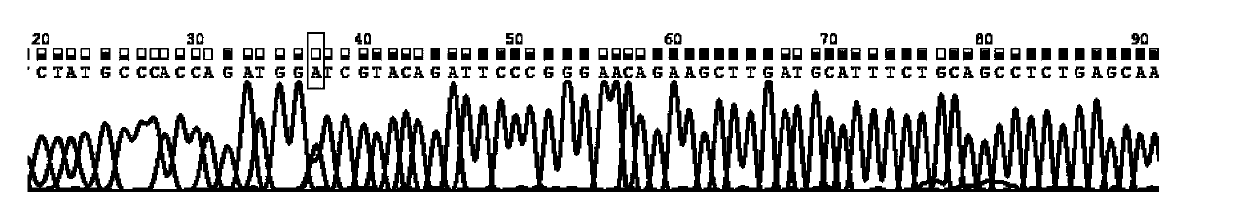
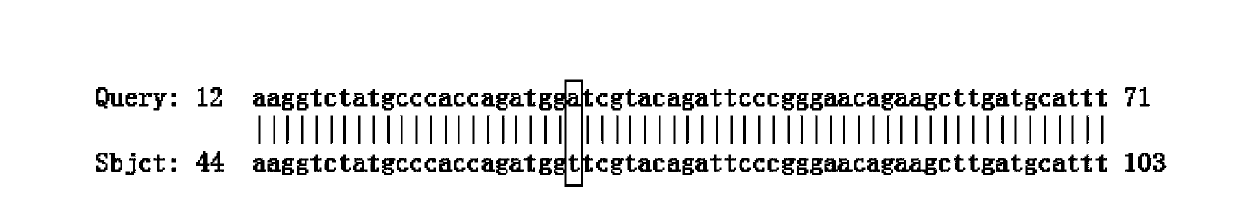
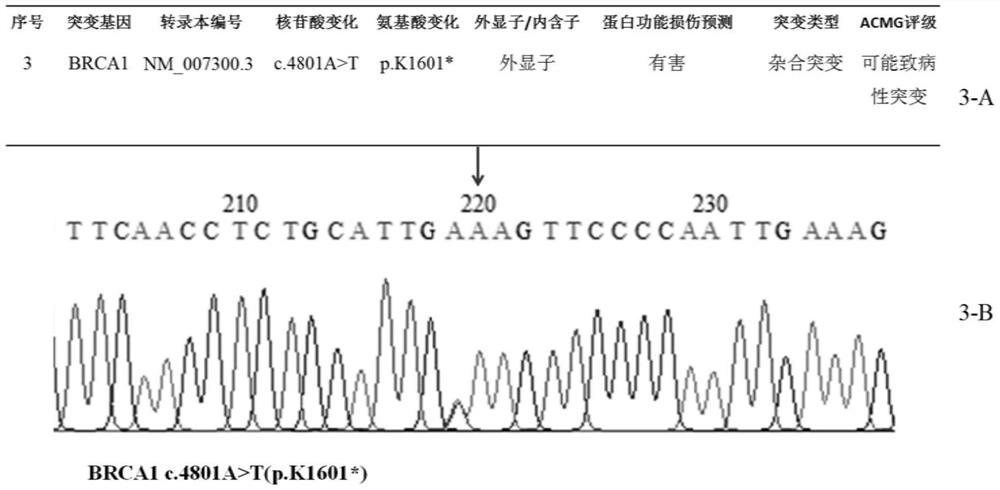
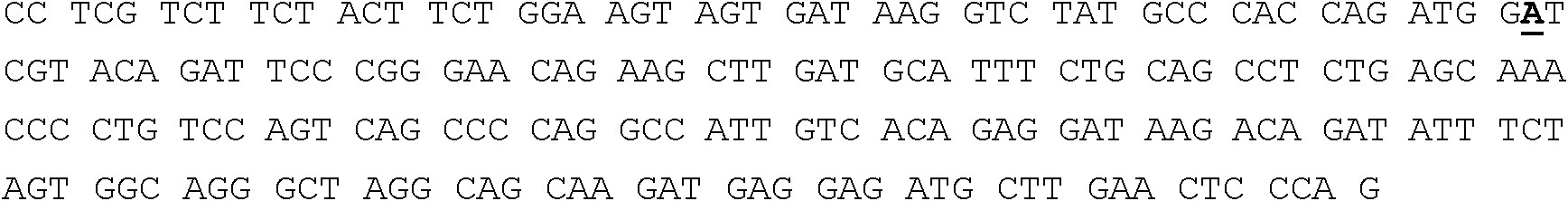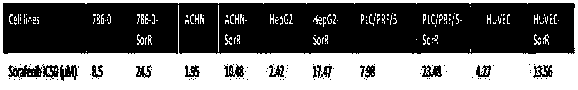Patents
Literature
47 results about "MLH1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
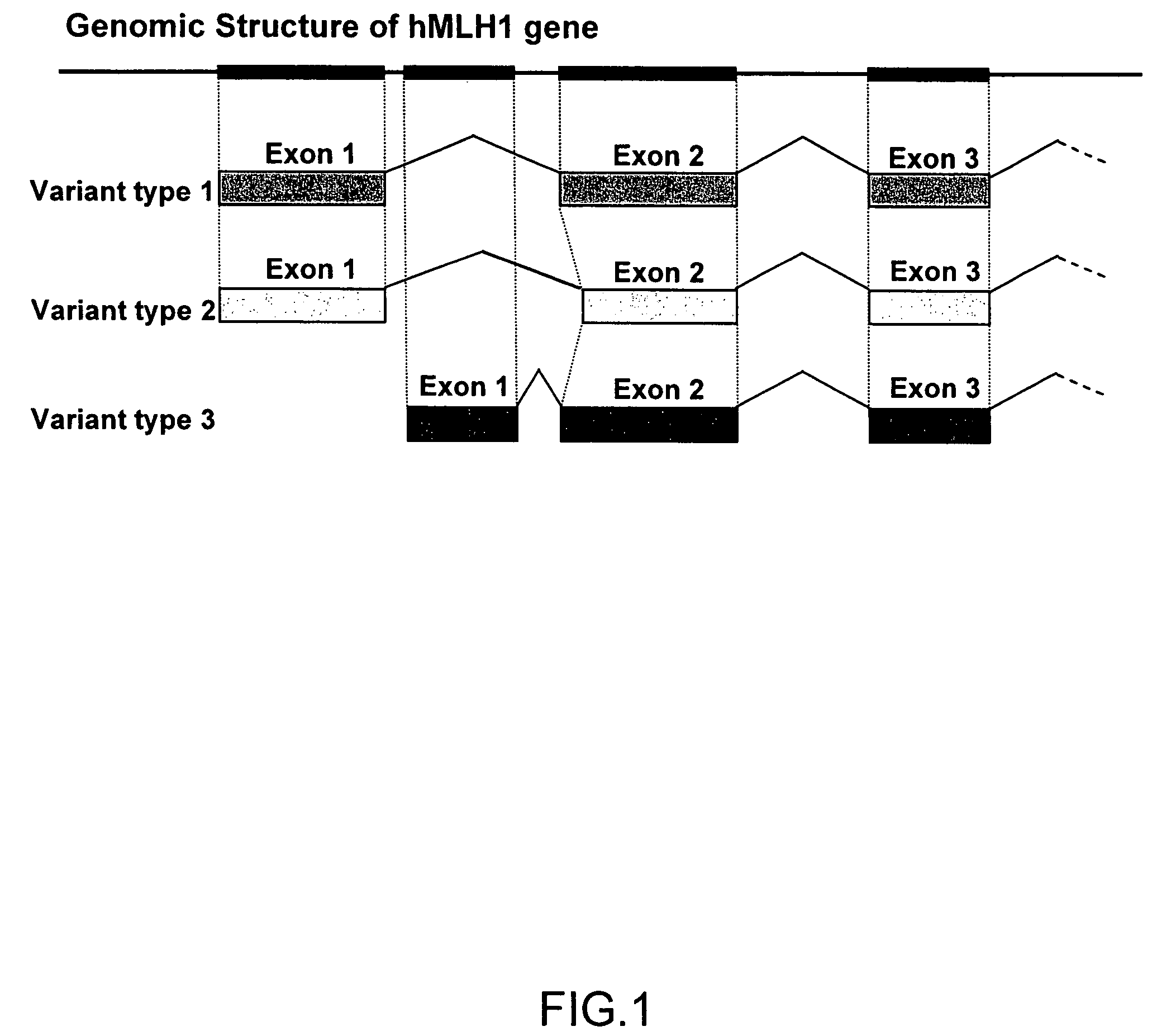
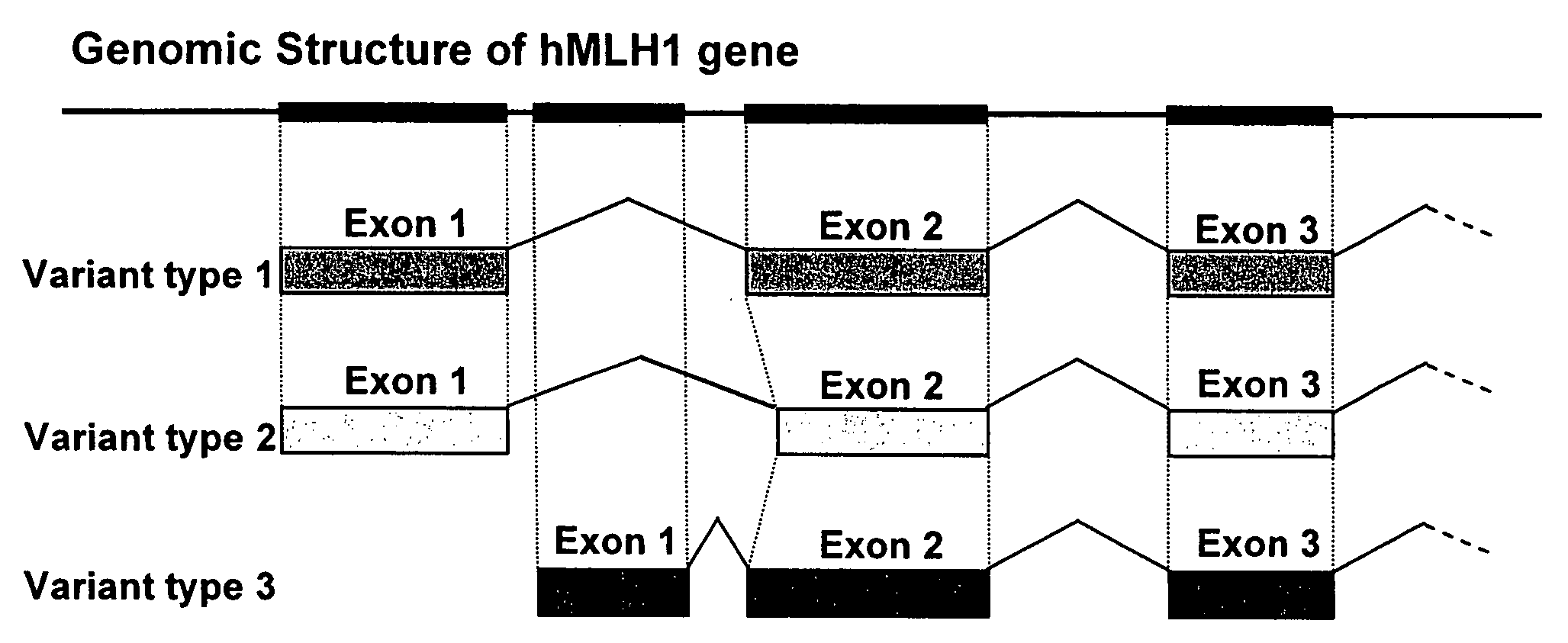
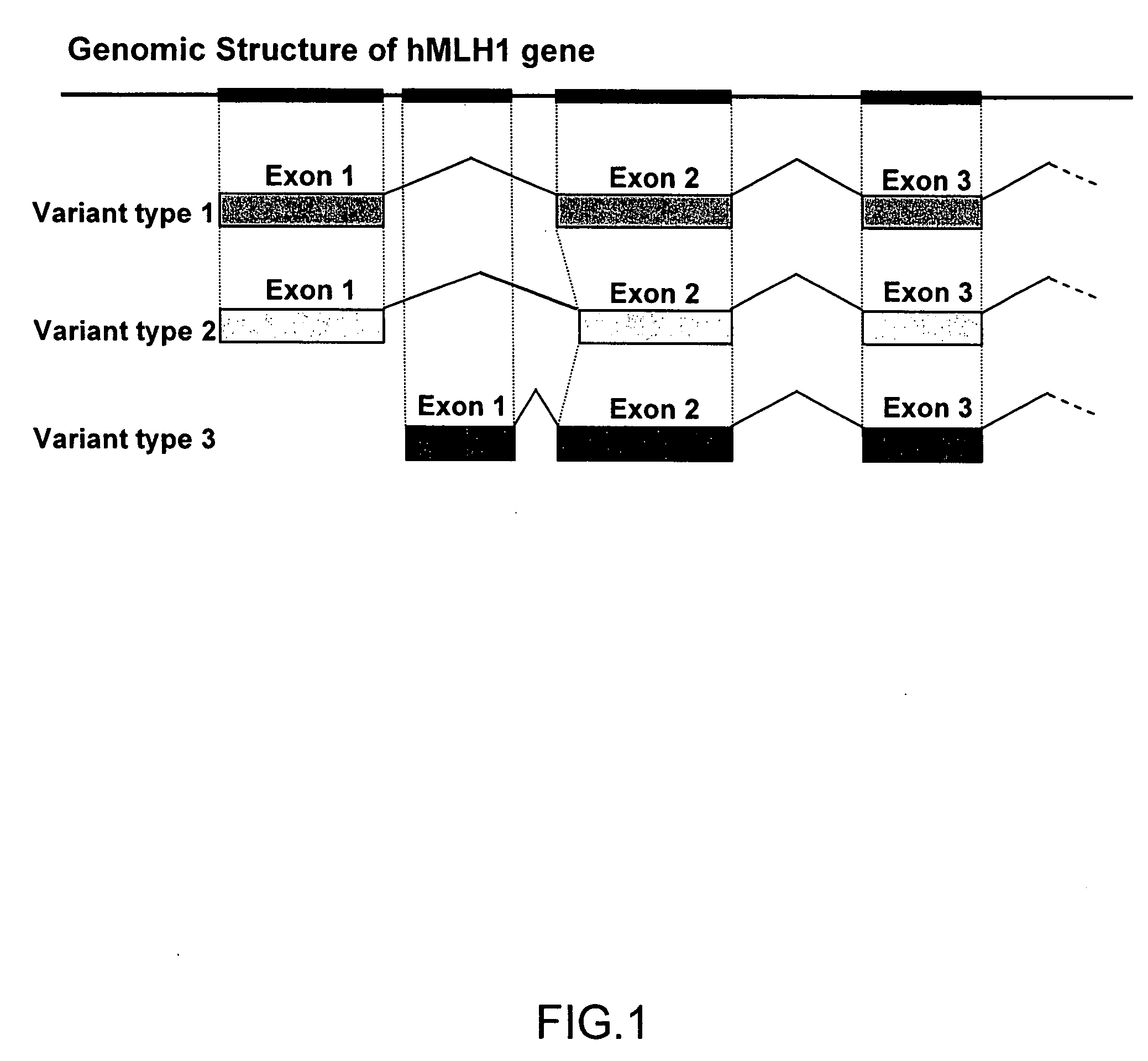
MutL homolog 1, colon cancer, nonpolyposis type 2 (E. coli) is a protein that in humans is encoded by the MLH1 gene located on chromosome 3. It is a gene commonly associated with hereditary nonpolyposis colorectal cancer. Orthologs of human MLH1 have also been studied in other organisms including mouse and the budding yeast Saccharomyces cerevisiae.
Mutations in human MLH1 and human MSH2 genes useful in diagnosing colorectal cancer
Variant human MLH1 and MSH2 genes are provided. Methods of using these variant genes to diagnose hereditary non-polyposis colorectal cancer (HNPCC) and / or determine a patient's susceptibility to developing HNPCC are also provided. Methods and compositions for identifying new variant MLH1 of MSH2 genes are also provided. In addition, experimental models for hereditary non-polyposis colorectal cancer comprising these variant genes are provided.
