Patents
Literature
41 results about "CD68" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD68 (Cluster of Differentiation 68) is a protein highly expressed by cells in the monocyte lineage (e.g., monocytic phagocytes, osteoclasts), by circulating macrophages, and by tissue macrophages (e.g., Kupffer cells, microglia).
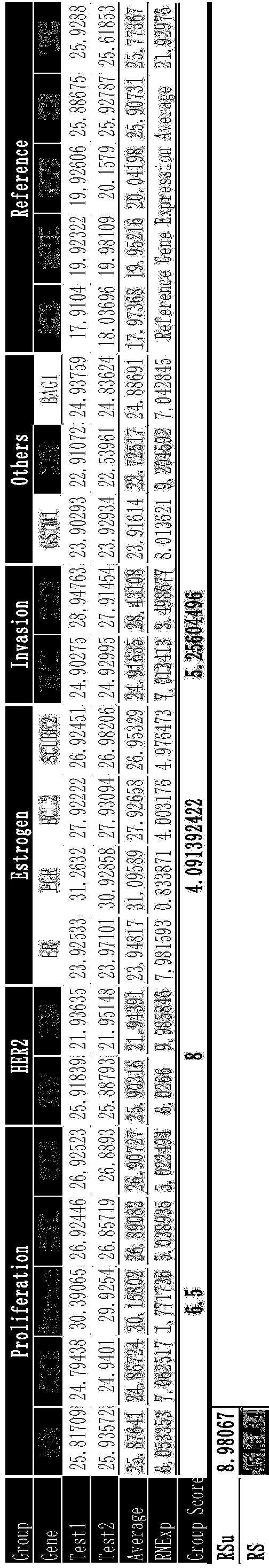
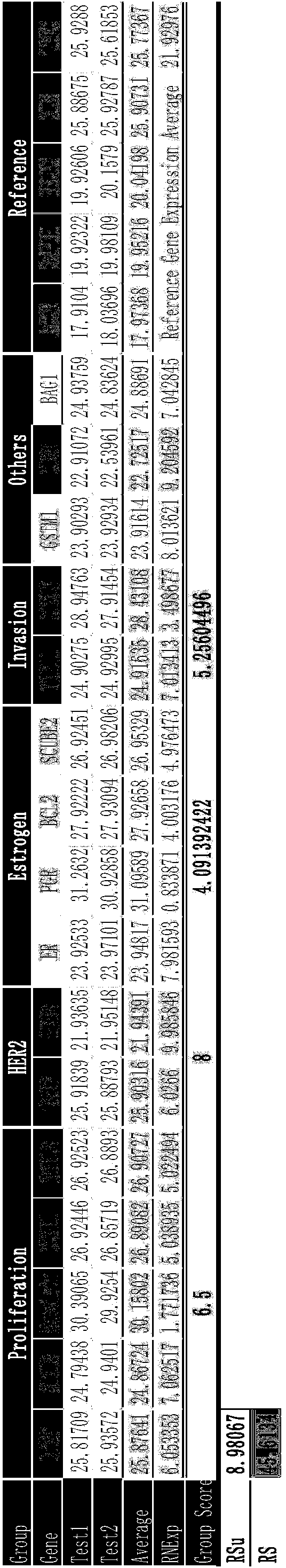
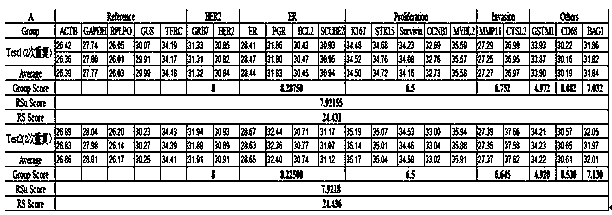
Kit for jointly detecting breast cancer 21 genes and preparation method of kit
InactiveCN104004844AEasy to operateShorten detection experiment timeMicrobiological testing/measurementSingle sampleBAG1
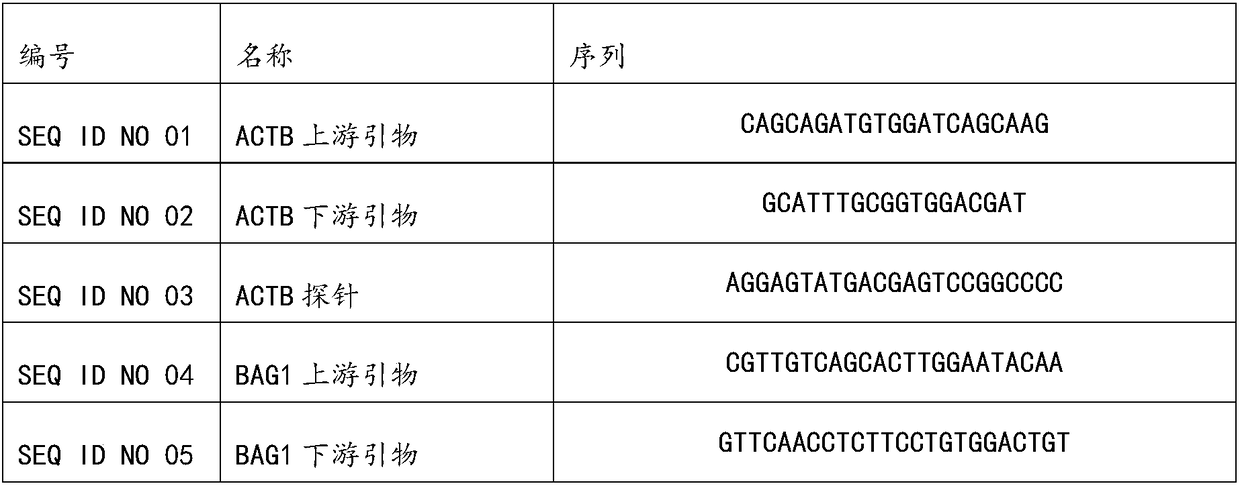
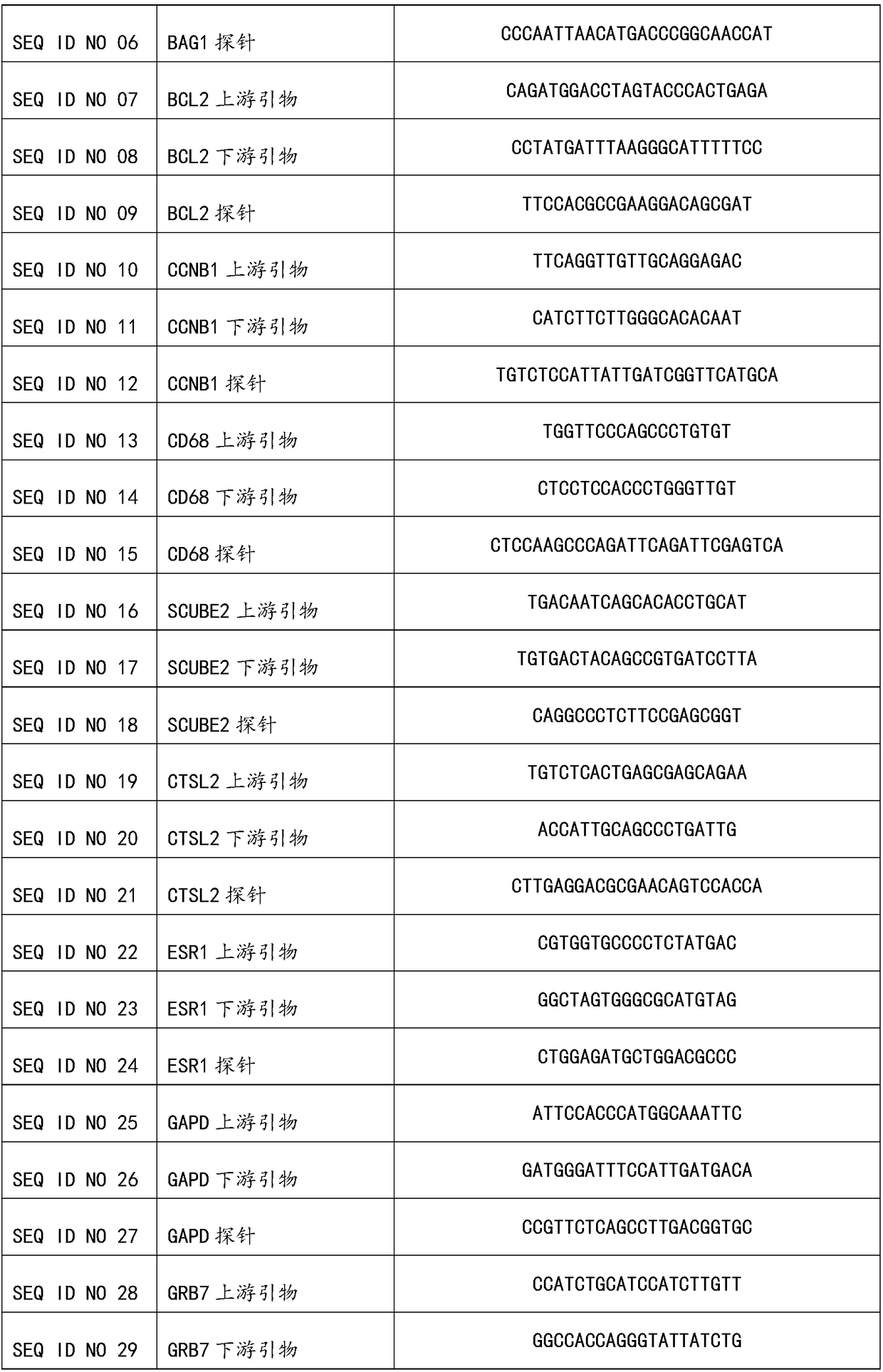
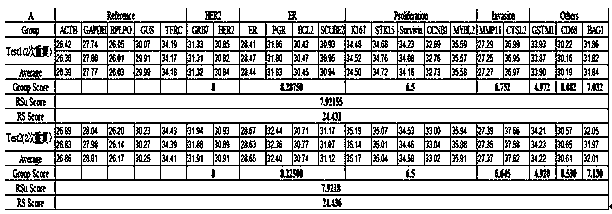
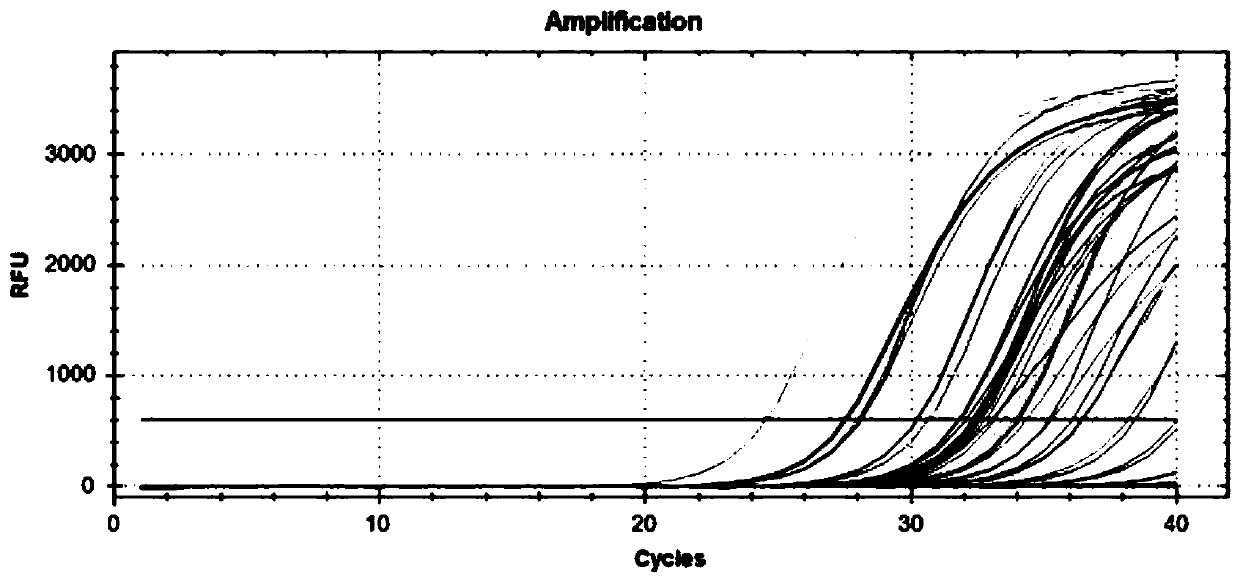
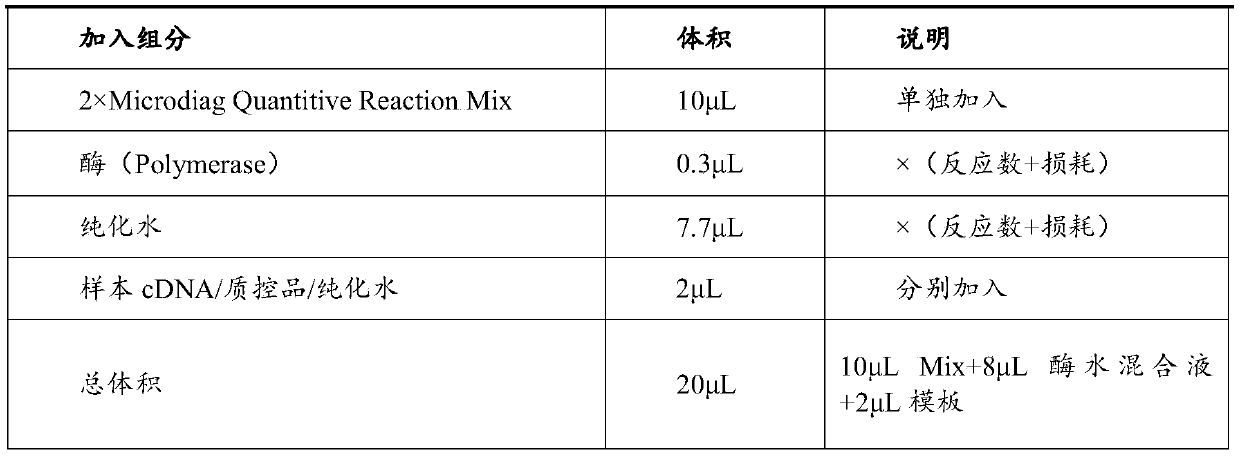
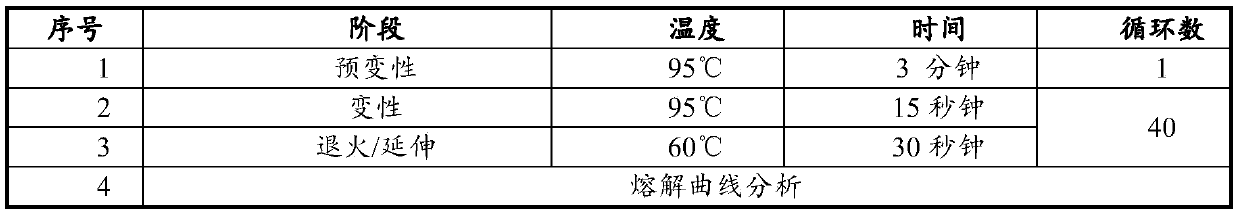
The invention discloses a kit for jointly detecting breast cancer 21 genes. A PCR (polymerase chain reaction) amplification primer consists of 21 primer pairs, and can be used for jointly detecting 21 gene-related expression quantities: Ki67, STK15, Survivin, CCNB1, MYBL2, MMP11, CTSL2, GRB7, HER2, GSTM1, CD68, BAG1, ER, PGR, BCL2, SCUBE2, ACTB, GAPDH, PRLPO, GUS, and TFRC. Output fragments of the 21 pairs of primers related in the invention are smaller than 100bp, so that PCR efficiency after reverse transcription of mRNA (messenger ribonucleic acid) extracted by a paraffin specimen is greatly improved, all primers of 21 genes are premixed, and time and labor for designing and synthesizing 21 pairs of genes by an experimenter are saved; the corresponding primers of the 21 genes are not needed to be added for premixing in mother liquor, and therefore, errors of reaction holes are greatly reduced. The kit disclosed by the invention is simple to operate, and capable of detecting on a quantitative PCR instrument by only adding a template, so that detection experimental time is greatly shortened. The kit disclosed by the invention is applicable to a single sample and multiple samplers, so that results can be quickly obtained, and therefore, scientific research and clinical detection requirements can be satisfied very well.
