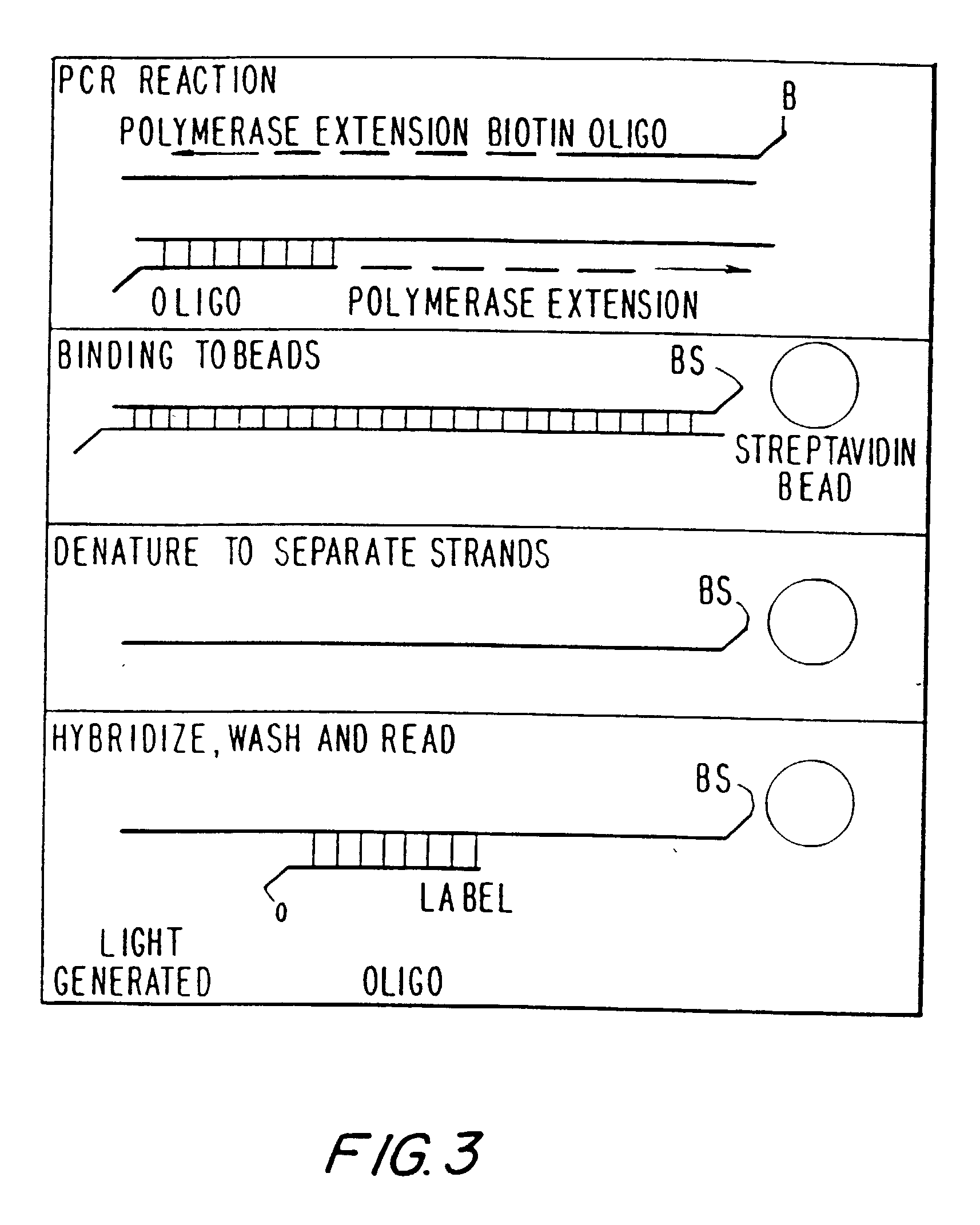
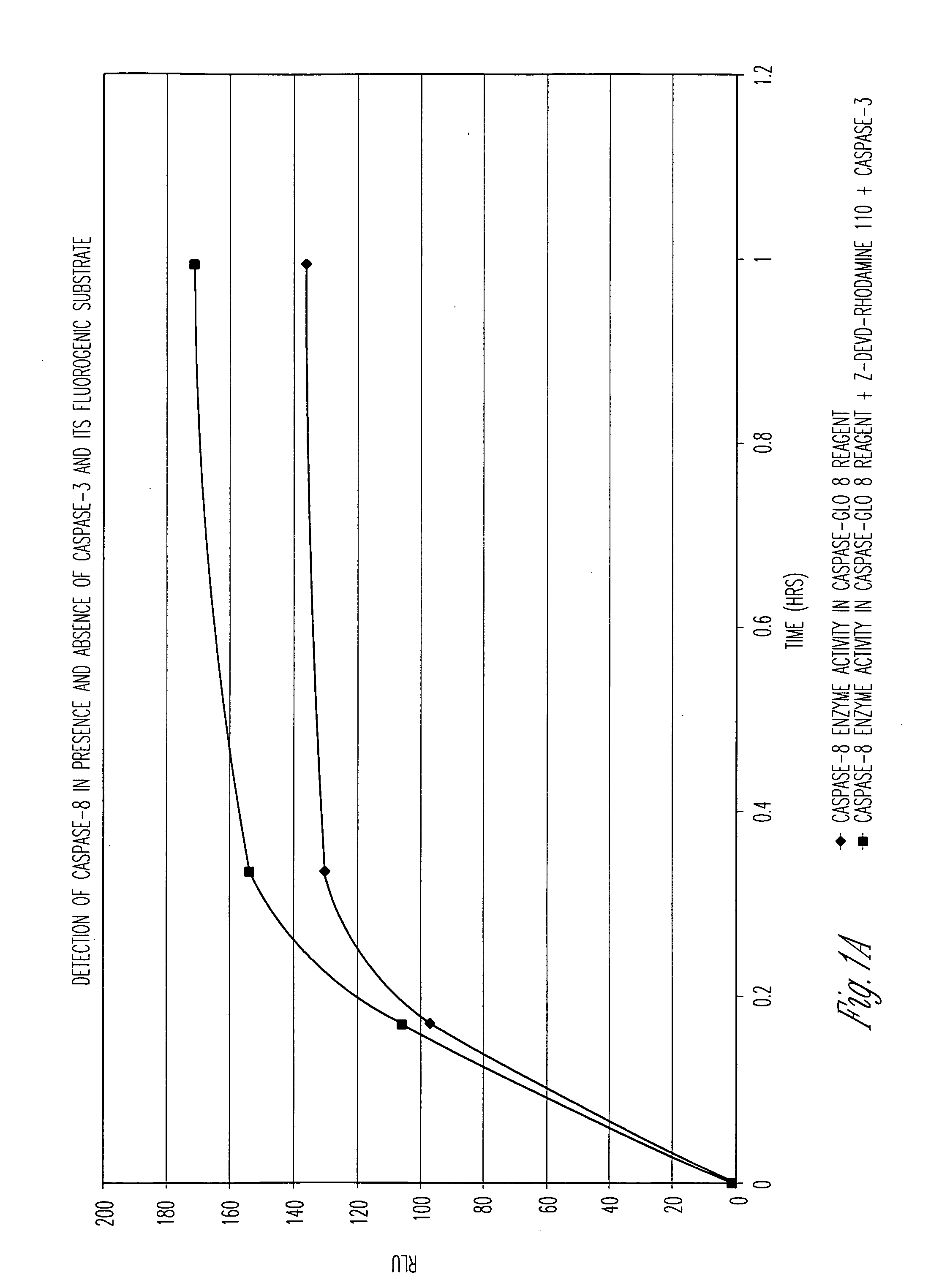
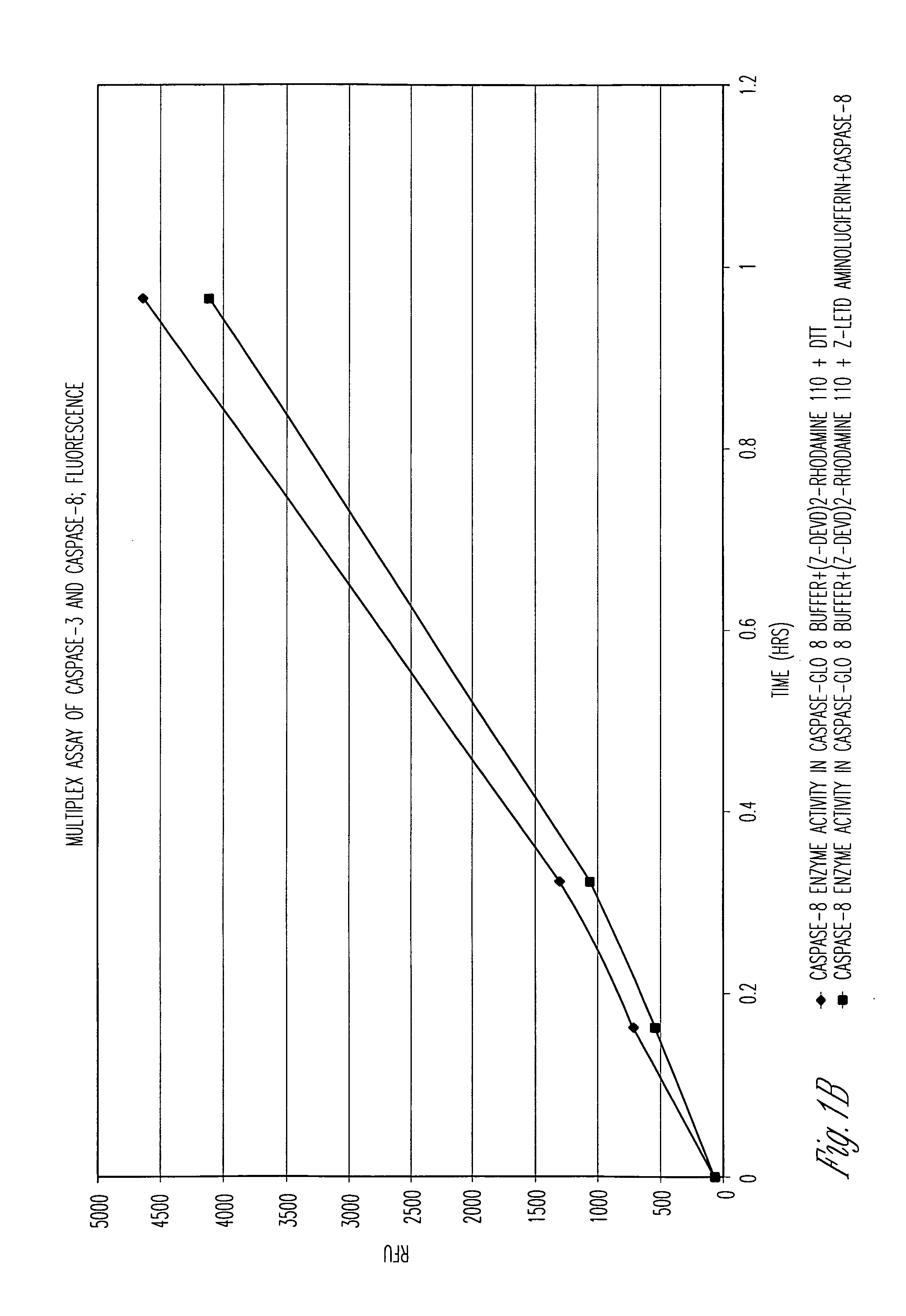
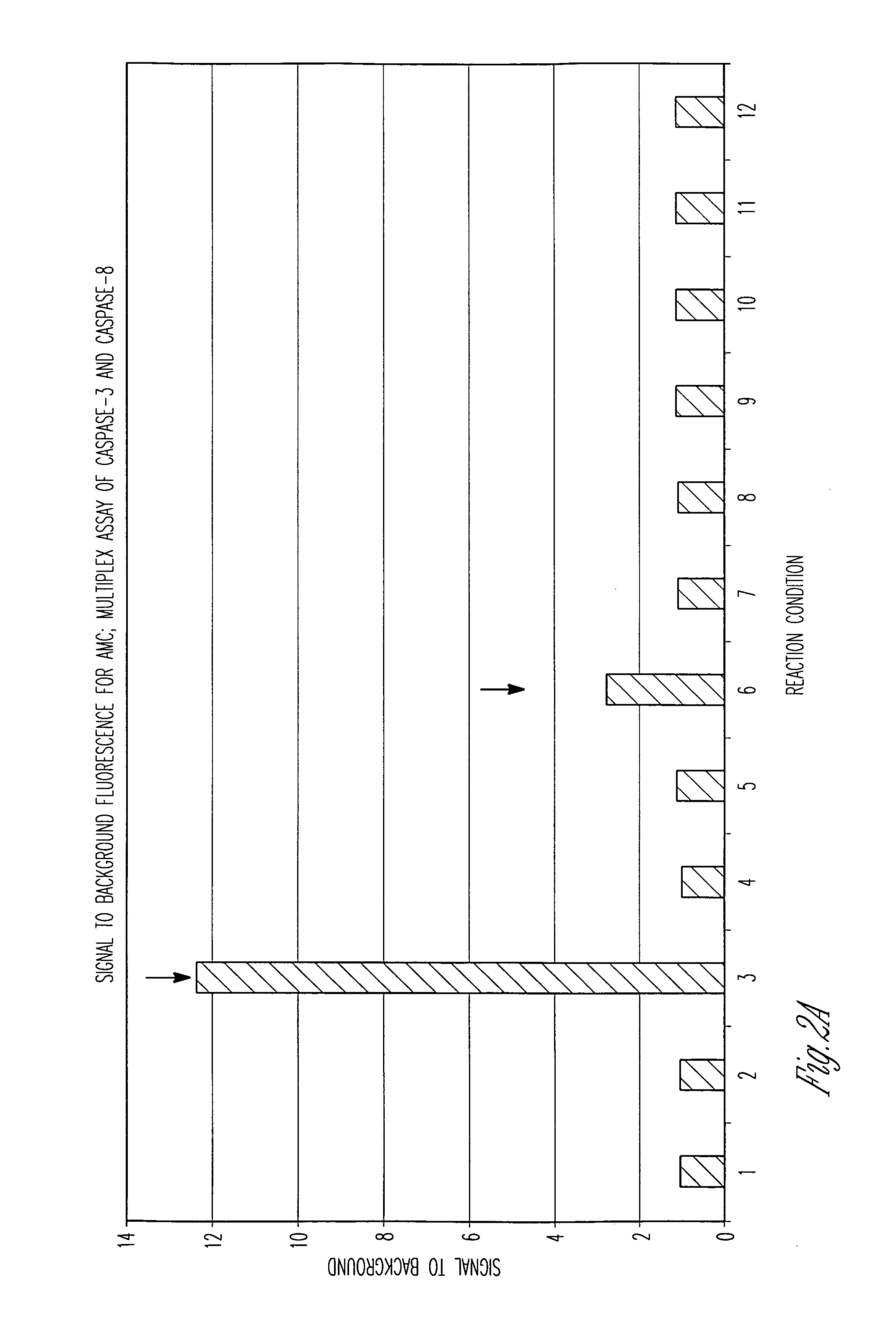
Patents
Literature
44 results about "Luminescent Assays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
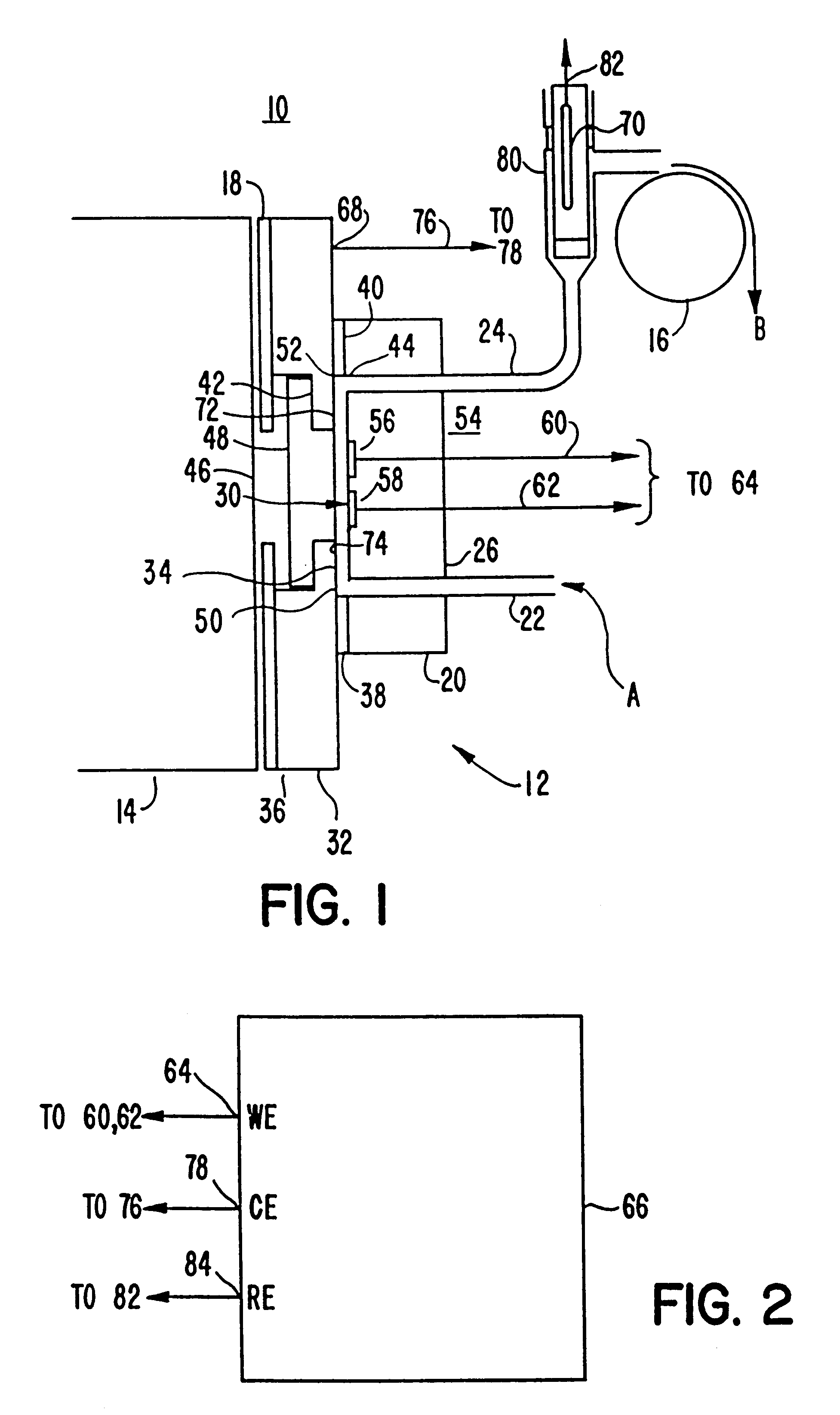
Methods and apparatus for improved luminescence assays
InactiveUS6325973B1High sensitivityFaster assay timeImmobilised enzymesCombination devicesAnalyteElectrochemiluminescence
An apparatus for performing a binding assay for an analyte of interest in a sample based upon measurement of clectrochemiluminescence at an electrode surface comprising: (a) a cell defining a sample; (b) an electrode adjacent a portion of the sample containing volume; (c) a voltage control device for impressing electrochemical energy upon the electrode sufficient to generate luminescence; (d) means for magnetically collecting particles along the electrode surface; and (e) a light detection device for measuring the luminescence.
Owner:BIOVERIS CORP
Stabilized two component system for chemiluminescent assay in immunodiagnostics
The present invention provides stabilized chemiluminescent formulations for use in in vitro diagnostics, including competitive as well as sandwich-type immunological assays. The stabilized assay system may be composed of two components, where the first component may contain a chemiluminescent organic compound, an enhancer, a homogenizing agent, and a suitable buffer with formulations having a pH range from about 7.2 to about 12, and optionally a solubilizing agent. The chemiluminescent system of the present invention is useful in immunoenzymatic analytical procedures, such as immunometric, competitive binding and sandwich type assays. In such immunoassays employing the chemiluminescent system of the present invention, the detectable light signal shows a proportional decay with time in the test samples and standards, so that the decay of the light emitted does not effect the concentration of the analyte measured over the entire analyte measurement range of the immunoassay. This allows accurate measurement of analyte concentrations in a test sample over extended periods of time.