Owner:DIADEXUS
Animal models of pancreatic adenocarcinoma and uses therefor
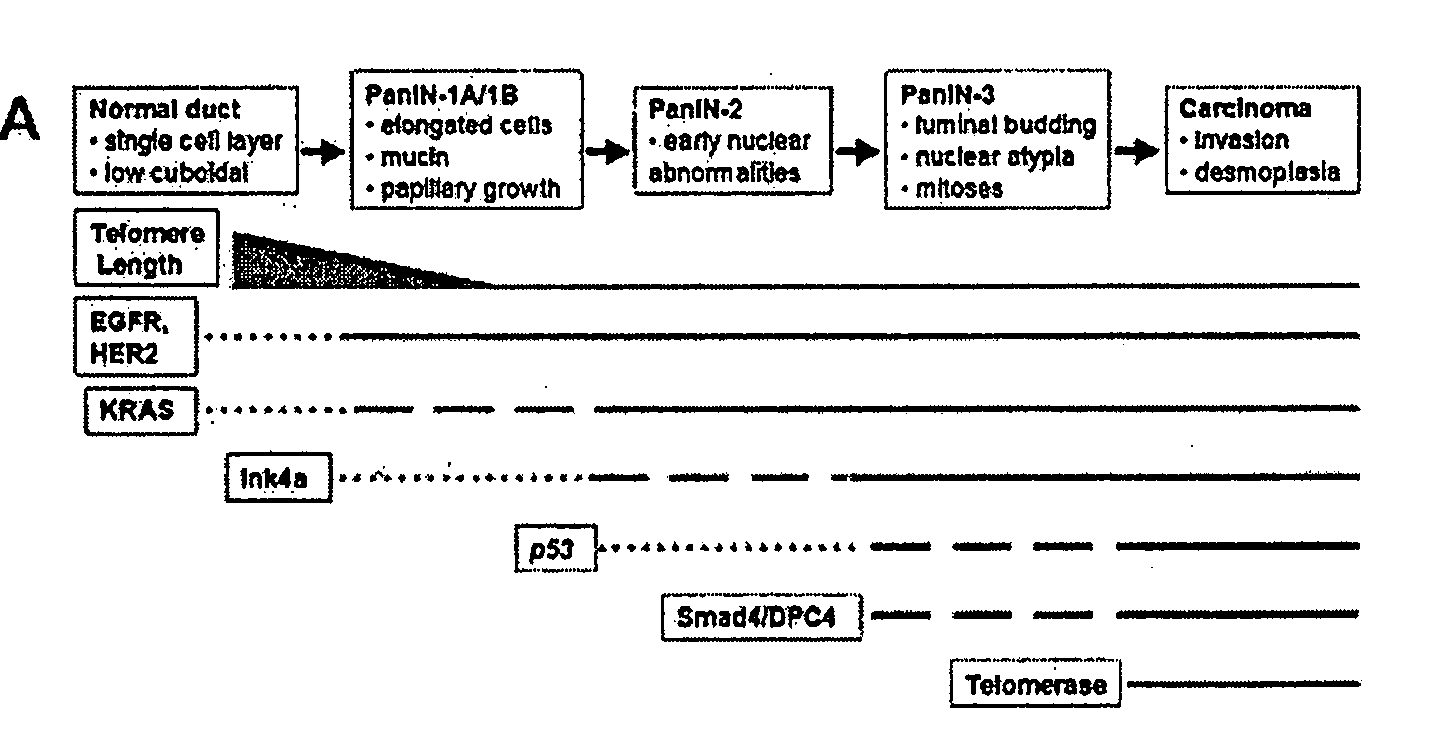
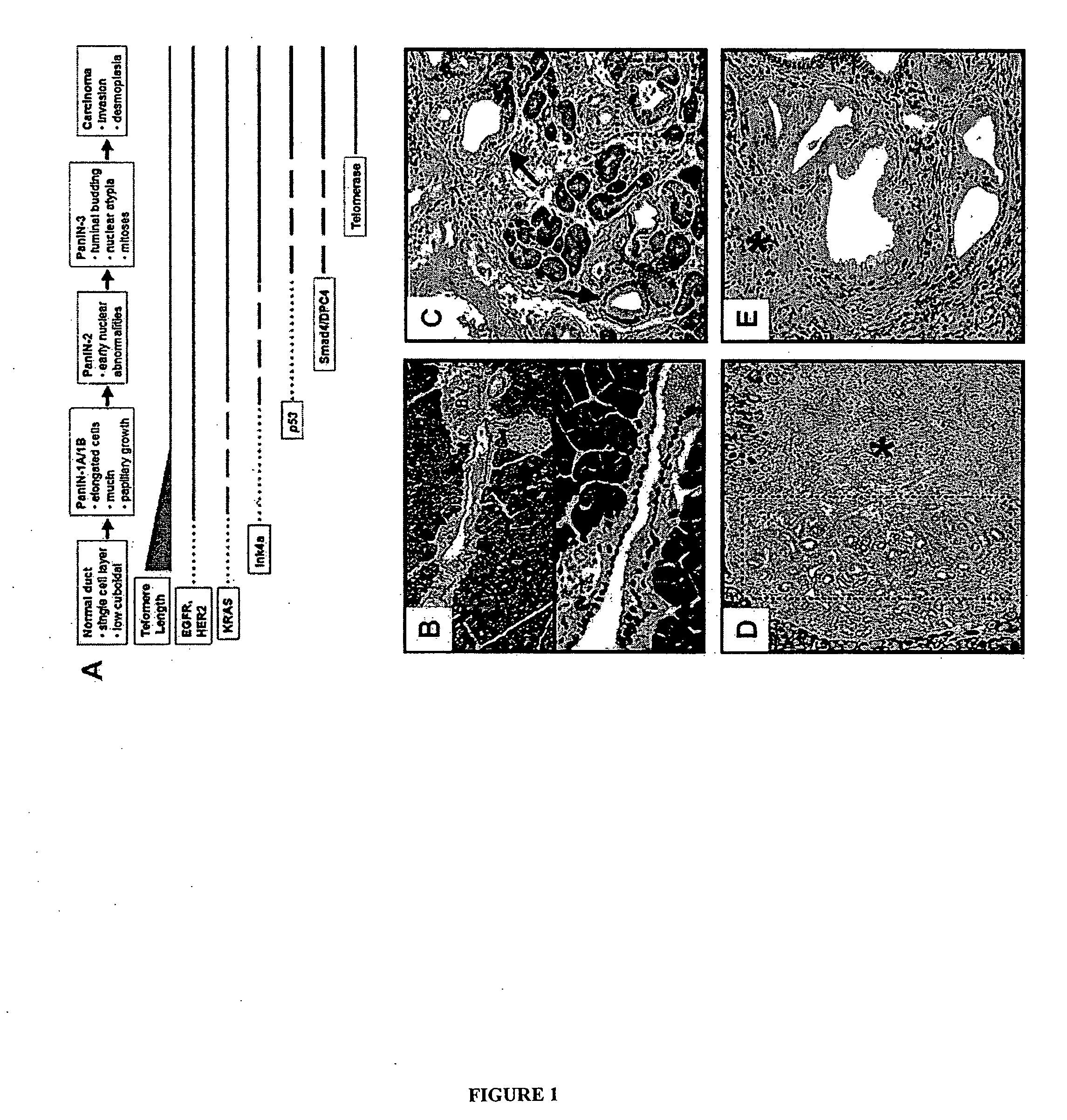
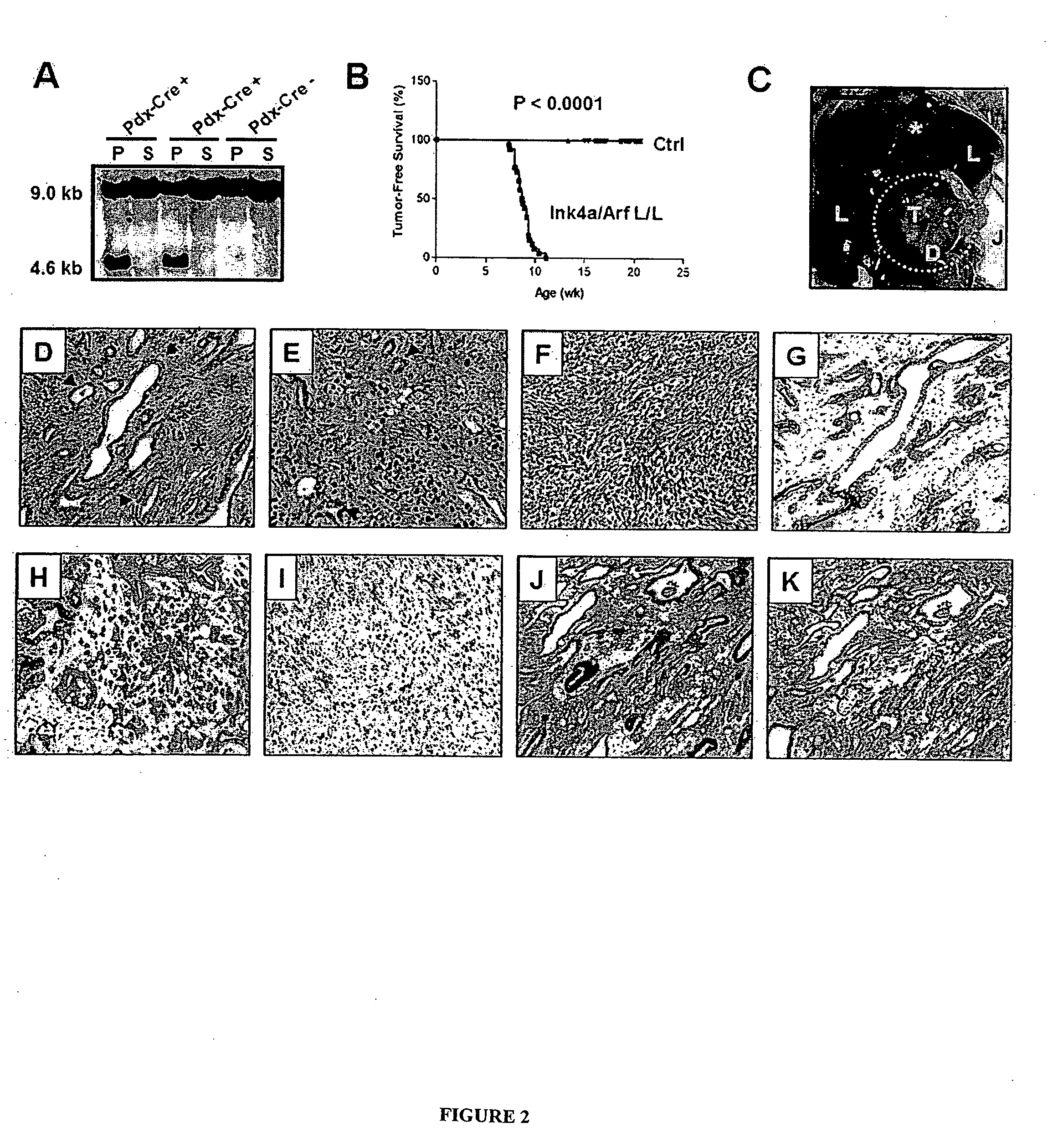
The present invention is based, at least in part, on the generation of an animal model of pancreatic adenocarcinoma which recapitulates the genetic and histological features of human pancreatic adenocarcinoma, including the initiation, maintenance, and progression of the disease. Accordingly, the present invention provides animal models of cancer, e.g., pancreatic adenocarcinoma, wherein an activating mutation of Kras has been introduced, and any one or more known or unknown tumor suppressor genes or loci, e.g., Ink4a / Arf, Ink4a, Arf, p53, Smad4 / Dpc, Lkb1, Brca2, or Mlh1, have been misexpressed, e.g., have been misexpressed leading to decreased expression or non-expression. The animal models of the invention may be used, for example, to identify biomarkers of pancreatic cancer, to identify agents for the treatment or prevention of pancreatic cancer, and to evaluate the effectiveness of potential therapeutic agents.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Homologous recombination in plants
InactiveUS20090031444A1Increase the number ofEasy to disassembleTissue cultureImmunoglobulinsMeiosisNucleic acid sequencing
The invention relates to the field of meiotic homologous recombination in plants. Provided are transgenic plants, cytological assays and MLH1 protein and nucleic acid sequences, as well anti-MLH1 antibodies, anti-SMC1, anti-SMC3 and anti-CENP-C antibodies.
Owner:WITTICH PETER EGBERTUS +4
Tumor susceptibility 62 genes and application thereof
InactiveCN105986031AImprove the detection rateEasy to identifyHealth-index calculationMicrobiological testing/measurementBAP1MAP2K4
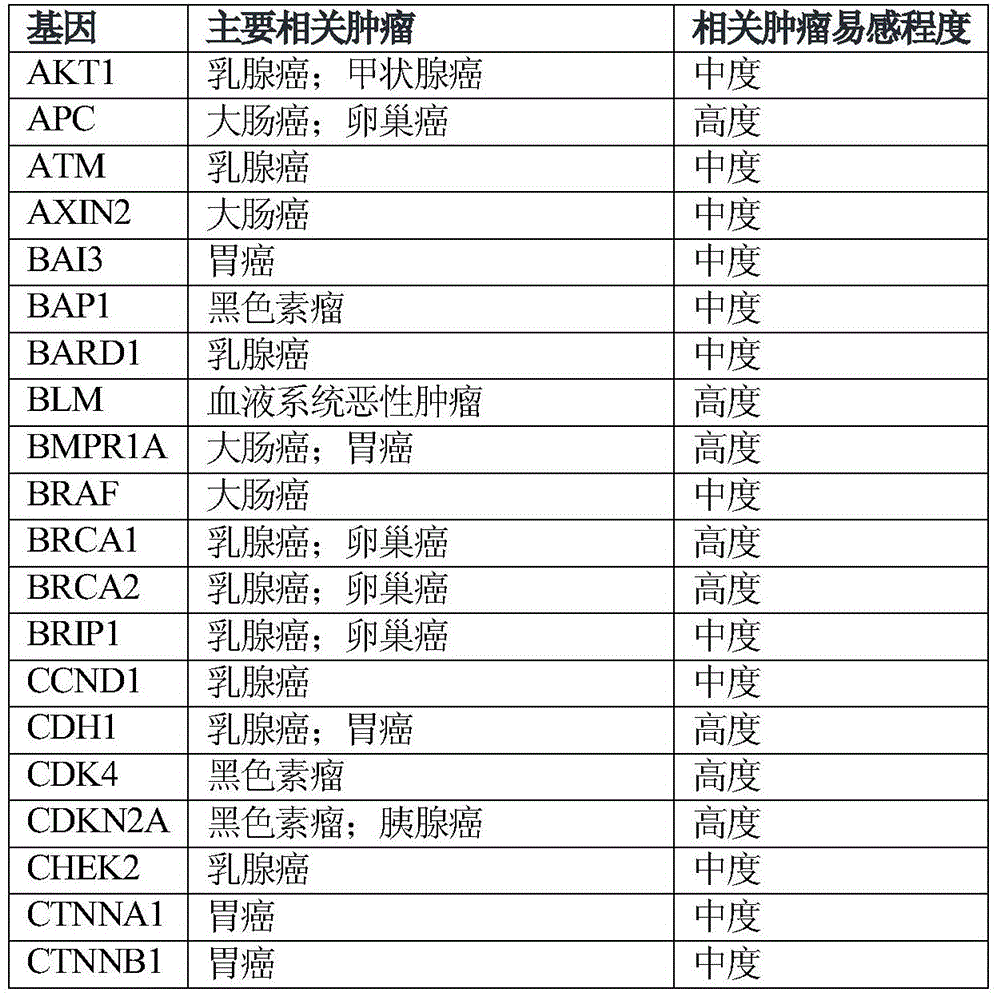
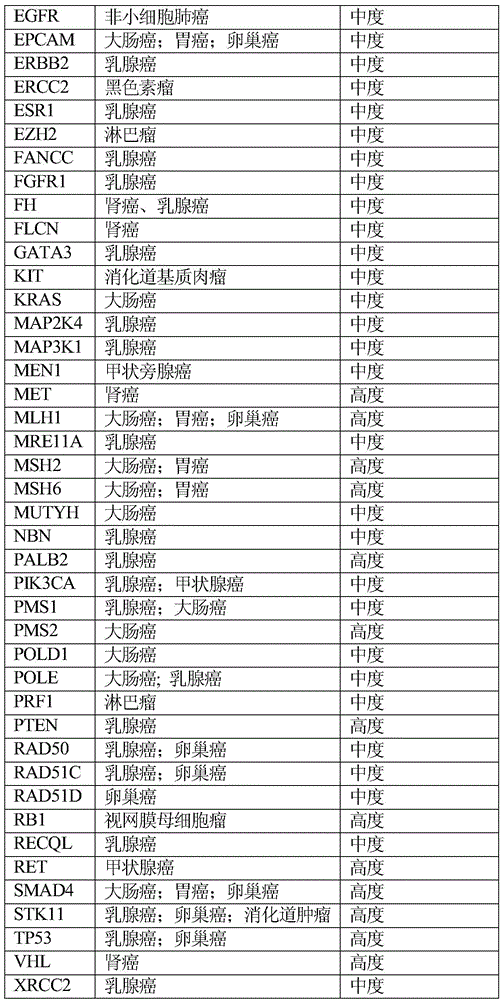
The invention relates to tumor susceptibility 62 genes and application thereof. The tumor susceptibility 62 genes comprise PTEN, STK11, CDH1, TP53, BRCA1, BRCA2, PALB2, CHEK2, ATM, BRIP1, NBN, RAD51C, MLH1, MSH2, MSH6, PMS2, BARD1, RAD51D, MRE11A, MUTYH, PMS1, RAD50, XRCC2, AKT1, PIK3CA, FANCC, RECQL, CCND1, ERBB2, ESR1, GATA3, FGFR1, MAP2K4, MAP3K1, BAI3, CTNNB1, BRAF, KRAS, CTNNA1, EPCAM, APC, BLM, SMAD4, BMPR1A, POLD1, POLE, AXIN2, MEN1, KIT, EGFR, EZH2, PRF1, CDKN2A, CDK4, BAP1, RB1, ERCC2, VHL, MET, FH, FLCN and RET. The detection of the genes can be used for evaluating tumor susceptibility.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Mismatch repair gene MLH1 mutation detection kit and application thereof
InactiveCN101974642AIncreased susceptibilityHigh mutation rateMicrobiological testing/measurementDissolutionRFLP - Restriction fragment length polymorphism
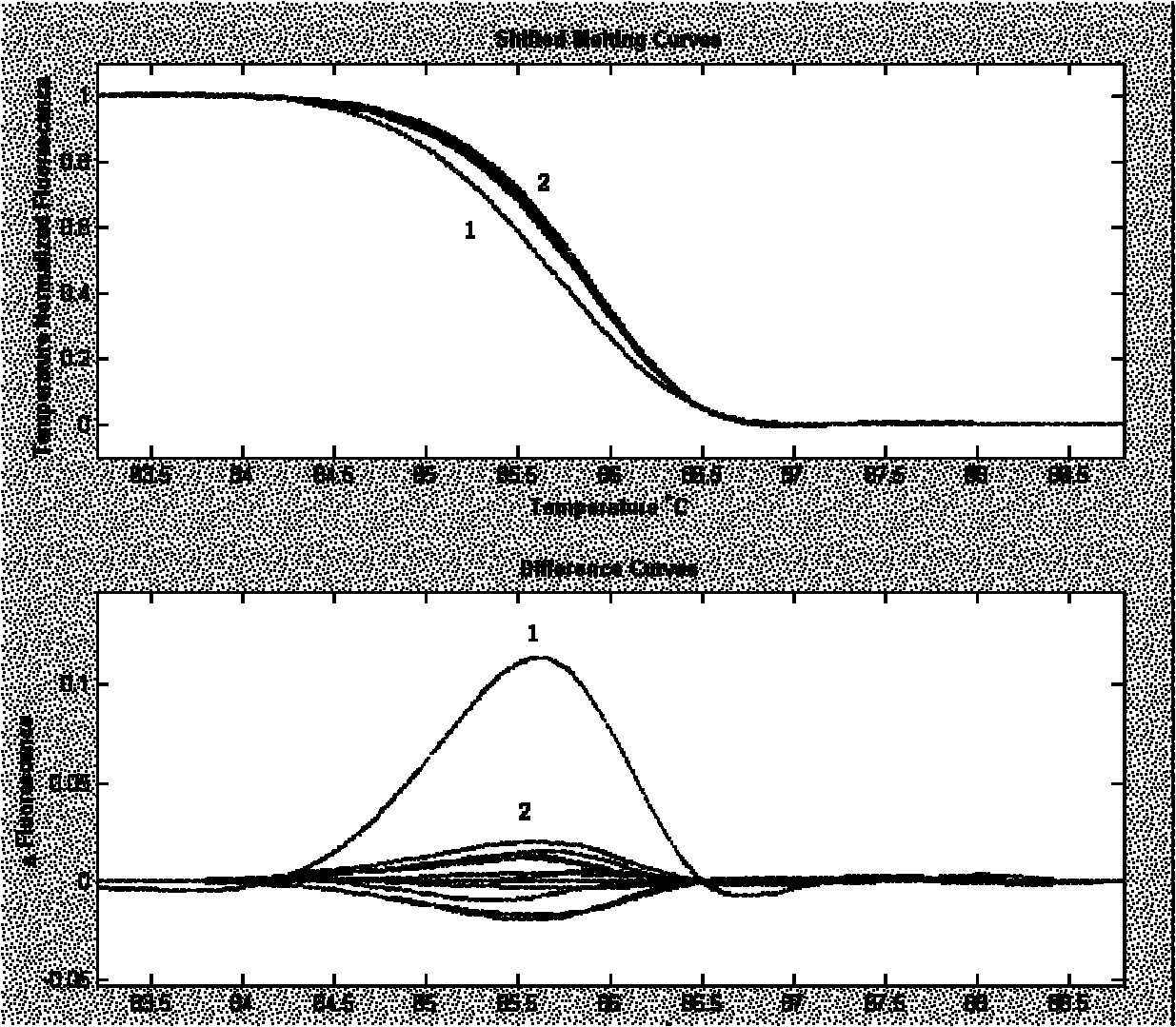
The invention relates to a mismatch repair gene MLH1 mutation detection kit and application thereof. The kit comprises the main components: firstly, 20 pairs of MLH1 gene PCR (Polymerase Chain Reaction) amplification and sequencing primers; and secondly, a reagent for PCR amplification. A PCR amplification product obtained through the kit can be used for screening gene micromutation through a high resolution dissolution curve method or carrying out restriction fragment length polymorphism analysis. The kit has high efficient MLH1 gene mutation detection and function evaluation actions and is used for mismatch repair gene MLH1 mutation detection.