Owner:杭州美中疾病基因研究院有限公司
Multi-immunohistochemical analysis kit of lung cancer, and using method and application thereof
ActiveCN109596831AGood treatment effectStrong detection signalBiological testingMonoclonal antibodyImmunohistochemistry technique
The invention provides a multi-immunohistochemical analysis kit of lung cancer, and a using method and application thereof, and relates to the field of the multi-immunohistochemical technology. The multi-immunohistochemical analysis kit defines that the monoclonal antibody group comprises more than two types of a CD163 monoclonal antibody, a CD68 monoclonal antibody, a PDL1 monoclonal antibody, aCD8 monoclonal antibody, a PD1 monoclonal antibody and a CD57 monoclonal antibody. The multi-immunohistochemical analysis kit uses the human or animal in vitro tissue, which is given the immune checkpoint inhibitor, as the sample to test, so as to judge the lung cancer effect validity of the multi-immunohistochemical analysis kit. The invention further provides a using method of the multi-immunohistochemical analysis kit; the method can effectively mark not less than 6 types of immune checkpoints on the same one tissue; and the cross reaction does not exist among multiple marks.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
Genes for prognosis of triple negative breast cancer and application thereof
ActiveCN105986024ATimely guidanceAccurate prognosisMicrobiological testing/measurementSpecial data processing applicationsBAG1Evaluation system
The invention discloses genes for prognosis of triple negative breast cancer and the application thereof. The genes include gene GSTM1, gene BAG1, gene CD68, gene HDGFRP3, gene MAOB and gene TMEFF2. The genes can be applied to a multi-parallel testing liquid-phase gene chip for prognosis of triple negative breast cancer, a kit for prognosis of triple negative breast cancer, and a gene evaluation system for prognosis of triple negative breast cancer. By the adoption of the genes, accurate prognosis and classification of the relapse conditions of early-stage triple negative breast cancer patients in China five years after surgery can be achieved, and clinical medication can be effectively and timely guided.
Owner:FUDAN UNIV SHANGHAI CANCER CENT +1
Pancreatic cancer detection reagent, pancreatic cancer detection kit, pancreatic cancer detection device and application
ActiveCN110456054AProlong lifeHigh detection sensitivityBiological material analysisBiological testingCD33Oncology
The invention provides a pancreatic cancer detection reagent, a pancreatic cancer detection kit, a pancreatic cancer detection device and application. The detection reagent comprises antibodies of anytwo or more of the following molecular markers, wherein CD3, CD4, CD8 and CD68 belong to a group A, CD11b, CD14, CD33, CD45RO, CD57, CD66b, CD163, FoxP3, LAG3, PD1, PDL1, TIGIT, panCK and TIM3 belongto a group B, and at least one molecular marker is selected from the group B. Detecting pancreatic cancer through the antibodies of two or more of the molecular markers provided by the invention hasthe advantages of high detection sensitivity and excellent specificity. Thus, the molecular markers are suitable for a multiple immunohistochemistry method to detect tumor microenvironment of a pancreatic cancer patient and are very advantageous, and consequently, whether the patient has a longer lifetime can be accurately predicted relatively, namely, prognosis is better.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
Double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis
The invention relates to a double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis. The double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis comprises a first antibody and a second antibody, wherein the first antibody is selected from the antibody combination of the following protein molecular markers:CD68 (cell differentiation antigen 68) and PD-L1 (procedural death ligand 1). The kit uses double-antibody combination indexes; the expression mode of the PD-L1 in tissues can be precisely reflected,so that the prognosis of a patient is better evaluated; the kit can help doctors to select the proper treatment user groups and modes, so that the powerful support is provided for effective diagnosisand treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
Breast cancer 21 gene detection kit and detection method thereof
ActiveCN108624697AIncrease the Tm valueAccurate dataMicrobiological testing/measurementBuffer solutionReaction tube
The invention discloses a breast cancer 21 gene detection kit and a detection method thereof. The kit comprises primers and probes for detecting genes ACTB, GAPDH, RPLPO, GUS, TFRC, CD68, BAG1, GSTM1,HER2, GRB7, MMP11, CTSL2, KI67, STK15, CCNB1, MYBL2, Survivin, ESR1, PGR, BCL2 and SCUBE2. Two pairs of primers and two specific probes are respectively designed for 21 genes in the kit, a primer andprobe liquid of each gene is placed in one reaction tube, a same buffer solution and a procedure are adopted for QPCR (Quantitative Polymerase Chain Reaction) amplification, the operation is simple and convenient to carry out, the detection time can be shortened, and clear and accurate results can be achieved; the kit is wide in application, and applicable to both scientific research and clinicaldetection and applicable to single or multiple samples, and rapid and accurate detection results can be achieved.
Owner:MICROPROBE MEDICAL TECH SHANGHAI CO LTD
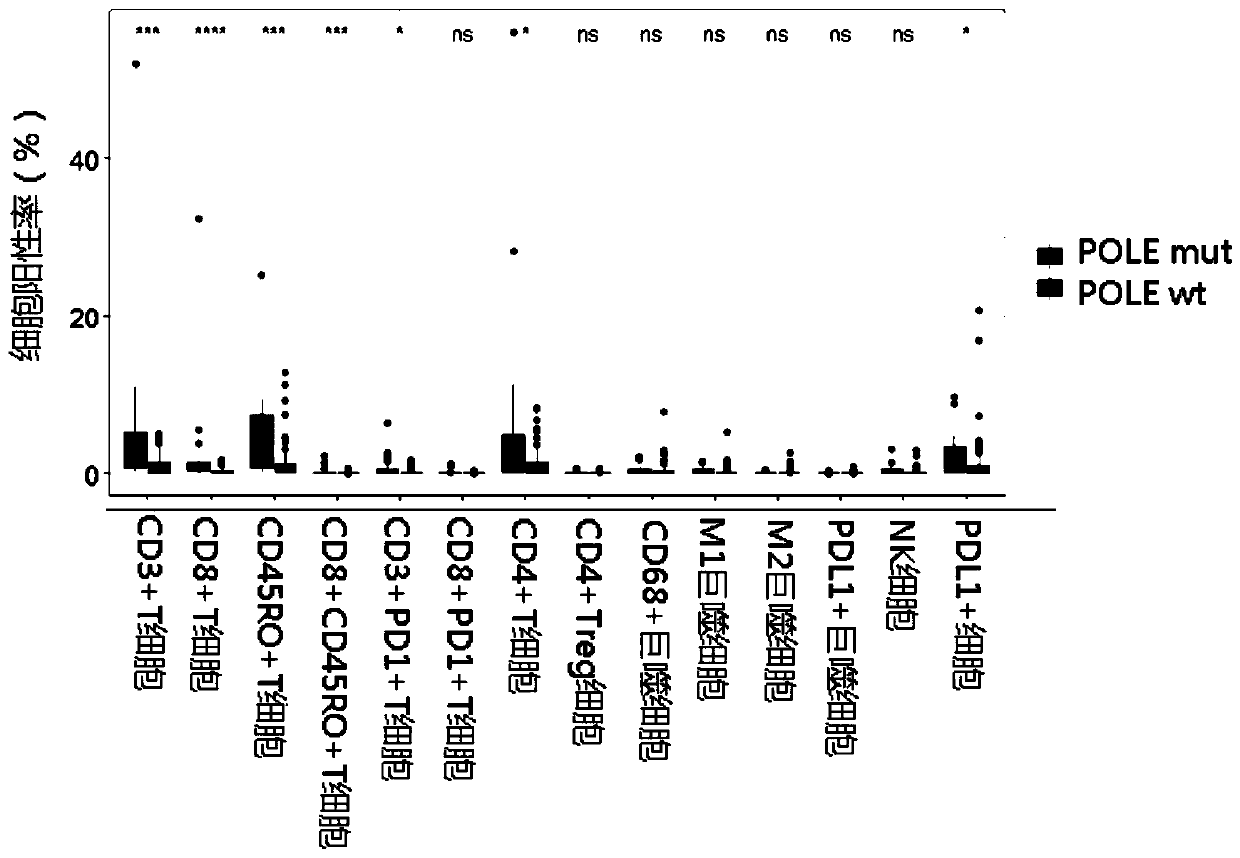
Reagent, kit and device for detecting tumor micro-environment of colorectal cancer as well as application of reagent
PendingCN111235273AAccurate predictionHigh detection sensitivityMicrobiological testing/measurementMaterial analysisOncologyCancer research
