Patents
Literature
33 results about "Associated substance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Manufacturing process, such as three dimensional printing, including binding of water-soluble material followed by softening and flowing and forming films of organic-solvent-soluble material
InactiveUS20070009606A1Effectively osteoinductiveHigh porosityAdditive manufacturing apparatusPhotosensitive materialsManufacturing technologyAdditive ingredient
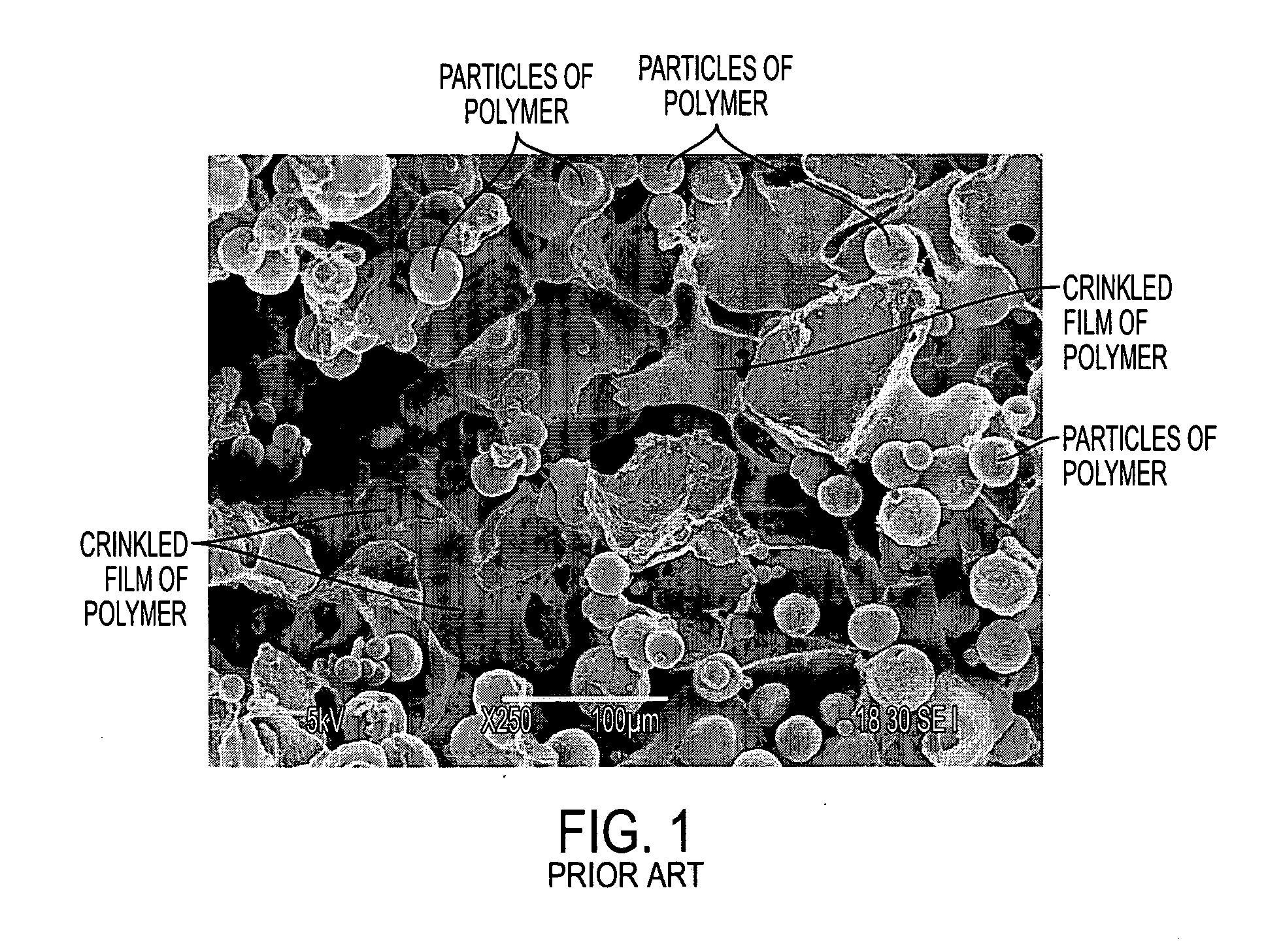
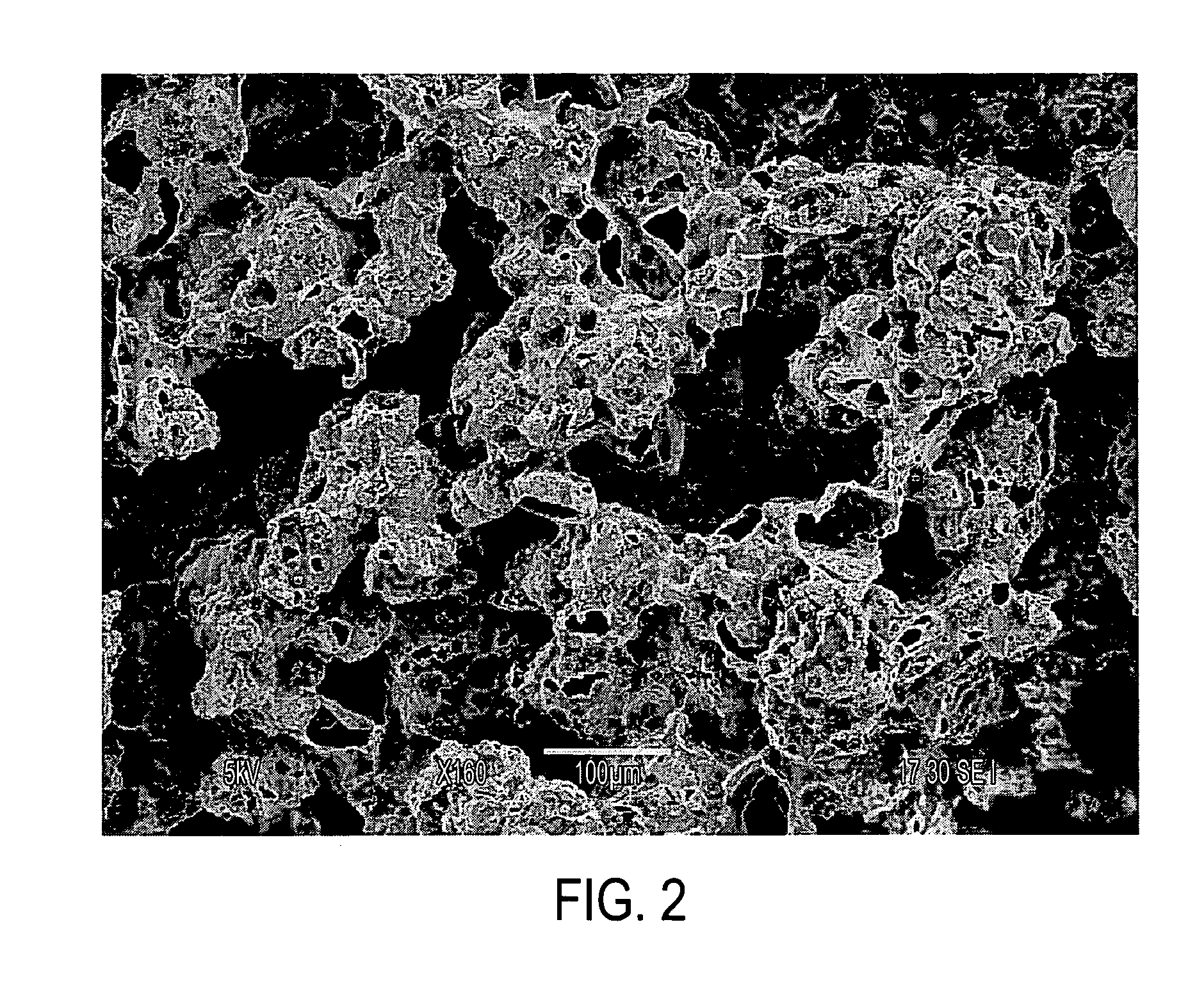
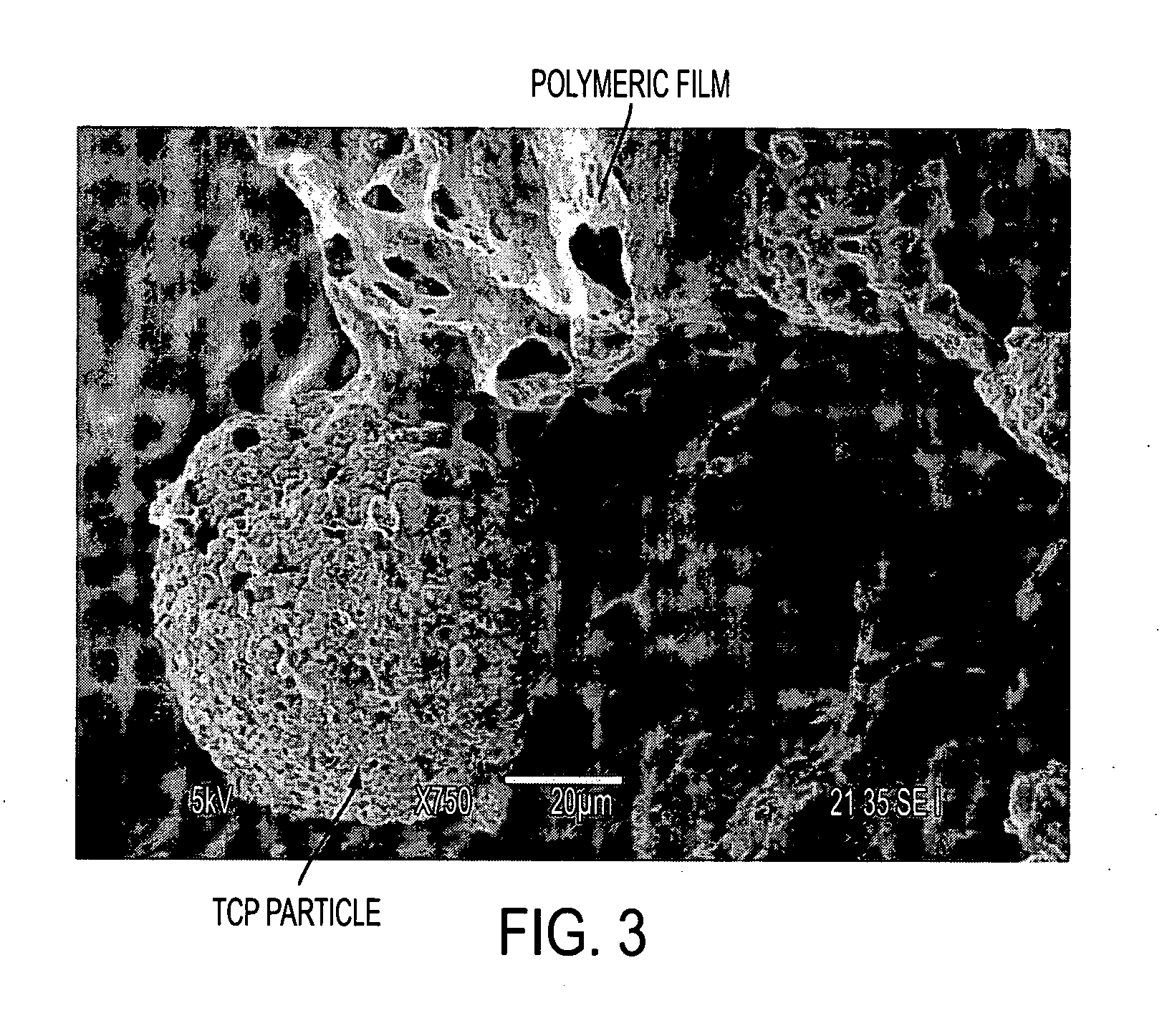
The invention includes biostructures which may be characterized as having substantially all of the organic-solvent-soluble material in the form of a network of irregularly shaped perforated films. The biostructure may further include particles of a substantially-insoluble material, which may be a member of the calcium phosphate family. The biostructure may be osteoconductive. The biostructure may further contain an Active Pharmaceutical Ingredient or other bioactive substance. The API may be a substance which stimulates the production of bone morphogenetic protein, such as Lovastatin or related substances, thereby making the biostructure effectively osteoinductive. One or more of the polymers may have a resorption rate in the human body such as to control the release of the API. Methods of manufacture are also disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Micro-array of organism-associated substance
InactiveUS20050063877A1Reducing noise lightEfficient productionBioreactor/fermenter combinationsMaterial nanotechnologyMedicineFluorescence
A microarray obtainable by slicing a block comprising a plurality of linear bodies or through-holes which carry an organism-related substance, in a direction intersecting the longitudinal direction of the linear bodies or through-holes, wherein the linear body and / or block comprise a substance that reduces the self-fluorescence thereof.