Owner:KALRA BHANU +1
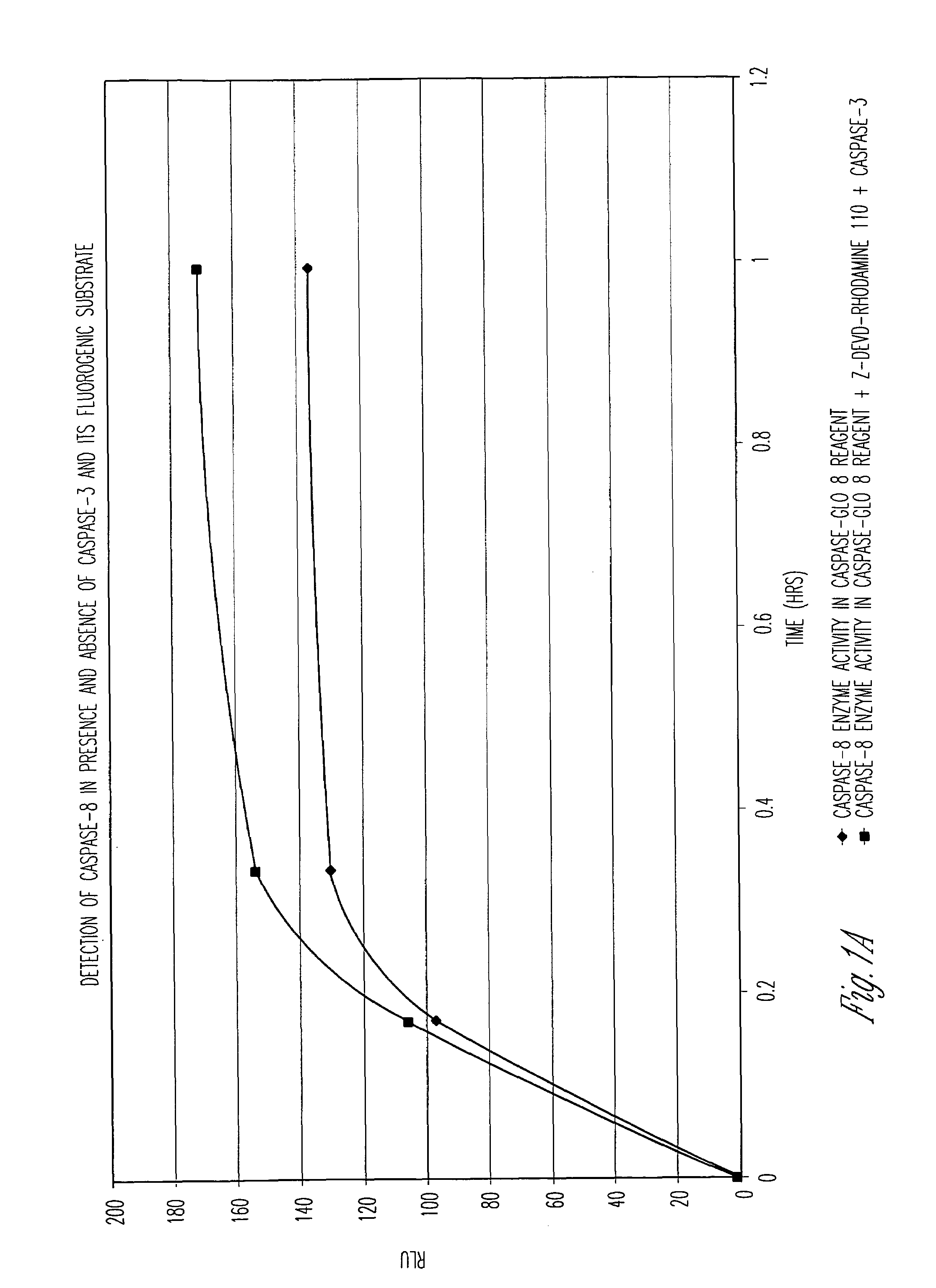
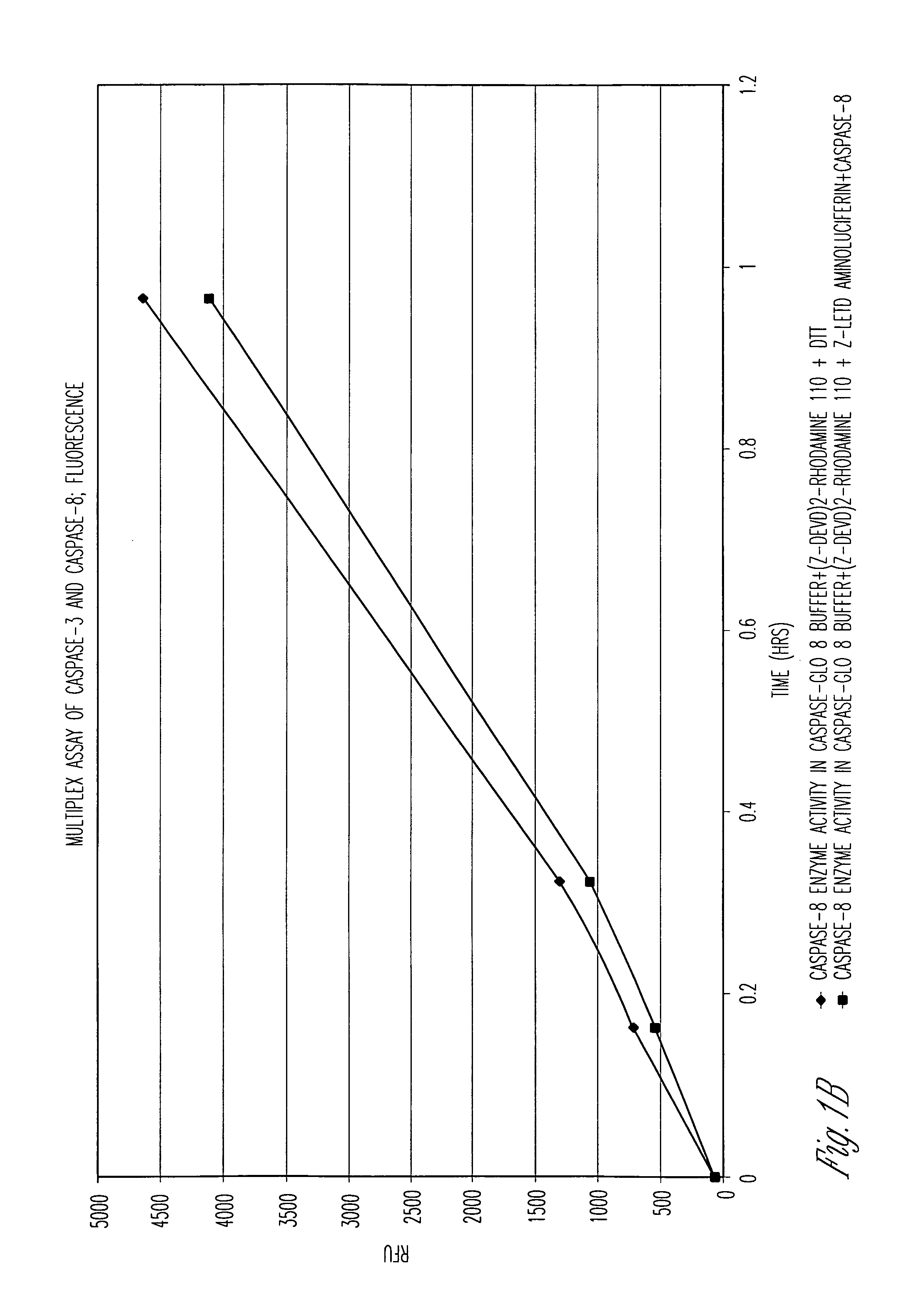
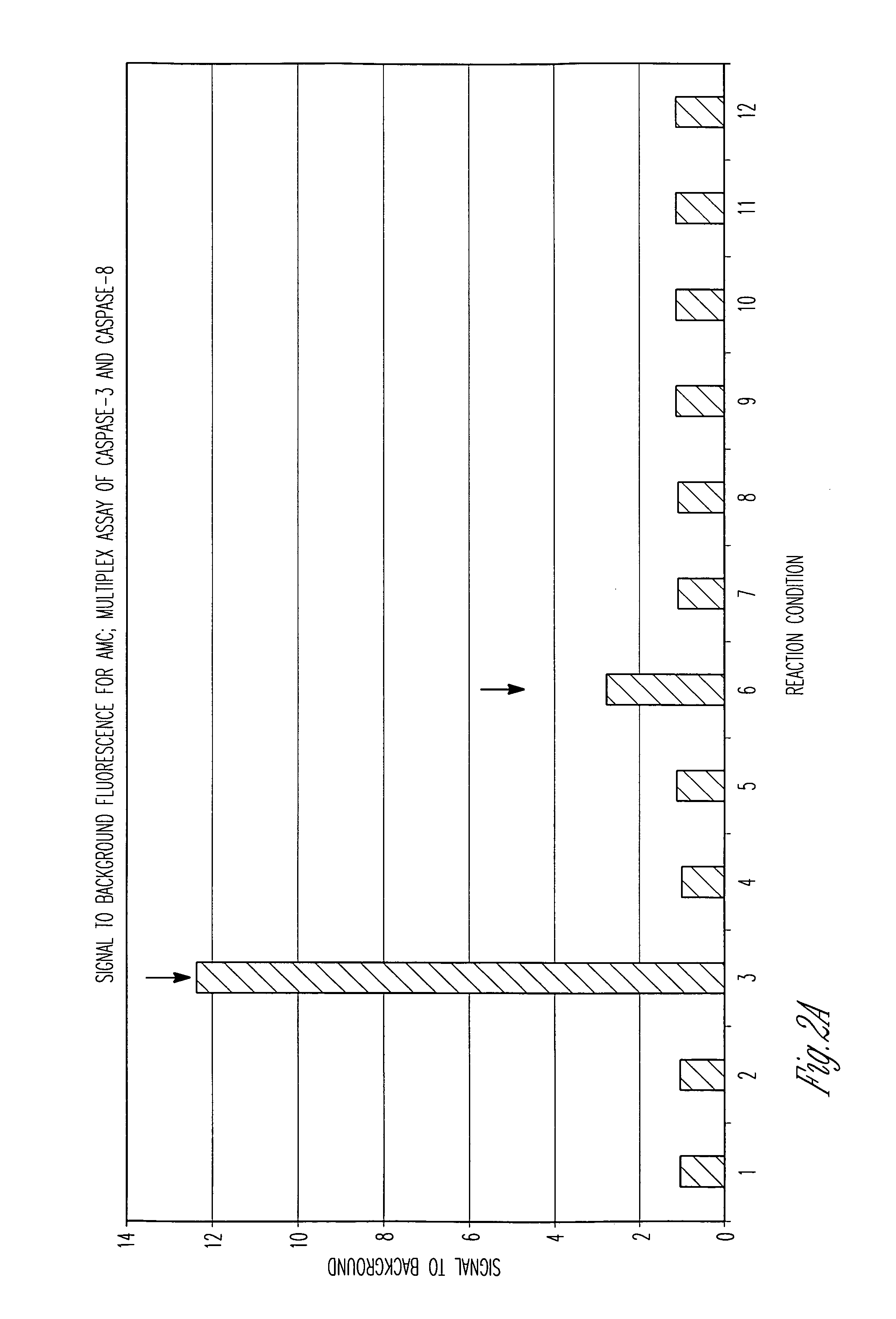
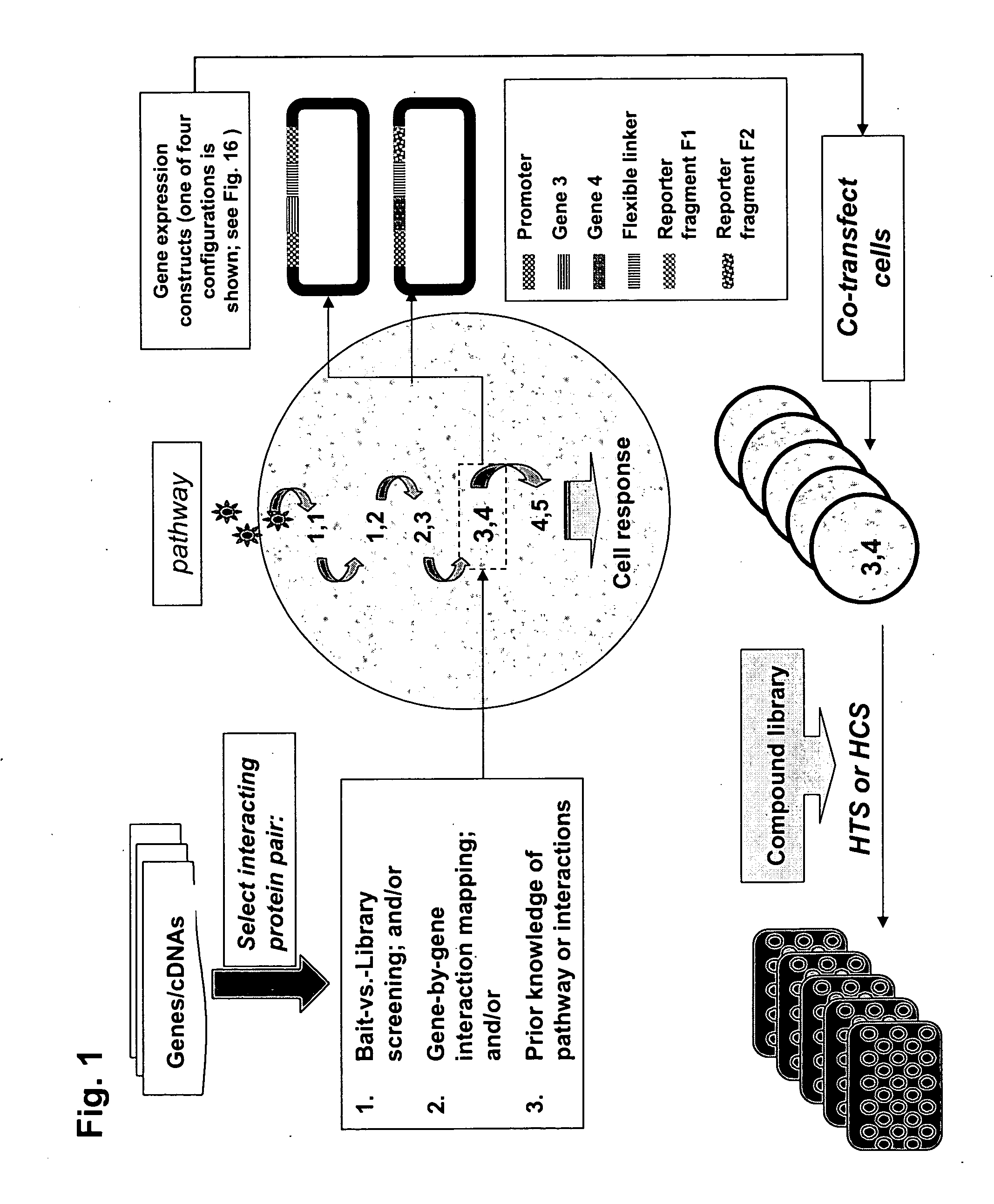
Protein fragment complementation assays for high-throughput and high-content screening
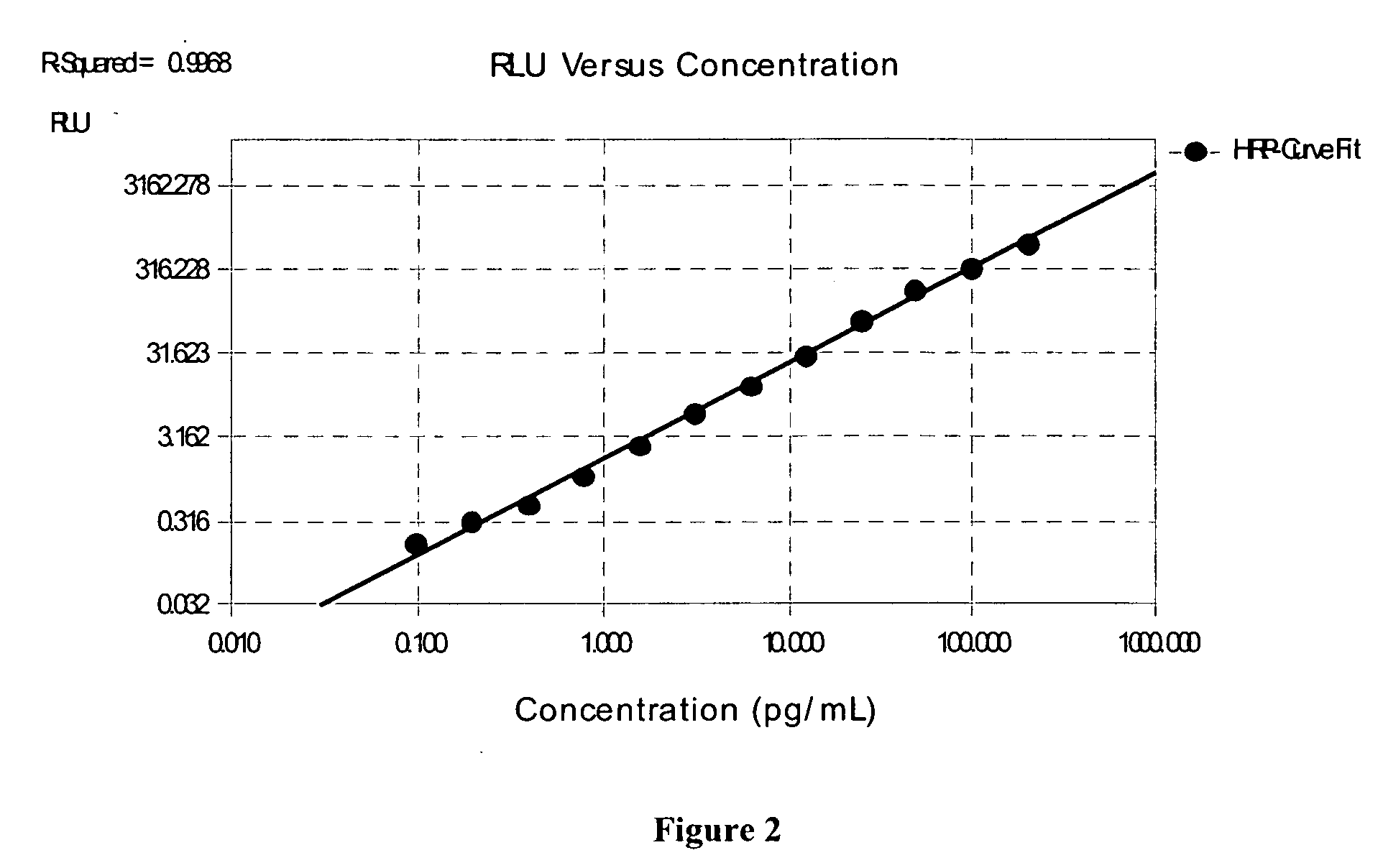
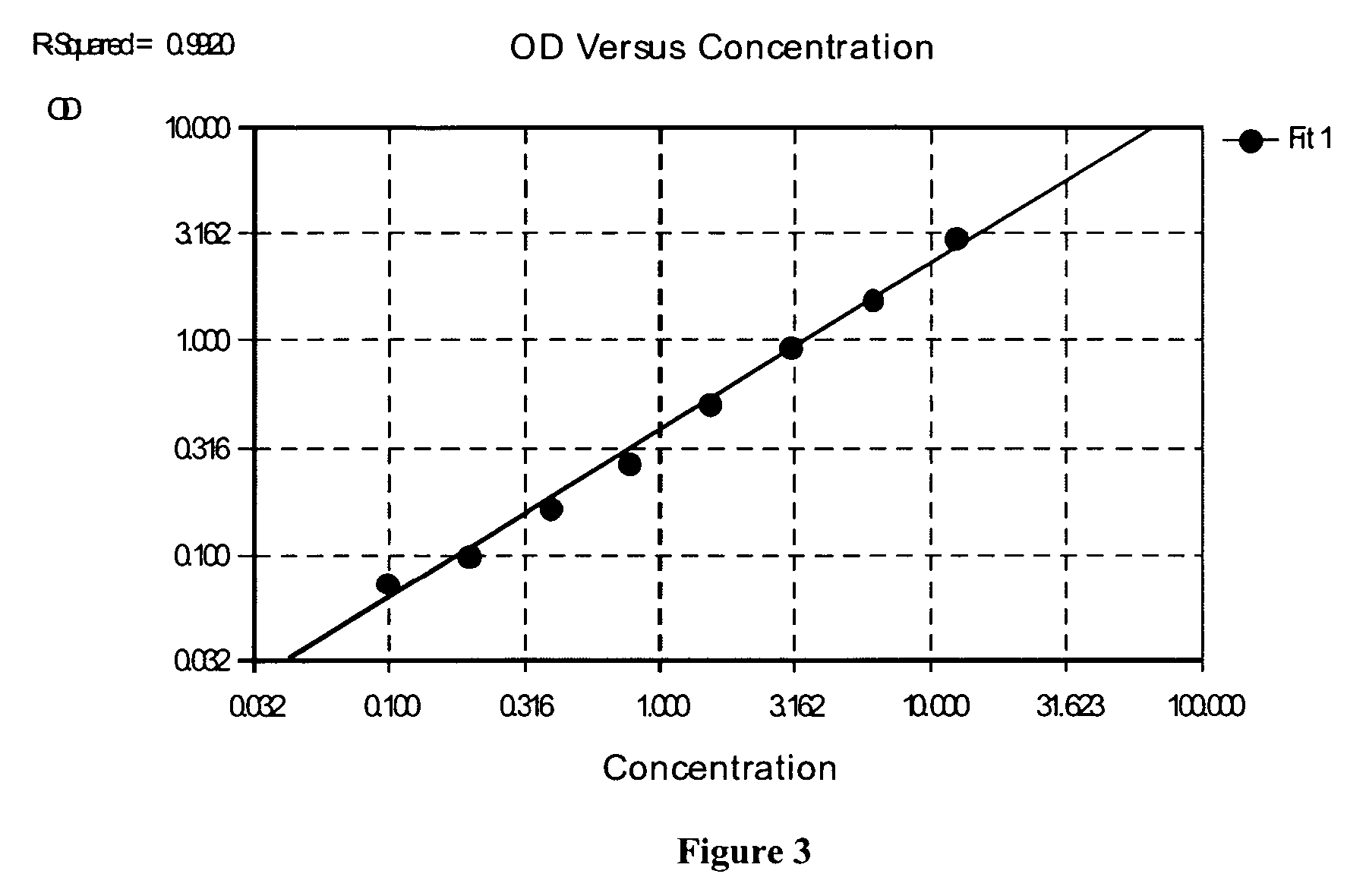
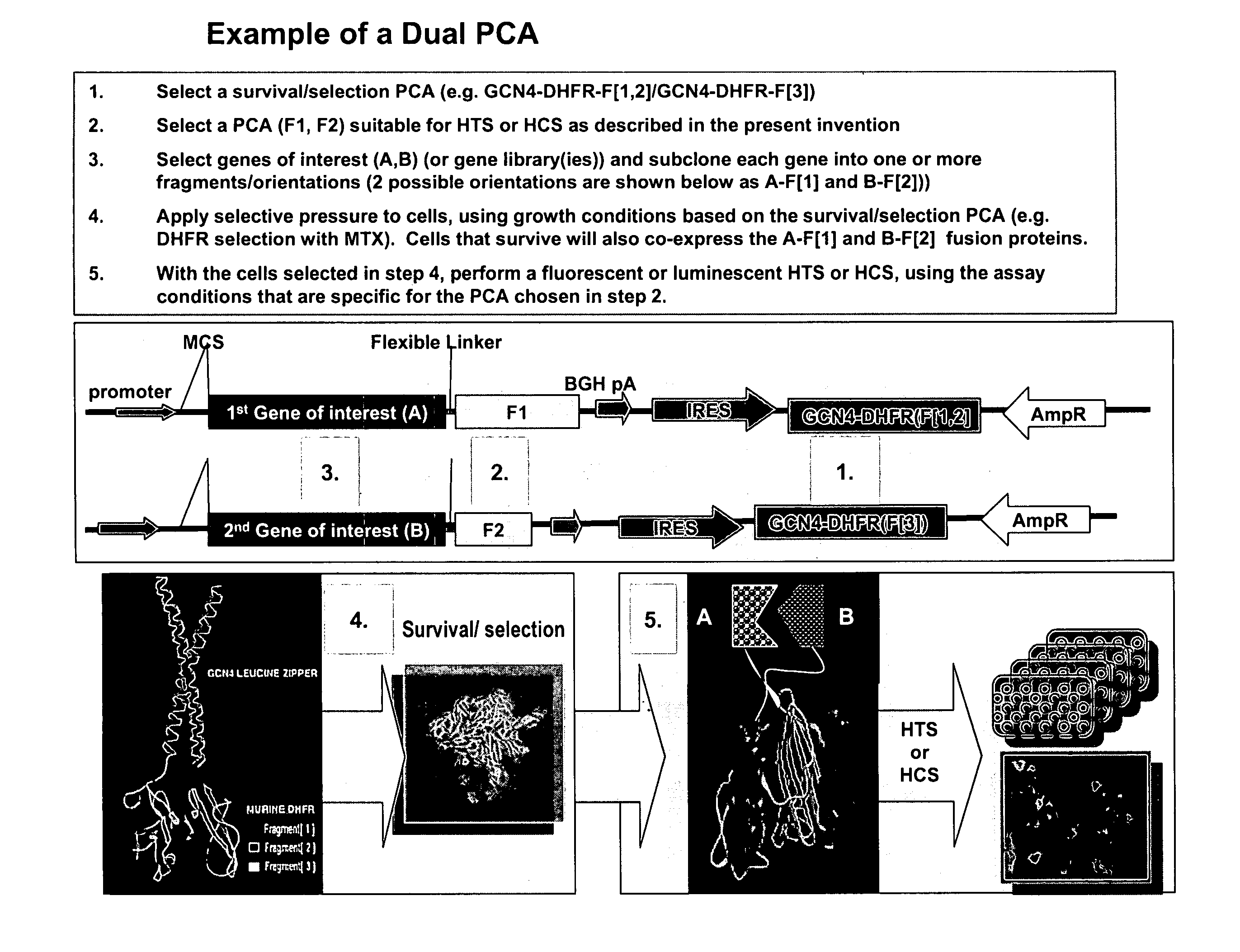
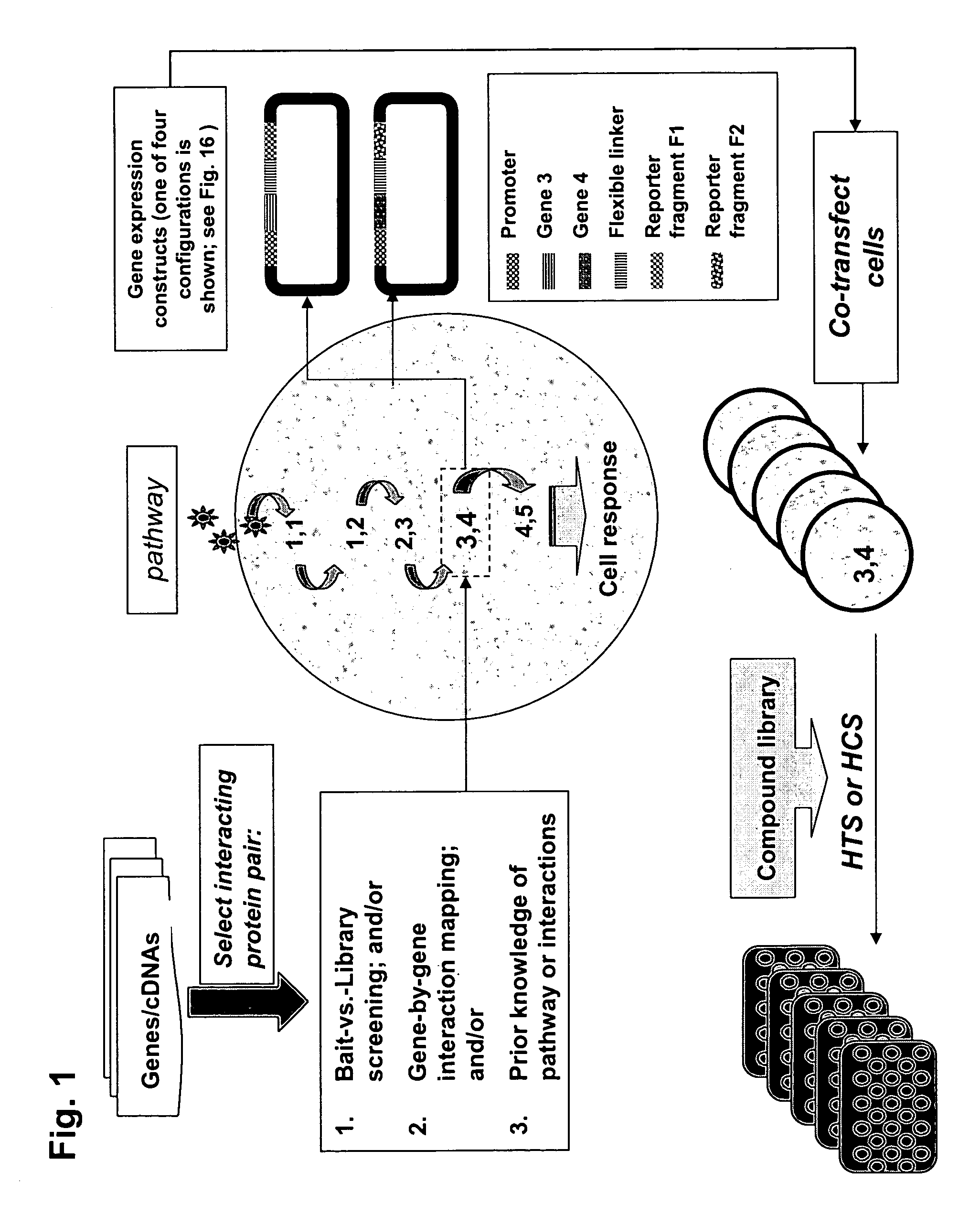
The present invention provides protein fragment complementation assays for drug discovery, in particular to identify compounds that activate or inhibit cellular pathways. Based on the selection of an interacting protein pair combined with an appropriate PCA reporter, the assays may be run in high-throughput or high-content mode and may be used in automated screening of libraries of compounds. The interacting pair may be selected by cDNA library screening; by gene-by-gene interaction mapping; or by prior knowledge of a pathway. Fluorescent and luminescent assays can be constructed using the methods provided herein. The selection of suitable PCA reporters for high-throughput or high-content (high-context) assay formats is described for a diversity of reporters, with particular detail provided for examples of monomeric enzymes and fluorescent proteins. Methods are described for constructing such assays for one or more steps in a biochemical pathway; testing the effects of compounds from combinatorial, natural product, peptide, antibody, nucleic acid or other diverse libraries on the protein or pathway(s) of interest; and using the results of the screening to identify specific compounds that activate or inhibit the protein or pathway(s) of interest. Single-color and multi-color assays are disclosed. Further disclosed are universal expression vectors with cassettes that allow the rapid construction of assays for a large and diverse number of gene / reporter combinations. The development of such assays is shown to be straightforward, providing for a broad, flexible and biologically relevant platform for drug discovery.
Owner:ODYSSEY THERA INC
Kits for increasing luminescence assay sensitivity
InactiveUS7078181B2High sensitivityDecrease in luminanceMicrobiological testing/measurementChemiluminescene/bioluminescenceAnalyteOrganic compound
Owner:PROMEGA CORP
Luminogenic and nonluminogenic multiplex assay
ActiveUS7553632B2Provides gene expression data quickly and easilyEliminate false resultMicrobiological testing/measurementBiological testingMultiplexAssay
Owner:PROMEGA CORP
Protein fragment complementation assays for high-throughput and high-content screening
Owner:ODYSSEY THERA INC
Methods and apparatus for improved luminescence assays
InactiveUS20030008339A1High sensitivityFaster assay timeSugar derivativesMicrobiological testing/measurementAnalyteLuminescence
What is described are methods and apparatus for performing a binding assay for an analyte of interest present in a sample. The methods include the steps of: forming a composition containing the sample, an assay-performance-substance which contains a component linked to a label compound capable of chemiluminescing when triggered, and a plurality of particles capable of specifically binding with the analyte and / or the assay-performance-substance; incubating the composition to form a complex which includes a particle and the labeled component; collecting the complex in a collection zone; introducing into the collection zone a trigger capable of triggering the label such that the label luminesces; and measuring the emitted luminescence to measure the presence of the analyte of interest in the sample.
