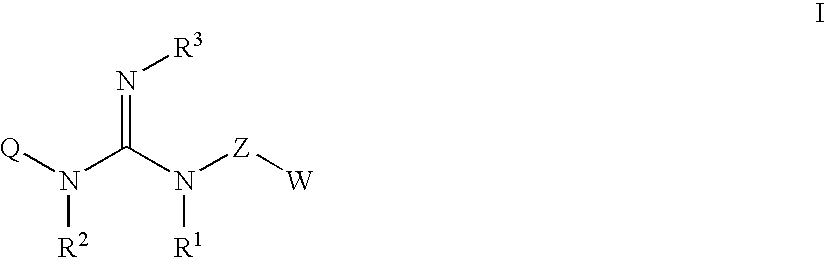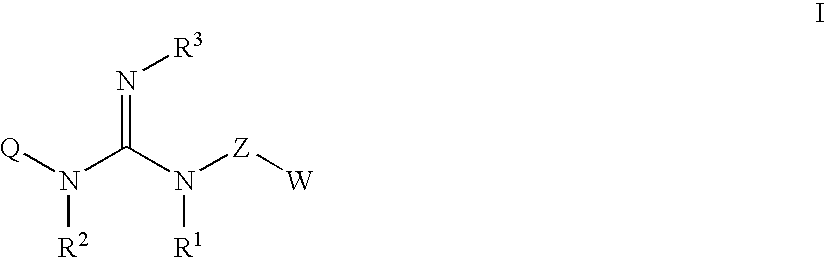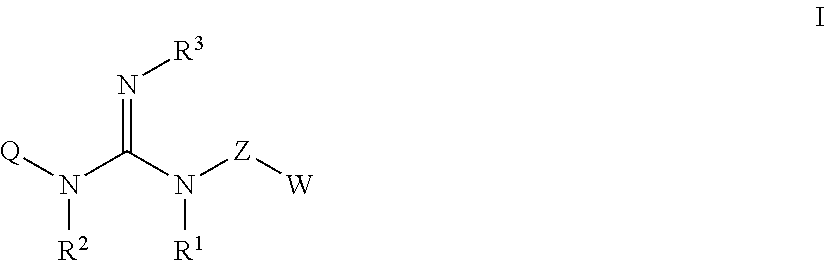Patents
Literature
32results about How to "Sufficient efficacy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
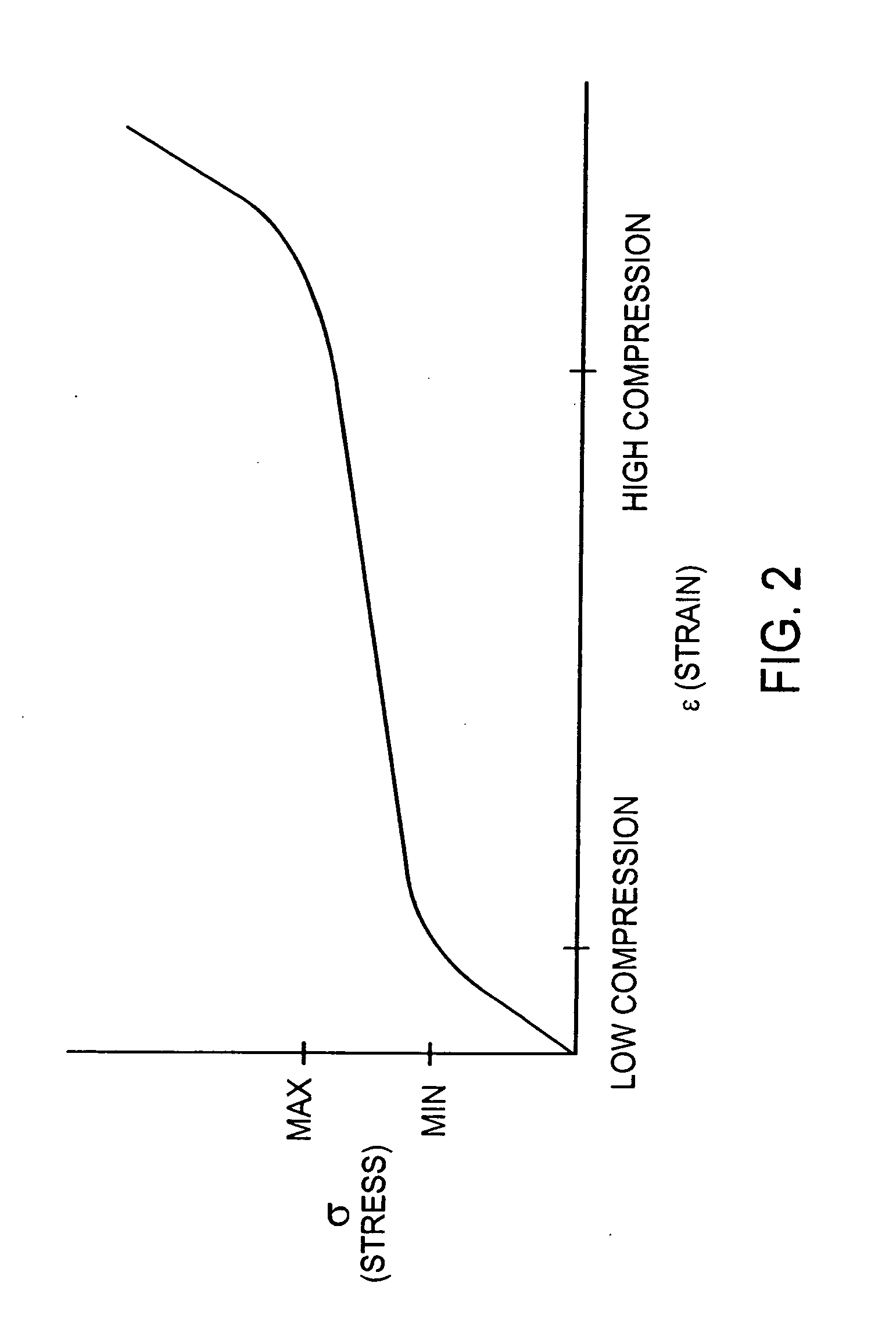
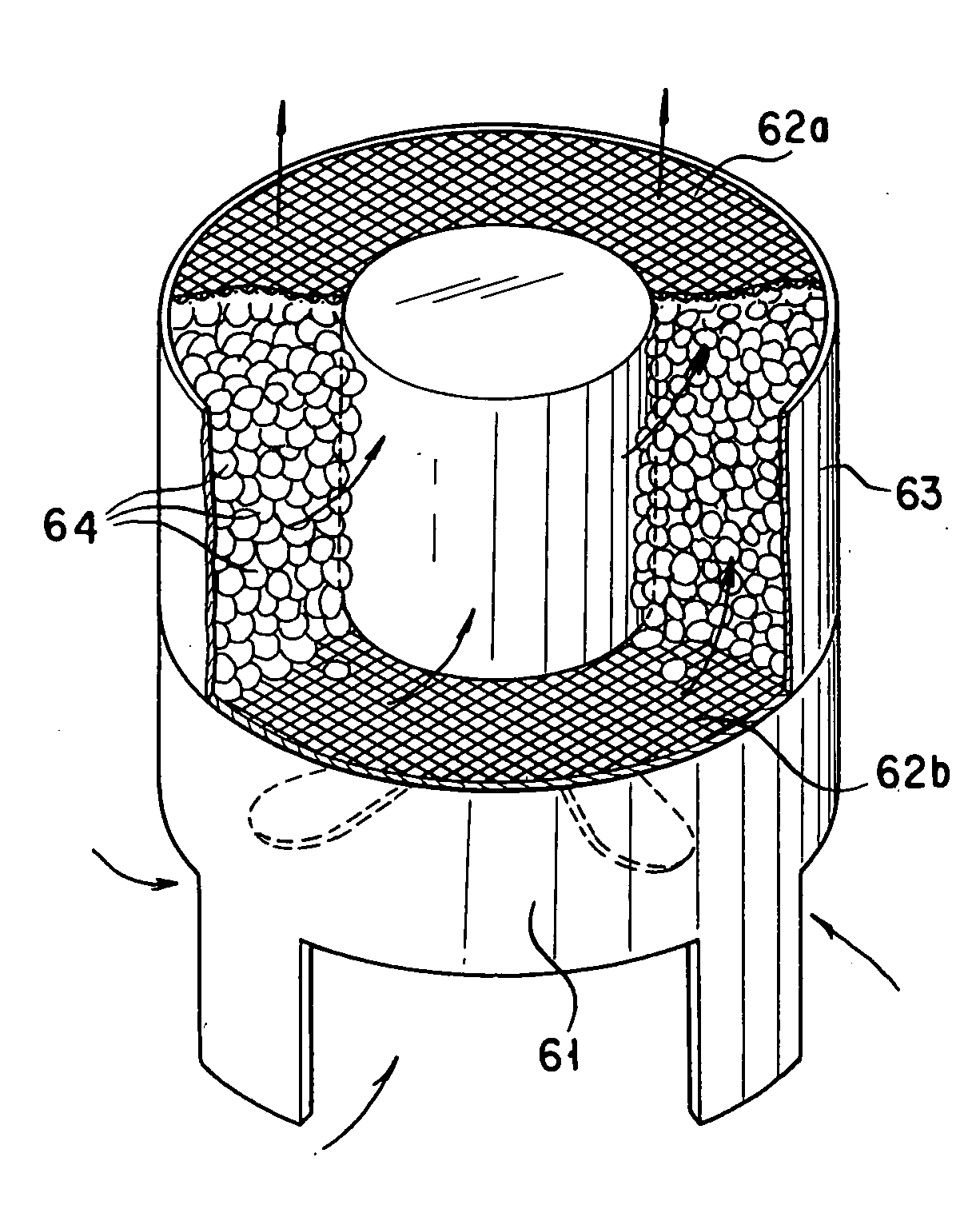
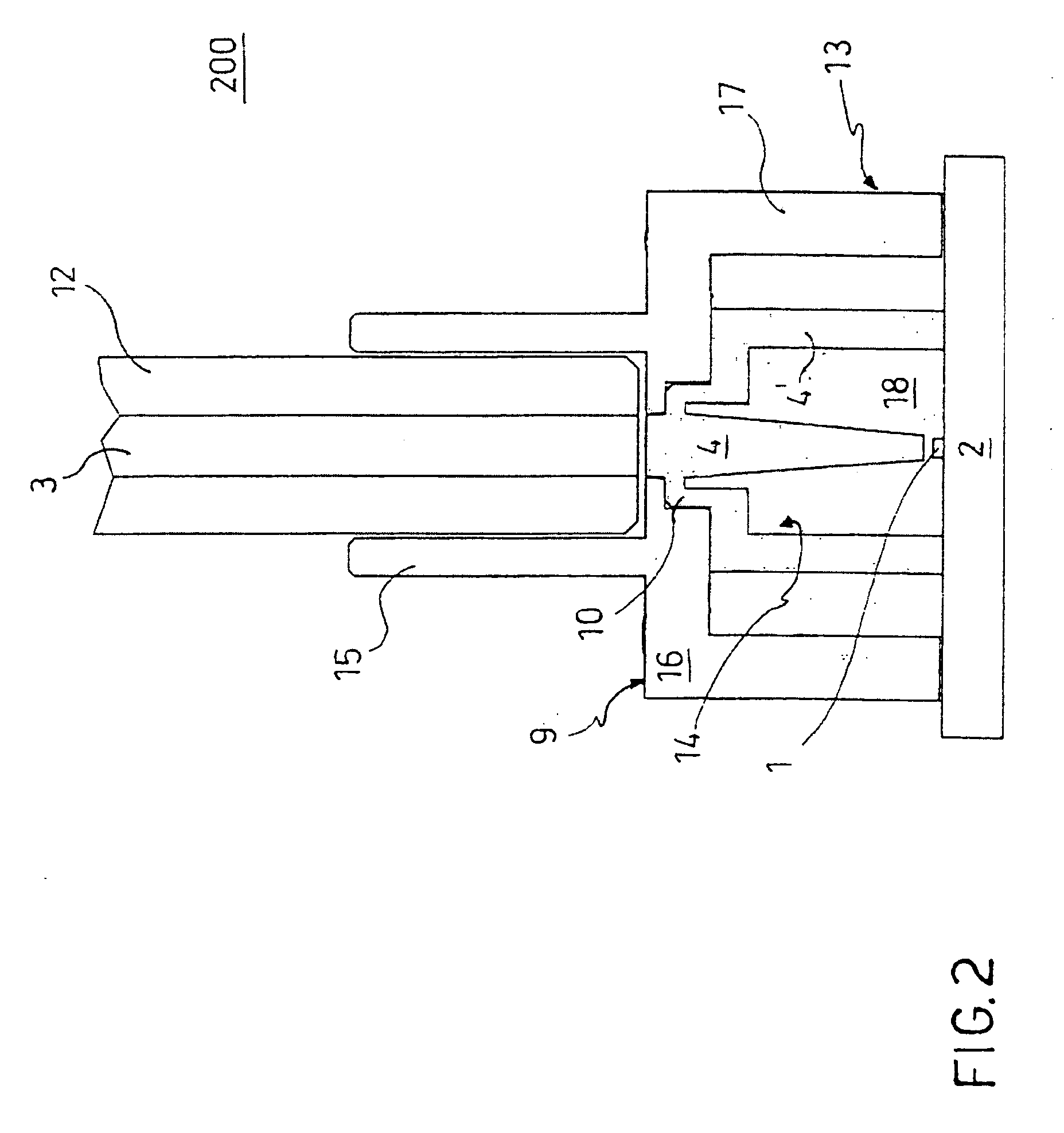
Chamber with adjustable volume for cell culture and organ assist
InactiveUS6855542B2Compromise liquid-tightnessCompromise sterilityBiocideBioreactor/fermenter combinationsEngineeringPerfusion
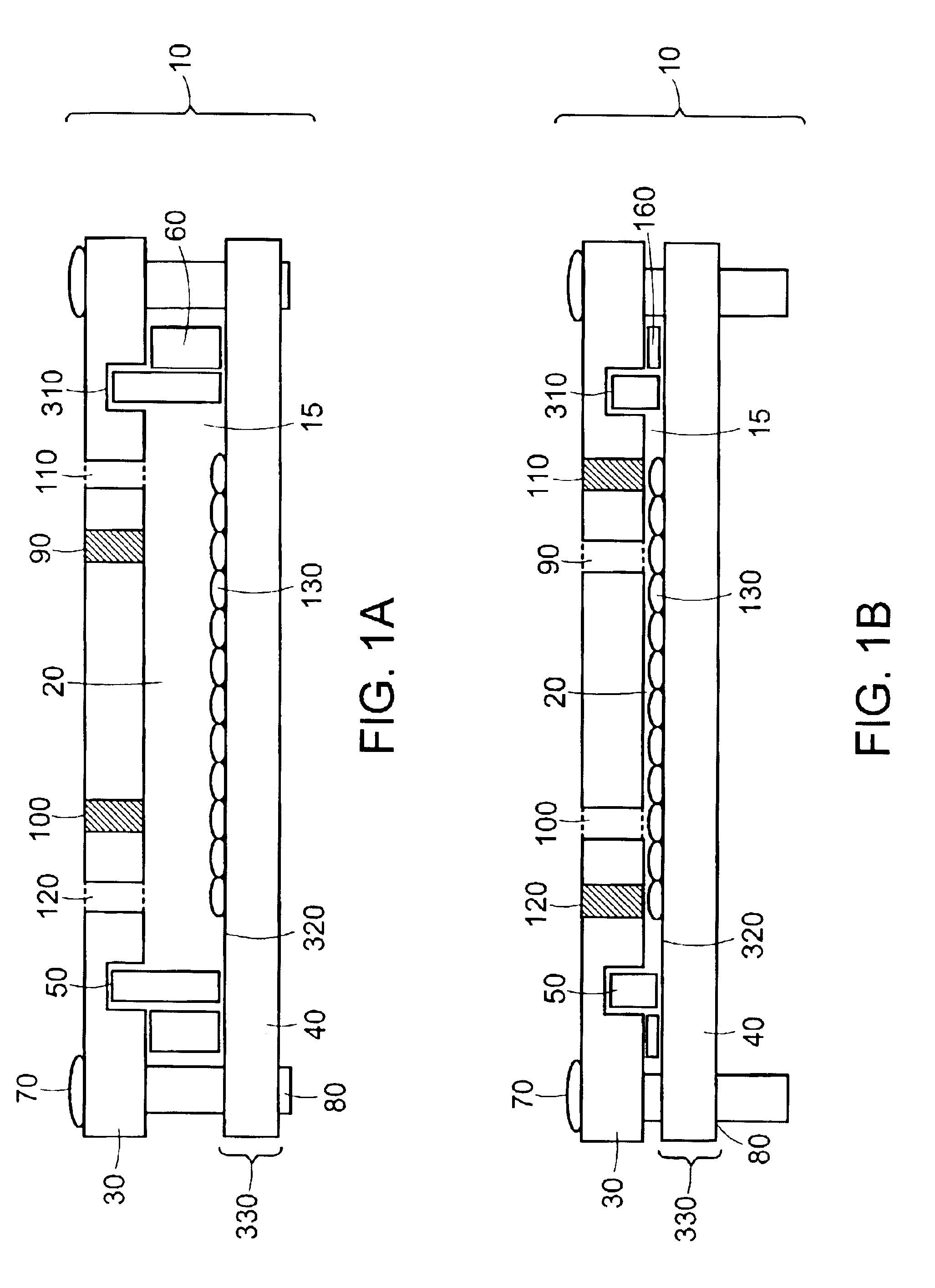
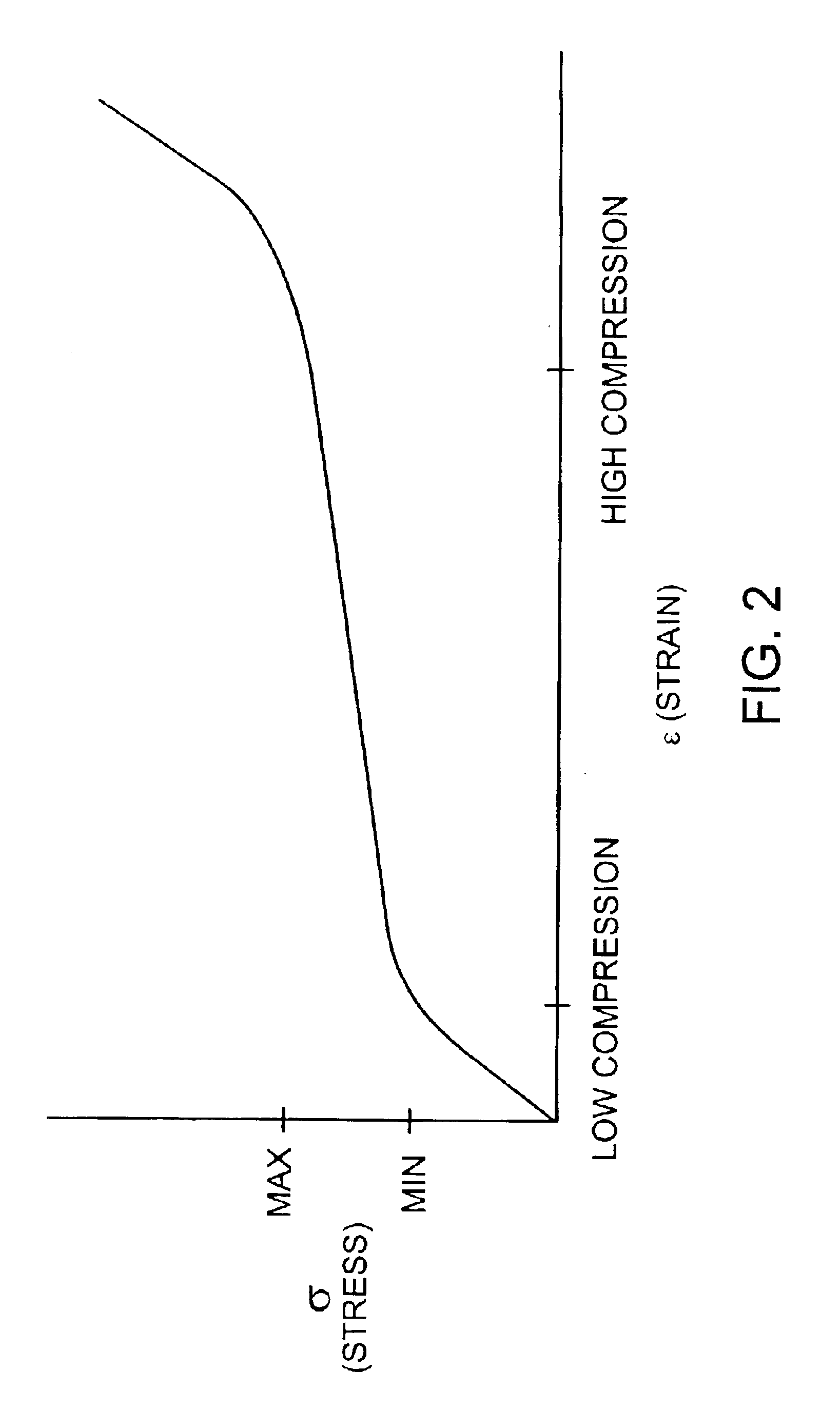
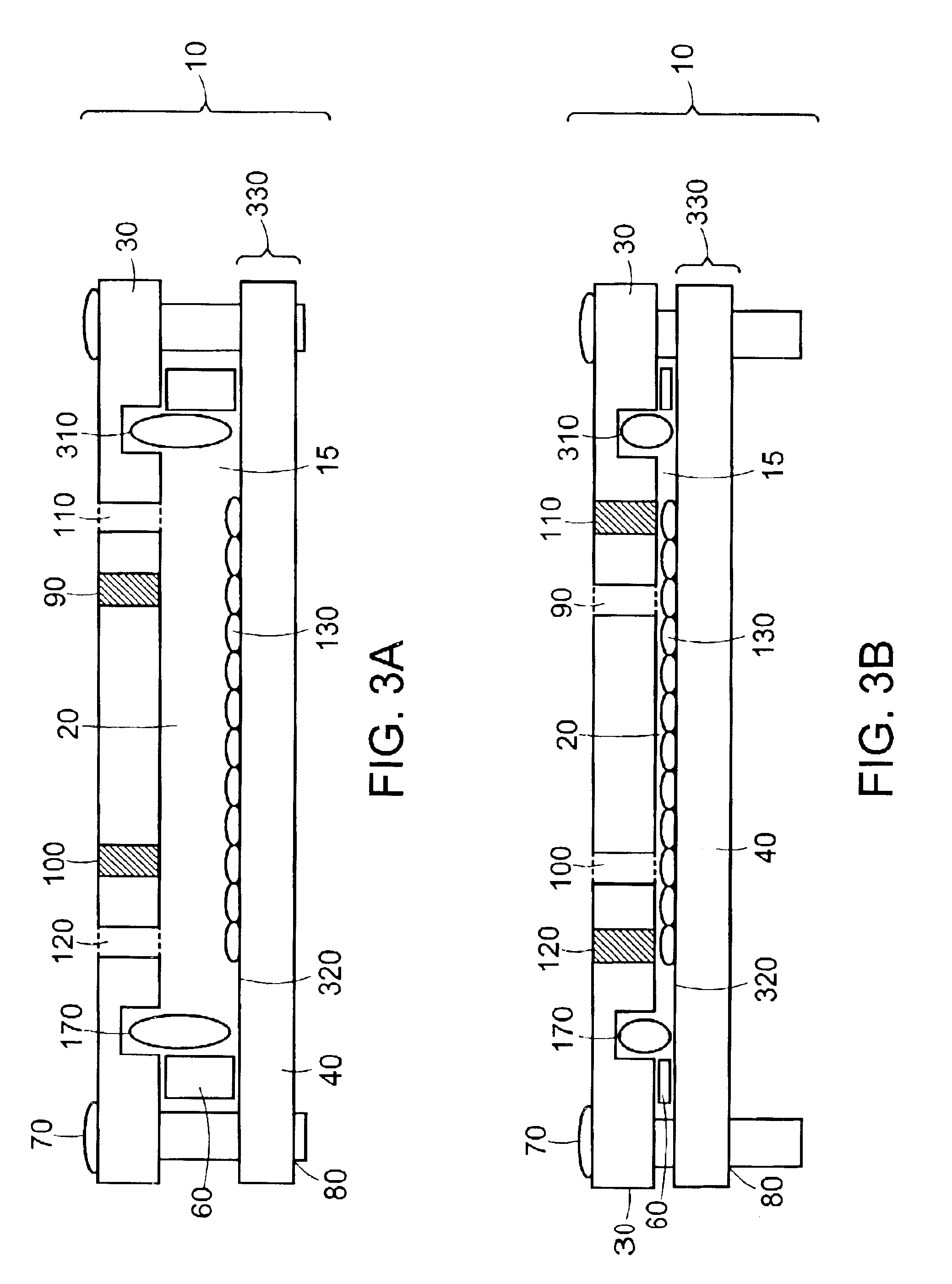
The invention features modular chambers for culturing cells in which the volume of a chamber can be adjusted without compromising the seal or sterility of the chamber. The invention is based on the principle that the volume of a chamber formed between two plates sandwiching a compressible gasket and a substantially incompressible stop can be adjusted using a gasket that forms a fluid-tight seal between the plates at a plurality of levels of compression. The invention enables the culture of cells between substantially parallel and rigid plates in which a relatively large volume can be used to seed the cells and the holdup volume reduced for perfusion without opening or otherwise disassembling the system to compromise its liquidtightness and sterility. The new closed, modular and scalable cell-culturing chamber can be thus perfused and used to culture cells (e.g., hepatocytes) with high levels of cell function in organ (e.g., liver) assist systems, for production of cells, for production of cell-derived products, such as proteins or viruses, or for systems to treat biological liquids to remove toxins, such as ammonia, add cell-synthesized products, or both.
Owner:ORGANOGENESIS
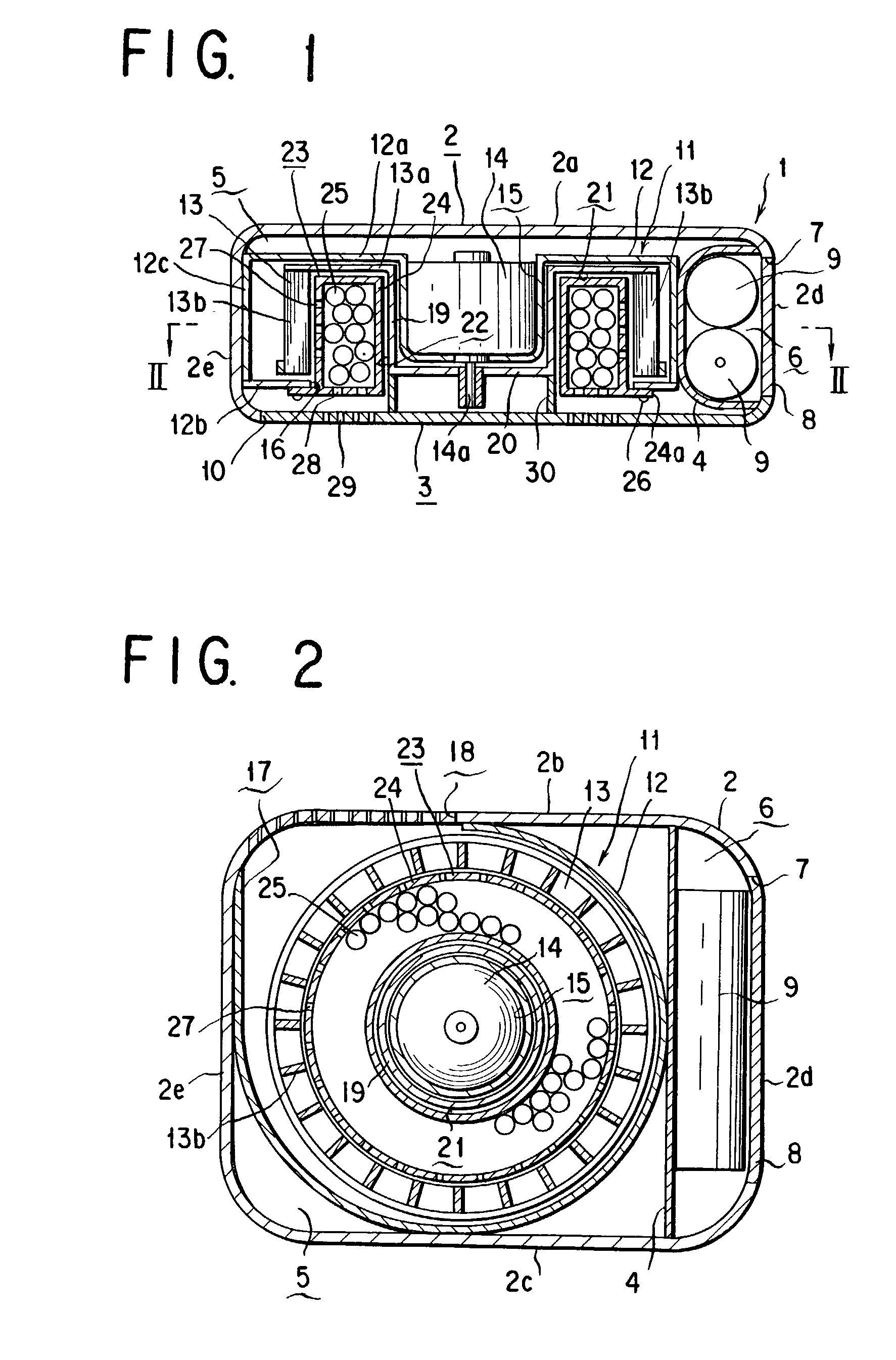
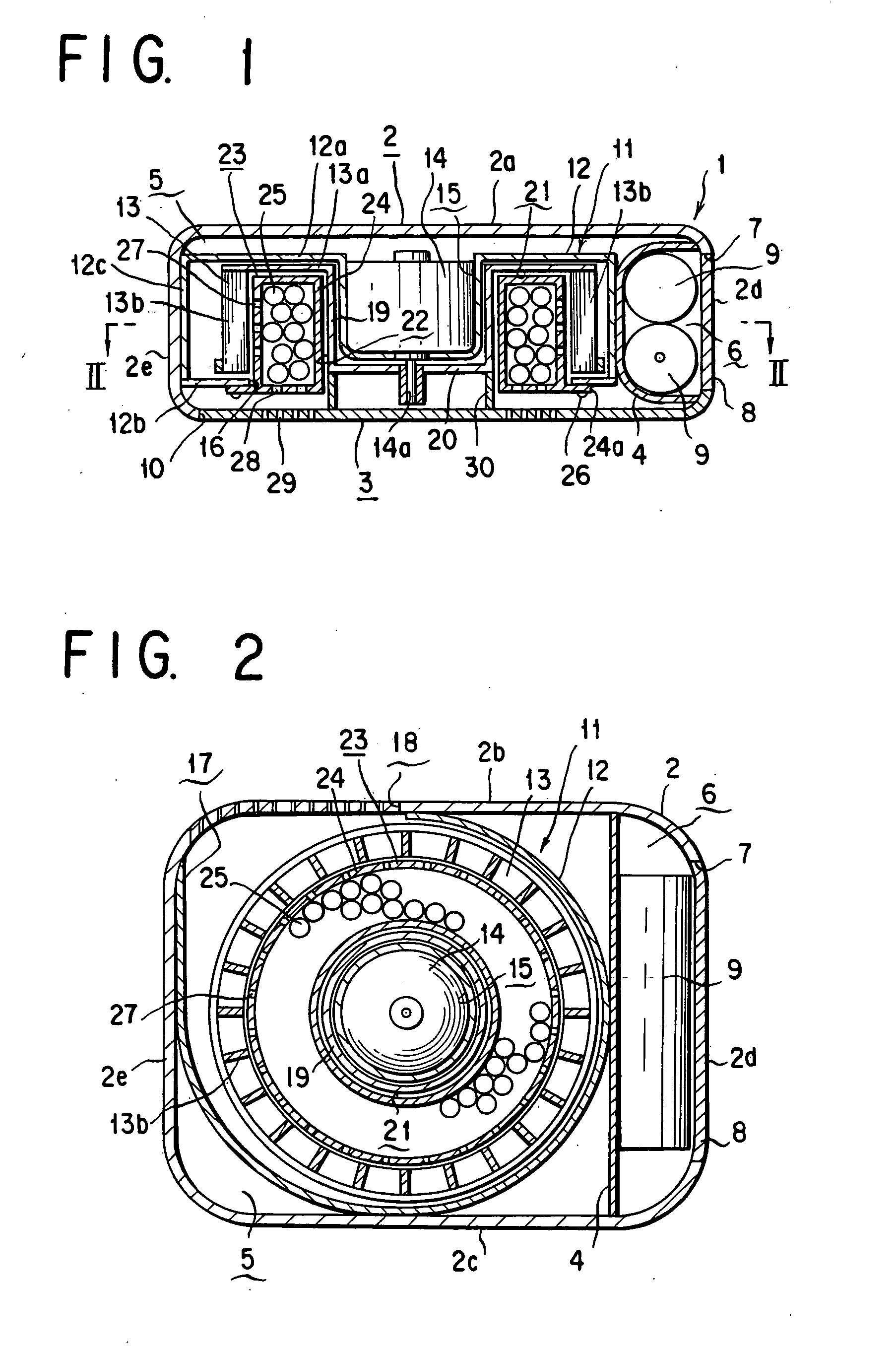
Fan type chemicals diffusing device
InactiveUS7175815B2Eliminate needSufficient efficacyBiocideMixing methodsEngineeringBULK ACTIVE INGREDIENT