Owner:NANJING UNIV
Gene detection kit for prognosing gastric cancer metastasis and use method of gene detection kit
InactiveCN106636366AThe result is objectiveOptimal treatment timeMicrobiological testing/measurementBreast cancer metastasisRAD51
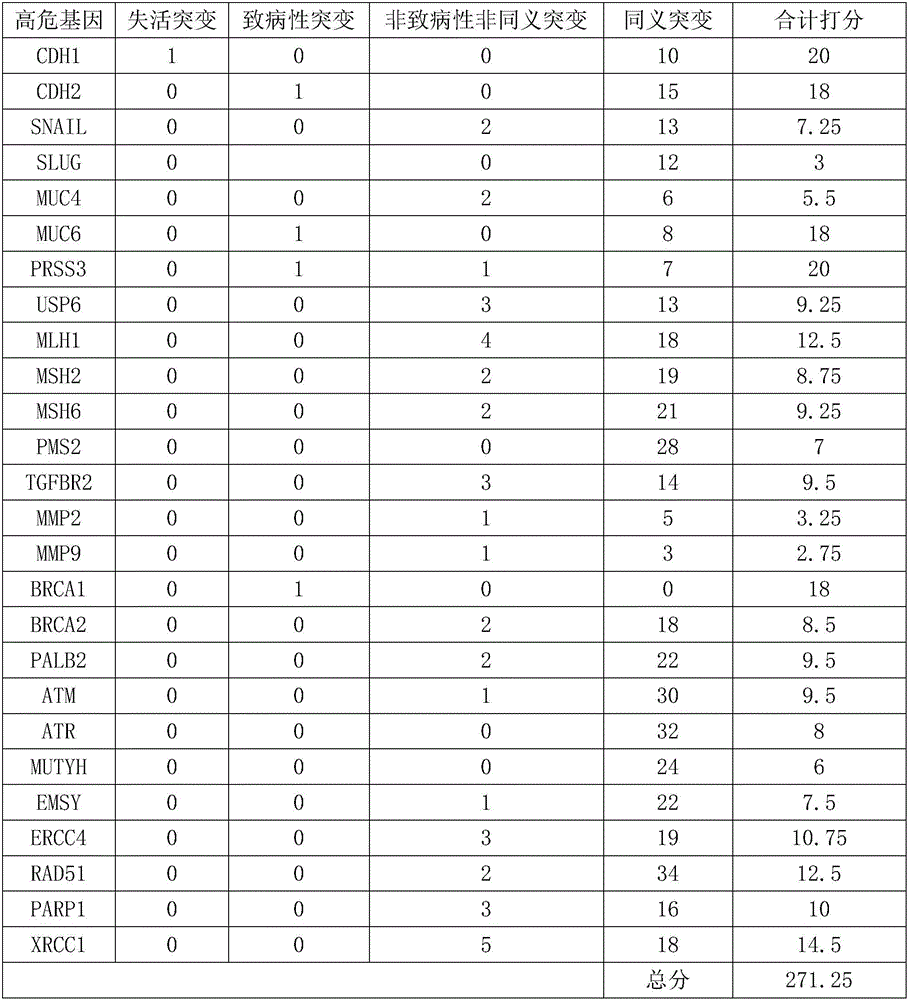
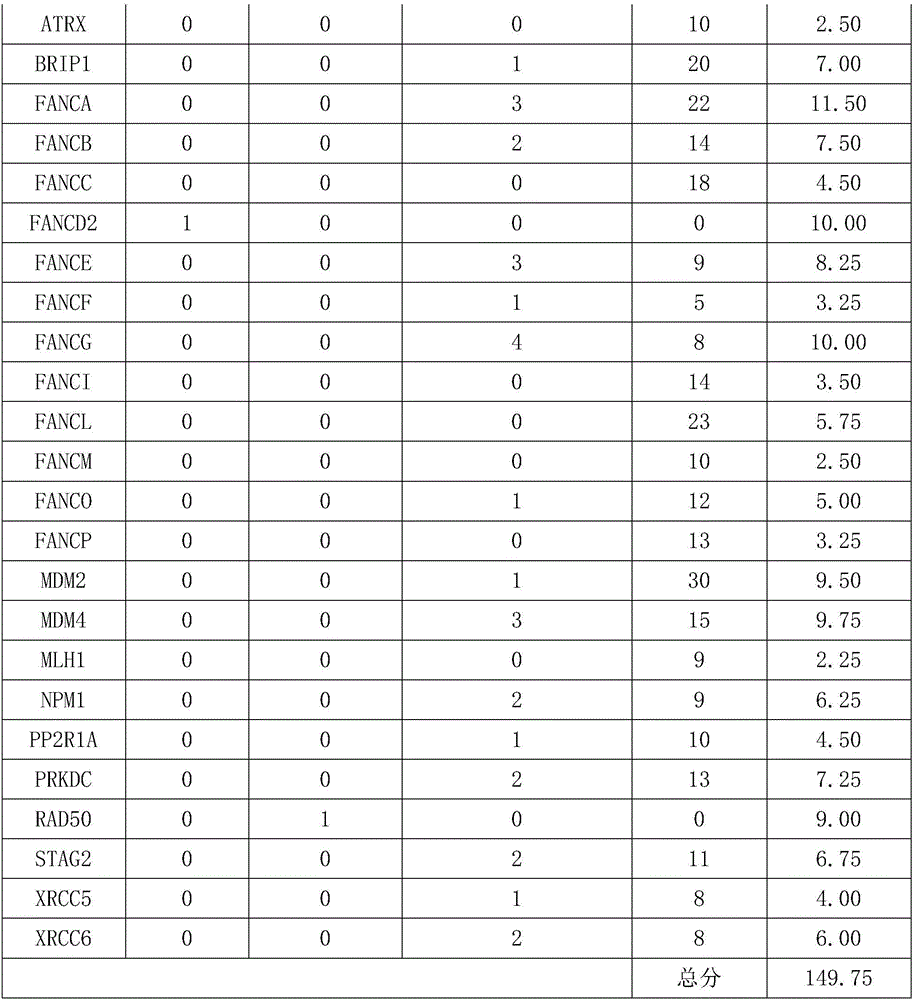
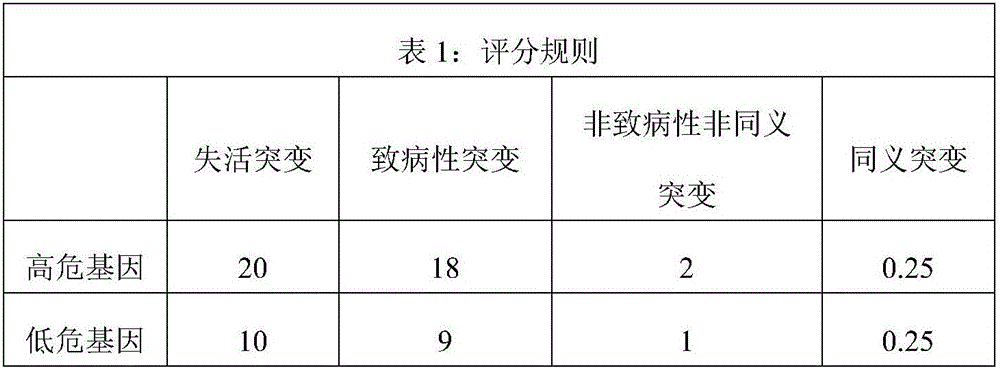
The invention relates to a gene detection kit for prognosing gastric cancer metastasis. The kit comprises a DNA library building kit, wherein the DNA library building kit comprises a high-risk gene probe and a low-risk gene probe; the high-risk gene probe comprises CDH1, CDH2, SNAIL, SLUG, MUC4, MUC6, PRSS3, USP6, MLH1, MSH2, MSH6, PMS2, TGFBR2, MMP2, MMP9, BRCA1, BRCA2, PALB2, ATM, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1 and XRCC1; the low-risk gene probe comprises ATRX, BRIP1, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FANCO, FANCP, MDM2, MDM4, MLH1, NPM1, PP2R1A, PRKDC, RAD50, STAG2, XRCC5 and CRCC6. The invention further discloses a use method of the kit. The use method comprises the following steps: extracting cfDNA in a blood sample; building a library for the cfDNA through the DNA library building kit, and then sequencing the DNA to obtain a gene overall length sequence; carrying out gene mutation analysis on the gene overall length sequence.
Owner:苏州首度基因科技有限责任公司 +1
Type 1 Diabetes Biomarkers
InactiveUS20160195546A1Improve risk prediction modelHigh detection sensitivityLibrary screeningDisease diagnosisAntigenPotential biomarkers
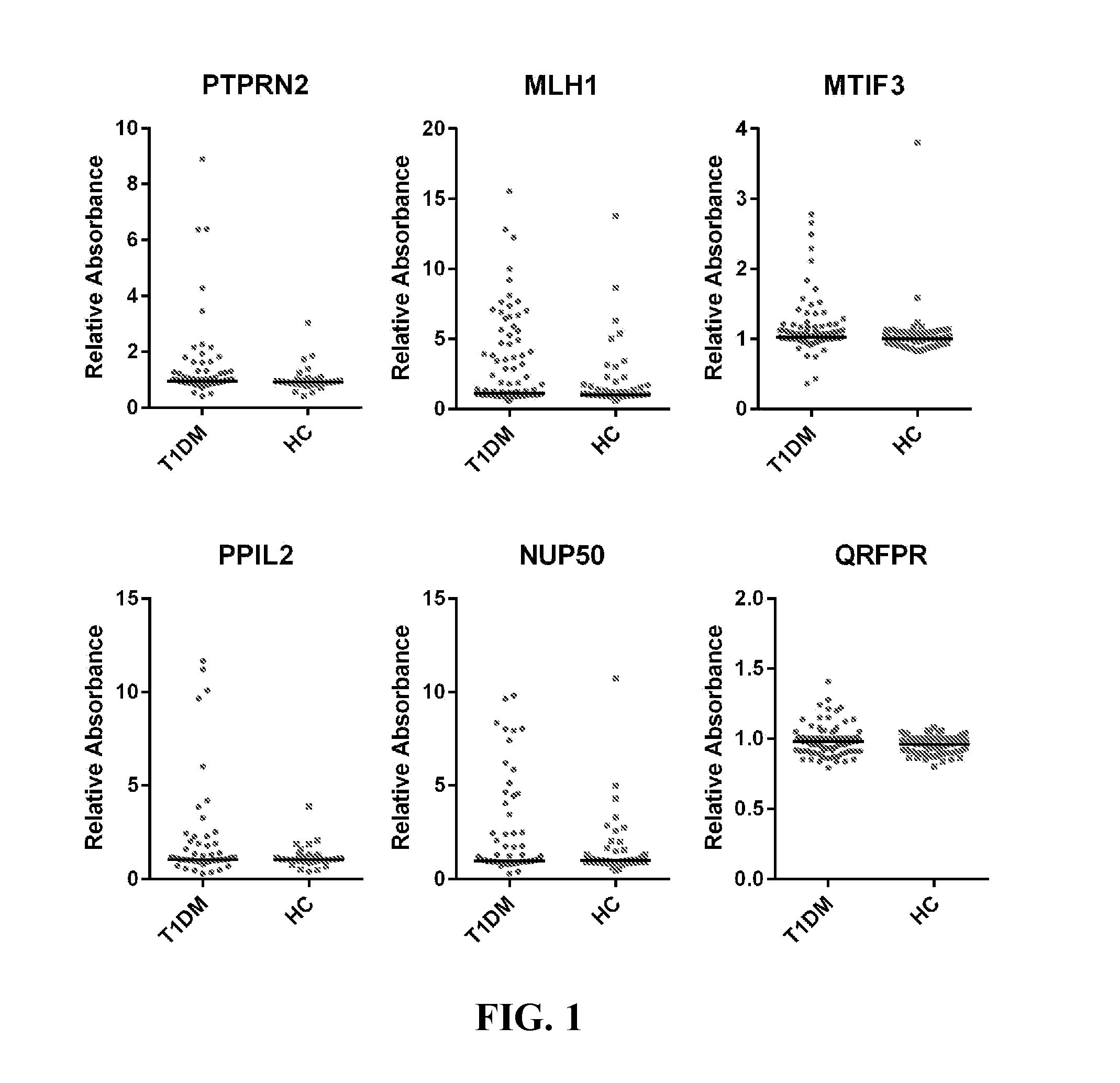
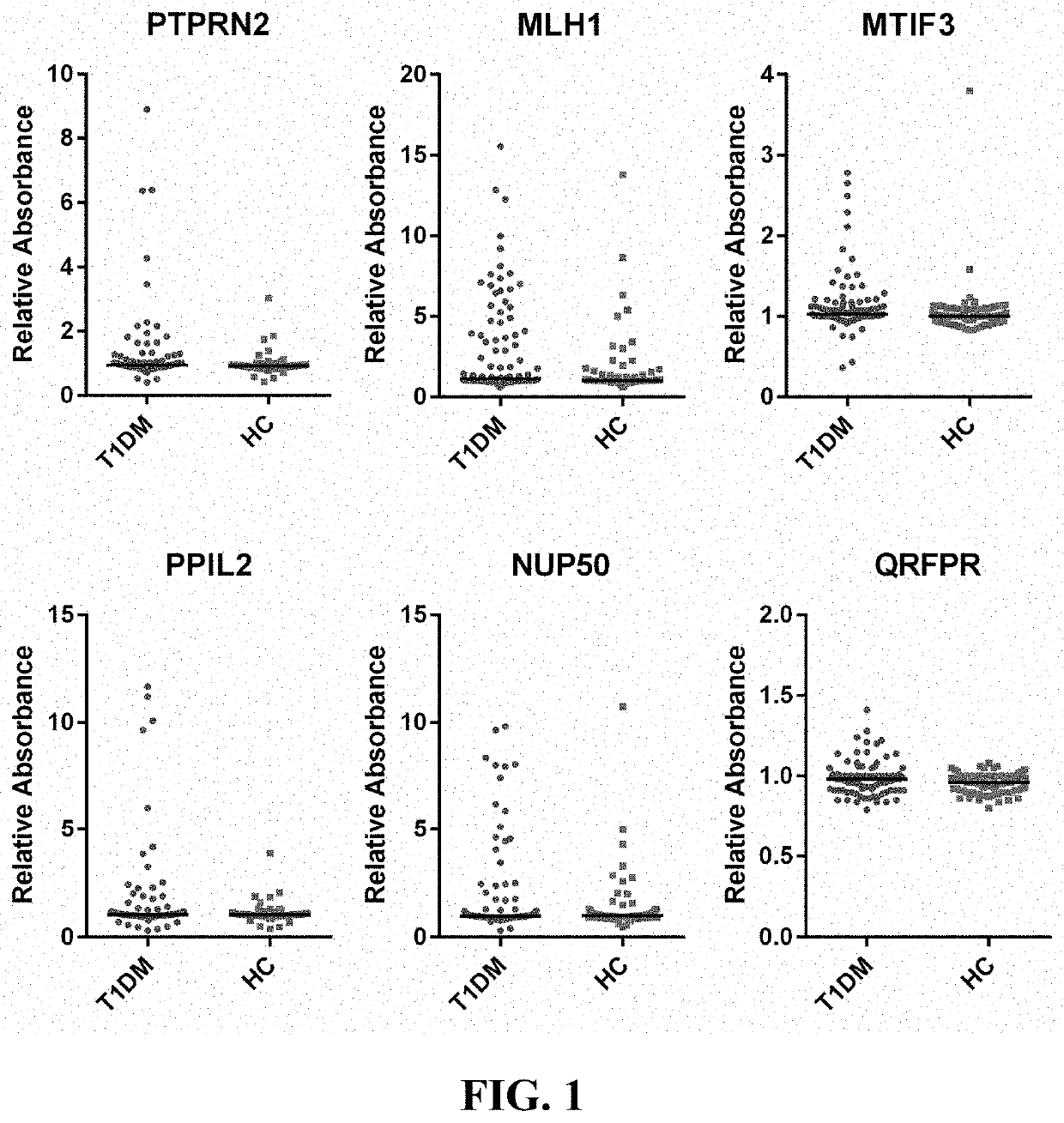
Type 1 diabetes (T1D) patients make antibodies to self-proteins that are potential biomarkers for early detection and risk prediction. We have identified seventeen antigens as biomarkers for early diagnosis and risk prediction of T1D, including the antigens MLH1, MTIF3, PPIL2, NUP50, TOX4, FIGN, C9orf142, ZNF280D, HES1, QRFPR, CTRC, SNX6, SYTL4, ELA2A, IGRP, PAX6, and HMGN3.