Owner:BIOVERIS CORP
Luminogenic and nonluminogenic multiplex assay
ActiveUS20070178545A1Simplicity flexibilitySensitivity simplicityMicrobiological testing/measurementBiological testingBiologyEnzyme
Owner:PROMEGA
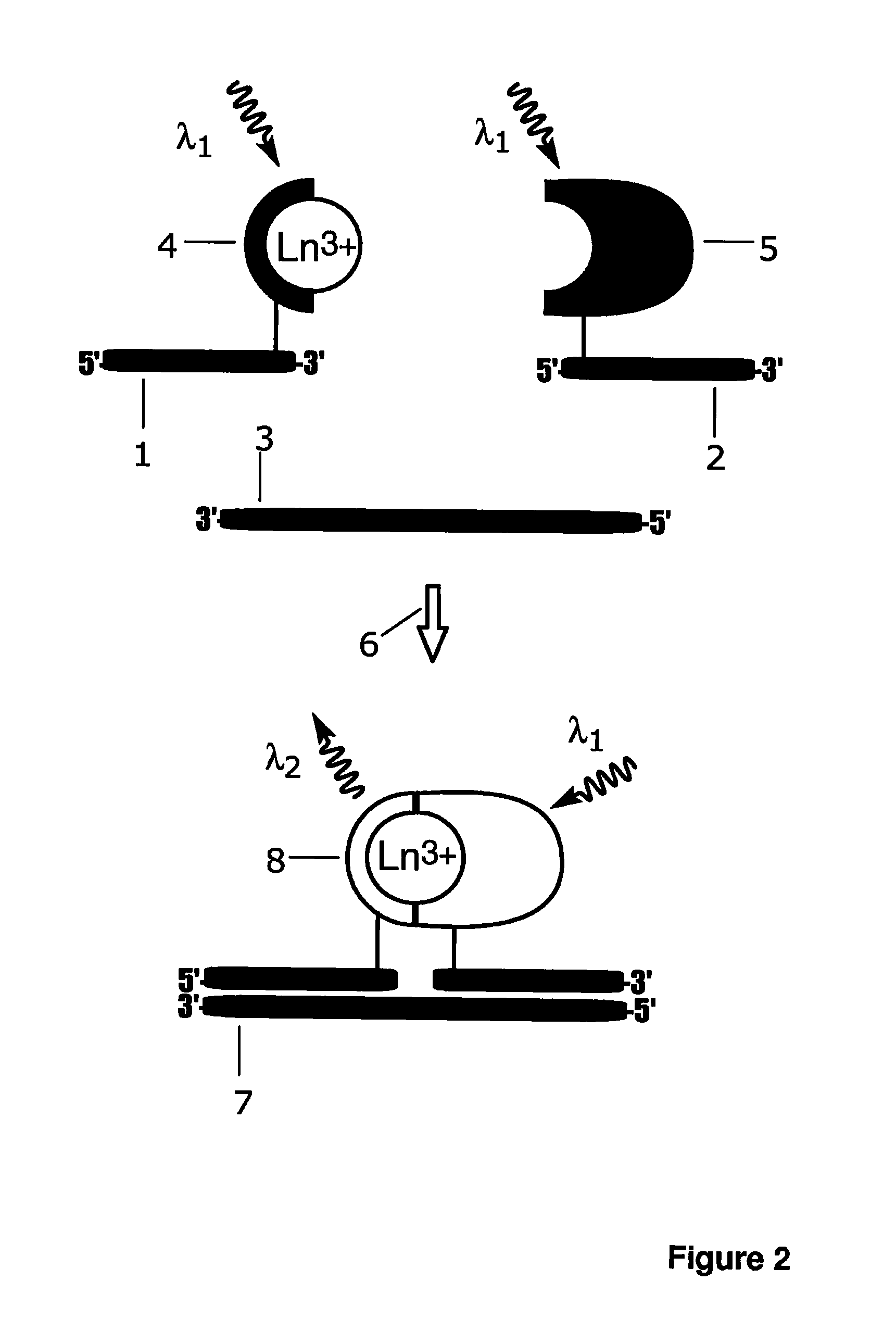
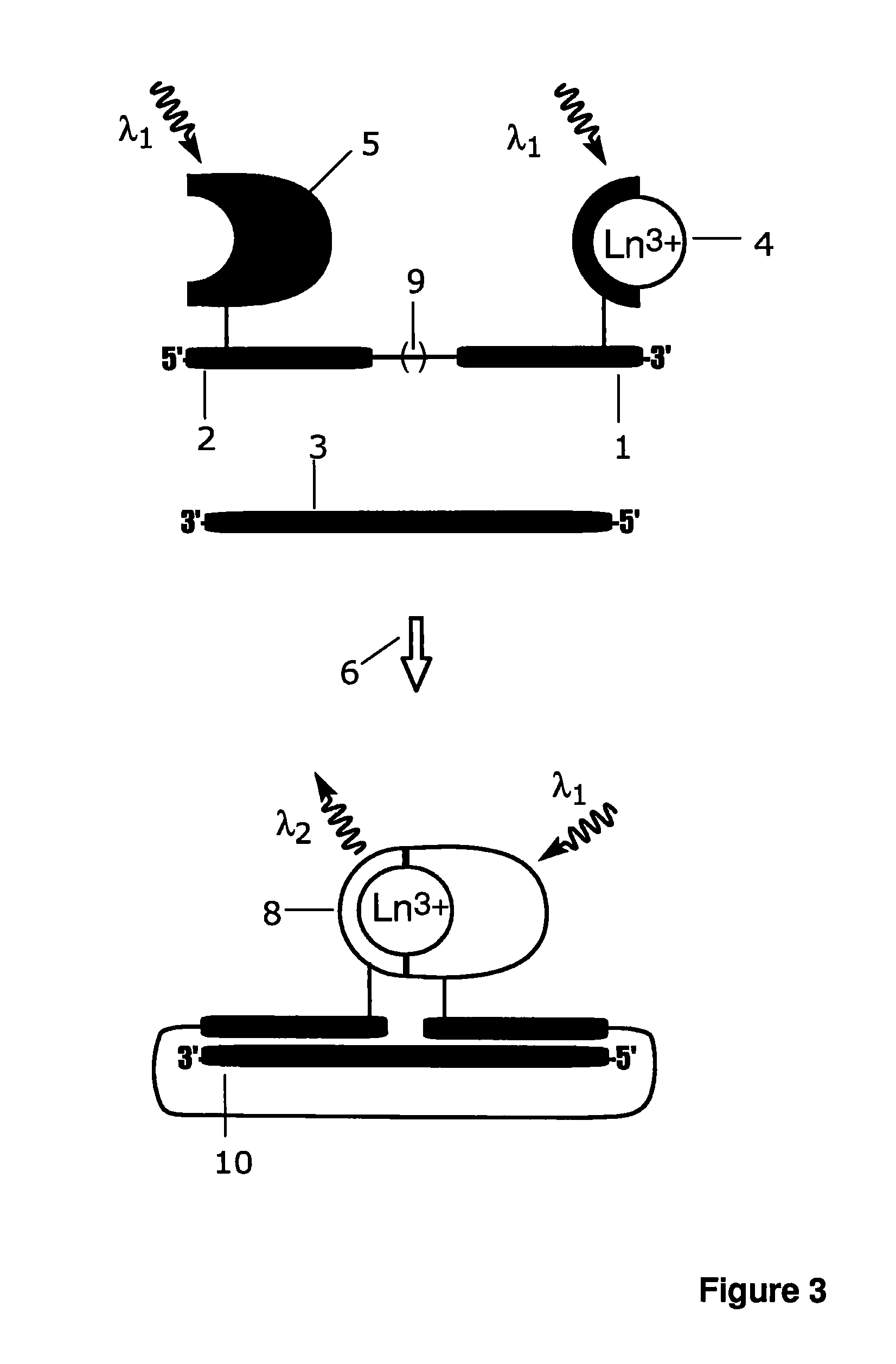
Luminescence assay method
ActiveUS20120009566A1Reduce fluorescenceMicrobiological testing/measurementFluorescence/phosphorescenceAnalyteFluorescence
A bioassay employing a first group including a lanthanide ion carrier chelate and a first recognition element, a second group including an antenna ligand and a second recognition element; where the lanthanide ion carrier chelate binds strongly to lanthanide, or the lanthanide ion carrier chelate binds moderately to lanthanide, and an agent complexing the lanthanide ion is additionally employed at a concentration of at least 1 pmol / l. The antenna ligand binds weakly to the lanthanide ion. Analyte recognition by the first recognition element and by the second recognition element results in either chelate complementation and increased fluorescence, or chelate discomplementation and decreased fluorescence.
Owner:OY ARCTIC PARTNERS
Methods and compositions for coupled luminescent assays
InactiveUS20080050762A1Inadequately sensitiveHigh degree of expertiseMicrobiological testing/measurementBiological material analysisInorganic phosphateHigh energy
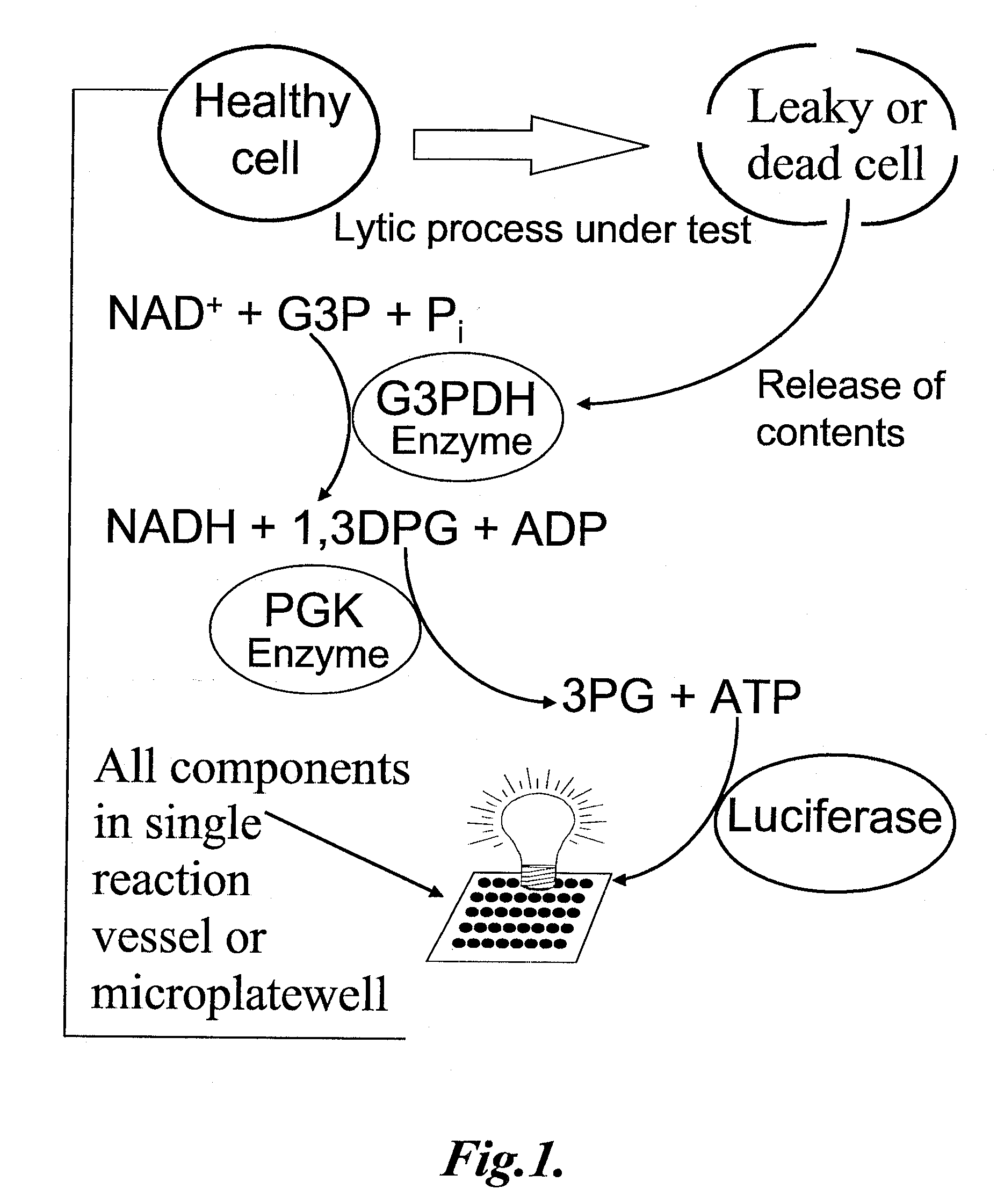
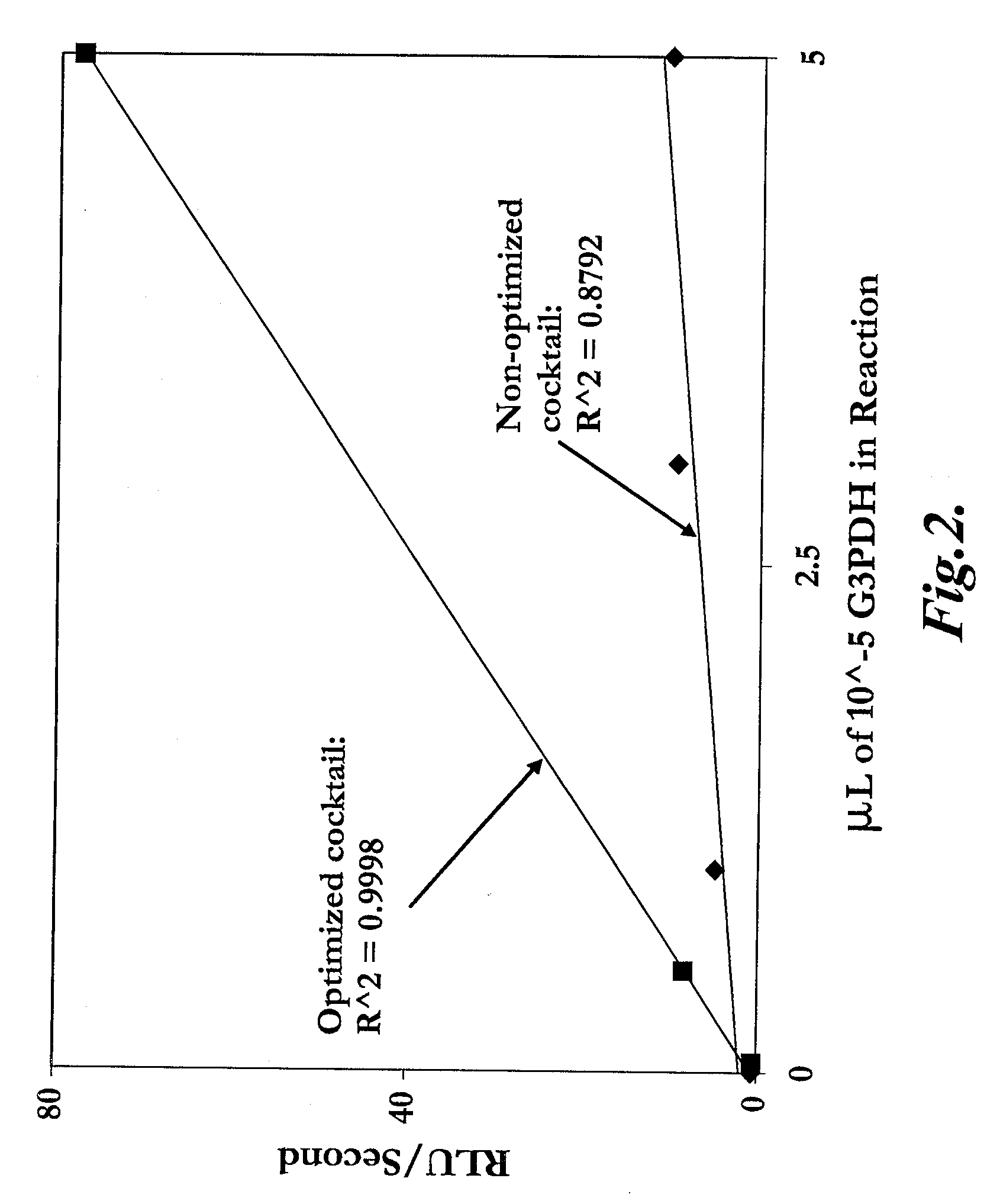
The invention provides methods for measuring the amount or activity of a component in a sample using a luminescent assay comprising a luciferase capable of generating light from a high-energy molecule. In one aspect, the invention provides methods for measuring the amount of NAD+ or the activity of an enzyme or enzyme series that results in the interconversion of NDA+ and NADH. In another aspect, the invention provides methods for measuring the amount of a kinase substrate, free inorganic phosphate, and phosphatase activity. In a further aspect, the invention provides methods for measuring the amount of cAMP in a sample.
Owner:COREY MICHAEL J +1
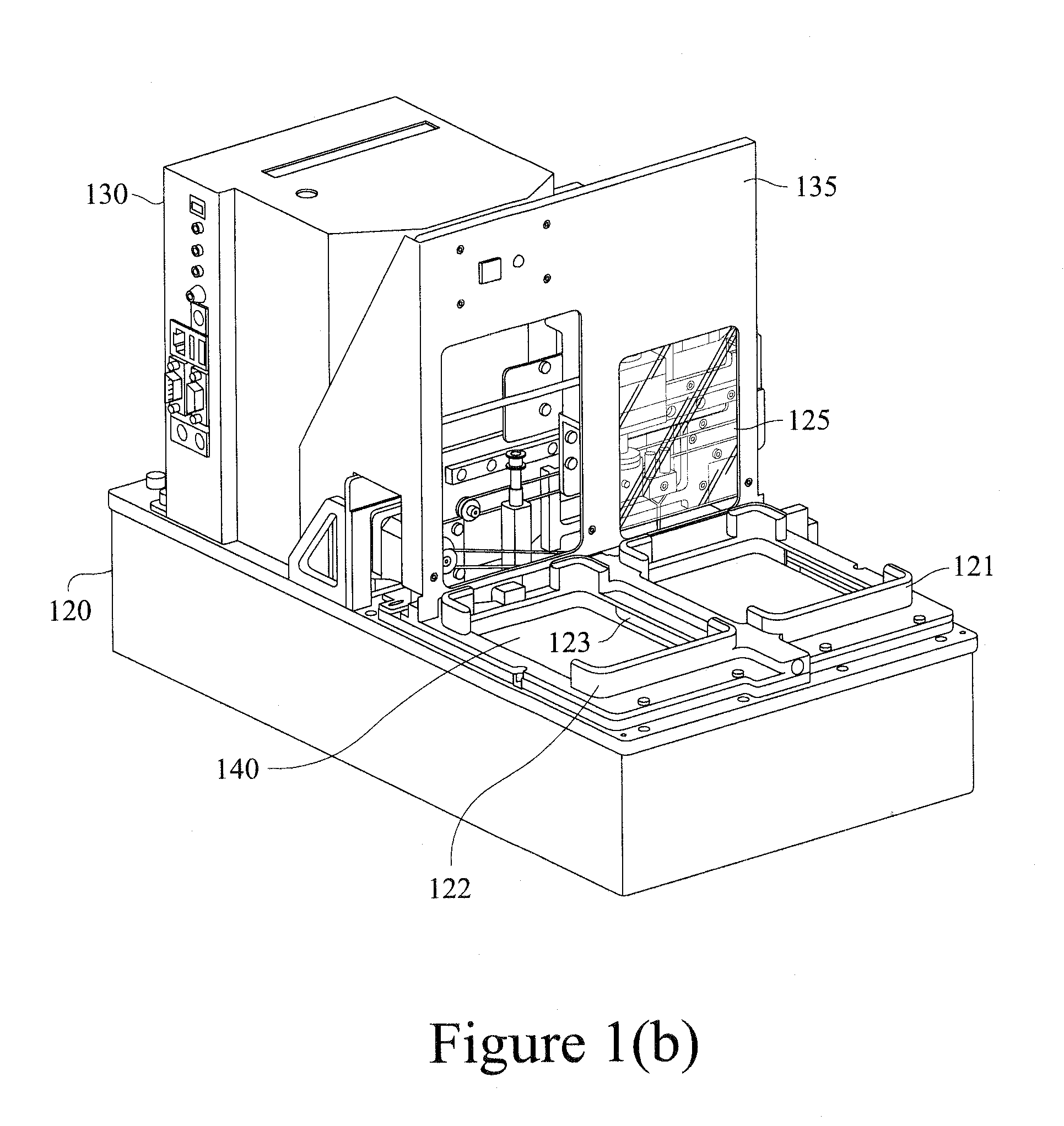
Detection module
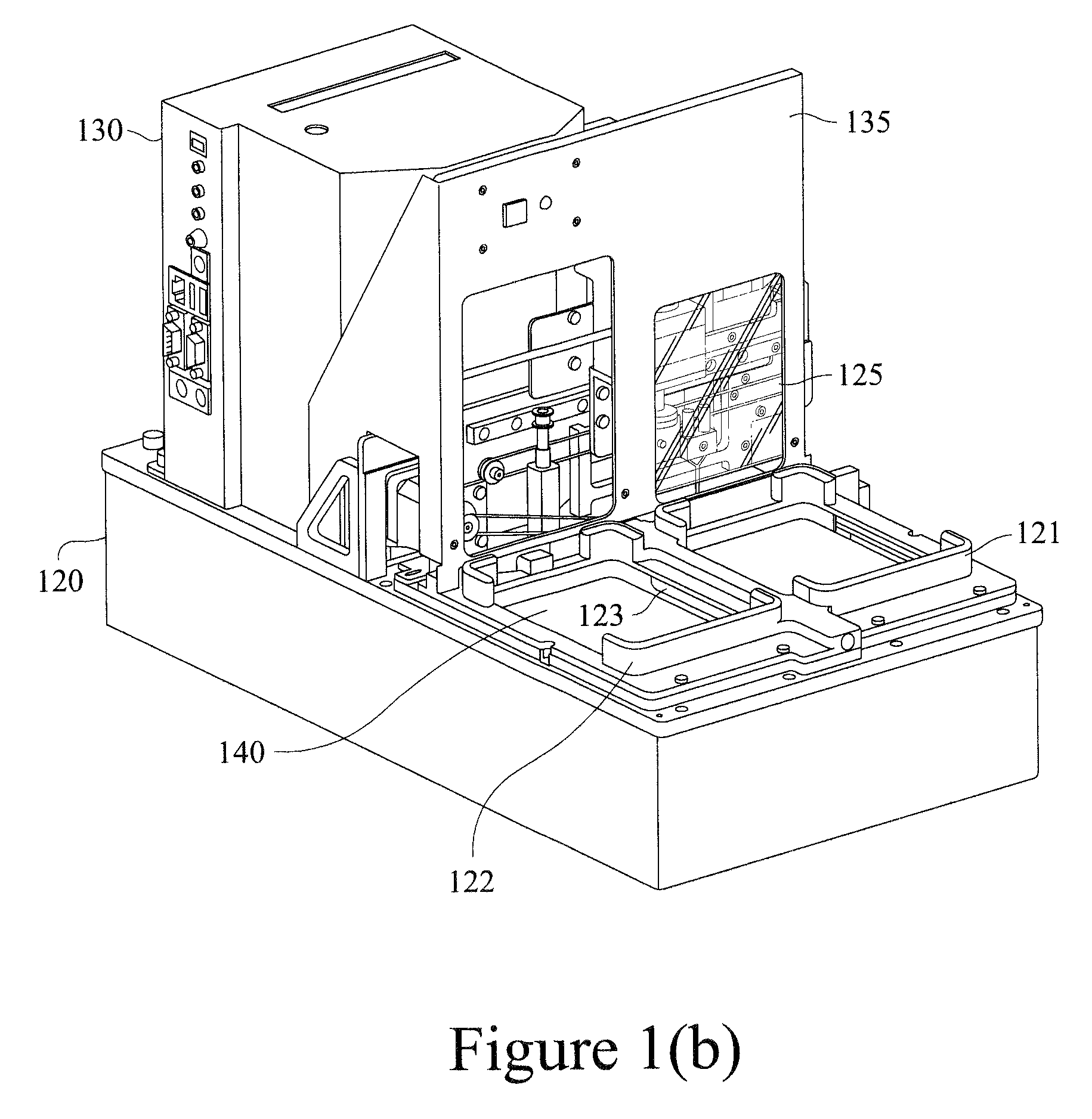
We describe a detection module useful with an apparatus and / or system for conducting luminescence assays.
