Patents
Literature
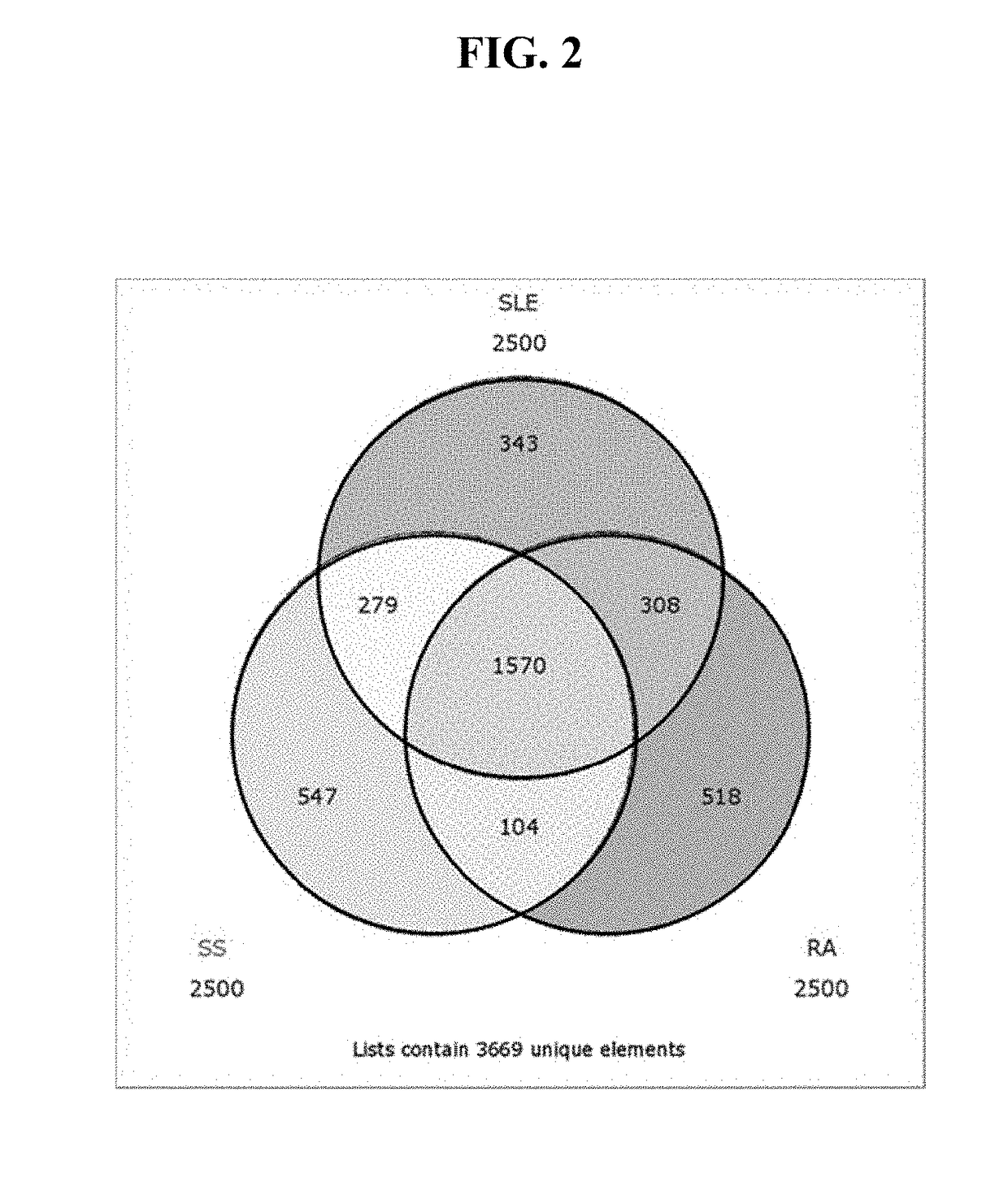
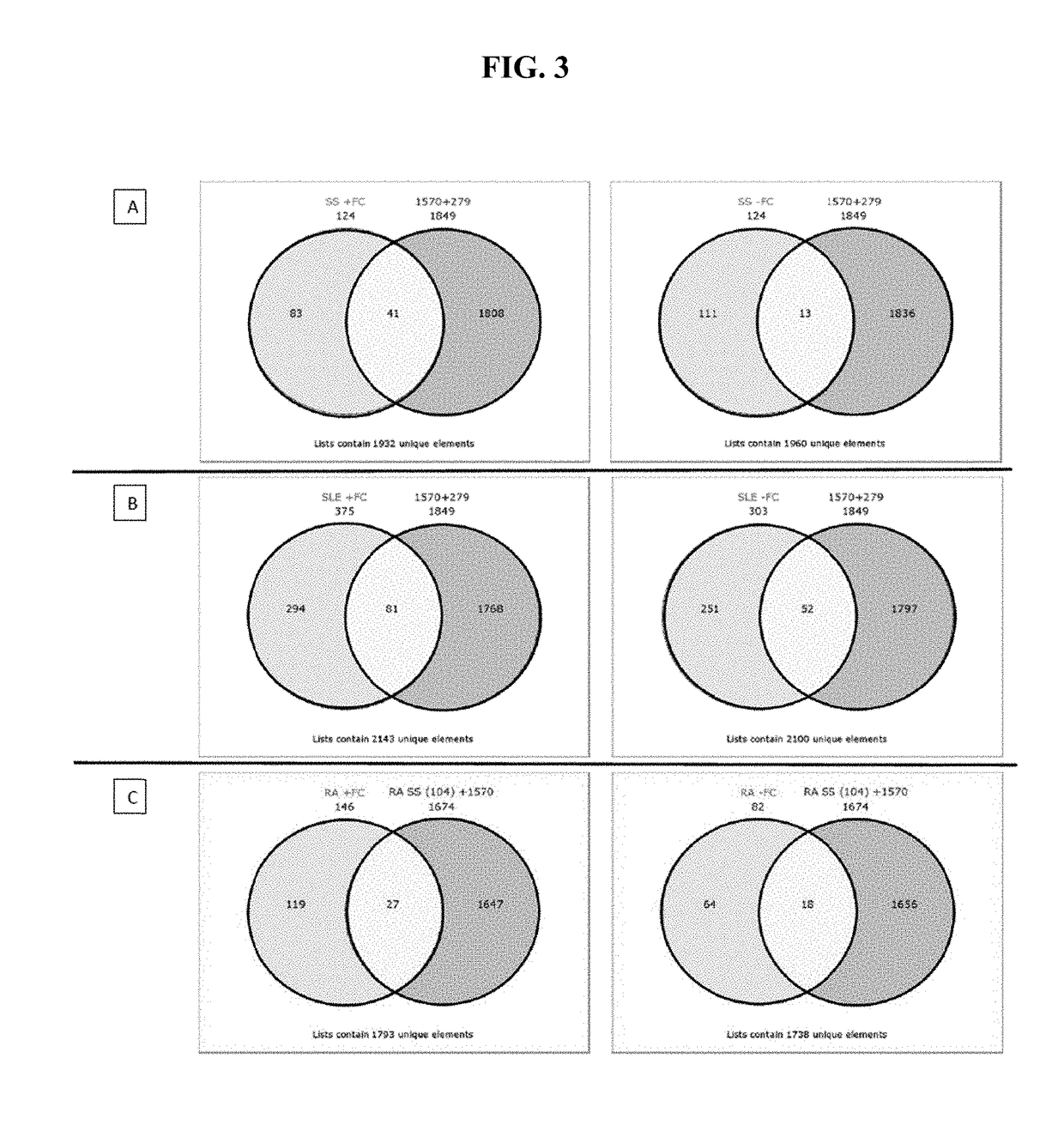
73 results about "Sjogrens syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
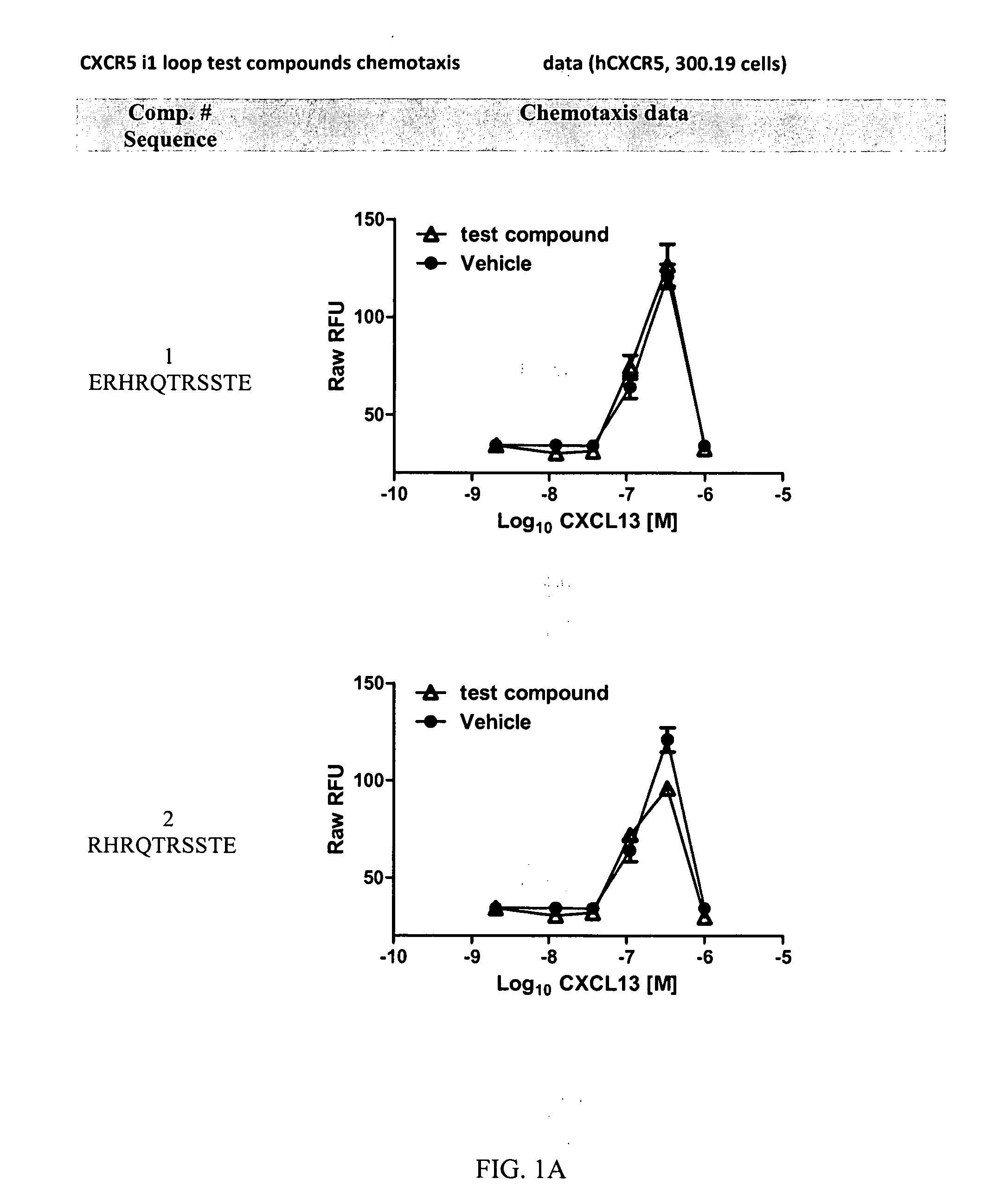
Cxcr5 receptor compounds
The invention relates generally to compounds which are allosteric modulators (e.g., negative and positive allosteric modulators, allosteric agonists, and ago-allosteric modulators) of the G protein coupled receptor CXCR5. The CXCR5 receptor compounds are derived from the intracellular loops and domains of the CXCR5 receptor. The invention also relates to the use of these CXCR5 receptor compounds and pharmaceutical compositions comprising the CXCR5 receptor compounds in the treatment of diseases and conditions associated with CXCR5 receptor modulation such as autoimmune diseases including lupus, HIV and rheumatoid arthritis, Primary Sjogren's Syndrome, chronic lymphocytic leukemia, Burkitt Lymphoma, colon and breast cancer tumor metastasis, Multiple Sclerosis and compromised immune function.
Owner:ANCHOR THERAPEUTICS
Method for treating Sjogren's syndrome
A method of treating Sjögren's syndrome in a patient eligible for treatment is provided involving administering an effective amount of an antagonist that binds to a B-cell surface marker to the patient to provide significant improvement over baseline in two or more of dryness, fatigue, and joint pain on a Visual Analogue Scale, and an article of manufacture therefor. Methods and articles are also provided involving treating Sjögren's syndrome in a subject eligible for treatment is provided involving administering an effective amount of an antibody that binds to a B-cell surface marker to the subject to provide an initial exposure and a subsequent exposure to the antibody within certain dosing regimens and an article of manufacture therefor.
Owner:GENENTECH INC
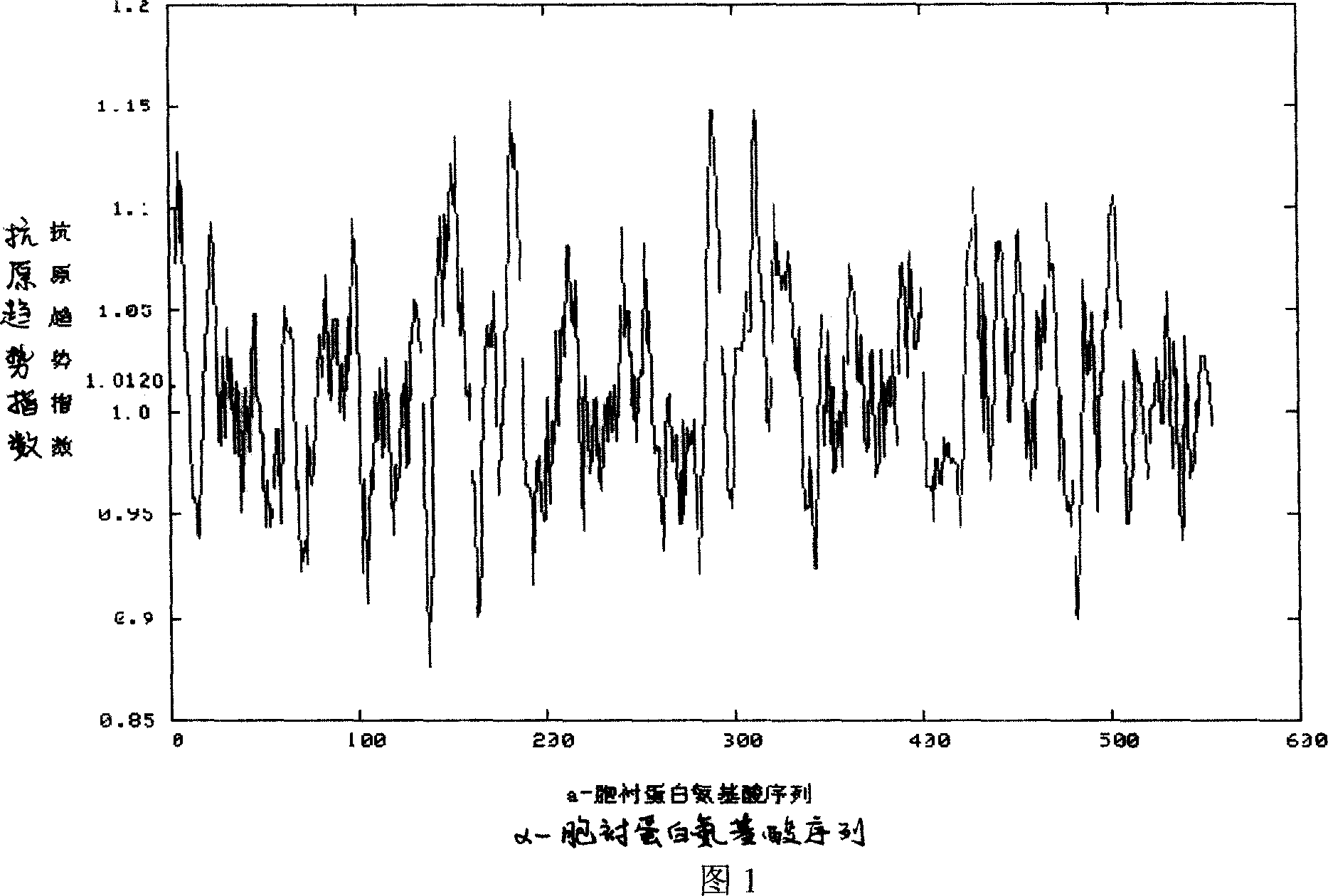
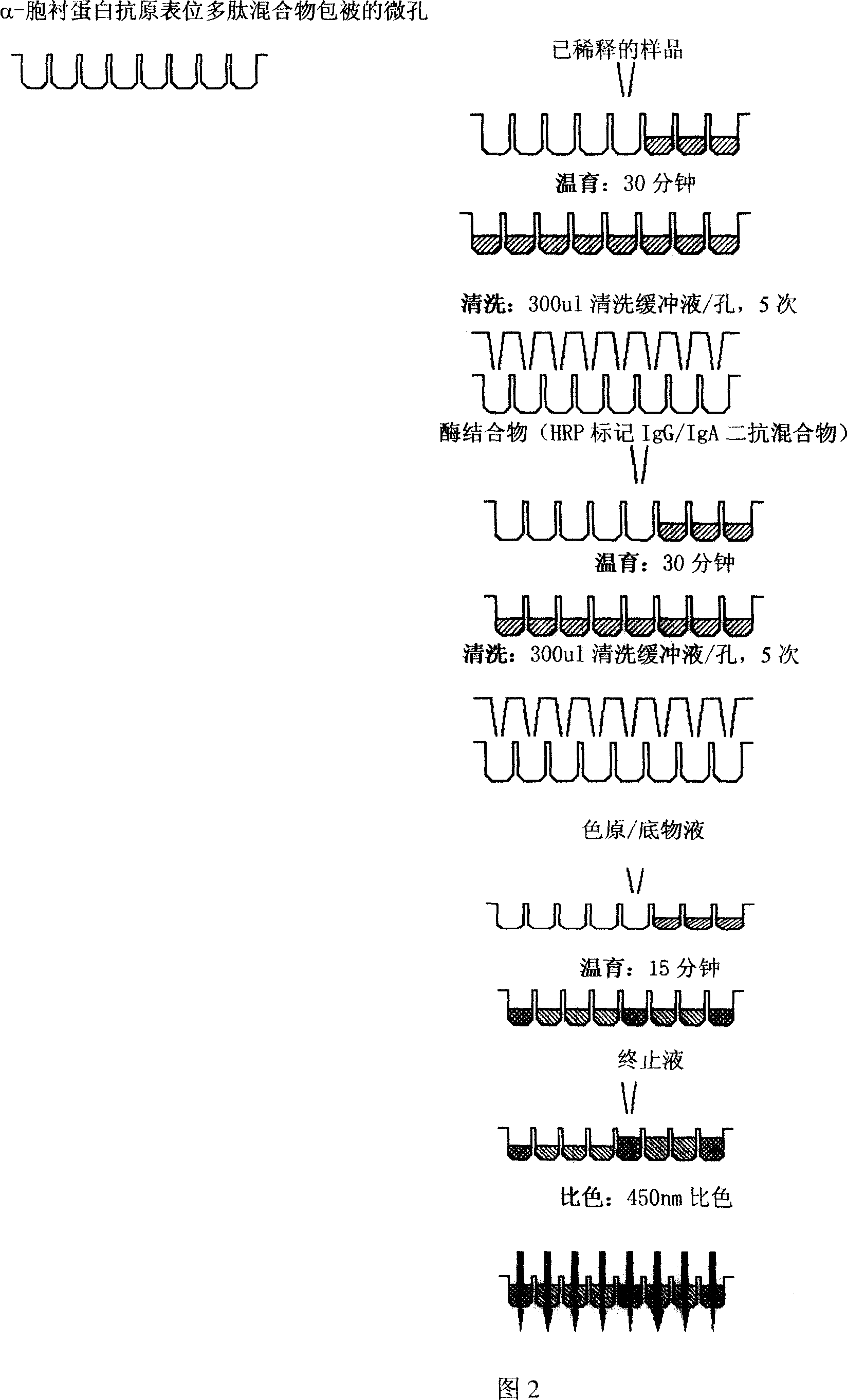
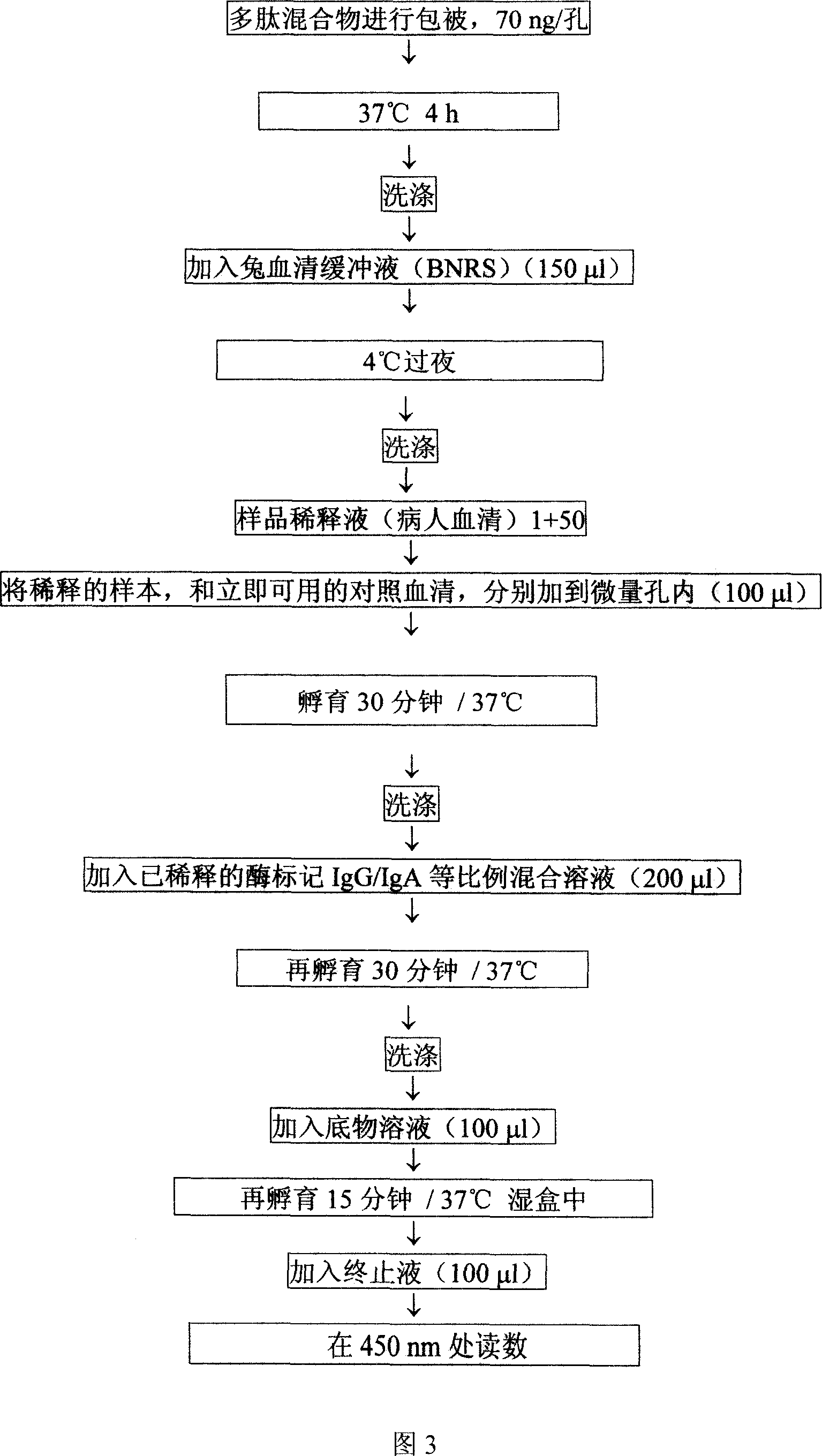
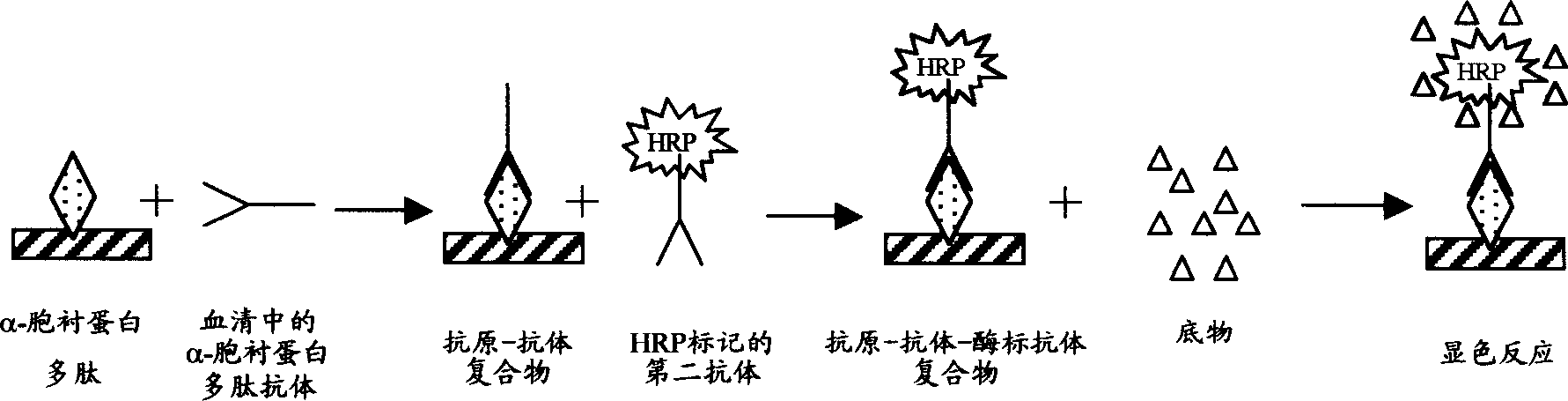
Alpha-fodrin antigen epi-position polypeptide mixture and its use
InactiveCN1979165AAdvantages of value assessmentBiological testingHybrid peptidesAntigen epitopeAntigen
The invention relates to screen for antigen epitope polypeptide, and building up of immunology detection method and the manufacturing and application for reagent for clinical diagnosis, especially for alpha-cyst albumen antigen epitope polypeptide.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Compositions and methods for sjögren's syndrome
ActiveUS20180080082A1High riskMicrobiological testing/measurementDisease diagnosisMedicineExpression gene
The present invention provides methods and compositions involving epigenetic and gene expression signatures and their association with Sjögren's syndrome.
