Patents
Literature
31results about How to "Laborious and time-consuming" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
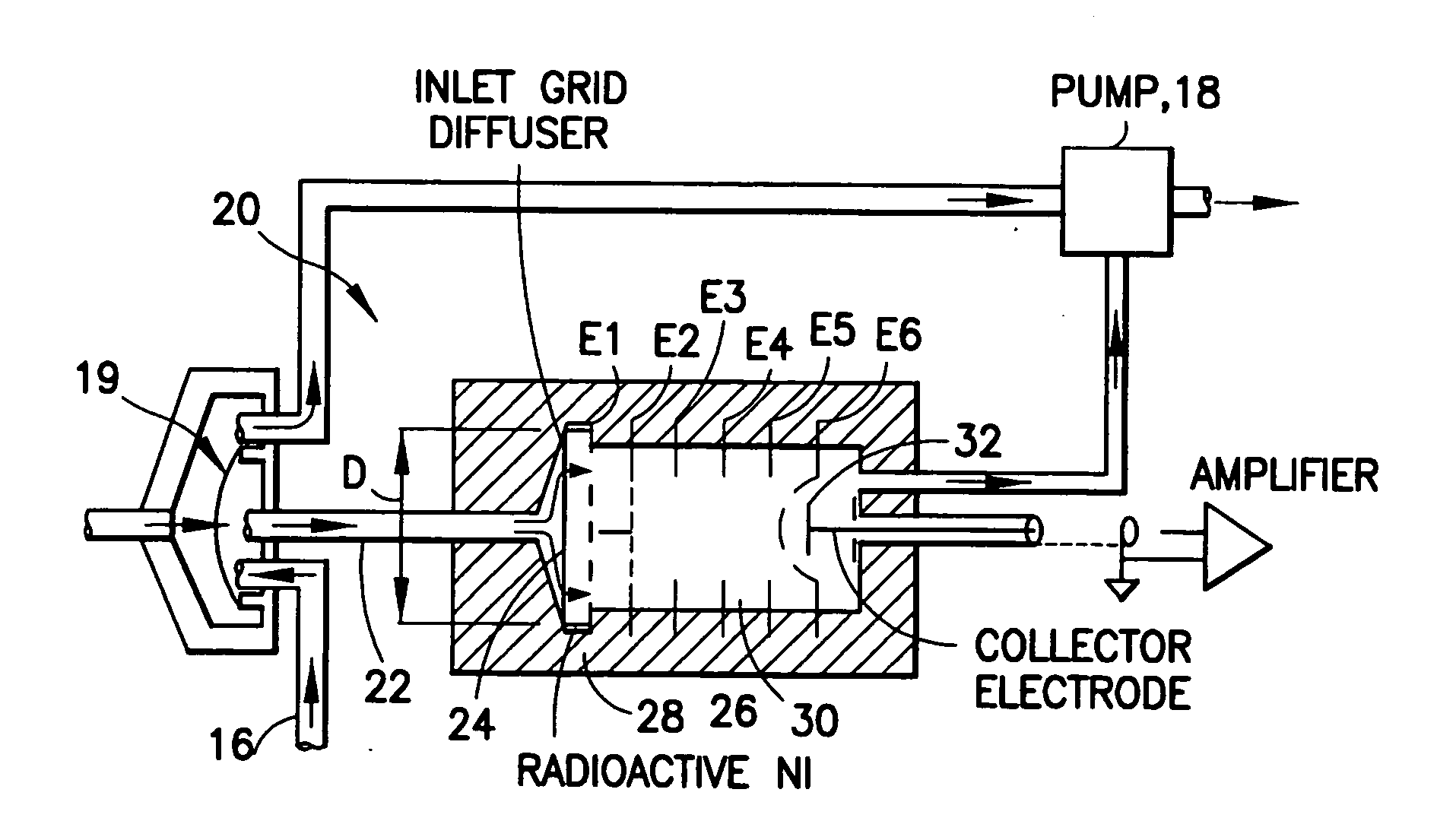
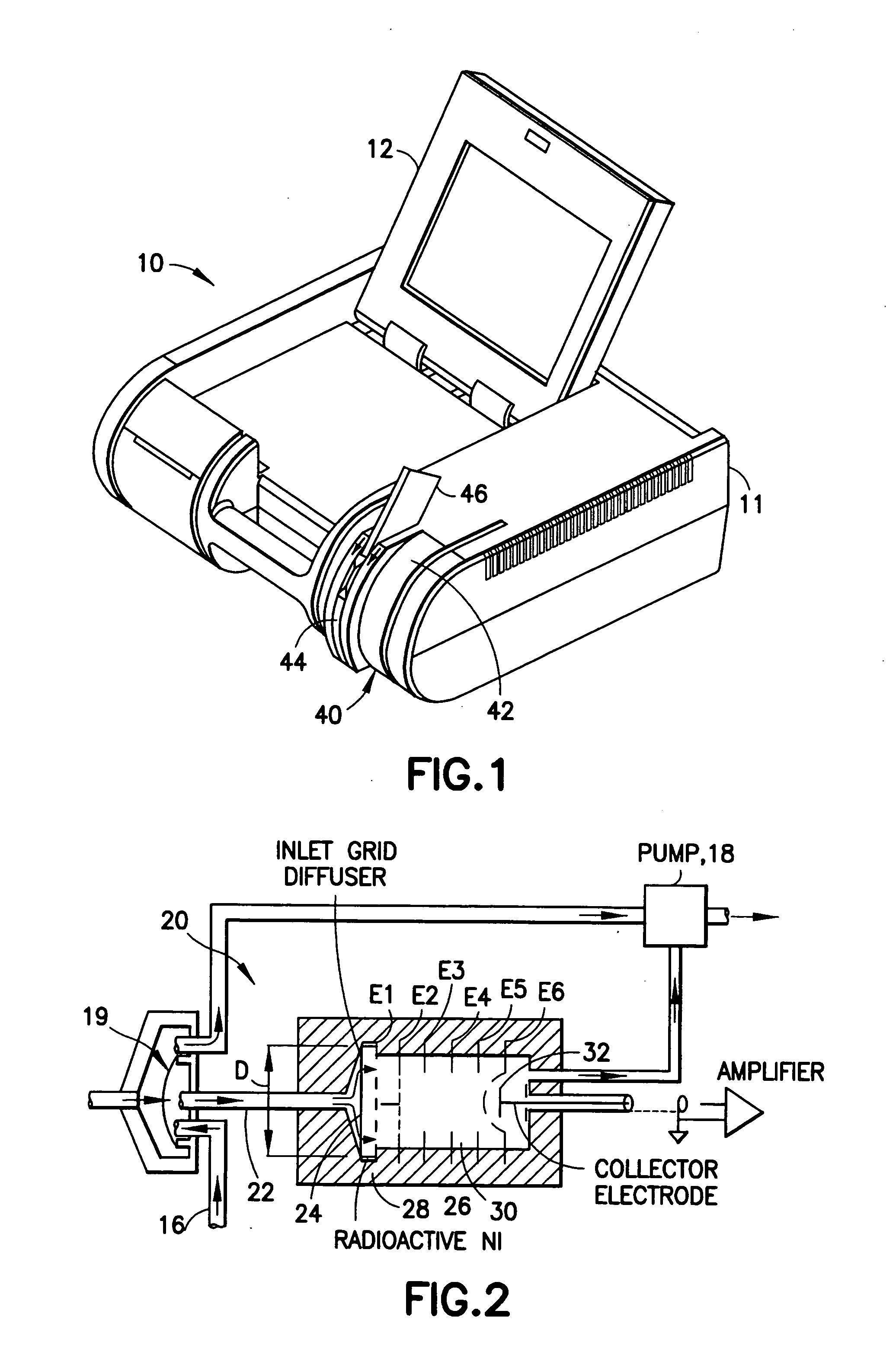
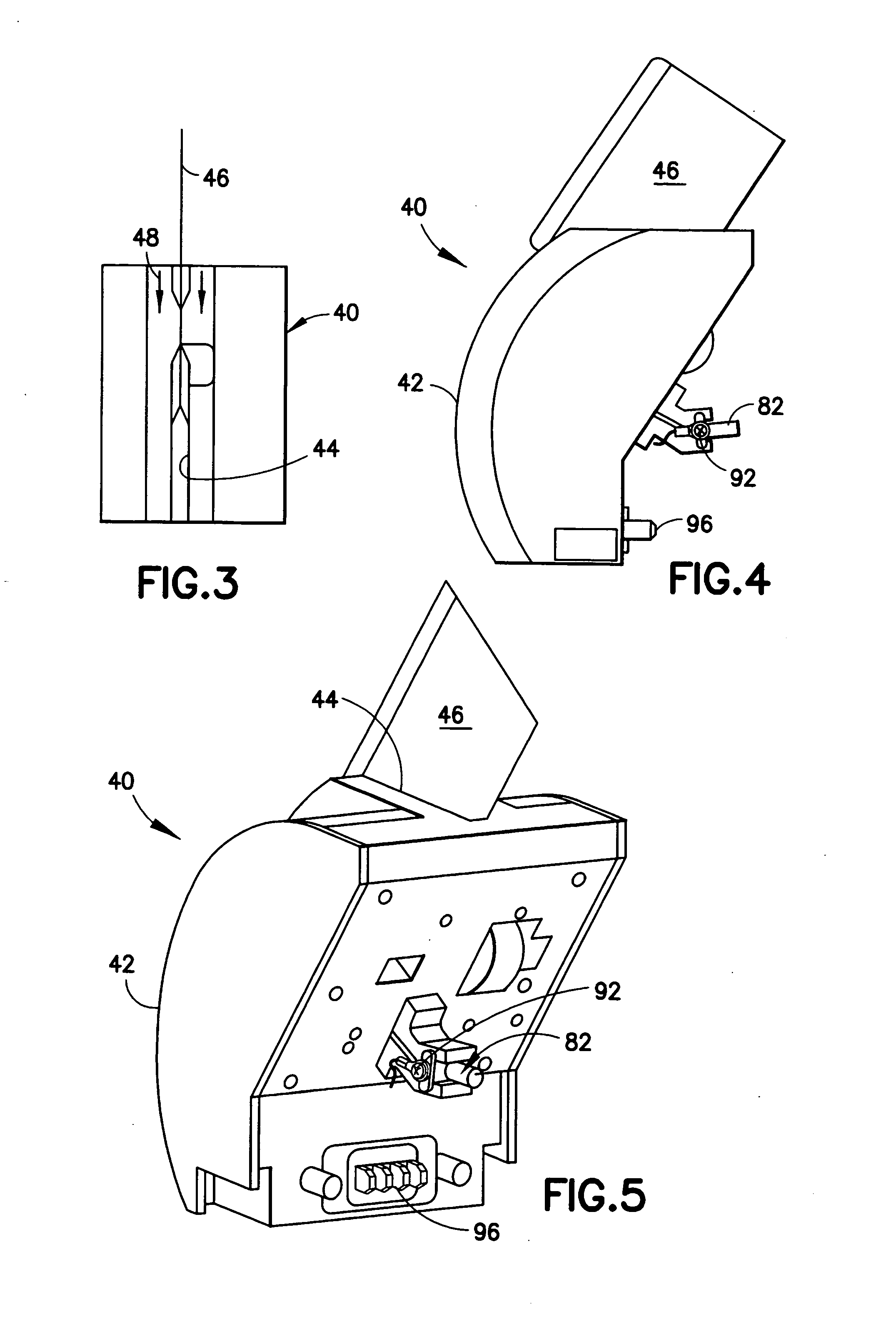
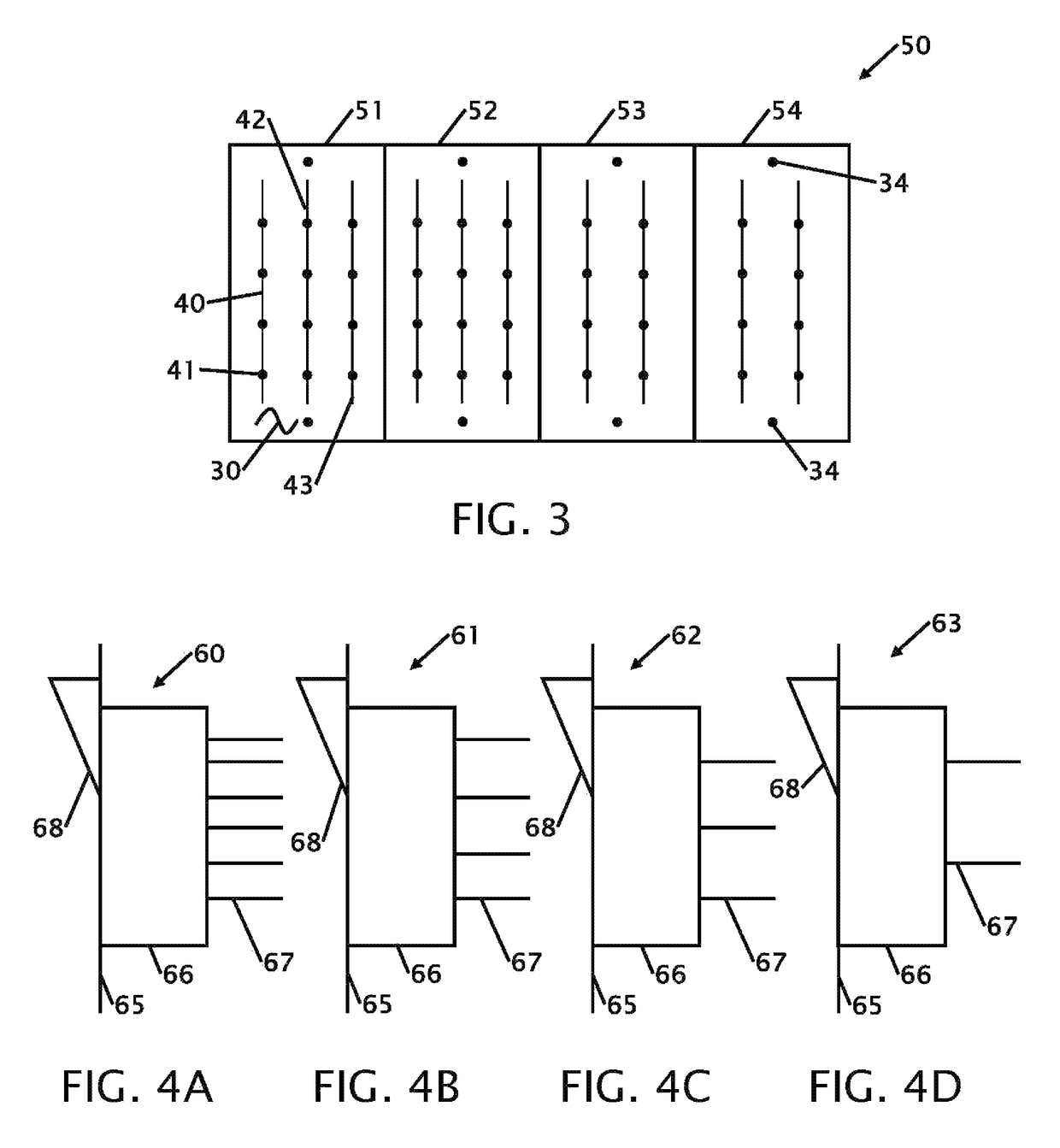
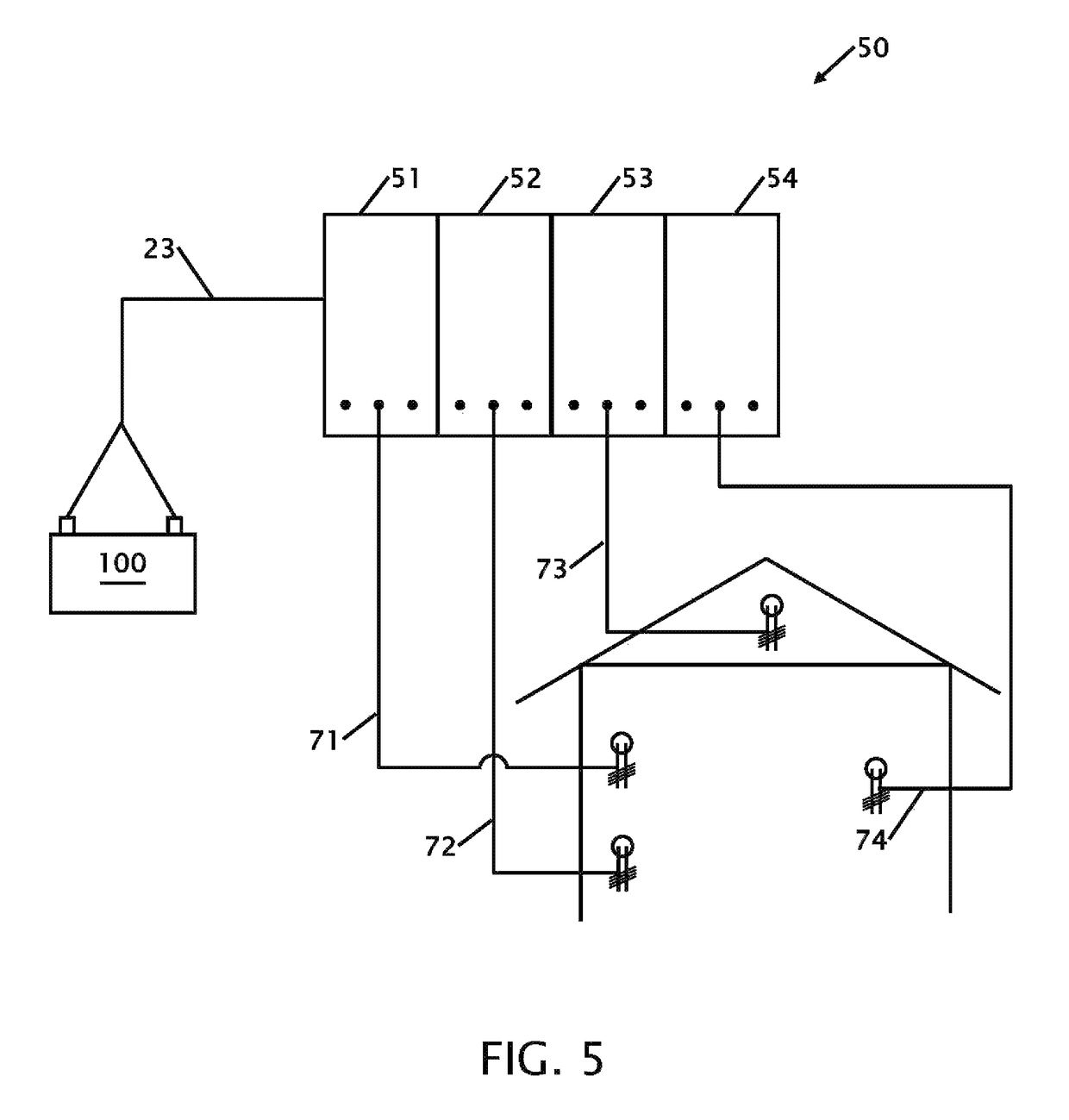
Device for testing surfaces of articles for traces of explosives and/or drugs
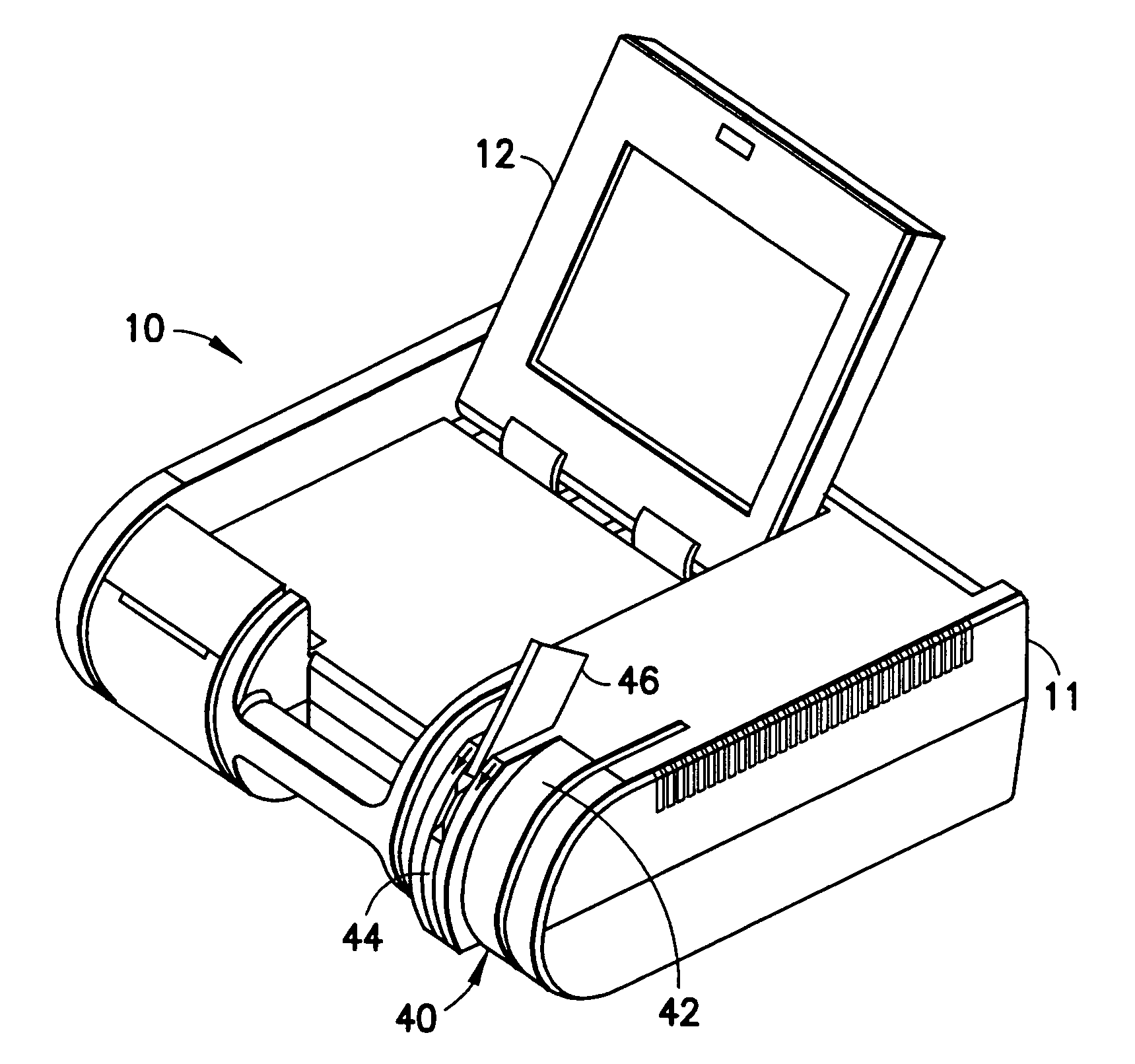
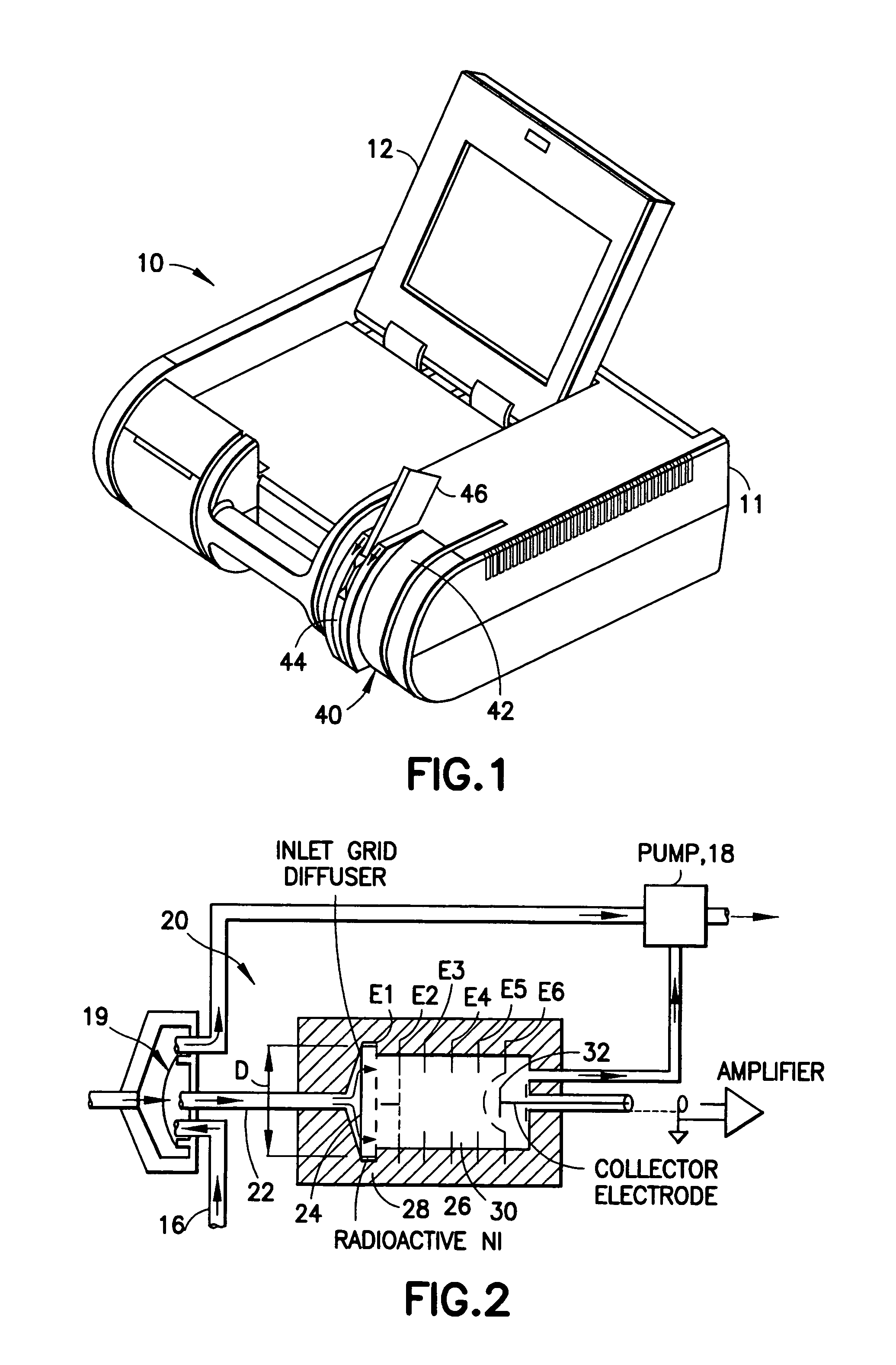
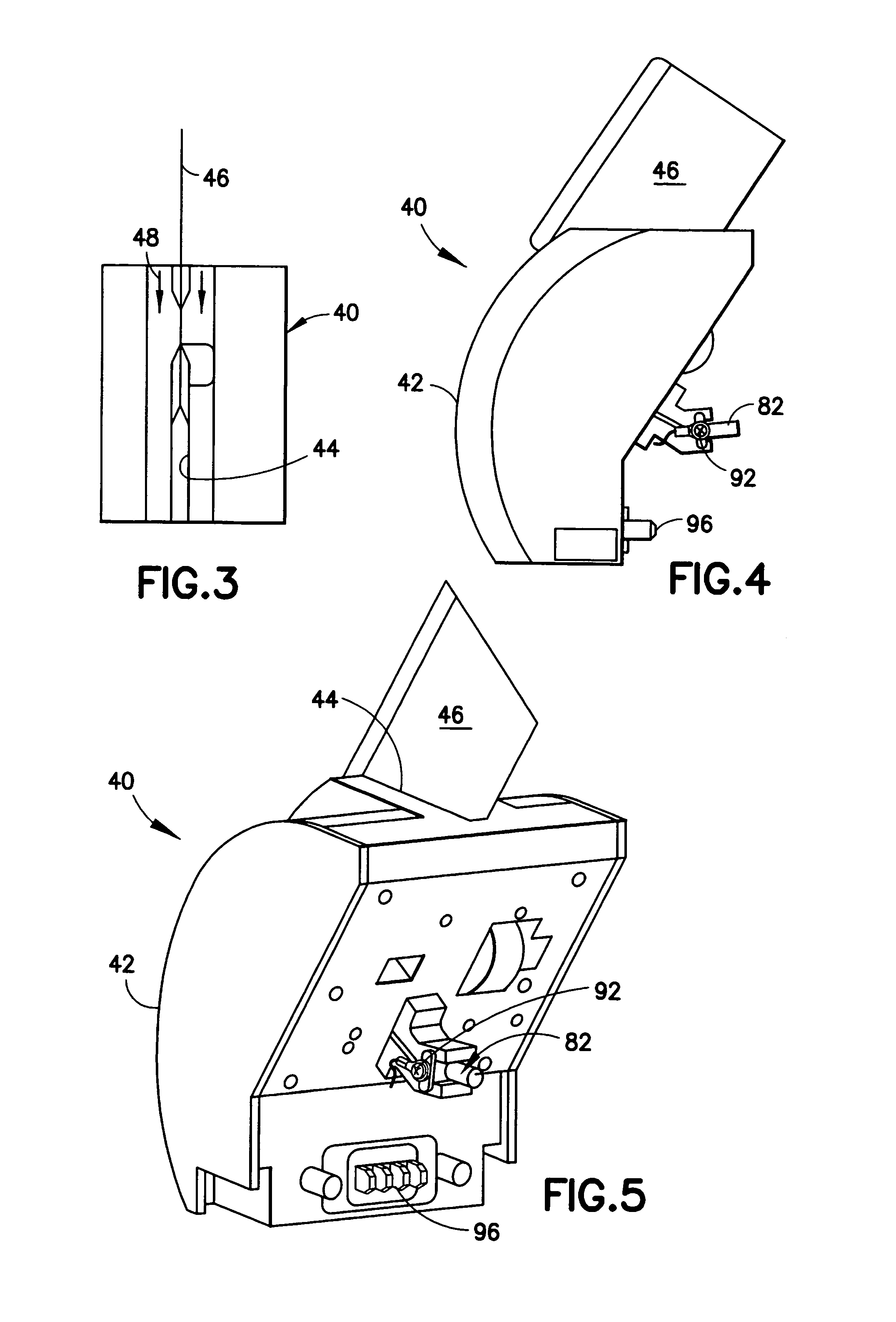
InactiveUS20050019220A1Laborious and time-consumingHeating fastMaterial analysis using wave/particle radiationTime-of-flight spectrometersExplosive materialMetal
A device is provided for testing surfaces of a card for the presence of explosives, drugs or other substances of interest. The device includes a slot for receiving the card. Thin metallic wiper blades are dispose in alignment with the slot and wipe over surfaces of the card as the card is passed through the slot. Thus, substances on the surface of the card are transferred to the wiper blade. The wiper blade then is enclosed and rapidly heated to desorb the material retrieved from the card. The enclosure then is placed in communication with a detector to test for the presence of substances of interest.