The invention provides a reagent, a kit and a device for detecting tumor micro-environment of colorectal cancer as well as application of the reagent. The detection reagent comprises a POLE gene mutation detection reagent and an antibody of at least one molecular marker of at least one of the following combinations such as a combination 1: CD3, CD8, CD45RO, PD-1 and PD-L1 and a combination 2: CD4,FOXP3, CD68, CD163, PD-L1 and CD57. By utilizing the reagent, the kit and the device for detecting tumor micro-environment of colorectal cancer as well as the application of the reagent disclosed bythe invention, findings prove that POLE gene mutation is obviously related with high expression of a part of the molecular markers, and a relation between the POLE gene mutation and the tumor micro-environment of the colorectal cancer is determined, so that a multi-immunohistochemical method is conveniently and comprehensively utilized, and simultaneously several molecular markers are marked, andthe advantages that the sensitivity of detection on the tumor micro-environment of a pancreatic patient is high and the specificity is high are also utilized, so that whether a detected object has 5-year survival rate of higher probability can be relatively and accurately predicted.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
Separation method of macrophage in thyroid papillary carcinoma tissue
InactiveCN103361312ASeparation succeededIncrease positive rateBlood/immune system cellsPhosphateDigestion
The invention provides a cell separation technology, and particularly relates to a separation method of macrophage in a thyroid papillary carcinoma tissue. The separation method comprises the following separation steps of: placing the collected thyroid papillary carcinoma tissue into D-hanks liquid; flushing; removing D-hanks liquid and connective tissues; cutting the collected thyroid papillary carcinoma tissue into blocks; adding II type collagenase into the blocked tissue; performing digestion reaction for 1 to 3 hours at temperature of 35 to 40 DEG C; adding an 1640 culture medium containing 10% of fetal calf serum for stopping the digestion reaction; filtering; centrifuging; removing supernatant; resuspending and planking cell sap through the 1640 culture medium; transferring into a cell incubator containing 5% of CO2 for incubating; fully washing through PBS (Phosphate Buffer Solution); re-culturing the obtained anchorage-dependent cells; collecting the supernatant; and freezing and storing. According to the separation method, the separated macrophage is circular and can be tightly adhered to a petri dish and shows positive by staining by CD68; the positive rate reaches more than 95%, namely, the macrophage is separated successfully.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Methods for assessing endometrium receptivity of a patient
ActiveUS20130072748A1Increasing the live birth potential of an in vitro fertilized mammalian embryoEnhanced hatching potential or complete hatching of the embryosMicrobiological testing/measurementLibrary screeningObstetricsPhysiology
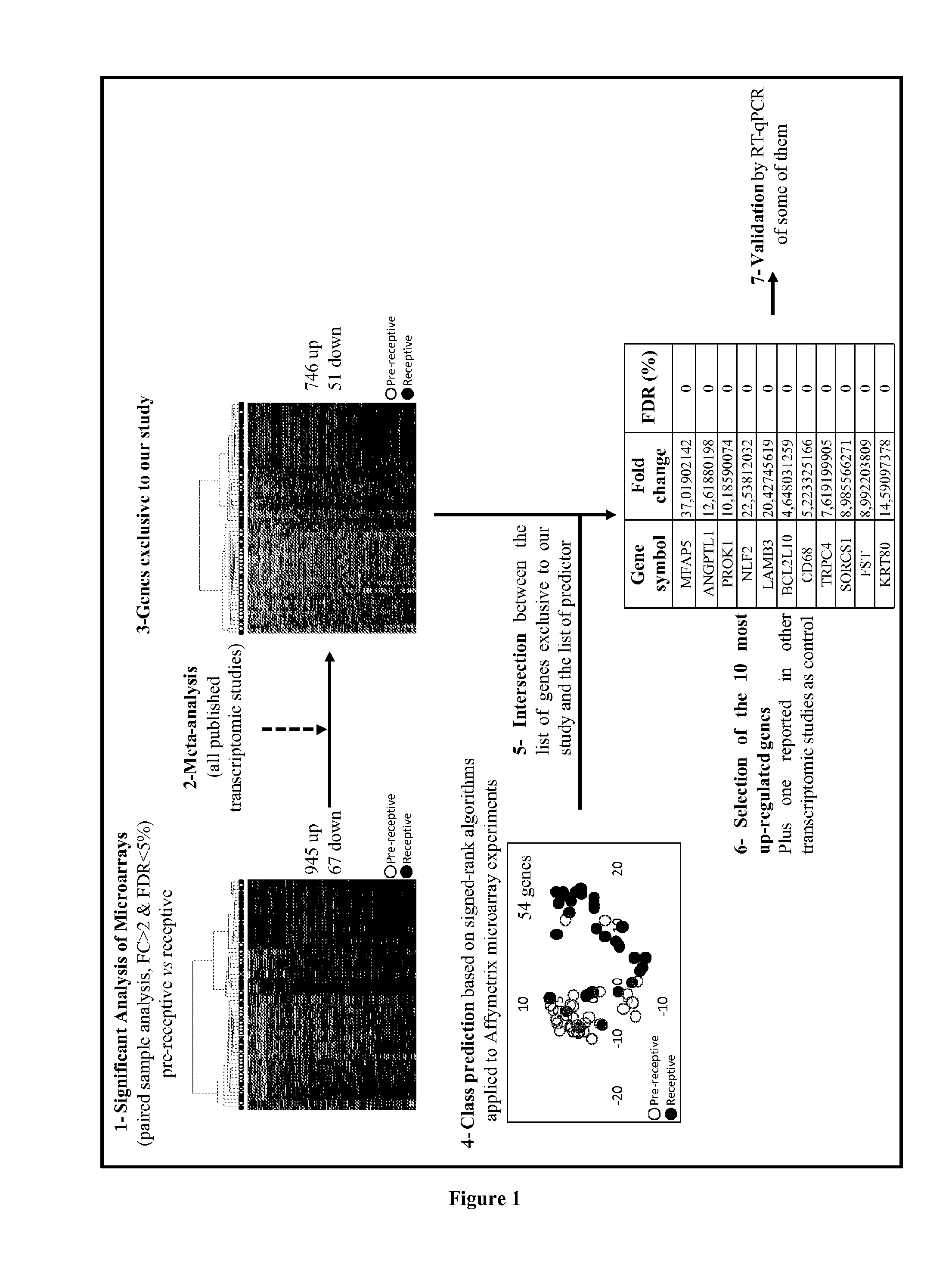
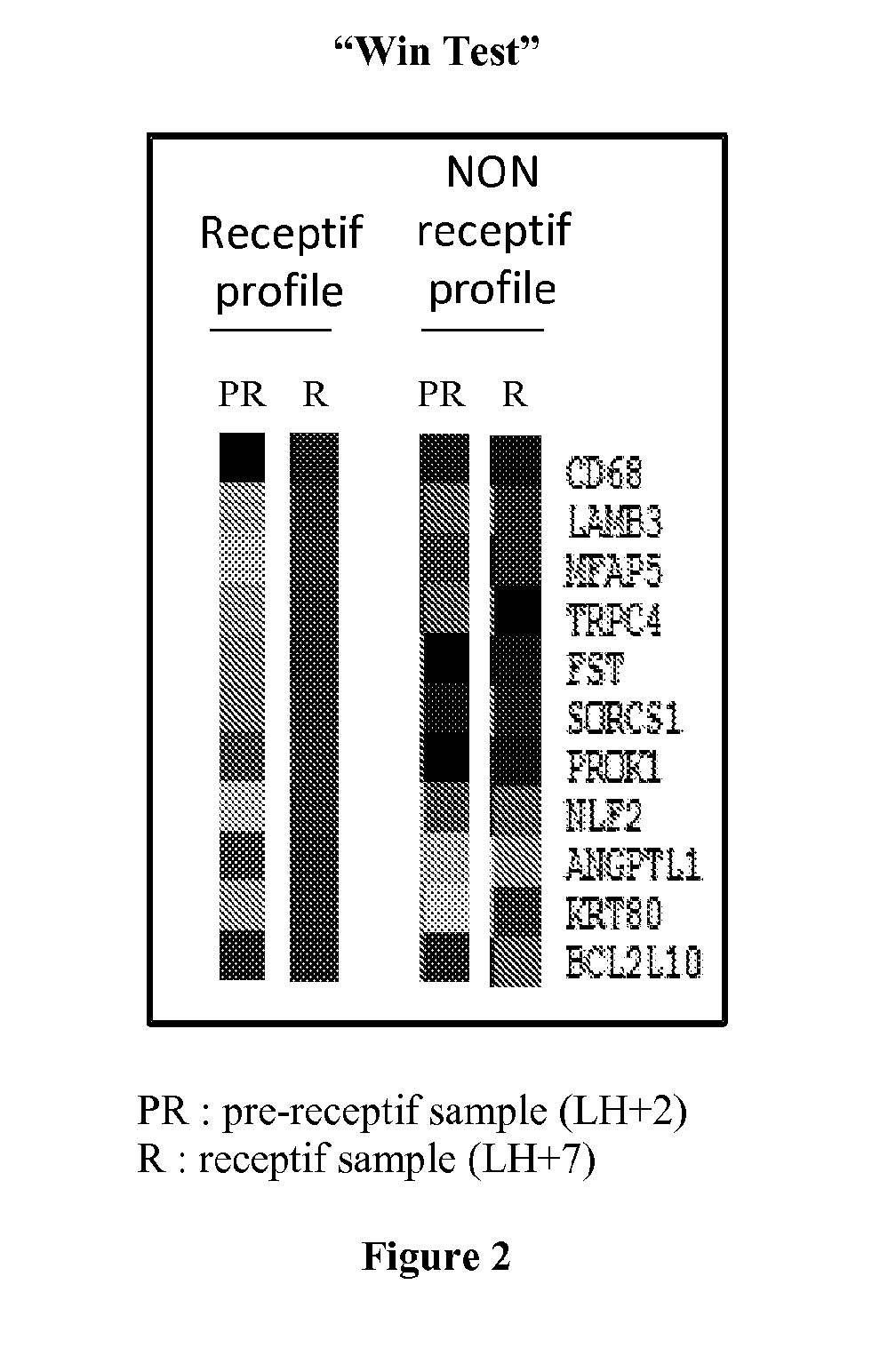
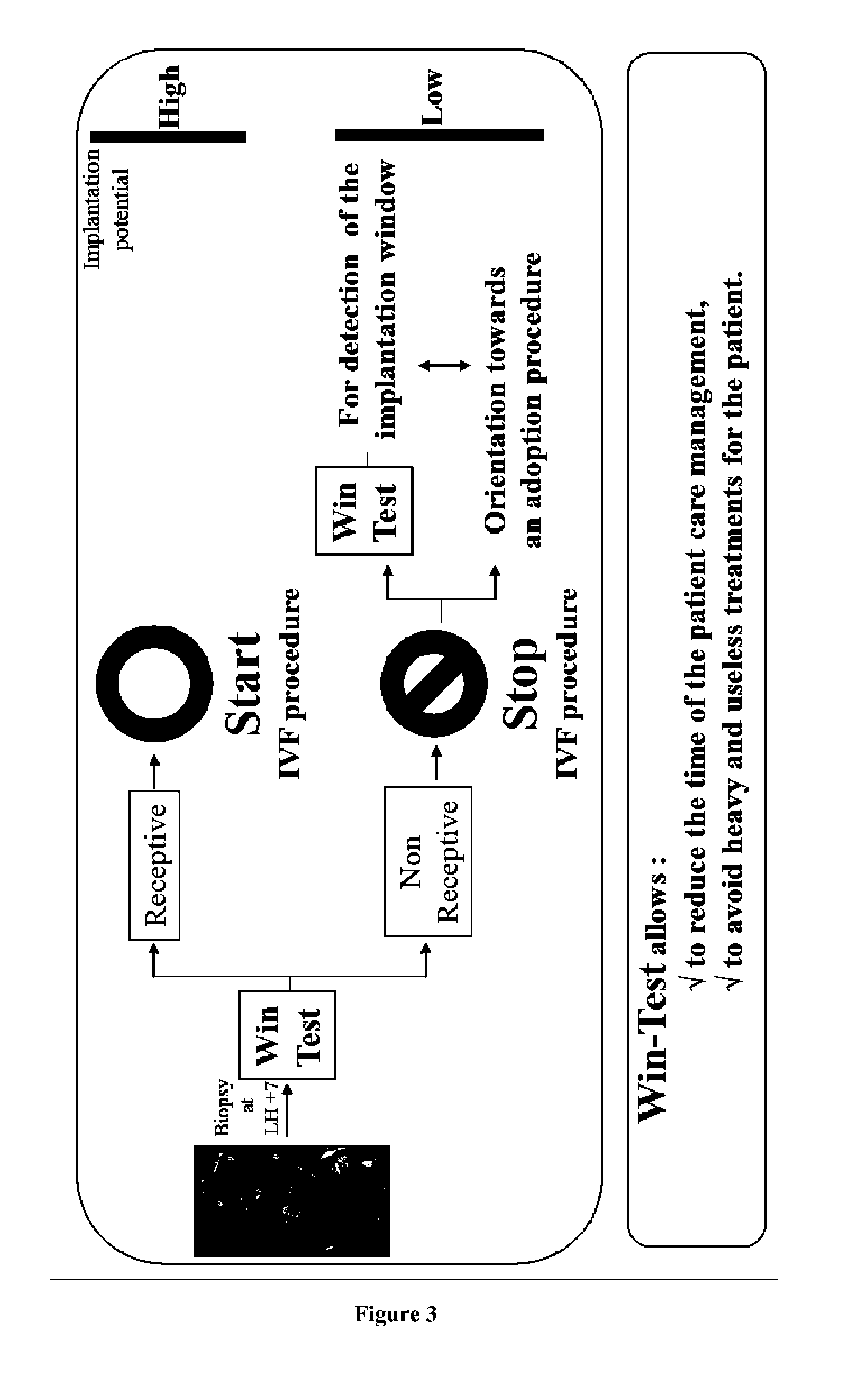
The present invention relates to a method for assessing the endometrium receptivity of a patient, comprising a step consisting of measuring the expression level of eleven genes in an endometrial biopsy sample obtained from said patient wherein said genes are MFAP5, ANGPTL1, PROK1, NLF2, LAMB3, BCL2L10, CD68, TRPC4, SORCS1, FST and KRT80.
Owner:UNIV DE MONTPELLIER +2
Methods of producing and using a human microglial cell line
InactiveUS20020064877A1Reaction is observedGenetic material ingredientsGenetically modified cellsDiseaseCD11c Antigen
An immortalized human cell line has the characteristics of human microglia. It expresses the CD 8 and CD11c antigens. The immortalized human cell line has at least three of the following attributes: CD11b (Mac1), CD68, HLA-ABC, HLA-DR, IL-1b, IL-6, IL-8, IL-12, IL-15, TGF-b, TNF-a, MIP-1a, MIP-1b, MCP-1, P2Y1R, P2Y2R. Also disclosed is a method of transforming human microglial cells into an immortalized cell line, a method of testing drugs for effects on human microglial cells and a method of treating individuals experiencing neurodegenerative disorders.
Owner:KIM SEUNG U
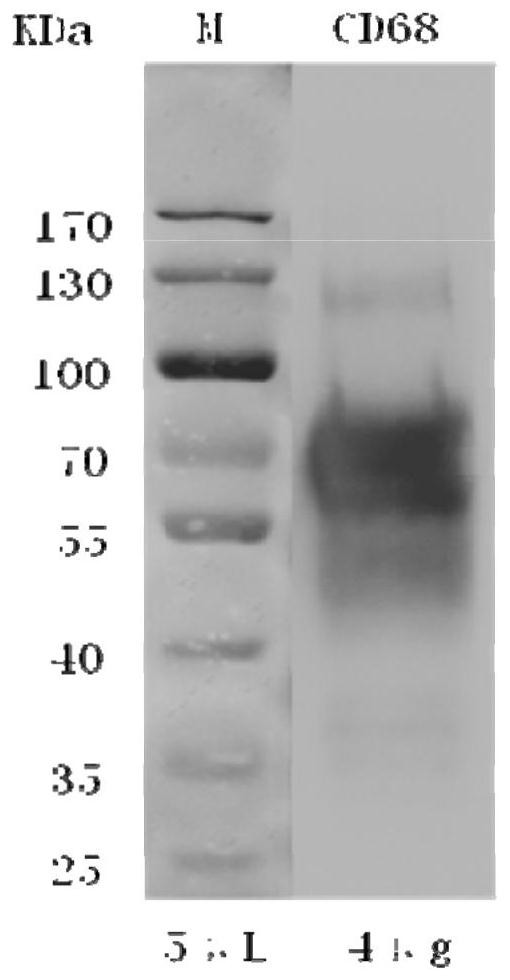
Hybridoma cell strain, CD68 monoclonal antibody, preparation method and application
ActiveCN108330107AGood dyeing effectIncrease positive rateBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsLymphocyteExtracellular proteins
The invention relates to a hybridoma cell strain, a CD68 monoclonal antibody, a preparation method and application. The preparation method of the monoclonal antibody comprises the following steps: taking purified CD68 recombinant extracellular proteins as immunogens for immunizing animal bodies; fusing myeloma cells SP2 / 0 with B lymphocytes of the immunized animal bodies to obtain hybridoma cells;screening specific hybridoma cell positive clones, carrying out cell cloning on the positive clones, screening hybridoma cells capable of stably secreting CD68 monoclonal antibodies to obtain the CD68 monoclonal antibody. The CD68 monoclonal antibody has the characteristics of high specificity, high affinity, high stability and high titer.
Owner:富恩生物技术(成都)有限公司
Kit for detecting expression level of breast cancer related genes
InactiveCN109439755AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBAG1Cell sensitivity
The invention relates to a kit for detecting the expression level of breast cancer related genes. The kit contains a detection reagent for detecting the expression level of one or more of BAG1, BCL2 CCNB1, CD68, SCUBE2, CTSL2, ESR1, GRB7, GSTM1, ERBB2, MKI67, MYBL2, PRG, AURKA, MMP11 and BIRC5 genes. With the adoption of the kit, the expression level of the breast cancer related genes can be subjected to high-throughput detection simultaneously under the same reaction condition. The detection is high in specificity and high in sensitivity and consumes short time, the lowest detection limit is1 ng / mu L, and the process from specimen inspection to result obtaining can be completed within 2 h.
