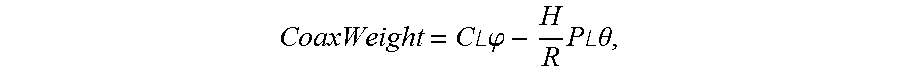Patents
Literature
34results about How to "Success rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
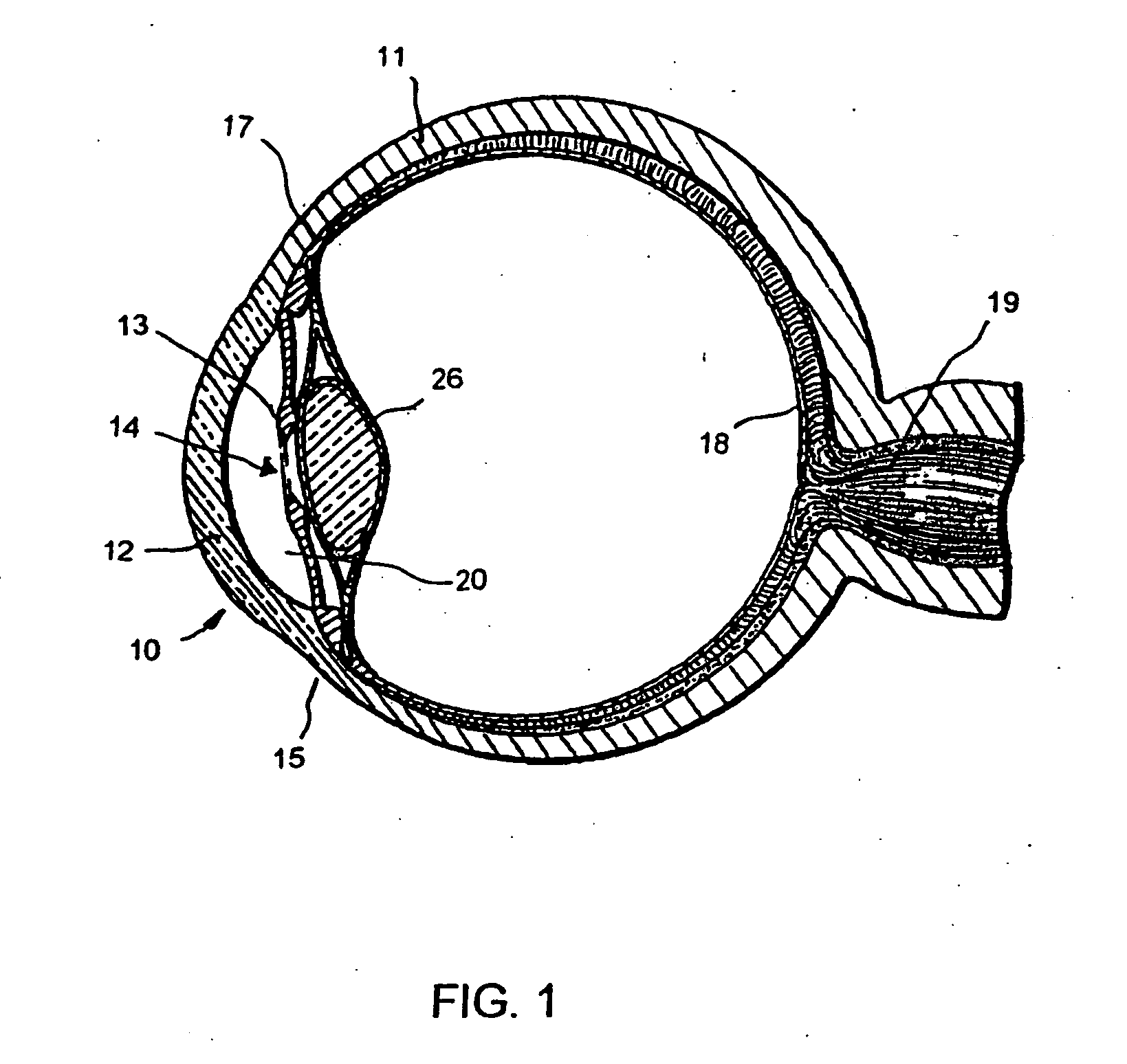
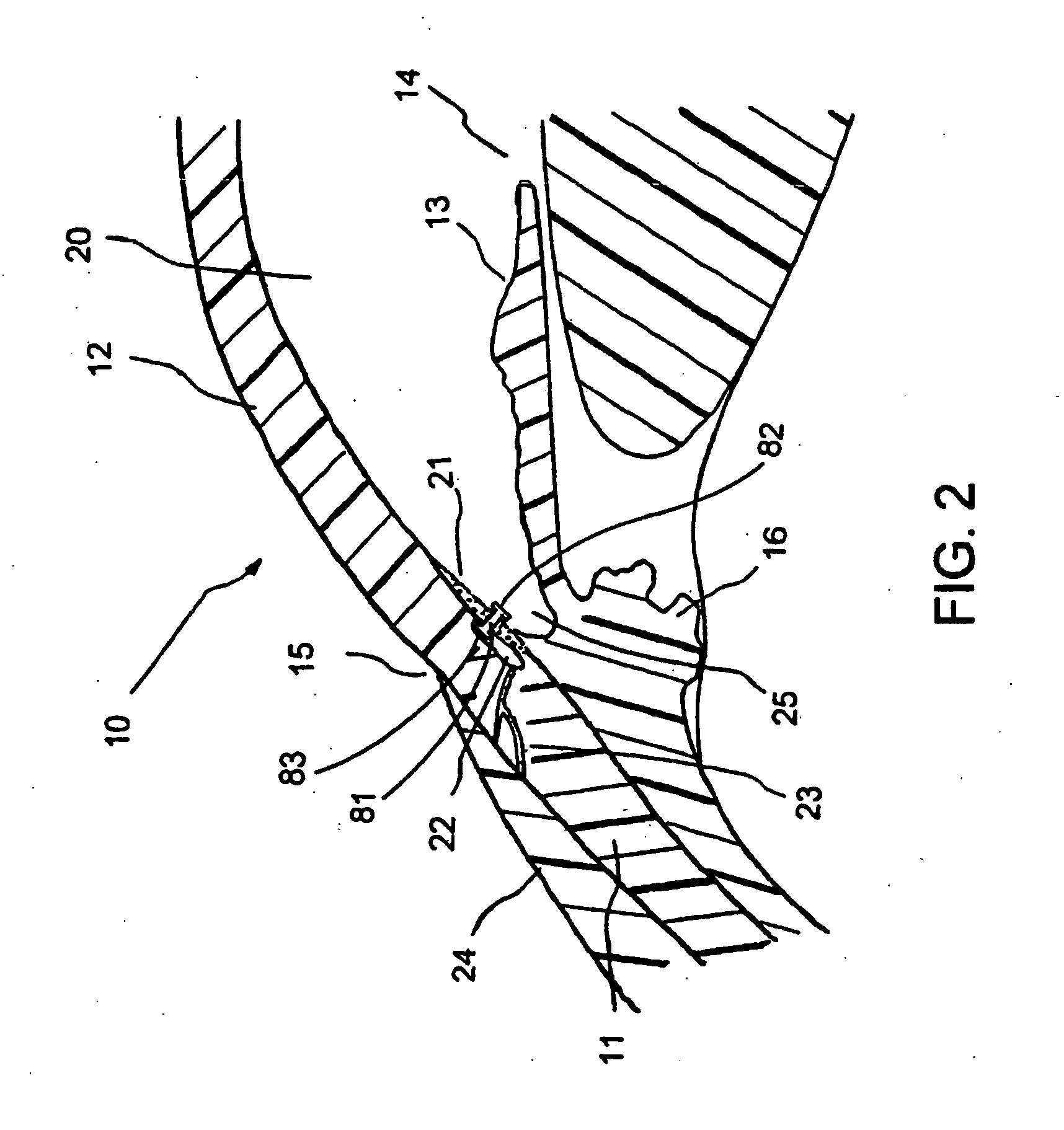
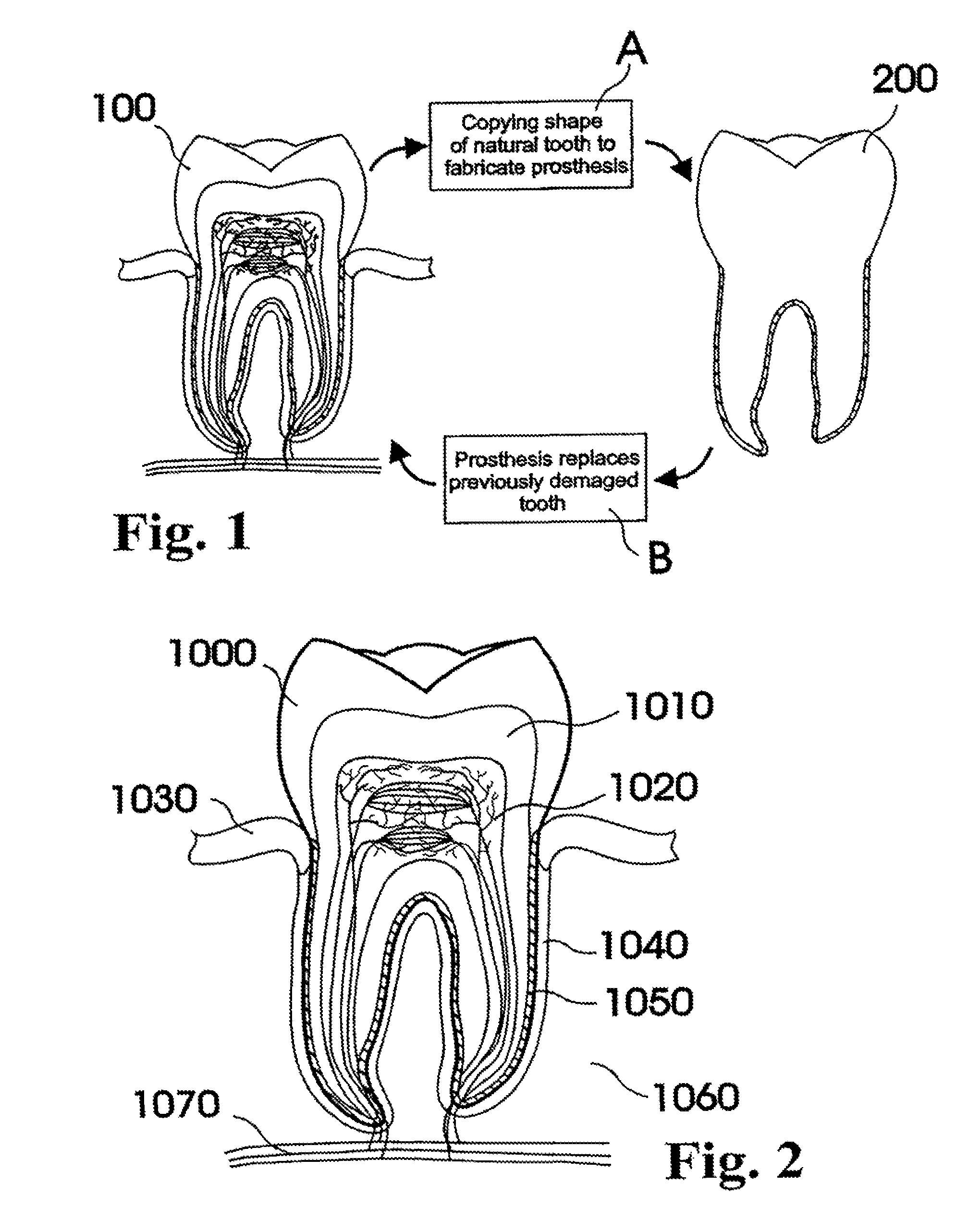
Aqueous outflow enhancement with vasodilated aqueous cavity
InactiveUS20050250788A1Promote recoverySuccess rateOrganic active ingredientsBiocideVeinAqueous outflow
A method for enhancing aqueous outflow and thereby lowering intraocular pressure is disclosed. The method comprises vasodilating aqueous veins by dilating or relaxing the smooth muscle of an aqueous cavity. In one embodiment, the step of dilating or relaxing the smooth muscle of the aqueous cavity is accomplished by slowly releasing loaded smooth muscle relaxing drug at an effective dose over time. In another embodiment, the step of dilating the smooth muscle of the aqueous cavity is accomplished by introducing a smooth muscle drug through an implant.
Owner:GLAUKOS CORP
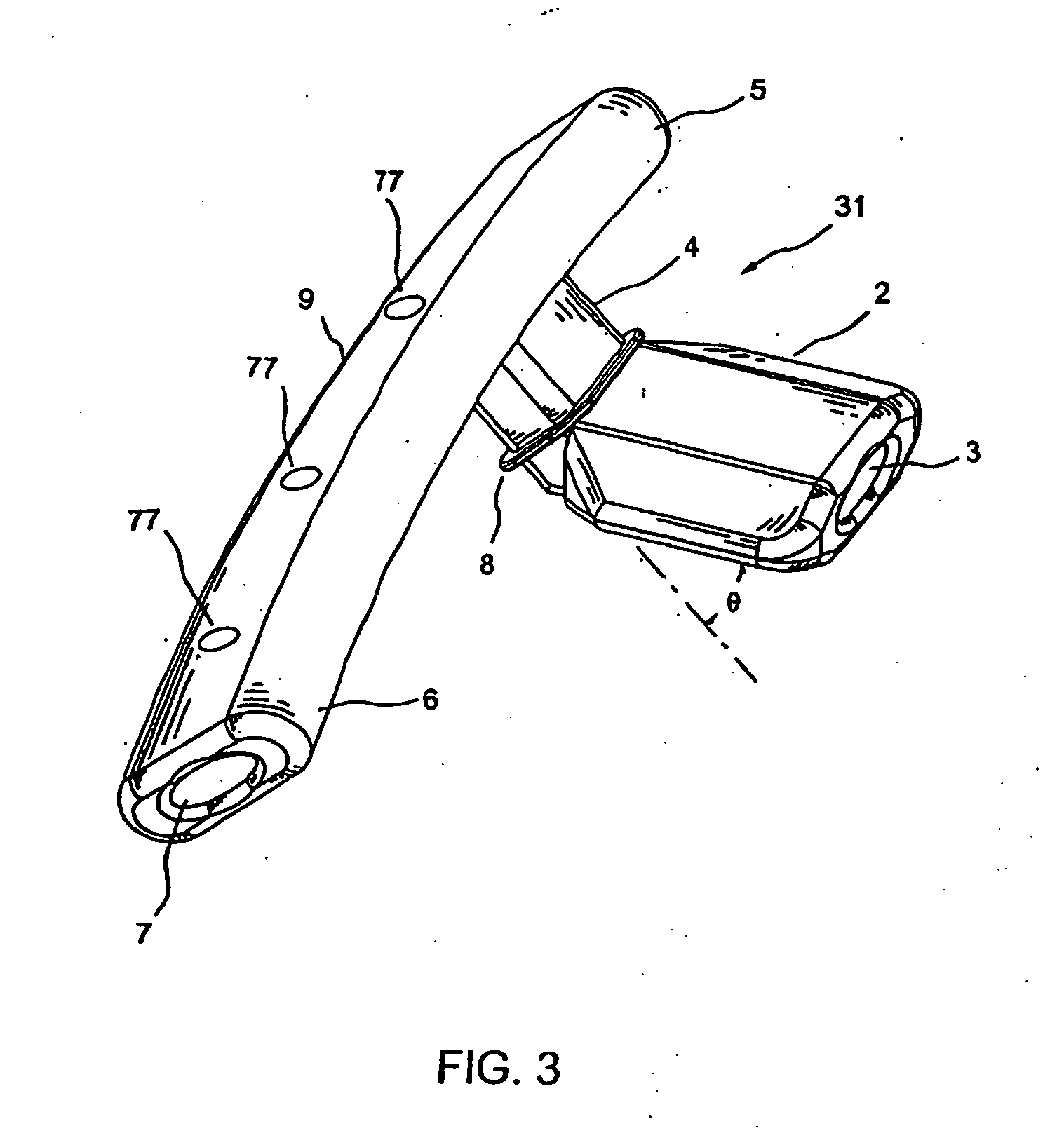
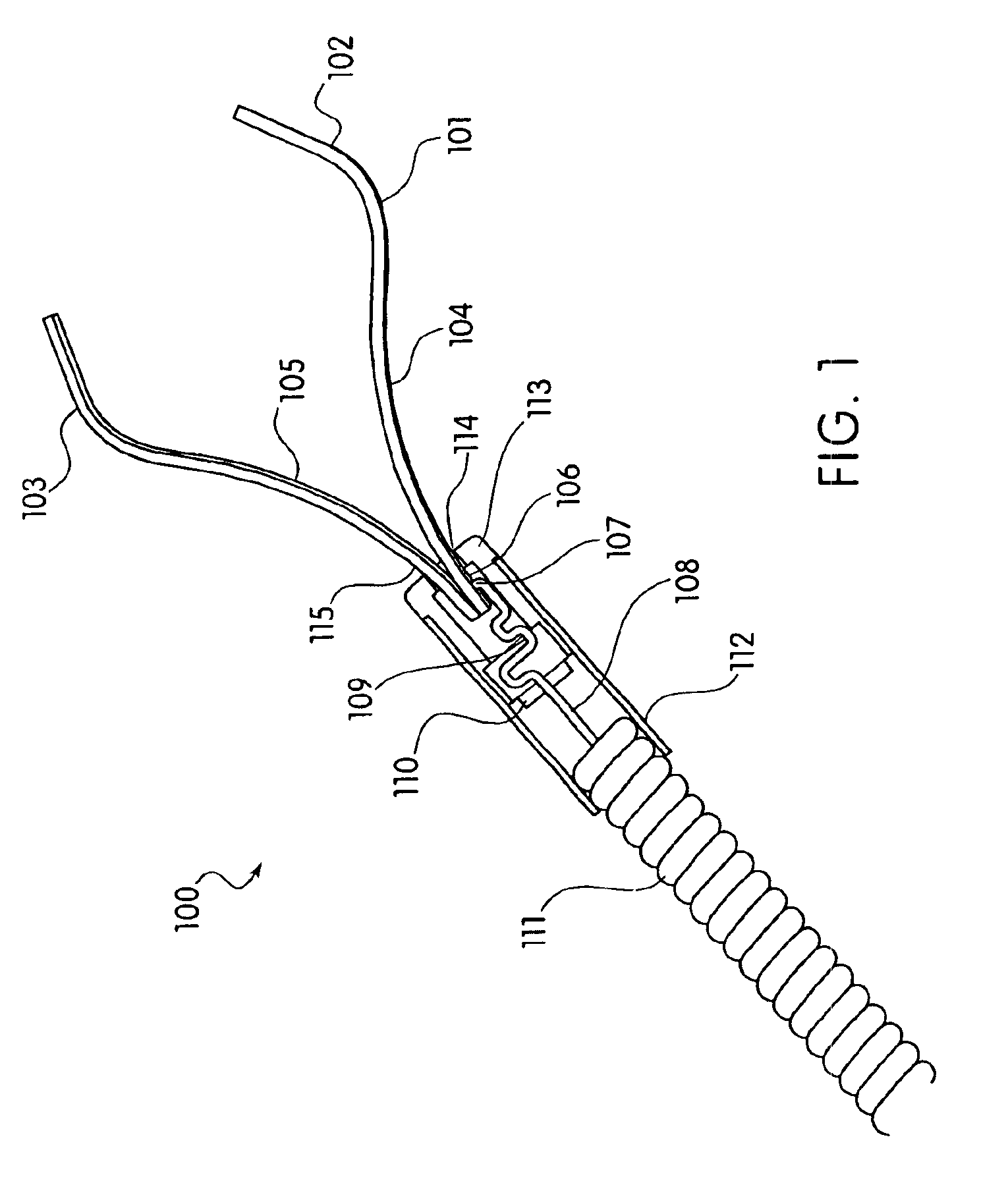
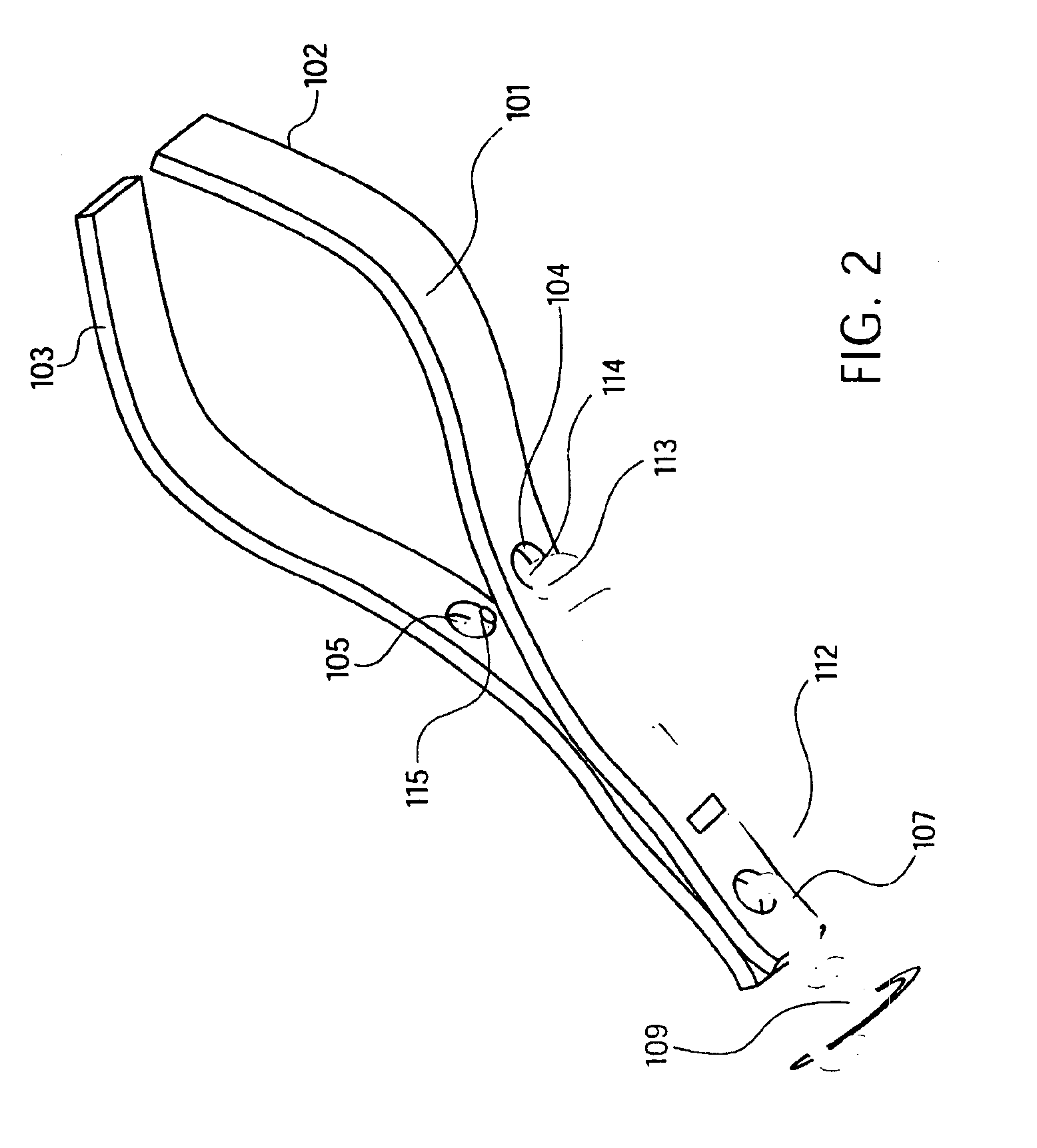
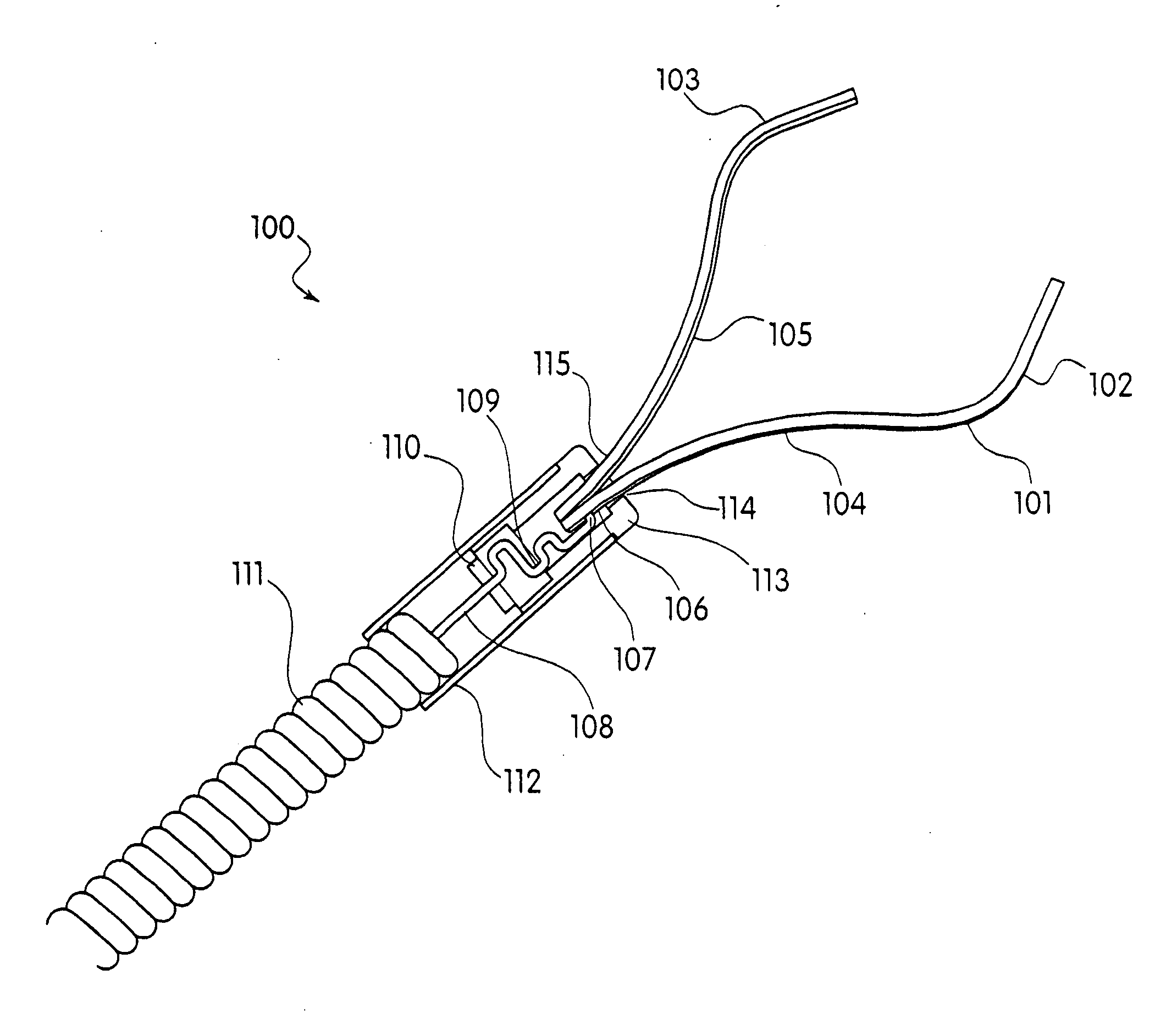
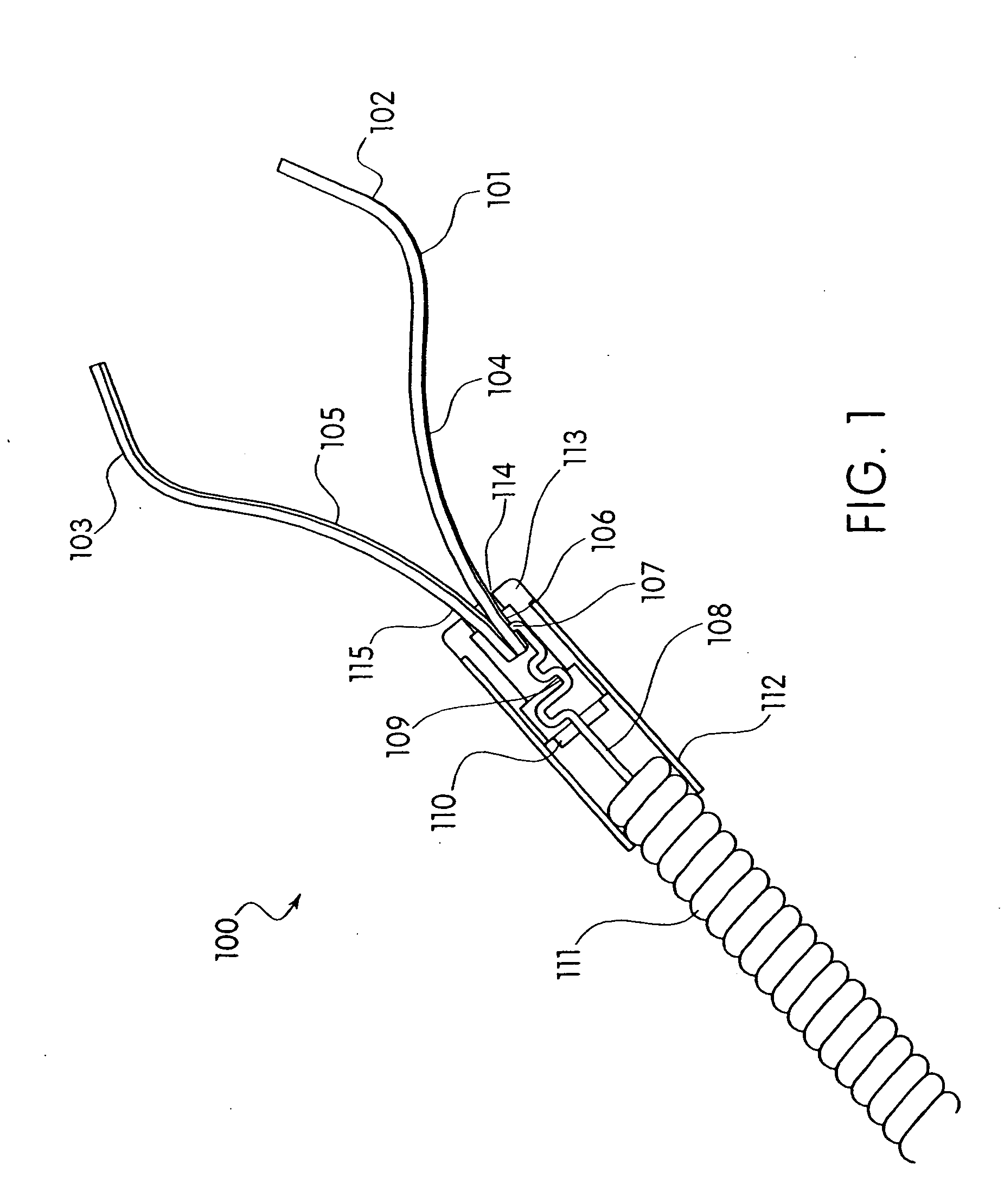
Device and method for through the scope endoscopic hemostatic clipping
Medical device used to cause hemostasis of blood vessels using a clip arrangement delivered to a target region through an endoscope. Method for using the device to cause hemostasis of a blood vessel through an endoscope. Medical device including a reversibly closeable clip, a locking arrangement, a control wire, a sheath, and a handle with an actuating trigger. Through the endoscope, hemostatic clipping device that is fully reversible and lockable. Hemostatic clip that reversibly targets and clips bleeding ulcers.
Owner:BOSTON SCI SCIMED INC
Device and method for through the scope endoscopic hemostatic clipping
ActiveUS20050182426A1Quicker procedureFew have been deployedDiagnosticsSurgical veterinaryEndoscopeMedical device
Medical device used to cause hemostasis of blood vessels using a clip arrangement delivered to a target region through an endoscope. Method for using the device to cause hemostasis of a blood vessel through an endoscope. Medical device including a reversibly closeable clip, a locking arrangement, a control wire, a sheath, and a handle with an actuating trigger. Through the endoscope, hemostatic clipping device that is fully reversible and lockable. Hemostatic clip that reversibly targets and clips bleeding ulcers.