A fan type chemical diffusing apparatus includes an apparatus main body having an air inlet and an air outlet, a fan type blower disposed in the apparatus main body, and an active ingredient impregnated body or mass for containing an active ingredient. The fan type blower includes a fan casing, a motor, and a fan including a rotary disk and a plurality of blades fastened to a peripheral portion of the rotary disk and provided with a hollow space that is on an interior of the fan with respect to the blades. The fan type blower is operable to send air from the air inlet through the hollow space and out through the air outlet.
Owner:FUMAKILLA LTD
Chamber with adjustable volume for cell culture and organ assist
InactiveUS20060003436A1Compromise liquid-tightnessCompromise sterilityBiocideBioreactor/fermenter combinationsCulture cellEngineering
The invention features modular chambers for culturing cells in which the volume of a chamber can be adjusted without compromising the seal or sterility of the chamber. The invention is based on the principle that the volume of a chamber formed between two plates sandwiching a compressible gasket and a substantially incompressible stop can be adjusted using a gasket that forms a fluid-tight seal between the plates at a plurality of levels of compression. The invention enables the culture of cells between substantially parallel and rigid plates in which a relatively large volume can be used to seed the cells and the holdup volume reduced for perfusion without opening or otherwise disassembling the system to compromise its liquidtightness and sterility. The new closed, modular and scalable cell-culturing chamber can be thus perfused and used to culture cells (e.g., hepatocytes) with high levels of cell function in organ (e.g., liver) assist systems, for production of cells, for production of cell-derived products, such as proteins or viruses, or for systems to treat biological liquids to remove toxins, such as ammonia, add cell-synthesized products, or both.