Owner:GUANGDONG ASCENDAS GENOMICS TECH CO LTD
Multiple immunohistochemical analysis kit for liver cancer, and using method and application thereof
ActiveCN109884303AImprove the effectiveness of treatmentEliminate distractionsBiological testingMonoclonal antibodyCD8
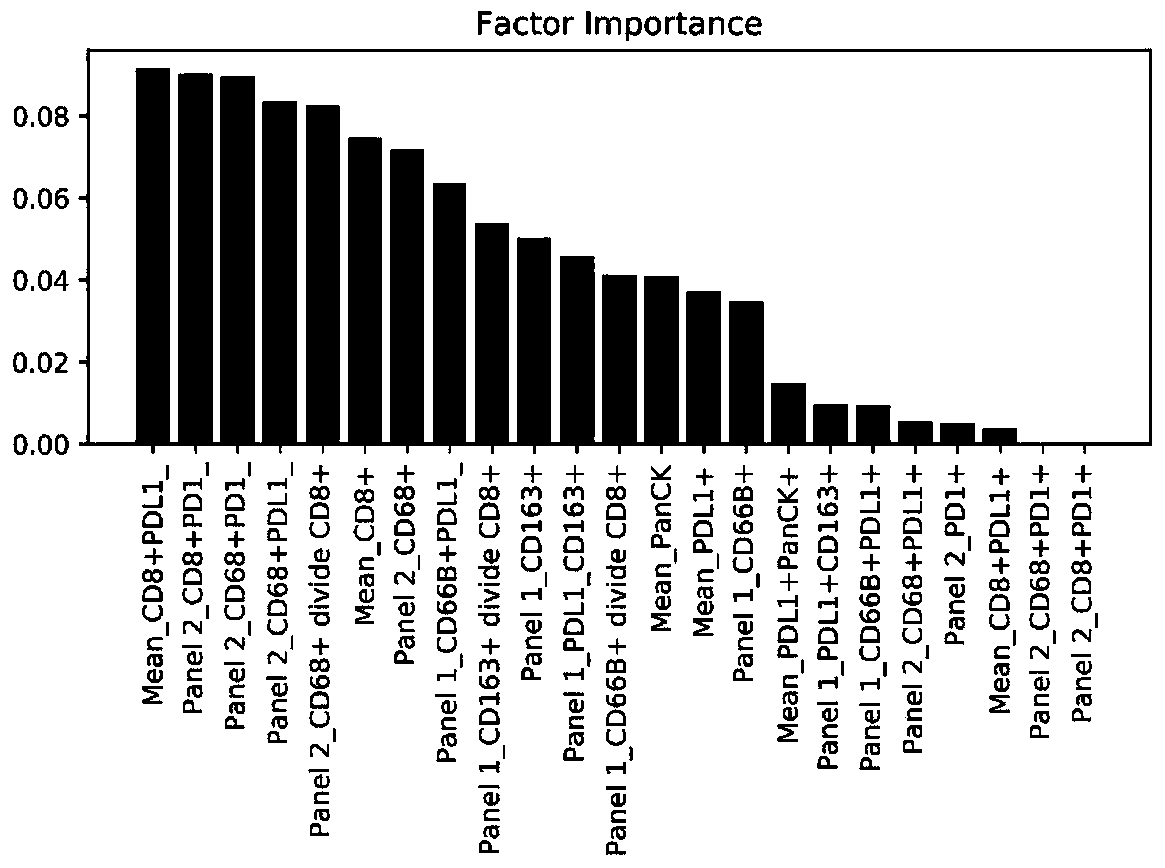
The invention provides a multiple immunohistochemical analysis kit for liver cancer, and a using method and application thereof, and relates to the technical field of multiple immunohistochemistry. The multiple immunohistochemical analysis kit limits that monoclonal antibody groups comprise a first monoclonal antibody group and a second monoclonal antibody group; the first monoclonal antibody group comprises a PanCK monoclonal antibody, a PDL1 monoclonal antibody, a CD8 monoclonal antibody, a CD66B monoclonal antibody and a CD163 monoclonal antibody; the second monoclonal antibody group comprises a PanCK monoclonal antibody, a PDL1 monoclonal antibody, a CD8 monoclonal antibody, a PD1 monoclonal antibody and a CD68 monoclonal antibody. According to the multiple immunohistochemical analysiskit provided by the invention, the paraffin sections of the human liver tissue in vitro are used as samples for detecting, and thus the effectiveness of the immune checkpoint inhibitor acting on theliver cancer is predicted.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD +2
Predictors for cancer treatment
InactiveCN103930785AMicrobiological testing/measurementBoron compound active ingredientsOncologyCancer treatment
Owner:JANSSEN PHARMA NV
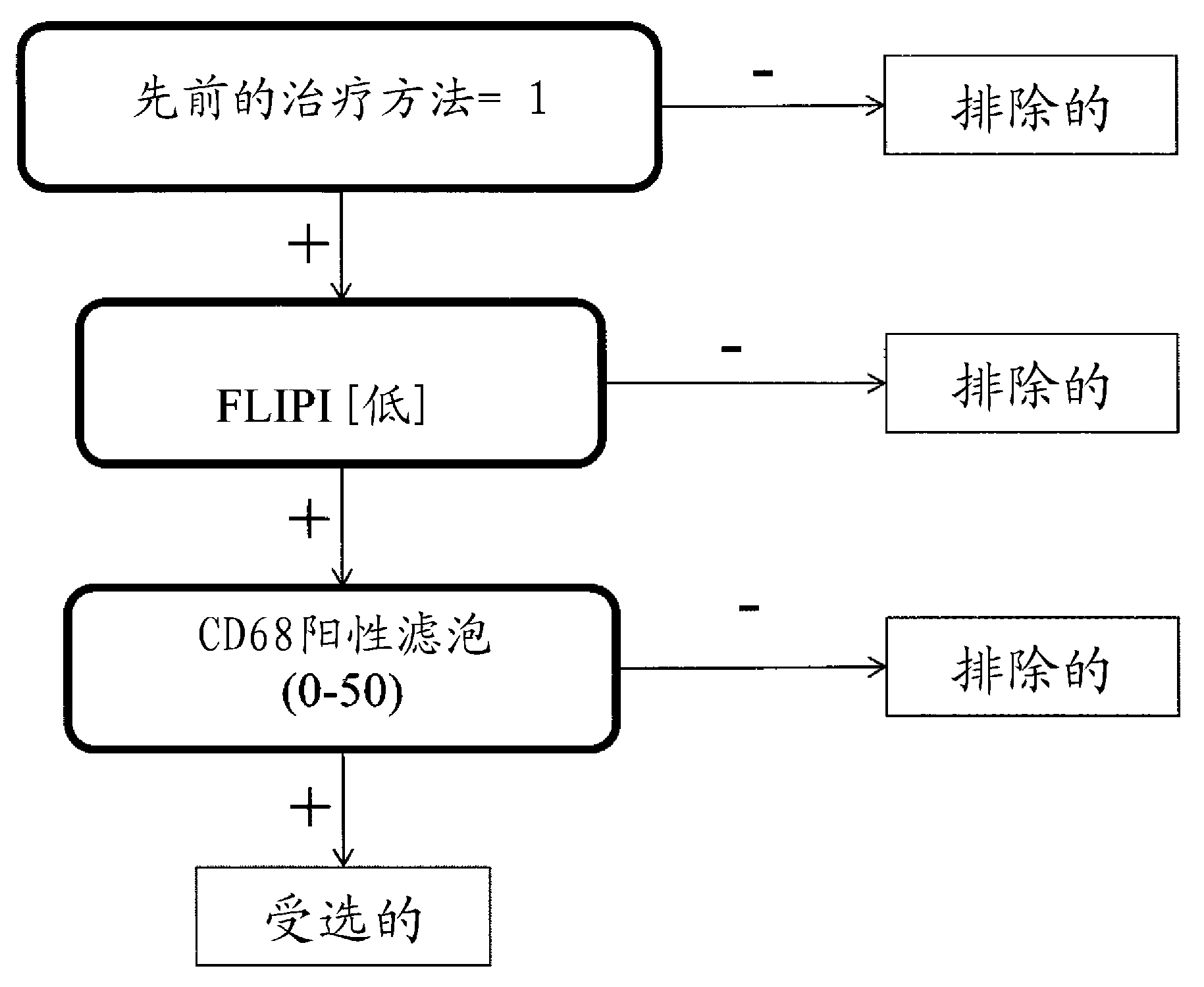
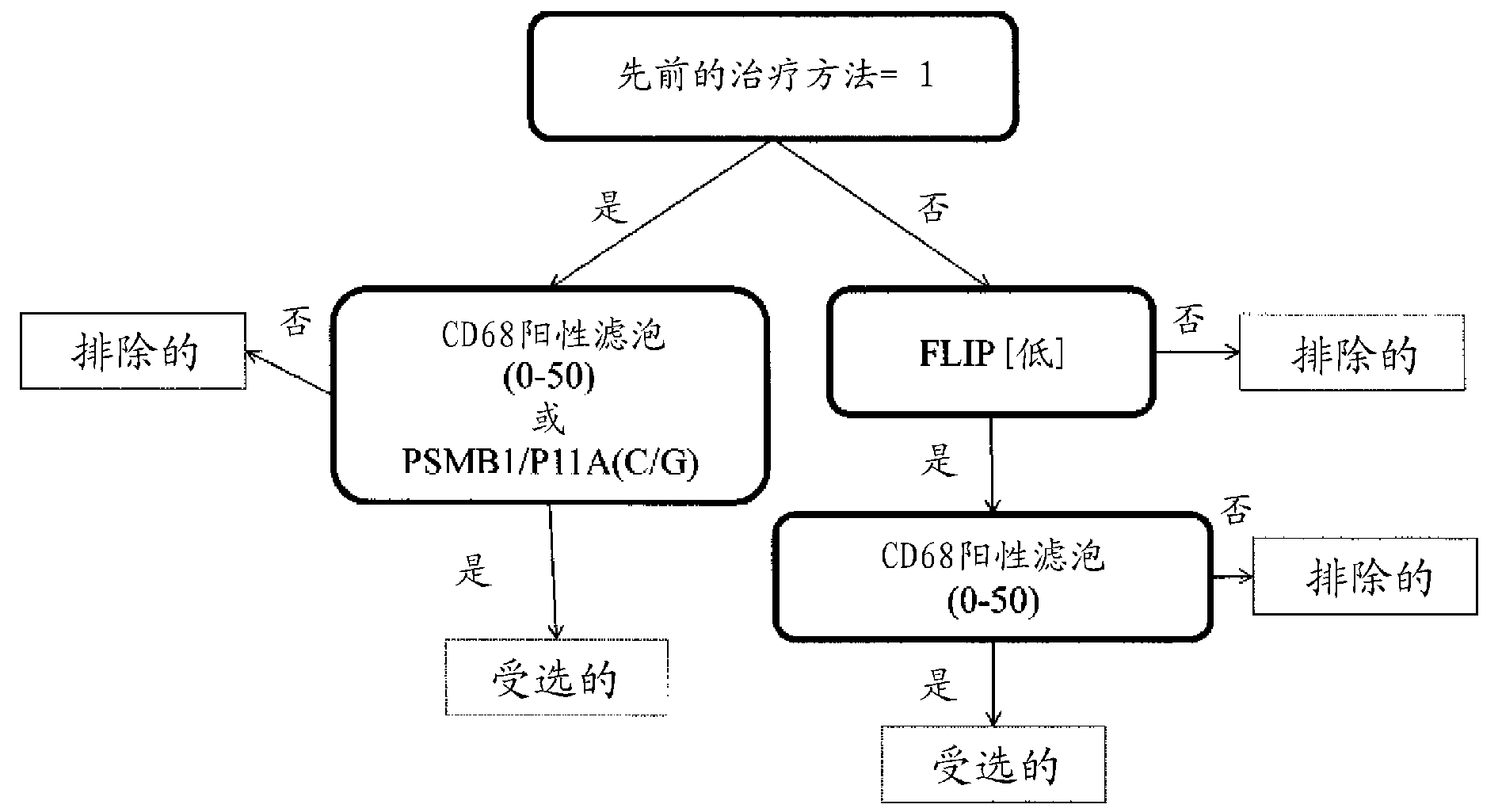
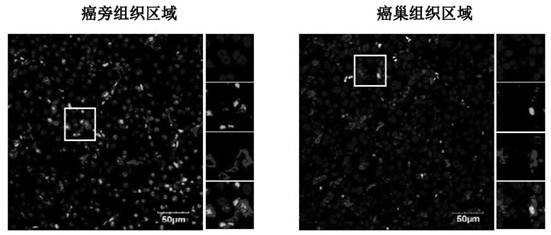
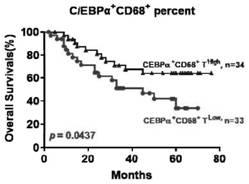
Application of C/EBP alpha and CD68 as prognostic markers in preparation of liver cancer prognostic prediction kit
InactiveCN112698035APredict prognosisThe test method is matureDisease diagnosisBiological testingIndividualized treatmentPrognostic prediction
The invention discloses an application of C / EBP alpha and CD68 as prognostic markers in preparation of a liver cancer prognostic prediction kit. Researches show that the in-situ differentiation and maturation state of mononuclear / macrophages in liver cancer tissues can be judged according to the in-situ co-localization condition of C / EBP alpha and CD68 in the tissues, so that the in-situ immune response condition of the tissues of liver cancer patients is determined, clinicians are helped to better judge the prognosis survival condition of the patients, and an immunological theoretical basis is provided for selecting proper individualized treatment. The invention also provides an application of the double-antibody combined fluorescence detection kit for detecting the differentiation maturity of mononuclear / macrophages in liver cancer tissues, the double-antibody combined fluorescence detection kit comprises a first antibody and a second antibody, the first antibody is a combination of C / EBP alpha and CD68 antibodies, and the second antibody is a fluorescently labeled second antibody.
Owner:SUN YAT SEN UNIV CANCER CENT
Application of METs as molecular marker in evaluation of clinical prognosis of glioma patient
PendingCN113820488APromote malignant progressionImprove clinical treatment effectDisease diagnosisOncologyMalignant progression
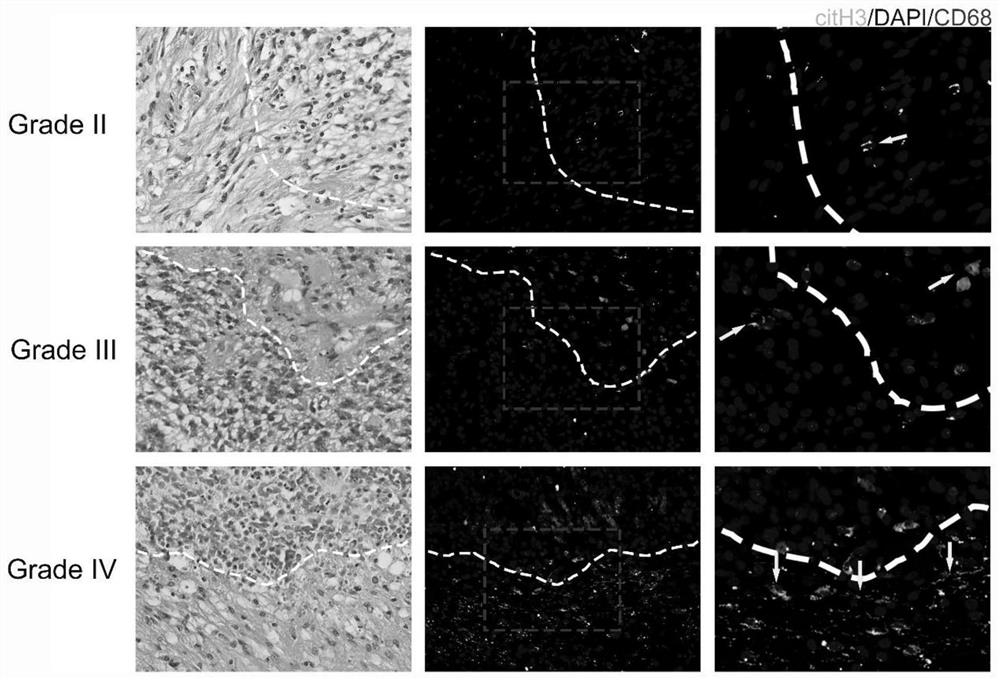
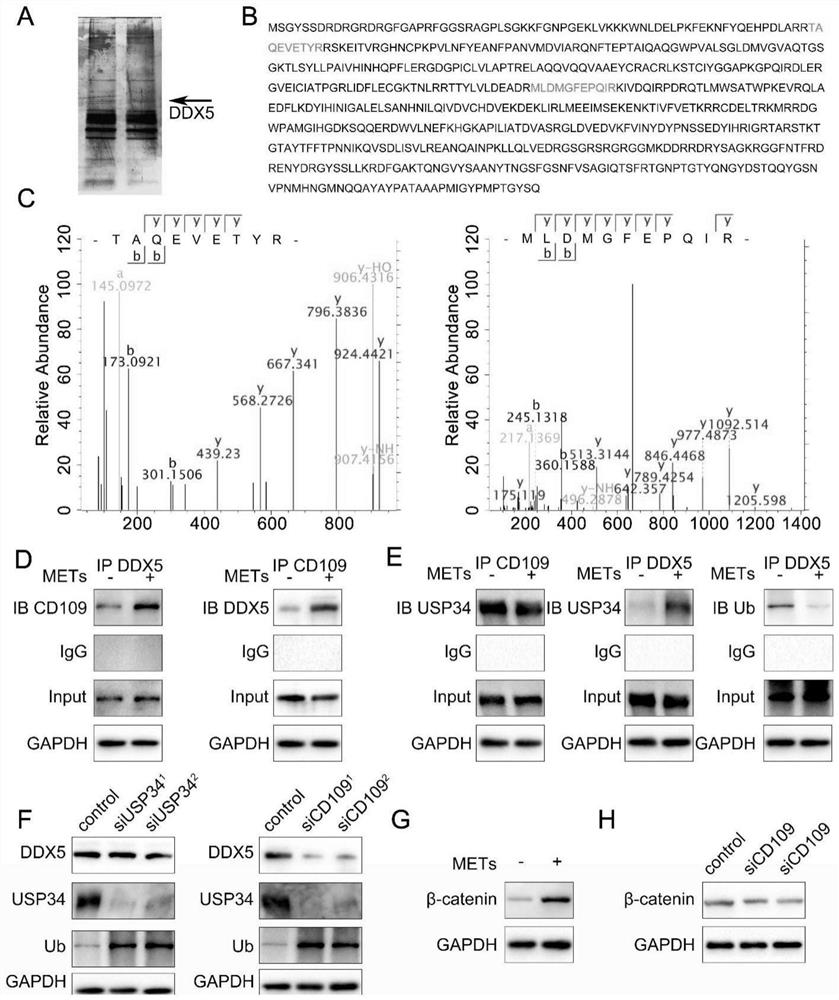
The invention discloses particularly discloses an application of METs as a molecular marker in evaluation of clinical prognosis of the patient suffering from brain glioma. According to clinical data in combination with experimental results, it is found that the level of METs in high-grade brain glioma is increased, METs can promote proliferation, invasion and migration ability of GBM, after METs stimulation, the ubiquitination level of DDX5 in GBM cells is reduced, beta-catenin nucleation is increased, and expression level changes of EMT related indexes and malignant progression of GBM cells can be regulated and controlled. Co-localized CD68 and citH3 in a biological sample are detected through technical methods such as immunofluorescence and immunoblotting experiments, and the level of METs can be indirectly detected so as to evaluate clinical prognosis of GBM patients. The invention provides a new technical means for guiding individualized diagnosis and treatment of the GBM patient, improving the clinical treatment effect of the patient and prolonging the overall lifetime of the GBM patient.
