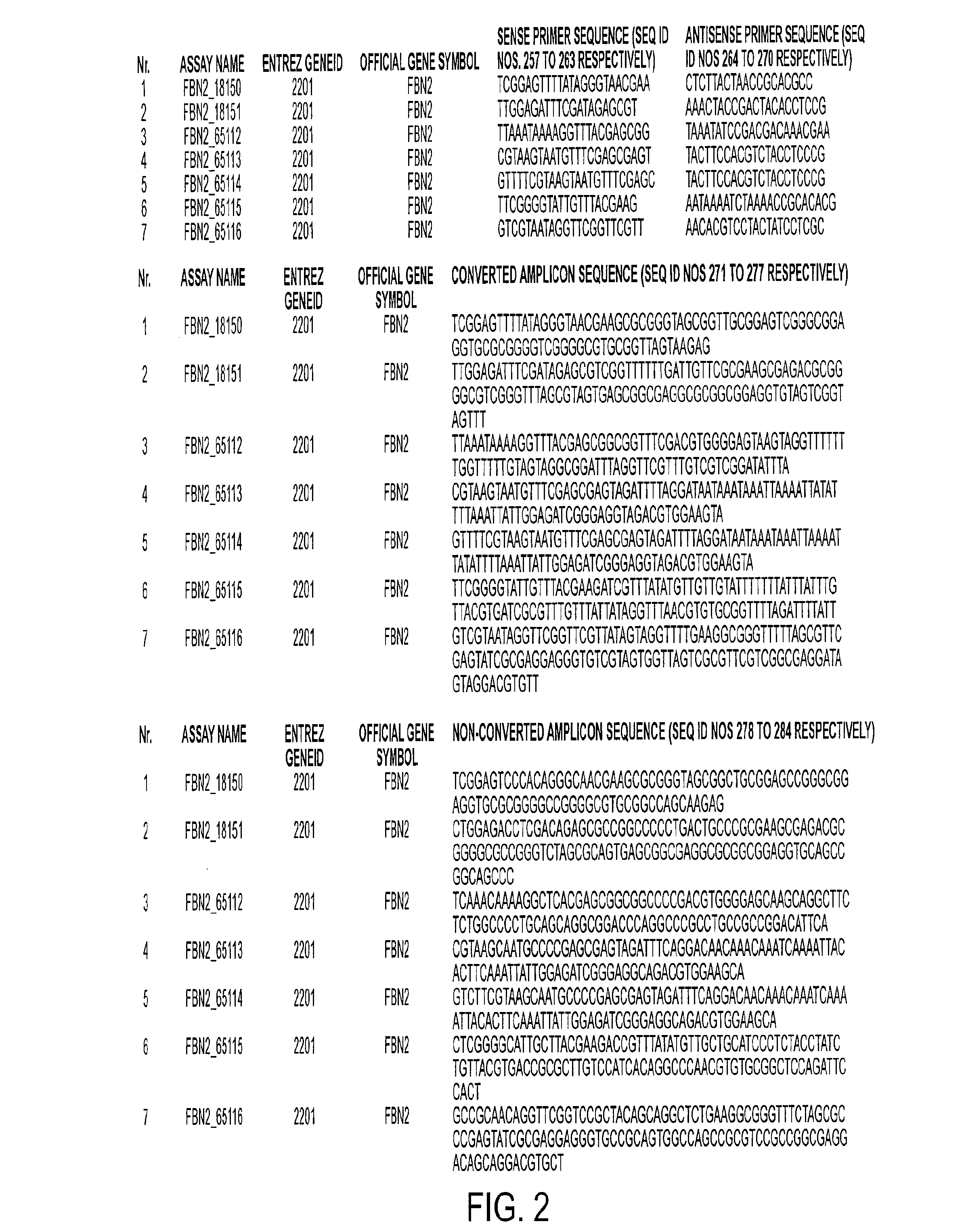
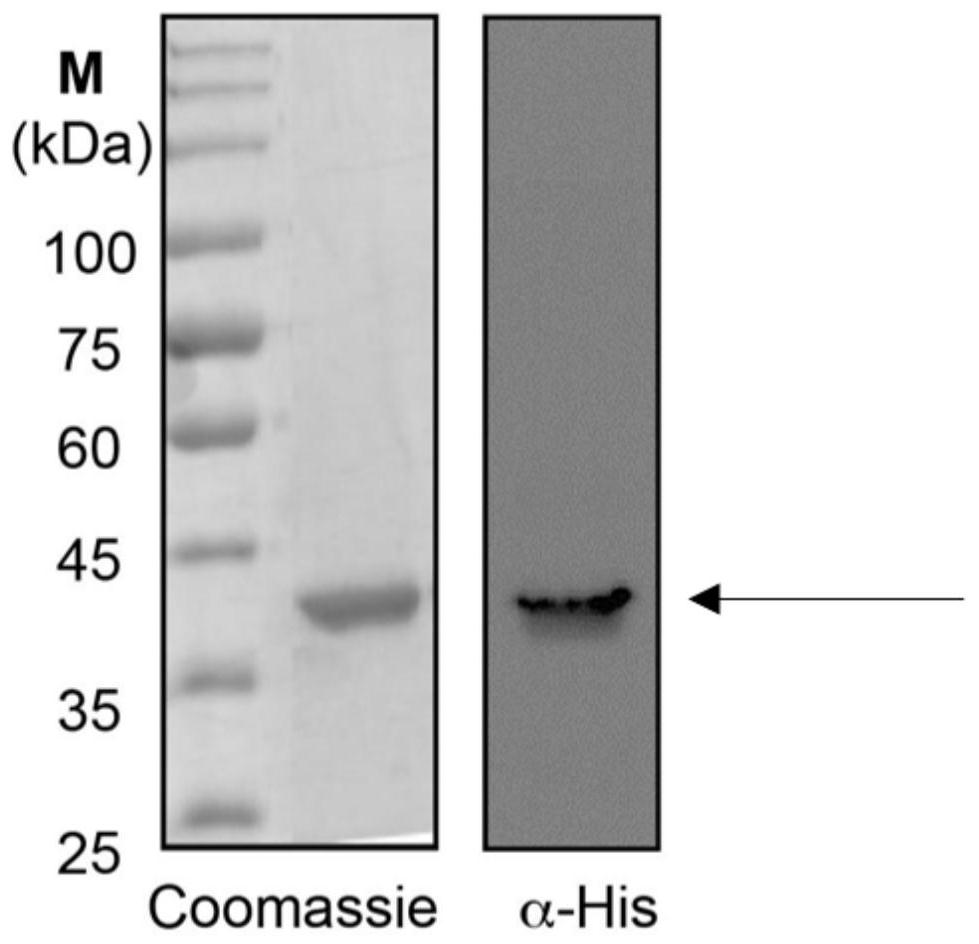
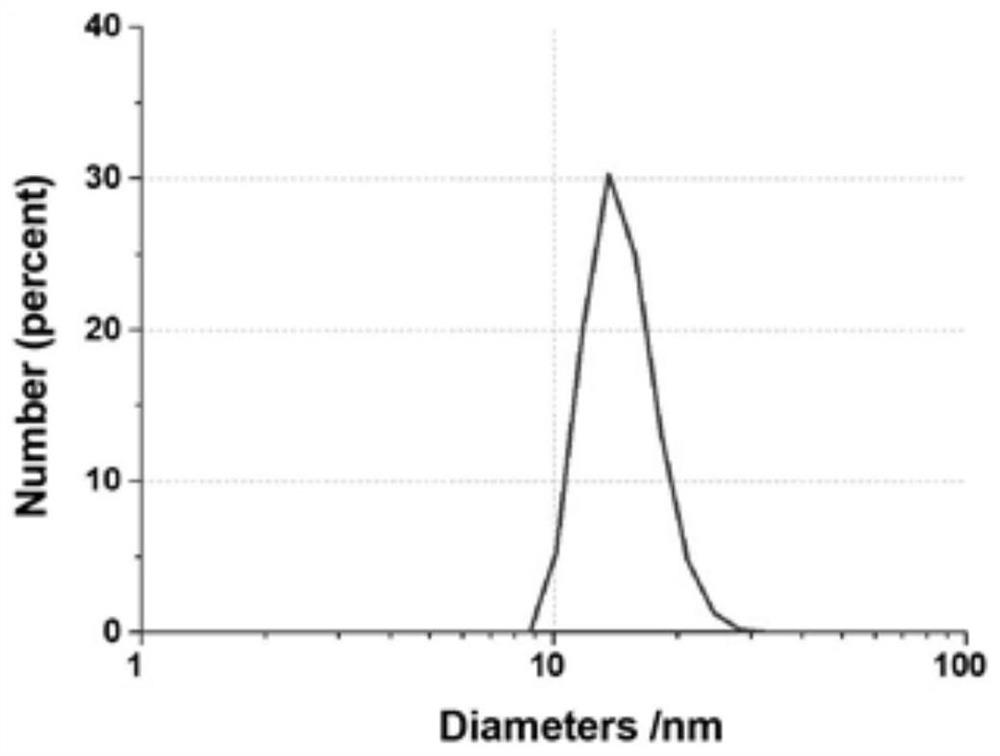
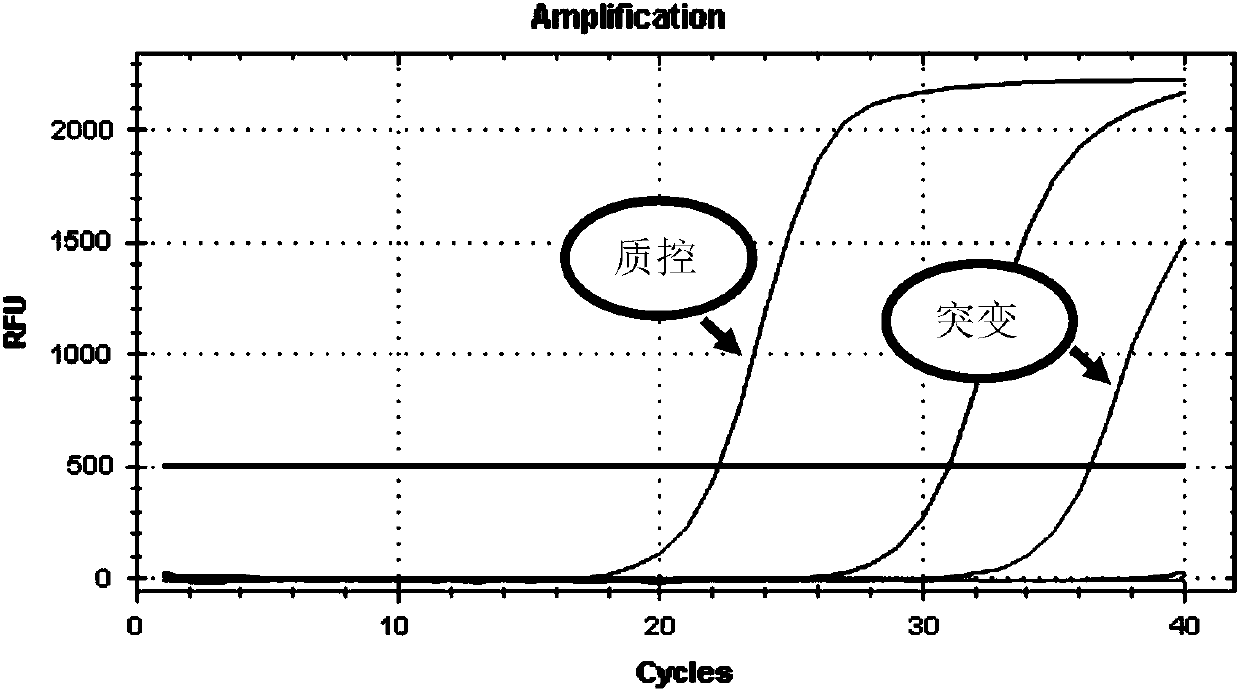
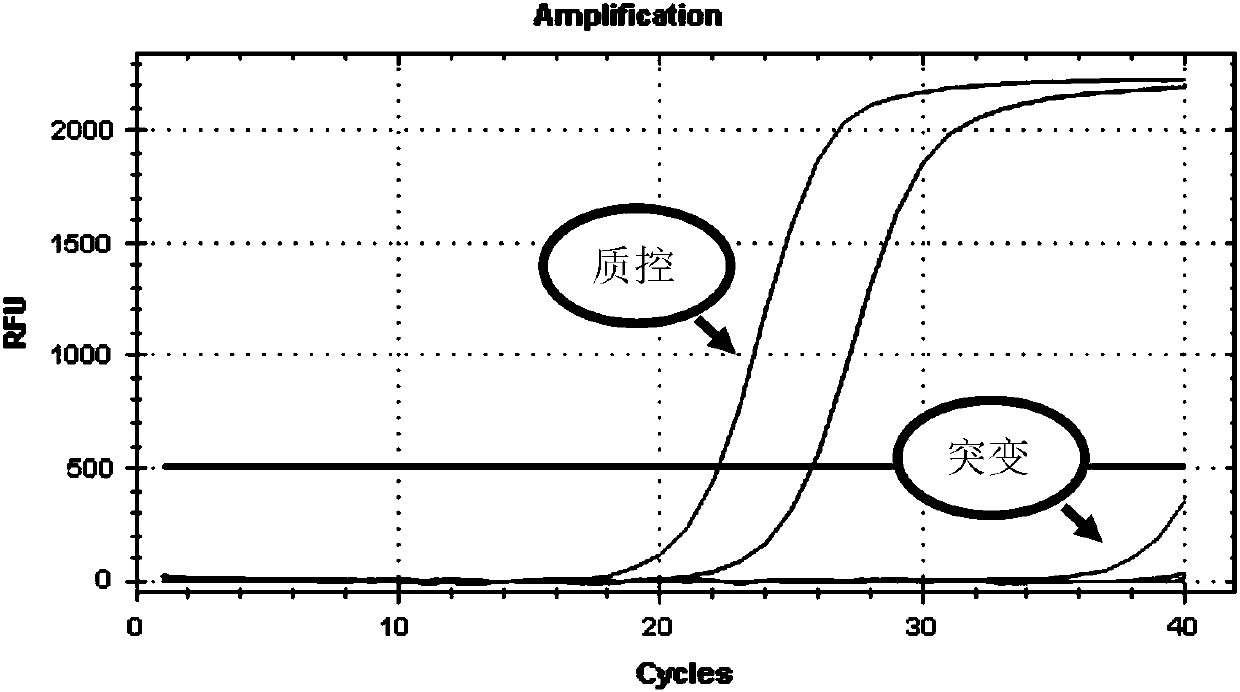
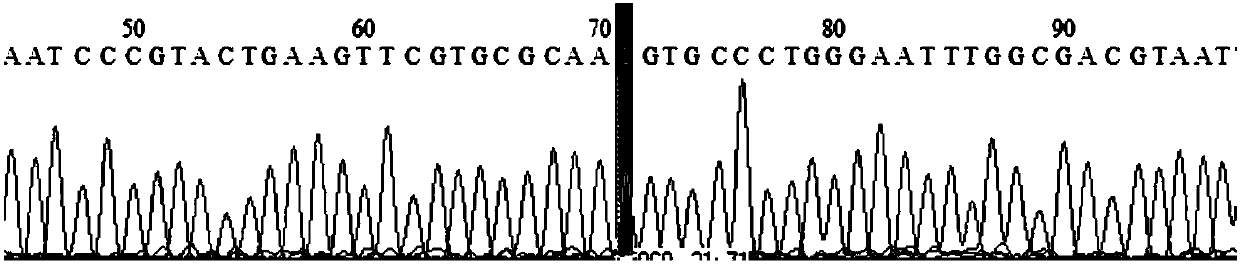
Patents
Literature
34 results about "GSTP1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
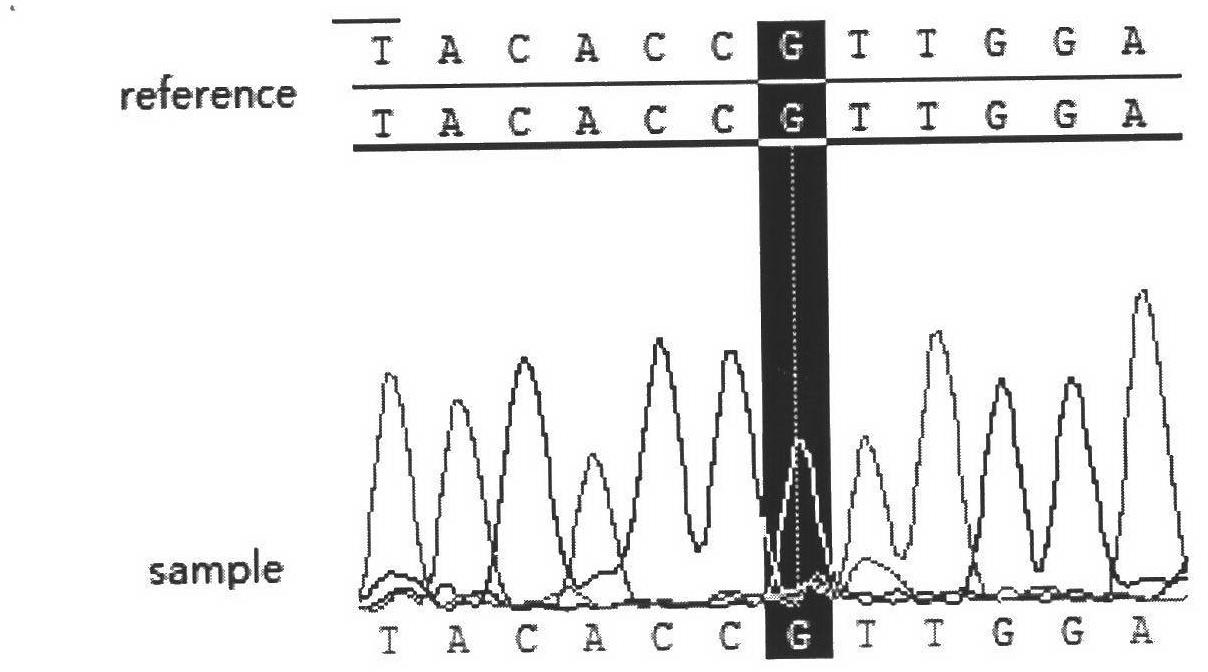
Glutathione S-transferase P is an enzyme that in humans is encoded by the GSTP1 gene.
