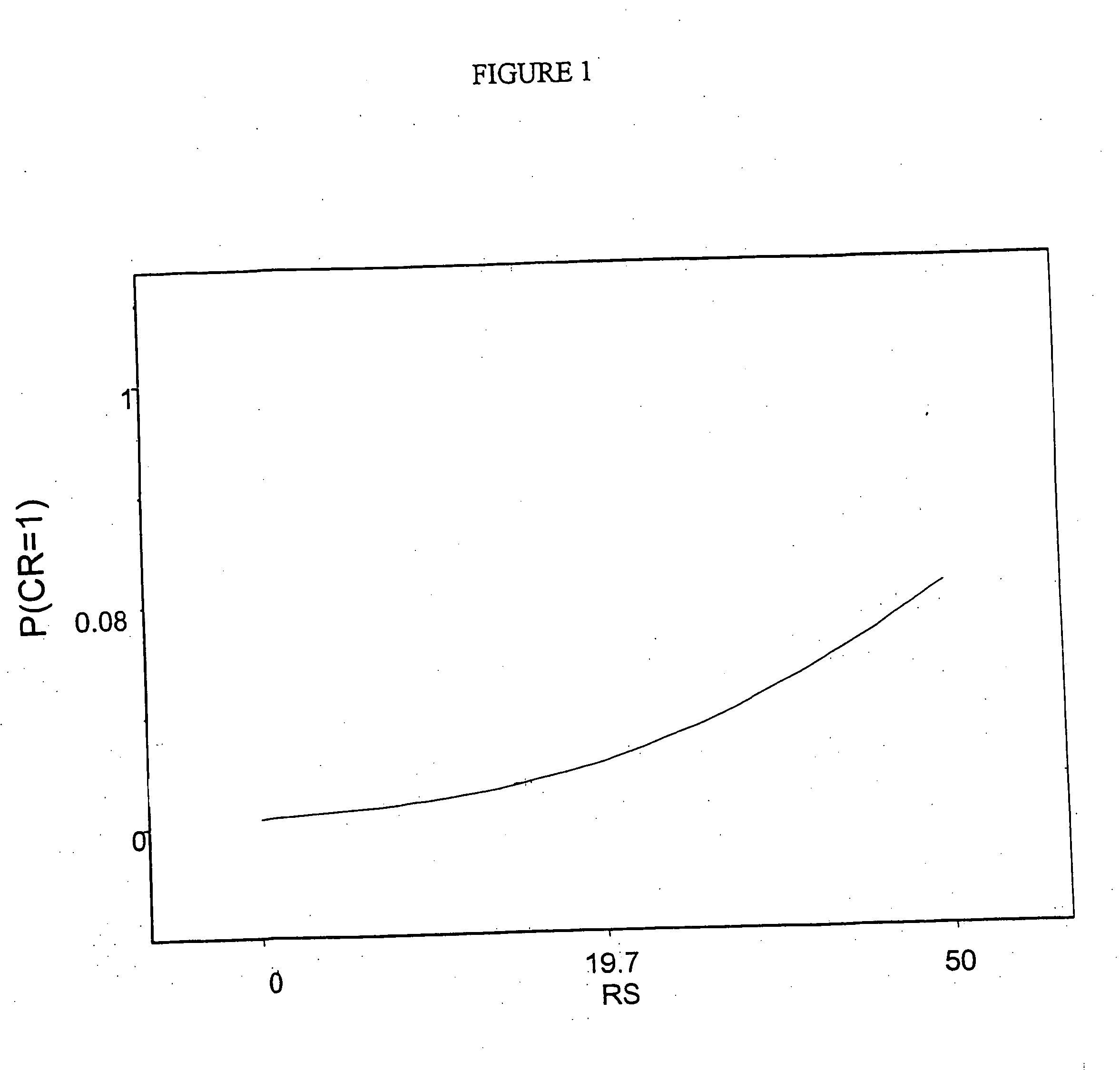
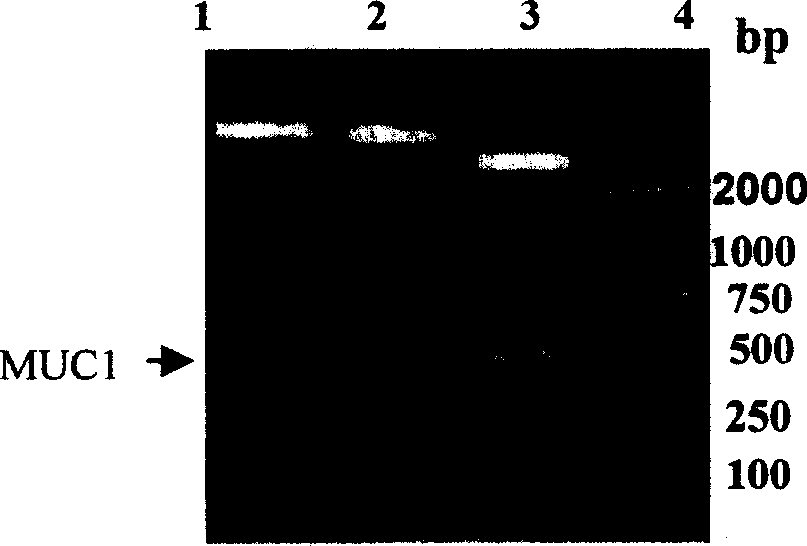
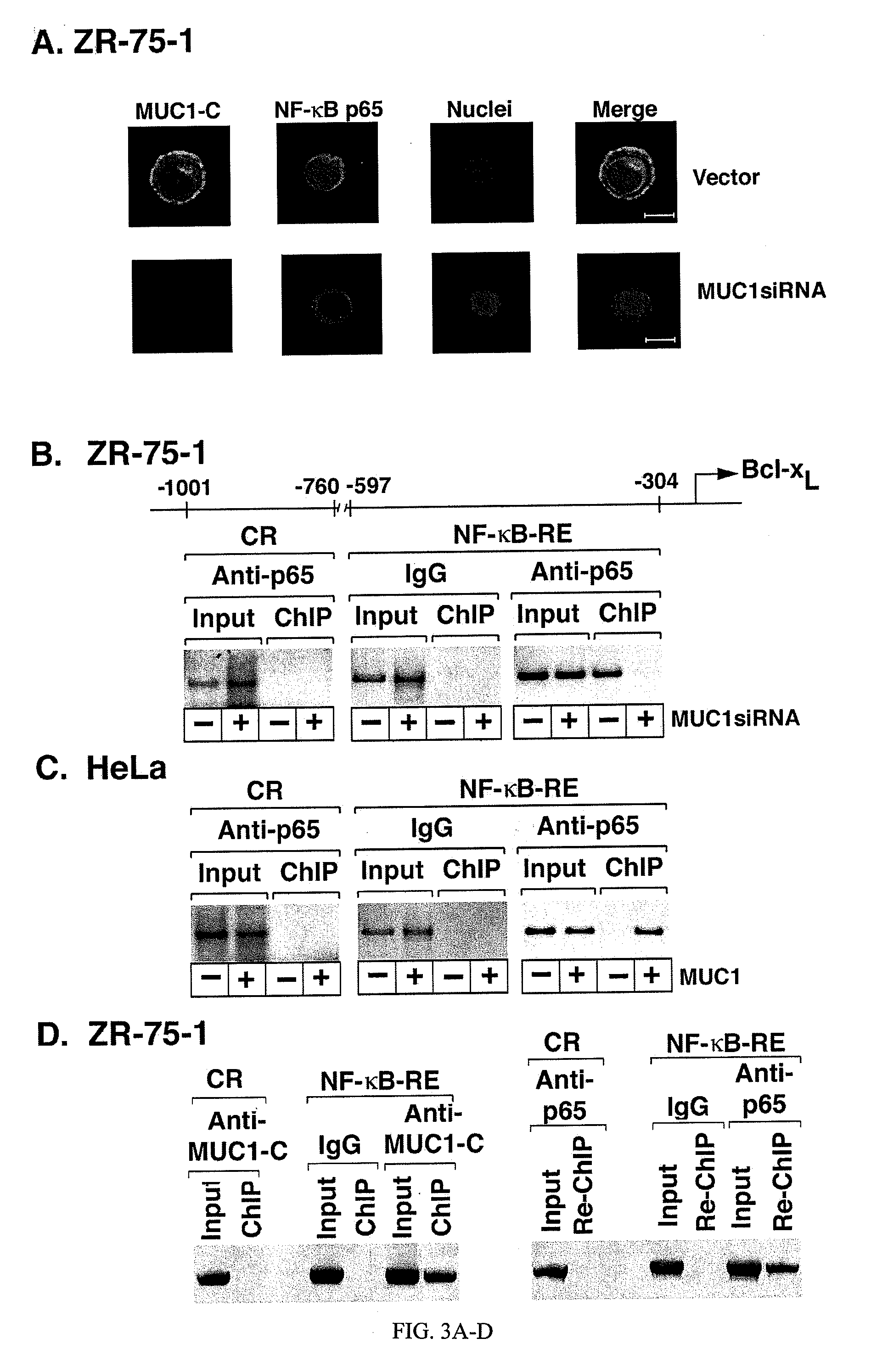
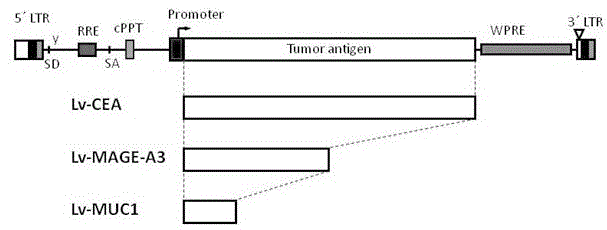
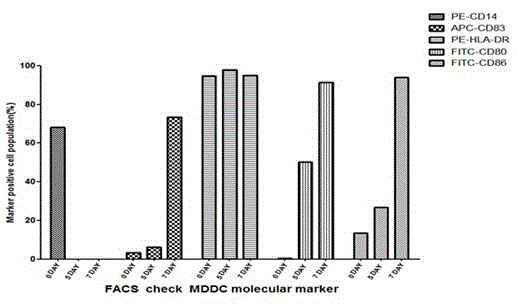
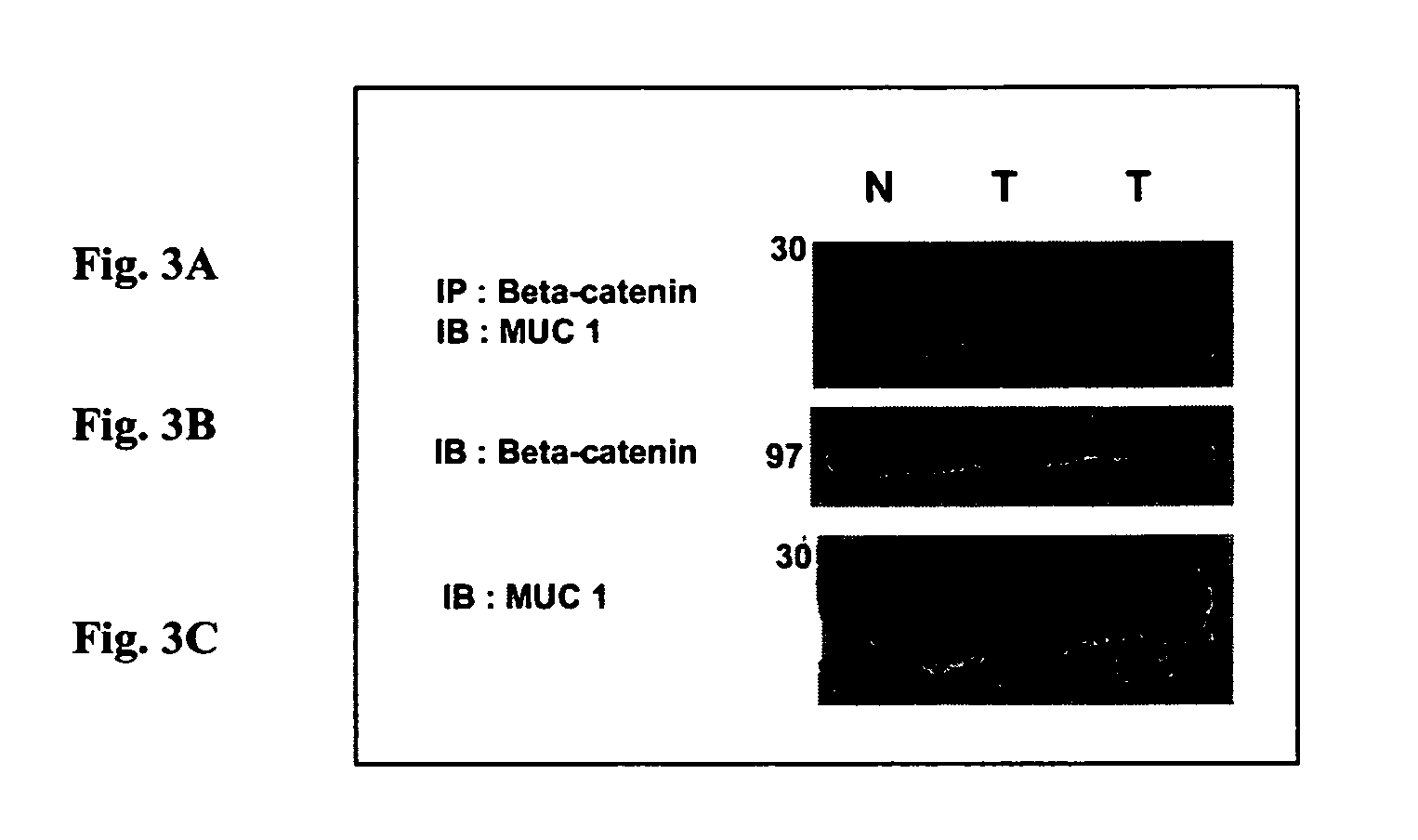
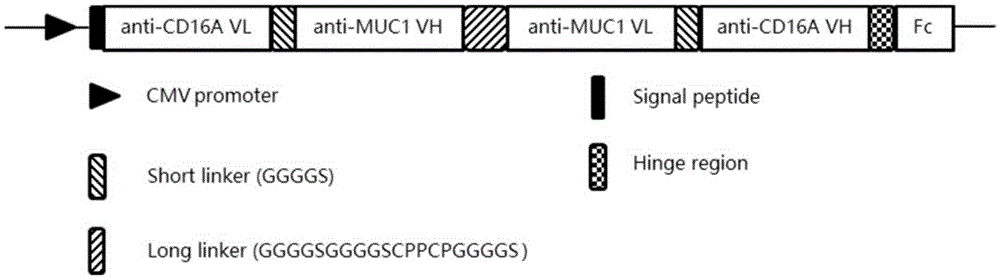
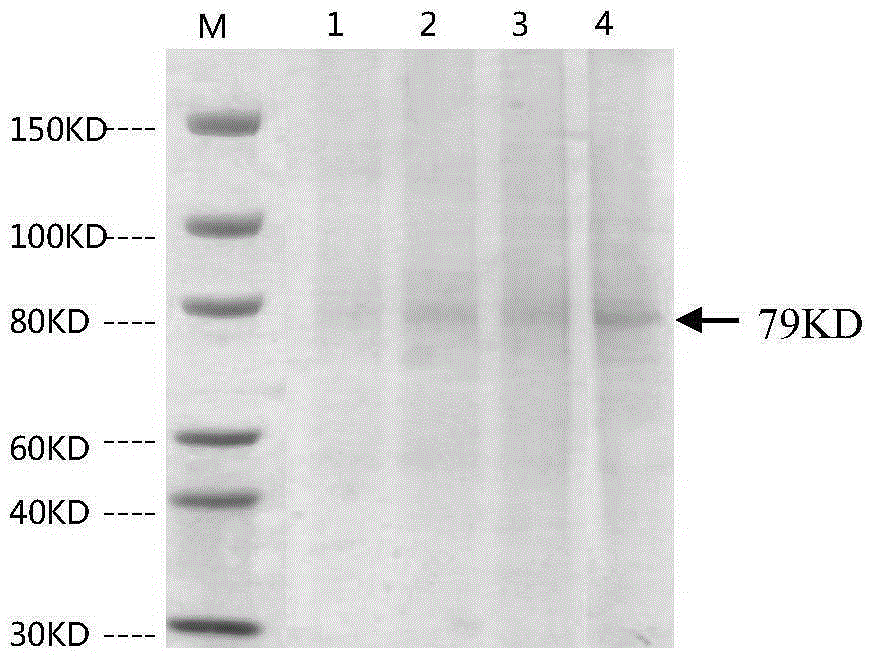
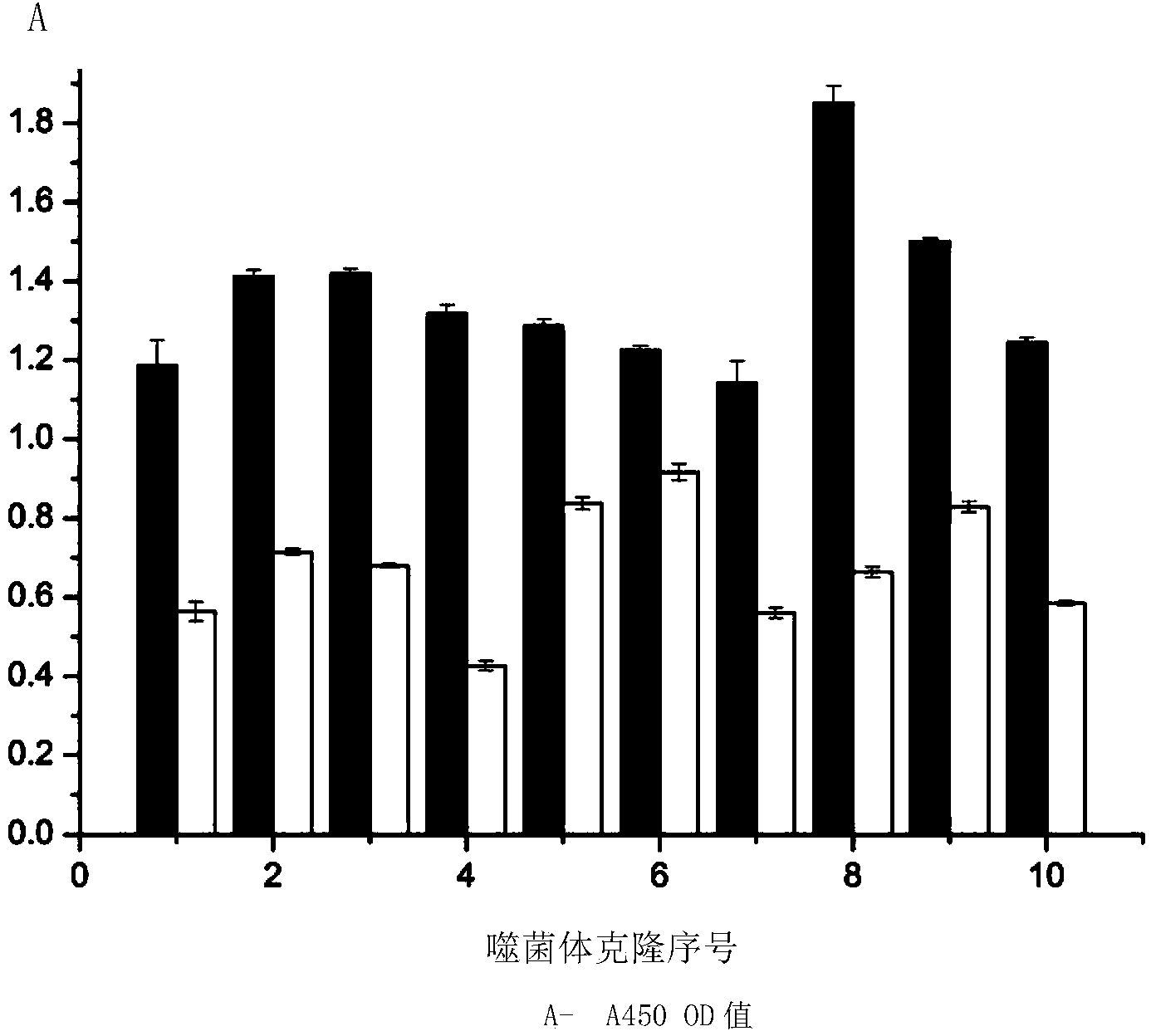
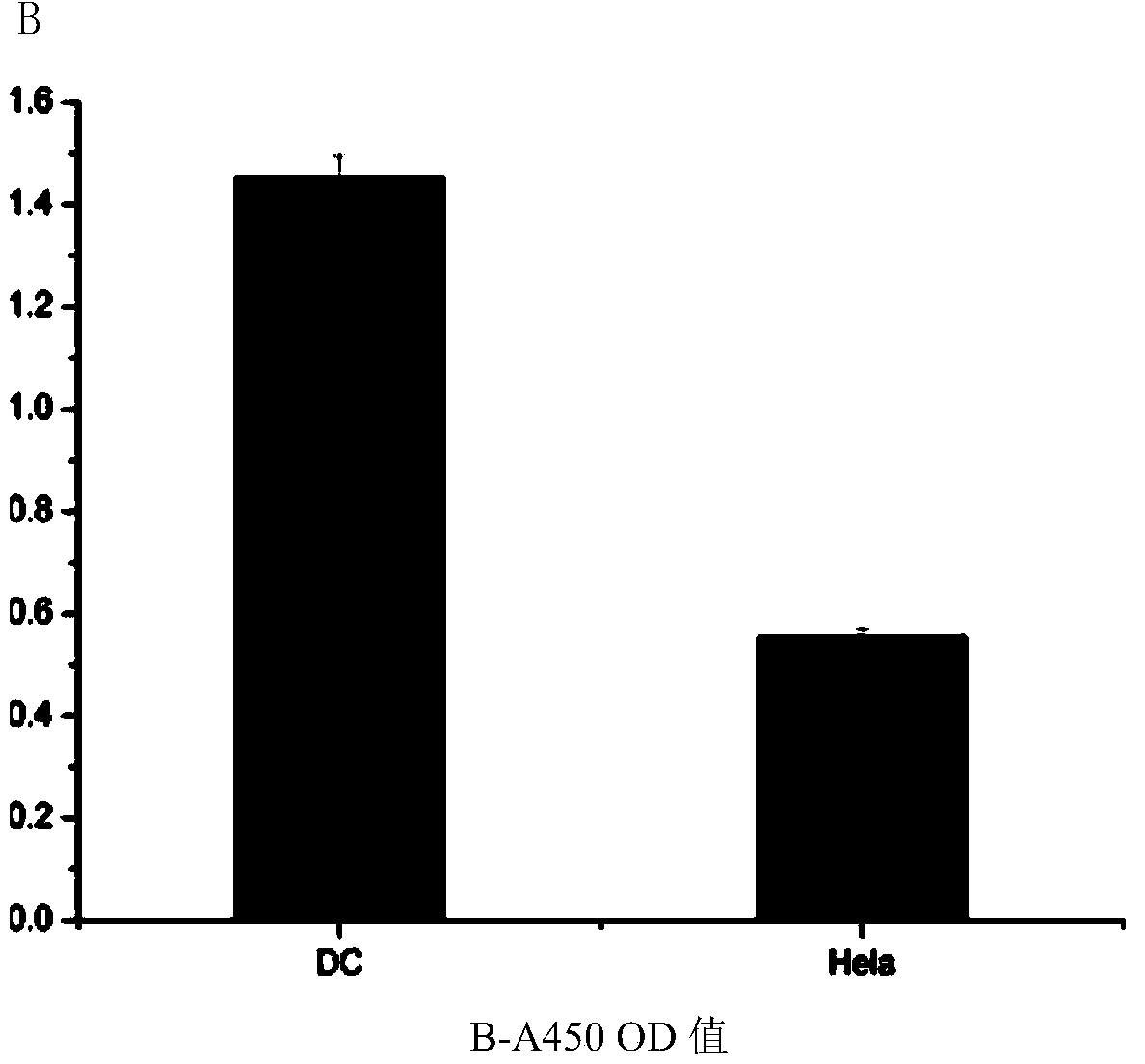
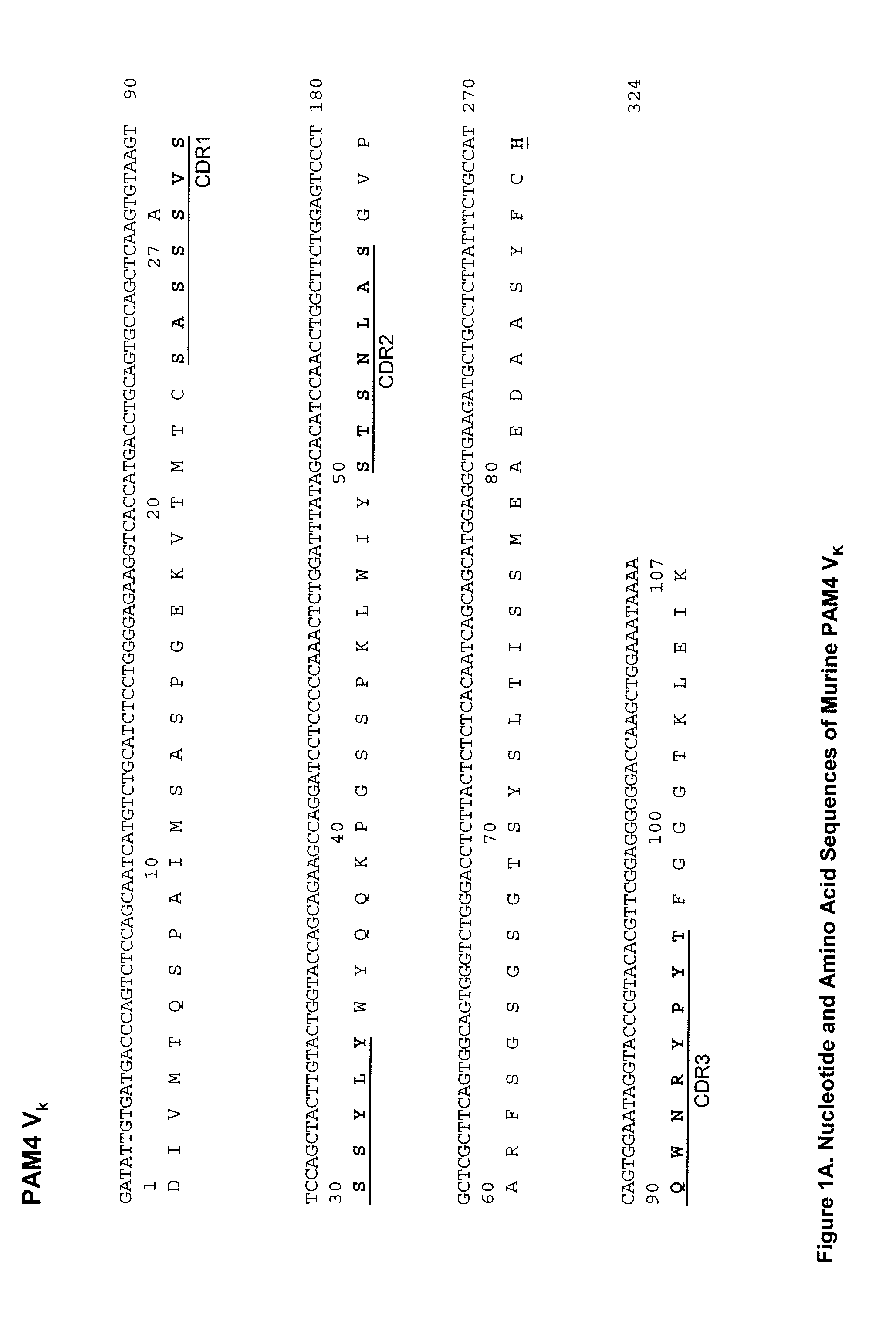
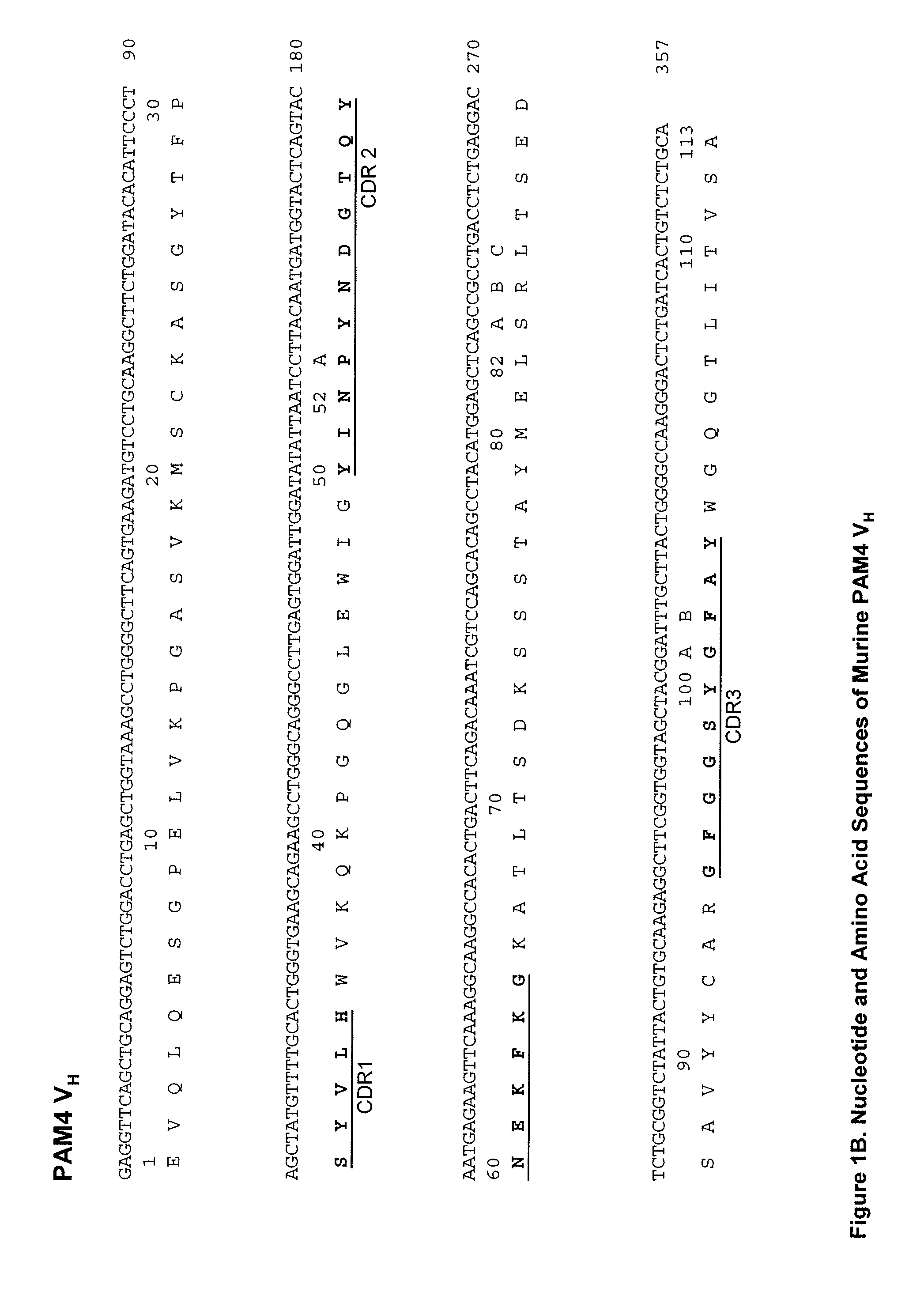
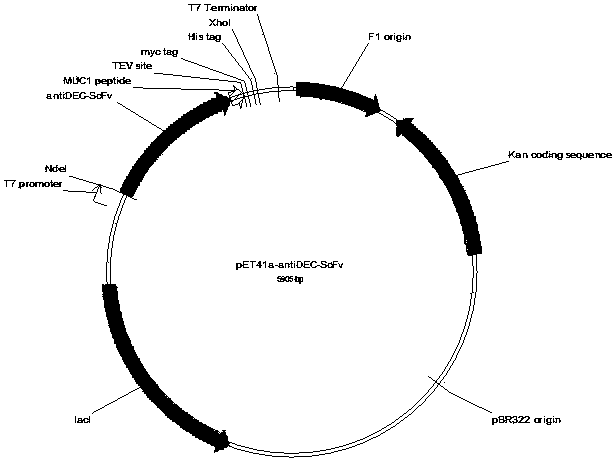
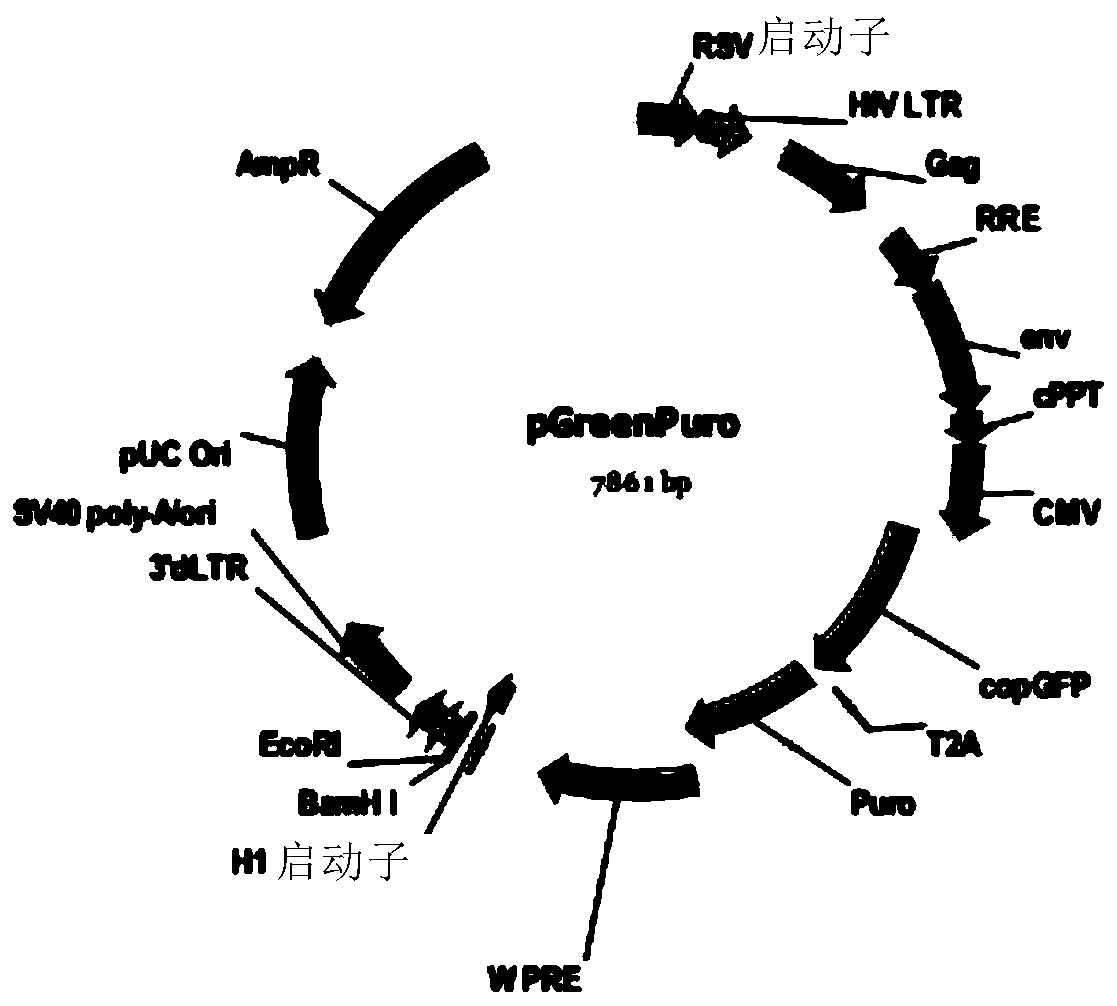
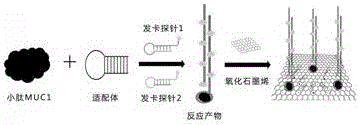
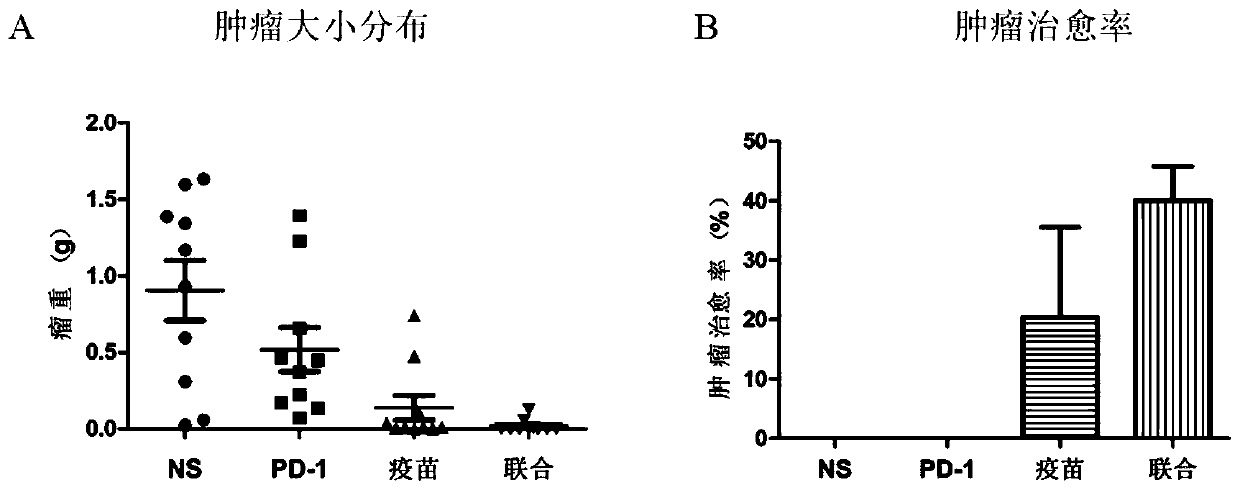
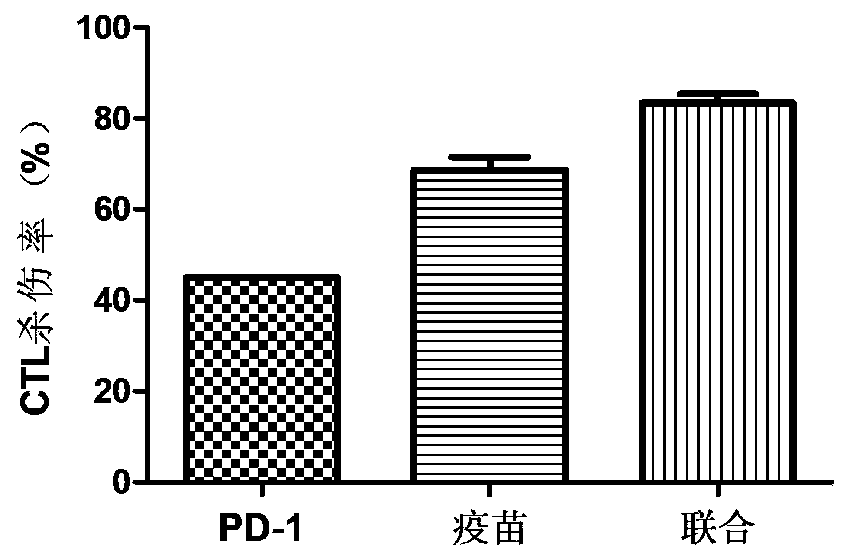
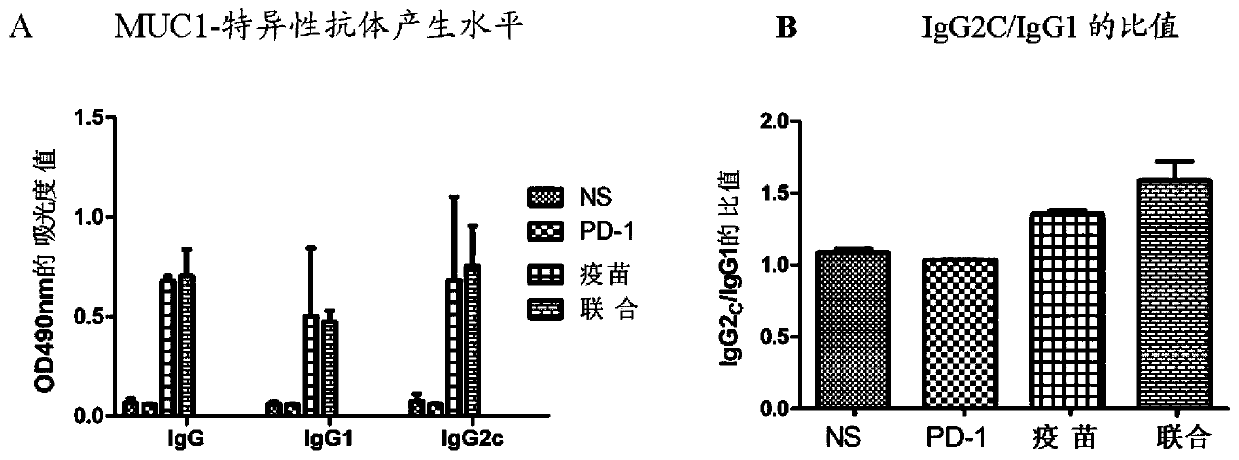
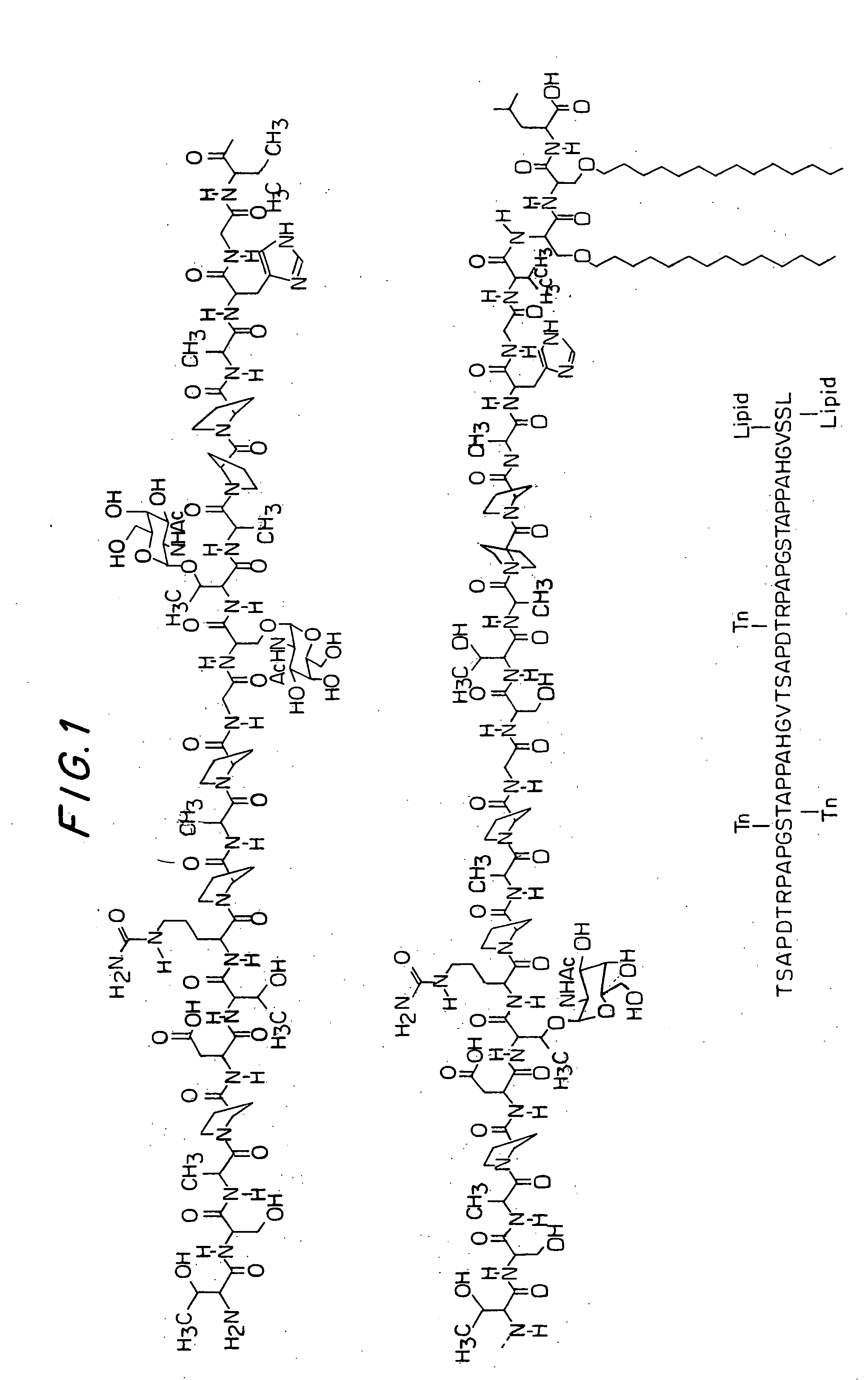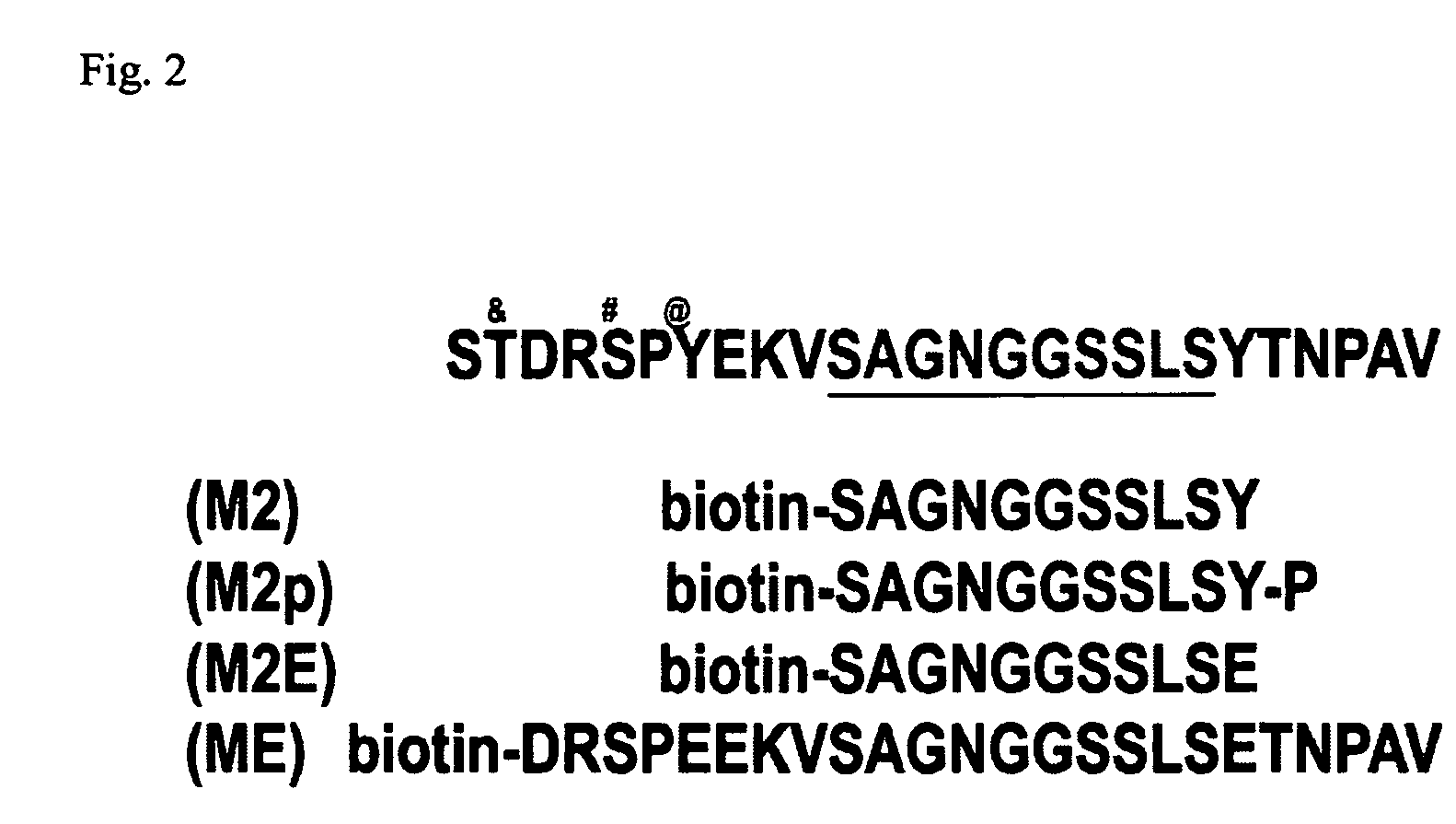Patents
Literature
133 results about "MUC1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
