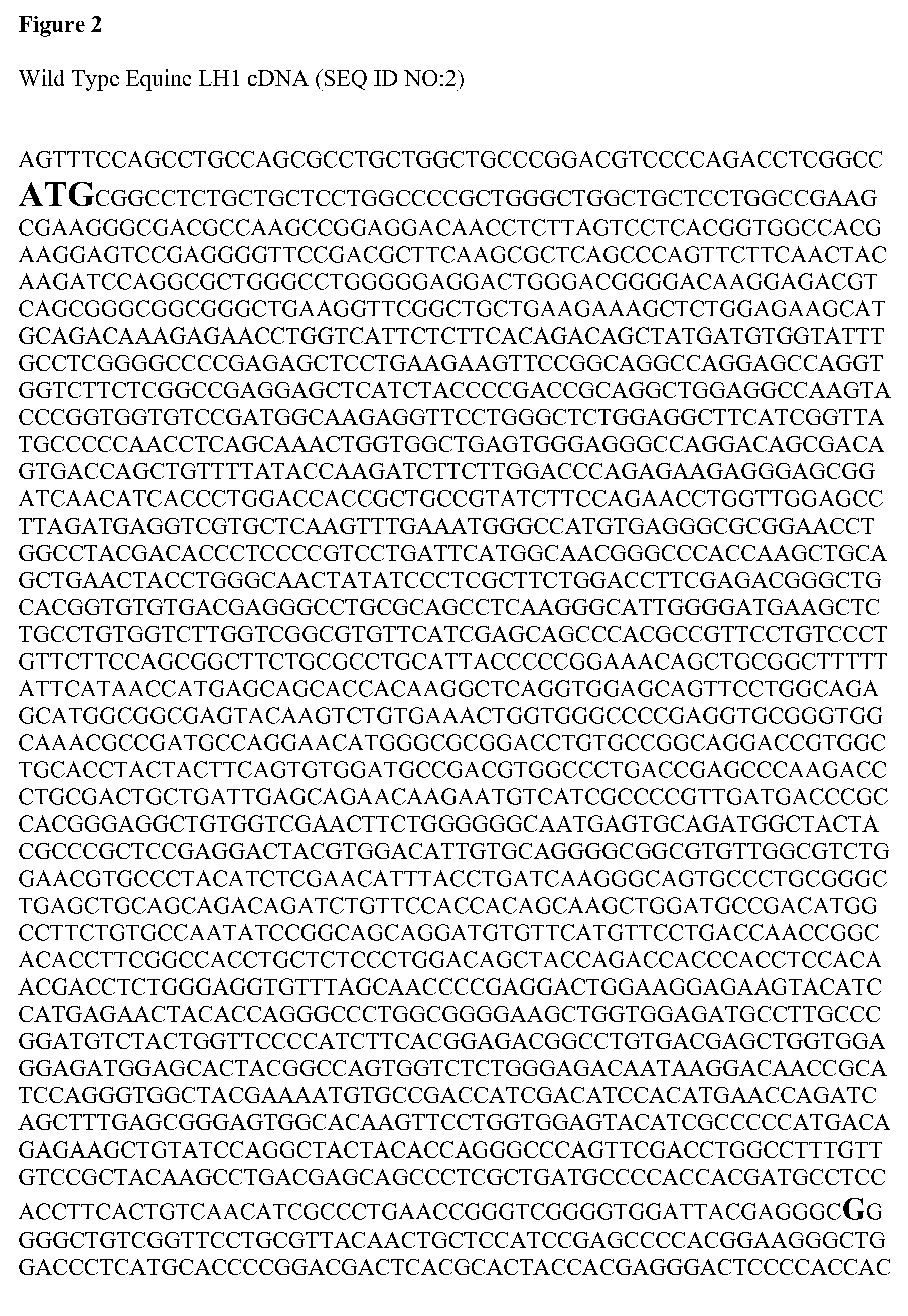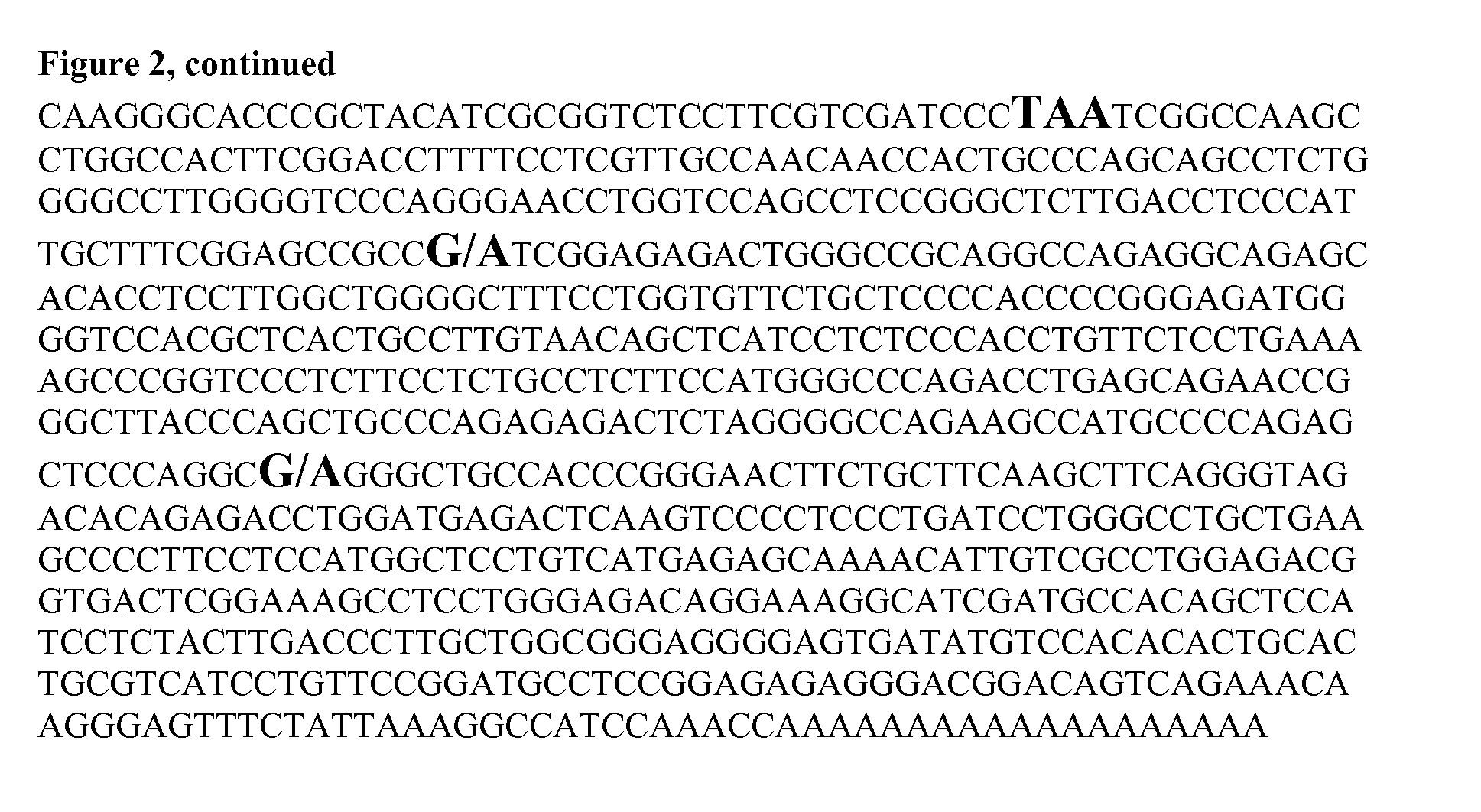Patents
Literature
37 results about "Connective Tissue Disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A disorder characterized by abnormalities in one or more of the elements of the connective tissues, typically associated with genetic defects.
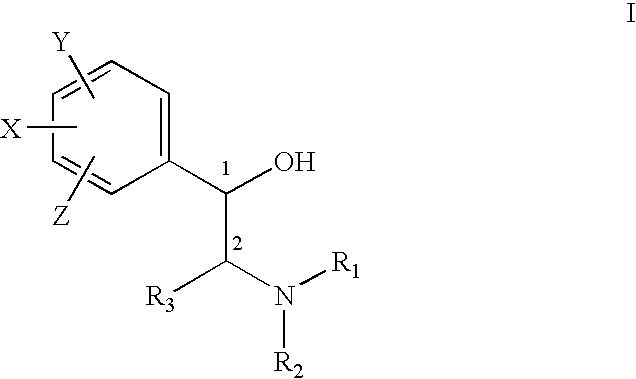
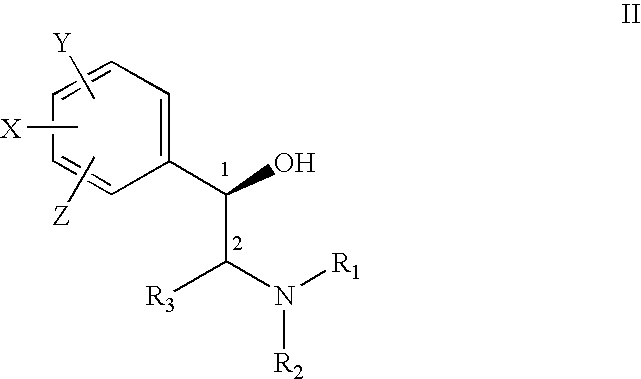
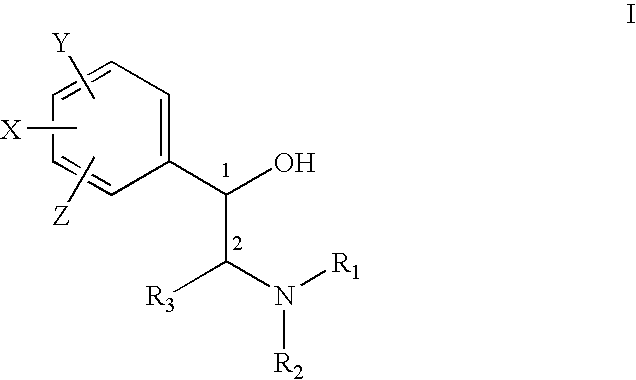
Pharmaceutical compositions and methods for managing connective tissue ailments
The present invention relates to compositions and methods for managing connective tissue disorders in a patient, a sugar compound that is converted to a glycosaminoglycan, a primary antioxidant component, at least one amino acid component, at least one transition metal component, at least one moisturizing agent, at least one fatty acid. In a preferred embodiment, the composition for topical administration to the patient's skin further included hydrogen peroxide in an amount sufficient to cleanse the skin.
Owner:MURAD HOWARD
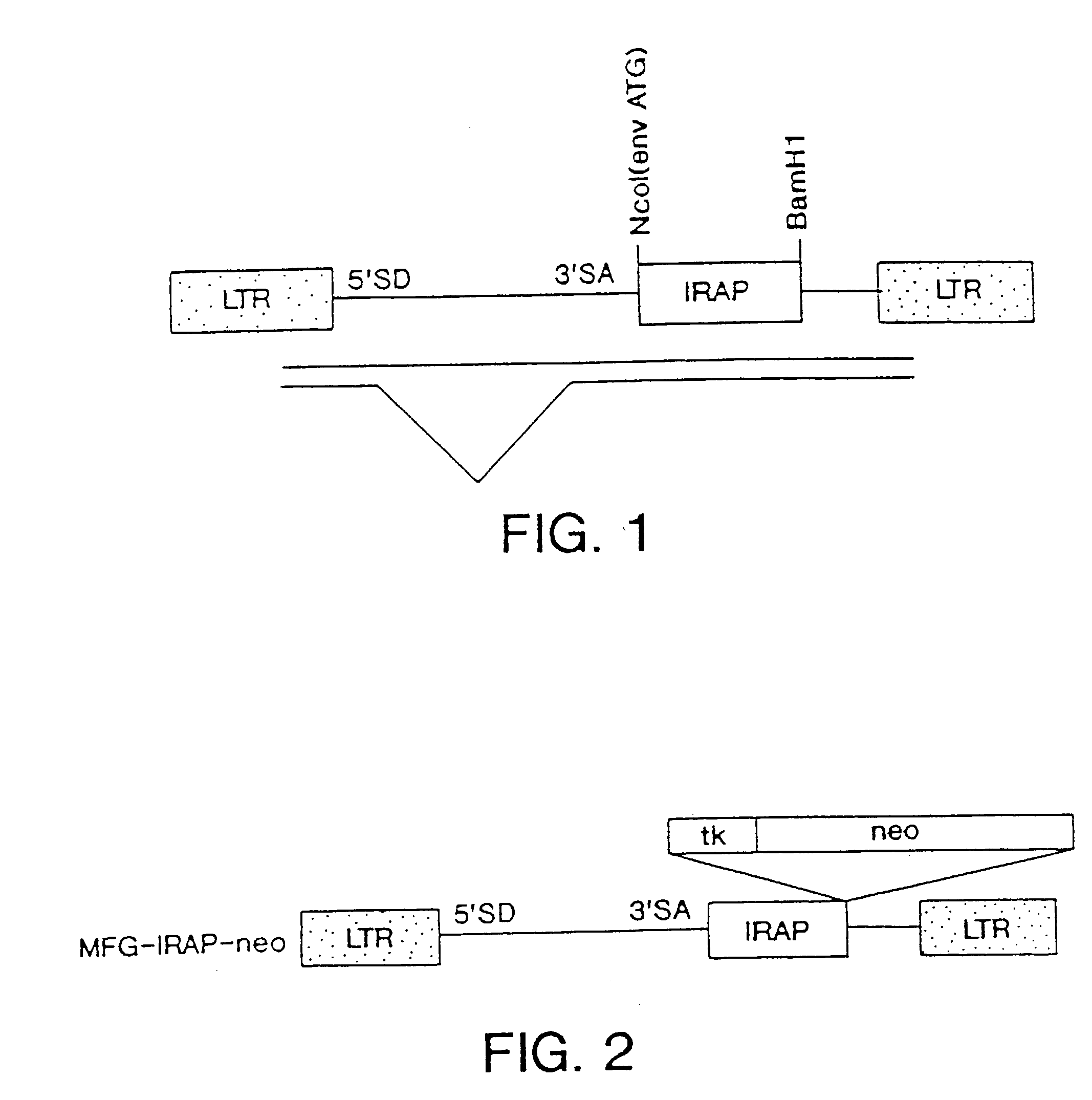
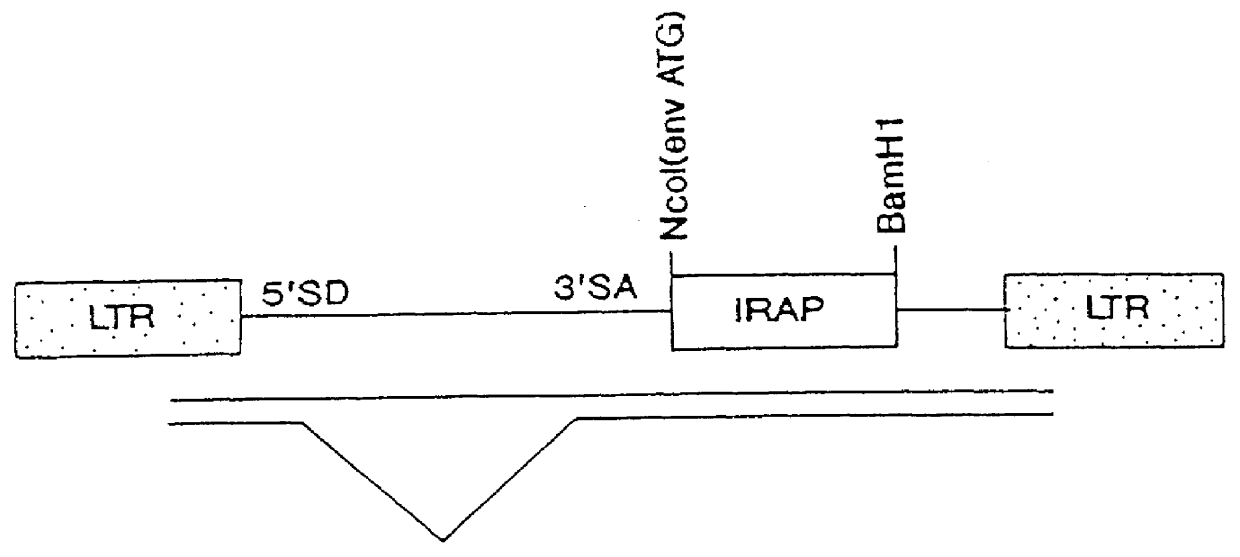
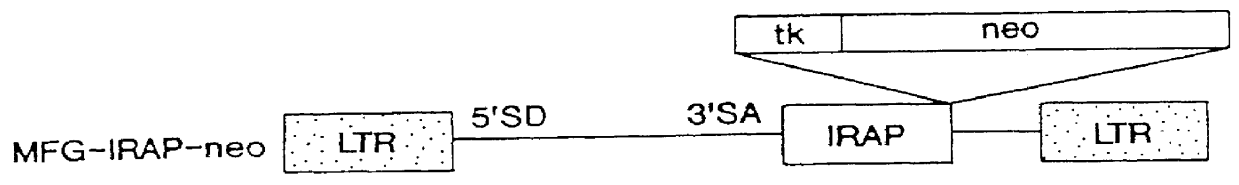
Gene transfer for treating a connective tissue of a mammalian host
Methods for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule containing the DNA sequence encoding for the product and infecting the target cell of the mammalian host using the vector. The injection can be done in vivo, by directly injecting the vector into the host, or can be done in vitro by transfecting a population of cultured target cells with the vector and transplanting them each into the host. Nonviral means can also be used to introduce the DNA sequence to the host. Administration of more than one gene of interest results in an enhanced therapeutic benefit. Also disclosed is a method for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a joint of a host for use in treating multiple joints of the host. Injection of a vector molecule containing the DNA sequence encoding for a product of interest, or non-viral introduction of such a DNA sequence, to one joint of a mammalian host results in a therapeutic benefit in that joint as well as other joints in the host.
