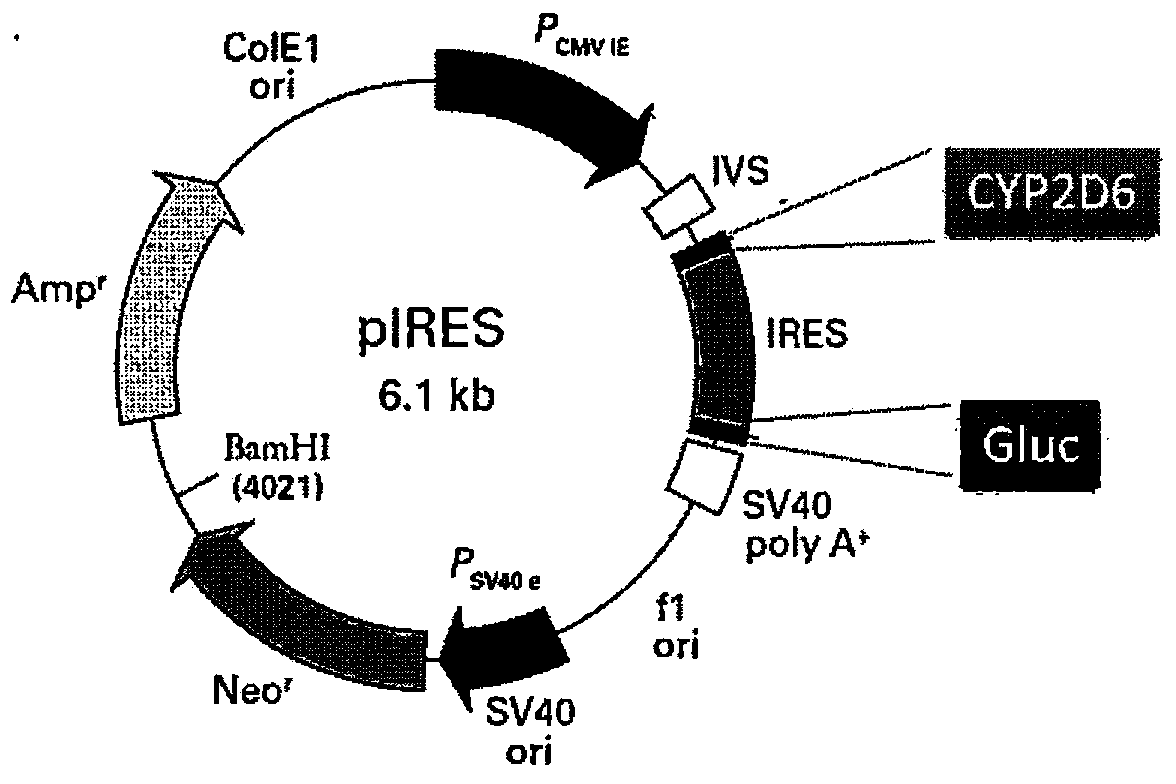
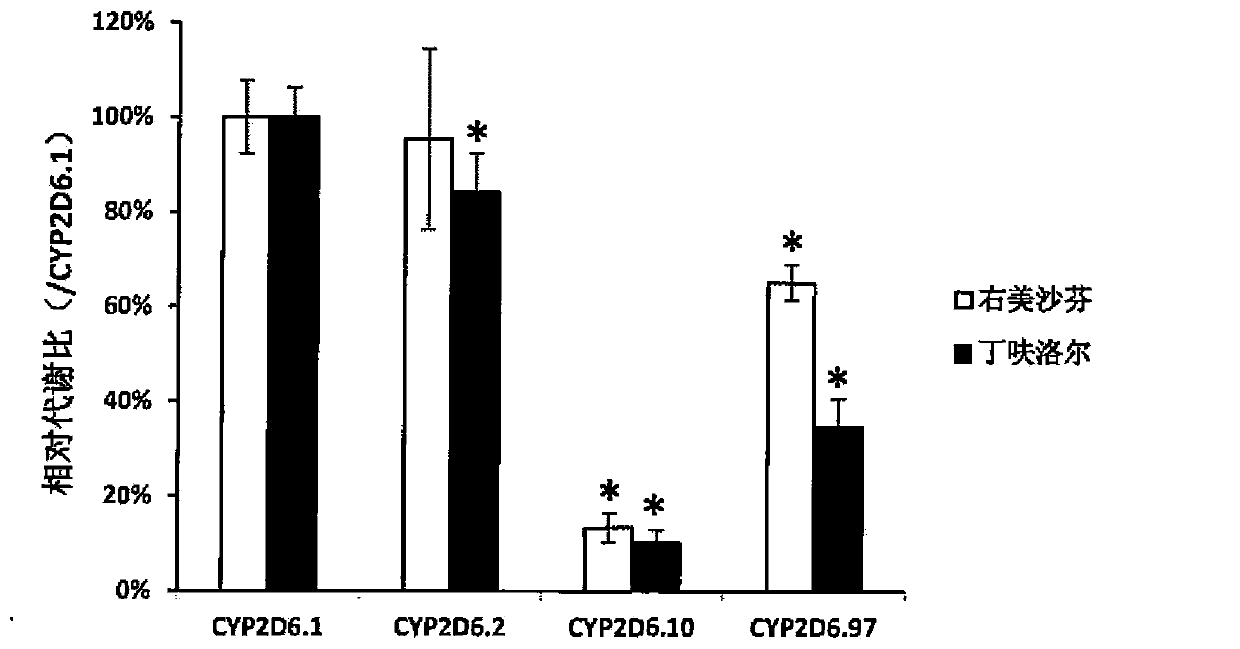
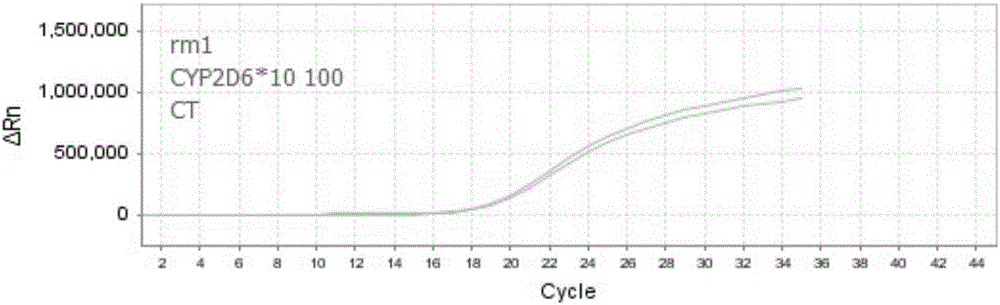
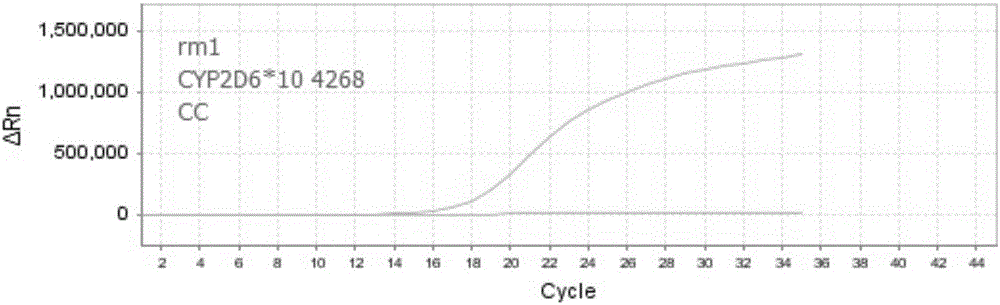
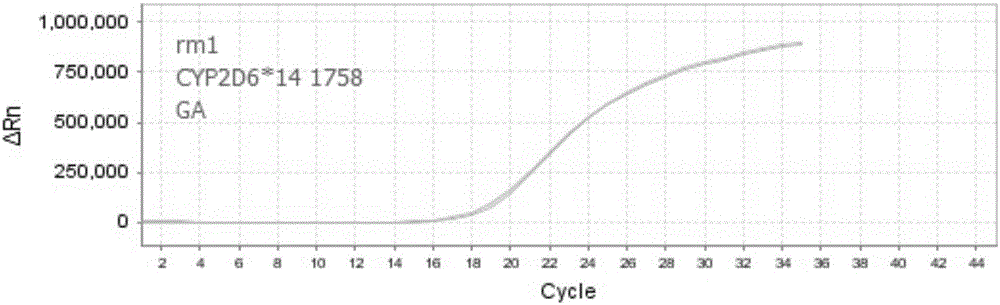
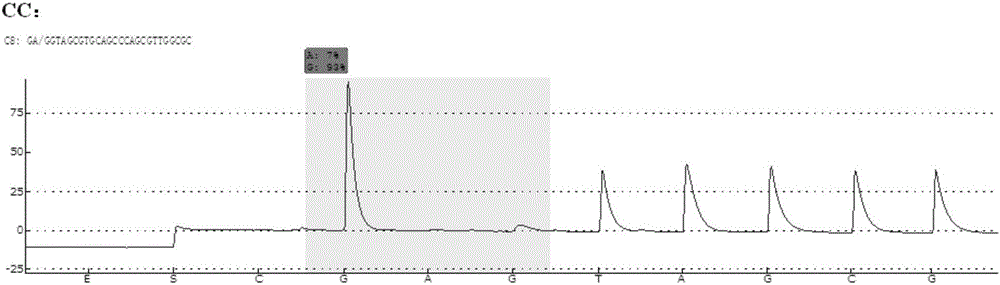
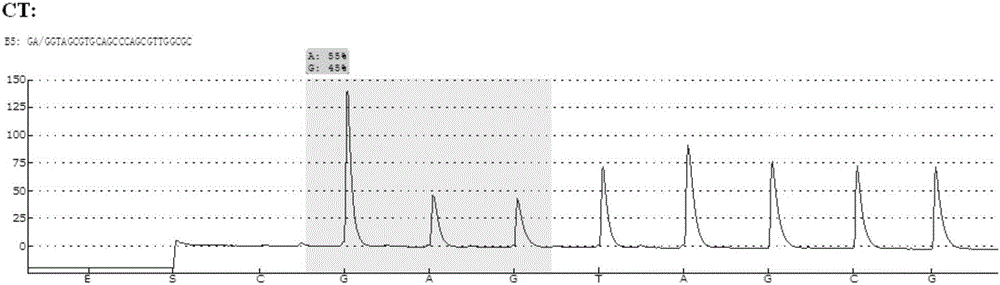
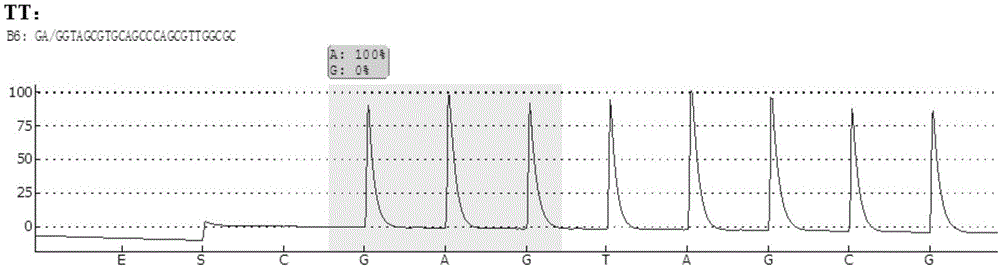
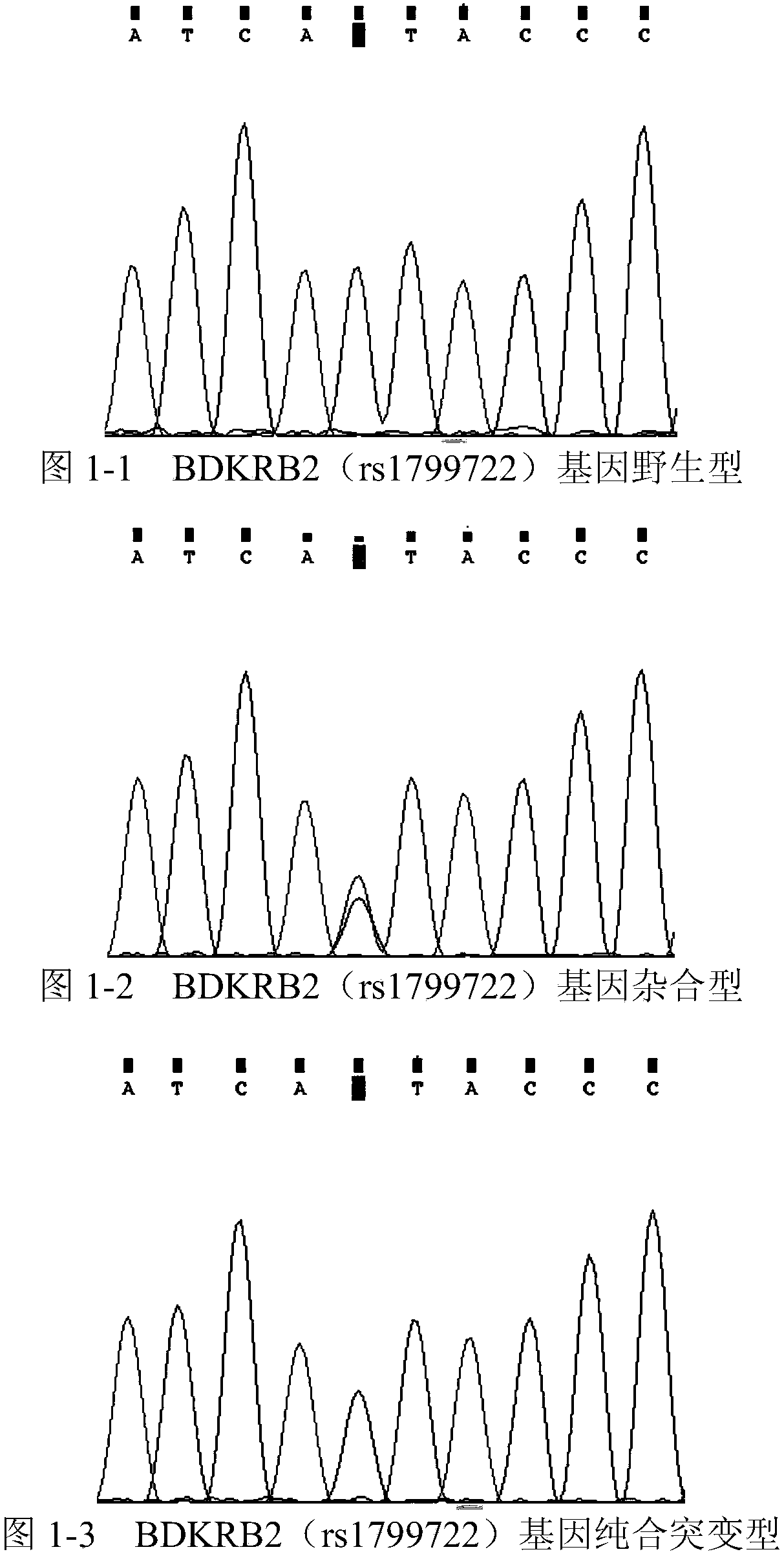
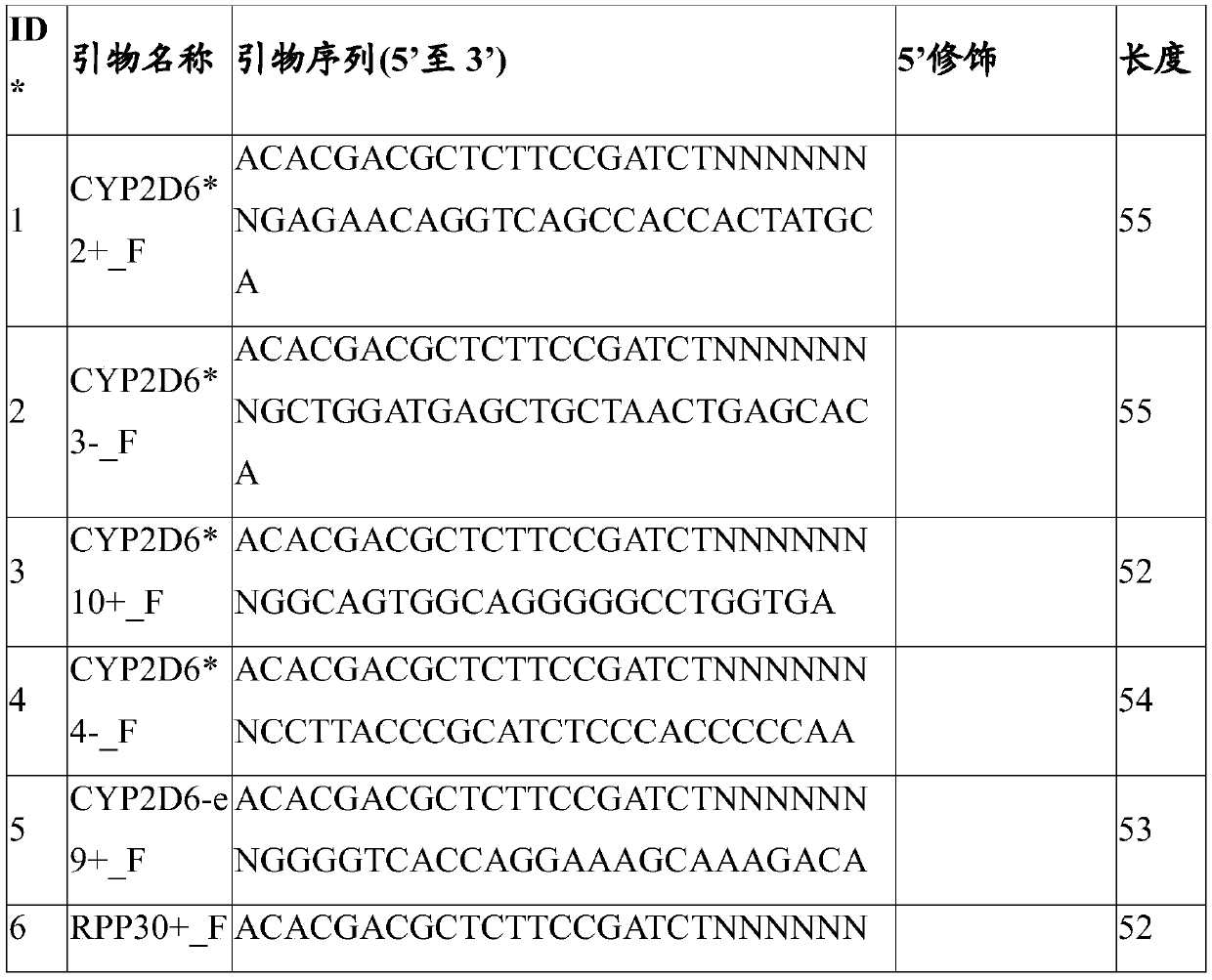
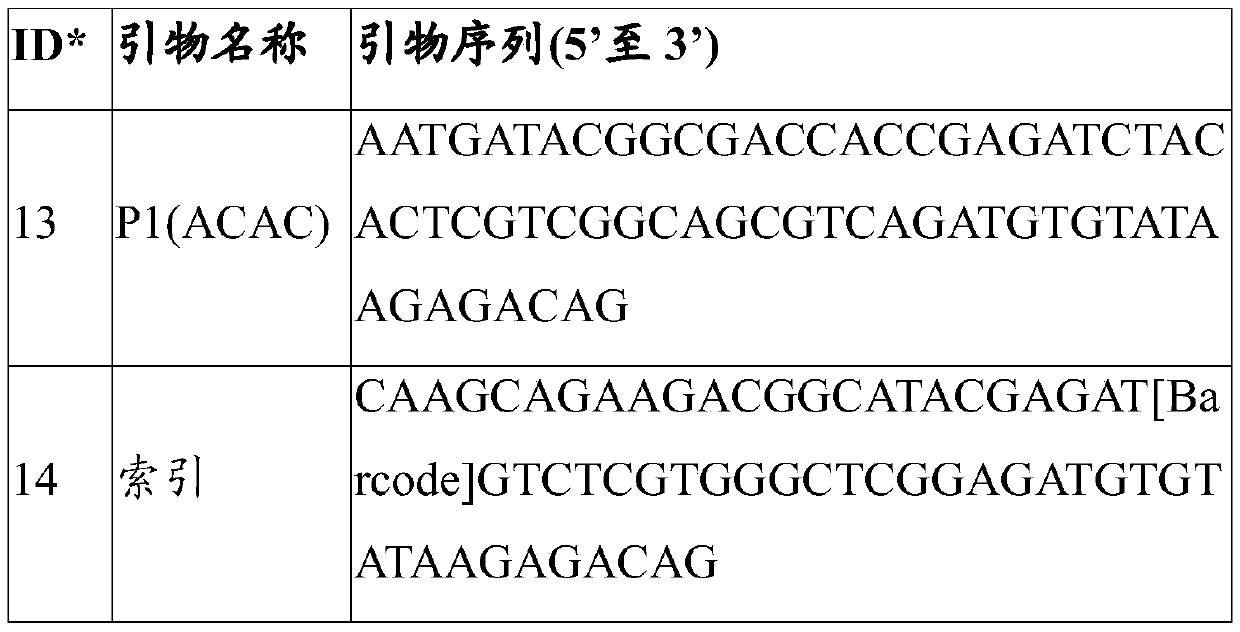
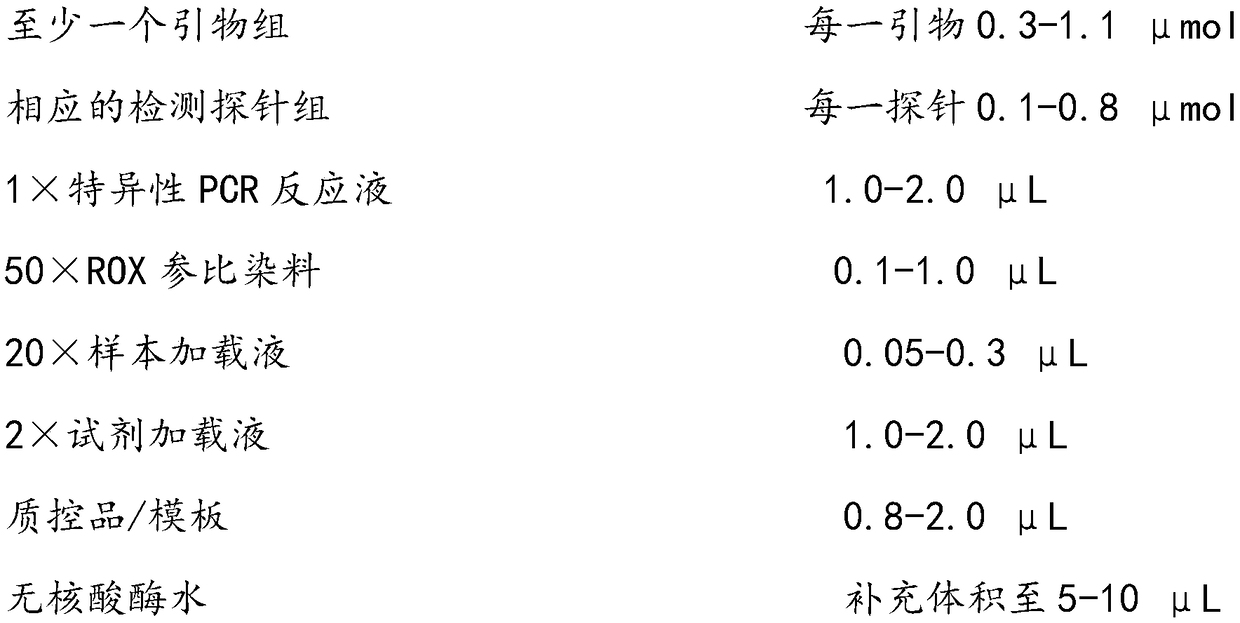
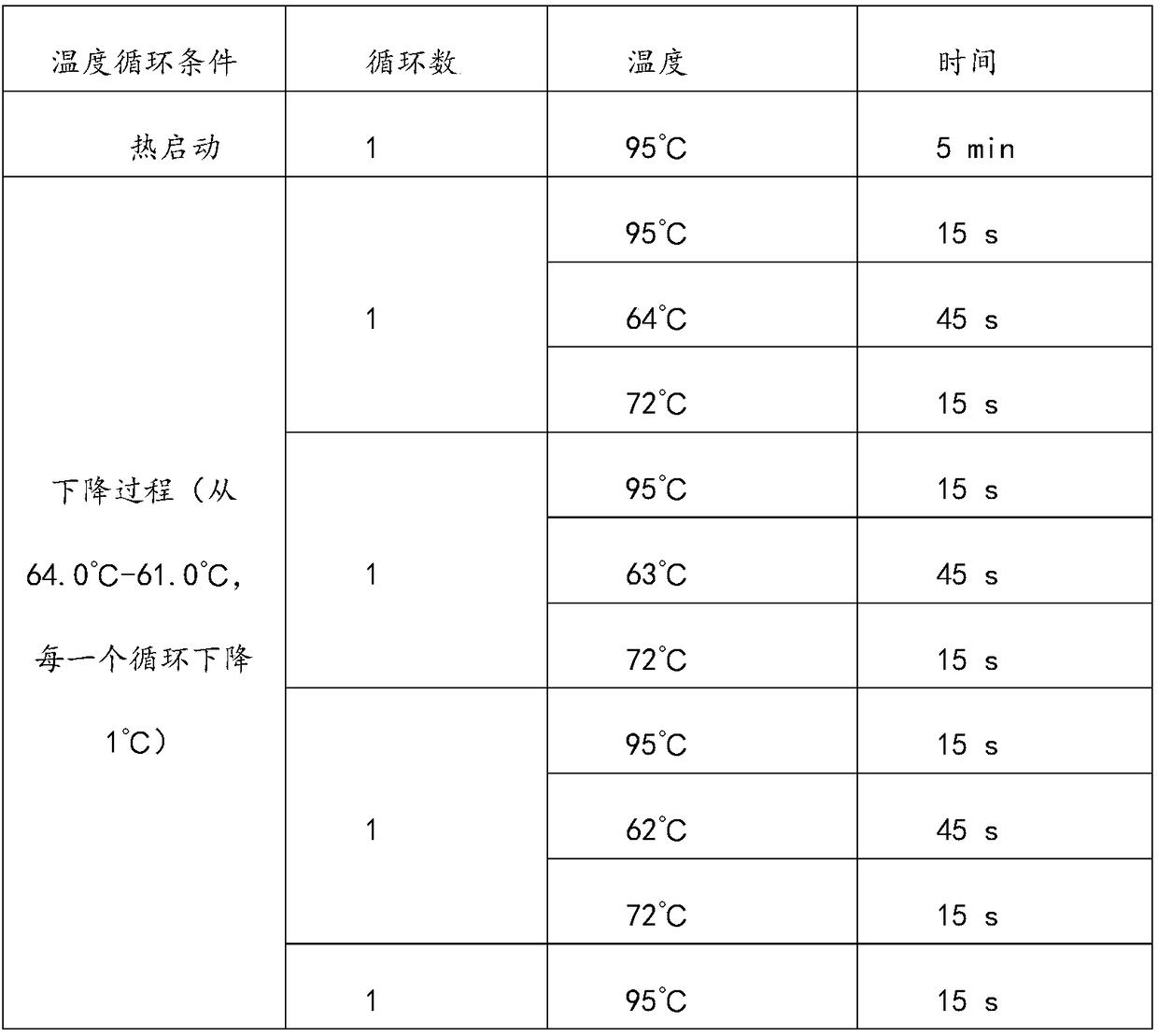
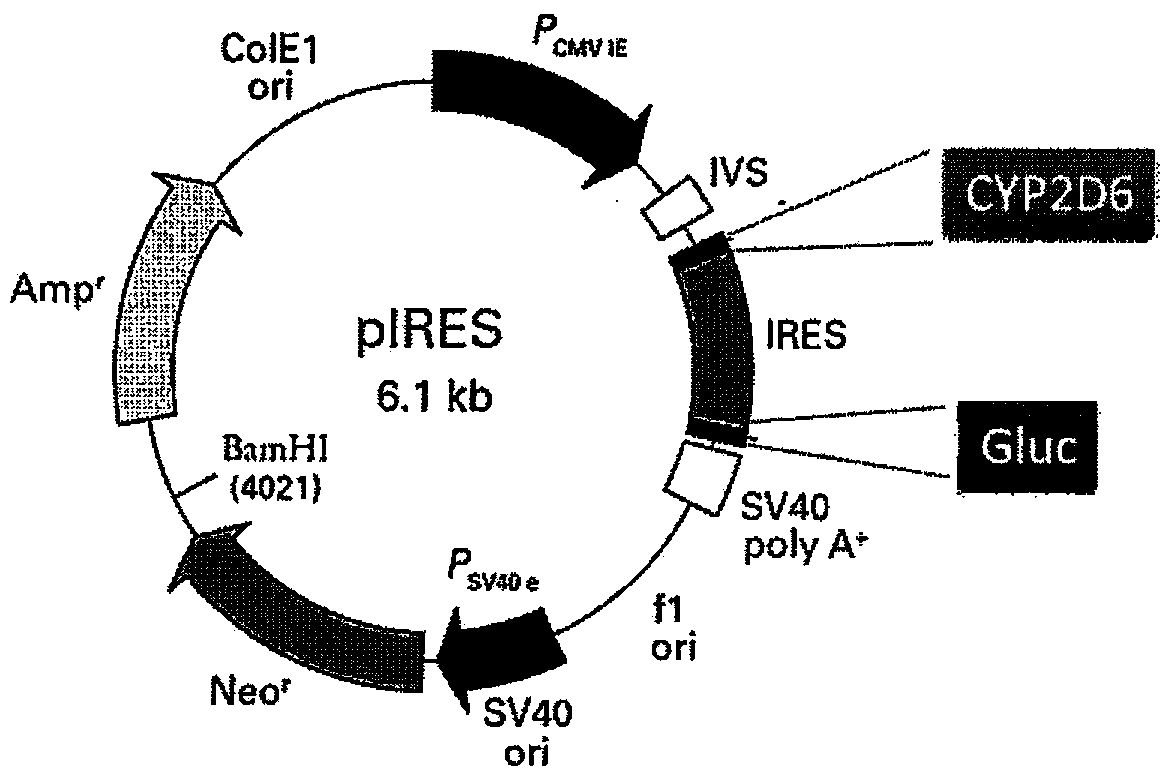
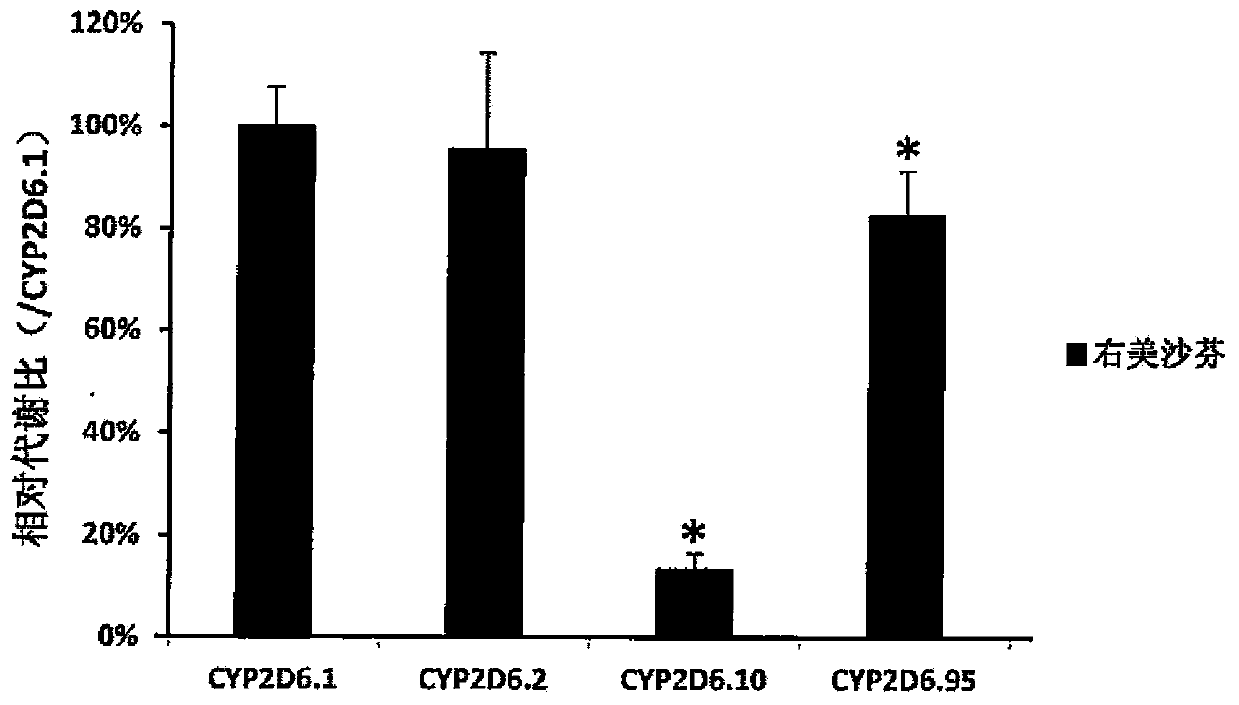
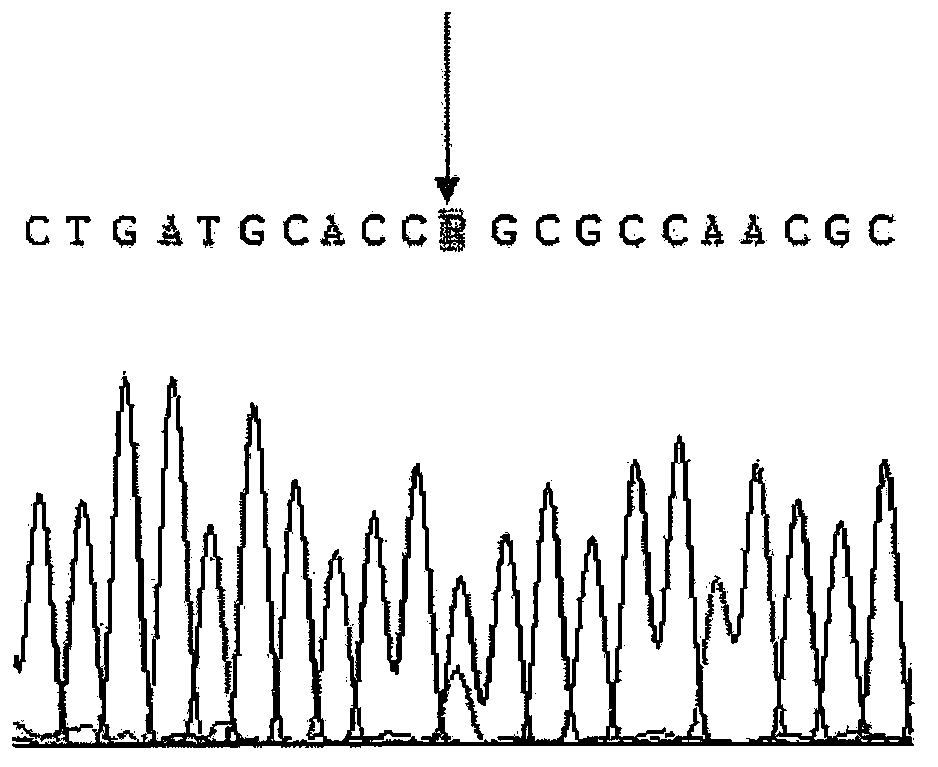
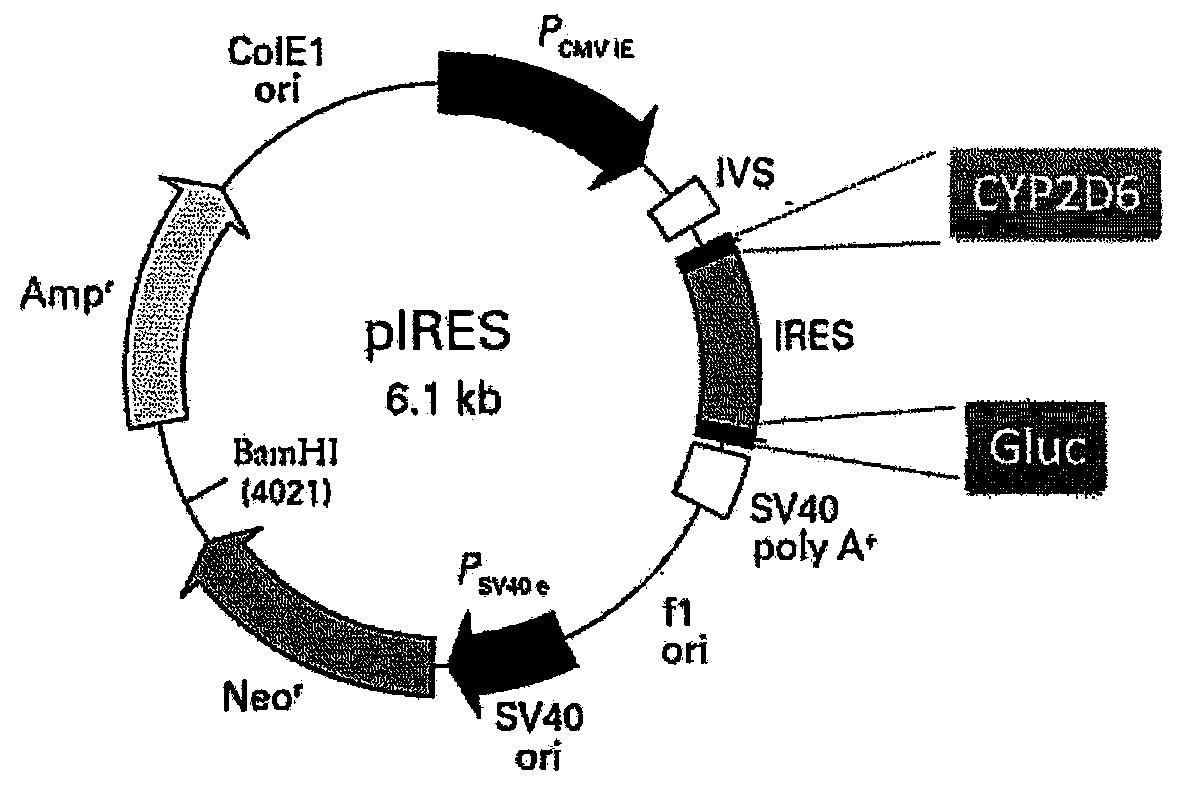
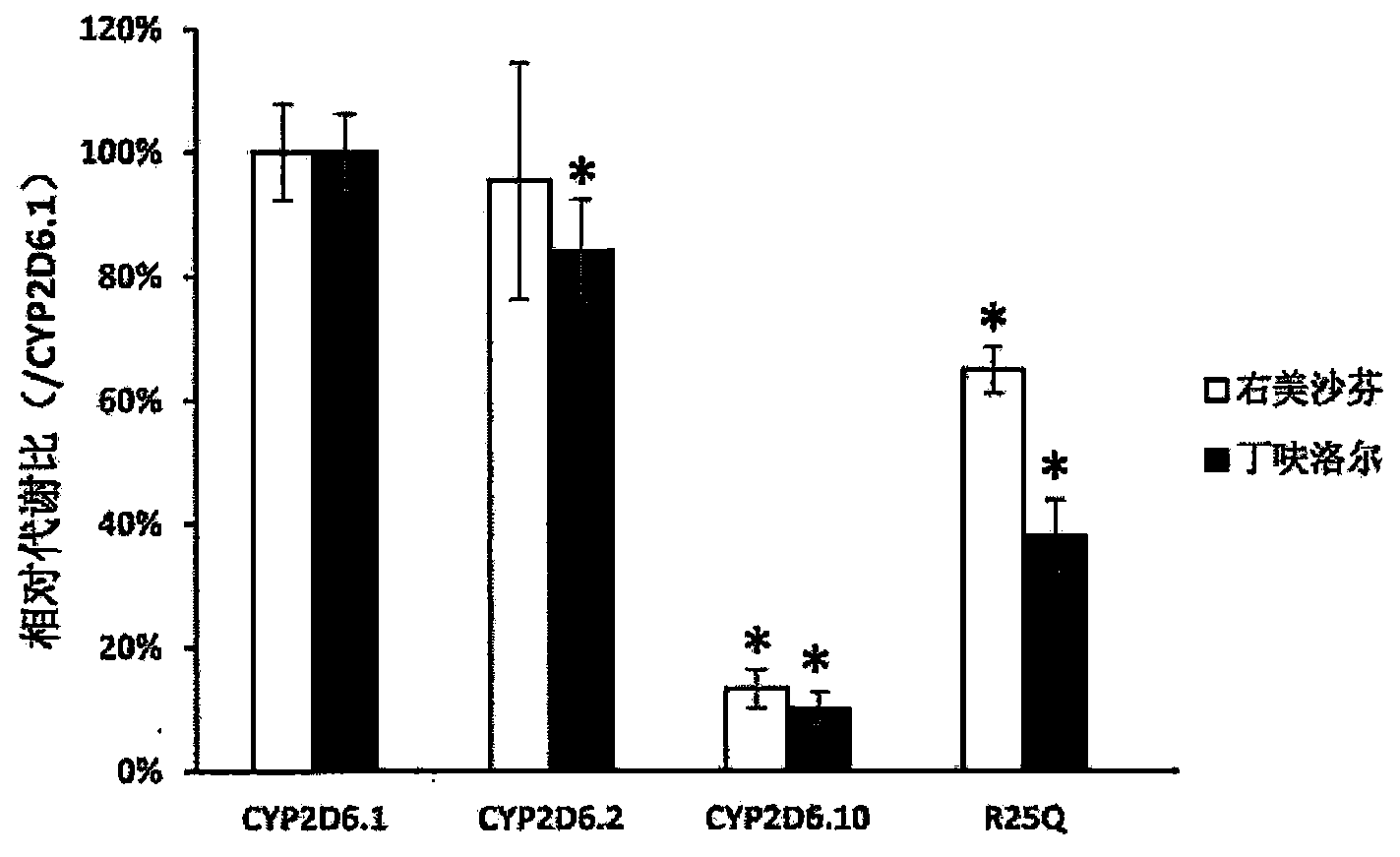
Patents
Literature
85 results about "CYP2D6 Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This gene plays a role in the metabolism of numerous clinically-relevant drugs. It is also involved in the oxidation of xenobiotics.
Medicine metabolic relevant loci detection method
InactiveCN101760528AAccurate and reliable metabolic strengthAvoid adverse reactionsMicrobiological testing/measurementDrug metabolismFluorescence
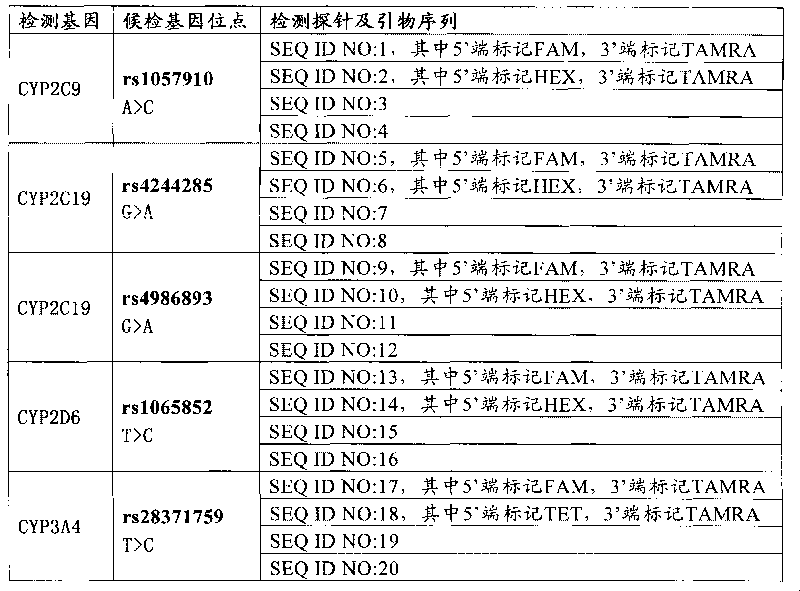
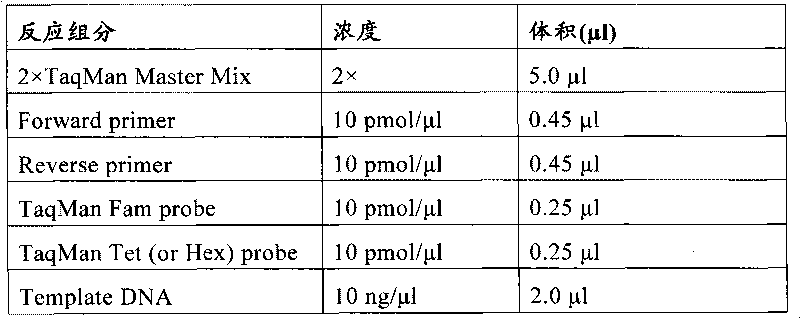