Owner:ARIZONA STATE UNIVERSITY +1
Kit for predicting colorectal cancer liver metastases and use method
InactiveCN106701920AImprove the quality of lifeProlong survival timeMicrobiological testing/measurementRAD51Lymphatic Spread
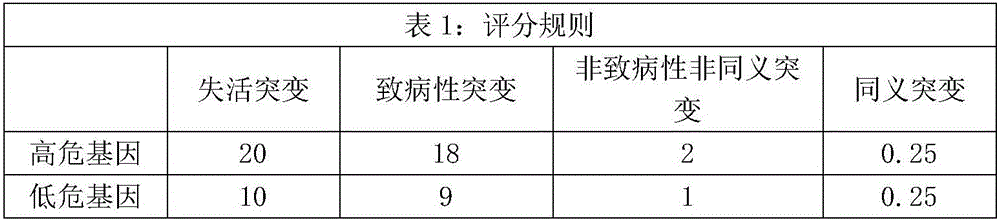
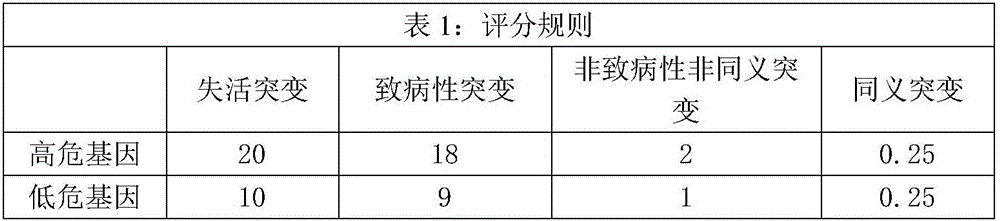
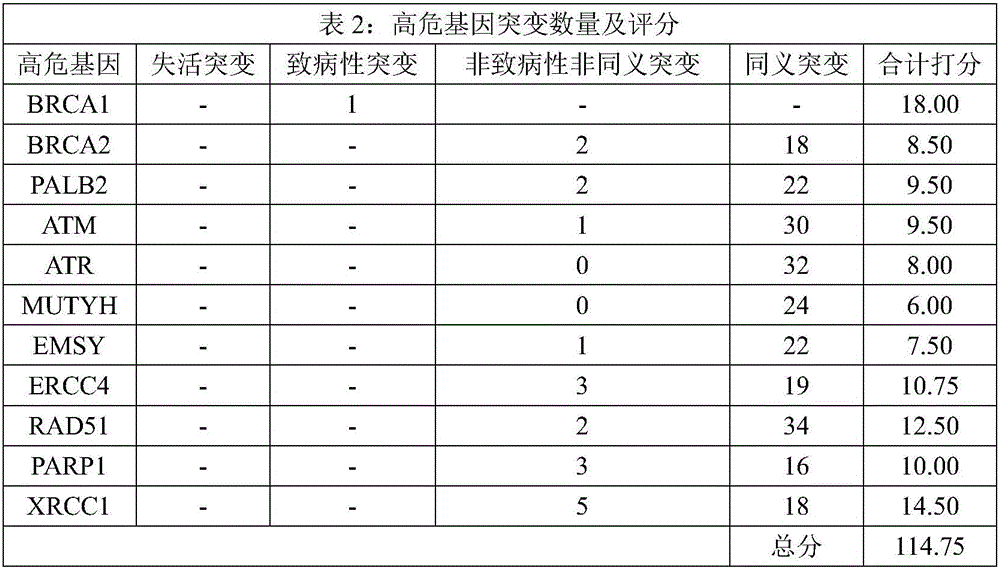
The invention relates to a kit for predicting colorectal cancer liver metastases and a use method. The kit includes a DNA database building kit, the DNA database building kit comprises probes of a plurality of genes, and the plurality of genes include: high risk genes: KRAS, BRAF, MLH1, NRAS, MSH2, PMS2, UGT1A1, MSH6 AKT1, PIK3CA, PTEN, SMAD4, TP53, NM23, TIAM1, MTS1; and low risk genes: PRKDC, RAD50, STAG2, XRCC5, XRCC6, FANCA, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1, XRCC1. The kit provided by the invention performs related mutation detection on colorectal cancer liver metastases related genes in peripheral blood, and combines specific scoring mechanism to rapidly and conveniently judge and predict colorectal cancer liver metastases.
Owner:苏州首度基因科技有限责任公司 +1
Assays, methods and compositions for diagnosing cancer
InactiveUS20140242583A1Improve the level ofScalable automationSugar derivativesMicrobiological testing/measurementActin genesDNA
The present invention provides a method and single-tube assay for identification and quantitative analysis of differentially methylated MLH1 promoter sequences that are associated with certain types of cancer in an individual by obtaining a biological sample comprising DNA from the individual, detecting the presence of and measuring the level of methylated MLH1 promoter sequences, and comparing the presence of and level of methylation in the sample to a normalization reference of “normal” beta-actin gene promoters, wherein a difference in the level or pattern of MLH1 methylation of the sample compared to the Actin gene reference level identifies abnormally methylated MLH1 promoter sequences associated with cancer.
Owner:GO PATH GLOBAL
Application of MLH1 gene, and kit for detecting gastrointestinal stromal tumor
ActiveCN103865981AAuxiliary diagnosisAuxiliary diagnosis, fromMicrobiological testing/measurementStromal tumorGene targets
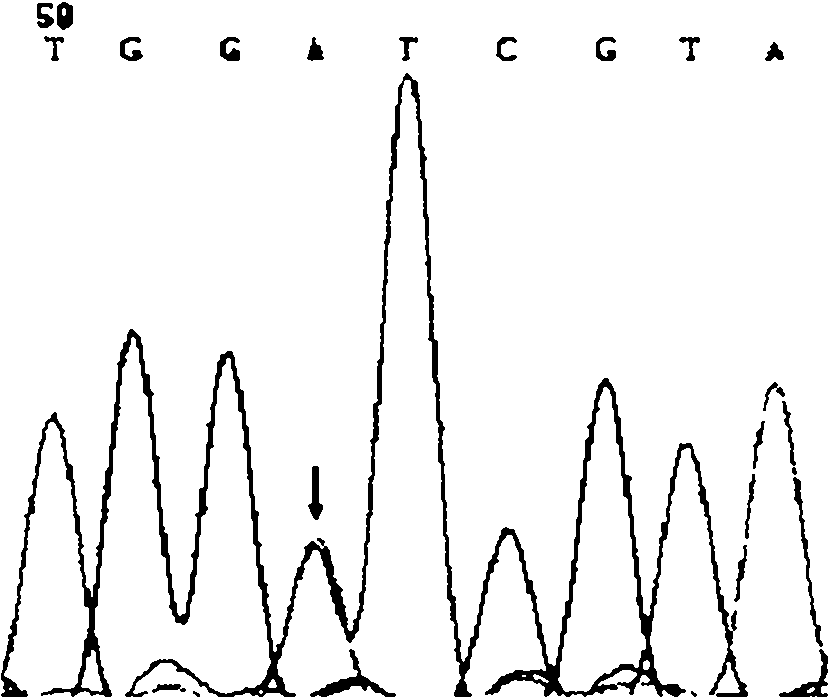
The present invention discloses an application of a MLH1 gene, and a kit for detecting a gastrointestinal stromal tumor, wherein the kit comprises MLH1 gene sequencing primers. According to the present invention, the inventor finds that: the gastrointestinal stromal tumor is caused by the common KIT and PDGFRA gene mutations, and the MLH1 (V384D) mutation is one of the causes of the gastrointestinal stromal tumor, such that the kit for detecting the gastrointestinal stromal tumor can be provided for rapidly and accurately detecting the MLH1 mutation site of the gastrointestinal stromal tumor and assistedly diagnosing the gastrointestinal stromal tumor so as to assistedly treat the gastrointestinal stromal tumor based on the finding; and after the MLH1 (V384D) mutation is detected by using the kit, the gene targeting drug can be adopted to specifically treat patients, such that the use of the targeting drug is specific.
Owner:北京圣谷智汇医学检验所有限公司
Method for constructing MLH1 gene knockout cell line
InactiveCN112501170AIncrease positive rateLow costGenetically modified cellsMicroorganism based processesTotal proteinGenetic engineering
The invention relates to a method for constructing an MLH1 gene knockout cell line, and relates to the technical field of gene engineering. According to the method, a CRISPR / Cas9 system is used to prepare an MLH1 gene knockout cell line, firstly designing two sgRNA sequences for the MLH1 gene, and constructing recombinant plasmids by utilizing molecular cloning technology; then transfecting the recombinant plasmids to HeLa cells, verifying the activity of sgRNA through PCR, carrying out puromycin drug screening and monoclonal treatment, extracting genome DNA and total protein of the monoclonalcell strain, and carrying out MSH1 gene level sequencing identification and expression detection of protein level to obtain an MSH1 gene knockout HeLa cell line. The MSH1 gene knockout HeLa cell lineconstructed by the invention is a cell line with stable inheritance of genes. The method provided by the invention can be used for directional knockout of the MSH1 gene to cause functional inactivation of the MSH1 gene, which has the characteristics of simplicity, convenience, high efficiency, quickness, low cost and the like, and has important significance for research on functions and related pathways of the MSH1 gene.
Owner:WUHAN AIBO TAIKE BIOTECH CO LTD
Kit, method and primers for analyzing methylation status of MLH1 promoter in DNA sample
InactiveCN105018476AMicrobiological testing/measurementDNA/RNA fragmentationAnalysis dnaOligonucleotide primers
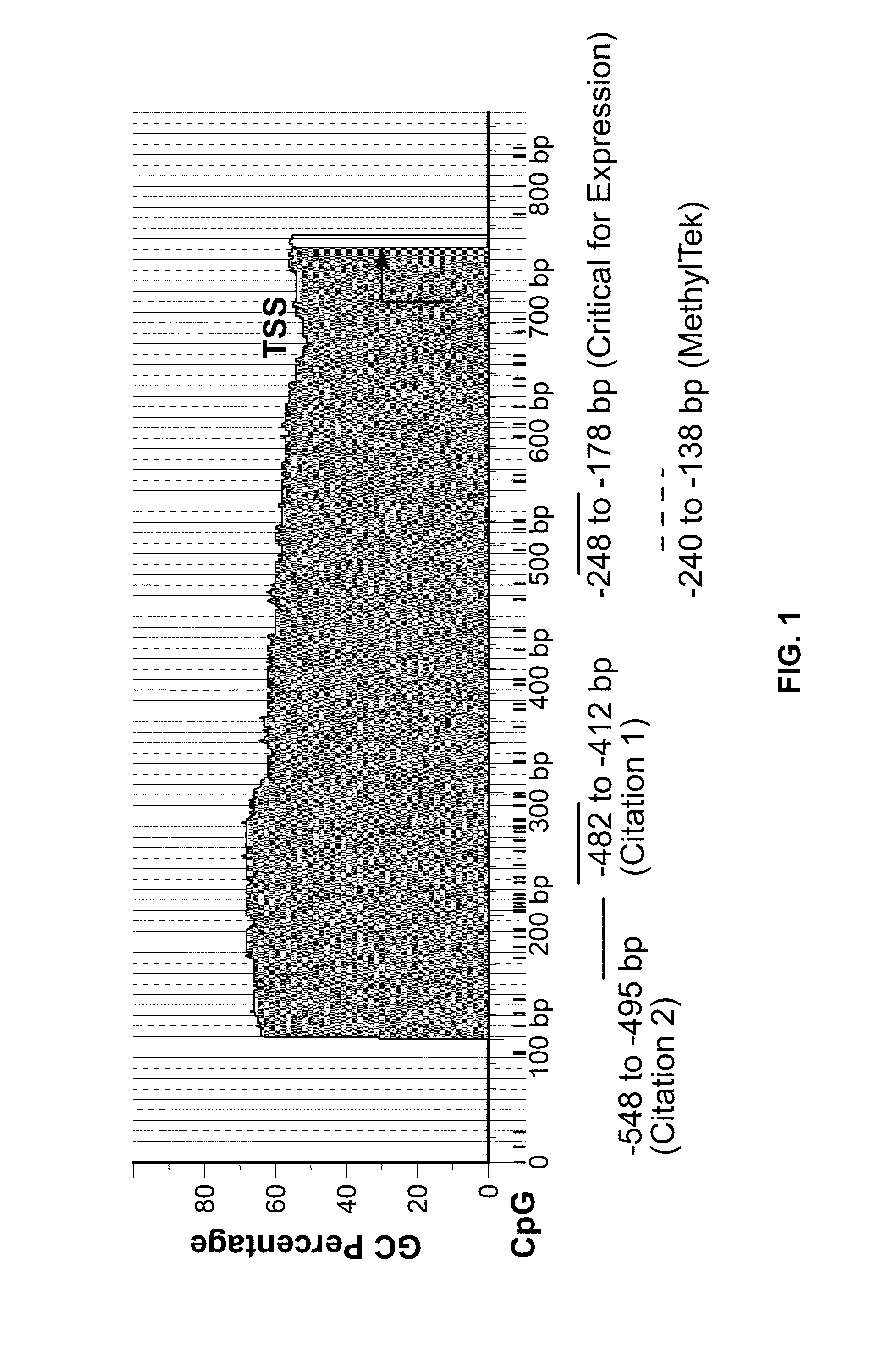
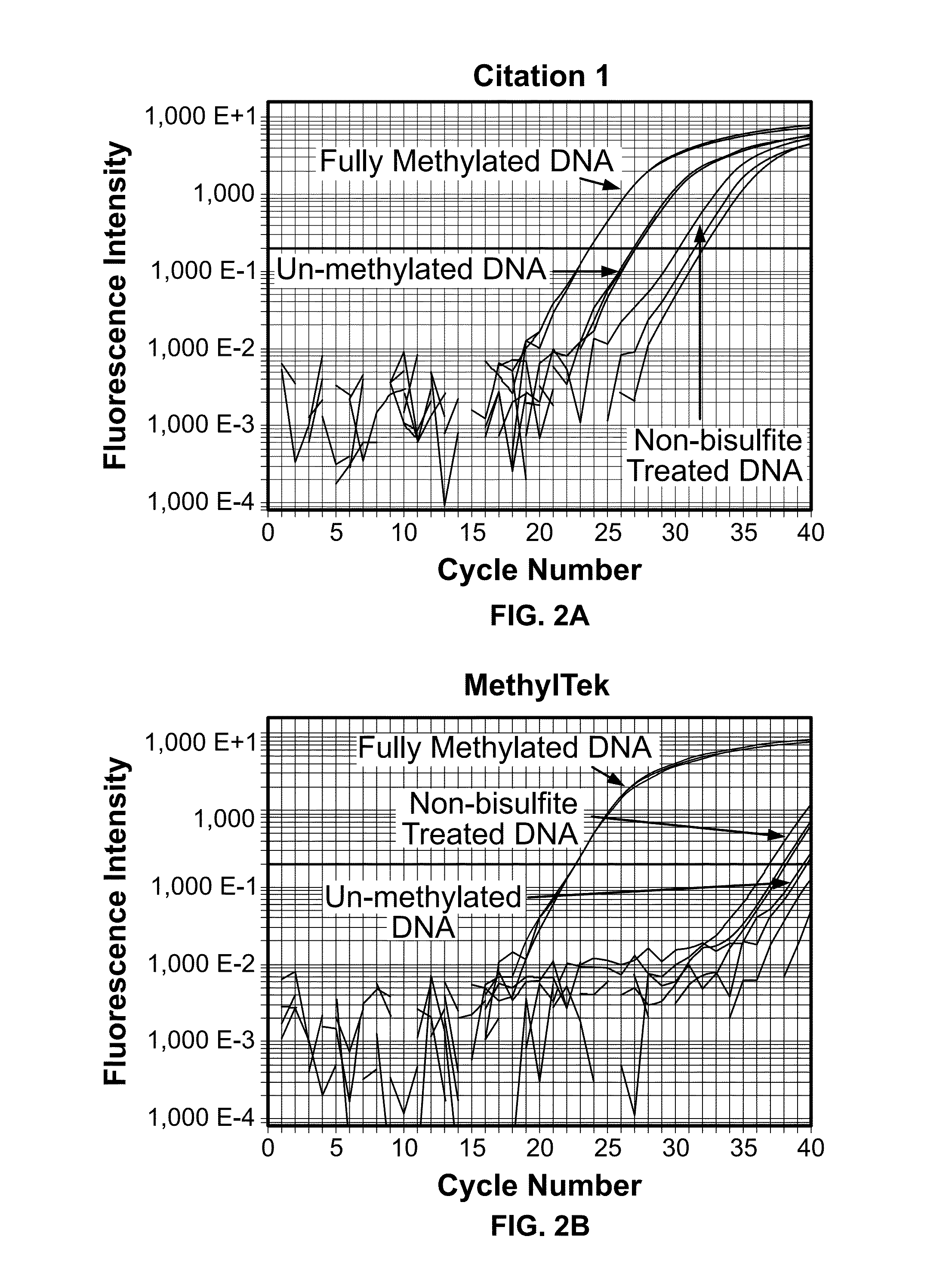
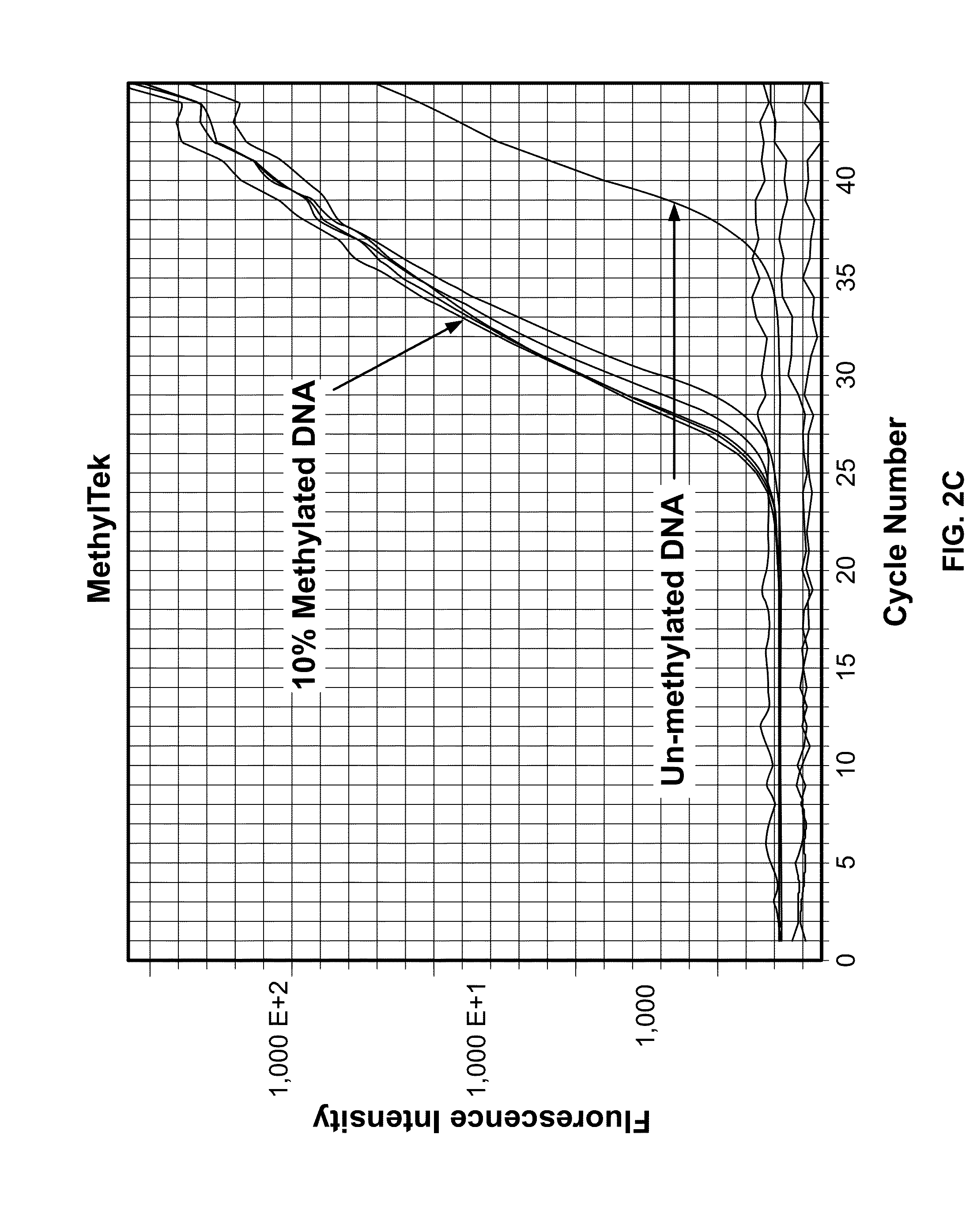
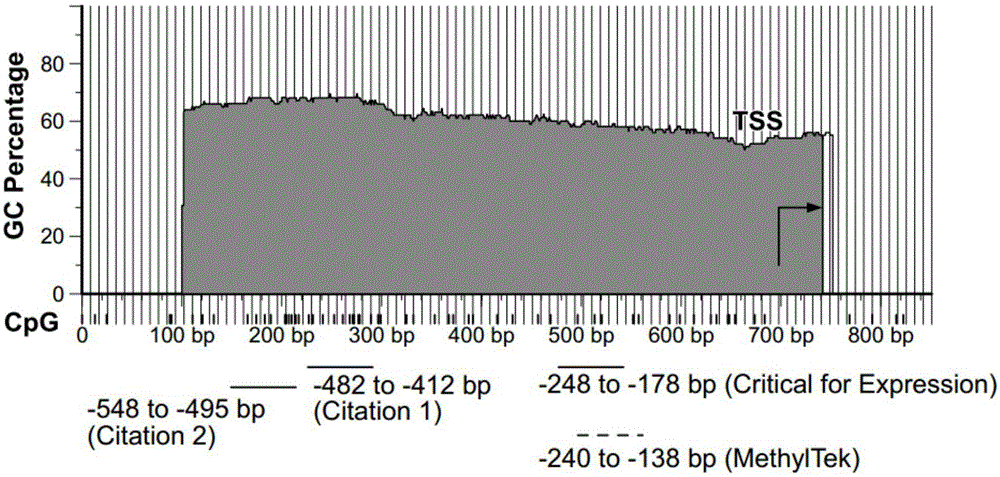
The present invention belongs to the field of biotechnology, and in particular relates to a kit, a method and primers for analyzing methylation status of an MLH1 promoter in a DNA sample. The invention provides a kit for analyzing the methylation status of a neoplastic disease associated MLH1 promoter in a DNA sample. The kit comprises oligonucleotide primers, the oligonucleotide primers are complementary with at least part of the sequence of the MLH1 promoter in a zone from -248bp to -178bp relative to a transcription start site and overlap with the methylation sites in the zone. The present invention discloses accurate and sensitive test, composition and method for detection of differential methylation of genomic MLH1 promoter DNA in clinical sample. The test, composition and method can be used in allow diagnosis and symptom method; and the applicable characteristic is that the presenting level of methylation genomic MLH1 promoter DNA in the absence of a specific disorder is distinguished from illness of methylation genomic MLH1 promoter DNA level.