Owner:MITSUBISHI CHEM CORP
Method and kit for detecting various related substance of CAH at same time
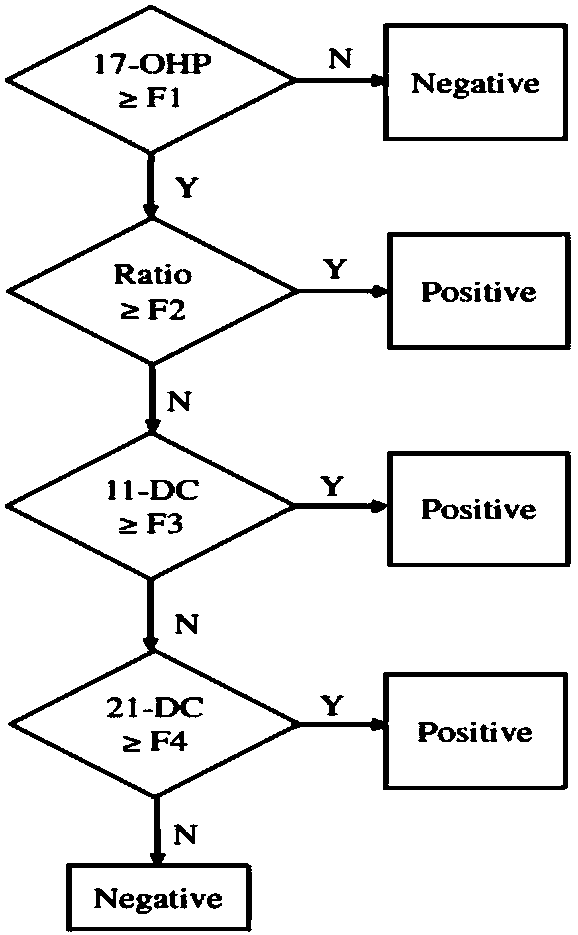
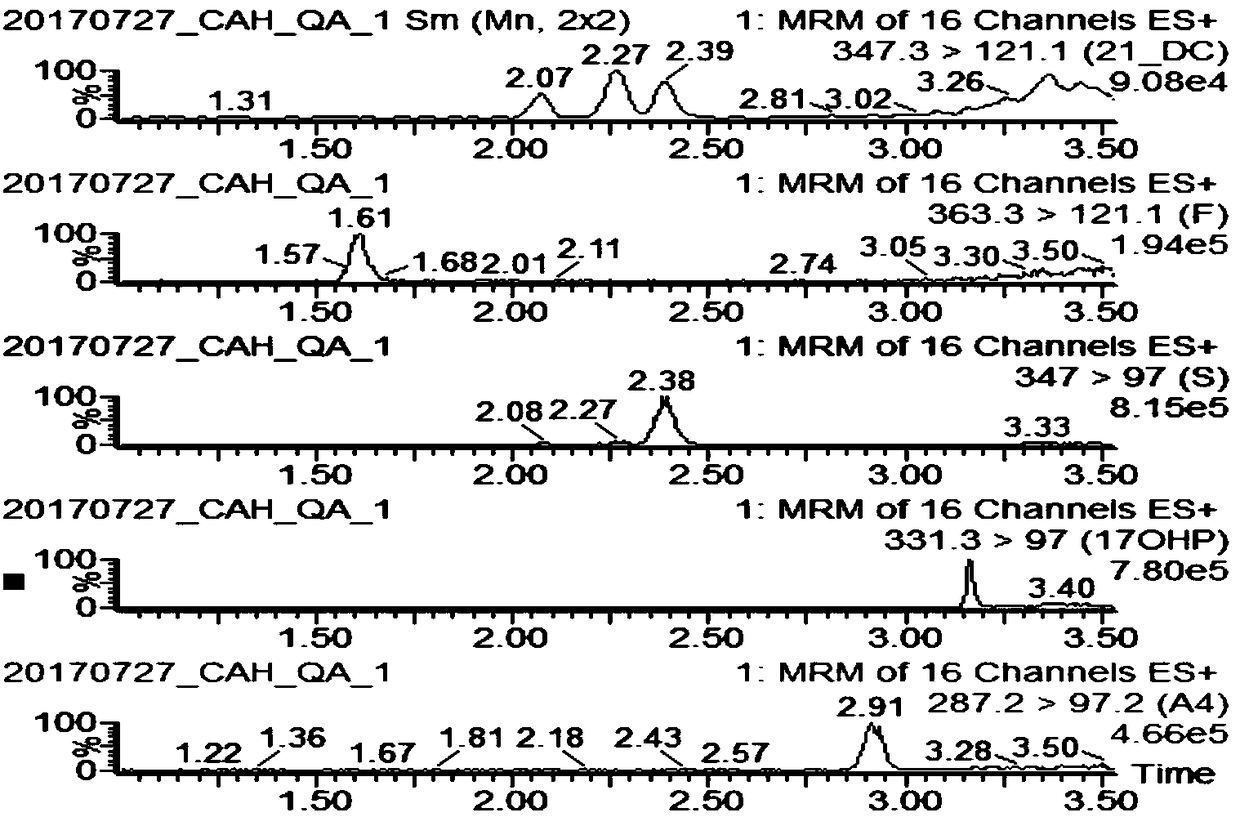
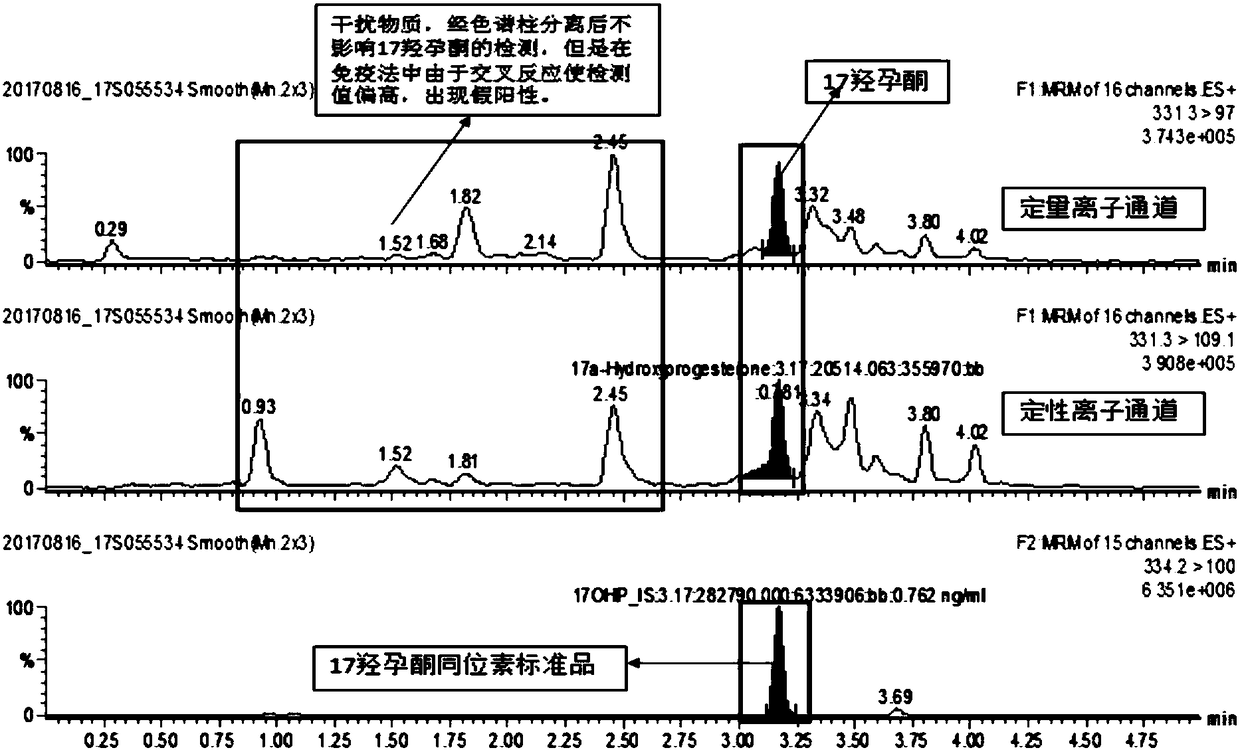
InactiveCN108088934AStrong specificityHigh sensitivityComponent separation11-DesoxycortisolChemistry
The invention discloses a method and a kit for detecting various related substance of CAH at the same time. The method is used for detecting 17 alpha progesterone, androstenedione, 11-deoxycortisol, 21-deoxycortisol and cortisol at the same time. The method includes: (1), adding an organic solvent into a mixed internal standard product to prepare a stock solution, and diluting to obtain extractionworking liquid; (2), using the extraction working liquid to incubate a to-be-detected blood spot sample; (3), centrifuging, taking supernate, concentrating, and using 45% methanol for re-dissolving;(4), using LC-MS / MS to detect a re-dissolving product, wherein the mixed internal standard product includes isotope internal standard products of 17 alpha progesterone, androstenedione, 11-deoxycortisol, 21-deoxycortisol and cortisol. The method can quantify five hormones in one time, can provide specific, sensitive, low-false positive and high-positive-prediction-value CAH screening and can screen CAH of 11-OHD and 21-OHD at the same time, thereby improving screening efficiency and quality.
Owner:SHENZHEN HUADA GENE INST
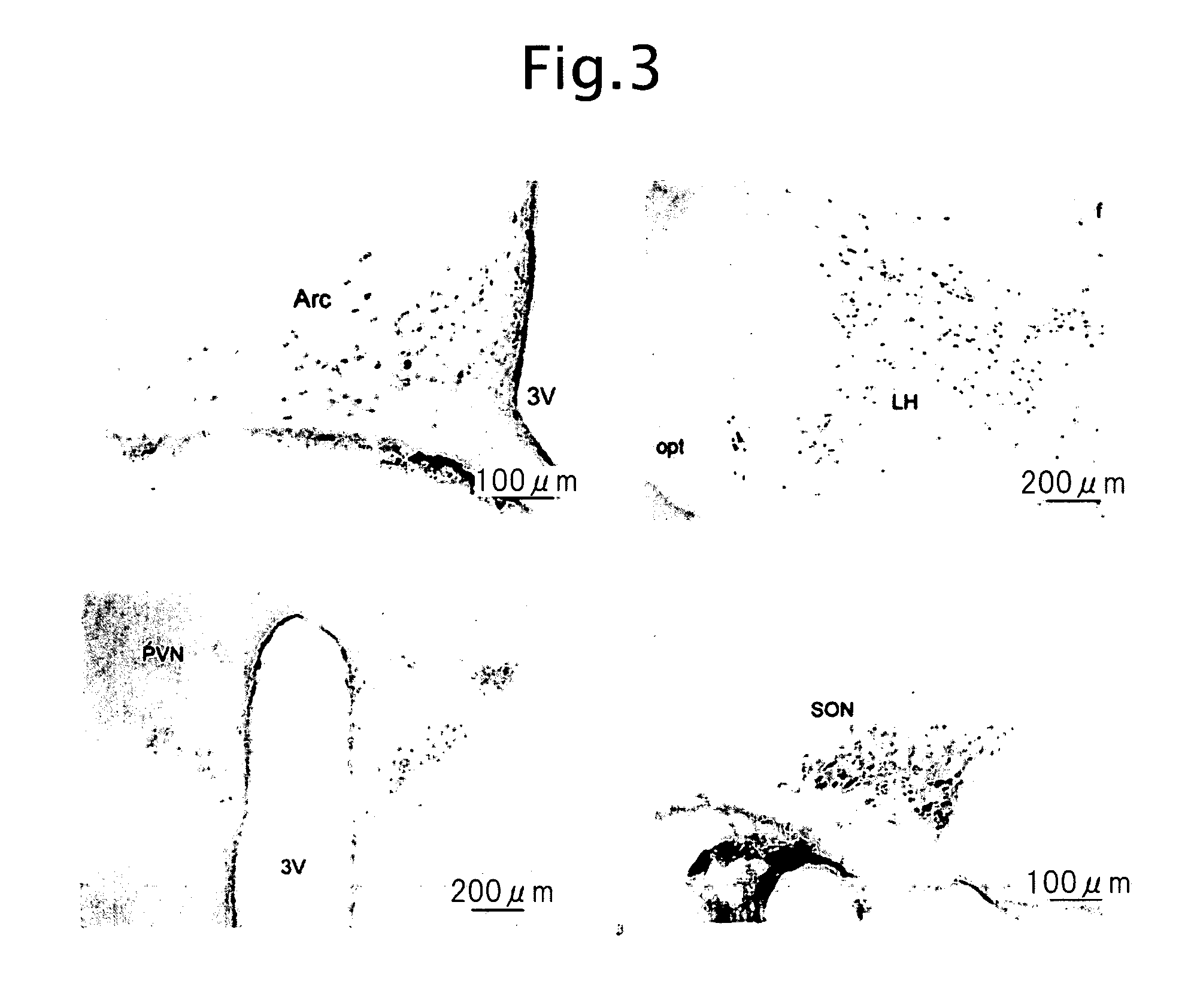
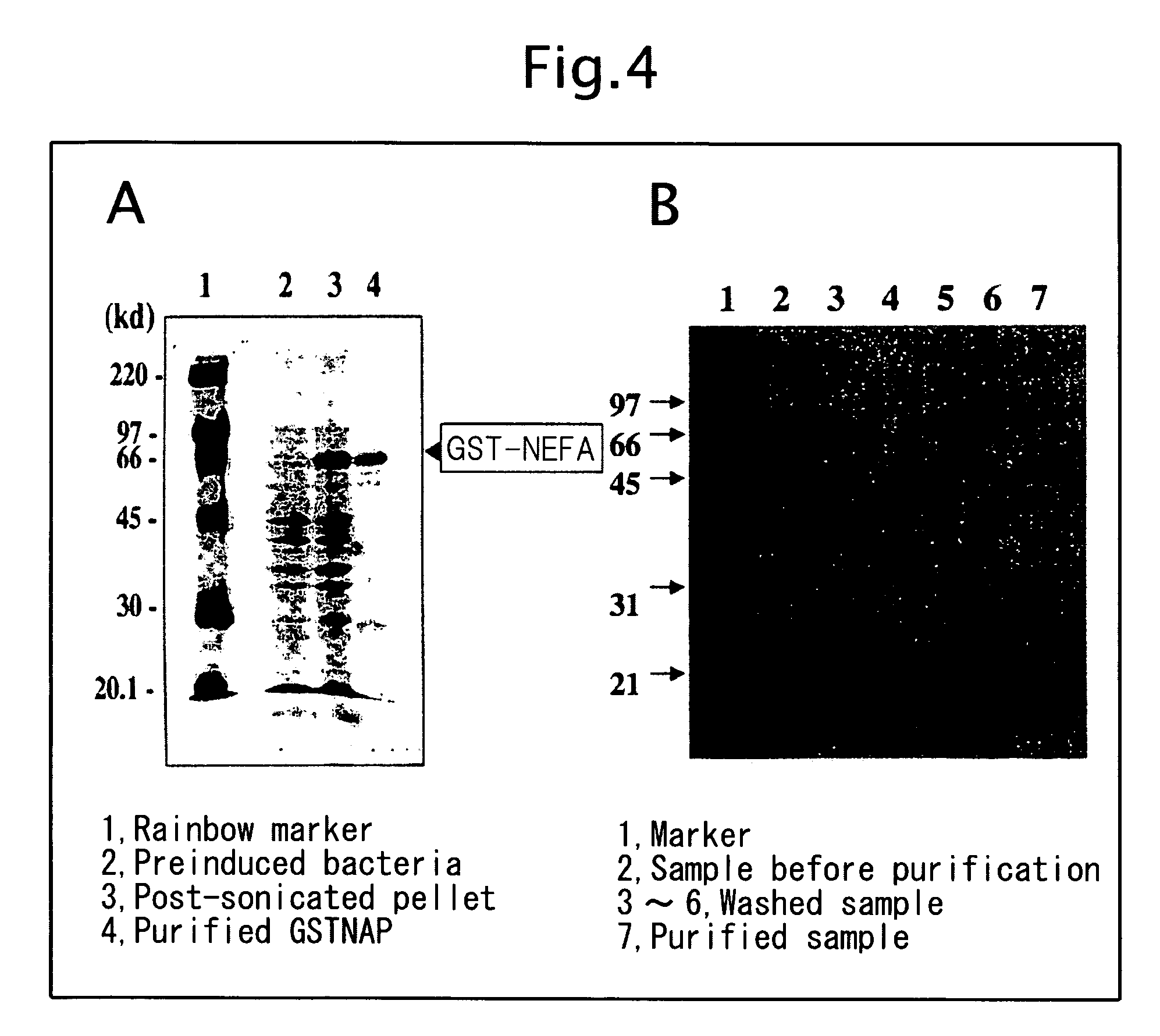
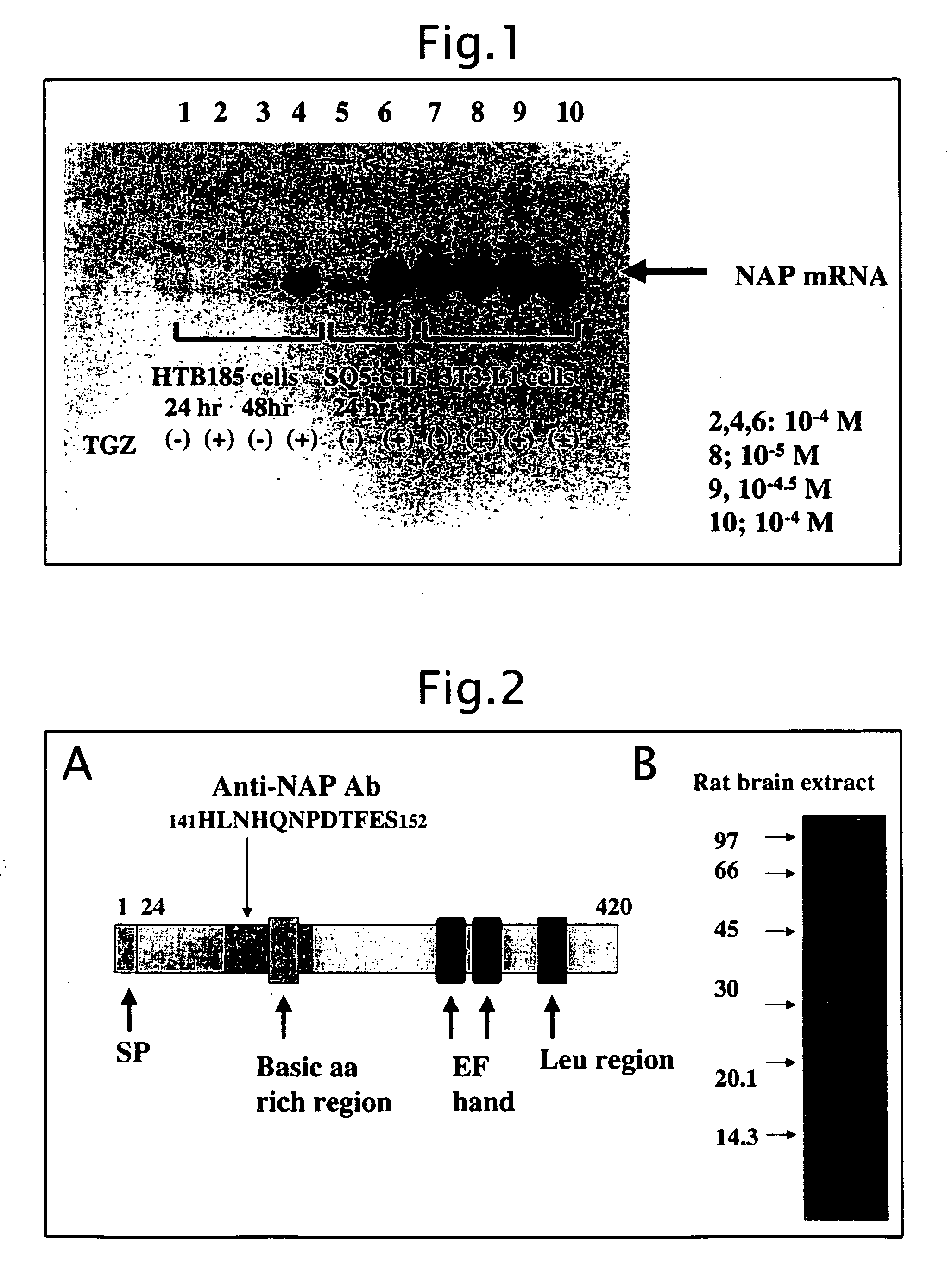
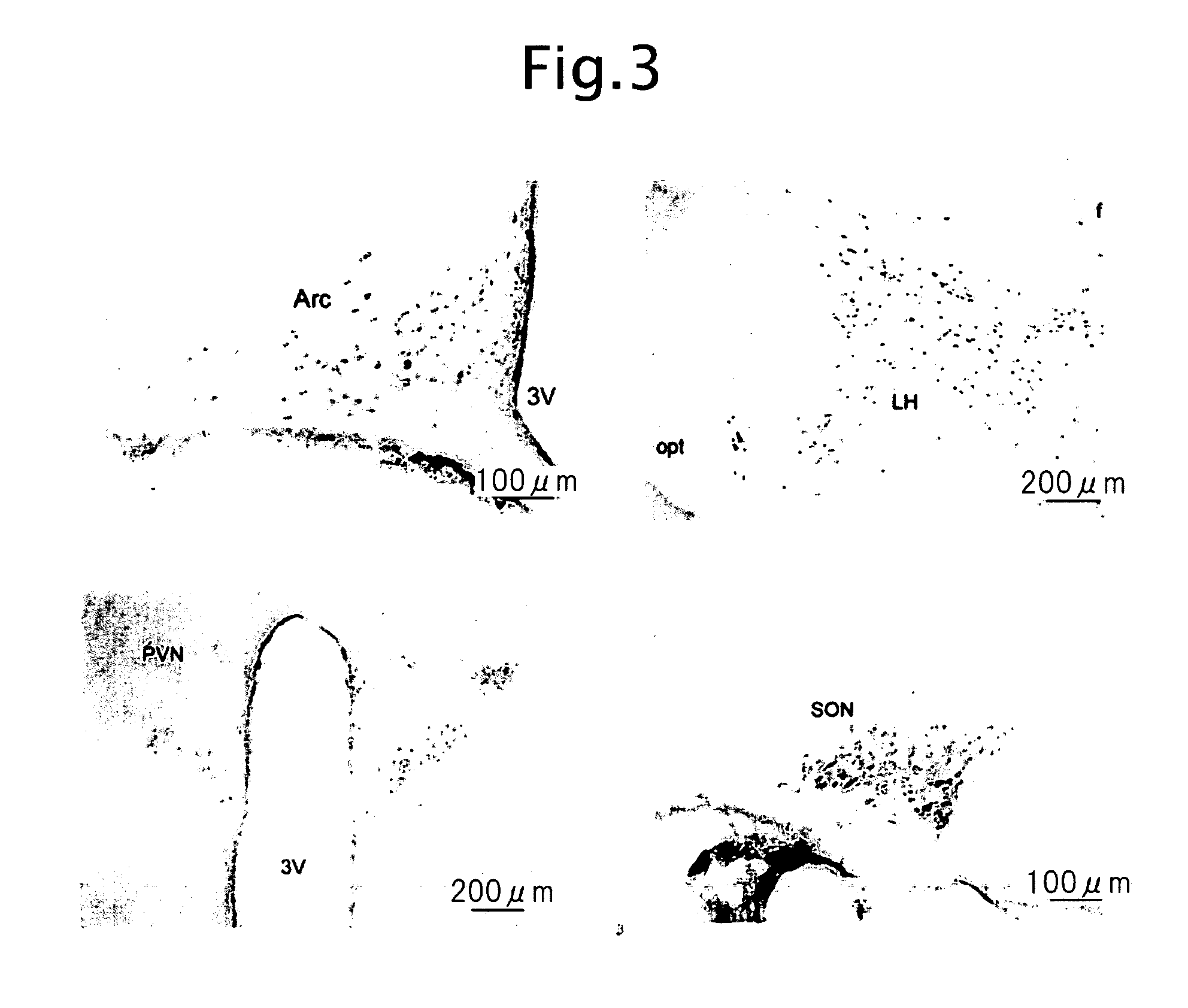
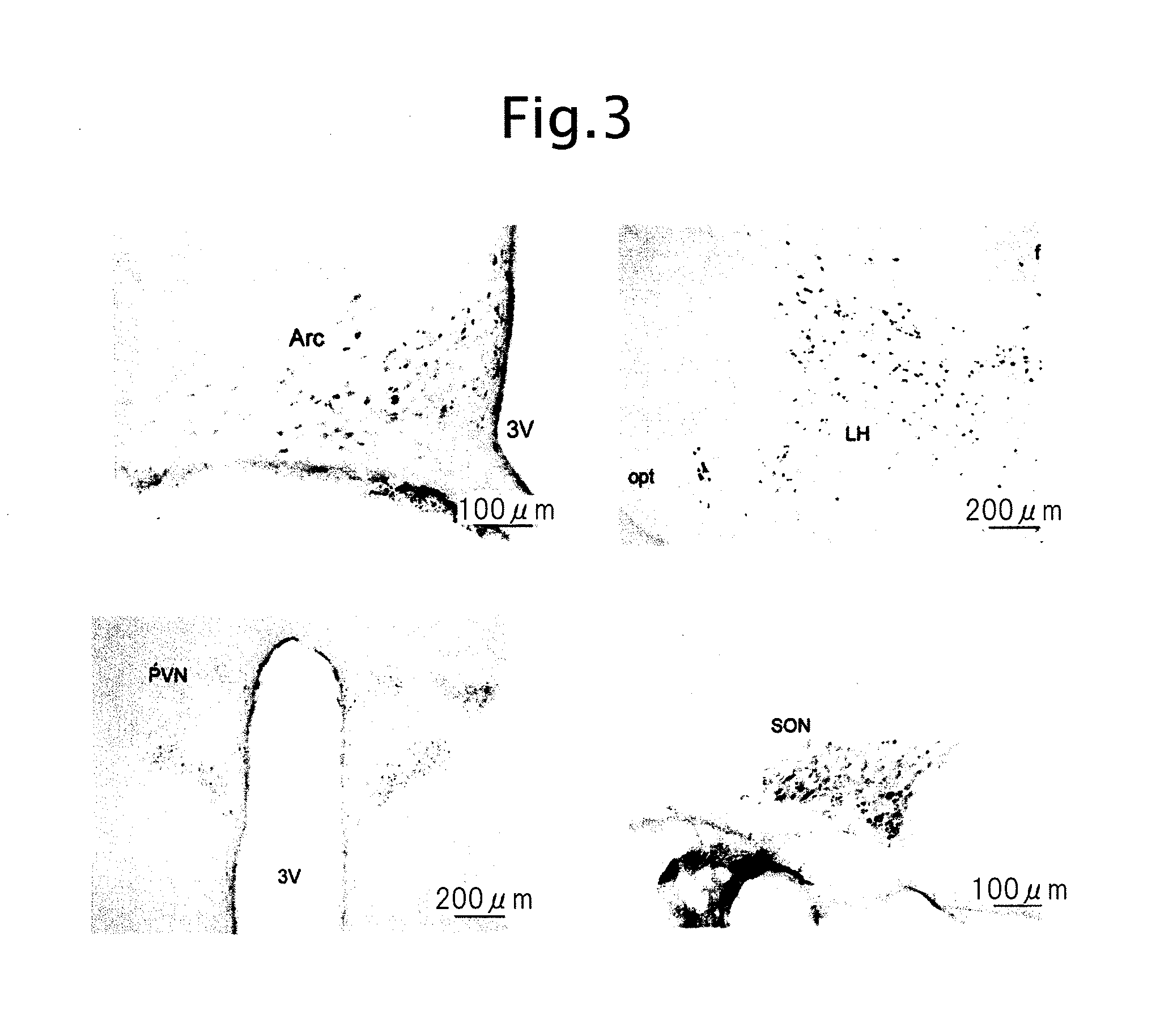
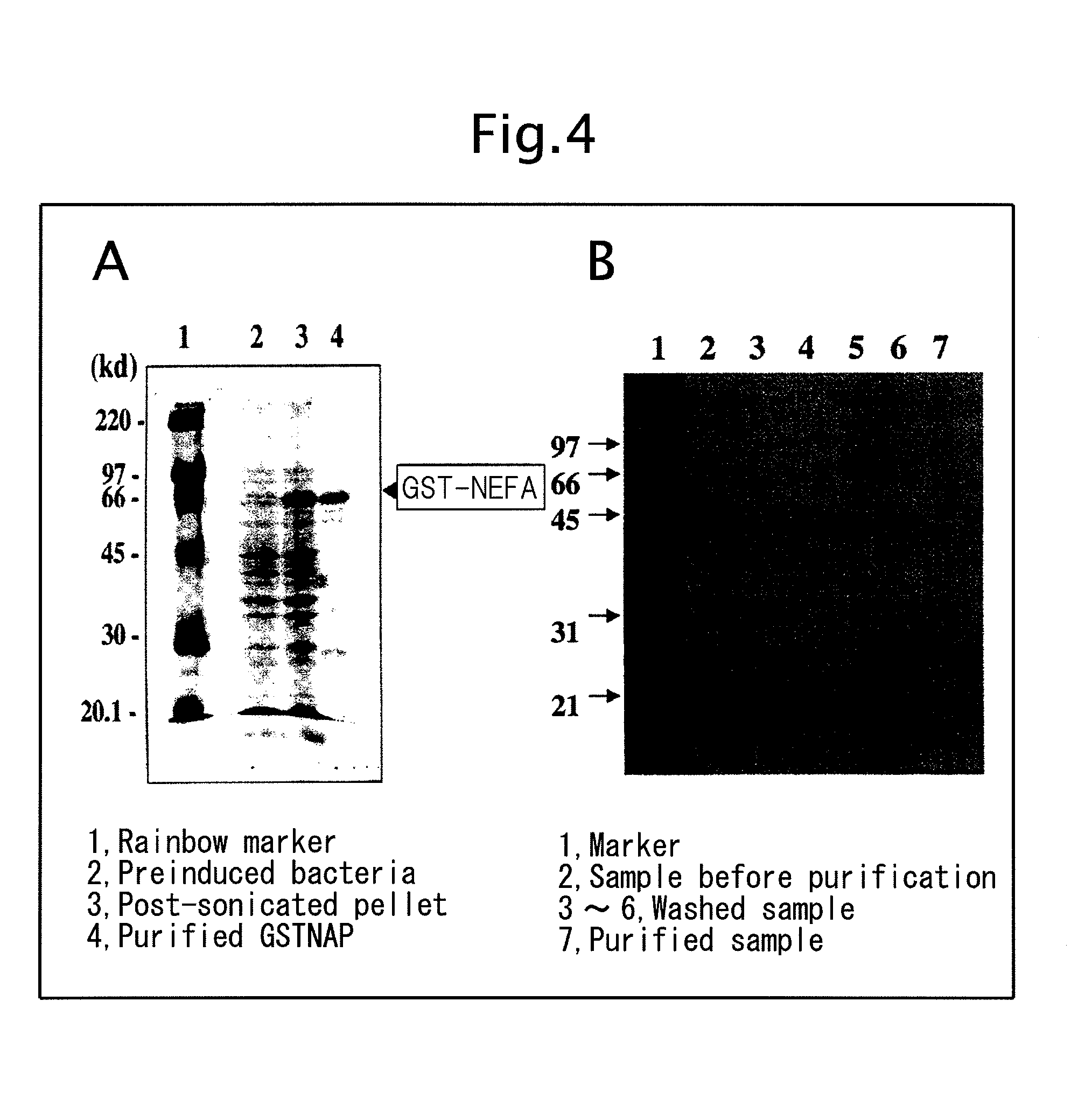
Biological substance nesfatin and its related substances and uses thereof
InactiveUS7795390B2Enhancing food intakeWeight increaseBacteriaPeptide/protein ingredientsAssociated substanceAppetite regulation
The present invention relates to a novel method of obtaining a factor involved in appetite control and / or body weight control, as well as genes obtained by said method, polypeptides encoded by said genes, or novel polypeptides obtained from the information on polypeptides encoded by said genes as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control. Also the present invention relates to substances that inhibit the effects of said genes or said polypeptides as a means for treating, controlling or diagnosing diseases associated with appetite control and / or body weight control. By using thiazolidine diones having a PPAR γ agonist activity, genes and polypeptides involved in appetite regulation and / or body weight reduction can be obtained. NESFATIN or the like obtained by said method can be used as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control.