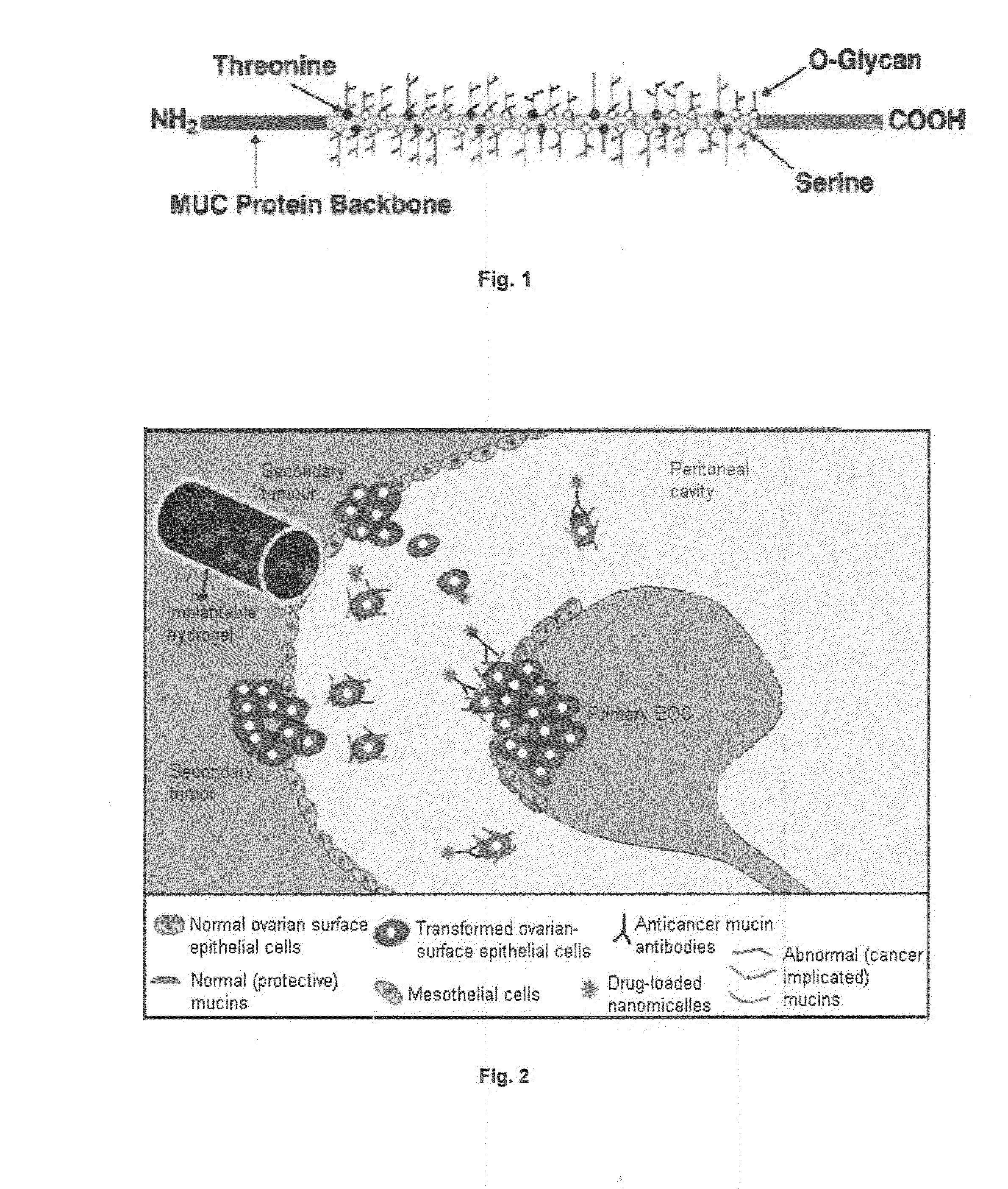
Mucin 1, cell surface associated (MUC1) or polymorphic epithelial mucin (PEM) is a mucin encoded by the MUC1 gene in humans. MUC1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Mucins line the apical surface of epithelial cells in the lungs, stomach, intestines, eyes and several other organs. Mucins protect the body from infection by pathogen binding to oligosaccharides in the extracellular domain, preventing the pathogen from reaching the cell surface. Overexpression of MUC1 is often associated with colon, breast, ovarian, lung and pancreatic cancers. Joyce Taylor-Papadimitriou identified and characterised the antigen during her work with breast and ovarian tumors.
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
Diagnostic panel of cancer antibodies and methods for use
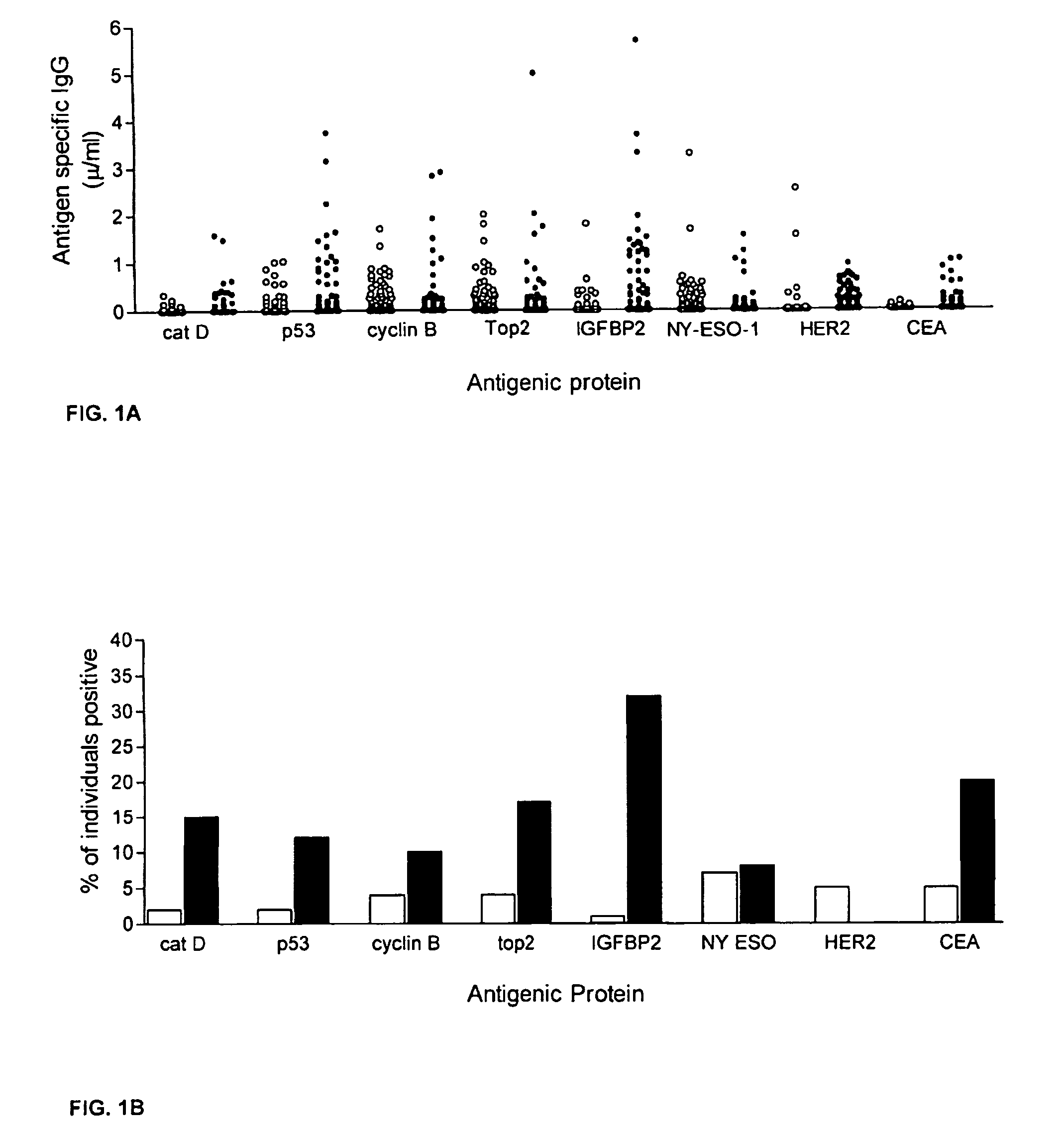
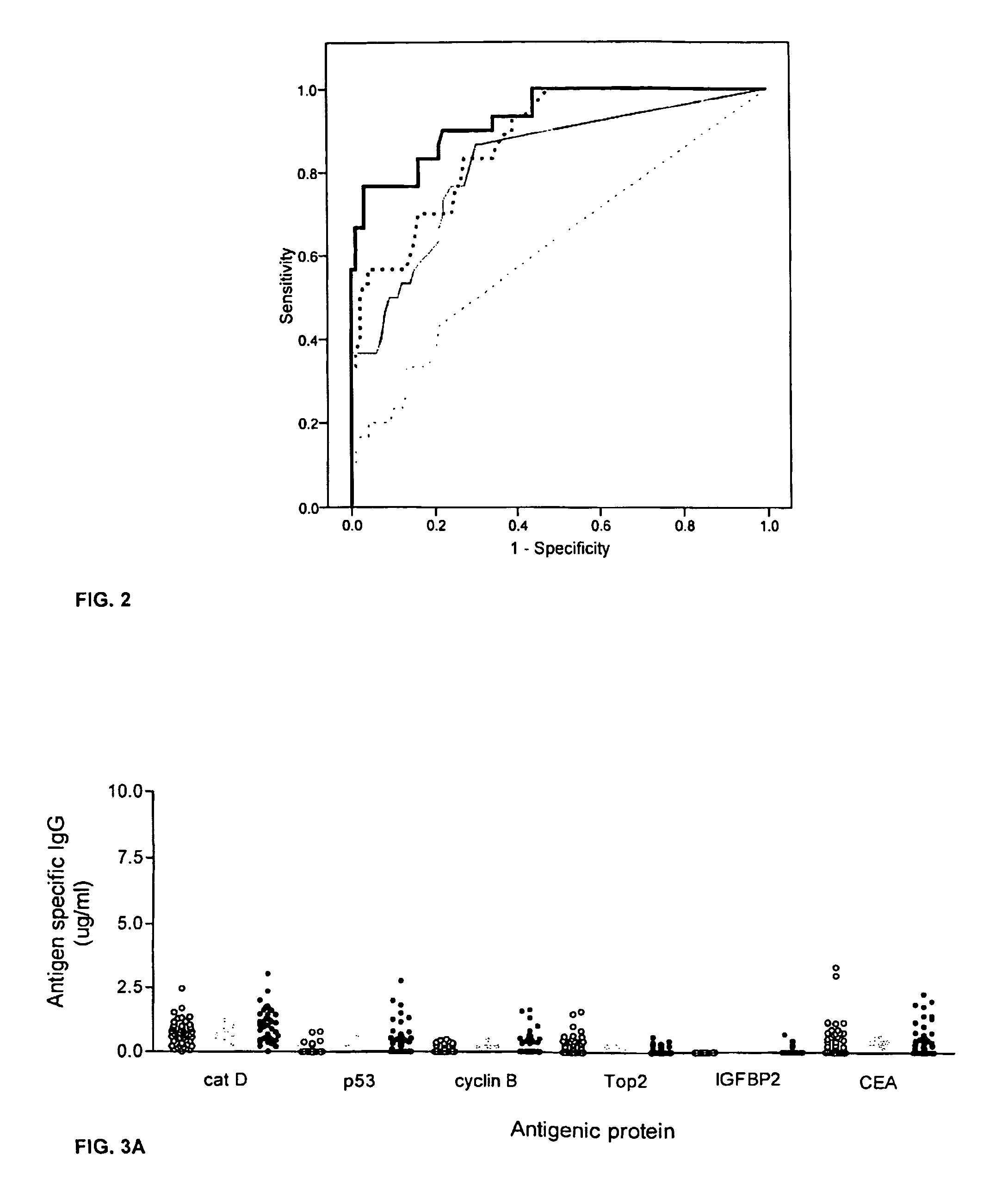
