Patents
Literature
42results about "Nickel ammonia complexes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
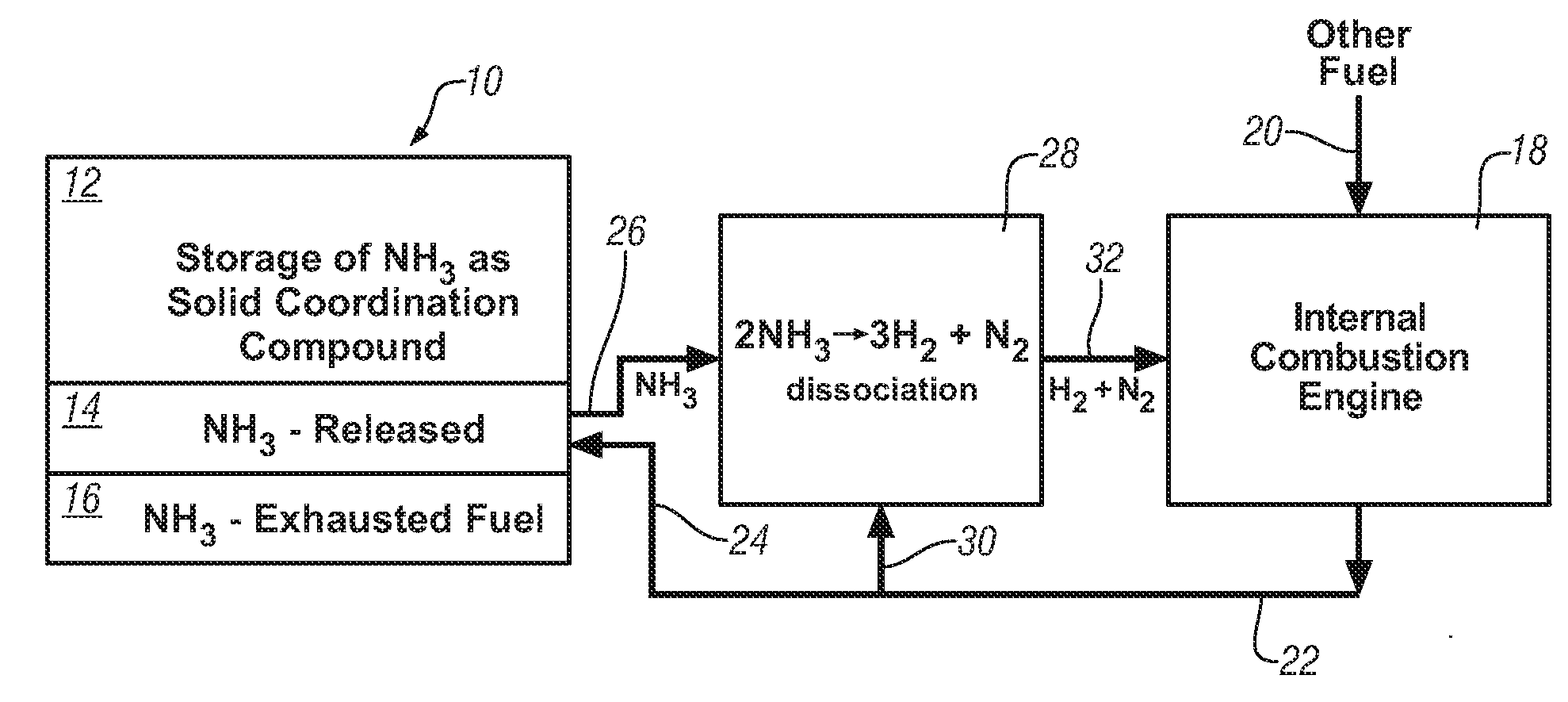
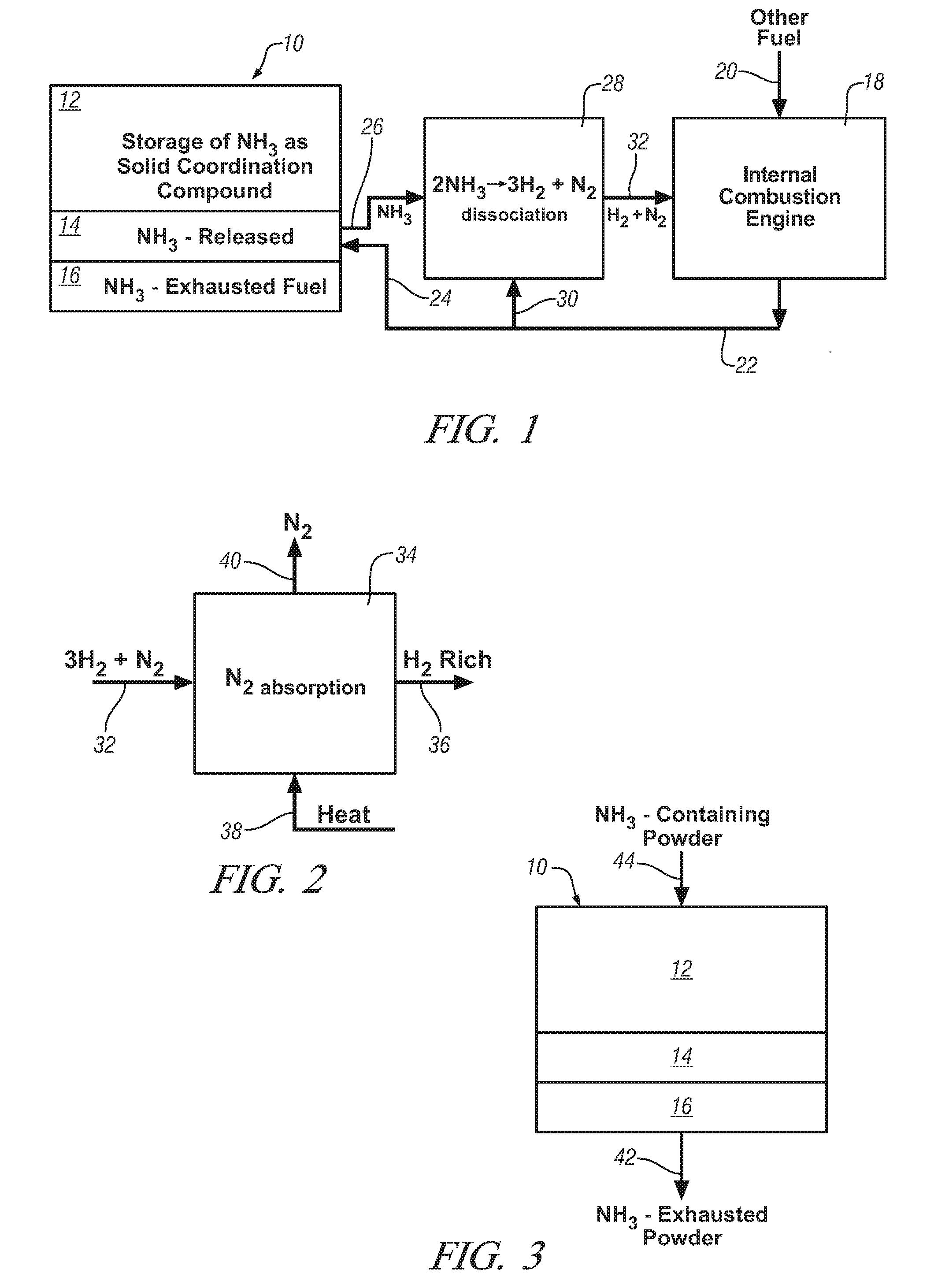
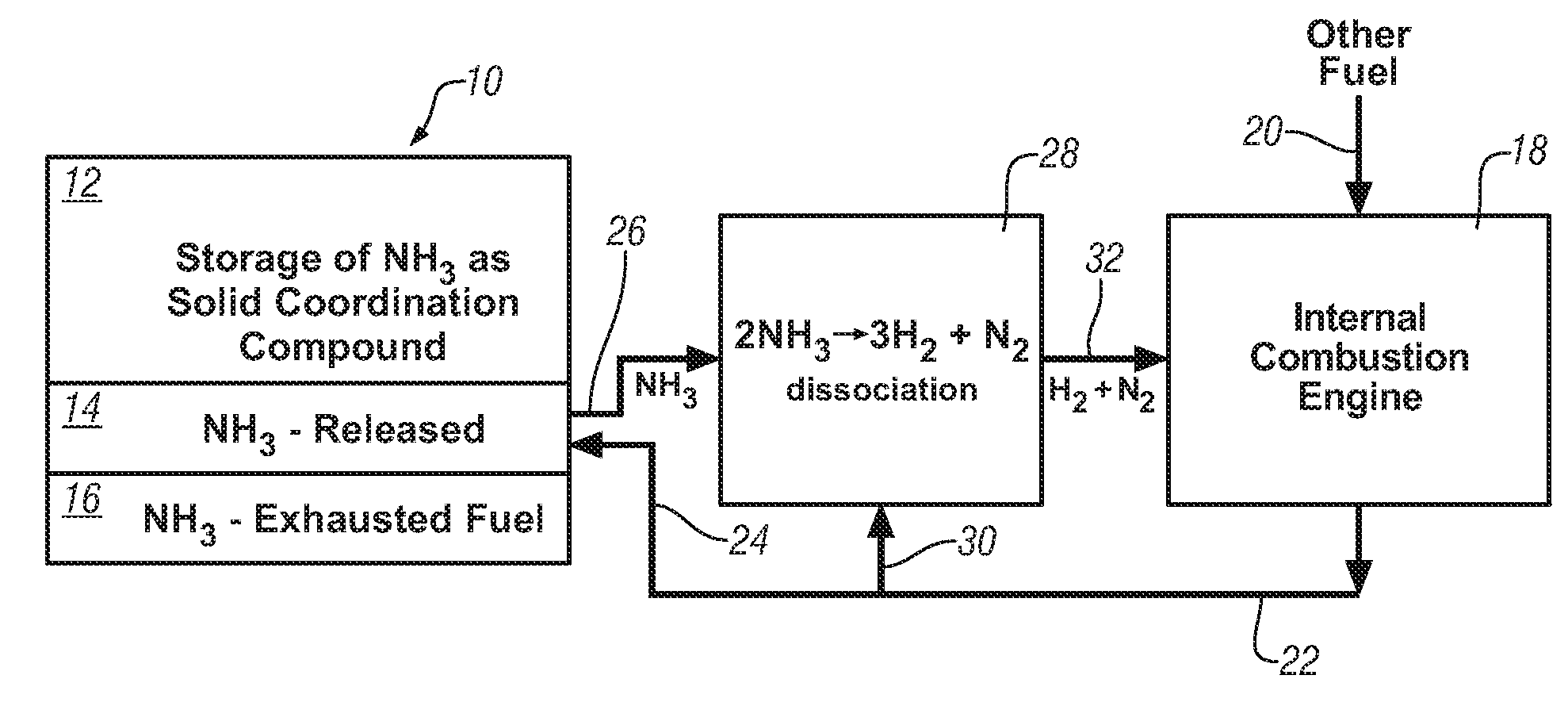
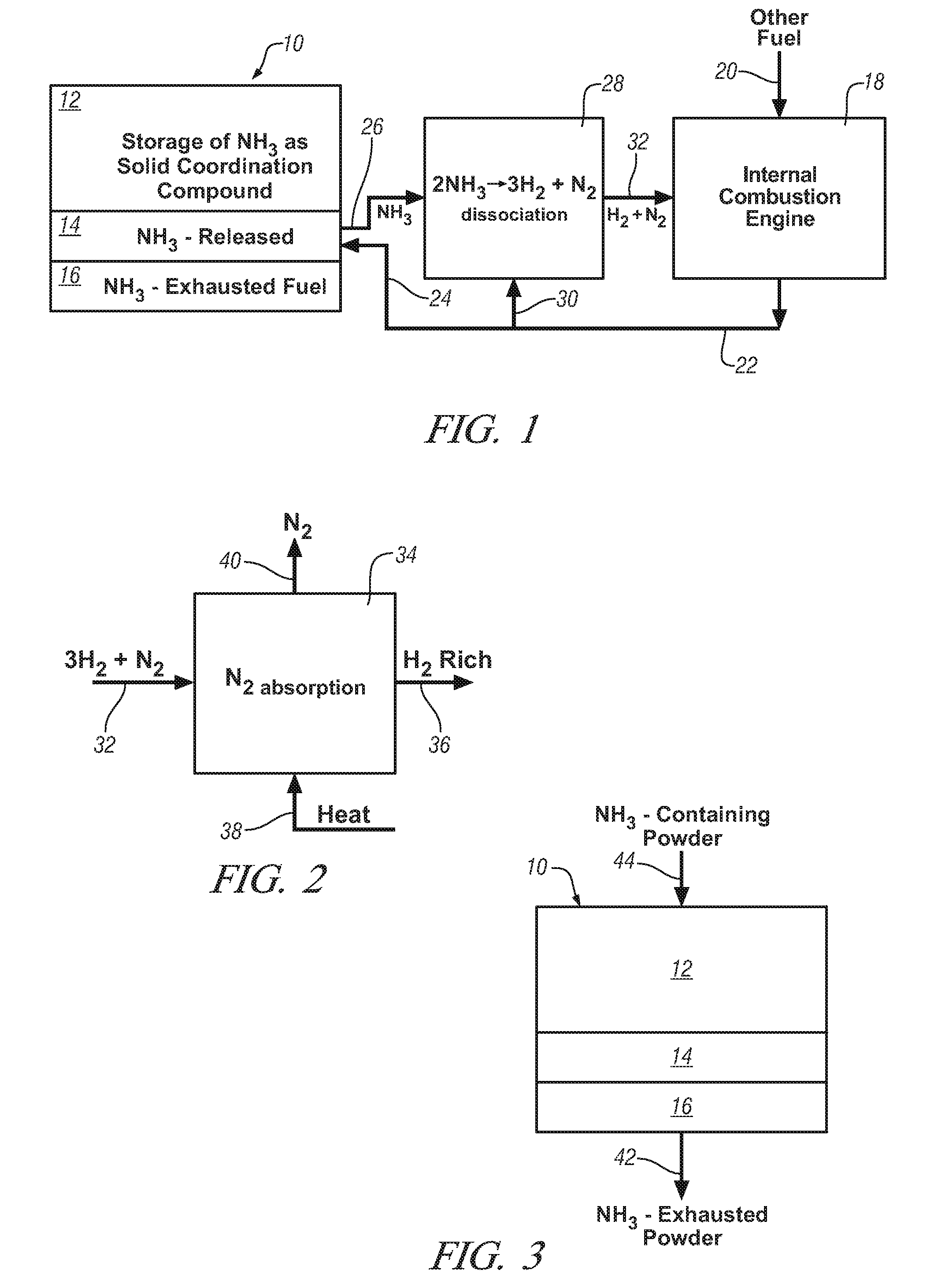
Ammonia storage for on-vehicle engine
InactiveUS20080241033A1Efficient and reversible processCobalt ammonia complexesCyanogen compoundsAmmonia storageExternal combustion engine
Ammonia is used as precursor source of hydrogen fuel in an on-vehicle internal combustion engine. Ammonia is stored as, for example, a ligand in an on-vehicle transition metal composition. Upon demand for hydrogen by the vehicle's engine control system, ammonia is expelled as a gas from some of the composition and the ammonia gas is dissociated into a mixture of hydrogen and nitrogen and delivered as a fuel-containing mixture to the engine. In a preferred embodiment, the hydrogen is used as a supplement to gasoline as a fuel for engine operation.
Owner:GM GLOBAL TECH OPERATIONS LLC
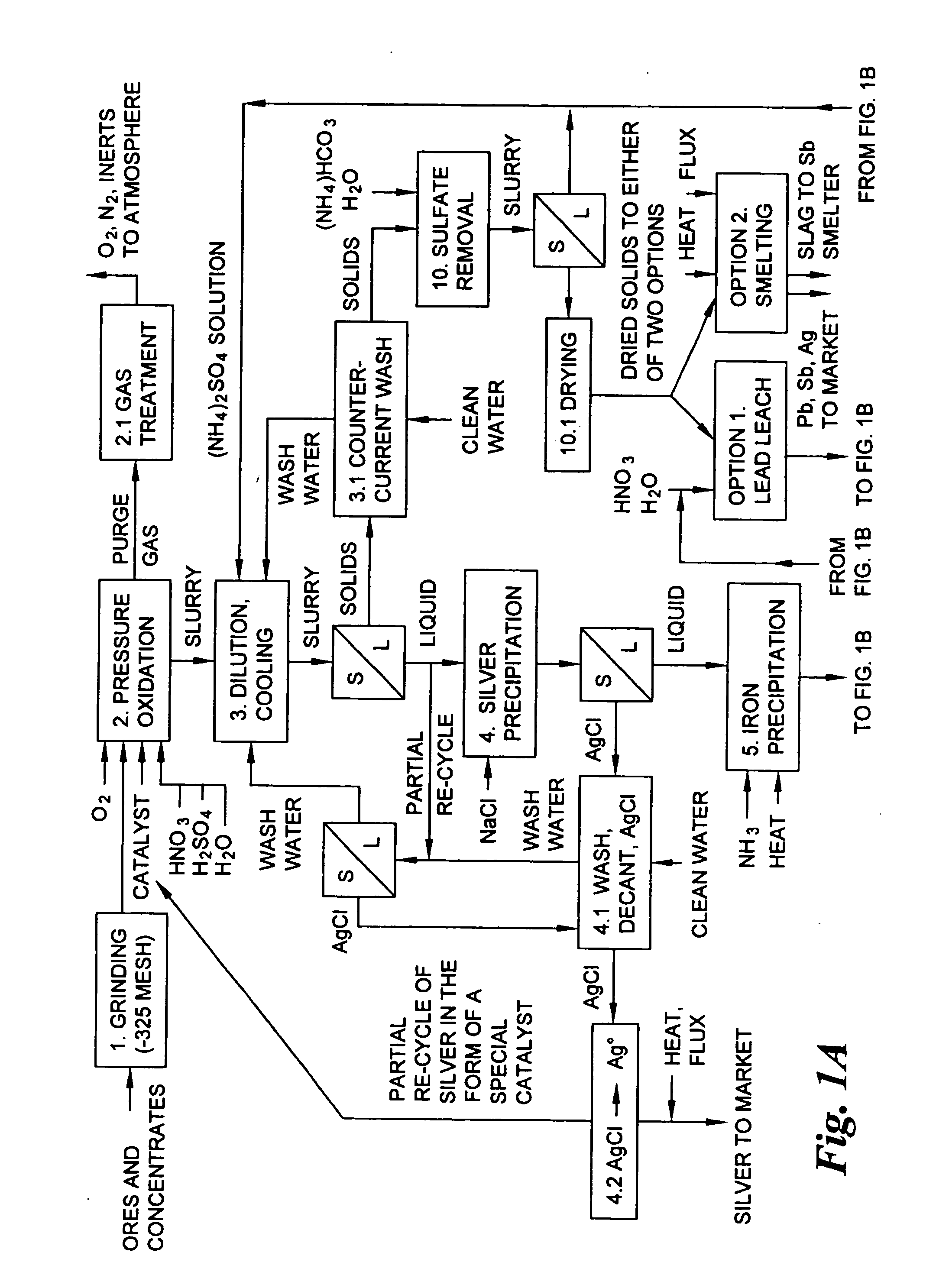
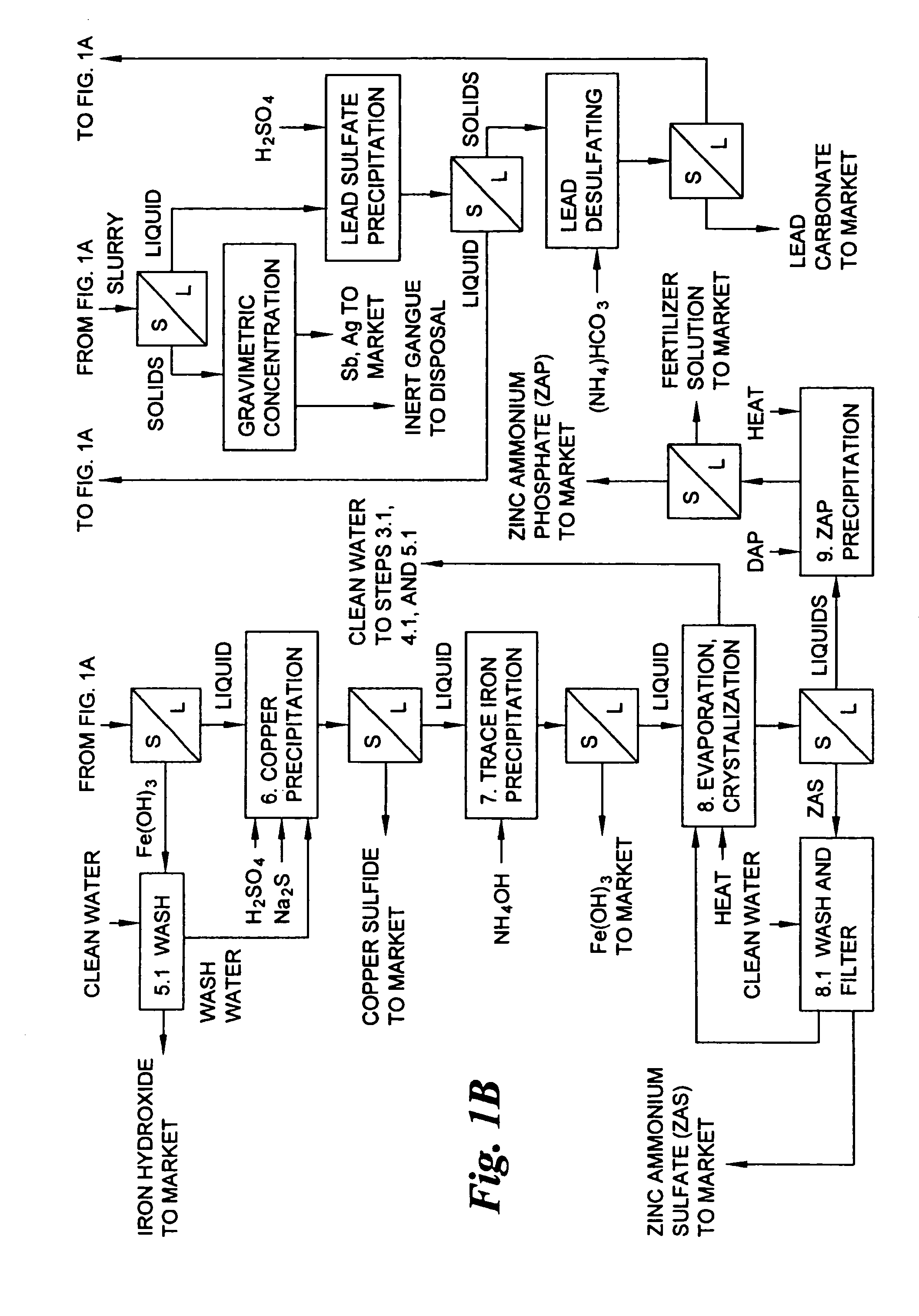
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS20070098609A1Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsMetallic sulfide
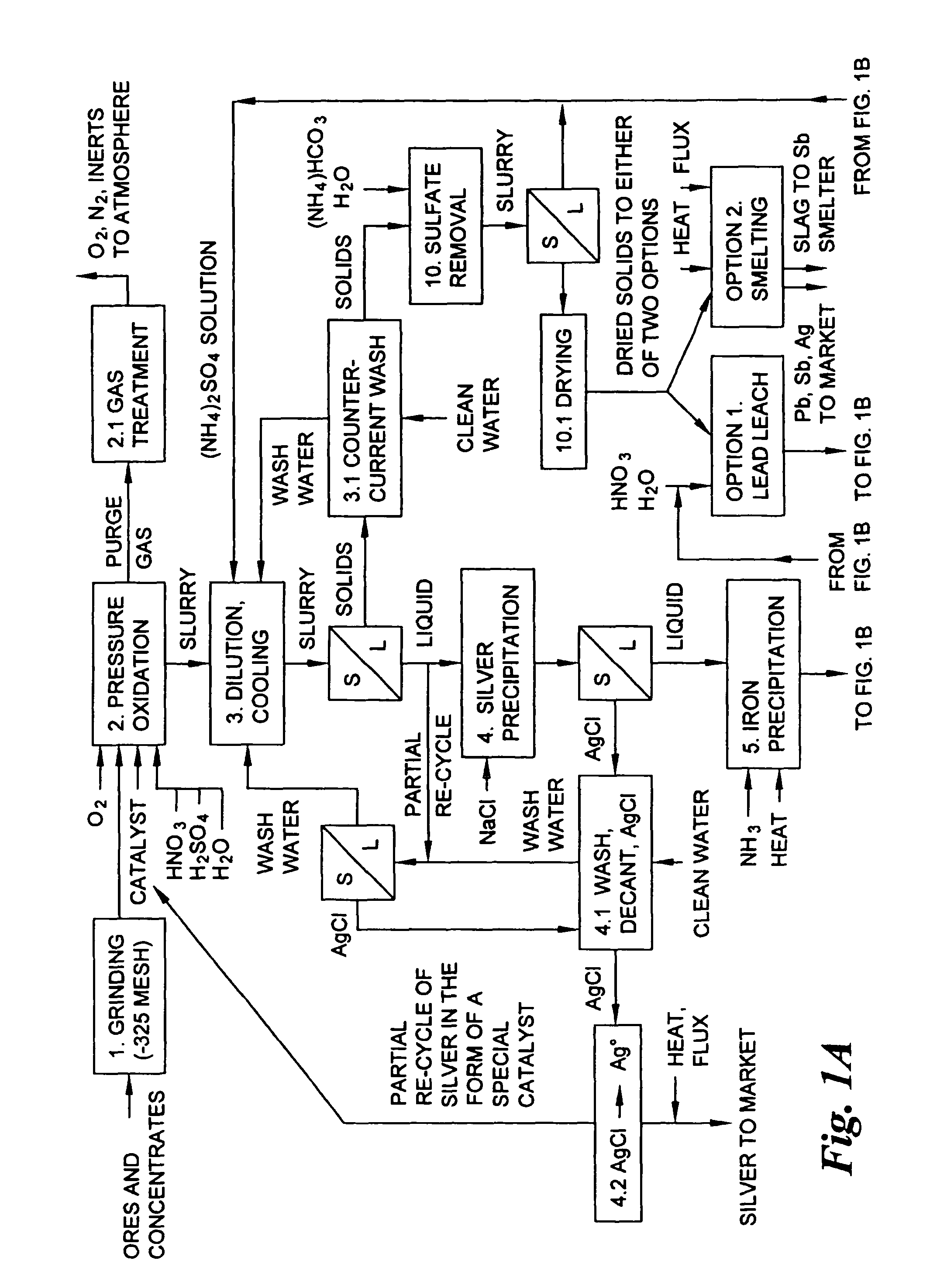
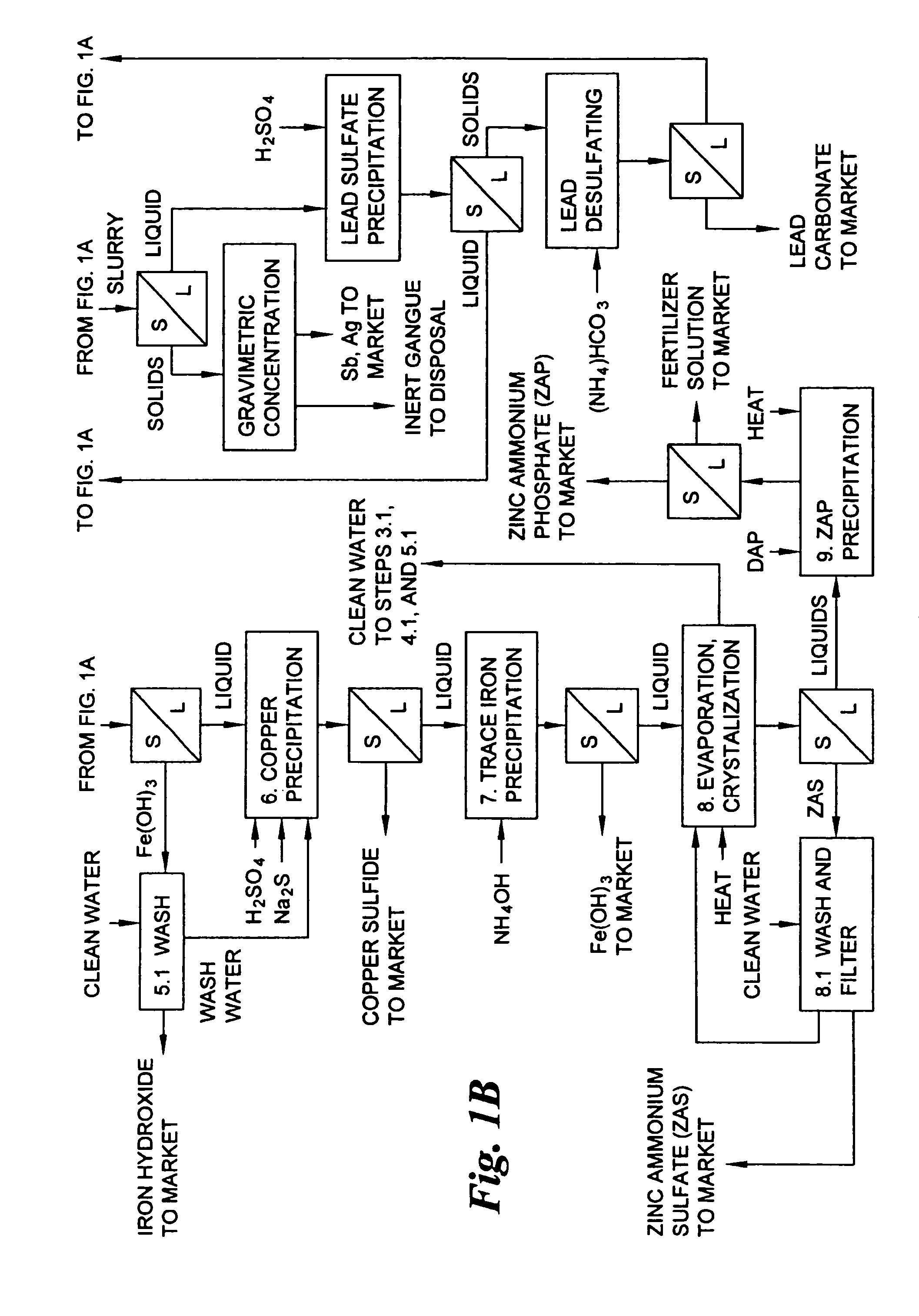
A hydrometallurgical process for the treatment of complex silver-bearing sulfide ores and concentrates that recovers substantially all silver, lead, antimony, zinc, copper and sulfur, along with the chemical reagents utilized during the process. Finely ground ores and concentrates are leached under heat and pressure with water, sulfuric acid, nitric acid, oxygen, and a catalyst, and are further treated to recover silver in the form of silver chloride; iron in the form of iron hydroxide; copper and all traces of soluble toxic metals as sulfides; zinc as zinc ammonium sulfate and specifically nitric acid, sulfuric acid, oxygen, ammonia, and ammonium compounds as valuable fertilizer products.
Owner:ROYAL SILVER PANAMA
In situ subsurface decontamination method
InactiveUS20020110509A1Control rateSimple methodCobalt ammonia complexesContaminated soil reclamationDivalent metalWater soluble
A method of decontaminating soil and ground water containing organic contaminants and divalent metal compounds. It comprises the steps of first treating such soils and ground water with an effective amount an aqueous solution containing a peroxide and a water soluble chelating agent for a time sufficient to have the water soluble chelating agent chelate at least one of the divalent metals of the divalent metal compounds present in the soil and ground water. Next, the chelated metals are brought into contact with the peroxide to catalytically convert the peroxide to an oxidizing agent. Finally, the last step is contacting the organic contaminants in the soil and ground water with the oxidizing agent to oxidize the organic contaminants to environmentally safe, non-toxic compounds.