Owner:TEIJIN LTD +1
Novel biological substance nesfatin and its related substances and uses thereof
InactiveUS20080115231A1Enhancing food intakeWeight increaseBacteriaPeptide/protein ingredientsAssociated substanceAppetite regulation
The present invention relates to a novel method of obtaining a factor involved in appetite control and / or body weight control, as well as genes obtained by said method, polypeptides encoded by said genes, or novel polypeptides obtained from the information on polypeptides encoded by said genes as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control. Also the present invention relates to substances that inhibit the effects of said genes or said polypeptides as a means for treating, controlling or diagnosing diseases associated with appetite control and / or body weight control. By using thiazolidine diones having a PPAR γ agonist activity, genes and polypeptides involved in appetite regulation and / or body weight reduction can be obtained. NESFATIN or the like obtained by said method can be used as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control.
Owner:TEIJIN LTD +1
Composition for cell proliferation
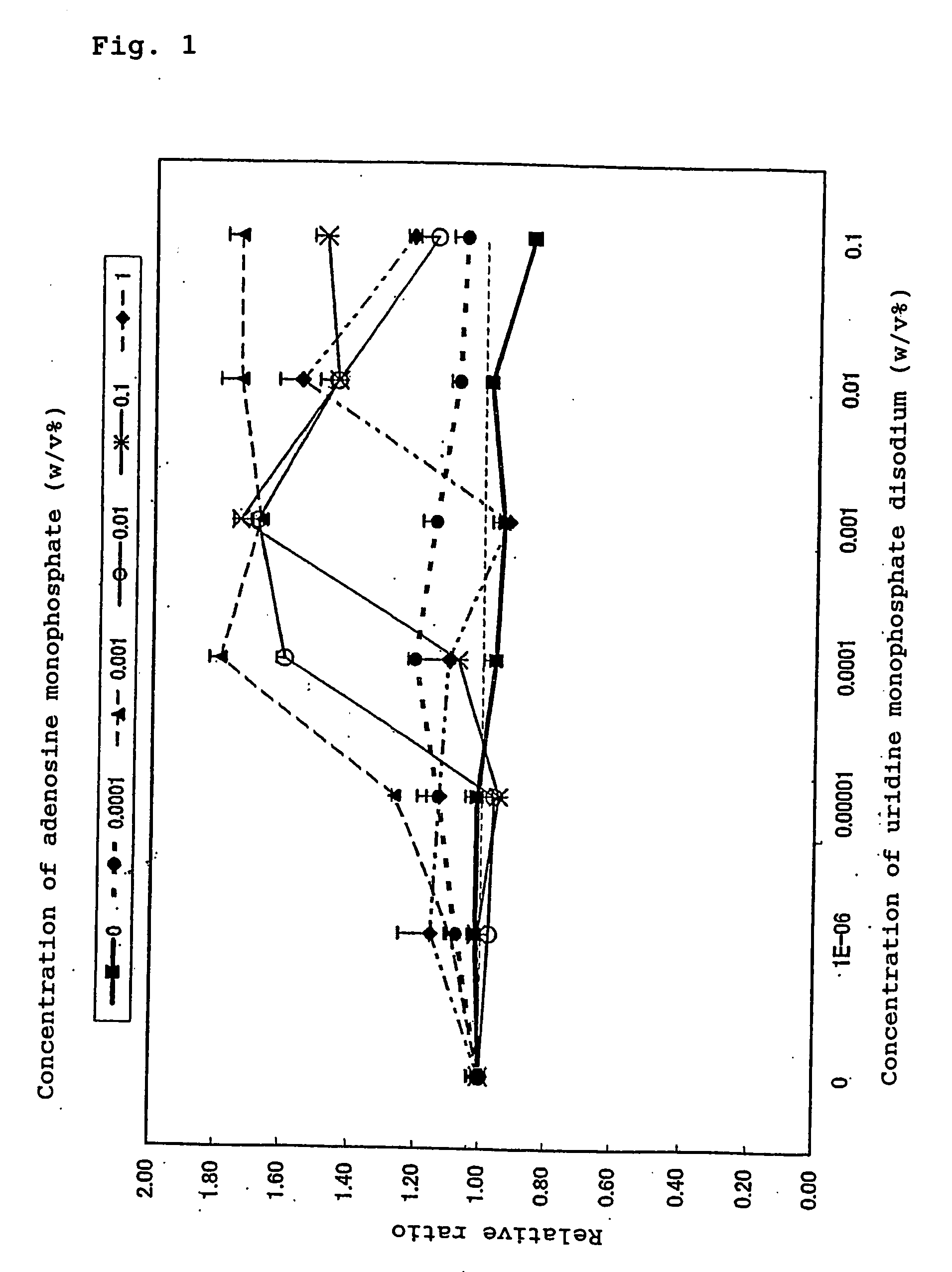
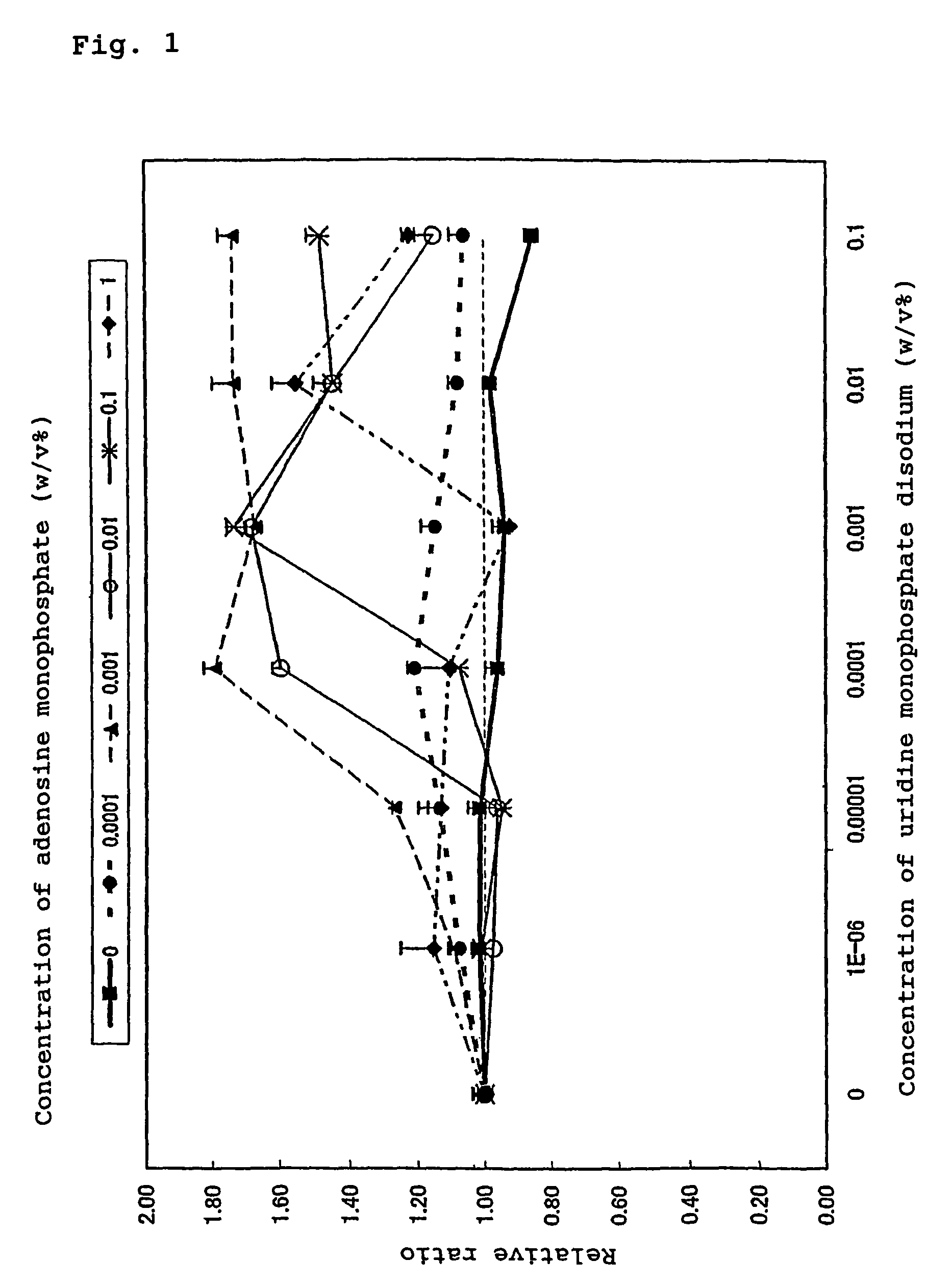
The present invention provides a method for effectively exerting a cell proliferation promoting effect of a purine nucleic acid-related substance. The present invention provides a composition for cell proliferation containing a purine nucleic acid-related substance and a pyrimidine nucleic acid-related substance. Further, the present invention provides a method for potentiating the cell proliferation promoting effect of the purine nucleic acid-related substance by using the purine nucleic acid-related substance in combination with the pyrimidine nucleic acid-related substance. Still further, the present invention provides a method for promoting cell proliferation, where the method comprising applying purine nucleic acid-related substance in combination with the pyrimidine nucleic acid-related substance to the skin or mucosa.