Method for determining susceptibility of an individual to allergen induced hypersensitivity
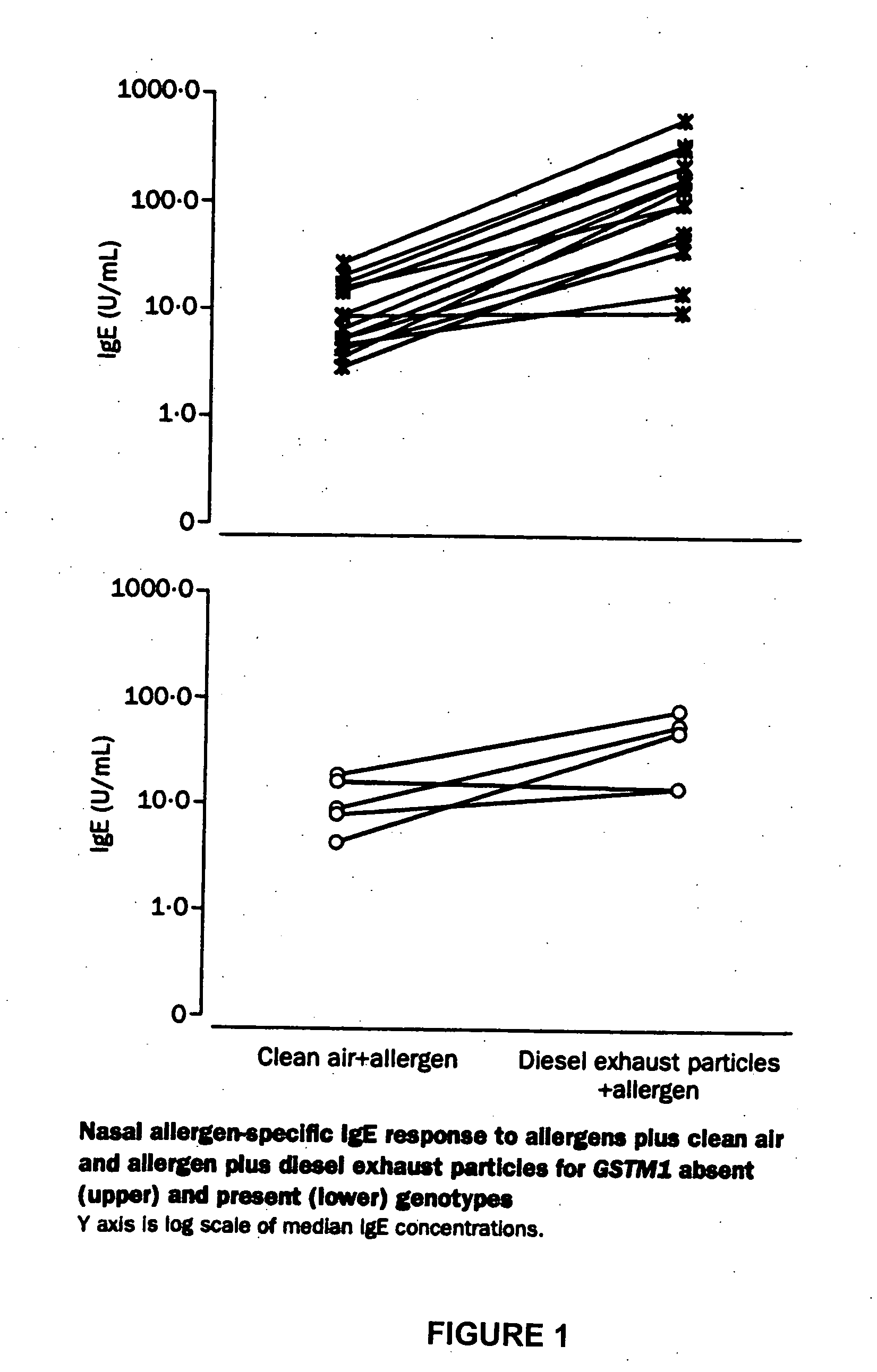
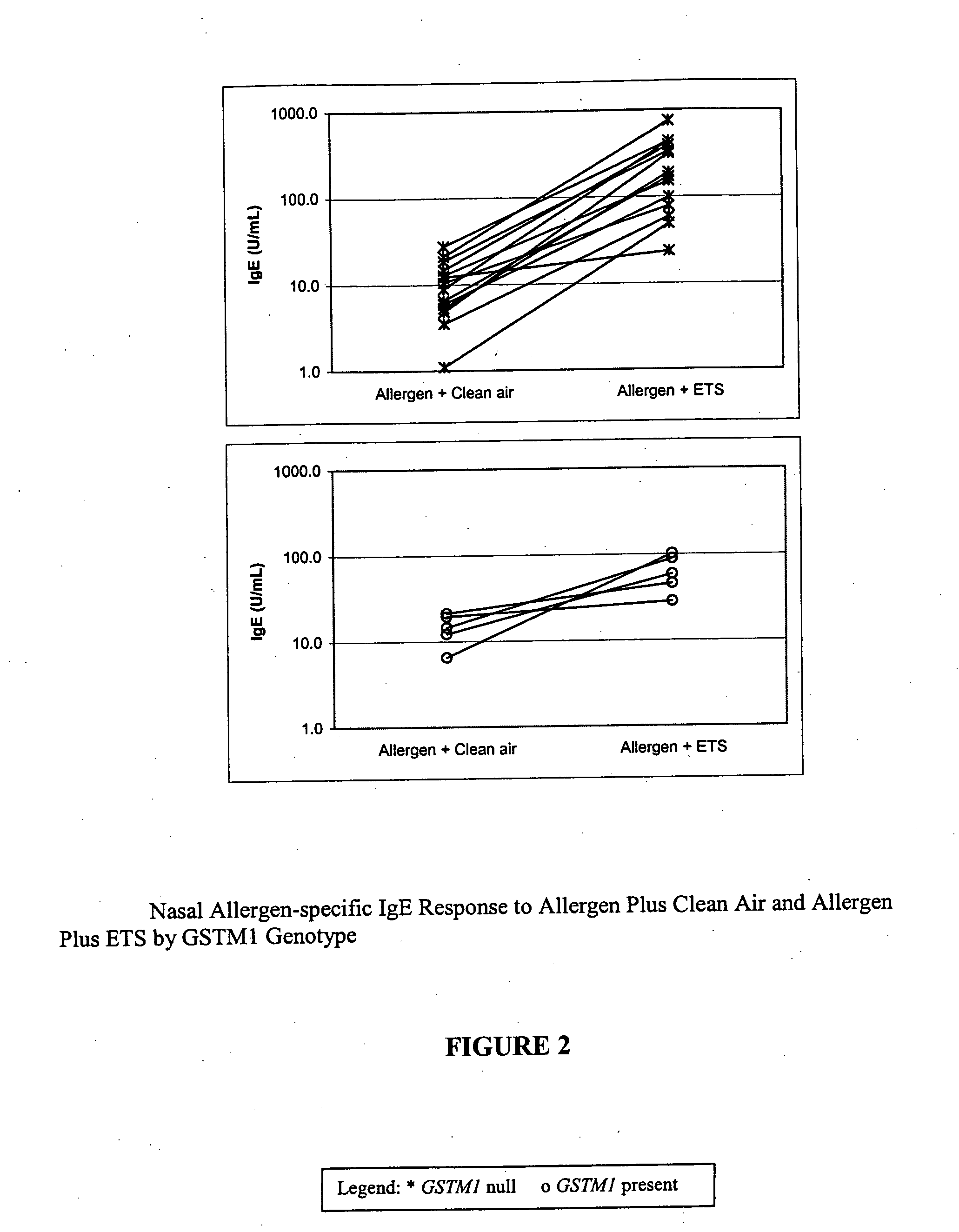
The present invention is related generally to a method for screening subjects to determine those subjects more likely to exhibit a hypersensitivity to allergen in the presence of airborne pollutants, such subject having a GSTM1 null genotype and / or a GSTP1 Ile / Ile genotype. This invention also provides novel or improved pharmaceutical compositions and therapeutic strategies for the treatment of immune diseases resulting from inappropriate or unwanted immune response such as allergic reactions.
Owner:RGT UNIV OF CALIFORNIA +2
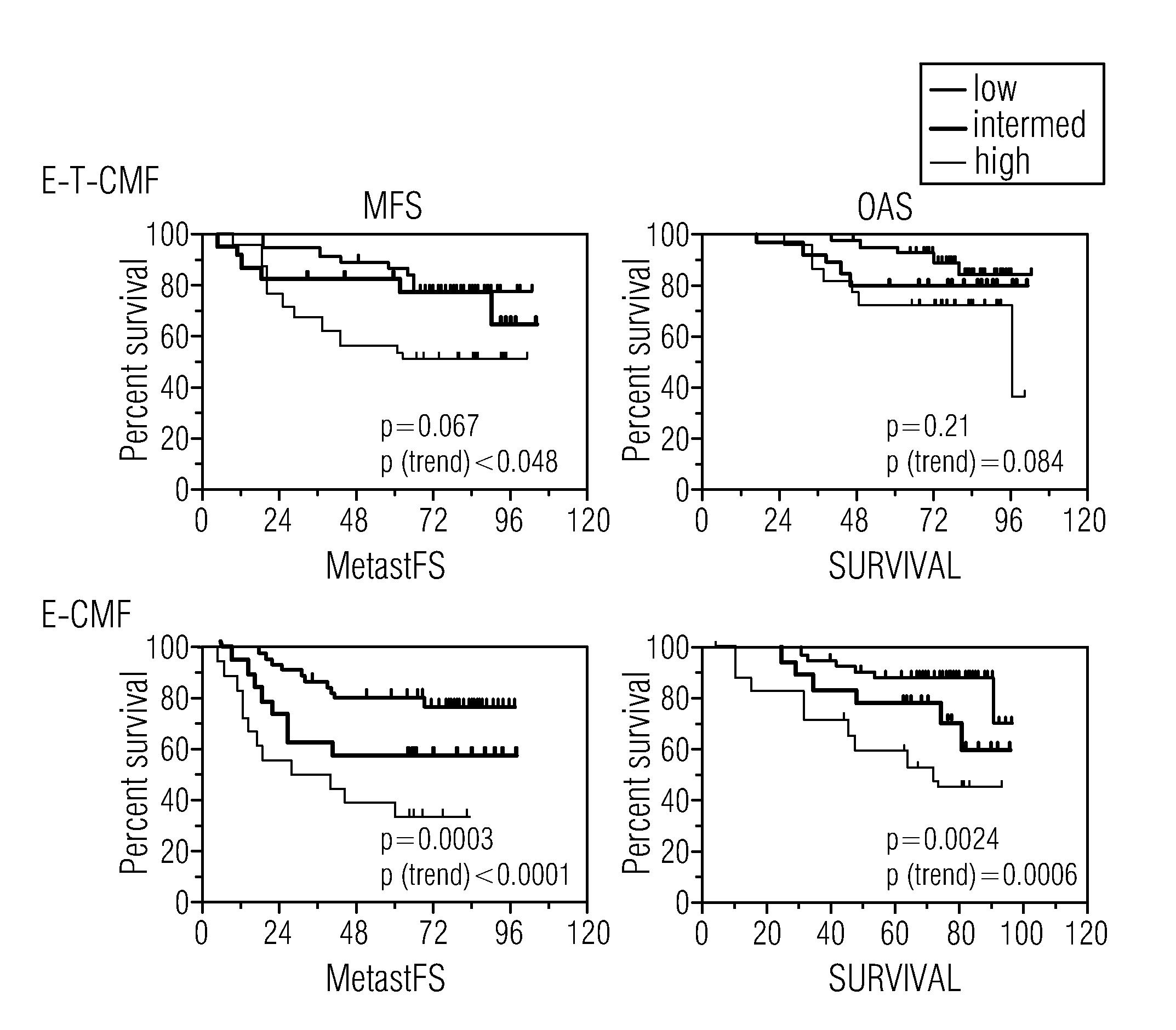
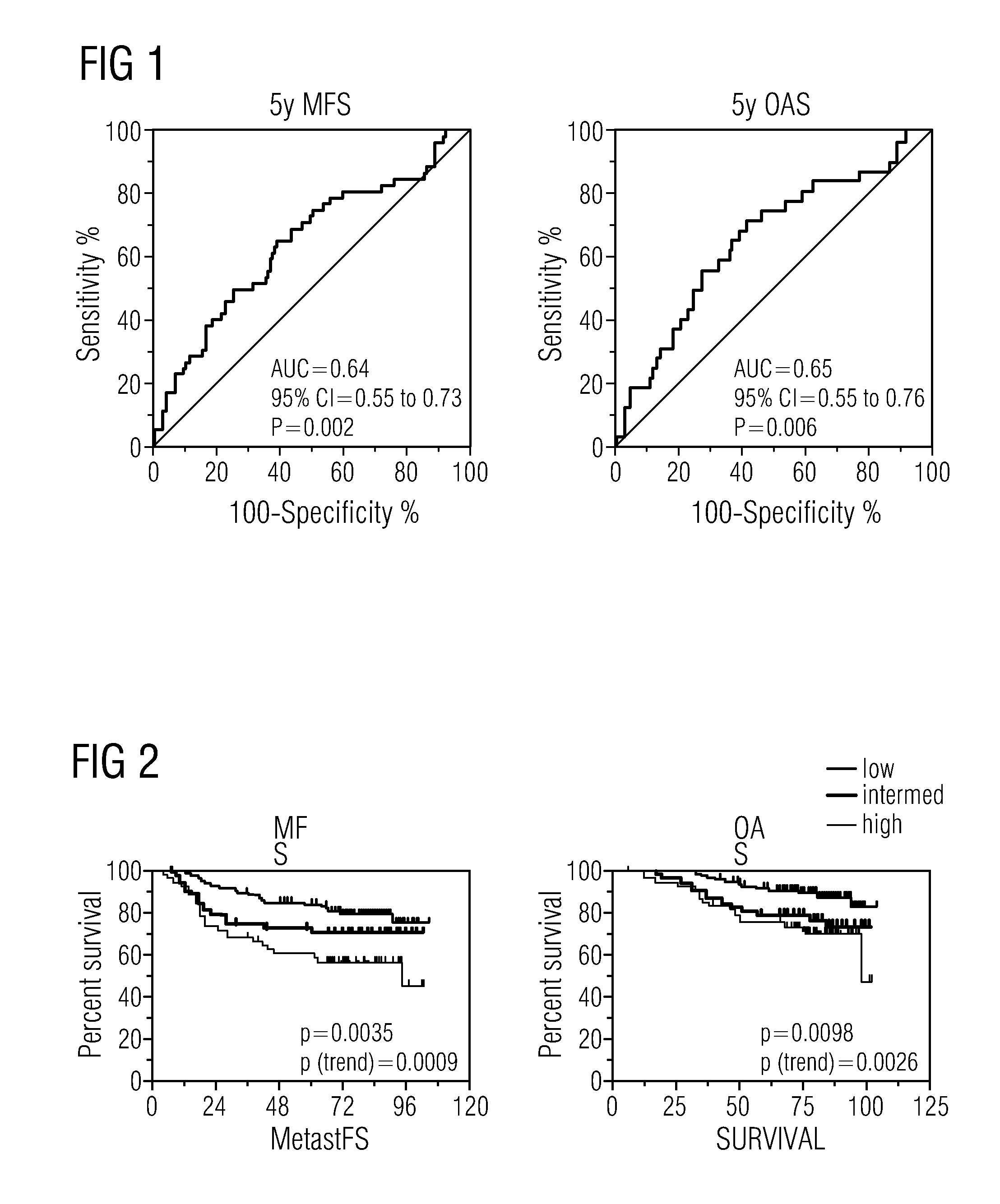
Algorithms for outcome prediction in patients with node-positive chemotherapy-treated breast cancer
InactiveUS20110166838A1Reduce chanceReduce deathMicrobiological testing/measurementBiostatisticsCentromere protein JSOX4
The invention relates to methods for predicting an outcome of cancer in a patient suffering from cancer, said patient having been previously diagnosed as node positive and treated with cytotoxic chemotherapy, said method comprising determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CAl2, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF; ABCB1, ABCG2, ADAM15, AKR1C1, AKR1C3, AKT1, BANF1, BCL2, BIRC5, BRMS1, CASP10, CCNE2, CENPJ, CHPT1, EGFR, CTTN, ERBB3, ERBB4, FBLN1, FIP1L1, FLT1, FLT4, FNTA, GATA3, GSTP1, Herstatin, IGF1R, IGHM, KDR, KIT, CKRT5, SLC39A6, MAPK3, MAPT, MKI67, MMP7, MTA1, FRAP1, MUC1, MYC, NCOA3, NFIB, OLFM1, TP53, PCNA, PI3K, PPERLD1, RAB31, RAD54B, RAF1, SCUBE2, STAU, TINF2, TMSL8, VGLL1, TRA@, TUBA1, TUBB, TUBB2A.