The invention relates to a medicine metabolic relevant loci detection method, which comprises the following steps: extracting genome DNA from human samples; respectively designing a Taqman probe pair and a primer pair according to at least two medicine metabolic relevant genes; respectively marking the 5' end and the 3'end of the Taqman probe pair with fluorescence reporting genes and fluorescence quenching genes; carrying out fluorescence quantitative PCR augmentation on the genome DNA; and judging whether the medicine metabolic relevant genes have the mutation according to the fluorescence quantitative PCR augmentation results. Preferably, the number of the medicine metabolic relevant genes is four, the Taqman probe pair and the primer pair are used for detecting a loci rs1057910 of a gene CYP2C9, a loci rs4244285 of a gene CYP2C19, a loci rs4986893of a gene CYP2C19, a loci rs1065852 of a gene CYP2D6 and a loci rs28371759 of a gene CYP3A4. The invention has the advantages of ingenious design, simple operation and accurate and reliable detection results, and provides the reference frame for determining whether professional doctors are needed to be consulted so as to make sure the medicine can be taken or not or the proper dosage and the like when a certain medicine is taken.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
Method of detecting mononucleotide pleomorphism of CYP2D6 gene ninth exon
InactiveCN101109018ASignificant practical valueSugar derivativesMicrobiological testing/measurementNucleotideExon
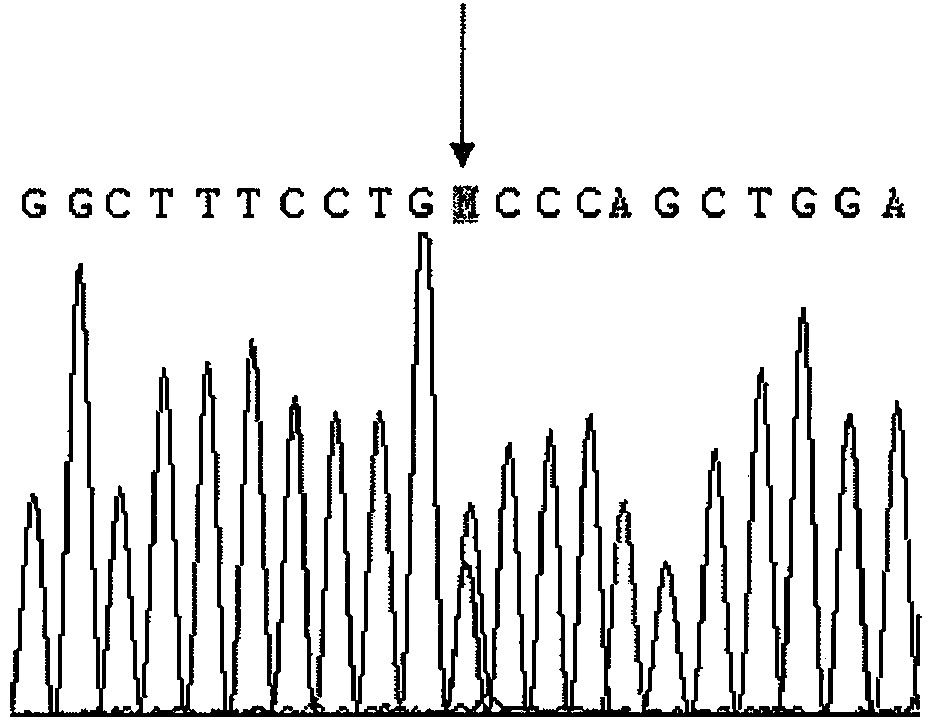
The invention relates to a testing method of CYP2D6 gene exon 9's single nucleotide polymorphism, and meanwhile relates to a separation nucleic acid and an allel-specific nucleic acid primer. The method comprises steps as described below: firstly the confirmation of the 1332 nucleic acid showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9, then test of the existence of the single nucleotide polymorphism, specifically a separation nucleic acid with the SEQ ID NO: 1 and the 1322 position is A, an allel-specific nucleic acid primer with the length of 15 to 50bp and specifically hybridizes and amplifies the amplified products of the 1322 nucleic acid polymorphism showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9. The invention can be used to research the relation between CYP2D6 gene polymorphism in Chinese people and the clinical drug safety, and provide guidance to the development of new drugs.