Owner:OTSUKA PHARM CO LTD
Propionyl-L-carnitine synthesis technology and detection method of related substance and its content
InactiveCN1651402ALow costOperational securityOrganic compound preparationComponent separationPropanoic acidSolvent
A process for synthesizing the propionyl-L-carnitine features the acylation reaction between L-carnitine and propionyl chloride in propionic acid as solvent under the catalysis of p-toluenesulfonic acid. The method for measuring the contents of propionyl-L-carnitine and associated substances features that the efficient liquid-phase chromatography is used to measure the content of propionyl-L-carnitine and the thin-layer chromatography is used to detect associated substances.
Owner:SUZHOU LANXITE BIOTECH
Separation purification method and microfluidic circuit
InactiveUS20110003285A1Easy to separate and purifyPerformed accurately and rapidlyImmobilised enzymesBioreactor/fermenter combinationsParticulatesPurification methods
Owner:ROHM CO LTD
Mefatinib composition, related compound, and preparation method and application of Mefatinib composition
ActiveCN108853109ASmall toxicityQuality assuranceOrganic active ingredientsOrganic chemistry methodsDiseaseAssociated substance
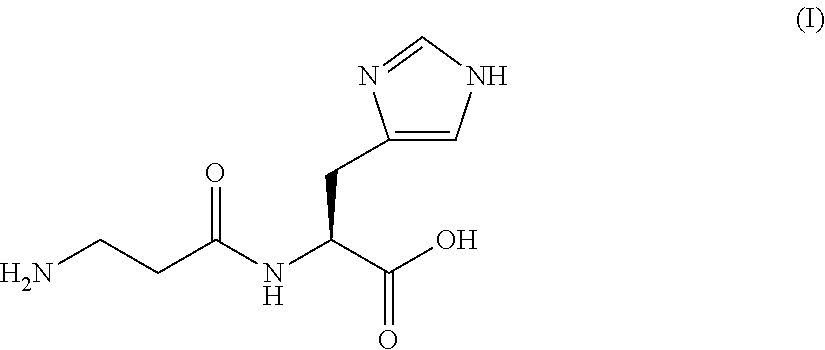
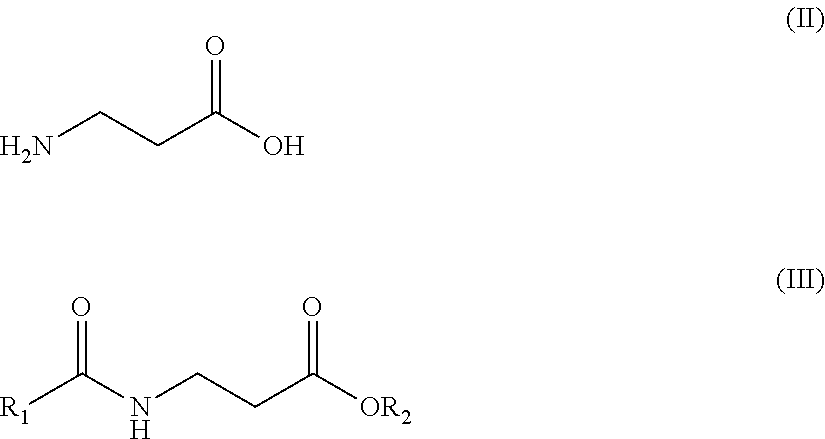
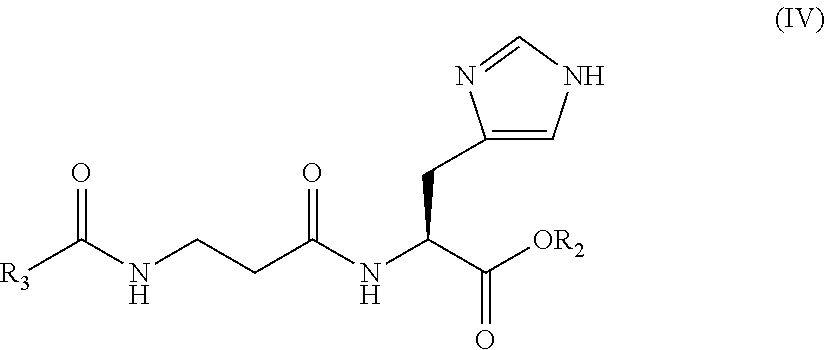
The invention discloses a Mefatinib composition, a related compound, and a preparation method and application of the Mefatinib composition. The Mefatinib composition comprises Mefatinib disclosed in aformula (I) and compound, wherein the normalized percentage content of the compound is 1.0% or lower, and the compound is disclosed by a formula (II) which is not zero. The Mefatinib composition canbe used for treating or preventing various adaptation diseases related to EGFR (Epidermal Growth Factor Receptor) and HER2 (Human Epidermal growth factor Receptor-2) kinase functions, and a toxic reaction occurrence rate is low. The compound disclosed by the formula (II) can be used as the standard substance of relevant substances in the Mefatinib, and is used for the quality control of Mefatinibraw material drugs or preparations. The formula (I) and the formula (II) are shown in the description.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
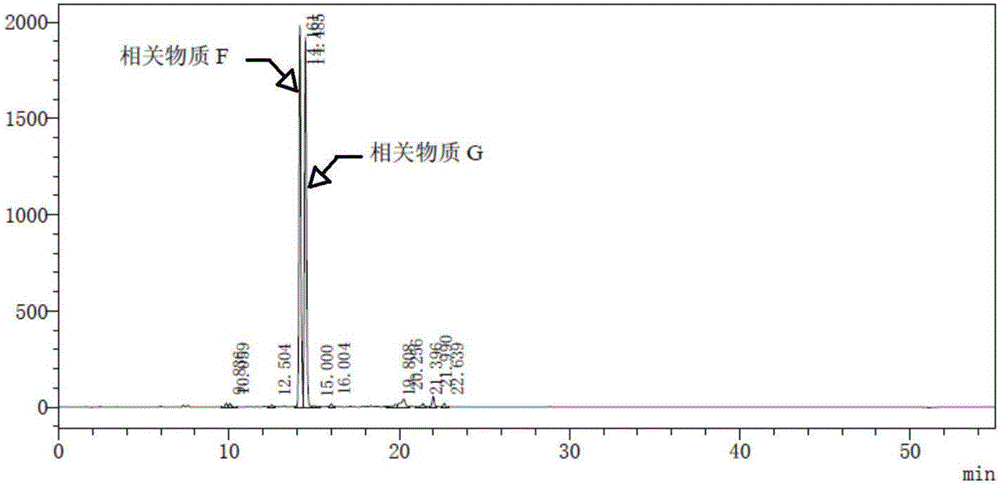
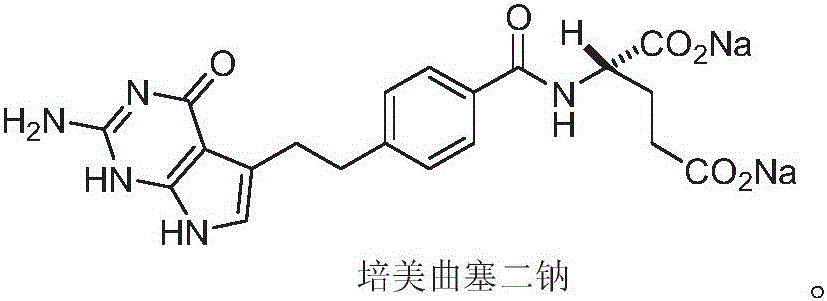
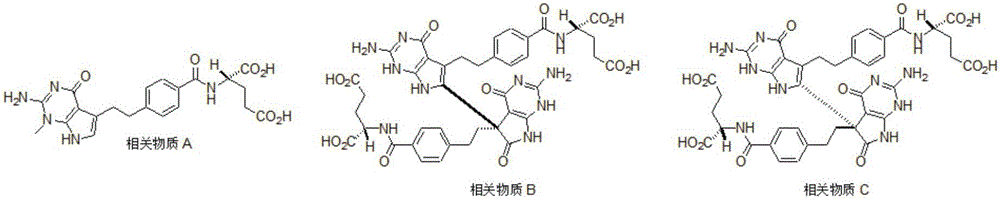
Related substances F and G of pemetrexed disodium as well as preparation and detection method thereof
ActiveCN106220634AImprove qualityImprove securityOrganic chemistryComponent separationDrug productPemetrexed disodium
The invention discloses related substances F and G of pemetrexed disodium as well as preparation and detection researches on the two substances. Remarkable economic and technical benefits can be made for improvement of the quality and the safety of medicines of pemetrexed disodium. The chemical formulae of the two substances are as shown in the specification.
Owner:SUZHOU LIXIN PHARMA
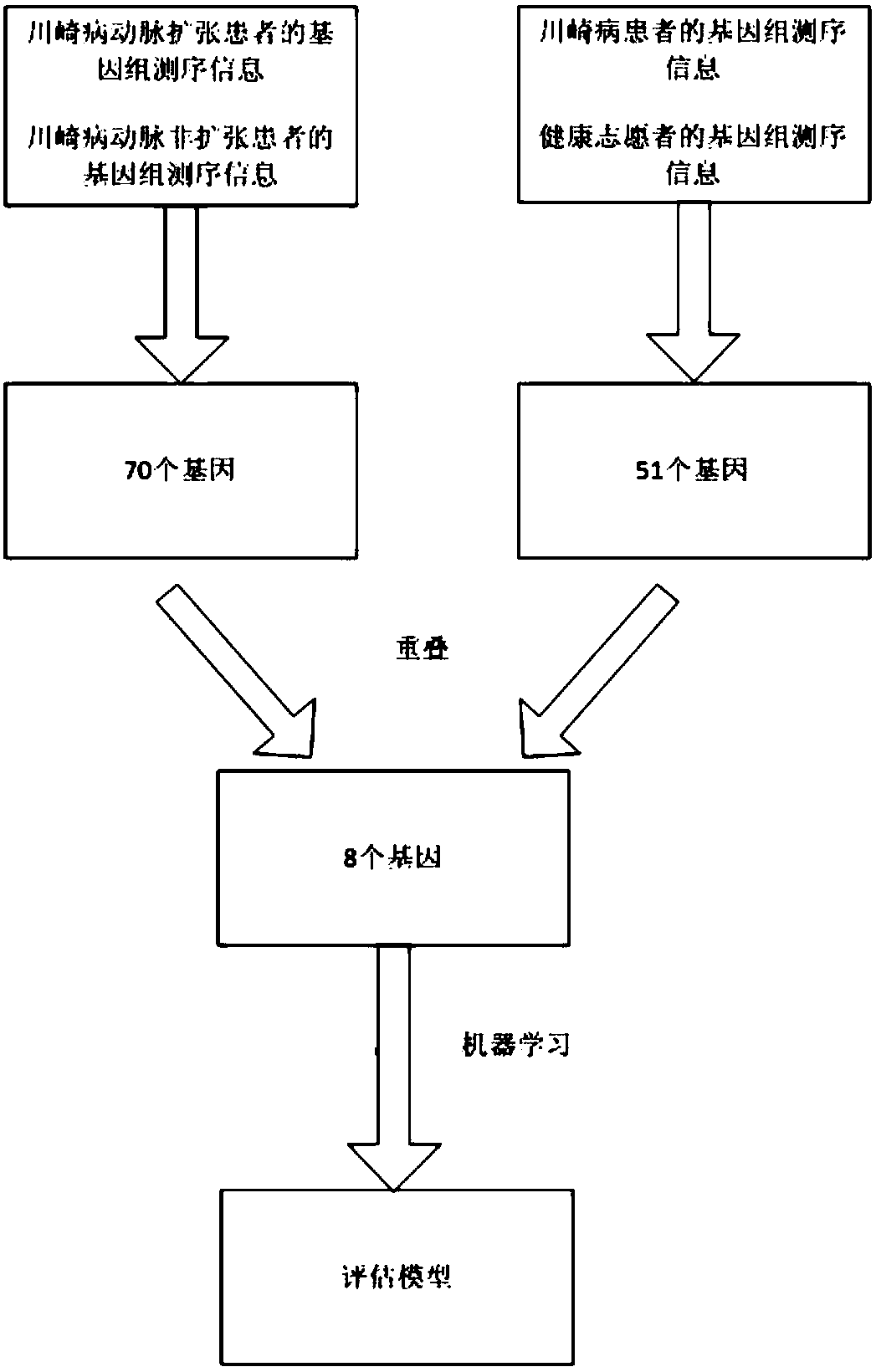
Kawasaki disease coronary artery disease risk diagnosis and detection kit
InactiveCN108034712AEarly treatmentEarly preventive measuresMicrobiological testing/measurementDisease diagnosisAssociated substanceKawasaki disease
The invention relates to the technical field of biology and particularly provides application of a Kawasaki disease-associated substance to preparation of a kit for Kawasaki disease coronary artery disease risk assessment as a biomarker. The Kawasaki disease-associated substance is selected from one or more of a Kawasaki disease-associated gene or fragments thereof, mRNA transcribed from the Kawasaki disease-associated gene or the fragments thereof and proteins encoded by the Kawasaki disease-associated gene or the fragments thereof. The Kawasaki disease-associated gene comprises one or eightof a CD247 gene, a TNFRSF1A gene, an SMAD2 gene, an MMP9 gene, a BMP2 gene, an ACVR2B gene, a CXCL14 gene and a CD14 gene. The Kawasaki disease coronary artery disease risk diagnosis and detection kitprovided by the invention can assess the Kawasaki disease coronary artery disease risk in Kawasaki disease patients and suspicious children, so that medical personnel can take appropriate treatment or preventive measures as soon as possible.
Owner:SHANGHAI CHILDRENS HOSPITAL +2
High performance liquid chromatography detection method for hydroxyiminobarbituric acid and related matters thereof
ActiveCN108414647AEasy to controlEfficient separationComponent separationCyanoacetic acidPhosphoric acid
The invention provides a method for determining contents of hydroxyiminobarbituric acid and related impurities of cyanoacetic acid, dimethylurea, dimethylacetamide and dimethyl 4AU(dihydrogen-6-imino-1,3-dimethylurea pyrimidine). A reverse high performance liquid chromatography peak area normalizing type quantitative analysis method is utilized, and adopts the chromatography conditions that a chromatography column is Phenyl C19 column; the length of the column is 150 to 250mm, the inner diameter of the column is 4.0 to 4.6mm, and the particle size of filler is 3.5 to 5mu m; the flow rate is 0.9 to 1.1ml / min; the detection wavelength is 205nm; the temperature of the column is 27 to 33 DEG C; the sample feeding amount is 20mul; the flowing phase comprises a 0.2% (V / V) triethylamine water solution A and acetonitrile B, the pH (potential of hydrogen) value of the 0.2% (V / V) triethylamine water solution A is adjusted to 2.8 to 3.2 by phosphoric acid, and the ratio of A to B is (96 to 98):(2to 4)(V / V). The method has the advantages that the product quality is conveniently controlled in the production and quality control process; the cost is low, the operation is simple, the implementingis easy, the accuracy and precision are high, and the stability and reproducibility are good.