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
Pharmaceutical composition for ophthalmic use
InactiveUS7732425B2Satisfied with the effectAdvantageous usefulness and safetyBiocideOrganic active ingredientsOphthalmologySjögren syndrome
An ophthalmic pharmaceutical composition comprising trehalose as an effective ingredient and a pharmaceutically-acceptable carrier. The pharmaceutical composition is a safe, long-term continuously-administrable, therapeutic and / or prophylactic agent for the ophthalmologic clinical symptoms and signs in Sjögren syndrome.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
New phenylalanine derivatives
Specific phenylalanine derivatives or pharmaceutically acceptable salts thereof have an antagonistic effect on the α4 integrins and, therefore, are usable as therapeutic agents or preventive agents for diseases in which α4 integrin-depending adhesion process participates in the pathology, such as inflammatory diseases, rheumatoid arthritis, inflammatory bowel diseases, systemic lupus erythematosus, multiple sclerosis, Sjögren's syndrome, asthma, psoriasis, allergy, diabetes, cardiovascular diseases, arterial sclerosis, restenosis, tumor proliferation, tumor metastasis and transplantation rejection.
Owner:EA PHARMA CO LTD
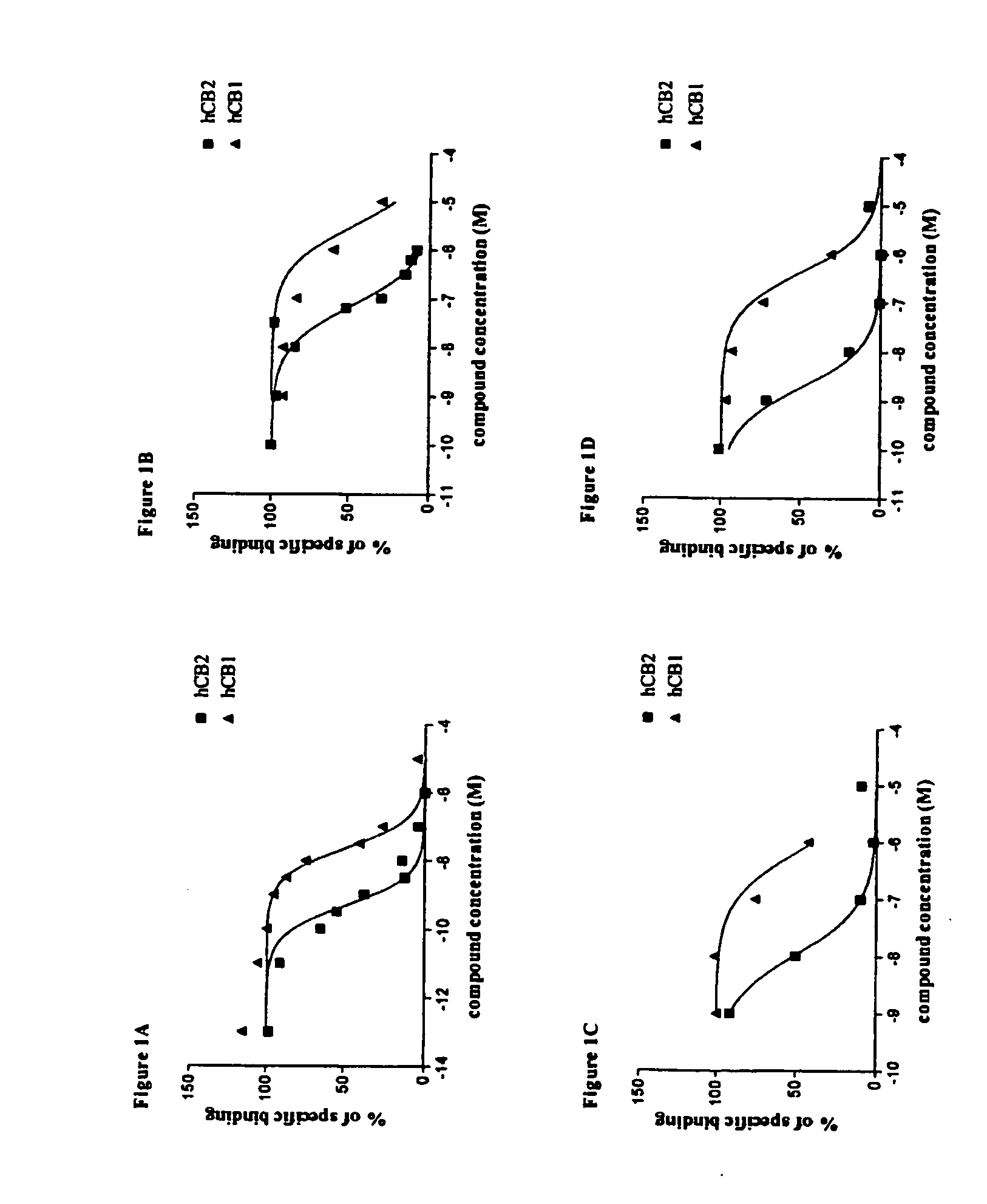
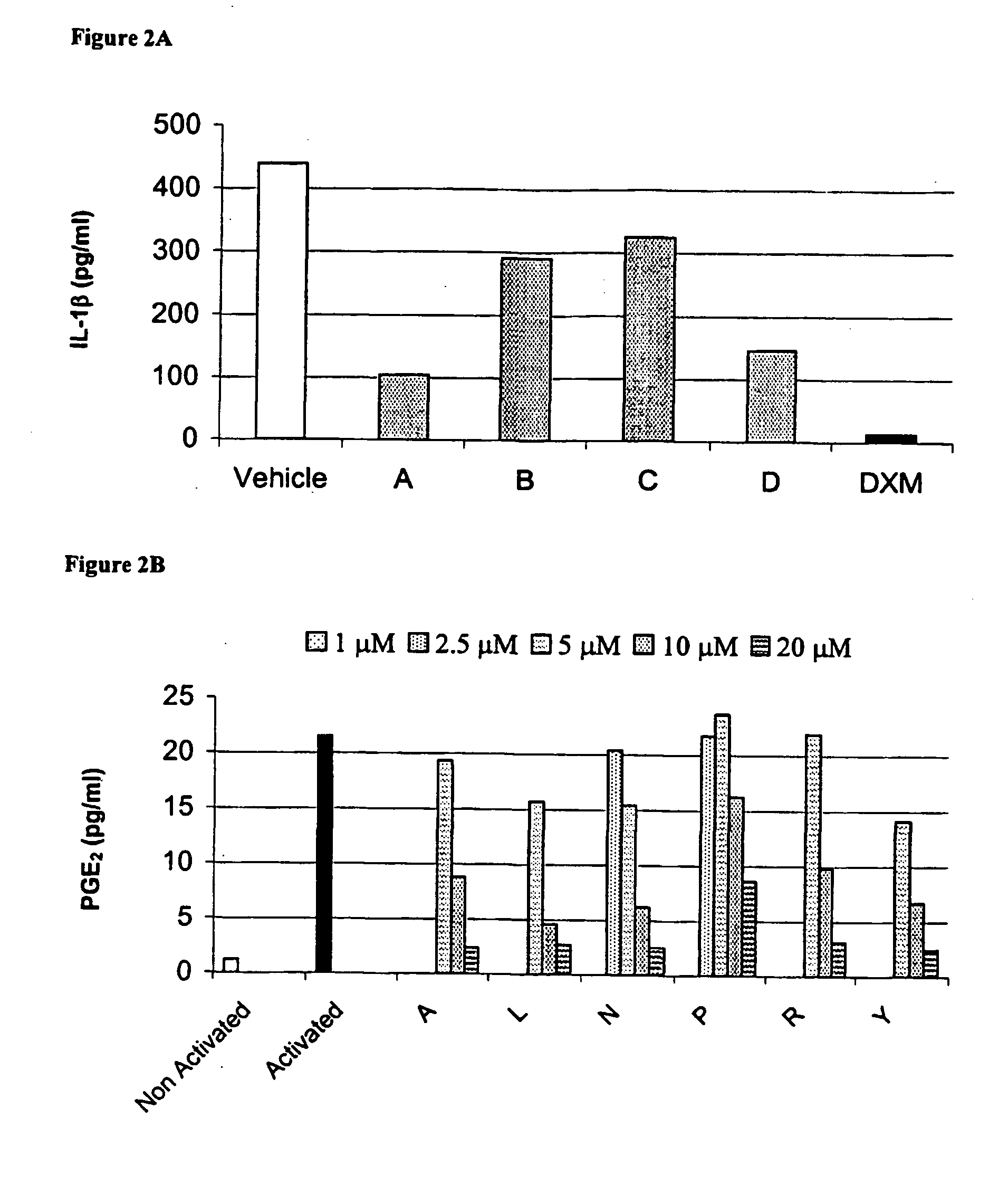
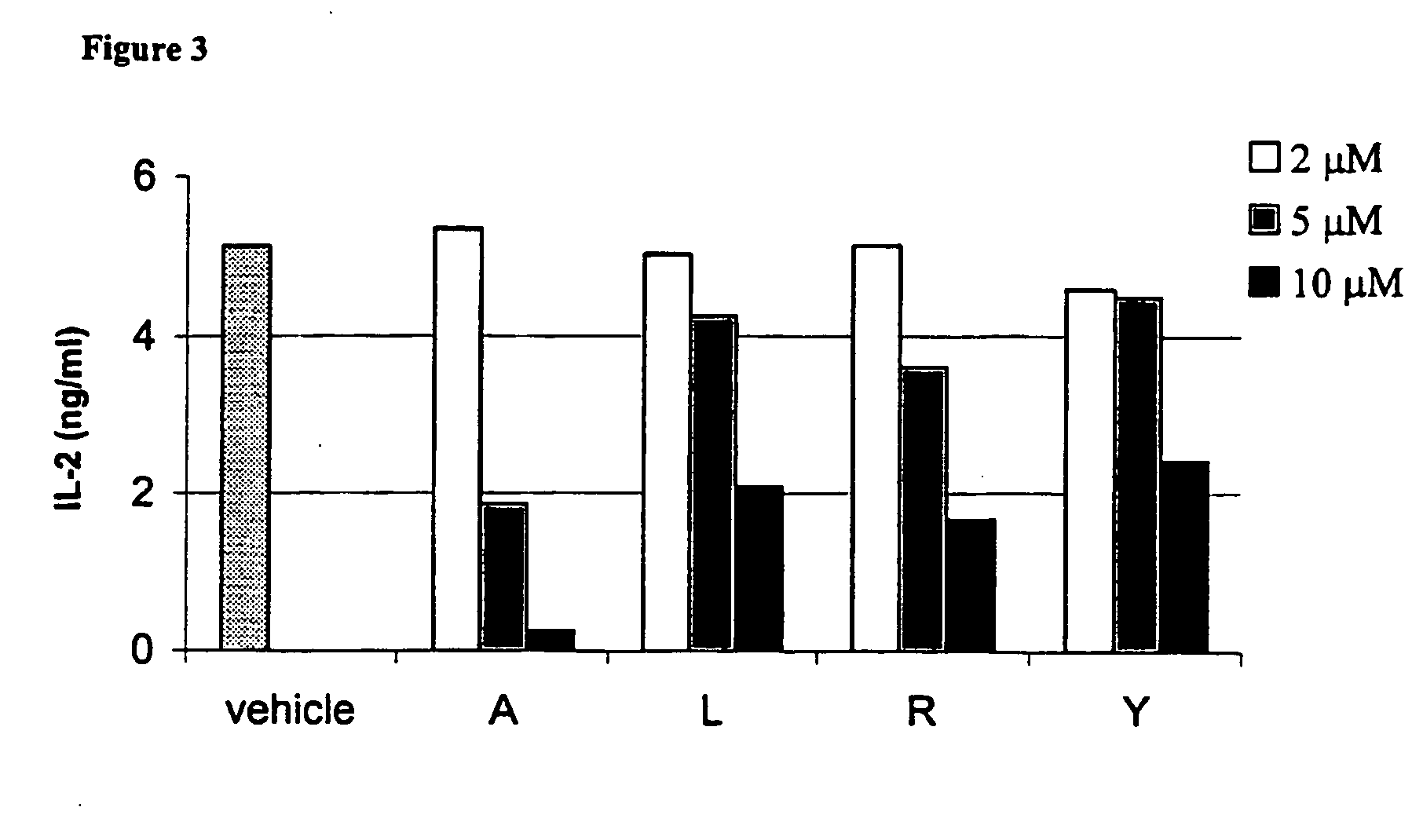
Bicyclic CB2 cannabinoid receptor ligands
The present invention relates to non-classical cannabinoids that are ligands of the peripheral cannabinoid receptor CB2, and to pharmaceutical compositions thereof comprising as an active ingredient novel (+) alpha-pinene derivatives, which are useful for prevention and treatment of autoimmune diseases including but not limited to rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, myasthenia gravis, diabetes mellitus type I, hepatitis, psoriasis, tissue rejection in organ transplants, malabsorption syndromes such as celiac disease, pulmonary diseases such as asthma and Sjögren's syndrome, inflammation including inflammatory bowel disease, pain including peripheral, visceral, neurophathic inflammatory and referred pain, muscle spasticity, cardiovascular disorders including arrhythmia, hypertension and myocardial ischemia, neurological disorders including stroke, migraine and cluster headaches, neurodegenerative diseases including Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's chorea, prion-associated neurodegeneration, CNS poisoning and certain types of cancer.
Owner:PHARMOS
Methods for the diagnosis and treatment of sjögren's syndrome
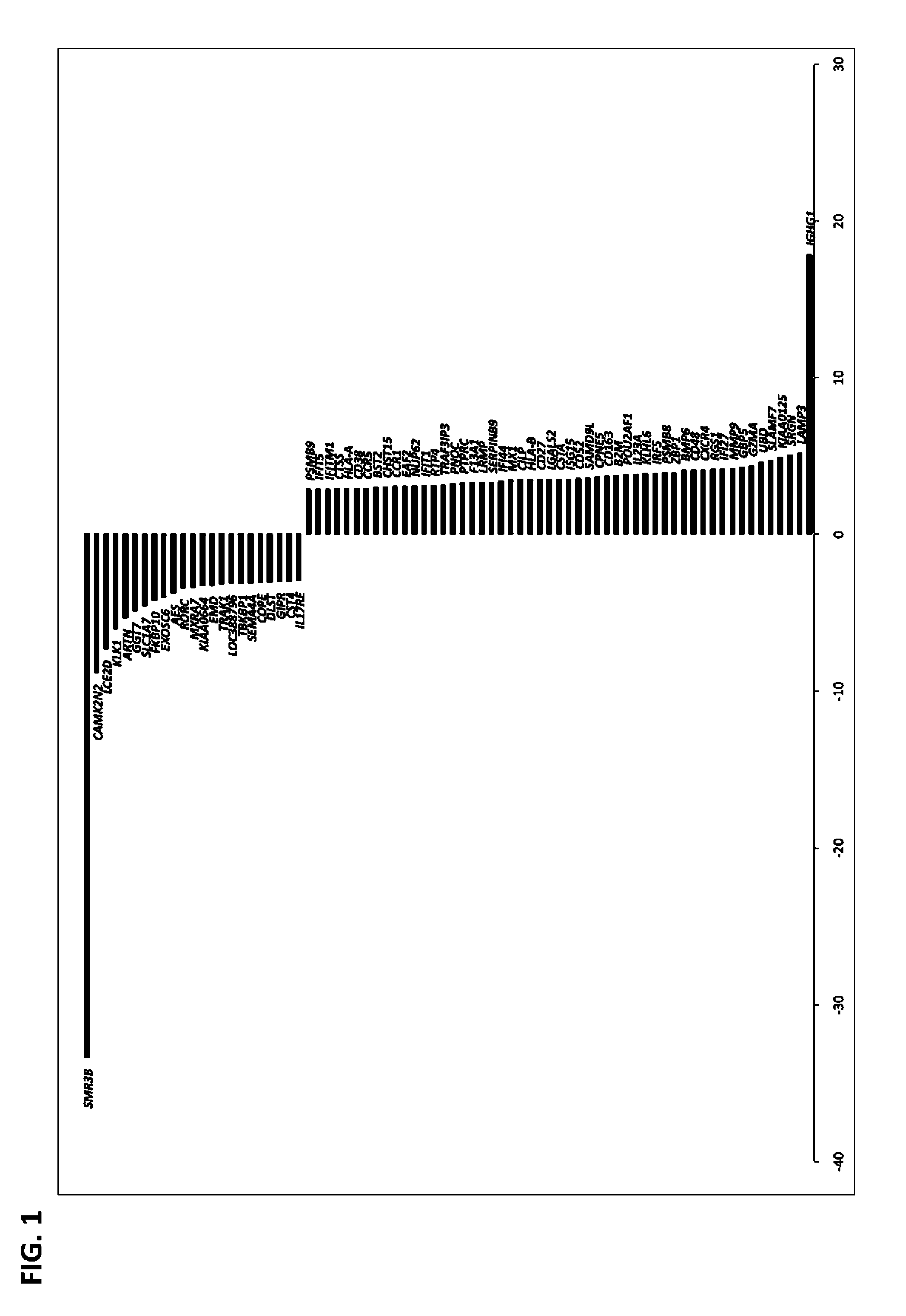
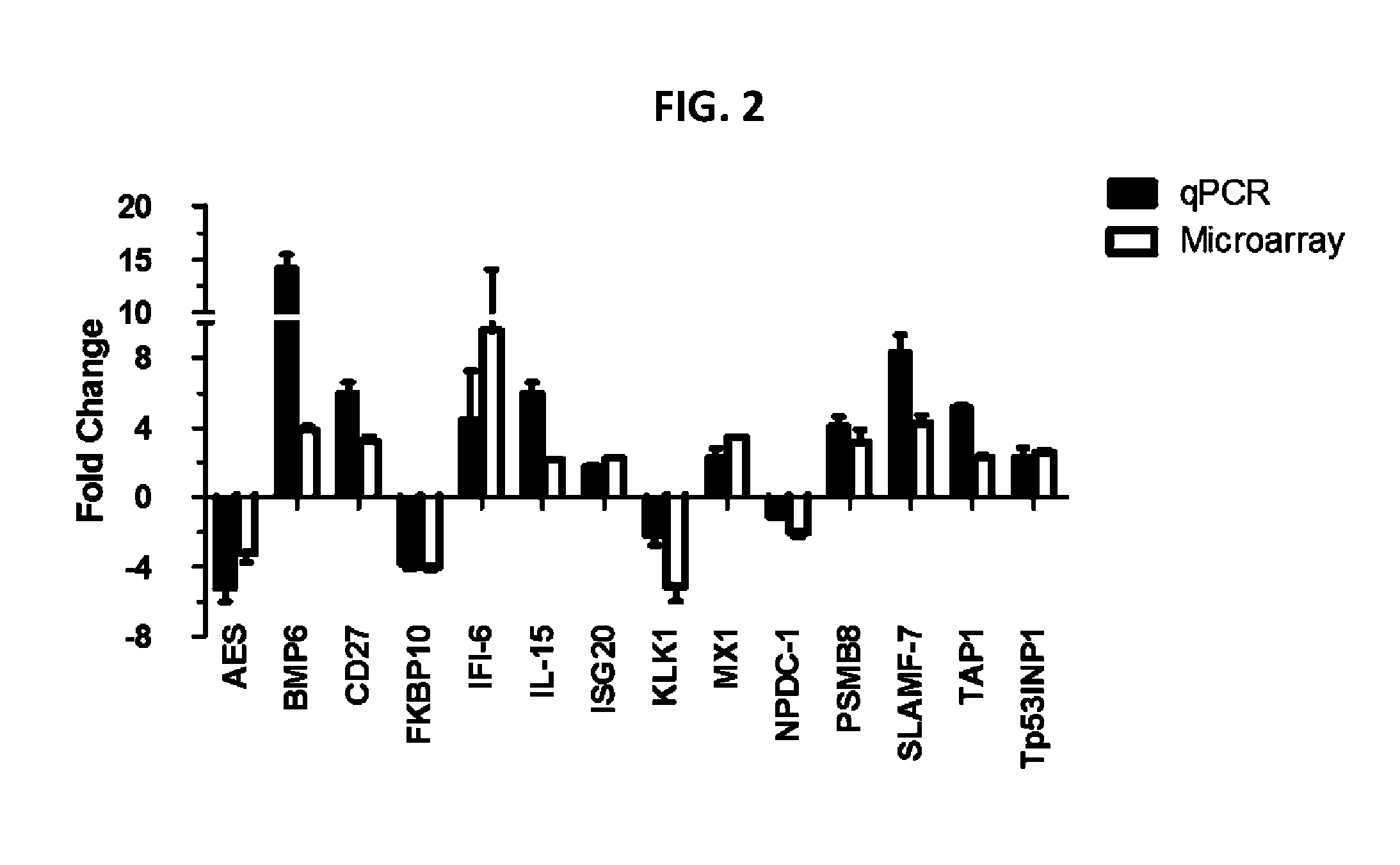
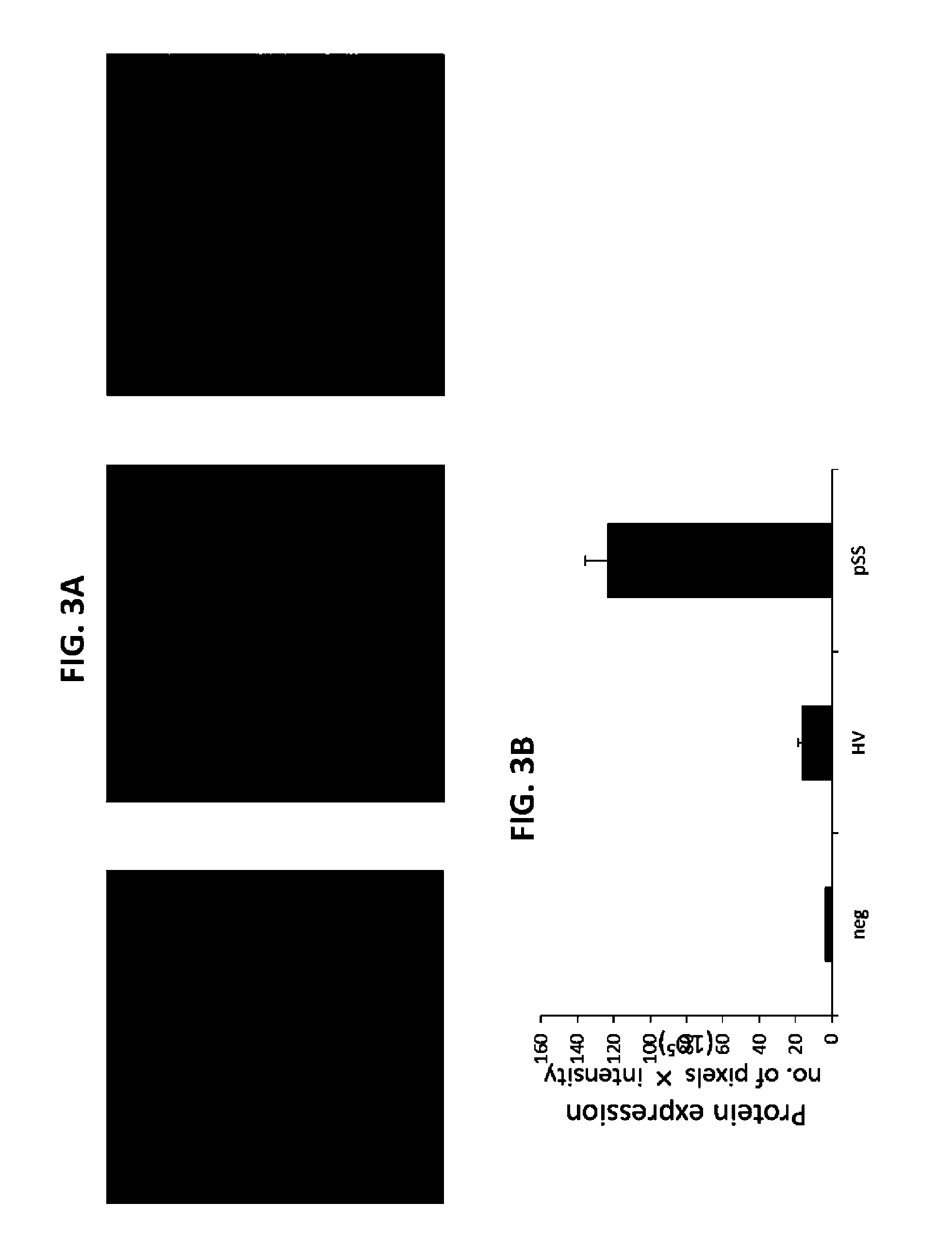
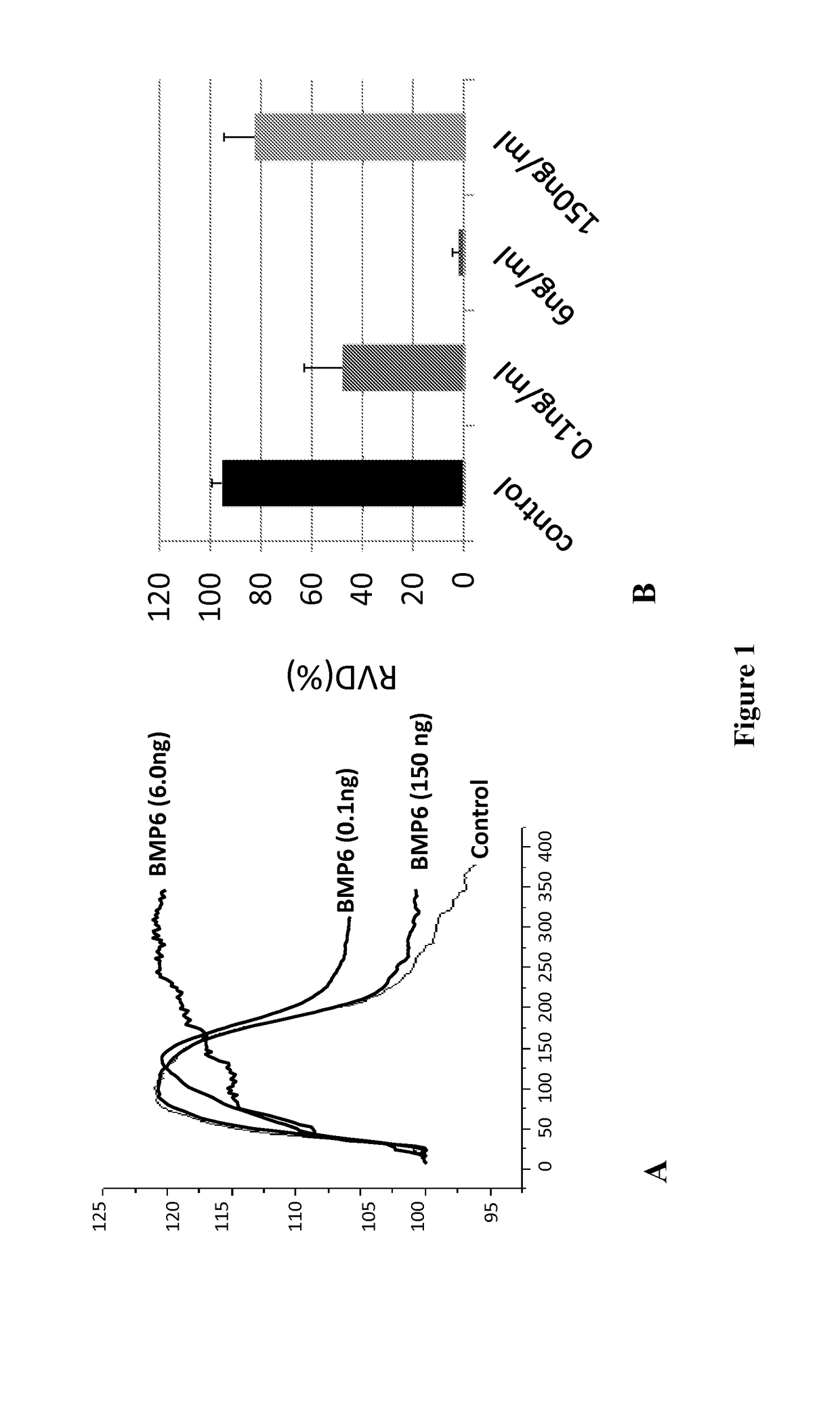
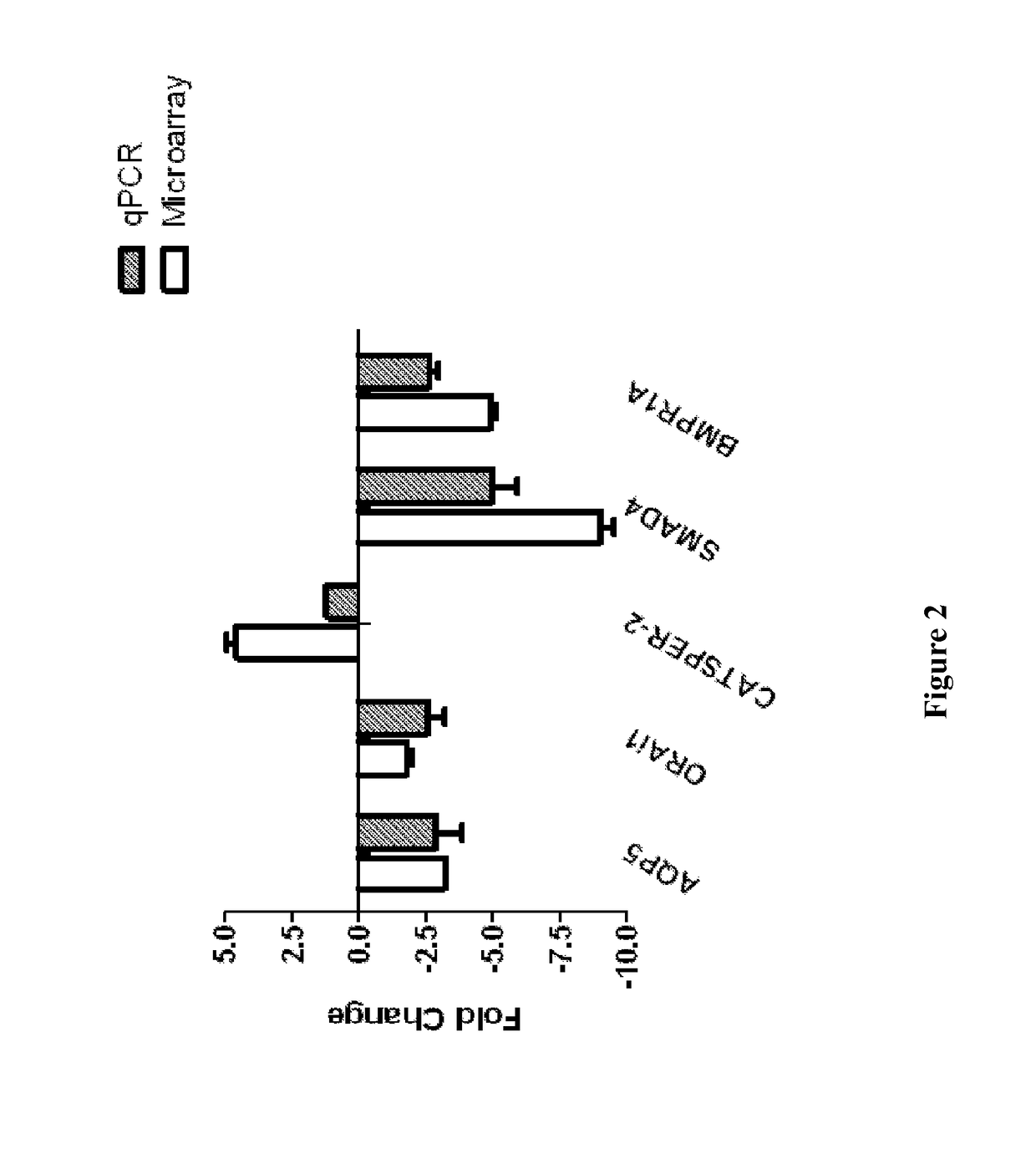
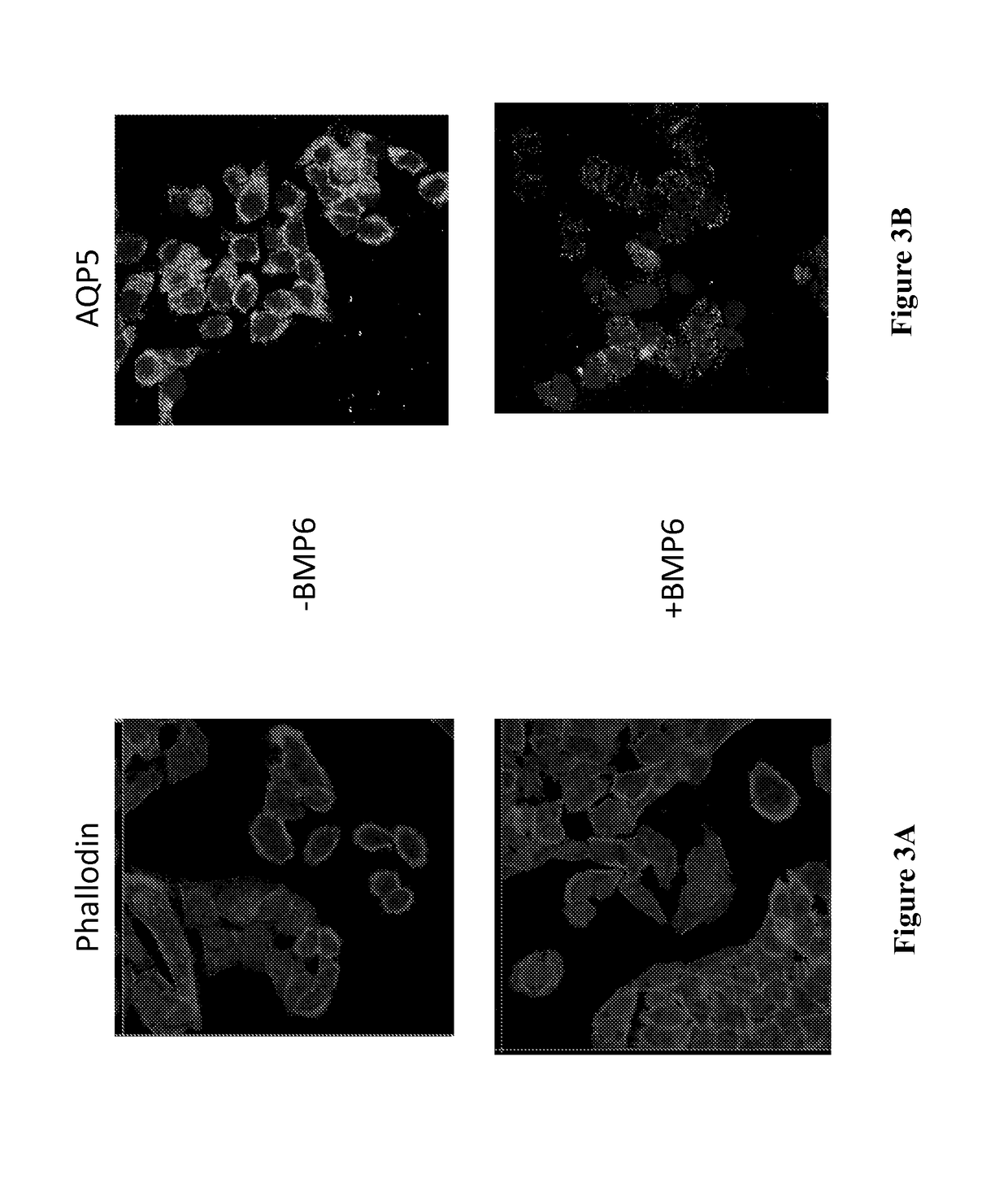