Owner:BOSTON SCI SCIMED INC
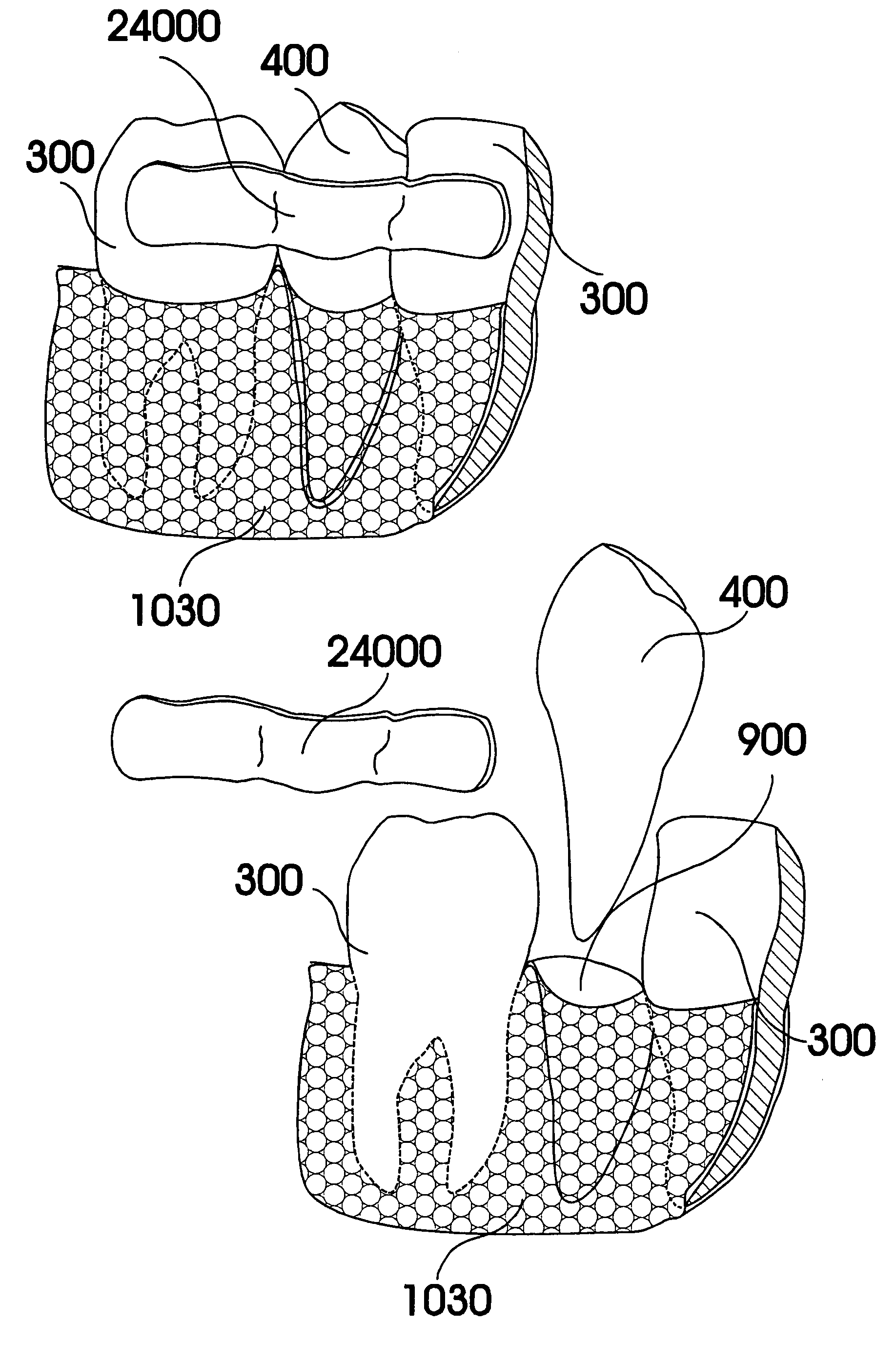
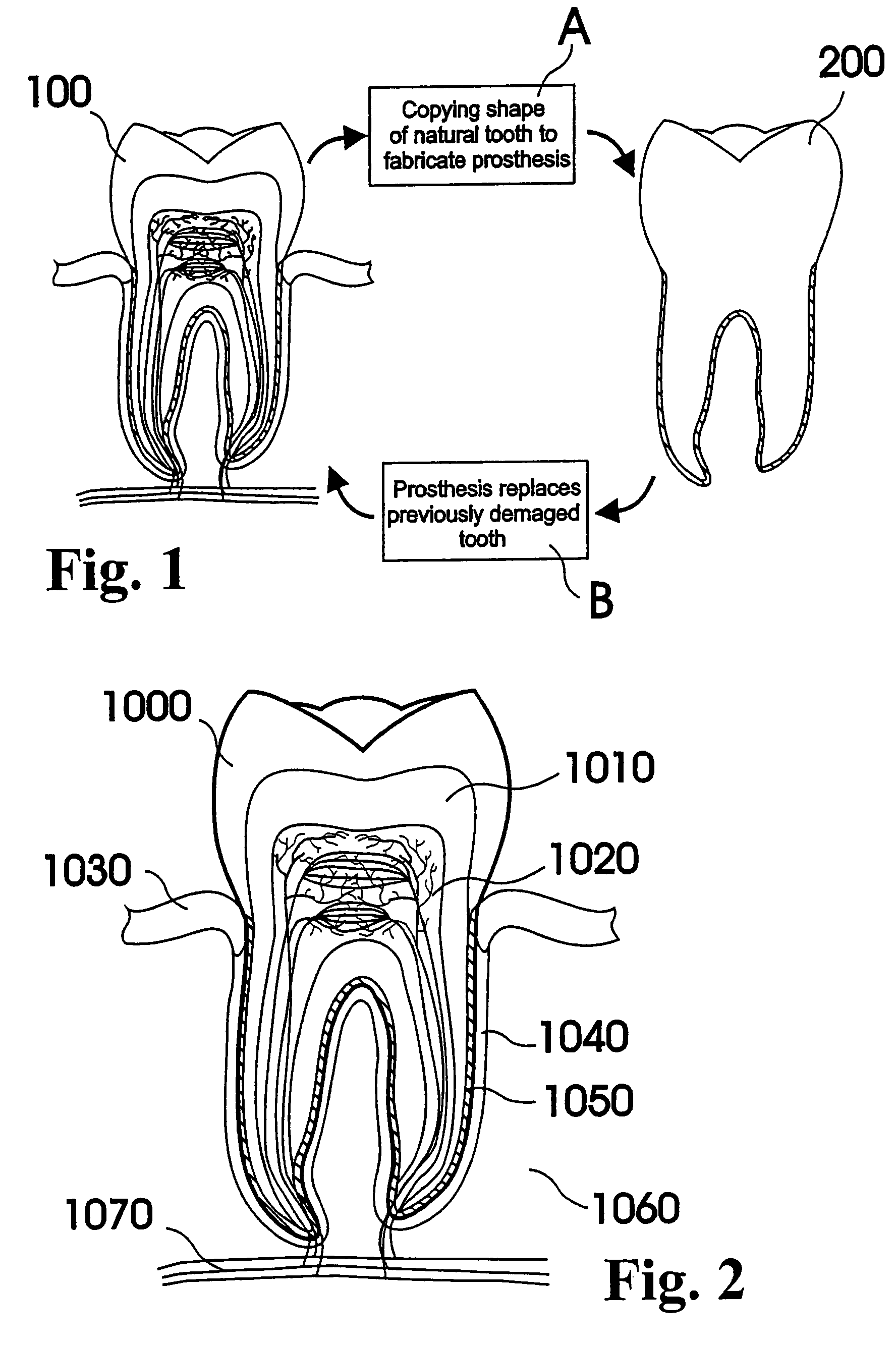
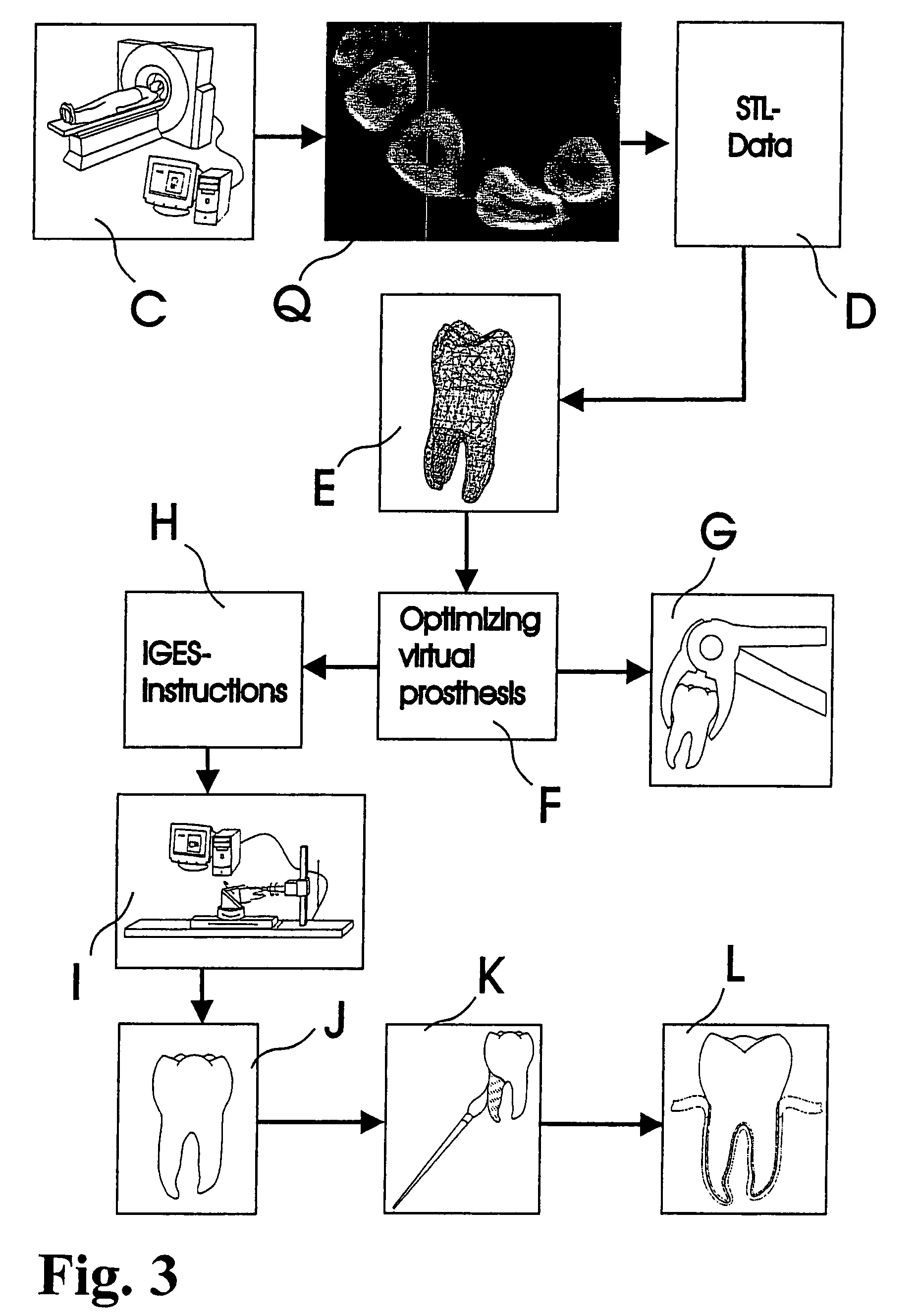
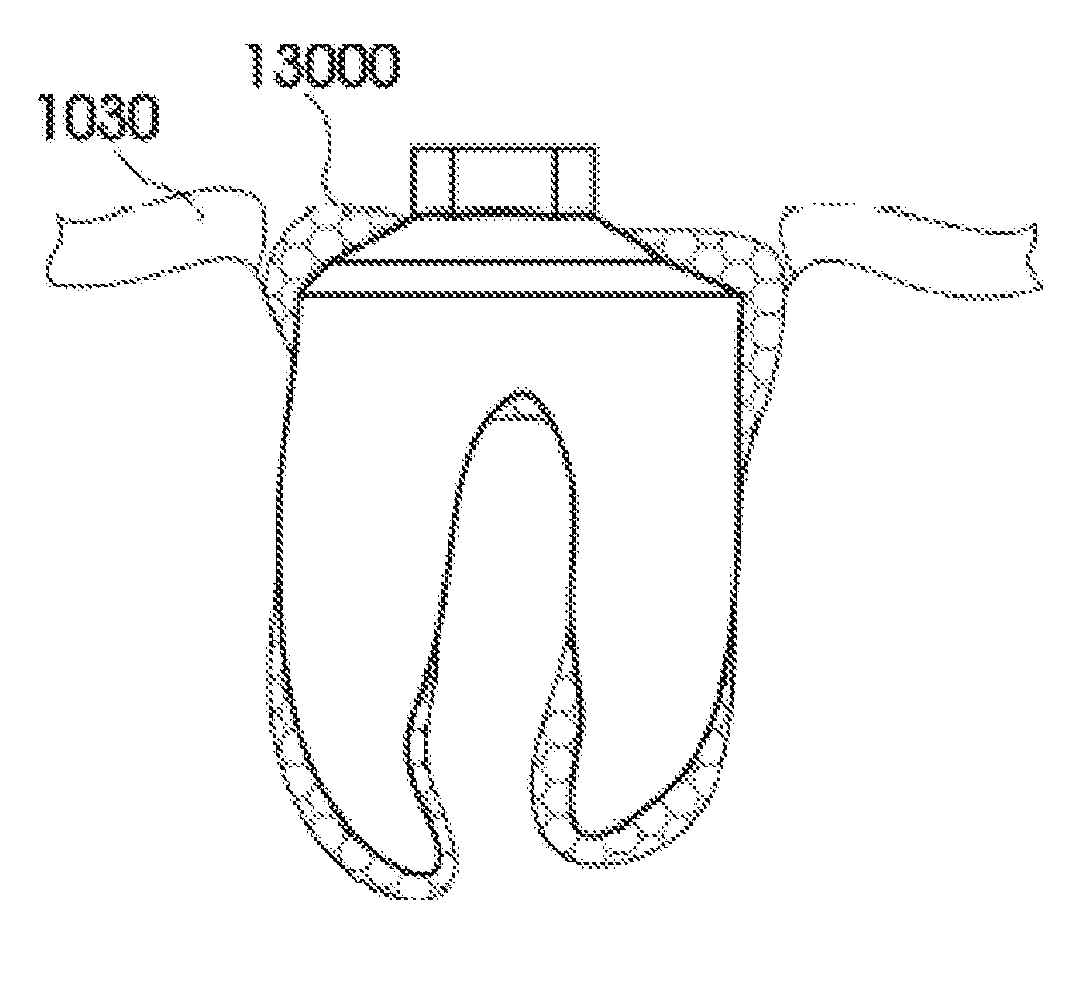
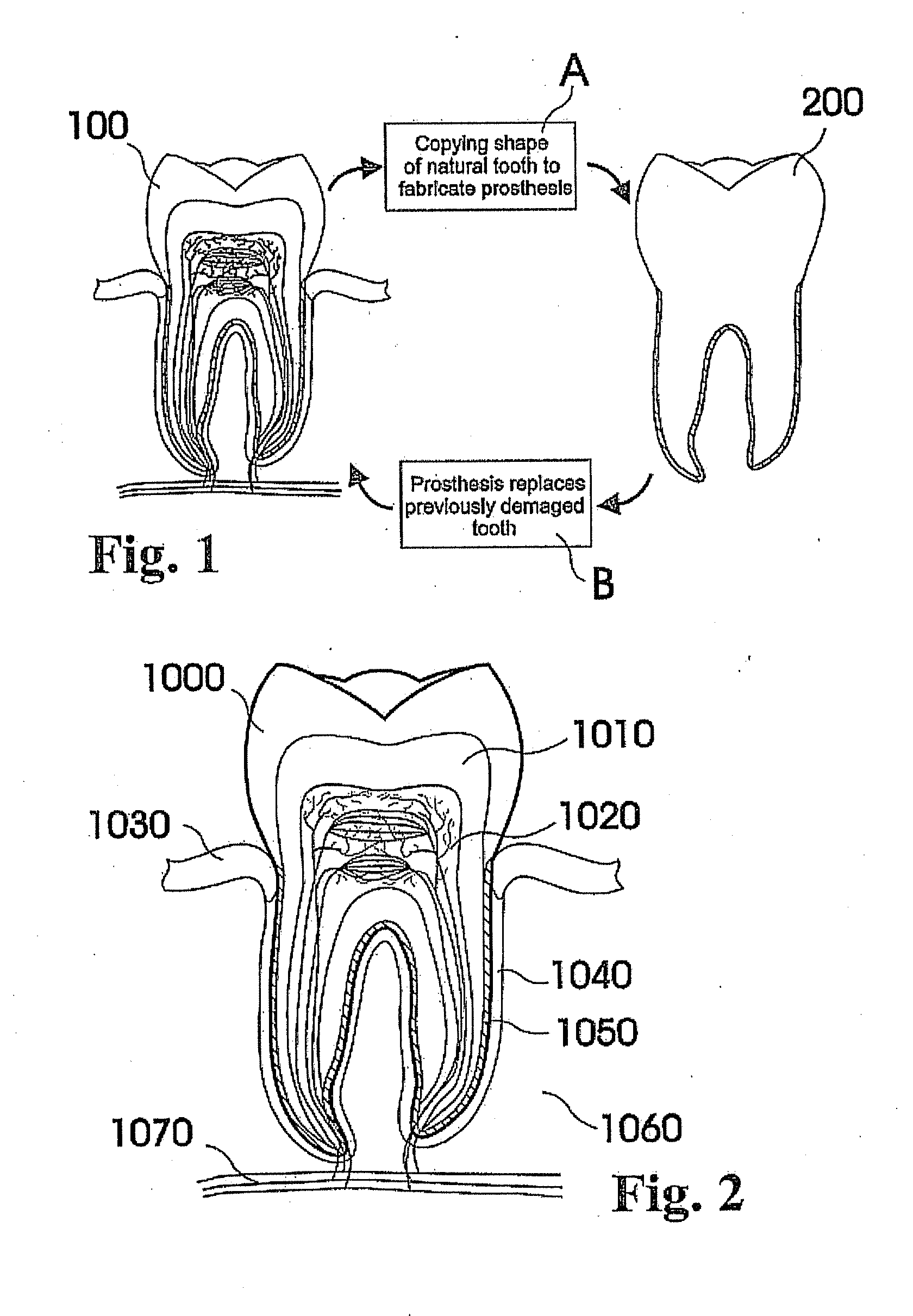
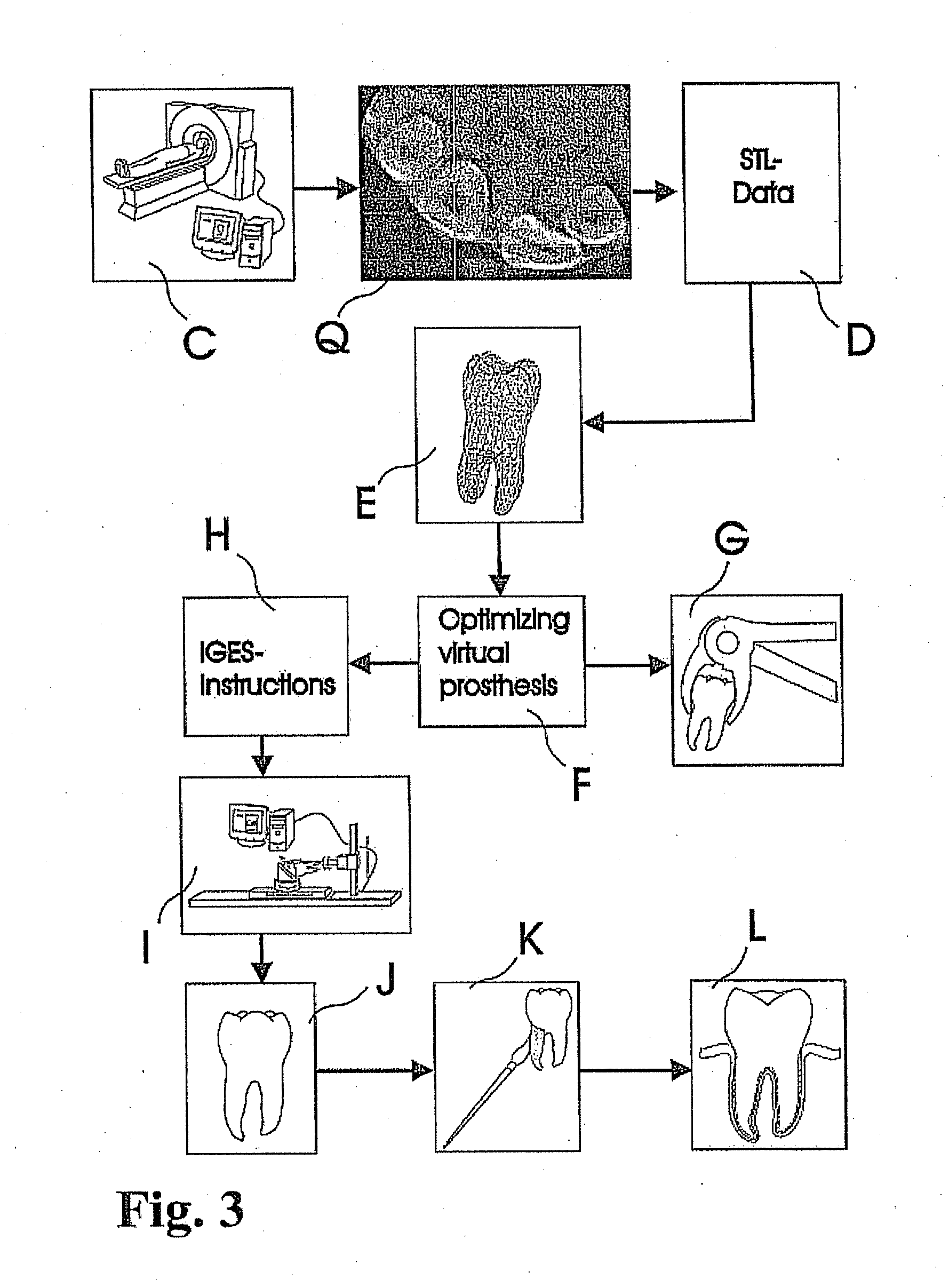
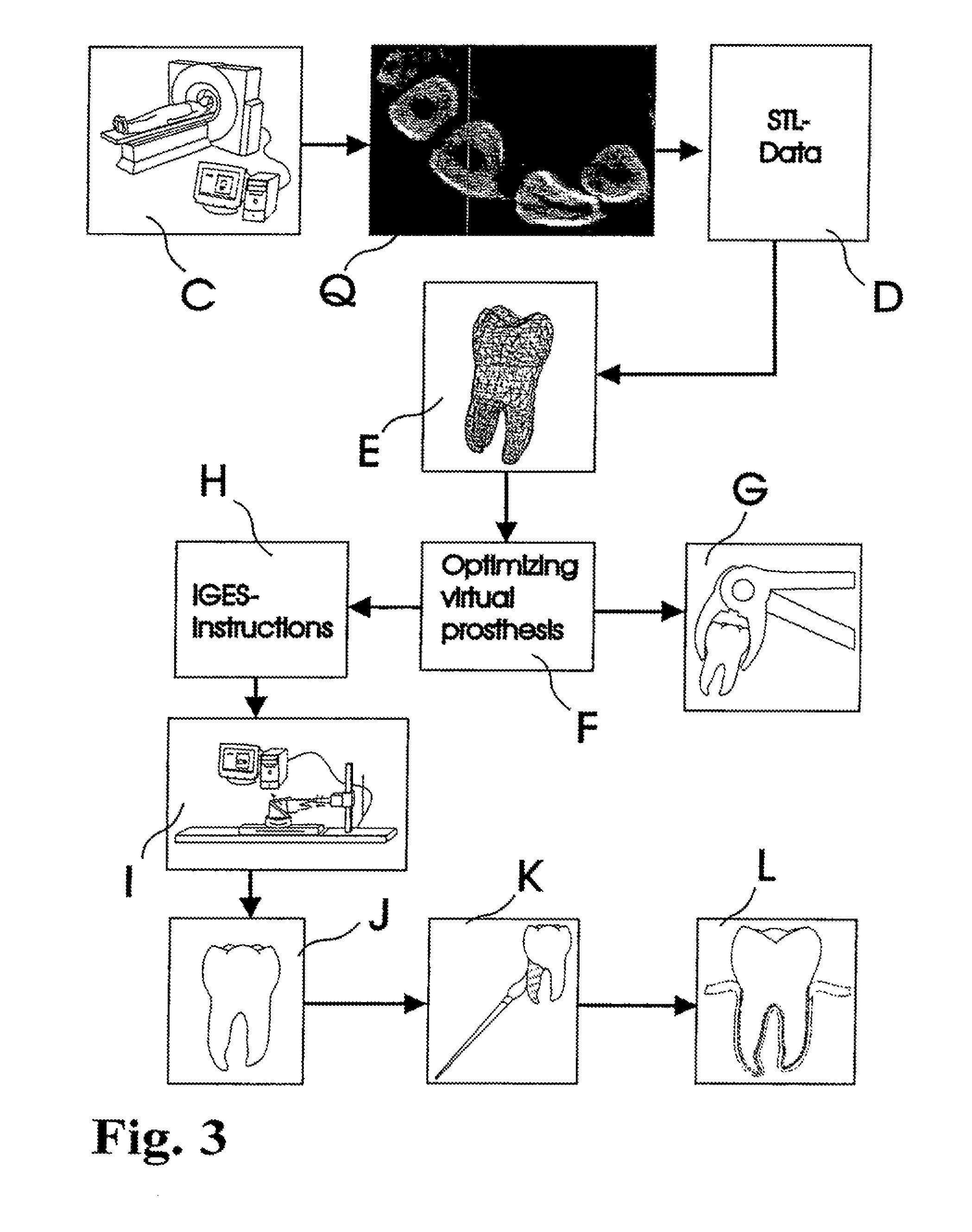
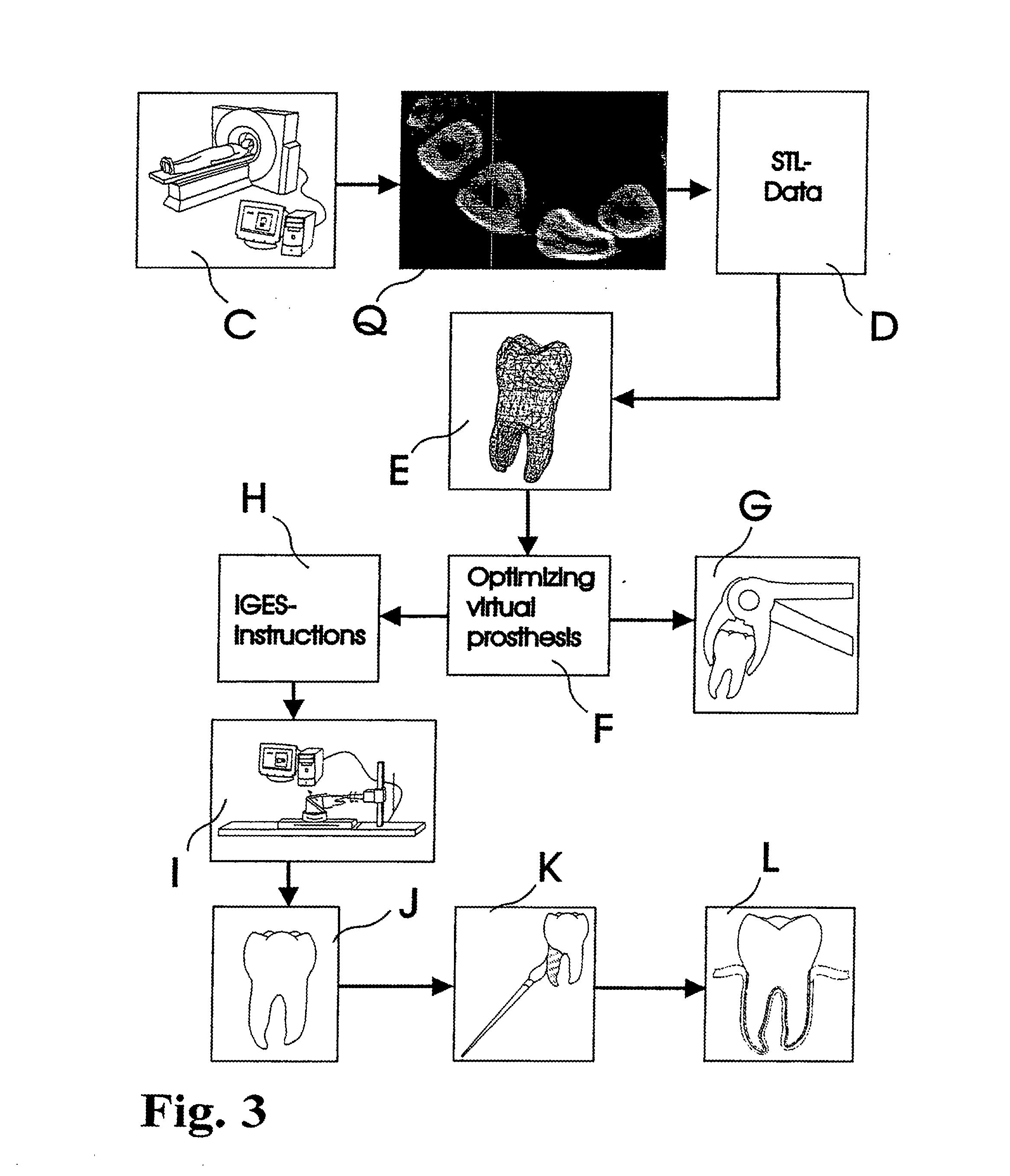
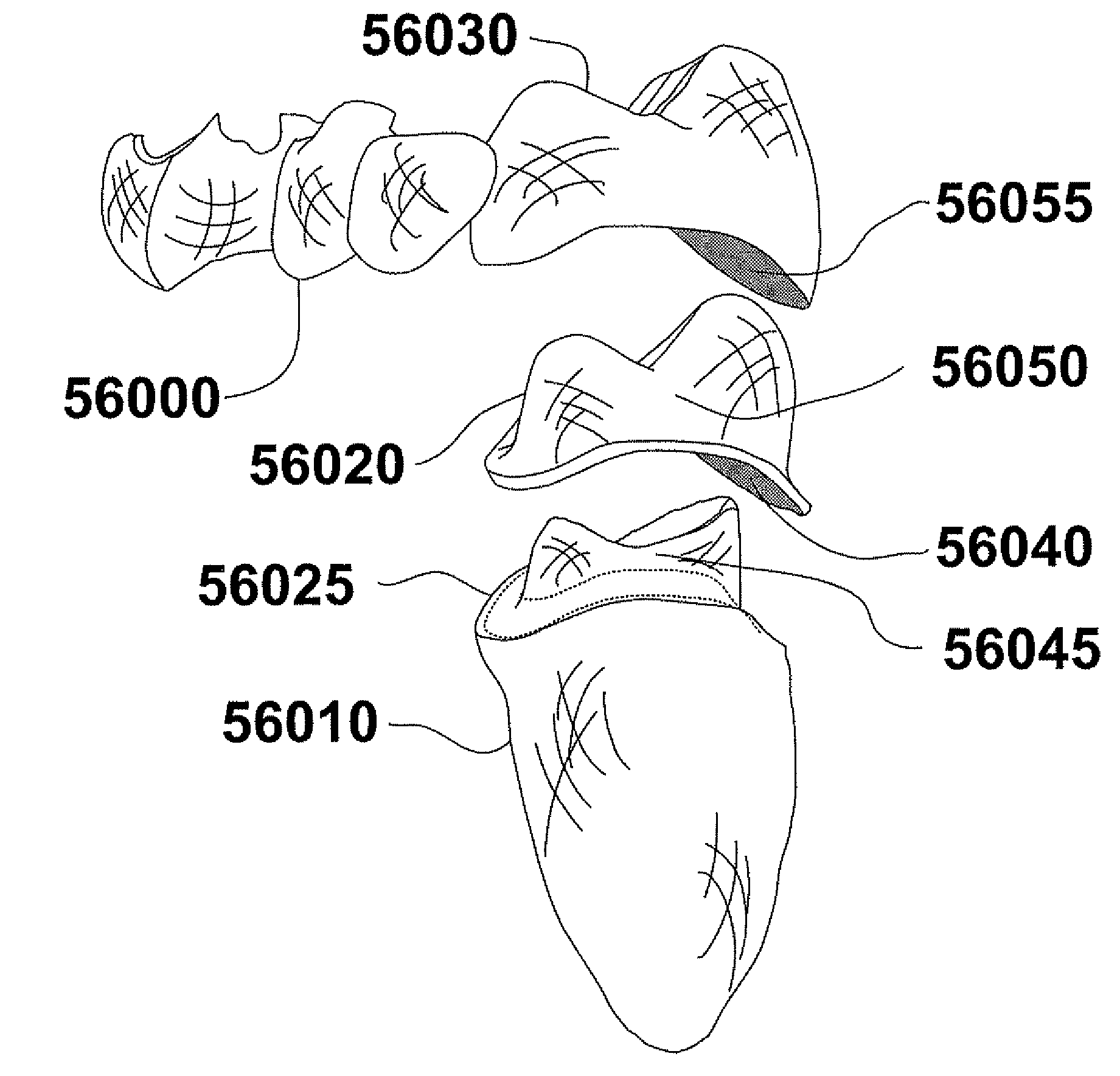
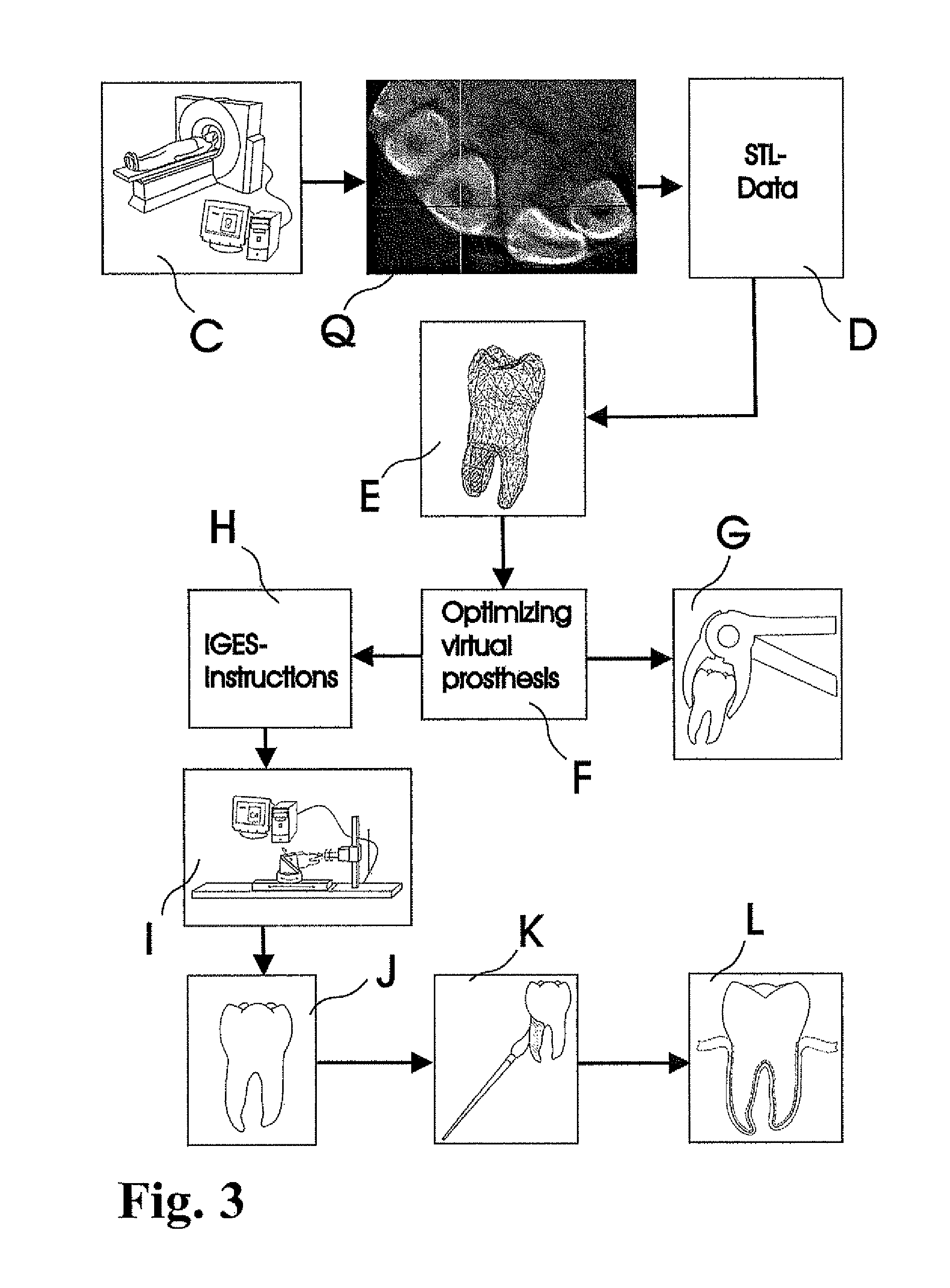
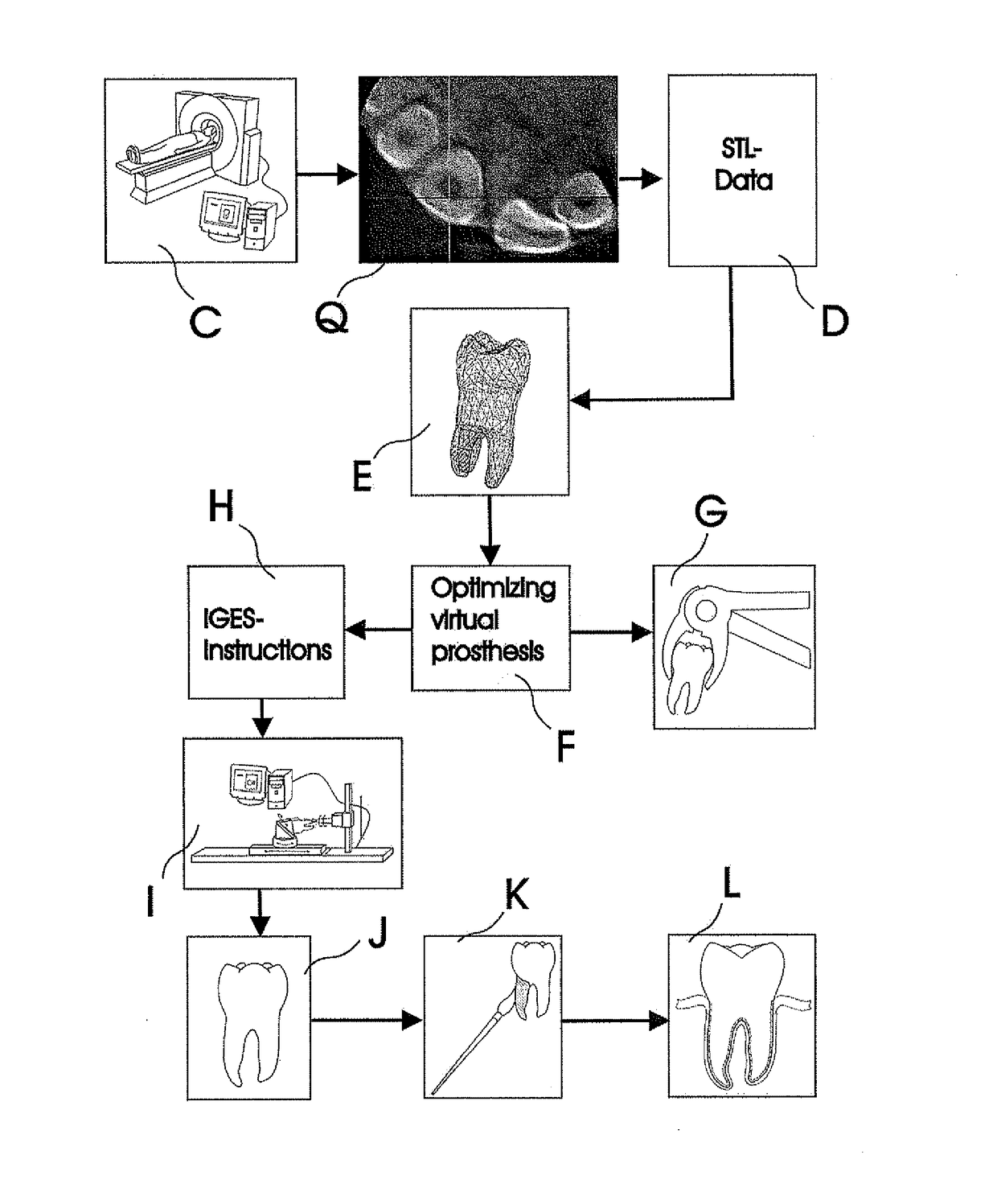
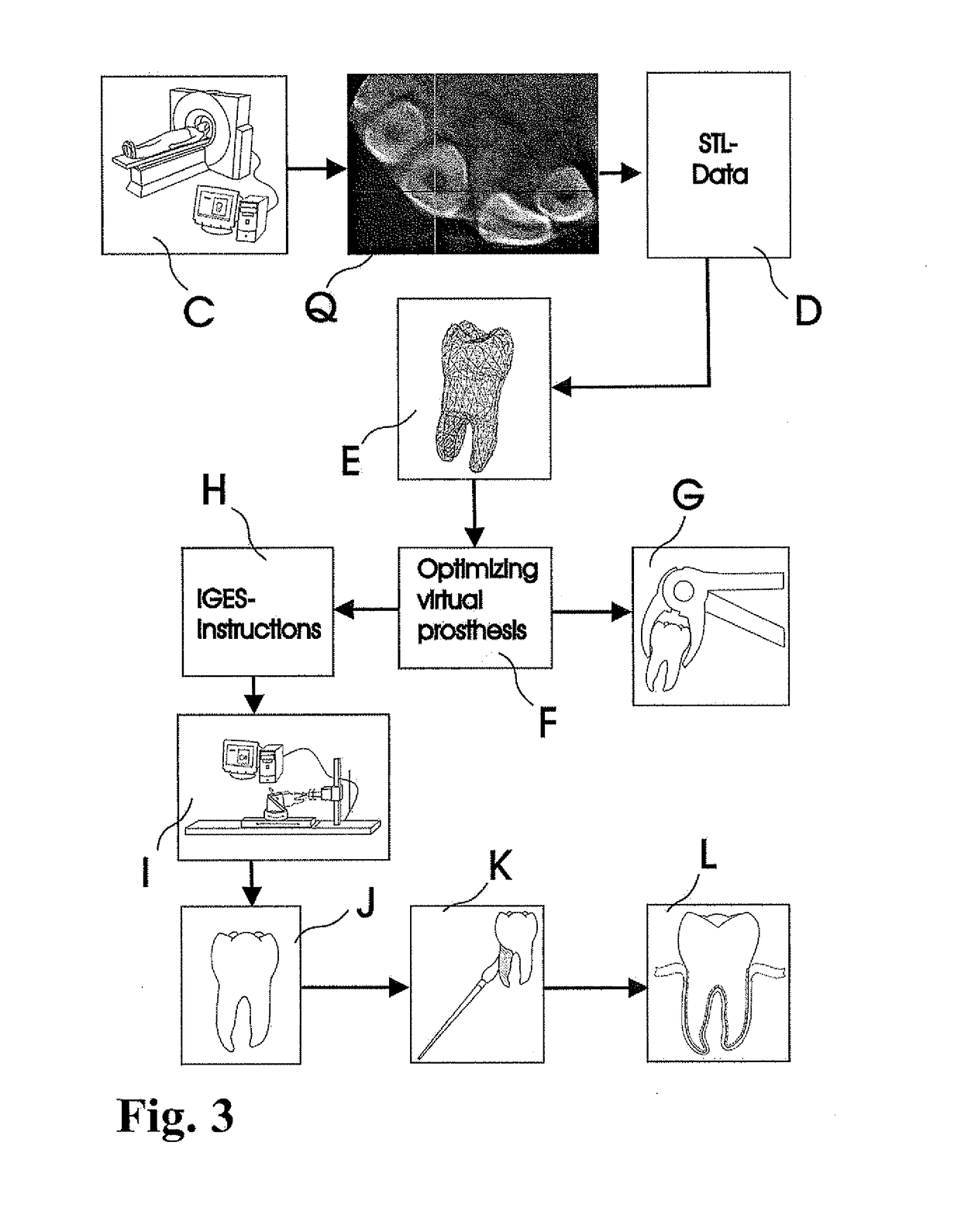
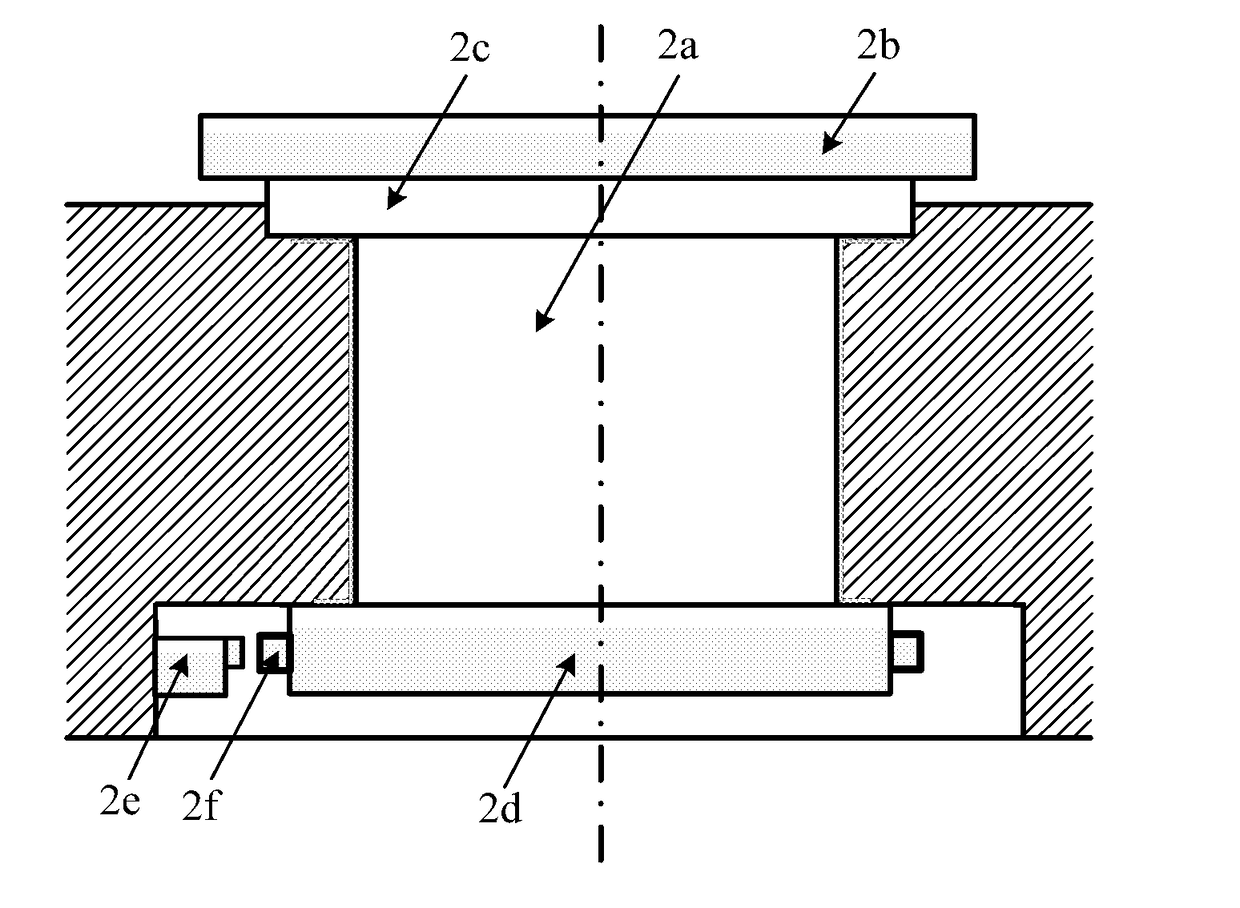
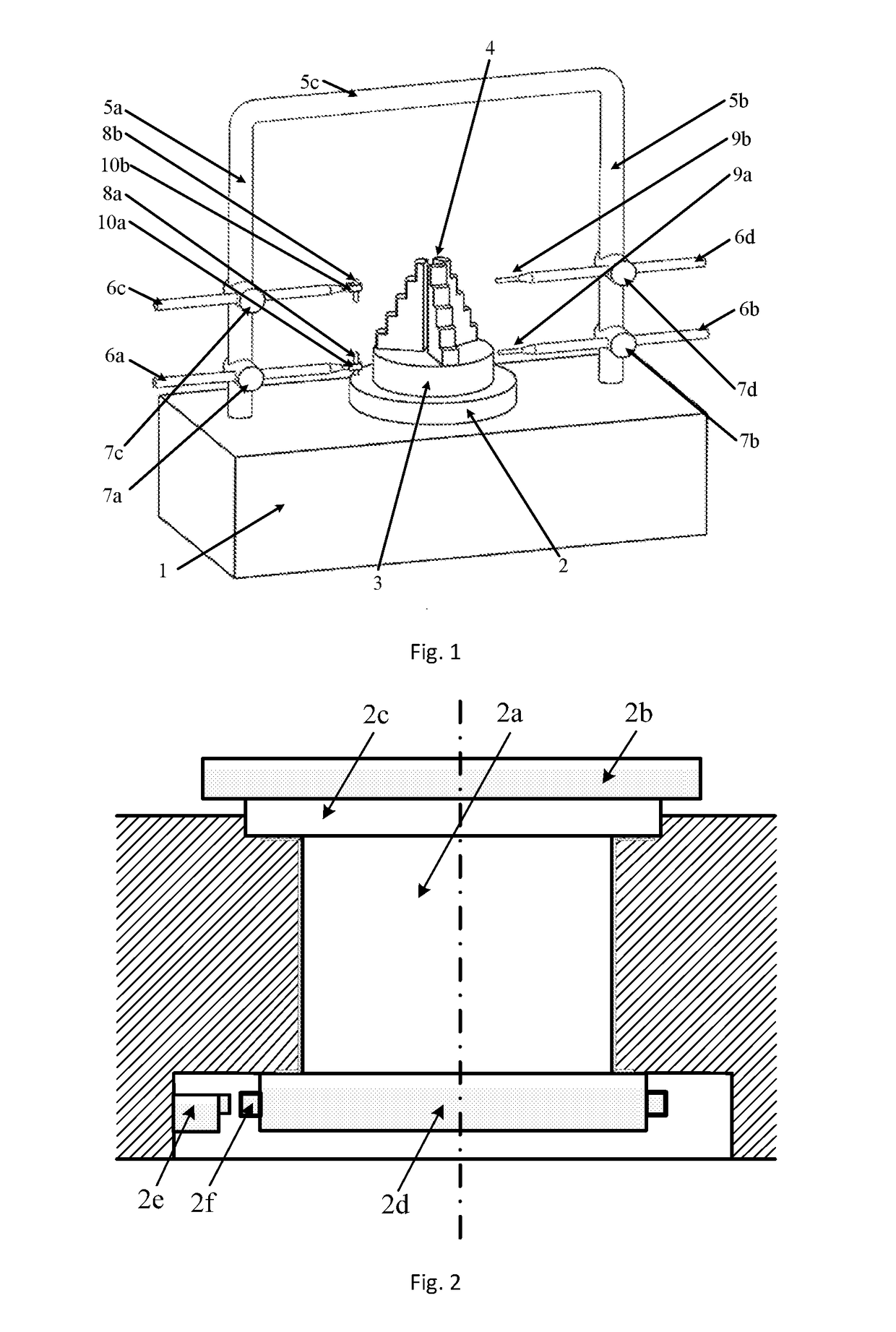
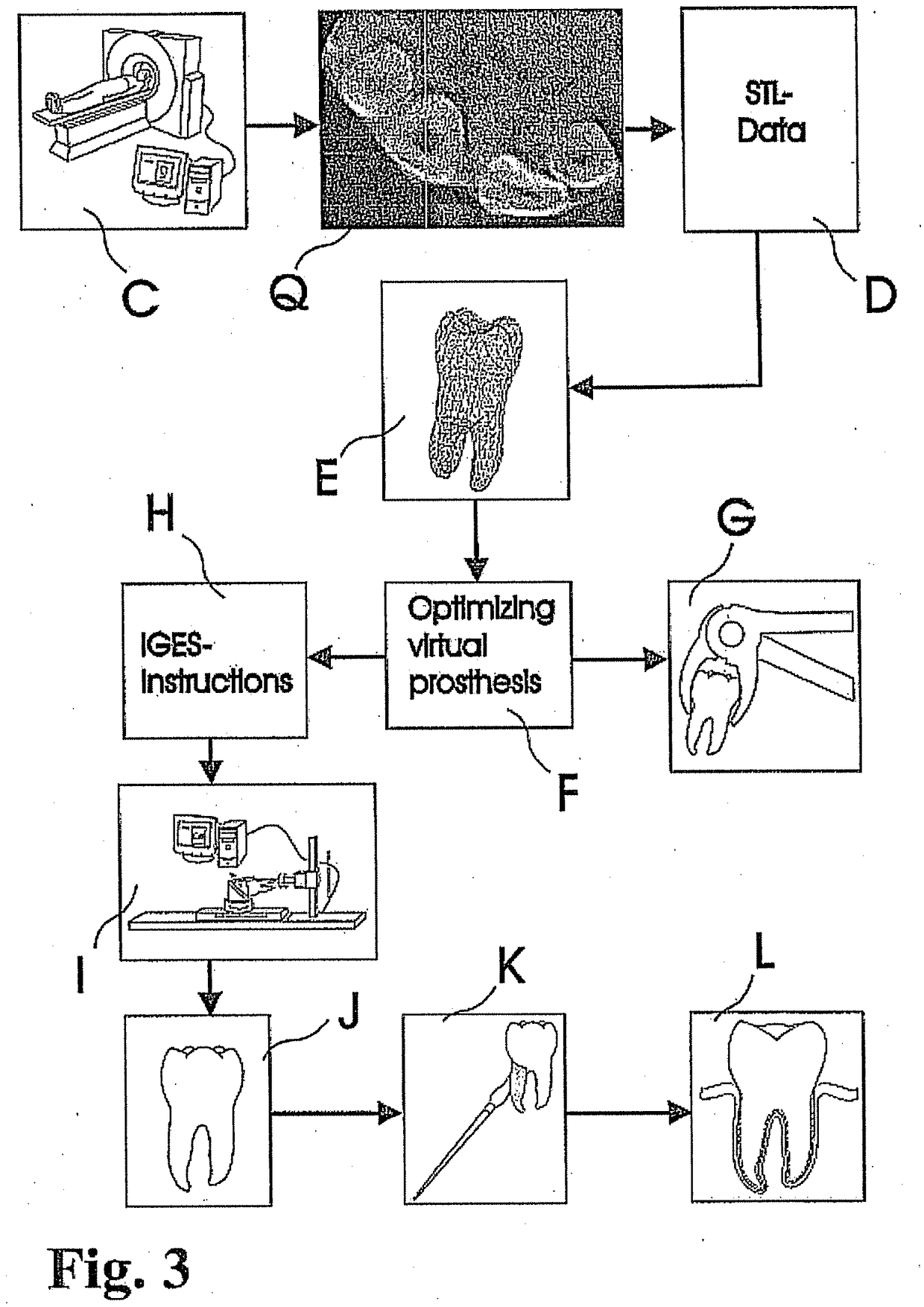
Methods of Designing and Manufacturing Customized Dental Prosthesis for Periodontal or Osseointegration and Related Systems
ActiveUS20120065756A1Great primary stabilityNone have achieved superiorDental implantsImpression capsX ray imageSurface scanning
