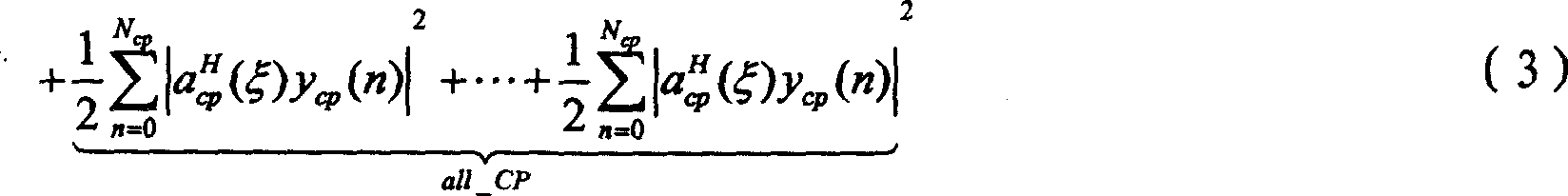Patents
Literature
277results about How to "Reduce insertion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
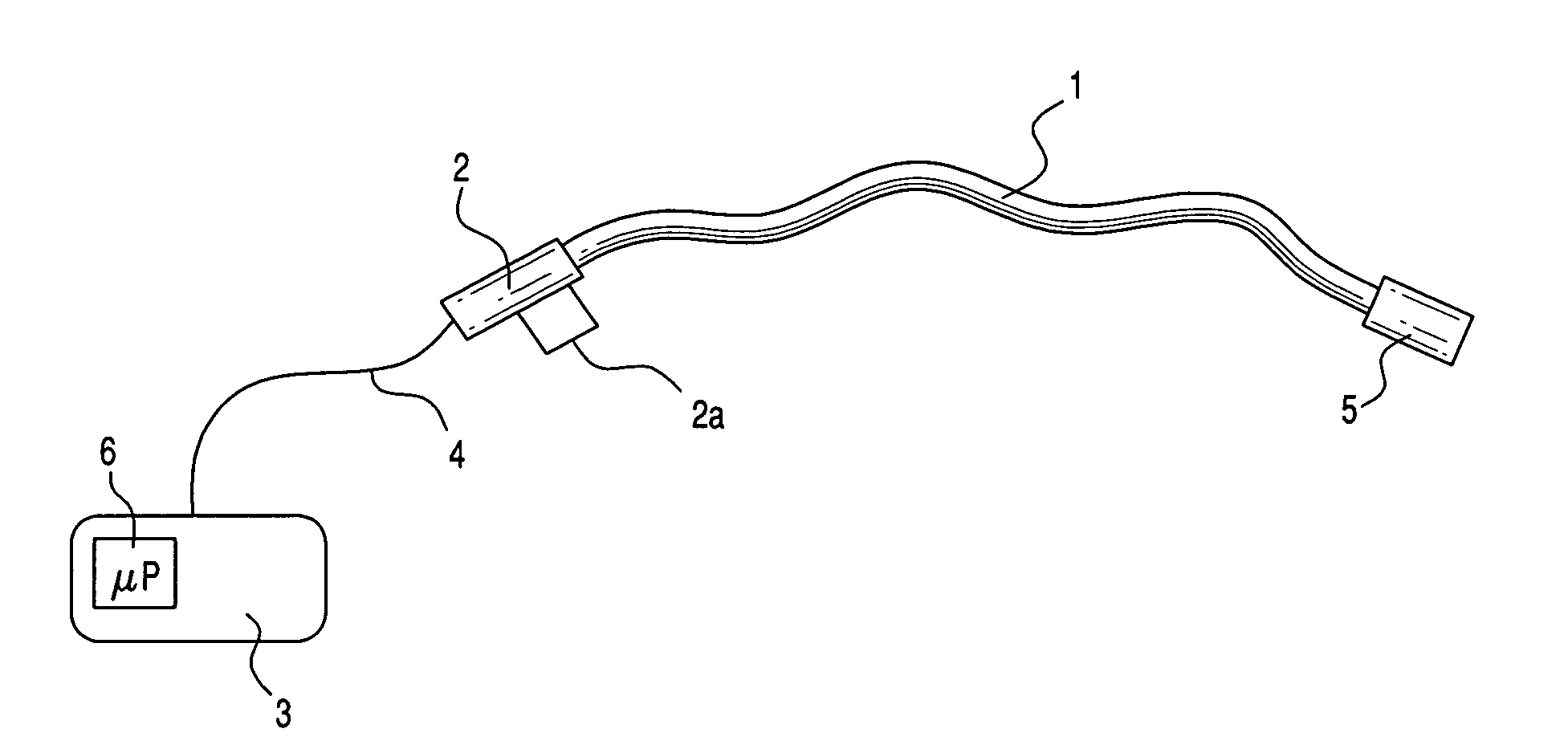
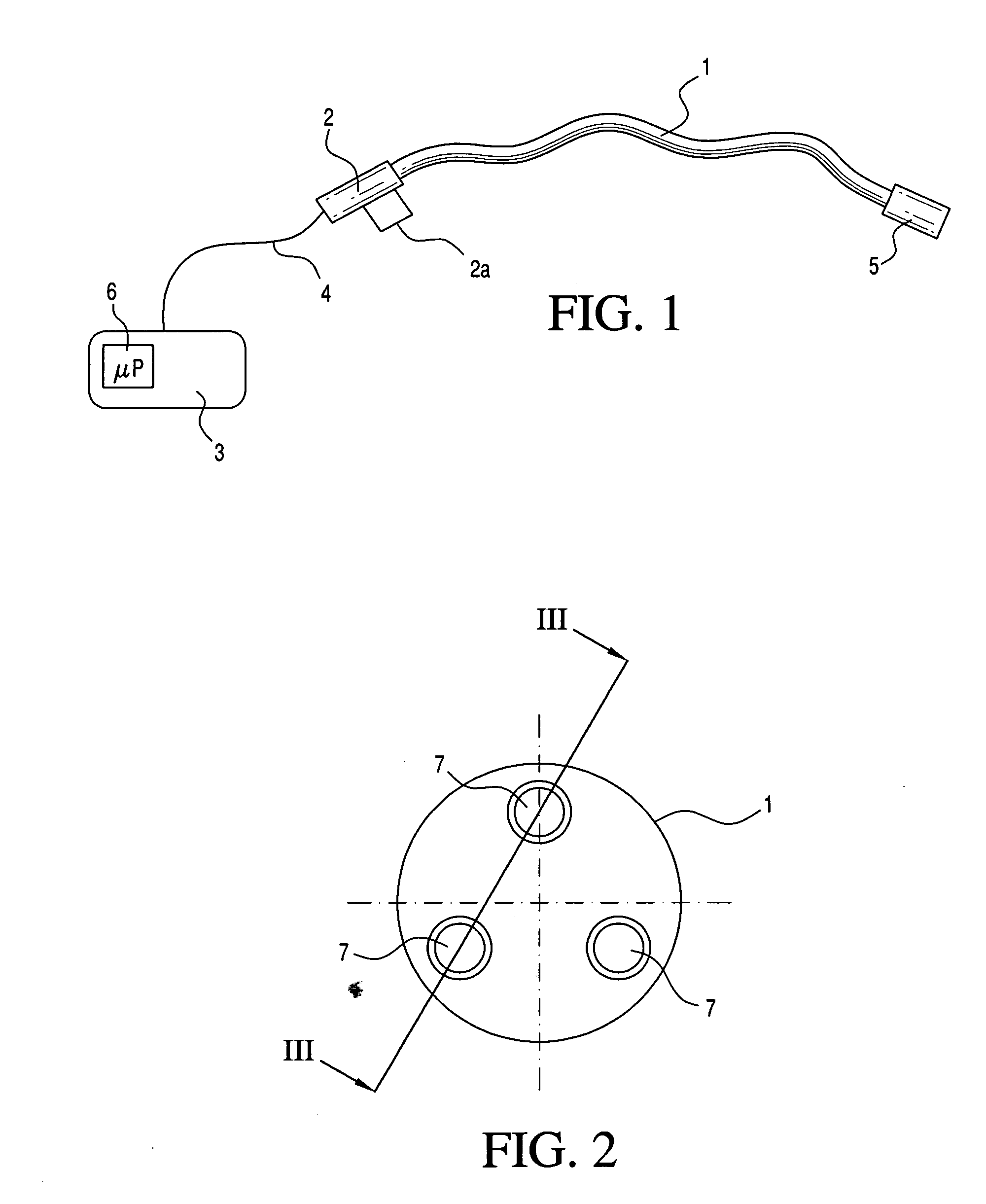
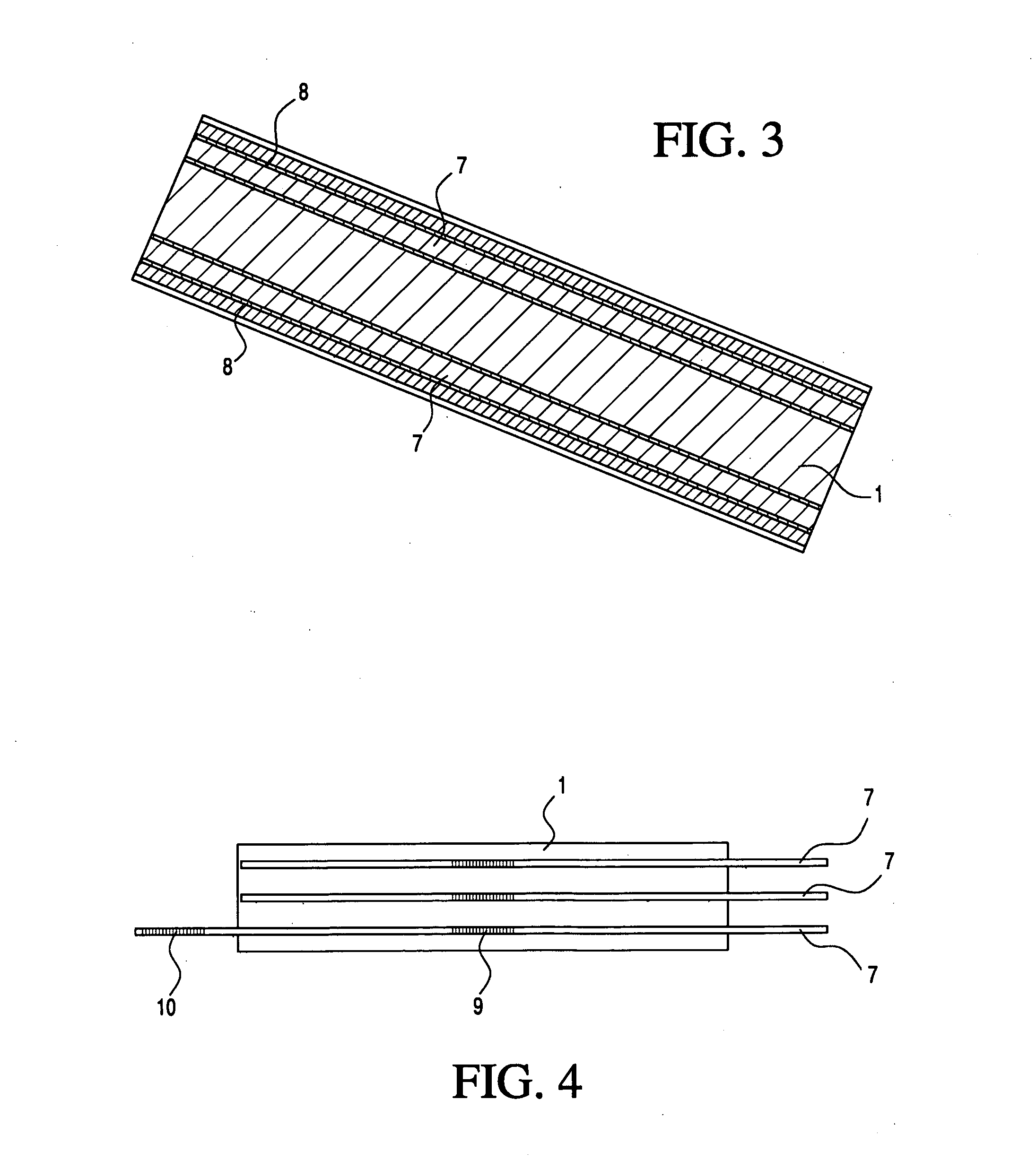
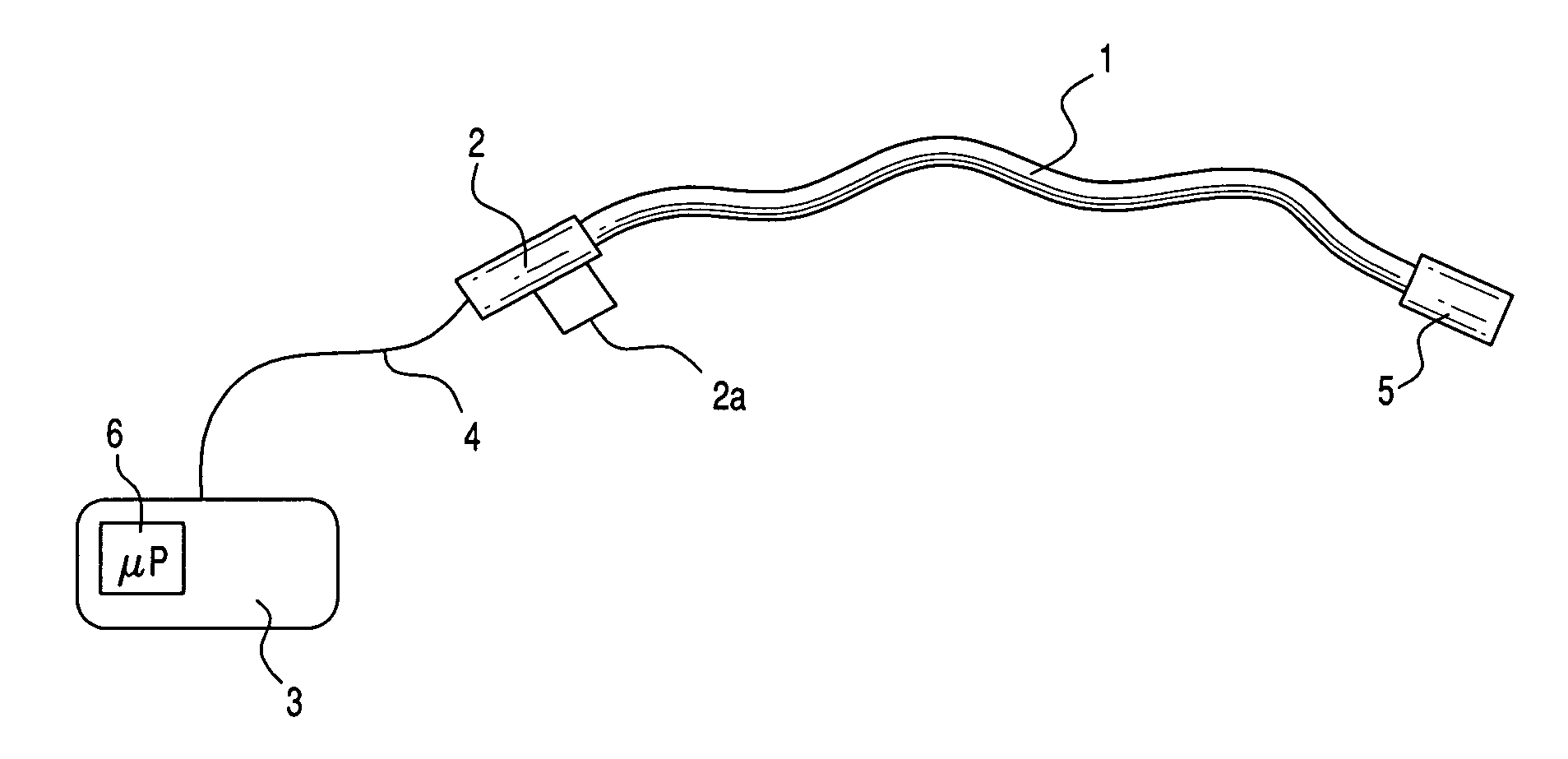
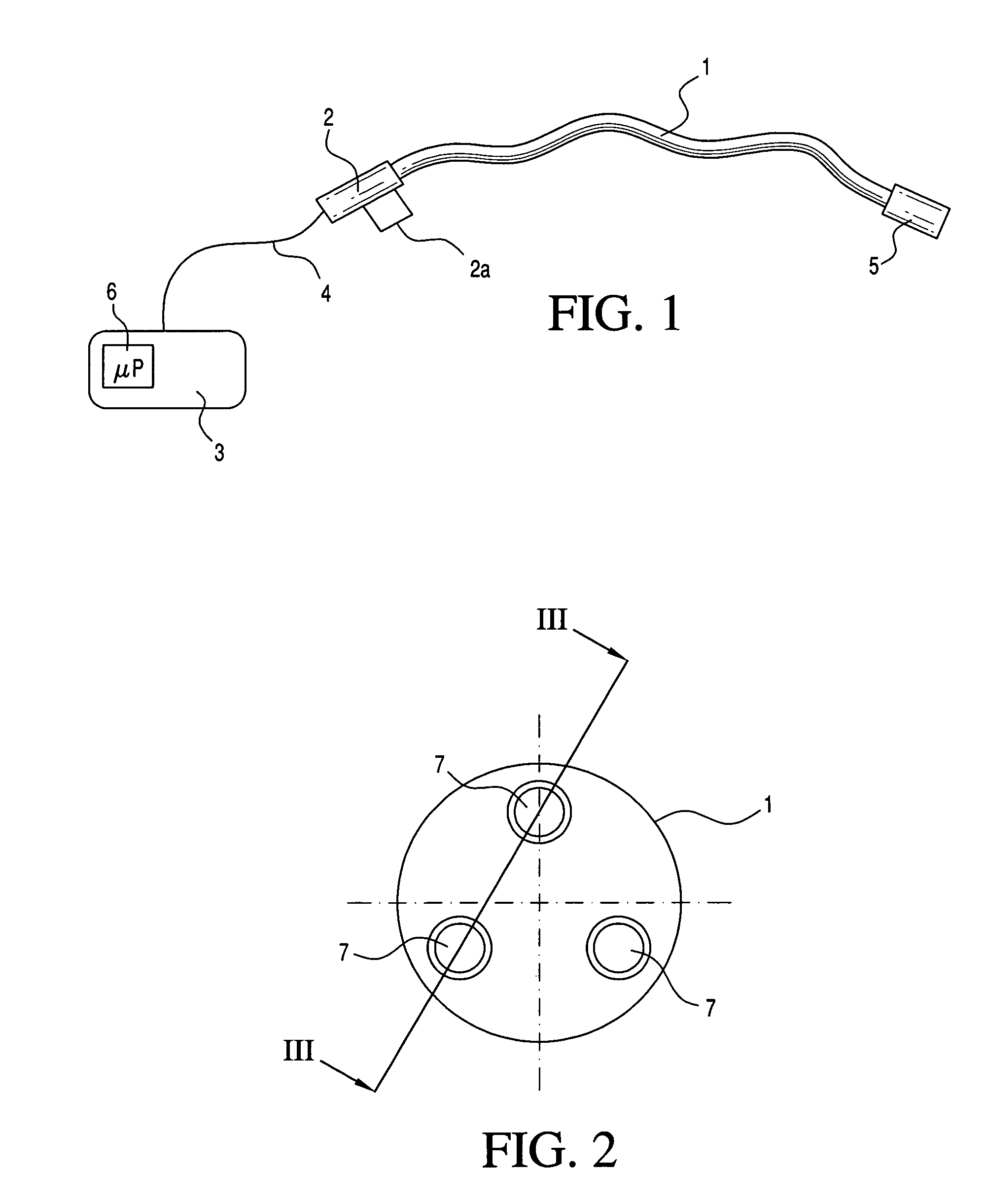
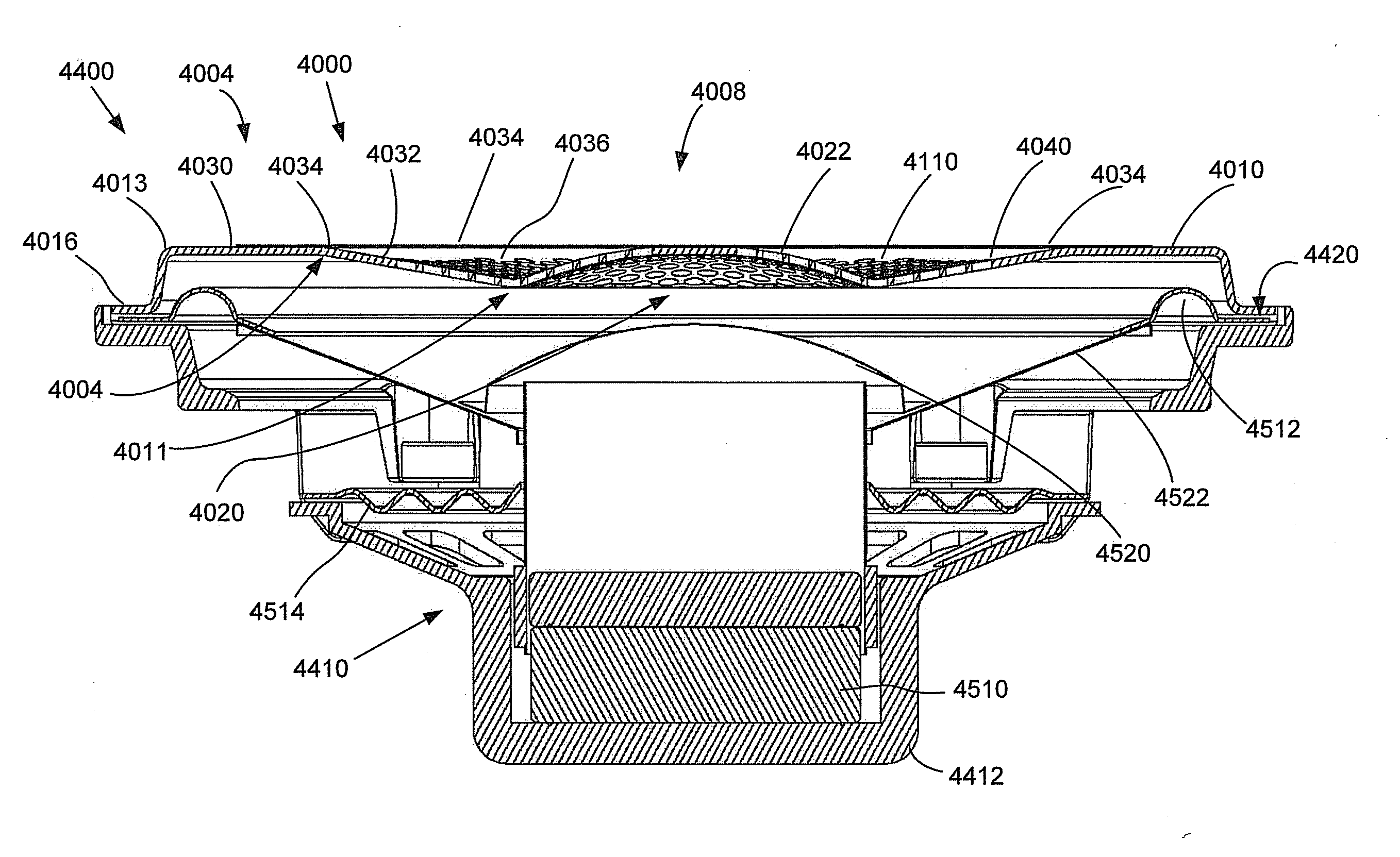
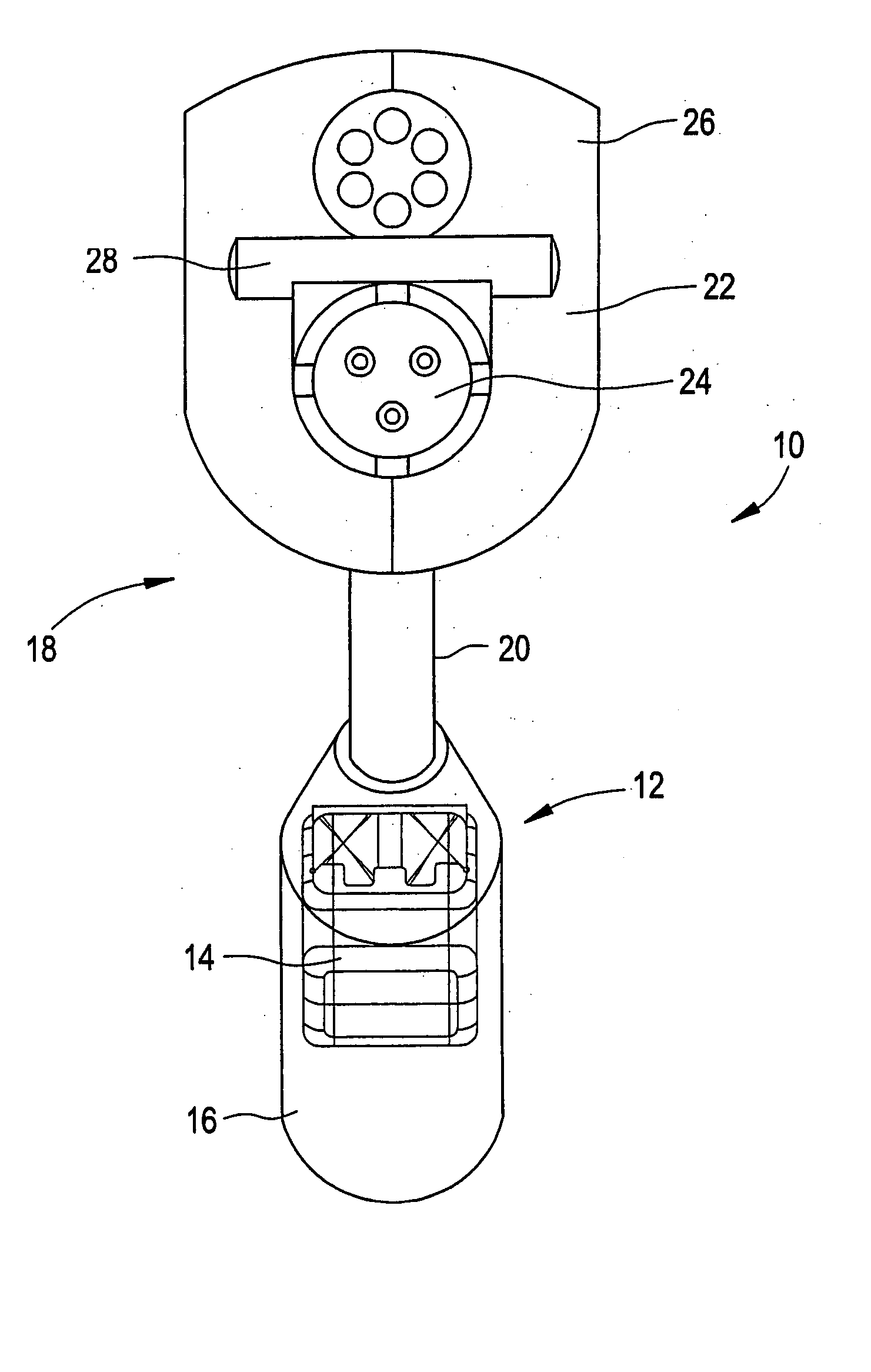
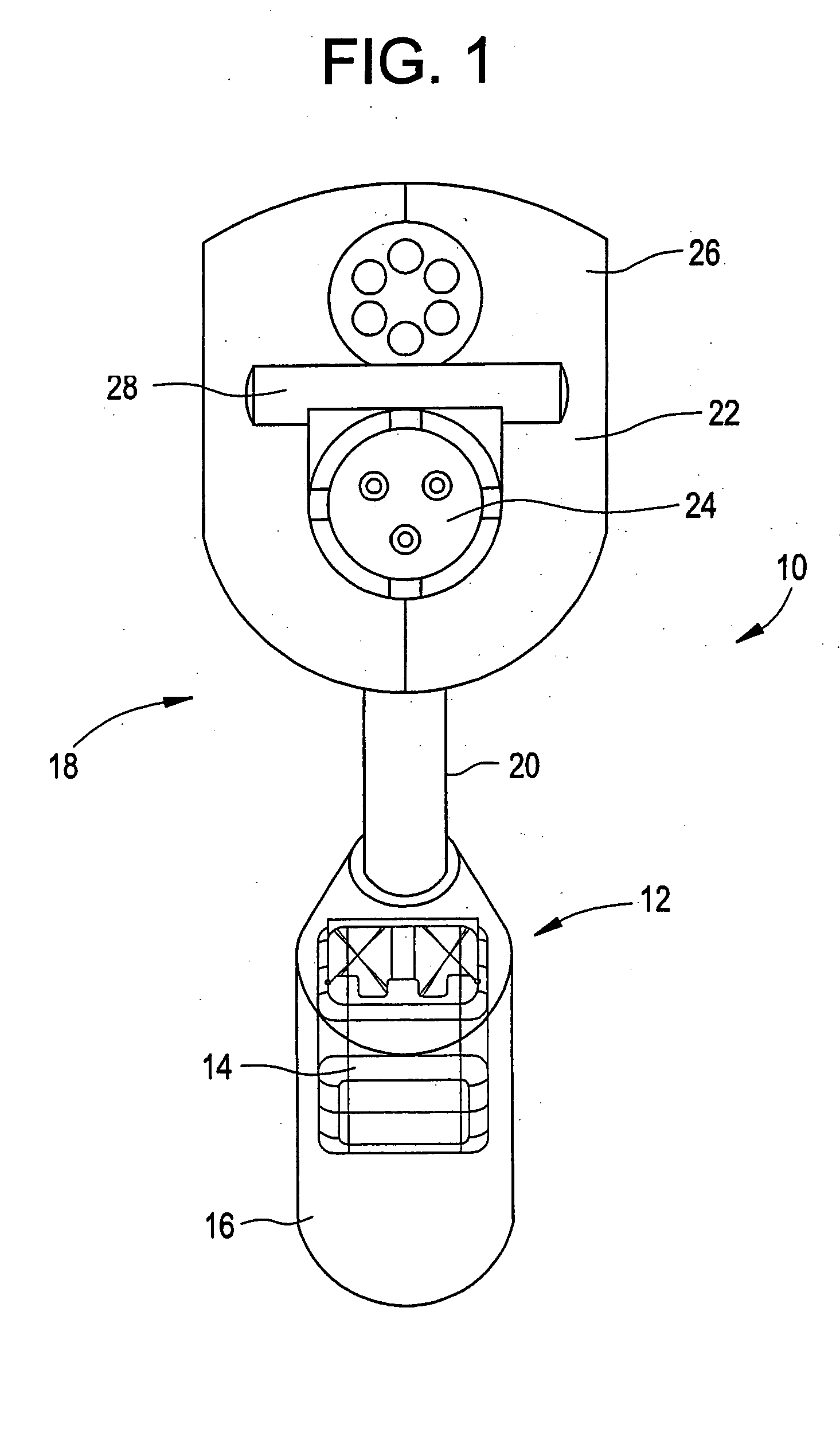
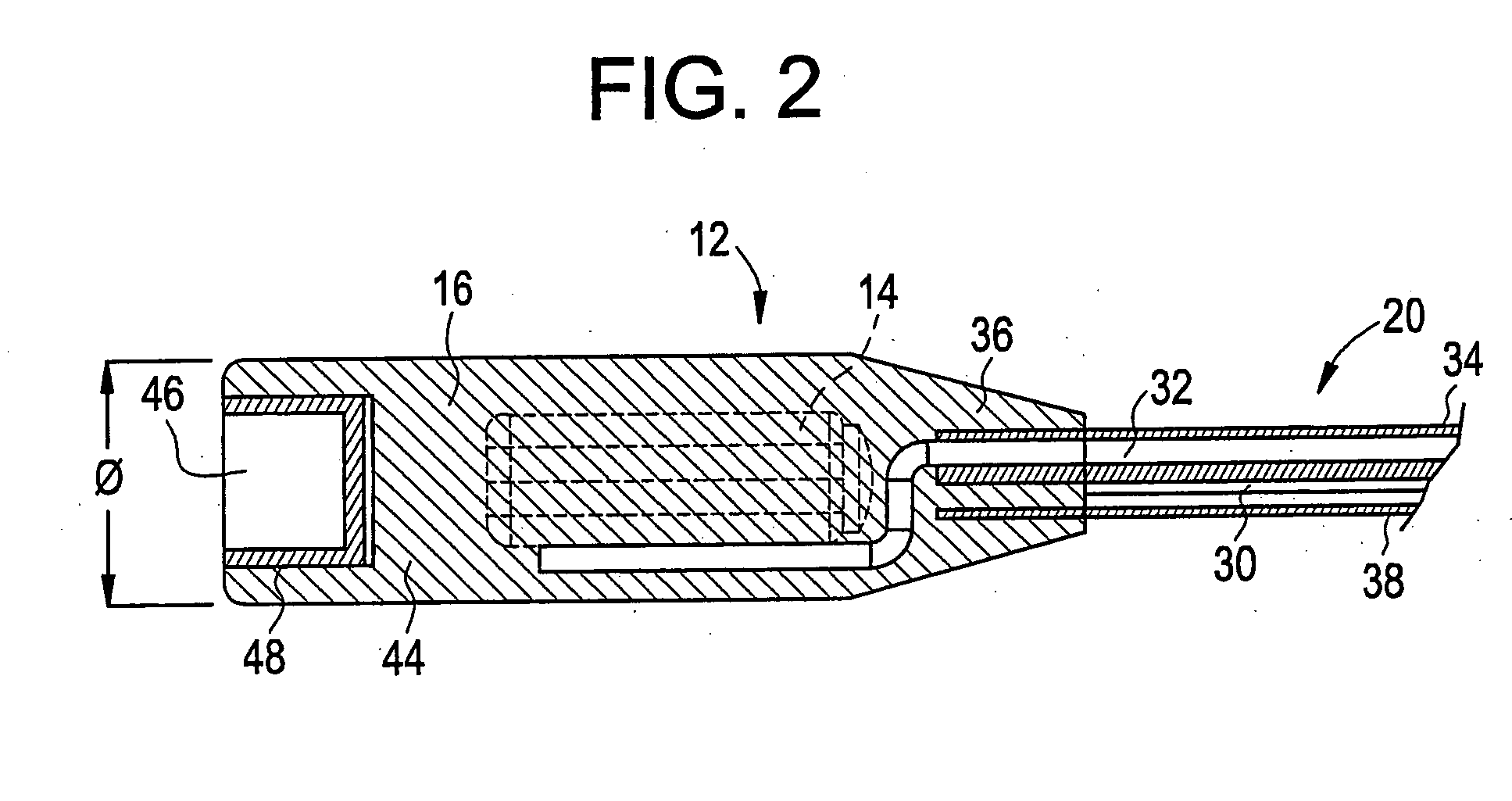
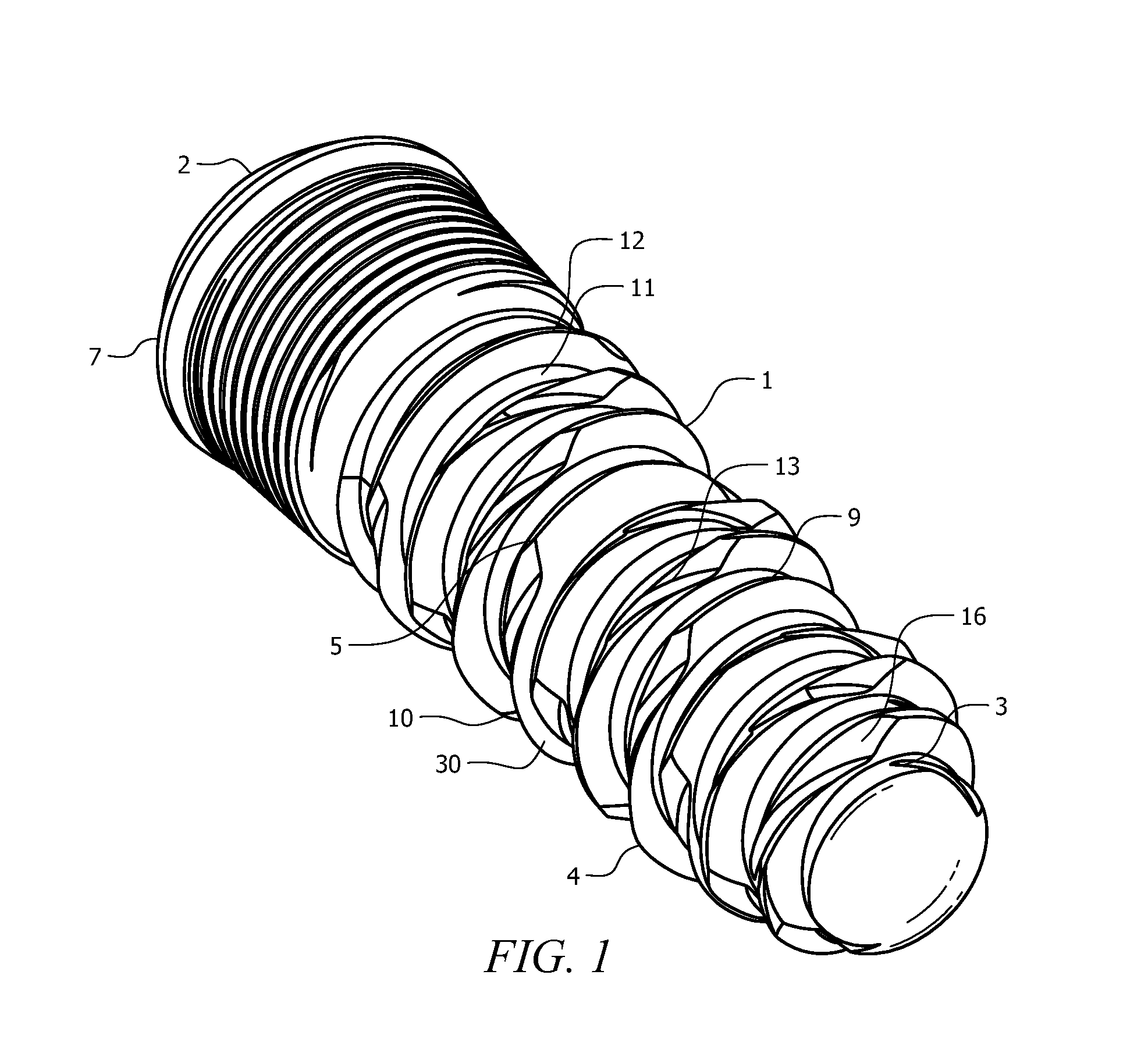
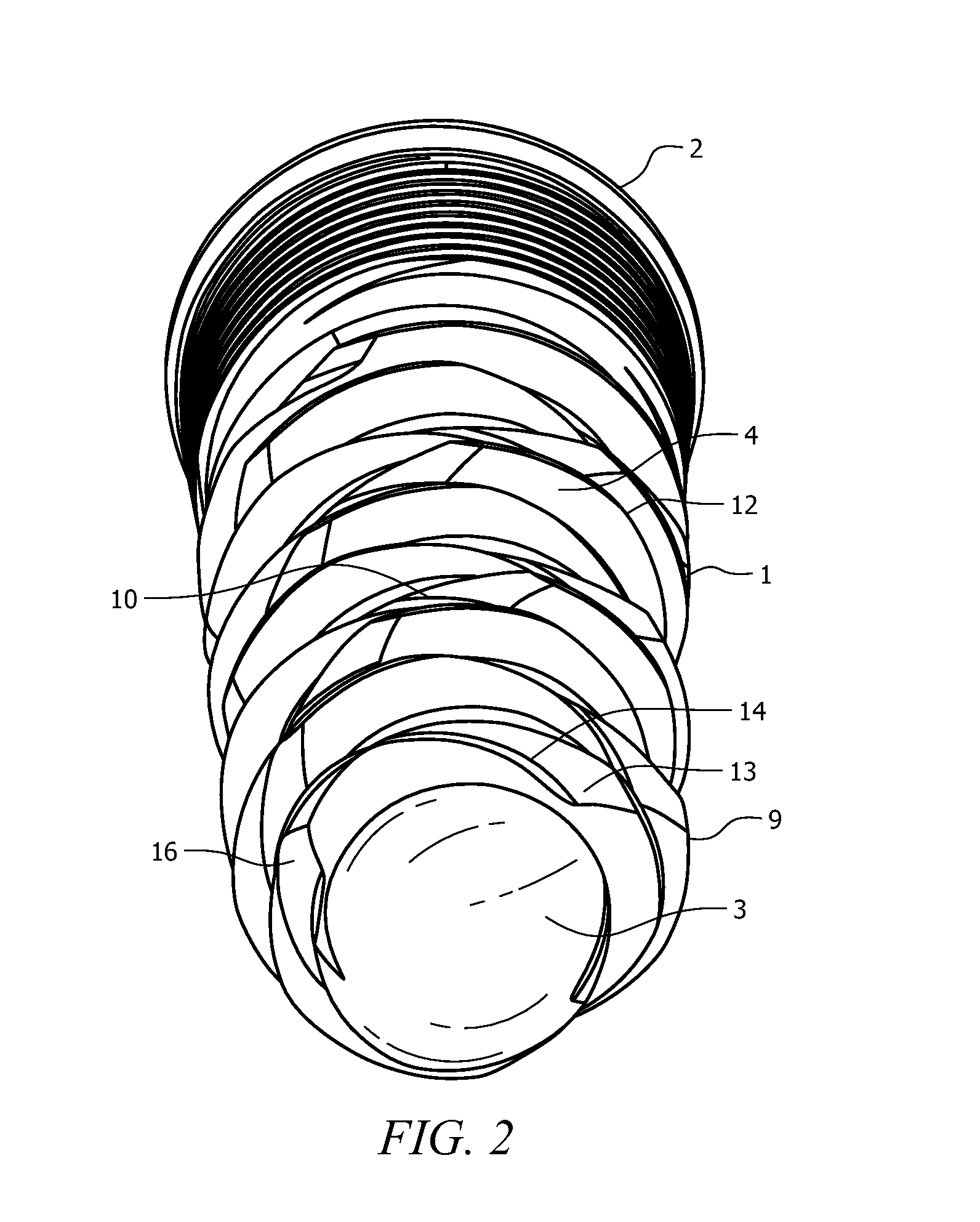
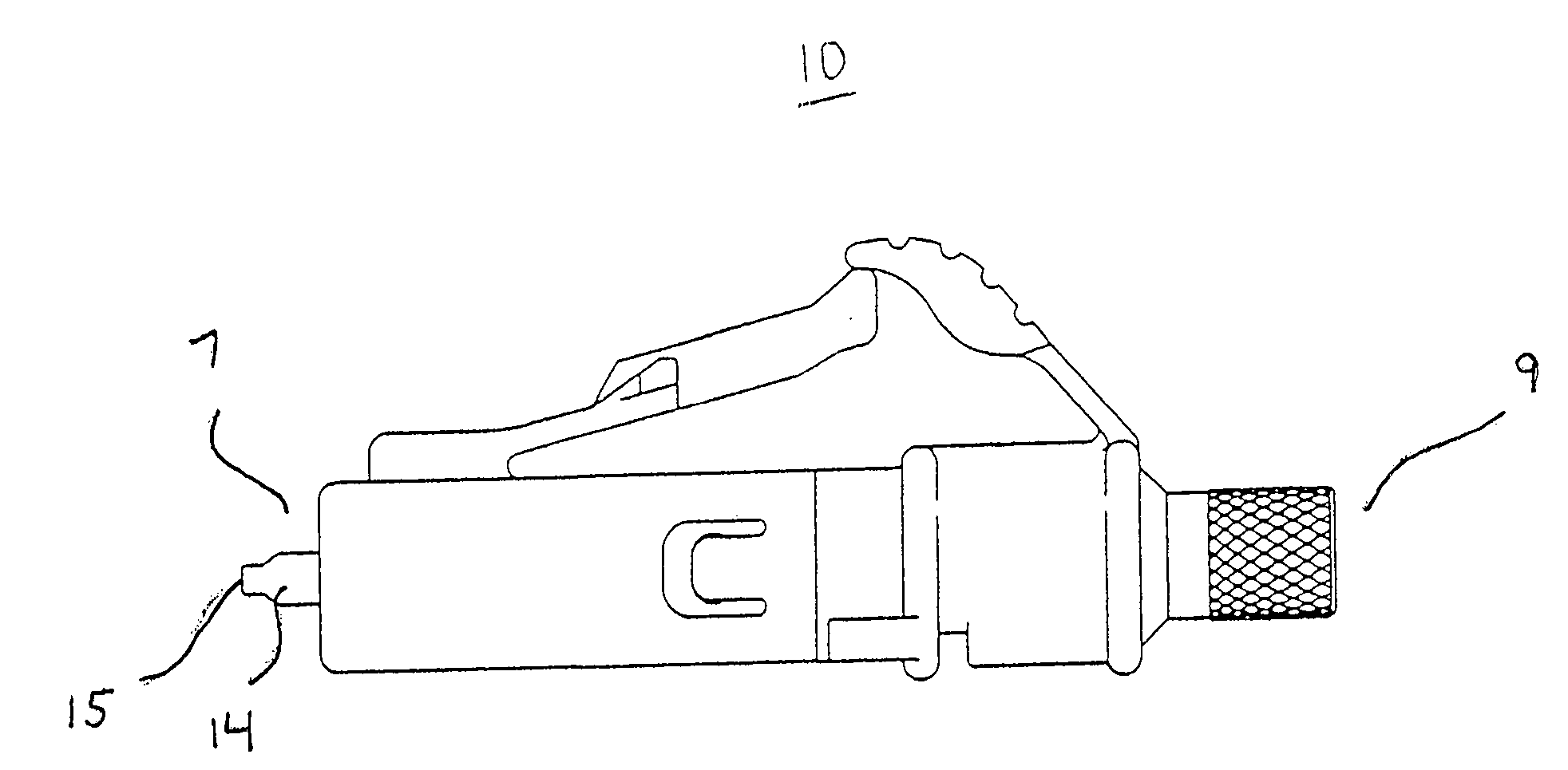
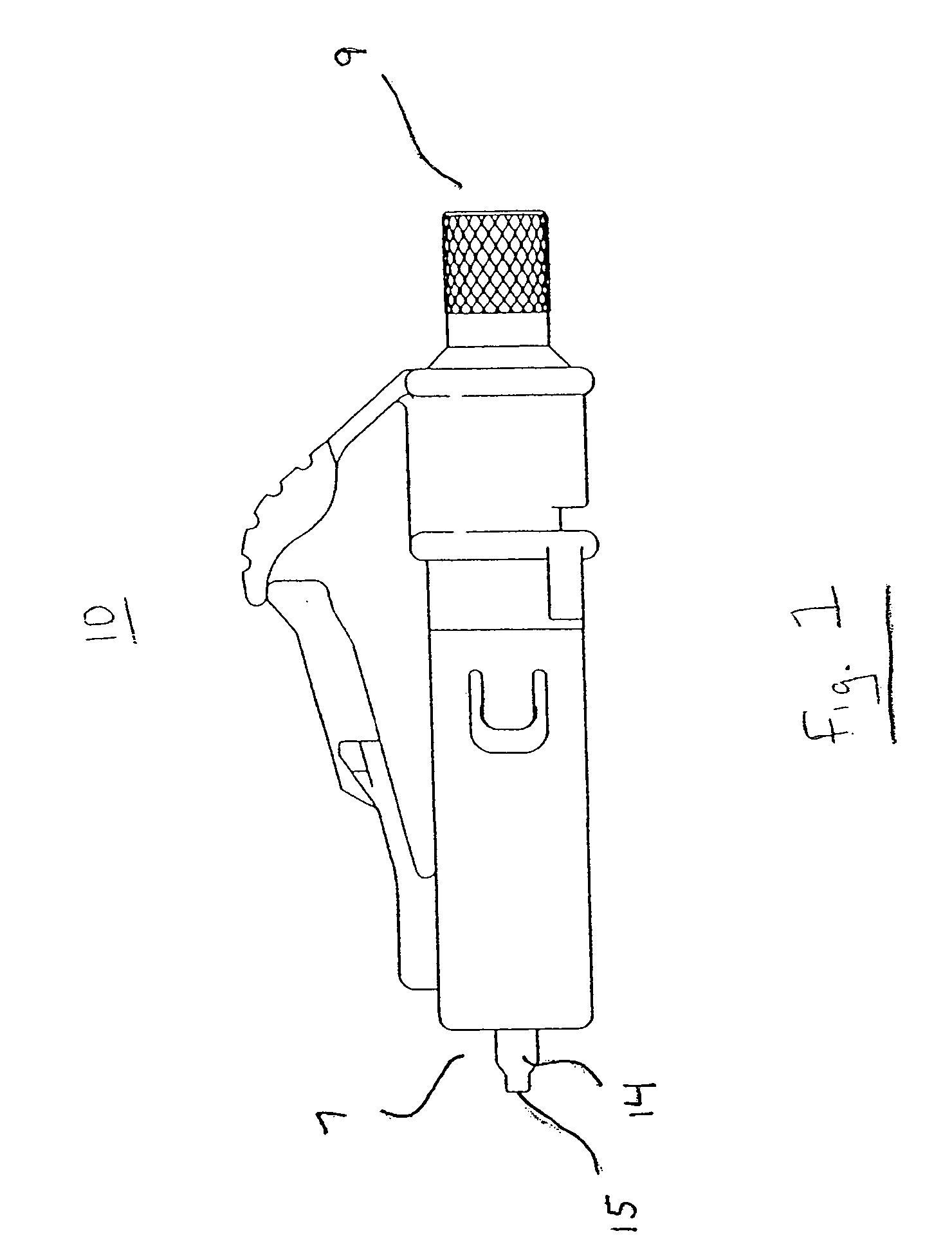
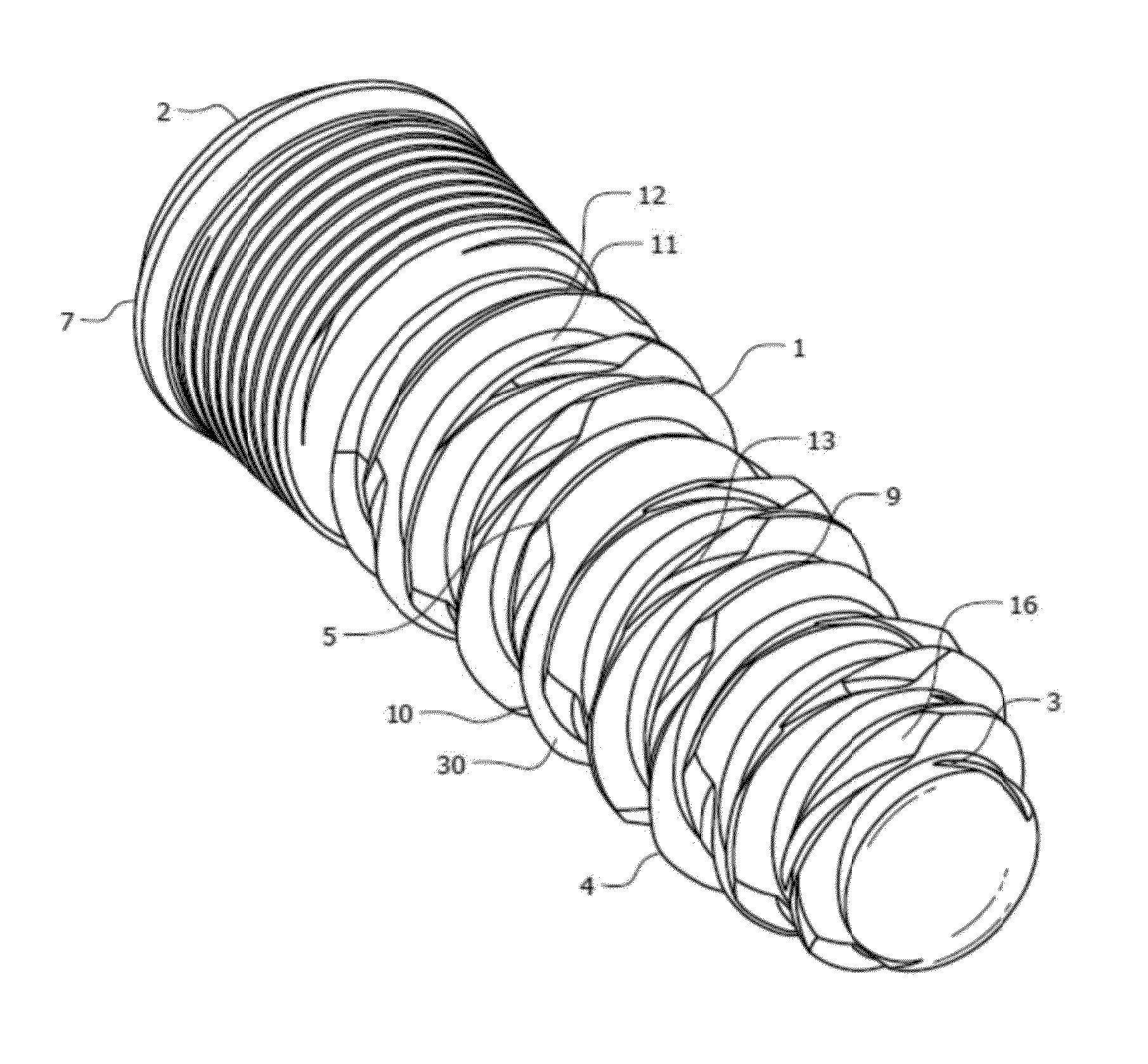
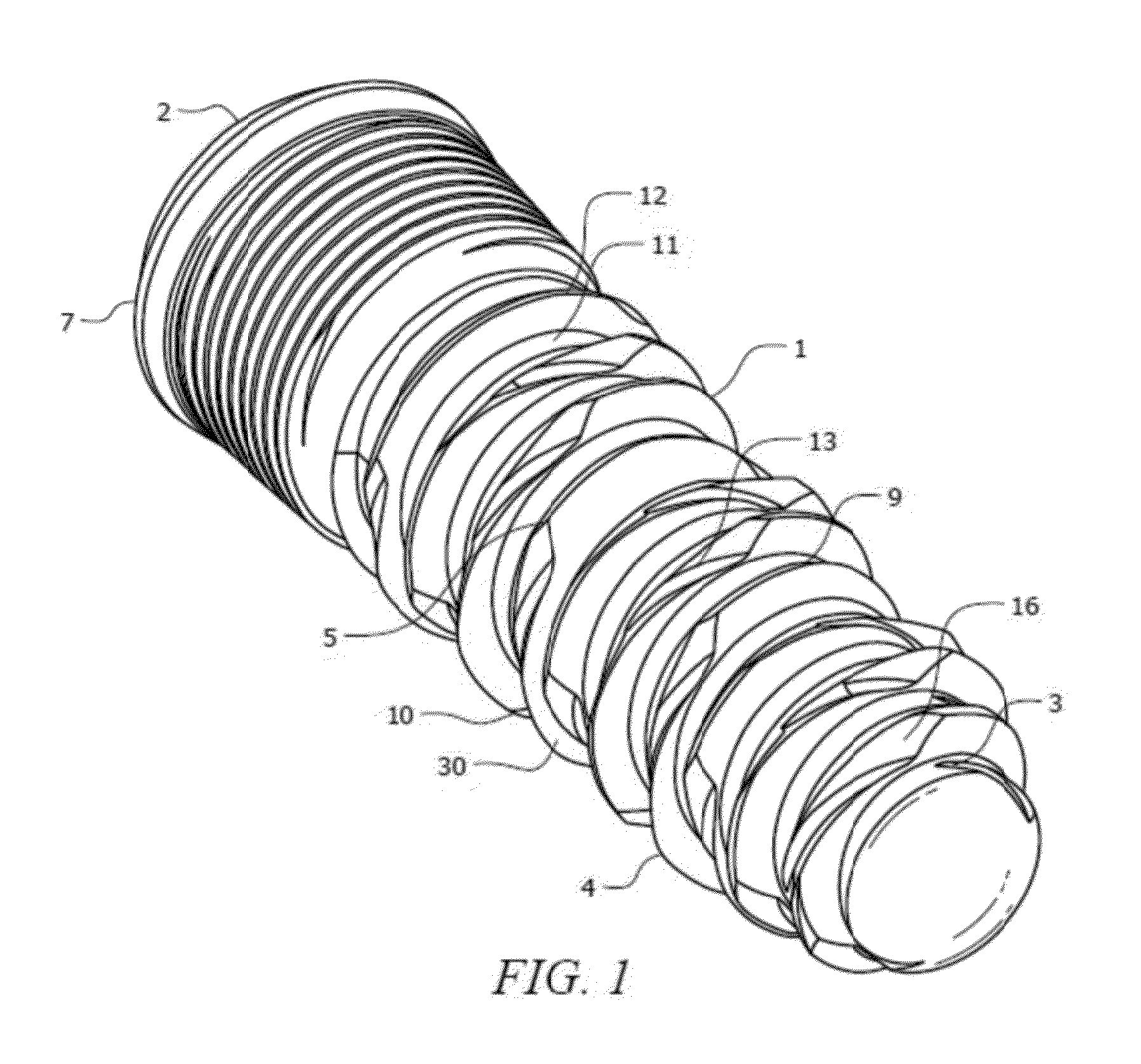
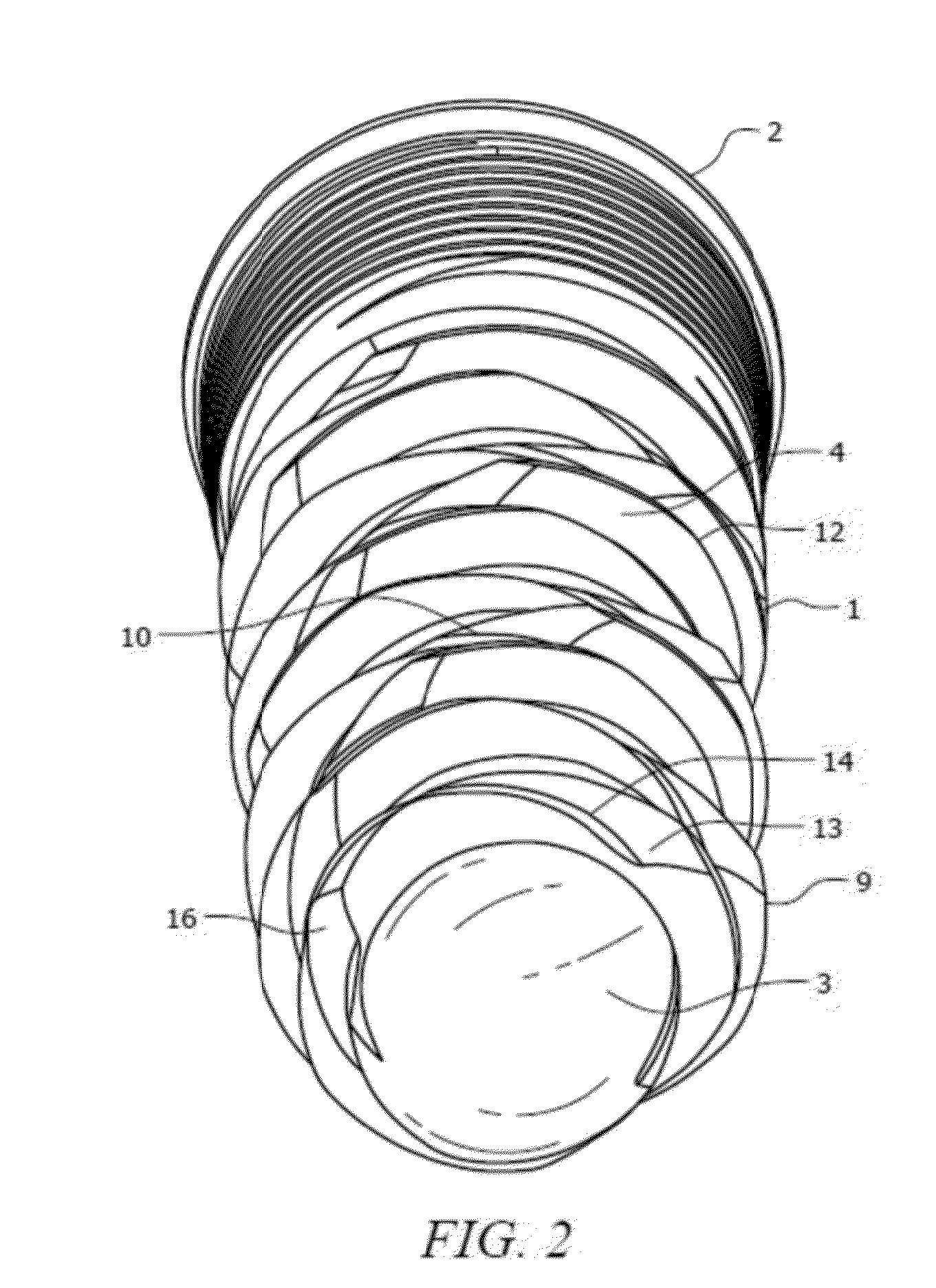
Medical apparatus system having optical fiber load sensing capability
ActiveUS20060200049A1Facilitate speedHelp accuracyStrain gaugePerson identificationRobotic systemsProcess logic
Apparatus is provided for diagnosing or treating an organ or vessel, wherein a deformable body having at least two optical fiber sensors disposed in a distal extremity thereof is coupled to processing logic programmed to compute a multi-dimensional force vector responsive to detected changes in the optical characteristics of the optical fiber sensors arising from deflection of the distal extremity resulting from contact with the tissue of the wall of the organ or vessel. The force vector may be used to facilitate manipulation of the deformable body either directly or automatically using a robotic system.