Owner:RAPISCAN SYST INC (US)
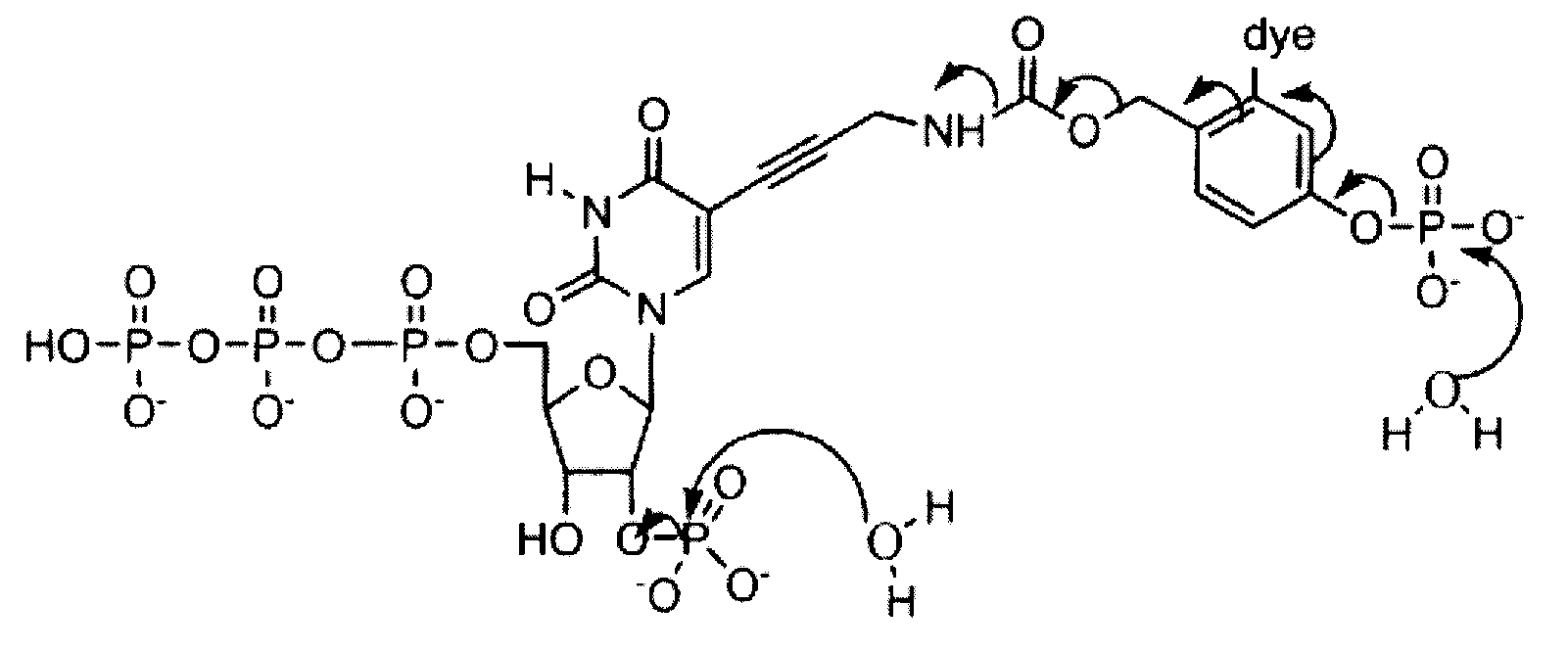
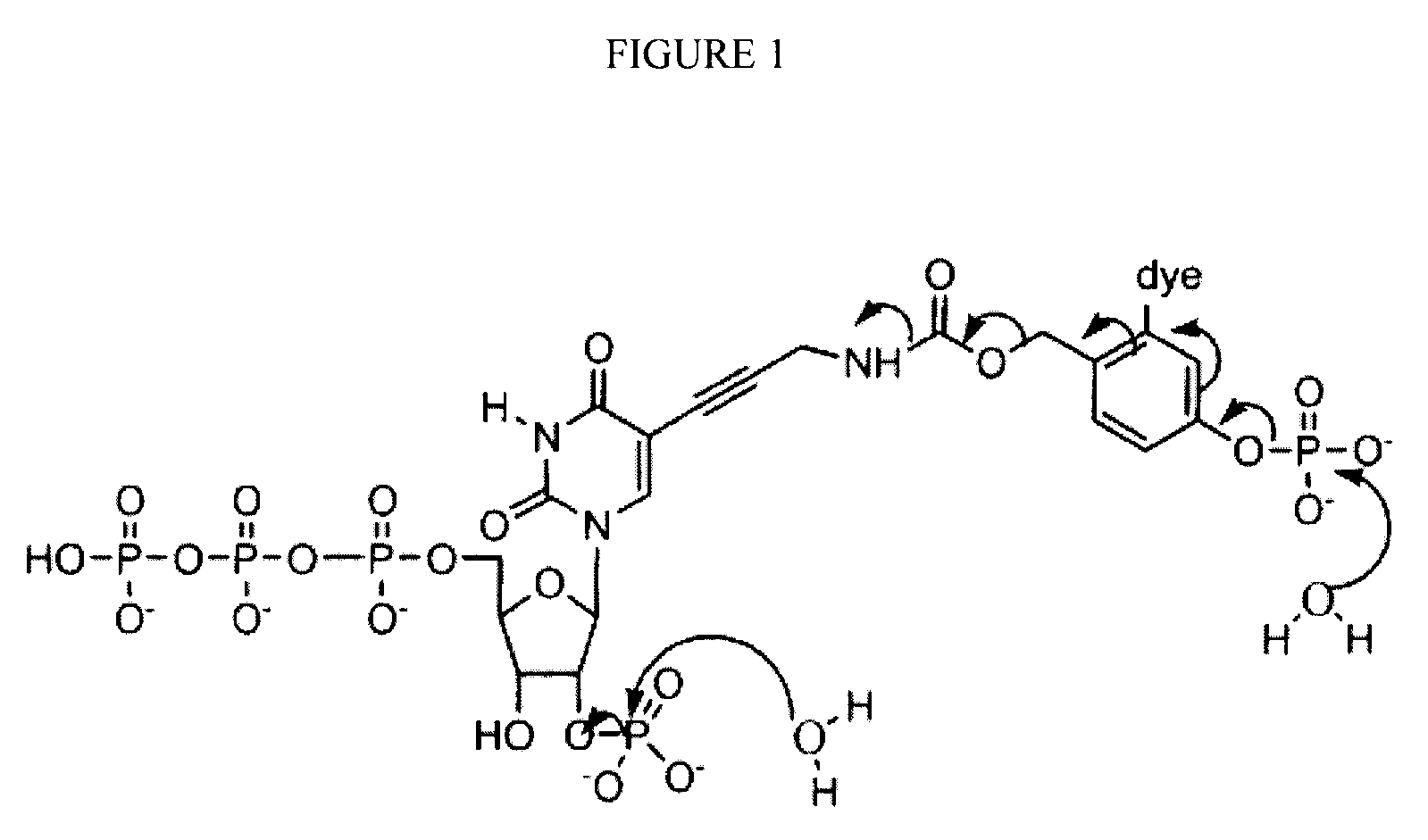
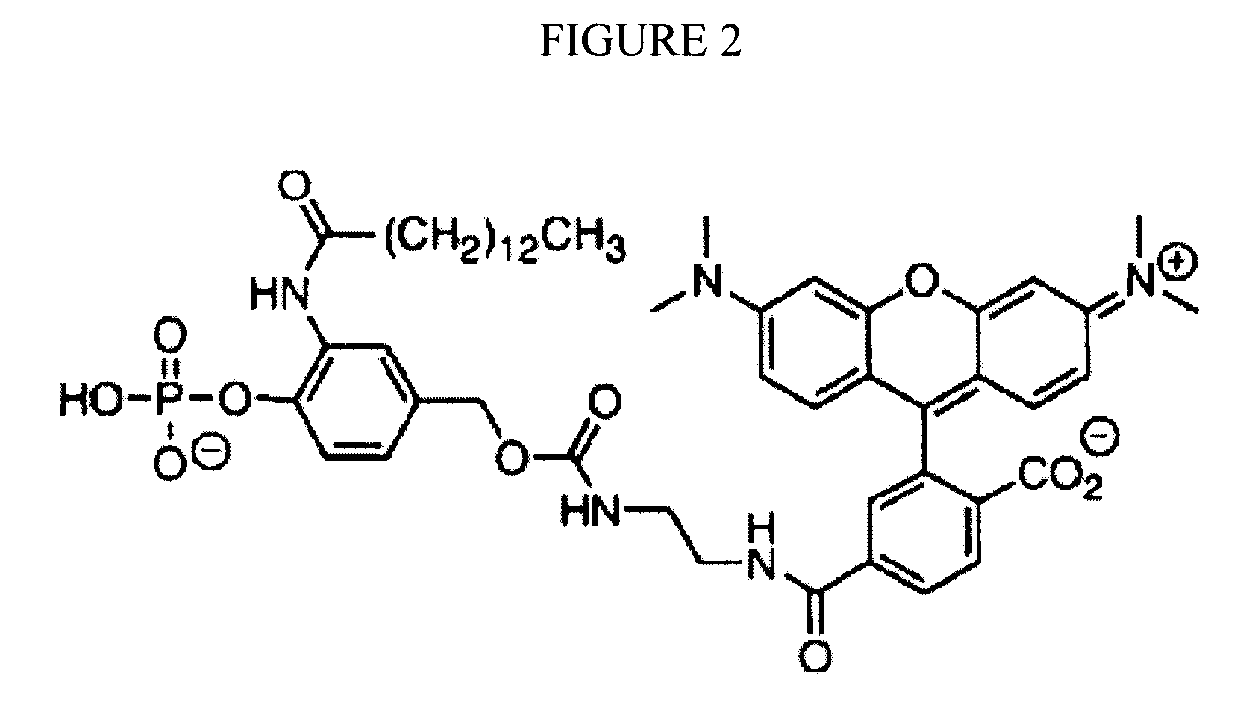
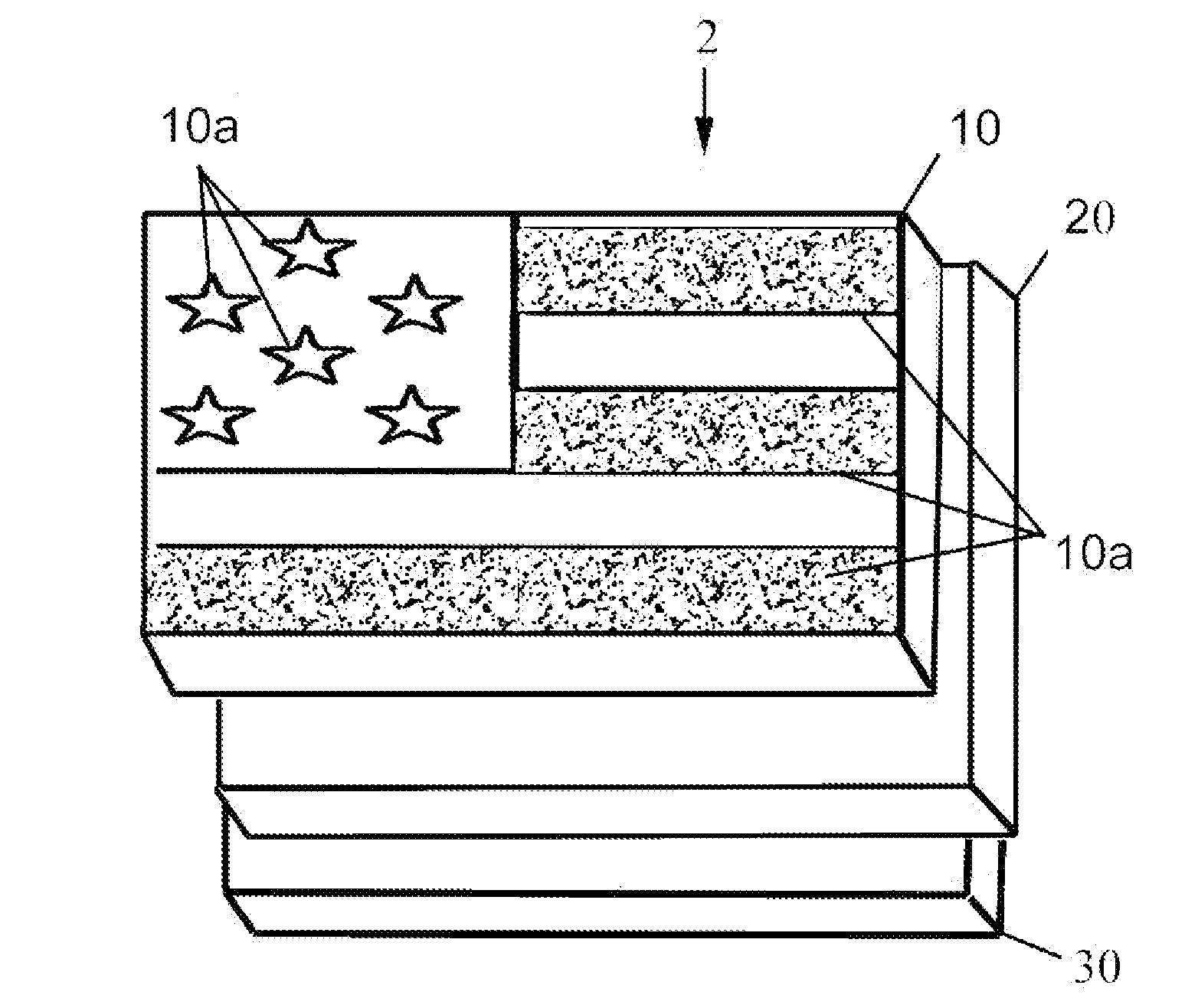
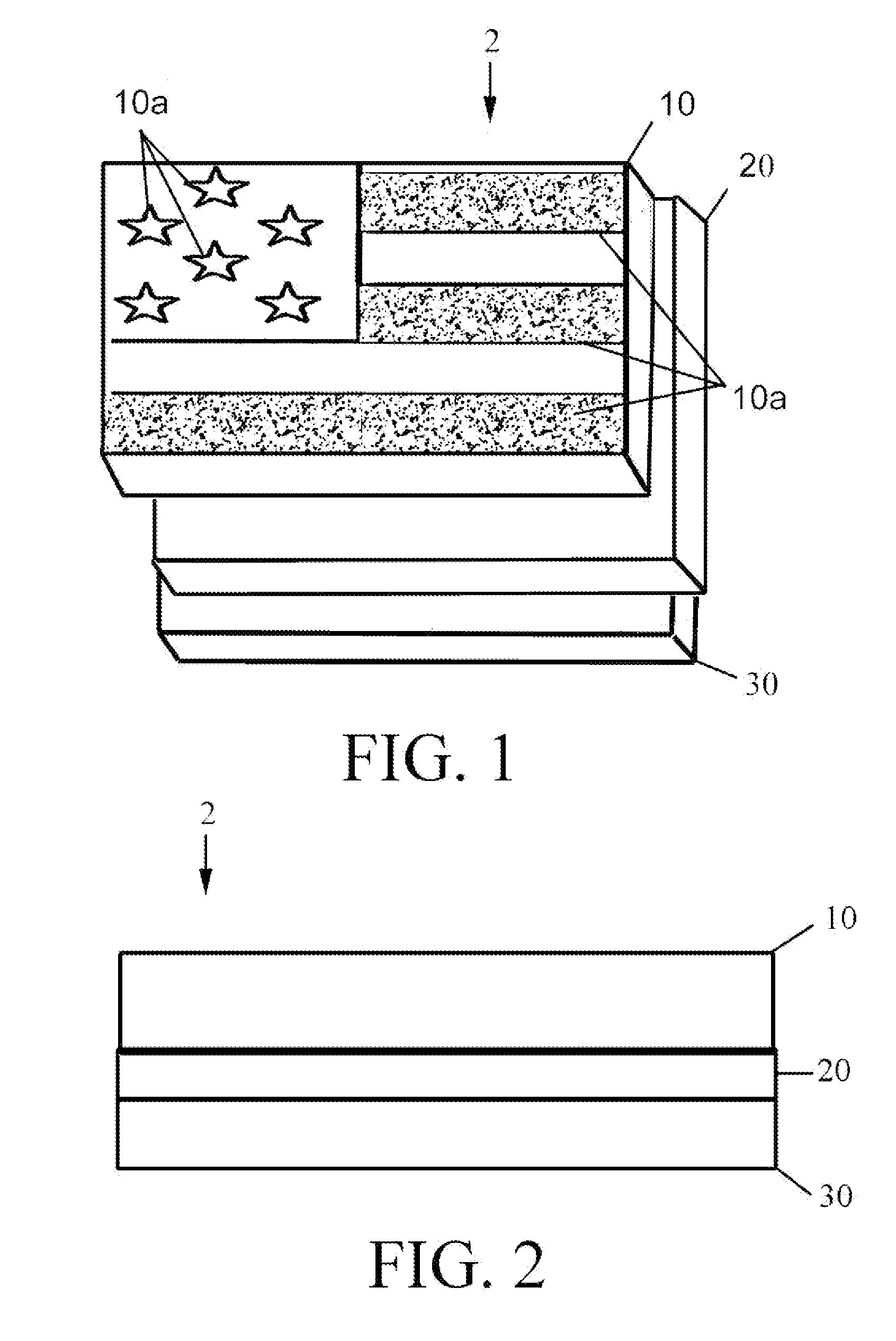
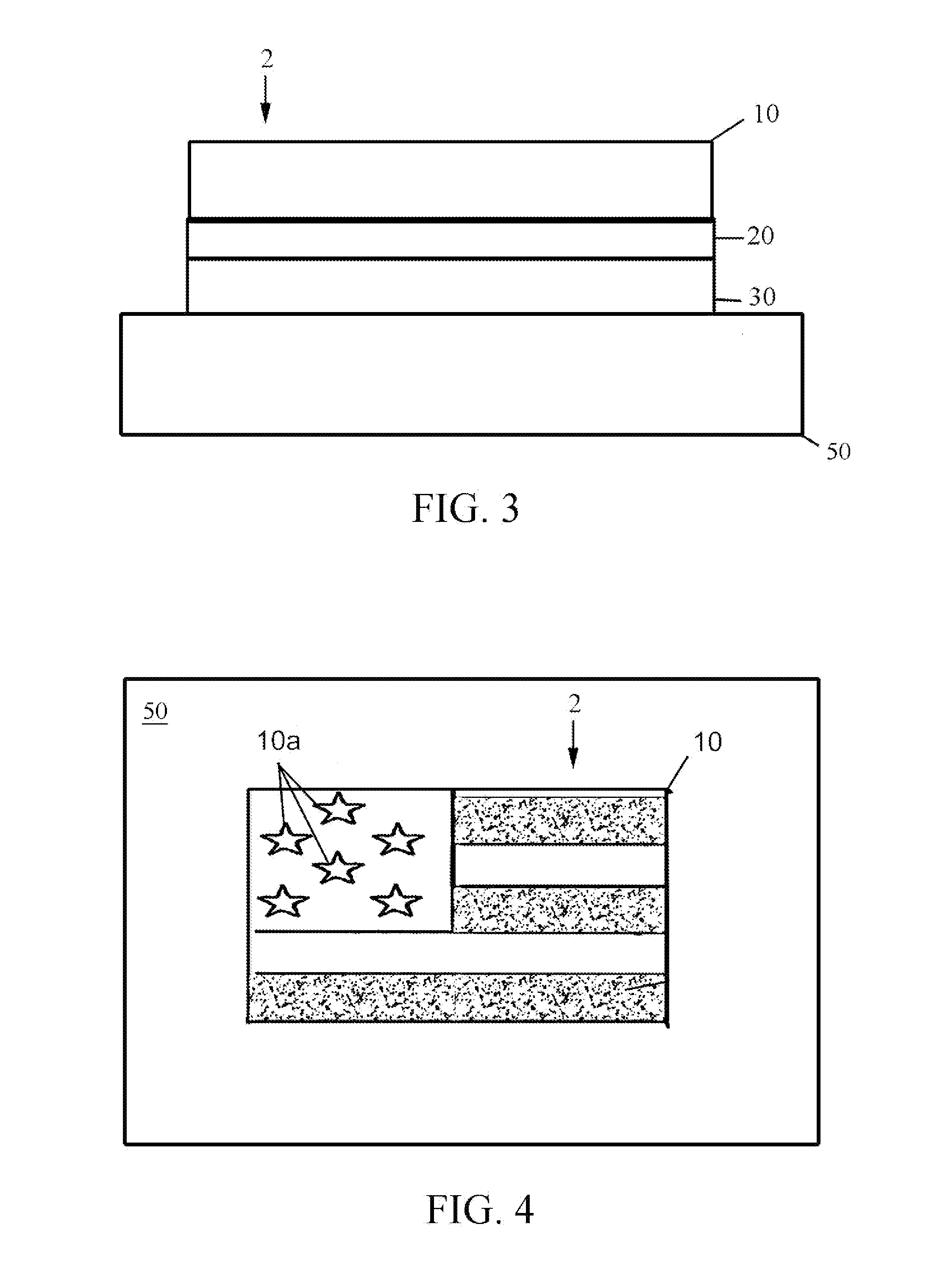
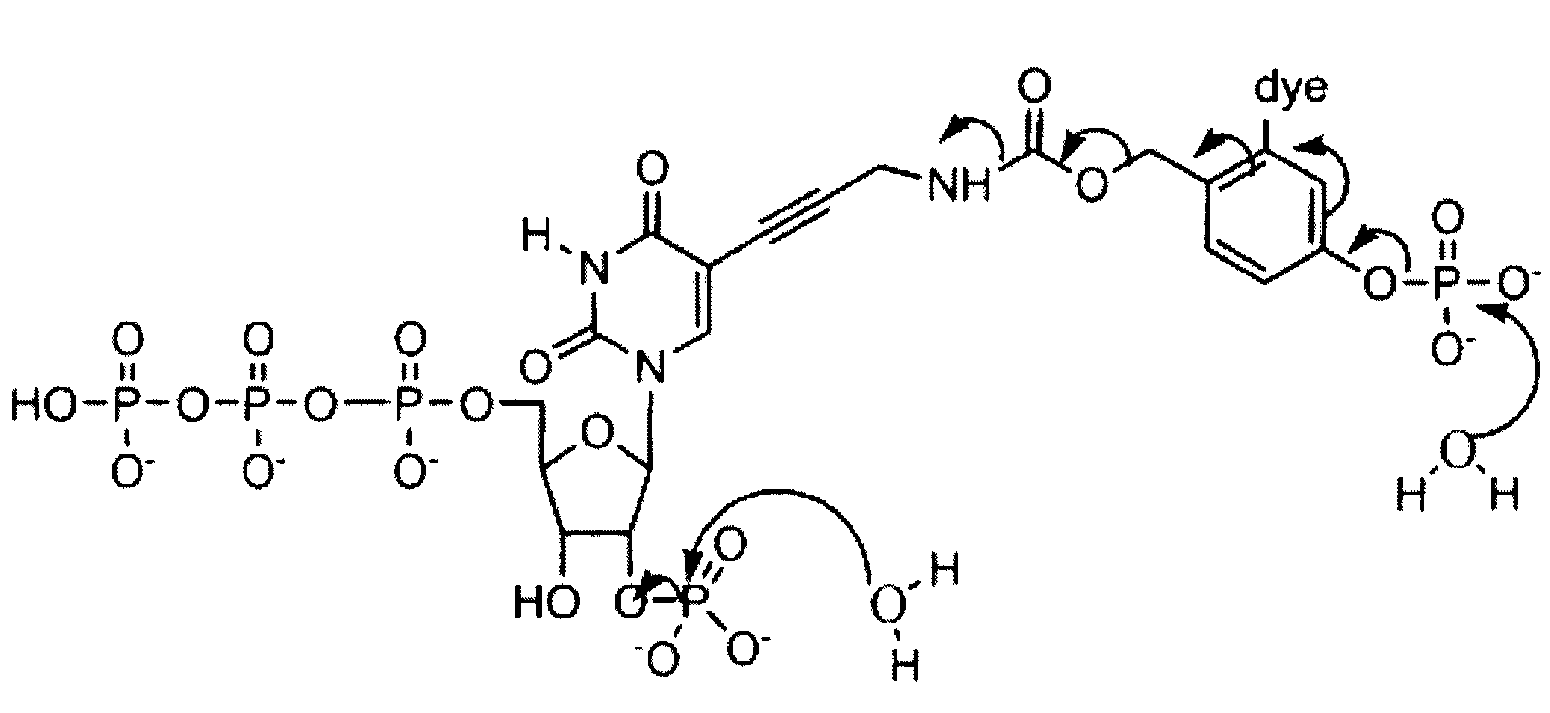
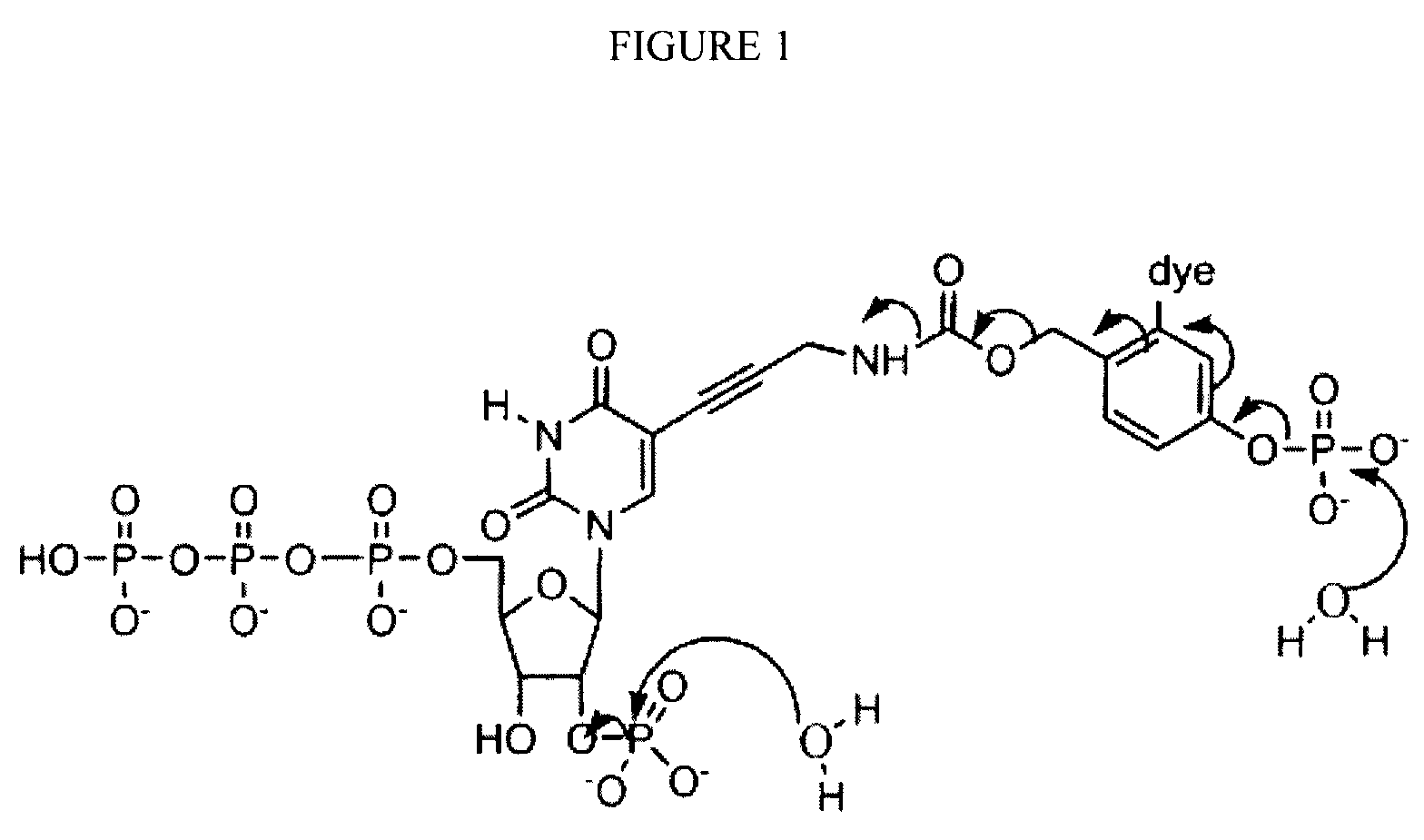
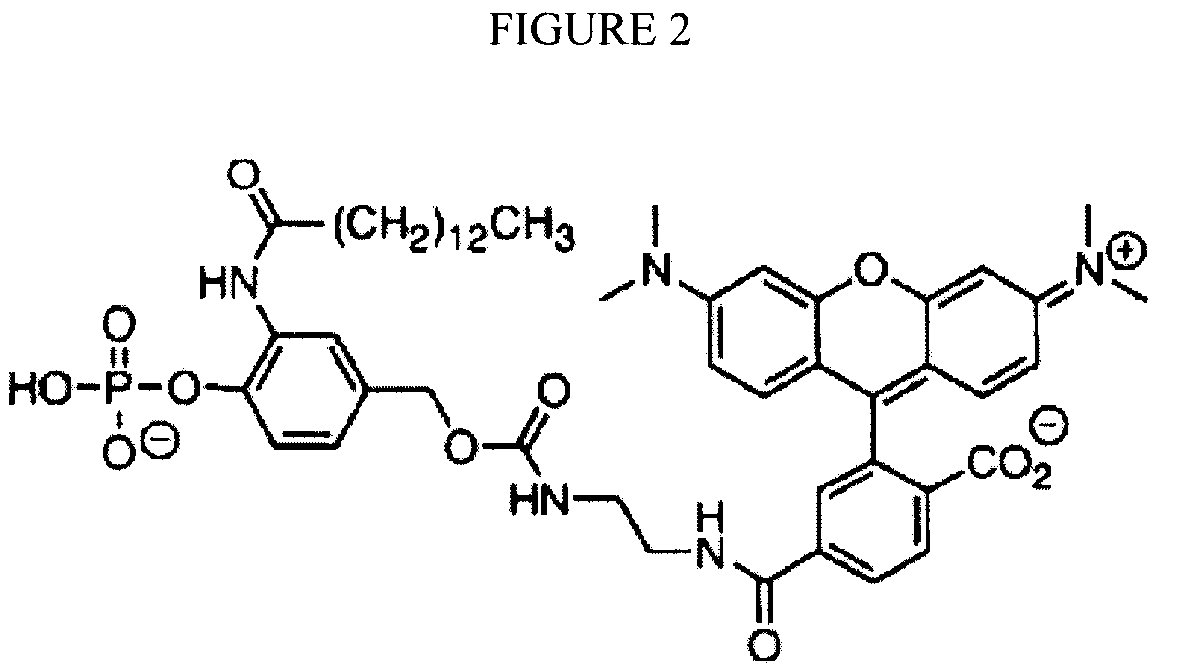
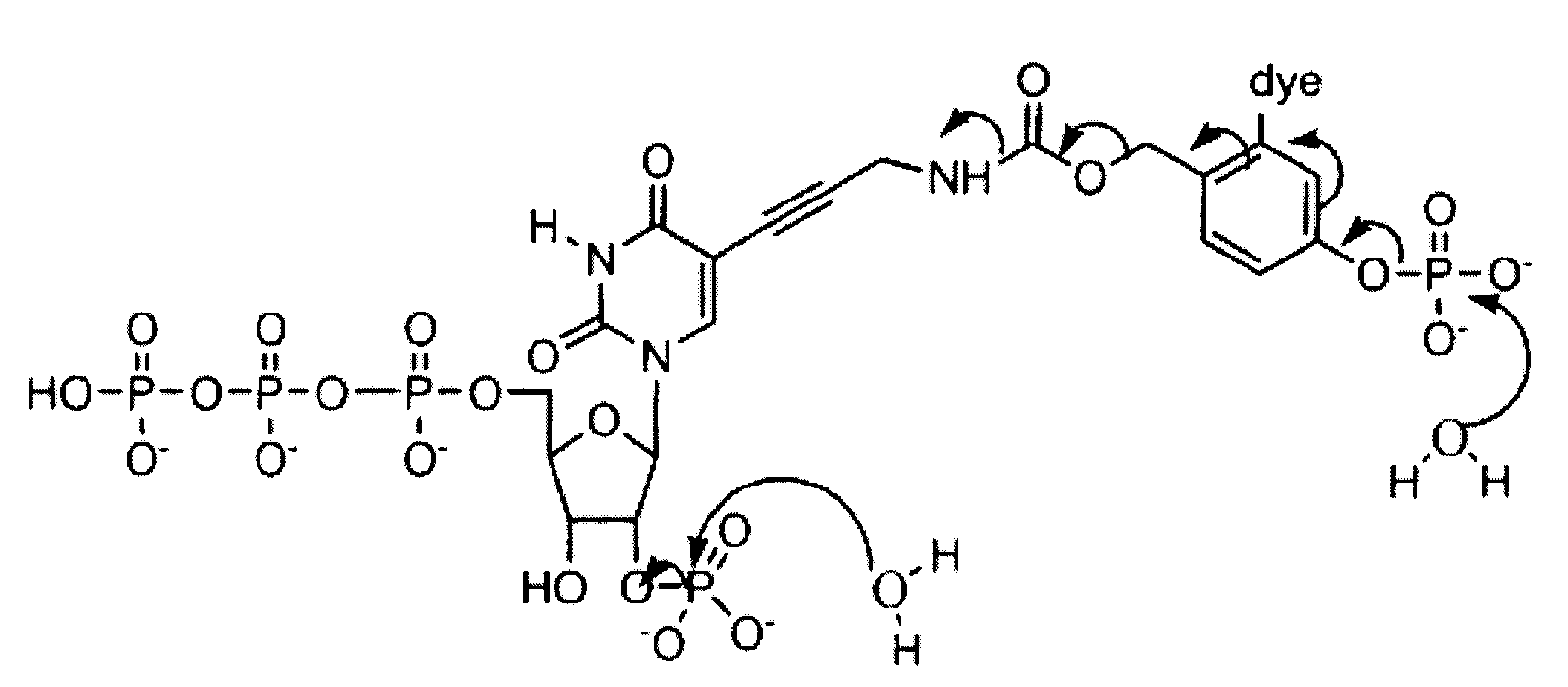
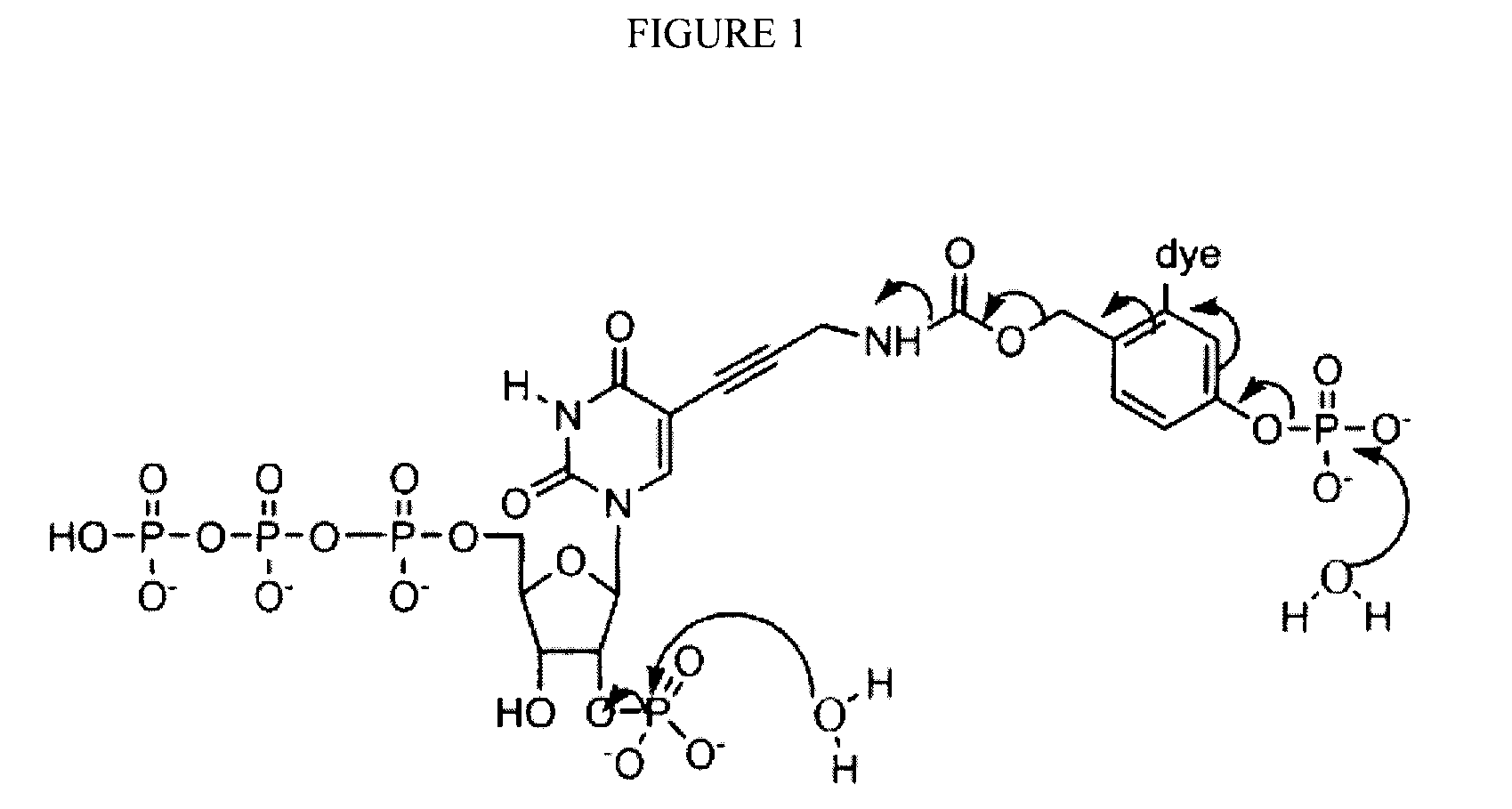
Reversible terminator nucleotides and methods of use
InactiveUS20080050780A1Laborious and time-consumingReduce the number of stepsSugar derivativesMicrobiological testing/measurementSugar nucleotideBiophysics
Owner:APPL BIOSYSTEMS INC
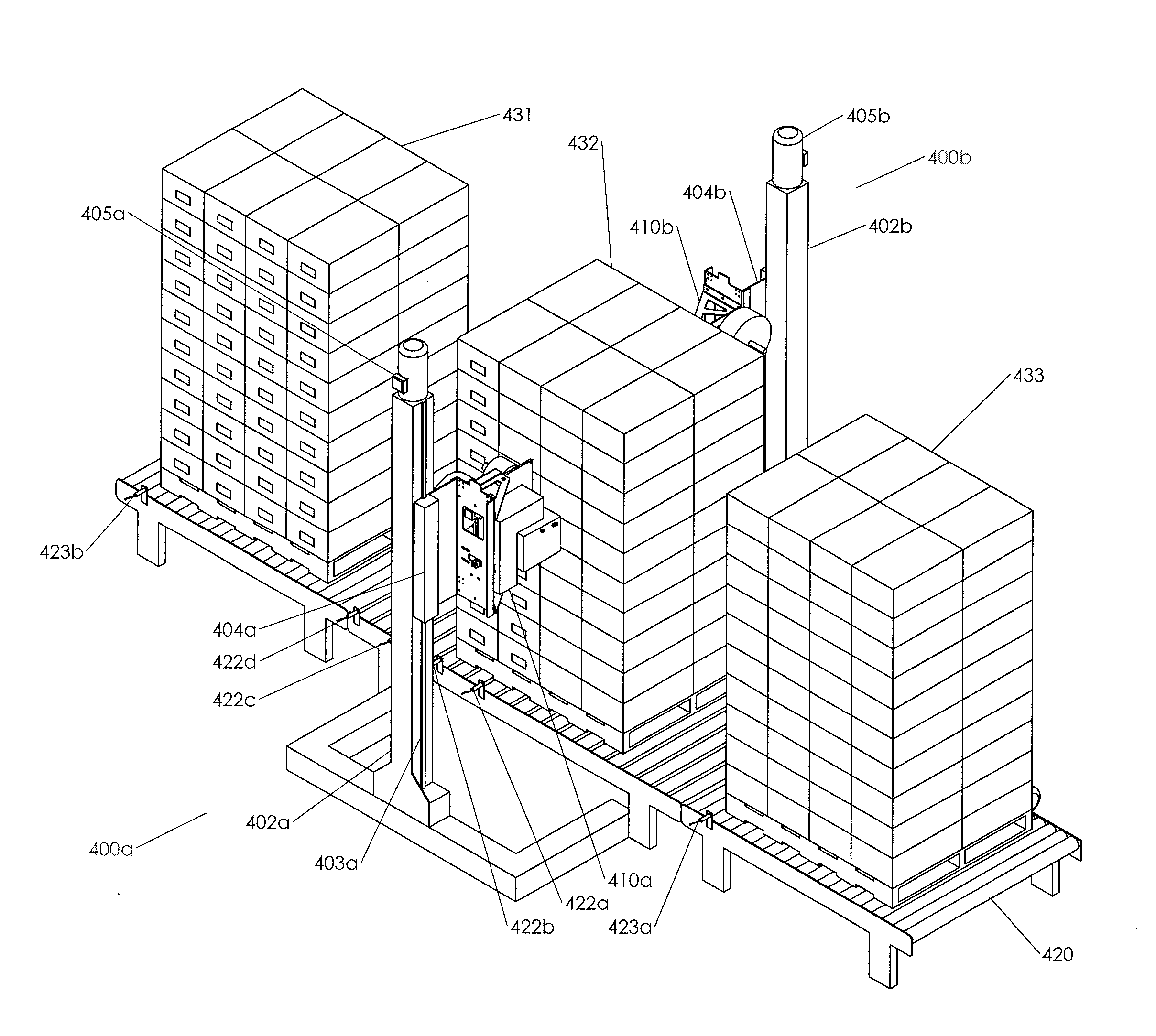
Container Labeling Systems and Methods of Use
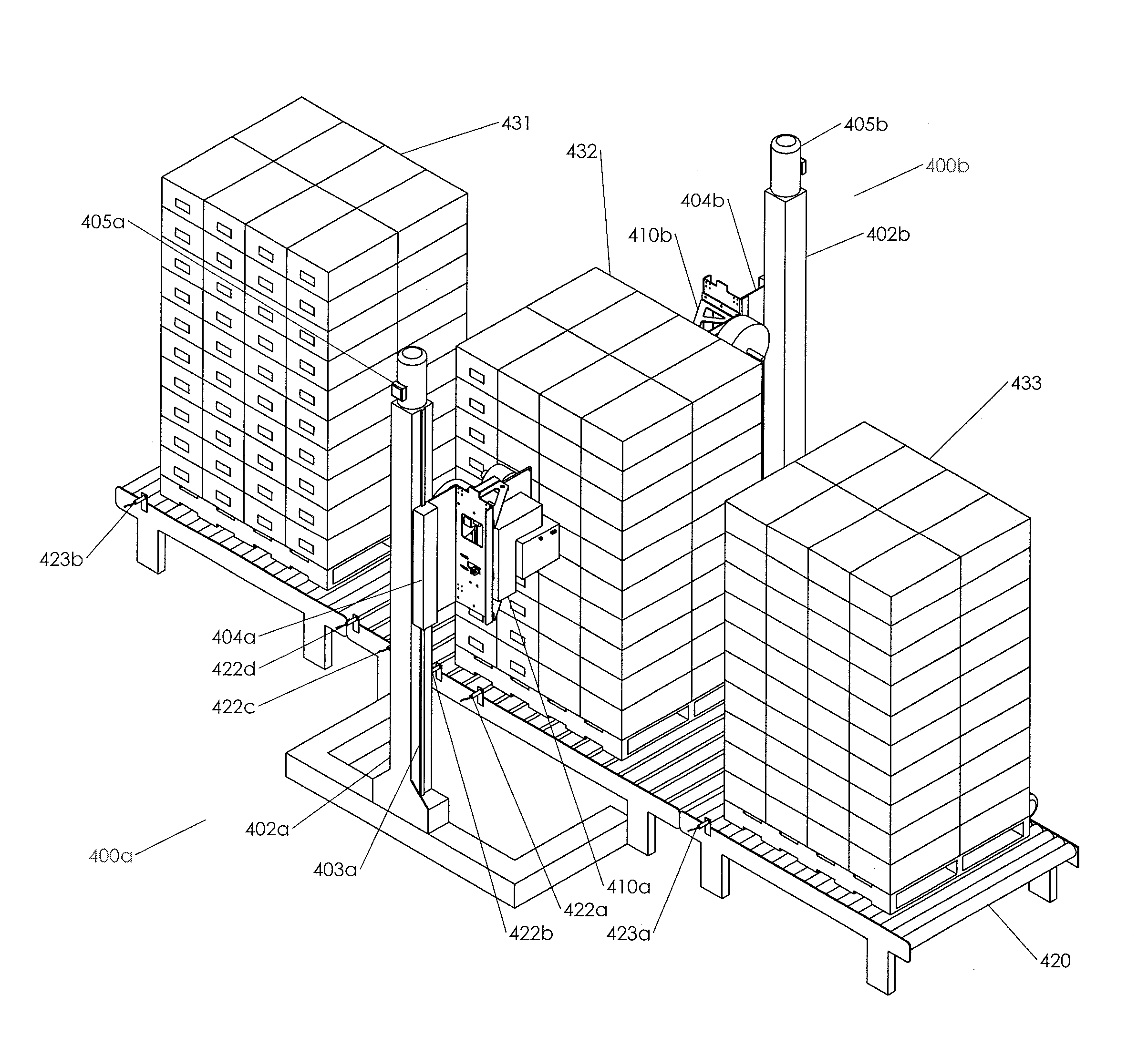
ActiveUS20150225104A1Quickly and efficiently labeledLaborious and time-consumingMechanical working/deformationTypewritersEngineeringPallet
The invention relates to labeling and / or printing devices, methods and systems for applying coding and / or labeling to containers that are stacked or otherwise organized in a group (e.g., containers stacked on a pallet). In embodiments of the invention, labeling and / or printing devices are mounted on carriages that are capable of moving in a vertical direction to apply labels as they move. Horizontal movement is imparted either by moving such carriages in a horizontal direction adjacent to the containers, or by