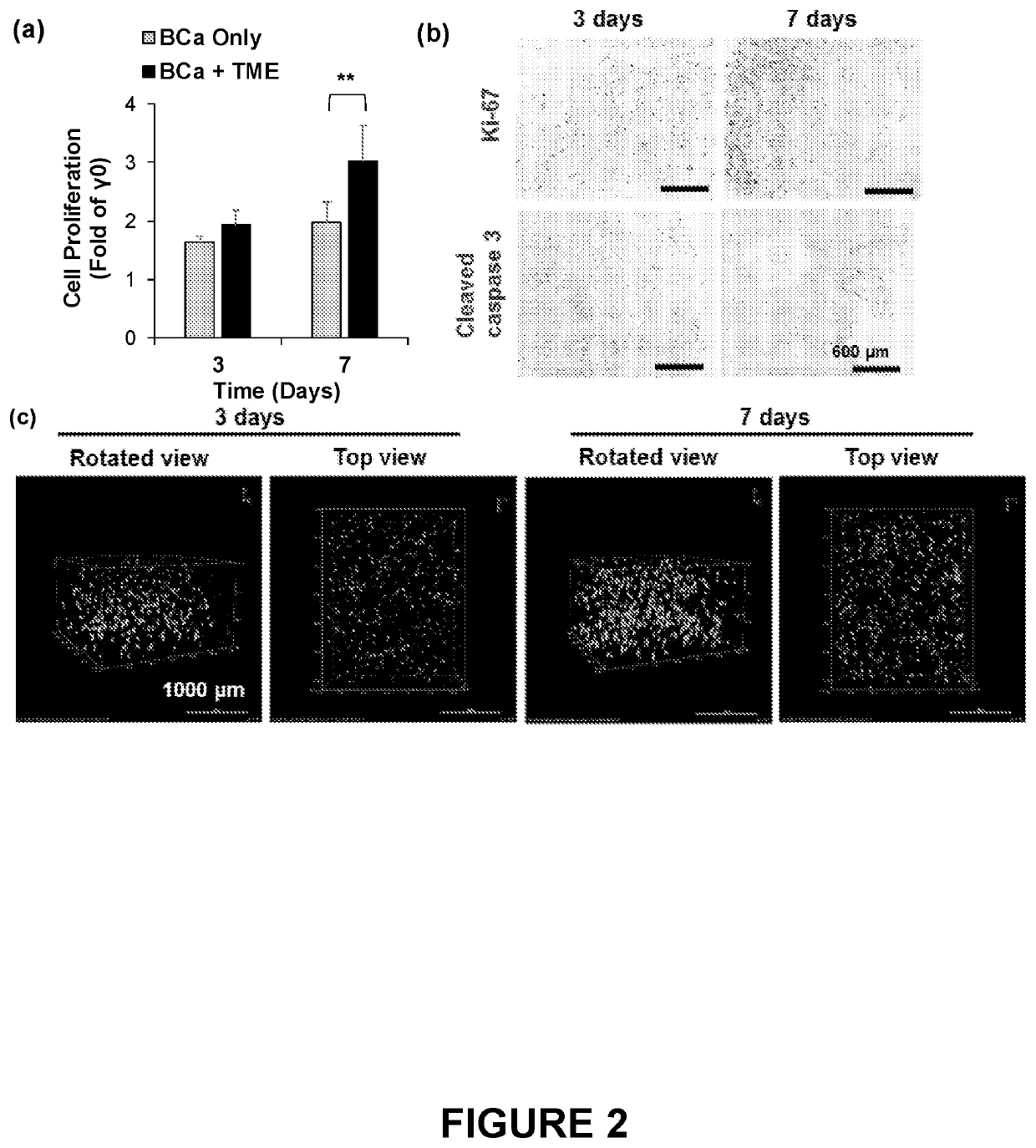
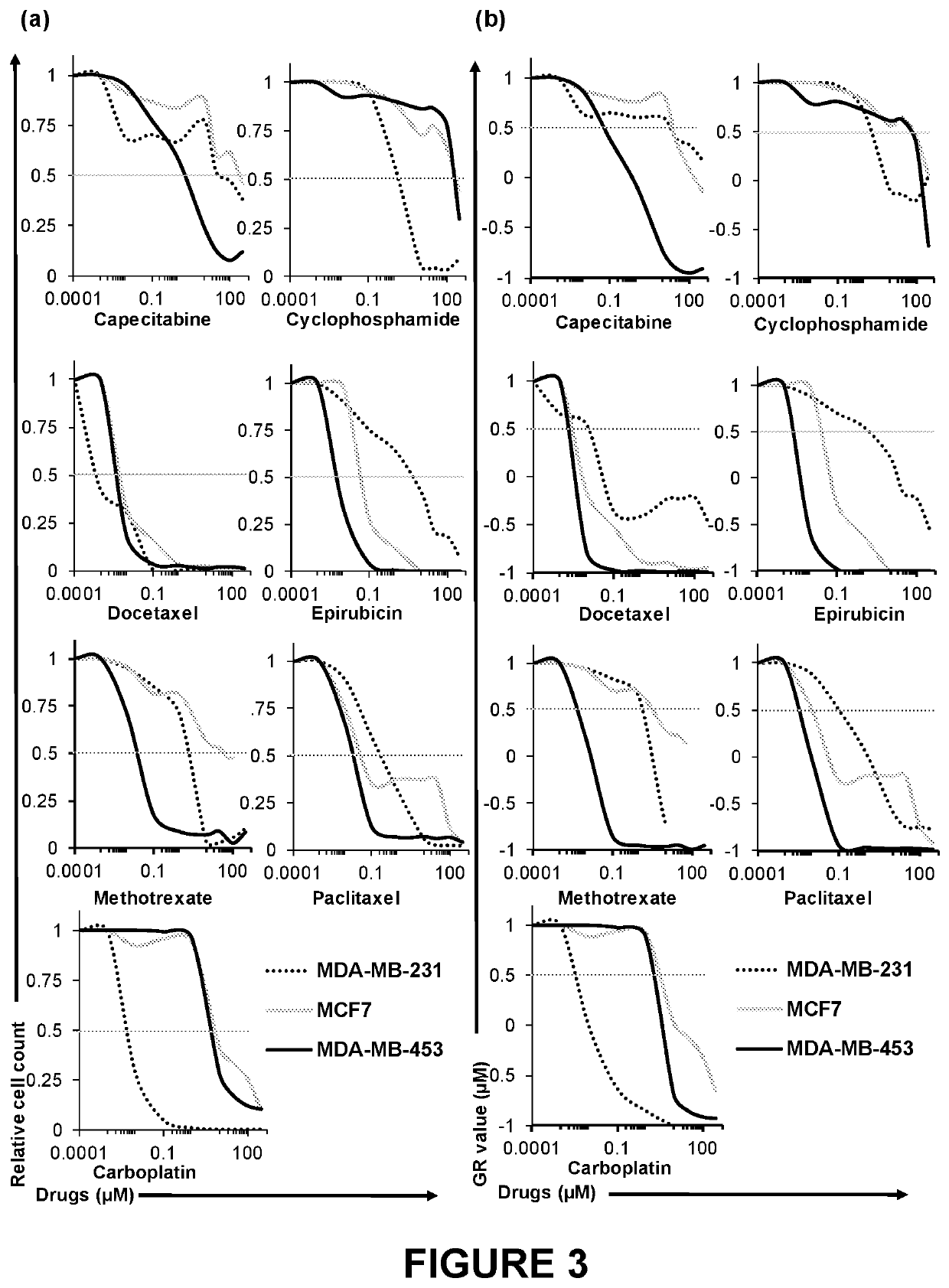
PendingUS20220228124A1Culture processCell culture supports/coatingBlood plasmaBiomedical engineering
The disclosure provides three-dimensional cross-linked scaffolds generated from peripheral blood plasma, and methods for making and using such scaffolds.