Owner:ST JUDE MEDICAL INT HLDG SARL
Artificial dielectric antenna elements
InactiveUS7379030B1Improve radiation performance and frequency bandwidthReduce total massAntenna feed intermediatesPhysicsHorn antenna
An artificial dielectric antenna structure for reducing the mass and insertion loss of an antenna is provided. The artificial dielectric antenna structure includes a plurality of layers of dielectric material. Each layer of dielectric material has a dielectric constant. The artificial dielectric antenna structure further includes a plurality of spacing layers interposed between the plurality of layers of dielectric material. Each of the plurality of spacing layers has a dielectric constant lower than the dielectric constant of any of the plurality of layers of dielectric material. The artificial dielectric antenna structure may be disposed within a horn antenna. The artificial dielectric antenna structure may alternately be disposed upon a transmission medium to form a dielectric resonator antenna.
Owner:LOCKHEED MARTIN CORP
Method and device for generating optical signals
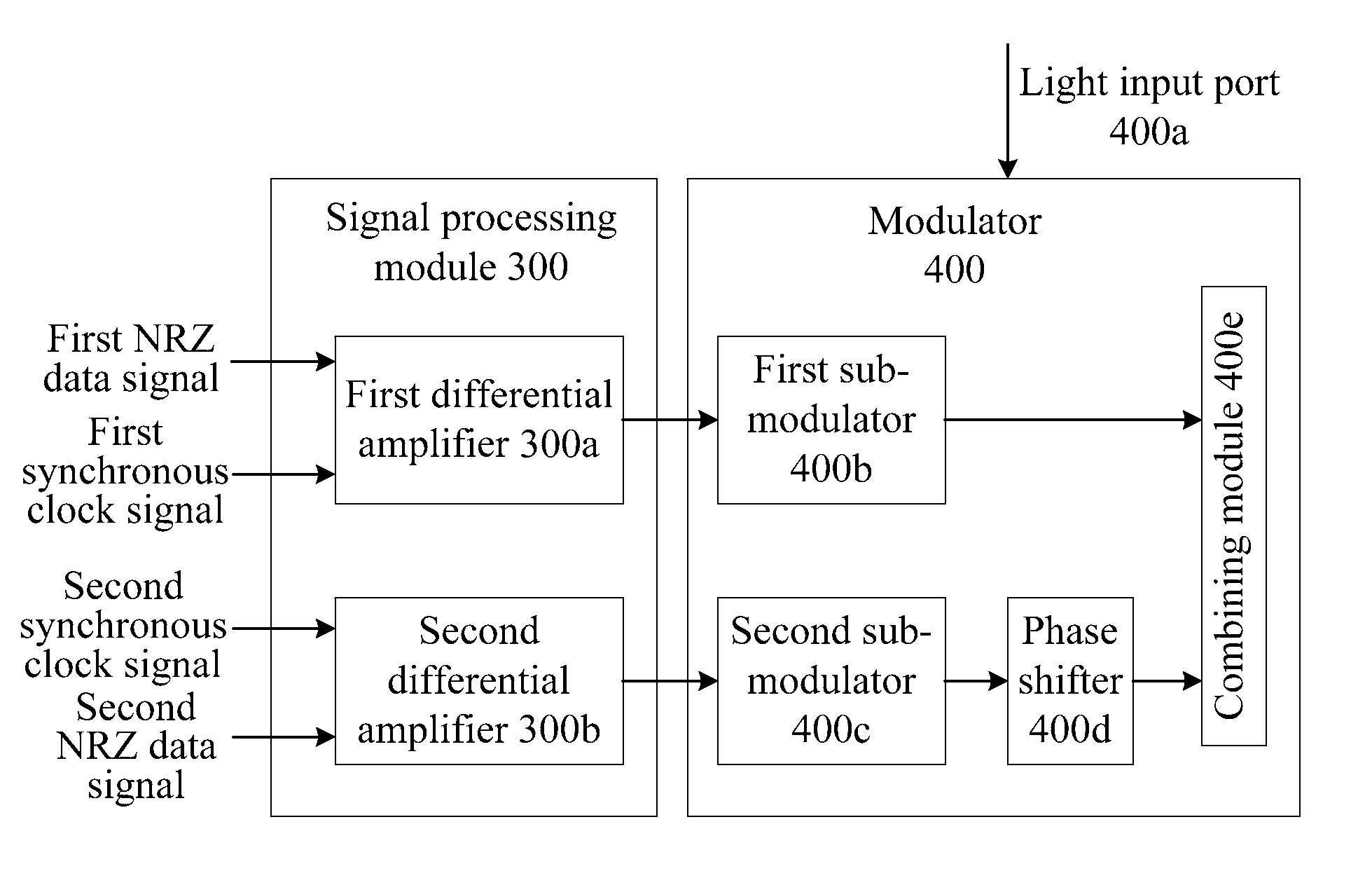
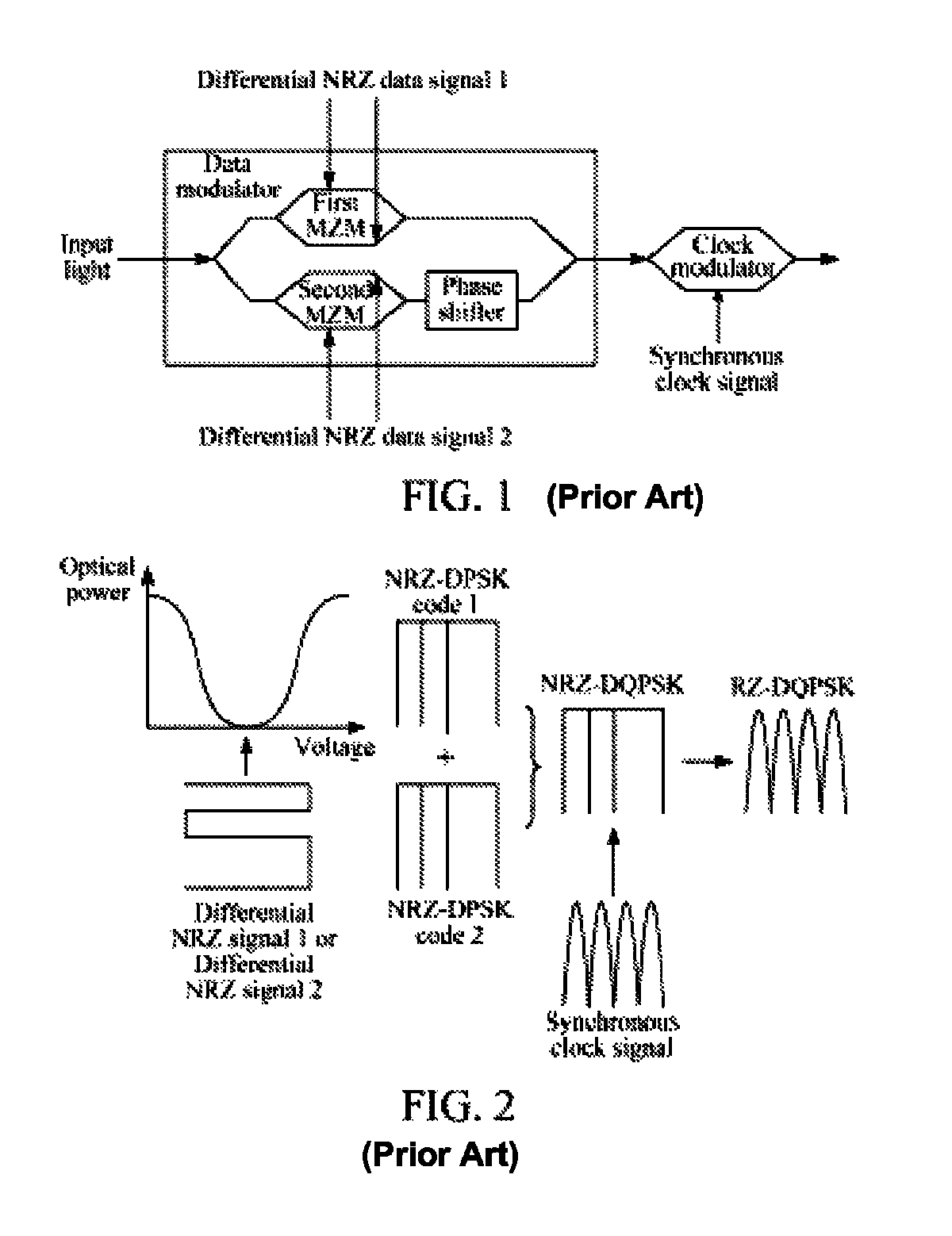
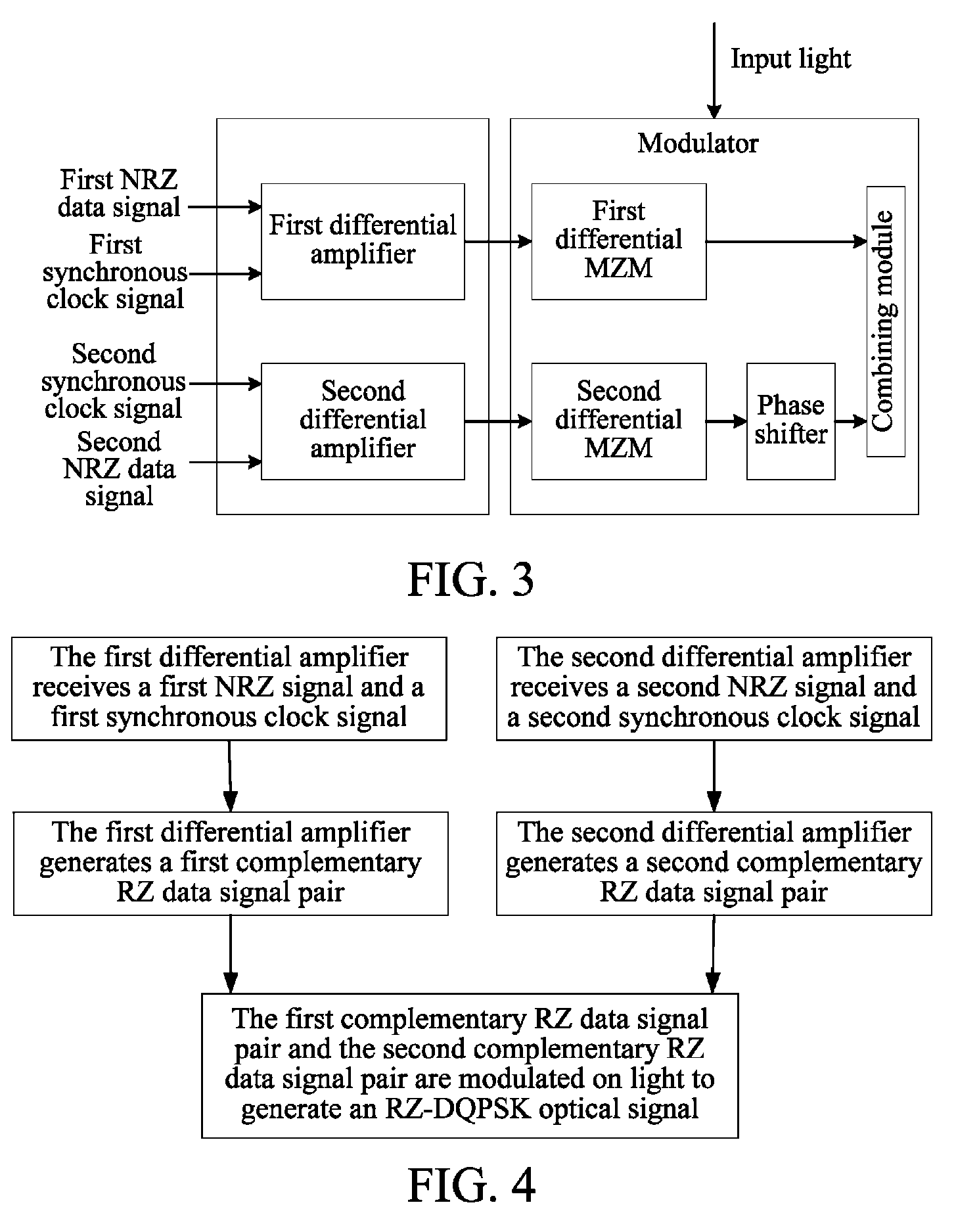
ActiveUS8831440B2Simple structureLow costElectromagnetic transmittersOptical NRZ to RZ conversionReturn-to-zeroSoftware engineering
The method includes: receiving a first Non Return to Zero (NRZ) data signal and a synchronous clock signal, and performing Return to Zero (RZ) processing to generate a first complementary RZ data signal pair; receiving a second NRZ data signal and a synchronous clock signal, and performing RZ processing to generate a second complementary RZ data signal pair; and modulating the first complementary RZ data signal pair and the second complementary RZ data signal pair on light to generate an RZ-Differential Quadrature Phase Shift Keying (RZ-DQPSK) optical signal. Through the method and device, RZ processing are performed on the NRZ data signals to generate the complementary RZ data signal pairs, and the complementary RZ data signal pairs are modulated on the light, thereby reducing the cost and the insertion loss of the entire device, lowering the requirements for input optical power and reducing the complexity of loop circuit control.
Owner:HUAWEI TECH CO LTD
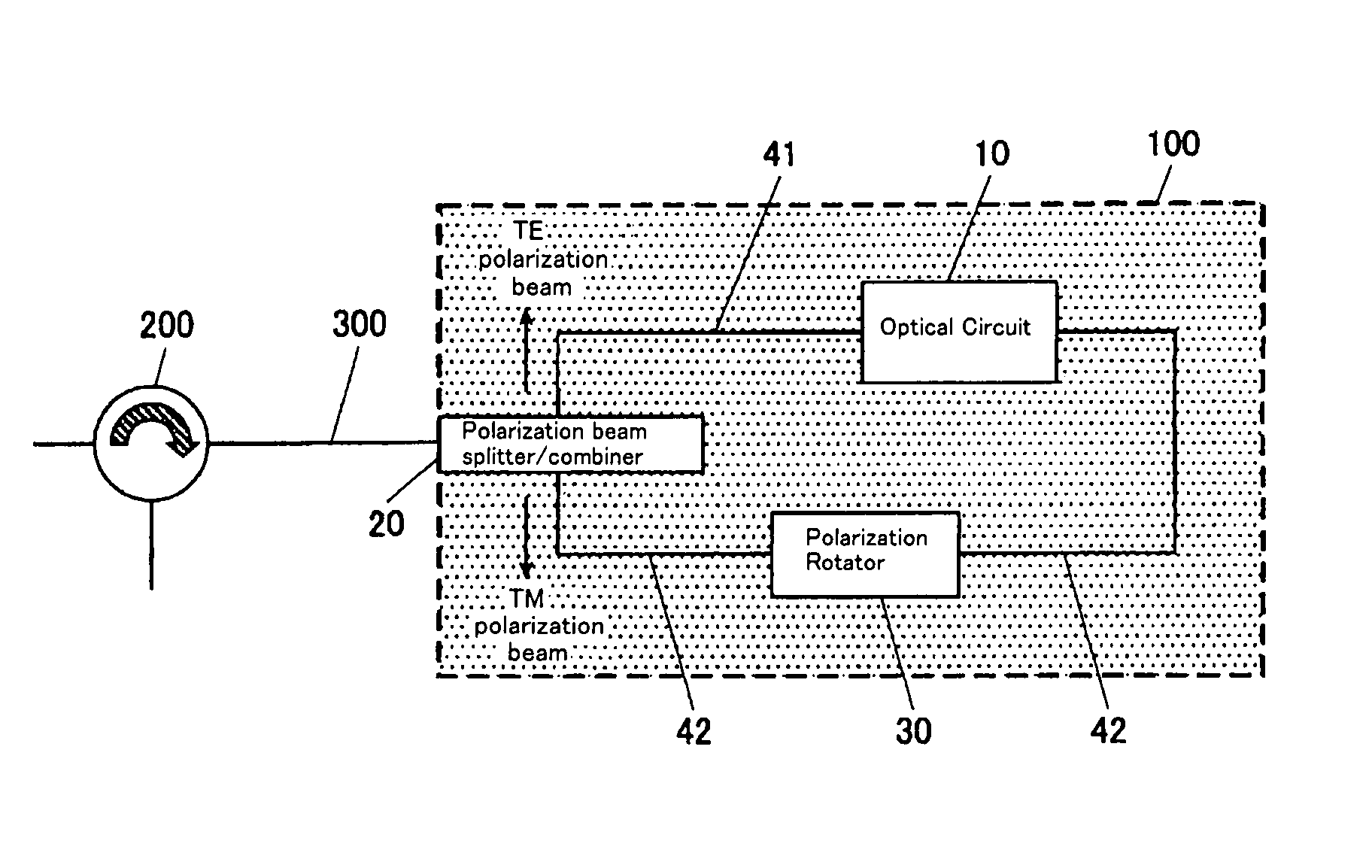
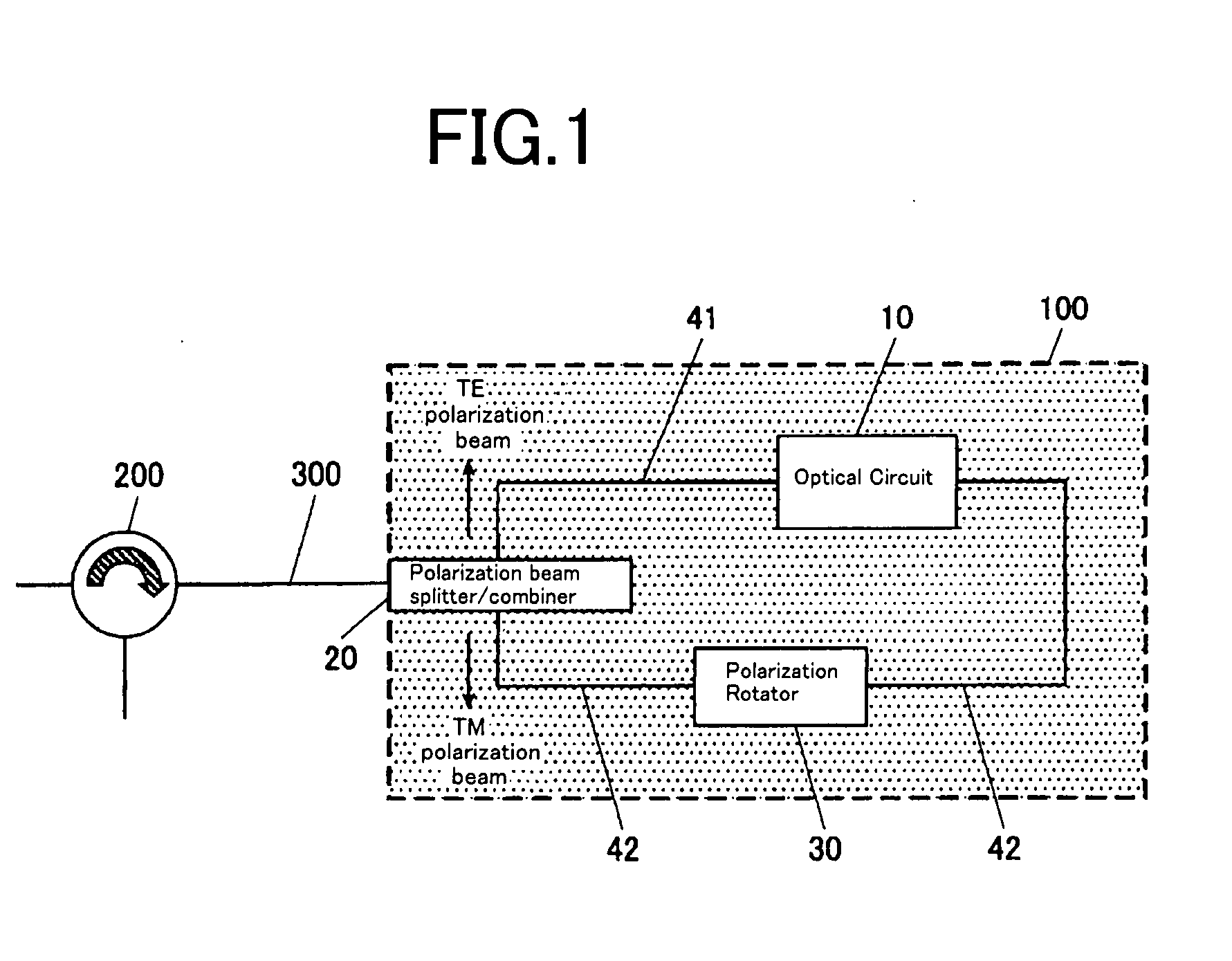
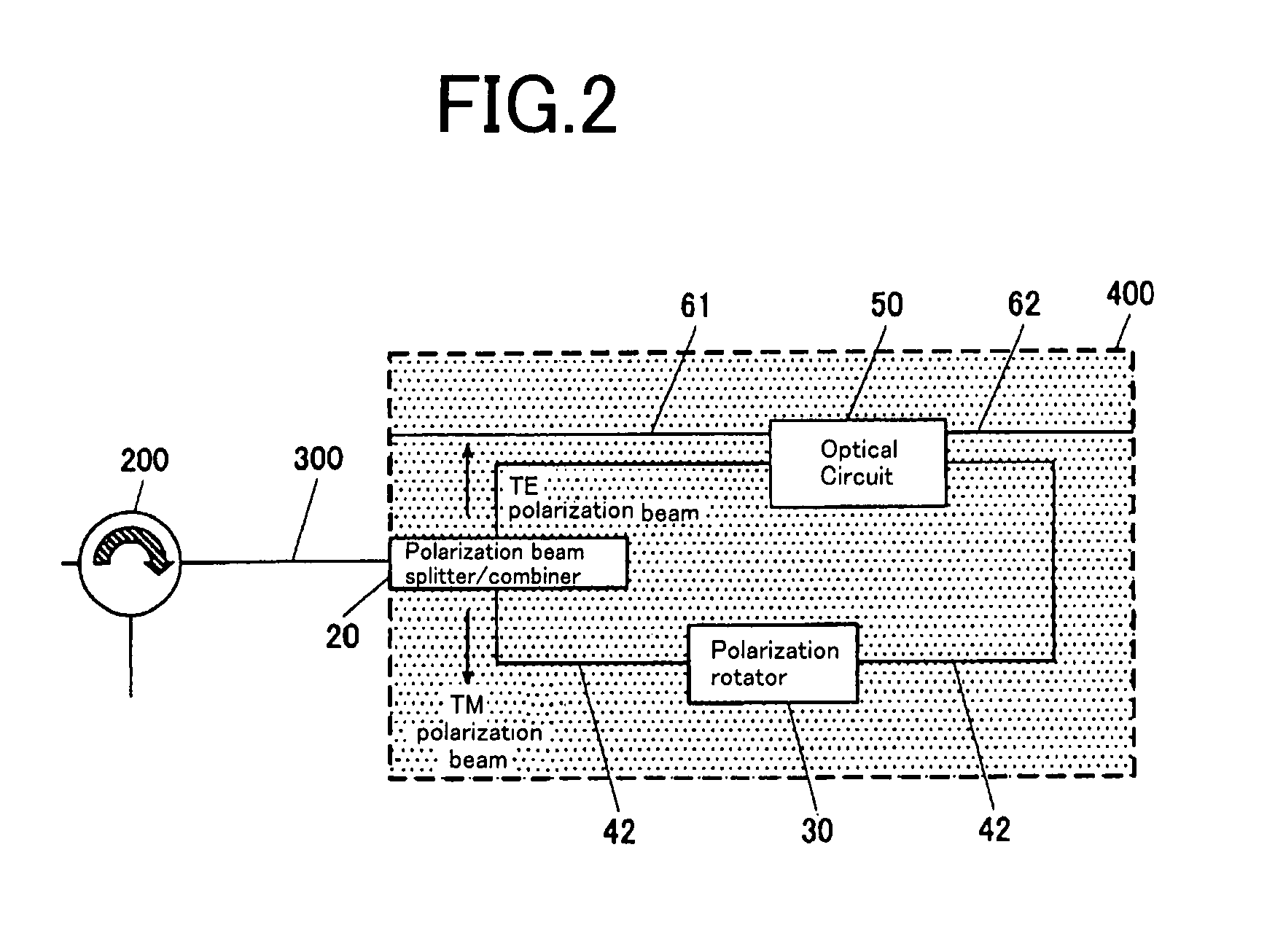
Optical Circuit Device
InactiveUS20080031566A1Reduce PDLExtinction ratio is not deterioratedOptical waveguide light guidePlanar substrateLight beam
The present invention provide an optical circuit device having: an optical circuit; a polarization beam splitter / combiner for splitting an incoming light beam into two polarization beams and combining the two polarization beams into an outgoing light beam; a first optical waveguide and a second optical waveguide for connecting the optical circuit and the polarization beam splitter / combiner and receiving the two polarization beams independently; and a polarization rotator, arranged on the first optical waveguide, for rotating a polarization plane of one of the two polarization beams split by the polarization beam splitter / combiner so as to match a polarization plane of the other of the two polarization beams, the optical circuit, the polarization beam splitter / combiner, the first optical waveguide, the second optical waveguide and the polarization rotator being integrated on a planar substrate.