Owner:MESO SCALE TECH LLC
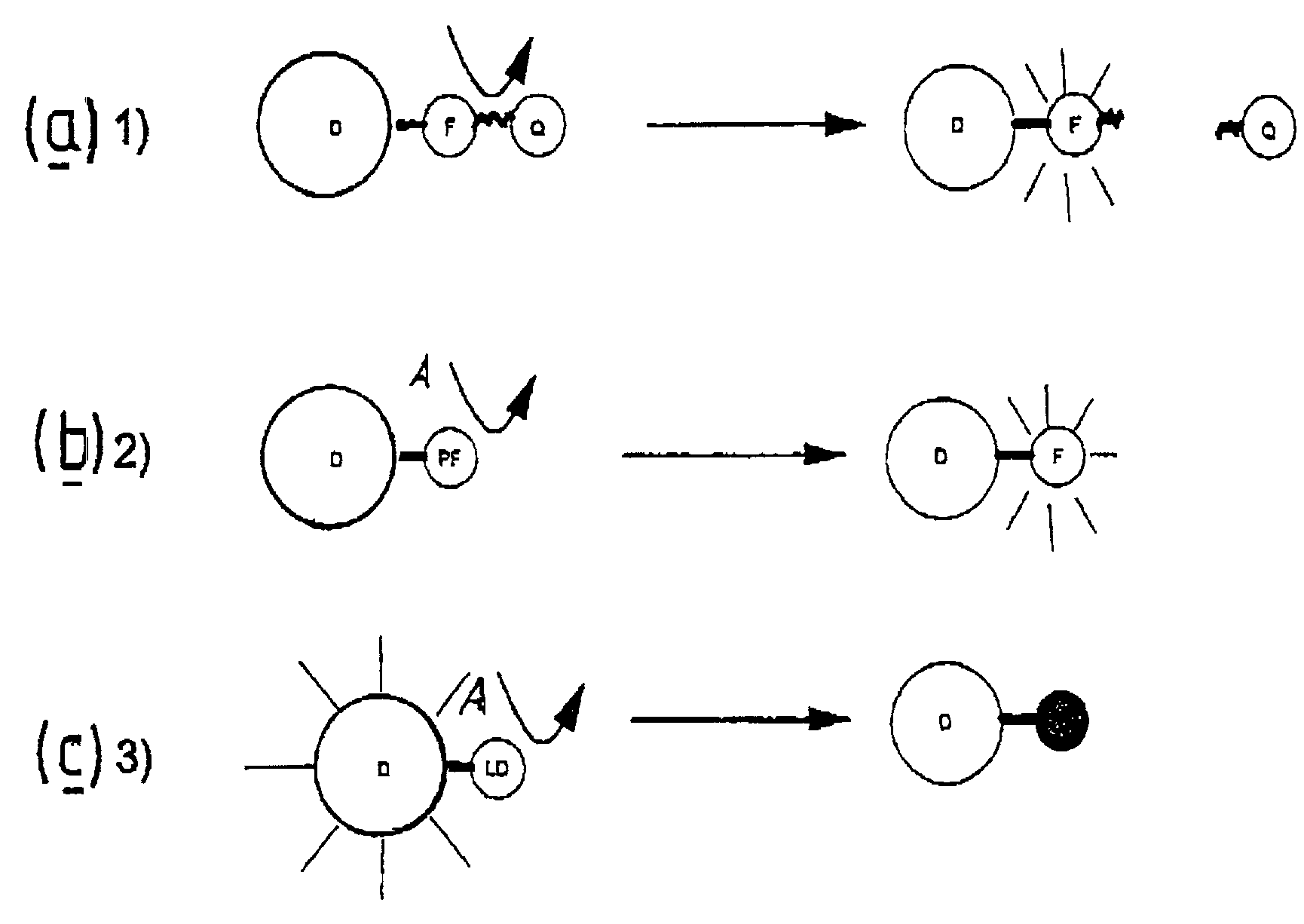
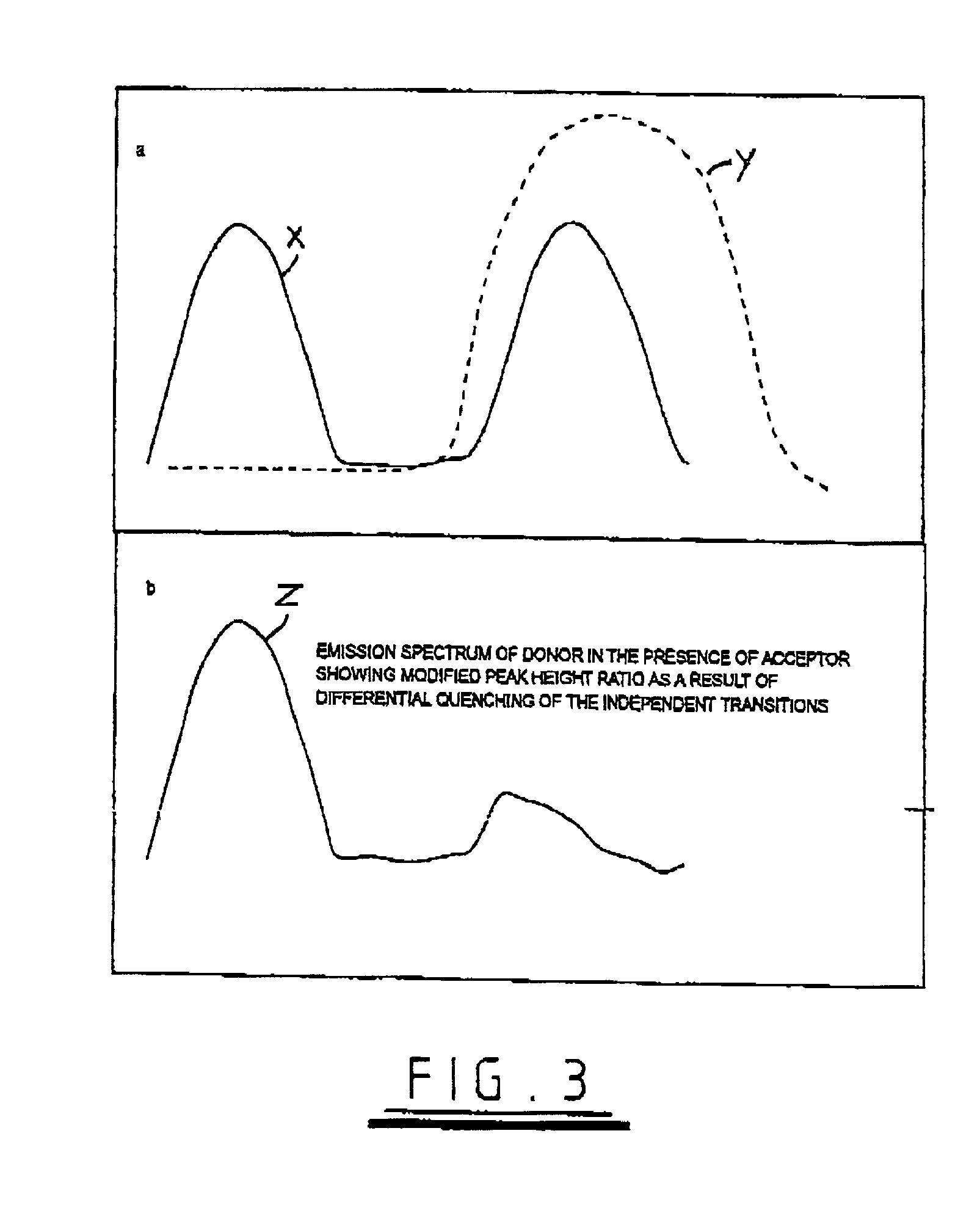
Luminescence assays
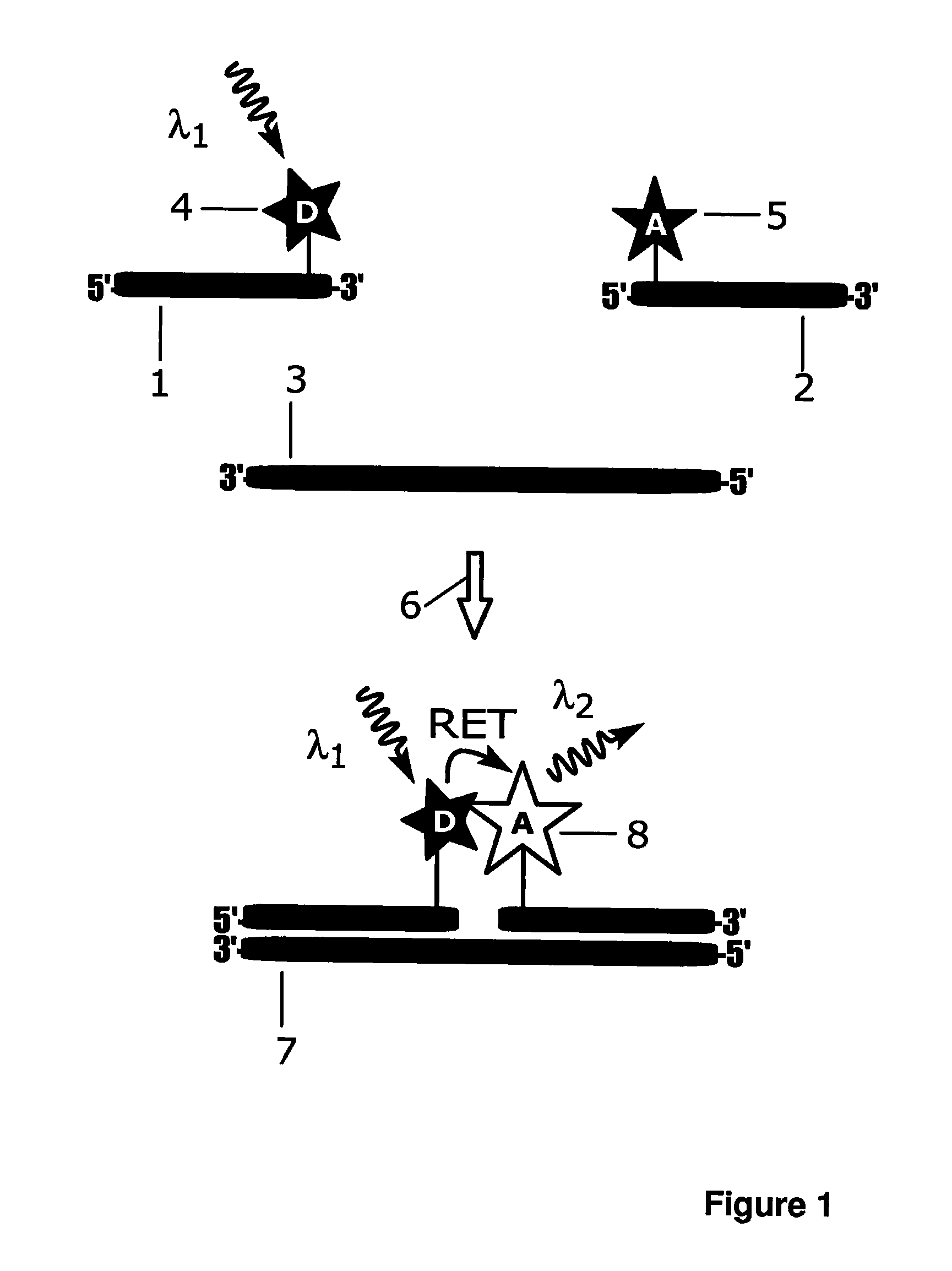
InactiveUS7332344B2Microbiological testing/measurementChemiluminescene/bioluminescenceEnergy transferUpconversion luminescence
A method of detecting an analyte is described in which an up-converting luminescent species that is excited by absorption of long wavelength light is provided as a donor of energy to an acceptor species that can be bound to, or generated in proximity to, the luminescent species. The analyte either alters the proximity between the luminescent species and the acceptor species or alters the absorbance or luminescence properties of the acceptor species. The luminescent energy donor is excited with long wavelength light and transfers up-converted energy to the proximate acceptor species and this transfer of energy is detected by measurement of appropriate luminescence properties of either the donor or of the acceptor or both.
Owner:PHOTONIC RES SYST
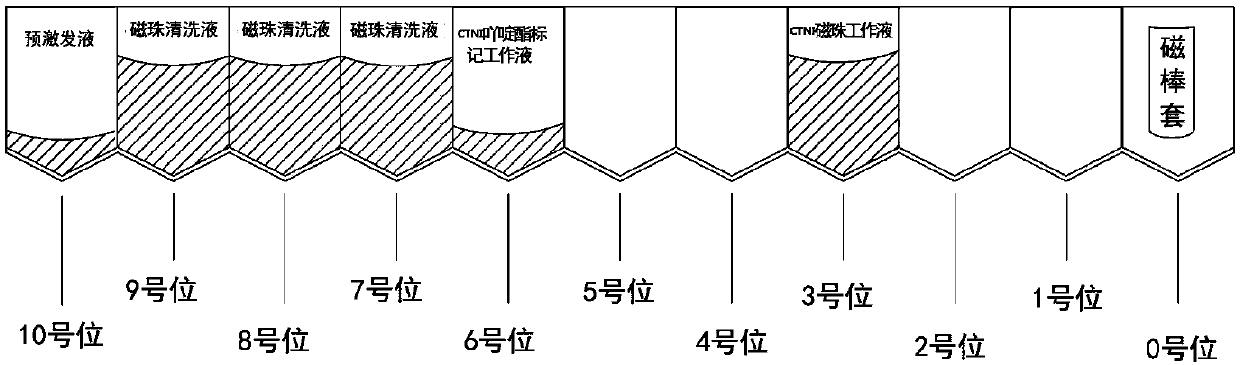
Cardiac troponin I magnetic particle chemiluminescence assay kit and usage method thereof
PendingCN107677833AHigh detection sensitivityImprove featuresBiological testingReagent stripMagnetic bead
The invention discloses a cardiac troponin I magnetic particle chemiluminescence assay kit and a usage method thereof. The kit includes a plurality of reagent strips composed of a plurality of side-by-side hole sites. The reagent strip includes a cTnI magnetic microparticle reagent and a cTnI labeled reagent and further includes a sample diluent, a calibration product, a magnetic bead cleaning solution and a pre-exciting solution. A magnetic rod sleeve is arranged in one hole site on the reagent strip. By combination of a chemiluminescence technology and immunomagnetic particles, the cardiac troponin I magnetic particle chemiluminescence assay kit has higher detection sensitivity and specificity and better performance parameters; the flexibility is high; different tests can be performed simultaneously, a single test can also be carried out, so that shorter test time can be obtained, and the product cost is greatly reduced.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
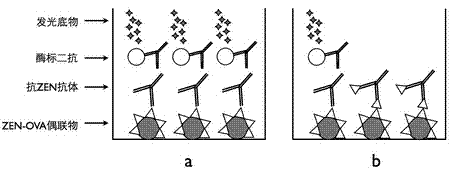
Chemiluminescent immunoassay method for rapidly detecting zearalenone toxin
InactiveCN102313810AStrong targetingImprove accuracyChemiluminescene/bioluminescenceChemiluminescent immunoassayChemiluminescence immunoassay
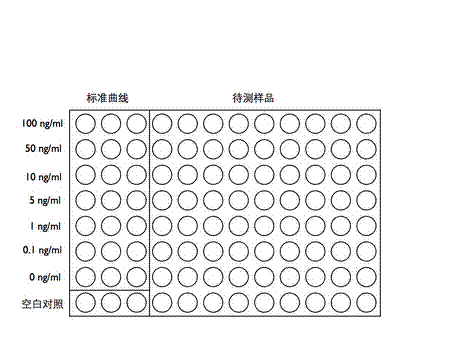
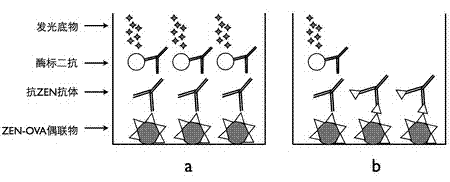
A chemiluminescence immunoassay method for detecting zearalenone toxin in the technical field of biological detection engineering comprises the following steps of: linking ZEN semiantigen with OVA to obtain ZEN-OVA; extracting a sample to be measured to obtain an extract; coating ZEN-OVA on a 96-well white plate at the optimum coating concentration, followed by sealing, respectively adding a known solution of the ZEN pure product and anti-zearalenone monoclonal antibody and a mixed solution of the extract of the sample to be measured and anti-zearalenone monoclonal antibody at different concentrations, followed by incubation; adding HRP-labeled secondary antibody after incubation, and adding a luminescence substrate for luminescent determination to obtain a result; making a standard curve; and acquiring the ZEN concentration in the extract of the sample to be measured through the standard curve. The method provided by the invention can be used to rapidly detect the amount of ZEN in the sample to be measured; in the meanwhile, the method has strong pertinency of detection object and results in high accuracy.
Owner:SHANGHAI JIAO TONG UNIV
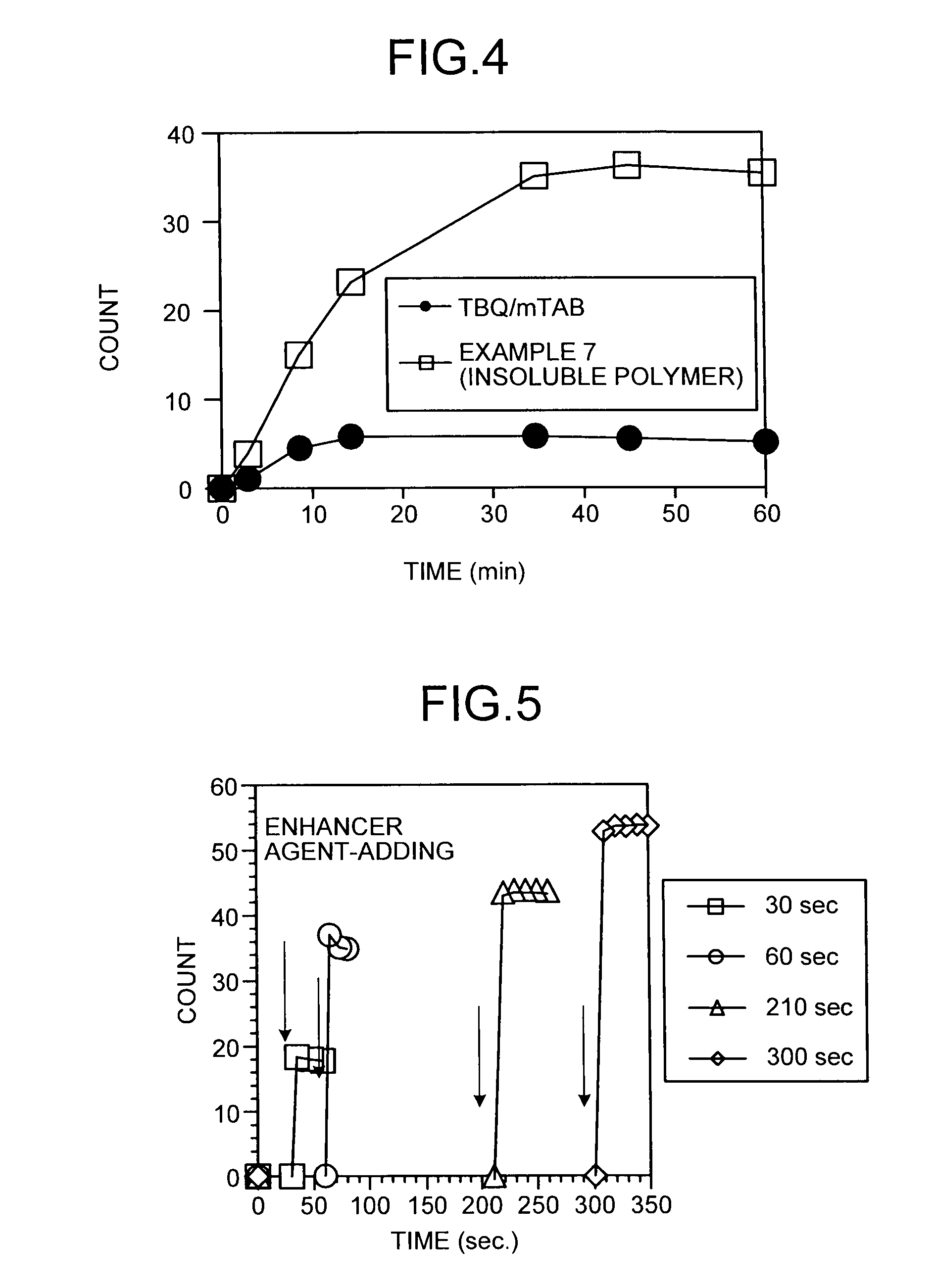
Luminescence enhancer and use thereof
InactiveUS20090081696A1Shorten the timeHigh sensitivityChemiluminescene/bioluminescenceBiological material analysisAntigenBiochemistry
An object of the present invention is to provide a luminescence enhancer by which a luminescence such as chemiluminescence can be enhanced with higher sensitivity and the luminescence can be continued over a prolonged period of time. That is, the present invention provides a luminescence enhancer containing a heteropolymer as an active ingredient, a method for measuring the luminescence using a luminescent substrate and said luminescence enhancer, and a method for analyzing a substance to be measured in a specimen wherein the specimen, an antigen and / or antibody corresponding to a substance to be measured, an antigen and / or antibody labeled-form with an activator and a luminescent substrate are reacted in the presence of said luminescence enhancer to measure the luminescence.
Owner:FUJIREBIO CO LTD
Nanometer magnetic particle chemiluminescent assay kit for cancer antigen CA15-3, and preparation method and detection method thereof
InactiveCN103048461ALow costAnalysis of small differences between batchesChemiluminescene/bioluminescenceAntigenCA15-3
The invention relates to a nanometer magnetic particle chemiluminescent assay kit for a cancer antigen CA15-3, and a preparation method and a detection method thereof. The kit comprises a solution containing a fluorescein-mark labeled cancer antigen CA15-3 antibody, suspension of fluorescein antibody coated magnetic particles and a solution containing an alkaline phosphatase labeled cancer antigen CA15-3 antibody, wherein the alkaline phosphatase labeled cancer antigen CA15-3 antibody is formed by connecting alkaline phosphatase and the cancer antigen CA15-3 antibody through a crosslinking agent SMCC and 2-IT. According to the invention, the cancer antigen CA15-3 can be quantitatively detected with low cost, high accuracy degree and high precision degree.
Owner:SUZHOU HAOOUBO BIOPHARML
Chemiluminescence assay kit for detecting C-peptide content in serum and detection method
InactiveCN109297953AStrong specificityImprove stabilityChemiluminescene/bioluminescenceBiological testingPh regulationMicroparticle
The invention belongs to the field of medical detection and discloses a chemiluminescence assay kit for detecting C-peptide content in serum and a detection method. The assay kit comprises: an M reagent, an R reagent, a C-P calibrator, a pH adjuster, a reaction enhancer, and a chemiluminescent substrate. The M reagent contains a magnetic particle coated with a C peptide-biotinylated antibody at aconcentration of 2[mu]g / mL, and a Tris buffer solution at a concentration of 0.2mol / L. The R reagent contains a C-P-AP labeled antibody at a concentration of 1[mu]g / mL, and a Tris buffer solution at aconcentration of 0.1mol / L. The C-P calibrator comprises two different concentrations of C-peptide samples and a 0.1mol / L concentration of Tris buffer solution. The assay kit for detecting C-peptide has advantages of strong specificity and good stability, thereby improving the sensitivity and specificity of the detection; has a good stability in the whole system; is simple in operation; and is suitable for use with a clinical full automatic chemiluminescence assay instrument.