Described herein is the finding that patients with Sjögren's syndrome exhibit a statistically significant increase in expression of BMP6 in the salivary gland, relative to healthy control subjects. Also described herein is the finding that overexpression of BMP6 in the salivary glands of mice results in an increase in electrical potential across the salivary gland. Thus, provided herein are methods of diagnosing a subject as having Sjögren's syndrome, or at risk for developing Sjögren's syndrome, by measuring the level of BMP6 expression in a salivary gland of a subject, measuring electrical potential in a salivary gland of a subject, or both. Also provided herein are methods of treating a subject with Sjögren's syndrome by administering an agent that inhibits expression of BMP6 expression or activity. Also described herein is the use of XIST and MECP2 as diagnostic and therapeutic targets for male Sjögren's syndrome patients.
Owner:UNITED STATES OF AMERICA
Beta-fodrin antigen epitope polypeptide, and its screening method and use
InactiveCN1935837AAdvantages of value assessmentBiological testingAnimals/human peptidesEpitopeSerum ige
The invention relates beta-fordin antigen epitope polypeptide screening for gaining amino acid sequence corresponded with the optimum beta-fordin polypeptide. The beta-fordin antigent epitope polypeptide includes the following two that one is formed by the amino acid sequence showed in SEQ ID NO.1; another is derived from the above amino acid sequence by replacing, missing or adding one or many amino acid with antigen epitope function. It can used to specially test beta-fordin polypeptide IgG antibody for Sjogren syndrome patient. The invention also supplies the beta-fordin polypeptide IgG antibody immunology method, and its application used as antigen in Sjogren syndrome medicine preparation.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
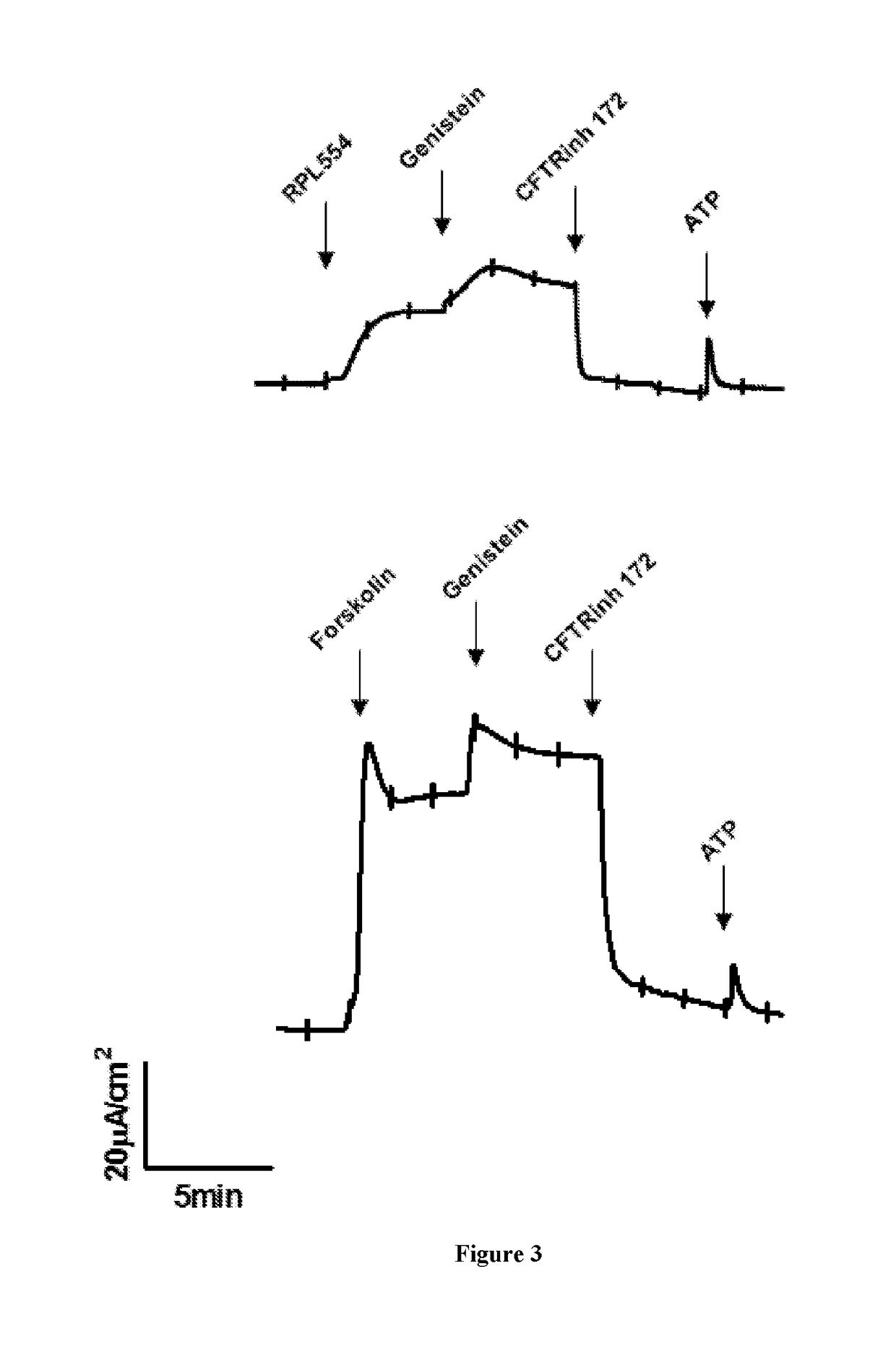
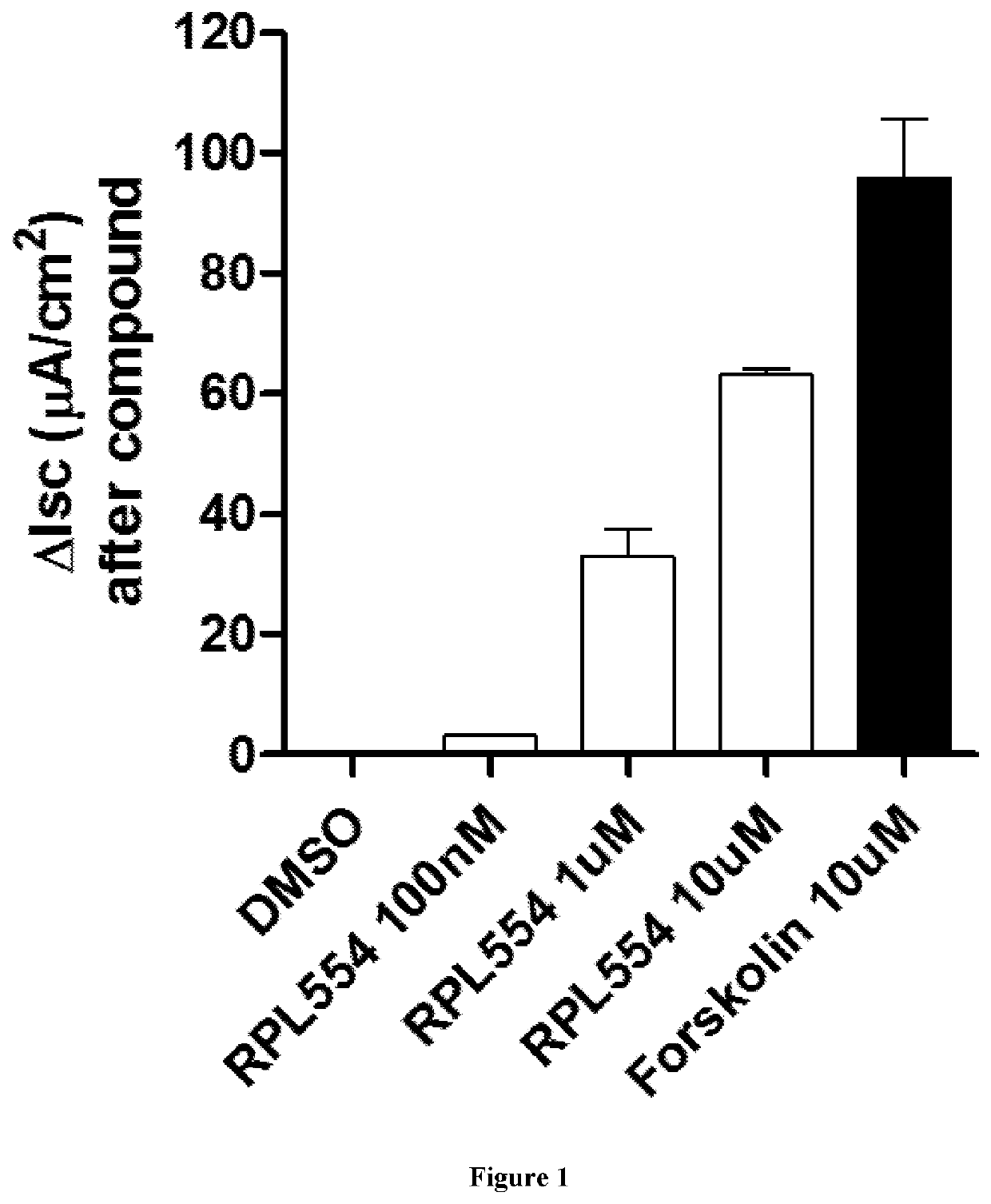
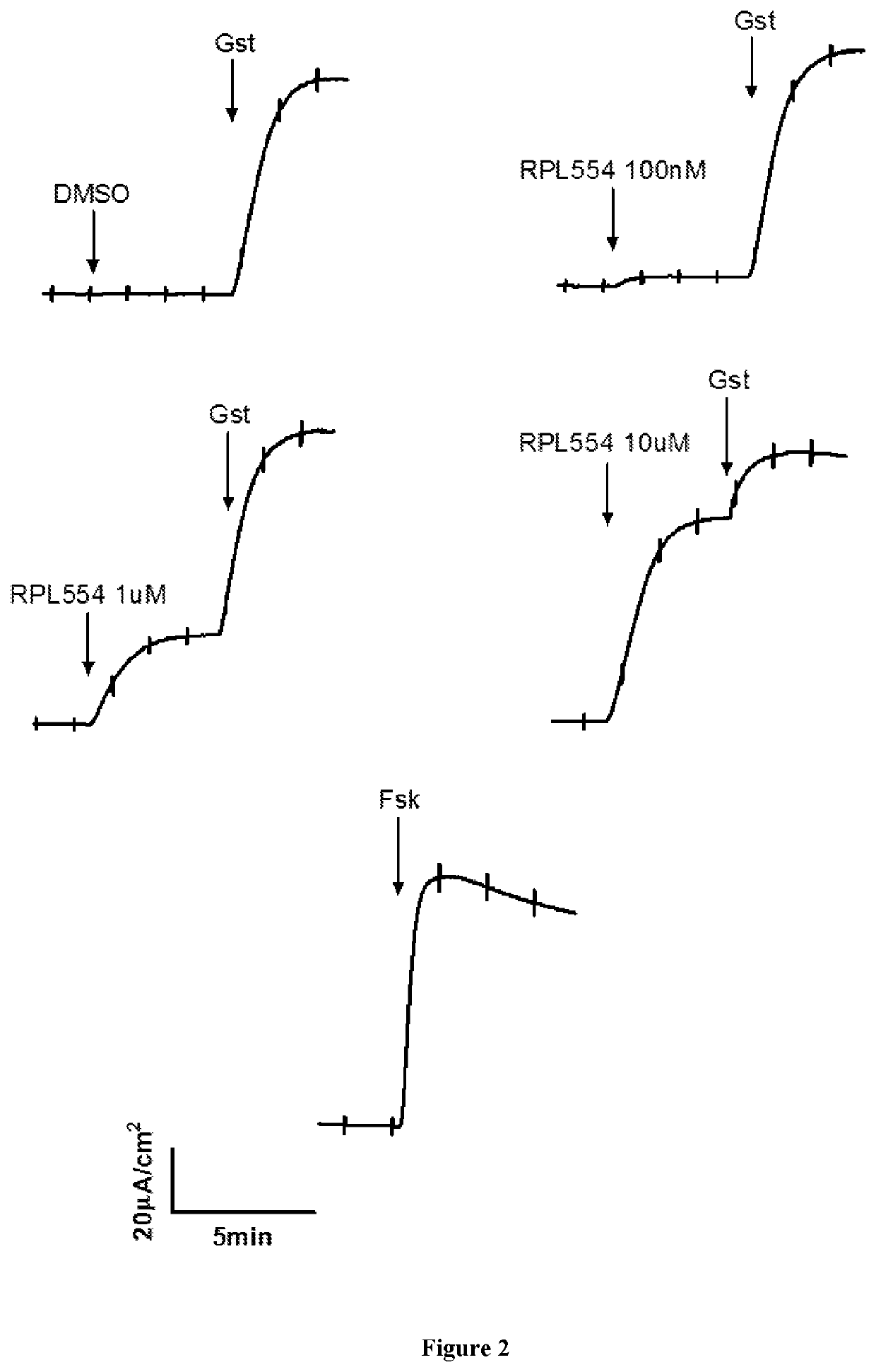
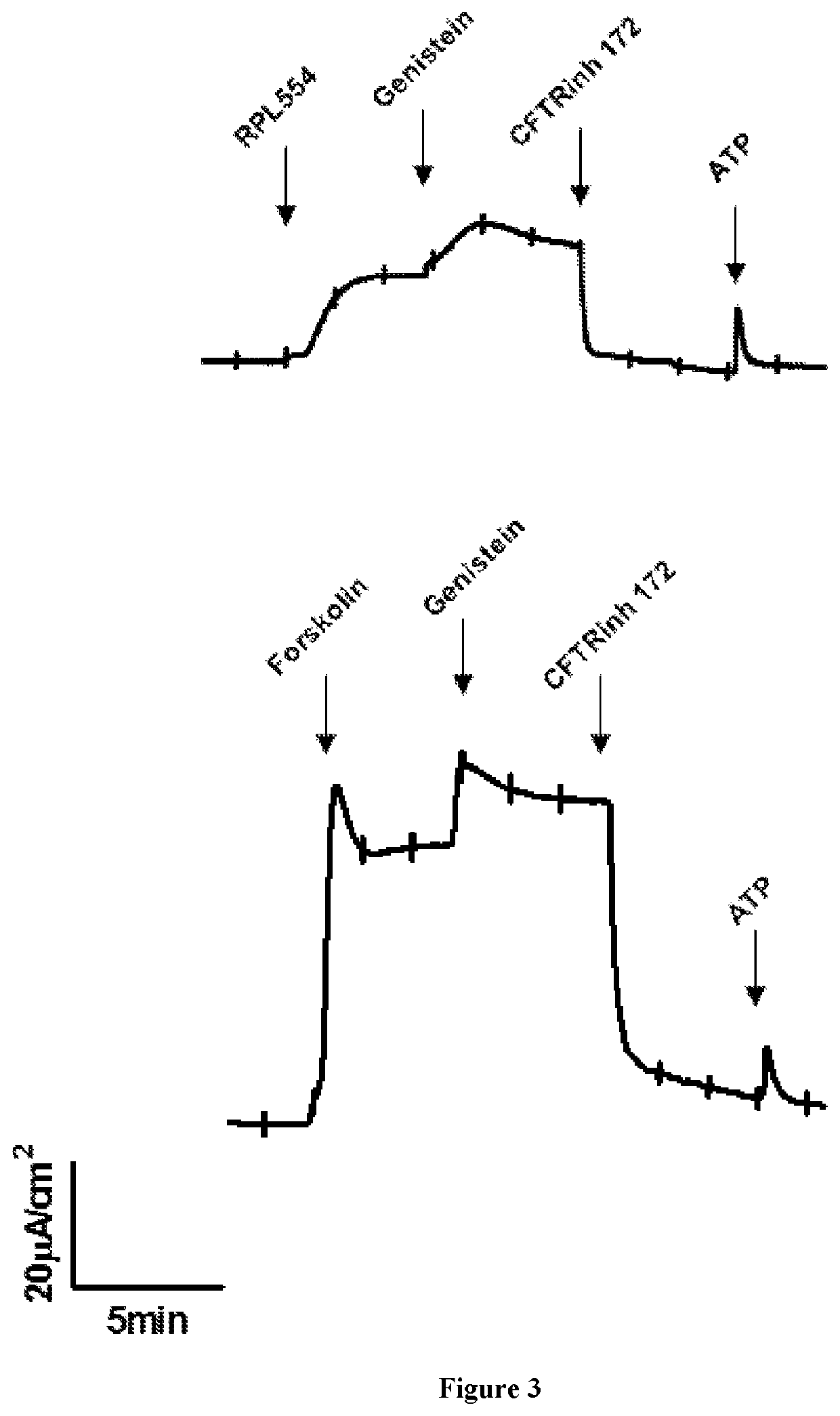
New treatment
The invention provides a compound for use in treating or preventing a disease or condition selected from cystic fibrosis, chronic obstructive pulmonary disease (COPD), asthma, mild pulmonary disease, bronchitis, bronchiectasis, idiopathic bronchiectasis, allergic bronchopulmonary aspergillosis, sinusitis, rhinosinusitis, CFTR-related metabolic syndrome (CRMS), pancreatitis, idiopathic chronic pancreatitis and Sjörgren's syndrome, or for use in preventing male infertility caused by congenital absence of the vas deferens, in a patient by modulating CFTR activity, which compound is 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a leukotriene receptor antagonist. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a CFTR potentiator or a CFTR corrector.