moving the containers themselves (or the pallet holding them) in a horizontal direction adjacent to such carriages. Multiple carriages with labeling devices may be provided to provide simultaneous labeling to more than one side of a group of stacked containers. Embodiments of the invention are capable of providing labeling of containers that are uniformly or non-uniformly grouped or stacked.
Owner:REED LORIN
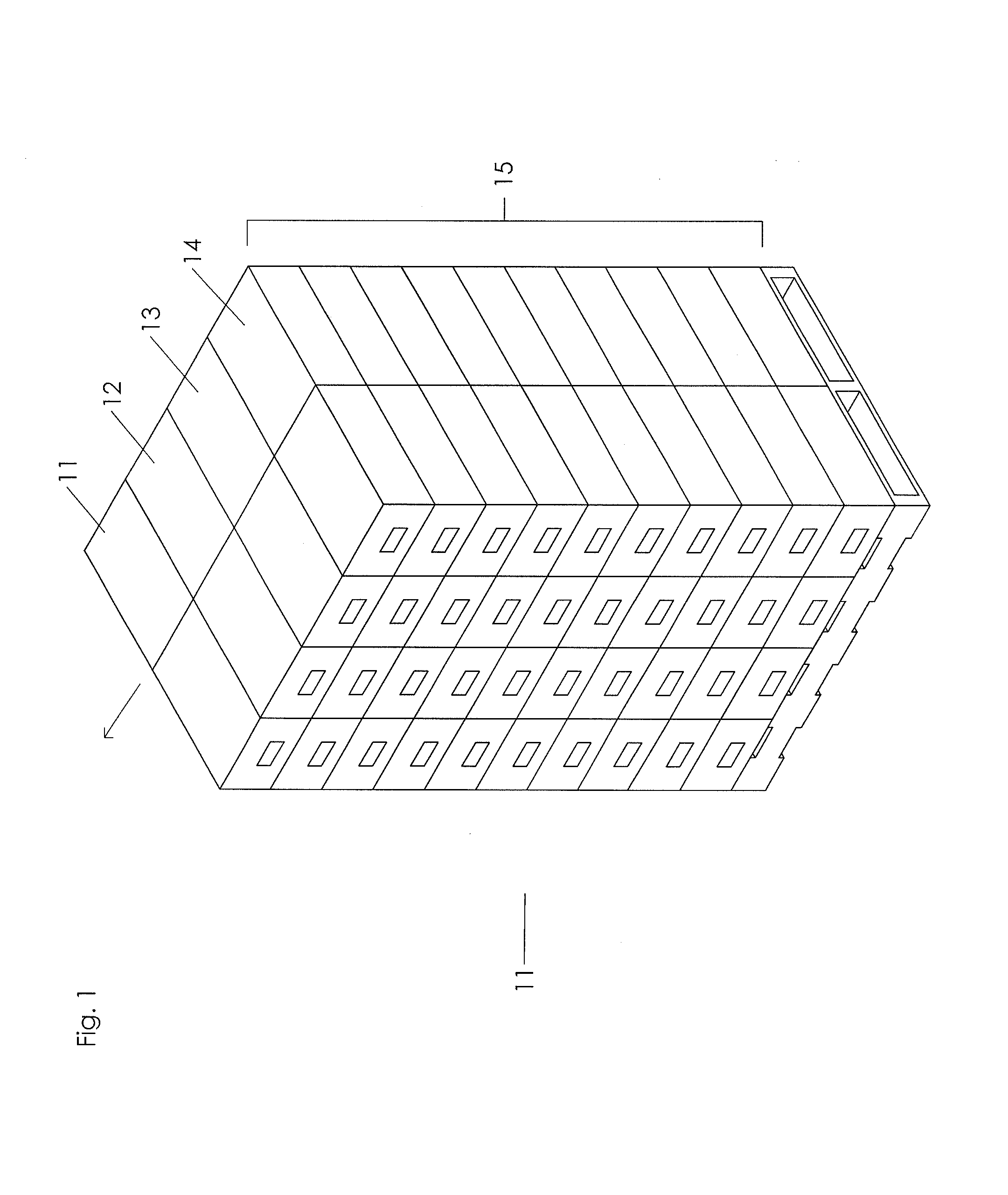
Cladding assembly
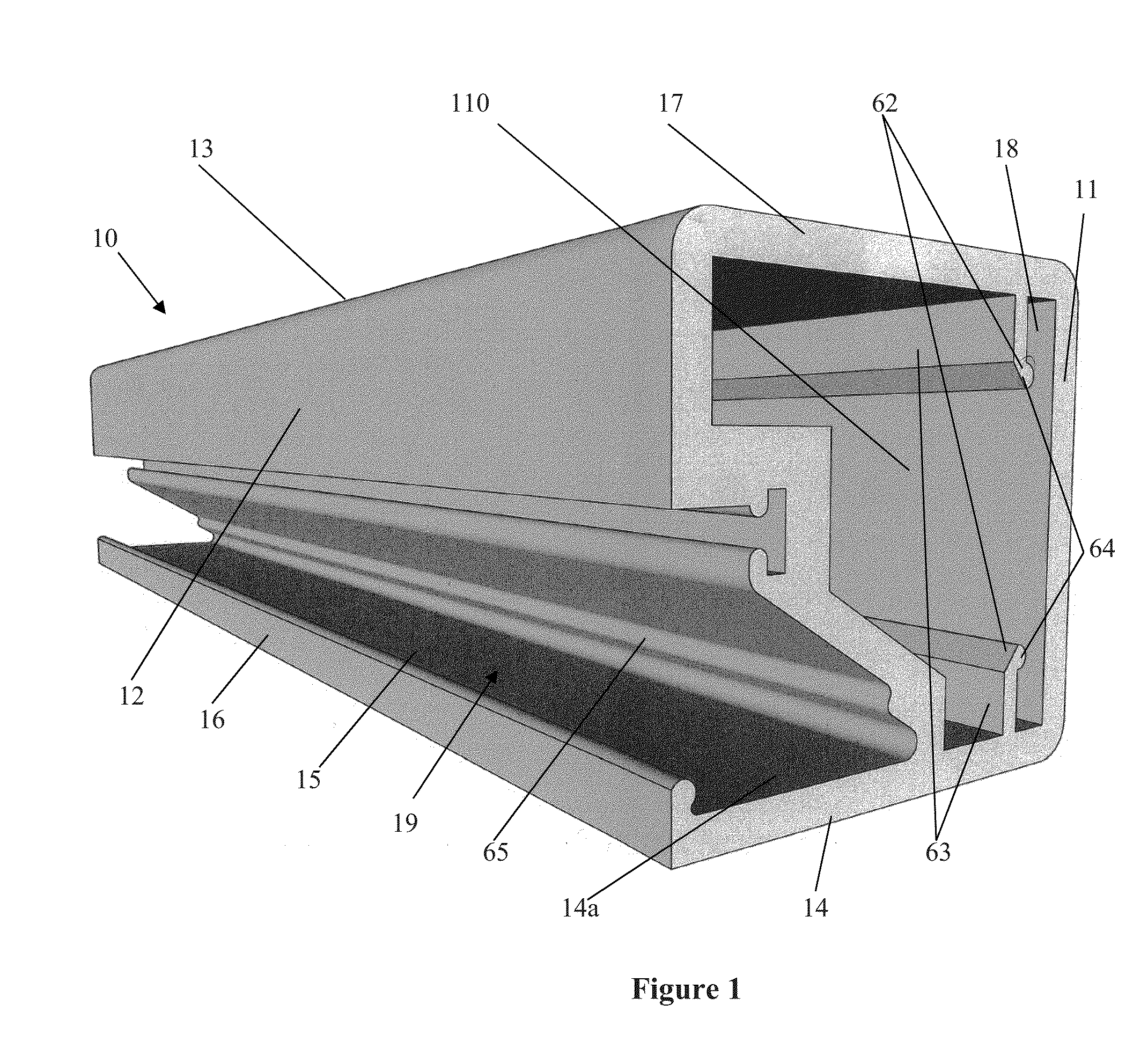
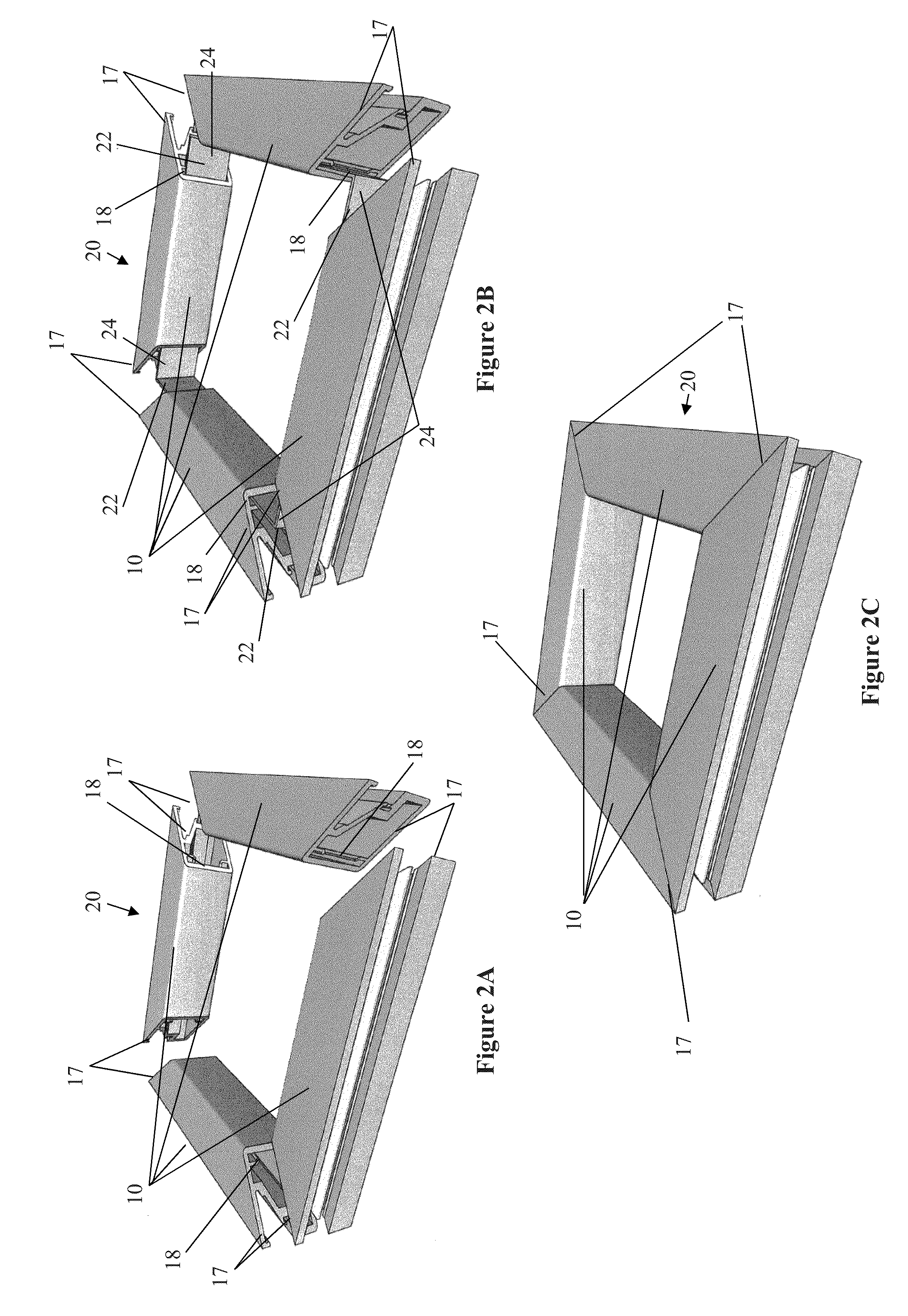
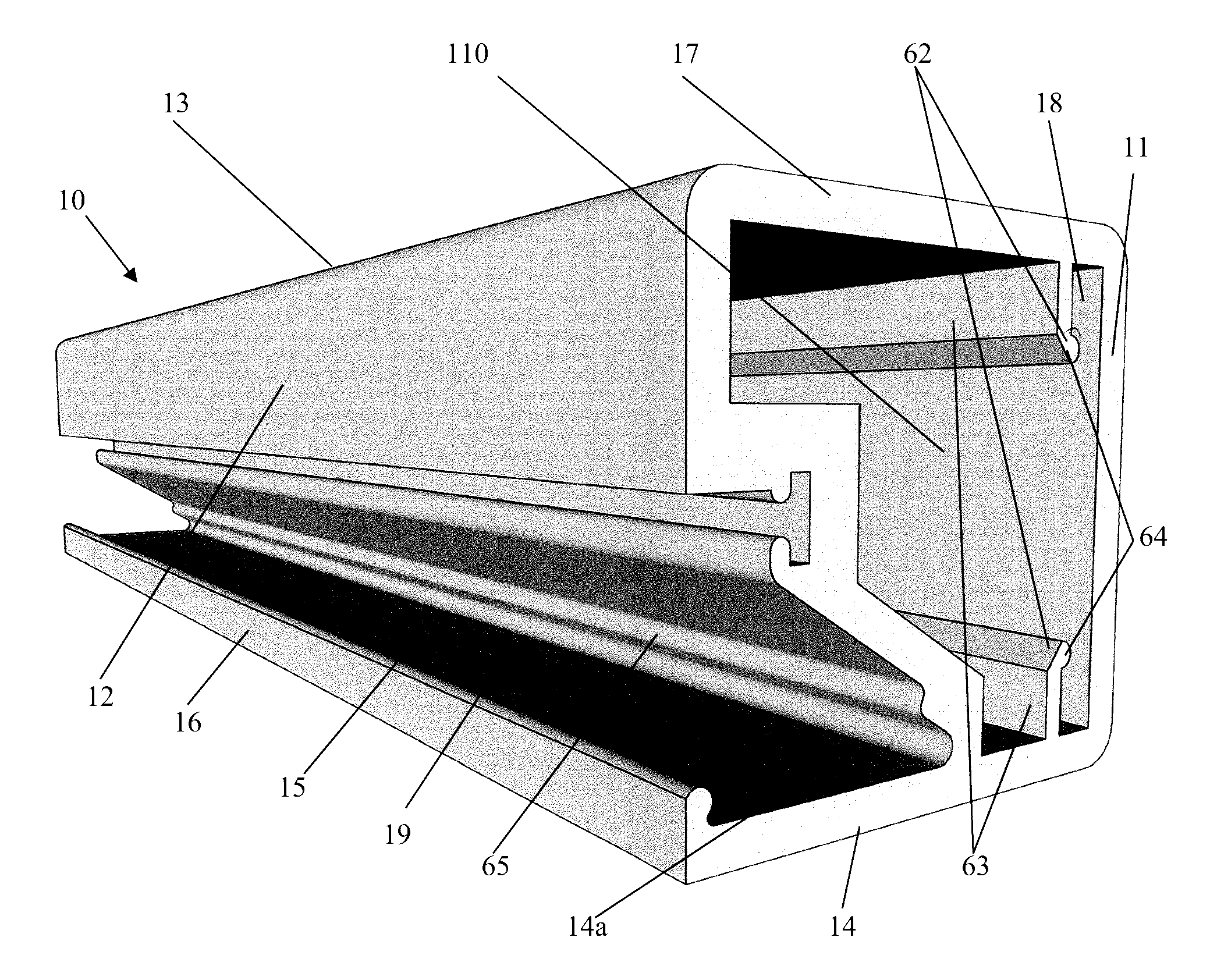
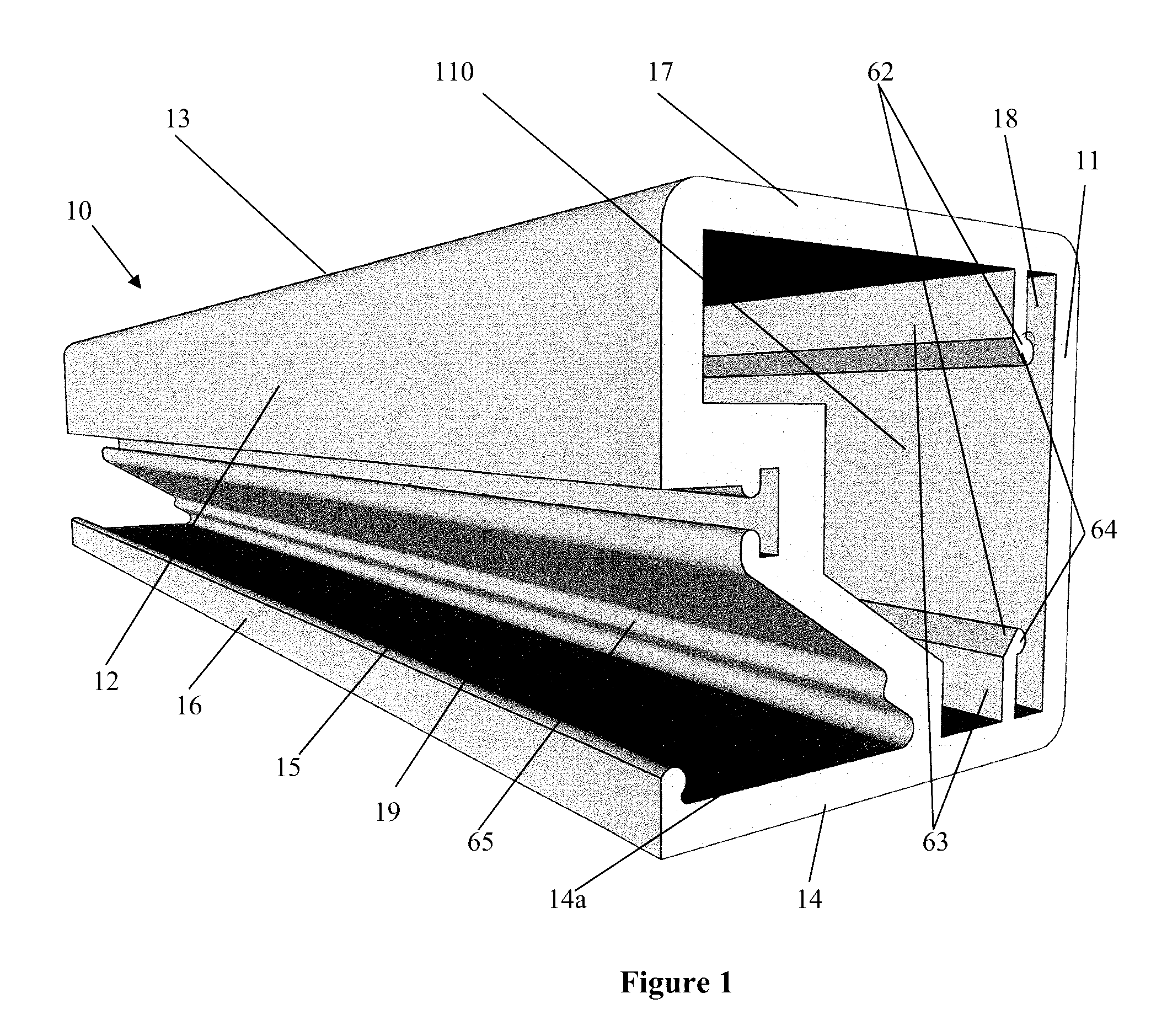
The present invention concerns a frame member (10), a frame formed from the frame member (10) and a panel including the frame for use in a cladding assembly for cladding a structure. The frame member (10) of the present invention is adapted to be joined together with other frame members (10) to form the frame, each frame member (10) includes at least four walls including an inner frame wall (11), an opposed outer frame wall (12), a panel abutting wall (13) and an opposed structure abutting wall (14) having a free end (15) with an outer edge protrusion (16). A receiving opening (19) is defined between a portion of the outer frame wall (12) and the outer edge protrusion (16) for receiving one or more fasteners which engage the outer edge protrusion (16) for, in use, fastening the frame member (10) and thereby the frame and preformed panel to the structure.