Owner:LUNDY WILLIAM L
Spherical tricobalt tetraoxide and method of preparing the same
ActiveUS20100135897A1Stable structureMaintain good propertiesIron oxides/hydroxidesCell electrodesCobalt(II,III) oxideHigh activity
A method of preparation of spherical tricobalt tetraoxide, including at least oxidizing a bivalent cobalt salt in a wet environment and in the presence of a precipitant, a complexing agent, and an oxidant to yield spherical cobalt oxyhydroxide.cobalt hydroxide according to the following equation Co2++3OH−+O→CoOOH.Co(OH)2; oxidizing the spherical hydroxy cobalt oxyhydroxide.cobalt hydroxide to yield spherical tricobalt tetraoxide according to the following equation 6 CoOOH.Co(OH)2+O→4 Co3O4+9 H2O; and roasting the spherical tricobalt tetraoxide at low or intermediate temperature to yield a black powder. The method is easily practiced and suitable for mass production, and the resultant spherical tricobalt tetraoxide has stable structure, reliable properties, and high activity.
Owner:NINGBO RONBAY LITHIUM BATTERY MATERIAL CO LTD
Solid ammonia storage and delivery material
InactiveUS20090280047A1Cobalt ammonia complexesNitrogen compoundsAlkaline earth metalAmmonia storage
A solid ammonia storage and delivery material A solid ammonia storage material comprising: an ammonia absorbing salt, wherein the ammonia absorbing salt is an ionic salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkaline earth metals, and / or one or more transition metals, such as Mn, Fe, Co, Ni, Cu, and / or Zn, X is one or more anions, a is the number of cations per salt molecule, z is the number of anions per salt molecule, and ri is the coordination number of 2 to 12, wherein M is Mg provides a safe, light-weight and cheap compact storage for ammonia to be used in the automotive industry.
Owner:AMMINEX EMISSIONS TECH
In situ subsurface decontamination method
InactiveUS6843618B2Simple methodCobalt ammonia complexesSolid waste disposalDivalent metalWater soluble