Owner:VERONA PHARMA
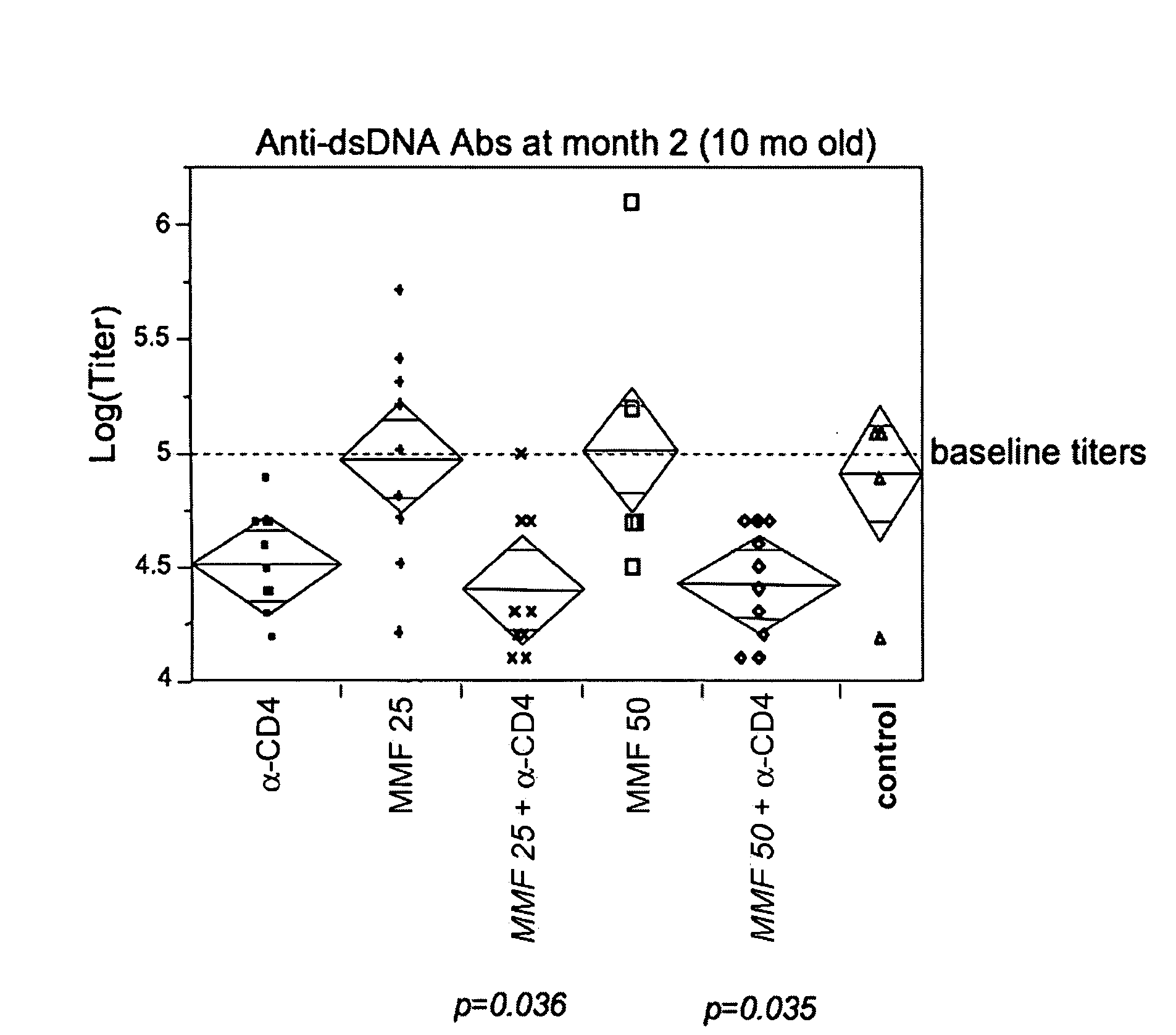
Methods of treating lupus using CD4 antibodies
InactiveUS20080279848A1Phosphorous compound active ingredientsAntibody ingredientsUlcerative colitisSystemic lupus erythematosus
Methods of treating lupus, including systemic lupus erythematosus, cutaneous lupus erythmetosus, and lupus nephritis, are provided. The methods involve administration of a combination of a non-depleting CD4 antibody and another compound used clinically or experimentally to treat lupus. Methods of treating lupus nephritis by administration of a non-depleting CD4 antibody that results in an improvement in renal function and / or a reduction in proteinuria or active urinary sediment are also provided. Methods of treating lupus or decreasing autoantibody titer by administration of a non-depleting CD4 antibody are also provided. Methods of treating multiple sclerosis by administration of a non-depleting CD4 antibody, optionally in combination with another compound used clinically or experimentally to treat MS, are described. Methods of treating transplant recipients and subjects with rheumatoid arthritis, asthma, psoriasis, Crohn's disease, ulcerative colitis, and Sjogren's syndrome are also provided.
Owner:GENENTECH INC
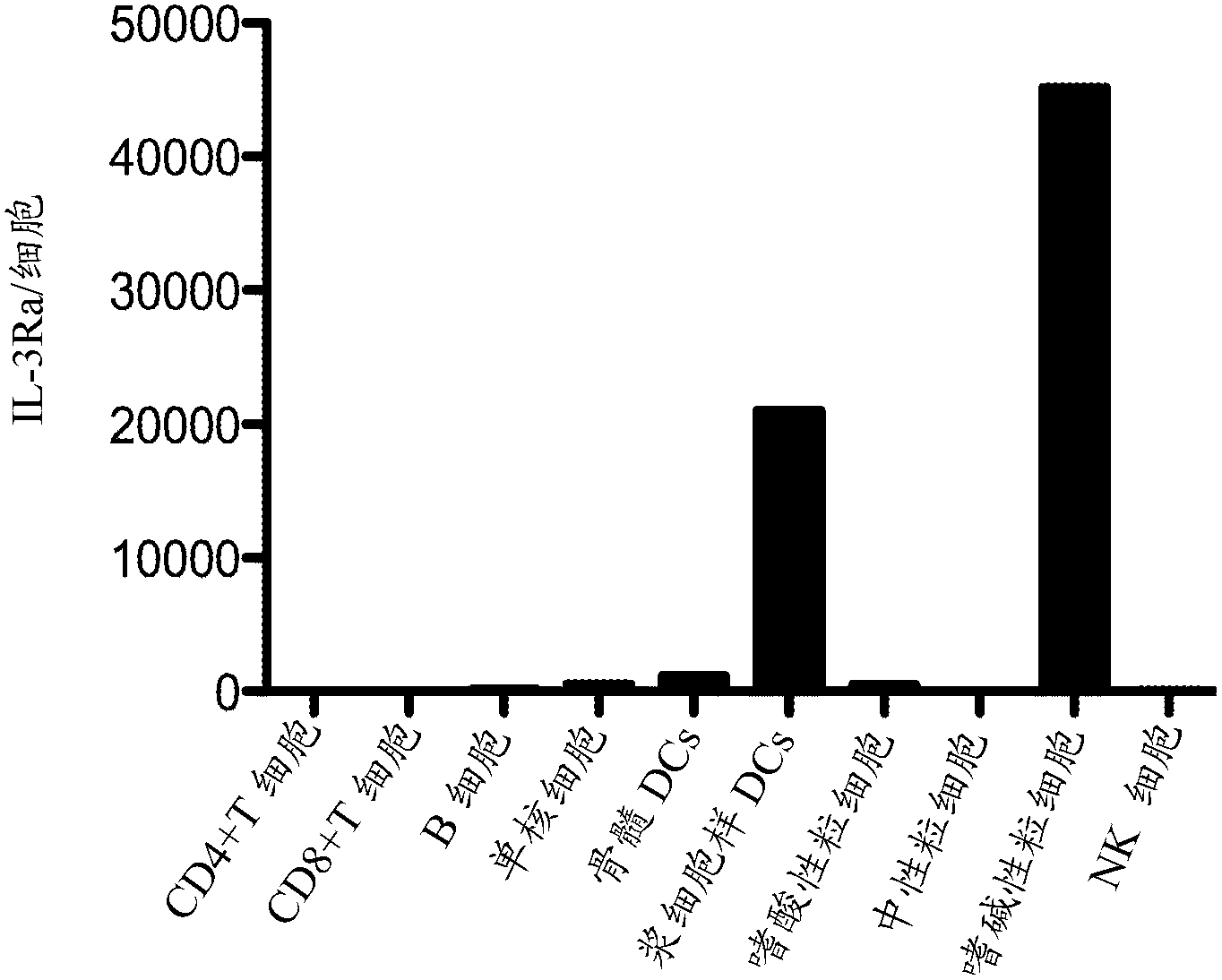
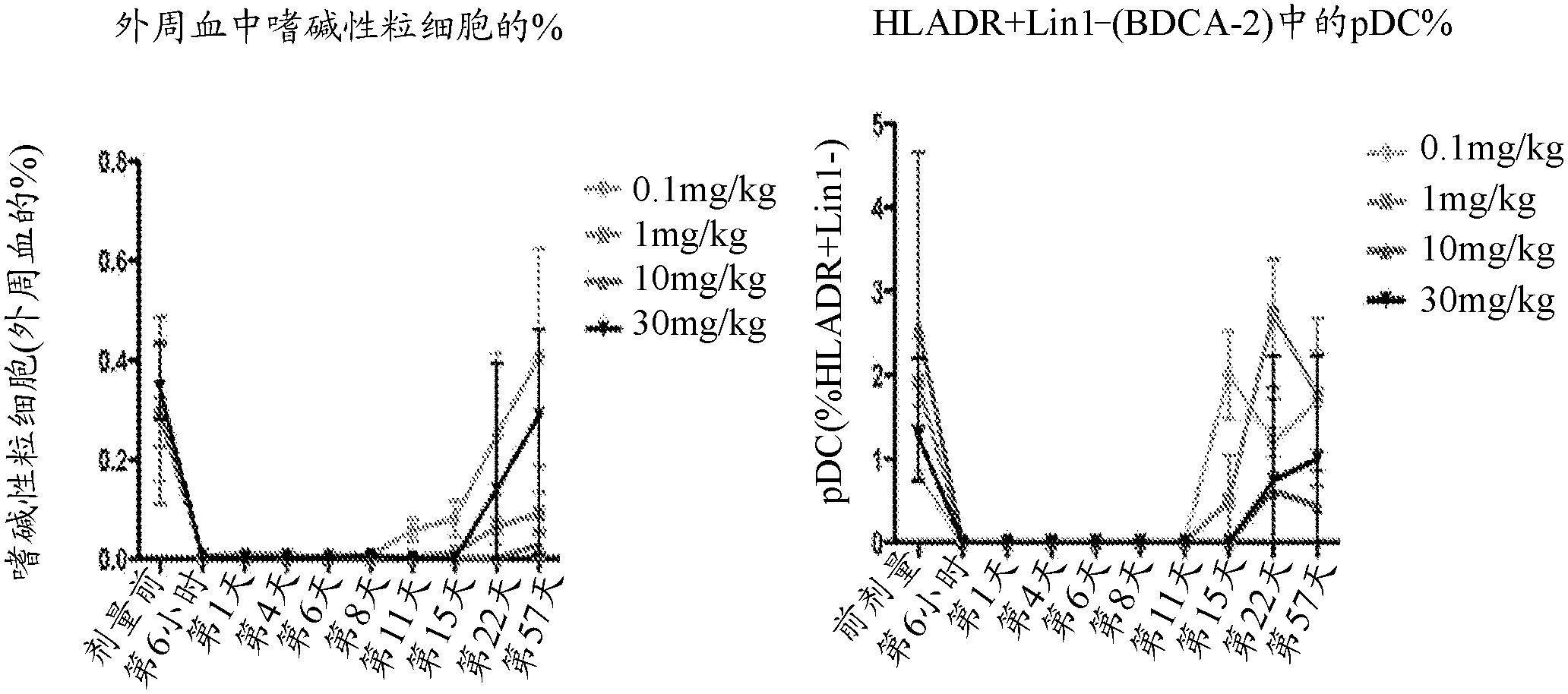
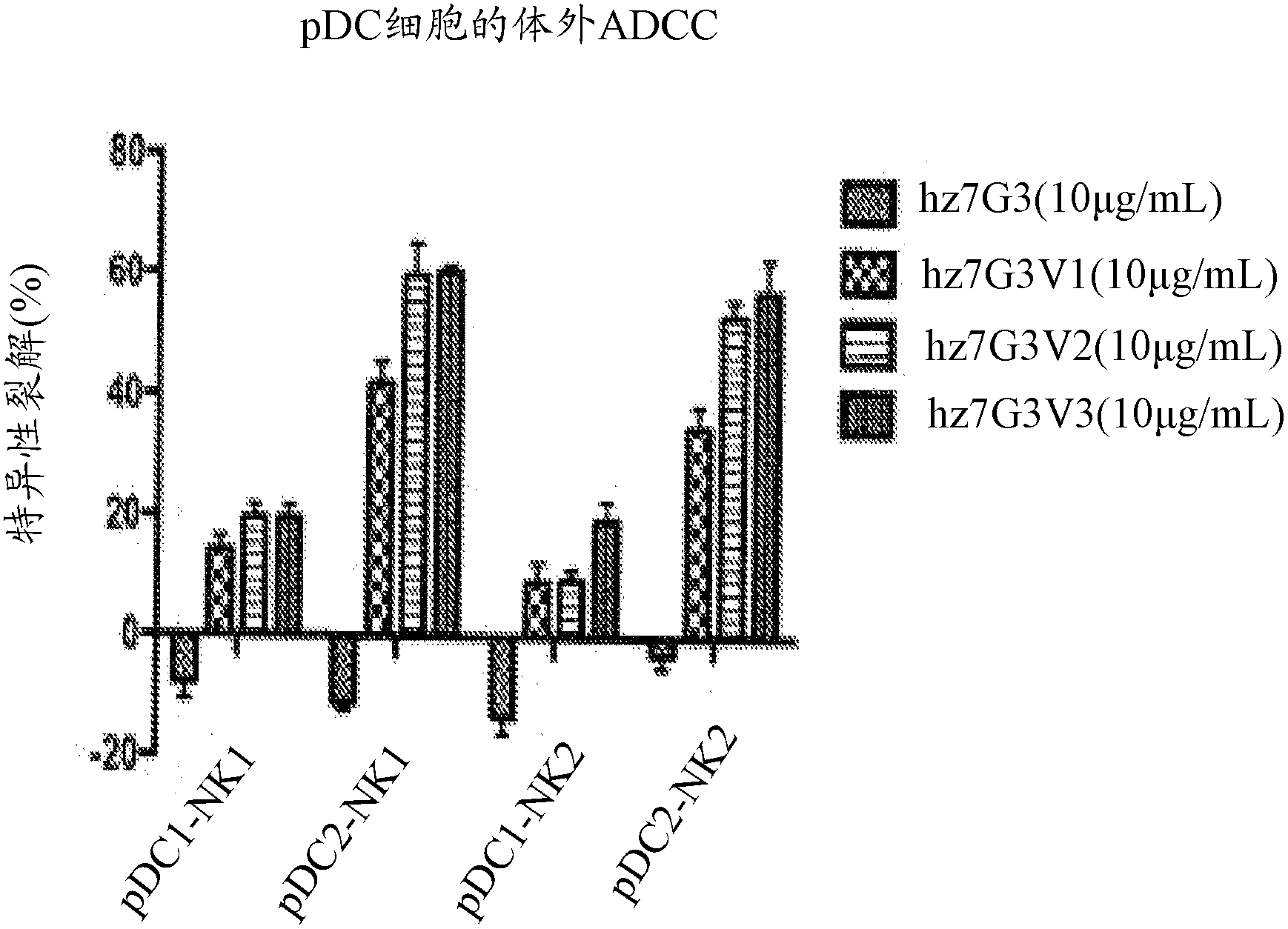
Compositions and methods for targeting type I interferon producing cells
The present disclosure provides a method for treating lupus, Sjogren's syndrome or scleroderma, the method comprising administering to the mammal an immunoglobulin which binds an interleukin 3 receptor alpha (IL-3R alpha) chain and which depletes or at least partly eliminates plasmacytoid dendritic cells (p DCs) and basophils to which it binds.
Owner:CSL LTD
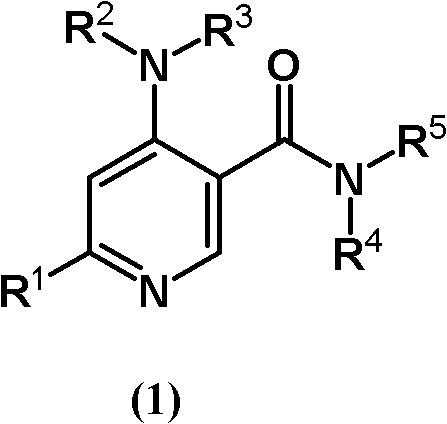
Pyridine-3-carboxyamide derivative
ActiveCN102227409AEnhanced inhibitory effectExcellent JAK3 inhibitionSenses disorderNervous disorderUveitisEczematous rash
Provided is a novel JAK3 inhibitor useful as a preventive and / or therapeutic agent for organ transplant rejection, graft versus host disease (GvHD), rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, Sjogren's syndrome, Behcet's disease, type I diabetes mellitus, autoimmune thyroiditis, idiopathic thrombocytopenic purpura, ulcerative colitis, Crohn's disease, asthma, allergic conjunctivitis, atopic dermatitis, contact dermatitis, urticaria, eczema, psoriasis, allergic conjunctivitis, uveitis, cancer, leukemia, etc. A pyridine-3-carboxyamide derivative represented by general formula (1) or a salt thereof or a solvate thereof are also provided.
