Patents
Literature
33results about How to "Easily material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
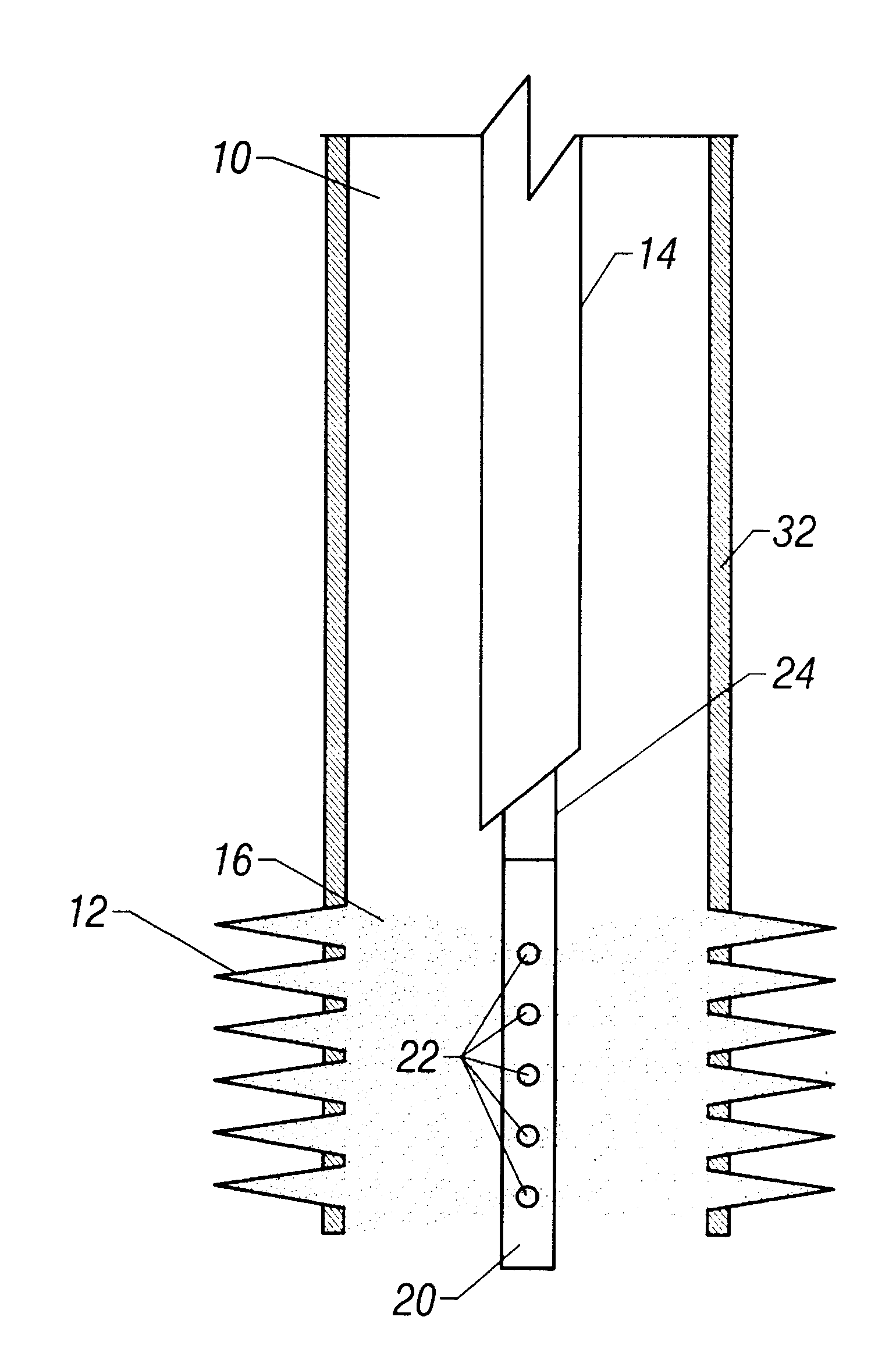
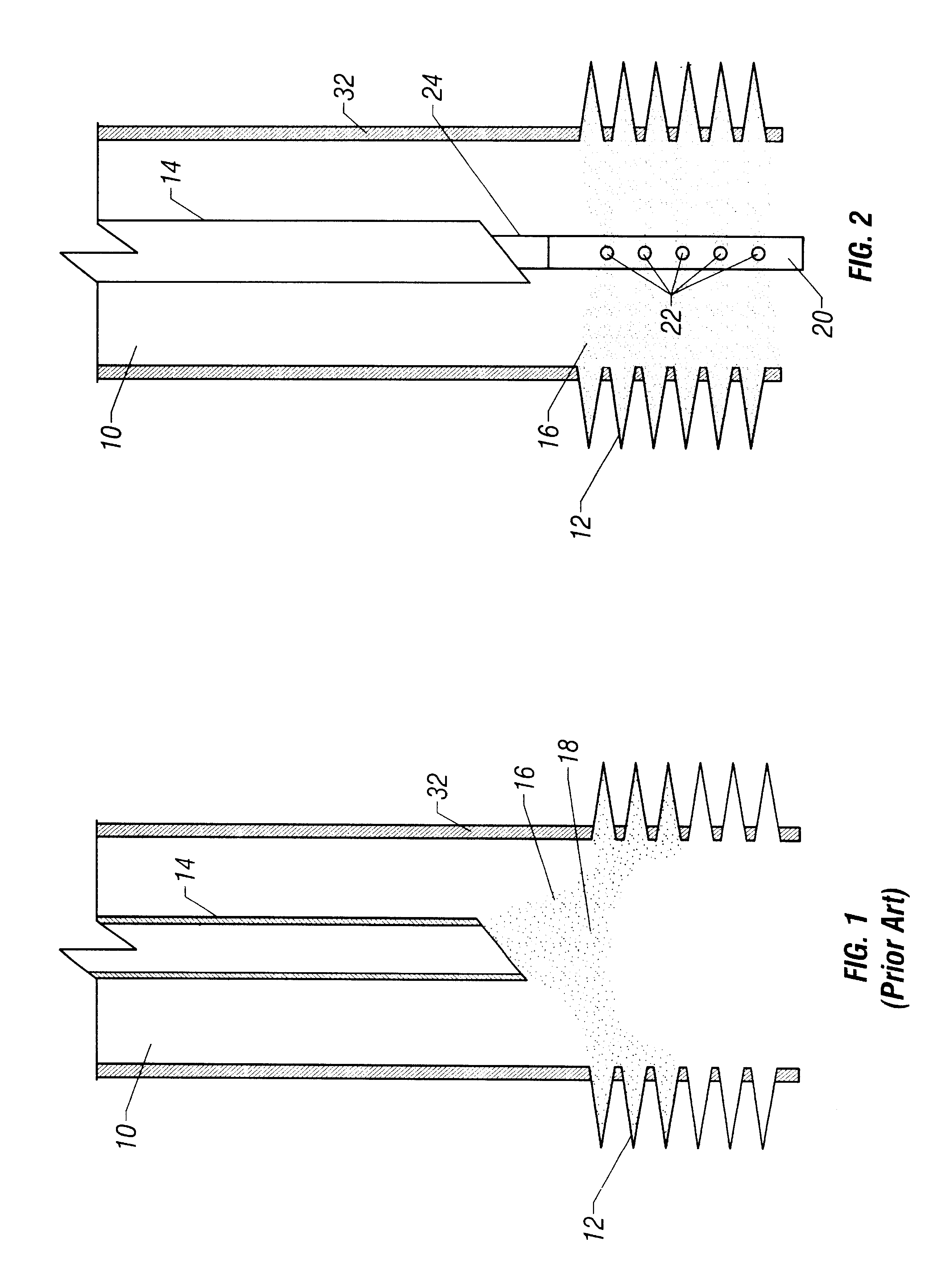
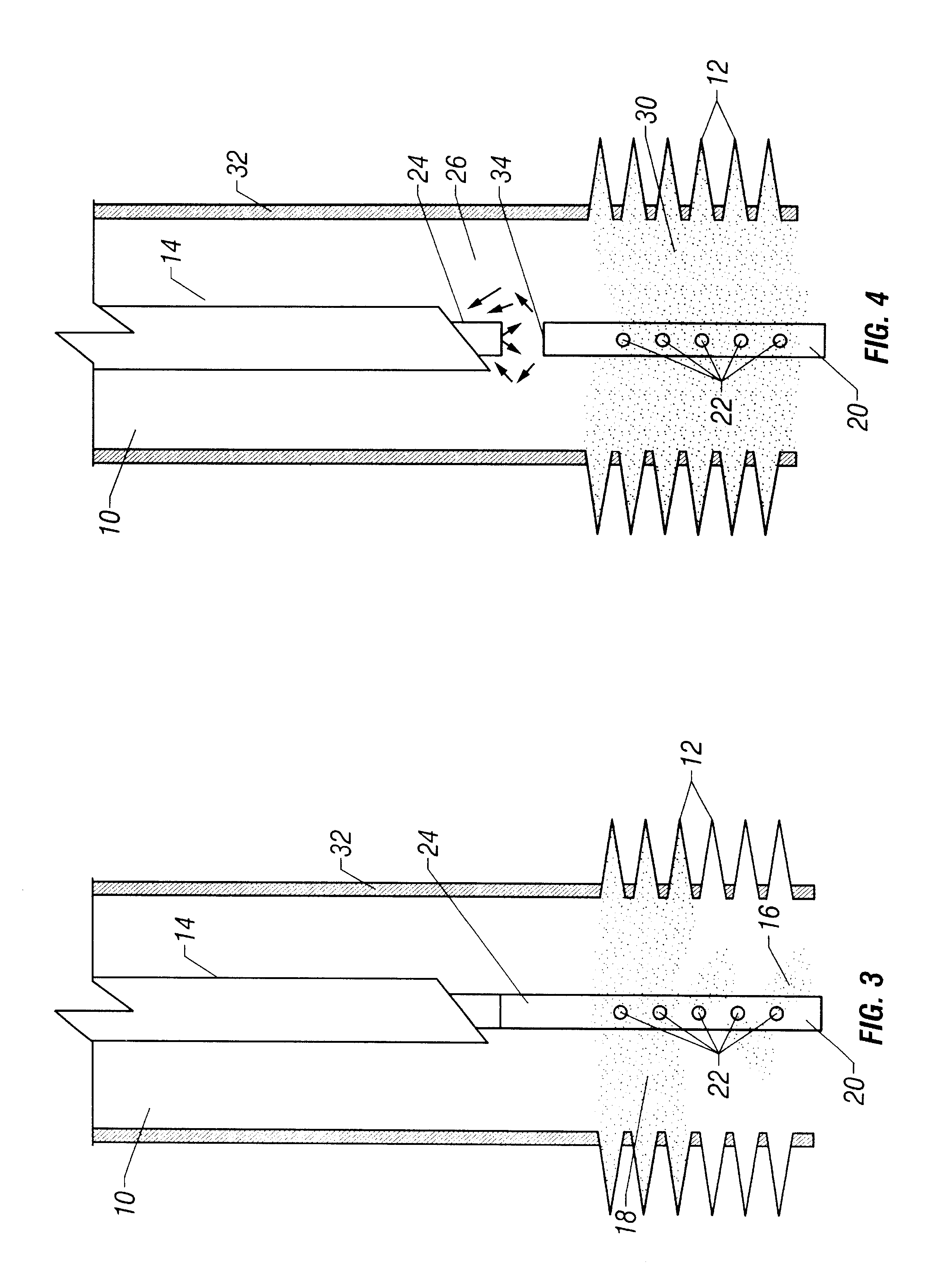
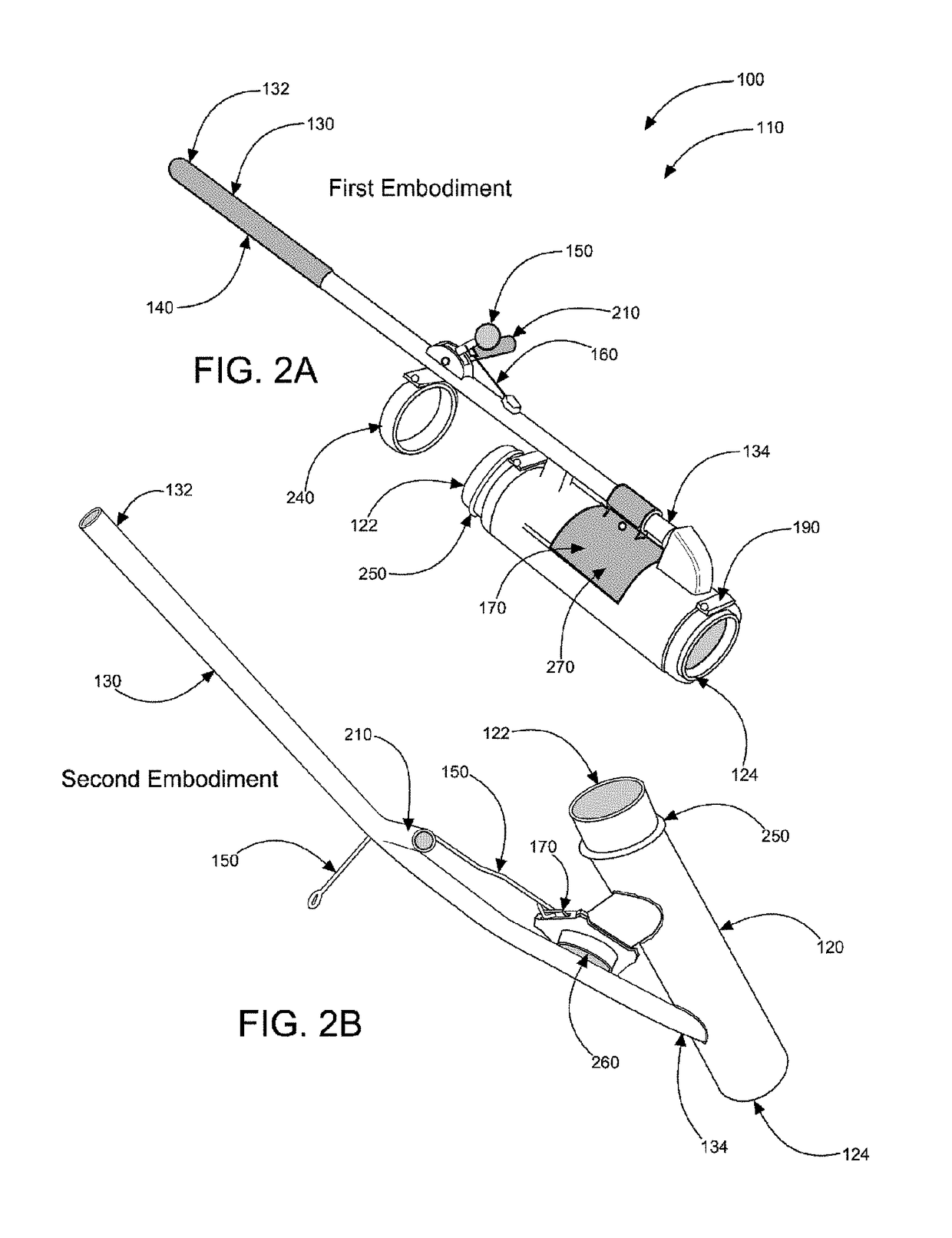
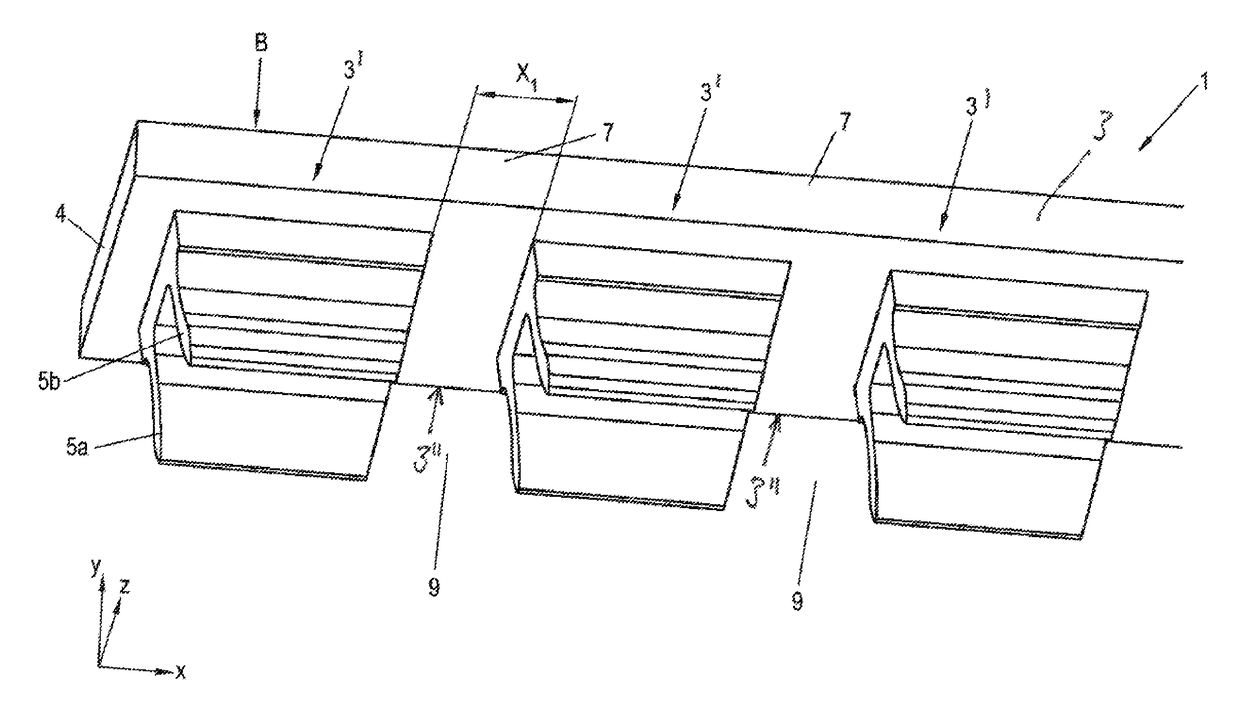
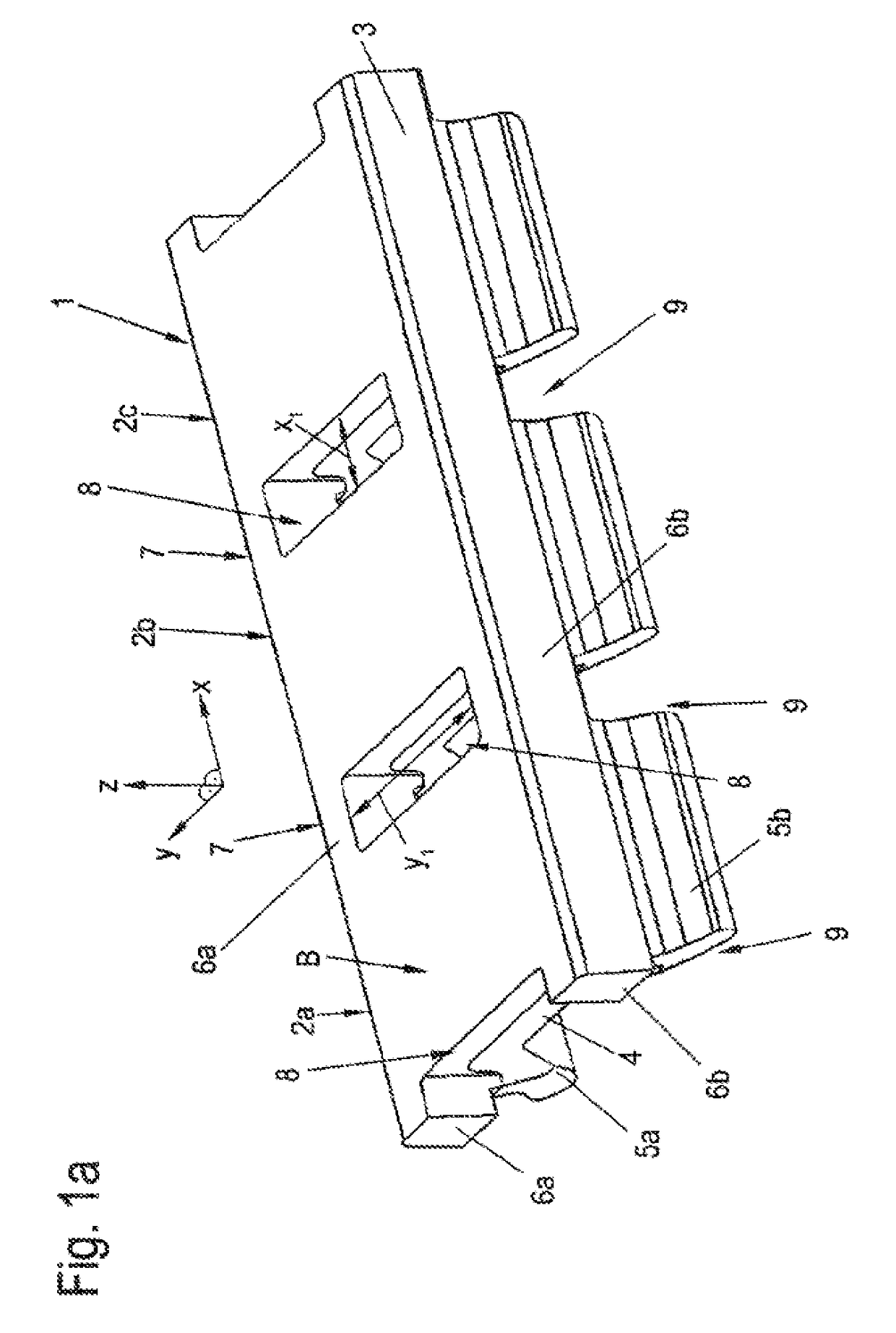
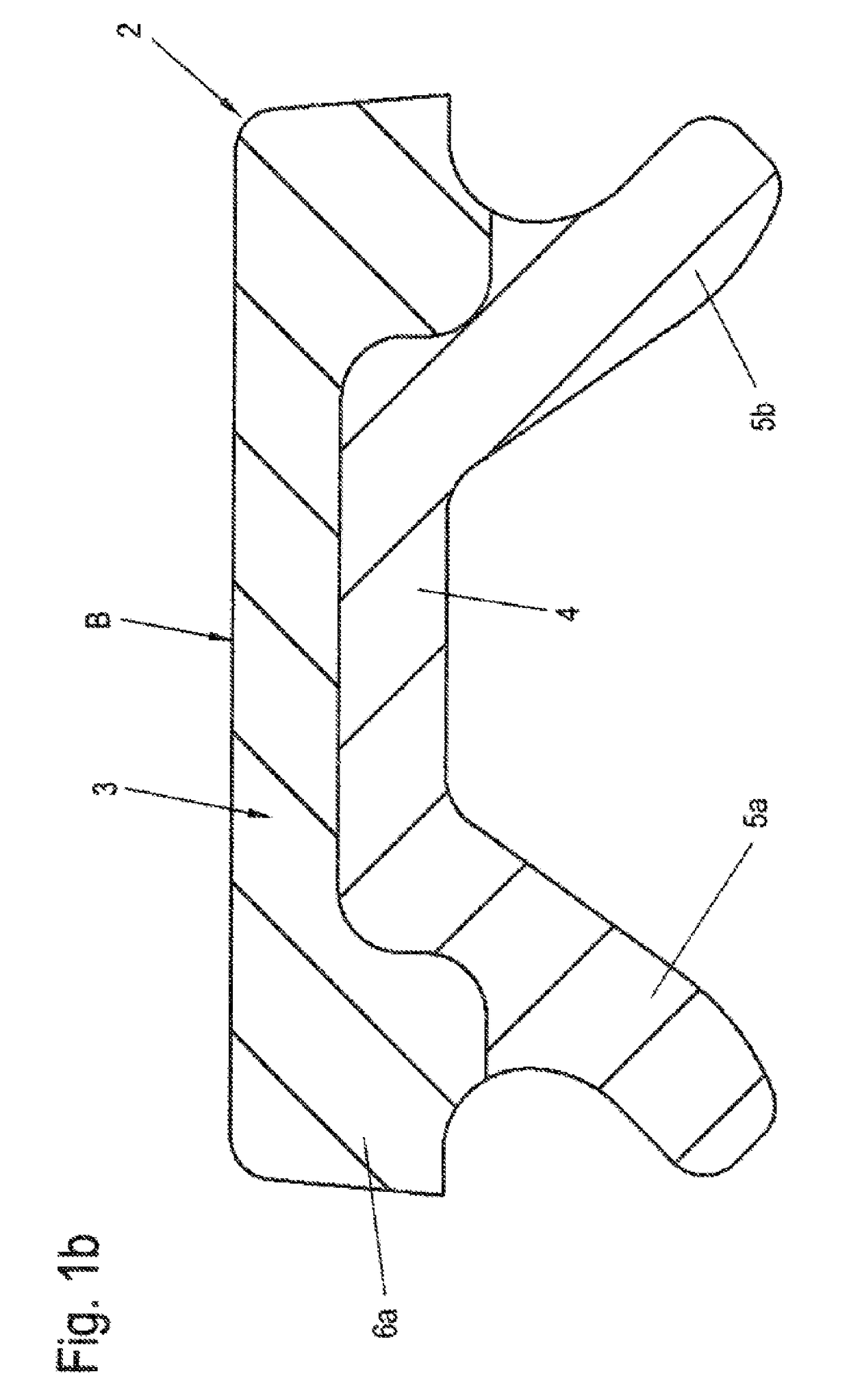
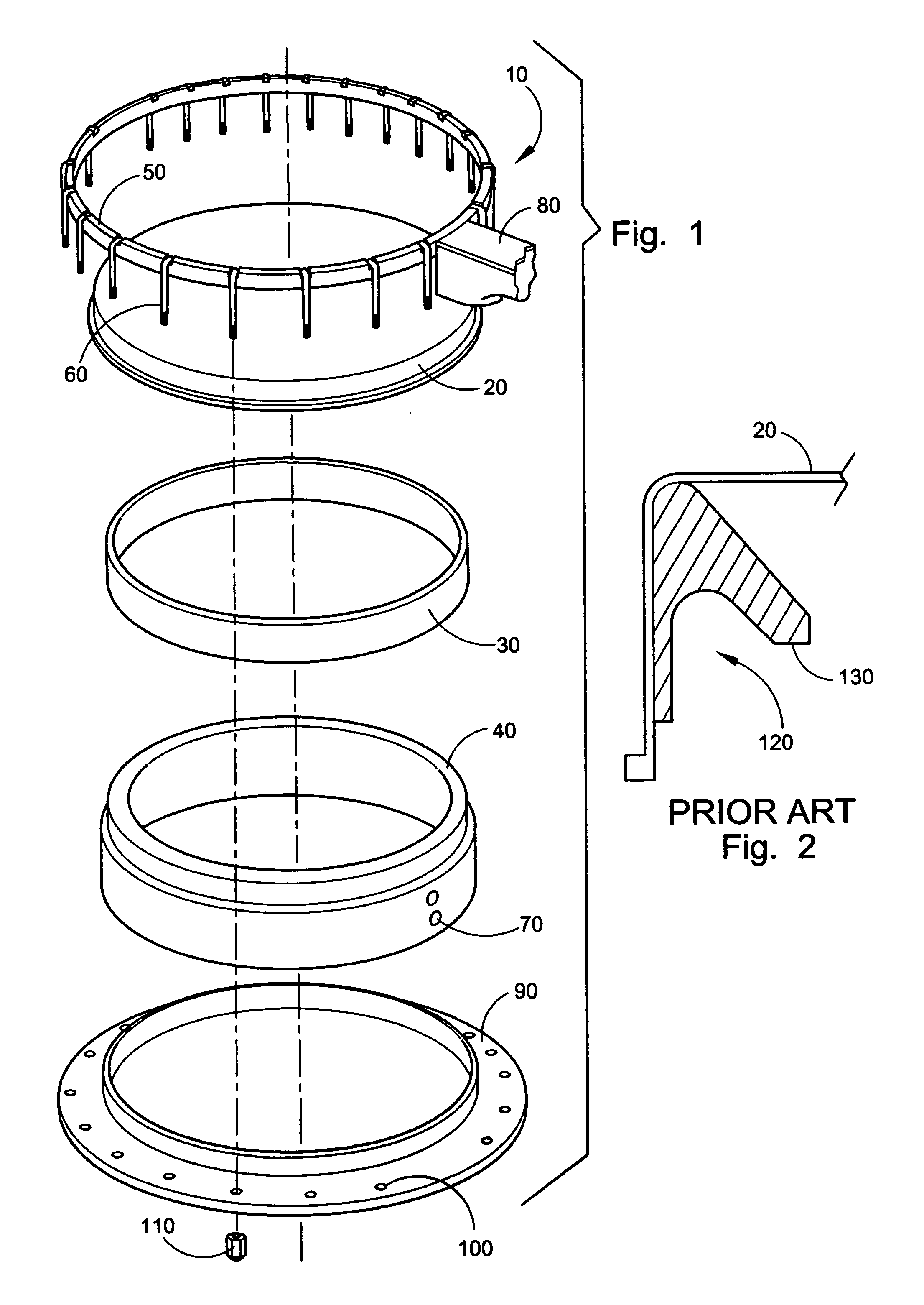
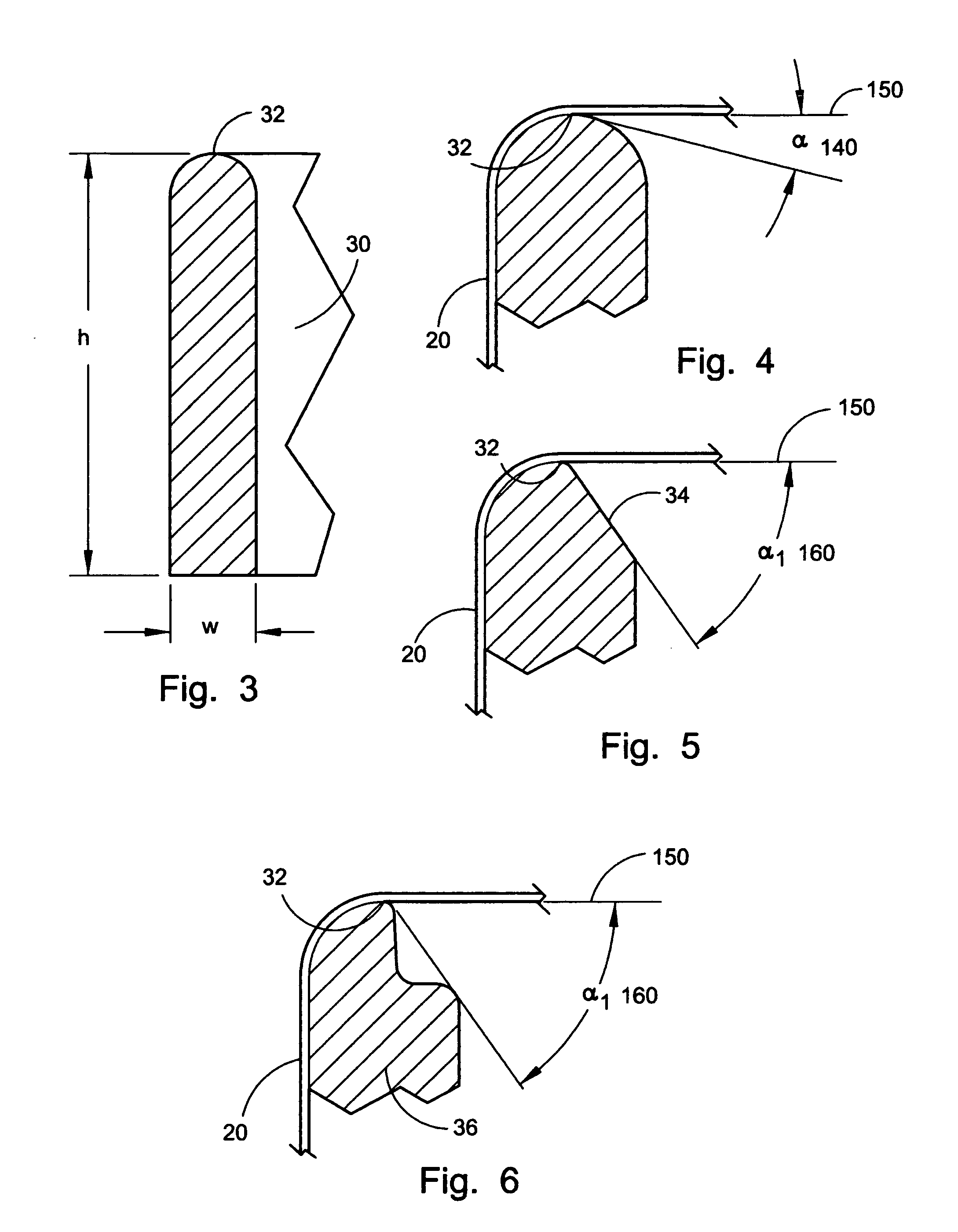
Sand control method and apparatus
InactiveUS6752206B2Reduce the possibilityIncrease pressureFluid removalDrinking water installationControl treatmentCoiled tubing
Owner:SCHLUMBERGER TECH CORP