Owner:UNIVERSITY OF PITTSBURGH
Viral vectors to inhibit leukocyte infiltration or cartilage degradation of joints
Methods for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule containing the DNA sequence encoding for the product and infecting the target cell of the mammalian host using the vector. The injection can be done in vivo, by directly injecting the vector into the host, or can be done in vitro by transfecting a population of cultured target cells with the vector and transplanting them each into the host. Nonviral means can also be used to introduce the DNA sequence to the host. Administration of more than one gene of interest results in an enhanced therapeutic benefit. Also disclosed is a method for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a joint of a host for use in treating multiple joints of the host. Injection of a vector molecule containing the DNA sequence encoding for a product of interest, or non-viral introduction of such a DNA sequence, to one join of a mammalian host results in a therapeutic benefit in that joint as well as other joints in the host
Owner:UNIVERSITY OF PITTSBURGH
Topical therapeutic formulations
ActiveUS20130209545A1Applied to skinReduce severityOrganic chemistryPeptide/protein ingredientsMuscle tissueDisease
The invention provides topical compositions and methods for using the compositions. The compositions can be used for the treatment of fibrotic or connective tissue disorders involving scarring, sub-dermal plaque accumulations, or fibrosis of muscle tissue. The disorders can be painlessly treated by the topical application of a composition described herein. One or more calcium channel blocker agents can serve as an active ingredient of the compositions, optionally in combination with, for example, one or more of emu oil and superoxide dismutase. The composition can further include pharmaceutically acceptable carriers that can facilitate the non-invasive transdermal delivery of the active(s) to subdermal sites.
Owner:HYBRID MEDICAL
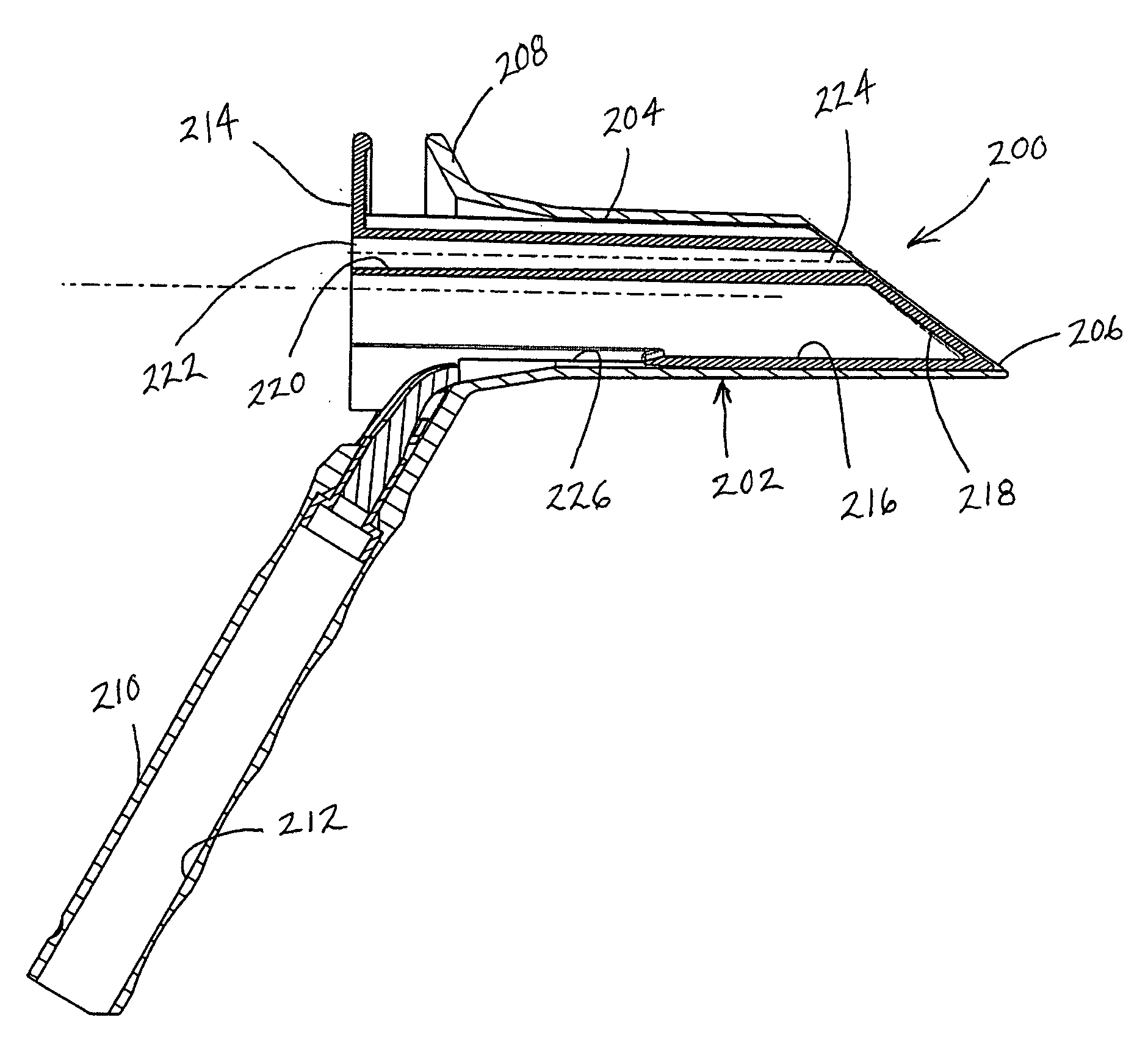
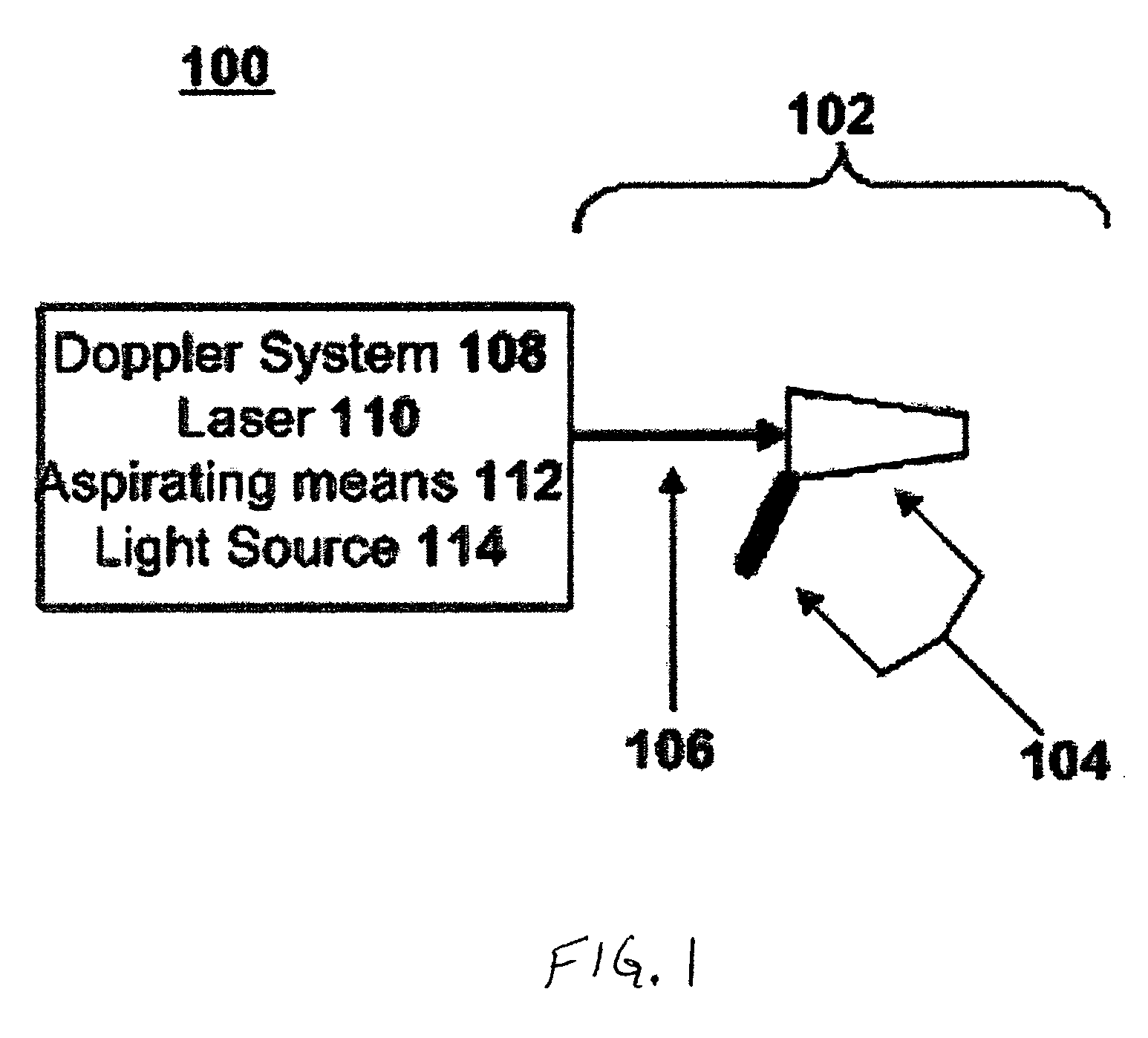
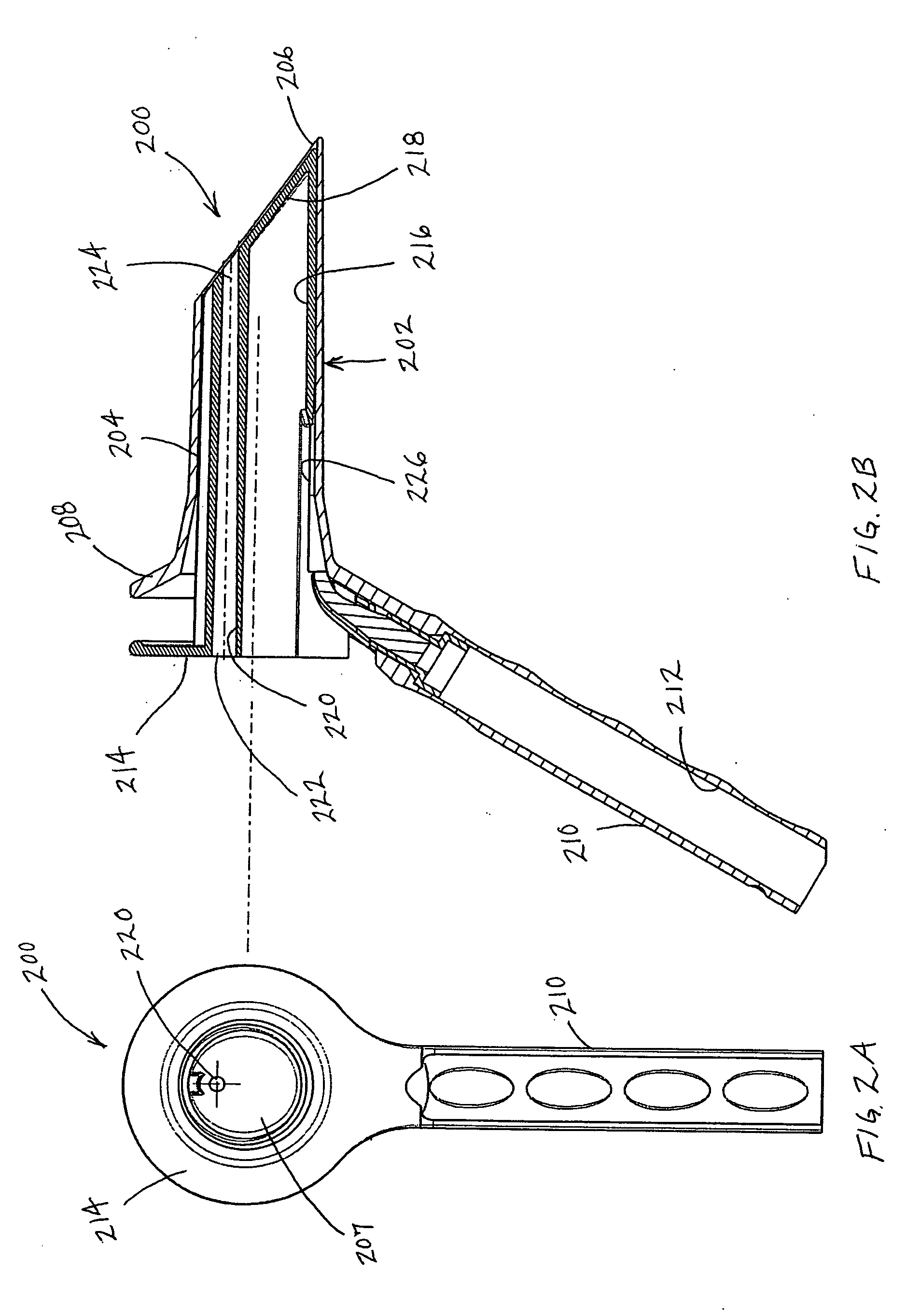
System and Method for Treating Hemorrhoids
InactiveUS20080281204A1Few and no complicationReduce complicationsOrgan movement/changes detectionDiagnostic recording/measuringWhole bodyGeneral anaesthesia
A system and method are presented for treating branches of the superior hemorrhoidal artery by photocoagulation using laser energy. The inflamed dilated blood vessels in and around the anal region, often called hemorrhoids or piles, are caused due to a connective tissue disorder, a relative increase in pressure in the superior hemorrhoidal artery and a weakening of the vessels' valves. A suitable treatment system and a method are given for treating such conditions in a minimally invasive manner. The treatment system photocoagulates the branches of a superior hemorrhoidal artery in the anal and rectal regions using laser energy while causing minimal pain or discomfort to the patient. The system is provided with a transparent operational window. The distal end of the fiber and Doppler system channel are at the operational window. The system also includes a viewing window. The post operative recovery is faster than alternative approaches with no complications. In contrast to most other successful methods of the prior art, general anesthesia is no longer required, thereby significantly reducing complications and simplifying treatment.