Owner:XINHUA PHARM (SHOUGUANG) CO LTD
Method and apparatus for automatic calibration of spectrometers in chemometry by means of a bayes iterative estimation method
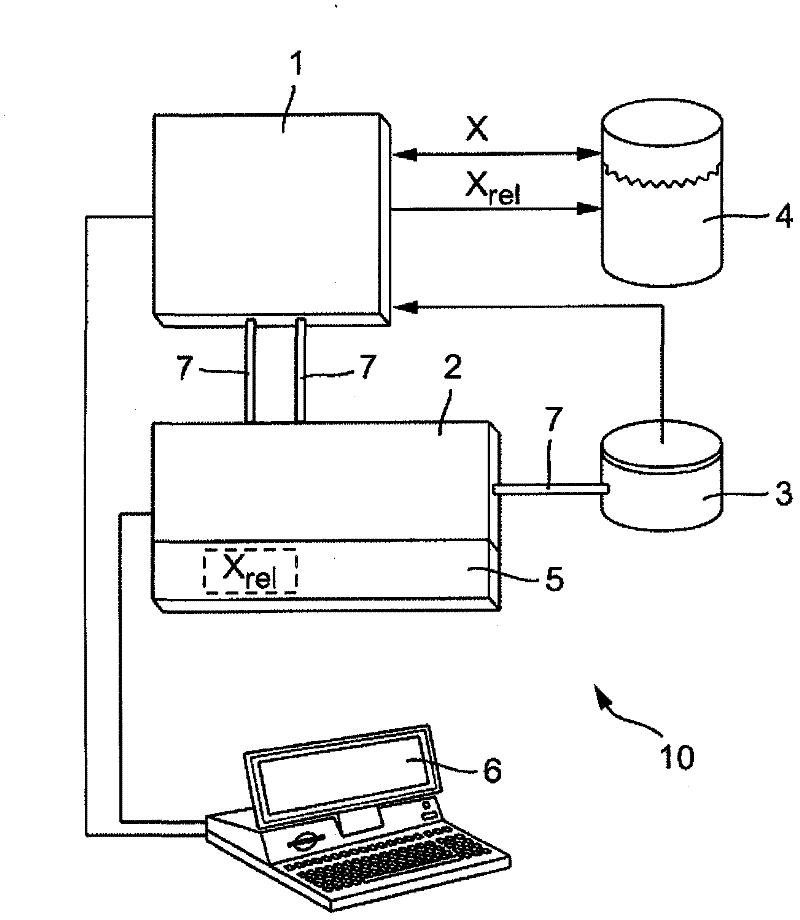
An apparatus and also a method for calibration of a spectrometer for the measurement of spectral fractions of a substance (4) as a part of chemometry or chemometric processes are proposed, with a spectrometer device (1) for measuring of spectral fractions of the substance (4) to be analysed, and also with a calibration unit (2) used to carry out a calibration of the spectrometer device and / or of the measured data on the basis of measured spectrometric data, characterized by the following steps: a) measuring the totality of spectral fractions X and / or of associated substance concentrations of the substance (4) under analysis; b) Saving the totality of measured spectral fractions X in a memory module (3) as spectrometric measured data in the form of a multi-dimensional coefficient vector; c) From the totality of measured spectral fractions X, automatic extraction of spectral fractions Xrel physically relevant to the particular measuring process by means of an automatically running, iterative estimation method saved in the calibration unit (2); d) Calibration of the measured spectrometric data for the analysed substance (4) on the basis of spectral fractions Xrel of the spectrometric data extracted in step c) and which are physically relevant to the substance (4) under analysis.
Owner:ENDRESS HAUSER CONDUCTA GESELLSCHAFT FUER MESS UND REGELTECHNIK MBH CO KG
Environmentally hazardous substance insurance system, computer program
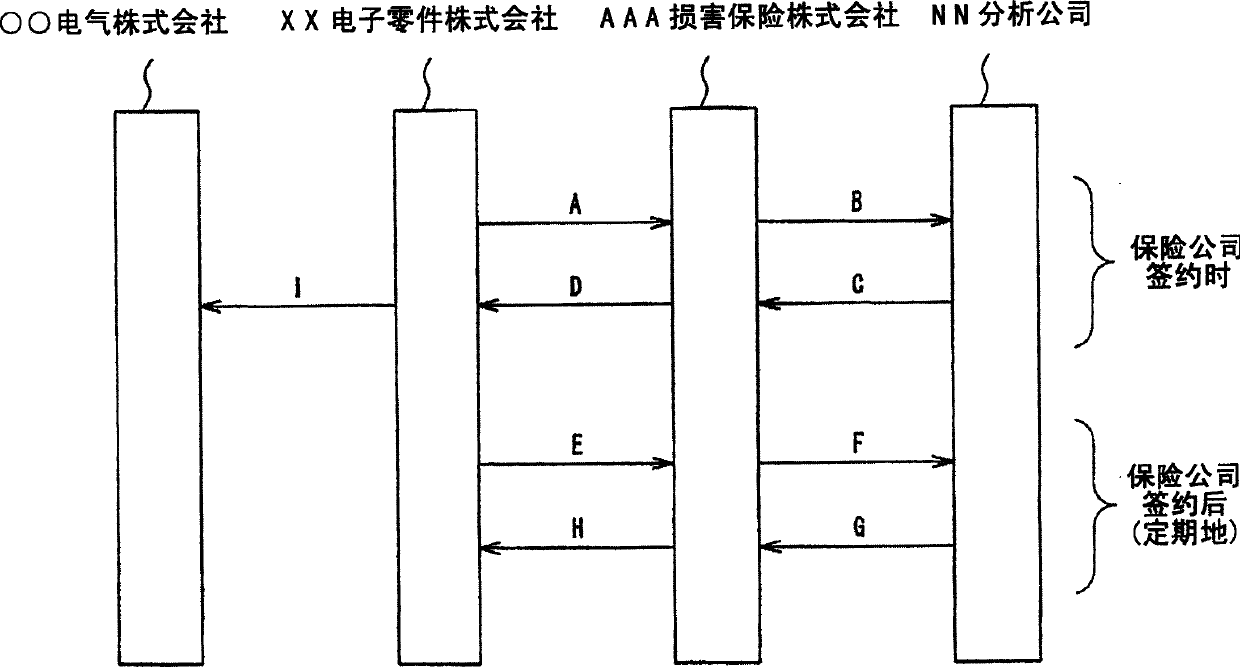
InactiveCN1637759AReduce the risk of compensation for damagesFinanceSpecial data processing applicationsAssociated substanceMedicine
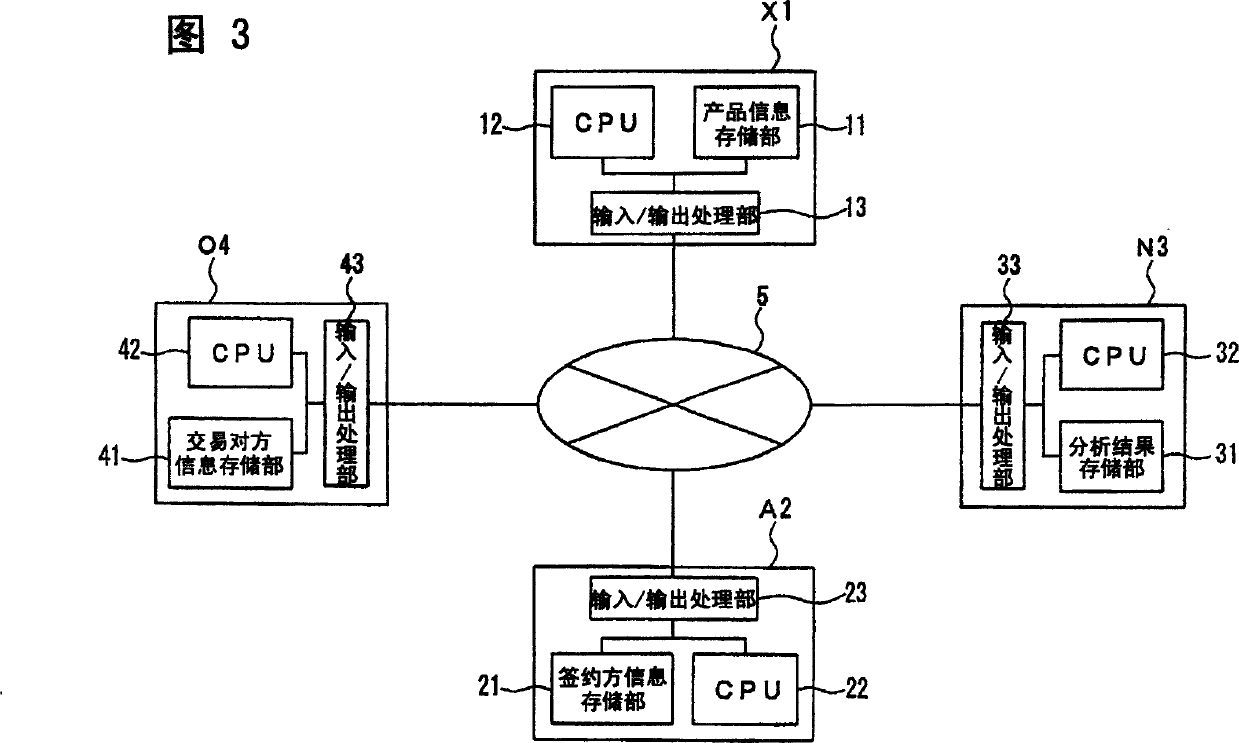
An environment-related substance insurance system and a computer program. Provides computer systems and computer programs for calculating appropriate insurance premiums to be paid by policyholders to insurers in order to realize environmental-related substance insurance that guarantees environmental-related substance-containing accidents. Provided is an environment-related substance insurance system that calculates insurance premiums for environment-related substance insurance that compensates for losses when products of an insured person contain environment-related substances due to accidents, characterized by having: an input / output processing unit (23) inputting at least information specifying environment-related substances as insurance targets and information on analysis results of products related to the contractor as contractor information related to the insured; 21); an insurance premium calculation unit (CPU22) that calculates an insurance premium based on the contracting party information stored in the contracting party information storage unit (21).
Owner:HITACHT MAXELL LTD
Enteric Coated Pharmaceutical Oral Formulations Comprising Acid-Labile Active Substances, and a Method Thereof
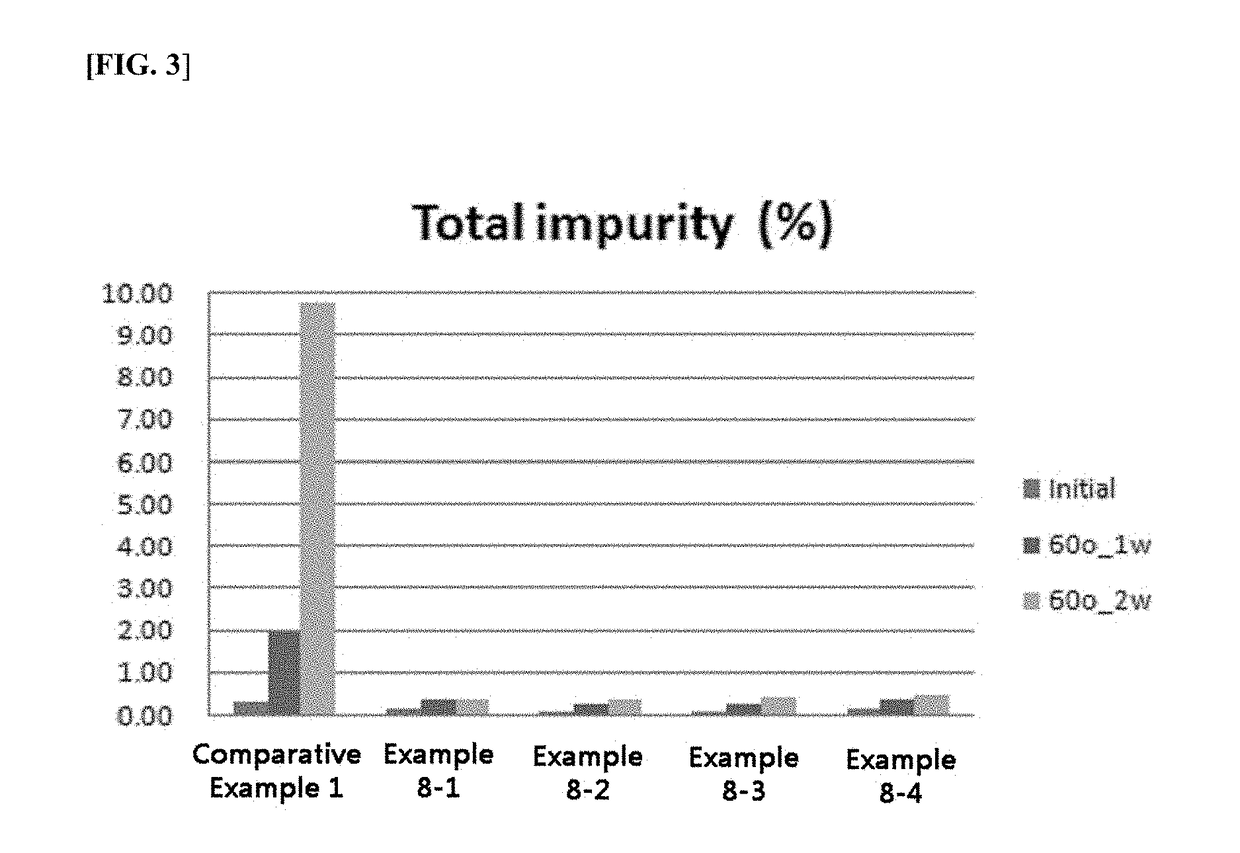
This invention relates to an oral pharmaceutical formulation formed by a direct coating of an enteric layer containing polyethylene glycol as a plasticizer on a core containing an acid-labile pantoprazole, and its manufacturing method. The enteric-coated oral pharmaceutical formulation of this invention, which combines directly a core containing an acid-labile pantoprazole and an enteric layer in the absence of an inert intermediate layer, is able to improve the storage stability of the acid-labile pantoprazole and maximize the bioavailability and oral absorption rates via preventing related substances from increasing.