Owner:KOWA CO LTD
Pyrrolopyrimidines as cftr potentiators
ActiveUS20190225621A1Ease of preparation and detectabilityGood metabolic stabilityOrganic active ingredientsSenses disorderDiseaseSinusitis
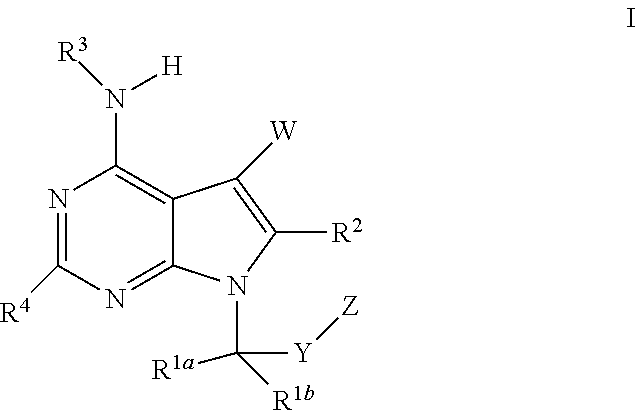
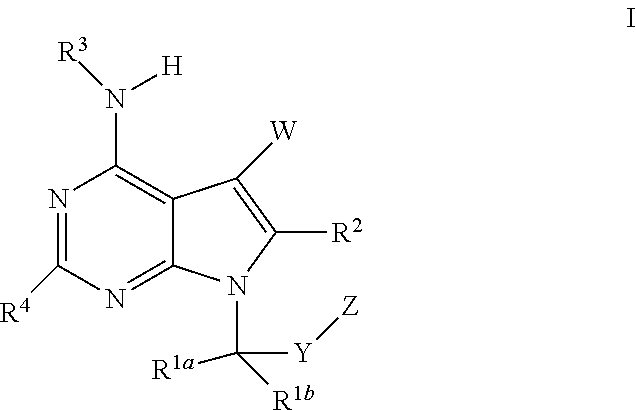
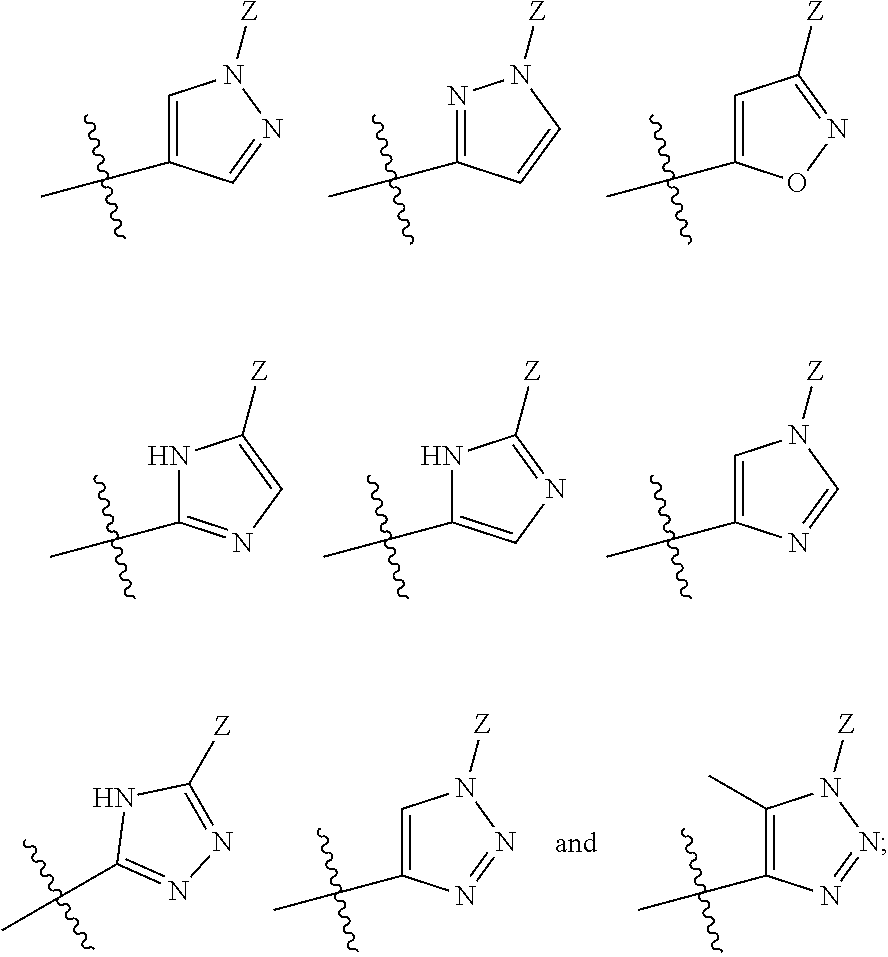
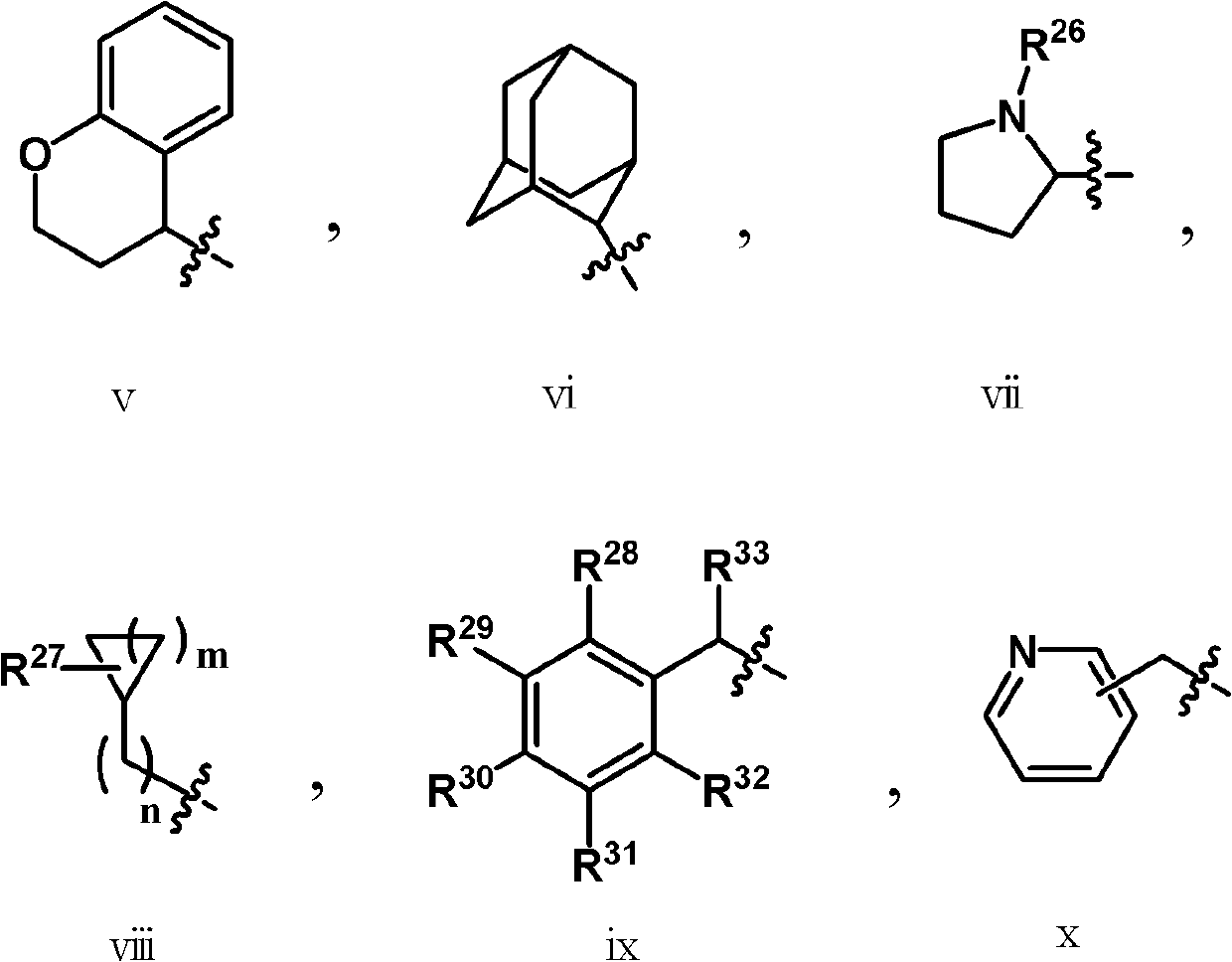
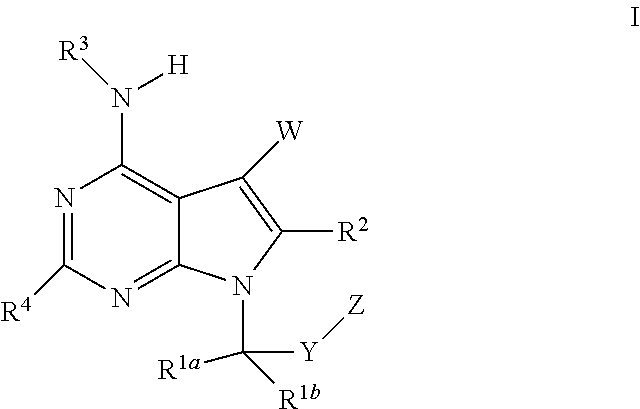
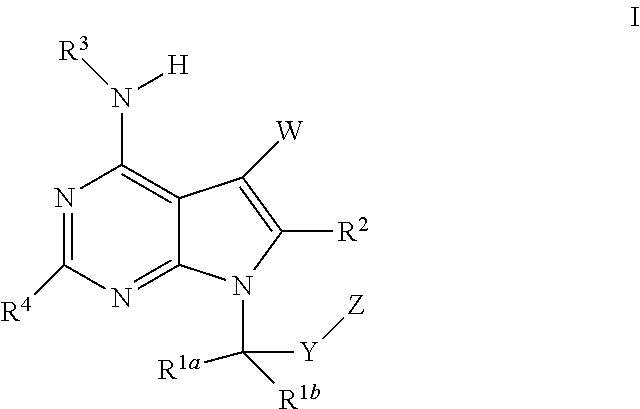
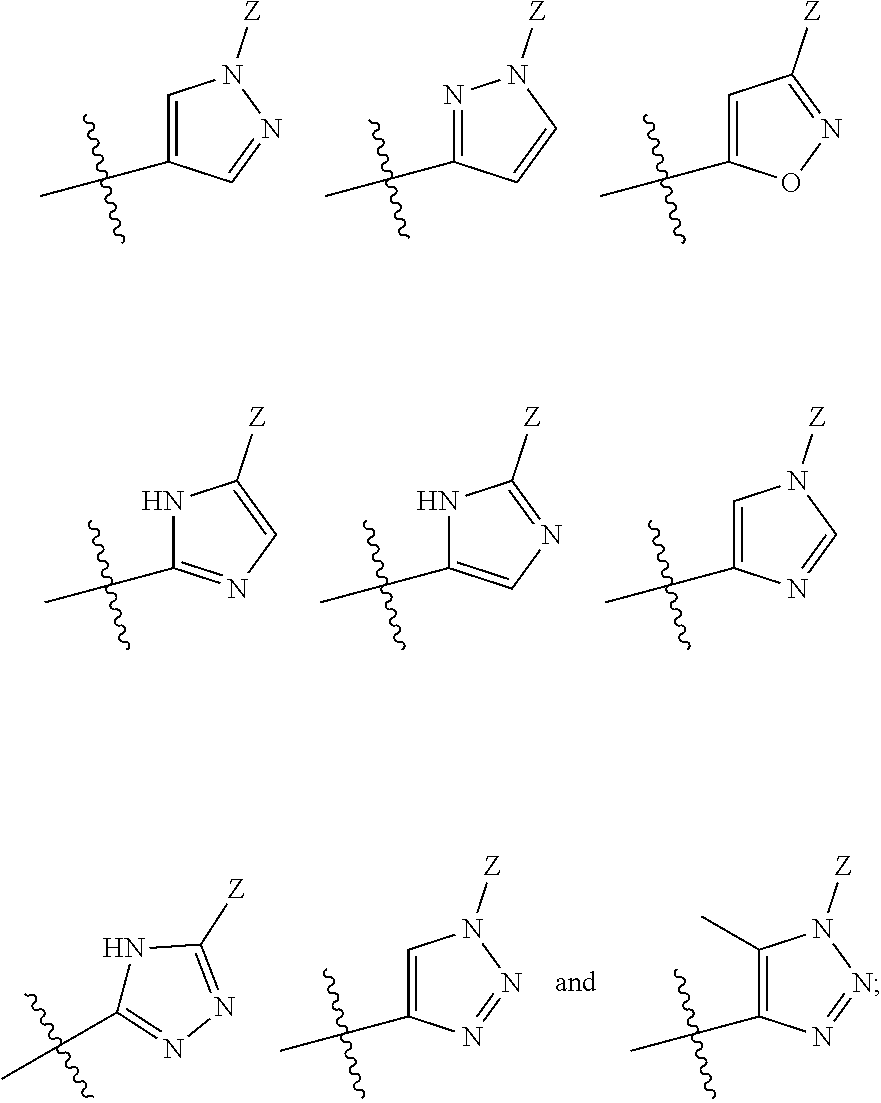
The present invention relates to methods of using compounds of Formula I,wherein R1a, R1b, R2, R3, R4, W, Y, and Z are as described herein, and pharmaceutically acceptable salts thereof. The compounds are potentiators of Cystic Fibrosis Transmembrane conductance Regulator (CFTR). The invention also discloses pharmaceutical compositions comprising the compound, optionally in combination with additional therapeutic agents, and methods of potentiating, in mammals, including humans, CFTR by administration of the compounds. These compounds are useful for the treatment of cystic fibrosis (CF), asthma, bronchiectasis, chronic obstructive pulmonary disease (COPD), constipation, Diabetes mellitus, dry eye disease, pancreatitis, rhinosinusitis, Sjögren's Syndrome, and other CFTR associated disorders.
Owner:CYSTIC FIBROSIS FOUND
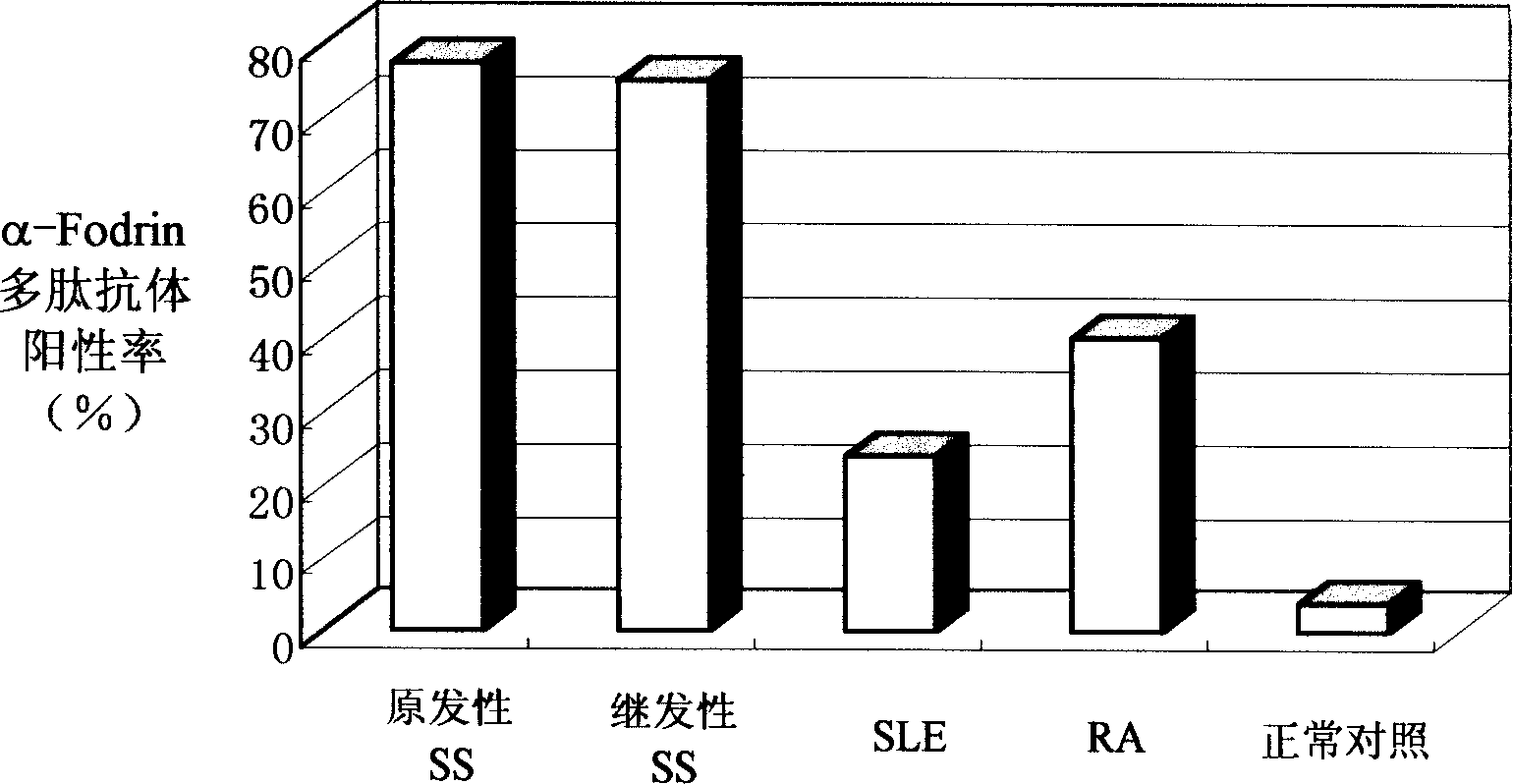
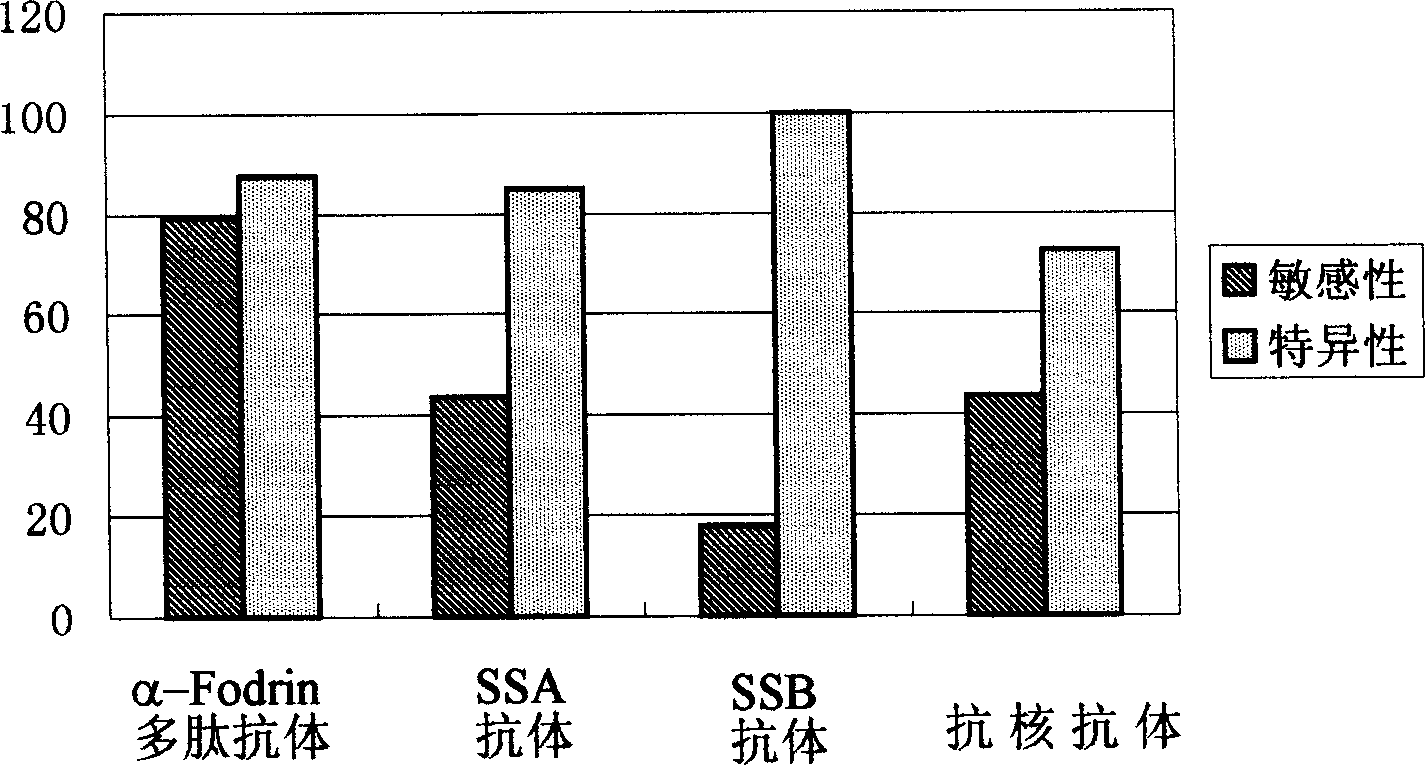
Application of alpha-fodrin polypeptide resisting antibody determination in the diagnosis of xerosis syndromes
The present invention provides one kind of alpha-fodrin polypeptide and its analog used for the detection of xerosis syndromes. This kind of polypeptide antigen may be combined specifically with the specific antibody in the body of xerosis syndromes patient and has very high sensitivity and specificity.