Owner:ORGANOGENESIS
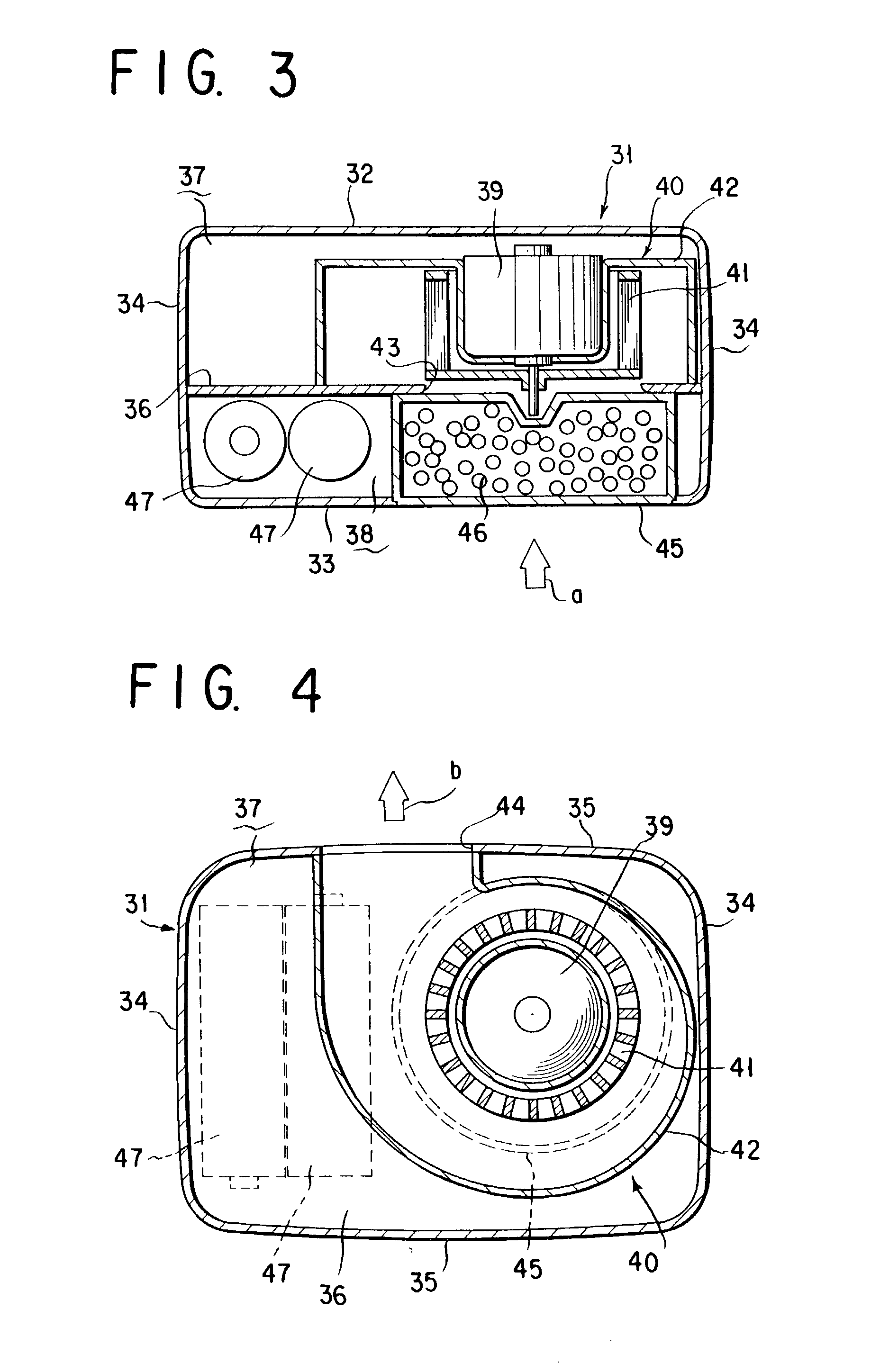
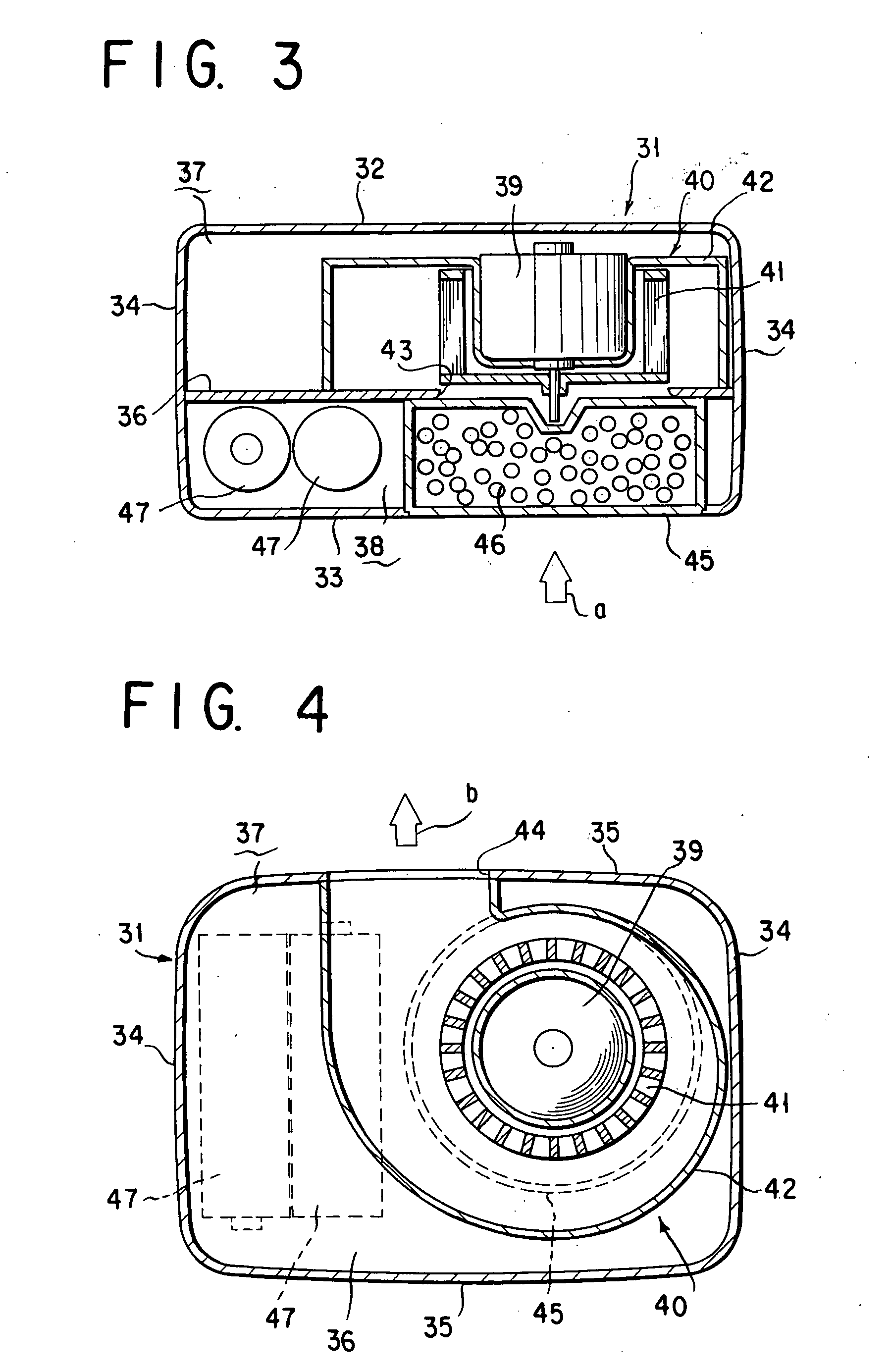
Fan type chemical diffusing apparatus
InactiveUS20060137241A1Eliminate needSufficient efficacyBiocideFire rescueCompound (substance)Current consumption
A fan type debugging apparatus is provided in which the active ingredient impregnated body or mass is disposed in a wind inlet side of the fan type blower and is designed to provide a wind force resistance which in terms of the proportion of current consumption by the motor in the presence of the active ingredient impregnated body or mass to current consumption by the motor in the absence of the active ingredient impregnated body or mass, ranges from 5 to 25%.
Owner:FUMAKILLA LTD
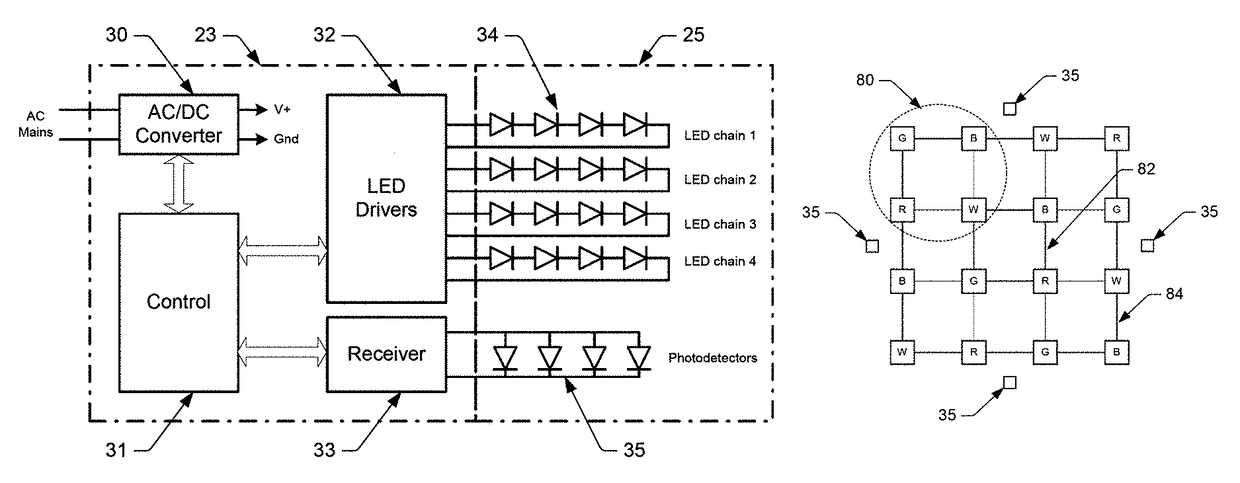
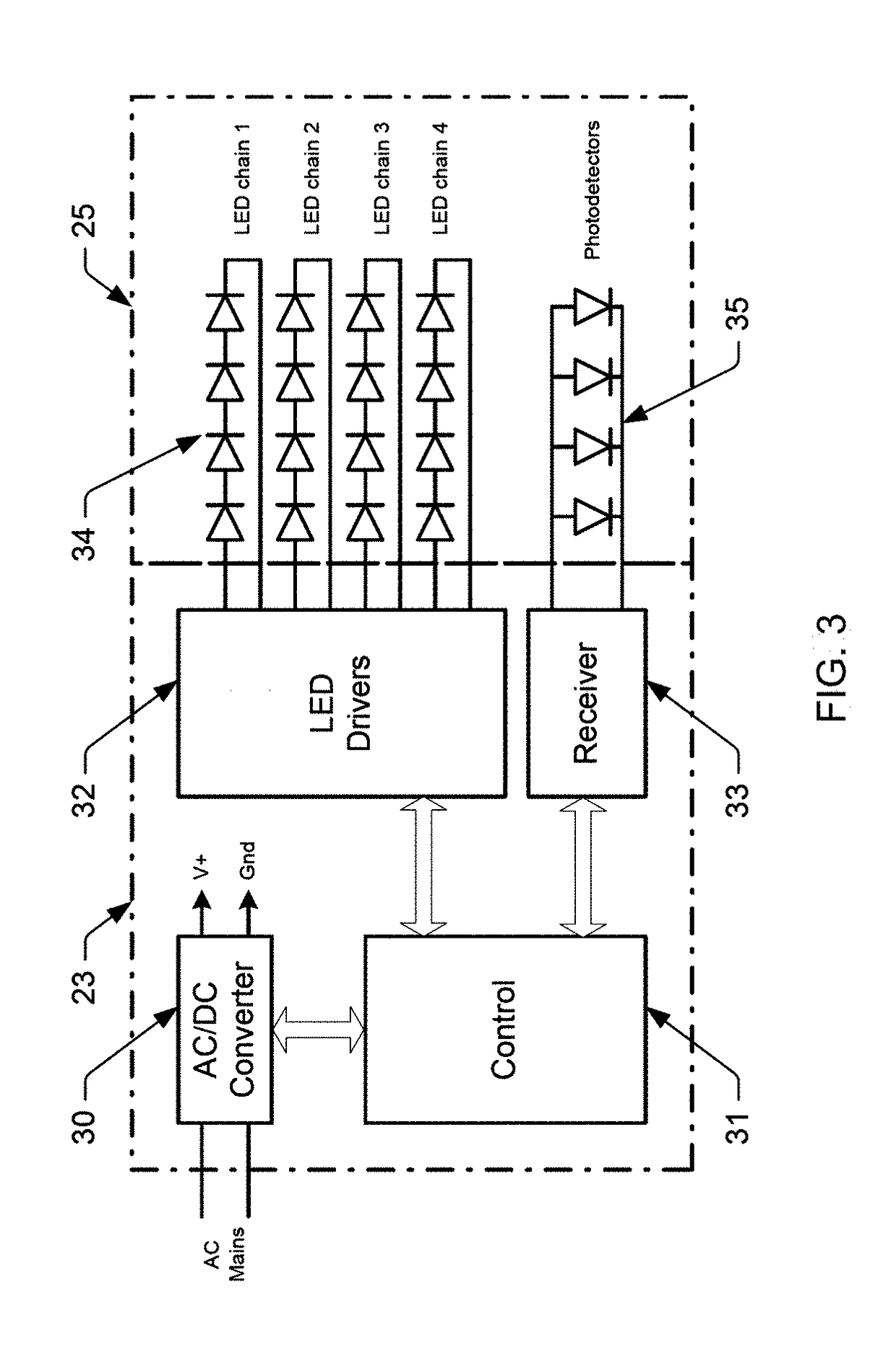
Color mixing optics for LED illumination device
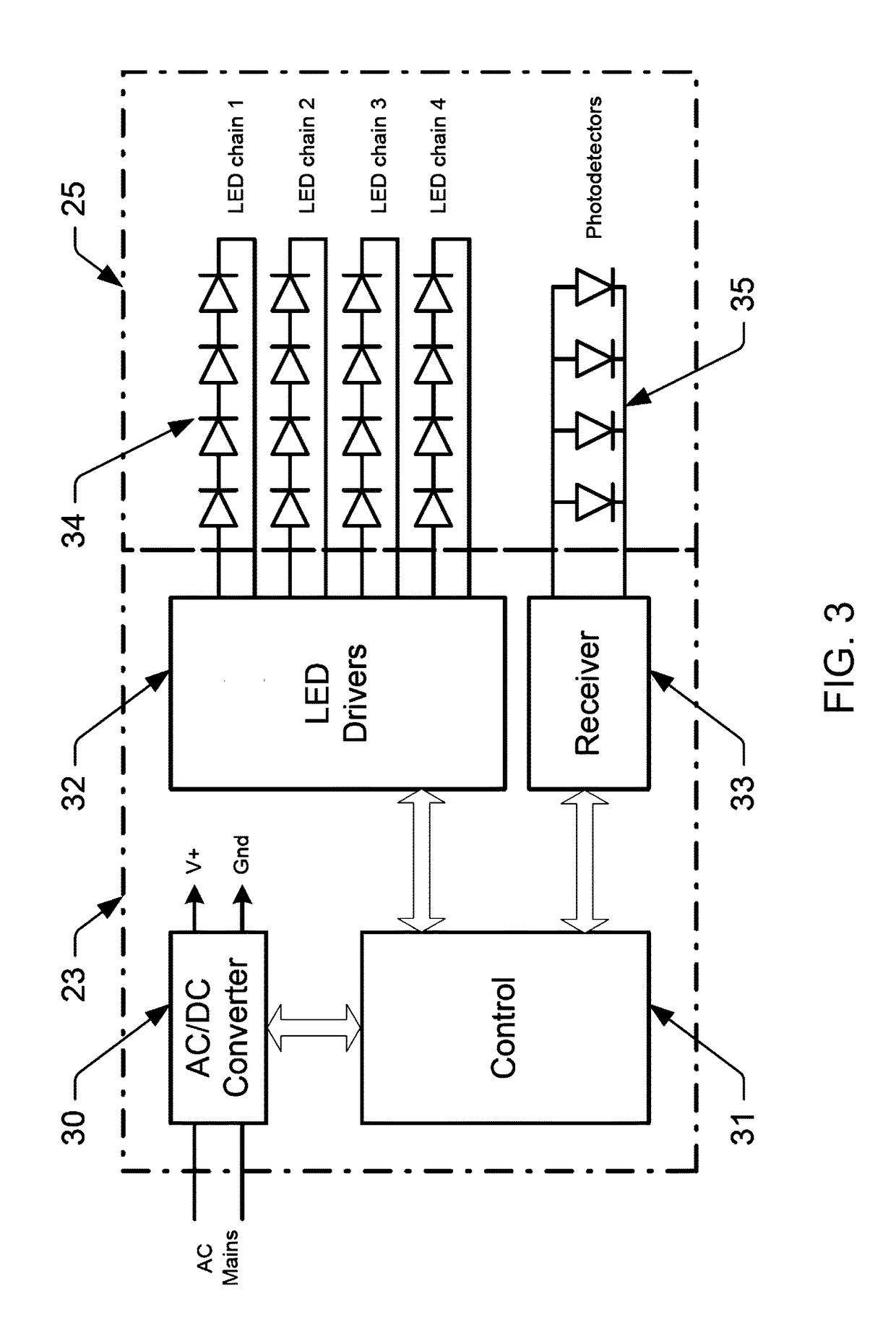
ActiveUS9736895B1Spatial variation be minimizeWell spatial uniformityPlanar light sourcesLight source combinationsPhotodetectorLenslet
Illumination devices with improved color mixing optics are disclosed herein for mixing the colors produced by a multi-colored LED emitter module to produce uniform color throughout the entire beam angle of the output light beam, along with smoother edges and improved center beam intensity. Embodiments disclosed herein include a unique arrangement of multi-color LEDs within an emitter module, a unique exit lens with different patterns of lenslets on opposing sides of the lens, and other associated optical features that thoroughly mix the different color components, and as such, provide uniform color across the output beam exiting the illumination device. Additional embodiments disclosed herein include a unique arrangement of photodetectors within the primary optics structure of the LED emitter module that ensure the optical feedback system properly measures the light produced by all similarly colored emission LEDs.
Owner:LUTRON TECH CO LLC
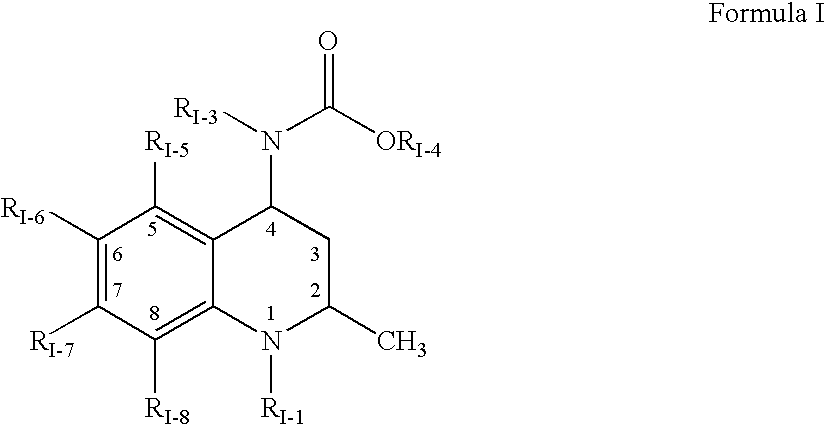
Guanidine compounds, and use thereof as binding partners for 5-HT5 receptors
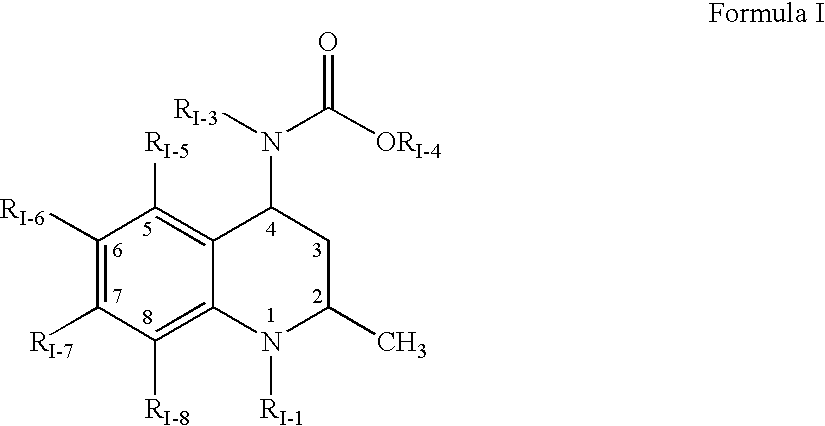
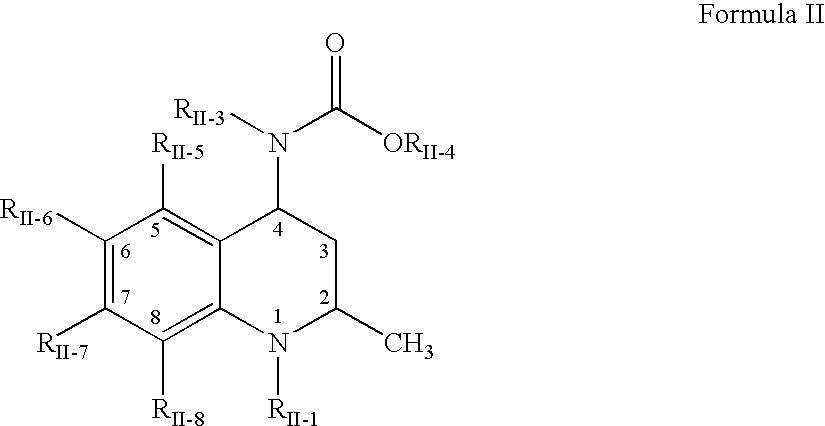
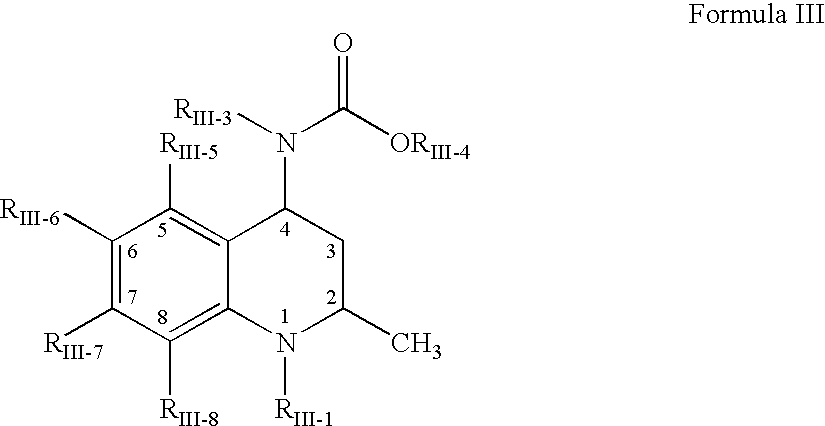
The present invention relates to guanidine compounds of the general formula Icorresponding enantiomeric, diastereomeric and / or tautomeric forms thereof as well as pharmaceutically acceptable salts thereof. The present compound further relates to the use of guanidine compounds as binding partners for 5-HT5 receptors for the treatment of diseases which are modulated by a 5-HT5 receptor activity, in particular for the treatment of neurodegenerative and neuropsychiatric disorders as well as the associated signs, symptoms and dysfunctions.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Optical radiation coupling module
InactiveUS20060263015A1Sufficient coupling efficacyEasy and cost-effective to manufactureCoupling light guidesOptical radiationWaveguide
An optical radiation coupling module includes a waveguide for the propagation of optical radiation, an optoelectronic device arranged on a substrate, and a mechanism for coupling the radiation between the waveguide and the optoelectronic device. The coupling mechanism includes a guiding element moulded and tapered in a radiation propagation direction.