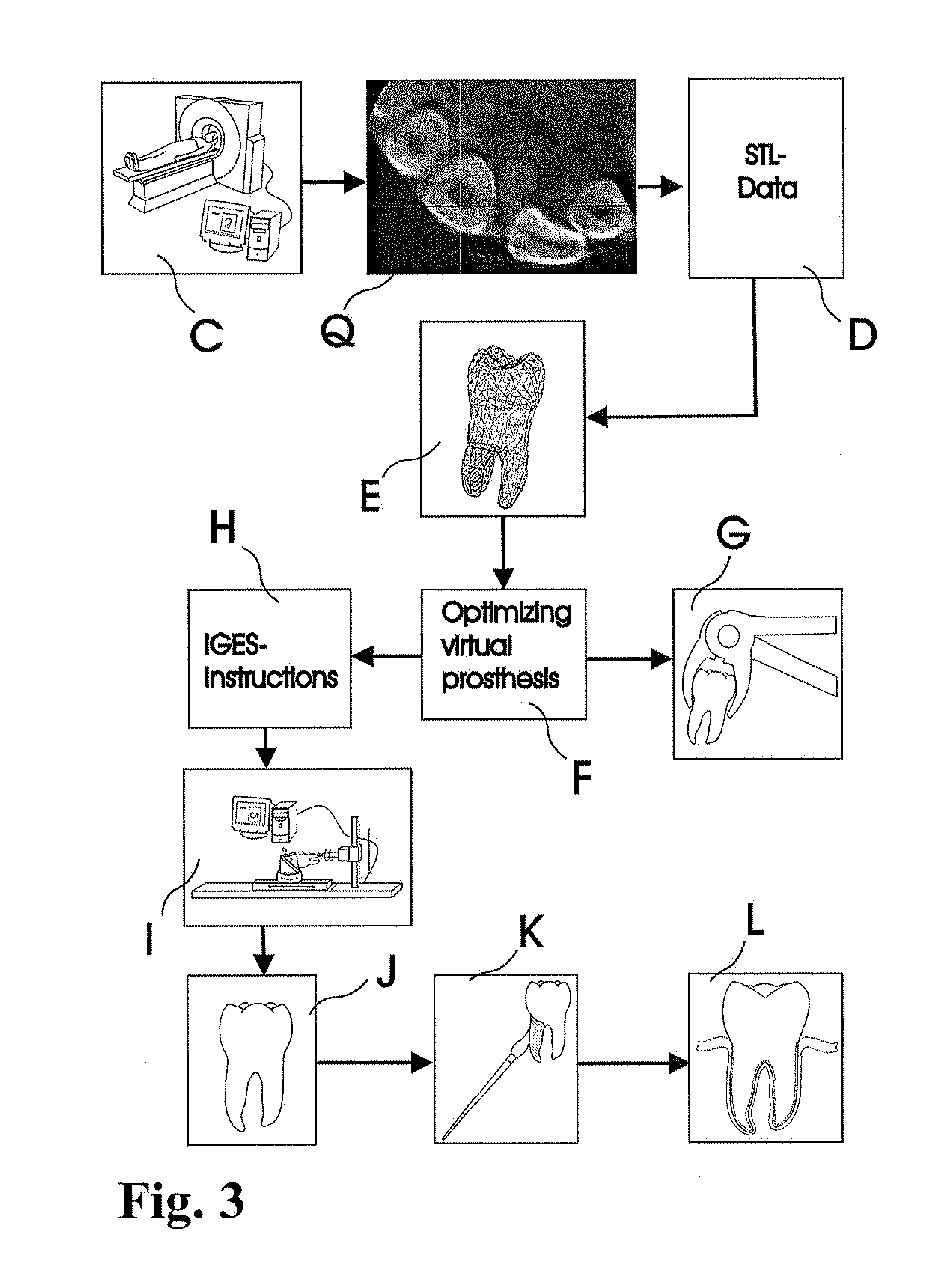
Methods of manufacturing dental prosthesis / implants each to replace a non-functional natural tooth positioned in a jawbone of a specific pre-identified patient are provided. An example method includes the steps of receiving imaging data such as x-ray image data and surface scan data of a dental anatomy and / or a physical impression of the dental anatomy of a specific preidentified patient. The steps can also include forming a three-dimensional virtual model of at least portions of a non-functional natural tooth positioned in the jawbone of the specific pre-identified patient based on the imaging and surface scan data, virtually designing a dental implant based upon the virtual model, exporting the data describing the designed dental implant to a manufacturing machine, and custom manufacturing the dental implant for the specific patient.