Owner:HONGKUI BIOLOGICAL CHINA CO LTD
Method for chemiluminescent detection
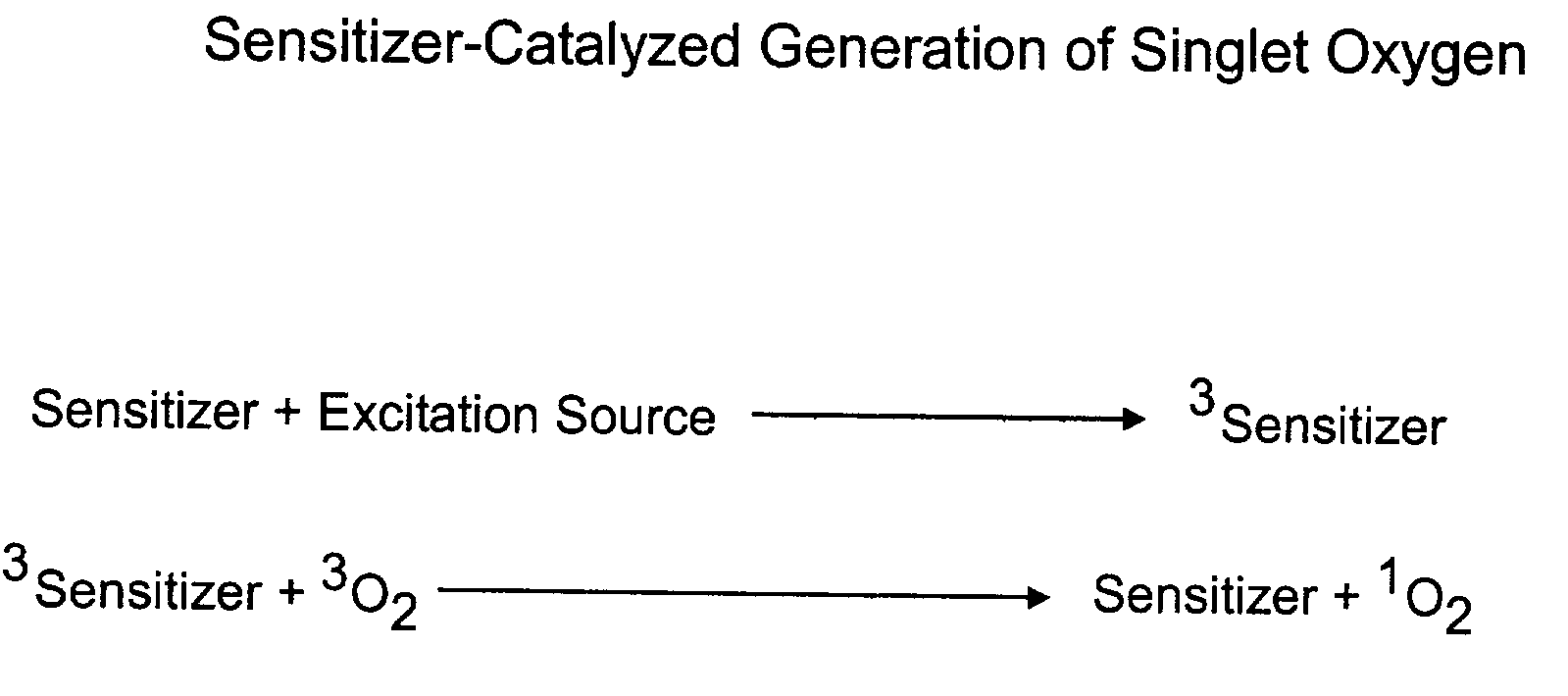
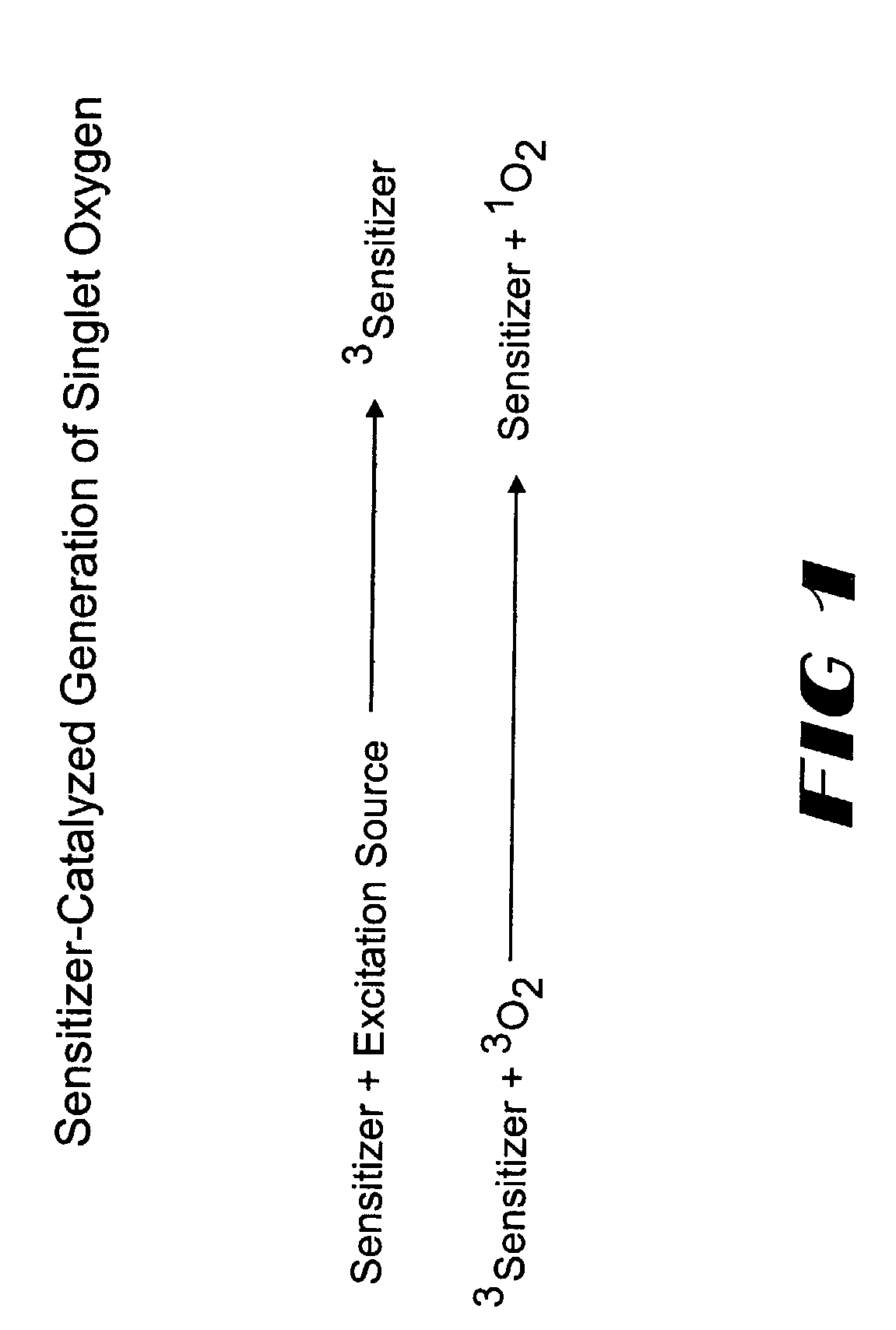
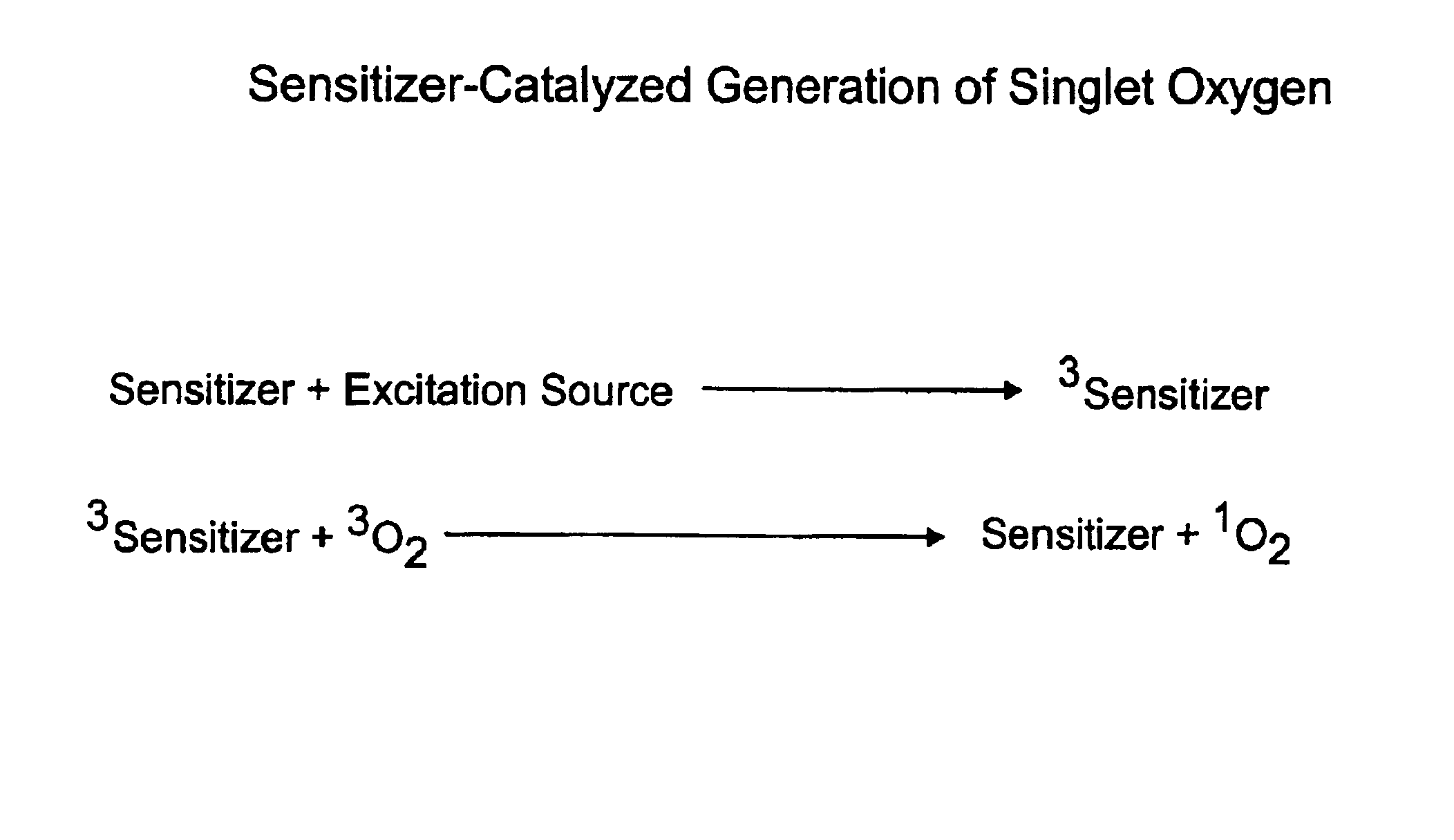
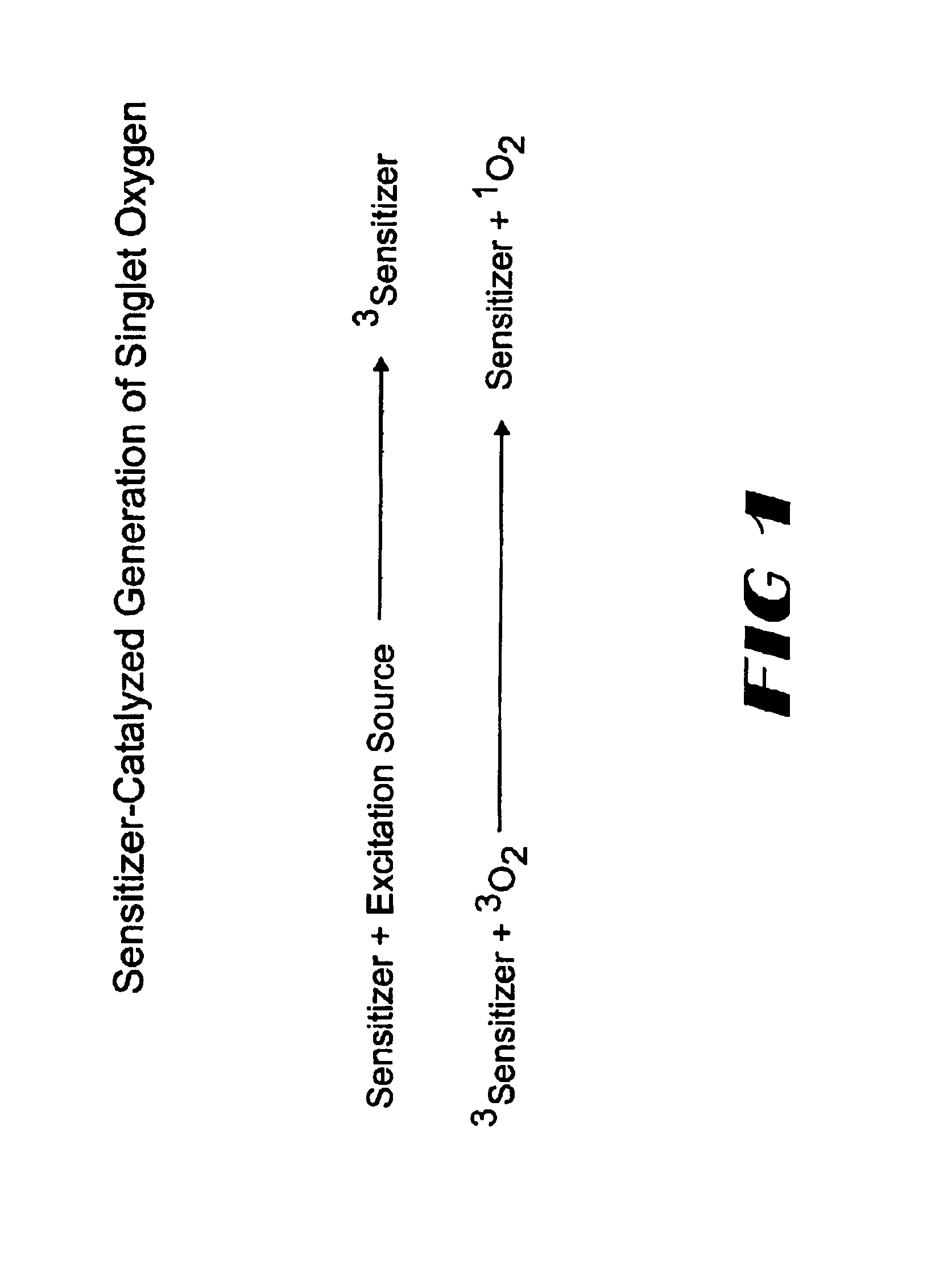
InactiveUS20040014200A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSinglet oxygen
The invention provides chemiluminescent assays that incorporate a film including at least one chemiluminescent precursor immobilized therewith which produces a triggerable chemiluminescent compound, the film being free of compounds which generate singlet oxygen and being adapted for use with a sensitizer-labeled agent or agent probative of the analyte.
Owner:EMP BIOTECH
Method for chemiluminescent detection
InactiveUS6764819B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSinglet oxygen
The invention provides chemiluminescent assays that incorporate a film including at least one chemiluminescent precursor immobilized therewith which produces a triggerable chemiluminescent compound, the film being free of compounds which generate singlet oxygen and being adapted for use with a sensitizer-labeled agent or agent probative of the analyte.
Owner:EMP BIOTECH
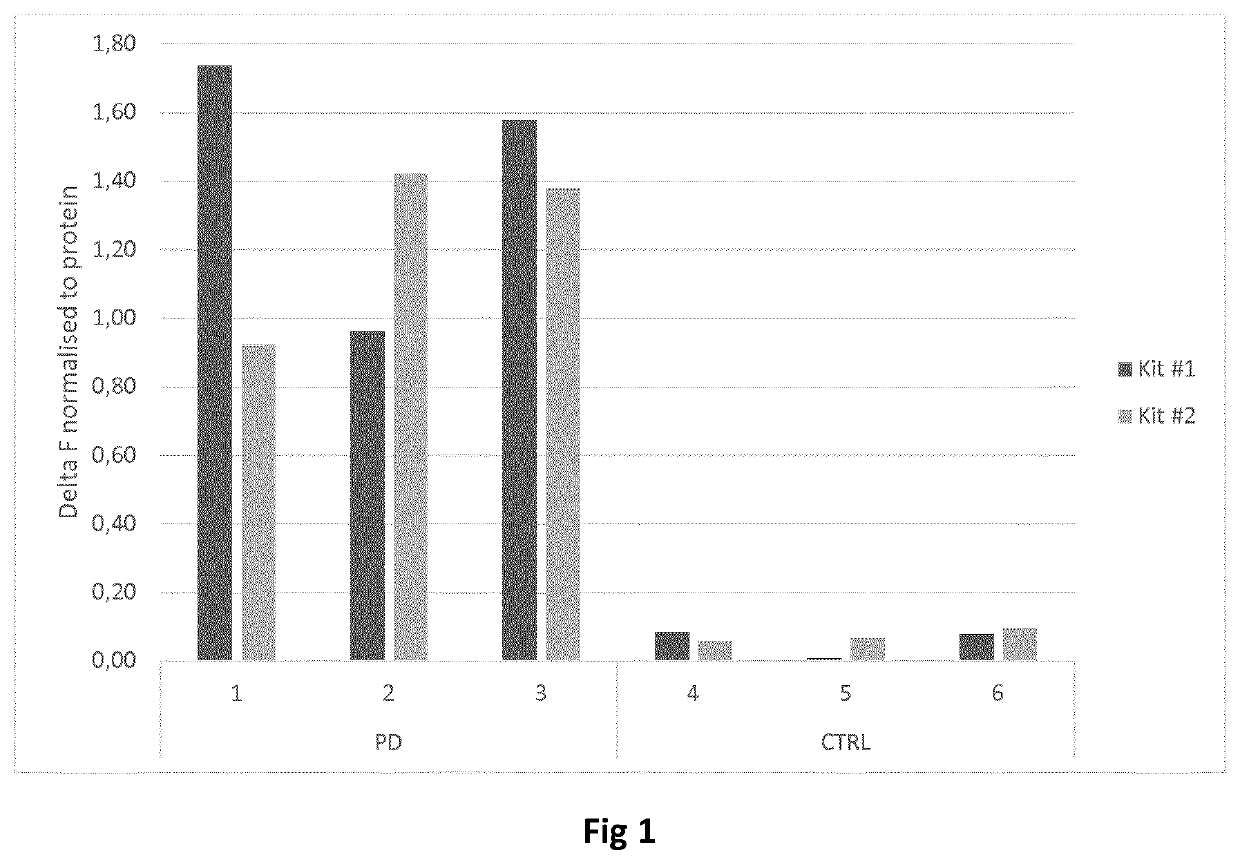
Assay, method and treatment of alpha-synucleinopathies
PendingUS20200309796A1Improve the level ofImmunoglobulins against animals/humansDisease diagnosisAssaySynucleinopathies
A method of detecting alpha-synuclein in a sample of a subject comprising the steps of a) Obtaining a sample from a subject,5 b) Applying the sample on a luminescence assay, and c) Optionally, comparing the results with a control
Owner:H LUNDBECK AS
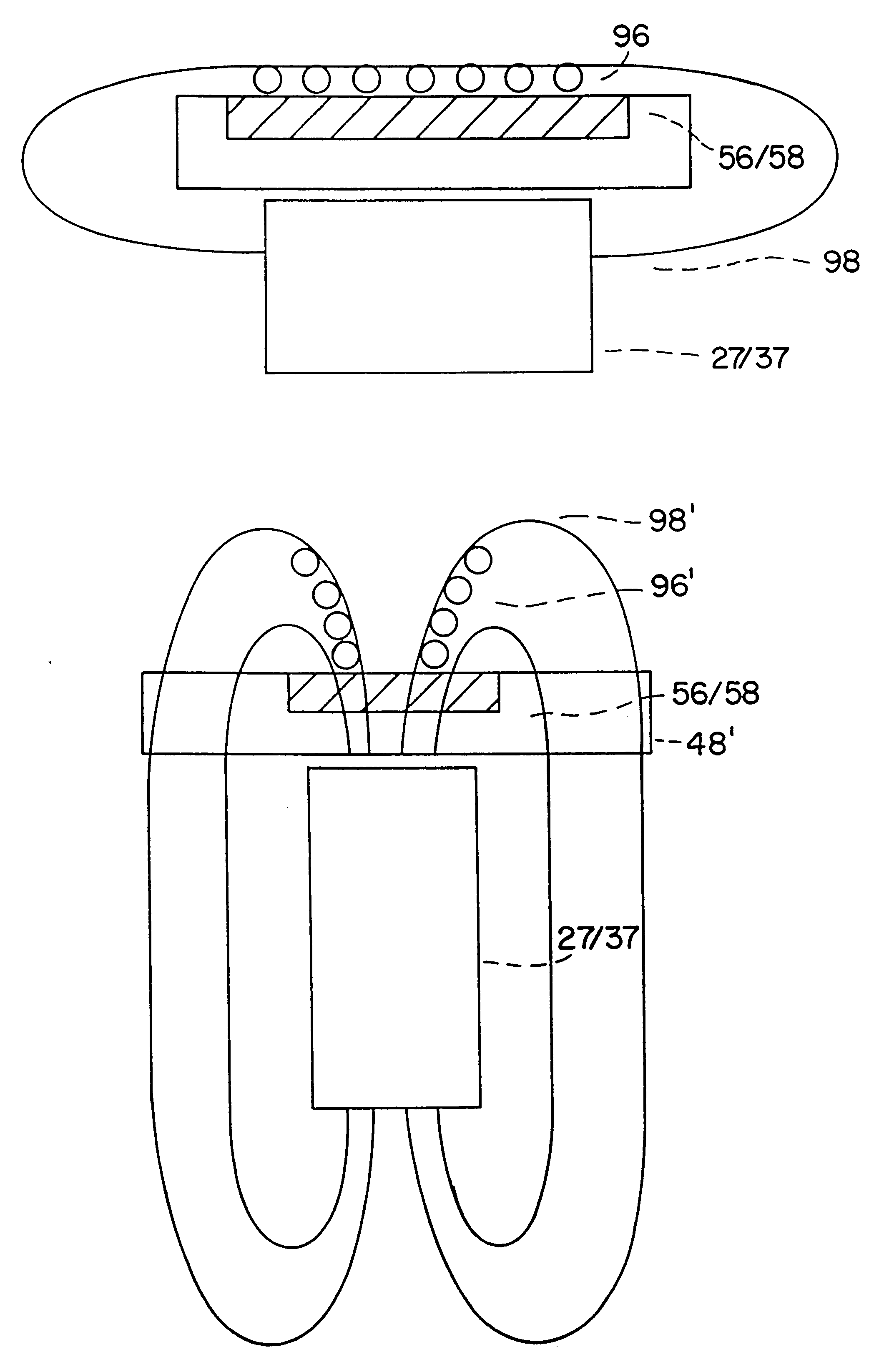
Replaceable detection module and an apparatus for conducting luminescence assays
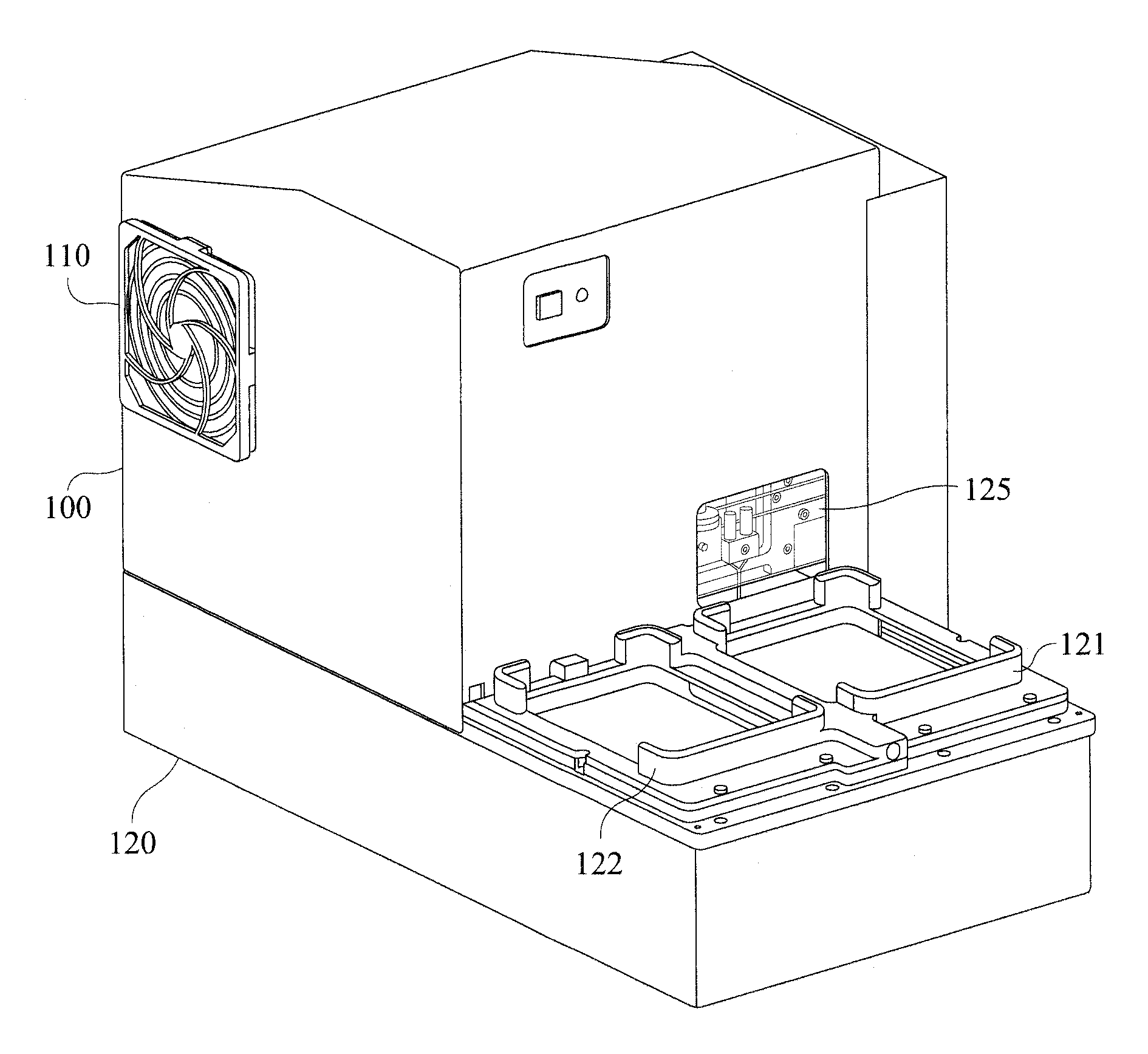
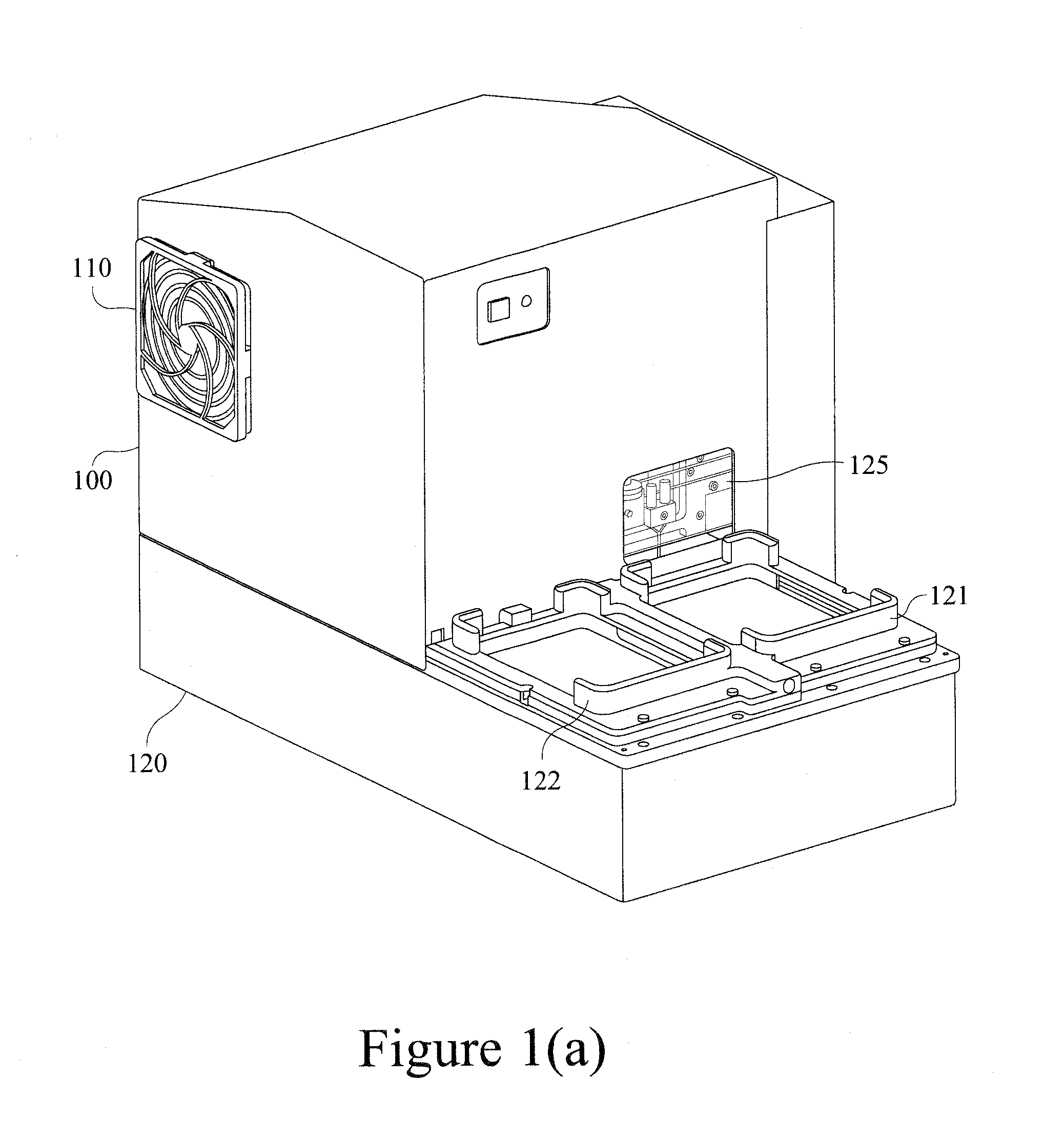
We describe a detection module used in an apparatus (and a system of the apparatus and the detection module) for conducting luminescence assays in multi-well plates, wherein the detection module includes a housing comprising a light detector and a control board. The apparatus and detection module each include engagement elements that align and engage the detection module with the apparatus in the system.