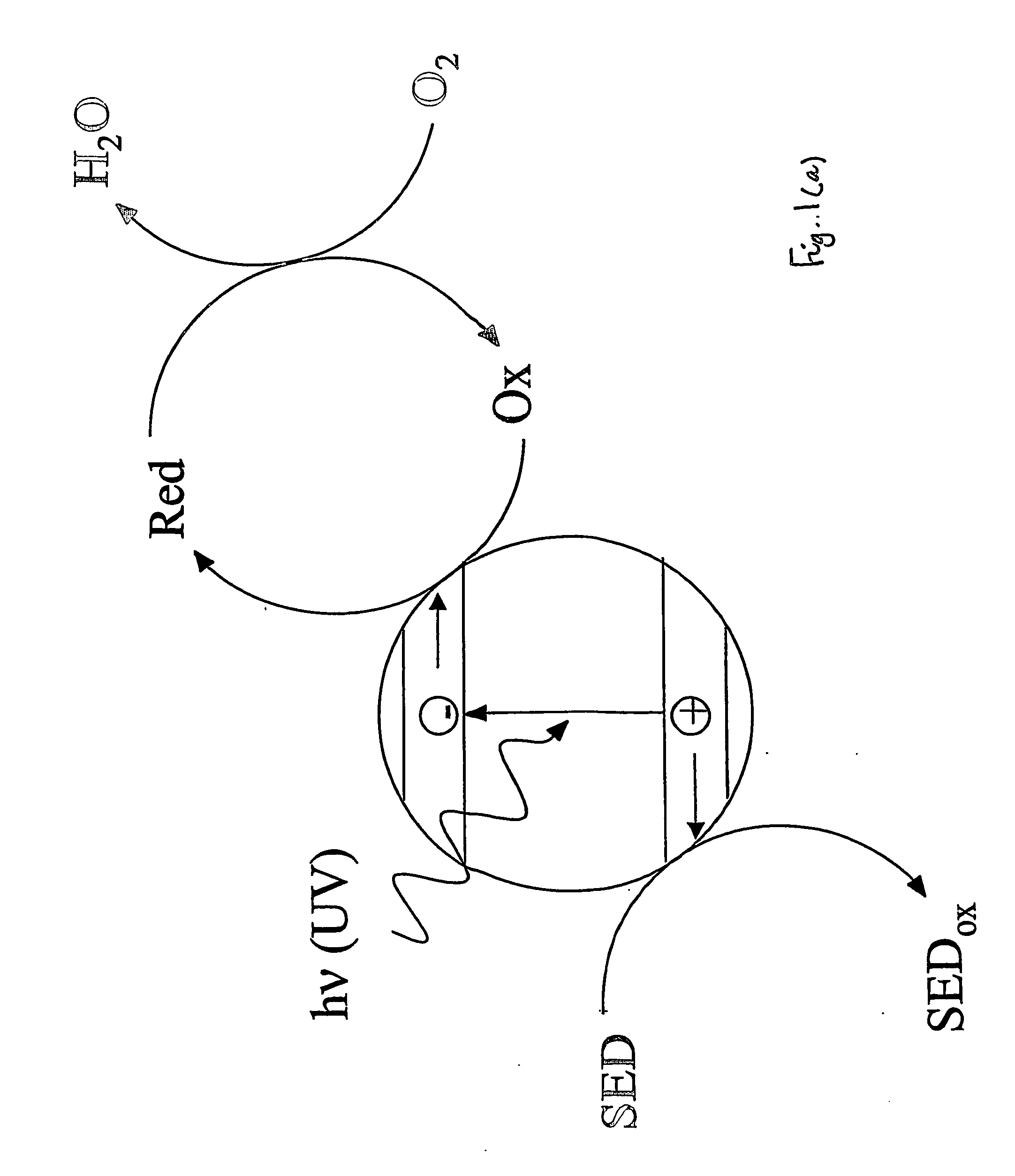
Indicator for detecting a photocatalyst
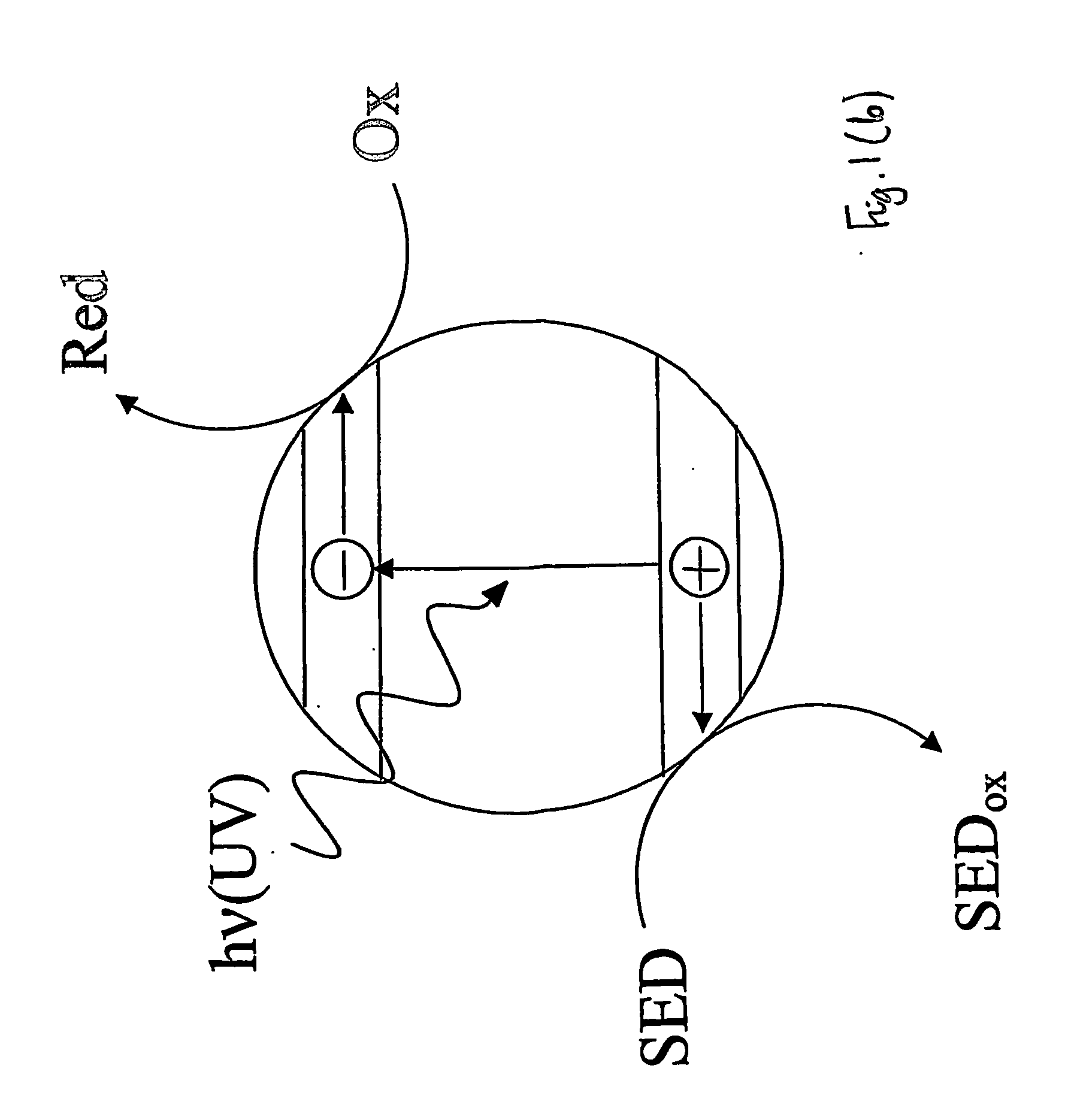
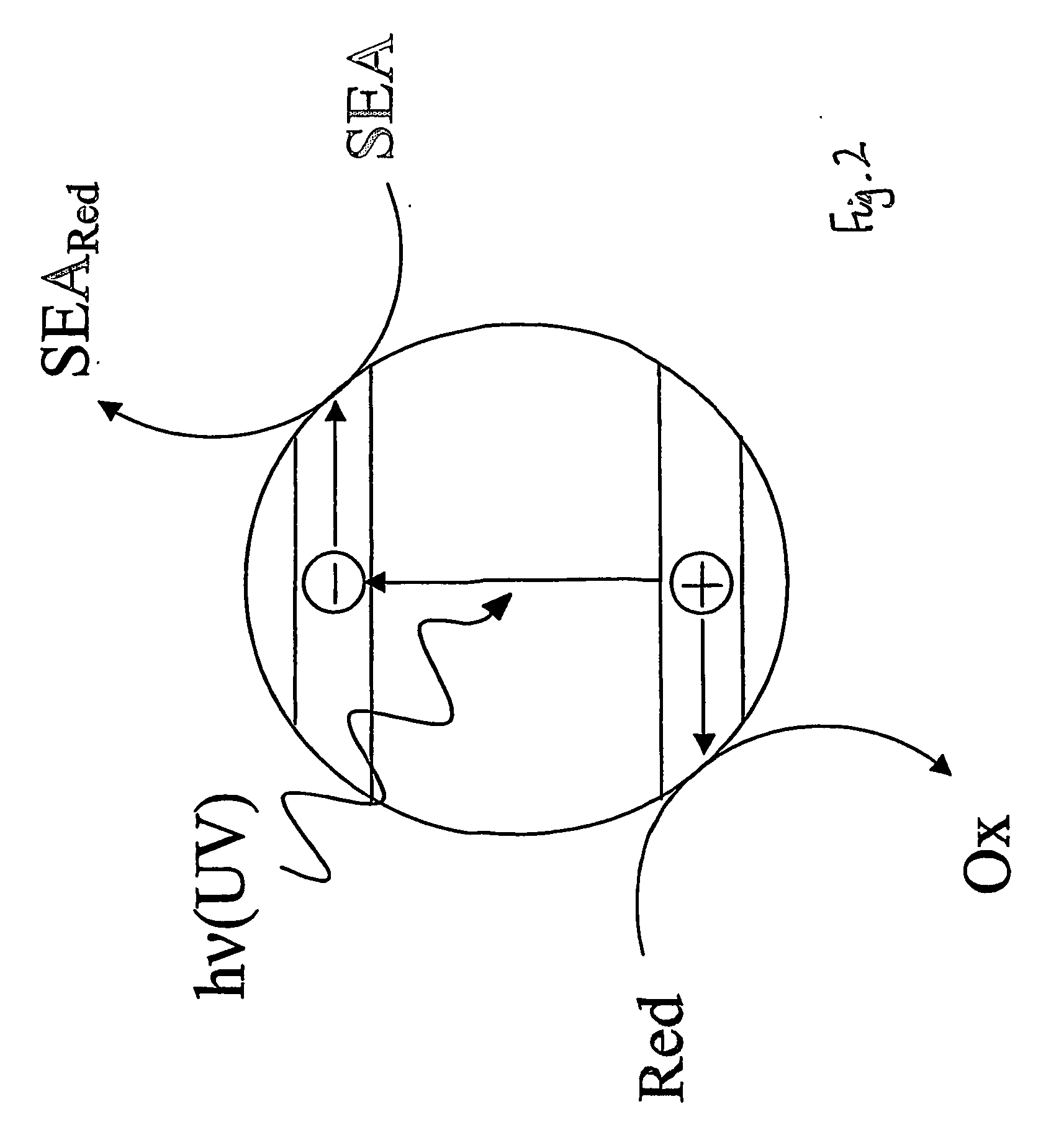
InactiveUS20070029527A1Readily oxidisableEasily materialAnalysis using chemical indicatorsOther chemical processesElectron donorSemiconductor
This invention relates to an indicator for detecting a photocatalyst wherein the indicator comprises at least one redox-sensitive material and either at least one electron donor or at least one electron acceptor. The invention also relates to a method for detecting a photocatalyst using an indicator. In particular, the photocatalyst may be a semiconductor such as titanium dioxide on a glass substrate.