Owner:HARBIN MEDICAL UNIVERSITY
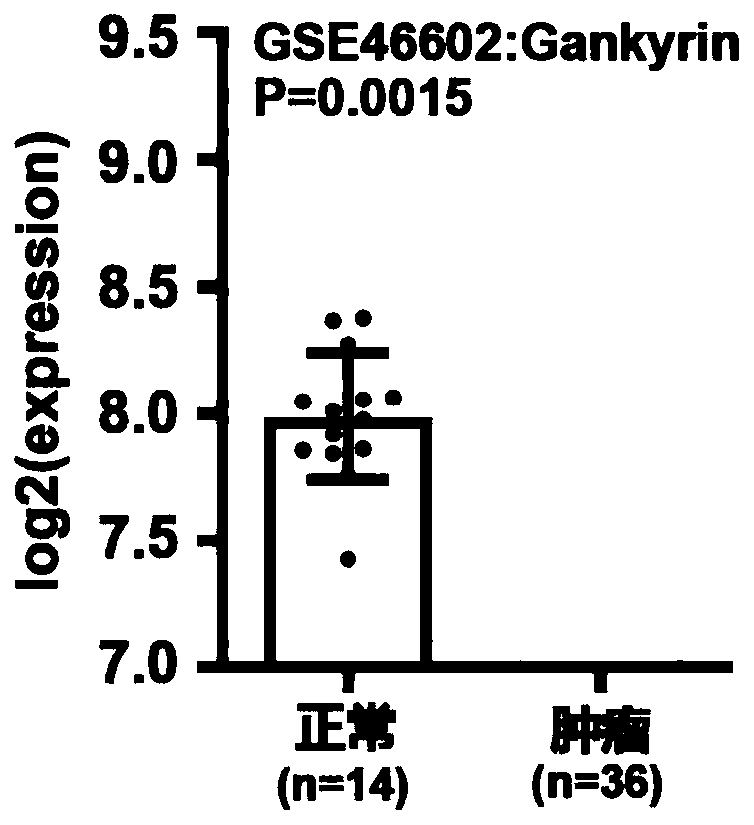
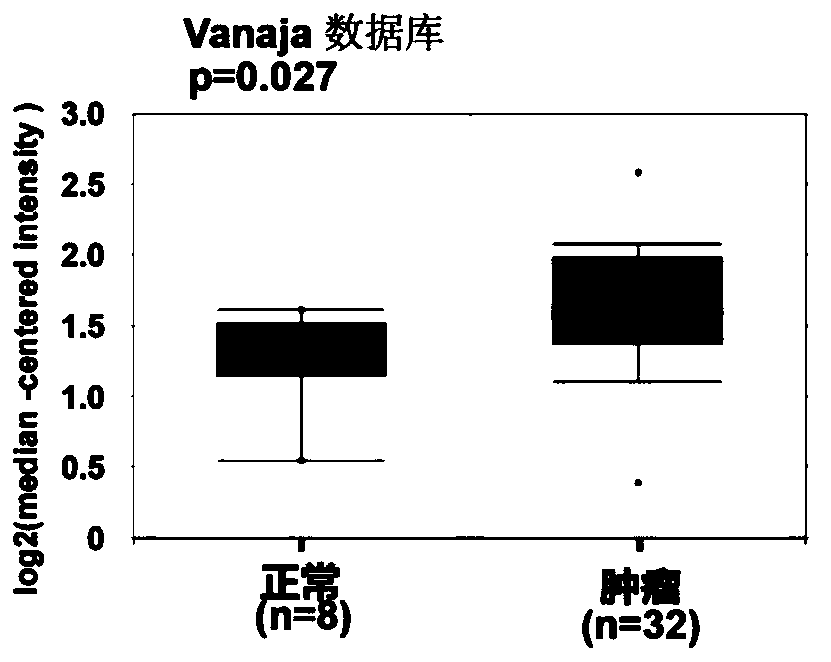
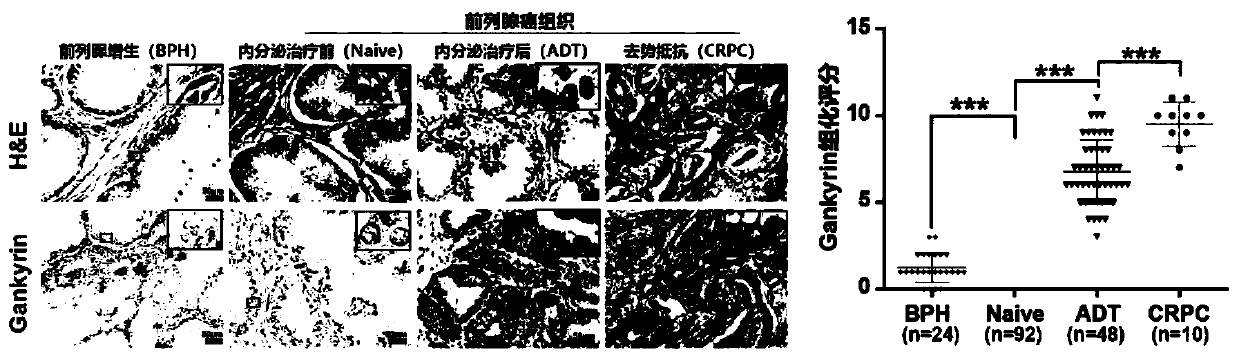
Application of Gankyrin protein as novel molecular marker in prognosis evaluation of prostate cancer
PendingCN111596059AEffectively predict treatment prognosisDisease diagnosisStainingAntiandrogen Treatment
The invention relates to the technical field of biology, in particular to a prognosis evaluation method of a Gankyrin protein and a tumor-associated macrophage marker CD68 combined with the Gankyrin protein as a novel molecular marker in a prostate cancer pathological specimen. According to the invention, the capability of Gankyrin as a prognostic typing molecular marker in prostate cancer is verified; gankyrin and CD68 immunohistochemical staining is carried out on a prostate biopsy pathological specimen of a patient; after immunohistochemical scoring, high expression and low expression of Gankyrin and CD68 are distinguished according to corresponding truncation values; patients are divided into three groups, i.e., a high risk (high expression of Gankyrin and high expression of CD68), a medium risk (high expression of Gankyrin or high expression of CD68) and a low risk (low expression of Gankyrin and low expression of CD68), for drug resistance and transfer risk of anti-androgen therapy.
Owner:SHANGHAI CHANGHAI HOSPITAL
A multiplex immunohistochemical analysis kit for liver cancer and its use and application
ActiveCN109884303BGood treatment effectStrong detection signalBiological testingMultiplexCARCINOMA LIVER
The invention provides a multiple immunohistochemical analysis kit for liver cancer, and a using method and application thereof, and relates to the technical field of multiple immunohistochemistry. The multiple immunohistochemical analysis kit limits that monoclonal antibody groups comprise a first monoclonal antibody group and a second monoclonal antibody group; the first monoclonal antibody group comprises a PanCK monoclonal antibody, a PDL1 monoclonal antibody, a CD8 monoclonal antibody, a CD66B monoclonal antibody and a CD163 monoclonal antibody; the second monoclonal antibody group comprises a PanCK monoclonal antibody, a PDL1 monoclonal antibody, a CD8 monoclonal antibody, a PD1 monoclonal antibody and a CD68 monoclonal antibody. According to the multiple immunohistochemical analysiskit provided by the invention, the paraffin sections of the human liver tissue in vitro are used as samples for detecting, and thus the effectiveness of the immune checkpoint inhibitor acting on theliver cancer is predicted.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD +2
Methods of treatement of cancer disease by targetting tumor associated macrophage
InactiveUS20190271702A1Reduce the number of cellsHigh frequencyOrganic active ingredientsImmunoglobulins against animals/humansAbnormal tissue growthTumor-associated macrophage
The present invention relates to methods of treatment of cancer by targeting tumor associated macrophages. The inventors investigated specific marker exposed on the surface of the macrophage associated tumor in order to detect and target TAMs. They showed that sideroflexin 3, which is absent in normal macrophage, is expressed by tumors associated macrophage. The relates to a method for identifying tumor-associated macrophage (TAM) in a sample comprising the steps of i) detecting the cell surface expression of CD163, CD68, and SFXN3 markers on the cell population contained in the sample and ii) concluding that the cells expressing CD163, CD68, and SFXN3 markers are the TAMs.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
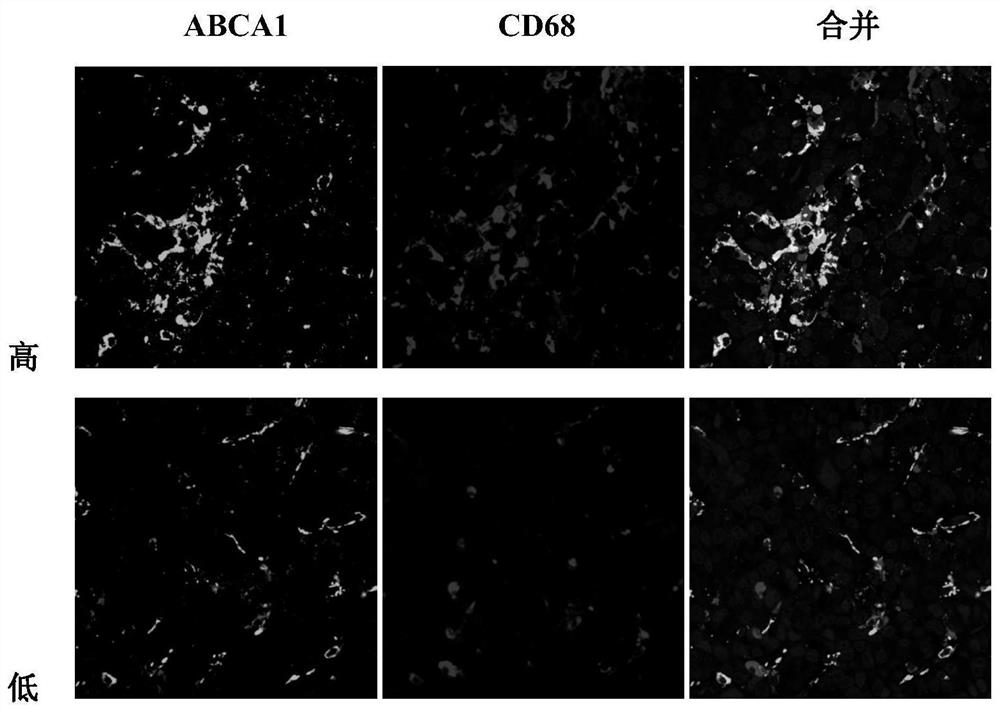
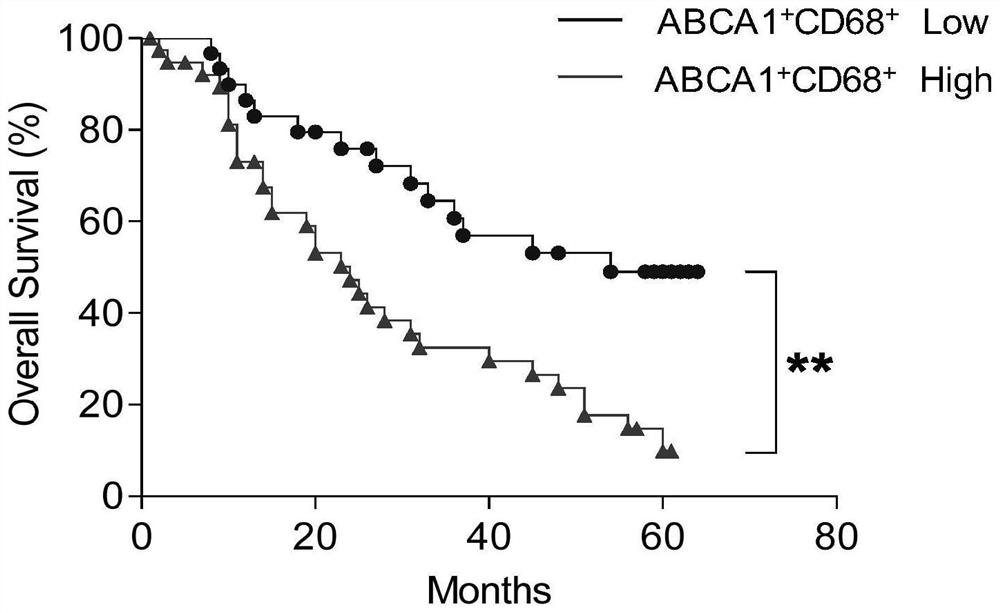
Application of ABCA1 and CD68 as prognosis markers in preparation of liver cancer prognosis prediction kit
ActiveCN112946269AThe test method is matureEasy to detectDisease diagnosisBiological testingAntiendomysial antibodiesHorse radish peroxidase
The invention discloses an application of ABCA1 and CD68 as prognosis markers in preparation of a liver cancer prognosis prediction kit. The research shows that the prognosis survival of a patient can be clinically and effectively predicted in an auxiliary manner by detecting the co-expression ratio of CD68 and ABCA1. The invention also provides a double-antibody combined fluorescence detection kit for prognosis prediction after liver cancer resection, which comprises a first antibody of the combination of ABCA1 and CD68 antibodies, a second antibody marked by horse radish peroxidase, and a tyramine signal amplification reagent. The kit can detect the expression proportion of macrophage ABCA1 in tissues by adopting a double-antibody combined tyramine signal amplification method, and has the characteristics of objectivity, accuracy and high efficiency. The kit is applied to detection of the expression mode of macrophages ABCA1 infiltrated in liver cancer tumor tissues, and can predict survival prognosis of patients.
Owner:SUN YAT SEN UNIV CANCER CENT
Hybridoma cell line, cd68 monoclonal antibody, preparation method and application
ActiveCN108330107BEasy to identifyClear stained backgroundBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsHigh titerMonoclonal
Owner:富恩生物技术(成都)有限公司
A breast cancer 21 gene detection kit and detection method thereof
ActiveCN108624697BIncrease the Tm valueAccurate dataMicrobiological testing/measurementBAG1Cancer research
The invention discloses a breast cancer 21 gene detection kit and a detection method thereof. The kit comprises primers and probes for detecting genes ACTB, GAPDH, RPLPO, GUS, TFRC, CD68, BAG1, GSTM1,HER2, GRB7, MMP11, CTSL2, KI67, STK15, CCNB1, MYBL2, Survivin, ESR1, PGR, BCL2 and SCUBE2. Two pairs of primers and two specific probes are respectively designed for 21 genes in the kit, a primer andprobe liquid of each gene is placed in one reaction tube, a same buffer solution and a procedure are adopted for QPCR (Quantitative Polymerase Chain Reaction) amplification, the operation is simple and convenient to carry out, the detection time can be shortened, and clear and accurate results can be achieved; the kit is wide in application, and applicable to both scientific research and clinicaldetection and applicable to single or multiple samples, and rapid and accurate detection results can be achieved.