Owner:NATURAL DENTAL IMPLANTS
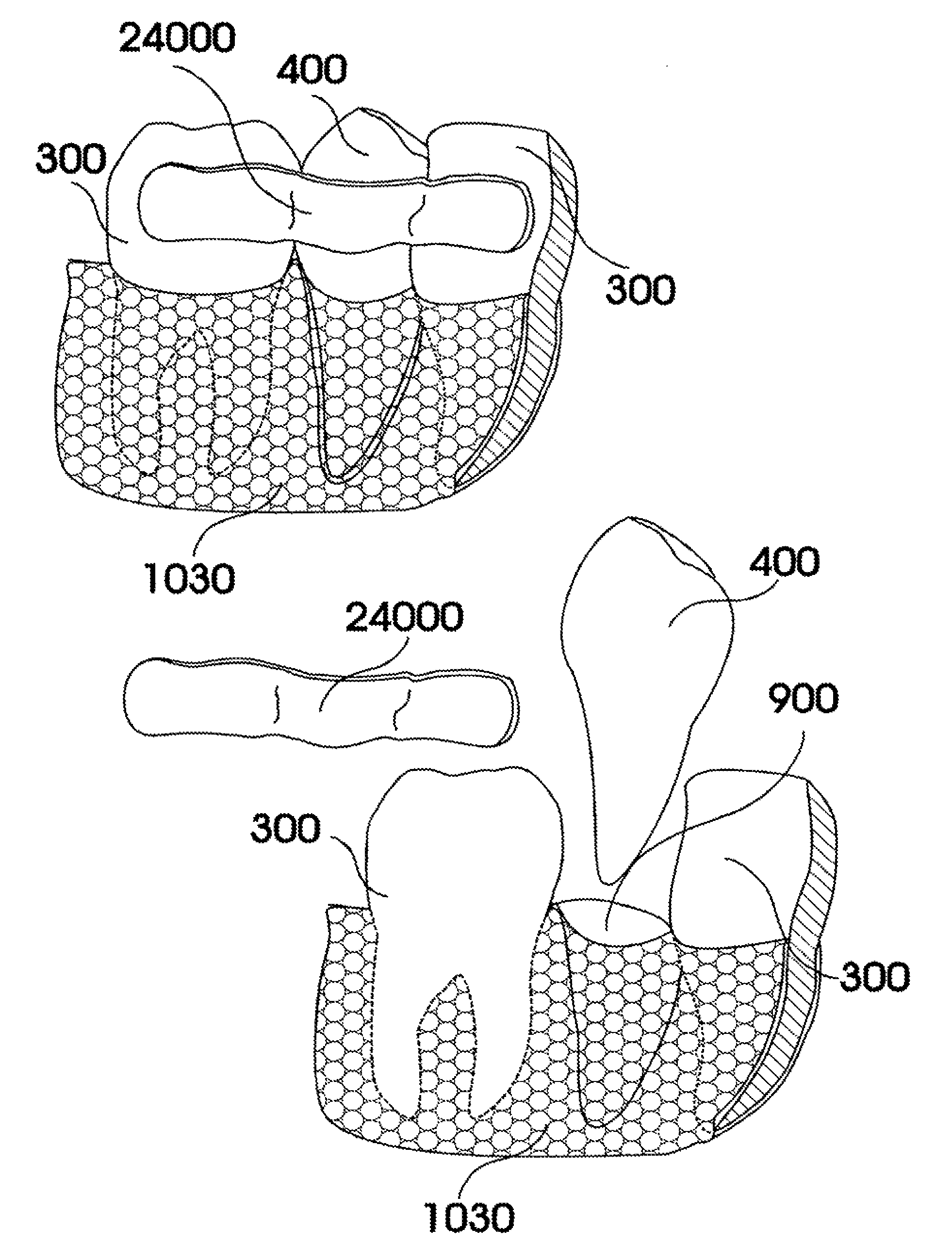
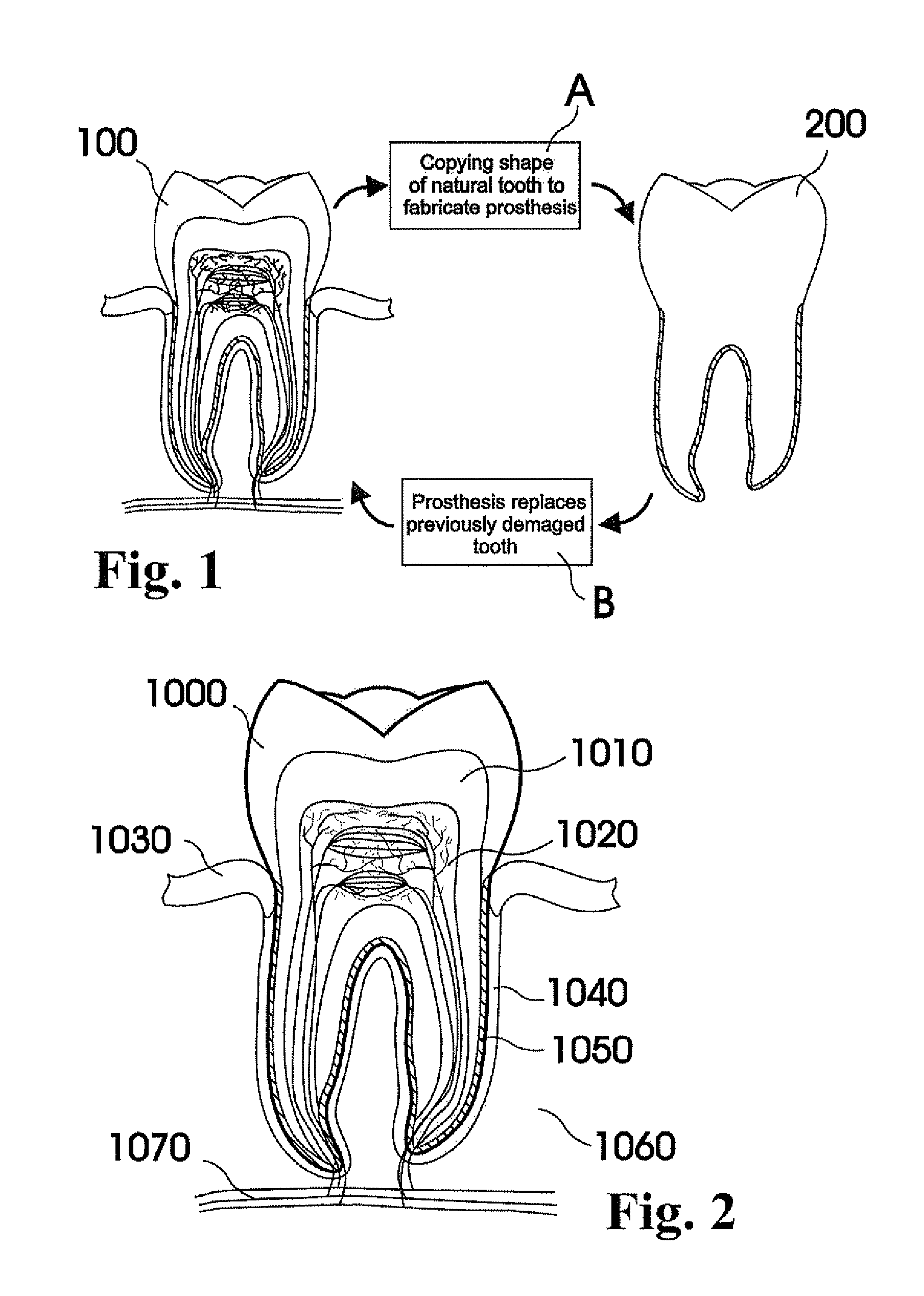
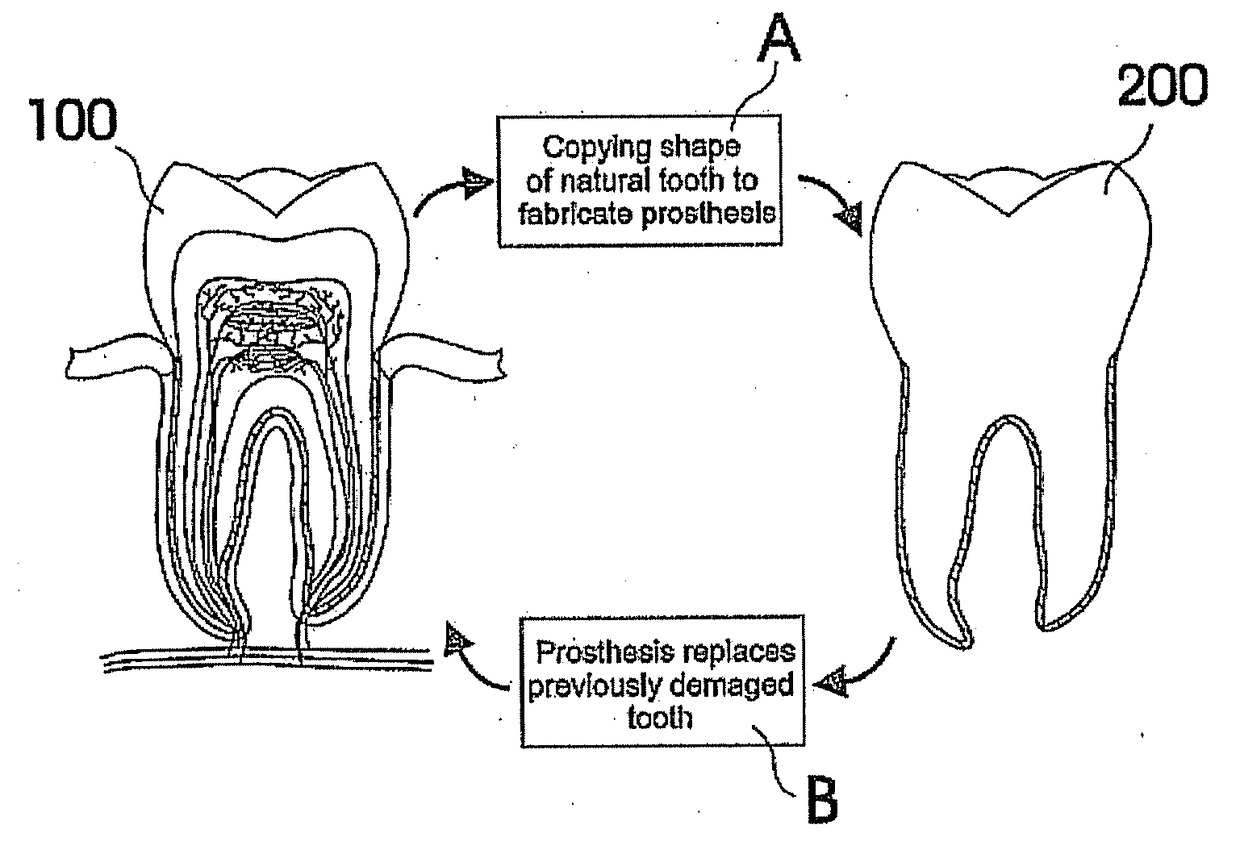
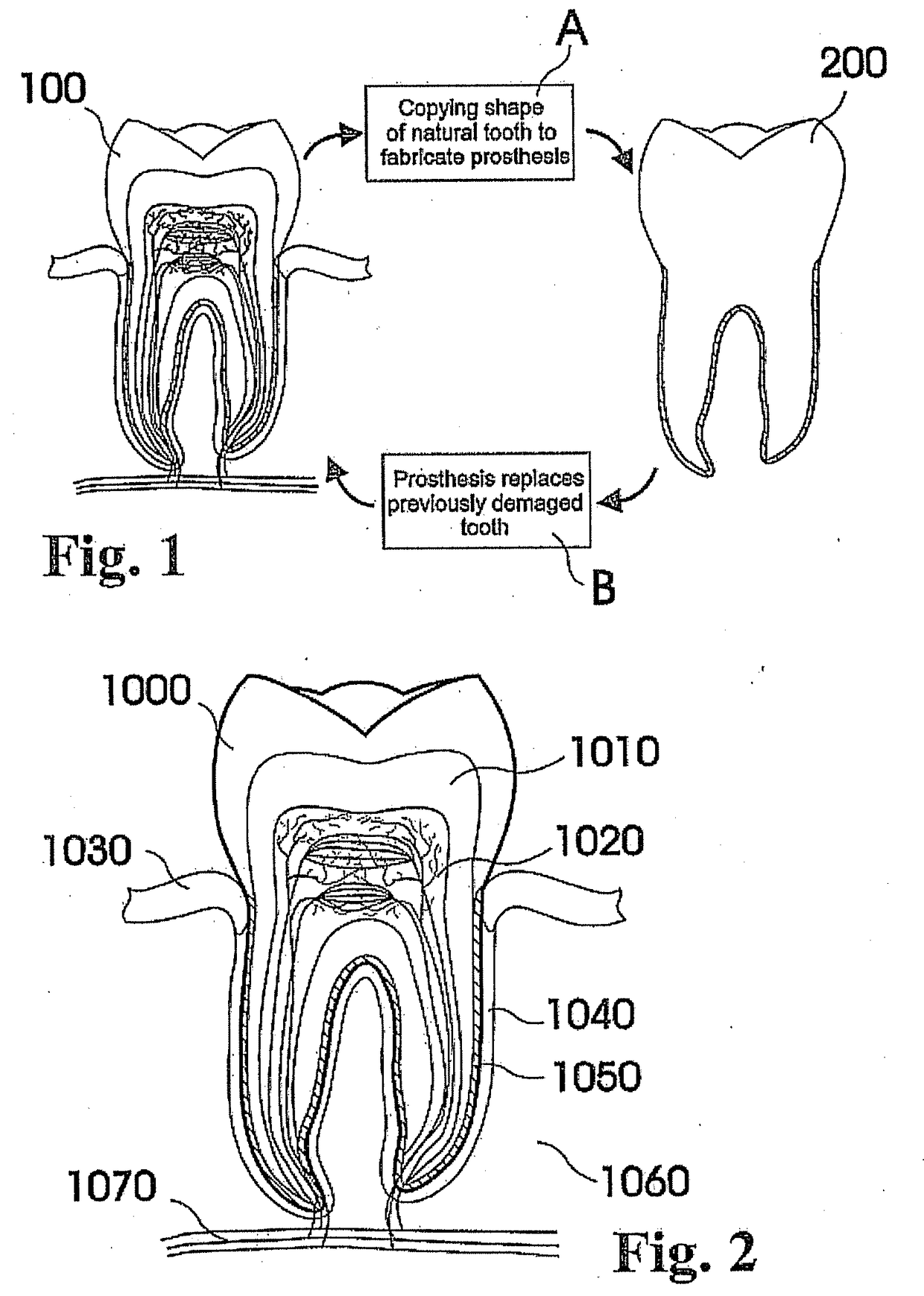
Customized dental prosthesis for periodontal- or osseointegration, and related systems and methods
ActiveUS7708557B2Quality improvementNone have achieved superiorDental implantsAdditive manufacturing apparatusOsseointegrationExtracted tooth
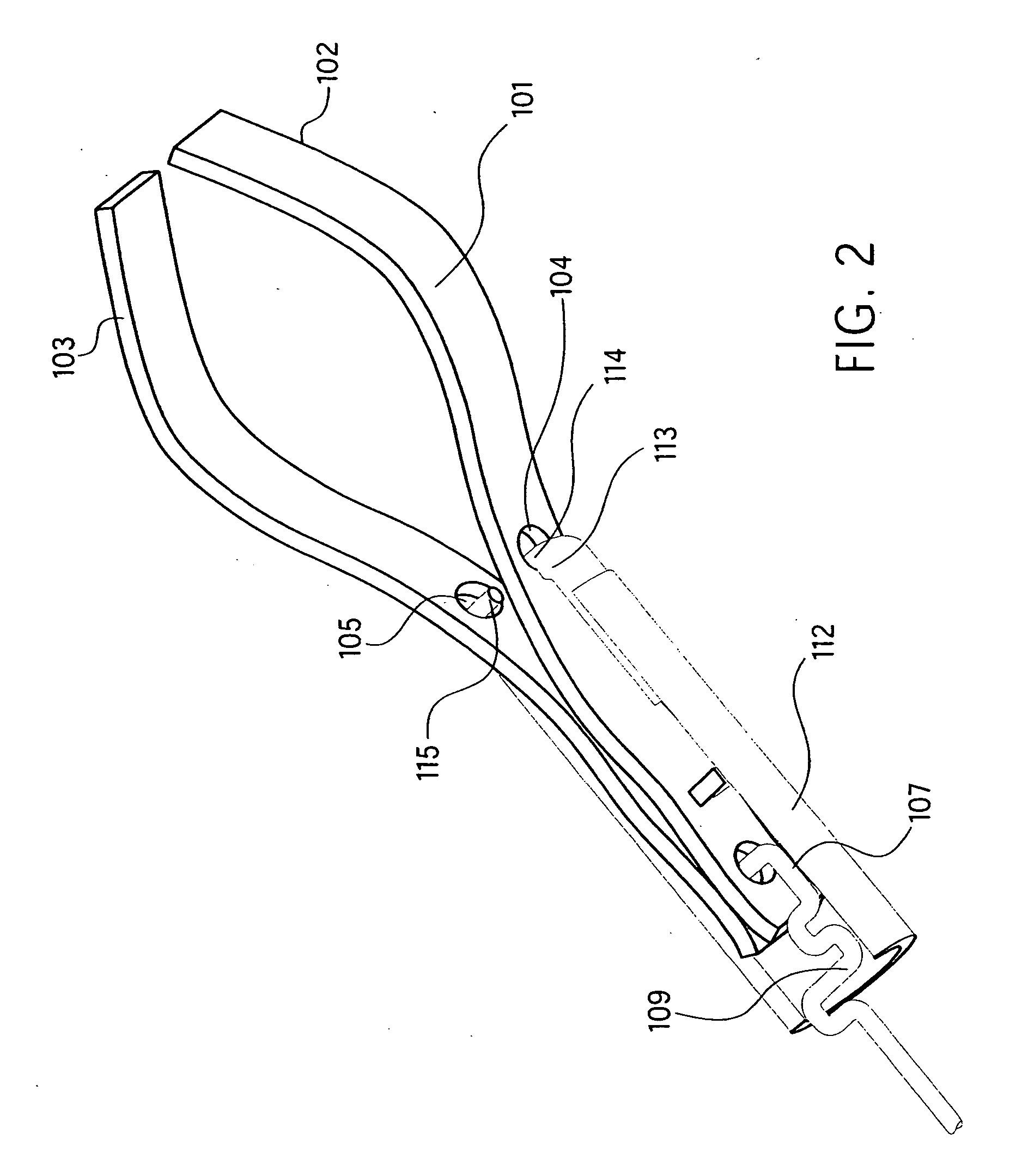
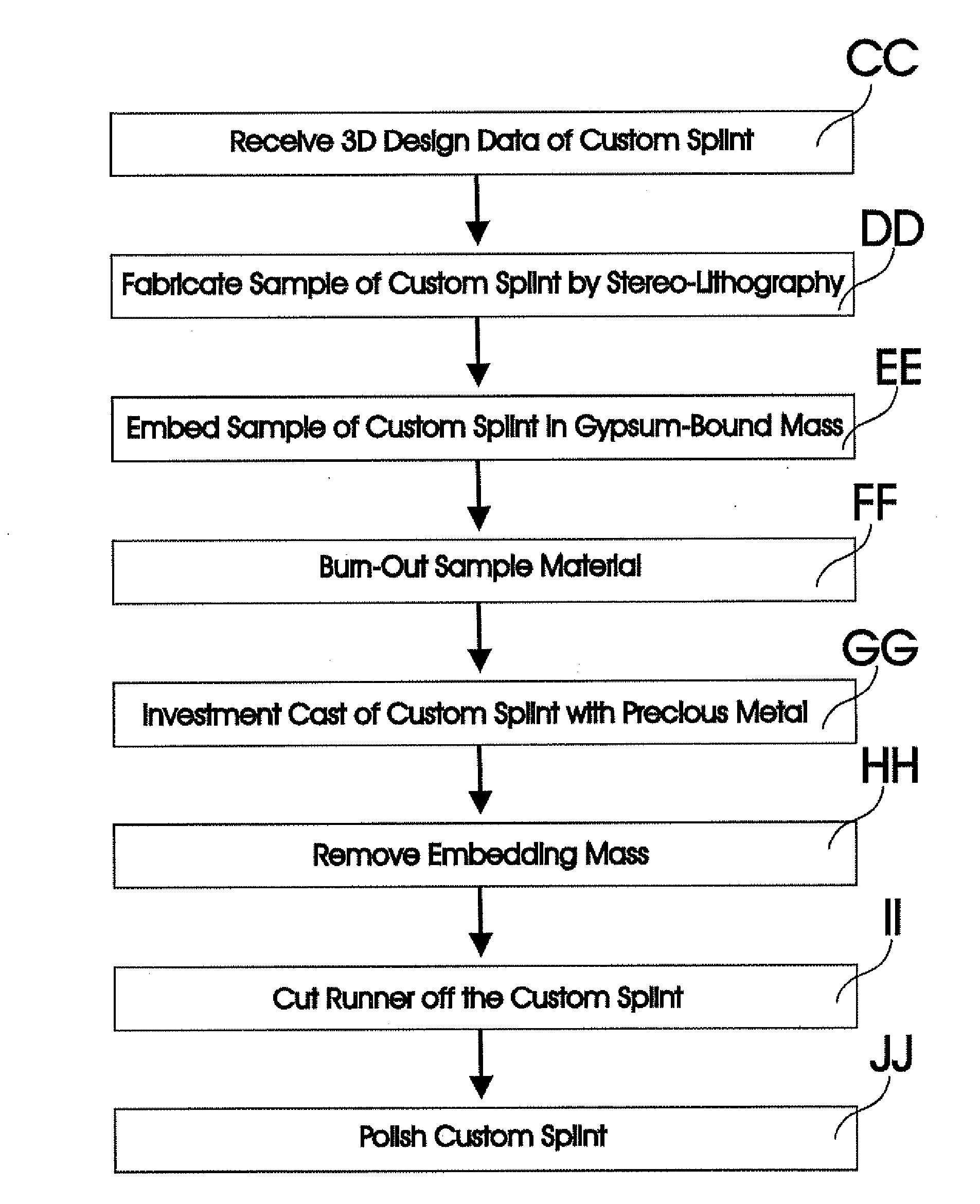
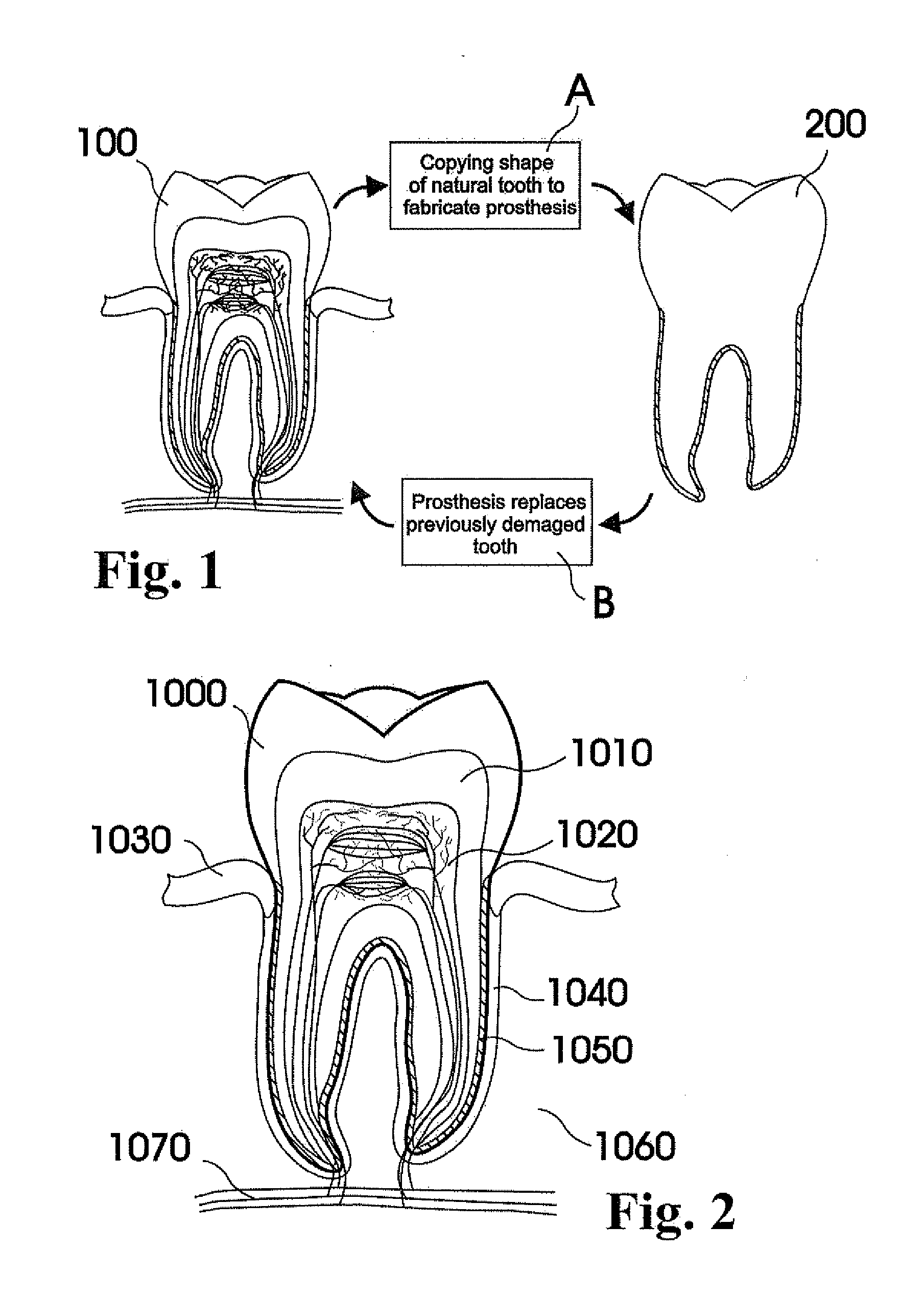
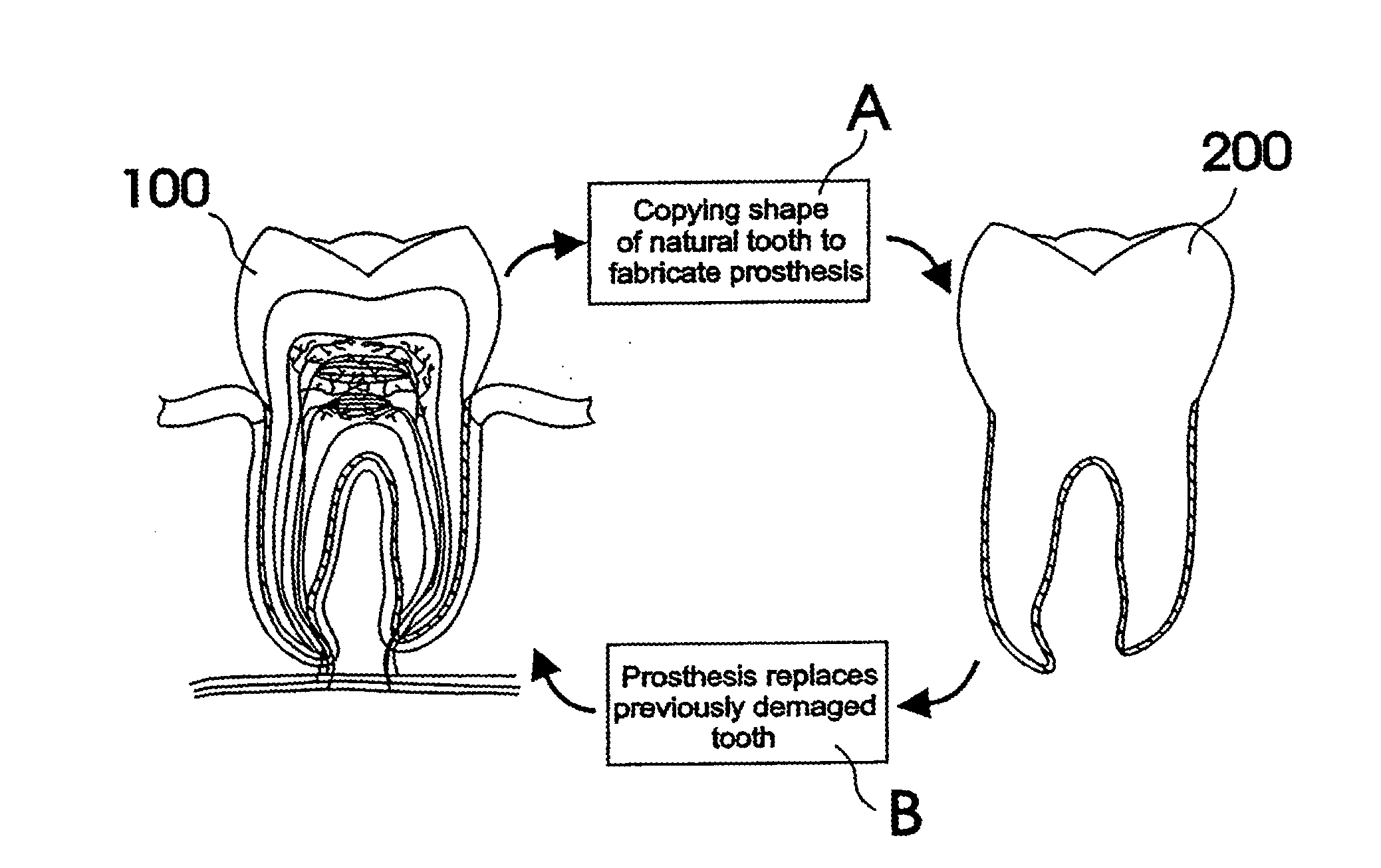
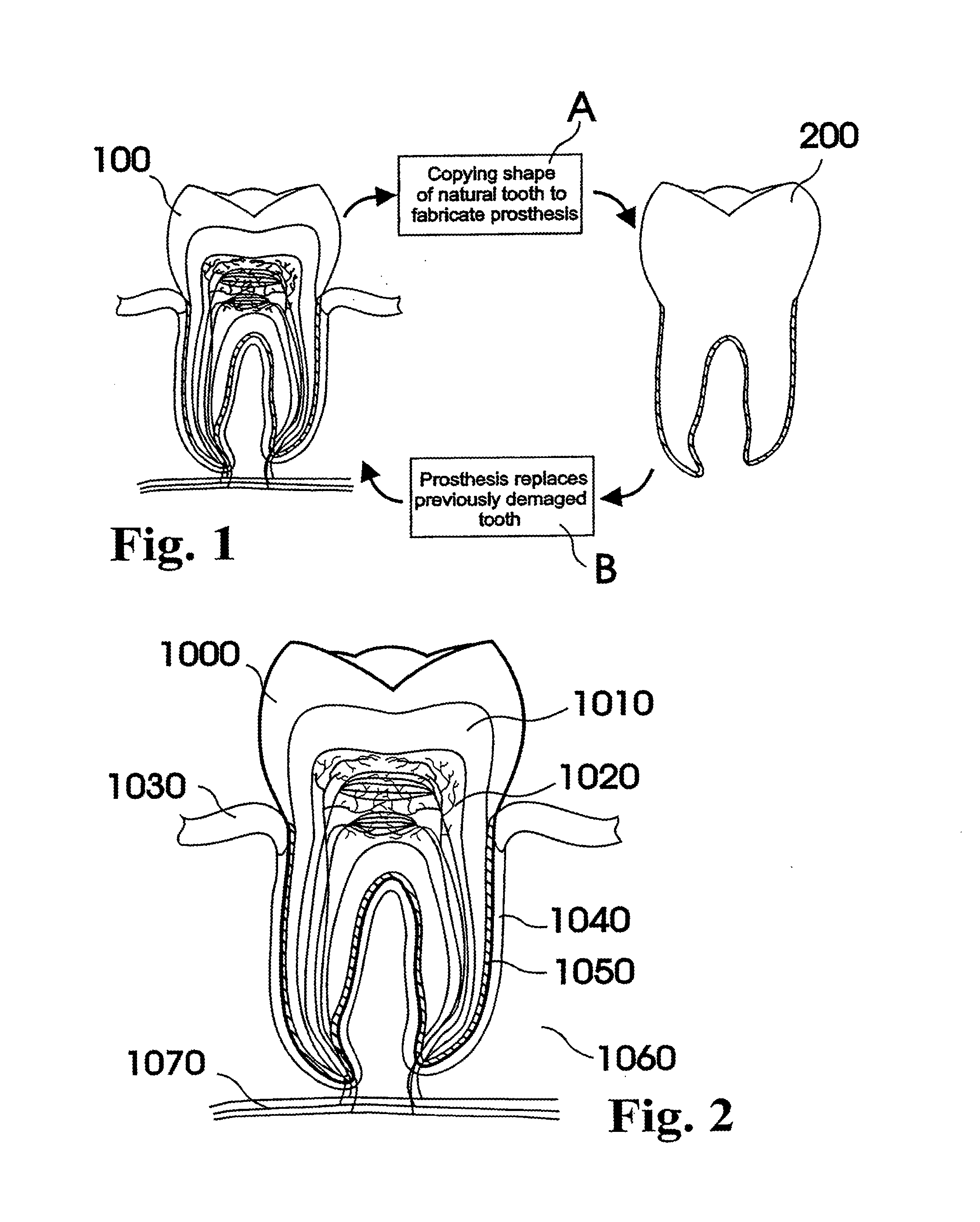
A dental prosthesis for periodontal integration is disclosed. Furthermore a customized dental prosthesis for osseointegration is disclosed having a first manufactured portion shaped to substantially conform to the three-dimensional surface of a root of a tooth to be replaced and a second manufactured portion shaped to substantially conform to the three-dimensional surface of a crown of a tooth to be replaced. Furthermore a customized manufactured splint is disclosed to position and fixate a tooth-shaped prosthesis. Furthermore a CAD / CAM based method of and a system for manufacturing a customized dental prosthesis replacing an extracted tooth is disclosed, where the extracted tooth is scanned regarding its three-dimensional shape and substantially copied using (a) an imaging system in-vitro like a 3D scanner or in-vivo like a cone beam CT system, (b) CNC machinery and (c) biocompatible material that is suitable to be integrated into the extraction socket and at least partially adopted by the existing tissue forming the socket.
Owner:NATURAL DENTAL IMPLANTS
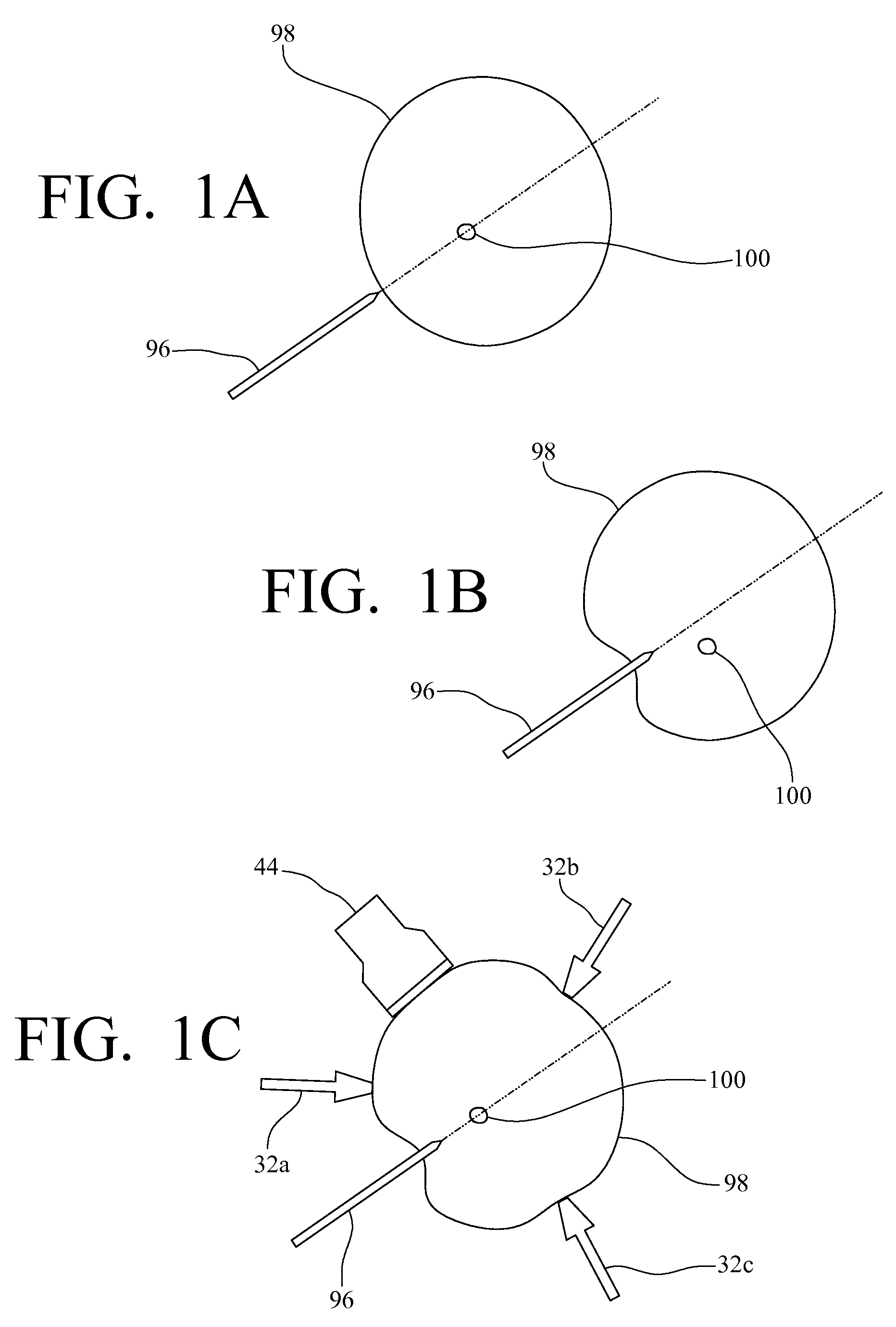
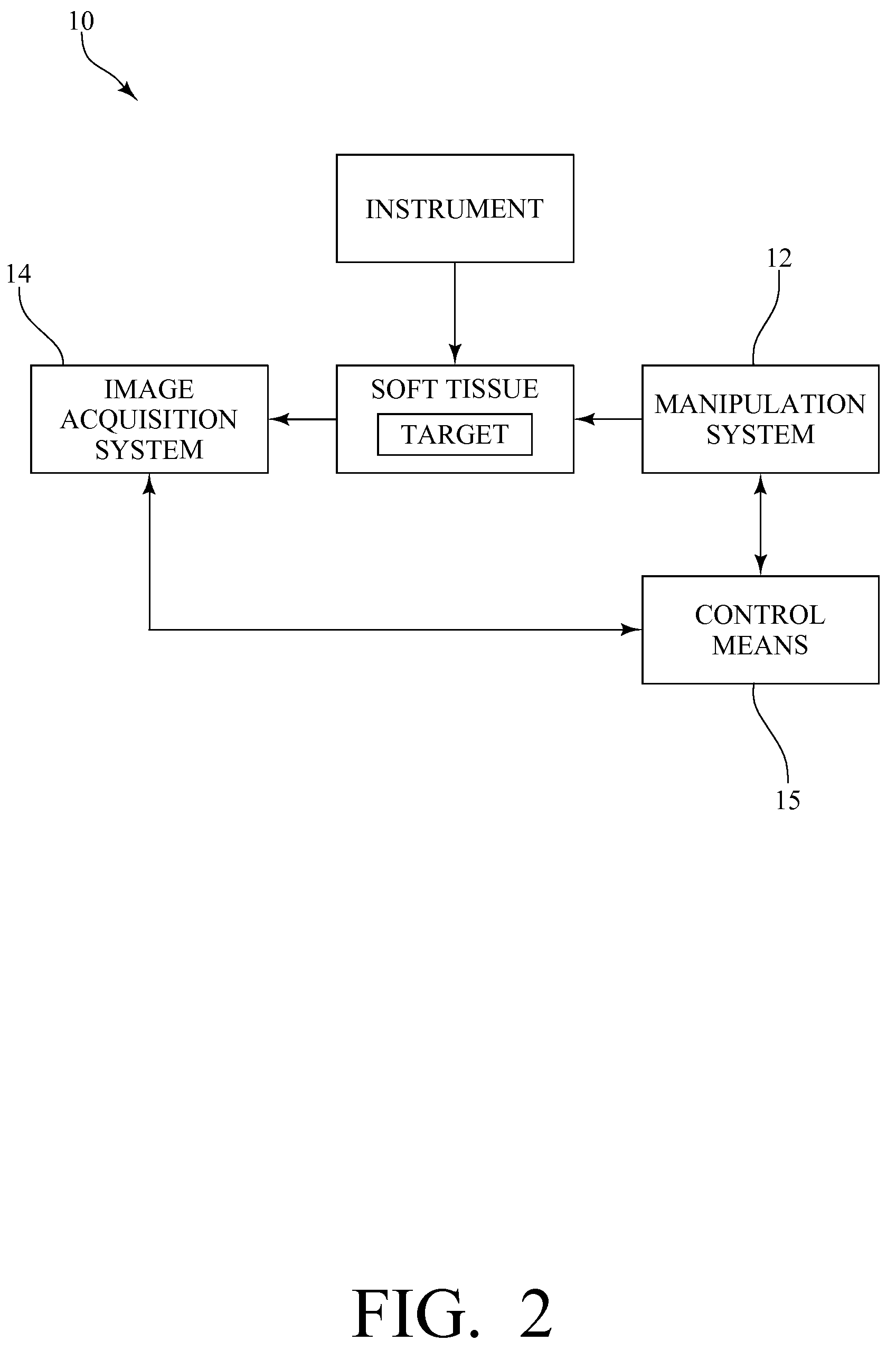
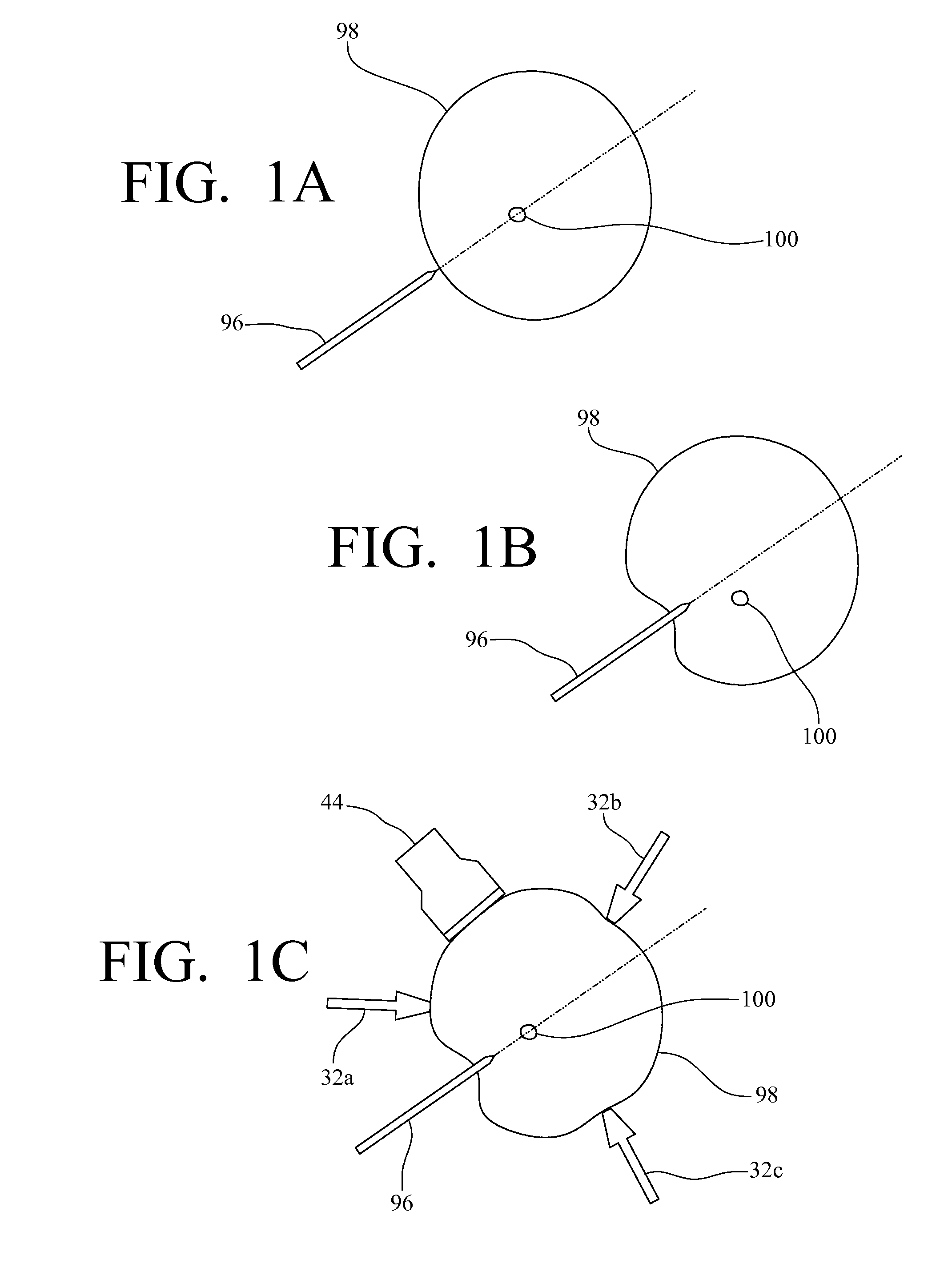
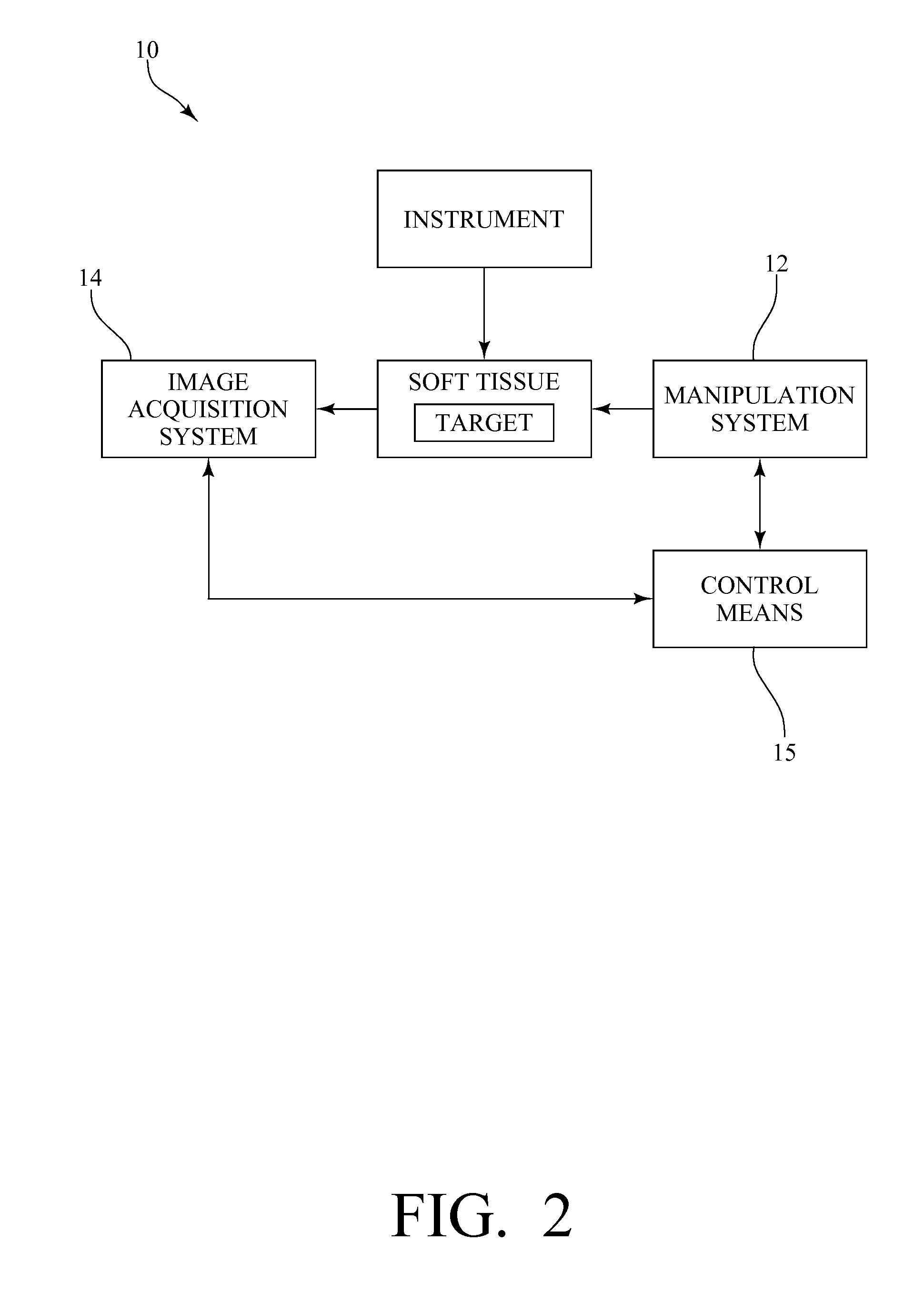
System, method and device for positioning a target located within soft tissue in a path of an instrument
InactiveUS8066644B2Minimize tracking errorReduce fatigueUltrasonic/sonic/infrasonic diagnosticsSurgical needlesUltrasound imagingRadiology
A system, method and device for positioning a target located within soft tissue in a path of an instrument inserted into the soft tissue includes: a manipulation system including a plurality of force applicators positioned around the soft tissue containing the target; an image acquisition system including an imaging probe for obtaining data for generating an image of the soft tissue containing the target; a detection means for detecting deflection of the target using the data from the imaging probe; and a control means for actuating the plurality of force applicators to apply forces on the soft tissue in response to a detected deflection of the target to move the target back in line with the path of the instrument. In an exemplary embodiment, the soft tissue is a breast, the imaging probe is an ultrasound imaging probe, and the instrument is a biopsy needle.
