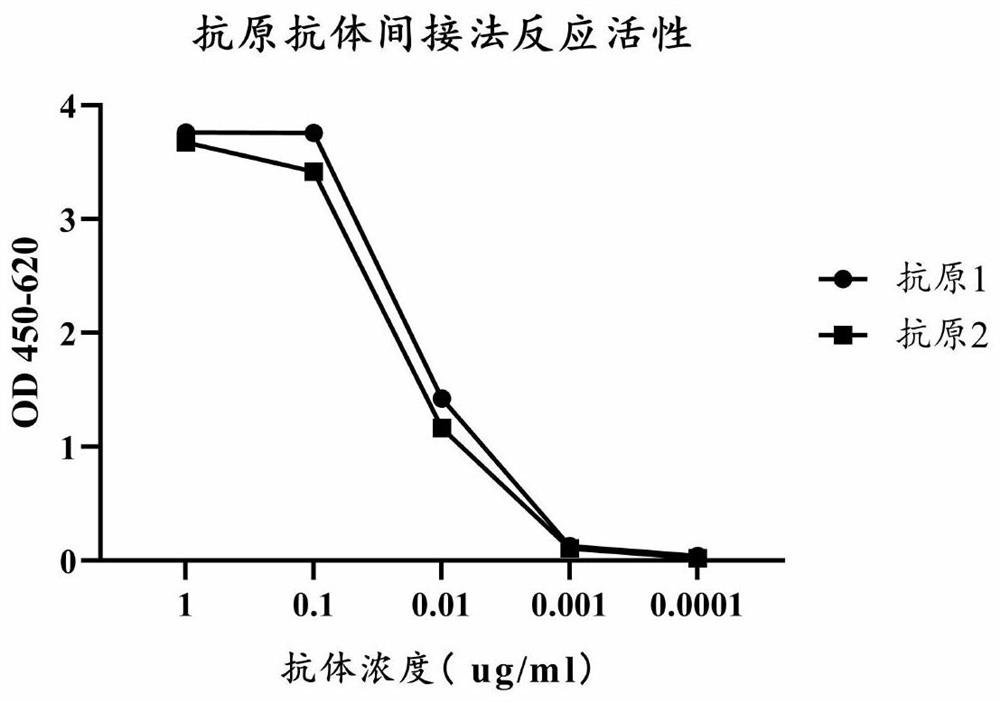
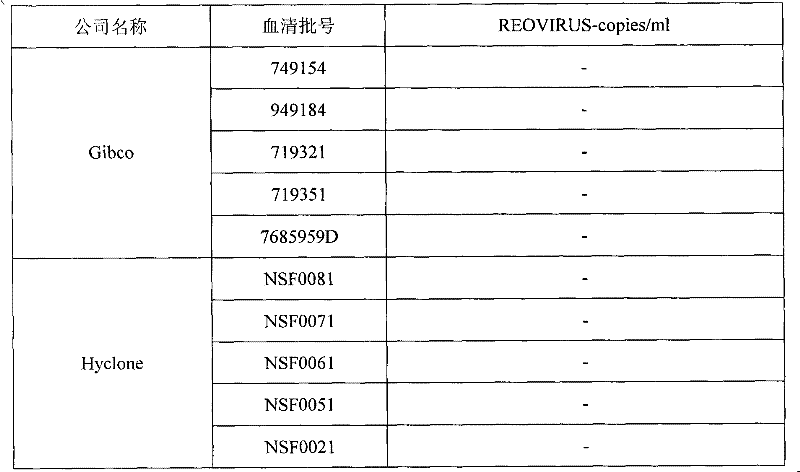
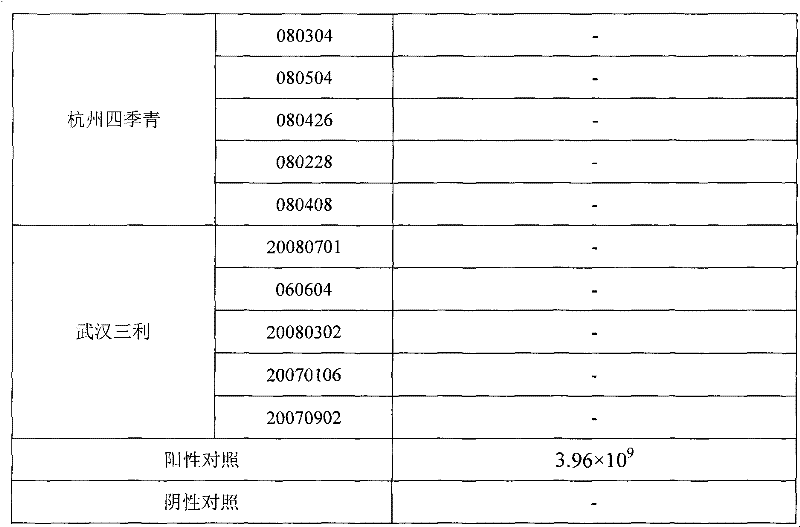
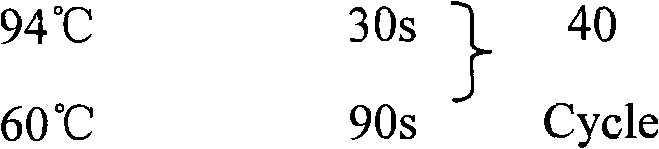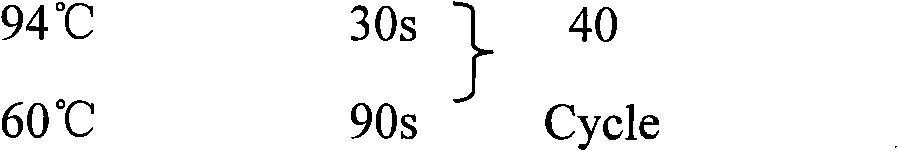Patents
Literature
30 results about "Serum product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and composition for conferring immunity in mammals
A composition and method for immunization of mammals, containing antibodies from the eggs / egg yolks of chickens, or other suitable avian source, hyperimmunized against a selected pathogen(s) or immunogens, and a quantity of non-specific mammalian antibodies, such as those from commercial colostrum or serum products, which are combined to produce a composition that provides protection against the selected pathogen. Applications include viral, bacterial and parasitic infections.
Owner:LA BELLE ASSOCS
Viral reduction method for plasma using a leukocyte-reduction filter and two virus-reduction filters of decreasing pore diameters
ActiveUS7592134B2Efficient methodEfficient removalOther blood circulation devicesHaemofiltrationWhite blood cellLeukocyte Reduction Filtration
The present invention relates to a plasma product or a serum product with an extremely low risk of viral contamination and a method for producing the same. Before treating plasma or serum to be used as a raw material for producing a plasma product or a serum product using a virus removal membrane, leucocytes contaminating the blood are removed. Thus, a plasma product or a serum product with an extremely low risk of viral contamination can be efficiently produced while preventing clogging. Since clogging scarcely arises, it is possible to carry out efficient filtration without applying an elevated pressure as the filtration proceeds.
Owner:ASAHI KASEI MEDICAL CO LTD
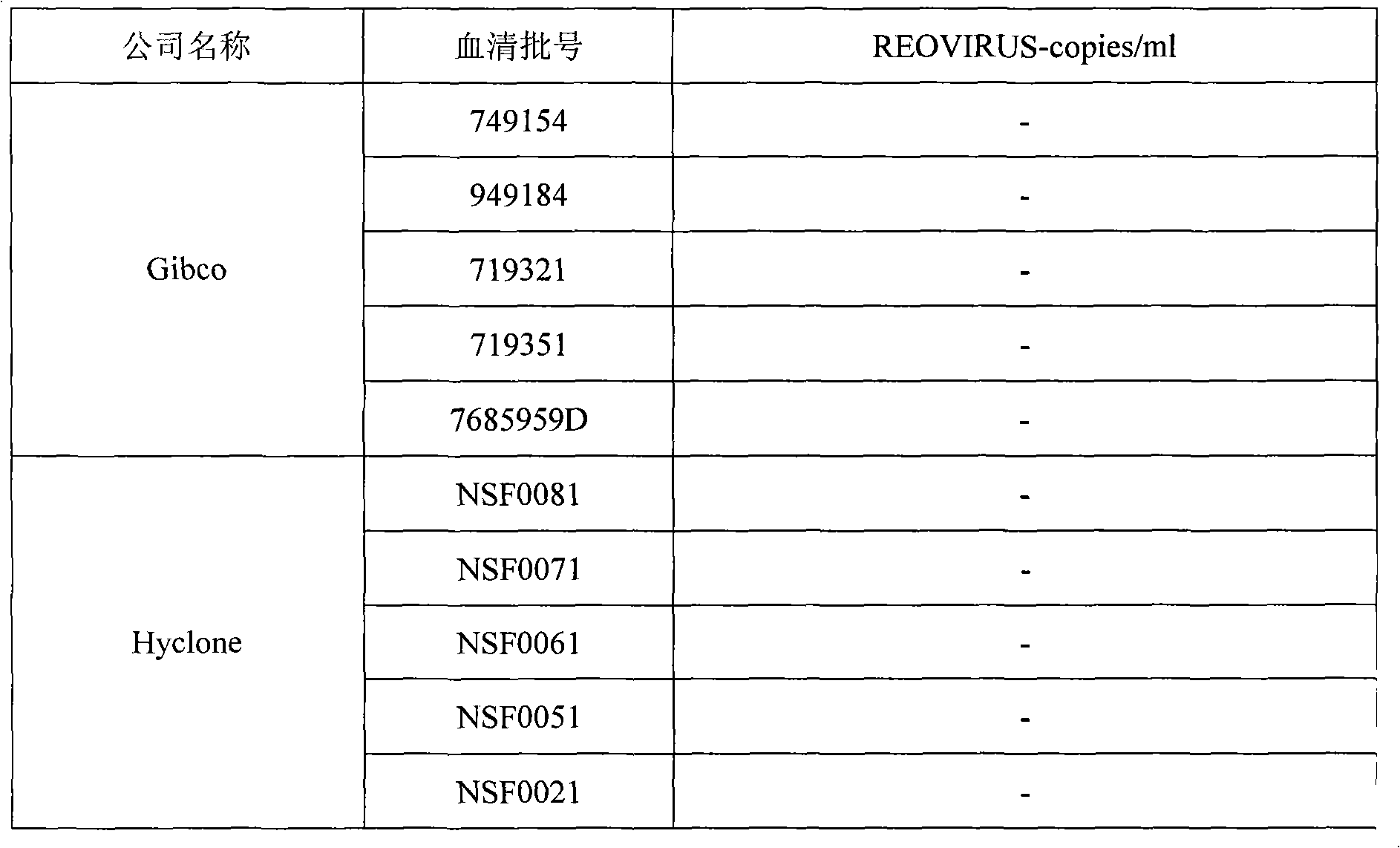
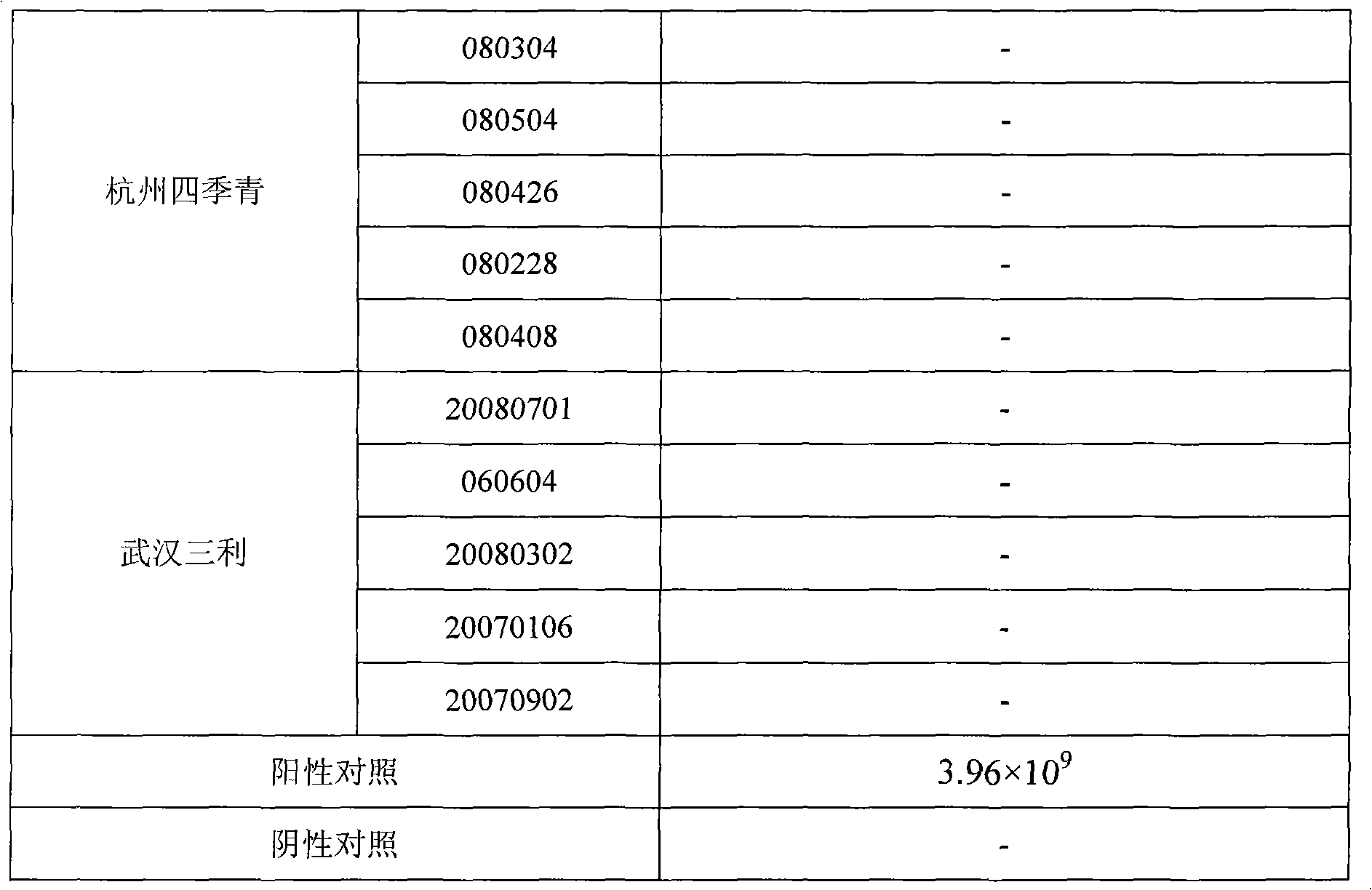
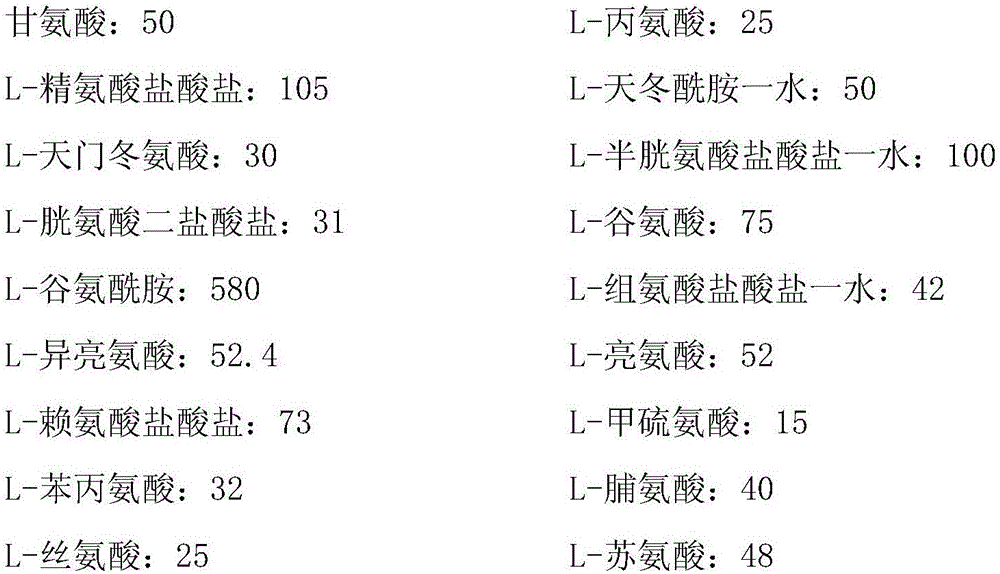
Reovirus-detecting fluorescence quantitative PCR kit and application thereof
ActiveCN101565757AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesRNA extractionReverse transcriptase
The invention discloses a reovirus-detecting fluorescence quantitative PCR kit and an application thereof. The kit comprises: a) an RNA extraction solution, b) a reverse transcriptase reaction solution, c) reverse transcriptase, d) an RNA enzyme inhibitor, e) a primer and a TaqMan probe, f) a standard positive DNA template and g) a PCR fluorescence quantitative reaction solution. The kit is characterized in that: primer sequence is a sense primer: 5'-TGCGCCTATCCTTGAGTTGA-3', and an antisense primer: 5'-TTGCCAGGAAATACGGGTCT-3', and the size of an amplicon is 138 bp; the sequence of a fluorescence probe is: 5'-FAM-TCAAAATGGTGGACTTCAGTTTCGATTT-TAMRA-3', the 5' end of the probe is marked with a fluorescence emission group FAM, the 3' end is marked with a fluorescence quenching group TAMRA, the standard positive DNA template transforms a colon bacillus DH5 alpha by a pGEM-T carrier inserted with reovirus S1 protein zone 361 bp segment, plasmid is extracted after proliferation, an A260 ration is measured in an ultraviolet spectrophotometer, and the plasmid is diluted by 10 times of gradient. The kit can efficiently and conveniently monitor the reovirus pollution in serum products in real time, can be applicable to epidemiology investigation of reovirus infection, and can provide technical support for relevant fundamental researches, thus having quite broad application prospect.