Owner:MICROPROBE MEDICAL TECH SHANGHAI CO LTD
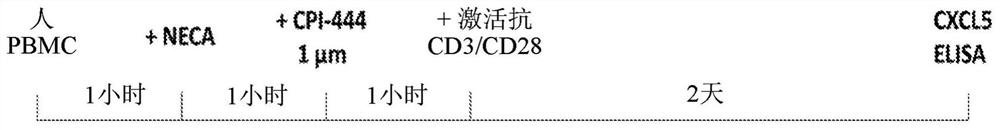
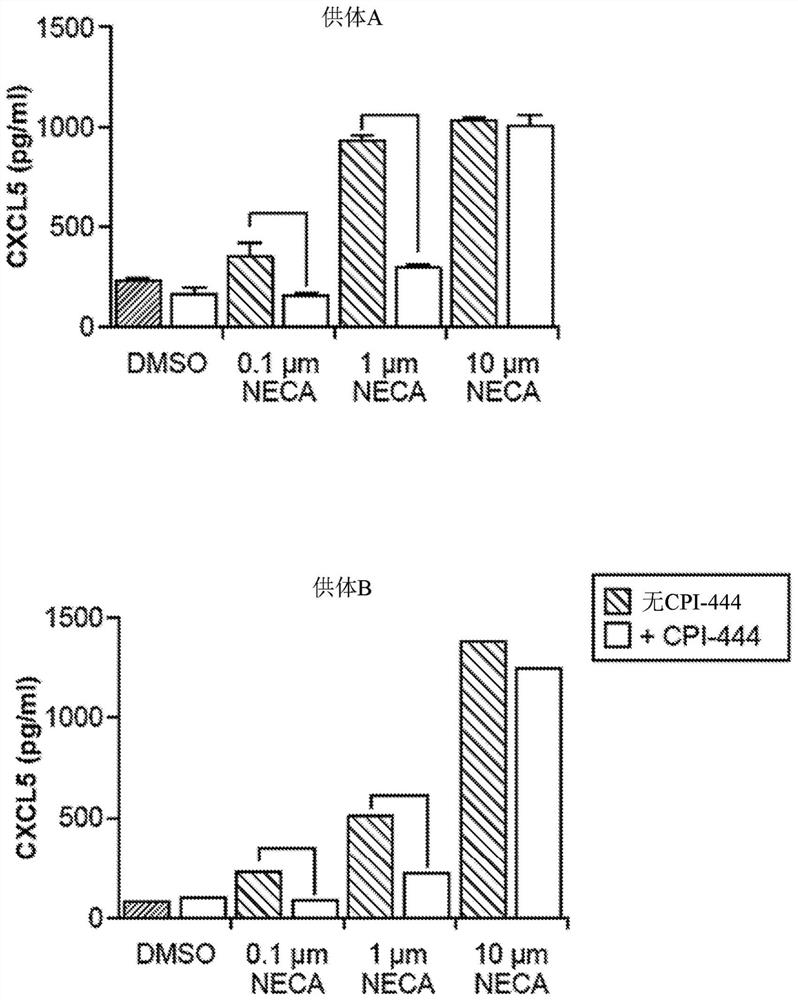
Methods for detecting and treating cancers having adenosine pathway activation
InactiveCN112601552AOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsRHCGCCL7
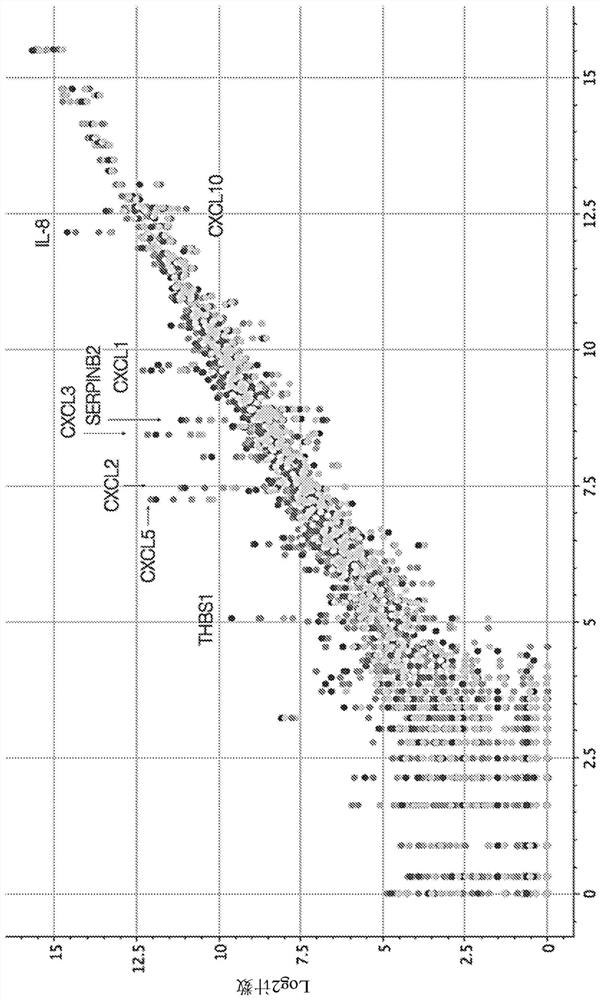
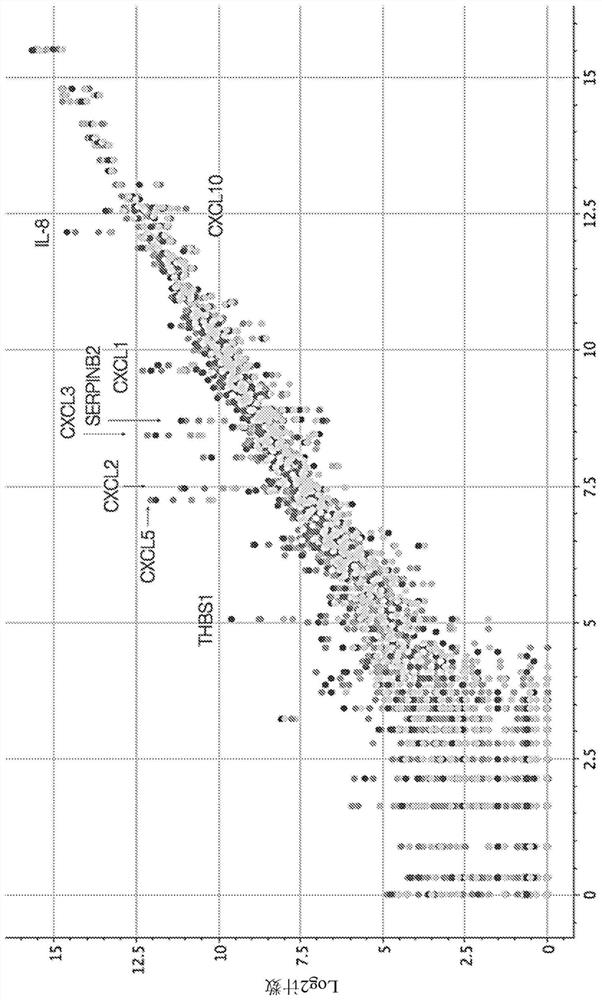
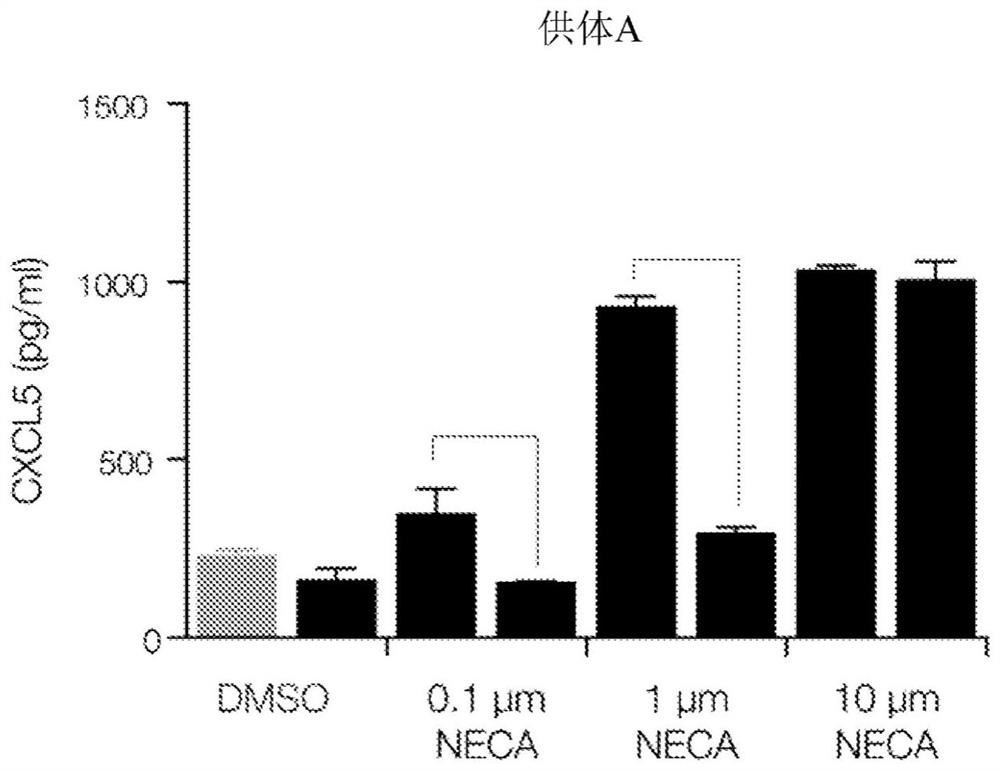
This disclosure relates to methods for detecting a level of expression of one or more genes (or proteins) in a subject having or suspected of having cancer, and optionally treating the subject with anadenosine pathway antagonist, for example an adenosine A2A receptor (ADORA2A) antagonist, to treat the cancer. The genes (or proteins) include, without limitation, CD68, CD163, LBP, CCL2, CCL3, CCL7,CCL24, CCNE1, CD 14, CD300E, CD86, CD93, CLEC5A, CSF3, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8, DFNA5, ECEL1, EPB41L3, EHF, FUT7, GALM, GBP6, GPR157, HAS1, IL1A, IL-1[beta], IL23, IL24, IL5, IL6, IL8, INHBA, LAP3, LAYN, LOC100505585, MRPL11, NID1, OST4, PADI2, PID1, PLAUR, PPBP, PTGS2, RHCG, SERPINB2, SLC11A1, SLC7A7, SPON1, ST6GALNAC2, TBX21, THBS1, C1R, C1S, C4BPA, CCL11, CCL20, CXCL16, CXCL2,HAMP, HSD11B1, ITGAM, LIF, SAA1, TFRC, TLR5, TNFSF14, TREM2, APP, ATG10, BCL2, CCL15, CD24, CD46, CD59, CREB5, CX3CL1, CXCL14, CYFIP2, DEFB1, DPP4, ECSIT, EPCAM, IFIT1, IGF1R, ITGA6, ITGB3, MAP2K4, MAPK1, MASP1, PPARG, RORC, SPA17, STAT5B, TOLLIP, AKT3, BMI1, CD 164, CD34, CDH5, CREB1, DOCK9, ENG, HMGB1, ITGA1, JAM3, MAF, MAPK3, MAPK8, MCAM, MFGE8, NOTCH1, NRP1, PRKCE, SMAD2, TAL1, THY1, TNFSF12,TRAF6, TXNIP, VEGFA, S100A8, and / or WDR83OS.
Owner:CORVUS PHARMACEUTICALS INC
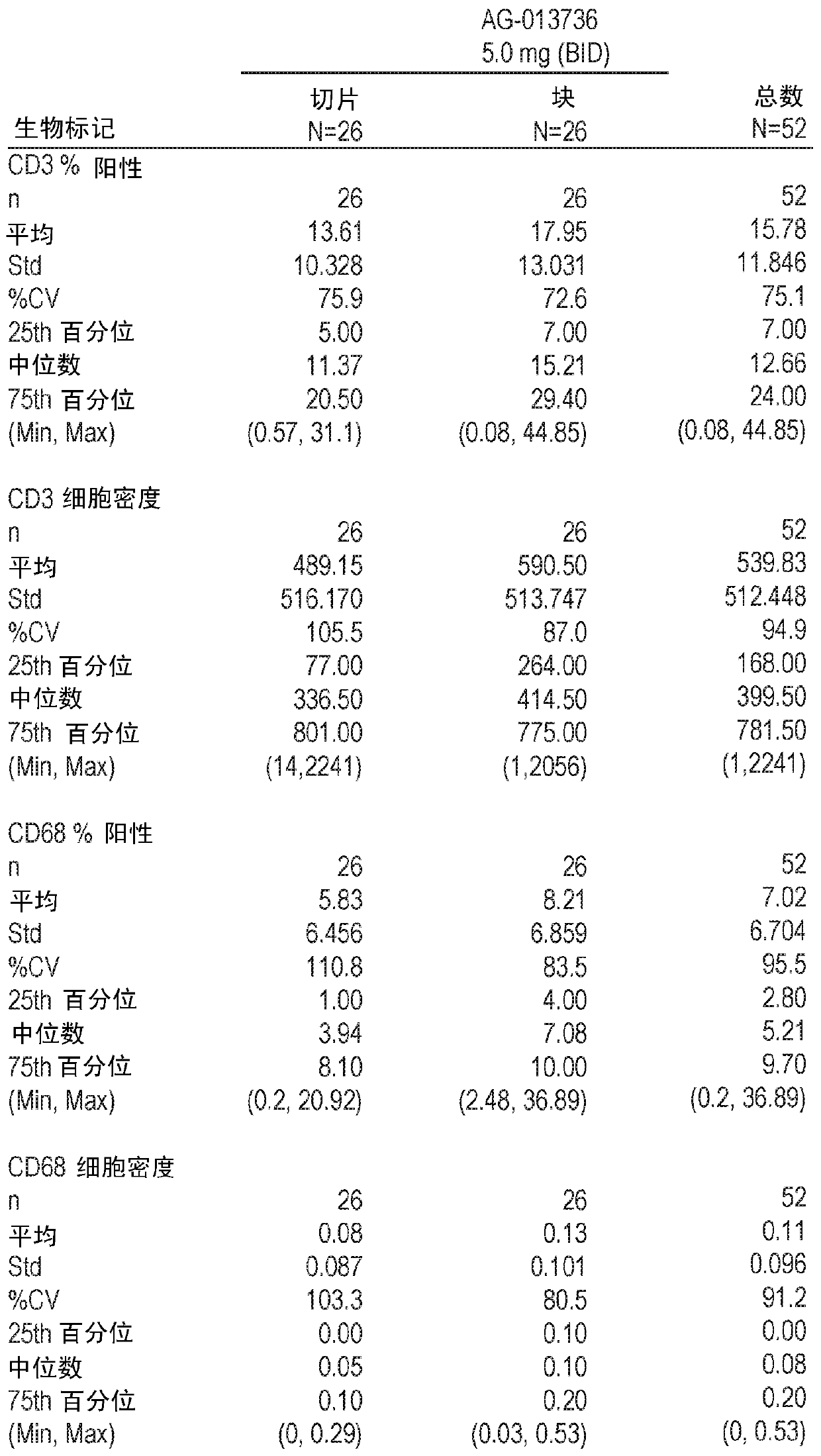
cancer treatment
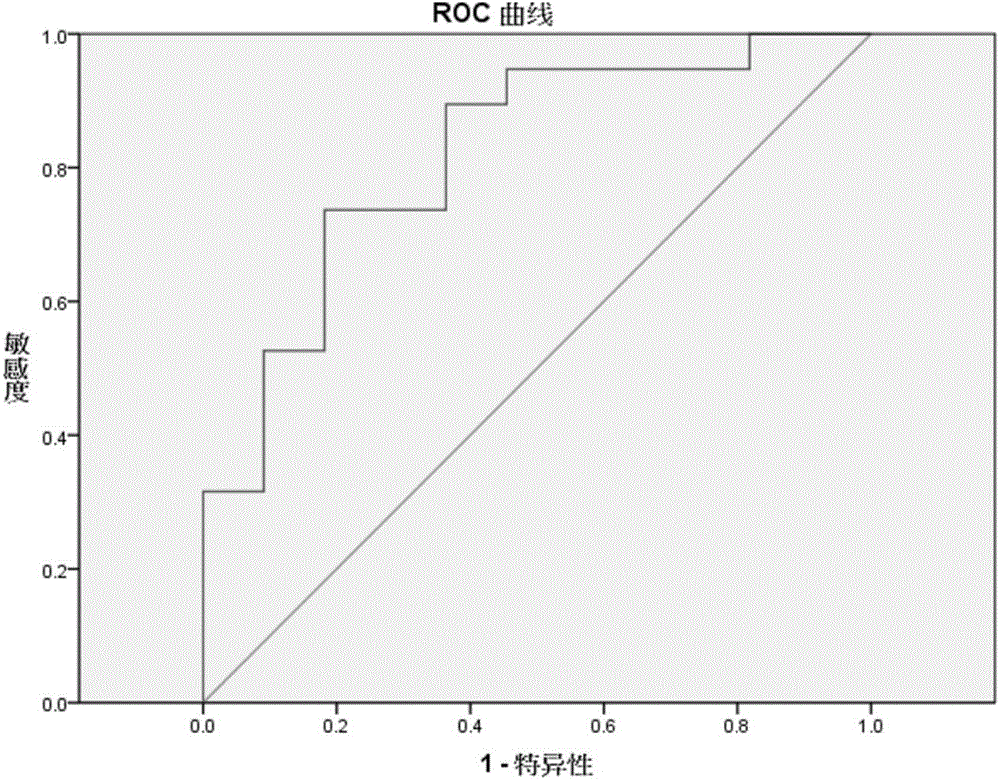
The invention discloses a diagnostic method for predicting whether human tumors are sensitive to axitinib treatment and a method for treating human tumors. These methods are based on measuring the expression level of the CD68 polypeptide in a tumor-derived tissue sample. CD68 expression levels can be measured using immunohistochemistry, wherein the percentage of CD68 positive cells in the tumor and the density of CD68 positive cells can be determined.