Owner:常州杰傲病理诊断技术有限公司
DNA methylation kit for colorectal cancer detection and detection method thereof
PendingCN110964823AImprove positive detection rateImprove featuresMicrobiological testing/measurementNucleotideA-DNA
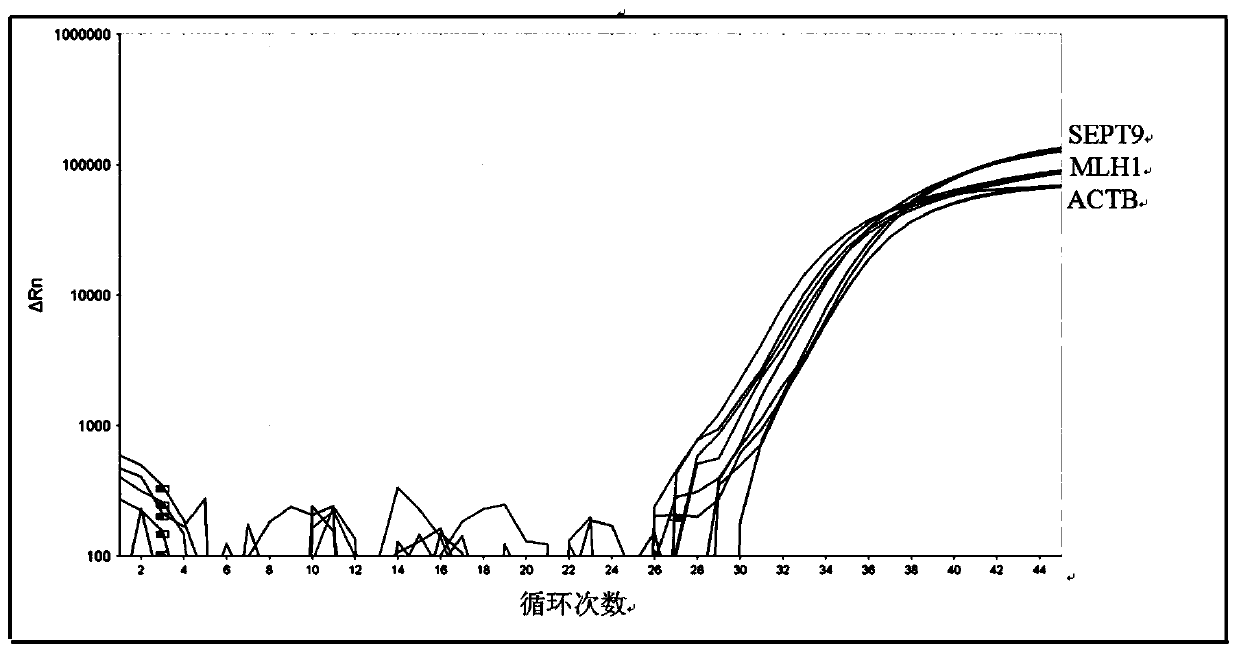
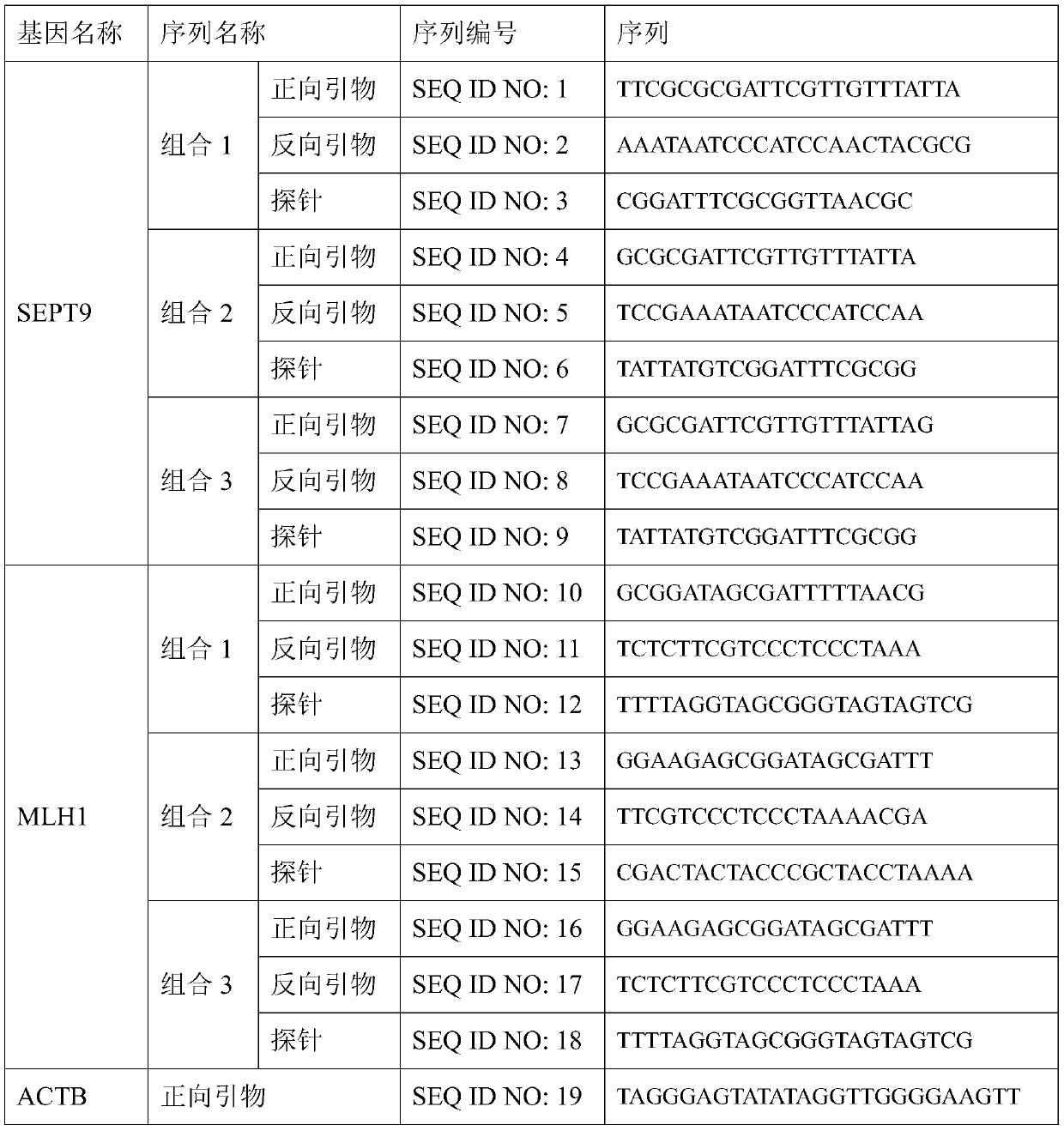
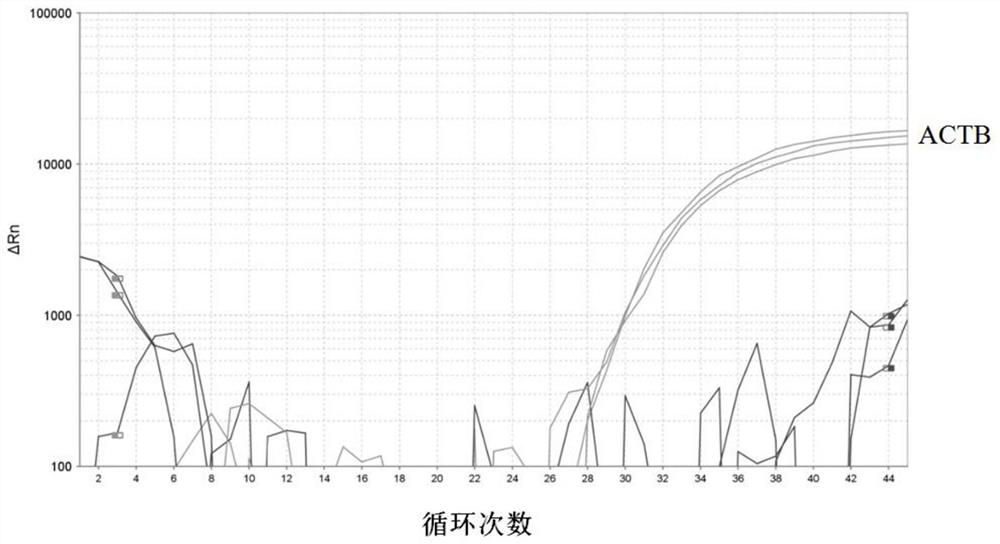
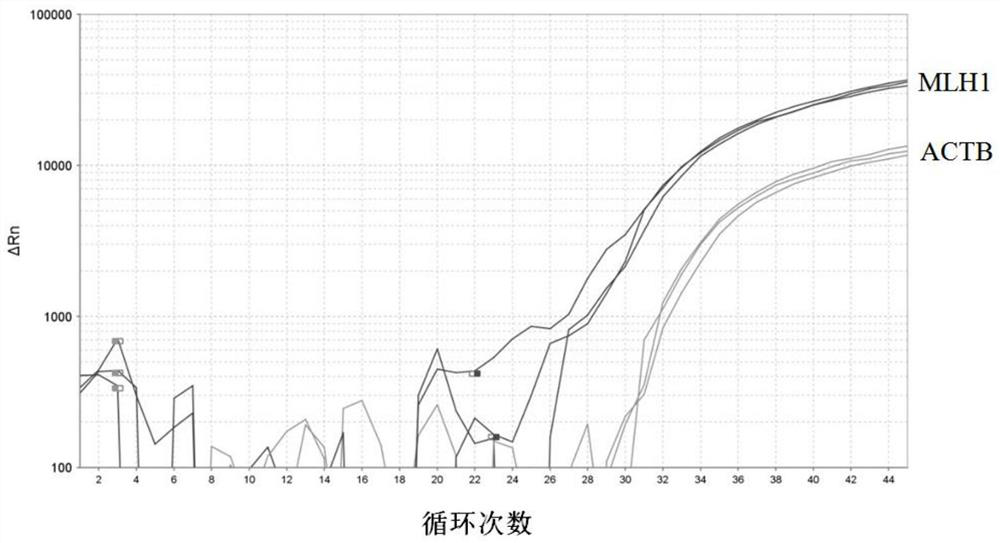
The invention discloses a DNA methylation kit for colorectal cancer detection and a detection method thereof. The kit comprises a specific primer and a probe which are used for detecting or measuringa methylation state or level of one or more specific genes in DNA of a tested sample, wherein the specific genes are SEPT9, MLH1 and ACTB. The kit provided by the invention can significantly improve the positive detection rate and specificity (real negative rate) of early colorectal cancer; the detection sensitivity of a biomarker can be improved to a pg / nanogram-grade DNA molecule, and the improvement of the detection sensitivity is realized by optimizing a specific nucleotide sequence primer and a DNA probe and improving a bisulfite treatment method of plasma free DNA.
Owner:深圳市新合生物医疗科技有限公司
Non-invasive high-throughput methylation in colorectal cancer diagnosis, research and treatment method
InactiveCN107164535AAccurately determineIncreased sensitivityMicrobiological testing/measurementNon invasiveImmunoprecipitation
A non-invasive high-throughput methylation in colorectal cancer diagnosis, research and treatment method is disclosed, and is characterized in that on the basis of high-throughput parallel methylation detection combinatorial sequencing and cfRNA information, DNA methylation of a subject is detected to produce a methylation spectrum of the subject and establish specific CpG island sites contained in SEPT9, MLH1, DKK2 and APC genes, methylation levels of CGI and CpG islands of which identification genes are in promoter regions are used as colorectal cancer diagnosis markers and clinical targets; a specific anti methylcytosine antibody is used for recognization and binding to methylated DNA fragments, a methylation second-generation sequencing library can be established by immunity precipitation, target sites are enriched, a methylation spectrum is compared with standard methylation spectrums, methylation site sequencing bioinformation is analyzed by the ''waterfall'' mechanism to determine whether a sample has cancer, and by combination of cfRNA and methylation site comprehensive analysis, the cancer can be determined and located.