Owner:UNIV OF STRATHCLYDE
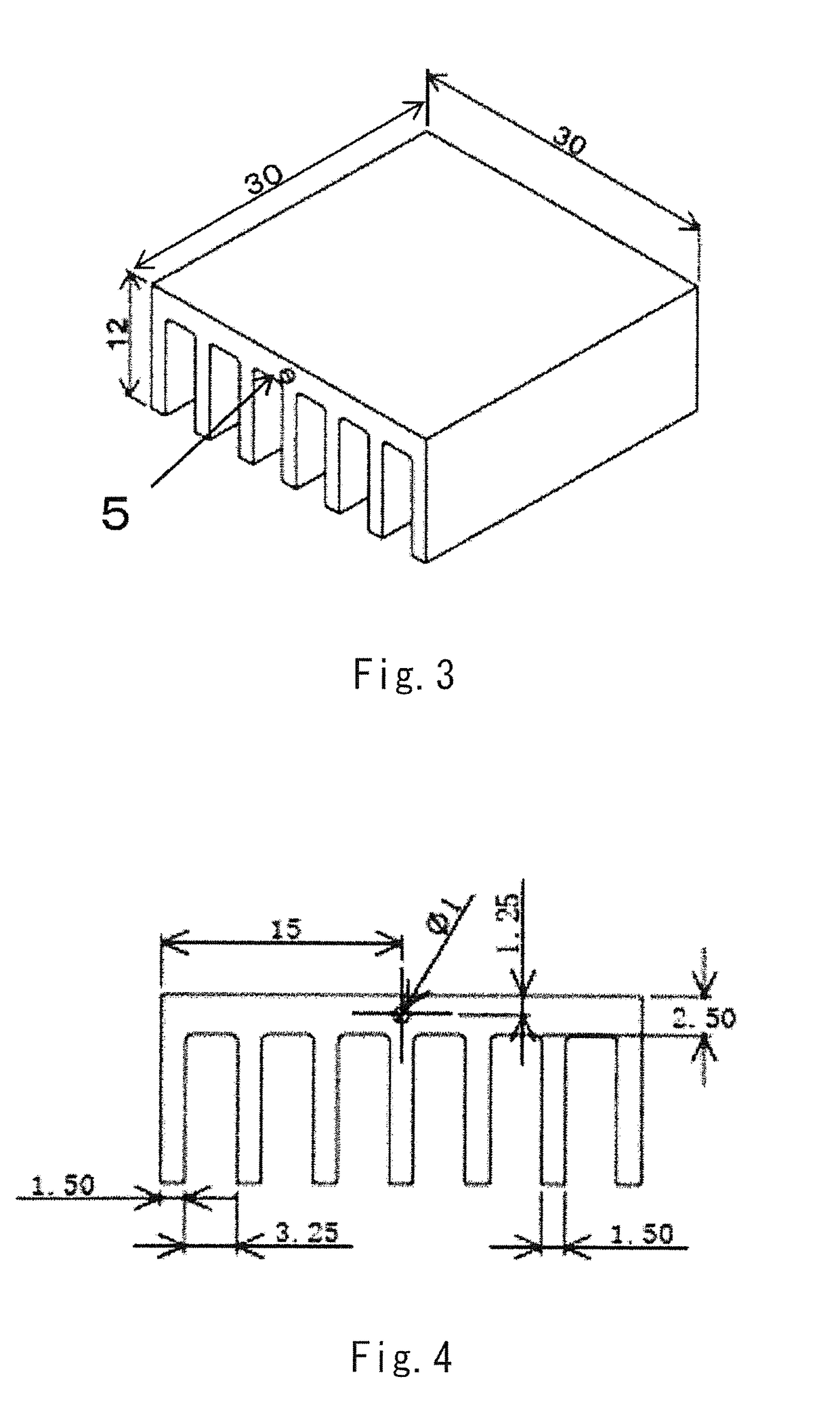
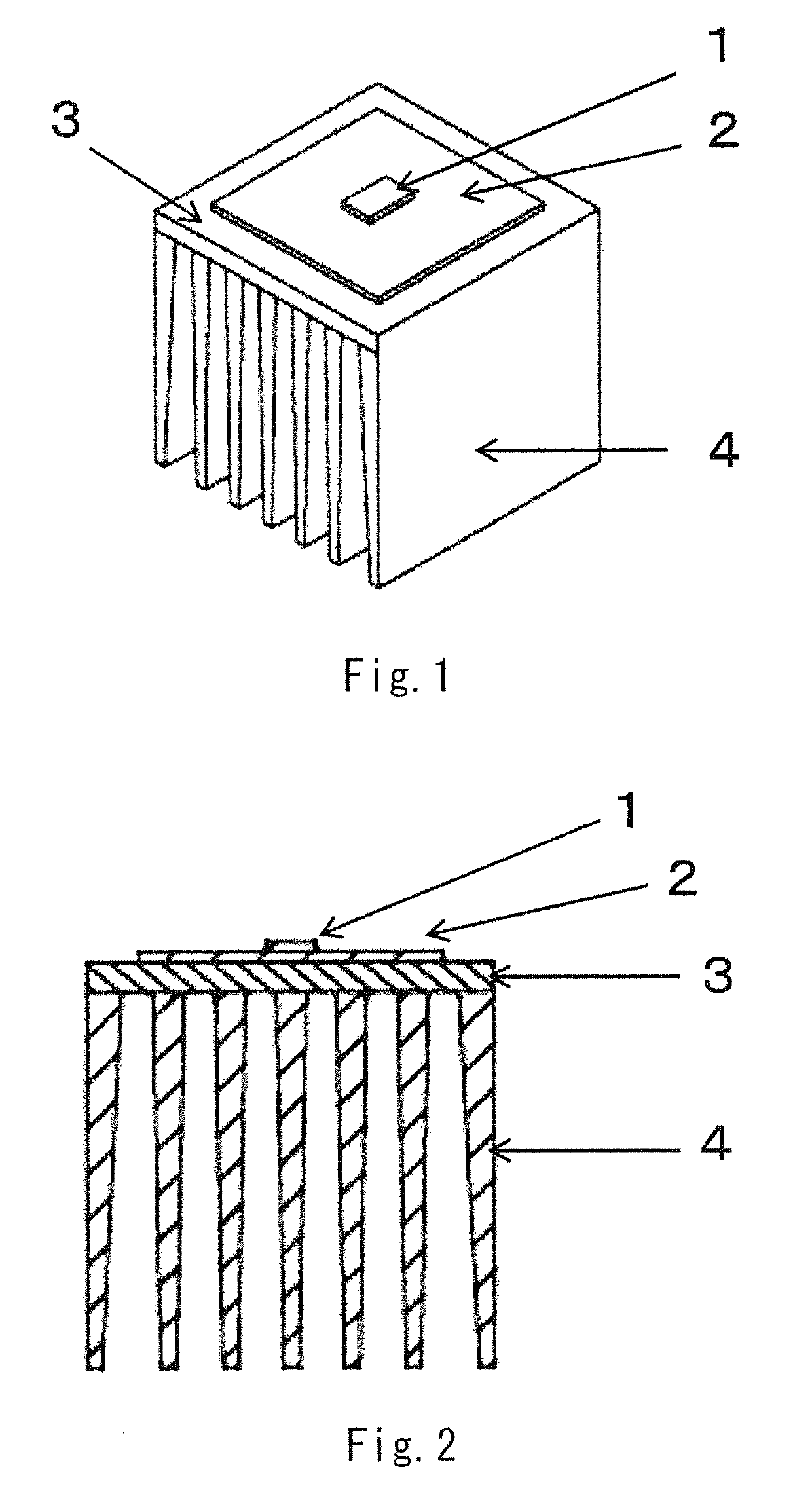
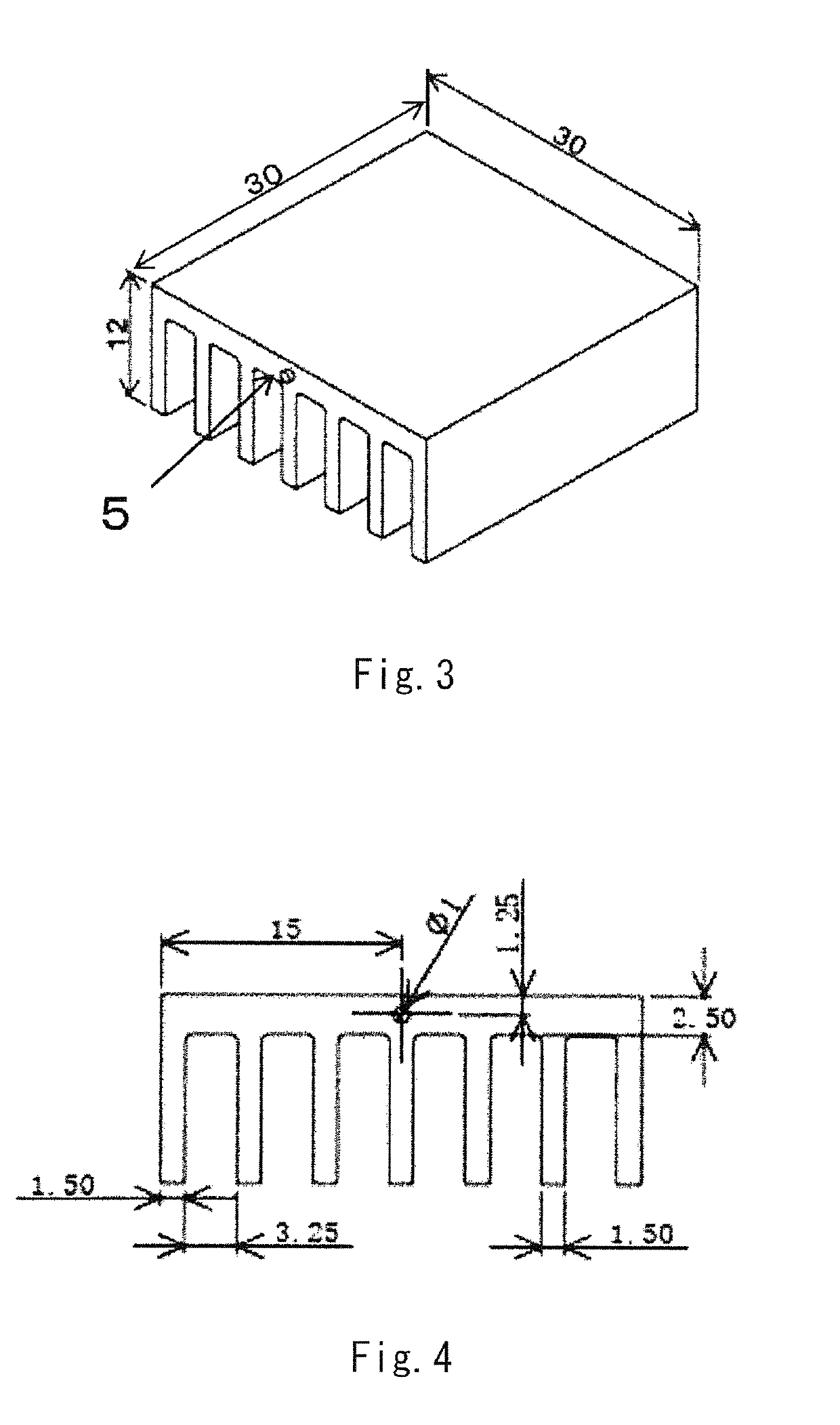
LED lamp heat sink
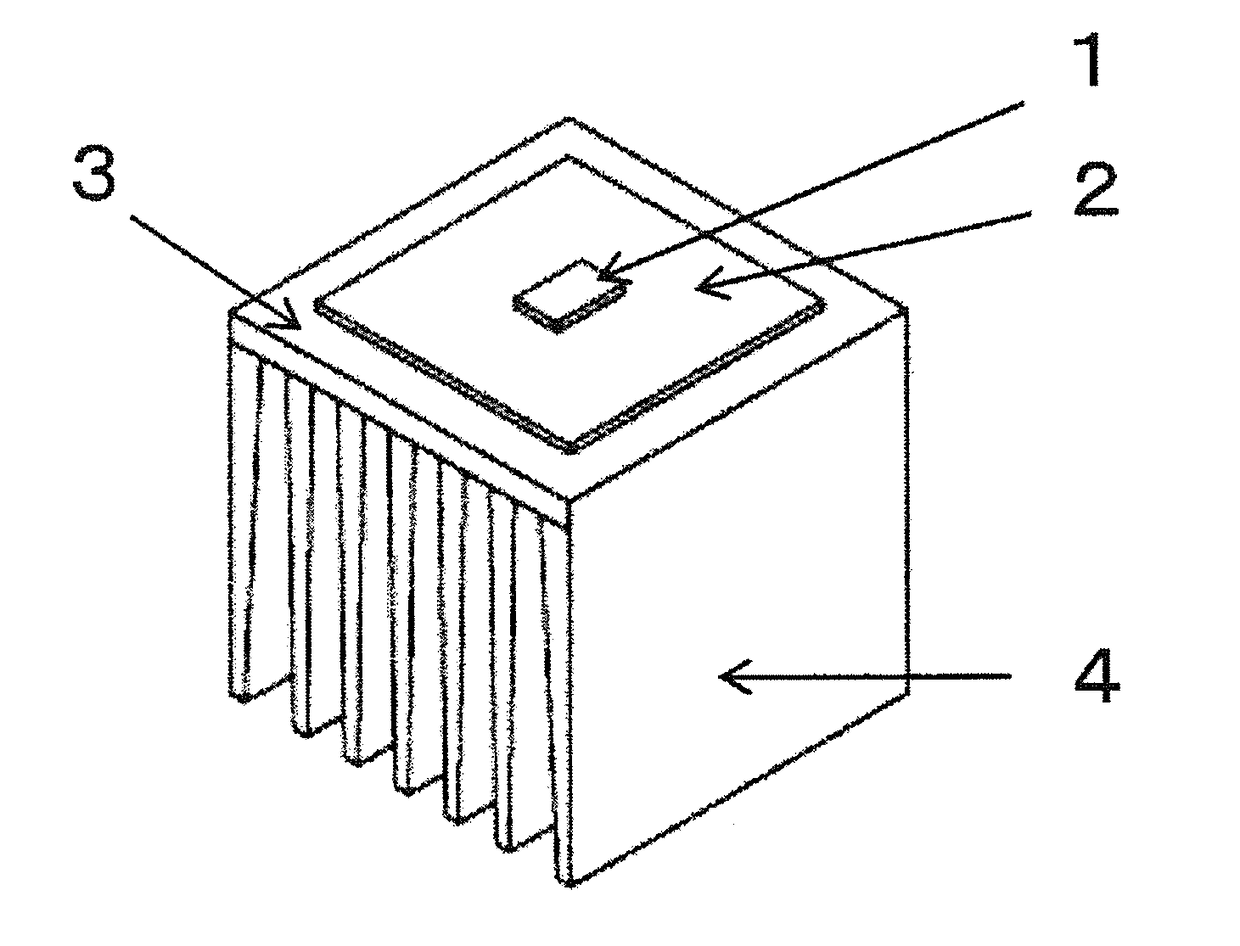
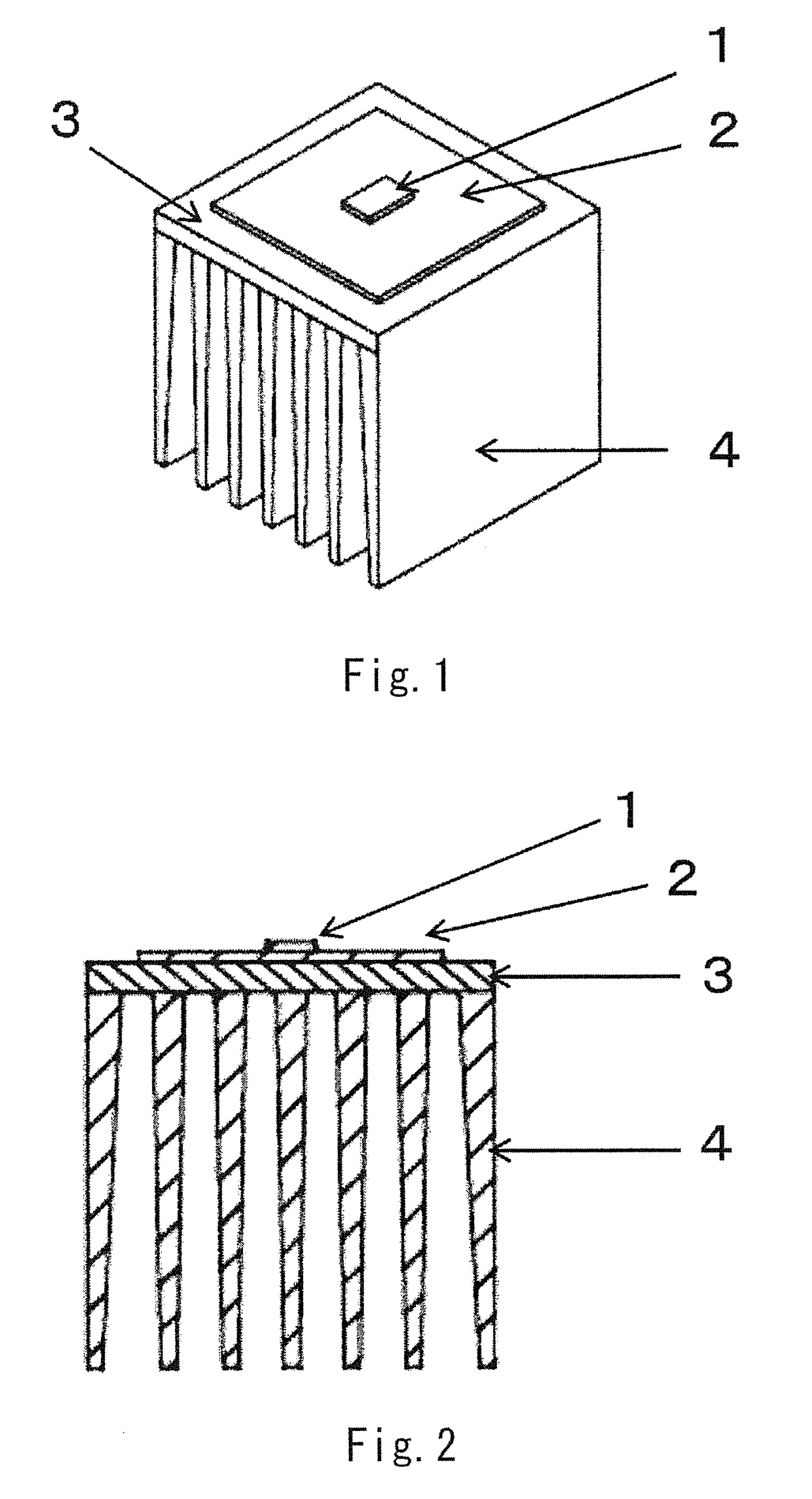
ActiveUS20170317257A1Improve conductivityGood moldabilitySemiconductor/solid-state device detailsSolid-state devicesPolyester resinGraphite
The present invention provides an LED lamp heat sink which has excellent thermal conductivity and moldability, is light in weight, and can be produced at low cost. The LED lamp heat sink is partially or wholly made of a thermally conductive resin composition and cools an LED module. The thermally conductive resin composition contains at least: 10 to 50 wt. % of thermoplastic polyester resin (A) having a number average molecular weight of 12,000 to 70,000; 10 to 50 wt. % of polyester-polyether copolymer (B); and 40 to 70 wt. % of scale-like graphite (C) having a fixed carbon content of 98 wt. % or more and an aspect ratio of 21 or more. Specific gravity of the thermally conductive resin composition is 1.7 to 2.0. Heat conductivity of the thermally conductive resin composition in a surface direction is 15 W / (m·K) or more.