Owner:MESO SCALE TECH LLC
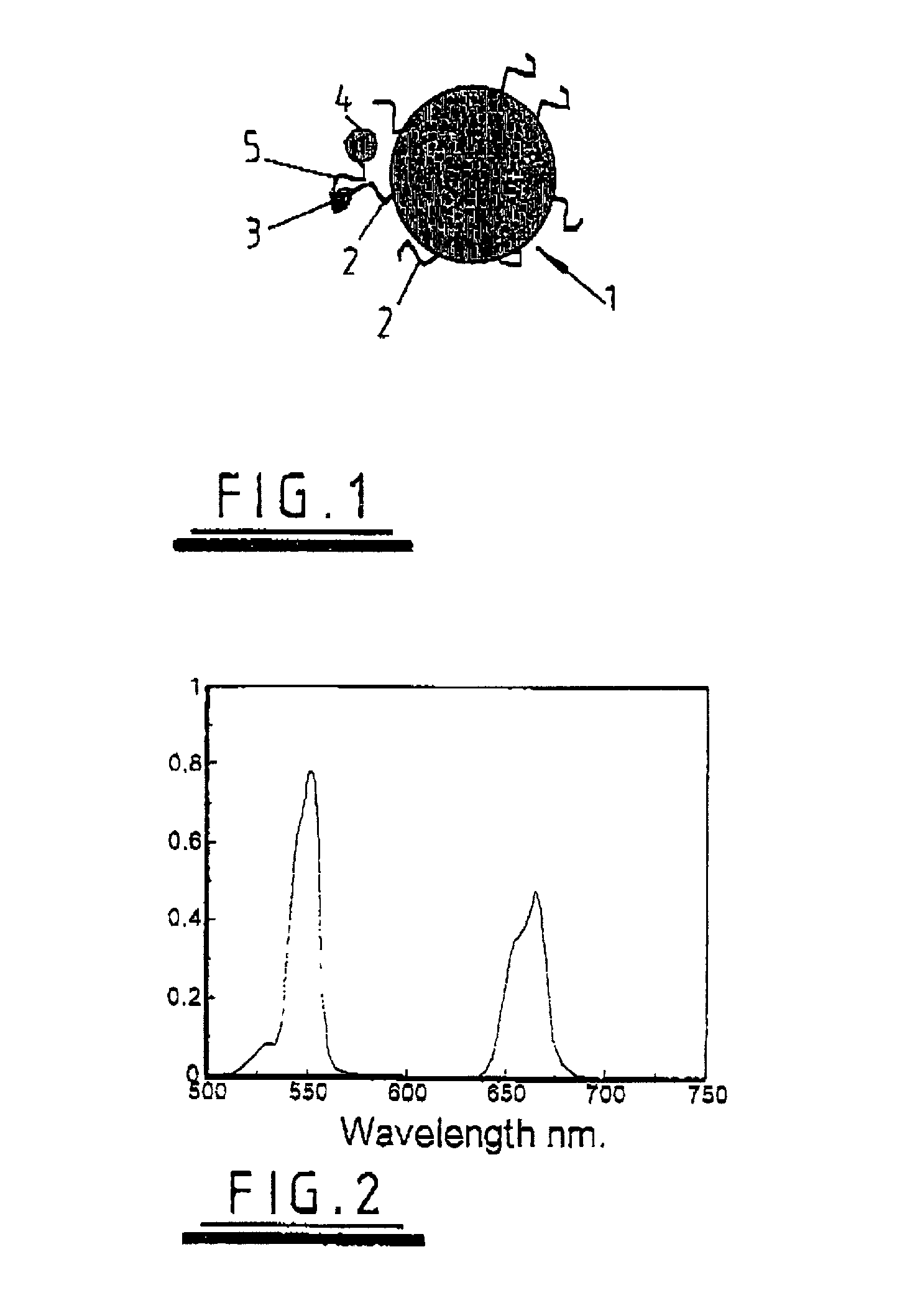
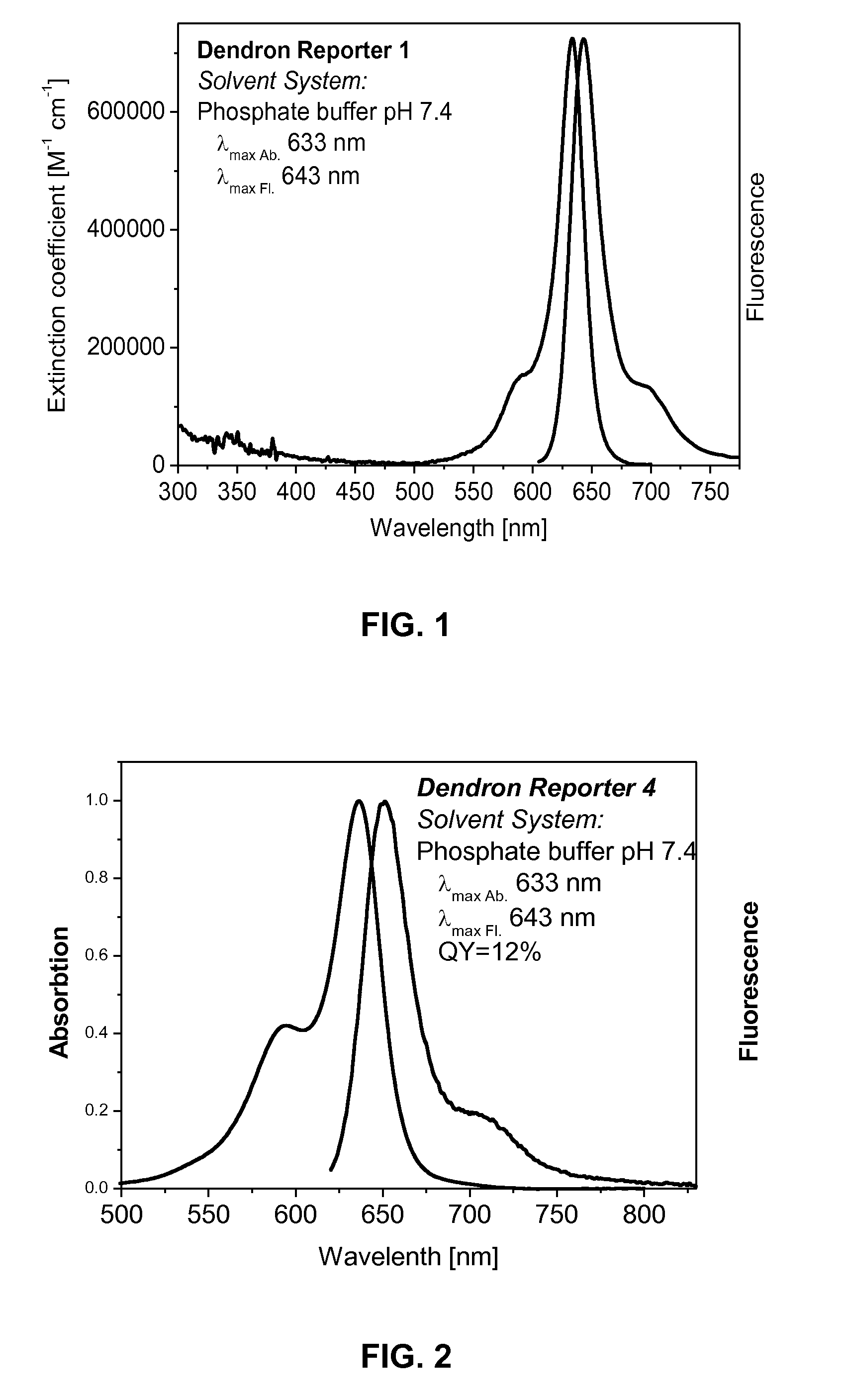
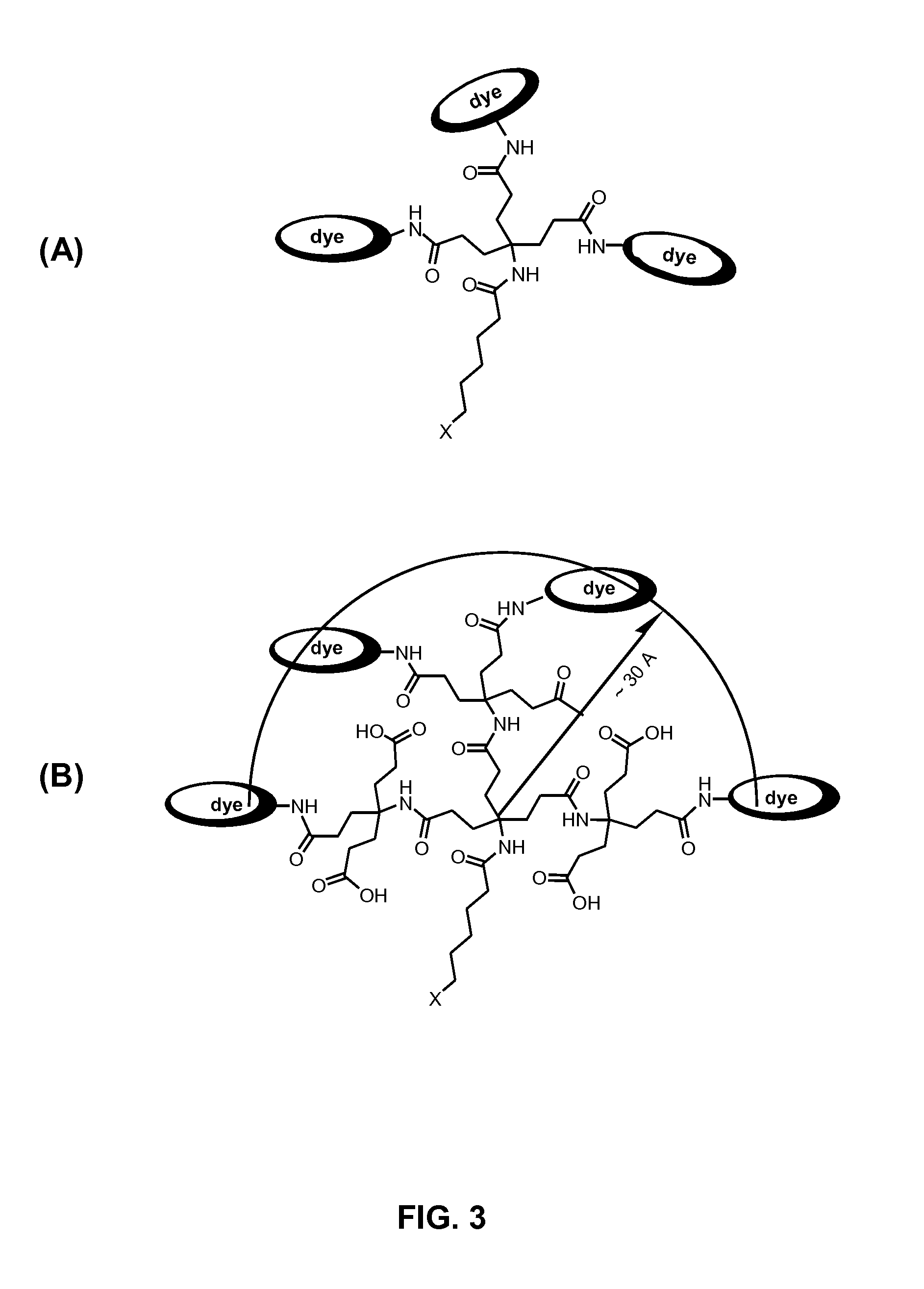
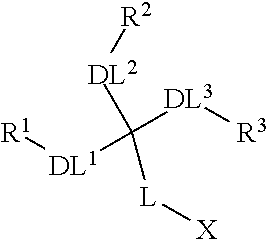
Dendron reporter molecules
InactiveUS20140200333A1Small volumeIncrease the measurable rangeImmunoglobulinsFermentationAnalyteHigh density
Dendronic reporters are described which incorporate a high density of luminescent or non-luminescent dyes at periphery sites and a focal point group that is reactive, ionic or a conjugated substance. Such dendronic reporters are capable of sensing analytes, or are otherwise useful in luminescent assays. Additionally, methods of synthesis are described.
Owner:SETA BIOMEDICALS
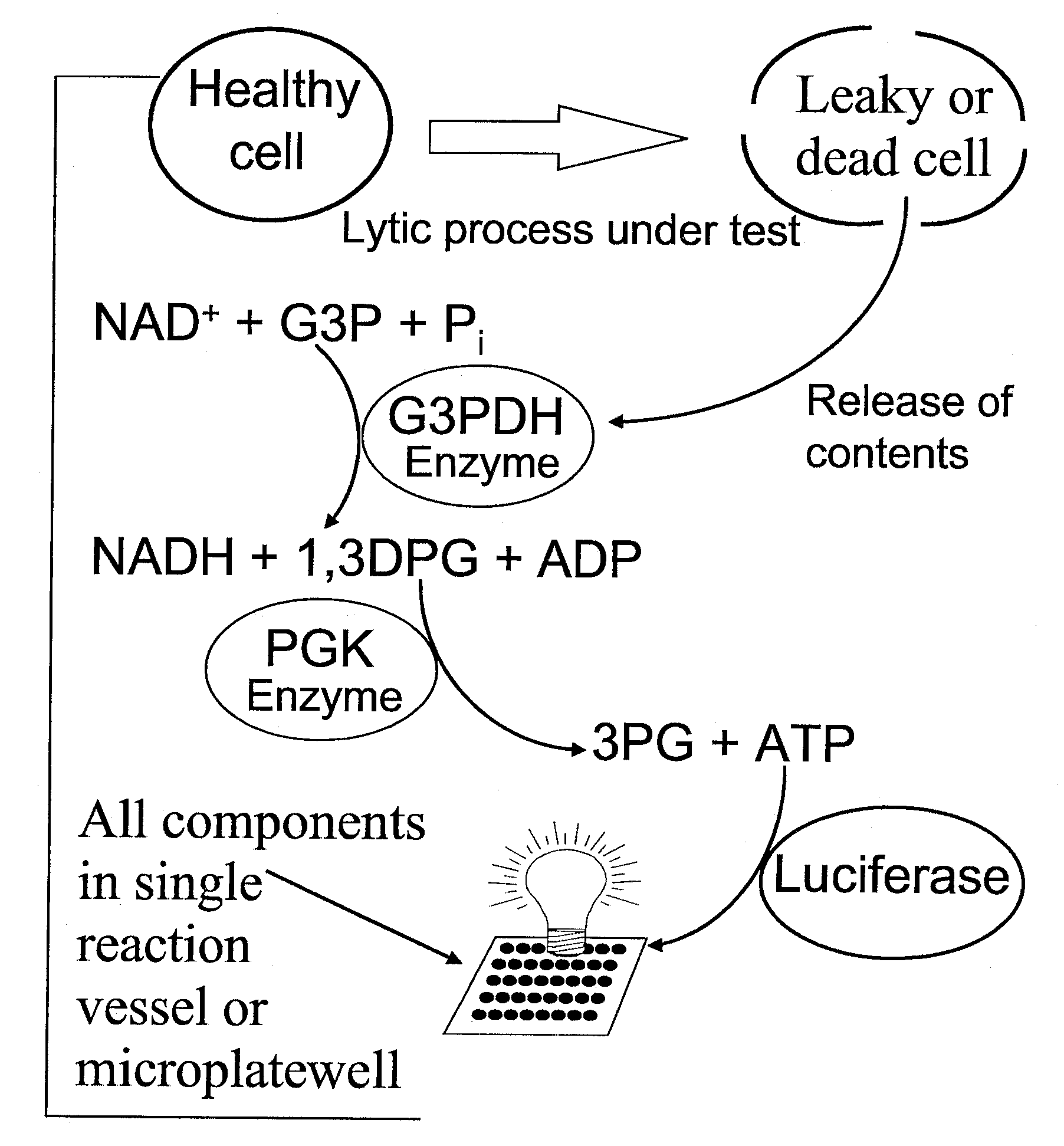
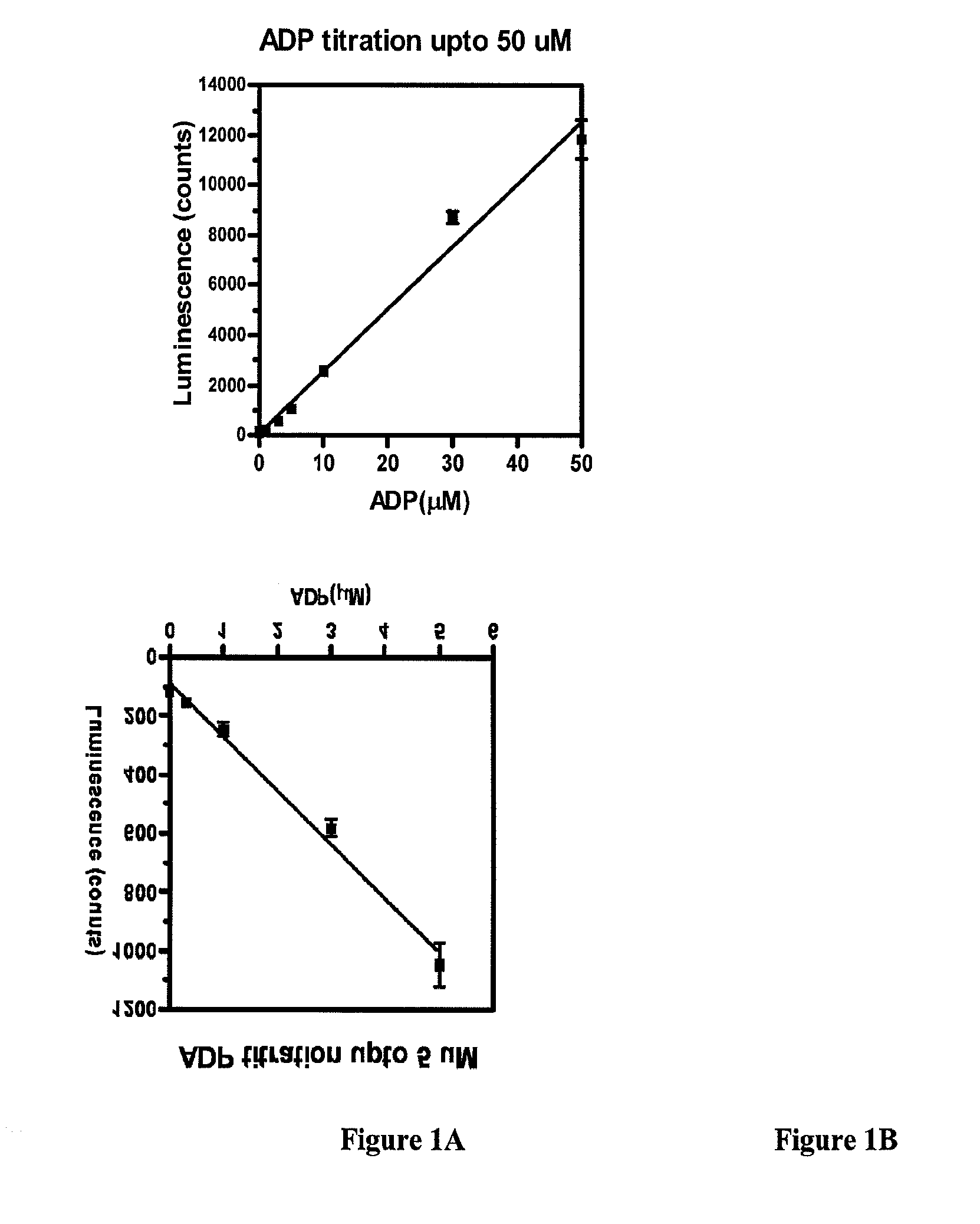
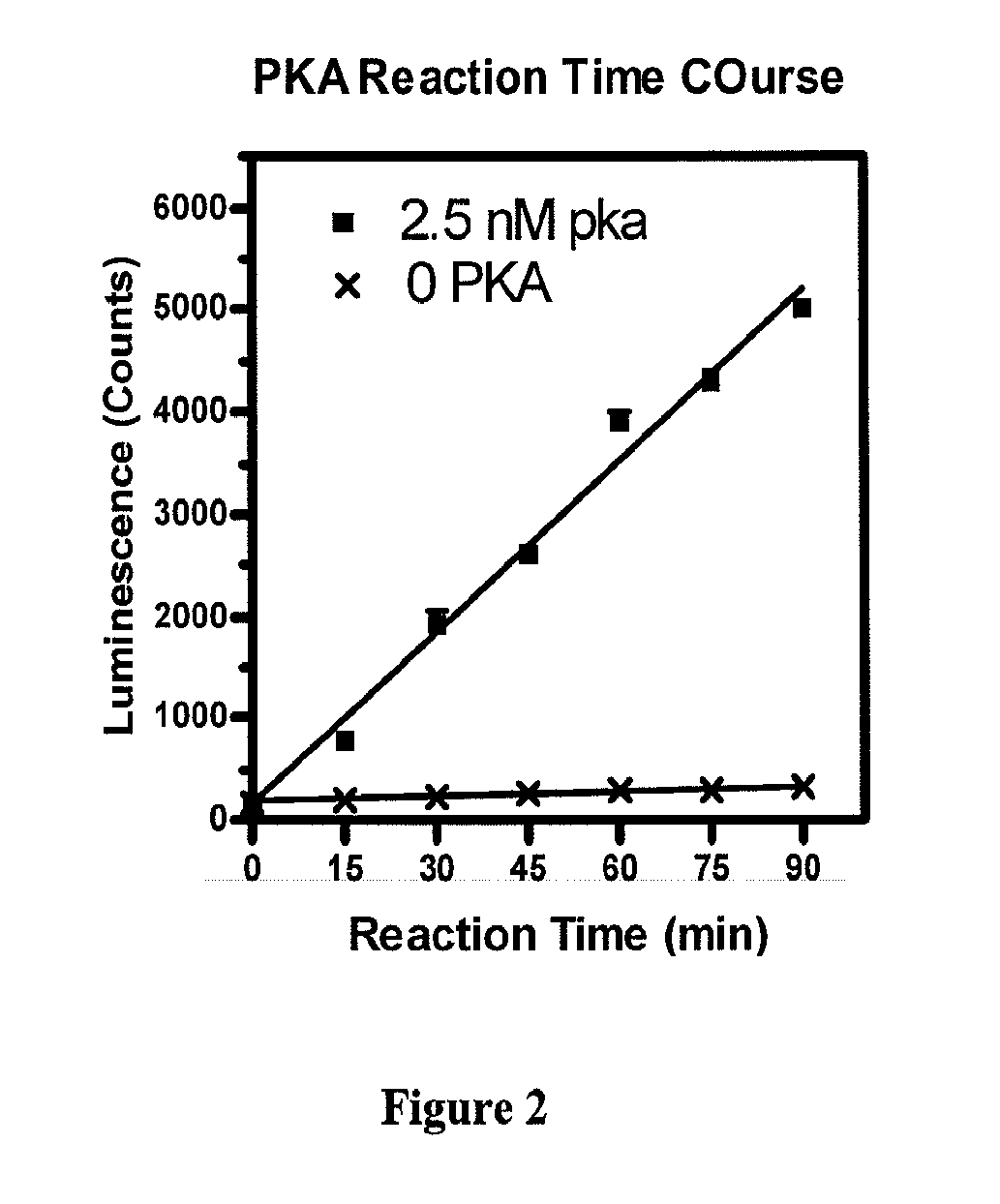
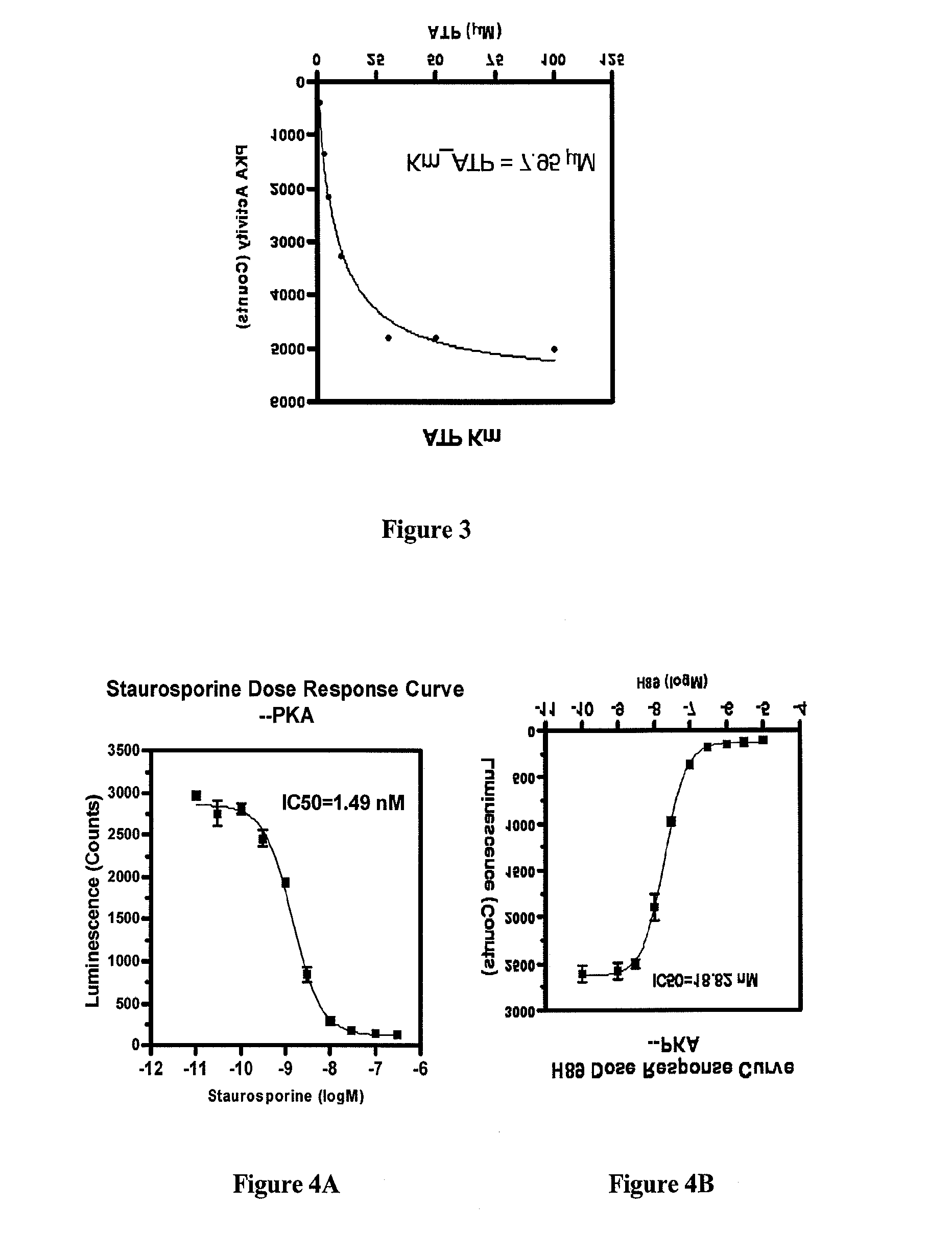
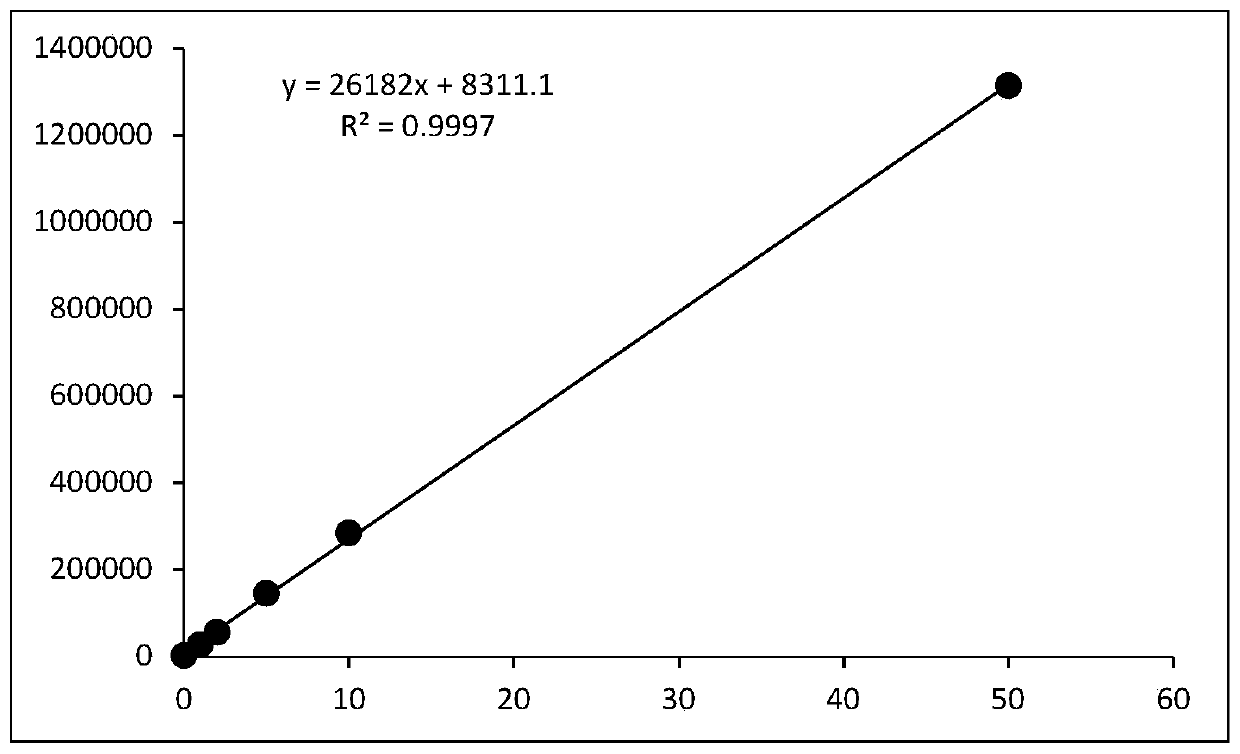
Methods for measuring adp
This invention relates to assays for detecting and measuring ADP. In particular, this invention provides homogeneous luminescent assays that detect ADP generation and measures ADP accumulation based on enzymatic coupling reactions. The assays of the present invention can be applied to all types of kinases and other ADP-generating enzymes, are antibody free, beads free, radioisotope free, and compatible with commonly used kinase buffers.