Owner:沈阳宁沪科技有限公司
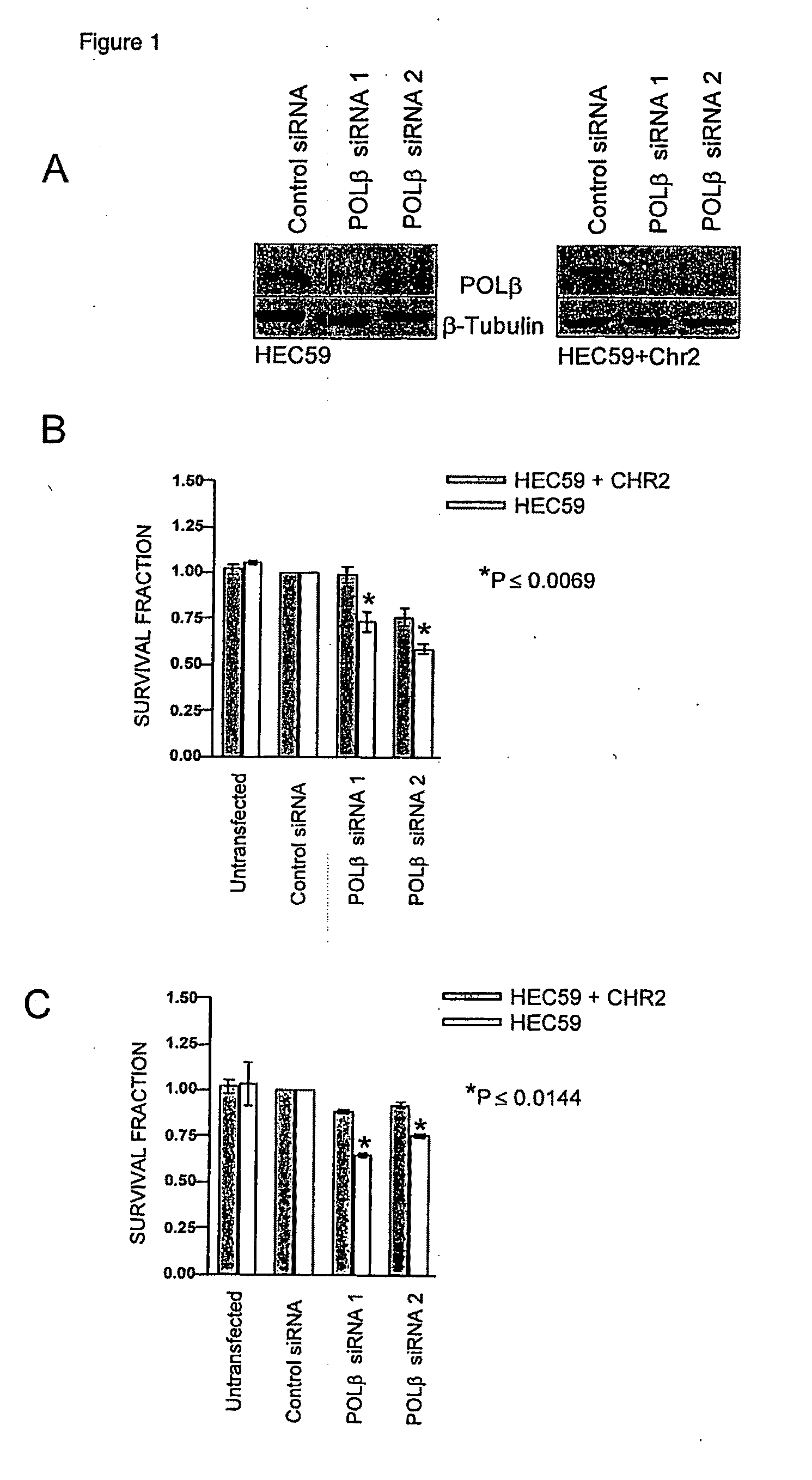
Materials and methods for exploiting synthetic lethality in mismatch repair-deficient cancers
InactiveUS20110212101A1Organic active ingredientsMicrobiological testing/measurementSynthetic lethalityCancer research
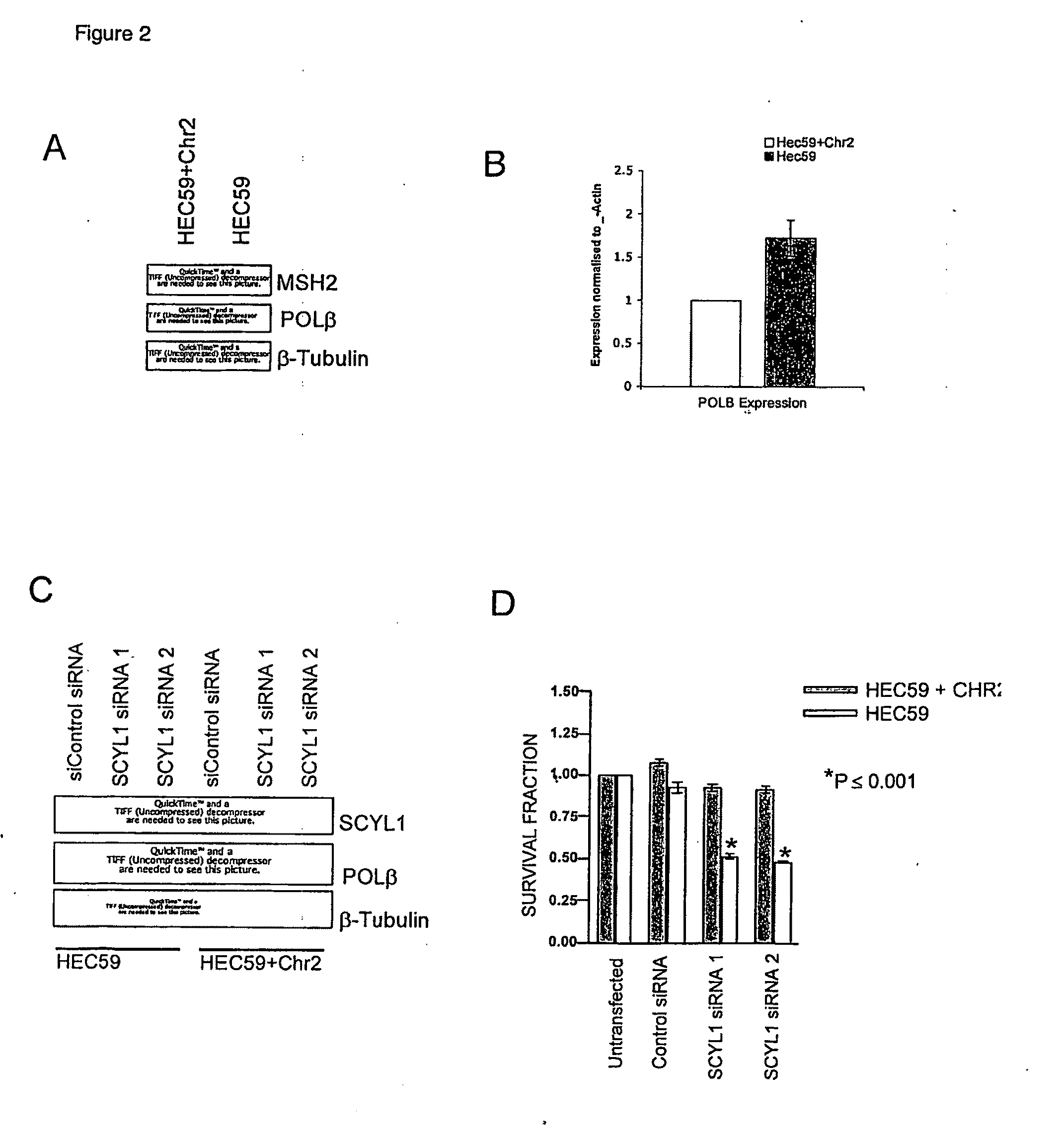
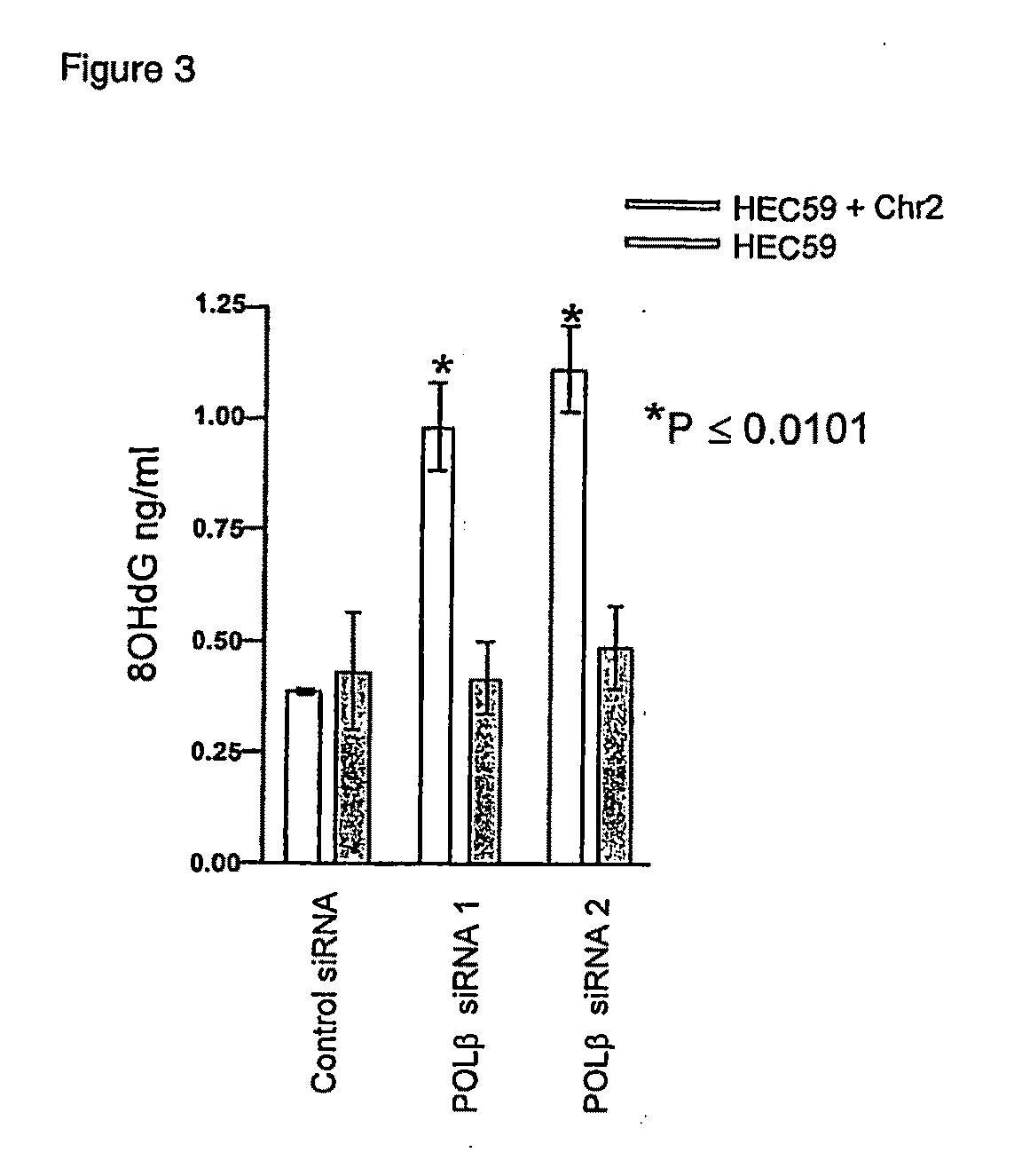
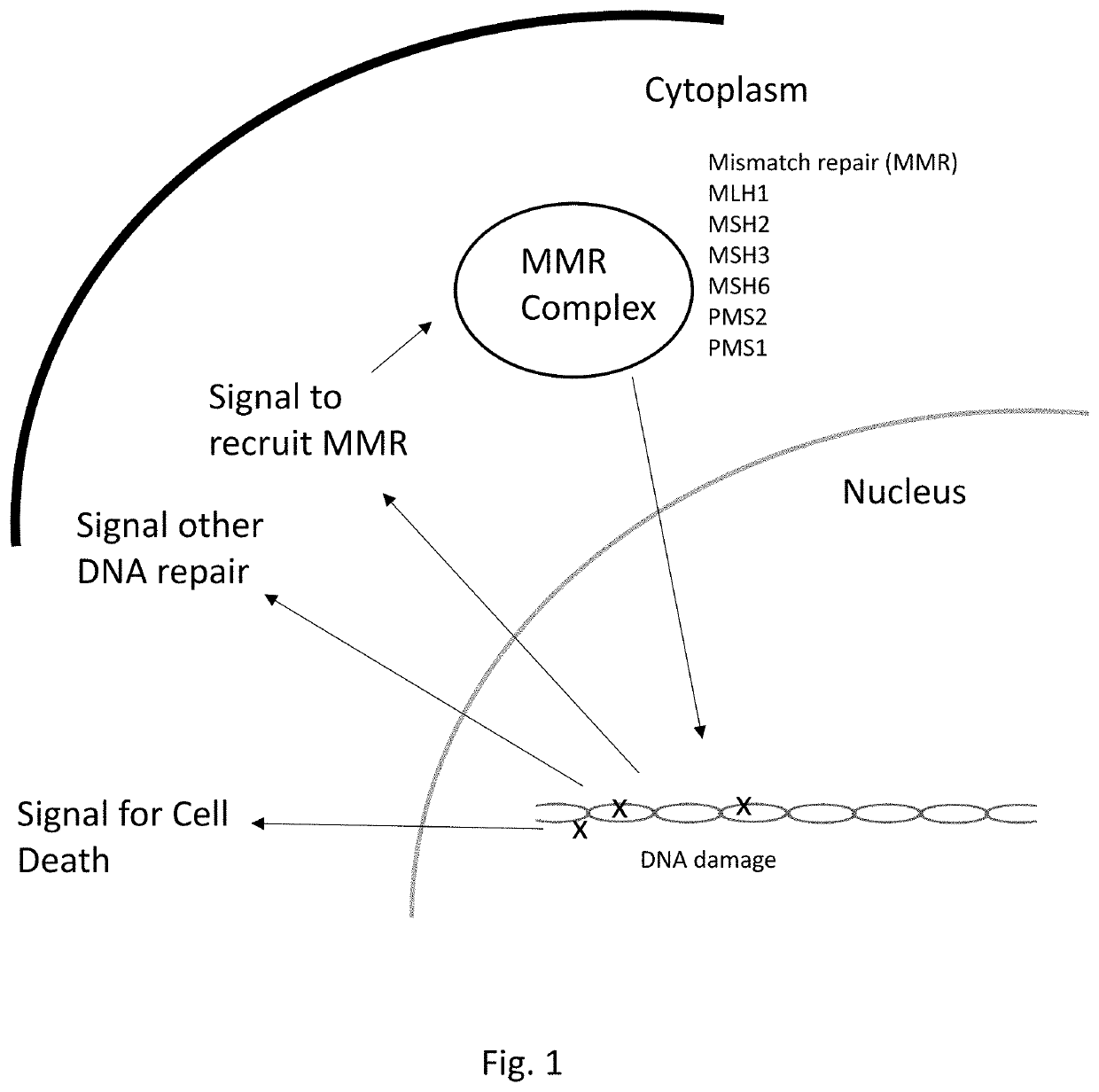
Therapeutic approaches to the treatment of DNA mismatch repair (MMR) deficient cancers are disclosed based on the use of complimentary gene-function and drug screening synthetic lethality approaches for designing therapies for the treatment of cancers where loss of tumour suppressor function has occurred. The work is based on experiments using human MSH2, an integral component of the MMR pathway, and is applicable to other genes in the MMR pathway, and in particular MLH1, MSH6, PMS1 and PMS2. In particular loss of MSH2 is synthetically lethal with inhibition of the DNA polymerase POLβ deficiency of MLH1 is synthetically lethal with DNA polymerase γ (POLG) inhibition.
Owner:MARTIN SARAH +2
MMR gene mutation detection kit
InactiveCN106367475AImprove throughputHigh speedMicrobiological testing/measurementDNA preparationMutation detectionA-DNA
The invention belongs to the field of molecular biology and medicine, and particularly relates to a DNA mismatch repair gene (MMR) mutation detection kit and uses thereof, more particularly to mutation detection of human MLH1 gene, human MSH2 gene, human MSH6 gene, human PMS1 gene and human PMS2 gene, and detection of the correlation with hereditary nonpolyposis colorectal cancer (HNPCC). According to the present invention, the kit can be used for detecting the genotype of the exon mutation of the individual MMR gene so as to provide the reference data for judging whether the individual has HNPCC and whether the suffering risk of family members is greater than the suffering risk of the general populations.
Owner:SHANGHAI CENT FOR BIOINFORMATION TECH +2
Kit for predicting lung cancer metastasis, and usage method of kit
InactiveCN106755322AGood repeatabilityMultiple treatment timesMicrobiological testing/measurementLymphatic SpreadLung cancer
The invention relates to a kit for predicting lung cancer metastasis, and a usage method of the kit. The kit comprises a DNA library kit which comprises probes of multiple genes, and the multiple genes comprise: high-risk genes: BRCA1, BRCA2, PALB2, ATM, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1 and XRCC1; and low-risk genes: ATRX, BRIP1, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FANCO, FANCP, MDM2, MDM4, MLH1, NPM1, PP2R1A, PRKDC, RAD50, STAG2, XRCC5, XRCC6. Through performing related mutation detection to the related genes of DNA repair system signal paths, lung cancer metastasis can be rapidly and conveniently judged and predicted by combining a special rating mechanism.
Owner:苏州首度基因科技有限责任公司 +1
Kit for homologous recombination repair defect detection
The invention discloses a kit for homologous recombination repair defect detection. A multi-probe targeted capture technology is combined with a next-generation sequencing technology to enrich and detect the variation conditions of 25 genes, wherein the 25 genes include ARID1A, BRCA1, BRCA2, POLG, ATR, BRIP1, MSH2, RAD51C, ATM, MLH1, MRE11A, RAD50, ATRX, CHECK1, MUTYH, RAD51D, BAP1, CHECK2, NBN, SMARCB1, BARD1, FANCE, PALB2, WRN and BLM.
Owner:宁波爱她基因科技有限公司
Mutant gene group, library and kit for evaluating risk of female malignant tumor
PendingCN111635942AImprove accuracyEarly interventionNucleotide librariesMicrobiological testing/measurementOncologyBiomedicine
The invention discloses a mutant gene group, library and kit for evaluating female malignant tumor risk, and belongs to the technical field of biomedical molecular detection. The mutant gene group forevaluating the risk of female malignant tumors comprises one or more selected from a group consisting of the following 25 genes: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53, BARD1, MRE11A, MUTYH, RAD50, XRCC2 and FANCC5. According to the invention, female malignant tumor related genetic markers can be widely screened andinspected, accuracy is higher, and a basis is provided for prediction, prevention and diagnosis of female malignant tumor onset risks.
Owner:AIR FORCE MEDICAL UNIV
C terminal specific human DNA mismatch repair protein MLH1 polypeptide and antibody preparation method
InactiveCN101318997AHigh potencyHigh affinityImmunoglobulins against animals/humansDiseaseCarrier protein
The invention discloses C-terminal specific human DNA mismatch repair protein MLH1 polypeptide, as well as an antibody and a preparation method thereof, belonging to the biomedicine technical field. The amino acid sequence of the C-terminal specific human MLH1 polypeptide is SLEGDTTKGTSEMSE. Antihuman MLH1 polypeptide antibody is prepared according to the following method that: (1) human MLH1 epitope is analyzed; (2) human MLH1 C-terminal polypeptide is synthesized; (3) synthesized polypeptide is crosslinked with carrier protein; (4) rabbit anti-human MLH1 polypeptide antibody is prepared; (5) serum containing the antibody is obtained through collection and separation, and the antibody is purified, and then the antihuman MLH1 polypeptide antibody can be obtained. The C-terminal specific antihuman MLH1 synthesized polypeptide antibody is high in potency, strong in affinity, good in specificity, capable of having specific binding reaction with natural human DNA mismatch repair protein MLH1 and low in preparation cost; purified antibody can be completely used for immunoblot and enzyme linked immunosorbent assay, as well as the establishment of in vitro immunization analytical method. The antibody provides a useful tool for the research on human MLH1 biological function as well as the relation between the human MLH1 and related diseases.