Owner:BIOLITEC UNTERNEHMENSBETEILLIGUNGS II AG
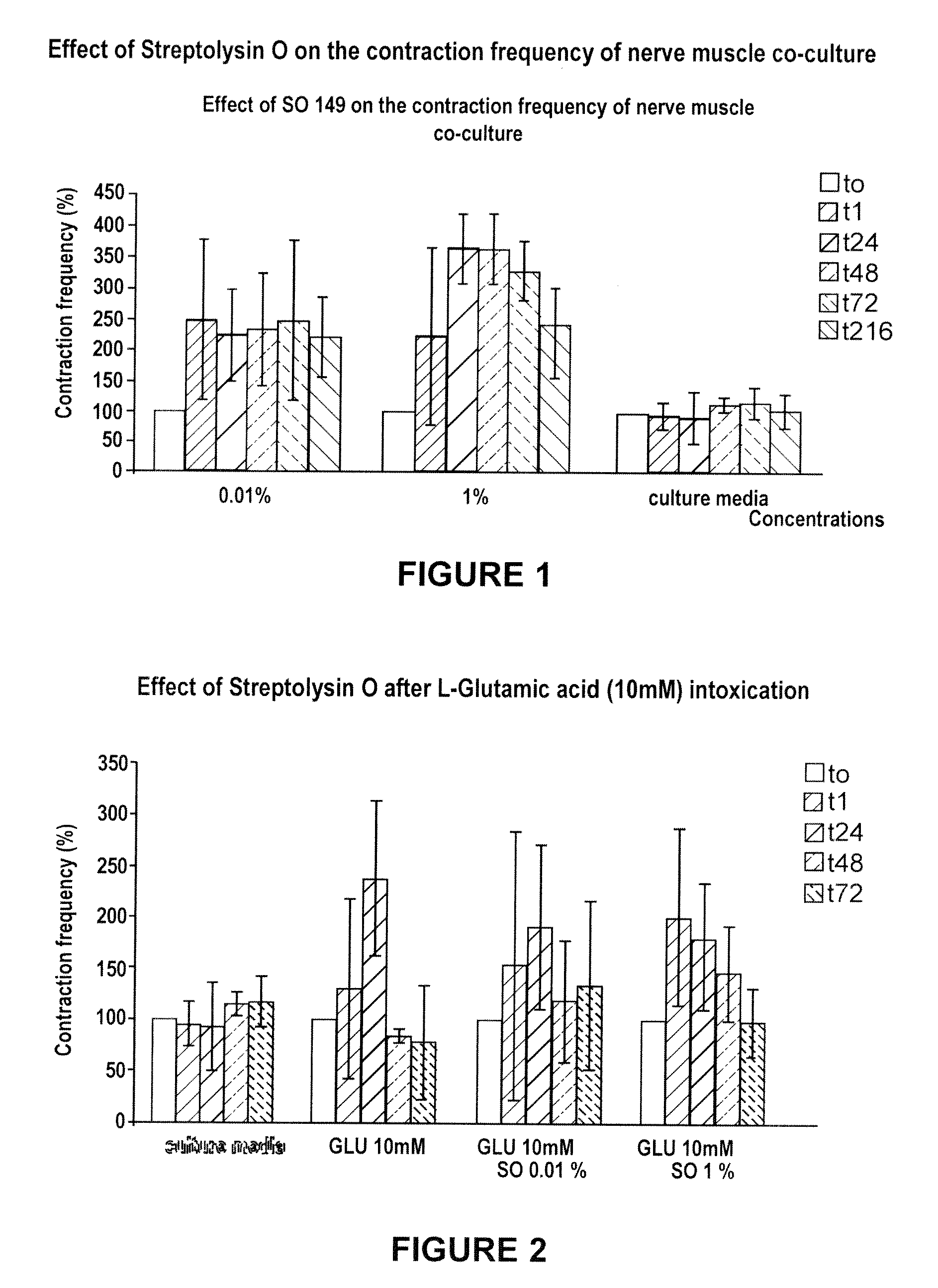
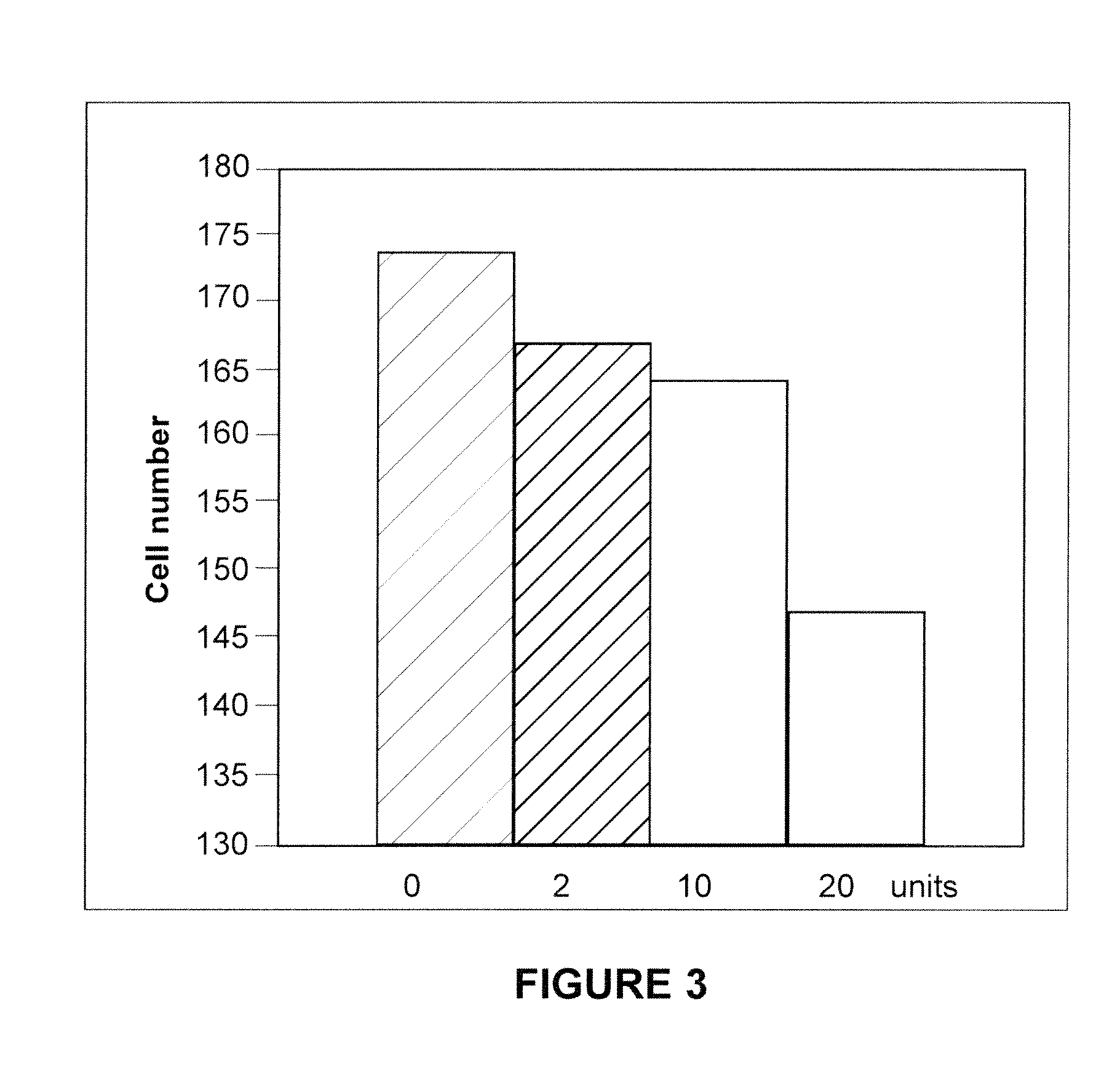
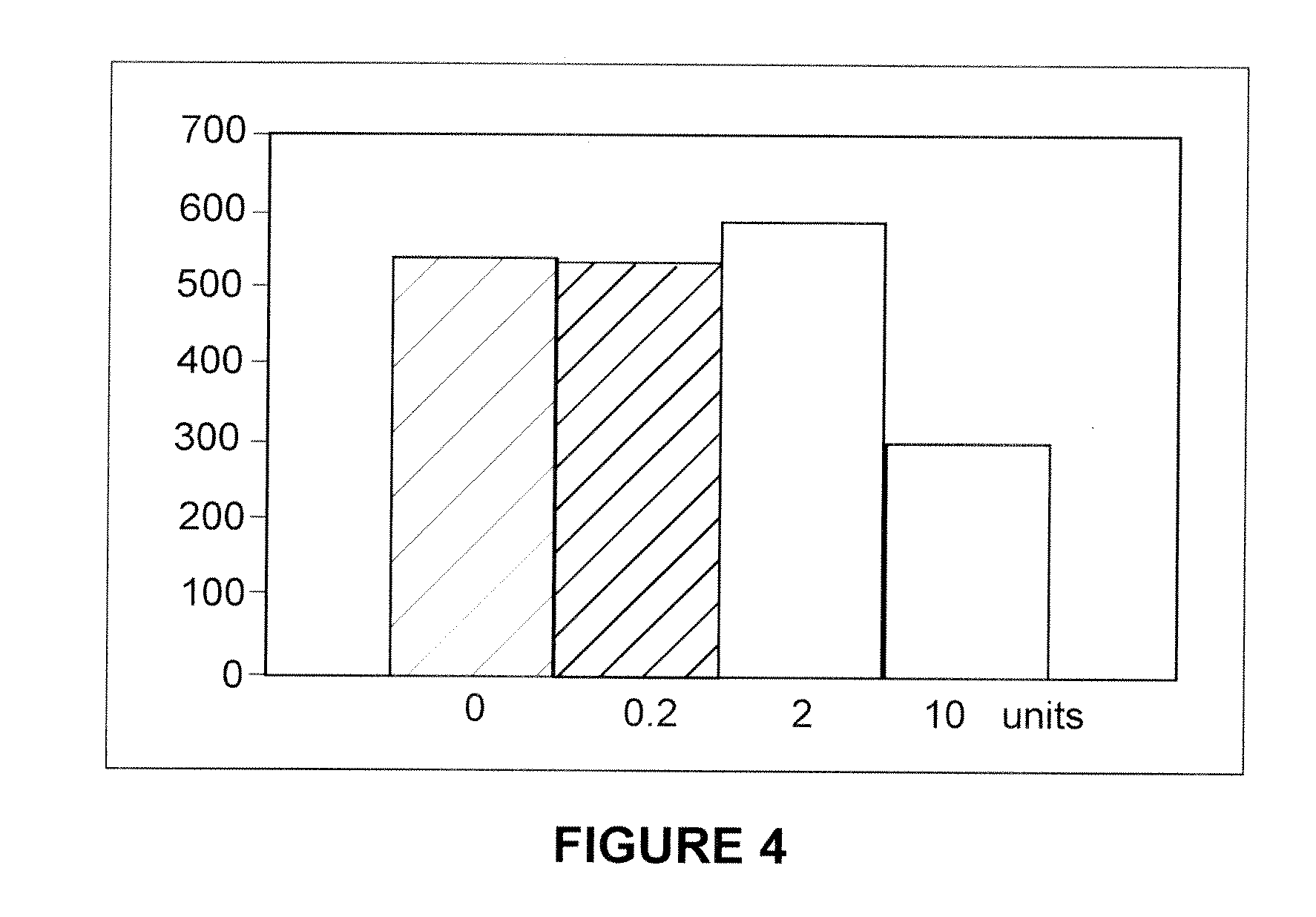
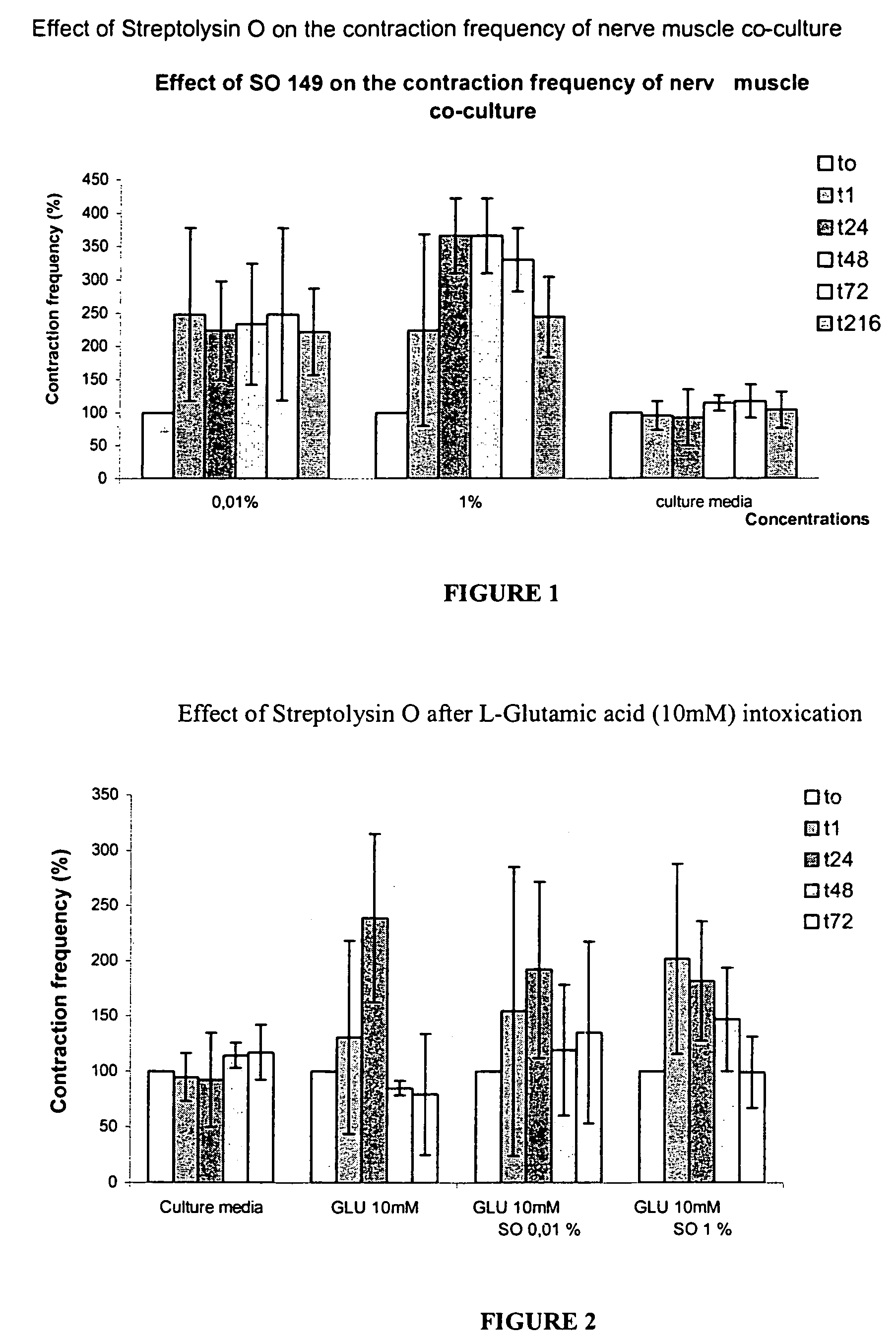
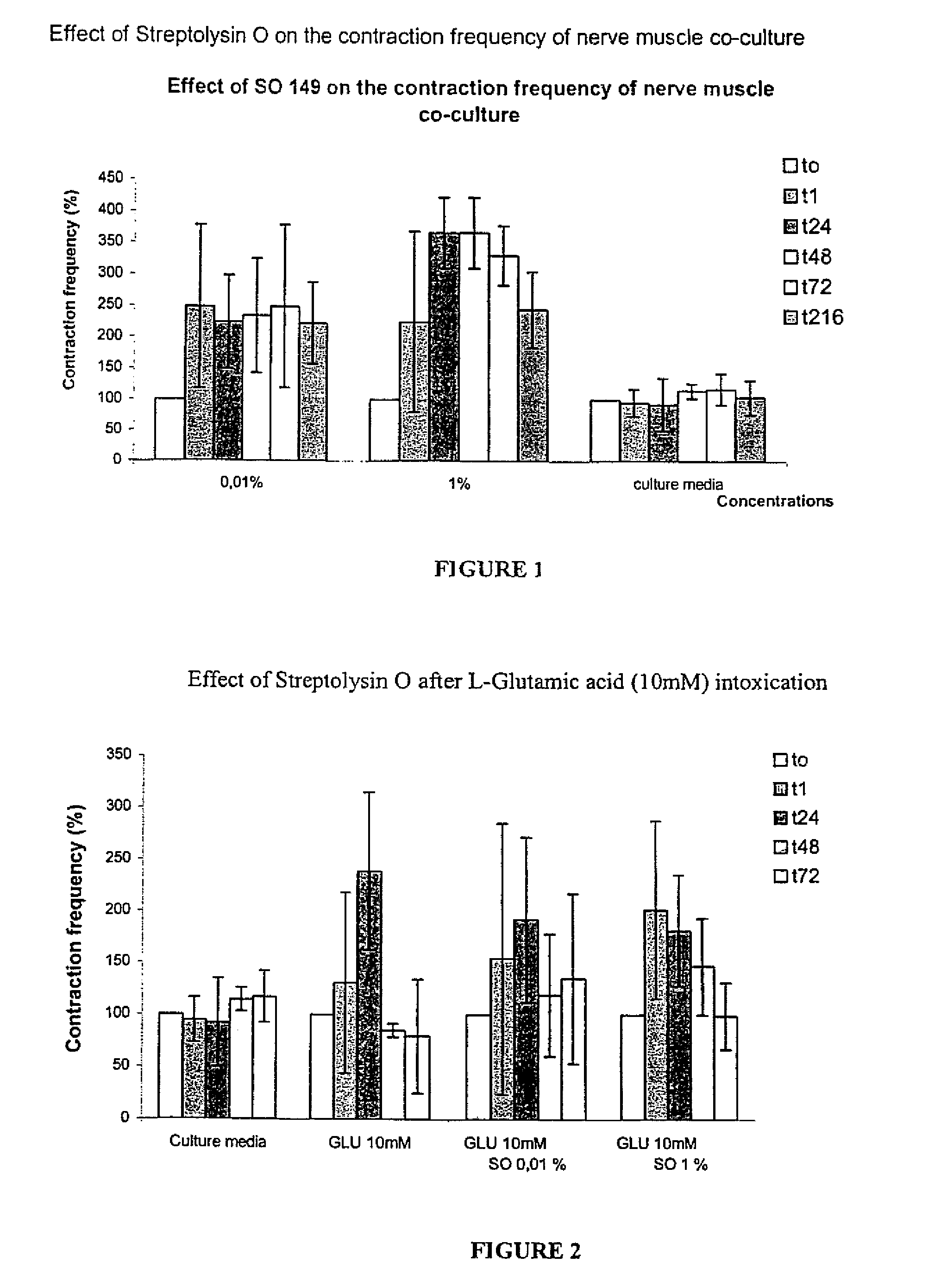
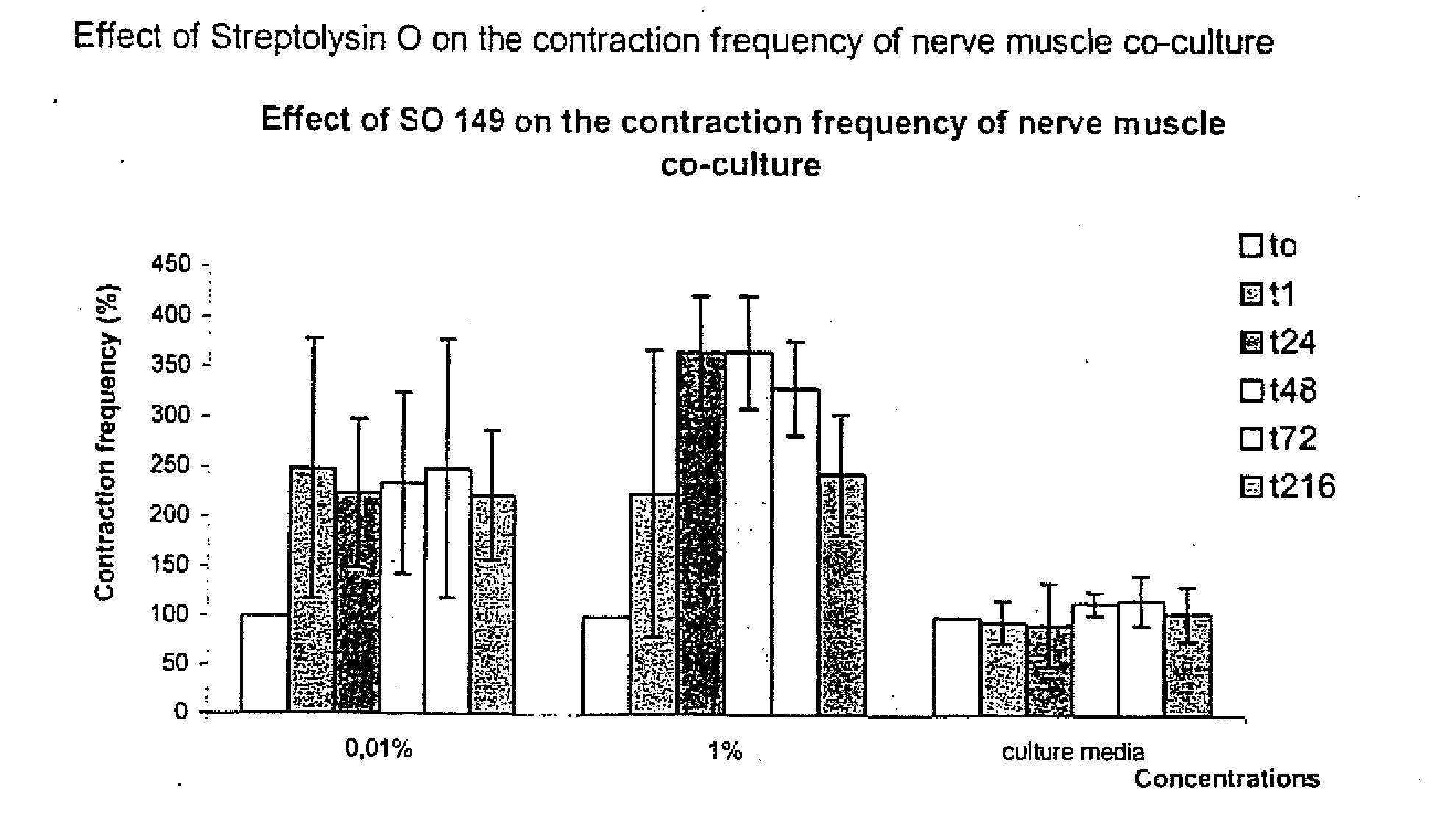
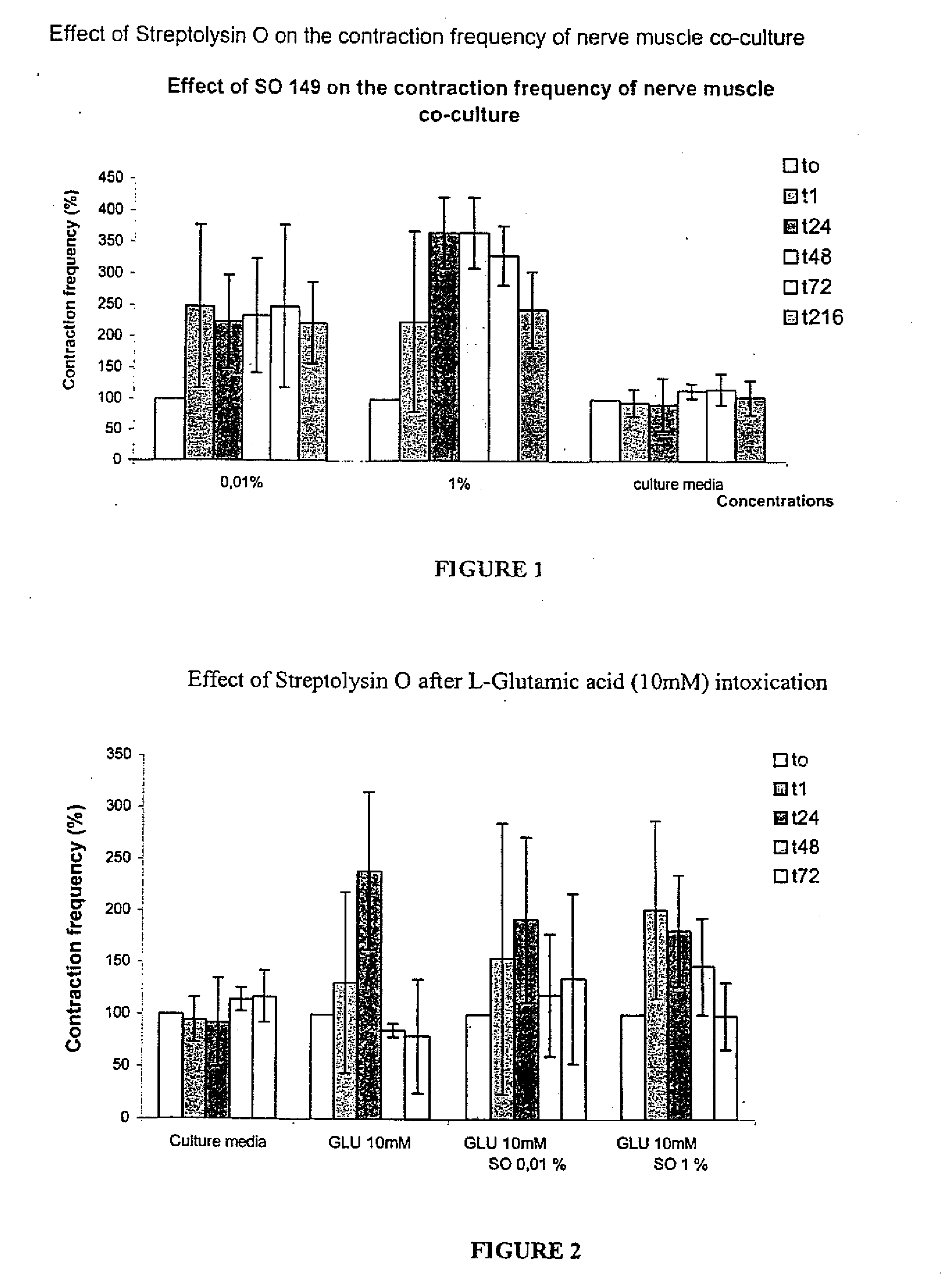
Methods of Inhibiting Metastatic Cancer by Administration of Streptolysin O
The invention provides a method for administering streptolysin O for treatment of various conditions including connective tissue disorders, reproductive fibroses and conditions mediated by the CD44 receptor. The invention also provides methods for protecting nerve cells from the effects of neurotoxic agents by the administration of streptolysin O.
Owner:BEECH TREE LABS
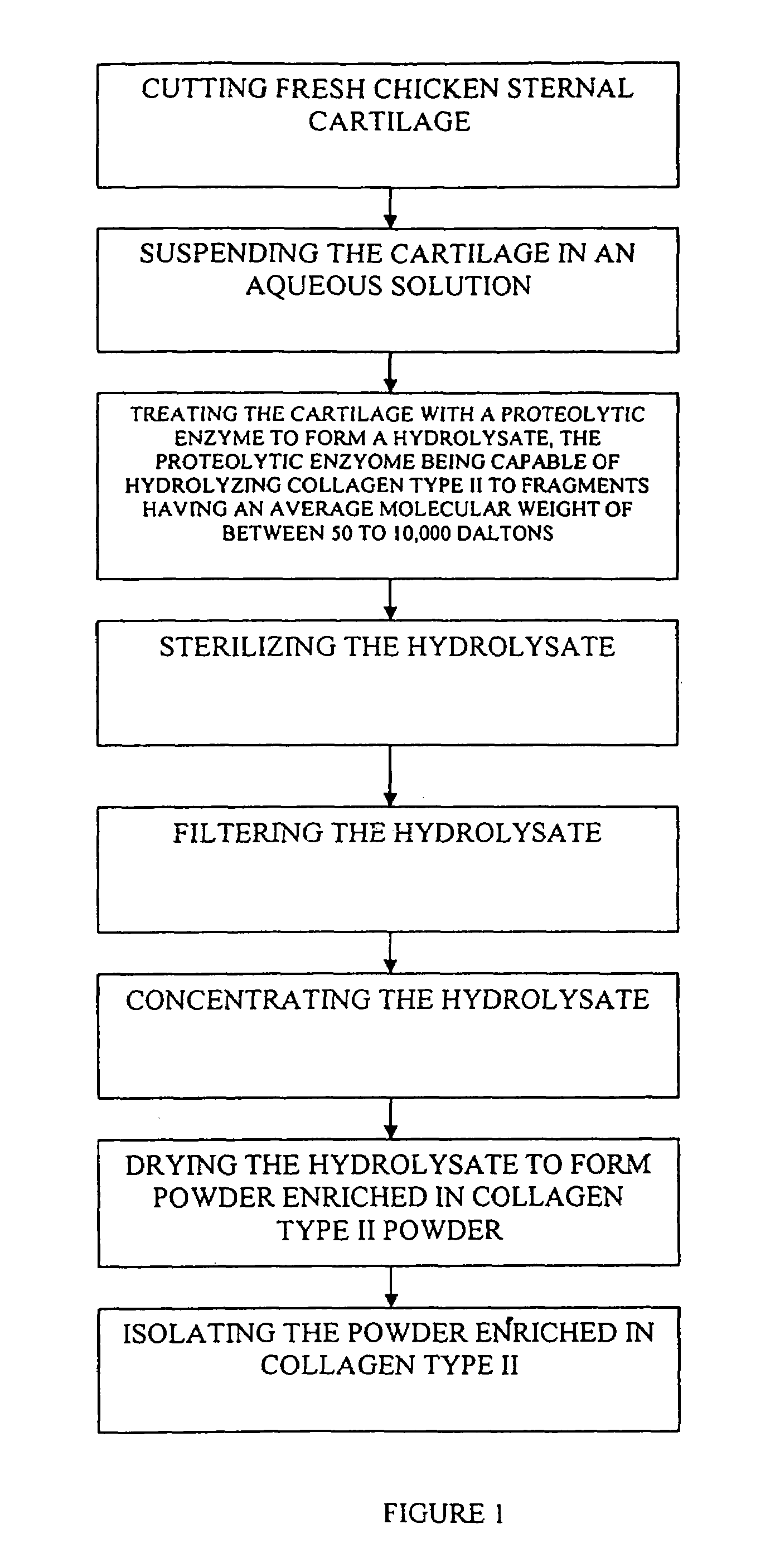
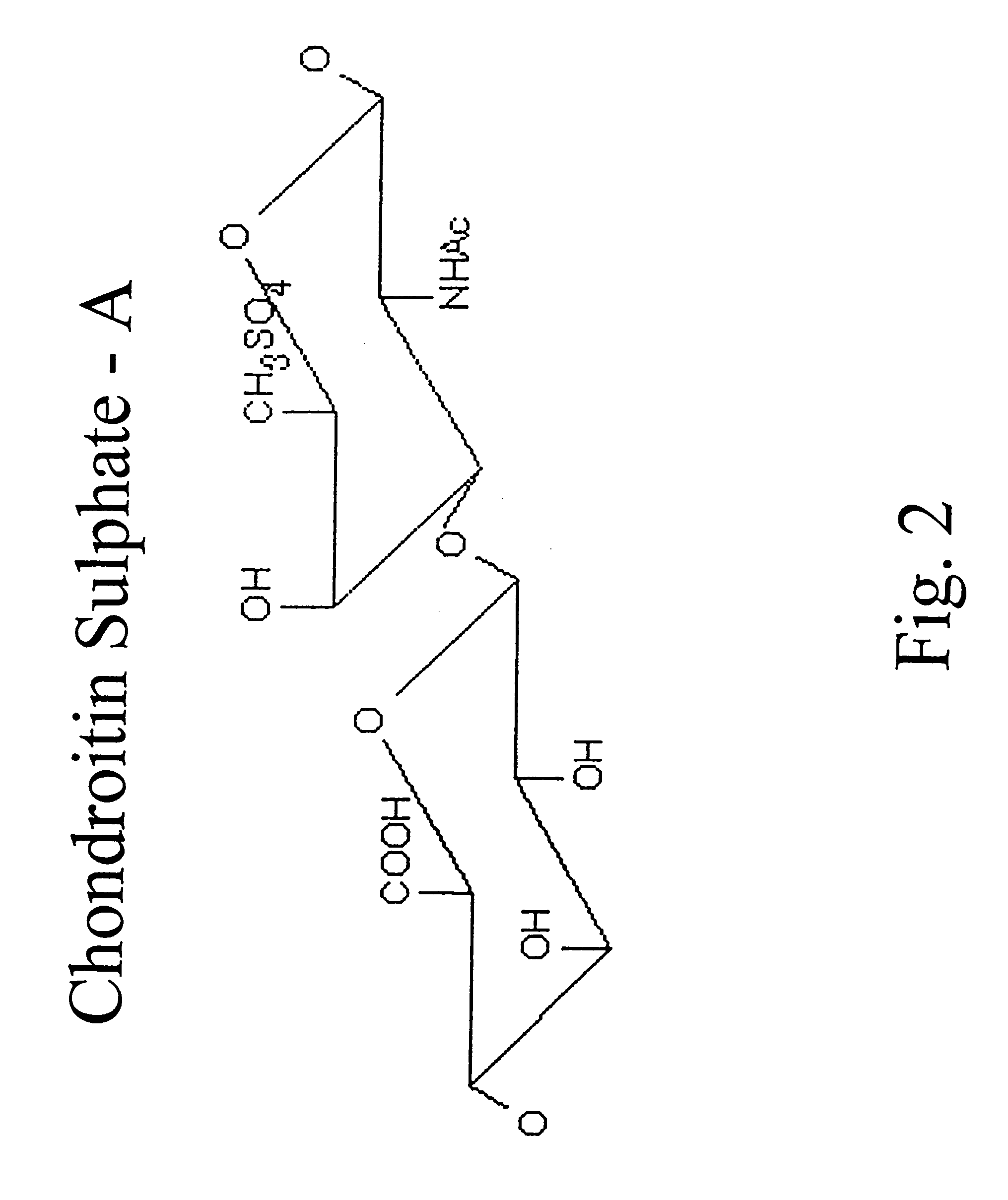
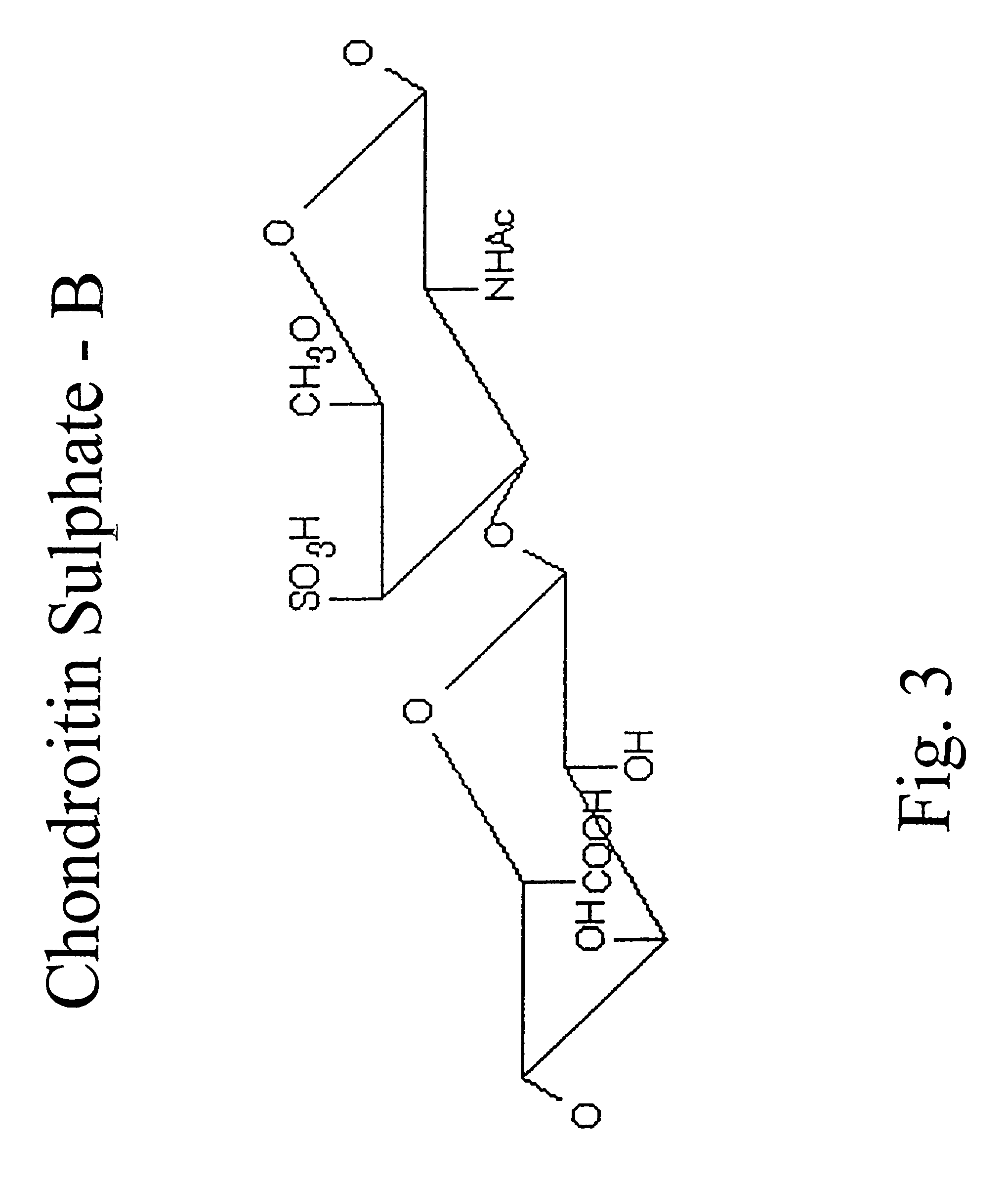
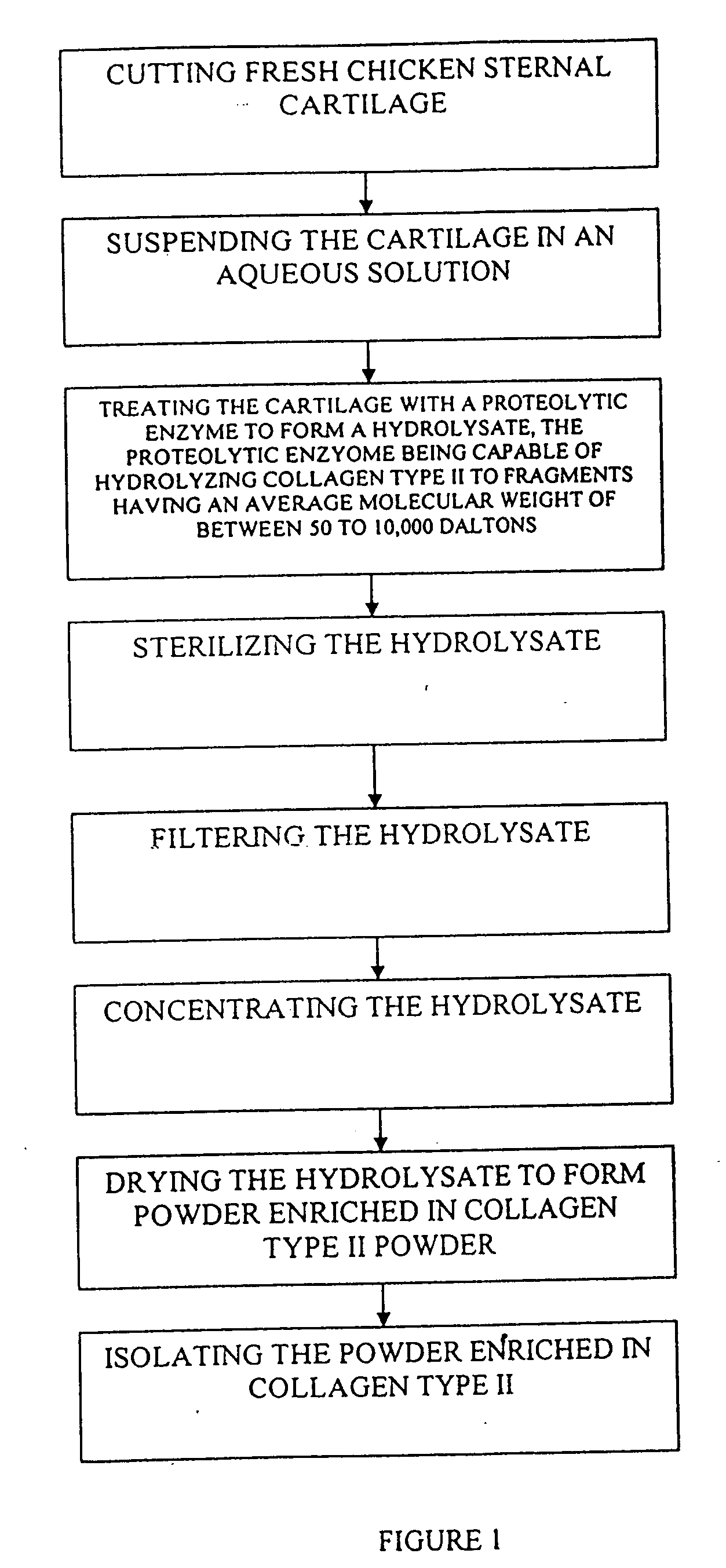