Owner:VANDERBILT UNIV
Customized dental prosthesis for periodontal or osseointegration and related systems
ActiveUS20120064489A1Great primary stabilityNone have achieved superiorDental implantsImpression capsNatural toothOsseointegration
Custom dental prosthesis or implants each individually designed and manufactured to replace nonfunctional natural teeth positioned in a jawbone of a specific pre-identified patient are provided. An example dental prosthesis / implant includes a dental implant body having a prosthesis interface formed therein to receive an occlusally-facing dental prosthesis component. The prosthesis interface has a custom three-dimensional surface shape positioned and formed to create a form locking fit with respect to the occlusally-facing dental prosthesis component when positioned thereon.
Owner:NATURAL DENTAL IMPLANTS
Customized dental prosthesis for periodontal or osseointegration and related systems and methods
ActiveUS8602780B2Quality improvementNone have achieved superiorDental implantsImpression capsOsseointegrationProsthesis
Dental prosthesis, systems, and methods of forming and using a dental prosthesis, are provided. An example of a dental prosthesis includes a first manufactured portion shaped to substantially conform to the three-dimensional surface of a root of a tooth to be replaced and a second manufactured portion shaped to substantially conform to the three-dimensional surface of a crown of the tooth. Furthermore, customized manufactured splints to position and fixate a tooth-shaped prosthesis, are provided. Furthermore, a CAD / CAM based methods of and a systems for manufacturing a customized dental prosthesis are provided. The tooth can be scanned to determine its three-dimensional shape and substantially copied using an imaging system in-vitro like a 3D scanner or in-vivo like a cone beam CT system and CNC machinery. Biocompatible material that is suitable to be integrated into the extraction socket and adopted by existing tissue forming the socket can be manufactured or engineered.
Owner:RTRS INVESTMENT LLC
Rock specimen and method for testing direct tensile strength of the same
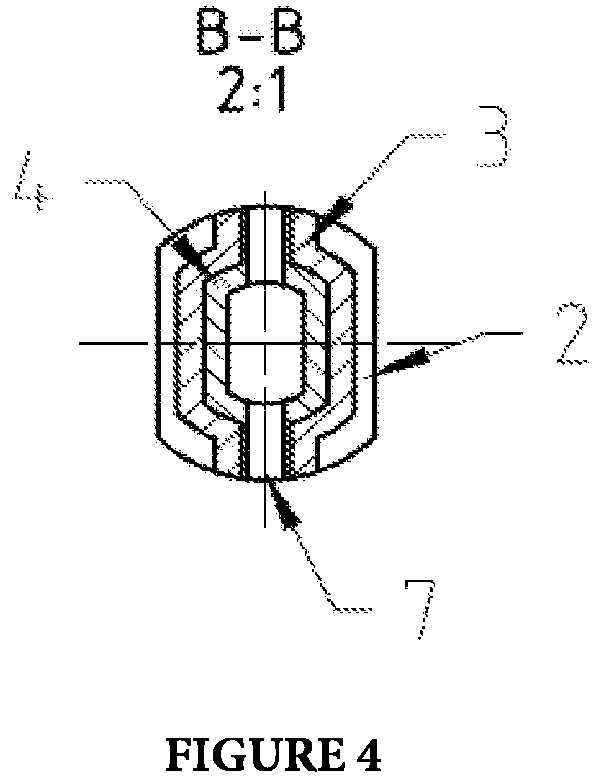
ActiveUS20160103047A1High measurement accuracyHigh success rateLayered productsMaterial strength using tensile/compressive forcesPrismRock sample
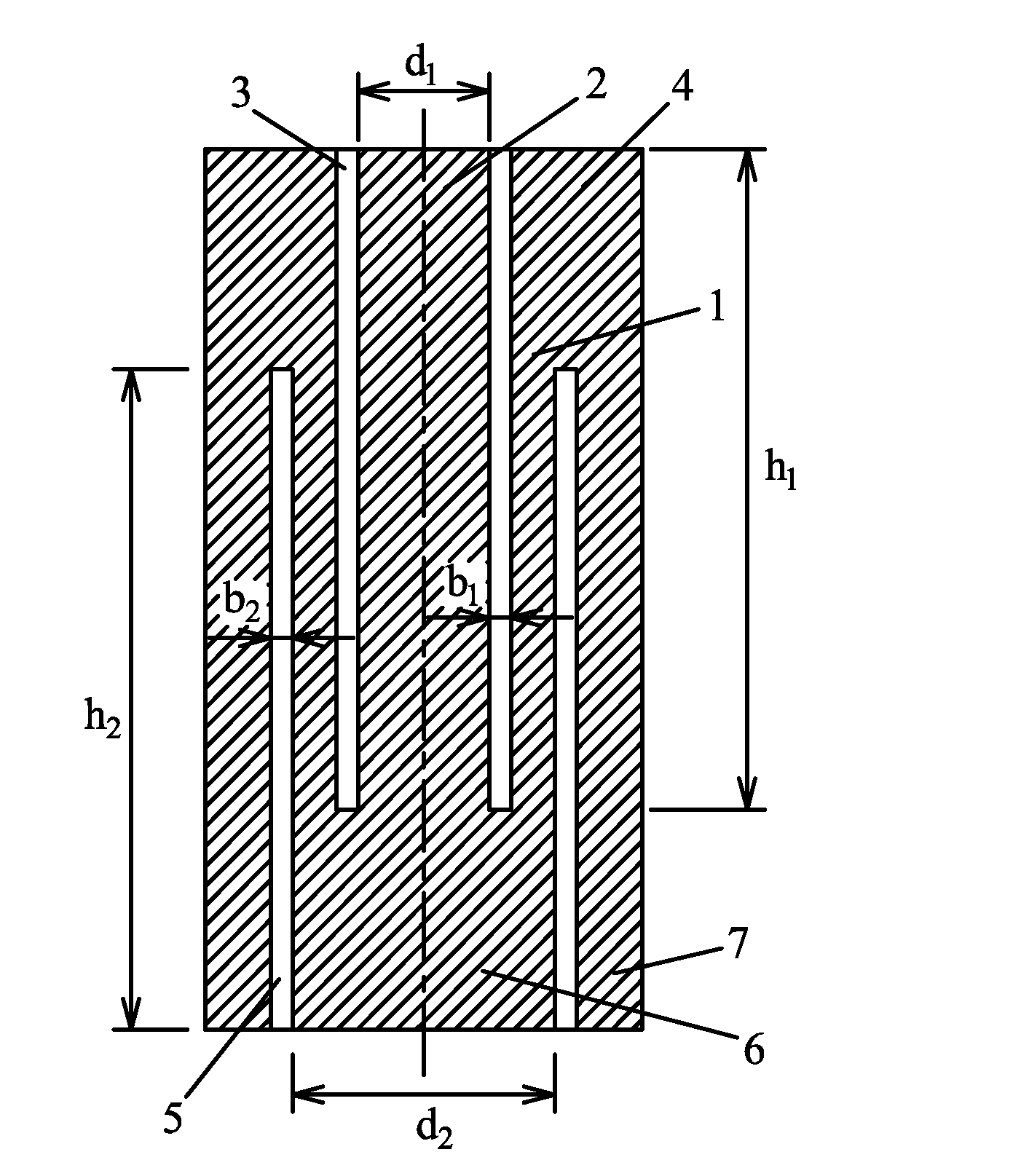
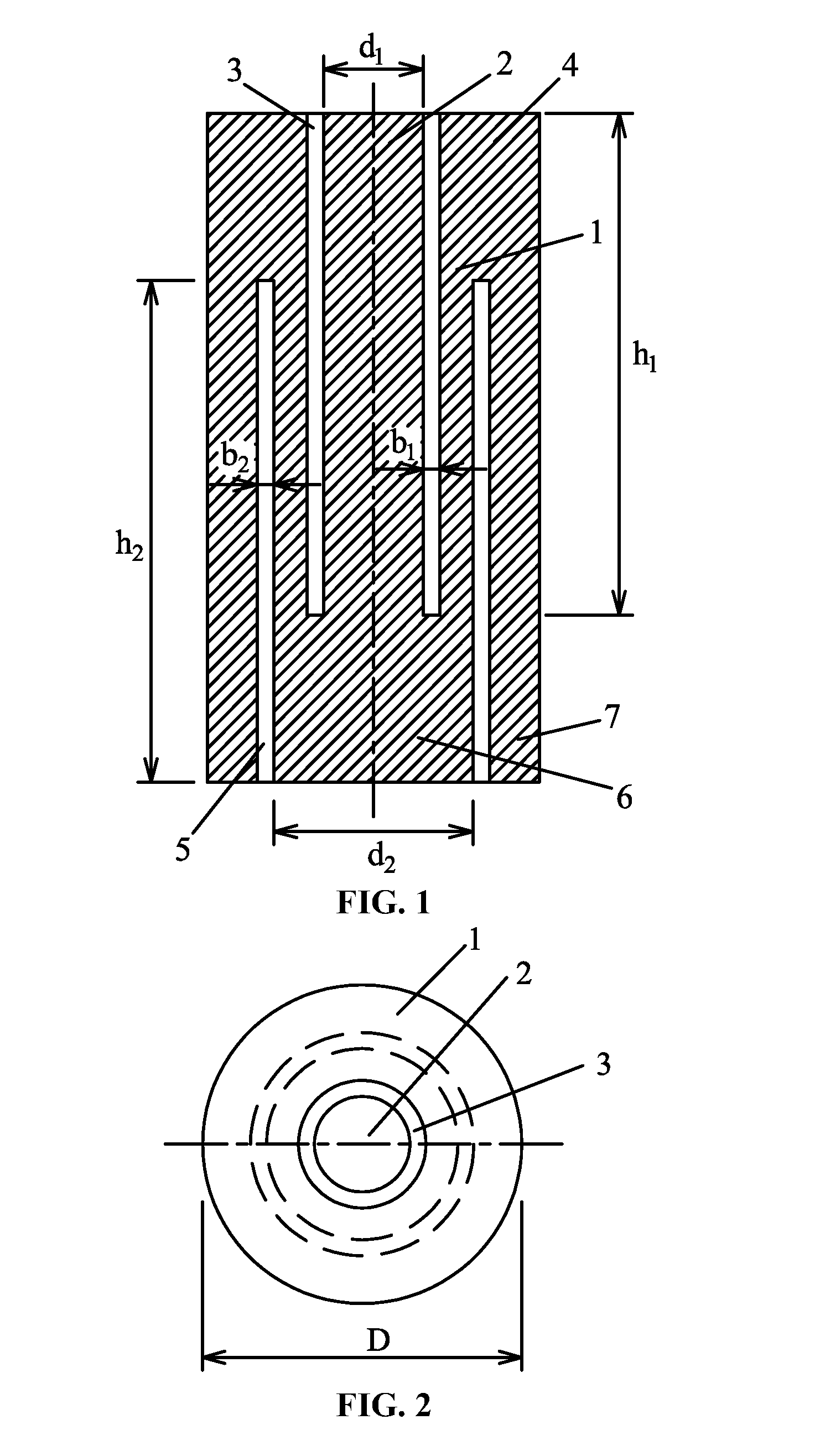
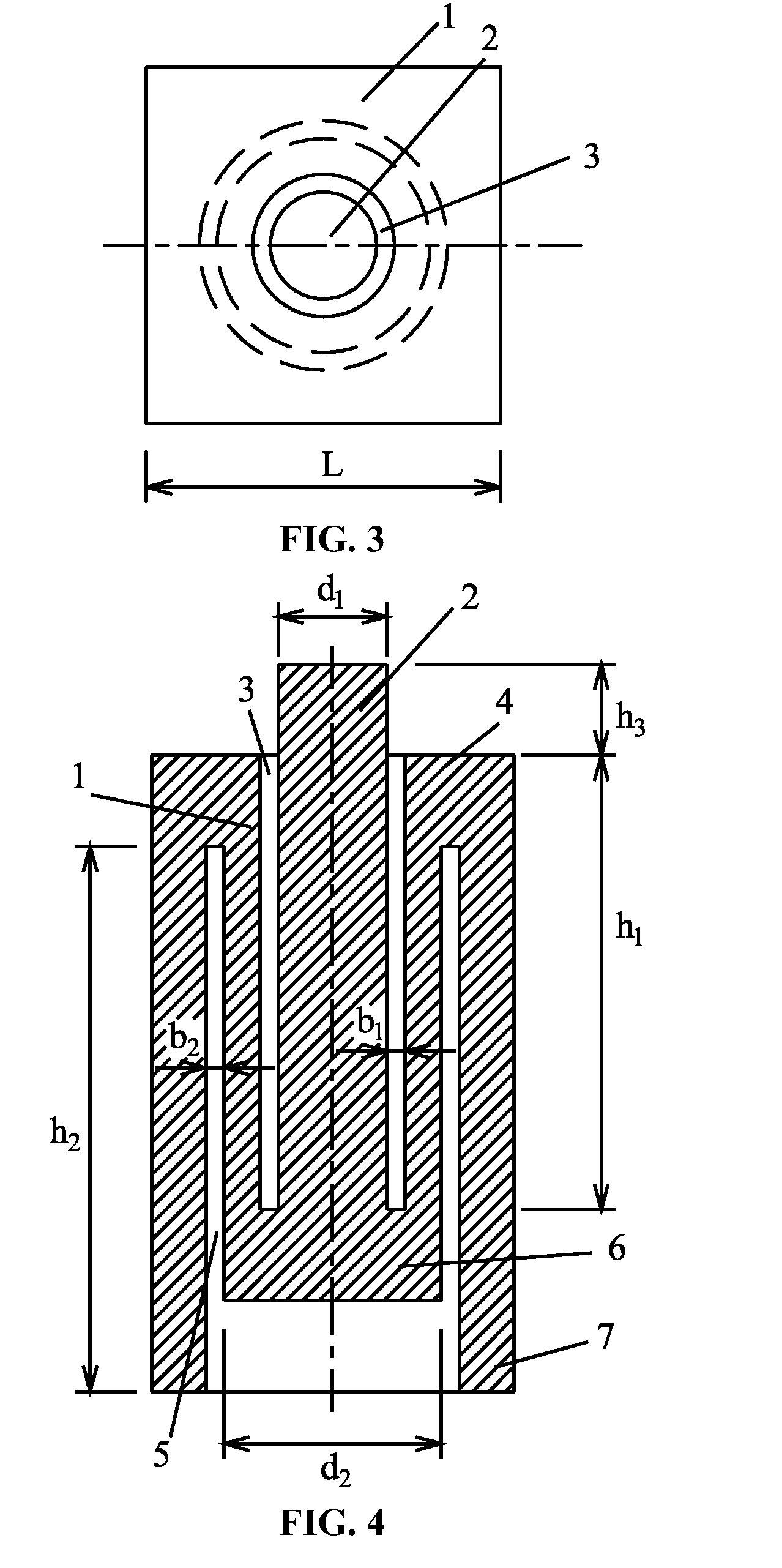
A rock specimen, including a rock body having the shape of a cylinder or a regular square prism. The rock body includes: an upper end face, a first circular groove, a first cylinder, a first circular body, a lower end face, a second circular groove, a second cylinder, and a second circular body. The first circular groove is disposed on the upper end face of the rock body and has a circle center coinciding with the center of the upper end face of the rock body. The second circular groove is disposed on the lower end face of the rock body and has a circle center coinciding with the center of the lower end face of the rock body. The outer diameter of the first circular groove is smaller than the inner diameter of the second circular groove, and the first circular groove and the second circular groove are staggered.
Owner:SICHUAN UNIV
System, method and device for positioning a target located within soft tissue in a path of an instrument
InactiveUS20080287827A1Minimize tracking errorReduce fatigueUltrasonic/sonic/infrasonic diagnosticsSurgical needlesUltrasound imagingSoft tissue
A system, method and device for positioning a target located within soft tissue in a path of an instrument inserted into the soft tissue includes: a manipulation system including a plurality of force applicators positioned around the soft tissue containing the target; an image acquisition system including an imaging probe for obtaining data for generating an image of the soft tissue containing the target; a detection means for detecting deflection of the target using the data from the imaging probe; and a control means for actuating the plurality of force applicators to apply forces on the soft tissue in response to a detected deflection of the target to move the target back in line with the path of the instrument. In an exemplary embodiment, the soft tissue is a breast, the imaging probe is an ultrasound imaging probe, and the instrument is a biopsy needle.
Owner:VANDERBILT UNIV
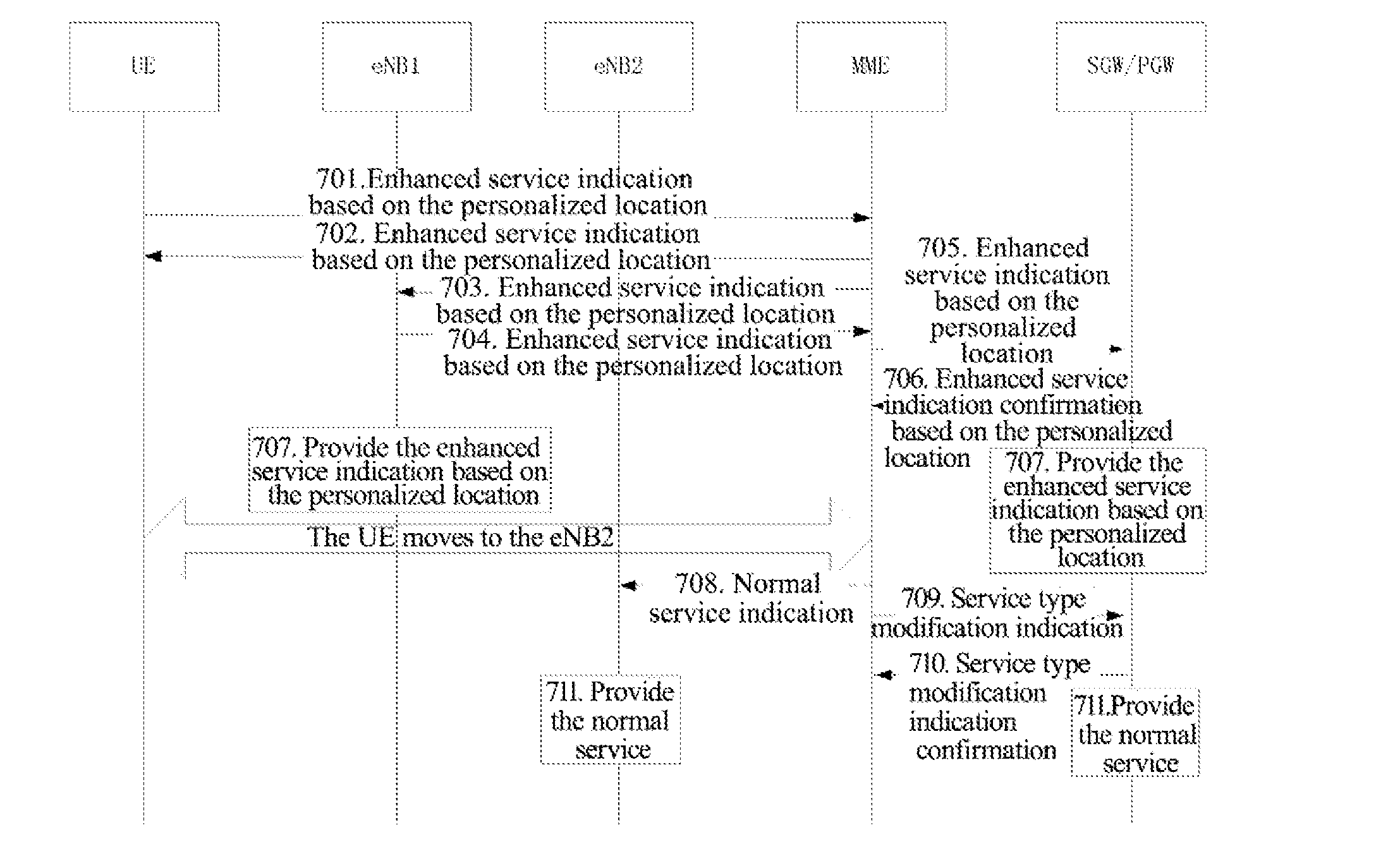
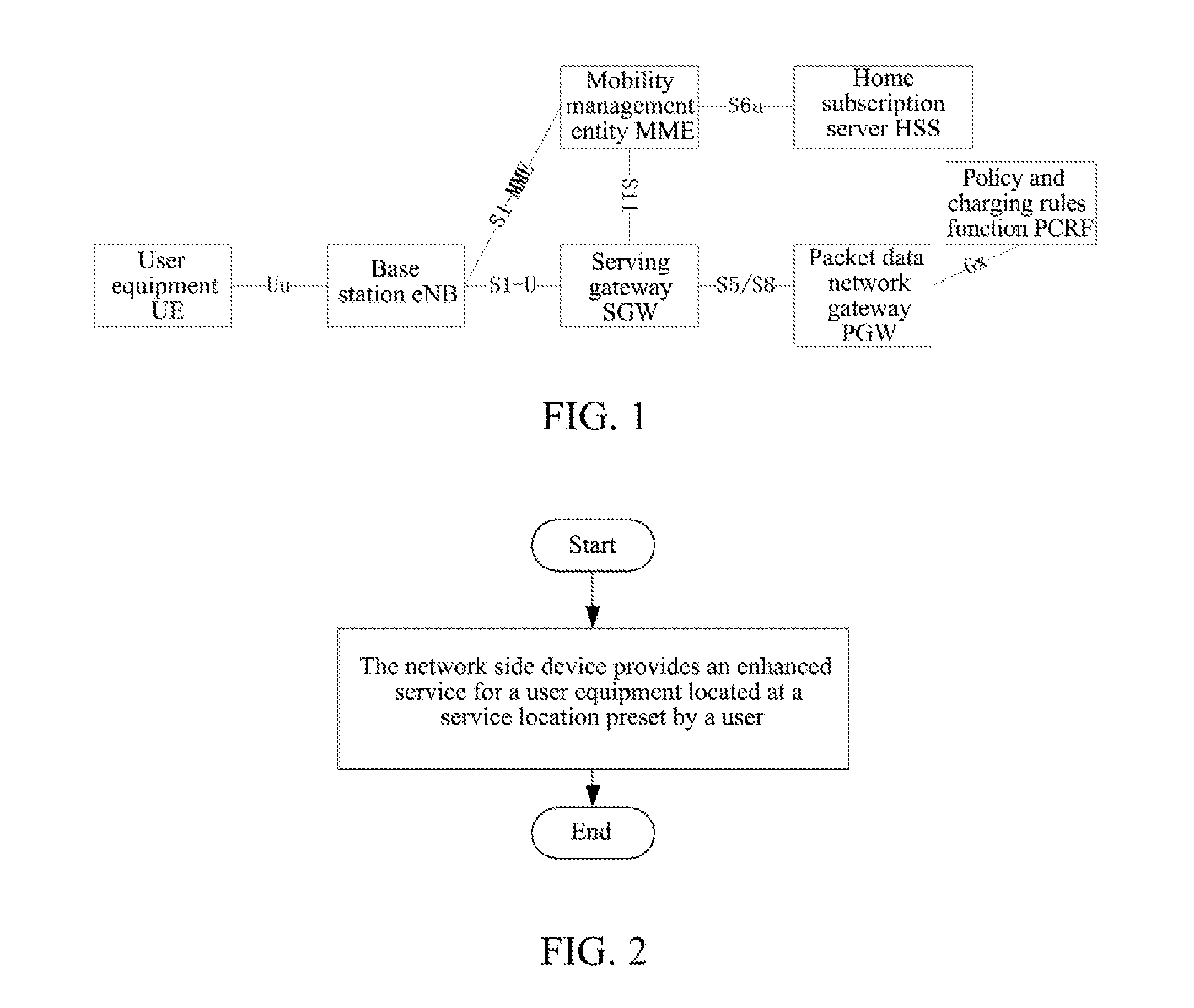
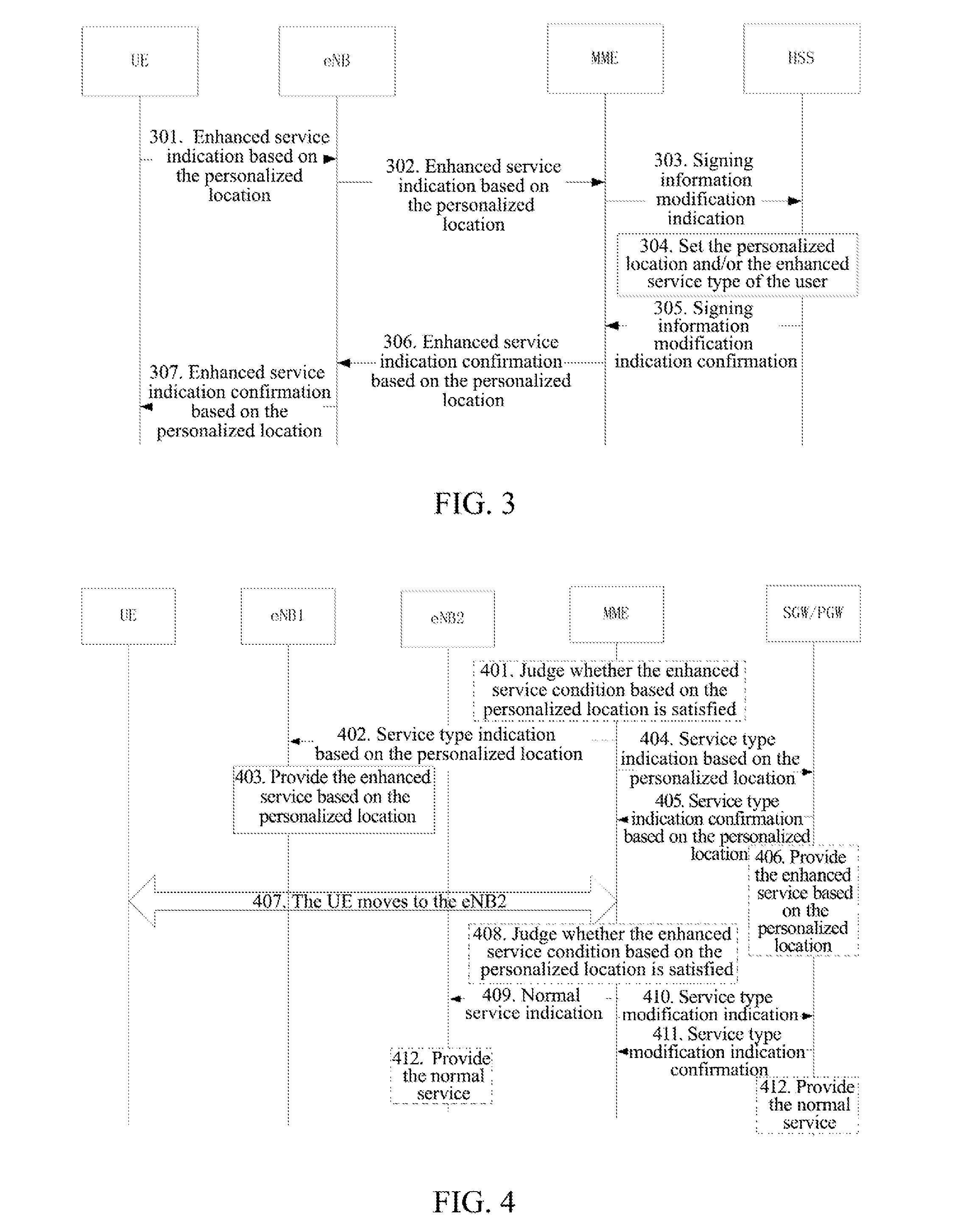
Personalized Method for Enhancing Service, Network Side Device, and Mobile User Equipment
ActiveUS20150181373A1Improve user experienceHigh communication rateAccounting/billing servicesTelephonic communicationPersonalizationService control
A personalized method for enhancing a service, a network side device and a mobile user equipment are provided. The network side device provides an enhanced service for a user equipment located at a service location preset by a user. The network side device comprises a service location determination module and an enhanced service control module. The service location determination module is configured to maintain the service location preset by the user. The enhanced service control module is configured to indicate to provide the enhanced service for the user equipment located at the service location preset by the user. The mobile user equipment comprises an enhanced service indication module. The enhanced service indication module is configured to send a user indication to the network side device to enable the network side device to provide the enhanced service for the user equipment located at the service location preset by the user.