Owner:WUHAN SANLI BIO TECH
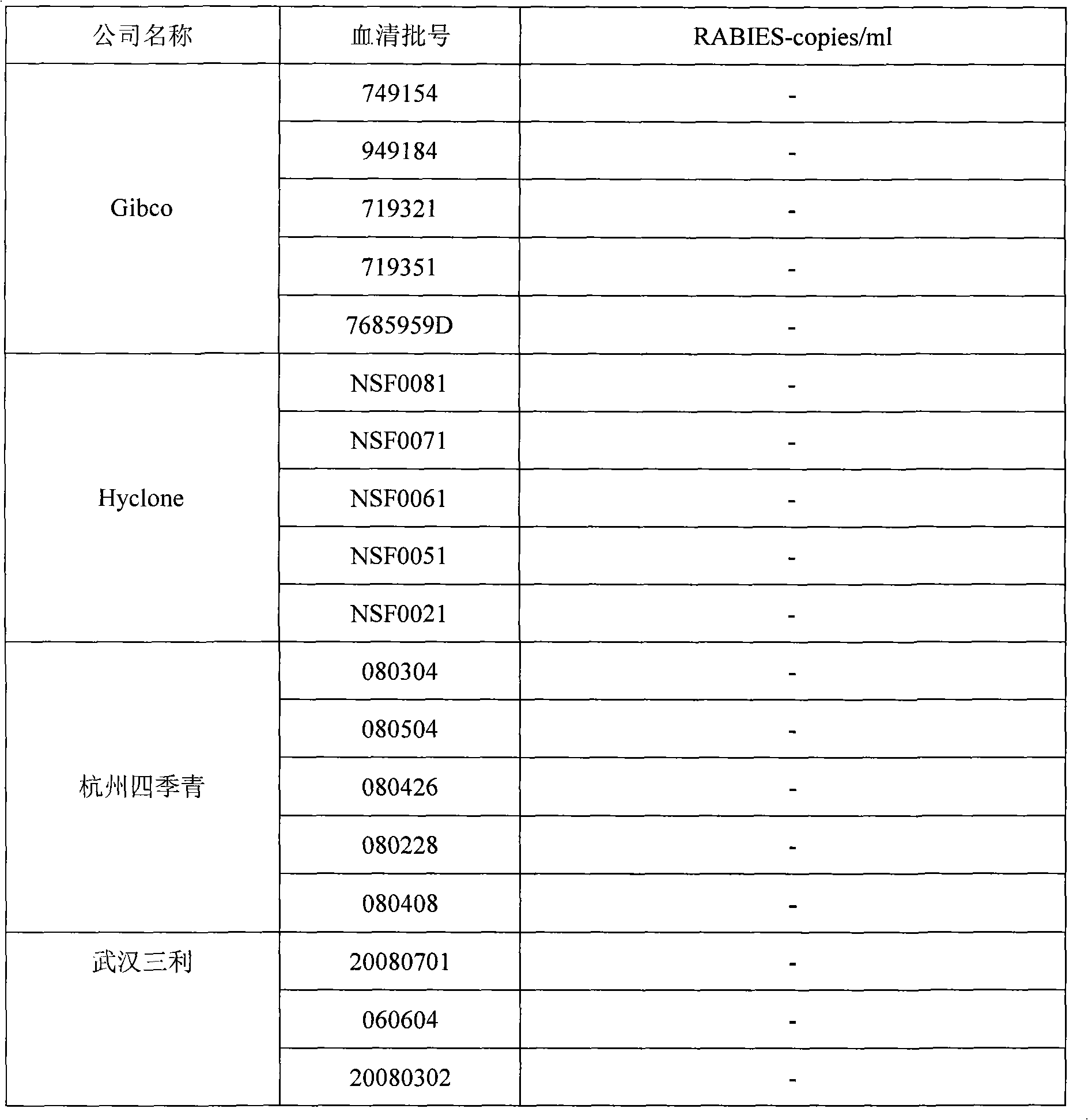
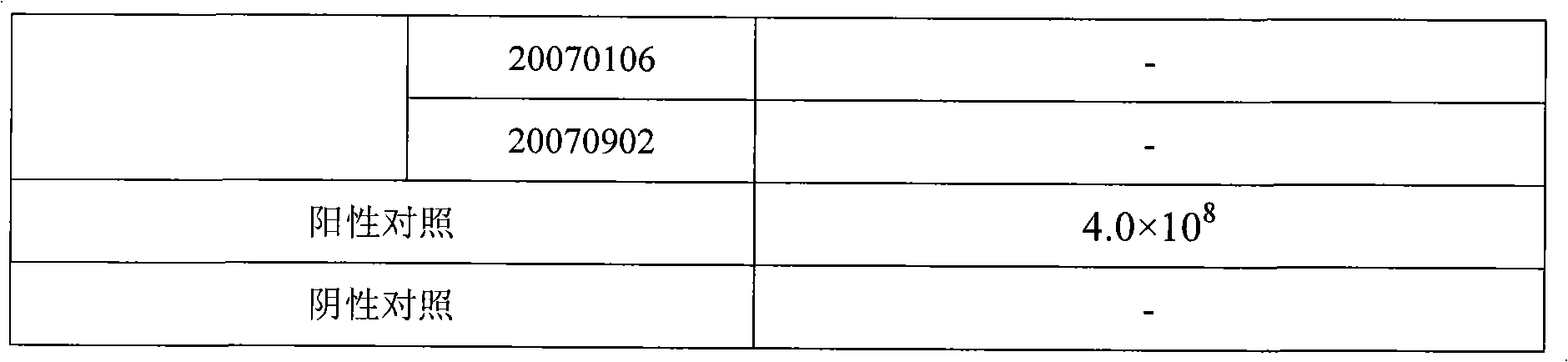
Rabies virus detecting fluorescence quantitative PCR kit and application thereof
ActiveCN101565758AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesRNA extractionReverse transcriptase
The invention discloses a rabies virus detecting fluorescence quantitative PCR kit and an application thereof. The kit comprises: a) an RNA extraction solution, b) a reverse transcriptase reaction solution, c) reverse transcriptase, d) an RNA enzyme inhibitor, e) a primer and a TaqMan probe, f) a standard positive DNA template, and g) a PCR fluorescence quantitative reaction solution. The kit is characterized in that: primer sequence is a sense primer: 5'-TAGGATGCTATATGGGTCAAGTCAGA-3', and an antisense primer: 5'-TTCAAATGTCCCTTTCCCGAAGAA-3', and the size of an amplicon is 125 bp; the sequence of a fluorescence probe is: 5'-FAM-CAACGGTTATTGCTGCATGTGCTCCTGA-TAMRA-3', the 5' end of the probe is marked with a fluorescence emission group FAM, the 3' end is marked with a fluorescence quenching group TAMRA, the standard positive DNA template transforms a colon bacillus DH5 alpha by a pGEM-T carrier inserted with rabies virus N protein zone 391 bp segment, plasmid is extracted after proliferation, an A260 ration is measured in an ultraviolet spectrophotometer, and the plasmid is diluted by 10 times of gradient. The kit can efficiently and conveniently monitor the rabies virus pollution in serum products in real time, and can provide technical support for relevant fundamental researches, thus having quite broad application prospect.
Owner:WUHAN SANLI BIO TECH
Serum-free medium and preparation method thereof
Owner:山东巨山能源科技有限公司
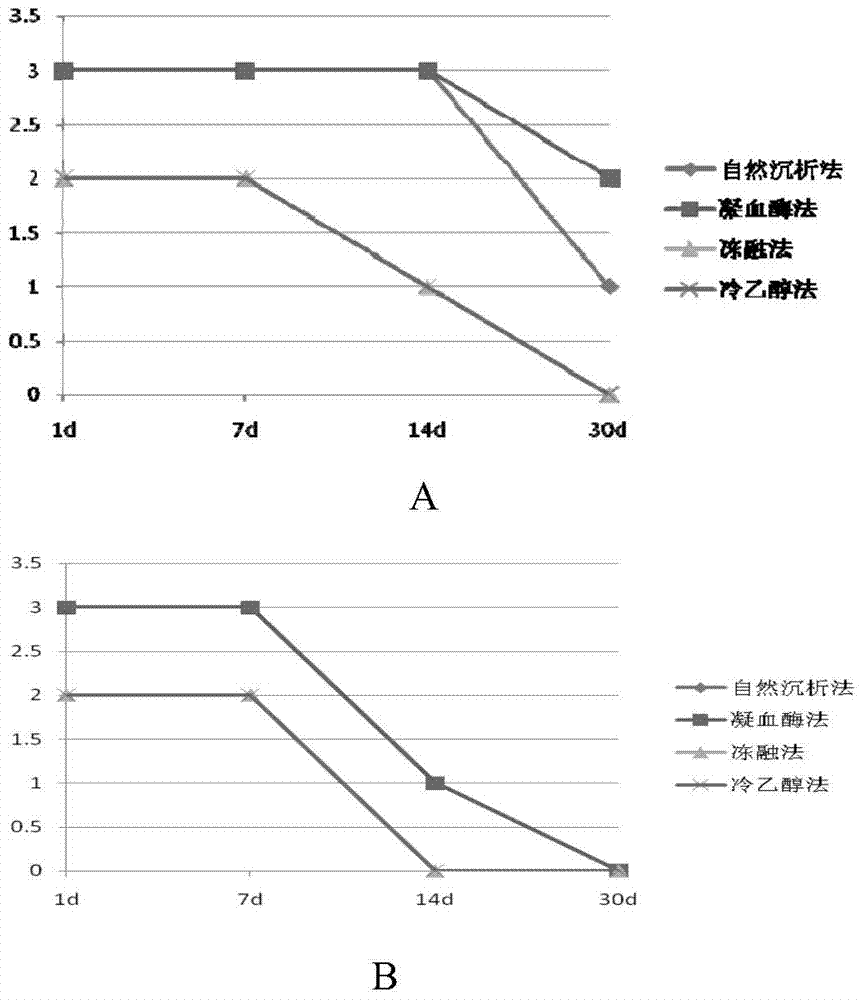
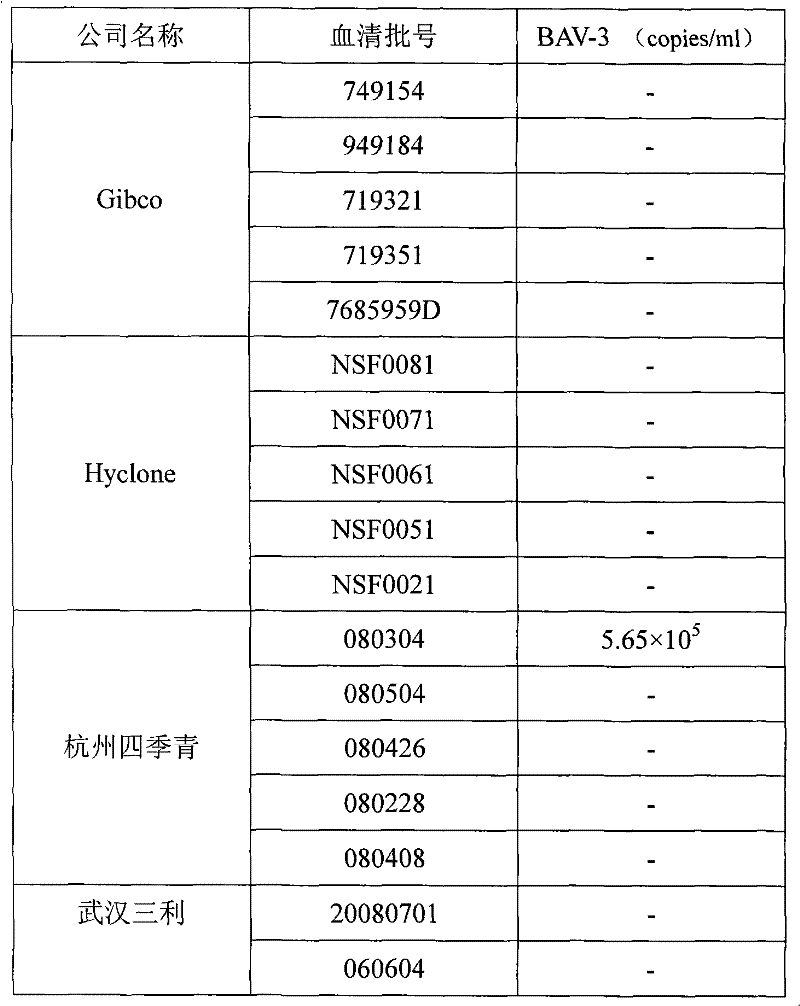
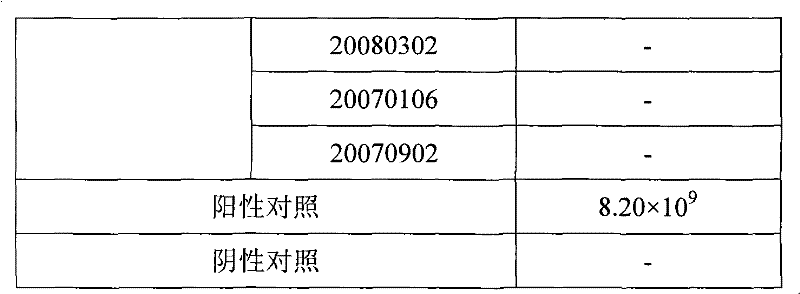
Fluorescence quantitative PCR kit for detecting type-3 cow adenovirus and application
ActiveCN101560573AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescenceBasic research
The invention discloses a fluorescence quantitative PCR kit for detecting type-3 cow adenovirus and application. The kit comprises a) DNA extraction reagent, b) hot starting Taq DNA polymerase, c) primers and TaqMan probe, d) standard positive DNA template, and e) PCR fluorescence quantitative reaction liquid. The kit is characterized in that the sequence of a positive primer is 5'-CCTGAATTCTCTTGCAGCCAGA-3', the sequence of a negative primer is 5'-CCTACCGAACCGACGCAGAT-3', the size of an amplicon is 100bp, the sequence of a fluorescence probe is 5'-FAM-TGAGAAGGTACTCCTCGTCGCTGGACCA-TAMRA-3', a 5' end of the probe marks a fluorescence emitting group FAM, a near 3' end of the probe marks a fluorescence quenching group TAMRA, the standard positive DNA template converts colon bacillus DH5a by a pGEM-T carrier inserted into a type-3 adenovirus pol protein 100bp fragment, plasmids are extracted after multiplication to prepare the kit, and A260 is measured by an ultraviolet spectrophotometer to definite quantity and is diluted by 10 times of gradient. The kit efficiently and conveniently monitors type-3 cow adenovirus pollution in a serum product in real time, can be widely applied to epidemiology research on adenovirus infection, can provide technical support for related basic research, and has wide application prospect.