Owner:PEOPLES HOSPITAL PEKING UNIV
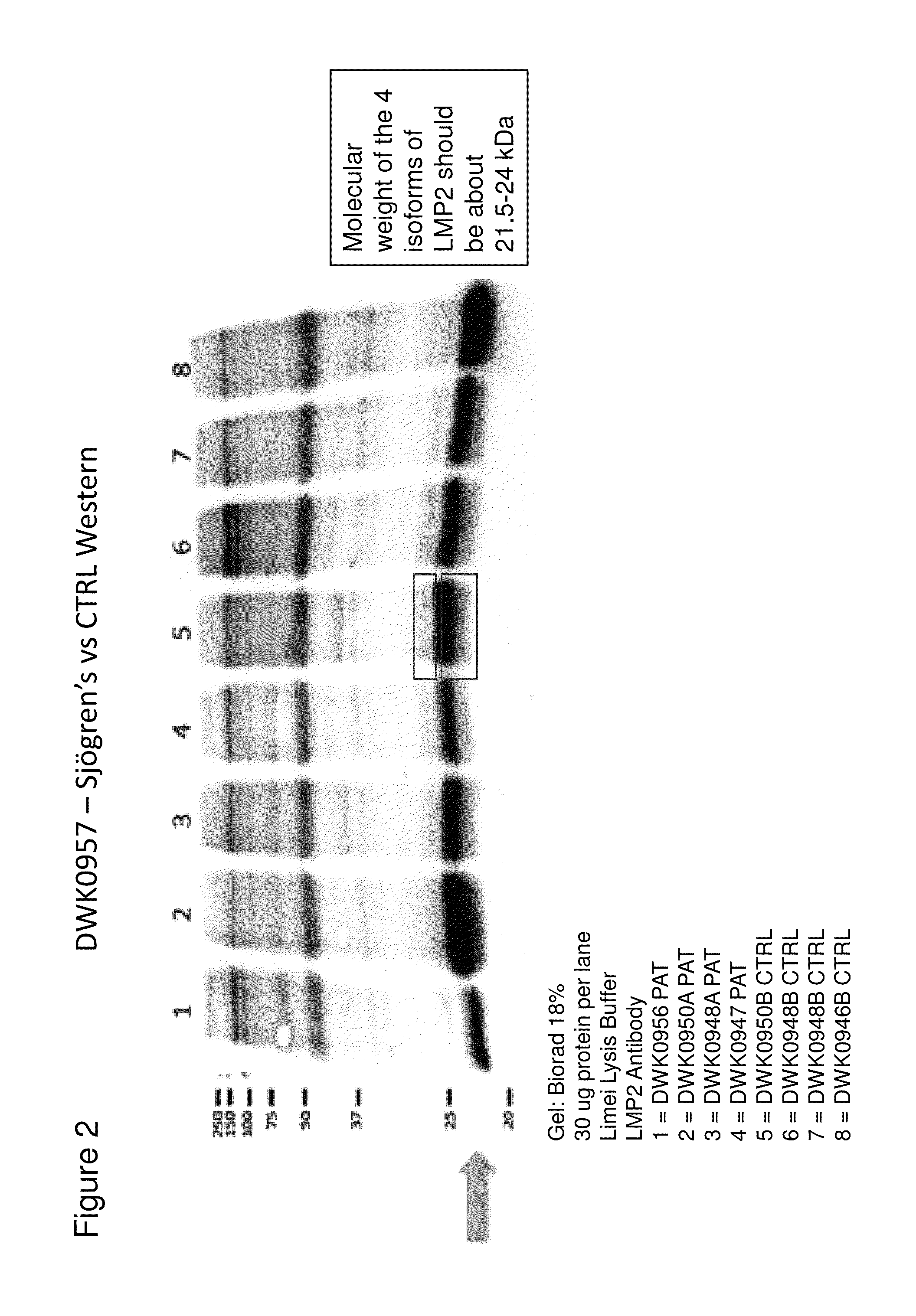
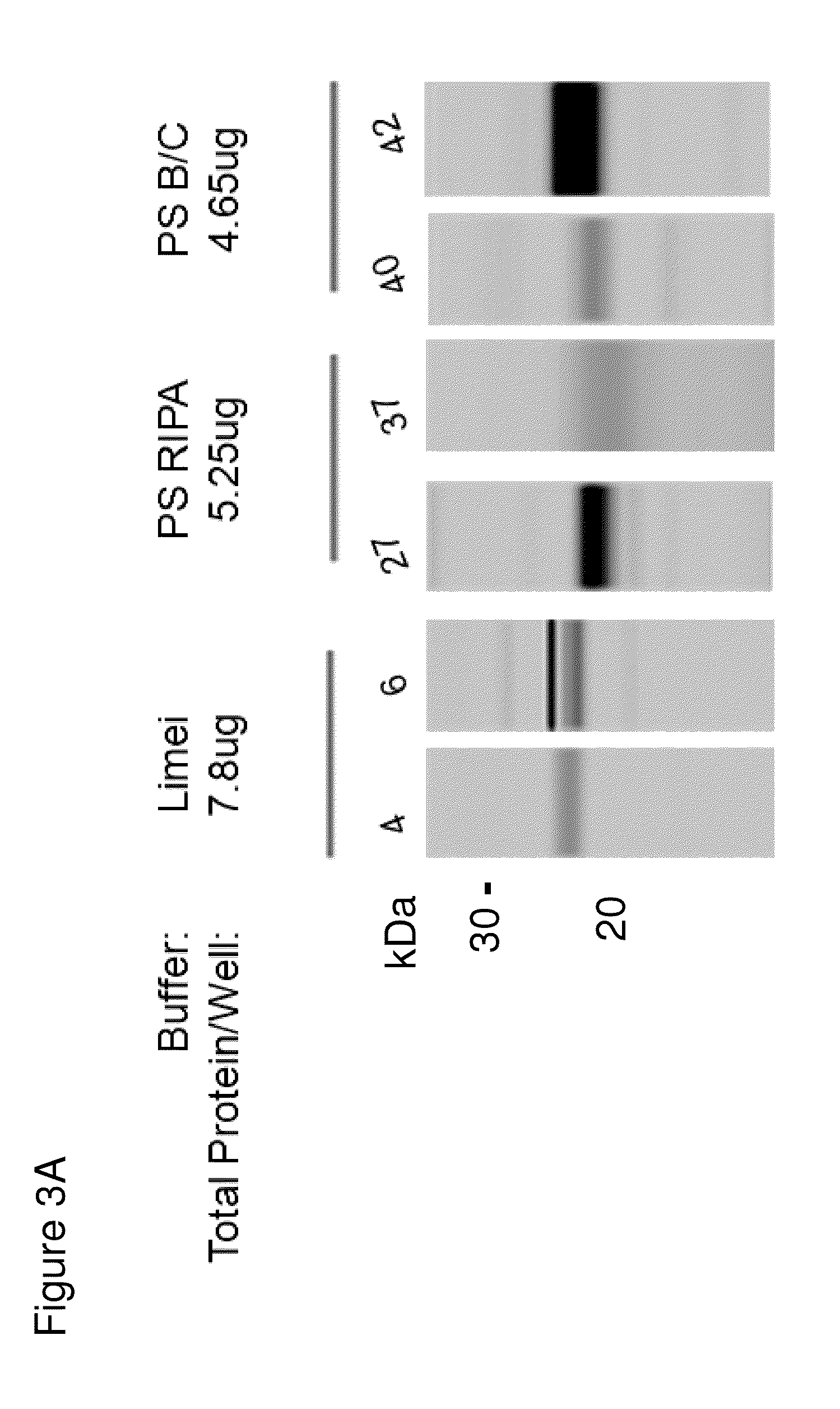
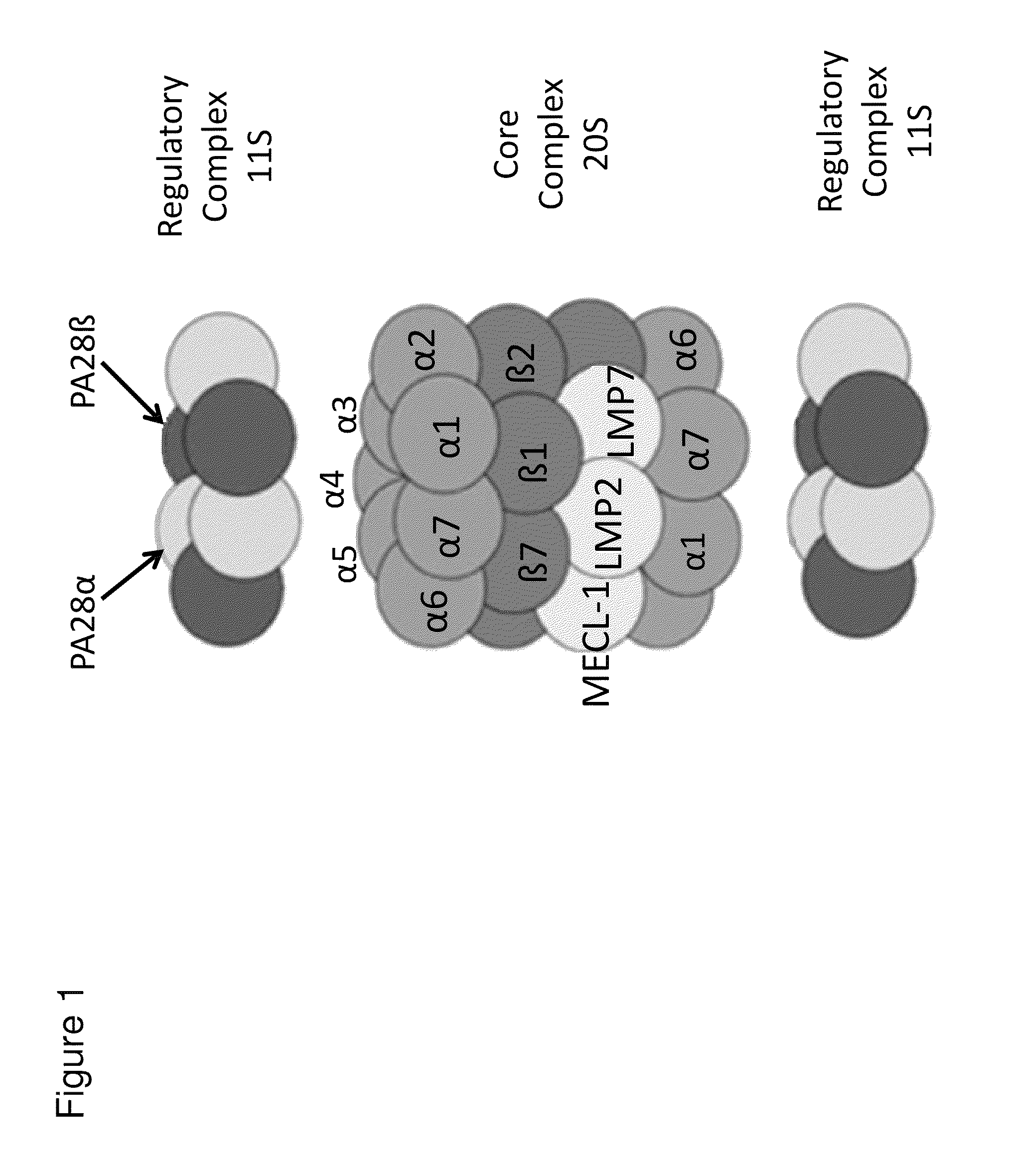
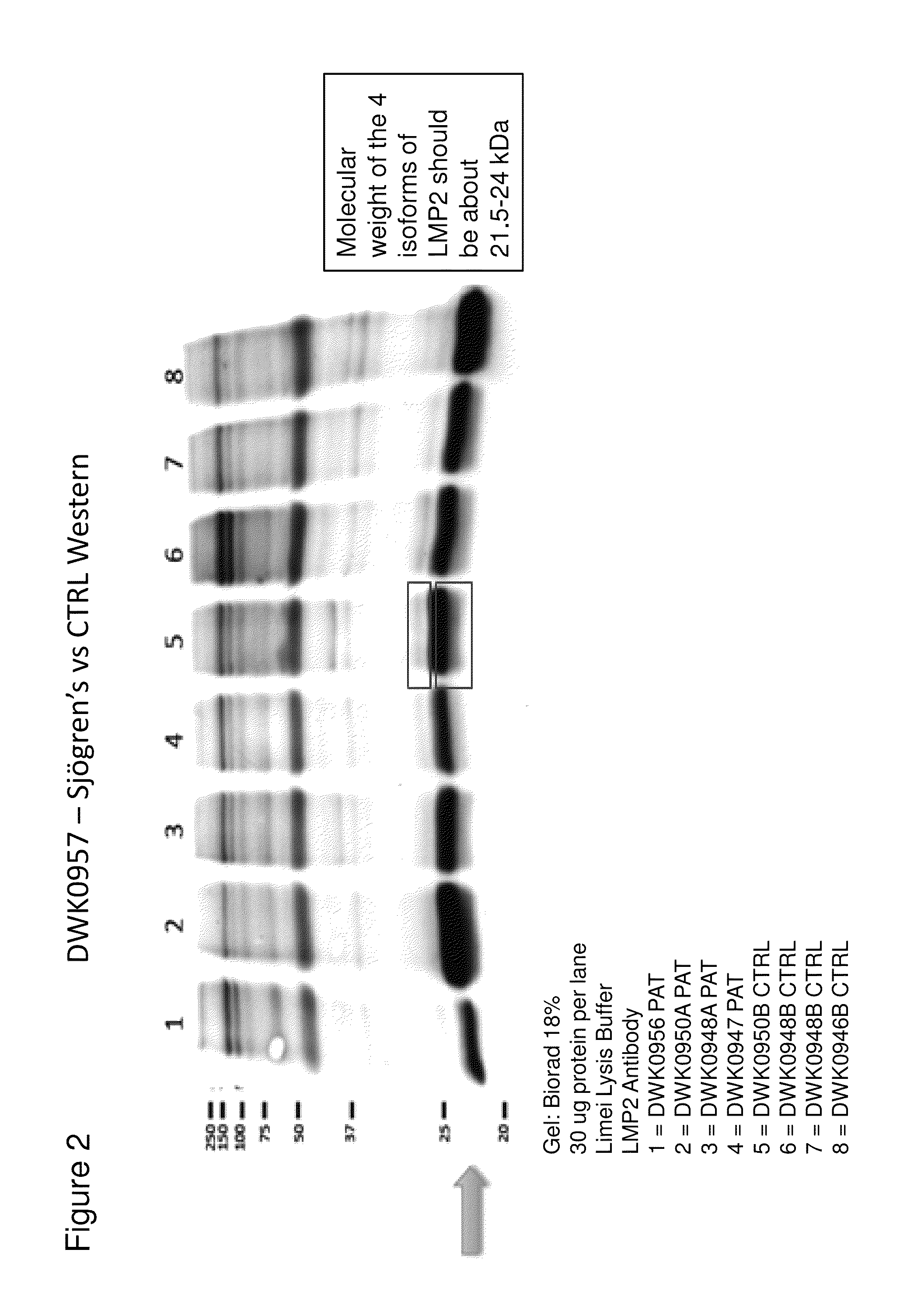
Methods and kits for diagnosing sjogren's syndrome
InactiveUS20140134644A1Reduce the amount requiredDisease diagnosisDetection of post translational modificationsPhosphorylationSjoegren's syndrome
The invention features methods and kits for determining the presence of, or a predisposition to develop, Sjögren's syndrome in humans. The invention features methods to detect changes in the levels of one or more LMP-2 protein isoforms, in particular phosphorylated isoforms of LMP-2, and to detect changes in cellular protein phosphorylation and ubiquitination in samples from Sjögren's patients.
Owner:THE GENERAL HOSPITAL CORP
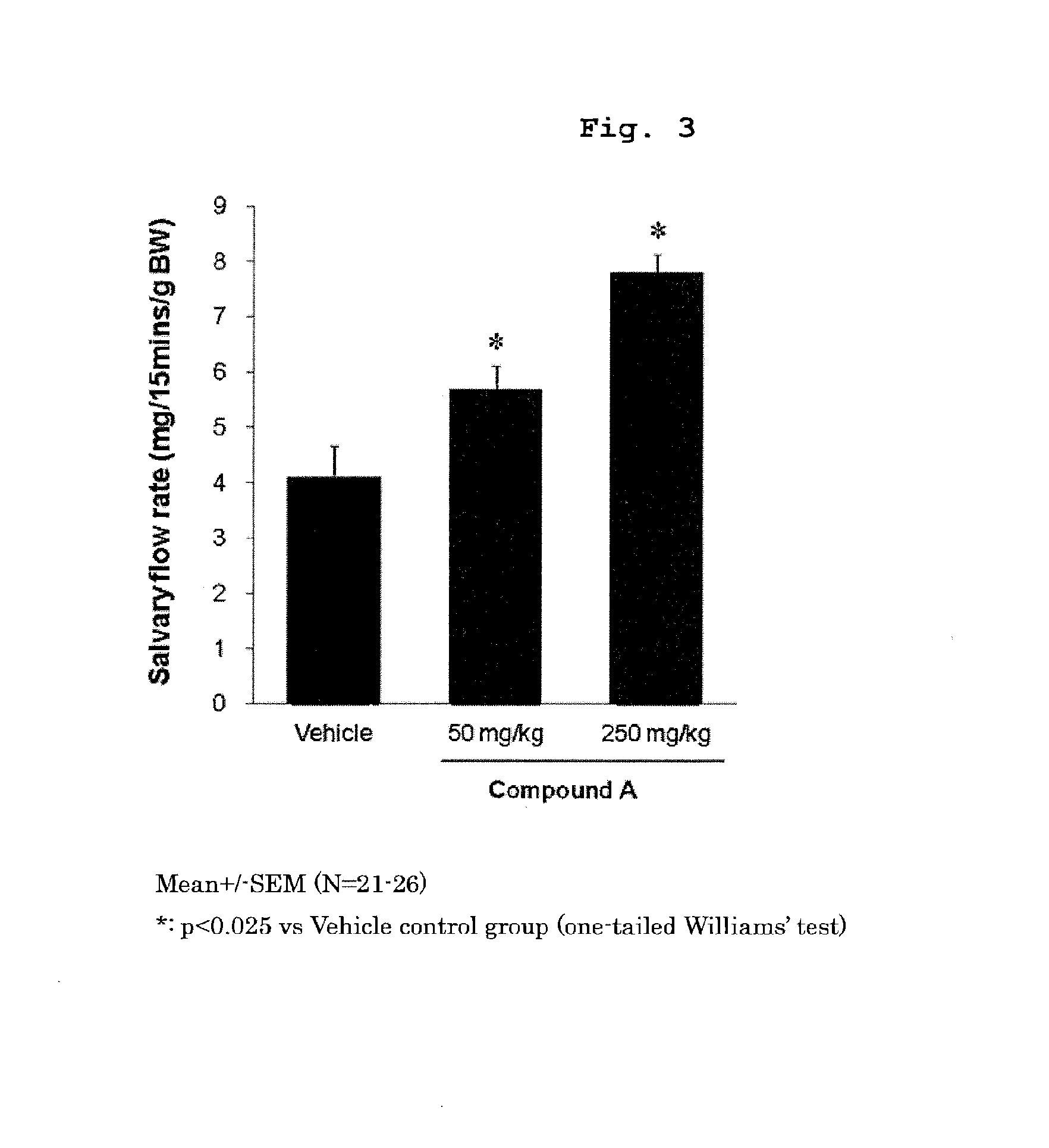
Prophylactic or therapeutic method for sjogren's syndrome
The present invention provides a prophylactic or therapeutic agent and a prophylactic or therapeutic method superior in the prophylaxis or treatment of Sjogren's syndrome. Provided are a prophylactic or therapeutic agent for Sjogren's syndrome, containing a compound represented by the formula (I):wherein each symbol is as defined in the Specification, or a pharmaceutically acceptable salt thereof, and a method for the prophylaxis or treatment of Sjogren's syndrome, including administering an effective amount of the compound represented by the formula (I) or a pharmaceutically acceptable salt thereof, to a subject in need thereof.
Owner:MILLENNIUM PHARMA INC
AAV mediated aquaporin gene transfer to treat Sjogren's syndrome
ActiveUS10166299B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsGene transferAquaporin 1
The invention relates to a gene transfer-based method to protect a subject from Sjogren's syndrome. The method comprises administering to the subject an AAV virion comprising an AAV vector that encodes aquaporin-1 (AQP-1) protein. Also provided are AQP-1 proteins and nucleic acid molecules that encode such proteins. Also provided are AAV vectors and AAV virions that encode an AQP-1 protein.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Treatment
The invention provides a compound for use in treating or preventing a disease or condition selected from cystic fibrosis, chronic obstructive pulmonary disease (COPD), asthma, mild pulmonary disease, bronchitis, bronchiectasis, idiopathic bronchiectasis, allergic bronchopulmonary aspergillosis, sinusitis, rhinosinusitis, CFTR-related metabolic syndrome (CRMS), pancreatitis, idiopathic chronic pancreatitis and Sjörgren's syndrome, or for use in preventing male infertility caused by congenital absence of the vas deferens, in a patient by modulating CFTR activity, which compound is 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a leukotriene receptor antagonist. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a CFTR potentiator or a CFTR corrector.
Owner:VERONA PHARMA
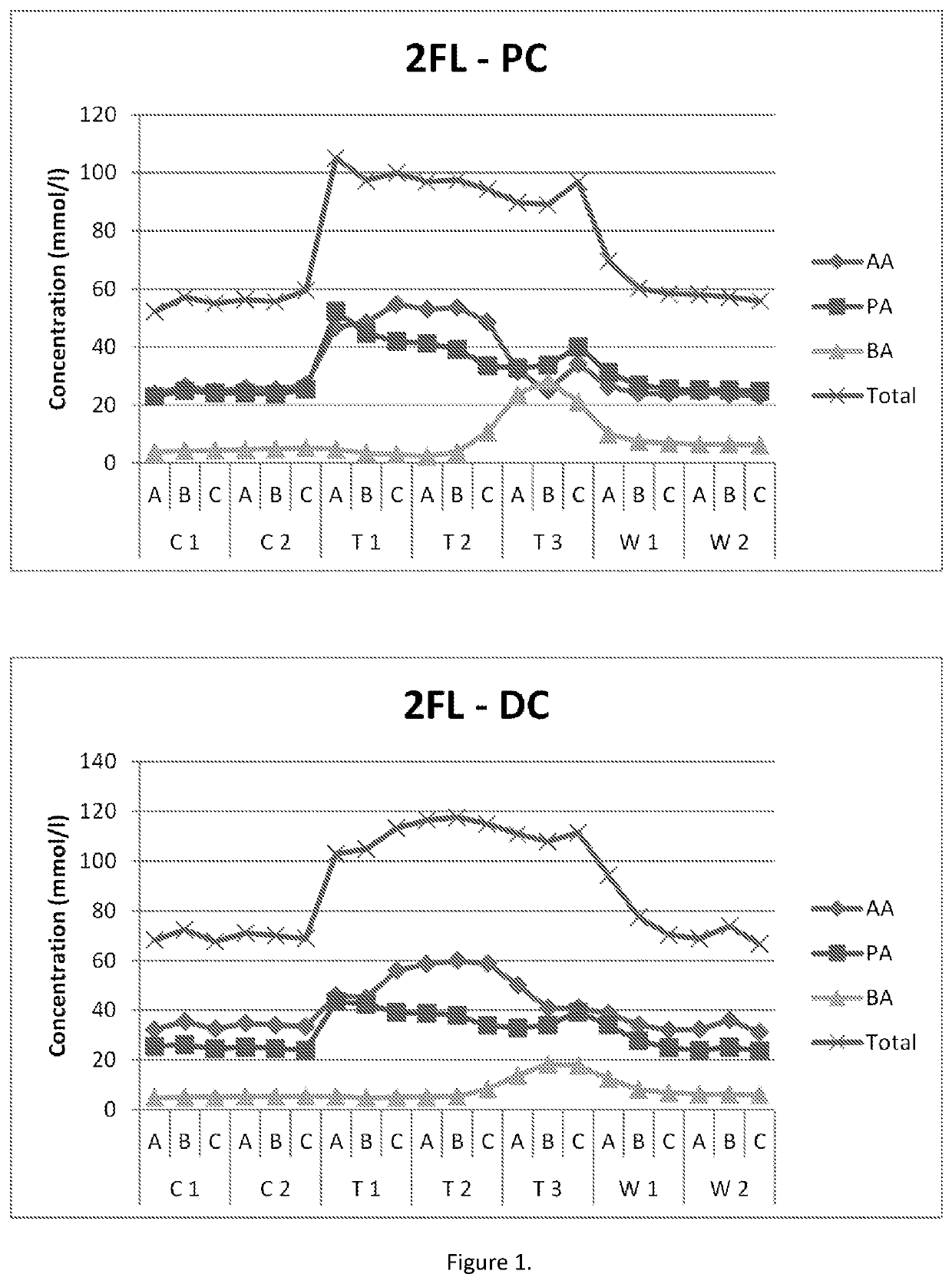
Mixture of hmos for treating autoimmune diseases
ActiveUS20210213038A1Impaired intestinal barrier functionOrganic active ingredientsVitamin food ingredientsImmunologic disordersSecondary Preventions
The invention relates to a method, compounds and compositions for the secondary prevention, treatment or dietary management of symptomatic and asymptomatic non-intestinal autoimmune diseases in a non-infant human including Sjogren's syndrome and type 1 diabetes. Said method, compounds and compositions for the secondary prevention, treatment or dietary management include human milk oligosaccharide (HMO), preferably mixtures of human milk oligosaccharides selected from the group of 2′-FL, LNnT, LNT, DFL, and 6′-SL.
Owner:GLYCOM AS
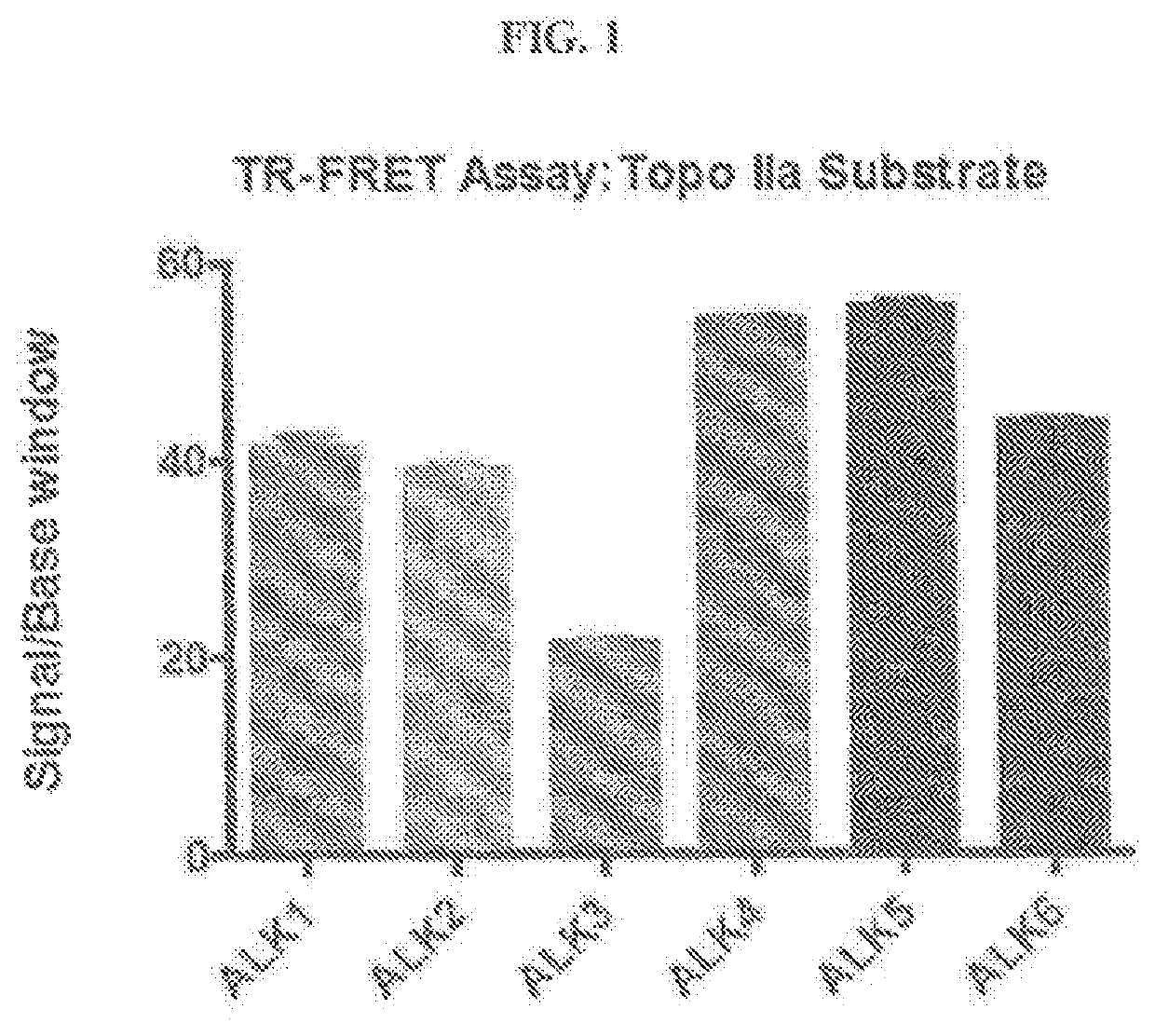
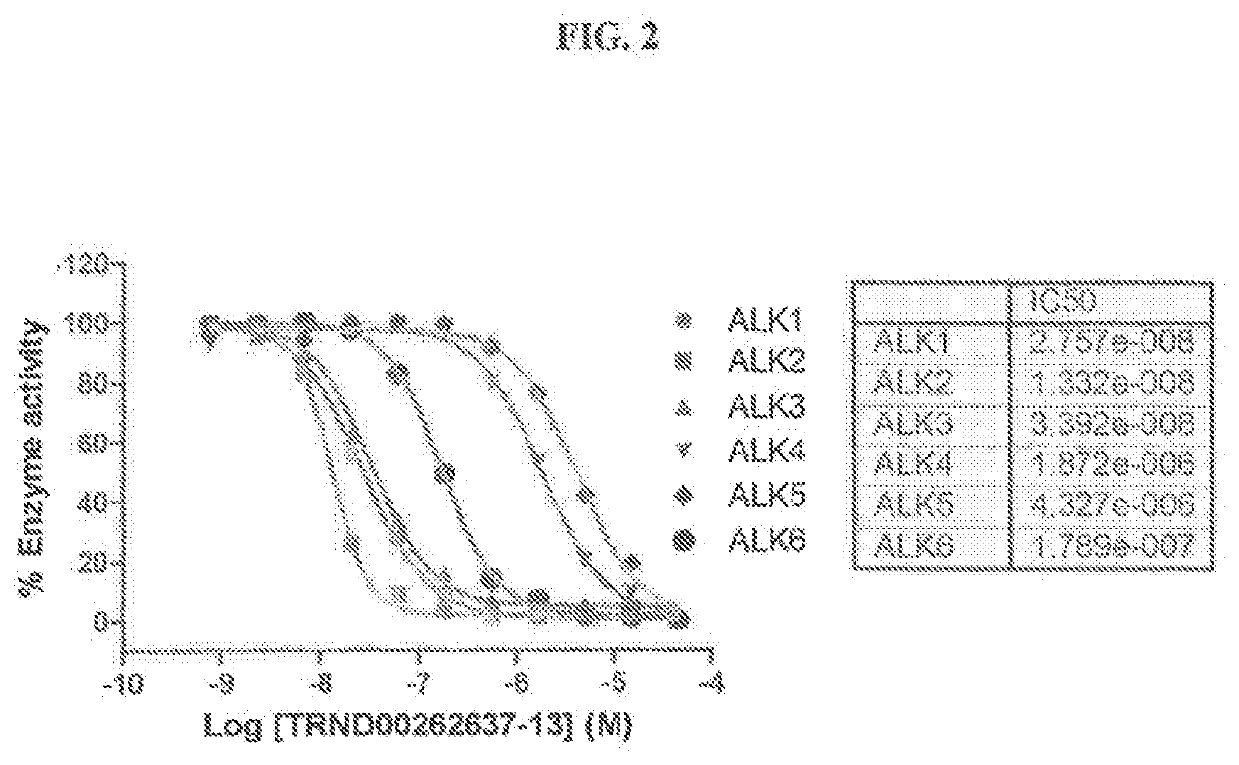
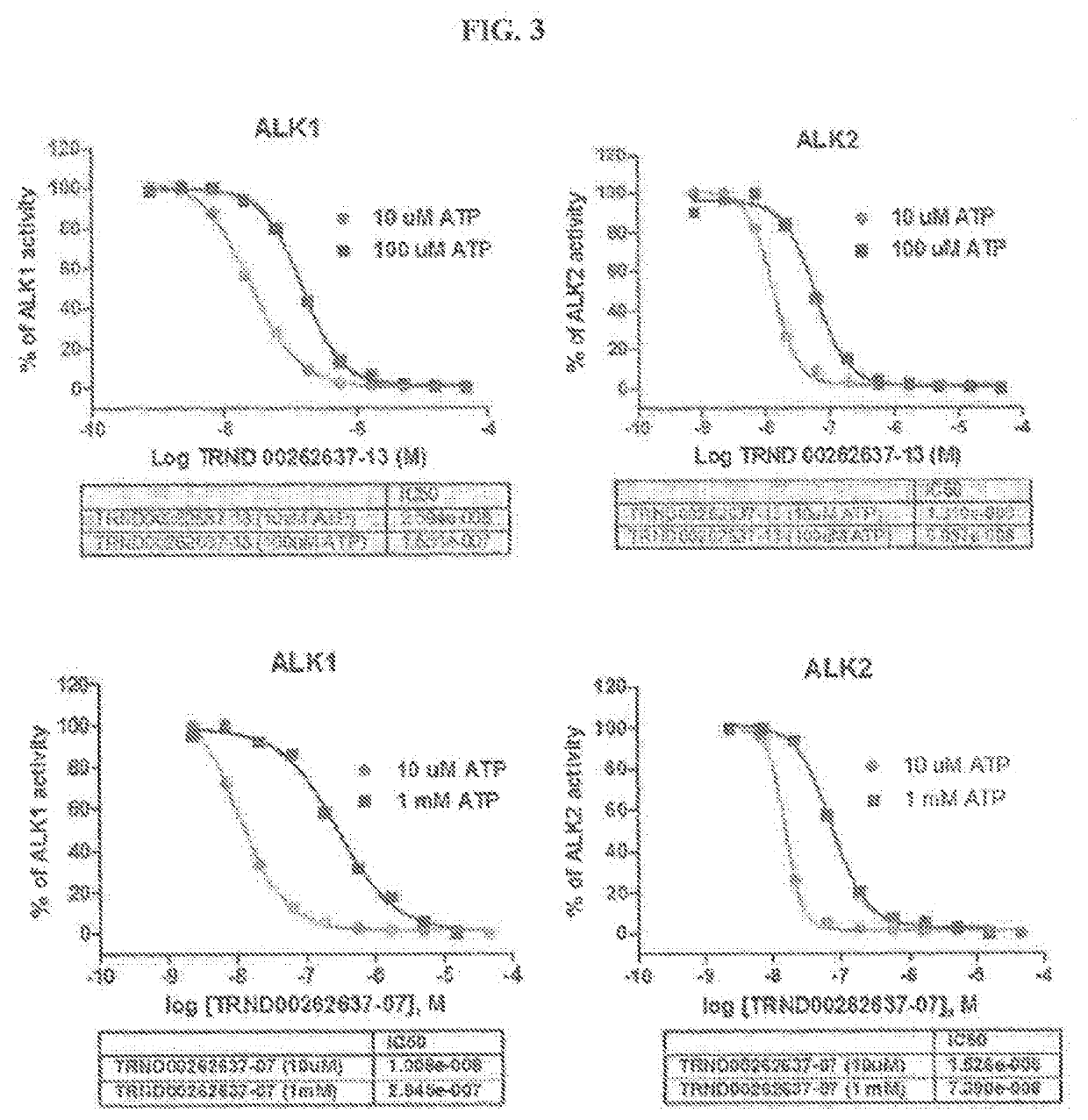
Novel alk2 inhibitors and methods for inhibiting bmp signaling
ActiveUS20200179389A1Inhibition is effectiveHigh expressionOrganic active ingredientsGroup 5/15 element organic compoundsApoptosisS syndrome
The present invention provides small-molecule inhibitors of BMP signaling and compositions and methods for inhibiting BMP signaling. These compounds and compositions may be used to modulate cell growth, differentiation, proliferation, and apoptosis, and thus may be useful for treating diseases or conditions associated with BMP signaling, including inflammation, cardiovascular disease, hematological disease, cancer, and bone disorders, as well as for modulating cellular differentiation and / or proliferation. These compounds and compositions may also be used to treat subjects with Sjögren's syndrome, or diffuse intrinsic pontine glioma (DIPG).