Owner:SIVIDON DIAGNOSTICS
Primer combination for detecting skin anti-aging capability genes, and detection method
InactiveCN106755390ASensitive highImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceLife habit
The invention discloses a primer combination for detecting skin anti-aging capability genes. The skin anti-aging capability genes comprises collagen multiplication capability gene COL1A1, a skin moisture retaining and keeping capability gene AQP3, a radiation protection capability gene ASIP, ultraviolet damage repair capability genes ERCC2 and LOC105374069, a skin detoxifying capability gene GSTP1, a skin sensitivity gene IL6R and an estrogen level gene DIAPH2, and corresponding PCR primers and single-base extension reaction primers are designed aiming at the eight genes. The invention further discloses a method for performing gene detection by utilizing the primer combination. The method comprises the following steps: carrying out sample DNA extraction, PCR amplification and single-base extension and nucleic acid mass spectrometric analysis to obtain corresponding genotypes, and then carrying out analysis and scoring, thereby scientifically evaluating the difference of individual skin aging processes, providing scientific and precise recommendations for daily skin care and life habits of individuals, and also being helpful to reasonable selection of skincare products of individuals according to skin types.
Owner:上海东方杰玛基因生物科技有限公司
Kit for genetic detection of breast cancer
InactiveCN101608225AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceEstrogen receptor
The invention discloses a kit for genetic detection of breast cancer. The kit comprises a specific primer pair and a specific probe pair which are used for detecting the mononucleotide polymorphism loci genotype of an estrogen receptor alpha gene (ER-alpha), a catechol-o-methyltransferas gene (COMT), a glutathione-s-transferase P1 gene (GSTP1), a human breast cancer susceptibility gene 1 (BRCA1), and a human breast cancer susceptibility gene 2(BRCA2), a fluorescent quantitative PCR general component and the like. The kit can evaluate the genetic risk of individuals suffered from the breast cancer by detecting the polymorphism loci genotype of the genes closely related to the genetic risk of the breast cancer at the same time.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Quantitative detection method and reagent kit of GSTP1 (Glutathione S-Transferase P1) methylation for predicting hepatic failure prognosis
InactiveCN103805699AGuaranteed specificityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceReference genesGenomic DNA
The invention provides a quantitative detection method and a reagent kit GSTP1 (Glutathione S-Transferase P1) methylation for predicting hepatic failure prognosis. The invention relates to quantitative detection for gene methylation, and in particular relates to a quantitative detection method and a reagent kit f GSTP1 (Glutathione S-Transferase P1) methylation for predicting hepatic failure prognosis. The reagent kit contains a reagent for extracting genomic DNA (Deoxyribonucleic Acid), a reagent for modifying the genomic DNA, a 5x premixing PCR (Polymerase Chain Reaction) system, a standard substance, positive and negative control, a methylation-specific primer pair aiming at a target gene GSTP1 promoter, a specific primer pair of a reference gene ALU-C4, and a corresponding Taqman fluorescent probe. By extracting peripheral blood genomic DNA and carrying out real-time fluorescence quantitative PCR, a quantitative value of the gene GSTP1 methylation is obtained through calculation. The reagent kit provided by the invention can help doctors to determine the pathogenetic condition and prognosis of hepatic failure patients so as to make effective treatment plans.
Owner:SHANDONG UNIV QILU HOSPITAL
Detection of GSTP1 hypermethylation in prostate cancer
InactiveUS20090186360A1Enhance clinical sensitivity sensitivityHigh analytical sensitivityMicrobiological testing/measurementProstate cancerCancer research
An assay for detecting prostate cancer includes reagents for detecting multiple methylation markers from within one gene such as GSTP1.
Owner:VERIDEX LCC
Prognostic and treatment response predictive method
PendingUS20200239968A1Avoiding unwanted side effectAggressive treatmentMicrobiological testing/measurementDisease diagnosisDPYDPredictive methods
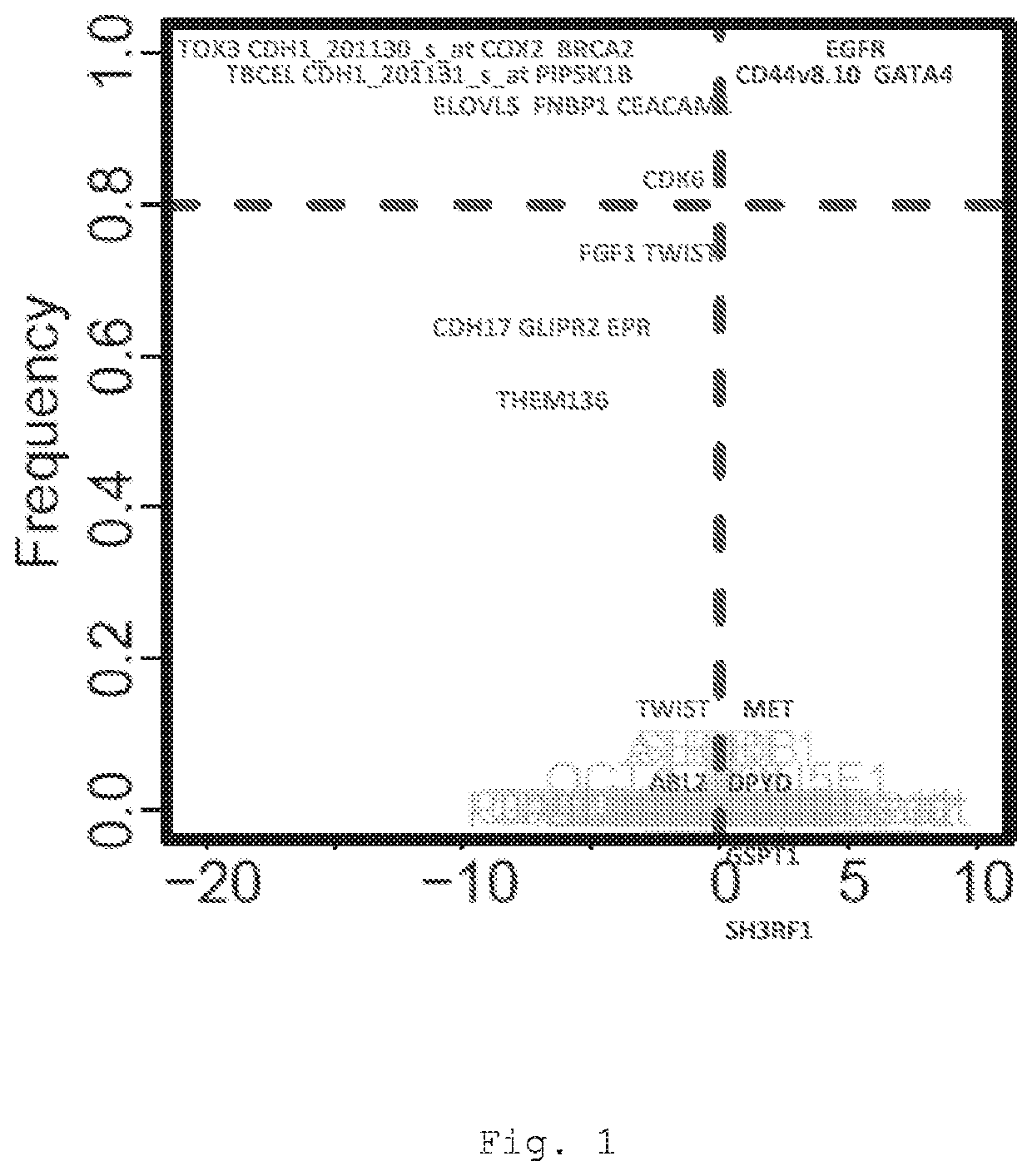
The present invention provides a method for predicting the treatment response of a human gastroesophageal cancer patient, the method comprising: a) measuring the gene expression of at least 3 of the following genes: CDH1, CDK6, COX2, ELOVL5, GATA4, EGFR, TBCEL, FGF7, CDH17, FNBP1, PIP5K1B, TWIST, CD44, MET, CEACAM1, TOX3, GLIPR2, GSTP1, RON, TMEM136, MYB, BRCA2, FGF1, POU5F1, EPR, DPYD, ABL2 and SH3RF1 in a sample obtained from the gastroesophageal tumour of the patient to obtain a sample gene expression profile of at least said genes; and b) making a prediction of the treatment response and / or prognosis of the patient based on the sample gene expression profile. Also provided are related computer-implemented methods and methods of treatment of gastroesophageal cancer.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +2
Gene combination used for guiding individual treatment of platinum medicines
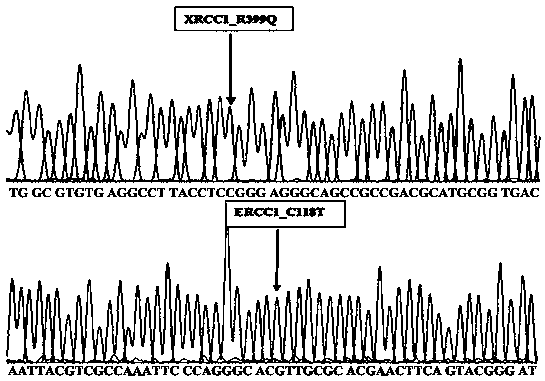
The invention belongs to the gene technical field, and provides a gene combination used for guiding individual treatment of platinum medicines. The gene combination comprises GSTP1, MRP2, XRCC1-Exon6, XRCC1-Exon10, ERCC1 and ERCC2. By detecting the mutation condition of gene sites of GSTP1, MRP2, XRCC1-Exon6, XRCC1-Exon10, ERCC1 and ERCC2 in tumor tissues of patients, the gene combination carries out a medicine sensitive guidance to the patients treated by platinum medicines for maximizing the curative effect and minimizing the side-effect.
Owner:SHANGHAI BIOTECAN PHARMA
Use of GSTP1
InactiveCN102573892APeptide/protein ingredientsMicrobiological testing/measurementHeart diseaseIschemic Heart Diseases
The invention discloses the use of glutathione S-transferase P1 (GSTP1) for the prevention or treatment of cardiomyopathies or ischemic heart diseases and for the diagnosis thereof.
Owner:MEDIZINISCHE UNIVET WIEN
Primer group, reagent and/or kit and system for detecting lung cancer chemotherapy related genes, and application
ActiveCN109517900AClustering is clearImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationCYP2C8Biology
The invention relates to a primer group, reagent and / or kit and system for detecting lung cancer chemotherapy related genes, and application. The primer group contains primers for detecting gene mutation of ERCC1, MTHFR, GSTP1, XRCC1, DYNC2H1, ABCB1, CYP2C8*3, TP53, NQO1, CBR3, SOD2, CYP2C19, UGT1A1*6, TYMS, NT5C2 and CDA. The primer group, the reagent and / or the kit, the system and the application have the advantages of high accuracy, high specificity, high sensitivity, good precision and the like, and meanwhile, further have the advantages of sample saving, short detection period, easy operation, convenient analysis and the like.