Owner:WUHAN SANLI BIO TECH
Medicament
InactiveUS20080207500A1Remarkable effectConvenient sourceSenses disorderNervous disorderDiseaseTreatment effect
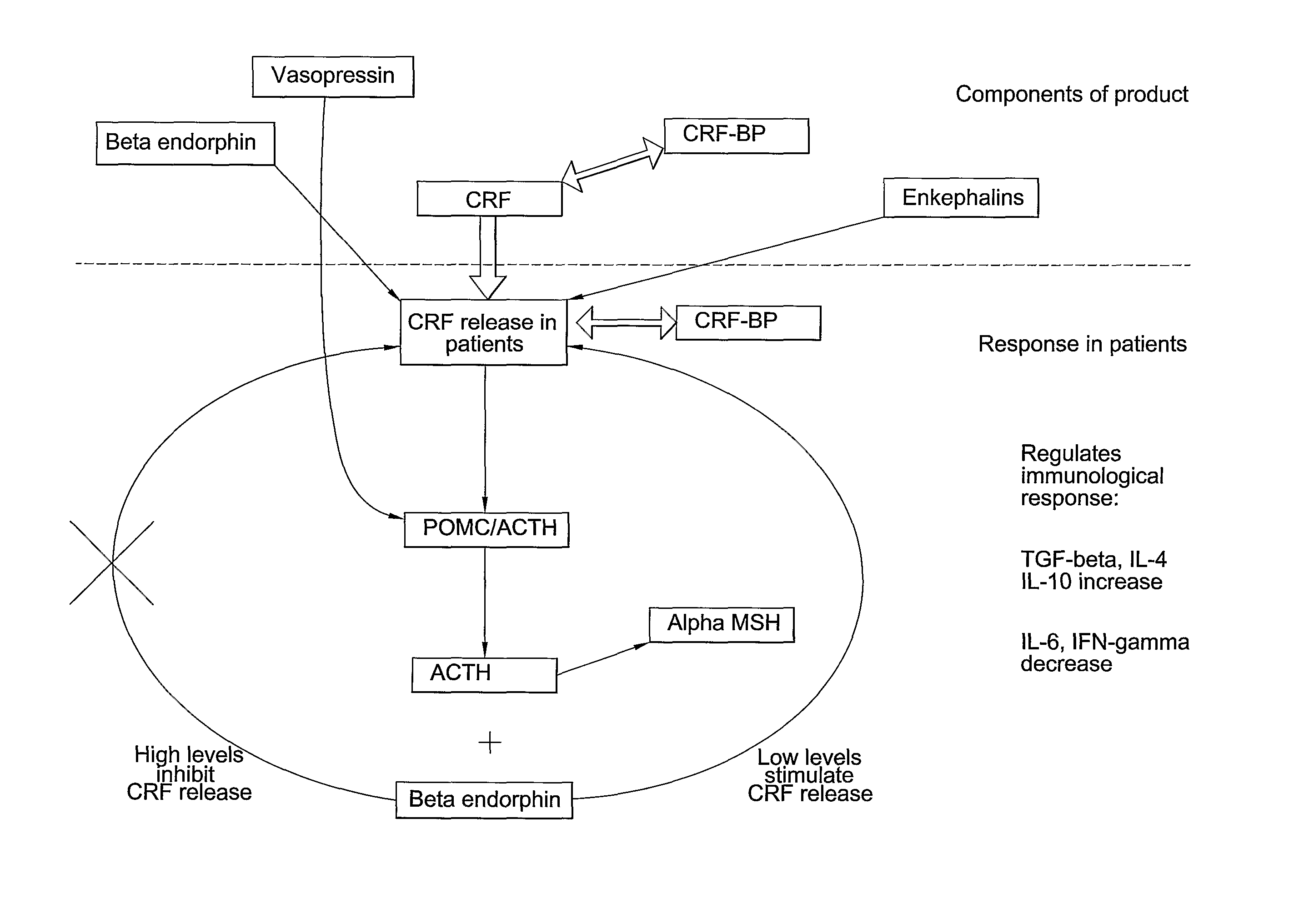
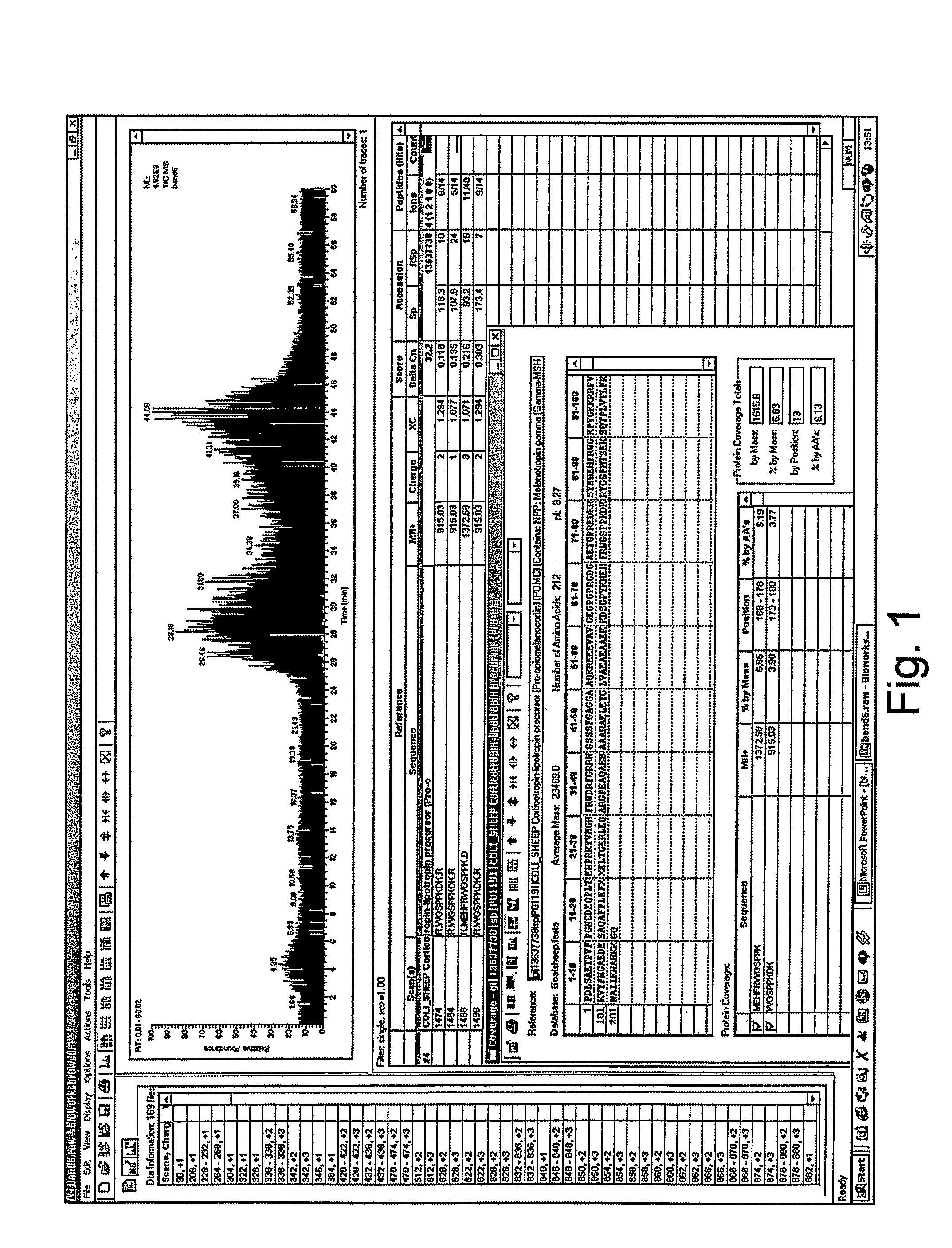
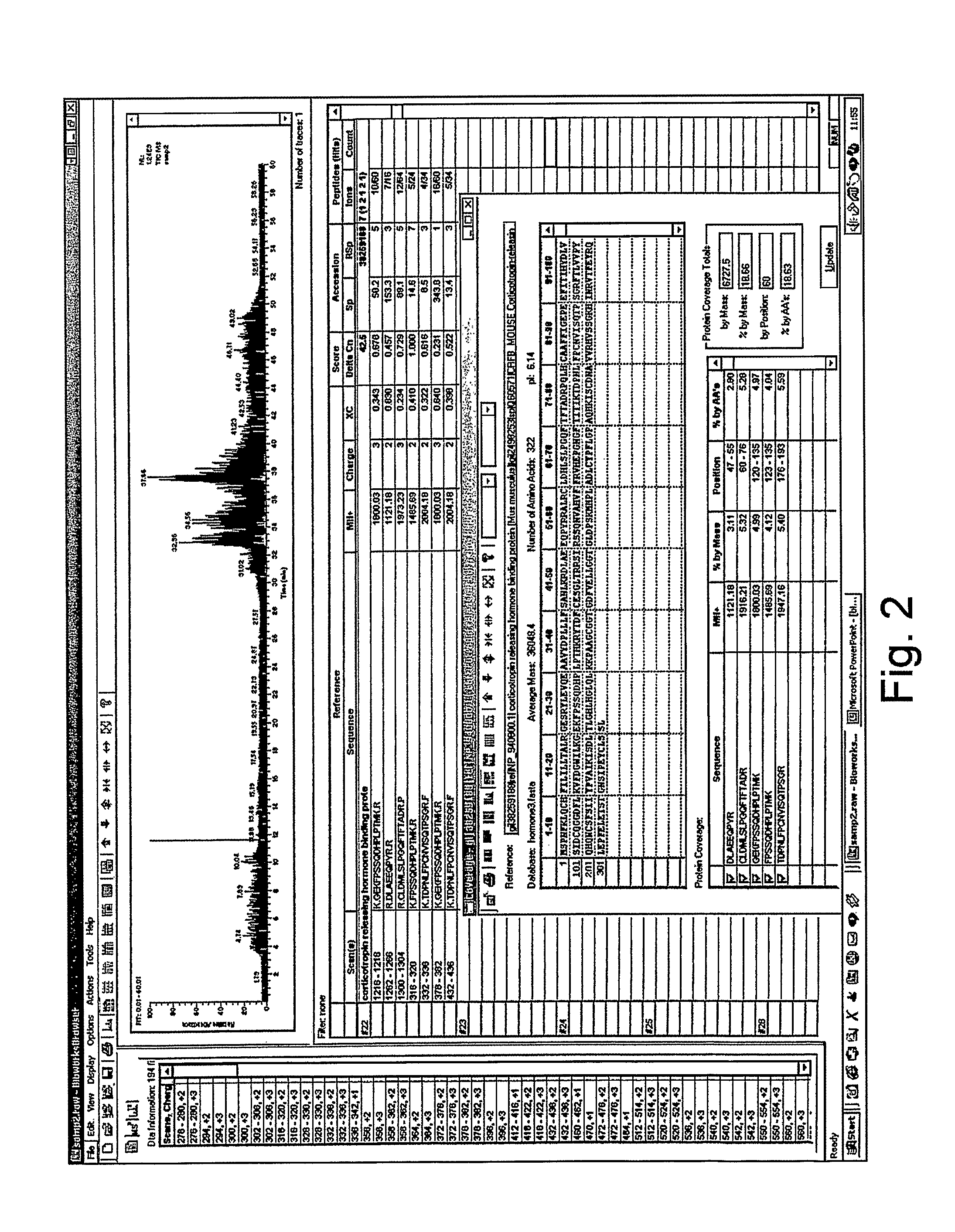
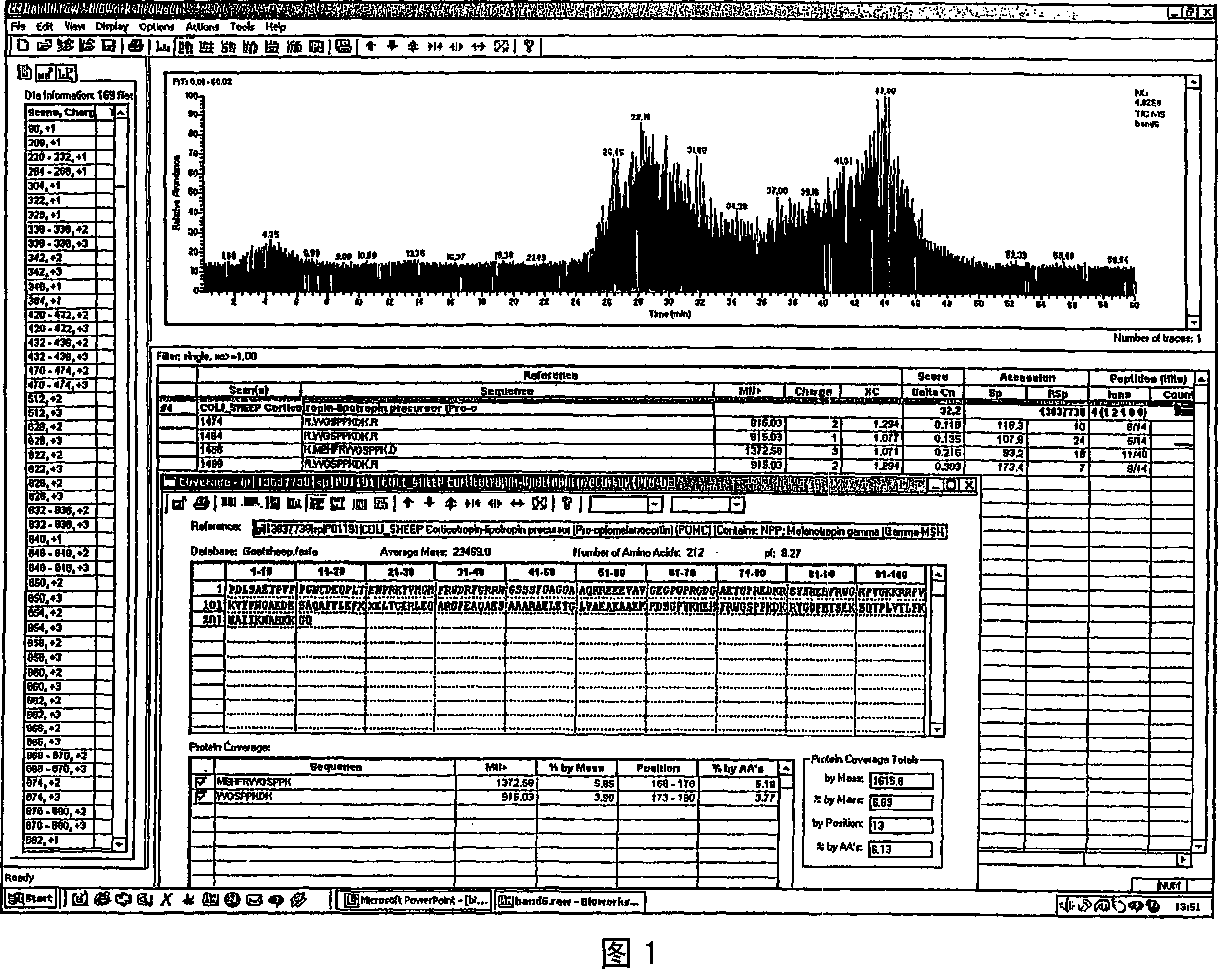
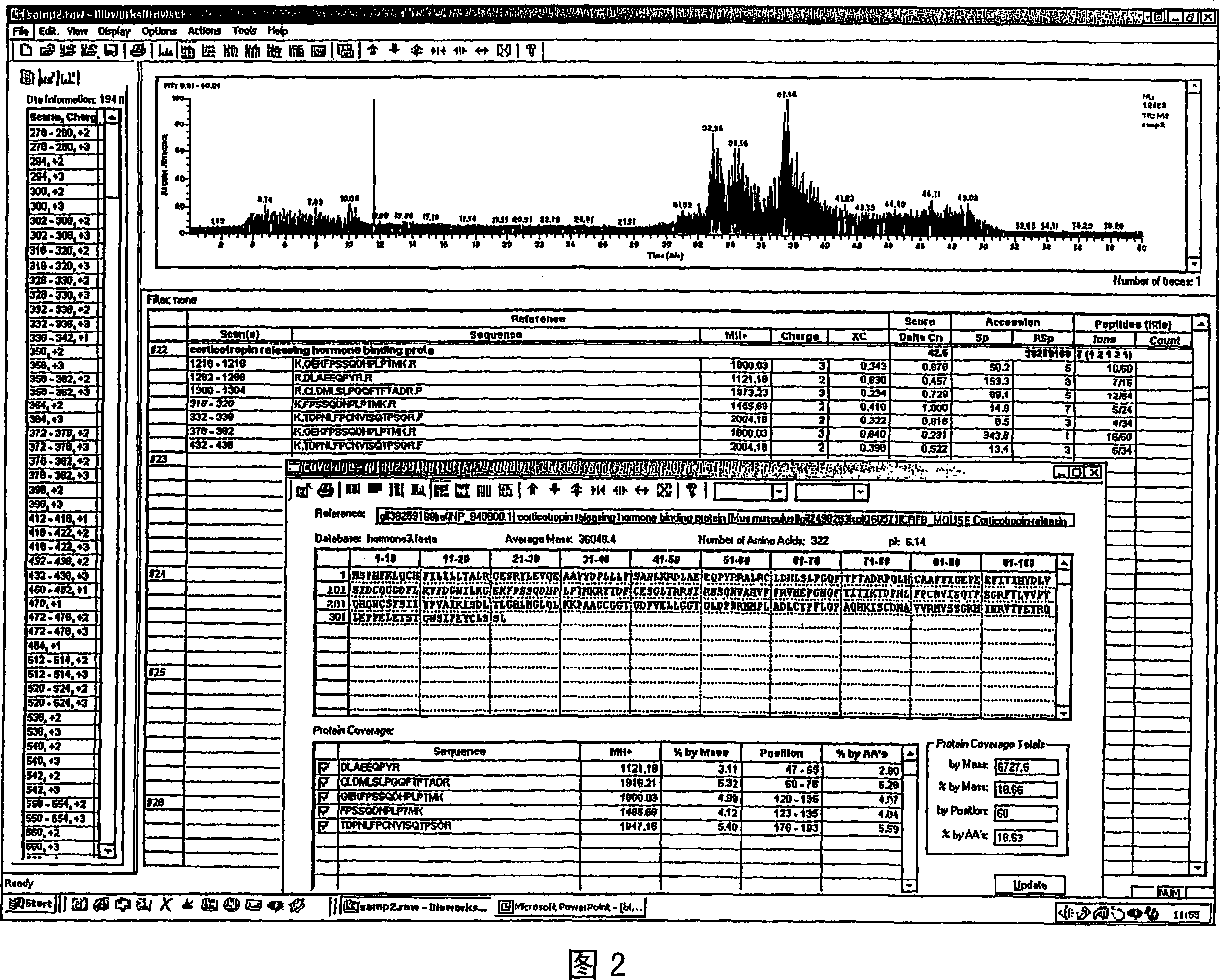