A method of decontaminating soil and ground water containing organic contaminants and divalent metal compounds. It comprises the steps of first treating such soils and ground water with an effective amount an aqueous solution containing a peroxide and a water soluble chelating agent for a time sufficient to have the water soluble chelating agent chelate at least one of the divalent metals of the divalent metal compounds present in the soil and ground water. Next, the chelated metals are brought into contact with the peroxide to catalytically convert the peroxide to an oxidizing agent. Finally, the last step is contacting the organic contaminants in the soil and ground water with the oxidizing agent to oxidize the organic contaminants to environmentally safe, non-toxic compounds.
Owner:LUNDY WILLIAM L
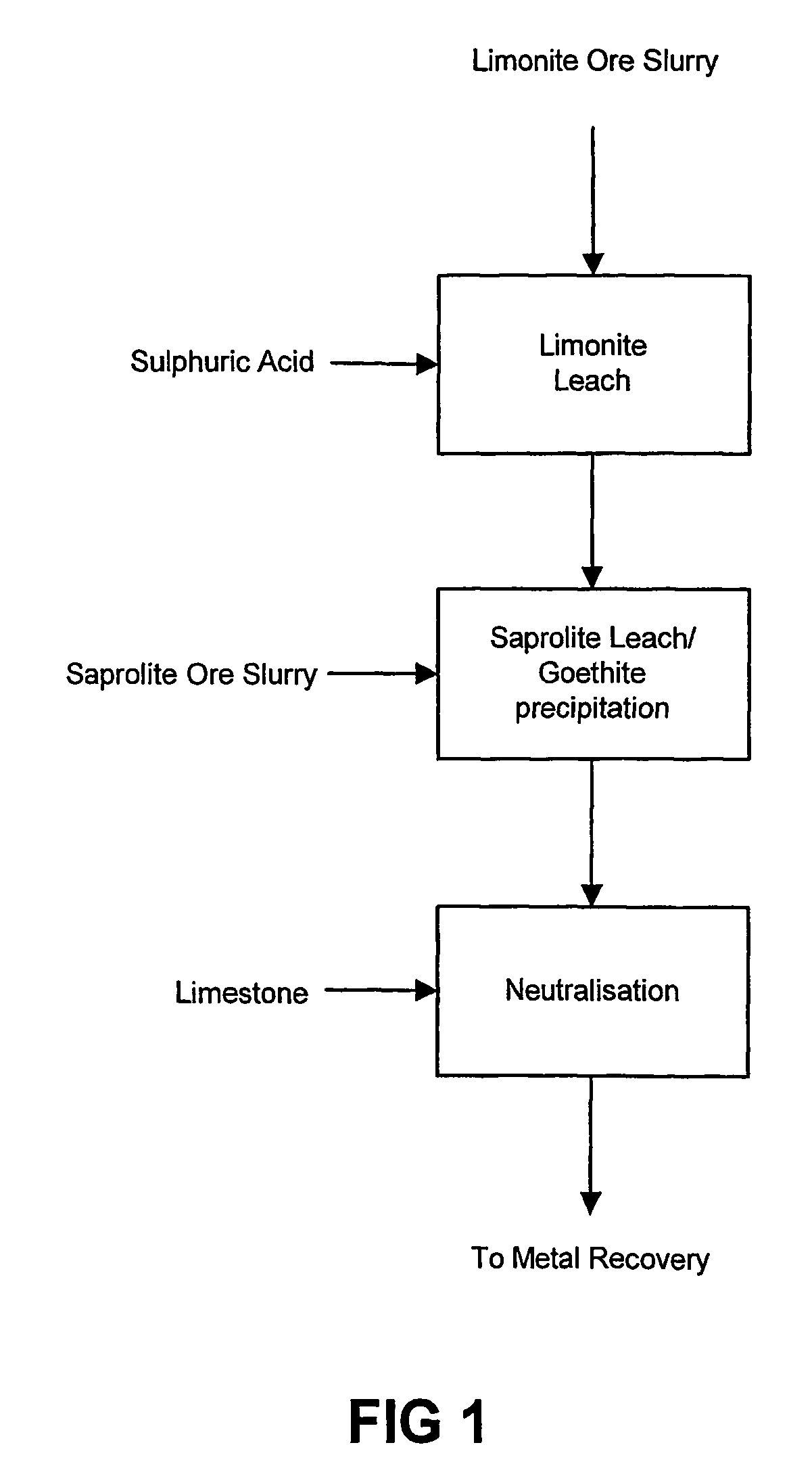
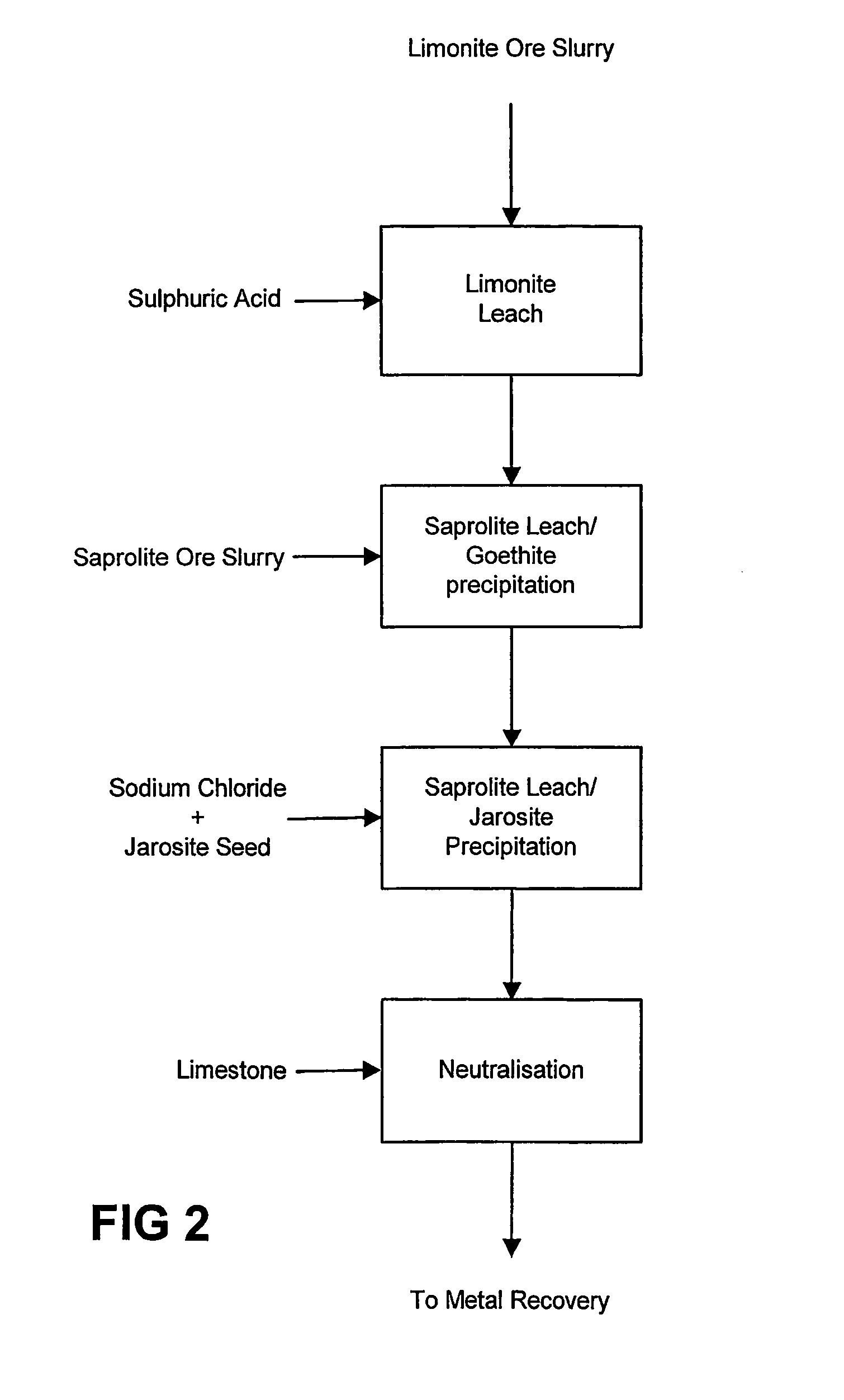
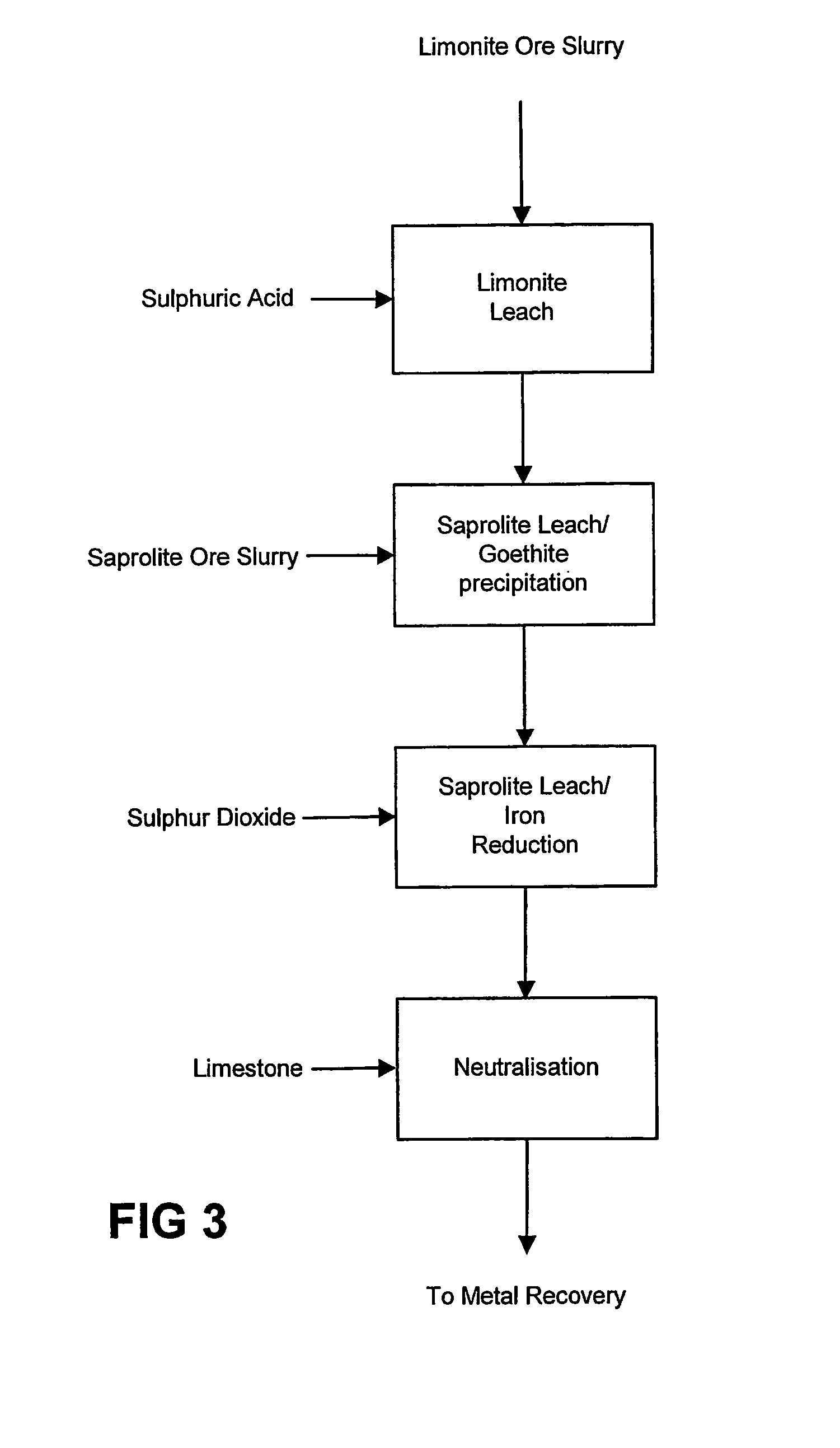
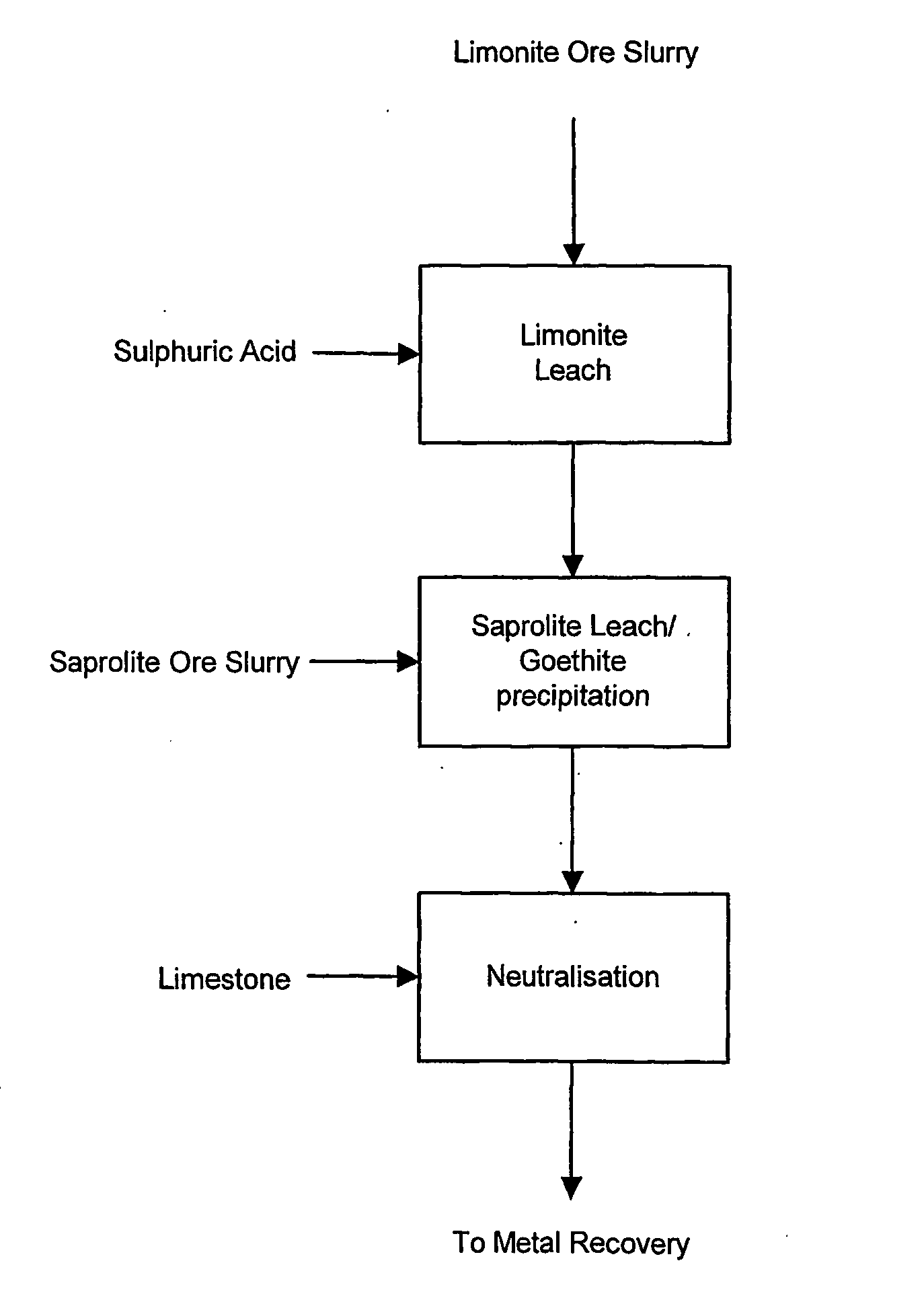
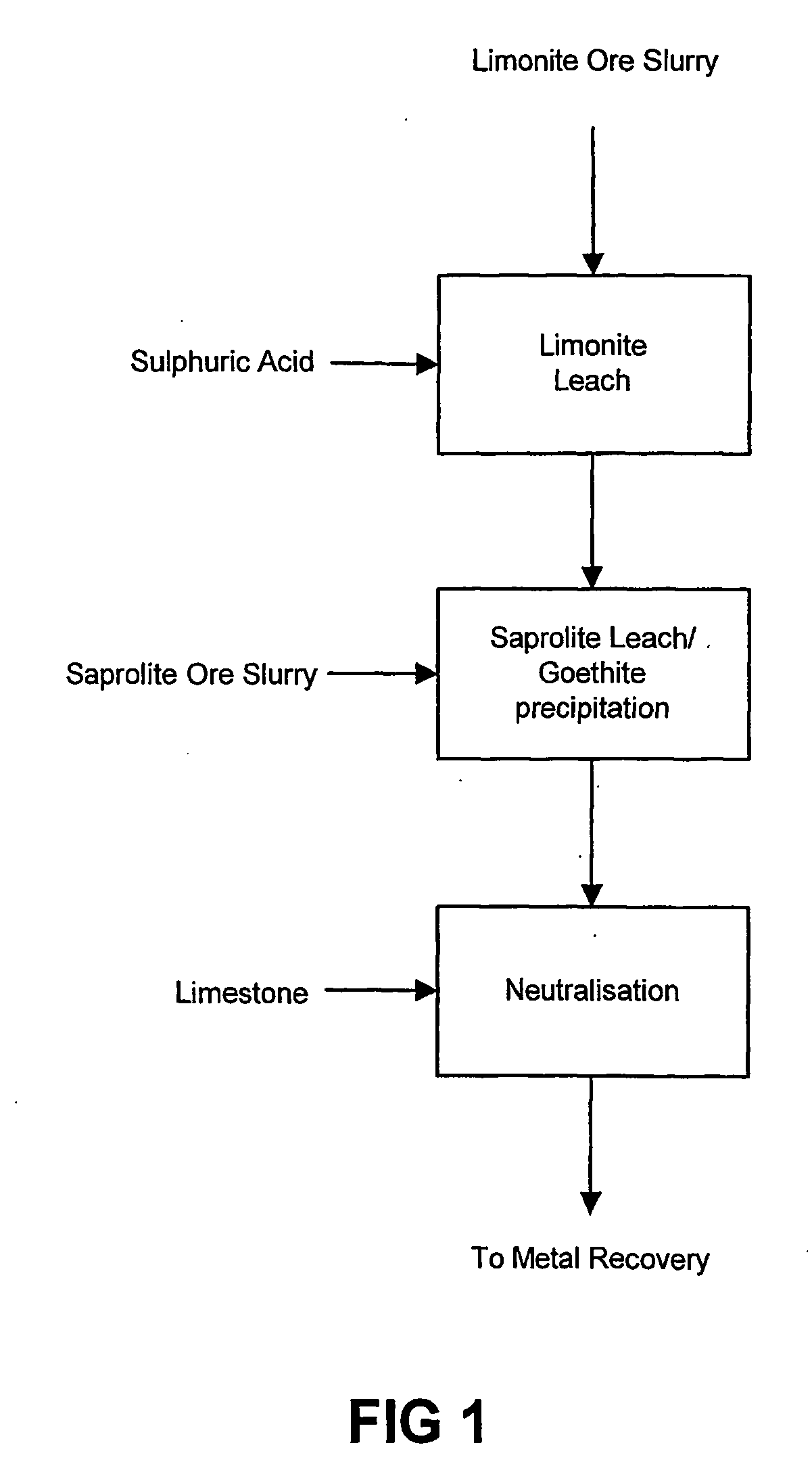
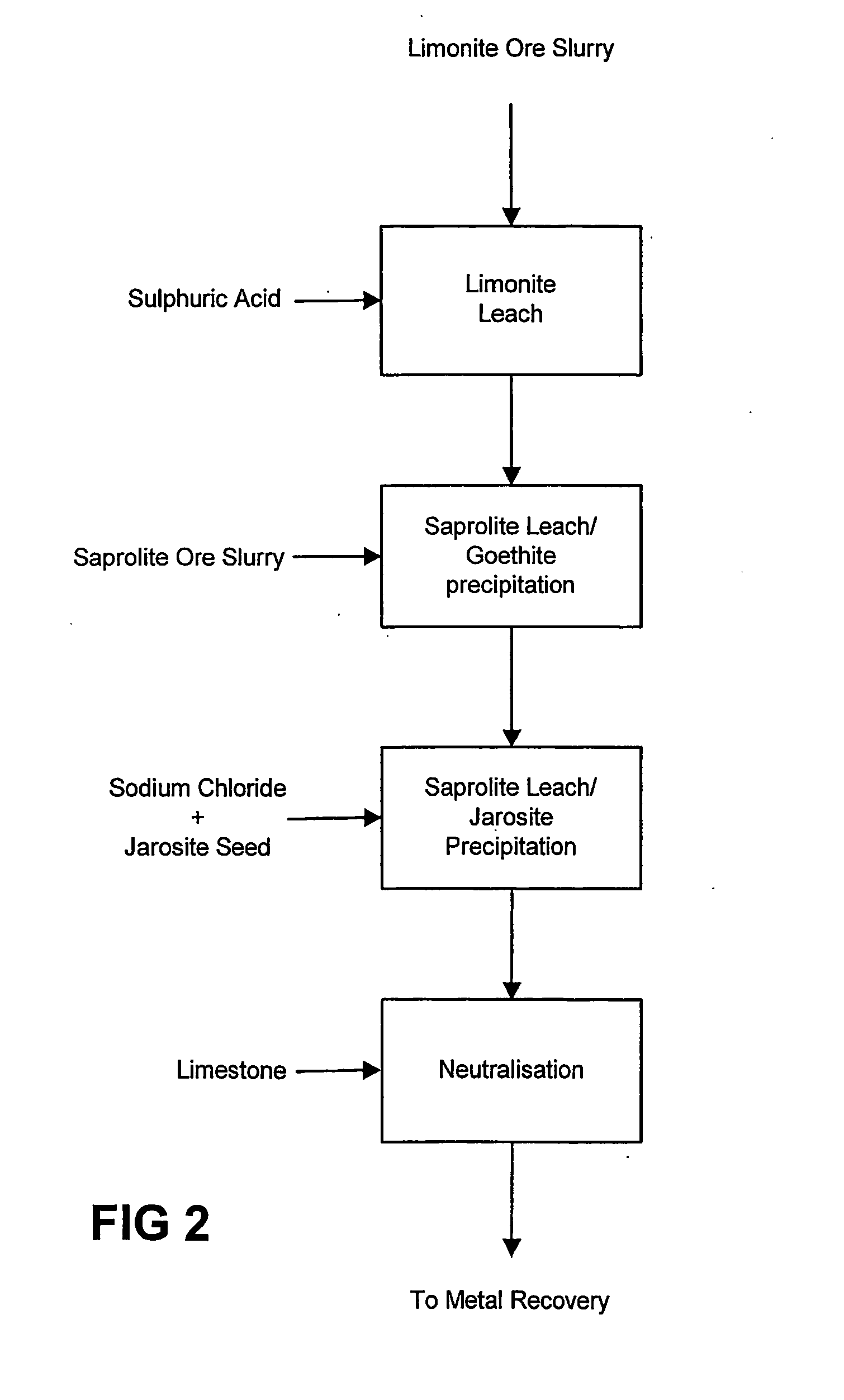
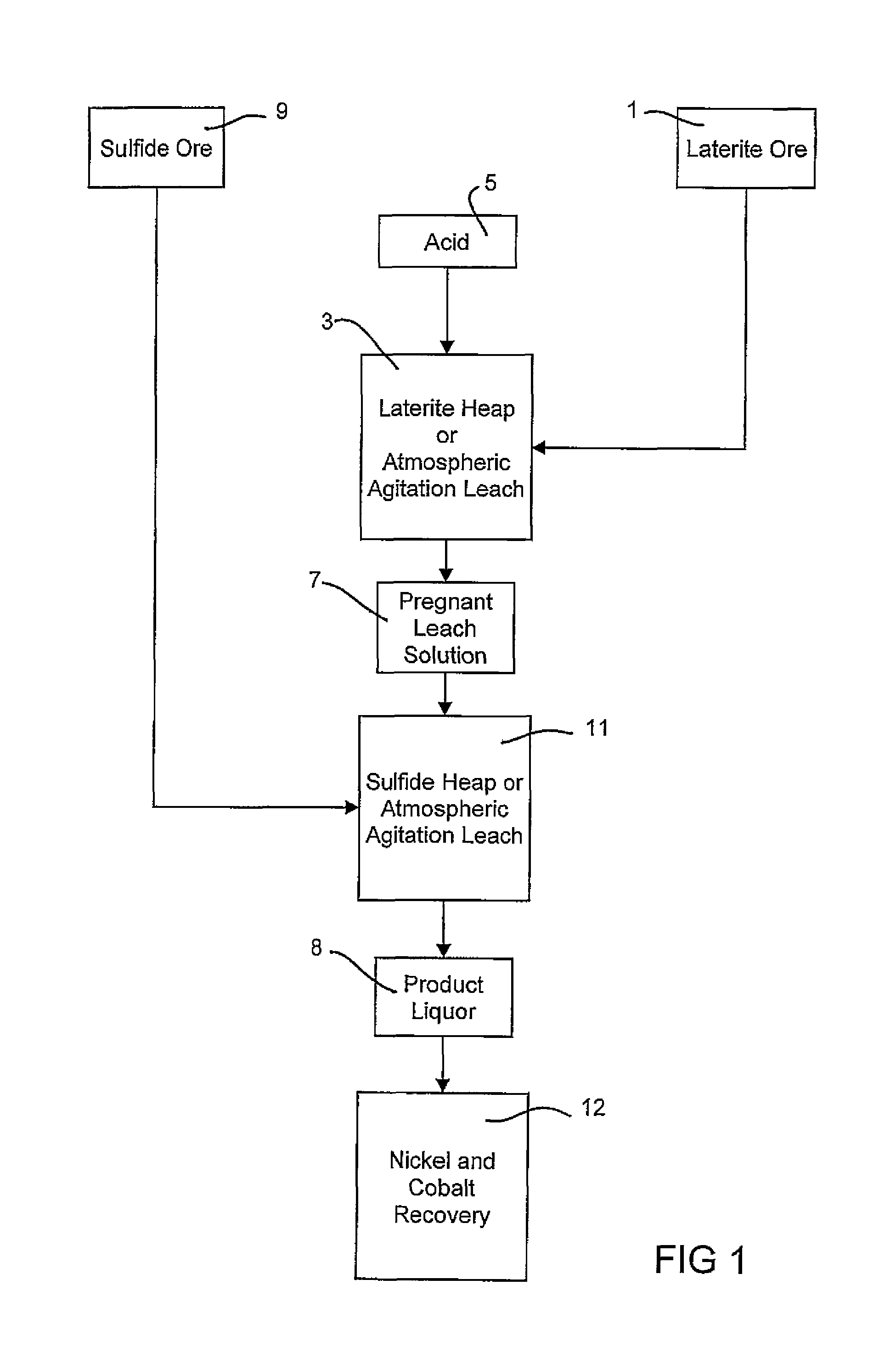
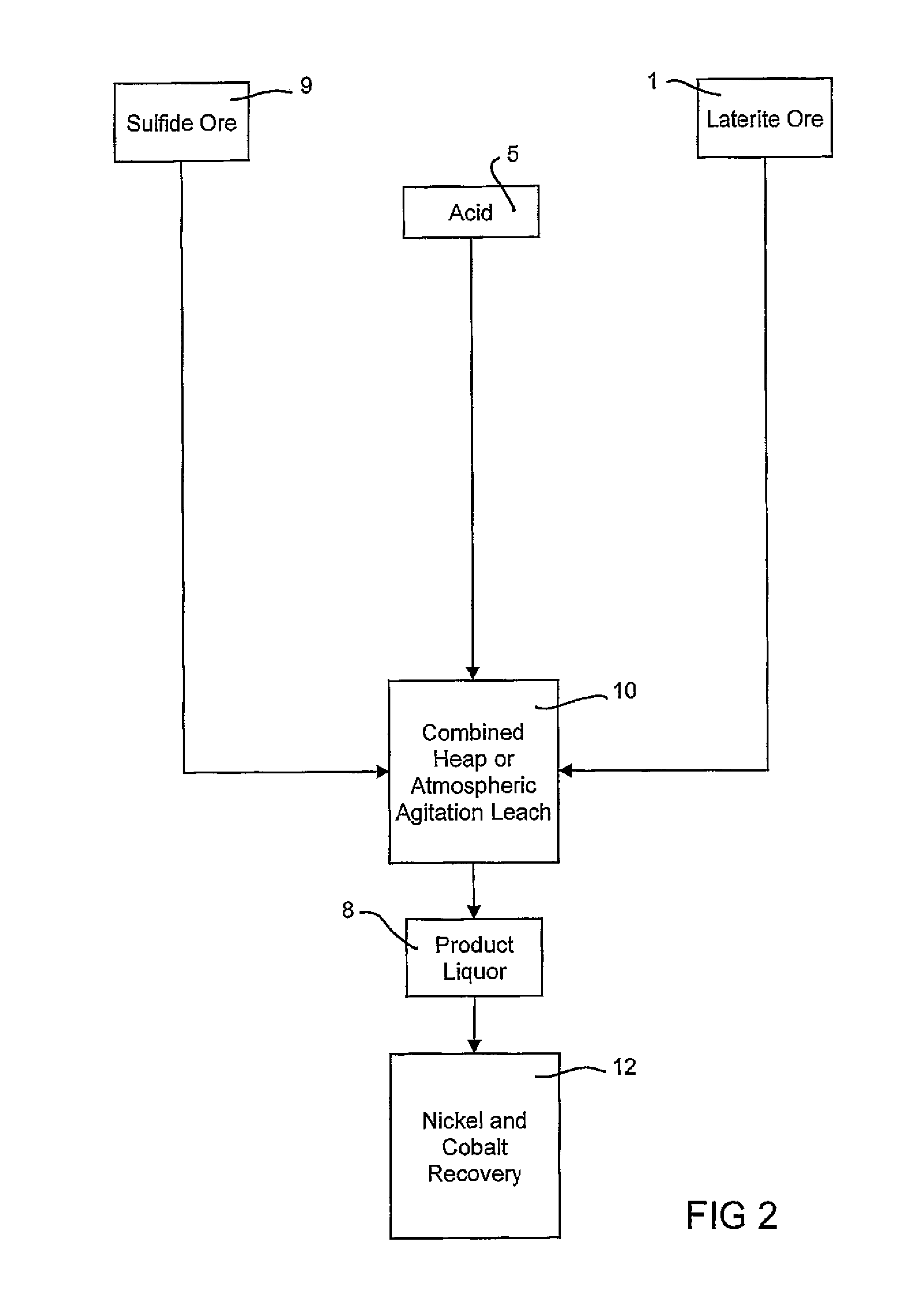
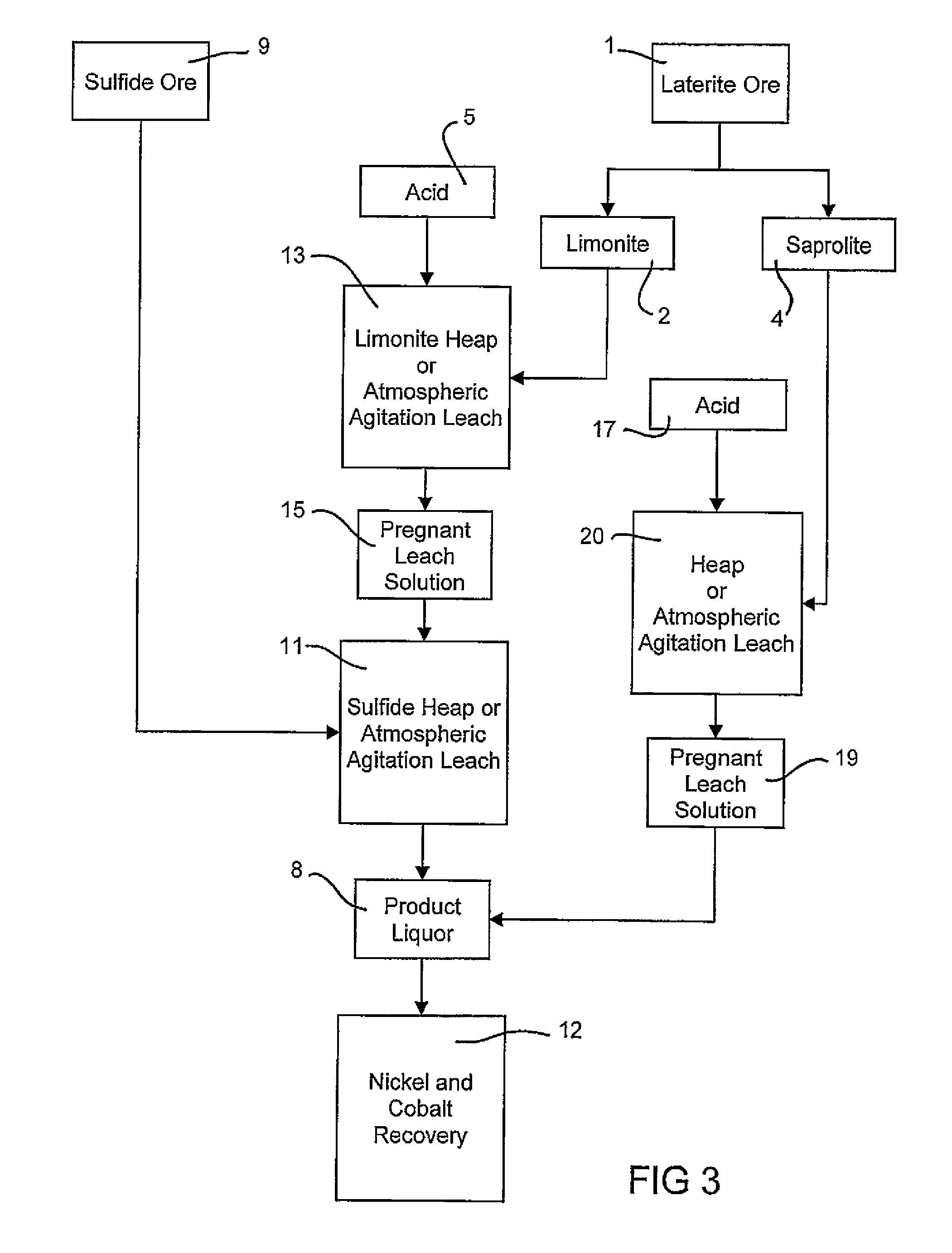
Atmospheric pressure leach process for lateritic nickel ore
An atmospheric leach process in the recovery of nickel and cobalt from lateritic ores, said processing including the steps of: a) separating the lateritic ore into a low magnesium containing ore fraction, and a high magnesium containing ore fraction by selective mining or post mining classification; b) separately slurrying the separated ore fractions; c) leaching the low magnesium containing ore fraction with concentrated sulphuric acid as a primary leach step; and d) introducing the high magnesium content ore slurry following substantial completion of the primary leach step and precipitating iron as goethite or another low sulphate containing form of iron oxide or iron hydroxide, wherein sulphuric acid released during iron precipitation is used to leach the high magnesium ore fraction as a secondary leach step.
Owner:CERRO MATOSO
Atmospheric pressure leach process for lateritic nickel ore
An atmospheric leach process in the recovery of nickel and cobalt from lateritic ores, said processing including the steps of: a) separating the lateritic ore into a low magnesium containing ore fraction, and a high magnesium containing ore fraction by selective mining or post mining classification; b) separately slurrying the separated ore fractions; c) leaching the low magnesium containing ore fraction with concentrated sulphuric acid as a primary leach step; and d) introducing the high magnesium content ore slurry following substantial completion of the primary leach step and precipitating iron as goethite or another low sulphate containing form of iron oxide or iron hydroxide, wherein sulphuric acid released during iron precipitation is used to leach the high magnesium ore fraction as a secondary leach step.