Owner:LEE DUANE
Textile embellishments that permanently bond to waterproof and/or waterproof-breathable fabrics
InactiveUS20110053450A1Excellent bonding compatibilityLaborious and time-consumingGarment special featuresDecorative surface effectsChemical LinkageSympaTex
A new product and process for producing an integrated textile embellishments such as patches, emblems, appliqués, labels and cut textile parts (including woven, knits and nonwoven structures made of both natural and or synthetic fibers) incorporating adhesives that permanently bond by heat-sealing to waterproof and / or waterproof-breathable fabrics including Gore-Tex®, Sympatex™ and other fabrics having durable water repellant finishes (DWR). This eliminates the tedious and cost intensive sewing operations previously needed to attach textile embellishments to such fabrics. The product and process includes a top layer decorative element, a second layer bonded to the top layer through lamination or chemical bonding to create a more uniform or smooth surface, and a thermal adhesive layer bonded to the second layer and capable of heat-application to textile based textile based products such as apparel and or accessories, headwear, outdoor furnishings and luggage. Products of this type can survive multiple washes and are desired by manufactures for their ease of application, saving time, money and reducing complexity in the application process
Owner:BAQAI NAVAID +1
Reversible terminator nucleotides and methods of use
InactiveUS7553949B2Laborious and time-consumingReduce the number of stepsSugar derivativesMicrobiological testing/measurementSugar nucleotideBiophysics
Owner:APPL BIOSYSTEMS INC
Pressure sensitive textile adhesive
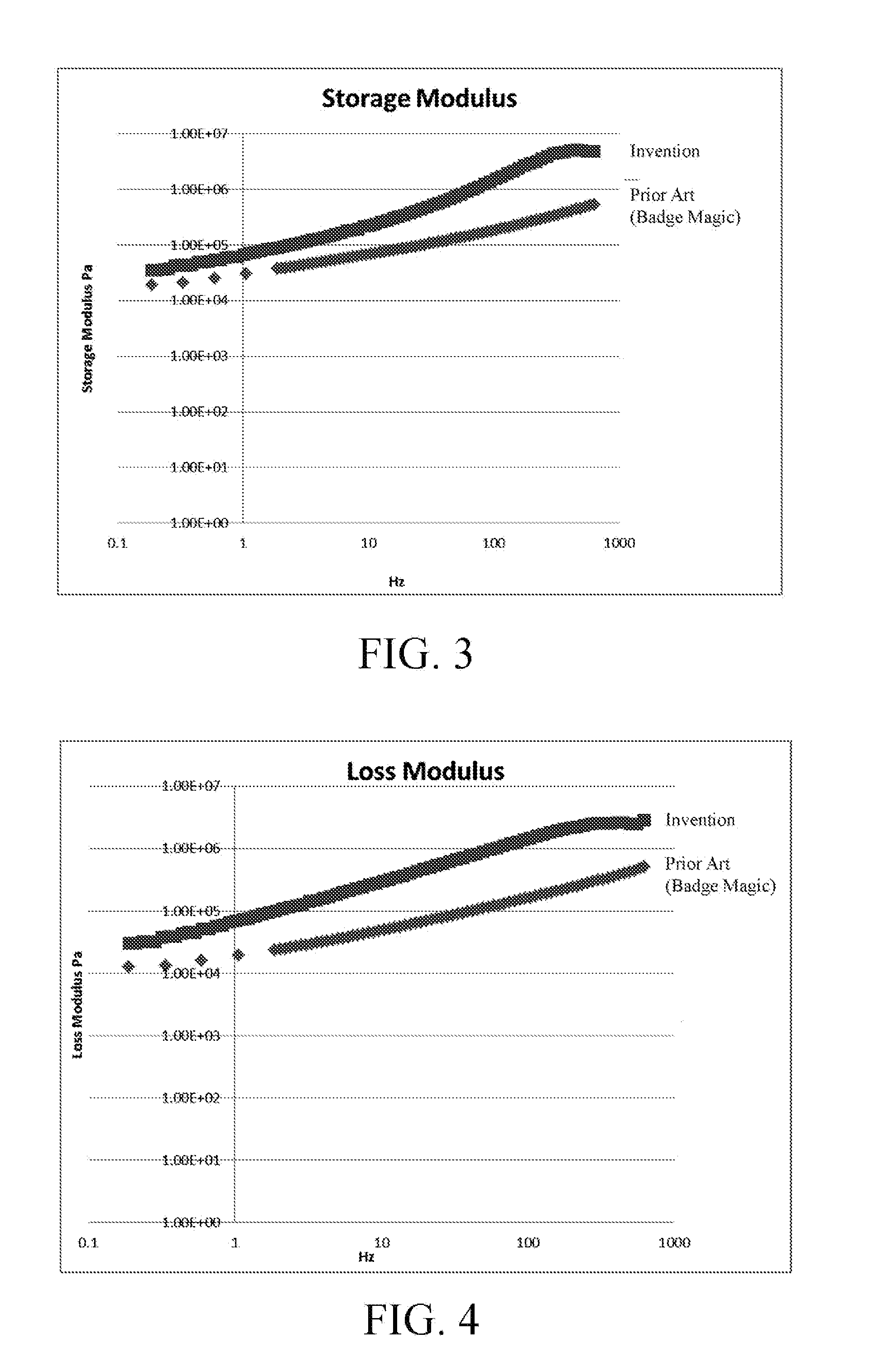
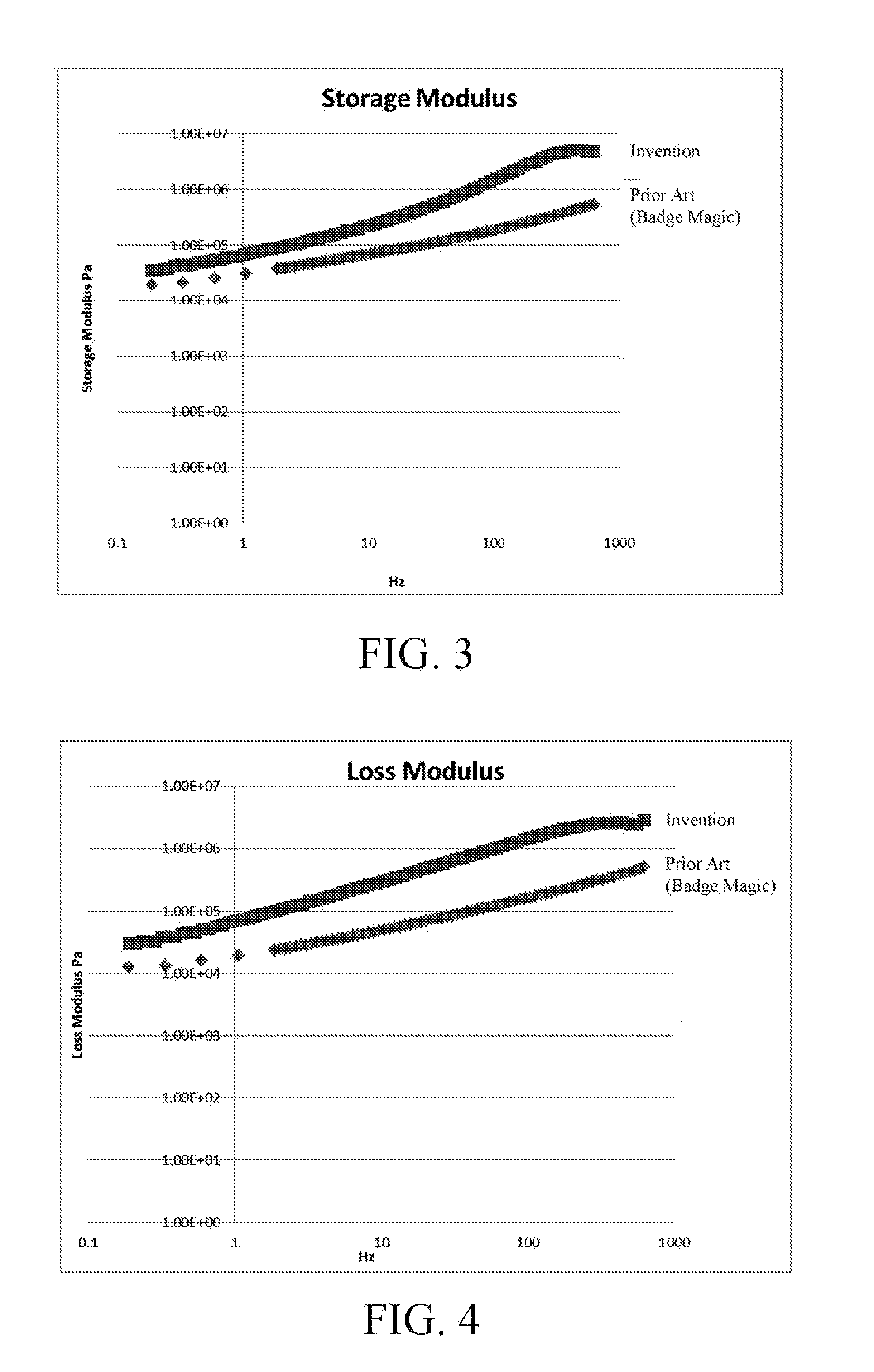
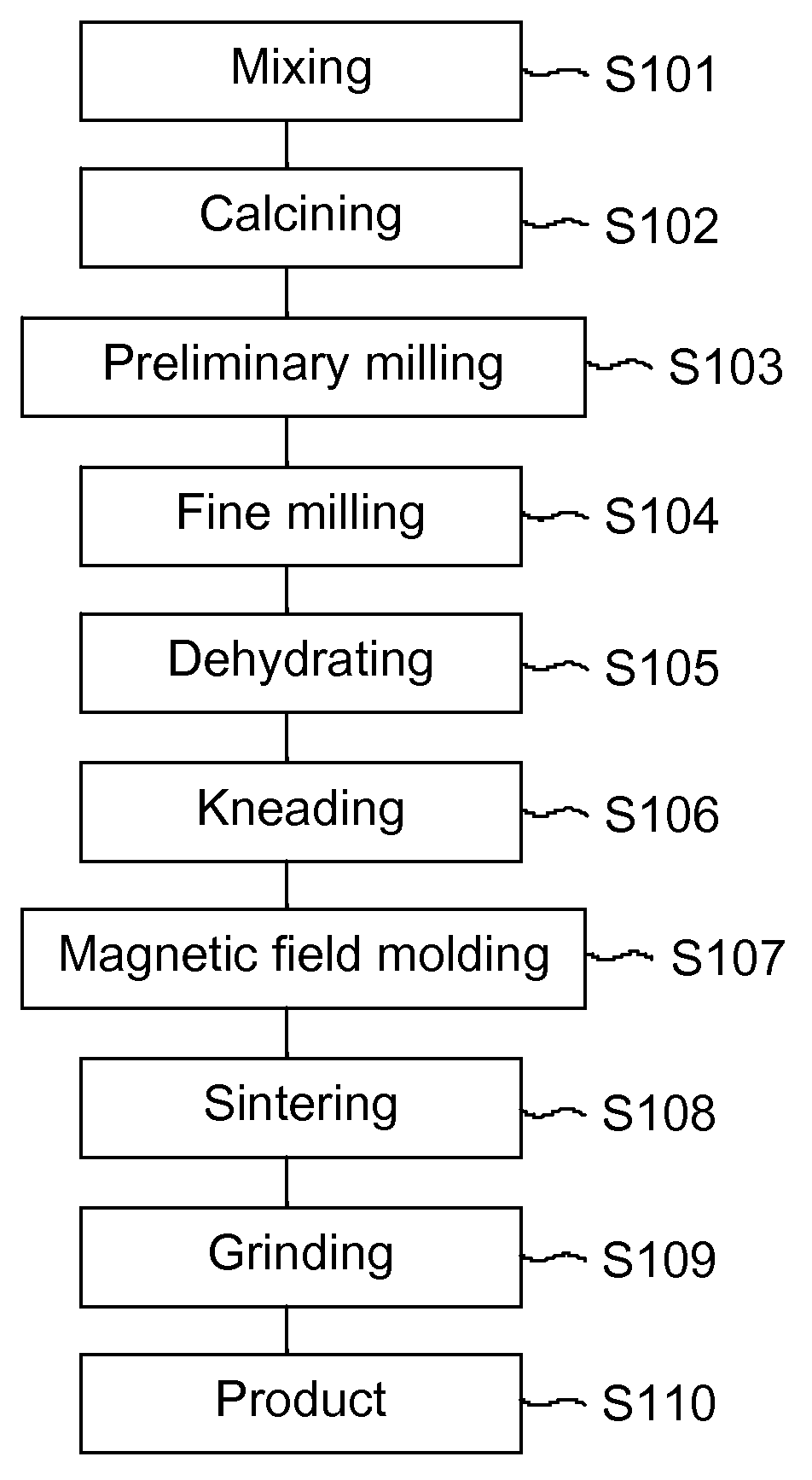
ActiveUS20110168319A1DurableSecure bondingLamination ancillary operationsDecorative surface effectsCross-linkHigh energy
A method of wash-durably bonding integrated textile emblems such as patches, emblems, labels and cut textile parts to another textile article by laminating a pressure sensitive acrylic polymer adhesive to the back surface of the textile emblem. The acrylic polymer adhesive has a storage modulus that is greater than a loss modulus throughout a frequency range of from 0.1885 Hz to 628 Hz where bonding and debonding are expected to occur, and is devoid of any cross linking additives or reagents. The integrated textile emblem with laminated pressure sensitive acrylic polymer adhesive has improved adhesion to low-to-high energy surfaces, is not water soluble and is of adequate thickness to provide a wash-durable textile-to-textile bond capable of seaming or permanently attaching items such as textile panels, appliqué bearing text, numbers, logos and other indicia for the apparel, accessory and other industries.
Owner:LION BROS CO INC
Device for testing surfaces of articles for traces of explosives and/or drugs
InactiveUS7456393B2Efficient scrapingEffectively wipedMaterial analysis using wave/particle radiationTime-of-flight spectrometersExplosive materialMetal
A device is provided for testing surfaces of a card for the presence of explosives, drugs or other substances of interest. The device includes a slot for receiving the card. Thin metallic wiper blades are dispose in alignment with the slot and wipe over surfaces of the card as the card is passed through the slot. Thus, substances on the surface of the card are transferred to the wiper blade. The wiper blade then is enclosed and rapidly heated to desorb the material retrieved from the card. The enclosure then is placed in communication with a detector to test for the presence of substances of interest.
Owner:RAPISCAN SYST INC (US)
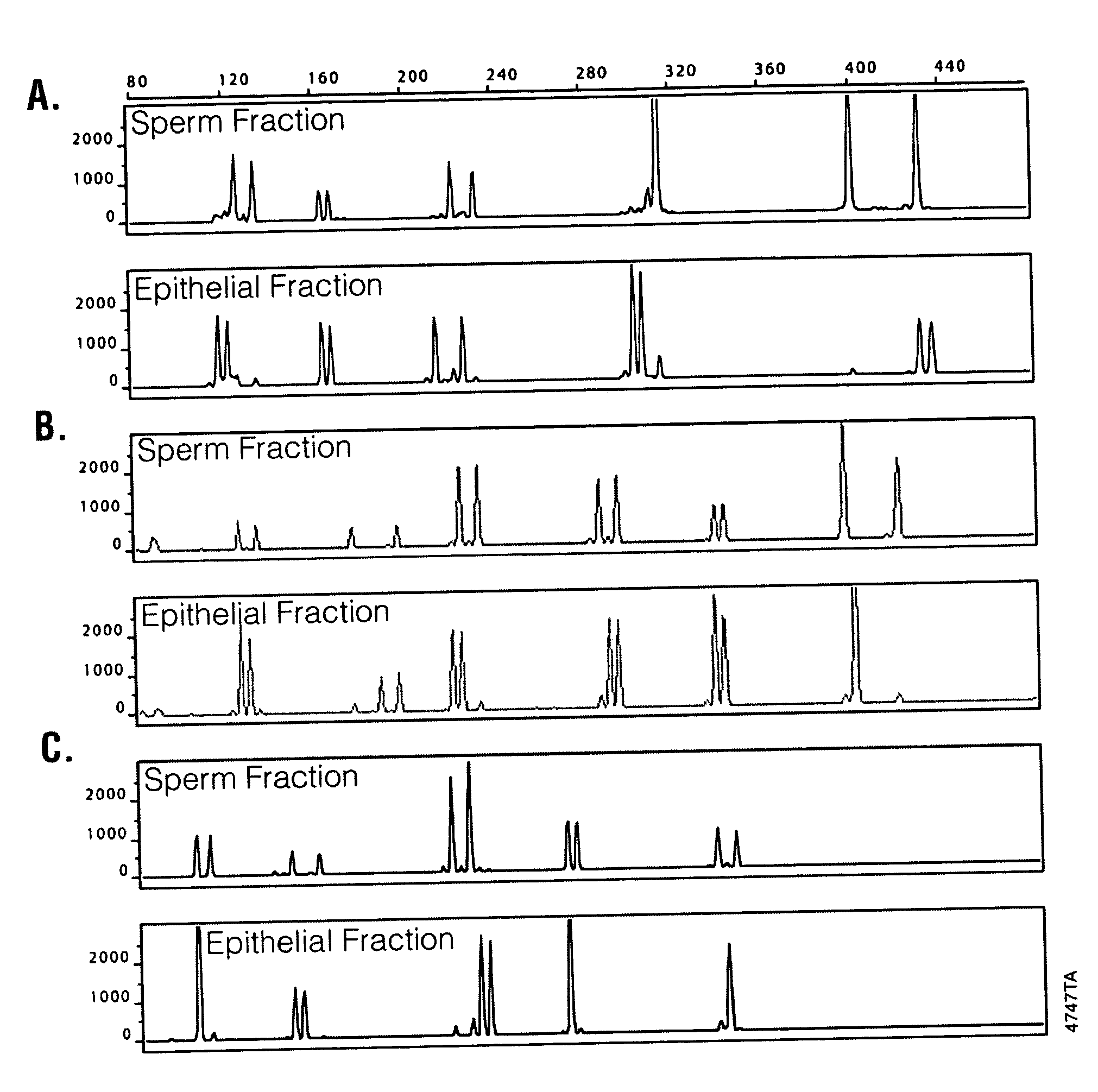
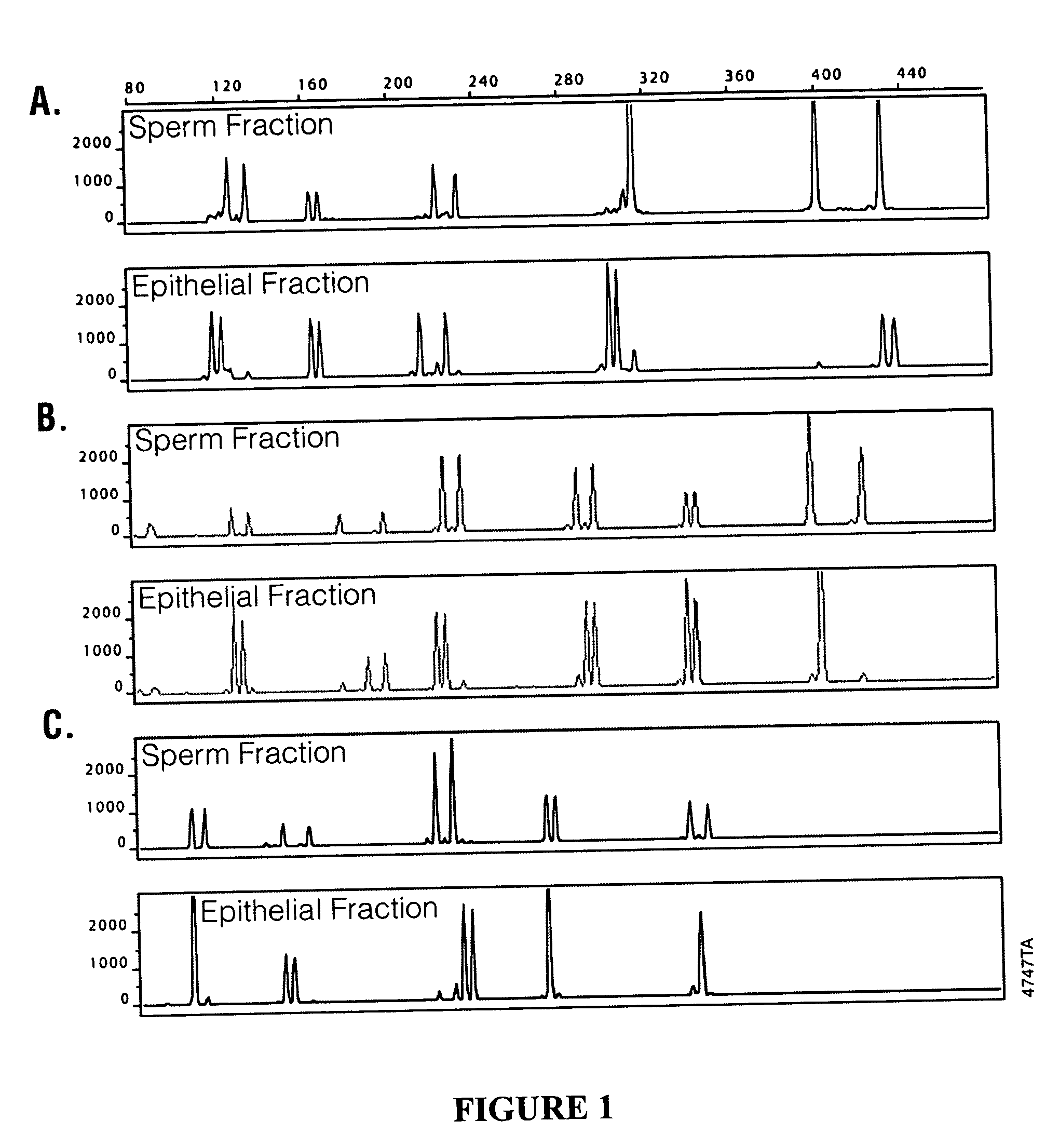
Methods and kits for isolating cells
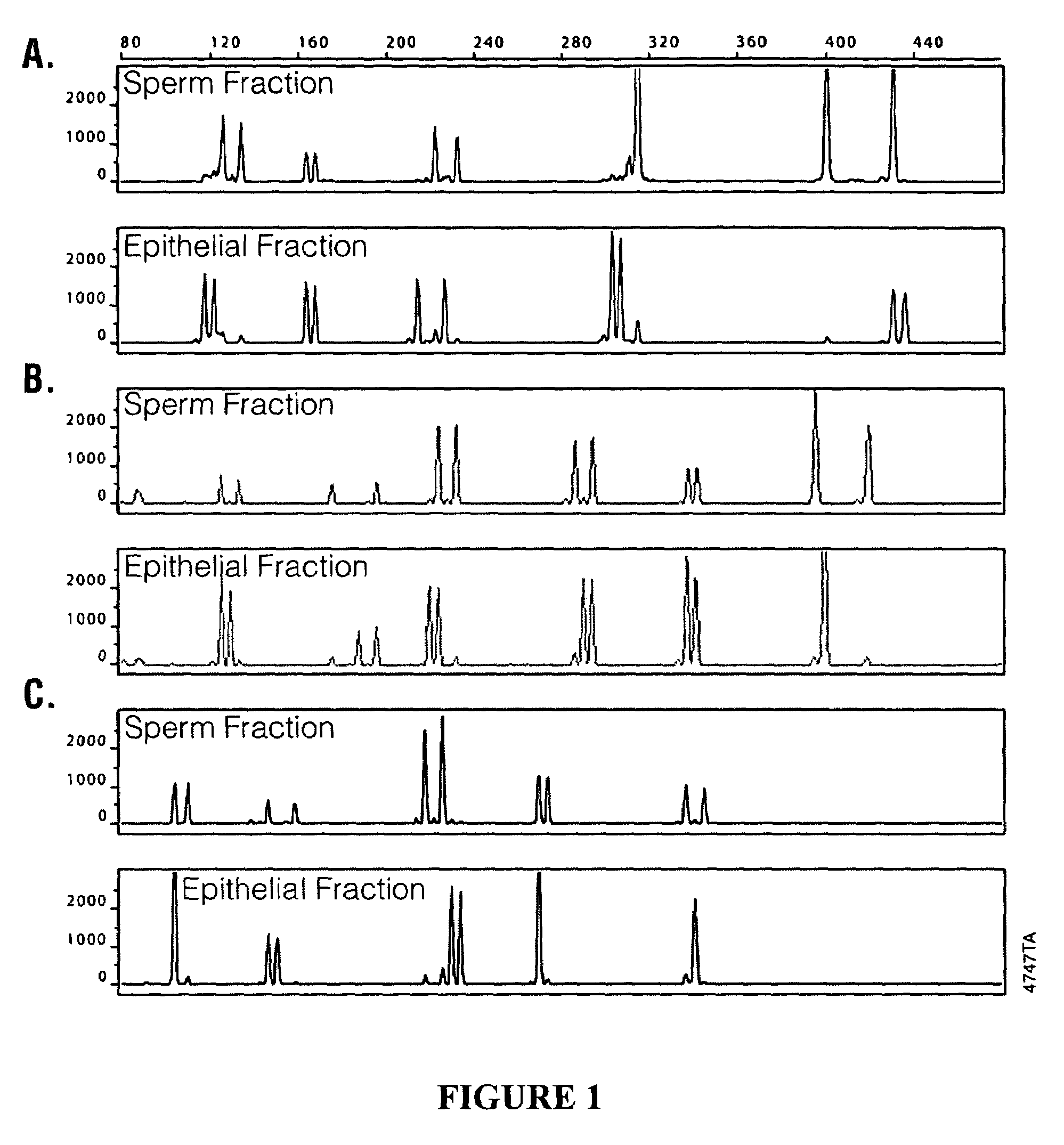
InactiveUS20100143878A1Reduce contributionEnhancing sensitivity and reliabilityMicroorganism lysisNucleic acid reductionDifferential extractionMicrobiology
Disclosed are methods for differential extraction of a target component from a sample which is predominantly composed of other types of non-target cells that can be lysed using methods that do not lyse the target cells, so that the target material can be purified away from the lysed non-target material. One exemplary method is directed to isolating sperm cells from an aqueous sample and kits for performing same.