Owner:FURUKAWA ELECTRIC CO LTD
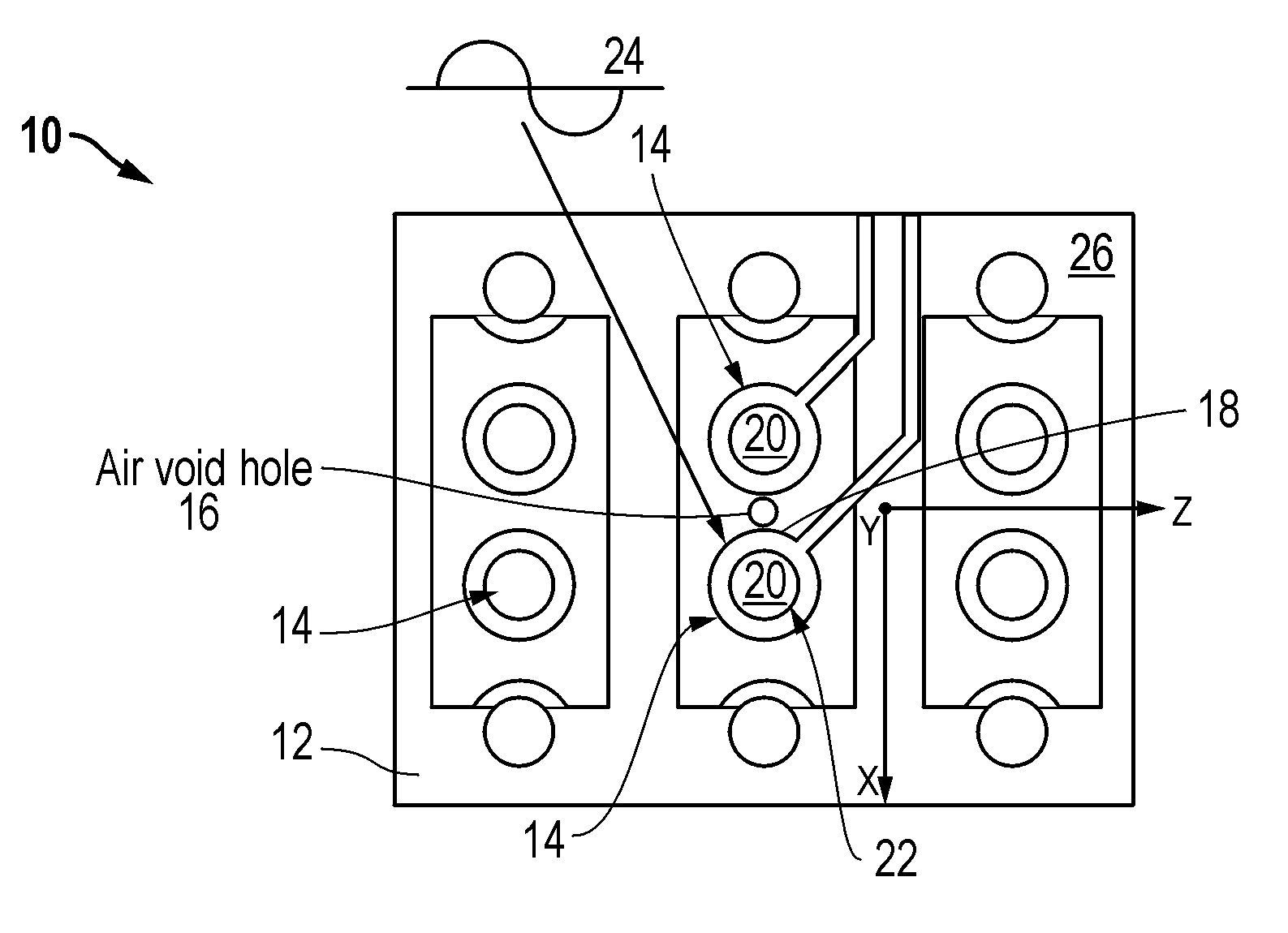
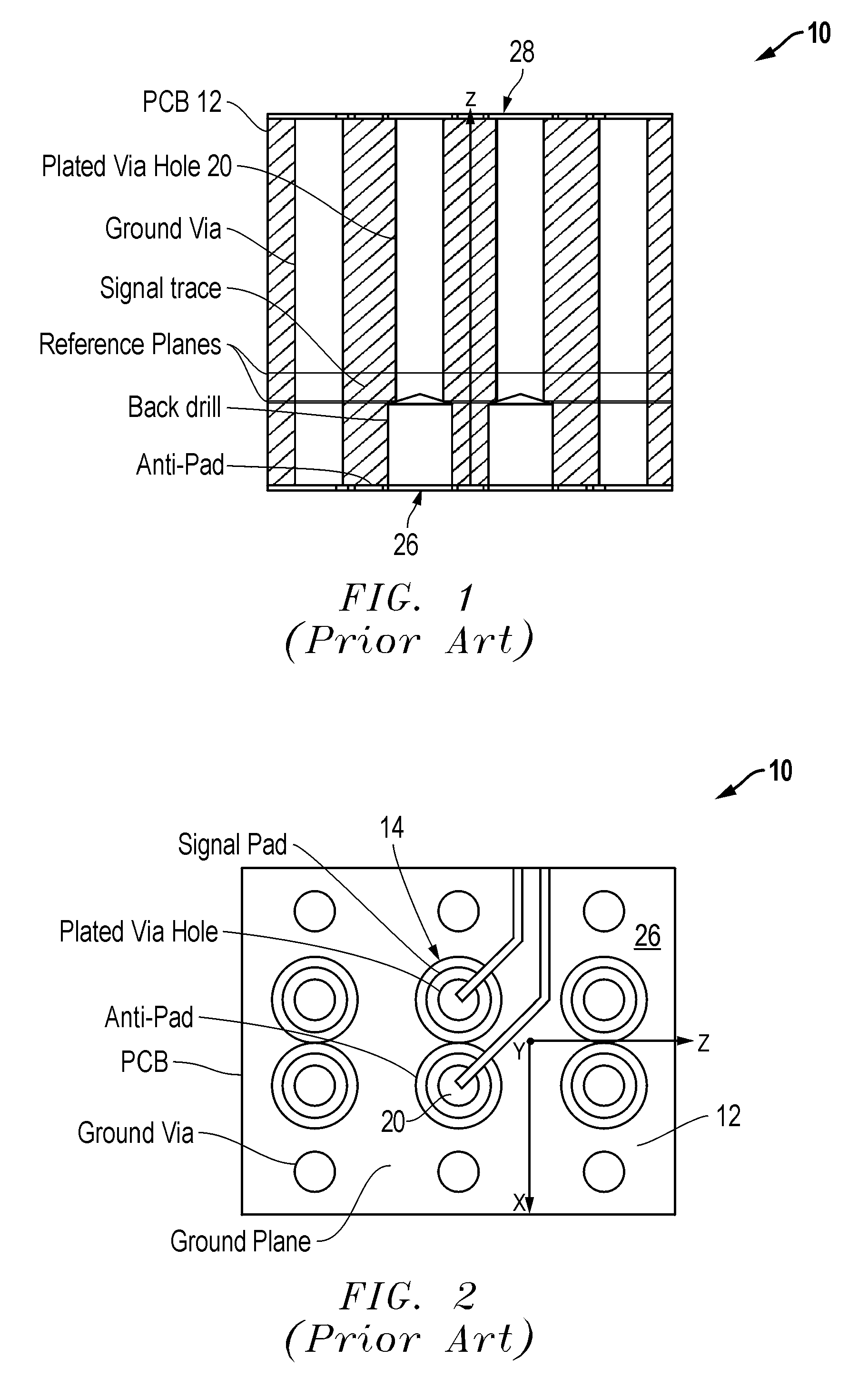
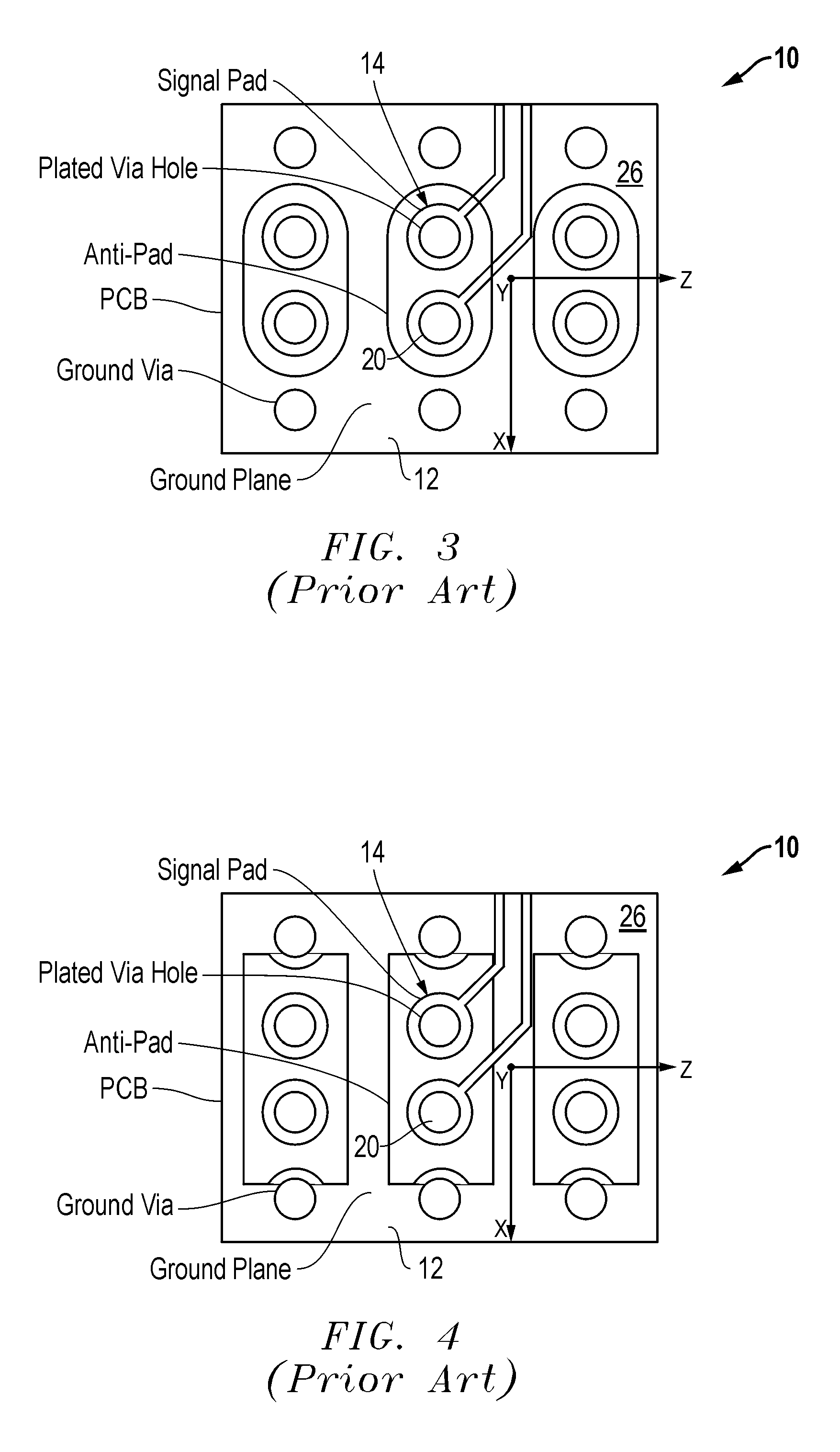
Air void via tuning
InactiveUS20060185890A1Improves main contributory factorImproves the main contributory factorsCross-talk/noise/interference reductionPrinted circuit aspectsElectromagnetic couplingEngineering
A wiring board (10) having reduced electromagnetic coupling between electronic devices includes a base board (12) that is adaptable to receive at least one electronic component (14) mounted on the base board (12). At least one hole or void (16) is formed in the base board (12). The hole (16) is separated from the selected electronic component (14) to be isolated against undesired electromagnetic radiation by a portion (18) of the base board (12).
Owner:SIMCLAR INTERCONNECT TECH
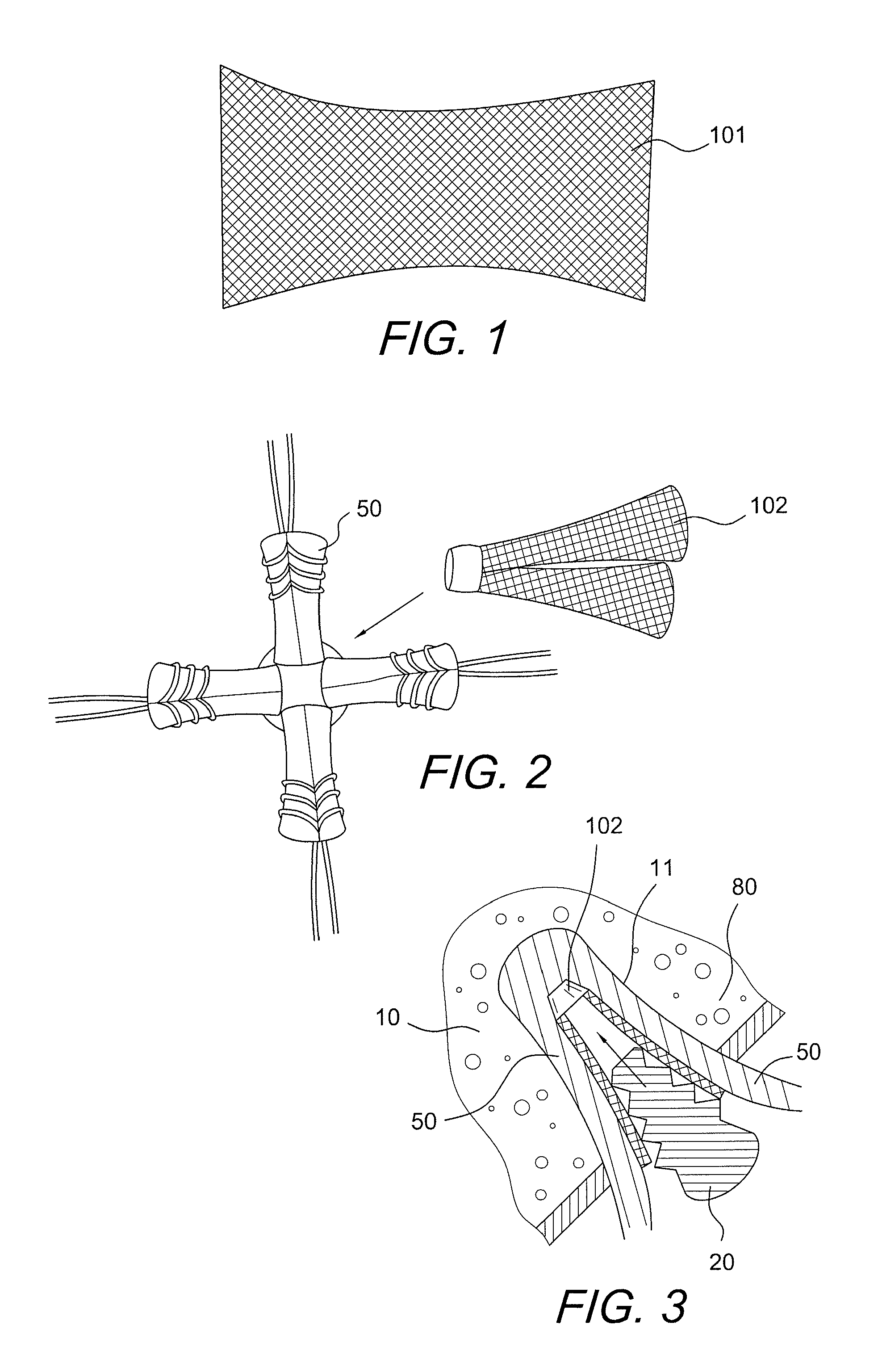
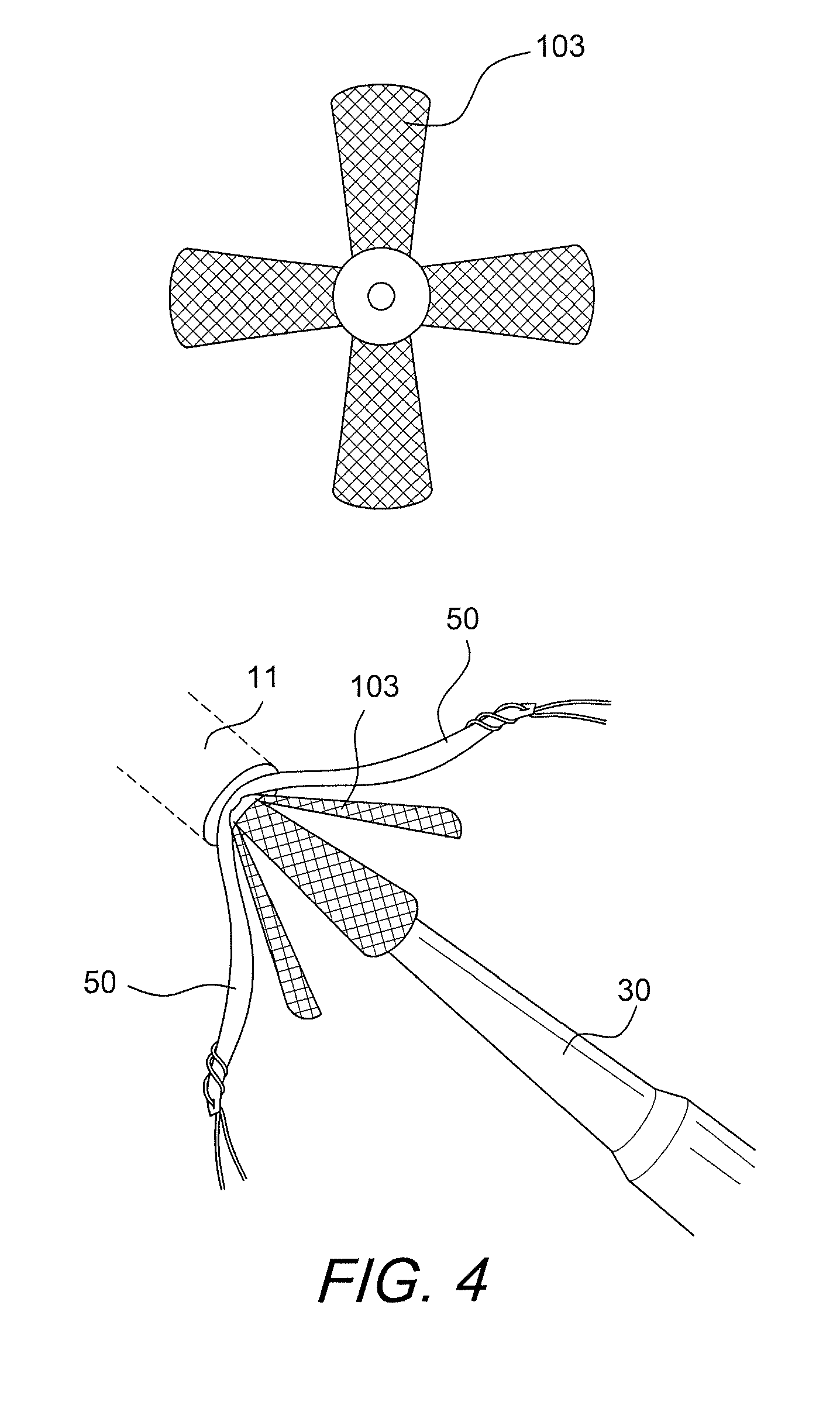
Graft protection mesh and fixation technique
ActiveUS8460350B2Improved strength and structural supportPromote resultsSuture equipmentsInternal osteosythesisCruciate ligamentDouble bundle
A three-dimensional mesh or screen in the shape of a simple flat piece of material that can be provided adjacent the graft (i.e., in between graft bundles, or around the graft, or between the graft and the fixation device) for improved strength and structural support for graft fixation. The mesh provides improved methods for installing and securing ligament grafts (such as double-bundle cruciate ligament grafts) with enhanced reconstruction results.
Owner:ARTHREX
Medical apparatus system having optical fiber load sensing capability
ActiveUS8182433B2Facilitate speedHelp accuracyStrain gaugePerson identificationRobotic systemsProcess logic
Owner:ST JUDE MEDICAL INT HLDG SARL
LiFePO4 FLAKES FOR Li-ION BATTERY AND METHOD FOR MANUFACTURING THE SAME
ActiveUS20120328947A1Charge-discharge efficiency can be improvedShort diffusion pathMaterial nanotechnologyPhosphatesCharge dischargeEngineering
LiFePO4 flakes for a Li-ion battery and a method for manufacturing the same are disclosed. The LiFePO4 flakes of the present invention have a thickness of 5 nm-200 nm, and the angle between the flat surface normal of the flake and the Li-ion diffusion channel is 0°-80°. In addition, according to the present invention, the LiFePO4 flakes with short Li ion diffusion path can be prepared through a simple process. Hence, not only the charge-discharge efficiency of the Li-ion battery can be improved by use of the LiFePO4 flakes of the present invention, but also the cost of the Li-ion battery can be further reduced.
Owner:NATIONAL TSING HUA UNIVERSITY
Phase plug and acoustic lens for direct radiating loudspeaker
ActiveUS20110168480A1Minimize distortionMinimize insertion lossLoudspeaker screensCabinetsEngineeringAcoustic lens
A phase plugs or acoustic lens improves the directional audio performance of a loudspeaker. Application of the improved directional audio performance to a sound system in a listening area may improve the performance of the audio system. Configuration of the acoustic lens or phase plug may include both symmetrical and asymmetrical features to provide an improved frequency response and directivity. The improved loudspeaker may provide improved an improved listing location, for example, in a vehicle.
Owner:HARMAN INT IND INC
Thin film bulk acoustic wave resonator structure and filter, and radio-frequency module using them
InactiveUS20080169884A1Reducing area of elementWidePiezoelectric/electrostriction/magnetostriction machinesImpedence networksThin-film bulk acoustic resonatorManufacturing technology
A thin film bulk acoustic wave (BAW) resonator structure and filter which can be fabricated by inexpensive manufacturing techniques and in smaller size than conventional such products are to be provided. The BAW resonator structure and filter have a substrate, a first BAW resonator placed over the substrate, an acoustic reflection layer placed over the first BAW resonator and a second BAW resonator placed over the acoustic reflection layer, and the acoustic reflection layer is electroconductive. Herein, the acoustic reflection layer constitutes a first electrode, and this first electrode electrically connects and acoustically separates the first BAW resonator and the second BAW resonator.
Owner:HITACHI MEDIA ELECTORONICS CO LTD
Stemless shoulder implant
ActiveUS8512410B2Improve pullout forceInsertion torque is minimizedJoint implantsShoulder jointsAnatomyBone humerus
Owner:ARTHREX
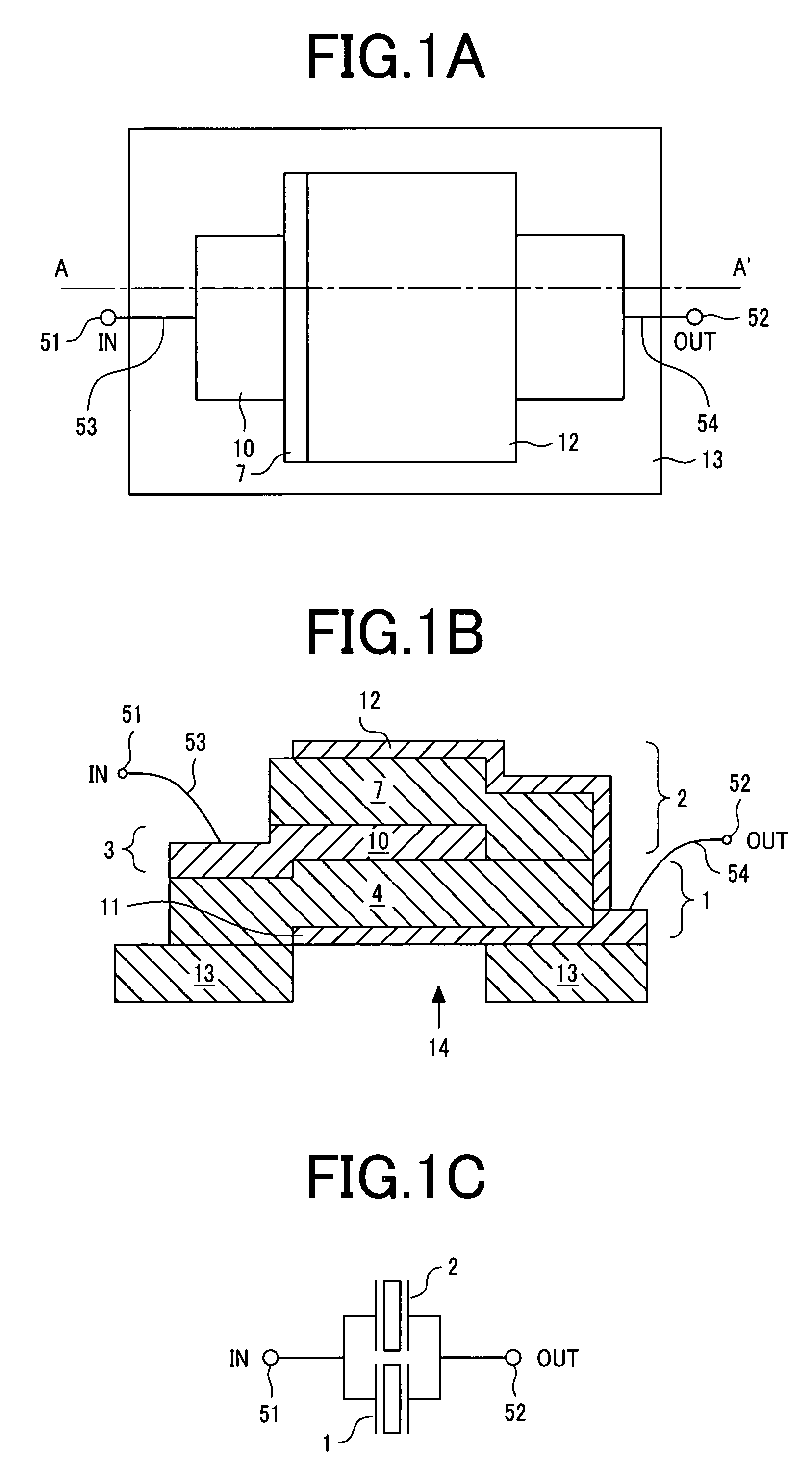
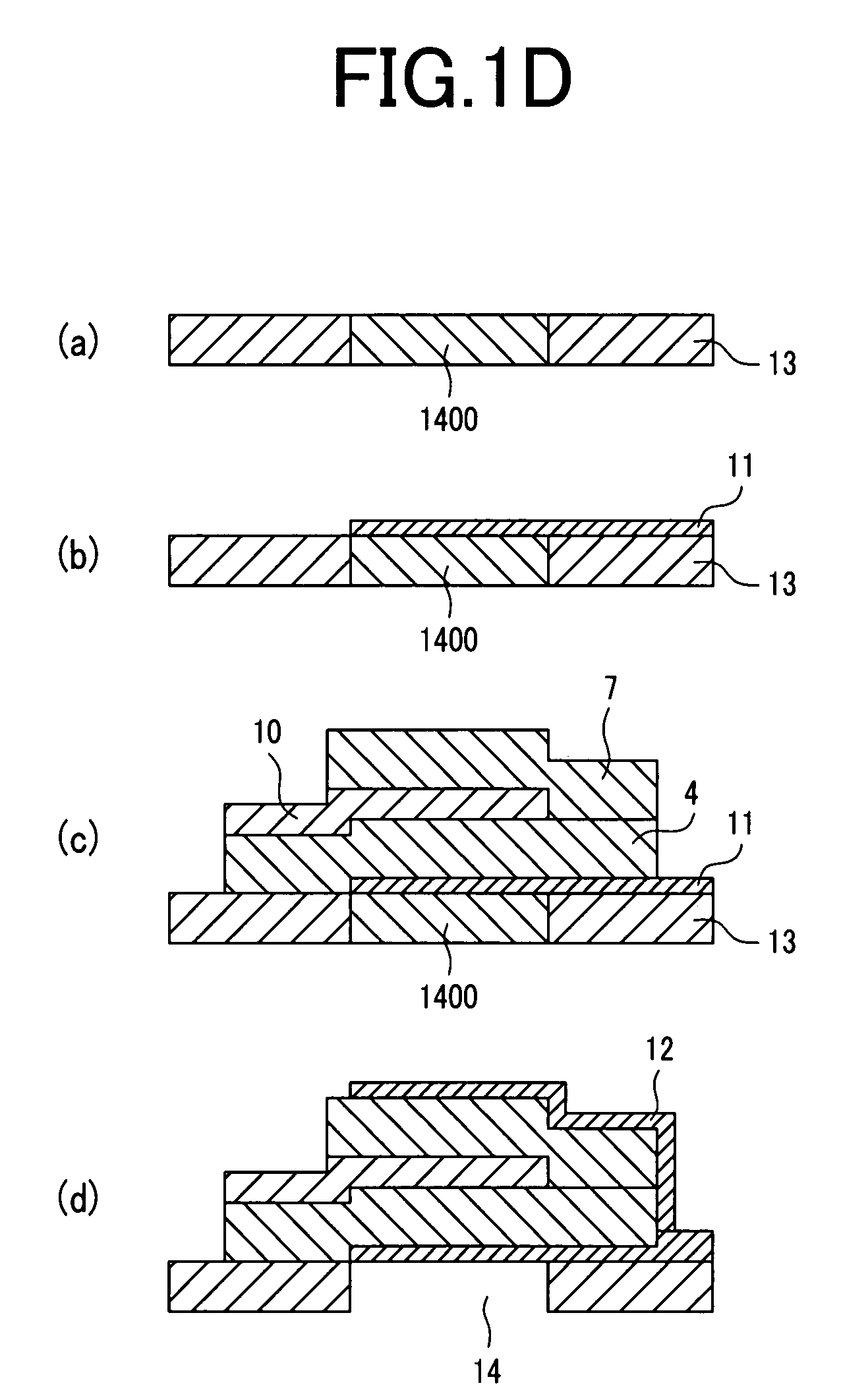
Piezoelectric thin-film filter
A piezoelectric thin-film filter reduces insertion loss and deterioration of steepness of a shoulder characteristic and reduces the ripple in the passband. In a first vibration portion, a piezoelectric thin film is disposed between a pair of electrodes along one main surface of a substrate. In a second vibration portion, the piezoelectric thin film is disposed between a pair of electrodes along the one main surface of the substrate. The vibration portions are both acoustically isolated from the substrate. In the first resonator, an additional film is disposed outside the electrode constituting half or more the overall length of the perimeter of the first vibration portion that is in contact with the electrode when seen from a thickness direction. In the second resonator, the external shape of the vibration portion when seen from a thickness direction is a polygon, and each side of the polygon is not parallel with any of the other sides thereof.