Owner:UNITED STATES OF AMERICA +1
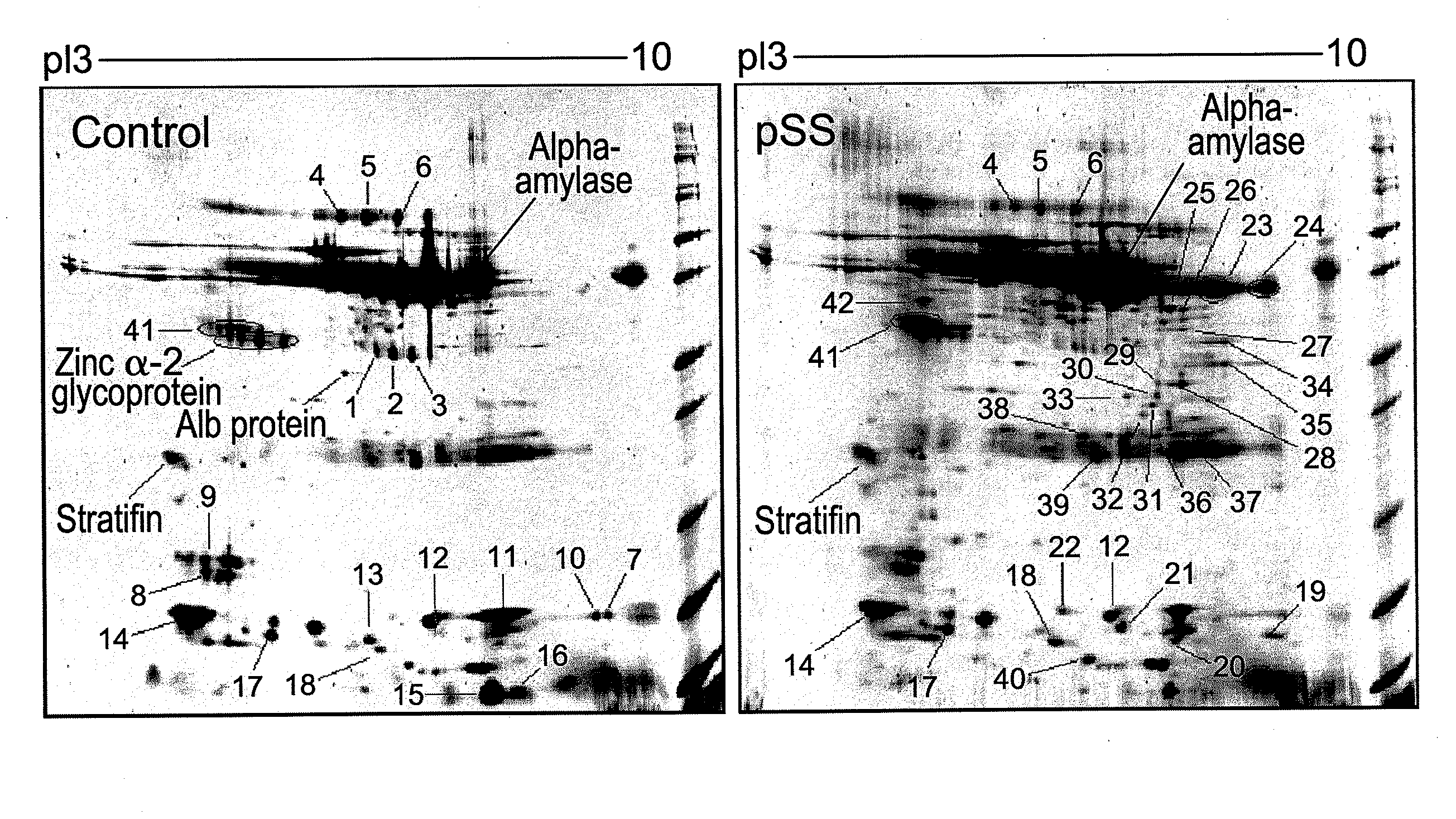
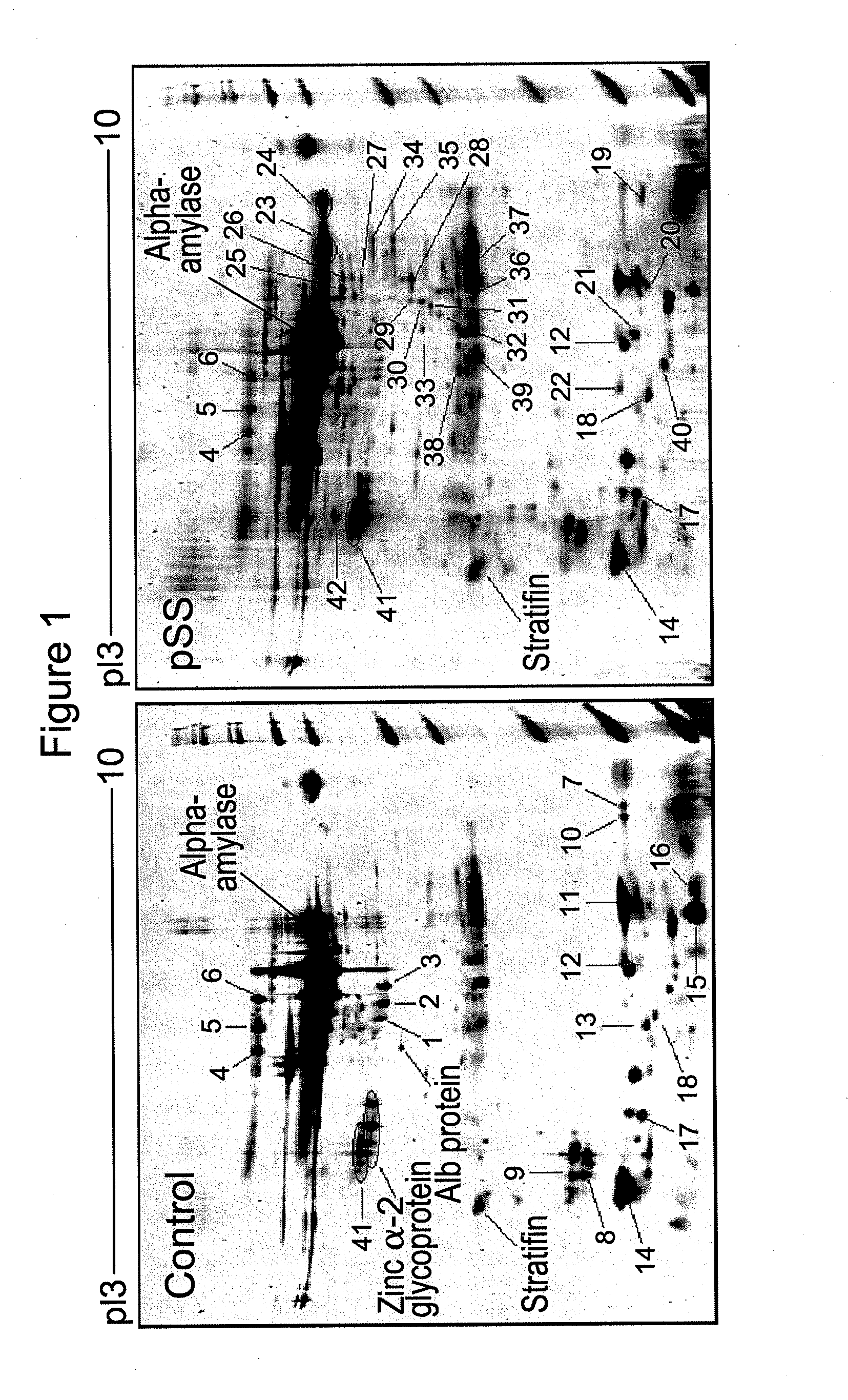
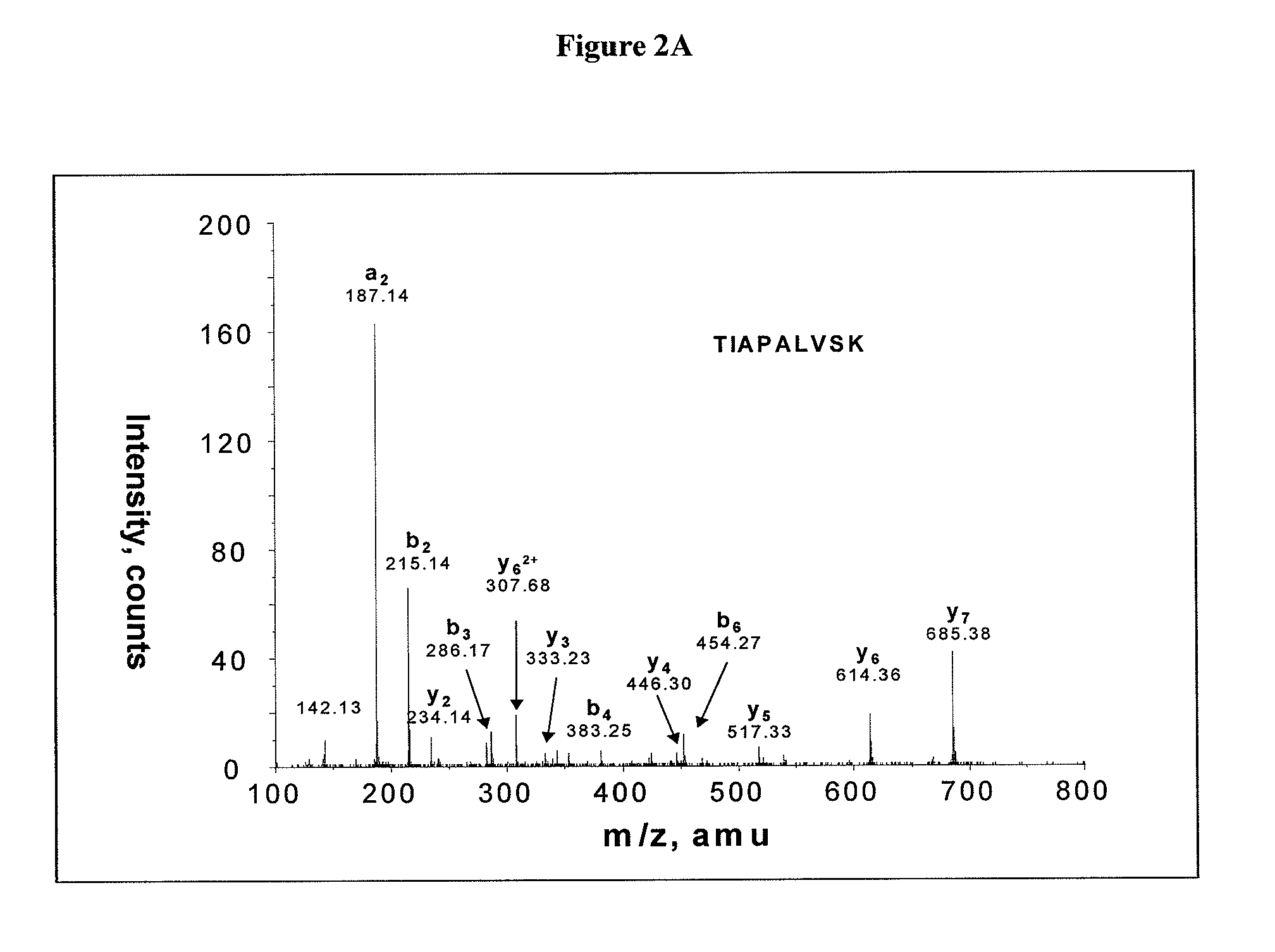
Salivary biomarkers for Sjögren's syndrome
The present invention provides for the first time the identification of salivary protein and RNA factors that can be used in the detection of primary Sjögren's Syndrome. The present invention therefore provides methods of diagnosing and providing a prognosis for Sjögren's Syndrome, by examining relevant proteins (including certain autoantigens and autoantibodies) and RNA in a patient's saliva.
Owner:RGT UNIV OF CALIFORNIA
Medicinal composition for treating sjogren syndrome and preparation method thereof and application
ActiveCN111759940ARelieve symptomsVisible and long-lasting drynessSenses disorderAntipyreticSjögren syndromeFlos chrysanthemi
The invention discloses a medicinal composition for treating sjogren syndrome and a preparation method thereof and an application. The medicinal composition is prepared from components including the following raw materials; 15-25 parts by weight of radix rehmanniae, 20-30 parts by weight of radix scrophulariae, 15-25 parts by weight of radix ophiopogonis, 25-35 parts by weight of radix paeoniae alba, 5-15 parts by weight of radix glycyrrhizae, 15-25 parts by weight of mulberry leaves, 10-20 parts by weight of flos chrysanthemi, 10-20 parts by weight of fructus aurantii, 15-25 parts by weight of fructus ligustri lucidi, 25-35 parts by weight of radix puerariae, and 15-25 parts by weight of caulis sinomenii. The medicinal composition of the present invention has significant effect on treating sjogren syndrome.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Pharmaceutical composition and method for the prevention and treatment of pathologies of the oral cavity
The invention relates to a pharmaceutical composition comprising lysozyme, dextranase and an essential oil of a plant belonging to the family Myrtaceae. The composition is preferably formulated, together with other ingredients, as a mouthwash.The invention also relates to a method for the prevention, treatment and follow-up of pathologies of the oral cavity of infectious, inflammatory, degenerative or autoimmune origin, the method comprising the administration of the composition of the invention to a patient affected by one or more of such pathologies, which are preferably selected in the group consisting of: gingivitis, periodontitis, aphthous stomatitis, herpetic stomatitis, prosthetic stomatitis, recurrent aphthosis, xerostomia, xerostomia due to Sjogren's syndrome, burning mouth syndrome (BMS), oral candidiasis, inflammatory dermatoses such as oral lichen planus, autoimmune disorders of the mucous membranes such as mucous membrane pemphigoid (MMP), graft-versus-host disease, white tongue, migratory glossitis, desquamative gingivitis due to oral lichen planus, herpes and combinations thereof.