Owner:PFIZER INC
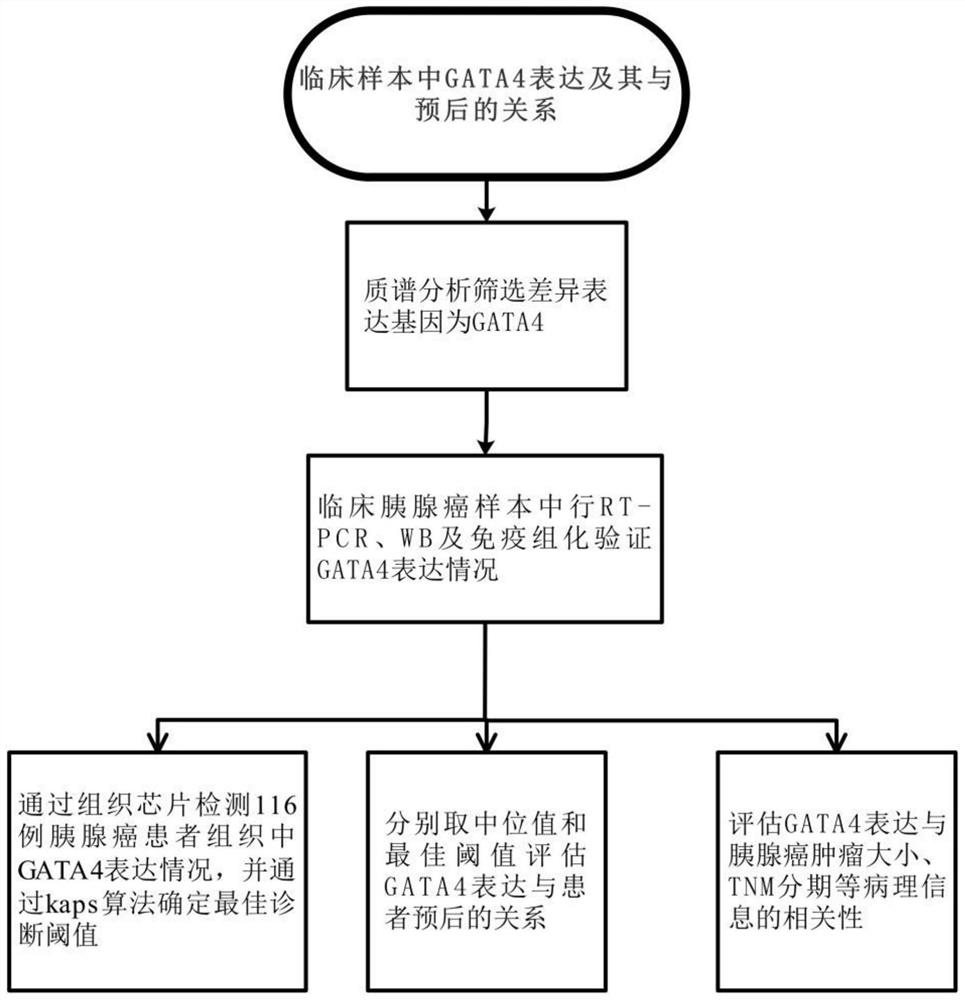
Application of diagnostic marker GATA4 in pancreas inflammatory cancer transformation
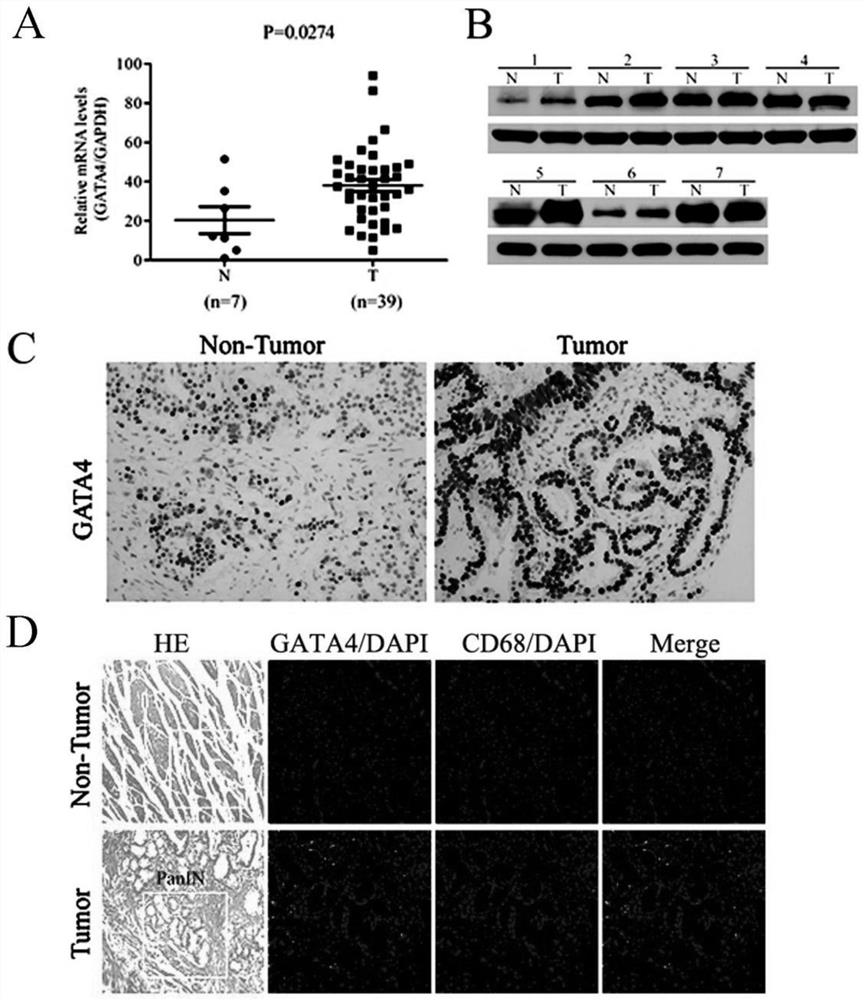
InactiveCN112626210AImprove accuracyImprove featuresMicrobiological testing/measurementDigestive systemPancreas CancersOncology
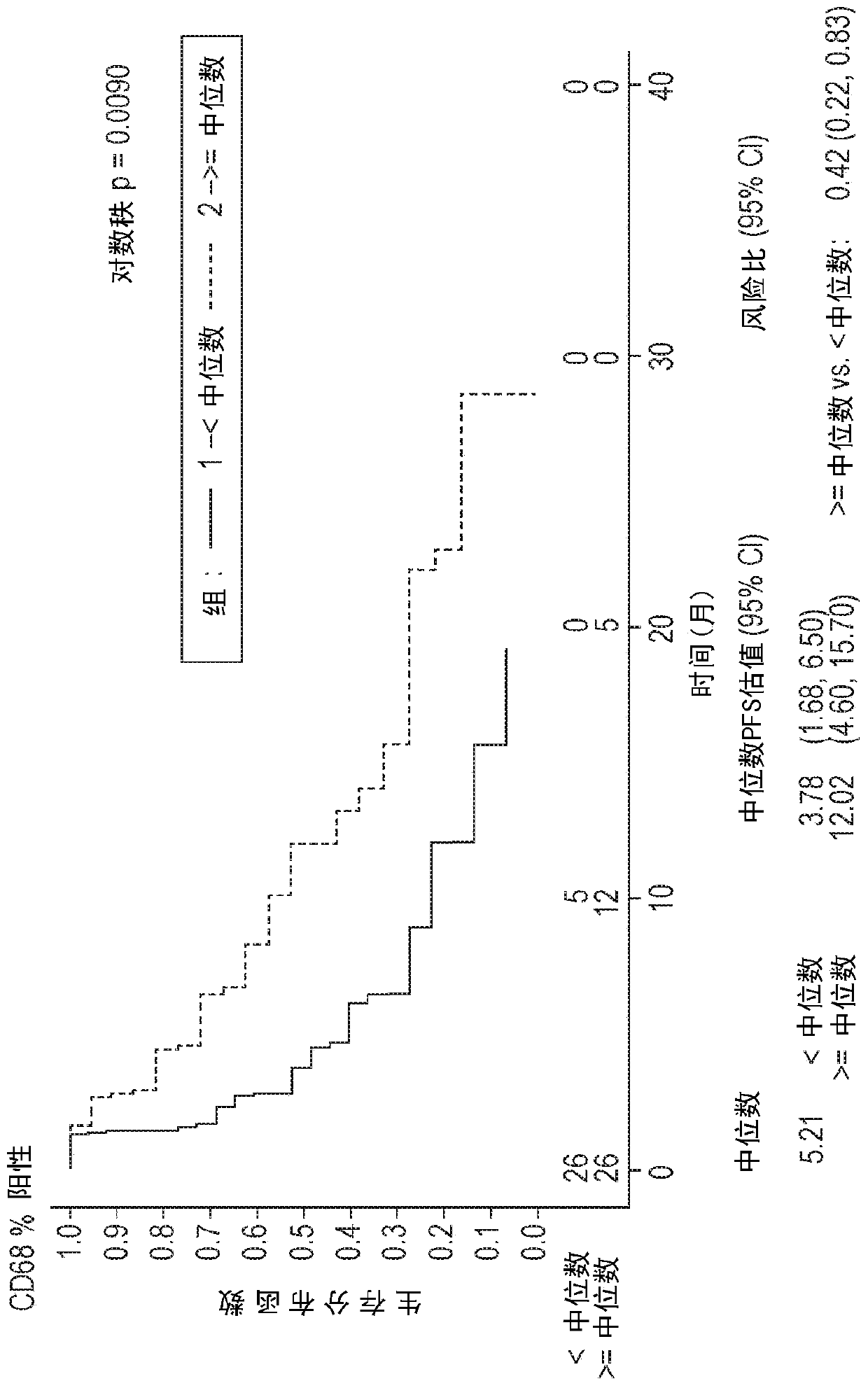
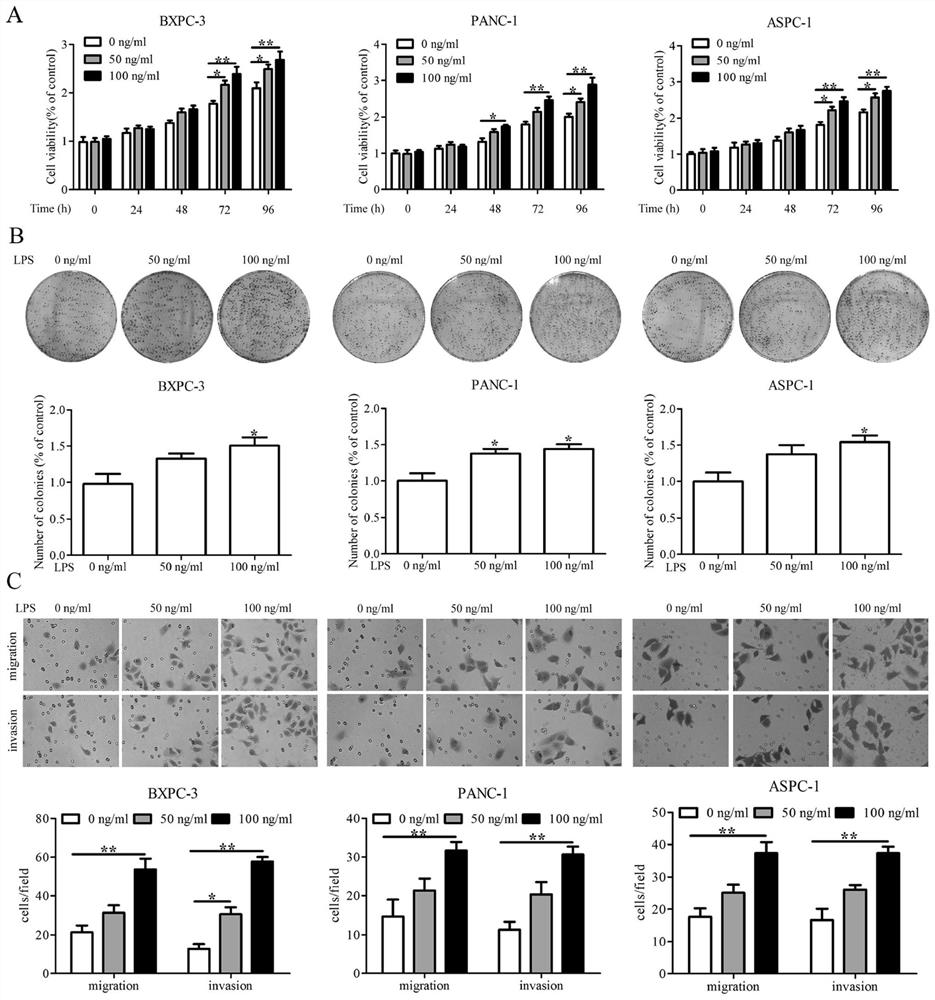
The invention relates to application of a diagnostic marker GATA4 in pancreas inflammatory cancer transformation. According to the invention, immunofluorescence detection finds that the level of the GATA4 is positively correlated with the CD68 level, and prompts that the GATA4 may participate in inflammatory regulation in the pancreatic cancer development process. According to the experiment, lipopolysaccharide (LPS) with different concentrations is added into a macrophage culture medium LMCM for inducing THP-1 differentiation to treat pancreatic cancer cells; the result shows that up-regulation of the GATA4 level in the pancreatic cancer cells can be promoted, and proliferation, migration and invasion of the pancreatic cancer cells can be promoted; and meanwhile, overexpression of the GATA4 can obviously promote proliferation, migration and invasion ability of the pancreatic cancer cells induced by the LMCM with different LPS concentrations. This prompts that the GATA4 may be a key factor in the pancreatitis cancer transformation process. The invention provides a method for diagnosing pancreas inflammatory cancer transformation through molecular detection; the diagnosis speed is high; the cost is low; and the result is accurate and reliable.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Breast cancer 21 genetic test reagent kit and application method thereof
InactiveCN111218512AHigh sensitivityStrong specificityMicrobiological testing/measurementOncologyHuman breast
The invention belongs to the fields of biological technology and medicines, and particularly relates to a breast cancer 21 genetic test reagent kit. The breast cancer 21 genetic test reagent kit comprises a specific primer for detecting Ki67,STK15, Survivin, CCNB1, MYBL2, MMP11, CTSL2, GRB7, HER2, ER, PGR, BCL2, SCUBE2, GSTM1, BAG1, CD68, beta-actin, GAPDH, RPLP0, GUS and TFRC genes, a probe, a positive quality control product, common detection assemblies for fluorescent quantitation PCR detection. The invention further provides an application method of the reagent kit. The reagent kit is usedfor assessing the recurring risk and chemotherapy benefit degrees of patients suffering from breast cancer by detecting 21 genes associated with human breast cancer, and the reagent kit has the characteristics of being high in sensitivity, high in specificity, simple in detection operation and the like.