Owner:KANEKA CORP
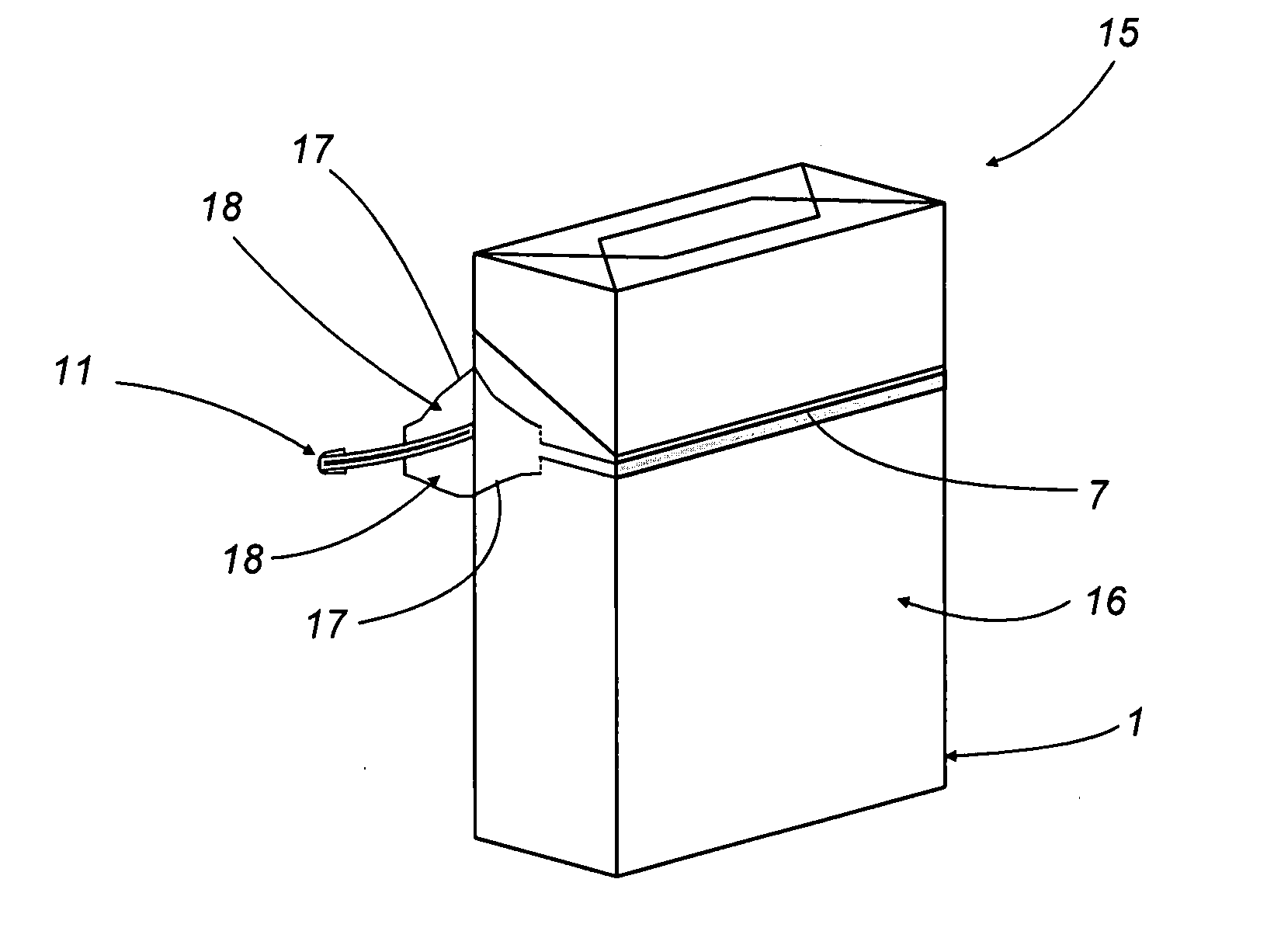
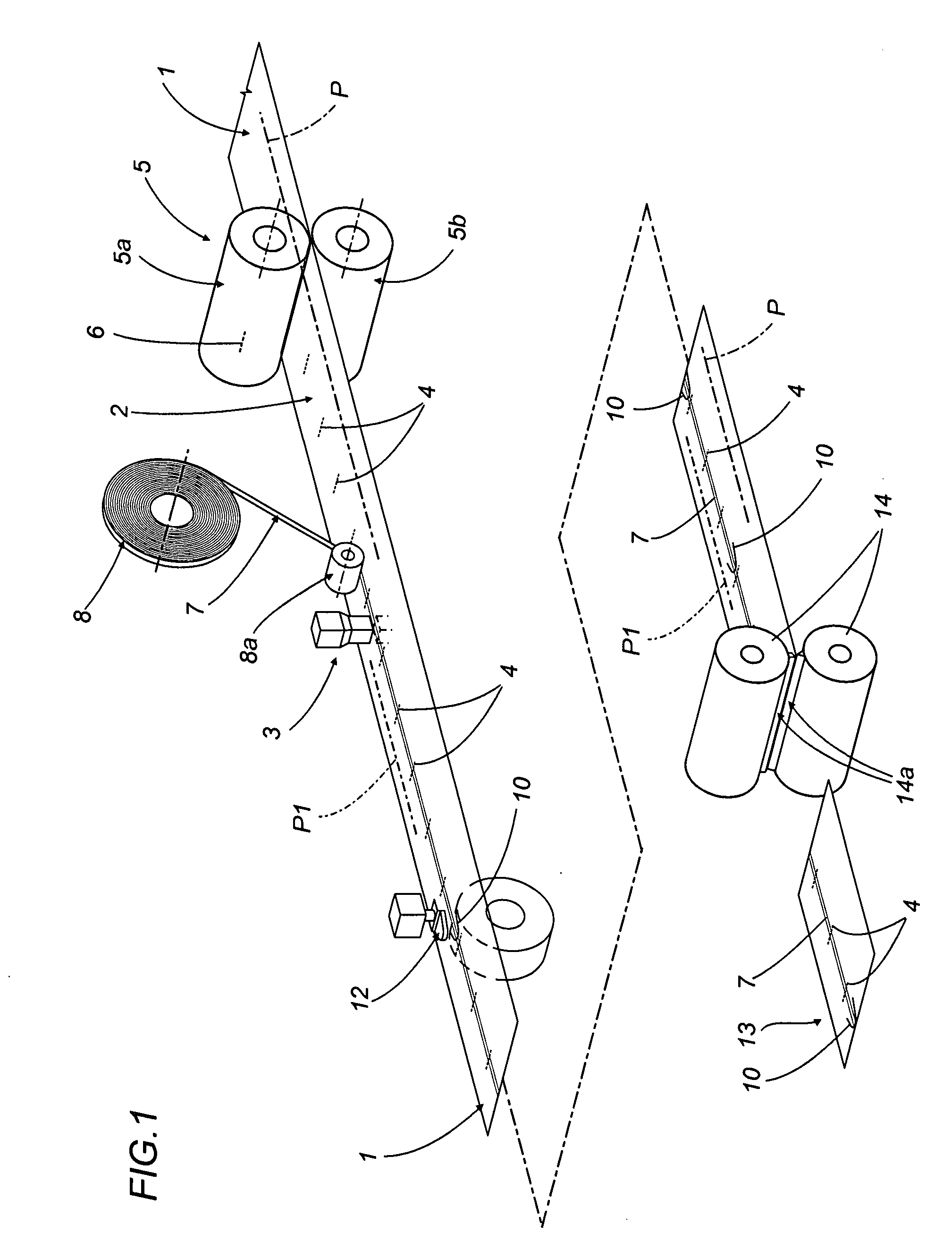
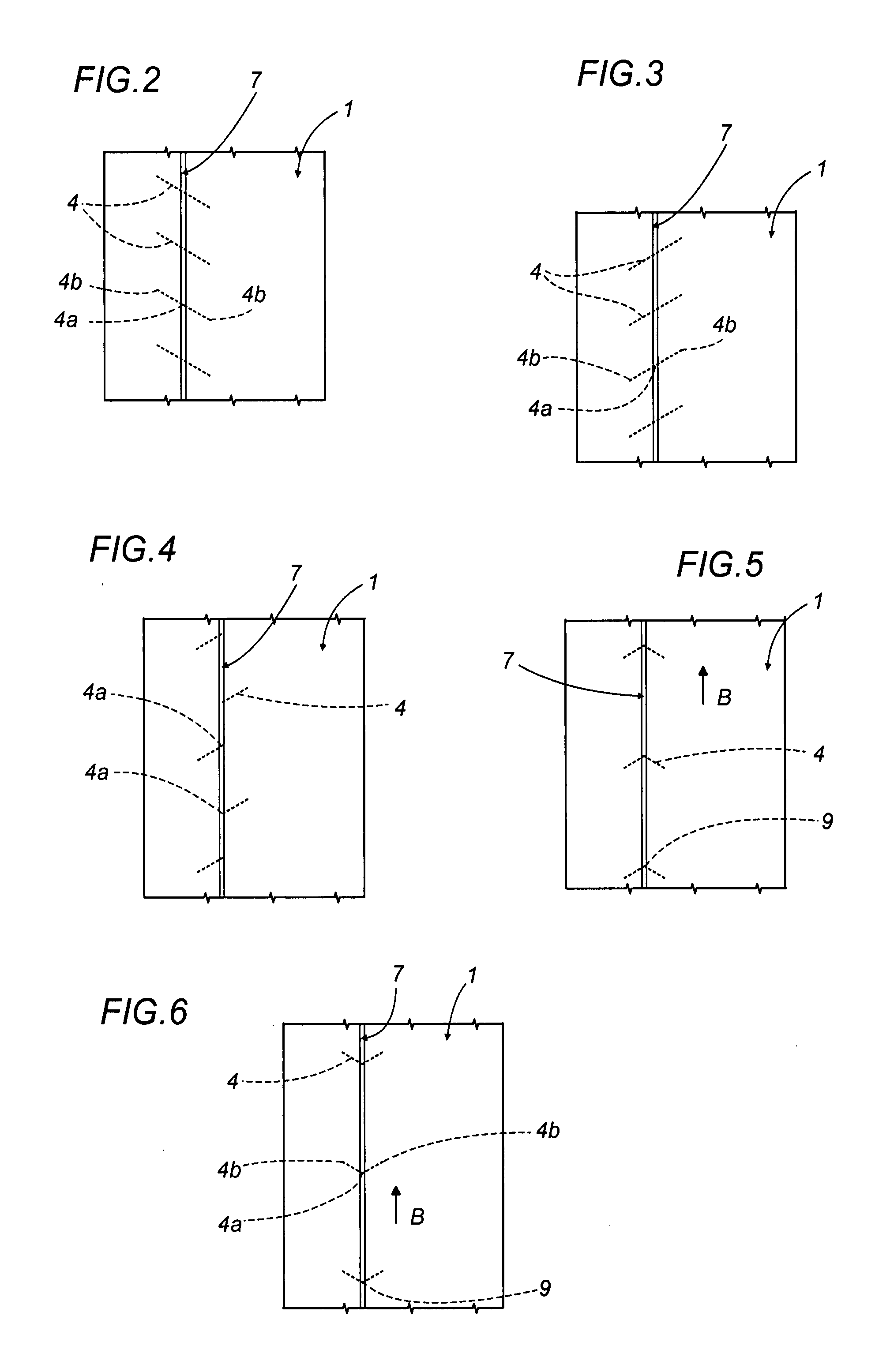
Method of producing overwrap material, and a pack furnished with the material produced
Transparent outer wrappers for cigarette packets are prepared by advancing a continuous strip of overwrap material along a given feed path in a given direction, piercing a predetermined area of the strip with a series of slits spaced apart one from another and extending nominally transverse to the feed direction, and bonding a narrow ribbon to the strip along the predetermined area, aligned on at least one portion of the slits; the strip then passes under a blade shaped so as to cut a series of “U” notches spaced apart one from the next, each creating a pull tab at the end of the ribbon, before being divided ultimately into leaves by a pair of contrarotating cutter rollers equipped with respective blades. Each single leaf serves as a wrapper for a relative pack, fashioned so that when torn open by pulling the ribbon, the ribbon encounters the slit and initiates two break lines extending away from the ribbon on either side to facilitate the removal of the wrapper in its entirety.
Owner:GD SPA
Graphite heat dissipation apparatus and clamping frame for clamping graphite heat dissipation fin module
InactiveUS7616441B2Improve cooling efficiencyLow costSemiconductor/solid-state device detailsSolid-state devicesGraphiteHeat sink
A graphite heat dissipation apparatus and a clamping frame for clamping a graphite heat dissipation fin module are provided. The graphite heat dissipation apparatus includes a graphite heat dissipation fin module and at least one clamping frame. The graphite heat dissipation fin module includes a substrate and a plurality of graphite fins which are positioned at and extend from one surface of the substrate. The clamping frame includes at least one support bracket, at least one retention plates and a frame body. The support brackets are positioned at top surface of a chassis. The frame body upwardly extends from the support brackets, and the retention plates traverse extend across the side surface of the frame body. The retention plates are used to clamp the substrate so that the graphite fins are positioned above the chassis. The graphite heat dissipation fin module is used to draw hear away from the electronic device which can be positioned below the substrate and at the surface of the chassis.
Owner:HORNG CHIN FU
Constant concentration delivery device and method for vaporized substances
InactiveUS6877724B1Reduce vapor phase chemicalReduce particulate matterLighting and heating apparatusTransportation and packagingCompound (substance)Biological substances
A zero emmission device and method for delivering constant concentration of a vaporized substance allows for the regulated use of chemical or biological substances, such as calibrating, exposure or therapeutic substances.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
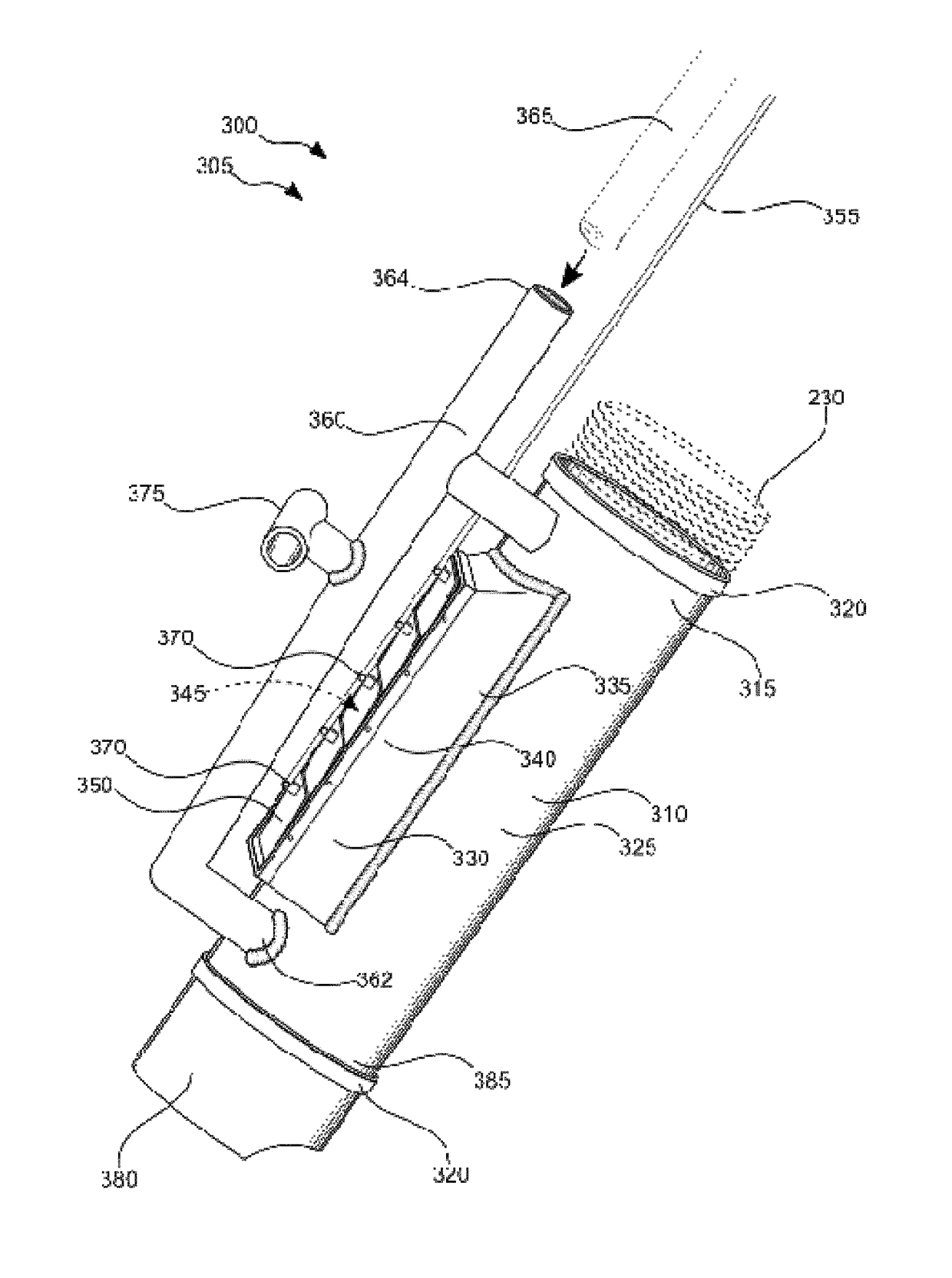
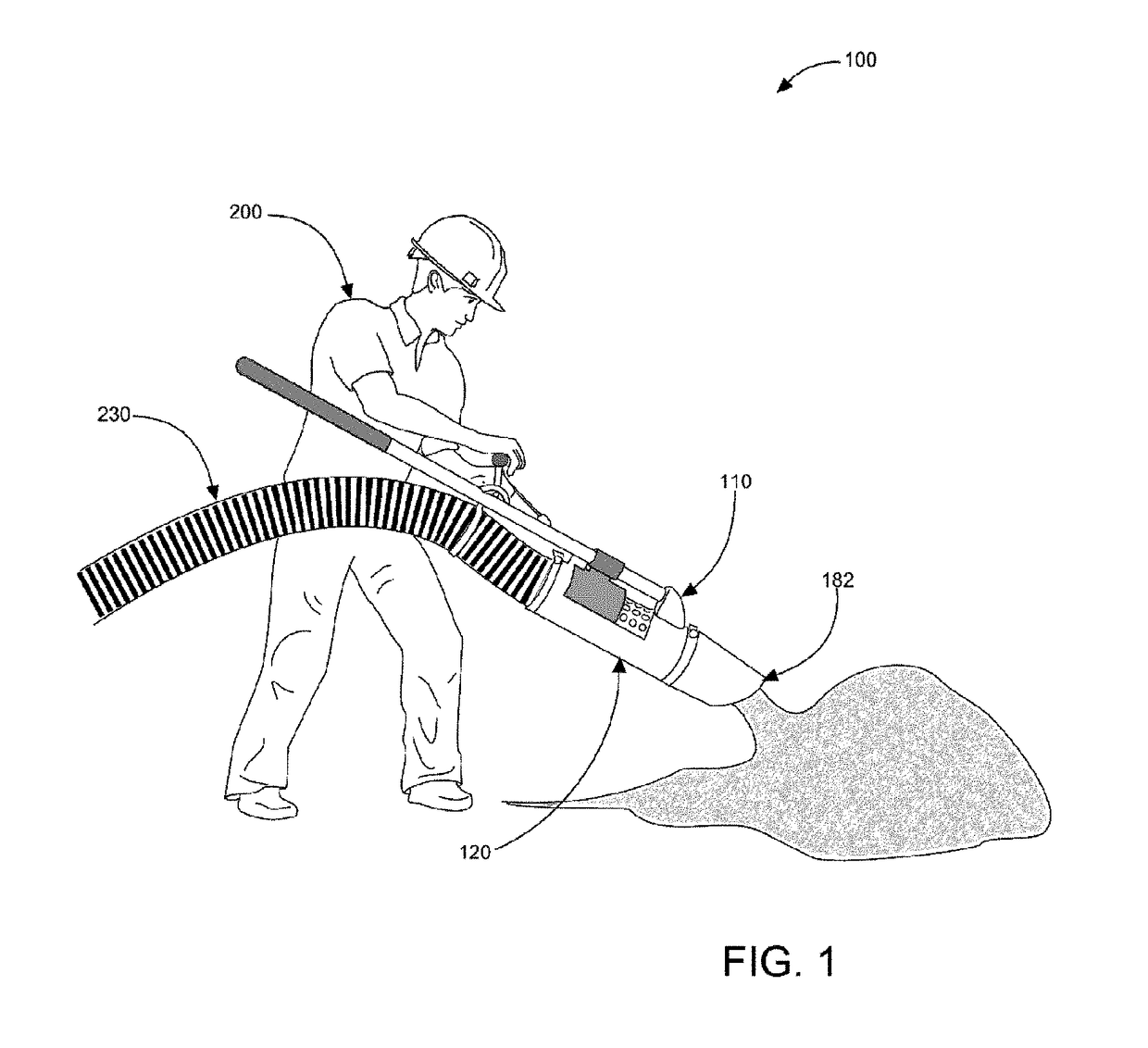
Vacuum hose handling and safety vacuum release system
ActiveUS9702101B1ConvenientlyEasily materialMechanical machines/dredgersMechanical suction controlEngineeringVacuum tube
A vacuum hose handling and safety vacuum release system, a tubular column attachable to an industrial vacuum hose end having a long handle and a short T-handle positioned at right angles to each other that will allow a user to strategically place the metallic vacuum tube conveniently and easily to vacuum debris in a safe manner. The tube has at least one bypass orifice that the user can open to reduce or eliminate the suction at the nozzle via a lever pivotally located adjacent the T-handle.
Owner:FICKS DAVID
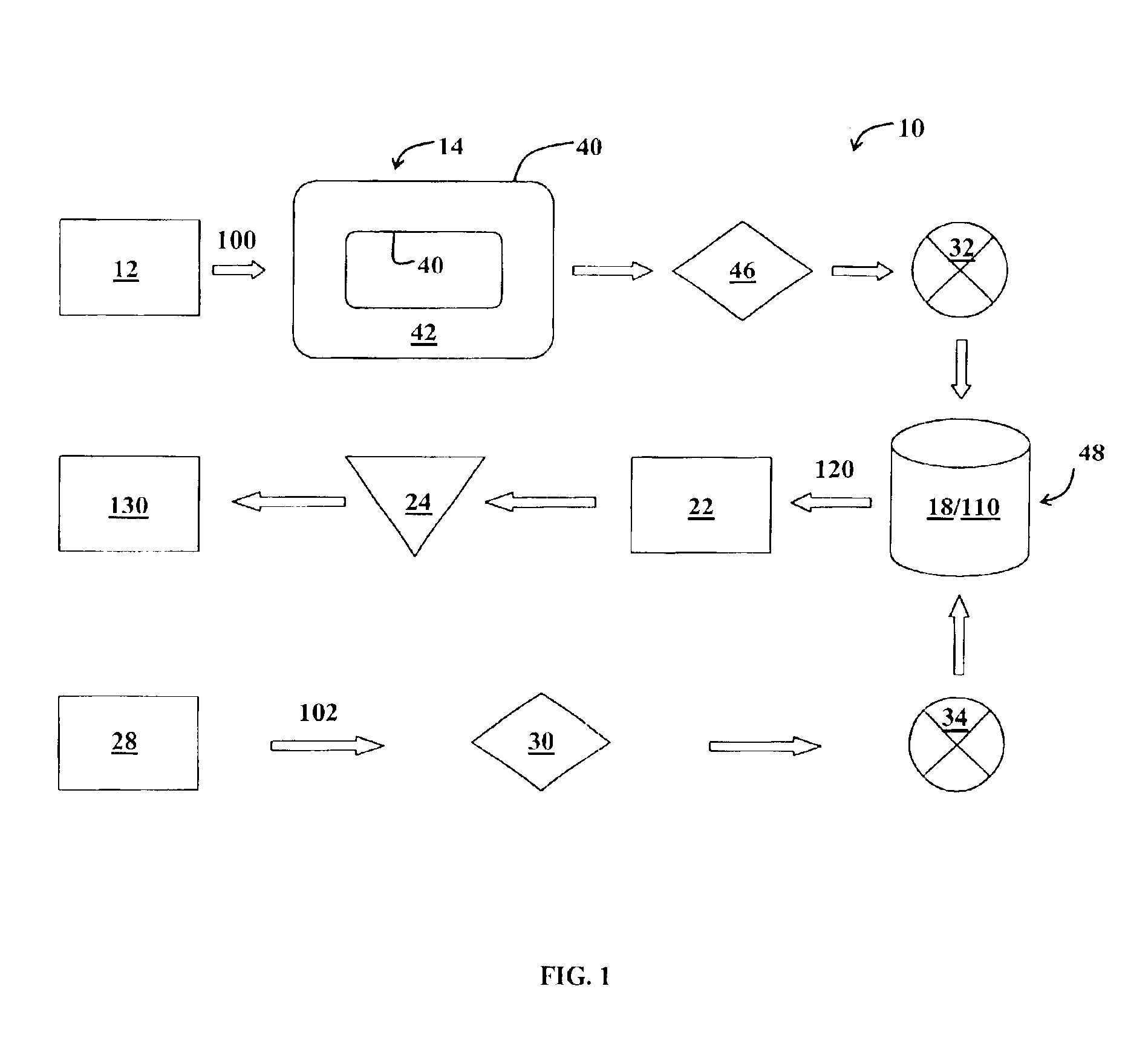
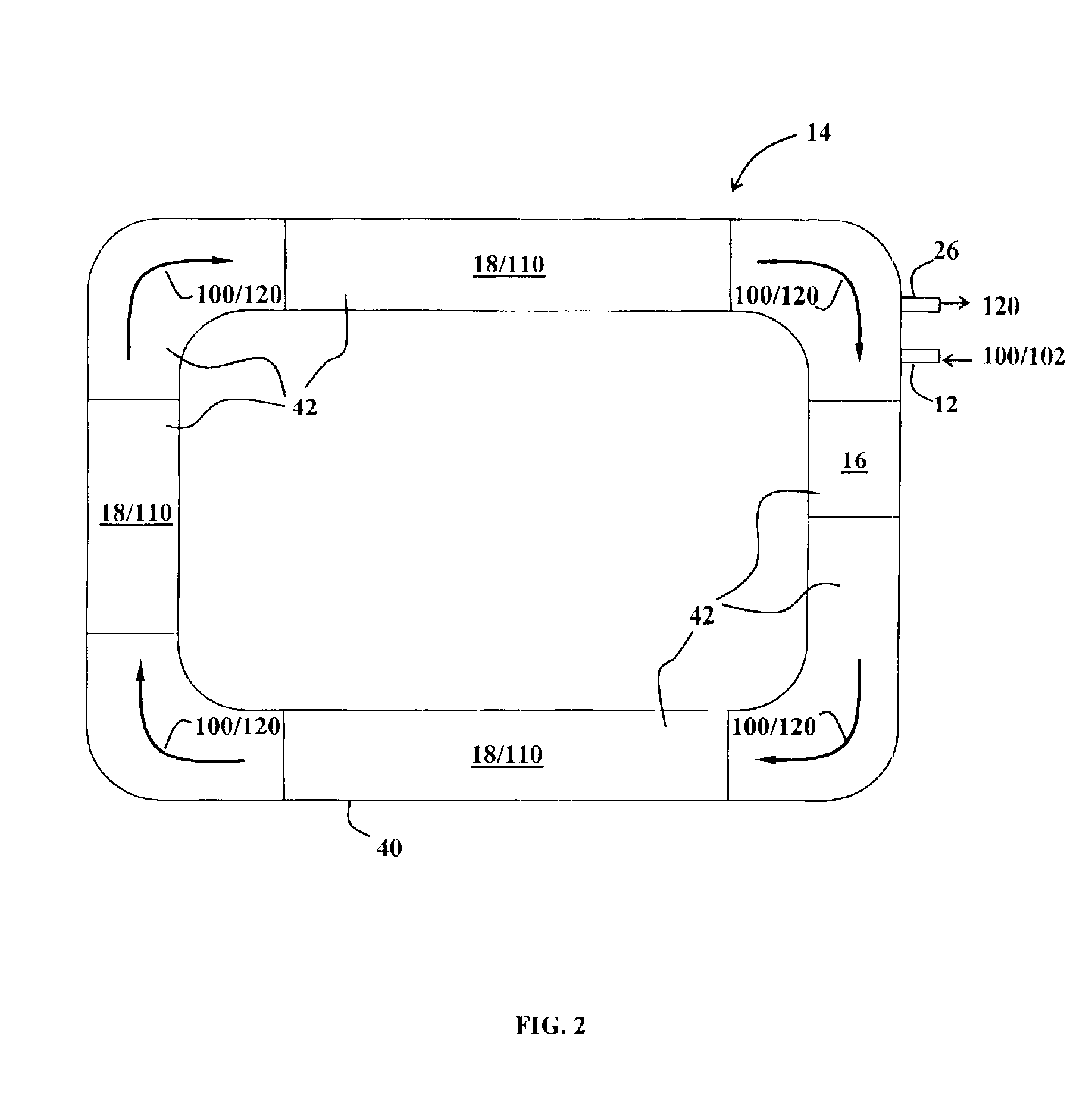
Sand control method and apparatus
InactiveUS20020033259A1Reduce the possibilityIncrease pressureFluid removalDrinking water installationControl treatmentCoiled tubing
The present invention discloses a system using a conduit run into a well on a service string. In one embodiment the conduit includes outlets along its length to permit a sand control treatment to exit the conduit along the length of the conduit and distribute the sand control treatment along the length of the conduit. The conduit can have a plurality of such outlets spaced to provide an even distribution of the sand control treatment. The conduit can be attached to the delivery tubing (such as coiled tubing or service string tubing) via a releasable connector. The conduit is deployed on the delivery tubing and connector (and is in fluid communication therewith) in the well adjacent to an area to be treated. The sand control treatment is pumped into the well. Once the sand control treatment is complete, the releasable connector is released to disconnect the conduit from the delivery tubing and the delivery tubing is removed from the well.