Owner:SANFORD HEALTH
Application of SIRT5 protein as marker in diagnosis or auxiliary diagnosis of acute myocardial infarction
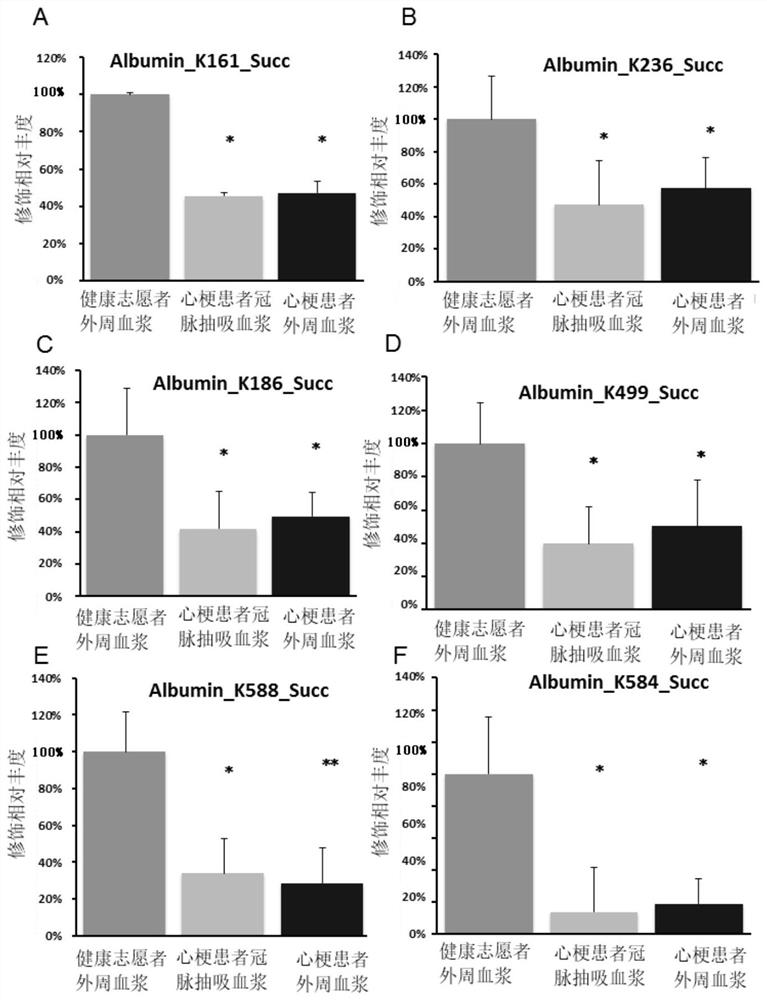
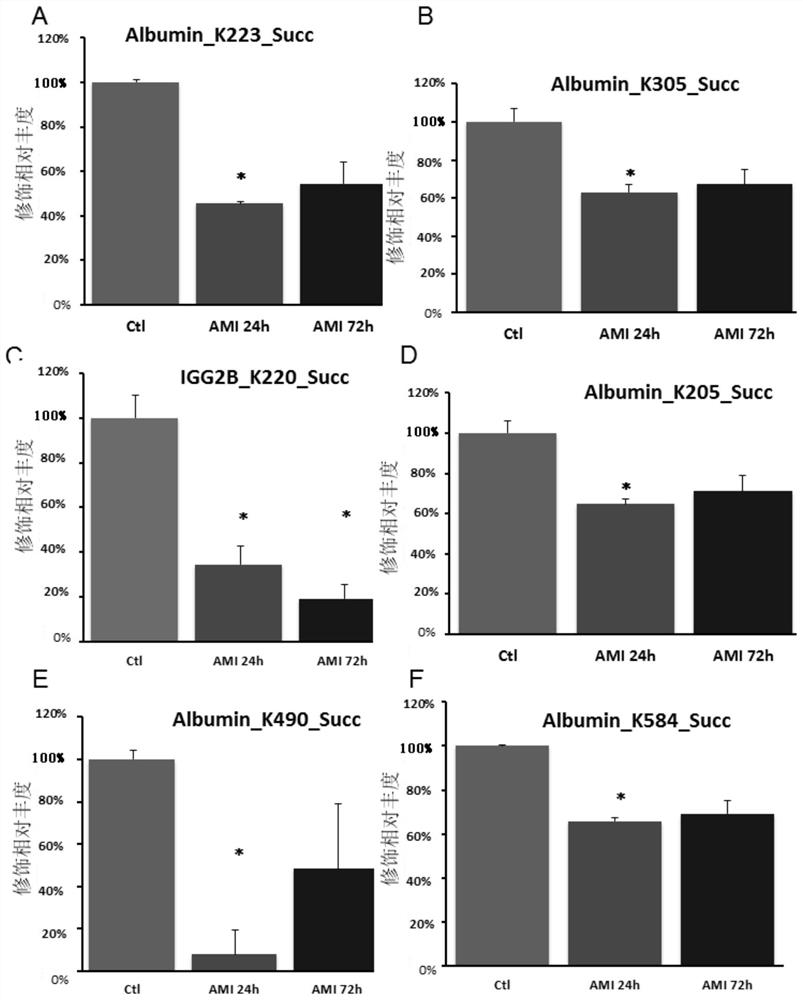
InactiveCN112753655AHigh expressionReduced succinylation modificationCompounds screening/testingDisease diagnosisIntravenous gammaglobulinSuccinylation
The invention discloses application of SIRT5 protein as a marker in diagnosis or auxiliary diagnosis of acute myocardial infarction. Experiments prove that the expression quantity of the SIRT5 protein in the heart of a C57BL / 6 mouse acute myocardial infarction model is remarkably increased compared with that of a sham-operation mouse; and compared with the peripheral plasma of the sham-operation