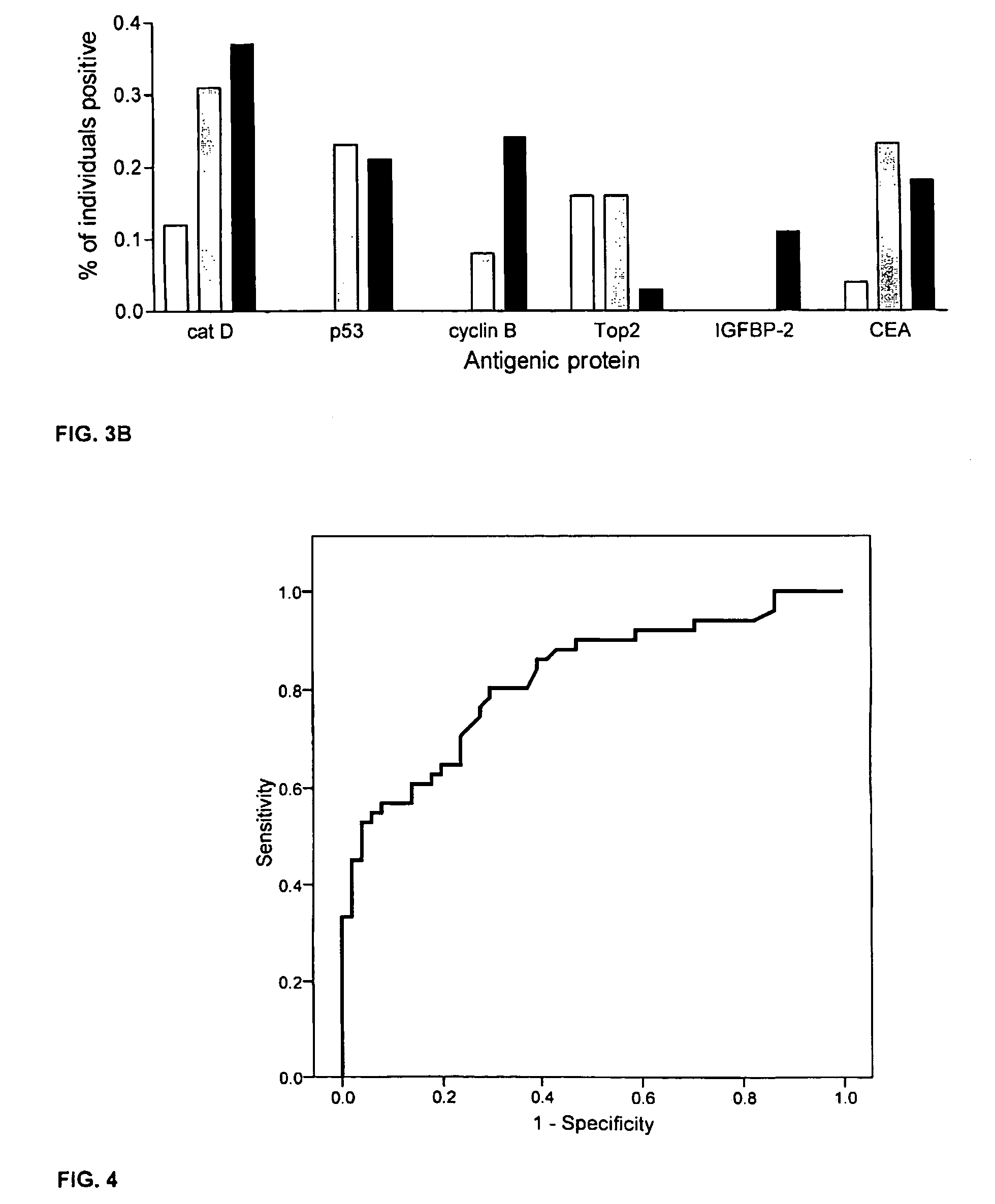
The invention provides a method for detection of a malignancy in a specimen of bodily fluid. The method comprises contacting the specimen with at least two antigens selected from the group consisting of p53, IGFBP2, Topo2α, cathepsin D, cyclin B, cyclin D1, MUC1, HER-2 / neu and CEA. The method further comprises incubating the specimen and the antigen for a duration and under conditions that are sufficient for the formation of immunocomplexes; and detecting the presence or absence of immunocomplex formation between the antigens and antibodies specific for the antigens in the specimen, thereby determining the presence or absence of the malignancy. Also provided is a method for monitoring the effectiveness of cancer therapy related to a malignancy in a warm-blooded animal, a method for distinguishing between Stage I and Stage II colorectal cancer in a specimen of bodily fluid.