Owner:GENEWIZ INC SZ
Thymidine kinase 1 magnetic particle chemiluminiscence assay kit and preparation method thereof
PendingCN111044724AQuickly measure concentrationDetermination of concentrationBiological material analysisBulk chemical productionAbzymeAntiendomysial antibodies
The invention relates to a thymidine kinase 1 magnetic particle chemiluminiscence assay kit and a preparation method thereof. The kit comprises a reagent A, a reagent B and a thymidine kinase 1 (TK1)calibrator, the reagent A is a magnetic particle suspension coated with an anti-thymidine kinase 1 (TK1) antibody, and the reagent B is an alkaline phosphatase labeled anti-thymidine kinase 1 (TK1) antibody enzyme conjugate reagent. Compared with the prior art, the invention provides the method for determining the content of thymidine kinase 1 (TK1), and by utilizing the kit provided by the invention, the content of thymidine kinase 1 (TK1) in a sample can be accurately, sensitively and quickly determined according to the principle of a double-antibody sandwich method.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
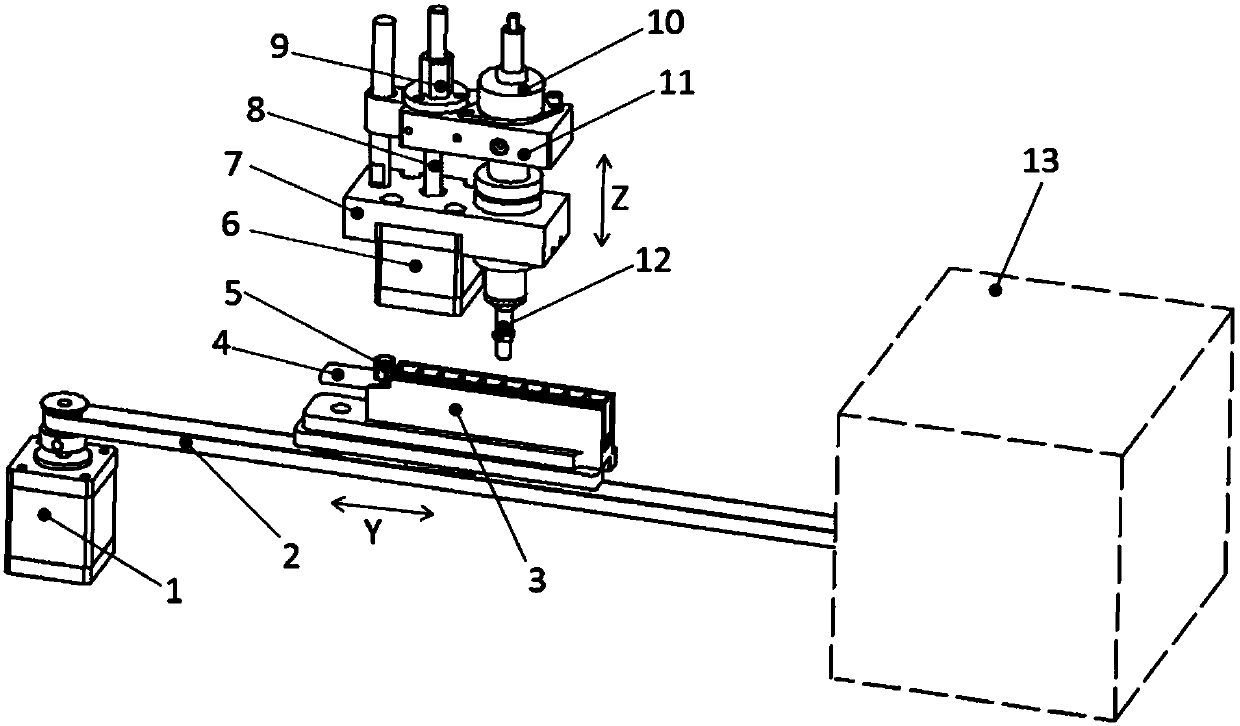
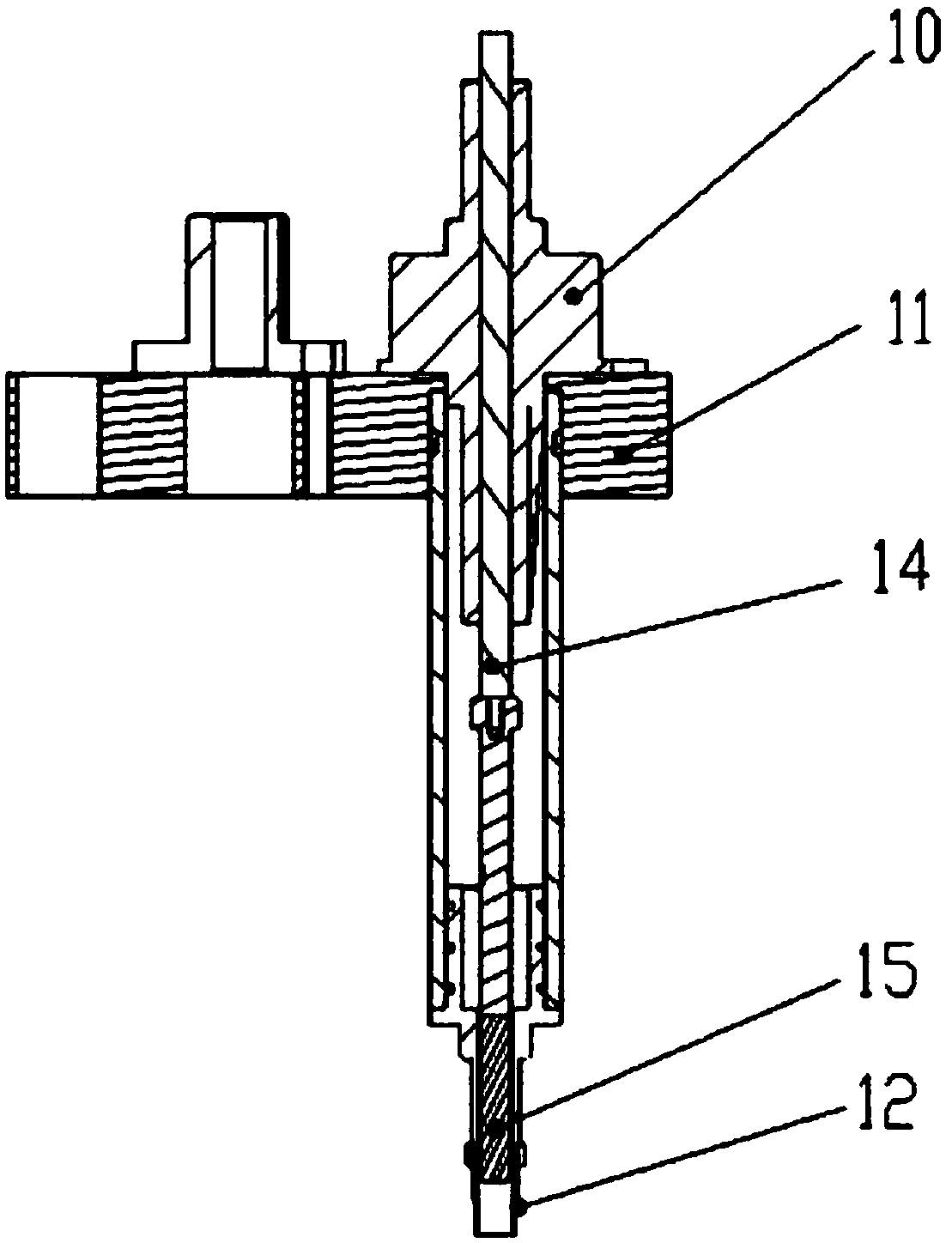
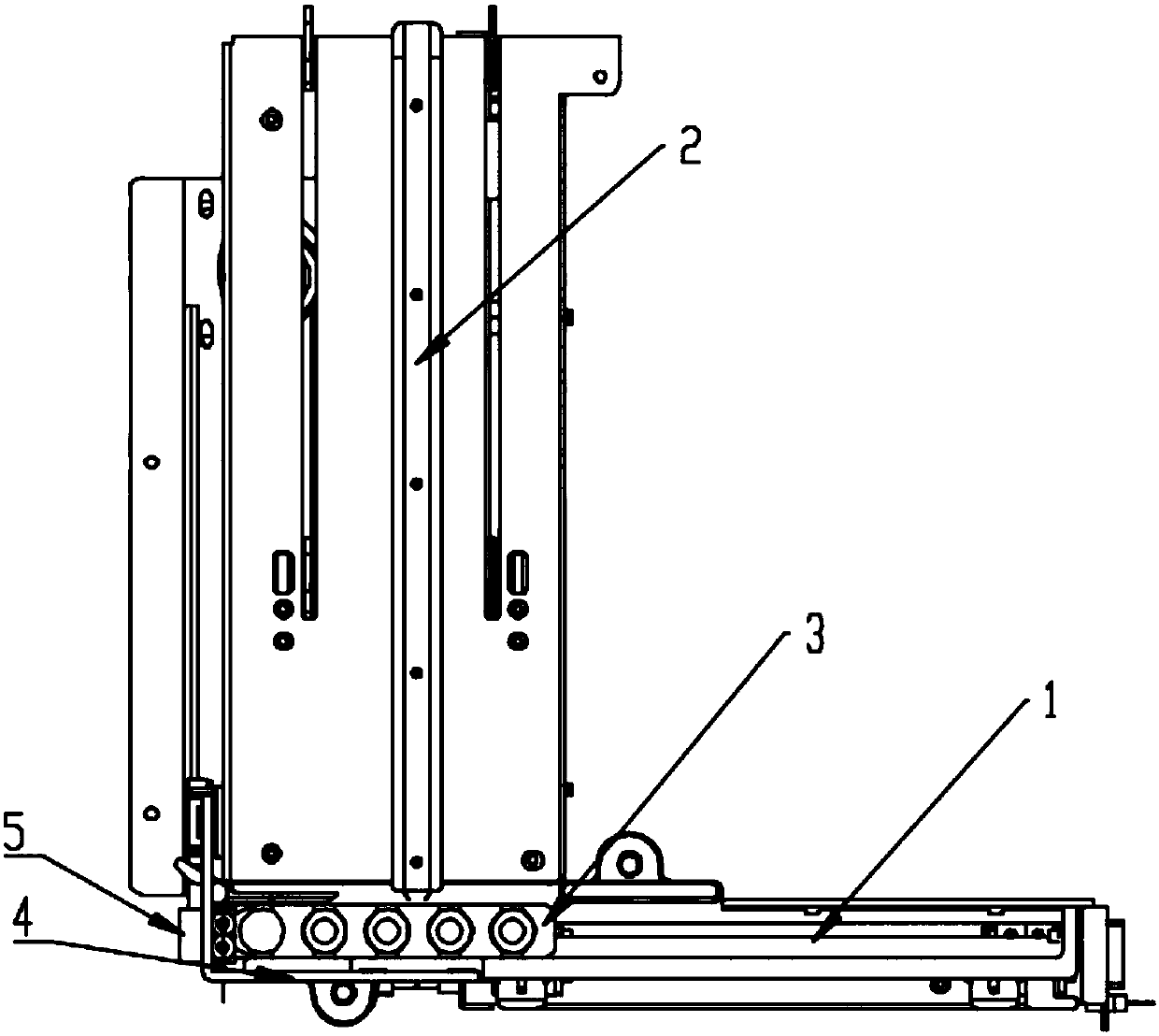
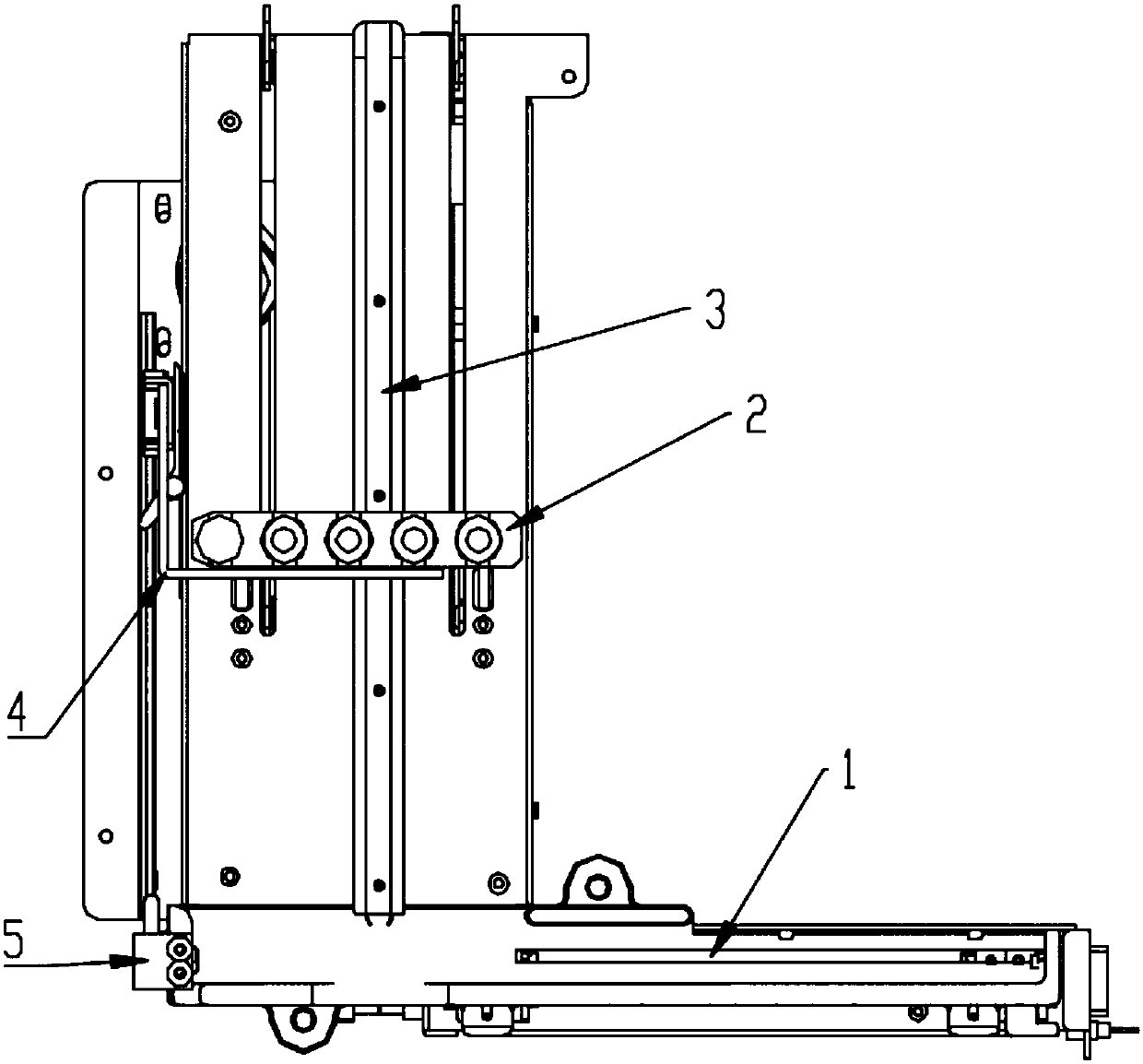
Sample frame positioning apparatus for full automatic luminescence measurement instrument
PendingCN107899631ASmooth initial movementPrecise positioningLab clamping meansMaterial analysisMechanical engineeringFully automated
The invention provides sample frame positioning apparatus for a full automatic luminescence measurement instrument. The sample frame positioning apparatus for the automatic luminescence measurement instrument comprises a first track, a second track and a sample frame; and the first track and the second track are located in a same plane, the starting end of the second track is perpendicularly connected to the tail end of the first track, the movement direction of the sample frame on the first track is parallel to the length direction of the sample frame, the movement direction of the sample frame on the second track is perpendicular to the length direction of the sample frame, a sample frame holding device is arranged on the side wall of the second track, and the sample frame holding deviceincludes a power section, a pushing section and a clamping section. The apparatus provided by the invention has the beneficial effects that because the above technical scheme is adopted, the startingmovement of the sample frame on the tracks is smooth and steady, and the starting positions are accurately positioned.
Owner:威海威高生物科技有限公司
Method for determining deoxynivalenol by chemiluminiscence
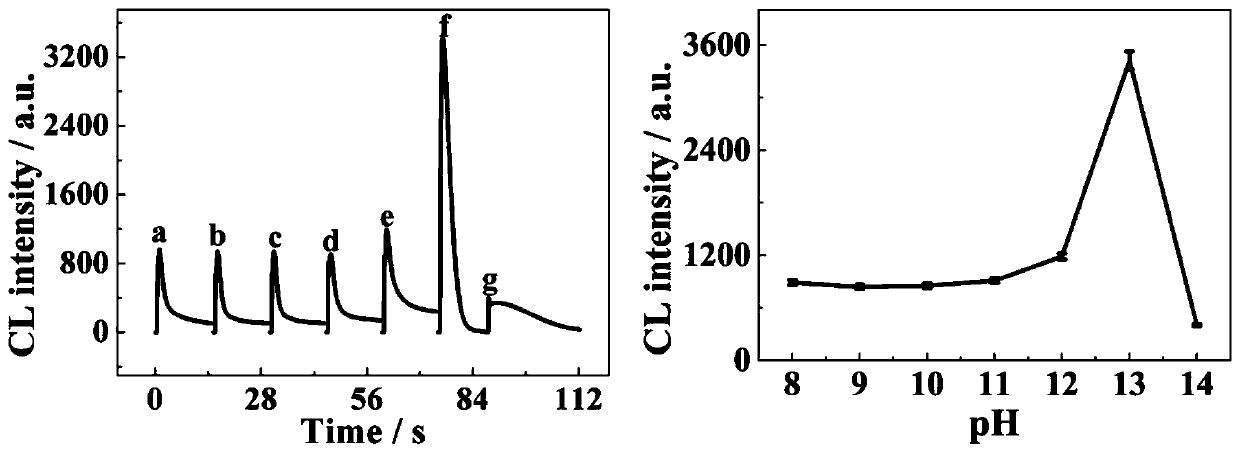
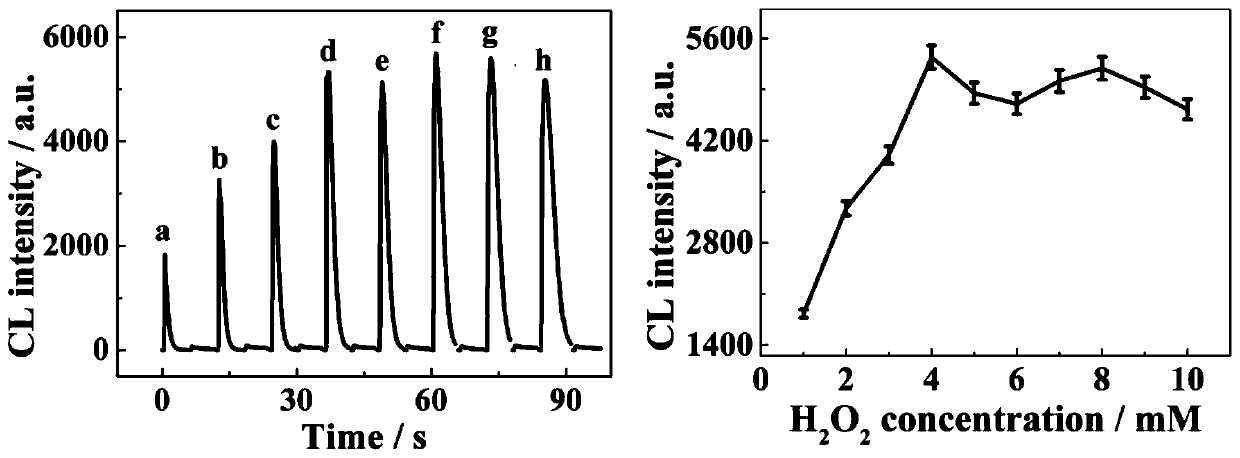
ActiveCN111413330AGood reproducibilityMaterial nanotechnologyTransportation and packagingBinary alloyAqueous solution
The invention belongs to the field of analytical chemistry and chemiluminiscence sensors. A PtCu binary alloy nano composite material is synthesized by taking (CH3COO)2Cu.H2O and K2PtCl6 as raw materials, starch centrifugate as a stabilizer and ascorbic acid as a reducing agent through an aqueous solution method. PtCu is used as a marker, and a novel method for determining deoxynivalenol is constructed by utilizing an aptamer recognition effect. The method is simple, high in sensitivity and low in cost.