Hyaluronic acid and chondroitin sulfate based hydrolyzed collagen type II and method of making same
InactiveUS7091180B2Readily absorb into the gastrointestinal tract of an individualReduce molecular weightConnective tissue peptidesHydrolysed protein ingredientsConnective tissueHydrolyzed collagen
Hydrolyzed collagen type II powder compositions for inducing cartilage formation in an individual, method of preparing the compositions and use of the compositions in treating connective tissue disorder, replenishing skin viscoelasticity. The compositions are administered through an orally ingestible delivery medium for absorption into the gastrointestinal tract. The compositions are administered through a topical delivery medium for absorption into a dermis of the individual.
Owner:BIOCELL TECH
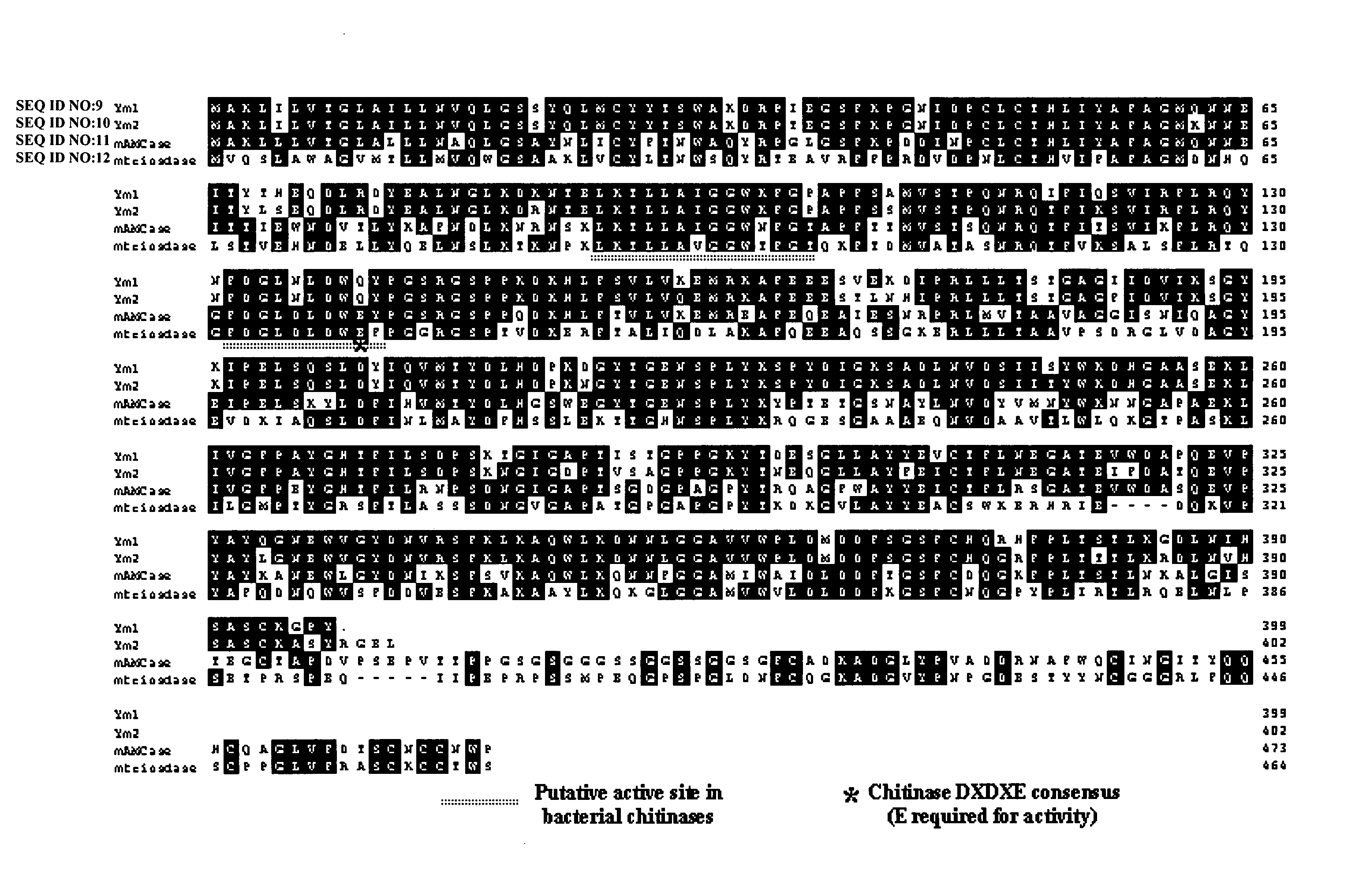
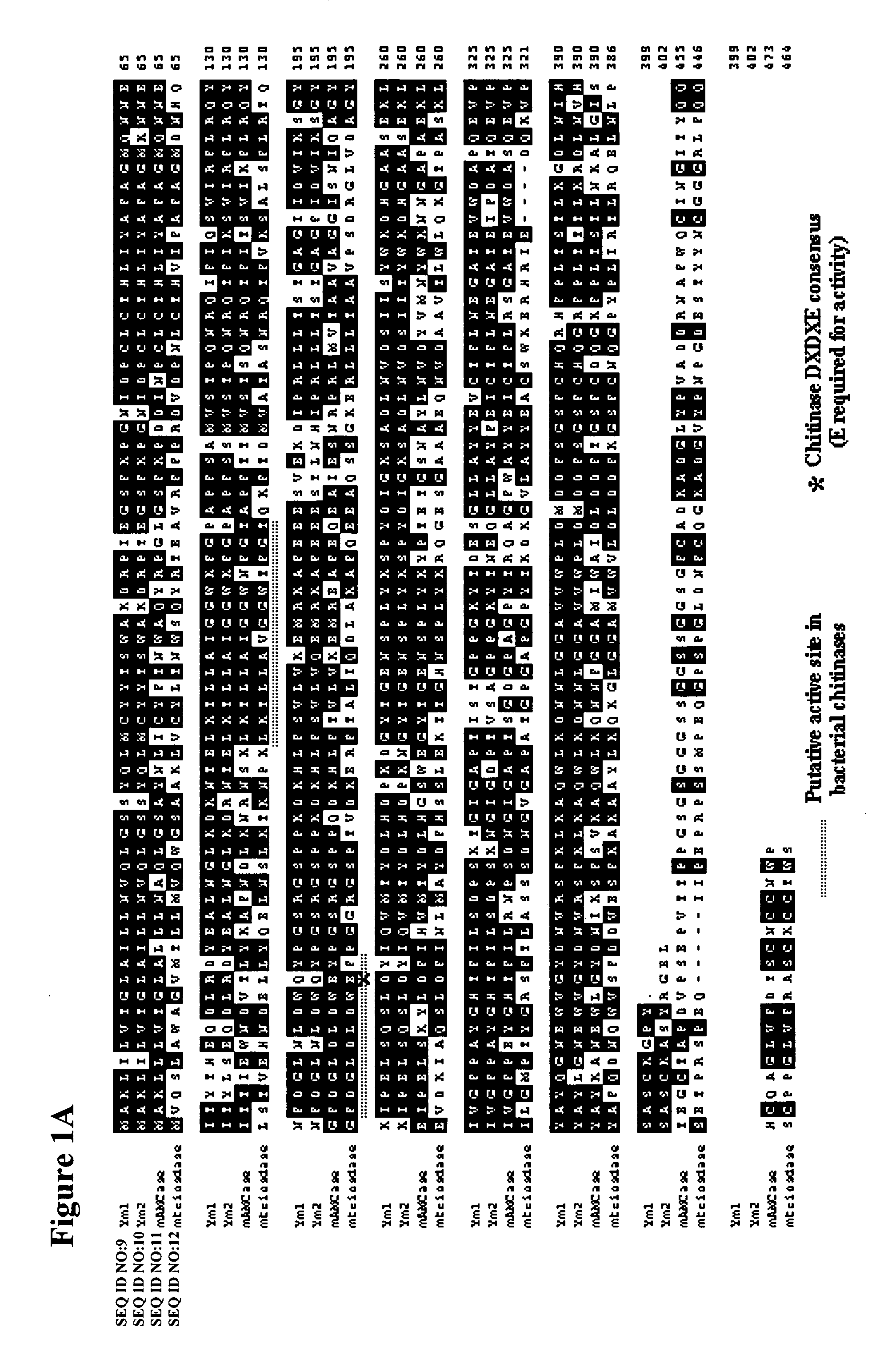
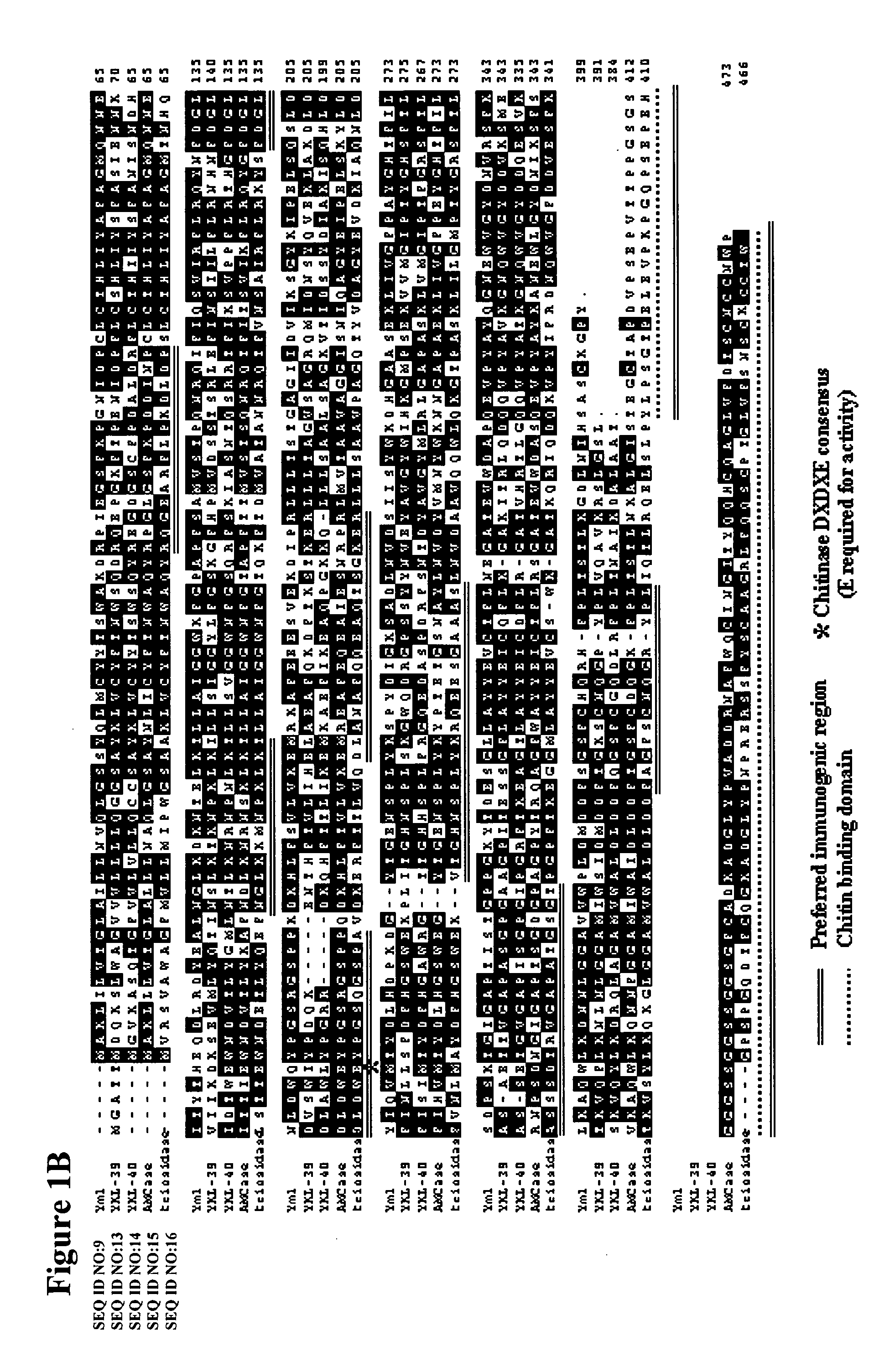
Methods and compositions relating to chitinases and chitinase-like molecules and modulation of osteoclasts
InactiveUS20050214297A1Antibody ingredientsImmunoglobulins against enzymesChitinaseOsteoclast maturation
The invention relates to the novel discovery that inhibiting a C / CLP modulates (e.g., inhibits) inflammation, as well as, bone and tissue destruction in arthritis. The present invention relates to compositions and methods for the treatment of bone metabolism and connective tissue disorders and diseases (e.g., bone metabolism disorders, osteoarthritis, psoriatic arthritis, rheumatoid arthritis, and osteoporosis) by modulating (e.g., inhibiting) at least one C / CLP. In particular where the inhibition of at least one C / CLP results in the inhibition of osteoclast activity, including but not limited to, osteoclast maturation, osteoclast differentiation, osteoclast proliferation, and osteoclastogenesis. One particular embodiment of the invention relates to inhibiting the expression or activity of one or more C / CLPs.