Owner:SIMCERE DIAGNOSTICS CO LTD +2
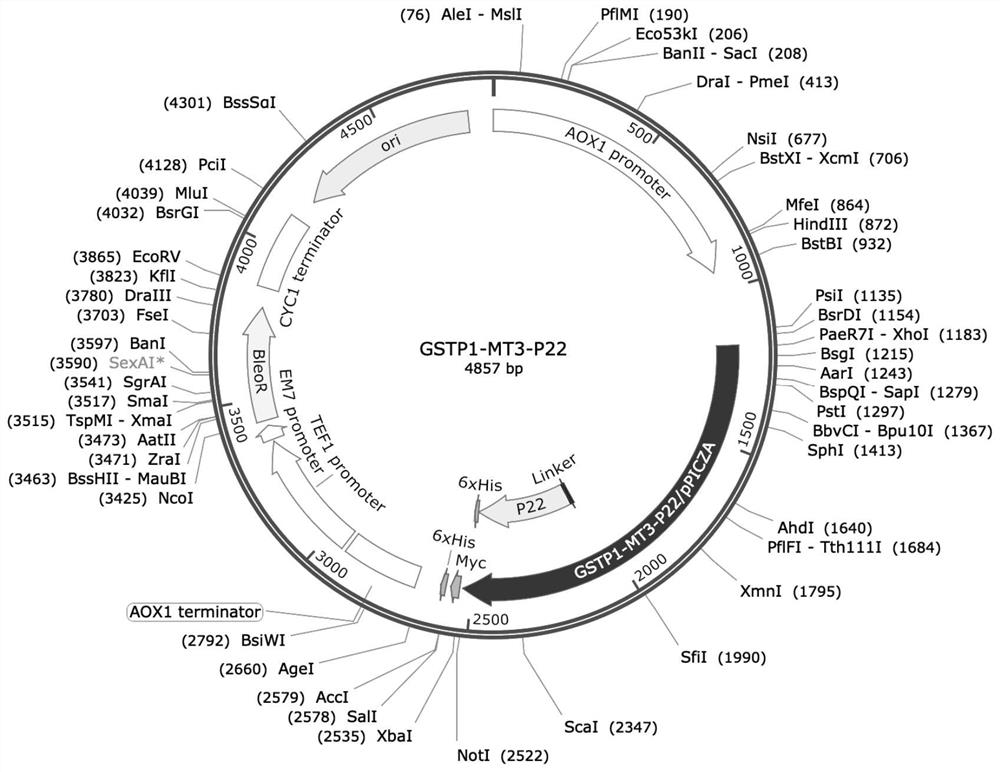
African swine fever virus P22 protein nanoparticle and preparation method and application thereof
ActiveCN112574318ASignificant progressStrong immune responsePolypeptide with localisation/targeting motifViral antigen ingredientsClassical swine fever virus CSFVImmunogenicity
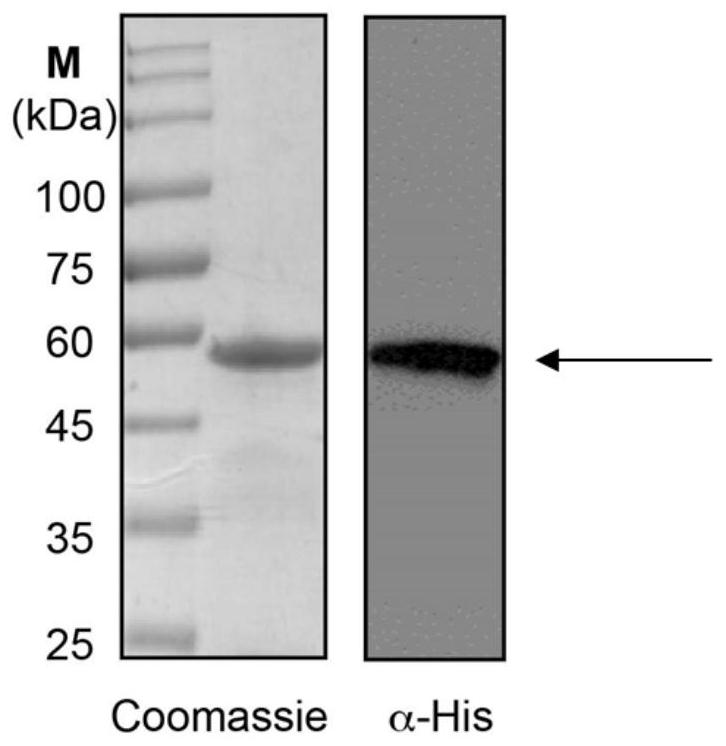
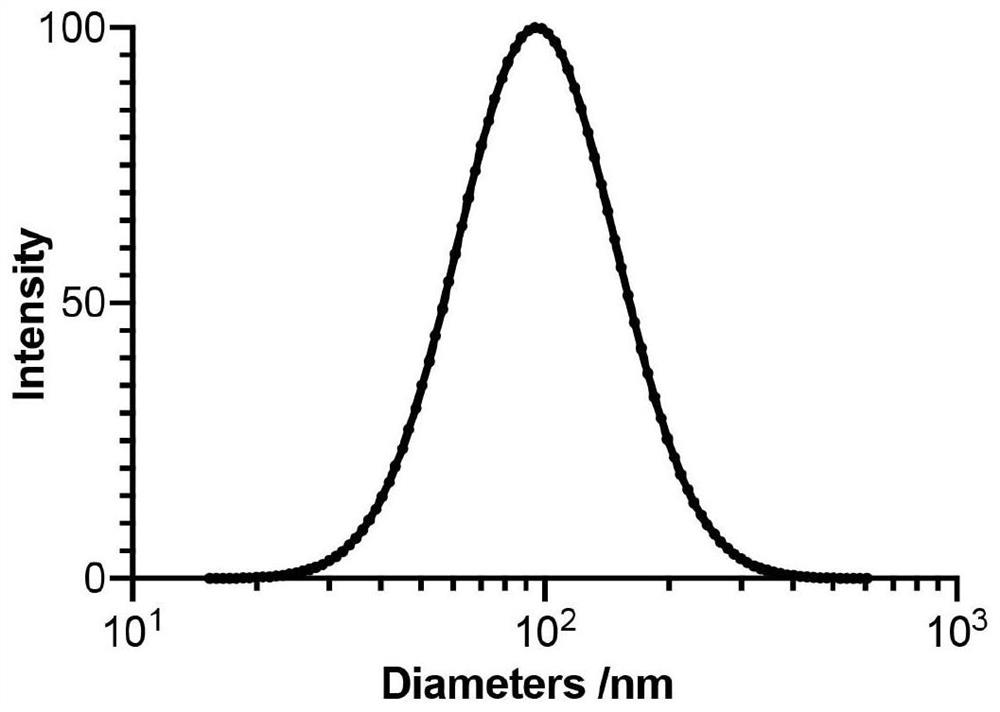
The invention relates to an African swine fever virus P22 protein nanoparticle and a preparation method and application thereof, the protein nanoparticle is formed by self-assembly of a protein monomer, and the protein monomer is a fusion protein sequentially containing metallothionein, glutathione thiotransferase and P22 protein from amino acid to a carboxyl terminal. The GSTP1-MT3 protein and the P22 antigen of the African swine fever virus are subjected to fusion expression, and in a pichia pastoris expression system, protein nanoparticles can be spontaneously formed through induction of ferrous ions. Immunogenicity determination is carried out in a mouse body, and the P22 protein nanoparticle can cause very strong immune response and has great potential to be developed into a new safeand effective African swine fever virus vaccine.
Owner:非零和(北京)投资管理有限公司
Detection and prognosis of lung cancer
Methods and tools are provided for detecting and predicting lung cancer. The methods and tools are based on epigenetic modification due to methylation of genes in lung cancer or pre-lung cancer. The tools can be assembled into kits or can be used seperately. Genes found to be epigentically silenced in association with lung cancer include ACSL6, ALS2CL, APC2, ART-S1, BEX1, BMP7, BNIP3, CBR3, CD248, CD44, CHD5, DLK1, DPYSL4, DSC2, EDNRB, EPB41L3, EPHB6, ERBB3, FBLN2, FBN2, FOXL2, GNAS, GSTP1, HS3ST2, HPN, IGFBP7, IRF7, JAM3, LOX, LY6D, LY6K, MACF1, MCAM, NCBP1, NEFH, NID2, PCDHB15, PCDHGA12, PFKP, PGRMC1, PHACTR3, PHKA2, POMC, PRKCA, PSEN1, RASSF1A, RASSF2, RBP1, RRAD, SFRP1, SGK, SOD3, SOX17, SULF2, TIMP3, TJP2, TRPV2, UCHL1, WDR69, ZFP42, ZNF442, and ZNF655.
Owner:MDXHEALTH +1
Kit for predicting prostate screening and lymphatic metastasis
InactiveCN107988365AReliable test resultsHigh clinical positive predictive valueMicrobiological testing/measurementDNA preparationFluorescenceProstate cancer
The invention relates to the molecular diagnosis field and relates to a molecular diagnosis method and reagent for non-invasive detection of prostate cancer and lymphatic metastasis of prostate cancerby virtue of urine. The method comprises the following steps: extracting and purifying urine nucleic acid; (2) carrying out DNA hydrosulphite conversion reaction and subsequent purification; (3) detecting a plurality of gene promoter regions by virtue of methylation-specific real-time fluorescence PCR; and (4) analyzing multiple gene results, wherein the steps (1) and (2) can be combined into onestep, and multiple genes contain the promoter regions of the following genes: CRMP4, PITX2, RASSF1A, SOSTDC, CYBA, EFEMP1, KNM5D, GSTP1, WFDC2, TACSTD2 and the like. By combining the detection results of the genes, the diagnosis of prostate cancer and the lymphatic metastasis of prostate cancer can be realized.
Owner:上海纽思格生物科技有限公司
Primer for simultaneously detecting XRCC1, ERCC, GSTP1 and GSTM1 gene polymorphism, and method thereof
InactiveCN105039528AStrong specificityImprove accuracyMicrobiological testing/measurementSpecific detectionMedicine
The invention provides a primer for simultaneously detecting the XRCC1, ERCC, GSTP1 and GSTM1 gene polymorphism. The primer comprises a PCR amplification primer and an SNaPshot PCR primer, and belongs to the technical field of biological detection. The primer provided by the invention can realize specific detection of the XRCC1, ERCC, GSTP1 and GSTM1 gene polymorphism, has no cross reaction and has good accuracy.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT +1
Methods and kits for diagnosing ulcerative colitis in a subject
The present invention relates to methods and kits for diagnosing ulcerative colitis in a subject. In particular, the present invention relates to a method for diagnosing ulcerative colitis in a subject comprising the steps consisting of determining in a sample obtained from the subject the expression level of at least one gene selected from the group consisting of ADH4, ADH6, ADHFE1, AKR1A1, AKR7A2, ALDH1A3, ALDH1L1, ALDH7A1, AOX1, BCHE, CBR3, CES1, CYP1B1, CYP2E1, CYP2W1, CYP4F11, CYP51A1, ESD, KCNAB2, COMT, GSTA4, GSTP1, INMT, MGST2, SULT2A1, TPMT, UGT1A4, UGT1A9, UGT2B7, ABCA1, ABCA2, ABCB1, ABCC1, ABCC10, ABCC5, ABCC6, ABCG2, ATP7A, SLC1A3, SLC7A5, SLC10A2, SLC15A1, SLC15A2, SLC19A2, SLC19A3, SLC22A3, SLC28A3, SLC29A2, SLC38A1, SLC38A5, SLC47A1, SLCO2B1, SLCO4C1, ARNT, FOXO1, HIF3A, NCOA2, NCOR2, NR1H3, NR3C1, PPARD, PPARGC1A, RARB, RXRB, and THRB.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Reagent kit for detecting lawful age females synthetic disease genetic susceptibility
InactiveCN101275911AMicrobiological testing/measurementChemiluminescene/bioluminescenceDiseaseFluorescence
The present invention discloses a reagent kit which detects the genetic susceptibility of the synthetic disease of adult woman. The reagent kit comprises a specific primer pair and a specific fluorescent probe pairs which simultaneously detects the SNP polymorphic genotype on the genes of CYBA, CAT, LPL, LEP, ADIPOQ, SOD3, IL3, NOS3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13, CBS, a routine component which is used for the fluorescent quantitative PCR testing and the like. The reagent kit of the invention evaluates the genetic susceptibility of the synthetic disease of adult woman through simultaneously detecting the mononucleotide polymorphism site genotype which is closely linked to the genetic susceptibility of the synthetic disease of adult woman.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
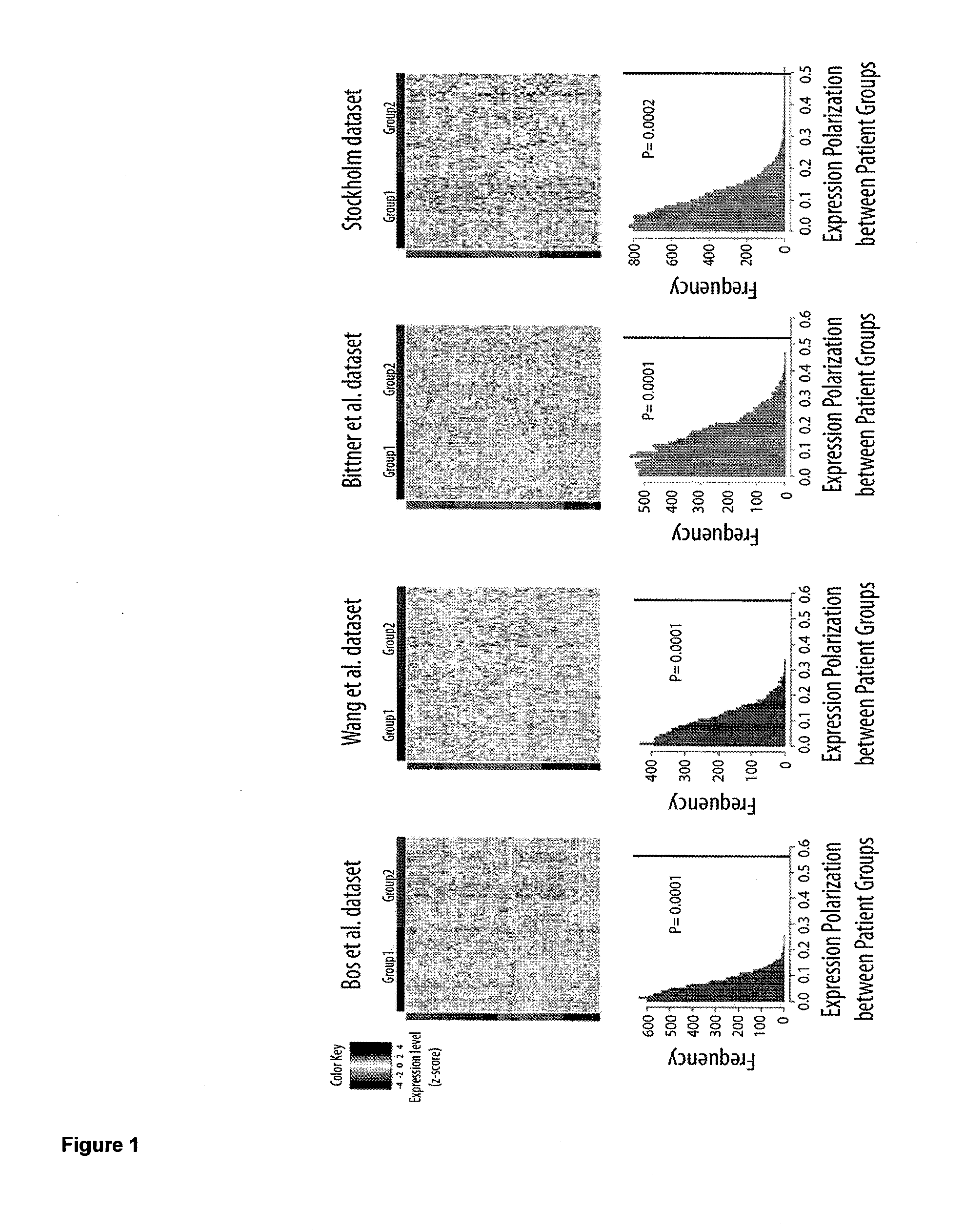
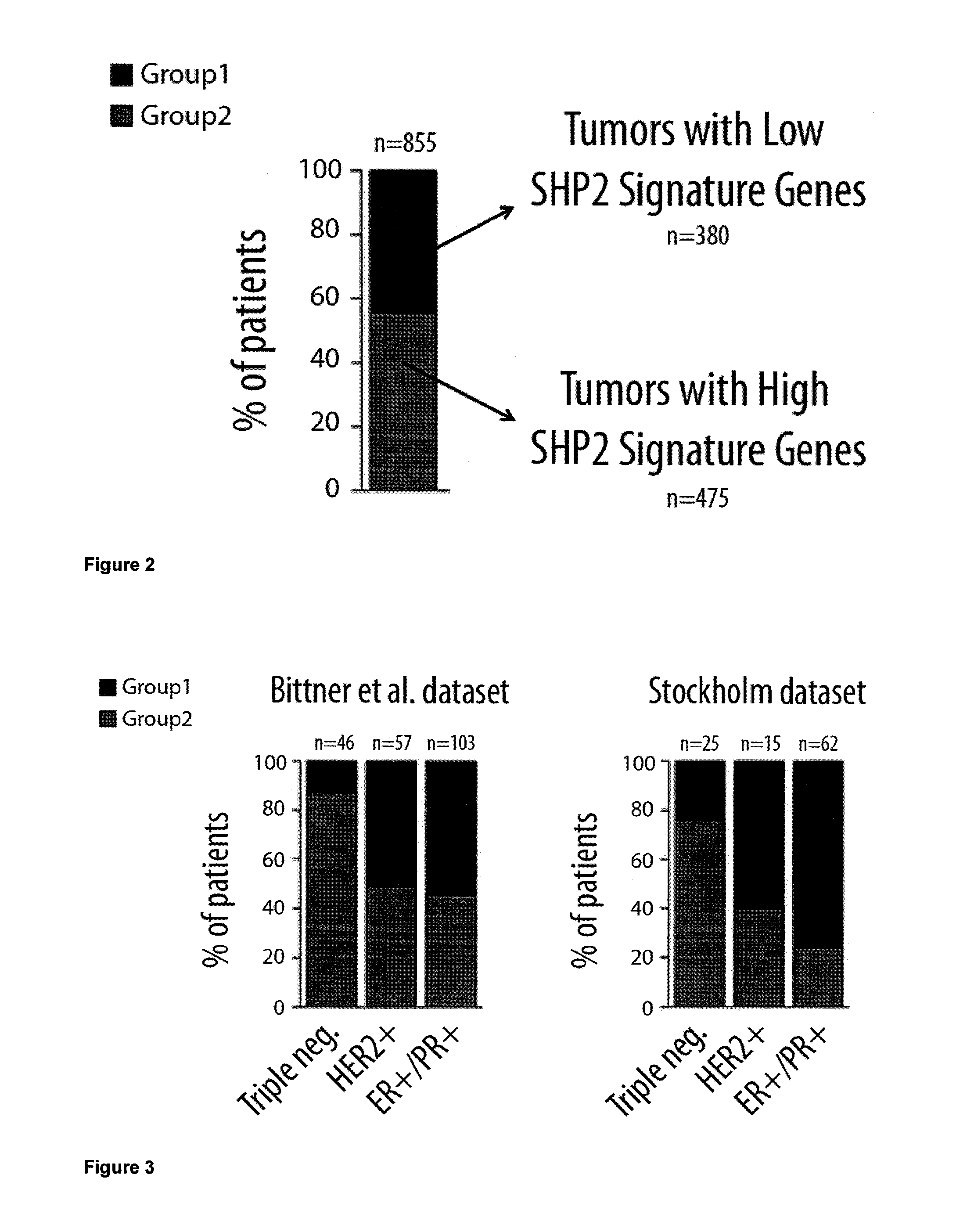
Protein tyrosine phosphatase, non-receptor type 11 (ptpn11) and triple-negative breast cancer
The present invention relates to a method for treating breast cancer in a subject having a breast cancer of the triple-negative type, which method comprises the step of administering to said subject a therapeutically effective amount of a modulator of the protein tyrosine phosphatase, non-receptor type 11 (PTPN11) gene or of its gene product (Shp2). The present invention also relates to a method for treating breast cancer in a subject having a breast cancer over-expressing the “SHP2 signature” genes, as compared to normal breast tissue samples, which method comprises the step of administering to said subject a therapeutically effective amount of a modulator of the protein tyrosine phosphatase, non-receptor type 11 (PTPN11) gene or of its gene product (Shp2), wherein said “SHP2 signature” genes consist of the genes SGCB, ZSCAN12, ID4, ZIC3, CPVL, HLA-A, MCOLN3, SPATA18, TMEM45A, GNAL, CYBRD1, TSPAN7, ZEB1, CNTLN, NEFL, CENPV, ARL6, HPRT1, LRRC34, PDPN, BEND7, SLC16A10, FAM27E1, PLEKHA1, HERC5, CHIC1, PHF6, ELOVL4, ANTXR1, PRAME, SCML1, CLIP4, CECR2, CNOT10, IGF2BP3, NAP1L3, GPC3, KIAA1804, DGKE, FAS, EPHA6, KDELC1, CRISPLD1, DOCK3, ACSL4, CNTNAP3, PLEKHM3, RDX, TBX18, RRAGD, HOXB5, SNCA, FUNDC2, ITGA8, HFM1, IGF2BP2, CCND2, SGTB, MKX, CRYBG3, WBP5, LPHN3, BEX4, CPNE8, GLDC, SLC35F1, HOXA13, SERPINF1, NEFM, SYCP2L, FHL1, APOBEC3C, CALD1, FKBP10, HOXD11, DENND2C, LRRC49, FAM55C, KIAA0408, HOXB9, C160RF62, ACN9, TUSC3, ELOVL2, SPOCK3, HOXB6, WDR35, MPP1, FBX038, PRKAA2, SLAIN1, NPHP3, KIAA1524, PRPS1, GJC1, AMOT, SLC9A6, KCTD12, NUP62CL, DZIP3, JAM3, HOXA9, ANKRD19, CDKN2A, BCAT1, OAT, LPHN2, CCDC82, HSD17B11, SAMHD1, WDR17, STK33, GSTP1, TRPC1, CKB, LIN28B, ALDH1L2, SACS, CLGN, MY03A, EPB41L3, SLC25A27, VCAN, GPX8, GALNT13, PVRL3, MOXD1, HEY1, MAP7D3, ESD, MPP6, EYA4, SPG20, ZDBF2, ZNF204, IFT57, AKR1B1, ADAT2, ZNF717, CCDC88A, ZNF215, MIDI, FBN2, LOC100130876, TCEAL8, IGF2BP1, ANKRD18B, PLAGL1, PM20D2, LDHB, C150RF51, PTPN11, EPB41L2, TLE4, GOLM1, C60RF192, HOXD13, SLIT2, UCHL1, DYNC2H1, CPS1, GPR180, PYGL, NRN1, PRTFDC1, SLC16A1, DSC3, TMC01, LRCH2, SLC6A15, DZIP1, HOXA5, HSPA4L, CDR1, PLS3, ECHDC1, SMARCA1, CXORF57, HOXD10, and IRS4.