Owner:GL PHARMTECH CO LTD
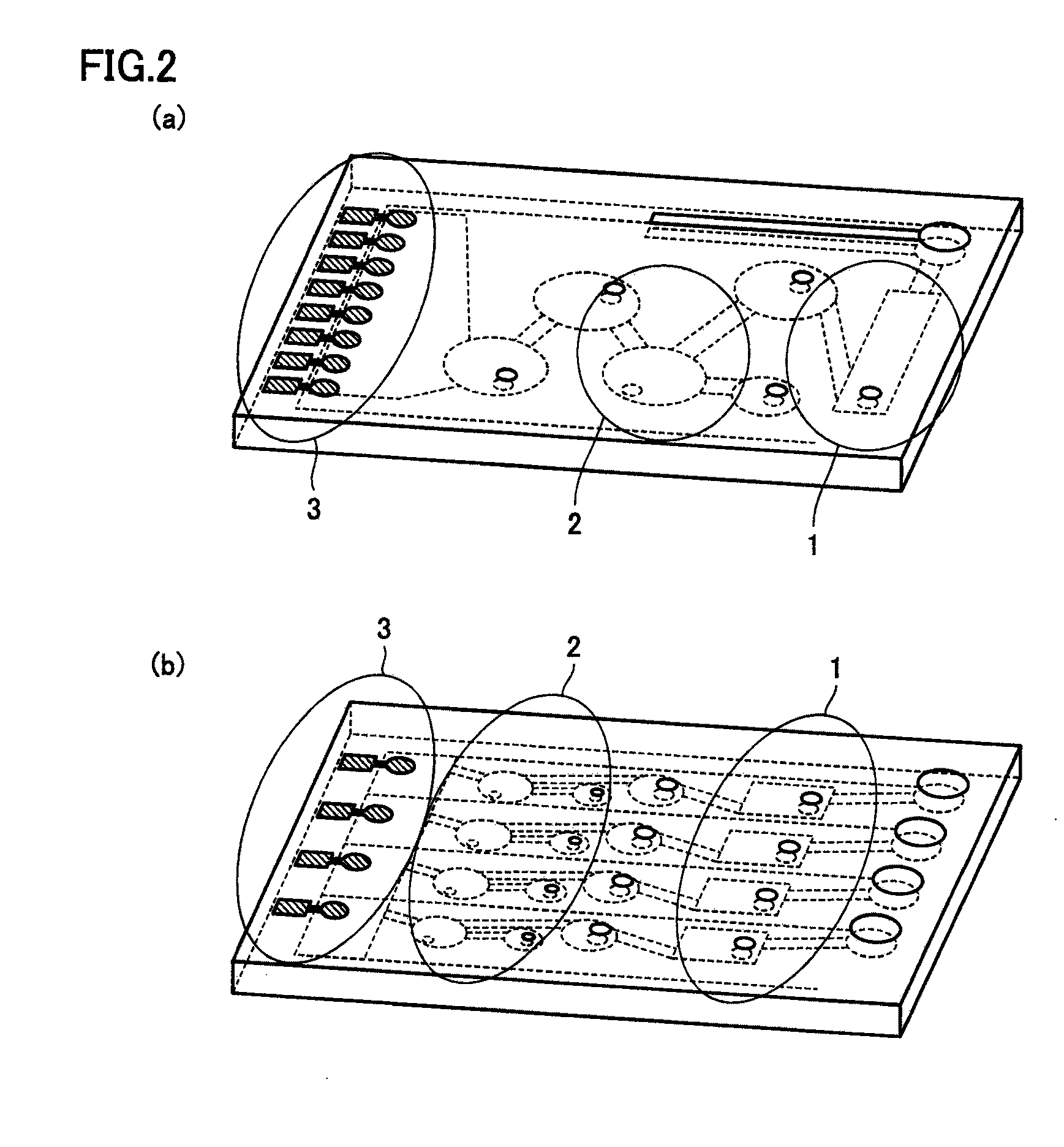
Device and method for manufacturing bead array, and method for detecting target substance
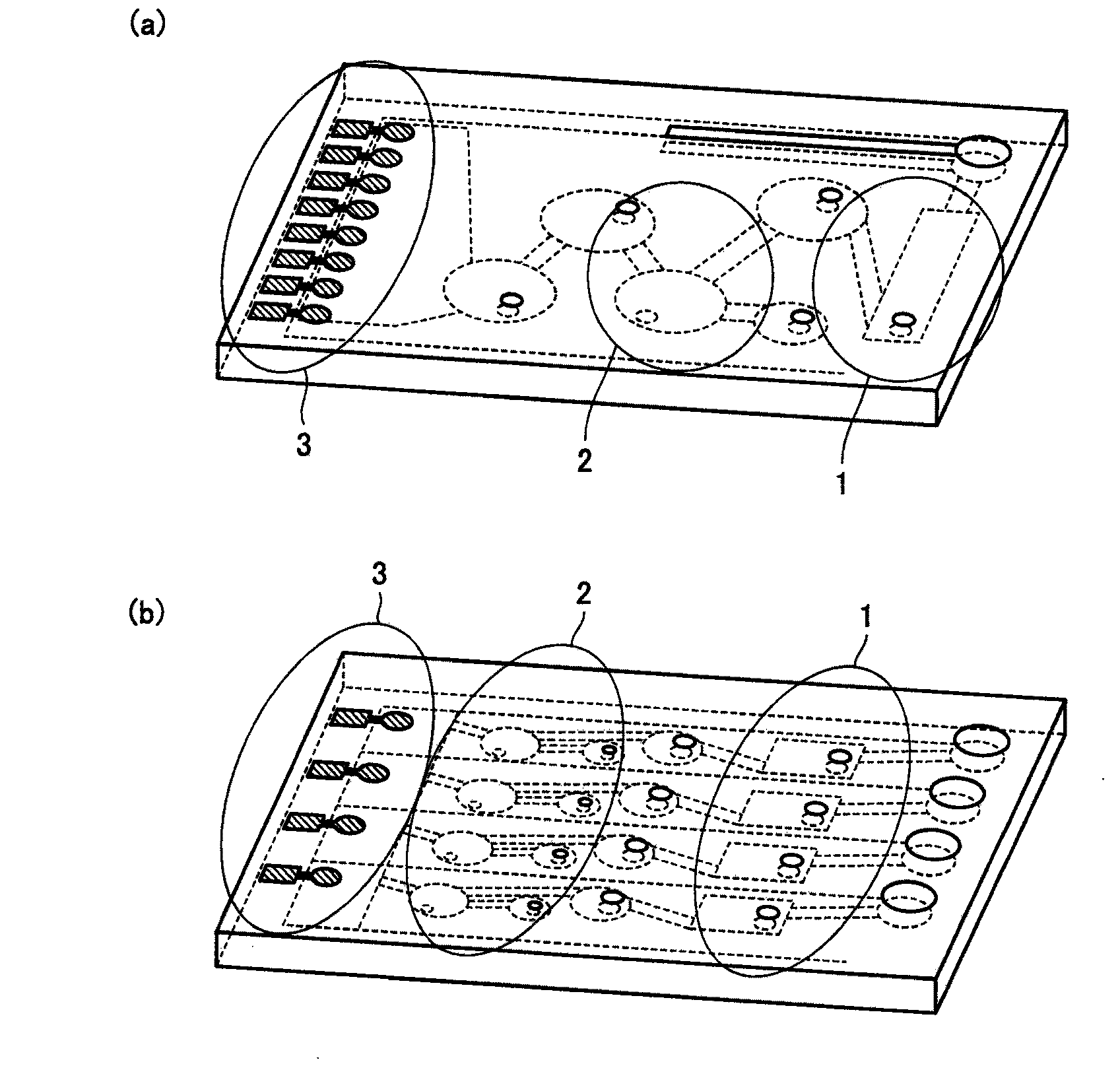
The invention provides a particle array manufacturing device and a particle array manufacturing method. The particle array manufacturing apparatus includes a mechanism for storing a liquid containing particles of a bio-related substance immobilized on the surface, and a mechanism for ejecting the liquid containing the particles to an arbitrary position on a solid-phase substrate. According to the particle array manufacturing apparatus of the present invention, the particles can be supplied to arbitrary reaction regions on the solid-phase substrate with better precision.
Owner:SEIKO EPSON CORP
Combined use of a carnosinase inhibitor with l-carnosine or its related substance and a composition containing the same
InactiveUS20140086855A1Good for healthImprove athletic abilityOrganic active ingredientsCosmetic preparationsDiseaseSide effect
The present invention provides combined use of a carnosinase inhibitor with L-carnosine and its related substance, and a composition containing the same, which are useful for treatment or prevention of various diseases, improvement of health conditions, improvement of exercise ability, improvement of skin health, prevention of the side effects of alcohol drinking and the like in mammals including human.
Owner:INNOVATIVE VISION PROD
Novel biological substance nesfatin and its related substances and uses thereof
InactiveUS20100317836A1Enhancing food intakeWeight increaseMicrobiological testing/measurementAntibody ingredientsAssociated substanceAppetite regulation
The present invention relates to a novel method of obtaining a factor involved in appetite control and / or body weight control, as well as genes obtained by said method, polypeptides encoded by said genes, or novel polypeptides obtained from the information on polypeptides encoded by said genes as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control. Also the present invention relates to substances that inhibit the effects of said genes or said polypeptides as a means for treating, controlling or diagnosing diseases associated with appetite control and / or body weight control. By using thiazolidine diones having a PPAR γ agonist activity, genes and polypeptides involved in appetite regulation and / or body weight reduction can be obtained. NESFATIN or the like obtained by said method can be used as a means for treating, controlling or diagnosing diseases associated with eating disorders and / or body weight control.
Owner:TEIJIN LTD +1
Method for analyzing related substances of a pharmaceutical composition containing a polymeric carrier
ActiveUS20180224405A1Reduce the amount requiredSubstance can be preventedOrganic active ingredientsOrganic chemistryAssociated substanceAnalysis method
A method for analyzing related substances in a pharmaceutical composition containing an amphiphilic block copolymer comprising a hydrophilic block and a hydrophobic block as a polymeric drug carrier, related substances identified thereby, and a method for evaluating a pharmaceutical composition by using the same are provided.
Owner:SAMYANG HLDG CORP
Substance searching method and device
InactiveCN107526797AImprove experienceBroad searchSpecial data processing applicationsSemantic tool creationRelevant informationRelational database
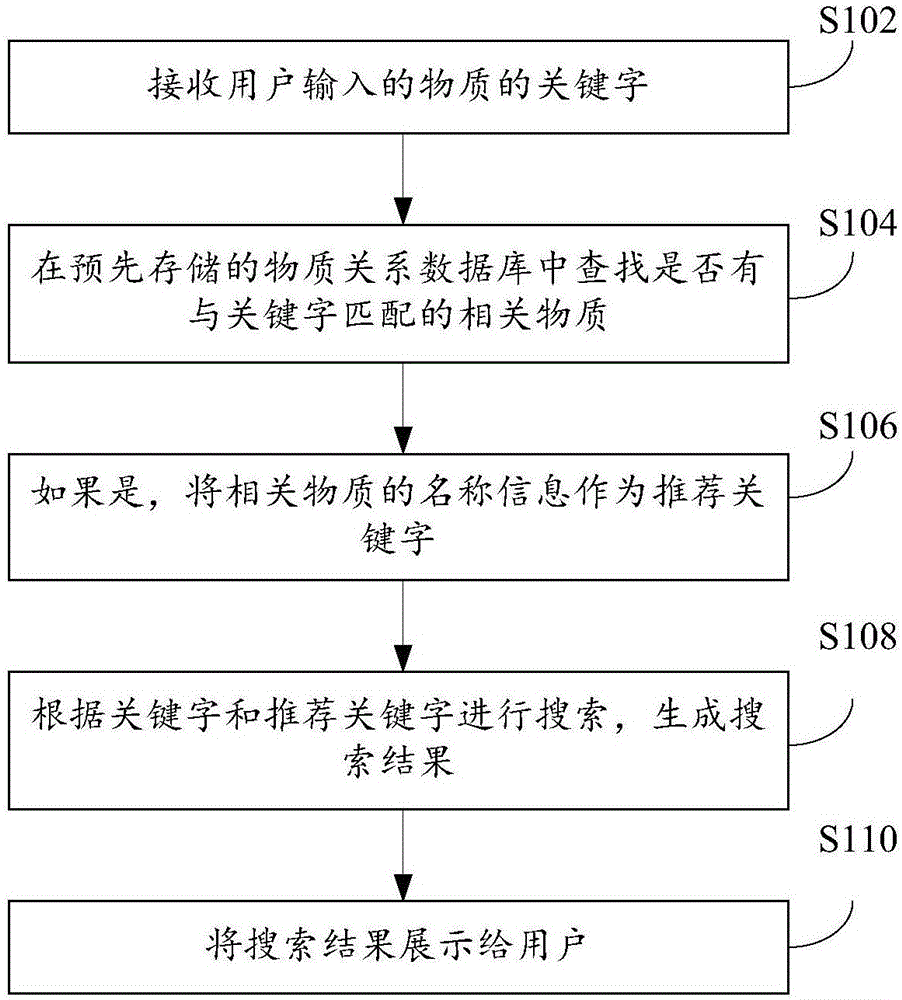
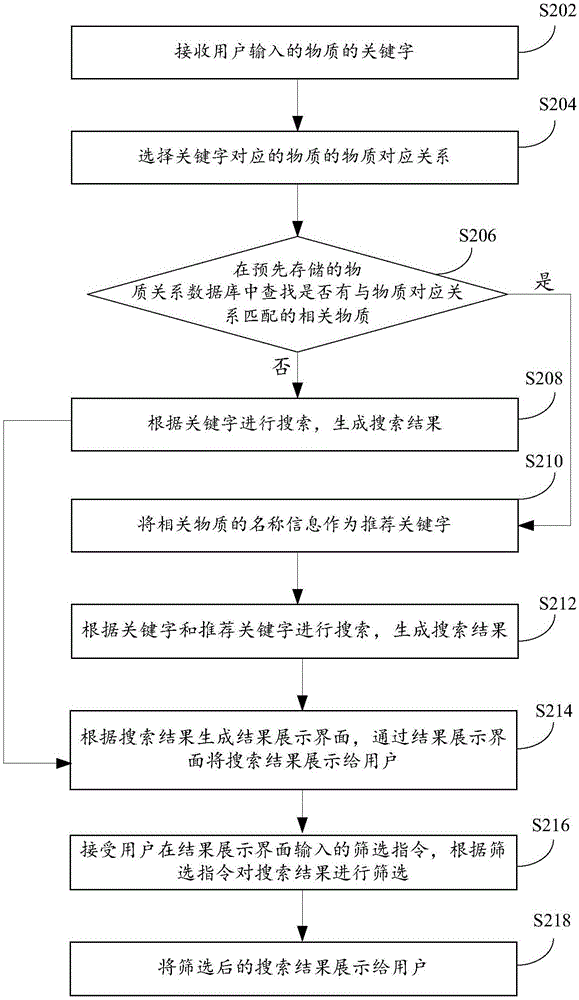
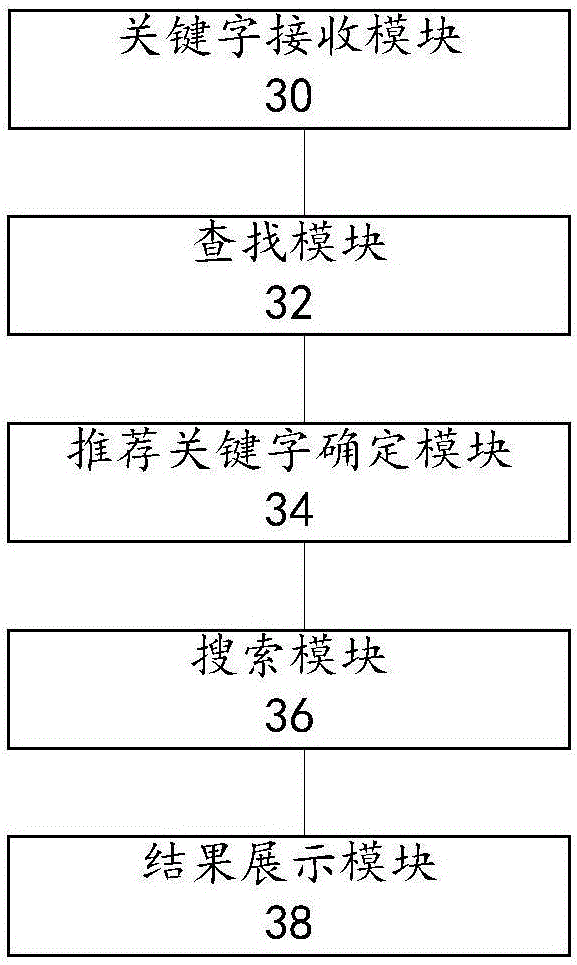
The invention provides a substance searching method and device, and relates to the technical field of substance search. The method comprises the following steps that: receiving a substance keyword input by a user; in a pre-stored substance relational database, searching whether a relevant substance matched with the keyword is in the presence or not; if the relevant substance matched with the keyword is in the presence, taking the name information of the relevant substance as a recommendation keyword; according to the keyword and the recommendation keyword, carrying out searching to generate a search result; and displaying the search result to the user. By use of the substance searching method and device provided by the embodiment of the invention, when the user carries out keyword search, a plurality of relevant recommendation keywords can be matched through the keyword for searching, more relevant information can be more widely searched and found, search efficiency can be improved, and the experience degree of the user is improved.