Owner:MEDIMMUNE LLC
Composition and method for treating and preventing musculoskeletal and connective tissue disorders
ActiveUS20090192201A1Promote angiogenesisPromote blood circulationBiocideInorganic active ingredientsEtiologyDisease
The present invention relates to a pharmaceutical composition and method for treating and preventing musculoskeletal and connective tissue diseases of unknown etiology, such as different forms of arthritis and other rheumatic conditions, comprising a combination of therapeutic agents that improve the processes of blood circulation and angiogenesis in the affected tissues, as well as other supporting therapies. Among the components of herein proposed pharmaceutical composition, are: vitamin K, polyunsaturated fatty acids (blood thinner), and niacin (vasodilator and hypolipidemic agent).
Owner:SELMAN HOUSEIN SOSA GUILLERMO
Treatment of connective tissue diseases of the skin
ActiveUS20060235048A1Strong therapeutic potentialEasy to optimizeBiocideHydroxy compound active ingredientsDiseaseSkin sensitization
The present invention provides effective and safe medicaments for the treatment of connective tissue diseases of the skin, particularly with respect to the treatment of cutaneous forms of Lupus Erythematous. The medicaments comprise as the therapeutically active ingredient a beta2 adrenoceptor agonist. The invention furthermore relates to dermatological compositions without skin sensitization properties and which contain enantiomerically pure or enriched R-enantiomers of a beta2 adrenoceptor agonist.
Owner:ASTION PHARMA AS
Hyaluronic acid and chondroitin sulfate based hydrolyzed collagen type II and method of making same
InactiveUS20060045920A1Reduce molecular weightReadily absorb into the gastrointestinal tract of an individualHydrolysed protein ingredientsSkeletal disorderDiseaseViscoelasticity
Hydrolyzed collagen type II powder compositions for inducing cartilage formation in an individual, method of preparing the compositions and use of the compositions in treating connective tissue disorder, replenishing skin viscoelasticity. The compositions are administered through an orally ingestible delivery medium for absorption into the gastrointestinal tract. The compositions are administered through a topical delivery medium for absorption into a dermis of the individual.
Owner:BIOCELL TECH
Method of treatment of conditions by administration of streptolysin O
Method for administering streptolysin O for treatment of various conditions including connective tissue disorders, reproductive fibroses and conditions mediated by the CD44 receptor. The invention also provides methods for protecting nerve cells from the effects of neurotoxic agents by the administration of streptolysin O.
Owner:MILKHAUS LAB
Identification of a novel retrovirus associated with primary biliary cirrhosis and autoimmune disorders
InactiveUS6468737B1Reduced responseReactivity is smallOrganic active ingredientsAntipyreticAutoimmune thyroiditisS syndrome
The present invention relates, first, to the discovery, identification, and characterization of novel nucleic acid molecules, that are associated with PBC, Sjögren's syndrome, scleroderma, SLE, autoimmune thyroiditis and various other connective tissue disorders. The novel nucleotide sequences of the present invention are retroviral in origin and are indicative of a PBC retrovirus which bears a strong correlation with PBC. The present invention is based, in part, on the Applicant's data which is the first evidence to suggest that PBC patient's tissue may harbor a transmissible agent. The association of a retroviral infectious agent with PBC was first demonstrated by Applicants in vitro by co-culture of periportal lymph nodes derived from patients at time of transplantation and healthy biliary epithelium cells. The Applicant's discoveries as described herein, report the characterization of PBC-associated infectious agent as retroviral as demonstrated by electron microscopy and immunoblot reactivity. In addition, Applicants have characterized novel nucleotide sequences which are associated with the PBC-associated retrovirus.
Owner:ALTON OCHSNER MEDICAL FOUND
Method of treatment of connective tissue disorders by administration of streptolysin O
ActiveUS6998121B2Relieve symptomsEffective treatmentNervous disorderPeptide/protein ingredientsSpinal claudicationConnective tissue
Method for administering streptolysin O to treat various connective tissue disorders in humans and animals such as Dupuytren's contracture, scleroderma, Peyronie's disease, mastistis in animals, and claudication due to peripheral arterial disease.
Owner:MILKHAUS LAB
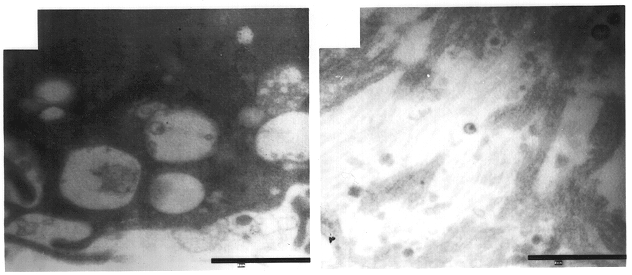
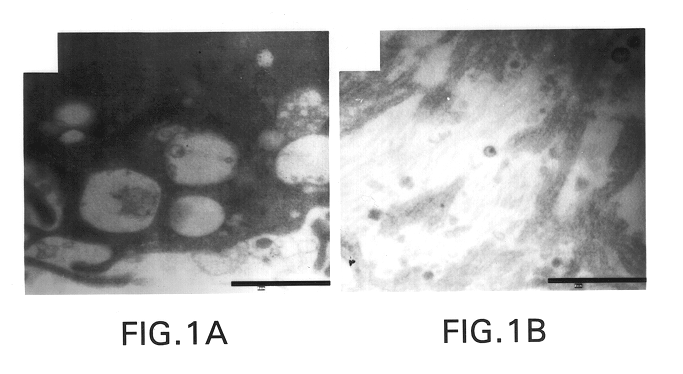
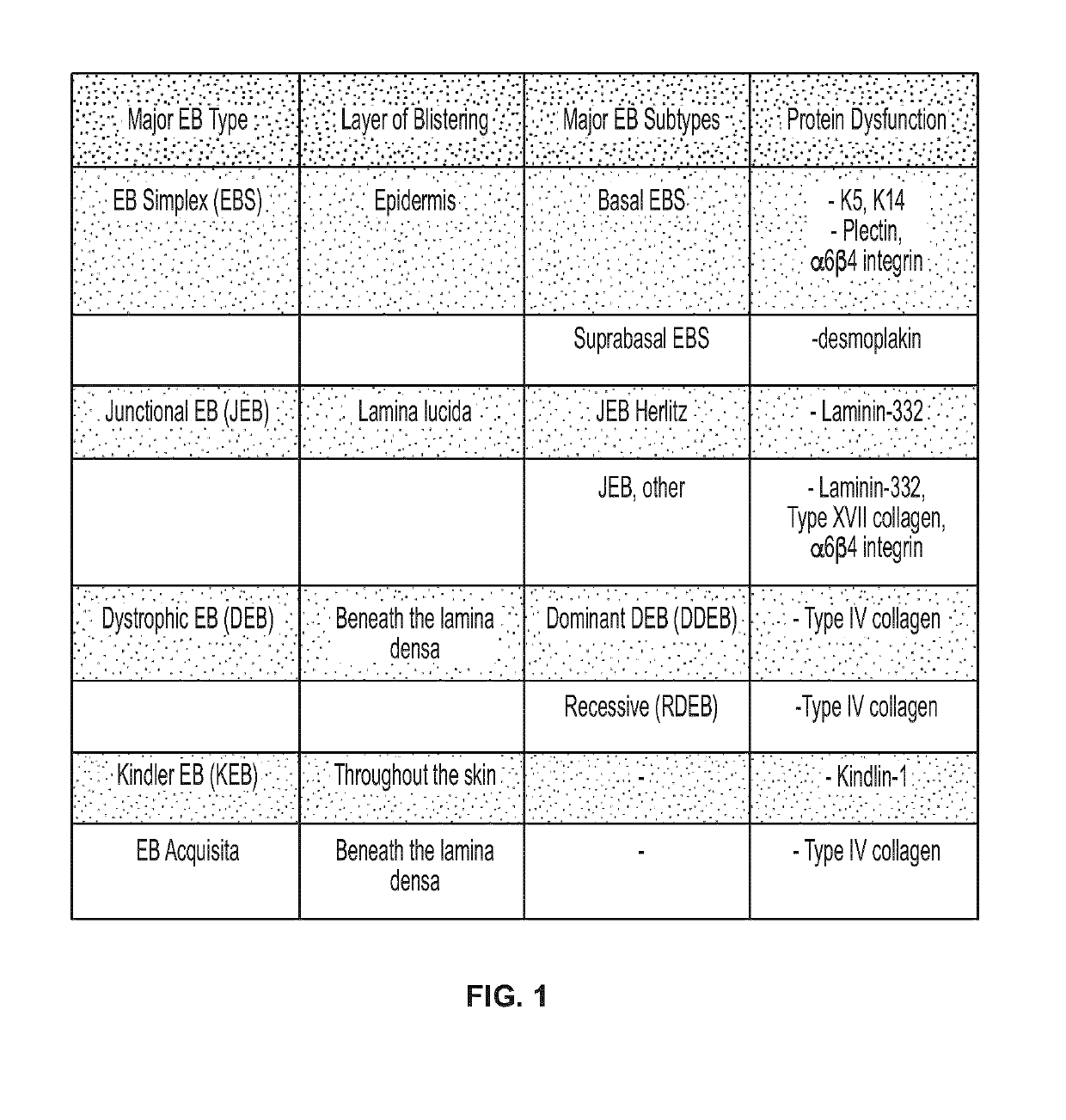
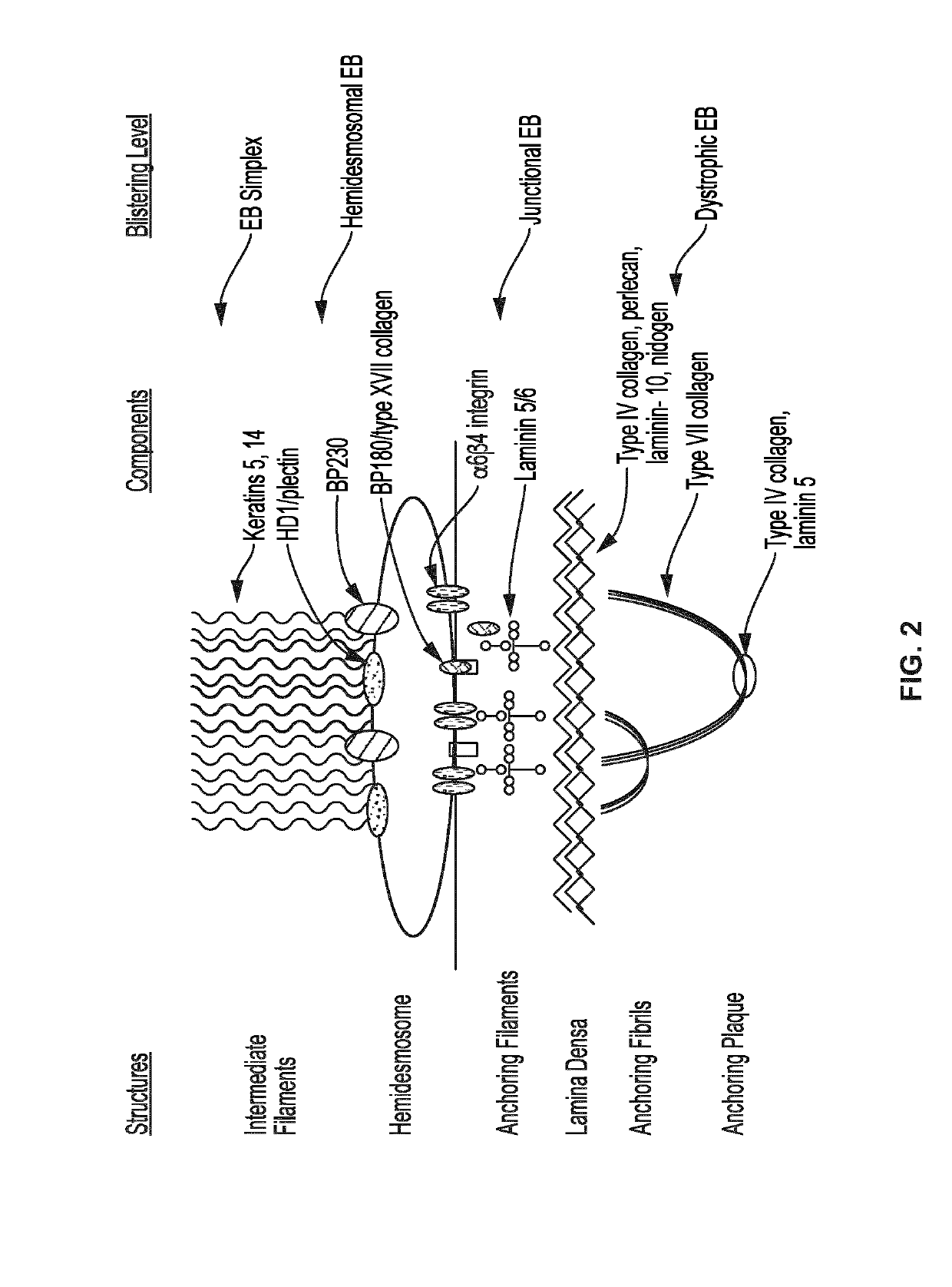
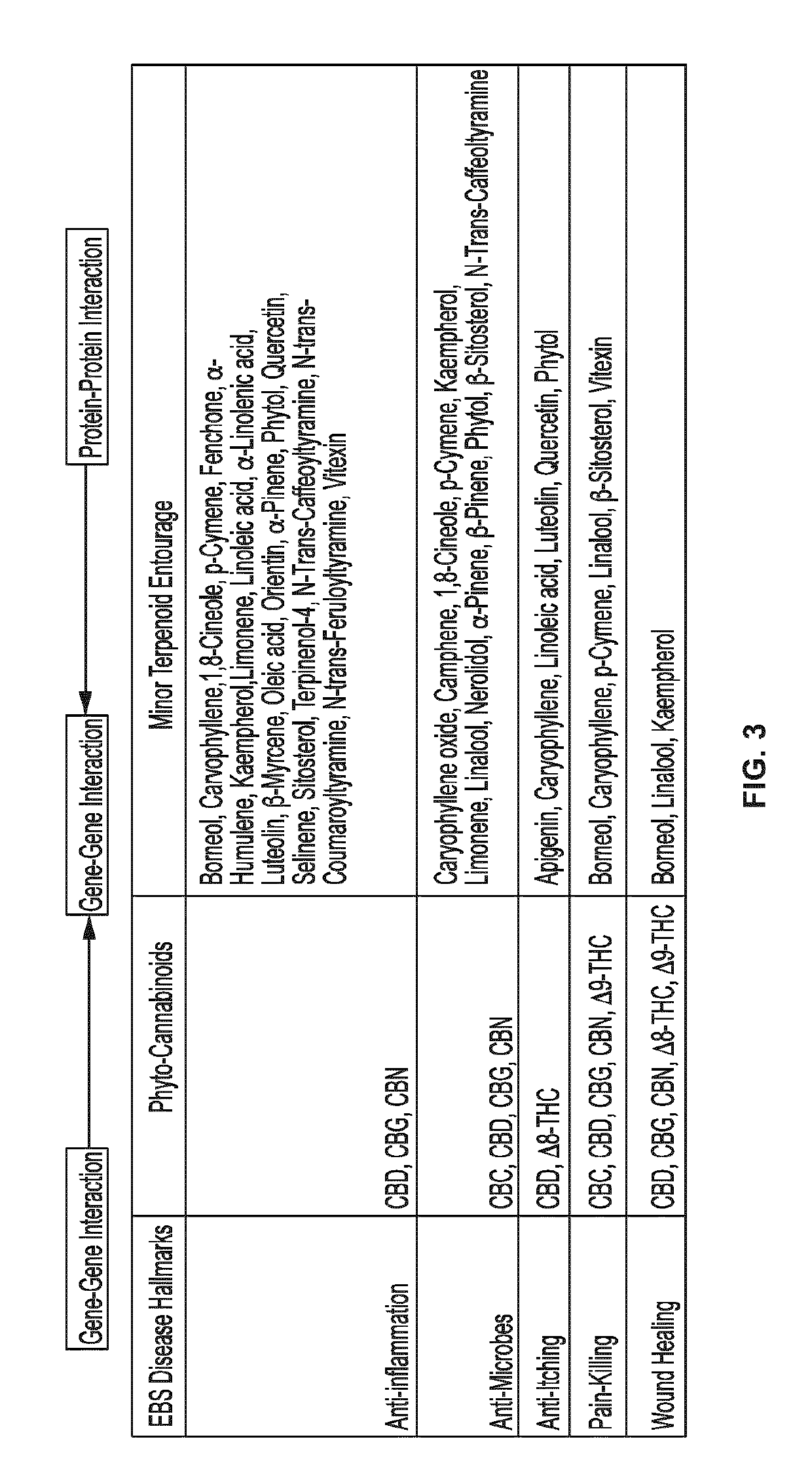
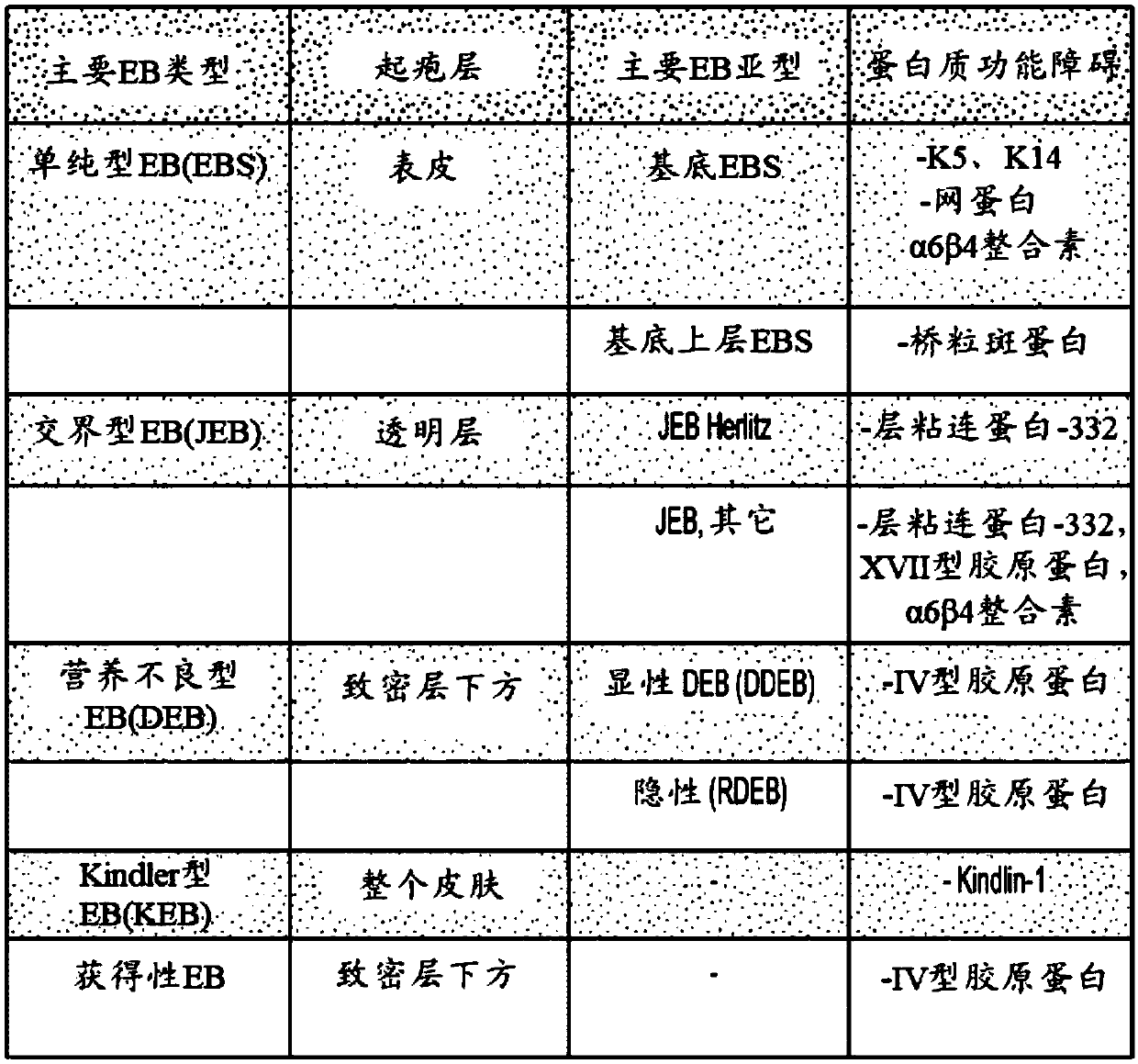
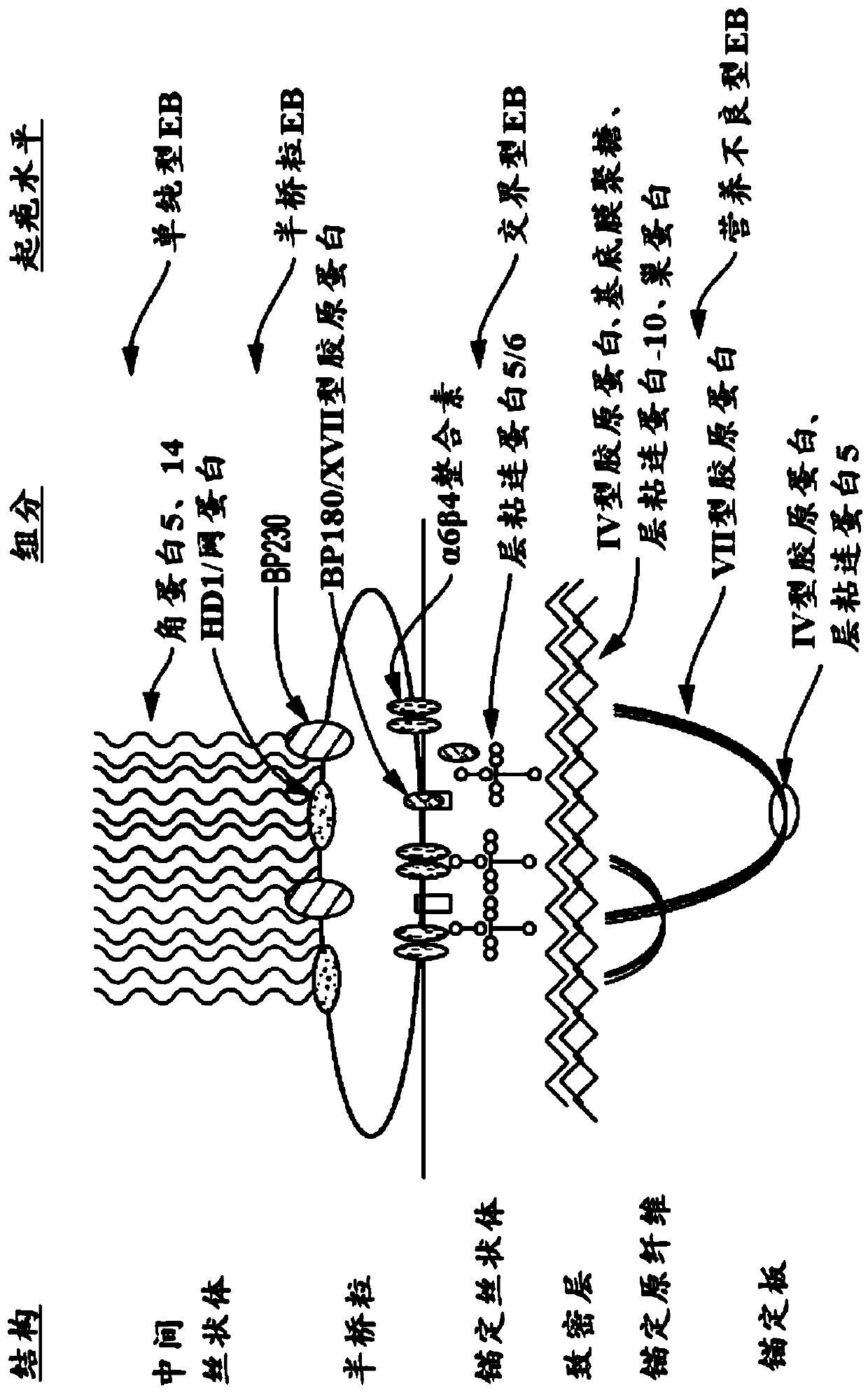
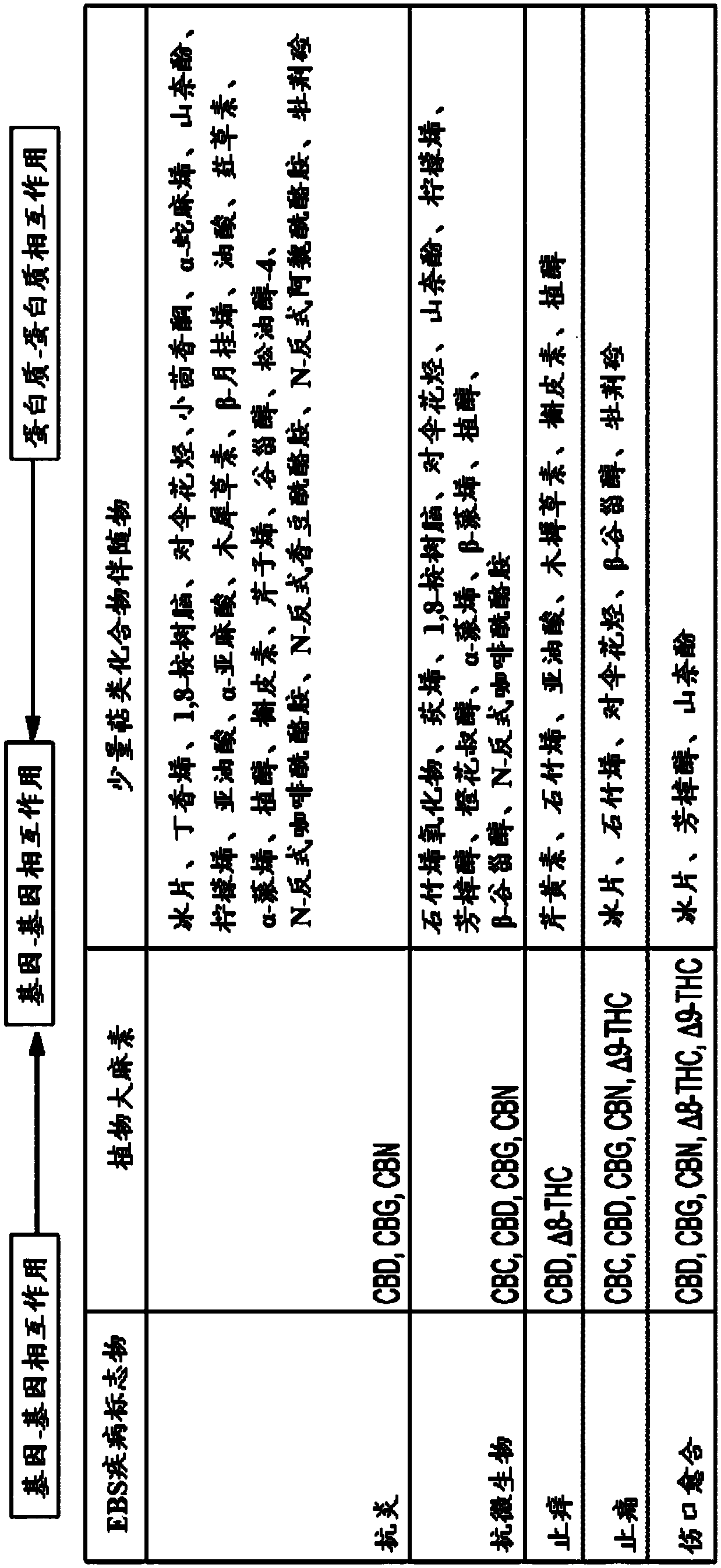
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
PendingUS20190142788A1Reduce inflammationPromote regenerationHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissueIntermediate filament
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such that loss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:INMED PHARMA INC
Composition and method for treating and preventing musculoskeletal and connective tissue disorders
ActiveUS8211947B2Increased riskPromote angiogenesisBiocideInorganic active ingredientsEtiologyDisease
The present invention relates to a pharmaceutical composition and method for treating and preventing musculoskeletal and connective tissue diseases of unknown etiology, such as different forms of arthritis and other rheumatic conditions, comprising a combination of therapeutic agents that improve the processes of blood circulation and angiogenesis in the affected tissues, as well as other supporting therapies. Among the components of herein proposed pharmaceutical composition, are: vitamin K, polyunsaturated fatty acids (blood thinner), and niacin (vasodilator and hypolipidemic agent).