Owner:STMICROELECTRONICS SRL
In-vitro method for producing oocytes or eggs having targeted genomic modification
InactiveUS20080113437A1High frequencyReduce frequencyHydrolasesStable introduction of DNAGenomeOocyte
Owner:INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE
Therapeutic agent for irritable bowel syndrome
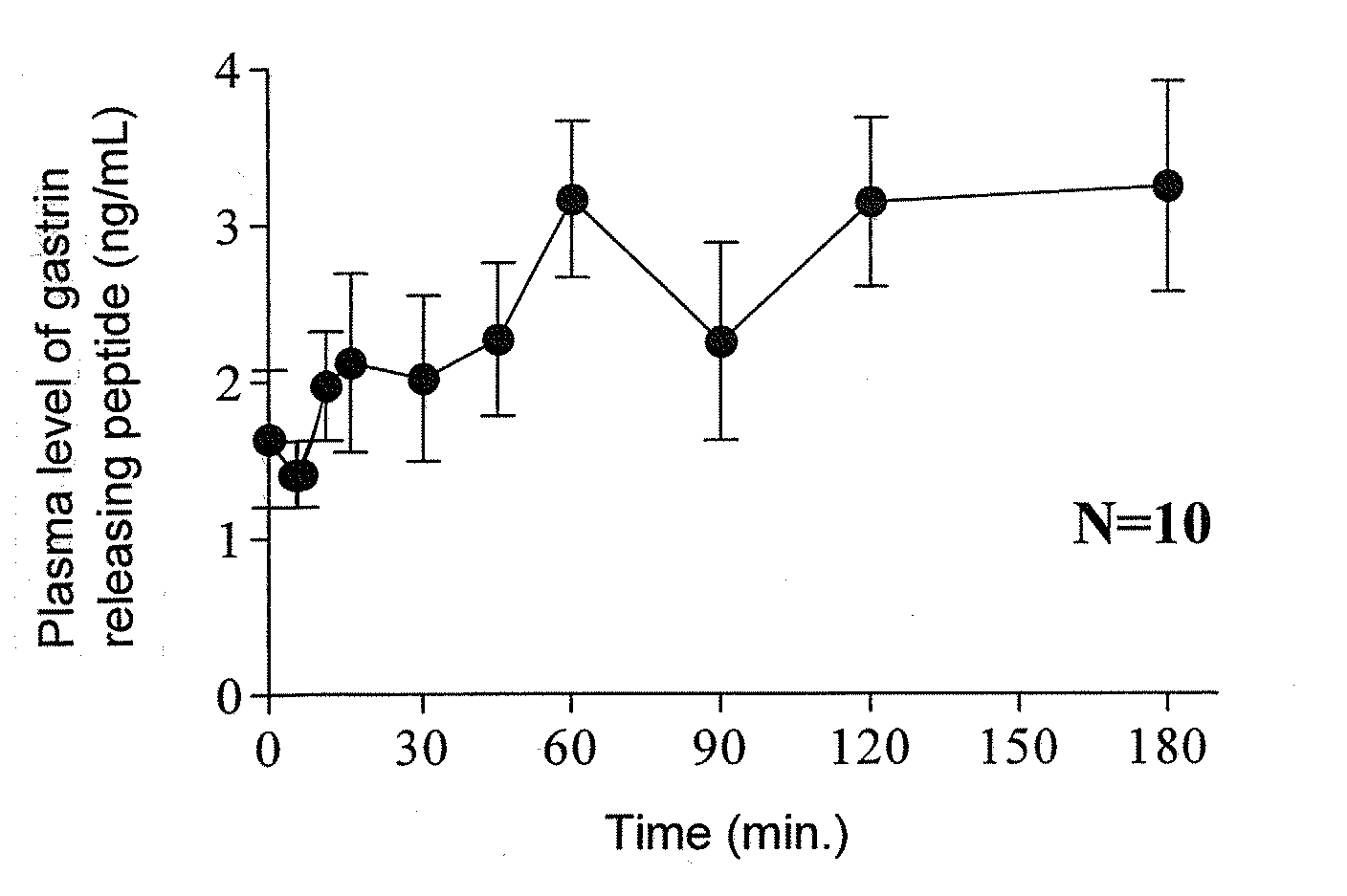
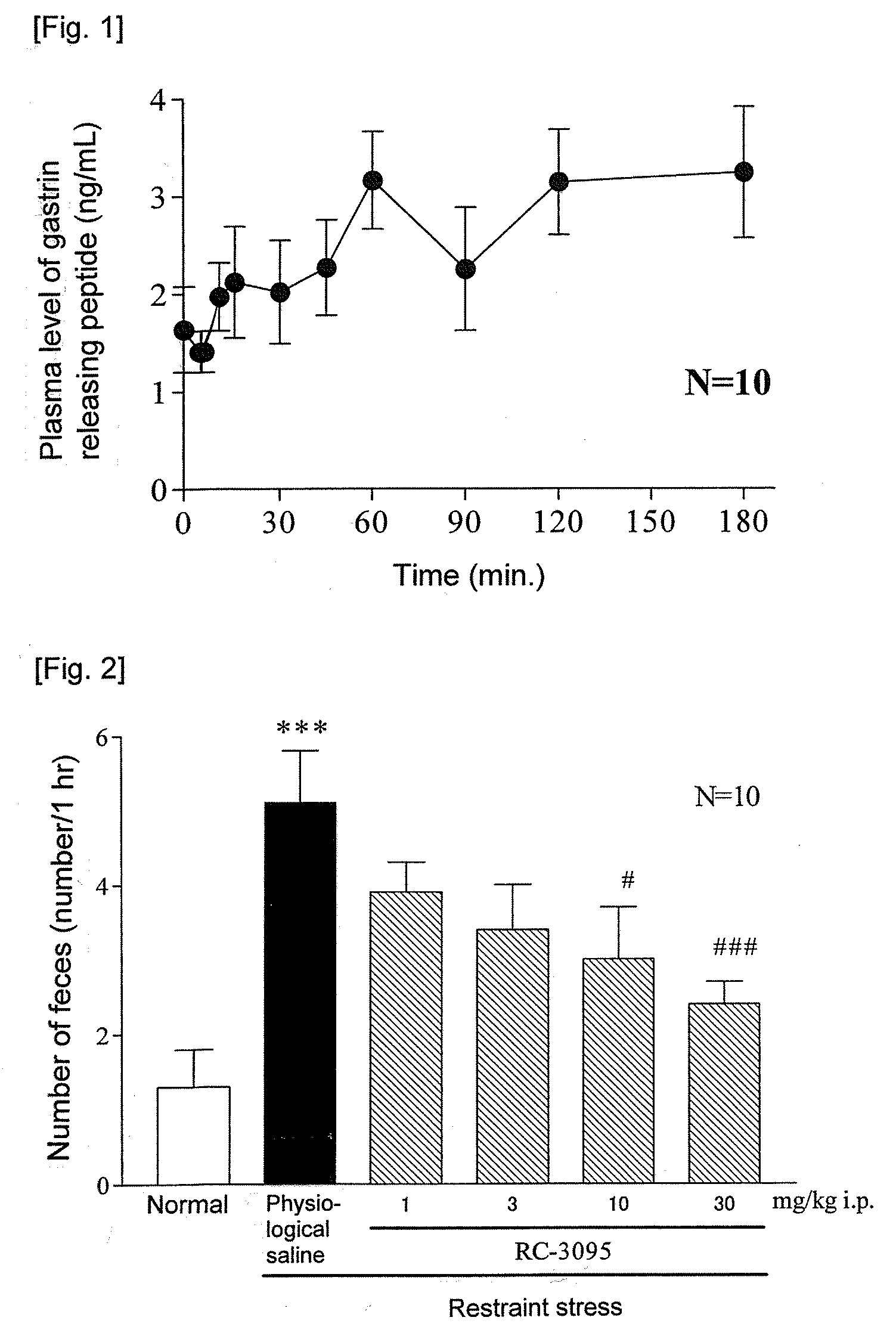
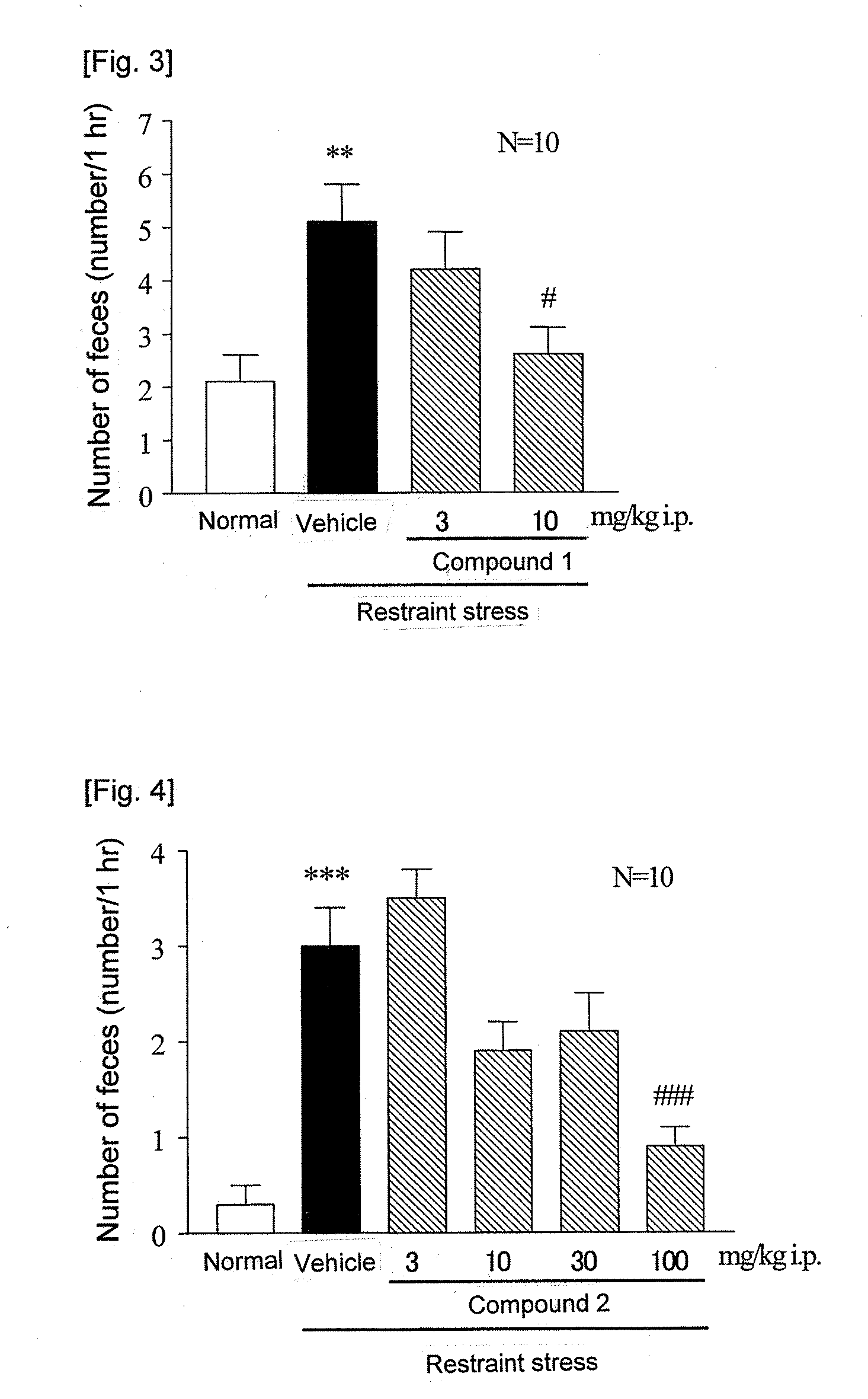
ActiveUS20090093415A1Excellent IBS-treating effectImprove efficacyBiocidePeptide/protein ingredientsIntestinal structureNK1 receptor antagonist
[Problem]To provide a therapeutic agent for irritable bowel syndrome (IBS), which is excellent in efficacy and safety.[Means for Resolution]It was shown that the bombesin 2 (BB2) receptor antagonists typified by RC-3095 are therapeutic agents for irritable bowel syndrome (IBS), which show excellent efficacy in both of an abdominal symptom and bowel movement disorder. Thus, according to the present invention, it became possible to provide a therapeutic agent for irritable bowel syndrome (IBS) which comprises, as an active ingredient, a bombesin 2 (BB2) receptor antagonist exerting an excellent efficacy in both an abdominal symptom and bowel movement disorder.
Owner:SELDAR PHARMA
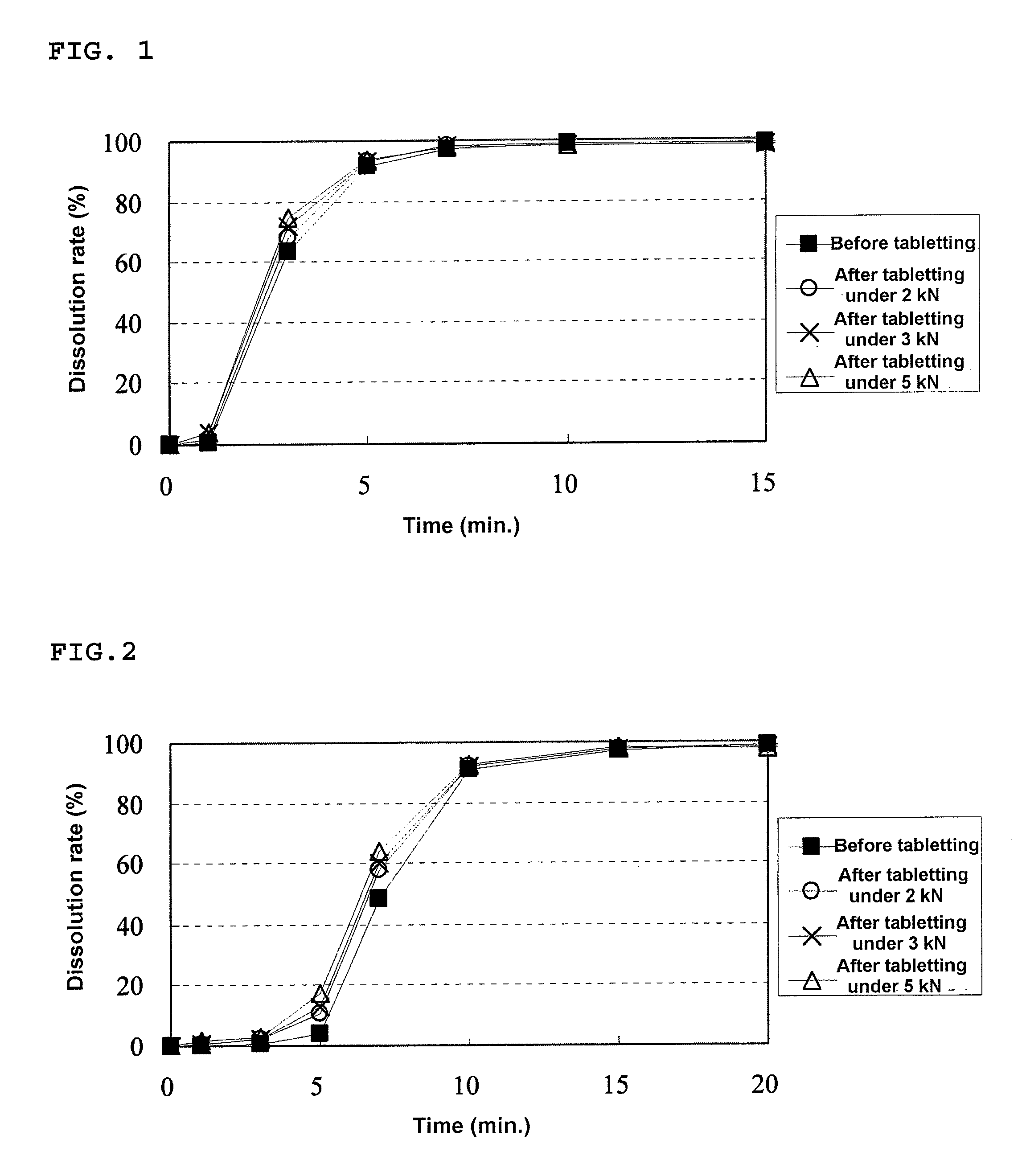
Granular pharmaceutical composition for oral administration
InactiveUS20100136110A1Improve complianceReduce unpleasantnessNervous disorderMetabolism disorderWater soluble polymersButyl methacrylate
A granular pharmaceutical composition for oral administration, wherein a drug-containing particle is coated with a coating comprising a methyl methacrylate-butyl methacrylate-dimethylaminoethyl methacrylate copolymer and a water-soluble polymer is disclosed.
Owner:ASTELLAS PHARMA INC
Pharmaceutical compositions of cholesteryl ester transfer protein inhibitors
InactiveUS7235259B2Improve aqueous concentrationEnhance solubilityPowder deliveryBiocideCholesteryl esterChemistry
A pharmaceutical composition comprises a solid amorphous dispersion of a cholesteryl ester transfer protein inhibitor and a concentration-enhancing polymer.