Owner:MURATA MFG CO LTD
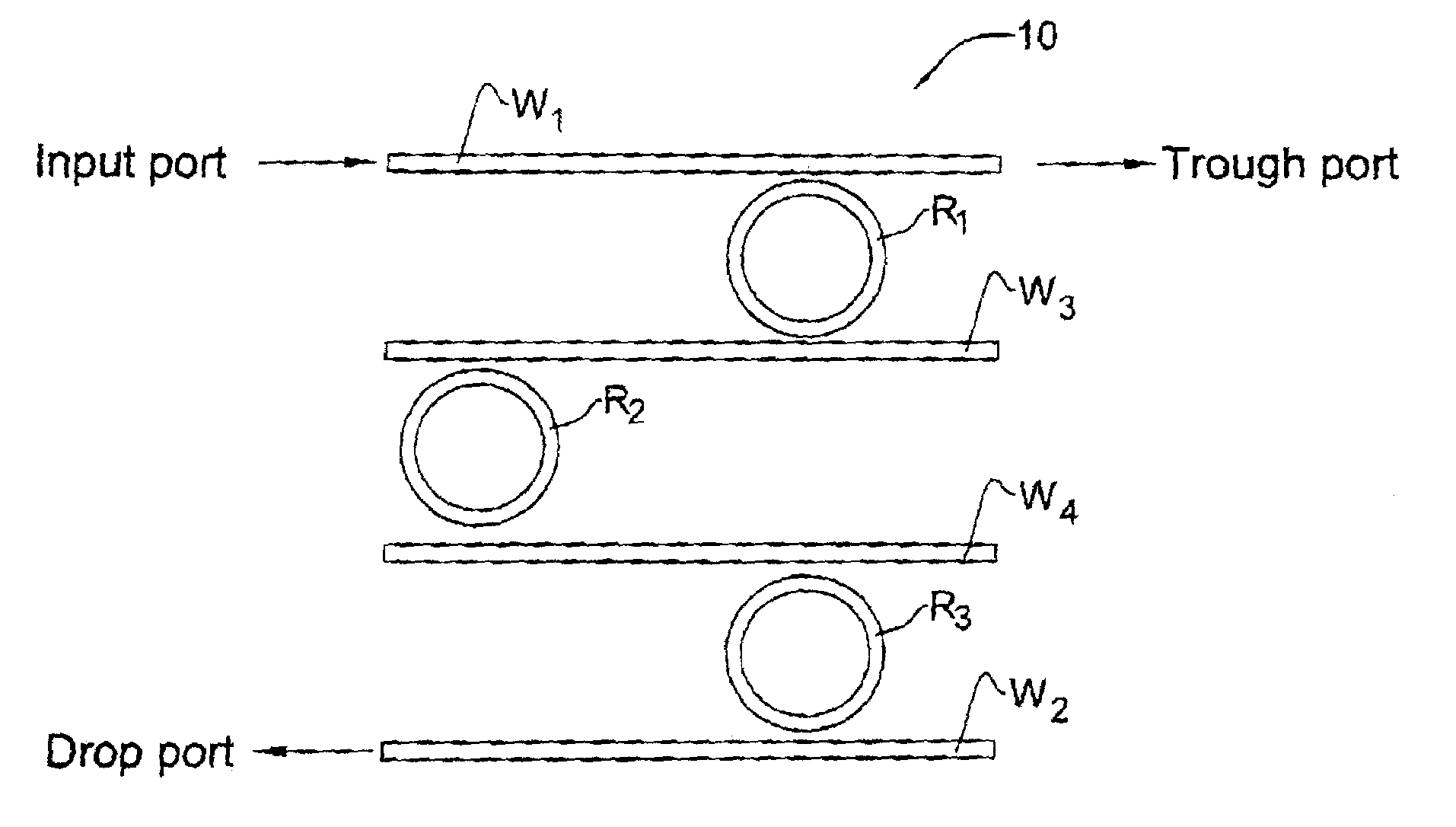
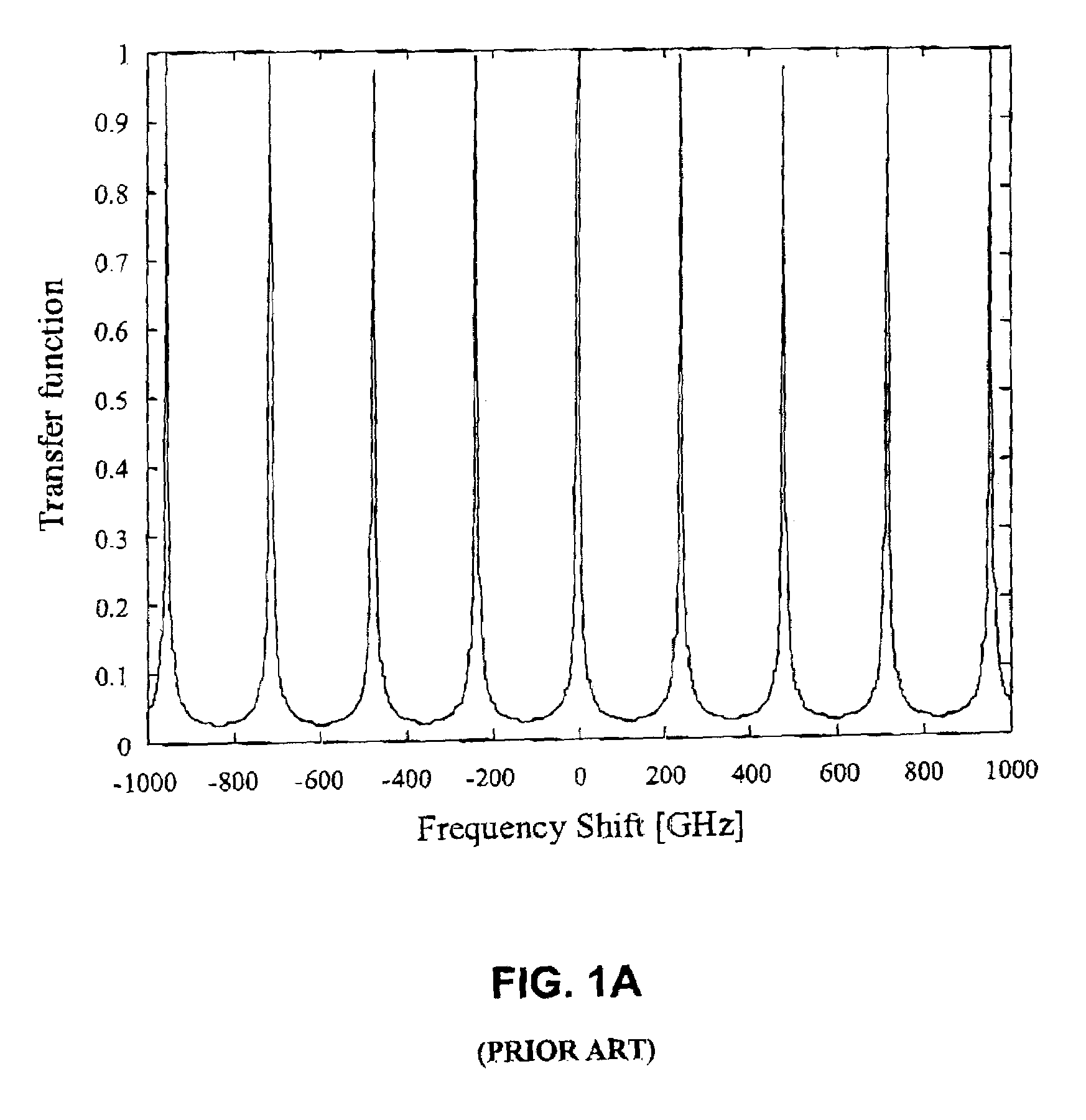
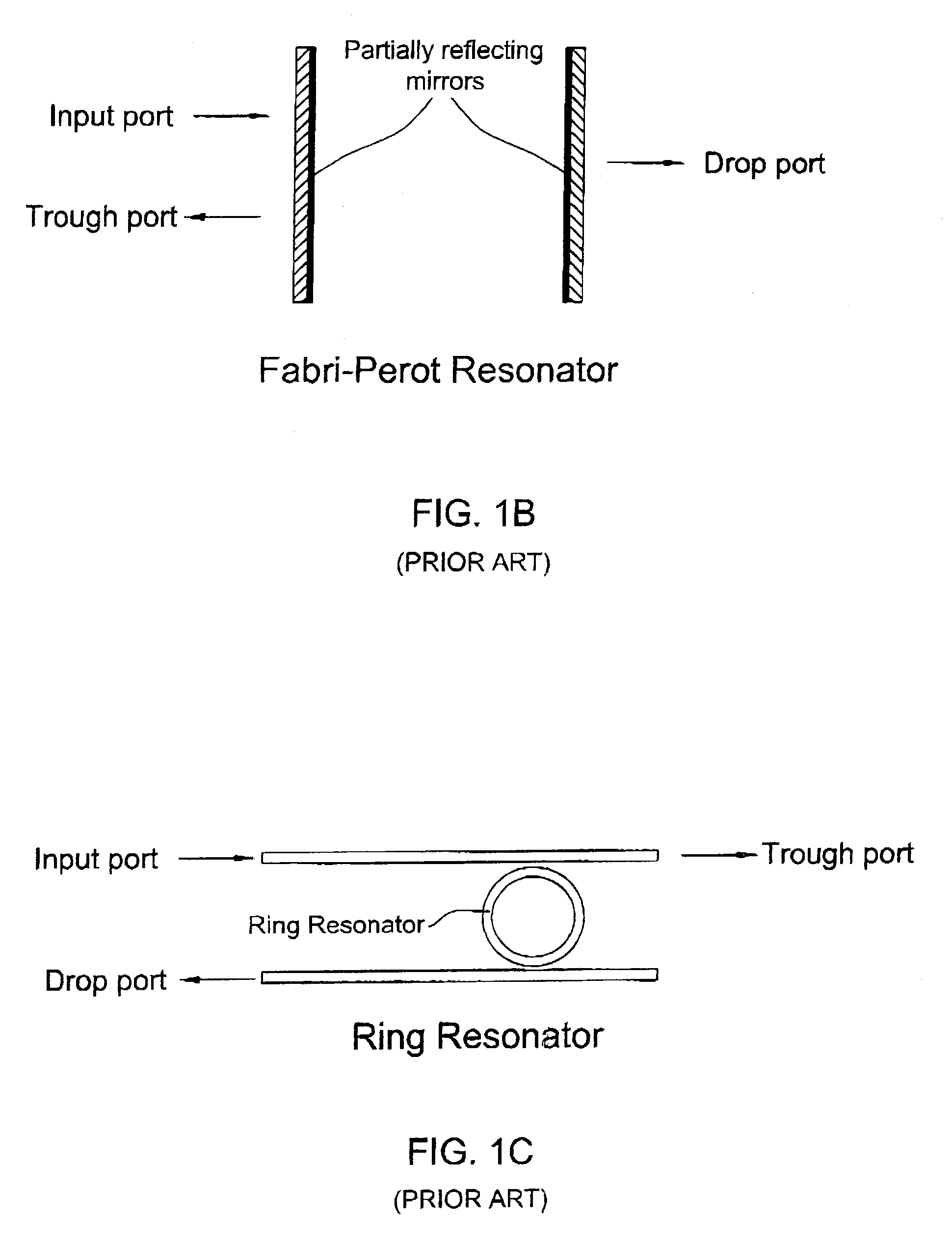
Integrated optical filters utilizing resonators
InactiveUS7065276B2Attenuation bandwidthReduced insertion lossCoupling light guidesOptical waveguide light guideCouplingClosed loop
A filtering method and optical filter structure are presented. The structure comprises an input waveguide, an output waveguide, and a filter stage formed by at least one closed loop resonator optically coupled to the input and output waveguides. A level of the coupling from each of the waveguides to the resonator is at least 5 times greater than a loss-per-revolution of the resonator. The filter structure thus provides for reducing a bandwidth and insertion loss while filtering at least one optical channel from a multi-channel light signal.
Owner:LAMBDA CROSSING
Optical connector
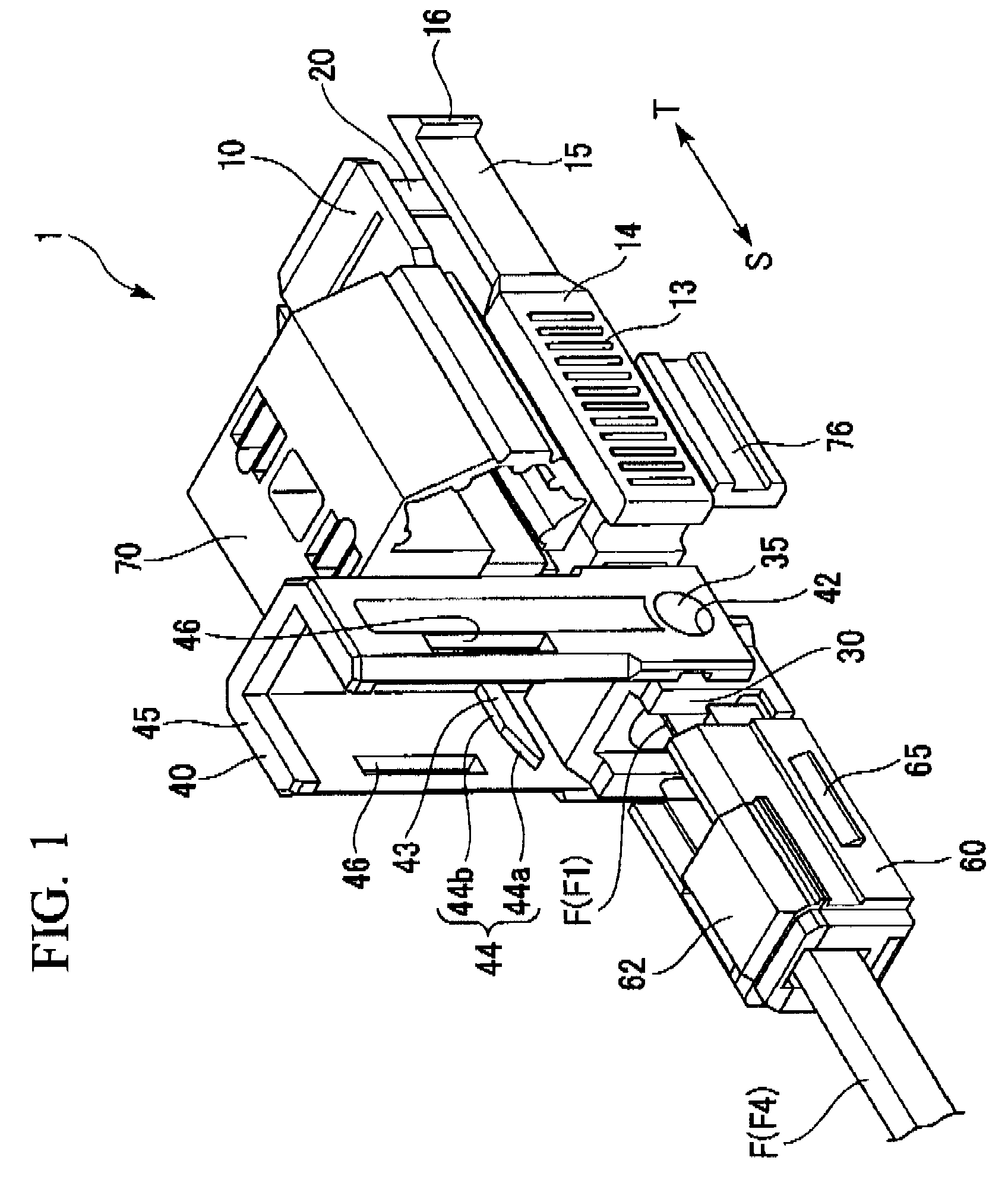
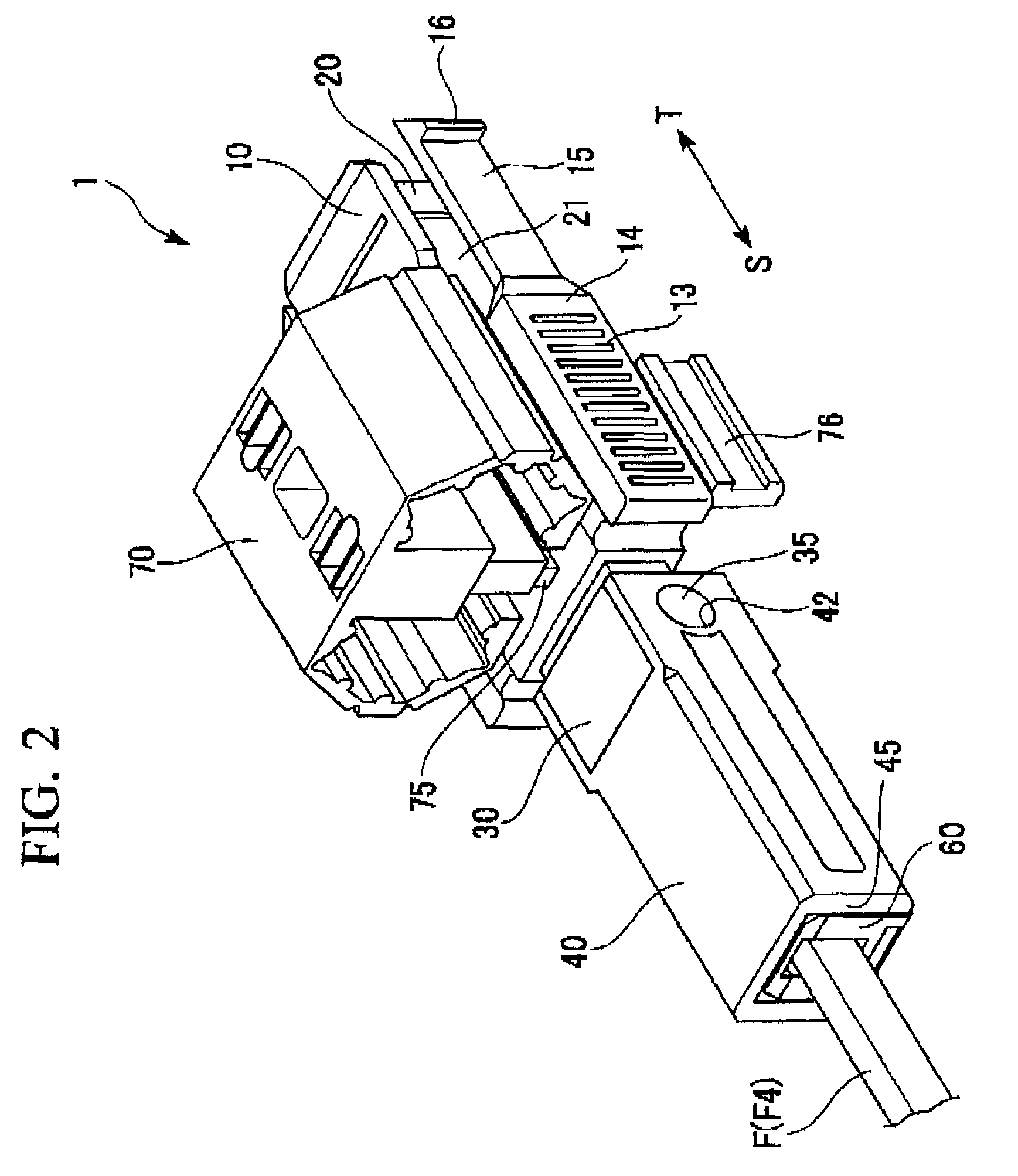
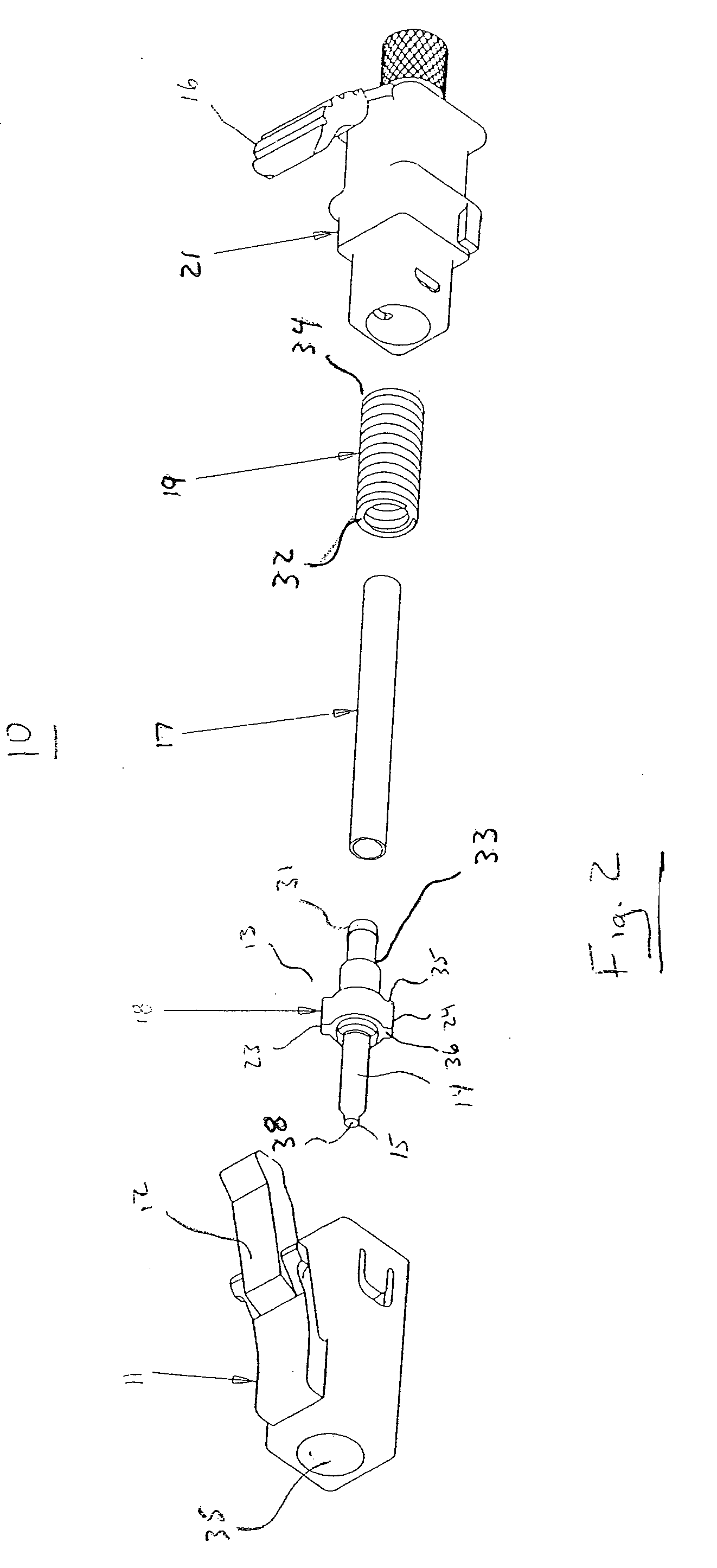
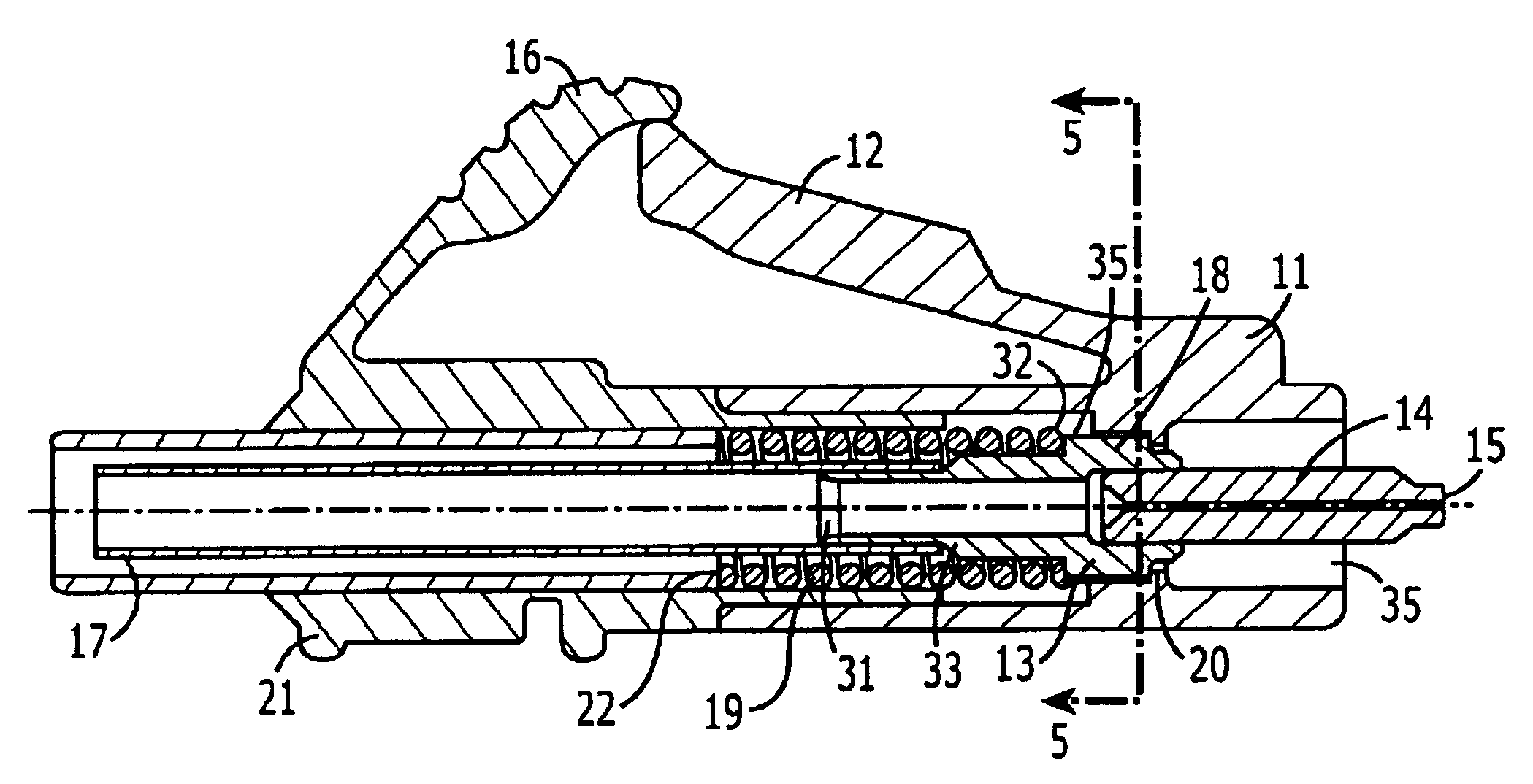
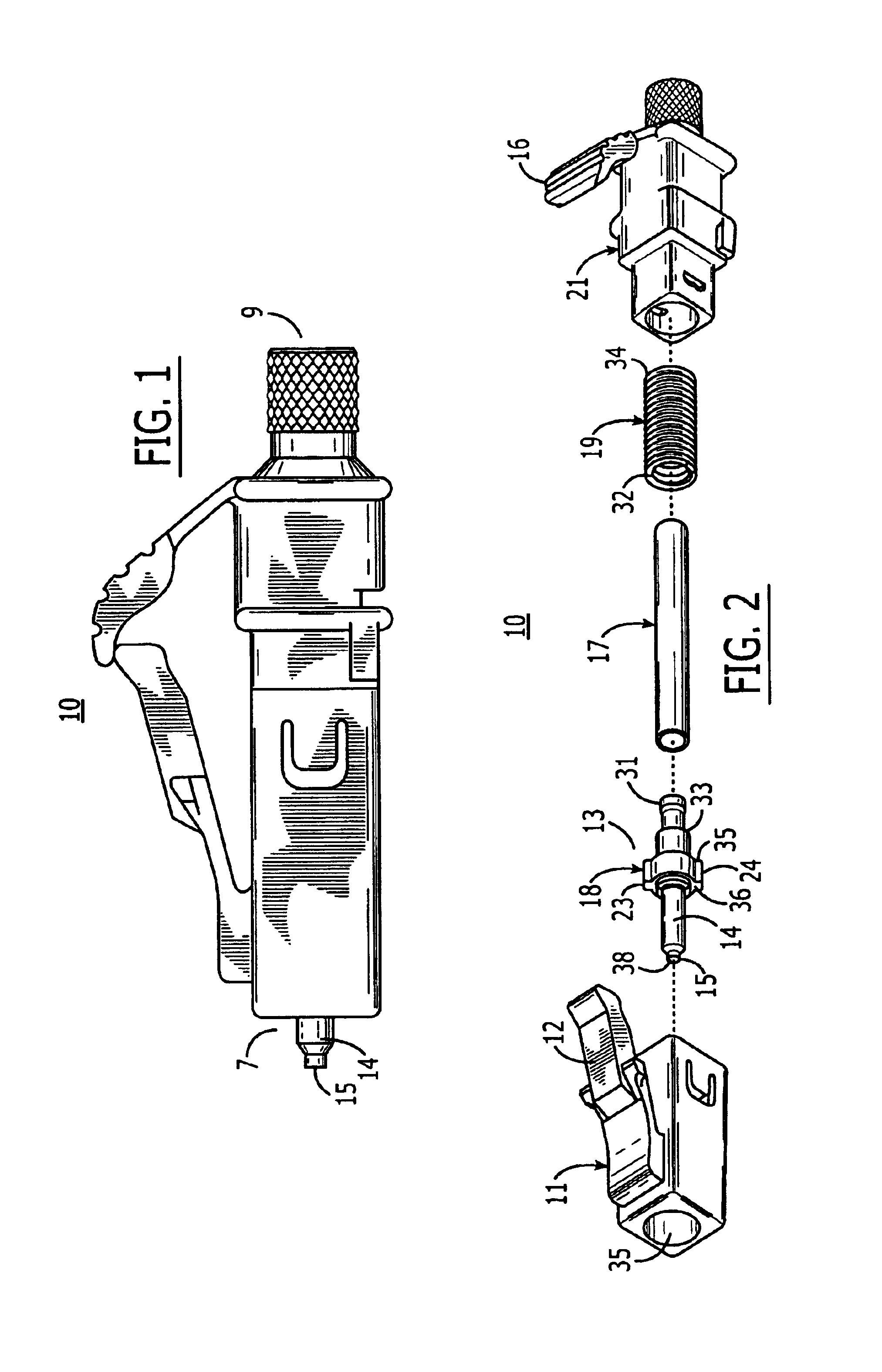
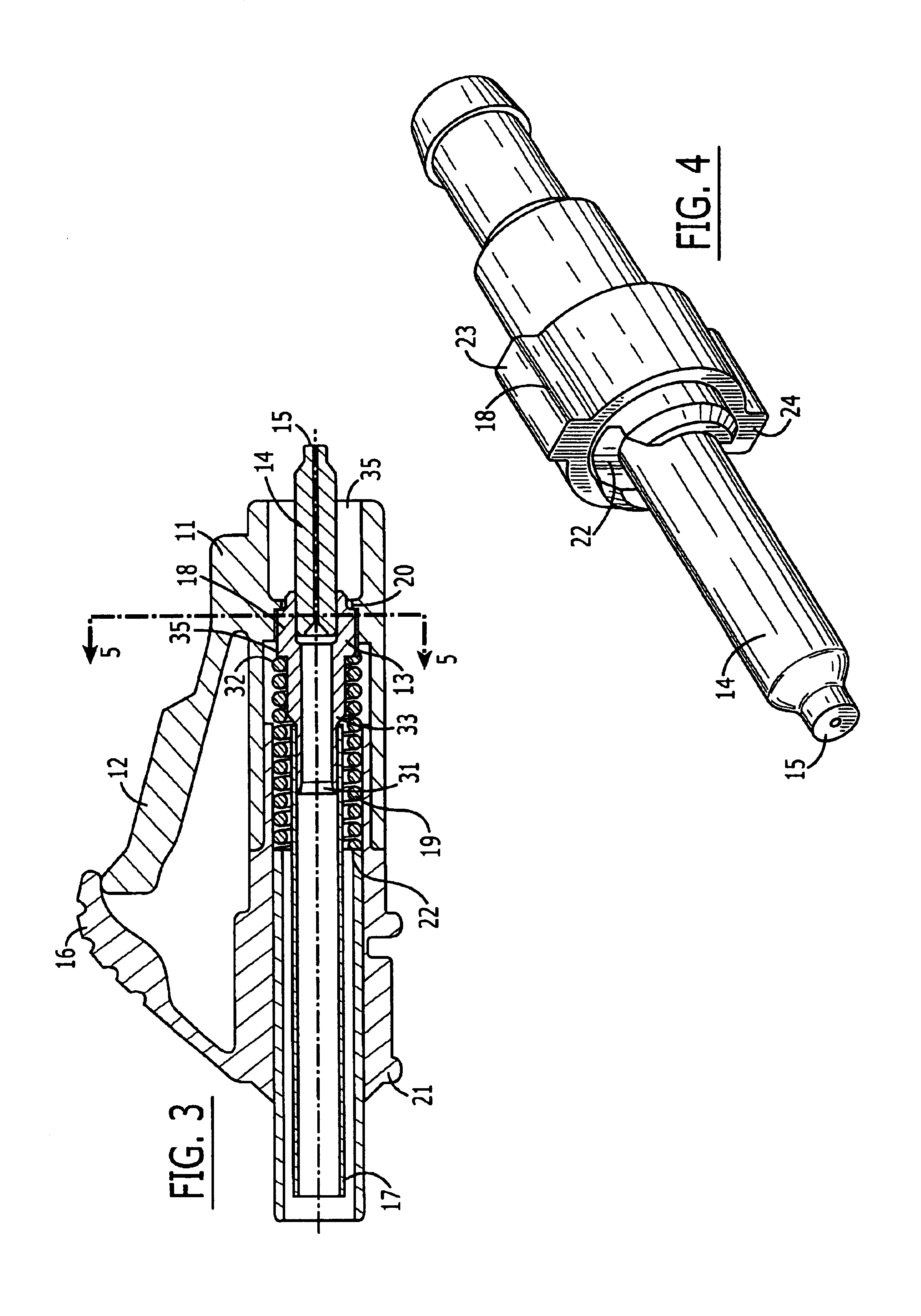
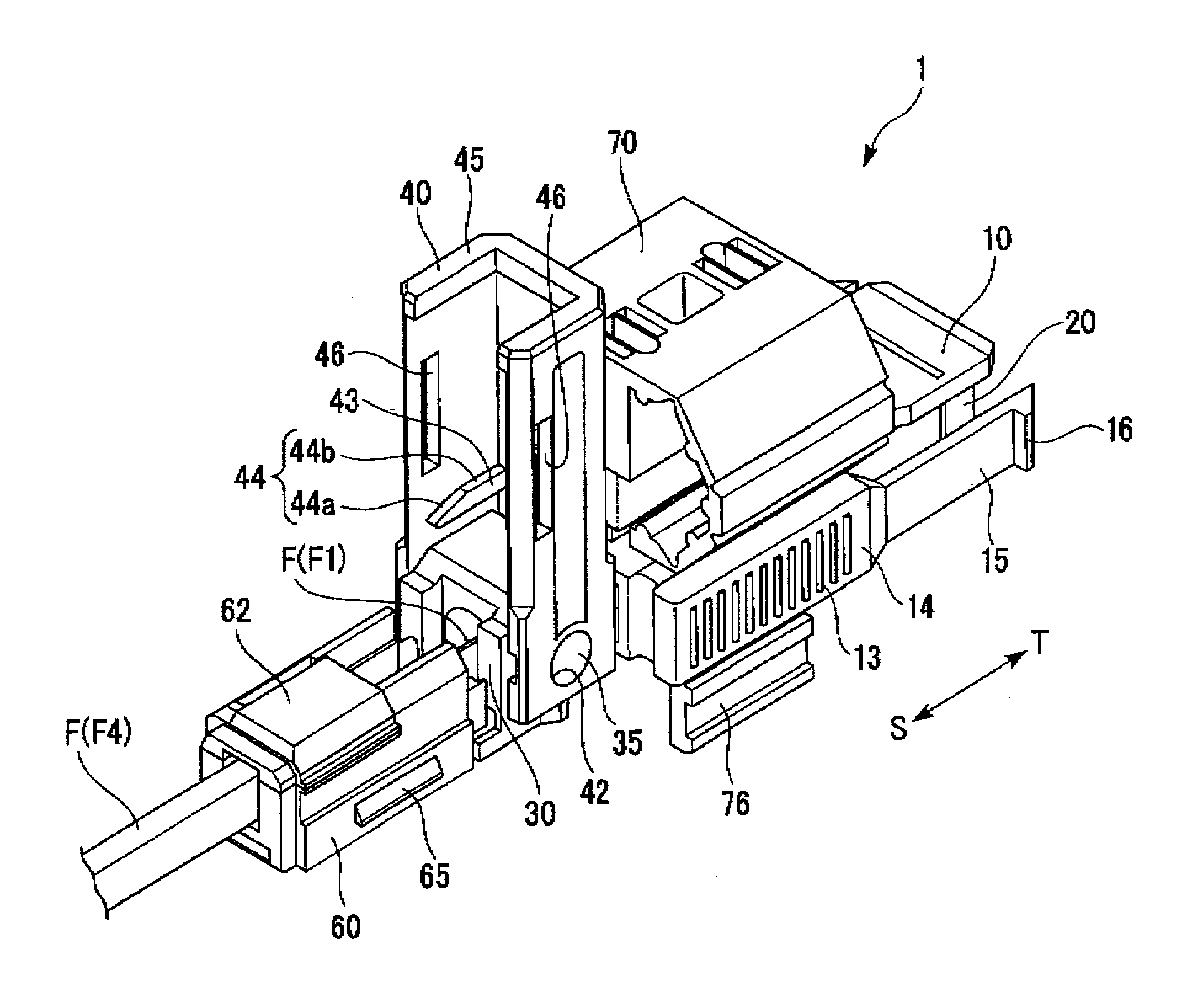
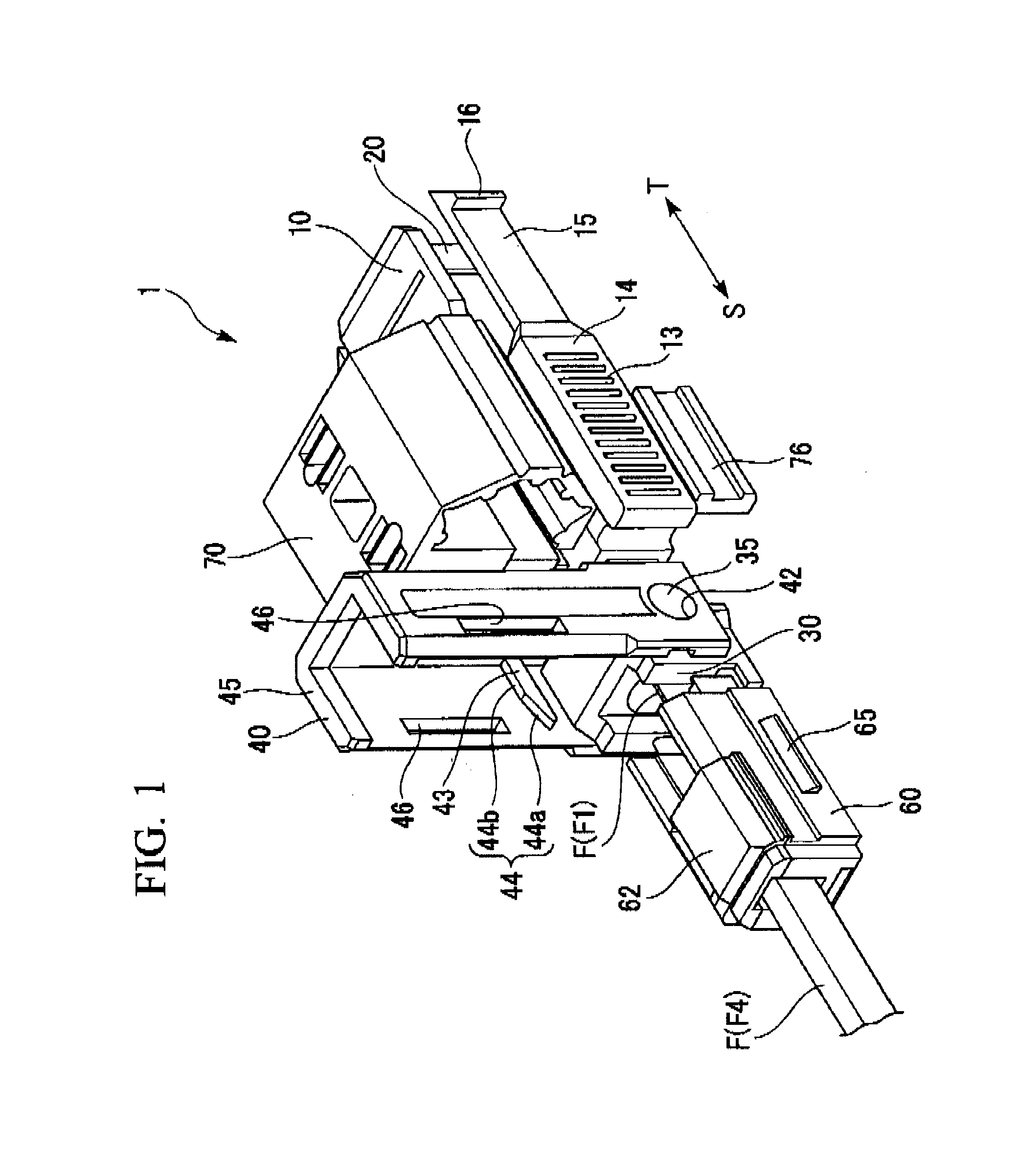
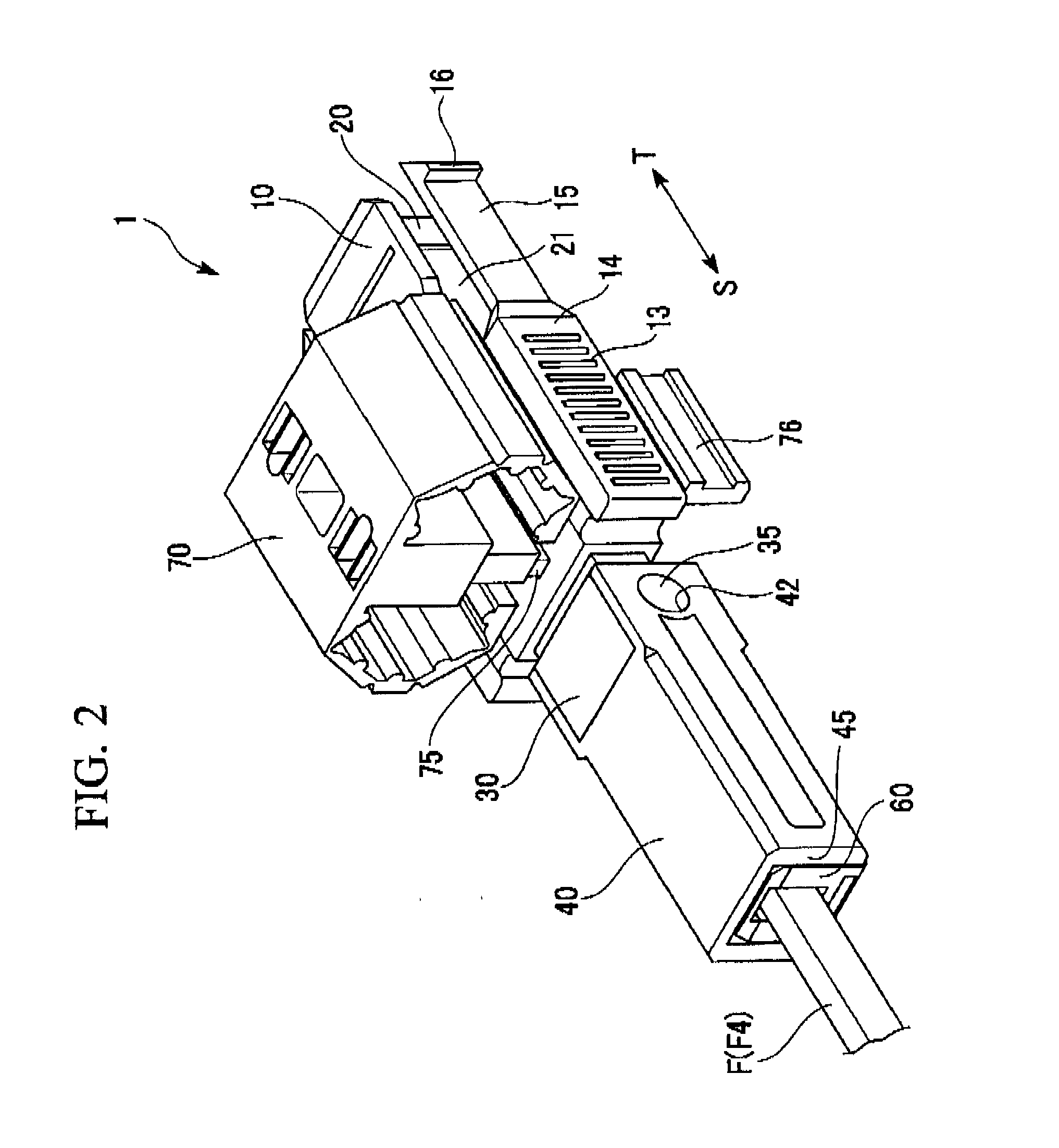
ActiveUS7347627B2Reduce insertionLess bendingCoupling light guidesEngineeringOptical fiber connector
An optical connector includes a connector body that has a first optical fiber housed in advance in a ferrule so as to project from a back end of the ferrule opposite to the connecting end surface and an anchoring fixture that anchors a second optical fiber that is to be optically connected to the first optical fiber, and by pressing the anchoring fixture into the connector body while the second optical fiber is anchored in this anchoring fixture, the anchoring fixture and the connector body are connected to optically connect the first optical fiber and the second optical fiber, and the connecting portion that connects the anchoring fixture and the connector body form a movable connecting portion that is adapted to vary the direction of the anchoring fixture with respect to the connector body.