Owner:CERRO MATOSO
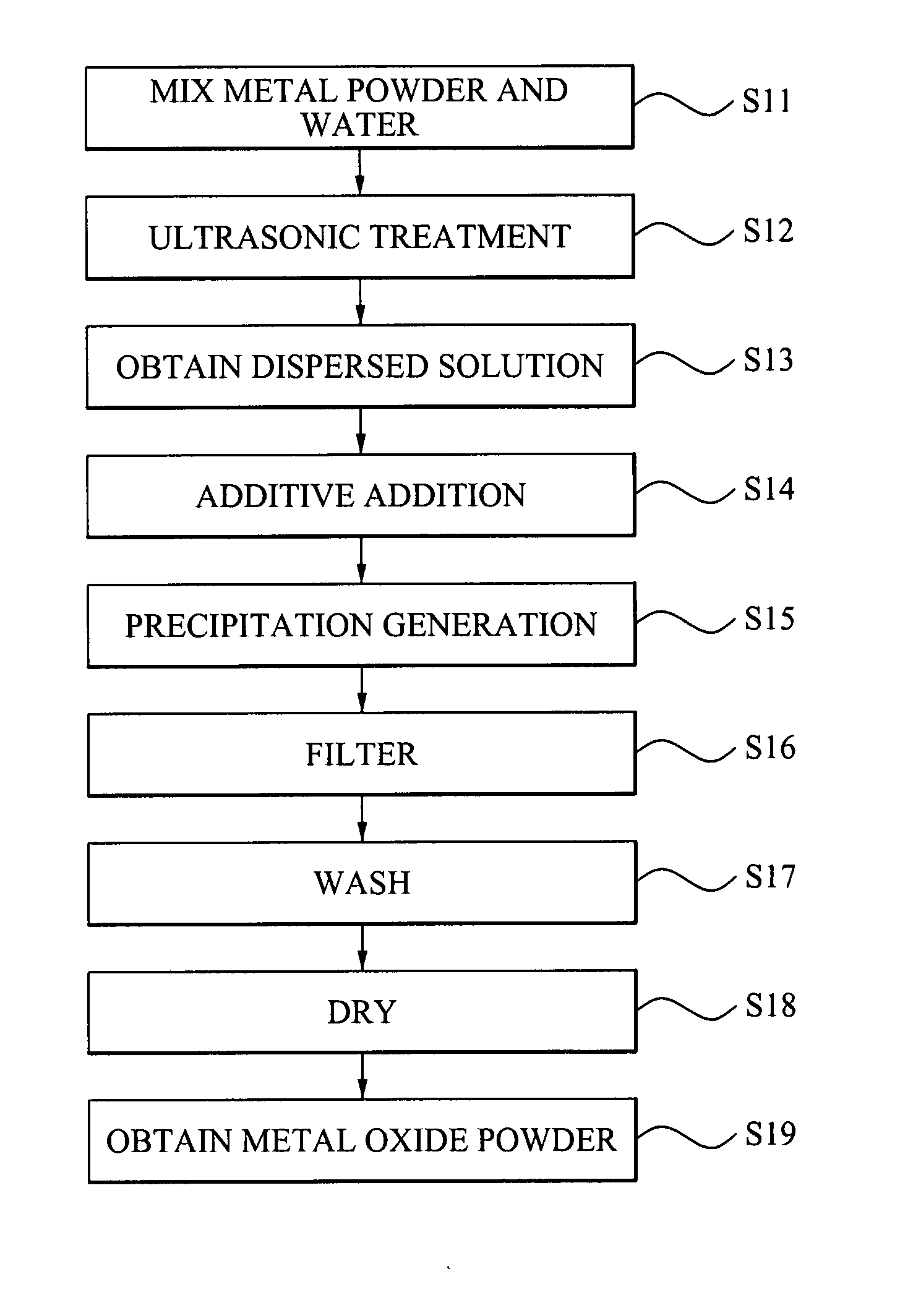
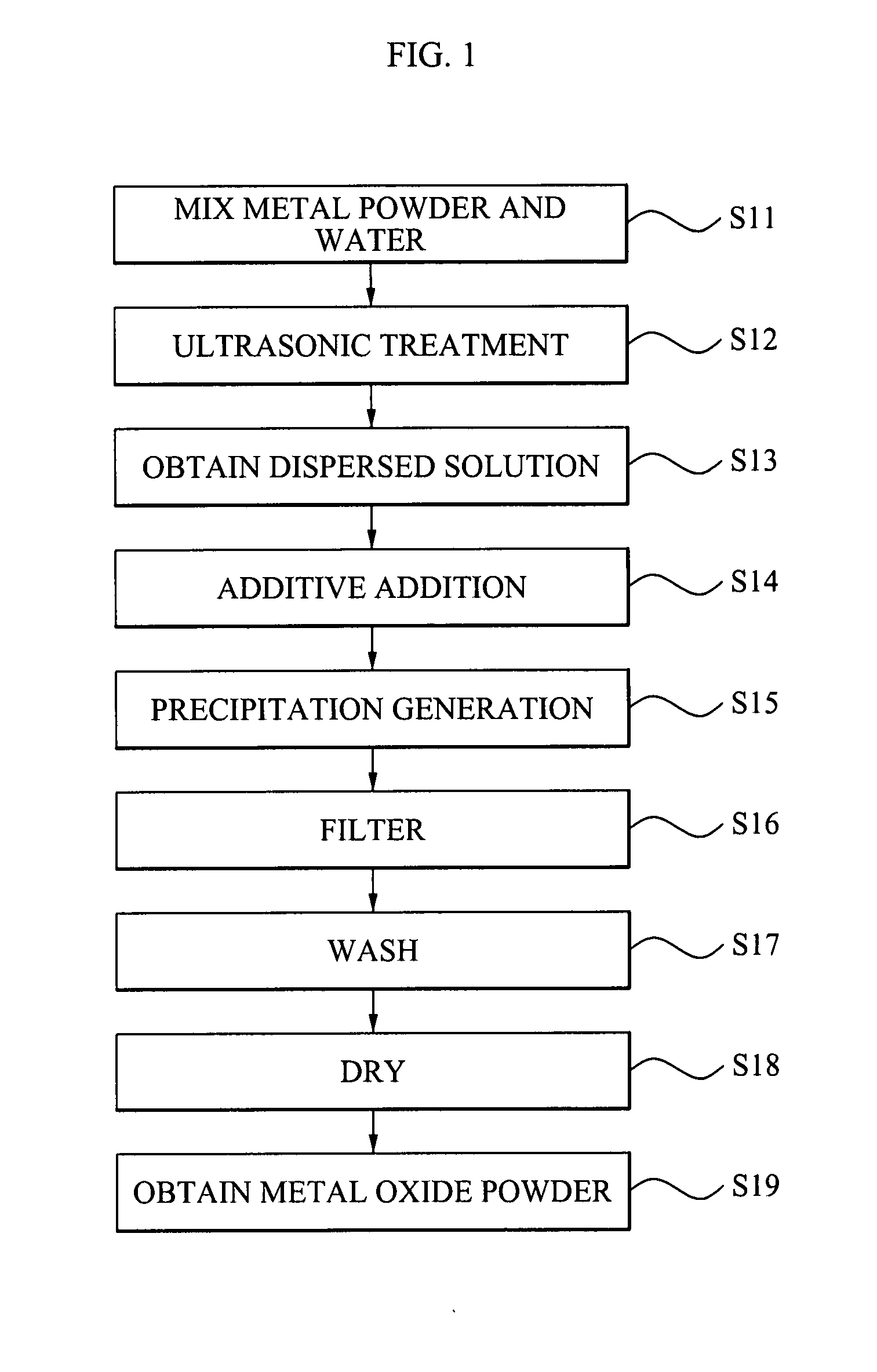
Metal oxide nano powder and manufacturing method of the same
Disclosed is a method of manufacturing a metal oxide nano powder comprising preparing a first dispersed solution by adding a nano-sized metal powder to water and dispersing the metal powder within the water, performing a hydration reaction of the first dispersed solution at a temperature of about 30 to about 70° C. to generate a precipitation, and filtering and drying the precipitation to prepare a metal oxide powder. Also, disclosed is a metal oxide nano powder manufactured by the method described above, and having any one of a bar-form, a cube-form, and a fiber-form.
Owner:RESINTECH CO LTD
Extraction of uranium from wet-process phosphoric acid
ActiveUS20100028226A1Lower iron levelsReduced valencyCobalt ammonia complexesPhotography auxillary processesFiltrationIon exchange
A process for the extraction of uranium compounds from wet-process phosphoric acid includes lowering the iron concentration of the wet-process phosphoric acid and reducing the valency of any remaining ferric iron in the wet-process phosphoric acid to ferrous iron, and then extracting uranium compounds from the wet-process phosphoric acid. The process can include separating a side stream from a feed stream of wet-process phosphoric acid, wherein the side stream has a greater concentration of the uranium compounds than the feed stream by filtration. Extracting uranium compounds from the wet-process phosphoric acid can be by ion exchange process or by solvent extraction.
Owner:URTEK
Nickel-contained materials abstraction and type-reverting production method of nickel salt
InactiveCN101139112AReduce demandRelieve production pressureNickel halidesNickel ammonia complexesNickel saltSulfamic acid
An extraction and transformation method to produce nickel salt with material containing nickel relates to an extraction and transformation technology with nickel salt medium solution in the preparation process of nickel salt products, in particular to the preparation of nickelous chloride and nickel sulfamic acid. The present invention is characterized in that the nickel salt solution after purified is extracted by P204 extracting agent and the main metal nickel is led into an organic phase and then the organic phase is back extracted by various acid solution. The nickel is transferred into outer nickel salt solution and then produced to other nickel salt products after the procedures of depth decontamination, evaporating and crystallization. The present invention can enlarge the material range of nickel salt products, greatly improve working environment and save production cost.