mouse, the succinylation modification of a plurality of lysine sites of albumin and immune globulin G in the peripheral plasma of the C57BL / 6 mouse acute myocardial infarction model is remarkably reduced. Therefore, the acute myocardial infarction can be diagnosed or diagnosed in an auxiliary manner by detecting the expression quantity and / or activity of the SIRT5 protein in the heart and / or the succinylation modification degree of lysine of the protein in the peripheral plasma. The application has an important application value.
Owner:BEIJING TSINGHUA CHANGGUNG HOSPITAL
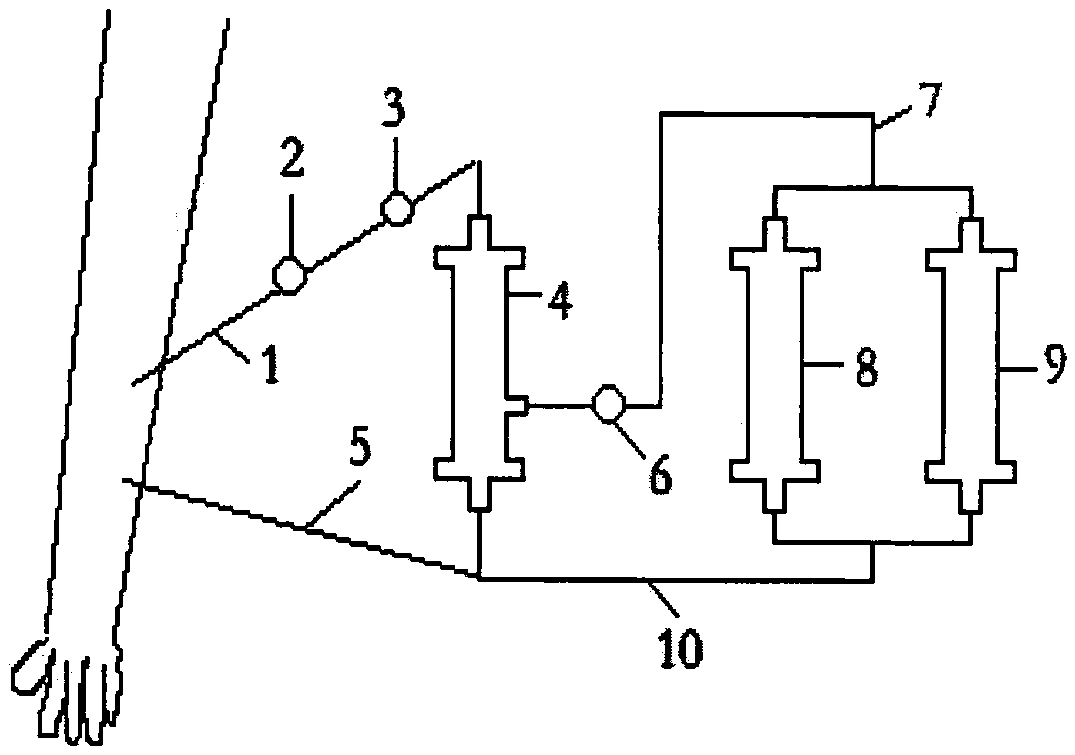
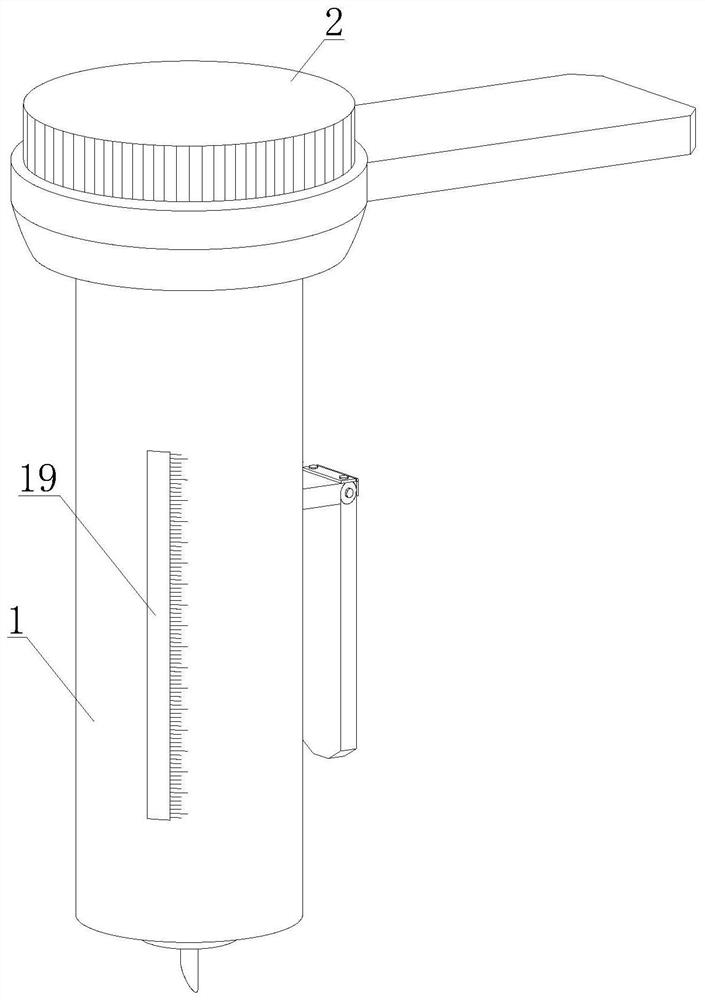
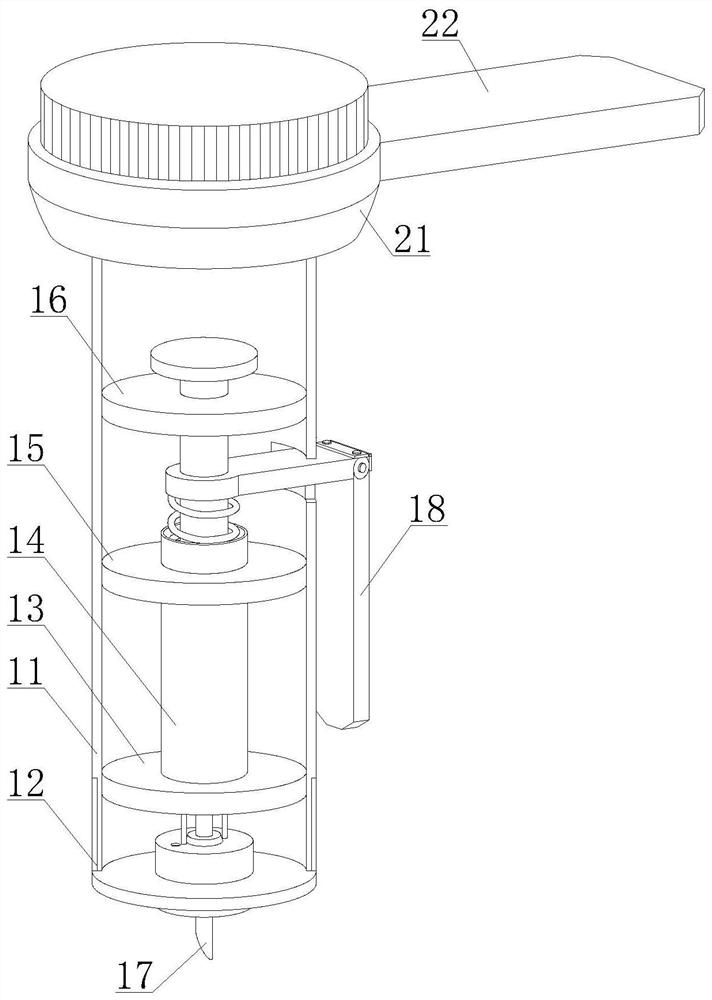
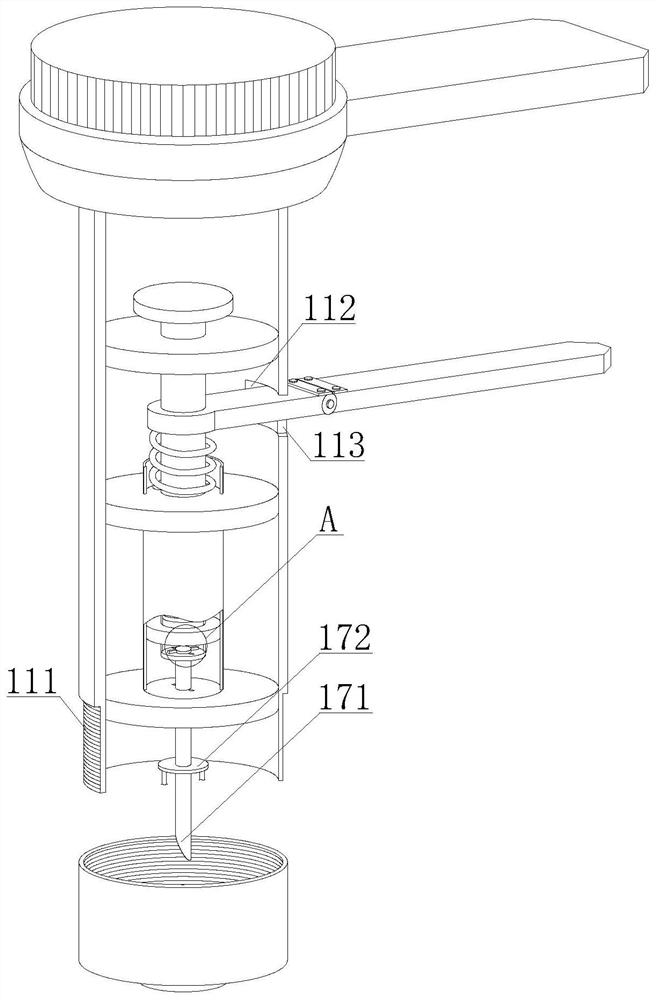
Rapid peripheral plasma collector and use method thereof
InactiveCN112914570AEasy to disinfect with alcoholEasy to disinfectDiagnostic recording/measuringSensorsRed blood cellEngineering
The invention discloses a rapid peripheral plasma collector which comprises a collection assembly and a mounting assembly, a disinfection box is filled with disinfection alcohol cotton through an injection hole, a mounting base and an outer plastic body are screwed together in a threaded mode, the lower end of the mounting base is aligned with a finger, a handle and a force application rod are tightly pressed, and the finger is punctured after a collecting needle is pointed, then taking down the mounting base and the collecting needle, putting a capillary aligned to the bleeding position for peripheral plasma collection, keeping the handle and the force application rod are continuously pressed, blood will enter the capillary, and the peripheral plasma collection work is completed through separation of a hemofilter. The invention further discloses a use method of the rapid peripheral plasma collector, blood is collected in the capillary through a seepage plate, a stop block can be used to prevent the blood from entering the collection tube, when the handle and the force application rod are loosened, a piston moves upwards, so that negative pressure is formed in the capillary and drive the blood to flow upwards, so that blood can be filtered by the hemofilter to separate plasma and red blood cells, and the operation is simple.