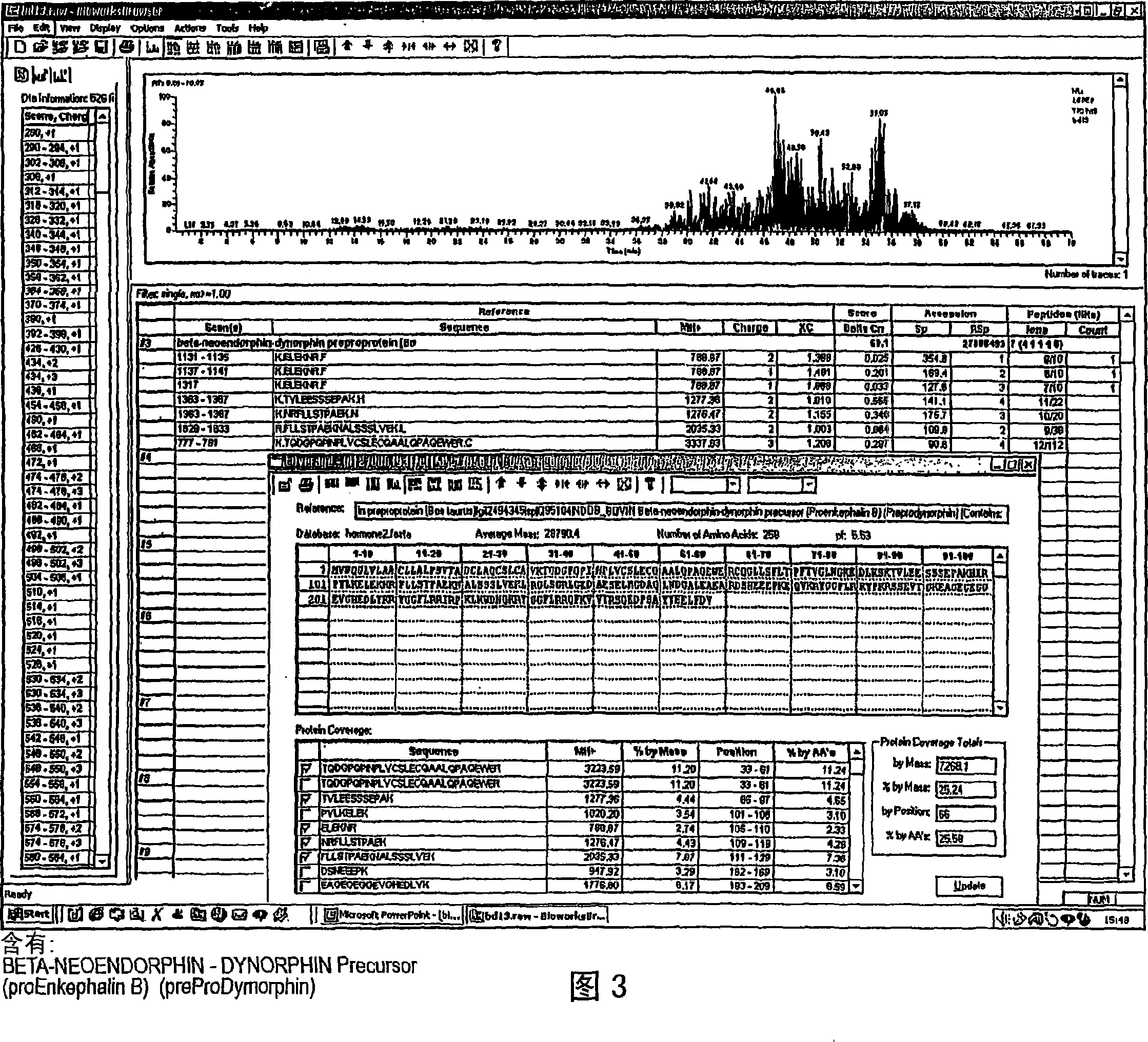
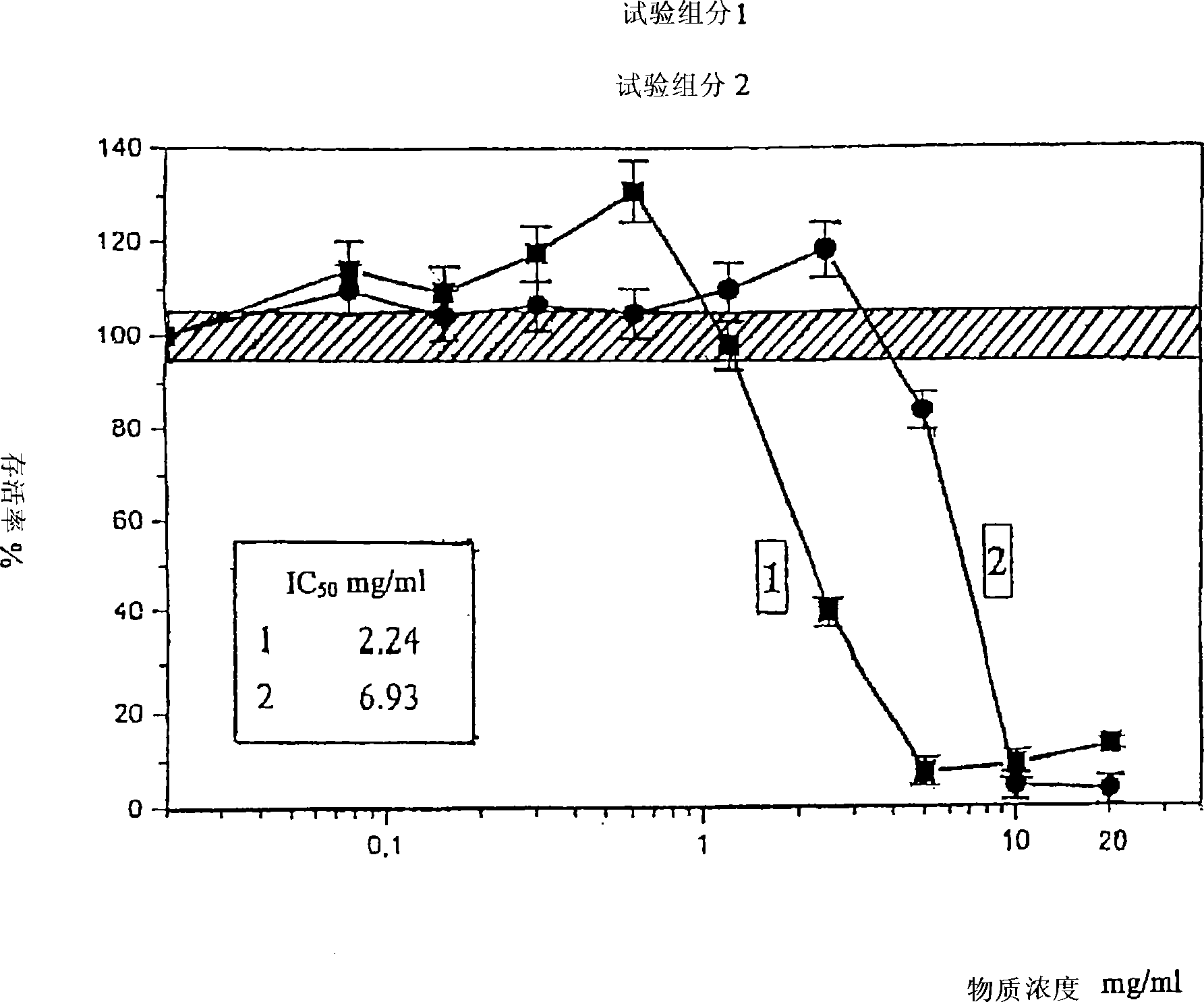
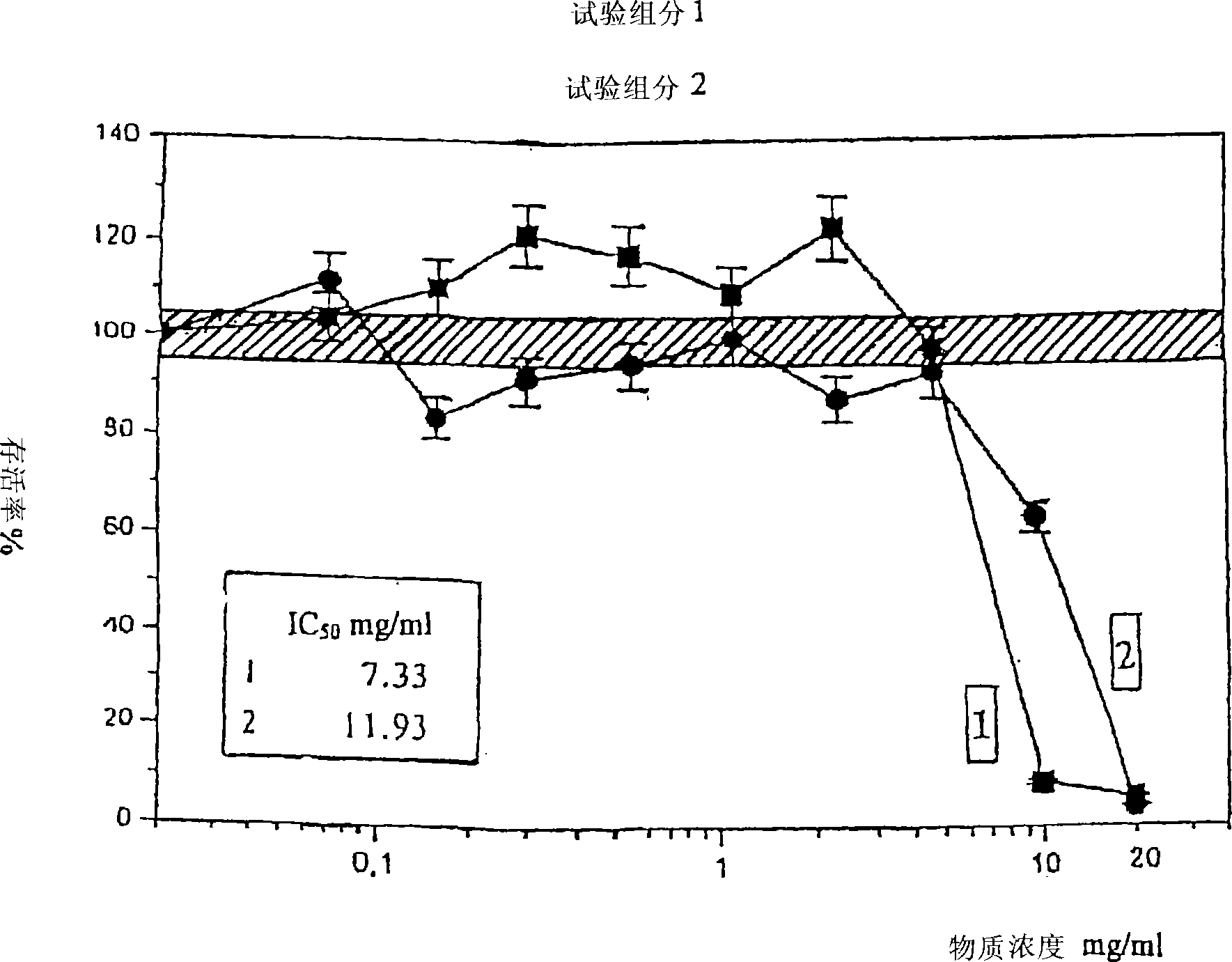
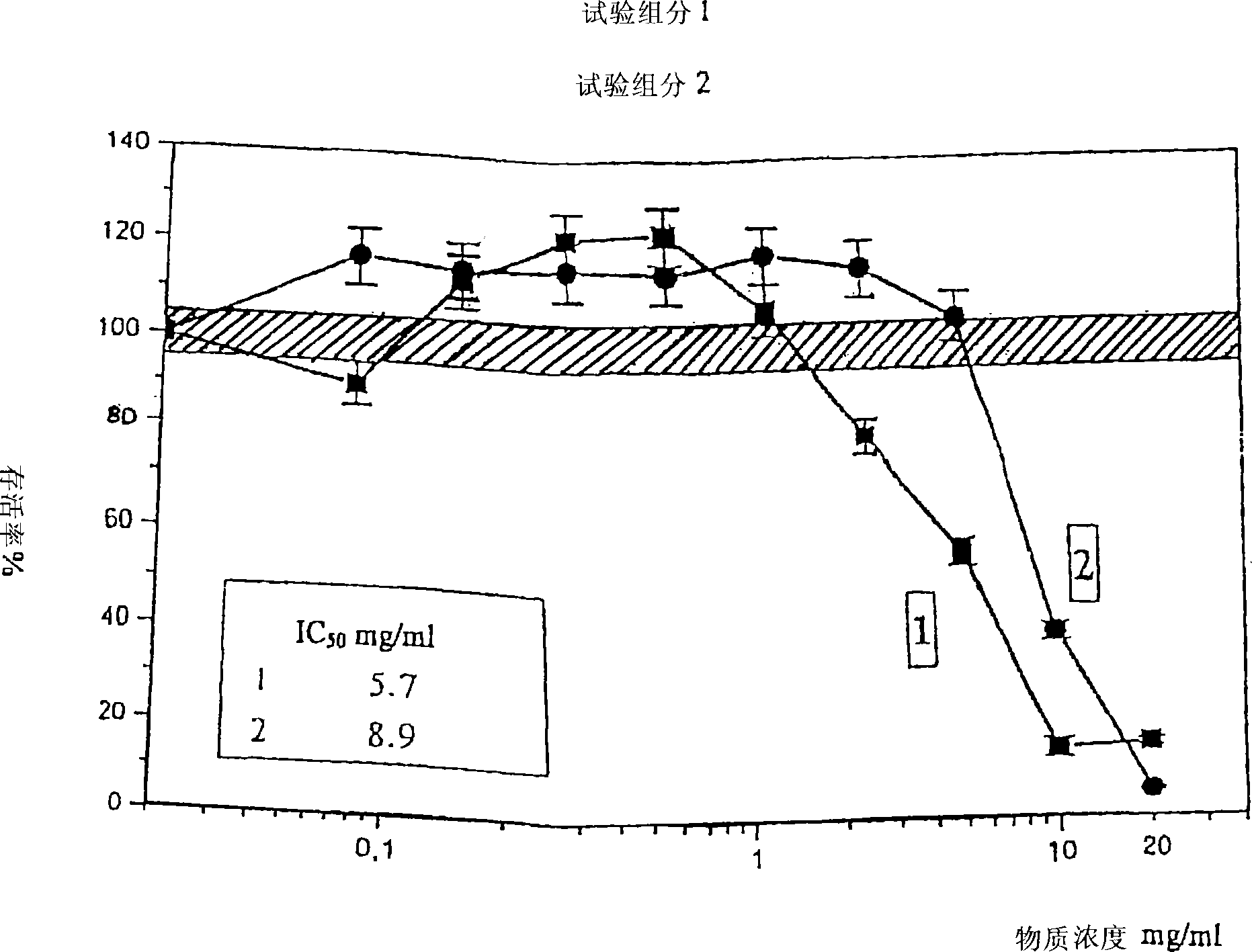
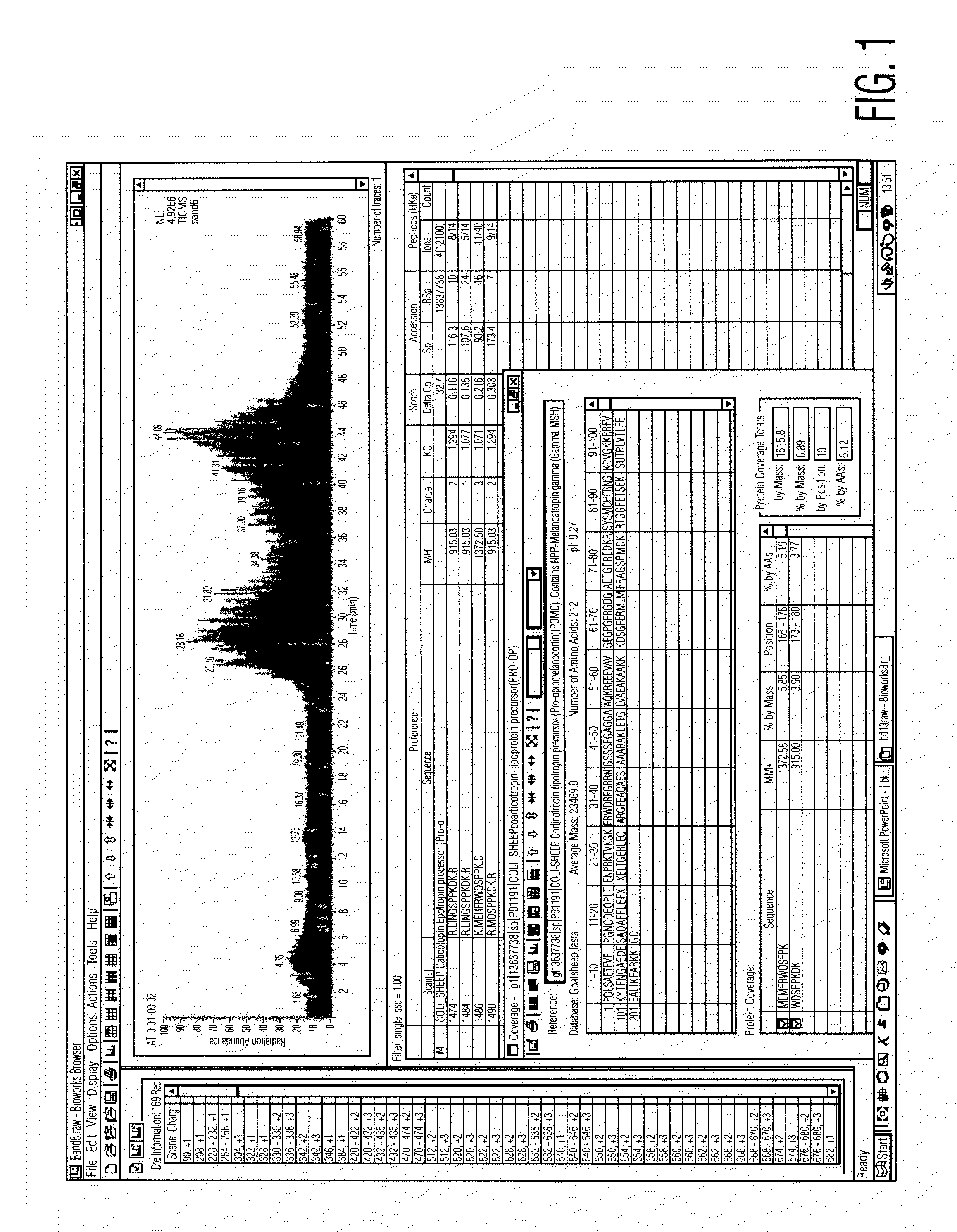
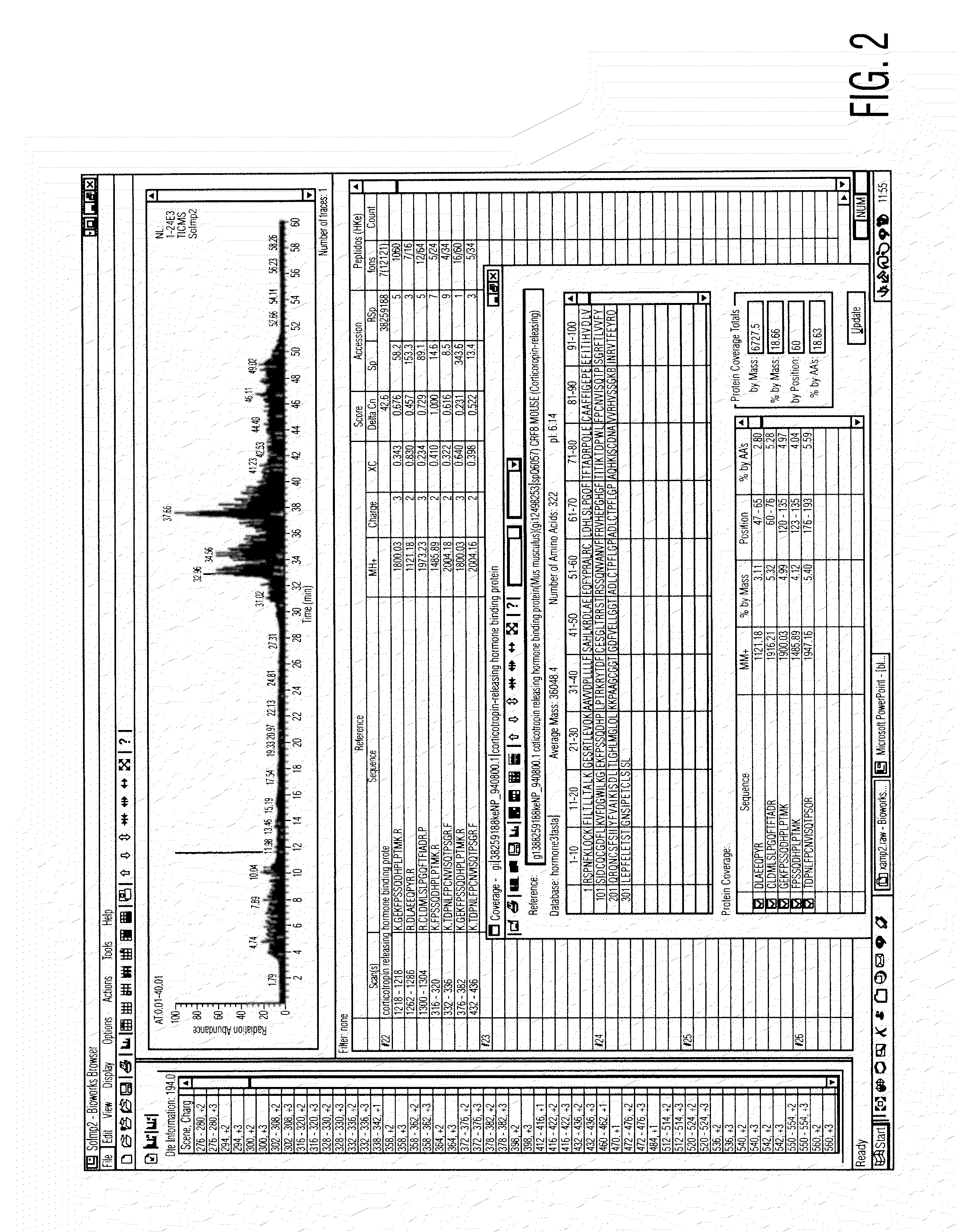
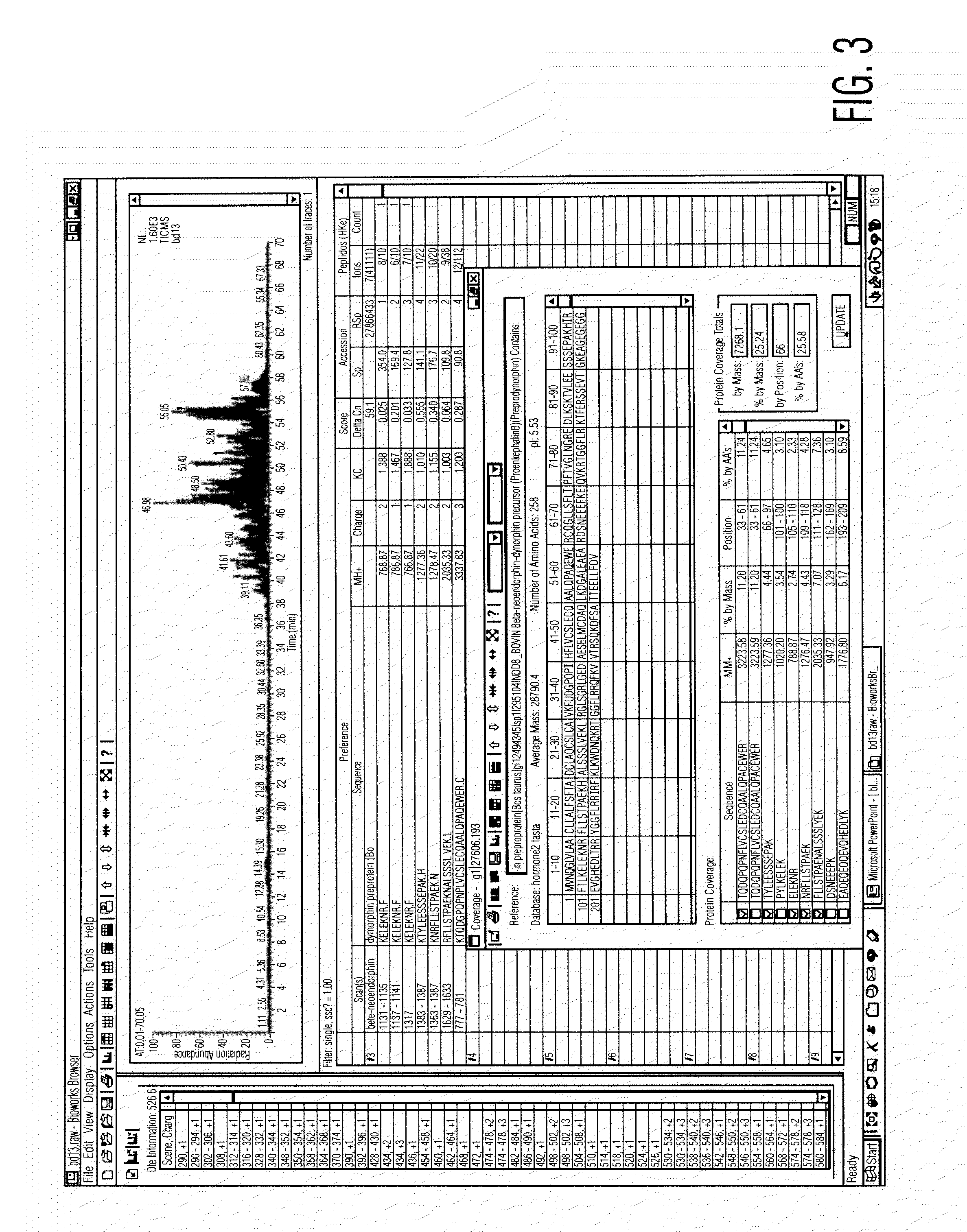
Analysis of a goat serum product with many therapeutic effects is described. The product is identified as containing proopiomelanocortin (POMC) and Corticotropin releasing factor (CRF) peptides, as well as breakdown products of these peptides. We describe methods of treatment of diseases including cancers, multiple sclerosis, and neural disorders using these peptides and their products, as well as medicaments including such peptides and methods of producing the peptides.