Owner:SCHLUMBERGER TECH CORP
Marking strip
ActiveUS20170282431A1Cost-effective and reliableEasy to produceStampsElectrically conductive connectionsPlastic materialsElectrical devices
A marking strip has a plurality of marking elements which are connected to each other by a connecting region. The marking strip having the marking elements is designed to mark electrical devices, in particular electrical devices that can be arranged next to each other such as terminal blocks. Each of the marking elements has a marking plate including at least one writing field which can be provided with information. On the side facing away from the writing field, each of the marking elements has a locking contour which is integrally connected with the marking plate. The marking strip is formed of at least two different plastic materials of different hardness. The locking contour of each marking element is formed of a harder plastic material, and the marking plate is formed of a softer plastic material at least in the region of the writing field. The connecting region between the marking elements is formed of the softer plastic material.
Owner:WEIDMULLER INTERFACE GMBH & CO KG
Iron base soft magnetic powder for powder magnetic cores, fabrication method for same, and powder magnetic core
InactiveUS20140002219A1Improve heat resistanceLow costTransportation and packagingMetal-working apparatusIron powderResin coating
This invention addresses the problem of providing an iron base soft magnetic powder for a powder magnetic core that does not use rare metals, that can maintain the electrical insulating properties between the iron powder particles even when subjected to high temperature thermal processing, and that has excellent thermal stability and mechanical strength. This invention also addresses the problem of providing a fabrication method for the iron base soft magnetic powder for the powder magnetic core, and providing the powder magnetic core. In this iron base soft magnetic powder for the powder magnetic core, a phosphatized coating film is formed on the surface of the iron base soft magnetic powder, and a silicon resin coating film is formed on the surface of the phosphatized coating film. The phosphatized coating film contains P, B, Mg, and Al.
Owner:KOBE STEEL LTD
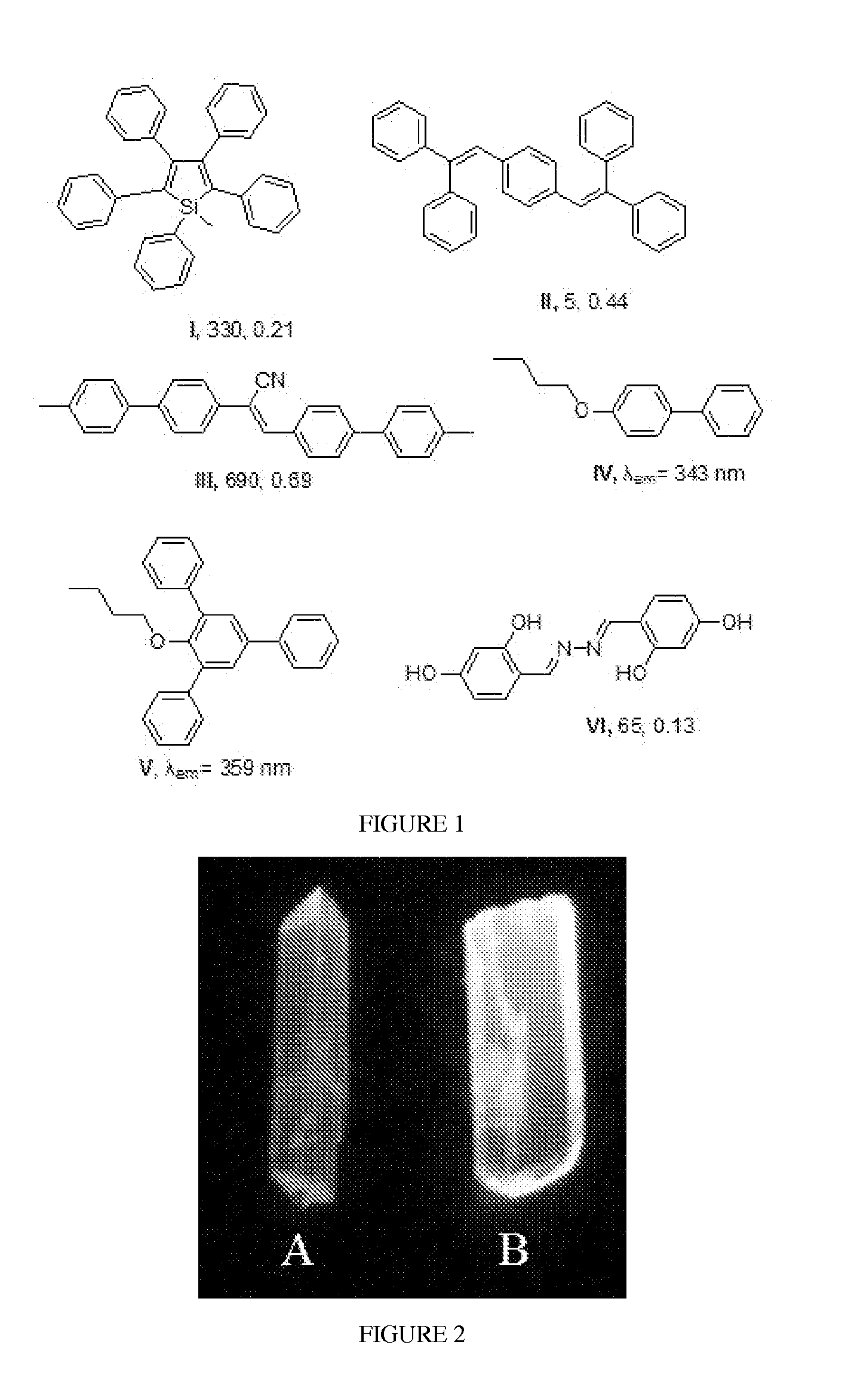
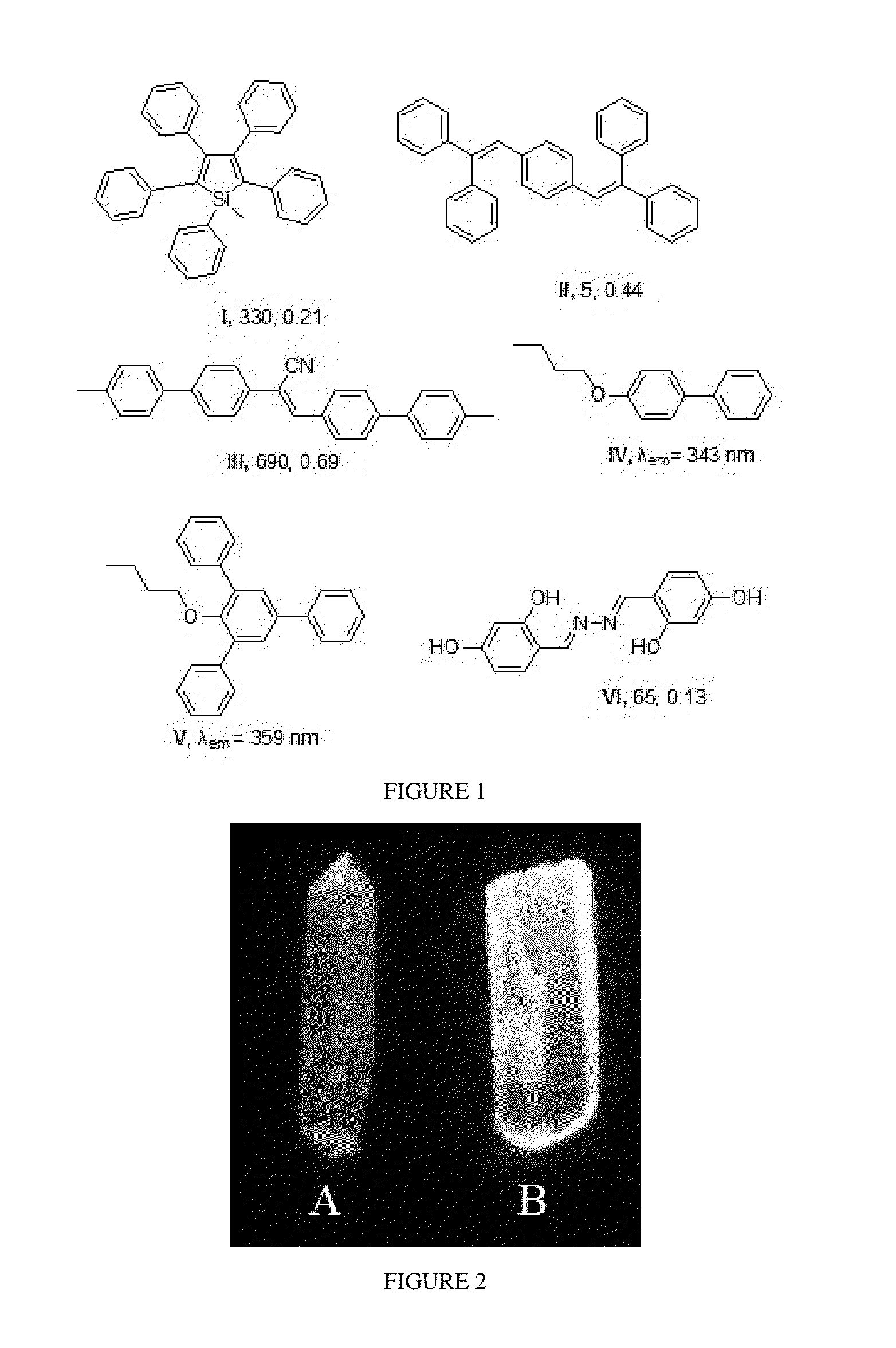
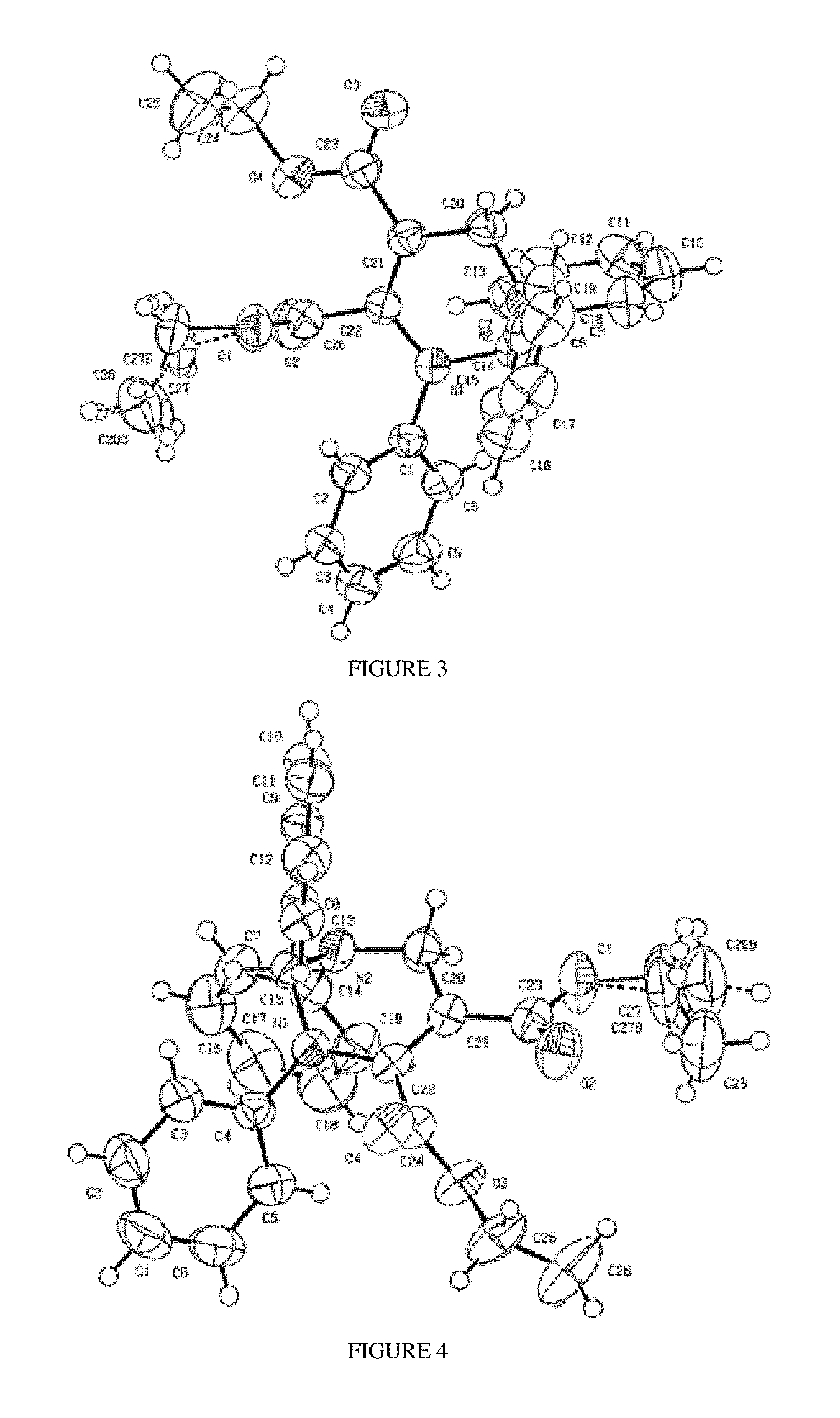
Penta-substituted tetrahydropyrimidines with aggregation-induced emission characteristics and preparation method and use thereof
InactiveUS20140051855A1Easy to getSimple and convenient stepOrganic chemistryLuminescent compositionsArylOrganic chemistry
The present invention provides a series of penta-substituted tetrahydropyrimidines with aggregation-induced emission (AIE) characteristics and preparation method and use thereof. The AIE penta-substituted tetrahydropyrimidines have structures shown as formula (I). R1 is selected from a group consisting of linear or branched alkyls and substituted alkyls. R2 and R4 is respectively selected from a group consisting of linear or branched alkyls, substituted alkyls, cycloalkyls, substituted cycloalkyls, aryls, substituted aryls, polycyclic aryls, substituted polycyclic aryls, heterocyclyls, substituted heterocyclyls, aromatic heterocyclyls and substituted aromatic heterocyclyls. R3 is selected from a group consisting of aryls, substituted aryls, polycyclic aryls, substituted polycyclic aryls, aromatic heterocyclyls and substituted aromatic heterocyclyls. The penta-substituted tetrahydropyrimidines can be prepared by multi-component reactions (MCR). There are 1˜3 aryls in the structure of the penta-substituted tetrahydropyrimidines. The penta-substituted tetrahydropyrimidines possess strong AIE properties and can be used for preparing organic electro-luminescence or photo-luminescence devices, or chemical and biological fluorescent sensors or probes.
Owner:SOUTHERN MEDICAL UNIVERSITY
Electrical contact element
ActiveUS20170342571A1Excellent corrosion protectionLow densityContact member manufacturingCoupling contact membersStandard electrode potentialMaterials science
An electrical contact element formed of sheet metal having a first region and a second region. Each one of the first and second regions is coated with a coating including a first layer containing a first material having a lower standard electrode potential than the sheet metal material. The coating includes a second layer in the first region which is absent in the second region. The second layer is arranged underneath the first layer and contains a second material that has a lower standard electrode potential than the first material.
Owner:APTIV TECH LTD
Bandage with a compressed layer that expands upon contact with liquid
InactiveUS20150223988A1Speed healingSpeeding healing processAdhesive dressingsPlaster of paris bandagesAdhesiveInfectious agent
A bandage is formed of a film layer, an adhesive applied to the film layer, and an absorbent layer connected to the film layer. The absorbent layer comprises a compressed fabric that upon saturation with liquid expands to a volume that is at least two or three times the volume of the compressed fabric. The film layer extends in at least two directions beyond edges of the absorbent layer, so that the film layer with adhesive can attach to skin around a wound with the absorbent layer covering the wound. The film layer has folded sections adjacent the absorbent layer, so that the folded sections unfold upon expansion of the compressed fabric. The expansion of the fabric layer upon contact with bodily fluids from a wound pulls the fluids and any infectious agents out of an away from the wound, thus speeding the healing process.
Owner:SPECTOR DONALD
Marking strip
ActiveUS10369735B2Cost-effective and reliableEasy to produceStampsElectrically conductive connectionsPlastic materialsMarking out
A marking strip has a plurality of marking elements which are connected to each other by a connecting region. The marking strip having the marking elements is designed to mark electrical devices, in particular electrical devices that can be arranged next to each other such as terminal blocks. Each of the marking elements has a marking plate including at least one writing field which can be provided with information. On the side facing away from the writing field, each of the marking elements has a locking contour which is integrally connected with the marking plate. The marking strip is formed of at least two different plastic materials of different hardness. The locking contour of each marking element is formed of a harder plastic material, and the marking plate is formed of a softer plastic material at least in the region of the writing field. The connecting region between the marking elements is formed of the softer plastic material.
Owner:WEIDMULLER INTERFACE GMBH & CO KG
Artificial dielectric lens
InactiveUS20150168601A1Facilitating arrangement of lensWithout requiring highly accurate processingAntennasOptical elementsOptical axisRefractive index
A z-axis is defined as an optical axis and axes perpendicular to the z-axis are defined as an x-axis and a y-axis. Multiple metal plate pieces each have multiple groove portions formed in the direction of the x-axis. An outer shape of the metal plate pieces are such that the respective cross sections of the metal plate pieces are parallel to x-z planes at given intervals of a lens along the y-axis from a lower edge to a center and from the center to an upper edge. A metal dielectric lens is formed by laminating the metal plate pieces such that the metal plate pieces are arranged parallel to the x-z planes at the given intervals. Thus a refractive index responsive to the number of the groove portions, and the width and the depth of the groove portion can be obtained. A resultant structure causes visible light to pass therethrough.