Owner:PFIZER INC +1
Optical radiation coupling module
InactiveUS7313302B2Easy and cost-effective to manufactureSufficient efficacyCoupling light guidesOptical radiationOpto electronic
Owner:STMICROELECTRONICS SRL
Synthetic glycolipid and use thereof
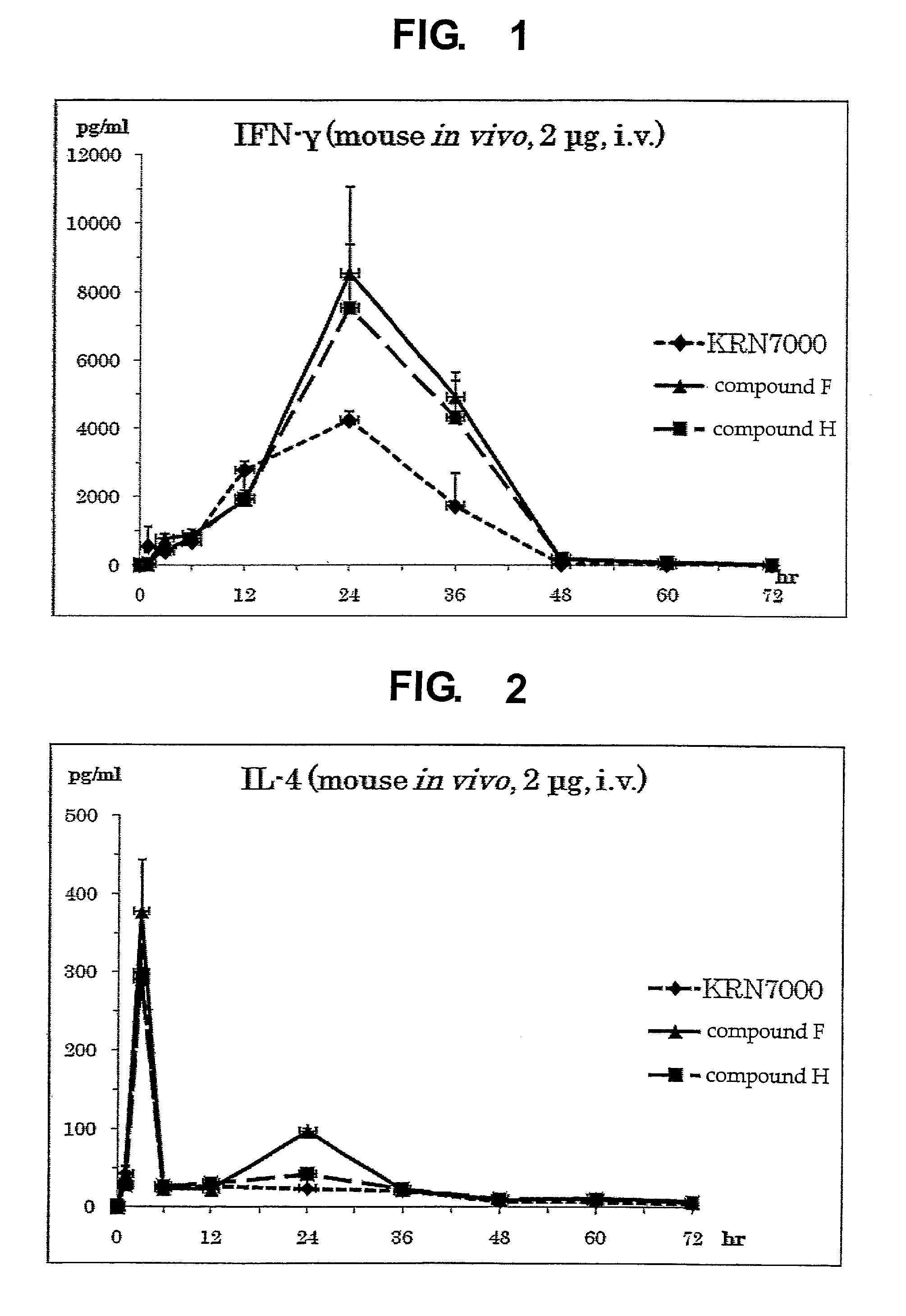
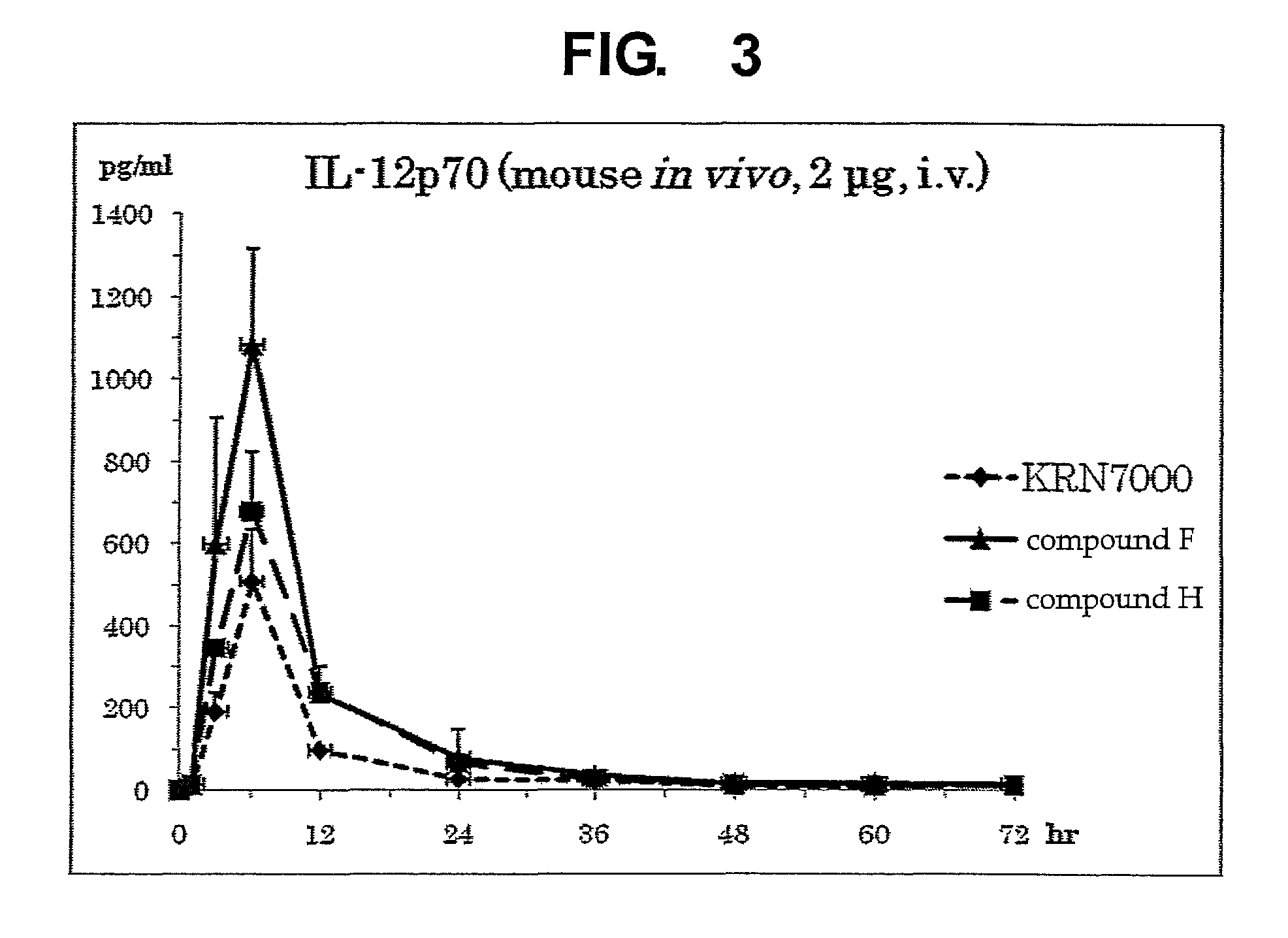
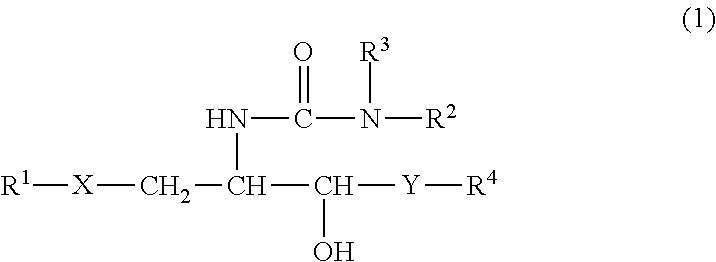
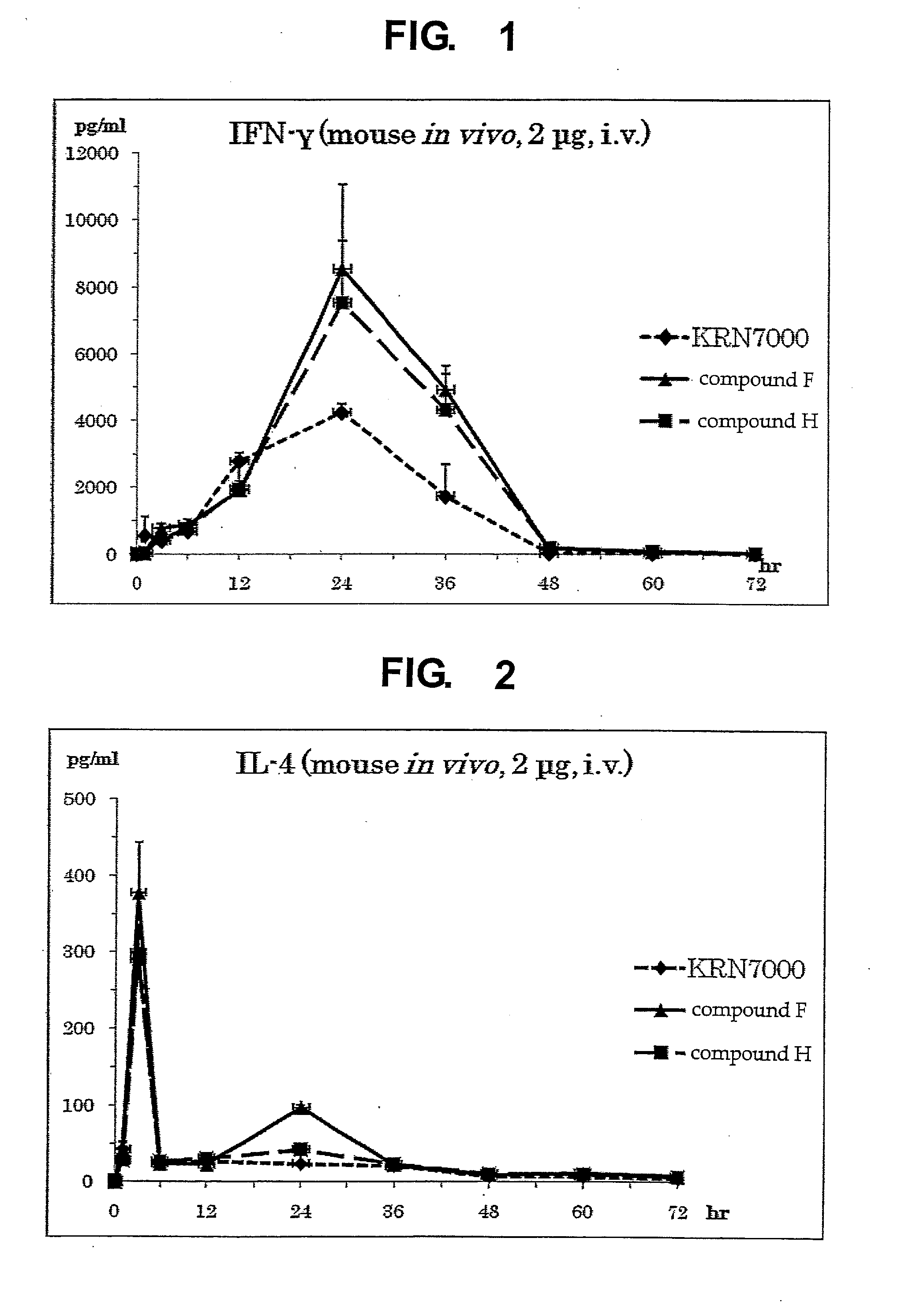
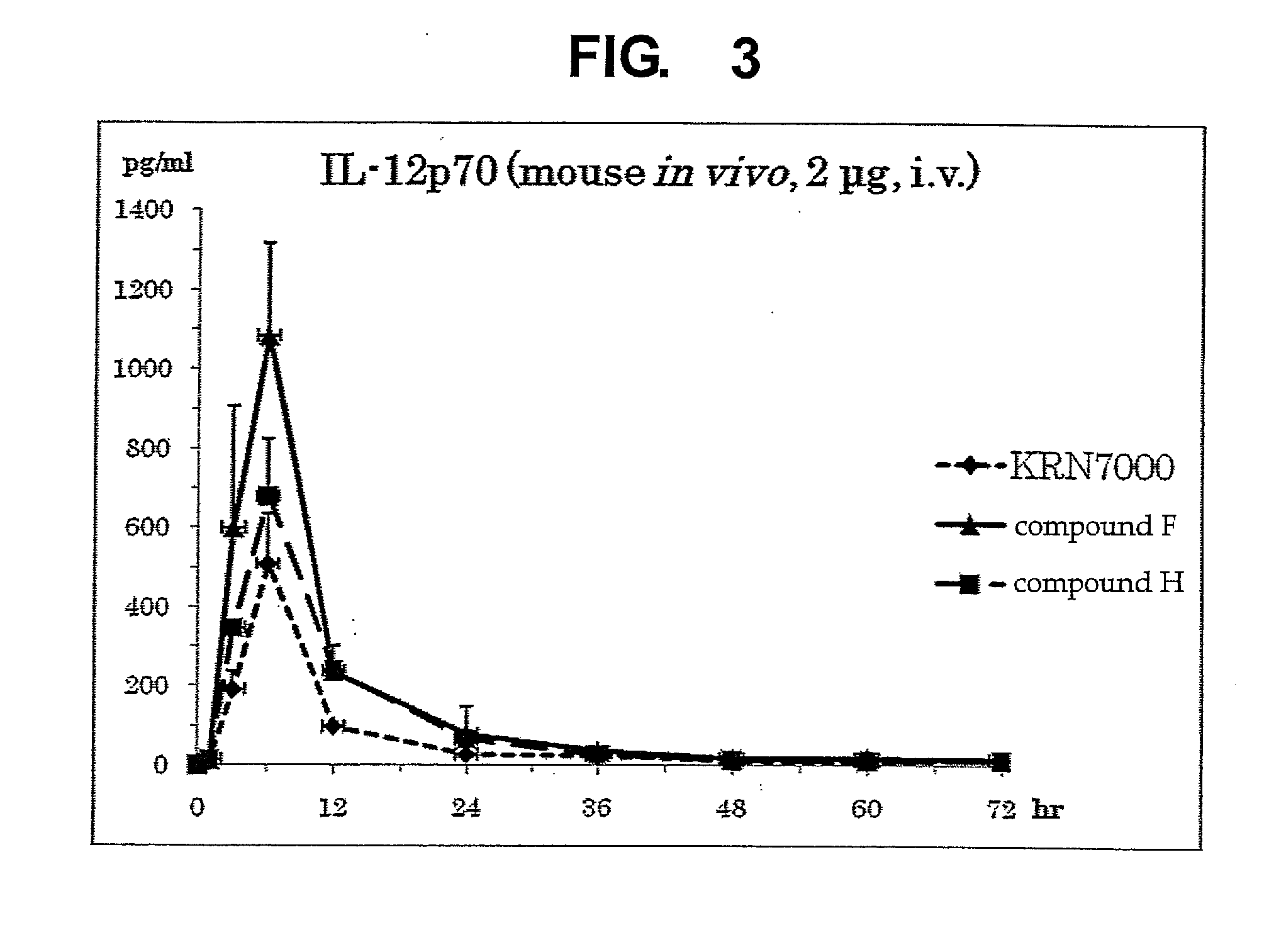
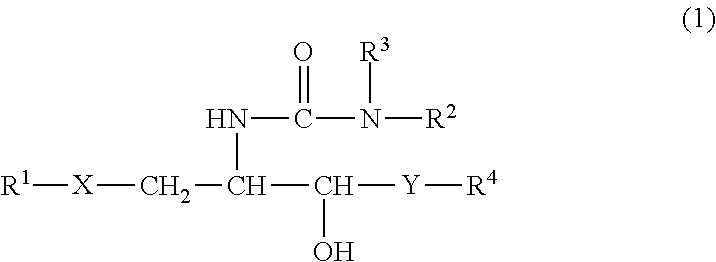
InactiveUS8853173B2Intensely activateSufficient efficacyAntibacterial agentsBiocideHydrogen atomNatural killer T cell
A compound represented by the following formula (1):wherein R1 is an aldopyranose residue wherein the 6-hydroxyl group is optionally alkylated, R2 is a C1-26 hydrocarbon group optionally having substituent(s), R3 is a hydrogen atom or a C1-26 hydrocarbon group optionally having substituent(s), R4 is a C1-21 hydrocarbon group optionally having substituent(s), X is an oxygen atom or —CH2—, and Y is —CH2—, —CH(OH)— or —CH═CH—, or a salt thereof is useful for the prophylaxis or treatment of cancer or infection, since it can preferentially induce production of IFN-γ of NKT cells.
Owner:RIKEN
Ultraviolet cold cathode florescent lamp
InactiveUS20120153804A1Uniformity and safetyEfficient curingDischarge tube luminescnet screensLamp detailsUltraviolet lightsEuropium
The present invention is related to a UV CCFL lamp, and more particularly, to a UV CCFL lamp for curing a nail gel with a UVA irradiation having a peak wavelength of such as 366 nm or 368 nm in the field of nail art. In order to provide a UV CCFL lamp capable generating ultraviolet light of high intensity and uniform lighting with great electrical safety and reliability, the UV CCFL lamp of the present invention comprises a translucent hermetic envelope configured to enclose a discharge medium and a UV-excited phosphor to generate the desired UV irradiation preferably in the UVA spectrum range. The discharge medium is preferably to be a mercury-ion vapor and distributed throughout and sealed within the lamp envelope to be excited to a plasma state and for producing a first emission spectrum of a UVC wavelength during operation; and the phosphor in contact with the mercury-ion vapor plasma preferably comprises a composition of either europium-doped boron strontium oxide (B4SrO7:Eu+) or europium-doped strontium fluoborate (SrFB2O3.5:Eu2+) for producing said UV irradiation in response to said first emission spectrum of the discharge medium. To provide a uniform UV irradiation output from the UV CCFL lamp of the present invention, the phosphor is advantageously prepared to be of a mean grain size of 8.5μm±3μm for SrFB2O3.5:Eu2+ and of 15μm±3μm for B4SrO7:Eu+.