Owner:UNIV OF WASHINGTON
Peptides and antibodies to MUC 1 proteins
InactiveUS7897351B2Improve the level ofHybrid immunoglobulinsPeptide/protein ingredientsCancer cellWilms' tumor
The invention relates to methods of inhibiting proliferation or growth of tumor cells and / or inducing cell death in cancer cells. This invention relates to the use of antibodies, hybridomas and pharmaceutical compositions containing same, for inhibiting growth or proliferation and inducing death in epithelial, colon, lung, breast and ovarian tumor cells or other cells which express MUC1 proteins.
Owner:RAMOT AT TEL AVIV UNIV LTD
Compositions and methods for preventing or treating cancer
The present invention relates to a MUC1 cytoplasmic tail peptide or portion thereof. These peptides are useful for inducing an immune response to MUC1-expressing tumor cells and thus for preventing or treating cancer.
Owner:UNIV OF NEBRASKA LINCOLN
Therapeutic peptides for the treatment of metastatic cancer
InactiveUS20060293234A1Invasiveness is thereby reduced and retardedVirusesPeptide/protein ingredientsLymphatic SpreadBinding site
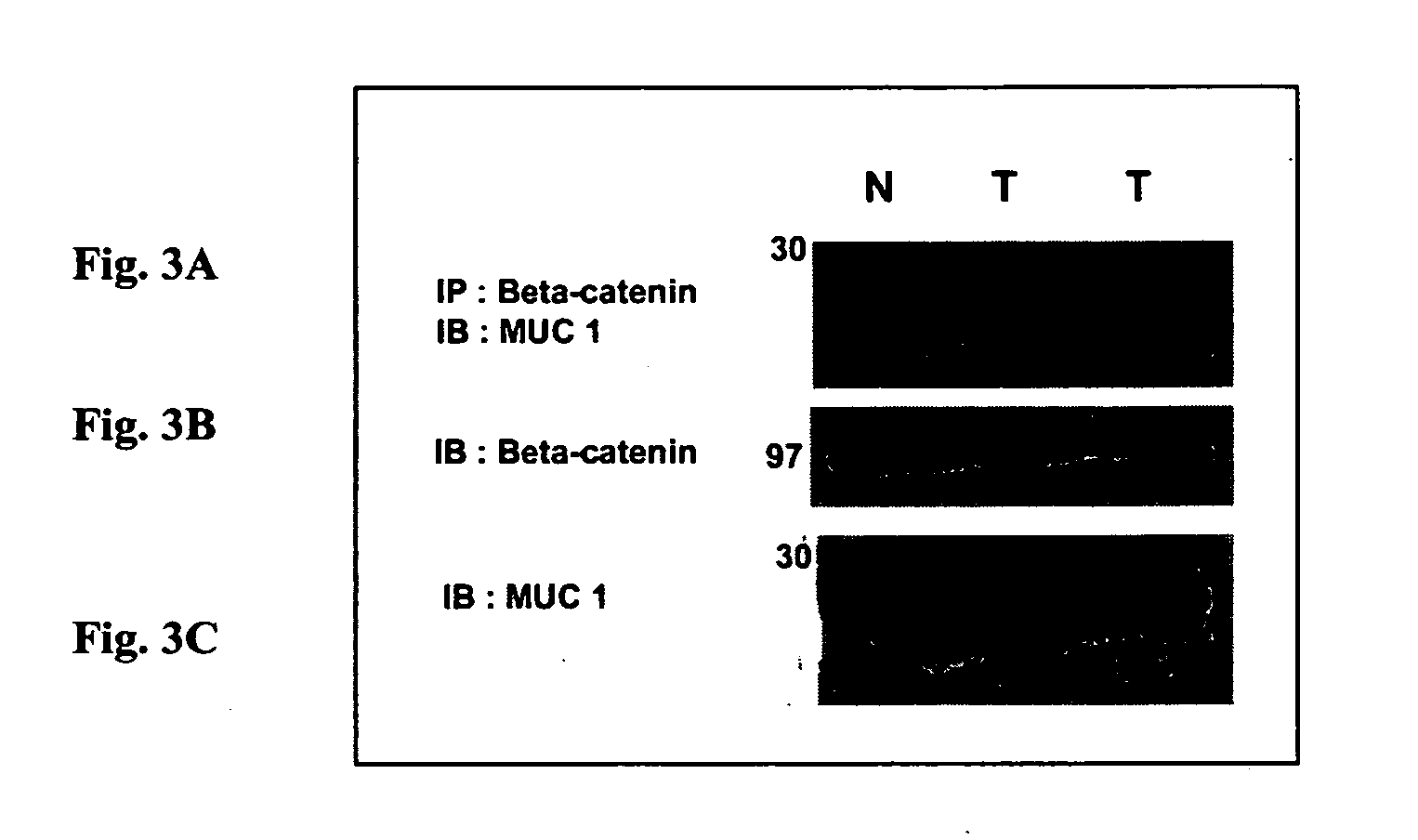
Interaction between MUC1 and β-catenin can be interrupted using polypeptides or antibodies that specifically bind to the binding site on MUC1. Interruption provides the beneficial effect of inhibiting, reducing, and / or retarding invasiveness and metastasis. Fusion polypeptides and antibodies are provided to achieve a therapeutic effect.
Owner:ARIZONA CANCER THERAPEUTICS
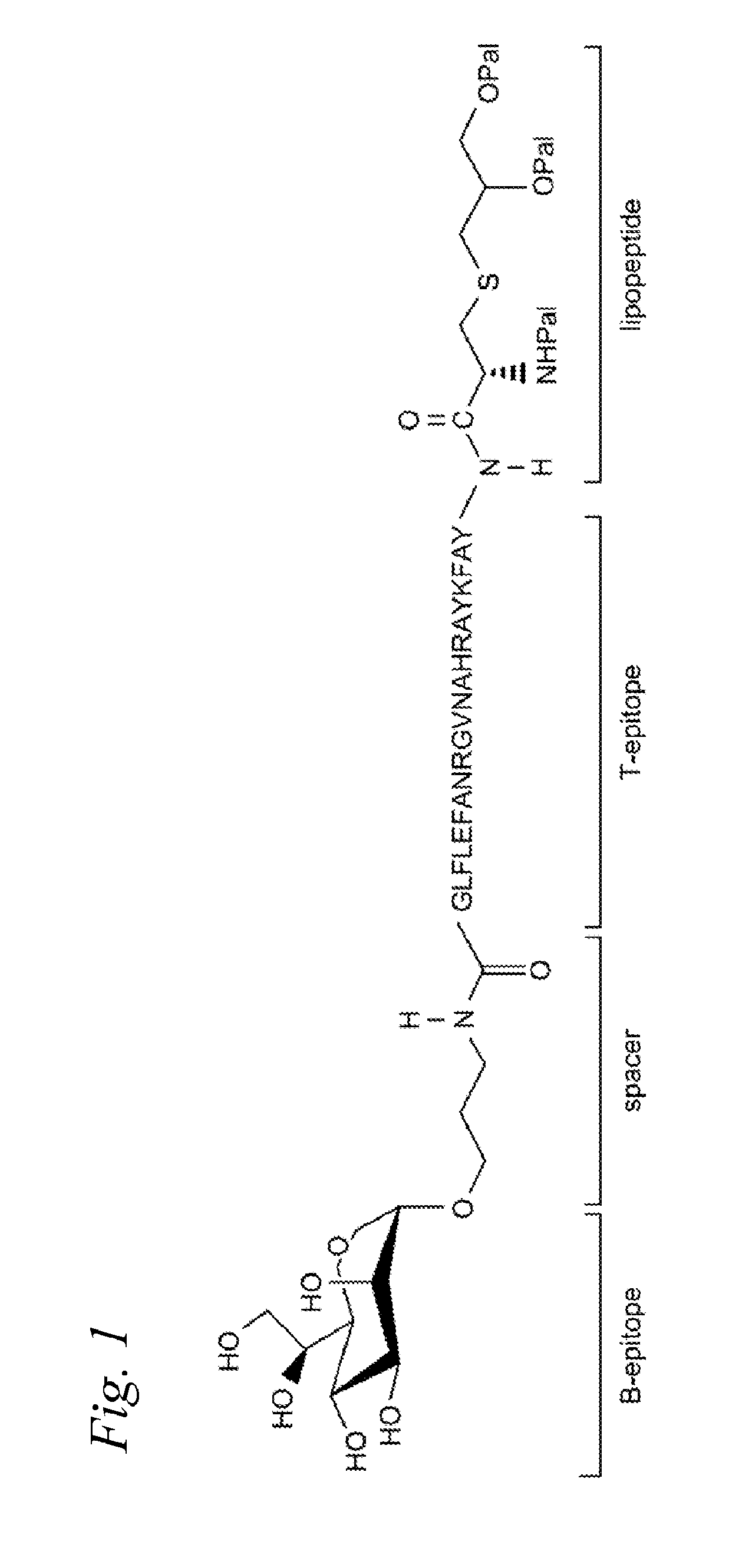
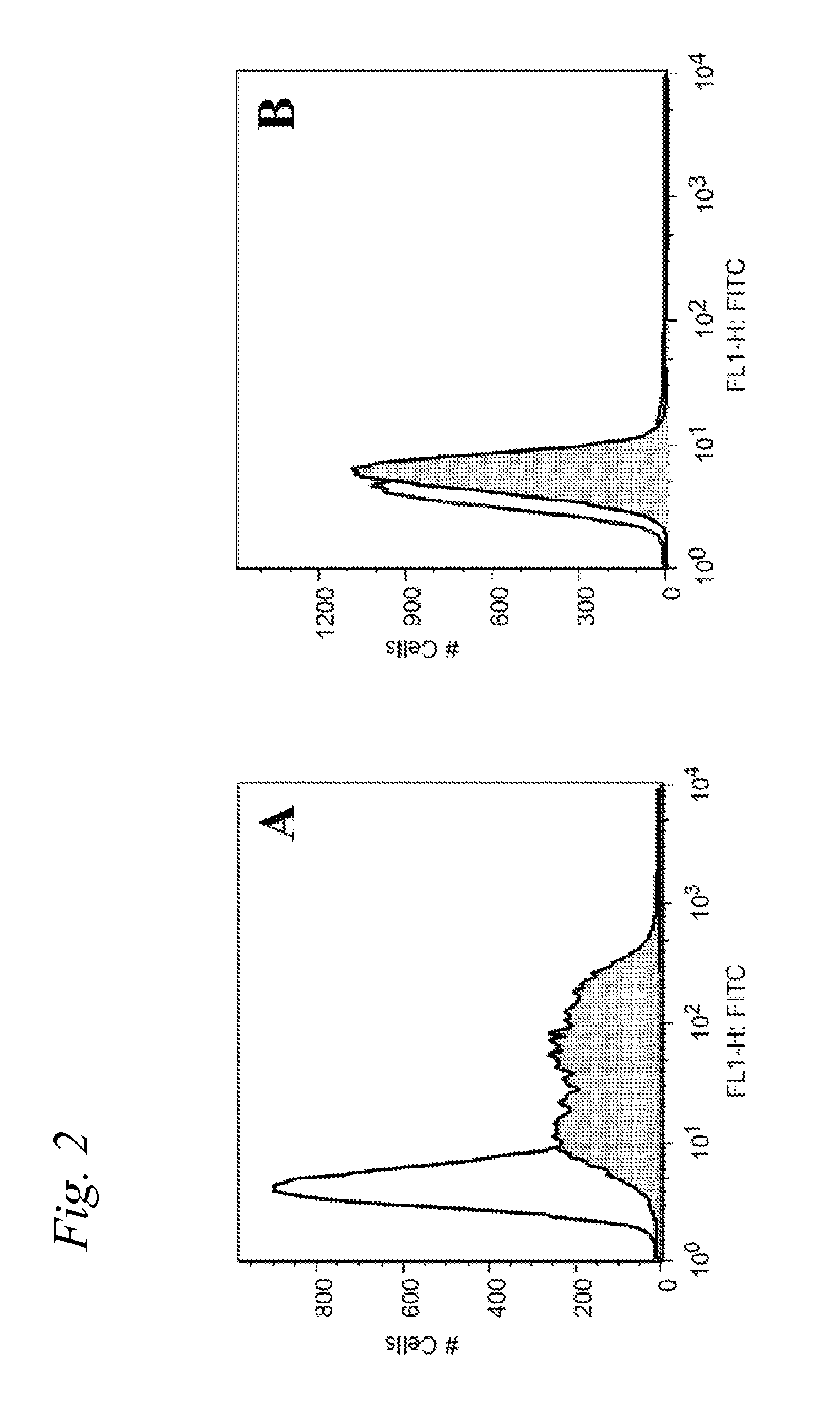
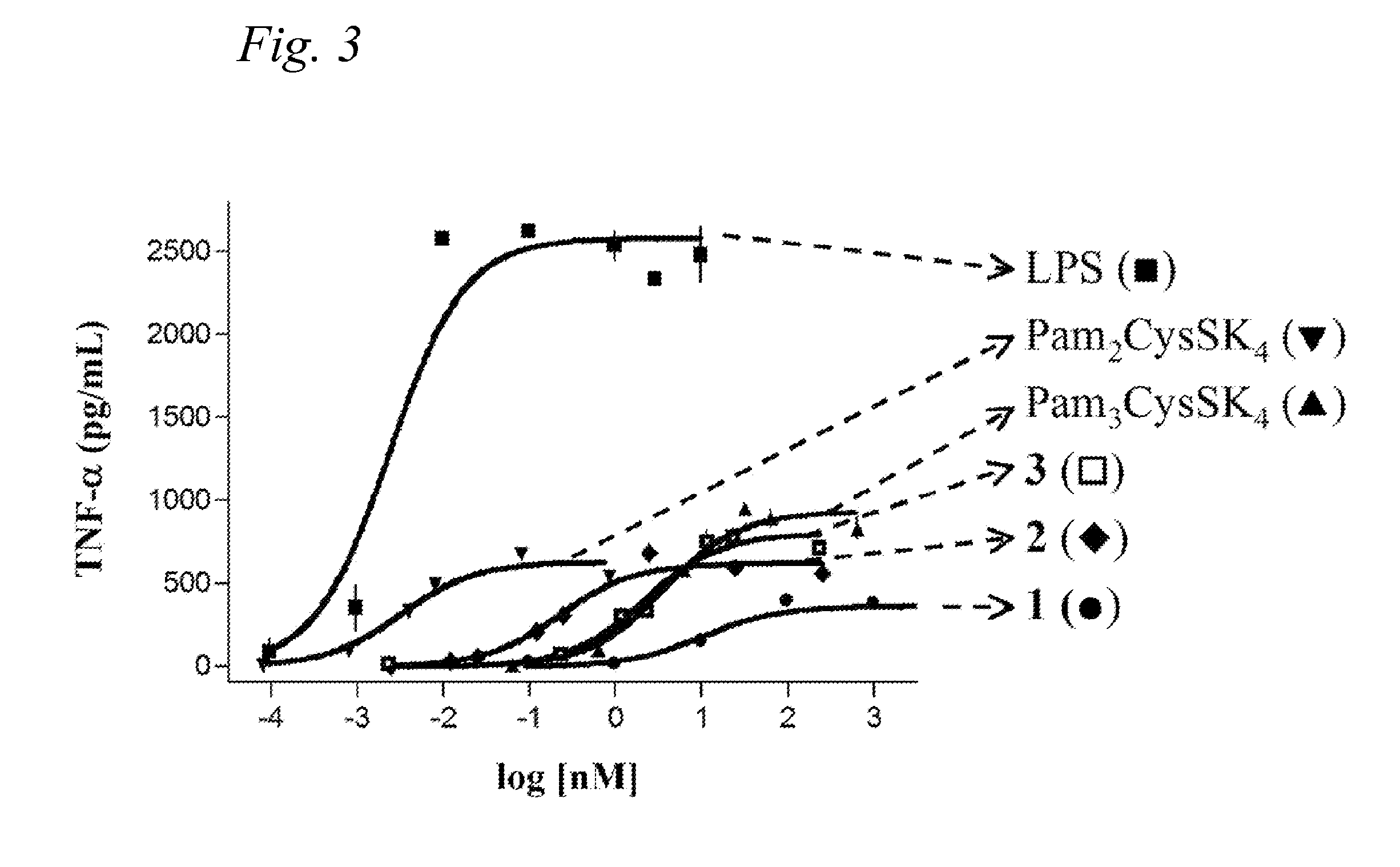
Synthetic glyco-lipo-peptides as vaccines
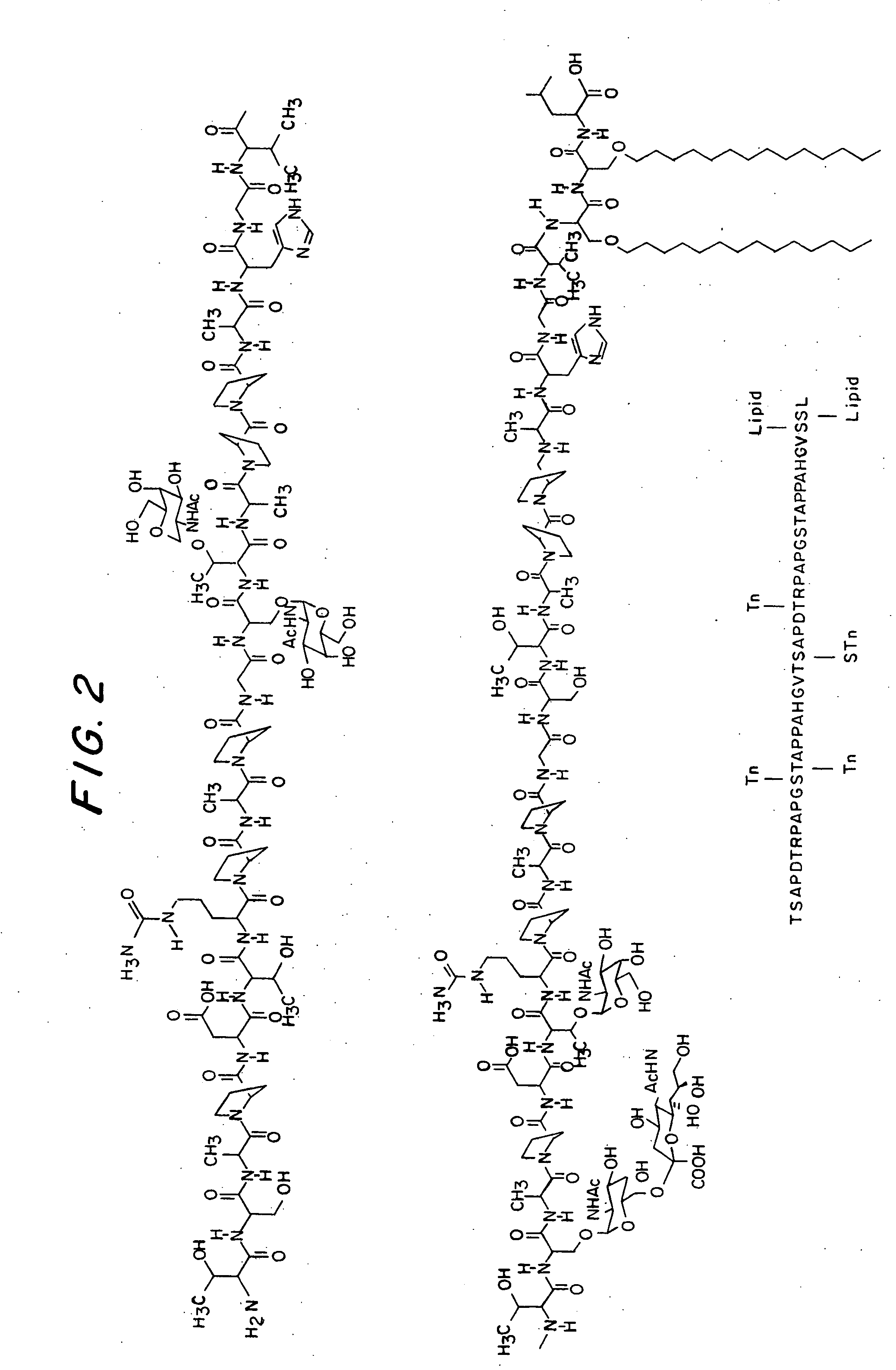
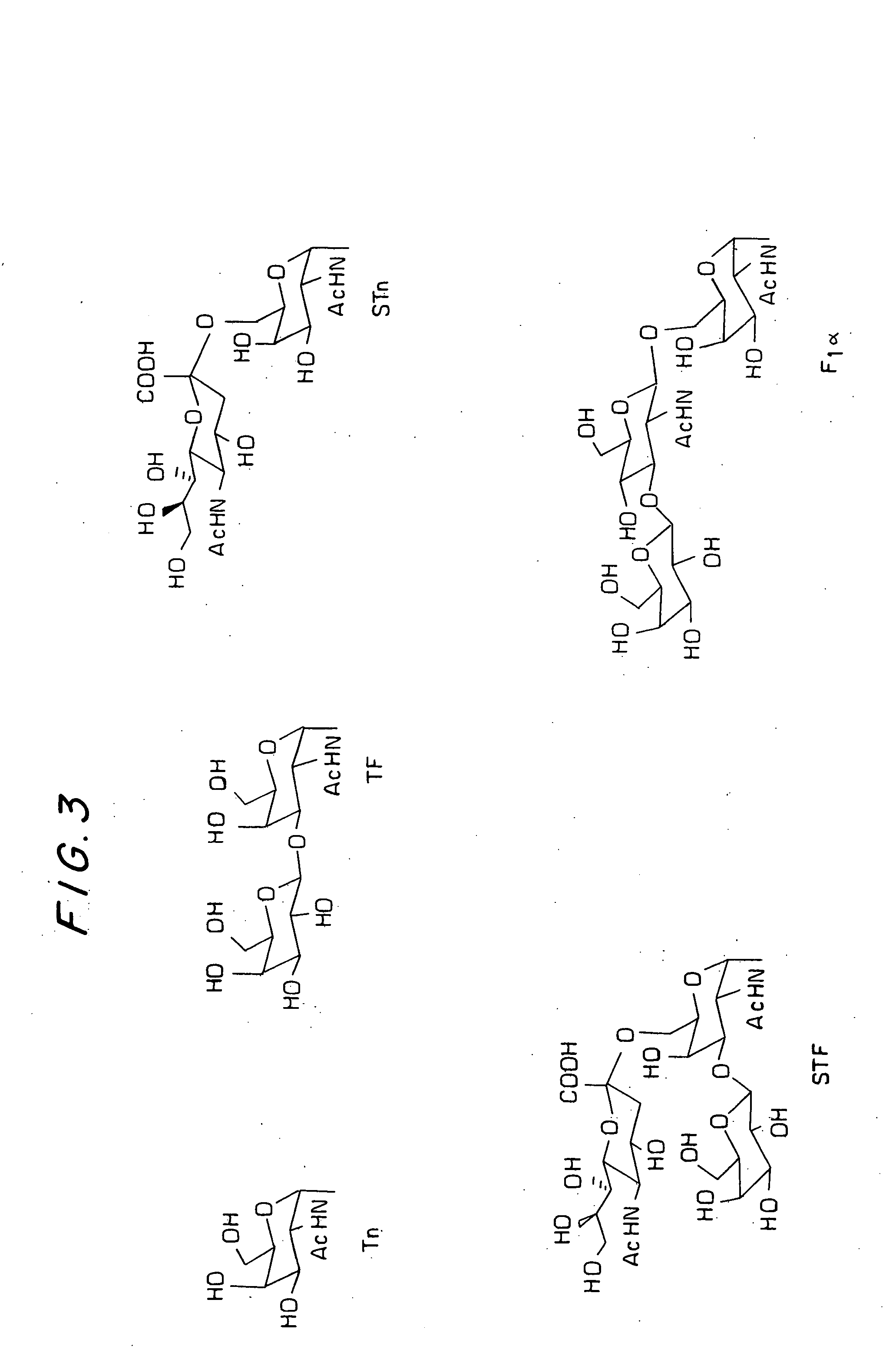
A glycolipopeptide comprising at least one disease-associated epitope, and characterized by at least one lipidated interior amino acid or by the presence of a MUC1 epitope, may be used in a vaccine, preferably in conjunction with a liposome.
Owner:ONCOTHYREON
Gene Expression Markers for Predicting Response to Chemotherapy
The present invention provides sets of genes the expression of which is important in the prognosis of cancer. In particular, the invention provides gene expression information useful for predicting whether cancer patients are likely to have a beneficial treatment response to chemotherapy. FHIT; MTA1; ErbB4; FUS; BBC3; IGF1R; CD9; TP53BP1; MUC1; IGFBP5; rhoC; RALBP1; STAT3; ERK1; SGCB; DHPS; MGMT; CRIP2; ErbB3; RAP1GDS1; CCND1; PRKCD; Hepsin; AK055699; ZNF38; SEMA3F; COL1A1; BAG1; AKT1; COL1A2; Wnt.5a; PTPD1; RAB6C; GSTM1, BCL2, ESR1; or the corresponding expression product, is determined, said report includes a prediction that said subject has a decreased likelihood of response to chemotherapy.
Owner:GENOMIC HEALTH INC +1
Recombination human Mucl-MBP fusion protein antitumour vaccine and production technology
InactiveCN1513556ATo achieve the purpose of anti-tumorLow costPeptide/protein ingredientsAntibody medical ingredientsEscherichia coliChemical synthesis