Owner:IBARAKI UNIVERSITY
Welding protection mask
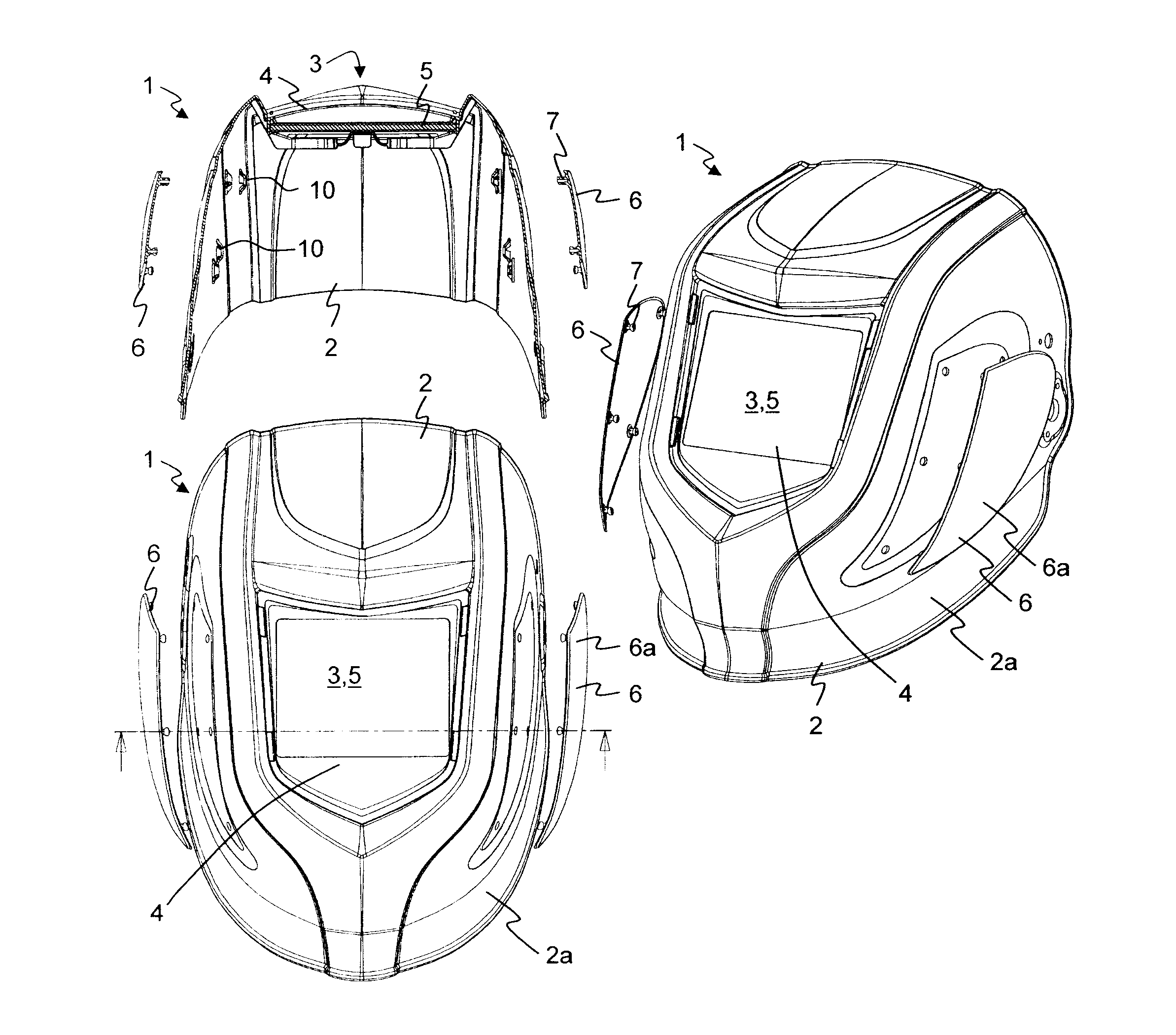
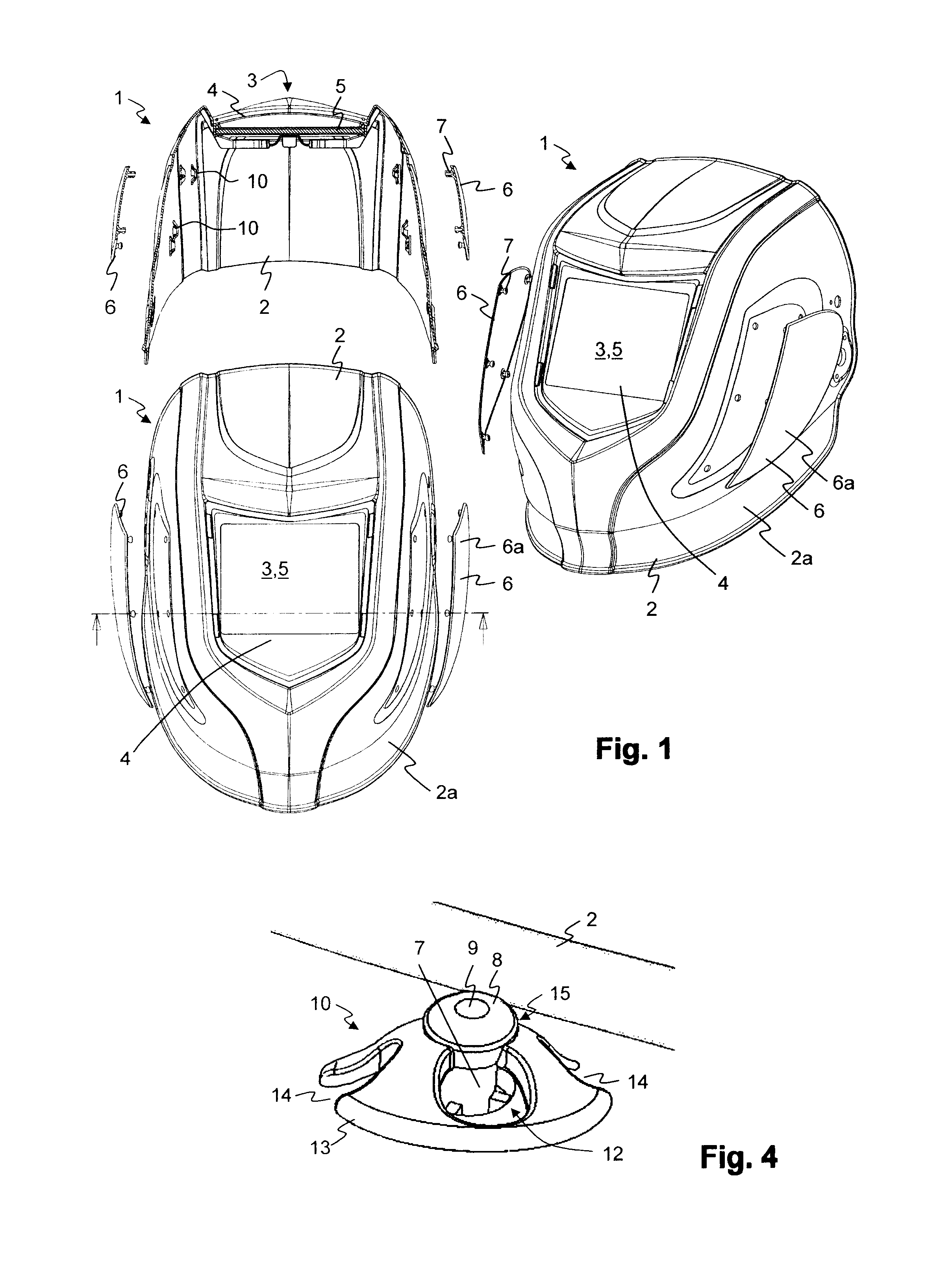
InactiveUS20110023205A1Simple individualizationEasy to changeEye-masksProtective garmentEngineeringMechanical engineering
A welding protection mask (1) includes a structural part (2) with a viewing opening (3) for the acceptance of a filter element (5) as a visor. To this end at least one insert (6) is designed at an outer surface of the structural part (2) other than the area of the viewing opening (3), which is insertable into the structural part (2) and which covers a section of the outer surface of the structural part. Thereby it is possible to individualize the welding protection masks (1) with limited effort and cost.
Owner:SPERIAN WELDING PROTECTION
Electrical contact element
ActiveUS9915003B2Avoid corrosionEasily materialContact member manufacturingCoupling contact membersStandard electrode potentialMaterials science
An electrical contact element formed of sheet metal having a first region and a second region. Each one of the first and second regions is coated with a coating including a first layer containing a first material having a lower standard electrode potential than the sheet metal material. The coating includes a second layer in the first region which is absent in the second region. The second layer is arranged underneath the first layer and contains a second material that has a lower standard electrode potential than the first material.
Owner:APTIV TECH LTD
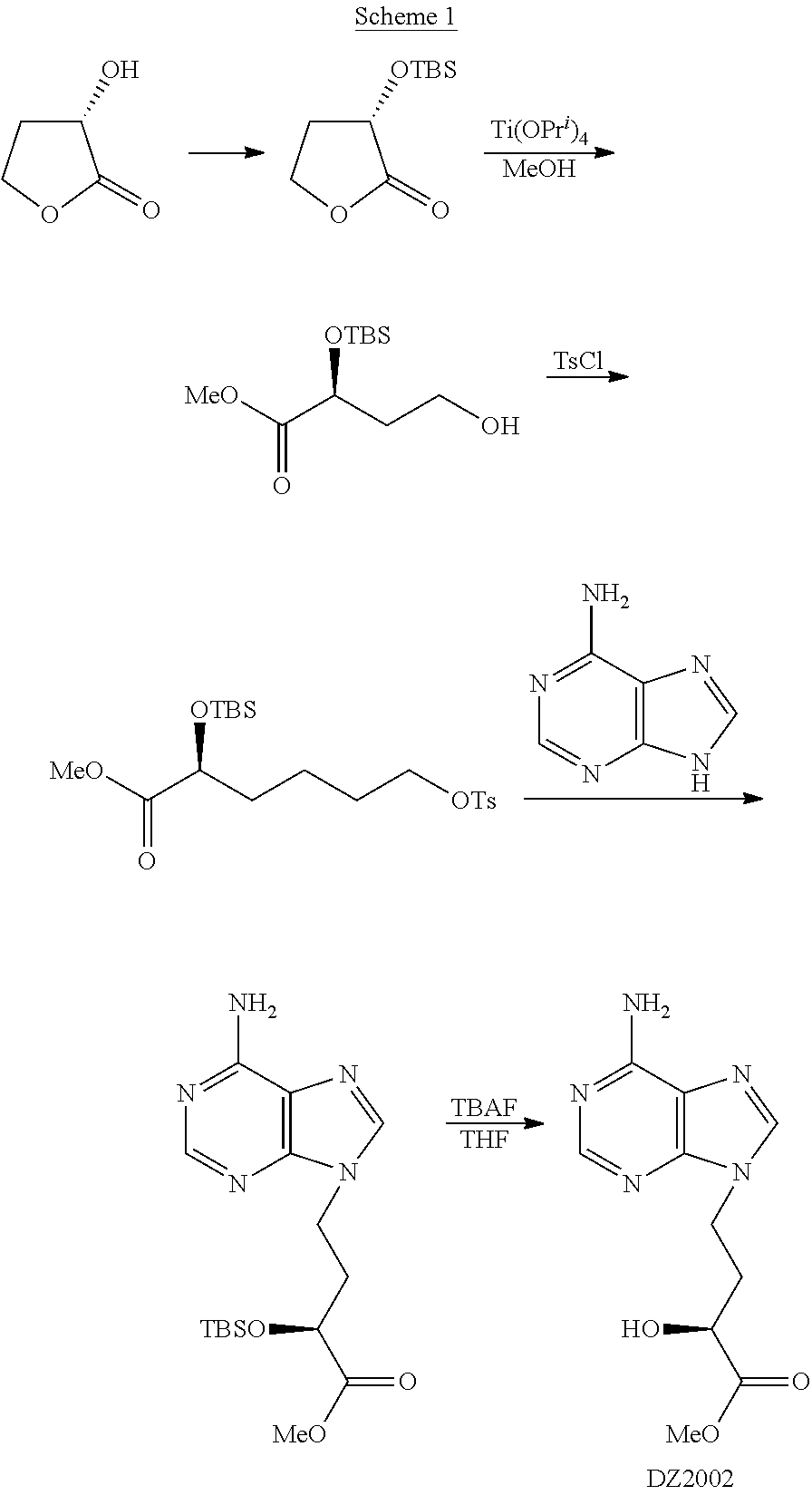
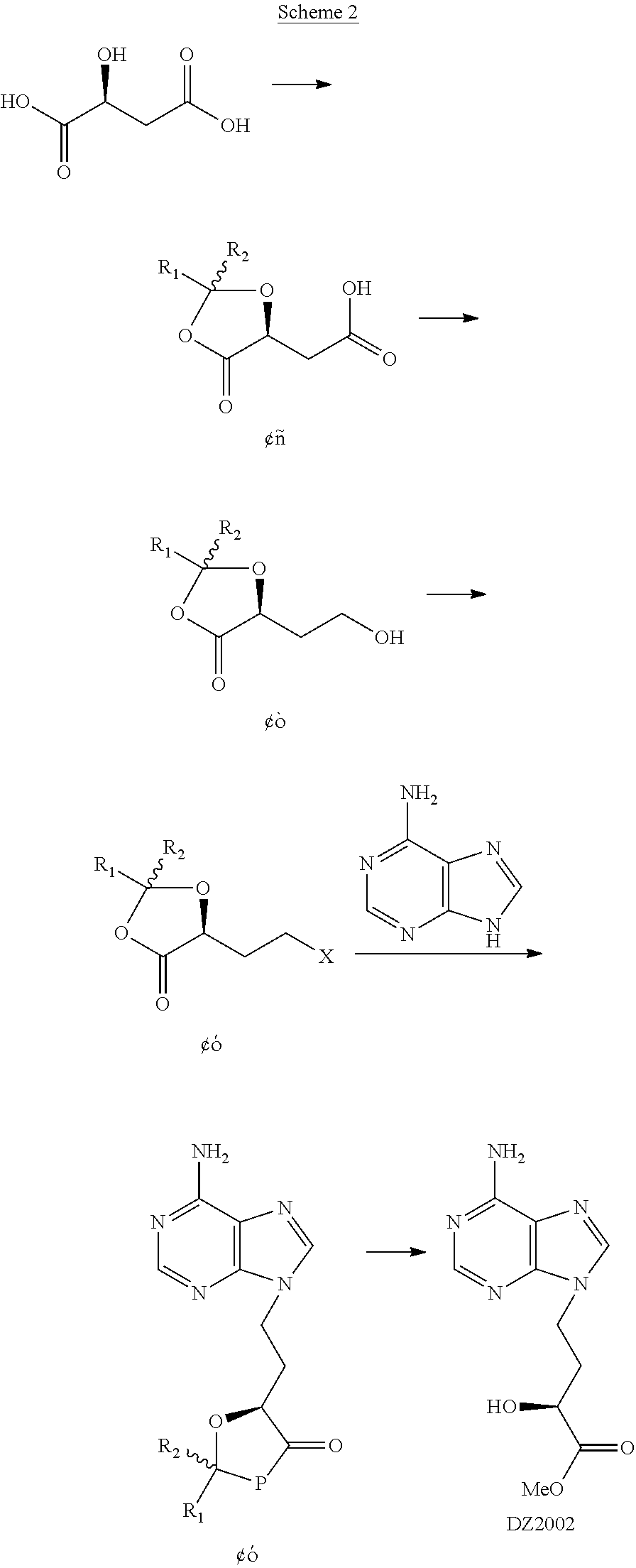
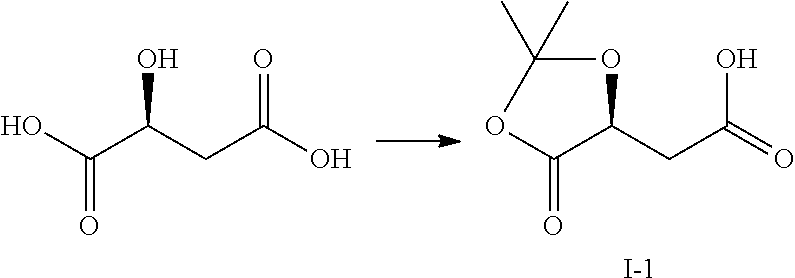
Method for preparing 4-(6-amino-purin-9-YL)-2(S)-hydroxy-butyric acid methyl ester
ActiveUS8247549B2Improve productivityEffectiveOrganic chemistryBulk chemical productionProduction ratePresent method
The present invention discloses a novel method for preparing and purifying 4-(6-Amino-purin-9-yl)-2(S)-hydroxy-butyric acid methyl ester. The preparation started from cheap and easily available L-malic acid, which was transformed to intermediate I after simultaneous protection of the groups of 1-carboxyl and 2-hydroxyl. The intermediate I was selectively reduced to intermediate alcohol II, whose hydroxyl group was further transformed to an easily leaving group to afford intermediate III. The intermediate III was nucleophilically substituted with adenine to afford intermediate IV. The intermediate IV was deprotected and methyl-esterified simultaneously in methanol in the presence of an acid or a base to afford crude 4-(6-Amino-purin-9-yl)-2(S)-hydroxy-butyric acid methyl ester, which was purified by recrystallization to afford the purified product. Comparing with the prior preparation methods, the present method has advantages in low cost, mild conditions, high retention of the chiral center during the reaction, high productivity, great improvement in the quality and yield of the product and great decrease in cost, and thus is suitable for the production on a large scale.
Owner:NINGBO ZIYUAN PHARMA INC
Penta-substituted tetrahydropyrimidines with aggregation-induced emission characteristics and preparation method and use thereof
InactiveUS8906927B2Easy to getNovel molecular structureBiocideOrganic chemistryAggregation-induced emissionPhotoluminescence