Owner:宁波美丽人生医药生物科技发展有限公司
Application of CXCL10+CD274+CD68 + cell population in preparation of chronic hepatitis B prognosis prediction product
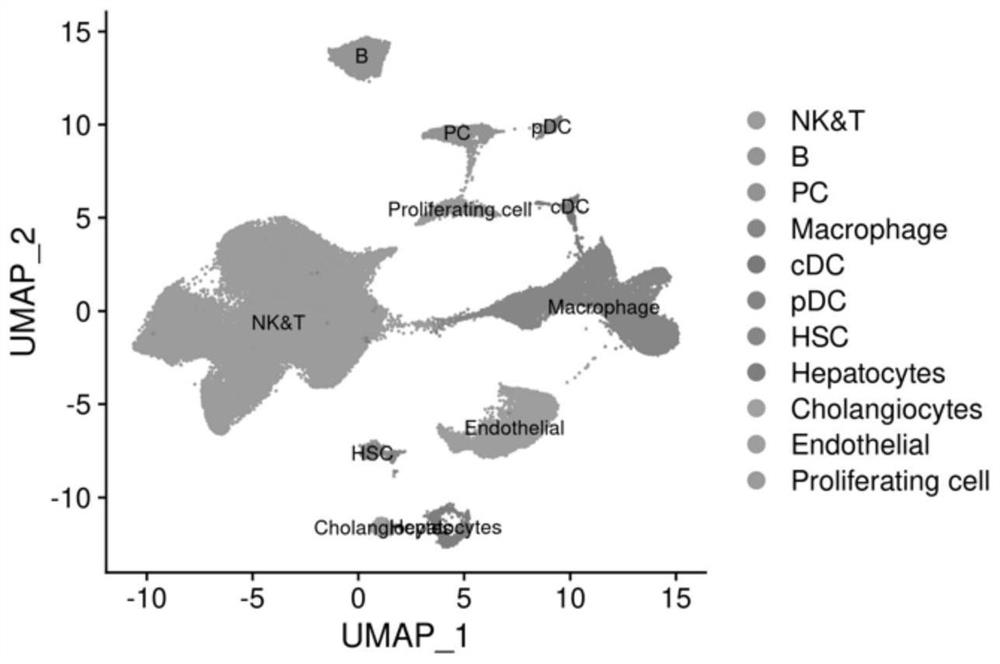
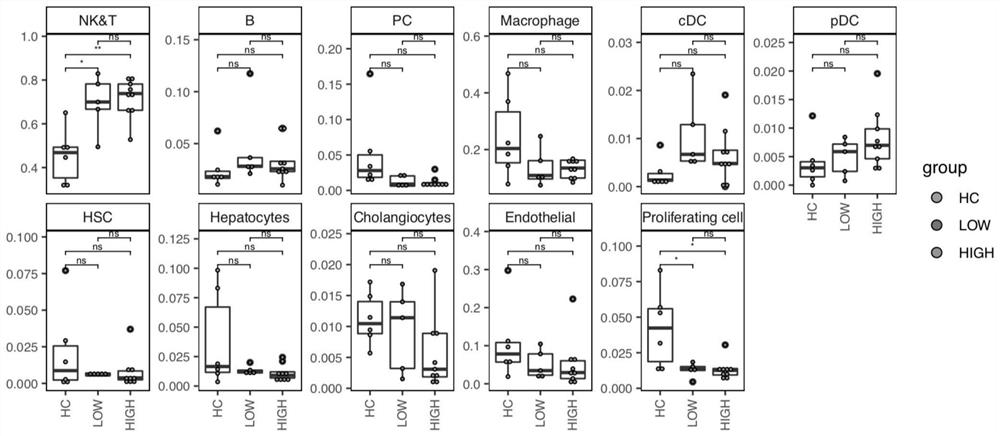
The invention discloses application of a substance for detecting the proportion of a CXCL10 < + > CD274 < + > CD68 < + > cell population in all CD68 < + > immune cells in a sample in preparation of a product for predicting prognosis of a chronic hepatitis B patient. The result of one embodiment of the invention shows that the proportion of CXCL10 < + > CD274 < + > CD68 < + > cell populations in human liver cells in all CD68 < + > immune cells is highly related to virus control and prognosis of chronic hepatitis B.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Methods for detecting and treating cancers having adenosine pathway activation
InactiveCN112654365AOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsRHCGCCL7
The present disclosure relates to methods for detecting expression levels of one or more genes in a subject having or suspected of having a cancer and optionally treating the subject with an adenosine pathway antagonist (e.g., an adenosine A2A receptor (ADORA2A) antagonist) in combination with a PD-1 inhibitor and / or a PD-L1 inhibitor to treat the cancer. The genes comprise, but are not limited to, CD68, CD163, LBP, CCL2, CCL3,CCL7,CCL24,CCNE1,CD14,CD300E,CD86,CD93,CLEC5A, CSF3,CXCL1,CXCL2,CXCL3,CXCL5,CXCL6,CXCL8,DFNA5,ECEL1,EPB41L3,EHF,FUT7,GALM,GBP6,GPR157,HAS1,IL1A,IL-1beta,IL23,IL24,IL5,IL6,IL8,INHBA,LAP3,LAYN,LOC100505585,MRPL11,NID1,OST4,PADI2,PID1,PLAUR,PPBP,PTGS2,RHCG,SERPINB2,SLC11A1,SLC7A7,SPON1,ST6GALNAC2,TBX21,THBS1,C1R,C1S,C4BPA,CCL11,CCL20,CXCL16,CXCL2,HAMP,HSD11B1,ITGAM,LIF,SAA1,TFRC,TLR5,TNFSF14,TREM2,APP,ATG10,BCL2,CCL15,CD24,CD46,CD59,CREB5,CX3CL1,CXCL14,CYFIP2,DEFB1,DPP4,ECSIT,EPCAM,IFIT1,IGF1R,ITGA6,ITGB3,MAP2K4,MAPK1,MASP1,PPARG,RORC,SPA17,STAT5B,TOLLIP,AKT3,BMI1,CD164,CD34,CDH5,CREB1,DOCK9,ENG,HMGB1,ITGA1,JAM3,MAF,MAPK3,MAPK8,MCAM,MFGE8,NOTCH1,NRP1,PRKCE,SMAD2,TAL1, THY1, TNFSF12,TRAF6, TXNIP,VEGFA,S100A8 and / or WDR83OS.
Owner:CORVUS PHARMACEUTICALS INC
Method for sorting macrophages in testis
PendingCN114438029AStrong specificityAccurate and efficient separationCell dissociation methodsBlood/immune system cellsProtein markersCD86
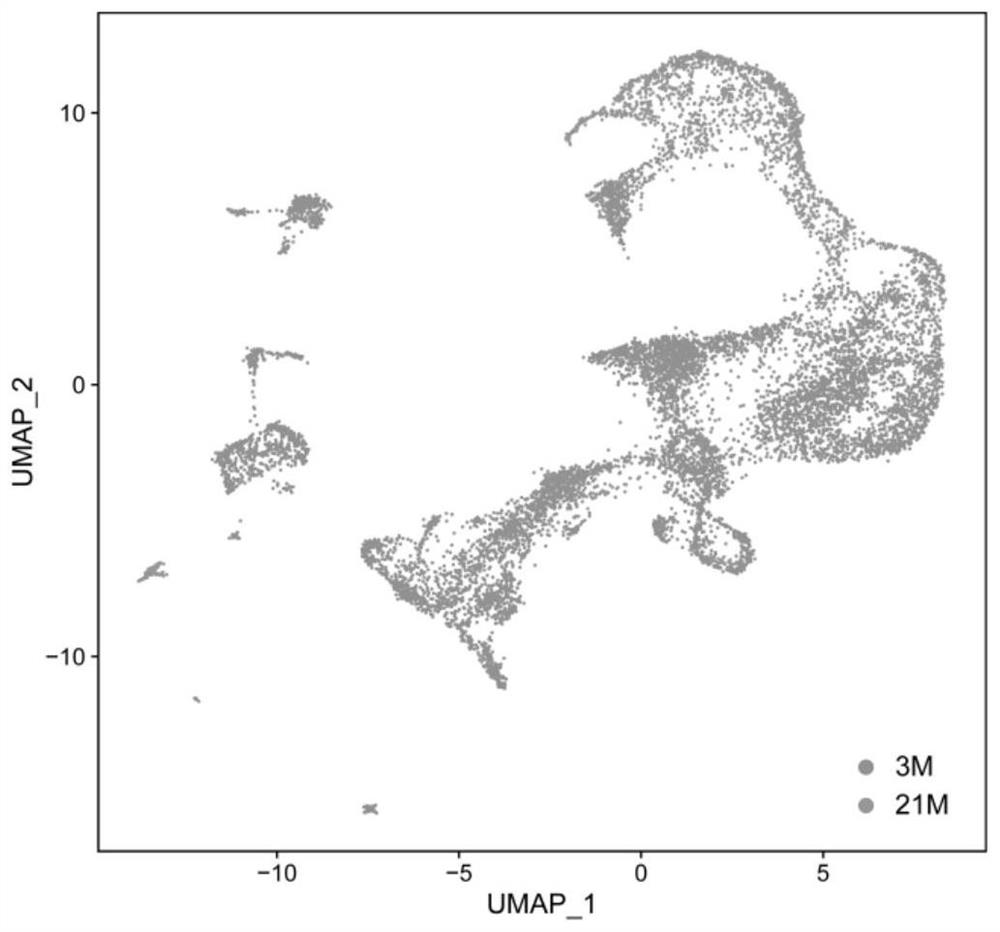
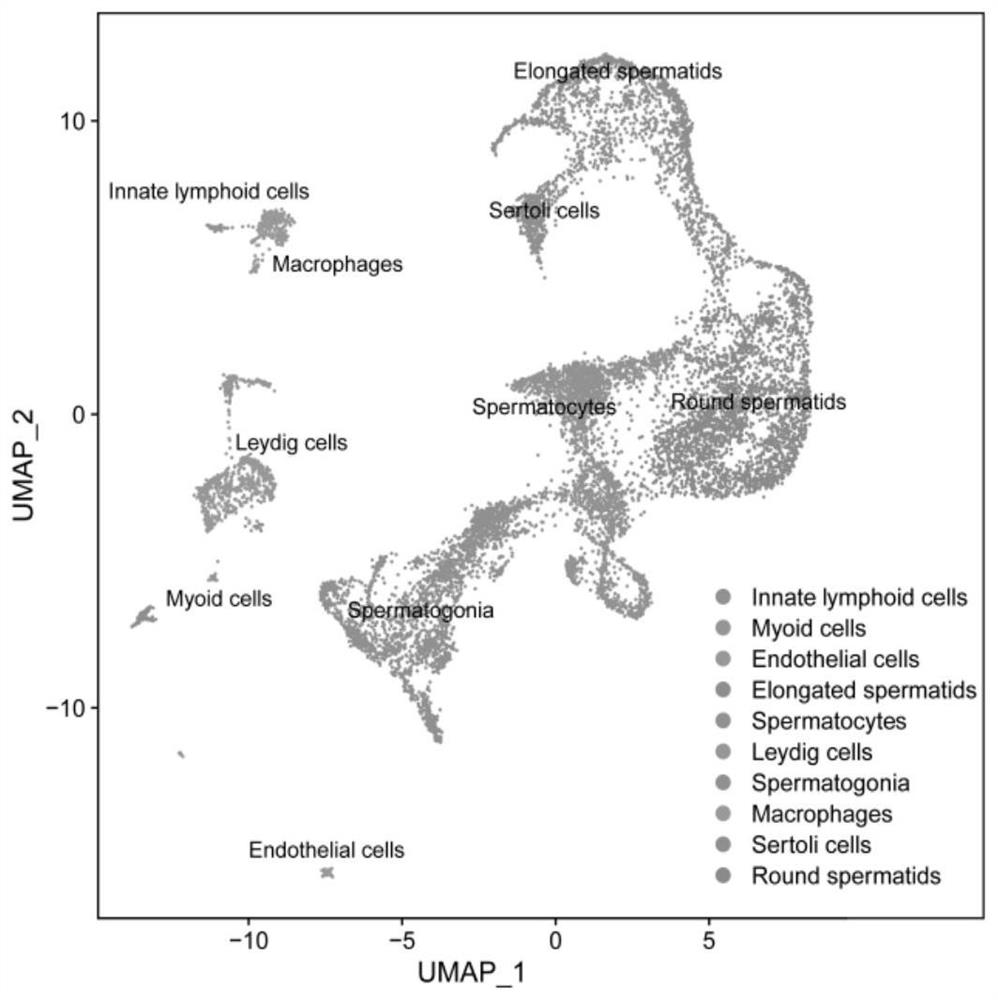
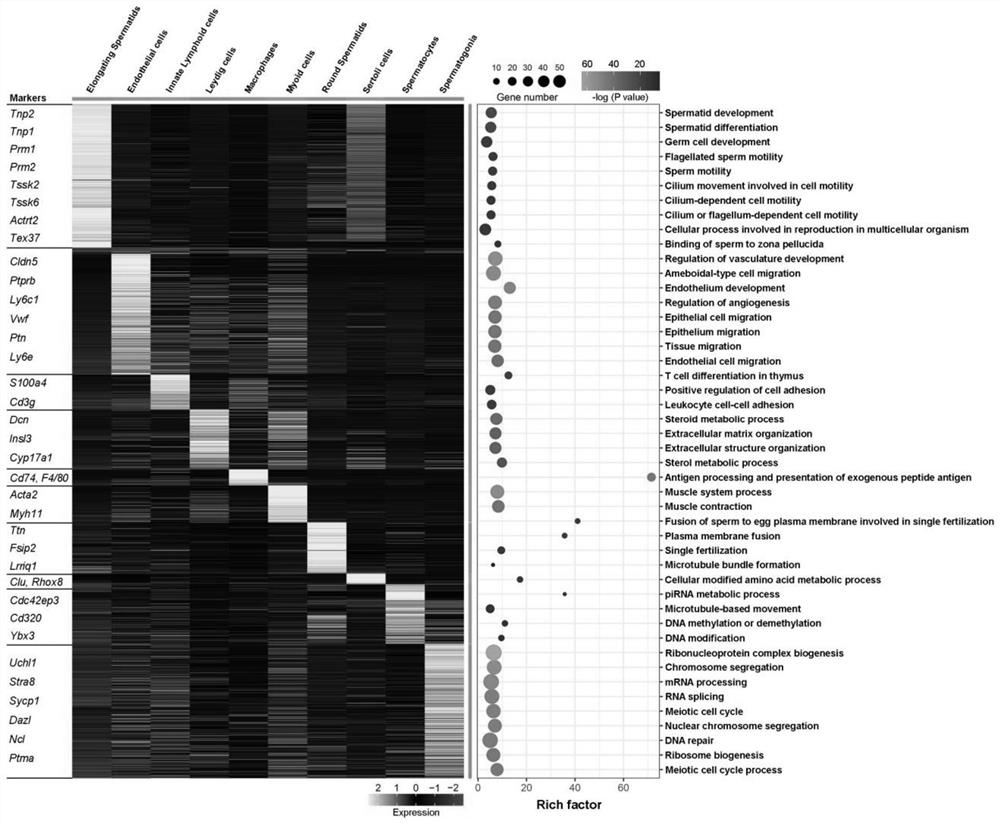
The invention discloses a sorting method of macrophages in testis, and relates to the technical field of biological cells. The invention provides an application of CD74 as a protein marker in macrophage sorting. Through a large number of experimental screening, the inventor finally finds that compared with existing common macrophage protein markers including F4 / 80, CD206, CD86, CD68, CD14, CD163 and CD11b, the testis macrophage protein marker CD74 disclosed by the invention has wider expression in a testis macrophage group, and shows the strongest testis specificity; by adopting the sorting method for the macrophages in the testis, the testis macrophages can be accurately and efficiently separated.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com