An anticancer vaccine of recombinant human MOC1-MBP fusion protein is disclosed, in which MBP is used as its adjuvant. The MBP gene and MUC1 gene are fused together. The MBP substituted for other fusion protein to induce CTL reaction. The pMAL-P2 is the carrier for effectively expressing maltose fusion protein. The serial repetitive sequence of MUC1 is inserted to downstream of malE gene.
Owner:台桂香
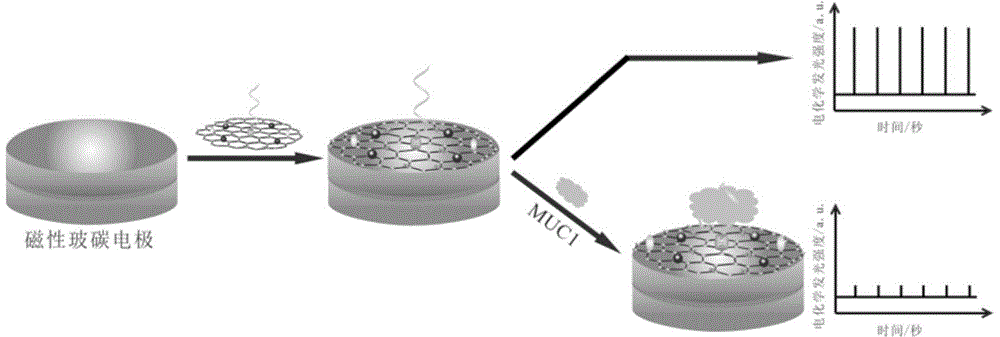
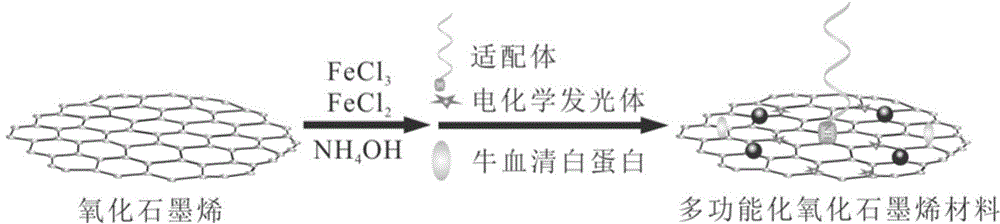
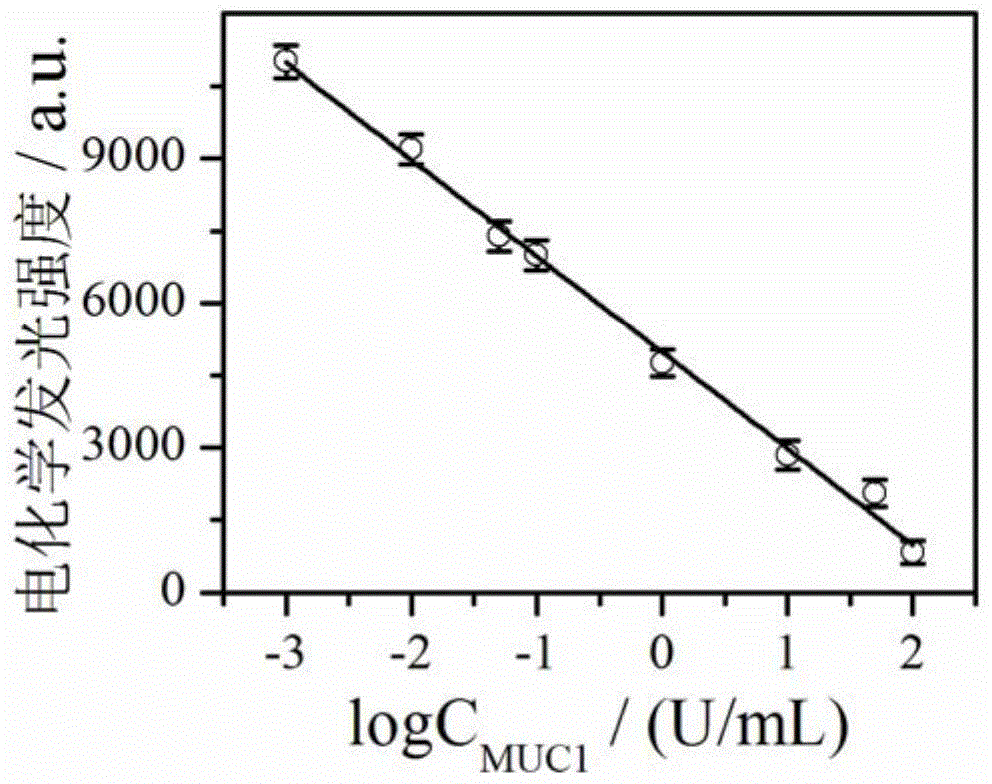
Preparation method and application of electrochemiluminescence aptamer sensor for detecting tumor marker MUC1
InactiveCN104655616ARealize quantitative detectionStrong and stable electrochemiluminescenceChemiluminescene/bioluminescenceAptamerElectrochemiluminescence
The invention discloses a preparation method and an application of an electrochemiluminescence aptamer sensor for detecting a tumor marker MUC1. The preparation method is characterized by comprising a step of synthesizing magnetic graphene oxide; simultaneously marking an electrochemiluminescence body and an aptamer on the surface of the magnetic graphene oxide material so as to obtain a multifunctional graphene oxide material; and a step of finally dropping and applying 5-10 mu L of multifunctional graphene oxide material to the surface of a magnetic glassy carbon electrode so as to obtain an electrochemiluminescence aptamer sensor for detecting the tumor marker MUC1. The electrochemiluminescence aptamer sensor can be used for determining the concentration of the tumor marker MUC1 in a to-be-detected sample on the basis of a quantitative relation between electrochemiluminescence intensity and the concentration of a tumor marker MUC1 solution, and the sensor has the advantages of being simple and rapid in detection method, sensitive and accurate in detection result, and high in specificity.
Owner:NINGBO UNIV
Inhibition of inflammation using antagonists of muc1
The invention provides for peptides from the MUC1 cytoplasmic domain and methods of use therefor. These peptides can inhibit MUC1 oligomerization, inhibit the interaction of MUC1 with NF-κB or a STAT, and block inflammatory response mediated by NF-κB or STAT signaling.
Owner:GENUS ONCOLOGY +1
Preparation method of antigen-specific cytotoxicity T lymphocytes
InactiveCN104004712AHigh purityHigh activity titerBlood/immune system cellsFermentationT lymphocyteT cell
The invention discloses a preparation method of antigen-specific cytotoxicity T lymphocytes. The method comprises the following steps: 1, constructing a recombinant vector carrying tumor antigen gene; 2, separating and culturing DC cells; 3, transducing immature DC cells in step 2 by using the recombinant vector in step 1; 4, inducing the immature DC cells in step 3 through cytokines to form mature DC cells; and 5, coculturing the mature DC cells in step 4 and T lymphocytes to obtain the antigen-specific cytotoxicity T lymphocytes. The method preferably selects a lentivirus mixed vector realizing transduction to be used in the expression of three wide spectrum tumor antigens comprising CEA, MAGE-A3 and MUC1 to obtain activated and proliferated tumor antigen-specific T cells, and has a wide application prospect.