Owner:SHANGHAI JIAO TONG UNIV
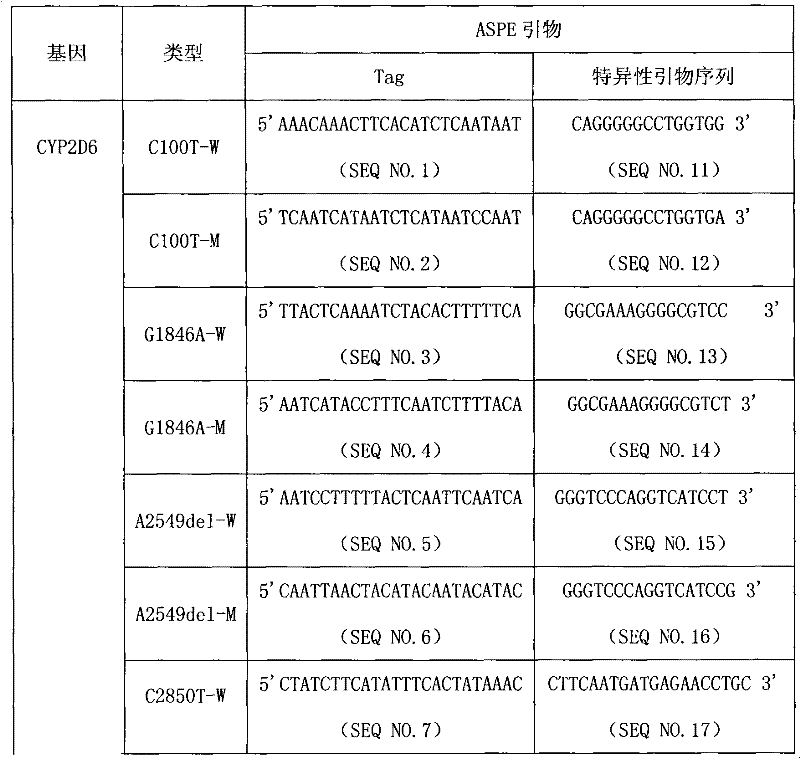
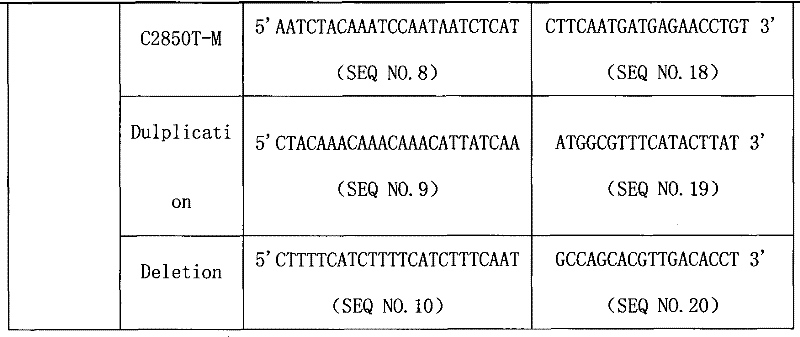
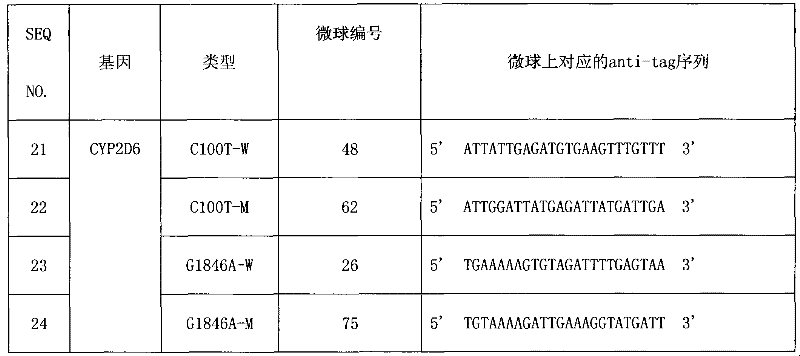
CYP2D6 gene mutation detection liquid-phase chip and detection method
ActiveCN101824467AImplement parallel detectionImprove signal-to-noise ratioMicrobiological testing/measurementSignal-to-noise ratio (imaging)Microsphere
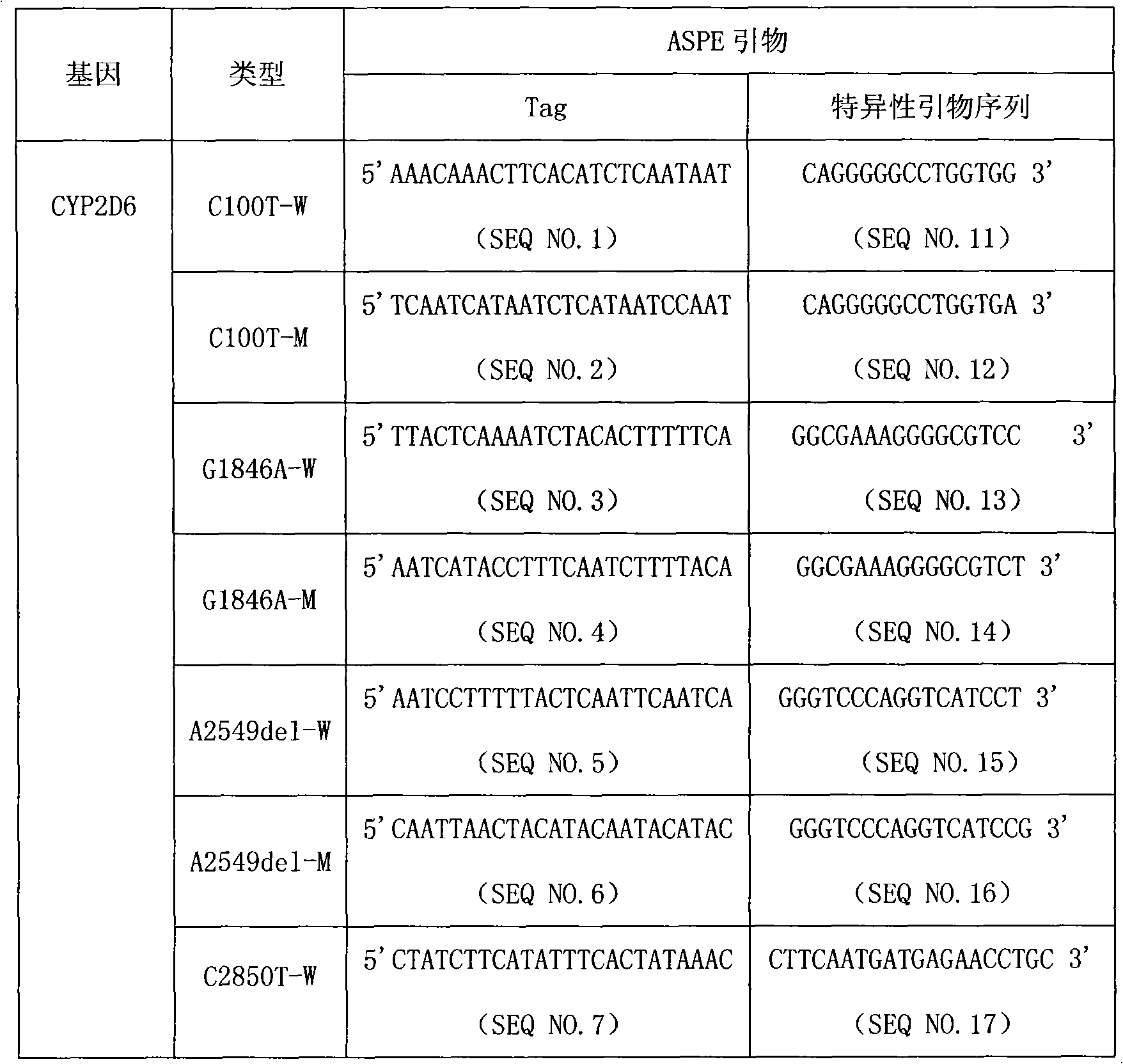
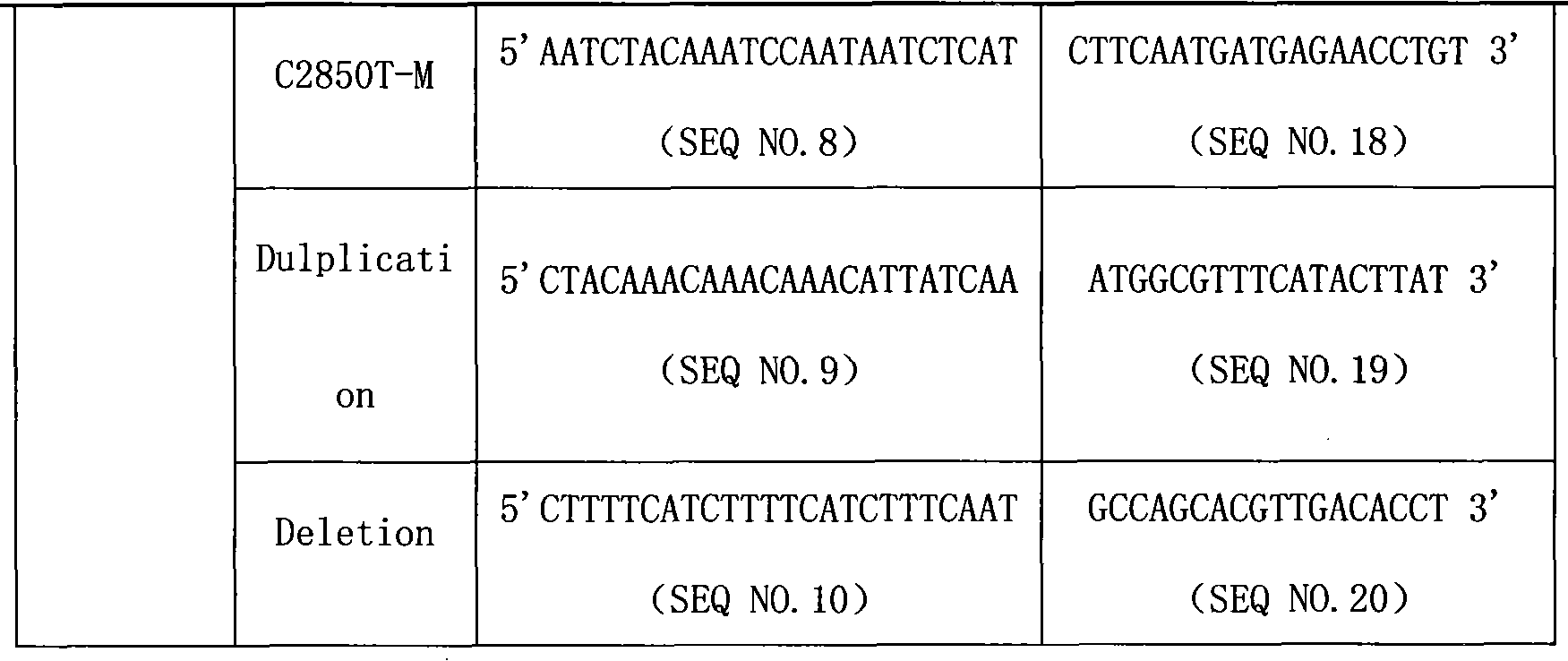
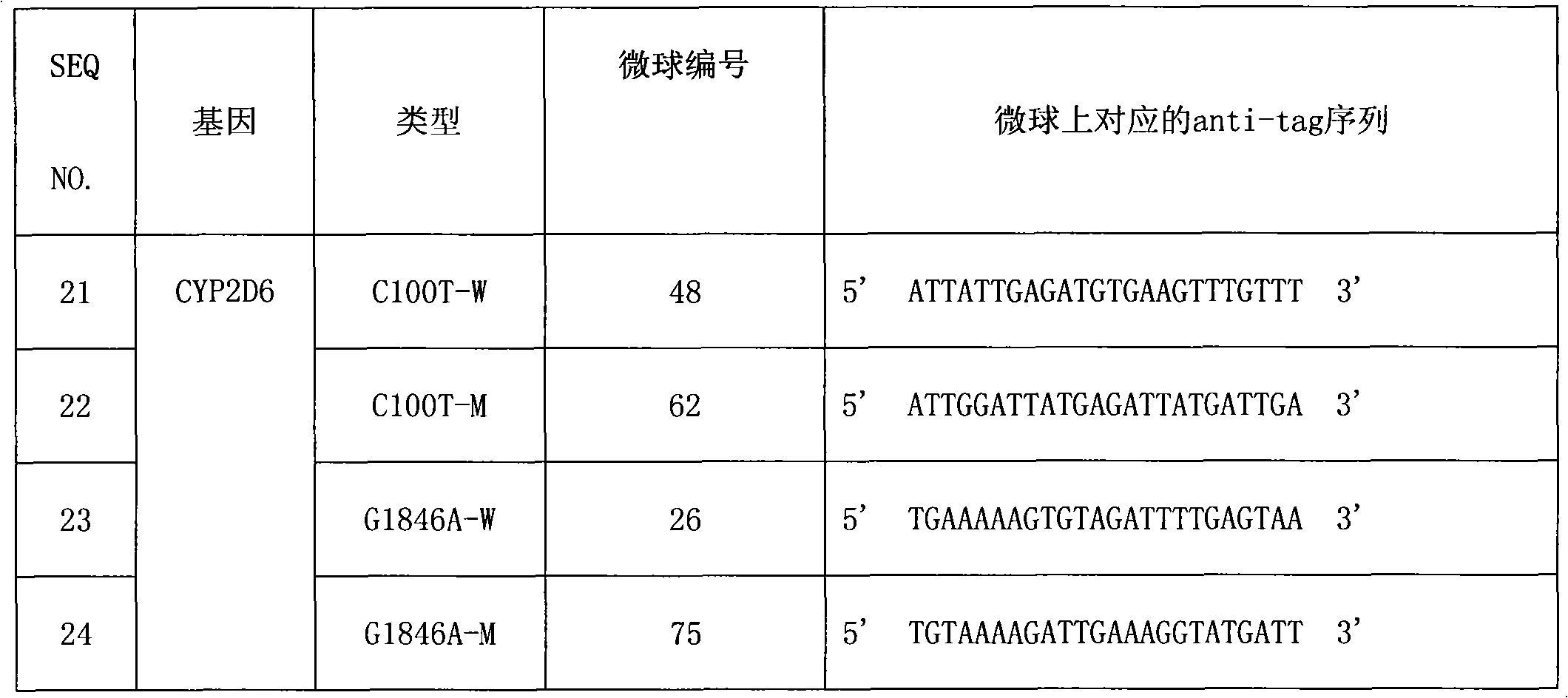
The invention provides a CYP2D6 gene mutation detection liquid-phase chip which comprises ASPE (Allele Specific Primer Extension) primers aiming at CYP2D6 C2850T and CYP2D6Deletion mutational sites, three microballoons respectively enveloped with a specific anti-tag sequence and amplification primers aiming at the CYP2D6C2850T and the CYP2D6 Deletion mutational sites. The CYP2D6 gene mutation detection liquid-phase chip can simultaneously detect aiming at the CYP2D6 C2850T and the CYP2D Deletion mutational sites and has excellent signal to noise ratio. The coincidence ratio with a sequencing method of the CYP2D6 gene mutation detection liquid-phase chip reaches up to 100 percent, and the CYP2D6 gene mutation detection liquid-phase chip has higher specificity and precision compared with intra-class correlation products.
Owner:SUREXAM BIO TECH
Kit for simultaneously detecting multisite mutation of genes CYP2C19 and CYP2D6
InactiveCN105861703AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDeletion mutationCoincidence
The invention discloses a kit for simultaneously detecting multisite mutation of genes CYP2C19 and CYP2D6. A group of Taqman allele resolution analysis method-based specific primers and probes is obtained by meticulous designing, multiple verification, screening and optimization, and 8 functional variations of 4 types, i.e. single base displacement, deletion mutation, gene deletion and duplication mutation, of genes CYP2C19 and CYP2D6 can be detected; from DNA extraction to fluorescent PCR and then to result acquisition, 4 hours is required only, and the manual operation time is shorter than 2 hours. The kit containing the primers has the advantages of time saving, convenience, high sensitivity, capability of ensuring that the positive coincidence rate and negative coincidence rate of a sample are both over 99 percent, and the like.
Owner:钟诗龙
Method for quickly and accurately detecting copy number variation of CYP2D6 gene
InactiveCN106498053AEasy to operateLow costMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceDNA extraction
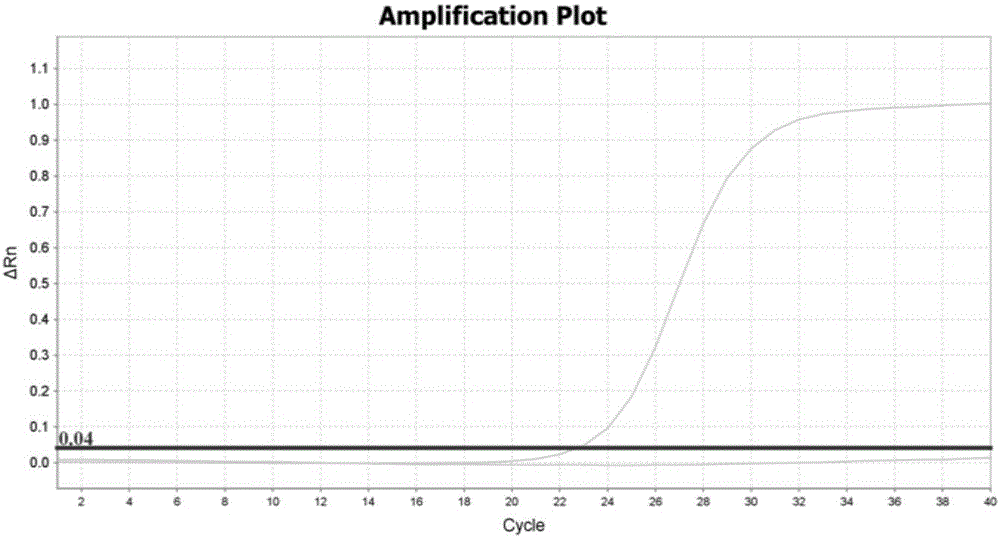
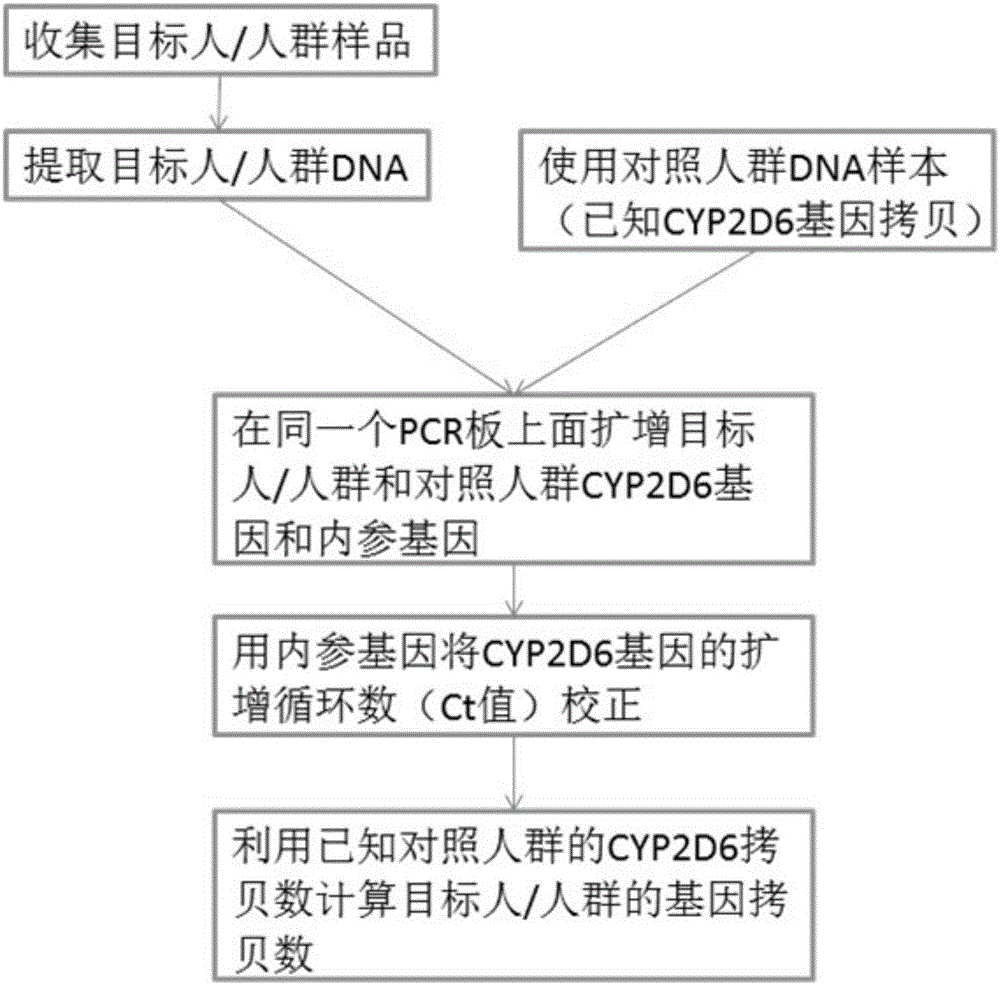
The invention discloses a method for quickly and accurately detecting a copy number variation of a CYP2D6 gene. The method comprises the steps of 1, preparing a primer which detects CYP2D6 gene polymorphism; 2, preparing a primer and a probe of a RPP30 gene; 3, conducting fluorescent quantitation PCR reaction, wherein a method of double quantitating PCR through a single tube is adopted to detect the copy number variation of the CYP2D6 gene; 4, taking the CYP2D6 gene as a sample of a single copy so as to be a check sample, wherein four repetitive PCR reactions are conducted on a detecting object and the check sample respectively to obtain a Ct value of real-time quantification PCR amplification; 5, analyzing a fluorescent quantitation PCR result. According to a real-time quantification fluorescence PCR detecting method, no PCR aftertreatment is conducted, and thus time and manpower can be greatly saved. From DNA extraction to the end of fluorescence PCR experience and data analysis, the whole process only takes 4 hours, and entity experiment operating time is less than 2 hours.
Owner:神州医疗科技股份有限公司 +1
Kit and method for detecting polymorphism of CYP2D6 gene
The invention relates to a kit and method for detecting the polymorphism of a CYP2D6 gene and belongs to the technical field of gene sequencing. The kit comprises primers for detection, wherein the primers for the detection include full-length amplification specific primers for a first exon to a ninth exon of the CYP2D6 gene, and at least one pair in forward and reverse sequencing primers for the first exon of the CYP2D6 gene, forward and reverse sequencing primers for the second exon of the CYP2D6 gene, forward and reverse sequencing primers for the third exon and the fourth exon of the CYP2D6 gene, forward and reverse sequencing primers of the fifth exon to the seventh exon of the CYP2D6 gene, and forward and reverse sequencing primers of the eighth exon and the ninth exon of the CYP2D6 gene. By the adoption of the kit and the method, the detection time is obviously shortened and the workload is remarkably reduced. The kit provides a brand-new quick and simple way for prediction of the dosage of a medicine which is metabolized by CYP2D6.