Owner:ZTE CORP
System and method for dynamic management of business processes
ActiveUS7516137B1Faster time-to-marketExcellent ease of useDigital data processing detailsOffice automationWeb serviceDatabase server
A computerized system is provided for modeling a business process, the computerized system comprising a web server and a database server. The web server implements a user interface to the system. The database server is in operable communication with the web server and comprises a data architecture representing the business process. The data architecture comprises an entity model representing an entity responsible for implementing at least a portion of the business process, a transaction model comprising at least one step defining a business process, a list model representing at least one step in the transaction, the list model comprising a list of at least one entity associated with the transaction; and a task model associated with the list, the task model defining at least one task associated with the at least one step in the transaction.
Owner:ARRAYWORKS
Stable dental analog
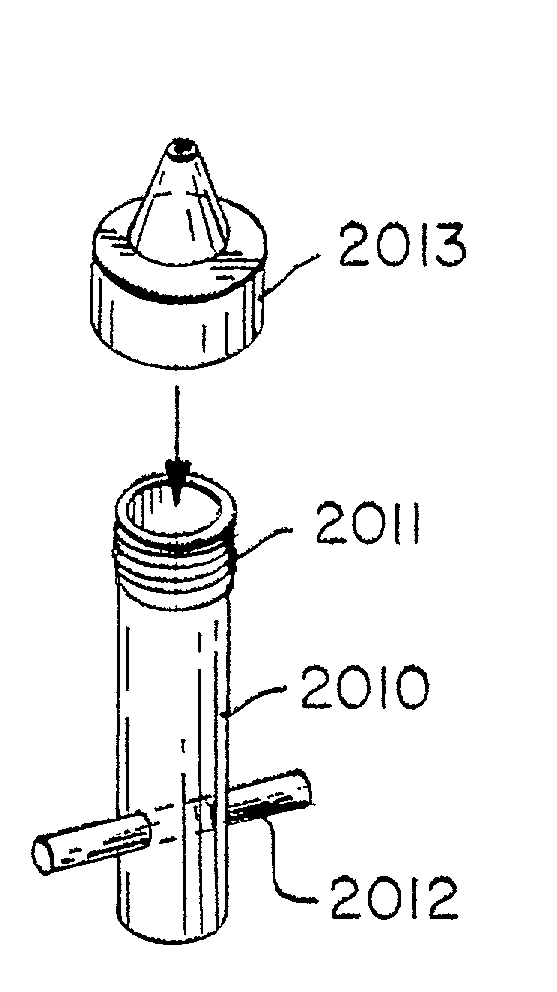
InactiveUS20030044753A1High heat toleranceSuccess rateDental implantsImpression capsTooth crownDental laboratory
An implant analog includes an abutment that can be mounted in the dental lab replica of the relevant section of a patient's mouth more securely than heretofore possible. Because of the implant analog, a crown will attach more accurately to the implant in the patient's mouth. The analogs have a pin or other protrusion that projects from the base of the analog. The analog has substantially the same height and dimensions as a conventional implant and abutment.
Owner:CLM ANALOGS LLC
Method for Providing Information in a Cellular Wireless Communication System
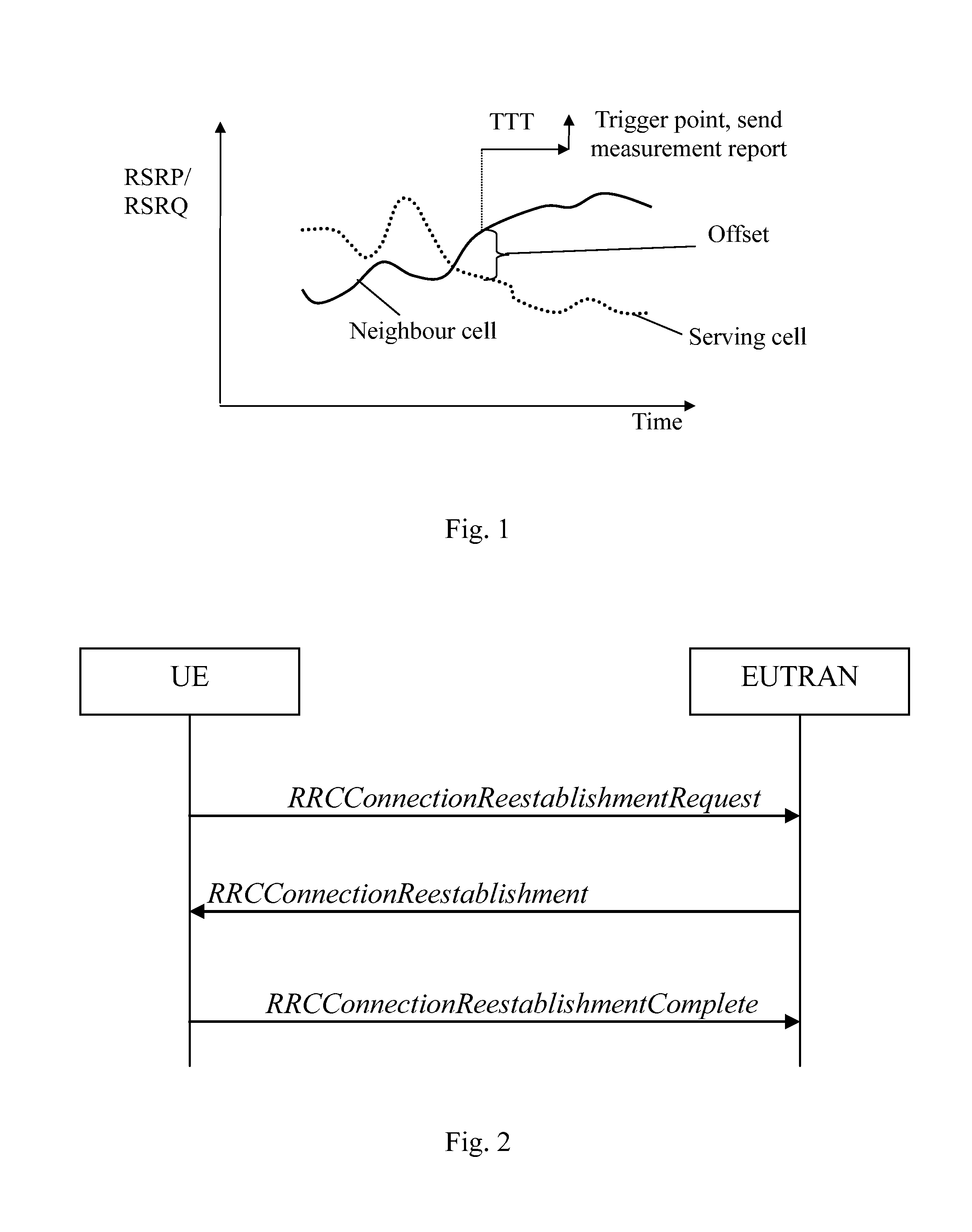
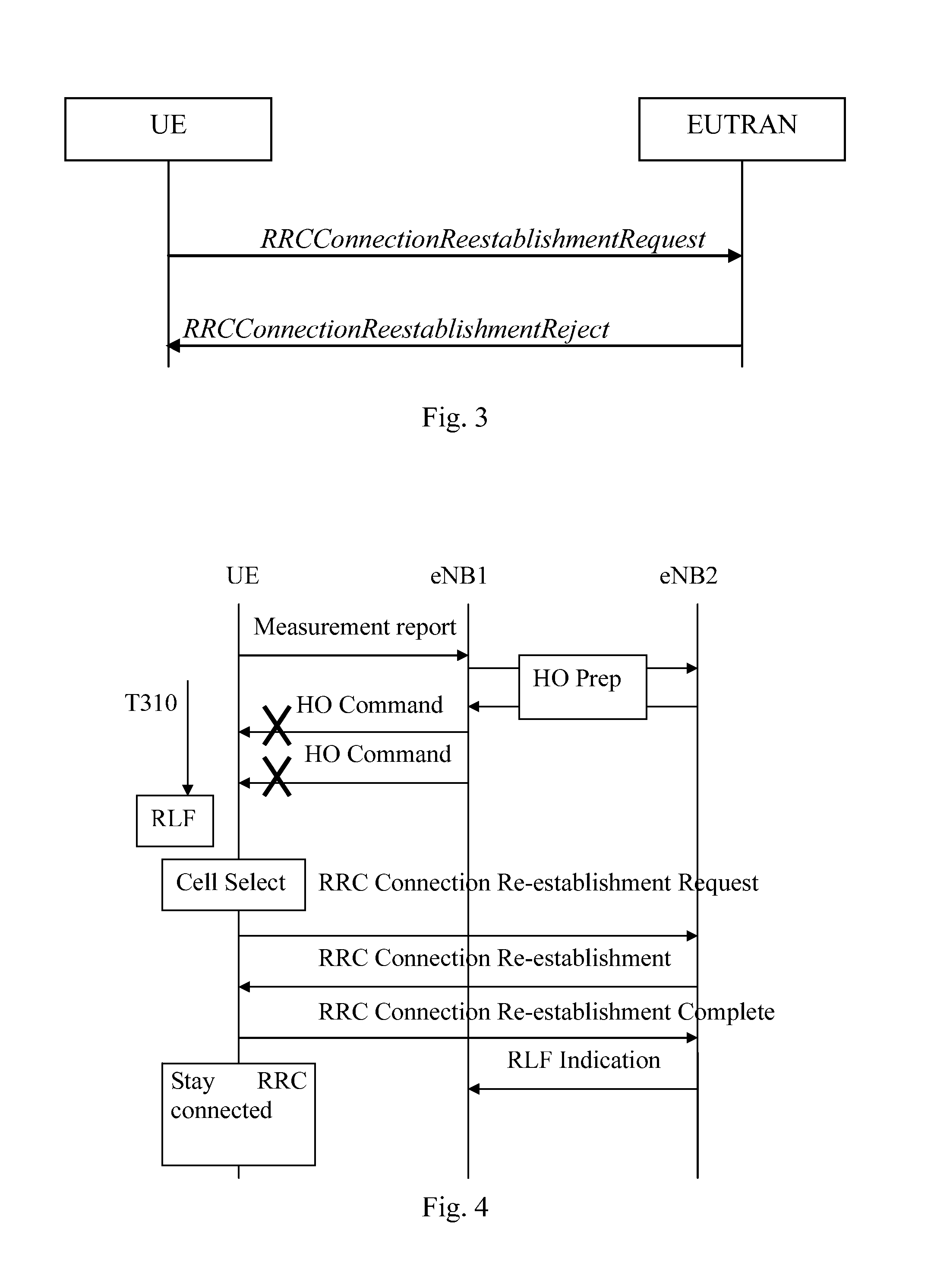
InactiveUS20140050197A1Small impactSuccess rateConnection managementWireless commuication servicesCommunications systemMobile station
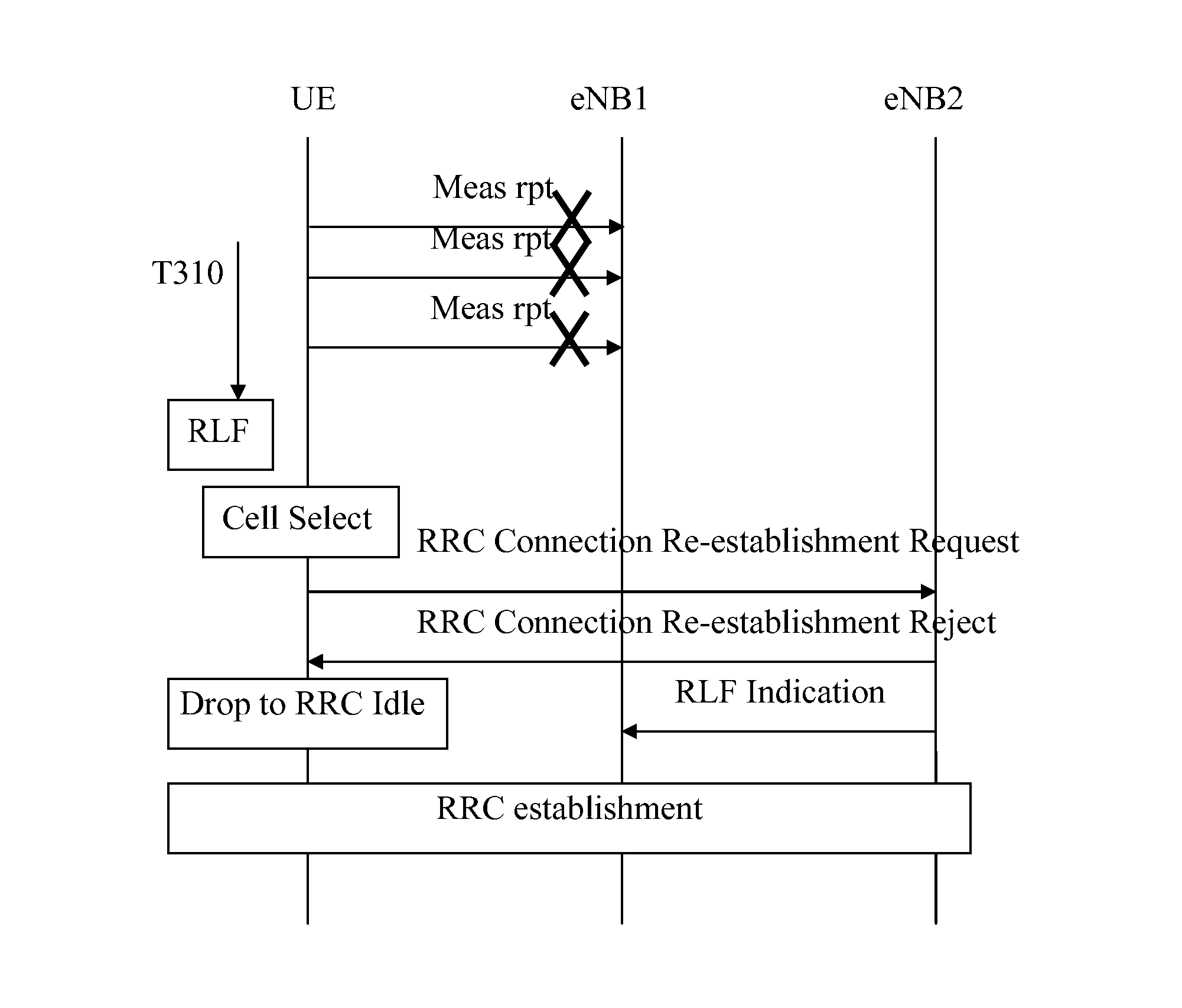
The present invention relates to a method for providing information in a cellular wireless communication system, wherein each cell in said cellular wireless communication system is served by a base station, and said cellular wireless communication system employs a procedure in which a mobile station suffering from a radio link failure (RLF), when being connected to a cell, may attempt to re-establish a connection in another cell, comprising the steps of: detecting a radio link failure (RLF) for said mobile station while connected to a first cell; requesting a radio resource control (RRC) re-establishment for said mobile station in a second cell after said radio link failure (RLF); and providing information, whether said radio resource control (RRC) re-establishment for said mobile station was successful or not, to said first cell and / or to a third cell, wherein said third cell is the cell to which said mobile station was connected before said first cell. Furthermore, the invention also relates to a method in a base station, a computer program, a computer program product, and a base station device.
Owner:HUAWEI TECH CO LTD
Customized dental prosthesis for periodontal or osseointegration, and related systems
InactiveUS20130244208A1Quality improvementNone have achieved superiorDental implantsImpression capsJaw boneOsseointegration
Owner:NATURAL DENTAL IMPLANTS
Methods of designing and manufacturing customized dental prosthesis for periodontal or osseointegration and related systems
ActiveUS9539062B2None have achieved superiorQuality improvementDental implantsImpression capsAnatomical structuresNatural tooth
Methods of manufacturing dental prosthesis / implants each to replace a non-functional natural tooth positioned in a jawbone of a specific pre-identified patient are provided. An example method includes the steps of receiving imaging data such as x-ray image data and surface scan data of a dental anatomy and / or a physical impression of the dental anatomy of a specific preidentified patient. The steps can also include forming a three-dimensional virtual model of at least portions of a non-functional natural tooth positioned in the jawbone of the specific pre-identified patient based on the imaging and surface scan data, virtually designing a dental implant based upon the virtual model, exporting the data describing the designed dental implant to a manufacturing machine, and custom manufacturing the dental implant for the specific patient.
Owner:NATURAL DENTAL IMPLANTS
Cell microinjection system with force feedback
ActiveUS20190292567A1Low costEasy to installAnimal reproductionProgramme-controlled manipulatorElectricityEmbryo
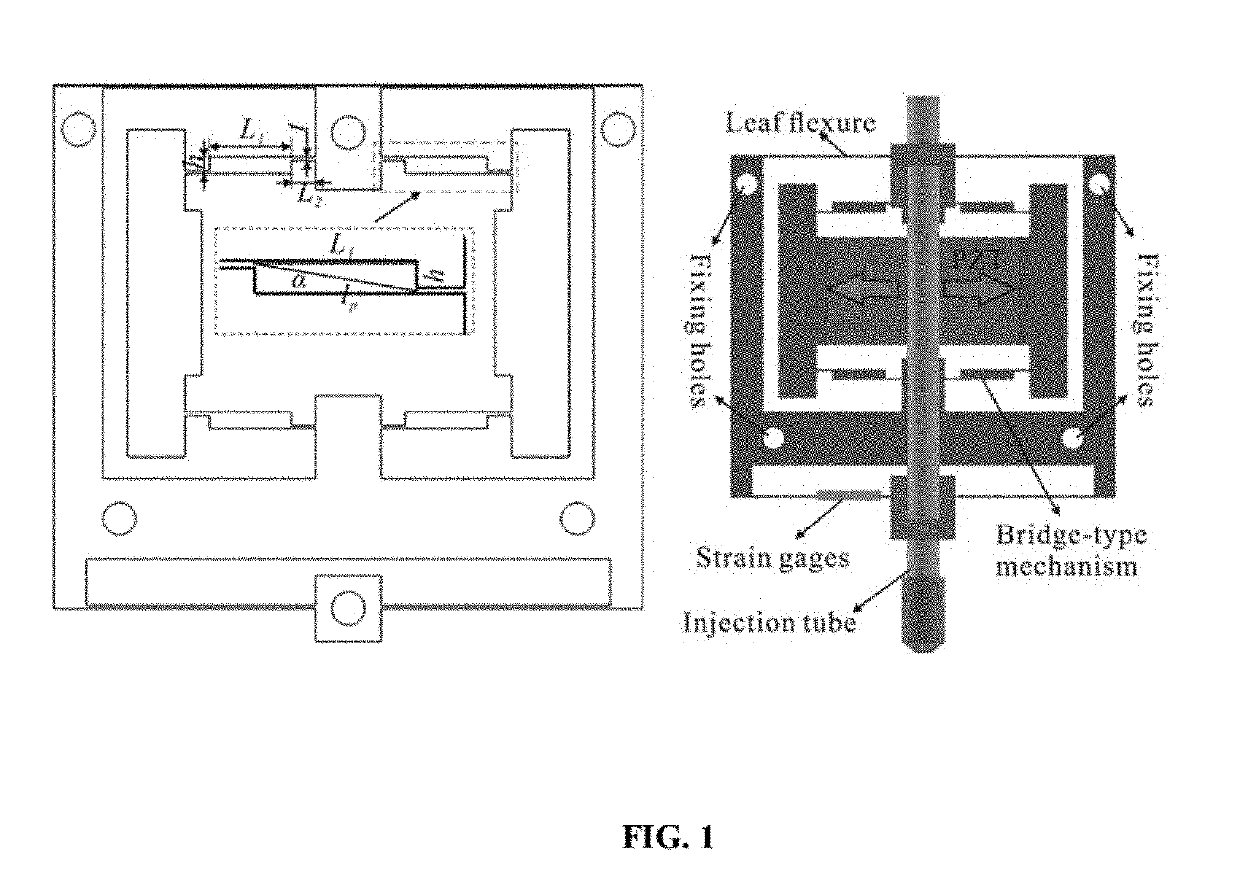
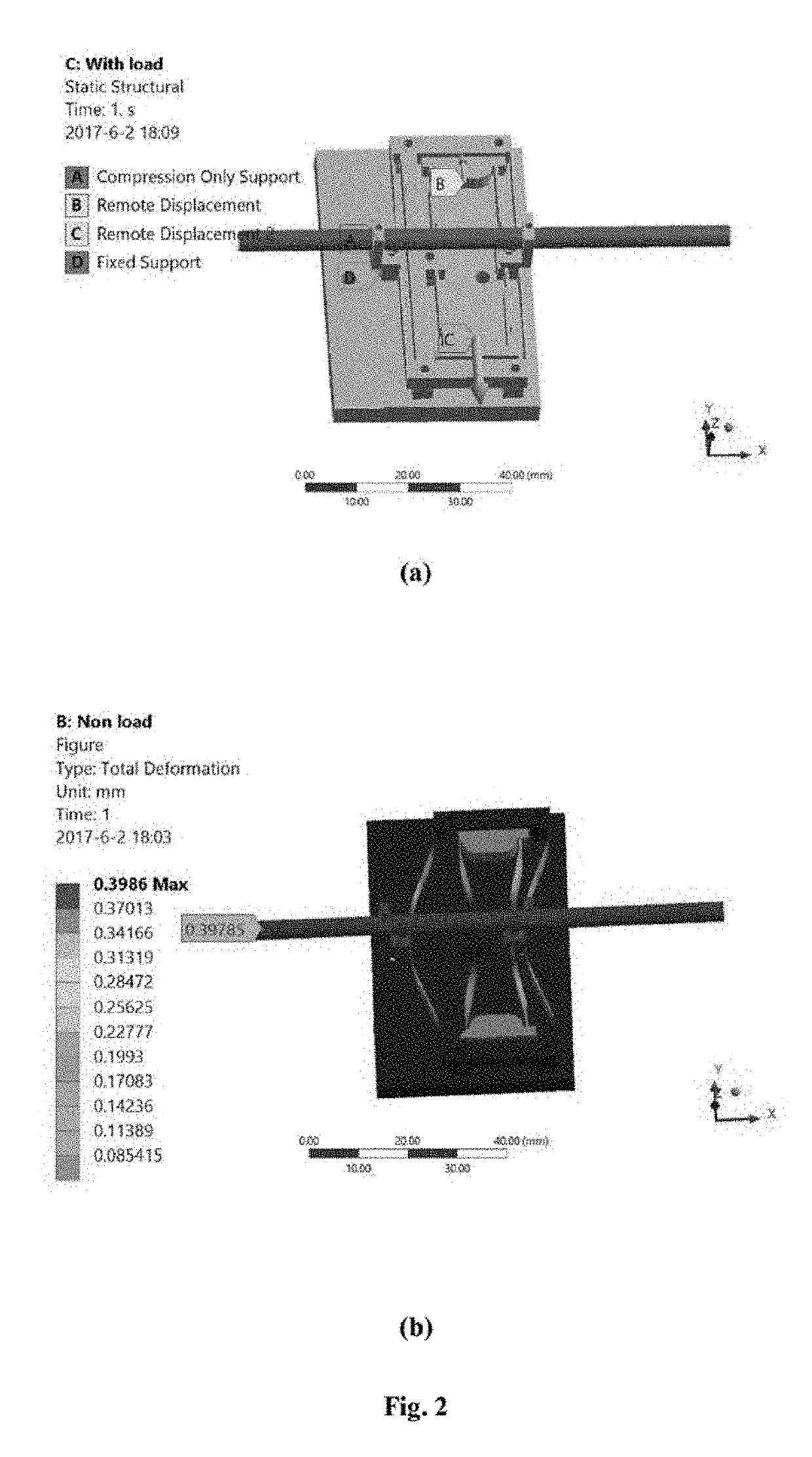
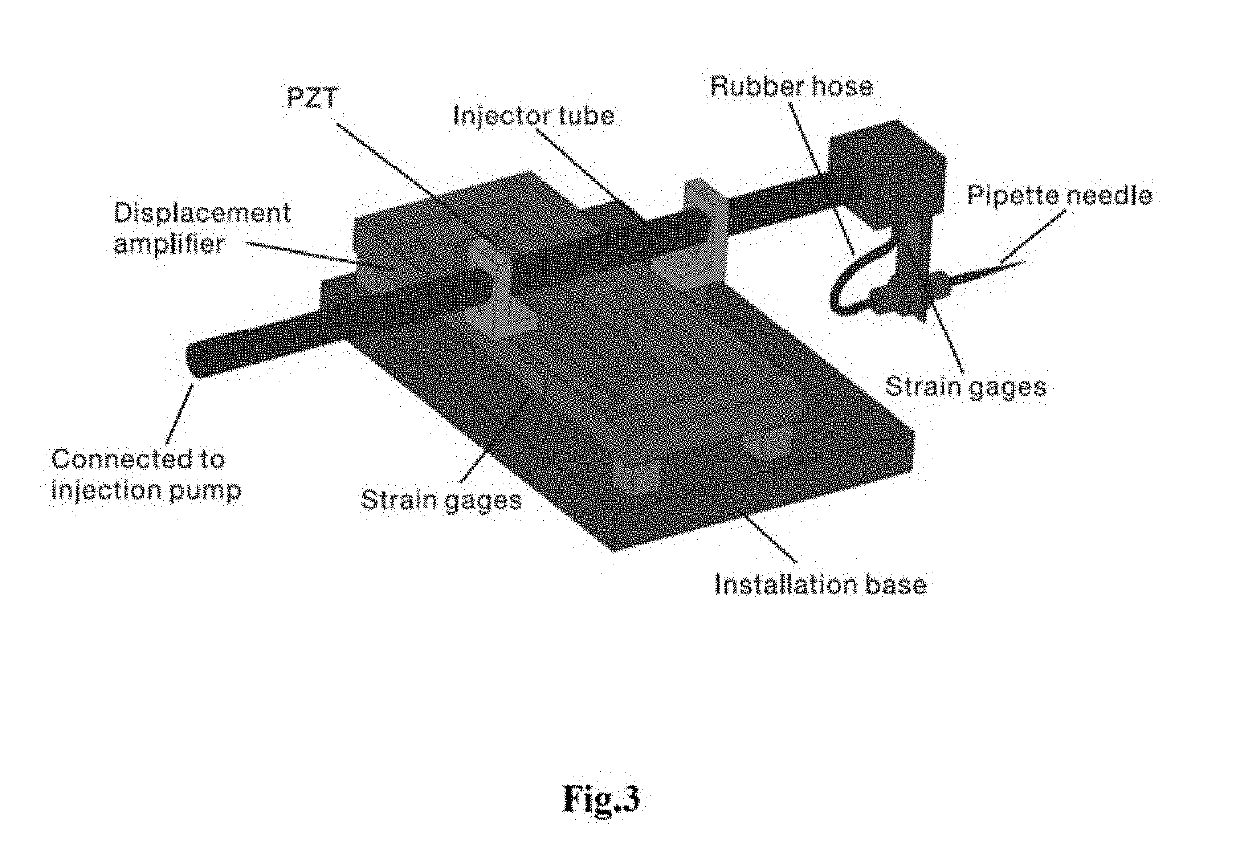
A novel piezo-driven cell injection system with force feedback overcomes the unsatisfied force interaction between the pipette needle and embryos in conventional position control. By integrating semiconductor strain-gage sensors for detecting the cell penetration force and the micropipette relative position in real time, the developed cell microinjection system features high operation speed, confident success rate, and high survival rate. The effectiveness of the developed cell injection system is experimentally verified by penetrating zebrafish embryos. The injection of 100 embryos are conducted with separate position control and force control. Results indicate that the force control enables a survival rate of 86%, which is higher than the survival rate of 82% produced by the position control in the same control environment. The experimental results quantitatively demonstrate the superiority of force control over conventional position control for the first time.
Owner:UNIVERSITY OF MACAU
System and Method for Dynamic Management of Business Processes
InactiveUS20090198546A1Success rateEasy to adaptOffice automationWebsite content managementWeb serviceDatabase server
The present invention provides a computerized system for modeling a business process, comprising a web server and a database server. The web server implements a user interface to the system. The database server is in operable communication with the web server and comprises a data architecture representing the business process. The data architecture comprises an entity model representing an entity responsible for implementing at least a portion of the business process, a transaction model comprising at least one step defining a business process, a list model representing at least one step in the transaction, the list model comprising a list of at least one entity associated with the transaction; and a task model associated with the list, the task model defining at least one task associated with the at least one step in the transaction.
Owner:EARLE GORDON +1
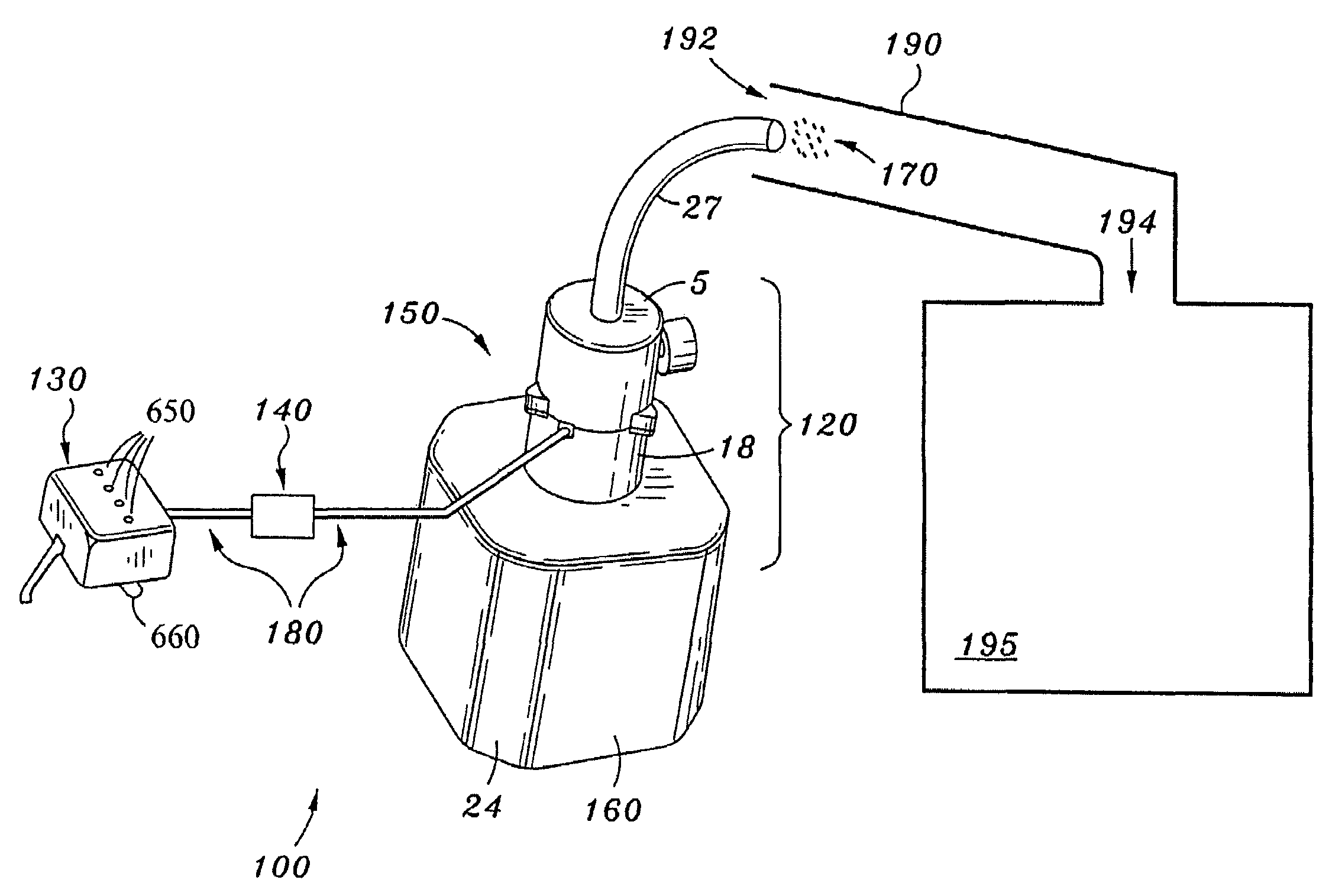
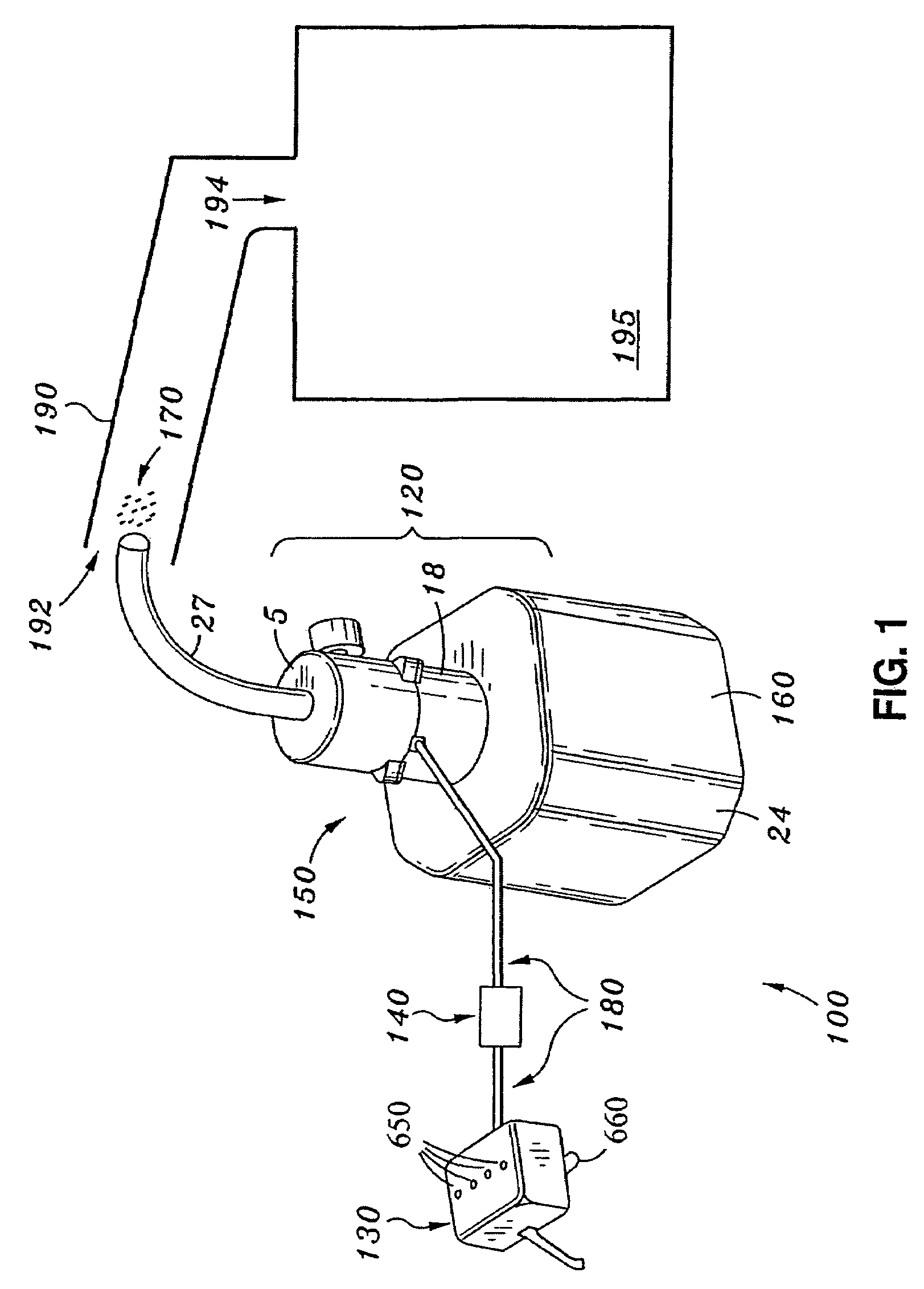
Fuel combustion catalyst microburst aerosol delivery device and continuous and consistent aerosol delivery device
InactiveUS7584905B2Improve fuel economyIncreases performance and efficiencyInternal combustion piston enginesNon-fuel substance addition to fuelCombustionNebulizer
Owner:EMISSIONS TECH
Methods of Designing and Manufacturing Customized Dental Prosthesis For Periodontal or Osseointegration and Related Systems
ActiveUS20170086953A1None have achieved superiorQuality improvementDental implantsImpression capsNatural toothAnatomical structures
Methods of manufacturing dental prosthesis / implants each to replace a non-functional natural tooth positioned in a jawbone of a specific pre-identified patient are provided. An example method includes the steps of receiving imaging data such as x-ray image data and surface scan data of a dental anatomy and / or a physical impression of the dental anatomy of a specific preidentified patient. The steps can also include forming a three-dimensional virtual model of at least portions of a non-functional natural tooth positioned in the jawbone of the specific pre-identified patient based on the imaging and surface scan data, virtually designing a dental implant based upon the virtual model, exporting the data describing the designed dental implant to a manufacturing machine, and custom manufacturing the dental implant for the specific patient.