Owner:SELMAN HOUSEIN SOSA GUILLERMO
Enoxaparin and methods of its use
InactiveUS20020128226A1Skeletal disorderCarbohydrate active ingredientsOsteoarthrosesLigament structure
This present invention discloses inhibitors of matrix metalloproteinases, and novel methods of their use. The inhibitors may be useful for treating conditions that involve enhanced activity of at least one of the matrix metalloproteinases neutrophil collagenase (MMP-8), aggrecanase, hADAMTS1, and gelatinase A (MMP-2). Such disorders may include, but are not limited to, degenerative joint disorders (e.g., osteoarthroses), spondyloses, chondrolysis associated with joint trauma or prolonged joint immobilization (often occurring after meniscus or patellar injuries or ligament tears), connective tissue disorders (e.g., collagenoses), wound healing disturbances, periodontal disorders, chronic disorders of the locomotor system (e.g., inflammatory, immunologically, or metabolism-related acute and chronic arthritides), arthropathies, myalgias, or disturbances of bone metabolism.
Owner:SANOFI AVENTIS DEUT GMBH
Topical therapeutic formulations
The invention provides topical compositions and methods for using the compositions. The compositions can be used for the treatment of fibrotic or connective tissue disorders involving scarring, sub-dermal plaque accumulations, or fibrosis of muscle tissue. The disorders can be painlessly treated by the topical application of a composition described herein. One or more calcium channel blocker agents can serve as an active ingredient of the compositions, optionally in combination with, for example, one or more of emu oil and superoxide dismutase. The composition can further include pharmaceutically acceptable carriers that can facilitate the non-invasive transdermal delivery of the active(s) to subdermal sites.
Owner:HYBRID MEDICAL
Method of treatment of tendonitis by administration of streptolysin O
InactiveUS7629312B2Relieve symptomsEffective treatmentBiocidePeptide/protein ingredientsCD44Neurotoxicity
The invention provides a method for administering streptolysin O for treatment of various conditions including connective tissue disorders, reproductive fibroses and conditions mediated by the CD44 receptor. The invention also provides methods for protecting nerve cells from the effects of neurotoxic agents by the administration of streptolysin O.
Owner:MILKHAUS LAB
Topical therapeutic formulations
The invention provides topical compositions and methods for using the compositions. The compositions can be used for the treatment of fibrotic or connective tissue disorders involving scarring, sub-dermal plaque accumulations, or fibrosis of muscle tissue. The disorders can be painlessly treated by the topical application of a composition described herein. One or more calcium channel blocker agents can serve as an active ingredient of the compositions, optionally in combination with, for example, one or more of emu oil and superoxide dismutase. The composition can further include pharmaceutically acceptable carriers that can facilitate the non-invasive transdermal delivery of the active(s) to subdermal sites.
Owner:HYBRID MEDICAL
Method of Treatment of Tendonitis by Administration of Streptolysin O
InactiveUS20070219119A1Many symptomRelieve symptomsBiocidePeptide/protein ingredientsConnective Tissue DisorderCD44
The invention provides a method for administering streptolysin O for treatment of various conditions including connective tissue disorders, reproductive fibroses and conditions mediated by the CD44 receptor. The invention also provides methods for protecting nerve cells from the effects of neurotoxic agents by the administration of streptolysin O.
Owner:MILKHAUS LAB
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
InactiveCN109689045AHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissue fiberMedicine
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such thatloss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:因美制药公司
Method for treating body tissue disease with acoustic waves
A method for treating inflammatory musculoskeletal connective tissue disorders by exposing the sufferer to acoustic waves from a transducer immersed in liquid. The person is preferably placed between one and twenty feet from the wave source, and is preferably exposed to waves at a frequency of about 600 Hertz for approximately twenty five minutes.
Owner:MCCALL JAMES B
Topical therapeutic formulations
The invention provides topical compositions and methods for using the compositions. The compositions can be used for the treatment of fibrotic or connective tissue disorders involving scarring, sub-dermal plaque accumulations, or fibrosis of muscle tissue. The disorders can be painlessly treated by the topical application of a composition described herein. One or more calcium channel blocker agents can serve as an active ingredient of the compositions, optionally in combination with, for example, one or more of emu oil and superoxide dismutase. The composition can further include pharmaceutically acceptable carriers that can facilitate the non-invasive transdermal delivery of the active(s) to subdermal sites.
Owner:HYBRID MEDICAL
Proteolytic extract from bromelain for the treatment of connective tissue disorders
ActiveUS20140154229A1Simplifying clinical procedurePatient compliance is goodAntibacterial agentsPowder deliveryDiseaseConnective tissue
The present invention relates to a proteolytic extract obtained from bromelain for the treatment of connective tissue diseases. In particular, the present invention relates to a pharmaceutical composition that includes proteolytic extract obtained from bromelain for the treatment of diseases such as Dupuytren's disease and Peyronie's disease.