Owner:刘辉
CYP2D6 gene segment containing 4094C>A mutant, protein segment coded by same and application thereof
ActiveCN103436545AReduced metabolic activityMicrobiological testing/measurementOxidoreductasesAlleleMutant
The invention belongs to the field of biology and relates to a single base mutant at the 4094th site of CYP2D6 allele, wherein C at the site is mutated into A. The invention particularly relates to a nucleic acid segment containing the mutant site and a corresponding protein segment coded by the nucleic acid segment, a reagent and detection method for identifying the mutant site, application of the site, and especially application of identification of the site in medicine instruction.
Owner:蔡剑平
Nucleic acid, kit and method for detecting polymorphism of human CYP2D6 gene
ActiveCN106755360AHigh degree of automationEasy to useMicrobiological testing/measurementDNA/RNA fragmentationBiologyPollution
The invention discloses nucleic acid, a kit and a method for quickly detecting polymorphism of a human CYP2D6 gene, which are particularly used for detecting site polymorphism of CYP2D6*10 genes C100T and G4268C as well as a CYP2D6*14 gene G1758A. The detection kit for quickly detecting the polymorphism of the CYP2D6 gene by using real-time fluorescent quantitative PCR, has remarkable advantages of high specificity, high sensitivity, short experiment cycle, simplicity in operation, safety, no toxicity, low cost and the like; the detection method is simple and convenient to operate and high in automation degree; the operation process is greatly simplified, pollution in the operation process is reduced, and the detection result is accurate and reliable.
Owner:武汉海吉力生物科技有限公司
Pyrosequencing primer pair and kit for qualitatively detecting CYP2D6 genotyping
InactiveCN106244708AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCapillary electrophoresisElectrophoresis
The invention relates to a pyrosequencing primer pair for qualitatively detecting CYP2D6 genotyping; the primer pair includes a forward amplification primer and a reverse amplification primer, and a sequencing primer, and 5' ends of the forward amplification primer and reverse amplification primer are subjected to biotin labeling respectively; the invention also relates to a pyrosequencing kit for qualitatively detecting CYP2D6 genotyping; the kit includes amplification primers, PCR (polymerase chain reaction) liquid 1, PCR liquid 2, sequencing primers, uracil DNA glycosylase and Taq polymerase. The pyrosequencing primer pair and kit have the advantages that detection results are accurate, the specificity is high, detection period is short, operation is simple and clinical examination requirements can be effectively met and additionally have the advantages that reaction process can be monitored in real time, reaction time is short, PCR products can be fed to a pyrosequencing instrument for sequencing and high-throughput sample detection just through simple treatment, and the sensitivity is higher than that of a golden standard method, namely capillary electrophoresis sequencing method.
Owner:CHANGSHA 3G BIOTECH
Primer group for detecting hypertension medicine metabolism related genes and reagent kit
ActiveCN109082464AReduce adverse reactionsAccurate typingMicrobiological testing/measurementDNA/RNA fragmentationApob geneAdrb2 gene
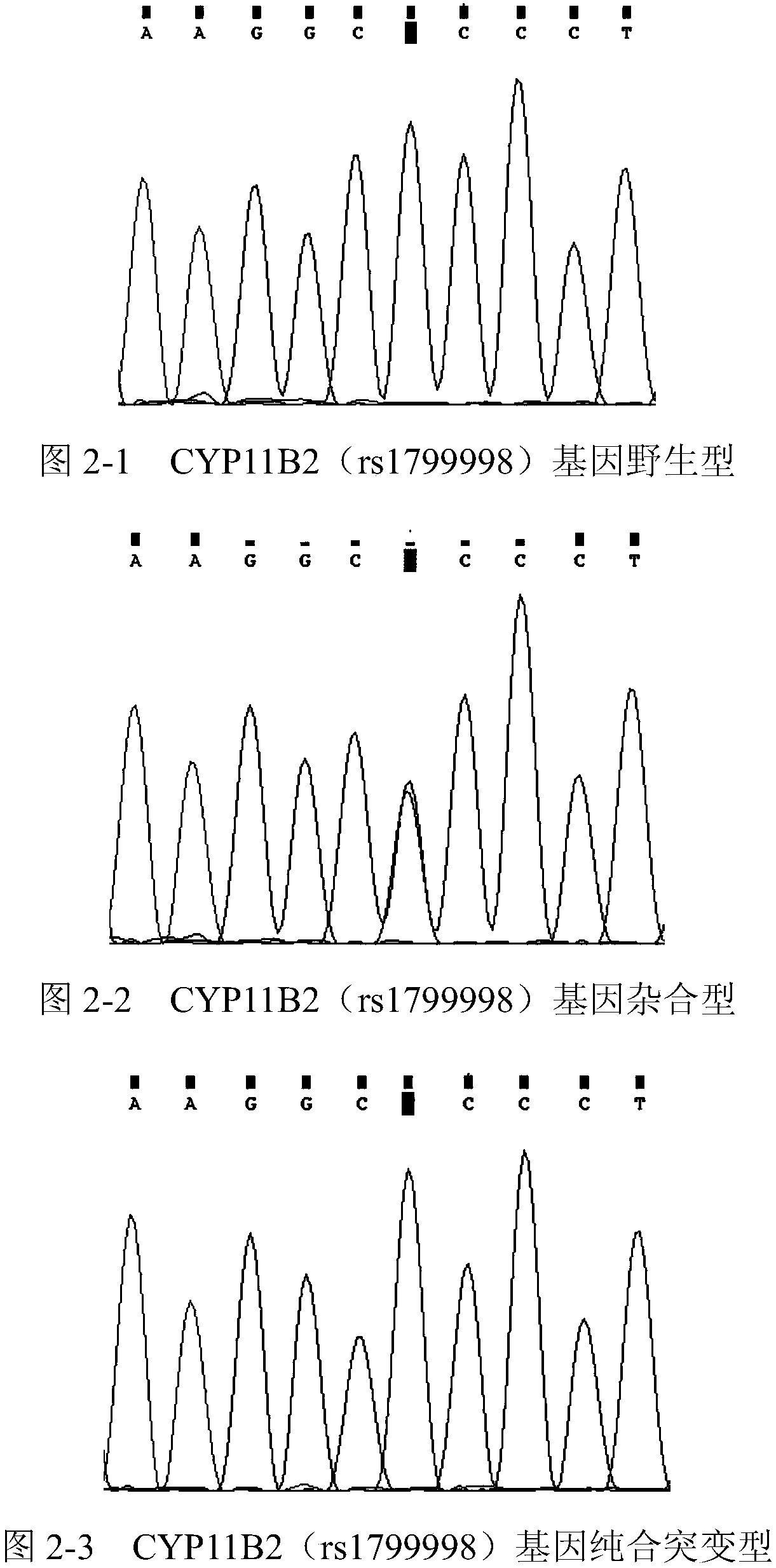
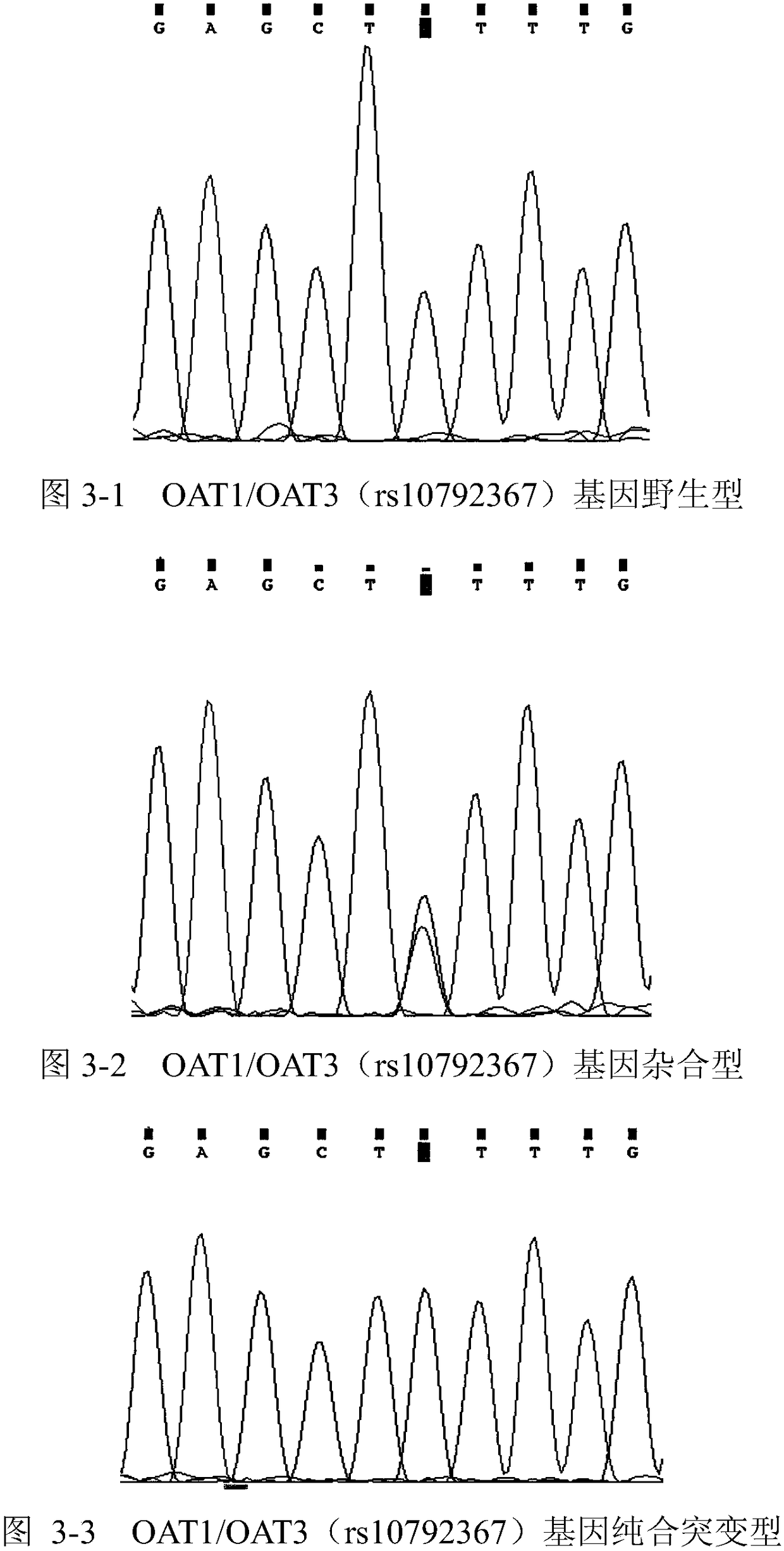
The invention relates to a primer group for detecting hypertension medicine metabolism related genes on the basis of Sanger sequencing methods and a reagent kit with the primer group. The primer groupis characterized by comprising primers for detecting at least one type of genes selected from BDKRB2 genes, CYP11B2 genes, OAT1 / OAT3 genes, AGT genes, KCNH2 genes, LDLR genes, APOB genes, CACNA1C genes, GRK4 genes, ACE genes, CYP2D6 genes, CYP2C9 genes, CYP2C19 genes, ACE2 genes, AGTR1 genes, ADRB2 genes and NR3C2 genes.
Owner:PRECEDO PHARMA CO LTD
Detection of curative effect of antihypertensive drug
InactiveCN101928757AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceCurative effect
The invention discloses a kit for detecting a curative effect of an individual antihypertensive drug. The kit comprises specific primer pairs of targeted locus CYP3A4 gene, CYP2D6 gene, CYP2C9 gene and CYP2C19 gene polymorphic locus for detecting the antihypertensive drug, specific fluorescent probe pairs, a fluorescent quantitive PCR conventional assembly and the like. The kit of the invention evaluates the curative effect of the individual antihypertensive drug by using the targeted locus CYP3A4 gene, CYP2D6 gene, CYP2C9 gene and CYP2C19 gene polymorphic locus for detecting the antihypertensive drug.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
CYP2D6 gene segment containing 2519A>C mutation, coded protein fragment thereby and applications thereof
ActiveCN103614390AReduced metabolic activityMicrobiological testing/measurementOxidoreductasesAlleleProtein Fragment
The invention belongs to the biological field, and relates to single base mutation of the 2519th locus of CYP2D6 allele. The locus is mutated into C from A. The invention concretely relates to a nucleic acid segment containing the mutation locus, a corresponding coded protein fragment, agents for identification of the mutation locus, a detection method and applications of the locus, especially applications of the identification of the locus in medication guidance.
Owner:蔡剑平
CYP2D6 gene segment containing 1735G>C mutation, coded protein fragment thereby and applications thereof
ActiveCN103614388AReduced metabolic activityMicrobiological testing/measurementOxidoreductasesAlleleProtein Fragment
The invention belongs to the biological field, and relates to single base mutation of the 1735th locus of CYP2D6 allele. The locus is mutated into C from G. The invention concretely relates to a nucleic acid segment containing the mutation locus, a corresponding coded protein fragment, agents for identification of the mutation locus, a detection method and applications of the locus, especially applications of the identification of the locus in medication guidance.
Owner:蔡剑平
Composition for detecting polymorphism and copy number of CYP2D6 gene, kit and method
ActiveCN110904220ALow costMicrobiological testing/measurementDNA/RNA fragmentationNucleotideOligonucleotide
The invention relates to the field of molecular biological detection, and more particularly to the field of detection of CYP2D6 gene polymorphism and copy number. The invention provides application ofan oligonucleotide combination in preparing a kit for detecting CYP2D6 gene polymorphism and copy number. Therefore, one or more of gene copy number variations of CYP2D6*3, CYP2D6*4, CYP2D6*5, CYP2D6*10 and CYP2D6 can be specifically detected. Meanwhile, the invention also provides an oligonucleotide pair composition, a kit containing the oligonucleotide pair composition, and a method for detecting the CYP2D6 gene polymorphism and copy number.
Owner:北京圣维尔医学检验实验室有限公司
Kit for detecting variation of copy number of CYP2D6 gene
InactiveCN109371132AHigh sensitivityStrong specificityMicrobiological testing/measurementReference genesExon
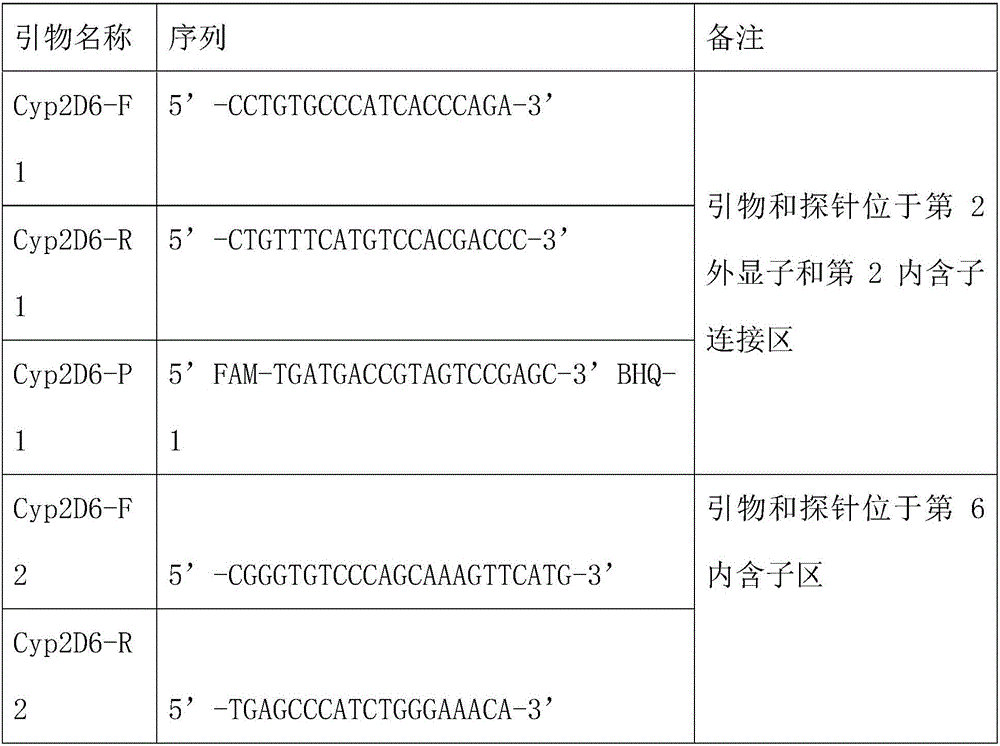
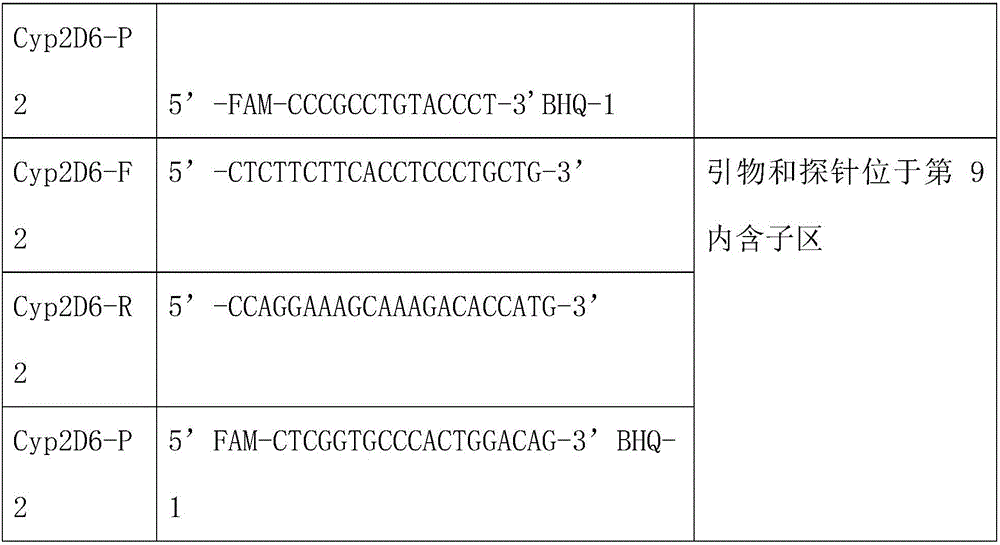
The invention relates to a kit for detecting variation of the copy number of a CYP2D6 gene. The kit comprises a second intron, a sixth exon, a fifth intron and a reference gene RPP30 which are used for detecting the CYP2D6 gene, a real-time fluorescent quantitation technology is adopted, and the change of the copy number of the CYP2D6 gene is determined quickly and accurately; . By using the kit,high-throughput detection can be simultaneously conducted on three loci of the gene related to CYP2D6 under the same reaction condition, detection specificity is high, sensitivity is high, the minimumlowest detection limit is 1 ng / [mu]L, the consumed time is short, and the process from specimen inspection to result obtaining can be completed within two hours.