Owner:SHENZHEN HORNETCORN BIOTECH
Anti-Mucin Antibodies for Early Detection and Treatment of Pancreatic Cancer
InactiveUS20120141375A1Strong specificityHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsBiocidePancreas CancersAntiendomysial antibodies
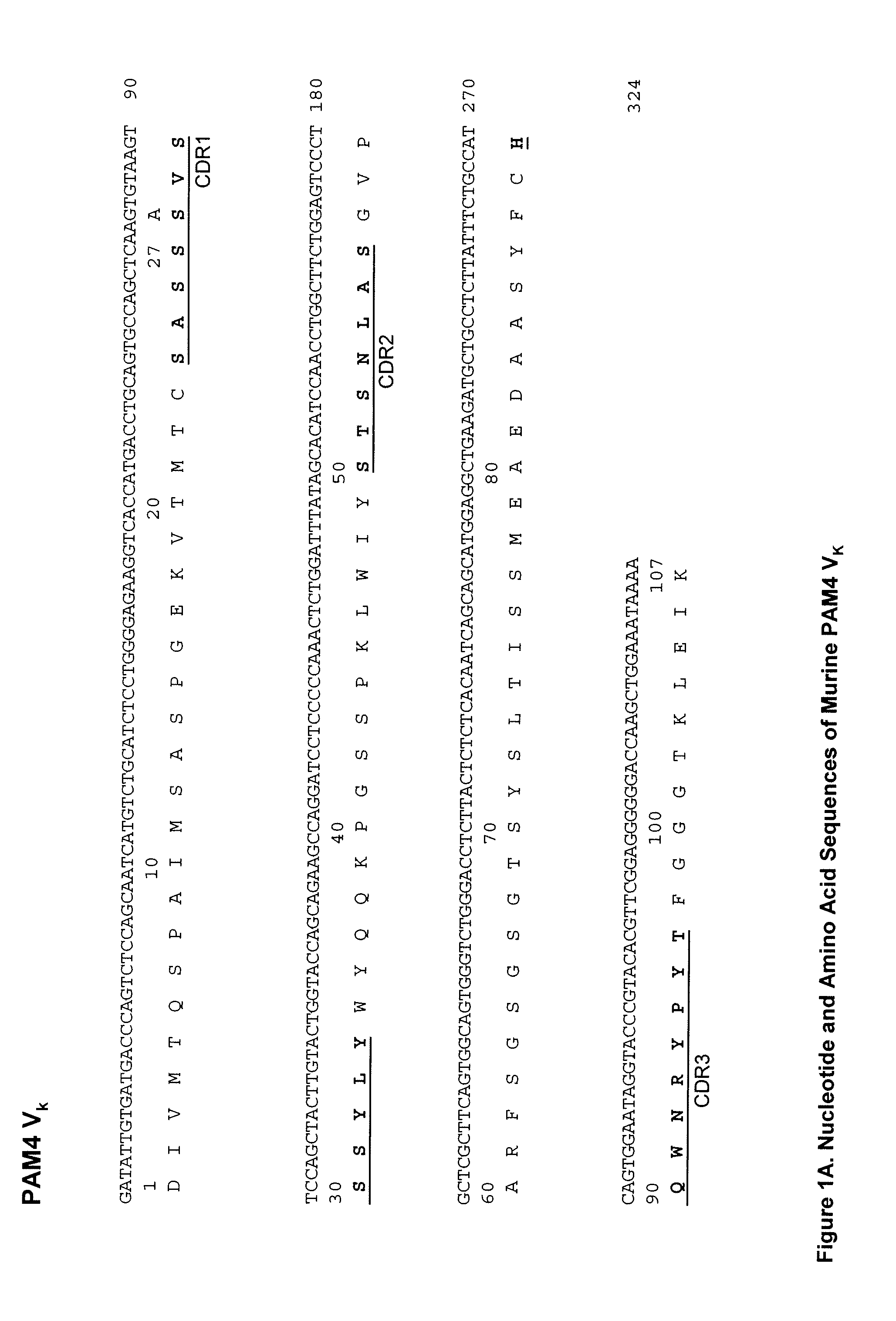
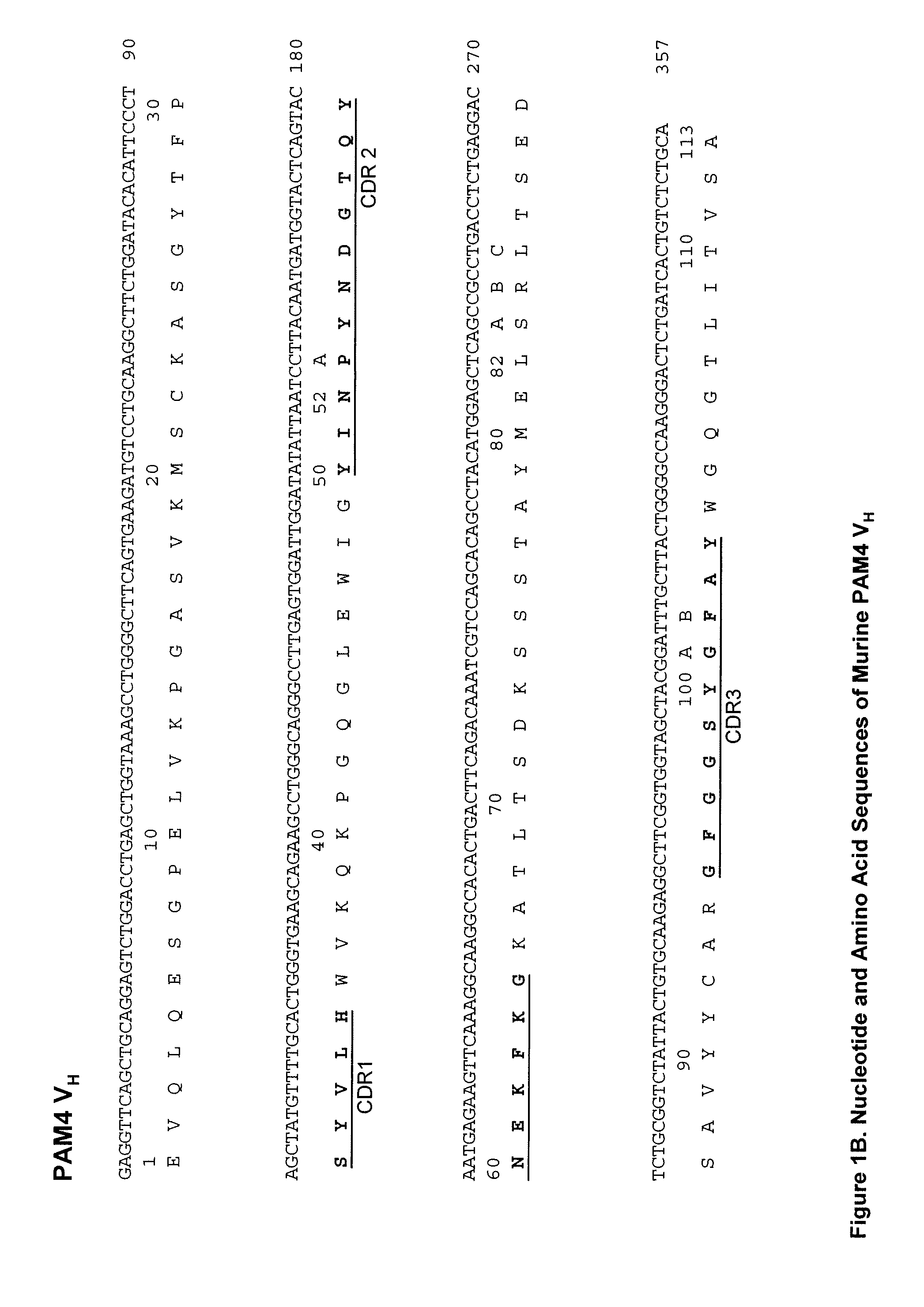
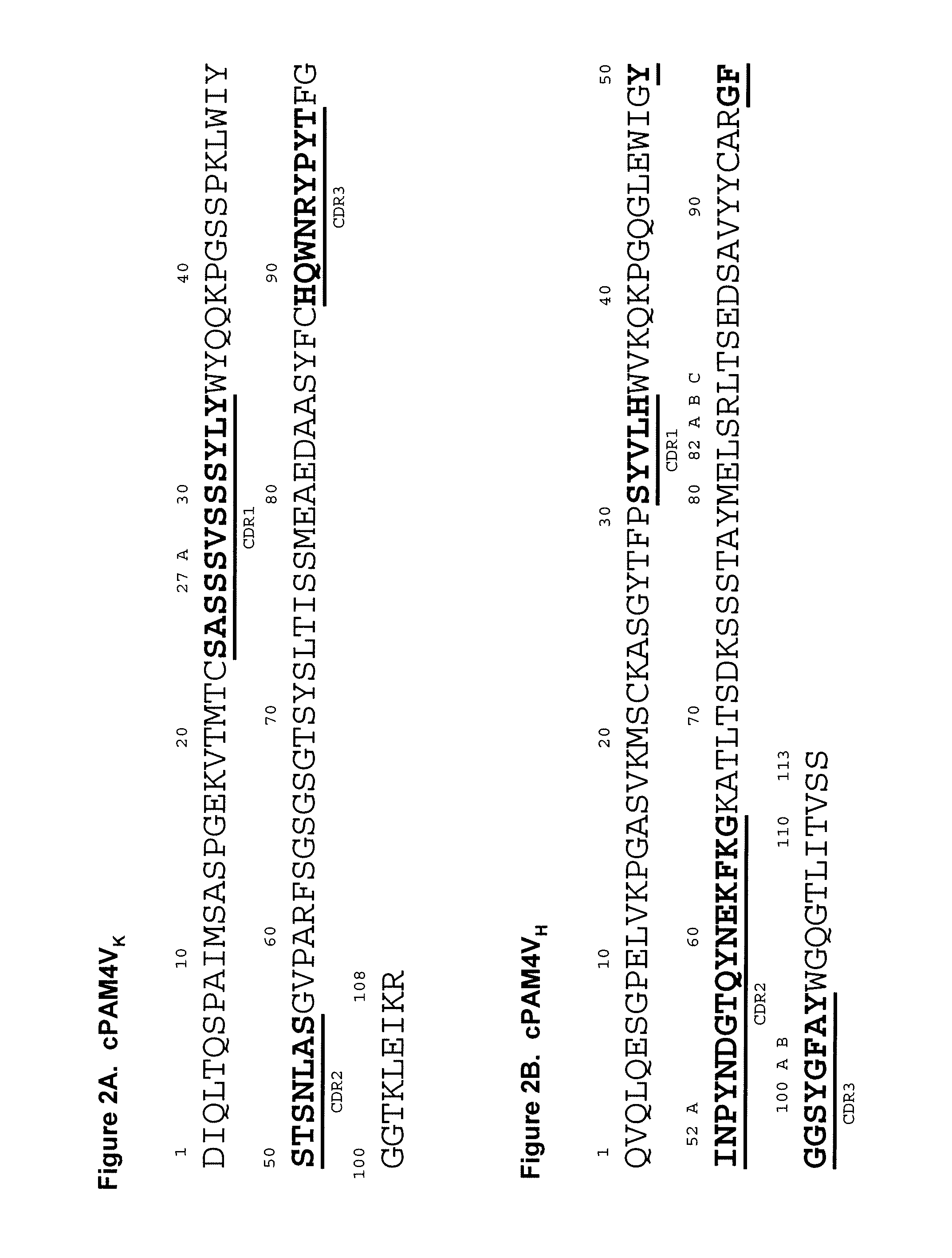
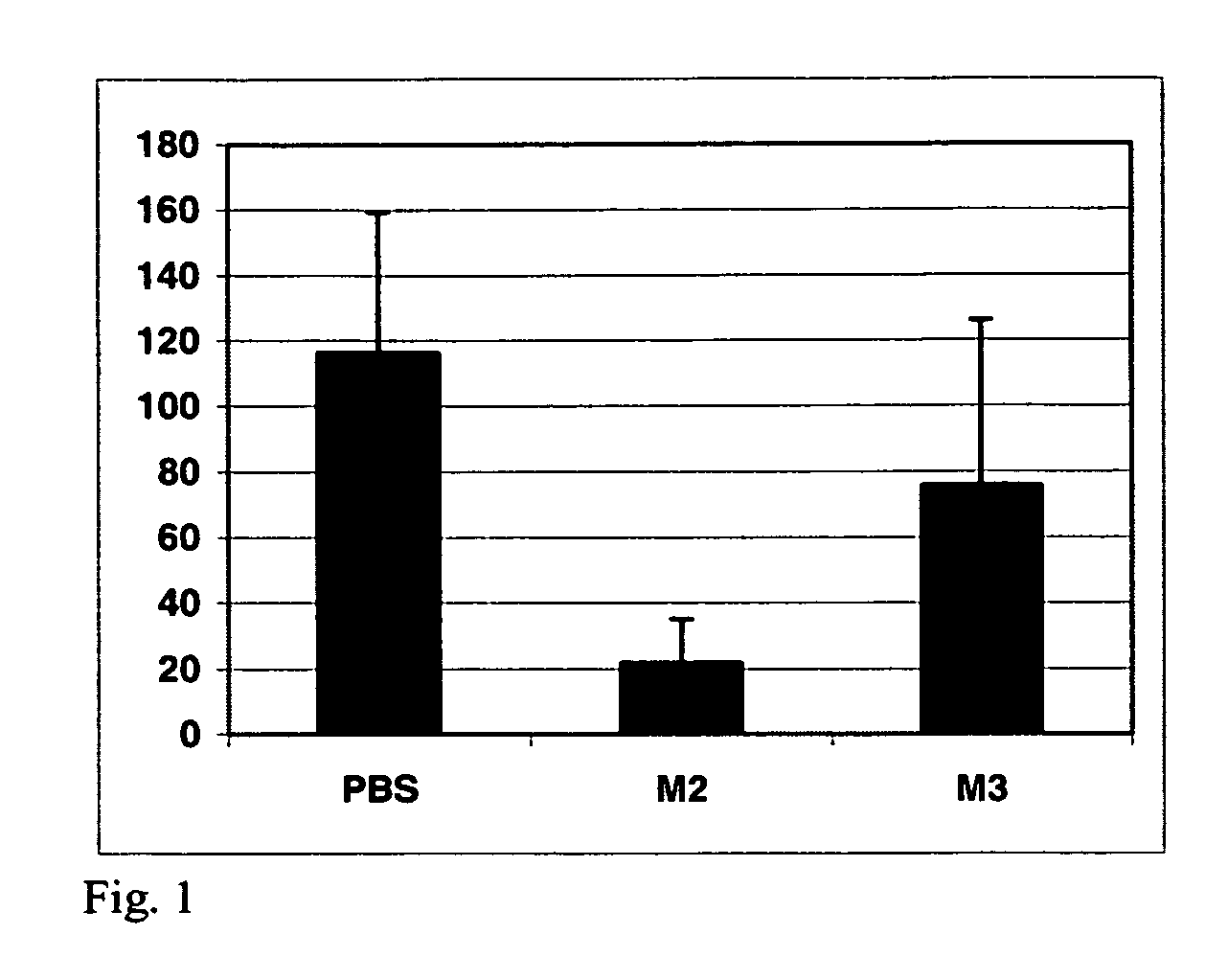
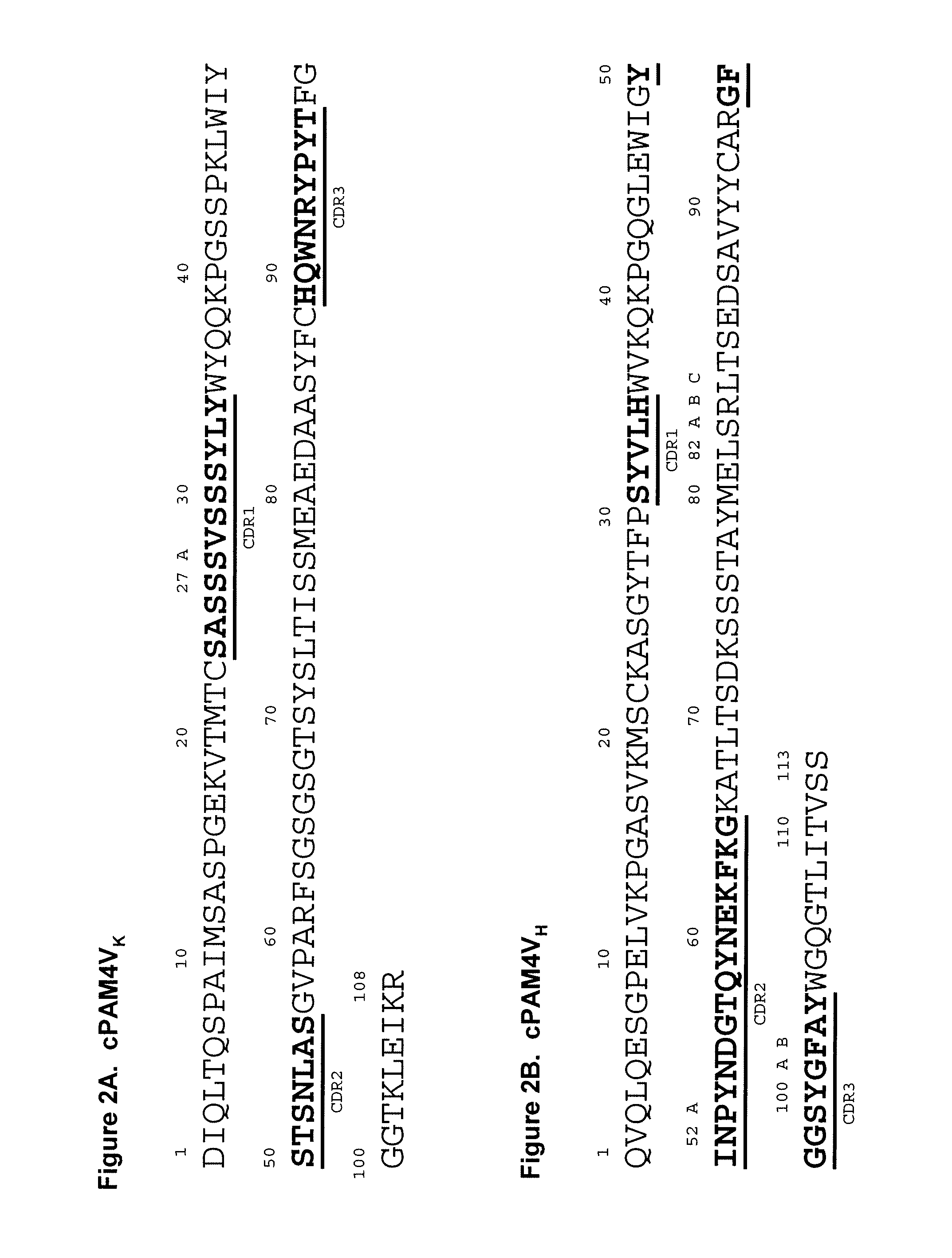
Described herein are compositions and methods of use of anti-pancreatic cancer antibodies or fragments thereof, such as murine, chimeric, humanized or human PAM4 antibodies. The antibodies show novel and useful diagnostic characteristics, such as binding with high specificity to pancreatic and other cancers, but not to normal or benign pancreatic tissues and binding to a high percentage of early stage pancreatic cancers. Preferably, the antibodies bind to pancreatic cancer mucins such as MUC1 or MUC5ac and are of use for the detection and diagnosis of early stage pancreatic cancer. In more preferred embodiments, the anti-pancreatic cancer antibodies can be used for immunoassay of serum samples, wherein the immunoassay detects a marker for early stage pancreatic cancer in serum. Most preferably, the serum is extracted with an organic phase, such as butanol, before immunoassay. Alternatively, immunoassay with PAM4 and anti-CA19.9 antibodies may be utilized to improve sensitivity for pancreatic cancer.
Owner:IMMUNOMEDICS INC
Therapeutic peptides for the treatment of metastatic cancer
InactiveUS7767642B2Invasiveness is thereby reduced and retardedPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesBinding site
Interaction between MUC1 and β-catenin can be interrupted using polypeptides or antibodies that specifically bind to the binding site on MUC1. Interruption provides the beneficial effect of inhibiting, reducing, and / or retarding invasiveness and metastasis. Fusion polypeptides and antibodies are provided to achieve a therapeutic effect.
Owner:ARIZONA CANCER THERAPEUTICS
Bispecific antibody resisting CD16A antigen and MUC1antigen
ActiveCN105367660AOvercoming inadequacyOvercoming the small molecular weightHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsEffector cellAntibody-dependent cell-mediated cytotoxicity
The invention provides a bispecific antibody resisting a CD16A antigen and an MUC1 antigen. The bispecific antibody comprises a first functional domain capable of specifically recognizing the CD16A antigen of immunologic effector cells, a second functional domain capable of specifically recognizing the MUC1 antigen of liver cancer cells and a linker linking the two functional domains. According to the technical scheme, the bispecific antibody can combine tumor-associated antigen and target molecules of immune cells, can recognize the MUC1 antigen of the liver cancer cells and induce ADCC (antibody-dependent cell-mediated cytotoxicity) and recognize the CD16A antigen of the immunologic effector cells, improves the killing capacity of NK (natural killer) cells on tumor cells, effectively guides the immunologic effector cells to perform specific killing on the tumor cells and has a stable structure and a longer half-life period.
Owner:SHENZHEN BEIKE BIOTECH
Treating muc1-expressing cancers with combination therapies
ActiveUS20130274198A1Improve survivalReduce the burden onOrganic active ingredientsPeptide/protein ingredientsSurgical treatmentCombined Modality Therapy
The invention provides method of treating cancers that express MUC1 by the administration of PI3-K inhibitors in combination with MUC1-directed cancer therapies. The PI3-K inhibition may advantageously be combined with peptides that inhibit MUC1 oligomerization, or further with other standard anticancer therapies such as chemo-, radio- and surgical therapies.