Owner:GUANGZHOU CCM INFORMATION SCI & TECH
Method and apparatus for automatic peak integration
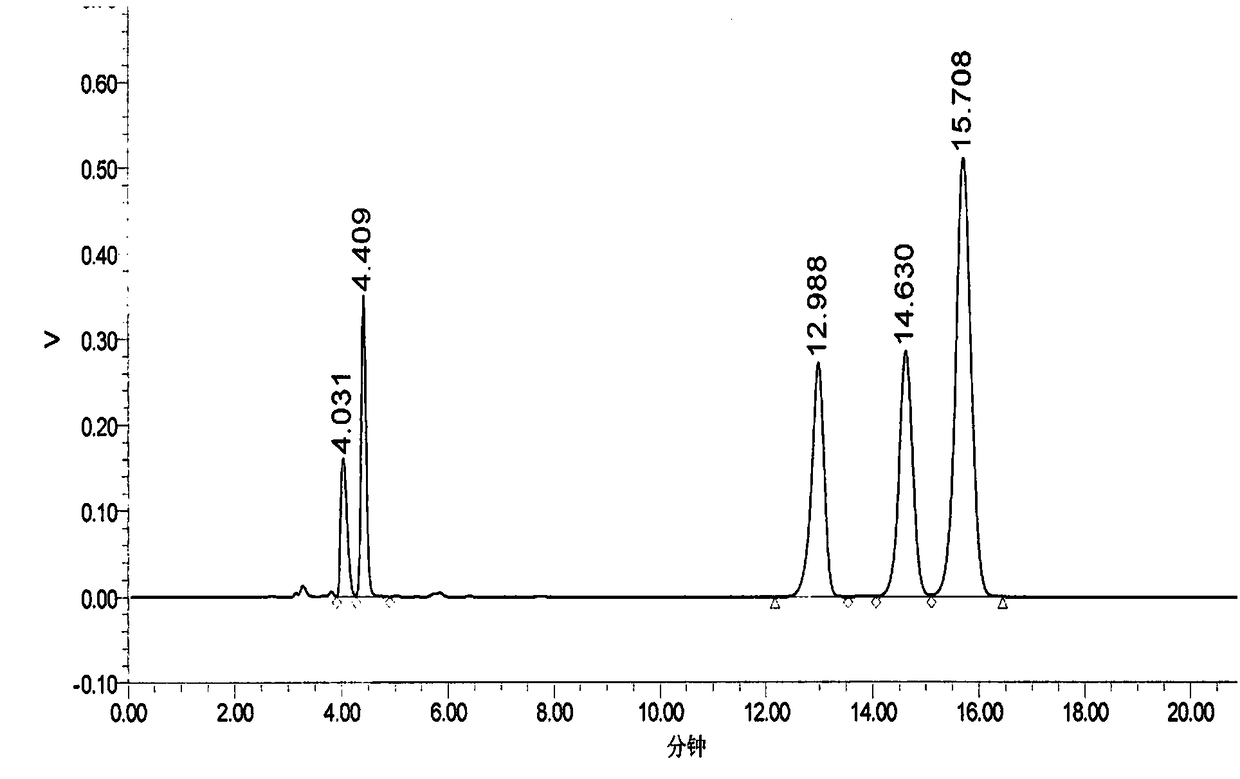
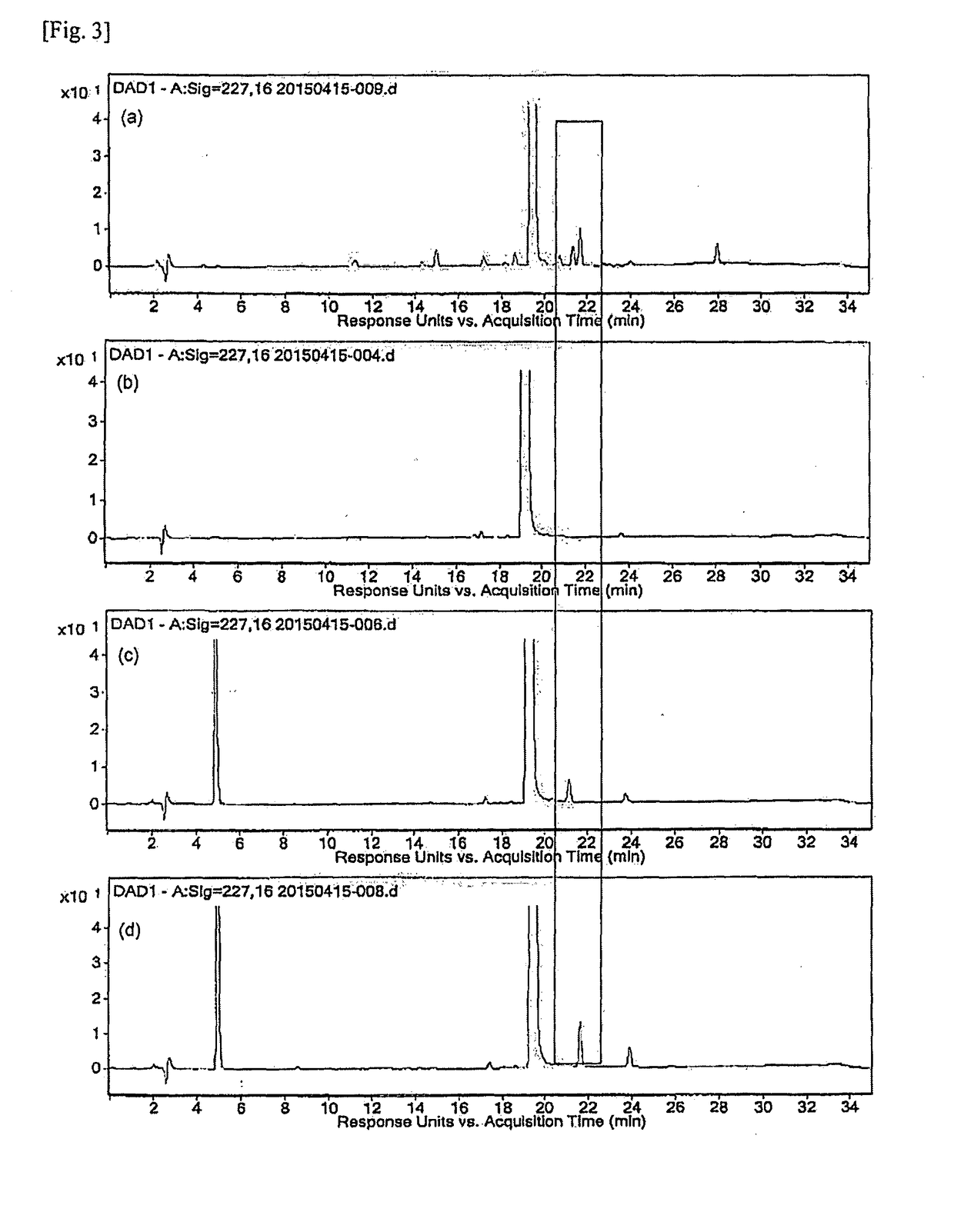
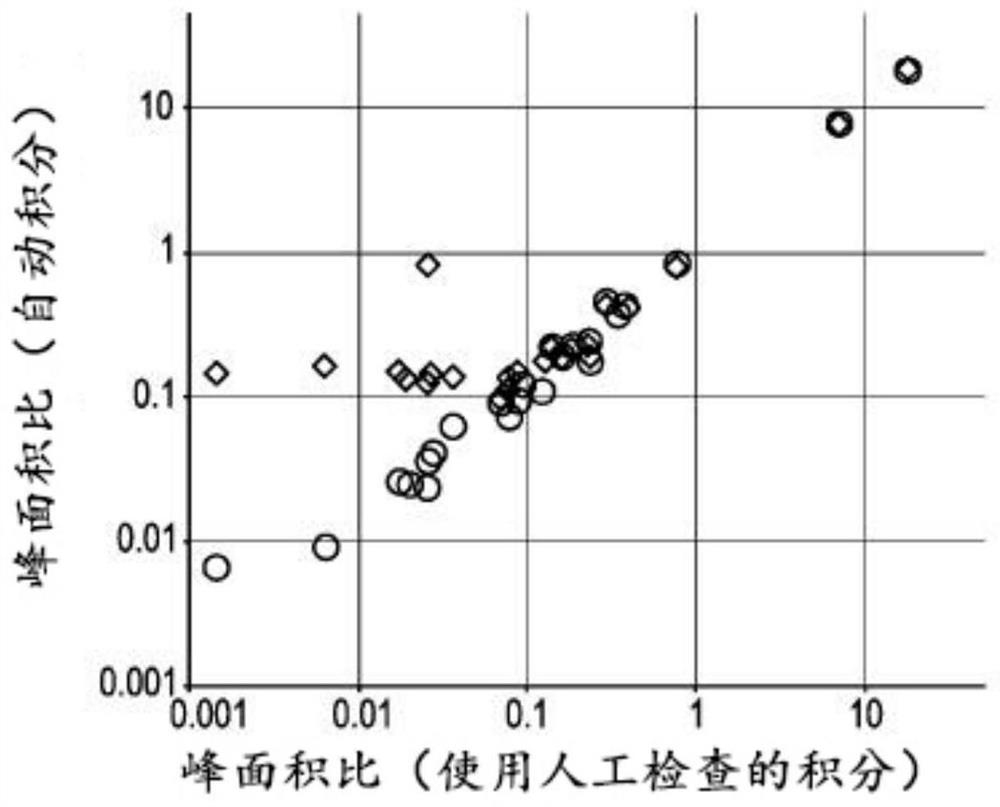
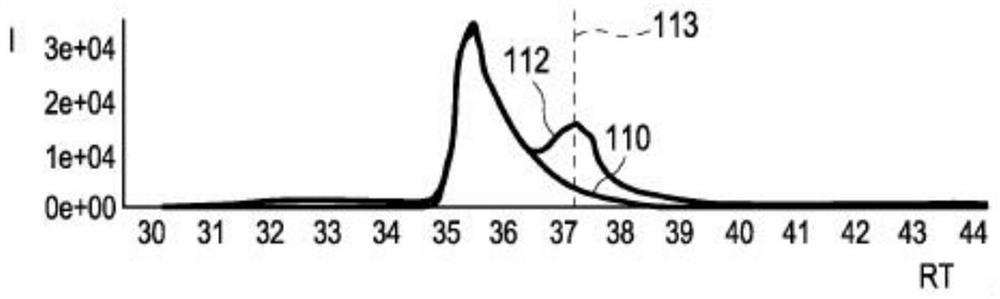
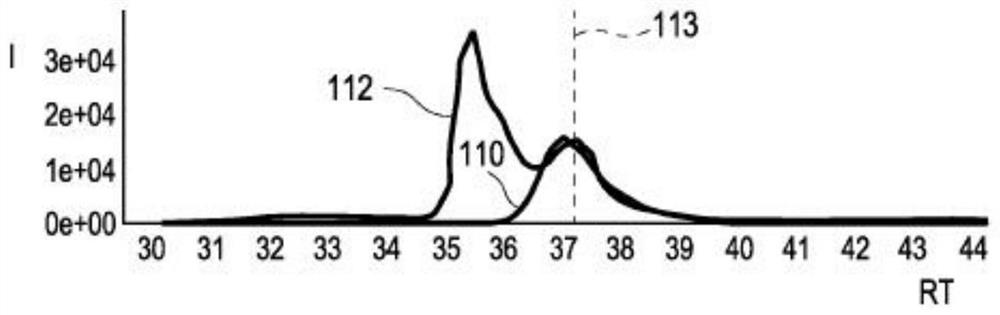
PendingCN114207435AReliable automatic peak integrationParticle separator tubesComponent separationAnalyteAssociated substance
A computer-implemented method for automatic peak integration of at least one chromatogram of at least one sample is presented. The sample comprises at least one analyte and at least one chemically related substance. The method comprises the steps of: a) retrieving at least one chromatogram of the chemically related substance and at least one chromatogram of the analyte; (114, 116) b) evaluating the chromatogram of the chemical related substance, wherein the evaluation comprises b1) determining a retention time (118) of the chemical-related substance and adding the retention time of the chemical-related substance to a predetermined or predefined constant offset and / or multiplying the retention time of the chemical-related substance by a predetermined or predefined constant factor (120), b) determining at least one initial value (117) of the analyte retention time by determining a peak shape parameter (121) of at least one peak of the chromatogram of the chemically relevant substance, b2) determining at least one initial value (122) of an analyte peak shape parameter by determining a peak shape parameter (121) of at least one peak of the chromatogram of the chemically relevant substance; c) evaluating the chromatogram of the analyte, where the evaluation comprises c1) at least one step (124) of position determination, where the analyte retention time is determined by taking into account the initial value of the retention time of the chemically relevant substance; c2) at least one peak integration step (126) in which at least one fitting analysis is applied to the chromatogram of the analyte by taking into account an initial value of an analyte peak shape parameter and the analyte retention time, thereby determining an analyte peak area and an analyte peak shape.
Owner:F HOFFMANN LA ROCHE & CO AG
HPLC method for the analysis of bosetan and related substances and use of these substances as reference standards and markers
InactiveUS8975402B2Selective, sensitive, linear, precise, accurate and robustSensitive highOrganic chemistryComponent separationHplc methodAssociated substance
The present invention relates to a new HPLC method for the analysis of the drug substance bosentan and related substances and to the use of said substances as reference standards and markers.
Owner:GENERICS UK LTD
Rotigotine-containing transdermal absorption preparation with improved stability
ActiveUS9877948B2Good storage stabilityPrevent crystallizationOrganic active ingredientsNervous disorderAssociated substanceAntioxidant
A method for preparing a transdermal absorption composition includes mixing rotigotine and an antioxidant at a weight ratio of 1:0.0001 to 0.1. A transdermal therapeutic system includes a substrate and a drug-containing adhesive layer disposed on the substrate and including an antioxidant at a weight ratio of 1:0.0001 to 0.1. The method and system of the present invention suppress the crystallization of rotigotine as well as the generation of related substances, thereby increasing the long-term storage stability of a therapeutic product containing rotigotine or related substances.
Owner:SK CHEM CO LTD
Combined therapy
The present invention provides a method of reducing tissue damage due to ischemia, the method comprising, administering to a mammal in need of such reduction an effective amount of an NHE-1 inhibitor and an agent selected from (a) a complement modulator, (b) A combination of a metabolic regulator, (c) an anti-apoptotic agent, (d) a nitric oxide synthase-related substance and (e) a second compound of an enzyme / protein regulator, or a pharmaceutical composition containing the combination. The present invention also provides a kit comprising an amount of a type 1 sodium-hydrogen exchanger inhibitor in a first unit dosage form and a pharmaceutically acceptable carrier, excipient or diluent; an amount of the second compound in a form and a pharmaceutically acceptable carrier, excipient or diluent; and a container.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
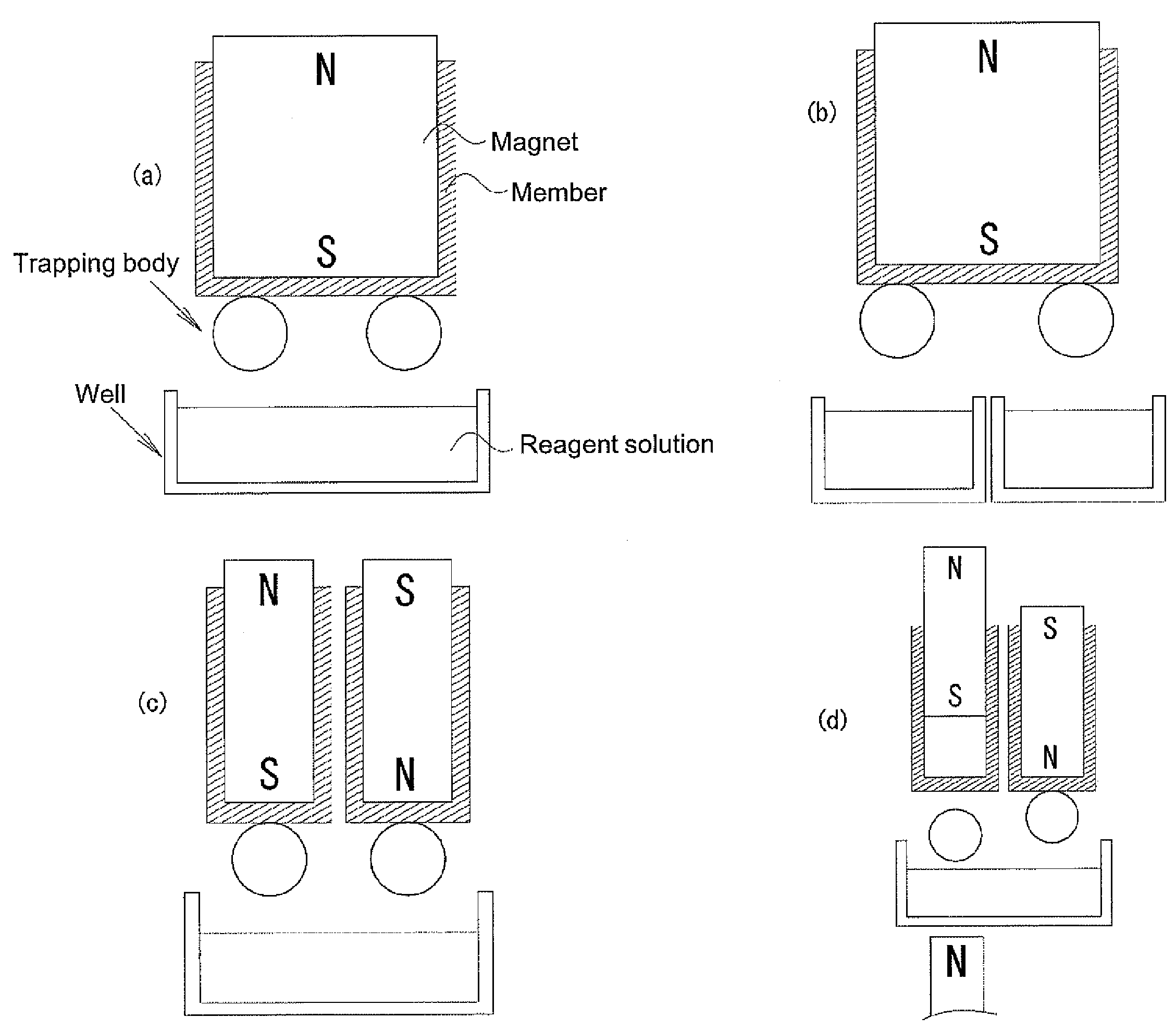
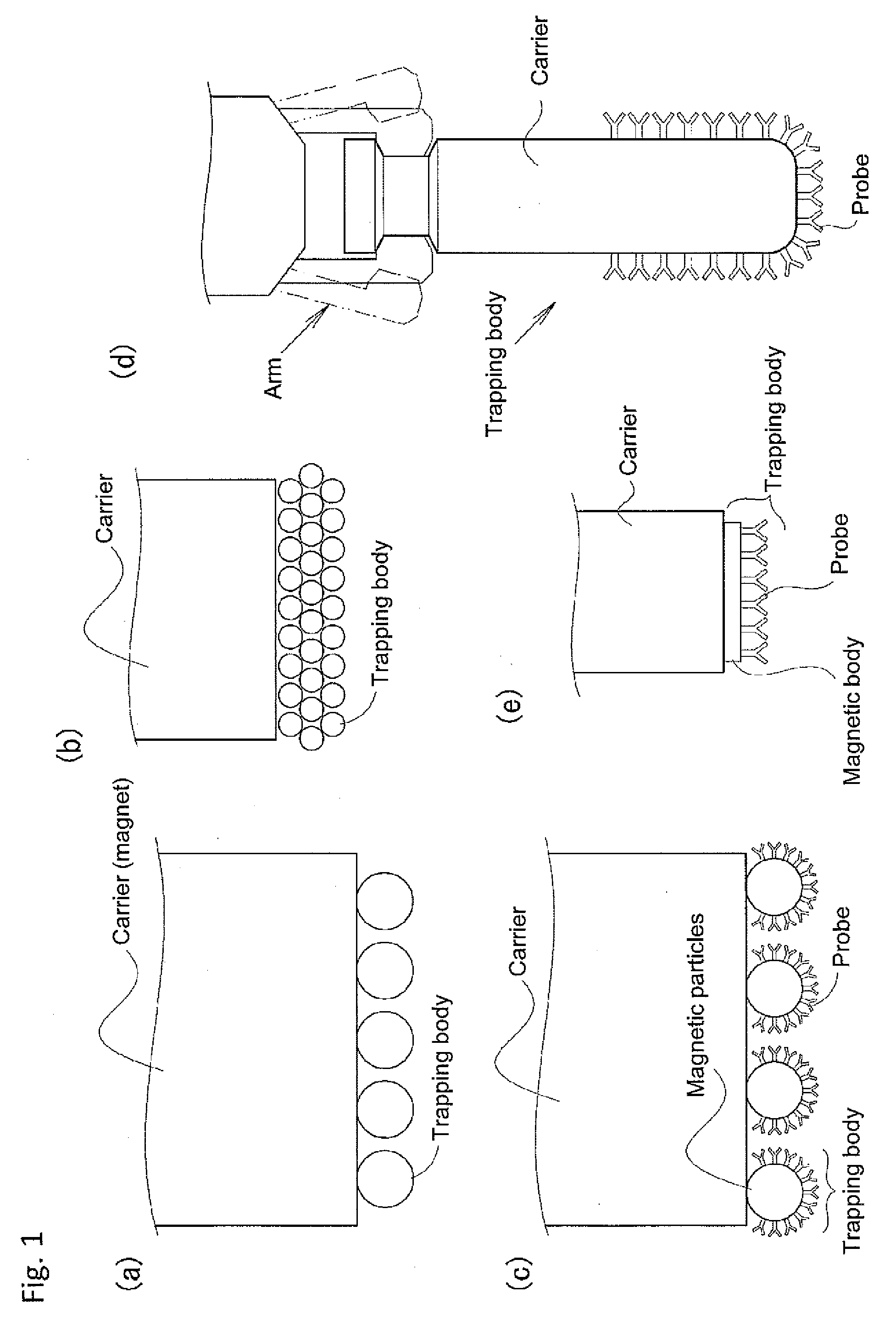
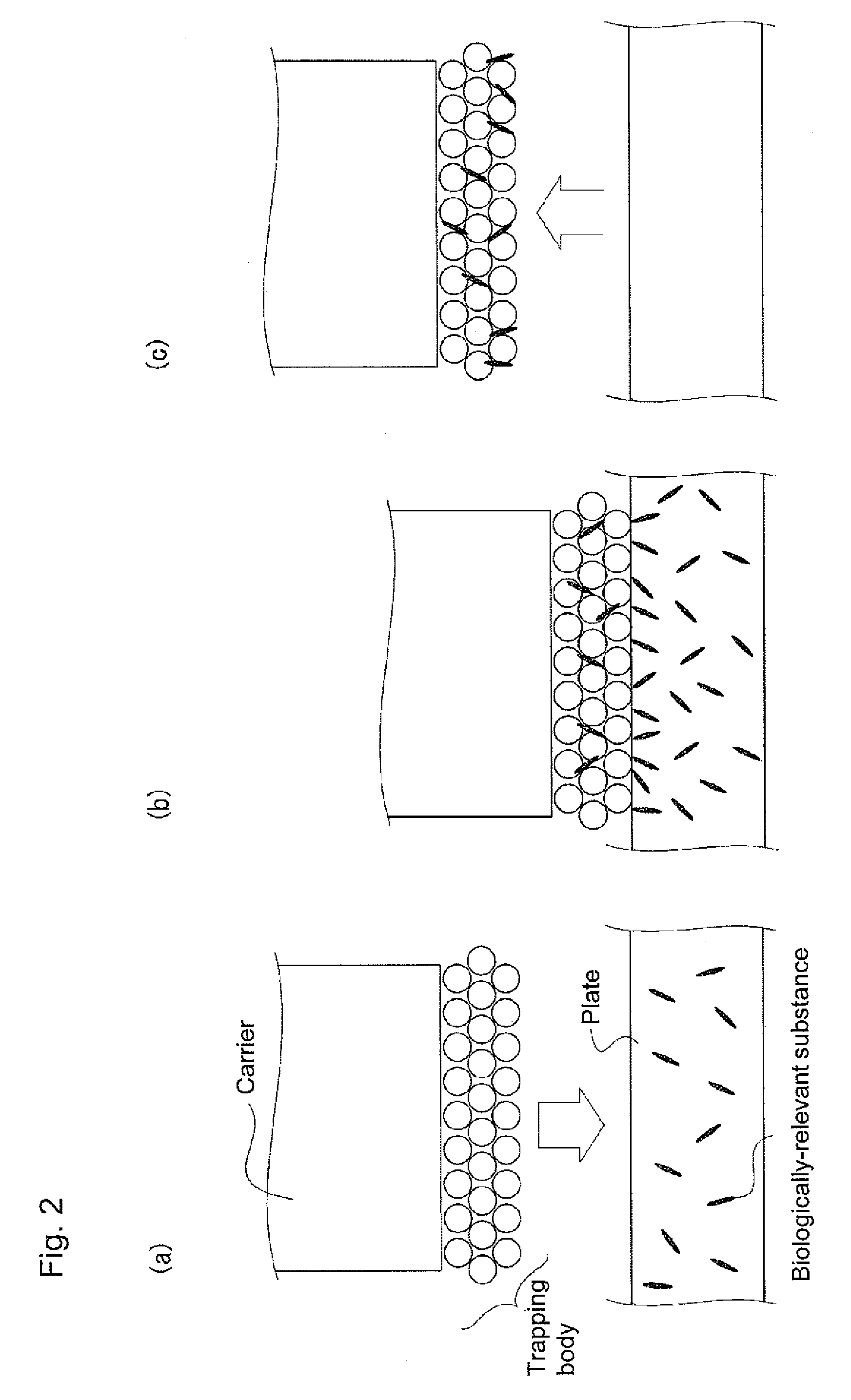
Device for trapping biologically-relevant substances and system for collecting biologically-relevant substances