Owner:JINCHUAN GROUP LIMITED
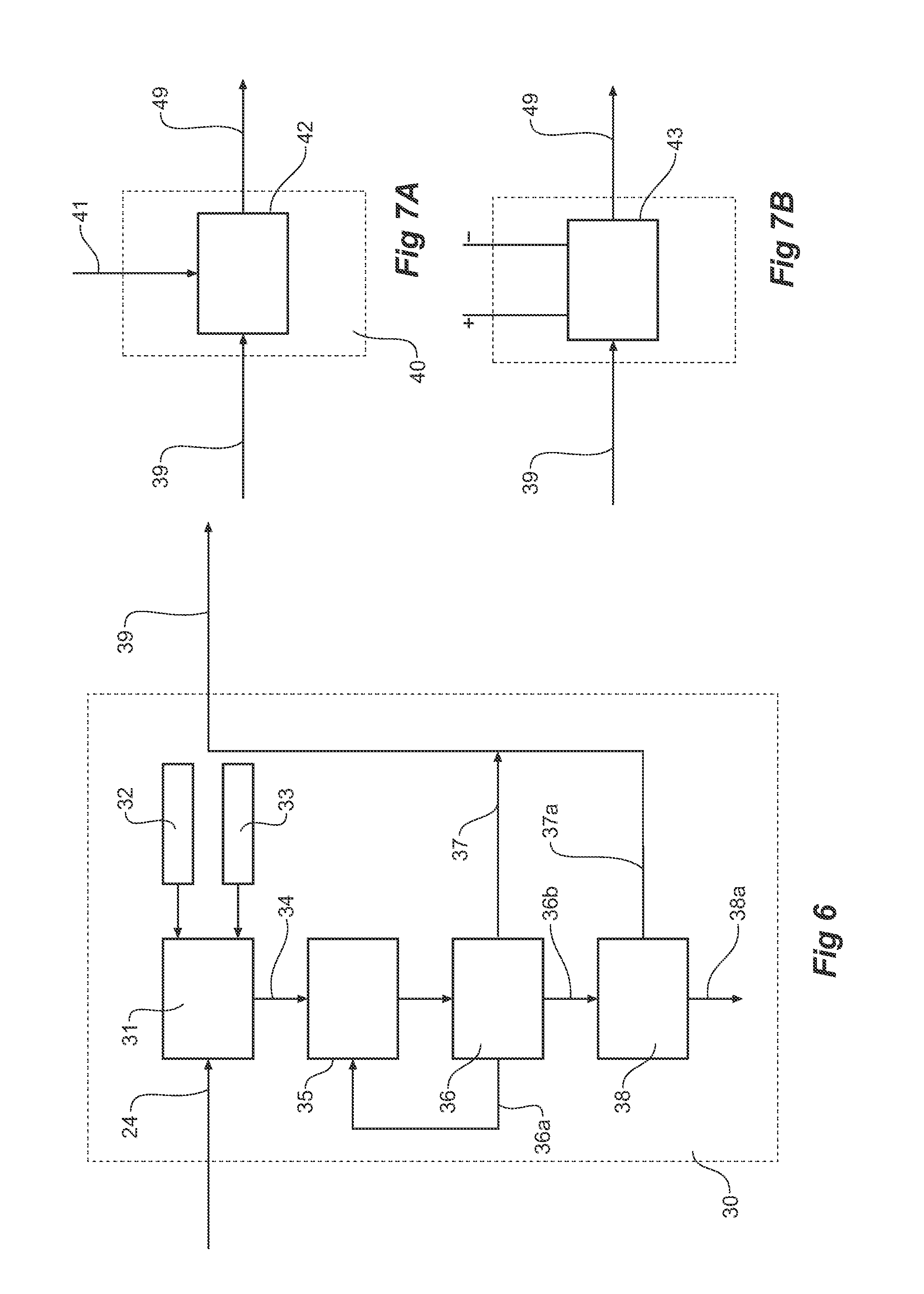
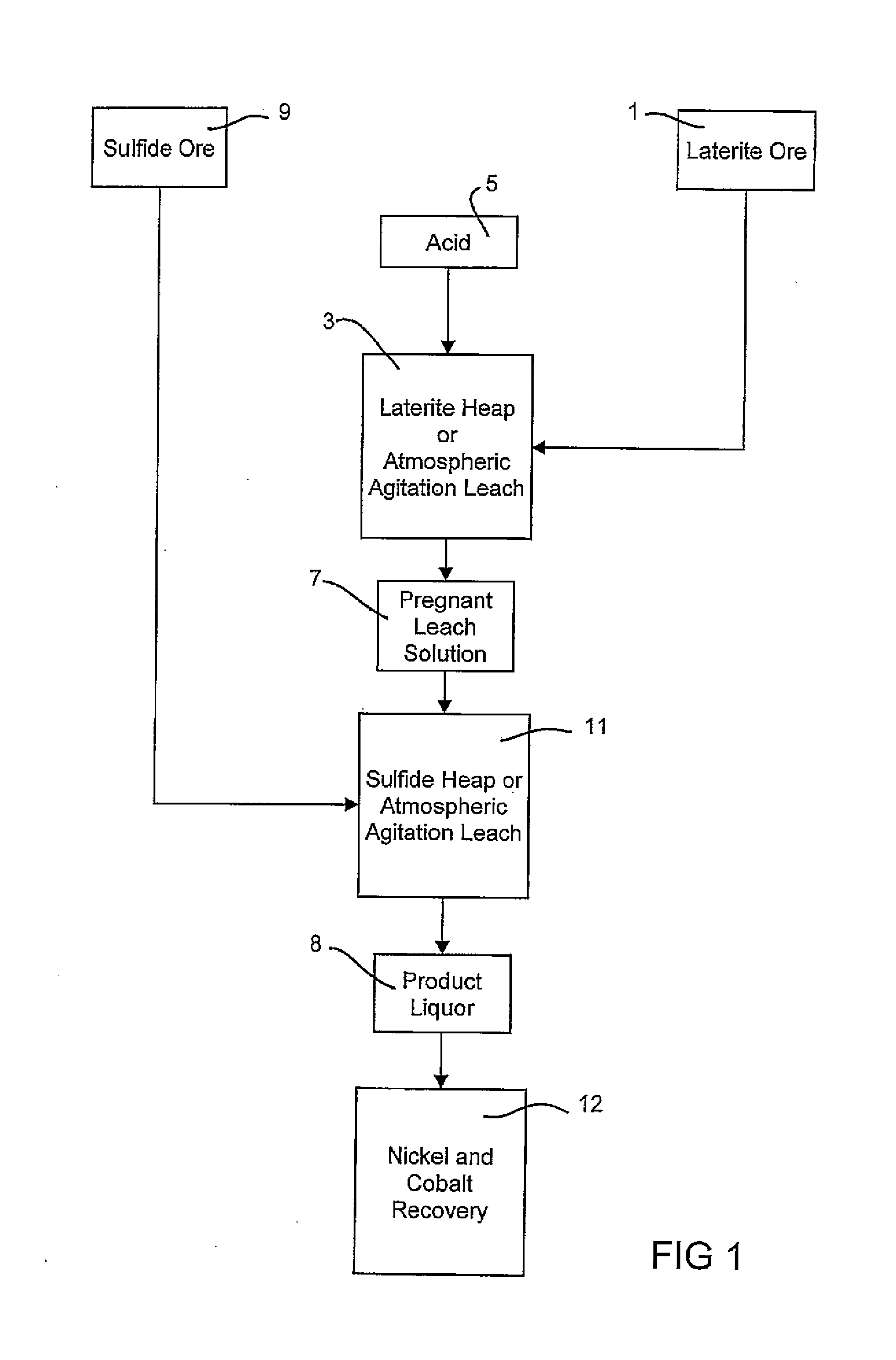
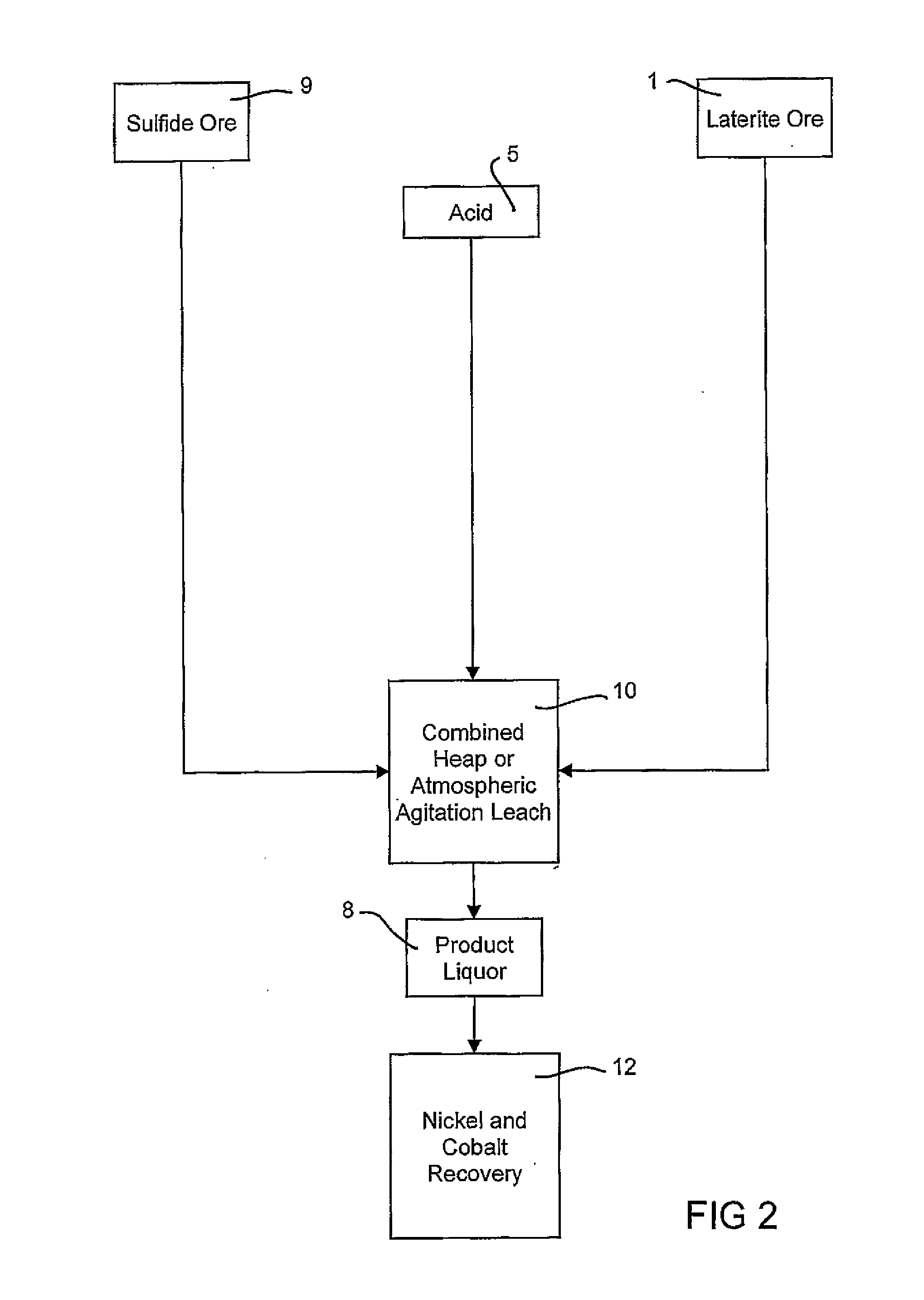
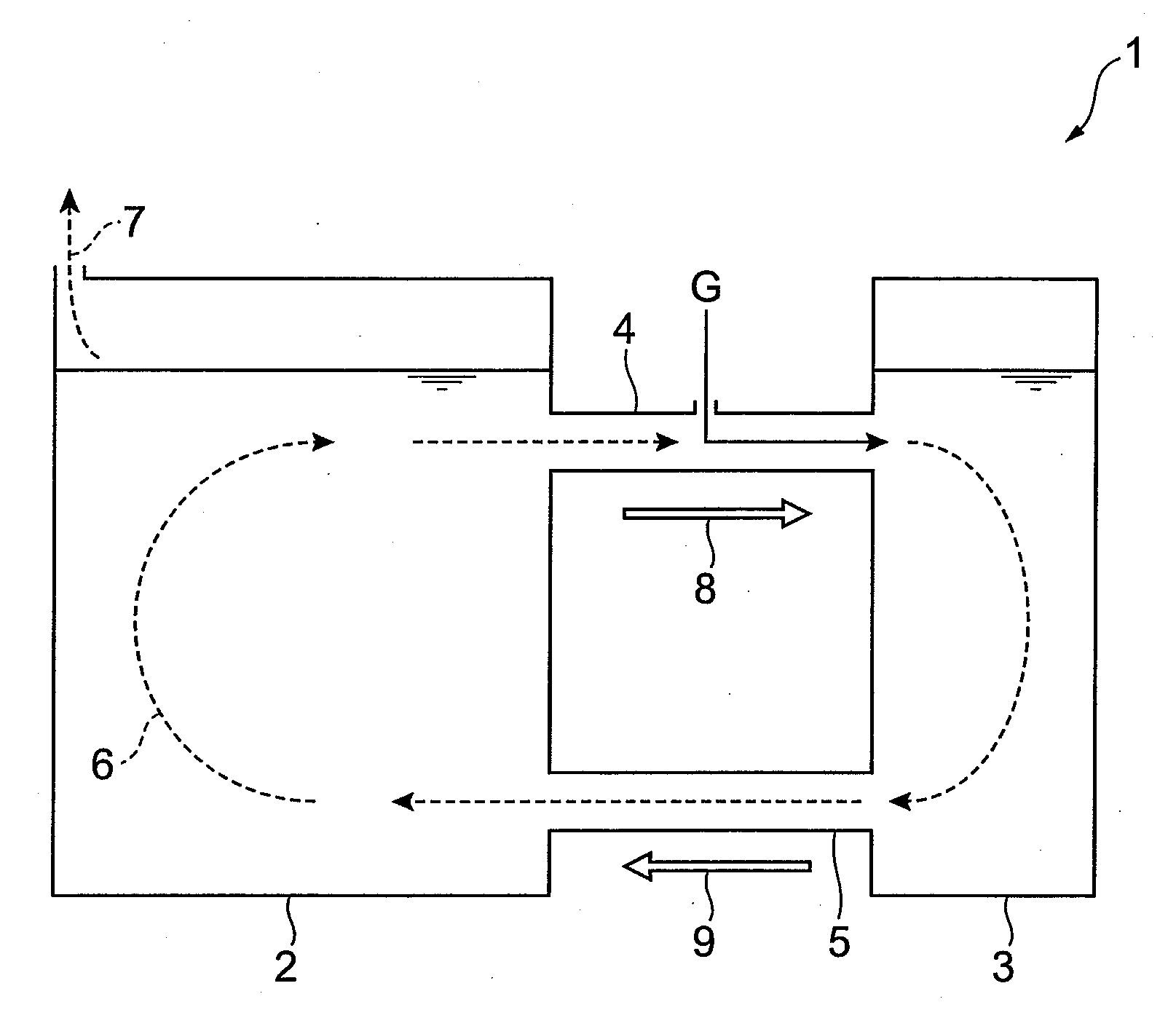
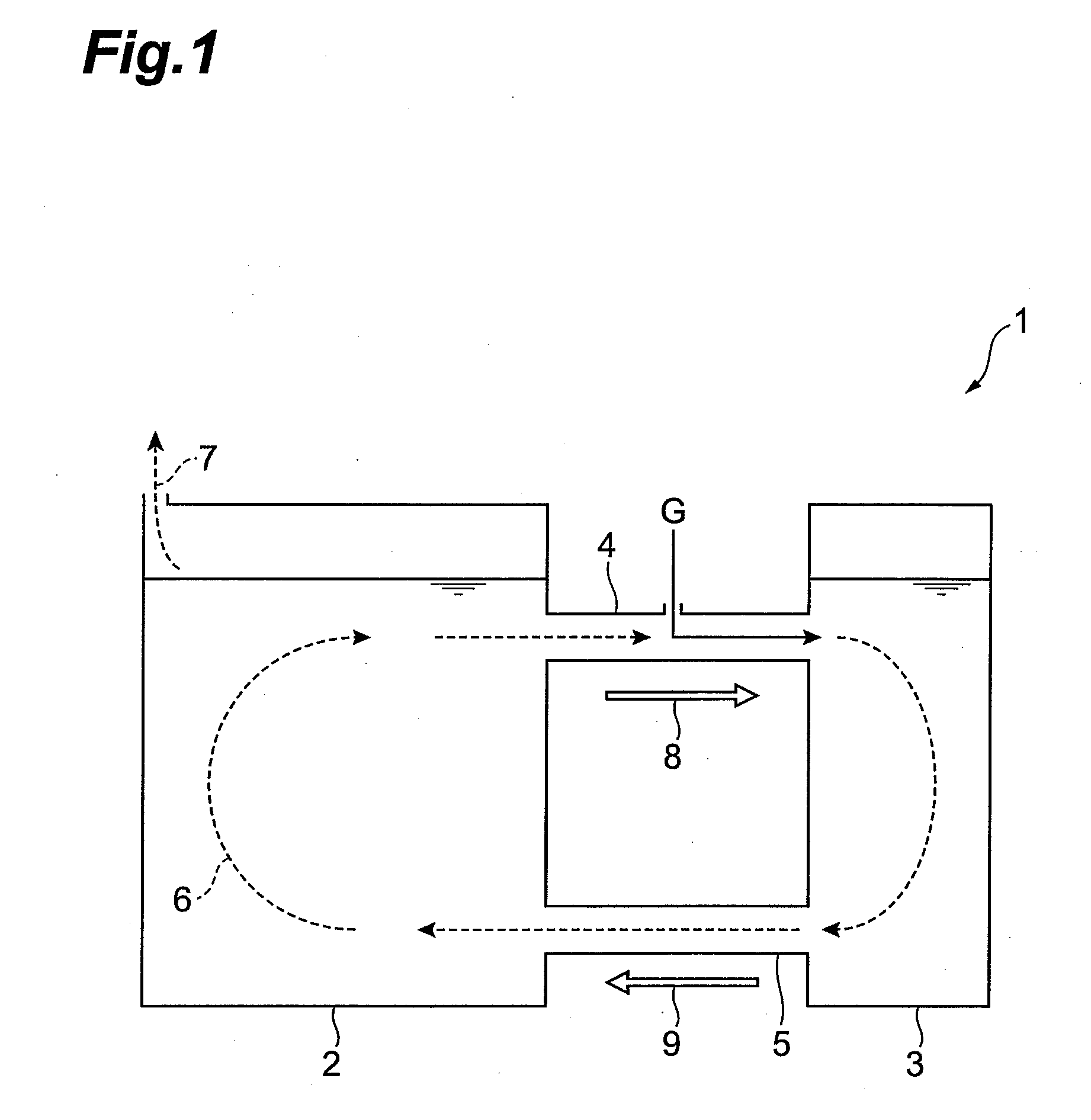
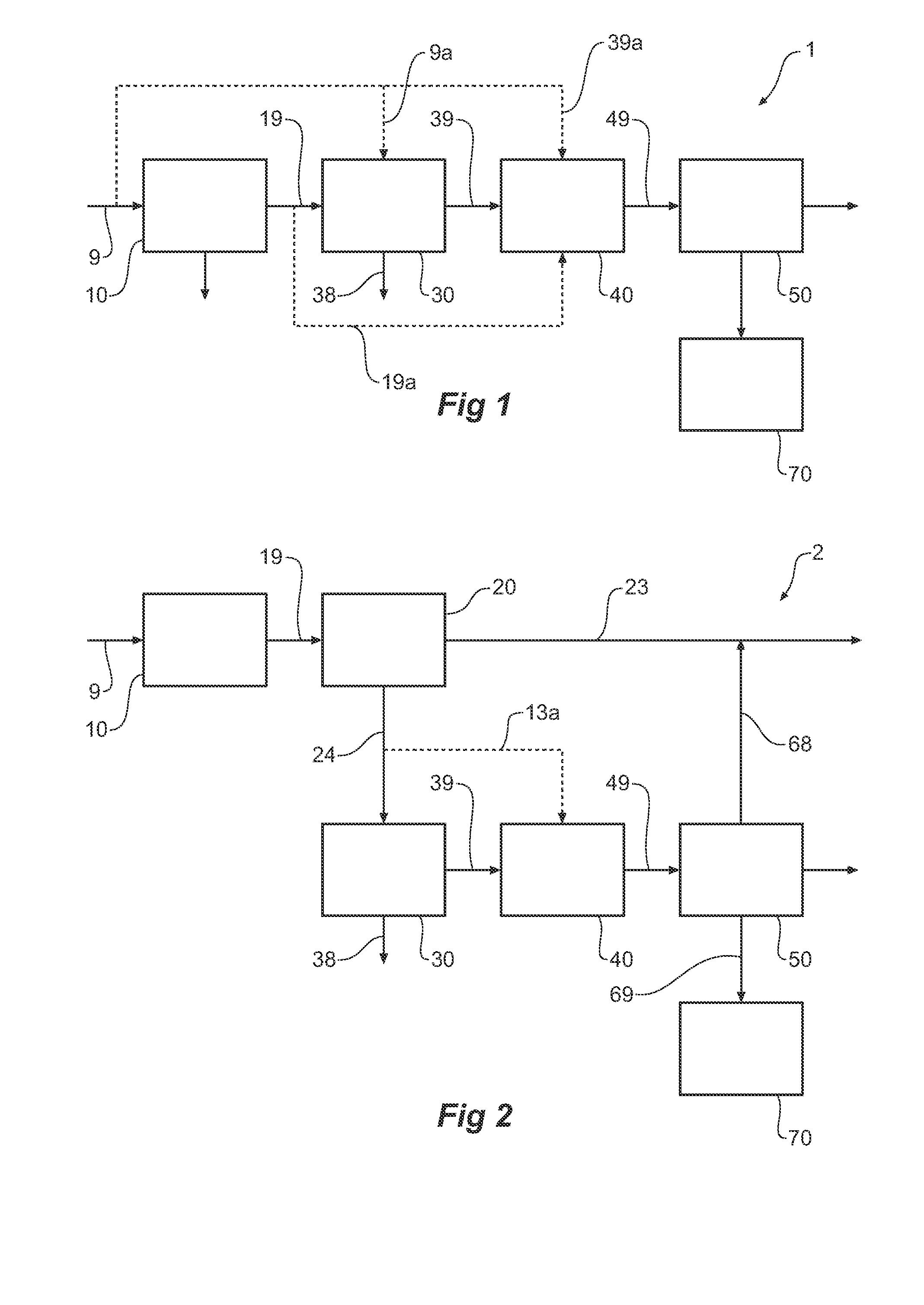
Consecutive or Simultaneous Leaching of Nickel and Cobalt Containing Ores
InactiveUS20080050294A1Minimize equipment sizeIncrease consumptionCobalt ammonia complexesIron oxides/hydroxidesPregnant leach solutionSulfide
A process for the recovery of nickel and cobalt from nickel and cobalt containing ores, including the steps of first leaching a laterite ore and / or a partially oxidised sulfide ore with an acid solution to produce a pregnant leach solution containing at least dissolved nickel, cobalt and ferric ions, and subsequently leaching a sulfide ore or concentrate with the pregnant leach solution to produce a product liquor. Alternatively, the laterite ore and / or partially oxidised sulfide ore can be leached in a combined leach with the sulfide ore or concentrate. The ferric ion content in the pregnant leach solution or in the combined leach is sufficient to maintain the oxidation and reduction potential in the sulfide leach high enough to assist in leaching nickel from the sulfide ore or concentrate.
Owner:BHP BILLITON SSM TECH PTY LTD
Process for production of iron oxyhydroxide particles
InactiveUS20090169470A1Small size changeUniform particle shapeMaterial nanotechnologyPigmenting treatmentIron oxyhydroxidePhotochemistry
The process for production of iron oxyhydroxide particles according to the invention is characterized by comprising a step (A) in which a suspension containing iron(II) is prepared, and a step (B) in which fine bubbles with diameters of 0.05-500 μm are generated in the suspension to form a reaction mixture, and the iron(II) in the reaction mixture is oxidized by the bubbles to produce iron oxyhydroxide particles.
Owner:TDK CORPARATION
Consecutive or simultaneous leaching of nickel and cobalt containing ores
InactiveUS7871584B2Minimize equipment sizeIncrease consumptionCobalt ammonia complexesIron oxides/hydroxidesPregnant leach solutionSulfide
A process for the recovery of nickel and cobalt from nickel and cobalt containing ores, including the steps of first leaching a laterite ore and / or a partially oxidized sulfide ore with an acid solution to produce a pregnant leach solution containing at least dissolved nickel, cobalt and ferric ions, and subsequently leaching a sulfide ore or concentrate with the pregnant leach solution to produce a product liquor. Alternatively, the laterite ore and / or partially oxidized sulfide ore can be leached in a combined leach with the sulfide ore or concentrate. The ferric ion content in the pregnant leach solution or in the combined leach is sufficient to maintain the oxidation and reduction potential in the sulfide leach high enough to assist in leaching nickel from the sulfide ore or concentrate.