Owner:PROMEGA CORP
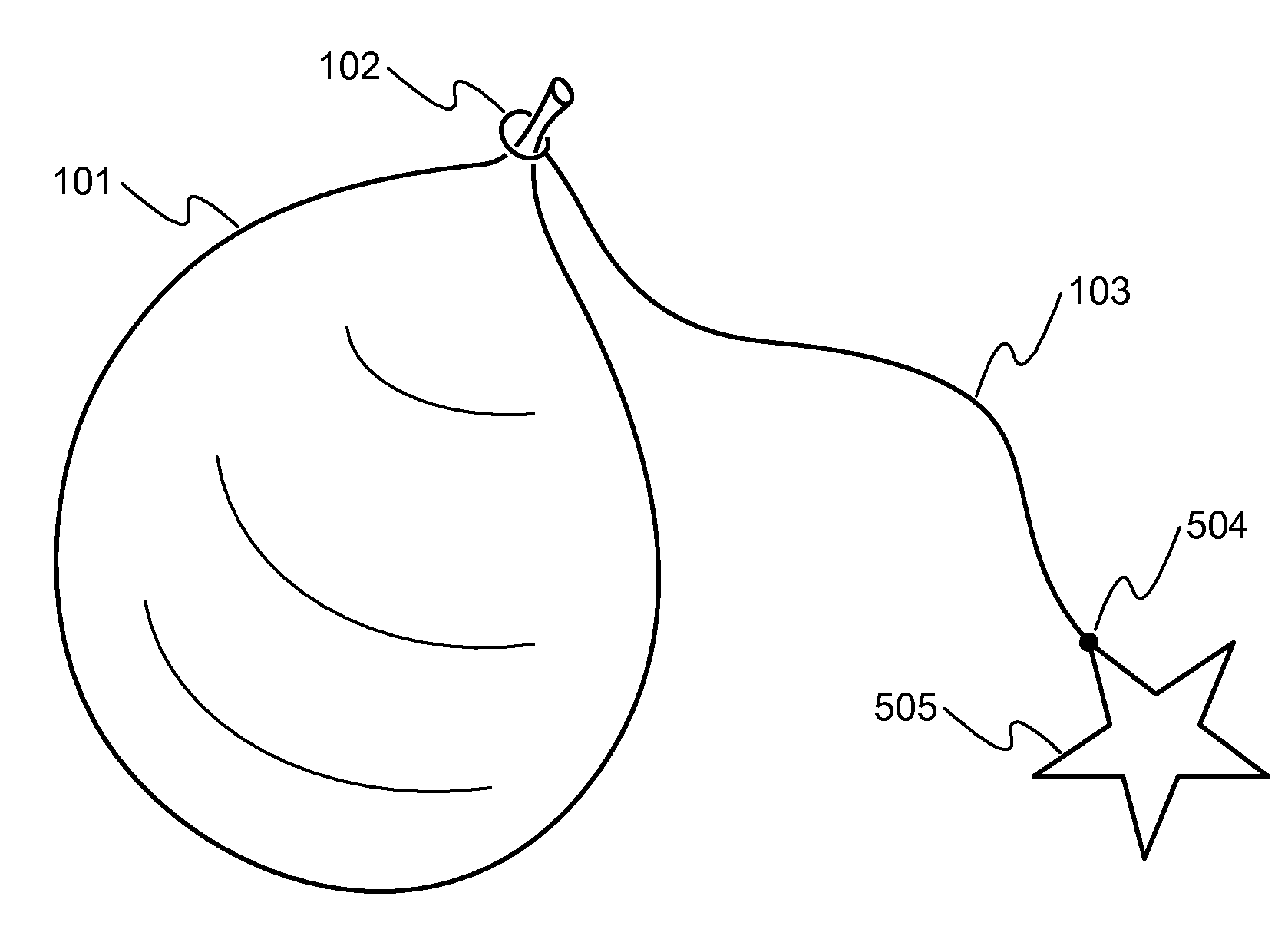
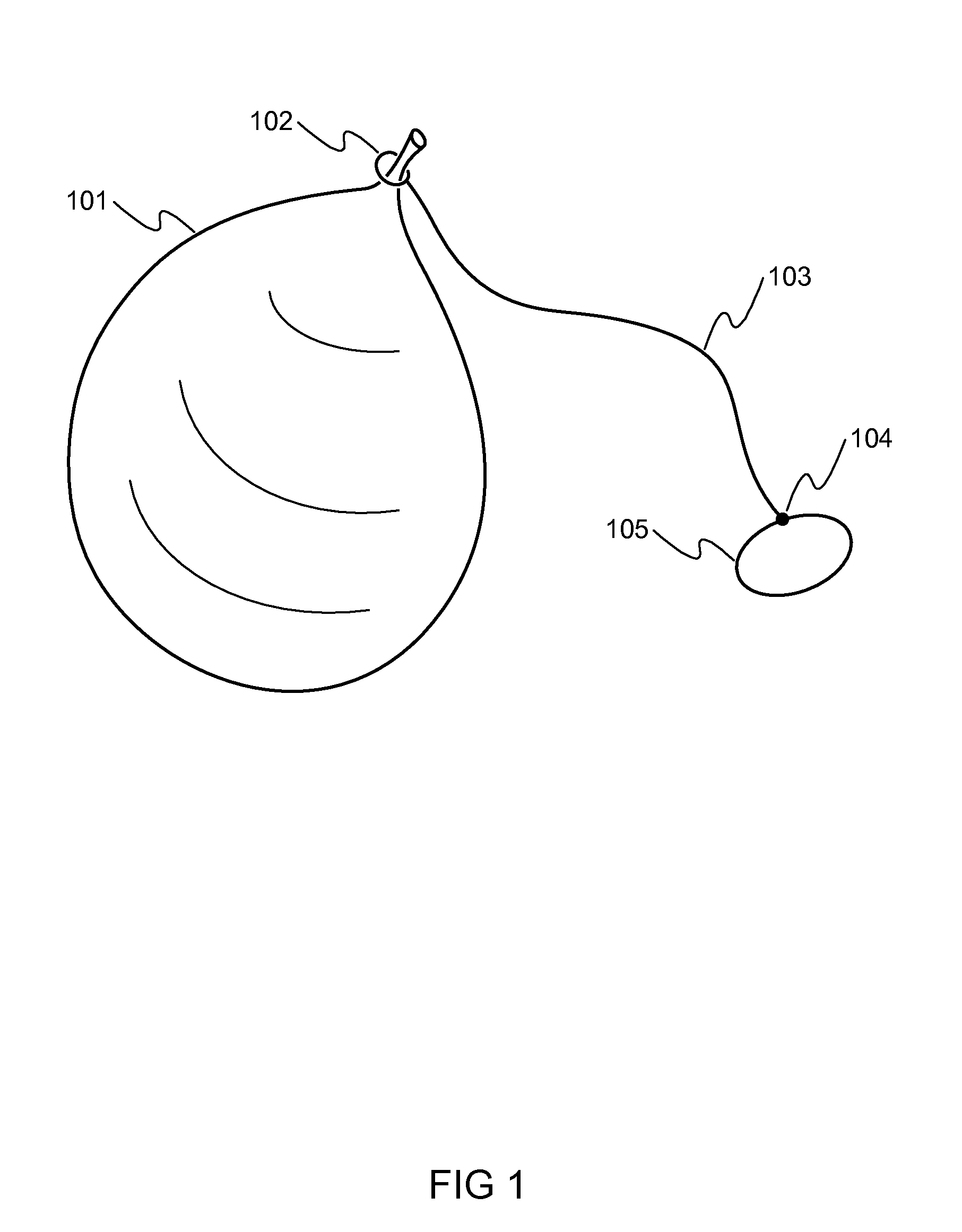
Method, system, and kit package for balloon weights and balloon stompers
This covers a game kit, system, method, and device for the balloon games. A game kit for the Balloon Stomper / popping / hitting / kicking / bursting game. The kit consists of a balloon, a tether for the balloon, and a device for attaching the balloon to a player, as an example. As one example, the game involves tying a balloon to the ankle of each player. Then the players try to stomp on each other's balloons, while protecting their own balloon. The winner is the last person or team with a balloon that has not been popped. There are many other variations for the game / rules as explained here in more details. One problem with the game is that preparing a large number of game set-ups is time consuming and labor intensive. This is intended to make both set-up and clean-up of the balloon stomper game simpler. It also deals with the balloon weight setup and system.
Owner:BOISE WAYNE SCOTT
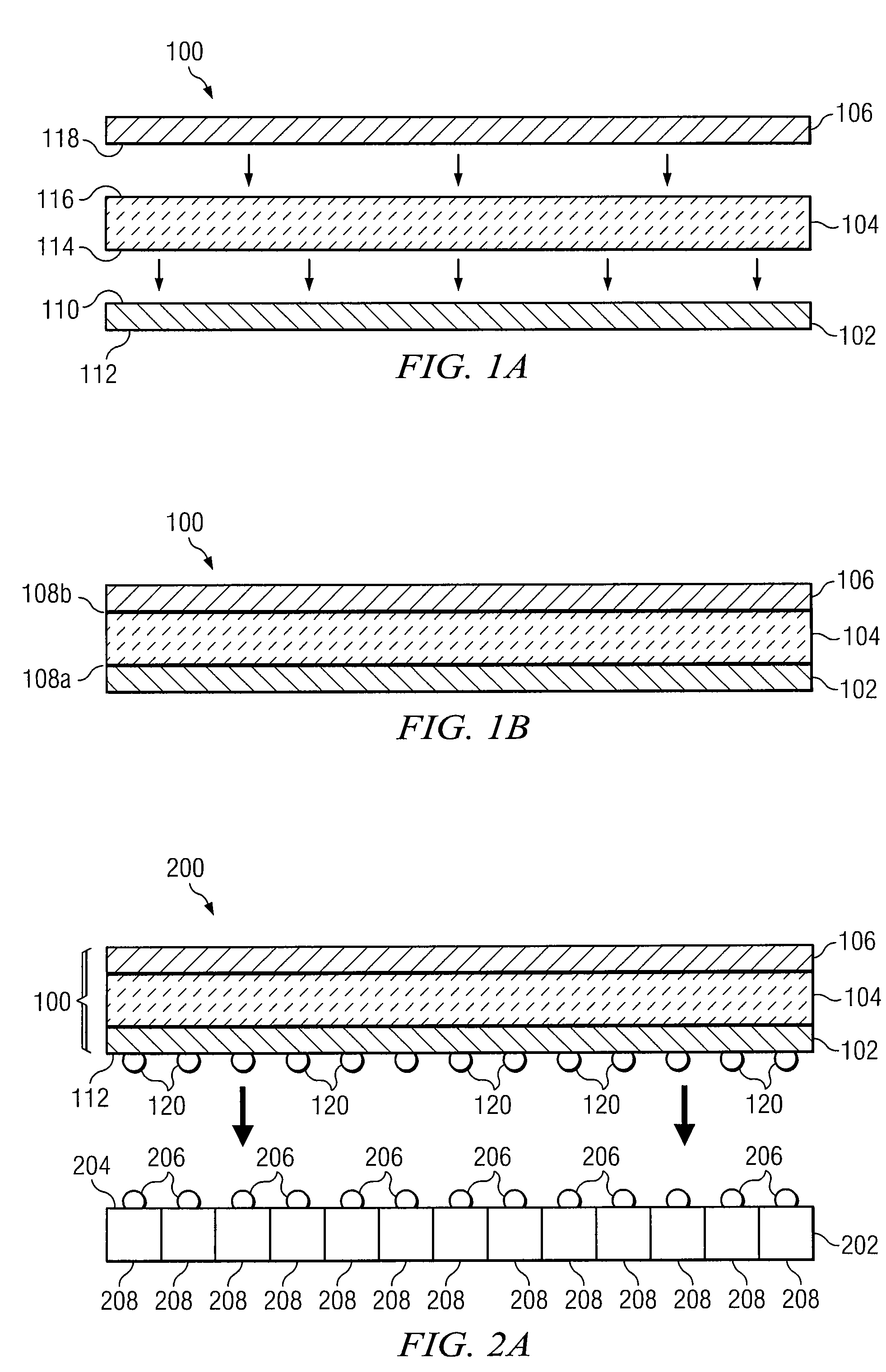
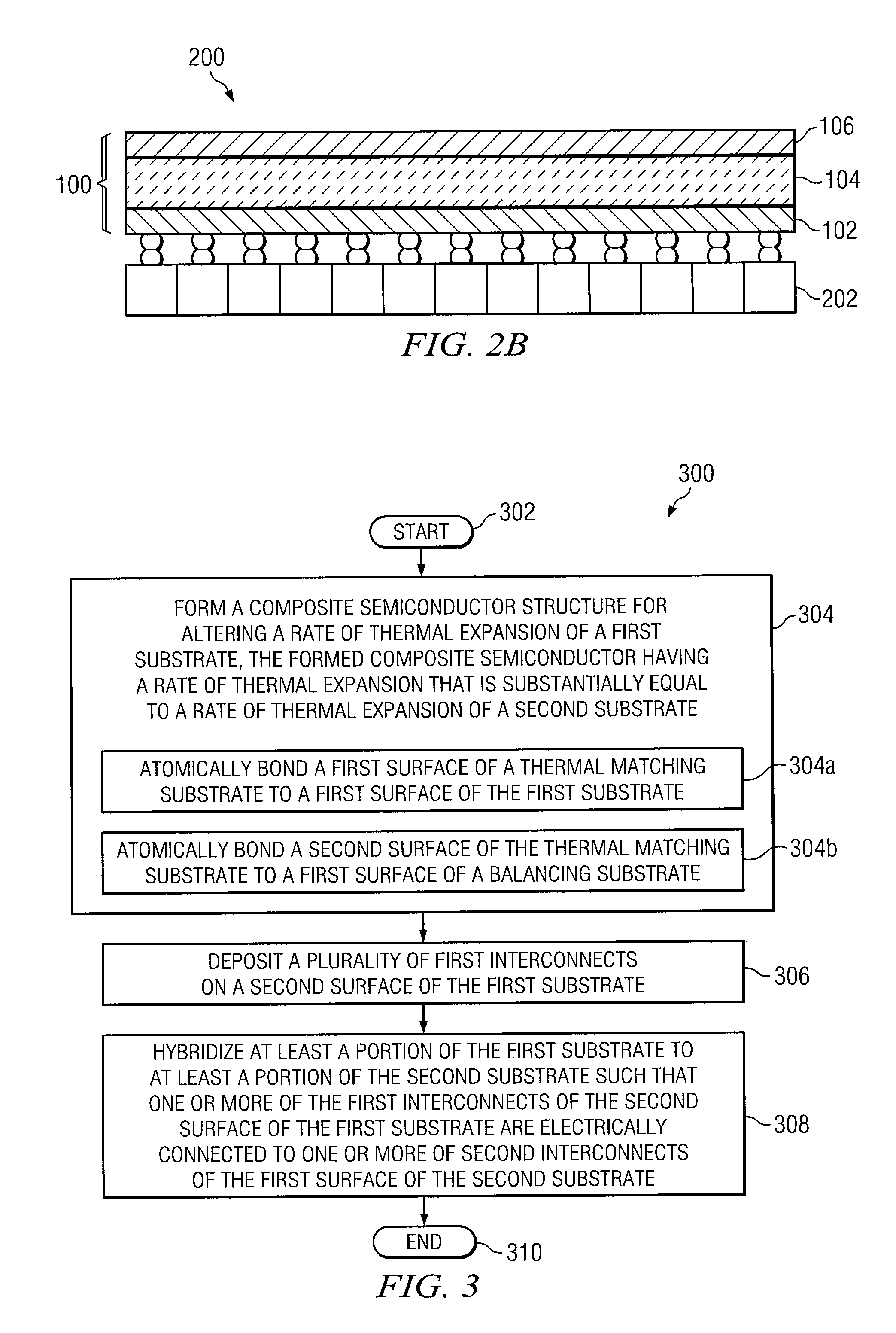
Composite Semiconductor Structure Formed Using Atomic Bonding and Adapted to Alter the Rate of Thermal Expansion of a Substrate
ActiveUS20110042772A1Avoid warpingLaborious and time-consumingSemiconductor/solid-state device detailsSolid-state devicesSemiconductor structureThermal expansion
In certain embodiments, a method includes forming a composite semiconductor structure for altering a rate of thermal expansion of a first substrate. The composite semiconductor structure is formed by atomically bonding a first surface of a thermal matching substrate to a first surface of the first substrate, and atomically bonding a second surface of the thermal matching substrate to a first surface of a balancing substrate. The thermal matching substrate is adapted to alter the rate of thermal expansion of the first substrate and the balancing substrate is adapted to substantially prevent warping of the composite semiconductor structure.
Owner:RAYTHEON CO
Audio and/or video generation apparatus and method of generating audio and/or video signals
ActiveUS20080040394A1Easy to createCreate additionalTelevision system detailsPulse modulation television signal transmissionSignal onAudio frequency
An audio and / or video generation apparatus configured to generate audio and / or video signals representative of an audio and / or video source, the audio and / or video generation apparatus comprising a recording unit configured to record the audio and / or video signals on a recording medium, wherein the audio and / or video generation apparatus is arranged to receive metadata associated with the audio and / or video signals generated by a data processor, the recording unit configured to record the metadata on the recording medium with the audio and / or video signals. The data processor may be arranged to receive signals representative of the time codes of the recorded audio / video signals, and the metadata may include time code data representative of the in and out points of a take of the audio / video signals generated by the data processor. The metadata may also include unique identification code for identifying the audio / video signals. The unique identification code may be a UMID or the like.
Owner:SONY CORP
Methods and kits for isolating sperm cells
ActiveUS7320891B2Laborious and time-consumingReduce capacityArtificial cell constructsDead animal preservationBiologySperm cell
Owner:PROMEGA CORP
Selection Methods of Self-Pollination and Normal Cross Pollination in Poplation, Variety of Crops
InactiveUS20080098492A1Laborious and time-consumingHybridization is not heavyPlant genotype modificationFemale groupSelf-pollination
The invention relates to the field of crop selection and crop breeding, particularly to selection methods for breeding colony varieties of crops involving self-pollination and normal cross-pollination. Selection methods include crossing male parents with female parents to obtain crop populations, wherein the female parents are individual plants in a segregation population or self-crossed descendants of early segregation generations, said segregation population obtained by hybridizing pairs of parental plants with different desired characteristics to produce population F1, and then hybridizing pairs of F1 one more time. The male parents are homozygous breeding lines, varieties, F1, heterozygous plants in the segregation generations or individual plants produced in the same manner as for the female group. Crop populations and colony varieties of crops display characteristics of consistency, stability, specificity, disease resistance, high yield, and high adaptability while permitting farmers to reserve seeds properly and companies to exploit pedigreed seed on a large scale.
Owner:RICE RES INST GUANGDONG ACADEMY OF AGRI SCI +1
Pressure sensitive textile adhesive
ActiveUS8398804B2Avoid the needLaborious and time-consumingLamination ancillary operationsDecorative surface effectsCross-linkHigh energy
A method of wash-durably bonding integrated textile emblems such as patches, emblems, labels and cut textile parts to another textile article by laminating a pressure sensitive acrylic polymer adhesive to the back surface of the textile emblem. The acrylic polymer adhesive has a storage modulus that is greater than a loss modulus throughout a frequency range of from 0.1885 Hz to 628 Hz where bonding and debonding are expected to occur, and is devoid of any cross linking additives or reagents. The integrated textile emblem with laminated pressure sensitive acrylic polymer adhesive has improved adhesion to low-to-high energy surfaces, is not water soluble and is of adequate thickness to provide a wash-durable textile-to-textile bond capable of seaming or permanently attaching items such as textile panels, appliqué bearing text, numbers, logos and other indicia for the apparel, accessory and other industries.
Owner:LION BROS CO INC
Magnetic field modling device, die and method for magnetic field molding
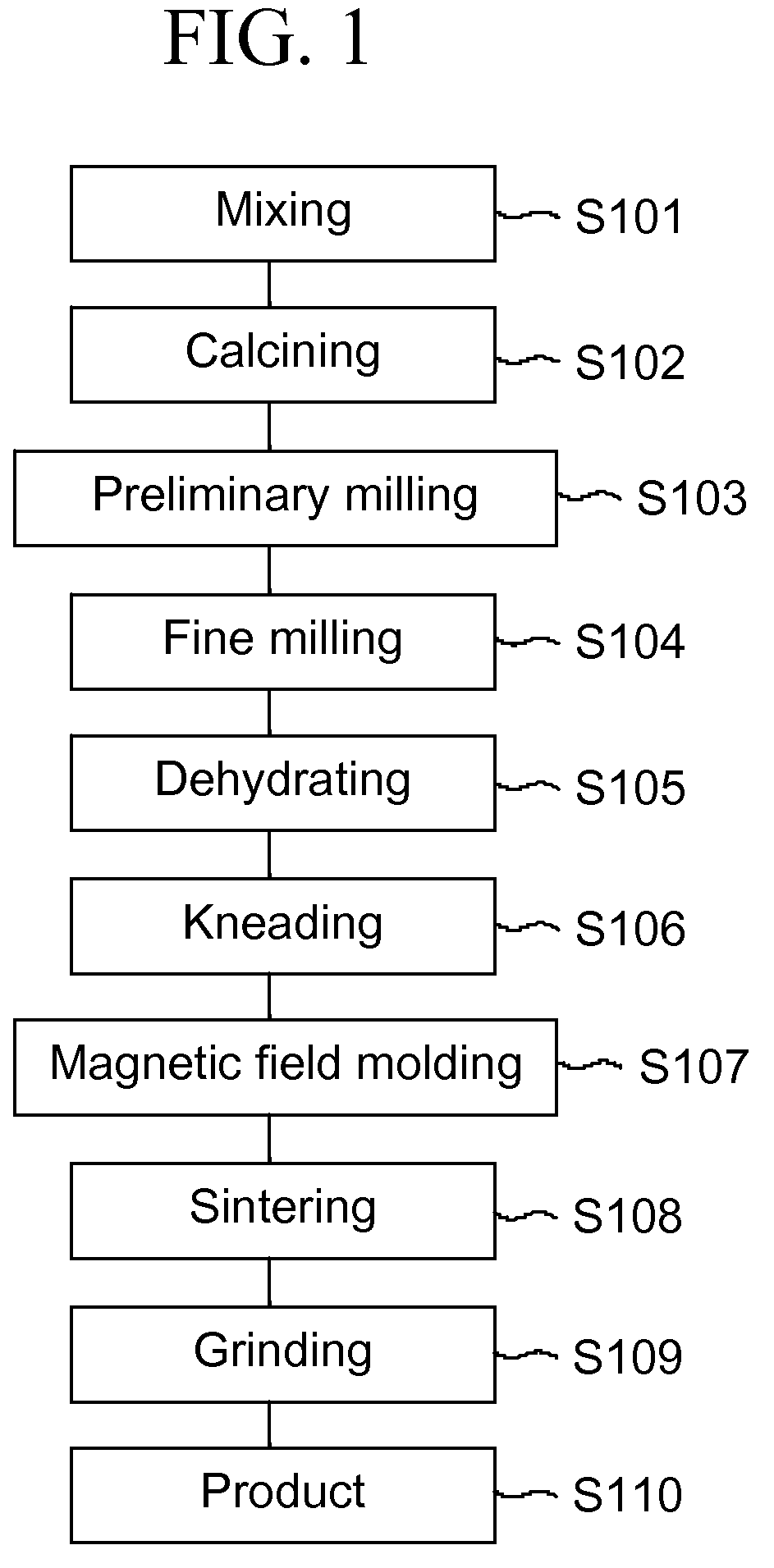
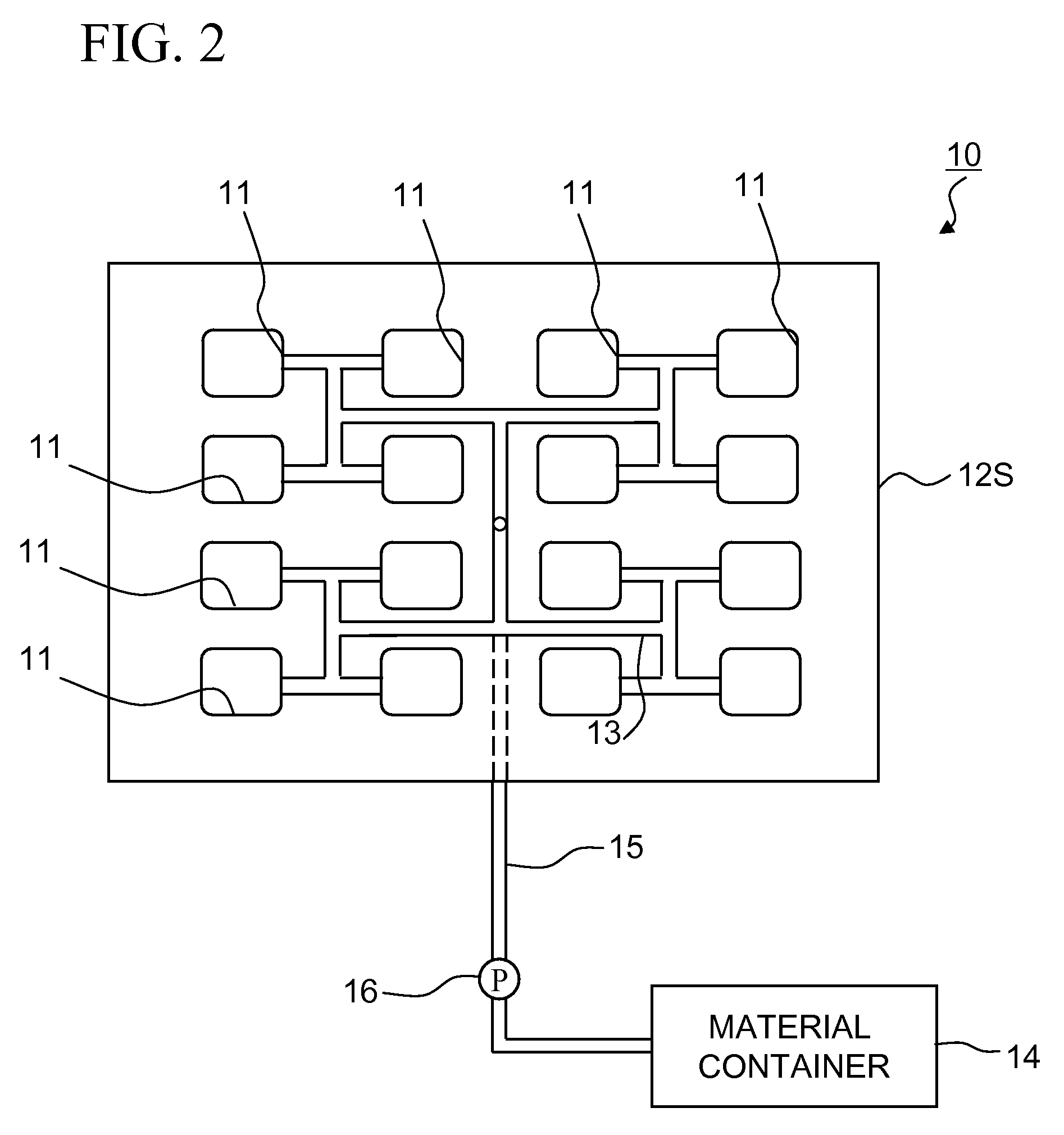
InactiveUS20070176329A1Improve the immunityIncreased durabilityTransportation and packagingMetal-working apparatusSolid componentSlurry
The objects of the present invention are to provide a durable magnetic field molding device for producing molded bodies for ferrite magnets, which needs a controlled amount of releasing agent, reduces production cost and improves productivity, and die and magnetic field molding method. The lower die 12B of the die 12 is coated, on the surface which defines the cavity 11, with the coating film 30 with a high hardness and a low friction coefficient, which improves resistance of the lower die 12B surface to wear by the solid component (fine powder) in the slurry, thereby greatly improving durability of the coating film 30 itself and reducing lubricant usage.