Owner:GENUS ONCOLOGY +1
Immunogenic vaccine
InactiveUS20150299290A1Promote formationCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsImmunogenicityGlycolipid
A glycolipopeptide comprising a carbohydrate component, a lipid component, and a MUC1 peptide component that induces both a humoral and a cellular immune response for use as a therapeutic or prophylactic vaccine.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
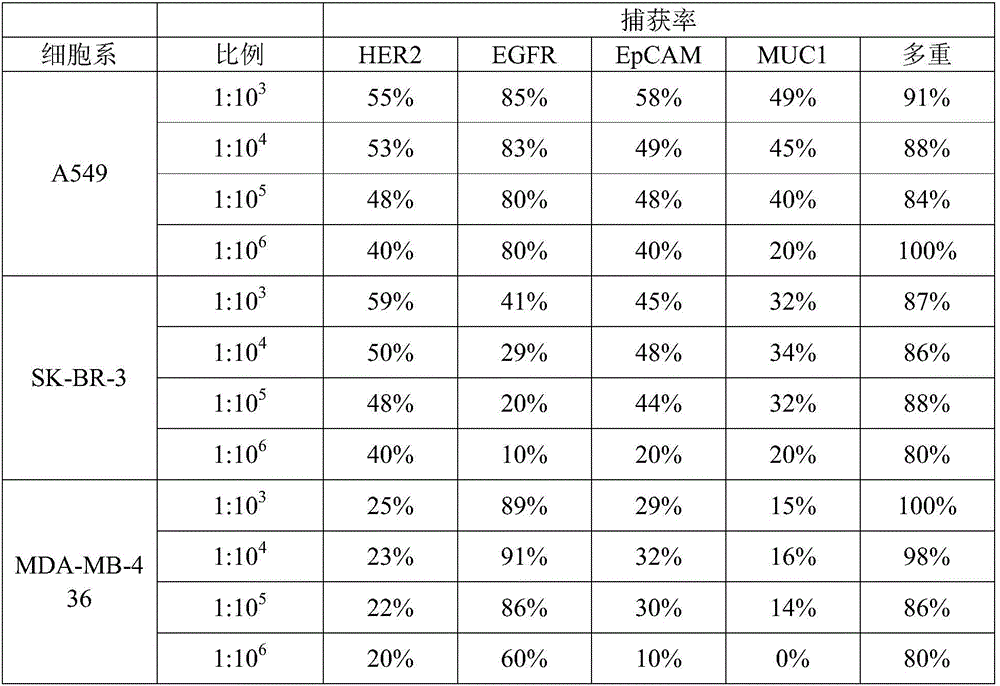
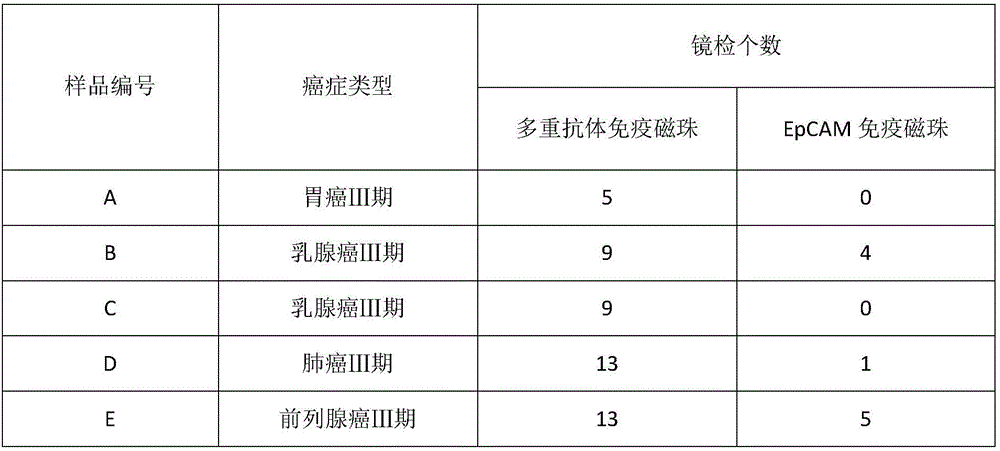
HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and preparation method thereof
ActiveCN106366197AAvoid damageStrong specificityInorganic material magnetismCarrier-bound/immobilised peptidesMedicineMicrosphere
The invention provides a HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and a preparation method thereof. The multiple antibody immunomagnetic bead includes a HER2 immunomagnetic bead, an EGFR immunomagnetic bead and an EpCAM immunomagnetic bead, and a HER2 antibody, an EGFR antibody and an EpCAM antibody are respectively coupled with magnetic microspheres to obtain the HER2 immunomagnetic bead, the EGFR immunomagnetic bead and the EpCAM immunomagnetic bead. The multiple antibody immunomagnetic bead is good in specificity and sensitivity, rapid in magnetic response, short in concentration time and high in capturing efficiency when being used for capturing CTCs (circulating tumor cells). The immunomagnetic bead is stable in nature, small in particle size, good in magnetic response and dispersibility, simple in preparation method and high in practicability.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Hla-a24 agonist epitopes of muc1-c oncoprotein and compositions and methods of use
ActiveUS20160251406A1Prevent metastatic progressionAvoid delayOrganic active ingredientsPeptide/protein ingredientsPeptideBiology
The invention provides a human cytotoxic T lymphocyte (CTL) agonist epitope from the C-terminal subunit of mucin 1 (MUC1-C), which can be used as a peptide, polypeptide (protein), and / or in vaccine or other composition for the prevention or therapy of cancer. The invention further provides a nucleic acid encoding the peptide, protein, or polypeptide, a vector comprising the nucleic acid, a cell comprising the peptide, polypeptide, nucleic acid, or vector, and compositions thereof.
Owner:UNITED STATES OF AMERICA
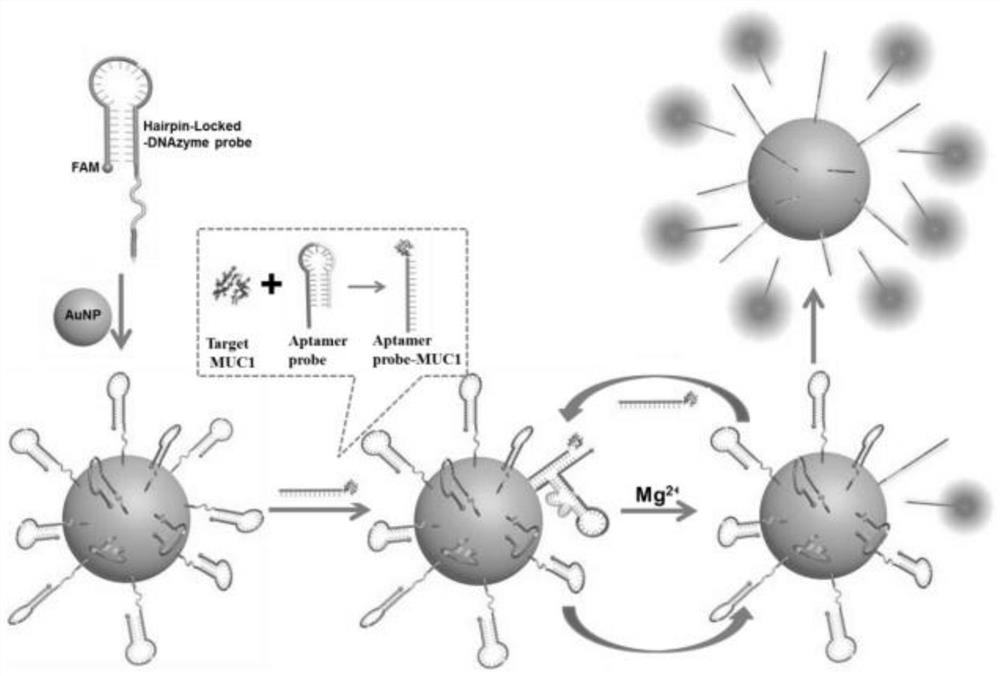
Gold nanoparticle sensor based on pin locking deoxyribozyme probe and application of gold nanoparticle sensor in detecting MUC1
ActiveCN111778315AMove quicklyContinuous signal amplificationMicrobiological testing/measurementAgainst vector-borne diseasesNanoparticleEngineering
The invention discloses a gold nanoparticle sensor based on a pin locking deoxyribozyme probe and application of the gold nanoparticle sensor in detecting MUC1. The gold nanoparticle sensor comprisesan MUC1 aptamer probe, the pin locking deoxyribozyme probe and gold nanoparticles, wherein the MUC1 aptamer probe can recognize MUC1 and release an aptamer DNA sequence; the pin locking deoxyribozymeprobe sequentially comprises a first connecting sequence, a first binding sequence, a substrate sequence, a second connecting sequence, a deoxyribozyme sequence and a second binding sequence; the first connecting sequence and the second connecting sequence are complementary to enable the pin locking deoxyribozyme probe to form a pin structure; the substrate sequence comprises a cutting site; afterthe first binding sequence, the second binding sequence and the aptamer DNA sequence are complementary, the deoxyribozyme sequence forms an active secondary structure in the catalytic core, and the 5'end of the pin locking deoxyribozyme probe is connected with a fluorophore which can be quenched by the gold nanoparticles; and the 3'end of the pin locking deoxyribozyme probe is connected with goldnanoparticles.
Owner:SHANDONG NORMAL UNIV
PD-1 gene knockout MUC1-targeting CAR-T cell as well as preparation method and application of PD-1 gene knockout MUC1-targeting CAR-T cell
PendingCN112940137AStrong specificityImprove securityNucleic acid vectorImmunoglobulinsIntracellular signallingAntigen binding
The invention belongs to the technical field of cellular immunotherapy, and particularly relates to a PD-1 gene knockout MUC1-targeting CAR-T cell as well as a preparation method and application thereof. An MUC1-targeting chimeric antigen receptor is prepared firstly, wherein the MUC1-targeting chimeric antigen receptor comprises an antigen binding structural domain, a transmembrane structural domain, a co-stimulation structural domain and an intracellular signal transduction structural domain. Tn glycosylation MUC1 is used as a target spot, the target spot specificity is high, off-target is not likely to occur, and the safety of the CAR-T cells is improved. On the basis, the PD-1 gene knockout MUC1-targeting CAR-T cell is obtained, and after the PD-1 gene is knocked out and the CAR-T cell is transfused back into a body, the CAR-T cell failure and inactivation caused by PD-L1 expressed by tumors cannot occur, so that the efficient specific cell killing effect on tumor cells is achieved, and the curative effect of the CAR-T cell is improved.
Owner:GUANGZHOU ANJIE BIOMEDICAL TECH CO LTD
Recombinant BCG vaccine based on human MUC1 repetitive sequence and GM-CSF fusion expression
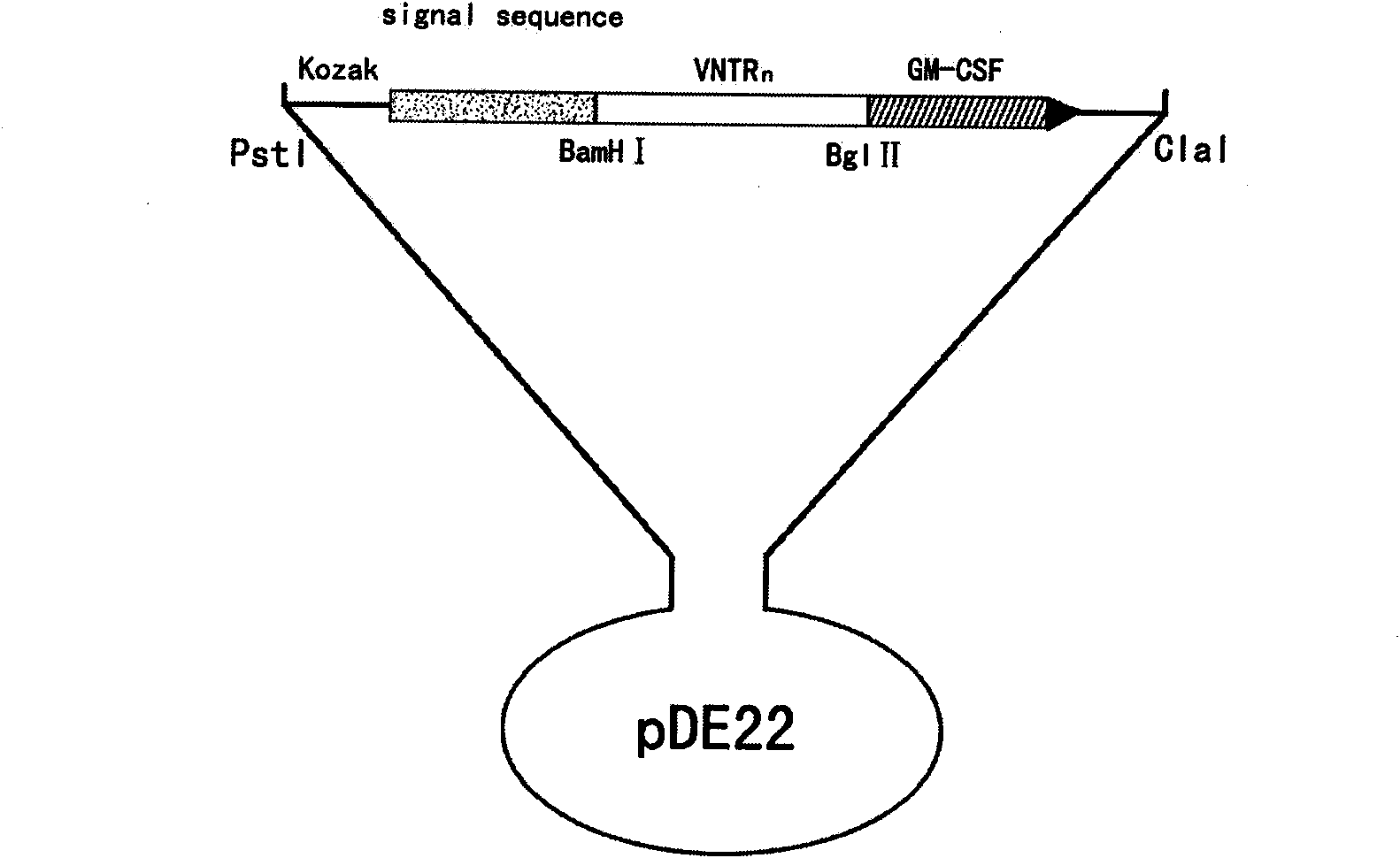
InactiveCN101575607AGood antigenicityMaintain secretory functionGenetic material ingredientsAntibody medical ingredientsSide effectAdjuvant
The invention discloses a recombinant BCG vaccine based on human MUC1 repetitive sequence and GM-CSF fusion expression. Firstly, MUC1 repetitive sequence multimers and fusion genes connected with GM-CSF genes by connecting DNA are constructed, a signal peptide sequence and a Kozak sequence are further connected, and an expression vector is constructed; and the constructed fusion genes are transfected to BCG vaccines, and applied to the preparation of anti-tumor vaccine or anti-tumor medicament by using MUC1 as a target spot. The recombinant BCG vaccine realizes effective combination of antibody, adjuvant and vaccine vector so that the immunogenic property of the MUC1 tumor vaccine achieves more effective and durable effect; and simultaneously the recombinant BCG vaccine is safe, has no toxic and side effects, and can induce specific T cell immune response and durable secretion expression of the human MUC1 repetitive sequence and GM-CSF fusion protein.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Pharmaceutical composition
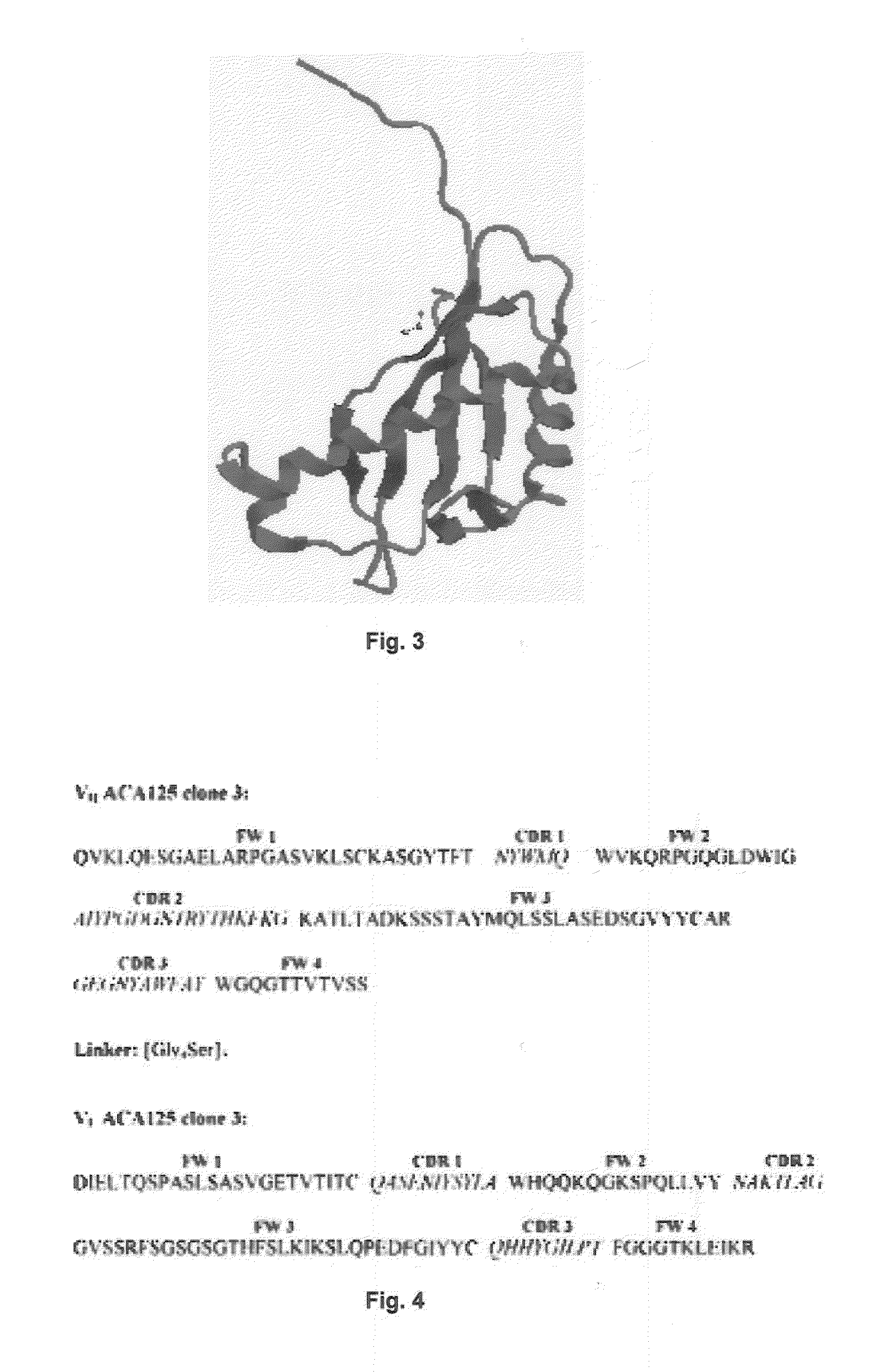
A pharmaceutical composition for intraperitoneal delivery of an anti-neoplastic agent is provided for treating cancers associated with aberrant mucin expression, preferably ovarian cancer and pancreatic, prostate, metastatic breast, bladder and lung cancers. The composition comprises nanomicelles loaded with the anti-neoplastic agent, and antibodies such as anti-MUC16, anti-MUC1 or anti-MUC4 are conjugated to these nanomicelles. The antibody-bound nanomicelles are optionally embedded in a biodegradable pH- and thermo-responsive hydrogel capable of sol-gel transition at body temperature. The pharmaceutical composition is implantable in the peritoneum, where it transforms into a semi-solid gel at the body's core temperature. In response to pH, the hydrogel swells and releases the antibody-bound nanomicelles. The nanomicelles specifically target mucin antigens on cancer cells. The anti-mucin antibodies can be internalized by the tumour cells, enabling the drug-loaded nanomicelles to gain entry and deliver the chemotherapeutic drugs inside the tumour cell.