Owner:COSMEX
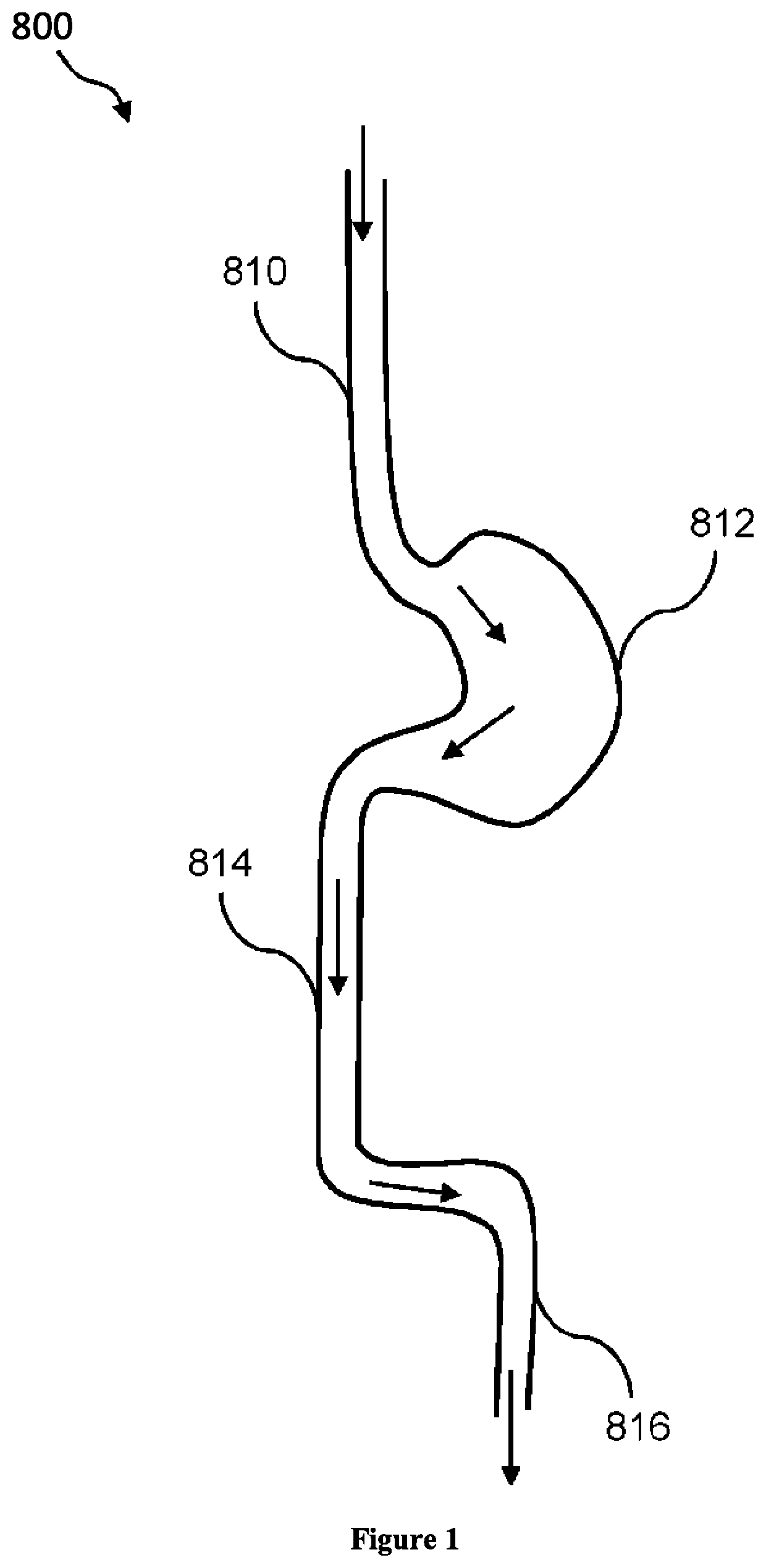
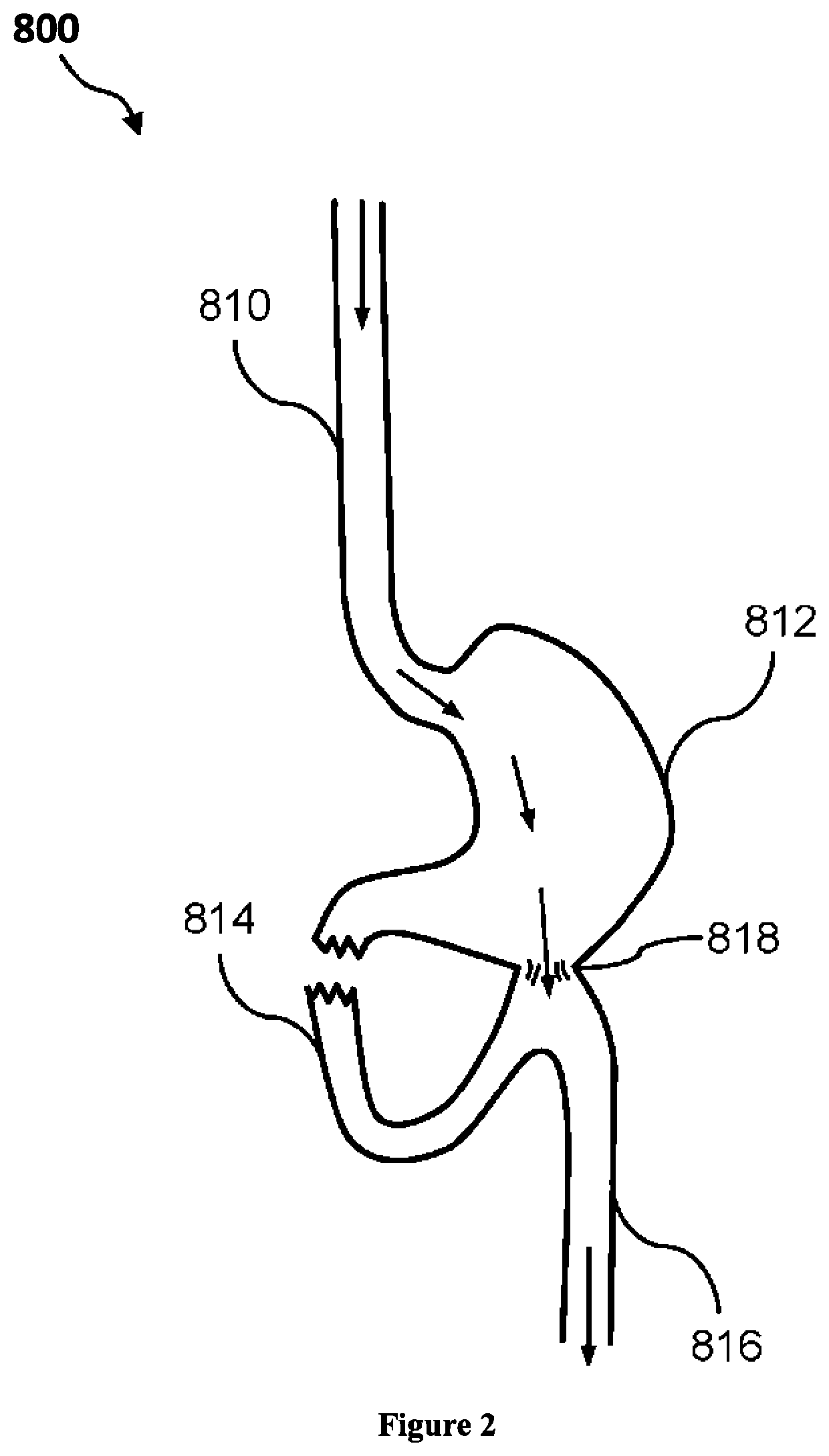
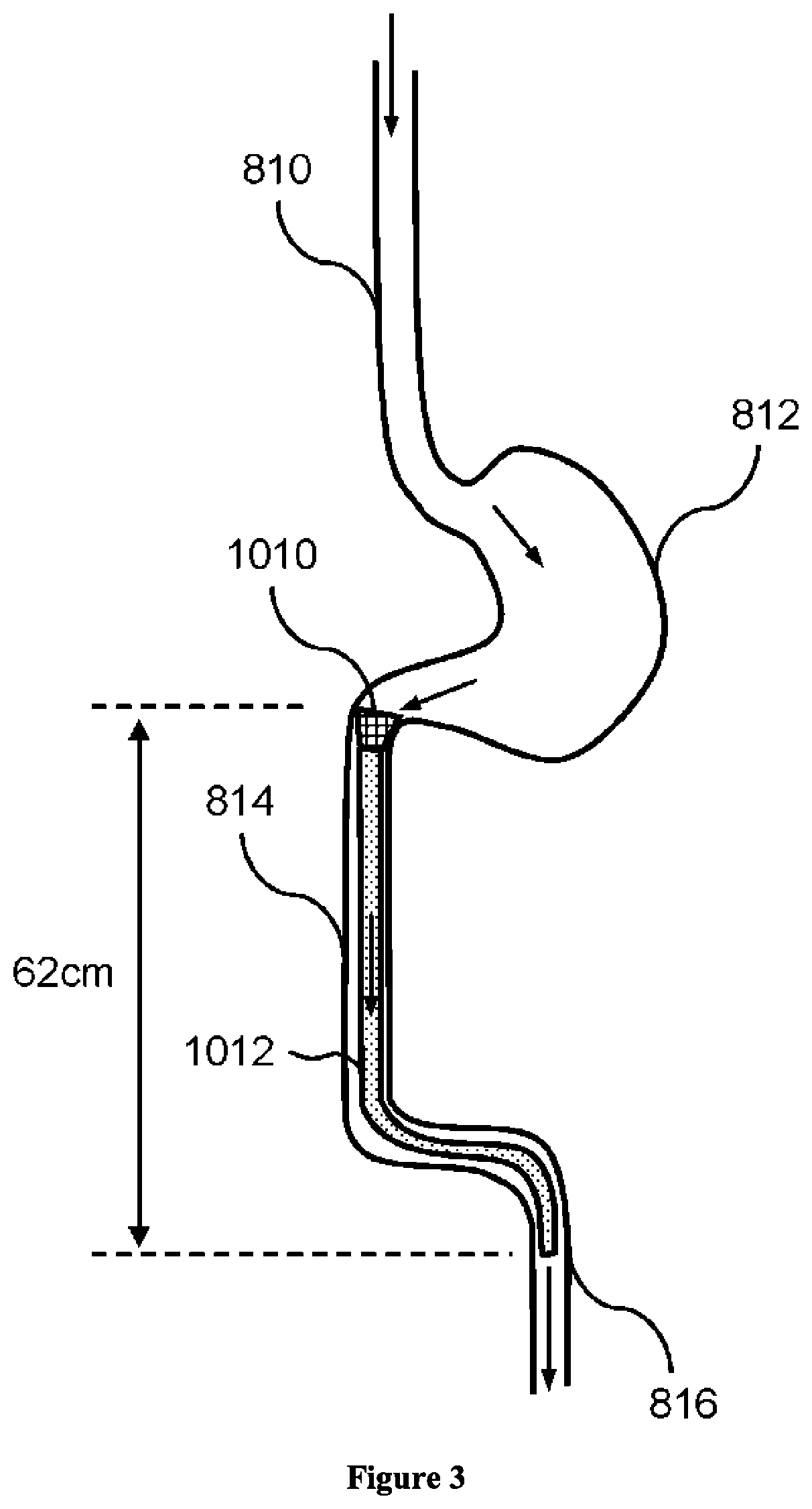
3D filter for prevention of stroke
ActiveUS10335259B2Avoid occlusionEfficiently deflectedStentsSurgeryPhysical medicine and rehabilitationProsthesis
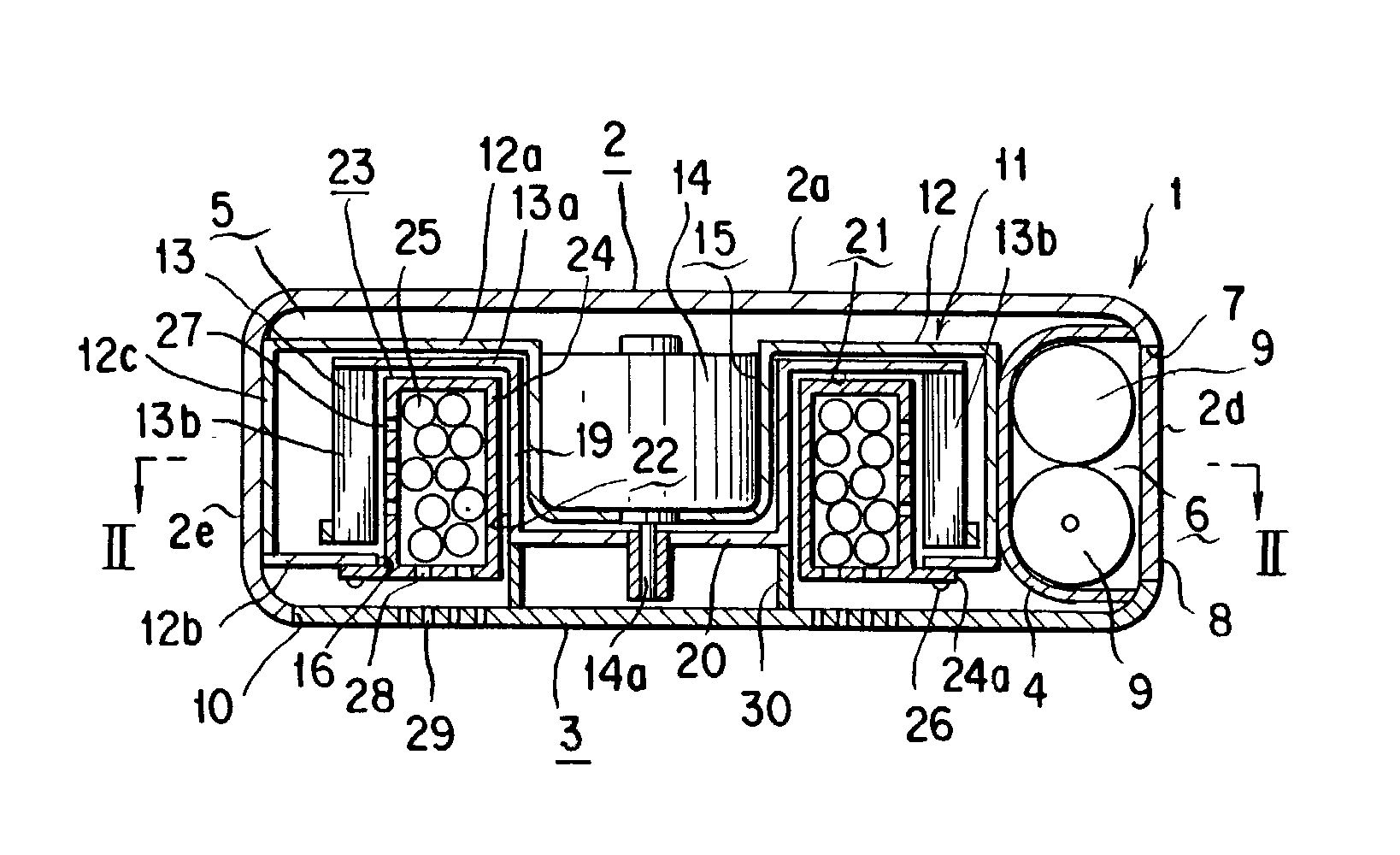
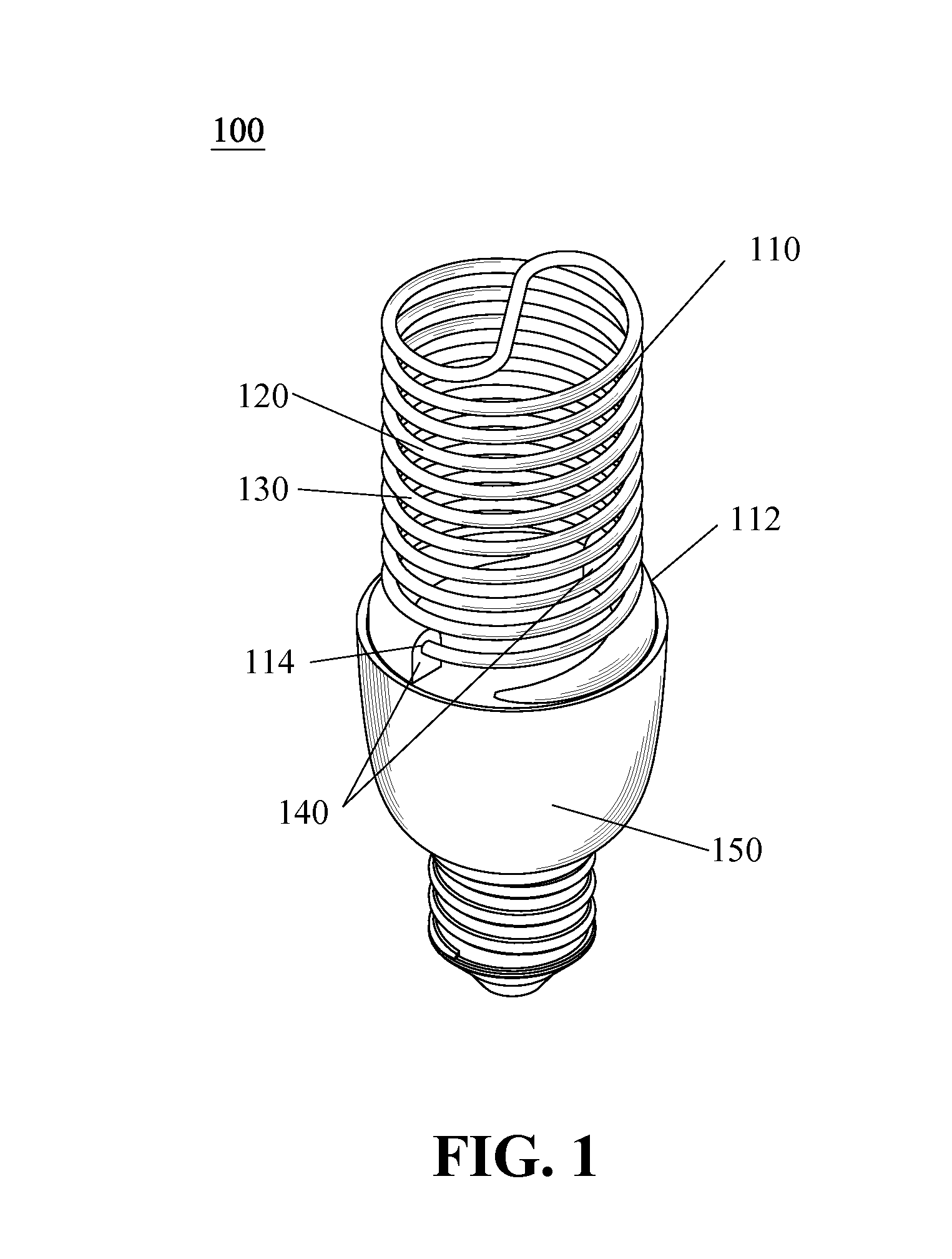
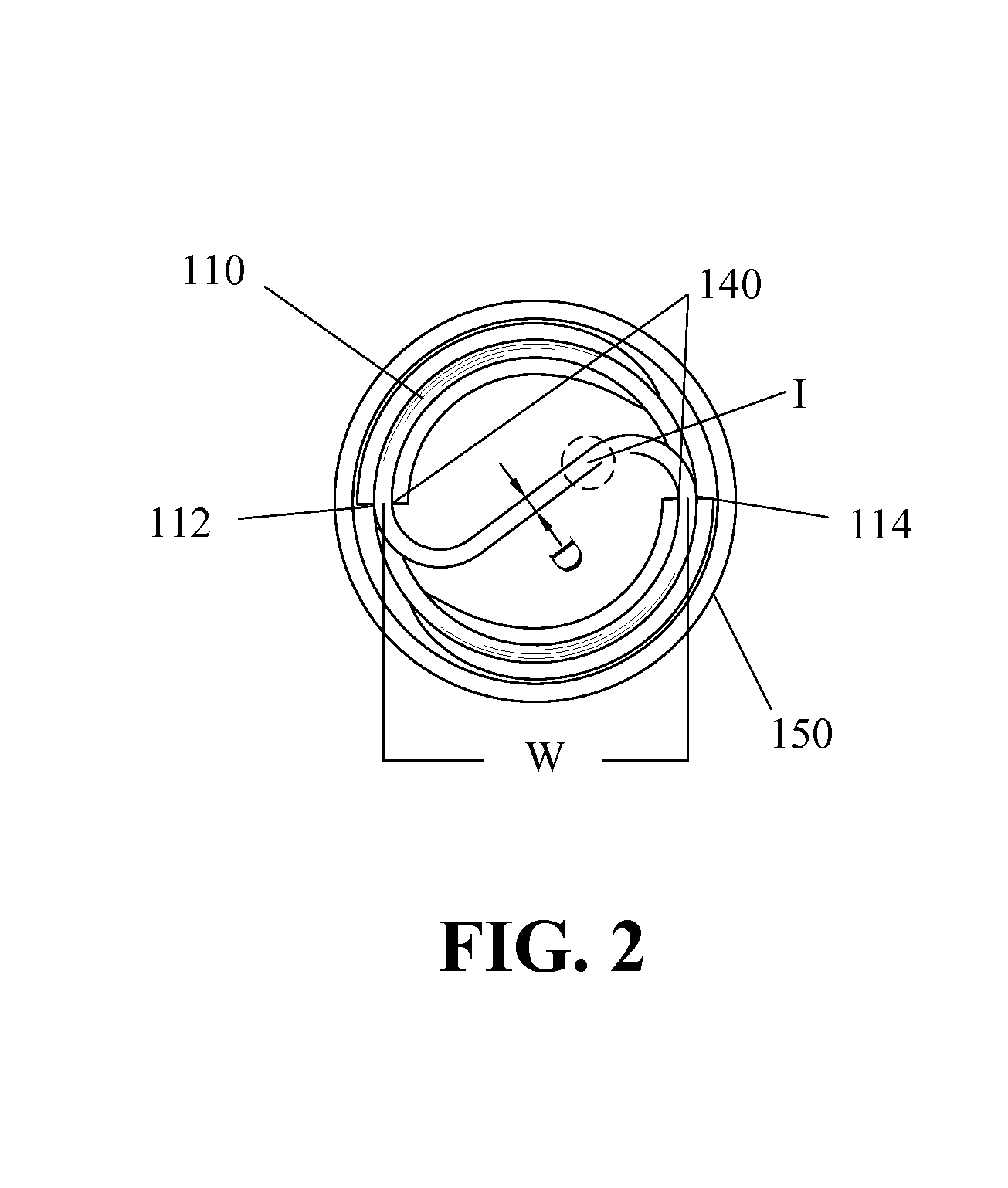
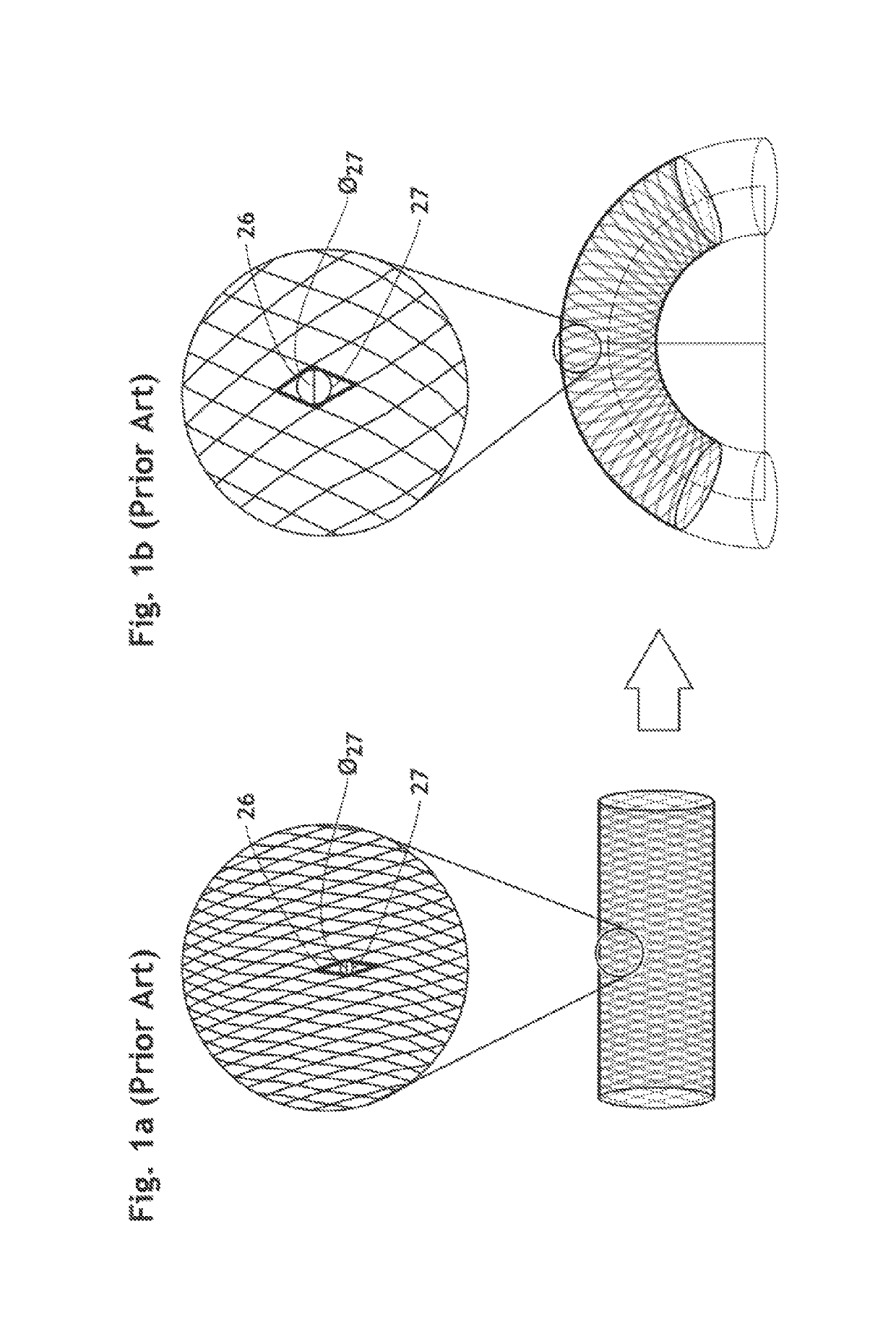
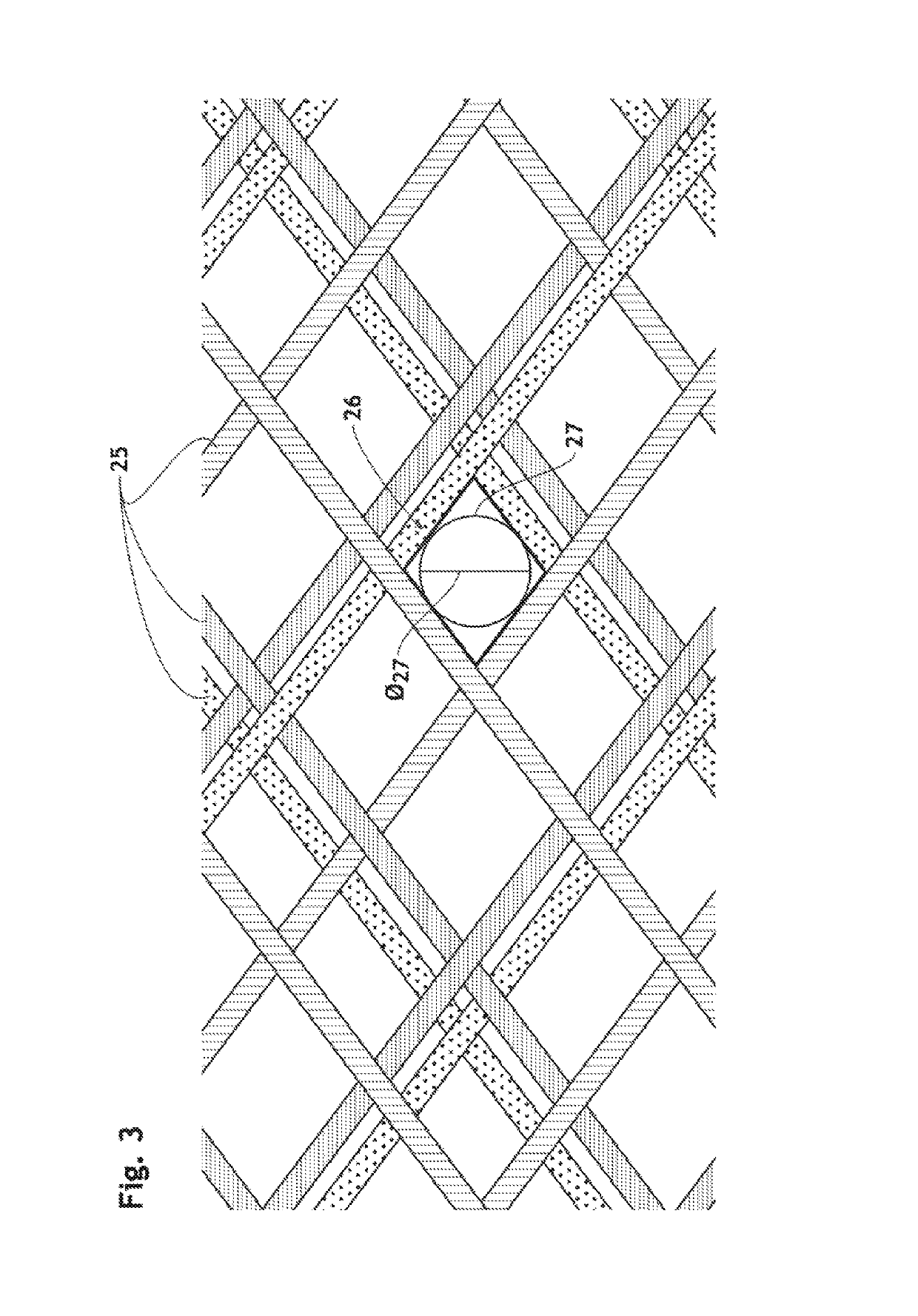
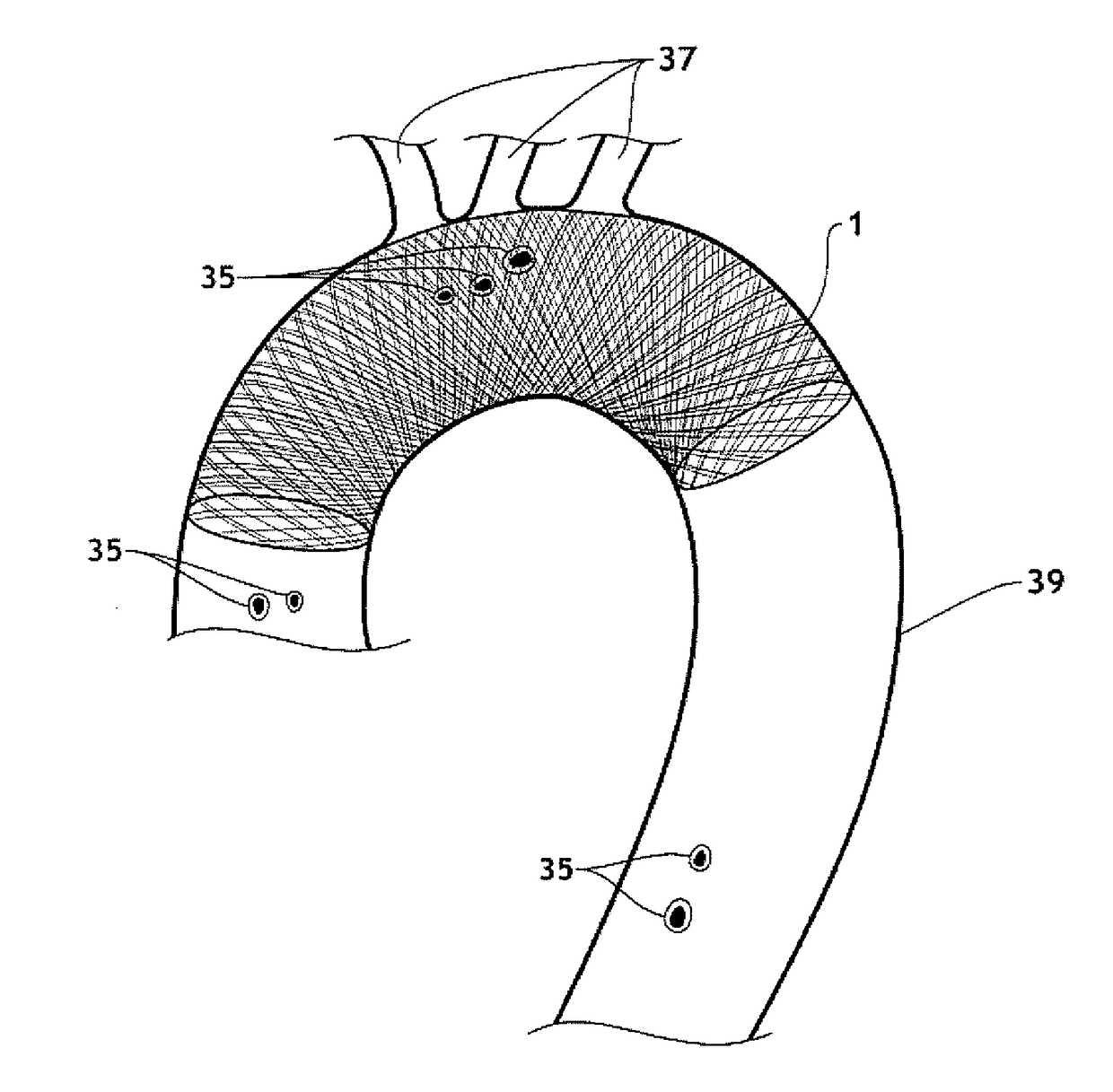
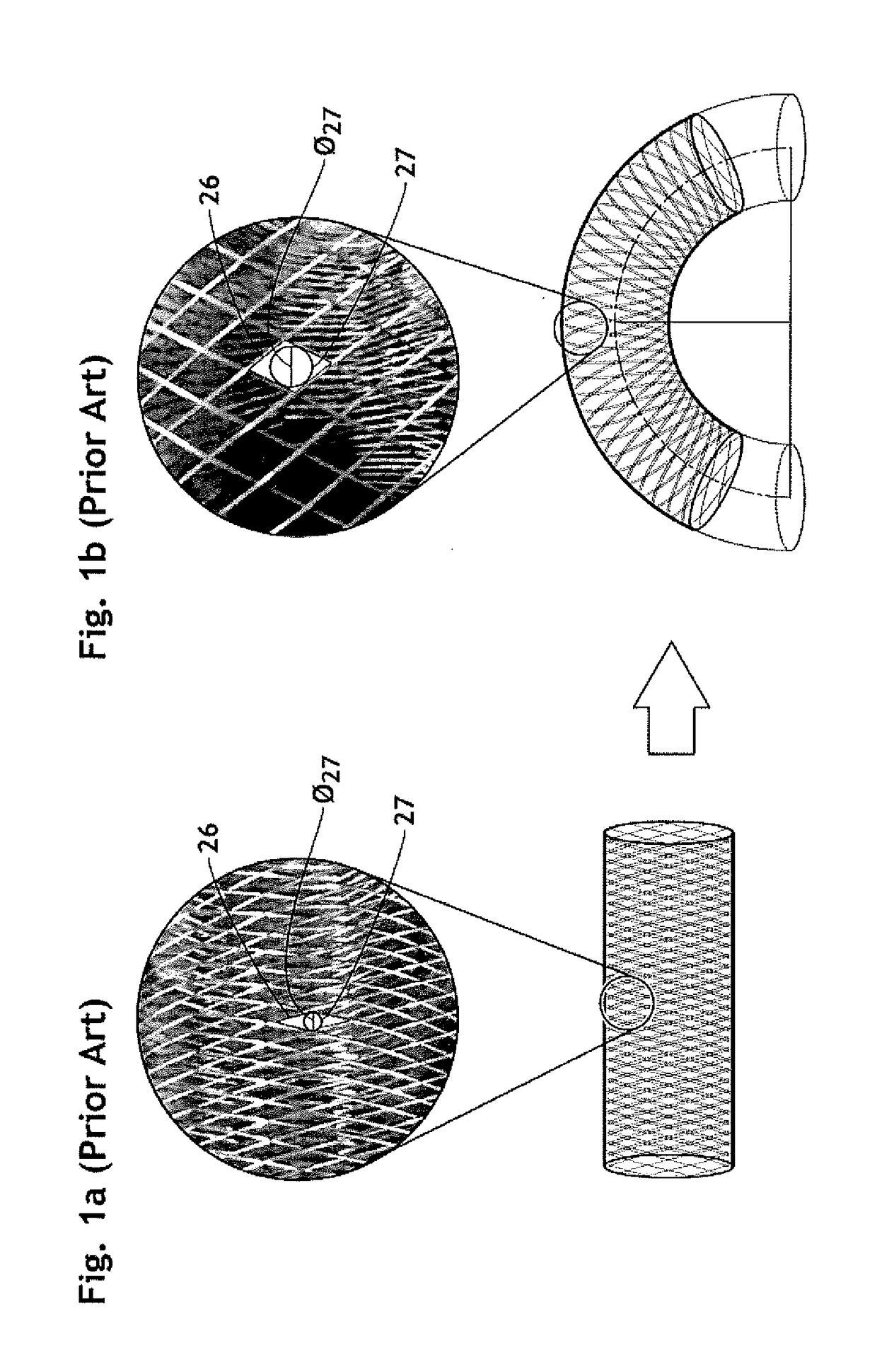
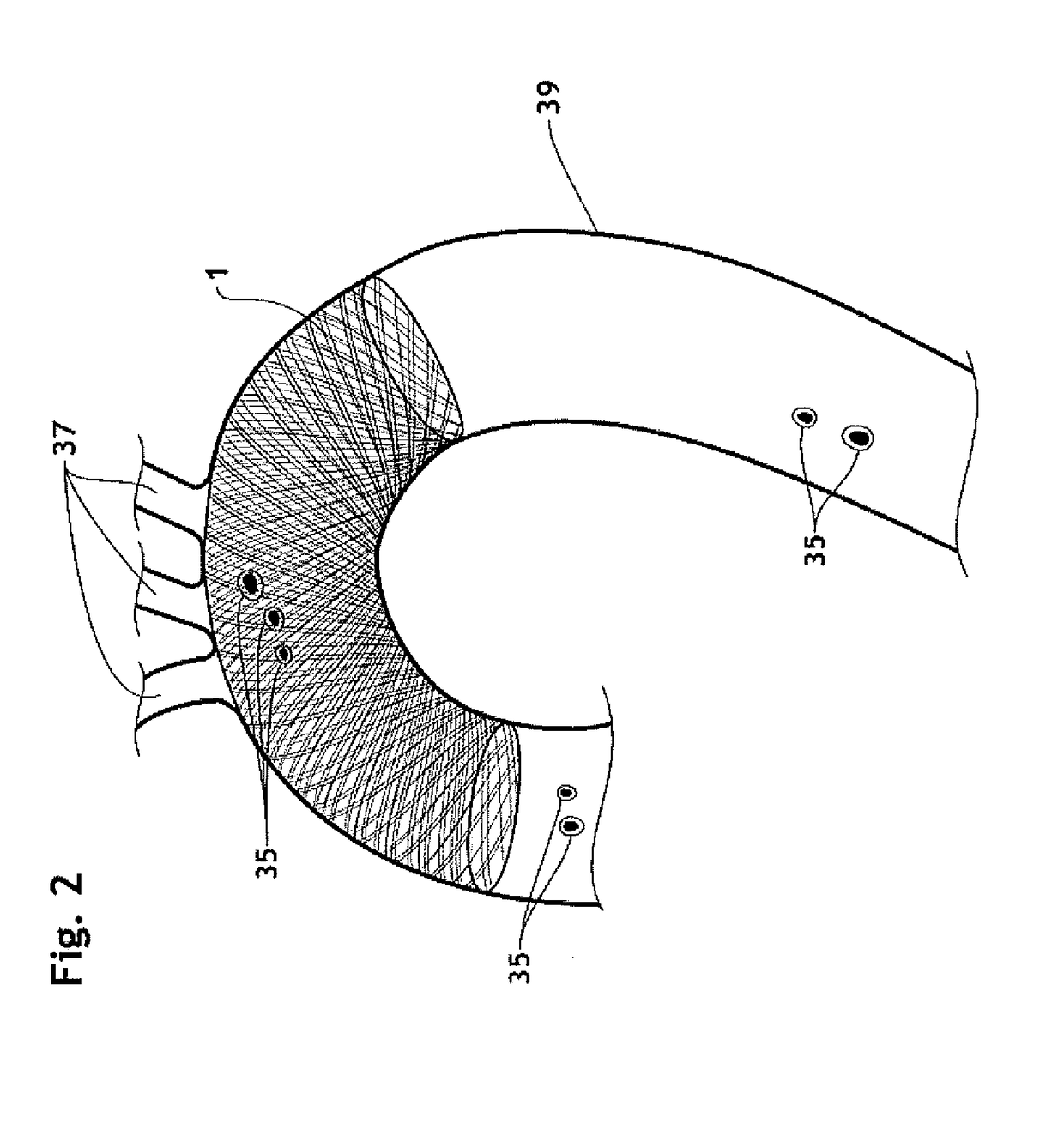
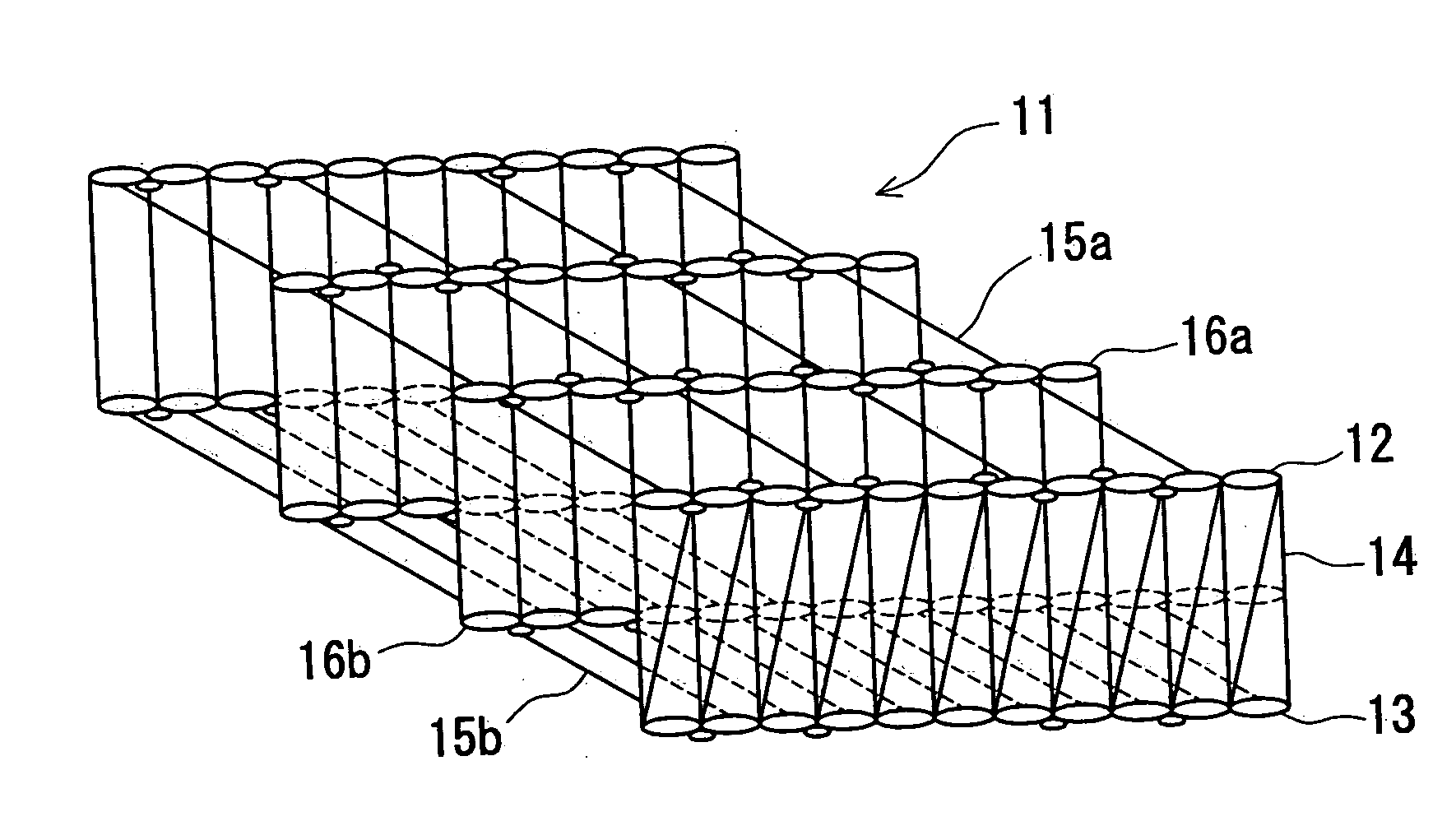
The present invention relates to implantable endoluminal prosthesis for preventing stroke. The endoluminal prosthesis (1) consists of a braided framework (20) defining a cylindrical lumen (21) devoid of impermeable membrane. Said braided framework (20) is self-expandable comprising a plurality of layers (22, 23, 24) of wires (25) made of biocompatible material. Each layer forms a mesh. The meshes form a lattice with a plurality of wires (2) of given layers (22, 23, 24). The lattice defines polygonal opening units (26) when observed normal to a wall of the implantable endoluminal prosthesis (1). The diameter (Ø25) of wire (25) being at least 30 μm and at most 150 μm, the mean diameter (Ø27) of the inscribed circle (27) of the polygonal opening units (26) being at least 75 μm and at most 200 μm in fully expanded state. The braided framework (20) consists of at least 128 and at most 512 wires (25). The ratio (T1 / Ø25) of the thickness (T1) of a wall of said implantable endoluminal prosthesis (1) to the diameter (Ø25) of wire (25) is at least 3.0. In a fully expanded state, the surface coverage ratio (SCR) of said braided framework (20) is more than 50% and less than 90%.
Owner:FRID MIND TECH
5a-ANDROSTANE (ALKYL)-3b, 5, 6b-TRIOL INJECTION AND PREPARATION METHOD THEREFOR
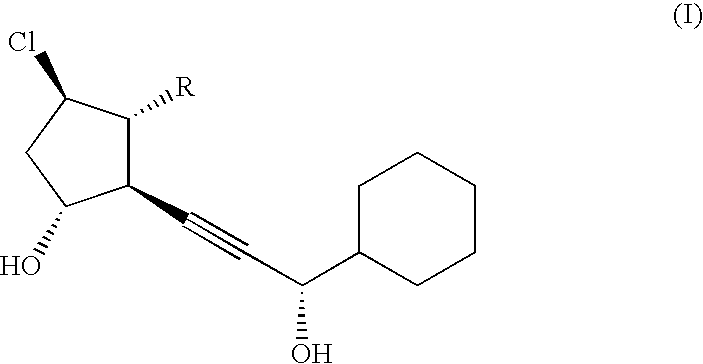
ActiveUS20130172307A1Improve solubilitySufficient efficacyOrganic active ingredientsNervous disorderDepyrogenationFreeze-drying
A 5α-androstane-3β,5,6β-triol injection and its preparation are disclosed. The injection uses hydroxypropyl-β-cyclodextrin as a solubilizing agent and the active ingredient is present at a weight ratio of 1-20:40-500 to the hydroxypropyl-β-cyclodextrin. The injection may also comprise, by weight, 1-100 parts of at least one isotonic adjusting agent, 0-200 parts of at least one freeze drying filler, and 0-2000 parts of at least one solvent. The preparation method comprises dissolving hydroxypropyl-β-cyclodextrin solution, 5α-androstane-3β,5,6β-triol and at least one additional soluble excipient in water for injection in sequence to obtain a raw injection solution, and subjecting the raw injection solution to decolorization, depyrogenation, filtration and sterilization to obtain the injection, Drying the filtrate yields a solid for injection.
Owner:GUANGZHOU CELLPROTEK PHARMA
Composition and method for treatment of metabolic disorders
ActiveUS20180228863A1Sufficient efficacySafely eliminated through excretionDispersion deliveryPeptide/protein ingredientsUnexpected therapeutic effectGastroenterology
The present invention provides compositions and methods for treatment of metabolic syndromes. Namely, the presently disclosed compositions and methods are provided for affecting the function of the gastrointestinal endocrine system in key regions of the gut, thereby, producing therapeutic effects on obesity, diabetes and other metabolic disorders. The compositions include components for forming luminal barriers within the gastrointestinal tract of a subject where the barrier is created in-situ via interaction of resident mucin with the mucin-interacting agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Composition and method for treatment of metabolic disorders
ActiveUS11090354B2Safely eliminated through excretionFast dissolutionDispersion deliveryPeptide/protein ingredientsTherapeutic effectEndocrine system
The present invention provides compositions and methods for treatment of metabolic syndromes. Namely, the presently disclosed compositions and methods are provided for affecting the function of the gastrointestinal endocrine system in key regions of the gut, thereby, producing therapeutic effects on obesity, diabetes and other metabolic disorders. The compositions include components for forming luminal barriers within the gastrointestinal tract of a subject where the barrier is created in-situ via interaction of resident mucin with the mucin-interacting agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pharmaceutical Compositions of Cholesteryl Ester Transfer Protein Inhibitor