Owner:TDK CORPARATION
Container labeling systems and methods of use
ActiveUS9440759B2Laborious and time-consumingEfficient importTypewritersPackaging automatic controlEngineeringPallet
The invention relates to labeling and / or printing devices, methods and systems for applying coding and / or labeling to containers that are stacked or otherwise organized in a group (e.g., containers stacked on a pallet). In embodiments of the invention, labeling and / or printing devices are mounted on carriages that are capable of moving in a vertical direction to apply labels as they move. Horizontal movement is imparted either by moving such carriages in a horizontal direction adjacent to the containers, or by moving the containers themselves (or the pallet holding them) in a horizontal direction adjacent to such carriages. Multiple carriages with labeling devices may be provided to provide simultaneous labeling to more than one side of a group of stacked containers. Embodiments of the invention are capable of providing labeling of containers that are uniformly or non-uniformly grouped or stacked.
Owner:REED LORIN
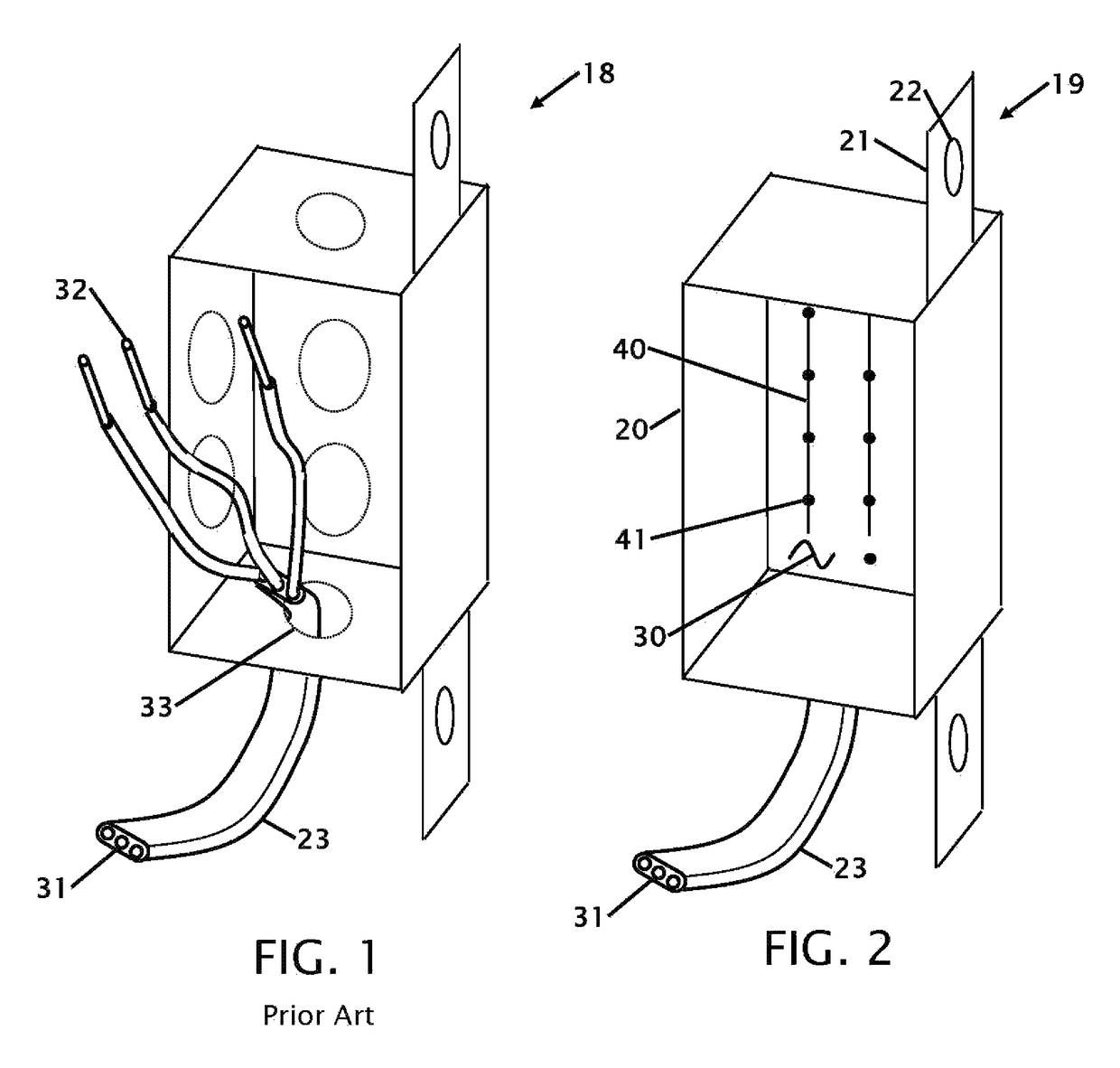
Junction box with an integrated connection circuit
InactiveUS10170878B1Laborious and time-consumingElectrically conductive connectionsTwo pole connectionsElectricityElectrical junction
Improvements to an electrical junction box is disclosed. The junction box is configured with integrated connections in the back of the junction box. Switches and outlets have tabs in specific locations that align with the desired integrated connections to provide the desired function. All of the outlets and switches align parallel with the back and front of the junction box. The junction box is configurable to accept a single switch, dimmer switch, 3-way or outlet to many more than one function. In addition, the function of a junction box can be easily changed as long as the wiring has been connected to the new junction box. Installation and changes can be made in seconds instead of minutes. The depth of the internal distribution receptacle is adjustable within the adjustable electrical box to accommodate the thickness of the outlet or switch.
Owner:REULMAN SR DAVID MARK
Methods and kits for isolating sperm cells
ActiveUS20060057715A1Low densityLaborious and time-consumingArtificial cell constructsDead animal preservationBiologySperm cell
Owner:PROMEGA
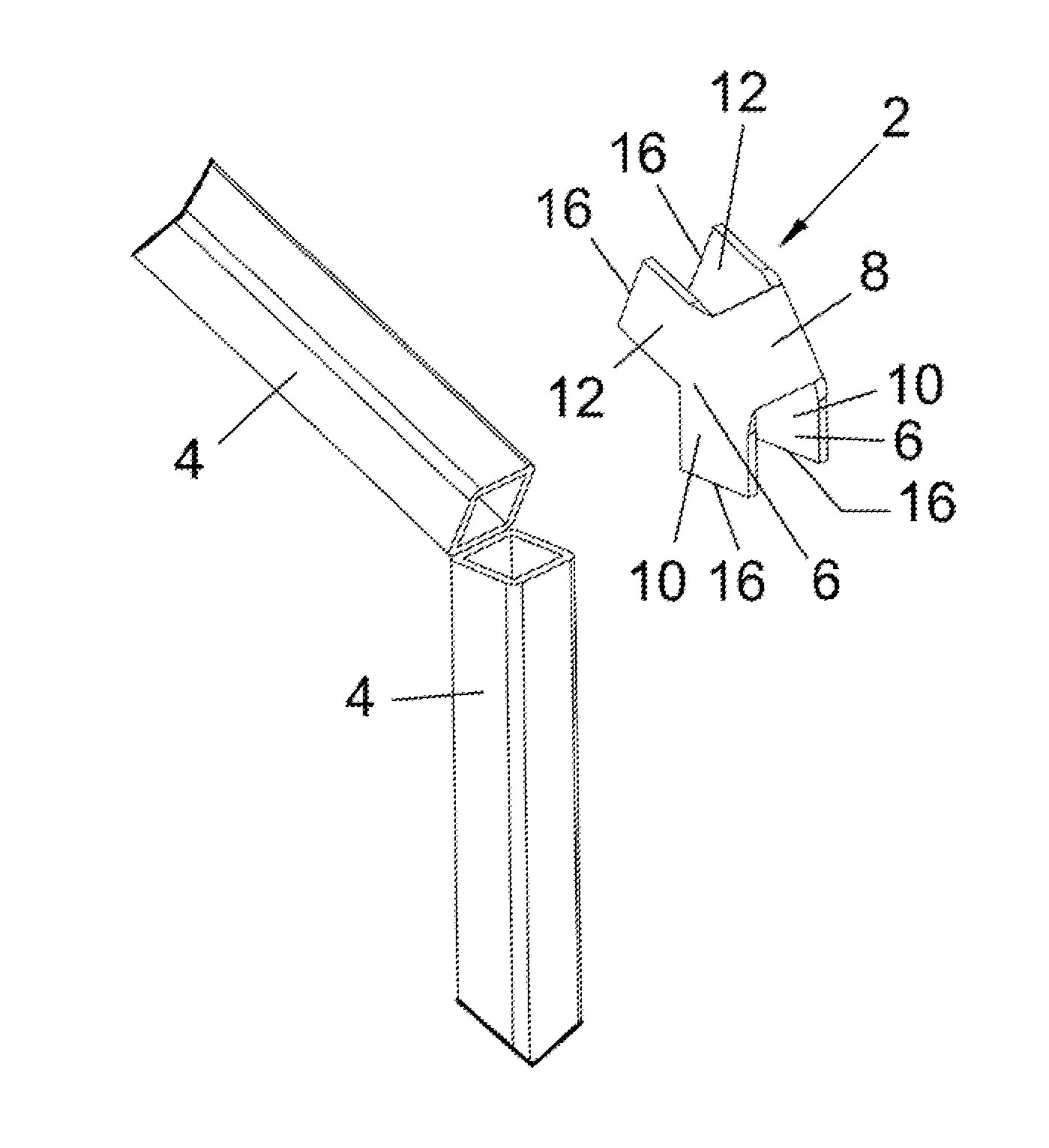
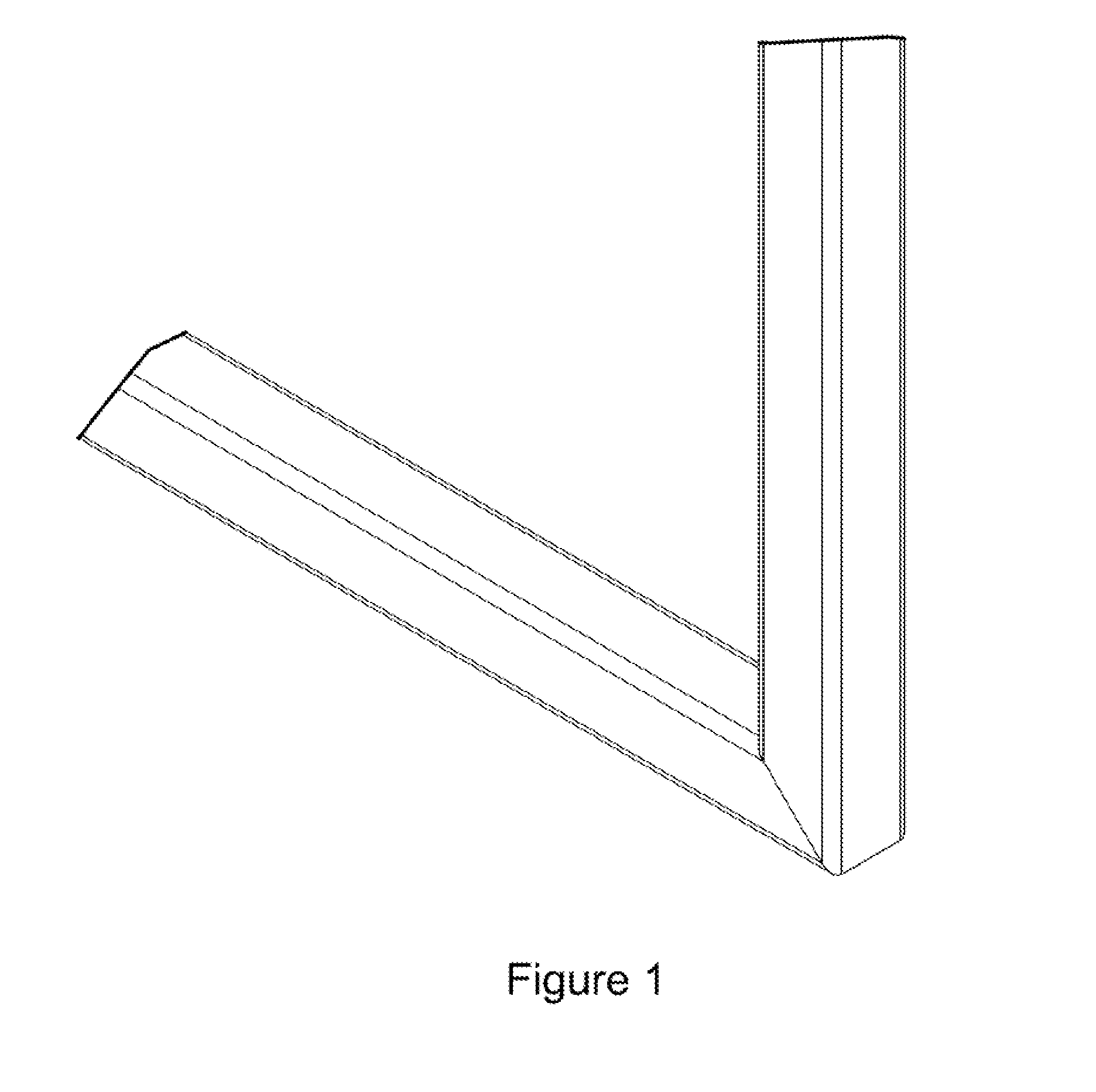
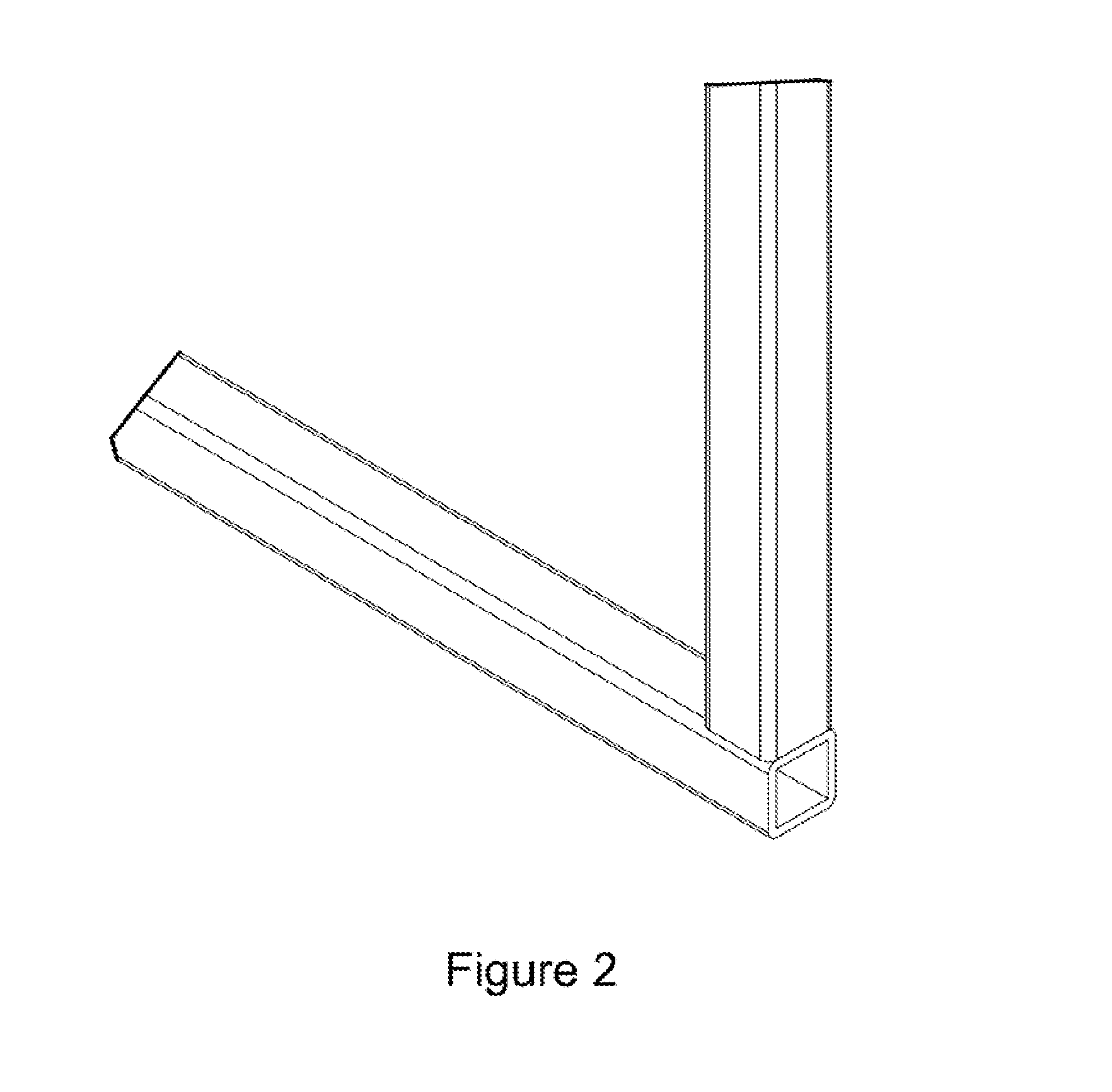
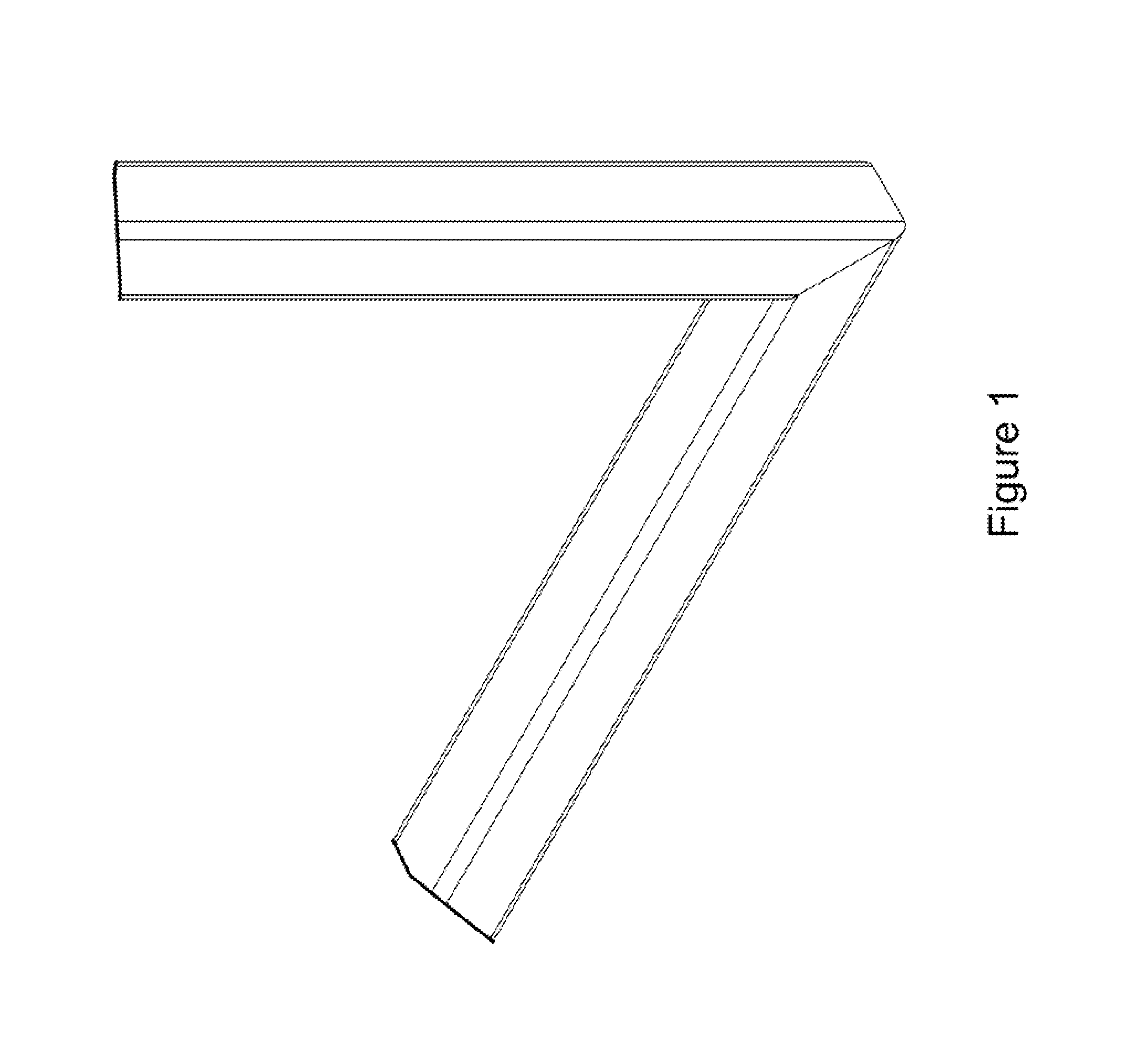
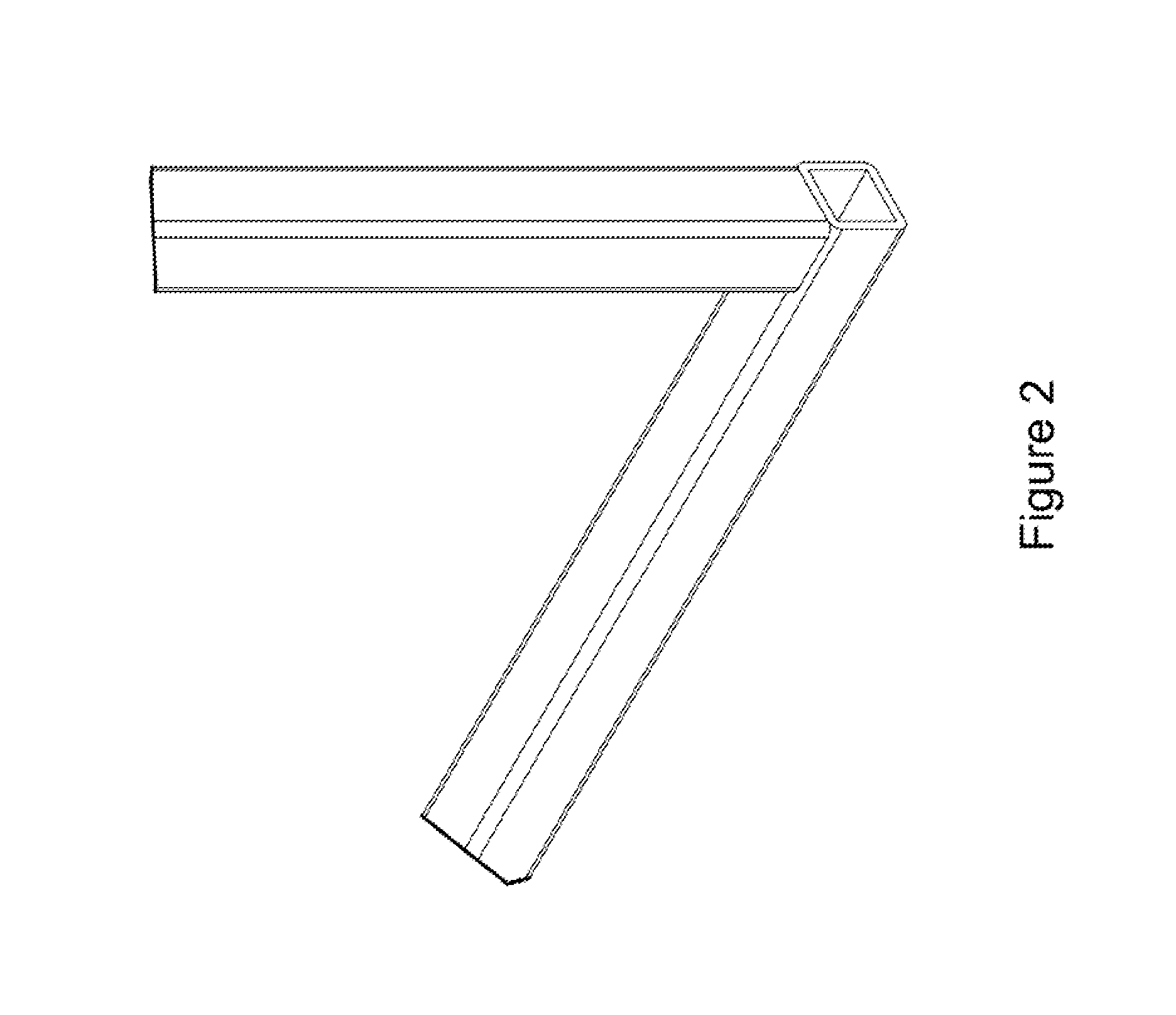
Miter coupling and method
InactiveUS20110299922A1Strong jointLaborious and time-consumingScaffold connectionsPicture framesCouplingEngineering
A miter coupling is provided, together with a method for using the miter coupling to join a pair of square metal tubes at a desired angle using a welding process. The miter coupling, in a preferred embodiment, comprises a single piece of metal formed into a shape that is adapted to receive the ends of two square metal tubes at a desired angle. The miter coupling is shaped to form a pair of parallel elbow members, where the elbow members are formed into a desired angle, and the elbow members are interconnected by a cross support. This arrangement provides a stronger joint, and eliminates the time consuming steps of cutting the square metal tubes at specific angles and grinding the welds to form a smooth seam.
Owner:TRIVETTE ROGER BLAINE
Method of producing textile emblems with pressure sensitive adhesive properties
InactiveUS20110014837A1Avoid the needLaborious and time-consumingDecorative surface effectsAdhesive articlesRoom temperatureHigh surface
A new device, pressure sensitive adhesive system for bonding textiles and a method for producing integrated textile emblems such as patches, emblems, emblems, labels and cut textile parts including wovens, knits and nonwoven structures made of both natural and or synthetic fibers incorporating a pressure sensitive adhesive. The resulting room temperature pressure sensitive-patch eliminates tedious and cost intensive sewing operations (embroidery) and or heat-sealing operations (heat transfers) to other textile products such as apparel and or accessories, headwear, crafts home furnishings and luggage. The room temperature pressure sensitive adhesive-patches comprise at least: 1) a top base layer which can be a knitted, woven or non-woven fabric sheet that is stitched, printed, embossed, etched, engraved, flocked or dyed to form a decorative element, and which may be cut into a desired shape; 2) a pressure-sensitive adhesive layer adhered to the top base layer that comprises an adhesive having good medium-to-high surface energy properties, is not water soluble and is of adequate thickness to provide a sustainable bond capable of surviving multiple washes to textile-based products such as apparel and or accessories, headwear, crafts home furnishings and luggage. The room temperature pressure sensitive-patches may be transferred by simple pressure, thereby eliminating the burden and expense of thermal or mechanical (stitched) bonding.