The present invention provides a series of penta-substituted tetrahydropyrimidines with aggregation-induced emission (AIE) characteristics and preparation method and use thereof. The AIE penta-substituted tetrahydropyrimidines have structures shown as formula (I). R1 is selected from a group consisting of linear or branched alkyls and substituted alkyls. R2 and R4 is respectively selected from a group consisting of linear or branched alkyls, substituted alkyls, cycloalkyls, substituted cycloalkyls, aryls, substituted aryls, polycyclic aryls, substituted polycyclic aryls, heterocyclyls, substituted heterocyclyls, aromatic heterocyclyls and substituted aromatic heterocyclyls. R3 is selected from a group consisting of aryls, substituted aryls, polycyclic aryls, substituted polycyclic aryls, aromatic heterocyclyls and substituted aromatic heterocyclyls. The penta-substituted tetrahydropyrimidines can be prepared by multi-component reactions (MCR). There are 1˜3 aryls in the structure of the penta-substituted tetrahydropyrimidines. The penta-substituted tetrahydropyrimidines possess strong AIE properties and can be used for preparing organic electro-luminescence or photo-luminescence devices, or chemical and biological fluorescent sensors or probes.
Owner:SOUTHERN MEDICAL UNIVERSITY
LED lamp heat sink
ActiveUS10355188B2Improve conductivityGood moldabilitySemiconductor/solid-state device detailsSolid-state devicesGraphitePolyester resin
Owner:KANEKA CORP
Process for producing single crystal
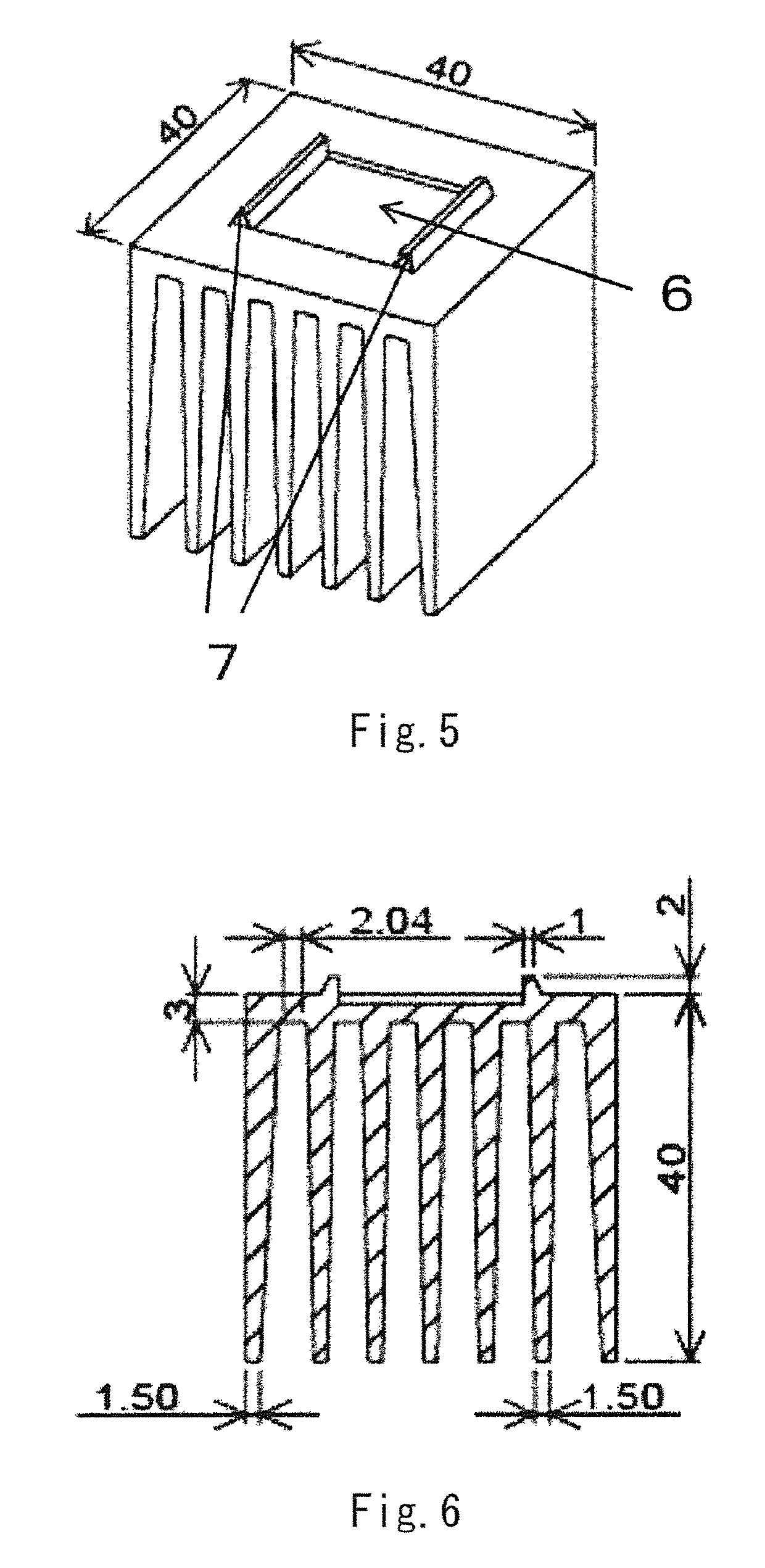
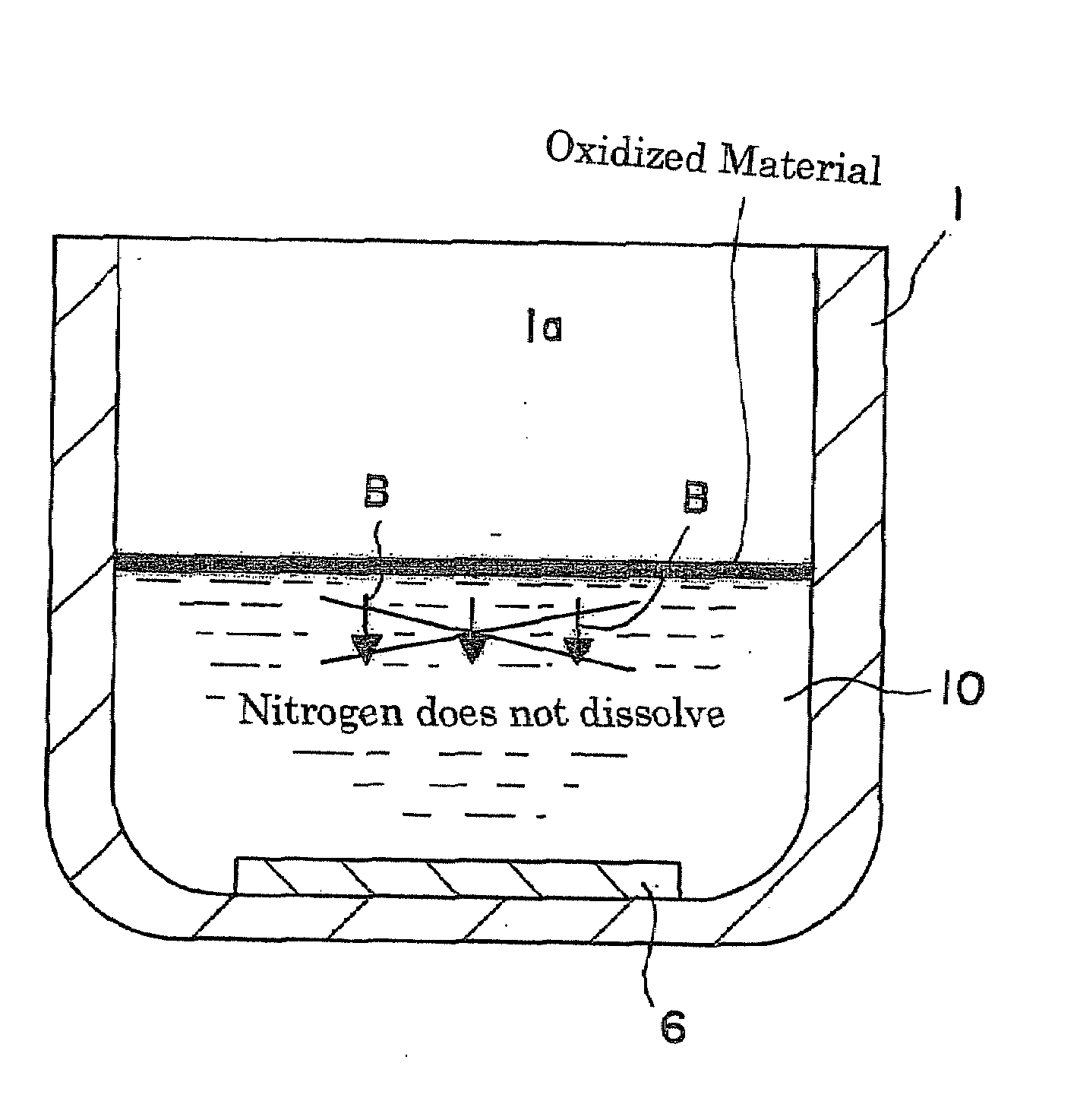
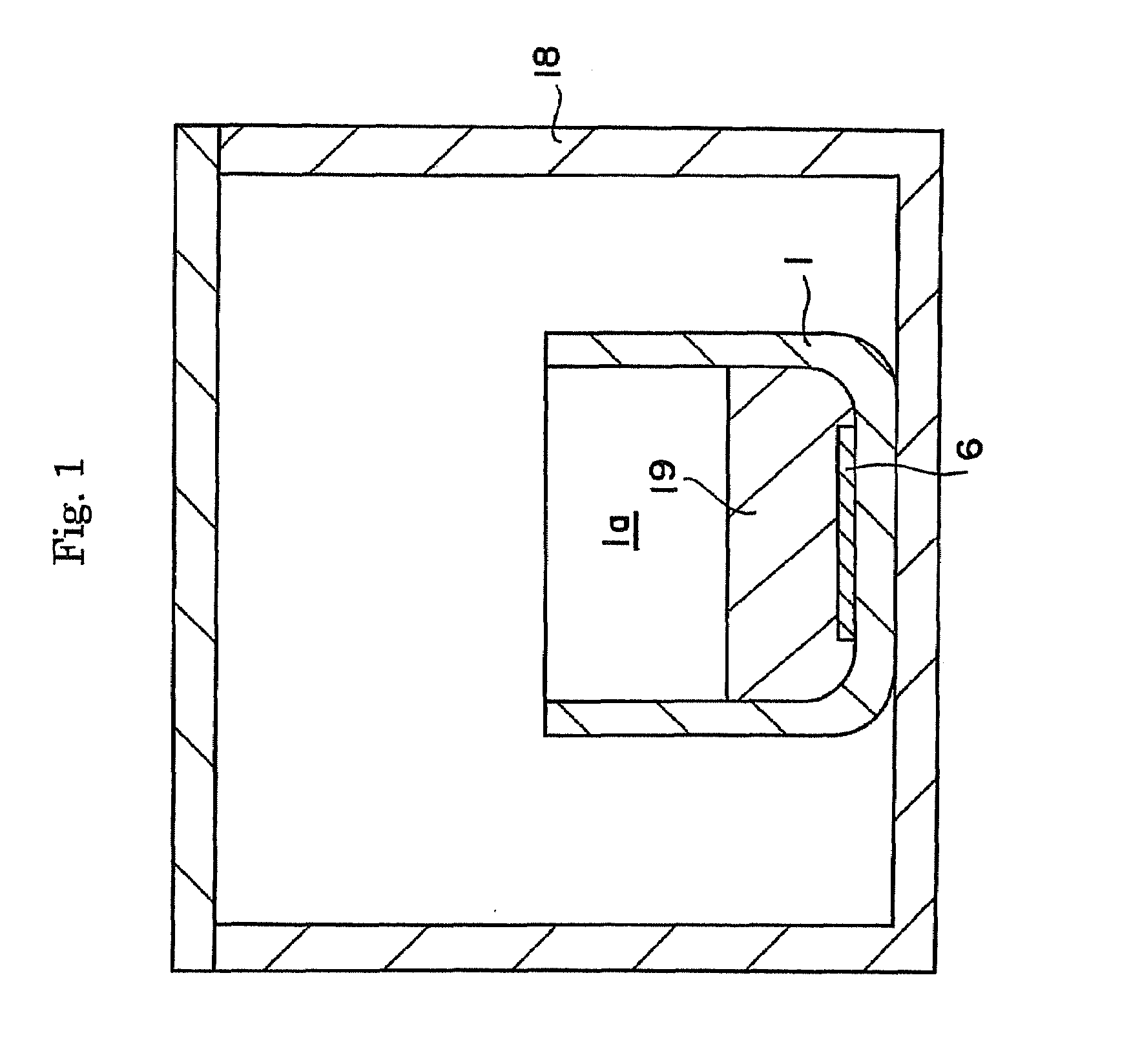
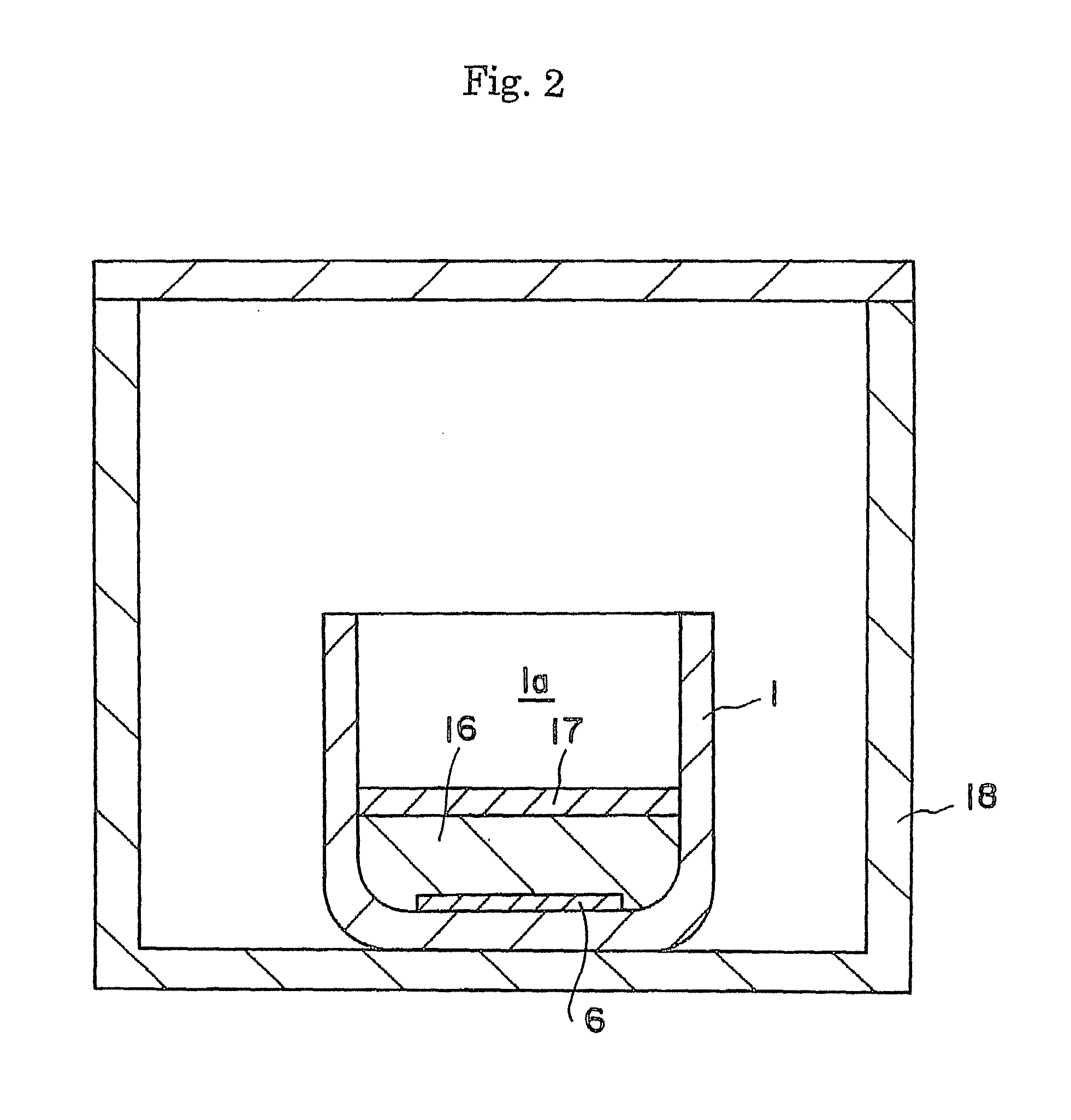
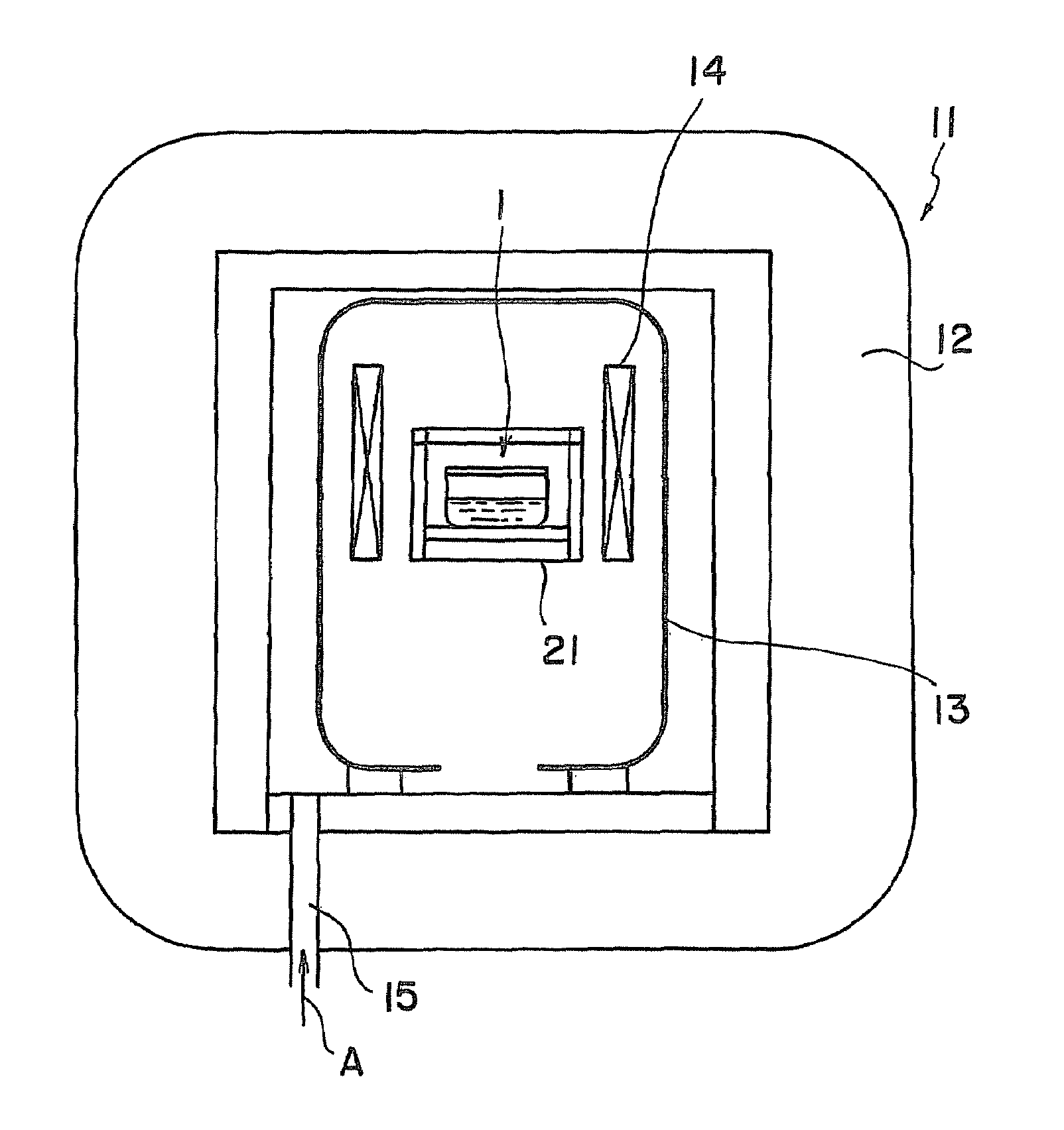
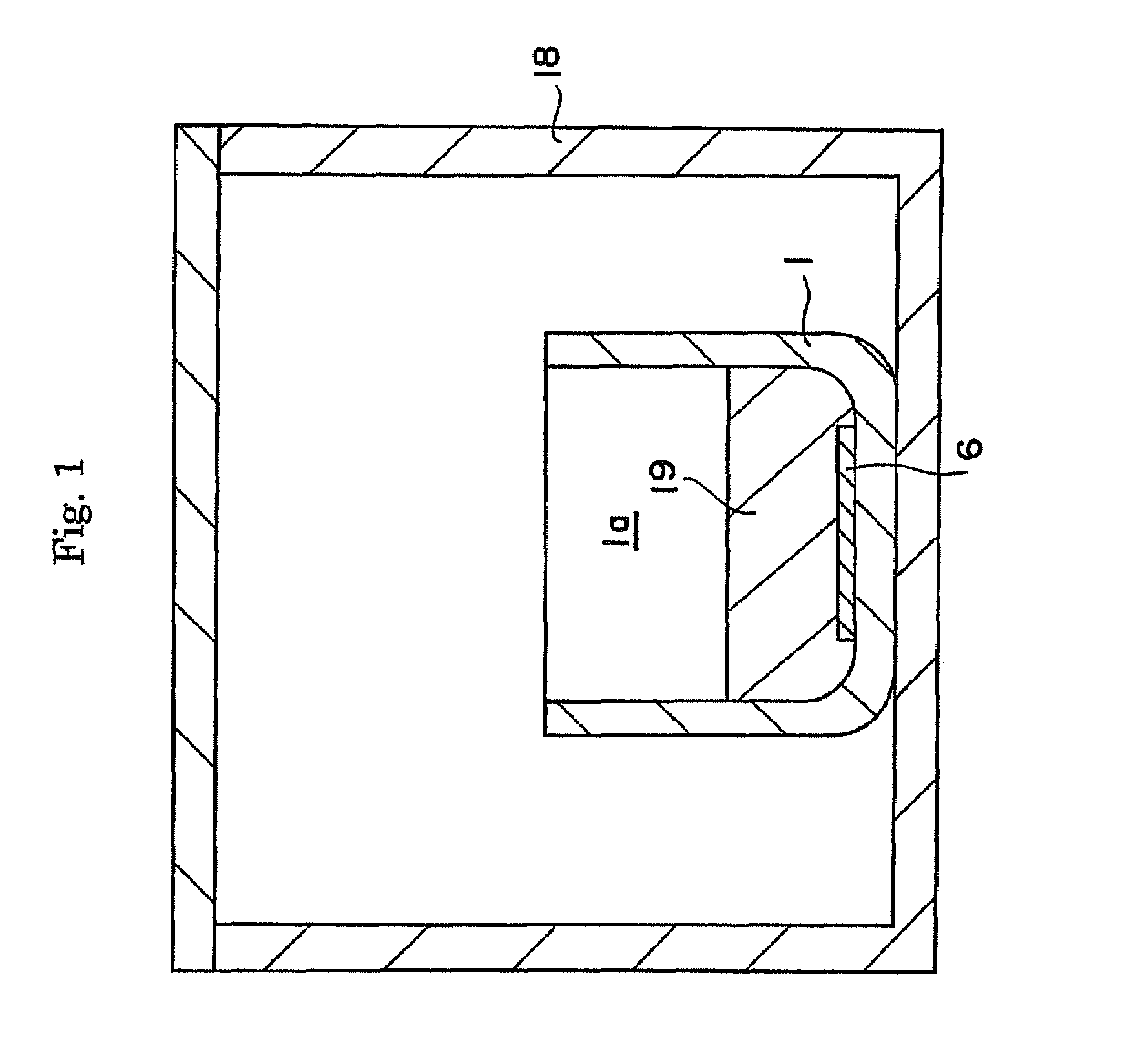
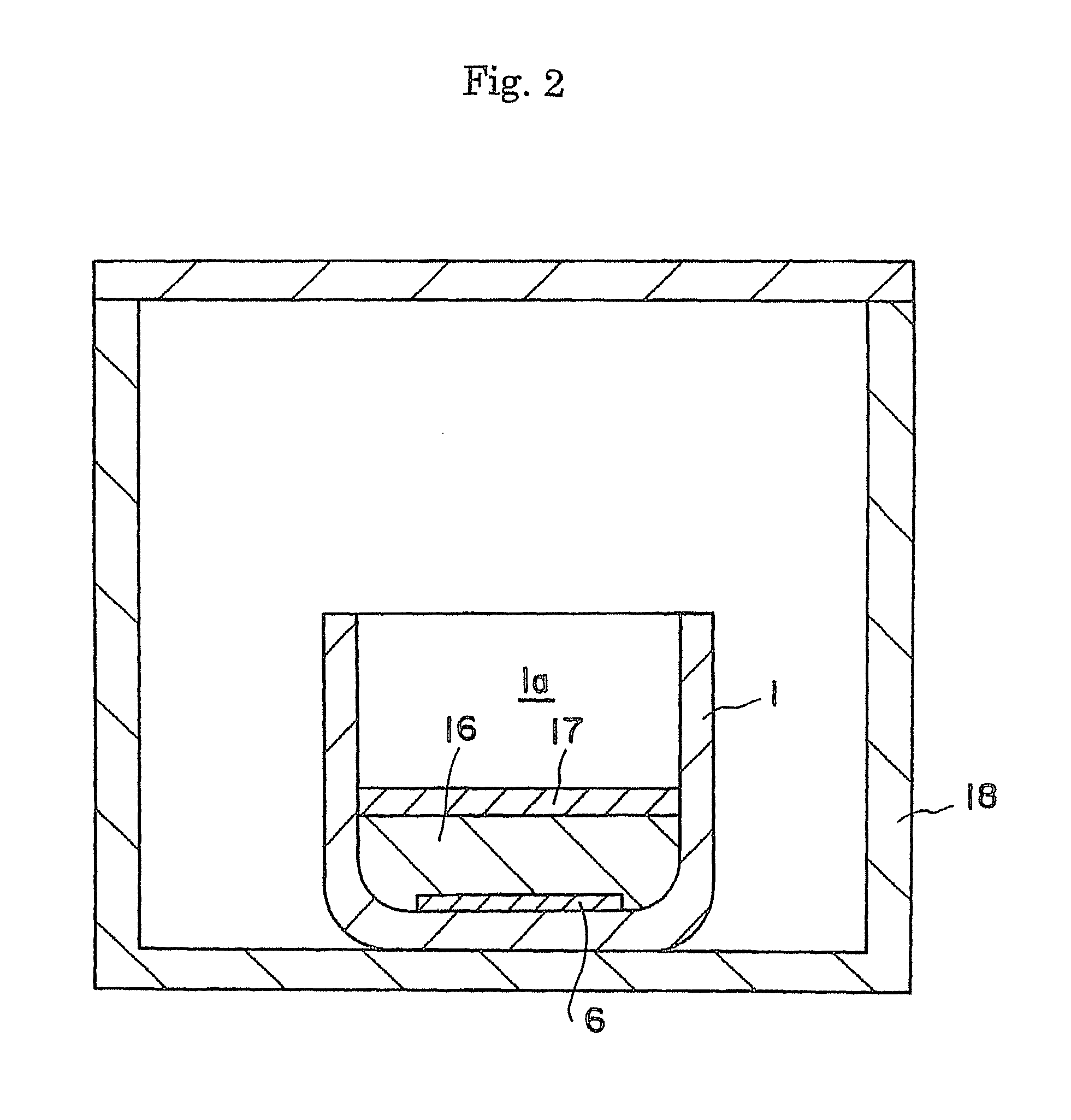
ActiveUS20090038539A1Inhibit coloringEasily materialPolycrystalline material growthFrom normal temperature solutionsSingle crystalCrystal growth
A raw material mixture containing an easily oxidizable material is weighed. The raw material mixture is melted and then solidified within a reaction vessel 1 set in a non-oxidizing atmosphere to thereby produce a solidified matter 19. The reaction vessel 1 and the solidified matter 19 are heated in a non-oxidizing atmosphere within a crystal growth apparatus to melt the solidified matter to thereby produce a solution. A single crystal is grown from the solution.
Owner:NGK INSULATORS LTD +1
Three-dimensional art and tool for creation of the same
ActiveUS20120128884A1Increasing the thicknessAdd featureHand artistic toolsOrnamental structuresEngineeringRotary union
A trowel for use in painting or sculpturing and having a handle enabling a user to hold the trowel, a rotating mechanism supported by the handle and an application member removably attached to and in rotary union with the rotating mechanism. The invention relates to a method for creating a three-dimensional artistic creation on a support member, with the method including distributing an image creating material on a palette, removing first portions of the image creating material and applying the image creating material with pressure across a path along and onto the support member with a twisting movement. Such application results in the first portions forming deposited panels that protrude from the support member at a thickness that is greater than that of the material on the palette. The invention also relates to a kit that includes a plurality of trowels with various size blades and image creating material.
Owner:MESAROS FRANCIS
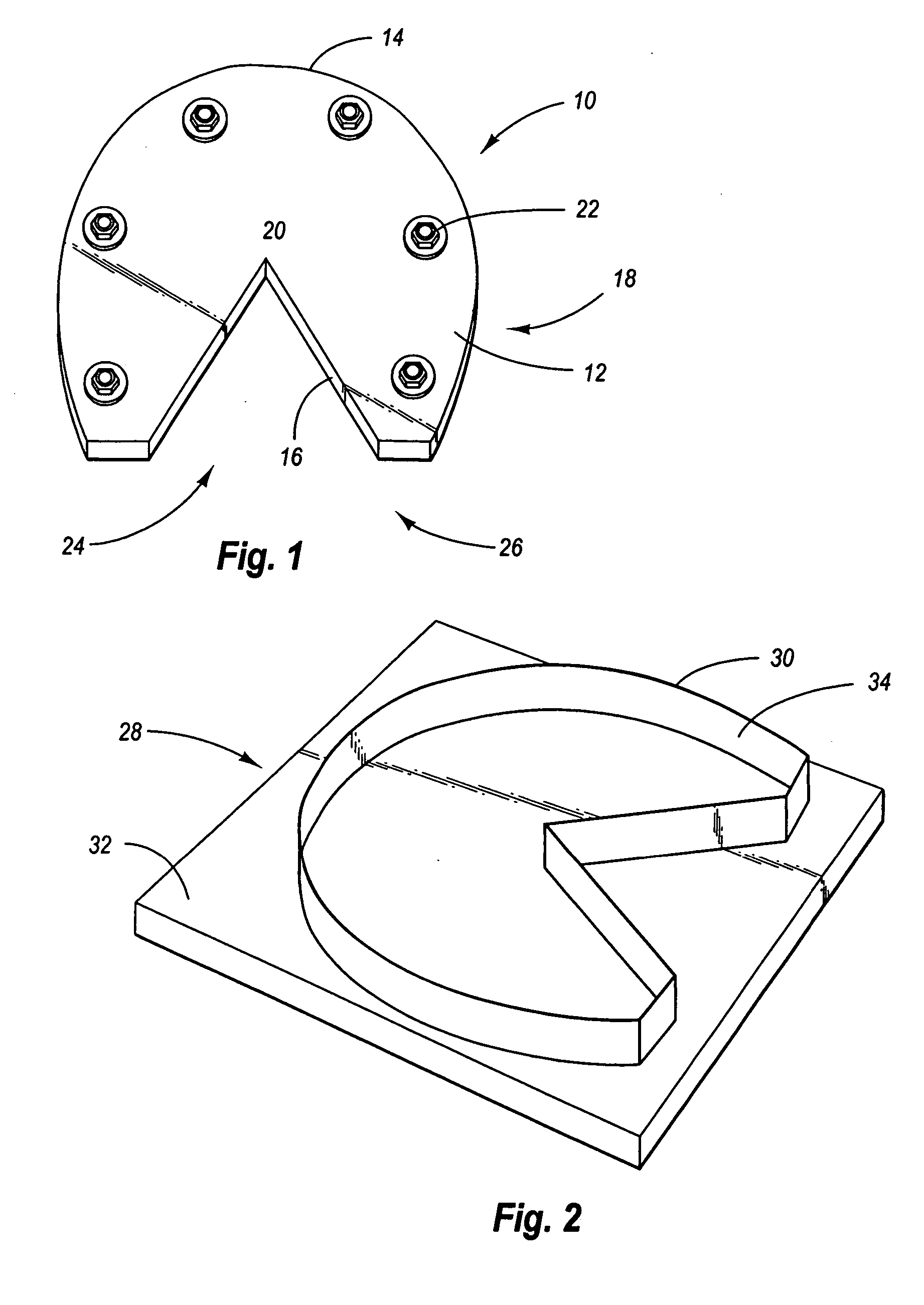
Flexible horseshoes, method for attaching and device for making
A flexible horseshoe which attaches to a horse hoof by threaded attachment screws. The invention also includes the device for cutting the flexible horseshoes, and the method of mounting the flexible horseshoes.