Owner:BHP BILLITON SSM TECH PTY LTD
Ammonia storage for on-vehicle engine
Ammonia is used as precursor source of hydrogen fuel in an on-vehicle internal combustion engine. Ammonia is stored as, for example, a ligand in an on-vehicle transition metal composition. Upon demand for hydrogen by the vehicle's engine control system, ammonia is expelled as a gas from some of the composition and the ammonia gas is dissociated into a mixture of hydrogen and nitrogen and delivered as a fuel-containing mixture to the engine. In a preferred embodiment, the hydrogen is used as a supplement to gasoline as a fuel for engine operation.
Owner:GM GLOBAL TECH OPERATIONS LLC
Extraction of uranium from wet-process phosphoric acid
ActiveUS8226910B2Reduced valencyLower iron levelsCobalt ammonia complexesPhotography auxillary processesFiltrationPhosphoric acid
Owner:URTEK
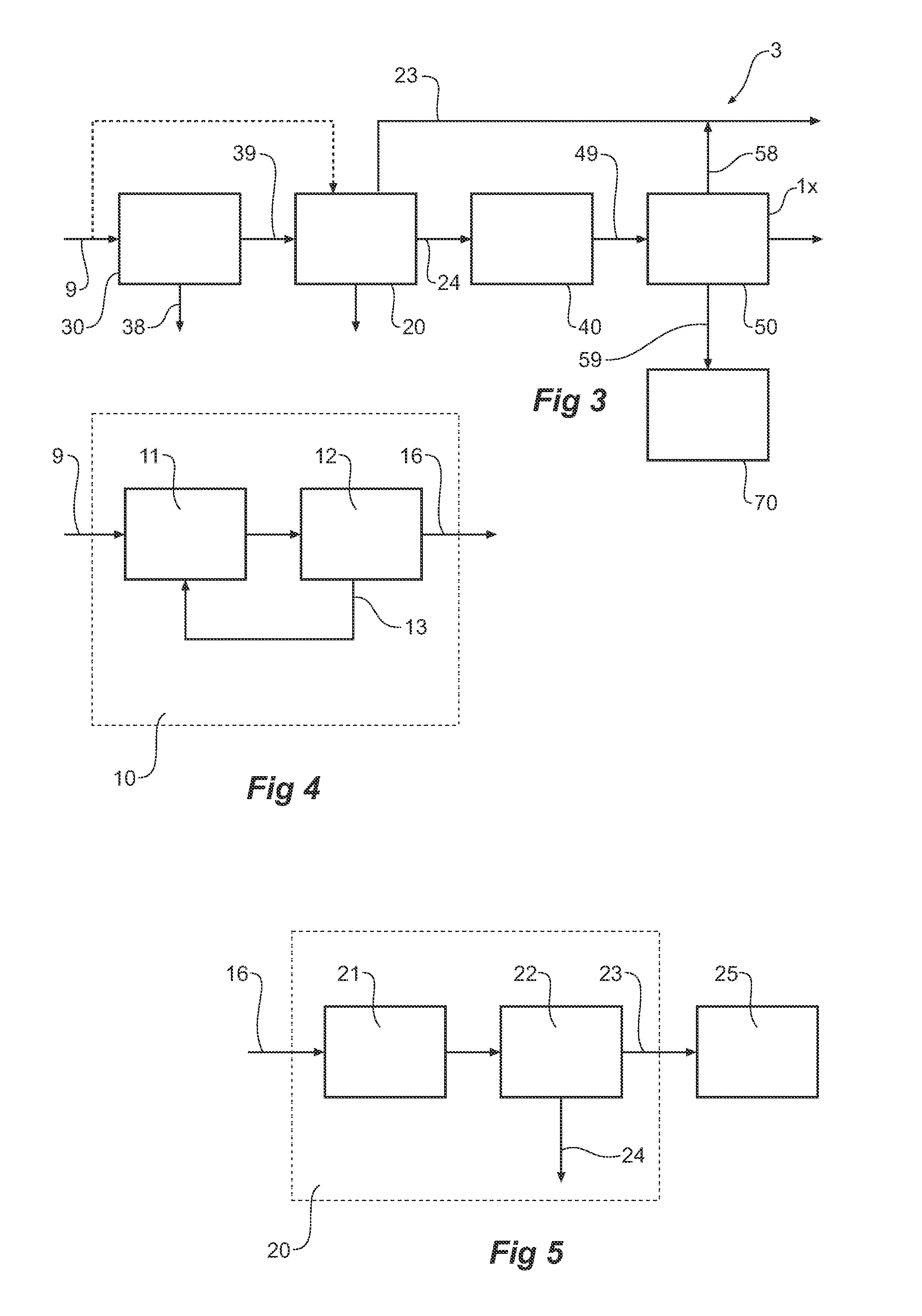
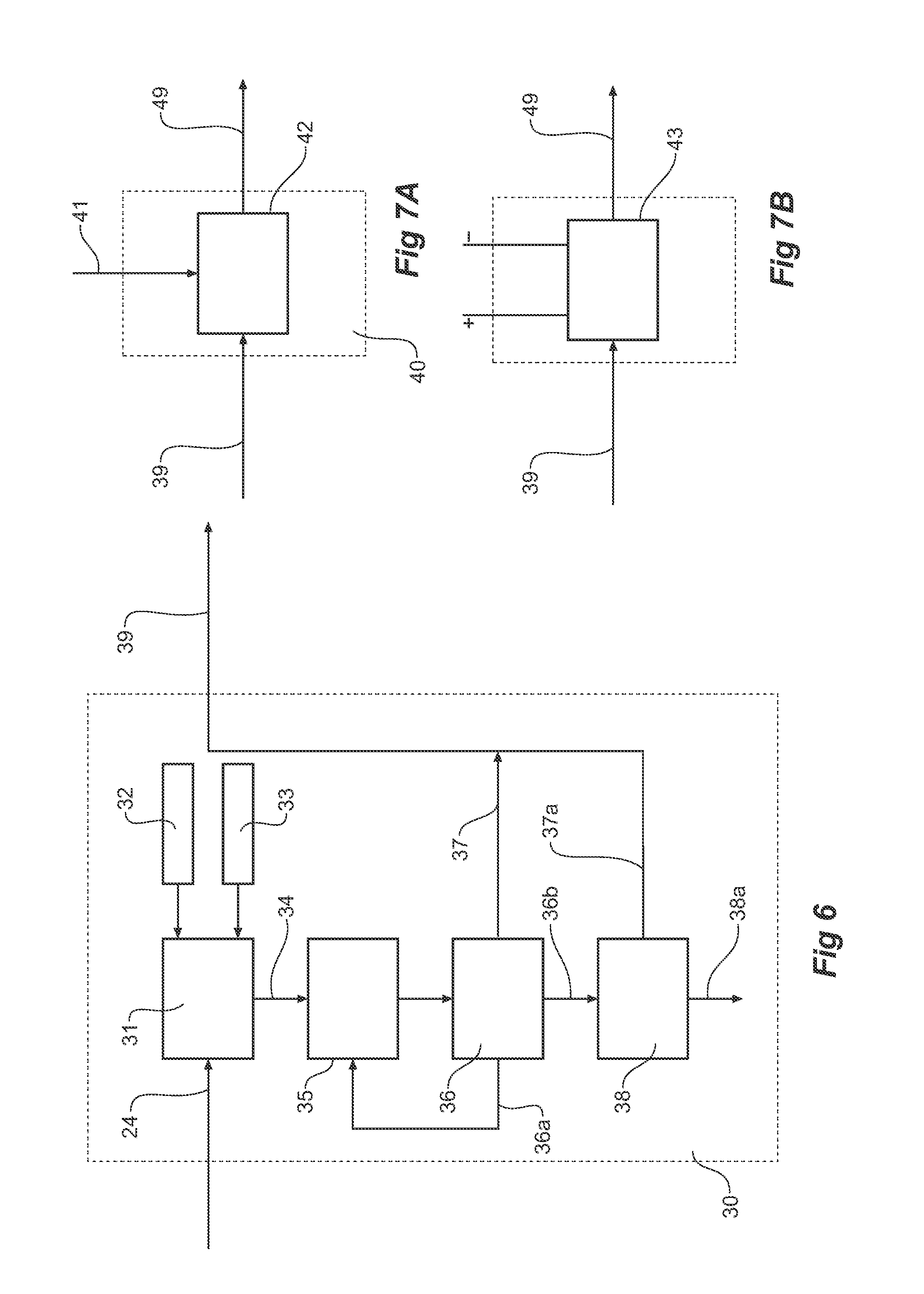
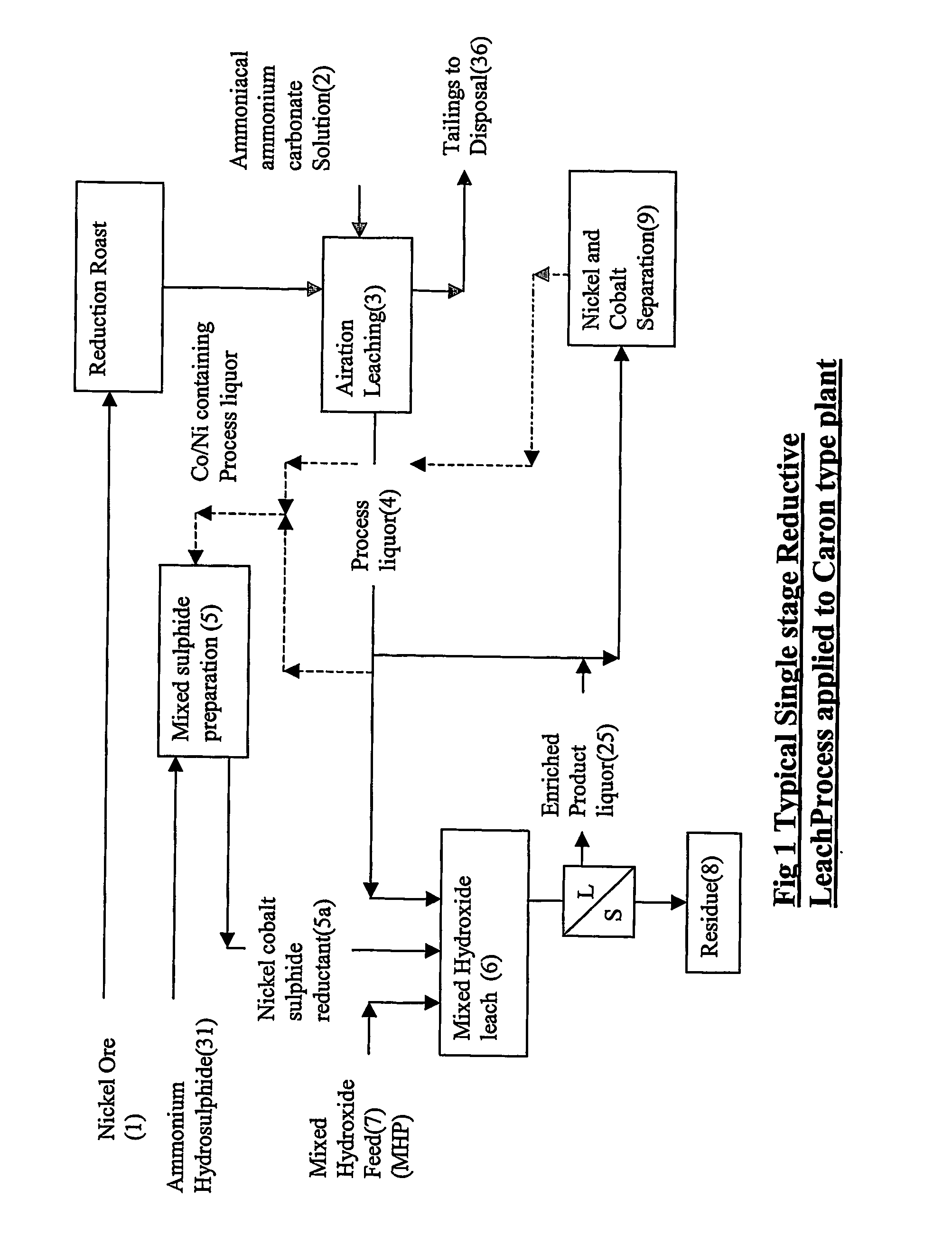
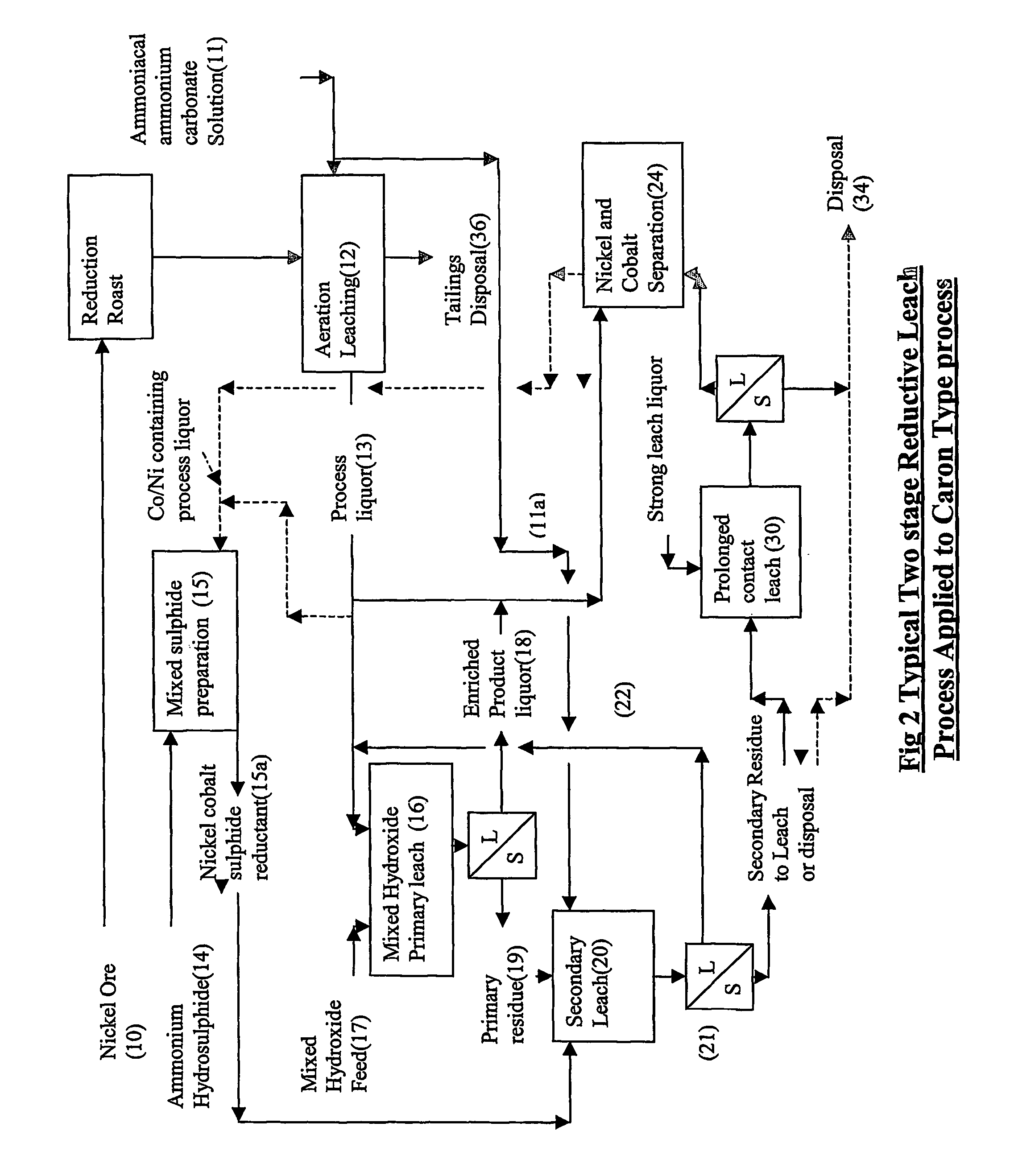
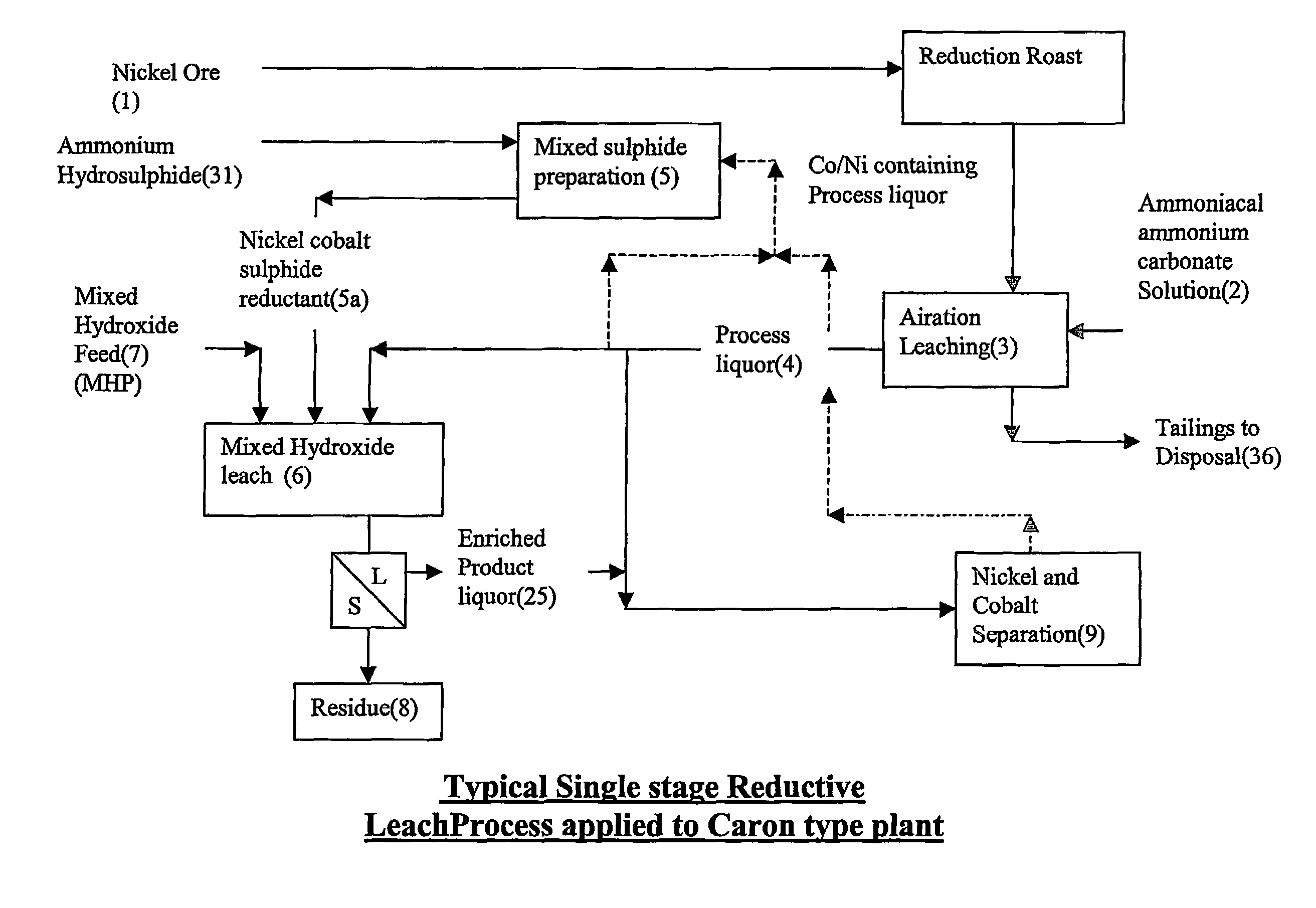
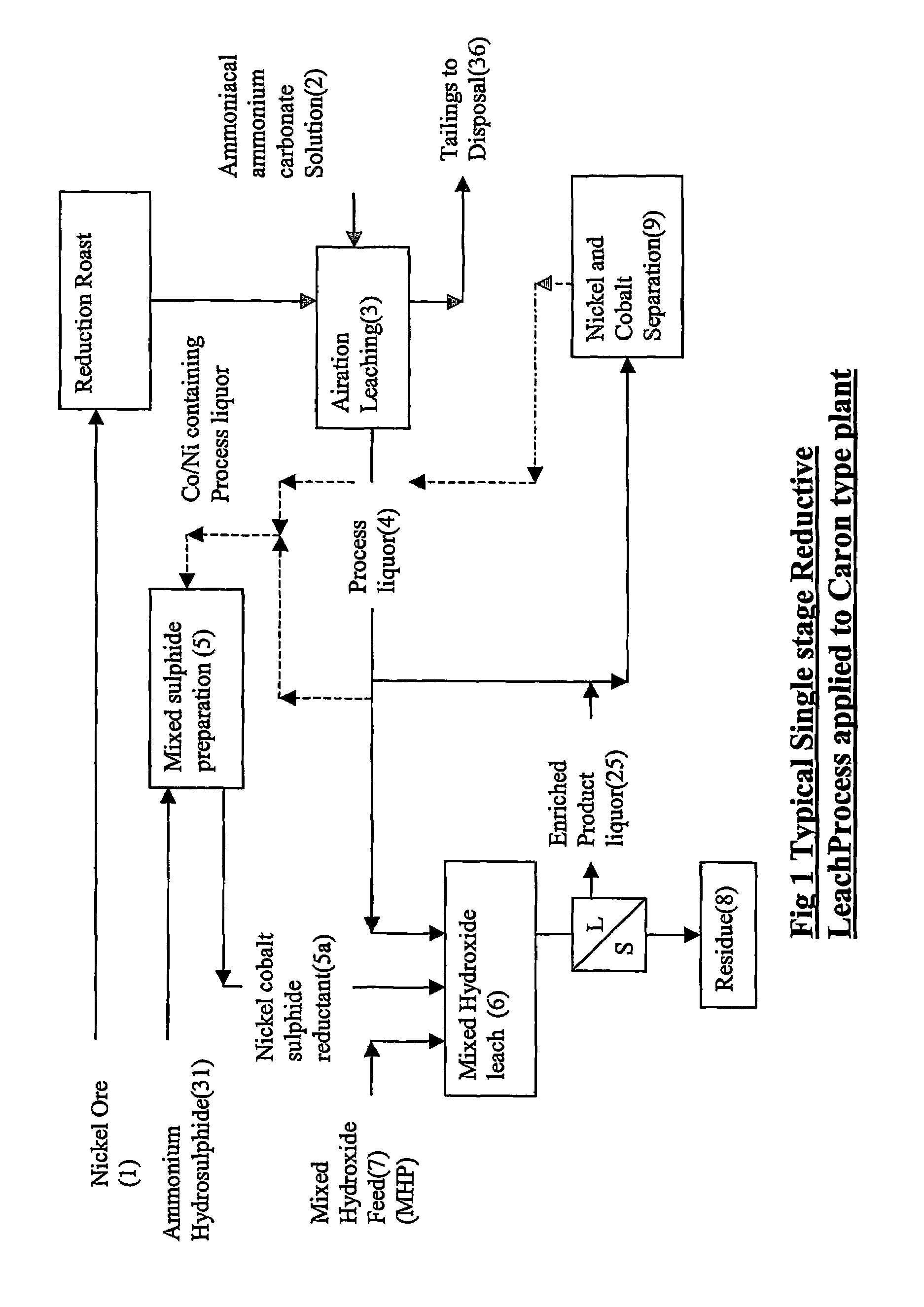
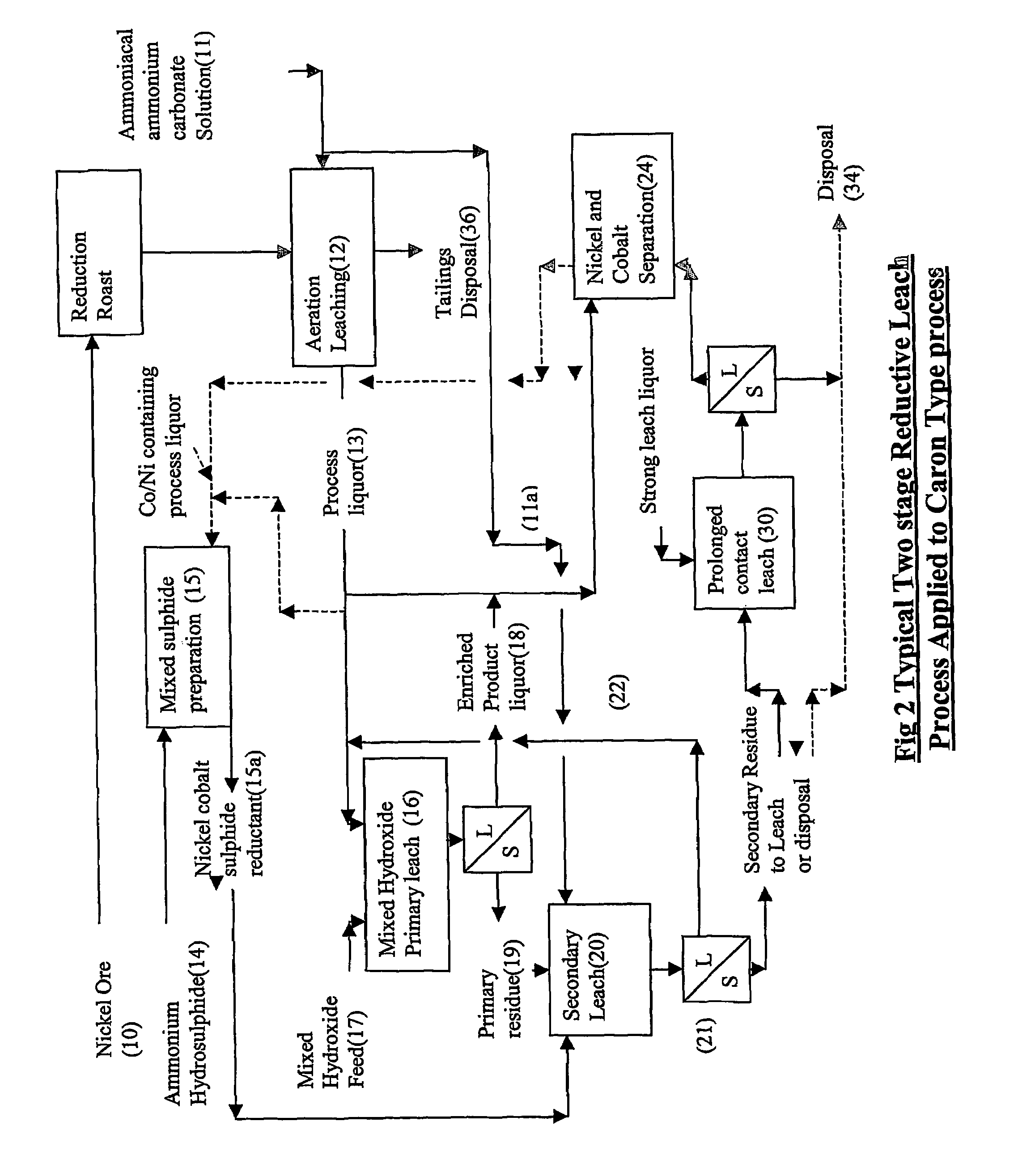
Reductive ammoniacal leaching of nickel and coblat bearing
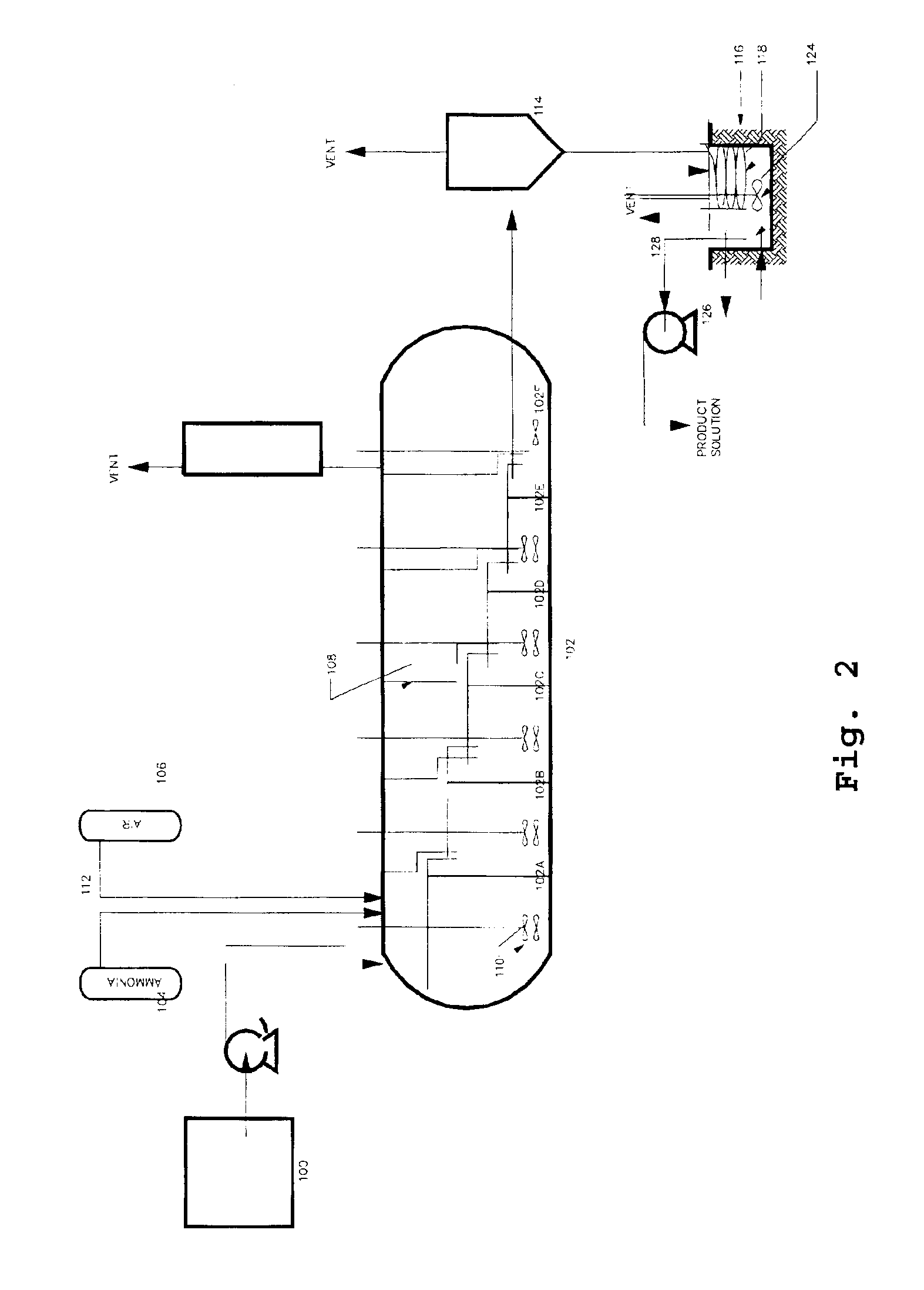
InactiveUS20070166214A1Efficient and economic separationImprove featuresCobalt ammonia complexesSolvent extractionAmmonium carbonateCobalt
A process for the recovery of nickel and / or cobalt from an impure nickel, cobalt or mixed nickel / cobalt material including the steps of: a) providing a nickel, cobalt or mixed nickel / cobalt material; and b) contacting the nickel, cobalt or mixed nickel / cobalt material with a feed ammoniacal ammonium carbonate solution and a reductant in a leach step.
Owner:BHP BILLITON SSM TECH PTY LTD
Solid ammonia storage and delivery material
InactiveUS20130230443A1Cobalt ammonia complexesNitrogen compoundsAlkaline earth metalAmmonia storage
Disclosed is a method for the selective catalytic reduction of NOx in waste / exhaust gas by using ammonia provides by heating one or more salts of formula Ma(NH3)nXz, wherein M represents one or more cations selected from alkaline earth metals and transition metals, X represents one or more anions, a represents the number of cations per salt molecule, z represents the number of anions per salt molecule, and n is a number of from 2 to 12, the one or more salts having been compressed to a bulk density above 70% of the skeleton density before use thereof.