Owner:BAQAI NAVAID +2
Computer rack attachment
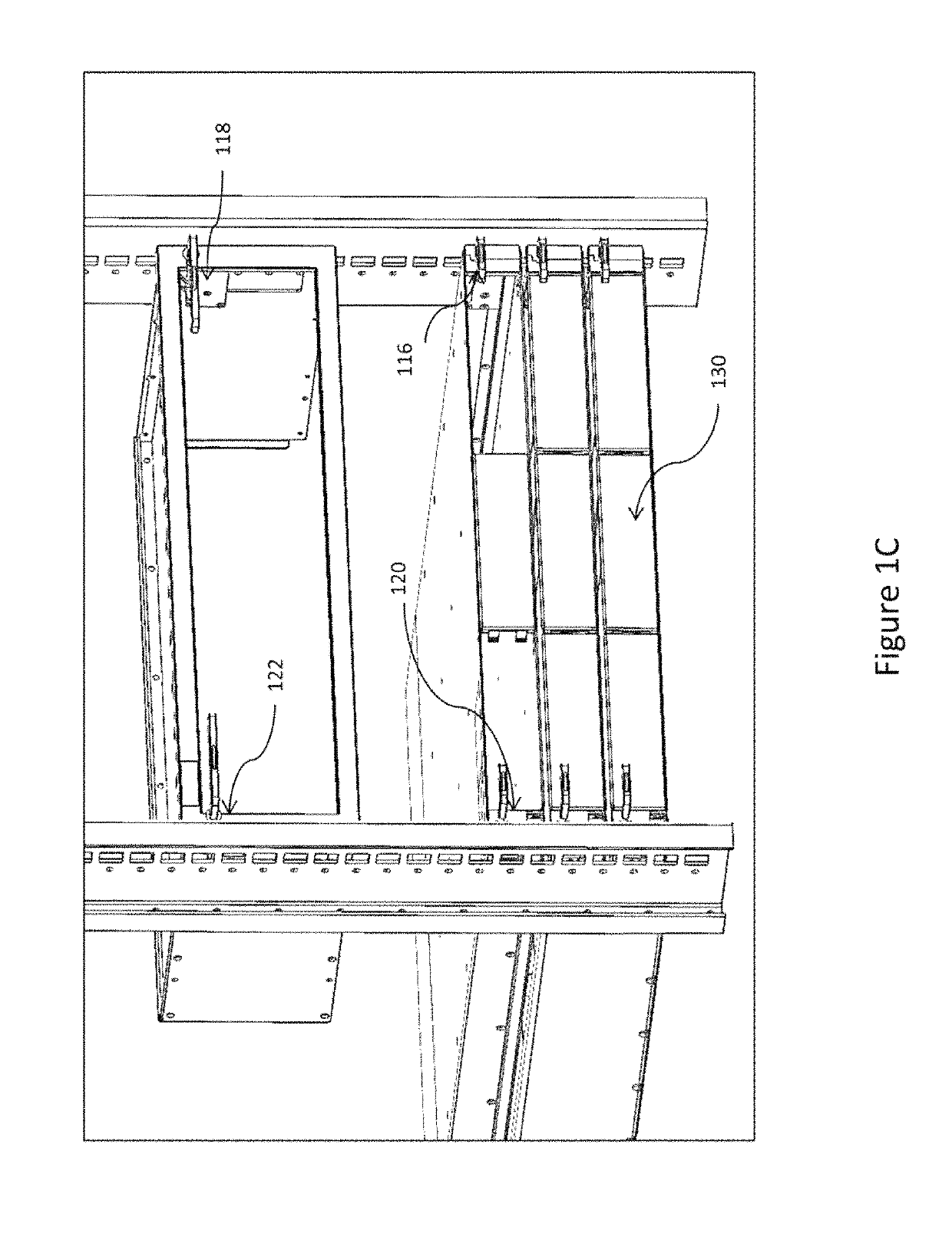
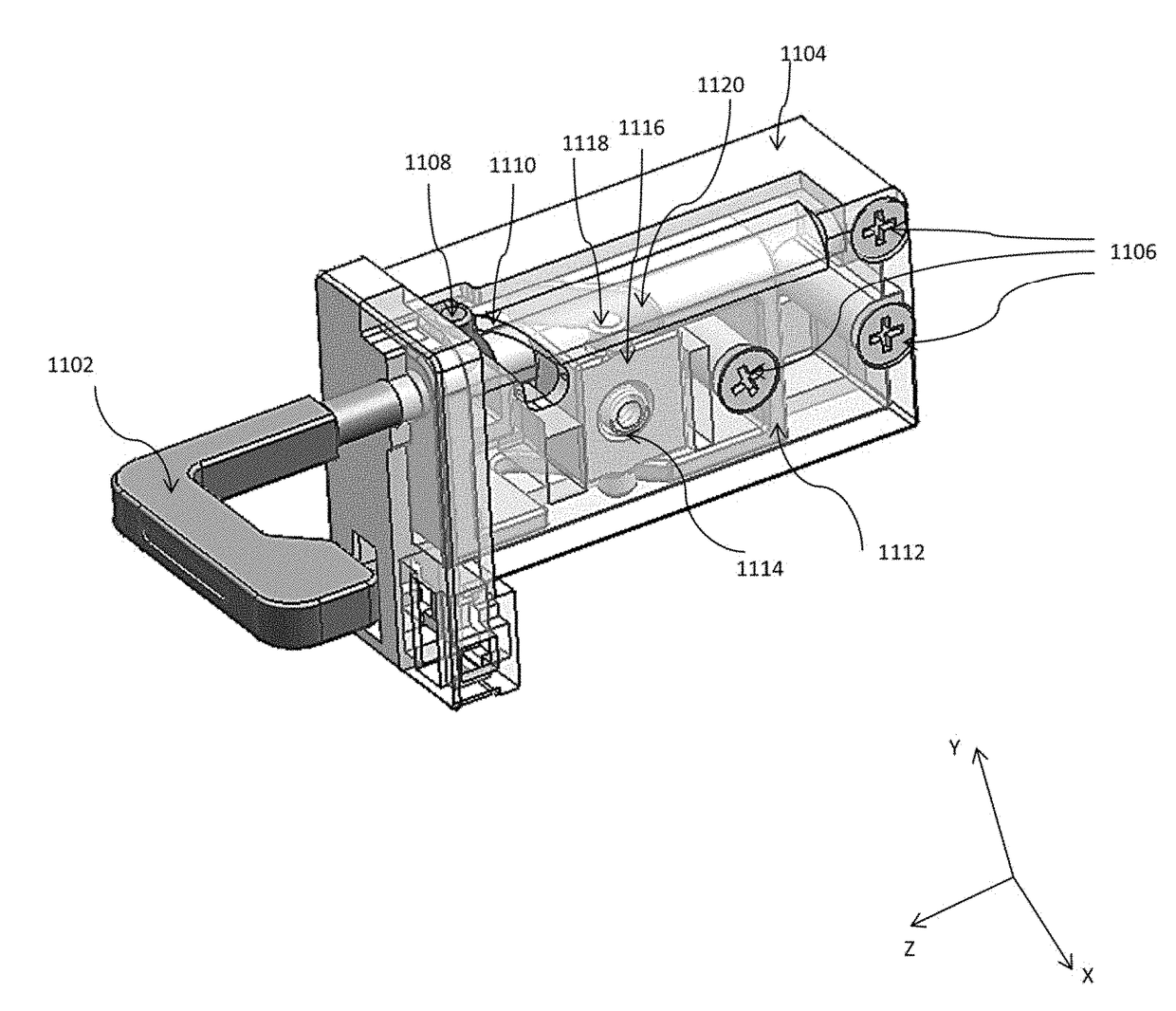
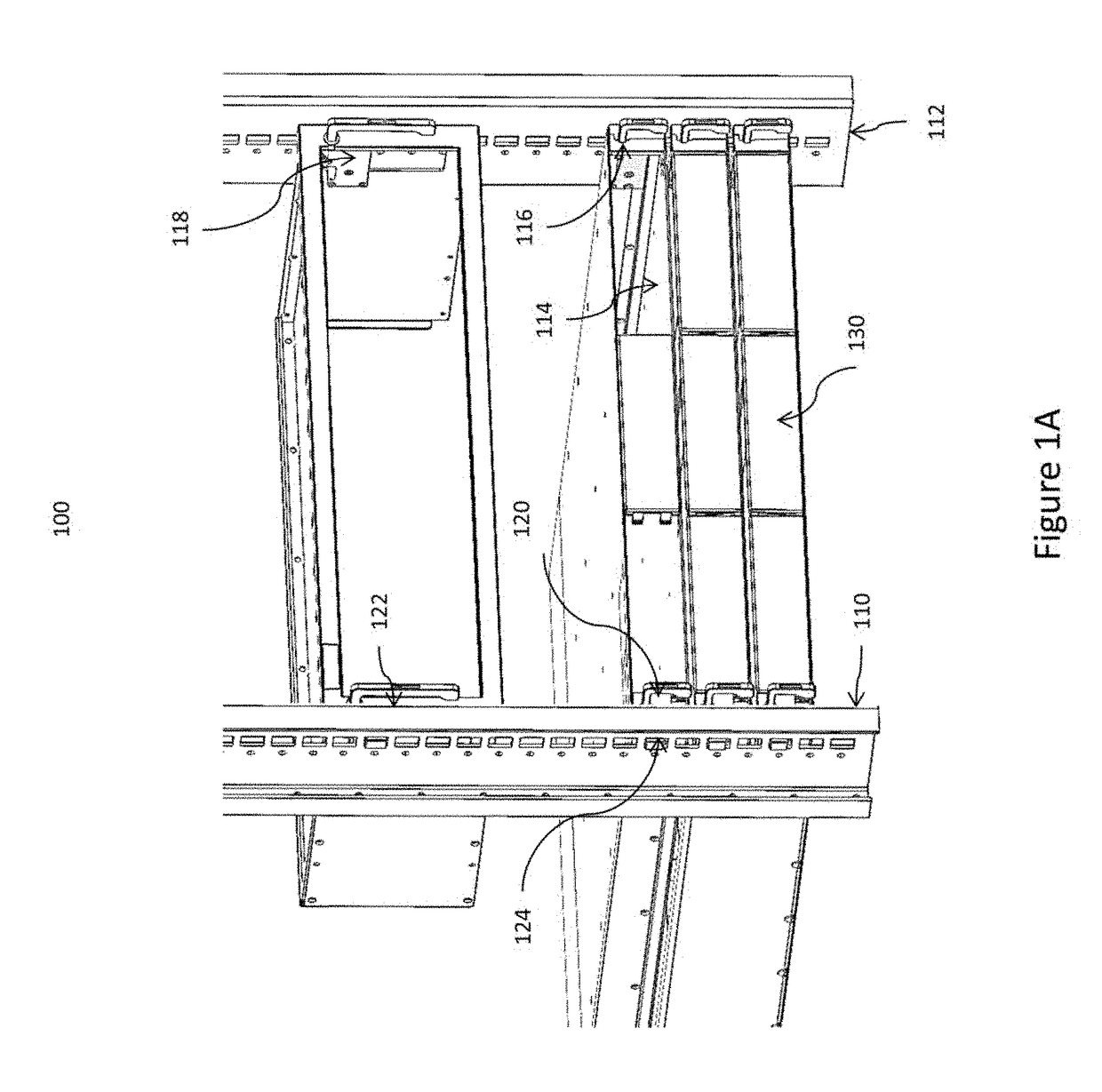
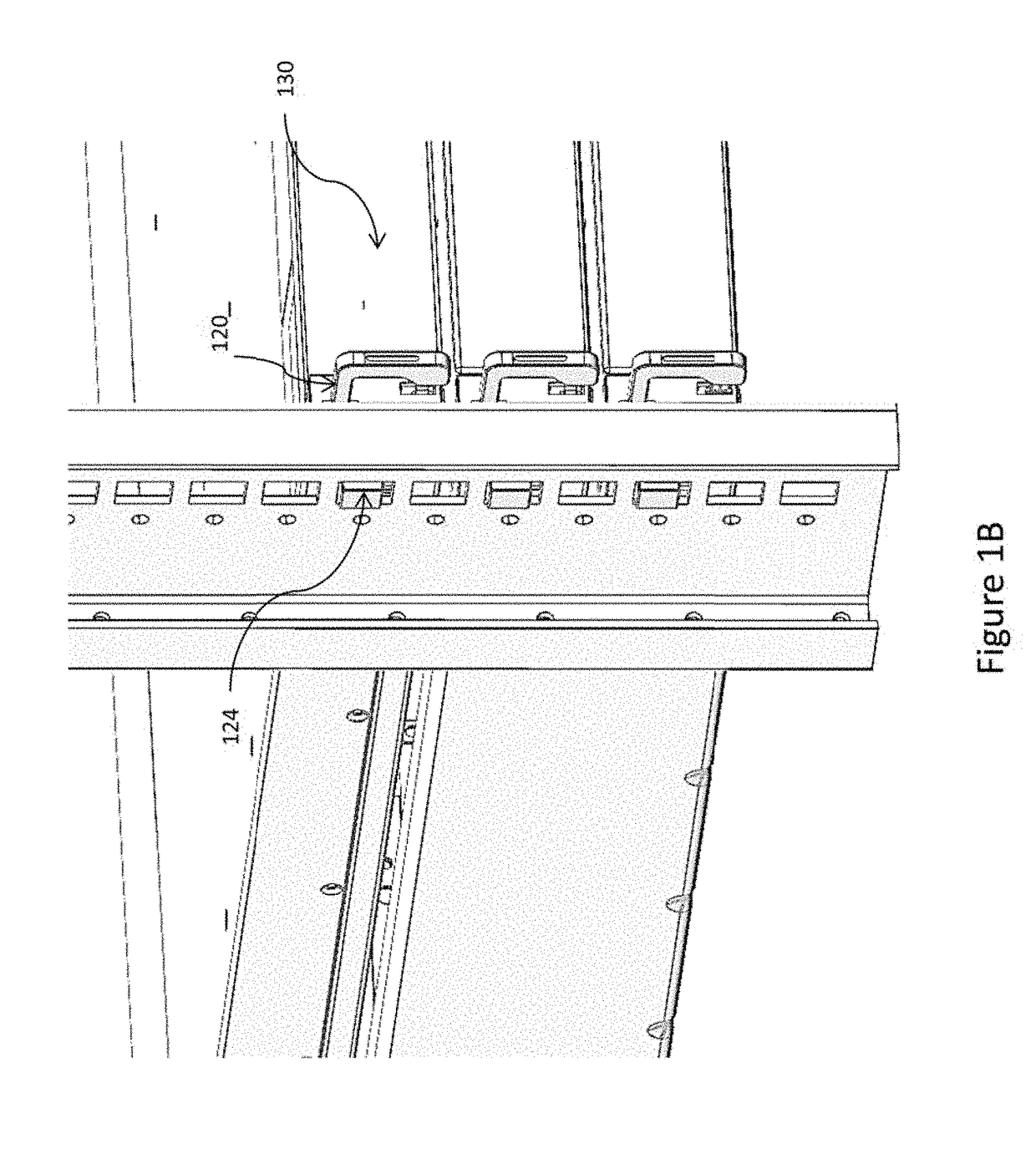
ActiveUS10499527B2Laborious and time-consumingServersDrawersMechanical engineeringElectronic equipment
Owner:SOUTHCO
Computer rack attachment
ActiveUS20180228049A1Prevent movementLaborious and time-consumingServersDrawersEngineeringMechanical engineering
A rack attachment device configured for attaching an electronic device to a rack. The rack attachment device includes a housing for coupling to the electronic device, a pawl mounted for movement relative to the housing, a slide mounted for movement relative to the housing, where the slide is positionable to contact the pawl, and a handle extending from the housing and coupled to the slide. In an unlocked position, the handle is in a first handle position and the slide is in a first slide position, such that the slide prevents the pawl from moving toward a rack aperture defined in the rack. In a locked position, the handle is in a second handle position and the slide is in a second slide position, such that the slide permits the pawl to enter the rack aperture when the slide is in the second slide position.
Owner:SOUTHCO
Cladding assembly
The present invention concerns a frame member (10), a frame formed from the frame member (10) and a panel including the frame for use in a cladding assembly for cladding a structure. The frame member (10) of the present invention is adapted to be joined together with other frame members (10) to form the frame, each frame member (10) includes at least four walls including an inner frame wall (11), an opposed outer frame wall (12), a panel abutting wall (13) and an opposed structure abutting wall (14) having a free end (15) with an outer edge protrusion (16). A receiving opening (19) is defined between a portion of the outer frame wall (12) and the outer edge protrusion (16) for receiving one or more fasteners which engage the outer edge protrusion (16) for, in use, fastening the frame member (10) and thereby the frame and preformed panel to the structure.
Owner:LEE DUANE
Miter coupling and method
InactiveUS8899868B2Less expensiveLaborious and time-consumingScaffold connectionsDoors/windowsCouplingEngineering
A miter coupling and method for using the miter coupling to join a pair of square metal tubes at a desired angle using a welding process. The miter coupling, in a preferred embodiment, comprises a single piece of metal formed into a shape that is adapted to receive the ends of two square metal tubes at a desired angle. The miter coupling is shaped to form a pair of parallel elbow members, where the elbow members are formed into a desired angle, and the elbow members are interconnected by a cross support.
Owner:TRIVETTE ROGER BLAINE
Reversible Terminator Nucleotides And Methods of Use
InactiveUS20100047796A1Laborious and time-consumingReduce the number of stepsSugar derivativesMicrobiological testing/measurementSugar nucleotideBiophysics
Owner:APPL BIOSYSTEMS INC
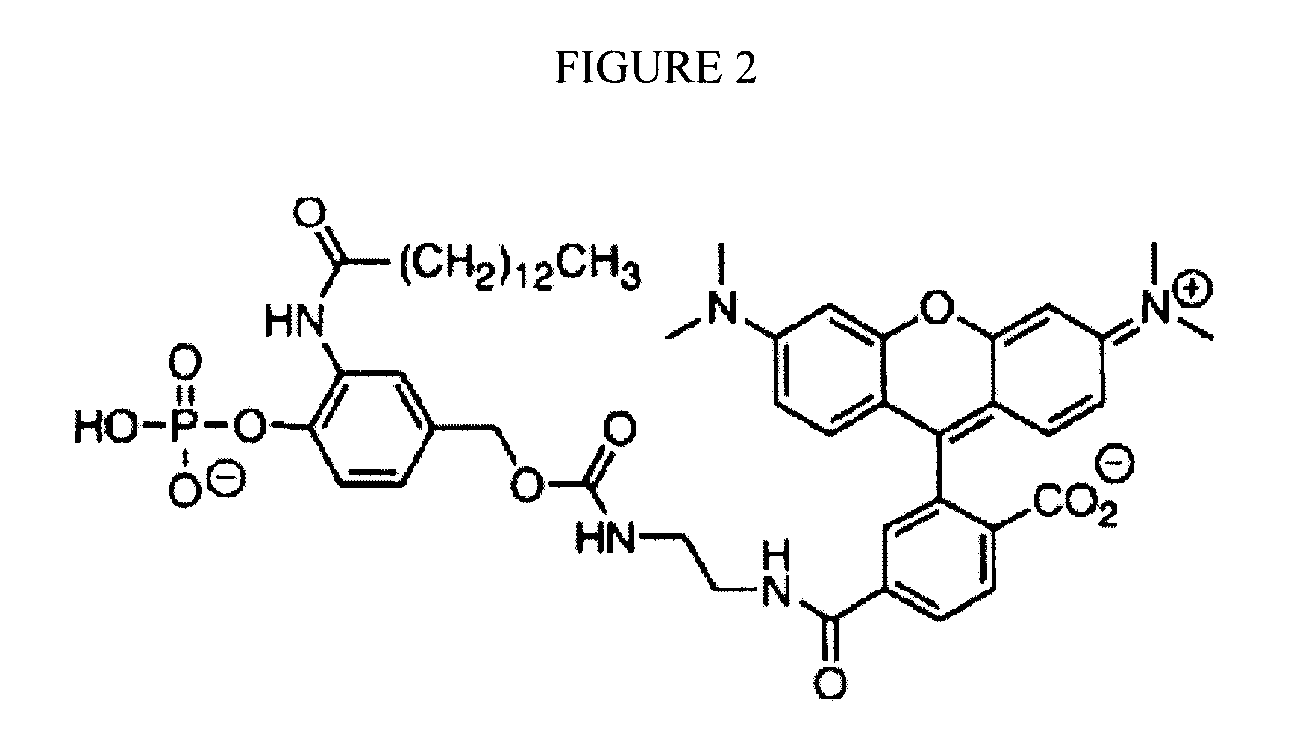
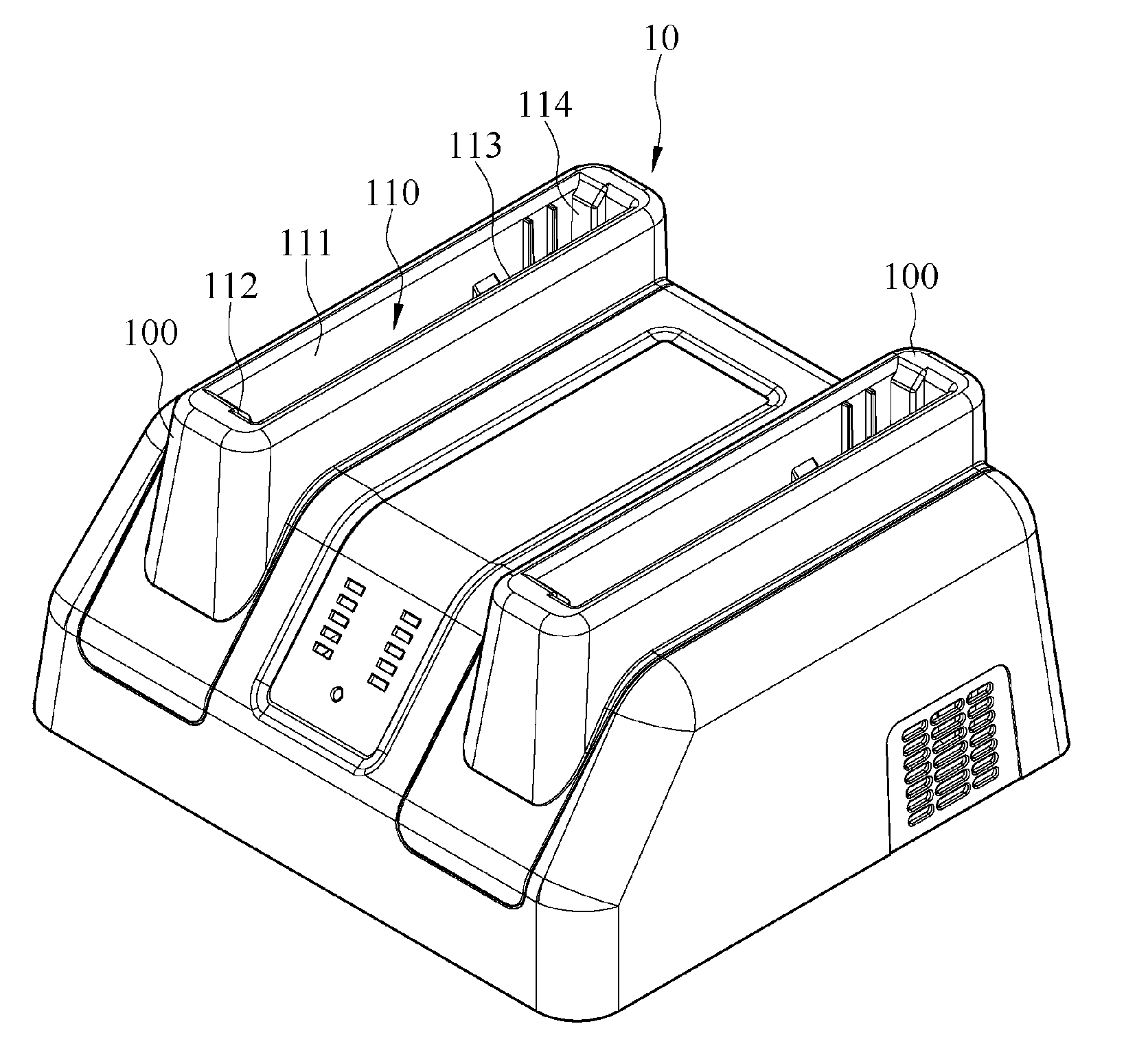
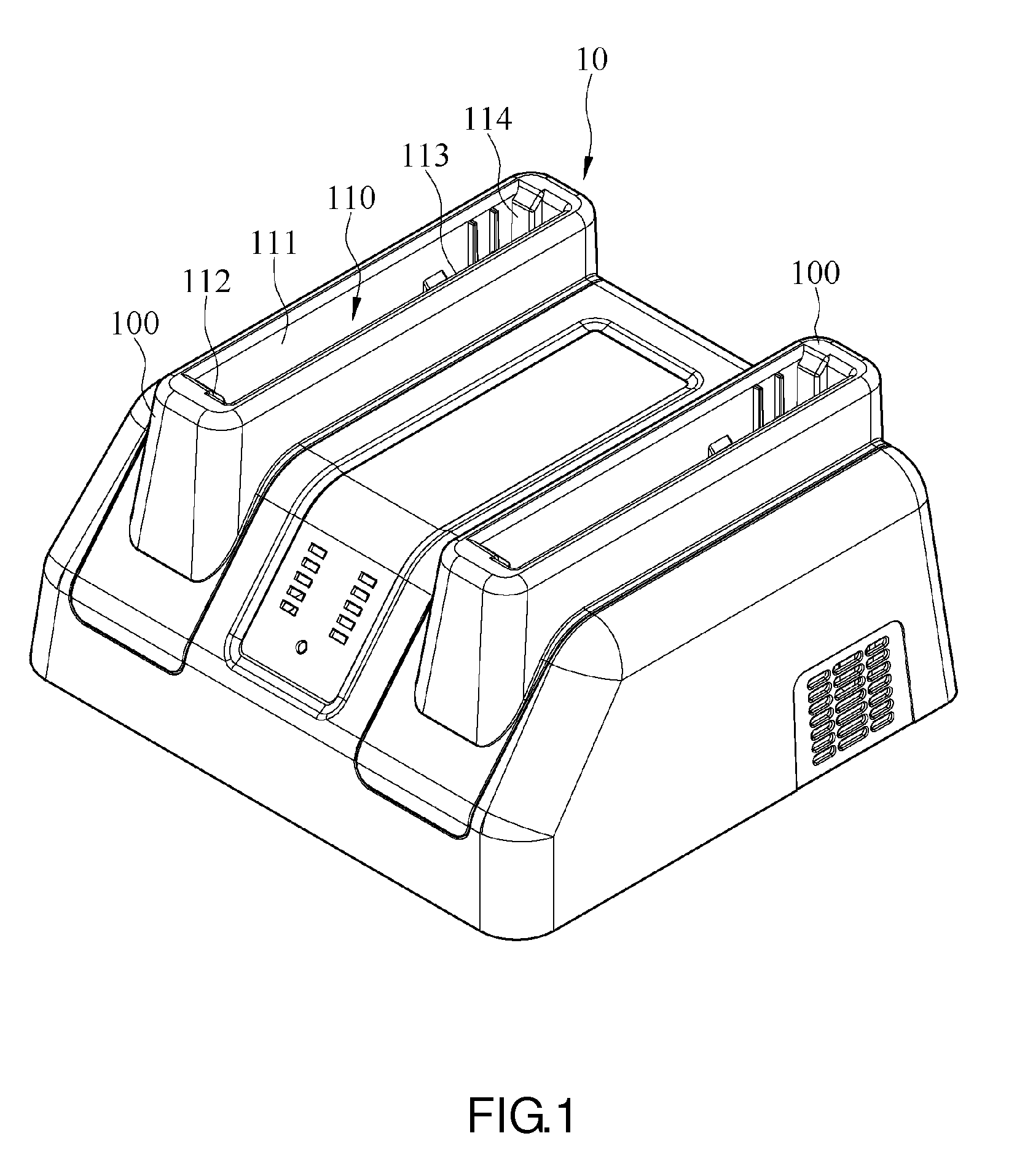
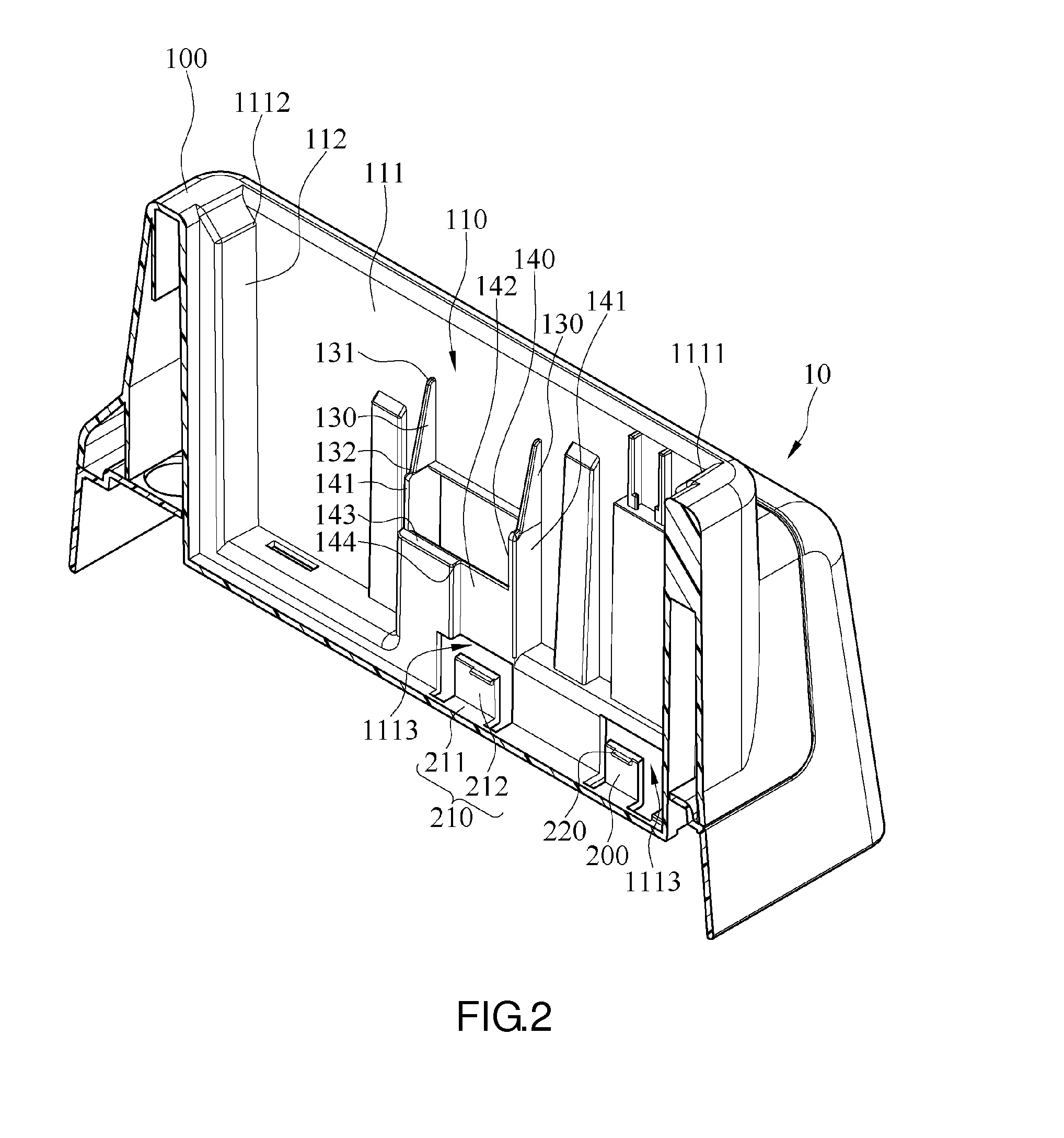
Charging device
ActiveUS10027152B2Easy to insert and take outLaborious and time-consumingElectric powerArrangements for several simultaneous batteriesEngineeringPlinth
Owner:GETAC TECH CORP
Stackable and stable bedding foundation
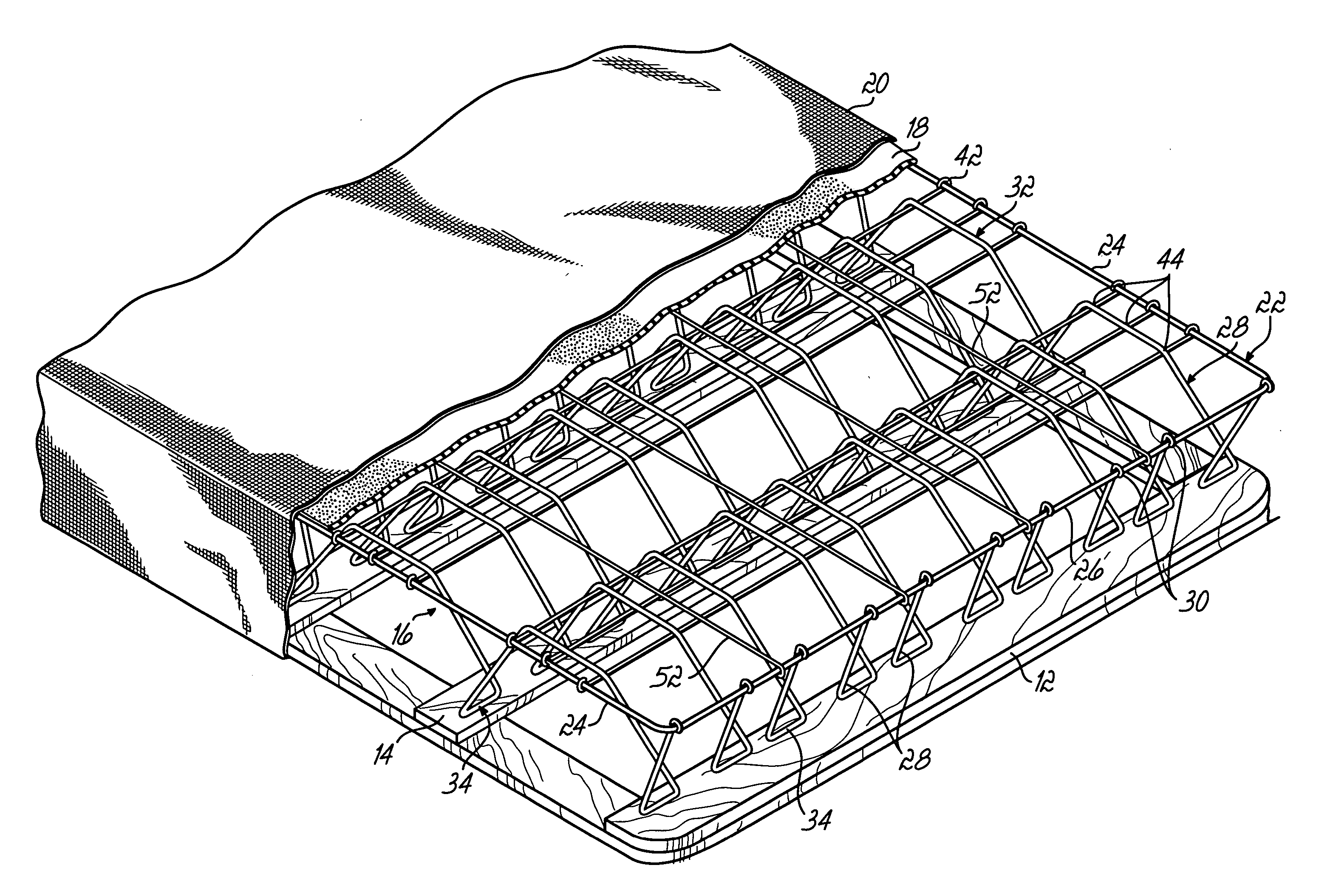
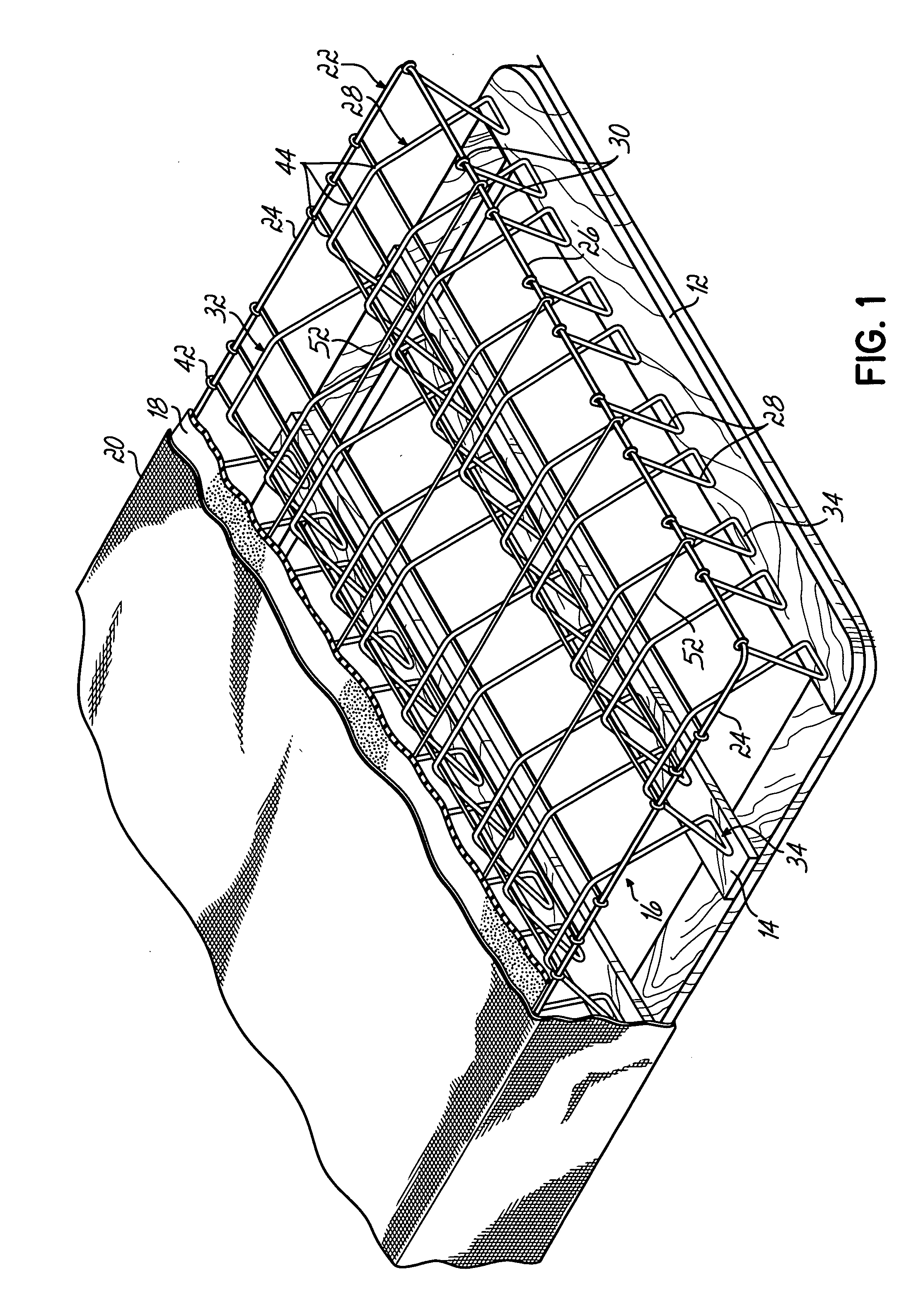
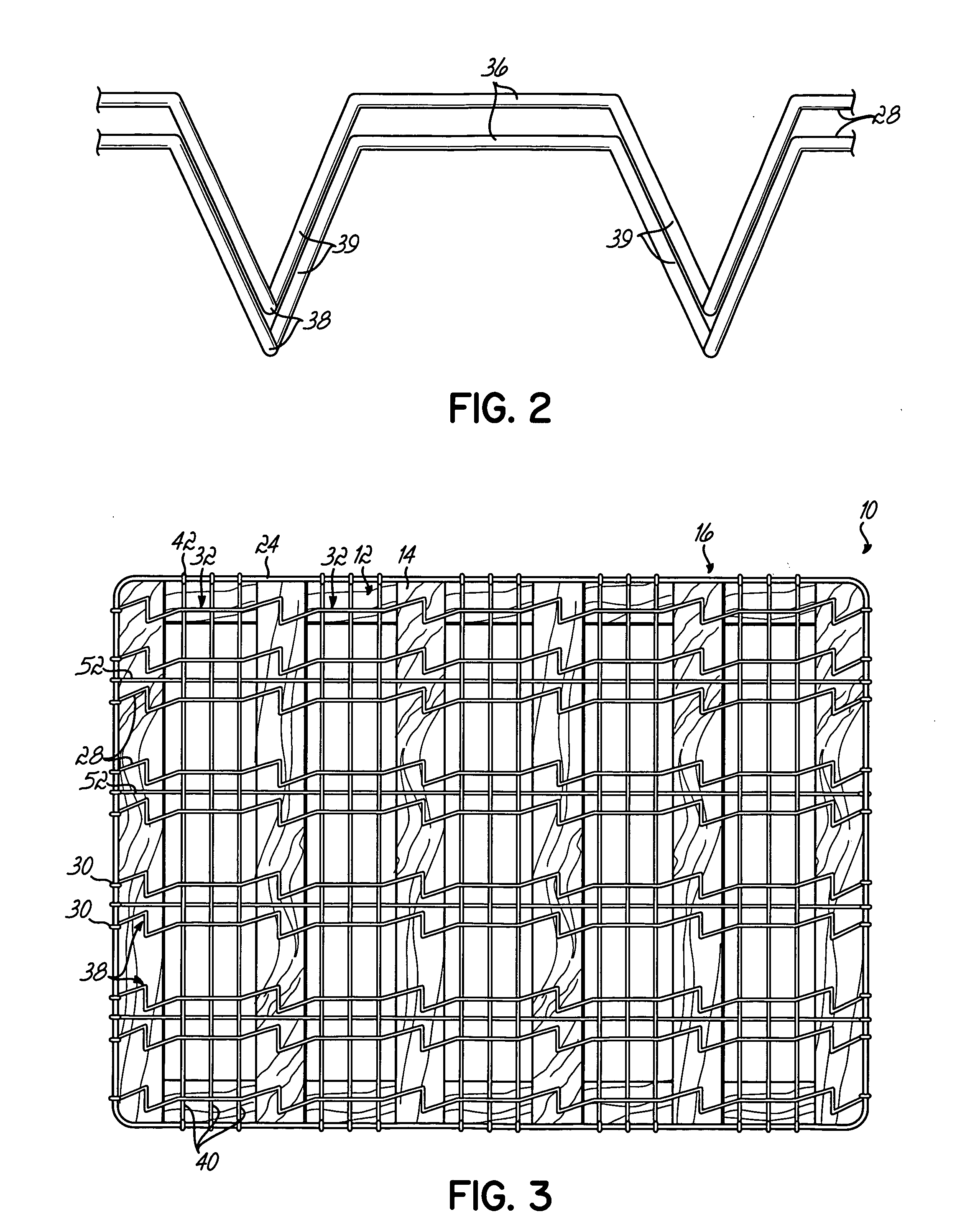