InactiveUS20060211654A1Increase concentrationImprove solubilityAntibacterial agentsPowder deliveryCholesterylester transfer proteinCholesteryl ester
A pharmaceutical composition comprises a solid amorphous dispersion of a cholesteryl ester transfer protein inhibitor and a concentration-enhancing polymer.
Owner:BEND RES
Anti-Calculus Oral Compositions
ActiveUS20160008238A1Sufficient efficacyReduce concentrationCosmetic preparationsToilet preparationsPhosphate ionMedicine
The present invention provides an oral composition comprising: (a) from 1 wt % to 60 wt % of a calcium-containing abrasive; (b) a calcium-catching phosphate source in an amount sufficient to provide at least 100 mM of phosphate ions, PO43−; (c) no more than 20 wt % of a humectant; and (d) at least 5 wt % of water; wherein the oral composition has a pH from 8 to 11.
Owner:THE PROCTER & GAMBLE COMPANY
Color Mixing Optics for LED Illumination Device
ActiveUS20170318635A1Sufficient colorSufficient efficacyPlanar light sourcesLight source combinationsBeam anglePhotovoltaic detectors
Illumination devices with improved color mixing optics are disclosed herein for mixing the colors produced by a multi-colored LED emitter module to produce uniform color throughout the entire beam angle of the output light beam, along with smoother edges and improved center beam intensity. Embodiments disclosed herein include a unique arrangement of multi-color LEDs within an emitter module, a unique exit lens with different patterns of lenslets on opposing sides of the lens, and other associated optical features that thoroughly mix the different color components, and as such, provide uniform color across the output beam exiting the illumination device. Additional embodiments disclosed herein include a unique arrangement of photodetectors within the primary optics structure of the LED emitter module that ensure the optical feedback system properly measures the light produced by all similarly colored emission LEDs.
Owner:LUTRON TECH CO LLC
Novel synthetic glycolipid and use thereof
InactiveUS20130005669A1Intensely activateSufficient efficacyAntibacterial agentsBiocideNatural killer T cellCancer
A compound represented by the following formula (1):wherein R1 is an aldopyranose residue wherein the 6-hydroxyl group is optionally alkylated, R2 is a C1-26 hydrocarbon group optionally having substituent(s), R3 is a hydrogen atom or a C1-26 hydrocarbon group optionally having substituent(s), R4 is a C1-21 hydrocarbon group optionally having substituent(s), X is an oxygen atom or —CH2—, and Y is —CH2—, —CH(OH)— or —CH═CH—, or a salt thereof is useful for the prophylaxis or treatment of cancer or infection, since it can preferentially induce production of IFN-γ of NKT cells.
Owner:RIKEN
3D filter for prevention of stroke
The present invention relates to implantable endoluminal prosthesis for preventing stroke. The endoluminal prosthesis (1) consists of a braided framework (20) defining a cylindrical lumen (21) devoid of impermeable membrane. Said braided framework (20) is self-expandable comprising a plurality of layers (22, 23, 24) of wires (25) made of biocompatible material. Each layer forms a mesh. The meshes form a lattice with a plurality of wires (2) of given layers (22, 23, 24). The lattice defines polygonal opening units (26) when observed normal to a wall of the implantable endoluminal prosthesis (1). The diameter (Ø25) of wire (25) being at least 30 μm and at most 150 μm, the mean diameter (Ø27) of the inscribed circle (27) of the polygonal opening units (26) being at least 75 μm and at most 200 μm in fully expanded state. The braided framework (20) consists of at least 128 and at most 512 wires (25). The ratio (T1 / Ø25) of the thickness (T1) of a wall of said implantable endoluminal prosthesis (1) to the diameter (Ø25) of wire (25) is at least 3.0. In a fully expanded state, the surface coverage ratio (SCR) of said braided framework (20) is more than 50% and less than 90%.
Owner:FRID MIND TECH
Particle and preparation containing the particle
InactiveUS20090022915A1High in vivo deliverySufficient efficacyPowder deliveryEnvelopes/bags making machineryEthyl phosphateCarboxylate
The present invention relates to a particle having a mean particle size of 0.01 to 20 μm, containing tert-butyl (4R)-4-{[((1R)-2-[(1-benzylpiperidin-4-yl)amino]-1-{[(cyclohexylmethyl)thio]methyl}-2-oxoethyl)amino]carbonyl}-1,3-thiazolidine-3-carboxylate. A preparation containing the particle is excellent in pulmonary delivery through inhalation and is easy to handle because of excellent dispersibility of the particle, and thus the present compound can be used as a pulmonary preparation.