Owner:GROSSI LIONELLO
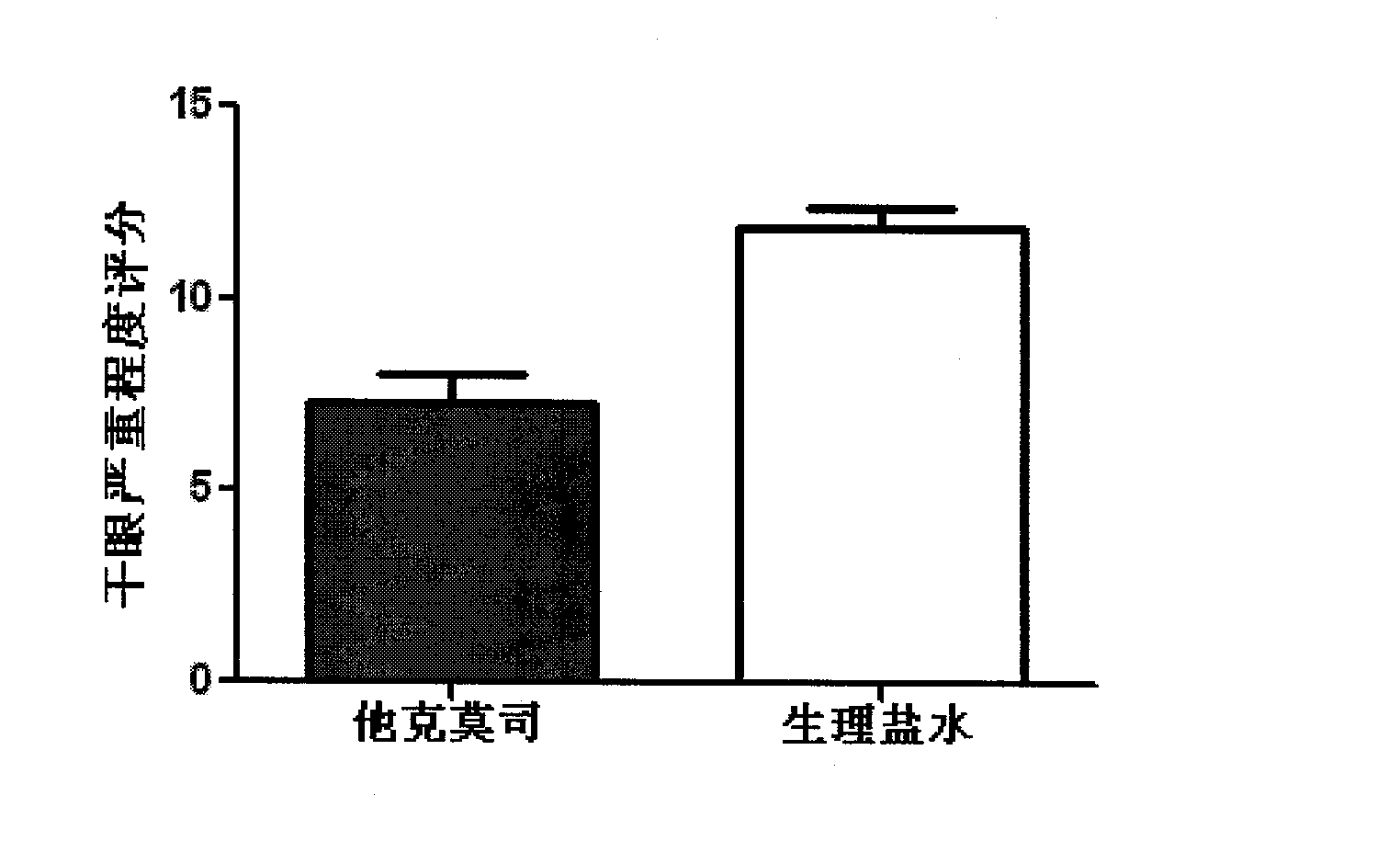
Tacrolimus eye drop for treatment of dry eyes
InactiveCN104042564ALittle side effectsSignificant effectOrganic active ingredientsSenses disorderSide effectSjögren syndrome
The invention relates to a novel treatment method for dry eyes, i.e. treatment of dry eyes by tacrolimus with a concentration of 0.02%-0.03%. Directed at the current situation of clinical absence of effective methods for treatment of dry eyes, the invention aims to provide an effective measure with low side effect to treat dry eyes. Specifically, tacrolimus with a concentration of 0.02%-0.03% is applied four times per day for one month continuously so as to treat chronic non-sjogren syndrome related dry eyes.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
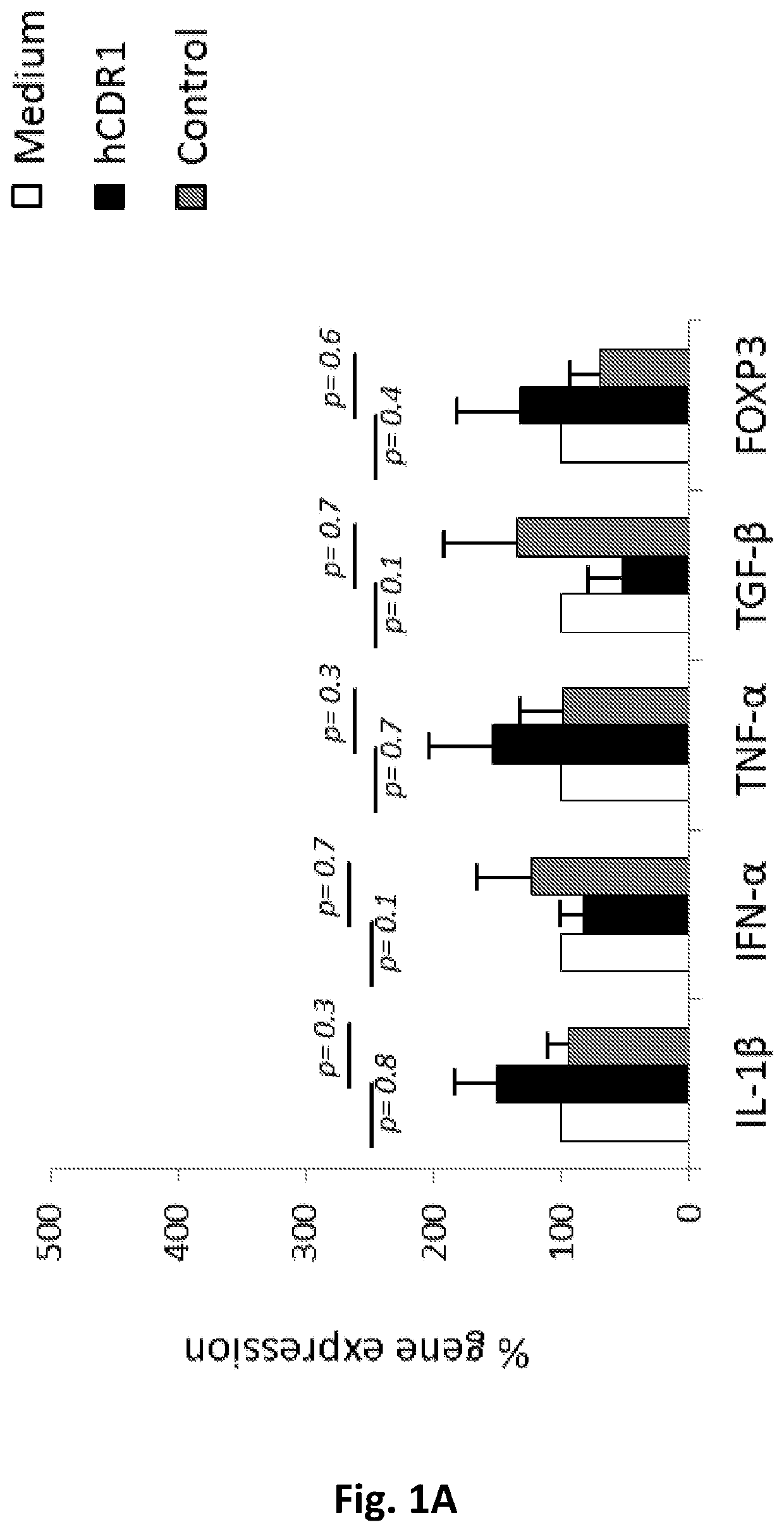
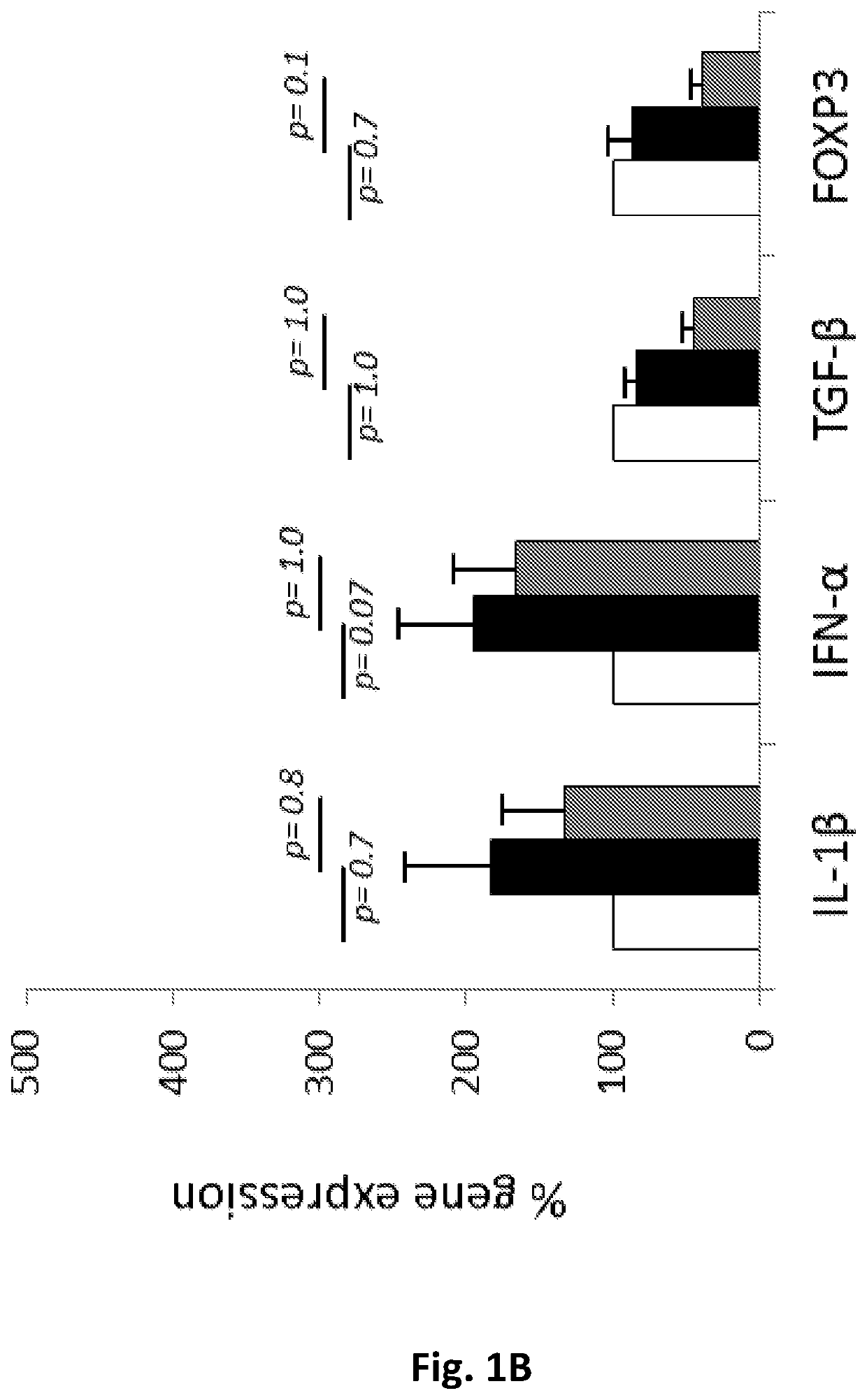
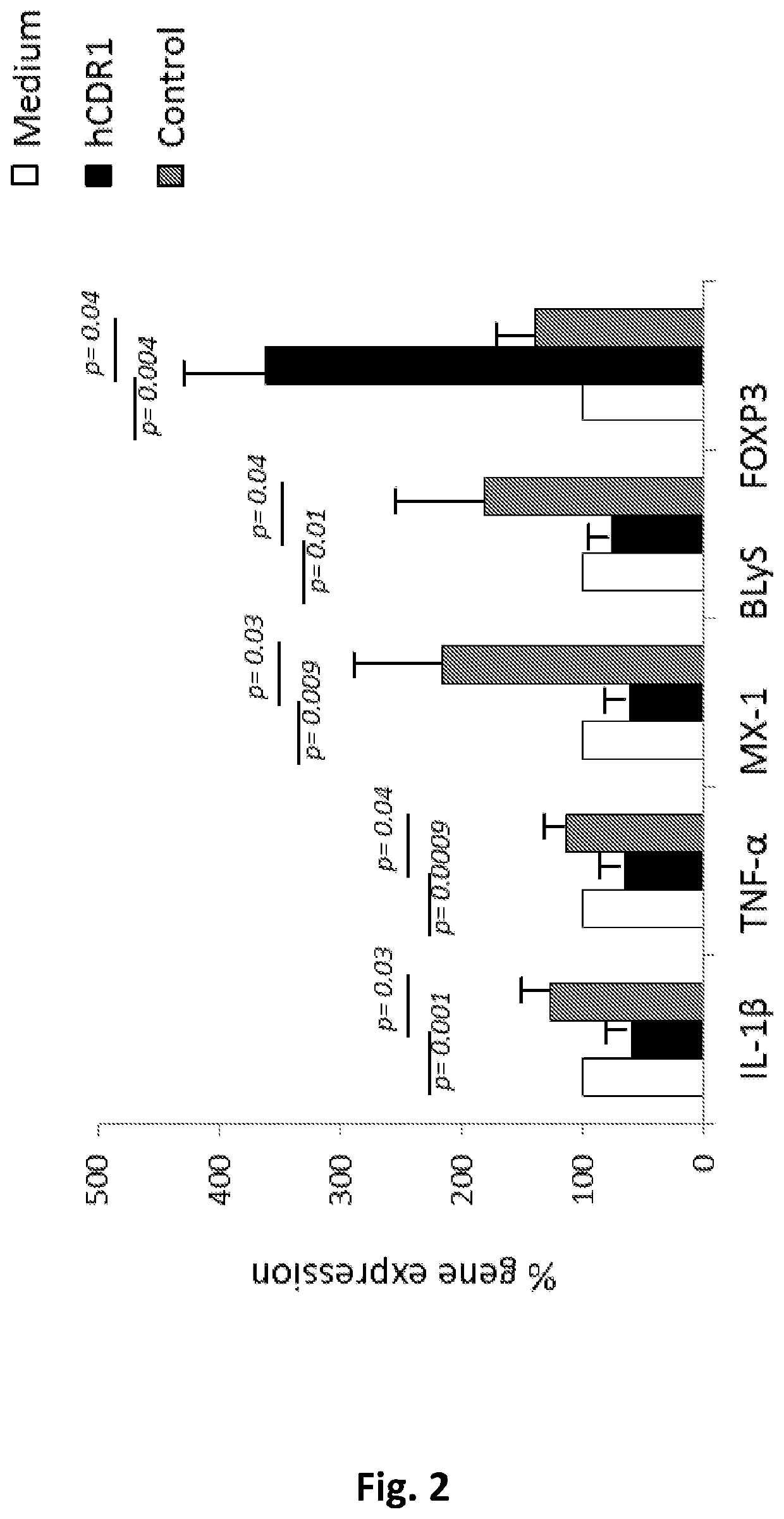
Peptides for treating sjogren's syndrome
ActiveUS20190351004A1Downregulating activity and expressionSenses disorderPeptide/protein ingredientsPeptideAnti dna
The present invention provides compositions and methods for the treatment of Sjogren's syndrome (SS) and SS-related symptoms in human subjects by synthetic peptides based on the sequence of CDR1 of an anti-DNA monoclonal antibody.
Owner:YEDA RES & DEV CO LTD
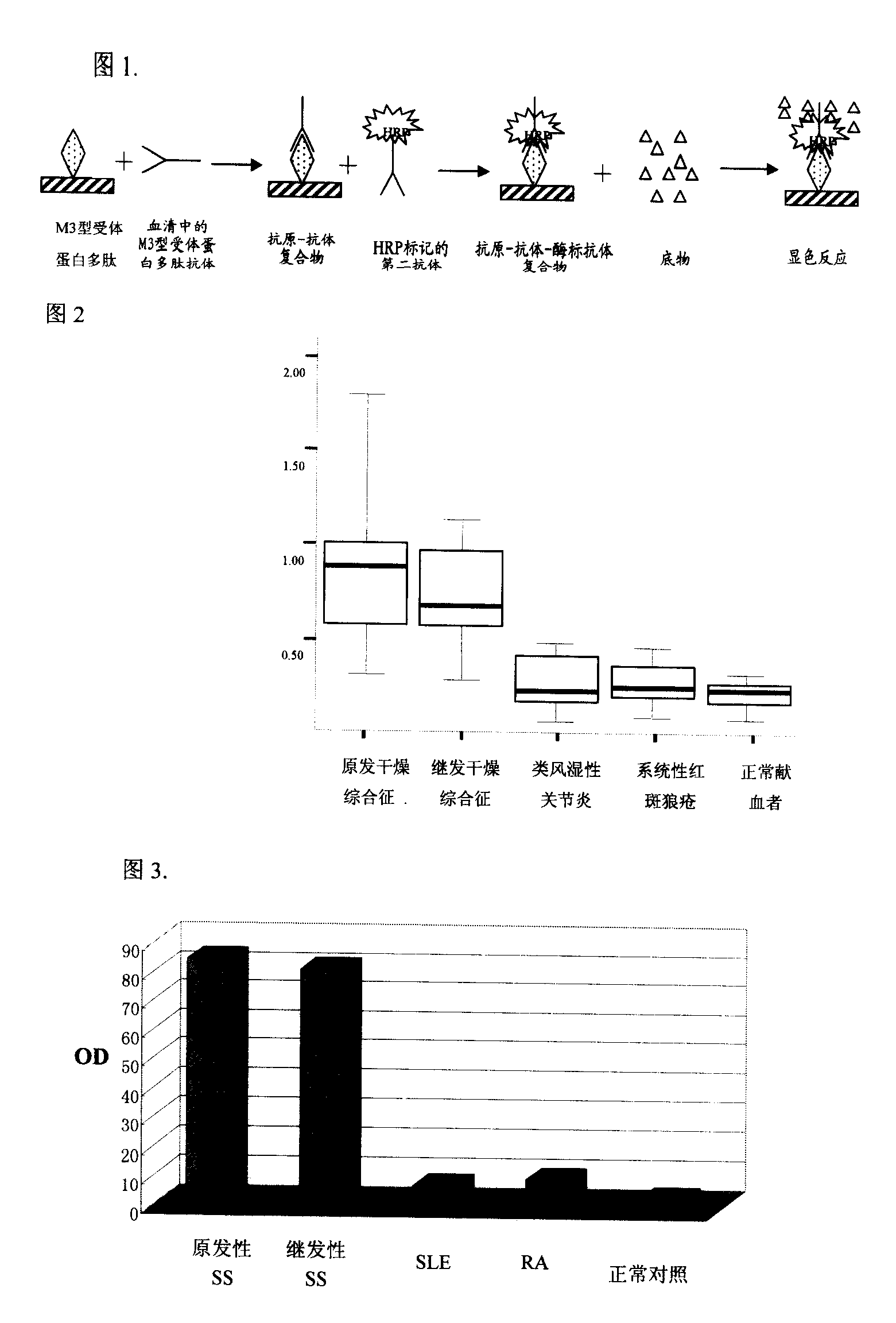
Application of M3 type receptor protein polypeptide resisting antibody measurement in Sjogren syndrome diagnosis
The invention provides a check of Sjogren syndrome by M3-type receptor protein polypeptide and analogues, wherein the peptide antigen can be specially combined with specific antibody in Sjogren syndrome patient, with high sensitivity and specificity.
Owner:PEOPLES HOSPITAL PEKING UNIV
Methods and kits for diagnosing Sjögren's syndrome
The invention features methods and kits for determining the presence of, or a predisposition to develop, Sjögren's syndrome in humans. The invention features methods to detect changes in the levels of one or more LMP-2 protein isoforms, in particular phosphorylated isoforms of LMP-2, and to detect changes in cellular protein phosphorylation and ubiquitination in samples from Sjögren's patients.
Owner:THE GENERAL HOSPITAL CORP
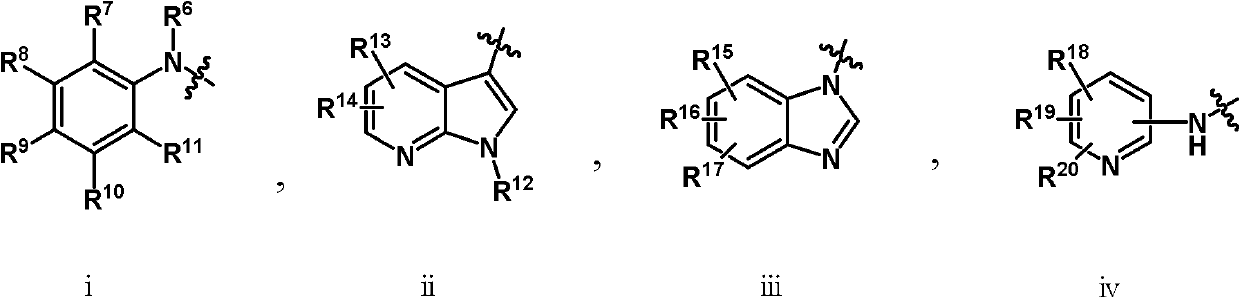
Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors
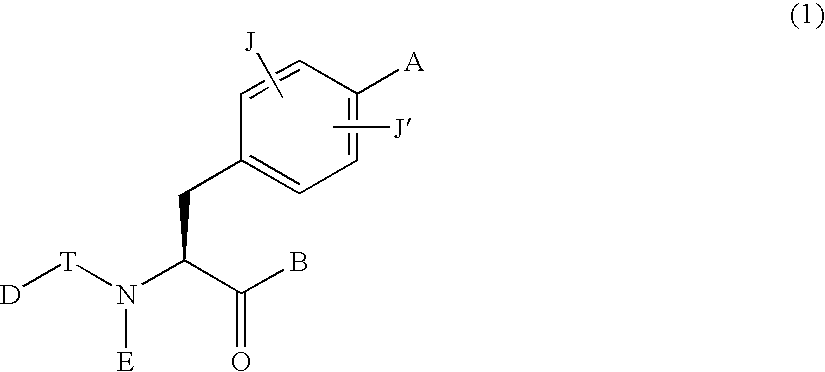
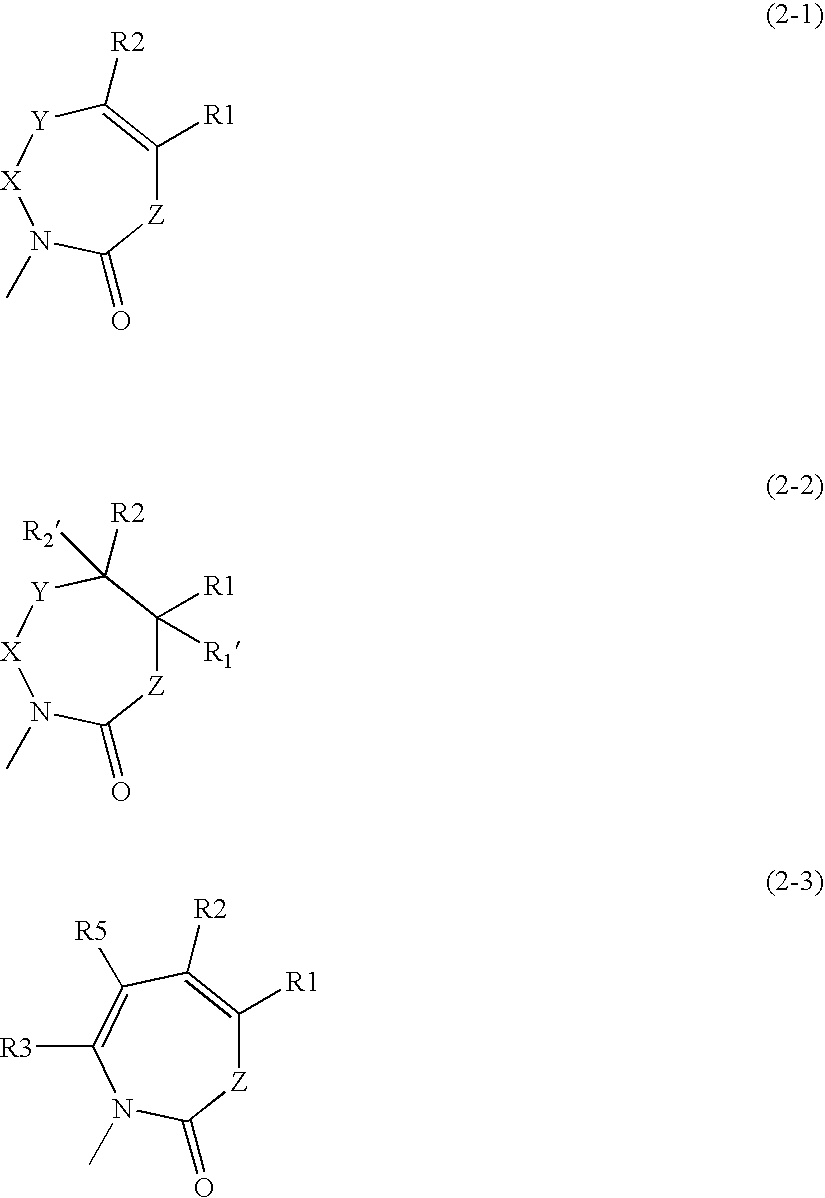
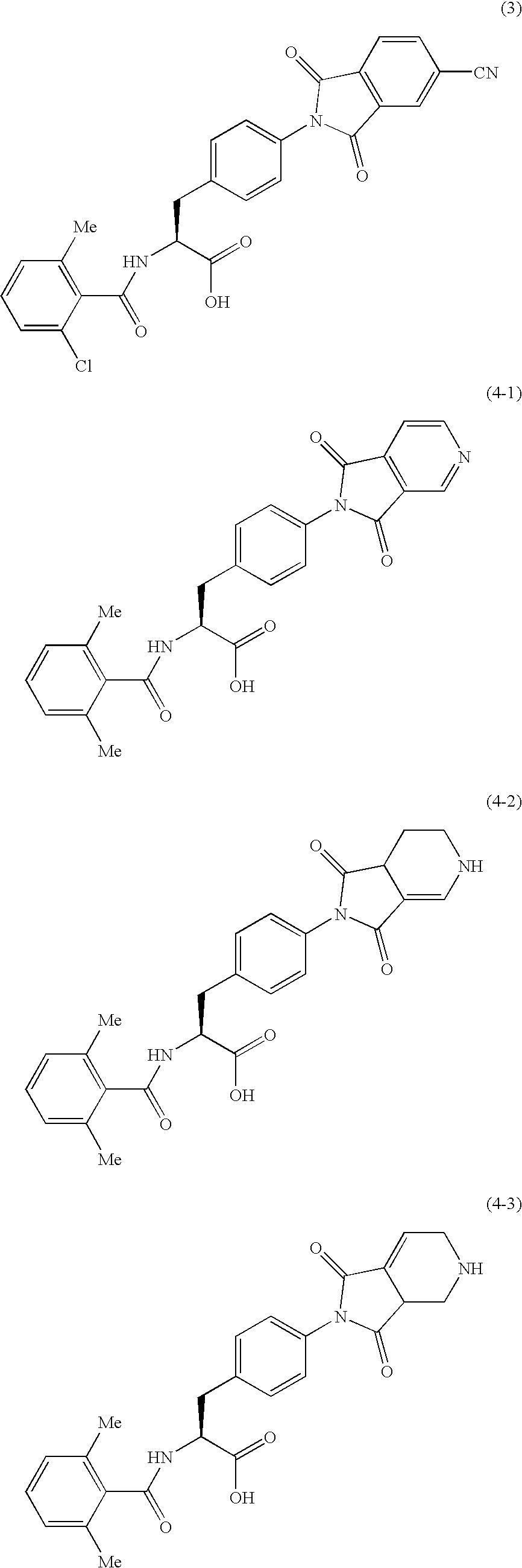
A first aspect of the invention relates to a compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof,wherein:one of R3 and R4 is H, and the other is selected from C1-6-alkyl, C1-6-haloalkyl, C1-6-alkoxy, and C6-12-aralkyl;or R3 and R4 are each independently selected from C1-6-alkyl and halo;R9 is a substituted 5 or 6-membered aryl or heteroaryl group or a 6,5- or 6,6-fused biaryl or heterobiaryl group.Compounds of formula (I) exhibit surprisingly high efficacies for human cathepsin S, excellent selectivity verses other mammalian cathepsins and are useful for treatment of diseases such as rheumatoid arthritis, multiple sclerosis, myasthenia gravis, transplant rejection, diabetes, Sjogrens syndrome, Grave's disease, systemic lupus erythematosis, osteoarthritis, psoriasis, idiopathic thrombocytopenic purpura, allergic rhinitis, asthma, atherosclerosis, obesity, chronic obstructive pulmonary disease and chronic pain.
Owner:AMURA THERAPEUTICS
Pyrrolopyrimidines as CFTR potentiators
ActiveUS10301315B2Ease of detectabilityEasy to prepareOrganic active ingredientsOrganic chemistryDiseaseFibrosis
The present invention relates to compounds of Formula I,wherein R1a, R1b, R2, R3, R4, W, Y, and Z are as described herein, and pharmaceutically acceptable salts thereof. The compounds are potentiators of Cystic Fibrosis Transmembrane conductance Regulator (CFTR). The invention also discloses pharmaceutical compositions comprising the compound, optionally in combination with additional therapeutic agents, and methods of potentiating, in mammals, including humans, CFTR by administration of the compounds. These compounds are useful for the treatment of cystic fibrosis (CF), asthma, bronchiectasis, chronic obstructive pulmonary disease (COPD), constipation, Diabetes mellitus, dry eye disease, pancreatitis, rhinosinusitis, Sjögren's Syndrome, and other CFTR associated disorders.
Owner:CYSTIC FIBROSIS FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors](https://images-eureka.patsnap.com/patent_img/a524b12a-8fd6-4ac9-8486-ec038ff18793/US08389737-20130305-C00001.png)
![Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors](https://images-eureka.patsnap.com/patent_img/a524b12a-8fd6-4ac9-8486-ec038ff18793/US08389737-20130305-C00002.png)
![Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors Furo[3, 2-B] pyrrol-3-ones as cathespin S inhibitors](https://images-eureka.patsnap.com/patent_img/a524b12a-8fd6-4ac9-8486-ec038ff18793/US08389737-20130305-C00003.png)