ActiveUS20060156470A1Lower unit costMinimal spaceMultiple spring combinationsSpring mattressesCoil springEngineering
A nestably stackable bedding foundation assembly replaces the traditional border wire and disposed coil spring foundation assembly in a so-called box spring. The foundation assembly may be nestably stacked with numerous other such assemblies for transportation, thereby avoiding the need to compress and tie the assembly for shipping. Each foundation assembly includes a number of corrugated support wires having alternating peaks and valleys. The valleys of selected support wires are twisted relative to their associated peaks to provide a more stable mounting to a base and offer a variety of firmness to specific zones of the assembly.
Owner:L & P PROPERTY MANAGEMENT CO
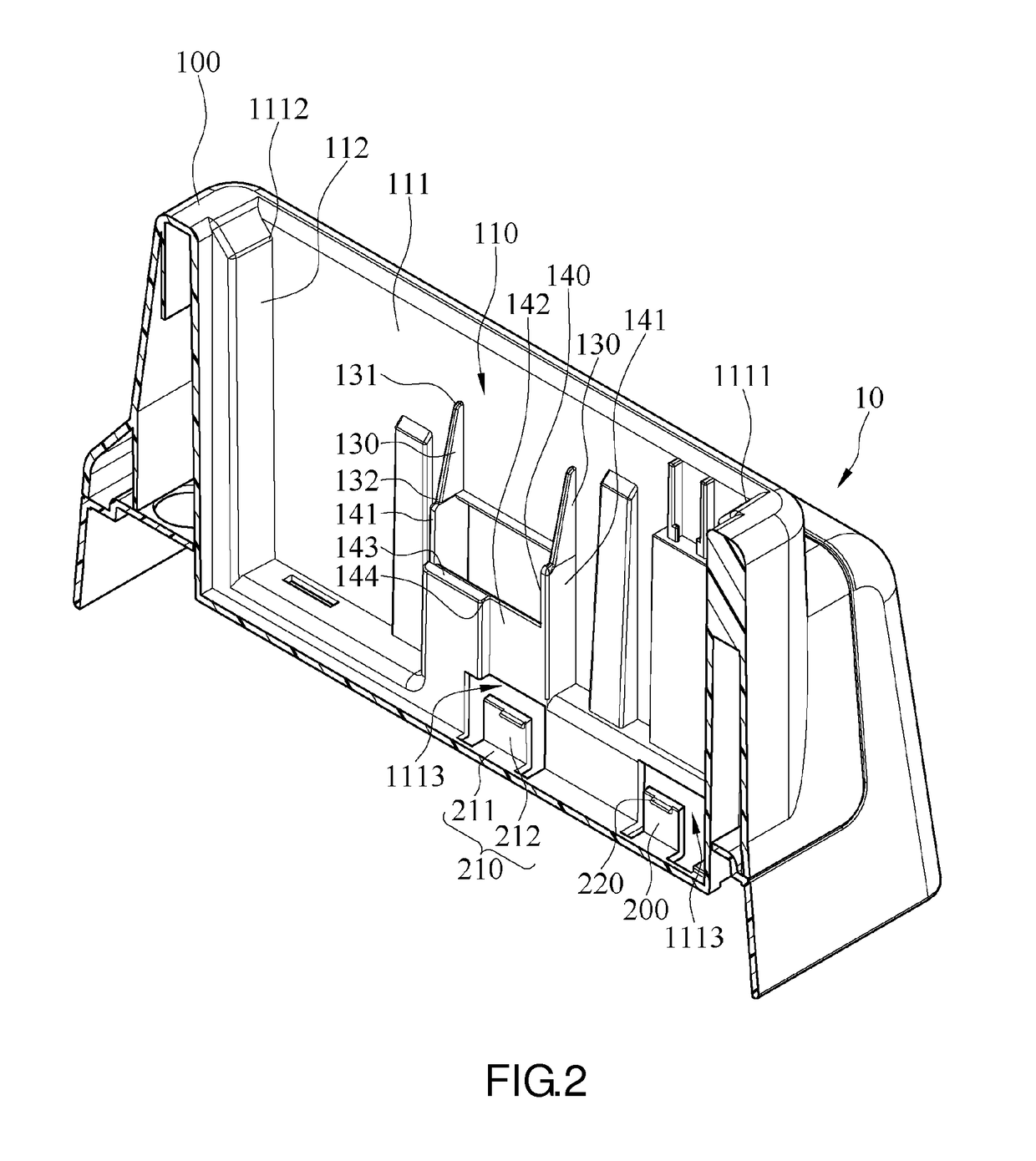
Charging device
ActiveUS20160156208A1Laborious and time-consumingLarge clamp forceElectric powerArrangements for several simultaneous batteriesEngineeringElectrical and Electronics engineering
A charging device including a charging base and at least one hook portion is provided. The charging base has a receiving chamber for receiving a battery. The receiving chamber includes a first sidewall. The first sidewall has a first side and a second side opposing the first side. The hook portion is disposed on the first sidewall, positioned proximate to the first side of the first sidewall, and adapted to fix the battery in place. When the battery begins to rotate under an applied force and therefore disconnect from the hook portion, the fulcrum of the rotating battery is positioned proximate to the second side of the first sidewall. The battery can be firmly inserted into the charging device. It is easy to insert and take out the battery.
Owner:GETAC TECH CORP
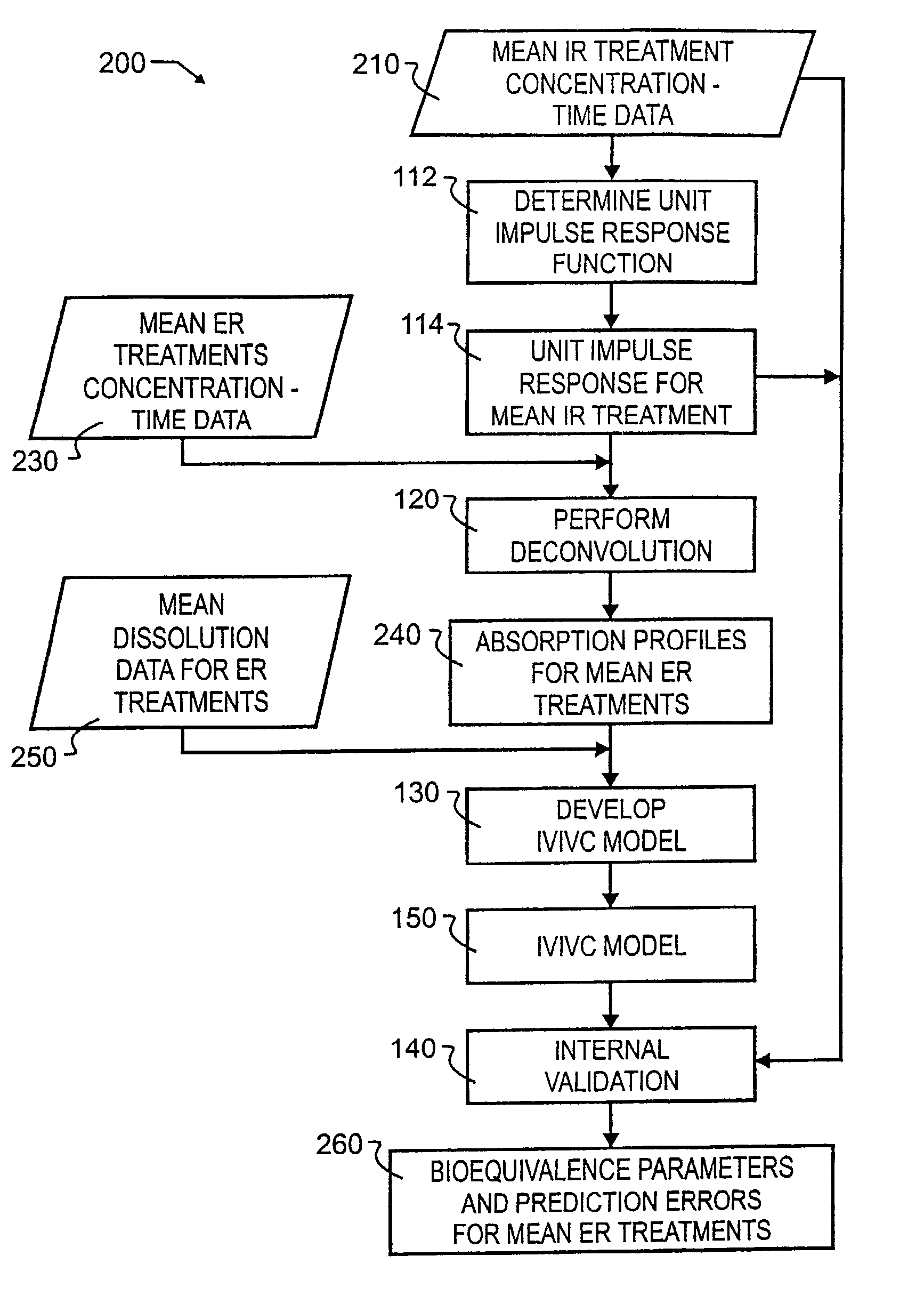
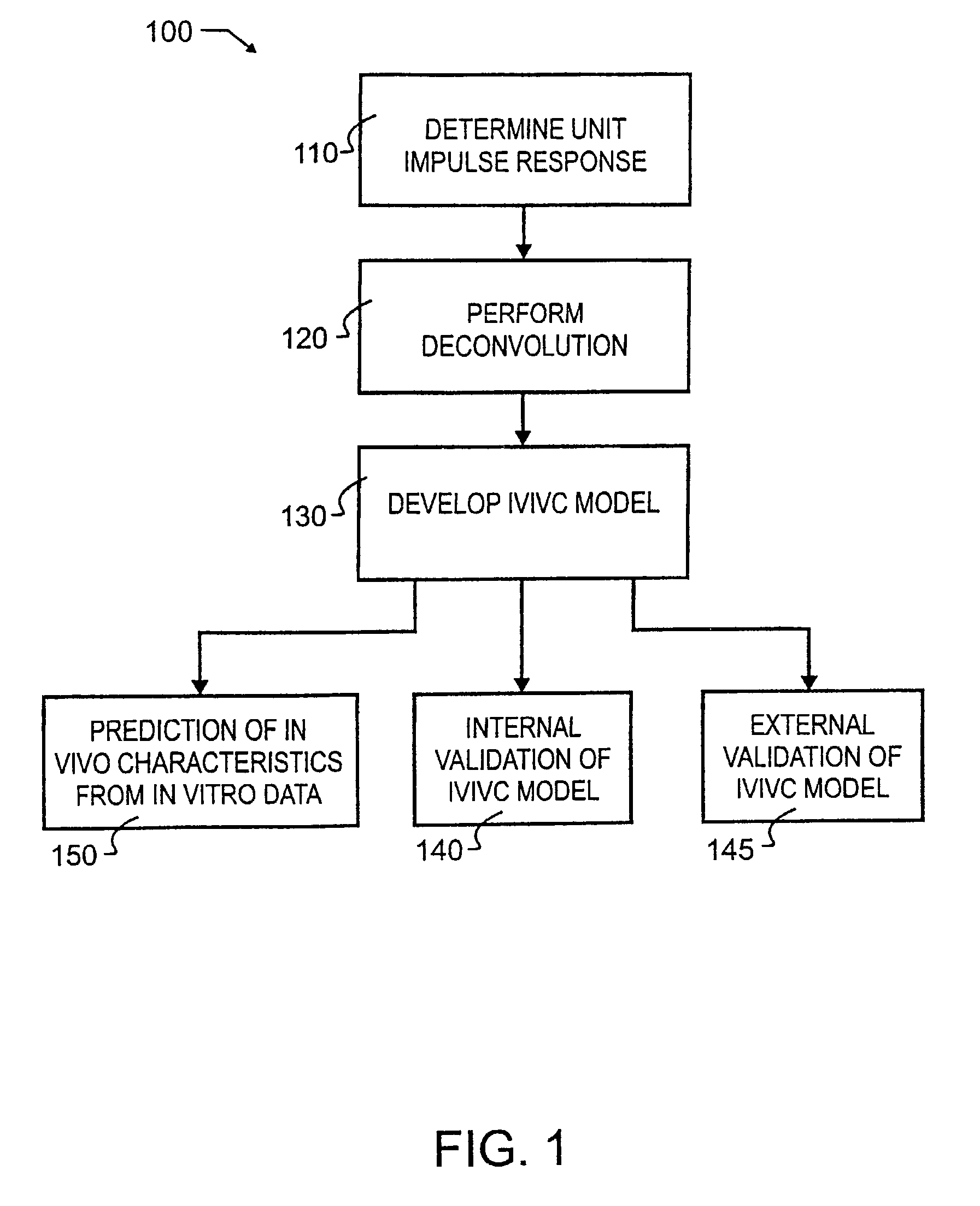
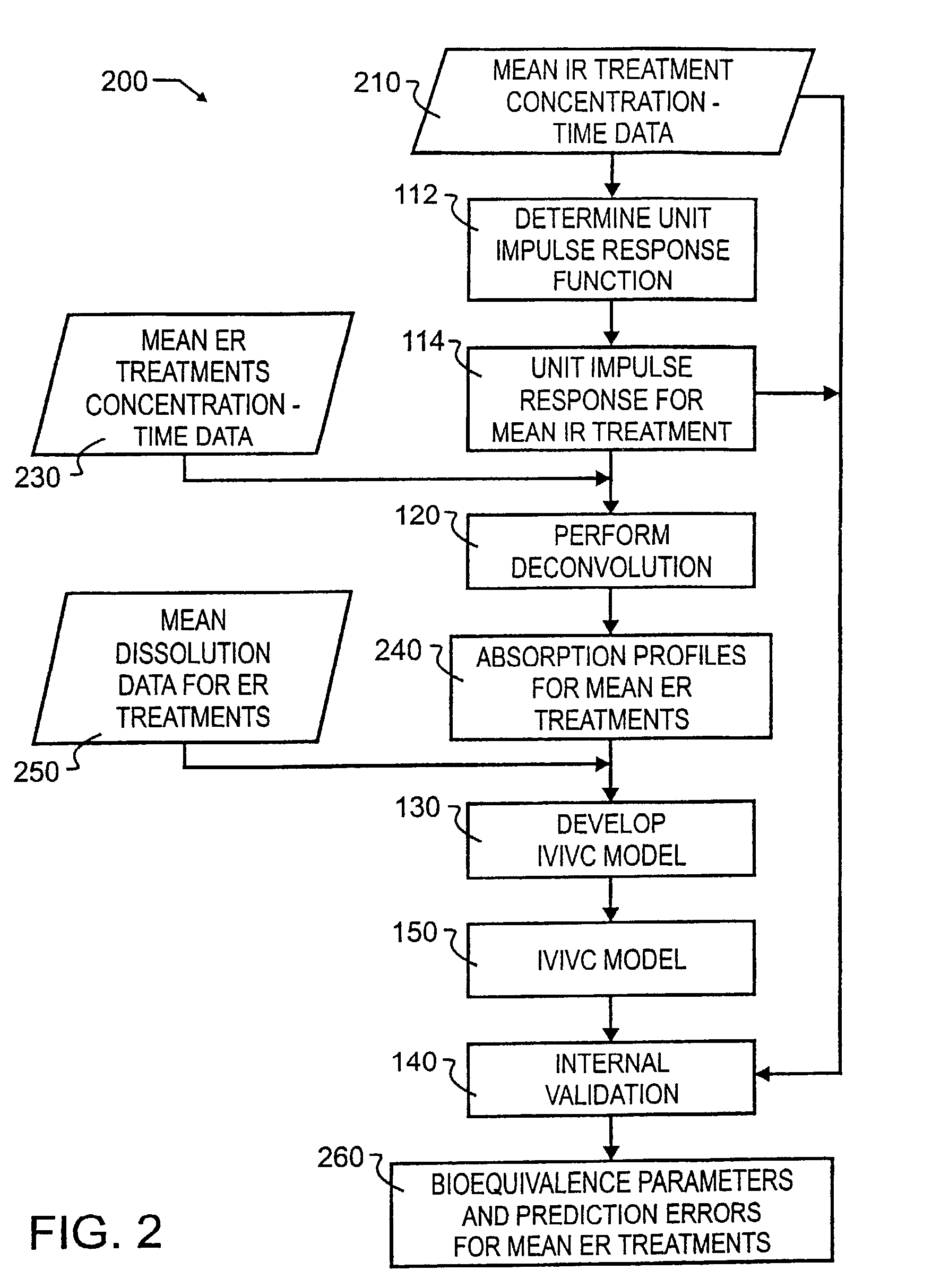
Tool for in vitro-in vivo correlation
InactiveUS7027970B2Accurately reflect observed phenomenonLaborious and time-consumingAnalogue computers for chemical processesBiostatisticsImmediate releaseComputer aid
A biological modeling system and method for enhanced computer-aided analysis of biological response data provides information synthesized from immediate and extended release in vivo data and in vitro data. An executable model of a biological system is developed from information and structures based on the data. In a preferred embodiment, a two stage approach to modeling is used in the development of an IVIVC. The first stage of the procedure is deconvolution, where the percentage of drug absorbed is determined. In the second stage, the in vivo percentage absorbed data is correlated to the in vitro fraction or percentage dissolved data. This correlation then represents a point-to-point relationship between the in vitro dissolution and the in vivo input rate of the drug from the dosage form. In such a correlation, the in vitro dissolution and in vivo absorption profiles are either directly superimposable or may be made to be superimposable by the use of a scaling factor. Prior to the deconvolution stage, a unit impulse response function can be determined from immediate-release concentration-time data. This impulse response function is used in the deconvolution process to determine the in vivo percent absorbed for the extended release formulations. A nonlinear IVIVC model is developed that can incorporate time-scaling and time-shifting into the model if needed. After the two-stage modeling is completed, the predictability of the developed IVIVC model is evaluated by both internal and external validation.
Owner:GLOBOMAX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com