Owner:FUJIKURA LTD +1
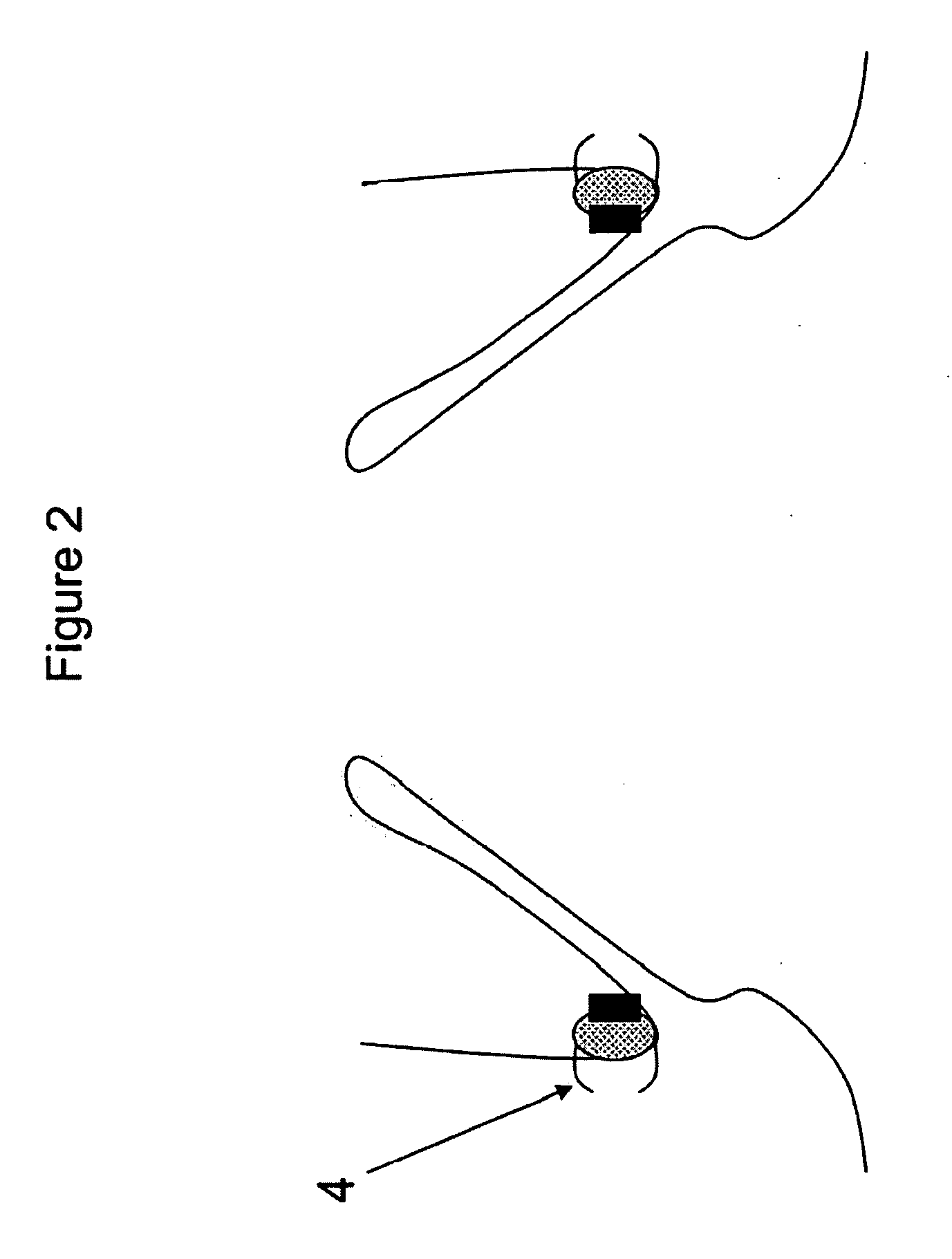
Method and apparatus for anchoring cardiovascular implants
InactiveUS20090030435A1Reduce risk of migrationReduce intrusionSuture equipmentsStentsVascular implantTherapeutic Devices
Methods, devices and systems facilitate retention of a variety of therapeutic devices. Devices generally include an anchoring element, which has been designed to promote fibrotic ingrowth, and an anchored device, which has been designed to firmly engage the complementary region of the anchoring element. The anchoring element may be placed in a minimally invasive procedure temporally separated from the deployment of the anchored device. Once enough time has passed to ensure appropriate fixation of the anchoring element by tissue and cellular ingrowth at the site of placement, the anchored device may then be deployed during which it firmly engages the complementary region of the anchoring element. In this manner, a firm attachment to the implantation site may be made with a minimum of required hardware. Some embodiments are delivered through a delivery tube or catheter and while some embodiments may require laparoscopy or open surgery for one or more of the placement procedures. Some embodiments anchor devices within the cardiovascular tree while others may anchor devices within the gastrointestinal, peritoneal, pleural, pulmonary, urogynecologic, nasopharyngeal or dermatologic regions of the body. An alternative embodiment provides for the placement of the anchoring element and anchored device simultaneously, but allows for their removal separately. This embodiment allows the device, which may be placed only temporarily and be designed to be removed, to experience significant fibrotic ingrowth, but then to be easily detached from the ingrowth-anchored region to allow for simple and quick device removal.