Owner:AIMSCO LTD
Preparation process of antitoxic serum for viral inactivation treatment
InactiveCN102526729AAdded inactivation stepComplete inactivationAntibacterial agentsSerum immunoglobulinsVirus inactivationUltrafiltration
A preparation process of antitoxic serum for viral inactivation treatment comprises the steps of pepsin digestion, first precipitation and heating degeneration treatment, second precipitation treatment, alum precipitation treatment, ultrafiltration concentration treatment, and stock solution preparation. Two viral inactivation methods are utilized in the steps and can be respectively and independently implemented or implemented in a combined mode to enable an antitoxic serum product to be safe and inactivation to be complete. Furthermore, the two viral inactivation methods are physical methods, are free of addition of other matters, do not bring about other practical problems to preparation of antitoxic serum stock solution do not happen, and have no special requirements for plants, facilities and personnel, thereby being convenient and easy to operate.
Owner:JIANGXI INST OF BIOLOGICAL PRODS
Plasma preparation or serum preparation and process for producing the same
ActiveUS20060127874A1Efficient methodEfficient removalOther blood circulation devicesHaemofiltrationWhite blood cellBlood plasma
The present invention relates to a plasma product or a serum product with an extremely low risk of viral contamination and a method for producing the same. Before treating plasma or serum to be used as a raw material for producing a plasma product or a serum product using a virus removal membrane, leucocytes contaminating the blood are removed. Thus, a plasma product or a serum product with an extremely low risk of viral contamination can be efficiently produced while preventing clogging. Since clogging scarcely arises, it is possible to carry out efficient filtration without applying an elevated pressure as the filtration proceeds.
Owner:ASAHI KASEI MEDICAL CO LTD
Medicament
Analysis of a goat serum product with many therapeutic effects is described. The product is identified as containing proopiomelanocortin (POMC) and Corticotropin releasing factor (CRF) peptides, as well as breakdown products of these peptides. We describe methods of treatment of diseases including cancers, multiple sclerosis, and neural disorders using these peptides and their products, as well as medicaments including such peptides and methods of producing the peptides.
Owner:AIMSCO LTD
Biologically active blood serum obtained by electroshock
The present invention relates to a method for preparing a blood serum product, the blood serum product and a pharmaceutical composition comprising said blood serum product as well as uses thereof in the treatment of various diseases and conditions, including epileptic seizures and apoplexy.
Owner:OWEN HLDG
Medicament
InactiveUS20130203669A1Effect in patientImprove the level ofSenses disorderNervous disorderDiseaseSerum ige
Owner:AIMSCO LTD
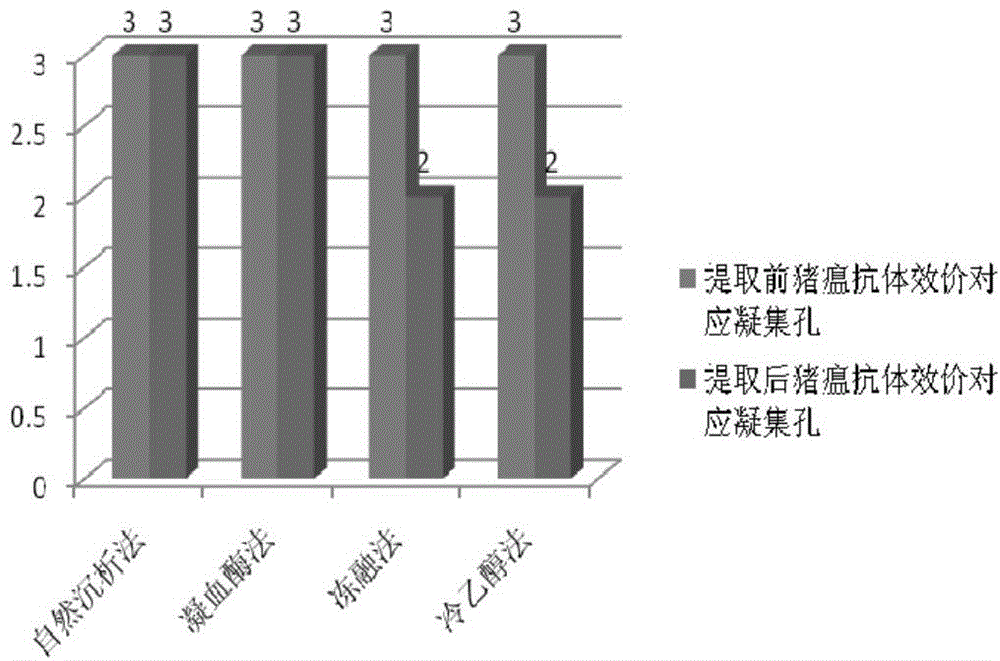
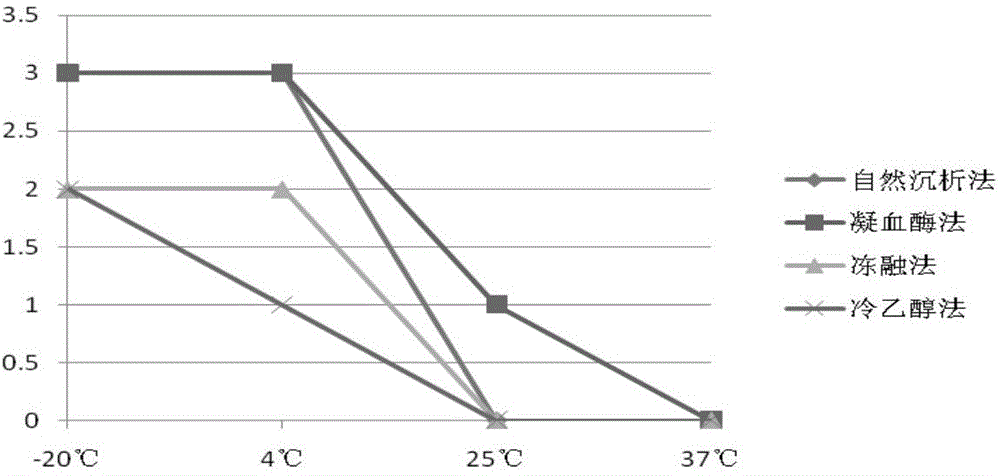
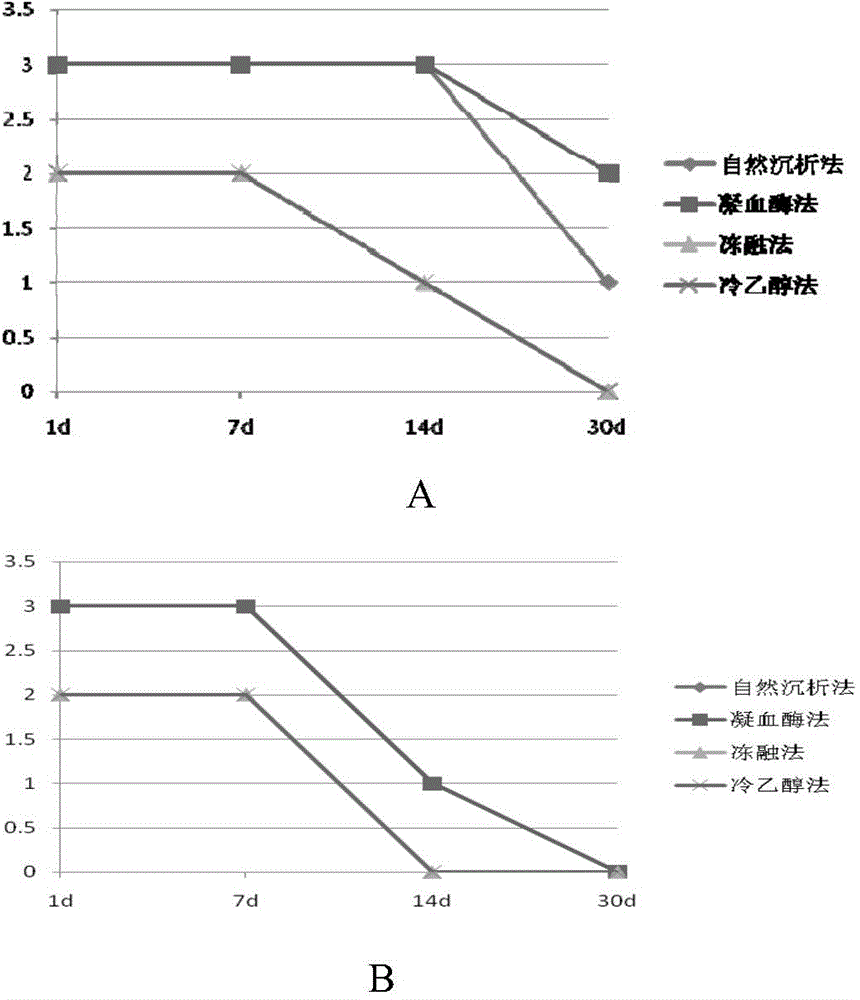
Preparation method of high-capacity serum antibody
The invention discloses a preparation method of a high-capacity serum antibody. The preparation method comprises the following steps of firstly, collecting immunized fresh blood, collecting blood plasma, and cryopreserving for later use; then, filtering the collected blood plasma, and collecting filtrate to obtain a primarily-filtered serum product; and adding thrombin into the prepared primarily-filtered serum product, uniformly stirring, warmly applying at the temperature of 20-37 DEG C for 0.5-4 hours, filtering by using filter paper with the aperture of 0.22-0.88mu m, and collecting filtrate to obtain the serum antibody. The serum antibody obtained by using the method disclosed by the invention is high in content and good in quality and stability; and the preparation method is suitable for preparing the high-capacity serum antibody and relatively good in market prospect.
Owner:SOUTHWEST UNIVERSITY
Agkistrodon halys venom identification method applying mass spectrometry and application of method
PendingCN111458420AQuality is easy to controlStrong targetingComponent separationMaterial analysis by electric/magnetic meansMass Spectrometry-Mass SpectrometryDiagnosis laboratory
The invention discloses a method for identifying agkistrodon halys venom by applying mass spectrometry. The method comprises the following steps of: (1) dissolving agkistrodon halys venom to prepare aprotein solution; (2) carrying out SDS-PAGE analysis; and (3) carrying out LC-MS / MS mass spectrometry to carry out agkistrodon halys venom component identification. In addition, the invention furtherdiscloses application of the identification method in the preparation of products for identifying the agkistrodon halys venom and application in the preparation of agkistrodon halys venom resistant serum for treating agkistrodon halys venom poisoning. According to the invention, the agkistrodon halys venom can be identified in a laboratory; agkistrodon halys venom is more accurately subjected toquality control, so that specific drug anti-agkistrodon halys poison serum for treating agkistrodon halys venom poisoning is produced; and the method is very important for ensuring the safety, effectiveness and quality controllability of anti-agkistrodon halys venom serum products and is of great significance for reference of the diagnosis and legal medical expert identification of the types of venom caused by snake venom poisoning. The method has the advantages of high detection sensitivity, high correct detection rate, no cross reaction and simple operation process.
Owner:SHANGHAI SERUM BIOTECH
Bovine parvovirus detecting fluorescence quantitative PCR kit and application thereof
ActiveCN101565759AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescenceA-DNA
The invention discloses a bovine parvovirus detecting fluorescence quantitative PCR kit and an application thereof. The kit comprises: a) a DNA extraction reagent, b) a hot start Taq DNA polymerase, c) a primer and a TaqMan probe, d) a standard positive DNA template, and e) a PCR fluorescence quantitative reaction solution. The kit is characterized in that: primer sequence is a sense primer: 5'-CCAGTACCAGGAAACGGAGAC-3', and an antisense primer: 5'-GCATGTATTCCGGTCTCCAA -3', and the size of an amplicon is 118 bp; the sequence of a fluorescence probe is: 5'-FAM-CCTCAACATCTACGTCACCGGACAA-TAMRA-3', the 5' end of the probe is marked with a fluorescence emission group FAM, the 3' end is marked with a fluorescence quenching group TAMRA, the standard positive DNA template transforms a colon bacillus DH5 alpha by a pGEM-T carrier inserted with bovine parvovirus VP3 protein coding zone 118 bp segment, plasmid is extracted after proliferation, an A260 ration is measured in an ultraviolet spectrophotometer, and the plasmid is diluted by 10 times of gradient. The fluorescence quantitative PCR kit, applied to the epidemiology investigation of cow bovine parvovirus infection, can efficiently and conveniently monitor the bovine parvovirus pollution in serum products in real time, and is widely applicable to the epidemiology investigation of bovine parvovirus infection.