Owner:NOVARTIS FORSCHUNGSSTIFTUNG ZWEIGNIEDERLASSUNG FRIEDRICH MIESCHER INSTITTUE FOR BIOMEDICAL RES
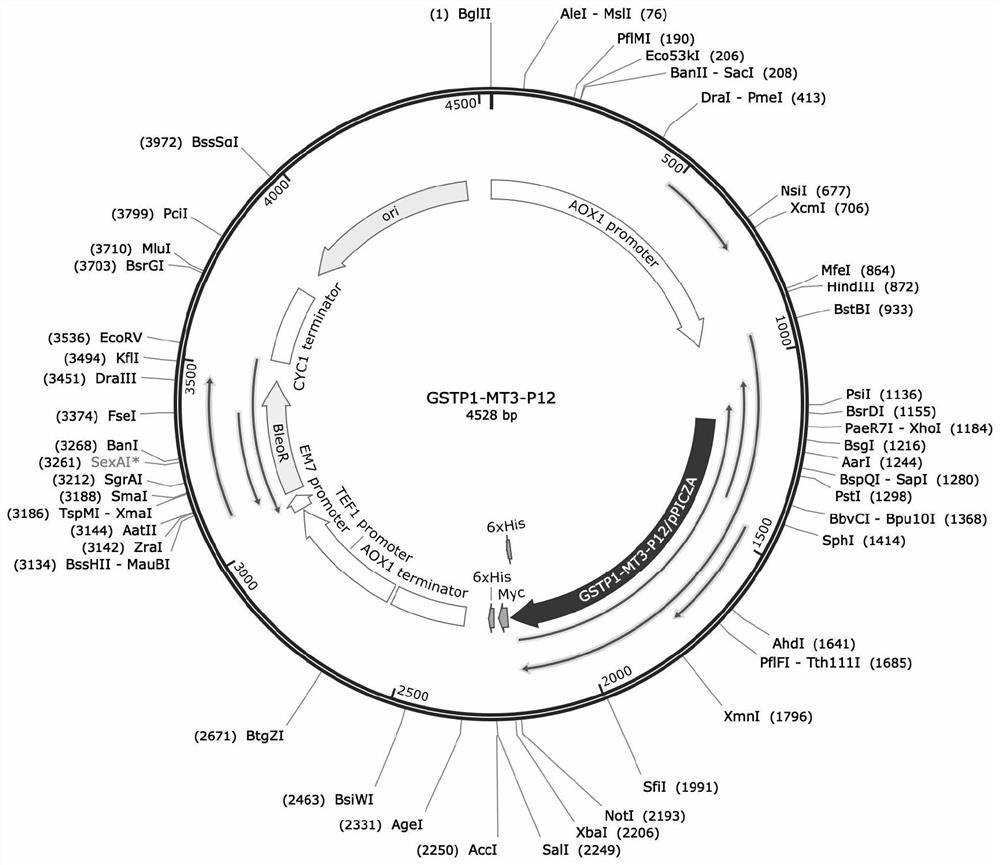
African swine fever virus P12 protein nanoparticle and preparation method and application thereof
ActiveCN112574319ASignificant progressStrong immune responsePowder deliveryAntibody mimetics/scaffoldsClassical swine fever virus CSFVImmunogenicity
The invention relates to an African swine fever virus P12 protein nanoparticle and a preparation method and application thereof, the protein nanoparticle is formed by self-assembly of a protein monomer, and the protein monomer is a fusion protein sequentially containing metallothionein, glutathione thiotransferase and P12 protein from amino acid to a carboxyl terminal. The GSTP1-MT3 protein and the P12 antigen of the African swine fever virus are subjected to fusion expression, and in a pichia pastoris expression system, the protein nanoparticle can be spontaneously formed through induction offerrous ions. Immunogenicity determination is carried out in the mouse body, and the P12 protein nanoparticle can cause very strong immune response and has great potential to be developed into a newsafe and effective African swine fever virus vaccine.
Owner:非零和(北京)投资管理有限公司
Reagent kit for detecting cigarette and wine damnification genetic susceptibility
InactiveCN101354342AMicrobiological testing/measurementMaterial analysis by optical meansFluorescenceXRCC1 Gene
The invention discloses a kit that is used for detecting the genetic predisposition of wine and tobacco damages. The kit comprises particularity primer pairs and particularity fluorescent probe pairs that are used for simultaneously detecting 16 SNP polymorphism genotypes on the genes of ADH2, ALDH2, CYP1A1, CYP2A13, CYP2E1, ENOS, ERCC2, GSTM1, GSTP1, GSTT1, MTHFR, NQO1 and XRCC1 and a routine component that is used for fluorescent quantitative PCR detection, etc. The kit of the invention evaluates the genetic predisposition of wine and tobacco damages by simultaneously detecting the 16 mononucleotide polymorphism locus genotypes that are closely related with the genetic predisposition of wine and tobacco damages.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Reagent kit for detecting man disease genetic susceptibility
The present invention provides a kit for detecting genetic predisposition of adult male syndrome. The kit includes specific primer pairs and specific fluorescent probe pairs for detecting 48 SNP polymorphism genotypes on the genes LPL, TNF-alpha, LEP, ADIPOQ, SOD3, IL3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13 at the same time, and normal components for FQ-PCR detection. The kit of the invention evaluates the genetic predisposition of adult male syndrome by detecting the genotypes of the 48 SNP polymorphism sites at the same time which is tightly relative to the genetic predisposition of adult male syndrome.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Drug paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphism detection kit and drug paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphism detection method
InactiveCN107841557AEfficient detectionSimple methodMicrobiological testing/measurementTreatment effectQuality control
The invention provides a drug paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphism detection kit and a drug paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphismdetection method, wherein the kit comprises a PCR buffer solution, specific ARMS detection primers and quality control primers. The present invention further provides the paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphism detection method. According to the present invention, with the method, the paclitaxel treatment effect-related gene (CYP2C8 and GSTP1) polymorphism can berapidly and accurately detected; and the kit has characteristics of high sensitivity, strong specificity, simple method, accurate result and the like.
Owner:宁波美丽人生医药生物科技发展有限公司
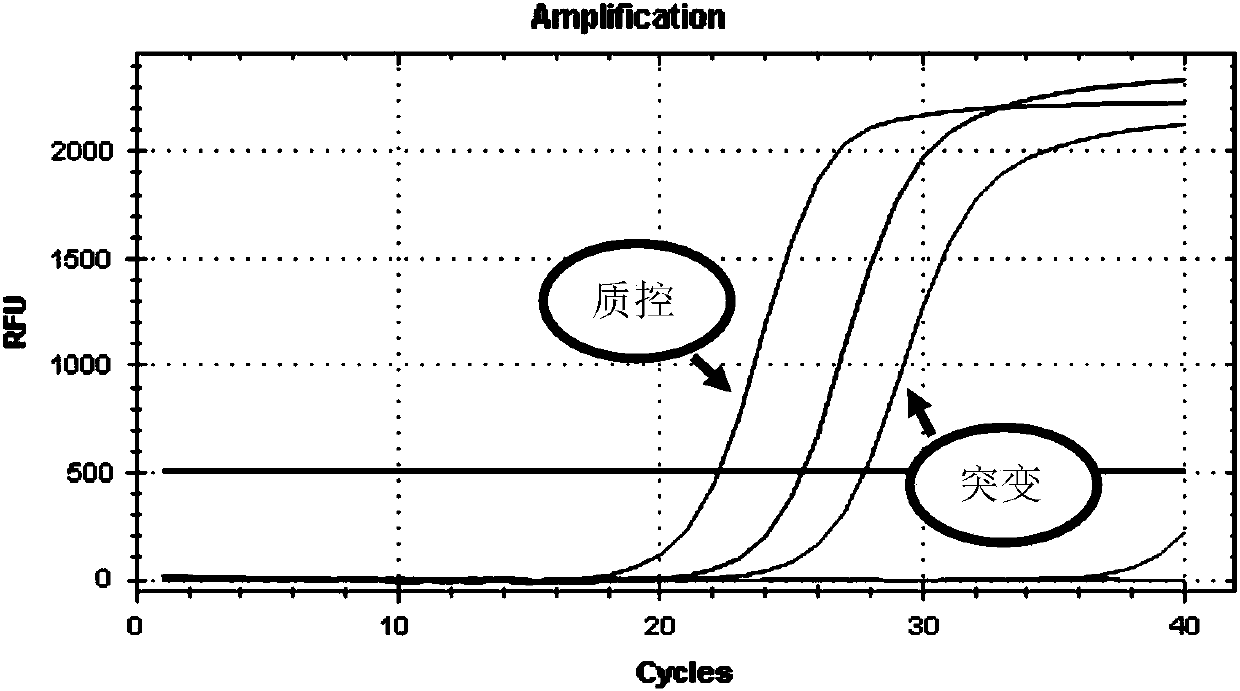
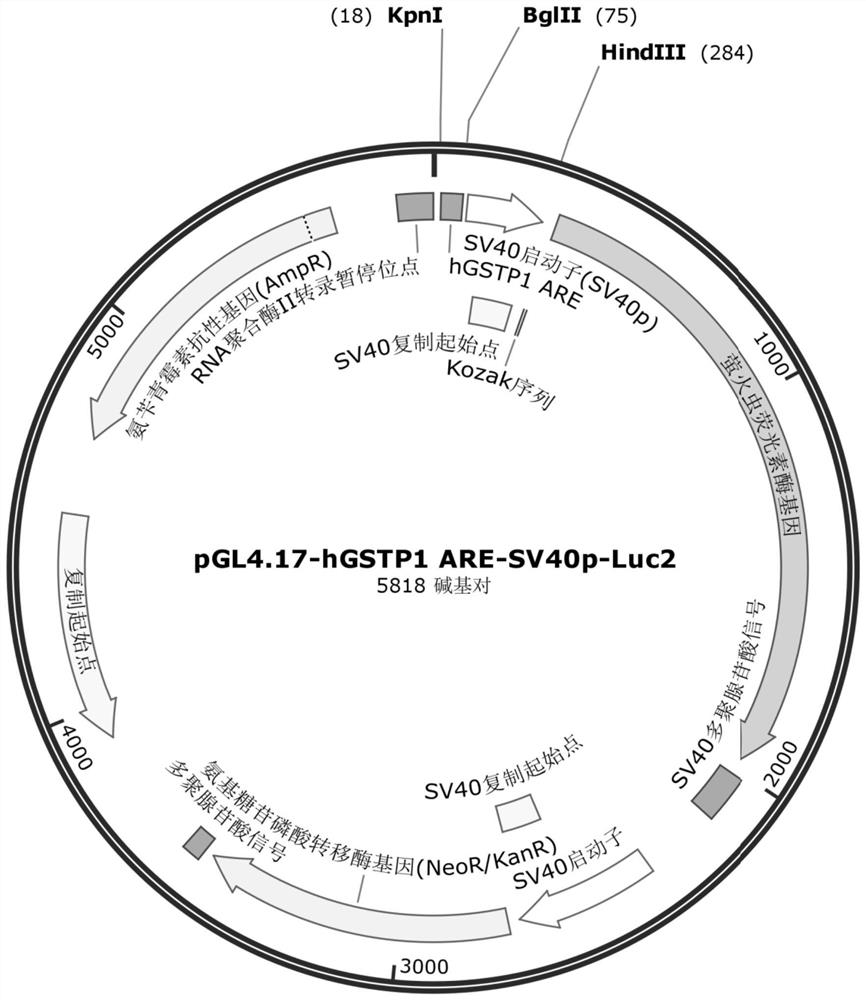
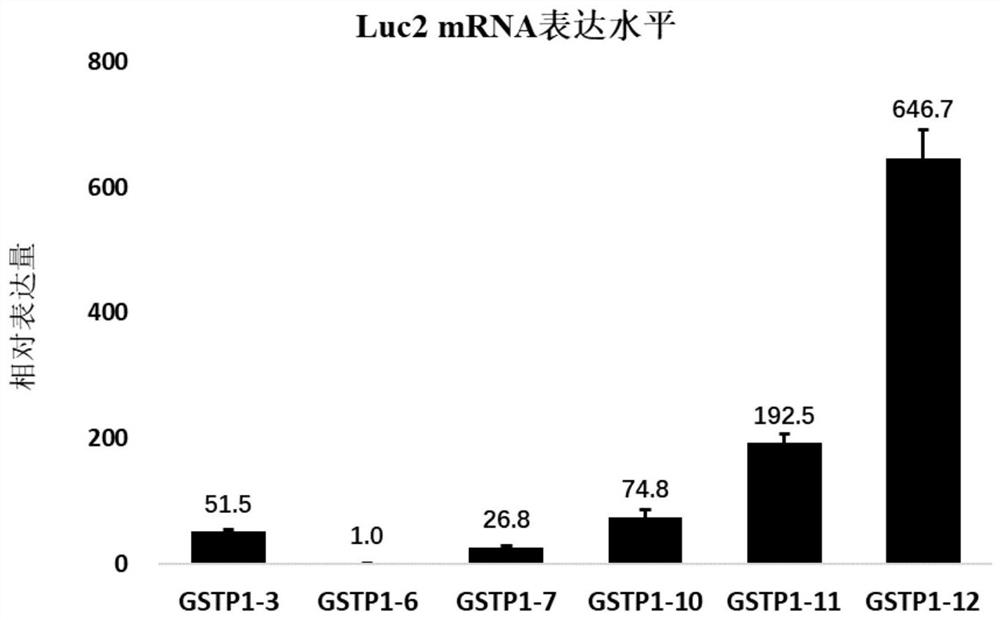
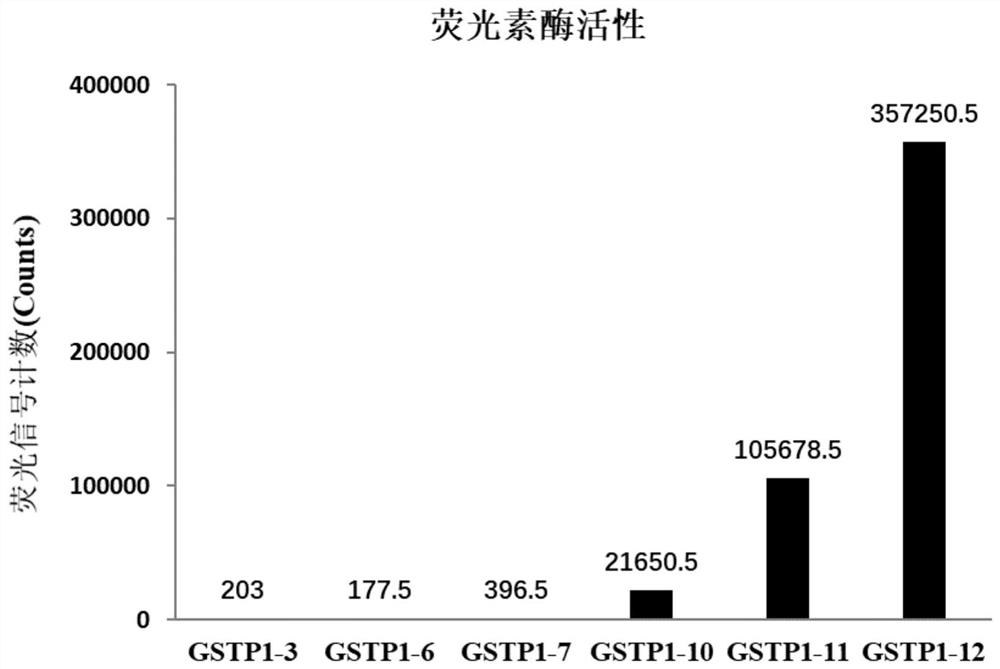
Construction of cell strain containing luciferase reporter gene of human GSTP1 ARE and application thereof
ActiveCN113265423AMicrobiological testing/measurementGenetically modified cellsSkin sensitizationNucleotide
The invention provides an overexpression vector of a luciferase reporter gene containing human GSTP1ARE. The overexpression vector comprises a nucleotide sequence as shown in SEQ ID NO: 2. The invention also provides a cell strain capable of stably expressing the luciferase reporter gene containing the human GSTP1ARE. The invention further relates to a method for evaluating the skin sensitization risk of a reagent or a product and an application of the cell strain stably expressing the luciferase reporter gene containing the human GSTP1ARE in evaluating the skin sensitization risk of the reagent or the product.