Owner:THERANOVA LLC
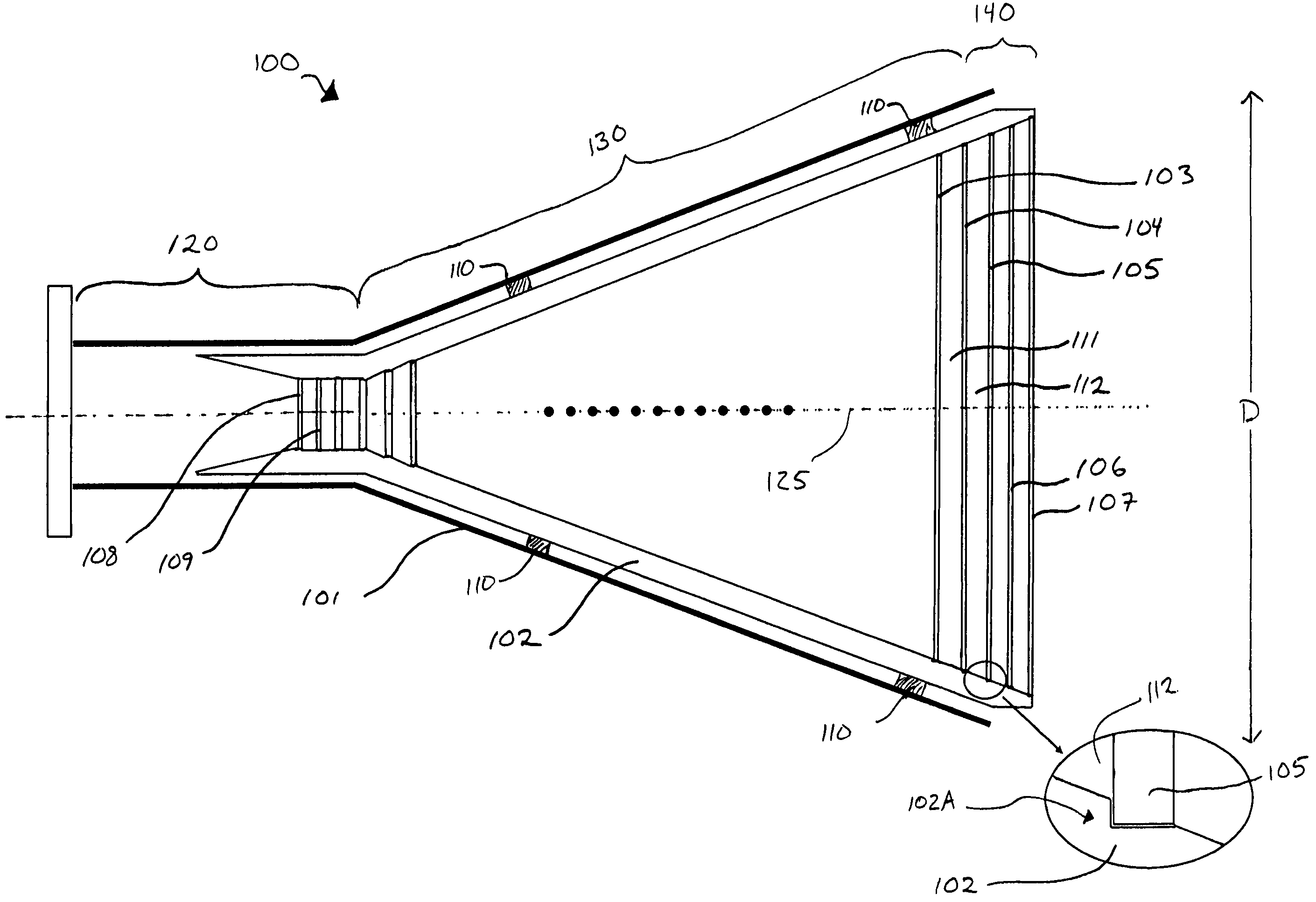
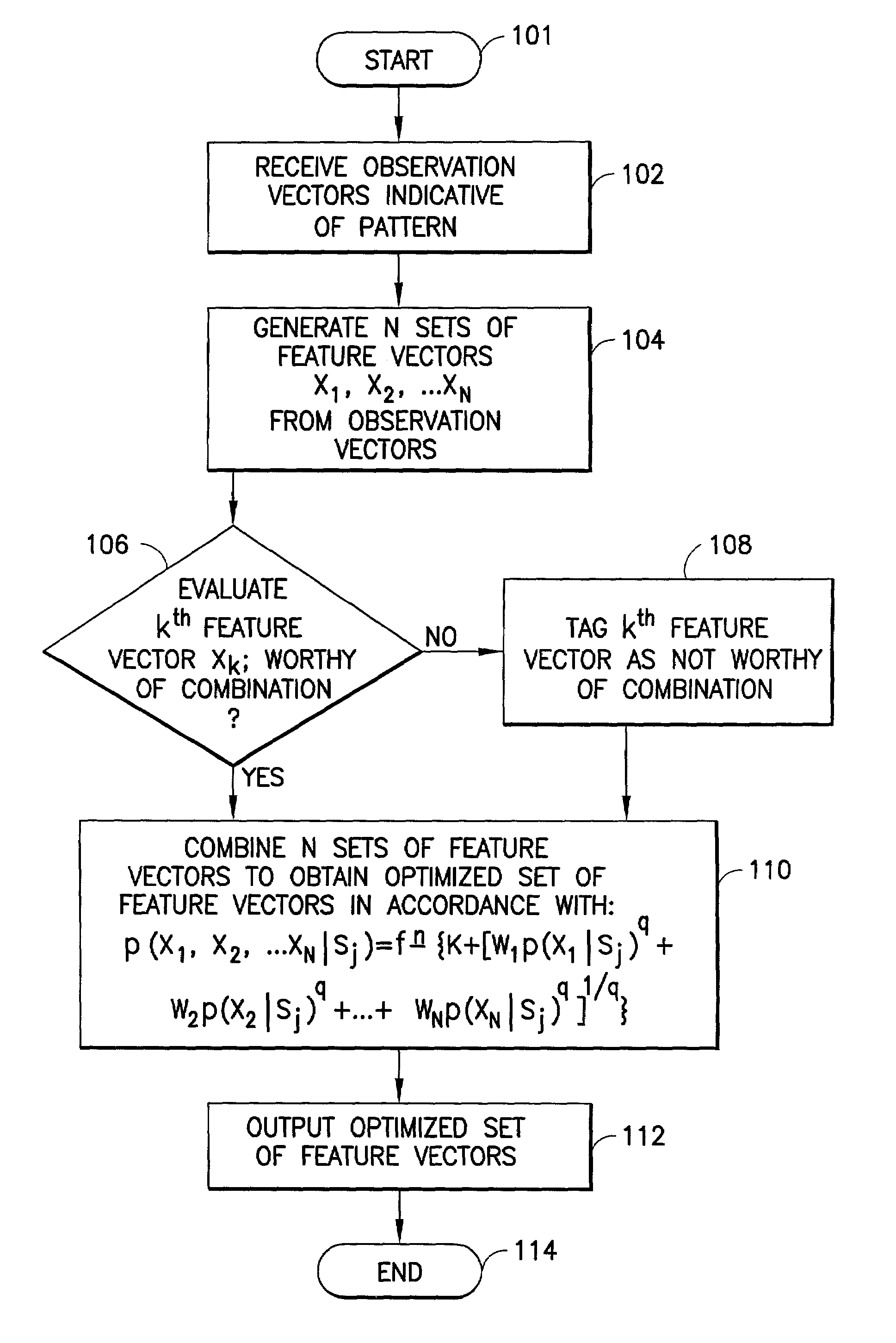
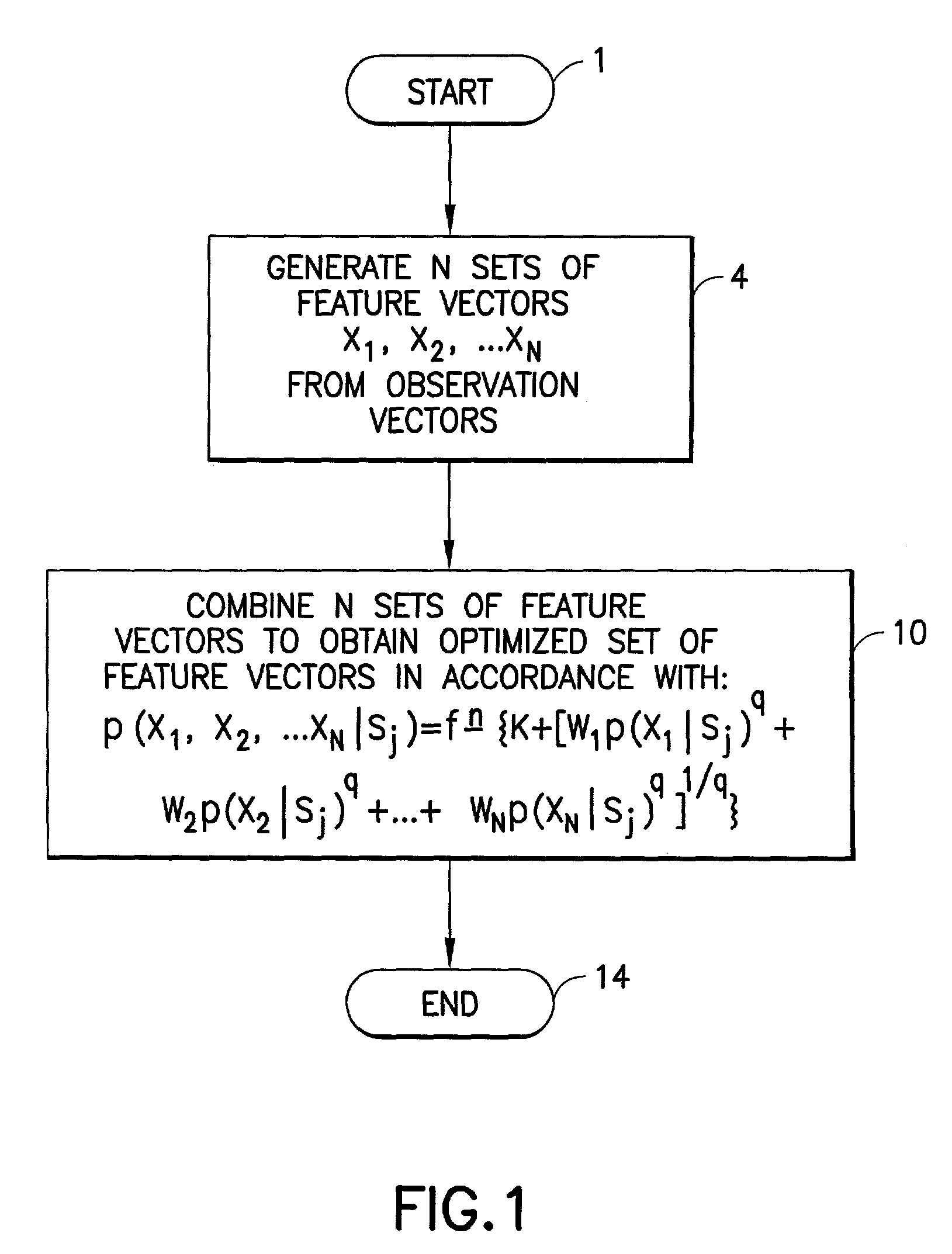
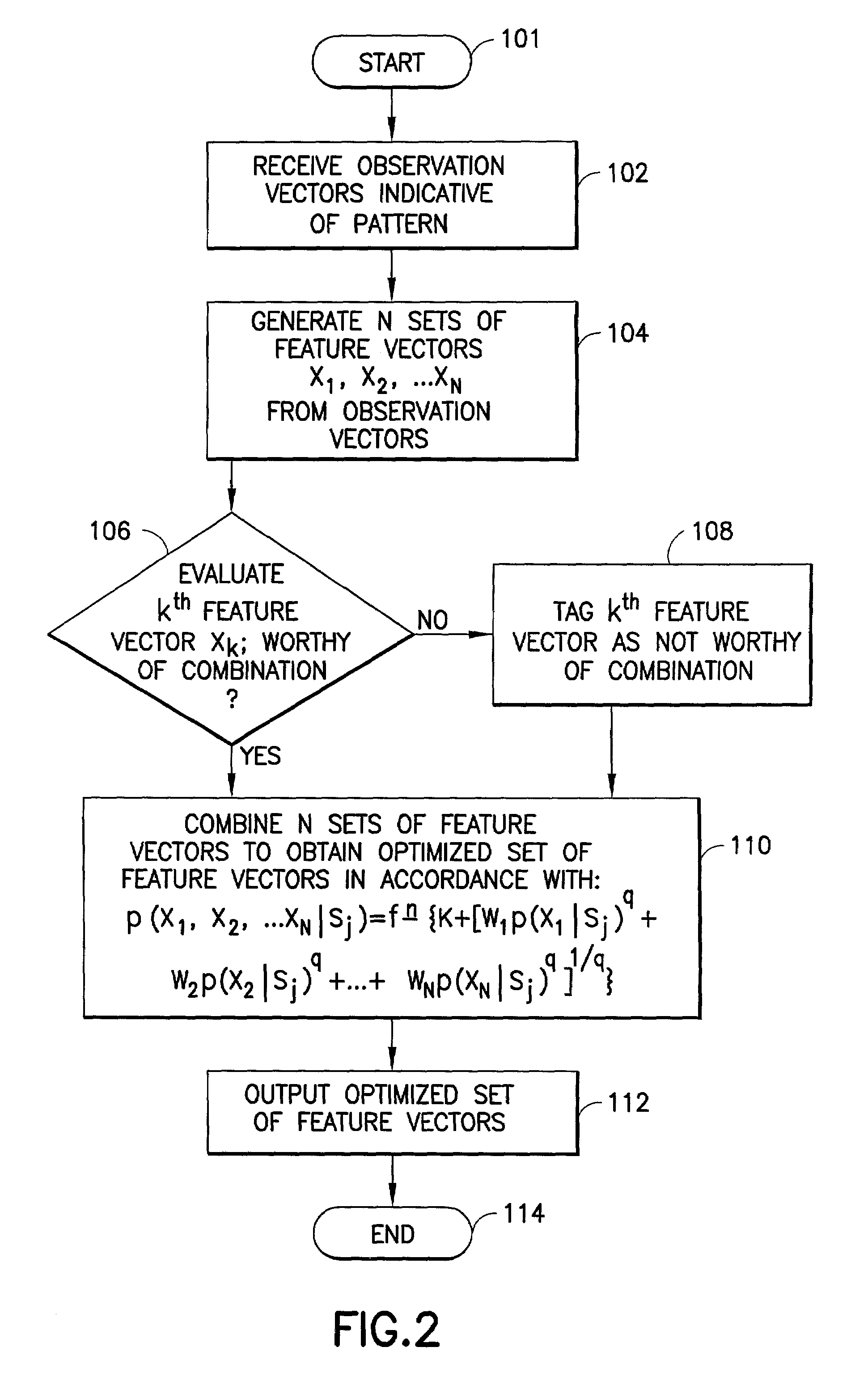
Feature vector-based apparatus and method for robust pattern recognition
N sets of feature vectors are generated from a set of observation vectors which are indicative of a pattern which it is desired to recognize. At least one of the sets of feature vectors is different than at least one other of the sets of feature vectors, and is preselected for purposes of containing at least some complimentary information with regard to the at least one other set of feature vectors. The N sets of feature vectors are combined in a manner to obtain an optimized set of feature vectors which best represents the pattern. The combination is performed via one of a weighted likelihood combination scheme and a rank-based state-selection scheme; preferably, it is done in accordance with an equation set forth herein. In one aspect, a weighted likelihood combination can be employed, while in another aspect, rank-based state selection can be employed. An apparatus suitable for performing the method is described, and implementation in a computer program product is also contemplated. The invention is applicable to any type of pattern recognition problem where robustness is important, such as, for example, recognition of speech, handwriting or optical characters under challenging conditions.
Owner:MICROSOFT TECH LICENSING LLC
Hearing aid system
InactiveUS20050078843A1Great stability and resiliencyReduce insertionNon-occlusive ear tipsHearing aids mounting/interconnectionLoudspeakerMembrane configuration
An exemplary hearing aid system includes a receiver configured so as to create an insertion loss over the audible range of hearing below about three decibels as compared to the unaided ear. An exemplary hearing aid system also includes one or more of: a micro-receiver positioned in an open-ear configuration within the ear canal of a user; an intermediate connecting portion extending between a sound processing unit and a receiver, wherein the intermediate connecting portion includes a stiffening wire provided on at least a portion of the intermediate connecting portion and / or within or on at least a portion of the receiver; a retaining wire extending from one of the stiffening wire and the receiver, the retaining wire configured to position within a portion of the concha of the ear; an electrical conducting component comprising two wires within distinct channels or otherwise isolated from one another within the intermediate connecting portion; and a speaker, at least partially enclosed within a casing having first and second end portions, the first end portion communicating with, the connection, the speaker communicating with a port provided at the second end portion of the casing, wherein the casing is sealed to fluids and wherein the port is sealed to fluids by a membrane or mesh material. The described hearing aid reduces the insertion and occlusion effects relative to comparison devices.
Owner:VIVATONE HEARING SYST
Self-Clearing Self-Cutting Implant
ActiveUS20110195380A1Reduce frettingReduce torqueDental implantsThread cutting machinesBone implantBiomedical engineering
Owner:INTRA LOCK INT
Optical fiber connector with ferrule radial orientation control
InactiveUS20040101254A1Minimize eccentricityPrecise alignmentCoupling light guidesFiberRadial position
A method of assembling an optical fiber assembly comprising the steps of: (a) providing a ferrule sub-assembly comprising a ferrule having a front end and a fiber secured therein and a positioning member; (b) positioning the ferrule sub-assembly in a housing adapted to receive the positioning member in a plurality of predetermined radial positions; (c) preparing the front end such that the fiber is suitable for optical coupling with a mating device; (d) rotating the ferrule sub-assembly to at least a portion of the plurality of predetermined radial positions within the housing while measuring the power loss associated with each radial position to determine the optimal radial position; and (e) optionally polishing the end of the ferrule into the desired configuration after the optimum radial position is determined.
Owner:COMMSCOPE TECH LLC
Electrical connector assembly having improved grounding means
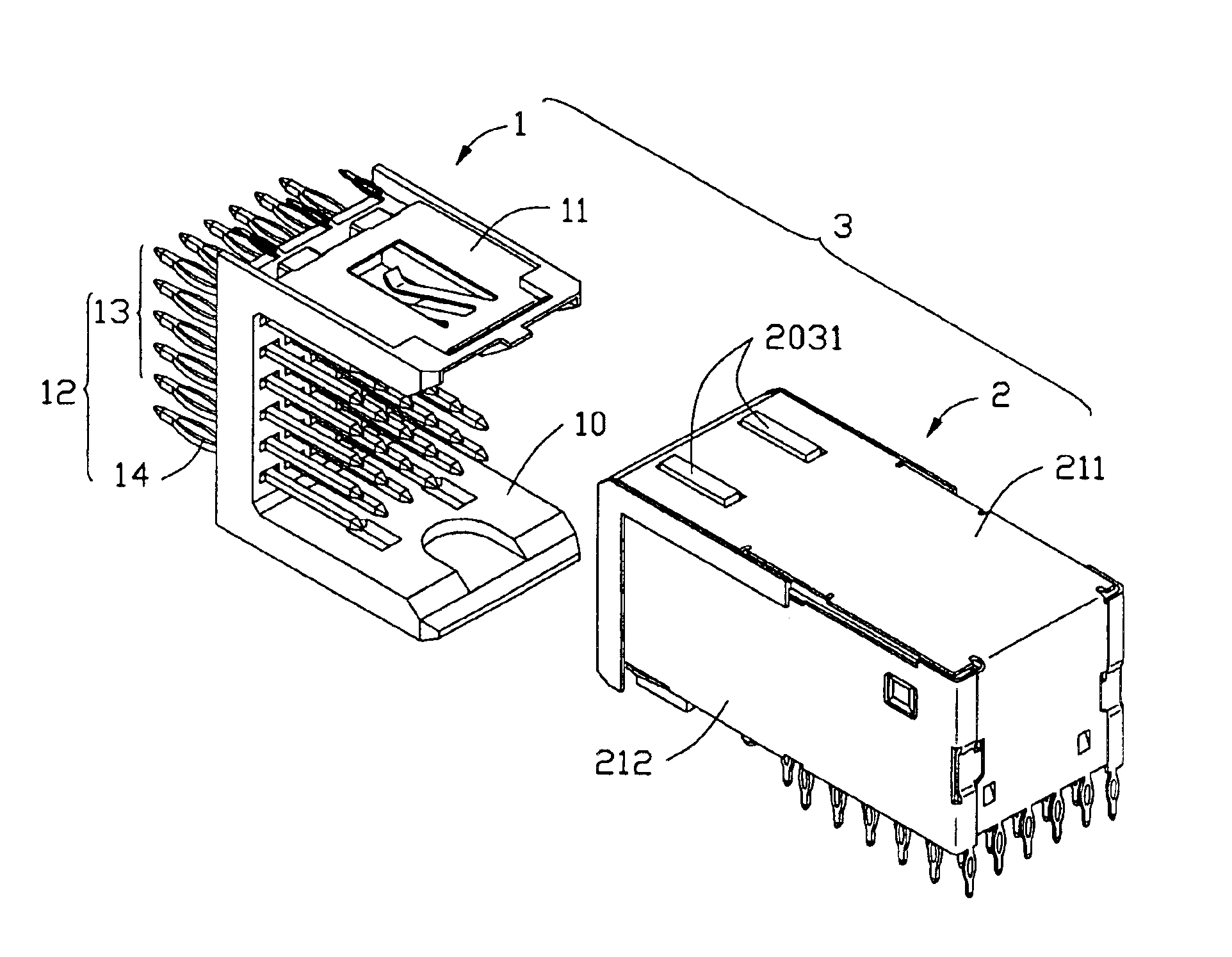
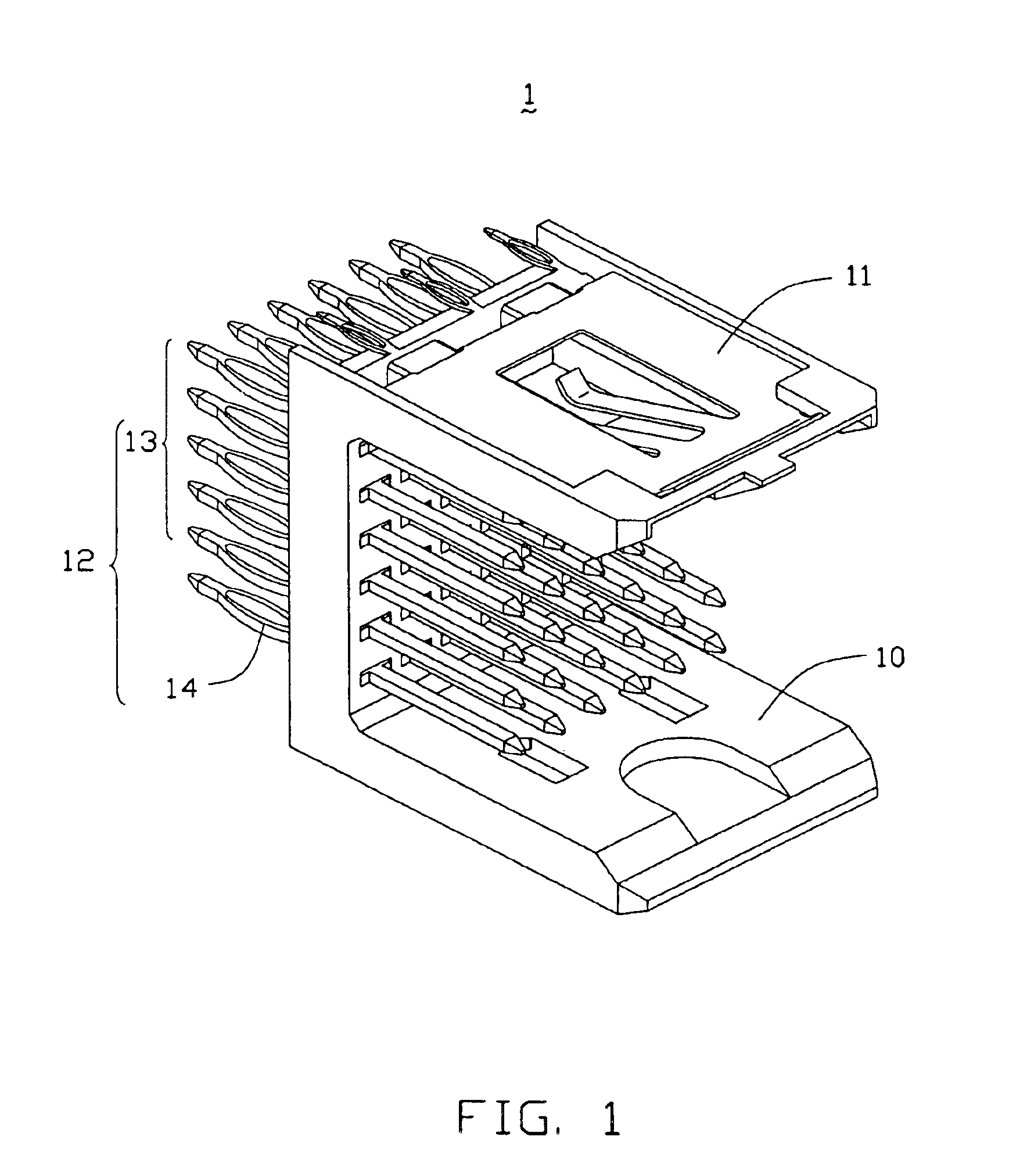
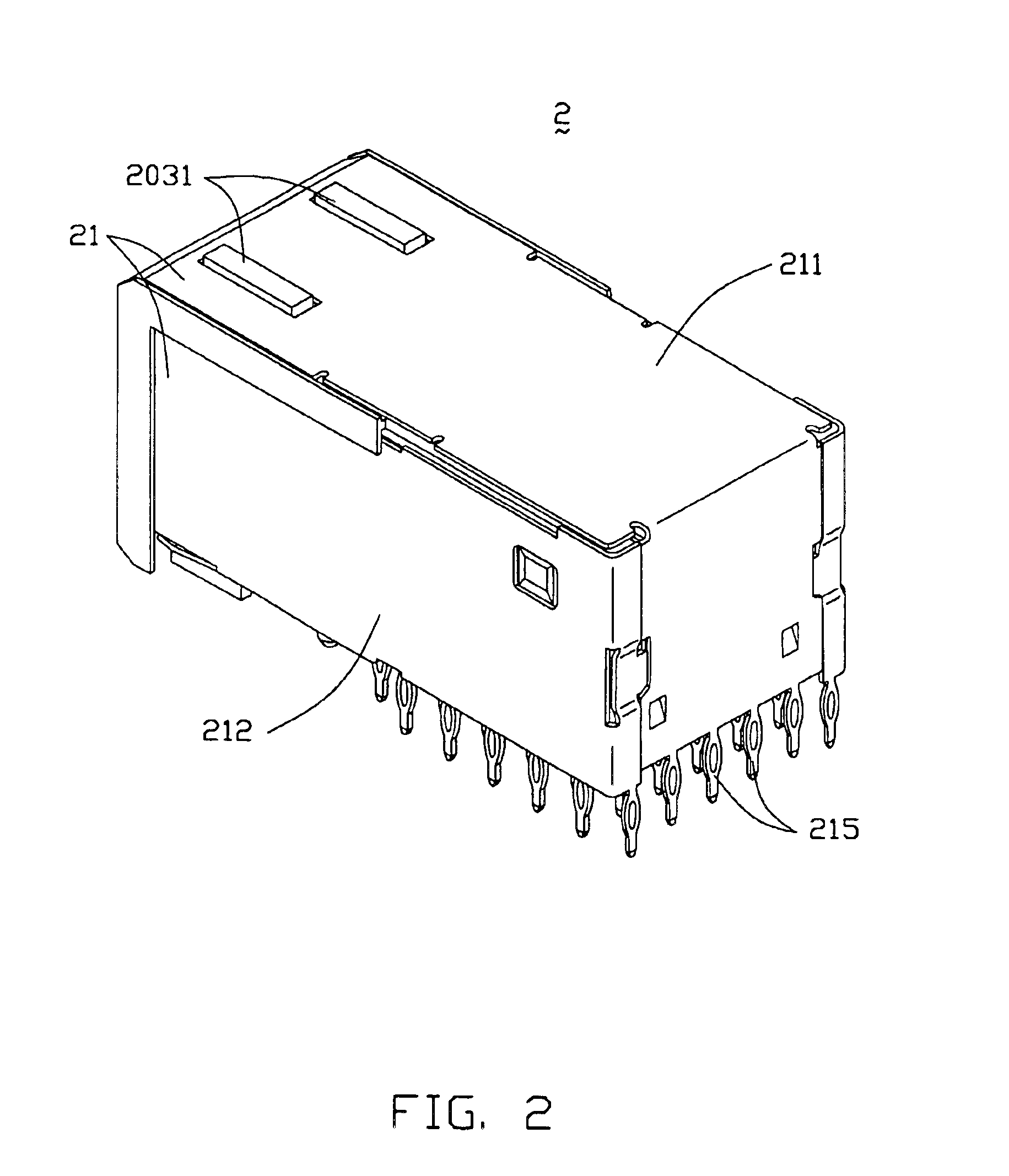
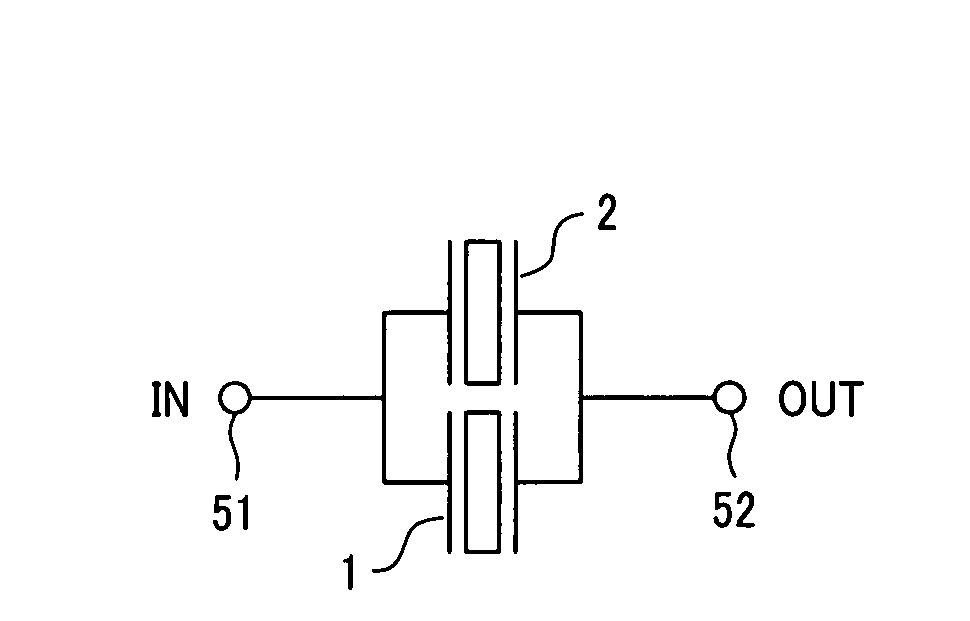
InactiveUS6893272B2Reduce insertionElectrically conductive connectionsPrinted circuitsGround contactEngineering
An electrical connector assembly (3) includes a header and a receptacle connectors (1,2). The header connector comprises a header housing (10) and a ground contact plate (11). The header housing has a base (100), a pair of sidewalls (101,102) extending from the base, and a mating space (103) formed between the sidewalls. The ground contact plate is attached to one of the sidewalls. The receptacle connector includes a receptacle housing (20) inserted into the mating space of the header housing, and a shield member (21) covering the receptacle housing. The ground contact plate has a contact beam (115) extending into the mating space through a notch (105) in the one sidewall along the insertion direction of the receptacle connector and contacting the shield member.
Owner:HON HAI PRECISION IND CO LTD
Optical fiber connector with ferrule radial orientation control
A method of assembling an optical fiber assembly comprising the steps of: (a) providing a ferrule sub-assembly comprising a ferrule having a front end and a fiber secured therein and a positioning member; (b) positioning the ferrule sub-assembly in a housing adapted to receive the positioning member in a plurality of predetermined radial positions; (c) preparing the front end such that the fiber is suitable for optical coupling with a mating device; (d) rotating the ferrule sub-assembly to at least a portion of the plurality of predetermined radial positions within the housing while measuring the power loss associated with each radial position to determine the optimal radial position; and (e) optionally polishing the end of the ferrule into the desired configuration after the optimum radial position is determined.
Owner:COMMSCOPE TECH LLC
Nonreciprocal circuit element
A nonreciprocal circuit element includes first and second center electrodes. On a ferrite to which a direct-current magnetic field is applied from a permanent magnet, the first and second center electrodes are insulated and intersect. First and second ends of the first center electrode are connected to an input port and an output port, respectively. First and second ends of the second center electrode are connected to the output port and a ground port, respectively. A first matching capacitor and a resistor are connected between the input port and the output port. A second matching capacitor is connected between the output port and the ground port. A parallel resonant circuit is connected in parallel to the resistor. A coupling element is connected between the parallel resonant circuit and another parallel resonant circuit including the first center electrode and the first matching capacitor so as to the parallel resonant circuits.