Owner:RTRS INVESTMENT LLC
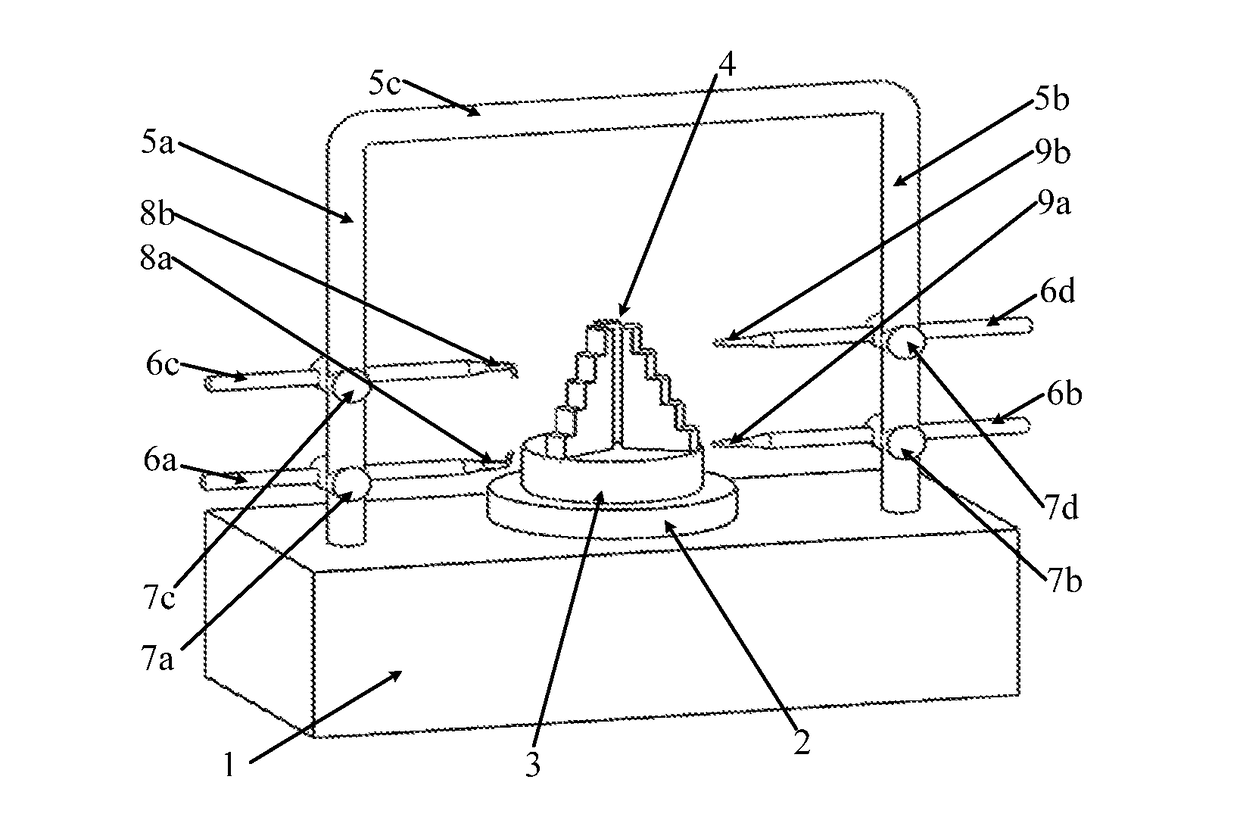
Aero engine rotor air floatation assembling method and device based on gantry structure
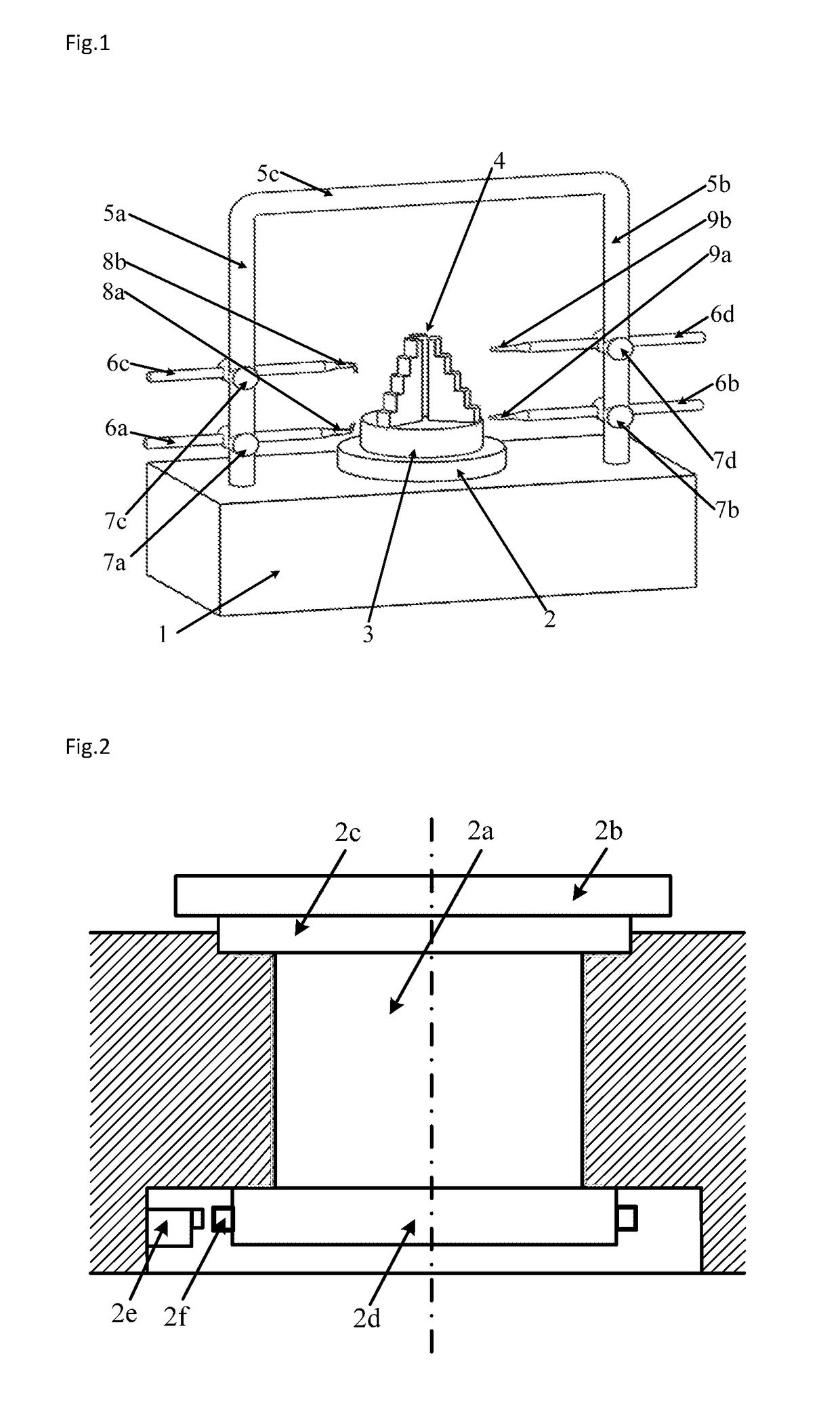
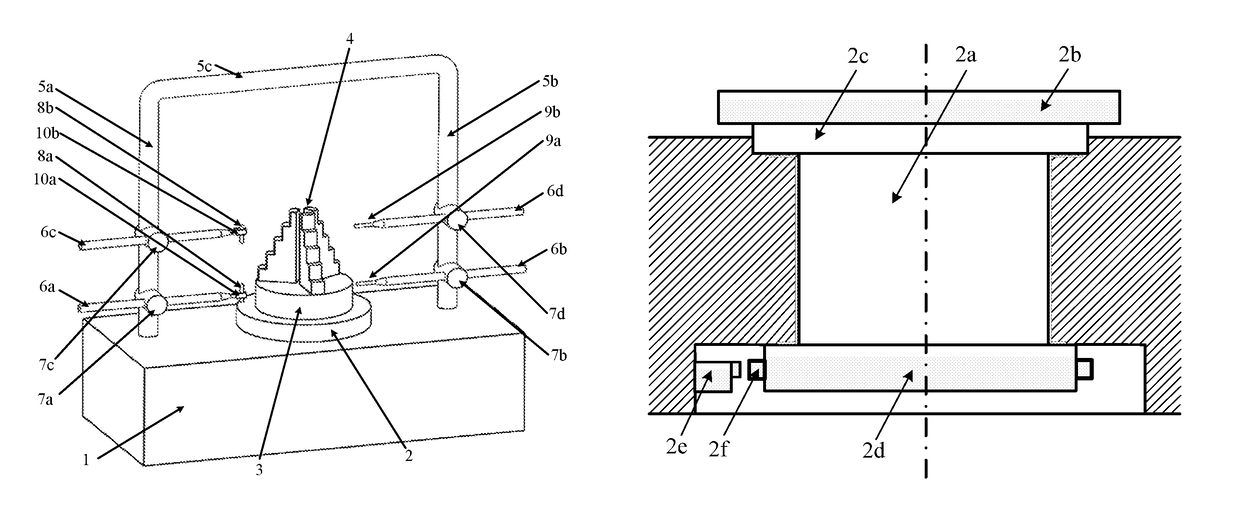
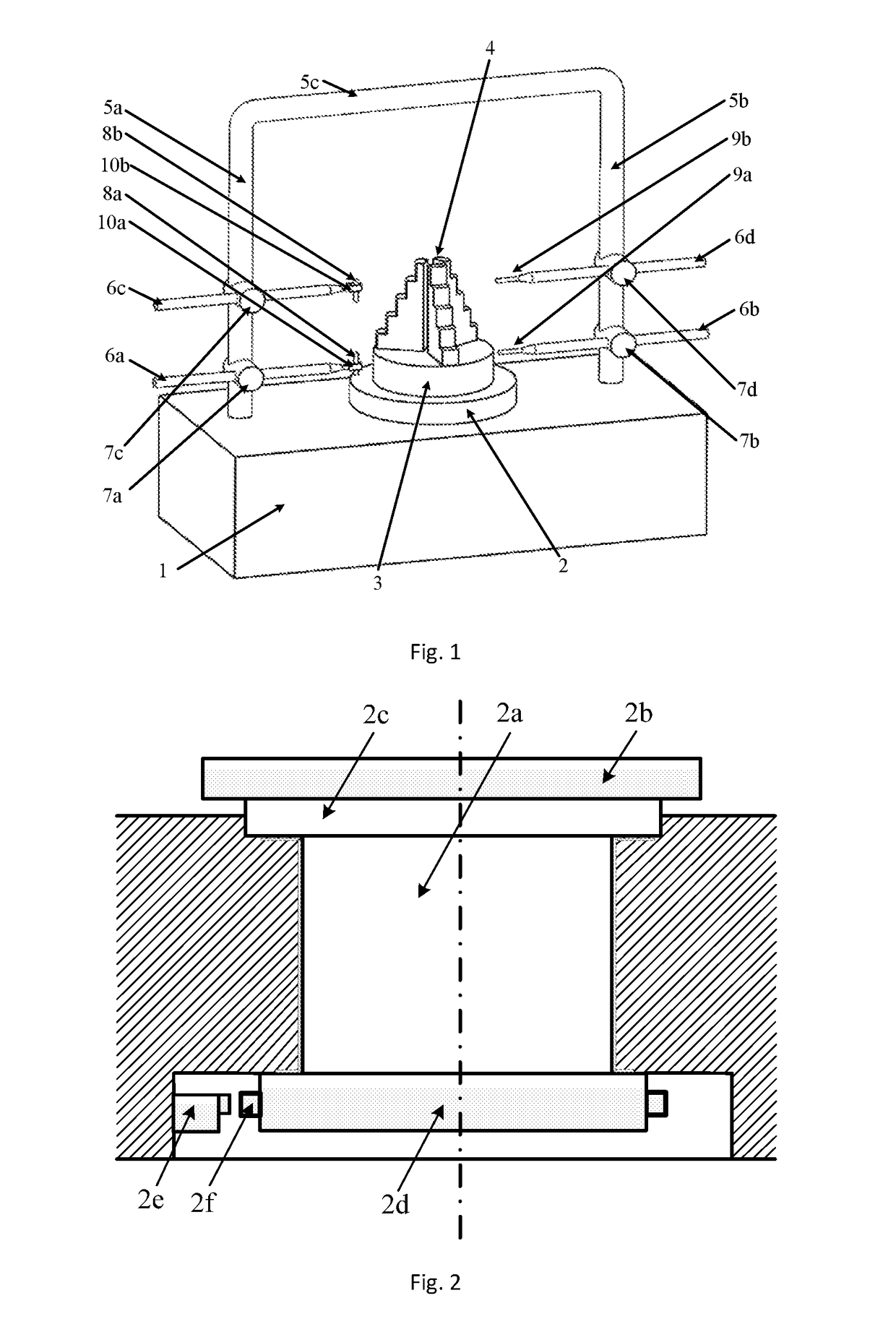
An aero engine rotor air floatation assembling method and device based on a gantry structure belong to mechanical assembling technology. The present invention can effectively solve the problem of poor coaxality after the aero engine rotor is assembled and has the characteristics of high coaxality after the rotor is assembled, reduced vibration, mounting easiness, high flexibility and improved engine performance. The measuring method and device are: determining rotary reference based on a rotary air bearing; determining the angular positioning of a rotary table according to a grating ruler; extracting the radial error of the radial mounting plane and the inclination error of the axial mounting plane of the rotor based on the four-probe measuring device to obtain the influencing weight of this rotor to the assembled rotor on coaxality; measuring respectively all the rotors required for assembling to obtain the influencing weight of each rotor to the assembled rotor on coaxality; vector optimizing the weight of each rotor to obtain the assembling angle of each rotor.
Owner:HARBIN INST OF TECH
Customized Dental Prosthesis For Periodontal or Osseointegration and Related Systems
InactiveUS20170156824A1None have achieved superiorQuality improvementDental implantsImpression capsNatural toothOsseointegration
Custom dental prosthesis or implants each individually designed and manufactured to replace nonfunctional natural teeth positioned in a jawbone of a specific pre-identified patient are provided. An example dental prosthesis / implant includes a dental implant body having a prosthesis interface formed therein to receive an occlusally-facing dental prosthesis component. The prosthesis interface has a custom three-dimensional surface shape positioned and formed to create a form locking fit with respect to the occlusally-facing dental prosthesis component when positioned thereon.
Owner:NATURAL DENTAL IMPLANTS
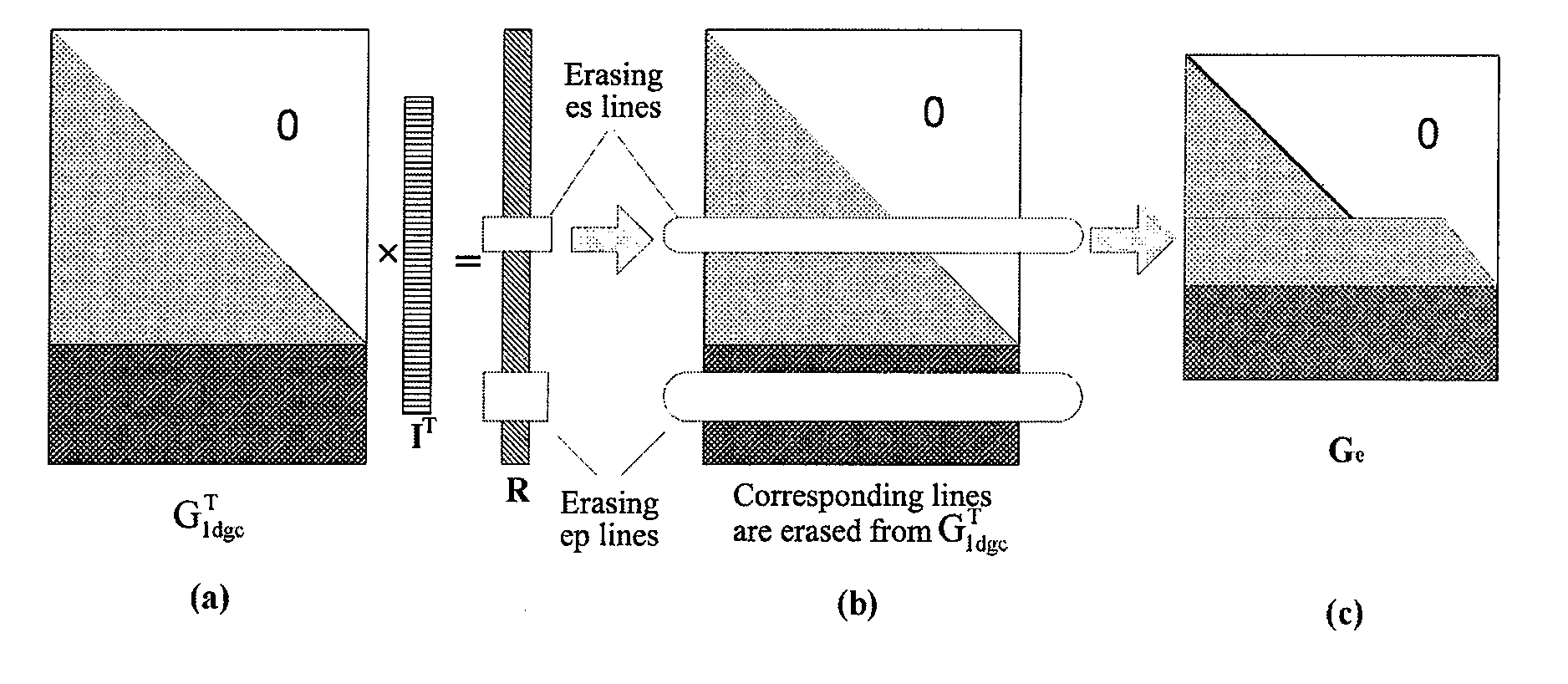
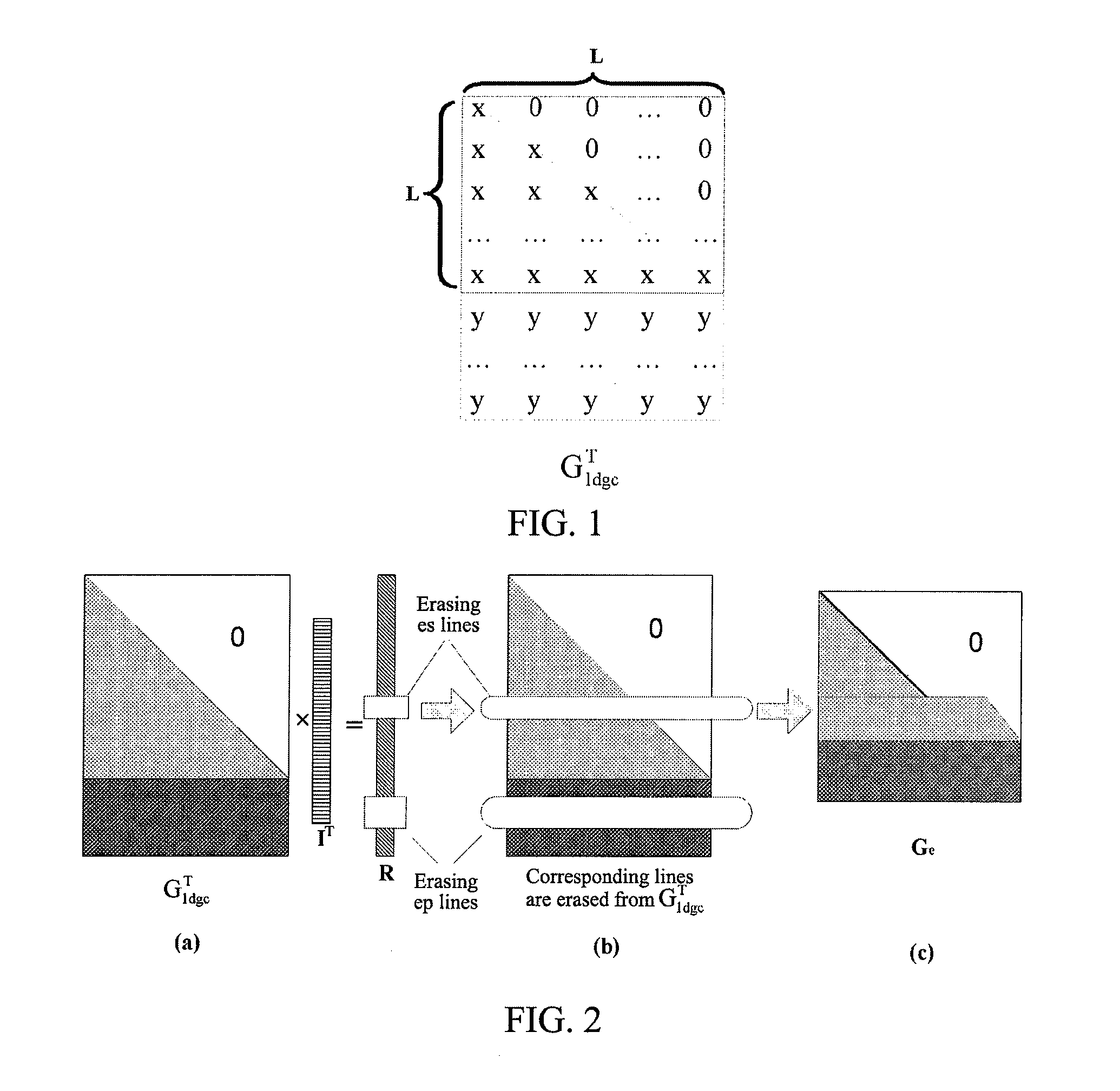
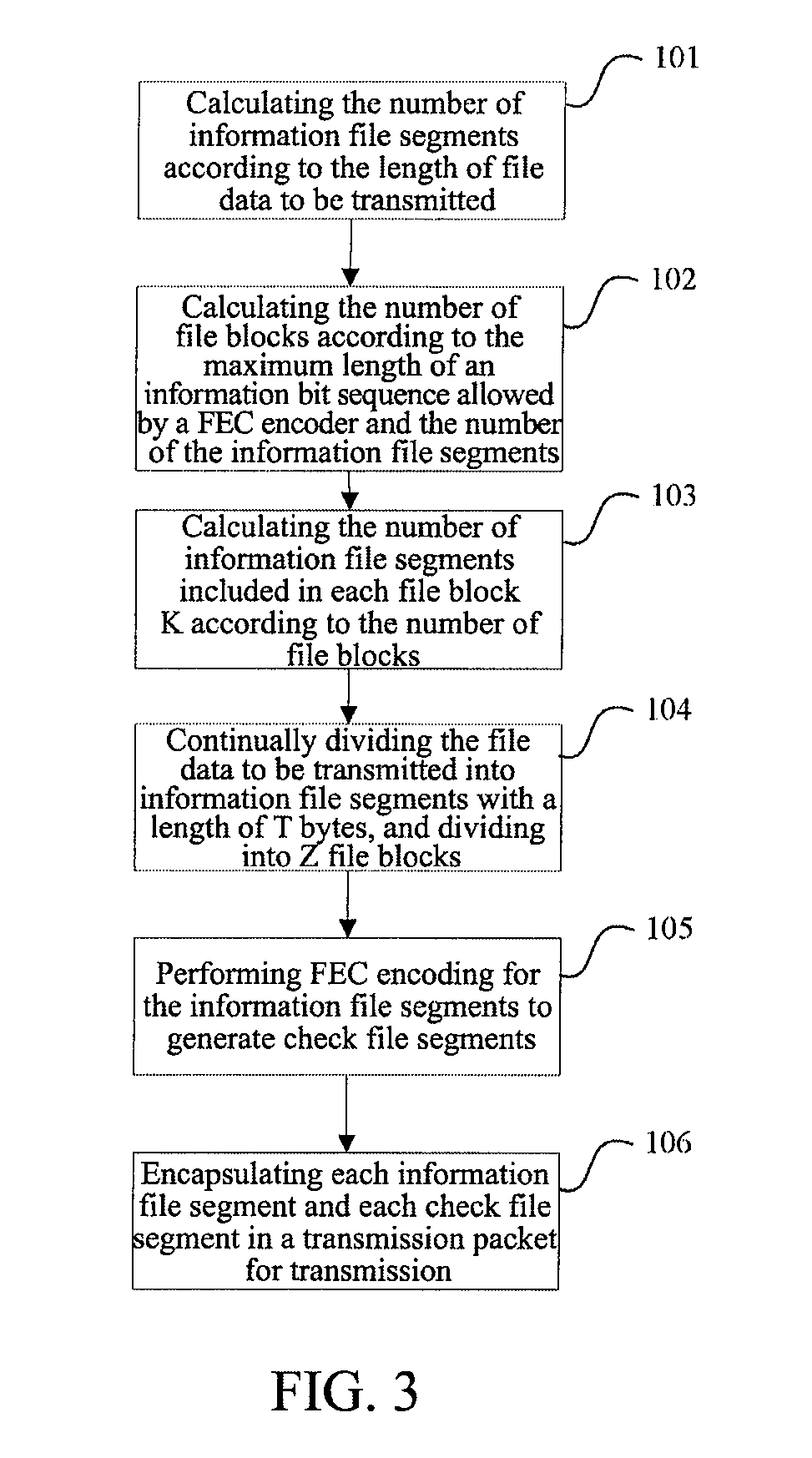
Method and Apparatus for Data Receiving
InactiveUS20110060959A1Improve data transfer efficiencyImprove processing speedError preventionCode conversionComputer hardwareForward error correction
A method and apparatus for receiving data is provided. A data receiving terminal processes each received file block as follows: performing forward error correction decoding for Tb bit sequences to be decoded of the file block respectively, obtaining Tb decoded information bit sequences with a length of K, wherein, the ith bit sequence to be decoded is composed of the ith bits of each unerased information file segment and check file segment of the file block in sequence according to the sequence of the information file segments and the check file segments; combing K decoded information file segments of the file block in sequence to generate original file data of the file block, wherein the Mth decoded information file segment is composed of the Mth bits of the Tb decoded information bit sequences in sequence according to the sequence of the information bit sequences.