The disclosed device for trapping biologically-relevant substances can easily collect biologically-relevant substances from a thin tissue section or a gel in which said biologically-relevant substance have been fractionated. Said device is provided with: trapping bodies for trapping biologically-relevant substances; carriers that hold the trapping bodies; and electrodes for charging the trapping bodies. The trapping bodies are charged and brought into contact with a prescribed position on a thin tissue section that contains biologically-relevant substances or a gel in which biologically-relevant substances have been fractionated, thereby trapping said biologically-relevant substances from said gel or thin tissue section.
Owner:UNIVERSAL BIO RESEARCH CO LTD
Nucleic acid based composition for cell proliferation
A method for effectively exerting a cell proliferation promoting effect of a purine nucleic acid-related substance, and a composition for cell proliferation containing a purine nucleic acid-related substance and a pyrimidine nucleic acid-related substance. Also a method for potentiating the cell proliferation promoting effect of the purine nucleic acid-related substance by using the purine nucleic acid-related substance in combination with the pyrimidine nucleic acid-related substance and a method for promoting cell proliferation, where the method includes the step of applying the purine nucleic acid-related substance in combination with the pyrimidine nucleic acid-related substance to the skin or mucosa.
Owner:OTSUKA PHARM CO LTD
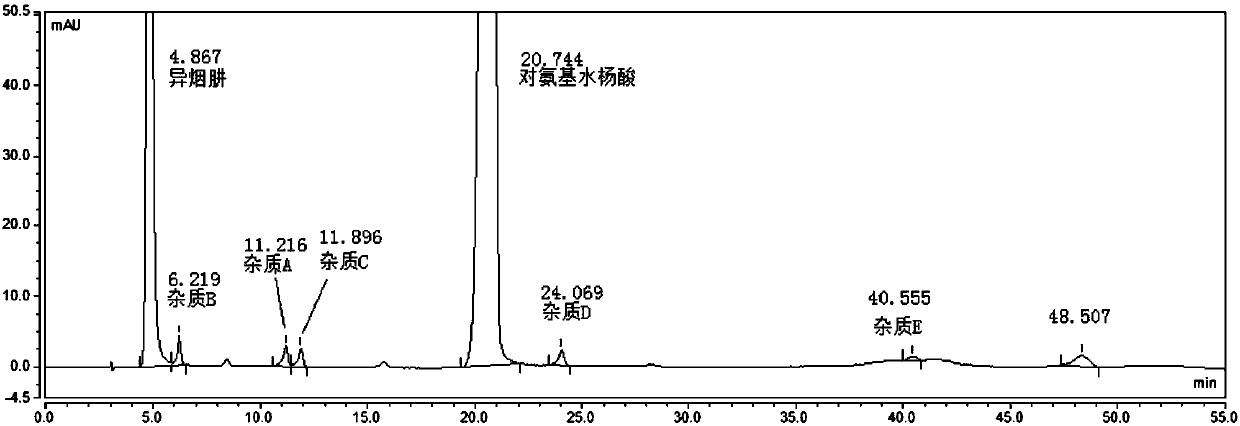
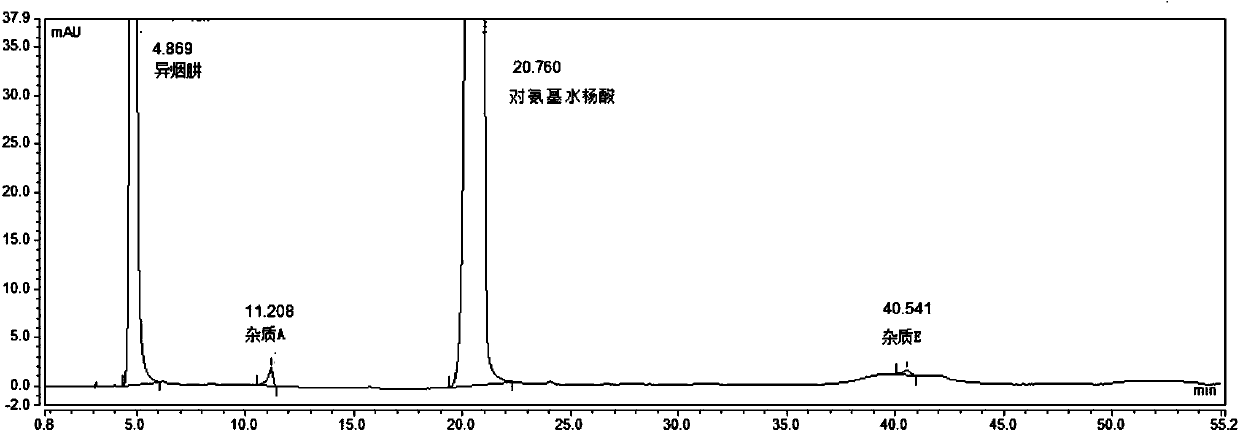
Method for separating and determining pasiniazide and related impurities thereof by HPLC method
ActiveCN107817307AQuality is easy to controlEfficient separationComponent separationHplc methodPhosphate
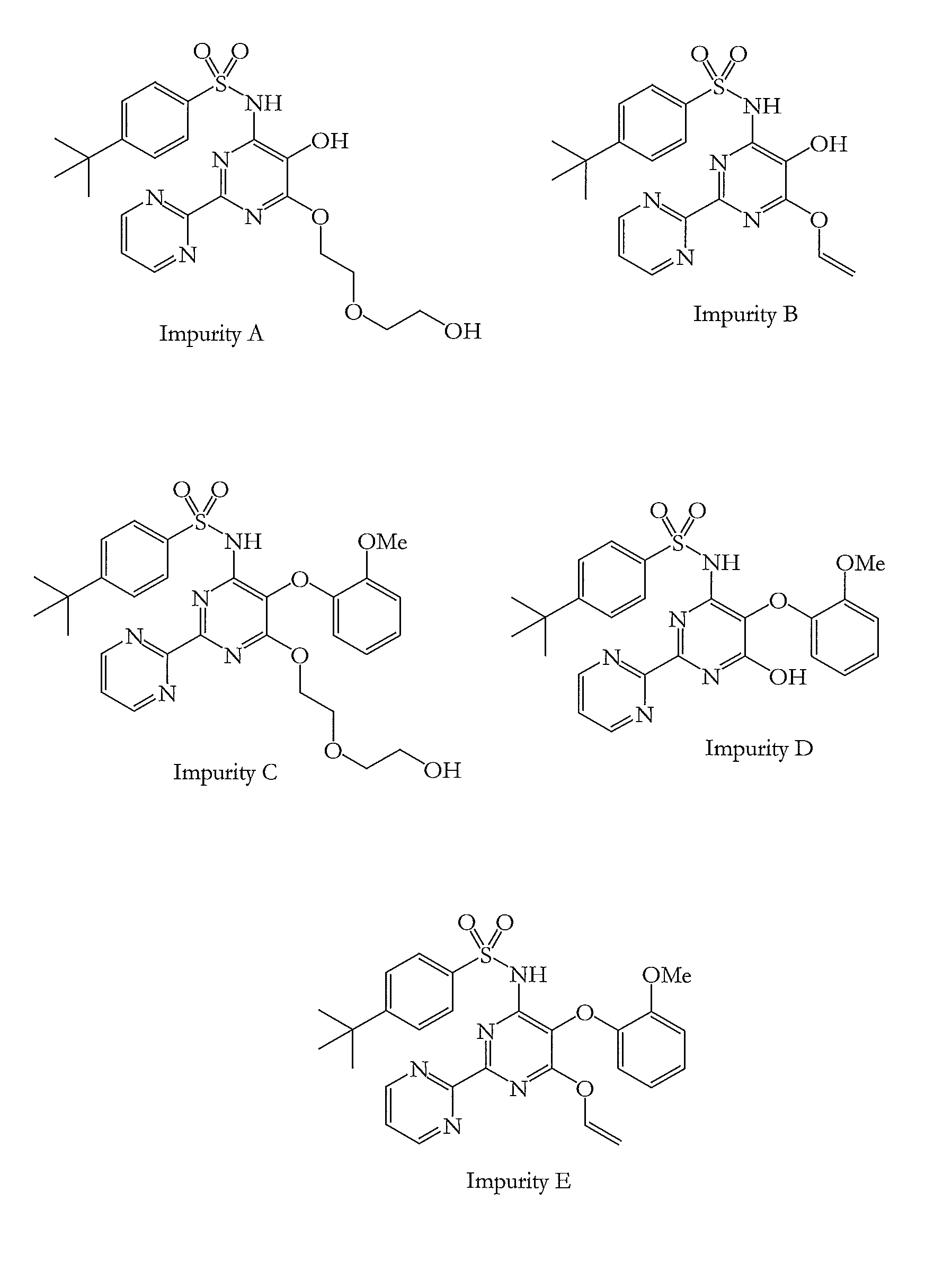
The invention belongs to the field of analytical chemistry and particularly relates to a method for separating and determining pasiniazide and related impurities thereof by an HPLC method. According to the method, an adopted chromatographic column takes octadecylsilane chemically bonded silica as filler, a mobile phase A and a mobile phase B are adopted to carry out gradient elution, and detectionis carried out in a detector; related substances comprise isoniazide, p-aminosalicylic acid and related impurities; the related impurities are one or more of A to E; the mobile phase A is a phosphatebuffer solution, and the mobile phase B is methanol. The method provided by the invention can be used for simultaneously separating and determining the isoniazide, the p-aminosalicylic acid and the related impurities A to E in the pasiniazide, and the efficiency of separation detection is increased compared with the prior art; the controllable quality of pasiniazide raw pharmaceutical materials and preparations thereof can be more accurately ensured, and the product can be safe and effective finally.
Owner:CHONGQING HUAPONT PHARMA
Propionyl-L-carnitine synthesis technology and detection method of related substance and its content
InactiveCN1290821CLow costOperational securityComponent separationOrganic compound preparationPropanoic acidSolvent
A process for synthesizing the propionyl-L-carnitine features the acylation reaction between L-carnitine and propionyl chloride in propionic acid as solvent under the catalysis of p-toluenesulfonic acid. The method for measuring the contents of propionyl-L-carnitine and associated substances features that the efficient liquid-phase chromatography is used to measure the content of propionyl-L-carnitine and the thin-layer chromatography is used to detect associated substances.
Owner:SUZHOU LANXITE BIOTECH
Process for preparing comparison solution for measuring polymeric substance in Cefepime, its salt raw material and preparation
ActiveCN100416272CComplete associationStable peak areaComponent separationAssociated substanceCefuroxime
This invention relates to process method to test cefuroxime and its salt materials and the method for polymer comparing product solution. The invention solves the comparing cefuroxime and dilutes it to the needed concentration and adjusts its Ph value above nine as alkalescence solution and to make it totally associated under certain temperature and time. The process method in the invention has complete cefuroxime and stable associated substance and stable peak area of associated substance.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Process for preparing comparison solution for measuring polymeric substance in Cefepime, its salt raw material and preparation
ActiveCN1670526AComplete associationStable peak areaComponent separationCefuroximeAssociated substance
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com