Owner:AMMINEX EMISSIONS TECH
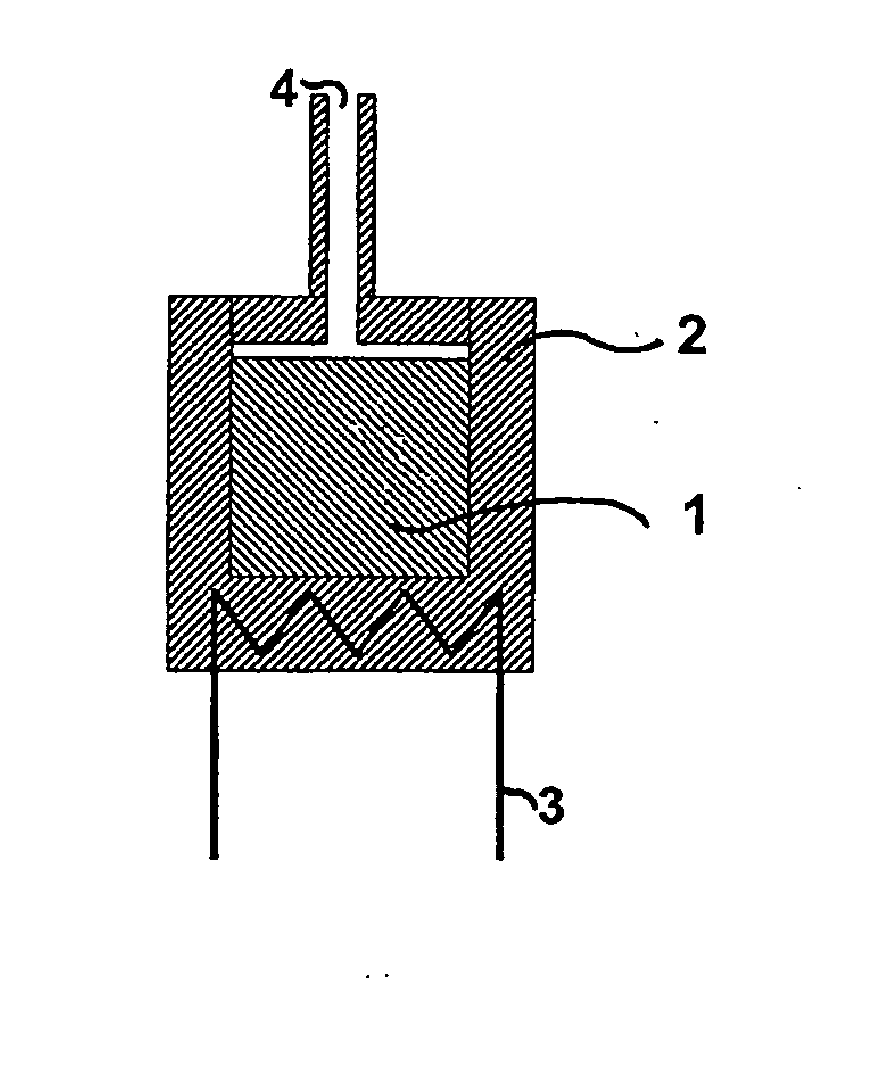
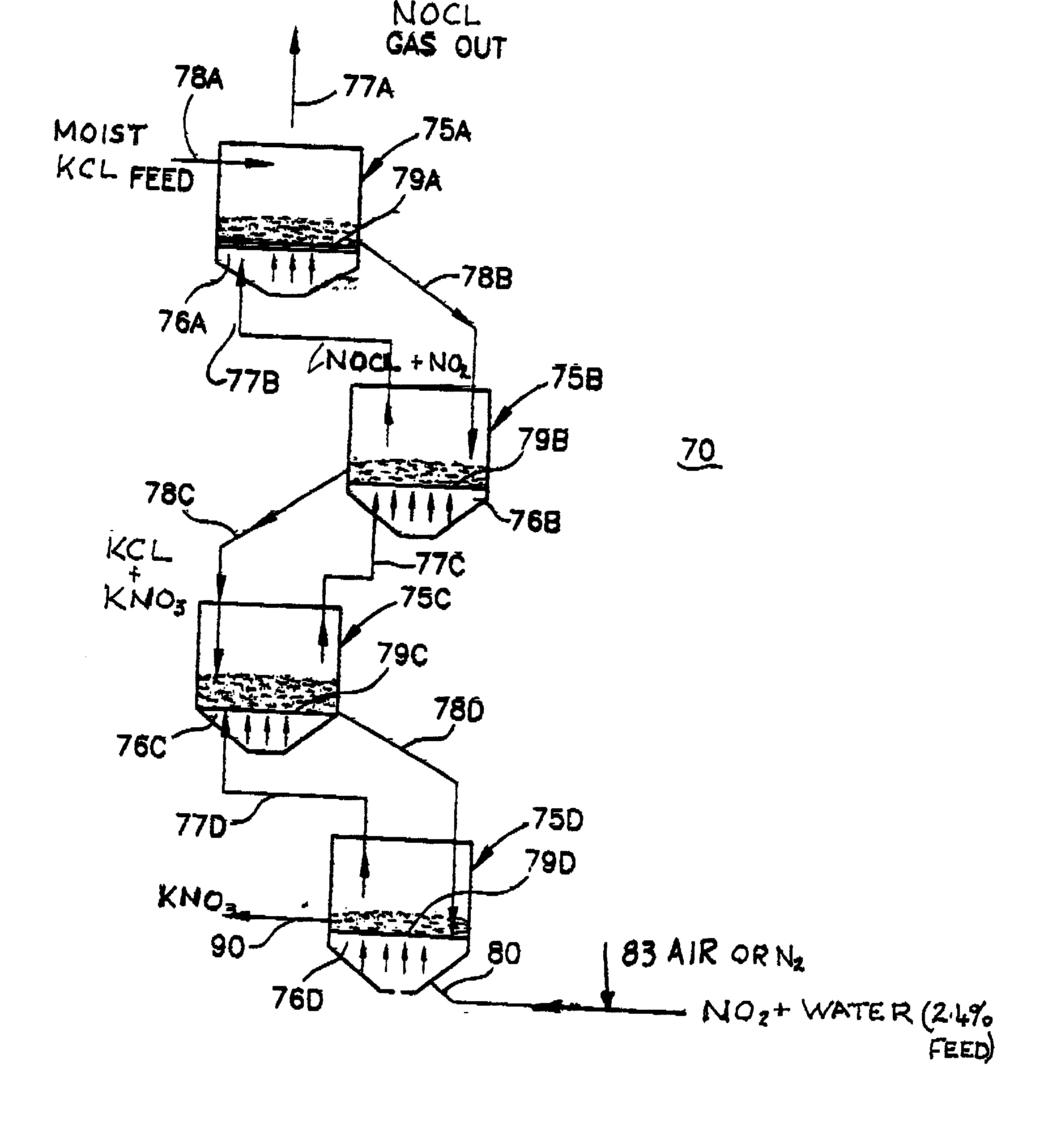
Process for manufacturing potassium nitrate fertilizer and other metal nitrates
InactiveUS20020110512A1Cost effectiveEfficient executionCalcareous fertilisersCobalt ammonia complexesWater vaporCounter current
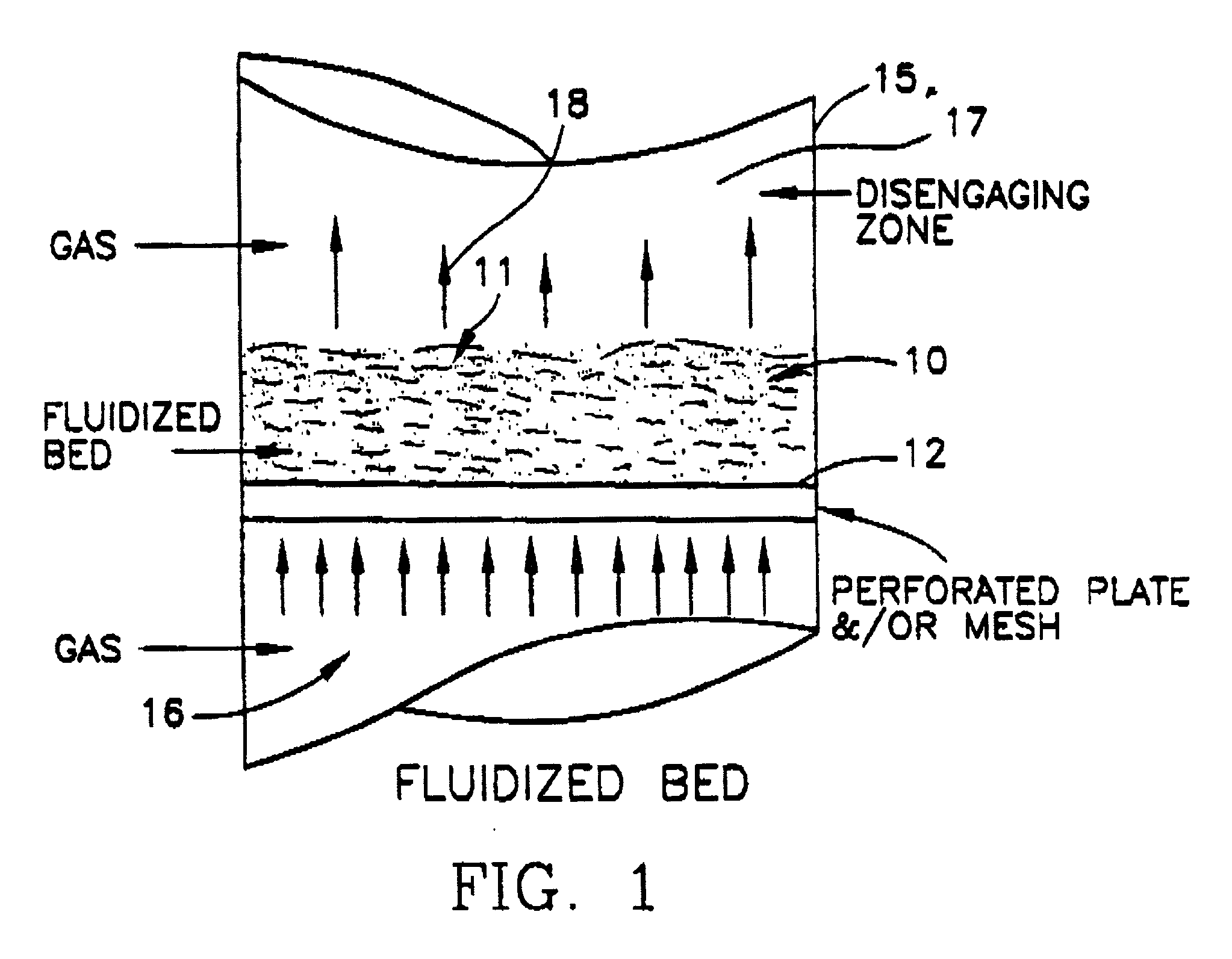
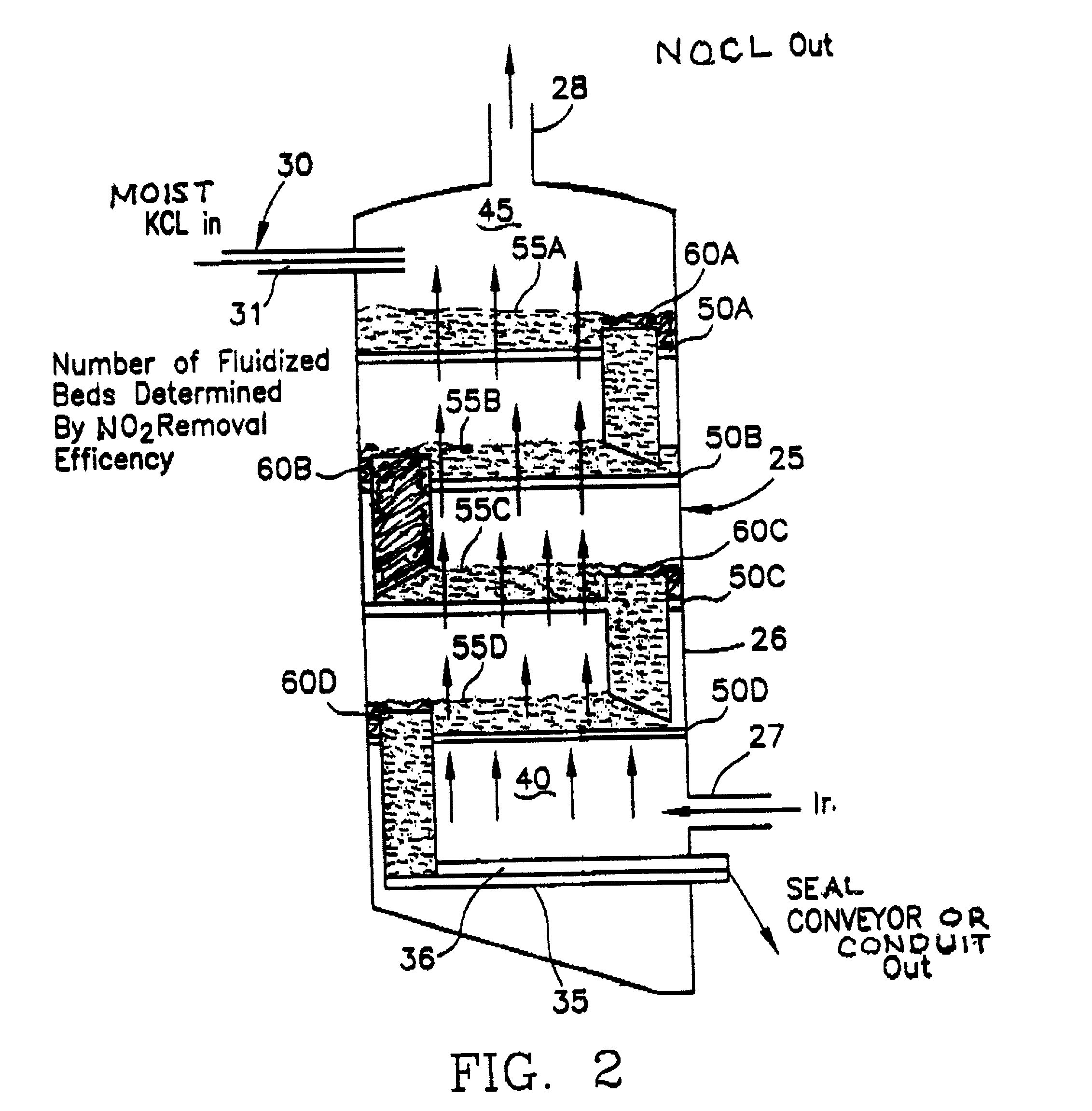
A process for producing potassium nitrate and other metal nitrates from the chlorides, sulfates, oxides of these metals. The process uses nitrogen dioxide as a true fluidizing medium in shallow beds of the aforementioned solids at moderately elevated temperatures in a continuous counter current process to convert the metal chlorides, sulfates, and oxides, into metal nitrates and effluent gas and water vapor. The process may be carried out in a series of true fluidized beds arranged in a vertical configuration so that the solids flow downward due to the fluidized process and the nitrogen dioxide gas flows counter currently in an upward direction producing pure metal nitrates at the bottom and nitrosyl chloride gas and / or water vapor at the top.
Owner:RIGBY WILLIAM J
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS7892505B2Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsHydrometallurgy
Owner:ROYAL SILVER PANAMA
Low-temperature reversible thermochromic material and preparation method and application thereof
InactiveCN104789206AReduce pollutionLow discoloration temperatureColor/spectral properties measurementsTenebresent compositionsDistilled waterSolvent
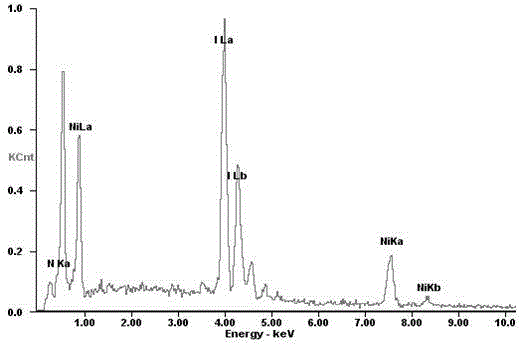
The invention discloses a low-temperature reversible thermochromic material and a preparation method and application thereof. The molecular formula of the thermochromic material is (Ni(NH3)6)I2.0.5H2O. The thermochromic material is prepared by adopting a low-temperature solvent-thermal method, and the specific preparation method is as follows: nickel powder: iodine elementary substance equal to (1.0-2.0): (0.8-1.5) according to molar ratio, 3.0-6.0mL of ammonia water is taken as a reactant, raw materials are loaded into a 25mL reaction kettle and placed at the temperature of 120-180 DEG C for reacting for 4-7 days, and then distilled water and ethanol are respectively used for washing. The thermochromic material is prepared. The discoloration principle is that gain and loss of crystal water can be used for detecting water. The thermochromic material disclosed by the invention has the advantages that the thermochromic material is prepared by adopting the one-step solvent-thermal method, the process flow is simple, the yield is high, the environmental pollution is little, the production cost is low, and the prepared thermochromic material has low discoloration temperature, short color recovery time, bright color and luster, sharp discoloration and good reversibility.
Owner:ZHEJIANG UNIV
Titania-Supported Hydrotreating Catalysts
TiO2-supported catalysts include at least molybdenum or tungsten as active components for hydrotreating processes, in particular for the removal of sulfur and nitrogen compounds as well as metals out of crude oil fractions and for the hydrogenation of sulfur oxides.
Owner:SACHTLEBEN CHEM GMBH
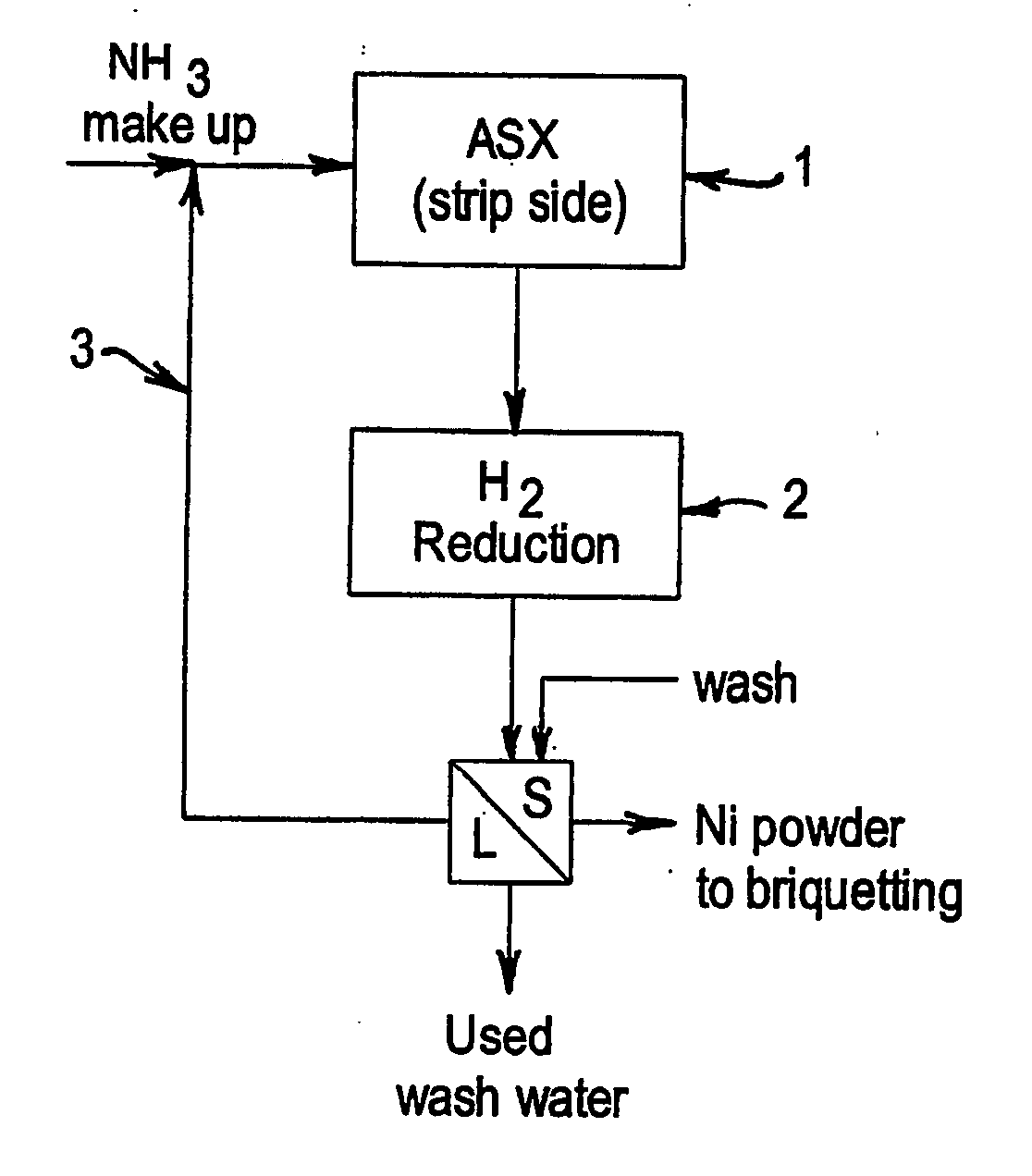
Integrated ammoniacal solvent extraction and hydrogen reduction of nickel
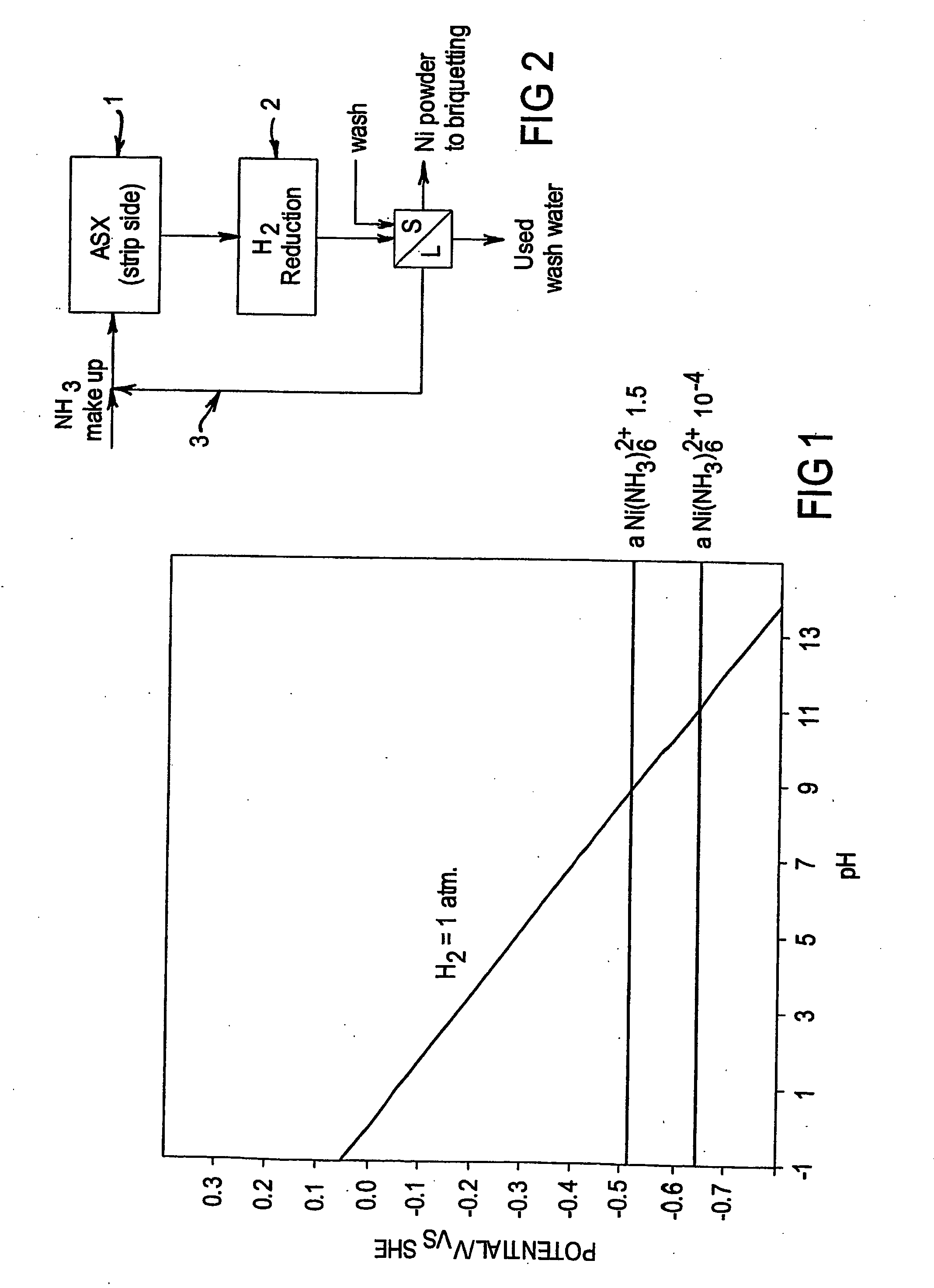
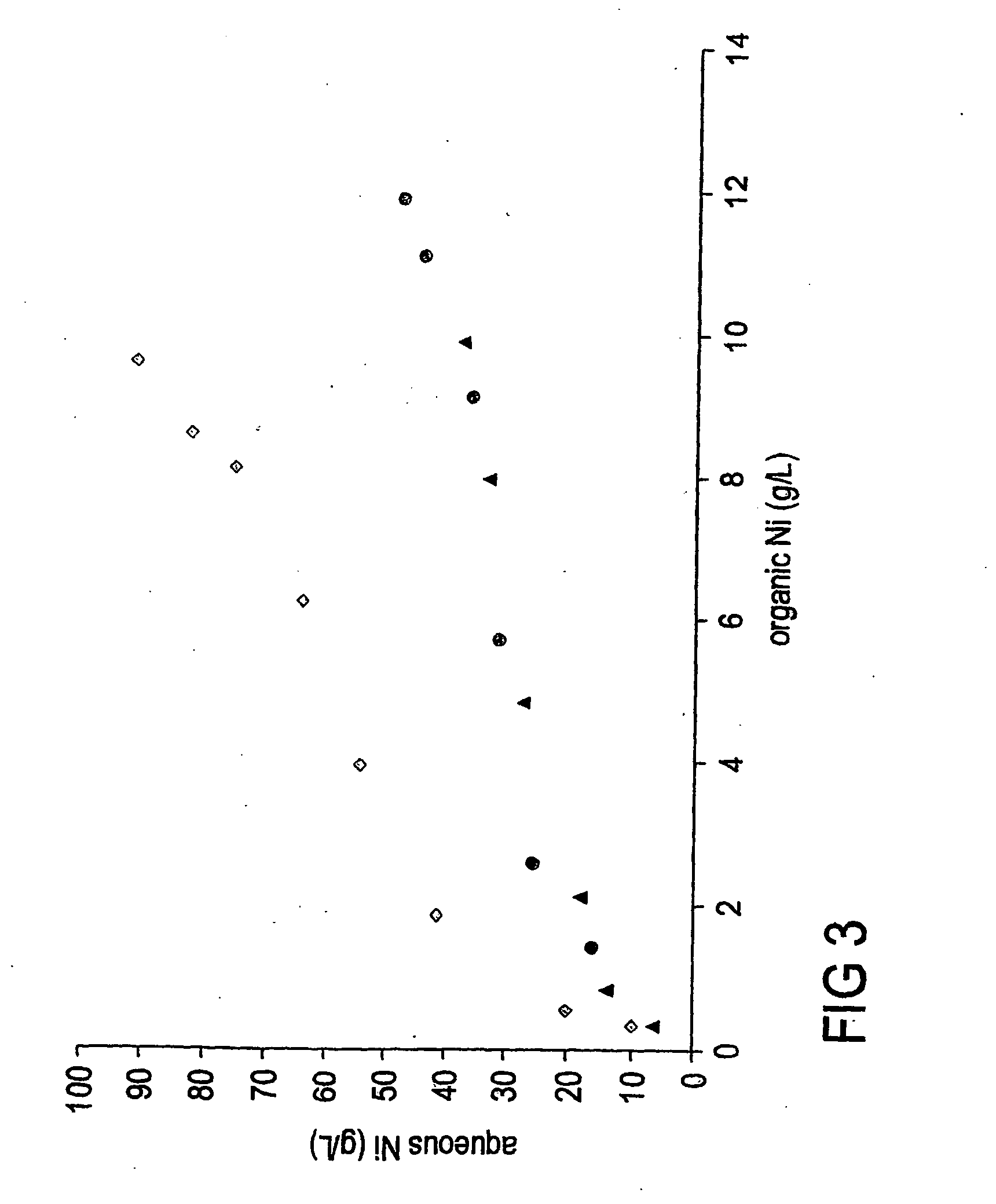
InactiveUS20050211022A1Quality improvementNumber of EliminationsCobalt ammonia complexesSolvent extractionHydrogenAmmonium hydroxide
A process is provided for the recovery of a metallic nickel product in a solvent extraction process which includes the steps of forming a nickel ammine complex by stripping nickel from a nickel loaded organic phase with a high strength ammonia solution and then reducing the nickel ammine complex with hydrogen from the high strength ammonia solution to produce a metallic nickel product. By virtue of the present process, a reductive precipitation of nickel by hydrogen is obtained so as to recover a metallic nickel product, and the process is advantageous in that it will allow for commercial processes wherein nickel may be recovered by hydrogen reduction based on high strength ammonia solutions.