Owner:QINGDAO UNIV OF SCI & TECH
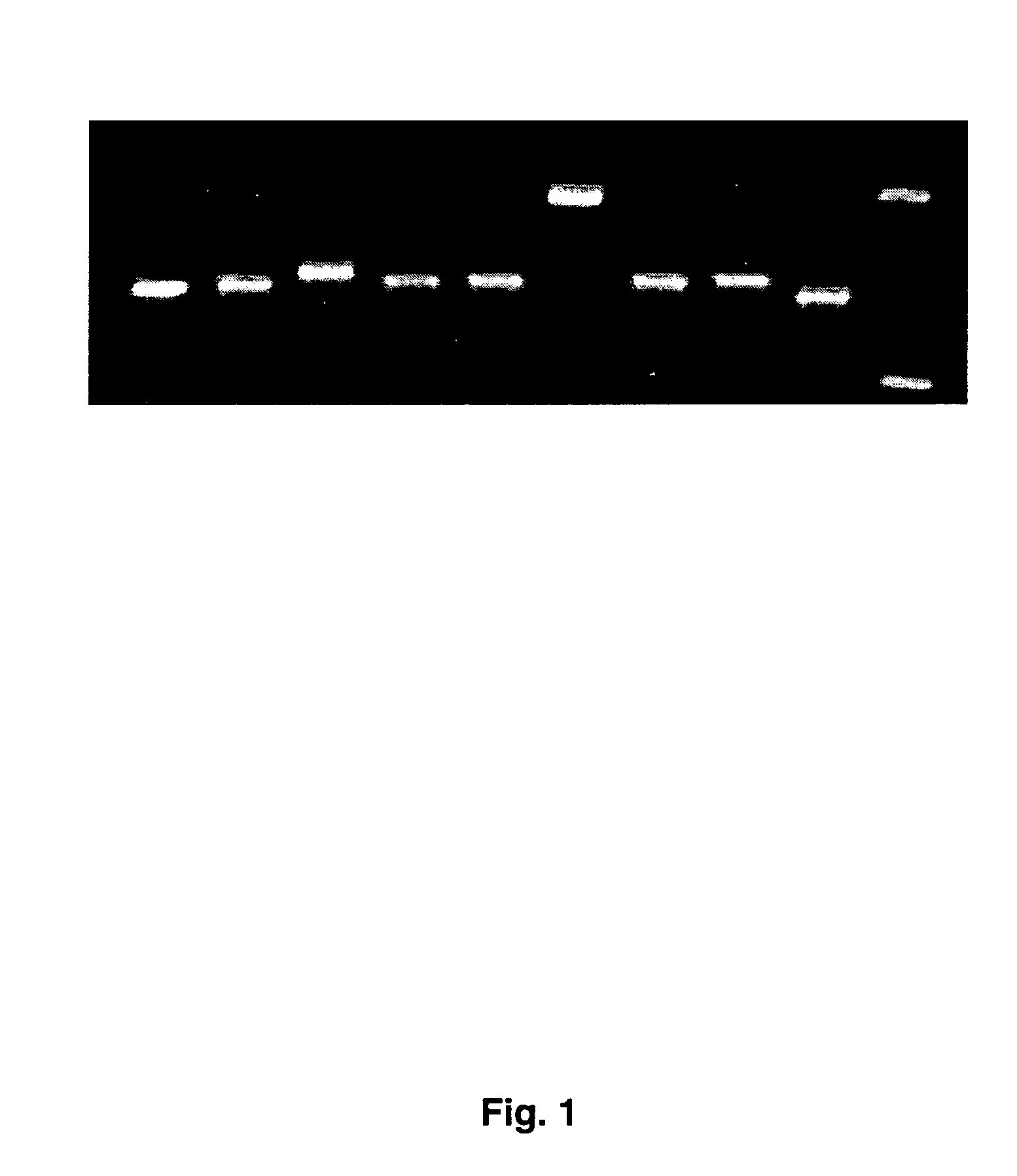
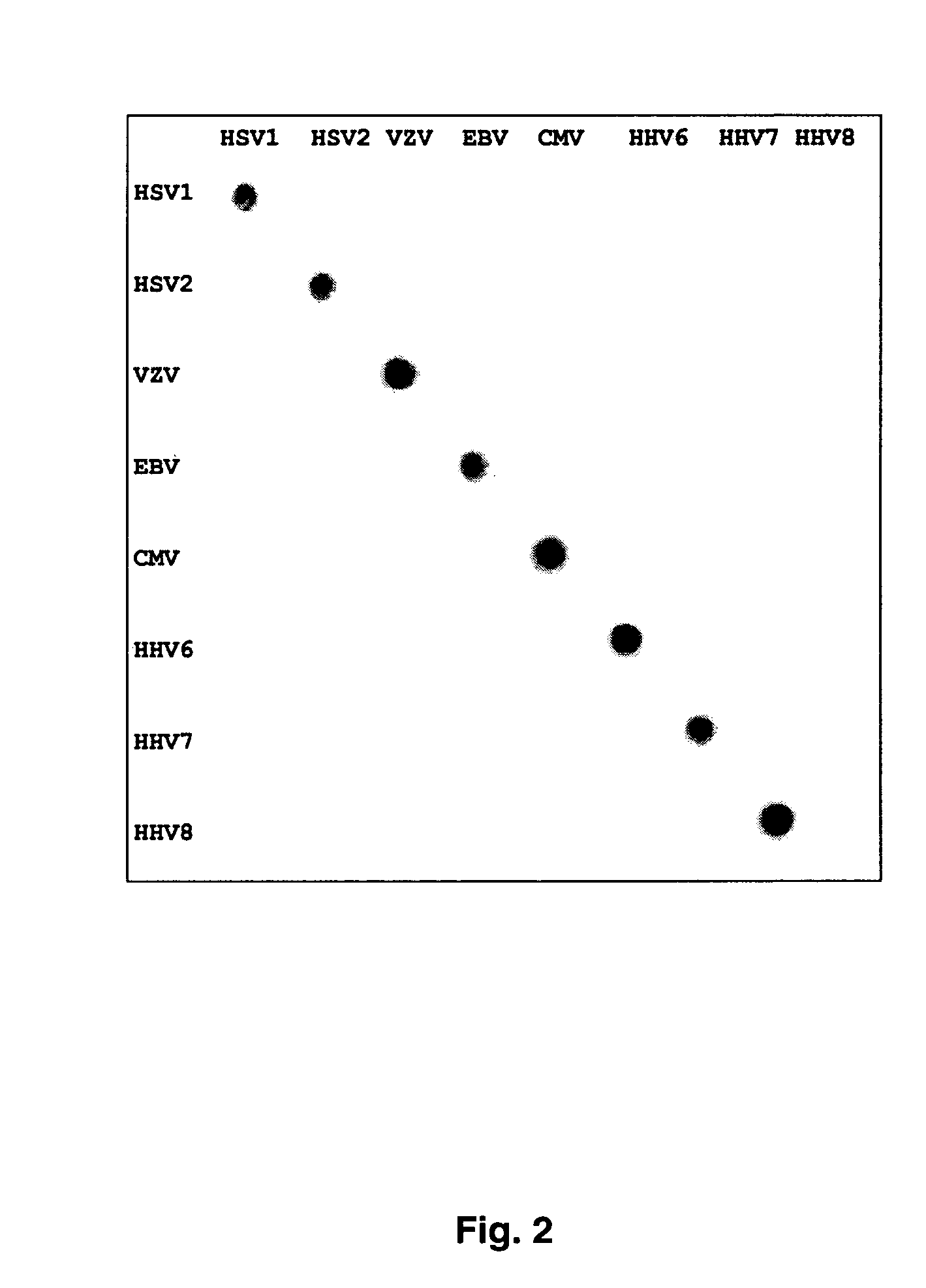
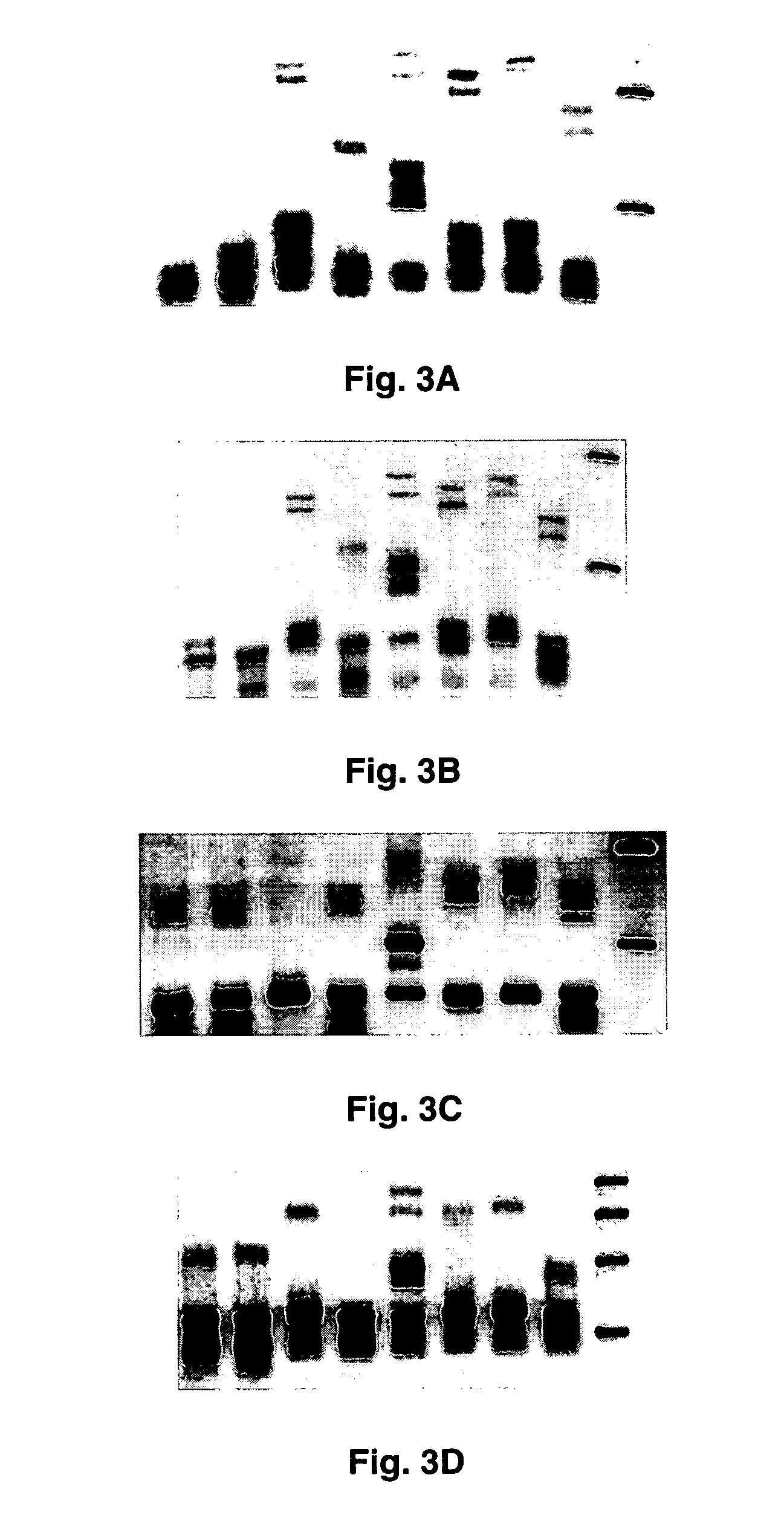
Clinical assays for the detection and typing of human herpesviruses
InactiveUS7348145B2Quick checkTest cycle time superiorSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
The present invention provides methods of unambiguously identifying a human herpesvirus in a sample. The assays, which allow for the detection and typing of all ten human herpesviruses, involve multiplex PCR assays using consensus primers to amplify conserved regions of the herpesvirus DNA. A dot blot / chemiluminescence assay and real time PCR assay ideal for clinical setting were disclosed. A heteroduplex mobility assay suitable for uses in research laboratory was also presented.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Microbial test kit, microbial test method and microbial test device
PendingUS20220170068A1Easy to testSamplingMicrobiological testing/measurementBiotechnologyMicroorganism
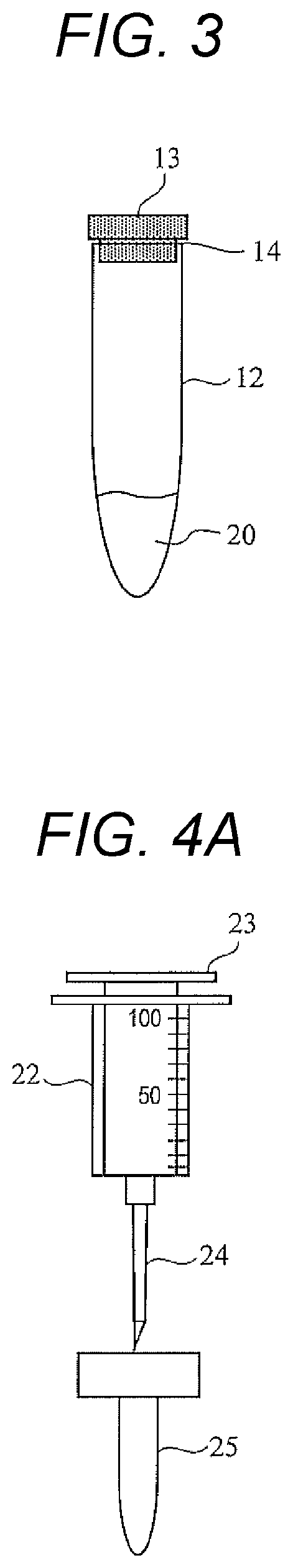
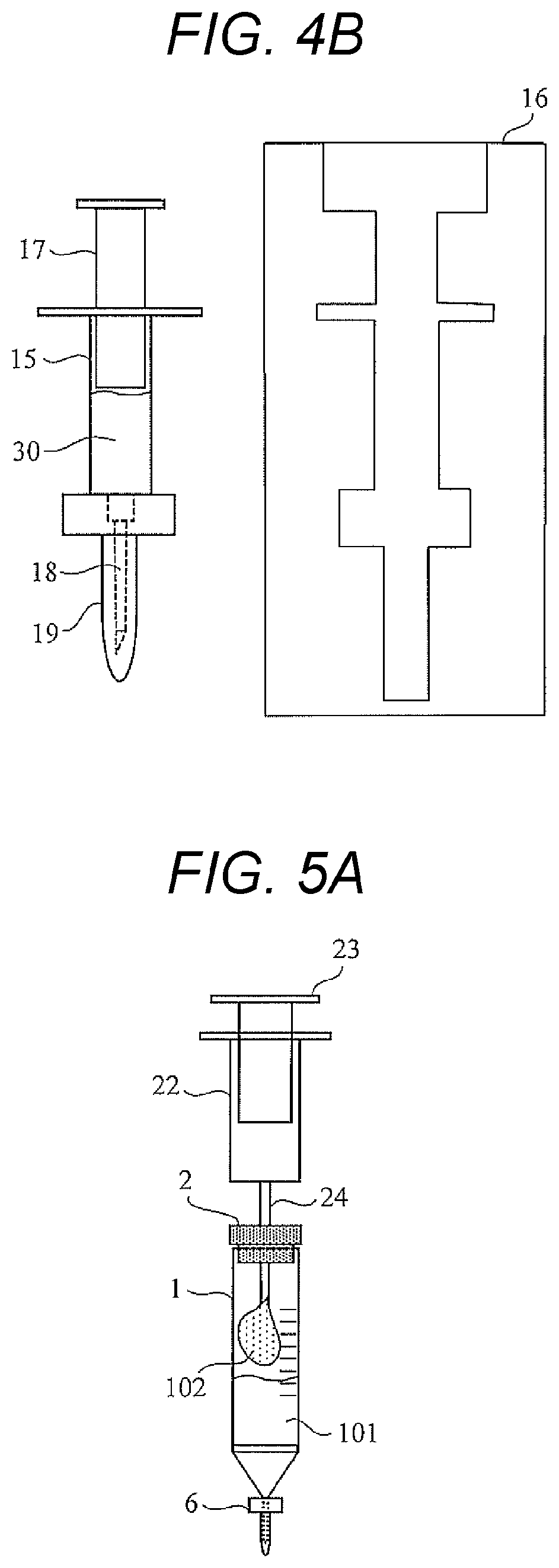
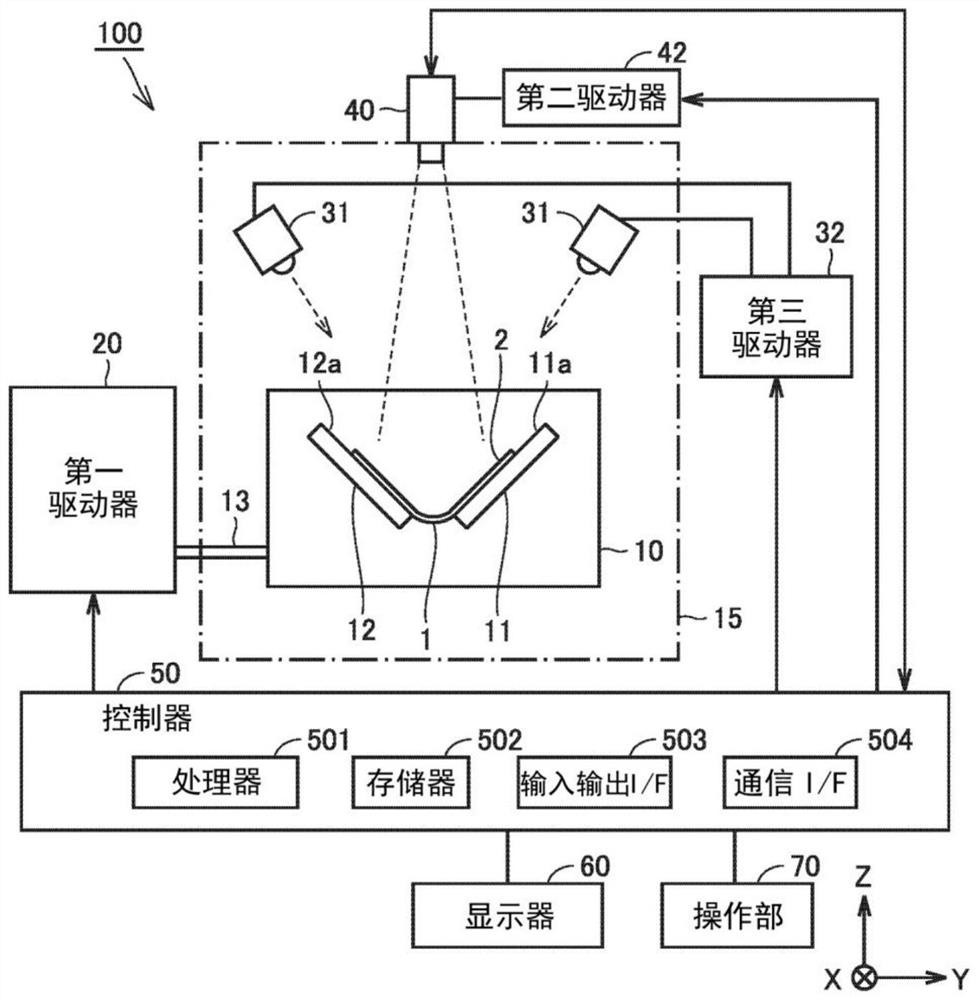
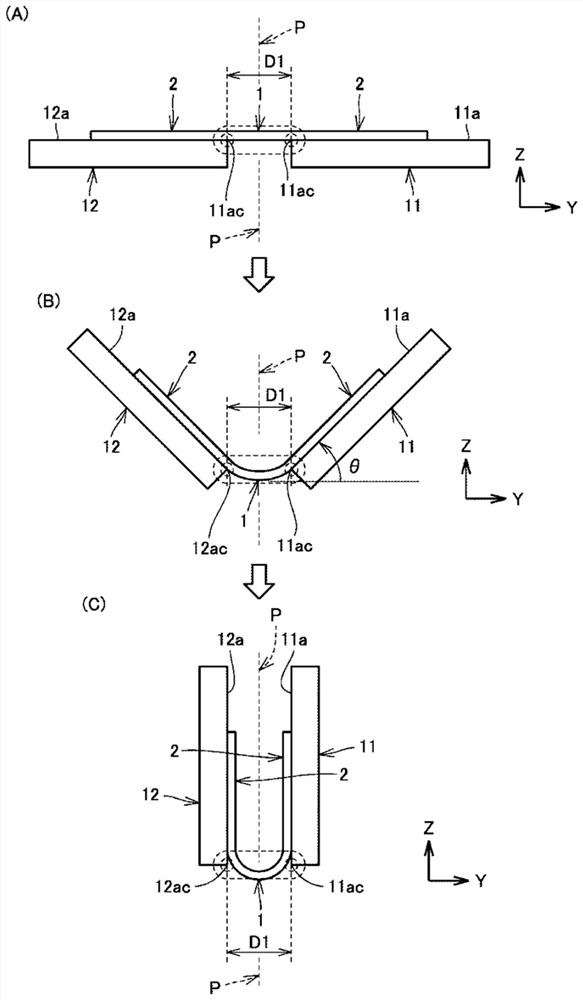
Provided is a technique capable of easily performing a microbial test with high accuracy. A microbial test kit of the present disclosure includes: a first syringe capable of collecting a specimen and having a first syringe needle; a second syringe containing an ATP extraction reagent and having a second syringe needle; a sealed reaction container having a first opening, a first sealing member fitted to the first opening, a nozzle, a nozzle cap covering the nozzle, and a filter disposed so as to partition the first opening and the nozzle; a waste liquid container including a second opening and a second sealing member fitted to the second opening, and sealed under reduced pressure; and a luminescence measurement container that includes a third opening and a third sealing member fitted to the third opening, stores a luminescent reagent that emits light in presence of ATP, and is sealed under reduced pressure.
Owner:HITACHI HIGH-TECH CORP
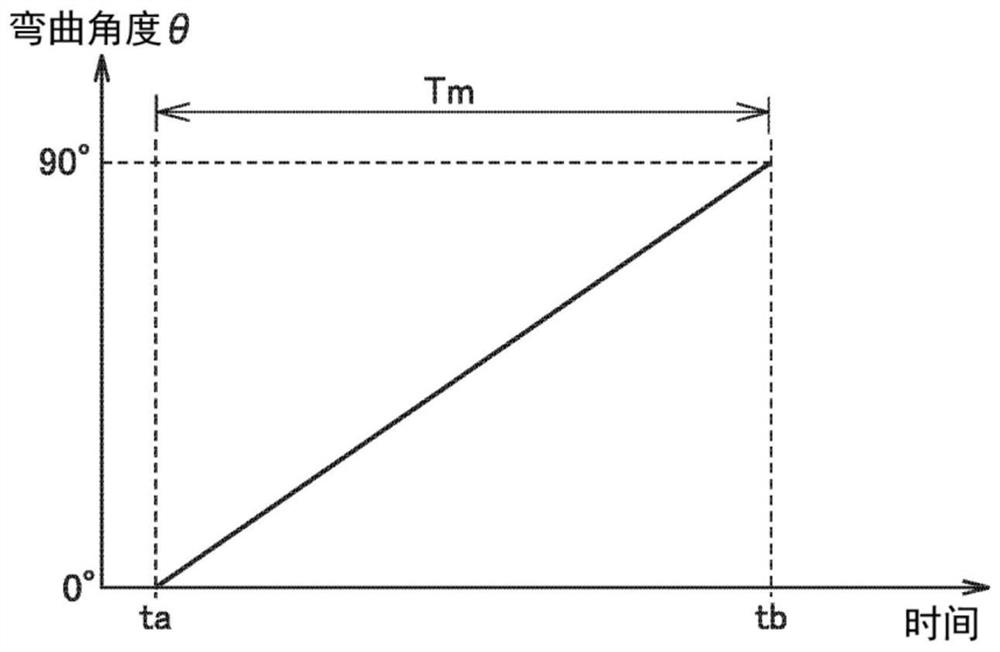
Stress luminescence measurement device and stress luminescence measurement method
InactiveCN113670707AForce measurement by measuring optical property variationMaterial strength using tensile/compressive forcesCamera controlImage capture
Owner:SHIMADZU SEISAKUSHO CO LTD
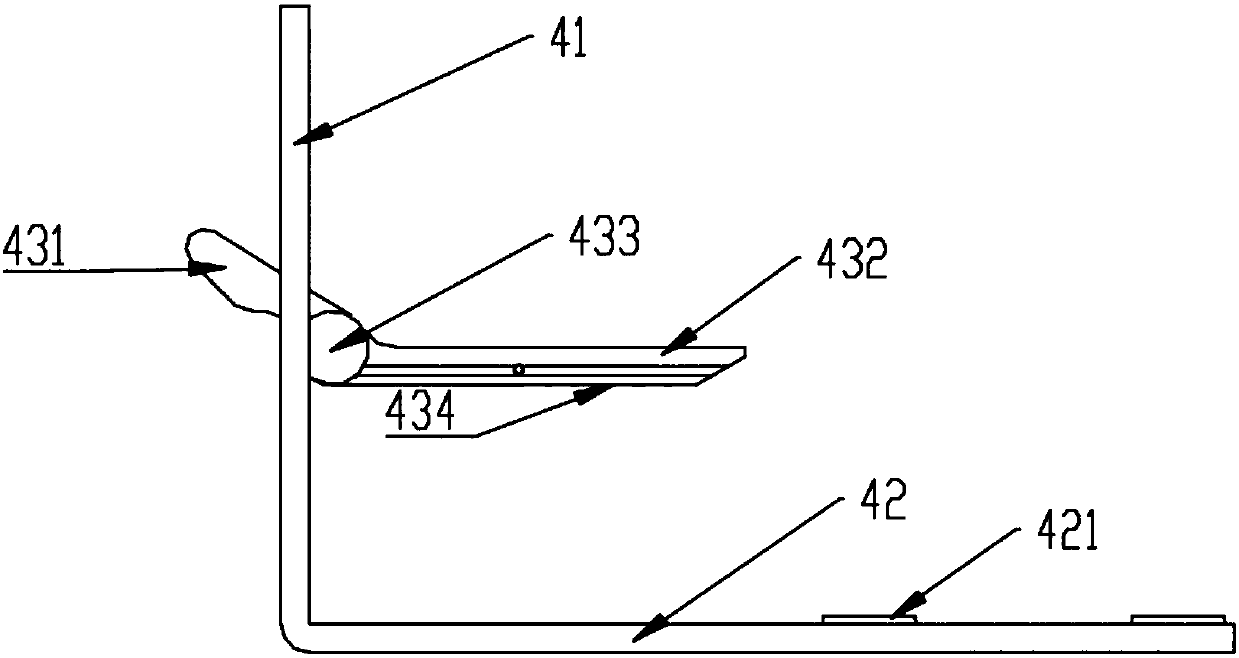
Fully automatic chemiluminescence analyzer
ActiveCN109406804BSimple structureEasy maintenanceChemiluminescene/bioluminescenceBiological testingReagent stripAssay
The invention belongs to the technical field of chemiluminescence measurement, and provides a full-automatic chemiluminescence measuring instrument. An incubating component is installed on the base of a transmission rack, and a shell breaking sampling mechanism, a magnetic separation mechanism and a testing mechanism are arranged above the base. , and set a plurality of tip slots for placing multiple tips on the incubation component, so that the incubation component is kept at a constant temperature and the limit is equipped with multiple reagent strips with multiple storage pools. The multiple storage pools seal different samples, and After the shell breaking sampling mechanism is plugged and removed, the gun head is controlled to puncture the capsule of the reagent strip and sampled, and the gun head is controlled to take samples to obtain magnetic beads and solutions for the magnetic separation mechanism to separate and leave the magnetic beads to put into multiple containers. The test pool in the object pool, and then the test mechanism is socketed with the test pool to form a light-shielding environment to read the light intensity of the solution to be tested in the test pool, so that it can automatically break the shell of multiple reagent strips and pick and place multiple tips. , Sampling, magnetic separation and testing operations, not only simple in structure, but also high in efficiency and low in cost.
Owner:深圳泰乐德医疗有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com