Owner:WUHAN SANLI BIO TECH
High-purity anti-snake-venom serum and preparation method thereof
ActiveCN111748031AHigh School and ValenceReduce proteinPeptide preparation methodsImmunoglobulinsCyanogen bromideSnake venom
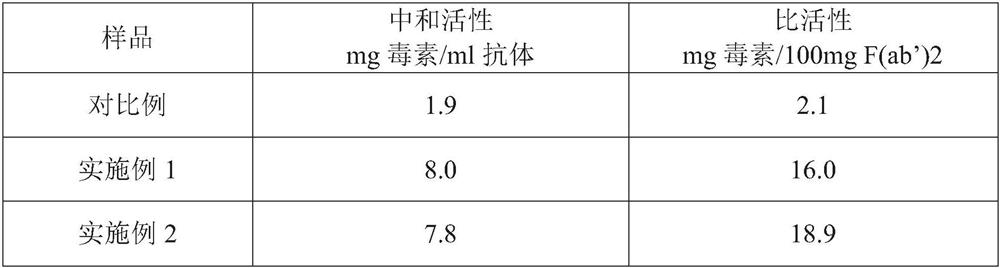
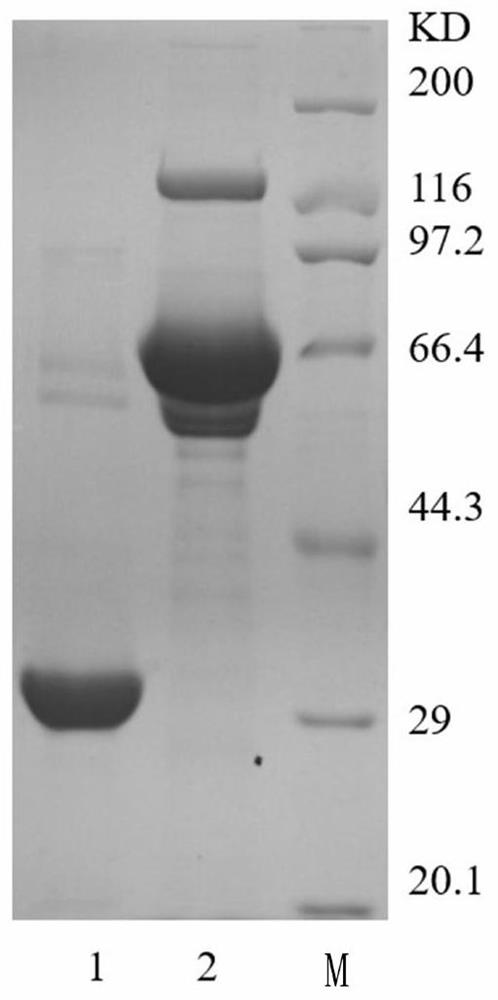
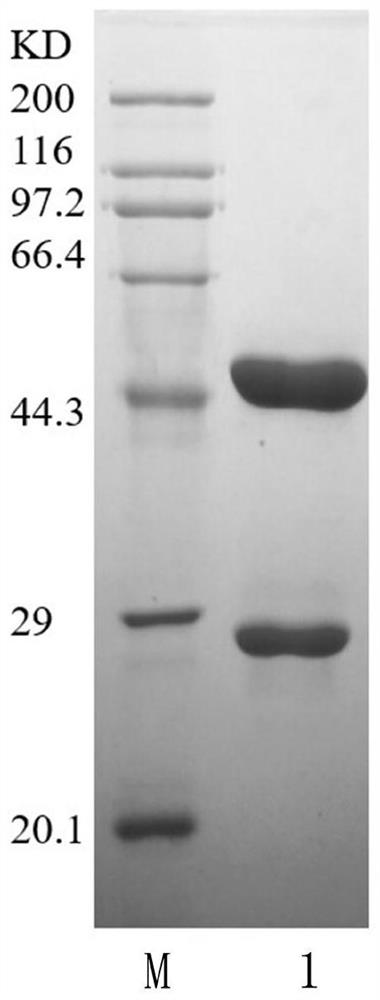
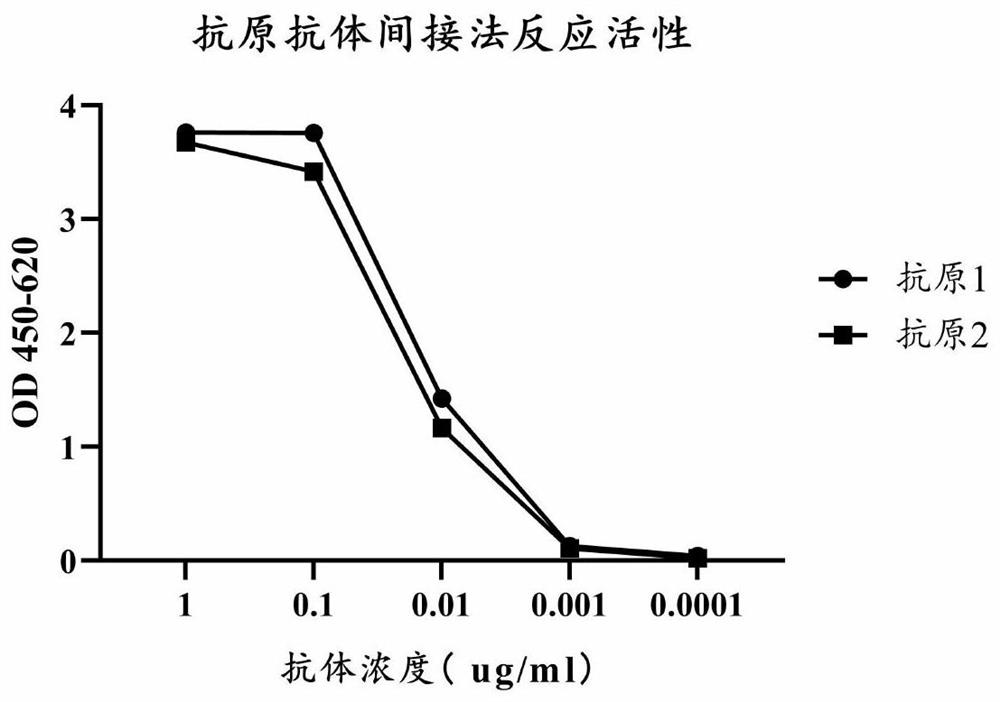
The invention discloses high-purity anti-snake-venom serum and a preparation method thereof. The high-purity anti-snake-venom serum is obtained mainly by the steps of activating a polysaccharide carrier by cyanogen bromide, coupling a toxic snake toxin to the cyanogen bromide activated polysaccharide carrier and purifying anti-snake-venom serum F (ab') 2 through an affinity chromatography method.The anti-snake-venom serum product with high neutralizing potency obtained by the preparation method has a good function of neutralizing snake venom, the amount of proteins without neutralizing toxiceffects in the product is greatly reduced, the occurrence rate of allergic reactions caused by heterogeneous proteins can be obviously reduced, and the severity of the allergic reactions can be reduced. In addition, the preparation method is simple, mild in condition and suitable for large-scale popularization and application.
Owner:浙江毓昌生物技术有限公司
Anti-human abnormal prothrombin antibody and its application
ActiveCN113817063BAvoid interferenceReduce difficultyGenetic engineeringFermentationZymogenAntiendomysial antibodies
The invention belongs to the field of antibodies, and in particular relates to an anti-human abnormal prothrombin antibody and the use of the antibody in preparing a hepatocellular carcinoma detection kit. The anti-human abnormal prothrombin antibody of the present invention can specifically bind human abnormal prothrombin, can further avoid interference from prothrombin in serum or plasma from the raw material level, and can further avoid abnormal blood coagulation from other species in animal serum products The interference of zymogens avoids related risks from the source, reduces the difficulty of reagent development, and has a good application prospect.
Owner:XIAMEN INNOBIOMAX BIOTECHNOLOGY CO LTD +1
Methods and compositions for use in the prevention, treatment and/or alleviation of cancer
The ratio of the metals zincand cadmium is of great significance for error-free proliferation and differentiation of cells. An understanding of the role of cadmium in the etiology of cancer offers possibilities to gain a better understanding of cancer as well as possibilities to prevent, treat and / or alleviate cancer. The concentration of Zn(II) in fetal serum, human serum from umbilical cord blood or healthy donors,and in avian serum including intact eggs and egg products has significance for the reliability and repeatability of cell and tissue culture method using said serum, or serum containing products,or eggs. Herein is disclosed a method step in the manufacture of serum products, in particular serum products intended for use in in vitroculture of mammalian cells or tissues, wherein the concentration of Zn(II) is determined, and preferably adjusted toa desired interval. Different products consisting of or containing serum or serum fractions are also disclosed.
Owner:马蒂 J 西伦
Anti-human abnormal prothrombin antibody and application thereof
ActiveCN113817063AAvoid interferenceReduce difficultyFermentationGenetic engineeringAntiendomysial antibodiesHepatocellular carcinoma
The invention belongs to the field of antibodies, and particularly relates to an anti-human abnormal prothrombin antibody and an application thereof to preparation of a hepatocellular carcinoma detection kit. The anti-human abnormal prothrombin antibody can specifically bind human abnormal prothrombin, can further avoid interference of prothrombin from serum or plasma from the raw material level, can further avoid interference of abnormal prothrombin from other species in animal serum products, avoids related risks from the source, reduces reagent development difficulty, and has a good application prospect.
Owner:XIAMEN INNOBIOMAX BIOTECHNOLOGY CO LTD +1
Reovirus-detecting fluorescence quantitative PCR kit and application thereof
ActiveCN101565757BIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesRNA extractionReverse transcriptase
The invention discloses a reovirus-detecting fluorescence quantitative PCR kit and an application thereof. The kit comprises: a) an RNA extraction solution, b) a reverse transcriptase reaction solution, c) reverse transcriptase, d) an RNA enzyme inhibitor, e) a primer and a TaqMan probe, f) a standard positive DNA template and g) a PCR fluorescence quantitative reaction solution. The kit is characterized in that: primer sequence is a sense primer: 5'-TGCGCCTATCCTTGAGTTGA-3', and an antisense primer: 5'-TTGCCAGGAAATACGGGTCT-3', and the size of an amplicon is 138 bp; the sequence of a fluorescence probe is: 5'-FAM-TCAAAATGGTGGACTTCAGTTTCGATTT-TAMRA-3', the 5' end of the probe is marked with a fluorescence emission group FAM, the 3' end is marked with a fluorescence quenching group TAMRA, thestandard positive DNA template transforms a colon bacillus DH5 alpha by a pGEM-T carrier inserted with reovirus S1 protein zone 361 bp segment, plasmid is extracted after proliferation, an A260 ration is measured in an ultraviolet spectrophotometer, and the plasmid is diluted by 10 times of gradient. The kit can efficiently and conveniently monitor the reovirus pollution in serum products in realtime, can be applicable to epidemiology investigation of reovirus infection, and can provide technical support for relevant fundamental researches, thus having quite broad application prospect.
Owner:WUHAN SANLI BIO TECH
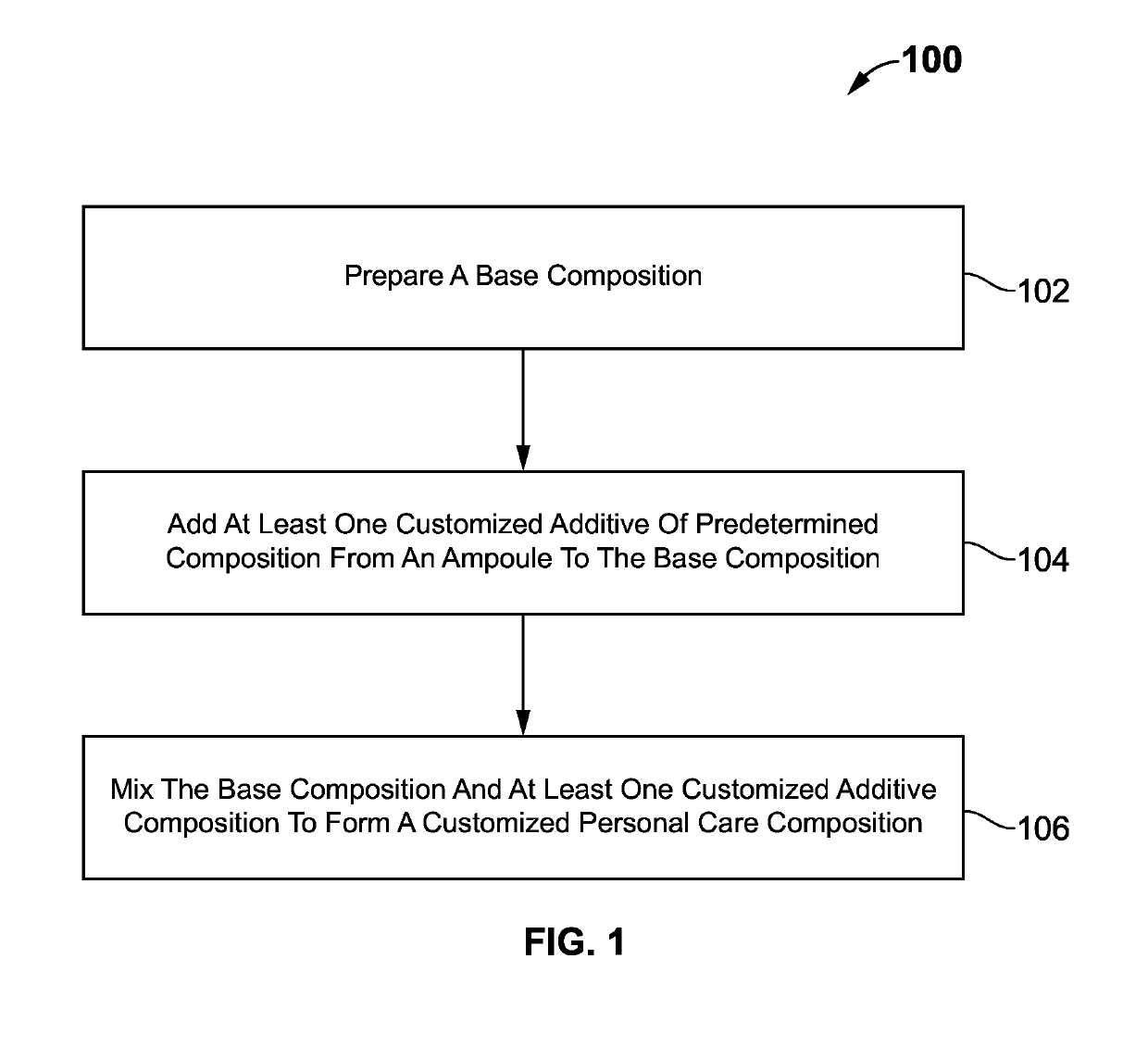
Composition and method for preparing a customized personal care product
The present invention provides a composition and method for preparing a customized personal care product. The composition comprising a base composition and at least one additive compositions. The base composition is at least one or combination of organic or inorganic ingredients and organic origin ingredients, and the additive compositions include at least one hair care based additive compositions. at least one customized additive of predetermined composition from an ampoule is added to the base composition. The base composition and at least one customized additive compositions are mixed to form a customized personal care composition. The personal care product could be a health care product, beauty product, face serum product, body lotion product, hair care product, or any other product.
Owner:HACATURJANCA JELENA
Anti-sea snake venom serum nano-membrane filtering method
PendingCN113801221AImprove filtration throughputEffective filteringSerum immunoglobulinsPeptide preparation methodsUltrafiltrationIon exchange
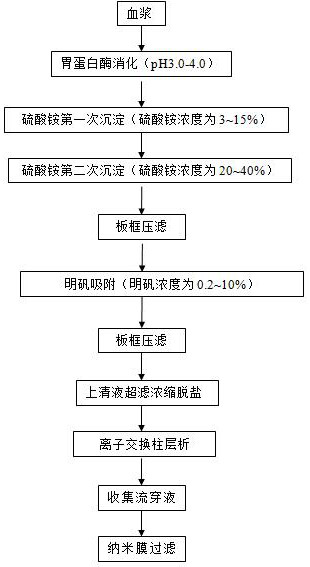
The invention discloses an anti-sea snake venom serum nano-membrane filtering method which comprises the following steps: (1) preparing an anti-sea snake venom serum chromatography flow-through liquid, namely obtaining plasma; digesting with pepsase; precipitating ammonium sulfate for the first time; precipitating ammonium sulfate for the second time; performing plate-frame pressure filtration; alum adsorption; performing plate-frame pressure filtration; performing supernate ultrafiltration, concentration and desalination; performing ion exchange column chromatography; collecting chromatography flow-through liquid; (2) controlling the environment temperature of nano-membrane filtration to be 20-28 DEG C, and preparing the chromatography flow-through liquid into a solution with a certain concentration and a certain prescription; and (3) carrying out nano-membrane filtration according to the conditions in the step (2). The chromatography flow-through liquid in the anti-sea snake venom serum process is subjected to nano-membrane filtration virus removal, optimal conditions are obtained through multiple tests, the flux of nano-membrane filtration is increased, the production cost is greatly reduced, the virus removal efficiency of anti-sea snake venom serum products is improved, and the application of nano-membrane filtration in anti-sea snake venom serum production is accelerated.