Owner:BEIJING IMMUNOHUNT CORP
Library construction method for detecting endometrial cancer related gene mutation based on high-throughput sequencing
ActiveCN112064122ALow costImplementing detection applicationsNucleotide librariesMicrobiological testing/measurementExonBlood plasma
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
Type 1 Diabetes Biomarkers
ActiveUS20200182887A1High detection sensitivityIncreased riskDisease diagnosisBiological testingAntigenAntiendomysial antibodies
Type 1 diabetes (T1D) patients make antibodies to self-proteins that are potential biomarkers for early detection and risk prediction. We have identified seventeen antigens as biomarkers for early diagnosis and risk prediction of T1D, including the antigens MLH1, MTIF3, PPIL2, NUP50, TOX4, FIGN, C9orf142, ZNF280D, HES1, QRFPR, CTRC, SNX6, SYTL4, ELA2A, IGRP, PAX6, and HMGN3.
Owner:ARIZONA STATE UNIVERSITY +1
Primer probe system for MLH1 (MutL homolog1) gene methylation detection and kit adopting primer probe system
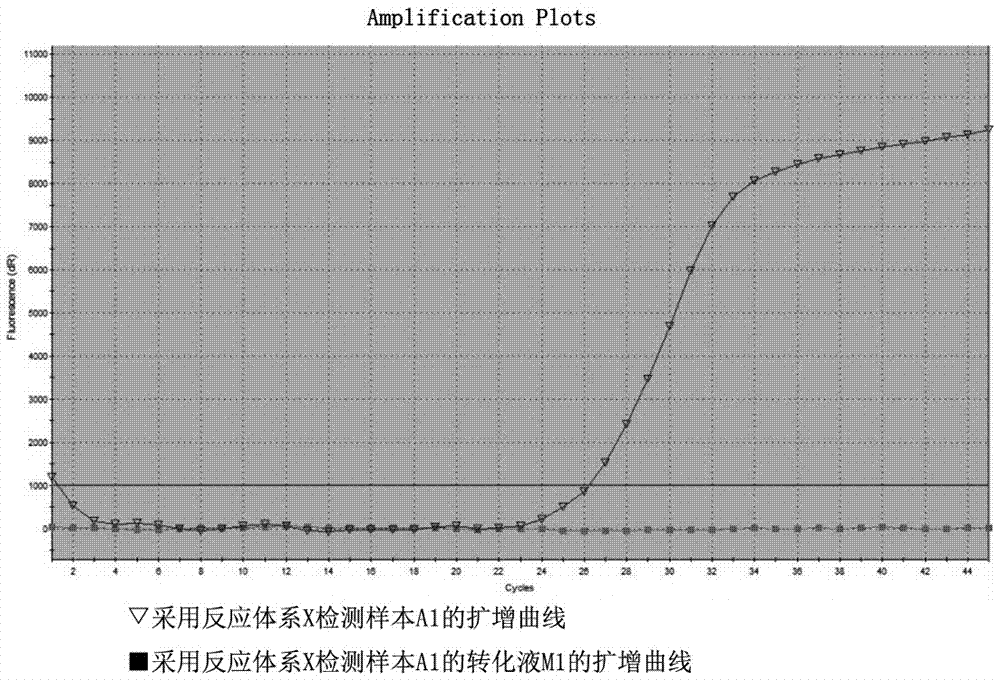
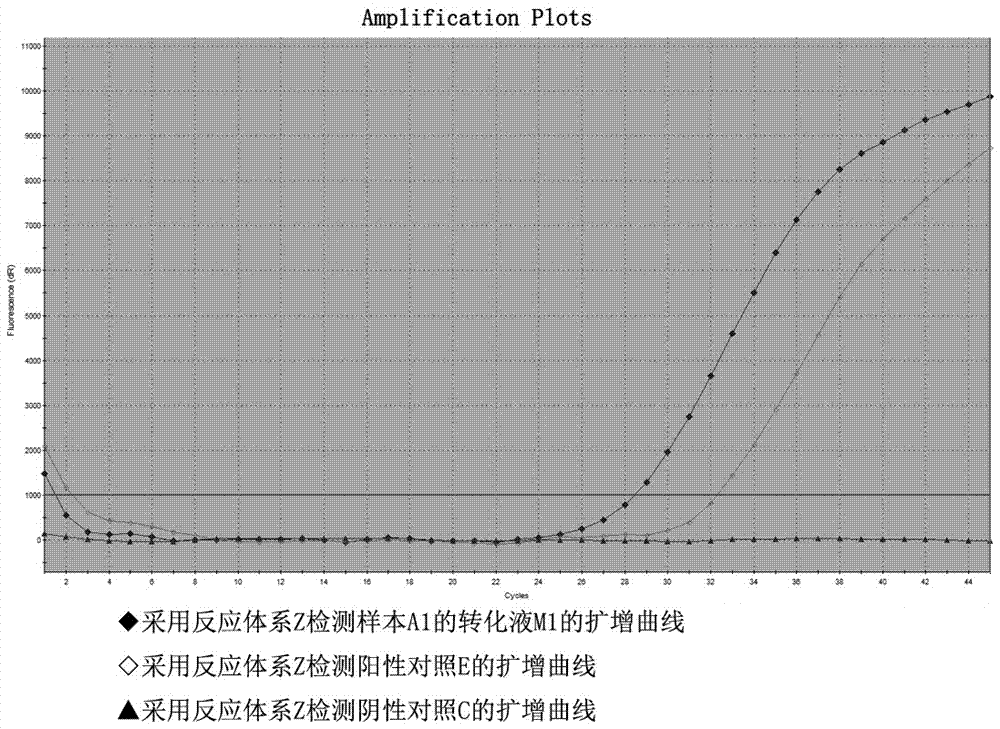
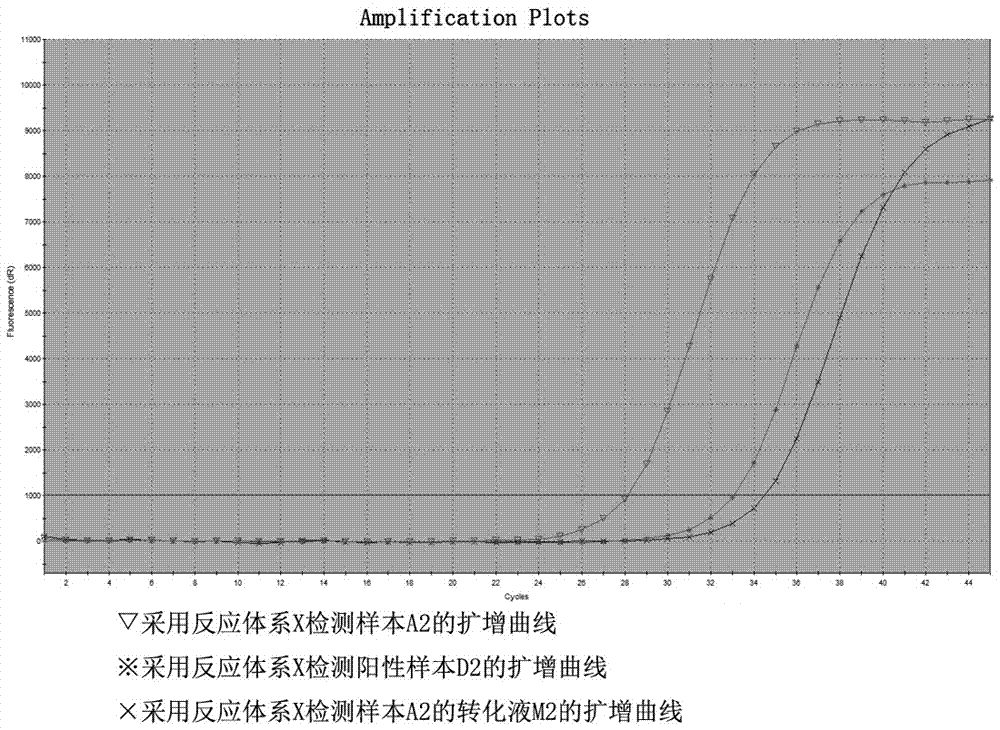
ActiveCN105441557AImprove bindingEliminate distractionsMicrobiological testing/measurementDNA/RNA fragmentationForward primerFluorescence
The invention relates to a primer probe system for MLH1 (MutL homolog1) gene methylation detection and a kit adopting the primer probe system. The primer probe system comprises a primer probe set X used for determining genomic DNA quality, a primer probe set Y used for determining a methylation conversion rate and a primer probe set Z used for detecting MLH1 gene promoter methylation conditions, wherein the primer probe set X comprises a forward primer a, a reverse primer a and a probe a; the primer probe set Y comprises a forward primer b, a reverse primer b and a probe b; the primer probe set Z comprises the forward primer b, the reverse primer b, a probe c and a probe d; fluorescence report groups are arranged at 5' ends of the probe a, the probe b and the probe c, and fluorescence quenching groups are arranged at 3' ends of the probe a, the probe b, the probe c and the probe d. According to the kit, the implementing scheme is concise, and the sensitivity and the accuracy rate are high.
Owner:上海达澈生物科技有限公司
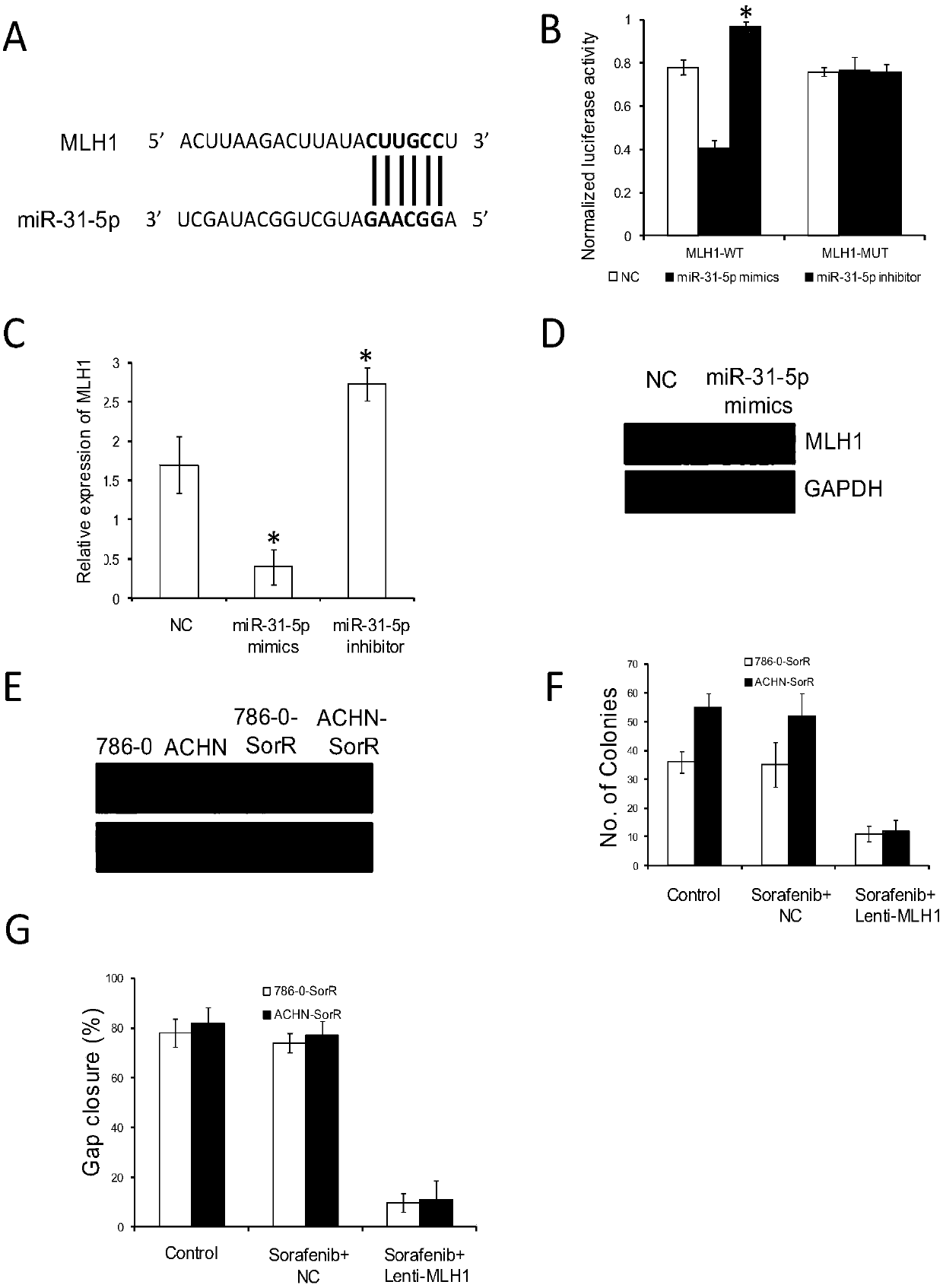
Application of reagent for detecting expression level of MLH1 to preparation of kit for detecting sensitivity of tumor targeted drug
ActiveCN108034726AIncreased sensitivityPredicting Drug Treatment EffectsMicrobiological testing/measurementAntineoplastic agentsTumor targetMedicine
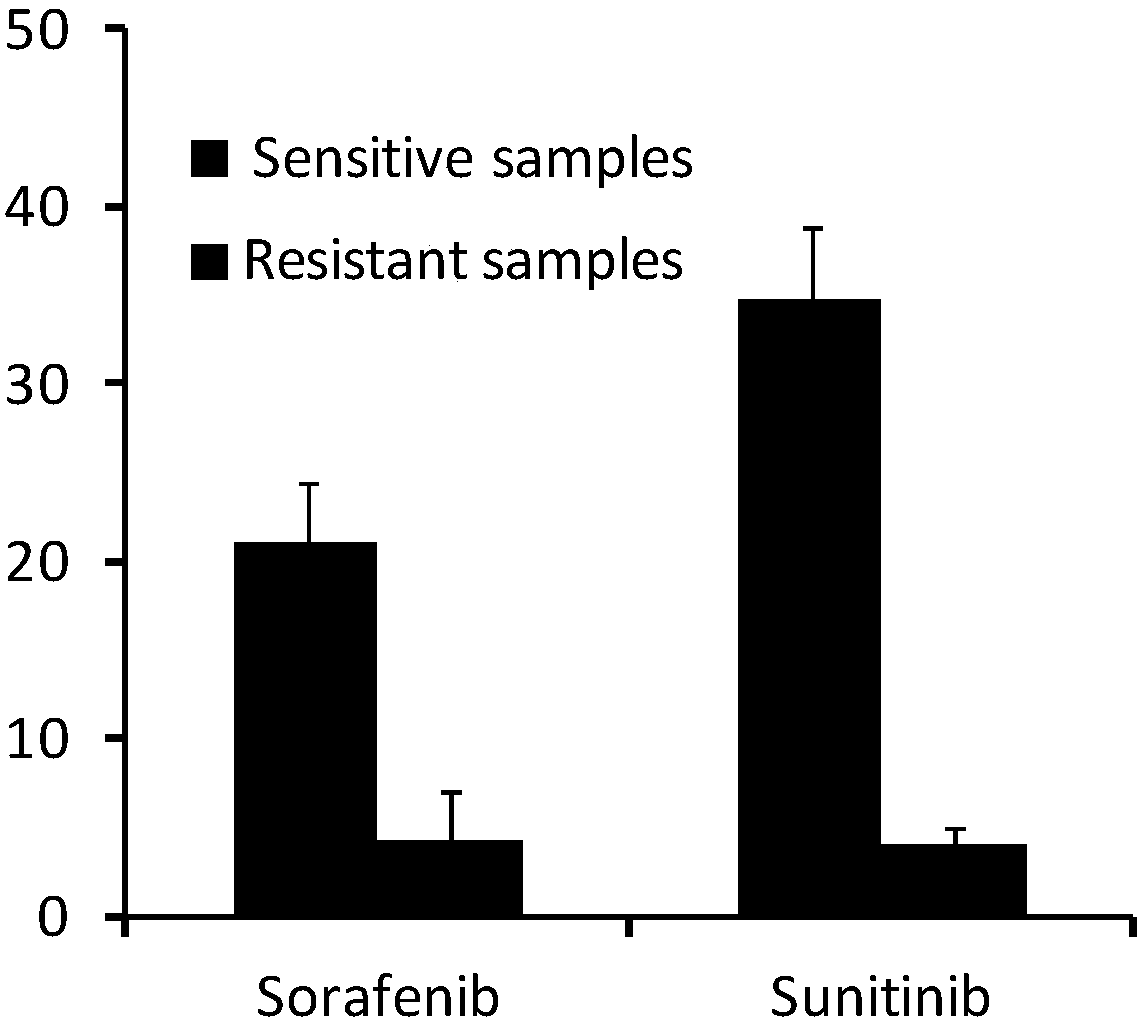
The invention discloses a kit for detecting sensitivity of a tumor targeted drug. The kit comprises an optional reagent for detecting the expression level of MLH1. The invention also discloses application of the reagent to detection of the expression level of the MLH1 in preparation of the kit for detecting the sensitivity of the tumor targeted drug. The kit has the advantages that by detecting the expression level of the MLH1, the sensitivity of a tumor patient to the targeted drugs (sorafenib and sunitinib) can be judged, the drug treatment effect on the tumor patient can be predicted in theclinical application, the basis is provided for the patient to take the related treatment measure or decision, and the clinical application prospect is good.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Methods and compositions for diagnosing and treating, germline mismatch repair deficiencies, lynch syndrome and assessing germline risks of cancer
PendingUS20220341935A1Change is minimalMicrobiological testing/measurementDisease diagnosisMolecular phenotypePhosphorylation
Owner:MORGAN & MENDEL GENOMICS INC +1
Method and kit for detection of microsatellite instability-positive cell
InactiveUS8828662B2High expressionSugar derivativesMicrobiological testing/measurementCancers diagnosisNucleotide
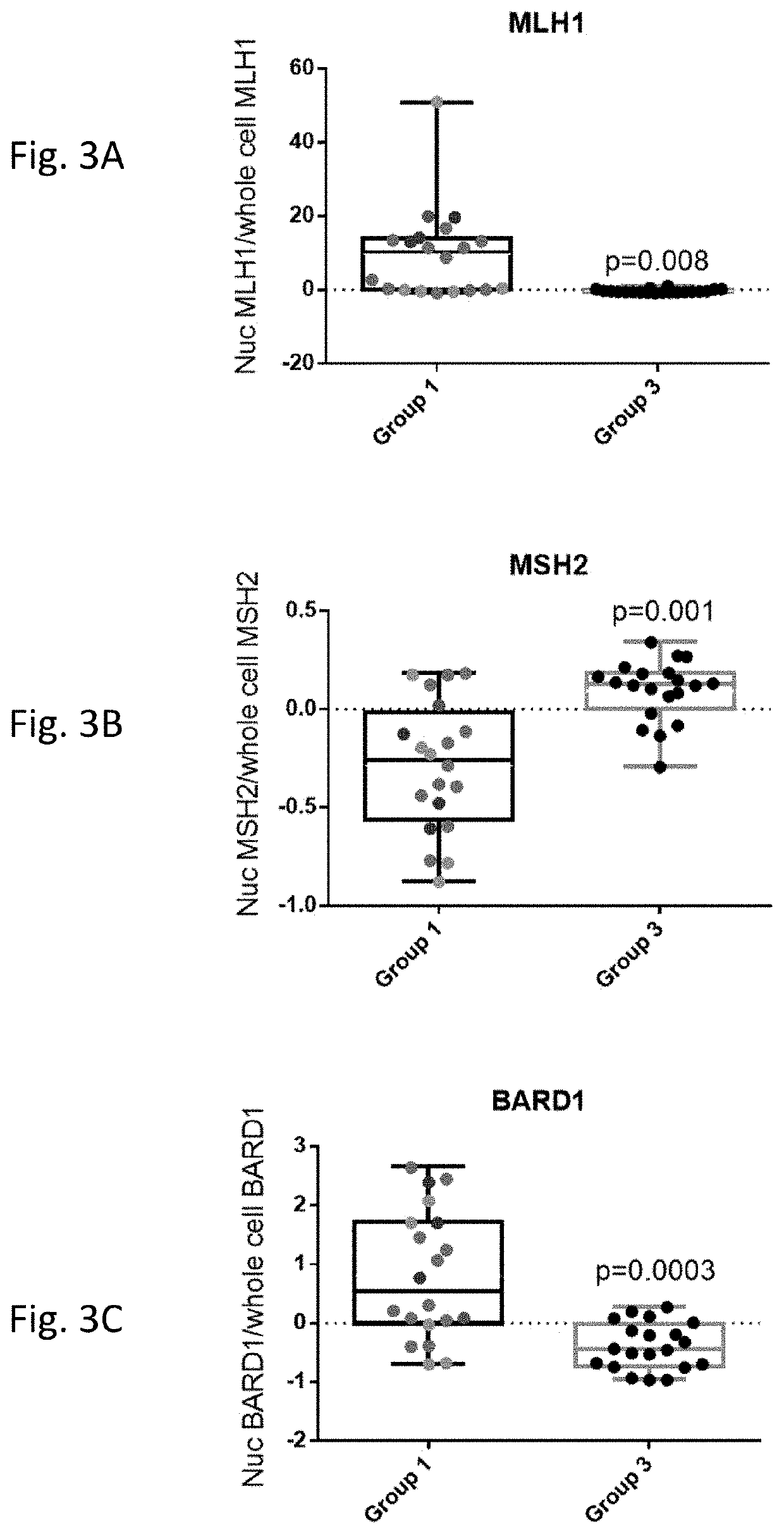
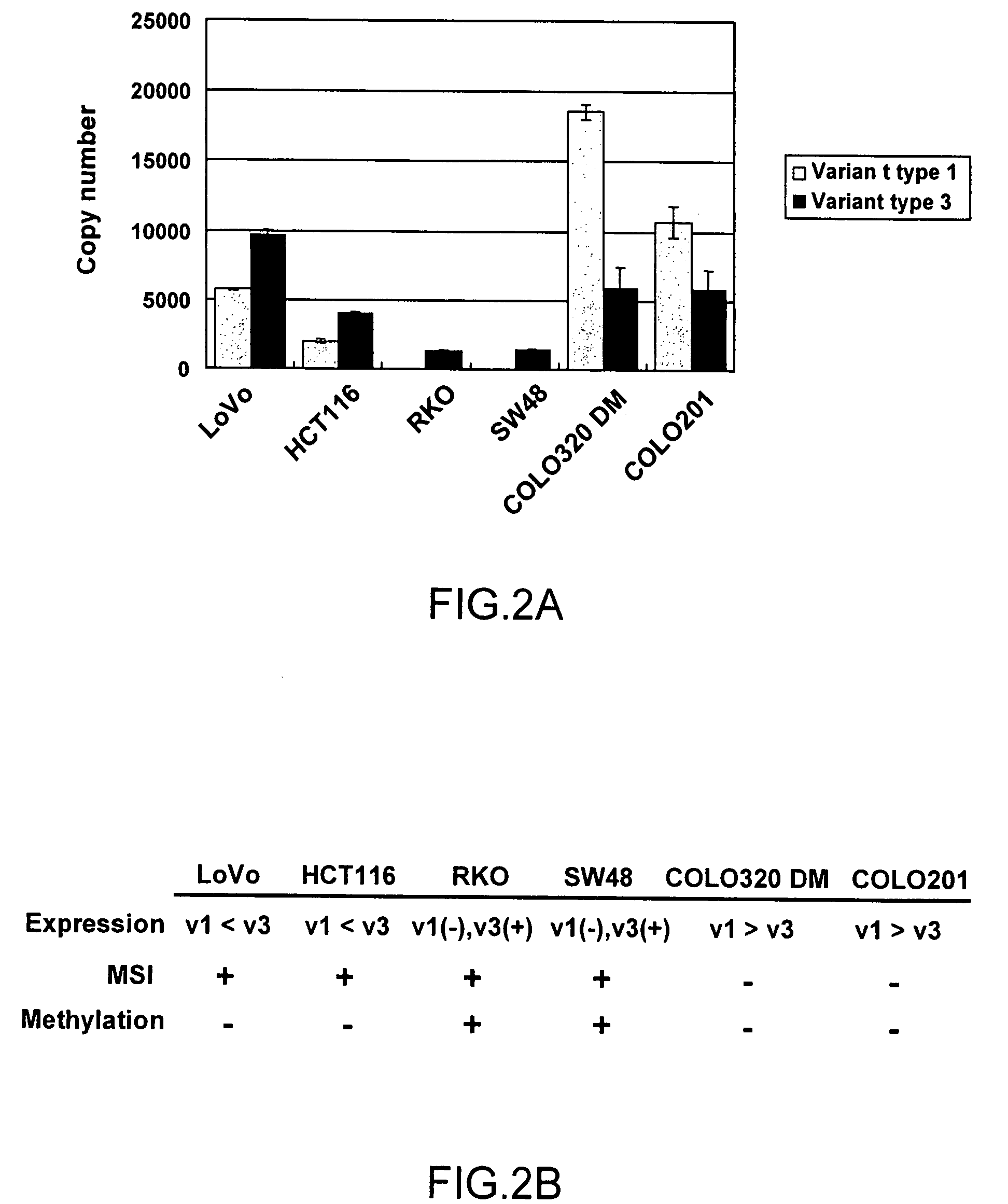
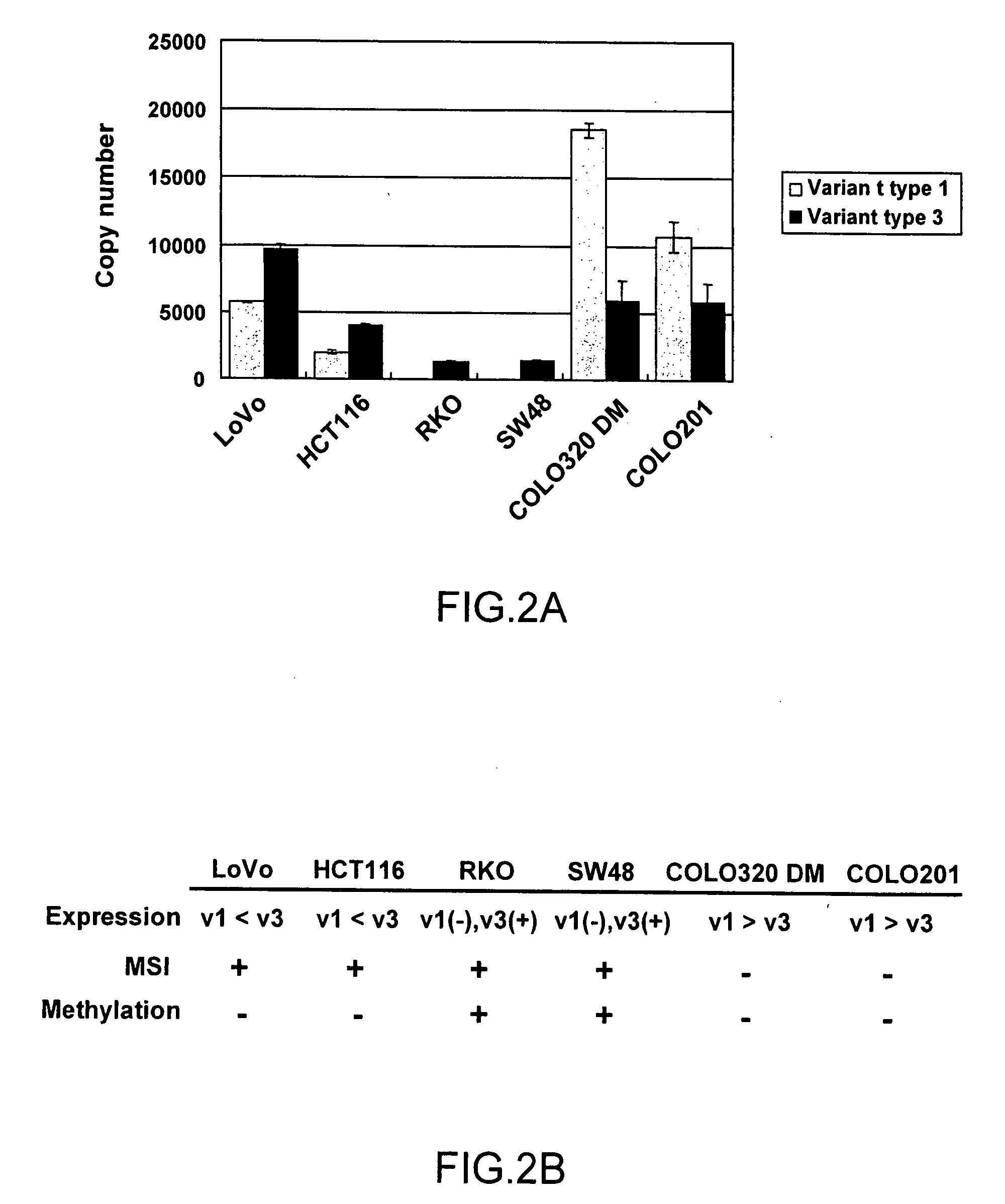
A method for detecting an abnormal cell based on gene expression analysis, which is useful for cancer diagnosis, is provided. A gene expression analysis method, comprising: measuring the expression level of a transcript of a human MLH1 gene containing the nucleotide sequence as shown in SEQ ID NO: 1 at the 5′-terminus thereof and the expression level of a transcript of a human MLH1 gene containing the nucleotide sequence as shown in SEQ ID NO: 2 at the 5′-terminus thereof, in a biological sample; and comparing the expression levels, thereby detecting a cell positive for microsatellite instability.