Owner:JIAMUSI UNIVERSITY
A method for increasing the proportion of fetal free DNA in the plasma free DNA sequencing library of pregnant women
ActiveCN105926043BIncrease the proportion of free DNA contentImprove throughputMicrobiological testing/measurementLibrary creationGeneticsBlood plasma
The invention discloses a method for improving a proportion of fetal free DNA in a maternal plasma free DNA sequencing library. The method comprises the following steps: extracting maternal plasma free DNA; adding specific sequence tags at two ends of the maternal plasma free DNA so as to obtain plasma free DNA with maternal individual identification markings; subjecting the plasma free DNA with the maternal individual identification markings to PCR amplification so as to obtain an original library of maternal individual plasma free DNA; and recovering the maternal original library in a selected length range so as to obtain a recovered library used for high-throughput sequencing, or mixing the maternal original libraries of a plurality of different individual pregnant women so as to build the recovered library. The method provided by the invention can accurately detect a sample with excessively low content of maternal peripheral plasma fetal free DNA, reduces the sequencing data volume of every sample, improves the sample throughput of a sequencing reaction, reduces detection cost, and facilitates large-scale popularization.
Owner:SUZHOU BASECARE MEDICAL DEVICE CO LTD
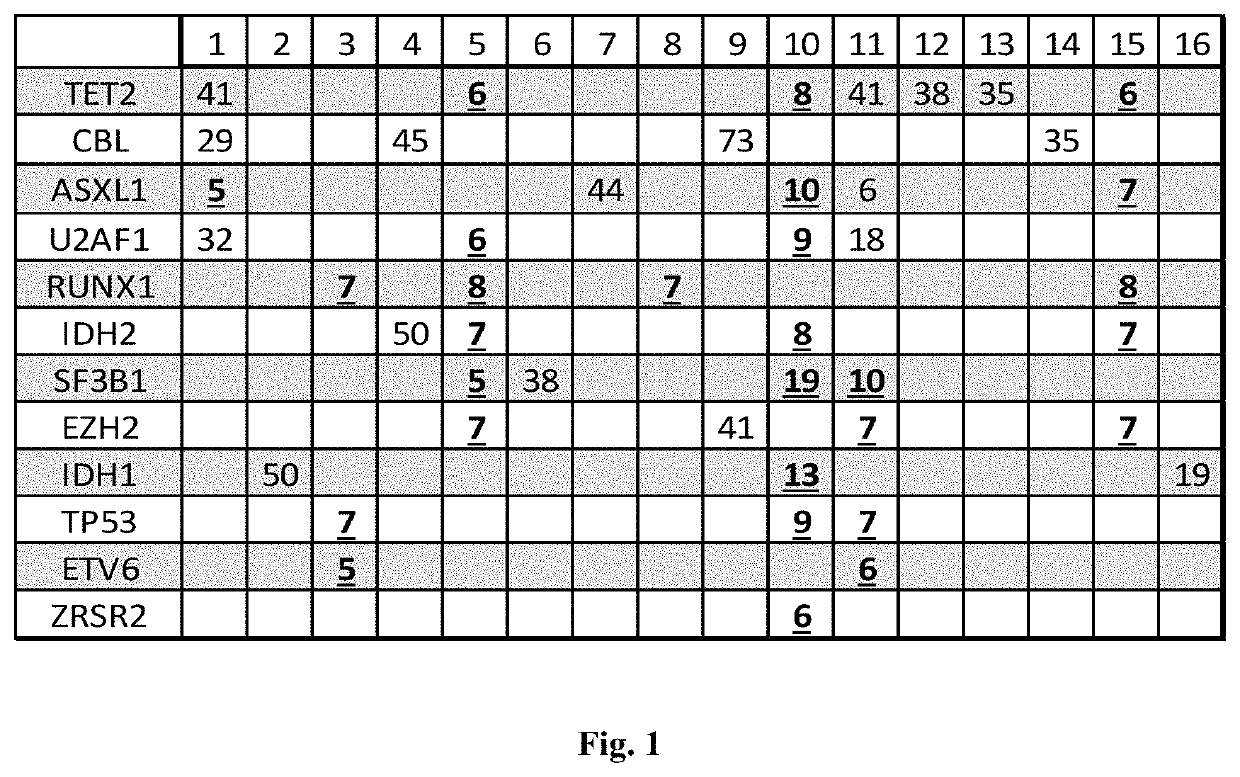
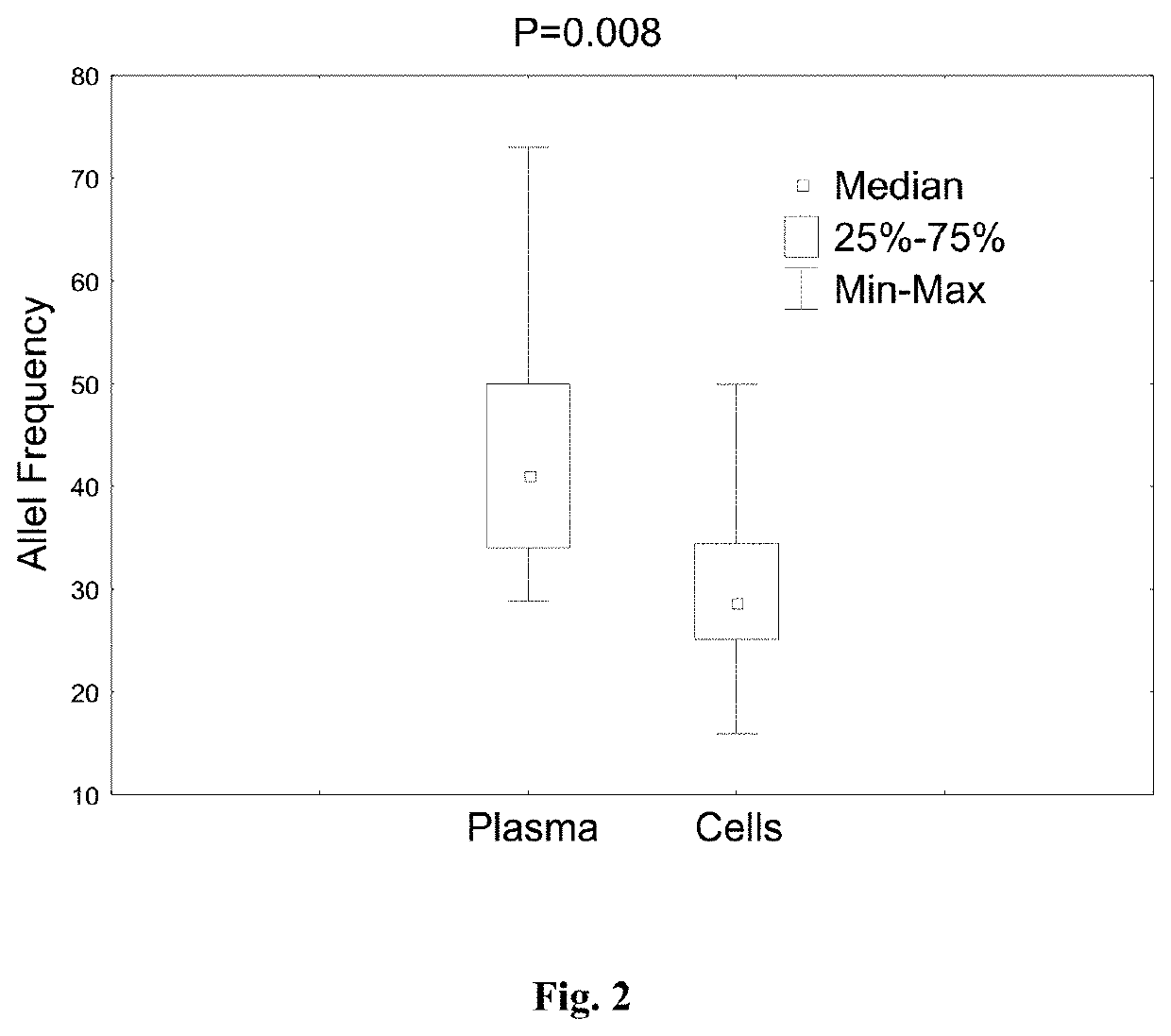
Deep sequecing of peripheral blood plasma DNA as a reliable test for confirming the diagnosis of myelodysplastic syndrome
ActiveUS10604801B2Increase in bone marrow blastsMicrobiological testing/measurementCell freeHematologic malignancy
Methods are provided for treating, managing, diagnosing and monitoring myelodysplastic syndrome and other hematologic malignancies. These methods comprise the next generation sequencing analysis conducted on cell-free DNA from peripheral blood plasma or serum.
Owner:NEOGENOMICS LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com