Owner:SHANGHAI JAHWA UNITED
Method and kit for identifying state of prostate cancer
PendingCN113234820AMicrobiological testing/measurementDNA/RNA fragmentationProstate cancerBiologic marker
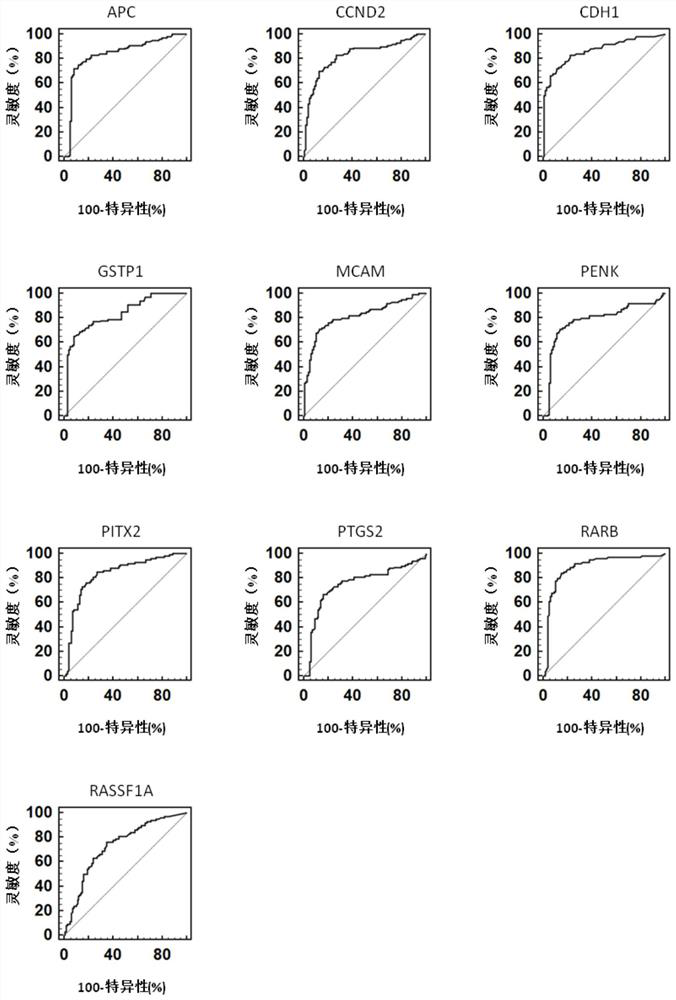
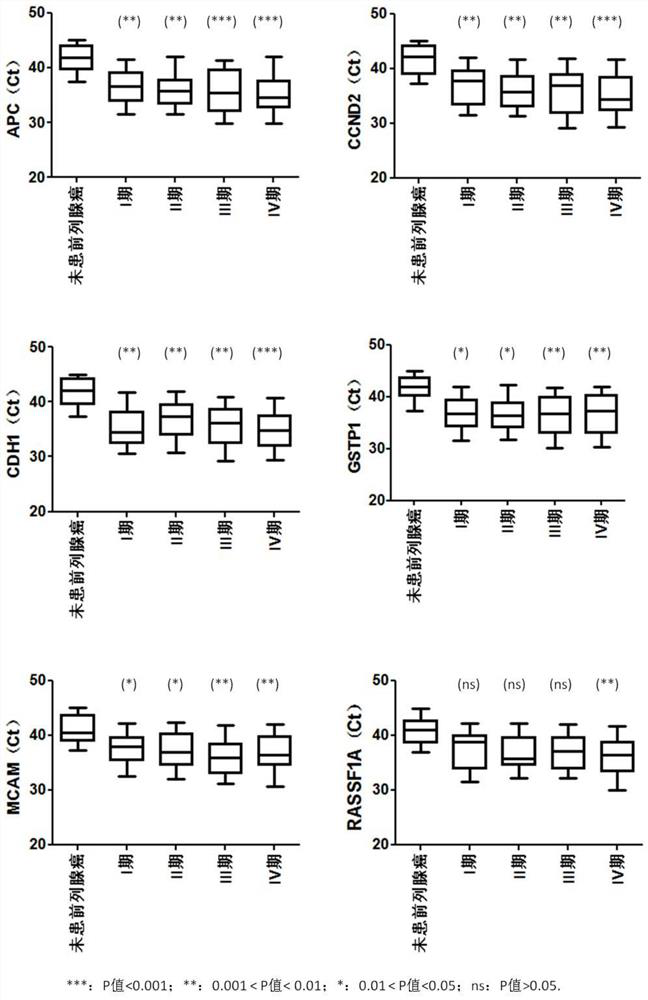
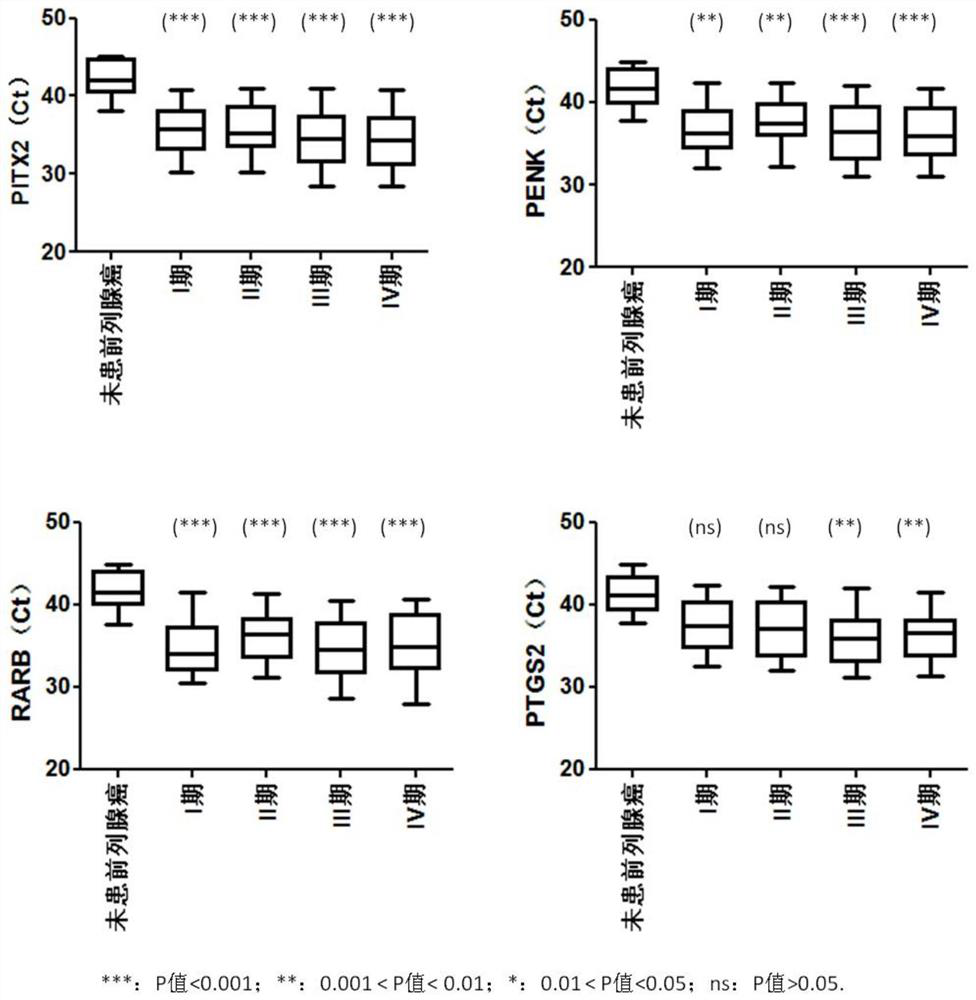
Provided herein is a method of identifying the state of prostate cancer in a subject, and the method comprises the steps: 1) detecting the methylation level of a biomarker gene in a biological sample from the subject wherein the biomarker gene is selected from one or more of the following genes: APC, CCND2, CDH1, GSTP1, MCAM, PENK, PITX2, PTGS2, RARB, and RASSF1A; 2) comparing the methylation level detected in the step 1) with the normal methylation level of the corresponding biomarker gene in the population to determine the state of prostate cancer in the subject. Also provided herein are kits for identifying the state of prostate cancer in a subject. The method and the kit provided by the invention provide a rapid, reliable and accurate new way for prediction, diagnosis and evaluation of prostate cancer.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Solid phase polymerase chain reaction (PCR) kit for chemotherapeutic medication guide gene detection
InactiveCN107663537ASimple and fast operationEasy to operateMicrobiological testing/measurementSanger sequencingBiology
The invention discloses a solid phase polymerase chain reaction (PCR) kit for chemotherapeutic medication guide gene detection, and a detection method, belonging to the technical field of PCR application. The invention also relates to a primer group for detecting ten chemotherapeutic medication guide; the primer group consists of GSTP1 SNP-I105V A313G, MTHFR SNP-C677T, MDR1 G2677AT, XRCC1 SNP-R399Q, CDA G208A, MDR1 SNP-C3435T, CDA A79C, CYP1B1C SNP-4326C / G, ERCC1 SNP-C118T, DPYD IVS14_1G / A; the solid phase PCR amplification kit can be used for detecting the ten gene sites at a time; computer amplification can be realized only by adding a corresponding nucleic acid template into the kit again; the PCR product, obtained after amplification is completed, is subjected to Sanger sequencing; chemotherapeutic medication guide can be realized according to the result of the Sanger sequencing. Compared with the prior art, the solid phase PCR kit adopts a specific primer sequence, so that the reliability of the detection results is ensured; the detection method is simple to operate, time-saving and labor-saving; the solid phase PCR kit is high in detection throughput and low in reagent consumable cost, can be directly used for detecting extracted nucleic acid, is low in requirements for a detection platform and detection personnel, and can be widely popularized in the clinical front line.