Owner:ONO PHARMA CO LTD
Method for surface treatment of a steel component by nitriding or nitrocarburising, oxidising and then impregnating
ActiveUS10774414B2Mitigate such drawbackIncrease resistanceSolid state diffusion coatingAlkaneIron nitride
Disclosed is a method for surface treatment of a steel component, providing high resistance to wear and corrosion, including nitriding or nitrocarburising to form a compound layer with a thickness of at least 8 micrometers made up of iron nitrides having phases ε and / or γ′, oxidizing to generate a layer of oxides with a thickness of 0.1-3 micrometers, and soaking in an impregnation bath during at least 5 minutes at room temperature, the bath being made up of at least 70 wt %, ±1%, of a solvent made up of a mixture of hydrocarbons formed by a C9 to C17 alkane fraction, 10 to 30 wt %, ±1%, of at least one paraffin oil formed by a C16 to C32 alkane fraction, and at least one additive such as a synthetic phenolic additive with a concentration of 0.01 to 3 wt %, ±0.1%.
Owner:H E F
Guanidine compounds, and use thereof as binding partners for 5-ht5 receptors
ActiveUS20110237589A1Sufficient efficacyEliminate side effectsBiocideNervous disorderDiseaseReceptor for activated C kinase 1
The present invention relates to guanidine compounds of the general formula Icorresponding enantiomeric, diastereomeric and / or tautomeric forms thereof as well as pharmaceutically acceptable salts thereof. The present compound further relates to the use of guanidine compounds as binding partners for 5-HT5 receptors for the treatment of diseases which are modulated by a 5-HT5 receptor activity, in particular for the treatment of neurodegenerative and neuropsychiatric disorders as well as the associated signs, symptoms and dysfunctions.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Support member and volatilizing apparatus
Owner:SUMITOMO CHEM CO LTD
External preparation comprising prostaglandin derivative
InactiveUS20100016427A1Good content uniformityGood storage stabilityBiocideElcosanoid active ingredientsCyclodextrinCompound (substance)
Provided is an external preparation comprising a complex containing: any one of a prostaglandin derivative, a pharmaceutically acceptable salt thereof and a hydrate thereof; and cyclodextrin, the prostaglandin derivative being represented by the following formula (I):(where R represents a group represented by the formula: —(CH2)4—S—CH2—CO2H, —(CH2)4—S—CH2—CO2CH3, —(CH2)4—C≡C—CO2H, —CH2—S—(CH2)2—S—CH2—CO2H, or —CH2—S— (CH2)4—CO2H), so that storage stability and content uniformity of the compound of the formula (I) are ensured.
Owner:TAISHO PHARMACEUTICAL CO LTD
5α-androstane (alkyl)-3β, 5, 6β-triol injection and preparation method therefor
ActiveUS9161985B2Improve solubilitySufficient efficacyOrganic active ingredientsNervous disorderDepyrogenationFreeze-drying
A 5α-androstane-3β,5,6β-triol injection and its preparation are disclosed. The injection uses hydroxypropyl-β-cyclodextrin as a solubilizing agent and the active ingredient is present at a weight ratio of 1-20:40-500 to the hydroxypropyl-β-cyclodextrin. The injection may also comprise, by weight, 1-100 parts of at least one isotonic adjusting agent, 0-200 parts of at least one freeze drying filler, and 0-2000 parts of at least one solvent. The preparation method comprises dissolving hydroxypropyl-β-cyclodextrin solution, 5α-androstane-3β,5,6β-triol and at least one additional soluble excipient in water for injection in sequence to obtain a raw injection solution, and subjecting the raw injection solution to decolorization, depyrogenation, filtration and sterilization to obtain the injection, Drying the filtrate yields a solid for injection.
Owner:GUANGZHOU CELLPROTEK PHARMA
Active halogen antimicrobial composition and method of use
ActiveUS9220272B2Sufficient efficacyImprove featuresBiocideInorganic active ingredientsDisinfectantElevated ph
Disclosed is an antimicrobial composition containing an active halogen-containing component having a source of an active halogen and having an elevated pH, which is mixed with an acidic component. An acidic component is added in an amount to the active halogen-containing component to reduce the pH of the antimicrobial composition. When the pH of the active halogen-containing composition is reduced, the resulting composition has been discovered to be effective as a disinfectant and, particularly, as a sporicide. Application methods for applying the composition are also described.
Owner:ARXADA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com