Owner:HITACHI LTD
Method and kit for detection of microsatellite instability-positive cell
InactiveUS20080118927A1High expressionMicrobiological testing/measurementNucleotideCancers diagnosis
A method for detecting an abnormal cell based on gene expression analysis, which is useful for cancer diagnosis, is provided. A gene expression analysis method, comprising: measuring the expression level of a transcript of a human MLH1 gene containing the nucleotide sequence as shown in SEQ ID NO: 1 at the 5′-terminus thereof and the expression level of a transcript of a human MLH1 gene containing the nucleotide sequence as shown in SEQ ID NO: 2 at the 5′-terminus thereof, in a biological sample; and comparing the expression levels, thereby detecting a cell positive for microsatellite instability.
Owner:HITACHI LTD
A kind of MMR gene mutation detection kit
InactiveCN106367475BImprove throughputHigh speedMicrobiological testing/measurementDNA preparationMutation detectionA-DNA
The invention belongs to the field of molecular biology and medicine, and particularly relates to a DNA mismatch repair gene (MMR) mutation detection kit and uses thereof, more particularly to mutation detection of human MLH1 gene, human MSH2 gene, human MSH6 gene, human PMS1 gene and human PMS2 gene, and detection of the correlation with hereditary nonpolyposis colorectal cancer (HNPCC). According to the present invention, the kit can be used for detecting the genotype of the exon mutation of the individual MMR gene so as to provide the reference data for judging whether the individual has HNPCC and whether the suffering risk of family members is greater than the suffering risk of the general populations.
Owner:SHANGHAI CENT FOR BIOINFORMATION TECH +2
Gene library constructing method for hereditary gastrointestinal tumor and kit
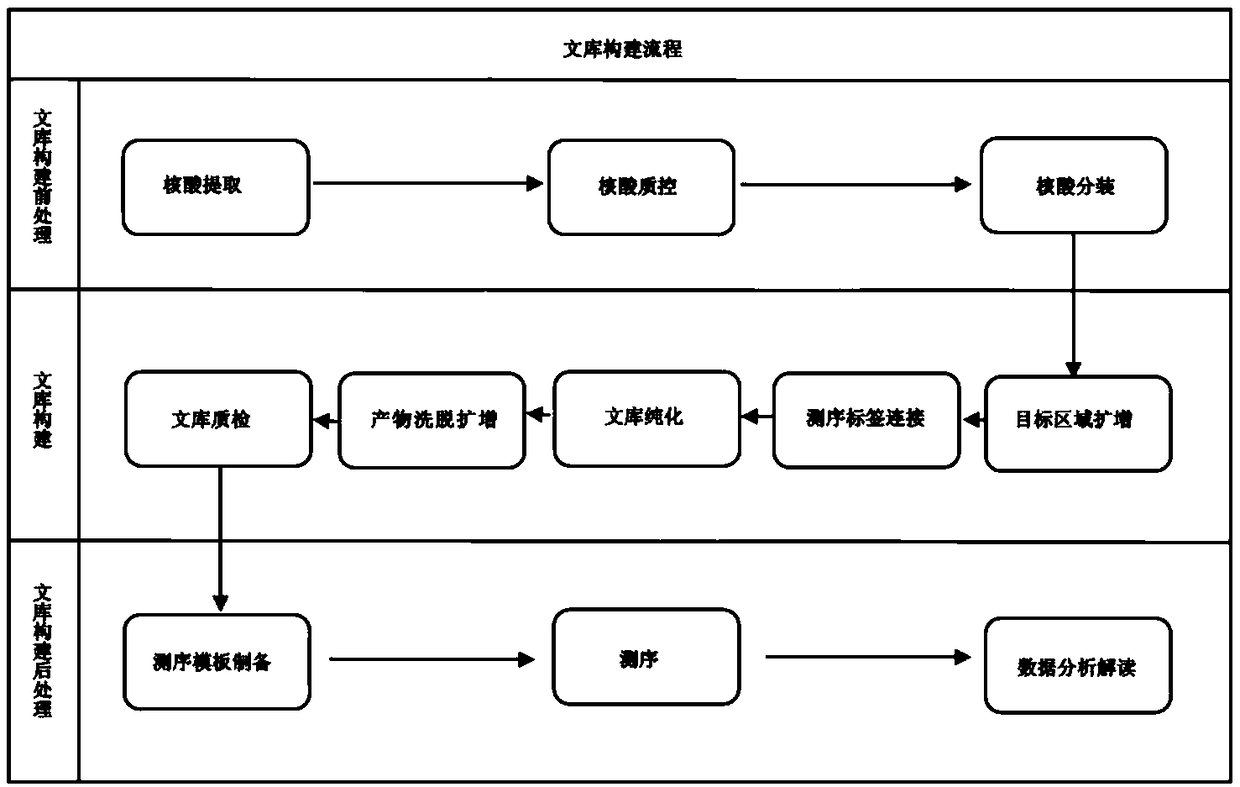
InactiveCN109468312AReduce demandEasy to operateMicrobiological testing/measurementLibrary creationExonWilms' tumor
The invention discloses a gene library constructing method for a hereditary gastrointestinal tumor, and relates to mutation of 7 genes, namely APC, CDH1, MSH2, MLH1, MSH6, PMS2 and EPCAM. Target regions are exon regions of encoding amino acids of the 7 genes and respective 20 base regions at the upstream and the downstream of exons. In order to ensure complete coverage of the target regions, a primer group necessary for PCR amplification is separated into two independent primer groups, and the target regions are amplified respectively. Then, sequencing tag connection, library elution and re-amplification are performed on a PCR product, and purification is performed after the amplification to obtain a sequencing library. The library constructing method is simple and rapid in steps, the costof library construction is effectively reduced and genes related to the gastrointestinal tumor are covered. Through combination with a high-throughput sequencing instrument, mutation of the related genes can be quickly and accurately obtained, so that the gene library constructing method is of great significance for the hereditary gastrointestinal tumor.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Kit for detecting human MLH1 gene methylation and use method of kit
PendingCN112391476AImprove positive detection rateImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationNucleotideA-DNA
The invention discloses a kit for detecting human MLH1 gene methylation and a use method of the kit. The kit comprises a specific primer and a probe which are used for detecting or measuring the methylation state or level of the MLH1 gene in DNA of a tested sample. The kit can significantly increase the positive detection rate and specificity (real negative rate) of early colorectal cancer and canimprove the detection sensitivity of a biomarker to a picogram / nanogram level DNA molecule, and the improvement of the detection sensitivity is realized by optimizing a specific nucleotide sequence primer and a DNA probe and improving a bisulfite treatment method of plasma free DNA.
Owner:深圳市新合生物医疗科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com