Owner:安徽安龙基因科技有限公司
Method for identifying breast cancer status and kit
PendingUS20210324479A1Fast and reliable and accurateFast and reliable and accurate newMicrobiological testing/measurementBiologic markerCancer research
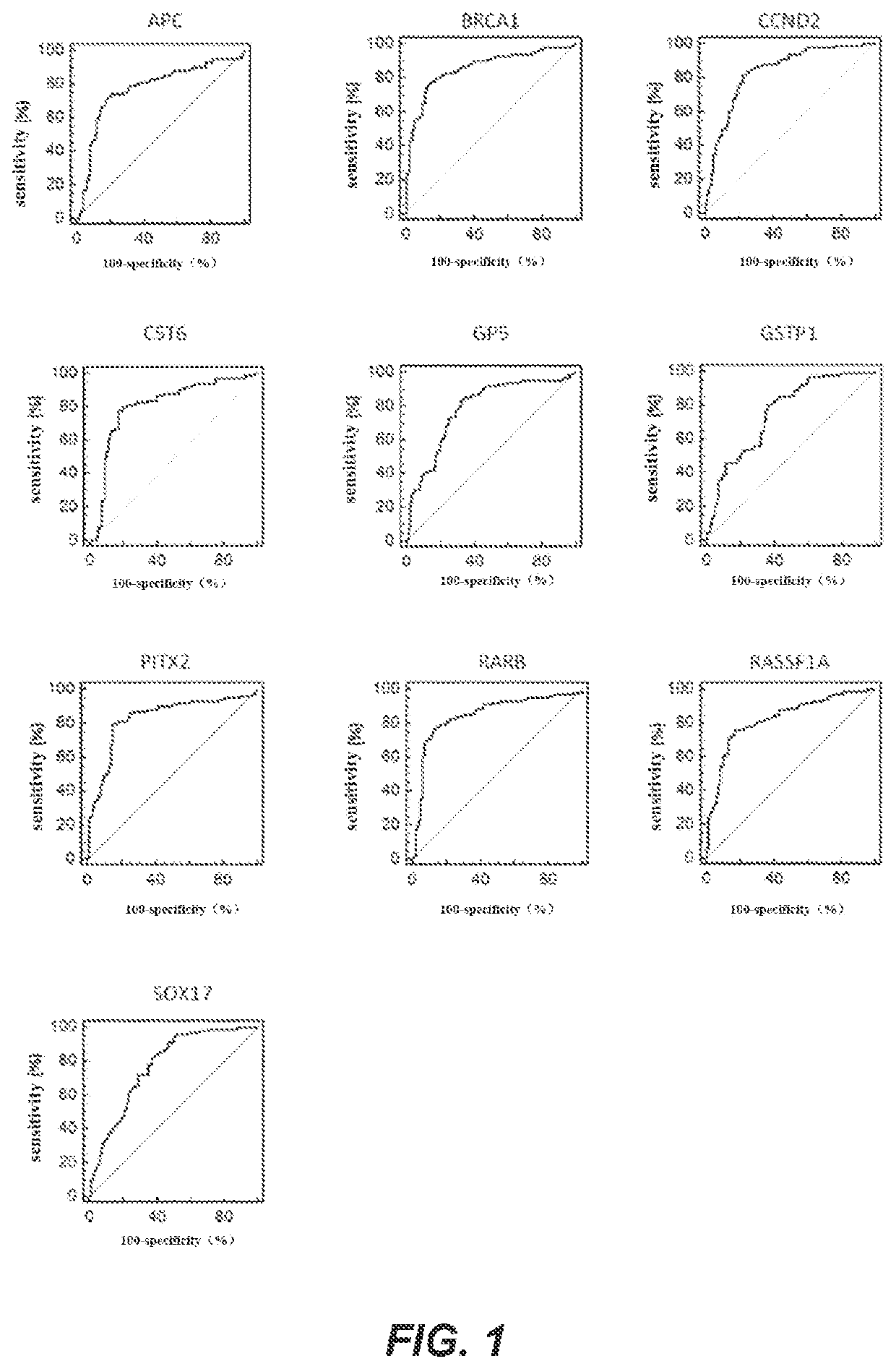
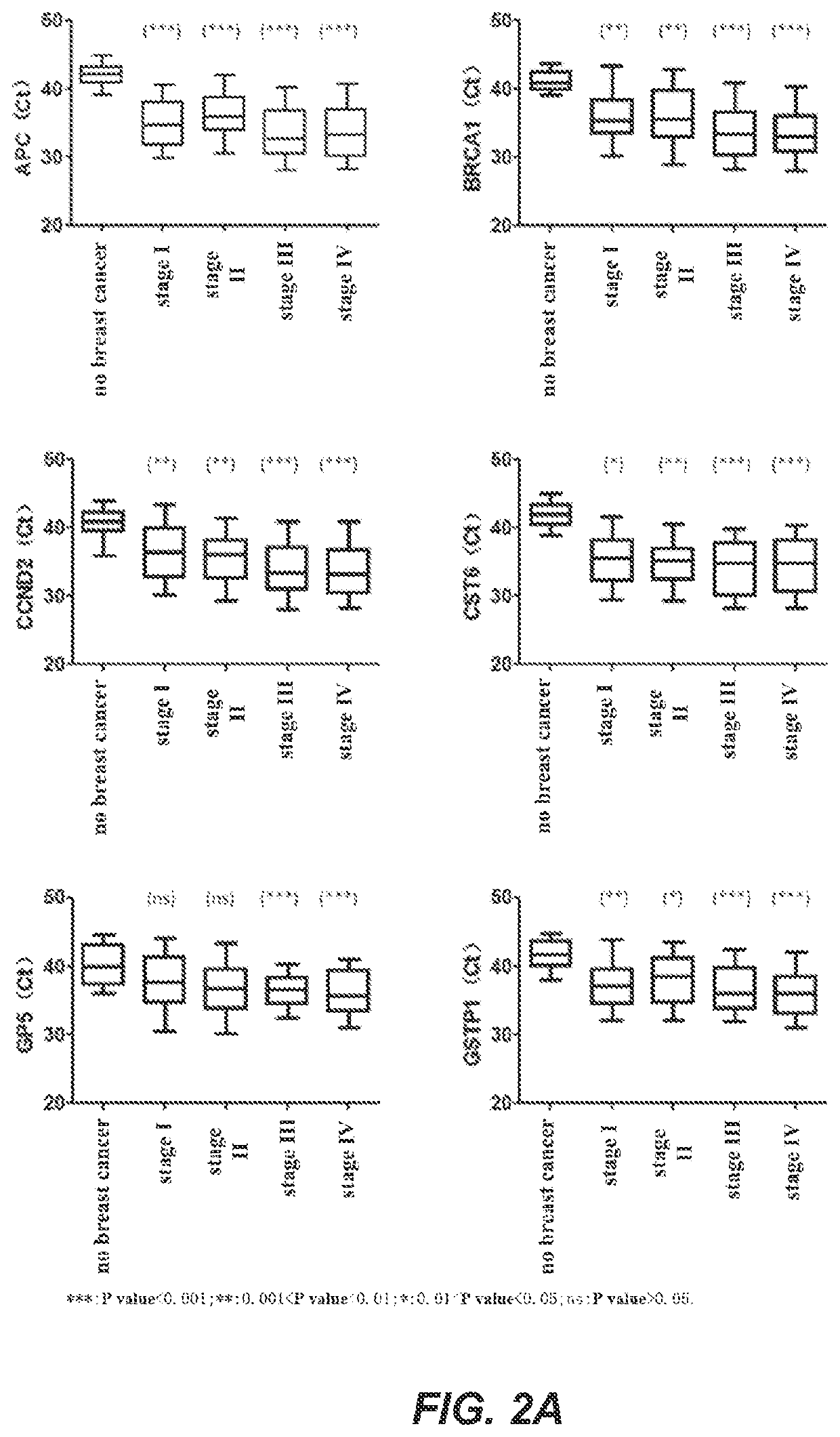
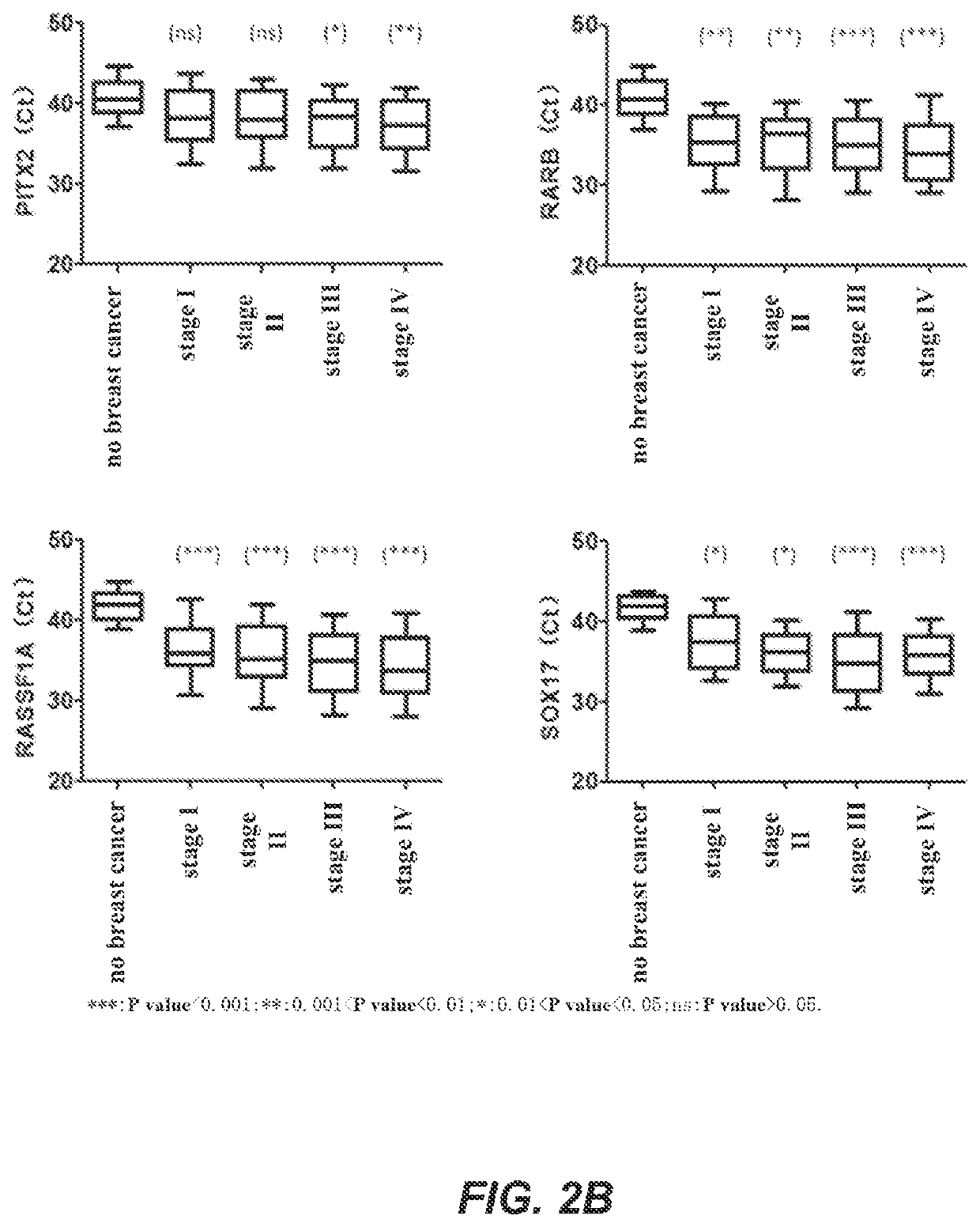
A method for identifying the state of breast cancer status in human subjects, comprising: (1) collecting biological samples from the human subjects; (2) detecting methylation level of biomarker genes in the biological samples, wherein the biomarker genes are one or more selected from the following genes: APC, BRCA1, CCND2, CST6, GP5, GSTP1, PITX2, RARB, RASSF1A and SOX17; and (3) comparing the methylation level detected in step (2) with the normal methylation level of the corresponding biomarker gene in the colony to determine the state of breast cancer in human subjects. Further provided is a kit for identifying the state of breast cancer in human subjects.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Kit for rapidly detecting malignant transformation of passage stem cells and application of kit
PendingCN111926079AEasy to operateImprove accuracyMicrobiological testing/measurementMalignancyMalignant transformation
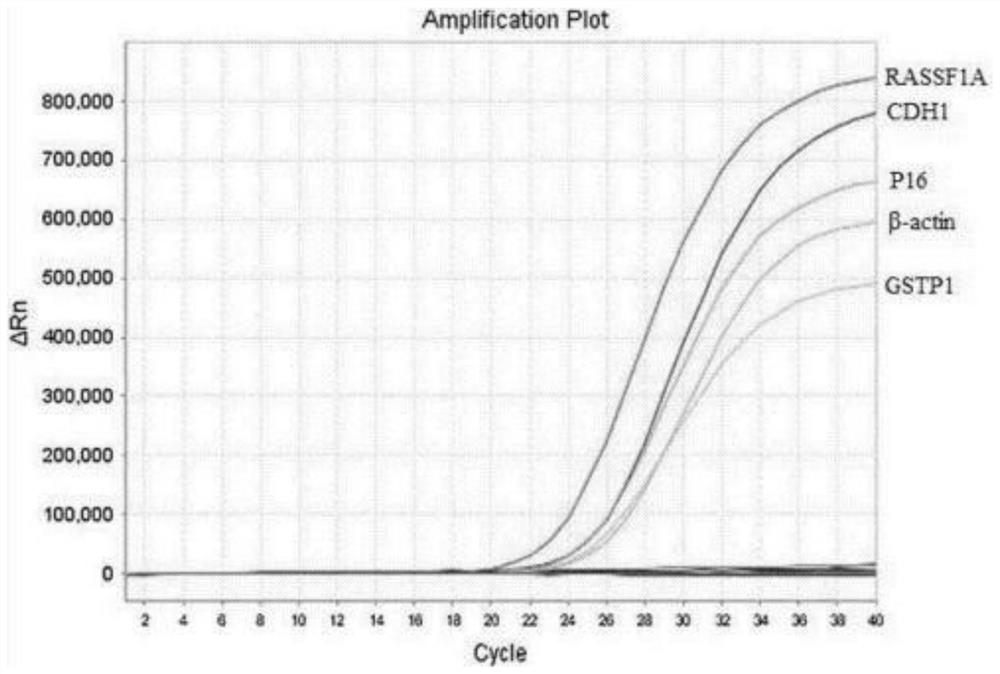
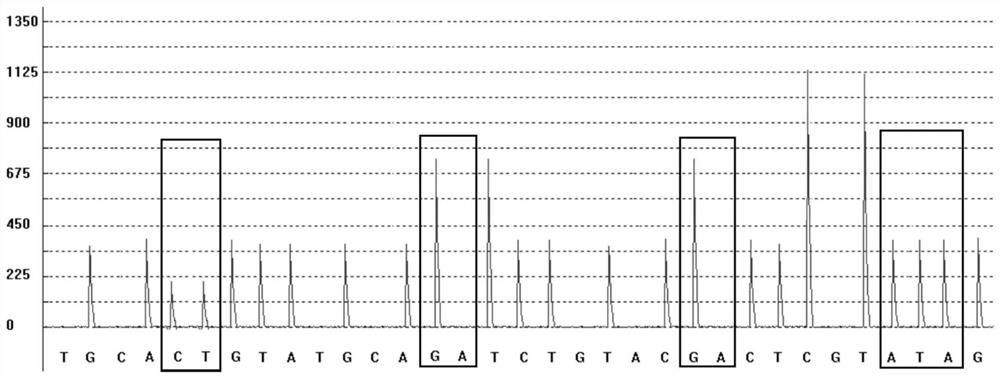
The invention provides a kit for rapidly detecting malignant transformation of passage stem cells and application of the kit, belongs to the technical field of stem cells, the kit comprises primers corresponding to four target genes of P16, RASSF1A, GSTP1 and CDH1, and a reference gene beta-actin primer, the kit is applied to rapid detection of malignant transformation of passage stem cells, and has the advantages of simple operation, good accuracy and high sensitivity in detection of malignant transformation of stem cells.
Owner:成都睿杰森生物科技有限公司
Gene polymorphism detection kit for predicting adverse reaction and curative effect of fluorouracil as well as detection method and application of gene polymorphism detection kit
PendingCN113755588ASimplify timeMicrobiological testing/measurementDNA/RNA fragmentationUracil in DNAFluorouracilum
The invention discloses a gene polymorphism detection kit for predicting adverse reaction and curative effect of fluorouracil as well as a detection method and application of the gene polymorphism detection kit. The detection kit is used for detecting gene polymorphism of fluorouracil metabolism related genes TYMS, GSTP1, NQO1 and MTHFR, the kit designs specific amplification primers and sequencing primers for the TYMS, GSTP1, NQO1 and MTHFR. The kit comprises the following components: a sample treating fluid, magnetic beads, an amplification reagent 1, an amplification reagent 2, the TYMS, the GSTP1, the NQO1, an MTHFR sequencing primer and a positive control. According to the gene polymorphism detection kit for predicting the adverse reaction and the curative effect of the fluorouracil as well as the detection method and application of the gene polymorphism detection kit, rapid DNA preparation, constant-temperature PCR amplification and pyrosequencing technologies are combined to detect the gene polymorphism for predicting the adverse reaction and curative effect of the fluorouracil, and suggestions from the gene perspective are provided for clinical personalized medication.