Owner:ZTE CORP
Console for operating actuating mechanism
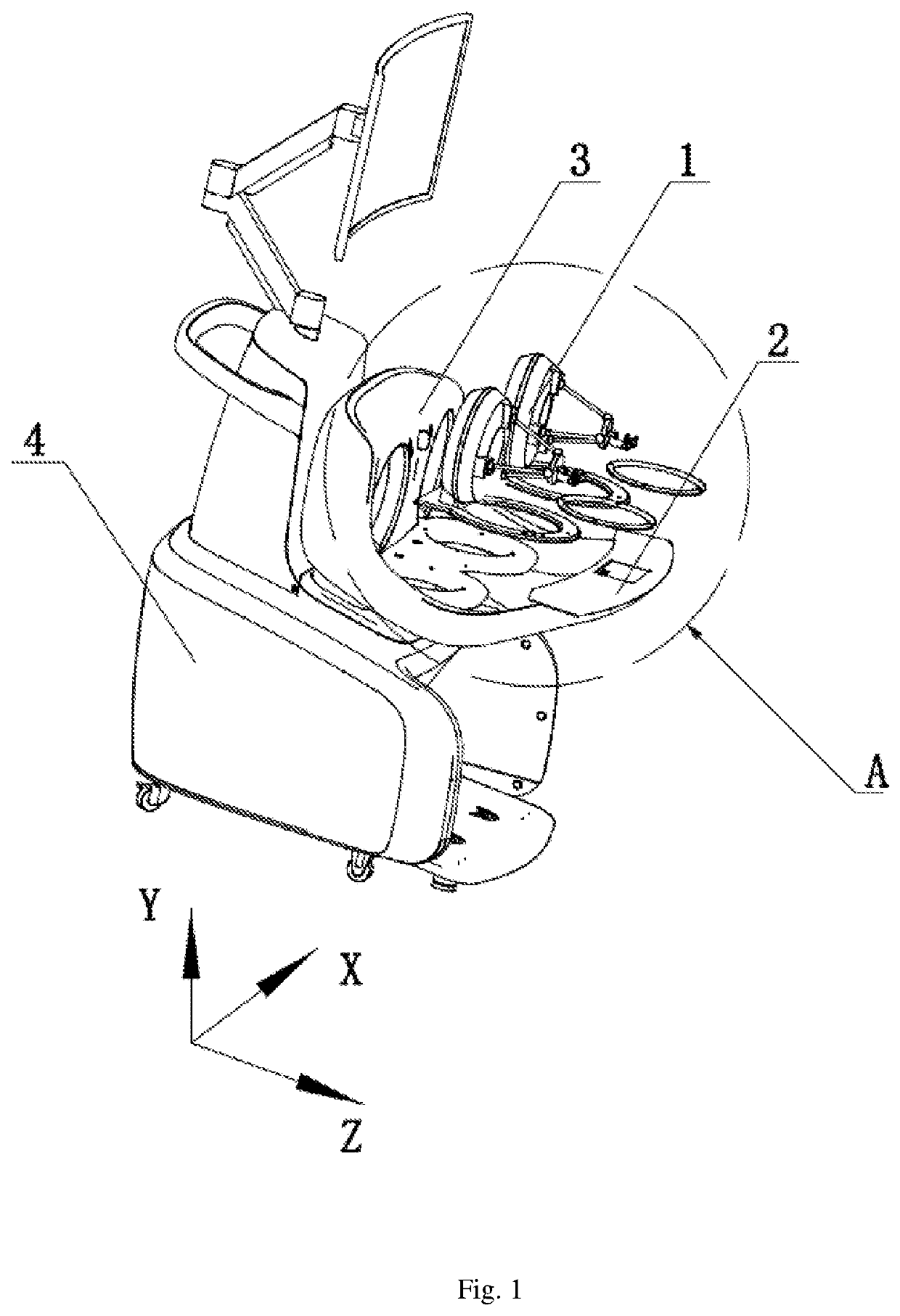
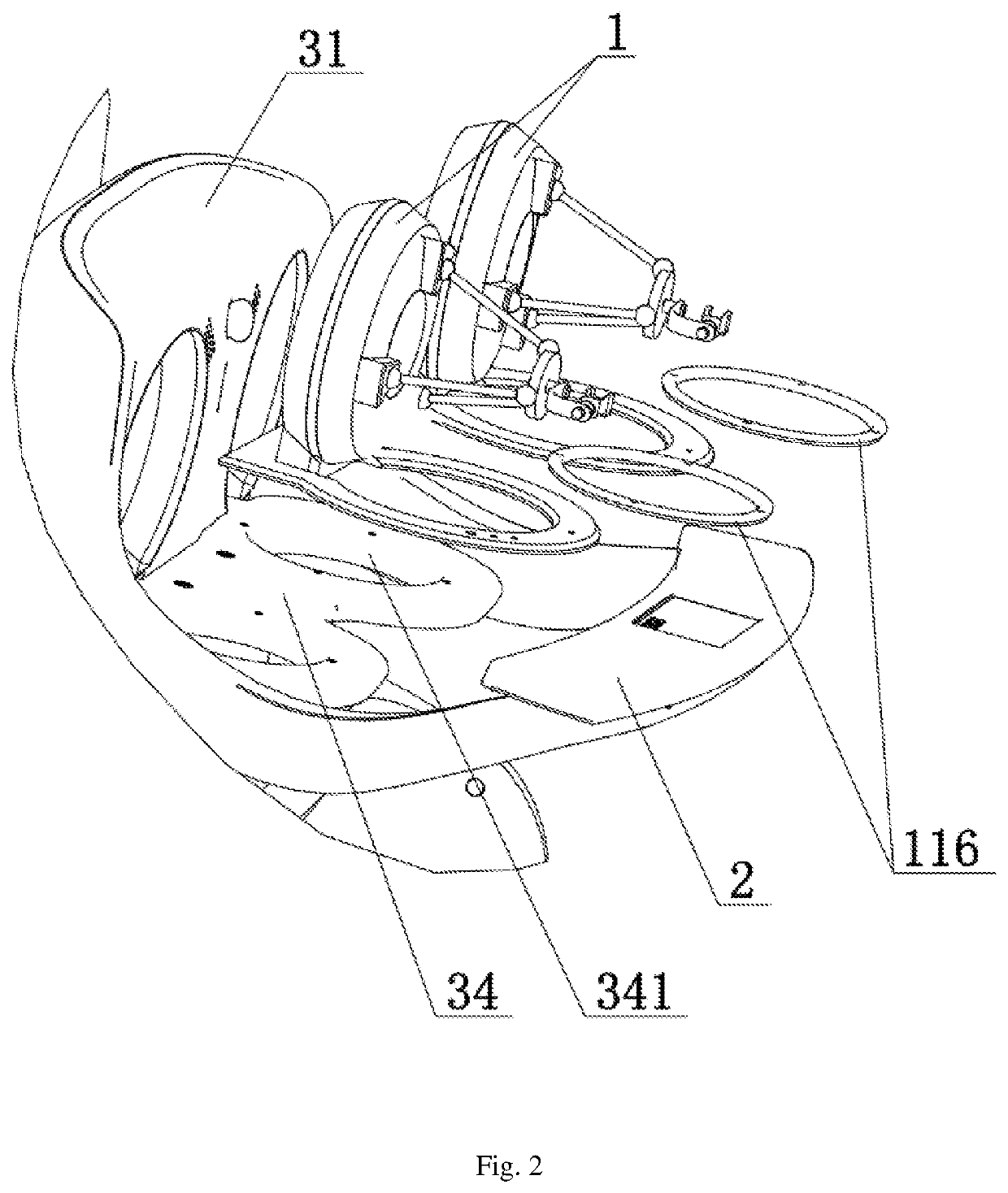
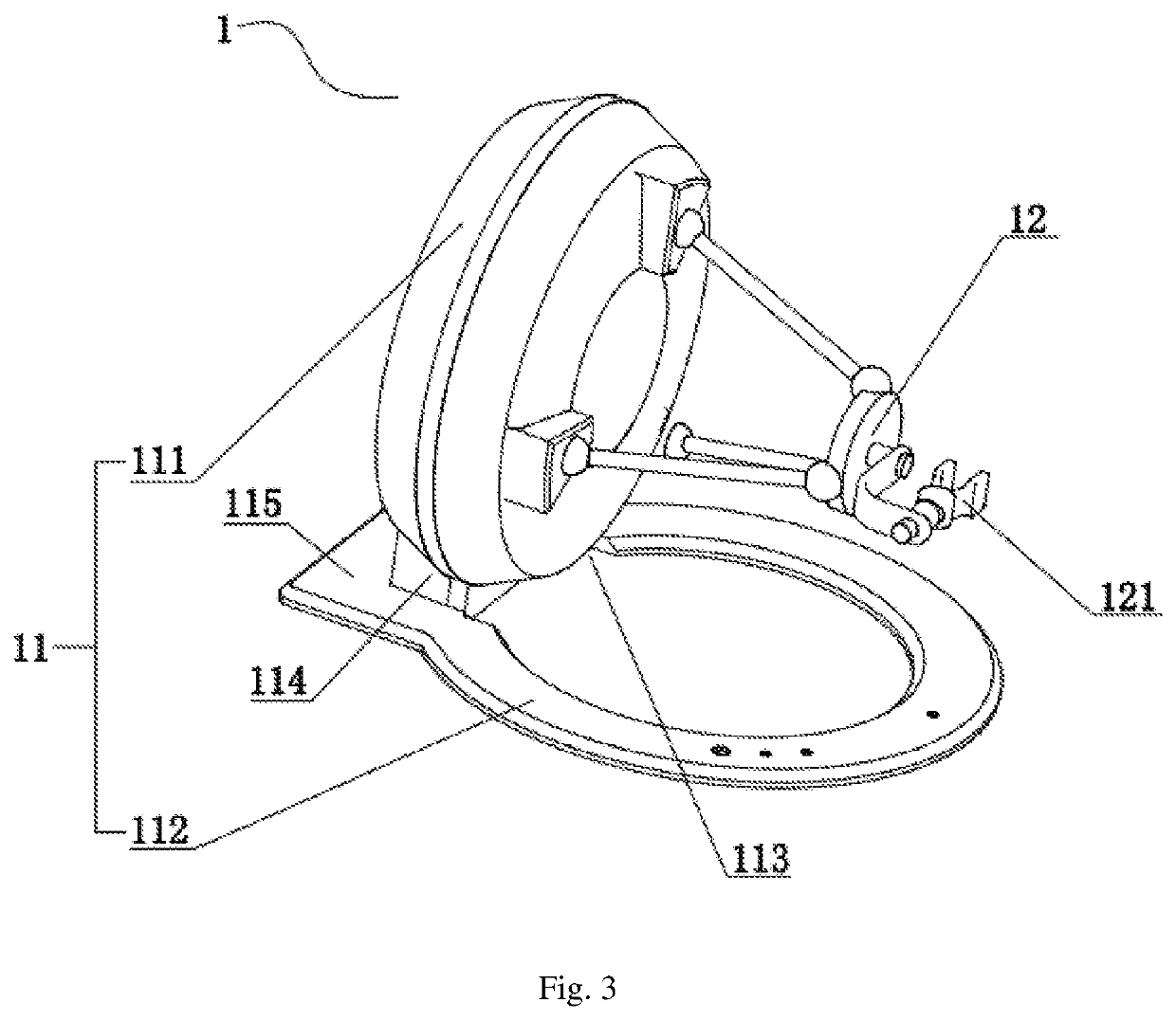
ActiveUS10993776B2Success rateAffects successIncision instrumentsDiagnosticsAutomatic controlEngineering
Disclosed is a console for operating an actuating mechanism, relating to the technical filed of automatic control, and being for use in resolving the technical problem of shift of a controller under the influence of gravity. The console for operating an actuating mechanism includes a controller and a support base. The controller is constrained in both the longitudinal direction and the vertical direction, so that connection between the controller and the support base is tight and reliable, and no displacement would occur in any of three directions (i.e., the longitudinal direction, the horizontal direction, and the vertical direction). Thus, the phenomenon that position information given by the controller is inaccurate due to shift of the controller under the influence of the gravity after long-term usage can be avoided, thereby removing the factors affecting success of surgery, and ensuring the success rate of the surgery.
Owner:CHENGDU BORNS MEDICAL ROBOTICS INC
Aero engine rotor assembling method and device based on concentricity and verticality measurement
ActiveUS9709392B2Improve coaxialityReduce vibrationEngine testingBlade accessoriesAviationRotary stage
An aero engine rotor assembling method and device based on concentricity and verticality measurement belongs to mechanical assembly technology. The present invention effectively solves the problem of poor coaxality after the aero engine rotor is assembled and has the characteristics of high coaxality after the rotor is assembled, reduced vibration, mounting easiness, high flexibility and improved engine performance. The measurement and device is: determining rotary reference; determining the angular positioning of a rotary table; extracting the radial error of the radial mounting plane and the inclination error of the axial mounting plane of the rotor based on the four-probe measuring device to obtain the influencing weight of this rotor to the assembled rotor on coaxality; measuring respectively all the rotors to obtain the influencing weight of each rotor to the assembled rotor on coaxality; vector optimizing the weight of each rotor to obtain the assembling angle of each rotor.
Owner:HARBIN INST OF TECH
Aero engine rotor air floatation assembling device based on gantry structure
InactiveUS9890661B2Improve coaxialityReduce vibrationEngine fuctionsBlade accessoriesAviationAir bearing
Owner:HARBIN INST OF TECH
Series clearance multi-point discharging sparking plug
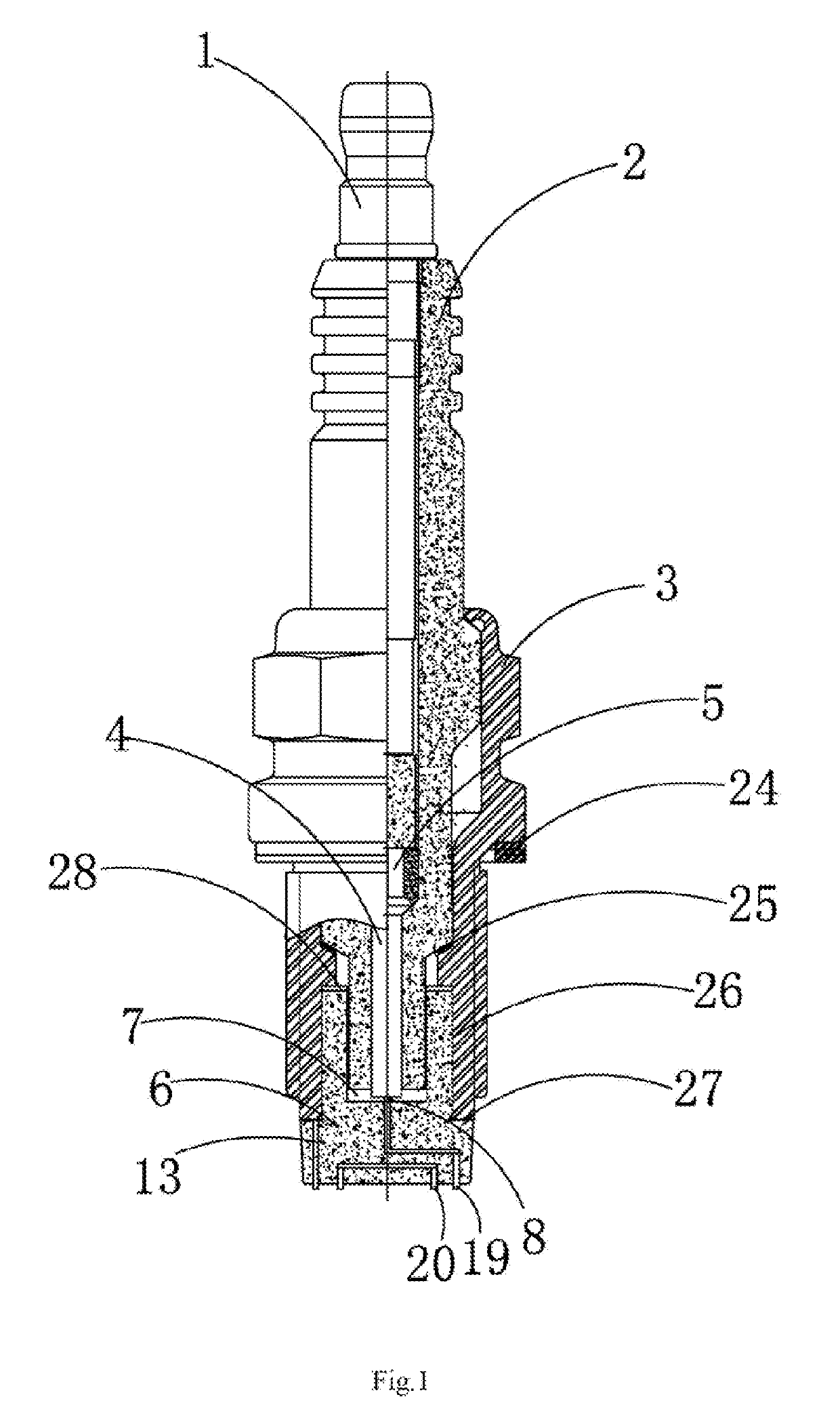
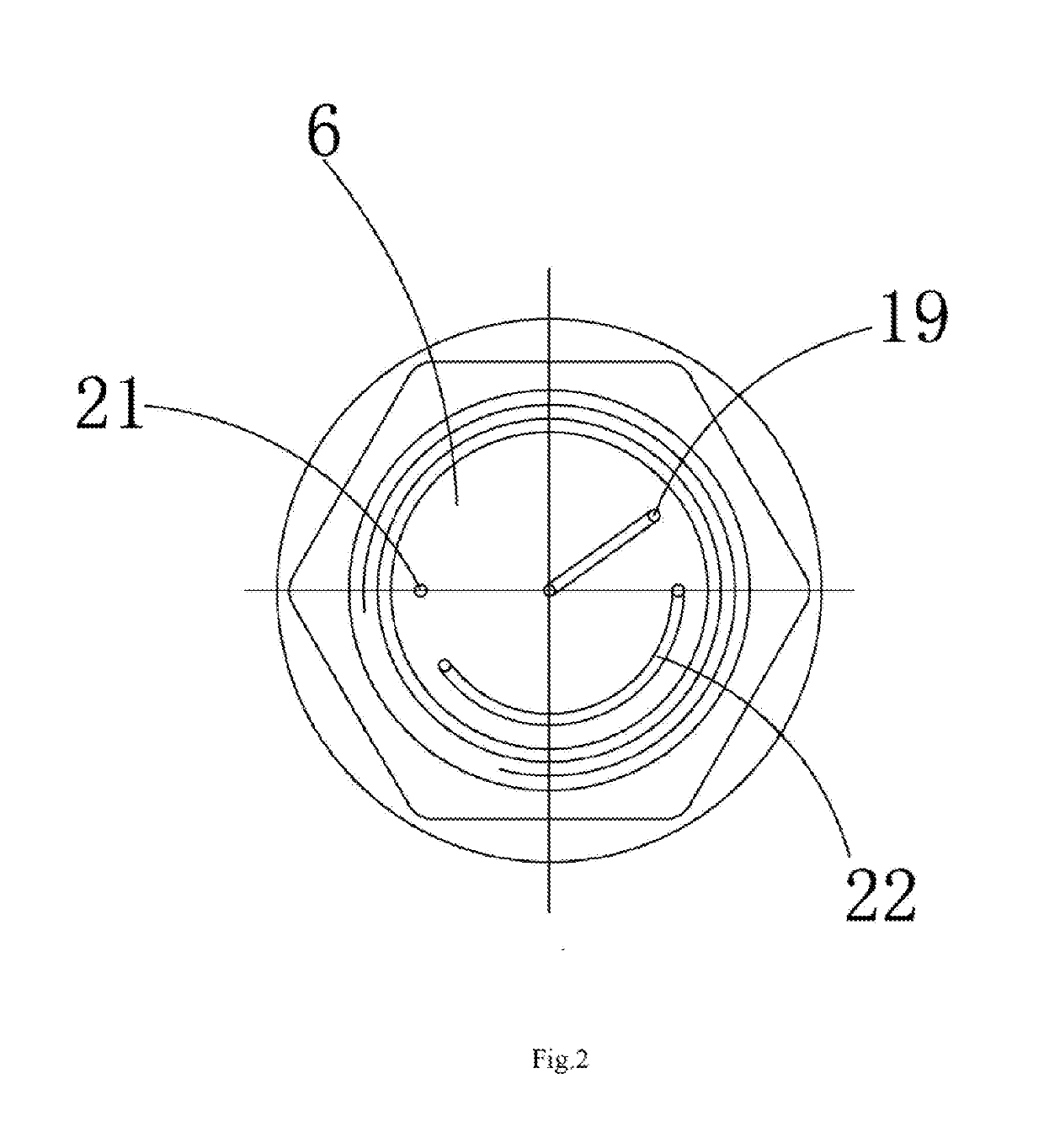
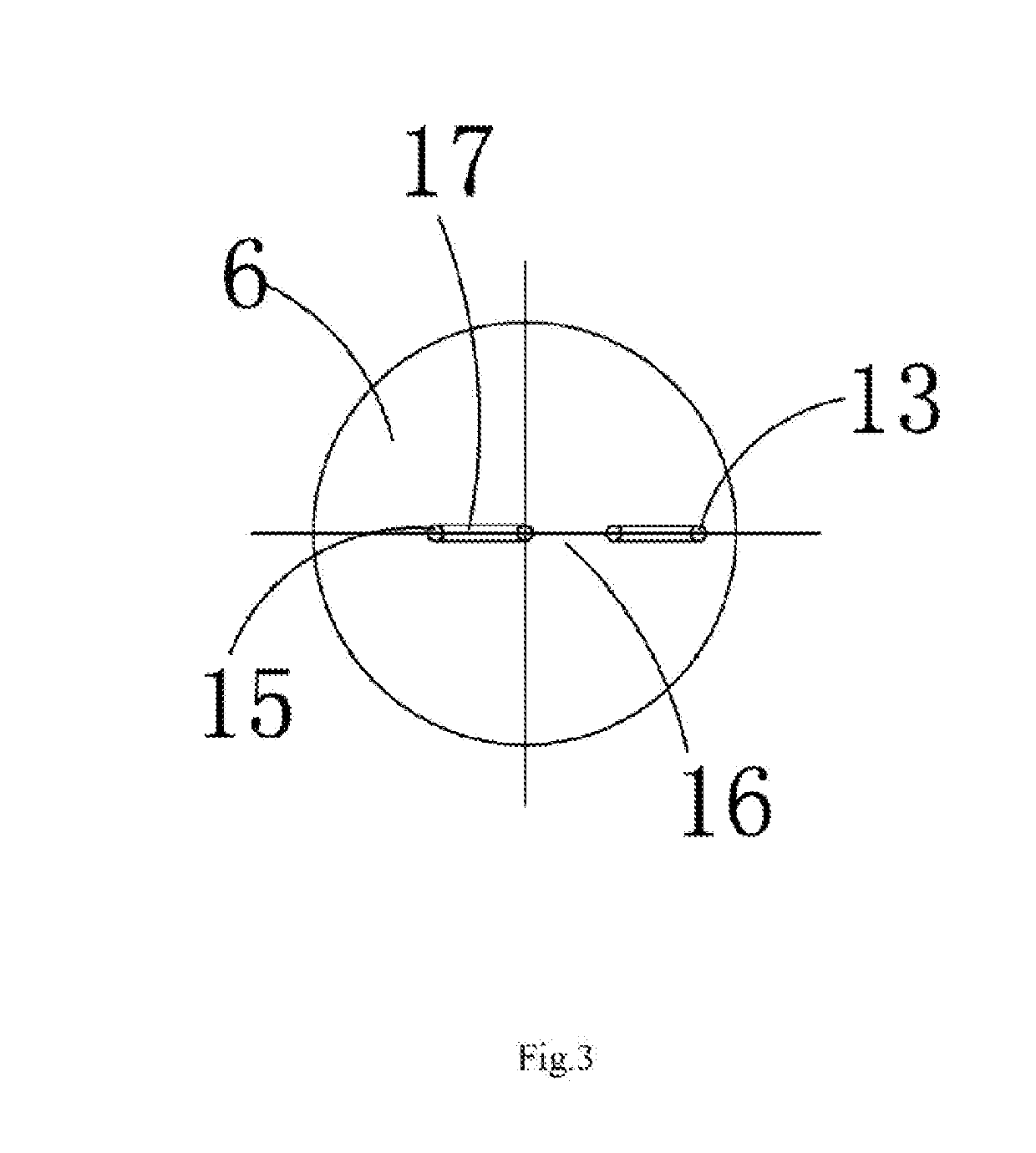
InactiveUS20190148920A1Diffusion fastLower performance requirementsSparking plugsEngineeringIncrease temperature
The present invention relates to a series clearance multi-point discharging spark plug for a spark ignition engine, including a wiring screw. The wiring screw is arranged in an insulator. A central electrode is arranged in a tip of the insulator. A built-in damping resistor is arranged between the central electrode and the wiring screw. A ceramic multi-point discharging ignition table fitted with the insulator is arranged at a bottom of the insulator. A cavity assembly is formed between the ceramic multi-point discharging ignition table and the insulator. An outer wall at an upper end of the ceramic multi-point discharging ignition table is fastened to the shell. An ignition electrode assembly is arranged at the bottom of the ceramic multi-point discharging ignition table. The series clearance multi-point discharging spark plug increases temperature and pressure of mixed gas during ignition, thereby improving ignition performance, shortening combustion duration and improving engine performance.
Owner:LOTUS SPARK PLUGS TECH HANGZHOU CO LTD
Protective film for under-screen ultrasonic fingerprint identification and preparation method thereof
A protective film for under-screen ultrasonic fingerprint identification includes a protective layer and a glue layer, and the thickness of the protective film within a fingerprint identification area is ≤0.70 mm. The protective film for under-screen ultrasonic fingerprint identification has low requirements for the shape of the protective layer. As the thickness of the fingerprint identification area is uniform and stable, it is easy to unlock the smartphone, or even achieve a 100% success rate by means of the supporting points.
Owner:ZHOU RUBIAO
High voltage ceramic electric heating body
PendingUS20220039210A1Reliability be ensureSuccess rate be 100 %Incandescent ignitionHeater elementsPhysicsElectric heating
A high-voltage ceramic electric heating element, comprising a body (9), the body (9) being hollow and having an open trailing portion, and a notch (7) being provided on the body (9) in the axial direction and extending through from left to right; a temperature control region (8) is provided at a position on an outer resistance layer (2) of the body (9), and the cross sectional area of the temperature control region (8) is smaller than the cross sectional area of the body (9). Using the high-voltage ceramic electric heating element can improve the ignition reliability and the service life.
Owner:CHONGQING LE MARK CERAMIC TECH CO LTD
Methods for amplifying hepatitis c virus nucleic acids
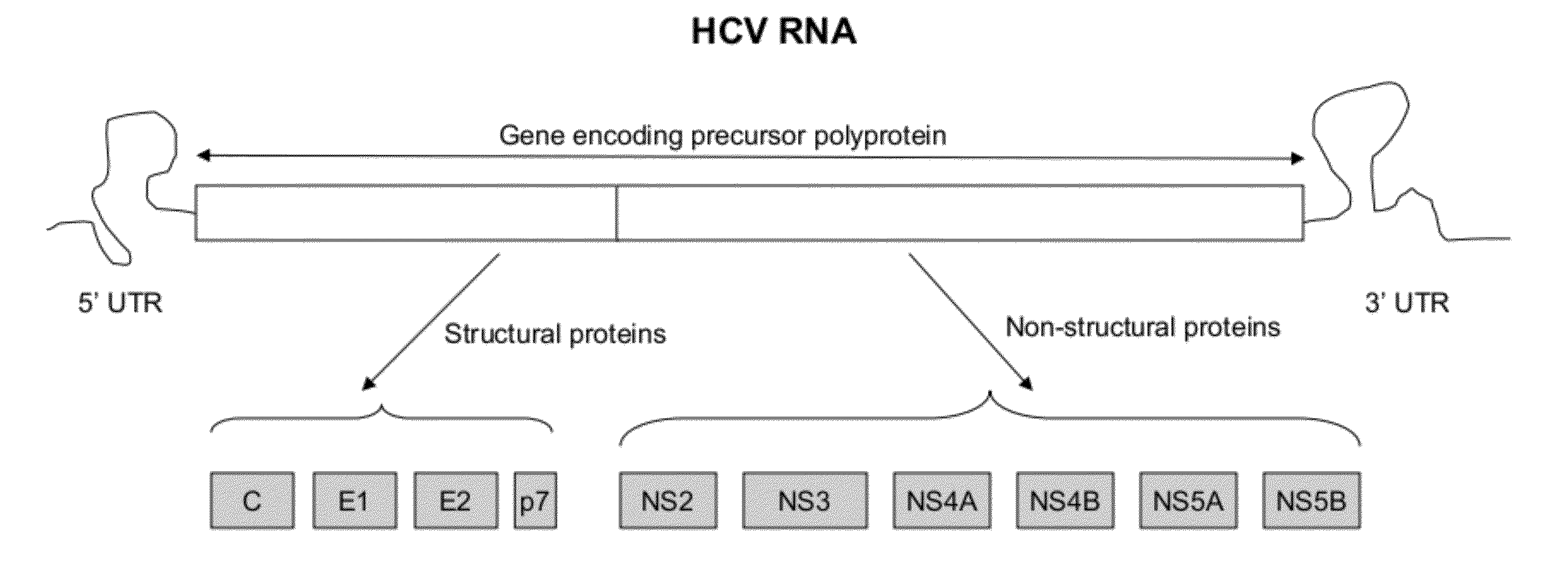
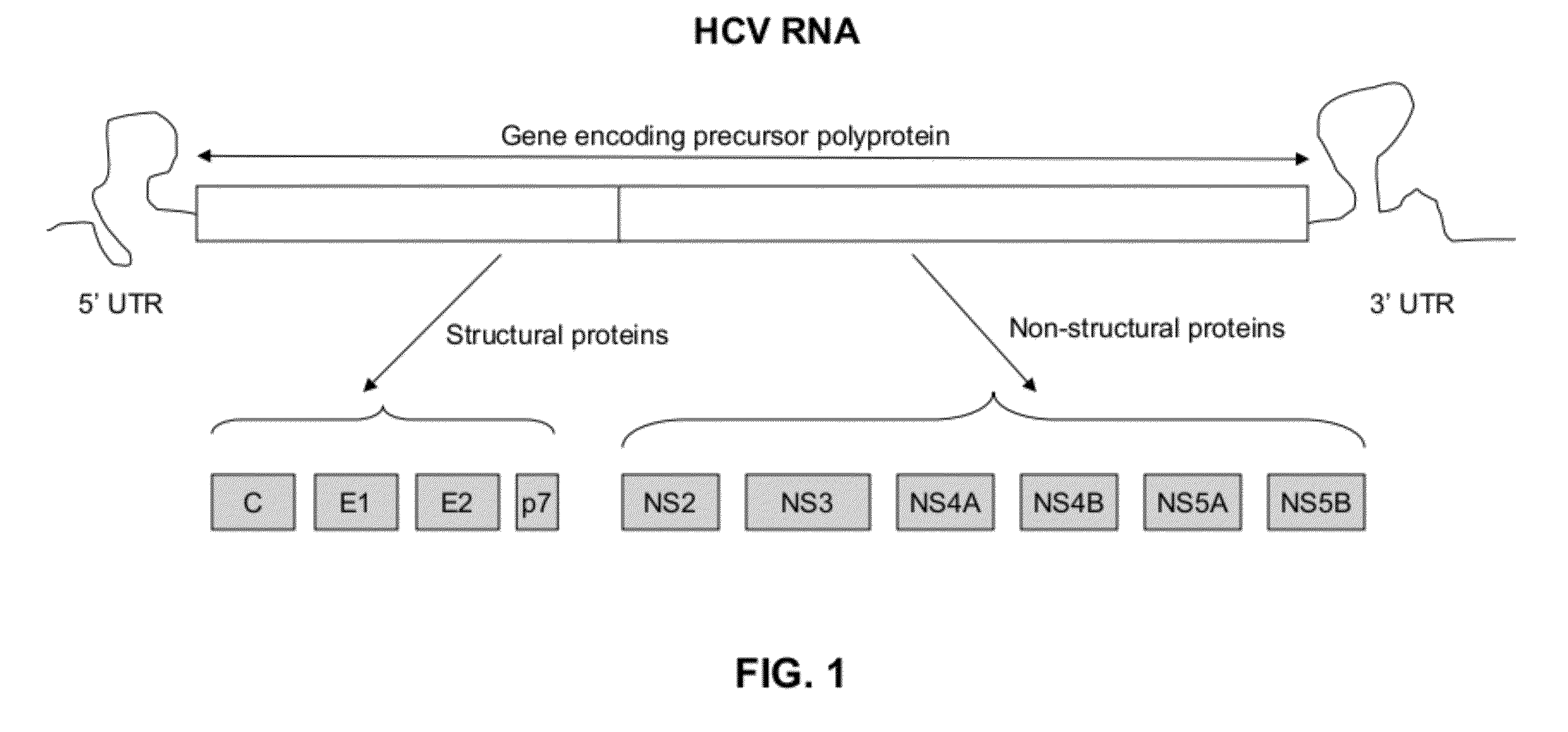
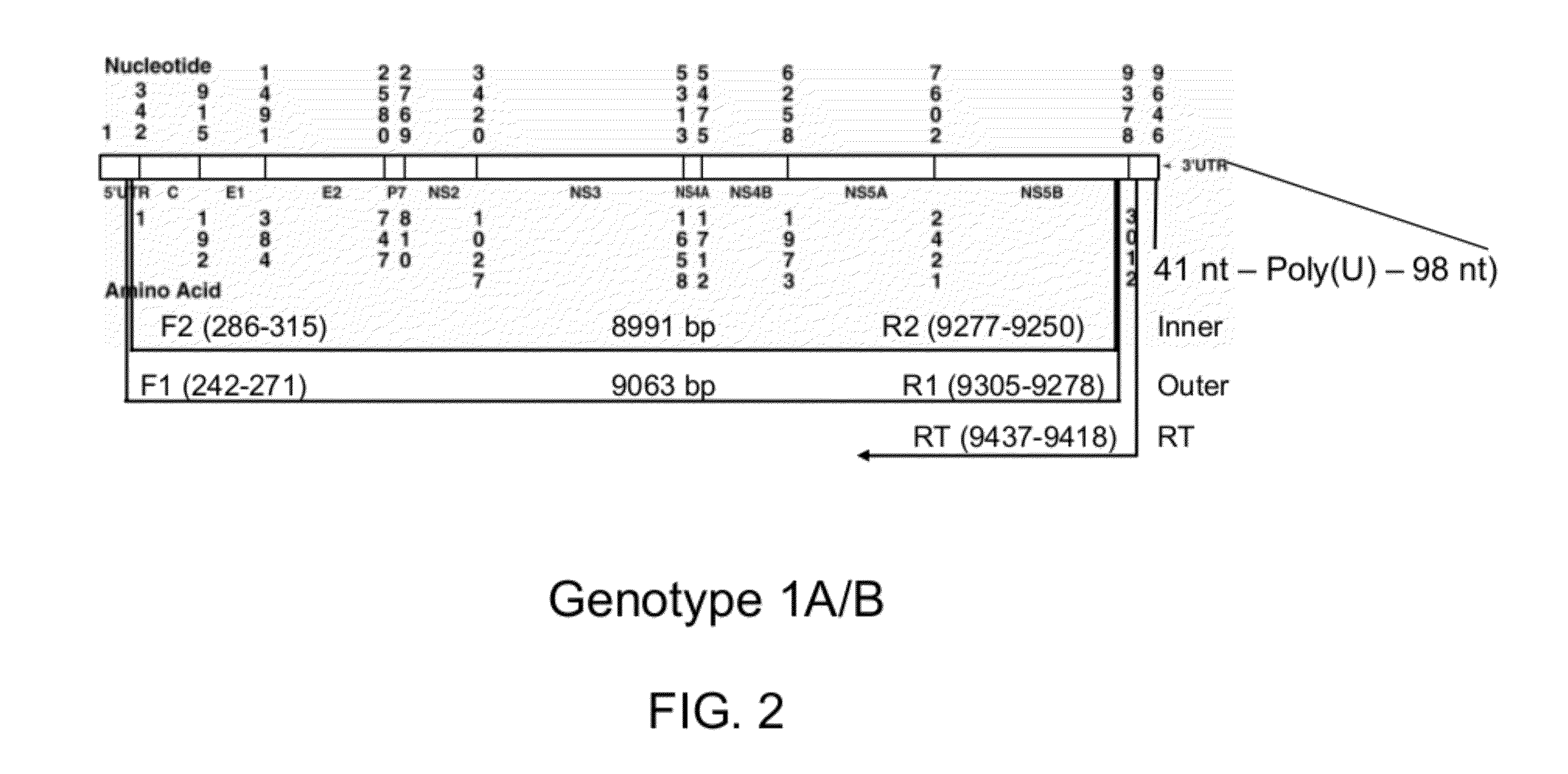
InactiveUS20120129155A1Good reproducibilitySuccess rateMicrobiological testing/measurementFermentationDna templateRNA
A method of amplifying an HCV nucleic acid in an HCV infected sample comprises amplifying a segment of a DNA template that is complementary to a genome of HCV RNA from the sample by a two-stage PCR, wherein a first stage PCR employs a first outer primer and a second outer primer, and a second stage PCR employs a first inner primer and a second inner primer. The nucleotide sequence of the first outer primer comprises a nucleotide sequence as set forth in SEQ ID NO: 2; or SEQ ID NO:9, wherein optionally 1, 2 or 3 nucleotides are other nucleotides than those of SEQ ID NO: 9. The nucleotide sequence of the second outer primer comprises a nucleotide sequence set forth in SEQ ID NO: 3 or 4; or a nucleotide sequence as set forth in SEQ ID NO: 10 or 11, wherein optionally 1, 2 or 3 nucleotides are other nucleotides than those of SEQ ID NO: 10 and 11. The nucleotide sequence of the first inner primer comprises a nucleotide sequence as set forth in SEQ ID NO: 5; or SEQ ID NO:12, wherein optionally 1, 2 or 3 nucleotides are other nucleotides than those of SEQ ID NO: 12. The nucleotide sequence of the second inner primer comprises a nucleotide sequence as set forth in SEQ ID NO: 6 or 7; or a nucleotide sequence as set forth in SEQ ID NO: 13 or 14, wherein optionally 1, 2 or 3 nucleotides are other nucleotides than those of SEQ ID NO: 13 and 14.
Owner:VERTEX PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com