Owner:UNIVERSITY OF THE WITWATERSRAND
Dendritic cell targeted peptide, coding gene and application
InactiveCN104387453APrecise positioningEfficient presentationMacromolecular non-active ingredientsHybrid peptidesDendritic cellRandom Peptide Library
The invention discloses a dendritic cell targeted peptide, a coding gene and an application. The dendritic cell targeted peptide is named NP. The amino acid sequence of the dendritic cell targeted peptide is shown in SEQ ID NO.1 in a sequence table. One efficiently targeted dendritic cell targeted peptide is screened out of numerous short peptides by using a dendritic cell as the target cell and reducing and screening phage random peptide libraries in a subtractive manner. The antigen presenting capacity of the dendritic cell and the capacity of the dendritic cell in killing most tumors, such as cervical cancers and breast cancers are obviously improved through fusion of the dendritic cell targeted peptide and a tumor-associated antigen MUC1. The dendritic cell targeted peptide has wide application value in diagnosis and treatment of most cancers.
Owner:SHENZHEN TONGKANG BIOLOGICAL PHARMA
Anti-mucin antibodies for early detection and treatment of pancreatic cancer
InactiveUS8574854B2Strong specificityHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsSurgeryPancreas CancersAntiendomysial antibodies
Described herein are compositions and methods of use of anti-pancreatic cancer antibodies or fragments thereof, such as murine, chimeric, humanized or human PAM4 antibodies. The antibodies show novel and useful diagnostic characteristics, such as binding with high specificity to pancreatic and other cancers, but not to normal or benign pancreatic tissues and binding to a high percentage of early stage pancreatic cancers. Preferably, the antibodies bind to pancreatic cancer mucins such as MUC1 or MUC5ac and are of use for the detection and diagnosis of early stage pancreatic cancer. In more preferred embodiments, the anti-pancreatic cancer antibodies can be used for immunoassay of serum samples, wherein the immunoassay detects a marker for early stage pancreatic cancer in serum. Most preferably, the serum is extracted with an organic phase, such as butanol, before immunoassay. Alternatively, immunoassay with PAM4 and anti-CA19.9 antibodies may be utilized to improve sensitivity for pancreatic cancer.
Owner:IMMUNOMEDICS INC
DNA tetrahedral nucleic acid framework type gastric cancer diagnosis-treatment integrated reagent and preparation method and application thereof
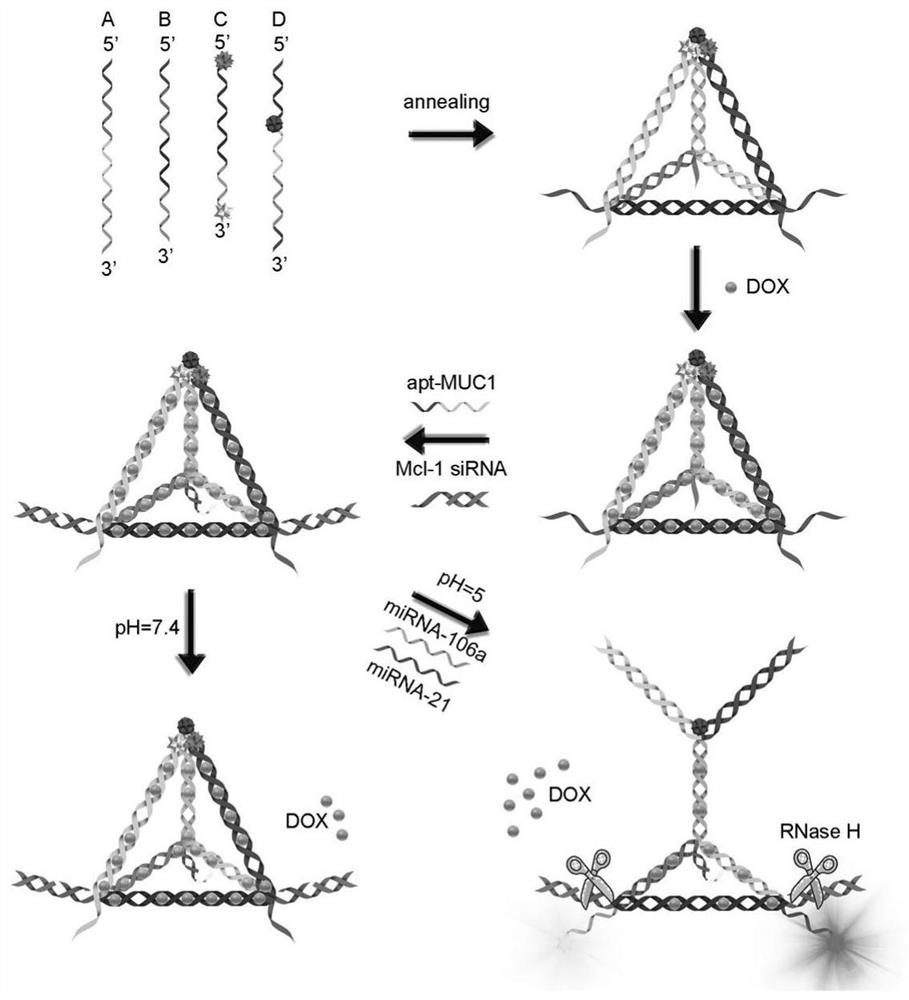
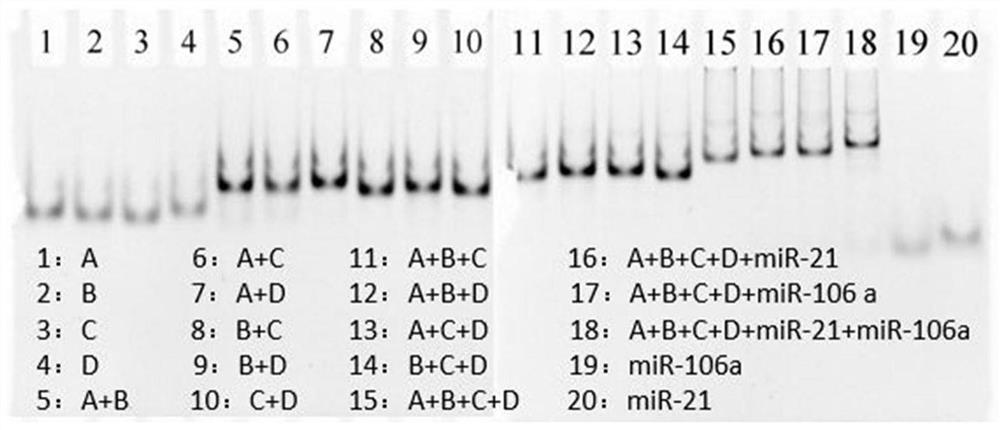
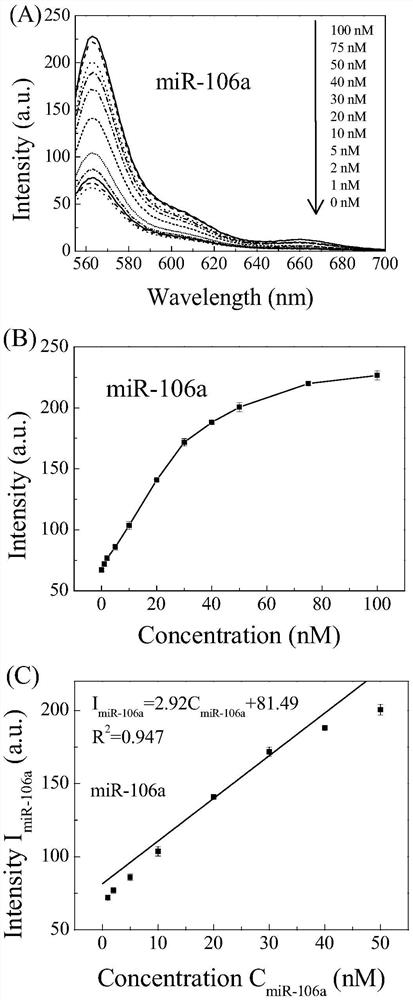
ActiveCN111803511AQuick releaseGood treatment effectOrganic active ingredientsGenetic material ingredientsCancers diagnosisTherapeutic effect
The invention discloses a DNA tetrahedral nucleic acid framework type gastric cancer diagnosis-treatment integrated reagent which includes a DNA tetrahedral nucleic acid framework which is loaded withchemotherapeutic drugs, and is obtained by connecting a MUC1 targeting aptamer with Mcl-1 siRNA. The invention further discloses a preparation method and application of the integrated reagent. The integrated reagent can perform targeting recognition of drug-resistant gastric cancer cells SGC-7901 / ADR, miRNA detection imaging and chemical drugs and gene silencing combined synergistic treatment, the internalization efficiency of the reagent in the drug-resistant gastric cancer cells and the detection efficiency of miRNA are improved, and by using the structural changes of the nucleic acid framework in a miRNA detection process, the rapid release of DOX loaded in a DNA double strand is realized. The reagent is further combined with Mcl-1 siRNA, the gene silencing of the Mcl-1 siRNA significantly improves the therapeutic effect of DOX, and the drug resistance problem in the application of nanomedicine in biological systems is solved.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for loading tumor antigen peptide to dendritic cell (DC) in targeting manner
InactiveCN103409451AImproving the ability to present tumor antigen peptide MUC1Blood/immune system cellsHybrid peptidesDendritic cellAntiendomysial antibodies
The invention provides a method for loading a tumor antigen peptide to a dendritic cell (DC) in a targeting manner, and belongs to the field of medicine technology. The method is characterized in that a fusion protein with antibody activity is tightly coupled with a DC surface receptor molecule DEC-205, and a tumor antigen peptide MUC1 is loaded to the DC in a targeting manner, so that the capacity of the DC in terms of the presentation of the tumor antigen peptide MUC1 can be greatly improved.
Owner:扬州维克斯生物科技有限公司
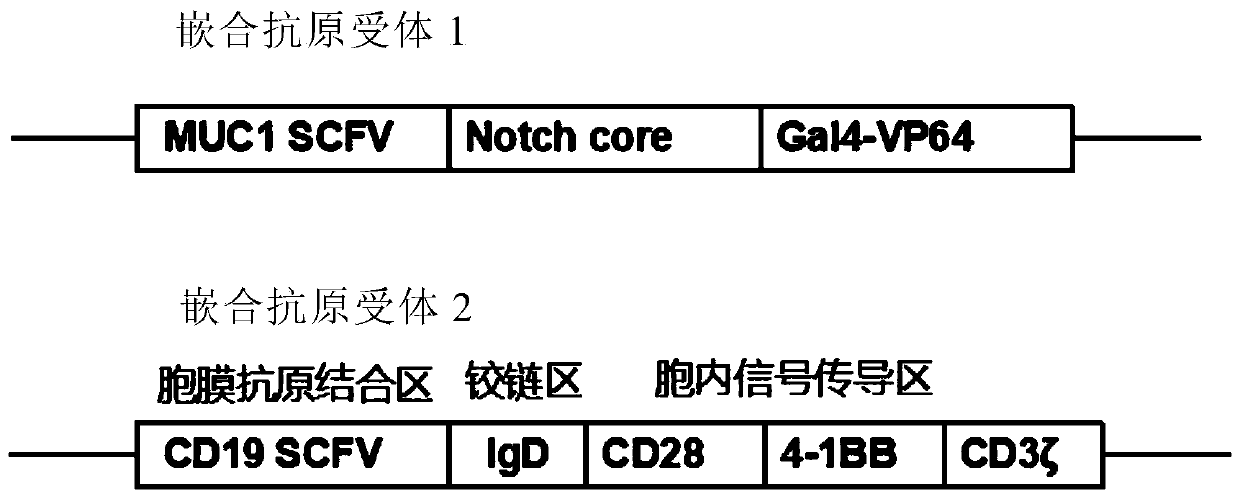
Chimeric antigen receptor combination expressed on T lymphocyte surface and application thereof
ActiveCN109748973AEfficient killingControllabilityMammal material medical ingredientsViruses/bacteriophagesHinge regionT lymphocyte
The invention provides a chimeric antigen receptor combination expressed on T lymphocyte surface and an application thereof. The chimeric antigen receptor combination comprises a chimeric antigen receptor 1 and a chimeric antigen receptor 2, wherein the chimeric antigen receptor 1, namely Anti MUC1 synNotch, comprises three parts linked sequentially as follows: SCFV, Notch core and transcription factor Gal4-VP64, which specifically recognize an MUC1 protein; the chimeric antigen receptor 2, namely Anti CD19 CAR, has an extracellular binding region, a hinge region and an intracellular signal region linked sequentially as follows: SCFV, IgD, CD28-4-1BB-CD3 zeta, which specifically recognize a CD19 protein. The chimeric antigen receptor combination is expressed on the surface of T lymphocytesand has the effect of specifically recognizing MUC1 and CD19 proteins.
Owner:深圳市芥至和生物科技有限公司
Method for rapidly assaying tumor-associated small peptide MUC1
InactiveCN105784658AAchieve specific identificationEasy to operateFluorescence/phosphorescenceFluoProbesSmall peptide
The invention discloses a method for rapidly assaying tumor-associated small peptide MUC1, which relates to the technical field of fluorescent probe assay. By utilizing the characteristic of specific recognition between the aptamer and the target small peptide, a special aptamer sequence is designed, and thereby the purpose of specific recognition of target objects is achieved; a signal amplification strategy is carried out in combination with the hybridization reaction of two types of hairpin probes, and thereby the sensitive assay of the target small peptide is realized. The method includes four steps, i.e. probe pre-denaturation, mixing reaction, background quenching and fluorescent assay, operation is easy and fast, time and labor can be saved, and a novel method is provided for the assay of tumor-associated small peptide MUC1.
Owner:UNIV OF JINAN
Medicine for treating and/or preventing cancer and application
PendingCN111298111AImprove anti-tumor effectHigh killing efficiencyAntibody ingredientsImmunological disordersCancer preventionAntiendomysial antibodies
The invention relates to a medicine for treating and / or preventing cancer and an application. The medicine of the invention includes a recombinant MUC1-MBP fusion protein vaccine and a PD-1 antibody.The medicine is expected to break an immune tolerance state of a tumor microenvironment in vivo and enhance the MUC1 specific anti-tumor immune response, and is expected to completely clear tumors tobring gospel to the majority of cancer patients.
Owner:长春康悦生物科技有限公司
MUC1 tandem repeat sequences polypeptide, preparation technique thereof and use as anti-tumor medicament
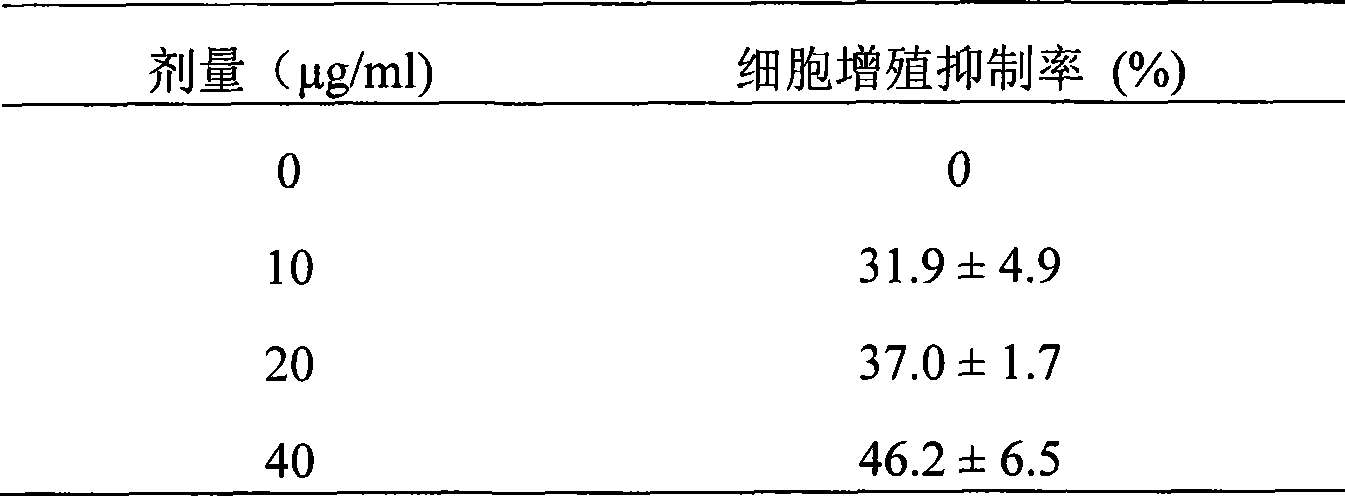
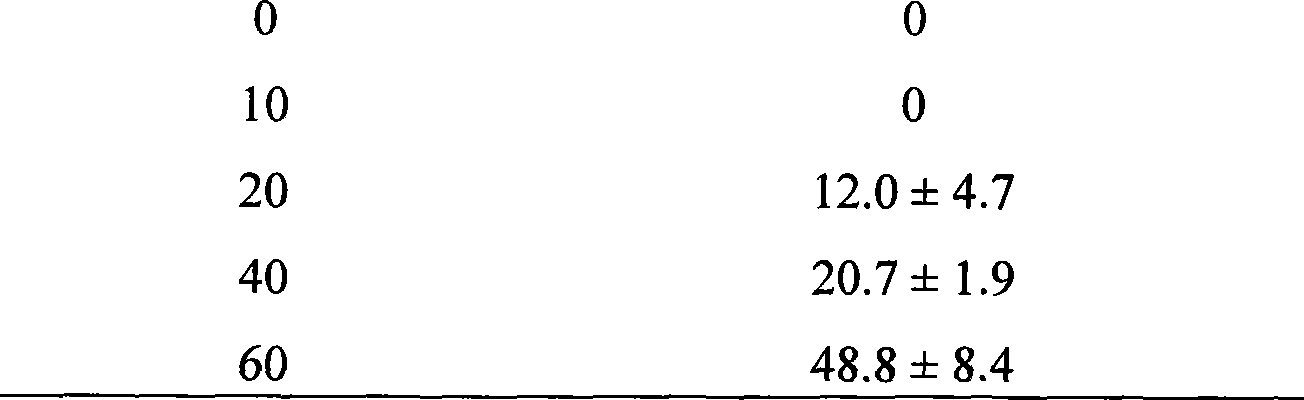
InactiveCN101412749APrevent proliferationGrowth inhibitionPeptide/protein ingredientsFermentationEscherichia coliEnzyme digestion
The invention provides polypeptide with tandem repeat sequences for MUC1 and a preparation technology and application as an anti-tumor drug thereof. Tandem repeat sequences of 20 amino acids of the MUC1 are adopted, cDNA fragments of 1 to 10 tandem repeat sequences are selected to link into a pGEX-4T-1 carrier, and a recombinant plasmid is transformed into colon bacillus DH5 alpha. A colony is transformed through the screening of penbritin, and the fragments are inserted through enzyme digestion and gene sequencing analysis. The recombinant plasmid is transformed into the colon bacillus DH5 alpha which is inducedly expressed through 0.3mM IPTG, and a steady expression strain pGEX-MUC1 / DH5 alpha is obtained through the screening. The bacteria pyrolysis is performed by boiling, the expression of MUC1-GST fusion protein is analyzed, and a specific expression band is appraised. Engineering bacteria are cultivated, a supernatant fluid is retained after the ultrasonication, and human MUC1-GST fusion protein is purified through a Glutathione-Sepharose 4B affinity layer. Then pure MUC1 polypeptide is obtained through thrombin pyrolysis and is prepared into a pharmaceutical preparation.
Owner:台桂香
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com