Owner:MURATA MFG CO LTD
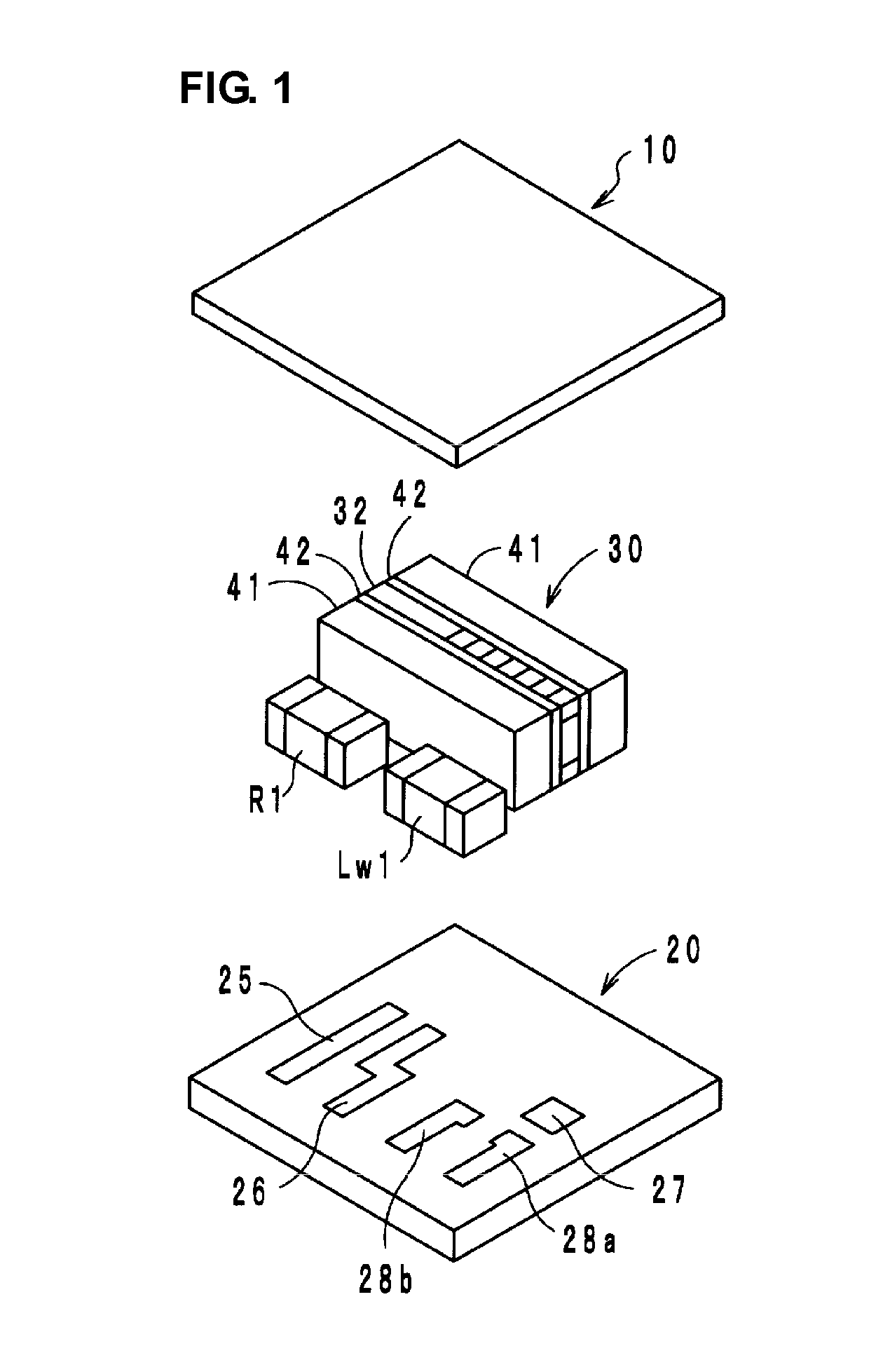
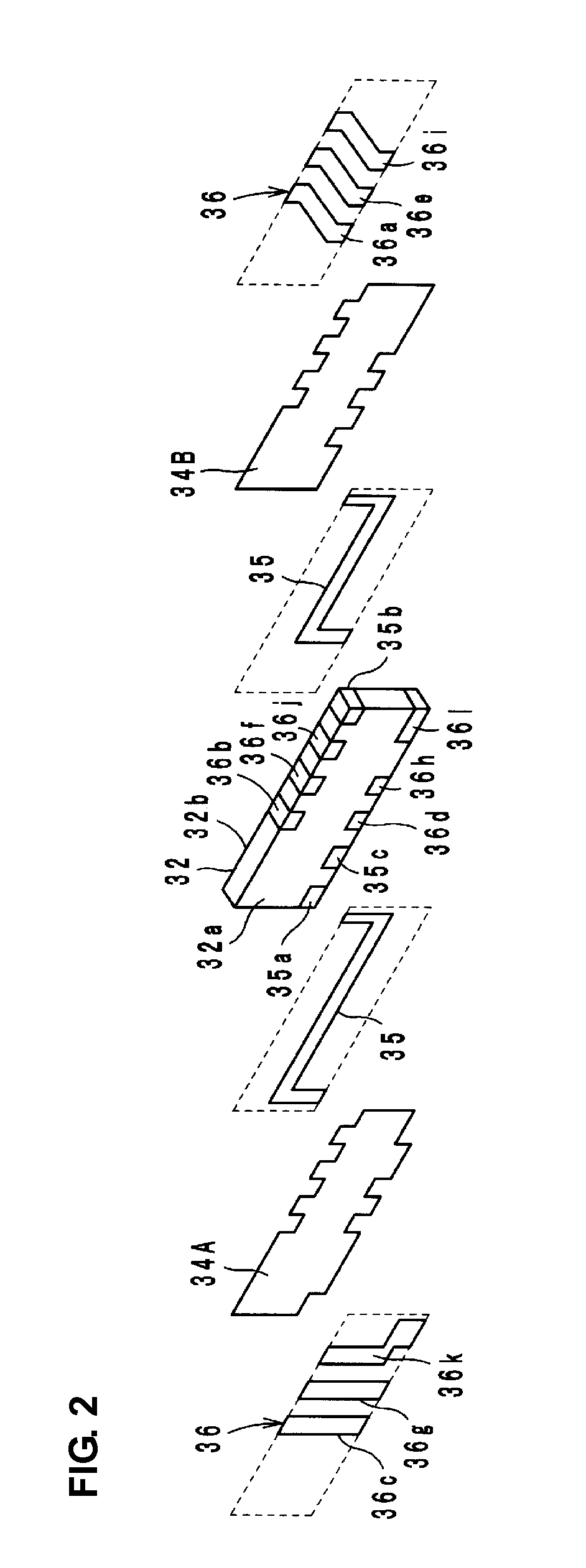
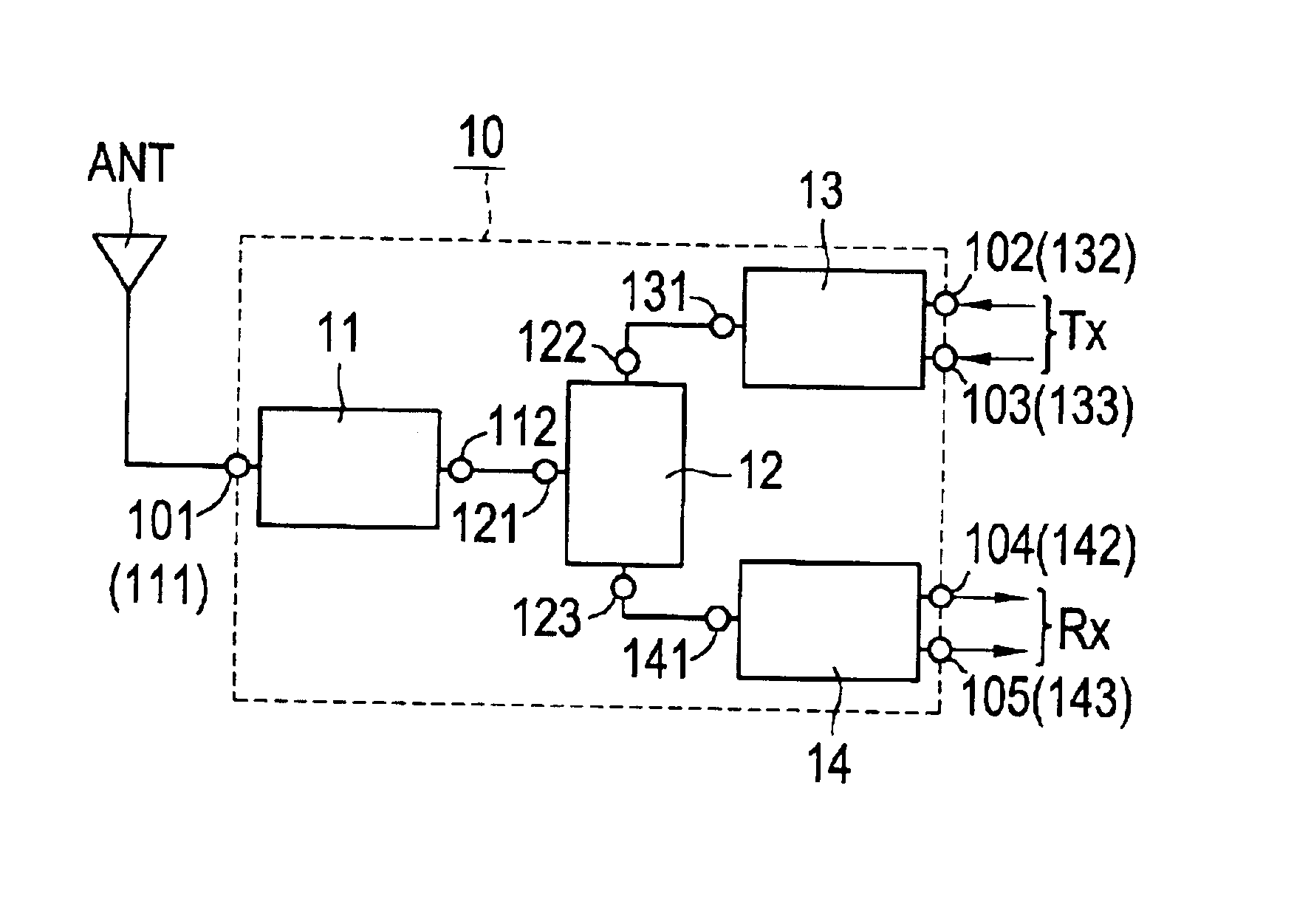
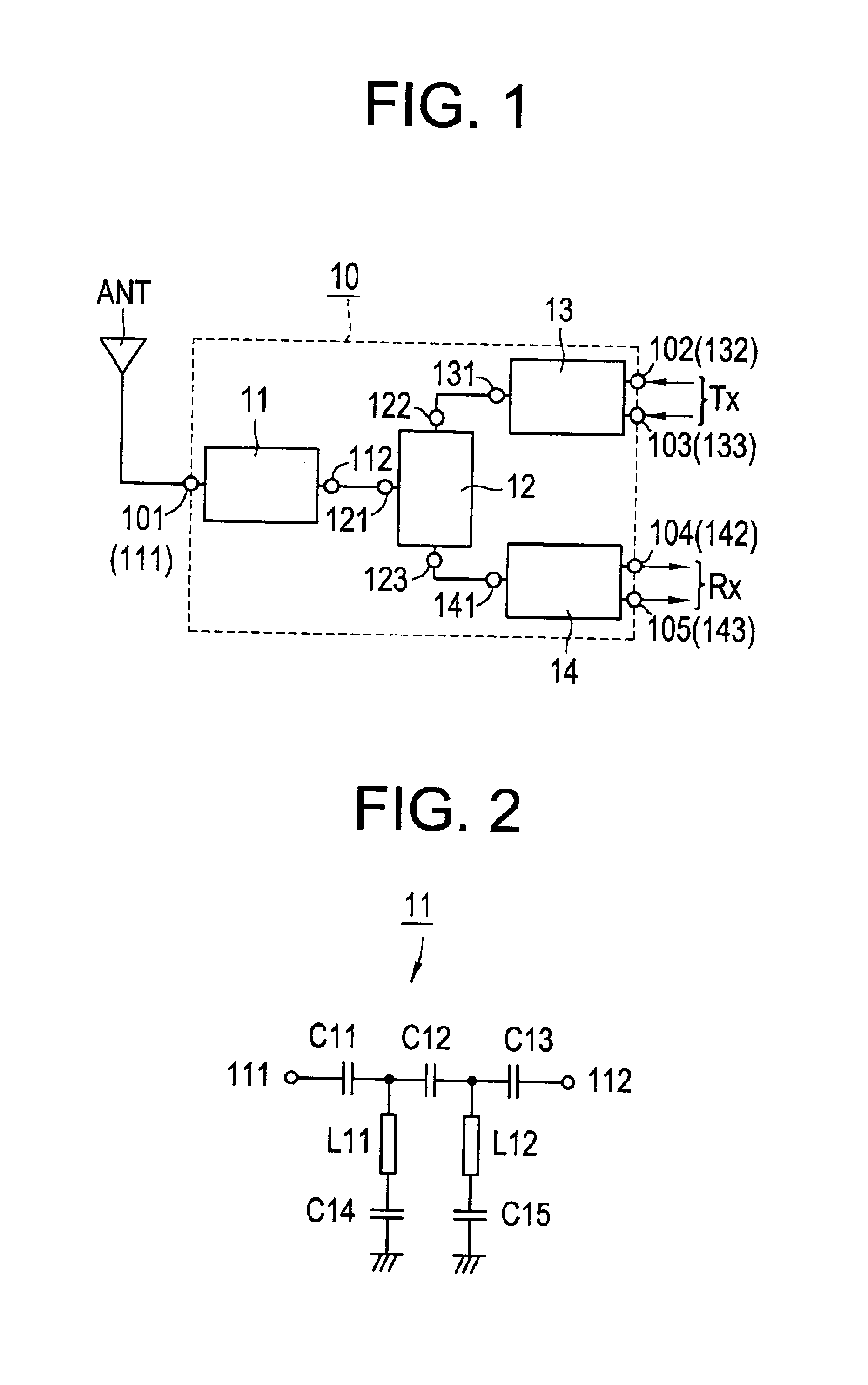
High-frequency module and radio device using the same
InactiveUS6937845B2Reduce insertion lossReduce insertionMultiple-port networksTransmissionHigh-pass filterEngineering
A high-frequency module includes first to fifth terminals, a high-pass filter, a high-frequency switch, a transmitter-side balun, and a receiver-side balun. The high-pass filter is connected to the high-frequency switch, and the high-frequency switch is also connected to the transmitter-side balun and to the receiver-side balun. The first terminal is connected to an antenna, the second and third terminals are connected to a transmitter circuit, and the fourth and fifth terminals are connected to a receiver circuit.
Owner:MURATA MFG CO LTD
Phase plug and acoustic lens for direct radiating loudspeaker
ActiveUS8181736B2Reduce distortionReduce insertionLoudspeaker screensCabinetsEngineeringAcoustic lens
A phase plugs or acoustic lens improves the directional audio performance of a loudspeaker. Application of the improved directional audio performance to a sound system in a listening area may improve the performance of the audio system. Configuration of the acoustic lens or phase plug may include both symmetrical and asymmetrical features to provide an improved frequency response and directivity. The improved loudspeaker may provide improved an improved listing location, for example, in a vehicle.
Owner:HARMAN INT IND INC
Optical connector
ActiveUS20070211997A1Reduce insertionLess bendingCoupling light guidesEngineeringOptical fiber connector
An optical connector includes a connector body that has a first optical fiber housed in advance in a ferrule so as to project from a back end of the ferrule opposite to the connecting end surface and an anchoring fixture that anchors a second optical fiber that is to be optically connected to the first optical fiber, and by pressing the anchoring fixture into the connector body while the second optical fiber is anchored in this anchoring fixture, the anchoring fixture and the connector body are connected to optically connect the first optical fiber and the second optical fiber, and the connecting portion that connects the anchoring fixture and the connector body form a movable connecting portion that is adapted to vary the direction of the anchoring fixture with respect to the connector body.
Owner:THE FUJIKURA CABLE WORKS LTD +1
Thin film bulk acoustic wave resonator and filter, and radio frequency module using them
InactiveUS7554427B2Low insertion lossLess costlyImpedence networksPiezoelectric/electrostriction/magnetostriction machinesThin-film bulk acoustic resonatorManufacturing technology
A thin film bulk acoustic wave (BAW) resonator structure and filter which can be fabricated by inexpensive manufacturing techniques and in smaller size than conventional such products are to be provided. The BAW resonator structure and filter have a substrate, a first BAW resonator placed over the substrate, an acoustic reflection layer placed over the first BAW resonator and a second BAW resonator placed over the acoustic reflection layer, and the acoustic reflection layer is electroconductive. Herein, the acoustic reflection layer constitutes a first electrode, and this first electrode electrically connects and acoustically separates the first BAW resonator and the second BAW resonator.
Owner:HITACHI MEDIA ELECTORONICS CO LTD
Transformer-free waveguide circulator
InactiveUS7242263B2Reduce insertionReduce the total massWaveguide type devicesTransformerImpedance matching
An improved waveguide circulator that eliminates the need for quarter-wave dielectric transformers or impedance steps in the interface waveguide for broadband operation is described. The circulator designs in the prior art all require impedance matching elements outside of the ferrite element in order to achieve acceptable performance. Through the use of this new invention, broadband circulator performance can be achieved without the addition of impedance matching elements in order to minimize the cost, size, mass, and loss of the circulator.
Owner:EMS TECHNOLOGIES
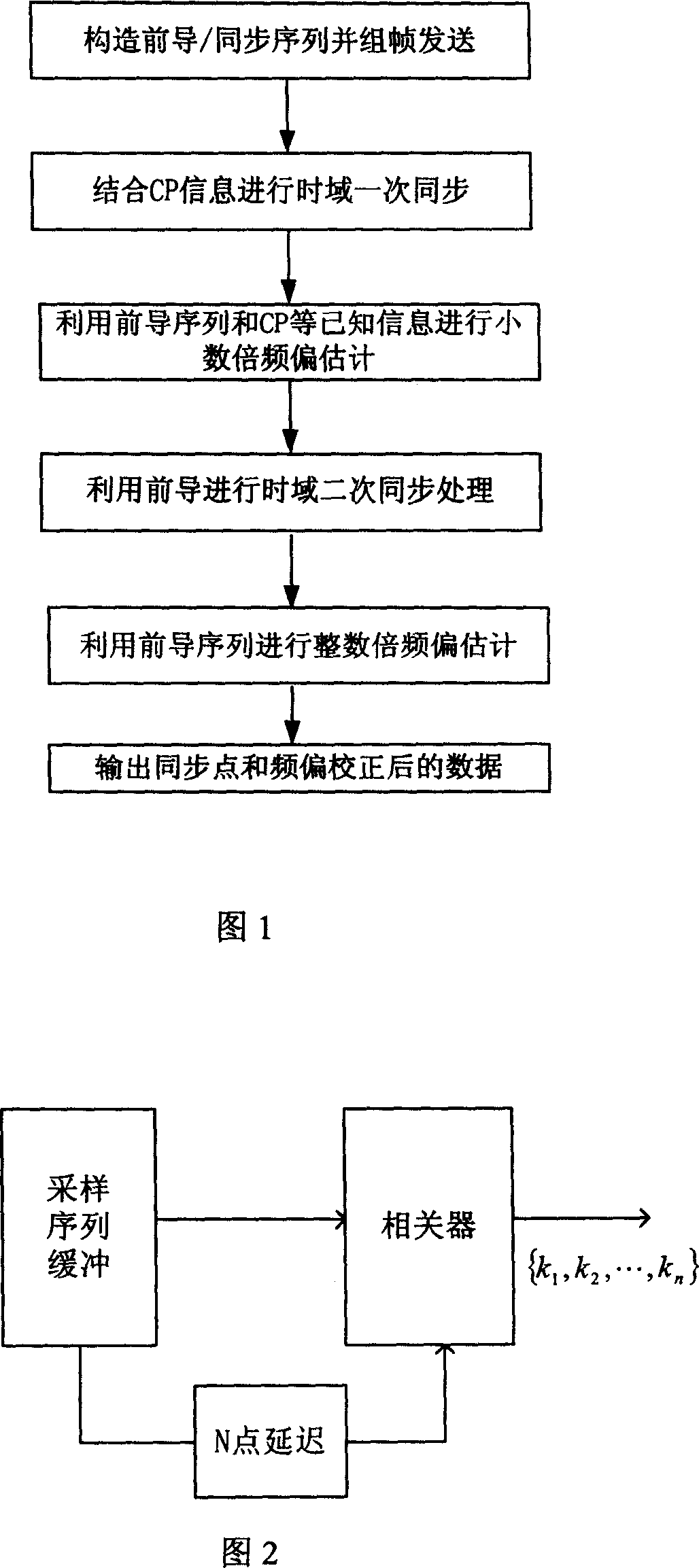
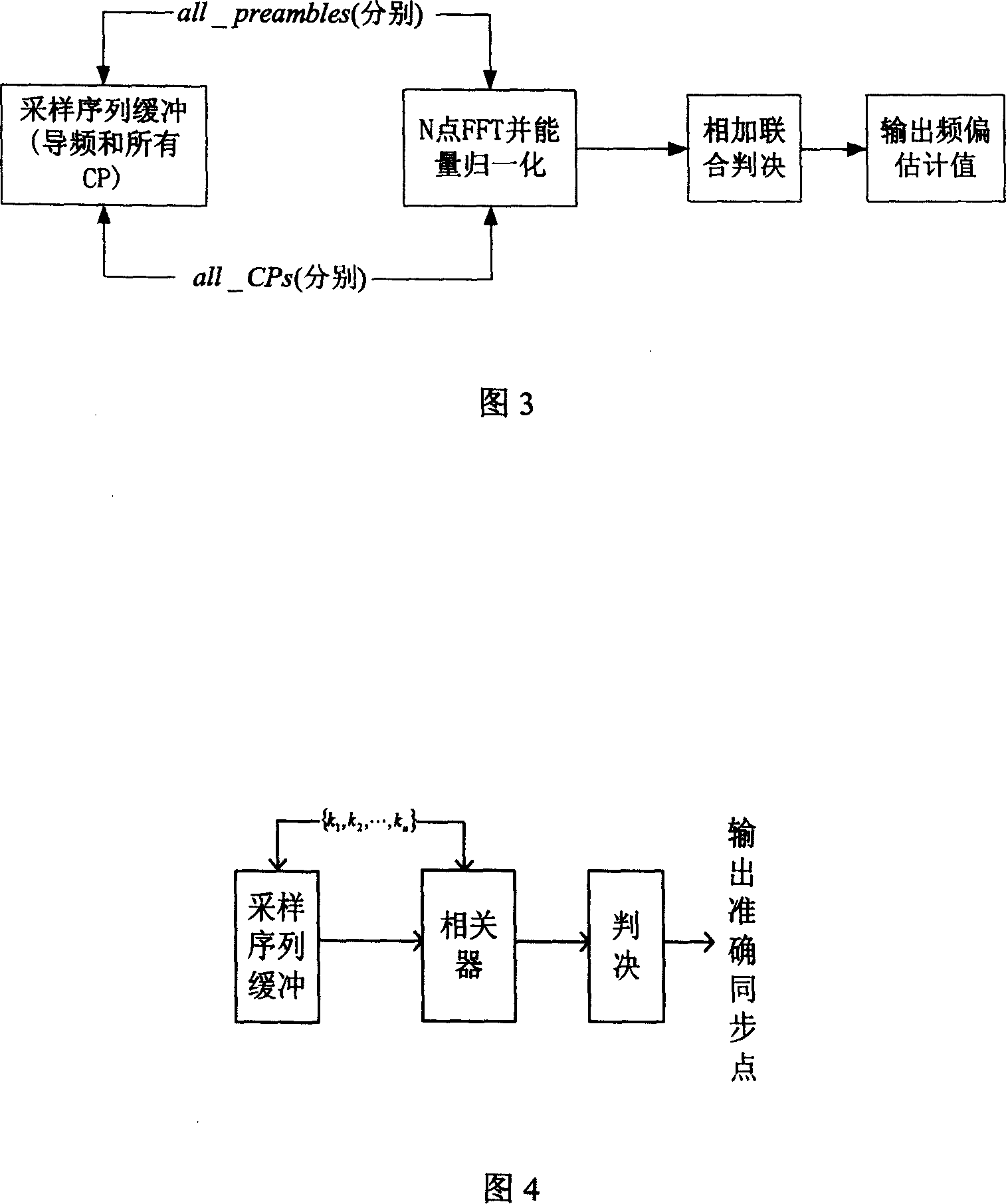
Synchronizing method for orthogonal frequency division multiplex system
InactiveCN1988525ASimple structureReduce computationMulti-frequency code systemsEffective lengthComputer science
The invention discloses a synchronization method for orthogonal frequency division multiplexing system, including: the launcher firstly constructs the originated / synchronous sequence that is sent out with OFDM data symbols together. Based on the correlation of CP effective length in OFDM symbols form the receiving end, the correlation result conducts a time domain processing to the correlation sequence energy to get the best synchronization points set after one synchronization. Based on the originated sequence and CP, the departure estimation and departure compensation of fractional part DF several times are processed. During a range of synchronization points set output at once, the long pre-symbol received after the compensation is made time-domain correlation with the symbol to find the second time synchronization point. Then after time-domain of the short pre-symbol and the received pre-sequence is made frequency departure compensation, time-domain-related operations is conduct to get the DF departure estimation of integer times, and departure compensation is conduct.
Owner:ZTE CORP
Dental Implant and Method for Rapid Integration
The invention relates to methods of stabilizing bone implants, comprising inserting a self tapping implant having at least two helical grooves running in opposite directions around the implant wherein the implant generates a minimum of bone debris during insertion and implant is integrated within 3-6 weeks.
Owner:INTRA LOCK INT
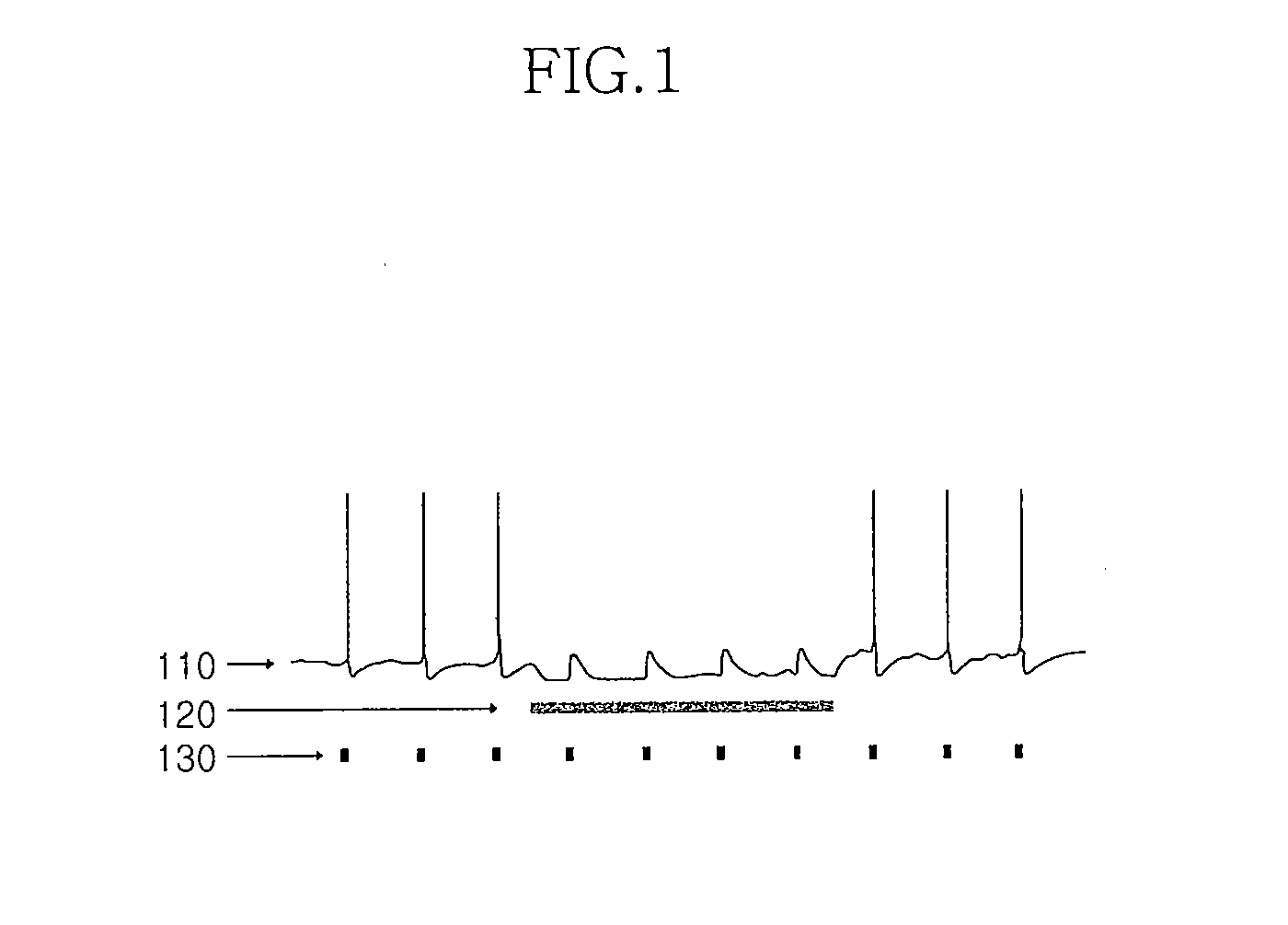
Apparatus for stimulating the brain and measuring the light induced neuronal activity and method for manufacturing the same
ActiveUS20100161017A1Lower the volumeReduce insertionInternal electrodesSensorsMedicineElectro physiology
Disclosed is an apparatus for stimulating the brain and measuring the light induced neuronal activity including a signal application unit which applies a signal to a living tissue to stimulate the neuronal cells in the living tissue; an electrode unit which detects an electrophysiological signal of the neuronal cell in response to the signal; and an insulation unit which controls an impedance of the electrode unit. The signal application unit is formed integrally with the electrode unit, so that the site where the signal is applied to the living tissue is approximated to the site where the response to the stimulation is measured.
Owner:DIGISONIC CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com