Owner:GUANGDONG ASCENDAS GENOMICS TECH CO LTD
Detection of curative effects of tamoxifen in auxiliary treatment of breast cancer
InactiveCN101928752AMicrobiological testing/measurementFluorescence/phosphorescenceMedicineFluorescence
The invention discloses a kit for detecting curative effects of individual tamoxifen in auxiliary treatment of breast cancer. The kit comprises a specific primer pair and a specific fluorescent probe pair for detecting the polymorphic site of a target site CYP2D6 gene of tamoxifen administration, a quantitative polymerase chain reaction (PCR) conventional component and the like. The kit of the invention evaluates the curative effects of the individual tamoxifen in the auxiliary treatment of breast cancer by detecting the polymorphic site of the target site CYP2D6 gene of tamoxifen administration.
Owner:HAINAN ZHUJIAN CELL MOLECULE GENETICS INSPECTION CENT
Kit for rapidly detecting CYP2D6 gene copy number by adopting pyrosequencing method and applications of kit
InactiveCN106868172AImprove efficiencyEasy to calculate ratioMicrobiological testing/measurementDNA/RNA fragmentationBiotinMicrobiology
The invention discloses primers for detecting the CYP2D6 gene copy number. The primers comprise: (1) a primer pair for amplifying the CYP2D6 gene, wherein the nucleotide sequence of the upstream primer is shown as SEQ ID NO:1, the nucleotide sequence of the downstream primer is shown as SEQ ID NO:2, and the 5' terminal of the downstream primer is labeled by biotin; and (2) a sequencing primer for the CYP2D6 gene copy number, wherein the nucleotide sequence of the sequencing primer is shown as SEQ ID NO:3. The invention further discloses applications of the primers in detecting the CYP2D6 gene copy number. The detection method is high in sensitivity, the result is visual, the interpretation is simple, accurate and rapid, the accurate, rapid and high-throughput detection for the CYP2D6 copy number can be realized, and thus the primers have great popularization and application values in the personalized medical treatment.
Owner:HANGZHOU D A GENETIC ENG
Composition for detecting human CYP2D6 gene polymorphism, kit, sample processing method and application
InactiveCN109321651ATo achieve the purpose of classificationOptimal use concentrationMicrobiological testing/measurementAgricultural scienceRepeatability
The invention relates to a composition for detecting human CYP2D6 gene polymorphism. The composition comprises primers for detecting the polymorphism of the C188T and / or G4268C locus of the CYP2D6 gene, and the primer for detecting C188T comprises a primer with the sequence as shown in SEQ ID NO. 1, SEQ ID NO. 2 and / or SEQ ID NO.3. The primer for detecting the G4268C locus comprises a primer withthe sequence as shown in SEQ ID NO. 4, SEQ ID NO. 5 and / or SEQ ID NO.6. The primer for detecting the C188T is combined with the primer for detecting G4268C, so as to detect the polymorphism of the C188T and the G4268C at the same time; the specificity is high; the sensitiveness is good; the repeatability is good.
Owner:湖南健基生物技术有限公司
CYP2D6 gene segment containing 3334G>A mutant, protein segment coded by same and application thereof
InactiveCN103436543AReduced metabolic activityMicrobiological testing/measurementOxidoreductasesAlleleProtein Fragment
The invention belongs to the field of biology and relates to a single base mutant at the 3334th site of CYP2D6 allele, wherein G at the site is mutated into A. The invention particularly relates to a nucleic acid segment containing the mutant site and a corresponding protein segment coded by the nucleic acid segment, a reagent and detection method for identifying the mutant site, application of the site, and especially application of identification of the site in medicine instruction.
Owner:蔡剑平
Method for detecting copy number variation of human CYP2D6 gene
ActiveCN113186266AEasy to operatePrevent false positive amplificationMicrobiological testing/measurementDNA/RNA fragmentationReference genesDNA extraction
The invention provides a method for detecting copy number variation of a human CYP2D6 gene, the method comprises the following steps of: selecting two DNA sequences which have certain copy number and do not have copy number variation in a known genome of a sample to be detected as reference genes, calculating the difference Ct0 of Ct values of the two reference genes, and judging whether the degradation degree of the sample reaches the inaccurate detection degree or not; if the Ct0 reaches a threshold value which cannot ensure the accuracy of the detection result, prompting to re-sample for DNA extraction; and if the Ct0 is within a threshold range for ensuring the accuracy of the detection result, calculating the difference value between the Ct value of the CYP2D6 gene and the Ct value of one reference gene to obtain Ct1, and further judging the CYP2D6 copy number variation condition of the sample to be detected. According to the invention, the quality problem of the sample is eliminated, the accuracy of the CNV detection result is ensured, the operation is convenient and fast, the detection time is shortened, and the high cost caused by sequencing is saved.
Owner:上海康黎诊断技术有限公司
Detection of curative effect of antilipemic and antiatherosclerotic drug
InactiveCN101928756AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceCurative effect
The invention discloses a kit for detecting the curative effect of an individual antilipemic and antiatherosclerotic drug. The kit comprises a specific primer pair, a specific fluorescent probe pair, a fluorescence quantitative PCR conventional assembly and the like, and the specific primer pair is used for detecting a medication target site, namely a CYP3A4 gene, CYP2D6 gene and CYP2C9 gene polymorphism site of the statin drug. The kit can be used for estimating the curative effect of the individual antilipemic and antiatherosclerotic drug by detecting the medication target site, namely CYP3A4 gene, CYP2D6 gene and CYP2C9 gene polymorphism siteCYP3A4 gene, CYP2D6 gene and CYP2C9 gene polymorphism site of the statin drug.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
CYP2D6 gene mutation detection primer and kit
InactiveCN109182474AEasy to operateHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionWild type