Owner:MEDIWOUND
Method of potentiating the therapeutic action of monoclonal and polyclonal antibodies
InactiveUS20060099208A1Improve anti-cancer effectImprove actionBiocideAntibody ingredientsDiseaseConjugated linoleic acid
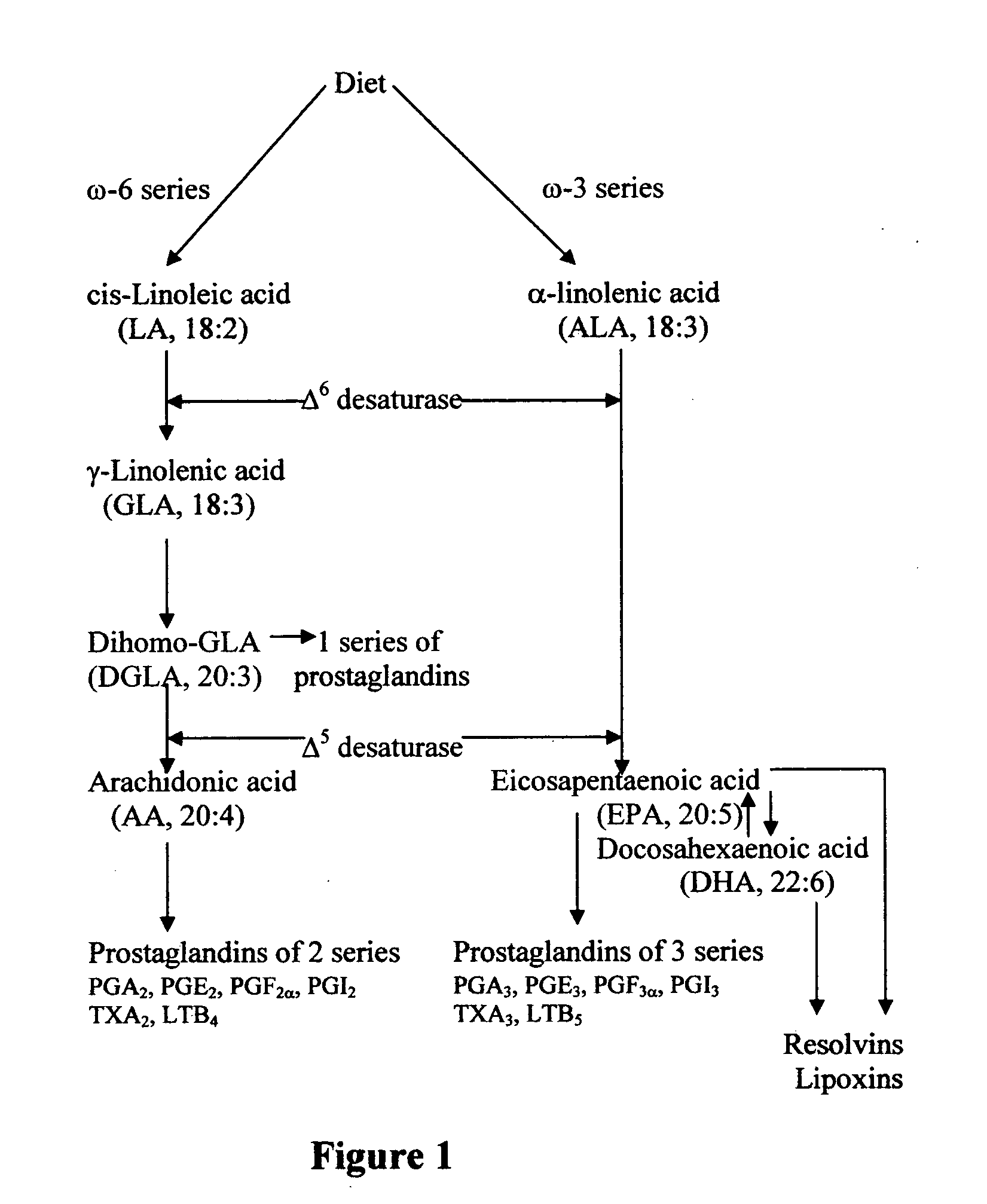
A method of potentiating the action of monoclonal / polyclonal antibodies against growth factors and their receptors by coupled conjugation with EFAs / PUFAs (optionally with a lymphographic agent) in the prevention and / or treatment of cancer, and inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, progressive systemic sclerosis, mixed connective tissue disorder, vasculitis. The disorders may be due to uncontrolled angiogenic activity causing proliferative diabetic retinopathy, other eye disorders such as macular degeneration, skin problems such as psoriasis, renal conditions such as proliferative glomerulonephritis, lymphoma and leukemias. The EFAs / PUFAs may be chosen from linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid, alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, cis-parinaric acid, docosapentaenoic acid and conjugated linoleic acid. The method selectively reduces blood supply to a neoplastic region, such as a tumor region, by causing occlusion of blood vessels feeding the tumor region. The invention also provides solutions / salts of PUFAs, in combination with a lymphographic agent.
Owner:UNDURTI DAS NARASIRMHA
Liposomal corticosteroids for treatment of inflammatory disorders in humans
InactiveCN104144679AEfficient removalOvercome inherent problemsOrganic active ingredientsAntipyreticLipid formationCorticosteroid preparation
The invention relates to a pharmaceutical composition comprising liposomes composed of non-charged vesicle-forming lipids, optionally including not more than 10 mole percent of negatively charged vesicle- forming lipids and / or not more than 10 mole percent of PEGylated lipids, the liposomes having a selected mean particle diameter in the size range of 40-200 nm and comprising a first corticosteroid in water soluble form, for the site-specific treatment of inflammatory disorders in humans, providing in human patients a fast, strong, and durable anti-inflammatory effect for at least 2 weeks at a dose of at most 5 mg / kg body weight of prednisolone or an equipotent dose corticosteroid other than prednisolone at a treatment frequency of at most once per two weeks. Furthermore the present invention relates to the application of the above-mentioned pharmaceutical composition given as intervention therapy in inflammatory disorders such as rheumatic disease or a related inflammatory connective tissue disorder, inflammatory diseases of the kidney or inflammatory bowel disorders, in combination with chronic therapy with a second free corticosteroid formulation or in combination with chronic treatment with a disease- modifying agent such as methotrexate.
Owner:ENCELADUS PHARMA BV
Treatment and/or prevention of cancer and/or arthritis
Owner:MERCK SERONO SA
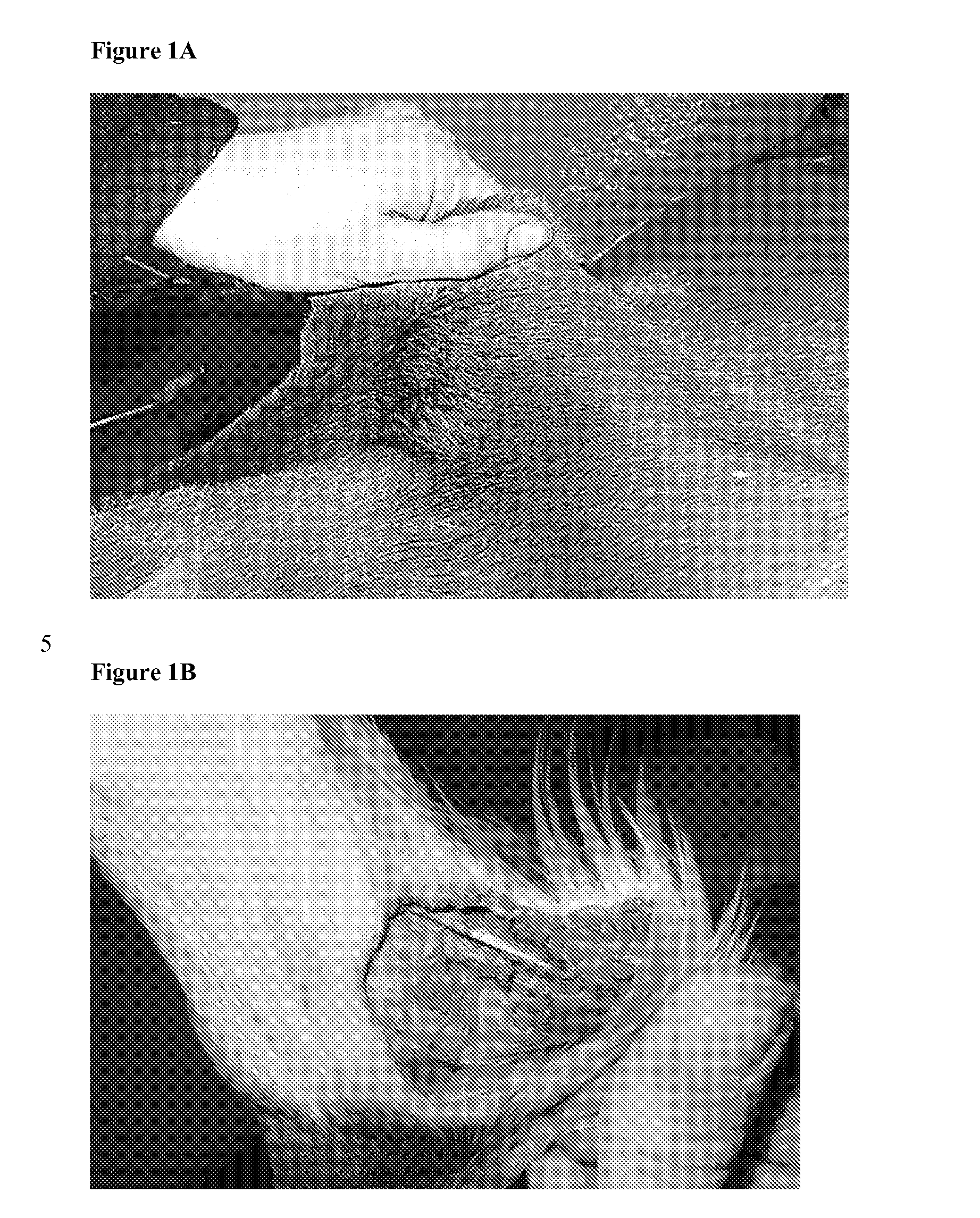
Identification of the causative mutation for inherited connective tissue disorders in equines and methods for testing for same
InactiveUS20140186835A1Sugar derivativesMicrobiological testing/measurementLYSYL HYDROXYLASE 1Connective tissue
Provided is a description of a mutation which is positively correlated with Warmblood Fragile Foal Syndrome Type 1 (WFFST1). The mutation is a G to A change at a specific location in the equine lysyl hydroxylase 1 (LH1) gene. Compositions and methods for use in diagnosing WFFST1 are provided.
Owner:CORNELL UNIVERSITY
Compositions and methods for treating cutaneous fibrosis
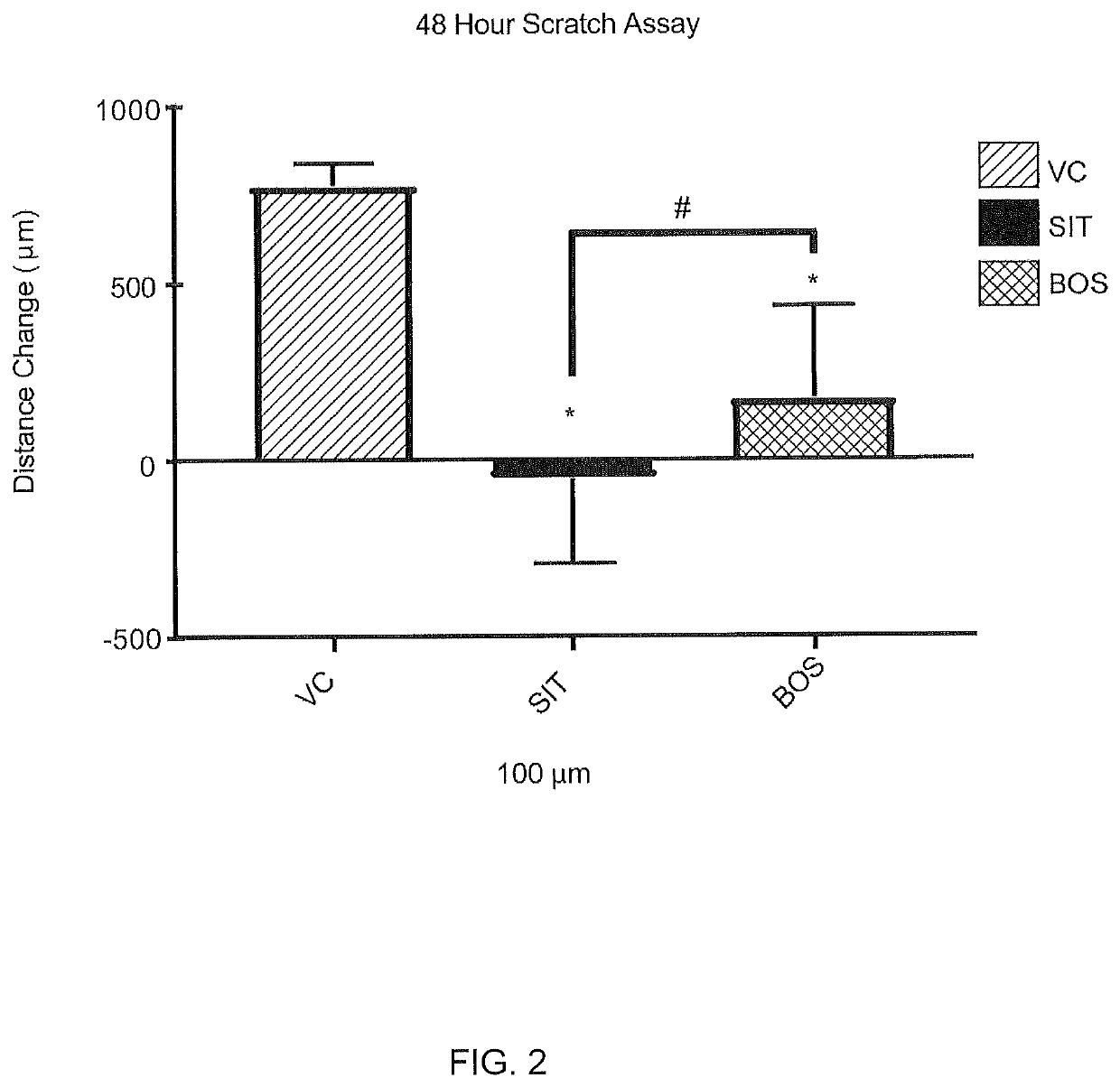
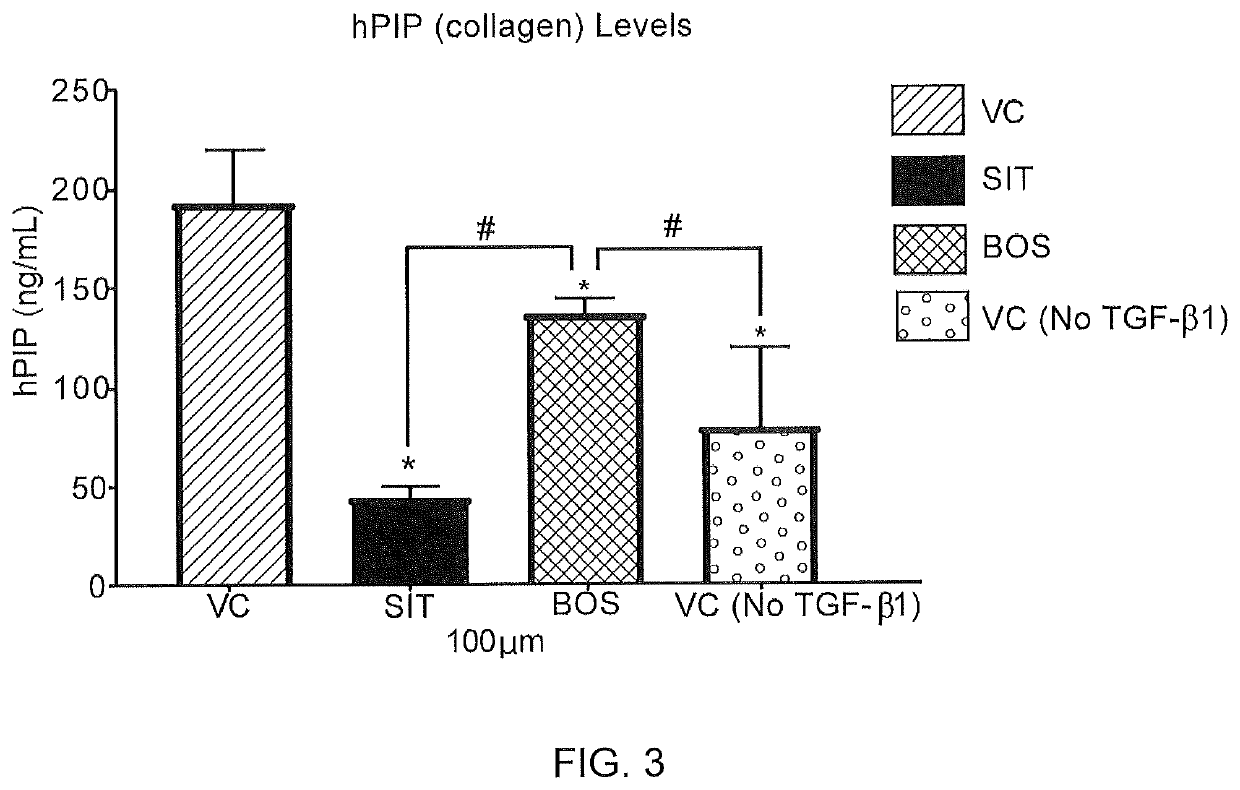
PendingUS20210038570A1Decreasing production of collagenReduced viabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseFibrosis
The present invention relates to local or topical compositions containing a therapeutically effective amount of a selective endothelin-A (ET-A) receptor antagonist or inhibitor, preferably sitaxentan, and pharmaceutically acceptable salts thereof. The compositions are useful for treating a patient that has a condition involving cutaneous fibrosis or connective tissue disease.
Owner:TIMBER PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com