Owner:QNI TECH
Producing cobalt (III) hexammine sulfate from nickel cobalt sulfides
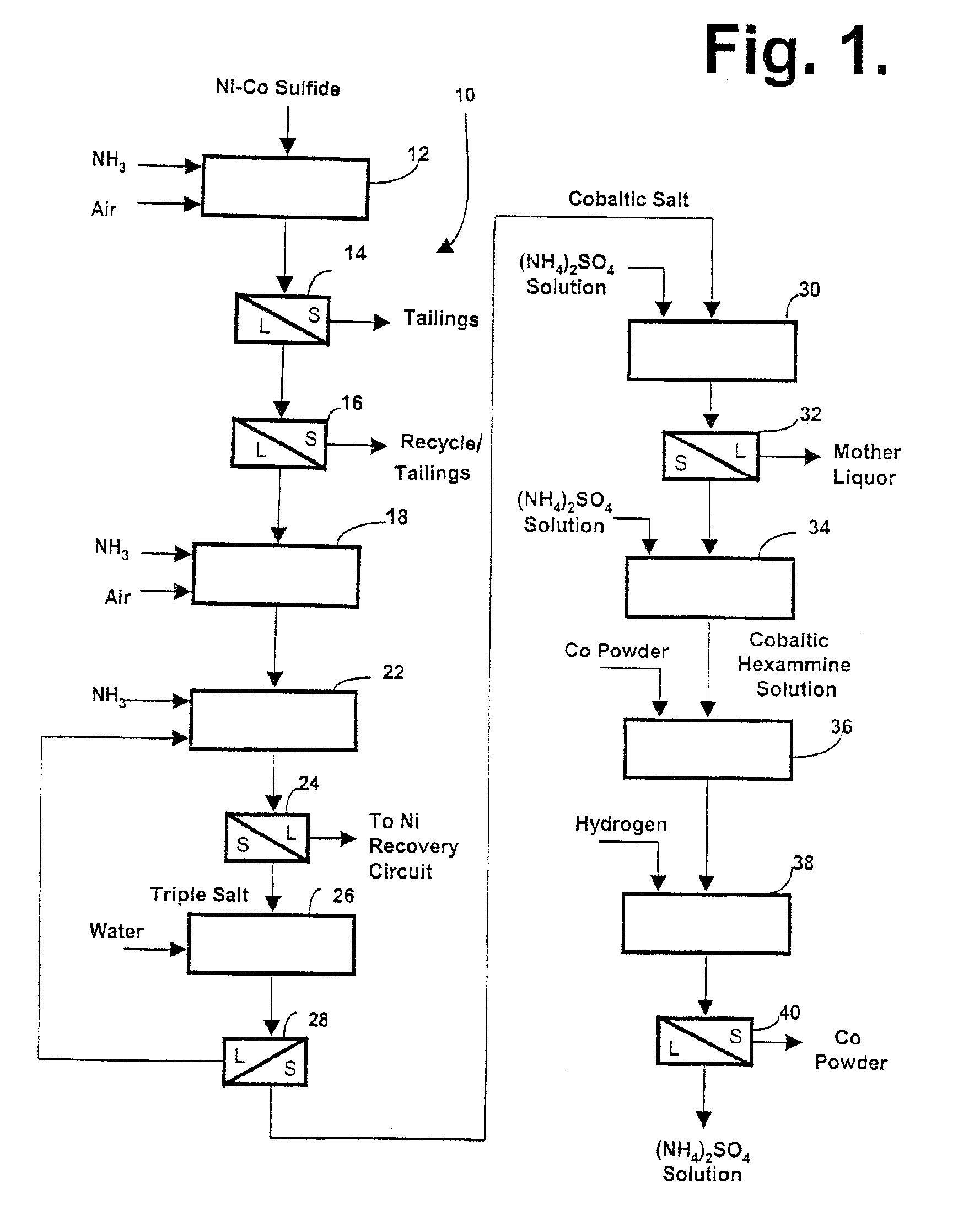
InactiveUS6949232B2Enhanced cobalt recoveryPromote recoveryCobalt ammonia complexesNitrogen compoundsNickel(II) sulfateSulfate
There is provided an improvement in a process for producing cobalt (III) hexammine sulphate which process comprises oxidatively pressure leaching nickel cobalt sulphides in an ammoniacal ammonium sulphate solution. The nickel and cobalt sulphides are oxidized to sulphates, and an ammoniacal leach liquor in which dissolved cobalt is predominantly in the (III) oxidation state is produced. The ammoniacal leach liquor is combined with ammonia to precipitate the triple salt of cobalt (III) hexammine sulphate, nickel (II) hexammine and ammonium sulphate which is further treated to produce a crystalline cobalt (III) hexammine sulphate and a nickel enriched leach liquor. The improvement involves the provision of a second oxidative pressure treatment effective to maximize the formation of the desired cobalt (III) hexammine ion, prior to the triple salt precipitation step.
Owner:SHERRITT INTERNATIONAL
Process for removal of zinc, iron and nickel from spent completion brines and produced water
Zinc, nickel and iron can be recovered from spent brines and produced water using a method that includes admixing an aqueous fluid with hydrazine to form a hydrazine complex and then filtering or otherwise removing the hydrazine complex from the aqueous fluid. Once treated, the aqueous fluid can then be recycled or at be the subject to an easier disposal. The isolated metal hydrazine complex may be recycled or discarded.
Owner:BAKER HUGHES INC
Process for production of iron oxyhydroxide particles
InactiveUS7910085B2Small size changeUniform particle shapeMaterial nanotechnologyPigmenting treatmentIron oxyhydroxidePhotochemistry
The process for production of iron oxyhydroxide particles according to the invention is characterized by comprising a step (A) in which a suspension containing iron(II) is prepared, and a step (B) in which fine bubbles with diameters of 0.05-500 μm are generated in the suspension to form a reaction mixture, and the iron(II) in the reaction mixture is oxidized by the bubbles to produce iron oxyhydroxide particles.
Owner:TDK CORPARATION
Methods for preparing and regenerating materials containing amorphous iron oxide hydroxide and desulfurizer comprising the same
ActiveUS8647600B2Reduce capacityHigh sulfur capacityOther chemical processesIron oxides/hydroxidesSulfurMaterials science
Methods for preparing a composition containing amorphous iron oxide hydroxide. Methods for regeneration of the amorphous iron oxide hydroxide after it has been used as desulfurizer. Regenerable desulfurizer with high sulfur capacity containing amorphous iron oxide hydroxide, not less than 88% w / w, and organic binder not less than 7% w / w. The organic binder is sodium carboxymethylcellulose, sesbania powder, cellulose powder, or a mixture thereof. A method for preparing the desulfurizer. A method for regenerating the waste agent produced after the desulfurizer and the composition containing the desulfurizer are used as desulfurizer. This method allows the desulfurizer and the composition containing the desulfurizer to be regenerated and reused avoiding the need for landfill disposal and environmental pollution.
Owner:BEIJING HAIXIN ENERGY TECH CO LTD
Method for selective precipitation of iron, arsenic and antimony
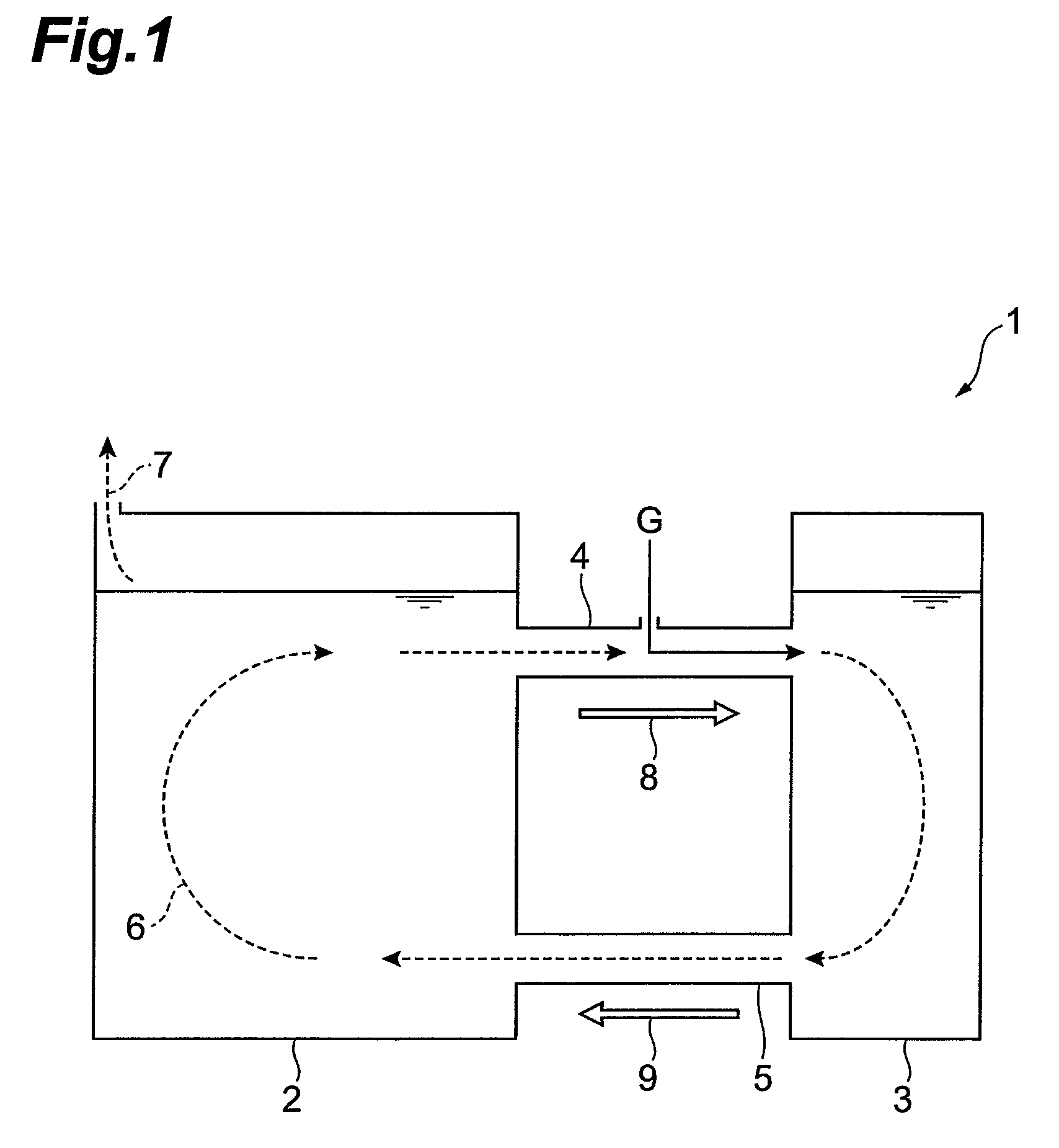
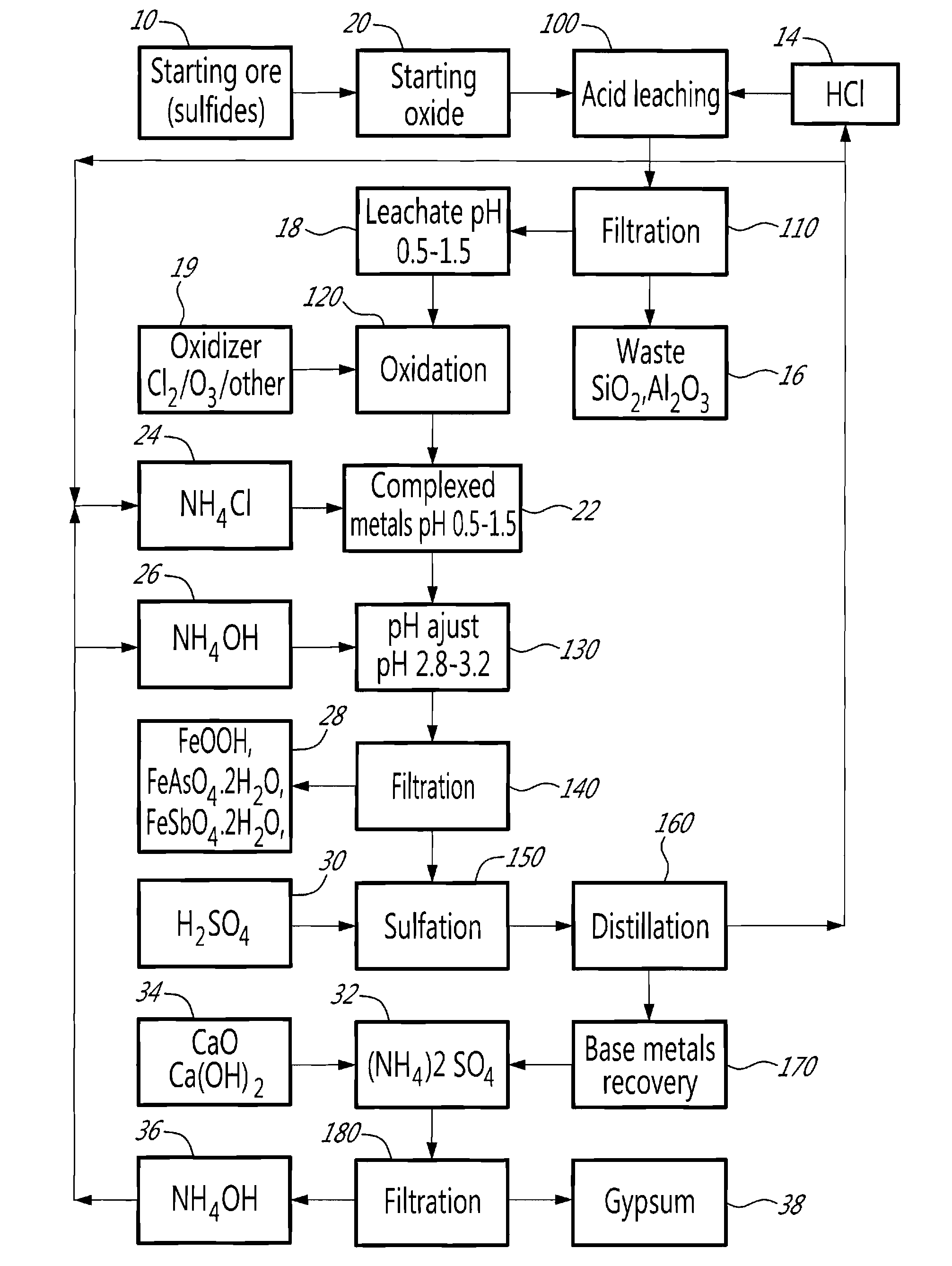
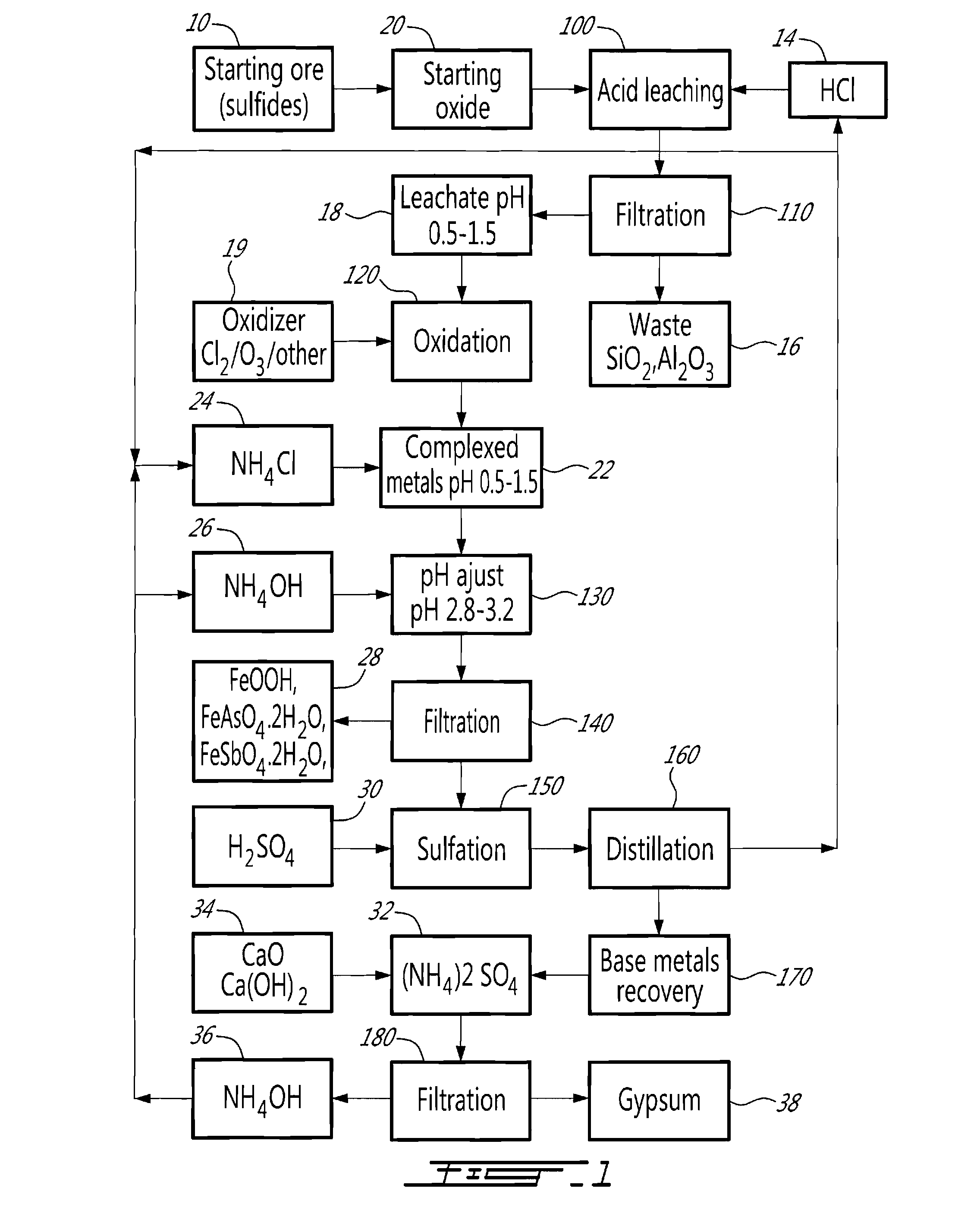
A method for selectively processing a polymetallic oxide solution containing a plurality of base metals comprising at least one of: Cu, Co, Ni, Zn associated with iron, comprising acid leaching the solution; recovering a filtered leachate; oxidizing the leachate; and adjusting the pH of the leachate in presence of a complexing agent; wherein the acidic solution is one of: i) a hydrochloric acid solution and ii) a sulfuric acid solution at a pH lower than about 1.5, and the complexing agent is one of: i) ammonium chloride and ii) ammonium sulfate, the step of adjusting the pH comprising raising the pH to a range between about 2.5 and about 3.5.
Owner:DUNDEE SUSTAINABLE TECH
Spherical tricobalt tetraoxide and method of preparing the same
ActiveUS7998452B2Stable structureMaintain good propertiesIron oxides/hydroxidesCell electrodesCobalt(II,III) oxideCobalt salt
A method of preparation of spherical tricobalt tetraoxide, including at least oxidizing a bivalent cobalt salt in a wet environment and in the presence of a precipitant, a complexing agent, and an oxidant to yield spherical cobalt oxyhydroxide.cobalt hydroxide according to the following equation Co2++3OH−+O→CoOOH.Co(OH)2; oxidizing the spherical hydroxy cobalt oxyhydroxide.cobalt hydroxide to yield spherical tricobalt tetraoxide according to the following equation 6CoOOH.Co(OH)2+O→4Co3O4+9H2O; and roasting the spherical tricobalt tetraoxide at low or intermediate temperature to yield a black powder. The method is easily practiced and suitable for mass production, and the resultant spherical tricobalt tetraoxide has stable structure, reliable properties, and high activity.
Owner:NINGBO RONBAY LITHIUM BATTERY MATERIAL CO LTD
Reductive ammoniacal leaching of nickel and cobalt bearing materials
InactiveUS7601314B2Efficient and economic separationImprove featuresCobalt ammonia complexesSolvent extractionAmmonium carbonateCobalt
A process for the recovery of nickel and / or cobalt from an impure nickel, cobalt or mixed nickel / cobalt material including the steps of: a) providing a nickel, cobalt or mixed nickel / cobalt material; and b) contacting the nickel, cobalt or mixed nickel / cobalt material with a feed ammoniacal ammonium carbonate solution and a reductant in a leach step.
Owner:BHP BILLITON SSM TECH PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com