Owner:SHANGHAI SERUM BIOTECH +1
Method for reducing complement level and protein precipitation by premixing bovine serum raw materials
ActiveCN111257072AReduced complement activityCultivation has no effectPreparing sample for investigationBiotechnologyComplement system
The invention relates to the technical field of bovine serum, and improves the quality of bovine serum products by fully mixing bovine serum at low temperature in advance. A bovine serum raw materiallow-temperature sufficient premixing process is innovatively adopted, and the problem that bovine serum effective components are severely damaged due to the fact that a bovine serum complement systemneeds to be treated at 56 DEG C for 30 minutes during elimination is solved. When bovine serum of different blood types is fully mixed by using the bovine serum premixing process, protein is separatedout, and if the bovine serum is only mixed before final filtration, the reaction time is insufficient, and the protein separation phenomenon of the final product is common, the bovine serum preparedby adopting the process can effectively avoid the protein separation. The method also has an advantage that the raw materials are fully premixed and then subjected to preliminary screening of detection items so that the method has better correspondence with the detection items of the final product.
Owner:RONG YE LANZHOU BIOLOGIC TECH
Preparation method of antitoxic serum for octanoic acid purification treatment
InactiveCN102526728AImprove quality indicatorsEasy to useAntibacterial agentsSerum immunoglobulinsOctanoic AcidsUltrafiltration
A preparation method of antitoxic serum for octanoic acid purification treatment comprises steps of pepsin digestion, first precipitation and heating degeneration treatment, second precipitation treatment, alum precipitation treatment, ultrafiltration concentration treatment, and stock solution preparation. An octanoic acid purification step is added in the preparation method, so that an antitoxic serum product has high quality indexes and safe usage. Compared with an existing preparation process, in main quality indexes of the antitoxic serum product, content of F(ab)2 is increased to above 80% from 50%-60%, purity specific activity (unit / g protein) is improved to above 78000 from 35000-45000, and allergic reaction incidence and serum sickness incidence of the antitoxic serum product are reduced by 30%-40% during clinical application.
Owner:JIANGXI INST OF BIOLOGICAL PRODS
Quality control method of sea snake venom and application thereof
PendingCN113804770AQuality is easy to controlEasy quality controlComponent separationSerum immunoglobulinsProtein solutionHydrophis cyanocinctus
The invention discloses a quality control method of sea snake venom and application thereof. The quality control method comprises the following steps of: (1) selecting freeze-dried powder of sea snake venom (containing Lapemis curtus venom and Hydrophis cyanocinctus venom) from the origin of China, dissolving the freeze-dried powder and preparing into a protein solution; and (2) performing high performance liquid chromatography analysis with different wavelengths. In addition, the invention further discloses application of the quality control method of the sea snake venom in preparation of anti-sea snake venom serum for treating sea snake venom poisoning. According to the quality control method, the quality of the sea snake venom can be controlled, the specific medicine anti-sea snake venom serum for treating sea snake venom poisoning is further produced, and the method has very important significance for ensuring that the anti-sea snake venom serum product is safe, effective and controllable in quality. The quality control method is good in detection repeatability, high in reliability, simple in operation process, quicker, more efficient and low in cost.
Owner:中国人民解放军海军特色医学中心 +1
Preparation method of large capacity serum antibody
The invention discloses a method for preparing a large-capacity serum antibody, which comprises the following steps: first collecting immunized fresh blood, collecting the plasma, and freezing and storing it for later use; then filtering the collected plasma, and collecting the filtrate to obtain a primary filtered serum product; Add thrombin to the prepared primary filtered serum product, stir evenly, and then apply it warmly for 0.5 to 4 hours at 20 to 37°C, then filter with filter paper with a pore size of 0.22 to 0.88 μm, collect the filtrate, and obtain serum antibodies; using the present invention The serum antibody prepared by the method has large quantity, good quality and good stability, is suitable for the preparation of large-capacity serum antibody, and has a good market prospect.
Owner:SOUTHWEST UNIV
A fluorescent quantitative PCR kit for detecting bovine adenovirus type 3
ActiveCN101560573BIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceBasic research
The invention discloses a fluorescent quantitative PCR kit for detecting bovine adenovirus type 3 and its application. The kit contains: a) a DNA extraction reagent, b) a hot-start Taq DNA polymerase, c) a primer and a TaqMan probe, d) ) Standard positive DNA template, e) PCR fluorescent quantitative reaction solution, characterized in that: the primer sequences are respectively sense primer: 5'-CCTGAATTCTCTTGCAGCCAGA-3', antisense primer: 5'-CCTACCGAACCGACGCAGAT-3', and the amplicon size is 100bp, the fluorescent probe sequence is: 5′-FAM-TGAGAAGGTACTCCTCGTCGCTGGACCA-TAMRA-3′, the 5′ end of the probe is labeled with the fluorescent emitting group FAM, and the 3′ end is labeled with the fluorescent quenching group TAMRA, and the standard positive DNA template is prepared by The pGEM-T vector inserted with the 100bp fragment of the adenovirus type 3 pol protein was transformed into Escherichia coli DH5α, and after multiplication, the plasmid was extracted and prepared, and quantified by measuring A260 with a UV spectrophotometer and 10-fold serial dilution. Efficient and convenient real-time monitoring of bovine adenovirus type 3 contamination in serum products can be widely used in epidemiological investigations of the virus infection, and can also provide technical support for related basic research, with broad application prospects.
Owner:WUHAN SANLI BIO TECH
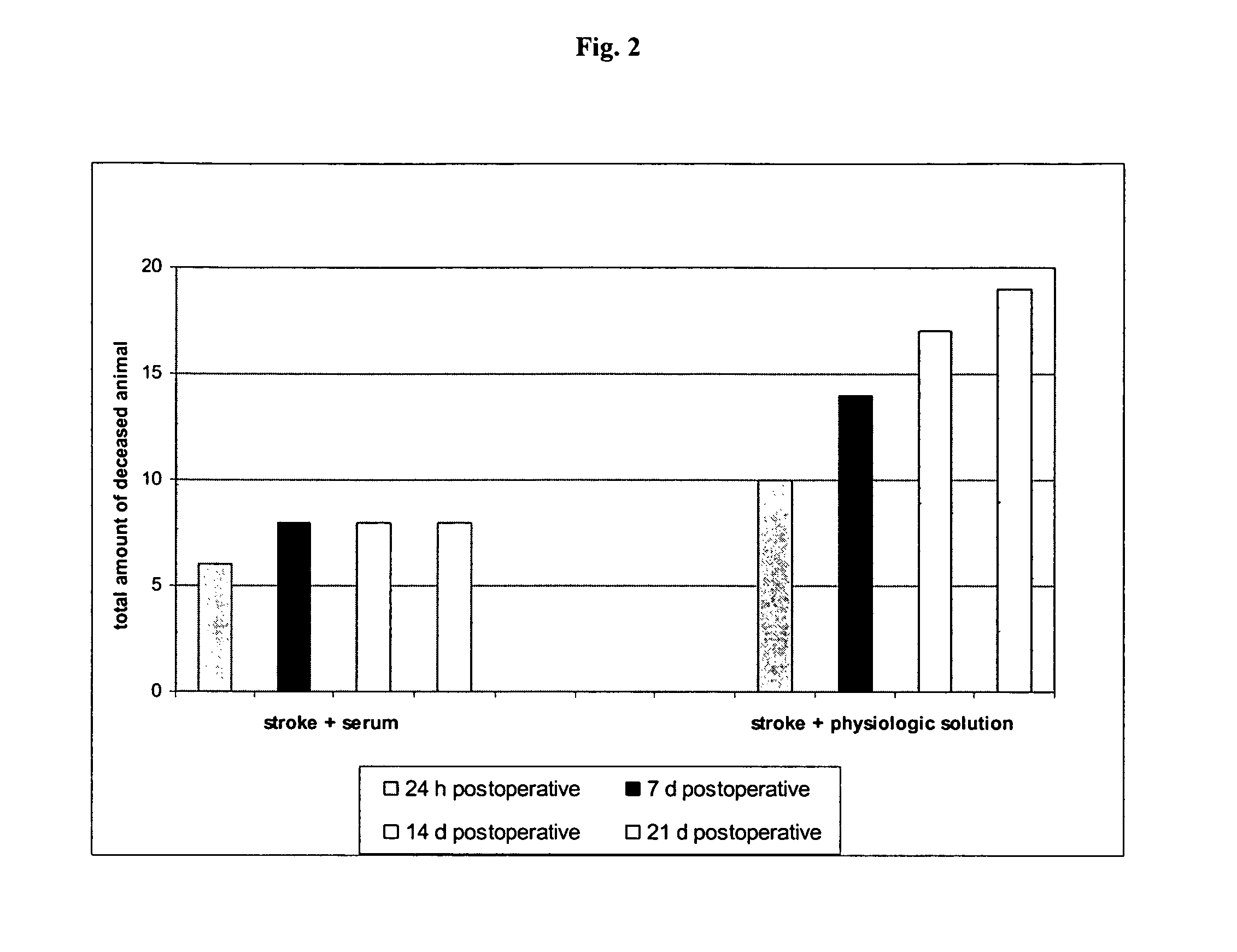
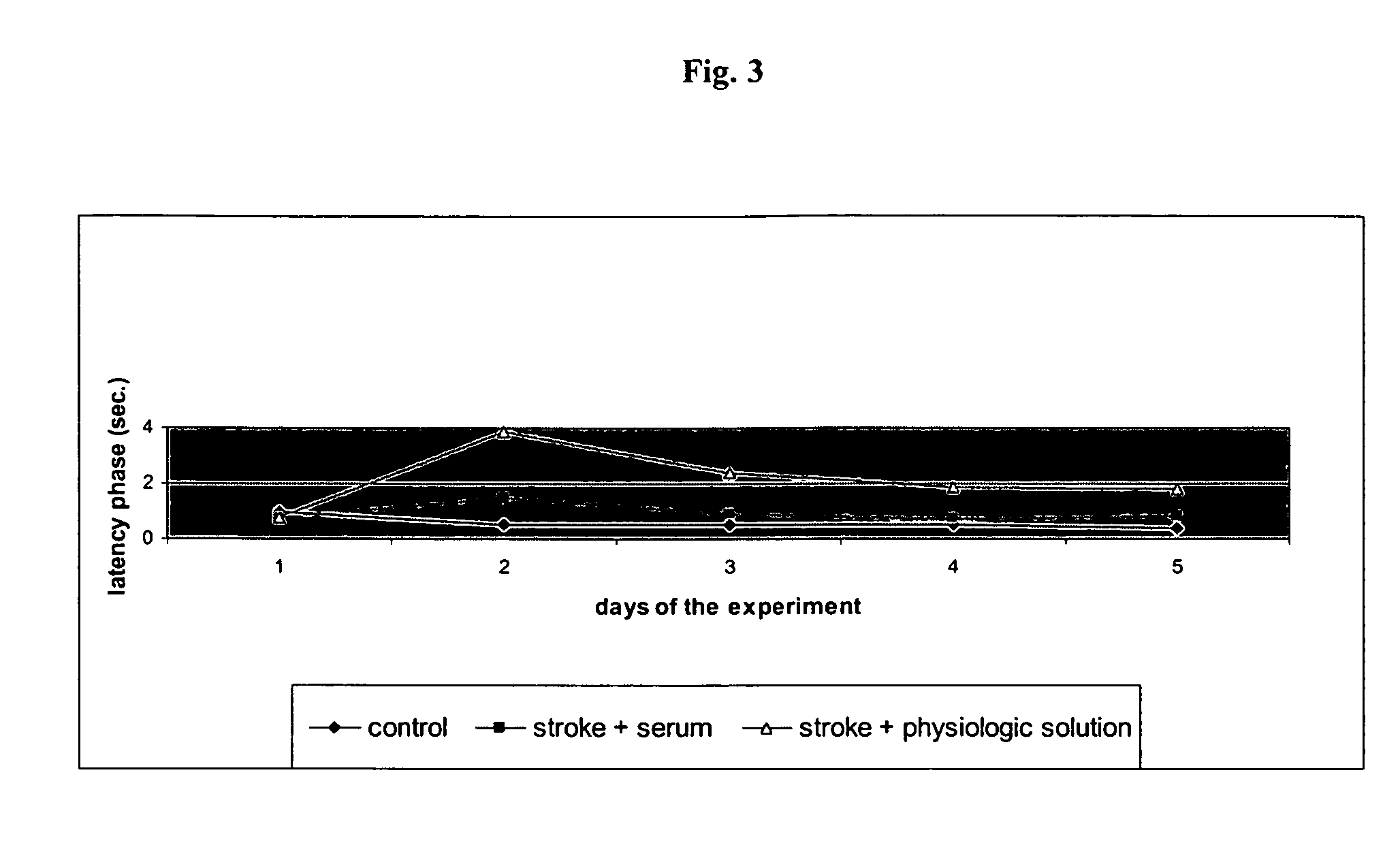
Use of a biologically active blood serum for the treatment of stroke
InactiveUS7744926B2Enhance body resistanceEasy to handleEnergy modified materialsMammal material medical ingredientsHuman animalGamma irradiation
The present invention relates to the use of a pharmacologically active blood serum product producible by a method comprising electrostimulation of a non-human animal, withdrawal of blood from said animal, isolation of serum from said blood, and gamma irradiation of said serum in the treatment of stroke, preferably ischemic stroke.
Owner:OWEN HLDG
Biologically active blood serum obtained by electroshock
Owner:OWEN HLDG
Bovine parvovirus detecting fluorescence quantitative PCR kit and application thereof
ActiveCN101565759BIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceA-DNA
The invention discloses a bovine parvovirus detecting fluorescence quantitative PCR kit and an application thereof. The kit comprises: a) a DNA extraction reagent, b) a hot start Taq DNA polymerase, c) a primer and a TaqMan probe, d) a standard positive DNA template, and e) a PCR fluorescence quantitative reaction solution. The kit is characterized in that: primer sequence is a sense primer: 5'-CCAGTACCAGGAAACGGAGAC-3', and an antisense primer: 5'-GCATGTATTCCGGTCTCCAA -3', and the size of an amplicon is 118 bp; the sequence of a fluorescence probe is: 5'-FAM-CCTCAACATCTACGTCACCGGACAA-TAMRA-3',the 5' end of the probe is marked with a fluorescence emission group FAM, the 3' end is marked with a fluorescence quenching group TAMRA, the standard positive DNA template transforms a colon bacillus DH5 alpha by a pGEM-T carrier inserted with bovine parvovirus VP3 protein coding zone 118 bp segment, plasmid is extracted after proliferation, an A260 ration is measured in an ultraviolet spectrophotometer, and the plasmid is diluted by 10 times of gradient. The fluorescence quantitative PCR kit, applied to the epidemiology investigation of cow bovine parvovirus infection, can efficiently and conveniently monitor the bovine parvovirus pollution in serum products in real time, and is widely applicable to the epidemiology investigation of bovine parvovirus infection.
Owner:WUHAN SANLI BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com