The invention discloses a CYP2D6 gene mutation detection primer, comprising an upstream primer as shown in SEQ ID No. 1, a downstream primer as shown in SEQ ID No. 2 and a probe as shown in SEQ ID No.3. The invention also discloses the use of the detection primer in preparing a CYP2D6 gene mutation detection reagent and a CYP2D6 gene mutation detection reagent kit. The detection primer provided by the invention can realize the simultaneous detection of wild type of CYP2D6*10 (rs1065852 C100T) site in the same tube PCR reaction, heterozygous mutation and homozygous mutation, and has the advantages of simple operation, high sensitivity, high specificity and low cost.
Owner:江门市妇幼保健院
Detection method of types of CYP2D6 gene
InactiveCN110157779AImprove detection efficiencyMicrobiological testing/measurementGenotypeMicrobiology
The invention provides a detection method of types of a CYP2D6 gene. The detection method comprises the steps of preparing a first polymerase chain reaction (PCR) amplification system comprising specific amplification primers shown in SEQ ID NO.1-14 and a second PCR amplification system comprising specific amplification primers shown in SEQ ID NO.15-28, carrying out amplification reactions in sequence, and then conducting digestive treatment; obtaining a first digestion reaction system and a second digestion reaction system; preparing a first single base extension reaction system comprising single base extension primers shown in SEQ ID NO.29-49 and a second single base extension reaction system comprising single base extension primers shown in SEQ ID NO.50-69; carrying out extension reactions on the first digestion reaction system joining in the first single base extension reaction system and the second digestion reaction system joining in the second single base extension reaction system, then conducting purification treatment, sequentially utilizing a detection instrument comprising a mass spectrometer for detection, and determining genotypes of SNP loci of the CYP2D6 gene of a to-be-detected sample. By means of the detection method, the detection efficiency of the types of the CYP2D6 gene can be improved.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
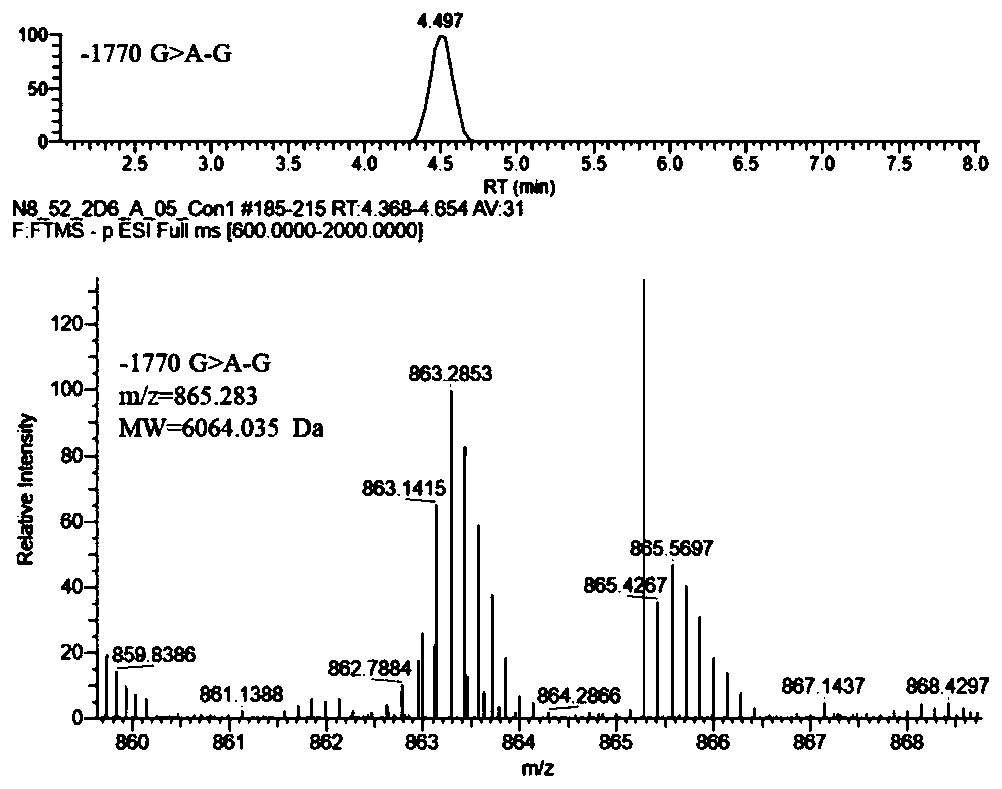
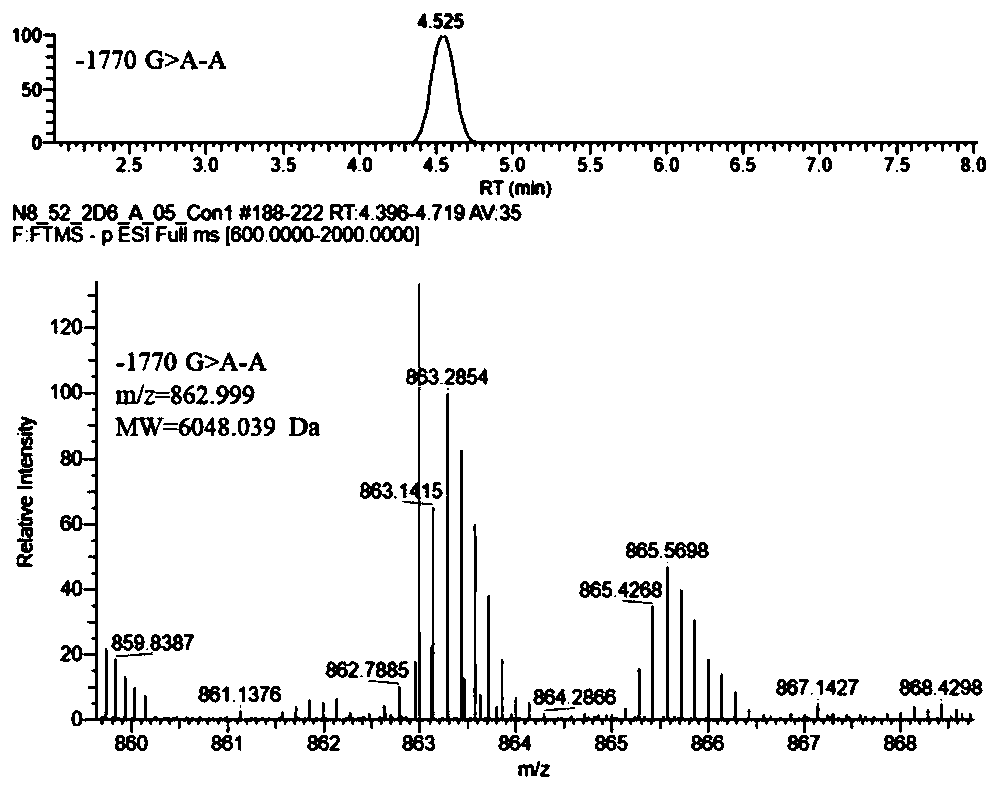
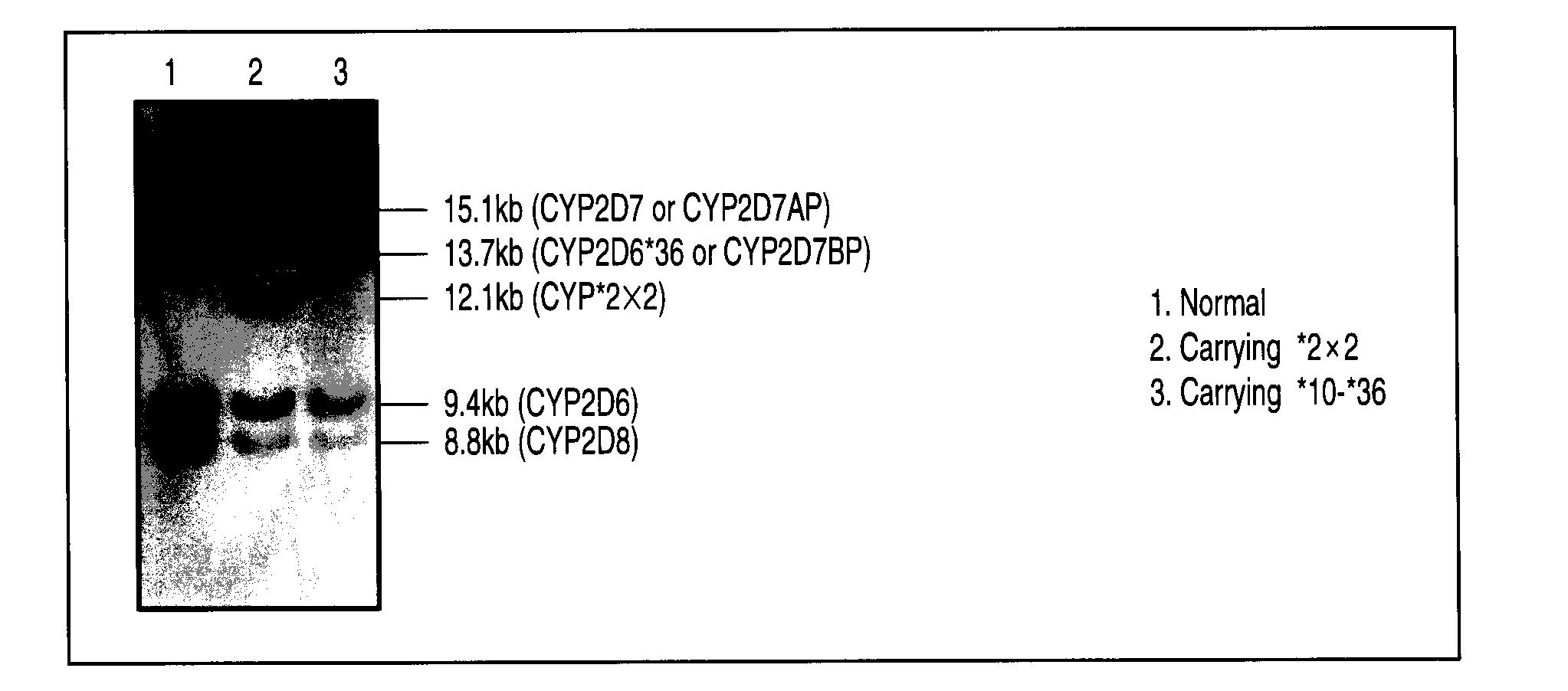
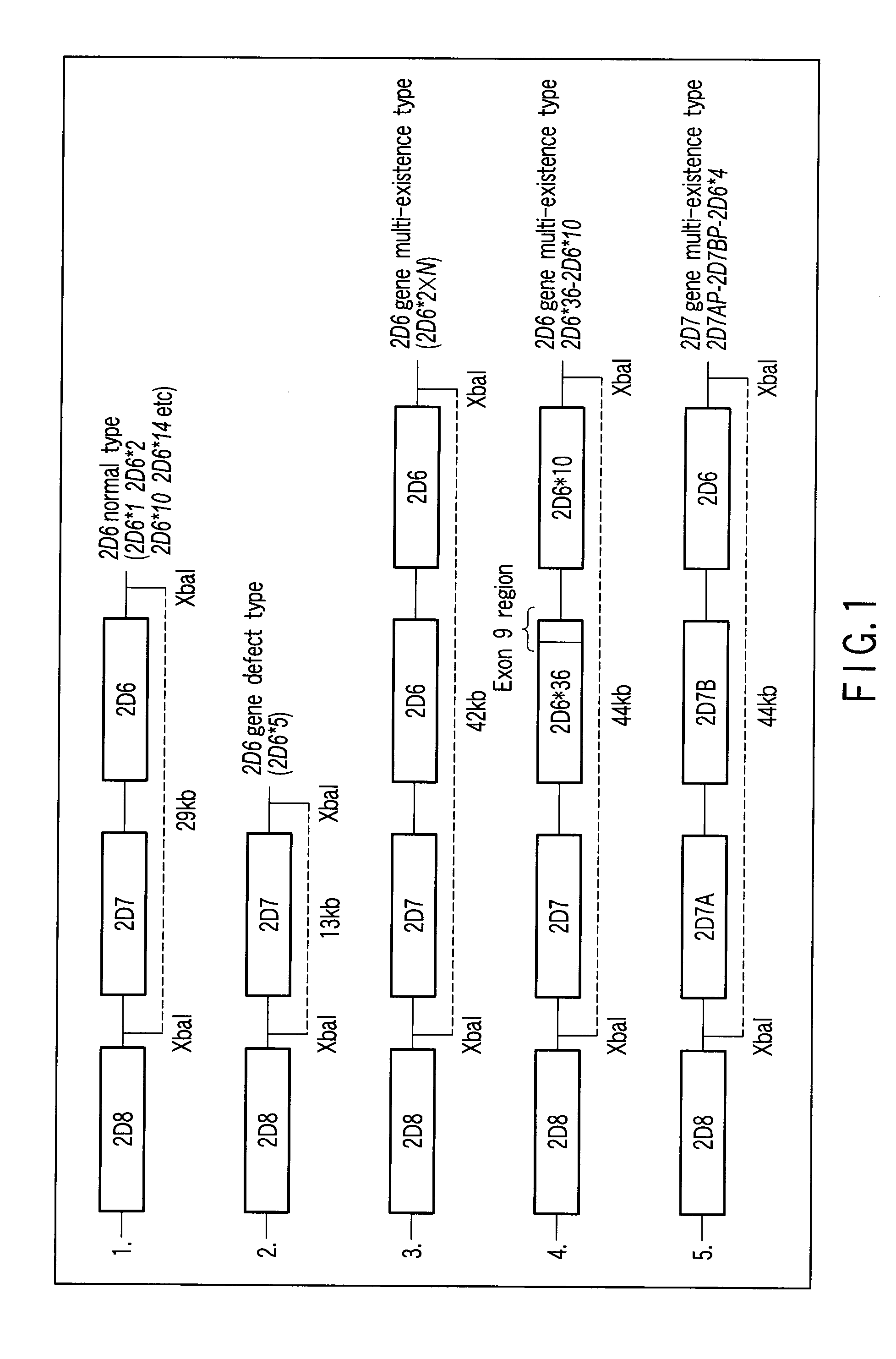
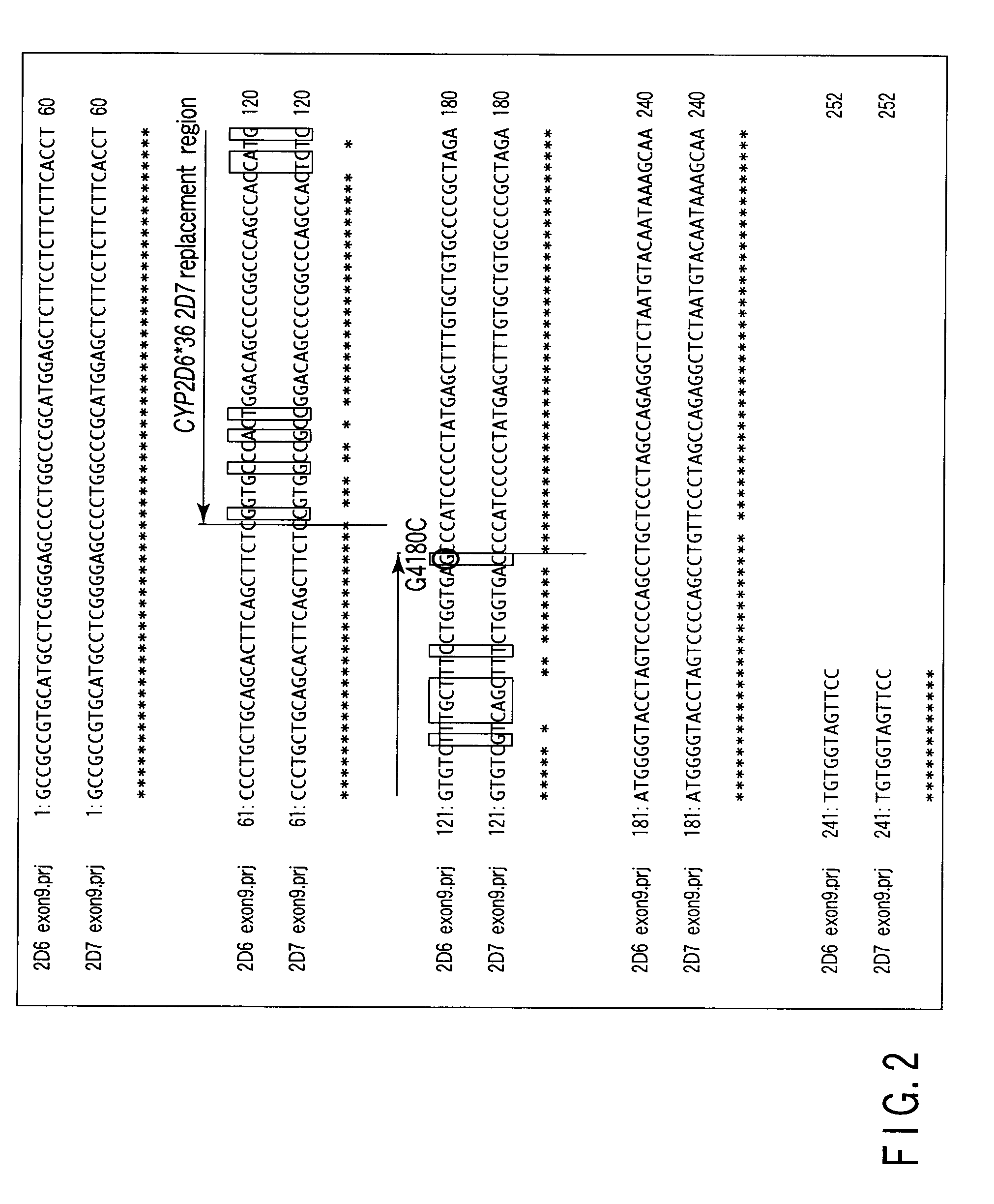
Method of detecting human cytochrome p450 (CYP) 2d6 gene mutation
A defect or multi-existence of a CYP2D6 gene is detected with a primer includes a complementary sequence to a sequence which is common between the CYP2D6 gene and a CYP2D8 gene but different from a CYP2D7 gene and which contains one or more of bases at the 86-, 90- and 93-positions in Exon 9 region of the CYP2D6 gene.
Owner:KK TOSHIBA +1
CYP2D6 gene mutation detection liquid-phase chip and detection method
ActiveCN101824467BImplement parallel detectionImprove signal-to-noise ratioMicrobiological testing/measurementSignal-to-noise ratio (imaging)Microsphere
The invention provides a CYP2D6 gene mutation detection liquid-phase chip which comprises ASPE (Allele Specific Primer Extension) primers aiming at CYP2D6 C2850T and CYP2D6Deletion mutational sites, three microballoons respectively enveloped with a specific anti-tag sequence and amplification primers aiming at the CYP2D6C2850T and the CYP2D6 Deletion mutational sites. The CYP2D6 gene mutation detection liquid-phase chip can simultaneously detect aiming at the CYP2D6 C2850T and the CYP2D Deletion mutational sites and has excellent signal to noise ratio. The coincidence ratio with a sequencing method of the CYP2D6 gene mutation detection liquid-phase chip reaches up to 100 percent, and the CYP2D6 gene mutation detection liquid-phase chip has higher specificity and precision compared with intra-class correlation products.
Owner:SUREXAM BIO TECH
Noninvasive detection kit for susceptibility genes for oral cancer
The invention provides a noninvasive detection kit for susceptibility genes for oral cancer. The kit comprises specific primers, DNA (deoxyribonucleic acid) sequencing primers, PCR (polymerase chain reaction) components, PCR product purification components, DNA sequencing reaction components and the like for detecting genotypes of three single nucleotide polymorphism (SNP) sites, including Ile462Val site polymorphism and MspI site polymorphism on CYP1A1 (cytochrome P4501A1) gene and C188T site polymorphism on CYP2D6 (cytochrome P450 2D6) gene. According to the invention, genotypes of three single nucleotide polymorphism sites closely related with oral cancer occurrence are detected by the kit, so as to estimate the risk level of the oral cancer of a subject, and finally the subject is instructed to specifically prevent occurrence of oral cancer at a genetic level according to a gene detection result of each subject, so as to lower the morbidity of oral cancer. The sampling method disclosed by the invention is oral mucosa sampling; the process is painless and non-invasive; and cross infection can be avoided.
Owner:解码(上海)生物医药科技有限公司
Oral eliglustat transmucosal delivery system
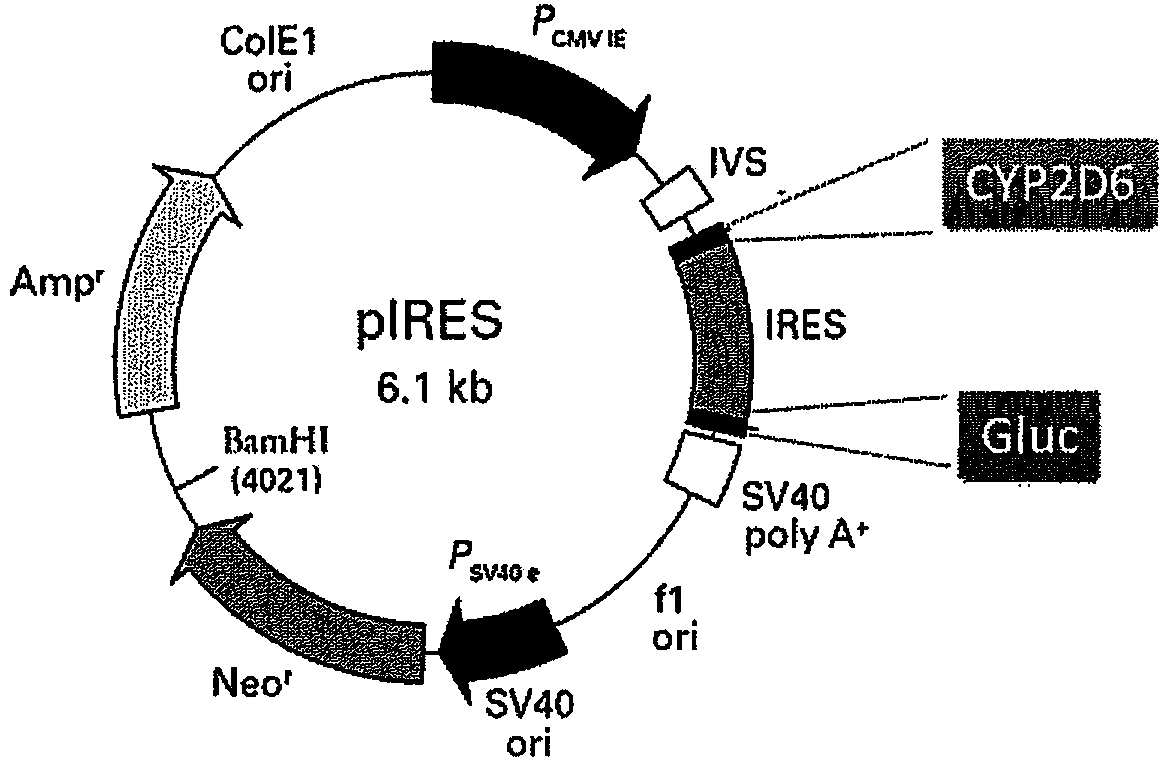
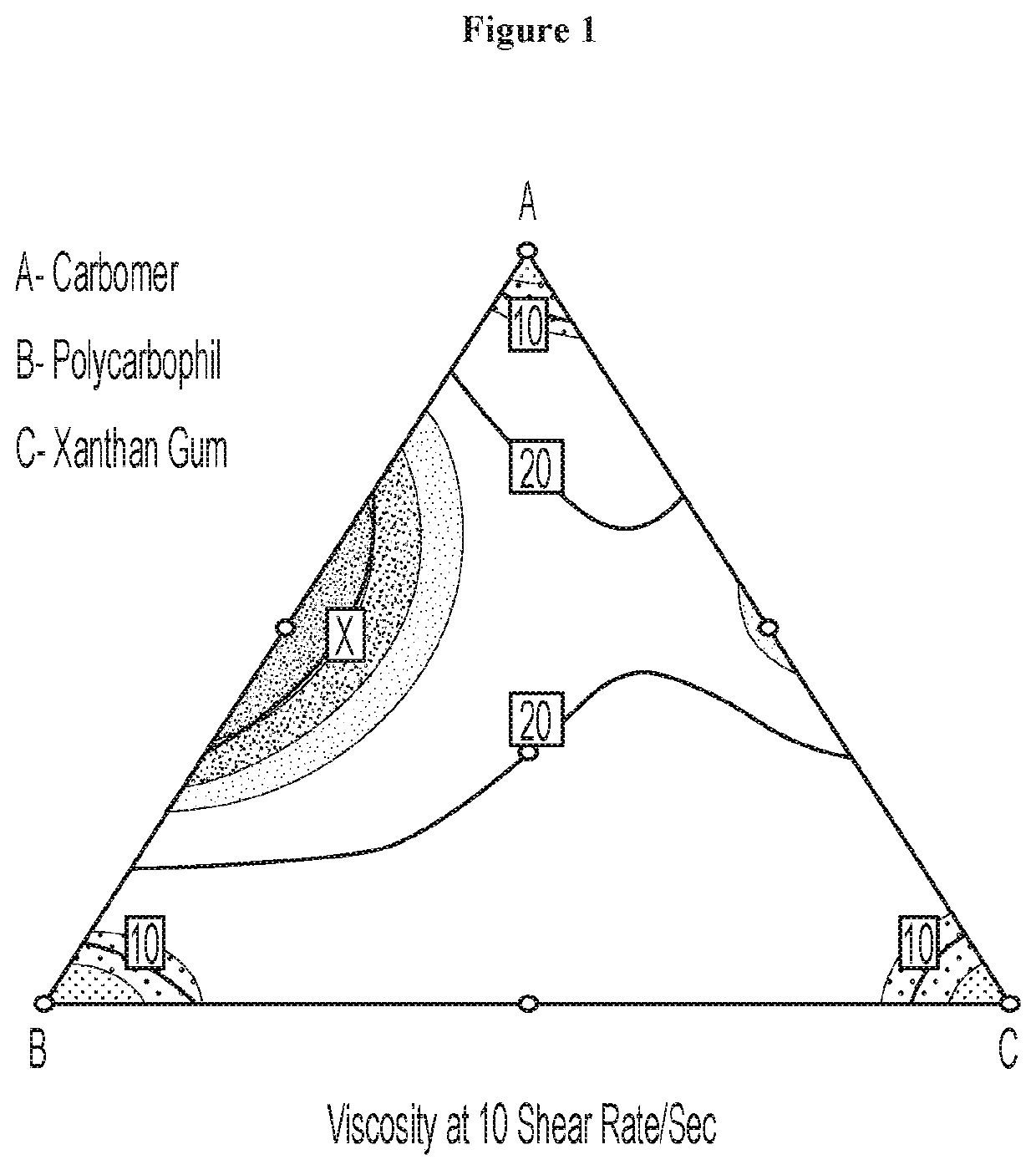
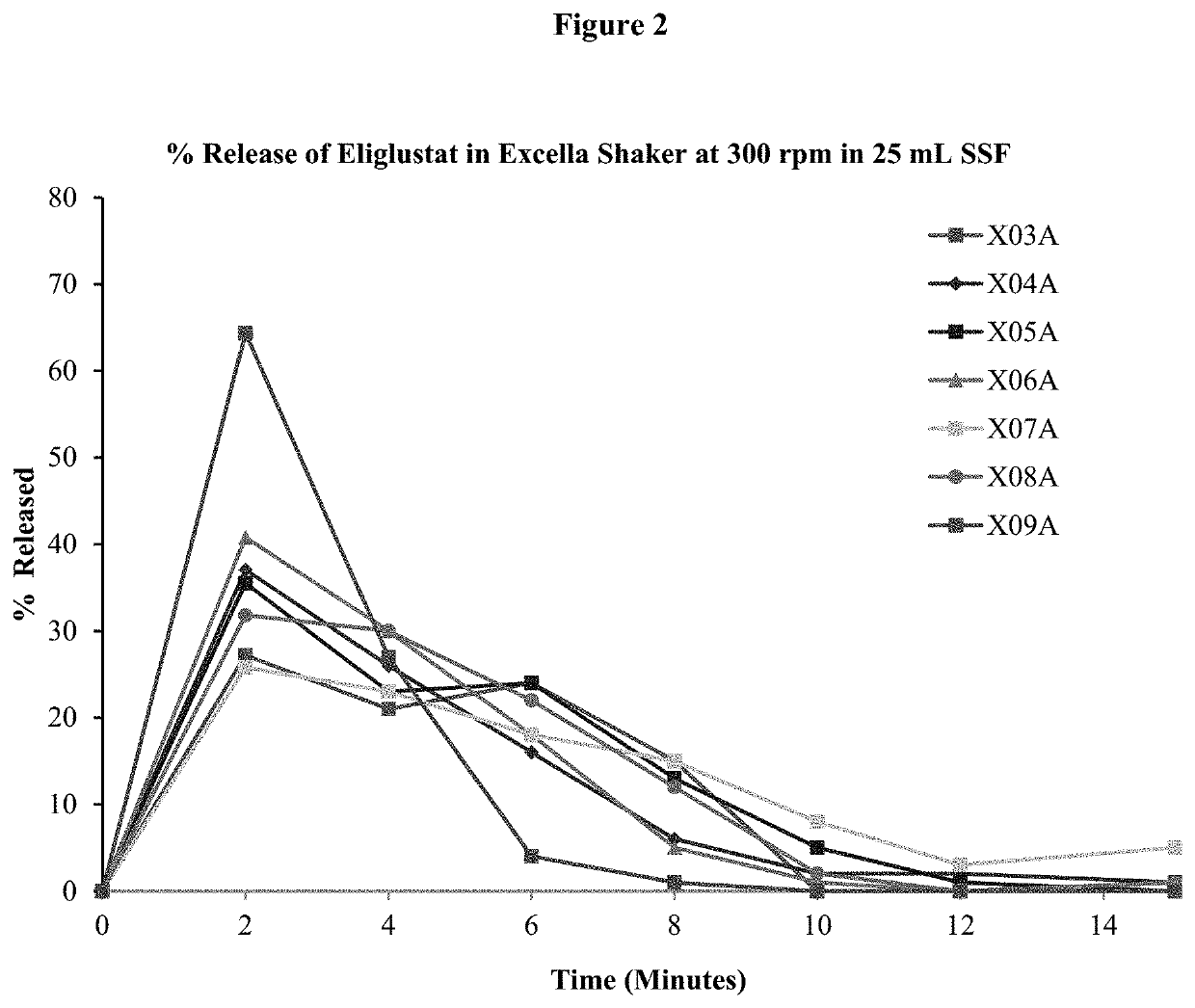
The present disclosure relates to an oral eliglustat transmucosal delivery system. More specifically, the present disclosure is related to an oral transmucosal dosage form comprising a non-disintegrating solid mass comprising (a) a hydrophilic viscosity modifying agent selected from a natural or a synthetic gum with a molecular weight of 10,000 Daltons or greater, (b) a low molecular weight water soluble component with a molecular weight of less than 10,000 Daltons and (c) not more than 70 mg eliglustat, wherein the dosage form is 500 mg or less and provides an oral cavity residence time of at least about 5 minutes, and wherein the dosage form generates a microenvironment inside the oral cavity exhibiting a thixotropic behavior with viscosities of at least 50 poises at 1 / sec shear rate and at least 10 poises at 10 / sec share rate. The dosage forms of as described herein have at least 30% dose reduction as compared to commercially available eliglustat capsules. In addition, unlike the dosing prerequisite for commercially available eliglustat capsules, the oral eliglustat transmucosal dosage form described herein can be administered to patients with Gaucher disease type 1 without pre-determination of patients' CYP2D6 genotype.