Owner:BALDWIN J GRANT
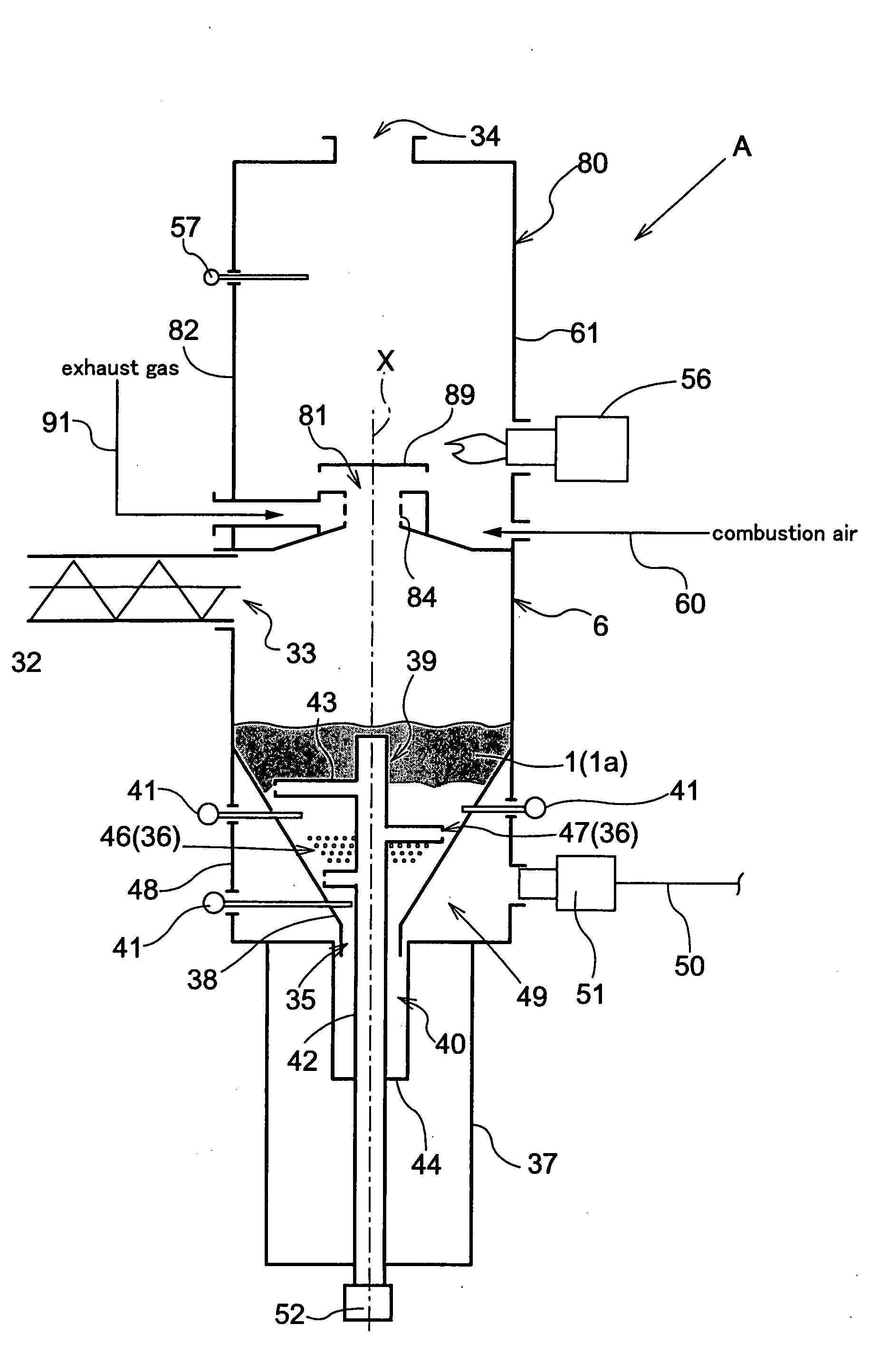
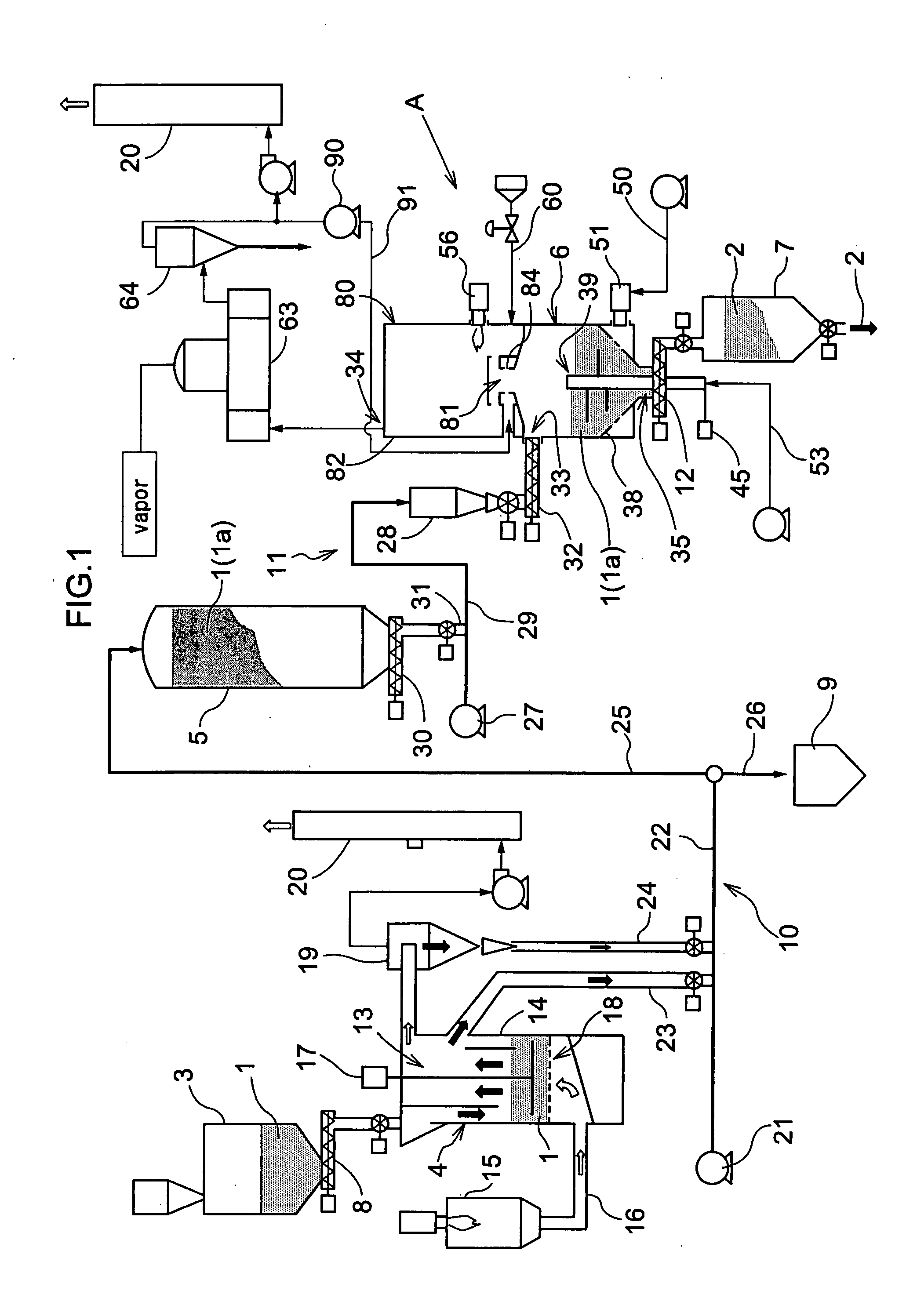
Carbonizing Apparatus
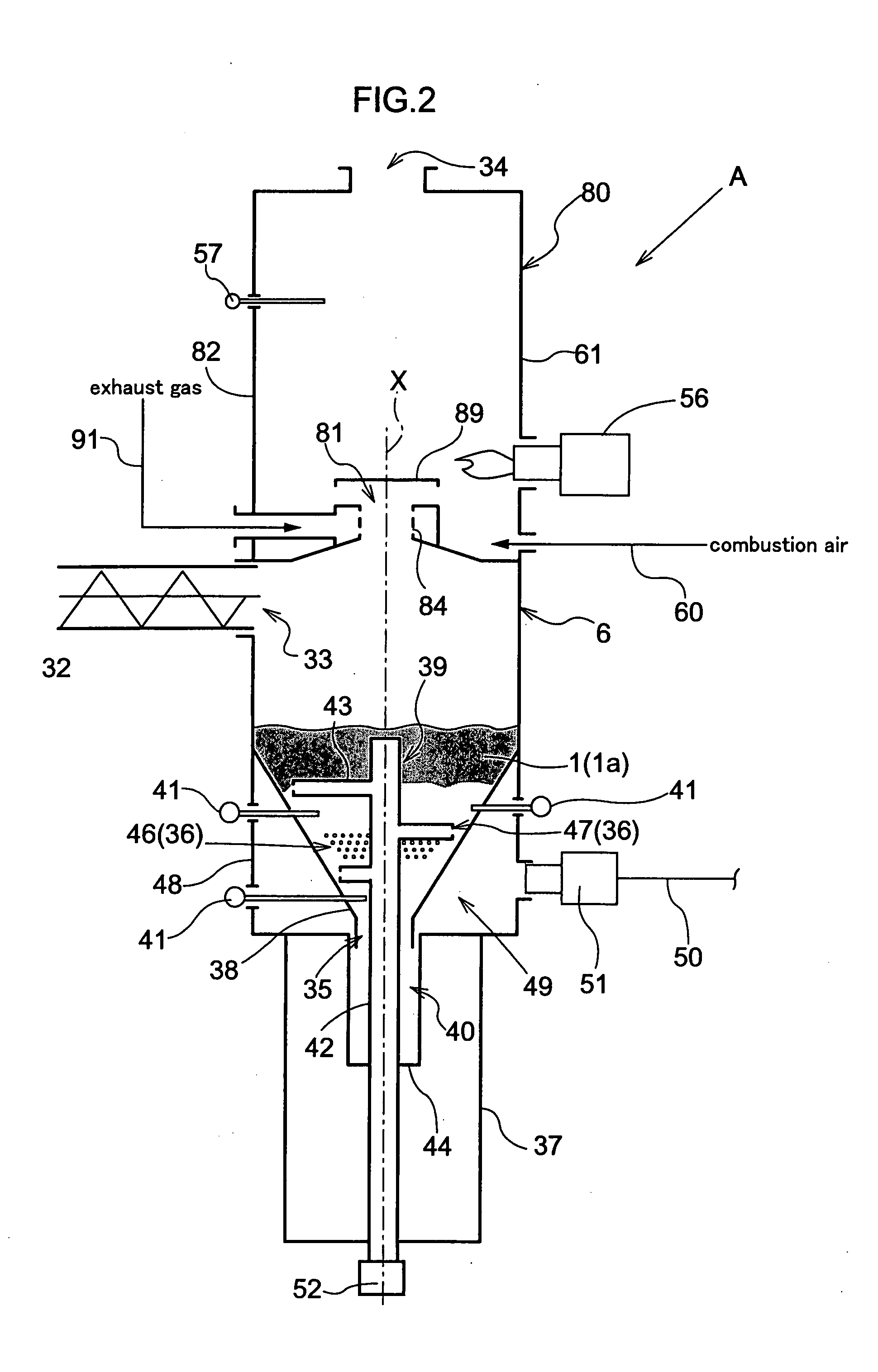
InactiveUS20080223710A1Easy to burnEasy to useBeehive ovensDirect heating destructive distillationSpontaneous combustionDistillation
This invention provides a carbonizing apparatus for easily carbonizing a material to a desired state of carbonization, which apparatus requires a reduced installation space, and lowers concentration of harmful gases contained in an exhaust gas. The interior of a cylindrical furnace body 82 having a vertical axis is divided by a partition wall 83 into an upper furnace body acting as a combustion section 80 and a lower furnace body acting as a carbonization section 6. The partition wall 83 defines a through hole for supplying distillation gas to the combustion section 80. The carbonization section 6 has a vertical structure, with a supply part 33 disposed in an upper portion for supplying a material under treatment 1, and a carbide take-out part 35 and a first blowoff part 36 of spontaneous combustion air disposed in a lower portion, so that the material under treatment 1 is movable by gravity toward the take-out part 35. An agitating device 39 includes agitating members 43 revolvable about a vertical axis. A premix combustion section 81 is provided on the combustion part 80 side of the partition wall 83 for mixing a distillation gas having passed through the through-hole with an exhaust gas discharged from a discharge part 34 and burning the mixture. Gas having passed through the premix combustion section 81 is supplied to the combustion section 80.
Owner:SUNTORY HLDG LTD
Process for producing single crystal
ActiveUS8486190B2Inhibit coloringEasily materialPolycrystalline material growthFrom normal temperature solutionsSingle crystalCrystal growth
Owner:NGK INSULATORS LTD +1
Three-dimensional art and tool for creation of the same
ActiveUS9085195B2Increasing the thicknessAdd featureHand artistic toolsOrnamental structuresEngineeringRotary union
Owner:MESAROS FRANCIS
Vertical sides banjo tone ring and methods
ActiveUS8759648B2Quality improvementHigh levelCasting safety devicesBanjosEngineeringSpecific weight
Owner:DEERING BANJO COMPANY
Cationic amino acid type lipid
InactiveUS7838685B2Easily materialEasy to synthesizeCosmetic preparationsToilet preparationsAmino acidHydrocarbon
The present invention provides a novel complex lipid having a cationic functional group derived from an amino acid. Namely, the present invention provides a cationic acid amino acid type lipid represented by the following formula:wherein, R1 is a hydrocarbon group having a cationic functional group derived from an amino acid, R2 and R3 are each independently a chain hydrocarbon group, A1 and A2 are each independently a linkage group selected from the group consisting of —COO—, —OCO—, —CONH— and NHCO—, and n is an integer of 2 to 4.
Owner:TAKEOKA SHINJI
Method and apparatus for producing forging by rotary forging
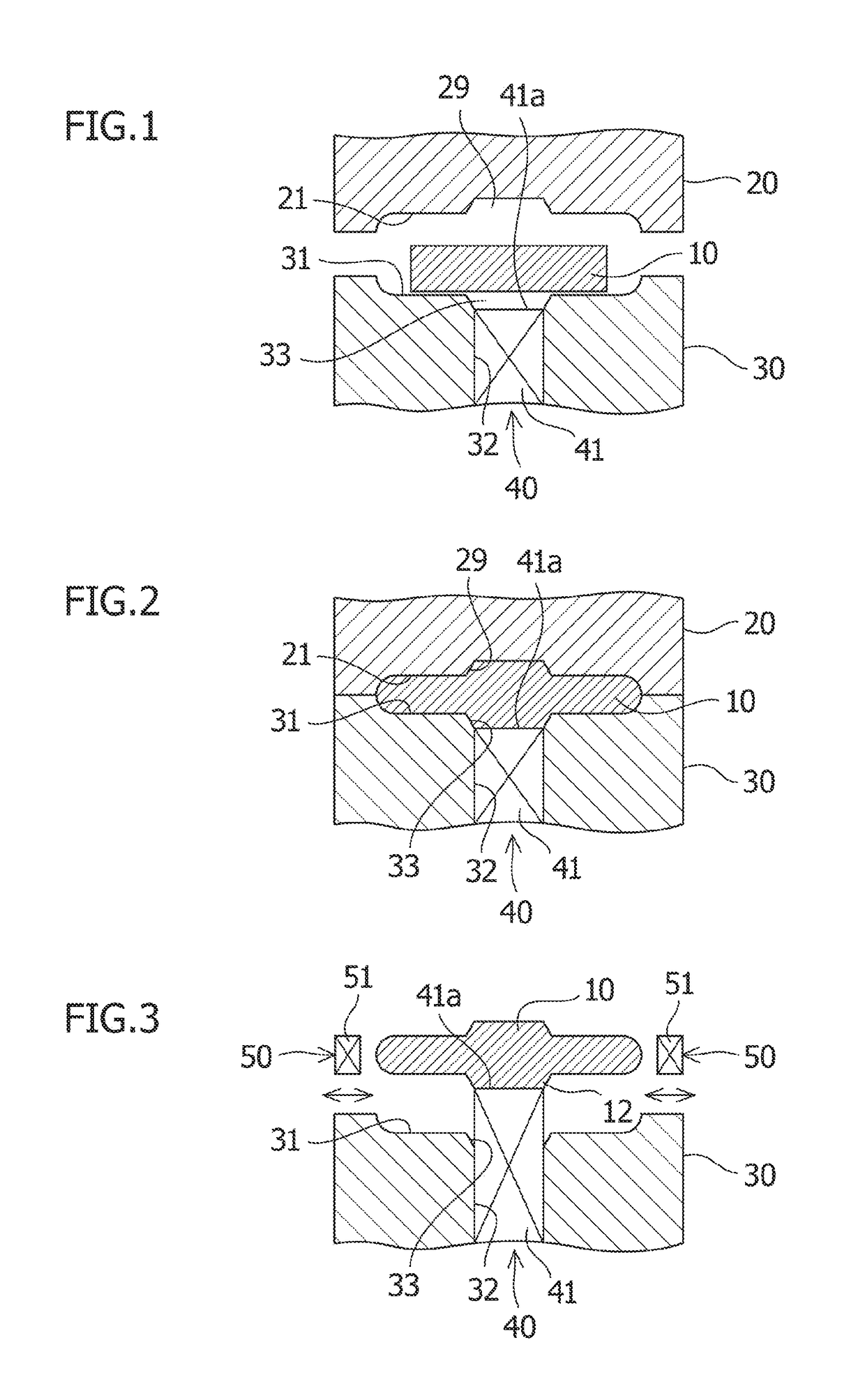
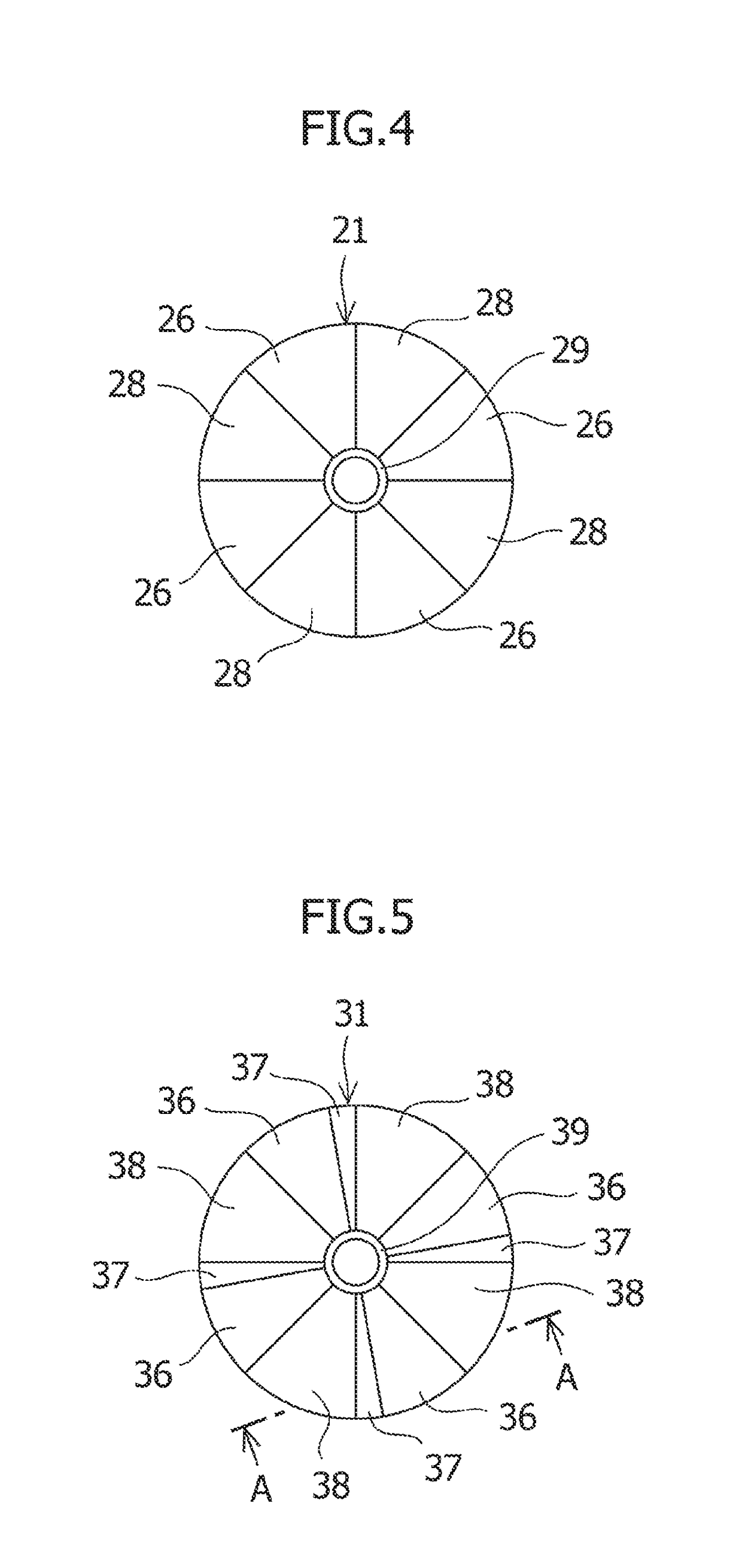
ActiveUS10576531B2Smooth rotationEasily materialForging/hammering/pressing machinesForging press detailsIndustrial engineeringMaterial Separation
A cycle is repeated a plurality of times, which includes a forging process for placing a material to be forged in a lower die and pressing the material to be forged in this state and then separating an upper die from the material to be forged; an elevation process for lifting the material to be forged by using an elevation device to separate the material to be forged from the lower die; a rotation process for rotating the material to be forged around its center by using a rotation device; and a lowering process for placing the material to be forged rotated by the elevation device in the lower die.
Owner:HITACHI METALS LTD
Method and apparatus for producing forging by rotary forging
ActiveUS20170100769A1Smooth rotationEfficient rotary forgingForging/hammering/pressing machinesForging press detailsMaterials scienceForging
A cycle is repeated a plurality of times, which includes a forging process for placing a material to be forged in a lower die and pressing the material to be forged in this state and then separating an upper die from the material to be forged; an elevation process for lifting the material to be forged by using an elevation device to separate the material to be forged from the lower die; a rotation process for rotating the material to be forged around its center by using a rotation device; and a lowering process for placing the material to be forged rotated by the elevation device in the lower die.
Owner:HITACHI METALS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com