Owner:菲思特(上海)生物科技有限公司
GSTP1 as teratogenic allele for autism and assays and methods based thereon
InactiveUS8722333B2Reduce the possibilityImprove stress conditionPeptide/protein ingredientsMicrobiological testing/measurementDiseasePrenatal diagnosis
The present invention provides novel markers and assays for autism based on the association of GSTP1 with prevalence for having a child or children with autism or autistic disease. The invention relates to the use and application of GSTP1 as a susceptibility marker and teratogenic allele for autism. In particular the genotype of GSTP1 at amino acids 105 and 114, corresponding to nucleotides 313 and 341 are determined. GSTP1 may be combined with other markers in methods and assays for monitoring, managing, diagnosis, prenatal diagnosis, and assessment of autism. In addition, the present invention discloses a novel method for identifying individuals who are genetically susceptible to have offspring with autism wherein the genotype of GSTP1, alone or in combination with other genetic markers or other indicators of oxidative stress, is determined.
Owner:NEW JERSEY UNIVESITY OF MEDICINE & DENTISTRY OF
Reagent kit for detecting male tumor disease genetic susceptibility
InactiveCN101354339AMicrobiological testing/measurementMaterial analysis by optical meansFluorescenceERCC2
The invention discloses a kit used for detecting the genetic susceptibility of male tumor diseases. The kit comprises a specific primer pair which detects genotypes of sixteen sites of single nucleotide polymorphism (SNP) in CCND1, CYP2A13, CYP1A1, CYP2E1, ERCC2, GSTM1, GSTP1, GSTT1, MTHFR, NQO1, PARP1, XRCC1 and ERCC1 simultaneously and specified fluorescent probe pairs, conventional components used in fluorescence quantitative PCR detecting, and the like. The kit of the invention evaluates the genetic susceptibility of male tumor diseases by detecting genotypes of sixteen sites of single nucleotide polymorphism which is closely related to the genetic susceptibility of male tumor diseases.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Nucleotide molecule combination for SNP (single nucleotide polymorphism) detection of fluorouracil metabolism-related genes
InactiveCN109337984AEasy to identifyAccurate readingMicrobiological testing/measurementDNA/RNA fragmentationDPYDDisease
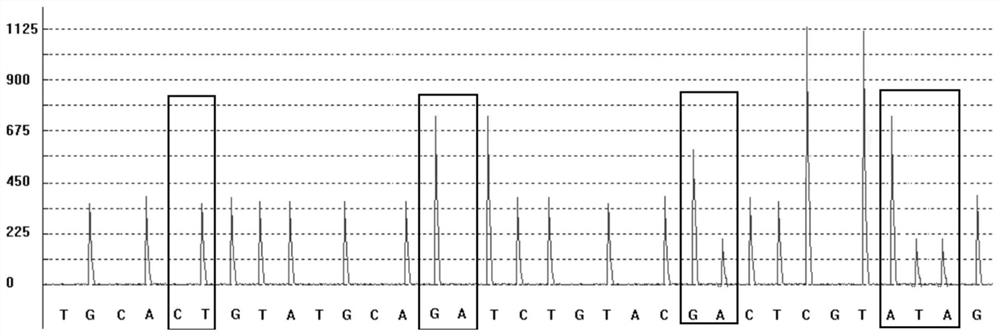
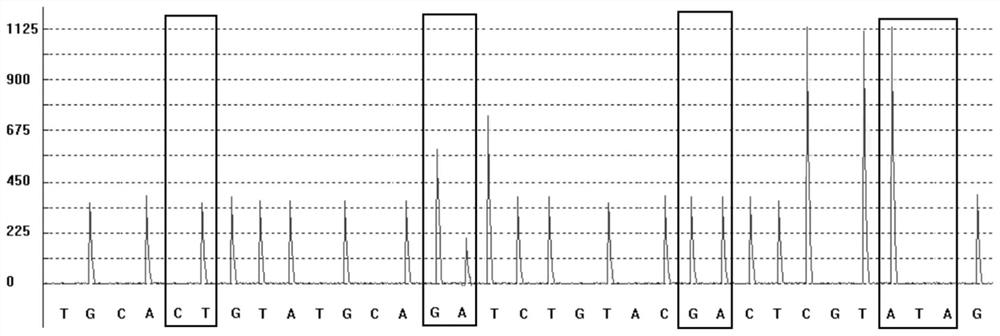
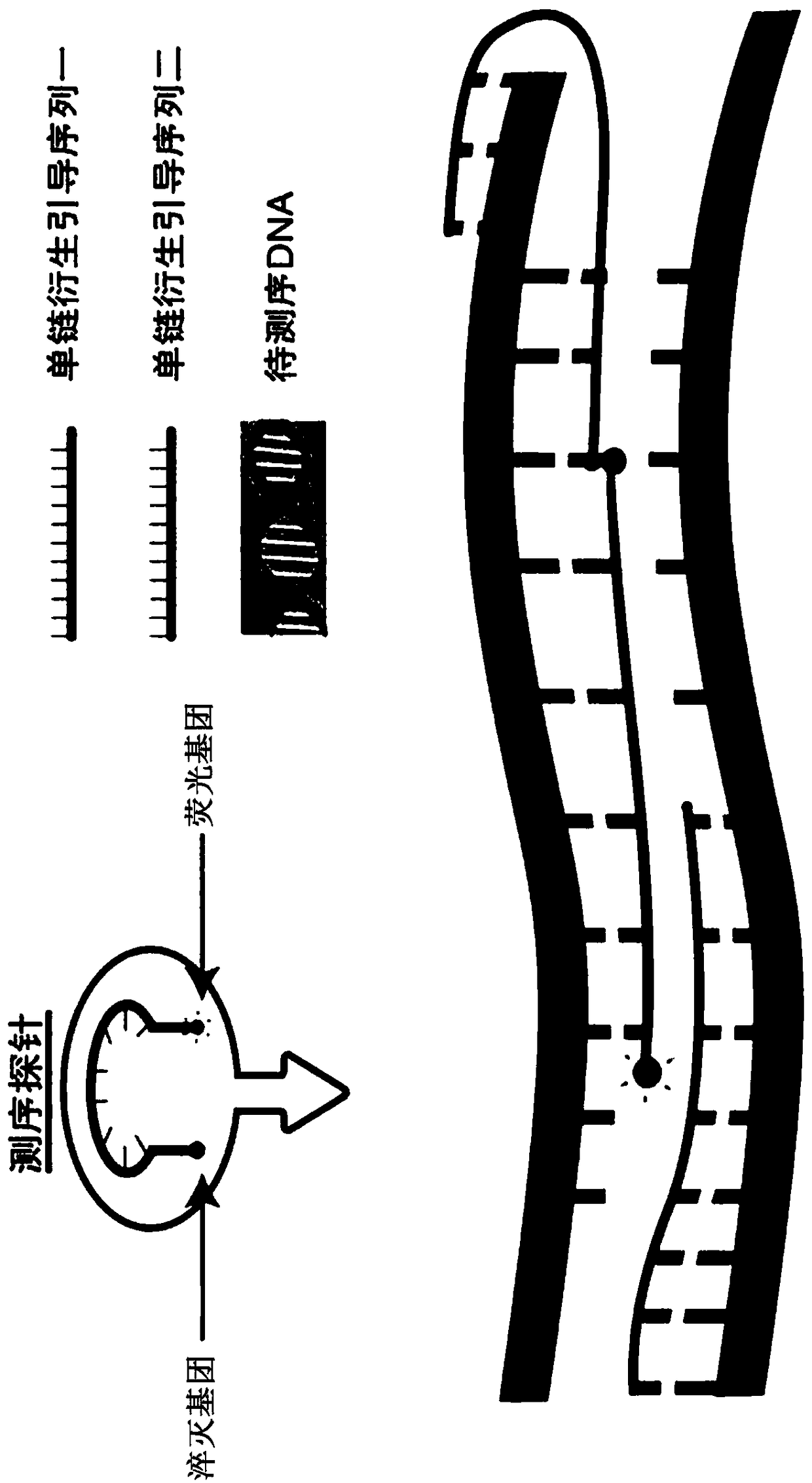
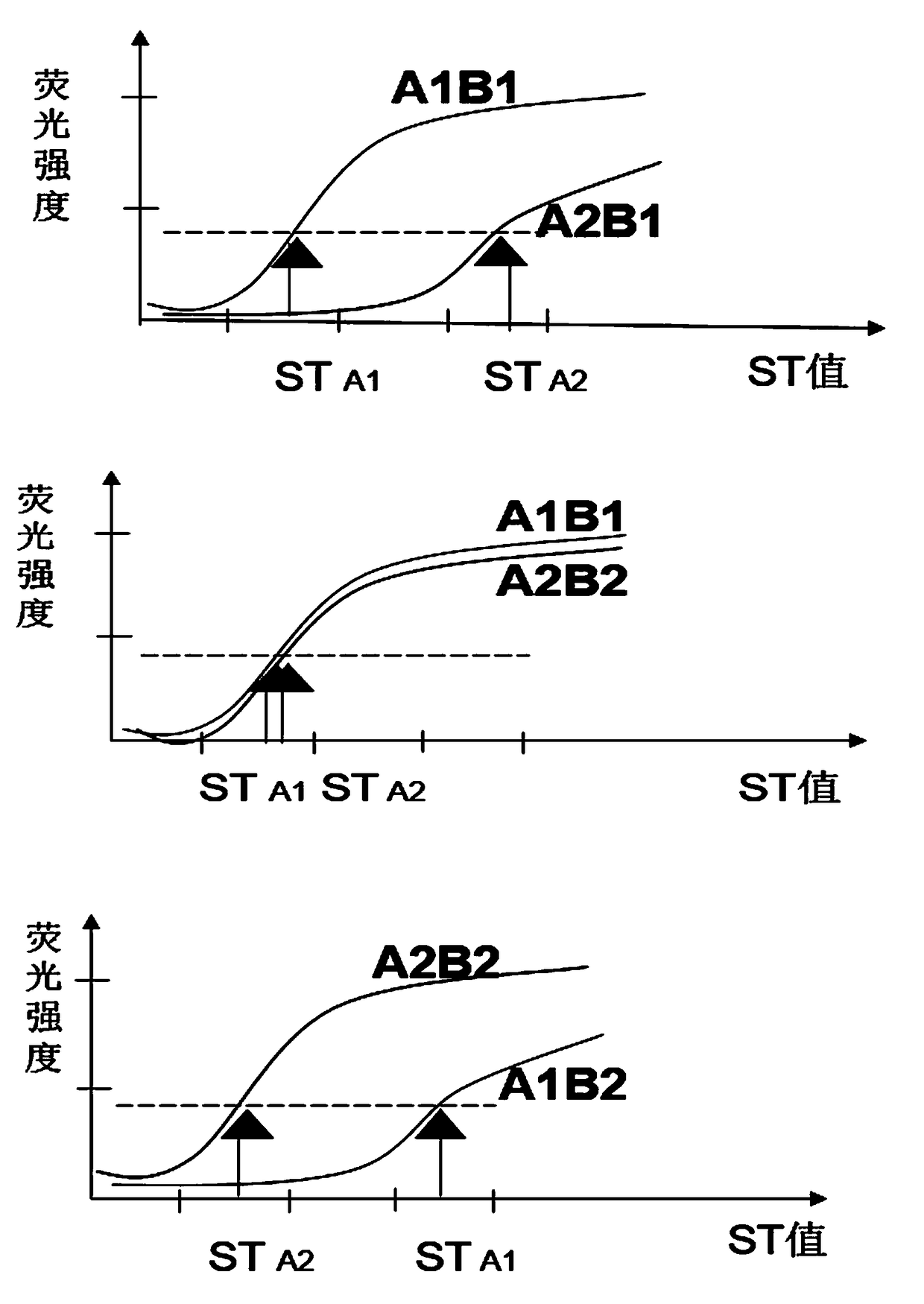
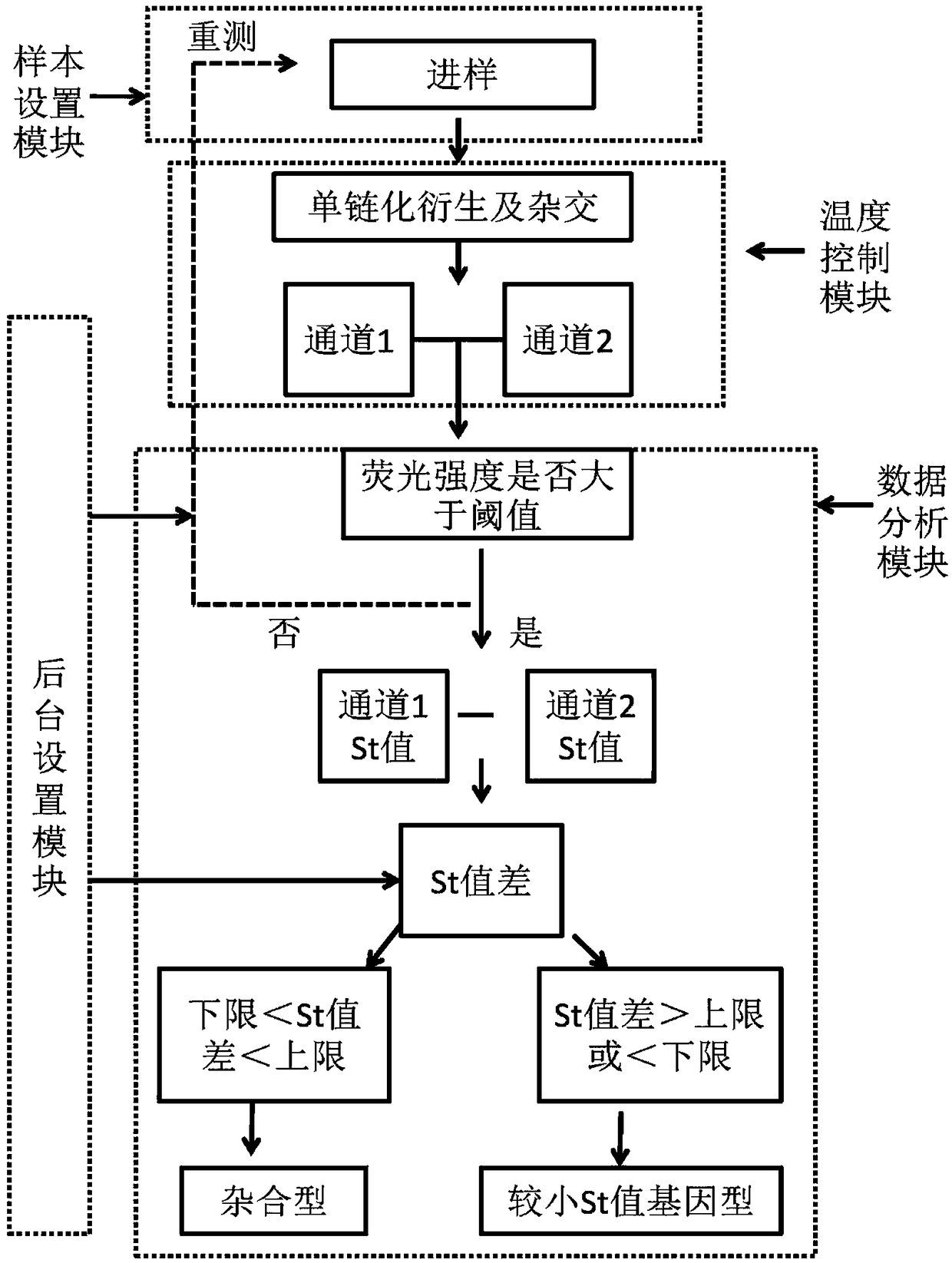
The invention discloses a group of nucleotide molecule combinations for SNP(single nucleotide polymorphism) detection of fluorouracil metabolism-related genes. The invention also provides an SNP fluorescence in-situ hybridization sequencing detection method for detecting fluorouracil and fluorouracil derivative drug metabolism-related genes GSTP1 and DPYD by the nucleotide molecule combinations. The method comprises the following steps: extracting DNA of a sample to be tested, performing single-stranded derivatization by taking the DNA as a template, then simultaneously adding a first sequencing probe and a second sequencing probe of different fluorescent labels to hybridize with a single-stranded derivative, and finally interpreting a hybridization result. The method is high in precision,good in stability, fast, safe and easy to operate automatically. By adopting the method, accurate classification of single nucleotide polymorphisms can be completed in the single-stranded derivatization and the hybridization reaction cycle, so that a genotype of a subject can be understood, and the prevention of diseases caused by risk factors and guidance for the use of drugs for clinical individuals can be achieved.
Owner:北京华夏时代基因科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com