Owner:ABON PHARMA
Juno<TM>-based safety medication detection kit for children and chip
InactiveCN108728524AGood curative effectReduce the risk of adverse reactionsMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCYP2C9
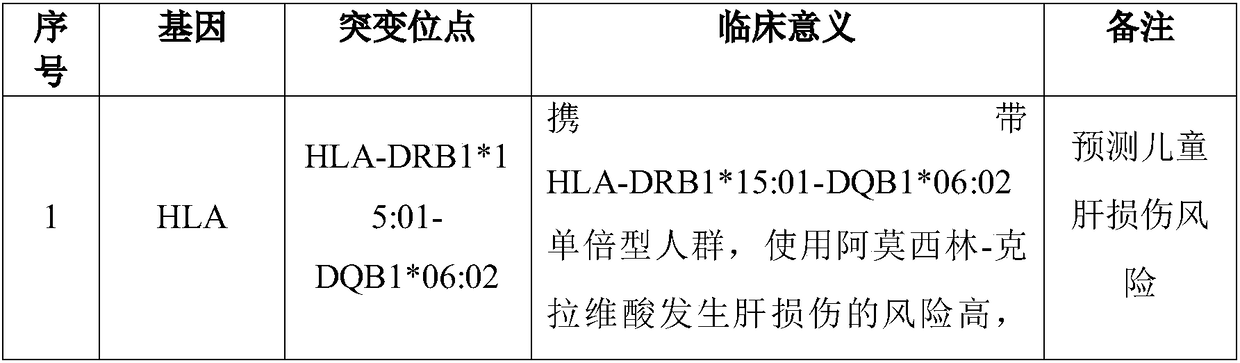
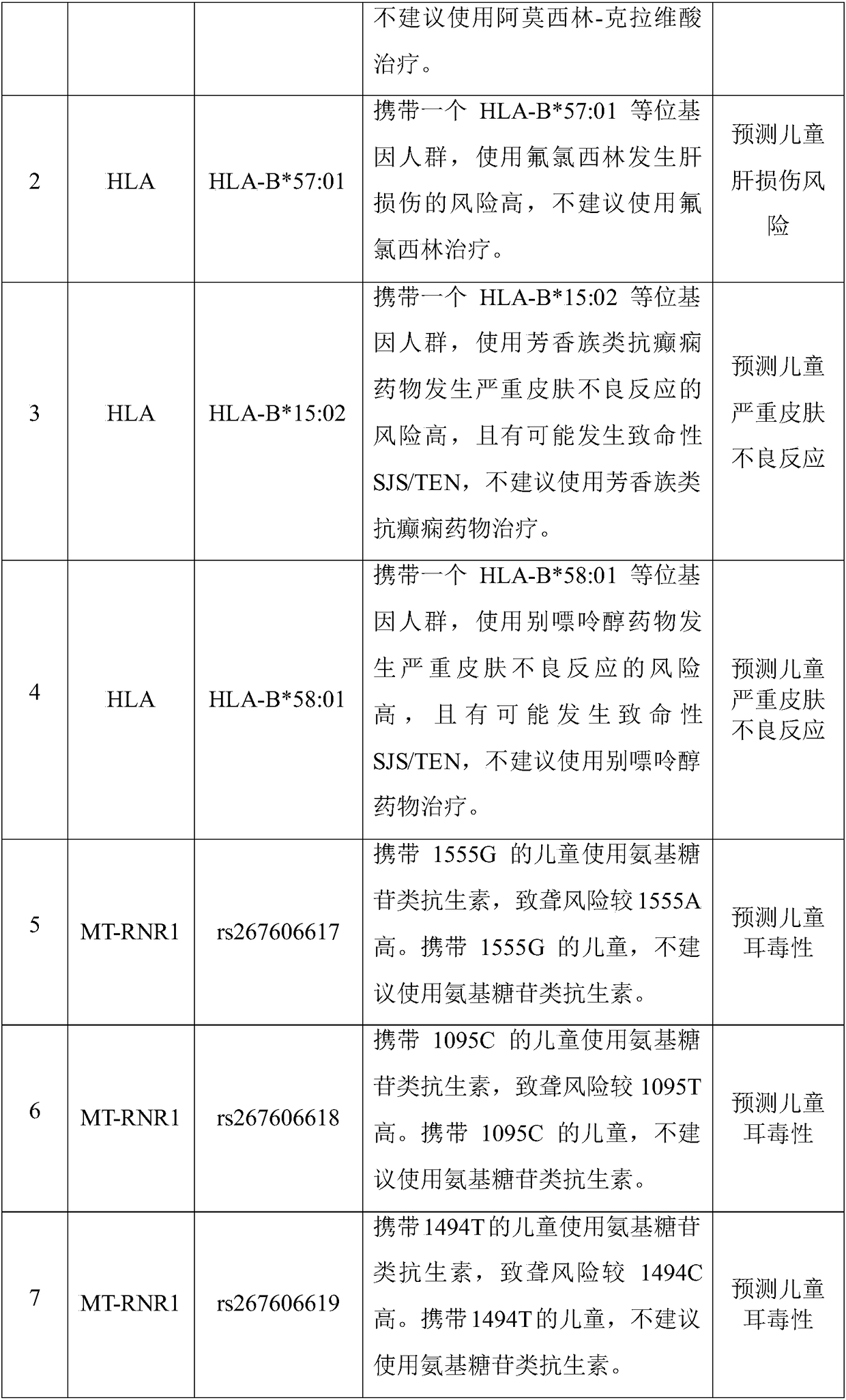
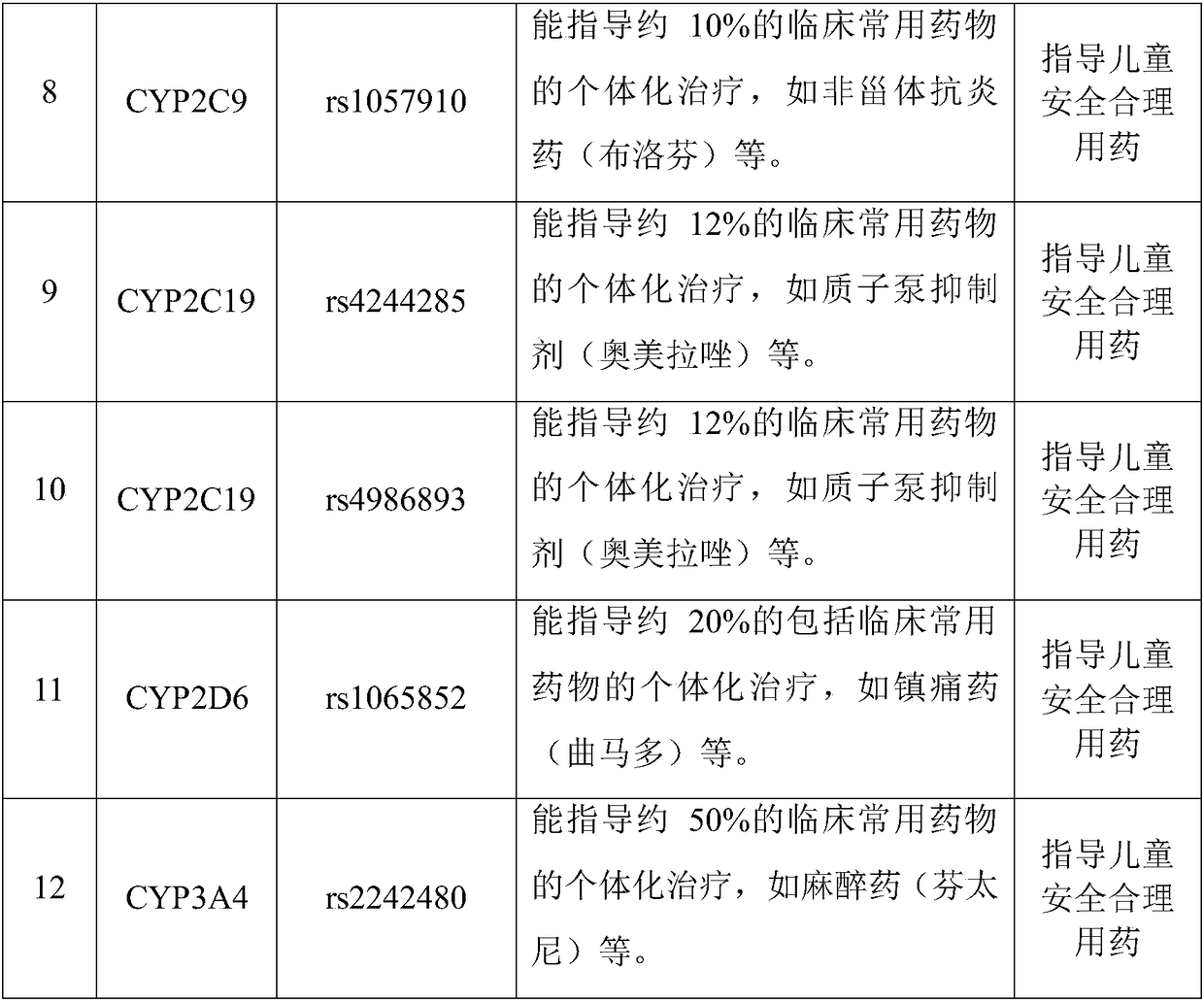
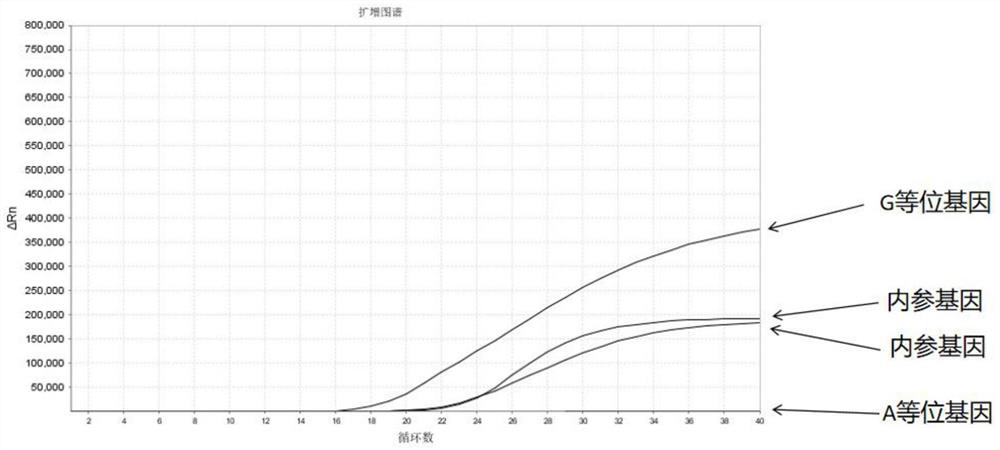
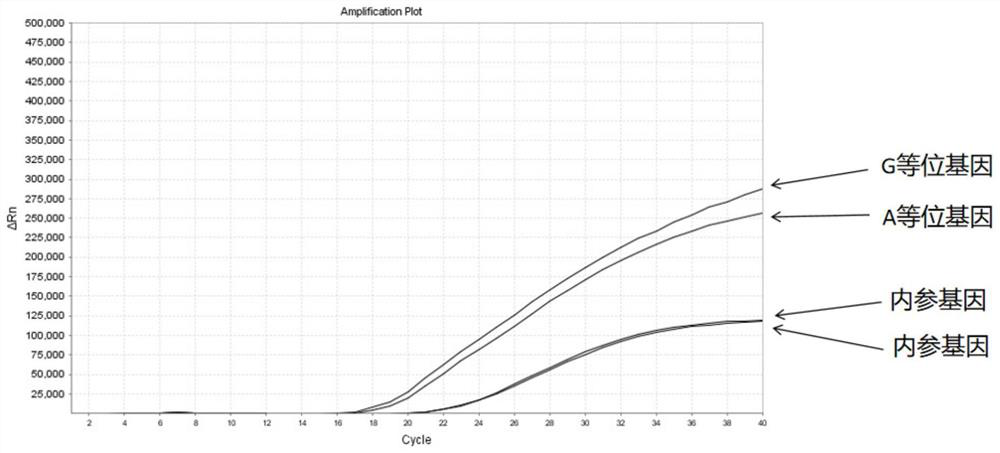
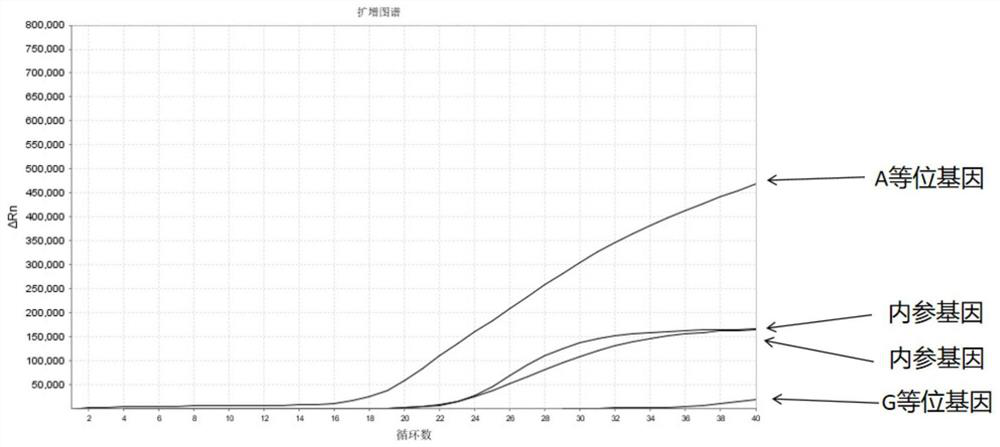
The invention discloses a Juno<TM>-based safety medication detection kit for children and a chip. The kit and the chip comprise primers at the following loci: HLA-DRB1*15:01-DQB1*06:02 loci of an HLAgene, an HLA-B*57:01 locus of the HLA gene, an HLA-B*15:02 locus of the HLA gene, an HLA-B*58:01 locus of the HLA gene, a rs267606617 locus of an MT-RNR1 gene,a rs267606618 locus of the MT-RNR1 gene,a rs267606619 locus of the MT-RNR1 gene, a rs1057910 locus of a CYP2C9 gene, a rs4244285 locus of a CYP2C19 gene, a rs4986893 locus of the CYP2C19 gene, a rs1065852 locus of a CYP2D6 gene, and a rs2242480 locus of a CYP3A4 gene.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Gene polymorphism detection kit for guiding psychiatric medication
PendingCN113584147AStrong specificityHigh sensitivityMicrobiological testing/measurementFluoProbesHTR2C gene
The invention discloses a gene polymorphism detection kit for guiding psychiatric medication. The kit comprises a specific primer group and a fluorescent probe group, wherein the specific primer group and the fluorescent probe group are used for detecting the rs1065852 site of a CYP2D6 gene, the rs1414334 site of an HTR2C gene, the rs1800497 site of an ANKK1 gene, the rs762551 site of a CYP1A2 gene, the rs489693 site of an MC4R gene and the rs1799978 site of a DRD2 gene. According to the invention, the primer group and the fluorescent probe group are utilized, and the template DNA is specifically amplified by using a fluorescent PCR amplification technology, so that the polymorphism of a plurality of sites of a plurality of genes related to psychotropic drugs can be accurately detected at the same time; ARMS primer design is adopted, mismatched bases are introduced, the specificity of primer amplification is improved, the sensitivity is high, and accurate genotyping can be conducted on human template DNA at the lowest DNA initial amount of 10 ng; the detection speed is high and the efficiency is high: the PCR reaction only needs less than 1 hour to complete the amplification reaction, the cost is lower, the required reagent consumables are all clinically common reagents, the cost is lower, and the clinical popularization is convenient.
Owner:ZHENGZHOU UNIV +1
CYP2D6 gene fragment comprising 74G>A mutation, coded protein fragment and applications thereof
ActiveCN103525839AReduced metabolic activityMicrobiological testing/measurementOxidoreductasesProtein FragmentReagent
The invention belongs to the fields of biology, and relates to a mutation of the single base in the 74th site of a CYP2D6 allelic gene, wherein the site is mutated from G to A. In specific, the invention relates to a nucleic acid fragment comprising the mutation site, a corresponding coded protein fragment thereof, a reagent for identifying the mutation site, a detecting method, and applications of the site, particularly applications of identification of the site for guiding pharmacy.
Owner:BEIJING HOSPITAL
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com